User login
Five steps to fewer heart failure hospitalizations
ESTES PARK, COLO. – Introducing five evidence-based interventions in patients with heart failure with reduced ejection fraction would dramatically cut admissions for heart failure, according to Dr. JoAnn Lindenfeld, vice president of the Heart Failure Society of America.
Here are the five interventions: recognizing when to switch from furosemide to another oral loop diuretic with far better bioavailability; up-titrating beta-blocker therapy to the maximum recommended dose as quickly as possible; adding a low-dose aldosterone antagonist to the treatment regimen; prescribing digoxin in symptomatic patients with a low ejection fraction; and identifying and treating iron deficiency, Dr. Lindenfeld said at a conference on internal medicine sponsored by the University of Colorado.
• Loop diuretics: Furosemide, everybody’s favorite low-cost loop diuretic, turns out to have an enormously variable oral bioavailability, ranging from 10% to 90% from patient to patient. It also varies substantially from day to day within the same individual. In contrast, torsemide (Demadex) and bumetanide (Bumex) have a consistently high oral bioavailability of roughly 90%. They are useful alternatives in poorly compensated heart failure patients.
"When your patient says they’re not diuresing and you’re pretty sure they’re taking their drugs, or if they’ve had more than one recent admission for heart failure and they’re having trouble with congestion and fluid retention, think about switching to bumetanide or torsemide," said Dr. Lindenfeld, professor of medicine and medical director of the heart transplant program at the university.
"In my own practice, when I have a patient admitted for acute decompensated heart failure with congestion and I don’t find another reversible cause, I will usually switch them," noted Dr. Lindenfeld, who also is codirector of the university’s Center for Women’s Health Research.
In a classic study, 234 patients hospitalized for acute decompensated heart failure were randomized at discharge to torsemide or furosemide in equivalent doses. The torsemide group subsequently had a 52% lower rate of heart failure hospitalization (Am. J. Med. 2001;111:513-21).
Bumetanide is now a pretty inexpensive drug, Dr. Lindenfeld noted. In making the switch, remember that 40 mg of furosemide is equivalent to 1 mg of bumetanide or 20 mg of torsemide.
• Beta-blocker up-titration: Beta-blocker and angiotensin-converting enzyme* (ACE) inhibitor therapy both have a class IA recommendation in heart failure. But what’s the best way to juggle the timing of dual dose increases?
"None of the guidelines says how to manage up-titration, but I strongly believe that once you have somebody on a reasonable dose of an ACE inhibitor – say, 5 mg of lisinopril or the equivalent – then you should go to the beta-blocker and up-titrate it to its maximum," she said. "Then later, come back to the ACE inhibitor and get the patient on the maximum dose of that."
The rationale for this approach is based on a comparison of the outcomes of the landmark beta-blocker trials versus ATLAS, a 3,104-patient trial conducted in the pre-beta-blocker era in which patients were randomized to low-dose lisinopril at 2.5-5 mg/day or high-dose therapy at 32.5-35 mg/day to determine which was better. After 4 years of follow-up, the high-dose group showed a 24% reduction in the risk of heart failure hospitalizations, but no significant advantage in terms of all-cause mortality (Circulation 1999;100:2312-8).
Contrast those results with the outcomes of the major clinical trials for carvedilol, metoprolol, and bisoprolol, each of which featured up-titration to the target dose within 8 weeks whenever possible. All three studies were halted within less than a year because of a roughly 35% reduction in mortality, compared with placebo. And that mortality benefit became apparent at 3 months.
"These are huge reductions in mortality," Dr. Lindenfeld noted. "You don’t want to have a patient come back every 4 weeks to up-titrate their ACE inhibitor for 5 months and miss the opportunity to get the patient on an effective dose of a beta-blocker, when the lifesaving benefit begins so early."
The recommended maximum doses in heart failure patients are carvedilol (Coreg) at 25 mg twice daily, or 50 mg twice daily for patients weighing more than 85 kg; 200 mg/day for extended-release metoprolol (Toprol XL); and 10 mg/day for bisoprolol (Zebeta). The three beta-blockers are similar in their efficacy for treating heart failure, Dr. Lindenfeld said. However, bisoprolol has the fewest pulmonary effects and is thus the best choice in patients with chronic obstructive pulmonary disease (COPD), even though it lacks a specific Food and Drug Administration (FDA)-approved indication for heart failure, she continued.
• Aldosterone antagonists: In terms of mortality benefit, the randomized trial data show that the aldosterone antagonists are nearly as good as beta-blockers. Yet they remain widely underutilized in the United States, according to Dr. Lindenfeld.
Indeed, three major randomized trials showed roughly a 25% reduction in total mortality, compared with placebo, in patients on standard background therapy including a beta-blocker and ACE inhibitor, along with a 20% decrease in risk of sudden cardiac death. The doses used were spironolactone at 12.5-25 mg/day or eplerenone (Inspra) at 25-50 mg/day.
An intriguing retrospective analysis conducted in close to 7,000 patients with heart failure following an acute myocardial infarction concluded that getting the aldosterone antagonist onboard early in that situation is key. Patients who started on the drug less than 7 days post MI had a 29% reduction in total mortality and a 47% decrease in sudden cardiac death, compared with those started on day 7 or later (Eur. J. Heart Fail. 2009;11:1099-1105). That benefit is believed to be the result of early left ventricular remodeling.
A definitive European prospective, randomized trial looking at the impact of starting an aldosterone antagonist within 7 days after acute MI is due to be presented later this year. The inside word is the results are highly favorable, she noted.
Hyperkalemia is a legitimate concern when prescribing an aldosterone antagonist. These agents should be avoided in a patient who has a creatinine level above 2.5 mg/dL or an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, or if other potassium-sparing drugs are onboard. Potassium levels should be checked after the first 3-7 days of therapy, again at 1 month, and then every 3 months, as well as anytime a patient becomes dehydrated.
• Digoxin: In the classic digoxin trial involving close to 7,000 patients, heart failure hospitalization was a prospectively defined endpoint. In patients with a left ventricular ejection fraction of 25%-45%, hospital admission for heart failure was reduced by 26% in patients assigned to digoxin. In those whose ejection fraction was less than 25%, the reduction in hospitalization was 39%.
"So don’t forget that digoxin is still a good drug in patients with low ejection fraction or who have substantial symptoms," Dr. Lindenfeld said. "If we had a drug approved today that didn’t change mortality but reduced hospital admissions by 39%, we’d all be giving it – and we’d be paying a lot for it."
• Role of iron deficiency in heart failure: A new European study is illuminating on this score: Among a cohort of 1,506 patients with chronic heart failure, fully 50% were determined to have iron deficiency, as defined by a ferritin level less than 100 mcg/L, or a ferritin level of 100-299 mcg/L with a transferring saturation lower than 20%. In a multivariate regression analysis, iron deficiency was a strong independent predictor for mortality, associated with a 42% increased risk (Am. Heart J. 2013;165:575-82).
"I think if you restricted the study to hospitalized heart failure patients, the iron deficiency rate would be even higher. It’s just appalling how many people we send home iron deficient without iron replacement therapy," Dr. Lindenfeld asserted.
She noted that in the European FAIR-HF trial involving 459 hospitalized iron-deficient heart failure patients randomized at discharge to intravenous iron corrective and maintenance therapy or to a matching placebo, the iron replacement group demonstrated significant improvement in quality of life and exercise capacity. The benefits were seen regardless of whether a patient’s baseline hemoglobin was high or low.
In addition, the rate of the combined endpoint of first hospitalization for worsening heart failure or death was 7.5% in the iron recipients, compared with 13.9% in placebo-treated controls – a difference that didn’t achieve statistical significance because the study was underpowered to evaluated that endpoint (N. Engl. J. Med. 2009;36:2436-48).
"Iron replacement is a distinct advantage for these patients, so you should be looking for iron deficiency. You probably don’t need to use IV iron, but if your patient is in the hospital anyway, IV iron is pretty benign and will get him iron-repleted almost immediately," Dr. Lindenfeld continued.
Before sending iron-deficient patients home on oral iron, make sure they can absorb it. Many older individuals can’t. Indeed, among patients hospitalized at the University of Colorado heart failure service, only 13% can actually absorb oral iron, she explained.
A simple way to tell is to draw a serum iron level, give the patient an iron tablet, and check the serum iron level again in 1-3 hours. It should roughly double, Dr. Lindenfeld explained.
Dr. Lindenfeld reported serving as a consultant for Medtronic, St. Jude, and other companies.
*Correction, 8/12/2013: An earlier version of this story misstated the meaning of ACE.
The five-point checklist as noted represents a reliable strategy for ensuring we apply evidence to our clinical practice. I would emphasize the following points:
•Pharmacodynamic issues such bioavailability of loop diuretics are often not well known, and the use of Bumex (bumetanide) can have a meaningful impact on those refractory to furosemide.
 |
| Dr. Hiren Shah |
•Up-titration to maximum beta-blockade prior to doing the same with ACE inhibitors is strongly supported by current evidence but not always done in clinical practice.
•Early use of aldosterone antagonists post MI is critical, but in those with chronic HF, it should still be restricted to patients with New York Heart Association (NYHA) class II HF and left ventricular ejection fraction (LVEF) less than or equal to 30% or class III to IV HF and EF less than or equal to 35%.
In addition, aldosterone antagonist therapy should be continued following hospital discharge only in patients who can be carefully monitored for hyperkalemia. Similarly, one should be aware of the occurrence of adverse drug reactions when using digoxin, owing to its narrow therapeutic index requiring serum level monitoring.
•Finally, the most overlooked aspect of HF management on this list is the importance of correcting iron-deficiency anemia, which occurs mainly through chronic renal insufficiency in HF patients.
We also now know that the anemia itself can worsen cardiac function, both because it causes cardiac stress through tachycardia and increased stroke volume, and because it can cause a reduced renal blood flow and fluid retention, adding further stress to the heart. Therefore, it is critical to correct the iron deficiency to break the vicious circle wherein CHF causes anemia, and the anemia causes more CHF, and both damage the kidneys, worsening the anemia and the CHF further.
We should all aim for the preferred strategy of intravenous iron for our hospitalized patients and then convert to oral iron, noting the absorption issues that should be evaluated prior to discharge. Of course, any new iron-deficiency anemia should still be worked up considering other causes such as GI losses.
Dr. Hiren Shah is an assistant professor of medicine in the Feinberg School of Medicine, Northwestern University, Chicago, and a medical director of the Medicine and Cardiac Telemetry Hospitalist Unit at Northwestern Memorial Hospital in Chicago.
The five-point checklist as noted represents a reliable strategy for ensuring we apply evidence to our clinical practice. I would emphasize the following points:
•Pharmacodynamic issues such bioavailability of loop diuretics are often not well known, and the use of Bumex (bumetanide) can have a meaningful impact on those refractory to furosemide.
 |
| Dr. Hiren Shah |
•Up-titration to maximum beta-blockade prior to doing the same with ACE inhibitors is strongly supported by current evidence but not always done in clinical practice.
•Early use of aldosterone antagonists post MI is critical, but in those with chronic HF, it should still be restricted to patients with New York Heart Association (NYHA) class II HF and left ventricular ejection fraction (LVEF) less than or equal to 30% or class III to IV HF and EF less than or equal to 35%.
In addition, aldosterone antagonist therapy should be continued following hospital discharge only in patients who can be carefully monitored for hyperkalemia. Similarly, one should be aware of the occurrence of adverse drug reactions when using digoxin, owing to its narrow therapeutic index requiring serum level monitoring.
•Finally, the most overlooked aspect of HF management on this list is the importance of correcting iron-deficiency anemia, which occurs mainly through chronic renal insufficiency in HF patients.
We also now know that the anemia itself can worsen cardiac function, both because it causes cardiac stress through tachycardia and increased stroke volume, and because it can cause a reduced renal blood flow and fluid retention, adding further stress to the heart. Therefore, it is critical to correct the iron deficiency to break the vicious circle wherein CHF causes anemia, and the anemia causes more CHF, and both damage the kidneys, worsening the anemia and the CHF further.
We should all aim for the preferred strategy of intravenous iron for our hospitalized patients and then convert to oral iron, noting the absorption issues that should be evaluated prior to discharge. Of course, any new iron-deficiency anemia should still be worked up considering other causes such as GI losses.
Dr. Hiren Shah is an assistant professor of medicine in the Feinberg School of Medicine, Northwestern University, Chicago, and a medical director of the Medicine and Cardiac Telemetry Hospitalist Unit at Northwestern Memorial Hospital in Chicago.
The five-point checklist as noted represents a reliable strategy for ensuring we apply evidence to our clinical practice. I would emphasize the following points:
•Pharmacodynamic issues such bioavailability of loop diuretics are often not well known, and the use of Bumex (bumetanide) can have a meaningful impact on those refractory to furosemide.
 |
| Dr. Hiren Shah |
•Up-titration to maximum beta-blockade prior to doing the same with ACE inhibitors is strongly supported by current evidence but not always done in clinical practice.
•Early use of aldosterone antagonists post MI is critical, but in those with chronic HF, it should still be restricted to patients with New York Heart Association (NYHA) class II HF and left ventricular ejection fraction (LVEF) less than or equal to 30% or class III to IV HF and EF less than or equal to 35%.
In addition, aldosterone antagonist therapy should be continued following hospital discharge only in patients who can be carefully monitored for hyperkalemia. Similarly, one should be aware of the occurrence of adverse drug reactions when using digoxin, owing to its narrow therapeutic index requiring serum level monitoring.
•Finally, the most overlooked aspect of HF management on this list is the importance of correcting iron-deficiency anemia, which occurs mainly through chronic renal insufficiency in HF patients.
We also now know that the anemia itself can worsen cardiac function, both because it causes cardiac stress through tachycardia and increased stroke volume, and because it can cause a reduced renal blood flow and fluid retention, adding further stress to the heart. Therefore, it is critical to correct the iron deficiency to break the vicious circle wherein CHF causes anemia, and the anemia causes more CHF, and both damage the kidneys, worsening the anemia and the CHF further.
We should all aim for the preferred strategy of intravenous iron for our hospitalized patients and then convert to oral iron, noting the absorption issues that should be evaluated prior to discharge. Of course, any new iron-deficiency anemia should still be worked up considering other causes such as GI losses.
Dr. Hiren Shah is an assistant professor of medicine in the Feinberg School of Medicine, Northwestern University, Chicago, and a medical director of the Medicine and Cardiac Telemetry Hospitalist Unit at Northwestern Memorial Hospital in Chicago.
ESTES PARK, COLO. – Introducing five evidence-based interventions in patients with heart failure with reduced ejection fraction would dramatically cut admissions for heart failure, according to Dr. JoAnn Lindenfeld, vice president of the Heart Failure Society of America.
Here are the five interventions: recognizing when to switch from furosemide to another oral loop diuretic with far better bioavailability; up-titrating beta-blocker therapy to the maximum recommended dose as quickly as possible; adding a low-dose aldosterone antagonist to the treatment regimen; prescribing digoxin in symptomatic patients with a low ejection fraction; and identifying and treating iron deficiency, Dr. Lindenfeld said at a conference on internal medicine sponsored by the University of Colorado.
• Loop diuretics: Furosemide, everybody’s favorite low-cost loop diuretic, turns out to have an enormously variable oral bioavailability, ranging from 10% to 90% from patient to patient. It also varies substantially from day to day within the same individual. In contrast, torsemide (Demadex) and bumetanide (Bumex) have a consistently high oral bioavailability of roughly 90%. They are useful alternatives in poorly compensated heart failure patients.
"When your patient says they’re not diuresing and you’re pretty sure they’re taking their drugs, or if they’ve had more than one recent admission for heart failure and they’re having trouble with congestion and fluid retention, think about switching to bumetanide or torsemide," said Dr. Lindenfeld, professor of medicine and medical director of the heart transplant program at the university.
"In my own practice, when I have a patient admitted for acute decompensated heart failure with congestion and I don’t find another reversible cause, I will usually switch them," noted Dr. Lindenfeld, who also is codirector of the university’s Center for Women’s Health Research.
In a classic study, 234 patients hospitalized for acute decompensated heart failure were randomized at discharge to torsemide or furosemide in equivalent doses. The torsemide group subsequently had a 52% lower rate of heart failure hospitalization (Am. J. Med. 2001;111:513-21).
Bumetanide is now a pretty inexpensive drug, Dr. Lindenfeld noted. In making the switch, remember that 40 mg of furosemide is equivalent to 1 mg of bumetanide or 20 mg of torsemide.
• Beta-blocker up-titration: Beta-blocker and angiotensin-converting enzyme* (ACE) inhibitor therapy both have a class IA recommendation in heart failure. But what’s the best way to juggle the timing of dual dose increases?
"None of the guidelines says how to manage up-titration, but I strongly believe that once you have somebody on a reasonable dose of an ACE inhibitor – say, 5 mg of lisinopril or the equivalent – then you should go to the beta-blocker and up-titrate it to its maximum," she said. "Then later, come back to the ACE inhibitor and get the patient on the maximum dose of that."
The rationale for this approach is based on a comparison of the outcomes of the landmark beta-blocker trials versus ATLAS, a 3,104-patient trial conducted in the pre-beta-blocker era in which patients were randomized to low-dose lisinopril at 2.5-5 mg/day or high-dose therapy at 32.5-35 mg/day to determine which was better. After 4 years of follow-up, the high-dose group showed a 24% reduction in the risk of heart failure hospitalizations, but no significant advantage in terms of all-cause mortality (Circulation 1999;100:2312-8).
Contrast those results with the outcomes of the major clinical trials for carvedilol, metoprolol, and bisoprolol, each of which featured up-titration to the target dose within 8 weeks whenever possible. All three studies were halted within less than a year because of a roughly 35% reduction in mortality, compared with placebo. And that mortality benefit became apparent at 3 months.
"These are huge reductions in mortality," Dr. Lindenfeld noted. "You don’t want to have a patient come back every 4 weeks to up-titrate their ACE inhibitor for 5 months and miss the opportunity to get the patient on an effective dose of a beta-blocker, when the lifesaving benefit begins so early."
The recommended maximum doses in heart failure patients are carvedilol (Coreg) at 25 mg twice daily, or 50 mg twice daily for patients weighing more than 85 kg; 200 mg/day for extended-release metoprolol (Toprol XL); and 10 mg/day for bisoprolol (Zebeta). The three beta-blockers are similar in their efficacy for treating heart failure, Dr. Lindenfeld said. However, bisoprolol has the fewest pulmonary effects and is thus the best choice in patients with chronic obstructive pulmonary disease (COPD), even though it lacks a specific Food and Drug Administration (FDA)-approved indication for heart failure, she continued.
• Aldosterone antagonists: In terms of mortality benefit, the randomized trial data show that the aldosterone antagonists are nearly as good as beta-blockers. Yet they remain widely underutilized in the United States, according to Dr. Lindenfeld.
Indeed, three major randomized trials showed roughly a 25% reduction in total mortality, compared with placebo, in patients on standard background therapy including a beta-blocker and ACE inhibitor, along with a 20% decrease in risk of sudden cardiac death. The doses used were spironolactone at 12.5-25 mg/day or eplerenone (Inspra) at 25-50 mg/day.
An intriguing retrospective analysis conducted in close to 7,000 patients with heart failure following an acute myocardial infarction concluded that getting the aldosterone antagonist onboard early in that situation is key. Patients who started on the drug less than 7 days post MI had a 29% reduction in total mortality and a 47% decrease in sudden cardiac death, compared with those started on day 7 or later (Eur. J. Heart Fail. 2009;11:1099-1105). That benefit is believed to be the result of early left ventricular remodeling.
A definitive European prospective, randomized trial looking at the impact of starting an aldosterone antagonist within 7 days after acute MI is due to be presented later this year. The inside word is the results are highly favorable, she noted.
Hyperkalemia is a legitimate concern when prescribing an aldosterone antagonist. These agents should be avoided in a patient who has a creatinine level above 2.5 mg/dL or an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, or if other potassium-sparing drugs are onboard. Potassium levels should be checked after the first 3-7 days of therapy, again at 1 month, and then every 3 months, as well as anytime a patient becomes dehydrated.
• Digoxin: In the classic digoxin trial involving close to 7,000 patients, heart failure hospitalization was a prospectively defined endpoint. In patients with a left ventricular ejection fraction of 25%-45%, hospital admission for heart failure was reduced by 26% in patients assigned to digoxin. In those whose ejection fraction was less than 25%, the reduction in hospitalization was 39%.
"So don’t forget that digoxin is still a good drug in patients with low ejection fraction or who have substantial symptoms," Dr. Lindenfeld said. "If we had a drug approved today that didn’t change mortality but reduced hospital admissions by 39%, we’d all be giving it – and we’d be paying a lot for it."
• Role of iron deficiency in heart failure: A new European study is illuminating on this score: Among a cohort of 1,506 patients with chronic heart failure, fully 50% were determined to have iron deficiency, as defined by a ferritin level less than 100 mcg/L, or a ferritin level of 100-299 mcg/L with a transferring saturation lower than 20%. In a multivariate regression analysis, iron deficiency was a strong independent predictor for mortality, associated with a 42% increased risk (Am. Heart J. 2013;165:575-82).
"I think if you restricted the study to hospitalized heart failure patients, the iron deficiency rate would be even higher. It’s just appalling how many people we send home iron deficient without iron replacement therapy," Dr. Lindenfeld asserted.
She noted that in the European FAIR-HF trial involving 459 hospitalized iron-deficient heart failure patients randomized at discharge to intravenous iron corrective and maintenance therapy or to a matching placebo, the iron replacement group demonstrated significant improvement in quality of life and exercise capacity. The benefits were seen regardless of whether a patient’s baseline hemoglobin was high or low.
In addition, the rate of the combined endpoint of first hospitalization for worsening heart failure or death was 7.5% in the iron recipients, compared with 13.9% in placebo-treated controls – a difference that didn’t achieve statistical significance because the study was underpowered to evaluated that endpoint (N. Engl. J. Med. 2009;36:2436-48).
"Iron replacement is a distinct advantage for these patients, so you should be looking for iron deficiency. You probably don’t need to use IV iron, but if your patient is in the hospital anyway, IV iron is pretty benign and will get him iron-repleted almost immediately," Dr. Lindenfeld continued.
Before sending iron-deficient patients home on oral iron, make sure they can absorb it. Many older individuals can’t. Indeed, among patients hospitalized at the University of Colorado heart failure service, only 13% can actually absorb oral iron, she explained.
A simple way to tell is to draw a serum iron level, give the patient an iron tablet, and check the serum iron level again in 1-3 hours. It should roughly double, Dr. Lindenfeld explained.
Dr. Lindenfeld reported serving as a consultant for Medtronic, St. Jude, and other companies.
*Correction, 8/12/2013: An earlier version of this story misstated the meaning of ACE.
ESTES PARK, COLO. – Introducing five evidence-based interventions in patients with heart failure with reduced ejection fraction would dramatically cut admissions for heart failure, according to Dr. JoAnn Lindenfeld, vice president of the Heart Failure Society of America.
Here are the five interventions: recognizing when to switch from furosemide to another oral loop diuretic with far better bioavailability; up-titrating beta-blocker therapy to the maximum recommended dose as quickly as possible; adding a low-dose aldosterone antagonist to the treatment regimen; prescribing digoxin in symptomatic patients with a low ejection fraction; and identifying and treating iron deficiency, Dr. Lindenfeld said at a conference on internal medicine sponsored by the University of Colorado.
• Loop diuretics: Furosemide, everybody’s favorite low-cost loop diuretic, turns out to have an enormously variable oral bioavailability, ranging from 10% to 90% from patient to patient. It also varies substantially from day to day within the same individual. In contrast, torsemide (Demadex) and bumetanide (Bumex) have a consistently high oral bioavailability of roughly 90%. They are useful alternatives in poorly compensated heart failure patients.
"When your patient says they’re not diuresing and you’re pretty sure they’re taking their drugs, or if they’ve had more than one recent admission for heart failure and they’re having trouble with congestion and fluid retention, think about switching to bumetanide or torsemide," said Dr. Lindenfeld, professor of medicine and medical director of the heart transplant program at the university.
"In my own practice, when I have a patient admitted for acute decompensated heart failure with congestion and I don’t find another reversible cause, I will usually switch them," noted Dr. Lindenfeld, who also is codirector of the university’s Center for Women’s Health Research.
In a classic study, 234 patients hospitalized for acute decompensated heart failure were randomized at discharge to torsemide or furosemide in equivalent doses. The torsemide group subsequently had a 52% lower rate of heart failure hospitalization (Am. J. Med. 2001;111:513-21).
Bumetanide is now a pretty inexpensive drug, Dr. Lindenfeld noted. In making the switch, remember that 40 mg of furosemide is equivalent to 1 mg of bumetanide or 20 mg of torsemide.
• Beta-blocker up-titration: Beta-blocker and angiotensin-converting enzyme* (ACE) inhibitor therapy both have a class IA recommendation in heart failure. But what’s the best way to juggle the timing of dual dose increases?
"None of the guidelines says how to manage up-titration, but I strongly believe that once you have somebody on a reasonable dose of an ACE inhibitor – say, 5 mg of lisinopril or the equivalent – then you should go to the beta-blocker and up-titrate it to its maximum," she said. "Then later, come back to the ACE inhibitor and get the patient on the maximum dose of that."
The rationale for this approach is based on a comparison of the outcomes of the landmark beta-blocker trials versus ATLAS, a 3,104-patient trial conducted in the pre-beta-blocker era in which patients were randomized to low-dose lisinopril at 2.5-5 mg/day or high-dose therapy at 32.5-35 mg/day to determine which was better. After 4 years of follow-up, the high-dose group showed a 24% reduction in the risk of heart failure hospitalizations, but no significant advantage in terms of all-cause mortality (Circulation 1999;100:2312-8).
Contrast those results with the outcomes of the major clinical trials for carvedilol, metoprolol, and bisoprolol, each of which featured up-titration to the target dose within 8 weeks whenever possible. All three studies were halted within less than a year because of a roughly 35% reduction in mortality, compared with placebo. And that mortality benefit became apparent at 3 months.
"These are huge reductions in mortality," Dr. Lindenfeld noted. "You don’t want to have a patient come back every 4 weeks to up-titrate their ACE inhibitor for 5 months and miss the opportunity to get the patient on an effective dose of a beta-blocker, when the lifesaving benefit begins so early."
The recommended maximum doses in heart failure patients are carvedilol (Coreg) at 25 mg twice daily, or 50 mg twice daily for patients weighing more than 85 kg; 200 mg/day for extended-release metoprolol (Toprol XL); and 10 mg/day for bisoprolol (Zebeta). The three beta-blockers are similar in their efficacy for treating heart failure, Dr. Lindenfeld said. However, bisoprolol has the fewest pulmonary effects and is thus the best choice in patients with chronic obstructive pulmonary disease (COPD), even though it lacks a specific Food and Drug Administration (FDA)-approved indication for heart failure, she continued.
• Aldosterone antagonists: In terms of mortality benefit, the randomized trial data show that the aldosterone antagonists are nearly as good as beta-blockers. Yet they remain widely underutilized in the United States, according to Dr. Lindenfeld.
Indeed, three major randomized trials showed roughly a 25% reduction in total mortality, compared with placebo, in patients on standard background therapy including a beta-blocker and ACE inhibitor, along with a 20% decrease in risk of sudden cardiac death. The doses used were spironolactone at 12.5-25 mg/day or eplerenone (Inspra) at 25-50 mg/day.
An intriguing retrospective analysis conducted in close to 7,000 patients with heart failure following an acute myocardial infarction concluded that getting the aldosterone antagonist onboard early in that situation is key. Patients who started on the drug less than 7 days post MI had a 29% reduction in total mortality and a 47% decrease in sudden cardiac death, compared with those started on day 7 or later (Eur. J. Heart Fail. 2009;11:1099-1105). That benefit is believed to be the result of early left ventricular remodeling.
A definitive European prospective, randomized trial looking at the impact of starting an aldosterone antagonist within 7 days after acute MI is due to be presented later this year. The inside word is the results are highly favorable, she noted.
Hyperkalemia is a legitimate concern when prescribing an aldosterone antagonist. These agents should be avoided in a patient who has a creatinine level above 2.5 mg/dL or an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, or if other potassium-sparing drugs are onboard. Potassium levels should be checked after the first 3-7 days of therapy, again at 1 month, and then every 3 months, as well as anytime a patient becomes dehydrated.
• Digoxin: In the classic digoxin trial involving close to 7,000 patients, heart failure hospitalization was a prospectively defined endpoint. In patients with a left ventricular ejection fraction of 25%-45%, hospital admission for heart failure was reduced by 26% in patients assigned to digoxin. In those whose ejection fraction was less than 25%, the reduction in hospitalization was 39%.
"So don’t forget that digoxin is still a good drug in patients with low ejection fraction or who have substantial symptoms," Dr. Lindenfeld said. "If we had a drug approved today that didn’t change mortality but reduced hospital admissions by 39%, we’d all be giving it – and we’d be paying a lot for it."
• Role of iron deficiency in heart failure: A new European study is illuminating on this score: Among a cohort of 1,506 patients with chronic heart failure, fully 50% were determined to have iron deficiency, as defined by a ferritin level less than 100 mcg/L, or a ferritin level of 100-299 mcg/L with a transferring saturation lower than 20%. In a multivariate regression analysis, iron deficiency was a strong independent predictor for mortality, associated with a 42% increased risk (Am. Heart J. 2013;165:575-82).
"I think if you restricted the study to hospitalized heart failure patients, the iron deficiency rate would be even higher. It’s just appalling how many people we send home iron deficient without iron replacement therapy," Dr. Lindenfeld asserted.
She noted that in the European FAIR-HF trial involving 459 hospitalized iron-deficient heart failure patients randomized at discharge to intravenous iron corrective and maintenance therapy or to a matching placebo, the iron replacement group demonstrated significant improvement in quality of life and exercise capacity. The benefits were seen regardless of whether a patient’s baseline hemoglobin was high or low.
In addition, the rate of the combined endpoint of first hospitalization for worsening heart failure or death was 7.5% in the iron recipients, compared with 13.9% in placebo-treated controls – a difference that didn’t achieve statistical significance because the study was underpowered to evaluated that endpoint (N. Engl. J. Med. 2009;36:2436-48).
"Iron replacement is a distinct advantage for these patients, so you should be looking for iron deficiency. You probably don’t need to use IV iron, but if your patient is in the hospital anyway, IV iron is pretty benign and will get him iron-repleted almost immediately," Dr. Lindenfeld continued.
Before sending iron-deficient patients home on oral iron, make sure they can absorb it. Many older individuals can’t. Indeed, among patients hospitalized at the University of Colorado heart failure service, only 13% can actually absorb oral iron, she explained.
A simple way to tell is to draw a serum iron level, give the patient an iron tablet, and check the serum iron level again in 1-3 hours. It should roughly double, Dr. Lindenfeld explained.
Dr. Lindenfeld reported serving as a consultant for Medtronic, St. Jude, and other companies.
*Correction, 8/12/2013: An earlier version of this story misstated the meaning of ACE.
EXPERT ANALYSIS FROM THE ANNUAL INTERNAL MEDICINE PROGRAM
Guidelines issued on radiation-induced heart disease
Cancer patients undergoing radiation therapy need to have baseline studies of cardiac function and routine screening for heart disease, according to recommendations from the European Society of Cardiology and the American Society of Echocardiography published July 16 in the European Heart Journal–Cardiovascular Imaging.
The groups recommend baseline preradiation echocardiography along with a cardiac exam as well as screening for risk factors. An annual cardiac history and physical should be performed to check for new-onset heart problems.
Within 10 years of treatment, 10%-30% of patients who undergo radiation therapy develop radiation-induced heart diseases (RIHD), including chronic pericarditis, myocardial fibrosis, coronary artery disease, aortic calcification, and valve regurgitation or stenosis. The hope of screening is to catch early RIHD, but screening is not currently routine.
"We wrote the expert consensus to raise the alarm that the risks of radiation-induced heart disease should not be ignored. The prevalence ... is increasing because the rate of cancer survival has improved," said Dr. Patrizio Lancellotti, who is a professor of cardiology at the University Hospital of Liège, Belgium, and led the recommendations task force.
Radiotherapy is given in more targeted form and at lower doses than it once was, but "patients are still at increased risk of RIHD, particularly when the heart is in the radiation field. This applies to patients treated for lymphoma, breast cancer, and esophageal cancer. Patients who receive radiotherapy for neck cancer are also at risk because lesions can develop on the carotid artery and increase the risk of stroke," Dr. Lancellotti said in a statement.
Using targeted radiation and alternate radiation fields, with avoidance and shielding of the heart, remain "the most important interventions to prevent" cardiac complications, the authors noted.
The task force advises that high-risk patients without evidence of heart disease on history and physical should have screening echocardiography every 5 years and noninvasive stress testing every 5-10 years; low-risk patients should have screening echocardiography every 10 years. If heart disorders are detected, routine monitoring should include echocardiography, cardiac magnetic resonance imaging, or carotid ultrasound as appropriate.
High-risk patients include those who received radiotherapy at younger ages; those who have cardiovascular risk factors or preexisting heart disease; and those who receive high-dose radiation (greater than 30 Gy), concomitant chemotherapy, radiation without shielding, or anterior or left chest radiation (Eur. Heart J. Cardiovasc. Imaging 2013;14:721-40).
The recommendations are based on an extensive literature review and analysis by Dr. Lancellotti and other specialists.
The authors reported no financial conflicts or outside funding for their work.
Cancer patients undergoing radiation therapy need to have baseline studies of cardiac function and routine screening for heart disease, according to recommendations from the European Society of Cardiology and the American Society of Echocardiography published July 16 in the European Heart Journal–Cardiovascular Imaging.
The groups recommend baseline preradiation echocardiography along with a cardiac exam as well as screening for risk factors. An annual cardiac history and physical should be performed to check for new-onset heart problems.
Within 10 years of treatment, 10%-30% of patients who undergo radiation therapy develop radiation-induced heart diseases (RIHD), including chronic pericarditis, myocardial fibrosis, coronary artery disease, aortic calcification, and valve regurgitation or stenosis. The hope of screening is to catch early RIHD, but screening is not currently routine.
"We wrote the expert consensus to raise the alarm that the risks of radiation-induced heart disease should not be ignored. The prevalence ... is increasing because the rate of cancer survival has improved," said Dr. Patrizio Lancellotti, who is a professor of cardiology at the University Hospital of Liège, Belgium, and led the recommendations task force.
Radiotherapy is given in more targeted form and at lower doses than it once was, but "patients are still at increased risk of RIHD, particularly when the heart is in the radiation field. This applies to patients treated for lymphoma, breast cancer, and esophageal cancer. Patients who receive radiotherapy for neck cancer are also at risk because lesions can develop on the carotid artery and increase the risk of stroke," Dr. Lancellotti said in a statement.
Using targeted radiation and alternate radiation fields, with avoidance and shielding of the heart, remain "the most important interventions to prevent" cardiac complications, the authors noted.
The task force advises that high-risk patients without evidence of heart disease on history and physical should have screening echocardiography every 5 years and noninvasive stress testing every 5-10 years; low-risk patients should have screening echocardiography every 10 years. If heart disorders are detected, routine monitoring should include echocardiography, cardiac magnetic resonance imaging, or carotid ultrasound as appropriate.
High-risk patients include those who received radiotherapy at younger ages; those who have cardiovascular risk factors or preexisting heart disease; and those who receive high-dose radiation (greater than 30 Gy), concomitant chemotherapy, radiation without shielding, or anterior or left chest radiation (Eur. Heart J. Cardiovasc. Imaging 2013;14:721-40).
The recommendations are based on an extensive literature review and analysis by Dr. Lancellotti and other specialists.
The authors reported no financial conflicts or outside funding for their work.
Cancer patients undergoing radiation therapy need to have baseline studies of cardiac function and routine screening for heart disease, according to recommendations from the European Society of Cardiology and the American Society of Echocardiography published July 16 in the European Heart Journal–Cardiovascular Imaging.
The groups recommend baseline preradiation echocardiography along with a cardiac exam as well as screening for risk factors. An annual cardiac history and physical should be performed to check for new-onset heart problems.
Within 10 years of treatment, 10%-30% of patients who undergo radiation therapy develop radiation-induced heart diseases (RIHD), including chronic pericarditis, myocardial fibrosis, coronary artery disease, aortic calcification, and valve regurgitation or stenosis. The hope of screening is to catch early RIHD, but screening is not currently routine.
"We wrote the expert consensus to raise the alarm that the risks of radiation-induced heart disease should not be ignored. The prevalence ... is increasing because the rate of cancer survival has improved," said Dr. Patrizio Lancellotti, who is a professor of cardiology at the University Hospital of Liège, Belgium, and led the recommendations task force.
Radiotherapy is given in more targeted form and at lower doses than it once was, but "patients are still at increased risk of RIHD, particularly when the heart is in the radiation field. This applies to patients treated for lymphoma, breast cancer, and esophageal cancer. Patients who receive radiotherapy for neck cancer are also at risk because lesions can develop on the carotid artery and increase the risk of stroke," Dr. Lancellotti said in a statement.
Using targeted radiation and alternate radiation fields, with avoidance and shielding of the heart, remain "the most important interventions to prevent" cardiac complications, the authors noted.
The task force advises that high-risk patients without evidence of heart disease on history and physical should have screening echocardiography every 5 years and noninvasive stress testing every 5-10 years; low-risk patients should have screening echocardiography every 10 years. If heart disorders are detected, routine monitoring should include echocardiography, cardiac magnetic resonance imaging, or carotid ultrasound as appropriate.
High-risk patients include those who received radiotherapy at younger ages; those who have cardiovascular risk factors or preexisting heart disease; and those who receive high-dose radiation (greater than 30 Gy), concomitant chemotherapy, radiation without shielding, or anterior or left chest radiation (Eur. Heart J. Cardiovasc. Imaging 2013;14:721-40).
The recommendations are based on an extensive literature review and analysis by Dr. Lancellotti and other specialists.
The authors reported no financial conflicts or outside funding for their work.
FROM THE EUROPEAN HEART JOURNAL – CARDIOVASCULAR IMAGING
Novel drug improved walk distance in pulmonary hypertension patients
Riociguat, an agent from a new class of compounds known as soluble guanylate cyclase stimulators, was found effective and safe for treating two different types of pulmonary hypertension in separate industry-sponsored phase III clinical trials reported online July 25 in the New England Journal of Medicine.
Compared with placebo, daily oral riociguat significantly improved exercise capacity as measured by 6-minute walk distance, and also improved pulmonary vascular resistance and World Health Organization (WHO) functional class. The results are from one international study involving 261 patients who had chronic inoperable thromboembolic pulmonary hypertension and another study involving 443 patients who had pulmonary arterial hypertension.
The magnitude of the improvement was greater than that reported for existing medications such as endothelin-receptor antagonists and prostanoids, both groups of researchers said.
The pathogenesis of pulmonary hypertension involves impairment of both nitric oxide synthesis and signaling through the nitric oxide–soluble guanylate cyclase–cyclic guanosine monophosphate pathway. Riociguat directly stimulates soluble guanylate cyclase independently of nitric oxide and also increases the enzyme’s sensitivity to nitric oxide. The drug also raises levels of cyclic guanosine monophosphate, which induces vasorelaxation and has additional antiproliferative and antifibrotic effects.
The CHEST-1 trial
In the CHEST-1 (Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase-Stimulator) trial, funded by Bayer HealthCare, the drug was assessed in adults with chronic thromboembolic pulmonary hypertension who were either ineligible for the standard surgical treatment (pulmonary endarterectomy) or whose condition persisted or recurred after they underwent the surgery. It is estimated that 63% of patients with this disorder are ineligible for endarterectomy, which is the only potentially curative treatment available, and that thromboembolic pulmonary hypertension persists or recurs in 35% of patients who do have the procedure. So an alternative approach is clearly needed, said Dr. Hossein-Ardeschir Ghofrani of University Hospital Giessen and Marburg, Germany, and his associates.
The CHEST-1 study subjects, who were treated and followed at 89 medical centers in 26 countries, were randomly assigned to receive either riociguat (173 patients) or matching placebo (88 patients) for 16 weeks.
The primary endpoint was change in 6-minute walk distance. In the intention-to-treat analysis, this increased by a mean of 39 m in patients taking riociguat, compared with a decrease of 6 m in those taking placebo. The benefit was similar in a per-protocol analysis, the investigators reported (N. Engl. J. Med. 2013 July 25 [doi: 10.1056/NEJMoa1209657]).
Riociguat improved 6-minute walk distance by 54 m in the patients who were ineligible for surgery and by 26 m in those who had persistent or recurrent disease after surgery. The drug’s beneficial effect on exercise capacity was consistent across several subgroups of patients.
In addition, the active drug significantly reduced pulmonary vascular resistance; improved other hemodynamic factors, including mean pulmonary artery pressure and cardiac output; improved WHO functional class, which is known to correlate with survival; and decreased levels of N-terminal pro-brain natriuretic peptide (NT-proBNP).
In exploratory analyses, riociguat also improved scores on the Borg dyspnea index when compared with placebo, and nominally improved scores on one measure of quality of life but not on a disease-specific QOL tool.
Drug-related serious adverse effects included three cases of syncope and one case each of gastritis, acute renal failure, and hypotension with riociguat, and one case each of syncope and trauma with placebo. Three percent of the riociguat group and 2% of the placebo group dropped out of the study owing to adverse events, and another 2% of each group dropped out because of serious adverse events that were not considered to be related to the study drug.
There were two deaths in the riociguat group and three in the placebo group.
A total of 237 of these study subjects elected to enroll in an extended study of the long-term safety and efficacy of riociguat (the CHEST-2 clinical trial). An exploratory analysis of data from the first 12 weeks of that study showed further increases of 51-63 m in the 6-minute walk distance, Dr. Ghofrani and his associates said.
The PATENT-1 trial
The other phase III clinical trial reported in the New England Journal of Medicine was the PATENT-1 (Pulmonary Arterial Hypertension Soluble Guanylate Cyclase-Stimulator) study. This trial also was funded by Bayer HealthCare and headed by Dr. Ghofrani.
In it, 443 patients who had symptomatic pulmonary arterial hypertension were treated and followed up at 124 medical centers in 30 countries. These subjects had idiopathic or familial disease, or pulmonary arterial hypertension associated with connective-tissue disease, congenital heart disease, portal hypertension with liver cirrhosis, anorexigen use, or amphetamine use.
A total of 44% of patients were already receiving an endothelin-receptor antagonist (usually bosentan), and 6% were taking prostanoids (usually inhaled iloprost) for the disorder, and the other half of the study population was not receiving any other treatments. Concomitant therapy with oral anticoagulants, diuretics, and supplemental oxygen was permitted during the study.
These study subjects were randomly assigned to receive high-dose riociguat capped at 2.5 mg three times daily (254 patients), low-dose riociguat capped at 1.5 mg three times daily (63 patients), or placebo (126 patients) for 12 weeks. The analysis of data from the low-dose group was considered exploratory and was reported separately.
The primary endpoint – change from baseline in 6-minute walk distance – increased by a mean of 30 m in the high-dose group but decreased by a mean of 6 m in the placebo group in the intention-to-treat analysis. The results were similar in the per-protocol analysis, the investigators reported (N. Engl. J. Med. 2013 July 25 [doi: 10.1056/NEJMoa1209655]).
This treatment benefit was consistent across several subgroups of patients. In addition, the active drug significantly decreased pulmonary vascular resistance, improved mean pulmonary artery pressure and cardiac output, lengthened the interval to clinical worsening, improved NT-proBNP levels, improved WHO functional class, and improved scores on the Borg dyspnea scale.
In exploratory analyses, riociguat did not improve scores on one measure of QOL but did so on a disease-specific QOL tool.
Drug-related serious adverse events occurred in 1.4 % of patients receiving high-dose riociguat and 1% of those receiving placebo. A total of 3% of patients in the riociguat group and 7% of those in the placebo group discontinued the study drug because of adverse events.
Serious or drug-related adverse events in both groups included increased hepatic enzyme levels, acute renal failure, syncope, esophageal pain and swelling, supraventricular tachycardia, hypotension, generalized edema, hypoxemia, dyspnea, and worsening of pulmonary hypertension. There were two deaths in the riociguat group and three in the placebo group, none of which were considered to be related to the study drug.
A total of 396 of the subjects in the PATENT-1 study elected to enroll in an extended study of the long-term safety and efficacy of riociguat (the PATENT-2 clinical trial). An exploratory analysis of data from the first 12 weeks of that study showed a further mean increase of 53 m in 6-minute walk distance with high-dose riociguat.
CHEST-1 and PATENT-1 were funded by Bayer HealthCare. Dr. Ghofrani reported ties to Actelion, Bayer, and other companies. His associates reported ties to numerous industry sources.
Riociguat "is poised for examination by the Food and Drug Administration as a therapy for pulmonary hypertension and, if approved, has the potential to generate substantial revenue" for the sponsor of these two clinical trials, said Dr. Stephen L. Archer.
Both CHEST-1 and PATENT-1 were sponsored by Bayer HealthCare, which also provided the statistician and editorial assistance for both trials. "In light of the financial stakes, both real and apparent investigator autonomy remain key to ensuring the delivery of new drugs for pulmonary hypertension for patients," he noted.
Although riociguat yielded only "modest" gains in exercise capacity, it looks promising and may prove to be the first effective oral therapy for inoperable Group 4 pulmonary hypertension, Dr. Archer added.
However, he noted limitations in both studies. The CHEST-1 trial was limited by its "failure to examine the effects of the study drug on the right ventricle. The 6-minute walk distance is as reflective of right ventricular or skeletal-muscle function as it is of reduction in pulmonary vascular resistance, the authors’ presumed mechanism of benefit."
PATENT-1 was limited by its modest effect size, he added. "This is particularly important, since 50% of the patients were receiving no other treatment for pulmonary arterial hypertension, and the rate of response to treatment among such patients is usually higher than the rate among patients who are receiving concomitant treatment for pulmonary arterial hypertension," Dr. Archer said.
Dr. Archer is in the department of medicine at Queen’s University, Kingston, Ont. He reported no financial conflicts of interest. These remarks were taken from his editorial accompanying Dr. Ghofrani’s reports (N. Engl. J. Med. 2013 July 25 [doi: 10.1056/NEJMe1306684]).
Riociguat "is poised for examination by the Food and Drug Administration as a therapy for pulmonary hypertension and, if approved, has the potential to generate substantial revenue" for the sponsor of these two clinical trials, said Dr. Stephen L. Archer.
Both CHEST-1 and PATENT-1 were sponsored by Bayer HealthCare, which also provided the statistician and editorial assistance for both trials. "In light of the financial stakes, both real and apparent investigator autonomy remain key to ensuring the delivery of new drugs for pulmonary hypertension for patients," he noted.
Although riociguat yielded only "modest" gains in exercise capacity, it looks promising and may prove to be the first effective oral therapy for inoperable Group 4 pulmonary hypertension, Dr. Archer added.
However, he noted limitations in both studies. The CHEST-1 trial was limited by its "failure to examine the effects of the study drug on the right ventricle. The 6-minute walk distance is as reflective of right ventricular or skeletal-muscle function as it is of reduction in pulmonary vascular resistance, the authors’ presumed mechanism of benefit."
PATENT-1 was limited by its modest effect size, he added. "This is particularly important, since 50% of the patients were receiving no other treatment for pulmonary arterial hypertension, and the rate of response to treatment among such patients is usually higher than the rate among patients who are receiving concomitant treatment for pulmonary arterial hypertension," Dr. Archer said.
Dr. Archer is in the department of medicine at Queen’s University, Kingston, Ont. He reported no financial conflicts of interest. These remarks were taken from his editorial accompanying Dr. Ghofrani’s reports (N. Engl. J. Med. 2013 July 25 [doi: 10.1056/NEJMe1306684]).
Riociguat "is poised for examination by the Food and Drug Administration as a therapy for pulmonary hypertension and, if approved, has the potential to generate substantial revenue" for the sponsor of these two clinical trials, said Dr. Stephen L. Archer.
Both CHEST-1 and PATENT-1 were sponsored by Bayer HealthCare, which also provided the statistician and editorial assistance for both trials. "In light of the financial stakes, both real and apparent investigator autonomy remain key to ensuring the delivery of new drugs for pulmonary hypertension for patients," he noted.
Although riociguat yielded only "modest" gains in exercise capacity, it looks promising and may prove to be the first effective oral therapy for inoperable Group 4 pulmonary hypertension, Dr. Archer added.
However, he noted limitations in both studies. The CHEST-1 trial was limited by its "failure to examine the effects of the study drug on the right ventricle. The 6-minute walk distance is as reflective of right ventricular or skeletal-muscle function as it is of reduction in pulmonary vascular resistance, the authors’ presumed mechanism of benefit."
PATENT-1 was limited by its modest effect size, he added. "This is particularly important, since 50% of the patients were receiving no other treatment for pulmonary arterial hypertension, and the rate of response to treatment among such patients is usually higher than the rate among patients who are receiving concomitant treatment for pulmonary arterial hypertension," Dr. Archer said.
Dr. Archer is in the department of medicine at Queen’s University, Kingston, Ont. He reported no financial conflicts of interest. These remarks were taken from his editorial accompanying Dr. Ghofrani’s reports (N. Engl. J. Med. 2013 July 25 [doi: 10.1056/NEJMe1306684]).
Riociguat, an agent from a new class of compounds known as soluble guanylate cyclase stimulators, was found effective and safe for treating two different types of pulmonary hypertension in separate industry-sponsored phase III clinical trials reported online July 25 in the New England Journal of Medicine.
Compared with placebo, daily oral riociguat significantly improved exercise capacity as measured by 6-minute walk distance, and also improved pulmonary vascular resistance and World Health Organization (WHO) functional class. The results are from one international study involving 261 patients who had chronic inoperable thromboembolic pulmonary hypertension and another study involving 443 patients who had pulmonary arterial hypertension.
The magnitude of the improvement was greater than that reported for existing medications such as endothelin-receptor antagonists and prostanoids, both groups of researchers said.
The pathogenesis of pulmonary hypertension involves impairment of both nitric oxide synthesis and signaling through the nitric oxide–soluble guanylate cyclase–cyclic guanosine monophosphate pathway. Riociguat directly stimulates soluble guanylate cyclase independently of nitric oxide and also increases the enzyme’s sensitivity to nitric oxide. The drug also raises levels of cyclic guanosine monophosphate, which induces vasorelaxation and has additional antiproliferative and antifibrotic effects.
The CHEST-1 trial
In the CHEST-1 (Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase-Stimulator) trial, funded by Bayer HealthCare, the drug was assessed in adults with chronic thromboembolic pulmonary hypertension who were either ineligible for the standard surgical treatment (pulmonary endarterectomy) or whose condition persisted or recurred after they underwent the surgery. It is estimated that 63% of patients with this disorder are ineligible for endarterectomy, which is the only potentially curative treatment available, and that thromboembolic pulmonary hypertension persists or recurs in 35% of patients who do have the procedure. So an alternative approach is clearly needed, said Dr. Hossein-Ardeschir Ghofrani of University Hospital Giessen and Marburg, Germany, and his associates.
The CHEST-1 study subjects, who were treated and followed at 89 medical centers in 26 countries, were randomly assigned to receive either riociguat (173 patients) or matching placebo (88 patients) for 16 weeks.
The primary endpoint was change in 6-minute walk distance. In the intention-to-treat analysis, this increased by a mean of 39 m in patients taking riociguat, compared with a decrease of 6 m in those taking placebo. The benefit was similar in a per-protocol analysis, the investigators reported (N. Engl. J. Med. 2013 July 25 [doi: 10.1056/NEJMoa1209657]).
Riociguat improved 6-minute walk distance by 54 m in the patients who were ineligible for surgery and by 26 m in those who had persistent or recurrent disease after surgery. The drug’s beneficial effect on exercise capacity was consistent across several subgroups of patients.
In addition, the active drug significantly reduced pulmonary vascular resistance; improved other hemodynamic factors, including mean pulmonary artery pressure and cardiac output; improved WHO functional class, which is known to correlate with survival; and decreased levels of N-terminal pro-brain natriuretic peptide (NT-proBNP).
In exploratory analyses, riociguat also improved scores on the Borg dyspnea index when compared with placebo, and nominally improved scores on one measure of quality of life but not on a disease-specific QOL tool.
Drug-related serious adverse effects included three cases of syncope and one case each of gastritis, acute renal failure, and hypotension with riociguat, and one case each of syncope and trauma with placebo. Three percent of the riociguat group and 2% of the placebo group dropped out of the study owing to adverse events, and another 2% of each group dropped out because of serious adverse events that were not considered to be related to the study drug.
There were two deaths in the riociguat group and three in the placebo group.
A total of 237 of these study subjects elected to enroll in an extended study of the long-term safety and efficacy of riociguat (the CHEST-2 clinical trial). An exploratory analysis of data from the first 12 weeks of that study showed further increases of 51-63 m in the 6-minute walk distance, Dr. Ghofrani and his associates said.
The PATENT-1 trial
The other phase III clinical trial reported in the New England Journal of Medicine was the PATENT-1 (Pulmonary Arterial Hypertension Soluble Guanylate Cyclase-Stimulator) study. This trial also was funded by Bayer HealthCare and headed by Dr. Ghofrani.
In it, 443 patients who had symptomatic pulmonary arterial hypertension were treated and followed up at 124 medical centers in 30 countries. These subjects had idiopathic or familial disease, or pulmonary arterial hypertension associated with connective-tissue disease, congenital heart disease, portal hypertension with liver cirrhosis, anorexigen use, or amphetamine use.
A total of 44% of patients were already receiving an endothelin-receptor antagonist (usually bosentan), and 6% were taking prostanoids (usually inhaled iloprost) for the disorder, and the other half of the study population was not receiving any other treatments. Concomitant therapy with oral anticoagulants, diuretics, and supplemental oxygen was permitted during the study.
These study subjects were randomly assigned to receive high-dose riociguat capped at 2.5 mg three times daily (254 patients), low-dose riociguat capped at 1.5 mg three times daily (63 patients), or placebo (126 patients) for 12 weeks. The analysis of data from the low-dose group was considered exploratory and was reported separately.
The primary endpoint – change from baseline in 6-minute walk distance – increased by a mean of 30 m in the high-dose group but decreased by a mean of 6 m in the placebo group in the intention-to-treat analysis. The results were similar in the per-protocol analysis, the investigators reported (N. Engl. J. Med. 2013 July 25 [doi: 10.1056/NEJMoa1209655]).
This treatment benefit was consistent across several subgroups of patients. In addition, the active drug significantly decreased pulmonary vascular resistance, improved mean pulmonary artery pressure and cardiac output, lengthened the interval to clinical worsening, improved NT-proBNP levels, improved WHO functional class, and improved scores on the Borg dyspnea scale.
In exploratory analyses, riociguat did not improve scores on one measure of QOL but did so on a disease-specific QOL tool.
Drug-related serious adverse events occurred in 1.4 % of patients receiving high-dose riociguat and 1% of those receiving placebo. A total of 3% of patients in the riociguat group and 7% of those in the placebo group discontinued the study drug because of adverse events.
Serious or drug-related adverse events in both groups included increased hepatic enzyme levels, acute renal failure, syncope, esophageal pain and swelling, supraventricular tachycardia, hypotension, generalized edema, hypoxemia, dyspnea, and worsening of pulmonary hypertension. There were two deaths in the riociguat group and three in the placebo group, none of which were considered to be related to the study drug.
A total of 396 of the subjects in the PATENT-1 study elected to enroll in an extended study of the long-term safety and efficacy of riociguat (the PATENT-2 clinical trial). An exploratory analysis of data from the first 12 weeks of that study showed a further mean increase of 53 m in 6-minute walk distance with high-dose riociguat.
CHEST-1 and PATENT-1 were funded by Bayer HealthCare. Dr. Ghofrani reported ties to Actelion, Bayer, and other companies. His associates reported ties to numerous industry sources.
Riociguat, an agent from a new class of compounds known as soluble guanylate cyclase stimulators, was found effective and safe for treating two different types of pulmonary hypertension in separate industry-sponsored phase III clinical trials reported online July 25 in the New England Journal of Medicine.
Compared with placebo, daily oral riociguat significantly improved exercise capacity as measured by 6-minute walk distance, and also improved pulmonary vascular resistance and World Health Organization (WHO) functional class. The results are from one international study involving 261 patients who had chronic inoperable thromboembolic pulmonary hypertension and another study involving 443 patients who had pulmonary arterial hypertension.
The magnitude of the improvement was greater than that reported for existing medications such as endothelin-receptor antagonists and prostanoids, both groups of researchers said.
The pathogenesis of pulmonary hypertension involves impairment of both nitric oxide synthesis and signaling through the nitric oxide–soluble guanylate cyclase–cyclic guanosine monophosphate pathway. Riociguat directly stimulates soluble guanylate cyclase independently of nitric oxide and also increases the enzyme’s sensitivity to nitric oxide. The drug also raises levels of cyclic guanosine monophosphate, which induces vasorelaxation and has additional antiproliferative and antifibrotic effects.
The CHEST-1 trial
In the CHEST-1 (Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase-Stimulator) trial, funded by Bayer HealthCare, the drug was assessed in adults with chronic thromboembolic pulmonary hypertension who were either ineligible for the standard surgical treatment (pulmonary endarterectomy) or whose condition persisted or recurred after they underwent the surgery. It is estimated that 63% of patients with this disorder are ineligible for endarterectomy, which is the only potentially curative treatment available, and that thromboembolic pulmonary hypertension persists or recurs in 35% of patients who do have the procedure. So an alternative approach is clearly needed, said Dr. Hossein-Ardeschir Ghofrani of University Hospital Giessen and Marburg, Germany, and his associates.
The CHEST-1 study subjects, who were treated and followed at 89 medical centers in 26 countries, were randomly assigned to receive either riociguat (173 patients) or matching placebo (88 patients) for 16 weeks.
The primary endpoint was change in 6-minute walk distance. In the intention-to-treat analysis, this increased by a mean of 39 m in patients taking riociguat, compared with a decrease of 6 m in those taking placebo. The benefit was similar in a per-protocol analysis, the investigators reported (N. Engl. J. Med. 2013 July 25 [doi: 10.1056/NEJMoa1209657]).
Riociguat improved 6-minute walk distance by 54 m in the patients who were ineligible for surgery and by 26 m in those who had persistent or recurrent disease after surgery. The drug’s beneficial effect on exercise capacity was consistent across several subgroups of patients.
In addition, the active drug significantly reduced pulmonary vascular resistance; improved other hemodynamic factors, including mean pulmonary artery pressure and cardiac output; improved WHO functional class, which is known to correlate with survival; and decreased levels of N-terminal pro-brain natriuretic peptide (NT-proBNP).
In exploratory analyses, riociguat also improved scores on the Borg dyspnea index when compared with placebo, and nominally improved scores on one measure of quality of life but not on a disease-specific QOL tool.
Drug-related serious adverse effects included three cases of syncope and one case each of gastritis, acute renal failure, and hypotension with riociguat, and one case each of syncope and trauma with placebo. Three percent of the riociguat group and 2% of the placebo group dropped out of the study owing to adverse events, and another 2% of each group dropped out because of serious adverse events that were not considered to be related to the study drug.
There were two deaths in the riociguat group and three in the placebo group.
A total of 237 of these study subjects elected to enroll in an extended study of the long-term safety and efficacy of riociguat (the CHEST-2 clinical trial). An exploratory analysis of data from the first 12 weeks of that study showed further increases of 51-63 m in the 6-minute walk distance, Dr. Ghofrani and his associates said.
The PATENT-1 trial
The other phase III clinical trial reported in the New England Journal of Medicine was the PATENT-1 (Pulmonary Arterial Hypertension Soluble Guanylate Cyclase-Stimulator) study. This trial also was funded by Bayer HealthCare and headed by Dr. Ghofrani.
In it, 443 patients who had symptomatic pulmonary arterial hypertension were treated and followed up at 124 medical centers in 30 countries. These subjects had idiopathic or familial disease, or pulmonary arterial hypertension associated with connective-tissue disease, congenital heart disease, portal hypertension with liver cirrhosis, anorexigen use, or amphetamine use.
A total of 44% of patients were already receiving an endothelin-receptor antagonist (usually bosentan), and 6% were taking prostanoids (usually inhaled iloprost) for the disorder, and the other half of the study population was not receiving any other treatments. Concomitant therapy with oral anticoagulants, diuretics, and supplemental oxygen was permitted during the study.
These study subjects were randomly assigned to receive high-dose riociguat capped at 2.5 mg three times daily (254 patients), low-dose riociguat capped at 1.5 mg three times daily (63 patients), or placebo (126 patients) for 12 weeks. The analysis of data from the low-dose group was considered exploratory and was reported separately.
The primary endpoint – change from baseline in 6-minute walk distance – increased by a mean of 30 m in the high-dose group but decreased by a mean of 6 m in the placebo group in the intention-to-treat analysis. The results were similar in the per-protocol analysis, the investigators reported (N. Engl. J. Med. 2013 July 25 [doi: 10.1056/NEJMoa1209655]).
This treatment benefit was consistent across several subgroups of patients. In addition, the active drug significantly decreased pulmonary vascular resistance, improved mean pulmonary artery pressure and cardiac output, lengthened the interval to clinical worsening, improved NT-proBNP levels, improved WHO functional class, and improved scores on the Borg dyspnea scale.
In exploratory analyses, riociguat did not improve scores on one measure of QOL but did so on a disease-specific QOL tool.
Drug-related serious adverse events occurred in 1.4 % of patients receiving high-dose riociguat and 1% of those receiving placebo. A total of 3% of patients in the riociguat group and 7% of those in the placebo group discontinued the study drug because of adverse events.
Serious or drug-related adverse events in both groups included increased hepatic enzyme levels, acute renal failure, syncope, esophageal pain and swelling, supraventricular tachycardia, hypotension, generalized edema, hypoxemia, dyspnea, and worsening of pulmonary hypertension. There were two deaths in the riociguat group and three in the placebo group, none of which were considered to be related to the study drug.
A total of 396 of the subjects in the PATENT-1 study elected to enroll in an extended study of the long-term safety and efficacy of riociguat (the PATENT-2 clinical trial). An exploratory analysis of data from the first 12 weeks of that study showed a further mean increase of 53 m in 6-minute walk distance with high-dose riociguat.
CHEST-1 and PATENT-1 were funded by Bayer HealthCare. Dr. Ghofrani reported ties to Actelion, Bayer, and other companies. His associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major finding: Riociguat significantly improved 6-minute walk distance by a mean of 39 m in patients with thromboembolic pulmonary hypertension and by a mean of 30 m in patients with pulmonary arterial hypertension.
Data source: Two separate, international, phase III, randomized controlled trials assessing the safety and efficacy of riociguat for thromboembolic pulmonary hypertension (261 patients) and pulmonary arterial hypertension (443 patients).
Disclosures: CHEST-1 and PATENT-1 were funded by Bayer HealthCare. Dr. Ghofrani reported ties to Actelion, Bayer, and other companies. His associates reported ties to numerous industry sources.
Copeptin helped differential diagnosis of hyponatremia
SAN FRANCISCO – Measuring plasma copeptin levels may provide an accurate and easier way to identify the cause of severe hyponatremia, compared with measuring arginine vasopressin levels, preliminary data on 175 patients suggested.
A plasma copeptin level greater than 70 pmol/L correlated with a diagnosis of hypovolemic or diuretic-induced hyponatremia requiring saline infusion with 91% specificity. A copeptin/urinary sodium ratio greater than 1.84 identified patients who clearly needed saline infusion with a specificity of 90%, Dr. Nicole Nigro reported at the annual meeting of the Endocrine Society.
"The differential diagnosis of hyponatremia is not easy to make" and often relies on detecting adequate or inadequate plasma levels of arginine vasopressin (AVP), she said in an interview. Blood samples for AVP measurements must be kept on ice, making handling and transportation to the lab more difficult. AVP mostly attaches to platelets, it clears rapidly, and AVP levels are known to be very unstable.
Copeptin, on the other hand, is very stable in plasma, easy to measure, and released by the body in an equimolar ratio with AVP, said Dr. Nigro of University Hospital, Basel, Switzerland.
She presented data on the first 175 patients in an ongoing study that has enrolled 290 of an expected 300 consecutive patients who present with severe hypo-osmolar hyponatremia at the emergency departments of two Swiss tertiary referral centers. All patients had a sodium level below 125 mmol/L. Three experts who were blinded to patients’ copeptin levels made the final diagnoses based on a clinical algorithm, a chart review, and response to therapy.
The median plasma copeptin level was 2.8 pmol/L in 17 patients diagnosed with primary polydipsia, 13.2 pmol/L in 45 patients with diuretic-induced hyponatremia, 13 pmol/L in 56 patients with syndrome of inappropriate antidiuretic hormone, 28 pmol/L in 25 patients with hypervolemic hyponatremia, and 55 pmol/L in 32 patients with hypovolemic hyponatremia, Dr. Nigro reported in an oral presentation and a featured poster at the meeting.
The 77 patients who required saline infusion had significantly higher copeptin levels (27 pmol/L), than patients who did not need saline (12 pmol/L).
Dr. Nigro’s hospital routinely measures copeptin levels but is not yet using them to guide the differential diagnosis of hyponatremia. If the current study produces clear findings when it’s finished, the investigators next may conduct a study that uses copeptin levels to guide treatment of patients with severe hyponatremia.
Ultimately, "copeptin may help us to guide the therapy and management of these patients with severe hyponatremia," she suggested.
Some of Dr. Nigro’s associates in the study have been speakers for B.R.A.H.M.S./Thermo Fisher Scientific. She reported having no other relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Measuring plasma copeptin levels may provide an accurate and easier way to identify the cause of severe hyponatremia, compared with measuring arginine vasopressin levels, preliminary data on 175 patients suggested.
A plasma copeptin level greater than 70 pmol/L correlated with a diagnosis of hypovolemic or diuretic-induced hyponatremia requiring saline infusion with 91% specificity. A copeptin/urinary sodium ratio greater than 1.84 identified patients who clearly needed saline infusion with a specificity of 90%, Dr. Nicole Nigro reported at the annual meeting of the Endocrine Society.
"The differential diagnosis of hyponatremia is not easy to make" and often relies on detecting adequate or inadequate plasma levels of arginine vasopressin (AVP), she said in an interview. Blood samples for AVP measurements must be kept on ice, making handling and transportation to the lab more difficult. AVP mostly attaches to platelets, it clears rapidly, and AVP levels are known to be very unstable.
Copeptin, on the other hand, is very stable in plasma, easy to measure, and released by the body in an equimolar ratio with AVP, said Dr. Nigro of University Hospital, Basel, Switzerland.
She presented data on the first 175 patients in an ongoing study that has enrolled 290 of an expected 300 consecutive patients who present with severe hypo-osmolar hyponatremia at the emergency departments of two Swiss tertiary referral centers. All patients had a sodium level below 125 mmol/L. Three experts who were blinded to patients’ copeptin levels made the final diagnoses based on a clinical algorithm, a chart review, and response to therapy.
The median plasma copeptin level was 2.8 pmol/L in 17 patients diagnosed with primary polydipsia, 13.2 pmol/L in 45 patients with diuretic-induced hyponatremia, 13 pmol/L in 56 patients with syndrome of inappropriate antidiuretic hormone, 28 pmol/L in 25 patients with hypervolemic hyponatremia, and 55 pmol/L in 32 patients with hypovolemic hyponatremia, Dr. Nigro reported in an oral presentation and a featured poster at the meeting.
The 77 patients who required saline infusion had significantly higher copeptin levels (27 pmol/L), than patients who did not need saline (12 pmol/L).
Dr. Nigro’s hospital routinely measures copeptin levels but is not yet using them to guide the differential diagnosis of hyponatremia. If the current study produces clear findings when it’s finished, the investigators next may conduct a study that uses copeptin levels to guide treatment of patients with severe hyponatremia.
Ultimately, "copeptin may help us to guide the therapy and management of these patients with severe hyponatremia," she suggested.
Some of Dr. Nigro’s associates in the study have been speakers for B.R.A.H.M.S./Thermo Fisher Scientific. She reported having no other relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Measuring plasma copeptin levels may provide an accurate and easier way to identify the cause of severe hyponatremia, compared with measuring arginine vasopressin levels, preliminary data on 175 patients suggested.
A plasma copeptin level greater than 70 pmol/L correlated with a diagnosis of hypovolemic or diuretic-induced hyponatremia requiring saline infusion with 91% specificity. A copeptin/urinary sodium ratio greater than 1.84 identified patients who clearly needed saline infusion with a specificity of 90%, Dr. Nicole Nigro reported at the annual meeting of the Endocrine Society.
"The differential diagnosis of hyponatremia is not easy to make" and often relies on detecting adequate or inadequate plasma levels of arginine vasopressin (AVP), she said in an interview. Blood samples for AVP measurements must be kept on ice, making handling and transportation to the lab more difficult. AVP mostly attaches to platelets, it clears rapidly, and AVP levels are known to be very unstable.
Copeptin, on the other hand, is very stable in plasma, easy to measure, and released by the body in an equimolar ratio with AVP, said Dr. Nigro of University Hospital, Basel, Switzerland.
She presented data on the first 175 patients in an ongoing study that has enrolled 290 of an expected 300 consecutive patients who present with severe hypo-osmolar hyponatremia at the emergency departments of two Swiss tertiary referral centers. All patients had a sodium level below 125 mmol/L. Three experts who were blinded to patients’ copeptin levels made the final diagnoses based on a clinical algorithm, a chart review, and response to therapy.
The median plasma copeptin level was 2.8 pmol/L in 17 patients diagnosed with primary polydipsia, 13.2 pmol/L in 45 patients with diuretic-induced hyponatremia, 13 pmol/L in 56 patients with syndrome of inappropriate antidiuretic hormone, 28 pmol/L in 25 patients with hypervolemic hyponatremia, and 55 pmol/L in 32 patients with hypovolemic hyponatremia, Dr. Nigro reported in an oral presentation and a featured poster at the meeting.
The 77 patients who required saline infusion had significantly higher copeptin levels (27 pmol/L), than patients who did not need saline (12 pmol/L).
Dr. Nigro’s hospital routinely measures copeptin levels but is not yet using them to guide the differential diagnosis of hyponatremia. If the current study produces clear findings when it’s finished, the investigators next may conduct a study that uses copeptin levels to guide treatment of patients with severe hyponatremia.
Ultimately, "copeptin may help us to guide the therapy and management of these patients with severe hyponatremia," she suggested.
Some of Dr. Nigro’s associates in the study have been speakers for B.R.A.H.M.S./Thermo Fisher Scientific. She reported having no other relevant financial disclosures.
On Twitter @sherryboschert
AT ENDO 2013
Major finding: A plasma copeptin level greater than 70 pmol/L in patients presenting with severe hypo-osmolar hyponatremia correlated with a diagnosis of hypovolemic or diuretic-induced hyponatremia requiring saline infusion with 91% specificity.
Data source: A prospective, multicenter observational study of 175 consecutive patients presenting to emergency departments at two Swiss tertiary referral centers.
Disclosures: Some of Dr. Nigro’s associates in the study have been speakers for B.R.A.H.M.S./Thermo Fisher Scientific. She reported having no other relevant financial disclosures.
Cardiac rehabilitation benefits elderly heart failure patients
ROME – A multiweek program of cardiac rehabilitation is as beneficial in elderly patients with chronic heart failure as it is in younger heart failure patients, according to a review of 243 patients at one Belgium center.
"Although they have lower exercise capacity at baseline, older patients have at least as much benefit from an exercise program as younger patients with chronic heart failure," Ms. Sofie Pardaens* reported in a poster at the annual meeting of the European Association for Cardiovascular Prevention and Rehabilitation.
A second analysis by Ms. Pardaens, a researcher at Ghent (Belgium) University, and her associates, reported in a separate poster, showed that a prolonged cardiac rehabilitation program was as effective in patients recently discharged from a heart failure hospitalization as it was in patients following cardiac surgery or after an acute coronary syndrome (ACS) event.
Their assessment of cardiac rehabilitation relative to a patient’s age included 243 patients who participated in a rehabilitation program at the University of Ghent, who had chronic heart failure, and who had an amino-terminal pro-B-type natriuretic peptide value of at least 400 pg/mL, a level very suggestive of heart failure (Circulation 2011;123:2015-9). The group included 43 patients (18%) who were at least 75 years old (average, 78 years) and 68 patients younger than 60 (average, 51 years), with the remaining 132 patients evenly distributed across the range of 60-74 years old.
All participants had just been hospitalized, for an ACS event, cardiac surgery, or heart failure.
The hospital-based rehabilitation program combined aerobic and strength training, and was designed to bring a patient’s heart rate to his anaerobic threshold during each session. Sessions occurred two or three times a week, and the full program included 45 sessions over a period of 4-5 months. The patients studied averaged 34 sessions each; patients aged 75 or older averaged 32 sessions each, while those younger than 60 averaged 35 sessions each.
The researchers measured peak exercise capacity using cardiopulmonary exercise testing at baseline and at the end of the rehabilitation session sequence, and found that the 16% average level of improvement among patients at least 75 years old closely matched the average 19% improvement among the patients younger than 60, and the 17% average improvement among everyone else, Ms. Pardaens and her associates reported. All age groups also showed similar improvements in their average ventilatory equivalence ratio, as well as their average 6-minute walk distance; however, the 29% average increased distance among patients younger than 60 years significantly exceeded the 19% average increase among those aged 75 or older.
The group’s second analysis focused on the 371 patients who underwent cardiac rehabilitation at the University of Ghent during January 2010 through May 2012 from among the 1,253 patients hospitalized during this period for an acute coronary syndrome event, cardiac surgery, or heart failure. In this pool of more than 1,000 patients who were potentially eligible to participate, only 30% actually enrolled in the rehabilitation program. The cardiac rehabilitation program again involved two to three sessions per week, with a goal for patients to complete 45 sessions within 5 months.
The sign-up rate for rehabilitation lagged even more among the 428 patients from the larger group whose index hospitalization had been for heart failure, with 37 of the acute heart failure patients (9%) actually engaging in rehabilitation. Rehabilitation participation was highest, a 56% rate, among the 358 patients who had been hospitalized for cardiac surgery, with a 28% uptake rate among 467 patients who had an ACS event.
Despite the low, 9% uptake of cardiac rehabilitation in heart failure patients, their benefit from participation closely tracked the benefit seen in surgery and ACS patients. Improvement in peak exercise capacity over baseline at the end of rehabilitation averaged 19% in the heart failure patients, 17% in the ACS patients, and 24% in the surgery patients, differences that were not statistically significant, reported Ms. Pardaens. All three subgroups also had similar average improvements in their 6-minute walk distance, which rose by an average of 21% in the heart failure patients and by averages of 27% and 28% in the other two subgroups.
Based on the efficacy but low usage of cardiac rehabilitation, future research should examine ways to boost its use by heart failure patients, concluded Ms. Pardaens.
Ms. Pardaens and her associates said they had no relevant financial disclosures.
On Twitter @mitchelzoler
*Correction 6/28/13: An earlier version of this article incorrectly reported researcher Sofie Pardaens' title. She is currently studying for her PhD in the department of internal medicine at Ghent University.
Cardiac rehabilitation is undoubtedly an essential component of the contemporary treatment of patients with coronary disease and heart failure.
Exercise training has the potential to act as a catalyst for promoting other aspects of rehabilitation, including risk factor modification through therapeutic lifestyle changes and optimization of psychosocial support. Similarly, among patients who are elderly, such outcome measures may include the achievement of functional independence, the prevention of premature disability, and a reduction in the need for custodial care.
Despite limited data, older patients have shown improvement in their exercise tolerance comparable to that of younger patients participating in equivalent exercise programs. In addition, the safety of exercise within cardiac rehabilitation programs is well accepted and established.
Dr. Jun Chiong is associate professor of medicine at Loma Linda (Calif.) University Medical Center. He is on the on the advisory board of CHEST Physician.
Cardiac rehabilitation is undoubtedly an essential component of the contemporary treatment of patients with coronary disease and heart failure.
Exercise training has the potential to act as a catalyst for promoting other aspects of rehabilitation, including risk factor modification through therapeutic lifestyle changes and optimization of psychosocial support. Similarly, among patients who are elderly, such outcome measures may include the achievement of functional independence, the prevention of premature disability, and a reduction in the need for custodial care.
Despite limited data, older patients have shown improvement in their exercise tolerance comparable to that of younger patients participating in equivalent exercise programs. In addition, the safety of exercise within cardiac rehabilitation programs is well accepted and established.
Dr. Jun Chiong is associate professor of medicine at Loma Linda (Calif.) University Medical Center. He is on the on the advisory board of CHEST Physician.
Cardiac rehabilitation is undoubtedly an essential component of the contemporary treatment of patients with coronary disease and heart failure.
Exercise training has the potential to act as a catalyst for promoting other aspects of rehabilitation, including risk factor modification through therapeutic lifestyle changes and optimization of psychosocial support. Similarly, among patients who are elderly, such outcome measures may include the achievement of functional independence, the prevention of premature disability, and a reduction in the need for custodial care.
Despite limited data, older patients have shown improvement in their exercise tolerance comparable to that of younger patients participating in equivalent exercise programs. In addition, the safety of exercise within cardiac rehabilitation programs is well accepted and established.
Dr. Jun Chiong is associate professor of medicine at Loma Linda (Calif.) University Medical Center. He is on the on the advisory board of CHEST Physician.
ROME – A multiweek program of cardiac rehabilitation is as beneficial in elderly patients with chronic heart failure as it is in younger heart failure patients, according to a review of 243 patients at one Belgium center.
"Although they have lower exercise capacity at baseline, older patients have at least as much benefit from an exercise program as younger patients with chronic heart failure," Ms. Sofie Pardaens* reported in a poster at the annual meeting of the European Association for Cardiovascular Prevention and Rehabilitation.
A second analysis by Ms. Pardaens, a researcher at Ghent (Belgium) University, and her associates, reported in a separate poster, showed that a prolonged cardiac rehabilitation program was as effective in patients recently discharged from a heart failure hospitalization as it was in patients following cardiac surgery or after an acute coronary syndrome (ACS) event.
Their assessment of cardiac rehabilitation relative to a patient’s age included 243 patients who participated in a rehabilitation program at the University of Ghent, who had chronic heart failure, and who had an amino-terminal pro-B-type natriuretic peptide value of at least 400 pg/mL, a level very suggestive of heart failure (Circulation 2011;123:2015-9). The group included 43 patients (18%) who were at least 75 years old (average, 78 years) and 68 patients younger than 60 (average, 51 years), with the remaining 132 patients evenly distributed across the range of 60-74 years old.
All participants had just been hospitalized, for an ACS event, cardiac surgery, or heart failure.
The hospital-based rehabilitation program combined aerobic and strength training, and was designed to bring a patient’s heart rate to his anaerobic threshold during each session. Sessions occurred two or three times a week, and the full program included 45 sessions over a period of 4-5 months. The patients studied averaged 34 sessions each; patients aged 75 or older averaged 32 sessions each, while those younger than 60 averaged 35 sessions each.
The researchers measured peak exercise capacity using cardiopulmonary exercise testing at baseline and at the end of the rehabilitation session sequence, and found that the 16% average level of improvement among patients at least 75 years old closely matched the average 19% improvement among the patients younger than 60, and the 17% average improvement among everyone else, Ms. Pardaens and her associates reported. All age groups also showed similar improvements in their average ventilatory equivalence ratio, as well as their average 6-minute walk distance; however, the 29% average increased distance among patients younger than 60 years significantly exceeded the 19% average increase among those aged 75 or older.
The group’s second analysis focused on the 371 patients who underwent cardiac rehabilitation at the University of Ghent during January 2010 through May 2012 from among the 1,253 patients hospitalized during this period for an acute coronary syndrome event, cardiac surgery, or heart failure. In this pool of more than 1,000 patients who were potentially eligible to participate, only 30% actually enrolled in the rehabilitation program. The cardiac rehabilitation program again involved two to three sessions per week, with a goal for patients to complete 45 sessions within 5 months.
The sign-up rate for rehabilitation lagged even more among the 428 patients from the larger group whose index hospitalization had been for heart failure, with 37 of the acute heart failure patients (9%) actually engaging in rehabilitation. Rehabilitation participation was highest, a 56% rate, among the 358 patients who had been hospitalized for cardiac surgery, with a 28% uptake rate among 467 patients who had an ACS event.
Despite the low, 9% uptake of cardiac rehabilitation in heart failure patients, their benefit from participation closely tracked the benefit seen in surgery and ACS patients. Improvement in peak exercise capacity over baseline at the end of rehabilitation averaged 19% in the heart failure patients, 17% in the ACS patients, and 24% in the surgery patients, differences that were not statistically significant, reported Ms. Pardaens. All three subgroups also had similar average improvements in their 6-minute walk distance, which rose by an average of 21% in the heart failure patients and by averages of 27% and 28% in the other two subgroups.
Based on the efficacy but low usage of cardiac rehabilitation, future research should examine ways to boost its use by heart failure patients, concluded Ms. Pardaens.
Ms. Pardaens and her associates said they had no relevant financial disclosures.
On Twitter @mitchelzoler
*Correction 6/28/13: An earlier version of this article incorrectly reported researcher Sofie Pardaens' title. She is currently studying for her PhD in the department of internal medicine at Ghent University.
ROME – A multiweek program of cardiac rehabilitation is as beneficial in elderly patients with chronic heart failure as it is in younger heart failure patients, according to a review of 243 patients at one Belgium center.
"Although they have lower exercise capacity at baseline, older patients have at least as much benefit from an exercise program as younger patients with chronic heart failure," Ms. Sofie Pardaens* reported in a poster at the annual meeting of the European Association for Cardiovascular Prevention and Rehabilitation.
A second analysis by Ms. Pardaens, a researcher at Ghent (Belgium) University, and her associates, reported in a separate poster, showed that a prolonged cardiac rehabilitation program was as effective in patients recently discharged from a heart failure hospitalization as it was in patients following cardiac surgery or after an acute coronary syndrome (ACS) event.
Their assessment of cardiac rehabilitation relative to a patient’s age included 243 patients who participated in a rehabilitation program at the University of Ghent, who had chronic heart failure, and who had an amino-terminal pro-B-type natriuretic peptide value of at least 400 pg/mL, a level very suggestive of heart failure (Circulation 2011;123:2015-9). The group included 43 patients (18%) who were at least 75 years old (average, 78 years) and 68 patients younger than 60 (average, 51 years), with the remaining 132 patients evenly distributed across the range of 60-74 years old.
All participants had just been hospitalized, for an ACS event, cardiac surgery, or heart failure.
The hospital-based rehabilitation program combined aerobic and strength training, and was designed to bring a patient’s heart rate to his anaerobic threshold during each session. Sessions occurred two or three times a week, and the full program included 45 sessions over a period of 4-5 months. The patients studied averaged 34 sessions each; patients aged 75 or older averaged 32 sessions each, while those younger than 60 averaged 35 sessions each.
The researchers measured peak exercise capacity using cardiopulmonary exercise testing at baseline and at the end of the rehabilitation session sequence, and found that the 16% average level of improvement among patients at least 75 years old closely matched the average 19% improvement among the patients younger than 60, and the 17% average improvement among everyone else, Ms. Pardaens and her associates reported. All age groups also showed similar improvements in their average ventilatory equivalence ratio, as well as their average 6-minute walk distance; however, the 29% average increased distance among patients younger than 60 years significantly exceeded the 19% average increase among those aged 75 or older.
The group’s second analysis focused on the 371 patients who underwent cardiac rehabilitation at the University of Ghent during January 2010 through May 2012 from among the 1,253 patients hospitalized during this period for an acute coronary syndrome event, cardiac surgery, or heart failure. In this pool of more than 1,000 patients who were potentially eligible to participate, only 30% actually enrolled in the rehabilitation program. The cardiac rehabilitation program again involved two to three sessions per week, with a goal for patients to complete 45 sessions within 5 months.
The sign-up rate for rehabilitation lagged even more among the 428 patients from the larger group whose index hospitalization had been for heart failure, with 37 of the acute heart failure patients (9%) actually engaging in rehabilitation. Rehabilitation participation was highest, a 56% rate, among the 358 patients who had been hospitalized for cardiac surgery, with a 28% uptake rate among 467 patients who had an ACS event.
Despite the low, 9% uptake of cardiac rehabilitation in heart failure patients, their benefit from participation closely tracked the benefit seen in surgery and ACS patients. Improvement in peak exercise capacity over baseline at the end of rehabilitation averaged 19% in the heart failure patients, 17% in the ACS patients, and 24% in the surgery patients, differences that were not statistically significant, reported Ms. Pardaens. All three subgroups also had similar average improvements in their 6-minute walk distance, which rose by an average of 21% in the heart failure patients and by averages of 27% and 28% in the other two subgroups.
Based on the efficacy but low usage of cardiac rehabilitation, future research should examine ways to boost its use by heart failure patients, concluded Ms. Pardaens.
Ms. Pardaens and her associates said they had no relevant financial disclosures.
On Twitter @mitchelzoler
*Correction 6/28/13: An earlier version of this article incorrectly reported researcher Sofie Pardaens' title. She is currently studying for her PhD in the department of internal medicine at Ghent University.
AT EUROPREVENT 2013
Major finding: Cardiac rehabilitation boosted exercise capacity by an average 16% in elderly patients and an average 19% in younger patients.
Data source: Data came from a review of 243 patients with chronic heart failure who participated in cardiac rehabilitation at one Belgium center.
Disclosures: Dr. Pardaens and her associates said they had no relevant financial disclosures.
Hospital revenue generated by cardiologists continues to drop
Average annual hospital revenue generated by invasive cardiologists dropped 3.2% from 2010 to 2013, while the revenue generated by noninvasive cardiologists dropped 6.6%, according to a survey by physician job placement firm Merritt Hawkins.
"In the last several years, some services provided by cardiologists have been considered reimbursement ‘outliers’ by Medicare and have been targeted for significant" cuts, the survey authors noted, adding that "revenues generated by invasive cardiologists for hospitals can be expected to decline in coming years."
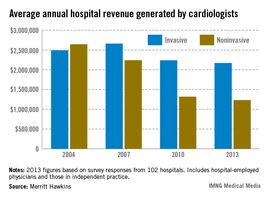
Average revenue of $2.17 million was reported for invasive cardiologists and just under $1.23 million for noninvasive cardiologists in 2013. That compares with almost $1.42 million for all specialists and $1.57 for all primary care physicians. The specialists’ average is the lowest Merritt Hawkins has reported since it started the survey in 2002 and is lower than the primary care average for the first time ever, the report noted.
The average net revenue generated for all physicians was $1.45 million – a drop of 6.2% from 2010 and, again, the lowest average since 2002, Merritt Hawkins said in its analysis.
The survey was sent to 5,500 hospitals in January 2013 and completed by 102 hospital chief financial officers.
Average annual hospital revenue generated by invasive cardiologists dropped 3.2% from 2010 to 2013, while the revenue generated by noninvasive cardiologists dropped 6.6%, according to a survey by physician job placement firm Merritt Hawkins.
"In the last several years, some services provided by cardiologists have been considered reimbursement ‘outliers’ by Medicare and have been targeted for significant" cuts, the survey authors noted, adding that "revenues generated by invasive cardiologists for hospitals can be expected to decline in coming years."

Average revenue of $2.17 million was reported for invasive cardiologists and just under $1.23 million for noninvasive cardiologists in 2013. That compares with almost $1.42 million for all specialists and $1.57 for all primary care physicians. The specialists’ average is the lowest Merritt Hawkins has reported since it started the survey in 2002 and is lower than the primary care average for the first time ever, the report noted.
The average net revenue generated for all physicians was $1.45 million – a drop of 6.2% from 2010 and, again, the lowest average since 2002, Merritt Hawkins said in its analysis.
The survey was sent to 5,500 hospitals in January 2013 and completed by 102 hospital chief financial officers.
Average annual hospital revenue generated by invasive cardiologists dropped 3.2% from 2010 to 2013, while the revenue generated by noninvasive cardiologists dropped 6.6%, according to a survey by physician job placement firm Merritt Hawkins.
"In the last several years, some services provided by cardiologists have been considered reimbursement ‘outliers’ by Medicare and have been targeted for significant" cuts, the survey authors noted, adding that "revenues generated by invasive cardiologists for hospitals can be expected to decline in coming years."

Average revenue of $2.17 million was reported for invasive cardiologists and just under $1.23 million for noninvasive cardiologists in 2013. That compares with almost $1.42 million for all specialists and $1.57 for all primary care physicians. The specialists’ average is the lowest Merritt Hawkins has reported since it started the survey in 2002 and is lower than the primary care average for the first time ever, the report noted.
The average net revenue generated for all physicians was $1.45 million – a drop of 6.2% from 2010 and, again, the lowest average since 2002, Merritt Hawkins said in its analysis.
The survey was sent to 5,500 hospitals in January 2013 and completed by 102 hospital chief financial officers.
Shock-Less trial improves physicians' ICD programming
DENVER – The rate of adherence to evidence-based implantable cardioverter-defibrillator programming strategies known to reduce the rate of unnecessary shocks climbed significantly in a large, prospective study in which physicians received detailed reports on their own performance and how it stacked up to that of others.
The Shock-Less study was a real-world international study involving 4,131 ICD recipients and their physicians at 118 sites. The improved adherence to evidence-based programming achieved through the use of the individualized, multipage therapy programming reports (TPRs) translated into a highly significant 27% reduction in the risk of all-cause ICD shocks during follow-up, Dr. Marc T. Silver reported at the annual meeting of the Heart Rhythm Society.
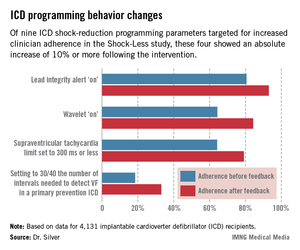
This is welcome news for patients. It means less shock-related morbidity and potentially less mortality, said Dr. Silver of WakeMed in Raleigh, N.C.
The study included 2,693 patients who received their Medtronic primary or secondary prevention ICD before a center received its first Shock-Less report and 1,438 implanted after the report. A total of 265 all-cause shocks occurred in the "before" group, 116 in the "after" cohort.
In a multivariate logistic regression analysis adjusted for factors known to affect shock rates, including patient age, smoking status, New York Heart Association functional class, and atrial fibrillation, patients in the "after" group had a 27% reduction in the relative risk of both appropriate and inappropriate shocks (P = .002).
The TPRs provided ICD centers and their individual physicians with detailed feedback on rates of adherence to nine evidence-based programming settings that help reduce shocks. Most ICDs don’t arrive from the manufacturer with these settings in place. Some of the changes in programming were quite impressive (see graphic), including a near-doubling of the rate of primary prevention ICDs programmed to 30/40 as the number of intervals to detect ventricular fibrillation; this rate improved from 18.5% to 33.1%.
Dr. Silver offered personal testimony as to the power of the TPRs as a behavior-modification tool.
"Having received TPRs myself, it is a character-building experience. If you can get hold of information like this on your own practices, I guarantee you will leave a little smaller afterwards, like I did. Bigger in some way, smaller in others," he said.
Yet there remains a clear opportunity for further improvement in physician performance, Dr. Silver added.
"Achieving numbers in the 33% range for adherence to 30/40 [the number of intervals needed to detect ventricular fibrillation] is not what I think many of us would hope for one day," he observed.
Along those lines, audience member Dr. Thomas F. Deering of the Piedmont Heart Institute, Atlanta, commented that while the changes in physician behavior achieved through the Shock-Less project were significant, they were not sweeping in magnitude. Given the negative clinical consequences that result from lack of adherence to evidence-based programming, isn’t it time to request that the device industry change the default settings on their ICDs in accord with the evidence-based guidelines?, he asked.
"As someone who’s become very interested in physician behavior," Dr. Silver replied, "I regret to say that changing nominal settings on the devices may be the best way to move our profession forward. I say that with some degree of regret, but I think that’s the truth."
Dr. Silver reported serving as a consultant to Medtronic, which sponsored the Shock-Less study.
DENVER – The rate of adherence to evidence-based implantable cardioverter-defibrillator programming strategies known to reduce the rate of unnecessary shocks climbed significantly in a large, prospective study in which physicians received detailed reports on their own performance and how it stacked up to that of others.
The Shock-Less study was a real-world international study involving 4,131 ICD recipients and their physicians at 118 sites. The improved adherence to evidence-based programming achieved through the use of the individualized, multipage therapy programming reports (TPRs) translated into a highly significant 27% reduction in the risk of all-cause ICD shocks during follow-up, Dr. Marc T. Silver reported at the annual meeting of the Heart Rhythm Society.

This is welcome news for patients. It means less shock-related morbidity and potentially less mortality, said Dr. Silver of WakeMed in Raleigh, N.C.
The study included 2,693 patients who received their Medtronic primary or secondary prevention ICD before a center received its first Shock-Less report and 1,438 implanted after the report. A total of 265 all-cause shocks occurred in the "before" group, 116 in the "after" cohort.
In a multivariate logistic regression analysis adjusted for factors known to affect shock rates, including patient age, smoking status, New York Heart Association functional class, and atrial fibrillation, patients in the "after" group had a 27% reduction in the relative risk of both appropriate and inappropriate shocks (P = .002).
The TPRs provided ICD centers and their individual physicians with detailed feedback on rates of adherence to nine evidence-based programming settings that help reduce shocks. Most ICDs don’t arrive from the manufacturer with these settings in place. Some of the changes in programming were quite impressive (see graphic), including a near-doubling of the rate of primary prevention ICDs programmed to 30/40 as the number of intervals to detect ventricular fibrillation; this rate improved from 18.5% to 33.1%.
Dr. Silver offered personal testimony as to the power of the TPRs as a behavior-modification tool.
"Having received TPRs myself, it is a character-building experience. If you can get hold of information like this on your own practices, I guarantee you will leave a little smaller afterwards, like I did. Bigger in some way, smaller in others," he said.
Yet there remains a clear opportunity for further improvement in physician performance, Dr. Silver added.
"Achieving numbers in the 33% range for adherence to 30/40 [the number of intervals needed to detect ventricular fibrillation] is not what I think many of us would hope for one day," he observed.
Along those lines, audience member Dr. Thomas F. Deering of the Piedmont Heart Institute, Atlanta, commented that while the changes in physician behavior achieved through the Shock-Less project were significant, they were not sweeping in magnitude. Given the negative clinical consequences that result from lack of adherence to evidence-based programming, isn’t it time to request that the device industry change the default settings on their ICDs in accord with the evidence-based guidelines?, he asked.
"As someone who’s become very interested in physician behavior," Dr. Silver replied, "I regret to say that changing nominal settings on the devices may be the best way to move our profession forward. I say that with some degree of regret, but I think that’s the truth."
Dr. Silver reported serving as a consultant to Medtronic, which sponsored the Shock-Less study.
DENVER – The rate of adherence to evidence-based implantable cardioverter-defibrillator programming strategies known to reduce the rate of unnecessary shocks climbed significantly in a large, prospective study in which physicians received detailed reports on their own performance and how it stacked up to that of others.
The Shock-Less study was a real-world international study involving 4,131 ICD recipients and their physicians at 118 sites. The improved adherence to evidence-based programming achieved through the use of the individualized, multipage therapy programming reports (TPRs) translated into a highly significant 27% reduction in the risk of all-cause ICD shocks during follow-up, Dr. Marc T. Silver reported at the annual meeting of the Heart Rhythm Society.

This is welcome news for patients. It means less shock-related morbidity and potentially less mortality, said Dr. Silver of WakeMed in Raleigh, N.C.
The study included 2,693 patients who received their Medtronic primary or secondary prevention ICD before a center received its first Shock-Less report and 1,438 implanted after the report. A total of 265 all-cause shocks occurred in the "before" group, 116 in the "after" cohort.
In a multivariate logistic regression analysis adjusted for factors known to affect shock rates, including patient age, smoking status, New York Heart Association functional class, and atrial fibrillation, patients in the "after" group had a 27% reduction in the relative risk of both appropriate and inappropriate shocks (P = .002).
The TPRs provided ICD centers and their individual physicians with detailed feedback on rates of adherence to nine evidence-based programming settings that help reduce shocks. Most ICDs don’t arrive from the manufacturer with these settings in place. Some of the changes in programming were quite impressive (see graphic), including a near-doubling of the rate of primary prevention ICDs programmed to 30/40 as the number of intervals to detect ventricular fibrillation; this rate improved from 18.5% to 33.1%.
Dr. Silver offered personal testimony as to the power of the TPRs as a behavior-modification tool.
"Having received TPRs myself, it is a character-building experience. If you can get hold of information like this on your own practices, I guarantee you will leave a little smaller afterwards, like I did. Bigger in some way, smaller in others," he said.
Yet there remains a clear opportunity for further improvement in physician performance, Dr. Silver added.
"Achieving numbers in the 33% range for adherence to 30/40 [the number of intervals needed to detect ventricular fibrillation] is not what I think many of us would hope for one day," he observed.
Along those lines, audience member Dr. Thomas F. Deering of the Piedmont Heart Institute, Atlanta, commented that while the changes in physician behavior achieved through the Shock-Less project were significant, they were not sweeping in magnitude. Given the negative clinical consequences that result from lack of adherence to evidence-based programming, isn’t it time to request that the device industry change the default settings on their ICDs in accord with the evidence-based guidelines?, he asked.
"As someone who’s become very interested in physician behavior," Dr. Silver replied, "I regret to say that changing nominal settings on the devices may be the best way to move our profession forward. I say that with some degree of regret, but I think that’s the truth."
Dr. Silver reported serving as a consultant to Medtronic, which sponsored the Shock-Less study.
AT HEART RHYTHM 2013
Major Finding: A 27% reduction in shocks from ICDs ensued after physicians received detailed, structured reports on their rates of adherence to evidence-based shock-reduction programming strategies.
Data Source: Shock-Less is an international prospective cohort study involving 4,131 ICD recipients and their physicians at 118 centers.
Disclosures: Dr. Silver reported serving as a consultant to Medtronic, which sponsored the Shock-Less study.
Heart failure guidelines: New hope in medical therapy
The newest heart failure management guidelines make a bold statement: Heart failure should no longer be considered a death sentence, but can instead be managed in a way that may add years of quality life for some patients.
“With optimal therapy applied to the right patient in the right manner at the right time, the risk of death can be markedly reduced, perhaps by as much as 50%. Treating fewer than 10 patients with all the correct therapies will easily save at least one life and one or more hospitalizations. Those are real benefits that dwarf the benefit of many of our other cardiovascular therapies,” said Dr. Clyde W. Yancy, chair of the joint guidelines writing committee. The document was published in the June 5 online edition of the Journal of the American College of Cardiology (2013 [doi:10.1016/j.jacc.2013.05.019]).
"For so long, we had assumed that, by definition, heart failure was a fatal diagnosis – that all we could do was tell patients to get their affairs in order and perhaps make them feel a little better, but that death was almost a fait accompli," said Dr. Yancy, the Magerstadt Professor of Medicine and chief of cardiology at Northwestern University, Chicago. "But, in the past few years, a lot of tough work has been done showing there are effective therapies and that when given correctly, major improvements in survival do occur."
A joint effort of the American College of Cardiology Foundation and the American Heart Association, the 2013 Heart Failure Guidelines represent the first update on the topic since 2009, Dr. Yancy said in an interview. Although the years between the documents are few, the strides in research have been many, he said.
"The emergence of new and important datasets generated the impetus for the 2013 guideline not as an update, but as a complete rewrite. All of the previously extant clinical practice guideline statements were subject to reanalysis, a change in level of evidence and most importantly, a change in the class of recommendation," he said.
The document is among the first in the United States to employ the concept of guideline-directed medical therapy (GDMT) – a new designation that allows clinicians to easily determine the best course of heart failure care for an individual patient. Schematic algorithms provide easy-to-follow treatment pathways that should be helpful for anyone who treats heart failure patients, from specialist to primary care provider, said Dr. Yancy.
A major focus of the guideline is treating heart failure with preserved ejection fraction (HFpEF), with the goal of preventing or delaying progression. HFpEF is "a real entity" that constitutes about half of heart failure diagnoses, Dr. Yancy said, but as yet, has no specific intervention.
Until research provides further answers, the best way to manage HFpEF is holistically. "About 90% of these patients have comorbid conditions like hypertension, coronary artery disease, diabetes, renal insufficiency and atrial fibrillation. In the absence of a specific intervention for HFpEF, focusing on these other conditions will provide us the opportunity to modify the natural history of this disease."
Dr. David E. Lanfear, a cardiologist specializing in advanced heart failure and transplantation at Henry Ford Hospital in Detroit, said the guidelines on HFpEF “are very reasonable. The recommendations appear similar to those in previous statements, on blood pressure control, volume control for symptoms, atrial fibrillation, and ischemia, without endorsing specific medications. The statement also eloquently points out the ways in which HFpEF represents a huge gap in the knowledge base.
The guidelines contain "critical" new indications for the use of aldosterone antagonists, Dr. Yancy said. The drugs saw a surge in use in the early 2000s, but the rush to embrace them brought challenges as well. "Some of the applications led to missteps resulting in elevated potassium levels and emergency admissions," Dr. Yancy said. Since then, additional trials have pinpointed the best ways to use aldosterone agonists in patients who have heart failure with reduced ejection fraction or cardiac injury after heart attack. Data now confirm their benefit in patients with mild and moderate disease, as well as those with more advanced disease.
"This is the first document in the United States to embrace the benefit of aldosterone antagonists for these patients," Dr. Yancy said. Provided that patient renal function is "reasonably intact," the drugs are a valuable addition to GDMT.
The guidelines also offer a refinement of the recommendations for cardiac resynchronization device therapy – another change supported by the results of recent, large-scale trials. "We now have three separate, well-done trials that suggest a significant benefit of cardiac resynchronization in patients with mild to moderate disease," Dr. Yancy said.
In addition to recommending the treatment for patients with mild to moderate disease, the guideline targets it more specifically. "We gave the greatest strength of recommendation for patients with a wide QRS interval and left bundle branch block, a modest recommendation for patients with a less wide interval, and an equivocal recommendation for those without left bundle branch block. We think this will allow better discrimination of those who are most likely to benefit from CRT from those unlikely to benefit."
There are also more plentiful data in favor of mechanical circulatory support for patients with advanced heart failure. "This is no longer a proof of concept strategy," Dr. Yancy said. "Left ventricular assist devices for advanced chronic heart failure represent an important component of a contemporary treatment algorithm for heart failure."
The guideline even reaches past the mechanics of heart failure into its possible genetic origins. "We’ve discovered that idiopathic dilated cardiomyopathy may not really be idiopathic, but instead related to genetic abnormality. We’ve coalesced observations and data from this emerging field to come up with recommendations about when we might consider genetic testing in patients and screening in family members. It’s something new, and we’re delighted that it’s presented in this document."
The guidelines also offer a new outlook on reducing heart failure readmissions – a problem that comes with a $25 billion/year price tag, Dr. Yancy said. Four simple, low-tech interventions stood out as practical and effective:
• Using in-hospital systems that identify heart failure patients appropriate for GDMT and prompt physicians to advance this care and assess response.
• Developing transitional care and discharge planning that emphasizes patient education to increase treatment compliance, manage comorbid conditions effectively, and tackle psychosocial barriers to care.
• Harnessing the cooperative power of a nurse-led multidisciplinary heart failure disease management program.
• Following up every patient with a phone call within 3 days of discharge and a physician appointment within 2 weeks.
"The beauty of this is that while everyone is looking for the silver bullet to decrease readmission – including high-tech interventions like device implantation and home electronic monitoring – we believe that these simple, straightforward, evidence-based approaches will work."
Finally, Dr. Yancy said, document places great importance on patient-centric outcomes like quality-of-life issues, shared decision-making, care coordination, and palliative care. Over the past decade, the physician/patient relationship has changed from almost paternalistic to an active partnership. "We need to include the patient’s point of view in this whole process. We need to put a greater emphasis on quality of life, and we need not fear a discussion on quality of death."
Dr. Yancy expressed a firm belief that integrating the guidelines into daily practice could have an enormous impact on the way heart failure patients are treated.
"We have come so far in our understanding and ability to treat these patients. These are dramatically effective interventions. We can shift the culture to the belief that heart failure is something that we can treat – to the idea that you can help your patients feel better and live longer. If we use this correctly, we can make a difference."
Dr. Yancy had no financial declarations.
The newest heart failure management guidelines make a bold statement: Heart failure should no longer be considered a death sentence, but can instead be managed in a way that may add years of quality life for some patients.
“With optimal therapy applied to the right patient in the right manner at the right time, the risk of death can be markedly reduced, perhaps by as much as 50%. Treating fewer than 10 patients with all the correct therapies will easily save at least one life and one or more hospitalizations. Those are real benefits that dwarf the benefit of many of our other cardiovascular therapies,” said Dr. Clyde W. Yancy, chair of the joint guidelines writing committee. The document was published in the June 5 online edition of the Journal of the American College of Cardiology (2013 [doi:10.1016/j.jacc.2013.05.019]).
"For so long, we had assumed that, by definition, heart failure was a fatal diagnosis – that all we could do was tell patients to get their affairs in order and perhaps make them feel a little better, but that death was almost a fait accompli," said Dr. Yancy, the Magerstadt Professor of Medicine and chief of cardiology at Northwestern University, Chicago. "But, in the past few years, a lot of tough work has been done showing there are effective therapies and that when given correctly, major improvements in survival do occur."
A joint effort of the American College of Cardiology Foundation and the American Heart Association, the 2013 Heart Failure Guidelines represent the first update on the topic since 2009, Dr. Yancy said in an interview. Although the years between the documents are few, the strides in research have been many, he said.
"The emergence of new and important datasets generated the impetus for the 2013 guideline not as an update, but as a complete rewrite. All of the previously extant clinical practice guideline statements were subject to reanalysis, a change in level of evidence and most importantly, a change in the class of recommendation," he said.
The document is among the first in the United States to employ the concept of guideline-directed medical therapy (GDMT) – a new designation that allows clinicians to easily determine the best course of heart failure care for an individual patient. Schematic algorithms provide easy-to-follow treatment pathways that should be helpful for anyone who treats heart failure patients, from specialist to primary care provider, said Dr. Yancy.
A major focus of the guideline is treating heart failure with preserved ejection fraction (HFpEF), with the goal of preventing or delaying progression. HFpEF is "a real entity" that constitutes about half of heart failure diagnoses, Dr. Yancy said, but as yet, has no specific intervention.
Until research provides further answers, the best way to manage HFpEF is holistically. "About 90% of these patients have comorbid conditions like hypertension, coronary artery disease, diabetes, renal insufficiency and atrial fibrillation. In the absence of a specific intervention for HFpEF, focusing on these other conditions will provide us the opportunity to modify the natural history of this disease."
Dr. David E. Lanfear, a cardiologist specializing in advanced heart failure and transplantation at Henry Ford Hospital in Detroit, said the guidelines on HFpEF “are very reasonable. The recommendations appear similar to those in previous statements, on blood pressure control, volume control for symptoms, atrial fibrillation, and ischemia, without endorsing specific medications. The statement also eloquently points out the ways in which HFpEF represents a huge gap in the knowledge base.
The guidelines contain "critical" new indications for the use of aldosterone antagonists, Dr. Yancy said. The drugs saw a surge in use in the early 2000s, but the rush to embrace them brought challenges as well. "Some of the applications led to missteps resulting in elevated potassium levels and emergency admissions," Dr. Yancy said. Since then, additional trials have pinpointed the best ways to use aldosterone agonists in patients who have heart failure with reduced ejection fraction or cardiac injury after heart attack. Data now confirm their benefit in patients with mild and moderate disease, as well as those with more advanced disease.
"This is the first document in the United States to embrace the benefit of aldosterone antagonists for these patients," Dr. Yancy said. Provided that patient renal function is "reasonably intact," the drugs are a valuable addition to GDMT.
The guidelines also offer a refinement of the recommendations for cardiac resynchronization device therapy – another change supported by the results of recent, large-scale trials. "We now have three separate, well-done trials that suggest a significant benefit of cardiac resynchronization in patients with mild to moderate disease," Dr. Yancy said.
In addition to recommending the treatment for patients with mild to moderate disease, the guideline targets it more specifically. "We gave the greatest strength of recommendation for patients with a wide QRS interval and left bundle branch block, a modest recommendation for patients with a less wide interval, and an equivocal recommendation for those without left bundle branch block. We think this will allow better discrimination of those who are most likely to benefit from CRT from those unlikely to benefit."
There are also more plentiful data in favor of mechanical circulatory support for patients with advanced heart failure. "This is no longer a proof of concept strategy," Dr. Yancy said. "Left ventricular assist devices for advanced chronic heart failure represent an important component of a contemporary treatment algorithm for heart failure."
The guideline even reaches past the mechanics of heart failure into its possible genetic origins. "We’ve discovered that idiopathic dilated cardiomyopathy may not really be idiopathic, but instead related to genetic abnormality. We’ve coalesced observations and data from this emerging field to come up with recommendations about when we might consider genetic testing in patients and screening in family members. It’s something new, and we’re delighted that it’s presented in this document."
The guidelines also offer a new outlook on reducing heart failure readmissions – a problem that comes with a $25 billion/year price tag, Dr. Yancy said. Four simple, low-tech interventions stood out as practical and effective:
• Using in-hospital systems that identify heart failure patients appropriate for GDMT and prompt physicians to advance this care and assess response.
• Developing transitional care and discharge planning that emphasizes patient education to increase treatment compliance, manage comorbid conditions effectively, and tackle psychosocial barriers to care.
• Harnessing the cooperative power of a nurse-led multidisciplinary heart failure disease management program.
• Following up every patient with a phone call within 3 days of discharge and a physician appointment within 2 weeks.
"The beauty of this is that while everyone is looking for the silver bullet to decrease readmission – including high-tech interventions like device implantation and home electronic monitoring – we believe that these simple, straightforward, evidence-based approaches will work."
Finally, Dr. Yancy said, document places great importance on patient-centric outcomes like quality-of-life issues, shared decision-making, care coordination, and palliative care. Over the past decade, the physician/patient relationship has changed from almost paternalistic to an active partnership. "We need to include the patient’s point of view in this whole process. We need to put a greater emphasis on quality of life, and we need not fear a discussion on quality of death."
Dr. Yancy expressed a firm belief that integrating the guidelines into daily practice could have an enormous impact on the way heart failure patients are treated.
"We have come so far in our understanding and ability to treat these patients. These are dramatically effective interventions. We can shift the culture to the belief that heart failure is something that we can treat – to the idea that you can help your patients feel better and live longer. If we use this correctly, we can make a difference."
Dr. Yancy had no financial declarations.
The newest heart failure management guidelines make a bold statement: Heart failure should no longer be considered a death sentence, but can instead be managed in a way that may add years of quality life for some patients.
“With optimal therapy applied to the right patient in the right manner at the right time, the risk of death can be markedly reduced, perhaps by as much as 50%. Treating fewer than 10 patients with all the correct therapies will easily save at least one life and one or more hospitalizations. Those are real benefits that dwarf the benefit of many of our other cardiovascular therapies,” said Dr. Clyde W. Yancy, chair of the joint guidelines writing committee. The document was published in the June 5 online edition of the Journal of the American College of Cardiology (2013 [doi:10.1016/j.jacc.2013.05.019]).
"For so long, we had assumed that, by definition, heart failure was a fatal diagnosis – that all we could do was tell patients to get their affairs in order and perhaps make them feel a little better, but that death was almost a fait accompli," said Dr. Yancy, the Magerstadt Professor of Medicine and chief of cardiology at Northwestern University, Chicago. "But, in the past few years, a lot of tough work has been done showing there are effective therapies and that when given correctly, major improvements in survival do occur."
A joint effort of the American College of Cardiology Foundation and the American Heart Association, the 2013 Heart Failure Guidelines represent the first update on the topic since 2009, Dr. Yancy said in an interview. Although the years between the documents are few, the strides in research have been many, he said.
"The emergence of new and important datasets generated the impetus for the 2013 guideline not as an update, but as a complete rewrite. All of the previously extant clinical practice guideline statements were subject to reanalysis, a change in level of evidence and most importantly, a change in the class of recommendation," he said.
The document is among the first in the United States to employ the concept of guideline-directed medical therapy (GDMT) – a new designation that allows clinicians to easily determine the best course of heart failure care for an individual patient. Schematic algorithms provide easy-to-follow treatment pathways that should be helpful for anyone who treats heart failure patients, from specialist to primary care provider, said Dr. Yancy.
A major focus of the guideline is treating heart failure with preserved ejection fraction (HFpEF), with the goal of preventing or delaying progression. HFpEF is "a real entity" that constitutes about half of heart failure diagnoses, Dr. Yancy said, but as yet, has no specific intervention.
Until research provides further answers, the best way to manage HFpEF is holistically. "About 90% of these patients have comorbid conditions like hypertension, coronary artery disease, diabetes, renal insufficiency and atrial fibrillation. In the absence of a specific intervention for HFpEF, focusing on these other conditions will provide us the opportunity to modify the natural history of this disease."
Dr. David E. Lanfear, a cardiologist specializing in advanced heart failure and transplantation at Henry Ford Hospital in Detroit, said the guidelines on HFpEF “are very reasonable. The recommendations appear similar to those in previous statements, on blood pressure control, volume control for symptoms, atrial fibrillation, and ischemia, without endorsing specific medications. The statement also eloquently points out the ways in which HFpEF represents a huge gap in the knowledge base.
The guidelines contain "critical" new indications for the use of aldosterone antagonists, Dr. Yancy said. The drugs saw a surge in use in the early 2000s, but the rush to embrace them brought challenges as well. "Some of the applications led to missteps resulting in elevated potassium levels and emergency admissions," Dr. Yancy said. Since then, additional trials have pinpointed the best ways to use aldosterone agonists in patients who have heart failure with reduced ejection fraction or cardiac injury after heart attack. Data now confirm their benefit in patients with mild and moderate disease, as well as those with more advanced disease.
"This is the first document in the United States to embrace the benefit of aldosterone antagonists for these patients," Dr. Yancy said. Provided that patient renal function is "reasonably intact," the drugs are a valuable addition to GDMT.
The guidelines also offer a refinement of the recommendations for cardiac resynchronization device therapy – another change supported by the results of recent, large-scale trials. "We now have three separate, well-done trials that suggest a significant benefit of cardiac resynchronization in patients with mild to moderate disease," Dr. Yancy said.
In addition to recommending the treatment for patients with mild to moderate disease, the guideline targets it more specifically. "We gave the greatest strength of recommendation for patients with a wide QRS interval and left bundle branch block, a modest recommendation for patients with a less wide interval, and an equivocal recommendation for those without left bundle branch block. We think this will allow better discrimination of those who are most likely to benefit from CRT from those unlikely to benefit."
There are also more plentiful data in favor of mechanical circulatory support for patients with advanced heart failure. "This is no longer a proof of concept strategy," Dr. Yancy said. "Left ventricular assist devices for advanced chronic heart failure represent an important component of a contemporary treatment algorithm for heart failure."
The guideline even reaches past the mechanics of heart failure into its possible genetic origins. "We’ve discovered that idiopathic dilated cardiomyopathy may not really be idiopathic, but instead related to genetic abnormality. We’ve coalesced observations and data from this emerging field to come up with recommendations about when we might consider genetic testing in patients and screening in family members. It’s something new, and we’re delighted that it’s presented in this document."
The guidelines also offer a new outlook on reducing heart failure readmissions – a problem that comes with a $25 billion/year price tag, Dr. Yancy said. Four simple, low-tech interventions stood out as practical and effective:
• Using in-hospital systems that identify heart failure patients appropriate for GDMT and prompt physicians to advance this care and assess response.
• Developing transitional care and discharge planning that emphasizes patient education to increase treatment compliance, manage comorbid conditions effectively, and tackle psychosocial barriers to care.
• Harnessing the cooperative power of a nurse-led multidisciplinary heart failure disease management program.
• Following up every patient with a phone call within 3 days of discharge and a physician appointment within 2 weeks.
"The beauty of this is that while everyone is looking for the silver bullet to decrease readmission – including high-tech interventions like device implantation and home electronic monitoring – we believe that these simple, straightforward, evidence-based approaches will work."
Finally, Dr. Yancy said, document places great importance on patient-centric outcomes like quality-of-life issues, shared decision-making, care coordination, and palliative care. Over the past decade, the physician/patient relationship has changed from almost paternalistic to an active partnership. "We need to include the patient’s point of view in this whole process. We need to put a greater emphasis on quality of life, and we need not fear a discussion on quality of death."
Dr. Yancy expressed a firm belief that integrating the guidelines into daily practice could have an enormous impact on the way heart failure patients are treated.
"We have come so far in our understanding and ability to treat these patients. These are dramatically effective interventions. We can shift the culture to the belief that heart failure is something that we can treat – to the idea that you can help your patients feel better and live longer. If we use this correctly, we can make a difference."
Dr. Yancy had no financial declarations.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Wearable defibrillator vest useful as bridge to ICD
DENVER – The LifeVest wearable automatic defibrillator provides a safe and highly effective bridging strategy while physicians decide whether a patient should get an implantable cardioverter defibrillator, according to findings from the WEARIT-II registry.
Experience gleaned from the first 882 of a planned 3,000 patients to be enrolled in this prospective, real-world registry indicates the defibrillator vest consistently recognizes and safely terminates life-threatening arrhythmias while avoiding unnecessary shocks for non–life-threatening arrhythmic events, Dr. Ilan Goldenberg reported at the annual meeting of the Heart Rhythm Society.
Indeed, the inappropriate shock rate in WEARIT-II was a mere 0.3%, far lower than with contemporary implantable cardioverter defibrillator (ICD) therapy, noted Dr. Goldenberg of the University of Rochester (N.Y.).
This bridging strategy deserves broad use in high-risk populations, he said. The WEARIT-II data show that bridging is particularly useful as a means of protecting patients with a transient arrhythmia risk as well as those whose long-term arrhythmia risk is undefined and requires further evaluation. Examples include patients who are post MI, or have new-onset heart failure with a depressed left ventricular ejection fraction (LVEF) of 35% or less, have recently undergone coronary revascularization, or are undergoing detailed evaluation of a possible inherited or congenital arrhythmic disorder.
Guidelines state that an ICD is indicated for primary prevention of sudden cardiac death in patients with an LVEF of 35% or less, but not within 40 days following an MI, or within 3 months after diagnosis of heart failure, or within 90 days following coronary revascularization. The reason for these mandatory delays is that many patients will experience improvement in LVEF in response to medical therapy such that they no longer qualify for ICD implantation. But if their physician is concerned about their arrhythmia risk during that waiting period, the wearable defibrillator is an excellent solution, Dr. Goldenberg continued.
There is a clear need for more selective prescription of ICDs for primary prevention of cardiac arrest. This was already evident more than a decade ago, when the MADIT-II (Multicenter Automatic Defibrillator Trial–II) demonstrated that only one-third of patients received appropriate ICD therapy during 4 years of follow-up (N. Engl. J. Med. 2002;346:877-83). More recently, the MADIT-Reduce Inappropriate Therapy trial reported that participating ICD recipients had a low appropriate shock rate of 3 shocks per 100 patient-years (N. Engl. J. Med. 2012;367:2275-83) . The wearable defibrillator bridging strategy offers a means of safely being more selective in ICD placement. Patients whose risk isn’t yet clearly defined can in effect have a nonpermanent trial run of up to 6 months’ duration using the LifeVest, the electrophysiologist explained.
The LifeVest is commercially available and routinely covered by insurers. It’s a thin, lightweight vest designed to be worn under clothes. It is attached to a waist battery pack. The LifeVest provides continuous heart rhythm monitoring and automatic defibrillation upon detection of a potentially fatal ventricular arrhythmia. The device has an override button that enables a patient experiencing a sustained ventricular tachyarrhythmia (VT) to allow the event to terminate spontaneously, thereby avoiding unnecessary shocks. Should the patient pass out while the VT continues, the LifeVest will deliver shock therapy.
The 882 patients in the WEAR-IT registry had a mean LVEF of 25%. A total of 771 patients had acquired heart conditions, most commonly nonischemic or ischemic cardiomyopathy. The other 111 patients had inherited or congenital conditions, such as long QT syndrome. Patients wore the LifeVest for an average of 81 days and for a mean of 21 hours daily.
Appropriate LifeVest shock therapy that terminated life-threatening fast VT or ventricular fibrillation occurred at a rate of 9 events per 100 patient-years. Sustained VT that was allowed to spontaneously terminate as a result of the patient’s use of the device’s override button occurred at a rate of 27 events per 100 patient-years.
The device also detected nonsustained VT at a rate of 47 events per 100 patient-years, atrial arrhythmias and other supraventricular tachycardias at 64 events per 100 patient-years, and asystole at 3 events per 100 patient-years.
Four deaths occurred. Three of those happened when the patient was not wearing the vest; the fourth was caused by asystole.
Upon ending their use of the LifeVest, 41% of patients did not receive an ICD because their LVEF improved. Moreover, the arrhythmias detected by the LifeVest affected patient disposition: 80% of patients who received an appropriate shock from the vest got an ICD, as did fewer than 40% of those with no arrhythmias detected during vest wear.
Patients within 40 days post MI or 90 days post revascularization had the highest arrhythmic event rates among those with acquired heart conditions; however, the event rate was even higher among those with inherited or congenital conditions.
Dr. Michael R. Gold commented that in his experience, another important group of candidates for the wearable defibrillator are arrhythmia-prone patients who develop a cardiac device infection requiring device removal.
"You’re worried about that patient yet you can’t implant another device because it may take weeks or months to clear the infection," noted Dr. Gold, professor of medicine, chief of cardiology, and medical director of the heart and vascular center at the Medical University of South Carolina, Charleston.
Dr. Goldenberg reported having received research grants from Zoll Medical, which sponsors the WEARIT-II registry and markets the LifeVest, as well as from Boston Scientific.
DENVER – The LifeVest wearable automatic defibrillator provides a safe and highly effective bridging strategy while physicians decide whether a patient should get an implantable cardioverter defibrillator, according to findings from the WEARIT-II registry.
Experience gleaned from the first 882 of a planned 3,000 patients to be enrolled in this prospective, real-world registry indicates the defibrillator vest consistently recognizes and safely terminates life-threatening arrhythmias while avoiding unnecessary shocks for non–life-threatening arrhythmic events, Dr. Ilan Goldenberg reported at the annual meeting of the Heart Rhythm Society.
Indeed, the inappropriate shock rate in WEARIT-II was a mere 0.3%, far lower than with contemporary implantable cardioverter defibrillator (ICD) therapy, noted Dr. Goldenberg of the University of Rochester (N.Y.).
This bridging strategy deserves broad use in high-risk populations, he said. The WEARIT-II data show that bridging is particularly useful as a means of protecting patients with a transient arrhythmia risk as well as those whose long-term arrhythmia risk is undefined and requires further evaluation. Examples include patients who are post MI, or have new-onset heart failure with a depressed left ventricular ejection fraction (LVEF) of 35% or less, have recently undergone coronary revascularization, or are undergoing detailed evaluation of a possible inherited or congenital arrhythmic disorder.
Guidelines state that an ICD is indicated for primary prevention of sudden cardiac death in patients with an LVEF of 35% or less, but not within 40 days following an MI, or within 3 months after diagnosis of heart failure, or within 90 days following coronary revascularization. The reason for these mandatory delays is that many patients will experience improvement in LVEF in response to medical therapy such that they no longer qualify for ICD implantation. But if their physician is concerned about their arrhythmia risk during that waiting period, the wearable defibrillator is an excellent solution, Dr. Goldenberg continued.
There is a clear need for more selective prescription of ICDs for primary prevention of cardiac arrest. This was already evident more than a decade ago, when the MADIT-II (Multicenter Automatic Defibrillator Trial–II) demonstrated that only one-third of patients received appropriate ICD therapy during 4 years of follow-up (N. Engl. J. Med. 2002;346:877-83). More recently, the MADIT-Reduce Inappropriate Therapy trial reported that participating ICD recipients had a low appropriate shock rate of 3 shocks per 100 patient-years (N. Engl. J. Med. 2012;367:2275-83) . The wearable defibrillator bridging strategy offers a means of safely being more selective in ICD placement. Patients whose risk isn’t yet clearly defined can in effect have a nonpermanent trial run of up to 6 months’ duration using the LifeVest, the electrophysiologist explained.
The LifeVest is commercially available and routinely covered by insurers. It’s a thin, lightweight vest designed to be worn under clothes. It is attached to a waist battery pack. The LifeVest provides continuous heart rhythm monitoring and automatic defibrillation upon detection of a potentially fatal ventricular arrhythmia. The device has an override button that enables a patient experiencing a sustained ventricular tachyarrhythmia (VT) to allow the event to terminate spontaneously, thereby avoiding unnecessary shocks. Should the patient pass out while the VT continues, the LifeVest will deliver shock therapy.
The 882 patients in the WEAR-IT registry had a mean LVEF of 25%. A total of 771 patients had acquired heart conditions, most commonly nonischemic or ischemic cardiomyopathy. The other 111 patients had inherited or congenital conditions, such as long QT syndrome. Patients wore the LifeVest for an average of 81 days and for a mean of 21 hours daily.
Appropriate LifeVest shock therapy that terminated life-threatening fast VT or ventricular fibrillation occurred at a rate of 9 events per 100 patient-years. Sustained VT that was allowed to spontaneously terminate as a result of the patient’s use of the device’s override button occurred at a rate of 27 events per 100 patient-years.
The device also detected nonsustained VT at a rate of 47 events per 100 patient-years, atrial arrhythmias and other supraventricular tachycardias at 64 events per 100 patient-years, and asystole at 3 events per 100 patient-years.
Four deaths occurred. Three of those happened when the patient was not wearing the vest; the fourth was caused by asystole.
Upon ending their use of the LifeVest, 41% of patients did not receive an ICD because their LVEF improved. Moreover, the arrhythmias detected by the LifeVest affected patient disposition: 80% of patients who received an appropriate shock from the vest got an ICD, as did fewer than 40% of those with no arrhythmias detected during vest wear.
Patients within 40 days post MI or 90 days post revascularization had the highest arrhythmic event rates among those with acquired heart conditions; however, the event rate was even higher among those with inherited or congenital conditions.
Dr. Michael R. Gold commented that in his experience, another important group of candidates for the wearable defibrillator are arrhythmia-prone patients who develop a cardiac device infection requiring device removal.
"You’re worried about that patient yet you can’t implant another device because it may take weeks or months to clear the infection," noted Dr. Gold, professor of medicine, chief of cardiology, and medical director of the heart and vascular center at the Medical University of South Carolina, Charleston.
Dr. Goldenberg reported having received research grants from Zoll Medical, which sponsors the WEARIT-II registry and markets the LifeVest, as well as from Boston Scientific.
DENVER – The LifeVest wearable automatic defibrillator provides a safe and highly effective bridging strategy while physicians decide whether a patient should get an implantable cardioverter defibrillator, according to findings from the WEARIT-II registry.
Experience gleaned from the first 882 of a planned 3,000 patients to be enrolled in this prospective, real-world registry indicates the defibrillator vest consistently recognizes and safely terminates life-threatening arrhythmias while avoiding unnecessary shocks for non–life-threatening arrhythmic events, Dr. Ilan Goldenberg reported at the annual meeting of the Heart Rhythm Society.
Indeed, the inappropriate shock rate in WEARIT-II was a mere 0.3%, far lower than with contemporary implantable cardioverter defibrillator (ICD) therapy, noted Dr. Goldenberg of the University of Rochester (N.Y.).
This bridging strategy deserves broad use in high-risk populations, he said. The WEARIT-II data show that bridging is particularly useful as a means of protecting patients with a transient arrhythmia risk as well as those whose long-term arrhythmia risk is undefined and requires further evaluation. Examples include patients who are post MI, or have new-onset heart failure with a depressed left ventricular ejection fraction (LVEF) of 35% or less, have recently undergone coronary revascularization, or are undergoing detailed evaluation of a possible inherited or congenital arrhythmic disorder.
Guidelines state that an ICD is indicated for primary prevention of sudden cardiac death in patients with an LVEF of 35% or less, but not within 40 days following an MI, or within 3 months after diagnosis of heart failure, or within 90 days following coronary revascularization. The reason for these mandatory delays is that many patients will experience improvement in LVEF in response to medical therapy such that they no longer qualify for ICD implantation. But if their physician is concerned about their arrhythmia risk during that waiting period, the wearable defibrillator is an excellent solution, Dr. Goldenberg continued.
There is a clear need for more selective prescription of ICDs for primary prevention of cardiac arrest. This was already evident more than a decade ago, when the MADIT-II (Multicenter Automatic Defibrillator Trial–II) demonstrated that only one-third of patients received appropriate ICD therapy during 4 years of follow-up (N. Engl. J. Med. 2002;346:877-83). More recently, the MADIT-Reduce Inappropriate Therapy trial reported that participating ICD recipients had a low appropriate shock rate of 3 shocks per 100 patient-years (N. Engl. J. Med. 2012;367:2275-83) . The wearable defibrillator bridging strategy offers a means of safely being more selective in ICD placement. Patients whose risk isn’t yet clearly defined can in effect have a nonpermanent trial run of up to 6 months’ duration using the LifeVest, the electrophysiologist explained.
The LifeVest is commercially available and routinely covered by insurers. It’s a thin, lightweight vest designed to be worn under clothes. It is attached to a waist battery pack. The LifeVest provides continuous heart rhythm monitoring and automatic defibrillation upon detection of a potentially fatal ventricular arrhythmia. The device has an override button that enables a patient experiencing a sustained ventricular tachyarrhythmia (VT) to allow the event to terminate spontaneously, thereby avoiding unnecessary shocks. Should the patient pass out while the VT continues, the LifeVest will deliver shock therapy.
The 882 patients in the WEAR-IT registry had a mean LVEF of 25%. A total of 771 patients had acquired heart conditions, most commonly nonischemic or ischemic cardiomyopathy. The other 111 patients had inherited or congenital conditions, such as long QT syndrome. Patients wore the LifeVest for an average of 81 days and for a mean of 21 hours daily.
Appropriate LifeVest shock therapy that terminated life-threatening fast VT or ventricular fibrillation occurred at a rate of 9 events per 100 patient-years. Sustained VT that was allowed to spontaneously terminate as a result of the patient’s use of the device’s override button occurred at a rate of 27 events per 100 patient-years.
The device also detected nonsustained VT at a rate of 47 events per 100 patient-years, atrial arrhythmias and other supraventricular tachycardias at 64 events per 100 patient-years, and asystole at 3 events per 100 patient-years.
Four deaths occurred. Three of those happened when the patient was not wearing the vest; the fourth was caused by asystole.
Upon ending their use of the LifeVest, 41% of patients did not receive an ICD because their LVEF improved. Moreover, the arrhythmias detected by the LifeVest affected patient disposition: 80% of patients who received an appropriate shock from the vest got an ICD, as did fewer than 40% of those with no arrhythmias detected during vest wear.
Patients within 40 days post MI or 90 days post revascularization had the highest arrhythmic event rates among those with acquired heart conditions; however, the event rate was even higher among those with inherited or congenital conditions.
Dr. Michael R. Gold commented that in his experience, another important group of candidates for the wearable defibrillator are arrhythmia-prone patients who develop a cardiac device infection requiring device removal.
"You’re worried about that patient yet you can’t implant another device because it may take weeks or months to clear the infection," noted Dr. Gold, professor of medicine, chief of cardiology, and medical director of the heart and vascular center at the Medical University of South Carolina, Charleston.
Dr. Goldenberg reported having received research grants from Zoll Medical, which sponsors the WEARIT-II registry and markets the LifeVest, as well as from Boston Scientific.
AT HEART RHYTHM 2013
Major finding: During an average of 81 days using the LifeVest wearable automatic defibrillator, patients experienced appropriate device shocks to terminate potentially fatal ventricular tachyarrhythmia/ventricular fibrillation at a rate of 9 events per 100 person-years while appropriately avoiding shocks for sustained VT with spontaneous termination at a rate of 27 events per 100 person-years. Only 0.3% of 882 vest users experienced an inappropriate shock.
Data source: The WEARIT-II registry, which to date includes 882 patients who have been prescribed the LifeVest wearable defibrillator.
Disclosures: The registry is sponsored by Zoll Medical, which markets the LifeVest. The presenter said he has received research grants from the company.
Biventricular pacing bests conventional tx in BLOCK HF trial
DENVER – Biventricular pacing rather than the right ventricular pacing recommended in current guidelines results in significantly better quality of life and symptom status, an analysis of secondary outcomes from the BLOCK HF trial have shown.
In a previous report of the trial’s primary outcome (N. Engl. J. Med. 2013;368:1585-93), the biventricular (BiV) pacing group showed a highly significant 26% reduction in risk of a composite of all-cause mortality, an urgent care visit for heart failure requiring intravenous therapy, or a 15% or greater increase in the left ventricular end-systolic volume index during an average of 37 months of follow-up, compared with patients who had right ventricular (RV) pacing.
The new report on prespecified secondary endpoints including quality of life and symptom status was undertaken because of researchers’ and regulatory agencies’ growing appreciation of the importance of such outcomes in patients with a chronic progressive disease such as heart failure, principal investigator Dr. Anne B. Curtis reported at the annual meeting of the Heart Rhythm Society.
BLOCK HF (Biventricular versus Right Ventricular Pacing in Patients with Left Ventricular Dysfunction and Atrioventricular Block) was a randomized, double-blind, prospective, 60-center clinical trial involving 691 patients in the United States and Canada. All had AV block warranting pacemaker therapy as well as New York Heart Association(NYHA) class I-III heart failure with a left ventricular ejection fraction of 50% or less. All participants got a cardiac resynchronization therapy device featuring BiV pacing. Patients were assigned in double-blind fashion for their device to run in BiV or conventional RV pacing mode, explained Dr. Curtis, professor and chair of the department of medicine at the University of Buffalo (N.Y.).
The new secondary analysis tabulated changes in the Packer clinical composite score, NYHA functional class, and quality of life, as measured by the Minnesota Living With Heart Failure Questionnaire, at 6, 12, 18, and 24 months.
The Packer clinical composite score classifies patients as improved, worsened, or unchanged based on patient global assessment, heart failure hospitalization, change in symptoms, and other factors. At all four time points through 24 months, significantly more patients in the BiV group were rated as improved and significantly fewer as worsened than in the RV group. For example, at 6 months 53% of the BiV group were categorized as improved and 23% as worsened, compared with 39% and 28% in the RV group, Dr. Curtis reported.
From a mean baseline quality of life score of 25, the BiV group improved by an average of 5 points at the 6-month assessment compared with a 0.3-point gain in the RV group. At 12 months, the BiV group still showed a 3.9-point improvement, significantly better than the average 0.9-point gain in the RV patients. At 18 and 24 months, however, there was no longer a significant difference between the two groups in terms of quality of life scores. Dr. Curtis attributed this drop-off to the fact that 86 patients in the RV arm crossed over to BiV pacing because of deteriorating heart failure, compared with 15 crossovers in the BiV group. In the intention-to-treat analysis employed in BLOCK HF, those crossovers to BiV pacing are still counted as being part of the RV group.
"If you could mandate that patients stayed in the same arm, I think you’d continue to see differences over time, but you can’t do that," she said.
An analysis of changes in NYHA functional class showed the BiV group had better outcomes at 12 months, but at not the other time points.
The BLOCK HF trial was undertaken in response to evidence suggesting that sustained RV apical pacing can degrade ventricular function, especially in patients with preexisting systolic dysfunction.
At present, cardiac resynchronization therapy devices aren’t approved by the Food and Drug Administration for patients with AV block with left ventricular dysfunction. Medtronic officials have indicated they plan to seek an expanded indication based on the BLOCK HF data.
One audience member, noting that the study outcomes were better with BiV pacing in patients across the full spectrum of depressed ejection fractions, asked Dr. Curtis if she expects an expansion of cardiac resynchronization therapy indications to include patients with heart block and a normal ejection fraction.
"I would anticipate that the guidelines will change for the type of patients studied here. But we didn’t study patients with a normal ejection fraction because the more normal the patient, the larger the sample size and longer the follow-up you’d need to show a difference. So I doubt that this study will change guidelines for patients with normal ejection fractions," she said.
Dr. Curtis reported that she serves as a consultant to Medtronic, which sponsored the BLOCK HF trial,
DENVER – Biventricular pacing rather than the right ventricular pacing recommended in current guidelines results in significantly better quality of life and symptom status, an analysis of secondary outcomes from the BLOCK HF trial have shown.
In a previous report of the trial’s primary outcome (N. Engl. J. Med. 2013;368:1585-93), the biventricular (BiV) pacing group showed a highly significant 26% reduction in risk of a composite of all-cause mortality, an urgent care visit for heart failure requiring intravenous therapy, or a 15% or greater increase in the left ventricular end-systolic volume index during an average of 37 months of follow-up, compared with patients who had right ventricular (RV) pacing.
The new report on prespecified secondary endpoints including quality of life and symptom status was undertaken because of researchers’ and regulatory agencies’ growing appreciation of the importance of such outcomes in patients with a chronic progressive disease such as heart failure, principal investigator Dr. Anne B. Curtis reported at the annual meeting of the Heart Rhythm Society.
BLOCK HF (Biventricular versus Right Ventricular Pacing in Patients with Left Ventricular Dysfunction and Atrioventricular Block) was a randomized, double-blind, prospective, 60-center clinical trial involving 691 patients in the United States and Canada. All had AV block warranting pacemaker therapy as well as New York Heart Association(NYHA) class I-III heart failure with a left ventricular ejection fraction of 50% or less. All participants got a cardiac resynchronization therapy device featuring BiV pacing. Patients were assigned in double-blind fashion for their device to run in BiV or conventional RV pacing mode, explained Dr. Curtis, professor and chair of the department of medicine at the University of Buffalo (N.Y.).
The new secondary analysis tabulated changes in the Packer clinical composite score, NYHA functional class, and quality of life, as measured by the Minnesota Living With Heart Failure Questionnaire, at 6, 12, 18, and 24 months.
The Packer clinical composite score classifies patients as improved, worsened, or unchanged based on patient global assessment, heart failure hospitalization, change in symptoms, and other factors. At all four time points through 24 months, significantly more patients in the BiV group were rated as improved and significantly fewer as worsened than in the RV group. For example, at 6 months 53% of the BiV group were categorized as improved and 23% as worsened, compared with 39% and 28% in the RV group, Dr. Curtis reported.
From a mean baseline quality of life score of 25, the BiV group improved by an average of 5 points at the 6-month assessment compared with a 0.3-point gain in the RV group. At 12 months, the BiV group still showed a 3.9-point improvement, significantly better than the average 0.9-point gain in the RV patients. At 18 and 24 months, however, there was no longer a significant difference between the two groups in terms of quality of life scores. Dr. Curtis attributed this drop-off to the fact that 86 patients in the RV arm crossed over to BiV pacing because of deteriorating heart failure, compared with 15 crossovers in the BiV group. In the intention-to-treat analysis employed in BLOCK HF, those crossovers to BiV pacing are still counted as being part of the RV group.
"If you could mandate that patients stayed in the same arm, I think you’d continue to see differences over time, but you can’t do that," she said.
An analysis of changes in NYHA functional class showed the BiV group had better outcomes at 12 months, but at not the other time points.
The BLOCK HF trial was undertaken in response to evidence suggesting that sustained RV apical pacing can degrade ventricular function, especially in patients with preexisting systolic dysfunction.
At present, cardiac resynchronization therapy devices aren’t approved by the Food and Drug Administration for patients with AV block with left ventricular dysfunction. Medtronic officials have indicated they plan to seek an expanded indication based on the BLOCK HF data.
One audience member, noting that the study outcomes were better with BiV pacing in patients across the full spectrum of depressed ejection fractions, asked Dr. Curtis if she expects an expansion of cardiac resynchronization therapy indications to include patients with heart block and a normal ejection fraction.
"I would anticipate that the guidelines will change for the type of patients studied here. But we didn’t study patients with a normal ejection fraction because the more normal the patient, the larger the sample size and longer the follow-up you’d need to show a difference. So I doubt that this study will change guidelines for patients with normal ejection fractions," she said.
Dr. Curtis reported that she serves as a consultant to Medtronic, which sponsored the BLOCK HF trial,
DENVER – Biventricular pacing rather than the right ventricular pacing recommended in current guidelines results in significantly better quality of life and symptom status, an analysis of secondary outcomes from the BLOCK HF trial have shown.
In a previous report of the trial’s primary outcome (N. Engl. J. Med. 2013;368:1585-93), the biventricular (BiV) pacing group showed a highly significant 26% reduction in risk of a composite of all-cause mortality, an urgent care visit for heart failure requiring intravenous therapy, or a 15% or greater increase in the left ventricular end-systolic volume index during an average of 37 months of follow-up, compared with patients who had right ventricular (RV) pacing.
The new report on prespecified secondary endpoints including quality of life and symptom status was undertaken because of researchers’ and regulatory agencies’ growing appreciation of the importance of such outcomes in patients with a chronic progressive disease such as heart failure, principal investigator Dr. Anne B. Curtis reported at the annual meeting of the Heart Rhythm Society.
BLOCK HF (Biventricular versus Right Ventricular Pacing in Patients with Left Ventricular Dysfunction and Atrioventricular Block) was a randomized, double-blind, prospective, 60-center clinical trial involving 691 patients in the United States and Canada. All had AV block warranting pacemaker therapy as well as New York Heart Association(NYHA) class I-III heart failure with a left ventricular ejection fraction of 50% or less. All participants got a cardiac resynchronization therapy device featuring BiV pacing. Patients were assigned in double-blind fashion for their device to run in BiV or conventional RV pacing mode, explained Dr. Curtis, professor and chair of the department of medicine at the University of Buffalo (N.Y.).
The new secondary analysis tabulated changes in the Packer clinical composite score, NYHA functional class, and quality of life, as measured by the Minnesota Living With Heart Failure Questionnaire, at 6, 12, 18, and 24 months.
The Packer clinical composite score classifies patients as improved, worsened, or unchanged based on patient global assessment, heart failure hospitalization, change in symptoms, and other factors. At all four time points through 24 months, significantly more patients in the BiV group were rated as improved and significantly fewer as worsened than in the RV group. For example, at 6 months 53% of the BiV group were categorized as improved and 23% as worsened, compared with 39% and 28% in the RV group, Dr. Curtis reported.
From a mean baseline quality of life score of 25, the BiV group improved by an average of 5 points at the 6-month assessment compared with a 0.3-point gain in the RV group. At 12 months, the BiV group still showed a 3.9-point improvement, significantly better than the average 0.9-point gain in the RV patients. At 18 and 24 months, however, there was no longer a significant difference between the two groups in terms of quality of life scores. Dr. Curtis attributed this drop-off to the fact that 86 patients in the RV arm crossed over to BiV pacing because of deteriorating heart failure, compared with 15 crossovers in the BiV group. In the intention-to-treat analysis employed in BLOCK HF, those crossovers to BiV pacing are still counted as being part of the RV group.
"If you could mandate that patients stayed in the same arm, I think you’d continue to see differences over time, but you can’t do that," she said.
An analysis of changes in NYHA functional class showed the BiV group had better outcomes at 12 months, but at not the other time points.
The BLOCK HF trial was undertaken in response to evidence suggesting that sustained RV apical pacing can degrade ventricular function, especially in patients with preexisting systolic dysfunction.
At present, cardiac resynchronization therapy devices aren’t approved by the Food and Drug Administration for patients with AV block with left ventricular dysfunction. Medtronic officials have indicated they plan to seek an expanded indication based on the BLOCK HF data.
One audience member, noting that the study outcomes were better with BiV pacing in patients across the full spectrum of depressed ejection fractions, asked Dr. Curtis if she expects an expansion of cardiac resynchronization therapy indications to include patients with heart block and a normal ejection fraction.
"I would anticipate that the guidelines will change for the type of patients studied here. But we didn’t study patients with a normal ejection fraction because the more normal the patient, the larger the sample size and longer the follow-up you’d need to show a difference. So I doubt that this study will change guidelines for patients with normal ejection fractions," she said.
Dr. Curtis reported that she serves as a consultant to Medtronic, which sponsored the BLOCK HF trial,
AT HEART RHYTHM 2013
Major finding: Fifty-three percent of patients with atrioventricular block and systolic dysfunction were rated as "improved" after 6 months of biventricular pacing, compared with just 39% of patients on conventional right ventricular pacing.
Data source: A prespecified secondary analysis of the BLOCK HF trial, a randomized, double-blind, prospective multicenter study involving 691 patients.
Disclosures: The BLOCK HF trial was sponsored by Medtronic. The presenter disclosed that she serves as a paid consultant to the device company.