User login
Raltegravir safe, effective in late pregnancy
SEATTLE – In HIV-positive pregnant women, an antiretroviral therapy (ART) regimen that included the integrase inhibitor raltegravir (RAL-ART) led to faster viral load (VL) reduction and a greater proportion of women with a VL of less than 200 copies/mL at delivery, compared with patients treated with an efavirenz-based ART (EFV-ART). There were no statistically significant differences between the two arms with respect to percentage of stillbirths, preterm delivery, or rates of HIV infection in the newborn.
“There are lots of advantages of these [integrase inhibitor] drugs, and we’d like to have pregnant women take advantage of them. The problem is, there’s no requirement of drug manufacturers to study the drugs in pregnancy. So these studies are put off until after the drug is licensed, and we’re playing catch-up,” Mark Mirochnick, MD, professor of pediatrics at Boston University, said in an interview. Dr. Mirochnick presented the results at the Conference on Retroviruses and Opportunistic Infections.
Another integrase inhibitor, dolutegravir, has a better resistance profile than that of raltegravir, but concerns over neural tube defects observed during a study in Botswana led both the Food and Drug Administration and the European Medicines Agency to issue safety warnings for that drug. The current study did not raise concern, since it began at 20 weeks’ gestation, well after the period when neural tube defects might occur. “I think it just demonstrates that [integrase inhibitors] are safe in mid- to late-pregnancy,” said Dr. Mirochnick.
It remains to be seen whether a potential link to neural tube defects, if it is a real effect, is due to a specific drug or the mechanism of action of integrase inhibitors more generally. “It’s a question we don’t have an answer to. So you have to balance the potential benefits and potential risks, and that’s probably a decision best made by an individual woman and her care provider. Some women do very well on a particular regimen and they don’t want to change, and you run the risk when you change that you’ll get a viral rebound. What do you tell women who are on dolutegravir and are thinking about becoming pregnant? That’s a controversial question. There are risks with both courses,” said Dr. Mirochnick.
The study comprised 408 patients recruited from centers in Brazil, Tanzania, South Africa, Thailand, Argentina, and the United States. The patients were between 20 and 37 weeks’ gestation and had not previously received ART. They were randomized to RAL-ART or EFV-ART. About 12% of patients were Asian, 36% were black, 52% were Hispanic, and 1% were white.
Overall, 94% of patients on RAL-ART had a VL less than 200 copies/mL at delivery, compared to 84% of EFV-ART patients (P = .001). The effect appeared to be driven by patients who enrolled later in pregnancy: There was no significant difference in those enrolled in weeks 20-28, but suppression occurred in 93% of the RAL-ART group versus 71% of the EFV-ART group among those enrolled in weeks 29-37 (P = .04).
Tolerability was slightly better in the RAL-ART group, with 99% versus 97% of patients staying on their assigned therapy (P = .05). In both groups, 30% of women experienced an adverse event of grade 3 or higher, as did 25% of live-born infants in both groups.
A total of 92% of women in the RAL-ART group had a sustained VL response through delivery, compared with 64% in the EFV-ART group (P less than .001). The median time to achieving a VL less than 200 copies/mL was 8 days in the RAL-ART group and 15 days in the EFV-ART group (generalized log-rank test P less than .001).
There was one stillbirth in the EFV-ART arm and three in the RAL-ART arm, with 11% in the EFV-ART group having preterm delivery compared with 12% in the RAL-ART group. In addition, the proportion of HIV-infected infants was lower in the RAL-ART arm (1% versus 3%). These differences were not significant.
The National Institutes of Health funded the study. Glaxo/ViiV, Merck, and Bristol-Myers Squibb supplied study drugs. Dr. Mirochnick has received research funding from those companies.
SOURCE: Mark Mirochnick et al. CROI 2019, Abstract 39 LB.
SEATTLE – In HIV-positive pregnant women, an antiretroviral therapy (ART) regimen that included the integrase inhibitor raltegravir (RAL-ART) led to faster viral load (VL) reduction and a greater proportion of women with a VL of less than 200 copies/mL at delivery, compared with patients treated with an efavirenz-based ART (EFV-ART). There were no statistically significant differences between the two arms with respect to percentage of stillbirths, preterm delivery, or rates of HIV infection in the newborn.
“There are lots of advantages of these [integrase inhibitor] drugs, and we’d like to have pregnant women take advantage of them. The problem is, there’s no requirement of drug manufacturers to study the drugs in pregnancy. So these studies are put off until after the drug is licensed, and we’re playing catch-up,” Mark Mirochnick, MD, professor of pediatrics at Boston University, said in an interview. Dr. Mirochnick presented the results at the Conference on Retroviruses and Opportunistic Infections.
Another integrase inhibitor, dolutegravir, has a better resistance profile than that of raltegravir, but concerns over neural tube defects observed during a study in Botswana led both the Food and Drug Administration and the European Medicines Agency to issue safety warnings for that drug. The current study did not raise concern, since it began at 20 weeks’ gestation, well after the period when neural tube defects might occur. “I think it just demonstrates that [integrase inhibitors] are safe in mid- to late-pregnancy,” said Dr. Mirochnick.
It remains to be seen whether a potential link to neural tube defects, if it is a real effect, is due to a specific drug or the mechanism of action of integrase inhibitors more generally. “It’s a question we don’t have an answer to. So you have to balance the potential benefits and potential risks, and that’s probably a decision best made by an individual woman and her care provider. Some women do very well on a particular regimen and they don’t want to change, and you run the risk when you change that you’ll get a viral rebound. What do you tell women who are on dolutegravir and are thinking about becoming pregnant? That’s a controversial question. There are risks with both courses,” said Dr. Mirochnick.
The study comprised 408 patients recruited from centers in Brazil, Tanzania, South Africa, Thailand, Argentina, and the United States. The patients were between 20 and 37 weeks’ gestation and had not previously received ART. They were randomized to RAL-ART or EFV-ART. About 12% of patients were Asian, 36% were black, 52% were Hispanic, and 1% were white.
Overall, 94% of patients on RAL-ART had a VL less than 200 copies/mL at delivery, compared to 84% of EFV-ART patients (P = .001). The effect appeared to be driven by patients who enrolled later in pregnancy: There was no significant difference in those enrolled in weeks 20-28, but suppression occurred in 93% of the RAL-ART group versus 71% of the EFV-ART group among those enrolled in weeks 29-37 (P = .04).
Tolerability was slightly better in the RAL-ART group, with 99% versus 97% of patients staying on their assigned therapy (P = .05). In both groups, 30% of women experienced an adverse event of grade 3 or higher, as did 25% of live-born infants in both groups.
A total of 92% of women in the RAL-ART group had a sustained VL response through delivery, compared with 64% in the EFV-ART group (P less than .001). The median time to achieving a VL less than 200 copies/mL was 8 days in the RAL-ART group and 15 days in the EFV-ART group (generalized log-rank test P less than .001).
There was one stillbirth in the EFV-ART arm and three in the RAL-ART arm, with 11% in the EFV-ART group having preterm delivery compared with 12% in the RAL-ART group. In addition, the proportion of HIV-infected infants was lower in the RAL-ART arm (1% versus 3%). These differences were not significant.
The National Institutes of Health funded the study. Glaxo/ViiV, Merck, and Bristol-Myers Squibb supplied study drugs. Dr. Mirochnick has received research funding from those companies.
SOURCE: Mark Mirochnick et al. CROI 2019, Abstract 39 LB.
SEATTLE – In HIV-positive pregnant women, an antiretroviral therapy (ART) regimen that included the integrase inhibitor raltegravir (RAL-ART) led to faster viral load (VL) reduction and a greater proportion of women with a VL of less than 200 copies/mL at delivery, compared with patients treated with an efavirenz-based ART (EFV-ART). There were no statistically significant differences between the two arms with respect to percentage of stillbirths, preterm delivery, or rates of HIV infection in the newborn.
“There are lots of advantages of these [integrase inhibitor] drugs, and we’d like to have pregnant women take advantage of them. The problem is, there’s no requirement of drug manufacturers to study the drugs in pregnancy. So these studies are put off until after the drug is licensed, and we’re playing catch-up,” Mark Mirochnick, MD, professor of pediatrics at Boston University, said in an interview. Dr. Mirochnick presented the results at the Conference on Retroviruses and Opportunistic Infections.
Another integrase inhibitor, dolutegravir, has a better resistance profile than that of raltegravir, but concerns over neural tube defects observed during a study in Botswana led both the Food and Drug Administration and the European Medicines Agency to issue safety warnings for that drug. The current study did not raise concern, since it began at 20 weeks’ gestation, well after the period when neural tube defects might occur. “I think it just demonstrates that [integrase inhibitors] are safe in mid- to late-pregnancy,” said Dr. Mirochnick.
It remains to be seen whether a potential link to neural tube defects, if it is a real effect, is due to a specific drug or the mechanism of action of integrase inhibitors more generally. “It’s a question we don’t have an answer to. So you have to balance the potential benefits and potential risks, and that’s probably a decision best made by an individual woman and her care provider. Some women do very well on a particular regimen and they don’t want to change, and you run the risk when you change that you’ll get a viral rebound. What do you tell women who are on dolutegravir and are thinking about becoming pregnant? That’s a controversial question. There are risks with both courses,” said Dr. Mirochnick.
The study comprised 408 patients recruited from centers in Brazil, Tanzania, South Africa, Thailand, Argentina, and the United States. The patients were between 20 and 37 weeks’ gestation and had not previously received ART. They were randomized to RAL-ART or EFV-ART. About 12% of patients were Asian, 36% were black, 52% were Hispanic, and 1% were white.
Overall, 94% of patients on RAL-ART had a VL less than 200 copies/mL at delivery, compared to 84% of EFV-ART patients (P = .001). The effect appeared to be driven by patients who enrolled later in pregnancy: There was no significant difference in those enrolled in weeks 20-28, but suppression occurred in 93% of the RAL-ART group versus 71% of the EFV-ART group among those enrolled in weeks 29-37 (P = .04).
Tolerability was slightly better in the RAL-ART group, with 99% versus 97% of patients staying on their assigned therapy (P = .05). In both groups, 30% of women experienced an adverse event of grade 3 or higher, as did 25% of live-born infants in both groups.
A total of 92% of women in the RAL-ART group had a sustained VL response through delivery, compared with 64% in the EFV-ART group (P less than .001). The median time to achieving a VL less than 200 copies/mL was 8 days in the RAL-ART group and 15 days in the EFV-ART group (generalized log-rank test P less than .001).
There was one stillbirth in the EFV-ART arm and three in the RAL-ART arm, with 11% in the EFV-ART group having preterm delivery compared with 12% in the RAL-ART group. In addition, the proportion of HIV-infected infants was lower in the RAL-ART arm (1% versus 3%). These differences were not significant.
The National Institutes of Health funded the study. Glaxo/ViiV, Merck, and Bristol-Myers Squibb supplied study drugs. Dr. Mirochnick has received research funding from those companies.
SOURCE: Mark Mirochnick et al. CROI 2019, Abstract 39 LB.
REPORTING FROM CROI 2019
Opioid overdose risk greater among HIV patients
SEATTLE – People with HIV are more likely to die from an opioid overdose than the general public, according to investigators from the Centers for Disease Control and Prevention.
“We looked into this because we know persons with HIV are more likely to have chronic pain and more likely to receive opioid analgesic treatments, and receive higher doses. In addition, they are more likely to have substance use disorders and mental illness than the U.S. general populations,” CDC epidemiologist Karin A. Bosh, PhD, said at the Conference on Retroviruses and Opportunistic Infections.
To see how that played out in terms of unintentional opioid overdose deaths, they turned to the National HIV Surveillance System and focused on overdose deaths during 2011-2015, the latest data available at the time of the work.
There were 1,363 overdose deaths among persons with HIV during that period, with the rate increasing 42.7% – from 23.2/100,000 HIV patients in 2011 to 33.1/100,000 in 2015.
Although the rate of increase was comparable to the general population, the crude rate was “actually substantially higher among persons with HIV,” Dr. Bosh said. Deaths were highest among persons aged 50-59 years (41.9/100,000), whites (49.1/100,000), injection drug users (137.4/100,000), and people who live in the Northeast (60.6/100,000).
Surprisingly, there was no increase in the rate of overdose deaths among HIV patients on the West Coast, possibly because heroin there was less likely to be cut with fentanyl.
Also, the rate of opioid overdose deaths was higher among women with HIV (35.2/100,000) than among men, perhaps because women are more likely to contract HIV by injection drug use, so they are more likely to be injection drug users at baseline, while the vast majority of men are infected through male-male sex, the investigators said.
The findings underscore the importance of intensifying overdose prevention in the HIV community, and better integrating HIV and substance use disorder treatment, they concluded.
That comes down to screening people for problems, especially in the subgroups identified in the study, and connecting them to drug treatment services. If HIV and substance disorder services were in the same clinic it would help, as would an increase in the number of buprenorphine providers, according to Sheryl B. Lyss, PhD, a coinvestigator and CDC epidemiologist.
“Obviously, when substance use is addressed, people can be much more adherent with their [HIV] medications,” she noted.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no relevant disclosures.
SOURCE: Bosh KA et al. CROI 2019, Abstract 147.
SEATTLE – People with HIV are more likely to die from an opioid overdose than the general public, according to investigators from the Centers for Disease Control and Prevention.
“We looked into this because we know persons with HIV are more likely to have chronic pain and more likely to receive opioid analgesic treatments, and receive higher doses. In addition, they are more likely to have substance use disorders and mental illness than the U.S. general populations,” CDC epidemiologist Karin A. Bosh, PhD, said at the Conference on Retroviruses and Opportunistic Infections.
To see how that played out in terms of unintentional opioid overdose deaths, they turned to the National HIV Surveillance System and focused on overdose deaths during 2011-2015, the latest data available at the time of the work.
There were 1,363 overdose deaths among persons with HIV during that period, with the rate increasing 42.7% – from 23.2/100,000 HIV patients in 2011 to 33.1/100,000 in 2015.
Although the rate of increase was comparable to the general population, the crude rate was “actually substantially higher among persons with HIV,” Dr. Bosh said. Deaths were highest among persons aged 50-59 years (41.9/100,000), whites (49.1/100,000), injection drug users (137.4/100,000), and people who live in the Northeast (60.6/100,000).
Surprisingly, there was no increase in the rate of overdose deaths among HIV patients on the West Coast, possibly because heroin there was less likely to be cut with fentanyl.
Also, the rate of opioid overdose deaths was higher among women with HIV (35.2/100,000) than among men, perhaps because women are more likely to contract HIV by injection drug use, so they are more likely to be injection drug users at baseline, while the vast majority of men are infected through male-male sex, the investigators said.
The findings underscore the importance of intensifying overdose prevention in the HIV community, and better integrating HIV and substance use disorder treatment, they concluded.
That comes down to screening people for problems, especially in the subgroups identified in the study, and connecting them to drug treatment services. If HIV and substance disorder services were in the same clinic it would help, as would an increase in the number of buprenorphine providers, according to Sheryl B. Lyss, PhD, a coinvestigator and CDC epidemiologist.
“Obviously, when substance use is addressed, people can be much more adherent with their [HIV] medications,” she noted.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no relevant disclosures.
SOURCE: Bosh KA et al. CROI 2019, Abstract 147.
SEATTLE – People with HIV are more likely to die from an opioid overdose than the general public, according to investigators from the Centers for Disease Control and Prevention.
“We looked into this because we know persons with HIV are more likely to have chronic pain and more likely to receive opioid analgesic treatments, and receive higher doses. In addition, they are more likely to have substance use disorders and mental illness than the U.S. general populations,” CDC epidemiologist Karin A. Bosh, PhD, said at the Conference on Retroviruses and Opportunistic Infections.
To see how that played out in terms of unintentional opioid overdose deaths, they turned to the National HIV Surveillance System and focused on overdose deaths during 2011-2015, the latest data available at the time of the work.
There were 1,363 overdose deaths among persons with HIV during that period, with the rate increasing 42.7% – from 23.2/100,000 HIV patients in 2011 to 33.1/100,000 in 2015.
Although the rate of increase was comparable to the general population, the crude rate was “actually substantially higher among persons with HIV,” Dr. Bosh said. Deaths were highest among persons aged 50-59 years (41.9/100,000), whites (49.1/100,000), injection drug users (137.4/100,000), and people who live in the Northeast (60.6/100,000).
Surprisingly, there was no increase in the rate of overdose deaths among HIV patients on the West Coast, possibly because heroin there was less likely to be cut with fentanyl.
Also, the rate of opioid overdose deaths was higher among women with HIV (35.2/100,000) than among men, perhaps because women are more likely to contract HIV by injection drug use, so they are more likely to be injection drug users at baseline, while the vast majority of men are infected through male-male sex, the investigators said.
The findings underscore the importance of intensifying overdose prevention in the HIV community, and better integrating HIV and substance use disorder treatment, they concluded.
That comes down to screening people for problems, especially in the subgroups identified in the study, and connecting them to drug treatment services. If HIV and substance disorder services were in the same clinic it would help, as would an increase in the number of buprenorphine providers, according to Sheryl B. Lyss, PhD, a coinvestigator and CDC epidemiologist.
“Obviously, when substance use is addressed, people can be much more adherent with their [HIV] medications,” she noted.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no relevant disclosures.
SOURCE: Bosh KA et al. CROI 2019, Abstract 147.
REPORTING FROM CROI 2019
Accessibility and Uptake of Pre-Exposure Prophylaxis for HIV Prevention in the VHA (FULL)
Despite important advances in treatment and prevention over the past 30 years, HIV remains a significant public health concern in the US, with nearly 40,000 new HIV infections. annually.1 Among the estimated 1.1 million Americans currently living with HIV, 1 in 8 remains undiagnosed, and only half (49%) are virally suppressed.2 Although data demonstrate that viral suppression virtually eliminates the risk of transmission among people living with HIV, pre-exposure prophylaxis (PrEP) for HIV remains an integral part of a coordinated effort to reduce transmission. Uptake of PrEP is particularly vital considering the large percentage of people in the US living with HIV who are not virally suppressed because they have not started, are unable to stay on HIV antiretroviral treatment, or have not been diagnosed.
The Department of Veterans Affairs (VA) is the largest single provider of care to HIV-infected individuals in the US, with more than 28,000 veterans in care with HIV in 2016 (data from the VA National HIV Clinical Registry Reports, written communication from Population Health Service, Office of Patient Care Services, January 2018).
The only FDA-approved medication for HIV pre-exposure prophylaxis is tenofovir disoproxil fumarate/emtricitabine (TDF/FTC), a fixed-dose combination of 2 antiretroviral medications that are also used to treat HIV. Its efficacy has been proven among numerous populations at risk for HIV, including those with sexual and injection drug use risk factors.4,5 Use of TDF/FTC for PrEP has been available at the VA since its July 2012 FDA approval. In May 2014, the US Public Health Service (PHS) and the US Department of Health and Human Services released the first comprehensive clinical practice guidelines for PrEP. Soon after, in September 2014, the VA released more formal guidance on the use of TDF/FTC for HIV PrEP as outlined by the PHS.6 Similar to patterns outside the VA, PrEP uptake across the Veterans Health Administration (VHA) has been modest and variable.
A recent VHA analysis of the variability in PrEP uptake identified about 1,600 patients who had been prescribed PrEP in the VA as of June 2017 among about 6 million veterans in care. Across VA medical facilities, the absolute number of PrEP initiations ranged from 0 to 109 with the maximum PrEP initiation rate at 146.4/100,000 veterans in care. Eight facilities did not initiate a single PrEP prescription over the 5-year period. This study presents strategicefforts undertaken by the VA to increase access to and uptake of PrEP across the health care system and to decrease disparities in HIV prevention care.
VA National Pr EP Working Group
In the beginning of 2017, the HIV, Hepatitis, and Related Conditions (HHRC) programs within the VHA Office of Specialty Care Services convened a national working group to better measure and address the gaps in PrEP usage across the health care system. This multidisciplinary PrEP Working Group was composed of more than 40 members with expertise in HIV clinical care and PrEP, including physicians, clinical pharmacists, advanced practice registered nurses (APRNs), physician assistants (PAs), social workers, psychologists, implementation scientists, and representatives from other VA programs with a relevant programmatic or policy interest in PrEP.
Implementation Targets
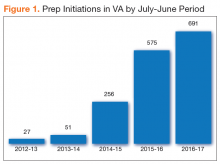
The National PrEP Working Group identified increased PrEP uptake across the VHA system as the primary implementation target with a specific focus on increasing PrEP use in primary care clinics and among those at highest risk. As noted earlier, overall uptake of PrEP across VHA medical facilities has been modest; however, new PrEP initiations have increased in each 12-month period since FDA approval (Figure 1).
To rapidly understand barriers to accessing PrEP, the National PrEP Working Group developed and deployed an informal survey to HIV clinicians at all VA facilities, with nearly half responding (n = 68). These frontline providers identified several important and common barriers inhibiting PrEP uptake, including knowledge gaps among providers without infectious diseases training
Patient adherence was not identified by providers as a significant barrier to PrEP uptake in this informal survey. A recent analysis of adherence among a national cohort of veterans on HIV PrEP in VA care between July 2012 and June 2016 found that adherence in the first year of PrEP was high with some differences detected by age, race, and gender.8
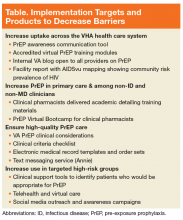
As an initial step in addressing these identified barriers to prescribing PrEP in the VHA, the National PrEP Working Group developed several provider education materials, trainings, and support tools to impact the overarching goal, and identified implementation targets of increasing access outside of primary care and among noninfectious disease and nonphysician clinicians, ensuring high-quality PrEP care in all settings, and targeting PrEP uptake to at-risk populations (Table).
Increasing PrEP Use in Primary Care and Women’s Health Clinics
As of June 2017, physicians (staff, interns, residents, and fellows) accounted for more than three-quarters of VA PrEP index prescriptions. Among staff physicians, infectious diseases specialists initiated 67% of all prescriptions. Clinical pharmacists prescribed only 6%; APRNs and PAs prescribed 16% of initiations. This is unsurprising, as the field survey identified lack of awareness and specific training on PrEP care among providers without infectious diseases training as a common barrier.
The VA is the largest US employer of nurses, including more than 5,500 APRNs. In December 2016, the VA granted full practice authority to APRNs across the health care system, regardless of state restrictions in most cases.9
In 2015, the VA employed about 7,700 clinical pharmacists, 3,200 of whom had an active SOP that allowed for prescribing authority. In fiscal year 2015, clinical pharmacists were responsible for at least 20% of all hepatitis C virus (HCV) prescriptions and 69% of prescriptions for anticoagulants across the system.10 Clinical pharmacists are increasingly recognized for their extensive contributions to increasing access to treatment in the VA across a broad spectrum of clinical issues. With this infrastructure and expertise, clinical pharmacists also are well positioned to expand their scope to include PrEP.
To that end, the National PrEP Working Group worked closely with clinical pharmacists in the field and from the VA Academic Detailing Service (ADS) within the VA Pharmacy Benefits Management Services office. The ADS supports the development of scholarly, balanced, evidence-based educational tools and information for frontline VA providers using one-on-one social marketing techniques to impact specific clinical targets. These interventions are delivered by clinical pharmacists to empower VA clinicians and promote evidence-based clinical care to help reduce variability in practice across the system.11 An ADS module for PrEP has been developed and will be available in 2018 across the VHA to facilities participating in the ADS.
A virtual accredited training program on prescribing PrEP and monitoring patients on PrEP designed for clinical pharmacists will be delivered early in 2018 to complement these materials and will be open to all prescribers interested in learning more about PrEP. By offering a complement of training and clinical support tools, most of which are detailed in other sections of this article, the National PrEP Working Group is creating educational opportunities that are accessible in a variety of different formats to decrease knowledge barriers over PrEP prescribing and build over time a broader pool of VA clinicians trained in PrEP care.
Ensuring High-Quality PrEP Care
One system-level concern about expanding PrEP to providers without infectious diseases training is the quality of follow-up care. In order to aid noninfectious diseases clinicians, and nonphysician providers who are not as familiar with PrEP, several clinical support tools have been created, including (1) VA’s Clinical Considerations for PrEP to Prevent HIV Infection, which is aligned with CDC clinical guidance12; (2) a PrEP clinical criterion check list; (3) clinical support tools, such as prepopulated electronic health record (EHR) templates and order menus to facilitate PrEP prescribing and monitoring in busy primary care clinical settings; and (4) PrEP-specific texts in the Annie App, an automated text-messaging application developed by the VA Office of Connected Care, which supports medication adherence, appointment attendance, vitals tracking, and education.13
Available evidence indicates that there is potential for disparities in PrEP effectiveness in the VA related to varying medication adherence. Analysis of pharmacy refill records found that adherence with TDF/FTC was high in the first year after PrEP initiation (median proportion of days covered in the first year was 74%), but adherence was lower among veterans in VA care who were African American, women, and/or under age 45 years.8 This highlights the importance of enhanced services, such as Annie, to support PrEP adherence in at-risk groups as well as monitoring of HIV risk factors to ensure PrEP is still indicated.
Targeting PrEP Uptake for High-Risk Veterans
Although the VA’s overarching goal is to increase access to and uptake of PrEP across the VHA, it also is important to direct resources to those at greatest risk of acquiring HIV infection. The National PrEP Working Group has focused on the following critical implementation issues in the VA’s strategic approach to HIV prevention, with a specific focus on the geographic disparities between PrEP uptake and HIV risk across the VHA as well as disparities based on rurality, race/ethnicity, and gender.
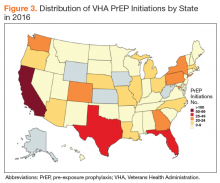
The majority of the VHA patient population is male (91% in 2016).14 A VHA analysis of PrEP initiations in the VA indicates that in June 2017, 97% of veterans in VA care receiving PrEP were male, 69% were white, 88% resided in urban areas, and the average age was 41.6 years. An analysis of PrEP initiation in the VA indicates that current PrEP uptake is clustered in a few geographic areas and that some areas with high HIV incidence had low uptake.15 States with the highest risk of HIV infection are in the Southeast, followed by parts of the West, Midwest, and Northeast (Figure 2).16,17
Rural areas are increasingly impacted by the HIV epidemic in the US, but access to PrEP is often limited in rural communities.19 Several rural counties in the Southeastern US now have rates of new HIV infection comparable with those historically seen in only the largest cities.1 In addition, recent outbreaks of HIV and hepatitis C virus infection related to needle sharing highlight the need for HIV prevention programs in rural areas impacted by the opioid epidemic.20
About 1 in 4 veterans overall—and 16% of veterans in care who are HIV-positive—reside in rural areas, but only 4.3% of veterans who had initiated PrEP through 2017 resided in rural areas.21,22 In order to address the need to improve access to PrEP in many rural-serving VHA facilities, the PrEP Working Group has emphasized the increased utilization of virtual care (telehealth, Annie App, the Virtual Medical Room) and broadening the pool of available PrEP prescribers to include noninfectious diseases physicians, pharmacists, and APRNs.
Important racial and ethnic disparities also exist in PrEP access nationally. For example, in the US as a whole, African American MSM, followed by Latino MSM continue to be at highest risk for HIV infection.1 In 2015, 45% of all new HIV infections in the US were among African Americans, 26% of whom were women and 58% identified as gay or bisexual.23 A recent analysis of US retail pharmacies that dispensed FTC/TDF analyzed the racial demographics of PrEP uptake and found that the majority of PrEP initiations were among whites (74%), followed by Hispanics (12%) and African Americans (10%); and females of all races made up 20.7%.24 The VA is performing better than these national averages. Of the 688 PrEP prescriptions in the VA in 2016, 64% of recipients identified themselves as white and 23% as African American. Hispanic ethnicity was reported by 13%.
There are several limitations to identifying a specific implementation target for PrEP across the VA system, including the challenge of accurately identifying the population at risk via the EHR or clinical informatics tools. For example, strong risk factors for HIV acquisition include IV drug use, receptive anal intercourse without a condom, and needlesticks.
Behaviors that pose lower risk, such as vaginal intercourse or insertive anal intercourse could contribute to a higher overall lifetime risk if these behaviors occur frequently.25 Behavioral risk factors are not well captured in the VA EHR, making it difficult to identify potential PrEP candidates through population health tools. Additionally, stigma and discrimination may make it difficult for a patient to disclose to their clinician and for a clinician to inquire into behavioral risk factors. The criminalization of HIV-related risk behaviors in some states also may complicate the identification of potential PrEP candidates.26,27 These issues contribute to the challenges that providers face in screening for HIV risk and that patients face in disclosing their personal risk.
To address these regional, rural, and ethnic disparities and enhance the identification of potential PrEP recipients, the National PrEP Working Group is developing a suite of tools to support frontline providers in identifying potential PrEP recipients and expanding care to those at highest risk and who may be more difficult to reach due to rurality, concerns about stigma, or other issues.
- Clinical support tools to identify potential PrEP recipients, such as a clinical reminder that identifies patients at high risk for HIV based on diagnosis codes, and a PrEP clinical dashboard;
- A telehealth protocol for PrEP care and promotion of the VA Virtual Medical Room, which allows providers to video conference with patients in their home; and
- Social media outreach and awareness campaigns targeted at veterans to increase PrEP awareness are being shared through VA Facebook and Twitter accounts, blog posts, and www.hiv.va.gov posts (Figure 4).
Implementation Strategy & Evaluation
During the calendar year 2017, the PrEP Working Group met monthly and in smaller subcommittees to develop the strategic plan, products, and tools described earlier. On World AIDS Day, a virtual live meeting on PrEP was made available to all providers across the system and will be made available for continuing education training through the VA online employee education system. During 2018, the primary focus of the PrEP Working Group will be the continued development and refinement of provider education materials, clinical tools, and data tracking as well as increasing veteran outreach through social media and other awareness campaigns planned throughout the year.
Annual assessment of PrEP uptake will evaluate progress on the primary implementation target and areas of clinical practice: (1) increase number of PrEP prescriptions overall; (2) ensure PrEP is prescribed at all VA facilities; (3) increase preciptions by noninfectious diseases provider; (4) increase prescriptions by clinical pharmacists and APRNs; (5) monitor quality of care, including by discipline/practice setting; (6) increase PrEP prescriptions in facilities in endemic areas; and (7) increase the proportion of PrEP prescriptions for veterans of color.
In 2019 and 2020, additional targeted intervention and outreach plans will be developed for sites with difficulty meeting implementation targets. Sites in highly HIV-endemic areas will be a priority, and outreach will be designed to assist in the identification of facility-level barriers to PrEP use.
Conclusion
HIV remains an important public health issue in the US and among veterans in VA care, and prevention is a critical component to combat the epidemic. The VHA is the largest single provider of HIV care in the US with facilities and community-based outpatient clinics in all states and US territories.
The VA seems to be performing better in terms of the proportion of PrEP uptake among racial groups at highest risk for HIV compared with a US sample from retail pharmacies, which may be, in part, driven by the cost of PrEP and follow-up sexually transmitted infection testing.24 However, a considerable gap remain in VHA PrEP uptake among populations at highest risk for HIV in the US.
With the investment of a National PrEP Working Group, the VA is charting a course to augment its HIV prevention services to exceed the US nationally. The National PrEP Working Group will continue to develop specific, measurable, and impactful targets guided by state-of-the-art scientific evidence and surveillance data and a suite of educational and clinical resources designed to assist frontline providers, facilities, and patients in meeting clearly defined implementation targets.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. HIV surveillance report, 2016; Vol 28. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance -report-2016-vol-28.pdf. Published November 2017. Accessed February 12, 2018.
2. Centers for Disease Control and Prevention. HIV continuum of care, US, 2014, overall and by age, race/ethnicity, transmission route and sex. https://www.cdc .gov/nchhstp/newsroom/2017/HIV-Continuum-of-Care.html. Updated September 12, 2017. Accessed February 12, 2018.
3. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
4. US Food and Drug Administration. FDA approves first medication to reduce HIV risk [press release]. https://aidsinfo.nih.gov/news/1254/fda-approves-first-drug -for-reducing-the-risk-of-sexually-acquired-hiv-infection. Published July 12, 2012. Accessed February 14, 2018.
5. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973-1983.
6. Centers for Disease Control and Prevention, US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014: a clinical practice guideline. http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf. Published 2014. Accessed February 12, 2018.
7. Smith DK, Mendoza MC, Stryker JE, Rose CE. PrEP awareness and attitudes in a national survey of primary care clinicians in the United States, 2009-2015. PLoS One. 2016;11(6):e0156592.
8. Van Epps P, Maier M, Lund B, et al. Medication adherence in a nationwide cohort of veterans initiating pre-exposure prophylaxis (PrEP) to prevent HIV infection. J Acquir Immune Defic Syndr. 2018;77(3):272-278.
9. US Department of Veterans Affairs. 38 CFR Part 17, RIN 2900-AP44. Advance Practice Registered Nurses. Federal Register, Rules and Regulations. 81(240) December 14, 2016
10. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
11. US Department of Veterans Affairs, Pharmacy Benefits Management Academic Detailing Service. VA academic detailing implementation guide. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/VA_Academic_Detailing_Implementation_Guide.pdf. Published September 2016. Accessed February 12, 2018.
12. Veterans Health Administration US Department of Veterans Affairs, Veterans Health Administration, Office of Specialty Services, HIV, Hepatitis, and Related Conditions Programs. Pre-exposure prophylaxis (PrEP) to prevent HIV infection: clinical considerations from the Department of Veterans Affairs National HIV Program. https://www.hiv.va.gov/pdf/PrEP-considerations.pdf. Published September 2016. Accessed January 4, 2018.
13. US Department of Veterans Affairs, VA Mobile Health. Annie app for clinicians. https://mobile.va.gov/app/annie-app-clinicians. Published September 2016. Accessed January 4, 2018.
14. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA utilization profile FY 2016. https://www.va.gov/vetdata/docs/Quickfacts/VA_Utilization_Profile.pdf. Published . November 2017. Accessed March 5, 2018.
15. Van Epps P. Pre-exposure prophylaxis for HIV prevention: the use and effectiveness of PrEP in the Veterans Health Administration (VHA). Abstract presented at: Infectious Diseases Week 2016; October 26-30, 2016; New Orleans, LA. https://idsa.confex.com/idsa/2016/webprogram/Paper60122.html. Accessed February 12, 2018.
16. Centers for Disease Control and Prevention. 2016 conference on retroviruses and opportunistic infections, lifetime risk of HIV diagnosis by state: https://www.cdc .gov/nchhstp/newsroom/images/2016/CROI_lifetime_risk_state.jpg. Published February 24, 2016. Accessed February 12, 2018.
17. Elopre L, Kudroff K, Westfall AO, Overton ET, Mugavero MJ. Brief report: the right people, right places, and right practices: disparities in PrEP access among African American men, women, and MSM in the Deep South. J Acquir Immune Defic Syndr. 2017;74(1):56-59.
18. Wu H, Mendoza MC, Huang YA, Hayes T, Smith DK, Hoover KW. Uptake of HIV preexposure prophylaxis among commercially insured persons-United States, 2010-2014. Clin Infect Dis. 2017;64(2):144-149.
19. Schafer KR, Albrecht H, Dillingham R, et al. The continuum of HIV care in rural communities in the United States and Canada: what is known and future research directions. J Acquir Immune Defic Syndr. 2017;75(1):355-344.
20. Conrad C, Bradley HM, Broz D, et al; Centers for Disease Control and Prevention (CDC). community outbreak of hiv infection linked to injection drug use of oxymorphone—Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(16):443-444.
21. Ohl ME, Richardson K, Kaboli P, Perencevich E, Vaughan-Sarrazin M. Geographic access and use of infectious diseases specialty and general primary care services by veterans with HIV infection: implications for telehealth and shared care programs. J Rural Health. 2014;30(4):412-421.
22. US Department of Veterans Affairs, Office of Rural Health. Rural veterans’ health care challenges. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Updated February 9, 2018. Accessed on February 12, 2018.
23. Centers for Disease Control and Prevention. HIV among African Americans. https://www.cdc.gov/hiv/group/racialethnic/africanamericans/index.html. Updated February 9, 2018. Accessed on February 12, 2018.
24. Bush S, Magnuson D, Rawlings K, et al. Racial characteristics of FTC/TDF for pre-exposure prophylaxis (PrEP) users in the US. Paper presented at: ASM Microbe Conference 2016; June 16-20, 2016; Boston, MA.
25. Centers for Disease Control and Prevention. HIV risk behaviors. https://www.cdc .gov/hiv/pdf/risk/estimates/cdc-hiv-risk-behaviors.pdf. Published December 2015. Accessed on February 12, 2018.
26. Lehman JS, Carr MH, Nichol AJ, et al. Prevalence and public health implications of state laws that criminalize potential HIV exposure in the United States. AIDS Behav. 2014;18(6):997-1006.
27. US Department of Justice, Civil Rights Division. Best practices guide to reform HIV-specific criminal laws to align with scientifically-supported factors. https://www.hivlawandpolicy.org/sites/default/files/DOj-HIV-Criminal-Law-Best-Practices-Guide.pdf. March 2014. Accessed on February 12, 2018.
28. Backus L, Czarnogorski M, Yip G, et al. HIV care continuum applied to the US Department of Veterans Affairs: HIV virologic outcomes in an integrated health care system. J Acquir Immune Defic Syndr. 2015;69(4):474-480.
Despite important advances in treatment and prevention over the past 30 years, HIV remains a significant public health concern in the US, with nearly 40,000 new HIV infections. annually.1 Among the estimated 1.1 million Americans currently living with HIV, 1 in 8 remains undiagnosed, and only half (49%) are virally suppressed.2 Although data demonstrate that viral suppression virtually eliminates the risk of transmission among people living with HIV, pre-exposure prophylaxis (PrEP) for HIV remains an integral part of a coordinated effort to reduce transmission. Uptake of PrEP is particularly vital considering the large percentage of people in the US living with HIV who are not virally suppressed because they have not started, are unable to stay on HIV antiretroviral treatment, or have not been diagnosed.
The Department of Veterans Affairs (VA) is the largest single provider of care to HIV-infected individuals in the US, with more than 28,000 veterans in care with HIV in 2016 (data from the VA National HIV Clinical Registry Reports, written communication from Population Health Service, Office of Patient Care Services, January 2018).
The only FDA-approved medication for HIV pre-exposure prophylaxis is tenofovir disoproxil fumarate/emtricitabine (TDF/FTC), a fixed-dose combination of 2 antiretroviral medications that are also used to treat HIV. Its efficacy has been proven among numerous populations at risk for HIV, including those with sexual and injection drug use risk factors.4,5 Use of TDF/FTC for PrEP has been available at the VA since its July 2012 FDA approval. In May 2014, the US Public Health Service (PHS) and the US Department of Health and Human Services released the first comprehensive clinical practice guidelines for PrEP. Soon after, in September 2014, the VA released more formal guidance on the use of TDF/FTC for HIV PrEP as outlined by the PHS.6 Similar to patterns outside the VA, PrEP uptake across the Veterans Health Administration (VHA) has been modest and variable.
A recent VHA analysis of the variability in PrEP uptake identified about 1,600 patients who had been prescribed PrEP in the VA as of June 2017 among about 6 million veterans in care. Across VA medical facilities, the absolute number of PrEP initiations ranged from 0 to 109 with the maximum PrEP initiation rate at 146.4/100,000 veterans in care. Eight facilities did not initiate a single PrEP prescription over the 5-year period. This study presents strategicefforts undertaken by the VA to increase access to and uptake of PrEP across the health care system and to decrease disparities in HIV prevention care.
VA National Pr EP Working Group
In the beginning of 2017, the HIV, Hepatitis, and Related Conditions (HHRC) programs within the VHA Office of Specialty Care Services convened a national working group to better measure and address the gaps in PrEP usage across the health care system. This multidisciplinary PrEP Working Group was composed of more than 40 members with expertise in HIV clinical care and PrEP, including physicians, clinical pharmacists, advanced practice registered nurses (APRNs), physician assistants (PAs), social workers, psychologists, implementation scientists, and representatives from other VA programs with a relevant programmatic or policy interest in PrEP.
Implementation Targets
The National PrEP Working Group identified increased PrEP uptake across the VHA system as the primary implementation target with a specific focus on increasing PrEP use in primary care clinics and among those at highest risk. As noted earlier, overall uptake of PrEP across VHA medical facilities has been modest; however, new PrEP initiations have increased in each 12-month period since FDA approval (Figure 1).
To rapidly understand barriers to accessing PrEP, the National PrEP Working Group developed and deployed an informal survey to HIV clinicians at all VA facilities, with nearly half responding (n = 68). These frontline providers identified several important and common barriers inhibiting PrEP uptake, including knowledge gaps among providers without infectious diseases training
Patient adherence was not identified by providers as a significant barrier to PrEP uptake in this informal survey. A recent analysis of adherence among a national cohort of veterans on HIV PrEP in VA care between July 2012 and June 2016 found that adherence in the first year of PrEP was high with some differences detected by age, race, and gender.8
As an initial step in addressing these identified barriers to prescribing PrEP in the VHA, the National PrEP Working Group developed several provider education materials, trainings, and support tools to impact the overarching goal, and identified implementation targets of increasing access outside of primary care and among noninfectious disease and nonphysician clinicians, ensuring high-quality PrEP care in all settings, and targeting PrEP uptake to at-risk populations (Table).
Increasing PrEP Use in Primary Care and Women’s Health Clinics
As of June 2017, physicians (staff, interns, residents, and fellows) accounted for more than three-quarters of VA PrEP index prescriptions. Among staff physicians, infectious diseases specialists initiated 67% of all prescriptions. Clinical pharmacists prescribed only 6%; APRNs and PAs prescribed 16% of initiations. This is unsurprising, as the field survey identified lack of awareness and specific training on PrEP care among providers without infectious diseases training as a common barrier.
The VA is the largest US employer of nurses, including more than 5,500 APRNs. In December 2016, the VA granted full practice authority to APRNs across the health care system, regardless of state restrictions in most cases.9
In 2015, the VA employed about 7,700 clinical pharmacists, 3,200 of whom had an active SOP that allowed for prescribing authority. In fiscal year 2015, clinical pharmacists were responsible for at least 20% of all hepatitis C virus (HCV) prescriptions and 69% of prescriptions for anticoagulants across the system.10 Clinical pharmacists are increasingly recognized for their extensive contributions to increasing access to treatment in the VA across a broad spectrum of clinical issues. With this infrastructure and expertise, clinical pharmacists also are well positioned to expand their scope to include PrEP.
To that end, the National PrEP Working Group worked closely with clinical pharmacists in the field and from the VA Academic Detailing Service (ADS) within the VA Pharmacy Benefits Management Services office. The ADS supports the development of scholarly, balanced, evidence-based educational tools and information for frontline VA providers using one-on-one social marketing techniques to impact specific clinical targets. These interventions are delivered by clinical pharmacists to empower VA clinicians and promote evidence-based clinical care to help reduce variability in practice across the system.11 An ADS module for PrEP has been developed and will be available in 2018 across the VHA to facilities participating in the ADS.
A virtual accredited training program on prescribing PrEP and monitoring patients on PrEP designed for clinical pharmacists will be delivered early in 2018 to complement these materials and will be open to all prescribers interested in learning more about PrEP. By offering a complement of training and clinical support tools, most of which are detailed in other sections of this article, the National PrEP Working Group is creating educational opportunities that are accessible in a variety of different formats to decrease knowledge barriers over PrEP prescribing and build over time a broader pool of VA clinicians trained in PrEP care.
Ensuring High-Quality PrEP Care
One system-level concern about expanding PrEP to providers without infectious diseases training is the quality of follow-up care. In order to aid noninfectious diseases clinicians, and nonphysician providers who are not as familiar with PrEP, several clinical support tools have been created, including (1) VA’s Clinical Considerations for PrEP to Prevent HIV Infection, which is aligned with CDC clinical guidance12; (2) a PrEP clinical criterion check list; (3) clinical support tools, such as prepopulated electronic health record (EHR) templates and order menus to facilitate PrEP prescribing and monitoring in busy primary care clinical settings; and (4) PrEP-specific texts in the Annie App, an automated text-messaging application developed by the VA Office of Connected Care, which supports medication adherence, appointment attendance, vitals tracking, and education.13
Available evidence indicates that there is potential for disparities in PrEP effectiveness in the VA related to varying medication adherence. Analysis of pharmacy refill records found that adherence with TDF/FTC was high in the first year after PrEP initiation (median proportion of days covered in the first year was 74%), but adherence was lower among veterans in VA care who were African American, women, and/or under age 45 years.8 This highlights the importance of enhanced services, such as Annie, to support PrEP adherence in at-risk groups as well as monitoring of HIV risk factors to ensure PrEP is still indicated.
Targeting PrEP Uptake for High-Risk Veterans
Although the VA’s overarching goal is to increase access to and uptake of PrEP across the VHA, it also is important to direct resources to those at greatest risk of acquiring HIV infection. The National PrEP Working Group has focused on the following critical implementation issues in the VA’s strategic approach to HIV prevention, with a specific focus on the geographic disparities between PrEP uptake and HIV risk across the VHA as well as disparities based on rurality, race/ethnicity, and gender.
The majority of the VHA patient population is male (91% in 2016).14 A VHA analysis of PrEP initiations in the VA indicates that in June 2017, 97% of veterans in VA care receiving PrEP were male, 69% were white, 88% resided in urban areas, and the average age was 41.6 years. An analysis of PrEP initiation in the VA indicates that current PrEP uptake is clustered in a few geographic areas and that some areas with high HIV incidence had low uptake.15 States with the highest risk of HIV infection are in the Southeast, followed by parts of the West, Midwest, and Northeast (Figure 2).16,17
Rural areas are increasingly impacted by the HIV epidemic in the US, but access to PrEP is often limited in rural communities.19 Several rural counties in the Southeastern US now have rates of new HIV infection comparable with those historically seen in only the largest cities.1 In addition, recent outbreaks of HIV and hepatitis C virus infection related to needle sharing highlight the need for HIV prevention programs in rural areas impacted by the opioid epidemic.20
About 1 in 4 veterans overall—and 16% of veterans in care who are HIV-positive—reside in rural areas, but only 4.3% of veterans who had initiated PrEP through 2017 resided in rural areas.21,22 In order to address the need to improve access to PrEP in many rural-serving VHA facilities, the PrEP Working Group has emphasized the increased utilization of virtual care (telehealth, Annie App, the Virtual Medical Room) and broadening the pool of available PrEP prescribers to include noninfectious diseases physicians, pharmacists, and APRNs.
Important racial and ethnic disparities also exist in PrEP access nationally. For example, in the US as a whole, African American MSM, followed by Latino MSM continue to be at highest risk for HIV infection.1 In 2015, 45% of all new HIV infections in the US were among African Americans, 26% of whom were women and 58% identified as gay or bisexual.23 A recent analysis of US retail pharmacies that dispensed FTC/TDF analyzed the racial demographics of PrEP uptake and found that the majority of PrEP initiations were among whites (74%), followed by Hispanics (12%) and African Americans (10%); and females of all races made up 20.7%.24 The VA is performing better than these national averages. Of the 688 PrEP prescriptions in the VA in 2016, 64% of recipients identified themselves as white and 23% as African American. Hispanic ethnicity was reported by 13%.
There are several limitations to identifying a specific implementation target for PrEP across the VA system, including the challenge of accurately identifying the population at risk via the EHR or clinical informatics tools. For example, strong risk factors for HIV acquisition include IV drug use, receptive anal intercourse without a condom, and needlesticks.
Behaviors that pose lower risk, such as vaginal intercourse or insertive anal intercourse could contribute to a higher overall lifetime risk if these behaviors occur frequently.25 Behavioral risk factors are not well captured in the VA EHR, making it difficult to identify potential PrEP candidates through population health tools. Additionally, stigma and discrimination may make it difficult for a patient to disclose to their clinician and for a clinician to inquire into behavioral risk factors. The criminalization of HIV-related risk behaviors in some states also may complicate the identification of potential PrEP candidates.26,27 These issues contribute to the challenges that providers face in screening for HIV risk and that patients face in disclosing their personal risk.
To address these regional, rural, and ethnic disparities and enhance the identification of potential PrEP recipients, the National PrEP Working Group is developing a suite of tools to support frontline providers in identifying potential PrEP recipients and expanding care to those at highest risk and who may be more difficult to reach due to rurality, concerns about stigma, or other issues.
- Clinical support tools to identify potential PrEP recipients, such as a clinical reminder that identifies patients at high risk for HIV based on diagnosis codes, and a PrEP clinical dashboard;
- A telehealth protocol for PrEP care and promotion of the VA Virtual Medical Room, which allows providers to video conference with patients in their home; and
- Social media outreach and awareness campaigns targeted at veterans to increase PrEP awareness are being shared through VA Facebook and Twitter accounts, blog posts, and www.hiv.va.gov posts (Figure 4).
Implementation Strategy & Evaluation
During the calendar year 2017, the PrEP Working Group met monthly and in smaller subcommittees to develop the strategic plan, products, and tools described earlier. On World AIDS Day, a virtual live meeting on PrEP was made available to all providers across the system and will be made available for continuing education training through the VA online employee education system. During 2018, the primary focus of the PrEP Working Group will be the continued development and refinement of provider education materials, clinical tools, and data tracking as well as increasing veteran outreach through social media and other awareness campaigns planned throughout the year.
Annual assessment of PrEP uptake will evaluate progress on the primary implementation target and areas of clinical practice: (1) increase number of PrEP prescriptions overall; (2) ensure PrEP is prescribed at all VA facilities; (3) increase preciptions by noninfectious diseases provider; (4) increase prescriptions by clinical pharmacists and APRNs; (5) monitor quality of care, including by discipline/practice setting; (6) increase PrEP prescriptions in facilities in endemic areas; and (7) increase the proportion of PrEP prescriptions for veterans of color.
In 2019 and 2020, additional targeted intervention and outreach plans will be developed for sites with difficulty meeting implementation targets. Sites in highly HIV-endemic areas will be a priority, and outreach will be designed to assist in the identification of facility-level barriers to PrEP use.
Conclusion
HIV remains an important public health issue in the US and among veterans in VA care, and prevention is a critical component to combat the epidemic. The VHA is the largest single provider of HIV care in the US with facilities and community-based outpatient clinics in all states and US territories.
The VA seems to be performing better in terms of the proportion of PrEP uptake among racial groups at highest risk for HIV compared with a US sample from retail pharmacies, which may be, in part, driven by the cost of PrEP and follow-up sexually transmitted infection testing.24 However, a considerable gap remain in VHA PrEP uptake among populations at highest risk for HIV in the US.
With the investment of a National PrEP Working Group, the VA is charting a course to augment its HIV prevention services to exceed the US nationally. The National PrEP Working Group will continue to develop specific, measurable, and impactful targets guided by state-of-the-art scientific evidence and surveillance data and a suite of educational and clinical resources designed to assist frontline providers, facilities, and patients in meeting clearly defined implementation targets.
Click here to read the digital edition.
Despite important advances in treatment and prevention over the past 30 years, HIV remains a significant public health concern in the US, with nearly 40,000 new HIV infections. annually.1 Among the estimated 1.1 million Americans currently living with HIV, 1 in 8 remains undiagnosed, and only half (49%) are virally suppressed.2 Although data demonstrate that viral suppression virtually eliminates the risk of transmission among people living with HIV, pre-exposure prophylaxis (PrEP) for HIV remains an integral part of a coordinated effort to reduce transmission. Uptake of PrEP is particularly vital considering the large percentage of people in the US living with HIV who are not virally suppressed because they have not started, are unable to stay on HIV antiretroviral treatment, or have not been diagnosed.
The Department of Veterans Affairs (VA) is the largest single provider of care to HIV-infected individuals in the US, with more than 28,000 veterans in care with HIV in 2016 (data from the VA National HIV Clinical Registry Reports, written communication from Population Health Service, Office of Patient Care Services, January 2018).
The only FDA-approved medication for HIV pre-exposure prophylaxis is tenofovir disoproxil fumarate/emtricitabine (TDF/FTC), a fixed-dose combination of 2 antiretroviral medications that are also used to treat HIV. Its efficacy has been proven among numerous populations at risk for HIV, including those with sexual and injection drug use risk factors.4,5 Use of TDF/FTC for PrEP has been available at the VA since its July 2012 FDA approval. In May 2014, the US Public Health Service (PHS) and the US Department of Health and Human Services released the first comprehensive clinical practice guidelines for PrEP. Soon after, in September 2014, the VA released more formal guidance on the use of TDF/FTC for HIV PrEP as outlined by the PHS.6 Similar to patterns outside the VA, PrEP uptake across the Veterans Health Administration (VHA) has been modest and variable.
A recent VHA analysis of the variability in PrEP uptake identified about 1,600 patients who had been prescribed PrEP in the VA as of June 2017 among about 6 million veterans in care. Across VA medical facilities, the absolute number of PrEP initiations ranged from 0 to 109 with the maximum PrEP initiation rate at 146.4/100,000 veterans in care. Eight facilities did not initiate a single PrEP prescription over the 5-year period. This study presents strategicefforts undertaken by the VA to increase access to and uptake of PrEP across the health care system and to decrease disparities in HIV prevention care.
VA National Pr EP Working Group
In the beginning of 2017, the HIV, Hepatitis, and Related Conditions (HHRC) programs within the VHA Office of Specialty Care Services convened a national working group to better measure and address the gaps in PrEP usage across the health care system. This multidisciplinary PrEP Working Group was composed of more than 40 members with expertise in HIV clinical care and PrEP, including physicians, clinical pharmacists, advanced practice registered nurses (APRNs), physician assistants (PAs), social workers, psychologists, implementation scientists, and representatives from other VA programs with a relevant programmatic or policy interest in PrEP.
Implementation Targets
The National PrEP Working Group identified increased PrEP uptake across the VHA system as the primary implementation target with a specific focus on increasing PrEP use in primary care clinics and among those at highest risk. As noted earlier, overall uptake of PrEP across VHA medical facilities has been modest; however, new PrEP initiations have increased in each 12-month period since FDA approval (Figure 1).
To rapidly understand barriers to accessing PrEP, the National PrEP Working Group developed and deployed an informal survey to HIV clinicians at all VA facilities, with nearly half responding (n = 68). These frontline providers identified several important and common barriers inhibiting PrEP uptake, including knowledge gaps among providers without infectious diseases training
Patient adherence was not identified by providers as a significant barrier to PrEP uptake in this informal survey. A recent analysis of adherence among a national cohort of veterans on HIV PrEP in VA care between July 2012 and June 2016 found that adherence in the first year of PrEP was high with some differences detected by age, race, and gender.8
As an initial step in addressing these identified barriers to prescribing PrEP in the VHA, the National PrEP Working Group developed several provider education materials, trainings, and support tools to impact the overarching goal, and identified implementation targets of increasing access outside of primary care and among noninfectious disease and nonphysician clinicians, ensuring high-quality PrEP care in all settings, and targeting PrEP uptake to at-risk populations (Table).
Increasing PrEP Use in Primary Care and Women’s Health Clinics
As of June 2017, physicians (staff, interns, residents, and fellows) accounted for more than three-quarters of VA PrEP index prescriptions. Among staff physicians, infectious diseases specialists initiated 67% of all prescriptions. Clinical pharmacists prescribed only 6%; APRNs and PAs prescribed 16% of initiations. This is unsurprising, as the field survey identified lack of awareness and specific training on PrEP care among providers without infectious diseases training as a common barrier.
The VA is the largest US employer of nurses, including more than 5,500 APRNs. In December 2016, the VA granted full practice authority to APRNs across the health care system, regardless of state restrictions in most cases.9
In 2015, the VA employed about 7,700 clinical pharmacists, 3,200 of whom had an active SOP that allowed for prescribing authority. In fiscal year 2015, clinical pharmacists were responsible for at least 20% of all hepatitis C virus (HCV) prescriptions and 69% of prescriptions for anticoagulants across the system.10 Clinical pharmacists are increasingly recognized for their extensive contributions to increasing access to treatment in the VA across a broad spectrum of clinical issues. With this infrastructure and expertise, clinical pharmacists also are well positioned to expand their scope to include PrEP.
To that end, the National PrEP Working Group worked closely with clinical pharmacists in the field and from the VA Academic Detailing Service (ADS) within the VA Pharmacy Benefits Management Services office. The ADS supports the development of scholarly, balanced, evidence-based educational tools and information for frontline VA providers using one-on-one social marketing techniques to impact specific clinical targets. These interventions are delivered by clinical pharmacists to empower VA clinicians and promote evidence-based clinical care to help reduce variability in practice across the system.11 An ADS module for PrEP has been developed and will be available in 2018 across the VHA to facilities participating in the ADS.
A virtual accredited training program on prescribing PrEP and monitoring patients on PrEP designed for clinical pharmacists will be delivered early in 2018 to complement these materials and will be open to all prescribers interested in learning more about PrEP. By offering a complement of training and clinical support tools, most of which are detailed in other sections of this article, the National PrEP Working Group is creating educational opportunities that are accessible in a variety of different formats to decrease knowledge barriers over PrEP prescribing and build over time a broader pool of VA clinicians trained in PrEP care.
Ensuring High-Quality PrEP Care
One system-level concern about expanding PrEP to providers without infectious diseases training is the quality of follow-up care. In order to aid noninfectious diseases clinicians, and nonphysician providers who are not as familiar with PrEP, several clinical support tools have been created, including (1) VA’s Clinical Considerations for PrEP to Prevent HIV Infection, which is aligned with CDC clinical guidance12; (2) a PrEP clinical criterion check list; (3) clinical support tools, such as prepopulated electronic health record (EHR) templates and order menus to facilitate PrEP prescribing and monitoring in busy primary care clinical settings; and (4) PrEP-specific texts in the Annie App, an automated text-messaging application developed by the VA Office of Connected Care, which supports medication adherence, appointment attendance, vitals tracking, and education.13
Available evidence indicates that there is potential for disparities in PrEP effectiveness in the VA related to varying medication adherence. Analysis of pharmacy refill records found that adherence with TDF/FTC was high in the first year after PrEP initiation (median proportion of days covered in the first year was 74%), but adherence was lower among veterans in VA care who were African American, women, and/or under age 45 years.8 This highlights the importance of enhanced services, such as Annie, to support PrEP adherence in at-risk groups as well as monitoring of HIV risk factors to ensure PrEP is still indicated.
Targeting PrEP Uptake for High-Risk Veterans
Although the VA’s overarching goal is to increase access to and uptake of PrEP across the VHA, it also is important to direct resources to those at greatest risk of acquiring HIV infection. The National PrEP Working Group has focused on the following critical implementation issues in the VA’s strategic approach to HIV prevention, with a specific focus on the geographic disparities between PrEP uptake and HIV risk across the VHA as well as disparities based on rurality, race/ethnicity, and gender.
The majority of the VHA patient population is male (91% in 2016).14 A VHA analysis of PrEP initiations in the VA indicates that in June 2017, 97% of veterans in VA care receiving PrEP were male, 69% were white, 88% resided in urban areas, and the average age was 41.6 years. An analysis of PrEP initiation in the VA indicates that current PrEP uptake is clustered in a few geographic areas and that some areas with high HIV incidence had low uptake.15 States with the highest risk of HIV infection are in the Southeast, followed by parts of the West, Midwest, and Northeast (Figure 2).16,17
Rural areas are increasingly impacted by the HIV epidemic in the US, but access to PrEP is often limited in rural communities.19 Several rural counties in the Southeastern US now have rates of new HIV infection comparable with those historically seen in only the largest cities.1 In addition, recent outbreaks of HIV and hepatitis C virus infection related to needle sharing highlight the need for HIV prevention programs in rural areas impacted by the opioid epidemic.20
About 1 in 4 veterans overall—and 16% of veterans in care who are HIV-positive—reside in rural areas, but only 4.3% of veterans who had initiated PrEP through 2017 resided in rural areas.21,22 In order to address the need to improve access to PrEP in many rural-serving VHA facilities, the PrEP Working Group has emphasized the increased utilization of virtual care (telehealth, Annie App, the Virtual Medical Room) and broadening the pool of available PrEP prescribers to include noninfectious diseases physicians, pharmacists, and APRNs.
Important racial and ethnic disparities also exist in PrEP access nationally. For example, in the US as a whole, African American MSM, followed by Latino MSM continue to be at highest risk for HIV infection.1 In 2015, 45% of all new HIV infections in the US were among African Americans, 26% of whom were women and 58% identified as gay or bisexual.23 A recent analysis of US retail pharmacies that dispensed FTC/TDF analyzed the racial demographics of PrEP uptake and found that the majority of PrEP initiations were among whites (74%), followed by Hispanics (12%) and African Americans (10%); and females of all races made up 20.7%.24 The VA is performing better than these national averages. Of the 688 PrEP prescriptions in the VA in 2016, 64% of recipients identified themselves as white and 23% as African American. Hispanic ethnicity was reported by 13%.
There are several limitations to identifying a specific implementation target for PrEP across the VA system, including the challenge of accurately identifying the population at risk via the EHR or clinical informatics tools. For example, strong risk factors for HIV acquisition include IV drug use, receptive anal intercourse without a condom, and needlesticks.
Behaviors that pose lower risk, such as vaginal intercourse or insertive anal intercourse could contribute to a higher overall lifetime risk if these behaviors occur frequently.25 Behavioral risk factors are not well captured in the VA EHR, making it difficult to identify potential PrEP candidates through population health tools. Additionally, stigma and discrimination may make it difficult for a patient to disclose to their clinician and for a clinician to inquire into behavioral risk factors. The criminalization of HIV-related risk behaviors in some states also may complicate the identification of potential PrEP candidates.26,27 These issues contribute to the challenges that providers face in screening for HIV risk and that patients face in disclosing their personal risk.
To address these regional, rural, and ethnic disparities and enhance the identification of potential PrEP recipients, the National PrEP Working Group is developing a suite of tools to support frontline providers in identifying potential PrEP recipients and expanding care to those at highest risk and who may be more difficult to reach due to rurality, concerns about stigma, or other issues.
- Clinical support tools to identify potential PrEP recipients, such as a clinical reminder that identifies patients at high risk for HIV based on diagnosis codes, and a PrEP clinical dashboard;
- A telehealth protocol for PrEP care and promotion of the VA Virtual Medical Room, which allows providers to video conference with patients in their home; and
- Social media outreach and awareness campaigns targeted at veterans to increase PrEP awareness are being shared through VA Facebook and Twitter accounts, blog posts, and www.hiv.va.gov posts (Figure 4).
Implementation Strategy & Evaluation
During the calendar year 2017, the PrEP Working Group met monthly and in smaller subcommittees to develop the strategic plan, products, and tools described earlier. On World AIDS Day, a virtual live meeting on PrEP was made available to all providers across the system and will be made available for continuing education training through the VA online employee education system. During 2018, the primary focus of the PrEP Working Group will be the continued development and refinement of provider education materials, clinical tools, and data tracking as well as increasing veteran outreach through social media and other awareness campaigns planned throughout the year.
Annual assessment of PrEP uptake will evaluate progress on the primary implementation target and areas of clinical practice: (1) increase number of PrEP prescriptions overall; (2) ensure PrEP is prescribed at all VA facilities; (3) increase preciptions by noninfectious diseases provider; (4) increase prescriptions by clinical pharmacists and APRNs; (5) monitor quality of care, including by discipline/practice setting; (6) increase PrEP prescriptions in facilities in endemic areas; and (7) increase the proportion of PrEP prescriptions for veterans of color.
In 2019 and 2020, additional targeted intervention and outreach plans will be developed for sites with difficulty meeting implementation targets. Sites in highly HIV-endemic areas will be a priority, and outreach will be designed to assist in the identification of facility-level barriers to PrEP use.
Conclusion
HIV remains an important public health issue in the US and among veterans in VA care, and prevention is a critical component to combat the epidemic. The VHA is the largest single provider of HIV care in the US with facilities and community-based outpatient clinics in all states and US territories.
The VA seems to be performing better in terms of the proportion of PrEP uptake among racial groups at highest risk for HIV compared with a US sample from retail pharmacies, which may be, in part, driven by the cost of PrEP and follow-up sexually transmitted infection testing.24 However, a considerable gap remain in VHA PrEP uptake among populations at highest risk for HIV in the US.
With the investment of a National PrEP Working Group, the VA is charting a course to augment its HIV prevention services to exceed the US nationally. The National PrEP Working Group will continue to develop specific, measurable, and impactful targets guided by state-of-the-art scientific evidence and surveillance data and a suite of educational and clinical resources designed to assist frontline providers, facilities, and patients in meeting clearly defined implementation targets.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. HIV surveillance report, 2016; Vol 28. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance -report-2016-vol-28.pdf. Published November 2017. Accessed February 12, 2018.
2. Centers for Disease Control and Prevention. HIV continuum of care, US, 2014, overall and by age, race/ethnicity, transmission route and sex. https://www.cdc .gov/nchhstp/newsroom/2017/HIV-Continuum-of-Care.html. Updated September 12, 2017. Accessed February 12, 2018.
3. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
4. US Food and Drug Administration. FDA approves first medication to reduce HIV risk [press release]. https://aidsinfo.nih.gov/news/1254/fda-approves-first-drug -for-reducing-the-risk-of-sexually-acquired-hiv-infection. Published July 12, 2012. Accessed February 14, 2018.
5. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973-1983.
6. Centers for Disease Control and Prevention, US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014: a clinical practice guideline. http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf. Published 2014. Accessed February 12, 2018.
7. Smith DK, Mendoza MC, Stryker JE, Rose CE. PrEP awareness and attitudes in a national survey of primary care clinicians in the United States, 2009-2015. PLoS One. 2016;11(6):e0156592.
8. Van Epps P, Maier M, Lund B, et al. Medication adherence in a nationwide cohort of veterans initiating pre-exposure prophylaxis (PrEP) to prevent HIV infection. J Acquir Immune Defic Syndr. 2018;77(3):272-278.
9. US Department of Veterans Affairs. 38 CFR Part 17, RIN 2900-AP44. Advance Practice Registered Nurses. Federal Register, Rules and Regulations. 81(240) December 14, 2016
10. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
11. US Department of Veterans Affairs, Pharmacy Benefits Management Academic Detailing Service. VA academic detailing implementation guide. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/VA_Academic_Detailing_Implementation_Guide.pdf. Published September 2016. Accessed February 12, 2018.
12. Veterans Health Administration US Department of Veterans Affairs, Veterans Health Administration, Office of Specialty Services, HIV, Hepatitis, and Related Conditions Programs. Pre-exposure prophylaxis (PrEP) to prevent HIV infection: clinical considerations from the Department of Veterans Affairs National HIV Program. https://www.hiv.va.gov/pdf/PrEP-considerations.pdf. Published September 2016. Accessed January 4, 2018.
13. US Department of Veterans Affairs, VA Mobile Health. Annie app for clinicians. https://mobile.va.gov/app/annie-app-clinicians. Published September 2016. Accessed January 4, 2018.
14. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA utilization profile FY 2016. https://www.va.gov/vetdata/docs/Quickfacts/VA_Utilization_Profile.pdf. Published . November 2017. Accessed March 5, 2018.
15. Van Epps P. Pre-exposure prophylaxis for HIV prevention: the use and effectiveness of PrEP in the Veterans Health Administration (VHA). Abstract presented at: Infectious Diseases Week 2016; October 26-30, 2016; New Orleans, LA. https://idsa.confex.com/idsa/2016/webprogram/Paper60122.html. Accessed February 12, 2018.
16. Centers for Disease Control and Prevention. 2016 conference on retroviruses and opportunistic infections, lifetime risk of HIV diagnosis by state: https://www.cdc .gov/nchhstp/newsroom/images/2016/CROI_lifetime_risk_state.jpg. Published February 24, 2016. Accessed February 12, 2018.
17. Elopre L, Kudroff K, Westfall AO, Overton ET, Mugavero MJ. Brief report: the right people, right places, and right practices: disparities in PrEP access among African American men, women, and MSM in the Deep South. J Acquir Immune Defic Syndr. 2017;74(1):56-59.
18. Wu H, Mendoza MC, Huang YA, Hayes T, Smith DK, Hoover KW. Uptake of HIV preexposure prophylaxis among commercially insured persons-United States, 2010-2014. Clin Infect Dis. 2017;64(2):144-149.
19. Schafer KR, Albrecht H, Dillingham R, et al. The continuum of HIV care in rural communities in the United States and Canada: what is known and future research directions. J Acquir Immune Defic Syndr. 2017;75(1):355-344.
20. Conrad C, Bradley HM, Broz D, et al; Centers for Disease Control and Prevention (CDC). community outbreak of hiv infection linked to injection drug use of oxymorphone—Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(16):443-444.
21. Ohl ME, Richardson K, Kaboli P, Perencevich E, Vaughan-Sarrazin M. Geographic access and use of infectious diseases specialty and general primary care services by veterans with HIV infection: implications for telehealth and shared care programs. J Rural Health. 2014;30(4):412-421.
22. US Department of Veterans Affairs, Office of Rural Health. Rural veterans’ health care challenges. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Updated February 9, 2018. Accessed on February 12, 2018.
23. Centers for Disease Control and Prevention. HIV among African Americans. https://www.cdc.gov/hiv/group/racialethnic/africanamericans/index.html. Updated February 9, 2018. Accessed on February 12, 2018.
24. Bush S, Magnuson D, Rawlings K, et al. Racial characteristics of FTC/TDF for pre-exposure prophylaxis (PrEP) users in the US. Paper presented at: ASM Microbe Conference 2016; June 16-20, 2016; Boston, MA.
25. Centers for Disease Control and Prevention. HIV risk behaviors. https://www.cdc .gov/hiv/pdf/risk/estimates/cdc-hiv-risk-behaviors.pdf. Published December 2015. Accessed on February 12, 2018.
26. Lehman JS, Carr MH, Nichol AJ, et al. Prevalence and public health implications of state laws that criminalize potential HIV exposure in the United States. AIDS Behav. 2014;18(6):997-1006.
27. US Department of Justice, Civil Rights Division. Best practices guide to reform HIV-specific criminal laws to align with scientifically-supported factors. https://www.hivlawandpolicy.org/sites/default/files/DOj-HIV-Criminal-Law-Best-Practices-Guide.pdf. March 2014. Accessed on February 12, 2018.
28. Backus L, Czarnogorski M, Yip G, et al. HIV care continuum applied to the US Department of Veterans Affairs: HIV virologic outcomes in an integrated health care system. J Acquir Immune Defic Syndr. 2015;69(4):474-480.
1. Centers for Disease Control and Prevention. HIV surveillance report, 2016; Vol 28. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance -report-2016-vol-28.pdf. Published November 2017. Accessed February 12, 2018.
2. Centers for Disease Control and Prevention. HIV continuum of care, US, 2014, overall and by age, race/ethnicity, transmission route and sex. https://www.cdc .gov/nchhstp/newsroom/2017/HIV-Continuum-of-Care.html. Updated September 12, 2017. Accessed February 12, 2018.
3. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
4. US Food and Drug Administration. FDA approves first medication to reduce HIV risk [press release]. https://aidsinfo.nih.gov/news/1254/fda-approves-first-drug -for-reducing-the-risk-of-sexually-acquired-hiv-infection. Published July 12, 2012. Accessed February 14, 2018.
5. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973-1983.
6. Centers for Disease Control and Prevention, US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014: a clinical practice guideline. http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf. Published 2014. Accessed February 12, 2018.
7. Smith DK, Mendoza MC, Stryker JE, Rose CE. PrEP awareness and attitudes in a national survey of primary care clinicians in the United States, 2009-2015. PLoS One. 2016;11(6):e0156592.
8. Van Epps P, Maier M, Lund B, et al. Medication adherence in a nationwide cohort of veterans initiating pre-exposure prophylaxis (PrEP) to prevent HIV infection. J Acquir Immune Defic Syndr. 2018;77(3):272-278.
9. US Department of Veterans Affairs. 38 CFR Part 17, RIN 2900-AP44. Advance Practice Registered Nurses. Federal Register, Rules and Regulations. 81(240) December 14, 2016
10. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
11. US Department of Veterans Affairs, Pharmacy Benefits Management Academic Detailing Service. VA academic detailing implementation guide. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/VA_Academic_Detailing_Implementation_Guide.pdf. Published September 2016. Accessed February 12, 2018.
12. Veterans Health Administration US Department of Veterans Affairs, Veterans Health Administration, Office of Specialty Services, HIV, Hepatitis, and Related Conditions Programs. Pre-exposure prophylaxis (PrEP) to prevent HIV infection: clinical considerations from the Department of Veterans Affairs National HIV Program. https://www.hiv.va.gov/pdf/PrEP-considerations.pdf. Published September 2016. Accessed January 4, 2018.
13. US Department of Veterans Affairs, VA Mobile Health. Annie app for clinicians. https://mobile.va.gov/app/annie-app-clinicians. Published September 2016. Accessed January 4, 2018.
14. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA utilization profile FY 2016. https://www.va.gov/vetdata/docs/Quickfacts/VA_Utilization_Profile.pdf. Published . November 2017. Accessed March 5, 2018.
15. Van Epps P. Pre-exposure prophylaxis for HIV prevention: the use and effectiveness of PrEP in the Veterans Health Administration (VHA). Abstract presented at: Infectious Diseases Week 2016; October 26-30, 2016; New Orleans, LA. https://idsa.confex.com/idsa/2016/webprogram/Paper60122.html. Accessed February 12, 2018.
16. Centers for Disease Control and Prevention. 2016 conference on retroviruses and opportunistic infections, lifetime risk of HIV diagnosis by state: https://www.cdc .gov/nchhstp/newsroom/images/2016/CROI_lifetime_risk_state.jpg. Published February 24, 2016. Accessed February 12, 2018.
17. Elopre L, Kudroff K, Westfall AO, Overton ET, Mugavero MJ. Brief report: the right people, right places, and right practices: disparities in PrEP access among African American men, women, and MSM in the Deep South. J Acquir Immune Defic Syndr. 2017;74(1):56-59.
18. Wu H, Mendoza MC, Huang YA, Hayes T, Smith DK, Hoover KW. Uptake of HIV preexposure prophylaxis among commercially insured persons-United States, 2010-2014. Clin Infect Dis. 2017;64(2):144-149.
19. Schafer KR, Albrecht H, Dillingham R, et al. The continuum of HIV care in rural communities in the United States and Canada: what is known and future research directions. J Acquir Immune Defic Syndr. 2017;75(1):355-344.
20. Conrad C, Bradley HM, Broz D, et al; Centers for Disease Control and Prevention (CDC). community outbreak of hiv infection linked to injection drug use of oxymorphone—Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(16):443-444.
21. Ohl ME, Richardson K, Kaboli P, Perencevich E, Vaughan-Sarrazin M. Geographic access and use of infectious diseases specialty and general primary care services by veterans with HIV infection: implications for telehealth and shared care programs. J Rural Health. 2014;30(4):412-421.
22. US Department of Veterans Affairs, Office of Rural Health. Rural veterans’ health care challenges. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Updated February 9, 2018. Accessed on February 12, 2018.
23. Centers for Disease Control and Prevention. HIV among African Americans. https://www.cdc.gov/hiv/group/racialethnic/africanamericans/index.html. Updated February 9, 2018. Accessed on February 12, 2018.
24. Bush S, Magnuson D, Rawlings K, et al. Racial characteristics of FTC/TDF for pre-exposure prophylaxis (PrEP) users in the US. Paper presented at: ASM Microbe Conference 2016; June 16-20, 2016; Boston, MA.
25. Centers for Disease Control and Prevention. HIV risk behaviors. https://www.cdc .gov/hiv/pdf/risk/estimates/cdc-hiv-risk-behaviors.pdf. Published December 2015. Accessed on February 12, 2018.
26. Lehman JS, Carr MH, Nichol AJ, et al. Prevalence and public health implications of state laws that criminalize potential HIV exposure in the United States. AIDS Behav. 2014;18(6):997-1006.
27. US Department of Justice, Civil Rights Division. Best practices guide to reform HIV-specific criminal laws to align with scientifically-supported factors. https://www.hivlawandpolicy.org/sites/default/files/DOj-HIV-Criminal-Law-Best-Practices-Guide.pdf. March 2014. Accessed on February 12, 2018.
28. Backus L, Czarnogorski M, Yip G, et al. HIV care continuum applied to the US Department of Veterans Affairs: HIV virologic outcomes in an integrated health care system. J Acquir Immune Defic Syndr. 2015;69(4):474-480.
Poor COPD management might increase MI risk in HIV
SEATTLE – Chronic obstructive pulmonary disease is independently associated with an increased risk of myocardial infarction in people with HIV, according to a report at the Conference on Retroviruses and Opportunistic Infections.
Chronic obstructive pulmonary disease (COPD) is known to increase the risk of myocardial infarction (MI) in the general population, but hadn’t been shown until now to do the same in HIV. The study raises the question of whether COPD is being managed adequately in patients with the virus, according to study lead Kristina Crothers, MD, associate professor in the division of pulmonary, critical care & sleep medicine at the University of Washington, Seattle.
The investigators reviewed 25,509 HIV patients in the Center for AIDS Research Network of Integrated Clinical Systems cohort, a large electronic database of HIV-infected people. They defined COPD by diagnostic codes and inhaler prescriptions. MIs were adjudicated by review.
The team identified 423 subjects with moderate to severe COPD, and 698 who had MIs, including 339 type 1 MIs (T1MI) from a ruptured plaque (54%), and 294 (46%) type 2 heart attacks (T2MI) from a supply-demand mismatch due to sepsis or some other problem. In general, T2MIs are far more common in people with HIV.
COPD was associated with a greater than twofold increased risk of MI after adjustment for age, sex, viral load, nadir CD4 count, hypertension, and other confounders. The risk dropped slightly when smoking – both current smoking and pack years – was added to the model (adjusted hazard ratio 1.88, 95% confidence interval, 1.34-2.63).
The association was particularly strong for T2MI, especially in the setting of bacteremia and sepsis, and unlike T1MI, it remained significant after adjustment for smoking.
The study establishes a link between COPD and MI in HIV, but it could not answer what’s going on. Chronic inflammation from the virus could be at play, but the team also found hints of inadequate COPD management.
“About 60% of patients were on inhalers ... but only about 25% of them were on long-acting inhalers. 75% were only on short-acting.” That’s a problem because long-acting inhalers are needed to control exacerbations, Dr. Crothers said.
The study didn’t capture exacerbation rates, but increased rates could help explain the MI risk. Increased rates of pneumonia could as well, since pneumonia is a common cause of sepsis.
“We need to better manage complications of COPD in this population. I think optimizing long-term COPD management could have many beneficial effects,” Dr. Crothers said.
The National Institutes of Health funded the work. Dr. Crothers had no disclosures.
SOURCE: Crothers K et al. CROI 2019, Abstract 31.
SEATTLE – Chronic obstructive pulmonary disease is independently associated with an increased risk of myocardial infarction in people with HIV, according to a report at the Conference on Retroviruses and Opportunistic Infections.
Chronic obstructive pulmonary disease (COPD) is known to increase the risk of myocardial infarction (MI) in the general population, but hadn’t been shown until now to do the same in HIV. The study raises the question of whether COPD is being managed adequately in patients with the virus, according to study lead Kristina Crothers, MD, associate professor in the division of pulmonary, critical care & sleep medicine at the University of Washington, Seattle.
The investigators reviewed 25,509 HIV patients in the Center for AIDS Research Network of Integrated Clinical Systems cohort, a large electronic database of HIV-infected people. They defined COPD by diagnostic codes and inhaler prescriptions. MIs were adjudicated by review.
The team identified 423 subjects with moderate to severe COPD, and 698 who had MIs, including 339 type 1 MIs (T1MI) from a ruptured plaque (54%), and 294 (46%) type 2 heart attacks (T2MI) from a supply-demand mismatch due to sepsis or some other problem. In general, T2MIs are far more common in people with HIV.
COPD was associated with a greater than twofold increased risk of MI after adjustment for age, sex, viral load, nadir CD4 count, hypertension, and other confounders. The risk dropped slightly when smoking – both current smoking and pack years – was added to the model (adjusted hazard ratio 1.88, 95% confidence interval, 1.34-2.63).
The association was particularly strong for T2MI, especially in the setting of bacteremia and sepsis, and unlike T1MI, it remained significant after adjustment for smoking.
The study establishes a link between COPD and MI in HIV, but it could not answer what’s going on. Chronic inflammation from the virus could be at play, but the team also found hints of inadequate COPD management.
“About 60% of patients were on inhalers ... but only about 25% of them were on long-acting inhalers. 75% were only on short-acting.” That’s a problem because long-acting inhalers are needed to control exacerbations, Dr. Crothers said.
The study didn’t capture exacerbation rates, but increased rates could help explain the MI risk. Increased rates of pneumonia could as well, since pneumonia is a common cause of sepsis.
“We need to better manage complications of COPD in this population. I think optimizing long-term COPD management could have many beneficial effects,” Dr. Crothers said.
The National Institutes of Health funded the work. Dr. Crothers had no disclosures.
SOURCE: Crothers K et al. CROI 2019, Abstract 31.
SEATTLE – Chronic obstructive pulmonary disease is independently associated with an increased risk of myocardial infarction in people with HIV, according to a report at the Conference on Retroviruses and Opportunistic Infections.
Chronic obstructive pulmonary disease (COPD) is known to increase the risk of myocardial infarction (MI) in the general population, but hadn’t been shown until now to do the same in HIV. The study raises the question of whether COPD is being managed adequately in patients with the virus, according to study lead Kristina Crothers, MD, associate professor in the division of pulmonary, critical care & sleep medicine at the University of Washington, Seattle.
The investigators reviewed 25,509 HIV patients in the Center for AIDS Research Network of Integrated Clinical Systems cohort, a large electronic database of HIV-infected people. They defined COPD by diagnostic codes and inhaler prescriptions. MIs were adjudicated by review.
The team identified 423 subjects with moderate to severe COPD, and 698 who had MIs, including 339 type 1 MIs (T1MI) from a ruptured plaque (54%), and 294 (46%) type 2 heart attacks (T2MI) from a supply-demand mismatch due to sepsis or some other problem. In general, T2MIs are far more common in people with HIV.
COPD was associated with a greater than twofold increased risk of MI after adjustment for age, sex, viral load, nadir CD4 count, hypertension, and other confounders. The risk dropped slightly when smoking – both current smoking and pack years – was added to the model (adjusted hazard ratio 1.88, 95% confidence interval, 1.34-2.63).
The association was particularly strong for T2MI, especially in the setting of bacteremia and sepsis, and unlike T1MI, it remained significant after adjustment for smoking.
The study establishes a link between COPD and MI in HIV, but it could not answer what’s going on. Chronic inflammation from the virus could be at play, but the team also found hints of inadequate COPD management.
“About 60% of patients were on inhalers ... but only about 25% of them were on long-acting inhalers. 75% were only on short-acting.” That’s a problem because long-acting inhalers are needed to control exacerbations, Dr. Crothers said.
The study didn’t capture exacerbation rates, but increased rates could help explain the MI risk. Increased rates of pneumonia could as well, since pneumonia is a common cause of sepsis.
“We need to better manage complications of COPD in this population. I think optimizing long-term COPD management could have many beneficial effects,” Dr. Crothers said.
The National Institutes of Health funded the work. Dr. Crothers had no disclosures.
SOURCE: Crothers K et al. CROI 2019, Abstract 31.
REPORTING FROM CROI 2019
Syphilis rates high in HIV-positive women
SEATTLE – The frequency of syphilis in HIV-infected women is unusually high, prompting concerns about congenital syphilis among newborns. The finding comes from a Centers for Disease Control analysis of the U.S. Center for Aids Research Clinical Network of Integrated Clinical Systems (CNICS) cohort.
“We found significant associations [between syphilis infection] and drug use and hepatitis C infection, which adds to some information from the CDC that what’s driving the epidemic in women is drug use – it’s a very different epidemic potentially in women with HIV than in men with HIV,” said Jodie Dionne-Odom, MD, chief of women’s health services at the University of Alabama, Birmingham’s 1917 Clinic. She described the results of her study at a press conference at the Conference on Retroviruses and Infectious Diseases.
“These predictors are important to understand so that we can come up with interventions to try to reduce the problem. Syphilis screening is relatively easy to do. USPSTF [U.S. Preventive Services Task Force] doesn’t even have a recommendation for that periodicity of screening frequency, so we have a ways to go to define how often we need to be looking, particularly in high-risk groups,” she said.
The likely force driving the increased incidence of syphilis is transactional sex. To explore that possibility, the researchers examined the number of sexual partners and found that more partners were linked to greater likelihood of having syphilis. “So I don’t think it’s the drug use itself – it’s the behavior that comes with the drug use,” Dr. Dionne-Odom said.
The findings shed more light on the interplay between drug use epidemics and disease epidemics. “As we have an increasing opioid epidemic and methamphetamine abuse that we’re all seeing in our clinics, it’s interesting to see this intersection between the drug use problem and the syphilis problem in this country. I think they’re not unrelated,” she said.
She believes the results should have broad implications for screening programs. Women admitted to drug treatment programs should be tested for syphilis. And women who abuse drugs and are pregnant should be screened repeatedly – at entry to care, at their 20-week scan, and at delivery. “We know we have about 6,000 women with HIV who deliver each year, so this is potentially very impactful,” Dr. Dionne-Odom said.
The researchers extracted data from the CNICS cohort, including 4,795 women with records between 2005 and 2016. They defined incident syphilis as a newly positive nontreponemal serologic test or a 300% titer increase, followed by a confirmatory test.
After adjustment, factors associated with syphilis included prior intravenous drug abuse (adjusted odds ratio, 2.3; 95% confidence interval, 1.3-3.9), hepatitis C antibody positivity (aOR, 2.1; 95% CI, 1.3-3.7), as well as black race (aOR, 2.3; 95% CI, 1.4-3.9). There was no association between age and HIV viral load with respect to risk of syphilis.
The frequency appears to be rising, with later entry into the CNICS cohort assorted with a more than doubled risk of syphilis (aOR, 2.3; 95% CI, 1.4-3.9 for 2011-2016, compared with 1994-2004).
The study was funded by the National Institute of Child Health and Human Development. Dr. Dionne-Odom reported no relevant conflicts of interest.
SOURCE: J Dionne-Odom et al. CROI 2019, Abstract 47
SEATTLE – The frequency of syphilis in HIV-infected women is unusually high, prompting concerns about congenital syphilis among newborns. The finding comes from a Centers for Disease Control analysis of the U.S. Center for Aids Research Clinical Network of Integrated Clinical Systems (CNICS) cohort.
“We found significant associations [between syphilis infection] and drug use and hepatitis C infection, which adds to some information from the CDC that what’s driving the epidemic in women is drug use – it’s a very different epidemic potentially in women with HIV than in men with HIV,” said Jodie Dionne-Odom, MD, chief of women’s health services at the University of Alabama, Birmingham’s 1917 Clinic. She described the results of her study at a press conference at the Conference on Retroviruses and Infectious Diseases.
“These predictors are important to understand so that we can come up with interventions to try to reduce the problem. Syphilis screening is relatively easy to do. USPSTF [U.S. Preventive Services Task Force] doesn’t even have a recommendation for that periodicity of screening frequency, so we have a ways to go to define how often we need to be looking, particularly in high-risk groups,” she said.
The likely force driving the increased incidence of syphilis is transactional sex. To explore that possibility, the researchers examined the number of sexual partners and found that more partners were linked to greater likelihood of having syphilis. “So I don’t think it’s the drug use itself – it’s the behavior that comes with the drug use,” Dr. Dionne-Odom said.
The findings shed more light on the interplay between drug use epidemics and disease epidemics. “As we have an increasing opioid epidemic and methamphetamine abuse that we’re all seeing in our clinics, it’s interesting to see this intersection between the drug use problem and the syphilis problem in this country. I think they’re not unrelated,” she said.
She believes the results should have broad implications for screening programs. Women admitted to drug treatment programs should be tested for syphilis. And women who abuse drugs and are pregnant should be screened repeatedly – at entry to care, at their 20-week scan, and at delivery. “We know we have about 6,000 women with HIV who deliver each year, so this is potentially very impactful,” Dr. Dionne-Odom said.
The researchers extracted data from the CNICS cohort, including 4,795 women with records between 2005 and 2016. They defined incident syphilis as a newly positive nontreponemal serologic test or a 300% titer increase, followed by a confirmatory test.
After adjustment, factors associated with syphilis included prior intravenous drug abuse (adjusted odds ratio, 2.3; 95% confidence interval, 1.3-3.9), hepatitis C antibody positivity (aOR, 2.1; 95% CI, 1.3-3.7), as well as black race (aOR, 2.3; 95% CI, 1.4-3.9). There was no association between age and HIV viral load with respect to risk of syphilis.
The frequency appears to be rising, with later entry into the CNICS cohort assorted with a more than doubled risk of syphilis (aOR, 2.3; 95% CI, 1.4-3.9 for 2011-2016, compared with 1994-2004).
The study was funded by the National Institute of Child Health and Human Development. Dr. Dionne-Odom reported no relevant conflicts of interest.
SOURCE: J Dionne-Odom et al. CROI 2019, Abstract 47
SEATTLE – The frequency of syphilis in HIV-infected women is unusually high, prompting concerns about congenital syphilis among newborns. The finding comes from a Centers for Disease Control analysis of the U.S. Center for Aids Research Clinical Network of Integrated Clinical Systems (CNICS) cohort.
“We found significant associations [between syphilis infection] and drug use and hepatitis C infection, which adds to some information from the CDC that what’s driving the epidemic in women is drug use – it’s a very different epidemic potentially in women with HIV than in men with HIV,” said Jodie Dionne-Odom, MD, chief of women’s health services at the University of Alabama, Birmingham’s 1917 Clinic. She described the results of her study at a press conference at the Conference on Retroviruses and Infectious Diseases.
“These predictors are important to understand so that we can come up with interventions to try to reduce the problem. Syphilis screening is relatively easy to do. USPSTF [U.S. Preventive Services Task Force] doesn’t even have a recommendation for that periodicity of screening frequency, so we have a ways to go to define how often we need to be looking, particularly in high-risk groups,” she said.
The likely force driving the increased incidence of syphilis is transactional sex. To explore that possibility, the researchers examined the number of sexual partners and found that more partners were linked to greater likelihood of having syphilis. “So I don’t think it’s the drug use itself – it’s the behavior that comes with the drug use,” Dr. Dionne-Odom said.
The findings shed more light on the interplay between drug use epidemics and disease epidemics. “As we have an increasing opioid epidemic and methamphetamine abuse that we’re all seeing in our clinics, it’s interesting to see this intersection between the drug use problem and the syphilis problem in this country. I think they’re not unrelated,” she said.
She believes the results should have broad implications for screening programs. Women admitted to drug treatment programs should be tested for syphilis. And women who abuse drugs and are pregnant should be screened repeatedly – at entry to care, at their 20-week scan, and at delivery. “We know we have about 6,000 women with HIV who deliver each year, so this is potentially very impactful,” Dr. Dionne-Odom said.
The researchers extracted data from the CNICS cohort, including 4,795 women with records between 2005 and 2016. They defined incident syphilis as a newly positive nontreponemal serologic test or a 300% titer increase, followed by a confirmatory test.
After adjustment, factors associated with syphilis included prior intravenous drug abuse (adjusted odds ratio, 2.3; 95% confidence interval, 1.3-3.9), hepatitis C antibody positivity (aOR, 2.1; 95% CI, 1.3-3.7), as well as black race (aOR, 2.3; 95% CI, 1.4-3.9). There was no association between age and HIV viral load with respect to risk of syphilis.
The frequency appears to be rising, with later entry into the CNICS cohort assorted with a more than doubled risk of syphilis (aOR, 2.3; 95% CI, 1.4-3.9 for 2011-2016, compared with 1994-2004).
The study was funded by the National Institute of Child Health and Human Development. Dr. Dionne-Odom reported no relevant conflicts of interest.
SOURCE: J Dionne-Odom et al. CROI 2019, Abstract 47
REPORTING FROM CROI 2019
Histoplasmosis Manifests After Decades
Immunocompromised patients can be at risk for complications long after the original health issue was resolved—a problem illustrated by a patient who had a heart transplant in 1986 but developed acute progressive disseminated histoplasmosis decades later.
The patient presented with altered mental status; a Mini-Mental State Exam showed confusion. A computed tomography scan of the patient’s head revealed lesions, raising the suspicion of metastatic malignancy, which was ruled out after biopsy of a medial right temporal brain lesion. MRIs of his chest, abdomen, and pelvis revealed bilateral masses on his adrenal glands. Guided adrenal biopsy showed necrotizing granulomas consistent with a diagnosis of disseminated histoplasmosis.
However, that diagnosis was questioned—the patient had lived in Arizona for years, not, for instance, the Midwest, where histoplasmosis is more common. Nor did he have a history of spelunking, prior exposure to bird or bat droppings. He did report a short visit to North Carolina 30 years earlier. And he had been on immunosuppressive drugs for years.
The patient was started on liposomal amphotericin B, which was discontinued when his renal function deteriorated. He was switched to itraconazole, then restarted on amphotericin B with close monitoring after the diagnosis was confirmed. His doses of immunosuppressive drugs were reduced.
The clinicians note that HIV/AIDS and use of immunosuppressive drugs are among the risk factors for disseminated infection. They cite 1 study that found immunosuppression was the single most common risk factor. In another study, the risk of histoplasmosis increased as CD4+ T cells dropped below 300/µL.
The patient’s case was complicated by the fact that it was > 30 years after his heart transplant, and he had made only a short visit to an endemic area. He also had no history of histoplasmosis—the clinicians say a database search turned up the fact that most reported cases were preceded by symptomatic infection.
When charting patient history, they advise placing emphasis on a history of travel to endemic areas and considering histoplasmosis in immunocompromised patients in nonendemic areas.
Immunocompromised patients can be at risk for complications long after the original health issue was resolved—a problem illustrated by a patient who had a heart transplant in 1986 but developed acute progressive disseminated histoplasmosis decades later.
The patient presented with altered mental status; a Mini-Mental State Exam showed confusion. A computed tomography scan of the patient’s head revealed lesions, raising the suspicion of metastatic malignancy, which was ruled out after biopsy of a medial right temporal brain lesion. MRIs of his chest, abdomen, and pelvis revealed bilateral masses on his adrenal glands. Guided adrenal biopsy showed necrotizing granulomas consistent with a diagnosis of disseminated histoplasmosis.
However, that diagnosis was questioned—the patient had lived in Arizona for years, not, for instance, the Midwest, where histoplasmosis is more common. Nor did he have a history of spelunking, prior exposure to bird or bat droppings. He did report a short visit to North Carolina 30 years earlier. And he had been on immunosuppressive drugs for years.
The patient was started on liposomal amphotericin B, which was discontinued when his renal function deteriorated. He was switched to itraconazole, then restarted on amphotericin B with close monitoring after the diagnosis was confirmed. His doses of immunosuppressive drugs were reduced.
The clinicians note that HIV/AIDS and use of immunosuppressive drugs are among the risk factors for disseminated infection. They cite 1 study that found immunosuppression was the single most common risk factor. In another study, the risk of histoplasmosis increased as CD4+ T cells dropped below 300/µL.
The patient’s case was complicated by the fact that it was > 30 years after his heart transplant, and he had made only a short visit to an endemic area. He also had no history of histoplasmosis—the clinicians say a database search turned up the fact that most reported cases were preceded by symptomatic infection.
When charting patient history, they advise placing emphasis on a history of travel to endemic areas and considering histoplasmosis in immunocompromised patients in nonendemic areas.
Immunocompromised patients can be at risk for complications long after the original health issue was resolved—a problem illustrated by a patient who had a heart transplant in 1986 but developed acute progressive disseminated histoplasmosis decades later.
The patient presented with altered mental status; a Mini-Mental State Exam showed confusion. A computed tomography scan of the patient’s head revealed lesions, raising the suspicion of metastatic malignancy, which was ruled out after biopsy of a medial right temporal brain lesion. MRIs of his chest, abdomen, and pelvis revealed bilateral masses on his adrenal glands. Guided adrenal biopsy showed necrotizing granulomas consistent with a diagnosis of disseminated histoplasmosis.
However, that diagnosis was questioned—the patient had lived in Arizona for years, not, for instance, the Midwest, where histoplasmosis is more common. Nor did he have a history of spelunking, prior exposure to bird or bat droppings. He did report a short visit to North Carolina 30 years earlier. And he had been on immunosuppressive drugs for years.
The patient was started on liposomal amphotericin B, which was discontinued when his renal function deteriorated. He was switched to itraconazole, then restarted on amphotericin B with close monitoring after the diagnosis was confirmed. His doses of immunosuppressive drugs were reduced.
The clinicians note that HIV/AIDS and use of immunosuppressive drugs are among the risk factors for disseminated infection. They cite 1 study that found immunosuppression was the single most common risk factor. In another study, the risk of histoplasmosis increased as CD4+ T cells dropped below 300/µL.
The patient’s case was complicated by the fact that it was > 30 years after his heart transplant, and he had made only a short visit to an endemic area. He also had no history of histoplasmosis—the clinicians say a database search turned up the fact that most reported cases were preceded by symptomatic infection.
When charting patient history, they advise placing emphasis on a history of travel to endemic areas and considering histoplasmosis in immunocompromised patients in nonendemic areas.
Point-of-care VL testing improves HIV suppression
SEATTLE – Point-of-care viral load testing improves HIV viral suppression and retention in care, according to a randomized trial of 390 subjects in South Africa.
Point-of-care (POC) testing delivers viral load results in about 2-3 hours, as opposed to the month or so patients wait to get results from a laboratory. The nearly instant turnaround gives clinicians the ability to identify patients who aren’t doing well – as indicated by high viral loads despite antiretroviral therapy (ART) – before they walk out the door, so immediate steps can be taken to address adherence or resistance problems.
However, POC viral load testing hasn’t really caught on in the United States, at least not yet, according to study leader Paul Drain, MD, assistant professor of global health at the University of Washington, Seattle.
To see if it would help, the team turned to a large public clinic in the city of Durban, and focused on adults who had been on ART for 6 months following HIV diagnosis. They randomized 195 to standard laboratory testing at study entrance, with a repeat at 6 months, at which point subjects had been on ART for 12 months; 195 others were randomized to POC testing with the Xpert HIV-1 Viral Load machine, from Cepheid, on the same schedule and with same-day counseling for those with high loads.
Treatment was otherwise similar between the groups, with clinic visits every 2 months and other measures as per South African HIV treatment guidelines.
POC testing made a difference. At study month 12,175 participants (89.7%) in the POC arm, but only 148 (75.9%) in the laboratory testing group, had reached the study’s primary endpoint: viral suppression with less than 200 copies/mL plus retention in care, meaning that subjects were still picking up their ART prescriptions.
Overall, POC testing increased viral load suppression by 10.3%, from 83.1% to 93.3% (P = .003) and retention by 7.7% from 84.6% to 92.3% (P = .03).
The investigators would like to evaluate the approach in the United States. Potentially, “POC testing will have a very important role in U.S. health care,” Dr. Drain said at the Conference on Retroviruses and Opportunistic Infections.
It “helps us identify those who are having problems right away, before they leave the clinic, because whether it’s in South Africa or Seattle, as soon as they leave, it’s very hard to get them back. The more you can do POC testing, the better we can intervene and help these people,” he said.
“You don’t need POC testing for everybody; a lot of people do just fine. They take their medications reliably. They don’t need to get their results back right away ... But there are people who have challenges and would benefit from additional adherence counseling” or who might need help overcoming drug resistance. “We want to identify” them quickly; POC testing may be the answer, he said.
The mean age in the study was 33 years, and 60% of the subjects were women. The median CD4 count at baseline was 468 cells/mm3. POC was $22 per test, versus $25 for lab testing.
The National Institutes of Health funded the work. Dr. Drain had no disclosures. Cepheid donated the POC testing machines.
SOURCE: Drain PK et al. CROI 2019, Abstract 53LB.
SEATTLE – Point-of-care viral load testing improves HIV viral suppression and retention in care, according to a randomized trial of 390 subjects in South Africa.
Point-of-care (POC) testing delivers viral load results in about 2-3 hours, as opposed to the month or so patients wait to get results from a laboratory. The nearly instant turnaround gives clinicians the ability to identify patients who aren’t doing well – as indicated by high viral loads despite antiretroviral therapy (ART) – before they walk out the door, so immediate steps can be taken to address adherence or resistance problems.
However, POC viral load testing hasn’t really caught on in the United States, at least not yet, according to study leader Paul Drain, MD, assistant professor of global health at the University of Washington, Seattle.
To see if it would help, the team turned to a large public clinic in the city of Durban, and focused on adults who had been on ART for 6 months following HIV diagnosis. They randomized 195 to standard laboratory testing at study entrance, with a repeat at 6 months, at which point subjects had been on ART for 12 months; 195 others were randomized to POC testing with the Xpert HIV-1 Viral Load machine, from Cepheid, on the same schedule and with same-day counseling for those with high loads.
Treatment was otherwise similar between the groups, with clinic visits every 2 months and other measures as per South African HIV treatment guidelines.
POC testing made a difference. At study month 12,175 participants (89.7%) in the POC arm, but only 148 (75.9%) in the laboratory testing group, had reached the study’s primary endpoint: viral suppression with less than 200 copies/mL plus retention in care, meaning that subjects were still picking up their ART prescriptions.
Overall, POC testing increased viral load suppression by 10.3%, from 83.1% to 93.3% (P = .003) and retention by 7.7% from 84.6% to 92.3% (P = .03).
The investigators would like to evaluate the approach in the United States. Potentially, “POC testing will have a very important role in U.S. health care,” Dr. Drain said at the Conference on Retroviruses and Opportunistic Infections.
It “helps us identify those who are having problems right away, before they leave the clinic, because whether it’s in South Africa or Seattle, as soon as they leave, it’s very hard to get them back. The more you can do POC testing, the better we can intervene and help these people,” he said.
“You don’t need POC testing for everybody; a lot of people do just fine. They take their medications reliably. They don’t need to get their results back right away ... But there are people who have challenges and would benefit from additional adherence counseling” or who might need help overcoming drug resistance. “We want to identify” them quickly; POC testing may be the answer, he said.
The mean age in the study was 33 years, and 60% of the subjects were women. The median CD4 count at baseline was 468 cells/mm3. POC was $22 per test, versus $25 for lab testing.
The National Institutes of Health funded the work. Dr. Drain had no disclosures. Cepheid donated the POC testing machines.
SOURCE: Drain PK et al. CROI 2019, Abstract 53LB.
SEATTLE – Point-of-care viral load testing improves HIV viral suppression and retention in care, according to a randomized trial of 390 subjects in South Africa.
Point-of-care (POC) testing delivers viral load results in about 2-3 hours, as opposed to the month or so patients wait to get results from a laboratory. The nearly instant turnaround gives clinicians the ability to identify patients who aren’t doing well – as indicated by high viral loads despite antiretroviral therapy (ART) – before they walk out the door, so immediate steps can be taken to address adherence or resistance problems.
However, POC viral load testing hasn’t really caught on in the United States, at least not yet, according to study leader Paul Drain, MD, assistant professor of global health at the University of Washington, Seattle.
To see if it would help, the team turned to a large public clinic in the city of Durban, and focused on adults who had been on ART for 6 months following HIV diagnosis. They randomized 195 to standard laboratory testing at study entrance, with a repeat at 6 months, at which point subjects had been on ART for 12 months; 195 others were randomized to POC testing with the Xpert HIV-1 Viral Load machine, from Cepheid, on the same schedule and with same-day counseling for those with high loads.
Treatment was otherwise similar between the groups, with clinic visits every 2 months and other measures as per South African HIV treatment guidelines.
POC testing made a difference. At study month 12,175 participants (89.7%) in the POC arm, but only 148 (75.9%) in the laboratory testing group, had reached the study’s primary endpoint: viral suppression with less than 200 copies/mL plus retention in care, meaning that subjects were still picking up their ART prescriptions.
Overall, POC testing increased viral load suppression by 10.3%, from 83.1% to 93.3% (P = .003) and retention by 7.7% from 84.6% to 92.3% (P = .03).
The investigators would like to evaluate the approach in the United States. Potentially, “POC testing will have a very important role in U.S. health care,” Dr. Drain said at the Conference on Retroviruses and Opportunistic Infections.
It “helps us identify those who are having problems right away, before they leave the clinic, because whether it’s in South Africa or Seattle, as soon as they leave, it’s very hard to get them back. The more you can do POC testing, the better we can intervene and help these people,” he said.
“You don’t need POC testing for everybody; a lot of people do just fine. They take their medications reliably. They don’t need to get their results back right away ... But there are people who have challenges and would benefit from additional adherence counseling” or who might need help overcoming drug resistance. “We want to identify” them quickly; POC testing may be the answer, he said.
The mean age in the study was 33 years, and 60% of the subjects were women. The median CD4 count at baseline was 468 cells/mm3. POC was $22 per test, versus $25 for lab testing.
The National Institutes of Health funded the work. Dr. Drain had no disclosures. Cepheid donated the POC testing machines.
SOURCE: Drain PK et al. CROI 2019, Abstract 53LB.
REPORTING FROM CROI 2019
CDC: United States has hit a plateau with HIV
The annual number of new HIV infections has remained stable in recent years, and the Centers for Disease Control and Prevention says that’s not good – but solutions are at hand.
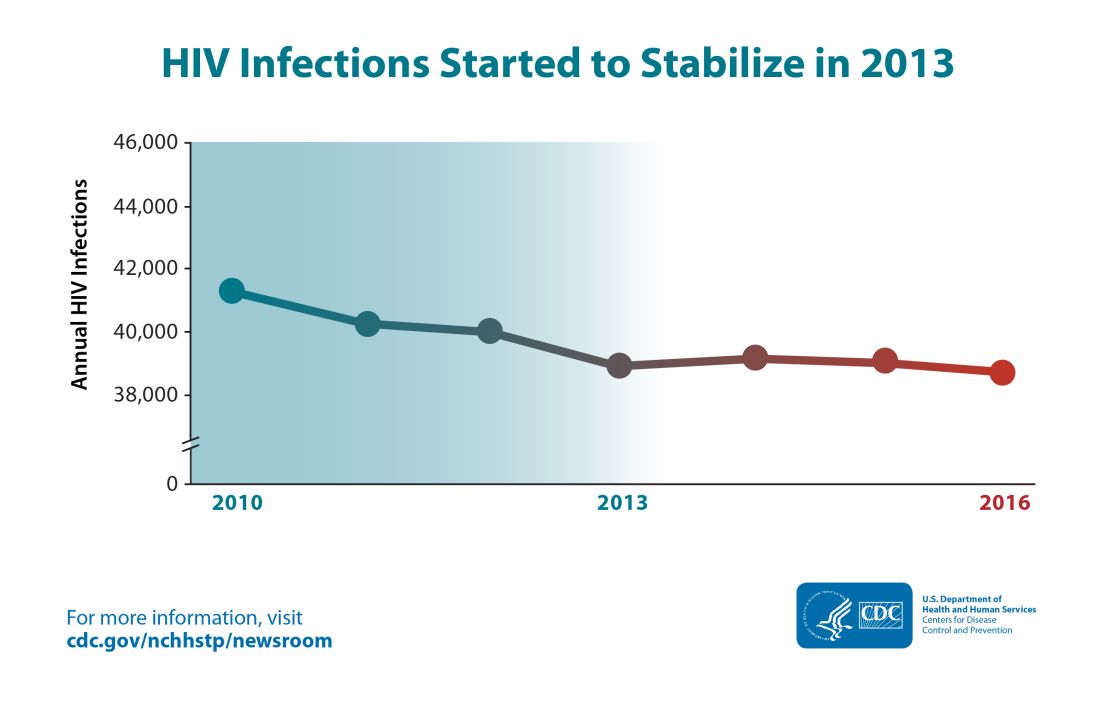
Though the estimated number of new HIV infections declined from just under 42,000 per year in 2010 to about 39,000 annually in 2013, that figure was essentially unchanged by 2016, with 38,700 new HIV infections seen that year.
“CDC estimates that the decline in HIV infections has plateaued because effective HIV prevention and treatment are not adequately reaching those who could most benefit from them. These gaps remain particularly troublesome in rural areas and in the South and among disproportionately affected populations like African Americans and Latinos,” said the CDC in a press release accompanying the report.
The report comes soon after President Trump’s State of the Union address, which announced a new multiagency initiative to eliminate the HIV epidemic in the United States, with the goal of reducing new HIV infections by 90% over the next 10 years. The multipronged initiative will implement geographically targeted HIV elimination teams in areas with high HIV prevalence, pulling together federal agencies, local and state governments, and community-level resources.
The initiative, called “Ending the Epidemic: A Plan for America” will combine an intensified approach to early diagnosis and treatment with efforts to boost uptake of pre-exposure prophylaxis for individuals at high risk for HIV infection.
The new CDC report used CD4 counts reported to the National HIV Surveillance System at the time of diagnosis to identify new (incident) cases and to track prevalence. Much of the report is devoted to finely detailed reporting of HIV incidence across sex, age, race/ethnicity, and transmission mode.
Though some groups, such as people who inject drugs, have seen a decrease of about 30% in the annual rate of new HIV cases, new cases have jumped for other groups. In particular, Latino gay and bisexual men saw new cases climb from 6,400 per year in 2010 to 8,300 in 2016. The incidence rate has stayed high and stable among African American gay and bisexual men, with 9,800 new cases reported in 2010; the same number was seen in 2016.
Among gay and bisexual men overall, the rate has also stayed stable, with about 26,000 new HIV infections reported at the beginning and end of the studied period. White heterosexual women saw about 1,000 new cases per year in 2010 and in 2016.
Some groups saw declines in new cases: African American and Latina heterosexual women each saw a falling incidence of new HIV cases. For the former group, new cases fell from 4,700 to 4,000, while the latter group of women saw new cases drop from 1,200 to 980 per year from 2010 to 2016.
Within these broad groups, HIV incidence also rose among some age groups and fell among others. Decreases were seen for younger African American gay and bisexual men (those aged 13-24 years), but rates increased by about two-thirds for men in this group aged 25-34 years. A similar increase was seen for Latino men in the 25-34 years age group, a change which drove the overall 30% increase in new infections for Latino gay and bisexual men.
White gay and bisexual men saw across-the-board decreases in new infections, though the overall decrease was less than 20%.
For heterosexual individuals as a group, new infections dropped by about 17%, from 10,900 to 9,100 annually. This change was driven mostly by decreases in women identifying as heterosexual.
“After a decades-long struggle, the path to eliminate America’s HIV epidemic is clear,” said Eugene McCray, MD, director of CDC’s Division of HIV/AIDS Prevention, in the press release. “Expanding efforts across the country will close gaps, overcome threats, and turn around troublesome trends.”
The press release cited local work in Washington and New York as evidence that targeted resources can make a difference in reducing new HIV cases. In these two areas, new infections dropped by 23% and 40% respectively from 2010 to 2016.
SOURCE: Centers for Disease Control. CDC Report: www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
The annual number of new HIV infections has remained stable in recent years, and the Centers for Disease Control and Prevention says that’s not good – but solutions are at hand.

Though the estimated number of new HIV infections declined from just under 42,000 per year in 2010 to about 39,000 annually in 2013, that figure was essentially unchanged by 2016, with 38,700 new HIV infections seen that year.
“CDC estimates that the decline in HIV infections has plateaued because effective HIV prevention and treatment are not adequately reaching those who could most benefit from them. These gaps remain particularly troublesome in rural areas and in the South and among disproportionately affected populations like African Americans and Latinos,” said the CDC in a press release accompanying the report.
The report comes soon after President Trump’s State of the Union address, which announced a new multiagency initiative to eliminate the HIV epidemic in the United States, with the goal of reducing new HIV infections by 90% over the next 10 years. The multipronged initiative will implement geographically targeted HIV elimination teams in areas with high HIV prevalence, pulling together federal agencies, local and state governments, and community-level resources.
The initiative, called “Ending the Epidemic: A Plan for America” will combine an intensified approach to early diagnosis and treatment with efforts to boost uptake of pre-exposure prophylaxis for individuals at high risk for HIV infection.
The new CDC report used CD4 counts reported to the National HIV Surveillance System at the time of diagnosis to identify new (incident) cases and to track prevalence. Much of the report is devoted to finely detailed reporting of HIV incidence across sex, age, race/ethnicity, and transmission mode.
Though some groups, such as people who inject drugs, have seen a decrease of about 30% in the annual rate of new HIV cases, new cases have jumped for other groups. In particular, Latino gay and bisexual men saw new cases climb from 6,400 per year in 2010 to 8,300 in 2016. The incidence rate has stayed high and stable among African American gay and bisexual men, with 9,800 new cases reported in 2010; the same number was seen in 2016.
Among gay and bisexual men overall, the rate has also stayed stable, with about 26,000 new HIV infections reported at the beginning and end of the studied period. White heterosexual women saw about 1,000 new cases per year in 2010 and in 2016.
Some groups saw declines in new cases: African American and Latina heterosexual women each saw a falling incidence of new HIV cases. For the former group, new cases fell from 4,700 to 4,000, while the latter group of women saw new cases drop from 1,200 to 980 per year from 2010 to 2016.
Within these broad groups, HIV incidence also rose among some age groups and fell among others. Decreases were seen for younger African American gay and bisexual men (those aged 13-24 years), but rates increased by about two-thirds for men in this group aged 25-34 years. A similar increase was seen for Latino men in the 25-34 years age group, a change which drove the overall 30% increase in new infections for Latino gay and bisexual men.
White gay and bisexual men saw across-the-board decreases in new infections, though the overall decrease was less than 20%.
For heterosexual individuals as a group, new infections dropped by about 17%, from 10,900 to 9,100 annually. This change was driven mostly by decreases in women identifying as heterosexual.
“After a decades-long struggle, the path to eliminate America’s HIV epidemic is clear,” said Eugene McCray, MD, director of CDC’s Division of HIV/AIDS Prevention, in the press release. “Expanding efforts across the country will close gaps, overcome threats, and turn around troublesome trends.”
The press release cited local work in Washington and New York as evidence that targeted resources can make a difference in reducing new HIV cases. In these two areas, new infections dropped by 23% and 40% respectively from 2010 to 2016.
SOURCE: Centers for Disease Control. CDC Report: www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
The annual number of new HIV infections has remained stable in recent years, and the Centers for Disease Control and Prevention says that’s not good – but solutions are at hand.

Though the estimated number of new HIV infections declined from just under 42,000 per year in 2010 to about 39,000 annually in 2013, that figure was essentially unchanged by 2016, with 38,700 new HIV infections seen that year.
“CDC estimates that the decline in HIV infections has plateaued because effective HIV prevention and treatment are not adequately reaching those who could most benefit from them. These gaps remain particularly troublesome in rural areas and in the South and among disproportionately affected populations like African Americans and Latinos,” said the CDC in a press release accompanying the report.
The report comes soon after President Trump’s State of the Union address, which announced a new multiagency initiative to eliminate the HIV epidemic in the United States, with the goal of reducing new HIV infections by 90% over the next 10 years. The multipronged initiative will implement geographically targeted HIV elimination teams in areas with high HIV prevalence, pulling together federal agencies, local and state governments, and community-level resources.
The initiative, called “Ending the Epidemic: A Plan for America” will combine an intensified approach to early diagnosis and treatment with efforts to boost uptake of pre-exposure prophylaxis for individuals at high risk for HIV infection.
The new CDC report used CD4 counts reported to the National HIV Surveillance System at the time of diagnosis to identify new (incident) cases and to track prevalence. Much of the report is devoted to finely detailed reporting of HIV incidence across sex, age, race/ethnicity, and transmission mode.
Though some groups, such as people who inject drugs, have seen a decrease of about 30% in the annual rate of new HIV cases, new cases have jumped for other groups. In particular, Latino gay and bisexual men saw new cases climb from 6,400 per year in 2010 to 8,300 in 2016. The incidence rate has stayed high and stable among African American gay and bisexual men, with 9,800 new cases reported in 2010; the same number was seen in 2016.
Among gay and bisexual men overall, the rate has also stayed stable, with about 26,000 new HIV infections reported at the beginning and end of the studied period. White heterosexual women saw about 1,000 new cases per year in 2010 and in 2016.
Some groups saw declines in new cases: African American and Latina heterosexual women each saw a falling incidence of new HIV cases. For the former group, new cases fell from 4,700 to 4,000, while the latter group of women saw new cases drop from 1,200 to 980 per year from 2010 to 2016.
Within these broad groups, HIV incidence also rose among some age groups and fell among others. Decreases were seen for younger African American gay and bisexual men (those aged 13-24 years), but rates increased by about two-thirds for men in this group aged 25-34 years. A similar increase was seen for Latino men in the 25-34 years age group, a change which drove the overall 30% increase in new infections for Latino gay and bisexual men.
White gay and bisexual men saw across-the-board decreases in new infections, though the overall decrease was less than 20%.
For heterosexual individuals as a group, new infections dropped by about 17%, from 10,900 to 9,100 annually. This change was driven mostly by decreases in women identifying as heterosexual.
“After a decades-long struggle, the path to eliminate America’s HIV epidemic is clear,” said Eugene McCray, MD, director of CDC’s Division of HIV/AIDS Prevention, in the press release. “Expanding efforts across the country will close gaps, overcome threats, and turn around troublesome trends.”
The press release cited local work in Washington and New York as evidence that targeted resources can make a difference in reducing new HIV cases. In these two areas, new infections dropped by 23% and 40% respectively from 2010 to 2016.
SOURCE: Centers for Disease Control. CDC Report: www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
Caring for aging HIV-infected patients requires close attention to unrelated diseases
A substantial proportion of non–AIDS-defining cancers, and other noninfectious comorbid diseases, could be prevented with interventions on traditional risk factors in HIV-infected patients, according to the results of large database analysis published online in The Lancet HIV.
The researchers analyzed traditional and HIV-related risk factors for four validated noncommunicable disease outcomes (non–AIDS-defining cancers, myocardial infarction, end-stage liver disease, and end-stage renal disease) among participants of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), according to Keri N. Althoff, PhD, of Johns Hopkins University, Baltimore, and her colleagues on behalf of the NA-ACCORD.
The study comprised individuals with the assessed disease conditions from among more than 180,000 adults (aged 18 years or older) with HIV from more than 200 sites who had at least two care visits within 12 months. The researchers used a population attributable fraction (PAF) approach to quantify the proportion of noncommunicable diseases that could be eliminated if particular risk factors were not present. According to the researchers, PAF can be used to inform prioritization of interventions.
Dr. Althoff and her colleagues found that, for non–AIDS-defining cancer, the significant preventable or modifiable risk factors were smoking, low CD4 cell count, detectable HIV RNA, a history of clinical AIDS diagnosis, and hepatitis B infection.
For myocardial infarction, the significant factors were smoking, elevated total cholesterol, hypertension, stage 4 chronic kidney disease, a low CD4 cell count, detectable HIV RNA, and hepatitis C infection.
For end-stage liver disease, the significant factors were low CD4 cell count, detectable HIV RNA, a history of a clinical AIDS diagnosis, and hepatitis B or C infection.
For end-stage renal disease, the significantly associated risk factors were elevated total cholesterol, hypertension, diabetes, low CD4 cell count, detectable HIV RNA, and a history of clinical AIDS diagnosis.
The most substantial PAF for each of the respective diseases was as follows: smoking for non–AIDS-related cancers (24%; 95% confidence interval, 13%-35%), elevated total cholesterol for myocardial infarction (44%; 95% CI, 30%-58%), and hepatitis C infection for end-stage liver disease (30%; 95% CI, 21%-39%). In addition, hypertension had the highest PAF for end-stage renal disease (39%; 95% CI, 26%-51%).
“Modifications to individual-level interventions and models of HIV care, and the implementation of structural and policy-level interventions that focus on prevention and modification of traditional risk factors are necessary to avoid noncommunicable diseases and preserve health among successfully antiretroviral-treated adults aging with HIV,” the researchers concluded.
The study was funded by the National Institutes of Health and the NA-ACCORD. Dr. Althoff reported having no relevant disclosures.
SOURCE: Althoff KN et al. The Lancet HIV. 2019 Jan 22. doi: 10.1016/S2352-3018(18)30295-9.
A substantial proportion of non–AIDS-defining cancers, and other noninfectious comorbid diseases, could be prevented with interventions on traditional risk factors in HIV-infected patients, according to the results of large database analysis published online in The Lancet HIV.
The researchers analyzed traditional and HIV-related risk factors for four validated noncommunicable disease outcomes (non–AIDS-defining cancers, myocardial infarction, end-stage liver disease, and end-stage renal disease) among participants of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), according to Keri N. Althoff, PhD, of Johns Hopkins University, Baltimore, and her colleagues on behalf of the NA-ACCORD.
The study comprised individuals with the assessed disease conditions from among more than 180,000 adults (aged 18 years or older) with HIV from more than 200 sites who had at least two care visits within 12 months. The researchers used a population attributable fraction (PAF) approach to quantify the proportion of noncommunicable diseases that could be eliminated if particular risk factors were not present. According to the researchers, PAF can be used to inform prioritization of interventions.
Dr. Althoff and her colleagues found that, for non–AIDS-defining cancer, the significant preventable or modifiable risk factors were smoking, low CD4 cell count, detectable HIV RNA, a history of clinical AIDS diagnosis, and hepatitis B infection.
For myocardial infarction, the significant factors were smoking, elevated total cholesterol, hypertension, stage 4 chronic kidney disease, a low CD4 cell count, detectable HIV RNA, and hepatitis C infection.
For end-stage liver disease, the significant factors were low CD4 cell count, detectable HIV RNA, a history of a clinical AIDS diagnosis, and hepatitis B or C infection.
For end-stage renal disease, the significantly associated risk factors were elevated total cholesterol, hypertension, diabetes, low CD4 cell count, detectable HIV RNA, and a history of clinical AIDS diagnosis.
The most substantial PAF for each of the respective diseases was as follows: smoking for non–AIDS-related cancers (24%; 95% confidence interval, 13%-35%), elevated total cholesterol for myocardial infarction (44%; 95% CI, 30%-58%), and hepatitis C infection for end-stage liver disease (30%; 95% CI, 21%-39%). In addition, hypertension had the highest PAF for end-stage renal disease (39%; 95% CI, 26%-51%).
“Modifications to individual-level interventions and models of HIV care, and the implementation of structural and policy-level interventions that focus on prevention and modification of traditional risk factors are necessary to avoid noncommunicable diseases and preserve health among successfully antiretroviral-treated adults aging with HIV,” the researchers concluded.
The study was funded by the National Institutes of Health and the NA-ACCORD. Dr. Althoff reported having no relevant disclosures.
SOURCE: Althoff KN et al. The Lancet HIV. 2019 Jan 22. doi: 10.1016/S2352-3018(18)30295-9.
A substantial proportion of non–AIDS-defining cancers, and other noninfectious comorbid diseases, could be prevented with interventions on traditional risk factors in HIV-infected patients, according to the results of large database analysis published online in The Lancet HIV.
The researchers analyzed traditional and HIV-related risk factors for four validated noncommunicable disease outcomes (non–AIDS-defining cancers, myocardial infarction, end-stage liver disease, and end-stage renal disease) among participants of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), according to Keri N. Althoff, PhD, of Johns Hopkins University, Baltimore, and her colleagues on behalf of the NA-ACCORD.
The study comprised individuals with the assessed disease conditions from among more than 180,000 adults (aged 18 years or older) with HIV from more than 200 sites who had at least two care visits within 12 months. The researchers used a population attributable fraction (PAF) approach to quantify the proportion of noncommunicable diseases that could be eliminated if particular risk factors were not present. According to the researchers, PAF can be used to inform prioritization of interventions.
Dr. Althoff and her colleagues found that, for non–AIDS-defining cancer, the significant preventable or modifiable risk factors were smoking, low CD4 cell count, detectable HIV RNA, a history of clinical AIDS diagnosis, and hepatitis B infection.
For myocardial infarction, the significant factors were smoking, elevated total cholesterol, hypertension, stage 4 chronic kidney disease, a low CD4 cell count, detectable HIV RNA, and hepatitis C infection.
For end-stage liver disease, the significant factors were low CD4 cell count, detectable HIV RNA, a history of a clinical AIDS diagnosis, and hepatitis B or C infection.
For end-stage renal disease, the significantly associated risk factors were elevated total cholesterol, hypertension, diabetes, low CD4 cell count, detectable HIV RNA, and a history of clinical AIDS diagnosis.
The most substantial PAF for each of the respective diseases was as follows: smoking for non–AIDS-related cancers (24%; 95% confidence interval, 13%-35%), elevated total cholesterol for myocardial infarction (44%; 95% CI, 30%-58%), and hepatitis C infection for end-stage liver disease (30%; 95% CI, 21%-39%). In addition, hypertension had the highest PAF for end-stage renal disease (39%; 95% CI, 26%-51%).
“Modifications to individual-level interventions and models of HIV care, and the implementation of structural and policy-level interventions that focus on prevention and modification of traditional risk factors are necessary to avoid noncommunicable diseases and preserve health among successfully antiretroviral-treated adults aging with HIV,” the researchers concluded.
The study was funded by the National Institutes of Health and the NA-ACCORD. Dr. Althoff reported having no relevant disclosures.
SOURCE: Althoff KN et al. The Lancet HIV. 2019 Jan 22. doi: 10.1016/S2352-3018(18)30295-9.
FROM THE LANCET HIV
Heart failure outcomes are worse in select HIV-infected individuals
People living with HIV (PLHIV) who have a low CD4 count or detectable viral load (VL) have an increased 30-day heart failure (HF) readmission rate as well as increased cardiovascular and all-cause mortality, compared with uninfected controls, according to Raza M. Alvi, MD, of Massachusetts General Hospital and Harvard Medical School, Boston, and his colleagues.
Overall, the 30-day HF hospital readmission rate was higher among PLHIV versus non-HIV–infected individuals (49% vs. 32%, P less than .001), according to the results of their cohort study of 2,308 individuals admitted to the hospital with decompensated HF.
PLHIV and the non-HIV control groups were both followed over 2 years with a median follow-up period of 19 months, the authors wrote in the American Heart Journal. Demographic make-up of the two groups was similar; in particular, there was no difference in blood pressure and heart rate between the PLHIV and non-HIV controls, suggesting that adherence with HF medications may be similar between groups. The cohorts differed primarily in that pulmonary artery systolic pressure was significantly higher (45 vs. 40 mm Hg; P less than .001) in the HIV group, as was cocaine use (36% vs. 19%; P less than .001) and hepatitis C virus (HCV) infection (13% vs. 7%; P less than .001).
The differing results between the two cohorts were primarily caused by the fact that, for PLHIV with HF, CD4 count and VL were risk factors for adverse outcomes among patients with all types of HF. (30-day HF readmission, cardiovascular [CV] mortality, and all-cause mortality), compared with those with CD4 count greater than or equal to 200 cells/mm3. In addition, a low CD4 count/high VL independently related to an increased 30-day HF readmission rate even after the researchers controlled for major traditional and nontraditional HF risk factors.
“Rates of 30-day HF readmission, CV mortality, and all-cause mortality are worse among individuals with a low CD4 count or nonsuppressed viral load,” the researchers concluded.
The National Institutes of Health sponsored the study. The authors reported that they had no conflicts.
SOURCE: Alvi RM et al. Am Heart J. 2019 Apr;210:39-48.
People living with HIV (PLHIV) who have a low CD4 count or detectable viral load (VL) have an increased 30-day heart failure (HF) readmission rate as well as increased cardiovascular and all-cause mortality, compared with uninfected controls, according to Raza M. Alvi, MD, of Massachusetts General Hospital and Harvard Medical School, Boston, and his colleagues.
Overall, the 30-day HF hospital readmission rate was higher among PLHIV versus non-HIV–infected individuals (49% vs. 32%, P less than .001), according to the results of their cohort study of 2,308 individuals admitted to the hospital with decompensated HF.
PLHIV and the non-HIV control groups were both followed over 2 years with a median follow-up period of 19 months, the authors wrote in the American Heart Journal. Demographic make-up of the two groups was similar; in particular, there was no difference in blood pressure and heart rate between the PLHIV and non-HIV controls, suggesting that adherence with HF medications may be similar between groups. The cohorts differed primarily in that pulmonary artery systolic pressure was significantly higher (45 vs. 40 mm Hg; P less than .001) in the HIV group, as was cocaine use (36% vs. 19%; P less than .001) and hepatitis C virus (HCV) infection (13% vs. 7%; P less than .001).
The differing results between the two cohorts were primarily caused by the fact that, for PLHIV with HF, CD4 count and VL were risk factors for adverse outcomes among patients with all types of HF. (30-day HF readmission, cardiovascular [CV] mortality, and all-cause mortality), compared with those with CD4 count greater than or equal to 200 cells/mm3. In addition, a low CD4 count/high VL independently related to an increased 30-day HF readmission rate even after the researchers controlled for major traditional and nontraditional HF risk factors.
“Rates of 30-day HF readmission, CV mortality, and all-cause mortality are worse among individuals with a low CD4 count or nonsuppressed viral load,” the researchers concluded.
The National Institutes of Health sponsored the study. The authors reported that they had no conflicts.
SOURCE: Alvi RM et al. Am Heart J. 2019 Apr;210:39-48.
People living with HIV (PLHIV) who have a low CD4 count or detectable viral load (VL) have an increased 30-day heart failure (HF) readmission rate as well as increased cardiovascular and all-cause mortality, compared with uninfected controls, according to Raza M. Alvi, MD, of Massachusetts General Hospital and Harvard Medical School, Boston, and his colleagues.
Overall, the 30-day HF hospital readmission rate was higher among PLHIV versus non-HIV–infected individuals (49% vs. 32%, P less than .001), according to the results of their cohort study of 2,308 individuals admitted to the hospital with decompensated HF.
PLHIV and the non-HIV control groups were both followed over 2 years with a median follow-up period of 19 months, the authors wrote in the American Heart Journal. Demographic make-up of the two groups was similar; in particular, there was no difference in blood pressure and heart rate between the PLHIV and non-HIV controls, suggesting that adherence with HF medications may be similar between groups. The cohorts differed primarily in that pulmonary artery systolic pressure was significantly higher (45 vs. 40 mm Hg; P less than .001) in the HIV group, as was cocaine use (36% vs. 19%; P less than .001) and hepatitis C virus (HCV) infection (13% vs. 7%; P less than .001).
The differing results between the two cohorts were primarily caused by the fact that, for PLHIV with HF, CD4 count and VL were risk factors for adverse outcomes among patients with all types of HF. (30-day HF readmission, cardiovascular [CV] mortality, and all-cause mortality), compared with those with CD4 count greater than or equal to 200 cells/mm3. In addition, a low CD4 count/high VL independently related to an increased 30-day HF readmission rate even after the researchers controlled for major traditional and nontraditional HF risk factors.
“Rates of 30-day HF readmission, CV mortality, and all-cause mortality are worse among individuals with a low CD4 count or nonsuppressed viral load,” the researchers concluded.
The National Institutes of Health sponsored the study. The authors reported that they had no conflicts.
SOURCE: Alvi RM et al. Am Heart J. 2019 Apr;210:39-48.
FROM THE AMERICAN HEART JOURNAL