User login
More states nix nonconsensual pelvic exams by med students
Performing intimate exams under anesthesia (EUA) is a standard part of medical training. Yet,
“Whenever I talk about this at conferences around the country, people always come up to me and say it’s still happening at their institutions,” Lori Bruce, MA, MBE, HEC-C, associate director of the Interdisciplinary Center for Bioethics at Yale University, New Haven, Conn., told this news organization.
Most think this is a women’s issue, which occurs only in unconscious patients, she said. But Ms. Bruce found otherwise in a survey last year in which she polled the general public about their intimate exam experiences.
“Unconsented exams happen much more than we imagined, and they happen as often to men [having] prostate exams without consent as to women. Black [respondents] were nearly four times more likely to have reported receiving an unconsented intimate pelvic or prostate exam,” she said, based on her research. And Ms. Bruce believes it can happen across the economic spectrum.
Concern about unconsented EUAs arose in the early 2000s. In a study at that time, 75% of medical students reported that their patients had not given consent to be examined during surgical procedures. An ethics committee of the American College of Obstetricians and Gynecologists published guidelines for EUAs and states began passing legislation with patient protections and medical training consent policies.
California is believed to be the first to adopt legislation outlawing unconsented pelvic exams for training purposes in 2003, followed by Virginia in 2007, along with a handful of other states.
In 2019, on the heels of the #MeToo movement and renewed calls to end unconsented exams, more patients and providers began to speak publicly about their experiences with the practice. Some posted on social media using the #MeTooPelvic hashtag. In 2022, an award-winning documentary was also released about consent, “At Your Cervix.”More states subsequently passed legislation, and some medical schools strengthened their EUA consent policies.
Today, nearly half the states in the country have enacted laws against unconsented intimate EUAs, with some carrying misdemeanor charges for both the individual conducting the exam and the supervising physician. Other states leave open the option to fine the physician and revoke or suspend medical licenses.
Much of the new legislation requires explicit consent for intimate exams involving the pelvis, prostate, and rectum, with exceptions for emergency procedures and, in some cases, the collection of court-ordered forensic evidence. In addition, several states, including Colorado, Indiana, and Ohio, have pending or recently introduced bills. Last month, sister bills in Missouri passed the House and Senate, gaining more traction than previous legislative attempts. A similar bill was introduced in the Kansas House several times, including this year, and is expected to be on the agenda again in the next session.
Intimate exams on patients without consent are “unethical and unacceptable,” said Alison Whelan, MD, chief academic officer of the Association of American Medical Colleges. Although medical students learn sensitive procedures through simulation labs and gynecological teaching associates – individuals specifically trained to help students develop physical exam skills – EUAs require strict adherence to widely accepted guidelines.
“Learners in the clinical setting should only perform such examinations for teaching purposes when the exam is explicitly consented to, related to the planned procedure, performed by a student who is recognized by the patient as a part of their care team, and done under direct supervision by an educator,” Dr. Whelan said.
Medical students bear moral burden
Arthur Caplan, PhD, director of medical ethics at New York University, has called unconsented intimate exams a “cousin issue” to abusive predatory behavior.
If the public is outraged that physicians “have misused their authority with athletes, then we should be equally outraged if that authority, even for a higher purpose [like] teaching and training, is still misused in terms of getting permission and consent,” he said in a video discussing Connecticut’s legislation to strengthen intimate exam requirements, which went into effect Jan. 1.
Advocates of stricter EUA consent policies say the variability in consent practices destroys patient trust by ignoring the basic principles of respect and autonomy. Because patients are usually unaware a violation has occurred, reporting typically depends on medical students raising questions with educators and attendings, which they may hesitate to do for fear of repercussions.
Current practices, such as patients signing consent documents in the outpatient setting where students aren’t always privy to the discussion, contribute to the lack of transparency, Karampreet Kaur, MD, a 2nd-year ob.gyn. resident at the Hospital of the University of Pennsylvania, Philadelphia, said.
A 2019 survey of medical students by Elle magazine found that nearly half did not meet patients before conducting an intimate EUA. Of the 92% who performed a pelvic EUA, 61% reported doing so without obtaining explicit patient consent.
Dr. Kaur recently coauthored a survey of students from six medical schools and found that 84% completed at least one pelvic EUA during their ob.gyn. clerkships. About half of the students surveyed observed patients giving informed consent most or every time. Of those, 67% reported they never or rarely witnessed an explicit explanation that a medical student may perform a pelvic EUA.
This burden weighs on the consciences of medical students. Respondents reported that they wanted to honor patient autonomy but felt they lacked the authority to object to pelvic EUAs when consent was unclear, which led to significant emotional distress.
“It’s not that physicians don’t care,” Dr. Kaur said. “I think most want to make sure patients feel safe and fully informed of the care they are receiving.”
To consent or not
Incorporating a separate EUA consent form, typically signed during a preoperative visit but occasionally on the day of surgery, offers one potential solution as it ensures “clear and consistent language is used and forces documentation of this conversation,” said Dr. Kaur. At her current institution, providers and medical students must review charted EUA documentation, then that information is “made clear to attendings, fellows, residents, students, and even the OR staff,” she said.
In Dr. Kaur’s survey, 11% of respondents supported a separate consent. Another study of 3rd- and 4th-year medical students published last year found that 45% agreed with having a separate signature line on the surgical consent form.
Legislation introduced recently in Colorado states that medical students must meet the patient, and patients must receive a written or electronic document titled, in at least 18-point bolded font, “consent for examination of breasts, pelvic region, rectum, and/or prostate.” The form must also include the names of medical students performing or observing an intimate exam for educational purposes.
Elizabeth Newman, MPP, public policy director at the Colorado Coalition Against Sexual Assault and supporter of the state’s intimate exam bill, said the legislation will allow medical students to learn the intricacies of these sensitive body systems and provide better patient care, particularly following the rollback of Roe v. Wade.
“Abortion is available and accessible in Colorado, and we are surrounded by states where it’s not,” said Ms. Newman. “Medical students in states where it’s outright banned are coming to Colorado to learn how to provide abortion care in their residencies and fellowships, so we want to maintain that access and not take those learning opportunities away with this law.”
Opponents of a separate form say it complicates the consent process. Dr. Kaur said she originally thought it would involve a lot of extra work, but it only takes 3-5 minutes. Few patients decline the exam after the conversation, and students benefit from the clear guidelines and transparency, she said.
“I had hoped that the many medical association guidelines [supporting] explicit consent would have influenced hospital policy, but it did not have that effect,” said Ms. Bruce, adding that recent legislative efforts have largely been driven by concerned bioethicists, lawmakers, and some medical students and physicians. “It all circles back to the patient having the right to refuse; it’s their body.”
A version of this article first appeared on Medscape.com.
Performing intimate exams under anesthesia (EUA) is a standard part of medical training. Yet,
“Whenever I talk about this at conferences around the country, people always come up to me and say it’s still happening at their institutions,” Lori Bruce, MA, MBE, HEC-C, associate director of the Interdisciplinary Center for Bioethics at Yale University, New Haven, Conn., told this news organization.
Most think this is a women’s issue, which occurs only in unconscious patients, she said. But Ms. Bruce found otherwise in a survey last year in which she polled the general public about their intimate exam experiences.
“Unconsented exams happen much more than we imagined, and they happen as often to men [having] prostate exams without consent as to women. Black [respondents] were nearly four times more likely to have reported receiving an unconsented intimate pelvic or prostate exam,” she said, based on her research. And Ms. Bruce believes it can happen across the economic spectrum.
Concern about unconsented EUAs arose in the early 2000s. In a study at that time, 75% of medical students reported that their patients had not given consent to be examined during surgical procedures. An ethics committee of the American College of Obstetricians and Gynecologists published guidelines for EUAs and states began passing legislation with patient protections and medical training consent policies.
California is believed to be the first to adopt legislation outlawing unconsented pelvic exams for training purposes in 2003, followed by Virginia in 2007, along with a handful of other states.
In 2019, on the heels of the #MeToo movement and renewed calls to end unconsented exams, more patients and providers began to speak publicly about their experiences with the practice. Some posted on social media using the #MeTooPelvic hashtag. In 2022, an award-winning documentary was also released about consent, “At Your Cervix.”More states subsequently passed legislation, and some medical schools strengthened their EUA consent policies.
Today, nearly half the states in the country have enacted laws against unconsented intimate EUAs, with some carrying misdemeanor charges for both the individual conducting the exam and the supervising physician. Other states leave open the option to fine the physician and revoke or suspend medical licenses.
Much of the new legislation requires explicit consent for intimate exams involving the pelvis, prostate, and rectum, with exceptions for emergency procedures and, in some cases, the collection of court-ordered forensic evidence. In addition, several states, including Colorado, Indiana, and Ohio, have pending or recently introduced bills. Last month, sister bills in Missouri passed the House and Senate, gaining more traction than previous legislative attempts. A similar bill was introduced in the Kansas House several times, including this year, and is expected to be on the agenda again in the next session.
Intimate exams on patients without consent are “unethical and unacceptable,” said Alison Whelan, MD, chief academic officer of the Association of American Medical Colleges. Although medical students learn sensitive procedures through simulation labs and gynecological teaching associates – individuals specifically trained to help students develop physical exam skills – EUAs require strict adherence to widely accepted guidelines.
“Learners in the clinical setting should only perform such examinations for teaching purposes when the exam is explicitly consented to, related to the planned procedure, performed by a student who is recognized by the patient as a part of their care team, and done under direct supervision by an educator,” Dr. Whelan said.
Medical students bear moral burden
Arthur Caplan, PhD, director of medical ethics at New York University, has called unconsented intimate exams a “cousin issue” to abusive predatory behavior.
If the public is outraged that physicians “have misused their authority with athletes, then we should be equally outraged if that authority, even for a higher purpose [like] teaching and training, is still misused in terms of getting permission and consent,” he said in a video discussing Connecticut’s legislation to strengthen intimate exam requirements, which went into effect Jan. 1.
Advocates of stricter EUA consent policies say the variability in consent practices destroys patient trust by ignoring the basic principles of respect and autonomy. Because patients are usually unaware a violation has occurred, reporting typically depends on medical students raising questions with educators and attendings, which they may hesitate to do for fear of repercussions.
Current practices, such as patients signing consent documents in the outpatient setting where students aren’t always privy to the discussion, contribute to the lack of transparency, Karampreet Kaur, MD, a 2nd-year ob.gyn. resident at the Hospital of the University of Pennsylvania, Philadelphia, said.
A 2019 survey of medical students by Elle magazine found that nearly half did not meet patients before conducting an intimate EUA. Of the 92% who performed a pelvic EUA, 61% reported doing so without obtaining explicit patient consent.
Dr. Kaur recently coauthored a survey of students from six medical schools and found that 84% completed at least one pelvic EUA during their ob.gyn. clerkships. About half of the students surveyed observed patients giving informed consent most or every time. Of those, 67% reported they never or rarely witnessed an explicit explanation that a medical student may perform a pelvic EUA.
This burden weighs on the consciences of medical students. Respondents reported that they wanted to honor patient autonomy but felt they lacked the authority to object to pelvic EUAs when consent was unclear, which led to significant emotional distress.
“It’s not that physicians don’t care,” Dr. Kaur said. “I think most want to make sure patients feel safe and fully informed of the care they are receiving.”
To consent or not
Incorporating a separate EUA consent form, typically signed during a preoperative visit but occasionally on the day of surgery, offers one potential solution as it ensures “clear and consistent language is used and forces documentation of this conversation,” said Dr. Kaur. At her current institution, providers and medical students must review charted EUA documentation, then that information is “made clear to attendings, fellows, residents, students, and even the OR staff,” she said.
In Dr. Kaur’s survey, 11% of respondents supported a separate consent. Another study of 3rd- and 4th-year medical students published last year found that 45% agreed with having a separate signature line on the surgical consent form.
Legislation introduced recently in Colorado states that medical students must meet the patient, and patients must receive a written or electronic document titled, in at least 18-point bolded font, “consent for examination of breasts, pelvic region, rectum, and/or prostate.” The form must also include the names of medical students performing or observing an intimate exam for educational purposes.
Elizabeth Newman, MPP, public policy director at the Colorado Coalition Against Sexual Assault and supporter of the state’s intimate exam bill, said the legislation will allow medical students to learn the intricacies of these sensitive body systems and provide better patient care, particularly following the rollback of Roe v. Wade.
“Abortion is available and accessible in Colorado, and we are surrounded by states where it’s not,” said Ms. Newman. “Medical students in states where it’s outright banned are coming to Colorado to learn how to provide abortion care in their residencies and fellowships, so we want to maintain that access and not take those learning opportunities away with this law.”
Opponents of a separate form say it complicates the consent process. Dr. Kaur said she originally thought it would involve a lot of extra work, but it only takes 3-5 minutes. Few patients decline the exam after the conversation, and students benefit from the clear guidelines and transparency, she said.
“I had hoped that the many medical association guidelines [supporting] explicit consent would have influenced hospital policy, but it did not have that effect,” said Ms. Bruce, adding that recent legislative efforts have largely been driven by concerned bioethicists, lawmakers, and some medical students and physicians. “It all circles back to the patient having the right to refuse; it’s their body.”
A version of this article first appeared on Medscape.com.
Performing intimate exams under anesthesia (EUA) is a standard part of medical training. Yet,
“Whenever I talk about this at conferences around the country, people always come up to me and say it’s still happening at their institutions,” Lori Bruce, MA, MBE, HEC-C, associate director of the Interdisciplinary Center for Bioethics at Yale University, New Haven, Conn., told this news organization.
Most think this is a women’s issue, which occurs only in unconscious patients, she said. But Ms. Bruce found otherwise in a survey last year in which she polled the general public about their intimate exam experiences.
“Unconsented exams happen much more than we imagined, and they happen as often to men [having] prostate exams without consent as to women. Black [respondents] were nearly four times more likely to have reported receiving an unconsented intimate pelvic or prostate exam,” she said, based on her research. And Ms. Bruce believes it can happen across the economic spectrum.
Concern about unconsented EUAs arose in the early 2000s. In a study at that time, 75% of medical students reported that their patients had not given consent to be examined during surgical procedures. An ethics committee of the American College of Obstetricians and Gynecologists published guidelines for EUAs and states began passing legislation with patient protections and medical training consent policies.
California is believed to be the first to adopt legislation outlawing unconsented pelvic exams for training purposes in 2003, followed by Virginia in 2007, along with a handful of other states.
In 2019, on the heels of the #MeToo movement and renewed calls to end unconsented exams, more patients and providers began to speak publicly about their experiences with the practice. Some posted on social media using the #MeTooPelvic hashtag. In 2022, an award-winning documentary was also released about consent, “At Your Cervix.”More states subsequently passed legislation, and some medical schools strengthened their EUA consent policies.
Today, nearly half the states in the country have enacted laws against unconsented intimate EUAs, with some carrying misdemeanor charges for both the individual conducting the exam and the supervising physician. Other states leave open the option to fine the physician and revoke or suspend medical licenses.
Much of the new legislation requires explicit consent for intimate exams involving the pelvis, prostate, and rectum, with exceptions for emergency procedures and, in some cases, the collection of court-ordered forensic evidence. In addition, several states, including Colorado, Indiana, and Ohio, have pending or recently introduced bills. Last month, sister bills in Missouri passed the House and Senate, gaining more traction than previous legislative attempts. A similar bill was introduced in the Kansas House several times, including this year, and is expected to be on the agenda again in the next session.
Intimate exams on patients without consent are “unethical and unacceptable,” said Alison Whelan, MD, chief academic officer of the Association of American Medical Colleges. Although medical students learn sensitive procedures through simulation labs and gynecological teaching associates – individuals specifically trained to help students develop physical exam skills – EUAs require strict adherence to widely accepted guidelines.
“Learners in the clinical setting should only perform such examinations for teaching purposes when the exam is explicitly consented to, related to the planned procedure, performed by a student who is recognized by the patient as a part of their care team, and done under direct supervision by an educator,” Dr. Whelan said.
Medical students bear moral burden
Arthur Caplan, PhD, director of medical ethics at New York University, has called unconsented intimate exams a “cousin issue” to abusive predatory behavior.
If the public is outraged that physicians “have misused their authority with athletes, then we should be equally outraged if that authority, even for a higher purpose [like] teaching and training, is still misused in terms of getting permission and consent,” he said in a video discussing Connecticut’s legislation to strengthen intimate exam requirements, which went into effect Jan. 1.
Advocates of stricter EUA consent policies say the variability in consent practices destroys patient trust by ignoring the basic principles of respect and autonomy. Because patients are usually unaware a violation has occurred, reporting typically depends on medical students raising questions with educators and attendings, which they may hesitate to do for fear of repercussions.
Current practices, such as patients signing consent documents in the outpatient setting where students aren’t always privy to the discussion, contribute to the lack of transparency, Karampreet Kaur, MD, a 2nd-year ob.gyn. resident at the Hospital of the University of Pennsylvania, Philadelphia, said.
A 2019 survey of medical students by Elle magazine found that nearly half did not meet patients before conducting an intimate EUA. Of the 92% who performed a pelvic EUA, 61% reported doing so without obtaining explicit patient consent.
Dr. Kaur recently coauthored a survey of students from six medical schools and found that 84% completed at least one pelvic EUA during their ob.gyn. clerkships. About half of the students surveyed observed patients giving informed consent most or every time. Of those, 67% reported they never or rarely witnessed an explicit explanation that a medical student may perform a pelvic EUA.
This burden weighs on the consciences of medical students. Respondents reported that they wanted to honor patient autonomy but felt they lacked the authority to object to pelvic EUAs when consent was unclear, which led to significant emotional distress.
“It’s not that physicians don’t care,” Dr. Kaur said. “I think most want to make sure patients feel safe and fully informed of the care they are receiving.”
To consent or not
Incorporating a separate EUA consent form, typically signed during a preoperative visit but occasionally on the day of surgery, offers one potential solution as it ensures “clear and consistent language is used and forces documentation of this conversation,” said Dr. Kaur. At her current institution, providers and medical students must review charted EUA documentation, then that information is “made clear to attendings, fellows, residents, students, and even the OR staff,” she said.
In Dr. Kaur’s survey, 11% of respondents supported a separate consent. Another study of 3rd- and 4th-year medical students published last year found that 45% agreed with having a separate signature line on the surgical consent form.
Legislation introduced recently in Colorado states that medical students must meet the patient, and patients must receive a written or electronic document titled, in at least 18-point bolded font, “consent for examination of breasts, pelvic region, rectum, and/or prostate.” The form must also include the names of medical students performing or observing an intimate exam for educational purposes.
Elizabeth Newman, MPP, public policy director at the Colorado Coalition Against Sexual Assault and supporter of the state’s intimate exam bill, said the legislation will allow medical students to learn the intricacies of these sensitive body systems and provide better patient care, particularly following the rollback of Roe v. Wade.
“Abortion is available and accessible in Colorado, and we are surrounded by states where it’s not,” said Ms. Newman. “Medical students in states where it’s outright banned are coming to Colorado to learn how to provide abortion care in their residencies and fellowships, so we want to maintain that access and not take those learning opportunities away with this law.”
Opponents of a separate form say it complicates the consent process. Dr. Kaur said she originally thought it would involve a lot of extra work, but it only takes 3-5 minutes. Few patients decline the exam after the conversation, and students benefit from the clear guidelines and transparency, she said.
“I had hoped that the many medical association guidelines [supporting] explicit consent would have influenced hospital policy, but it did not have that effect,” said Ms. Bruce, adding that recent legislative efforts have largely been driven by concerned bioethicists, lawmakers, and some medical students and physicians. “It all circles back to the patient having the right to refuse; it’s their body.”
A version of this article first appeared on Medscape.com.
Vaginal microbiome does not affect infant gut microbiome
The findings suggest that practices such as vaginal seeding are ineffective.
A longitudinal, prospective cohort study of more than 600 pregnant Canadian women and their newborns showed significant differences in an infant’s stool composition by delivery mode at 10 days post partum, but the differences could not be explained by the mother’s vaginal microbiome, and they effectively disappeared by 3 months.
The findings were surprising, Scott Dos Santos, a PhD candidate at the University of Saskatchewan in Saskatoon, told this news organization. “The bacteria living in the maternal vagina are the first microbes that vaginally delivered infants are exposed to. … so it sounds intuitive that different kinds of vaginal microbiomes could end up influencing the development of a baby’s gut microbiome in different ways. But the maternal vaginal microbiome didn’t seem to have any role in predicting what the infant stool microbiome looked like.”
Therefore, women should not be concerned about cesarean delivery having an adverse effect on their baby’s gut microbiome, said Mr. Dos Santos. Moreover, “vaginal seeding is not safe or advised. Professional bodies, including the Society of Obstetricians and Gynecologists of Canada and the American College of Obstetricians and Gynecologists, strongly advise against this practice.”
The study was published online in Frontiers in Cellular and Infection Microbiology.
Independent communities
The investigators analyzed vaginal and stool microbiome profiles from 442 mother-infant dyads. The mothers were healthy, low-risk women who delivered at term. They were recruited into the Maternal Microbiome LEGACY Project from three hospitals in British Columbia.
The mean age of the mothers at delivery was 34.6 years, which is typical of the study hospitals’ delivery populations. Participants identified themselves as White (54.7%), Asian (21.2%), South Asian (8.3%), and of other ethnicities.
A nurse, midwife, or clinician collected maternal vaginal swabs of the posterior fornix and lateral vaginal wall at first presentation to the labor and delivery area. Neonatal meconium, which was defined as the first stool specimen collected within 72 hours of birth, and two infant stool samples were collected at follow-up visits at 10 days and 3 months post partum.
A principal component analysis of infant stool microbiomes showed no significant clustering of microbiome profiles at 10 days or 3 months by maternal community state types (that is, microbial species).
Correspondence analyses also showed no coclustering of maternal and infant clusters at either time. In addition, there were no differences in the distribution of maternal vaginal microbiome clusters among infant stool microbiome clusters, regardless of delivery mode.
Vaginal microbiome clusters were distributed across infant stool clusters in proportion to their frequency in the overall maternal population, indicating that the two communities were independent of each other.
Intrapartum antibiotic administration was identified as a confounder of infant stool microbiome differences and was associated with lower abundances of Escherichia coli, Bacteroides vulgatus, Bifidobacterium longum, and Parabacteroides distasonis.
“Our findings demonstrate that maternal vaginal microbiome composition at delivery does not affect infant stool microbiome composition and development, suggesting that practices to amend infant stool microbiome composition focus on factors other than maternal vaginal microbes,” the authors conclude.
More evidence needed
Commenting on the study, Emily H. Adhikari, MD, assistant professor of obstetrics and gynecology at UT Southwestern Medical Center in Dallas, and medical director of perinatal infectious diseases for the Parkland Health and Hospital System, said, “These findings contribute significantly more data to an understudied area of research into factors that affect the infant gut microbiome from the earliest hours of life. Prior studies have been small and often conflicting, and the authors reference recent larger studies, which corroborate their findings.”
The data regarding whether delivery mode or antibiotic-associated differences in infant microbiomes persist remain controversial, said Dr. Adhikari. “More evidence is needed involving a more ethnically diverse sampling of patients.” In addition, prospectively evaluating vaginal seeding in a rigorously designed clinical trial setting is “imperative to understand any potential benefit and certainly to understand the potential harms of the practice. To date, this does not exist.”
The study was funded by a Canadian Institutes of Health Research grant. Mr. Dos Santos and Dr. Adhikari have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The findings suggest that practices such as vaginal seeding are ineffective.
A longitudinal, prospective cohort study of more than 600 pregnant Canadian women and their newborns showed significant differences in an infant’s stool composition by delivery mode at 10 days post partum, but the differences could not be explained by the mother’s vaginal microbiome, and they effectively disappeared by 3 months.
The findings were surprising, Scott Dos Santos, a PhD candidate at the University of Saskatchewan in Saskatoon, told this news organization. “The bacteria living in the maternal vagina are the first microbes that vaginally delivered infants are exposed to. … so it sounds intuitive that different kinds of vaginal microbiomes could end up influencing the development of a baby’s gut microbiome in different ways. But the maternal vaginal microbiome didn’t seem to have any role in predicting what the infant stool microbiome looked like.”
Therefore, women should not be concerned about cesarean delivery having an adverse effect on their baby’s gut microbiome, said Mr. Dos Santos. Moreover, “vaginal seeding is not safe or advised. Professional bodies, including the Society of Obstetricians and Gynecologists of Canada and the American College of Obstetricians and Gynecologists, strongly advise against this practice.”
The study was published online in Frontiers in Cellular and Infection Microbiology.
Independent communities
The investigators analyzed vaginal and stool microbiome profiles from 442 mother-infant dyads. The mothers were healthy, low-risk women who delivered at term. They were recruited into the Maternal Microbiome LEGACY Project from three hospitals in British Columbia.
The mean age of the mothers at delivery was 34.6 years, which is typical of the study hospitals’ delivery populations. Participants identified themselves as White (54.7%), Asian (21.2%), South Asian (8.3%), and of other ethnicities.
A nurse, midwife, or clinician collected maternal vaginal swabs of the posterior fornix and lateral vaginal wall at first presentation to the labor and delivery area. Neonatal meconium, which was defined as the first stool specimen collected within 72 hours of birth, and two infant stool samples were collected at follow-up visits at 10 days and 3 months post partum.
A principal component analysis of infant stool microbiomes showed no significant clustering of microbiome profiles at 10 days or 3 months by maternal community state types (that is, microbial species).
Correspondence analyses also showed no coclustering of maternal and infant clusters at either time. In addition, there were no differences in the distribution of maternal vaginal microbiome clusters among infant stool microbiome clusters, regardless of delivery mode.
Vaginal microbiome clusters were distributed across infant stool clusters in proportion to their frequency in the overall maternal population, indicating that the two communities were independent of each other.
Intrapartum antibiotic administration was identified as a confounder of infant stool microbiome differences and was associated with lower abundances of Escherichia coli, Bacteroides vulgatus, Bifidobacterium longum, and Parabacteroides distasonis.
“Our findings demonstrate that maternal vaginal microbiome composition at delivery does not affect infant stool microbiome composition and development, suggesting that practices to amend infant stool microbiome composition focus on factors other than maternal vaginal microbes,” the authors conclude.
More evidence needed
Commenting on the study, Emily H. Adhikari, MD, assistant professor of obstetrics and gynecology at UT Southwestern Medical Center in Dallas, and medical director of perinatal infectious diseases for the Parkland Health and Hospital System, said, “These findings contribute significantly more data to an understudied area of research into factors that affect the infant gut microbiome from the earliest hours of life. Prior studies have been small and often conflicting, and the authors reference recent larger studies, which corroborate their findings.”
The data regarding whether delivery mode or antibiotic-associated differences in infant microbiomes persist remain controversial, said Dr. Adhikari. “More evidence is needed involving a more ethnically diverse sampling of patients.” In addition, prospectively evaluating vaginal seeding in a rigorously designed clinical trial setting is “imperative to understand any potential benefit and certainly to understand the potential harms of the practice. To date, this does not exist.”
The study was funded by a Canadian Institutes of Health Research grant. Mr. Dos Santos and Dr. Adhikari have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The findings suggest that practices such as vaginal seeding are ineffective.
A longitudinal, prospective cohort study of more than 600 pregnant Canadian women and their newborns showed significant differences in an infant’s stool composition by delivery mode at 10 days post partum, but the differences could not be explained by the mother’s vaginal microbiome, and they effectively disappeared by 3 months.
The findings were surprising, Scott Dos Santos, a PhD candidate at the University of Saskatchewan in Saskatoon, told this news organization. “The bacteria living in the maternal vagina are the first microbes that vaginally delivered infants are exposed to. … so it sounds intuitive that different kinds of vaginal microbiomes could end up influencing the development of a baby’s gut microbiome in different ways. But the maternal vaginal microbiome didn’t seem to have any role in predicting what the infant stool microbiome looked like.”
Therefore, women should not be concerned about cesarean delivery having an adverse effect on their baby’s gut microbiome, said Mr. Dos Santos. Moreover, “vaginal seeding is not safe or advised. Professional bodies, including the Society of Obstetricians and Gynecologists of Canada and the American College of Obstetricians and Gynecologists, strongly advise against this practice.”
The study was published online in Frontiers in Cellular and Infection Microbiology.
Independent communities
The investigators analyzed vaginal and stool microbiome profiles from 442 mother-infant dyads. The mothers were healthy, low-risk women who delivered at term. They were recruited into the Maternal Microbiome LEGACY Project from three hospitals in British Columbia.
The mean age of the mothers at delivery was 34.6 years, which is typical of the study hospitals’ delivery populations. Participants identified themselves as White (54.7%), Asian (21.2%), South Asian (8.3%), and of other ethnicities.
A nurse, midwife, or clinician collected maternal vaginal swabs of the posterior fornix and lateral vaginal wall at first presentation to the labor and delivery area. Neonatal meconium, which was defined as the first stool specimen collected within 72 hours of birth, and two infant stool samples were collected at follow-up visits at 10 days and 3 months post partum.
A principal component analysis of infant stool microbiomes showed no significant clustering of microbiome profiles at 10 days or 3 months by maternal community state types (that is, microbial species).
Correspondence analyses also showed no coclustering of maternal and infant clusters at either time. In addition, there were no differences in the distribution of maternal vaginal microbiome clusters among infant stool microbiome clusters, regardless of delivery mode.
Vaginal microbiome clusters were distributed across infant stool clusters in proportion to their frequency in the overall maternal population, indicating that the two communities were independent of each other.
Intrapartum antibiotic administration was identified as a confounder of infant stool microbiome differences and was associated with lower abundances of Escherichia coli, Bacteroides vulgatus, Bifidobacterium longum, and Parabacteroides distasonis.
“Our findings demonstrate that maternal vaginal microbiome composition at delivery does not affect infant stool microbiome composition and development, suggesting that practices to amend infant stool microbiome composition focus on factors other than maternal vaginal microbes,” the authors conclude.
More evidence needed
Commenting on the study, Emily H. Adhikari, MD, assistant professor of obstetrics and gynecology at UT Southwestern Medical Center in Dallas, and medical director of perinatal infectious diseases for the Parkland Health and Hospital System, said, “These findings contribute significantly more data to an understudied area of research into factors that affect the infant gut microbiome from the earliest hours of life. Prior studies have been small and often conflicting, and the authors reference recent larger studies, which corroborate their findings.”
The data regarding whether delivery mode or antibiotic-associated differences in infant microbiomes persist remain controversial, said Dr. Adhikari. “More evidence is needed involving a more ethnically diverse sampling of patients.” In addition, prospectively evaluating vaginal seeding in a rigorously designed clinical trial setting is “imperative to understand any potential benefit and certainly to understand the potential harms of the practice. To date, this does not exist.”
The study was funded by a Canadian Institutes of Health Research grant. Mr. Dos Santos and Dr. Adhikari have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM FRONTIERS IN CELLULAR AND INFECTION MICROBIOLOGY
IUD-released levonorgestrel eases heavy menstrual periods
Median blood loss decreased by more than 90% in the first three cycles. Overall, treatment was successful in 81.8% of 99 patients (95% confidence interval, 74.2%-89.4%), according to findings published in Obstetrics & Gynecology.
Already approved for contraception, the IUD (Liletta) had substantial benefits for quality of life in measures such as sleep, pain/cramping, and daily functioning, wrote a group led by Mitchell D. Creinin, MD, a professor in the department of obstetrics and gynecology at University of California, Davis.
“This study provides evidence of high efficacy, as expected, for the Liletta levonorgestrel 52 mg IUD for heavy menstrual bleeding treatment,” Dr. Creinin said in an interview.
Racially diverse cohort
Conducted at 29 U.S. sites prior to seeking FDA registration for this new use, the phase 3 open-label trial of the 52 mg progestin-releasing IUD enrolled 105 participants with a mean age of 35.4 years. Unlike previous trials, this one included obese or severely obese women (44.8%), with 42 participants having a body mass index (BMI) of more than 35 kg/m2, and also 28 nulliparous women (27.6%).
Those with abnormalities such as fibroids or coagulopathies were excluded. Although most of the cohort was White (n = 68), the study included Black (n = 25), Asian (n = 4), and Hispanic (n = 10) women, plus 7 from other minorities, suggesting the results would be widely applicable.
Mean baseline blood loss in the cohort ranged from 73 mL to 520 mL (median, 143 mL). Of 89 treated women with follow-up, participants had a median absolute blood-loss decreases of 93.3% (86.1%-97.8%) at cycle three and 97.6% (90.4%-100%) at cycle six. Median bleeding reductions at cycle six were similar between women with and without obesity at 97.6% and 97.5%, respectively, and between nulliparous and parous women at 97.0% and 98.1%, respectively (P = .43). The study, however, was not sufficiently powered to fully analyze these subgroups, the authors acknowledged.
Although results were overall comparable with those of a previous study on a different IUD, the expulsion rate was somewhat higher, at 9%, than the 6% reported in the earlier study.
“Although this strategy for reducing blood loss is not new, this study is notable because it looked at high-BMI women and nulliparous women,” said Kathryn J. Gray, MD, PhD, an attending physician in the department of obstetrics and gynecology at Brigham and Women’s Hospital in Boston, who was not involved in the research.“No prior trials have included patients with BMIs exceeding 35 kg/m2 or nulliparous patients, while this study enrolled a full array of patients, which allowed exploratory analyses of these subpopulations,” Dr. Creinin confirmed.
According to Dr. Gray, the IUD approach has advantages over systemic treatment with oral medication. “First, treatment is not user-dependent so the user doesn’t have to remember to take it. In addition, because the medication is locally targeted in the uterus, it is more effective and there is less fluctuation and variability in drug levels than when taken orally.”
As to treatment durability, Dr. Creinin said, “Long-term studies in a population being treated for heavy menstrual bleeding would be helpful to have an idea of how long this effect lasts. Still, there is no reason to expect that the effect will not last for many years.”
And with this treatment, he added, both patient and clinician can readily detect its effect. “If bleeding begins to increase, they will know!”
Would there be a lingering residual effect even after removal of the IUD? “That is an excellent question that remains to be answered,” Dr. Creinin said. “There are no data on when the heavy bleeding returns, but it would be expected to do so.”
This study was funded, designed, and supervised by Medicines360, which also provided the study treatment. Dr. Creinin disclosed financial relationships with various private-sector companies, including Medicines360, Organon, Fuji Pharma, GlaxoSmithKline, and Merck & Co. Multiple study coauthors disclosed similar financial ties to industry partners, including Medicines360. Dr. Gray had no potential conflicts of interest with regard to her comments.
Median blood loss decreased by more than 90% in the first three cycles. Overall, treatment was successful in 81.8% of 99 patients (95% confidence interval, 74.2%-89.4%), according to findings published in Obstetrics & Gynecology.
Already approved for contraception, the IUD (Liletta) had substantial benefits for quality of life in measures such as sleep, pain/cramping, and daily functioning, wrote a group led by Mitchell D. Creinin, MD, a professor in the department of obstetrics and gynecology at University of California, Davis.
“This study provides evidence of high efficacy, as expected, for the Liletta levonorgestrel 52 mg IUD for heavy menstrual bleeding treatment,” Dr. Creinin said in an interview.
Racially diverse cohort
Conducted at 29 U.S. sites prior to seeking FDA registration for this new use, the phase 3 open-label trial of the 52 mg progestin-releasing IUD enrolled 105 participants with a mean age of 35.4 years. Unlike previous trials, this one included obese or severely obese women (44.8%), with 42 participants having a body mass index (BMI) of more than 35 kg/m2, and also 28 nulliparous women (27.6%).
Those with abnormalities such as fibroids or coagulopathies were excluded. Although most of the cohort was White (n = 68), the study included Black (n = 25), Asian (n = 4), and Hispanic (n = 10) women, plus 7 from other minorities, suggesting the results would be widely applicable.
Mean baseline blood loss in the cohort ranged from 73 mL to 520 mL (median, 143 mL). Of 89 treated women with follow-up, participants had a median absolute blood-loss decreases of 93.3% (86.1%-97.8%) at cycle three and 97.6% (90.4%-100%) at cycle six. Median bleeding reductions at cycle six were similar between women with and without obesity at 97.6% and 97.5%, respectively, and between nulliparous and parous women at 97.0% and 98.1%, respectively (P = .43). The study, however, was not sufficiently powered to fully analyze these subgroups, the authors acknowledged.
Although results were overall comparable with those of a previous study on a different IUD, the expulsion rate was somewhat higher, at 9%, than the 6% reported in the earlier study.
“Although this strategy for reducing blood loss is not new, this study is notable because it looked at high-BMI women and nulliparous women,” said Kathryn J. Gray, MD, PhD, an attending physician in the department of obstetrics and gynecology at Brigham and Women’s Hospital in Boston, who was not involved in the research.“No prior trials have included patients with BMIs exceeding 35 kg/m2 or nulliparous patients, while this study enrolled a full array of patients, which allowed exploratory analyses of these subpopulations,” Dr. Creinin confirmed.
According to Dr. Gray, the IUD approach has advantages over systemic treatment with oral medication. “First, treatment is not user-dependent so the user doesn’t have to remember to take it. In addition, because the medication is locally targeted in the uterus, it is more effective and there is less fluctuation and variability in drug levels than when taken orally.”
As to treatment durability, Dr. Creinin said, “Long-term studies in a population being treated for heavy menstrual bleeding would be helpful to have an idea of how long this effect lasts. Still, there is no reason to expect that the effect will not last for many years.”
And with this treatment, he added, both patient and clinician can readily detect its effect. “If bleeding begins to increase, they will know!”
Would there be a lingering residual effect even after removal of the IUD? “That is an excellent question that remains to be answered,” Dr. Creinin said. “There are no data on when the heavy bleeding returns, but it would be expected to do so.”
This study was funded, designed, and supervised by Medicines360, which also provided the study treatment. Dr. Creinin disclosed financial relationships with various private-sector companies, including Medicines360, Organon, Fuji Pharma, GlaxoSmithKline, and Merck & Co. Multiple study coauthors disclosed similar financial ties to industry partners, including Medicines360. Dr. Gray had no potential conflicts of interest with regard to her comments.
Median blood loss decreased by more than 90% in the first three cycles. Overall, treatment was successful in 81.8% of 99 patients (95% confidence interval, 74.2%-89.4%), according to findings published in Obstetrics & Gynecology.
Already approved for contraception, the IUD (Liletta) had substantial benefits for quality of life in measures such as sleep, pain/cramping, and daily functioning, wrote a group led by Mitchell D. Creinin, MD, a professor in the department of obstetrics and gynecology at University of California, Davis.
“This study provides evidence of high efficacy, as expected, for the Liletta levonorgestrel 52 mg IUD for heavy menstrual bleeding treatment,” Dr. Creinin said in an interview.
Racially diverse cohort
Conducted at 29 U.S. sites prior to seeking FDA registration for this new use, the phase 3 open-label trial of the 52 mg progestin-releasing IUD enrolled 105 participants with a mean age of 35.4 years. Unlike previous trials, this one included obese or severely obese women (44.8%), with 42 participants having a body mass index (BMI) of more than 35 kg/m2, and also 28 nulliparous women (27.6%).
Those with abnormalities such as fibroids or coagulopathies were excluded. Although most of the cohort was White (n = 68), the study included Black (n = 25), Asian (n = 4), and Hispanic (n = 10) women, plus 7 from other minorities, suggesting the results would be widely applicable.
Mean baseline blood loss in the cohort ranged from 73 mL to 520 mL (median, 143 mL). Of 89 treated women with follow-up, participants had a median absolute blood-loss decreases of 93.3% (86.1%-97.8%) at cycle three and 97.6% (90.4%-100%) at cycle six. Median bleeding reductions at cycle six were similar between women with and without obesity at 97.6% and 97.5%, respectively, and between nulliparous and parous women at 97.0% and 98.1%, respectively (P = .43). The study, however, was not sufficiently powered to fully analyze these subgroups, the authors acknowledged.
Although results were overall comparable with those of a previous study on a different IUD, the expulsion rate was somewhat higher, at 9%, than the 6% reported in the earlier study.
“Although this strategy for reducing blood loss is not new, this study is notable because it looked at high-BMI women and nulliparous women,” said Kathryn J. Gray, MD, PhD, an attending physician in the department of obstetrics and gynecology at Brigham and Women’s Hospital in Boston, who was not involved in the research.“No prior trials have included patients with BMIs exceeding 35 kg/m2 or nulliparous patients, while this study enrolled a full array of patients, which allowed exploratory analyses of these subpopulations,” Dr. Creinin confirmed.
According to Dr. Gray, the IUD approach has advantages over systemic treatment with oral medication. “First, treatment is not user-dependent so the user doesn’t have to remember to take it. In addition, because the medication is locally targeted in the uterus, it is more effective and there is less fluctuation and variability in drug levels than when taken orally.”
As to treatment durability, Dr. Creinin said, “Long-term studies in a population being treated for heavy menstrual bleeding would be helpful to have an idea of how long this effect lasts. Still, there is no reason to expect that the effect will not last for many years.”
And with this treatment, he added, both patient and clinician can readily detect its effect. “If bleeding begins to increase, they will know!”
Would there be a lingering residual effect even after removal of the IUD? “That is an excellent question that remains to be answered,” Dr. Creinin said. “There are no data on when the heavy bleeding returns, but it would be expected to do so.”
This study was funded, designed, and supervised by Medicines360, which also provided the study treatment. Dr. Creinin disclosed financial relationships with various private-sector companies, including Medicines360, Organon, Fuji Pharma, GlaxoSmithKline, and Merck & Co. Multiple study coauthors disclosed similar financial ties to industry partners, including Medicines360. Dr. Gray had no potential conflicts of interest with regard to her comments.
FROM OBSTETRICS & GYNECOLOGY
Spotting STIs: Vaginal swabs work best
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Product updates and reviews
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort
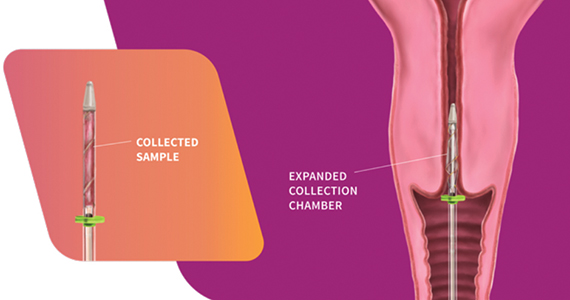
The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort

The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort

The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
CarePostRoe.com: Study seeks to document poor quality medical care due to new abortion bans
In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.

In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.

In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.
Risk of expulsion low after early postpartum IUD placement
Intrauterine device (IUD) placement at 2-4 weeks postpartum was noninferior to placement at 6-8 weeks postpartum for complete expulsion, and carried only a slightly higher risk of partial expulsion. A randomized study of expulsion rates reports the risk of expulsion at these points may help patients and clinicians make informed choices about the timing of IUD insertion, wrote the study authors, led by Sarah H. Averbach, MD, MAS, an obstetrician-gynecologist at the University of California, San Diego. “We found that the risk of complete IUD expulsion was low at 2% after early IUD placement 2-4 weeks after delivery, and was noninferior to interval placement 6-8 weeks after delivery at 0%,” Dr. Averbach said in an interview.
Although the risks of partial expulsion and malposition were modestly greater after early placement, “the possibility of a small increase in the risk of IUD expulsion or malposition with early IUD placement should be weighed against the risk of undesired pregnancy and short-interval pregnancy by delaying placement.”
The timing of IUD placement in the postpartum period should be guided by patients’ goals and preferences, she added. The early postpartum period 2-4 weeks after birth has the advantage of convenience since it coincides with early-postpartum or well-baby visits. The absolute risk differences observed between early and interval placement were small for both complete or partial expulsion at 3.8%, and the rate for complete expulsion after early placement was much lower than historical expulsion rates for immediate postpartum placement within in few days of delivery.
Last year, a large study showed an increase in expulsion risk with IUD insertion within 3 days of delivery. Current guidelines, however, support immediate insertion as a safe practice.
The study
Enrolling 404 participants from diverse settings during the period of 2018 to July 2021, researchers for the noninferiority trial randomly assigned 203 to early IUD placement 14-28 days postpartum and 201 to standard-interval placement at 42-56 days. Patients had a mean age of 29.9 years, 11.4% were Black, 56.4% were White, and 43.3% were Hispanic (some Hispanic participants self-identified as White and some as Black). By 6 months postpartum, 73% of the cohort had received an IUD and completed 6-months of follow-up, while 13% had never received an IUD and 14% were lost to follow-up. Complete expulsion rates were 3 of 149, or 2.0% (95% confidence interval [CI], 0.4-5.8) in the early group and 0 of 145, or 0% (95% CI, 0.0-2.5) in the standard group, for a between-group difference of 2.0 percentage points (95% CI, −0.5 to 5.7, P = .04). Two women chose to replace their IUDs.
Partial expulsion occurred in 14, or 9.4% (95% CI, 5.2-15.3) of patients in the early group and 11, or 7.6% (95% CI, 3.9-13.2) in the standard-interval group, for a between-group difference of 1.8 (95% CI, −4.8 to 8.6) percentage points (P = .22).
The small absolute increase in risk of partial expulsion in the early arm did not meet the prespecified criterion for noninferiority of 6%. Three pelvic infections occurred in the early placement arm.
There were 42 IUD removals: 25 in the early placement group and 17 in the standard interval group. Thirteen participants had their IUDs removed for symptoms such as cramping and bothersome vaginal bleeding.
No perforations were identified in either group at 6 months, suggesting that the rate of uterine perforations is low when IUDs are placed in the early and standard-interval postpartum periods. IUD use at 6 months remained comparable between arms: 69.5% in the early group vs. 67.2% in the standard-interval group.
Commenting on the trial but not involved in it, Maureen K. Baldwin, MD, MPH, associate professor of obstetrics and gynecology at Oregon Health & Science University in Portland, said it provides further data on the prevalence of expulsion and malposition after placements using ultrasonography as needed. While two failures occurred with asymptomatic malposition, she added, “It should be noted that IUD position can change as a result of pregnancy, so it was not determined that malposition occurred prior to contraceptive failure.”
According to Dr. Baldwin, one strategy to reduce concerns is to use transvaginal ultrasonography at a later time or in the presence of unusual symptoms.
Overall, the study establishes that postpartum placement is an option equivalent to standard timing and it should be incorporated into patient preferences, she said. “Pain may be lowest at early placement compared to other timings, particularly for those who had vaginal birth.”
The study was supported by the Society of Family Planning research fund and the National Institutes of Health - National Institute of Child Health and Human Development. Dr. Averbach reported personal fees from Bayer Pharmaceuticals for advice on postpartum IUD placement as well as grants from the NIH outside of the submitted work. Dr. Baldwin disclosed no potential conflicts of interest with regard to her comments.
Intrauterine device (IUD) placement at 2-4 weeks postpartum was noninferior to placement at 6-8 weeks postpartum for complete expulsion, and carried only a slightly higher risk of partial expulsion. A randomized study of expulsion rates reports the risk of expulsion at these points may help patients and clinicians make informed choices about the timing of IUD insertion, wrote the study authors, led by Sarah H. Averbach, MD, MAS, an obstetrician-gynecologist at the University of California, San Diego. “We found that the risk of complete IUD expulsion was low at 2% after early IUD placement 2-4 weeks after delivery, and was noninferior to interval placement 6-8 weeks after delivery at 0%,” Dr. Averbach said in an interview.
Although the risks of partial expulsion and malposition were modestly greater after early placement, “the possibility of a small increase in the risk of IUD expulsion or malposition with early IUD placement should be weighed against the risk of undesired pregnancy and short-interval pregnancy by delaying placement.”
The timing of IUD placement in the postpartum period should be guided by patients’ goals and preferences, she added. The early postpartum period 2-4 weeks after birth has the advantage of convenience since it coincides with early-postpartum or well-baby visits. The absolute risk differences observed between early and interval placement were small for both complete or partial expulsion at 3.8%, and the rate for complete expulsion after early placement was much lower than historical expulsion rates for immediate postpartum placement within in few days of delivery.
Last year, a large study showed an increase in expulsion risk with IUD insertion within 3 days of delivery. Current guidelines, however, support immediate insertion as a safe practice.
The study
Enrolling 404 participants from diverse settings during the period of 2018 to July 2021, researchers for the noninferiority trial randomly assigned 203 to early IUD placement 14-28 days postpartum and 201 to standard-interval placement at 42-56 days. Patients had a mean age of 29.9 years, 11.4% were Black, 56.4% were White, and 43.3% were Hispanic (some Hispanic participants self-identified as White and some as Black). By 6 months postpartum, 73% of the cohort had received an IUD and completed 6-months of follow-up, while 13% had never received an IUD and 14% were lost to follow-up. Complete expulsion rates were 3 of 149, or 2.0% (95% confidence interval [CI], 0.4-5.8) in the early group and 0 of 145, or 0% (95% CI, 0.0-2.5) in the standard group, for a between-group difference of 2.0 percentage points (95% CI, −0.5 to 5.7, P = .04). Two women chose to replace their IUDs.
Partial expulsion occurred in 14, or 9.4% (95% CI, 5.2-15.3) of patients in the early group and 11, or 7.6% (95% CI, 3.9-13.2) in the standard-interval group, for a between-group difference of 1.8 (95% CI, −4.8 to 8.6) percentage points (P = .22).
The small absolute increase in risk of partial expulsion in the early arm did not meet the prespecified criterion for noninferiority of 6%. Three pelvic infections occurred in the early placement arm.
There were 42 IUD removals: 25 in the early placement group and 17 in the standard interval group. Thirteen participants had their IUDs removed for symptoms such as cramping and bothersome vaginal bleeding.
No perforations were identified in either group at 6 months, suggesting that the rate of uterine perforations is low when IUDs are placed in the early and standard-interval postpartum periods. IUD use at 6 months remained comparable between arms: 69.5% in the early group vs. 67.2% in the standard-interval group.
Commenting on the trial but not involved in it, Maureen K. Baldwin, MD, MPH, associate professor of obstetrics and gynecology at Oregon Health & Science University in Portland, said it provides further data on the prevalence of expulsion and malposition after placements using ultrasonography as needed. While two failures occurred with asymptomatic malposition, she added, “It should be noted that IUD position can change as a result of pregnancy, so it was not determined that malposition occurred prior to contraceptive failure.”
According to Dr. Baldwin, one strategy to reduce concerns is to use transvaginal ultrasonography at a later time or in the presence of unusual symptoms.
Overall, the study establishes that postpartum placement is an option equivalent to standard timing and it should be incorporated into patient preferences, she said. “Pain may be lowest at early placement compared to other timings, particularly for those who had vaginal birth.”
The study was supported by the Society of Family Planning research fund and the National Institutes of Health - National Institute of Child Health and Human Development. Dr. Averbach reported personal fees from Bayer Pharmaceuticals for advice on postpartum IUD placement as well as grants from the NIH outside of the submitted work. Dr. Baldwin disclosed no potential conflicts of interest with regard to her comments.
Intrauterine device (IUD) placement at 2-4 weeks postpartum was noninferior to placement at 6-8 weeks postpartum for complete expulsion, and carried only a slightly higher risk of partial expulsion. A randomized study of expulsion rates reports the risk of expulsion at these points may help patients and clinicians make informed choices about the timing of IUD insertion, wrote the study authors, led by Sarah H. Averbach, MD, MAS, an obstetrician-gynecologist at the University of California, San Diego. “We found that the risk of complete IUD expulsion was low at 2% after early IUD placement 2-4 weeks after delivery, and was noninferior to interval placement 6-8 weeks after delivery at 0%,” Dr. Averbach said in an interview.
Although the risks of partial expulsion and malposition were modestly greater after early placement, “the possibility of a small increase in the risk of IUD expulsion or malposition with early IUD placement should be weighed against the risk of undesired pregnancy and short-interval pregnancy by delaying placement.”
The timing of IUD placement in the postpartum period should be guided by patients’ goals and preferences, she added. The early postpartum period 2-4 weeks after birth has the advantage of convenience since it coincides with early-postpartum or well-baby visits. The absolute risk differences observed between early and interval placement were small for both complete or partial expulsion at 3.8%, and the rate for complete expulsion after early placement was much lower than historical expulsion rates for immediate postpartum placement within in few days of delivery.
Last year, a large study showed an increase in expulsion risk with IUD insertion within 3 days of delivery. Current guidelines, however, support immediate insertion as a safe practice.
The study
Enrolling 404 participants from diverse settings during the period of 2018 to July 2021, researchers for the noninferiority trial randomly assigned 203 to early IUD placement 14-28 days postpartum and 201 to standard-interval placement at 42-56 days. Patients had a mean age of 29.9 years, 11.4% were Black, 56.4% were White, and 43.3% were Hispanic (some Hispanic participants self-identified as White and some as Black). By 6 months postpartum, 73% of the cohort had received an IUD and completed 6-months of follow-up, while 13% had never received an IUD and 14% were lost to follow-up. Complete expulsion rates were 3 of 149, or 2.0% (95% confidence interval [CI], 0.4-5.8) in the early group and 0 of 145, or 0% (95% CI, 0.0-2.5) in the standard group, for a between-group difference of 2.0 percentage points (95% CI, −0.5 to 5.7, P = .04). Two women chose to replace their IUDs.
Partial expulsion occurred in 14, or 9.4% (95% CI, 5.2-15.3) of patients in the early group and 11, or 7.6% (95% CI, 3.9-13.2) in the standard-interval group, for a between-group difference of 1.8 (95% CI, −4.8 to 8.6) percentage points (P = .22).
The small absolute increase in risk of partial expulsion in the early arm did not meet the prespecified criterion for noninferiority of 6%. Three pelvic infections occurred in the early placement arm.
There were 42 IUD removals: 25 in the early placement group and 17 in the standard interval group. Thirteen participants had their IUDs removed for symptoms such as cramping and bothersome vaginal bleeding.
No perforations were identified in either group at 6 months, suggesting that the rate of uterine perforations is low when IUDs are placed in the early and standard-interval postpartum periods. IUD use at 6 months remained comparable between arms: 69.5% in the early group vs. 67.2% in the standard-interval group.
Commenting on the trial but not involved in it, Maureen K. Baldwin, MD, MPH, associate professor of obstetrics and gynecology at Oregon Health & Science University in Portland, said it provides further data on the prevalence of expulsion and malposition after placements using ultrasonography as needed. While two failures occurred with asymptomatic malposition, she added, “It should be noted that IUD position can change as a result of pregnancy, so it was not determined that malposition occurred prior to contraceptive failure.”
According to Dr. Baldwin, one strategy to reduce concerns is to use transvaginal ultrasonography at a later time or in the presence of unusual symptoms.
Overall, the study establishes that postpartum placement is an option equivalent to standard timing and it should be incorporated into patient preferences, she said. “Pain may be lowest at early placement compared to other timings, particularly for those who had vaginal birth.”
The study was supported by the Society of Family Planning research fund and the National Institutes of Health - National Institute of Child Health and Human Development. Dr. Averbach reported personal fees from Bayer Pharmaceuticals for advice on postpartum IUD placement as well as grants from the NIH outside of the submitted work. Dr. Baldwin disclosed no potential conflicts of interest with regard to her comments.
FROM JAMA
Ectopic pregnancy risk and levonorgestrel-releasing IUDs
Researchers report that use of any levonorgestrel-releasing intrauterine system was associated with a significantly increased risk of ectopic pregnancy, compared with other hormonal contraceptives, in a study published in JAMA.
A national health database analysis headed by Amani Meaidi, MD, PhD, of the Danish Cancer Society Research Center, Cancer Surveillance and Pharmacoepidemiology, in Copenhagen, compared the 13.5-mg with the 19.5-mg and 52-mg dosages of levonorgestrel-releasing intrauterine systems (IUSs).
The hormone content in levonorgestrel-releasing IUSs must be high enough to maintain optimal contraceptive effect but sufficiently low to minimize progestin-related adverse events, Dr. Meaidi and colleagues noted; they advised using the middle dosage of 19.5 mg. All dosages are recommended for contraception, with the highest dosage also recommended for heavy menstrual bleeding.
“If 10,000 women using the hormonal IUD for 1 year were given the 19.5-mg hormonal IUD instead of the 13.5-mg hormonal IUD, around nine ectopic pregnancies would be avoided,” Dr. Meaidi said in an interview.
“Ectopic pregnancy is an acknowledged adverse event of hormonal IUD use. Although a rare event, it is a serious one, and a difference in ectopic pregnancy safety between the two low-dose hormonal IUDs would impact my recommendations to women.”
The study
Dr. Meaidi’s group followed 963,964 women for 7.8 million person-years. For users of levonorgestrel IUS dosages 52 mg, 19.5 mg, and 13.5 mg, and other hormonal contraceptives, the median ages were 24, 22, 22, and 21 years, respectively.
Eligible women were nulliparous with no previous ectopic pregnancy, abdominal or pelvic surgery, infertility treatment, endometriosis, or use of a levonorgestrel IUS. They were followed from Jan. 1, 2001, or their 15th birthday, until July 1, 2021, age 35, pregnancy, death, emigration, or the occurrence of any exclusion criterion.
During the study period, the cohort registered 2,925 ectopic pregnancies, including 35 at 52 mg, 32 at 19.5 mg, and 80 at 13.5 mg of levonorgestrel. For all other types of hormonal contraception, there were 763 ectopic pregnancies.
In terms of adjusted absolute rates of ectopic pregnancy per 10,000 person-years, compared with other hormonal contraceptives (rate = 2.4), these were 7.7 with 52 mg levonorgestrel IUS, 7.1 with 19.5 mg, and 15.7 with 13.5 mg. They translated to comparative differences of 5.3 (95% confidence interval, 1.9-8.7), 4.8 (95% CI, 1.5-8.0), and 13.4 (95% CI, 8.8-18.1), respectively.
Corresponding adjusted relative rate ratios were 3.4, 4.1, and 7.9. For each levonorgestrel IUS dosage; the ectopic pregnancy rate increased with duration of use.
The adjusted ectopic pregnancy rate difference per 10,000 person-years between the 19.5-mg and 52-mg levonorgestrel dosages was −0.6 , and between the 13.5-mg and 52-mg doses, 8.0, with a rate ratio of 2.3. The rate difference between the 13.5-mg and 19.5-mg levonorgestrel IUS was 8.6, with a rate ratio of 1.9.
An outsider’s perspective
Offering an outsider’s perspective on the study, Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital in New York, said these data should spark further evaluation of risk of ectopic pregnancy with levonorgestrel-releasing IUDs. “The best advice for clinicians is to individualize the choice of which contraceptive to use, and when levonorgestrel IUD is selected, to individualize the appropriate dose and timing of placement,” he said in an interview.
Several additional factors may determine the best choice, Dr. Bornstein added, including medical conditions that contraindicate other contraceptives and those conditions that justify avoidance of pregnancy, as well as uterine myomas or malformation, the ability of the patient to comply with other options, and informed patient choice. “It is important to remember the potential risk for expulsion and ectopic pregnancy, maintain alertness, and use ultrasound to exclude these potential complications if suspected,” he said.
Dr. Meaidi said the mechanism of ectopic pregnancy with hormonal IUDs is unclear, but in vitro and animal studies have observed that levonorgestrel reduces the ciliary beat frequency in the fallopian tubes. “Thus, it could be hypothesized that if a woman was unfortunate enough to become pregnant using a hormonal IUD, the hormone could inhibit or slow down the movement of the zygote into the uterus for rightful intrauterine implantation and thereby increase the risk of ectopic pregnancy.”
Two coauthors of the study reported financial support from private-sector companies. Dr. Meaidi had no conflicts of interest. Dr. Bornstein disclosed no competing interests.
Researchers report that use of any levonorgestrel-releasing intrauterine system was associated with a significantly increased risk of ectopic pregnancy, compared with other hormonal contraceptives, in a study published in JAMA.
A national health database analysis headed by Amani Meaidi, MD, PhD, of the Danish Cancer Society Research Center, Cancer Surveillance and Pharmacoepidemiology, in Copenhagen, compared the 13.5-mg with the 19.5-mg and 52-mg dosages of levonorgestrel-releasing intrauterine systems (IUSs).
The hormone content in levonorgestrel-releasing IUSs must be high enough to maintain optimal contraceptive effect but sufficiently low to minimize progestin-related adverse events, Dr. Meaidi and colleagues noted; they advised using the middle dosage of 19.5 mg. All dosages are recommended for contraception, with the highest dosage also recommended for heavy menstrual bleeding.
“If 10,000 women using the hormonal IUD for 1 year were given the 19.5-mg hormonal IUD instead of the 13.5-mg hormonal IUD, around nine ectopic pregnancies would be avoided,” Dr. Meaidi said in an interview.
“Ectopic pregnancy is an acknowledged adverse event of hormonal IUD use. Although a rare event, it is a serious one, and a difference in ectopic pregnancy safety between the two low-dose hormonal IUDs would impact my recommendations to women.”
The study
Dr. Meaidi’s group followed 963,964 women for 7.8 million person-years. For users of levonorgestrel IUS dosages 52 mg, 19.5 mg, and 13.5 mg, and other hormonal contraceptives, the median ages were 24, 22, 22, and 21 years, respectively.
Eligible women were nulliparous with no previous ectopic pregnancy, abdominal or pelvic surgery, infertility treatment, endometriosis, or use of a levonorgestrel IUS. They were followed from Jan. 1, 2001, or their 15th birthday, until July 1, 2021, age 35, pregnancy, death, emigration, or the occurrence of any exclusion criterion.
During the study period, the cohort registered 2,925 ectopic pregnancies, including 35 at 52 mg, 32 at 19.5 mg, and 80 at 13.5 mg of levonorgestrel. For all other types of hormonal contraception, there were 763 ectopic pregnancies.
In terms of adjusted absolute rates of ectopic pregnancy per 10,000 person-years, compared with other hormonal contraceptives (rate = 2.4), these were 7.7 with 52 mg levonorgestrel IUS, 7.1 with 19.5 mg, and 15.7 with 13.5 mg. They translated to comparative differences of 5.3 (95% confidence interval, 1.9-8.7), 4.8 (95% CI, 1.5-8.0), and 13.4 (95% CI, 8.8-18.1), respectively.
Corresponding adjusted relative rate ratios were 3.4, 4.1, and 7.9. For each levonorgestrel IUS dosage; the ectopic pregnancy rate increased with duration of use.
The adjusted ectopic pregnancy rate difference per 10,000 person-years between the 19.5-mg and 52-mg levonorgestrel dosages was −0.6 , and between the 13.5-mg and 52-mg doses, 8.0, with a rate ratio of 2.3. The rate difference between the 13.5-mg and 19.5-mg levonorgestrel IUS was 8.6, with a rate ratio of 1.9.
An outsider’s perspective
Offering an outsider’s perspective on the study, Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital in New York, said these data should spark further evaluation of risk of ectopic pregnancy with levonorgestrel-releasing IUDs. “The best advice for clinicians is to individualize the choice of which contraceptive to use, and when levonorgestrel IUD is selected, to individualize the appropriate dose and timing of placement,” he said in an interview.
Several additional factors may determine the best choice, Dr. Bornstein added, including medical conditions that contraindicate other contraceptives and those conditions that justify avoidance of pregnancy, as well as uterine myomas or malformation, the ability of the patient to comply with other options, and informed patient choice. “It is important to remember the potential risk for expulsion and ectopic pregnancy, maintain alertness, and use ultrasound to exclude these potential complications if suspected,” he said.
Dr. Meaidi said the mechanism of ectopic pregnancy with hormonal IUDs is unclear, but in vitro and animal studies have observed that levonorgestrel reduces the ciliary beat frequency in the fallopian tubes. “Thus, it could be hypothesized that if a woman was unfortunate enough to become pregnant using a hormonal IUD, the hormone could inhibit or slow down the movement of the zygote into the uterus for rightful intrauterine implantation and thereby increase the risk of ectopic pregnancy.”
Two coauthors of the study reported financial support from private-sector companies. Dr. Meaidi had no conflicts of interest. Dr. Bornstein disclosed no competing interests.
Researchers report that use of any levonorgestrel-releasing intrauterine system was associated with a significantly increased risk of ectopic pregnancy, compared with other hormonal contraceptives, in a study published in JAMA.
A national health database analysis headed by Amani Meaidi, MD, PhD, of the Danish Cancer Society Research Center, Cancer Surveillance and Pharmacoepidemiology, in Copenhagen, compared the 13.5-mg with the 19.5-mg and 52-mg dosages of levonorgestrel-releasing intrauterine systems (IUSs).
The hormone content in levonorgestrel-releasing IUSs must be high enough to maintain optimal contraceptive effect but sufficiently low to minimize progestin-related adverse events, Dr. Meaidi and colleagues noted; they advised using the middle dosage of 19.5 mg. All dosages are recommended for contraception, with the highest dosage also recommended for heavy menstrual bleeding.
“If 10,000 women using the hormonal IUD for 1 year were given the 19.5-mg hormonal IUD instead of the 13.5-mg hormonal IUD, around nine ectopic pregnancies would be avoided,” Dr. Meaidi said in an interview.
“Ectopic pregnancy is an acknowledged adverse event of hormonal IUD use. Although a rare event, it is a serious one, and a difference in ectopic pregnancy safety between the two low-dose hormonal IUDs would impact my recommendations to women.”
The study
Dr. Meaidi’s group followed 963,964 women for 7.8 million person-years. For users of levonorgestrel IUS dosages 52 mg, 19.5 mg, and 13.5 mg, and other hormonal contraceptives, the median ages were 24, 22, 22, and 21 years, respectively.
Eligible women were nulliparous with no previous ectopic pregnancy, abdominal or pelvic surgery, infertility treatment, endometriosis, or use of a levonorgestrel IUS. They were followed from Jan. 1, 2001, or their 15th birthday, until July 1, 2021, age 35, pregnancy, death, emigration, or the occurrence of any exclusion criterion.
During the study period, the cohort registered 2,925 ectopic pregnancies, including 35 at 52 mg, 32 at 19.5 mg, and 80 at 13.5 mg of levonorgestrel. For all other types of hormonal contraception, there were 763 ectopic pregnancies.
In terms of adjusted absolute rates of ectopic pregnancy per 10,000 person-years, compared with other hormonal contraceptives (rate = 2.4), these were 7.7 with 52 mg levonorgestrel IUS, 7.1 with 19.5 mg, and 15.7 with 13.5 mg. They translated to comparative differences of 5.3 (95% confidence interval, 1.9-8.7), 4.8 (95% CI, 1.5-8.0), and 13.4 (95% CI, 8.8-18.1), respectively.
Corresponding adjusted relative rate ratios were 3.4, 4.1, and 7.9. For each levonorgestrel IUS dosage; the ectopic pregnancy rate increased with duration of use.
The adjusted ectopic pregnancy rate difference per 10,000 person-years between the 19.5-mg and 52-mg levonorgestrel dosages was −0.6 , and between the 13.5-mg and 52-mg doses, 8.0, with a rate ratio of 2.3. The rate difference between the 13.5-mg and 19.5-mg levonorgestrel IUS was 8.6, with a rate ratio of 1.9.
An outsider’s perspective
Offering an outsider’s perspective on the study, Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital in New York, said these data should spark further evaluation of risk of ectopic pregnancy with levonorgestrel-releasing IUDs. “The best advice for clinicians is to individualize the choice of which contraceptive to use, and when levonorgestrel IUD is selected, to individualize the appropriate dose and timing of placement,” he said in an interview.
Several additional factors may determine the best choice, Dr. Bornstein added, including medical conditions that contraindicate other contraceptives and those conditions that justify avoidance of pregnancy, as well as uterine myomas or malformation, the ability of the patient to comply with other options, and informed patient choice. “It is important to remember the potential risk for expulsion and ectopic pregnancy, maintain alertness, and use ultrasound to exclude these potential complications if suspected,” he said.
Dr. Meaidi said the mechanism of ectopic pregnancy with hormonal IUDs is unclear, but in vitro and animal studies have observed that levonorgestrel reduces the ciliary beat frequency in the fallopian tubes. “Thus, it could be hypothesized that if a woman was unfortunate enough to become pregnant using a hormonal IUD, the hormone could inhibit or slow down the movement of the zygote into the uterus for rightful intrauterine implantation and thereby increase the risk of ectopic pregnancy.”
Two coauthors of the study reported financial support from private-sector companies. Dr. Meaidi had no conflicts of interest. Dr. Bornstein disclosed no competing interests.
FROM JAMA
Iron deficiency and anemia in patients with heavy menstrual bleeding: Mechanisms and management
Recurrent episodic blood loss from normal menstruation is not expected to result in anemia. But without treatment, chronic heavy periods will progress through the stages of low iron stores to iron deficiency and then to anemia. When iron storage levels are low, the bone marrow’s blood cell factory cannot keep up with continued losses. Every patient with heavy menstrual bleeding (HMB) or prolonged menstrual episodes should be tested and treated for iron deficiency and anemia.1,2
Particular attention should be paid to assessment of iron storage levels with serum ferritin, recognizing that low iron levels progress to anemia once the storage is depleted. Recovery from anemia is much slower in individuals with iron deficiency, so assessment for iron storage also should be included in preoperative assessments and following a diagnosis of acute blood loss anemia.
The mechanics of erythropoiesis, hemoglobin, and oxygen transport
Red blood cells (erythrocytes) have a short life cycle and require constant replacement. Erythrocytes are generated on demand in erythropoiesis by a hormonal signaling process, regardless of whether sufficient components are available.3 Hemoglobin, the main intracellular component of erythrocytes, is comprised of 4 globin chains, which each contain 1 iron atom bound to a heme molecule. After erythrocytes are assembled, they are sent out into circulation for approximately 120 days. A hemoglobin level measures the oxygen-carrying capacity of erythrocytes, and anemia is defined as hemoglobin less than 12 g/dL.
Unless erythrocytes are lost from bleeding, they are decommissioned—that is, the heme molecule is metabolized into bilirubin and excreted, and the iron atoms are recycled back to the bone marrow or to storage.4 Ferritin is the storage molecule that binds to iron, a glycoprotein with numerous subunits around a core that can contain about 4,000 iron atoms. Most ferritin is intracellular, but a small proportion is present in serum, where it can be measured.
Serum ferritin is a good marker for the iron supply in healthy individuals because it has high correlation to iron in the bone marrow and correlates to total intracellular storage unless there is inflammation, when mobilization to serum increases. The ferritin level at which the iron supply is deficient to meet demand, defined as iron deficiency, is hotly debated and ranges from less than 15 to 50 ng/mL in menstruating individuals, with higher thresholds based on onset of erythropoiesis signaling and the lower threshold being the World Health Organization recommendation.5-7 When iron atoms are in short supply, erythrocytes still are generated but they have lower amounts of intracellular hemoglobin, which makes them thinner, smaller, and paler—and less effective at oxygen transport.
CASE Patient seeks treatment for HMB-associated symptoms
A 17-year-old patient presents with HMB, fatigue, and difficulty with concentration. She reports that her periods have been regular and lasting 7 days since menarche at age 13. While they are manageable, they seem to be getting heavier, soaking pads in 2 to 3 hours. The patient reports that she would like to start treatment for her progressively heavy bleeding and prefers lighter scheduled bleeding; she currently does not desire contraception. The patient has no family history of bleeding problems and self-reports no personal history of epistaxis or bleeding with tooth extraction or tonsillectomy. Laboratory tests confirm iron deficiency with a hemoglobin level of 12.5 g/dL (reference range, 12.0–17.5 g/dL) and a serum ferritin level of 8 ng/mL (reference range, 50–420 ng/mL). Results from a coagulopathy panel are normal, as are von Willebrand factor levels.
Untreated iron deficiency will progress to anemia
This patient has iron deficiency without anemia, which warrants significant attention in HMB because without treatment it eventually will progress to anemia. The prevalence of iron deficiency, which makes up half of all causes of anemia, is at least double that of iron deficiency anemia.3
Adult bodies usually contain about 3 to 4 g of iron, with two-thirds in erythrocytes as hemoglobin.8 Approximately 40 to 60 mg of iron is recycled daily, 1 to 2 mg/day is lost from sloughed cells and sweat, and at least 1 mg/day is lost during normal menstruation. These losses are balanced with gastrointestinal uptake of 1 to 2 mg/day until bleeding exceeds about 10 mL/day. In this 17-year-old patient, iron stores have likely been on a progressive decline since menarche.
For normally menstruating individuals to maintain iron homeostasis, the daily dietary iron requirement is 18 mg/day. Iron requirements also increase during periods of illness or inflammation due to hormonal signaling in the iron absorption and transport pathway, in athletes due to sweating, foot strike hemolysis and bruising, and during growth spurts.9
Continue to: Managing iron deficiency and anemia...
Managing iron deficiency and anemia
Management of iron deficiency and iron deficiency anemia in the setting of HMB includes:
- workup for the etiology of the abnormal uterine bleeding (TABLE)
- reducing the source of blood loss, and
- iron supplementation to correct the iron deficiency state.
In most cases, workup, reduction, and repletion can occur simultaneously. The goal is not always complete cessation of menstrual bleeding; even short-term therapy can allow time to replenish iron storage. Use a shared decision-making process to assess what is important to the patient, and provide information about relative amounts of bleeding cessation that can be expected with various therapies.10
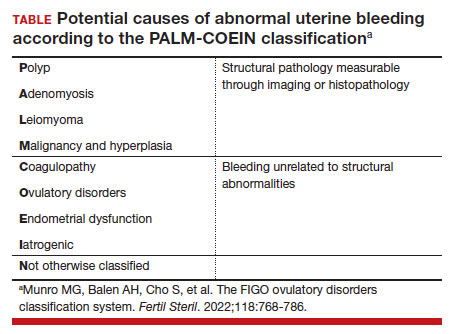
Treatment options
Medical treatments to decrease menstrual iron losses are recommended prior to proceeding with surgical interventions.11 Hormonal treatments are the most consistently recommended, with many guidelines citing the 52-mg levonorgestrel-releasing intrauterine device (LNG IUD) as first-line treatment due to its substantial reduction in the amount of bleeding, HMB treatment indication approved by the US Food and Drug Administration (FDA), and evidence of success in those with HMB.12
Any progestin or combined hormonal medication with estrogen and a progestin will result in an approximately 60% to 90% bleeding reduction, thus providing many effective options for blood loss while considering patient preferences for bleeding pattern, route of administration, and concomitant benefits. While only 1 oral product (estradiol valerate/dienogest) is FDA approved for managementof HMB, use of any of the commercially available contraceptive products will provide substantial benefit.11,13
Nonhormonal options, such as antifibrinolytics and nonsteroidal anti-inflammatory drugs (NSAIDs), tend to be listed as second-line therapies or for those who want to avoid hormonal medications. Antifibrinolytics, such as tranexamic acid, require frequent dosing of large pills and result in approximately 40% blood loss reduction, but they are a very successful and well-tolerated method for those seeking on-demand therapy.14 NSAIDs may result in a slight bleeding reduction, but they are far less effective than other therapies.15 Antifibrinolytics have a theoretical risk of thrombosis and a contraindication to use with hormonal contraceptives; therefore, concomitant use with estrogen-containing medications is reserved for patients with refractory heavy bleeding or for heavy bleeding days during the hormone-free interval, when benefits likely outweigh potential risk.16,17
Guidelines for medical management of acute HMB typically cite 3 small comparative studies with high-dose regimens of parenteral conjugated estrogen, combined ethinyl estradiol and progestin, or oral medroxyprogesterone acetate.18,19 Dosing recommendations for the oral medications include a loading dose followed by a taper regimen that is poorly tolerated and for which there is no evidence of superior effectiveness over the standard dose.20,21In most cases, initiation of the preferred long-term hormonal medication plan will reduce bleeding significantly within 2 to 3 days. Many clinicians who commonly treat acute HMB prescribe norethindrone acetate 5 mg daily (up to 3 times daily, if needed) for effective and safe menstrual suppression.22
Iron replenishment: Dosing frequency, dietary iron, and multivitamins
Iron repletion is usually via the oral route unless surgery is imminent, anemia is severe, or the oral route is not tolerated or effective.23 Oral iron has substantial adverse effects that limit tolerance, including nausea, epigastric pain, diarrhea, and constipation. Fortunately, evidence supports lower oral iron doses than previously used.4
Iron homeostasis is controlled by the peptide hormone hepcidin, produced by the liver, which controls mobilization of iron from the gut and spleen and aids iron absorption from the diet and supplements.24 Hepcidin levels decrease in response to high circulating levels of iron, so the ideal iron repletion dose in iron-deficient nonanemic women was determined by assessing the dose response of hepcidin. Researchers compared iron 60 mg daily for 14 days versus every other day for 28 days and found that iron absorption was greater in the every-other-day group (21.8% vs 16.3%).25They concluded that changing iron administration to 60 mg or more in a single dose every other day is most efficient in those with iron deficiency without anemia. Since study participants did not have anemia, research is pending on whether different strategies (such as daily dosing) are more effective for more severe cases. The bottom line is that conventional high-dose divided daily oral iron administration results in reduced iron bioavailability compared with alternate-day dosing.
Increasing dietary iron is insufficient to treat low iron storage, iron deficiency, and iron deficiency anemia. Likewise, multivitamins, which contain very little elemental iron, are not recommended for repletion. Any iron salt with 60 to 120 mg of elemental iron can be used (for examples, ferrous sulfate, ferrous gluconate).25 Once ingested, stomach and pancreatic acids release elemental iron from its bound form. For that reason, absorption may be improved by administering iron at least 1 hour before a meal and avoiding antacids, including milk. Meat proteins and ascorbic acid help maintain the soluble ferrous form and also aid absorption. Tea, coffee, and tannins prevent absorption when polyphenol compounds form an insoluble complex with iron (see box at end of article). Gastrointestinal adverse effects can be minimized by decreasing the dose and taking after meals, although with reduced efficacy.
Intravenous iron treatment raises hemoglobin levels significantly faster than oral administration but is limited by cost and availability, so it is reserved for individuals with a hemoglobin level less than 9 g/dL, prior gastrointestinal or bariatric surgery, imminent surgery, and intolerance, poor adherence, or nonresponse to oral iron therapy. Several approved formulations are available, all with equivalent effectiveness and similar safety profiles. Lower-dose formulations (such as iron sucrose) may require several infusions, but higher-dose intravenous iron products (ferric carboxymaltose, low-molecular weight iron dextran, etc) have a stable carbohydrate shell that inhibits free iron release and improves safety, allowing a single administration.26
Common adverse effects of intravenous iron treatment include a metallic taste and headache during administration. More serious adverse effects, such as hypotension, arthralgia, malaise, and nausea, are usually self-limited. With mild infusion reactions (1 in 200), the infusion can be stopped until symptoms improve and can be resumed at a slower rate.27
Continue to: The role of blood transfusion...
The role of blood transfusion
Blood transfusion is expensive and potentially hazardous, so its use is limited to treatment of acute blood loss or severe anemia.
A one-time red blood cell transfusion does not impact diagnostic criteria to assess for iron deficiency with ferritin, and it does not improve underlying iron deficiency.28Patients with acute blood loss anemia superimposed on chronic blood loss should be screened and treated for iron deficiency even after receiving a transfusion.
Since ferritin levels can rise significantly as an acute phase reactant, even following a hemorrhage, iron deficiency during inflammation is defined as ferritin less than 70 ng/mL.
The potential for iron overload
Since iron is never metabolized or excreted, it is possible to have iron overload following accidental overdose, transfusion dependency, and disorders of iron transport, such as hemochromatosis and thalassemia.
While a low ferritin level always indicates iron deficiency, high ferritin levels can be an acute phase reactant. Ferritin levels greater than 150 ng/mL in healthy menstruating individuals and greater than 500 ng/mL in unhealthy individuals should raise concern for excess iron and should prompt discontinuation of iron intake or workup for conditions at risk for overload.5
Oral iron supplements should be stored away from small children, who are at particular risk of toxicity.
How long to treat?
Treatment duration depends on the individual’s degree of iron deficiency, whether anemia is present, and the amount of ongoing blood loss. The main treatment goal is normalization and maintenance of serum ferritin.
Successful treatment should be confirmed with a complete blood count and ferritin level. Hemoglobin levels improve 2 g/dL after 3 weeks of oral iron therapy, but repletion may take 4 to 6 months.23,29 The American College of Obstetricians and Gynecologists recommends 3 to 6 months of continued iron therapy after resolution of HMB.19
In a comparative study of treatment for HMB with the 52-mg LNG IUD versus hysterectomy, hemoglobin levels increased in both treatment groups but stayed lower in those with initial anemia.8 Ferritin levels normalized only after 5 years and were still lower in individuals with initial anemia.
Increase in hemoglobin is faster after intravenous iron administration but is equivalent to oral therapy by 12 weeks. If management to reduce menstrual losses is discontinued, periodic or maintenance iron repletion will be necessary.
CASE Management plan initiated
This 17-year-old patient with iron deficiency resulting from HMB requests management to reduce menstrual iron losses with a preference for predictable menses. We have already completed a basic workup, which could also include assessment for hypermobility with a Beighton score, as connective tissue disorders also are associated with HMB.30 We discuss the options of cyclic hormonal therapy, antifibrinolytic treatment, and an LNG IUD. The patient is concerned about adherence and wants to avoid unscheduled bleeding, so she opts for a trial of tranexamic acid 1,300 mg 3 times daily for 5 days during menses. This regimen results in a 50% reduction in bleeding amount, which the patient finds satisfactory. Iron repletion with oral ferrous sulfate 325 mg (containing 65 mg of elemental iron) is administered on alternating days with vitamin C taken 1 hour prior to dinner. Repeat laboratory test results at 3 weeks show improvement to a hemoglobin level of 14.2 g/dL and a ferritin level of 12 ng/mL. By 3 months, her ferritin levels are greater than 30 ng/mL and oral iron is administered only during menses.
Summing up
Chronic HMB results in a progressive net loss of iron and eventual anemia. Screening with complete blood count and ferritin and early treatment of low iron storage when ferritin is less than 30 ng/mL will help avoid symptoms. Any amount of reduction of menstrual blood loss can be beneficial, allowing a variety of effective hormonal and nonhormonal treatment options. ●
- Take 60 to 120 mg elemental iron every other day.
- To help with absorption:
—Take 1 hour before a meal, but not with coffee, tea, tannins, antacids, or milk
—Take with vitamin C or other acidic fruit juice
- Recheck complete blood count and ferritin in 2 to 3 weeks to confirm initial response.
- Continue treatment for up to 3 to 6 months until ferritin levels are greater than 30 to 50 ng/mL.
- Munro MG, Mast AE, Powers JM, et al. The relationship between heavy menstrual bleeding, iron deficiency, and iron deficiency anemia. Am J Obstet Gynecol. 2023;S00029378(23)00024-8.
- Tsakiridis I, Giouleka S, Koutsouki G, et al. Investigation and management of abnormal uterine bleeding in reproductive aged women: a descriptive review of national and international recommendations. Eur J Contracept Reprod Health Care. 2022;27:504-517.
- Camaschella C. Iron deficiency. Blood. 2019;133:30-39.
- Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272.
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. April 21, 2020. Accessed February 17, 2023. https://www.who.int/publications/i/item/9789240000124
- Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8: e572-e582.
- Galetti V, Stoffel NU, Sieber C, et al. Threshold ferritin and hepcidin concentrations indicating early iron deficiency in young women based on upregulation of iron absorption. EClinicalMedicine. 2021;39:101052.
- Percy L, Mansour D, Fraser I. Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol. 2017;40:55-67.
- Brittenham GM. Short-term periods of strenuous physical activity lower iron absorption. Am J Clin Nutr. 2021;113:261-262.
- Chen M, Lindley A, Kimport K, et al. An in-depth analysis of the use of shared decision making in contraceptive counseling. Contraception. 2019;99:187-191.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180.
- Mansour D, Hofmann A, Gemzell-Danielsson K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv Ther. 2021;38:201-225.
- Micks EA, Jensen JT. Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill. Adv Ther. 2013;30:1-13.
- Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;4:CD000249.
- Bofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;9:CD000400.
- Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract T hromb Haemost. 2021;5:e12546.
- Reid RL, Westhoff C, Mansour D, et al. Oral contraceptives and venous thromboembolism consensus opinion from an international workshop held in Berlin, Germany in December 2009. J Fam Plann Reprod Health Care. 2010;36:117-122.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 785: screening and management of bleeding disorders in adolescents with heavy menstrual bleeding. Obstet Gynecol. 2019;134:e71-e83.
- Haamid F, Sass AE, Dietrich JE. Heavy menstrual bleeding in adolescents. J Pediatr Adolesc Gynecol. 2017;30:335-340.
- Roth LP, Haley KM, Baldwin MK. A retrospective comparison of time to cessation of acute heavy menstrual bleeding in adolescents following two dose regimens of combined oral hormonal therapy. J Pediatr Adolesc Gynecol. 2022;35:294-298.
- Huguelet PS, Buyers EM, Lange-Liss JH, et al. Treatment of acute abnormal uterine bleeding in adolescents: what are providers doing in various specialties? J Pediatr Adolesc Gynecol. 2016;29:286-291.
- Elstrott B, Khan L, Olson S, et al. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. 2020;104:153-161.
- Pagani A, Nai A, Silvestri L, et al. Hepcidin and anemia: a tight relationship. Front Physiol. 2019;10:1294.
- Stoffel NU, von Siebenthal HK, Moretti D, et al. Oral iron supplementation in iron-deficient women: how much and how often? Mol Aspects Med. 2020;75:100865.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Dave CV, Brittenham GM, Carson JL, et al. Risks for anaphylaxis with intravenous iron formulations: a retrospective cohort study. Ann Intern Med. 2022;175:656-664.
- Froissart A, Rossi B, Ranque B, et al; SiMFI Group. Effect of a red blood cell transfusion on biological markers used to determine the cause of anemia: a prospective study. Am J Med. 2018;131:319-322.
- Carson JL, Brittenham GM. How I treat anemia with red blood cell transfusion and iron. Blood. 2022;blood.2022018521.
- Borzutzky C, Jaffray J. Diagnosis and management of heavy menstrual bleeding and bleeding disorders in adolescents. JAMA Pediatr. 2020;174:186-194.
Recurrent episodic blood loss from normal menstruation is not expected to result in anemia. But without treatment, chronic heavy periods will progress through the stages of low iron stores to iron deficiency and then to anemia. When iron storage levels are low, the bone marrow’s blood cell factory cannot keep up with continued losses. Every patient with heavy menstrual bleeding (HMB) or prolonged menstrual episodes should be tested and treated for iron deficiency and anemia.1,2
Particular attention should be paid to assessment of iron storage levels with serum ferritin, recognizing that low iron levels progress to anemia once the storage is depleted. Recovery from anemia is much slower in individuals with iron deficiency, so assessment for iron storage also should be included in preoperative assessments and following a diagnosis of acute blood loss anemia.
The mechanics of erythropoiesis, hemoglobin, and oxygen transport
Red blood cells (erythrocytes) have a short life cycle and require constant replacement. Erythrocytes are generated on demand in erythropoiesis by a hormonal signaling process, regardless of whether sufficient components are available.3 Hemoglobin, the main intracellular component of erythrocytes, is comprised of 4 globin chains, which each contain 1 iron atom bound to a heme molecule. After erythrocytes are assembled, they are sent out into circulation for approximately 120 days. A hemoglobin level measures the oxygen-carrying capacity of erythrocytes, and anemia is defined as hemoglobin less than 12 g/dL.
Unless erythrocytes are lost from bleeding, they are decommissioned—that is, the heme molecule is metabolized into bilirubin and excreted, and the iron atoms are recycled back to the bone marrow or to storage.4 Ferritin is the storage molecule that binds to iron, a glycoprotein with numerous subunits around a core that can contain about 4,000 iron atoms. Most ferritin is intracellular, but a small proportion is present in serum, where it can be measured.
Serum ferritin is a good marker for the iron supply in healthy individuals because it has high correlation to iron in the bone marrow and correlates to total intracellular storage unless there is inflammation, when mobilization to serum increases. The ferritin level at which the iron supply is deficient to meet demand, defined as iron deficiency, is hotly debated and ranges from less than 15 to 50 ng/mL in menstruating individuals, with higher thresholds based on onset of erythropoiesis signaling and the lower threshold being the World Health Organization recommendation.5-7 When iron atoms are in short supply, erythrocytes still are generated but they have lower amounts of intracellular hemoglobin, which makes them thinner, smaller, and paler—and less effective at oxygen transport.

CASE Patient seeks treatment for HMB-associated symptoms
A 17-year-old patient presents with HMB, fatigue, and difficulty with concentration. She reports that her periods have been regular and lasting 7 days since menarche at age 13. While they are manageable, they seem to be getting heavier, soaking pads in 2 to 3 hours. The patient reports that she would like to start treatment for her progressively heavy bleeding and prefers lighter scheduled bleeding; she currently does not desire contraception. The patient has no family history of bleeding problems and self-reports no personal history of epistaxis or bleeding with tooth extraction or tonsillectomy. Laboratory tests confirm iron deficiency with a hemoglobin level of 12.5 g/dL (reference range, 12.0–17.5 g/dL) and a serum ferritin level of 8 ng/mL (reference range, 50–420 ng/mL). Results from a coagulopathy panel are normal, as are von Willebrand factor levels.
Untreated iron deficiency will progress to anemia
This patient has iron deficiency without anemia, which warrants significant attention in HMB because without treatment it eventually will progress to anemia. The prevalence of iron deficiency, which makes up half of all causes of anemia, is at least double that of iron deficiency anemia.3
Adult bodies usually contain about 3 to 4 g of iron, with two-thirds in erythrocytes as hemoglobin.8 Approximately 40 to 60 mg of iron is recycled daily, 1 to 2 mg/day is lost from sloughed cells and sweat, and at least 1 mg/day is lost during normal menstruation. These losses are balanced with gastrointestinal uptake of 1 to 2 mg/day until bleeding exceeds about 10 mL/day. In this 17-year-old patient, iron stores have likely been on a progressive decline since menarche.
For normally menstruating individuals to maintain iron homeostasis, the daily dietary iron requirement is 18 mg/day. Iron requirements also increase during periods of illness or inflammation due to hormonal signaling in the iron absorption and transport pathway, in athletes due to sweating, foot strike hemolysis and bruising, and during growth spurts.9
Continue to: Managing iron deficiency and anemia...
Managing iron deficiency and anemia
Management of iron deficiency and iron deficiency anemia in the setting of HMB includes:
- workup for the etiology of the abnormal uterine bleeding (TABLE)
- reducing the source of blood loss, and
- iron supplementation to correct the iron deficiency state.
In most cases, workup, reduction, and repletion can occur simultaneously. The goal is not always complete cessation of menstrual bleeding; even short-term therapy can allow time to replenish iron storage. Use a shared decision-making process to assess what is important to the patient, and provide information about relative amounts of bleeding cessation that can be expected with various therapies.10

Treatment options
Medical treatments to decrease menstrual iron losses are recommended prior to proceeding with surgical interventions.11 Hormonal treatments are the most consistently recommended, with many guidelines citing the 52-mg levonorgestrel-releasing intrauterine device (LNG IUD) as first-line treatment due to its substantial reduction in the amount of bleeding, HMB treatment indication approved by the US Food and Drug Administration (FDA), and evidence of success in those with HMB.12
Any progestin or combined hormonal medication with estrogen and a progestin will result in an approximately 60% to 90% bleeding reduction, thus providing many effective options for blood loss while considering patient preferences for bleeding pattern, route of administration, and concomitant benefits. While only 1 oral product (estradiol valerate/dienogest) is FDA approved for managementof HMB, use of any of the commercially available contraceptive products will provide substantial benefit.11,13
Nonhormonal options, such as antifibrinolytics and nonsteroidal anti-inflammatory drugs (NSAIDs), tend to be listed as second-line therapies or for those who want to avoid hormonal medications. Antifibrinolytics, such as tranexamic acid, require frequent dosing of large pills and result in approximately 40% blood loss reduction, but they are a very successful and well-tolerated method for those seeking on-demand therapy.14 NSAIDs may result in a slight bleeding reduction, but they are far less effective than other therapies.15 Antifibrinolytics have a theoretical risk of thrombosis and a contraindication to use with hormonal contraceptives; therefore, concomitant use with estrogen-containing medications is reserved for patients with refractory heavy bleeding or for heavy bleeding days during the hormone-free interval, when benefits likely outweigh potential risk.16,17
Guidelines for medical management of acute HMB typically cite 3 small comparative studies with high-dose regimens of parenteral conjugated estrogen, combined ethinyl estradiol and progestin, or oral medroxyprogesterone acetate.18,19 Dosing recommendations for the oral medications include a loading dose followed by a taper regimen that is poorly tolerated and for which there is no evidence of superior effectiveness over the standard dose.20,21In most cases, initiation of the preferred long-term hormonal medication plan will reduce bleeding significantly within 2 to 3 days. Many clinicians who commonly treat acute HMB prescribe norethindrone acetate 5 mg daily (up to 3 times daily, if needed) for effective and safe menstrual suppression.22
Iron replenishment: Dosing frequency, dietary iron, and multivitamins
Iron repletion is usually via the oral route unless surgery is imminent, anemia is severe, or the oral route is not tolerated or effective.23 Oral iron has substantial adverse effects that limit tolerance, including nausea, epigastric pain, diarrhea, and constipation. Fortunately, evidence supports lower oral iron doses than previously used.4
Iron homeostasis is controlled by the peptide hormone hepcidin, produced by the liver, which controls mobilization of iron from the gut and spleen and aids iron absorption from the diet and supplements.24 Hepcidin levels decrease in response to high circulating levels of iron, so the ideal iron repletion dose in iron-deficient nonanemic women was determined by assessing the dose response of hepcidin. Researchers compared iron 60 mg daily for 14 days versus every other day for 28 days and found that iron absorption was greater in the every-other-day group (21.8% vs 16.3%).25They concluded that changing iron administration to 60 mg or more in a single dose every other day is most efficient in those with iron deficiency without anemia. Since study participants did not have anemia, research is pending on whether different strategies (such as daily dosing) are more effective for more severe cases. The bottom line is that conventional high-dose divided daily oral iron administration results in reduced iron bioavailability compared with alternate-day dosing.
Increasing dietary iron is insufficient to treat low iron storage, iron deficiency, and iron deficiency anemia. Likewise, multivitamins, which contain very little elemental iron, are not recommended for repletion. Any iron salt with 60 to 120 mg of elemental iron can be used (for examples, ferrous sulfate, ferrous gluconate).25 Once ingested, stomach and pancreatic acids release elemental iron from its bound form. For that reason, absorption may be improved by administering iron at least 1 hour before a meal and avoiding antacids, including milk. Meat proteins and ascorbic acid help maintain the soluble ferrous form and also aid absorption. Tea, coffee, and tannins prevent absorption when polyphenol compounds form an insoluble complex with iron (see box at end of article). Gastrointestinal adverse effects can be minimized by decreasing the dose and taking after meals, although with reduced efficacy.
Intravenous iron treatment raises hemoglobin levels significantly faster than oral administration but is limited by cost and availability, so it is reserved for individuals with a hemoglobin level less than 9 g/dL, prior gastrointestinal or bariatric surgery, imminent surgery, and intolerance, poor adherence, or nonresponse to oral iron therapy. Several approved formulations are available, all with equivalent effectiveness and similar safety profiles. Lower-dose formulations (such as iron sucrose) may require several infusions, but higher-dose intravenous iron products (ferric carboxymaltose, low-molecular weight iron dextran, etc) have a stable carbohydrate shell that inhibits free iron release and improves safety, allowing a single administration.26
Common adverse effects of intravenous iron treatment include a metallic taste and headache during administration. More serious adverse effects, such as hypotension, arthralgia, malaise, and nausea, are usually self-limited. With mild infusion reactions (1 in 200), the infusion can be stopped until symptoms improve and can be resumed at a slower rate.27
Continue to: The role of blood transfusion...
The role of blood transfusion
Blood transfusion is expensive and potentially hazardous, so its use is limited to treatment of acute blood loss or severe anemia.
A one-time red blood cell transfusion does not impact diagnostic criteria to assess for iron deficiency with ferritin, and it does not improve underlying iron deficiency.28Patients with acute blood loss anemia superimposed on chronic blood loss should be screened and treated for iron deficiency even after receiving a transfusion.
Since ferritin levels can rise significantly as an acute phase reactant, even following a hemorrhage, iron deficiency during inflammation is defined as ferritin less than 70 ng/mL.
The potential for iron overload
Since iron is never metabolized or excreted, it is possible to have iron overload following accidental overdose, transfusion dependency, and disorders of iron transport, such as hemochromatosis and thalassemia.
While a low ferritin level always indicates iron deficiency, high ferritin levels can be an acute phase reactant. Ferritin levels greater than 150 ng/mL in healthy menstruating individuals and greater than 500 ng/mL in unhealthy individuals should raise concern for excess iron and should prompt discontinuation of iron intake or workup for conditions at risk for overload.5
Oral iron supplements should be stored away from small children, who are at particular risk of toxicity.
How long to treat?
Treatment duration depends on the individual’s degree of iron deficiency, whether anemia is present, and the amount of ongoing blood loss. The main treatment goal is normalization and maintenance of serum ferritin.
Successful treatment should be confirmed with a complete blood count and ferritin level. Hemoglobin levels improve 2 g/dL after 3 weeks of oral iron therapy, but repletion may take 4 to 6 months.23,29 The American College of Obstetricians and Gynecologists recommends 3 to 6 months of continued iron therapy after resolution of HMB.19
In a comparative study of treatment for HMB with the 52-mg LNG IUD versus hysterectomy, hemoglobin levels increased in both treatment groups but stayed lower in those with initial anemia.8 Ferritin levels normalized only after 5 years and were still lower in individuals with initial anemia.
Increase in hemoglobin is faster after intravenous iron administration but is equivalent to oral therapy by 12 weeks. If management to reduce menstrual losses is discontinued, periodic or maintenance iron repletion will be necessary.
CASE Management plan initiated
This 17-year-old patient with iron deficiency resulting from HMB requests management to reduce menstrual iron losses with a preference for predictable menses. We have already completed a basic workup, which could also include assessment for hypermobility with a Beighton score, as connective tissue disorders also are associated with HMB.30 We discuss the options of cyclic hormonal therapy, antifibrinolytic treatment, and an LNG IUD. The patient is concerned about adherence and wants to avoid unscheduled bleeding, so she opts for a trial of tranexamic acid 1,300 mg 3 times daily for 5 days during menses. This regimen results in a 50% reduction in bleeding amount, which the patient finds satisfactory. Iron repletion with oral ferrous sulfate 325 mg (containing 65 mg of elemental iron) is administered on alternating days with vitamin C taken 1 hour prior to dinner. Repeat laboratory test results at 3 weeks show improvement to a hemoglobin level of 14.2 g/dL and a ferritin level of 12 ng/mL. By 3 months, her ferritin levels are greater than 30 ng/mL and oral iron is administered only during menses.
Summing up
Chronic HMB results in a progressive net loss of iron and eventual anemia. Screening with complete blood count and ferritin and early treatment of low iron storage when ferritin is less than 30 ng/mL will help avoid symptoms. Any amount of reduction of menstrual blood loss can be beneficial, allowing a variety of effective hormonal and nonhormonal treatment options. ●
- Take 60 to 120 mg elemental iron every other day.
- To help with absorption:
—Take 1 hour before a meal, but not with coffee, tea, tannins, antacids, or milk
—Take with vitamin C or other acidic fruit juice
- Recheck complete blood count and ferritin in 2 to 3 weeks to confirm initial response.
- Continue treatment for up to 3 to 6 months until ferritin levels are greater than 30 to 50 ng/mL.
Recurrent episodic blood loss from normal menstruation is not expected to result in anemia. But without treatment, chronic heavy periods will progress through the stages of low iron stores to iron deficiency and then to anemia. When iron storage levels are low, the bone marrow’s blood cell factory cannot keep up with continued losses. Every patient with heavy menstrual bleeding (HMB) or prolonged menstrual episodes should be tested and treated for iron deficiency and anemia.1,2
Particular attention should be paid to assessment of iron storage levels with serum ferritin, recognizing that low iron levels progress to anemia once the storage is depleted. Recovery from anemia is much slower in individuals with iron deficiency, so assessment for iron storage also should be included in preoperative assessments and following a diagnosis of acute blood loss anemia.
The mechanics of erythropoiesis, hemoglobin, and oxygen transport
Red blood cells (erythrocytes) have a short life cycle and require constant replacement. Erythrocytes are generated on demand in erythropoiesis by a hormonal signaling process, regardless of whether sufficient components are available.3 Hemoglobin, the main intracellular component of erythrocytes, is comprised of 4 globin chains, which each contain 1 iron atom bound to a heme molecule. After erythrocytes are assembled, they are sent out into circulation for approximately 120 days. A hemoglobin level measures the oxygen-carrying capacity of erythrocytes, and anemia is defined as hemoglobin less than 12 g/dL.
Unless erythrocytes are lost from bleeding, they are decommissioned—that is, the heme molecule is metabolized into bilirubin and excreted, and the iron atoms are recycled back to the bone marrow or to storage.4 Ferritin is the storage molecule that binds to iron, a glycoprotein with numerous subunits around a core that can contain about 4,000 iron atoms. Most ferritin is intracellular, but a small proportion is present in serum, where it can be measured.
Serum ferritin is a good marker for the iron supply in healthy individuals because it has high correlation to iron in the bone marrow and correlates to total intracellular storage unless there is inflammation, when mobilization to serum increases. The ferritin level at which the iron supply is deficient to meet demand, defined as iron deficiency, is hotly debated and ranges from less than 15 to 50 ng/mL in menstruating individuals, with higher thresholds based on onset of erythropoiesis signaling and the lower threshold being the World Health Organization recommendation.5-7 When iron atoms are in short supply, erythrocytes still are generated but they have lower amounts of intracellular hemoglobin, which makes them thinner, smaller, and paler—and less effective at oxygen transport.

CASE Patient seeks treatment for HMB-associated symptoms
A 17-year-old patient presents with HMB, fatigue, and difficulty with concentration. She reports that her periods have been regular and lasting 7 days since menarche at age 13. While they are manageable, they seem to be getting heavier, soaking pads in 2 to 3 hours. The patient reports that she would like to start treatment for her progressively heavy bleeding and prefers lighter scheduled bleeding; she currently does not desire contraception. The patient has no family history of bleeding problems and self-reports no personal history of epistaxis or bleeding with tooth extraction or tonsillectomy. Laboratory tests confirm iron deficiency with a hemoglobin level of 12.5 g/dL (reference range, 12.0–17.5 g/dL) and a serum ferritin level of 8 ng/mL (reference range, 50–420 ng/mL). Results from a coagulopathy panel are normal, as are von Willebrand factor levels.
Untreated iron deficiency will progress to anemia
This patient has iron deficiency without anemia, which warrants significant attention in HMB because without treatment it eventually will progress to anemia. The prevalence of iron deficiency, which makes up half of all causes of anemia, is at least double that of iron deficiency anemia.3
Adult bodies usually contain about 3 to 4 g of iron, with two-thirds in erythrocytes as hemoglobin.8 Approximately 40 to 60 mg of iron is recycled daily, 1 to 2 mg/day is lost from sloughed cells and sweat, and at least 1 mg/day is lost during normal menstruation. These losses are balanced with gastrointestinal uptake of 1 to 2 mg/day until bleeding exceeds about 10 mL/day. In this 17-year-old patient, iron stores have likely been on a progressive decline since menarche.
For normally menstruating individuals to maintain iron homeostasis, the daily dietary iron requirement is 18 mg/day. Iron requirements also increase during periods of illness or inflammation due to hormonal signaling in the iron absorption and transport pathway, in athletes due to sweating, foot strike hemolysis and bruising, and during growth spurts.9
Continue to: Managing iron deficiency and anemia...
Managing iron deficiency and anemia
Management of iron deficiency and iron deficiency anemia in the setting of HMB includes:
- workup for the etiology of the abnormal uterine bleeding (TABLE)
- reducing the source of blood loss, and
- iron supplementation to correct the iron deficiency state.
In most cases, workup, reduction, and repletion can occur simultaneously. The goal is not always complete cessation of menstrual bleeding; even short-term therapy can allow time to replenish iron storage. Use a shared decision-making process to assess what is important to the patient, and provide information about relative amounts of bleeding cessation that can be expected with various therapies.10

Treatment options
Medical treatments to decrease menstrual iron losses are recommended prior to proceeding with surgical interventions.11 Hormonal treatments are the most consistently recommended, with many guidelines citing the 52-mg levonorgestrel-releasing intrauterine device (LNG IUD) as first-line treatment due to its substantial reduction in the amount of bleeding, HMB treatment indication approved by the US Food and Drug Administration (FDA), and evidence of success in those with HMB.12
Any progestin or combined hormonal medication with estrogen and a progestin will result in an approximately 60% to 90% bleeding reduction, thus providing many effective options for blood loss while considering patient preferences for bleeding pattern, route of administration, and concomitant benefits. While only 1 oral product (estradiol valerate/dienogest) is FDA approved for managementof HMB, use of any of the commercially available contraceptive products will provide substantial benefit.11,13
Nonhormonal options, such as antifibrinolytics and nonsteroidal anti-inflammatory drugs (NSAIDs), tend to be listed as second-line therapies or for those who want to avoid hormonal medications. Antifibrinolytics, such as tranexamic acid, require frequent dosing of large pills and result in approximately 40% blood loss reduction, but they are a very successful and well-tolerated method for those seeking on-demand therapy.14 NSAIDs may result in a slight bleeding reduction, but they are far less effective than other therapies.15 Antifibrinolytics have a theoretical risk of thrombosis and a contraindication to use with hormonal contraceptives; therefore, concomitant use with estrogen-containing medications is reserved for patients with refractory heavy bleeding or for heavy bleeding days during the hormone-free interval, when benefits likely outweigh potential risk.16,17
Guidelines for medical management of acute HMB typically cite 3 small comparative studies with high-dose regimens of parenteral conjugated estrogen, combined ethinyl estradiol and progestin, or oral medroxyprogesterone acetate.18,19 Dosing recommendations for the oral medications include a loading dose followed by a taper regimen that is poorly tolerated and for which there is no evidence of superior effectiveness over the standard dose.20,21In most cases, initiation of the preferred long-term hormonal medication plan will reduce bleeding significantly within 2 to 3 days. Many clinicians who commonly treat acute HMB prescribe norethindrone acetate 5 mg daily (up to 3 times daily, if needed) for effective and safe menstrual suppression.22
Iron replenishment: Dosing frequency, dietary iron, and multivitamins
Iron repletion is usually via the oral route unless surgery is imminent, anemia is severe, or the oral route is not tolerated or effective.23 Oral iron has substantial adverse effects that limit tolerance, including nausea, epigastric pain, diarrhea, and constipation. Fortunately, evidence supports lower oral iron doses than previously used.4
Iron homeostasis is controlled by the peptide hormone hepcidin, produced by the liver, which controls mobilization of iron from the gut and spleen and aids iron absorption from the diet and supplements.24 Hepcidin levels decrease in response to high circulating levels of iron, so the ideal iron repletion dose in iron-deficient nonanemic women was determined by assessing the dose response of hepcidin. Researchers compared iron 60 mg daily for 14 days versus every other day for 28 days and found that iron absorption was greater in the every-other-day group (21.8% vs 16.3%).25They concluded that changing iron administration to 60 mg or more in a single dose every other day is most efficient in those with iron deficiency without anemia. Since study participants did not have anemia, research is pending on whether different strategies (such as daily dosing) are more effective for more severe cases. The bottom line is that conventional high-dose divided daily oral iron administration results in reduced iron bioavailability compared with alternate-day dosing.
Increasing dietary iron is insufficient to treat low iron storage, iron deficiency, and iron deficiency anemia. Likewise, multivitamins, which contain very little elemental iron, are not recommended for repletion. Any iron salt with 60 to 120 mg of elemental iron can be used (for examples, ferrous sulfate, ferrous gluconate).25 Once ingested, stomach and pancreatic acids release elemental iron from its bound form. For that reason, absorption may be improved by administering iron at least 1 hour before a meal and avoiding antacids, including milk. Meat proteins and ascorbic acid help maintain the soluble ferrous form and also aid absorption. Tea, coffee, and tannins prevent absorption when polyphenol compounds form an insoluble complex with iron (see box at end of article). Gastrointestinal adverse effects can be minimized by decreasing the dose and taking after meals, although with reduced efficacy.
Intravenous iron treatment raises hemoglobin levels significantly faster than oral administration but is limited by cost and availability, so it is reserved for individuals with a hemoglobin level less than 9 g/dL, prior gastrointestinal or bariatric surgery, imminent surgery, and intolerance, poor adherence, or nonresponse to oral iron therapy. Several approved formulations are available, all with equivalent effectiveness and similar safety profiles. Lower-dose formulations (such as iron sucrose) may require several infusions, but higher-dose intravenous iron products (ferric carboxymaltose, low-molecular weight iron dextran, etc) have a stable carbohydrate shell that inhibits free iron release and improves safety, allowing a single administration.26
Common adverse effects of intravenous iron treatment include a metallic taste and headache during administration. More serious adverse effects, such as hypotension, arthralgia, malaise, and nausea, are usually self-limited. With mild infusion reactions (1 in 200), the infusion can be stopped until symptoms improve and can be resumed at a slower rate.27
Continue to: The role of blood transfusion...
The role of blood transfusion
Blood transfusion is expensive and potentially hazardous, so its use is limited to treatment of acute blood loss or severe anemia.
A one-time red blood cell transfusion does not impact diagnostic criteria to assess for iron deficiency with ferritin, and it does not improve underlying iron deficiency.28Patients with acute blood loss anemia superimposed on chronic blood loss should be screened and treated for iron deficiency even after receiving a transfusion.
Since ferritin levels can rise significantly as an acute phase reactant, even following a hemorrhage, iron deficiency during inflammation is defined as ferritin less than 70 ng/mL.
The potential for iron overload
Since iron is never metabolized or excreted, it is possible to have iron overload following accidental overdose, transfusion dependency, and disorders of iron transport, such as hemochromatosis and thalassemia.
While a low ferritin level always indicates iron deficiency, high ferritin levels can be an acute phase reactant. Ferritin levels greater than 150 ng/mL in healthy menstruating individuals and greater than 500 ng/mL in unhealthy individuals should raise concern for excess iron and should prompt discontinuation of iron intake or workup for conditions at risk for overload.5
Oral iron supplements should be stored away from small children, who are at particular risk of toxicity.
How long to treat?
Treatment duration depends on the individual’s degree of iron deficiency, whether anemia is present, and the amount of ongoing blood loss. The main treatment goal is normalization and maintenance of serum ferritin.
Successful treatment should be confirmed with a complete blood count and ferritin level. Hemoglobin levels improve 2 g/dL after 3 weeks of oral iron therapy, but repletion may take 4 to 6 months.23,29 The American College of Obstetricians and Gynecologists recommends 3 to 6 months of continued iron therapy after resolution of HMB.19
In a comparative study of treatment for HMB with the 52-mg LNG IUD versus hysterectomy, hemoglobin levels increased in both treatment groups but stayed lower in those with initial anemia.8 Ferritin levels normalized only after 5 years and were still lower in individuals with initial anemia.
Increase in hemoglobin is faster after intravenous iron administration but is equivalent to oral therapy by 12 weeks. If management to reduce menstrual losses is discontinued, periodic or maintenance iron repletion will be necessary.
CASE Management plan initiated
This 17-year-old patient with iron deficiency resulting from HMB requests management to reduce menstrual iron losses with a preference for predictable menses. We have already completed a basic workup, which could also include assessment for hypermobility with a Beighton score, as connective tissue disorders also are associated with HMB.30 We discuss the options of cyclic hormonal therapy, antifibrinolytic treatment, and an LNG IUD. The patient is concerned about adherence and wants to avoid unscheduled bleeding, so she opts for a trial of tranexamic acid 1,300 mg 3 times daily for 5 days during menses. This regimen results in a 50% reduction in bleeding amount, which the patient finds satisfactory. Iron repletion with oral ferrous sulfate 325 mg (containing 65 mg of elemental iron) is administered on alternating days with vitamin C taken 1 hour prior to dinner. Repeat laboratory test results at 3 weeks show improvement to a hemoglobin level of 14.2 g/dL and a ferritin level of 12 ng/mL. By 3 months, her ferritin levels are greater than 30 ng/mL and oral iron is administered only during menses.
Summing up
Chronic HMB results in a progressive net loss of iron and eventual anemia. Screening with complete blood count and ferritin and early treatment of low iron storage when ferritin is less than 30 ng/mL will help avoid symptoms. Any amount of reduction of menstrual blood loss can be beneficial, allowing a variety of effective hormonal and nonhormonal treatment options. ●
- Take 60 to 120 mg elemental iron every other day.
- To help with absorption:
—Take 1 hour before a meal, but not with coffee, tea, tannins, antacids, or milk
—Take with vitamin C or other acidic fruit juice
- Recheck complete blood count and ferritin in 2 to 3 weeks to confirm initial response.
- Continue treatment for up to 3 to 6 months until ferritin levels are greater than 30 to 50 ng/mL.
- Munro MG, Mast AE, Powers JM, et al. The relationship between heavy menstrual bleeding, iron deficiency, and iron deficiency anemia. Am J Obstet Gynecol. 2023;S00029378(23)00024-8.
- Tsakiridis I, Giouleka S, Koutsouki G, et al. Investigation and management of abnormal uterine bleeding in reproductive aged women: a descriptive review of national and international recommendations. Eur J Contracept Reprod Health Care. 2022;27:504-517.
- Camaschella C. Iron deficiency. Blood. 2019;133:30-39.
- Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272.
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. April 21, 2020. Accessed February 17, 2023. https://www.who.int/publications/i/item/9789240000124
- Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8: e572-e582.
- Galetti V, Stoffel NU, Sieber C, et al. Threshold ferritin and hepcidin concentrations indicating early iron deficiency in young women based on upregulation of iron absorption. EClinicalMedicine. 2021;39:101052.
- Percy L, Mansour D, Fraser I. Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol. 2017;40:55-67.
- Brittenham GM. Short-term periods of strenuous physical activity lower iron absorption. Am J Clin Nutr. 2021;113:261-262.
- Chen M, Lindley A, Kimport K, et al. An in-depth analysis of the use of shared decision making in contraceptive counseling. Contraception. 2019;99:187-191.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180.
- Mansour D, Hofmann A, Gemzell-Danielsson K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv Ther. 2021;38:201-225.
- Micks EA, Jensen JT. Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill. Adv Ther. 2013;30:1-13.
- Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;4:CD000249.
- Bofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;9:CD000400.
- Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract T hromb Haemost. 2021;5:e12546.
- Reid RL, Westhoff C, Mansour D, et al. Oral contraceptives and venous thromboembolism consensus opinion from an international workshop held in Berlin, Germany in December 2009. J Fam Plann Reprod Health Care. 2010;36:117-122.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 785: screening and management of bleeding disorders in adolescents with heavy menstrual bleeding. Obstet Gynecol. 2019;134:e71-e83.
- Haamid F, Sass AE, Dietrich JE. Heavy menstrual bleeding in adolescents. J Pediatr Adolesc Gynecol. 2017;30:335-340.
- Roth LP, Haley KM, Baldwin MK. A retrospective comparison of time to cessation of acute heavy menstrual bleeding in adolescents following two dose regimens of combined oral hormonal therapy. J Pediatr Adolesc Gynecol. 2022;35:294-298.
- Huguelet PS, Buyers EM, Lange-Liss JH, et al. Treatment of acute abnormal uterine bleeding in adolescents: what are providers doing in various specialties? J Pediatr Adolesc Gynecol. 2016;29:286-291.
- Elstrott B, Khan L, Olson S, et al. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. 2020;104:153-161.
- Pagani A, Nai A, Silvestri L, et al. Hepcidin and anemia: a tight relationship. Front Physiol. 2019;10:1294.
- Stoffel NU, von Siebenthal HK, Moretti D, et al. Oral iron supplementation in iron-deficient women: how much and how often? Mol Aspects Med. 2020;75:100865.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Dave CV, Brittenham GM, Carson JL, et al. Risks for anaphylaxis with intravenous iron formulations: a retrospective cohort study. Ann Intern Med. 2022;175:656-664.
- Froissart A, Rossi B, Ranque B, et al; SiMFI Group. Effect of a red blood cell transfusion on biological markers used to determine the cause of anemia: a prospective study. Am J Med. 2018;131:319-322.
- Carson JL, Brittenham GM. How I treat anemia with red blood cell transfusion and iron. Blood. 2022;blood.2022018521.
- Borzutzky C, Jaffray J. Diagnosis and management of heavy menstrual bleeding and bleeding disorders in adolescents. JAMA Pediatr. 2020;174:186-194.
- Munro MG, Mast AE, Powers JM, et al. The relationship between heavy menstrual bleeding, iron deficiency, and iron deficiency anemia. Am J Obstet Gynecol. 2023;S00029378(23)00024-8.
- Tsakiridis I, Giouleka S, Koutsouki G, et al. Investigation and management of abnormal uterine bleeding in reproductive aged women: a descriptive review of national and international recommendations. Eur J Contracept Reprod Health Care. 2022;27:504-517.
- Camaschella C. Iron deficiency. Blood. 2019;133:30-39.
- Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272.
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. April 21, 2020. Accessed February 17, 2023. https://www.who.int/publications/i/item/9789240000124
- Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8: e572-e582.
- Galetti V, Stoffel NU, Sieber C, et al. Threshold ferritin and hepcidin concentrations indicating early iron deficiency in young women based on upregulation of iron absorption. EClinicalMedicine. 2021;39:101052.
- Percy L, Mansour D, Fraser I. Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol. 2017;40:55-67.
- Brittenham GM. Short-term periods of strenuous physical activity lower iron absorption. Am J Clin Nutr. 2021;113:261-262.
- Chen M, Lindley A, Kimport K, et al. An in-depth analysis of the use of shared decision making in contraceptive counseling. Contraception. 2019;99:187-191.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180.
- Mansour D, Hofmann A, Gemzell-Danielsson K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv Ther. 2021;38:201-225.
- Micks EA, Jensen JT. Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill. Adv Ther. 2013;30:1-13.
- Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;4:CD000249.
- Bofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;9:CD000400.
- Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract T hromb Haemost. 2021;5:e12546.
- Reid RL, Westhoff C, Mansour D, et al. Oral contraceptives and venous thromboembolism consensus opinion from an international workshop held in Berlin, Germany in December 2009. J Fam Plann Reprod Health Care. 2010;36:117-122.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 785: screening and management of bleeding disorders in adolescents with heavy menstrual bleeding. Obstet Gynecol. 2019;134:e71-e83.
- Haamid F, Sass AE, Dietrich JE. Heavy menstrual bleeding in adolescents. J Pediatr Adolesc Gynecol. 2017;30:335-340.
- Roth LP, Haley KM, Baldwin MK. A retrospective comparison of time to cessation of acute heavy menstrual bleeding in adolescents following two dose regimens of combined oral hormonal therapy. J Pediatr Adolesc Gynecol. 2022;35:294-298.
- Huguelet PS, Buyers EM, Lange-Liss JH, et al. Treatment of acute abnormal uterine bleeding in adolescents: what are providers doing in various specialties? J Pediatr Adolesc Gynecol. 2016;29:286-291.
- Elstrott B, Khan L, Olson S, et al. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. 2020;104:153-161.
- Pagani A, Nai A, Silvestri L, et al. Hepcidin and anemia: a tight relationship. Front Physiol. 2019;10:1294.
- Stoffel NU, von Siebenthal HK, Moretti D, et al. Oral iron supplementation in iron-deficient women: how much and how often? Mol Aspects Med. 2020;75:100865.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Dave CV, Brittenham GM, Carson JL, et al. Risks for anaphylaxis with intravenous iron formulations: a retrospective cohort study. Ann Intern Med. 2022;175:656-664.
- Froissart A, Rossi B, Ranque B, et al; SiMFI Group. Effect of a red blood cell transfusion on biological markers used to determine the cause of anemia: a prospective study. Am J Med. 2018;131:319-322.
- Carson JL, Brittenham GM. How I treat anemia with red blood cell transfusion and iron. Blood. 2022;blood.2022018521.
- Borzutzky C, Jaffray J. Diagnosis and management of heavy menstrual bleeding and bleeding disorders in adolescents. JAMA Pediatr. 2020;174:186-194.
Prepare for endometriosis excision surgery with a multidisciplinary approach
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.