User login
TRED-HF: Despite recovery, dilated cardiomyopathy returns after halting HF drugs
CHICAGO – Phased withdrawal of guideline-directed medical therapy in patients who seemed to have recovered from dilated cardiomyopathy resulted in relapses in 40% of patients within 6 months in the TRED-HF trial.
The clinical implications of this small pilot randomized trial are clear: “Withdrawal of therapy should not usually be attempted, at least until we can predict who’s going to relapse and who’s not,” Brian P. Halliday, MD, PhD, said at the American Heart Association scientific sessions.
“Improvement in function represents remission rather than permanent recovery for many patients,” added Dr. Halliday of Imperial College London.
The study was performed to address a question that arises with increasing frequency in clinical practice as a result of the impressive advances in heart failure therapy in recent years, he said. “Patients frequently come to us in clinic and ask us, ‘Do I need to continue to take these medications forever?’ They’re frequently young, and they want to know if they really need to be subject to 40 or 50 years of medication. Some are concerned about side effects, others are interested in pregnancy, and then there is the financial cost.”
Simultaneously published in The Lancet, TRED-HF was a single-center, open-label study of 51 patients who had prior dilated cardiomyopathy (DCM) and a median left ventricular ejection fraction (LVEF) of 25% at the time of diagnosis 4.9 years earlier and who subsequently recovered in response to therapy. That is, they became symptom-free with an LVEF greater than 50%, a normal left ventricular end diastolic volume index, and a reassuringly low median N-terminal pro b-type natriuretic peptide (NP-pro-BNP) level of 72 ng/L.
For the study, 25 patients were randomized to phased withdrawal of their heart failure drugs over a 16-week period: First they reduced or stopped loop diuretics, then mineralocorticoid antagonists, then beta-blockers, and finally their ACE inhibitor or angiotensin receptor blocker. The other 26 participants continued therapy during the first 6 months of the study, then 25 of the 26 crossed over to phased withdrawal. The outlier didn’t cross over because of atrial fibrillation.
The primary endpoint was relapse of DCM within 6 months of the start of the study. Relapse was defined as either a drop in LVEF of more than 10% to a level below 50%, at least a doubling of NT-pro-BNP to greater than 400 ng/L, clinical evidence of heart failure, or a greater than 10% increase in LV end diastolic volume as assessed by cardiac MRI.
The results
During the first half of the study, 11 of 25 patients (44%) relapsed during or after medication withdrawal. None of the controls relapsed. In the crossover phase, 9 of 25 patients (35%) relapsed in response to treatment withdrawal. Of the 20 patients who relapsed, 13 did so within 16 weeks of beginning medication withdrawal. Indeed, most patients relapsed within 8 weeks of their last medication. Ten of the twenty fulfilled multiple criteria for relapse.
Medication withdrawal was accompanied not only by a mean 9.5% reduction in LVEF, compared with baseline, but by a 15.4-bpm rise in heart rate, a 7.0–mm Hg increase in diastolic blood pressure, and 5.1-point deterioration in Kansas City Cardiomyopathy Questionnaire scores, demonstrating that what happened off treatment was true DCM recurrence and not simply an imaging artifact.
Everyone who relapsed immediately restarted treatment. At their next follow-up visit, all were once again asymptomatic, and 17 of the 20 (85%) had an LVEF greater than 50%. Two of the other three had an LVEF of 45%-50%, and the other had an LVEF of 43%.
“So they did seem to recover when they went back on medication,” Dr. Halliday observed.
Underpowered exploratory analyses designed for hypothesis generation identified several potential baseline predictors of DCM relapse, including older age, being on three or more heart failure drugs, and use of a mineralocorticoid antagonist.
Experts react
Designated discussant Jane E. Wilcox, MD, commented, “Currently, in 2018, we have no true signature of recovery. These patients are indeed in cardiac remission and have an indefinite indication for continuing their evidence-based medical therapy without interruption.”
“The clinical implication here is, I think, we should TRED-lightly,” quipped Dr. Wilcox of Northwestern University in Chicago.
Her own research indicates that even patients who have recovered their LVEF and no longer seem to have a heart failure phenotype still have an abnormal myocardial substrate as evidenced by persistent dysfunctional cardiac mechanics on echocardiography. Nonetheless, she remains optimistic.
“I don’t think [TRED-HF] squelches the future of myocardial recovery. I think it actually invigorates the field for an assessment of genomics, proteomics, and metabolomics looking for that true signature of cardiac recovery,” she said.
Donald Lloyd-Jones, MD, who chaired a press conference where Dr. Halliday presented the TRED-HF results, complimented the investigators for tackling what he termed “an incredibly important clinical question that comes up all the time.”
“I really want to commend the investigators for taking on what, on its face, might be an ethically challenging question by taking treatment away when we don’t know what the answer is likely to be. But they really checked all the boxes to make sure this was done in a very safe and monitored way, so that even though the outcome was what it turned out to be, the harm to patients was minimalized,” said Dr. Lloyd-Jones, professor and chair of the department of preventive medicine and director of the Northwestern University Clinical and Translational Sciences Institute, Chicago.
“No patient wants to be on more medication than they need to be, but I think for the time being this class of patients is going to have to be maintained on medications until we understand a little more,” Dr. Lloyd-Jones concluded.
Dr. Halliday reported having no financial conflicts regarding the study, funded by the British Heart Foundation.
SOURCE: Halliday BP. AHA scientific sessions, Abstract 18621. Simulpub The Lancet. 2018 Nov 11. doi: 10.1016/S0140-6736(18)32484-X.
CHICAGO – Phased withdrawal of guideline-directed medical therapy in patients who seemed to have recovered from dilated cardiomyopathy resulted in relapses in 40% of patients within 6 months in the TRED-HF trial.
The clinical implications of this small pilot randomized trial are clear: “Withdrawal of therapy should not usually be attempted, at least until we can predict who’s going to relapse and who’s not,” Brian P. Halliday, MD, PhD, said at the American Heart Association scientific sessions.
“Improvement in function represents remission rather than permanent recovery for many patients,” added Dr. Halliday of Imperial College London.
The study was performed to address a question that arises with increasing frequency in clinical practice as a result of the impressive advances in heart failure therapy in recent years, he said. “Patients frequently come to us in clinic and ask us, ‘Do I need to continue to take these medications forever?’ They’re frequently young, and they want to know if they really need to be subject to 40 or 50 years of medication. Some are concerned about side effects, others are interested in pregnancy, and then there is the financial cost.”
Simultaneously published in The Lancet, TRED-HF was a single-center, open-label study of 51 patients who had prior dilated cardiomyopathy (DCM) and a median left ventricular ejection fraction (LVEF) of 25% at the time of diagnosis 4.9 years earlier and who subsequently recovered in response to therapy. That is, they became symptom-free with an LVEF greater than 50%, a normal left ventricular end diastolic volume index, and a reassuringly low median N-terminal pro b-type natriuretic peptide (NP-pro-BNP) level of 72 ng/L.
For the study, 25 patients were randomized to phased withdrawal of their heart failure drugs over a 16-week period: First they reduced or stopped loop diuretics, then mineralocorticoid antagonists, then beta-blockers, and finally their ACE inhibitor or angiotensin receptor blocker. The other 26 participants continued therapy during the first 6 months of the study, then 25 of the 26 crossed over to phased withdrawal. The outlier didn’t cross over because of atrial fibrillation.
The primary endpoint was relapse of DCM within 6 months of the start of the study. Relapse was defined as either a drop in LVEF of more than 10% to a level below 50%, at least a doubling of NT-pro-BNP to greater than 400 ng/L, clinical evidence of heart failure, or a greater than 10% increase in LV end diastolic volume as assessed by cardiac MRI.
The results
During the first half of the study, 11 of 25 patients (44%) relapsed during or after medication withdrawal. None of the controls relapsed. In the crossover phase, 9 of 25 patients (35%) relapsed in response to treatment withdrawal. Of the 20 patients who relapsed, 13 did so within 16 weeks of beginning medication withdrawal. Indeed, most patients relapsed within 8 weeks of their last medication. Ten of the twenty fulfilled multiple criteria for relapse.
Medication withdrawal was accompanied not only by a mean 9.5% reduction in LVEF, compared with baseline, but by a 15.4-bpm rise in heart rate, a 7.0–mm Hg increase in diastolic blood pressure, and 5.1-point deterioration in Kansas City Cardiomyopathy Questionnaire scores, demonstrating that what happened off treatment was true DCM recurrence and not simply an imaging artifact.
Everyone who relapsed immediately restarted treatment. At their next follow-up visit, all were once again asymptomatic, and 17 of the 20 (85%) had an LVEF greater than 50%. Two of the other three had an LVEF of 45%-50%, and the other had an LVEF of 43%.
“So they did seem to recover when they went back on medication,” Dr. Halliday observed.
Underpowered exploratory analyses designed for hypothesis generation identified several potential baseline predictors of DCM relapse, including older age, being on three or more heart failure drugs, and use of a mineralocorticoid antagonist.
Experts react
Designated discussant Jane E. Wilcox, MD, commented, “Currently, in 2018, we have no true signature of recovery. These patients are indeed in cardiac remission and have an indefinite indication for continuing their evidence-based medical therapy without interruption.”
“The clinical implication here is, I think, we should TRED-lightly,” quipped Dr. Wilcox of Northwestern University in Chicago.
Her own research indicates that even patients who have recovered their LVEF and no longer seem to have a heart failure phenotype still have an abnormal myocardial substrate as evidenced by persistent dysfunctional cardiac mechanics on echocardiography. Nonetheless, she remains optimistic.
“I don’t think [TRED-HF] squelches the future of myocardial recovery. I think it actually invigorates the field for an assessment of genomics, proteomics, and metabolomics looking for that true signature of cardiac recovery,” she said.
Donald Lloyd-Jones, MD, who chaired a press conference where Dr. Halliday presented the TRED-HF results, complimented the investigators for tackling what he termed “an incredibly important clinical question that comes up all the time.”
“I really want to commend the investigators for taking on what, on its face, might be an ethically challenging question by taking treatment away when we don’t know what the answer is likely to be. But they really checked all the boxes to make sure this was done in a very safe and monitored way, so that even though the outcome was what it turned out to be, the harm to patients was minimalized,” said Dr. Lloyd-Jones, professor and chair of the department of preventive medicine and director of the Northwestern University Clinical and Translational Sciences Institute, Chicago.
“No patient wants to be on more medication than they need to be, but I think for the time being this class of patients is going to have to be maintained on medications until we understand a little more,” Dr. Lloyd-Jones concluded.
Dr. Halliday reported having no financial conflicts regarding the study, funded by the British Heart Foundation.
SOURCE: Halliday BP. AHA scientific sessions, Abstract 18621. Simulpub The Lancet. 2018 Nov 11. doi: 10.1016/S0140-6736(18)32484-X.
CHICAGO – Phased withdrawal of guideline-directed medical therapy in patients who seemed to have recovered from dilated cardiomyopathy resulted in relapses in 40% of patients within 6 months in the TRED-HF trial.
The clinical implications of this small pilot randomized trial are clear: “Withdrawal of therapy should not usually be attempted, at least until we can predict who’s going to relapse and who’s not,” Brian P. Halliday, MD, PhD, said at the American Heart Association scientific sessions.
“Improvement in function represents remission rather than permanent recovery for many patients,” added Dr. Halliday of Imperial College London.
The study was performed to address a question that arises with increasing frequency in clinical practice as a result of the impressive advances in heart failure therapy in recent years, he said. “Patients frequently come to us in clinic and ask us, ‘Do I need to continue to take these medications forever?’ They’re frequently young, and they want to know if they really need to be subject to 40 or 50 years of medication. Some are concerned about side effects, others are interested in pregnancy, and then there is the financial cost.”
Simultaneously published in The Lancet, TRED-HF was a single-center, open-label study of 51 patients who had prior dilated cardiomyopathy (DCM) and a median left ventricular ejection fraction (LVEF) of 25% at the time of diagnosis 4.9 years earlier and who subsequently recovered in response to therapy. That is, they became symptom-free with an LVEF greater than 50%, a normal left ventricular end diastolic volume index, and a reassuringly low median N-terminal pro b-type natriuretic peptide (NP-pro-BNP) level of 72 ng/L.
For the study, 25 patients were randomized to phased withdrawal of their heart failure drugs over a 16-week period: First they reduced or stopped loop diuretics, then mineralocorticoid antagonists, then beta-blockers, and finally their ACE inhibitor or angiotensin receptor blocker. The other 26 participants continued therapy during the first 6 months of the study, then 25 of the 26 crossed over to phased withdrawal. The outlier didn’t cross over because of atrial fibrillation.
The primary endpoint was relapse of DCM within 6 months of the start of the study. Relapse was defined as either a drop in LVEF of more than 10% to a level below 50%, at least a doubling of NT-pro-BNP to greater than 400 ng/L, clinical evidence of heart failure, or a greater than 10% increase in LV end diastolic volume as assessed by cardiac MRI.
The results
During the first half of the study, 11 of 25 patients (44%) relapsed during or after medication withdrawal. None of the controls relapsed. In the crossover phase, 9 of 25 patients (35%) relapsed in response to treatment withdrawal. Of the 20 patients who relapsed, 13 did so within 16 weeks of beginning medication withdrawal. Indeed, most patients relapsed within 8 weeks of their last medication. Ten of the twenty fulfilled multiple criteria for relapse.
Medication withdrawal was accompanied not only by a mean 9.5% reduction in LVEF, compared with baseline, but by a 15.4-bpm rise in heart rate, a 7.0–mm Hg increase in diastolic blood pressure, and 5.1-point deterioration in Kansas City Cardiomyopathy Questionnaire scores, demonstrating that what happened off treatment was true DCM recurrence and not simply an imaging artifact.
Everyone who relapsed immediately restarted treatment. At their next follow-up visit, all were once again asymptomatic, and 17 of the 20 (85%) had an LVEF greater than 50%. Two of the other three had an LVEF of 45%-50%, and the other had an LVEF of 43%.
“So they did seem to recover when they went back on medication,” Dr. Halliday observed.
Underpowered exploratory analyses designed for hypothesis generation identified several potential baseline predictors of DCM relapse, including older age, being on three or more heart failure drugs, and use of a mineralocorticoid antagonist.
Experts react
Designated discussant Jane E. Wilcox, MD, commented, “Currently, in 2018, we have no true signature of recovery. These patients are indeed in cardiac remission and have an indefinite indication for continuing their evidence-based medical therapy without interruption.”
“The clinical implication here is, I think, we should TRED-lightly,” quipped Dr. Wilcox of Northwestern University in Chicago.
Her own research indicates that even patients who have recovered their LVEF and no longer seem to have a heart failure phenotype still have an abnormal myocardial substrate as evidenced by persistent dysfunctional cardiac mechanics on echocardiography. Nonetheless, she remains optimistic.
“I don’t think [TRED-HF] squelches the future of myocardial recovery. I think it actually invigorates the field for an assessment of genomics, proteomics, and metabolomics looking for that true signature of cardiac recovery,” she said.
Donald Lloyd-Jones, MD, who chaired a press conference where Dr. Halliday presented the TRED-HF results, complimented the investigators for tackling what he termed “an incredibly important clinical question that comes up all the time.”
“I really want to commend the investigators for taking on what, on its face, might be an ethically challenging question by taking treatment away when we don’t know what the answer is likely to be. But they really checked all the boxes to make sure this was done in a very safe and monitored way, so that even though the outcome was what it turned out to be, the harm to patients was minimalized,” said Dr. Lloyd-Jones, professor and chair of the department of preventive medicine and director of the Northwestern University Clinical and Translational Sciences Institute, Chicago.
“No patient wants to be on more medication than they need to be, but I think for the time being this class of patients is going to have to be maintained on medications until we understand a little more,” Dr. Lloyd-Jones concluded.
Dr. Halliday reported having no financial conflicts regarding the study, funded by the British Heart Foundation.
SOURCE: Halliday BP. AHA scientific sessions, Abstract 18621. Simulpub The Lancet. 2018 Nov 11. doi: 10.1016/S0140-6736(18)32484-X.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: The heart failure relapse rate is high after medication withdrawal.
Major finding: Of patients who were seemingly recovered from dilated cardiomyopathy, 40% experienced early relapse following structured medication withdrawal.
Study details: This randomized crossover trial included 51 patients whose medications were withdrawn after their apparent recovery from dilated cardiomyopathy.
Disclosures: The study was funded by the British Heart Foundation. The presenter reported having no financial conflicts.
Source: Halliday BP. AHA scientific sessions, Abstract 18621. Simulpub The Lancet. 2018 Nov 11. doi: 10.1016/S0140-6736(18)32484-X.
Heart disease remains the leading cause of death in U.S.
The 10 leading causes of death in the United States remained unchanged over the past year, according to a new report from the Centers for Disease Control (CDC). Though life expectancy at birth decreased to 78.6 years in 2017, down from 78.7 years in 2016, that change was driven primarily by suicide and drug overdose.
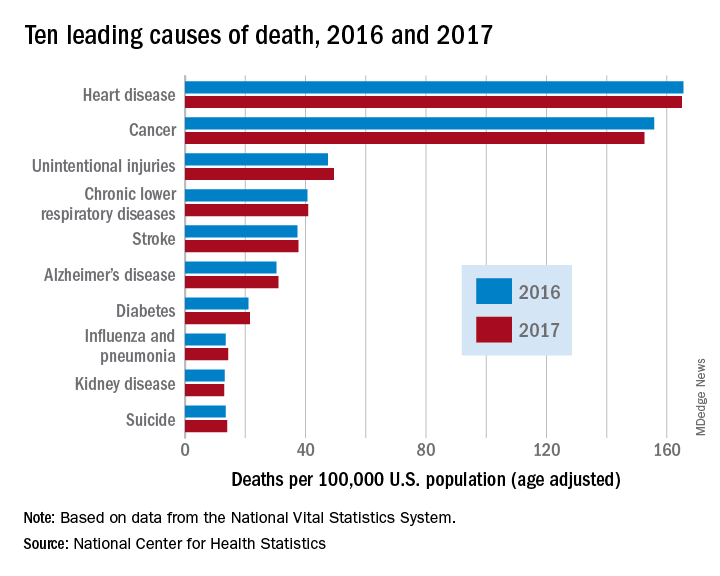
However, heart disease remains the leading cause of death in the United States, at 165 deaths per 100,000 individuals in 2017. This represents a slight, statistically nonsignificant, decrease from the 165.5 deaths per 100,000 caused by heart disease in the previous year.
Other diseases related to cardiometabolic health saw increases. Stroke and diabetes each caused a small but significant increase in deaths in 2017, which saw a 1-year increase to 37.6 from 37.3 stroke deaths per 100,000 people. Diabetes deaths increased to 21.5 from 21 per 100,000 the previous year. Stroke was the fifth and diabetes the seventh most common cause of death, according to the data brief published by the CDC’s National Center for Health Statistics (NCHS).
Alzheimer’s disease deaths also increased significantly, from 30.3 per 100,000 in 2016 to 31 per 100,000 in 2017. Although Alzheimer’s exact etiology remains under study, cardiovascular disease factors and Alzheimer’s disease share many risk factors and are often comorbid .
“With a slight decrease in deaths from heart disease in 2017 and a slight increase in deaths from stroke, this lack of any major movement in these areas has been a trend we’ve seen the last couple of years,” said Ivor Benjamin, MD, president of the American Heart Association, in a press release. “It is discouraging after experiencing decades when heart disease and stroke death rates both dropped more dramatically.”
Infant deaths from congenital malformations decreased from 2016 to 2017, from 122.1 to 118.8 deaths per 100,000 live births. “While the report doesn’t specify death rates for specific types of congenital malformations, this is heartening news as it could reflect fewer deaths from congenital heart defects,” said the AHA in its release.
According to the CDC, the 10 leading causes of death together account for about three quarters of United States deaths. Cancer caused nearly as many deaths as heart disease – 152.5 per 100,000. This represented a significant decrease from the 155.8 cancer deaths per 100,000 seen in 2016. The remaining top 10 causes of death, in decreasing order, were unintentional injuries, chronic lower respiratory diseases, influenza and pneumonia, kidney disease, and suicide.
The 10 leading causes of death in the United States remained unchanged over the past year, according to a new report from the Centers for Disease Control (CDC). Though life expectancy at birth decreased to 78.6 years in 2017, down from 78.7 years in 2016, that change was driven primarily by suicide and drug overdose.

However, heart disease remains the leading cause of death in the United States, at 165 deaths per 100,000 individuals in 2017. This represents a slight, statistically nonsignificant, decrease from the 165.5 deaths per 100,000 caused by heart disease in the previous year.
Other diseases related to cardiometabolic health saw increases. Stroke and diabetes each caused a small but significant increase in deaths in 2017, which saw a 1-year increase to 37.6 from 37.3 stroke deaths per 100,000 people. Diabetes deaths increased to 21.5 from 21 per 100,000 the previous year. Stroke was the fifth and diabetes the seventh most common cause of death, according to the data brief published by the CDC’s National Center for Health Statistics (NCHS).
Alzheimer’s disease deaths also increased significantly, from 30.3 per 100,000 in 2016 to 31 per 100,000 in 2017. Although Alzheimer’s exact etiology remains under study, cardiovascular disease factors and Alzheimer’s disease share many risk factors and are often comorbid .
“With a slight decrease in deaths from heart disease in 2017 and a slight increase in deaths from stroke, this lack of any major movement in these areas has been a trend we’ve seen the last couple of years,” said Ivor Benjamin, MD, president of the American Heart Association, in a press release. “It is discouraging after experiencing decades when heart disease and stroke death rates both dropped more dramatically.”
Infant deaths from congenital malformations decreased from 2016 to 2017, from 122.1 to 118.8 deaths per 100,000 live births. “While the report doesn’t specify death rates for specific types of congenital malformations, this is heartening news as it could reflect fewer deaths from congenital heart defects,” said the AHA in its release.
According to the CDC, the 10 leading causes of death together account for about three quarters of United States deaths. Cancer caused nearly as many deaths as heart disease – 152.5 per 100,000. This represented a significant decrease from the 155.8 cancer deaths per 100,000 seen in 2016. The remaining top 10 causes of death, in decreasing order, were unintentional injuries, chronic lower respiratory diseases, influenza and pneumonia, kidney disease, and suicide.
The 10 leading causes of death in the United States remained unchanged over the past year, according to a new report from the Centers for Disease Control (CDC). Though life expectancy at birth decreased to 78.6 years in 2017, down from 78.7 years in 2016, that change was driven primarily by suicide and drug overdose.

However, heart disease remains the leading cause of death in the United States, at 165 deaths per 100,000 individuals in 2017. This represents a slight, statistically nonsignificant, decrease from the 165.5 deaths per 100,000 caused by heart disease in the previous year.
Other diseases related to cardiometabolic health saw increases. Stroke and diabetes each caused a small but significant increase in deaths in 2017, which saw a 1-year increase to 37.6 from 37.3 stroke deaths per 100,000 people. Diabetes deaths increased to 21.5 from 21 per 100,000 the previous year. Stroke was the fifth and diabetes the seventh most common cause of death, according to the data brief published by the CDC’s National Center for Health Statistics (NCHS).
Alzheimer’s disease deaths also increased significantly, from 30.3 per 100,000 in 2016 to 31 per 100,000 in 2017. Although Alzheimer’s exact etiology remains under study, cardiovascular disease factors and Alzheimer’s disease share many risk factors and are often comorbid .
“With a slight decrease in deaths from heart disease in 2017 and a slight increase in deaths from stroke, this lack of any major movement in these areas has been a trend we’ve seen the last couple of years,” said Ivor Benjamin, MD, president of the American Heart Association, in a press release. “It is discouraging after experiencing decades when heart disease and stroke death rates both dropped more dramatically.”
Infant deaths from congenital malformations decreased from 2016 to 2017, from 122.1 to 118.8 deaths per 100,000 live births. “While the report doesn’t specify death rates for specific types of congenital malformations, this is heartening news as it could reflect fewer deaths from congenital heart defects,” said the AHA in its release.
According to the CDC, the 10 leading causes of death together account for about three quarters of United States deaths. Cancer caused nearly as many deaths as heart disease – 152.5 per 100,000. This represented a significant decrease from the 155.8 cancer deaths per 100,000 seen in 2016. The remaining top 10 causes of death, in decreasing order, were unintentional injuries, chronic lower respiratory diseases, influenza and pneumonia, kidney disease, and suicide.
FROM A CDC DATA BRIEF
Mylan issues voluntary recall of certain valsartan-containing products
Mylan has announced that it is voluntarily recalling 15 lots of products containing valsartan because of the detection of trace amounts of N-nitrosodiethylamine within the active ingredient, according to a company announcement posted on the website of the Food and Drug Administration.
The affected products include six lots of amlodipine/valsartan tablets (5-mg/160-mg, 10-mg/160-mg, and 10-mg/320-mg strengths), seven lots of valsartan tablets (40-mg, 80-mg, 160-mg, and 320-mg strengths), and two lots of valsartan/hydrochlorothiazide tablets (320-mg/25-mg strength). All products were distributed between March 2017 and November 2018.
“Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication. Patients who are on valsartan should continue taking their medication, as the risk of harm to the patient’s health may be higher if the treatment is stopped immediately without any alternative treatment,” the company statement said.
N-nitrosodiethylamine is a naturally occurring substance that has been identified as a human carcinogen by the International Agency for Research on Cancer.
The lot and NDC (National Drug Code) numbers of the affected products can be found in the full press release on the FDA website.
Mylan is notifying its distributors and customers by letter and is arranging for return of all recalled products. Wholesalers, retailers, and consumers in possession of recalled product should contact Stericycle at 1-888-406-9305 for the return of the recalled product. Normal business hours are Monday through Friday, 8 a.m. to 5 p.m. EST, according to the statement.
Mylan has announced that it is voluntarily recalling 15 lots of products containing valsartan because of the detection of trace amounts of N-nitrosodiethylamine within the active ingredient, according to a company announcement posted on the website of the Food and Drug Administration.
The affected products include six lots of amlodipine/valsartan tablets (5-mg/160-mg, 10-mg/160-mg, and 10-mg/320-mg strengths), seven lots of valsartan tablets (40-mg, 80-mg, 160-mg, and 320-mg strengths), and two lots of valsartan/hydrochlorothiazide tablets (320-mg/25-mg strength). All products were distributed between March 2017 and November 2018.
“Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication. Patients who are on valsartan should continue taking their medication, as the risk of harm to the patient’s health may be higher if the treatment is stopped immediately without any alternative treatment,” the company statement said.
N-nitrosodiethylamine is a naturally occurring substance that has been identified as a human carcinogen by the International Agency for Research on Cancer.
The lot and NDC (National Drug Code) numbers of the affected products can be found in the full press release on the FDA website.
Mylan is notifying its distributors and customers by letter and is arranging for return of all recalled products. Wholesalers, retailers, and consumers in possession of recalled product should contact Stericycle at 1-888-406-9305 for the return of the recalled product. Normal business hours are Monday through Friday, 8 a.m. to 5 p.m. EST, according to the statement.
Mylan has announced that it is voluntarily recalling 15 lots of products containing valsartan because of the detection of trace amounts of N-nitrosodiethylamine within the active ingredient, according to a company announcement posted on the website of the Food and Drug Administration.
The affected products include six lots of amlodipine/valsartan tablets (5-mg/160-mg, 10-mg/160-mg, and 10-mg/320-mg strengths), seven lots of valsartan tablets (40-mg, 80-mg, 160-mg, and 320-mg strengths), and two lots of valsartan/hydrochlorothiazide tablets (320-mg/25-mg strength). All products were distributed between March 2017 and November 2018.
“Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication. Patients who are on valsartan should continue taking their medication, as the risk of harm to the patient’s health may be higher if the treatment is stopped immediately without any alternative treatment,” the company statement said.
N-nitrosodiethylamine is a naturally occurring substance that has been identified as a human carcinogen by the International Agency for Research on Cancer.
The lot and NDC (National Drug Code) numbers of the affected products can be found in the full press release on the FDA website.
Mylan is notifying its distributors and customers by letter and is arranging for return of all recalled products. Wholesalers, retailers, and consumers in possession of recalled product should contact Stericycle at 1-888-406-9305 for the return of the recalled product. Normal business hours are Monday through Friday, 8 a.m. to 5 p.m. EST, according to the statement.
PIONEER-HF called “practice changing” for acute decompensated heart failure
CHICAGO – Initiation of angiotensin-neprilysin inhibition using sacubitril/valsartan during hospitalization for acute decompensated heart failure, instead of relying upon enalapril, resulted in a substantially greater reduction in N-terminal of the prohormone brain natriuretic peptide concentration and a markedly lower rate of rehospitalization with no safety downside in the PIONEER-HF trial, Eric J. Velazquez, MD, reported at the American Heart Association scientific sessions.
“We believe these results have clinical implications that support the in-hospital initiation of sacubitril/valsartan in stabilized patients with acute decompensated heart failure and reduced ejection fraction irrespective of prior ACE inhibitor or ARB [angiotensin II receptor blocker] use or prior diagnosis of heart failure,” said Dr. Velazquez, a professor of medicine and chief of the section of cardiovascular medicine at Yale University, New Haven, Conn., and physician in chief of the Heart and Vascular Center for the Yale-New Haven Health System.
Sacubitril/valsartan (Entresto) has a class I indication for treatment of symptomatic heart failure with reduced ejection fraction (HFrEF) in the AHA/American College of Cardiology guidelines. This strong recommendation is based largely upon the impressive results of the PARADIGM-HF trial, which in ambulatory outpatients demonstrated a lower risk of cardiovascular mortality or hospitalization for heart failure than enalapril (N Engl J Med. 2014 Sep 11;371[11]:993-1004).
However, since patients with acute decompensated heart failure (ADHF) were excluded from PARADIGM-HF, the safety and effectiveness of starting such patients on the drug while hospitalized for acute decompensation was unknown.
PIONEER-HF was carried out to shed light on that issue and thereby address a major unmet need for better treatments for ADHF. Even though this condition accounts for more than 1 million hospitalizations annually in the United States, short-term rehospitalization and mortality rates in affected patients remain unacceptably high at 21% and 12%, respectively. And the standard-of-care treatment – decongestion with intravenous diuretics and hemodynamic support with inotropes and vasodilators – hasn’t changed in nearly half a century, Dr. Velazquez noted.
The trial included 881 patients hospitalized for acute decompensated HFrEF at 129 U.S. centers. The study population was diverse: 36% of participants were black and one-third of subjects had no diagnosis of heart failure prior to their hospitalization. After achieving hemodynamic stabilization, patients were randomized to receive sacubitril/valsartan or enalapril.
Key outcomes
The primary endpoint was change in N-terminal of the prohormone brain natriuretic peptide concentration from baseline to week 8. There was a 25% reduction in the enalapril group and a 45% reduction with sacubitril/valsartan. This translated to a highly significant 29% greater relative risk reduction with sacubitril/valsartan.
More eye opening was the between-group difference in the prespecified composite clinical endpoint comprising death, rehospitalization for heart failure, implantation of a left ventricular assist device, or listing for heart transplant during the 8-week study.
The rate was 16.8% in the enalapril group and 9.3% with sacubitril/valsartan. This worked out to a 46% relative risk reduction, with a number needed to treat of 13.
The composite result was driven by a 44% reduction in risk of heart failure rehospitalization in the sacubitril/valsartan group: 8.0% versus 13.8%. The sacubitril/valsartan group also had a numerically lower mortality rate: 2.3% versus 3.4%, although the number of fatalities was small and this 34% relative risk reduction didn’t achieve statistical significance.
Rates of the key safety outcomes – symptomatic hypotension, worsening renal function, hyperkalemia, and angioedema – didn’t differ between the two study arms. Of interest, however, all six cases of angioedema in the enalapril group occurred in black patients, while the only case in the sacubitril/valsartan group was in a white patient.
PIONEER-HF treatment strategy
Hemodynamic stabilization as a prelude to randomization to sacubitril/valsartan or enalapril required maintaining a systolic blood pressure of at least 100 mm Hg in the previous 6 hours, with no symptomatic hypotension, intensification of intravenous diuretics, or use of intravenous vasodilators during that time period, and no intravenous inotropes in the previous 24 hours.
Enalapril was titrated to a target dose of 10 mg twice daily. Sacubitril/valsartan was titrated to a target dose of 97/103 mg twice daily. Titration was carried out using an algorithm based upon systolic BP. If the SBP was at least 100 and less than 120 mm Hg at baseline, sacubitril/valsartan was initiated at 24/26 mg twice daily, enalapril at 2.5 mg b.i.d. If the SBP at randomization was 120 mm Hg or higher, the initial dosing was sacubitril/valsartan at 49/51 mm Hg b.i.d. or enalapril at 5 mg b.i.d. Up-titration occurred after 1 week, then biweekly through week 8.
PIONEER in perspective
Discussant Larry A. Allen, MD, a heart failure specialist at the University of Colorado at Denver, Aurora, predicted that this will be a practice-changing study.
“There has been a need for a study like PIONEER in heart failure,” he observed. While multiple randomized trials have advanced the treatment of ambulatory HFrEF patients, demonstrating benefit for initiation and intensification of treatment with ACE inhibitors, angiotensin II receptor blockers, beta-blockers, and mineralocorticoid receptor antagonists, the treatment of patients with ADHF has remained relatively static, marked by failed trials of once-promising novel agents including tolvaptan, nesiritide, and serelaxin.
“All the data is in ambulatory patients, but the action for the care of heart failure patients actually occurs largely in the hospital. Seventy percent of care provided in the U.S. to patients with heart failure occurs in the hospital setting. These patients are a captive audience at that time, and the transitions from inpatient to outpatient care are fragile,” Dr. Allen said.
He noted that the use of sacubitril/valsartan in routine practice as reflected in national registries has been “extremely low” – less than 15% among eligible patients – despite the drug having been approved more than 3 years ago. One major reason for the low uptake, in his view, is clinical inertia. That should melt away in what he termed “the post-PIONEER world.”
“I think one of the great things about this study is it keeps it simple. We now have a simpler algorithm for inpatient and subsequent outpatient management of heart failure with reduced ejection fraction. It’s easier for us to start with the treatment we want patients to be on, and it’s better for patients, too. Most importantly, this study reinforces the importance and safety of aggressive guideline-directed medical therapy starting from the beginning in most patients,” Dr. Allen said.
The study findings were published simultaneously in the New England Journal of Medicine (2018 Nov 11. doi: 10.1056/NEJMoa1812851).
PIONEER-HF was sponsored by Novartis. Dr. Velazquez reported receiving research grants from and serving as a consultant to that company and others. Dr. Allen reported having no financial conflicts.
CHICAGO – Initiation of angiotensin-neprilysin inhibition using sacubitril/valsartan during hospitalization for acute decompensated heart failure, instead of relying upon enalapril, resulted in a substantially greater reduction in N-terminal of the prohormone brain natriuretic peptide concentration and a markedly lower rate of rehospitalization with no safety downside in the PIONEER-HF trial, Eric J. Velazquez, MD, reported at the American Heart Association scientific sessions.
“We believe these results have clinical implications that support the in-hospital initiation of sacubitril/valsartan in stabilized patients with acute decompensated heart failure and reduced ejection fraction irrespective of prior ACE inhibitor or ARB [angiotensin II receptor blocker] use or prior diagnosis of heart failure,” said Dr. Velazquez, a professor of medicine and chief of the section of cardiovascular medicine at Yale University, New Haven, Conn., and physician in chief of the Heart and Vascular Center for the Yale-New Haven Health System.
Sacubitril/valsartan (Entresto) has a class I indication for treatment of symptomatic heart failure with reduced ejection fraction (HFrEF) in the AHA/American College of Cardiology guidelines. This strong recommendation is based largely upon the impressive results of the PARADIGM-HF trial, which in ambulatory outpatients demonstrated a lower risk of cardiovascular mortality or hospitalization for heart failure than enalapril (N Engl J Med. 2014 Sep 11;371[11]:993-1004).
However, since patients with acute decompensated heart failure (ADHF) were excluded from PARADIGM-HF, the safety and effectiveness of starting such patients on the drug while hospitalized for acute decompensation was unknown.
PIONEER-HF was carried out to shed light on that issue and thereby address a major unmet need for better treatments for ADHF. Even though this condition accounts for more than 1 million hospitalizations annually in the United States, short-term rehospitalization and mortality rates in affected patients remain unacceptably high at 21% and 12%, respectively. And the standard-of-care treatment – decongestion with intravenous diuretics and hemodynamic support with inotropes and vasodilators – hasn’t changed in nearly half a century, Dr. Velazquez noted.
The trial included 881 patients hospitalized for acute decompensated HFrEF at 129 U.S. centers. The study population was diverse: 36% of participants were black and one-third of subjects had no diagnosis of heart failure prior to their hospitalization. After achieving hemodynamic stabilization, patients were randomized to receive sacubitril/valsartan or enalapril.
Key outcomes
The primary endpoint was change in N-terminal of the prohormone brain natriuretic peptide concentration from baseline to week 8. There was a 25% reduction in the enalapril group and a 45% reduction with sacubitril/valsartan. This translated to a highly significant 29% greater relative risk reduction with sacubitril/valsartan.
More eye opening was the between-group difference in the prespecified composite clinical endpoint comprising death, rehospitalization for heart failure, implantation of a left ventricular assist device, or listing for heart transplant during the 8-week study.
The rate was 16.8% in the enalapril group and 9.3% with sacubitril/valsartan. This worked out to a 46% relative risk reduction, with a number needed to treat of 13.
The composite result was driven by a 44% reduction in risk of heart failure rehospitalization in the sacubitril/valsartan group: 8.0% versus 13.8%. The sacubitril/valsartan group also had a numerically lower mortality rate: 2.3% versus 3.4%, although the number of fatalities was small and this 34% relative risk reduction didn’t achieve statistical significance.
Rates of the key safety outcomes – symptomatic hypotension, worsening renal function, hyperkalemia, and angioedema – didn’t differ between the two study arms. Of interest, however, all six cases of angioedema in the enalapril group occurred in black patients, while the only case in the sacubitril/valsartan group was in a white patient.
PIONEER-HF treatment strategy
Hemodynamic stabilization as a prelude to randomization to sacubitril/valsartan or enalapril required maintaining a systolic blood pressure of at least 100 mm Hg in the previous 6 hours, with no symptomatic hypotension, intensification of intravenous diuretics, or use of intravenous vasodilators during that time period, and no intravenous inotropes in the previous 24 hours.
Enalapril was titrated to a target dose of 10 mg twice daily. Sacubitril/valsartan was titrated to a target dose of 97/103 mg twice daily. Titration was carried out using an algorithm based upon systolic BP. If the SBP was at least 100 and less than 120 mm Hg at baseline, sacubitril/valsartan was initiated at 24/26 mg twice daily, enalapril at 2.5 mg b.i.d. If the SBP at randomization was 120 mm Hg or higher, the initial dosing was sacubitril/valsartan at 49/51 mm Hg b.i.d. or enalapril at 5 mg b.i.d. Up-titration occurred after 1 week, then biweekly through week 8.
PIONEER in perspective
Discussant Larry A. Allen, MD, a heart failure specialist at the University of Colorado at Denver, Aurora, predicted that this will be a practice-changing study.
“There has been a need for a study like PIONEER in heart failure,” he observed. While multiple randomized trials have advanced the treatment of ambulatory HFrEF patients, demonstrating benefit for initiation and intensification of treatment with ACE inhibitors, angiotensin II receptor blockers, beta-blockers, and mineralocorticoid receptor antagonists, the treatment of patients with ADHF has remained relatively static, marked by failed trials of once-promising novel agents including tolvaptan, nesiritide, and serelaxin.
“All the data is in ambulatory patients, but the action for the care of heart failure patients actually occurs largely in the hospital. Seventy percent of care provided in the U.S. to patients with heart failure occurs in the hospital setting. These patients are a captive audience at that time, and the transitions from inpatient to outpatient care are fragile,” Dr. Allen said.
He noted that the use of sacubitril/valsartan in routine practice as reflected in national registries has been “extremely low” – less than 15% among eligible patients – despite the drug having been approved more than 3 years ago. One major reason for the low uptake, in his view, is clinical inertia. That should melt away in what he termed “the post-PIONEER world.”
“I think one of the great things about this study is it keeps it simple. We now have a simpler algorithm for inpatient and subsequent outpatient management of heart failure with reduced ejection fraction. It’s easier for us to start with the treatment we want patients to be on, and it’s better for patients, too. Most importantly, this study reinforces the importance and safety of aggressive guideline-directed medical therapy starting from the beginning in most patients,” Dr. Allen said.
The study findings were published simultaneously in the New England Journal of Medicine (2018 Nov 11. doi: 10.1056/NEJMoa1812851).
PIONEER-HF was sponsored by Novartis. Dr. Velazquez reported receiving research grants from and serving as a consultant to that company and others. Dr. Allen reported having no financial conflicts.
CHICAGO – Initiation of angiotensin-neprilysin inhibition using sacubitril/valsartan during hospitalization for acute decompensated heart failure, instead of relying upon enalapril, resulted in a substantially greater reduction in N-terminal of the prohormone brain natriuretic peptide concentration and a markedly lower rate of rehospitalization with no safety downside in the PIONEER-HF trial, Eric J. Velazquez, MD, reported at the American Heart Association scientific sessions.
“We believe these results have clinical implications that support the in-hospital initiation of sacubitril/valsartan in stabilized patients with acute decompensated heart failure and reduced ejection fraction irrespective of prior ACE inhibitor or ARB [angiotensin II receptor blocker] use or prior diagnosis of heart failure,” said Dr. Velazquez, a professor of medicine and chief of the section of cardiovascular medicine at Yale University, New Haven, Conn., and physician in chief of the Heart and Vascular Center for the Yale-New Haven Health System.
Sacubitril/valsartan (Entresto) has a class I indication for treatment of symptomatic heart failure with reduced ejection fraction (HFrEF) in the AHA/American College of Cardiology guidelines. This strong recommendation is based largely upon the impressive results of the PARADIGM-HF trial, which in ambulatory outpatients demonstrated a lower risk of cardiovascular mortality or hospitalization for heart failure than enalapril (N Engl J Med. 2014 Sep 11;371[11]:993-1004).
However, since patients with acute decompensated heart failure (ADHF) were excluded from PARADIGM-HF, the safety and effectiveness of starting such patients on the drug while hospitalized for acute decompensation was unknown.
PIONEER-HF was carried out to shed light on that issue and thereby address a major unmet need for better treatments for ADHF. Even though this condition accounts for more than 1 million hospitalizations annually in the United States, short-term rehospitalization and mortality rates in affected patients remain unacceptably high at 21% and 12%, respectively. And the standard-of-care treatment – decongestion with intravenous diuretics and hemodynamic support with inotropes and vasodilators – hasn’t changed in nearly half a century, Dr. Velazquez noted.
The trial included 881 patients hospitalized for acute decompensated HFrEF at 129 U.S. centers. The study population was diverse: 36% of participants were black and one-third of subjects had no diagnosis of heart failure prior to their hospitalization. After achieving hemodynamic stabilization, patients were randomized to receive sacubitril/valsartan or enalapril.
Key outcomes
The primary endpoint was change in N-terminal of the prohormone brain natriuretic peptide concentration from baseline to week 8. There was a 25% reduction in the enalapril group and a 45% reduction with sacubitril/valsartan. This translated to a highly significant 29% greater relative risk reduction with sacubitril/valsartan.
More eye opening was the between-group difference in the prespecified composite clinical endpoint comprising death, rehospitalization for heart failure, implantation of a left ventricular assist device, or listing for heart transplant during the 8-week study.
The rate was 16.8% in the enalapril group and 9.3% with sacubitril/valsartan. This worked out to a 46% relative risk reduction, with a number needed to treat of 13.
The composite result was driven by a 44% reduction in risk of heart failure rehospitalization in the sacubitril/valsartan group: 8.0% versus 13.8%. The sacubitril/valsartan group also had a numerically lower mortality rate: 2.3% versus 3.4%, although the number of fatalities was small and this 34% relative risk reduction didn’t achieve statistical significance.
Rates of the key safety outcomes – symptomatic hypotension, worsening renal function, hyperkalemia, and angioedema – didn’t differ between the two study arms. Of interest, however, all six cases of angioedema in the enalapril group occurred in black patients, while the only case in the sacubitril/valsartan group was in a white patient.
PIONEER-HF treatment strategy
Hemodynamic stabilization as a prelude to randomization to sacubitril/valsartan or enalapril required maintaining a systolic blood pressure of at least 100 mm Hg in the previous 6 hours, with no symptomatic hypotension, intensification of intravenous diuretics, or use of intravenous vasodilators during that time period, and no intravenous inotropes in the previous 24 hours.
Enalapril was titrated to a target dose of 10 mg twice daily. Sacubitril/valsartan was titrated to a target dose of 97/103 mg twice daily. Titration was carried out using an algorithm based upon systolic BP. If the SBP was at least 100 and less than 120 mm Hg at baseline, sacubitril/valsartan was initiated at 24/26 mg twice daily, enalapril at 2.5 mg b.i.d. If the SBP at randomization was 120 mm Hg or higher, the initial dosing was sacubitril/valsartan at 49/51 mm Hg b.i.d. or enalapril at 5 mg b.i.d. Up-titration occurred after 1 week, then biweekly through week 8.
PIONEER in perspective
Discussant Larry A. Allen, MD, a heart failure specialist at the University of Colorado at Denver, Aurora, predicted that this will be a practice-changing study.
“There has been a need for a study like PIONEER in heart failure,” he observed. While multiple randomized trials have advanced the treatment of ambulatory HFrEF patients, demonstrating benefit for initiation and intensification of treatment with ACE inhibitors, angiotensin II receptor blockers, beta-blockers, and mineralocorticoid receptor antagonists, the treatment of patients with ADHF has remained relatively static, marked by failed trials of once-promising novel agents including tolvaptan, nesiritide, and serelaxin.
“All the data is in ambulatory patients, but the action for the care of heart failure patients actually occurs largely in the hospital. Seventy percent of care provided in the U.S. to patients with heart failure occurs in the hospital setting. These patients are a captive audience at that time, and the transitions from inpatient to outpatient care are fragile,” Dr. Allen said.
He noted that the use of sacubitril/valsartan in routine practice as reflected in national registries has been “extremely low” – less than 15% among eligible patients – despite the drug having been approved more than 3 years ago. One major reason for the low uptake, in his view, is clinical inertia. That should melt away in what he termed “the post-PIONEER world.”
“I think one of the great things about this study is it keeps it simple. We now have a simpler algorithm for inpatient and subsequent outpatient management of heart failure with reduced ejection fraction. It’s easier for us to start with the treatment we want patients to be on, and it’s better for patients, too. Most importantly, this study reinforces the importance and safety of aggressive guideline-directed medical therapy starting from the beginning in most patients,” Dr. Allen said.
The study findings were published simultaneously in the New England Journal of Medicine (2018 Nov 11. doi: 10.1056/NEJMoa1812851).
PIONEER-HF was sponsored by Novartis. Dr. Velazquez reported receiving research grants from and serving as a consultant to that company and others. Dr. Allen reported having no financial conflicts.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The rate of rehospitalization for heart failure during the next 2 months after initiation of sacubitril/valsartan during hospitalization for acute decompensated heart failure was 44% lower than with enalapril.
Study details: A randomized, multicenter trial involving 881 patients hospitalized for acute decompensated heart failure.
Disclosures: The PIONEER-HF trial was sponsored by Novartis. The presenter reported receiving research grants from and serving as a consultant to that company and others.
PIONEER-HF secures place for sacubitril/valsartan in this heart failure doc’s practice
CHICAGO – Dr. Larry A. Allen will now have an easier time of treating hospitalized patients with acute decompensated heart failure because of the results of the PIONEER-HF trial.
That study examined whether in-hospital initiation of sacubitril/valsartan compared to enalapril is safe and effective in ADHF, a treatment that hasn’t been studied well or taken up in clinical practice.
, showed comparable safety, and reduced composite endpoint of death, rehospitalization for heart failure, implantation of a left-ventricular implant device, and need for a transplant by 46%.
Dr. Allen, of the University of Colorado, Denver, was the designated discussant for the PIONEER-HF presentation at the American Heart Association scientific sessions. In an interview, he explained how these results will change his practice as a heart failure specialist. “It simplifies things: I don’t have to start on an old therapy in the hospital, get the patients back in clinic, and switch the over to this newer therapy. I can just start from the beginning with the therapy that I think will be most effective.”
To find out why, watch the complete interview.
CHICAGO – Dr. Larry A. Allen will now have an easier time of treating hospitalized patients with acute decompensated heart failure because of the results of the PIONEER-HF trial.
That study examined whether in-hospital initiation of sacubitril/valsartan compared to enalapril is safe and effective in ADHF, a treatment that hasn’t been studied well or taken up in clinical practice.
, showed comparable safety, and reduced composite endpoint of death, rehospitalization for heart failure, implantation of a left-ventricular implant device, and need for a transplant by 46%.
Dr. Allen, of the University of Colorado, Denver, was the designated discussant for the PIONEER-HF presentation at the American Heart Association scientific sessions. In an interview, he explained how these results will change his practice as a heart failure specialist. “It simplifies things: I don’t have to start on an old therapy in the hospital, get the patients back in clinic, and switch the over to this newer therapy. I can just start from the beginning with the therapy that I think will be most effective.”
To find out why, watch the complete interview.
CHICAGO – Dr. Larry A. Allen will now have an easier time of treating hospitalized patients with acute decompensated heart failure because of the results of the PIONEER-HF trial.
That study examined whether in-hospital initiation of sacubitril/valsartan compared to enalapril is safe and effective in ADHF, a treatment that hasn’t been studied well or taken up in clinical practice.
, showed comparable safety, and reduced composite endpoint of death, rehospitalization for heart failure, implantation of a left-ventricular implant device, and need for a transplant by 46%.
Dr. Allen, of the University of Colorado, Denver, was the designated discussant for the PIONEER-HF presentation at the American Heart Association scientific sessions. In an interview, he explained how these results will change his practice as a heart failure specialist. “It simplifies things: I don’t have to start on an old therapy in the hospital, get the patients back in clinic, and switch the over to this newer therapy. I can just start from the beginning with the therapy that I think will be most effective.”
To find out why, watch the complete interview.
REPORTING FROM AHA 2018
Exercise improves outcomes for patients with heart failure and OSA
Exercise may be as effective as CPAP in improving obstructive sleep apnea and quality of life in patients with heart failure, according to a study published in the October issue of Chest [https://journal.chestnet.org/article/S0012-3692(18)30790-6/fulltext].
Researchers undertook a randomized, four-arm trial in 65 patients with heart failure and obstructive sleep apnea, which compared the effects of CPAP alone, exercise alone – consisting of three supervised sessions per week for three months, or CPAP plus exercise. A control group received education sessions on the importance of exercise.
The greatest reduction in mean apnea-hypopnea index was seen in the CPAP group, who experienced a mean decrease of 24 events per hour. The exercise plus CPAP group and the exercise only groups showed a mean decrease of 10 events per hour. In contrast, the control group showed no significant decrease in the number of events per hour of sleep.
The authors commented that the change in apnea-hypopnea index was due to reduction in obstructive apneas and hypopneas, and noted the “difficulty of accurately distinguishing obstructive from central hypopneas”.
All the active interventions were associated with significant decreases in arousal index and improvements in sleep-related saturation compared to the control intervention.
Exercise – both alone and with CPAP – was associated with an increase in maximum heart rate and peak VO2, and decrease in VE/VCO2 slope compared to the CPAP-alone and control groups.
“We found that peak oxygen consumption and muscle performance improved significantly only in the exercise groups, but not with CPAP alone, even though CPAP was most effective in attenuating OSA severity,” wrote Dr. Denise M. Servantes, from the Departamento de Psicobiologia at the Universidade Federal de São Paul in Brazil, and co-authors. “Because peak VO2 is an independent predictor of survival and crucial to the optimal timing of cardiac transplantation, these findings have important clinical implications, even in patients who are adherent to CPAP.”
A significant number of participants in the active intervention groups changed New York Heart Association functional class; the number of patients in the exercise group in class I went from 0%-88% by three months, in the CPAP group it increased from 0% to 47%, and in the CPAP plus exercise group, it increased from 0% to 73%.
The study also found evidence of a trend towards improved sexual function in the participants who undertook both exercise plus CPAP.
All patients in the intervention groups showed improvements in subjective daytime sleepiness and quality of life, although improvements in the Minnesota Living with Heart Failure Questionnaire and Short Form Health Survey (SF-36) were significant only in the two groups that did exercise.
“The data suggest that exercise could be a therapeutic option for patients with HF and OSA who refuse CPAP or are intolerant to it,” the authors wrote. “In this regard, a considerable number of patients with HF and OSA do not experience subjective excessive daytime sleepiness and consequently observe no immediate benefit from using CPAP, which could contribute to poor long-term adherence.”
Individuals in the exercise group showed a slight but significant weight reduction, and those who undertook the exercise program also showed significant improvements in muscle strength and endurance compared to the control group.
The authors commented that another study examining the impact of weight loss program in people with moderate to severe obstructive sleep apnea found weight loss only or combined interventions achieved benefits for C-reactive protein levels, insulin resistance, and serum triglyceride levels. But these benefits weren’t seen with CPAP alone.
“The results of that study, and the present one emphasize the importance of adjunctive therapy of OSA with weight loss and exercise when applicable.”
However they acknowledged that the short duration of the study, and small sample size were limitations, and that this was only a preliminary investigation.
No conflicts of interest were declared.
SOURCE: Servantes D et al. Chest, 2018; 154:808-817. https://doi.org/10.1016/j.chest.2018.05.011. https://journal.chestnet.org/article/S0012-3692(18)30790-6/fulltext
Exercise may be as effective as CPAP in improving obstructive sleep apnea and quality of life in patients with heart failure, according to a study published in the October issue of Chest [https://journal.chestnet.org/article/S0012-3692(18)30790-6/fulltext].
Researchers undertook a randomized, four-arm trial in 65 patients with heart failure and obstructive sleep apnea, which compared the effects of CPAP alone, exercise alone – consisting of three supervised sessions per week for three months, or CPAP plus exercise. A control group received education sessions on the importance of exercise.
The greatest reduction in mean apnea-hypopnea index was seen in the CPAP group, who experienced a mean decrease of 24 events per hour. The exercise plus CPAP group and the exercise only groups showed a mean decrease of 10 events per hour. In contrast, the control group showed no significant decrease in the number of events per hour of sleep.
The authors commented that the change in apnea-hypopnea index was due to reduction in obstructive apneas and hypopneas, and noted the “difficulty of accurately distinguishing obstructive from central hypopneas”.
All the active interventions were associated with significant decreases in arousal index and improvements in sleep-related saturation compared to the control intervention.
Exercise – both alone and with CPAP – was associated with an increase in maximum heart rate and peak VO2, and decrease in VE/VCO2 slope compared to the CPAP-alone and control groups.
“We found that peak oxygen consumption and muscle performance improved significantly only in the exercise groups, but not with CPAP alone, even though CPAP was most effective in attenuating OSA severity,” wrote Dr. Denise M. Servantes, from the Departamento de Psicobiologia at the Universidade Federal de São Paul in Brazil, and co-authors. “Because peak VO2 is an independent predictor of survival and crucial to the optimal timing of cardiac transplantation, these findings have important clinical implications, even in patients who are adherent to CPAP.”
A significant number of participants in the active intervention groups changed New York Heart Association functional class; the number of patients in the exercise group in class I went from 0%-88% by three months, in the CPAP group it increased from 0% to 47%, and in the CPAP plus exercise group, it increased from 0% to 73%.
The study also found evidence of a trend towards improved sexual function in the participants who undertook both exercise plus CPAP.
All patients in the intervention groups showed improvements in subjective daytime sleepiness and quality of life, although improvements in the Minnesota Living with Heart Failure Questionnaire and Short Form Health Survey (SF-36) were significant only in the two groups that did exercise.
“The data suggest that exercise could be a therapeutic option for patients with HF and OSA who refuse CPAP or are intolerant to it,” the authors wrote. “In this regard, a considerable number of patients with HF and OSA do not experience subjective excessive daytime sleepiness and consequently observe no immediate benefit from using CPAP, which could contribute to poor long-term adherence.”
Individuals in the exercise group showed a slight but significant weight reduction, and those who undertook the exercise program also showed significant improvements in muscle strength and endurance compared to the control group.
The authors commented that another study examining the impact of weight loss program in people with moderate to severe obstructive sleep apnea found weight loss only or combined interventions achieved benefits for C-reactive protein levels, insulin resistance, and serum triglyceride levels. But these benefits weren’t seen with CPAP alone.
“The results of that study, and the present one emphasize the importance of adjunctive therapy of OSA with weight loss and exercise when applicable.”
However they acknowledged that the short duration of the study, and small sample size were limitations, and that this was only a preliminary investigation.
No conflicts of interest were declared.
SOURCE: Servantes D et al. Chest, 2018; 154:808-817. https://doi.org/10.1016/j.chest.2018.05.011. https://journal.chestnet.org/article/S0012-3692(18)30790-6/fulltext
Exercise may be as effective as CPAP in improving obstructive sleep apnea and quality of life in patients with heart failure, according to a study published in the October issue of Chest [https://journal.chestnet.org/article/S0012-3692(18)30790-6/fulltext].
Researchers undertook a randomized, four-arm trial in 65 patients with heart failure and obstructive sleep apnea, which compared the effects of CPAP alone, exercise alone – consisting of three supervised sessions per week for three months, or CPAP plus exercise. A control group received education sessions on the importance of exercise.
The greatest reduction in mean apnea-hypopnea index was seen in the CPAP group, who experienced a mean decrease of 24 events per hour. The exercise plus CPAP group and the exercise only groups showed a mean decrease of 10 events per hour. In contrast, the control group showed no significant decrease in the number of events per hour of sleep.
The authors commented that the change in apnea-hypopnea index was due to reduction in obstructive apneas and hypopneas, and noted the “difficulty of accurately distinguishing obstructive from central hypopneas”.
All the active interventions were associated with significant decreases in arousal index and improvements in sleep-related saturation compared to the control intervention.
Exercise – both alone and with CPAP – was associated with an increase in maximum heart rate and peak VO2, and decrease in VE/VCO2 slope compared to the CPAP-alone and control groups.
“We found that peak oxygen consumption and muscle performance improved significantly only in the exercise groups, but not with CPAP alone, even though CPAP was most effective in attenuating OSA severity,” wrote Dr. Denise M. Servantes, from the Departamento de Psicobiologia at the Universidade Federal de São Paul in Brazil, and co-authors. “Because peak VO2 is an independent predictor of survival and crucial to the optimal timing of cardiac transplantation, these findings have important clinical implications, even in patients who are adherent to CPAP.”
A significant number of participants in the active intervention groups changed New York Heart Association functional class; the number of patients in the exercise group in class I went from 0%-88% by three months, in the CPAP group it increased from 0% to 47%, and in the CPAP plus exercise group, it increased from 0% to 73%.
The study also found evidence of a trend towards improved sexual function in the participants who undertook both exercise plus CPAP.
All patients in the intervention groups showed improvements in subjective daytime sleepiness and quality of life, although improvements in the Minnesota Living with Heart Failure Questionnaire and Short Form Health Survey (SF-36) were significant only in the two groups that did exercise.
“The data suggest that exercise could be a therapeutic option for patients with HF and OSA who refuse CPAP or are intolerant to it,” the authors wrote. “In this regard, a considerable number of patients with HF and OSA do not experience subjective excessive daytime sleepiness and consequently observe no immediate benefit from using CPAP, which could contribute to poor long-term adherence.”
Individuals in the exercise group showed a slight but significant weight reduction, and those who undertook the exercise program also showed significant improvements in muscle strength and endurance compared to the control group.
The authors commented that another study examining the impact of weight loss program in people with moderate to severe obstructive sleep apnea found weight loss only or combined interventions achieved benefits for C-reactive protein levels, insulin resistance, and serum triglyceride levels. But these benefits weren’t seen with CPAP alone.
“The results of that study, and the present one emphasize the importance of adjunctive therapy of OSA with weight loss and exercise when applicable.”
However they acknowledged that the short duration of the study, and small sample size were limitations, and that this was only a preliminary investigation.
No conflicts of interest were declared.
SOURCE: Servantes D et al. Chest, 2018; 154:808-817. https://doi.org/10.1016/j.chest.2018.05.011. https://journal.chestnet.org/article/S0012-3692(18)30790-6/fulltext
FROM CHEST
Key clinical point: Exercise alone or with CPAP achieves additional improvements to quality of life in patients with heart failure and obstructive sleep apnea.
Major finding: Individuals with heart failure and obstructive sleep apnea showed significant improvements to quality of life with exercise.
Study details: Randomized controlled trial in 65 patients with heart failure and obstructive sleep apnea.
Disclosures: The study was supported by the Associacao Fundo de Incentivo a Pesquisa, Sao Paulo Research Foundation. No conflicts of interest were declared.
Source: Servantes D et al.Chest 2018;154:808-817.doi:10.1016/j.chest.2018.05.011
Palliative care update highlights role of nonspecialists
The new edition of providing care for critically ill patients, not just those clinicians actively specialized in palliative care.
The Clinical Practice Guidelines for Quality Palliative Care, 4th Edition, emphasizes the importance of palliative care provided by “clinicians in primary care and specialty care practices, such as oncologists,” the guideline authors stated.
The latest revision of the guideline aims to establish a foundation for “gold-standard” palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to the National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology and the Oncology Nurses Society.
One key reason for the update, according to the NCP, was to acknowledge that today’s health care system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on all clinicians who are not palliative specialists to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
This approach differs from the way palliative care is traditionally practiced, often by fellowship-trained physicians, trained nurses, and other specialists who provide that support.
The guidelines are organized into sections covering palliative care structure and processes, care for the patient nearing the end of life, and specific aspects of palliative care, including physical, psychological, and psychiatric; social; cultural, ethical, and legal; and spiritual, religious, and existential aspects.
“The expectation is that all clinicians caring for seriously ill patients will integrate palliative care competencies, such as safe and effective pain and symptom management and expert communication skills in their practice, and palliative care specialists will provide expertise for those with the most complex needs,” the guideline authors wrote.
Implications for treatment of oncology patients
These new guidelines represent a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, who is a medical oncologist, palliative care physician, and patient experience researcher at Duke University, Durham, N.C.
“Part of this report to is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” said Dr. LeBlanc.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who needs us the most, and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said in an interview.
That’s a major driver behind the emphasis in these latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, he added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists, and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Palliative care in surgical care
These guidelines are particularly useful to surgeons in part because of their focus on what’s known as primary palliative care, said to Geoffrey P. Dunn, MD, former chair of the American College of Surgeons Committee on Surgical Palliative Care. Palliative care, the new guidelines suggest, can be implemented by nonspecialists.
Primary palliative care includes diverse skills such as breaking adverse news to patients, managing uncomplicated pain, and being able to recognize signs and symptoms of imminent demise. “These are the minimum deliverables for all people dealing with seriously ill patients,” Dr. Dunn said in an interview. “It’s palliative care that any practicing physician should be able to handle.”
Dr. Dunn concurred with Dr. LaBlanc about the workforce shortage in the palliative field. The traditional model has created a shortage of specialized clinicians to meet palliative care needs. Across the board, “staffing for palliative teams is very inconsistent,” said Dr. Dunn. “It’s a classic unfunded mandate.”
While these guidelines are a step forward in recognizing the importance of palliative care outside of the palliative care specialty, there is no reference to surgery anywhere in the text of the 141-page prepublication draft provided by the NCP, Dr. Dunn noted in the interview.
“There’s still a danger of parallel universes, where surgery is developing its own understanding of this in parallel with the more general national palliative care movement,” he said. Despite that, there is a growing connection between surgery and the broader palliative care community. That linkage is especially important given the number of seriously ill patients with high symptom burden that are seen in surgery.
“I think where surgeons are beginning to find [palliative principles] very helpful is dealing with these protracted serial discussions with families in difficult circumstances, such as how long is the life support going to be prolonged in someone with a devastating head injury, or multiple system organ failure in the elderly,” Dr. Dunn added.
The new edition of providing care for critically ill patients, not just those clinicians actively specialized in palliative care.
The Clinical Practice Guidelines for Quality Palliative Care, 4th Edition, emphasizes the importance of palliative care provided by “clinicians in primary care and specialty care practices, such as oncologists,” the guideline authors stated.
The latest revision of the guideline aims to establish a foundation for “gold-standard” palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to the National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology and the Oncology Nurses Society.
One key reason for the update, according to the NCP, was to acknowledge that today’s health care system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on all clinicians who are not palliative specialists to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
This approach differs from the way palliative care is traditionally practiced, often by fellowship-trained physicians, trained nurses, and other specialists who provide that support.
The guidelines are organized into sections covering palliative care structure and processes, care for the patient nearing the end of life, and specific aspects of palliative care, including physical, psychological, and psychiatric; social; cultural, ethical, and legal; and spiritual, religious, and existential aspects.
“The expectation is that all clinicians caring for seriously ill patients will integrate palliative care competencies, such as safe and effective pain and symptom management and expert communication skills in their practice, and palliative care specialists will provide expertise for those with the most complex needs,” the guideline authors wrote.
Implications for treatment of oncology patients
These new guidelines represent a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, who is a medical oncologist, palliative care physician, and patient experience researcher at Duke University, Durham, N.C.
“Part of this report to is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” said Dr. LeBlanc.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who needs us the most, and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said in an interview.
That’s a major driver behind the emphasis in these latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, he added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists, and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Palliative care in surgical care
These guidelines are particularly useful to surgeons in part because of their focus on what’s known as primary palliative care, said to Geoffrey P. Dunn, MD, former chair of the American College of Surgeons Committee on Surgical Palliative Care. Palliative care, the new guidelines suggest, can be implemented by nonspecialists.
Primary palliative care includes diverse skills such as breaking adverse news to patients, managing uncomplicated pain, and being able to recognize signs and symptoms of imminent demise. “These are the minimum deliverables for all people dealing with seriously ill patients,” Dr. Dunn said in an interview. “It’s palliative care that any practicing physician should be able to handle.”
Dr. Dunn concurred with Dr. LaBlanc about the workforce shortage in the palliative field. The traditional model has created a shortage of specialized clinicians to meet palliative care needs. Across the board, “staffing for palliative teams is very inconsistent,” said Dr. Dunn. “It’s a classic unfunded mandate.”
While these guidelines are a step forward in recognizing the importance of palliative care outside of the palliative care specialty, there is no reference to surgery anywhere in the text of the 141-page prepublication draft provided by the NCP, Dr. Dunn noted in the interview.
“There’s still a danger of parallel universes, where surgery is developing its own understanding of this in parallel with the more general national palliative care movement,” he said. Despite that, there is a growing connection between surgery and the broader palliative care community. That linkage is especially important given the number of seriously ill patients with high symptom burden that are seen in surgery.
“I think where surgeons are beginning to find [palliative principles] very helpful is dealing with these protracted serial discussions with families in difficult circumstances, such as how long is the life support going to be prolonged in someone with a devastating head injury, or multiple system organ failure in the elderly,” Dr. Dunn added.
The new edition of providing care for critically ill patients, not just those clinicians actively specialized in palliative care.
The Clinical Practice Guidelines for Quality Palliative Care, 4th Edition, emphasizes the importance of palliative care provided by “clinicians in primary care and specialty care practices, such as oncologists,” the guideline authors stated.
The latest revision of the guideline aims to establish a foundation for “gold-standard” palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to the National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology and the Oncology Nurses Society.
One key reason for the update, according to the NCP, was to acknowledge that today’s health care system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on all clinicians who are not palliative specialists to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
This approach differs from the way palliative care is traditionally practiced, often by fellowship-trained physicians, trained nurses, and other specialists who provide that support.
The guidelines are organized into sections covering palliative care structure and processes, care for the patient nearing the end of life, and specific aspects of palliative care, including physical, psychological, and psychiatric; social; cultural, ethical, and legal; and spiritual, religious, and existential aspects.
“The expectation is that all clinicians caring for seriously ill patients will integrate palliative care competencies, such as safe and effective pain and symptom management and expert communication skills in their practice, and palliative care specialists will provide expertise for those with the most complex needs,” the guideline authors wrote.
Implications for treatment of oncology patients
These new guidelines represent a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, who is a medical oncologist, palliative care physician, and patient experience researcher at Duke University, Durham, N.C.
“Part of this report to is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” said Dr. LeBlanc.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who needs us the most, and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said in an interview.
That’s a major driver behind the emphasis in these latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, he added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists, and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Palliative care in surgical care
These guidelines are particularly useful to surgeons in part because of their focus on what’s known as primary palliative care, said to Geoffrey P. Dunn, MD, former chair of the American College of Surgeons Committee on Surgical Palliative Care. Palliative care, the new guidelines suggest, can be implemented by nonspecialists.
Primary palliative care includes diverse skills such as breaking adverse news to patients, managing uncomplicated pain, and being able to recognize signs and symptoms of imminent demise. “These are the minimum deliverables for all people dealing with seriously ill patients,” Dr. Dunn said in an interview. “It’s palliative care that any practicing physician should be able to handle.”
Dr. Dunn concurred with Dr. LaBlanc about the workforce shortage in the palliative field. The traditional model has created a shortage of specialized clinicians to meet palliative care needs. Across the board, “staffing for palliative teams is very inconsistent,” said Dr. Dunn. “It’s a classic unfunded mandate.”
While these guidelines are a step forward in recognizing the importance of palliative care outside of the palliative care specialty, there is no reference to surgery anywhere in the text of the 141-page prepublication draft provided by the NCP, Dr. Dunn noted in the interview.
“There’s still a danger of parallel universes, where surgery is developing its own understanding of this in parallel with the more general national palliative care movement,” he said. Despite that, there is a growing connection between surgery and the broader palliative care community. That linkage is especially important given the number of seriously ill patients with high symptom burden that are seen in surgery.
“I think where surgeons are beginning to find [palliative principles] very helpful is dealing with these protracted serial discussions with families in difficult circumstances, such as how long is the life support going to be prolonged in someone with a devastating head injury, or multiple system organ failure in the elderly,” Dr. Dunn added.
Heart cell transplant rejections
The concept that cell transplantation is the answer to the treatment of heart failure may have suffered a major setback as a result of a research scam perpetrated at Harvard University’s Brigham and Women’s Hospital in Boston.
The fraudulent cardiac research at that institution was from the lab of Piero Anversa, MD, which falsified or fabricated data. Dr. Anversa was one of the leaders in pursuing the concept that adult cardiomyocytes can not only regenerate by cell division but can also be transplanted from one animal to another, in his case a mouse.
The original reports over a decade ago caused a major stir in heart failure research. They also were met with considerable skepticism in the research field and have not been reproduced in other laboratories. Numerous small trials in humans have been unsuccessful in demonstrating the survival of autologous cells transplanted in both animal and humans. Dr. Anversa’s research has been under scrutiny by Harvard and Brigham and Women’s since 2013, ultimately resulting in the call to retract 31 published studies in mid-October. In the meantime, admission of fraud in regard to the original Anversa papers resulted in a fine of $10 million paid by the institutions to the National Institutes of Health in a 2017 settlement. Dr. Anversa left Harvard in 2015.
Nevertheless Dr. Anversa’s concepts led to the initiation of an NIH-supported multicenter trial in humans of the implantation of autologous bone marrow cells using endocardial devices to transplant cells in 144 patients with heart failure. The Combination of Autologous Bone Marrow Derived Mesenchymal and C-Kit+ Cardiac Stem Cells as Regenerative Therapy for Heart Failure (CONCERT-HF) was started in 2015 and was still recruiting until Oct. 29, when the National Heart, Lung, and Blood Institute announced that a pause in recruitment was called in order to review the fraudulent data that led to the trial’s initiation. Patients were to be followed over a 1-year period using delayed-enhanced magnetic resonance imaging (DEMRI) scans to assess scar size and left ventricular function and structure at baseline and at 6 and 12 months post study product ad-ministration. For the purpose of the endpoint analysis and safety evaluations, the investigators planned to use an intention-to-treat study population evaluating a number of clinical parameters.
Anatomists and physiologists have long been of the opinion that adult human and mammalian cardiomyocytes are terminally differentiated and do not undergo cell division. However, over time, cardiomyocytes can undergo hypertrophy as a result of increased workload. Cells can die as a result of ischemia, infarction, or stress mediated through unchecked inflammatory processes. It also has been shown that cells can die as a result of apoptosis, particularly in areas in proximity to myocardial scars. It is generally believed that these three methods of cell depletion contribute to the progression of heart failure. Dr. Anversa also proposed that there is some degree of replication of cardiomyocytes but not to a degree that can have a meaningful replacement value.
The concept of cell transplantation in medicine certainly has been in the forefront of clinical research in medicine for the last decade based in part on research by Dr. Anversa and others in cardiology and numerous investigators in other medical disciplines. With few exceptions, there has been little support for the clinical benefit of these clinical studies. We are unfortunately now faced with the release of the tainted fraudulent research that led to CONCERT-HF. Whether there is anything of scientific value to come of the trial is highly unlikely.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
This article was updated Oct. 30, 2018.
The concept that cell transplantation is the answer to the treatment of heart failure may have suffered a major setback as a result of a research scam perpetrated at Harvard University’s Brigham and Women’s Hospital in Boston.
The fraudulent cardiac research at that institution was from the lab of Piero Anversa, MD, which falsified or fabricated data. Dr. Anversa was one of the leaders in pursuing the concept that adult cardiomyocytes can not only regenerate by cell division but can also be transplanted from one animal to another, in his case a mouse.
The original reports over a decade ago caused a major stir in heart failure research. They also were met with considerable skepticism in the research field and have not been reproduced in other laboratories. Numerous small trials in humans have been unsuccessful in demonstrating the survival of autologous cells transplanted in both animal and humans. Dr. Anversa’s research has been under scrutiny by Harvard and Brigham and Women’s since 2013, ultimately resulting in the call to retract 31 published studies in mid-October. In the meantime, admission of fraud in regard to the original Anversa papers resulted in a fine of $10 million paid by the institutions to the National Institutes of Health in a 2017 settlement. Dr. Anversa left Harvard in 2015.
Nevertheless Dr. Anversa’s concepts led to the initiation of an NIH-supported multicenter trial in humans of the implantation of autologous bone marrow cells using endocardial devices to transplant cells in 144 patients with heart failure. The Combination of Autologous Bone Marrow Derived Mesenchymal and C-Kit+ Cardiac Stem Cells as Regenerative Therapy for Heart Failure (CONCERT-HF) was started in 2015 and was still recruiting until Oct. 29, when the National Heart, Lung, and Blood Institute announced that a pause in recruitment was called in order to review the fraudulent data that led to the trial’s initiation. Patients were to be followed over a 1-year period using delayed-enhanced magnetic resonance imaging (DEMRI) scans to assess scar size and left ventricular function and structure at baseline and at 6 and 12 months post study product ad-ministration. For the purpose of the endpoint analysis and safety evaluations, the investigators planned to use an intention-to-treat study population evaluating a number of clinical parameters.
Anatomists and physiologists have long been of the opinion that adult human and mammalian cardiomyocytes are terminally differentiated and do not undergo cell division. However, over time, cardiomyocytes can undergo hypertrophy as a result of increased workload. Cells can die as a result of ischemia, infarction, or stress mediated through unchecked inflammatory processes. It also has been shown that cells can die as a result of apoptosis, particularly in areas in proximity to myocardial scars. It is generally believed that these three methods of cell depletion contribute to the progression of heart failure. Dr. Anversa also proposed that there is some degree of replication of cardiomyocytes but not to a degree that can have a meaningful replacement value.
The concept of cell transplantation in medicine certainly has been in the forefront of clinical research in medicine for the last decade based in part on research by Dr. Anversa and others in cardiology and numerous investigators in other medical disciplines. With few exceptions, there has been little support for the clinical benefit of these clinical studies. We are unfortunately now faced with the release of the tainted fraudulent research that led to CONCERT-HF. Whether there is anything of scientific value to come of the trial is highly unlikely.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
This article was updated Oct. 30, 2018.
The concept that cell transplantation is the answer to the treatment of heart failure may have suffered a major setback as a result of a research scam perpetrated at Harvard University’s Brigham and Women’s Hospital in Boston.
The fraudulent cardiac research at that institution was from the lab of Piero Anversa, MD, which falsified or fabricated data. Dr. Anversa was one of the leaders in pursuing the concept that adult cardiomyocytes can not only regenerate by cell division but can also be transplanted from one animal to another, in his case a mouse.
The original reports over a decade ago caused a major stir in heart failure research. They also were met with considerable skepticism in the research field and have not been reproduced in other laboratories. Numerous small trials in humans have been unsuccessful in demonstrating the survival of autologous cells transplanted in both animal and humans. Dr. Anversa’s research has been under scrutiny by Harvard and Brigham and Women’s since 2013, ultimately resulting in the call to retract 31 published studies in mid-October. In the meantime, admission of fraud in regard to the original Anversa papers resulted in a fine of $10 million paid by the institutions to the National Institutes of Health in a 2017 settlement. Dr. Anversa left Harvard in 2015.
Nevertheless Dr. Anversa’s concepts led to the initiation of an NIH-supported multicenter trial in humans of the implantation of autologous bone marrow cells using endocardial devices to transplant cells in 144 patients with heart failure. The Combination of Autologous Bone Marrow Derived Mesenchymal and C-Kit+ Cardiac Stem Cells as Regenerative Therapy for Heart Failure (CONCERT-HF) was started in 2015 and was still recruiting until Oct. 29, when the National Heart, Lung, and Blood Institute announced that a pause in recruitment was called in order to review the fraudulent data that led to the trial’s initiation. Patients were to be followed over a 1-year period using delayed-enhanced magnetic resonance imaging (DEMRI) scans to assess scar size and left ventricular function and structure at baseline and at 6 and 12 months post study product ad-ministration. For the purpose of the endpoint analysis and safety evaluations, the investigators planned to use an intention-to-treat study population evaluating a number of clinical parameters.
Anatomists and physiologists have long been of the opinion that adult human and mammalian cardiomyocytes are terminally differentiated and do not undergo cell division. However, over time, cardiomyocytes can undergo hypertrophy as a result of increased workload. Cells can die as a result of ischemia, infarction, or stress mediated through unchecked inflammatory processes. It also has been shown that cells can die as a result of apoptosis, particularly in areas in proximity to myocardial scars. It is generally believed that these three methods of cell depletion contribute to the progression of heart failure. Dr. Anversa also proposed that there is some degree of replication of cardiomyocytes but not to a degree that can have a meaningful replacement value.
The concept of cell transplantation in medicine certainly has been in the forefront of clinical research in medicine for the last decade based in part on research by Dr. Anversa and others in cardiology and numerous investigators in other medical disciplines. With few exceptions, there has been little support for the clinical benefit of these clinical studies. We are unfortunately now faced with the release of the tainted fraudulent research that led to CONCERT-HF. Whether there is anything of scientific value to come of the trial is highly unlikely.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
This article was updated Oct. 30, 2018.
Antimalarial-induced cardiomyopathy in lupus may be underrecognized
Cardiomyopathy induced by antimalarial treatment for systematic lupus erythematosus may not be as rare as previously thought, according to the authors of a case series published Oct. 15 in The Journal of Rheumatology.
The paper describes eight patients attending a lupus clinic, who were diagnosed with definite or possible antimalarial-induced cardiomyopathy over the course of 2 years.
Konstantinos Tselios, MD, PhD, a clinical research fellow at the University of Toronto Lupus Clinic, and his coauthors wrote that antimalarial-induced cardiomyopathy was thought to be relatively rare, with only 47 previous isolated reports, but they suggested the complication may be significantly underrecognized.
“Hypertrophic cardiomyopathy and heart failure, the most common clinical features of AM-induced cardiomyopathy (AMIC), may be falsely attributed to other causes, such as arterial hypertension or ischemic cardiomyopathy,” the authors wrote. “Consequently, nonspecific therapeutic approaches with diuretics and/or antihypertensives will exert minimum or even deleterious effects on such patients.”
All eight patients in this series were female, with a median age of 62.5 years, median disease duration of 35 years, and median antimalarial use of 22 years. They presented with conditions such as heart failure, exertional dyspnea, and pedal edema. Several patients were asymptomatic but had been found to have elevated heart biomarker levels that prompted further investigation.
All patients showed abnormal cardiac troponin I and brain natriuretic peptide levels, and seven of the eight also had chronically elevated creatine phosphokinase.
In three patients, endomyocardial biopsy showed cardiomyocyte vacuolation, intracytoplasmic myelinoid inclusions, and curvilinear bodies.
Four patients were diagnosed based on cardiac MRI, which showed features suggestive of antimalarial-induced cardiomyopathy, including ventricular hypertrophy with or without atrial enlargement and late gadolinium enhancement in a nonvascular pattern.
All patients had left ventricular hypertrophy, and four also had right ventricular hypertrophy. Only one patient showed impaired systolic function, compared with around half of patients in the literature with antimalarial-induced cardiomyopathy, but seven patients showed a restrictive filling pattern of the left ventricle.
“It seems possible that AMIC is a chronic process and systolic dysfunction will become apparent only in late stages,” the authors suggested.
One patient showed complete atrioventricular block, left ventricular and septal hypertrophy, and concomitant ocular toxicity.
After patients stopped antimalarials, the hypertrophy regressed and heart biomarkers decreased in seven patients, but one patient died from refractory heart failure.
Based on their findings, the authors proposed that heart-specific biomarkers be used as a regular screening tool for detecting myocardial injury, followed by more thorough investigations, such as cardiac MRI, in patients with positive biomarker findings.
“However, drug cessation should be prompt and probably upon suspicion of AMIC, because complete investigation may be delayed significantly.”
One author was supported by the Geoff Carr Fellowship from Lupus Ontario. The University of Toronto Lupus Research Program is supported by the University Health Network, Lou and Marissa Rocca, and the Lupus Foundation of Ontario.
SOURCE: Tselios K et al. J Rheumatol, 2018 Oct 15. doi: 10.3899/jrheum.180124.
Cardiomyopathy induced by antimalarial treatment for systematic lupus erythematosus may not be as rare as previously thought, according to the authors of a case series published Oct. 15 in The Journal of Rheumatology.
The paper describes eight patients attending a lupus clinic, who were diagnosed with definite or possible antimalarial-induced cardiomyopathy over the course of 2 years.
Konstantinos Tselios, MD, PhD, a clinical research fellow at the University of Toronto Lupus Clinic, and his coauthors wrote that antimalarial-induced cardiomyopathy was thought to be relatively rare, with only 47 previous isolated reports, but they suggested the complication may be significantly underrecognized.
“Hypertrophic cardiomyopathy and heart failure, the most common clinical features of AM-induced cardiomyopathy (AMIC), may be falsely attributed to other causes, such as arterial hypertension or ischemic cardiomyopathy,” the authors wrote. “Consequently, nonspecific therapeutic approaches with diuretics and/or antihypertensives will exert minimum or even deleterious effects on such patients.”
All eight patients in this series were female, with a median age of 62.5 years, median disease duration of 35 years, and median antimalarial use of 22 years. They presented with conditions such as heart failure, exertional dyspnea, and pedal edema. Several patients were asymptomatic but had been found to have elevated heart biomarker levels that prompted further investigation.
All patients showed abnormal cardiac troponin I and brain natriuretic peptide levels, and seven of the eight also had chronically elevated creatine phosphokinase.
In three patients, endomyocardial biopsy showed cardiomyocyte vacuolation, intracytoplasmic myelinoid inclusions, and curvilinear bodies.
Four patients were diagnosed based on cardiac MRI, which showed features suggestive of antimalarial-induced cardiomyopathy, including ventricular hypertrophy with or without atrial enlargement and late gadolinium enhancement in a nonvascular pattern.
All patients had left ventricular hypertrophy, and four also had right ventricular hypertrophy. Only one patient showed impaired systolic function, compared with around half of patients in the literature with antimalarial-induced cardiomyopathy, but seven patients showed a restrictive filling pattern of the left ventricle.
“It seems possible that AMIC is a chronic process and systolic dysfunction will become apparent only in late stages,” the authors suggested.
One patient showed complete atrioventricular block, left ventricular and septal hypertrophy, and concomitant ocular toxicity.
After patients stopped antimalarials, the hypertrophy regressed and heart biomarkers decreased in seven patients, but one patient died from refractory heart failure.
Based on their findings, the authors proposed that heart-specific biomarkers be used as a regular screening tool for detecting myocardial injury, followed by more thorough investigations, such as cardiac MRI, in patients with positive biomarker findings.
“However, drug cessation should be prompt and probably upon suspicion of AMIC, because complete investigation may be delayed significantly.”
One author was supported by the Geoff Carr Fellowship from Lupus Ontario. The University of Toronto Lupus Research Program is supported by the University Health Network, Lou and Marissa Rocca, and the Lupus Foundation of Ontario.
SOURCE: Tselios K et al. J Rheumatol, 2018 Oct 15. doi: 10.3899/jrheum.180124.
Cardiomyopathy induced by antimalarial treatment for systematic lupus erythematosus may not be as rare as previously thought, according to the authors of a case series published Oct. 15 in The Journal of Rheumatology.
The paper describes eight patients attending a lupus clinic, who were diagnosed with definite or possible antimalarial-induced cardiomyopathy over the course of 2 years.
Konstantinos Tselios, MD, PhD, a clinical research fellow at the University of Toronto Lupus Clinic, and his coauthors wrote that antimalarial-induced cardiomyopathy was thought to be relatively rare, with only 47 previous isolated reports, but they suggested the complication may be significantly underrecognized.
“Hypertrophic cardiomyopathy and heart failure, the most common clinical features of AM-induced cardiomyopathy (AMIC), may be falsely attributed to other causes, such as arterial hypertension or ischemic cardiomyopathy,” the authors wrote. “Consequently, nonspecific therapeutic approaches with diuretics and/or antihypertensives will exert minimum or even deleterious effects on such patients.”
All eight patients in this series were female, with a median age of 62.5 years, median disease duration of 35 years, and median antimalarial use of 22 years. They presented with conditions such as heart failure, exertional dyspnea, and pedal edema. Several patients were asymptomatic but had been found to have elevated heart biomarker levels that prompted further investigation.
All patients showed abnormal cardiac troponin I and brain natriuretic peptide levels, and seven of the eight also had chronically elevated creatine phosphokinase.
In three patients, endomyocardial biopsy showed cardiomyocyte vacuolation, intracytoplasmic myelinoid inclusions, and curvilinear bodies.
Four patients were diagnosed based on cardiac MRI, which showed features suggestive of antimalarial-induced cardiomyopathy, including ventricular hypertrophy with or without atrial enlargement and late gadolinium enhancement in a nonvascular pattern.
All patients had left ventricular hypertrophy, and four also had right ventricular hypertrophy. Only one patient showed impaired systolic function, compared with around half of patients in the literature with antimalarial-induced cardiomyopathy, but seven patients showed a restrictive filling pattern of the left ventricle.
“It seems possible that AMIC is a chronic process and systolic dysfunction will become apparent only in late stages,” the authors suggested.
One patient showed complete atrioventricular block, left ventricular and septal hypertrophy, and concomitant ocular toxicity.
After patients stopped antimalarials, the hypertrophy regressed and heart biomarkers decreased in seven patients, but one patient died from refractory heart failure.
Based on their findings, the authors proposed that heart-specific biomarkers be used as a regular screening tool for detecting myocardial injury, followed by more thorough investigations, such as cardiac MRI, in patients with positive biomarker findings.
“However, drug cessation should be prompt and probably upon suspicion of AMIC, because complete investigation may be delayed significantly.”
One author was supported by the Geoff Carr Fellowship from Lupus Ontario. The University of Toronto Lupus Research Program is supported by the University Health Network, Lou and Marissa Rocca, and the Lupus Foundation of Ontario.
SOURCE: Tselios K et al. J Rheumatol, 2018 Oct 15. doi: 10.3899/jrheum.180124.
FROM THE JOURNAL OF RHEUMATOLOGY
Key clinical point:
Major finding: Elevated cardiac troponin I and brain natriuretic peptide, and abnormal cardiac MRI may indicate antimalarial-induced cardiomyopathy.
Study details: Case series of eight patients with antimalarial-induced cardiomyopathy.
Disclosures: One author was supported by the Geoff Carr Fellowship from Lupus Ontario. The University of Toronto Lupus Research Program is supported by the University Health Network, Lou and Marissa Rocca, and the Lupus Foundation of Ontario.
Source: Tselios K et al. J Rheumatol. 2018 Oct 15. doi: 10.3899/jrheum.180124.
HF unlikely in pregnant cancer survivors without history of cardiotoxicity
The risk of adverse cardiac events in female cancer survivors during pregnancy is low unless there is a history of cardiotoxicity, according to Shiying Liu, MD, of the University of Toronto, and her associates.
In a research letter published in the Journal of the American College of Cardiology, Dr. Liu and her associates reported on a retrospective chart review of 78 women with 94 pregnancies who had previously received cancer therapy who were seen at Mount Sinai Hospital between 2005 and 2015. Of these, 15 pregnancies occurred in 13 women with a prior history of cardiotoxicity. The primary outcome was a composite of cardiac events including cardiac death, heart failure (HF), acute coronary syndrome, and sustained arrhythmia.
HF occurred during five pregnancies in four women; no other adverse cardiac events occurred during the study period. All four of the women who experienced HF had a history of cardiotoxicity. There was no difference in age at cancer diagnosis, age at pregnancy, cancer type, or exposure to anthracyclines between those who did and did not experience HF, but women who developed HF were more likely to have left ventricular systolic dysfunction at the first antenatal visit (75% vs. 8%; P = .004) and to be on cardiac medications (50% vs. 8%; P = .026).
“The risk of developing [HF] during pregnancy is rare in female cancer survivors without a history of cardiotoxicity. These women can be reassured that they are at a very low risk of developing [HF] during pregnancy. Women who have a history of cardiotoxicity have an approximately one in three chance of developing [HF] during pregnancy and should receive close cardiac surveillance during pregnancy at a center with expertise in cardiac disease in pregnancy,” the authors concluded.
Coauthor Paaladinesh Thavendiranathan, MD, reported support from the Canadian Institutes of Health Research New Investigator Award. None of the other authors had any relevant financial disclosures.
SOURCE: Liu S et al. J Am Coll Cardiol. 2018 Oct 15. doi: 10.1016/j.jacc.2018.07.085.
The risk of adverse cardiac events in female cancer survivors during pregnancy is low unless there is a history of cardiotoxicity, according to Shiying Liu, MD, of the University of Toronto, and her associates.
In a research letter published in the Journal of the American College of Cardiology, Dr. Liu and her associates reported on a retrospective chart review of 78 women with 94 pregnancies who had previously received cancer therapy who were seen at Mount Sinai Hospital between 2005 and 2015. Of these, 15 pregnancies occurred in 13 women with a prior history of cardiotoxicity. The primary outcome was a composite of cardiac events including cardiac death, heart failure (HF), acute coronary syndrome, and sustained arrhythmia.
HF occurred during five pregnancies in four women; no other adverse cardiac events occurred during the study period. All four of the women who experienced HF had a history of cardiotoxicity. There was no difference in age at cancer diagnosis, age at pregnancy, cancer type, or exposure to anthracyclines between those who did and did not experience HF, but women who developed HF were more likely to have left ventricular systolic dysfunction at the first antenatal visit (75% vs. 8%; P = .004) and to be on cardiac medications (50% vs. 8%; P = .026).
“The risk of developing [HF] during pregnancy is rare in female cancer survivors without a history of cardiotoxicity. These women can be reassured that they are at a very low risk of developing [HF] during pregnancy. Women who have a history of cardiotoxicity have an approximately one in three chance of developing [HF] during pregnancy and should receive close cardiac surveillance during pregnancy at a center with expertise in cardiac disease in pregnancy,” the authors concluded.
Coauthor Paaladinesh Thavendiranathan, MD, reported support from the Canadian Institutes of Health Research New Investigator Award. None of the other authors had any relevant financial disclosures.
SOURCE: Liu S et al. J Am Coll Cardiol. 2018 Oct 15. doi: 10.1016/j.jacc.2018.07.085.
The risk of adverse cardiac events in female cancer survivors during pregnancy is low unless there is a history of cardiotoxicity, according to Shiying Liu, MD, of the University of Toronto, and her associates.
In a research letter published in the Journal of the American College of Cardiology, Dr. Liu and her associates reported on a retrospective chart review of 78 women with 94 pregnancies who had previously received cancer therapy who were seen at Mount Sinai Hospital between 2005 and 2015. Of these, 15 pregnancies occurred in 13 women with a prior history of cardiotoxicity. The primary outcome was a composite of cardiac events including cardiac death, heart failure (HF), acute coronary syndrome, and sustained arrhythmia.
HF occurred during five pregnancies in four women; no other adverse cardiac events occurred during the study period. All four of the women who experienced HF had a history of cardiotoxicity. There was no difference in age at cancer diagnosis, age at pregnancy, cancer type, or exposure to anthracyclines between those who did and did not experience HF, but women who developed HF were more likely to have left ventricular systolic dysfunction at the first antenatal visit (75% vs. 8%; P = .004) and to be on cardiac medications (50% vs. 8%; P = .026).
“The risk of developing [HF] during pregnancy is rare in female cancer survivors without a history of cardiotoxicity. These women can be reassured that they are at a very low risk of developing [HF] during pregnancy. Women who have a history of cardiotoxicity have an approximately one in three chance of developing [HF] during pregnancy and should receive close cardiac surveillance during pregnancy at a center with expertise in cardiac disease in pregnancy,” the authors concluded.
Coauthor Paaladinesh Thavendiranathan, MD, reported support from the Canadian Institutes of Health Research New Investigator Award. None of the other authors had any relevant financial disclosures.
SOURCE: Liu S et al. J Am Coll Cardiol. 2018 Oct 15. doi: 10.1016/j.jacc.2018.07.085.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY