User login
Should you prescribe bioidentical hormones for menopause?
BALTIMORE – according to an expert at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Clinicians write an estimated 26 to 33 million prescriptions for compounded bioidentical hormone therapy (cBHT) every year, and almost 41% of menopausal women who need treatment try cBHT during their lives. But these drugs lack the approval for this indication from the Food and Drug Administration.
“There is a public perception that this is natural, safer, and anti-aging,” said Robert Kauffman, MD, a professor of obstetrics and gynecology and assistant dean for research at Texas Tech University Health Sciences Center in Amarillo.
Following the 2002 Women’s Health Initiative report showing a link between hormone therapy (HT) and an increase in the incidence of breast cancer, medical schools have slowed or paused instructing trainees on the traditional treatment, Dr. Kauffman said. The association was later determined to be spurious: HT is not associated with a risk for all-cause mortality or deaths from cardiovascular disease or cancer. However, HT still is largely ignored by younger physicians, Dr. Kauffman said, because of unsubstantiated “dangers” such as heart attack, stroke, and deep vein thrombosis.
The lack of education on HT for medical school students and residents has “opened the door to unsubstantiated marketing claims and practices” for cBHT, Dr. Kauffman said. “Hence, the use of compounded bioidentical hormone therapy has increased” as clinicians look for alternatives.
Groups including ACOG, the North American Menopause Society (NAMS), and the U.S. Preventive Services Task Force recommend against the use of Non–FDA-approved therapies such as cBHT, except for narrow indications. Dr. Kauffman said that drug manufacturers have not conducted randomized controlled trials or observational studies on cBHT in treating menopause.
He cited studies showing quality problems with the compounding process of these drugs, and wide variations in the amount of actual ingredients from product labels. One 2021 study published in Menopause comparing patients taking cBHT or FDA-approved HT found that side effects were significantly higher in the cBHT group (57.6% vs. 14.8%; P < .0001).
But manufacturers of cBHT claim that their products prevent cardiovascular disease and Alzheimer’s disease and decrease the risk for breast cancer and stroke – assertions that are at best unproven, according to Dr. Kauffman.
The National Academies of Sciences, Engineering, and Medicine in 2020 said that clinicians have a duty to inform patients of the insufficient evidence to support clinical use of cBHT and should prescribe the products only to patients with documented allergies to an active ingredient in an FDA-approved agent or who require an alternative dosage.
Patients may also have to pay much more out of pocket for cBHT products because they often are not covered by insurance. Generic HT products, meanwhile, are relatively inexpensive and typically are covered, he noted.
“We have to be careful to avoid financial harm to patients by prescribing things, which are much more expensive than those which are usually available,” Dr. Kauffman said.
Prescribing any non–FDA-approved product, especially when biosimilars are available, places physicians at legal risk, Dr. Kauffman said. Physicians who recommend cBHT should inform patients that the products are not FDA approved and carefully document this discussion in the patient’s electronic health record. State boards of medicine can sanction physicians for “coercion” for prescribing cBHT products without mentioning alternatives, he added.
JoAnn Pinkerton, MD, professor of obstetrics and gynecology at the University of Virginia, Charlottesville, and executive director emeritus of NAMS, who attended the session, praised Dr. Kauffman for providing a balanced and evidence-based overview of the subject.
“There are issues concerning safety, contaminants, and not knowing exactly what dose you’re getting,” with compounded hormones, Dr. Pinkerton said. “They’re being hyped as safer and more effective when in reality, we don’t have any studies that show that information.”
Dr. Pinkerton noted that while a compounded form of physiological testosterone might be relatively reliable, “if you’re using something like a pellet that is super physiologic with incredibly high doses, that you really don’t have any information to stand on that it’s safe or effective ... it might be putting your license at risk.”
A version of this article first appeared on Medscape.com.
BALTIMORE – according to an expert at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Clinicians write an estimated 26 to 33 million prescriptions for compounded bioidentical hormone therapy (cBHT) every year, and almost 41% of menopausal women who need treatment try cBHT during their lives. But these drugs lack the approval for this indication from the Food and Drug Administration.
“There is a public perception that this is natural, safer, and anti-aging,” said Robert Kauffman, MD, a professor of obstetrics and gynecology and assistant dean for research at Texas Tech University Health Sciences Center in Amarillo.
Following the 2002 Women’s Health Initiative report showing a link between hormone therapy (HT) and an increase in the incidence of breast cancer, medical schools have slowed or paused instructing trainees on the traditional treatment, Dr. Kauffman said. The association was later determined to be spurious: HT is not associated with a risk for all-cause mortality or deaths from cardiovascular disease or cancer. However, HT still is largely ignored by younger physicians, Dr. Kauffman said, because of unsubstantiated “dangers” such as heart attack, stroke, and deep vein thrombosis.
The lack of education on HT for medical school students and residents has “opened the door to unsubstantiated marketing claims and practices” for cBHT, Dr. Kauffman said. “Hence, the use of compounded bioidentical hormone therapy has increased” as clinicians look for alternatives.
Groups including ACOG, the North American Menopause Society (NAMS), and the U.S. Preventive Services Task Force recommend against the use of Non–FDA-approved therapies such as cBHT, except for narrow indications. Dr. Kauffman said that drug manufacturers have not conducted randomized controlled trials or observational studies on cBHT in treating menopause.
He cited studies showing quality problems with the compounding process of these drugs, and wide variations in the amount of actual ingredients from product labels. One 2021 study published in Menopause comparing patients taking cBHT or FDA-approved HT found that side effects were significantly higher in the cBHT group (57.6% vs. 14.8%; P < .0001).
But manufacturers of cBHT claim that their products prevent cardiovascular disease and Alzheimer’s disease and decrease the risk for breast cancer and stroke – assertions that are at best unproven, according to Dr. Kauffman.
The National Academies of Sciences, Engineering, and Medicine in 2020 said that clinicians have a duty to inform patients of the insufficient evidence to support clinical use of cBHT and should prescribe the products only to patients with documented allergies to an active ingredient in an FDA-approved agent or who require an alternative dosage.
Patients may also have to pay much more out of pocket for cBHT products because they often are not covered by insurance. Generic HT products, meanwhile, are relatively inexpensive and typically are covered, he noted.
“We have to be careful to avoid financial harm to patients by prescribing things, which are much more expensive than those which are usually available,” Dr. Kauffman said.
Prescribing any non–FDA-approved product, especially when biosimilars are available, places physicians at legal risk, Dr. Kauffman said. Physicians who recommend cBHT should inform patients that the products are not FDA approved and carefully document this discussion in the patient’s electronic health record. State boards of medicine can sanction physicians for “coercion” for prescribing cBHT products without mentioning alternatives, he added.
JoAnn Pinkerton, MD, professor of obstetrics and gynecology at the University of Virginia, Charlottesville, and executive director emeritus of NAMS, who attended the session, praised Dr. Kauffman for providing a balanced and evidence-based overview of the subject.
“There are issues concerning safety, contaminants, and not knowing exactly what dose you’re getting,” with compounded hormones, Dr. Pinkerton said. “They’re being hyped as safer and more effective when in reality, we don’t have any studies that show that information.”
Dr. Pinkerton noted that while a compounded form of physiological testosterone might be relatively reliable, “if you’re using something like a pellet that is super physiologic with incredibly high doses, that you really don’t have any information to stand on that it’s safe or effective ... it might be putting your license at risk.”
A version of this article first appeared on Medscape.com.
BALTIMORE – according to an expert at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Clinicians write an estimated 26 to 33 million prescriptions for compounded bioidentical hormone therapy (cBHT) every year, and almost 41% of menopausal women who need treatment try cBHT during their lives. But these drugs lack the approval for this indication from the Food and Drug Administration.
“There is a public perception that this is natural, safer, and anti-aging,” said Robert Kauffman, MD, a professor of obstetrics and gynecology and assistant dean for research at Texas Tech University Health Sciences Center in Amarillo.
Following the 2002 Women’s Health Initiative report showing a link between hormone therapy (HT) and an increase in the incidence of breast cancer, medical schools have slowed or paused instructing trainees on the traditional treatment, Dr. Kauffman said. The association was later determined to be spurious: HT is not associated with a risk for all-cause mortality or deaths from cardiovascular disease or cancer. However, HT still is largely ignored by younger physicians, Dr. Kauffman said, because of unsubstantiated “dangers” such as heart attack, stroke, and deep vein thrombosis.
The lack of education on HT for medical school students and residents has “opened the door to unsubstantiated marketing claims and practices” for cBHT, Dr. Kauffman said. “Hence, the use of compounded bioidentical hormone therapy has increased” as clinicians look for alternatives.
Groups including ACOG, the North American Menopause Society (NAMS), and the U.S. Preventive Services Task Force recommend against the use of Non–FDA-approved therapies such as cBHT, except for narrow indications. Dr. Kauffman said that drug manufacturers have not conducted randomized controlled trials or observational studies on cBHT in treating menopause.
He cited studies showing quality problems with the compounding process of these drugs, and wide variations in the amount of actual ingredients from product labels. One 2021 study published in Menopause comparing patients taking cBHT or FDA-approved HT found that side effects were significantly higher in the cBHT group (57.6% vs. 14.8%; P < .0001).
But manufacturers of cBHT claim that their products prevent cardiovascular disease and Alzheimer’s disease and decrease the risk for breast cancer and stroke – assertions that are at best unproven, according to Dr. Kauffman.
The National Academies of Sciences, Engineering, and Medicine in 2020 said that clinicians have a duty to inform patients of the insufficient evidence to support clinical use of cBHT and should prescribe the products only to patients with documented allergies to an active ingredient in an FDA-approved agent or who require an alternative dosage.
Patients may also have to pay much more out of pocket for cBHT products because they often are not covered by insurance. Generic HT products, meanwhile, are relatively inexpensive and typically are covered, he noted.
“We have to be careful to avoid financial harm to patients by prescribing things, which are much more expensive than those which are usually available,” Dr. Kauffman said.
Prescribing any non–FDA-approved product, especially when biosimilars are available, places physicians at legal risk, Dr. Kauffman said. Physicians who recommend cBHT should inform patients that the products are not FDA approved and carefully document this discussion in the patient’s electronic health record. State boards of medicine can sanction physicians for “coercion” for prescribing cBHT products without mentioning alternatives, he added.
JoAnn Pinkerton, MD, professor of obstetrics and gynecology at the University of Virginia, Charlottesville, and executive director emeritus of NAMS, who attended the session, praised Dr. Kauffman for providing a balanced and evidence-based overview of the subject.
“There are issues concerning safety, contaminants, and not knowing exactly what dose you’re getting,” with compounded hormones, Dr. Pinkerton said. “They’re being hyped as safer and more effective when in reality, we don’t have any studies that show that information.”
Dr. Pinkerton noted that while a compounded form of physiological testosterone might be relatively reliable, “if you’re using something like a pellet that is super physiologic with incredibly high doses, that you really don’t have any information to stand on that it’s safe or effective ... it might be putting your license at risk.”
A version of this article first appeared on Medscape.com.
AT ACOG 2023
Female sexual pleasure: Is it in the water?
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. In a secondary analysis also presented at the meeting, the lubricants were found not to alter the vaginal microbiome.
Using these types of lubricants during vaginal intercourse at least once a week over a 4-week period resulted in a statistically significant increase of over four points in the 36-point Female Sexual Function Index (FSFI), a self-reported measure of sexual functioning, for participants, said Michael Krychman, MD, executive director of the Southern California Center for Sexual Health and Survivorship Medicine, Newport Beach, the senior author of the study. Statistically significant improvements also were observed in individual areas such as sexual desire and arousal, orgasm, and satisfaction. Results of the study have been published in the Journal of Sexual Medicine.
In the open-label, five-arm, parallel study conducted in Germany, 174 women aged 18-65 years were randomly assigned to use one of five lubricants from three popular brands. After a 4-week run-in period with no use of lubricants, participants were shown how to apply the products and instructed to use the substances during vaginal intercourse at least once a week over a 4-week period.
Participants reported experiencing mild to moderate vaginal dryness and dyspareunia during vaginal intercourse within the previous 3 months.
Statistically significant improvements were seen across all six individual domain scores of the FSFI (desire, arousal, lubrication, orgasm, satisfaction, and pain reduction) from baseline to week 4 with all five lubricants (P < .0001 for lubrication and pain reduction; P < .05 for desire, arousal, orgasm, and satisfaction), according to the researchers.
After 4 weeks, a clinically meaningful improvement in the total FSFI score was observed for four lubricants among premenopausal women and for all lubricants among postmenopausal women. The percentage of participants with sexual function as defined as a score of at least 26.55 on the FSFI was significantly greater after treatment (76.9%) than before treatment (20.8%; P < .0001).
“You would assume if you’re using lubricant it would improve the dryness, but what was very exciting for us is that it improved desire, it improved orgasm, it improved arousal,” Dr. Krychman said in an interview. Like concentric overlapping circles of female sexual function, he said, “if you improve one aspect, you improve the other.”
Nearly 80 nonserious adverse effects occurred in 43 participants, five of which were thought to be possibly attributed to the products, such as vulvovaginal burning, itching, or discomfort. In questionnaires, most women agreed that using the lubricants made sex more enjoyable and provided an overall pleasant experience.
One limitation of the study is that because most participants were Caucasian, the results may not be generalizable to all populations, according to the researchers. Further research is required to fully determine safety and efficacy in patients of all races and ethnicities, they reported, especially given that vaginal dryness has been reported more frequently in non-White ethnic groups.
In a companion presentation, Dr. Krychman discussed another aspect of the study looking at the lubricants’ effects on the vaginal microbiome. Repeated application of the products did not significantly alter the vaginal microbiome for up to 4 weeks, and vaginal pH slightly increased in all treatment groups shortly after use but was restored in most cases after a day.
Water-based lubricants are recommended by the WHO for use with condoms because they do not erode latex, said Karen Adams, MD, professor emeritus of obstetrics and gynecology and founding director of the Menopause and Sexual Medicine Program at Oregon Health & Science University, Portland. Guidelines from the group recommend lubricants should have an osmolality that is as close to normal vaginal secretions as possible to decrease the likelihood of irritation or other side effects, she said. Some available lubricants have four to six times that osmolality, which potentially could dehydrate cells, achieving the opposite of the desired effect.
“The reason this is important is they’re trying to develop lubricants that are more ‘vaginal friendly’ and more in line with the WHO guidelines,” said Dr. Adams, who is joining Stanford (Calif.) University in July to create and lead a new program in menopause and healthy aging. “They came up with four formulas consistent with WHO guidelines to see if these new ones worked at least as well [as commercially available products with higher osmolality], and it turns out they did,” she said. “They worked just fine.”
The study was funded by Reckitt Healthcare. Dr. Krychman is a paid medical consultant for the company. Dr. Adams disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. In a secondary analysis also presented at the meeting, the lubricants were found not to alter the vaginal microbiome.
Using these types of lubricants during vaginal intercourse at least once a week over a 4-week period resulted in a statistically significant increase of over four points in the 36-point Female Sexual Function Index (FSFI), a self-reported measure of sexual functioning, for participants, said Michael Krychman, MD, executive director of the Southern California Center for Sexual Health and Survivorship Medicine, Newport Beach, the senior author of the study. Statistically significant improvements also were observed in individual areas such as sexual desire and arousal, orgasm, and satisfaction. Results of the study have been published in the Journal of Sexual Medicine.
In the open-label, five-arm, parallel study conducted in Germany, 174 women aged 18-65 years were randomly assigned to use one of five lubricants from three popular brands. After a 4-week run-in period with no use of lubricants, participants were shown how to apply the products and instructed to use the substances during vaginal intercourse at least once a week over a 4-week period.
Participants reported experiencing mild to moderate vaginal dryness and dyspareunia during vaginal intercourse within the previous 3 months.
Statistically significant improvements were seen across all six individual domain scores of the FSFI (desire, arousal, lubrication, orgasm, satisfaction, and pain reduction) from baseline to week 4 with all five lubricants (P < .0001 for lubrication and pain reduction; P < .05 for desire, arousal, orgasm, and satisfaction), according to the researchers.
After 4 weeks, a clinically meaningful improvement in the total FSFI score was observed for four lubricants among premenopausal women and for all lubricants among postmenopausal women. The percentage of participants with sexual function as defined as a score of at least 26.55 on the FSFI was significantly greater after treatment (76.9%) than before treatment (20.8%; P < .0001).
“You would assume if you’re using lubricant it would improve the dryness, but what was very exciting for us is that it improved desire, it improved orgasm, it improved arousal,” Dr. Krychman said in an interview. Like concentric overlapping circles of female sexual function, he said, “if you improve one aspect, you improve the other.”
Nearly 80 nonserious adverse effects occurred in 43 participants, five of which were thought to be possibly attributed to the products, such as vulvovaginal burning, itching, or discomfort. In questionnaires, most women agreed that using the lubricants made sex more enjoyable and provided an overall pleasant experience.
One limitation of the study is that because most participants were Caucasian, the results may not be generalizable to all populations, according to the researchers. Further research is required to fully determine safety and efficacy in patients of all races and ethnicities, they reported, especially given that vaginal dryness has been reported more frequently in non-White ethnic groups.
In a companion presentation, Dr. Krychman discussed another aspect of the study looking at the lubricants’ effects on the vaginal microbiome. Repeated application of the products did not significantly alter the vaginal microbiome for up to 4 weeks, and vaginal pH slightly increased in all treatment groups shortly after use but was restored in most cases after a day.
Water-based lubricants are recommended by the WHO for use with condoms because they do not erode latex, said Karen Adams, MD, professor emeritus of obstetrics and gynecology and founding director of the Menopause and Sexual Medicine Program at Oregon Health & Science University, Portland. Guidelines from the group recommend lubricants should have an osmolality that is as close to normal vaginal secretions as possible to decrease the likelihood of irritation or other side effects, she said. Some available lubricants have four to six times that osmolality, which potentially could dehydrate cells, achieving the opposite of the desired effect.
“The reason this is important is they’re trying to develop lubricants that are more ‘vaginal friendly’ and more in line with the WHO guidelines,” said Dr. Adams, who is joining Stanford (Calif.) University in July to create and lead a new program in menopause and healthy aging. “They came up with four formulas consistent with WHO guidelines to see if these new ones worked at least as well [as commercially available products with higher osmolality], and it turns out they did,” she said. “They worked just fine.”
The study was funded by Reckitt Healthcare. Dr. Krychman is a paid medical consultant for the company. Dr. Adams disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. In a secondary analysis also presented at the meeting, the lubricants were found not to alter the vaginal microbiome.
Using these types of lubricants during vaginal intercourse at least once a week over a 4-week period resulted in a statistically significant increase of over four points in the 36-point Female Sexual Function Index (FSFI), a self-reported measure of sexual functioning, for participants, said Michael Krychman, MD, executive director of the Southern California Center for Sexual Health and Survivorship Medicine, Newport Beach, the senior author of the study. Statistically significant improvements also were observed in individual areas such as sexual desire and arousal, orgasm, and satisfaction. Results of the study have been published in the Journal of Sexual Medicine.
In the open-label, five-arm, parallel study conducted in Germany, 174 women aged 18-65 years were randomly assigned to use one of five lubricants from three popular brands. After a 4-week run-in period with no use of lubricants, participants were shown how to apply the products and instructed to use the substances during vaginal intercourse at least once a week over a 4-week period.
Participants reported experiencing mild to moderate vaginal dryness and dyspareunia during vaginal intercourse within the previous 3 months.
Statistically significant improvements were seen across all six individual domain scores of the FSFI (desire, arousal, lubrication, orgasm, satisfaction, and pain reduction) from baseline to week 4 with all five lubricants (P < .0001 for lubrication and pain reduction; P < .05 for desire, arousal, orgasm, and satisfaction), according to the researchers.
After 4 weeks, a clinically meaningful improvement in the total FSFI score was observed for four lubricants among premenopausal women and for all lubricants among postmenopausal women. The percentage of participants with sexual function as defined as a score of at least 26.55 on the FSFI was significantly greater after treatment (76.9%) than before treatment (20.8%; P < .0001).
“You would assume if you’re using lubricant it would improve the dryness, but what was very exciting for us is that it improved desire, it improved orgasm, it improved arousal,” Dr. Krychman said in an interview. Like concentric overlapping circles of female sexual function, he said, “if you improve one aspect, you improve the other.”
Nearly 80 nonserious adverse effects occurred in 43 participants, five of which were thought to be possibly attributed to the products, such as vulvovaginal burning, itching, or discomfort. In questionnaires, most women agreed that using the lubricants made sex more enjoyable and provided an overall pleasant experience.
One limitation of the study is that because most participants were Caucasian, the results may not be generalizable to all populations, according to the researchers. Further research is required to fully determine safety and efficacy in patients of all races and ethnicities, they reported, especially given that vaginal dryness has been reported more frequently in non-White ethnic groups.
In a companion presentation, Dr. Krychman discussed another aspect of the study looking at the lubricants’ effects on the vaginal microbiome. Repeated application of the products did not significantly alter the vaginal microbiome for up to 4 weeks, and vaginal pH slightly increased in all treatment groups shortly after use but was restored in most cases after a day.
Water-based lubricants are recommended by the WHO for use with condoms because they do not erode latex, said Karen Adams, MD, professor emeritus of obstetrics and gynecology and founding director of the Menopause and Sexual Medicine Program at Oregon Health & Science University, Portland. Guidelines from the group recommend lubricants should have an osmolality that is as close to normal vaginal secretions as possible to decrease the likelihood of irritation or other side effects, she said. Some available lubricants have four to six times that osmolality, which potentially could dehydrate cells, achieving the opposite of the desired effect.
“The reason this is important is they’re trying to develop lubricants that are more ‘vaginal friendly’ and more in line with the WHO guidelines,” said Dr. Adams, who is joining Stanford (Calif.) University in July to create and lead a new program in menopause and healthy aging. “They came up with four formulas consistent with WHO guidelines to see if these new ones worked at least as well [as commercially available products with higher osmolality], and it turns out they did,” she said. “They worked just fine.”
The study was funded by Reckitt Healthcare. Dr. Krychman is a paid medical consultant for the company. Dr. Adams disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT ACOG 2023
Scheduled bleeding may boost tolerability of hormone implants
BALTIMORE – The bleeding causes some women to have the device removed, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a randomized, double-blinded, placebo-controlled trial of 51 patients desiring the implants – which suppress ovulation by releasing progestin over a 3-year period – taking norethindrone acetate for 1 week every 4 weeks led to 80% of participants in the treatment group reporting satisfactory bleeding patterns with the etonogestrel implants in place.
Rates of early discontinuation have been variable, according to published literature, ranging from 13% to 21.1%, said Jordan Gray, MD, a fourth-year resident in ob.gyn. at Baylor Scott and White Medical Center, Temple, Tex., who helped conduct the new study. Reasons included bothersome bleeding. Dr. Gray and colleagues found that 24% of women in the placebo group requested removal of the implant, compared with 9% of those in the treatment group. Among these women, none requested removal for bothersome bleeding but rather for reasons such as wanting to get pregnant. One person requested removal because she did not like amenorrhea.
While the results of the study did not achieve statistical significance, owing to its size and noncompliance among some participants, it does indicate that norethindrone acetate may be helpful, Dr. Gray said.
During the study, participants in the treatment group (n = 22) received a monthly treatment regimen of 5 mg of oral norethindrone acetate daily for 7 days each month for the first 6 months after placement of an etonogestrel implant. The placebo group (n = 29) was given inert tablets prescribed in the same regimen. Both groups received products from a mail-order pharmacy.
Participants were women aged 18-48 years who desired an implant or those aged 14 years who had permission from a parent or guardian to receive the contraceptive. The study excluded people with known or suspected pregnancy, those less than 8 weeks’ post partum, those who experienced menarche less than 2 years ago, those with body mass index greater than 40, and those who received depot medroxyprogesterone acetate within the previous 12 weeks. Excessive bleeding was defined as bleeding or spotting on more than 7 consecutive days or a fifth episode of bleeding in 90 days.
Overall, 11 patients (38%) in the placebo group and 10 (45%) in the treatment arm withdrew from the study. Reasons included wanting to get pregnant, mood changes, or noncompliance with study parameters, which included not responding or returning bleeding diaries, Dr. Gray said.
A limitation of the study was that compliance was less than expected. In addition, there were challenges with rates of responses, Dr. Gray said. The study was conducted during the COVID-19 pandemic, when all in-person visits were transitioned to telehealth. Although the investigators offered payment to participants, not all returned text-message surveys. The researchers had intended to enroll 124 participants but curtailed the study early, owing to the limited number of participants.
Given that there is no standard approach to treating prolonged or excessive bleeding with etonogestrel implants, Dr. Gray said, “Our data suggests that this regimen is a simple and acceptable method to treat bothersome bleeding and that predictable bleeding may be more satisfactory than unpredictable bleeding.”
Veronica Maria Pimentel, MD, moderator of the session and a maternal-fetal medicine specialist and director of research for the ob.gyn. residency program at St. Francis Hospital, part of Trinity Health of New England in Hartford, Conn., praised the researchers for a well-designed study.
“However, unfortunately, they were not able to recruit the number of patients that they needed in order to achieve the power to show the difference [between treatment arms], so another study would have to be done to show if there is a difference,” Dr. Pimentel said.
Dr. Pimentel complimented Dr. Gray following her presentation, congratulating her for conducting a randomized, controlled trial: “That’s not easy, as you have shown, but it’s also a good try, so you can actually see how hard it is to obtain quality data from research.”
The study was supported in part by a research grant from the Investigator-Initiated Studies Program of Organon. Dr. Gray is a consultant for Johnson & Johnson. Dr. Pimentel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BALTIMORE – The bleeding causes some women to have the device removed, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a randomized, double-blinded, placebo-controlled trial of 51 patients desiring the implants – which suppress ovulation by releasing progestin over a 3-year period – taking norethindrone acetate for 1 week every 4 weeks led to 80% of participants in the treatment group reporting satisfactory bleeding patterns with the etonogestrel implants in place.
Rates of early discontinuation have been variable, according to published literature, ranging from 13% to 21.1%, said Jordan Gray, MD, a fourth-year resident in ob.gyn. at Baylor Scott and White Medical Center, Temple, Tex., who helped conduct the new study. Reasons included bothersome bleeding. Dr. Gray and colleagues found that 24% of women in the placebo group requested removal of the implant, compared with 9% of those in the treatment group. Among these women, none requested removal for bothersome bleeding but rather for reasons such as wanting to get pregnant. One person requested removal because she did not like amenorrhea.
While the results of the study did not achieve statistical significance, owing to its size and noncompliance among some participants, it does indicate that norethindrone acetate may be helpful, Dr. Gray said.
During the study, participants in the treatment group (n = 22) received a monthly treatment regimen of 5 mg of oral norethindrone acetate daily for 7 days each month for the first 6 months after placement of an etonogestrel implant. The placebo group (n = 29) was given inert tablets prescribed in the same regimen. Both groups received products from a mail-order pharmacy.
Participants were women aged 18-48 years who desired an implant or those aged 14 years who had permission from a parent or guardian to receive the contraceptive. The study excluded people with known or suspected pregnancy, those less than 8 weeks’ post partum, those who experienced menarche less than 2 years ago, those with body mass index greater than 40, and those who received depot medroxyprogesterone acetate within the previous 12 weeks. Excessive bleeding was defined as bleeding or spotting on more than 7 consecutive days or a fifth episode of bleeding in 90 days.
Overall, 11 patients (38%) in the placebo group and 10 (45%) in the treatment arm withdrew from the study. Reasons included wanting to get pregnant, mood changes, or noncompliance with study parameters, which included not responding or returning bleeding diaries, Dr. Gray said.
A limitation of the study was that compliance was less than expected. In addition, there were challenges with rates of responses, Dr. Gray said. The study was conducted during the COVID-19 pandemic, when all in-person visits were transitioned to telehealth. Although the investigators offered payment to participants, not all returned text-message surveys. The researchers had intended to enroll 124 participants but curtailed the study early, owing to the limited number of participants.
Given that there is no standard approach to treating prolonged or excessive bleeding with etonogestrel implants, Dr. Gray said, “Our data suggests that this regimen is a simple and acceptable method to treat bothersome bleeding and that predictable bleeding may be more satisfactory than unpredictable bleeding.”
Veronica Maria Pimentel, MD, moderator of the session and a maternal-fetal medicine specialist and director of research for the ob.gyn. residency program at St. Francis Hospital, part of Trinity Health of New England in Hartford, Conn., praised the researchers for a well-designed study.
“However, unfortunately, they were not able to recruit the number of patients that they needed in order to achieve the power to show the difference [between treatment arms], so another study would have to be done to show if there is a difference,” Dr. Pimentel said.
Dr. Pimentel complimented Dr. Gray following her presentation, congratulating her for conducting a randomized, controlled trial: “That’s not easy, as you have shown, but it’s also a good try, so you can actually see how hard it is to obtain quality data from research.”
The study was supported in part by a research grant from the Investigator-Initiated Studies Program of Organon. Dr. Gray is a consultant for Johnson & Johnson. Dr. Pimentel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BALTIMORE – The bleeding causes some women to have the device removed, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a randomized, double-blinded, placebo-controlled trial of 51 patients desiring the implants – which suppress ovulation by releasing progestin over a 3-year period – taking norethindrone acetate for 1 week every 4 weeks led to 80% of participants in the treatment group reporting satisfactory bleeding patterns with the etonogestrel implants in place.
Rates of early discontinuation have been variable, according to published literature, ranging from 13% to 21.1%, said Jordan Gray, MD, a fourth-year resident in ob.gyn. at Baylor Scott and White Medical Center, Temple, Tex., who helped conduct the new study. Reasons included bothersome bleeding. Dr. Gray and colleagues found that 24% of women in the placebo group requested removal of the implant, compared with 9% of those in the treatment group. Among these women, none requested removal for bothersome bleeding but rather for reasons such as wanting to get pregnant. One person requested removal because she did not like amenorrhea.
While the results of the study did not achieve statistical significance, owing to its size and noncompliance among some participants, it does indicate that norethindrone acetate may be helpful, Dr. Gray said.
During the study, participants in the treatment group (n = 22) received a monthly treatment regimen of 5 mg of oral norethindrone acetate daily for 7 days each month for the first 6 months after placement of an etonogestrel implant. The placebo group (n = 29) was given inert tablets prescribed in the same regimen. Both groups received products from a mail-order pharmacy.
Participants were women aged 18-48 years who desired an implant or those aged 14 years who had permission from a parent or guardian to receive the contraceptive. The study excluded people with known or suspected pregnancy, those less than 8 weeks’ post partum, those who experienced menarche less than 2 years ago, those with body mass index greater than 40, and those who received depot medroxyprogesterone acetate within the previous 12 weeks. Excessive bleeding was defined as bleeding or spotting on more than 7 consecutive days or a fifth episode of bleeding in 90 days.
Overall, 11 patients (38%) in the placebo group and 10 (45%) in the treatment arm withdrew from the study. Reasons included wanting to get pregnant, mood changes, or noncompliance with study parameters, which included not responding or returning bleeding diaries, Dr. Gray said.
A limitation of the study was that compliance was less than expected. In addition, there were challenges with rates of responses, Dr. Gray said. The study was conducted during the COVID-19 pandemic, when all in-person visits were transitioned to telehealth. Although the investigators offered payment to participants, not all returned text-message surveys. The researchers had intended to enroll 124 participants but curtailed the study early, owing to the limited number of participants.
Given that there is no standard approach to treating prolonged or excessive bleeding with etonogestrel implants, Dr. Gray said, “Our data suggests that this regimen is a simple and acceptable method to treat bothersome bleeding and that predictable bleeding may be more satisfactory than unpredictable bleeding.”
Veronica Maria Pimentel, MD, moderator of the session and a maternal-fetal medicine specialist and director of research for the ob.gyn. residency program at St. Francis Hospital, part of Trinity Health of New England in Hartford, Conn., praised the researchers for a well-designed study.
“However, unfortunately, they were not able to recruit the number of patients that they needed in order to achieve the power to show the difference [between treatment arms], so another study would have to be done to show if there is a difference,” Dr. Pimentel said.
Dr. Pimentel complimented Dr. Gray following her presentation, congratulating her for conducting a randomized, controlled trial: “That’s not easy, as you have shown, but it’s also a good try, so you can actually see how hard it is to obtain quality data from research.”
The study was supported in part by a research grant from the Investigator-Initiated Studies Program of Organon. Dr. Gray is a consultant for Johnson & Johnson. Dr. Pimentel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACOG 2023
Mailed HPV test kits boost cervical cancer screening
The self-sampling kits, which detect human papillomavirus (HPV), are available only for use in clinical trials, but the researchers hope that eventually these kits will be approved for use by the general public.
The researchers, from the University of North Carolina, explored use of these kits in the My Body, My Test-3 study, which was published online in The Lancet Public Health.
In a commentary published with the study, Runzhi Wang, MD, and Jennell Coleman, MD, MPH, both of Johns Hopkins University, Baltimore, said it “provides the required evidence that ... self-collected samples can be an effective strategy for hard-to-reach populations.”
The study involved 665 women (aged 25-64) in North Carolina who were either uninsured or enrolled in Medicaid or Medicare. The patients had low-income backgrounds and lived in urban areas. More than half self-reported as Black or Hispanic (55%), uninsured (78%) or unemployed (57%). None had a Pap smear in at least 4 years or a high-risk HPV test in the last 6 years.
Two-thirds of the women were mailed an HPV self-collection kit and received assistance with scheduling an in-person screening appointment. The kit included a Viba-Brush device, which is inserted into the vagina like a tampon to collect the sample.
The other third of women, the control group, only received scheduling assistance.
The team found that mailing the self-collection tests along with helping women book in-clinic appointments improved screening rates twofold, compared with just assisting patients to schedule an appointment.
Screening success among those who received the at-home collection kit was 72%, compared with 37% in the control group.
Of those who received the kits, 78% returned them. This is “impressive,” said Dr. Wang and Dr. Coleman, as previous studies have reported return rates of only 8%-20%.
About 23% of eligible women are overdue for cervical cancer screening by at least a year, according to the National Cancer Institute. Jennifer Smith, PhD, MPH, professor of epidemiology at the University of North Carolina at Chapel Hill and an author of the study, believes every woman deserves equal access to cervical screening.
“I think we really need to make efforts to increase cervical cancer screening among women who are overdue for screening by a year or more from the recommended guidelines,” Dr. Smith said. “We’ve proven along with the wide evidence both in the U.S. and globally that self-collection intervention works well and can motivate screening uptake by breaking down barriers for populations that have less access to care.”
“We’re hoping this research in combination with all of the extensive evidence on the positive performance of HPV self-collection will provide additional information to be considered by the FDA for approval of the kits for primary screening,” Dr. Smith said.
“Government approval of at-home HPV tests would have a huge impact,” said coauthor Noel Brewer, PhD, also of UNC Chapel Hill. “We could better reach those in rural areas where cervical cancer screening is hard to come by.”
Dr. Smith has received research grants, supply donations, and consultancies for Hologic and BD Diagnostics. Dr. Brewer, Dr. Wang, and Dr. Coleman reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
The self-sampling kits, which detect human papillomavirus (HPV), are available only for use in clinical trials, but the researchers hope that eventually these kits will be approved for use by the general public.
The researchers, from the University of North Carolina, explored use of these kits in the My Body, My Test-3 study, which was published online in The Lancet Public Health.
In a commentary published with the study, Runzhi Wang, MD, and Jennell Coleman, MD, MPH, both of Johns Hopkins University, Baltimore, said it “provides the required evidence that ... self-collected samples can be an effective strategy for hard-to-reach populations.”
The study involved 665 women (aged 25-64) in North Carolina who were either uninsured or enrolled in Medicaid or Medicare. The patients had low-income backgrounds and lived in urban areas. More than half self-reported as Black or Hispanic (55%), uninsured (78%) or unemployed (57%). None had a Pap smear in at least 4 years or a high-risk HPV test in the last 6 years.
Two-thirds of the women were mailed an HPV self-collection kit and received assistance with scheduling an in-person screening appointment. The kit included a Viba-Brush device, which is inserted into the vagina like a tampon to collect the sample.
The other third of women, the control group, only received scheduling assistance.
The team found that mailing the self-collection tests along with helping women book in-clinic appointments improved screening rates twofold, compared with just assisting patients to schedule an appointment.
Screening success among those who received the at-home collection kit was 72%, compared with 37% in the control group.
Of those who received the kits, 78% returned them. This is “impressive,” said Dr. Wang and Dr. Coleman, as previous studies have reported return rates of only 8%-20%.
About 23% of eligible women are overdue for cervical cancer screening by at least a year, according to the National Cancer Institute. Jennifer Smith, PhD, MPH, professor of epidemiology at the University of North Carolina at Chapel Hill and an author of the study, believes every woman deserves equal access to cervical screening.
“I think we really need to make efforts to increase cervical cancer screening among women who are overdue for screening by a year or more from the recommended guidelines,” Dr. Smith said. “We’ve proven along with the wide evidence both in the U.S. and globally that self-collection intervention works well and can motivate screening uptake by breaking down barriers for populations that have less access to care.”
“We’re hoping this research in combination with all of the extensive evidence on the positive performance of HPV self-collection will provide additional information to be considered by the FDA for approval of the kits for primary screening,” Dr. Smith said.
“Government approval of at-home HPV tests would have a huge impact,” said coauthor Noel Brewer, PhD, also of UNC Chapel Hill. “We could better reach those in rural areas where cervical cancer screening is hard to come by.”
Dr. Smith has received research grants, supply donations, and consultancies for Hologic and BD Diagnostics. Dr. Brewer, Dr. Wang, and Dr. Coleman reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
The self-sampling kits, which detect human papillomavirus (HPV), are available only for use in clinical trials, but the researchers hope that eventually these kits will be approved for use by the general public.
The researchers, from the University of North Carolina, explored use of these kits in the My Body, My Test-3 study, which was published online in The Lancet Public Health.
In a commentary published with the study, Runzhi Wang, MD, and Jennell Coleman, MD, MPH, both of Johns Hopkins University, Baltimore, said it “provides the required evidence that ... self-collected samples can be an effective strategy for hard-to-reach populations.”
The study involved 665 women (aged 25-64) in North Carolina who were either uninsured or enrolled in Medicaid or Medicare. The patients had low-income backgrounds and lived in urban areas. More than half self-reported as Black or Hispanic (55%), uninsured (78%) or unemployed (57%). None had a Pap smear in at least 4 years or a high-risk HPV test in the last 6 years.
Two-thirds of the women were mailed an HPV self-collection kit and received assistance with scheduling an in-person screening appointment. The kit included a Viba-Brush device, which is inserted into the vagina like a tampon to collect the sample.
The other third of women, the control group, only received scheduling assistance.
The team found that mailing the self-collection tests along with helping women book in-clinic appointments improved screening rates twofold, compared with just assisting patients to schedule an appointment.
Screening success among those who received the at-home collection kit was 72%, compared with 37% in the control group.
Of those who received the kits, 78% returned them. This is “impressive,” said Dr. Wang and Dr. Coleman, as previous studies have reported return rates of only 8%-20%.
About 23% of eligible women are overdue for cervical cancer screening by at least a year, according to the National Cancer Institute. Jennifer Smith, PhD, MPH, professor of epidemiology at the University of North Carolina at Chapel Hill and an author of the study, believes every woman deserves equal access to cervical screening.
“I think we really need to make efforts to increase cervical cancer screening among women who are overdue for screening by a year or more from the recommended guidelines,” Dr. Smith said. “We’ve proven along with the wide evidence both in the U.S. and globally that self-collection intervention works well and can motivate screening uptake by breaking down barriers for populations that have less access to care.”
“We’re hoping this research in combination with all of the extensive evidence on the positive performance of HPV self-collection will provide additional information to be considered by the FDA for approval of the kits for primary screening,” Dr. Smith said.
“Government approval of at-home HPV tests would have a huge impact,” said coauthor Noel Brewer, PhD, also of UNC Chapel Hill. “We could better reach those in rural areas where cervical cancer screening is hard to come by.”
Dr. Smith has received research grants, supply donations, and consultancies for Hologic and BD Diagnostics. Dr. Brewer, Dr. Wang, and Dr. Coleman reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
FROM THE LANCET PUBLIC HEALTH
Vulvar syringoma
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
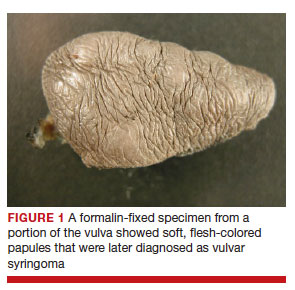
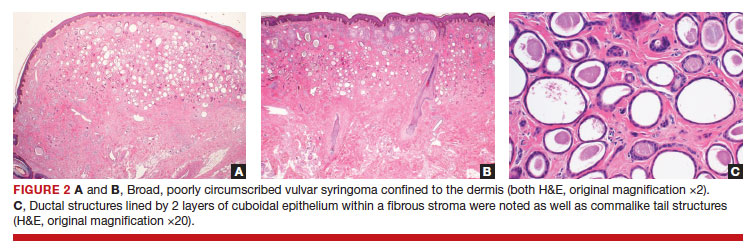
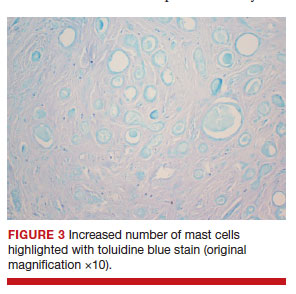
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (FIGURE 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (FIGURES 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (FIGURE 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (FIGURE 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.
Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulvar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (FIGURES 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (FIGURE 3).Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems. ●
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas DermoSifiliográficas. 2008; 99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995; 22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinomaaggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369372.
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (FIGURE 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (FIGURES 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (FIGURE 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (FIGURE 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.



Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulvar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (FIGURES 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (FIGURE 3).Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems. ●
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (FIGURE 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (FIGURES 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (FIGURE 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (FIGURE 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.



Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulvar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (FIGURES 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (FIGURE 3).Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems. ●
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas DermoSifiliográficas. 2008; 99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995; 22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinomaaggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369372.
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas DermoSifiliográficas. 2008; 99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995; 22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinomaaggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369372.
Does the current age cutoff for screening miss too many cases of cervical cancer in older women?
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
Federal rules don’t require period product ingredients on packaging labels. States are stepping in.
Tens of millions of Americans use menstrual products, and while manufacturers contend they are safe, most disclose little about the chemicals they contain. Now, amid calls for more disclosure and research into the health effects of these products, some states require more transparency.
The manufacture and sale of period and related products is a big business, with revenue expected to top $4.5 billion in the United States this year. On average, a person uses up to 17,000 tampons or pads in their lifetime, and they might also use rubber or silicone cups, or absorbent period underwear.
The FDA regulates and classifies menstrual products as medical devices, meaning they are not subject to the same labeling laws as other consumer items. But companies can voluntarily disclose what’s in their products.
Now, some states are stepping into the breach. In 2021, New York became the first state to enact a menstrual product disclosure law requiring companies to list all intentionally added ingredients on packaging. California’s governor signed a similar law that took effect this year, but it gives manufacturers trade secret protections, so not all ingredients are necessarily disclosed. At least six other states have introduced legislation to address safety and disclosure of ingredients in these products.
Shruthi Mahalingaiah, an assistant professor of environmental, reproductive, and women’s health at Harvard University, Boston, evaluates endocrine disruptors in personal care products and studies menstrual health. She said the health risk depends on the dose, duration, and sensitivity of a person to the ingredients and their mixtures.
Harmful chemicals could come from manufacturing processes, through materials and shipping, from equipment cleaners, from contact with contaminants, or from companies adding them intentionally, said Alexandra Scranton, director of science and research for Women’s Voices for the Earth, a Montana-based nonprofit focused on eliminating toxic chemicals that affect women’s health.
Vaginal and vulvar tissues are capable of absorbing fluids at a higher rate than skin, which can lead to rapid chemical exposure. Ms. Scranton said scarcity of clinical studies and funding for vaginal health research limits understanding about the long-term effects of the ingredients and additives in period products.
“We think manufacturers should do better and be more careful with the ingredients they choose to use,” Ms. Scranton said. “The presence of toxic and hormone-disrupting chemicals in menstrual products is unsettling. We know that chemicals can cause disease, and exposures do add up over time.”
Ms. Scranton’s organization advocates for labels to include the chemical name of the ingredient, the component in which the ingredient is used, and the function of the ingredient.
K. Malaika Walton, operations director for the Center for Baby and Adult Hygiene Products, a trade industry group, said in an email, “BAHP supports accurate and transparent information for users of period products and many of our member companies list ingredients on their packages and websites.”
In a written statement, Procter & Gamble, a major manufacturer of menstrual products, said that ingredients it uses go through rigorous safety evaluations and are continuously tested, and that all fragrance components are added at levels the industry considers safe.
Even though manufacturing of scented tampons for the U.S. market has mostly stopped, companies still use fragrances in other menstrual products. Laws protecting trade secrets keep details about fragrances in pads and tampons confidential so competitors can’t copy the formulas. The Children’s Environmental Health Network lists phthalates, a group of chemicals commonly called plasticizers, that are suspected hormone disruptors, as an ingredient found in fragrances.
Manufacturers follow regulatory guidance issued in 2005 by registering with the Food and Drug Administration and submitting a detailed risk assessment of their products’ components and design, and a safety profile, before being cleared to sell in the United States.
Pads and menstrual cups are considered exempt from regulatory guidance and do not require premarket review, according to FDA spokesperson Carly Kempler. While tampons do require review, the FDA “does not clear or approve individual materials that are used in the fabrication of medical devices.”
“There’s an understanding that the FDA is regulating these products, and they are; it’s just not very adequate,” said Laura Strausfeld, an attorney and a cofounder of Period Law, an organization working to advance state and federal period-equity policies that would stop taxation of products and make them freely available in places like schools and prisons. “The consumer is supposed to trust that when these products are put on shelves they’ve been vetted by the government. But it’s basically a rubber stamp.”
In a 2022 report, a congressional committee directed the FDA to update its guidance for menstrual products to recommend that labels disclose intentionally added ingredients, such as fragrances, and test for contaminants. The FDA is reviewing the directives outlined by the House Appropriations Committee and will update the 2005 guidance as soon as possible, Ms. Kempler said. “We will share additional details when we are able to.”
At least one period product company makes disclosure of its ingredients a selling point. Alex Friedman, cofounder of Lola, said a lack of knowledge is a problem, and more action and awareness are needed to keep people safe.
“The hardest part to swallow is why this is even up for debate. We should all know what’s in these products,” Ms. Friedman said.
New York’s law requires companies to disclose all intentionally added ingredients no matter how much is used, with no trade secret protections for fragrances. Though it applies only to products sold in that state, similar detailed labeling is appearing elsewhere, advocates said.
“We’re also seeing similar or identical disclosure on packaging in other states outside of New York, which is a testament to the power of the law,” said Jamie McConnell, deputy director of Women’s Voices for the Earth.
Manufacturers have 18 months from the passage of the New York law to comply, and some products on shelves in New York still list few ingredients other than “absorbent material,” “surfactant,” “ink,” and “adhesive.”
“We’re like, ‘OK, what is that exactly?’ ” Ms. McConnell said.
Her organization is calling for a federal law at least as strong as New York’s. Previous federal legislation failed to advance, including the most recent, the Menstrual Products Right to Know Act, introduced in 2022.
BAHP, the trade group, supported the federal legislation and the California law. Ms. McConnell said she opposed both bills because they didn’t require companies to list all fragrance ingredients.
“I think what it boiled down to at the federal level was the support of corporate interests over public health,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Tens of millions of Americans use menstrual products, and while manufacturers contend they are safe, most disclose little about the chemicals they contain. Now, amid calls for more disclosure and research into the health effects of these products, some states require more transparency.
The manufacture and sale of period and related products is a big business, with revenue expected to top $4.5 billion in the United States this year. On average, a person uses up to 17,000 tampons or pads in their lifetime, and they might also use rubber or silicone cups, or absorbent period underwear.
The FDA regulates and classifies menstrual products as medical devices, meaning they are not subject to the same labeling laws as other consumer items. But companies can voluntarily disclose what’s in their products.
Now, some states are stepping into the breach. In 2021, New York became the first state to enact a menstrual product disclosure law requiring companies to list all intentionally added ingredients on packaging. California’s governor signed a similar law that took effect this year, but it gives manufacturers trade secret protections, so not all ingredients are necessarily disclosed. At least six other states have introduced legislation to address safety and disclosure of ingredients in these products.
Shruthi Mahalingaiah, an assistant professor of environmental, reproductive, and women’s health at Harvard University, Boston, evaluates endocrine disruptors in personal care products and studies menstrual health. She said the health risk depends on the dose, duration, and sensitivity of a person to the ingredients and their mixtures.
Harmful chemicals could come from manufacturing processes, through materials and shipping, from equipment cleaners, from contact with contaminants, or from companies adding them intentionally, said Alexandra Scranton, director of science and research for Women’s Voices for the Earth, a Montana-based nonprofit focused on eliminating toxic chemicals that affect women’s health.
Vaginal and vulvar tissues are capable of absorbing fluids at a higher rate than skin, which can lead to rapid chemical exposure. Ms. Scranton said scarcity of clinical studies and funding for vaginal health research limits understanding about the long-term effects of the ingredients and additives in period products.
“We think manufacturers should do better and be more careful with the ingredients they choose to use,” Ms. Scranton said. “The presence of toxic and hormone-disrupting chemicals in menstrual products is unsettling. We know that chemicals can cause disease, and exposures do add up over time.”
Ms. Scranton’s organization advocates for labels to include the chemical name of the ingredient, the component in which the ingredient is used, and the function of the ingredient.
K. Malaika Walton, operations director for the Center for Baby and Adult Hygiene Products, a trade industry group, said in an email, “BAHP supports accurate and transparent information for users of period products and many of our member companies list ingredients on their packages and websites.”
In a written statement, Procter & Gamble, a major manufacturer of menstrual products, said that ingredients it uses go through rigorous safety evaluations and are continuously tested, and that all fragrance components are added at levels the industry considers safe.
Even though manufacturing of scented tampons for the U.S. market has mostly stopped, companies still use fragrances in other menstrual products. Laws protecting trade secrets keep details about fragrances in pads and tampons confidential so competitors can’t copy the formulas. The Children’s Environmental Health Network lists phthalates, a group of chemicals commonly called plasticizers, that are suspected hormone disruptors, as an ingredient found in fragrances.
Manufacturers follow regulatory guidance issued in 2005 by registering with the Food and Drug Administration and submitting a detailed risk assessment of their products’ components and design, and a safety profile, before being cleared to sell in the United States.
Pads and menstrual cups are considered exempt from regulatory guidance and do not require premarket review, according to FDA spokesperson Carly Kempler. While tampons do require review, the FDA “does not clear or approve individual materials that are used in the fabrication of medical devices.”
“There’s an understanding that the FDA is regulating these products, and they are; it’s just not very adequate,” said Laura Strausfeld, an attorney and a cofounder of Period Law, an organization working to advance state and federal period-equity policies that would stop taxation of products and make them freely available in places like schools and prisons. “The consumer is supposed to trust that when these products are put on shelves they’ve been vetted by the government. But it’s basically a rubber stamp.”
In a 2022 report, a congressional committee directed the FDA to update its guidance for menstrual products to recommend that labels disclose intentionally added ingredients, such as fragrances, and test for contaminants. The FDA is reviewing the directives outlined by the House Appropriations Committee and will update the 2005 guidance as soon as possible, Ms. Kempler said. “We will share additional details when we are able to.”
At least one period product company makes disclosure of its ingredients a selling point. Alex Friedman, cofounder of Lola, said a lack of knowledge is a problem, and more action and awareness are needed to keep people safe.
“The hardest part to swallow is why this is even up for debate. We should all know what’s in these products,” Ms. Friedman said.
New York’s law requires companies to disclose all intentionally added ingredients no matter how much is used, with no trade secret protections for fragrances. Though it applies only to products sold in that state, similar detailed labeling is appearing elsewhere, advocates said.
“We’re also seeing similar or identical disclosure on packaging in other states outside of New York, which is a testament to the power of the law,” said Jamie McConnell, deputy director of Women’s Voices for the Earth.
Manufacturers have 18 months from the passage of the New York law to comply, and some products on shelves in New York still list few ingredients other than “absorbent material,” “surfactant,” “ink,” and “adhesive.”
“We’re like, ‘OK, what is that exactly?’ ” Ms. McConnell said.
Her organization is calling for a federal law at least as strong as New York’s. Previous federal legislation failed to advance, including the most recent, the Menstrual Products Right to Know Act, introduced in 2022.
BAHP, the trade group, supported the federal legislation and the California law. Ms. McConnell said she opposed both bills because they didn’t require companies to list all fragrance ingredients.
“I think what it boiled down to at the federal level was the support of corporate interests over public health,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Tens of millions of Americans use menstrual products, and while manufacturers contend they are safe, most disclose little about the chemicals they contain. Now, amid calls for more disclosure and research into the health effects of these products, some states require more transparency.
The manufacture and sale of period and related products is a big business, with revenue expected to top $4.5 billion in the United States this year. On average, a person uses up to 17,000 tampons or pads in their lifetime, and they might also use rubber or silicone cups, or absorbent period underwear.
The FDA regulates and classifies menstrual products as medical devices, meaning they are not subject to the same labeling laws as other consumer items. But companies can voluntarily disclose what’s in their products.
Now, some states are stepping into the breach. In 2021, New York became the first state to enact a menstrual product disclosure law requiring companies to list all intentionally added ingredients on packaging. California’s governor signed a similar law that took effect this year, but it gives manufacturers trade secret protections, so not all ingredients are necessarily disclosed. At least six other states have introduced legislation to address safety and disclosure of ingredients in these products.
Shruthi Mahalingaiah, an assistant professor of environmental, reproductive, and women’s health at Harvard University, Boston, evaluates endocrine disruptors in personal care products and studies menstrual health. She said the health risk depends on the dose, duration, and sensitivity of a person to the ingredients and their mixtures.
Harmful chemicals could come from manufacturing processes, through materials and shipping, from equipment cleaners, from contact with contaminants, or from companies adding them intentionally, said Alexandra Scranton, director of science and research for Women’s Voices for the Earth, a Montana-based nonprofit focused on eliminating toxic chemicals that affect women’s health.
Vaginal and vulvar tissues are capable of absorbing fluids at a higher rate than skin, which can lead to rapid chemical exposure. Ms. Scranton said scarcity of clinical studies and funding for vaginal health research limits understanding about the long-term effects of the ingredients and additives in period products.
“We think manufacturers should do better and be more careful with the ingredients they choose to use,” Ms. Scranton said. “The presence of toxic and hormone-disrupting chemicals in menstrual products is unsettling. We know that chemicals can cause disease, and exposures do add up over time.”
Ms. Scranton’s organization advocates for labels to include the chemical name of the ingredient, the component in which the ingredient is used, and the function of the ingredient.
K. Malaika Walton, operations director for the Center for Baby and Adult Hygiene Products, a trade industry group, said in an email, “BAHP supports accurate and transparent information for users of period products and many of our member companies list ingredients on their packages and websites.”
In a written statement, Procter & Gamble, a major manufacturer of menstrual products, said that ingredients it uses go through rigorous safety evaluations and are continuously tested, and that all fragrance components are added at levels the industry considers safe.
Even though manufacturing of scented tampons for the U.S. market has mostly stopped, companies still use fragrances in other menstrual products. Laws protecting trade secrets keep details about fragrances in pads and tampons confidential so competitors can’t copy the formulas. The Children’s Environmental Health Network lists phthalates, a group of chemicals commonly called plasticizers, that are suspected hormone disruptors, as an ingredient found in fragrances.
Manufacturers follow regulatory guidance issued in 2005 by registering with the Food and Drug Administration and submitting a detailed risk assessment of their products’ components and design, and a safety profile, before being cleared to sell in the United States.
Pads and menstrual cups are considered exempt from regulatory guidance and do not require premarket review, according to FDA spokesperson Carly Kempler. While tampons do require review, the FDA “does not clear or approve individual materials that are used in the fabrication of medical devices.”
“There’s an understanding that the FDA is regulating these products, and they are; it’s just not very adequate,” said Laura Strausfeld, an attorney and a cofounder of Period Law, an organization working to advance state and federal period-equity policies that would stop taxation of products and make them freely available in places like schools and prisons. “The consumer is supposed to trust that when these products are put on shelves they’ve been vetted by the government. But it’s basically a rubber stamp.”
In a 2022 report, a congressional committee directed the FDA to update its guidance for menstrual products to recommend that labels disclose intentionally added ingredients, such as fragrances, and test for contaminants. The FDA is reviewing the directives outlined by the House Appropriations Committee and will update the 2005 guidance as soon as possible, Ms. Kempler said. “We will share additional details when we are able to.”
At least one period product company makes disclosure of its ingredients a selling point. Alex Friedman, cofounder of Lola, said a lack of knowledge is a problem, and more action and awareness are needed to keep people safe.
“The hardest part to swallow is why this is even up for debate. We should all know what’s in these products,” Ms. Friedman said.
New York’s law requires companies to disclose all intentionally added ingredients no matter how much is used, with no trade secret protections for fragrances. Though it applies only to products sold in that state, similar detailed labeling is appearing elsewhere, advocates said.
“We’re also seeing similar or identical disclosure on packaging in other states outside of New York, which is a testament to the power of the law,” said Jamie McConnell, deputy director of Women’s Voices for the Earth.
Manufacturers have 18 months from the passage of the New York law to comply, and some products on shelves in New York still list few ingredients other than “absorbent material,” “surfactant,” “ink,” and “adhesive.”
“We’re like, ‘OK, what is that exactly?’ ” Ms. McConnell said.
Her organization is calling for a federal law at least as strong as New York’s. Previous federal legislation failed to advance, including the most recent, the Menstrual Products Right to Know Act, introduced in 2022.
BAHP, the trade group, supported the federal legislation and the California law. Ms. McConnell said she opposed both bills because they didn’t require companies to list all fragrance ingredients.
“I think what it boiled down to at the federal level was the support of corporate interests over public health,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Wireless neurostimulation safe for urge incontinence
CHICAGO – , according to new findings presented at the 2023 annual meeting of the American Urological Association.
As many as half of women in the United States aged 60 and older will experience urinary incontinence. Of those, roughly one in four experience urge urinary incontinence, marked by a sudden need to void that cannot be fully suppressed.
Researchers studied the benefits of the RENOVA iStim (BlueWind Medical) implantable tibial neuromodulation system for the treatment of overactive bladder in the OASIS trial.
Study investigator Roger R. Dmochowski, MD, MMHC, professor of urology and surgery and associate surgeon-in-chief at Vanderbilt University Medical Center, Nashville, Tenn., said the first-line treatment of urinary incontinence is lifestyle changes to retrain the bladder or physical therapy, including pelvic floor and Kegel exercises, per AUA guidelines. He said the success rate is about 30% and is not sustained. Second-line treatments include medications, which most (60%) patients stop taking by 6 months.
More than three-quarters of the 151 women who received the device responded to therapy at 1 year, and 84.6% of the patients showed improvement, according to Dr. Dmochowski.
The participants (mean age, 58.8) demonstrated a mean baseline of 4.8 urge incidents per day (standard deviation, 2.9) and 10 voids/day (SD, 3.3). No device or procedure-related serious adverse events were reported at 12 months. Half of the women no longer had symptoms on three consecutive days, Dr. Dmochowski said.
Because urge urinary incontinence is a chronic condition, “treatment with the BlueWind System will be ongoing, with frequency determined based on the patient’s response,” Dr. Dmochowski said. “The patient is then empowered to control when and where they perform therapy.”
“The device is activated by the external wearable. It’s like an on-off switch. It has a receiver within it that basically has the capacity to be turned on and off by the wearable, which is the control device. The device is in an off-position until the wearable is applied,” he said.
He said the device should be worn twice a day for about 20 minutes, with many patients using it less.
Only one implanted tibial neuromodulation device has been approved by the Food and Drug Administration – eCOIN (Valencia Technologies). The RENOVA iStim is an investigational device under review by the FDA, Dr. Dmochowski said.
In installing the device, Dr. Dmochowski said urologists use a subfascial technique to enable direct visualization of the tibial nerve and suture fixation that increases the possibility of a predictable placement. Patients use an external wearable, which activates the implant, without concern for battery longevity or replacement.
“This therapy is not associated with any adverse effects and may be beneficial for patients who do not respond to other treatments for OAB such as medications or Botox,” said Carol E. Bretschneider, MD, a urogynecologic and pelvic surgeon at Northwestern Medicine Central DuPage Hospital, outside Chicago. “Neurostimulators can be a great advanced therapy option for patients who do not respond to more conservative treatments or cannot take or tolerate a medication.”
The devices do not stimulate or strengthen muscles but act by modulating the reflexes that influence the bladder, sphincter, and pelvic floor, added Dr. Bretschneider, who was not involved in the study.
Other treatments for urge incontinence can include acupuncture, or percutaneous tibial nerve stimulation, to target the posterior tibial nerve in the ankle, which shares the same nerve root that controls the bladder, according to Aron Liaw, MD, a reconstructive urologist and assistant professor of urology at Wayne State University in Detroit. This treatment has been shown to be at least as effective as available medications, but with fewer side effects, he said.
But regular stimulation is necessary to achieve and preserve efficacy, he said.
Dr. Liaw, who was not involved in the neuromodulation study, said the benefits of a device like Renova iStim are that implantation is relatively easy and can be performed in office settings, and patients can then treat themselves at home. However, because the new study did not compare the device to other treatments or a placebo device, its relative benefits are unclear, he said,
Other treatments for urge urinary incontinence, such as bladder Botox and sacral neuromodulation, also are minimally invasive and have proven benefit, “so a device like this could well be less effective with little other advantage,” he said.
“Lifestyle changes can make a big difference, but making big lifestyle changes is not always easy,” added Dr. Liaw. “I have found neuromodulation [to be] very effective, especially in conjunction with lifestyle changes.”
BlueWind Medical funds the OASIS trial. Dr. Dmochowski reported he received no grants nor has any relevant financial relationships. Dr. Bretschneider and Dr. Liaw report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – , according to new findings presented at the 2023 annual meeting of the American Urological Association.
As many as half of women in the United States aged 60 and older will experience urinary incontinence. Of those, roughly one in four experience urge urinary incontinence, marked by a sudden need to void that cannot be fully suppressed.
Researchers studied the benefits of the RENOVA iStim (BlueWind Medical) implantable tibial neuromodulation system for the treatment of overactive bladder in the OASIS trial.
Study investigator Roger R. Dmochowski, MD, MMHC, professor of urology and surgery and associate surgeon-in-chief at Vanderbilt University Medical Center, Nashville, Tenn., said the first-line treatment of urinary incontinence is lifestyle changes to retrain the bladder or physical therapy, including pelvic floor and Kegel exercises, per AUA guidelines. He said the success rate is about 30% and is not sustained. Second-line treatments include medications, which most (60%) patients stop taking by 6 months.
More than three-quarters of the 151 women who received the device responded to therapy at 1 year, and 84.6% of the patients showed improvement, according to Dr. Dmochowski.
The participants (mean age, 58.8) demonstrated a mean baseline of 4.8 urge incidents per day (standard deviation, 2.9) and 10 voids/day (SD, 3.3). No device or procedure-related serious adverse events were reported at 12 months. Half of the women no longer had symptoms on three consecutive days, Dr. Dmochowski said.
Because urge urinary incontinence is a chronic condition, “treatment with the BlueWind System will be ongoing, with frequency determined based on the patient’s response,” Dr. Dmochowski said. “The patient is then empowered to control when and where they perform therapy.”
“The device is activated by the external wearable. It’s like an on-off switch. It has a receiver within it that basically has the capacity to be turned on and off by the wearable, which is the control device. The device is in an off-position until the wearable is applied,” he said.
He said the device should be worn twice a day for about 20 minutes, with many patients using it less.
Only one implanted tibial neuromodulation device has been approved by the Food and Drug Administration – eCOIN (Valencia Technologies). The RENOVA iStim is an investigational device under review by the FDA, Dr. Dmochowski said.
In installing the device, Dr. Dmochowski said urologists use a subfascial technique to enable direct visualization of the tibial nerve and suture fixation that increases the possibility of a predictable placement. Patients use an external wearable, which activates the implant, without concern for battery longevity or replacement.
“This therapy is not associated with any adverse effects and may be beneficial for patients who do not respond to other treatments for OAB such as medications or Botox,” said Carol E. Bretschneider, MD, a urogynecologic and pelvic surgeon at Northwestern Medicine Central DuPage Hospital, outside Chicago. “Neurostimulators can be a great advanced therapy option for patients who do not respond to more conservative treatments or cannot take or tolerate a medication.”
The devices do not stimulate or strengthen muscles but act by modulating the reflexes that influence the bladder, sphincter, and pelvic floor, added Dr. Bretschneider, who was not involved in the study.
Other treatments for urge incontinence can include acupuncture, or percutaneous tibial nerve stimulation, to target the posterior tibial nerve in the ankle, which shares the same nerve root that controls the bladder, according to Aron Liaw, MD, a reconstructive urologist and assistant professor of urology at Wayne State University in Detroit. This treatment has been shown to be at least as effective as available medications, but with fewer side effects, he said.
But regular stimulation is necessary to achieve and preserve efficacy, he said.
Dr. Liaw, who was not involved in the neuromodulation study, said the benefits of a device like Renova iStim are that implantation is relatively easy and can be performed in office settings, and patients can then treat themselves at home. However, because the new study did not compare the device to other treatments or a placebo device, its relative benefits are unclear, he said,
Other treatments for urge urinary incontinence, such as bladder Botox and sacral neuromodulation, also are minimally invasive and have proven benefit, “so a device like this could well be less effective with little other advantage,” he said.
“Lifestyle changes can make a big difference, but making big lifestyle changes is not always easy,” added Dr. Liaw. “I have found neuromodulation [to be] very effective, especially in conjunction with lifestyle changes.”
BlueWind Medical funds the OASIS trial. Dr. Dmochowski reported he received no grants nor has any relevant financial relationships. Dr. Bretschneider and Dr. Liaw report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – , according to new findings presented at the 2023 annual meeting of the American Urological Association.
As many as half of women in the United States aged 60 and older will experience urinary incontinence. Of those, roughly one in four experience urge urinary incontinence, marked by a sudden need to void that cannot be fully suppressed.
Researchers studied the benefits of the RENOVA iStim (BlueWind Medical) implantable tibial neuromodulation system for the treatment of overactive bladder in the OASIS trial.
Study investigator Roger R. Dmochowski, MD, MMHC, professor of urology and surgery and associate surgeon-in-chief at Vanderbilt University Medical Center, Nashville, Tenn., said the first-line treatment of urinary incontinence is lifestyle changes to retrain the bladder or physical therapy, including pelvic floor and Kegel exercises, per AUA guidelines. He said the success rate is about 30% and is not sustained. Second-line treatments include medications, which most (60%) patients stop taking by 6 months.
More than three-quarters of the 151 women who received the device responded to therapy at 1 year, and 84.6% of the patients showed improvement, according to Dr. Dmochowski.
The participants (mean age, 58.8) demonstrated a mean baseline of 4.8 urge incidents per day (standard deviation, 2.9) and 10 voids/day (SD, 3.3). No device or procedure-related serious adverse events were reported at 12 months. Half of the women no longer had symptoms on three consecutive days, Dr. Dmochowski said.
Because urge urinary incontinence is a chronic condition, “treatment with the BlueWind System will be ongoing, with frequency determined based on the patient’s response,” Dr. Dmochowski said. “The patient is then empowered to control when and where they perform therapy.”
“The device is activated by the external wearable. It’s like an on-off switch. It has a receiver within it that basically has the capacity to be turned on and off by the wearable, which is the control device. The device is in an off-position until the wearable is applied,” he said.
He said the device should be worn twice a day for about 20 minutes, with many patients using it less.
Only one implanted tibial neuromodulation device has been approved by the Food and Drug Administration – eCOIN (Valencia Technologies). The RENOVA iStim is an investigational device under review by the FDA, Dr. Dmochowski said.
In installing the device, Dr. Dmochowski said urologists use a subfascial technique to enable direct visualization of the tibial nerve and suture fixation that increases the possibility of a predictable placement. Patients use an external wearable, which activates the implant, without concern for battery longevity or replacement.
“This therapy is not associated with any adverse effects and may be beneficial for patients who do not respond to other treatments for OAB such as medications or Botox,” said Carol E. Bretschneider, MD, a urogynecologic and pelvic surgeon at Northwestern Medicine Central DuPage Hospital, outside Chicago. “Neurostimulators can be a great advanced therapy option for patients who do not respond to more conservative treatments or cannot take or tolerate a medication.”
The devices do not stimulate or strengthen muscles but act by modulating the reflexes that influence the bladder, sphincter, and pelvic floor, added Dr. Bretschneider, who was not involved in the study.
Other treatments for urge incontinence can include acupuncture, or percutaneous tibial nerve stimulation, to target the posterior tibial nerve in the ankle, which shares the same nerve root that controls the bladder, according to Aron Liaw, MD, a reconstructive urologist and assistant professor of urology at Wayne State University in Detroit. This treatment has been shown to be at least as effective as available medications, but with fewer side effects, he said.
But regular stimulation is necessary to achieve and preserve efficacy, he said.
Dr. Liaw, who was not involved in the neuromodulation study, said the benefits of a device like Renova iStim are that implantation is relatively easy and can be performed in office settings, and patients can then treat themselves at home. However, because the new study did not compare the device to other treatments or a placebo device, its relative benefits are unclear, he said,
Other treatments for urge urinary incontinence, such as bladder Botox and sacral neuromodulation, also are minimally invasive and have proven benefit, “so a device like this could well be less effective with little other advantage,” he said.
“Lifestyle changes can make a big difference, but making big lifestyle changes is not always easy,” added Dr. Liaw. “I have found neuromodulation [to be] very effective, especially in conjunction with lifestyle changes.”
BlueWind Medical funds the OASIS trial. Dr. Dmochowski reported he received no grants nor has any relevant financial relationships. Dr. Bretschneider and Dr. Liaw report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AUA 2023
Neuropsychiatric side effects of hormonal contraceptives: More common than you think!
Since its introduction in 1950, the combined oral contraceptive pill has been used by countless women as a method for birth control (Liao P. Can Fam Physician. 2012 Dec; 58[12]:e757-e760).
Hormonal contraception (HC) provides women with both contraceptive and noncontraceptive benefits, most notably a method for avoiding unintended pregnancy. In addition to being an effective method of contraception, oral contraceptive pills (OCPs) are well established for treating conditions such as hirsutism, pain symptoms associated with endometriosis and adenomyosis, and pelvic inflammatory disease, among others (Schindler A. Int J Endocrinol Metab. 2013 Winter;11[1]:41-7).
Combined hormonal contraceptives are also first-line treatment for women with menstrual disorders, and in women with polycystic ovary syndrome, can offer an effective long-term method to regulate their menstrual cycle, decrease androgens, clear up oily skin and acne, and reduce facial hair while also providing them with effective contraception (de Melo et al. Open Access J Contracept. 2017;8:13-23).
Associations between ‘the pill’ and mood effects remain controversial
More than 100 million women worldwide use hormonal contraceptives today, yet despite this, the data are mixed regarding the prevalence and extent of neuropsychiatric symptoms and mood changes associated with use of “the pill.” Some studies show combined oral contraceptives are associated with a decrease in general well-being, but had no effect on depression, in women compared with placebo (Zethraeus N et al. Fertil Steril. 2017 May;107[5]:1238-45).
However, a large Danish study published in JAMA Psychiatry of more than 1 million women found a significant association between use of hormonal contraception and antidepressant use or first diagnosis of depression, with adolescents having a higher rate of first depression diagnosis and antidepressant use compared with women 20–30 years old (Skovlund C et al. JAMA Psychiatry. 2016 Nov 1;73[11]:1154-62).
Studies have also shown long-term exposure to levonorgestrel is significantly associated with anxiety and sleep problems in women without a history of these issues (Slattery J et al. Drug Saf. 2018 Oct;41[10]:951-8). A recent small nationwide cohort study in France suggests this may also be true of levonorgestrel delivered by intrauterine devices (IUD) and the association may be dose-dependent (Roland N et al. JAMA. 2023;329[3]:257-9).
Of note, a study published in the American Journal of Psychiatry found a nearly twofold risk of suicide attempt and over threefold risk of suicide among women taking hormonal contraception compared with women who had never used hormonal contraceptives (Skovlund et al. Am J Psychiatry. 2017 Nov 17:appiajp201717060616).
Knowledge gaps make drawing conclusions difficult
The latest information on use of antidepressant and antianxiety medications in women of reproductive age (18-44 years) is sparse and, in some cases, outdated. According to data from the National Health and Nutrition Examination Survey, 18.6% of adult women 18 years or older reported using antidepressant medications within the last 30 days in 2017-2018, an increase from 13.8% in 2009-2010. Among women aged 15-44 year with private employer–sponsored insurance surveyed during 2008-2013, the results showed 15.4% of women filled a prescription for an antidepressant. We must look back further to find data on antianxiety medication use among women aged 18-44 years where use of antianxiety drugs (anxiolytics, sedatives, and hypnotics) was 4.3% between 2005 and 2008.
A lack of literature in this area is likely due to significant underreporting, and an inability to select patients who are sensitive to or at risk of developing neuropsychiatric symptoms resulting from hormonal contraception use because the true pathophysiology is unknown. Existing studies tend to use varying methods to assess mood changes, and do not usually specify hormonal contraceptive use type in their analyses (Schaffir J et al. Eur J Contracept Reprod Health Care. 2016 Oct;21[5]:347-55).
Studies of this nature also require large sample sizes, but the percentage of women who develop neuropsychiatric symptoms from hormonal contraceptive use has historically been relatively small. In the late 1990s, Rosenberg and colleagues found 46% of 1,657 women discontinued oral contraceptives due to side effects within 6 months of starting a new prescription; of these women, 5% reported mood changes as their reason for discontinuing oral contraceptives (Rosenberg M et al. Am J Obstet Gynecol. 1998 Sep;179[3 Pt 1]:577-82).
One might expect that, as lower dosage combined hormonal contraceptives were developed in the 1980s, that the rate of reporting psychological side effects would continue to decrease as well. Yet greater awareness of the potential for mood changes while on “the pill” as outlined by the lay press and social media may be leading to increased reporting of neuropsychiatric effects in women. In a recent cross-sectional survey of 188 women in New York, 43.6% said they experienced mood changes while on hormonal contraceptives, and 61.2% of women with histories of psychiatric illness reported mood changes they attributed to hormonal contraceptives (Martell S et al. Contracept Reprod Med. 2023;8:9).
Martell and colleagues found 48.3% of women cited side effects as a reason for discontinuing hormonal contraception, and 43 participants mentioned psychological side effects unprompted, including 2 patients with suicidal thoughts. The authors said this suggests “psychological side effects, at least in part, may have impacted” HC users’ decisions to switch from OCPs to an alternative method of contraception.
It is also not clear what risk factors exist for women who develop neuropsychiatric symptoms from hormonal contraceptive use. First, it is important to note that both progestin-only contraceptives and combined hormonal contraceptives are classified by the Centers for Disease Control and Prevention’s US Medical Eligibility Criteria for Contraceptive Use, 2016 as having no restrictions for use, including among patients with depression. While women in a smaller subgroup have significant neuropsychiatric symptoms related to their hormonal contraceptives, the underlying mechanism is unknown, and is thought to be largely related to the progestogen component of combined hormonal contraceptives or progestogen-only contraceptives (Mu E. Aust Prescr. 2022 Jun; 45[3]:75-9). We know that some women are hormone sensitive, while others are less so, and some not at all. Progestogens could affect mood as a direct action of the progestogen, because progestogens can be neurosteroids, or the progestogen effect could be mediated secondarily through a change in that woman’s own production of or bioavailability of androgens or naturally occurring estrogens (Giatti S. J Mol Endocrinol. 2016 Aug;57[2]:R109-26).
Here, we also find that currently available evidence limits our ability to draw firm conclusions. A study by Berry-Bibee and colleagues found a “low concern for clinically significant interactions” between hormonal contraception and psychotropic drugs, but was limited by quality/quantity of evidence (Berry-Bibee E et al. Contraception. 2016 Dec;94[6]:650-67). Interestingly, a study by Robinson and colleagues from the mid-2000s posited based on low evidence that “psychological response to the practice of contraception” was a potential explanation for the side effect profile of hormonal contraception (Robinson S et al. Med Hypotheses. 2004;63[2]:268-73).
Further, it may be that women with premenstrual dysphoric disorder (PMDD) might be selected for oral contraceptives, and they are predisposed to other neuropsychiatric problems. Estimates have placed the prevalence of comorbid psychiatric disorders such as anxiety, major depression, bipolar disorder, and posttraumatic stress disorder as high as 70% for women with PMDD (Sepede G et al. Neuropsychiatr Dis Treat. 2020;16:415-26). This phenomenon is not new, having been characterized in the lay literature nearly 20 years ago, by endocrinologist Geoffrey P. Redmond, MD (Redmond GP. The Hormonally Vulnerable Woman. New York: HarperCollins; 2005).
While the cause is not exactly idiosyncratic, They tend to have an entire spectrum of responses to the progestogens in combined or progestin-only contraceptives, ranging from just a flattened affect – which could easily be explained by their flattened level of endogenous hormones – to frank depression. Their frank depression, in turn, can be demonstrated to include suicidal ideation and actual suicide.
Compounding this issue is a woman’s perception of her sexuality. Some women with low sexual desire or sexual problems who are younger may have more distress about their problems compared with women of older reproductive age. While the reason for that is not clear, it may be that in the sexual arena, it is more important for some younger women to be a sexual person than in perimenopausal women, or that women who are younger are more likely to be partnered than women of older reproductive age. While the European Society of Sexual Medicine concluded in a 2019 position statement that there is inconclusive evidence whether hormonal contraception may be contributing to changes in sexual desire and sexual dysfunction, it appears that “a minority of women” experience “better or worse sexual functioning” from taking combined oral contraceptives (Both S et al. J Sex Med. 2019 Nov;16[11]:1681-95), suggesting that the majority of women report no significant changes.
Practitioners should discuss mood effects during consultation
An ob.gyn., primary care physicians, or others with prescriptive authority (i.e. nurse practitioners and physician assistants) in clinical practice may encounter a patient who seems to have mood side effects owing to progestogen-containing contraceptives that they prescribe. However, many ob.gyns. are likely unaware of the prevalence, or that some of those same patients can have such significant mood effects that they would become or are suicidal.
I believe questioning patients about mood effects during consultation and particularly during follow-up following the initiation of any hormonal contraceptive is worth a passing comment for every patient, which should include mood effects in broader discussion for anyone currently using an antidepressant, patients with a history of antidepressant use, and patients who have considered suicide. As we do with other drugs, these questions can be posed in the form of a questionnaire followed up by the practitioner in counseling.
Practitioners who encounter a patient with mood changes as a result of hormonal contraceptive use can consider changing to a nonhormonal method of birth control, or recommending the patient use a barrier method during sexual activity, as none of these options have neuropsychiatric side effects.
Ultimately, practitioners of all types need to engage in shared decision-making to identify the key benefits and risks of hormonal contraceptive use for each patient, which may involve trial and error to determine the ideal treatment. It is critical that practitioners of all types strike a balance between alleviating patient concerns about potential mood changes, monitoring patients with an appreciable risk of mood changes, and continuing patients on hormonal contraception for whom the benefits outweigh the risks.
Dr. Simon is a clinical professor at George Washington University and the medical director and founder of IntimMedicine Specialists in Washington, which provides patient-focused care for women across the reproductive life cycle. He is a past president of the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Dr. Simon has been a consultant to, received grant and research support from, and served on the speakers bureau for various pharmaceutical companies that develop combination hormonal contraceptives. Email Dr. Simon at obnews@mdedge.com.
Since its introduction in 1950, the combined oral contraceptive pill has been used by countless women as a method for birth control (Liao P. Can Fam Physician. 2012 Dec; 58[12]:e757-e760).
Hormonal contraception (HC) provides women with both contraceptive and noncontraceptive benefits, most notably a method for avoiding unintended pregnancy. In addition to being an effective method of contraception, oral contraceptive pills (OCPs) are well established for treating conditions such as hirsutism, pain symptoms associated with endometriosis and adenomyosis, and pelvic inflammatory disease, among others (Schindler A. Int J Endocrinol Metab. 2013 Winter;11[1]:41-7).
Combined hormonal contraceptives are also first-line treatment for women with menstrual disorders, and in women with polycystic ovary syndrome, can offer an effective long-term method to regulate their menstrual cycle, decrease androgens, clear up oily skin and acne, and reduce facial hair while also providing them with effective contraception (de Melo et al. Open Access J Contracept. 2017;8:13-23).
Associations between ‘the pill’ and mood effects remain controversial
More than 100 million women worldwide use hormonal contraceptives today, yet despite this, the data are mixed regarding the prevalence and extent of neuropsychiatric symptoms and mood changes associated with use of “the pill.” Some studies show combined oral contraceptives are associated with a decrease in general well-being, but had no effect on depression, in women compared with placebo (Zethraeus N et al. Fertil Steril. 2017 May;107[5]:1238-45).
However, a large Danish study published in JAMA Psychiatry of more than 1 million women found a significant association between use of hormonal contraception and antidepressant use or first diagnosis of depression, with adolescents having a higher rate of first depression diagnosis and antidepressant use compared with women 20–30 years old (Skovlund C et al. JAMA Psychiatry. 2016 Nov 1;73[11]:1154-62).
Studies have also shown long-term exposure to levonorgestrel is significantly associated with anxiety and sleep problems in women without a history of these issues (Slattery J et al. Drug Saf. 2018 Oct;41[10]:951-8). A recent small nationwide cohort study in France suggests this may also be true of levonorgestrel delivered by intrauterine devices (IUD) and the association may be dose-dependent (Roland N et al. JAMA. 2023;329[3]:257-9).
Of note, a study published in the American Journal of Psychiatry found a nearly twofold risk of suicide attempt and over threefold risk of suicide among women taking hormonal contraception compared with women who had never used hormonal contraceptives (Skovlund et al. Am J Psychiatry. 2017 Nov 17:appiajp201717060616).
Knowledge gaps make drawing conclusions difficult
The latest information on use of antidepressant and antianxiety medications in women of reproductive age (18-44 years) is sparse and, in some cases, outdated. According to data from the National Health and Nutrition Examination Survey, 18.6% of adult women 18 years or older reported using antidepressant medications within the last 30 days in 2017-2018, an increase from 13.8% in 2009-2010. Among women aged 15-44 year with private employer–sponsored insurance surveyed during 2008-2013, the results showed 15.4% of women filled a prescription for an antidepressant. We must look back further to find data on antianxiety medication use among women aged 18-44 years where use of antianxiety drugs (anxiolytics, sedatives, and hypnotics) was 4.3% between 2005 and 2008.
A lack of literature in this area is likely due to significant underreporting, and an inability to select patients who are sensitive to or at risk of developing neuropsychiatric symptoms resulting from hormonal contraception use because the true pathophysiology is unknown. Existing studies tend to use varying methods to assess mood changes, and do not usually specify hormonal contraceptive use type in their analyses (Schaffir J et al. Eur J Contracept Reprod Health Care. 2016 Oct;21[5]:347-55).
Studies of this nature also require large sample sizes, but the percentage of women who develop neuropsychiatric symptoms from hormonal contraceptive use has historically been relatively small. In the late 1990s, Rosenberg and colleagues found 46% of 1,657 women discontinued oral contraceptives due to side effects within 6 months of starting a new prescription; of these women, 5% reported mood changes as their reason for discontinuing oral contraceptives (Rosenberg M et al. Am J Obstet Gynecol. 1998 Sep;179[3 Pt 1]:577-82).
One might expect that, as lower dosage combined hormonal contraceptives were developed in the 1980s, that the rate of reporting psychological side effects would continue to decrease as well. Yet greater awareness of the potential for mood changes while on “the pill” as outlined by the lay press and social media may be leading to increased reporting of neuropsychiatric effects in women. In a recent cross-sectional survey of 188 women in New York, 43.6% said they experienced mood changes while on hormonal contraceptives, and 61.2% of women with histories of psychiatric illness reported mood changes they attributed to hormonal contraceptives (Martell S et al. Contracept Reprod Med. 2023;8:9).
Martell and colleagues found 48.3% of women cited side effects as a reason for discontinuing hormonal contraception, and 43 participants mentioned psychological side effects unprompted, including 2 patients with suicidal thoughts. The authors said this suggests “psychological side effects, at least in part, may have impacted” HC users’ decisions to switch from OCPs to an alternative method of contraception.
It is also not clear what risk factors exist for women who develop neuropsychiatric symptoms from hormonal contraceptive use. First, it is important to note that both progestin-only contraceptives and combined hormonal contraceptives are classified by the Centers for Disease Control and Prevention’s US Medical Eligibility Criteria for Contraceptive Use, 2016 as having no restrictions for use, including among patients with depression. While women in a smaller subgroup have significant neuropsychiatric symptoms related to their hormonal contraceptives, the underlying mechanism is unknown, and is thought to be largely related to the progestogen component of combined hormonal contraceptives or progestogen-only contraceptives (Mu E. Aust Prescr. 2022 Jun; 45[3]:75-9). We know that some women are hormone sensitive, while others are less so, and some not at all. Progestogens could affect mood as a direct action of the progestogen, because progestogens can be neurosteroids, or the progestogen effect could be mediated secondarily through a change in that woman’s own production of or bioavailability of androgens or naturally occurring estrogens (Giatti S. J Mol Endocrinol. 2016 Aug;57[2]:R109-26).
Here, we also find that currently available evidence limits our ability to draw firm conclusions. A study by Berry-Bibee and colleagues found a “low concern for clinically significant interactions” between hormonal contraception and psychotropic drugs, but was limited by quality/quantity of evidence (Berry-Bibee E et al. Contraception. 2016 Dec;94[6]:650-67). Interestingly, a study by Robinson and colleagues from the mid-2000s posited based on low evidence that “psychological response to the practice of contraception” was a potential explanation for the side effect profile of hormonal contraception (Robinson S et al. Med Hypotheses. 2004;63[2]:268-73).
Further, it may be that women with premenstrual dysphoric disorder (PMDD) might be selected for oral contraceptives, and they are predisposed to other neuropsychiatric problems. Estimates have placed the prevalence of comorbid psychiatric disorders such as anxiety, major depression, bipolar disorder, and posttraumatic stress disorder as high as 70% for women with PMDD (Sepede G et al. Neuropsychiatr Dis Treat. 2020;16:415-26). This phenomenon is not new, having been characterized in the lay literature nearly 20 years ago, by endocrinologist Geoffrey P. Redmond, MD (Redmond GP. The Hormonally Vulnerable Woman. New York: HarperCollins; 2005).
While the cause is not exactly idiosyncratic, They tend to have an entire spectrum of responses to the progestogens in combined or progestin-only contraceptives, ranging from just a flattened affect – which could easily be explained by their flattened level of endogenous hormones – to frank depression. Their frank depression, in turn, can be demonstrated to include suicidal ideation and actual suicide.
Compounding this issue is a woman’s perception of her sexuality. Some women with low sexual desire or sexual problems who are younger may have more distress about their problems compared with women of older reproductive age. While the reason for that is not clear, it may be that in the sexual arena, it is more important for some younger women to be a sexual person than in perimenopausal women, or that women who are younger are more likely to be partnered than women of older reproductive age. While the European Society of Sexual Medicine concluded in a 2019 position statement that there is inconclusive evidence whether hormonal contraception may be contributing to changes in sexual desire and sexual dysfunction, it appears that “a minority of women” experience “better or worse sexual functioning” from taking combined oral contraceptives (Both S et al. J Sex Med. 2019 Nov;16[11]:1681-95), suggesting that the majority of women report no significant changes.
Practitioners should discuss mood effects during consultation
An ob.gyn., primary care physicians, or others with prescriptive authority (i.e. nurse practitioners and physician assistants) in clinical practice may encounter a patient who seems to have mood side effects owing to progestogen-containing contraceptives that they prescribe. However, many ob.gyns. are likely unaware of the prevalence, or that some of those same patients can have such significant mood effects that they would become or are suicidal.
I believe questioning patients about mood effects during consultation and particularly during follow-up following the initiation of any hormonal contraceptive is worth a passing comment for every patient, which should include mood effects in broader discussion for anyone currently using an antidepressant, patients with a history of antidepressant use, and patients who have considered suicide. As we do with other drugs, these questions can be posed in the form of a questionnaire followed up by the practitioner in counseling.
Practitioners who encounter a patient with mood changes as a result of hormonal contraceptive use can consider changing to a nonhormonal method of birth control, or recommending the patient use a barrier method during sexual activity, as none of these options have neuropsychiatric side effects.
Ultimately, practitioners of all types need to engage in shared decision-making to identify the key benefits and risks of hormonal contraceptive use for each patient, which may involve trial and error to determine the ideal treatment. It is critical that practitioners of all types strike a balance between alleviating patient concerns about potential mood changes, monitoring patients with an appreciable risk of mood changes, and continuing patients on hormonal contraception for whom the benefits outweigh the risks.
Dr. Simon is a clinical professor at George Washington University and the medical director and founder of IntimMedicine Specialists in Washington, which provides patient-focused care for women across the reproductive life cycle. He is a past president of the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Dr. Simon has been a consultant to, received grant and research support from, and served on the speakers bureau for various pharmaceutical companies that develop combination hormonal contraceptives. Email Dr. Simon at obnews@mdedge.com.
Since its introduction in 1950, the combined oral contraceptive pill has been used by countless women as a method for birth control (Liao P. Can Fam Physician. 2012 Dec; 58[12]:e757-e760).
Hormonal contraception (HC) provides women with both contraceptive and noncontraceptive benefits, most notably a method for avoiding unintended pregnancy. In addition to being an effective method of contraception, oral contraceptive pills (OCPs) are well established for treating conditions such as hirsutism, pain symptoms associated with endometriosis and adenomyosis, and pelvic inflammatory disease, among others (Schindler A. Int J Endocrinol Metab. 2013 Winter;11[1]:41-7).
Combined hormonal contraceptives are also first-line treatment for women with menstrual disorders, and in women with polycystic ovary syndrome, can offer an effective long-term method to regulate their menstrual cycle, decrease androgens, clear up oily skin and acne, and reduce facial hair while also providing them with effective contraception (de Melo et al. Open Access J Contracept. 2017;8:13-23).
Associations between ‘the pill’ and mood effects remain controversial
More than 100 million women worldwide use hormonal contraceptives today, yet despite this, the data are mixed regarding the prevalence and extent of neuropsychiatric symptoms and mood changes associated with use of “the pill.” Some studies show combined oral contraceptives are associated with a decrease in general well-being, but had no effect on depression, in women compared with placebo (Zethraeus N et al. Fertil Steril. 2017 May;107[5]:1238-45).
However, a large Danish study published in JAMA Psychiatry of more than 1 million women found a significant association between use of hormonal contraception and antidepressant use or first diagnosis of depression, with adolescents having a higher rate of first depression diagnosis and antidepressant use compared with women 20–30 years old (Skovlund C et al. JAMA Psychiatry. 2016 Nov 1;73[11]:1154-62).
Studies have also shown long-term exposure to levonorgestrel is significantly associated with anxiety and sleep problems in women without a history of these issues (Slattery J et al. Drug Saf. 2018 Oct;41[10]:951-8). A recent small nationwide cohort study in France suggests this may also be true of levonorgestrel delivered by intrauterine devices (IUD) and the association may be dose-dependent (Roland N et al. JAMA. 2023;329[3]:257-9).
Of note, a study published in the American Journal of Psychiatry found a nearly twofold risk of suicide attempt and over threefold risk of suicide among women taking hormonal contraception compared with women who had never used hormonal contraceptives (Skovlund et al. Am J Psychiatry. 2017 Nov 17:appiajp201717060616).
Knowledge gaps make drawing conclusions difficult
The latest information on use of antidepressant and antianxiety medications in women of reproductive age (18-44 years) is sparse and, in some cases, outdated. According to data from the National Health and Nutrition Examination Survey, 18.6% of adult women 18 years or older reported using antidepressant medications within the last 30 days in 2017-2018, an increase from 13.8% in 2009-2010. Among women aged 15-44 year with private employer–sponsored insurance surveyed during 2008-2013, the results showed 15.4% of women filled a prescription for an antidepressant. We must look back further to find data on antianxiety medication use among women aged 18-44 years where use of antianxiety drugs (anxiolytics, sedatives, and hypnotics) was 4.3% between 2005 and 2008.
A lack of literature in this area is likely due to significant underreporting, and an inability to select patients who are sensitive to or at risk of developing neuropsychiatric symptoms resulting from hormonal contraception use because the true pathophysiology is unknown. Existing studies tend to use varying methods to assess mood changes, and do not usually specify hormonal contraceptive use type in their analyses (Schaffir J et al. Eur J Contracept Reprod Health Care. 2016 Oct;21[5]:347-55).
Studies of this nature also require large sample sizes, but the percentage of women who develop neuropsychiatric symptoms from hormonal contraceptive use has historically been relatively small. In the late 1990s, Rosenberg and colleagues found 46% of 1,657 women discontinued oral contraceptives due to side effects within 6 months of starting a new prescription; of these women, 5% reported mood changes as their reason for discontinuing oral contraceptives (Rosenberg M et al. Am J Obstet Gynecol. 1998 Sep;179[3 Pt 1]:577-82).
One might expect that, as lower dosage combined hormonal contraceptives were developed in the 1980s, that the rate of reporting psychological side effects would continue to decrease as well. Yet greater awareness of the potential for mood changes while on “the pill” as outlined by the lay press and social media may be leading to increased reporting of neuropsychiatric effects in women. In a recent cross-sectional survey of 188 women in New York, 43.6% said they experienced mood changes while on hormonal contraceptives, and 61.2% of women with histories of psychiatric illness reported mood changes they attributed to hormonal contraceptives (Martell S et al. Contracept Reprod Med. 2023;8:9).
Martell and colleagues found 48.3% of women cited side effects as a reason for discontinuing hormonal contraception, and 43 participants mentioned psychological side effects unprompted, including 2 patients with suicidal thoughts. The authors said this suggests “psychological side effects, at least in part, may have impacted” HC users’ decisions to switch from OCPs to an alternative method of contraception.
It is also not clear what risk factors exist for women who develop neuropsychiatric symptoms from hormonal contraceptive use. First, it is important to note that both progestin-only contraceptives and combined hormonal contraceptives are classified by the Centers for Disease Control and Prevention’s US Medical Eligibility Criteria for Contraceptive Use, 2016 as having no restrictions for use, including among patients with depression. While women in a smaller subgroup have significant neuropsychiatric symptoms related to their hormonal contraceptives, the underlying mechanism is unknown, and is thought to be largely related to the progestogen component of combined hormonal contraceptives or progestogen-only contraceptives (Mu E. Aust Prescr. 2022 Jun; 45[3]:75-9). We know that some women are hormone sensitive, while others are less so, and some not at all. Progestogens could affect mood as a direct action of the progestogen, because progestogens can be neurosteroids, or the progestogen effect could be mediated secondarily through a change in that woman’s own production of or bioavailability of androgens or naturally occurring estrogens (Giatti S. J Mol Endocrinol. 2016 Aug;57[2]:R109-26).
Here, we also find that currently available evidence limits our ability to draw firm conclusions. A study by Berry-Bibee and colleagues found a “low concern for clinically significant interactions” between hormonal contraception and psychotropic drugs, but was limited by quality/quantity of evidence (Berry-Bibee E et al. Contraception. 2016 Dec;94[6]:650-67). Interestingly, a study by Robinson and colleagues from the mid-2000s posited based on low evidence that “psychological response to the practice of contraception” was a potential explanation for the side effect profile of hormonal contraception (Robinson S et al. Med Hypotheses. 2004;63[2]:268-73).
Further, it may be that women with premenstrual dysphoric disorder (PMDD) might be selected for oral contraceptives, and they are predisposed to other neuropsychiatric problems. Estimates have placed the prevalence of comorbid psychiatric disorders such as anxiety, major depression, bipolar disorder, and posttraumatic stress disorder as high as 70% for women with PMDD (Sepede G et al. Neuropsychiatr Dis Treat. 2020;16:415-26). This phenomenon is not new, having been characterized in the lay literature nearly 20 years ago, by endocrinologist Geoffrey P. Redmond, MD (Redmond GP. The Hormonally Vulnerable Woman. New York: HarperCollins; 2005).
While the cause is not exactly idiosyncratic, They tend to have an entire spectrum of responses to the progestogens in combined or progestin-only contraceptives, ranging from just a flattened affect – which could easily be explained by their flattened level of endogenous hormones – to frank depression. Their frank depression, in turn, can be demonstrated to include suicidal ideation and actual suicide.
Compounding this issue is a woman’s perception of her sexuality. Some women with low sexual desire or sexual problems who are younger may have more distress about their problems compared with women of older reproductive age. While the reason for that is not clear, it may be that in the sexual arena, it is more important for some younger women to be a sexual person than in perimenopausal women, or that women who are younger are more likely to be partnered than women of older reproductive age. While the European Society of Sexual Medicine concluded in a 2019 position statement that there is inconclusive evidence whether hormonal contraception may be contributing to changes in sexual desire and sexual dysfunction, it appears that “a minority of women” experience “better or worse sexual functioning” from taking combined oral contraceptives (Both S et al. J Sex Med. 2019 Nov;16[11]:1681-95), suggesting that the majority of women report no significant changes.
Practitioners should discuss mood effects during consultation
An ob.gyn., primary care physicians, or others with prescriptive authority (i.e. nurse practitioners and physician assistants) in clinical practice may encounter a patient who seems to have mood side effects owing to progestogen-containing contraceptives that they prescribe. However, many ob.gyns. are likely unaware of the prevalence, or that some of those same patients can have such significant mood effects that they would become or are suicidal.
I believe questioning patients about mood effects during consultation and particularly during follow-up following the initiation of any hormonal contraceptive is worth a passing comment for every patient, which should include mood effects in broader discussion for anyone currently using an antidepressant, patients with a history of antidepressant use, and patients who have considered suicide. As we do with other drugs, these questions can be posed in the form of a questionnaire followed up by the practitioner in counseling.
Practitioners who encounter a patient with mood changes as a result of hormonal contraceptive use can consider changing to a nonhormonal method of birth control, or recommending the patient use a barrier method during sexual activity, as none of these options have neuropsychiatric side effects.
Ultimately, practitioners of all types need to engage in shared decision-making to identify the key benefits and risks of hormonal contraceptive use for each patient, which may involve trial and error to determine the ideal treatment. It is critical that practitioners of all types strike a balance between alleviating patient concerns about potential mood changes, monitoring patients with an appreciable risk of mood changes, and continuing patients on hormonal contraception for whom the benefits outweigh the risks.
Dr. Simon is a clinical professor at George Washington University and the medical director and founder of IntimMedicine Specialists in Washington, which provides patient-focused care for women across the reproductive life cycle. He is a past president of the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Dr. Simon has been a consultant to, received grant and research support from, and served on the speakers bureau for various pharmaceutical companies that develop combination hormonal contraceptives. Email Dr. Simon at obnews@mdedge.com.
Premenopausal women benefit from ovarian conservation with benign hysterectomies
Although bilateral salpingo-oophorectomy (BSO) with hysterectomy has been shown to reduce the risk for ovarian cancer in women at increased risk, current guidelines are touting ovarian conservation, especially in premenopausal women, wrote Mathilde Gottschau, MD, of the Danish Cancer Society Research Center, Copenhagen, and colleagues. However, post-hysterectomy outcomes in women with and without BSO have not been well examined.
In a study published in the Annals of Internal Medicine, the researchers reviewed data from a nationwide registry of women in Denmark aged 20 years and older who underwent benign hysterectomies with BSO (22,974 women) and without BSO (120,011 women) between 1977 and 2017. The women were divided into subgroups based on age; those younger than 45 years were defined as premenopausal, those aged 45-54 years were defined as perimenopausal, those aged 55-64 were defined as early postmenopausal, and those aged 65 and older were defined as late menopausal.
The primary outcomes were hospitalization for cardiovascular disease, cancer incidence, and all-cause mortality over a median follow-up period of 22 years.
For women younger than 45 years, the 10-year cumulative risk for all cancer was lower with BSO than without, but the risk of overall cardiovascular disease was higher with BSO, with higher levels of ischemic heart disease and stroke, compared with women without BSO. The 10-year cumulative mortality was higher with BSO than without (2.16% vs. 1.94%).
For women aged 45-54 years, the 10-year cumulative cancer risk was higher in those with BSO than those without BSO (risk difference, 0.73 percentage points) associated mainly with nonbreast cancer, and both 10-year and 20-year mortality were higher in those with BSO than those without.
For women aged 55-65 years, the 10-year cumulative cancer risk was higher in those with BSO than those without BSO. Cumulative overall mortality was higher at 10 years for those with BSO, but lower at 20 years.
For women aged 65 years and older, both 10-year and 20-year cumulative overall cancer risk was higher with BSO than without (RD, 2.54 and 4.57 percentage points, respectively). Cumulative mortality was higher in the BSO group at 10 years, but lower at 20 years.
The study findings were limited by several factors including the use of age to determine menopausal status and the lack of genetic predisposition data, and the focus only on a relatively homogeneous population that may not be generalizable to other populations, the researchers noted.
However, the results were strengthened by the use of a nationwide registry and the long-term follow-up period, they said. The current study indicates that the health risks outweigh the potential benefits of BSO with benign hysterectomy for premenopausal women and supports the current guidelines for ovarian conservation in these women with low lifetime ovarian cancer risk, they said. For postmenopausal women, the data support a cautious approach to BSO given the lack of a clear survival benefit and cancer excess, they concluded.
Delayed diagnosis of ovarian cancers favors BSO
“The question of removing ovaries at the time of benign hysterectomy to prevent ovarian cancer in low-risk women has been widely debated,” which has contributed to the variation in incidence rates of unilateral and bilateral oophorectomy over time, wrote Elizabeth Casiano Evans, MD, of the University of Texas, San Antonio, and Deslyn T.G. Hobson, MD, of Wayne State University, Detroit, in an accompanying editorial.
Ovarian cancer often goes undiagnosed until an advanced stage, and BSO can significantly reduce risk in women with BRCA1 and BRCA2 mutations, they noted.
For women without increased risk, those who are premenopausal may wish to preserve ovarian function, but women also may benefit from improvements in a range of menopause-related symptoms including vasomotor and urogenital symptoms, sexual dysfunction, and psychiatric and cognitive symptoms, they said.
“In addition, salpingectomy alone has a role in significantly reducing ovarian cancer incidence without compromising ovarian function because the fallopian tube has been found to be at the origin of many ovarian cancer cases,” they noted. In the current study, “the crude ovarian cancer risk was lower with BSO” across all age groups, the editorialists said.
The choice of whether to include BSO at the time of benign hysterectomy is complicated, with many factors to consider, the editorialists wrote, and the current study supports the need for informed, shared decision-making between clinicians and patients.
The study was supported by the Danish Cancer Society’s Scientific Committee and the Mermaid Project. The researchers had no financial conflicts to disclose. The editorial authors had no financial conflicts to disclose.
Although bilateral salpingo-oophorectomy (BSO) with hysterectomy has been shown to reduce the risk for ovarian cancer in women at increased risk, current guidelines are touting ovarian conservation, especially in premenopausal women, wrote Mathilde Gottschau, MD, of the Danish Cancer Society Research Center, Copenhagen, and colleagues. However, post-hysterectomy outcomes in women with and without BSO have not been well examined.
In a study published in the Annals of Internal Medicine, the researchers reviewed data from a nationwide registry of women in Denmark aged 20 years and older who underwent benign hysterectomies with BSO (22,974 women) and without BSO (120,011 women) between 1977 and 2017. The women were divided into subgroups based on age; those younger than 45 years were defined as premenopausal, those aged 45-54 years were defined as perimenopausal, those aged 55-64 were defined as early postmenopausal, and those aged 65 and older were defined as late menopausal.
The primary outcomes were hospitalization for cardiovascular disease, cancer incidence, and all-cause mortality over a median follow-up period of 22 years.
For women younger than 45 years, the 10-year cumulative risk for all cancer was lower with BSO than without, but the risk of overall cardiovascular disease was higher with BSO, with higher levels of ischemic heart disease and stroke, compared with women without BSO. The 10-year cumulative mortality was higher with BSO than without (2.16% vs. 1.94%).
For women aged 45-54 years, the 10-year cumulative cancer risk was higher in those with BSO than those without BSO (risk difference, 0.73 percentage points) associated mainly with nonbreast cancer, and both 10-year and 20-year mortality were higher in those with BSO than those without.
For women aged 55-65 years, the 10-year cumulative cancer risk was higher in those with BSO than those without BSO. Cumulative overall mortality was higher at 10 years for those with BSO, but lower at 20 years.
For women aged 65 years and older, both 10-year and 20-year cumulative overall cancer risk was higher with BSO than without (RD, 2.54 and 4.57 percentage points, respectively). Cumulative mortality was higher in the BSO group at 10 years, but lower at 20 years.
The study findings were limited by several factors including the use of age to determine menopausal status and the lack of genetic predisposition data, and the focus only on a relatively homogeneous population that may not be generalizable to other populations, the researchers noted.
However, the results were strengthened by the use of a nationwide registry and the long-term follow-up period, they said. The current study indicates that the health risks outweigh the potential benefits of BSO with benign hysterectomy for premenopausal women and supports the current guidelines for ovarian conservation in these women with low lifetime ovarian cancer risk, they said. For postmenopausal women, the data support a cautious approach to BSO given the lack of a clear survival benefit and cancer excess, they concluded.
Delayed diagnosis of ovarian cancers favors BSO
“The question of removing ovaries at the time of benign hysterectomy to prevent ovarian cancer in low-risk women has been widely debated,” which has contributed to the variation in incidence rates of unilateral and bilateral oophorectomy over time, wrote Elizabeth Casiano Evans, MD, of the University of Texas, San Antonio, and Deslyn T.G. Hobson, MD, of Wayne State University, Detroit, in an accompanying editorial.
Ovarian cancer often goes undiagnosed until an advanced stage, and BSO can significantly reduce risk in women with BRCA1 and BRCA2 mutations, they noted.
For women without increased risk, those who are premenopausal may wish to preserve ovarian function, but women also may benefit from improvements in a range of menopause-related symptoms including vasomotor and urogenital symptoms, sexual dysfunction, and psychiatric and cognitive symptoms, they said.
“In addition, salpingectomy alone has a role in significantly reducing ovarian cancer incidence without compromising ovarian function because the fallopian tube has been found to be at the origin of many ovarian cancer cases,” they noted. In the current study, “the crude ovarian cancer risk was lower with BSO” across all age groups, the editorialists said.
The choice of whether to include BSO at the time of benign hysterectomy is complicated, with many factors to consider, the editorialists wrote, and the current study supports the need for informed, shared decision-making between clinicians and patients.
The study was supported by the Danish Cancer Society’s Scientific Committee and the Mermaid Project. The researchers had no financial conflicts to disclose. The editorial authors had no financial conflicts to disclose.
Although bilateral salpingo-oophorectomy (BSO) with hysterectomy has been shown to reduce the risk for ovarian cancer in women at increased risk, current guidelines are touting ovarian conservation, especially in premenopausal women, wrote Mathilde Gottschau, MD, of the Danish Cancer Society Research Center, Copenhagen, and colleagues. However, post-hysterectomy outcomes in women with and without BSO have not been well examined.
In a study published in the Annals of Internal Medicine, the researchers reviewed data from a nationwide registry of women in Denmark aged 20 years and older who underwent benign hysterectomies with BSO (22,974 women) and without BSO (120,011 women) between 1977 and 2017. The women were divided into subgroups based on age; those younger than 45 years were defined as premenopausal, those aged 45-54 years were defined as perimenopausal, those aged 55-64 were defined as early postmenopausal, and those aged 65 and older were defined as late menopausal.
The primary outcomes were hospitalization for cardiovascular disease, cancer incidence, and all-cause mortality over a median follow-up period of 22 years.
For women younger than 45 years, the 10-year cumulative risk for all cancer was lower with BSO than without, but the risk of overall cardiovascular disease was higher with BSO, with higher levels of ischemic heart disease and stroke, compared with women without BSO. The 10-year cumulative mortality was higher with BSO than without (2.16% vs. 1.94%).
For women aged 45-54 years, the 10-year cumulative cancer risk was higher in those with BSO than those without BSO (risk difference, 0.73 percentage points) associated mainly with nonbreast cancer, and both 10-year and 20-year mortality were higher in those with BSO than those without.
For women aged 55-65 years, the 10-year cumulative cancer risk was higher in those with BSO than those without BSO. Cumulative overall mortality was higher at 10 years for those with BSO, but lower at 20 years.
For women aged 65 years and older, both 10-year and 20-year cumulative overall cancer risk was higher with BSO than without (RD, 2.54 and 4.57 percentage points, respectively). Cumulative mortality was higher in the BSO group at 10 years, but lower at 20 years.
The study findings were limited by several factors including the use of age to determine menopausal status and the lack of genetic predisposition data, and the focus only on a relatively homogeneous population that may not be generalizable to other populations, the researchers noted.
However, the results were strengthened by the use of a nationwide registry and the long-term follow-up period, they said. The current study indicates that the health risks outweigh the potential benefits of BSO with benign hysterectomy for premenopausal women and supports the current guidelines for ovarian conservation in these women with low lifetime ovarian cancer risk, they said. For postmenopausal women, the data support a cautious approach to BSO given the lack of a clear survival benefit and cancer excess, they concluded.
Delayed diagnosis of ovarian cancers favors BSO
“The question of removing ovaries at the time of benign hysterectomy to prevent ovarian cancer in low-risk women has been widely debated,” which has contributed to the variation in incidence rates of unilateral and bilateral oophorectomy over time, wrote Elizabeth Casiano Evans, MD, of the University of Texas, San Antonio, and Deslyn T.G. Hobson, MD, of Wayne State University, Detroit, in an accompanying editorial.
Ovarian cancer often goes undiagnosed until an advanced stage, and BSO can significantly reduce risk in women with BRCA1 and BRCA2 mutations, they noted.
For women without increased risk, those who are premenopausal may wish to preserve ovarian function, but women also may benefit from improvements in a range of menopause-related symptoms including vasomotor and urogenital symptoms, sexual dysfunction, and psychiatric and cognitive symptoms, they said.
“In addition, salpingectomy alone has a role in significantly reducing ovarian cancer incidence without compromising ovarian function because the fallopian tube has been found to be at the origin of many ovarian cancer cases,” they noted. In the current study, “the crude ovarian cancer risk was lower with BSO” across all age groups, the editorialists said.
The choice of whether to include BSO at the time of benign hysterectomy is complicated, with many factors to consider, the editorialists wrote, and the current study supports the need for informed, shared decision-making between clinicians and patients.
The study was supported by the Danish Cancer Society’s Scientific Committee and the Mermaid Project. The researchers had no financial conflicts to disclose. The editorial authors had no financial conflicts to disclose.
FROM THE ANNALS OF INTERNAL MEDICINE











