User login
RVD With Weekly Bortezomib Has a Favorable Toxicity and Effectiveness Profile in a Large Cohort of US Veterans With Multiple Myeloma
Background
Lenalidomide, bortezomib, and dexamethasone (RVD) is standard triplet induction for fit newly-diagnosed myeloma (NDMM) patients, with response rate (RR)>90%. A 21-day cycle with bortezomib given days 1, 4, 8, and 11 (2x/w) is standard. However, up to 80% of patients develop neuropathy. Weekly bortezomib dosing (1x/w), subcutaneous route, and 28- to 35-day cycle length may optimize tolerance. We present an effectiveness and toxicity analysis of Veterans who received RVD with 1x/w and 2x/w bortezomib for NDMM.
Methods
The VA Corporate Data Warehouse identified 1499 Veterans with NDMM given RVD ≤42 days of treatment start. 840 Veterans were grouped for initial analysis based on criteria: 1) lenalidomide and ≥ 3 bortezomib doses during cycle 1; 2) ≥6 mean days between bortezomib treatments=1x/w); and 3) number of lenalidomide days informed cycle length (21d, 28d, or 35d; default 7-day rest). Investigators reviewed algorithm results to finalize group assignments. Endpoints included depth of response, time to next treatment (TTNT), overall survival (OS), and neuropathy. Neuropathy was defined as use of neuropathy medications and neuropathy ICD-10 codes.
Results
Our algorithm correctly assigned 82% of 840 cycle 1 RVD schedules. The largest groups were 21d 1x/w (n=291), 21d 2x/w (n=193), 28d 1x/w (n=188), and 28d 2x/w (n=82). Median age was 68.3; 53% were non-Hispanic White. Demographics and ISS stage of groups were similar. 30% underwent autologous transplant. Tolerability. Median number of bortezomib doses ranged from 22.5-25.5 (p=0.57). Neuropathy favored 1x/w, 17.7 vs 30.2% (p=0.0001) and was highest (34.7%) in 21d 2x/w. Effectiveness. Response was assessable for 28% of patients. RR (72%, p=0.68) and median TTNT (19.3 months, p=0.20) were similar, including 1x/w vs 2x/w comparison (p=0.79). 21d regimens optimized TTNT (21.4 vs 13.9 months, p=0.045) and trended to better OS (73 vs 65 months, p=0.06).
Conclusions
1x/w RVD preserved effectiveness compared to “standard” RVD in a large Veteran cohort. 1x/w reduced neuropathy incidence. 28d regimens demonstrated inferior longer-term outcomes. Certain endpoints, such as RR and neuropathy, appear underestimated due to data source limitations. 21d 1x/w RVD optimizes effectiveness, tolerability, and administration and should be considered for broader utilization in Veterans with NDMM.
Background
Lenalidomide, bortezomib, and dexamethasone (RVD) is standard triplet induction for fit newly-diagnosed myeloma (NDMM) patients, with response rate (RR)>90%. A 21-day cycle with bortezomib given days 1, 4, 8, and 11 (2x/w) is standard. However, up to 80% of patients develop neuropathy. Weekly bortezomib dosing (1x/w), subcutaneous route, and 28- to 35-day cycle length may optimize tolerance. We present an effectiveness and toxicity analysis of Veterans who received RVD with 1x/w and 2x/w bortezomib for NDMM.
Methods
The VA Corporate Data Warehouse identified 1499 Veterans with NDMM given RVD ≤42 days of treatment start. 840 Veterans were grouped for initial analysis based on criteria: 1) lenalidomide and ≥ 3 bortezomib doses during cycle 1; 2) ≥6 mean days between bortezomib treatments=1x/w); and 3) number of lenalidomide days informed cycle length (21d, 28d, or 35d; default 7-day rest). Investigators reviewed algorithm results to finalize group assignments. Endpoints included depth of response, time to next treatment (TTNT), overall survival (OS), and neuropathy. Neuropathy was defined as use of neuropathy medications and neuropathy ICD-10 codes.
Results
Our algorithm correctly assigned 82% of 840 cycle 1 RVD schedules. The largest groups were 21d 1x/w (n=291), 21d 2x/w (n=193), 28d 1x/w (n=188), and 28d 2x/w (n=82). Median age was 68.3; 53% were non-Hispanic White. Demographics and ISS stage of groups were similar. 30% underwent autologous transplant. Tolerability. Median number of bortezomib doses ranged from 22.5-25.5 (p=0.57). Neuropathy favored 1x/w, 17.7 vs 30.2% (p=0.0001) and was highest (34.7%) in 21d 2x/w. Effectiveness. Response was assessable for 28% of patients. RR (72%, p=0.68) and median TTNT (19.3 months, p=0.20) were similar, including 1x/w vs 2x/w comparison (p=0.79). 21d regimens optimized TTNT (21.4 vs 13.9 months, p=0.045) and trended to better OS (73 vs 65 months, p=0.06).
Conclusions
1x/w RVD preserved effectiveness compared to “standard” RVD in a large Veteran cohort. 1x/w reduced neuropathy incidence. 28d regimens demonstrated inferior longer-term outcomes. Certain endpoints, such as RR and neuropathy, appear underestimated due to data source limitations. 21d 1x/w RVD optimizes effectiveness, tolerability, and administration and should be considered for broader utilization in Veterans with NDMM.
Background
Lenalidomide, bortezomib, and dexamethasone (RVD) is standard triplet induction for fit newly-diagnosed myeloma (NDMM) patients, with response rate (RR)>90%. A 21-day cycle with bortezomib given days 1, 4, 8, and 11 (2x/w) is standard. However, up to 80% of patients develop neuropathy. Weekly bortezomib dosing (1x/w), subcutaneous route, and 28- to 35-day cycle length may optimize tolerance. We present an effectiveness and toxicity analysis of Veterans who received RVD with 1x/w and 2x/w bortezomib for NDMM.
Methods
The VA Corporate Data Warehouse identified 1499 Veterans with NDMM given RVD ≤42 days of treatment start. 840 Veterans were grouped for initial analysis based on criteria: 1) lenalidomide and ≥ 3 bortezomib doses during cycle 1; 2) ≥6 mean days between bortezomib treatments=1x/w); and 3) number of lenalidomide days informed cycle length (21d, 28d, or 35d; default 7-day rest). Investigators reviewed algorithm results to finalize group assignments. Endpoints included depth of response, time to next treatment (TTNT), overall survival (OS), and neuropathy. Neuropathy was defined as use of neuropathy medications and neuropathy ICD-10 codes.
Results
Our algorithm correctly assigned 82% of 840 cycle 1 RVD schedules. The largest groups were 21d 1x/w (n=291), 21d 2x/w (n=193), 28d 1x/w (n=188), and 28d 2x/w (n=82). Median age was 68.3; 53% were non-Hispanic White. Demographics and ISS stage of groups were similar. 30% underwent autologous transplant. Tolerability. Median number of bortezomib doses ranged from 22.5-25.5 (p=0.57). Neuropathy favored 1x/w, 17.7 vs 30.2% (p=0.0001) and was highest (34.7%) in 21d 2x/w. Effectiveness. Response was assessable for 28% of patients. RR (72%, p=0.68) and median TTNT (19.3 months, p=0.20) were similar, including 1x/w vs 2x/w comparison (p=0.79). 21d regimens optimized TTNT (21.4 vs 13.9 months, p=0.045) and trended to better OS (73 vs 65 months, p=0.06).
Conclusions
1x/w RVD preserved effectiveness compared to “standard” RVD in a large Veteran cohort. 1x/w reduced neuropathy incidence. 28d regimens demonstrated inferior longer-term outcomes. Certain endpoints, such as RR and neuropathy, appear underestimated due to data source limitations. 21d 1x/w RVD optimizes effectiveness, tolerability, and administration and should be considered for broader utilization in Veterans with NDMM.
Chronic Myeloid Leukemia Presenting as Priapism: A Rare and Acute Initial Presentation in a Young Male
Introduction
Priapism, defined as a prolonged and often painful penile erection without sexual arousal, constitutes a urological emergency requiring immediate intervention. While commonly associated with conditions like sickle cell anemia and certain medications, malignancy-related priapism is rare and frequently overlooked. Herein, we present a unique case of a 31-year-old male with no significant medical history, who developed persistent priapism as the initial presentation of chronic myeloid leukemia (CML).
Case Presentation
A 31-year-old male without significant medical history, presented to the emergency department with painless priapism, was evaluated by urology and discharged home with precautions. He returned the following day with persistent, now painful priapism. Upon examination, his vital signs were stable. Urology performed aspiration and injection with Sudafed, resulting in mild symptom improvement. Laboratory findings revealed elevated white blood cell count (563.64 k/mcL), anemia (hemoglobin 8.4 g/dL), and a peripheral blood smear showed immature circulating cells with blast forms. He was transferred to a tertiary care center where conservative management addressed bleeding from the penile injection site, with subsequent treatment including leukapheresis and hydroxyurea for cytoreduction. Imaging revealed severe splenomegaly (36 cm) with abdominal mass effect. Peripheral flow cytometry didn’t show malignancy, but cytogenetic analysis showed a BCR/ABL1 fusion gene, confirming chronic myeloid leukemia (CML). Bone marrow biopsy showed hypercellularity without increased blasts. Treatment with dasatinib reduced the white count to 52,000 k/mcL, and was discharged home.
Discussion
Priapism is a urological emergency necessitating immediate intervention to prevent erectile dysfunction and permanent impotence. Management aims to achieve detumescence and typically involves methods such as irrigation or injection of vasoconstrictors into the penis. Malignancy-associated priapism (MAP) often results from venous obstruction due to hyperviscosity. Studies show that CML accounts for approximately 50% cases presenting with MAP, predominantly affecting younger individuals with a mean onset around 27 years of age. Priapism can occur before, during, or after treatment initiation or splenectomy in these patients. Providers should keep a high threshold of suspicion for MAP in patients with no other risk factors as prompt identification and treatment are needed to avoid permanent injury.
Introduction
Priapism, defined as a prolonged and often painful penile erection without sexual arousal, constitutes a urological emergency requiring immediate intervention. While commonly associated with conditions like sickle cell anemia and certain medications, malignancy-related priapism is rare and frequently overlooked. Herein, we present a unique case of a 31-year-old male with no significant medical history, who developed persistent priapism as the initial presentation of chronic myeloid leukemia (CML).
Case Presentation
A 31-year-old male without significant medical history, presented to the emergency department with painless priapism, was evaluated by urology and discharged home with precautions. He returned the following day with persistent, now painful priapism. Upon examination, his vital signs were stable. Urology performed aspiration and injection with Sudafed, resulting in mild symptom improvement. Laboratory findings revealed elevated white blood cell count (563.64 k/mcL), anemia (hemoglobin 8.4 g/dL), and a peripheral blood smear showed immature circulating cells with blast forms. He was transferred to a tertiary care center where conservative management addressed bleeding from the penile injection site, with subsequent treatment including leukapheresis and hydroxyurea for cytoreduction. Imaging revealed severe splenomegaly (36 cm) with abdominal mass effect. Peripheral flow cytometry didn’t show malignancy, but cytogenetic analysis showed a BCR/ABL1 fusion gene, confirming chronic myeloid leukemia (CML). Bone marrow biopsy showed hypercellularity without increased blasts. Treatment with dasatinib reduced the white count to 52,000 k/mcL, and was discharged home.
Discussion
Priapism is a urological emergency necessitating immediate intervention to prevent erectile dysfunction and permanent impotence. Management aims to achieve detumescence and typically involves methods such as irrigation or injection of vasoconstrictors into the penis. Malignancy-associated priapism (MAP) often results from venous obstruction due to hyperviscosity. Studies show that CML accounts for approximately 50% cases presenting with MAP, predominantly affecting younger individuals with a mean onset around 27 years of age. Priapism can occur before, during, or after treatment initiation or splenectomy in these patients. Providers should keep a high threshold of suspicion for MAP in patients with no other risk factors as prompt identification and treatment are needed to avoid permanent injury.
Introduction
Priapism, defined as a prolonged and often painful penile erection without sexual arousal, constitutes a urological emergency requiring immediate intervention. While commonly associated with conditions like sickle cell anemia and certain medications, malignancy-related priapism is rare and frequently overlooked. Herein, we present a unique case of a 31-year-old male with no significant medical history, who developed persistent priapism as the initial presentation of chronic myeloid leukemia (CML).
Case Presentation
A 31-year-old male without significant medical history, presented to the emergency department with painless priapism, was evaluated by urology and discharged home with precautions. He returned the following day with persistent, now painful priapism. Upon examination, his vital signs were stable. Urology performed aspiration and injection with Sudafed, resulting in mild symptom improvement. Laboratory findings revealed elevated white blood cell count (563.64 k/mcL), anemia (hemoglobin 8.4 g/dL), and a peripheral blood smear showed immature circulating cells with blast forms. He was transferred to a tertiary care center where conservative management addressed bleeding from the penile injection site, with subsequent treatment including leukapheresis and hydroxyurea for cytoreduction. Imaging revealed severe splenomegaly (36 cm) with abdominal mass effect. Peripheral flow cytometry didn’t show malignancy, but cytogenetic analysis showed a BCR/ABL1 fusion gene, confirming chronic myeloid leukemia (CML). Bone marrow biopsy showed hypercellularity without increased blasts. Treatment with dasatinib reduced the white count to 52,000 k/mcL, and was discharged home.
Discussion
Priapism is a urological emergency necessitating immediate intervention to prevent erectile dysfunction and permanent impotence. Management aims to achieve detumescence and typically involves methods such as irrigation or injection of vasoconstrictors into the penis. Malignancy-associated priapism (MAP) often results from venous obstruction due to hyperviscosity. Studies show that CML accounts for approximately 50% cases presenting with MAP, predominantly affecting younger individuals with a mean onset around 27 years of age. Priapism can occur before, during, or after treatment initiation or splenectomy in these patients. Providers should keep a high threshold of suspicion for MAP in patients with no other risk factors as prompt identification and treatment are needed to avoid permanent injury.
The First Female Patient in the Veteran Affairs System to Receive Chimeric Antigen Receptors (CAR) T-cell Therapy for Refractory Multiple Myeloma and the Role of CAR T-cell Therapy in Penta-refractory Disease
Background
In 2024, the first two veterans, both from the Michael E. DeBakey Veteran Affairs (VA) Medical Center, received chimeric antigen receptors (CAR) T-cell therapy for refractory multiple myeloma through the Tennessee Valley Healthcare System (TVHS). Currently, TVHS is the only VA where this treatment is available. One of these patients also had penta-refractory multiple myeloma (P-RMM), which is associated with significantly worse progression-free survival and overall survival (OS) (Gill et al, 2021). P-RMM is defined as resistance to at least two immunomodulatory drugs, two different proteasome inhibitors, and one CD38 monoclonal antibody.
Case Presentation
A 71-year-old female veteran was diagnosed with high-risk multiple myeloma and received induction therapy with carfilzomib, lenalidomide, and dexamethasone in 2017. She underwent autologous stem cell transplant (SCT) in 4/2018. The veteran subsequently received maintenance therapy with lenalidomide, bortezomib, and dexamethasone. Her disease recurred in 1/2022. The patient then received two more lines of treatments with daratumumab and pomalidomide followed by selinexor. She had another autologous SCT in 5/2023, to which she was refractory. Her fifth line therapy included addition of bortezomib to her selinexor regimen. She eventually underwent CAR T-cell therapy at THVS on 5/1/2024 with good tolerance of therapy. At her follow-up visit, the patient had significant response to CAR T-cell treatment, based on her symptoms and improvement in free light chains and serum protein electrophoresis.
Discussion
CAR T-cell therapy is one of the newest and most cutting-edge therapies for patients with refractory multiple myeloma. Access to this therapy has been limited throughout the country. However, as shown by our case, this life-saving treatment is now available to patients within the VA. According to a retrospective study on P-RMM patients, the OS in patients who received B-cell maturation antigen (BCMA) targeted therapy was significantly higher than in those who did not (17 vs. 6 months, p < 0.0001). Among the BCMA-targeted therapies, CAR T-cell therapy is associated with the highest OS (29 months) compared to antibody-drug conjugates and bispecific T-cell engagers (Atrash et al, 2023). Thus, accessibility to CAR T-cell therapy was essential in our patient with P-RMM in ensuring her best survival outcomes.
Background
In 2024, the first two veterans, both from the Michael E. DeBakey Veteran Affairs (VA) Medical Center, received chimeric antigen receptors (CAR) T-cell therapy for refractory multiple myeloma through the Tennessee Valley Healthcare System (TVHS). Currently, TVHS is the only VA where this treatment is available. One of these patients also had penta-refractory multiple myeloma (P-RMM), which is associated with significantly worse progression-free survival and overall survival (OS) (Gill et al, 2021). P-RMM is defined as resistance to at least two immunomodulatory drugs, two different proteasome inhibitors, and one CD38 monoclonal antibody.
Case Presentation
A 71-year-old female veteran was diagnosed with high-risk multiple myeloma and received induction therapy with carfilzomib, lenalidomide, and dexamethasone in 2017. She underwent autologous stem cell transplant (SCT) in 4/2018. The veteran subsequently received maintenance therapy with lenalidomide, bortezomib, and dexamethasone. Her disease recurred in 1/2022. The patient then received two more lines of treatments with daratumumab and pomalidomide followed by selinexor. She had another autologous SCT in 5/2023, to which she was refractory. Her fifth line therapy included addition of bortezomib to her selinexor regimen. She eventually underwent CAR T-cell therapy at THVS on 5/1/2024 with good tolerance of therapy. At her follow-up visit, the patient had significant response to CAR T-cell treatment, based on her symptoms and improvement in free light chains and serum protein electrophoresis.
Discussion
CAR T-cell therapy is one of the newest and most cutting-edge therapies for patients with refractory multiple myeloma. Access to this therapy has been limited throughout the country. However, as shown by our case, this life-saving treatment is now available to patients within the VA. According to a retrospective study on P-RMM patients, the OS in patients who received B-cell maturation antigen (BCMA) targeted therapy was significantly higher than in those who did not (17 vs. 6 months, p < 0.0001). Among the BCMA-targeted therapies, CAR T-cell therapy is associated with the highest OS (29 months) compared to antibody-drug conjugates and bispecific T-cell engagers (Atrash et al, 2023). Thus, accessibility to CAR T-cell therapy was essential in our patient with P-RMM in ensuring her best survival outcomes.
Background
In 2024, the first two veterans, both from the Michael E. DeBakey Veteran Affairs (VA) Medical Center, received chimeric antigen receptors (CAR) T-cell therapy for refractory multiple myeloma through the Tennessee Valley Healthcare System (TVHS). Currently, TVHS is the only VA where this treatment is available. One of these patients also had penta-refractory multiple myeloma (P-RMM), which is associated with significantly worse progression-free survival and overall survival (OS) (Gill et al, 2021). P-RMM is defined as resistance to at least two immunomodulatory drugs, two different proteasome inhibitors, and one CD38 monoclonal antibody.
Case Presentation
A 71-year-old female veteran was diagnosed with high-risk multiple myeloma and received induction therapy with carfilzomib, lenalidomide, and dexamethasone in 2017. She underwent autologous stem cell transplant (SCT) in 4/2018. The veteran subsequently received maintenance therapy with lenalidomide, bortezomib, and dexamethasone. Her disease recurred in 1/2022. The patient then received two more lines of treatments with daratumumab and pomalidomide followed by selinexor. She had another autologous SCT in 5/2023, to which she was refractory. Her fifth line therapy included addition of bortezomib to her selinexor regimen. She eventually underwent CAR T-cell therapy at THVS on 5/1/2024 with good tolerance of therapy. At her follow-up visit, the patient had significant response to CAR T-cell treatment, based on her symptoms and improvement in free light chains and serum protein electrophoresis.
Discussion
CAR T-cell therapy is one of the newest and most cutting-edge therapies for patients with refractory multiple myeloma. Access to this therapy has been limited throughout the country. However, as shown by our case, this life-saving treatment is now available to patients within the VA. According to a retrospective study on P-RMM patients, the OS in patients who received B-cell maturation antigen (BCMA) targeted therapy was significantly higher than in those who did not (17 vs. 6 months, p < 0.0001). Among the BCMA-targeted therapies, CAR T-cell therapy is associated with the highest OS (29 months) compared to antibody-drug conjugates and bispecific T-cell engagers (Atrash et al, 2023). Thus, accessibility to CAR T-cell therapy was essential in our patient with P-RMM in ensuring her best survival outcomes.
Is Location a Risk Factor for Early-Onset Cancer?
Early-onset cancer—diagnosed in adults aged ≤ 50 years—is on the rise. Researchers have studied a variety of factors driving the trend, such as type of cancer. However, geographic locality might have as much, if not more, to do with it, according to a study by researchers at Fox Chase Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center research facility.
Using the US Cancer Statistics Public Use Research Database, the researchers collected data from adults aged 20 to 49 years with invasive cancer (excluding in situ cases) diagnosed from 2015 through 2020. They calculated the incidence for each state using the national rate as the reference. Then, they calculated a second set of rates, comparing each state to the US in terms of overall incidence and advanced-stage incidence for all early-onset cancers.
The resulting maps indicated that early-onset cancer cases are not evenly distributed. States with worse-than-national rates are frequently near each other geographically. For instance, the rate of early-onset female breast cancer was worse than the national rate in 17 states, 16 of which were located in the eastern half of the US (Hawaii was the 17th state). Similarly, most states with worse-than-national rates of digestive cancers were located in the eastern half of the US, with a concentration in the South. Rates of male genital cancers were worse than national rates in 18 states, primarily in the eastern half of the country (plus Montana, Nebraska, and Puerto Rico).
Three states in the Southeast, 7 in the Northeast, and Puerto Rico had the highest incidence of lymphohematopoietic cancers. Incidence rates of endocrine cancers were worse than national rates in 25 states, which the researchers found formed “a horizontal core of the country running from east to west,” plus Puerto Rico. Rates of urinary system cancers were worse than national rates in 17 contiguous states, from New Mexico to Pennsylvania.
Rates of female genital cancers were worse than national rates in 16 states, largely in the Midwest and South, plus California and Puerto Rico. Skin cancer, on the other hand, was a great leveler, with worse-than-national rates in 32 states, mostly in the northern portion of the country.
Kentucky and West Virginia had the highest overall and advanced-stage incidence rates of early-onset cancer for all cancer sites combined. They were followed by Arkansas, Connecticut, Florida, Georgia, Iowa, Louisiana, Maine, Missouri, New Jersey, New York, North Carolina, Ohio, and Pennsylvania.
According to the researchers, this study provides the first analysis of age-adjusted rates of early-onset cancer based on state-level population and case numbers. Geographic patterns in early-onset cancer, they suggest, indicate possible similarities that could relate to demographic, socioeconomic, behavioral, or environmental risks. “Focusing prevention efforts on the highest-incidence states for the most prevalent sites may reduce the rate of early-onset cancer nationally.”
Early-onset cancer—diagnosed in adults aged ≤ 50 years—is on the rise. Researchers have studied a variety of factors driving the trend, such as type of cancer. However, geographic locality might have as much, if not more, to do with it, according to a study by researchers at Fox Chase Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center research facility.
Using the US Cancer Statistics Public Use Research Database, the researchers collected data from adults aged 20 to 49 years with invasive cancer (excluding in situ cases) diagnosed from 2015 through 2020. They calculated the incidence for each state using the national rate as the reference. Then, they calculated a second set of rates, comparing each state to the US in terms of overall incidence and advanced-stage incidence for all early-onset cancers.
The resulting maps indicated that early-onset cancer cases are not evenly distributed. States with worse-than-national rates are frequently near each other geographically. For instance, the rate of early-onset female breast cancer was worse than the national rate in 17 states, 16 of which were located in the eastern half of the US (Hawaii was the 17th state). Similarly, most states with worse-than-national rates of digestive cancers were located in the eastern half of the US, with a concentration in the South. Rates of male genital cancers were worse than national rates in 18 states, primarily in the eastern half of the country (plus Montana, Nebraska, and Puerto Rico).
Three states in the Southeast, 7 in the Northeast, and Puerto Rico had the highest incidence of lymphohematopoietic cancers. Incidence rates of endocrine cancers were worse than national rates in 25 states, which the researchers found formed “a horizontal core of the country running from east to west,” plus Puerto Rico. Rates of urinary system cancers were worse than national rates in 17 contiguous states, from New Mexico to Pennsylvania.
Rates of female genital cancers were worse than national rates in 16 states, largely in the Midwest and South, plus California and Puerto Rico. Skin cancer, on the other hand, was a great leveler, with worse-than-national rates in 32 states, mostly in the northern portion of the country.
Kentucky and West Virginia had the highest overall and advanced-stage incidence rates of early-onset cancer for all cancer sites combined. They were followed by Arkansas, Connecticut, Florida, Georgia, Iowa, Louisiana, Maine, Missouri, New Jersey, New York, North Carolina, Ohio, and Pennsylvania.
According to the researchers, this study provides the first analysis of age-adjusted rates of early-onset cancer based on state-level population and case numbers. Geographic patterns in early-onset cancer, they suggest, indicate possible similarities that could relate to demographic, socioeconomic, behavioral, or environmental risks. “Focusing prevention efforts on the highest-incidence states for the most prevalent sites may reduce the rate of early-onset cancer nationally.”
Early-onset cancer—diagnosed in adults aged ≤ 50 years—is on the rise. Researchers have studied a variety of factors driving the trend, such as type of cancer. However, geographic locality might have as much, if not more, to do with it, according to a study by researchers at Fox Chase Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center research facility.
Using the US Cancer Statistics Public Use Research Database, the researchers collected data from adults aged 20 to 49 years with invasive cancer (excluding in situ cases) diagnosed from 2015 through 2020. They calculated the incidence for each state using the national rate as the reference. Then, they calculated a second set of rates, comparing each state to the US in terms of overall incidence and advanced-stage incidence for all early-onset cancers.
The resulting maps indicated that early-onset cancer cases are not evenly distributed. States with worse-than-national rates are frequently near each other geographically. For instance, the rate of early-onset female breast cancer was worse than the national rate in 17 states, 16 of which were located in the eastern half of the US (Hawaii was the 17th state). Similarly, most states with worse-than-national rates of digestive cancers were located in the eastern half of the US, with a concentration in the South. Rates of male genital cancers were worse than national rates in 18 states, primarily in the eastern half of the country (plus Montana, Nebraska, and Puerto Rico).
Three states in the Southeast, 7 in the Northeast, and Puerto Rico had the highest incidence of lymphohematopoietic cancers. Incidence rates of endocrine cancers were worse than national rates in 25 states, which the researchers found formed “a horizontal core of the country running from east to west,” plus Puerto Rico. Rates of urinary system cancers were worse than national rates in 17 contiguous states, from New Mexico to Pennsylvania.
Rates of female genital cancers were worse than national rates in 16 states, largely in the Midwest and South, plus California and Puerto Rico. Skin cancer, on the other hand, was a great leveler, with worse-than-national rates in 32 states, mostly in the northern portion of the country.
Kentucky and West Virginia had the highest overall and advanced-stage incidence rates of early-onset cancer for all cancer sites combined. They were followed by Arkansas, Connecticut, Florida, Georgia, Iowa, Louisiana, Maine, Missouri, New Jersey, New York, North Carolina, Ohio, and Pennsylvania.
According to the researchers, this study provides the first analysis of age-adjusted rates of early-onset cancer based on state-level population and case numbers. Geographic patterns in early-onset cancer, they suggest, indicate possible similarities that could relate to demographic, socioeconomic, behavioral, or environmental risks. “Focusing prevention efforts on the highest-incidence states for the most prevalent sites may reduce the rate of early-onset cancer nationally.”
Cancer Data Trends 2024
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
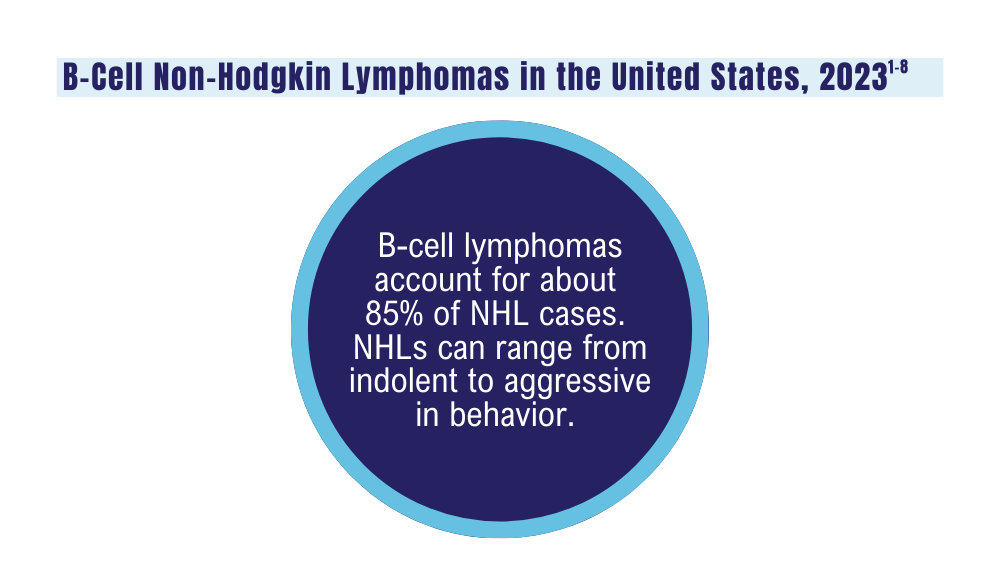
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
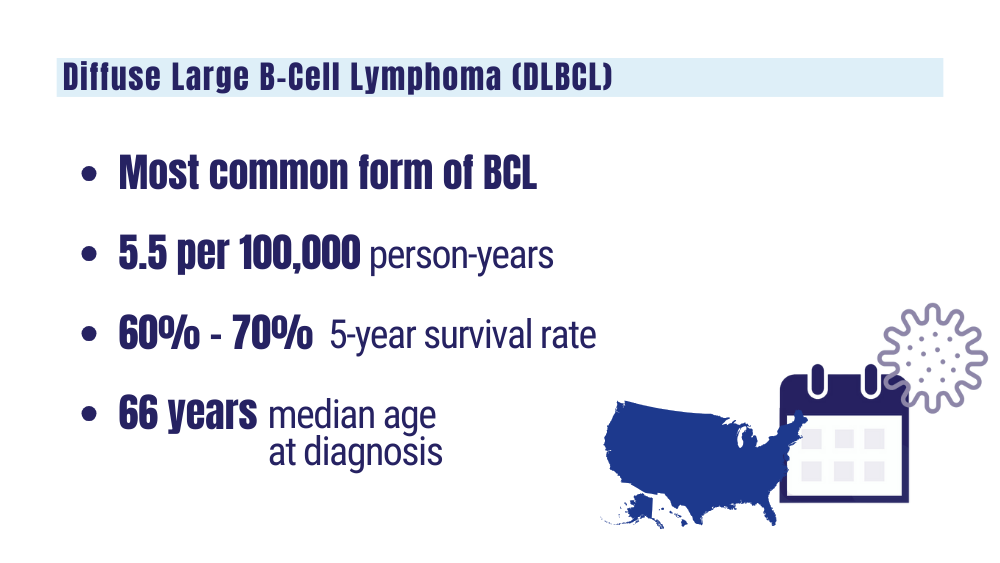
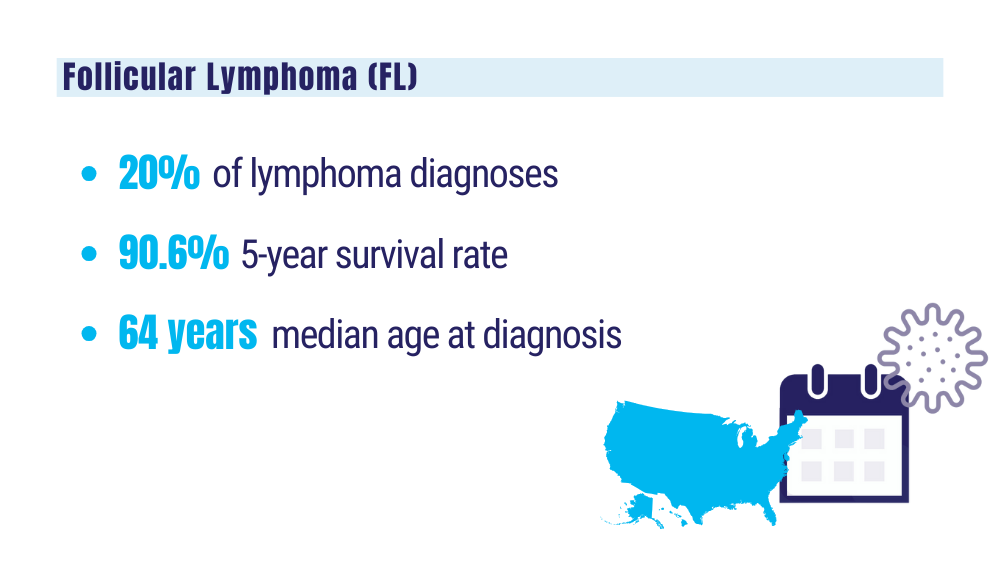
Cancer Data Trends 2024: B-Cell Lymphoma
Lu W, Chen W, Zhou Y, et al. A model to predict the prognosis of diffuse large B-cell lymphoma based on ultrasound images. Sci Rep. 2023;13(1):3346. doi:10.1038/s41598-023-30533-y
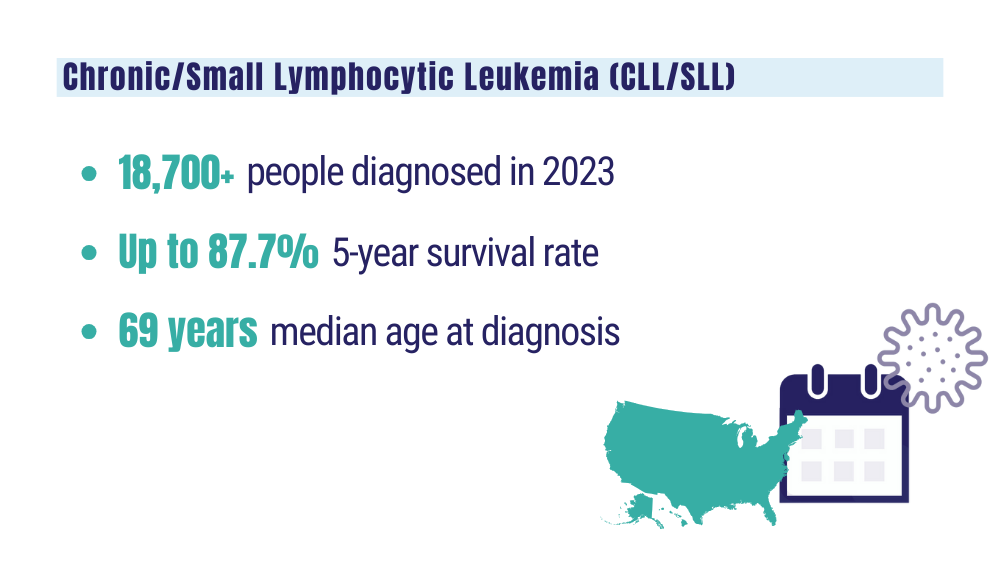
Leukemia - Chronic lymphocytic - CLL: statistics. Cancer.net. Published February 2023. Accessed January 24, 2024. https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics
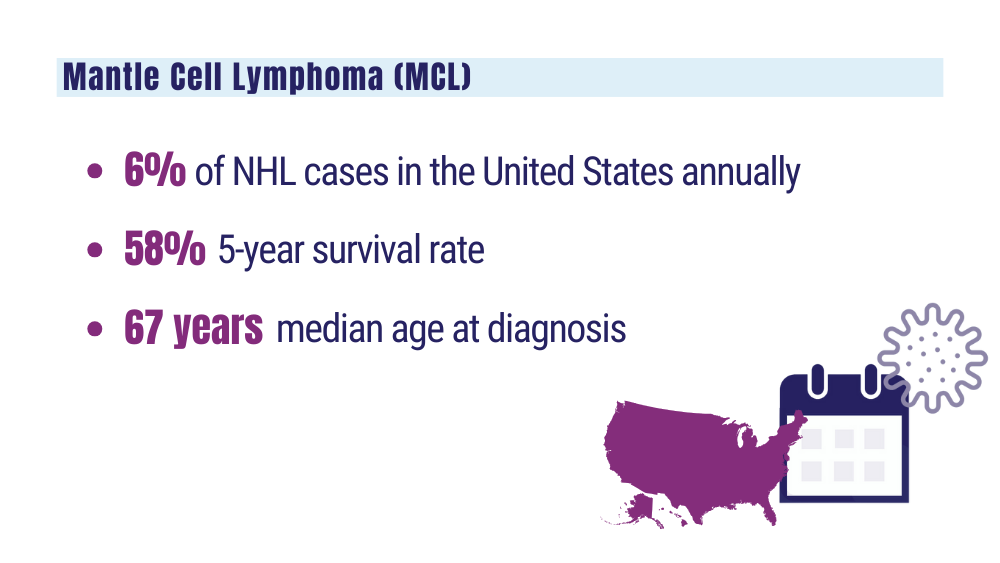
Harmanen M, Hujo M, Sund R, et al. Survival of patients with mantle cell lymphoma in the rituximab era: retrospective binational analysis between 2000 and 2020. Br J Haematol. 2023;201(1):64-74. doi:10.1111/bjh.18597
Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481. doi:10.1007/s40266-020-00765-y
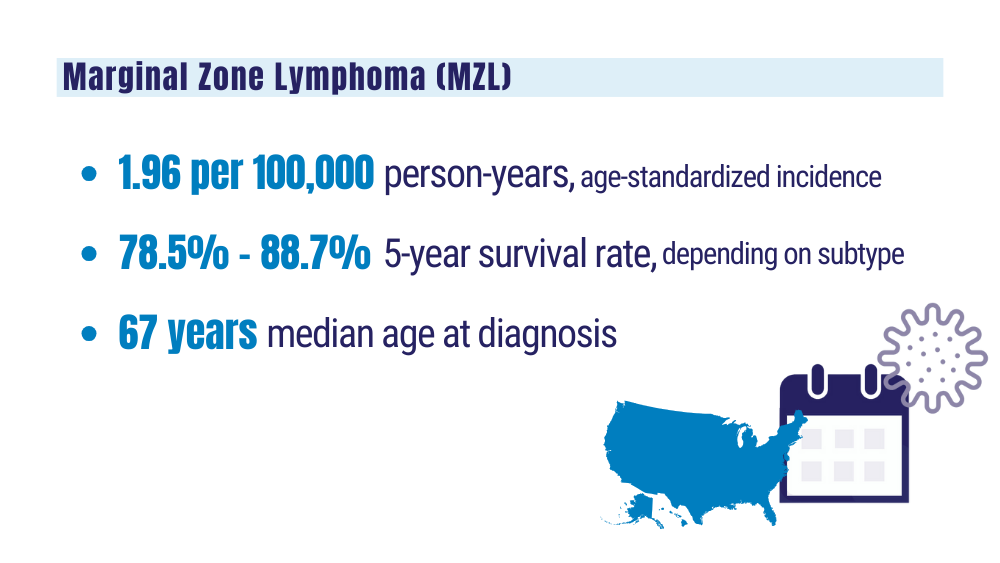
Marginal zone lymphoma (MZL). Leukemia and Lymphoma Society. Accessed January 24, 2024. https://www.lls.org/research/marginal-zone-lymphoma-mzl
Understanding lymphoma: diffuse large B-cell lymphoma. Lymphoma Research Foundation fact sheet. Updated 2023. Accessed January 24, 2024 https://lymphoma.org/wp-content/uploads/2023/10/LRF_Understanding_Lymphoma_Diffuse_Large_B_Cell_Lymphoma_Fact_Sheet.pdf
Key statistics for non-Hodgkin lymphoma. American Cancer Society. Updated January 17, 2024. Accessed January 24, 2024. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html
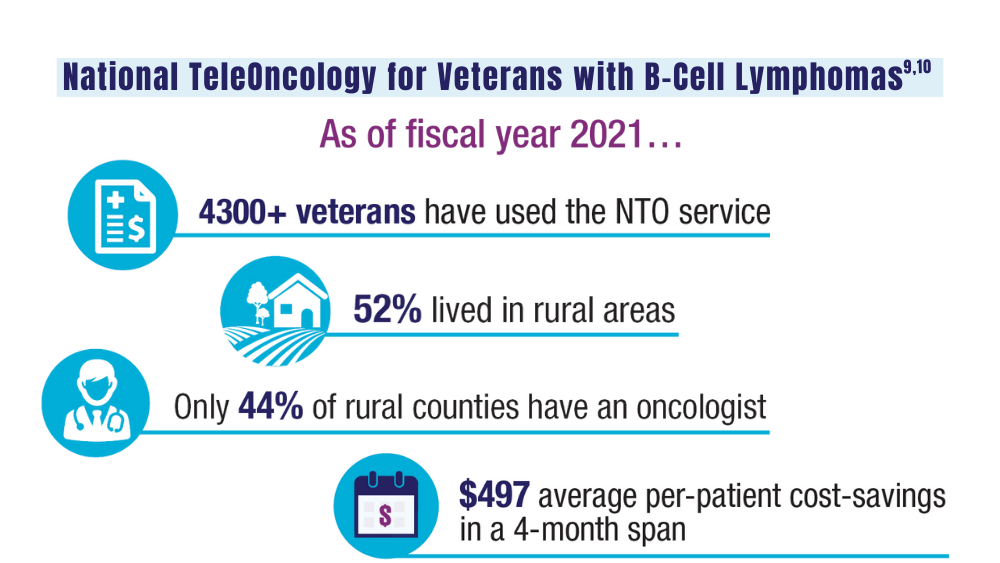
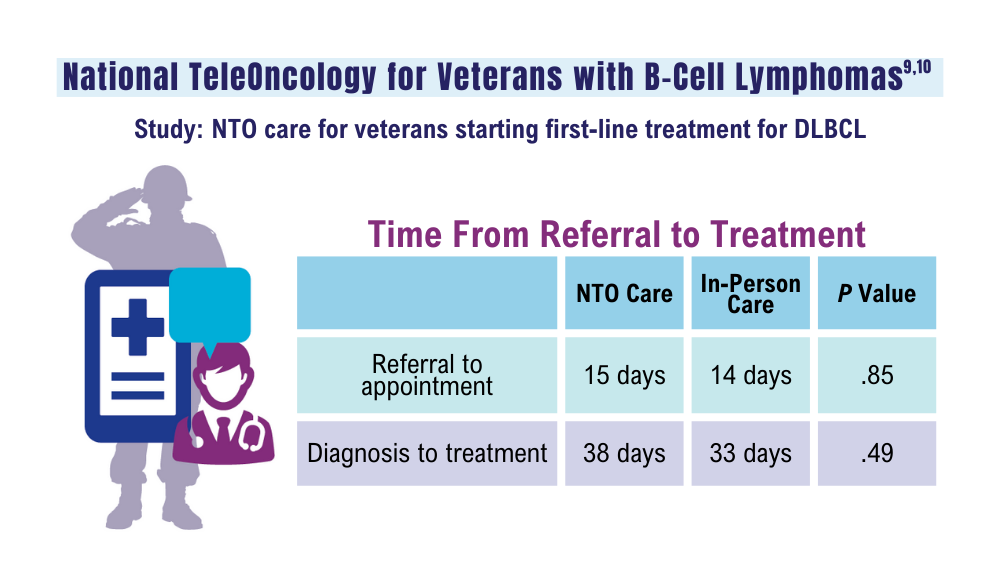
Zullig LL, Raska W, McWhirter G, et al. Veterans Health Administration National TeleOncology Service. JCO Oncol Pract. 2023;19(4):e504-e510. doi:10.1200/OP.22.00455
Lin C, Zhou KI, Burningham ZR, et al. Telemedicine-supervised cancer therapy for patients with an aggressive lymphoma and metastatic lung cancer in the U.S. Veterans Affairs National TeleOncology Service. J Clin Oncol. 2023;41(16 suppl)16:Abstract 1602. doi:10.1200/JCO.2023.41.16_suppl.1602
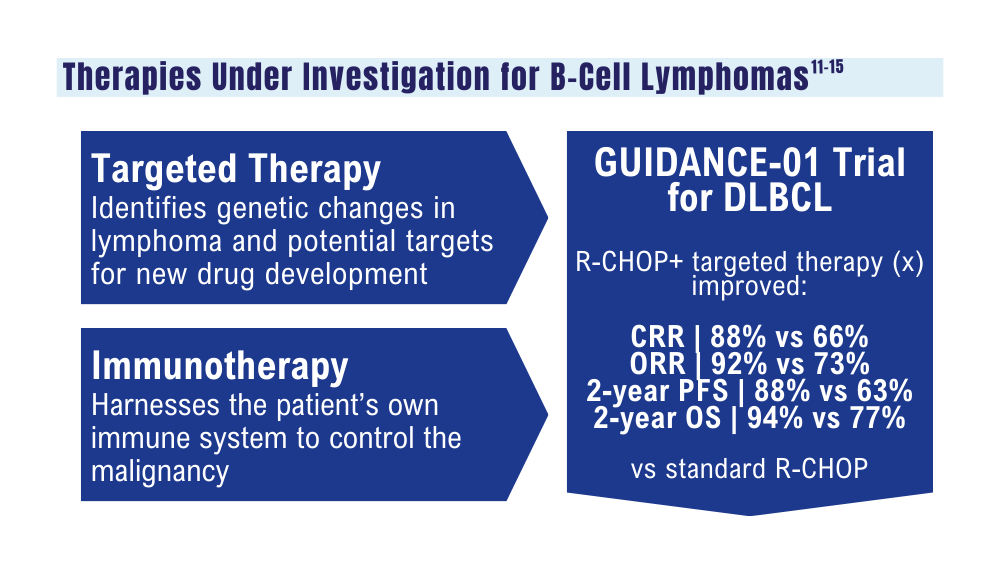
Zhang MC, Tian S, Fu D, et al. Genetic subtype-guided immunochemotherapy in diffuse large B-cell lymphoma: the randomized GUIDANCE-01 trial. Cancer Cell. 2023;41(10):1705-1716.e5. doi:10.1016/j.ccell.2023.09.004
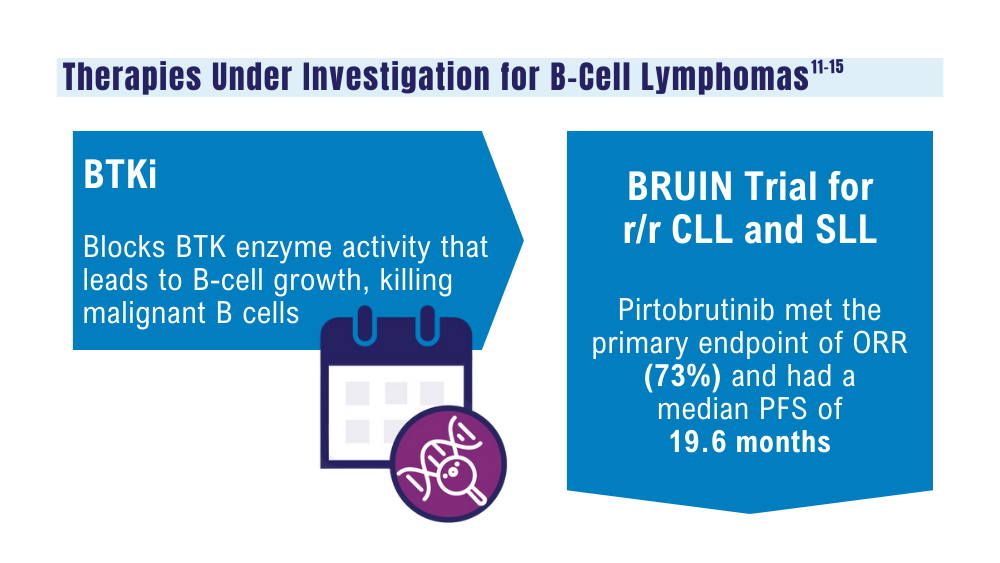
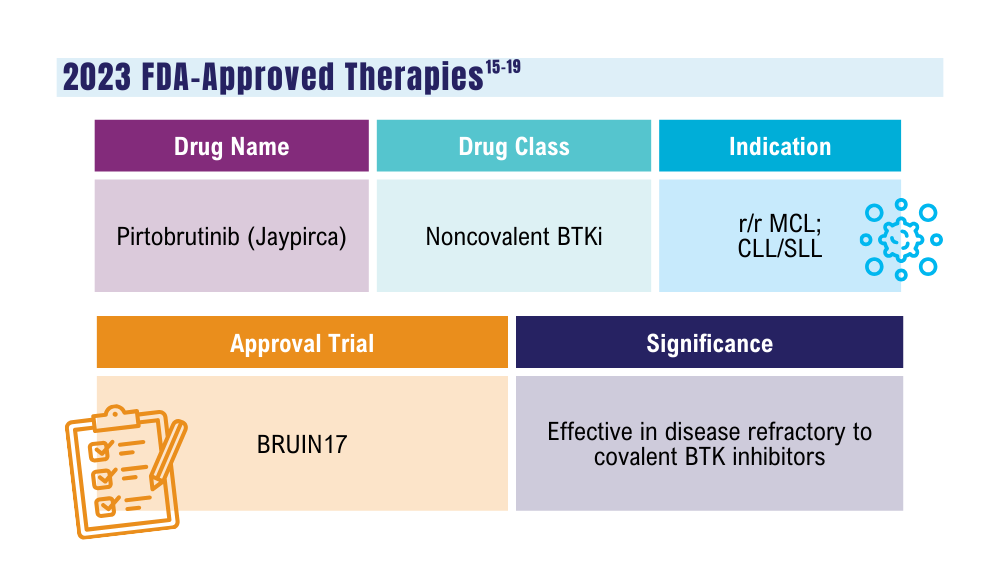
Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33-44. doi:10.1056/NEJMoa2300696
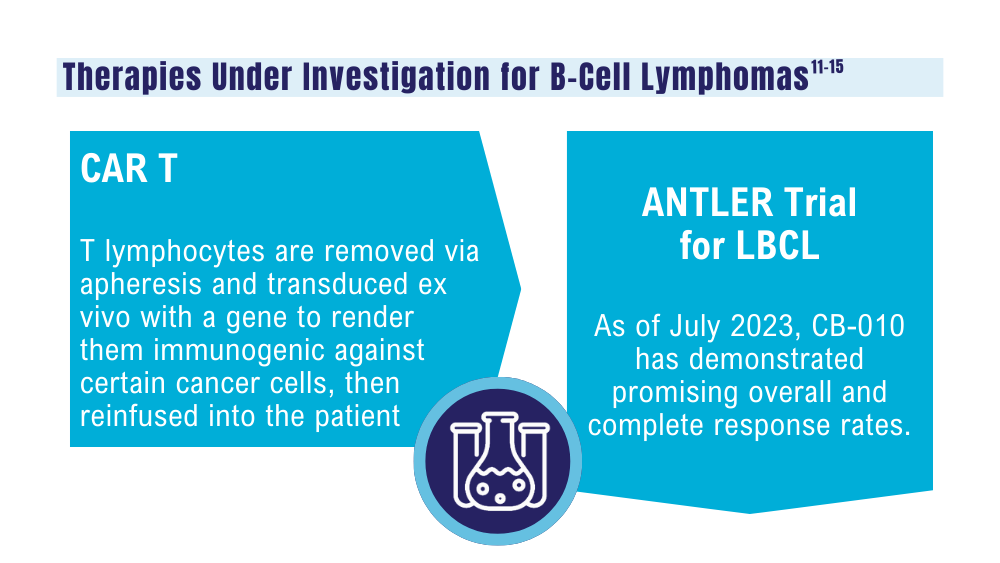
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
Caribou Biosciences reports positive clinical data from dose escalation of CB-010 ANTLER phase 1 trial in r/r B-NHL [news release]. Globalnewswire.com. Published July 13, 2023. Accessed January 24, 2024. https://www.globenewswire.com/news-release/2023/07/13/2704702/0/en/Caribou-Biosciences-Reports-Positive-Clinical-Data-from-Dose-Escalation-of-CB-010-ANTLER-Phase-1-Trial-in-r-r-B-NHL.html
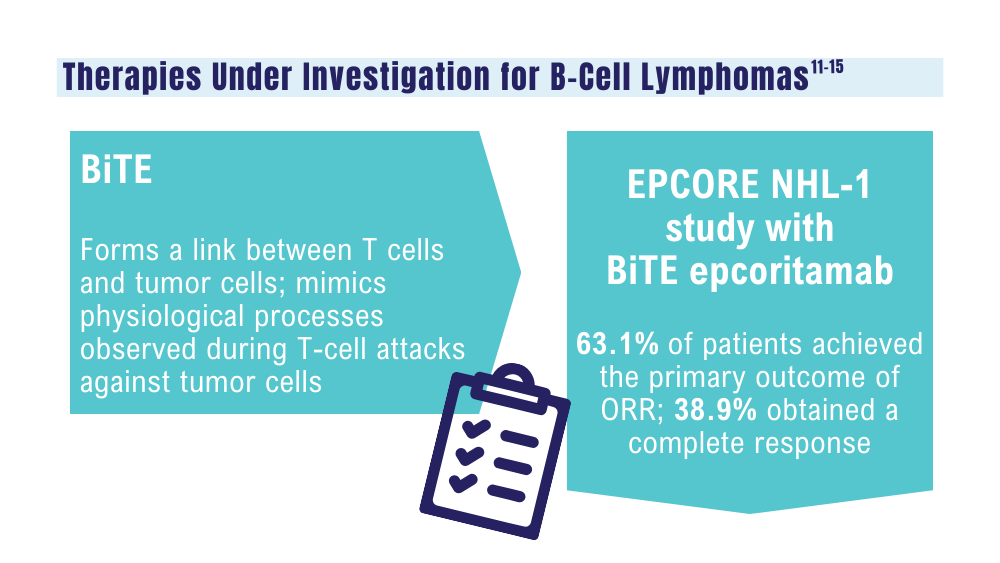
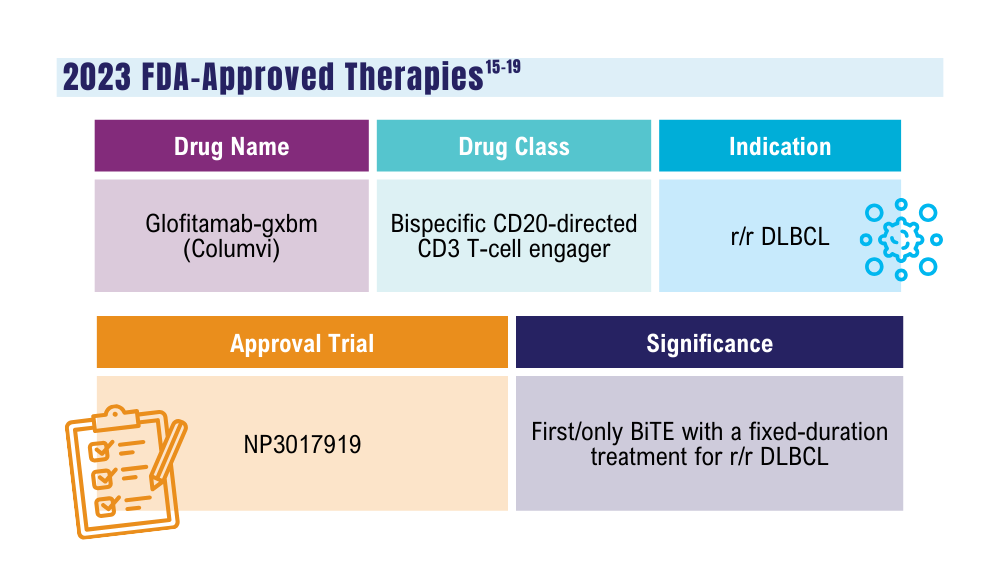
Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi:10.3389/fimmu.2021.626616
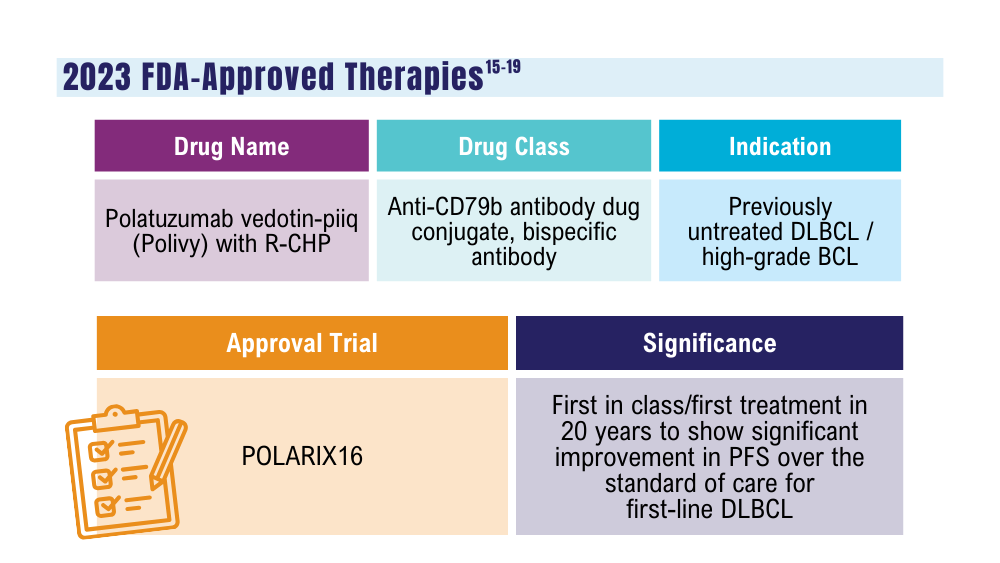
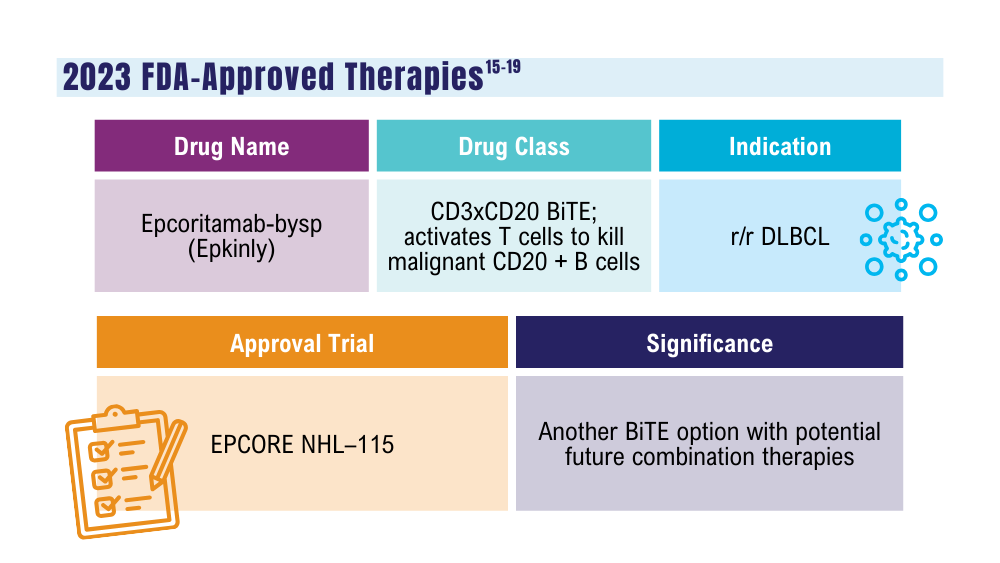
Davis JA, Granger K, Sakowski A, et al. Dual target dilemma: navigating epcoritamab vs. glofitamab in relapsed refractory diffuse large B-cell lymphoma. Expert Rev Hematol. 2023;16(12):915-918. doi:10.1080/17474086.2023.2285978
Lynch RC, Poh C, Ujjani CS, et al. Polatuzumab vedotin with infusional chemotherapy for untreated aggressive B-cell non-Hodgkin lymphomas. Blood Adv. 2023;7(11):2449-2458. doi:10.1182/bloodadvances.2022009145
Thompson PA, Tam CS. Pirtobrutinib: a new hope for patients with BTK inhibitor-refractory lymphoproliferative disorders. Blood. 2023;141(26):3137-3142. doi:10.1182/blood.2023020240
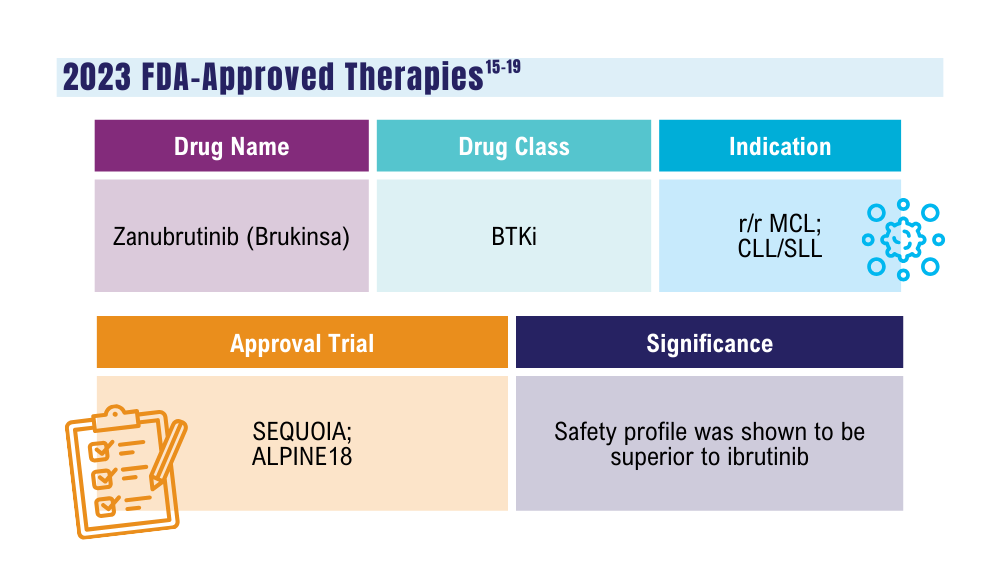
US Food and Drug Administration. FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma. Published January 19, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma
US Food and Drug Administration. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. Published June 16, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-glofitamab-gxbm-selected-relapsed-or-refractory-large-b-cell
Lu W, Chen W, Zhou Y, et al. A model to predict the prognosis of diffuse large B-cell lymphoma based on ultrasound images. Sci Rep. 2023;13(1):3346. doi:10.1038/s41598-023-30533-y
Leukemia - Chronic lymphocytic - CLL: statistics. Cancer.net. Published February 2023. Accessed January 24, 2024. https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics
Harmanen M, Hujo M, Sund R, et al. Survival of patients with mantle cell lymphoma in the rituximab era: retrospective binational analysis between 2000 and 2020. Br J Haematol. 2023;201(1):64-74. doi:10.1111/bjh.18597
Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481. doi:10.1007/s40266-020-00765-y
Marginal zone lymphoma (MZL). Leukemia and Lymphoma Society. Accessed January 24, 2024. https://www.lls.org/research/marginal-zone-lymphoma-mzl
Understanding lymphoma: diffuse large B-cell lymphoma. Lymphoma Research Foundation fact sheet. Updated 2023. Accessed January 24, 2024 https://lymphoma.org/wp-content/uploads/2023/10/LRF_Understanding_Lymphoma_Diffuse_Large_B_Cell_Lymphoma_Fact_Sheet.pdf
Key statistics for non-Hodgkin lymphoma. American Cancer Society. Updated January 17, 2024. Accessed January 24, 2024. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html
Zullig LL, Raska W, McWhirter G, et al. Veterans Health Administration National TeleOncology Service. JCO Oncol Pract. 2023;19(4):e504-e510. doi:10.1200/OP.22.00455
Lin C, Zhou KI, Burningham ZR, et al. Telemedicine-supervised cancer therapy for patients with an aggressive lymphoma and metastatic lung cancer in the U.S. Veterans Affairs National TeleOncology Service. J Clin Oncol. 2023;41(16 suppl)16:Abstract 1602. doi:10.1200/JCO.2023.41.16_suppl.1602
Zhang MC, Tian S, Fu D, et al. Genetic subtype-guided immunochemotherapy in diffuse large B-cell lymphoma: the randomized GUIDANCE-01 trial. Cancer Cell. 2023;41(10):1705-1716.e5. doi:10.1016/j.ccell.2023.09.004
Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33-44. doi:10.1056/NEJMoa2300696
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
Caribou Biosciences reports positive clinical data from dose escalation of CB-010 ANTLER phase 1 trial in r/r B-NHL [news release]. Globalnewswire.com. Published July 13, 2023. Accessed January 24, 2024. https://www.globenewswire.com/news-release/2023/07/13/2704702/0/en/Caribou-Biosciences-Reports-Positive-Clinical-Data-from-Dose-Escalation-of-CB-010-ANTLER-Phase-1-Trial-in-r-r-B-NHL.html
Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi:10.3389/fimmu.2021.626616
Davis JA, Granger K, Sakowski A, et al. Dual target dilemma: navigating epcoritamab vs. glofitamab in relapsed refractory diffuse large B-cell lymphoma. Expert Rev Hematol. 2023;16(12):915-918. doi:10.1080/17474086.2023.2285978
Lynch RC, Poh C, Ujjani CS, et al. Polatuzumab vedotin with infusional chemotherapy for untreated aggressive B-cell non-Hodgkin lymphomas. Blood Adv. 2023;7(11):2449-2458. doi:10.1182/bloodadvances.2022009145
Thompson PA, Tam CS. Pirtobrutinib: a new hope for patients with BTK inhibitor-refractory lymphoproliferative disorders. Blood. 2023;141(26):3137-3142. doi:10.1182/blood.2023020240
US Food and Drug Administration. FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma. Published January 19, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma
US Food and Drug Administration. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. Published June 16, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-glofitamab-gxbm-selected-relapsed-or-refractory-large-b-cell
Lu W, Chen W, Zhou Y, et al. A model to predict the prognosis of diffuse large B-cell lymphoma based on ultrasound images. Sci Rep. 2023;13(1):3346. doi:10.1038/s41598-023-30533-y
Leukemia - Chronic lymphocytic - CLL: statistics. Cancer.net. Published February 2023. Accessed January 24, 2024. https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics
Harmanen M, Hujo M, Sund R, et al. Survival of patients with mantle cell lymphoma in the rituximab era: retrospective binational analysis between 2000 and 2020. Br J Haematol. 2023;201(1):64-74. doi:10.1111/bjh.18597
Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481. doi:10.1007/s40266-020-00765-y
Marginal zone lymphoma (MZL). Leukemia and Lymphoma Society. Accessed January 24, 2024. https://www.lls.org/research/marginal-zone-lymphoma-mzl
Understanding lymphoma: diffuse large B-cell lymphoma. Lymphoma Research Foundation fact sheet. Updated 2023. Accessed January 24, 2024 https://lymphoma.org/wp-content/uploads/2023/10/LRF_Understanding_Lymphoma_Diffuse_Large_B_Cell_Lymphoma_Fact_Sheet.pdf
Key statistics for non-Hodgkin lymphoma. American Cancer Society. Updated January 17, 2024. Accessed January 24, 2024. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html
Zullig LL, Raska W, McWhirter G, et al. Veterans Health Administration National TeleOncology Service. JCO Oncol Pract. 2023;19(4):e504-e510. doi:10.1200/OP.22.00455
Lin C, Zhou KI, Burningham ZR, et al. Telemedicine-supervised cancer therapy for patients with an aggressive lymphoma and metastatic lung cancer in the U.S. Veterans Affairs National TeleOncology Service. J Clin Oncol. 2023;41(16 suppl)16:Abstract 1602. doi:10.1200/JCO.2023.41.16_suppl.1602
Zhang MC, Tian S, Fu D, et al. Genetic subtype-guided immunochemotherapy in diffuse large B-cell lymphoma: the randomized GUIDANCE-01 trial. Cancer Cell. 2023;41(10):1705-1716.e5. doi:10.1016/j.ccell.2023.09.004
Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33-44. doi:10.1056/NEJMoa2300696
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
Caribou Biosciences reports positive clinical data from dose escalation of CB-010 ANTLER phase 1 trial in r/r B-NHL [news release]. Globalnewswire.com. Published July 13, 2023. Accessed January 24, 2024. https://www.globenewswire.com/news-release/2023/07/13/2704702/0/en/Caribou-Biosciences-Reports-Positive-Clinical-Data-from-Dose-Escalation-of-CB-010-ANTLER-Phase-1-Trial-in-r-r-B-NHL.html
Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi:10.3389/fimmu.2021.626616
Davis JA, Granger K, Sakowski A, et al. Dual target dilemma: navigating epcoritamab vs. glofitamab in relapsed refractory diffuse large B-cell lymphoma. Expert Rev Hematol. 2023;16(12):915-918. doi:10.1080/17474086.2023.2285978
Lynch RC, Poh C, Ujjani CS, et al. Polatuzumab vedotin with infusional chemotherapy for untreated aggressive B-cell non-Hodgkin lymphomas. Blood Adv. 2023;7(11):2449-2458. doi:10.1182/bloodadvances.2022009145
Thompson PA, Tam CS. Pirtobrutinib: a new hope for patients with BTK inhibitor-refractory lymphoproliferative disorders. Blood. 2023;141(26):3137-3142. doi:10.1182/blood.2023020240
US Food and Drug Administration. FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma. Published January 19, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma
US Food and Drug Administration. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. Published June 16, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-glofitamab-gxbm-selected-relapsed-or-refractory-large-b-cell
How Does Military Service Impact Cancer Risk? It’s Complicated
CHICAGO—While it’s extremely difficult to link cancer rates to military service, researchers are starting to get some initial inklings of possible connections, a US Department of Veterans Affairs (VA) oncologist told an audience at the 2023 annual meeting of the Association of VA Hematology/Oncology.
One study found surprising levels of abnormal proteins and cancer in the blood of service members, said Christin DeStefano, MD, of David Grant US Air Force Medical Center at Travis Air Force Base in California. Another may have uncovered a link between military trauma and lymphoma. And an analysis of pilots found they have higher rates of certain kinds of cancer— but lower levels of other cancer types.
“It is hard to tell if service-related exposures heighten the risk of cancer. Some aspects of military service might increase cancer risk,” DeStefano said. “But other aspects of military service might decrease cancer risk.”
The VA has been especially focused on the possible link between military service and cancer since the passage of the PACT Act in 2022. The legislation prioritizes claims for cancer, terminal illnesses, and homelessness, and it’s sparked more than 4.1 million free toxic-exposure screenings for veterans.
VA Under Secretary for Health Shereef Elnahal, MD, MBA, noted in the keynote address at the 2023 AVAHO annual meeting that “Every type of solid tumor is now considered a presumptive condition associated with burden of exposure to veterans deployed anywhere in Central Command, either in the Persian Gulf War or the post-9/11 conflicts.”
DeStefano noted that there are a variety of challenges to analyzing data regarding connections between military exposure and cancer. For one, “it’s hard to include people from the time they enter the military to postmilitary service. Some are getting their health care in the civilian health care systems.” In addition, “There are a lot of problems with ICD-9 and ICD-10 codes, which can be very erroneous. Maybe somebody came in with a mass, the doctor or the nurse practitioner was busy and they put down ‘suspected cancer,’ and now they have that ICD code in their chart where they never actually had cancer.” This is why more reliable cancer registries are so important, she said.
Another challenge is figuring out when exposures occurred and whether they actually occurred in the military at all. “There are multiple studies suggesting that a first driver event is often acquired 20 to 40 years before a cancer diagnosis, often in one’s 20s and 30s,” she said. “It's hard to quantify an exposure, since there can be exposures before military service and exposures after military service. The amount of exposure and duration of exposure might differ, and individuals might metabolize the exposures differently from each other.”
To make matters more complicated, research is pointing in surprising directions. DeStefano highlighted her not-yet-published study of monoclonal gammopathy (MG), a condition in which abnormal proteins are found in the blood, in 534 service members. MG can be a cancer precursor. Those exposed to burn pits in Iraq had similar risks of MG (6.7%) vs an unexposed, matched control group (5.4%; P = .22), Dr. DeStefano said. Over a mean follow-up of 14 years and 10 years, respectively, 7% of participants in each group developed cancer.
“You might think, ‘this is a negative study. There's no difference.’ However, it is very notable that the prevalence of monoclonal gammopathy was 6.1%. That is 3 times as high as we would expect in somebody in their 40s,” she said. “Also, 7% having a cancer diagnosis is not insignificant.” She added: “It is very possible that many of these service members already have full-blown multiple myeloma or something associated with monoclonal gammopathy that just has not been diagnosed yet.”
In another study, this one published in 2021, Dr. DeStefano and colleagues tracked 8834 injured Iraq/Afghanistan veterans and compared them with matched controls to see if there was a link between severe trauma and cancer. There wasn’t except for lymphoma (22 vs 7 cases, respectively; odds ratio = 3.1; 95% CI, 1.34-7.37; P = .008). The connection remained after adjustment for confounders.
What’s going on? “It’s possible that blast injury might induce some alterations to the immune system that might set the stage for lymphoma genesis,” she said. “Or maybe that blast injury is a surrogate for a toxic exposure: Maybe carcinogens are released during a blast injury.”
Dr. DeStefano also highlighted a 2022 study that tracked 386,190 Air Force officers. The study found that combat pilots (9.1% of the total) had greater adjusted odds of testicular and prostate cancers and melanoma than the other officers. Why? “Military pilots have exposure to cosmic ionizing radiation as well as ultraviolet radiation,” she said.
But while “these are scary, sobering things,” she noted that combat pilots were less likely to develop several cancers than the general population, including kidney, testicular, colorectal, bladder and thyroid cancer, and they were less likely to die from colorectal cancer.
CHICAGO—While it’s extremely difficult to link cancer rates to military service, researchers are starting to get some initial inklings of possible connections, a US Department of Veterans Affairs (VA) oncologist told an audience at the 2023 annual meeting of the Association of VA Hematology/Oncology.
One study found surprising levels of abnormal proteins and cancer in the blood of service members, said Christin DeStefano, MD, of David Grant US Air Force Medical Center at Travis Air Force Base in California. Another may have uncovered a link between military trauma and lymphoma. And an analysis of pilots found they have higher rates of certain kinds of cancer— but lower levels of other cancer types.
“It is hard to tell if service-related exposures heighten the risk of cancer. Some aspects of military service might increase cancer risk,” DeStefano said. “But other aspects of military service might decrease cancer risk.”
The VA has been especially focused on the possible link between military service and cancer since the passage of the PACT Act in 2022. The legislation prioritizes claims for cancer, terminal illnesses, and homelessness, and it’s sparked more than 4.1 million free toxic-exposure screenings for veterans.
VA Under Secretary for Health Shereef Elnahal, MD, MBA, noted in the keynote address at the 2023 AVAHO annual meeting that “Every type of solid tumor is now considered a presumptive condition associated with burden of exposure to veterans deployed anywhere in Central Command, either in the Persian Gulf War or the post-9/11 conflicts.”
DeStefano noted that there are a variety of challenges to analyzing data regarding connections between military exposure and cancer. For one, “it’s hard to include people from the time they enter the military to postmilitary service. Some are getting their health care in the civilian health care systems.” In addition, “There are a lot of problems with ICD-9 and ICD-10 codes, which can be very erroneous. Maybe somebody came in with a mass, the doctor or the nurse practitioner was busy and they put down ‘suspected cancer,’ and now they have that ICD code in their chart where they never actually had cancer.” This is why more reliable cancer registries are so important, she said.
Another challenge is figuring out when exposures occurred and whether they actually occurred in the military at all. “There are multiple studies suggesting that a first driver event is often acquired 20 to 40 years before a cancer diagnosis, often in one’s 20s and 30s,” she said. “It's hard to quantify an exposure, since there can be exposures before military service and exposures after military service. The amount of exposure and duration of exposure might differ, and individuals might metabolize the exposures differently from each other.”
To make matters more complicated, research is pointing in surprising directions. DeStefano highlighted her not-yet-published study of monoclonal gammopathy (MG), a condition in which abnormal proteins are found in the blood, in 534 service members. MG can be a cancer precursor. Those exposed to burn pits in Iraq had similar risks of MG (6.7%) vs an unexposed, matched control group (5.4%; P = .22), Dr. DeStefano said. Over a mean follow-up of 14 years and 10 years, respectively, 7% of participants in each group developed cancer.
“You might think, ‘this is a negative study. There's no difference.’ However, it is very notable that the prevalence of monoclonal gammopathy was 6.1%. That is 3 times as high as we would expect in somebody in their 40s,” she said. “Also, 7% having a cancer diagnosis is not insignificant.” She added: “It is very possible that many of these service members already have full-blown multiple myeloma or something associated with monoclonal gammopathy that just has not been diagnosed yet.”
In another study, this one published in 2021, Dr. DeStefano and colleagues tracked 8834 injured Iraq/Afghanistan veterans and compared them with matched controls to see if there was a link between severe trauma and cancer. There wasn’t except for lymphoma (22 vs 7 cases, respectively; odds ratio = 3.1; 95% CI, 1.34-7.37; P = .008). The connection remained after adjustment for confounders.
What’s going on? “It’s possible that blast injury might induce some alterations to the immune system that might set the stage for lymphoma genesis,” she said. “Or maybe that blast injury is a surrogate for a toxic exposure: Maybe carcinogens are released during a blast injury.”
Dr. DeStefano also highlighted a 2022 study that tracked 386,190 Air Force officers. The study found that combat pilots (9.1% of the total) had greater adjusted odds of testicular and prostate cancers and melanoma than the other officers. Why? “Military pilots have exposure to cosmic ionizing radiation as well as ultraviolet radiation,” she said.
But while “these are scary, sobering things,” she noted that combat pilots were less likely to develop several cancers than the general population, including kidney, testicular, colorectal, bladder and thyroid cancer, and they were less likely to die from colorectal cancer.
CHICAGO—While it’s extremely difficult to link cancer rates to military service, researchers are starting to get some initial inklings of possible connections, a US Department of Veterans Affairs (VA) oncologist told an audience at the 2023 annual meeting of the Association of VA Hematology/Oncology.
One study found surprising levels of abnormal proteins and cancer in the blood of service members, said Christin DeStefano, MD, of David Grant US Air Force Medical Center at Travis Air Force Base in California. Another may have uncovered a link between military trauma and lymphoma. And an analysis of pilots found they have higher rates of certain kinds of cancer— but lower levels of other cancer types.
“It is hard to tell if service-related exposures heighten the risk of cancer. Some aspects of military service might increase cancer risk,” DeStefano said. “But other aspects of military service might decrease cancer risk.”
The VA has been especially focused on the possible link between military service and cancer since the passage of the PACT Act in 2022. The legislation prioritizes claims for cancer, terminal illnesses, and homelessness, and it’s sparked more than 4.1 million free toxic-exposure screenings for veterans.
VA Under Secretary for Health Shereef Elnahal, MD, MBA, noted in the keynote address at the 2023 AVAHO annual meeting that “Every type of solid tumor is now considered a presumptive condition associated with burden of exposure to veterans deployed anywhere in Central Command, either in the Persian Gulf War or the post-9/11 conflicts.”
DeStefano noted that there are a variety of challenges to analyzing data regarding connections between military exposure and cancer. For one, “it’s hard to include people from the time they enter the military to postmilitary service. Some are getting their health care in the civilian health care systems.” In addition, “There are a lot of problems with ICD-9 and ICD-10 codes, which can be very erroneous. Maybe somebody came in with a mass, the doctor or the nurse practitioner was busy and they put down ‘suspected cancer,’ and now they have that ICD code in their chart where they never actually had cancer.” This is why more reliable cancer registries are so important, she said.
Another challenge is figuring out when exposures occurred and whether they actually occurred in the military at all. “There are multiple studies suggesting that a first driver event is often acquired 20 to 40 years before a cancer diagnosis, often in one’s 20s and 30s,” she said. “It's hard to quantify an exposure, since there can be exposures before military service and exposures after military service. The amount of exposure and duration of exposure might differ, and individuals might metabolize the exposures differently from each other.”
To make matters more complicated, research is pointing in surprising directions. DeStefano highlighted her not-yet-published study of monoclonal gammopathy (MG), a condition in which abnormal proteins are found in the blood, in 534 service members. MG can be a cancer precursor. Those exposed to burn pits in Iraq had similar risks of MG (6.7%) vs an unexposed, matched control group (5.4%; P = .22), Dr. DeStefano said. Over a mean follow-up of 14 years and 10 years, respectively, 7% of participants in each group developed cancer.
“You might think, ‘this is a negative study. There's no difference.’ However, it is very notable that the prevalence of monoclonal gammopathy was 6.1%. That is 3 times as high as we would expect in somebody in their 40s,” she said. “Also, 7% having a cancer diagnosis is not insignificant.” She added: “It is very possible that many of these service members already have full-blown multiple myeloma or something associated with monoclonal gammopathy that just has not been diagnosed yet.”
In another study, this one published in 2021, Dr. DeStefano and colleagues tracked 8834 injured Iraq/Afghanistan veterans and compared them with matched controls to see if there was a link between severe trauma and cancer. There wasn’t except for lymphoma (22 vs 7 cases, respectively; odds ratio = 3.1; 95% CI, 1.34-7.37; P = .008). The connection remained after adjustment for confounders.
What’s going on? “It’s possible that blast injury might induce some alterations to the immune system that might set the stage for lymphoma genesis,” she said. “Or maybe that blast injury is a surrogate for a toxic exposure: Maybe carcinogens are released during a blast injury.”
Dr. DeStefano also highlighted a 2022 study that tracked 386,190 Air Force officers. The study found that combat pilots (9.1% of the total) had greater adjusted odds of testicular and prostate cancers and melanoma than the other officers. Why? “Military pilots have exposure to cosmic ionizing radiation as well as ultraviolet radiation,” she said.
But while “these are scary, sobering things,” she noted that combat pilots were less likely to develop several cancers than the general population, including kidney, testicular, colorectal, bladder and thyroid cancer, and they were less likely to die from colorectal cancer.
When to treat DLBCL with radiotherapy?
SAN DIEGO –
For example, radiation may not be needed for advanced-stage patients who’ve received at least four cycles of R-CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisolone plus rituximab), and whose PET scans show no sign of disease at interim or end-of treatment phases, said Joanna Yang, MD, MPH, of Washington University in St. Louis, in a presentation at the annual meeting of the American Society for Radiation Oncology.
These patients “may be able to omit radiotherapy without sacrificing good outcomes,” Dr. Yang said. In contrast, those whose PET scans show signs of disease at interim and end-of-treatment points may benefit from radiotherapy to selected sites, she said.
Dr. Yang highlighted a 2021 study in Blood that tracked 723 patients with advanced-stage DLBCL who were diagnosed from 2005 to 2017. All were treated with R-CHOP, and some of those who were PET-positive – that is, showing signs of malignant disease – were treated with radiotherapy.
Over a mean follow-up of 4.3 years, the study reported “time to progression and overall survival at 3 years were 83% vs. 56% and 87% vs. 64% in patients with PET-NEG and PET-POS scans, respectively.”
These findings aren’t surprising, Dr. Yang said. But “the PET-positive patients who got radiation actually had outcomes that came close to the outcomes that the PET-negative patients were able to achieve.” Their 3-year overall survival was 80% vs. 87% in the PET-negative, no-radiation group vs. 44% in the PET-positive, no-radiation group.
Dr. Yang cautioned, however, that withholding radiation in PET-negative patients isn’t right for everyone: “This doesn’t mean this should be the approach for every single patient.”
What about early-stage DLBCL? In patients without risk factors, Dr. Yang recommends PET scans after four treatments with R-CHOP. “Getting that end-of-treatment PET is going to be super-critical because that’s going to help guide you in terms of the patients who you may feel comfortable omitting radiation versus the patients who remain PET-positive at the end of chemotherapy. Many places will also add an interim PET as well.”
According to her, radiotherapy is appropriate in patients who are PET-positive, based on the findings of the FLYER and LYSA-GOELAMS 02-03 trials.
In early-stage patients who have risk factors such as advanced age or bulky or extra-nodal disease, Dr. Yang suggests examining interim PET scans after three treatments with R-CHOP. If they are negative, another R-CHOP treatment is appropriate – with or without radiotherapy.
“There’s a lot that goes into that decision. The first thing I think about in patients who have risk factors is: What salvage options are available for my patient? Can they tolerate these salvage option? If they’re older, they might not be eligible for auto [autologous hematopoietic cell transplantation]. If they’re frail, they might not be eligible for auto or CAR T cells. If they have bulk, it’s certainly an area of concern. It seems like radiation does help control disease in areas of bulk for patients with DLBCL.”
If these patients are PET-positive, go directly to radiotherapy, Dr. Yang advised. Trials that support this approach include S1001, LYSA-GOELAMS 02-03, and RICOVER-noRTH, she said.
What about double-hit and triple-hit lymphomas, which are especially aggressive due to genetic variations? Research suggests that “even if double hit/triple hit is not responding to chemo, it still responds to radiation,” Dr. Yang said.
In regard to advanced-stage disease, “if patients are receiving full-dose chemo for least six cycles, I use that end-of-treatment PET to help guide me. And then I make an individualized decision based on how bulky that disease is, where the location is, how morbid a relapse would be. If they’re older or receiving reduced-dose chemotherapy, then I’ll more seriously consider radiation just because there are limited options for these patients. And we know that DLCBL is most commonly a disease of the elderly.”
In an adjoining presentation at ASTRO, Andrea Ng, MD, MPH, of Harvard Medical School/Dana-Farber Brigham Cancer Center, Boston, discussed which patients with incomplete response or refractory/relapsed DLCBL can benefit from radiotherapy.
She highlighted patients with good partial response and end-of-treatment PET-positive with evidence of residual 18F-fluorodeoxyglucose activity via PET scan (Deauville 4/5) – a group that “we’re increasingly seeing.” In these patients, “radiation can be quite effective” at doses of 36-45 Gy. She highlighted a study from 2011 that linked consolidation radiotherapy to 5-year event-free survival in 65% of patients.
As for relapsed/refractory disease in patients who aren’t candidates for further systemic therapy – the “frail without good options” – Dr. Ng said data about salvage radiotherapy is limited. However, a 2015 study tracked 65 patients who were treated with a median dose of 40 Gy with “curative” intent. Local control was “not great” at 72% at 2 years, Dr. Ng said, while overall survival was 60% and progress-free survival was 46%.
Dr. Ng, who was one of this study’s authors, said several groups did better: Those with refractory vs. relapsed disease and those who were responsive to chemotherapy vs. those who were not.
She also highlighted a similar 2019 study of 32 patients with refractory/relapsed disease treated with salvage radiotherapy (median dose of 42.7 Gy) found that 61.8% reached progress-free survival at 5 years – a better outcome.
Dr. Yang has no disclosures. Dr. Ng discloses royalties from UpToDate and Elsevier.
SAN DIEGO –
For example, radiation may not be needed for advanced-stage patients who’ve received at least four cycles of R-CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisolone plus rituximab), and whose PET scans show no sign of disease at interim or end-of treatment phases, said Joanna Yang, MD, MPH, of Washington University in St. Louis, in a presentation at the annual meeting of the American Society for Radiation Oncology.
These patients “may be able to omit radiotherapy without sacrificing good outcomes,” Dr. Yang said. In contrast, those whose PET scans show signs of disease at interim and end-of-treatment points may benefit from radiotherapy to selected sites, she said.
Dr. Yang highlighted a 2021 study in Blood that tracked 723 patients with advanced-stage DLBCL who were diagnosed from 2005 to 2017. All were treated with R-CHOP, and some of those who were PET-positive – that is, showing signs of malignant disease – were treated with radiotherapy.
Over a mean follow-up of 4.3 years, the study reported “time to progression and overall survival at 3 years were 83% vs. 56% and 87% vs. 64% in patients with PET-NEG and PET-POS scans, respectively.”
These findings aren’t surprising, Dr. Yang said. But “the PET-positive patients who got radiation actually had outcomes that came close to the outcomes that the PET-negative patients were able to achieve.” Their 3-year overall survival was 80% vs. 87% in the PET-negative, no-radiation group vs. 44% in the PET-positive, no-radiation group.
Dr. Yang cautioned, however, that withholding radiation in PET-negative patients isn’t right for everyone: “This doesn’t mean this should be the approach for every single patient.”
What about early-stage DLBCL? In patients without risk factors, Dr. Yang recommends PET scans after four treatments with R-CHOP. “Getting that end-of-treatment PET is going to be super-critical because that’s going to help guide you in terms of the patients who you may feel comfortable omitting radiation versus the patients who remain PET-positive at the end of chemotherapy. Many places will also add an interim PET as well.”
According to her, radiotherapy is appropriate in patients who are PET-positive, based on the findings of the FLYER and LYSA-GOELAMS 02-03 trials.
In early-stage patients who have risk factors such as advanced age or bulky or extra-nodal disease, Dr. Yang suggests examining interim PET scans after three treatments with R-CHOP. If they are negative, another R-CHOP treatment is appropriate – with or without radiotherapy.
“There’s a lot that goes into that decision. The first thing I think about in patients who have risk factors is: What salvage options are available for my patient? Can they tolerate these salvage option? If they’re older, they might not be eligible for auto [autologous hematopoietic cell transplantation]. If they’re frail, they might not be eligible for auto or CAR T cells. If they have bulk, it’s certainly an area of concern. It seems like radiation does help control disease in areas of bulk for patients with DLBCL.”
If these patients are PET-positive, go directly to radiotherapy, Dr. Yang advised. Trials that support this approach include S1001, LYSA-GOELAMS 02-03, and RICOVER-noRTH, she said.
What about double-hit and triple-hit lymphomas, which are especially aggressive due to genetic variations? Research suggests that “even if double hit/triple hit is not responding to chemo, it still responds to radiation,” Dr. Yang said.
In regard to advanced-stage disease, “if patients are receiving full-dose chemo for least six cycles, I use that end-of-treatment PET to help guide me. And then I make an individualized decision based on how bulky that disease is, where the location is, how morbid a relapse would be. If they’re older or receiving reduced-dose chemotherapy, then I’ll more seriously consider radiation just because there are limited options for these patients. And we know that DLCBL is most commonly a disease of the elderly.”
In an adjoining presentation at ASTRO, Andrea Ng, MD, MPH, of Harvard Medical School/Dana-Farber Brigham Cancer Center, Boston, discussed which patients with incomplete response or refractory/relapsed DLCBL can benefit from radiotherapy.
She highlighted patients with good partial response and end-of-treatment PET-positive with evidence of residual 18F-fluorodeoxyglucose activity via PET scan (Deauville 4/5) – a group that “we’re increasingly seeing.” In these patients, “radiation can be quite effective” at doses of 36-45 Gy. She highlighted a study from 2011 that linked consolidation radiotherapy to 5-year event-free survival in 65% of patients.
As for relapsed/refractory disease in patients who aren’t candidates for further systemic therapy – the “frail without good options” – Dr. Ng said data about salvage radiotherapy is limited. However, a 2015 study tracked 65 patients who were treated with a median dose of 40 Gy with “curative” intent. Local control was “not great” at 72% at 2 years, Dr. Ng said, while overall survival was 60% and progress-free survival was 46%.
Dr. Ng, who was one of this study’s authors, said several groups did better: Those with refractory vs. relapsed disease and those who were responsive to chemotherapy vs. those who were not.
She also highlighted a similar 2019 study of 32 patients with refractory/relapsed disease treated with salvage radiotherapy (median dose of 42.7 Gy) found that 61.8% reached progress-free survival at 5 years – a better outcome.
Dr. Yang has no disclosures. Dr. Ng discloses royalties from UpToDate and Elsevier.
SAN DIEGO –
For example, radiation may not be needed for advanced-stage patients who’ve received at least four cycles of R-CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisolone plus rituximab), and whose PET scans show no sign of disease at interim or end-of treatment phases, said Joanna Yang, MD, MPH, of Washington University in St. Louis, in a presentation at the annual meeting of the American Society for Radiation Oncology.
These patients “may be able to omit radiotherapy without sacrificing good outcomes,” Dr. Yang said. In contrast, those whose PET scans show signs of disease at interim and end-of-treatment points may benefit from radiotherapy to selected sites, she said.
Dr. Yang highlighted a 2021 study in Blood that tracked 723 patients with advanced-stage DLBCL who were diagnosed from 2005 to 2017. All were treated with R-CHOP, and some of those who were PET-positive – that is, showing signs of malignant disease – were treated with radiotherapy.
Over a mean follow-up of 4.3 years, the study reported “time to progression and overall survival at 3 years were 83% vs. 56% and 87% vs. 64% in patients with PET-NEG and PET-POS scans, respectively.”
These findings aren’t surprising, Dr. Yang said. But “the PET-positive patients who got radiation actually had outcomes that came close to the outcomes that the PET-negative patients were able to achieve.” Their 3-year overall survival was 80% vs. 87% in the PET-negative, no-radiation group vs. 44% in the PET-positive, no-radiation group.
Dr. Yang cautioned, however, that withholding radiation in PET-negative patients isn’t right for everyone: “This doesn’t mean this should be the approach for every single patient.”
What about early-stage DLBCL? In patients without risk factors, Dr. Yang recommends PET scans after four treatments with R-CHOP. “Getting that end-of-treatment PET is going to be super-critical because that’s going to help guide you in terms of the patients who you may feel comfortable omitting radiation versus the patients who remain PET-positive at the end of chemotherapy. Many places will also add an interim PET as well.”
According to her, radiotherapy is appropriate in patients who are PET-positive, based on the findings of the FLYER and LYSA-GOELAMS 02-03 trials.
In early-stage patients who have risk factors such as advanced age or bulky or extra-nodal disease, Dr. Yang suggests examining interim PET scans after three treatments with R-CHOP. If they are negative, another R-CHOP treatment is appropriate – with or without radiotherapy.
“There’s a lot that goes into that decision. The first thing I think about in patients who have risk factors is: What salvage options are available for my patient? Can they tolerate these salvage option? If they’re older, they might not be eligible for auto [autologous hematopoietic cell transplantation]. If they’re frail, they might not be eligible for auto or CAR T cells. If they have bulk, it’s certainly an area of concern. It seems like radiation does help control disease in areas of bulk for patients with DLBCL.”
If these patients are PET-positive, go directly to radiotherapy, Dr. Yang advised. Trials that support this approach include S1001, LYSA-GOELAMS 02-03, and RICOVER-noRTH, she said.
What about double-hit and triple-hit lymphomas, which are especially aggressive due to genetic variations? Research suggests that “even if double hit/triple hit is not responding to chemo, it still responds to radiation,” Dr. Yang said.
In regard to advanced-stage disease, “if patients are receiving full-dose chemo for least six cycles, I use that end-of-treatment PET to help guide me. And then I make an individualized decision based on how bulky that disease is, where the location is, how morbid a relapse would be. If they’re older or receiving reduced-dose chemotherapy, then I’ll more seriously consider radiation just because there are limited options for these patients. And we know that DLCBL is most commonly a disease of the elderly.”
In an adjoining presentation at ASTRO, Andrea Ng, MD, MPH, of Harvard Medical School/Dana-Farber Brigham Cancer Center, Boston, discussed which patients with incomplete response or refractory/relapsed DLCBL can benefit from radiotherapy.
She highlighted patients with good partial response and end-of-treatment PET-positive with evidence of residual 18F-fluorodeoxyglucose activity via PET scan (Deauville 4/5) – a group that “we’re increasingly seeing.” In these patients, “radiation can be quite effective” at doses of 36-45 Gy. She highlighted a study from 2011 that linked consolidation radiotherapy to 5-year event-free survival in 65% of patients.
As for relapsed/refractory disease in patients who aren’t candidates for further systemic therapy – the “frail without good options” – Dr. Ng said data about salvage radiotherapy is limited. However, a 2015 study tracked 65 patients who were treated with a median dose of 40 Gy with “curative” intent. Local control was “not great” at 72% at 2 years, Dr. Ng said, while overall survival was 60% and progress-free survival was 46%.
Dr. Ng, who was one of this study’s authors, said several groups did better: Those with refractory vs. relapsed disease and those who were responsive to chemotherapy vs. those who were not.
She also highlighted a similar 2019 study of 32 patients with refractory/relapsed disease treated with salvage radiotherapy (median dose of 42.7 Gy) found that 61.8% reached progress-free survival at 5 years – a better outcome.
Dr. Yang has no disclosures. Dr. Ng discloses royalties from UpToDate and Elsevier.
FROM ASTRO 2023
Metastatic Urothelial Carcinoma Presenting as Mediastinal Lymphadenopathy Without Appreciable Bladder Mass in a Patient With Chronic Lymphocytic Leukemia
INTRODUCTION
Lymphadenopathy in Chronic Lymphocytic Leukemia (CLL) is a very common feature. However, sudden increase in lymphadenopathy or other symptoms like weight loss should be evaluated for possible metastatic malignancy. We describe a CLL patient with diffuse mediastinal lymphadenopathy who was diagnosed with metastatic bladder cancer without a primary bladder tumor mass on imaging.
CASE DESCRIPTION
A 60-year-old man with a 60 pack-year smoking history, alcoholic cirrhosis, and a 5-year history of stage 1 CLL presented with 3 months of progressive shortness of breath; persistent cough; chills; hemoptysis; and a steady weight loss of 35 lbs. Notably, he had no bladder symptoms. Initial labs showed leukocytosis of 35.8k with a lymphocytic predominance. Screening low-dose chest CT was positive for diffuse mediastinal lymphadenopathy. Subsequent PET/CT revealed numerous hypermetabolic lymph nodes in the neck, mediastinum, left hilum, and right periaortic abdominal region. CT Chest, Abdomen, Pelvis revealed progressive lymphadenopathy as seen in prior imaging, stable pulmonary nodules up to 4 mm in size, and splenomegaly. No distant primary sites, including of the bladder, were identified. Mediastinal lymph node biopsy confirmed metastatic poorly differentiated carcinoma with immunohistochemical staining negative for p40, p63, CK20, TTF-1, Napsin A, CDX2, CA19- 9, Calretinin, and D2-40 and positive for CK7, GATA3, Ber-EP4, and Uroplakin, supporting bladder as primary origin. Urology deferred a cystoscopy given his lack of urinary symptoms and positive biopsy and was started on Carboplatin/Gemcitabine for his metastatic disease. He was ineligible for Cisplatin given his cirrhosis and hearing impairment.
DISCUSSION
In patients with CLL, new onset mediastinal lymphadenopathy is concerning for disease progression and possible transformation to a diffuse b-cell lymphoma. However, this symptom has a broad differential, including primary lung carcinomas, sarcomas, and metastatic disease. While our patient’s PET/CT and pan-CT failed to identify a distant primary site, maintaining a low clinical suspicion for metastatic disease and doing a thorough work-up was paramount. Only through immunohistochemical staining were we able to diagnosis this patient with urothelial carcinoma.
CONCLUSIONS
Biopsy with immunohistochemical staining and maintaining a low suspicion for worsening lymphadenopathy can identify unusually presenting urothelial carcinomas in CLL patients.
INTRODUCTION
Lymphadenopathy in Chronic Lymphocytic Leukemia (CLL) is a very common feature. However, sudden increase in lymphadenopathy or other symptoms like weight loss should be evaluated for possible metastatic malignancy. We describe a CLL patient with diffuse mediastinal lymphadenopathy who was diagnosed with metastatic bladder cancer without a primary bladder tumor mass on imaging.
CASE DESCRIPTION
A 60-year-old man with a 60 pack-year smoking history, alcoholic cirrhosis, and a 5-year history of stage 1 CLL presented with 3 months of progressive shortness of breath; persistent cough; chills; hemoptysis; and a steady weight loss of 35 lbs. Notably, he had no bladder symptoms. Initial labs showed leukocytosis of 35.8k with a lymphocytic predominance. Screening low-dose chest CT was positive for diffuse mediastinal lymphadenopathy. Subsequent PET/CT revealed numerous hypermetabolic lymph nodes in the neck, mediastinum, left hilum, and right periaortic abdominal region. CT Chest, Abdomen, Pelvis revealed progressive lymphadenopathy as seen in prior imaging, stable pulmonary nodules up to 4 mm in size, and splenomegaly. No distant primary sites, including of the bladder, were identified. Mediastinal lymph node biopsy confirmed metastatic poorly differentiated carcinoma with immunohistochemical staining negative for p40, p63, CK20, TTF-1, Napsin A, CDX2, CA19- 9, Calretinin, and D2-40 and positive for CK7, GATA3, Ber-EP4, and Uroplakin, supporting bladder as primary origin. Urology deferred a cystoscopy given his lack of urinary symptoms and positive biopsy and was started on Carboplatin/Gemcitabine for his metastatic disease. He was ineligible for Cisplatin given his cirrhosis and hearing impairment.
DISCUSSION
In patients with CLL, new onset mediastinal lymphadenopathy is concerning for disease progression and possible transformation to a diffuse b-cell lymphoma. However, this symptom has a broad differential, including primary lung carcinomas, sarcomas, and metastatic disease. While our patient’s PET/CT and pan-CT failed to identify a distant primary site, maintaining a low clinical suspicion for metastatic disease and doing a thorough work-up was paramount. Only through immunohistochemical staining were we able to diagnosis this patient with urothelial carcinoma.
CONCLUSIONS
Biopsy with immunohistochemical staining and maintaining a low suspicion for worsening lymphadenopathy can identify unusually presenting urothelial carcinomas in CLL patients.
INTRODUCTION
Lymphadenopathy in Chronic Lymphocytic Leukemia (CLL) is a very common feature. However, sudden increase in lymphadenopathy or other symptoms like weight loss should be evaluated for possible metastatic malignancy. We describe a CLL patient with diffuse mediastinal lymphadenopathy who was diagnosed with metastatic bladder cancer without a primary bladder tumor mass on imaging.
CASE DESCRIPTION
A 60-year-old man with a 60 pack-year smoking history, alcoholic cirrhosis, and a 5-year history of stage 1 CLL presented with 3 months of progressive shortness of breath; persistent cough; chills; hemoptysis; and a steady weight loss of 35 lbs. Notably, he had no bladder symptoms. Initial labs showed leukocytosis of 35.8k with a lymphocytic predominance. Screening low-dose chest CT was positive for diffuse mediastinal lymphadenopathy. Subsequent PET/CT revealed numerous hypermetabolic lymph nodes in the neck, mediastinum, left hilum, and right periaortic abdominal region. CT Chest, Abdomen, Pelvis revealed progressive lymphadenopathy as seen in prior imaging, stable pulmonary nodules up to 4 mm in size, and splenomegaly. No distant primary sites, including of the bladder, were identified. Mediastinal lymph node biopsy confirmed metastatic poorly differentiated carcinoma with immunohistochemical staining negative for p40, p63, CK20, TTF-1, Napsin A, CDX2, CA19- 9, Calretinin, and D2-40 and positive for CK7, GATA3, Ber-EP4, and Uroplakin, supporting bladder as primary origin. Urology deferred a cystoscopy given his lack of urinary symptoms and positive biopsy and was started on Carboplatin/Gemcitabine for his metastatic disease. He was ineligible for Cisplatin given his cirrhosis and hearing impairment.
DISCUSSION
In patients with CLL, new onset mediastinal lymphadenopathy is concerning for disease progression and possible transformation to a diffuse b-cell lymphoma. However, this symptom has a broad differential, including primary lung carcinomas, sarcomas, and metastatic disease. While our patient’s PET/CT and pan-CT failed to identify a distant primary site, maintaining a low clinical suspicion for metastatic disease and doing a thorough work-up was paramount. Only through immunohistochemical staining were we able to diagnosis this patient with urothelial carcinoma.
CONCLUSIONS
Biopsy with immunohistochemical staining and maintaining a low suspicion for worsening lymphadenopathy can identify unusually presenting urothelial carcinomas in CLL patients.
FDA approves motixafortide for stem cell mobilization in myeloma
The success of autologous stem cell transplantation (ASCT) depends on adequate mobilization of stem cells during the treatment process. Collection of a sufficient number of stem cells to perform two transplantations is recommended. However, in up to 47% of patients, collecting target numbers of hematopoietic stem cells for ASCT after one apheresis session remains a challenge, BioLineRx explained in a press release today, announcing the approval.
The goal of combining motixafortide with filgrastim is to mobilize stem cells more reliably than filgrastim can alone, with fewer days of apheresis sessions and fewer doses of filgrastim.
“We believe [motixafortide] will play a critical role in addressing unmet needs and introduce a new treatment paradigm for” patients with multiple myeloma, CEO Philip Serlin said in the release.
The drug approval was based on the GENESIS trial, which randomized 122 patients to either motixafortide plus filgrastim or placebo plus filgrastim.
BioLineRx said the trial included patients considered representative of the typical multiple myeloma population undergoing ASCT, with a median age of 63 years and with about 70% of patients in both arms of the trial receiving lenalidomide-containing induction therapy.
Motixafortide plus filgrastim enabled 67.5% of patients to achieve the stem cell collection goal of 6 million or more CD34+ cells/kg within two apheresis sessions, versus 9.5% of patients receiving the placebo plus filgrastim regimen. Additionally, 92.5% of patients reached the stem cell collection goal in up to two apheresis sessions in the motixafortide arm and 21.4% in the placebo arm.
However, “the data are descriptive and were not statistically powered nor prespecified. The information should be cautiously interpreted,” the company said.
Serious adverse reactions occurred in 5.4% of patients in the motixafortide arm, including vomiting, injection-site reaction, hypersensitivity reaction, injection-site cellulitis, hypokalemia, and hypoxia. The most common adverse reactions, occurring in more than 20% of patients, were injection site reactions (pain, erythema, and pruritus), pruritus, flushing, and back pain.
Labeling for the subcutaneous injection is available online.
A version of this article first appeared on Medscape.com.
The success of autologous stem cell transplantation (ASCT) depends on adequate mobilization of stem cells during the treatment process. Collection of a sufficient number of stem cells to perform two transplantations is recommended. However, in up to 47% of patients, collecting target numbers of hematopoietic stem cells for ASCT after one apheresis session remains a challenge, BioLineRx explained in a press release today, announcing the approval.
The goal of combining motixafortide with filgrastim is to mobilize stem cells more reliably than filgrastim can alone, with fewer days of apheresis sessions and fewer doses of filgrastim.
“We believe [motixafortide] will play a critical role in addressing unmet needs and introduce a new treatment paradigm for” patients with multiple myeloma, CEO Philip Serlin said in the release.
The drug approval was based on the GENESIS trial, which randomized 122 patients to either motixafortide plus filgrastim or placebo plus filgrastim.
BioLineRx said the trial included patients considered representative of the typical multiple myeloma population undergoing ASCT, with a median age of 63 years and with about 70% of patients in both arms of the trial receiving lenalidomide-containing induction therapy.
Motixafortide plus filgrastim enabled 67.5% of patients to achieve the stem cell collection goal of 6 million or more CD34+ cells/kg within two apheresis sessions, versus 9.5% of patients receiving the placebo plus filgrastim regimen. Additionally, 92.5% of patients reached the stem cell collection goal in up to two apheresis sessions in the motixafortide arm and 21.4% in the placebo arm.
However, “the data are descriptive and were not statistically powered nor prespecified. The information should be cautiously interpreted,” the company said.
Serious adverse reactions occurred in 5.4% of patients in the motixafortide arm, including vomiting, injection-site reaction, hypersensitivity reaction, injection-site cellulitis, hypokalemia, and hypoxia. The most common adverse reactions, occurring in more than 20% of patients, were injection site reactions (pain, erythema, and pruritus), pruritus, flushing, and back pain.
Labeling for the subcutaneous injection is available online.
A version of this article first appeared on Medscape.com.
The success of autologous stem cell transplantation (ASCT) depends on adequate mobilization of stem cells during the treatment process. Collection of a sufficient number of stem cells to perform two transplantations is recommended. However, in up to 47% of patients, collecting target numbers of hematopoietic stem cells for ASCT after one apheresis session remains a challenge, BioLineRx explained in a press release today, announcing the approval.
The goal of combining motixafortide with filgrastim is to mobilize stem cells more reliably than filgrastim can alone, with fewer days of apheresis sessions and fewer doses of filgrastim.
“We believe [motixafortide] will play a critical role in addressing unmet needs and introduce a new treatment paradigm for” patients with multiple myeloma, CEO Philip Serlin said in the release.
The drug approval was based on the GENESIS trial, which randomized 122 patients to either motixafortide plus filgrastim or placebo plus filgrastim.
BioLineRx said the trial included patients considered representative of the typical multiple myeloma population undergoing ASCT, with a median age of 63 years and with about 70% of patients in both arms of the trial receiving lenalidomide-containing induction therapy.
Motixafortide plus filgrastim enabled 67.5% of patients to achieve the stem cell collection goal of 6 million or more CD34+ cells/kg within two apheresis sessions, versus 9.5% of patients receiving the placebo plus filgrastim regimen. Additionally, 92.5% of patients reached the stem cell collection goal in up to two apheresis sessions in the motixafortide arm and 21.4% in the placebo arm.
However, “the data are descriptive and were not statistically powered nor prespecified. The information should be cautiously interpreted,” the company said.
Serious adverse reactions occurred in 5.4% of patients in the motixafortide arm, including vomiting, injection-site reaction, hypersensitivity reaction, injection-site cellulitis, hypokalemia, and hypoxia. The most common adverse reactions, occurring in more than 20% of patients, were injection site reactions (pain, erythema, and pruritus), pruritus, flushing, and back pain.
Labeling for the subcutaneous injection is available online.
A version of this article first appeared on Medscape.com.