User login
<p>For ClinicalEdge sponsored section only</p>
Communicating Statin Safety to Patients With Hypercholesterolemia
Dr James de Lemos, professor of internal medicine at the University of Texas Southwestern Medical Center, presents a framework to address the most common questions that patients have about the reported muscular, cognitive, and hepatotoxic side effects of statin therapy.
To start, he presents clinical data to address areas of patient concern. Next, he discusses ways to develop — from the first visit — a partnership with patients and encourage their informed decision-making by guiding them to reliable medical sources.
Finally, Dr de Lemos presents strategies that clinicians can use to improve adherence to statin therapy to reach LDL-C treatment goals
--
James de Lemos, MD, PhD
Professor, Internal Medicine, Division of Cardiology, University of Texas Southwestern Medical Center, Dallas, Texas
James de Lemos, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Amgen; Regeneron; AstraZeneca
Dr James de Lemos, professor of internal medicine at the University of Texas Southwestern Medical Center, presents a framework to address the most common questions that patients have about the reported muscular, cognitive, and hepatotoxic side effects of statin therapy.
To start, he presents clinical data to address areas of patient concern. Next, he discusses ways to develop — from the first visit — a partnership with patients and encourage their informed decision-making by guiding them to reliable medical sources.
Finally, Dr de Lemos presents strategies that clinicians can use to improve adherence to statin therapy to reach LDL-C treatment goals
--
James de Lemos, MD, PhD
Professor, Internal Medicine, Division of Cardiology, University of Texas Southwestern Medical Center, Dallas, Texas
James de Lemos, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Amgen; Regeneron; AstraZeneca
Dr James de Lemos, professor of internal medicine at the University of Texas Southwestern Medical Center, presents a framework to address the most common questions that patients have about the reported muscular, cognitive, and hepatotoxic side effects of statin therapy.
To start, he presents clinical data to address areas of patient concern. Next, he discusses ways to develop — from the first visit — a partnership with patients and encourage their informed decision-making by guiding them to reliable medical sources.
Finally, Dr de Lemos presents strategies that clinicians can use to improve adherence to statin therapy to reach LDL-C treatment goals
--
James de Lemos, MD, PhD
Professor, Internal Medicine, Division of Cardiology, University of Texas Southwestern Medical Center, Dallas, Texas
James de Lemos, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Amgen; Regeneron; AstraZeneca

Prominent clinical guidelines fall short of conflict of interest standards
Two recent clinical practice guidelines – one for cholesterol management and another for treatment of chronic hepatitis C – did not meet the Institute of Medicine’s standards for limiting commercial conflicts of interest, according to results of a new analysis.
In research published online Jan. 17, Akilah A. Jefferson, MD, and Steven D. Pearson, MD, both of the National Institutes of Health in Bethesda, Md., re-examined conflict of interest disclosures for the 2013 American College of Cardiology and American Heart Association cholesterol management guideline, as well as for the American Association for the Study of Liver Diseases and Infectious Diseases Society of America’s joint 2014 guideline related to novel drug treatments for chronic hepatitis C virus (HCV) infection.
The IOM standards for conflicts of interest in guidelines, introduced in 2011, require that less than half the members of any guideline writing committee have a commercial conflict, which can include consultancies, board memberships, and stock in manufacturers of devices or treatments. Guideline writing committee chairs and cochairs should have no commercial conflicts of interest, according to the IOM.
For the cholesterol guidelines, the investigators found that while the committee members fell below the threshold with only 44% reporting commercial conflicts, one writing chair had multiple conflicts until about 1 year before guideline development began, an analysis of concurrent publications suggested. For the HCV guidelines, meanwhile, 72% of the committee members reported commercial conflicts, along with four out of six committee cochairs. An analysis of concurrent publications revealed incomplete disclosure of conflicts among authors of both guidelines (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.8439).
“Management of levels of commercial [conflict of interest] among guideline committees remains an important problem 5 years after the IOM standards were published,” the investigators wrote. They recommended “broader and more explicit adoption” of the IOM’s framework for conflict of interest.
The study notes that the HCV guideline met all nine of the additional IOM guideline development and evidence standards, while the cholesterol guideline met five of those standards.
The study was funded by an NIH grant. Dr. Pearson reported receiving research funding from foundations and membership dues paid by insurance and pharmaceutical companies. No other disclosures were reported.
Two recent clinical practice guidelines – one for cholesterol management and another for treatment of chronic hepatitis C – did not meet the Institute of Medicine’s standards for limiting commercial conflicts of interest, according to results of a new analysis.
In research published online Jan. 17, Akilah A. Jefferson, MD, and Steven D. Pearson, MD, both of the National Institutes of Health in Bethesda, Md., re-examined conflict of interest disclosures for the 2013 American College of Cardiology and American Heart Association cholesterol management guideline, as well as for the American Association for the Study of Liver Diseases and Infectious Diseases Society of America’s joint 2014 guideline related to novel drug treatments for chronic hepatitis C virus (HCV) infection.
The IOM standards for conflicts of interest in guidelines, introduced in 2011, require that less than half the members of any guideline writing committee have a commercial conflict, which can include consultancies, board memberships, and stock in manufacturers of devices or treatments. Guideline writing committee chairs and cochairs should have no commercial conflicts of interest, according to the IOM.
For the cholesterol guidelines, the investigators found that while the committee members fell below the threshold with only 44% reporting commercial conflicts, one writing chair had multiple conflicts until about 1 year before guideline development began, an analysis of concurrent publications suggested. For the HCV guidelines, meanwhile, 72% of the committee members reported commercial conflicts, along with four out of six committee cochairs. An analysis of concurrent publications revealed incomplete disclosure of conflicts among authors of both guidelines (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.8439).
“Management of levels of commercial [conflict of interest] among guideline committees remains an important problem 5 years after the IOM standards were published,” the investigators wrote. They recommended “broader and more explicit adoption” of the IOM’s framework for conflict of interest.
The study notes that the HCV guideline met all nine of the additional IOM guideline development and evidence standards, while the cholesterol guideline met five of those standards.
The study was funded by an NIH grant. Dr. Pearson reported receiving research funding from foundations and membership dues paid by insurance and pharmaceutical companies. No other disclosures were reported.
Two recent clinical practice guidelines – one for cholesterol management and another for treatment of chronic hepatitis C – did not meet the Institute of Medicine’s standards for limiting commercial conflicts of interest, according to results of a new analysis.
In research published online Jan. 17, Akilah A. Jefferson, MD, and Steven D. Pearson, MD, both of the National Institutes of Health in Bethesda, Md., re-examined conflict of interest disclosures for the 2013 American College of Cardiology and American Heart Association cholesterol management guideline, as well as for the American Association for the Study of Liver Diseases and Infectious Diseases Society of America’s joint 2014 guideline related to novel drug treatments for chronic hepatitis C virus (HCV) infection.
The IOM standards for conflicts of interest in guidelines, introduced in 2011, require that less than half the members of any guideline writing committee have a commercial conflict, which can include consultancies, board memberships, and stock in manufacturers of devices or treatments. Guideline writing committee chairs and cochairs should have no commercial conflicts of interest, according to the IOM.
For the cholesterol guidelines, the investigators found that while the committee members fell below the threshold with only 44% reporting commercial conflicts, one writing chair had multiple conflicts until about 1 year before guideline development began, an analysis of concurrent publications suggested. For the HCV guidelines, meanwhile, 72% of the committee members reported commercial conflicts, along with four out of six committee cochairs. An analysis of concurrent publications revealed incomplete disclosure of conflicts among authors of both guidelines (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.8439).
“Management of levels of commercial [conflict of interest] among guideline committees remains an important problem 5 years after the IOM standards were published,” the investigators wrote. They recommended “broader and more explicit adoption” of the IOM’s framework for conflict of interest.
The study notes that the HCV guideline met all nine of the additional IOM guideline development and evidence standards, while the cholesterol guideline met five of those standards.
The study was funded by an NIH grant. Dr. Pearson reported receiving research funding from foundations and membership dues paid by insurance and pharmaceutical companies. No other disclosures were reported.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: In total, 72% of members of the hepatitis C virus guideline committee disclosed conflicts of interest, while 44% of members of the cholesterol guideline committee reported commercial conflicts.
Data source: A retrospective review of the ACA/AHA’s cholesterol guideline and the AASLD/IDSA’s hepatitis C virus guideline.
Disclosures: The research was funded by a grant from the National Institutes of Health. Dr. Pearson reported research grants from foundations and membership dues paid by insurance and pharmaceutical companies. No other disclosures were reported.
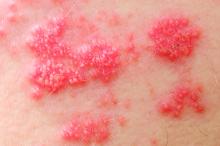
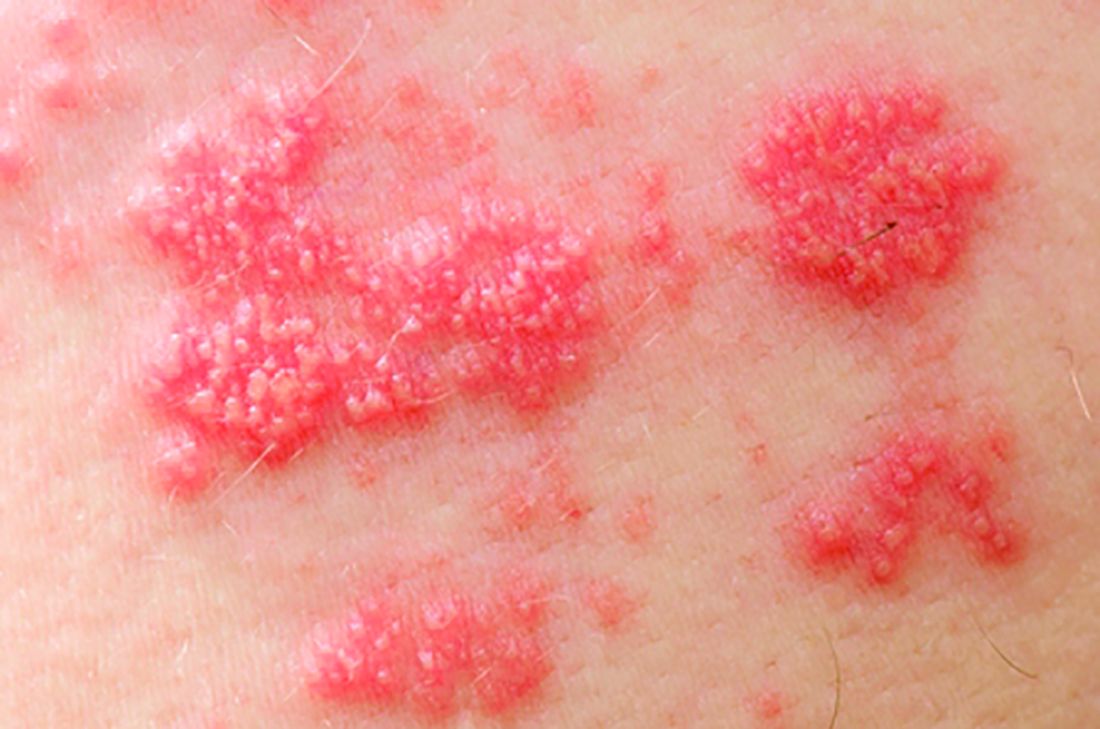
Statin use increases risk of herpes zoster
Exposure to statins can significantly increase the odds of developing herpes zoster (HZ), according to a study published in the British Journal of Dermatology.
“The mechanisms by which statins may increase the risk of HZ are not established. As statins are commonly prescribed worldwide, any adverse effects may have substantial public health implications,” wrote Anthony Matthews of the London School of Hygiene and Tropical Medicine, and his colleagues. “An increased risk of HZ would not only have an impact on the quality of life of affected patients, but could also add to the burden on health services, given the high cost of treatment for PHN [postherpetic neuralgia].”
Each subject was matched with no more than four control patients – all of whom had no prior history of HZ or PHN – selected from the same databases. Matching was based on the following criteria: subject’s general practitioner (GP) practice, age (within 1 year of the HZ patient), sex, and lack of prior statin exposure. Ultimately 144,959 subjects were matched with 549,336 controls (Br J Dermatol. 2016 Dec;175[6]:1183-94).
The primary outcome was defined as incident diagnoses of HZ, which was measured via odds ratios (ORs) calculated from conditional logistic regression models and adjusted for any potential confounding factors. In addition, ORs were calculated to “explore the association between the risk of HZ and the dosage of the latest prescription (low, medium, high), stratified by time since last exposure,” the investigators noted.
Results showed an adjusted OR of 1.13 for HZ after any kind of statin treatment, indicating a significant increase in risk. There was also a higher adjusted OR for those who had stopped using statins 12-36 months prior to the study than those whose last exposure was greater than 36 months prior: 1.11 and 1.06, respectively (P less than .001). Those whose current statin usage was “high” had an adjusted OR of 1.27 versus 1.16 for those whose usage was “medium,” and 1.14 for those whose usage was “low” (P less than .001).
“It is clear that the preventive benefits of statin therapy are likely to outweigh the limited increase in HZ risk in many cases [but] this evidence should be taken into account by GPs when prescribing statins to those at high risk of HZ,” Mr. Matthews and his associates concluded. “We would also suggest that there may be an extra motivation to maximize HZ vaccine uptake among eligible patients who are also receiving a statin.”
Three coauthors disclosed individual funding sources: the Wellcome Trust/Royal Society Sir Henry Dale fellowship, the Senior Wellcome Fellowship in Clinical Science, and the National Institute for Health Research Clinician Scientist fellowship. The authors did not report any relevant financial disclosures.
“The present study by Matthews et al. observed a dose-response relationship between statin exposure and zoster risk. [It] is the first to explore the association between time since last statin use and the occurrence of zoster. As the time since last statin exposure increased, the risk of zoster decreased, suggesting that the risk for zoster is reduced after termination of statin treatment.
“Zoster is a significant contributor to morbidity, disability, and chronic pain, and constitutes a major public health concern. Zoster vaccination is recommended in most persons who are 50 years of age or older. Vaccination reduces the risk of zoster and postherpetic neuralgia. However, the price of zoster vaccine limits its use worldwide.
“The ‘take home’ message from the article is that in the setting of older age or immune suppression, dermatologists should consider recommending to their patients the use of a zoster vaccine, particularly in patients who receive statin therapy.”
Guy Shalom, MD, is a member of the department of dermatology and venereology, Soroka Medical Center in Beer-Sheva, Israel, and reported no relevant financial conflicts. Arnon D. Cohen, MD, is a member of the Siaal Research Center for Family Medicine and Primary Care at Ben-Gurion University of the Negev, Beer-Sheva, and reported financial ties to AbbVie, Dexcel Pharma, Janssen, Novartis, Perrigo, Pfizer, and Rafa as a consultant, adviser, or speaker. These comments were excerpted from their commentary accompanying Mr. Matthews’ article ( Br J Dermatol. 2017;175:1137-8 ).
“The present study by Matthews et al. observed a dose-response relationship between statin exposure and zoster risk. [It] is the first to explore the association between time since last statin use and the occurrence of zoster. As the time since last statin exposure increased, the risk of zoster decreased, suggesting that the risk for zoster is reduced after termination of statin treatment.
“Zoster is a significant contributor to morbidity, disability, and chronic pain, and constitutes a major public health concern. Zoster vaccination is recommended in most persons who are 50 years of age or older. Vaccination reduces the risk of zoster and postherpetic neuralgia. However, the price of zoster vaccine limits its use worldwide.
“The ‘take home’ message from the article is that in the setting of older age or immune suppression, dermatologists should consider recommending to their patients the use of a zoster vaccine, particularly in patients who receive statin therapy.”
Guy Shalom, MD, is a member of the department of dermatology and venereology, Soroka Medical Center in Beer-Sheva, Israel, and reported no relevant financial conflicts. Arnon D. Cohen, MD, is a member of the Siaal Research Center for Family Medicine and Primary Care at Ben-Gurion University of the Negev, Beer-Sheva, and reported financial ties to AbbVie, Dexcel Pharma, Janssen, Novartis, Perrigo, Pfizer, and Rafa as a consultant, adviser, or speaker. These comments were excerpted from their commentary accompanying Mr. Matthews’ article ( Br J Dermatol. 2017;175:1137-8 ).
“The present study by Matthews et al. observed a dose-response relationship between statin exposure and zoster risk. [It] is the first to explore the association between time since last statin use and the occurrence of zoster. As the time since last statin exposure increased, the risk of zoster decreased, suggesting that the risk for zoster is reduced after termination of statin treatment.
“Zoster is a significant contributor to morbidity, disability, and chronic pain, and constitutes a major public health concern. Zoster vaccination is recommended in most persons who are 50 years of age or older. Vaccination reduces the risk of zoster and postherpetic neuralgia. However, the price of zoster vaccine limits its use worldwide.
“The ‘take home’ message from the article is that in the setting of older age or immune suppression, dermatologists should consider recommending to their patients the use of a zoster vaccine, particularly in patients who receive statin therapy.”
Guy Shalom, MD, is a member of the department of dermatology and venereology, Soroka Medical Center in Beer-Sheva, Israel, and reported no relevant financial conflicts. Arnon D. Cohen, MD, is a member of the Siaal Research Center for Family Medicine and Primary Care at Ben-Gurion University of the Negev, Beer-Sheva, and reported financial ties to AbbVie, Dexcel Pharma, Janssen, Novartis, Perrigo, Pfizer, and Rafa as a consultant, adviser, or speaker. These comments were excerpted from their commentary accompanying Mr. Matthews’ article ( Br J Dermatol. 2017;175:1137-8 ).
Exposure to statins can significantly increase the odds of developing herpes zoster (HZ), according to a study published in the British Journal of Dermatology.
“The mechanisms by which statins may increase the risk of HZ are not established. As statins are commonly prescribed worldwide, any adverse effects may have substantial public health implications,” wrote Anthony Matthews of the London School of Hygiene and Tropical Medicine, and his colleagues. “An increased risk of HZ would not only have an impact on the quality of life of affected patients, but could also add to the burden on health services, given the high cost of treatment for PHN [postherpetic neuralgia].”
Each subject was matched with no more than four control patients – all of whom had no prior history of HZ or PHN – selected from the same databases. Matching was based on the following criteria: subject’s general practitioner (GP) practice, age (within 1 year of the HZ patient), sex, and lack of prior statin exposure. Ultimately 144,959 subjects were matched with 549,336 controls (Br J Dermatol. 2016 Dec;175[6]:1183-94).
The primary outcome was defined as incident diagnoses of HZ, which was measured via odds ratios (ORs) calculated from conditional logistic regression models and adjusted for any potential confounding factors. In addition, ORs were calculated to “explore the association between the risk of HZ and the dosage of the latest prescription (low, medium, high), stratified by time since last exposure,” the investigators noted.
Results showed an adjusted OR of 1.13 for HZ after any kind of statin treatment, indicating a significant increase in risk. There was also a higher adjusted OR for those who had stopped using statins 12-36 months prior to the study than those whose last exposure was greater than 36 months prior: 1.11 and 1.06, respectively (P less than .001). Those whose current statin usage was “high” had an adjusted OR of 1.27 versus 1.16 for those whose usage was “medium,” and 1.14 for those whose usage was “low” (P less than .001).
“It is clear that the preventive benefits of statin therapy are likely to outweigh the limited increase in HZ risk in many cases [but] this evidence should be taken into account by GPs when prescribing statins to those at high risk of HZ,” Mr. Matthews and his associates concluded. “We would also suggest that there may be an extra motivation to maximize HZ vaccine uptake among eligible patients who are also receiving a statin.”
Three coauthors disclosed individual funding sources: the Wellcome Trust/Royal Society Sir Henry Dale fellowship, the Senior Wellcome Fellowship in Clinical Science, and the National Institute for Health Research Clinician Scientist fellowship. The authors did not report any relevant financial disclosures.
Exposure to statins can significantly increase the odds of developing herpes zoster (HZ), according to a study published in the British Journal of Dermatology.
“The mechanisms by which statins may increase the risk of HZ are not established. As statins are commonly prescribed worldwide, any adverse effects may have substantial public health implications,” wrote Anthony Matthews of the London School of Hygiene and Tropical Medicine, and his colleagues. “An increased risk of HZ would not only have an impact on the quality of life of affected patients, but could also add to the burden on health services, given the high cost of treatment for PHN [postherpetic neuralgia].”
Each subject was matched with no more than four control patients – all of whom had no prior history of HZ or PHN – selected from the same databases. Matching was based on the following criteria: subject’s general practitioner (GP) practice, age (within 1 year of the HZ patient), sex, and lack of prior statin exposure. Ultimately 144,959 subjects were matched with 549,336 controls (Br J Dermatol. 2016 Dec;175[6]:1183-94).
The primary outcome was defined as incident diagnoses of HZ, which was measured via odds ratios (ORs) calculated from conditional logistic regression models and adjusted for any potential confounding factors. In addition, ORs were calculated to “explore the association between the risk of HZ and the dosage of the latest prescription (low, medium, high), stratified by time since last exposure,” the investigators noted.
Results showed an adjusted OR of 1.13 for HZ after any kind of statin treatment, indicating a significant increase in risk. There was also a higher adjusted OR for those who had stopped using statins 12-36 months prior to the study than those whose last exposure was greater than 36 months prior: 1.11 and 1.06, respectively (P less than .001). Those whose current statin usage was “high” had an adjusted OR of 1.27 versus 1.16 for those whose usage was “medium,” and 1.14 for those whose usage was “low” (P less than .001).
“It is clear that the preventive benefits of statin therapy are likely to outweigh the limited increase in HZ risk in many cases [but] this evidence should be taken into account by GPs when prescribing statins to those at high risk of HZ,” Mr. Matthews and his associates concluded. “We would also suggest that there may be an extra motivation to maximize HZ vaccine uptake among eligible patients who are also receiving a statin.”
Three coauthors disclosed individual funding sources: the Wellcome Trust/Royal Society Sir Henry Dale fellowship, the Senior Wellcome Fellowship in Clinical Science, and the National Institute for Health Research Clinician Scientist fellowship. The authors did not report any relevant financial disclosures.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point:
Major finding: There is an odds ratio of 1.13 of contracting HZ if given a statin previously, with a significant increase (P less than .001) in risk in patients who used statins more recently.
Data source: Matched case-control study of 144,959 adults with HZ from 2000 through 2011.
Disclosures: Dr. Matthews and colleagues reported no relevant financial disclosures.
Nutrition expert to heart patients: ‘Eat some cheese’
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
FROM THE AHA SCIENTIFIC SESSIONS
VIDEO: New antisense inhibitor nets impressive reductions in lipids
NEW ORLEANS – A new antisense inhibitor to angiopoietinlike protein 3 (ANGPTL3) reduces lipids in healthy adults with elevated triglyceride levels, according to results of a phase I/IIa ascending-dose trial reported at the American Heart Association scientific sessions.
ANGPTL3 regulates lipid and possibly general metabolism through actions in the liver, gut, muscle, and adipose tissue, explained presenting author Sotirios Tsimikas, MD, an investigator with Ionis Pharmaceuticals, Carlsbad, Calif., and a professor of medicine and director of vascular medicine at the University of California, San Diego. Individuals having loss-of-function mutations in the gene encoding this protein have very low plasma levels of cholesterol and triglycerides.
In the trial, sponsored by Ionis Pharmaceuticals, 32 healthy volunteers with elevated triglyceride levels were treated with various doses of the antisense inhibitor, called IONIS-ANGPTL3-LRx, or a placebo, given by weekly subcutaneous injections for 6 weeks.
Results showed that participants treated at the higher-dose levels had a reduction from baseline of 66% in triglycerides, 68% in apoliprotein C-III (ApoC-III), 35% in LDL cholesterol, 36% in total cholesterol, and 40% in non-HDL cholesterol, as well as 25% in HDL cholesterol, Dr. Tsimikas reported. They also had reductions in apolipoprotein B (ApoB).
“All these lipid parameters are really going in the right direction in terms of postulating potential clinical benefit,” he commented.
Safety and tolerability results showed that only a single participant experienced a local injection site adverse event related to the inhibitor (erythema and pruritus). None experienced flulike symptoms, platelet reductions, or serious adverse events, and none left the study because of adverse events.
“Among all known therapies that lower triglycerides, this [IONIS-ANGPTL3-LRx] is also associated with a reduction not only in LDL cholesterol levels, but also in ApoB as well, which portends well for future outcomes trials,” noted Dr. Tsimikas.
“We think this is going to be a promising candidate for patients who have uncontrolled LDL cholesterol, elevated triglycerides, and possibly patients who have hepatic steatosis and NASH [nonalcoholic steatohepatitis],” he concluded.
Dr. Tsimikas, who is an employee of and shareholder in the trial’s sponsor, Ionis Pharmaceuticals, discussed his findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The trial shows a very clear proof of concept in regards to the effects on lipids.
In contrast to agents that increase LDL receptor levels (statins, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors, bile acid–binding resins, and ezetimibe), IONIS-ANGPTL3-LRx produces greater reductions in non-HDL cholesterol than in LDL cholesterol, suggesting different mechanisms are at work.
But the key issue at this point is to ascertain the longer-term safety of IONIS-ANGPTL3-LRx in the liver and other organs.
One of the most impressive aspects of the combination of biotechnology and genetics is how rapidly you can come to a proof of concept in humans, and this is unprecedented, compared with what we have seen over the last 20 or 30 years. Unfortunately, the determination of safety is not quite as rapid, and it takes large numbers and long duration of follow-up. Obviously, we need that in terms of understanding benefits and risks.
Christie M. Ballantyne, MD, director of the Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart Center, Baylor College of Medicine in Houston, made these comments as the invited discussant. He has been a consultant to and received research support from trial sponsor Ionis Pharmaceuticals.
The trial shows a very clear proof of concept in regards to the effects on lipids.
In contrast to agents that increase LDL receptor levels (statins, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors, bile acid–binding resins, and ezetimibe), IONIS-ANGPTL3-LRx produces greater reductions in non-HDL cholesterol than in LDL cholesterol, suggesting different mechanisms are at work.
But the key issue at this point is to ascertain the longer-term safety of IONIS-ANGPTL3-LRx in the liver and other organs.
One of the most impressive aspects of the combination of biotechnology and genetics is how rapidly you can come to a proof of concept in humans, and this is unprecedented, compared with what we have seen over the last 20 or 30 years. Unfortunately, the determination of safety is not quite as rapid, and it takes large numbers and long duration of follow-up. Obviously, we need that in terms of understanding benefits and risks.
Christie M. Ballantyne, MD, director of the Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart Center, Baylor College of Medicine in Houston, made these comments as the invited discussant. He has been a consultant to and received research support from trial sponsor Ionis Pharmaceuticals.
The trial shows a very clear proof of concept in regards to the effects on lipids.
In contrast to agents that increase LDL receptor levels (statins, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors, bile acid–binding resins, and ezetimibe), IONIS-ANGPTL3-LRx produces greater reductions in non-HDL cholesterol than in LDL cholesterol, suggesting different mechanisms are at work.
But the key issue at this point is to ascertain the longer-term safety of IONIS-ANGPTL3-LRx in the liver and other organs.
One of the most impressive aspects of the combination of biotechnology and genetics is how rapidly you can come to a proof of concept in humans, and this is unprecedented, compared with what we have seen over the last 20 or 30 years. Unfortunately, the determination of safety is not quite as rapid, and it takes large numbers and long duration of follow-up. Obviously, we need that in terms of understanding benefits and risks.
Christie M. Ballantyne, MD, director of the Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart Center, Baylor College of Medicine in Houston, made these comments as the invited discussant. He has been a consultant to and received research support from trial sponsor Ionis Pharmaceuticals.
NEW ORLEANS – A new antisense inhibitor to angiopoietinlike protein 3 (ANGPTL3) reduces lipids in healthy adults with elevated triglyceride levels, according to results of a phase I/IIa ascending-dose trial reported at the American Heart Association scientific sessions.
ANGPTL3 regulates lipid and possibly general metabolism through actions in the liver, gut, muscle, and adipose tissue, explained presenting author Sotirios Tsimikas, MD, an investigator with Ionis Pharmaceuticals, Carlsbad, Calif., and a professor of medicine and director of vascular medicine at the University of California, San Diego. Individuals having loss-of-function mutations in the gene encoding this protein have very low plasma levels of cholesterol and triglycerides.
In the trial, sponsored by Ionis Pharmaceuticals, 32 healthy volunteers with elevated triglyceride levels were treated with various doses of the antisense inhibitor, called IONIS-ANGPTL3-LRx, or a placebo, given by weekly subcutaneous injections for 6 weeks.
Results showed that participants treated at the higher-dose levels had a reduction from baseline of 66% in triglycerides, 68% in apoliprotein C-III (ApoC-III), 35% in LDL cholesterol, 36% in total cholesterol, and 40% in non-HDL cholesterol, as well as 25% in HDL cholesterol, Dr. Tsimikas reported. They also had reductions in apolipoprotein B (ApoB).
“All these lipid parameters are really going in the right direction in terms of postulating potential clinical benefit,” he commented.
Safety and tolerability results showed that only a single participant experienced a local injection site adverse event related to the inhibitor (erythema and pruritus). None experienced flulike symptoms, platelet reductions, or serious adverse events, and none left the study because of adverse events.
“Among all known therapies that lower triglycerides, this [IONIS-ANGPTL3-LRx] is also associated with a reduction not only in LDL cholesterol levels, but also in ApoB as well, which portends well for future outcomes trials,” noted Dr. Tsimikas.
“We think this is going to be a promising candidate for patients who have uncontrolled LDL cholesterol, elevated triglycerides, and possibly patients who have hepatic steatosis and NASH [nonalcoholic steatohepatitis],” he concluded.
Dr. Tsimikas, who is an employee of and shareholder in the trial’s sponsor, Ionis Pharmaceuticals, discussed his findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – A new antisense inhibitor to angiopoietinlike protein 3 (ANGPTL3) reduces lipids in healthy adults with elevated triglyceride levels, according to results of a phase I/IIa ascending-dose trial reported at the American Heart Association scientific sessions.
ANGPTL3 regulates lipid and possibly general metabolism through actions in the liver, gut, muscle, and adipose tissue, explained presenting author Sotirios Tsimikas, MD, an investigator with Ionis Pharmaceuticals, Carlsbad, Calif., and a professor of medicine and director of vascular medicine at the University of California, San Diego. Individuals having loss-of-function mutations in the gene encoding this protein have very low plasma levels of cholesterol and triglycerides.
In the trial, sponsored by Ionis Pharmaceuticals, 32 healthy volunteers with elevated triglyceride levels were treated with various doses of the antisense inhibitor, called IONIS-ANGPTL3-LRx, or a placebo, given by weekly subcutaneous injections for 6 weeks.
Results showed that participants treated at the higher-dose levels had a reduction from baseline of 66% in triglycerides, 68% in apoliprotein C-III (ApoC-III), 35% in LDL cholesterol, 36% in total cholesterol, and 40% in non-HDL cholesterol, as well as 25% in HDL cholesterol, Dr. Tsimikas reported. They also had reductions in apolipoprotein B (ApoB).
“All these lipid parameters are really going in the right direction in terms of postulating potential clinical benefit,” he commented.
Safety and tolerability results showed that only a single participant experienced a local injection site adverse event related to the inhibitor (erythema and pruritus). None experienced flulike symptoms, platelet reductions, or serious adverse events, and none left the study because of adverse events.
“Among all known therapies that lower triglycerides, this [IONIS-ANGPTL3-LRx] is also associated with a reduction not only in LDL cholesterol levels, but also in ApoB as well, which portends well for future outcomes trials,” noted Dr. Tsimikas.
“We think this is going to be a promising candidate for patients who have uncontrolled LDL cholesterol, elevated triglycerides, and possibly patients who have hepatic steatosis and NASH [nonalcoholic steatohepatitis],” he concluded.
Dr. Tsimikas, who is an employee of and shareholder in the trial’s sponsor, Ionis Pharmaceuticals, discussed his findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Treatment was associated with reductions in levels of triglycerides (–66%), ApoC-III (–68%), LDL cholesterol (–35%), total cholesterol (–36%), and non-HDL cholesterol (–40%), as well as levels of HDL cholesterol (–25%).
Data source: A phase I/IIa ascending-dose trial among 32 healthy adults with hypertriglyceridemia.
Disclosures: Dr. Tsimikas is an employee of and shareholder in Ionis Pharmaceuticals, which sponsored the trial.
Lupus patients may be shortchanged on lipid management
WASHINGTON – Many patients with systemic lupus erythematosus may not be getting lipid testing or statin prescriptions despite having risk factors and even frank cardiovascular conditions, according to a large Medicaid database study presented at the annual meeting of the American College of Rheumatology.
The study found that systemic lupus erythematosus (SLE) patients were half as likely to get a lipid screening and 77% less likely to get a statin prescription, compared with patients with diabetes, a group that has a similarly elevated baseline CV disease risk.
This is concerning because it has been shown in several studies that SLE patients have higher rates of CV events than do age- and sex-matched patients with diabetes, and hyperlipidemia has been associated with CV disease in SLE patients. However, no studies have directly demonstrated reduced CV disease risk with statin use in SLE patients, unlike the general population and in those with diabetes, and so no clear guidelines exist, said lead study author Sarah Chen, MD, a rheumatology fellow at Brigham and Women’s Hospital, Boston.
Analyses of 1 year of billing records for each patient showed that SLE patients visited their doctor significantly more often than did the diabetes patients during their index year (mean of 4.7 vs. 3.2 visits). They were more likely to have a renal manifestation of their disease (13% vs. 4%). Although hypertension was similar among lupus and diabetes patients (35% vs. 40%), lupus patients were less likely to have hyperlipidemia (11% vs. 24%) but more likely to have concurrent cardiovascular disease, defined as angina, heart attack, atherosclerosis, percutaneous coronary intervention, coronary artery bypass grafting, stroke, or peripheral artery disease (13% vs. 10%).
Overall, 27% of lupus patients and 42% of diabetes patients had at least one lipid screen during their study year. This proportion stayed steady among diabetes patients as they aged, but rose significantly among lupus patients from 23% in the youngest group of 18-39 years to 32% of the oldest group of 50-65 years.
“This suggests that diabetes patients are getting consistent cardiovascular assessment as they age, while lupus patients are not,” Dr. Chen said at the meeting.
Statin prescriptions were also significantly less common among lupus patients overall (12% vs. 33%). The proportion of lupus patients who got at least one statin prescription increased with age, from 7% in the youngest group to 21% in the oldest group. But it increased more among diabetes patients, from 23% among the youngest to 42% among the oldest, Dr. Chen noted.
She conducted a multivariate analysis that controlled for age, gender, race, ethnicity, region of residence, socioeconomic status, Charlson Comorbidity Index, and the presence of cardiovascular disease. This determined that lupus patients were 54% less likely to have lipid testing and 77% less likely to get a statin.
A subanalysis found some significant predictors of SLE patients’ receipt of lipid screening. Increasing age increased the odds of screening by 2% per year. Lipid screening was 6% more likely for each additional medication they took. Lupus patients with nephritis were 36% more likely to have a screening than were those without nephritis. Those with existing cardiovascular disease were 11% more likely to have a test than were those without it, although this odds ratio (OR) had a nonsignificant 95% confidence interval of 1.00-1.24.
Hispanic and Asian patients were significantly more likely to receive lipid testing than were whites.
A second subanalysis found significant predictors of lupus patients receiving a statin prescription among lupus patients. With every passing year, the chance of getting a statin increased by 5%; it also increased by 7% for every medication that a patient was taking. Being a male increased the likelihood of receiving a statin prescription by 23%. Lupus nephritis more than doubled the odds of getting a statin (OR, 2.34), and the presence of cardiovascular disease was a similarly strong predictor (OR, 1.95). Hispanics and Asians were more likely to get the medication than were whites.
One significant limitation of the study is a lack of results on lipid testing, particularly in light of the potential effect the results might have had on decisions to prescribe statins.
Dr. Chen had no financial disclosures. The study was supported by a grant from the National Institutes of Health.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Many patients with systemic lupus erythematosus may not be getting lipid testing or statin prescriptions despite having risk factors and even frank cardiovascular conditions, according to a large Medicaid database study presented at the annual meeting of the American College of Rheumatology.
The study found that systemic lupus erythematosus (SLE) patients were half as likely to get a lipid screening and 77% less likely to get a statin prescription, compared with patients with diabetes, a group that has a similarly elevated baseline CV disease risk.
This is concerning because it has been shown in several studies that SLE patients have higher rates of CV events than do age- and sex-matched patients with diabetes, and hyperlipidemia has been associated with CV disease in SLE patients. However, no studies have directly demonstrated reduced CV disease risk with statin use in SLE patients, unlike the general population and in those with diabetes, and so no clear guidelines exist, said lead study author Sarah Chen, MD, a rheumatology fellow at Brigham and Women’s Hospital, Boston.
Analyses of 1 year of billing records for each patient showed that SLE patients visited their doctor significantly more often than did the diabetes patients during their index year (mean of 4.7 vs. 3.2 visits). They were more likely to have a renal manifestation of their disease (13% vs. 4%). Although hypertension was similar among lupus and diabetes patients (35% vs. 40%), lupus patients were less likely to have hyperlipidemia (11% vs. 24%) but more likely to have concurrent cardiovascular disease, defined as angina, heart attack, atherosclerosis, percutaneous coronary intervention, coronary artery bypass grafting, stroke, or peripheral artery disease (13% vs. 10%).
Overall, 27% of lupus patients and 42% of diabetes patients had at least one lipid screen during their study year. This proportion stayed steady among diabetes patients as they aged, but rose significantly among lupus patients from 23% in the youngest group of 18-39 years to 32% of the oldest group of 50-65 years.
“This suggests that diabetes patients are getting consistent cardiovascular assessment as they age, while lupus patients are not,” Dr. Chen said at the meeting.
Statin prescriptions were also significantly less common among lupus patients overall (12% vs. 33%). The proportion of lupus patients who got at least one statin prescription increased with age, from 7% in the youngest group to 21% in the oldest group. But it increased more among diabetes patients, from 23% among the youngest to 42% among the oldest, Dr. Chen noted.
She conducted a multivariate analysis that controlled for age, gender, race, ethnicity, region of residence, socioeconomic status, Charlson Comorbidity Index, and the presence of cardiovascular disease. This determined that lupus patients were 54% less likely to have lipid testing and 77% less likely to get a statin.
A subanalysis found some significant predictors of SLE patients’ receipt of lipid screening. Increasing age increased the odds of screening by 2% per year. Lipid screening was 6% more likely for each additional medication they took. Lupus patients with nephritis were 36% more likely to have a screening than were those without nephritis. Those with existing cardiovascular disease were 11% more likely to have a test than were those without it, although this odds ratio (OR) had a nonsignificant 95% confidence interval of 1.00-1.24.
Hispanic and Asian patients were significantly more likely to receive lipid testing than were whites.
A second subanalysis found significant predictors of lupus patients receiving a statin prescription among lupus patients. With every passing year, the chance of getting a statin increased by 5%; it also increased by 7% for every medication that a patient was taking. Being a male increased the likelihood of receiving a statin prescription by 23%. Lupus nephritis more than doubled the odds of getting a statin (OR, 2.34), and the presence of cardiovascular disease was a similarly strong predictor (OR, 1.95). Hispanics and Asians were more likely to get the medication than were whites.
One significant limitation of the study is a lack of results on lipid testing, particularly in light of the potential effect the results might have had on decisions to prescribe statins.
Dr. Chen had no financial disclosures. The study was supported by a grant from the National Institutes of Health.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Many patients with systemic lupus erythematosus may not be getting lipid testing or statin prescriptions despite having risk factors and even frank cardiovascular conditions, according to a large Medicaid database study presented at the annual meeting of the American College of Rheumatology.
The study found that systemic lupus erythematosus (SLE) patients were half as likely to get a lipid screening and 77% less likely to get a statin prescription, compared with patients with diabetes, a group that has a similarly elevated baseline CV disease risk.
This is concerning because it has been shown in several studies that SLE patients have higher rates of CV events than do age- and sex-matched patients with diabetes, and hyperlipidemia has been associated with CV disease in SLE patients. However, no studies have directly demonstrated reduced CV disease risk with statin use in SLE patients, unlike the general population and in those with diabetes, and so no clear guidelines exist, said lead study author Sarah Chen, MD, a rheumatology fellow at Brigham and Women’s Hospital, Boston.
Analyses of 1 year of billing records for each patient showed that SLE patients visited their doctor significantly more often than did the diabetes patients during their index year (mean of 4.7 vs. 3.2 visits). They were more likely to have a renal manifestation of their disease (13% vs. 4%). Although hypertension was similar among lupus and diabetes patients (35% vs. 40%), lupus patients were less likely to have hyperlipidemia (11% vs. 24%) but more likely to have concurrent cardiovascular disease, defined as angina, heart attack, atherosclerosis, percutaneous coronary intervention, coronary artery bypass grafting, stroke, or peripheral artery disease (13% vs. 10%).
Overall, 27% of lupus patients and 42% of diabetes patients had at least one lipid screen during their study year. This proportion stayed steady among diabetes patients as they aged, but rose significantly among lupus patients from 23% in the youngest group of 18-39 years to 32% of the oldest group of 50-65 years.
“This suggests that diabetes patients are getting consistent cardiovascular assessment as they age, while lupus patients are not,” Dr. Chen said at the meeting.
Statin prescriptions were also significantly less common among lupus patients overall (12% vs. 33%). The proportion of lupus patients who got at least one statin prescription increased with age, from 7% in the youngest group to 21% in the oldest group. But it increased more among diabetes patients, from 23% among the youngest to 42% among the oldest, Dr. Chen noted.
She conducted a multivariate analysis that controlled for age, gender, race, ethnicity, region of residence, socioeconomic status, Charlson Comorbidity Index, and the presence of cardiovascular disease. This determined that lupus patients were 54% less likely to have lipid testing and 77% less likely to get a statin.
A subanalysis found some significant predictors of SLE patients’ receipt of lipid screening. Increasing age increased the odds of screening by 2% per year. Lipid screening was 6% more likely for each additional medication they took. Lupus patients with nephritis were 36% more likely to have a screening than were those without nephritis. Those with existing cardiovascular disease were 11% more likely to have a test than were those without it, although this odds ratio (OR) had a nonsignificant 95% confidence interval of 1.00-1.24.
Hispanic and Asian patients were significantly more likely to receive lipid testing than were whites.
A second subanalysis found significant predictors of lupus patients receiving a statin prescription among lupus patients. With every passing year, the chance of getting a statin increased by 5%; it also increased by 7% for every medication that a patient was taking. Being a male increased the likelihood of receiving a statin prescription by 23%. Lupus nephritis more than doubled the odds of getting a statin (OR, 2.34), and the presence of cardiovascular disease was a similarly strong predictor (OR, 1.95). Hispanics and Asians were more likely to get the medication than were whites.
One significant limitation of the study is a lack of results on lipid testing, particularly in light of the potential effect the results might have had on decisions to prescribe statins.
Dr. Chen had no financial disclosures. The study was supported by a grant from the National Institutes of Health.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Lupus patients were 54% less likely to have lipid level testing and 77% less likely to have a statin prescribed than were diabetes patients.
Data source: A Medicaid database review comprising nearly 20,000 lupus patients and 40,000 diabetes patients during 2007-2010.
Disclosures: Dr. Chen had no financial disclosures. The study was supported by a grant from the National Institutes of Health.
Tocilizumab raises cholesterol, but not cardiovascular events
WASHINGTON – The lipid changes induced by tocilizumab do not translate into an increased risk of heart attack or stroke in patients with rheumatoid arthritis, a 5-year postmarketing study has determined.
Patients taking the drug experienced quick and dramatic increases in low-density lipoprotein cholesterol: 12% in just 4 weeks. But when compared against patients taking etanercept, they showed no significant increases in stroke, heart attack, heart failure hospitalization, or cardiac death, Jon Giles, MD, said at the annual meeting of the American College of Rheumatology.
“This has been a lingering question,” he said. “These data are very reassuring. Practitioners can feel comfortable that they’re not inducing harm with this drug in patients with high cardiovascular risk. Now when we’re evaluating RA patients for the best drug, we can make a choice based on other parameters ... how the patient wants to take the drug, for example, or other safety indicators. This study takes away one element of uncertainty.”
Tocilizumab’s profound effects on lipids have been confirmed in all of its pivotal trials, with average across-the-board increases of 17%-25% in total cholesterol, low-and high-density cholesterol, and triglycerides. ENTRACTE is the first trial, however, to compare its cardiovascular safety profile to that of another RA medication.
The study comprised 3,080 patients with active, seropositive RA from 353 centers across 31 countries. The mean age of the patients was 61 years. All patients had at least one cardiovascular risk factor, including current smoking (30%), hypertension (71%), or diabetes (18%). About a quarter were already taking a statin, and about 5% had a history of heart attack, stroke, or coronary revascularization.
They were randomized to open-label tocilizumab 8 mg/kg infused every 4 weeks or etanercept 50 mg injected weekly. The mean follow-up reported was 3.5 years, but some patients had full 5-year data. “This was plenty of time to see early atherothrombotic complications if they were going to develop,” Dr. Giles said.
The primary endpoint was a composite of major cardiovascular outcomes including cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. Secondary outcomes included individual components of the primary endpoint, as well as all-cause mortality, heart failure hospitalization, fatal and nonfatal myocardial infarction and stroke, and lipid levels.
By week 4, LDL-cholesterol levels had risen by almost 12% in the tocilizumab group. This moderated somewhat, but stayed elevated, reaching 9% above baseline by week 144. In contrast, LDL-cholesterol in the etanercept group was virtually unchanged throughout the study.
In the intent-to-treat analysis, there were 161 occurrences of the composite outcome: 83 in the tocilizumab group and 78 in the etanercept group (hazard ratio, 1.05). This was not a significant difference. The event rate was 1.70 per 100 person-years in the etanercept group and 1.82 in the tocilizumab group – also not significantly different.
The individual secondary endpoints were almost identical in each group: cardiovascular death, 35 with etanercept vs. 36 with tocilizumab; nonfatal MI, 31 vs. 28; nonfatal stroke, 15 vs. 24; and all-cause mortality, 64 in each group. A multivariate regression analysis showed no significantly increased risks associated with tocilizumab in any of the secondary endpoints. The rates and types of adverse events were also similar.
Because this was a safety study, the investigators were interested in the uncertainty in their estimate of risk, Dr. Giles said in an interview.
“The study was designed to exclude at most an 80% increase in cardiovascular events for the patients treated with tocilizumab, compared with etanercept. What we determined was that we could exclude with 95% certainty that if a much larger sample of similar patients was studied, the risk of such events in the tocilizumab group would not exceed a 43% higher rate at the extreme. Notably, the degree of uncertainty included the possibility that the event rate could be lower in the tocilizumab group, compared with those treated with etanercept.”
Dr. Giles reported receiving consulting fees from Genentech, which markets tocilizumab. All other authors also disclosed financial relationships with Genentech or its parent company, Roche. Four authors were employees of Roche/Genentech.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – The lipid changes induced by tocilizumab do not translate into an increased risk of heart attack or stroke in patients with rheumatoid arthritis, a 5-year postmarketing study has determined.
Patients taking the drug experienced quick and dramatic increases in low-density lipoprotein cholesterol: 12% in just 4 weeks. But when compared against patients taking etanercept, they showed no significant increases in stroke, heart attack, heart failure hospitalization, or cardiac death, Jon Giles, MD, said at the annual meeting of the American College of Rheumatology.
“This has been a lingering question,” he said. “These data are very reassuring. Practitioners can feel comfortable that they’re not inducing harm with this drug in patients with high cardiovascular risk. Now when we’re evaluating RA patients for the best drug, we can make a choice based on other parameters ... how the patient wants to take the drug, for example, or other safety indicators. This study takes away one element of uncertainty.”
Tocilizumab’s profound effects on lipids have been confirmed in all of its pivotal trials, with average across-the-board increases of 17%-25% in total cholesterol, low-and high-density cholesterol, and triglycerides. ENTRACTE is the first trial, however, to compare its cardiovascular safety profile to that of another RA medication.
The study comprised 3,080 patients with active, seropositive RA from 353 centers across 31 countries. The mean age of the patients was 61 years. All patients had at least one cardiovascular risk factor, including current smoking (30%), hypertension (71%), or diabetes (18%). About a quarter were already taking a statin, and about 5% had a history of heart attack, stroke, or coronary revascularization.
They were randomized to open-label tocilizumab 8 mg/kg infused every 4 weeks or etanercept 50 mg injected weekly. The mean follow-up reported was 3.5 years, but some patients had full 5-year data. “This was plenty of time to see early atherothrombotic complications if they were going to develop,” Dr. Giles said.
The primary endpoint was a composite of major cardiovascular outcomes including cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. Secondary outcomes included individual components of the primary endpoint, as well as all-cause mortality, heart failure hospitalization, fatal and nonfatal myocardial infarction and stroke, and lipid levels.
By week 4, LDL-cholesterol levels had risen by almost 12% in the tocilizumab group. This moderated somewhat, but stayed elevated, reaching 9% above baseline by week 144. In contrast, LDL-cholesterol in the etanercept group was virtually unchanged throughout the study.
In the intent-to-treat analysis, there were 161 occurrences of the composite outcome: 83 in the tocilizumab group and 78 in the etanercept group (hazard ratio, 1.05). This was not a significant difference. The event rate was 1.70 per 100 person-years in the etanercept group and 1.82 in the tocilizumab group – also not significantly different.
The individual secondary endpoints were almost identical in each group: cardiovascular death, 35 with etanercept vs. 36 with tocilizumab; nonfatal MI, 31 vs. 28; nonfatal stroke, 15 vs. 24; and all-cause mortality, 64 in each group. A multivariate regression analysis showed no significantly increased risks associated with tocilizumab in any of the secondary endpoints. The rates and types of adverse events were also similar.
Because this was a safety study, the investigators were interested in the uncertainty in their estimate of risk, Dr. Giles said in an interview.
“The study was designed to exclude at most an 80% increase in cardiovascular events for the patients treated with tocilizumab, compared with etanercept. What we determined was that we could exclude with 95% certainty that if a much larger sample of similar patients was studied, the risk of such events in the tocilizumab group would not exceed a 43% higher rate at the extreme. Notably, the degree of uncertainty included the possibility that the event rate could be lower in the tocilizumab group, compared with those treated with etanercept.”
Dr. Giles reported receiving consulting fees from Genentech, which markets tocilizumab. All other authors also disclosed financial relationships with Genentech or its parent company, Roche. Four authors were employees of Roche/Genentech.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – The lipid changes induced by tocilizumab do not translate into an increased risk of heart attack or stroke in patients with rheumatoid arthritis, a 5-year postmarketing study has determined.
Patients taking the drug experienced quick and dramatic increases in low-density lipoprotein cholesterol: 12% in just 4 weeks. But when compared against patients taking etanercept, they showed no significant increases in stroke, heart attack, heart failure hospitalization, or cardiac death, Jon Giles, MD, said at the annual meeting of the American College of Rheumatology.
“This has been a lingering question,” he said. “These data are very reassuring. Practitioners can feel comfortable that they’re not inducing harm with this drug in patients with high cardiovascular risk. Now when we’re evaluating RA patients for the best drug, we can make a choice based on other parameters ... how the patient wants to take the drug, for example, or other safety indicators. This study takes away one element of uncertainty.”
Tocilizumab’s profound effects on lipids have been confirmed in all of its pivotal trials, with average across-the-board increases of 17%-25% in total cholesterol, low-and high-density cholesterol, and triglycerides. ENTRACTE is the first trial, however, to compare its cardiovascular safety profile to that of another RA medication.
The study comprised 3,080 patients with active, seropositive RA from 353 centers across 31 countries. The mean age of the patients was 61 years. All patients had at least one cardiovascular risk factor, including current smoking (30%), hypertension (71%), or diabetes (18%). About a quarter were already taking a statin, and about 5% had a history of heart attack, stroke, or coronary revascularization.
They were randomized to open-label tocilizumab 8 mg/kg infused every 4 weeks or etanercept 50 mg injected weekly. The mean follow-up reported was 3.5 years, but some patients had full 5-year data. “This was plenty of time to see early atherothrombotic complications if they were going to develop,” Dr. Giles said.
The primary endpoint was a composite of major cardiovascular outcomes including cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. Secondary outcomes included individual components of the primary endpoint, as well as all-cause mortality, heart failure hospitalization, fatal and nonfatal myocardial infarction and stroke, and lipid levels.
By week 4, LDL-cholesterol levels had risen by almost 12% in the tocilizumab group. This moderated somewhat, but stayed elevated, reaching 9% above baseline by week 144. In contrast, LDL-cholesterol in the etanercept group was virtually unchanged throughout the study.
In the intent-to-treat analysis, there were 161 occurrences of the composite outcome: 83 in the tocilizumab group and 78 in the etanercept group (hazard ratio, 1.05). This was not a significant difference. The event rate was 1.70 per 100 person-years in the etanercept group and 1.82 in the tocilizumab group – also not significantly different.
The individual secondary endpoints were almost identical in each group: cardiovascular death, 35 with etanercept vs. 36 with tocilizumab; nonfatal MI, 31 vs. 28; nonfatal stroke, 15 vs. 24; and all-cause mortality, 64 in each group. A multivariate regression analysis showed no significantly increased risks associated with tocilizumab in any of the secondary endpoints. The rates and types of adverse events were also similar.
Because this was a safety study, the investigators were interested in the uncertainty in their estimate of risk, Dr. Giles said in an interview.
“The study was designed to exclude at most an 80% increase in cardiovascular events for the patients treated with tocilizumab, compared with etanercept. What we determined was that we could exclude with 95% certainty that if a much larger sample of similar patients was studied, the risk of such events in the tocilizumab group would not exceed a 43% higher rate at the extreme. Notably, the degree of uncertainty included the possibility that the event rate could be lower in the tocilizumab group, compared with those treated with etanercept.”
Dr. Giles reported receiving consulting fees from Genentech, which markets tocilizumab. All other authors also disclosed financial relationships with Genentech or its parent company, Roche. Four authors were employees of Roche/Genentech.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: LDL-cholesterol levels rose by almost 12% in the tocilizumab group, but there was no corresponding increase in cardiovascular events.
Data source: ENTRACTE, which randomized 3,080 patients with RA to either tocilizumab or etanercept.
Disclosures: Dr. Giles reported receiving consulting fees from Genentech, which markets tocilizumab. All other authors also disclosed financial relationships with Genentech or its parent company, Roche. Four authors were employees of Roche/Genentech.
VIDEO: Statins cut mortality in ankylosing spondylitis, psoriatic arthritis
WASHINGTON – Statins lowered all-cause mortality by 32% in patients with ankylosing spondylitis (AS) and psoriatic arthritis (PsA) in a retrospective cohort study.
The magnitude of benefit from statins in these two disease states is greater than that found in the general population (estimated 9%-14% reduction in all-cause mortality) and than that reported in patients with rheumatoid arthritis (RA, 21% reduction), said Amar Oza, MD, a second-year rheumatology fellow at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“This is a unique study. The benefit of statins has not been looked at in AS and PsA, specifically,” Dr. Oza explained. “More data are needed” to establish this benefit with certainty, he added.
The data were presented at the annual meeting of the American College of Rheumatology, and Dr. Oza discussed the findings in a video interview.
The study compared 2,904 patients with AS or PsA who initiated statins between 2000 and 2014 with 2,904 propensity-matched AS or PsA patients who did not initiate statins during that period. Patients were drawn from a United Kingdom general population database.
The investigators used a propensity score that accounted for 50 confounding variables to match the two cohorts. These variables included, but were not limited to, disease duration, socioeconomic status, body mass index, lifestyle factors, and medication use.
“This study is the first step in elucidating the benefit of statins in AS and PsA. It is a good step forward. If additional data substantiate that AS and PsA patients have a low threshold for statins, I can envision statins for both primary and secondary prevention in this patient population,” Dr. Oza stated.
The authors had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Statins lowered all-cause mortality by 32% in patients with ankylosing spondylitis (AS) and psoriatic arthritis (PsA) in a retrospective cohort study.
The magnitude of benefit from statins in these two disease states is greater than that found in the general population (estimated 9%-14% reduction in all-cause mortality) and than that reported in patients with rheumatoid arthritis (RA, 21% reduction), said Amar Oza, MD, a second-year rheumatology fellow at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“This is a unique study. The benefit of statins has not been looked at in AS and PsA, specifically,” Dr. Oza explained. “More data are needed” to establish this benefit with certainty, he added.
The data were presented at the annual meeting of the American College of Rheumatology, and Dr. Oza discussed the findings in a video interview.
The study compared 2,904 patients with AS or PsA who initiated statins between 2000 and 2014 with 2,904 propensity-matched AS or PsA patients who did not initiate statins during that period. Patients were drawn from a United Kingdom general population database.
The investigators used a propensity score that accounted for 50 confounding variables to match the two cohorts. These variables included, but were not limited to, disease duration, socioeconomic status, body mass index, lifestyle factors, and medication use.
“This study is the first step in elucidating the benefit of statins in AS and PsA. It is a good step forward. If additional data substantiate that AS and PsA patients have a low threshold for statins, I can envision statins for both primary and secondary prevention in this patient population,” Dr. Oza stated.
The authors had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Statins lowered all-cause mortality by 32% in patients with ankylosing spondylitis (AS) and psoriatic arthritis (PsA) in a retrospective cohort study.
The magnitude of benefit from statins in these two disease states is greater than that found in the general population (estimated 9%-14% reduction in all-cause mortality) and than that reported in patients with rheumatoid arthritis (RA, 21% reduction), said Amar Oza, MD, a second-year rheumatology fellow at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“This is a unique study. The benefit of statins has not been looked at in AS and PsA, specifically,” Dr. Oza explained. “More data are needed” to establish this benefit with certainty, he added.
The data were presented at the annual meeting of the American College of Rheumatology, and Dr. Oza discussed the findings in a video interview.
The study compared 2,904 patients with AS or PsA who initiated statins between 2000 and 2014 with 2,904 propensity-matched AS or PsA patients who did not initiate statins during that period. Patients were drawn from a United Kingdom general population database.
The investigators used a propensity score that accounted for 50 confounding variables to match the two cohorts. These variables included, but were not limited to, disease duration, socioeconomic status, body mass index, lifestyle factors, and medication use.
“This study is the first step in elucidating the benefit of statins in AS and PsA. It is a good step forward. If additional data substantiate that AS and PsA patients have a low threshold for statins, I can envision statins for both primary and secondary prevention in this patient population,” Dr. Oza stated.
The authors had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ACR ANNUAL MEETING
CSL112 enhances cholesterol efflux capacity after acute MI
CSL112, a plasma-derived apolipoprotein A-1 (apoA-1) that enhances cholesterol efflux capacity, was found safe for use after acute MI in a manufacturer-sponsored phase IIb trial, Michael Gibson, MD, reported at the American Heart Association scientific sessions.
Cholesterol efflux capacity is a measure of HDL cholesterol’s ability to remove excess cholesterol from atherosclerotic plaque for transport to the liver. Drugs that improve this capacity rather than simply raising HDL cholesterol are expected to reduce plaque burden and stabilize vulnerable plaque immediately following acute MI, when cholesterol efflux is significantly impaired. But they have not been tested in this patient population until now, said Dr. Gibson of Beth Israel Deaconess Medical Center and Harvard, both in Boston.
An earlier prototype formulation of CSL112 was discontinued because of concerns about transient elevations in liver enzymes and the potential for renal toxicity due to the agent’s high sucrose content. Dr. Gibson and his associates in the AEGIS-1 trial (ApoA-1 Event Reducing in Ischemic Syndromes 1) now report their findings for the current formulation of CSL112, which contains lower phosphatidylcholine and lower sucrose levels, in 1,258 patients who had MI during the preceding week and who had normal or only mildly impaired renal function. These results were reported at the meeting and simultaneously published online in Circulation (2016, Nov 15. doi: 10.1161/CIRCULATION AHA.116.025687).
The study participants were enrolled in 16 countries during an 11-month period. They were randomly assigned to receive low-dose (2 g) CSL112 (419 patients), high-dose (6 g) CSL112 (421 patients), or a matching placebo (418 patients) via IV infusion every week for 4 consecutive weeks. The median duration of follow-up at the time of this report was 7.5 months.
The two primary safety end points – the rate of hepatic impairment and the rate of renal impairment during treatment – were not significantly different across the three study groups. Hepatic impairment developed in 1.0% of the low-dose group, 0.5% of the high-dose group, and 0.0% of the placebo group. Renal impairment developed in 0.0% of the low-dose group, 0.7% of the high-dose group, and 0.2% of the placebo group.
Similarly, rates of major adverse cardiovascular events were comparable across the three study groups, as were rates of any grade of bleeding, rates of serious and life-threatening adverse events, and rates of adverse events leading to drug discontinuation.
This study focused on safety and tolerability and was not designed or powered to assess efficacy. Nevertheless, CSL112 caused a substantial and dose-dependent increase in both apoA-1 and cholesterol efflux capacity. The low-dose drug raised total cholesterol efflux capacity by 1.87-fold, and the high-dose drug raised it 2.45-fold.
As the first study to establish the safety and feasibility of adding CSL112 to standard care for acute MI, this trial demonstrates that an adequately powered, multicenter, phase III trial is warranted, Dr. Gibson said.
The current formulation of CSL112 didn’t provoke hepatic toxicity, and even though it was given shortly after contrast studies in MI patients, it also didn’t provoke renal toxicity. “This demonstrates the feasibility of administering CSL112 to patients with MI who have normal renal function or mild renal impairment shortly after angiography. A study in MI patients who have moderate renal impairment is now under way,” he noted.
This study was sponsored by CSL Behring, maker of CSL112. Dr. Gibson and his associates reported financial ties to Behring and numerous other industry sources.
CSL112, a plasma-derived apolipoprotein A-1 (apoA-1) that enhances cholesterol efflux capacity, was found safe for use after acute MI in a manufacturer-sponsored phase IIb trial, Michael Gibson, MD, reported at the American Heart Association scientific sessions.
Cholesterol efflux capacity is a measure of HDL cholesterol’s ability to remove excess cholesterol from atherosclerotic plaque for transport to the liver. Drugs that improve this capacity rather than simply raising HDL cholesterol are expected to reduce plaque burden and stabilize vulnerable plaque immediately following acute MI, when cholesterol efflux is significantly impaired. But they have not been tested in this patient population until now, said Dr. Gibson of Beth Israel Deaconess Medical Center and Harvard, both in Boston.
An earlier prototype formulation of CSL112 was discontinued because of concerns about transient elevations in liver enzymes and the potential for renal toxicity due to the agent’s high sucrose content. Dr. Gibson and his associates in the AEGIS-1 trial (ApoA-1 Event Reducing in Ischemic Syndromes 1) now report their findings for the current formulation of CSL112, which contains lower phosphatidylcholine and lower sucrose levels, in 1,258 patients who had MI during the preceding week and who had normal or only mildly impaired renal function. These results were reported at the meeting and simultaneously published online in Circulation (2016, Nov 15. doi: 10.1161/CIRCULATION AHA.116.025687).
The study participants were enrolled in 16 countries during an 11-month period. They were randomly assigned to receive low-dose (2 g) CSL112 (419 patients), high-dose (6 g) CSL112 (421 patients), or a matching placebo (418 patients) via IV infusion every week for 4 consecutive weeks. The median duration of follow-up at the time of this report was 7.5 months.
The two primary safety end points – the rate of hepatic impairment and the rate of renal impairment during treatment – were not significantly different across the three study groups. Hepatic impairment developed in 1.0% of the low-dose group, 0.5% of the high-dose group, and 0.0% of the placebo group. Renal impairment developed in 0.0% of the low-dose group, 0.7% of the high-dose group, and 0.2% of the placebo group.
Similarly, rates of major adverse cardiovascular events were comparable across the three study groups, as were rates of any grade of bleeding, rates of serious and life-threatening adverse events, and rates of adverse events leading to drug discontinuation.
This study focused on safety and tolerability and was not designed or powered to assess efficacy. Nevertheless, CSL112 caused a substantial and dose-dependent increase in both apoA-1 and cholesterol efflux capacity. The low-dose drug raised total cholesterol efflux capacity by 1.87-fold, and the high-dose drug raised it 2.45-fold.
As the first study to establish the safety and feasibility of adding CSL112 to standard care for acute MI, this trial demonstrates that an adequately powered, multicenter, phase III trial is warranted, Dr. Gibson said.
The current formulation of CSL112 didn’t provoke hepatic toxicity, and even though it was given shortly after contrast studies in MI patients, it also didn’t provoke renal toxicity. “This demonstrates the feasibility of administering CSL112 to patients with MI who have normal renal function or mild renal impairment shortly after angiography. A study in MI patients who have moderate renal impairment is now under way,” he noted.
This study was sponsored by CSL Behring, maker of CSL112. Dr. Gibson and his associates reported financial ties to Behring and numerous other industry sources.
CSL112, a plasma-derived apolipoprotein A-1 (apoA-1) that enhances cholesterol efflux capacity, was found safe for use after acute MI in a manufacturer-sponsored phase IIb trial, Michael Gibson, MD, reported at the American Heart Association scientific sessions.
Cholesterol efflux capacity is a measure of HDL cholesterol’s ability to remove excess cholesterol from atherosclerotic plaque for transport to the liver. Drugs that improve this capacity rather than simply raising HDL cholesterol are expected to reduce plaque burden and stabilize vulnerable plaque immediately following acute MI, when cholesterol efflux is significantly impaired. But they have not been tested in this patient population until now, said Dr. Gibson of Beth Israel Deaconess Medical Center and Harvard, both in Boston.
An earlier prototype formulation of CSL112 was discontinued because of concerns about transient elevations in liver enzymes and the potential for renal toxicity due to the agent’s high sucrose content. Dr. Gibson and his associates in the AEGIS-1 trial (ApoA-1 Event Reducing in Ischemic Syndromes 1) now report their findings for the current formulation of CSL112, which contains lower phosphatidylcholine and lower sucrose levels, in 1,258 patients who had MI during the preceding week and who had normal or only mildly impaired renal function. These results were reported at the meeting and simultaneously published online in Circulation (2016, Nov 15. doi: 10.1161/CIRCULATION AHA.116.025687).
The study participants were enrolled in 16 countries during an 11-month period. They were randomly assigned to receive low-dose (2 g) CSL112 (419 patients), high-dose (6 g) CSL112 (421 patients), or a matching placebo (418 patients) via IV infusion every week for 4 consecutive weeks. The median duration of follow-up at the time of this report was 7.5 months.
The two primary safety end points – the rate of hepatic impairment and the rate of renal impairment during treatment – were not significantly different across the three study groups. Hepatic impairment developed in 1.0% of the low-dose group, 0.5% of the high-dose group, and 0.0% of the placebo group. Renal impairment developed in 0.0% of the low-dose group, 0.7% of the high-dose group, and 0.2% of the placebo group.
Similarly, rates of major adverse cardiovascular events were comparable across the three study groups, as were rates of any grade of bleeding, rates of serious and life-threatening adverse events, and rates of adverse events leading to drug discontinuation.
This study focused on safety and tolerability and was not designed or powered to assess efficacy. Nevertheless, CSL112 caused a substantial and dose-dependent increase in both apoA-1 and cholesterol efflux capacity. The low-dose drug raised total cholesterol efflux capacity by 1.87-fold, and the high-dose drug raised it 2.45-fold.
As the first study to establish the safety and feasibility of adding CSL112 to standard care for acute MI, this trial demonstrates that an adequately powered, multicenter, phase III trial is warranted, Dr. Gibson said.
The current formulation of CSL112 didn’t provoke hepatic toxicity, and even though it was given shortly after contrast studies in MI patients, it also didn’t provoke renal toxicity. “This demonstrates the feasibility of administering CSL112 to patients with MI who have normal renal function or mild renal impairment shortly after angiography. A study in MI patients who have moderate renal impairment is now under way,” he noted.
This study was sponsored by CSL Behring, maker of CSL112. Dr. Gibson and his associates reported financial ties to Behring and numerous other industry sources.
FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: CSL112, a plasma-derived apolipoprotein A-1 that enhances cholesterol efflux capacity, was found safe for use after acute MI in an international phase IIb trial.
Major finding: Hepatic impairment developed in 1.0% of the low-dose group, 0.5% of the high-dose group, and 0.0% of the placebo group, while renal impairment developed in 0.0% of the low-dose group, 0.7% of the high-dose group, and 0.2% of the placebo group – all nonsignificant differences.
Data source: A manufacturer-sponsored randomized double-blind placebo-controlled phase IIb trial involving 1,258 patients in 16 countries.
Disclosures: This study was sponsored by CSL Behring, maker of CSL112. Dr. Gibson and his associates reported financial ties to Behring and numerous other industry sources.
USPSTF: Expand statin use beyond lipids to CVD risk
Use of statins for the primary prevention of cardiovascular disease should be based on overall cardiovascular disease risk, not necessarily blood lipid levels, according to new recommendations from the United States Preventive Services Task Force.
Despite widespread use in the elderly, USPSTF also said there’s insufficient evidence to recommend starting statins for cardiovascular disease (CVD) prevention in patients 76 years or older (JAMA. 2016 Nov;316[19]:1997-2007).
USPSTF now recommends low- to moderate-dose statins for primary prevention in adults 40-75 years old who have at least one CVD risk factor – dyslipidemia, diabetes, hypertension, or smoking – and a 10-year CVD event risk of at least 10% (B grade). It also recommended “selectively” offering low- to moderate-dose statins if patients have a 7.5%-10% risk (C grade). The task force recommends using the online American College of Cardiology/American Heart Association risk calculator.
A B grade denotes that USPSTF recommends the service as one where there is “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.” With a C grade, the task force recommends that the service be provided subject to professional judgment and patient preferences.
The new advices from USPSTF is consistent with the task force’s 2015 draft proposals, and is based on a pooled analysis of 19 randomized trials in 71,344 adults at risk for CVD but without prior events; 17 of the studies were sponsored at least in part by the companies that make statins. The median duration of follow-up was 3 years. Trials included atorvastatin (Lipitor) and six other statins from 1994-2016.
Few of the 19 trials enrolled patients older than 75 years, so “the balance of benefits and harms of initiating statin use for” primary prevention in elderly adults “cannot be determined.” The task force did not make a recommendation either way, and called for further investigation.
For younger patients, the scales tipped toward benefit when the group found no overall increased risk of cancer, liver damage, diabetes, or cognitive problems with statins for primary prevention. “Although muscle pain, soreness, or weakness are commonly reported with statin use, there were no statistically significant differences between the intervention and control groups for myalgia, myopathy, or rhabdomyolysis,” according to the task force.
Across the 19 trials, statin therapy was associated with a statistically significant 14% reduction in all-cause mortality; 31% reduction in cardiovascular mortality; 29% reduction in stroke; and a 36% reduction in myocardial infarction; 250 patients needed to be treated to prevent one death from any cause after 1-6 years, and 233 to prevent one cardiovascular death after 2-6 years.
The recommendations call for low to moderate doses because that’s what most of the studies used, and there were no clear benefits when trials stratified patients by dose.
USPSTF cautioned that “reliance on a risk calculator ... alone as a basis for prevention may be problematic, given its possible overestimation of risk in some populations” and noted that the benefits “of statin use may be linear according to a patient’s absolute risk level, and any cut points used are only population estimates of benefits.”
The recommendations do not pertain to adults with very high CVD risk, such as those with familial hypercholesterolemia or an LDL level greater than 190mg/dL, since they were excluded from primary prevention trials.
While the USPSTF was careful in its evidence review, “ the limitations of the evidence were not considered sufficiently, given the serious concerns about the harms of statins for primary prevention,” Rita Redberg, MD, and Mitchell Katz, MD, wrote in an editorial accompanying the recommendations.
“USPSTF also did not have access to patient-level data; they had to rely on peer-reviewed published reports,” according to the editorialists. “The actual trial data are largely held by the Cholesterol Treatment Trialists’ Collaboration on behalf of industry sponsors and have not been made available to other researchers, despite multiple requests over many years.” Further, many of the trials were industry sponsored.
Using the USPSTF data, only 2% of patients who take statins for 5 years will avoid a myocardial infarction; virtually all (98%) will not experience any benefit. At the same time, 5%-20% will experience side effects including rhabdomyolysis, cognitive dysfunction, and increased risk of diabetes (JAMA. 2016 Nov;316[19]:1979-81).
There are unintended consequences of widespread statin use in healthy persons, Dr. Redberg and Dr. Katz wrote. People taking statins are more likely to become obese and more sedentary over time, likely because they mistakenly think they do not need to eat a healthy diet and exercise.
The USPSTF analysis was funded by the Agency for Healthcare Research and Quality. Task force lead Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco, has advised the Institute for Clinical and Economic Review, which is partly funded by industry, on the cost-effectiveness of lipid-lowering drugs. Another member reported comparing how well they worked. The other 15 task force members had no disclosures.
Dare cardiovascular experts consider a future in which there are near-universal statin recommendations for middle-aged adults?
[USPSTF analysis suggests] that statin recommendations could be based primarily on a patient’s underlying CVD risk rather than on his or her cholesterol level. Disseminating a treatment strategy based largely on CVD risk alone has been a difficult message for the clinical community to accept and implement.
Nearly a generation of physicians has considered high-cholesterol levels, rather than generalized CVD risk, the target for statin treatment. Only a minority of physicians consistently use complex, risk-based probabilistic calculations to determine therapy.
Several key questions deserve careful consideration. Should LDL be considered in treatment recommendations beyond CVD risk? The decision to use absolute risk to guide statin recommendations is based on the finding that the relative risk reduction seen with statin therapy is independent of baseline risk; thus, those with the highest absolute baseline CVD risk experience the greatest reduction in CVD events.
However, the relative risk reduction of lipid-lowering therapy is also proportional to mmol/dL reduction in LDL level. This supports the contention that those with the highest baseline LDL levels should benefit the most from treatment because they have the most potential decline in LDL with intervention. One way to reconcile these findings is to incorporate both LDL levels and CVD risk into treatment recommendations, as has been done in the European guidelines. This approach recognizes that the relative benefit of statins is proportional to LDL lowering but that the absolute treatment benefit is largely driven by baseline risk.
From the resource perspective, the vast majority of statins are now available as generic products and require limited monitoring, leading to quite modest therapeutic costs. For patients in the gray area not covered by the guidelines, clinicians should be cautioned against adopting either a “treat none” or a “treat all” strategy. Rather, gaps in the evidence provide opportunities for clinicians to practice the art of medicine and engage with patients in shared decision-making regarding strategies for CVD prevention.
Ann Marie Navar, MD, PhD, and Eric Peterson, MD, MPH, are both at the Duke Clinical Research Institute in Durham, N.C. Dr. Navar reported funding from Regeneron and Sanofi. Dr. Peterson reported funding from Merck, Sanofi, Regeneron, and AstraZeneca. They made their comments in an editorial to the USPSTF report (JAMA. 2016 Nov;316[19]:1981-3).
Dare cardiovascular experts consider a future in which there are near-universal statin recommendations for middle-aged adults?
[USPSTF analysis suggests] that statin recommendations could be based primarily on a patient’s underlying CVD risk rather than on his or her cholesterol level. Disseminating a treatment strategy based largely on CVD risk alone has been a difficult message for the clinical community to accept and implement.
Nearly a generation of physicians has considered high-cholesterol levels, rather than generalized CVD risk, the target for statin treatment. Only a minority of physicians consistently use complex, risk-based probabilistic calculations to determine therapy.
Several key questions deserve careful consideration. Should LDL be considered in treatment recommendations beyond CVD risk? The decision to use absolute risk to guide statin recommendations is based on the finding that the relative risk reduction seen with statin therapy is independent of baseline risk; thus, those with the highest absolute baseline CVD risk experience the greatest reduction in CVD events.
However, the relative risk reduction of lipid-lowering therapy is also proportional to mmol/dL reduction in LDL level. This supports the contention that those with the highest baseline LDL levels should benefit the most from treatment because they have the most potential decline in LDL with intervention. One way to reconcile these findings is to incorporate both LDL levels and CVD risk into treatment recommendations, as has been done in the European guidelines. This approach recognizes that the relative benefit of statins is proportional to LDL lowering but that the absolute treatment benefit is largely driven by baseline risk.
From the resource perspective, the vast majority of statins are now available as generic products and require limited monitoring, leading to quite modest therapeutic costs. For patients in the gray area not covered by the guidelines, clinicians should be cautioned against adopting either a “treat none” or a “treat all” strategy. Rather, gaps in the evidence provide opportunities for clinicians to practice the art of medicine and engage with patients in shared decision-making regarding strategies for CVD prevention.
Ann Marie Navar, MD, PhD, and Eric Peterson, MD, MPH, are both at the Duke Clinical Research Institute in Durham, N.C. Dr. Navar reported funding from Regeneron and Sanofi. Dr. Peterson reported funding from Merck, Sanofi, Regeneron, and AstraZeneca. They made their comments in an editorial to the USPSTF report (JAMA. 2016 Nov;316[19]:1981-3).
Dare cardiovascular experts consider a future in which there are near-universal statin recommendations for middle-aged adults?
[USPSTF analysis suggests] that statin recommendations could be based primarily on a patient’s underlying CVD risk rather than on his or her cholesterol level. Disseminating a treatment strategy based largely on CVD risk alone has been a difficult message for the clinical community to accept and implement.
Nearly a generation of physicians has considered high-cholesterol levels, rather than generalized CVD risk, the target for statin treatment. Only a minority of physicians consistently use complex, risk-based probabilistic calculations to determine therapy.
Several key questions deserve careful consideration. Should LDL be considered in treatment recommendations beyond CVD risk? The decision to use absolute risk to guide statin recommendations is based on the finding that the relative risk reduction seen with statin therapy is independent of baseline risk; thus, those with the highest absolute baseline CVD risk experience the greatest reduction in CVD events.
However, the relative risk reduction of lipid-lowering therapy is also proportional to mmol/dL reduction in LDL level. This supports the contention that those with the highest baseline LDL levels should benefit the most from treatment because they have the most potential decline in LDL with intervention. One way to reconcile these findings is to incorporate both LDL levels and CVD risk into treatment recommendations, as has been done in the European guidelines. This approach recognizes that the relative benefit of statins is proportional to LDL lowering but that the absolute treatment benefit is largely driven by baseline risk.
From the resource perspective, the vast majority of statins are now available as generic products and require limited monitoring, leading to quite modest therapeutic costs. For patients in the gray area not covered by the guidelines, clinicians should be cautioned against adopting either a “treat none” or a “treat all” strategy. Rather, gaps in the evidence provide opportunities for clinicians to practice the art of medicine and engage with patients in shared decision-making regarding strategies for CVD prevention.
Ann Marie Navar, MD, PhD, and Eric Peterson, MD, MPH, are both at the Duke Clinical Research Institute in Durham, N.C. Dr. Navar reported funding from Regeneron and Sanofi. Dr. Peterson reported funding from Merck, Sanofi, Regeneron, and AstraZeneca. They made their comments in an editorial to the USPSTF report (JAMA. 2016 Nov;316[19]:1981-3).
Use of statins for the primary prevention of cardiovascular disease should be based on overall cardiovascular disease risk, not necessarily blood lipid levels, according to new recommendations from the United States Preventive Services Task Force.
Despite widespread use in the elderly, USPSTF also said there’s insufficient evidence to recommend starting statins for cardiovascular disease (CVD) prevention in patients 76 years or older (JAMA. 2016 Nov;316[19]:1997-2007).
USPSTF now recommends low- to moderate-dose statins for primary prevention in adults 40-75 years old who have at least one CVD risk factor – dyslipidemia, diabetes, hypertension, or smoking – and a 10-year CVD event risk of at least 10% (B grade). It also recommended “selectively” offering low- to moderate-dose statins if patients have a 7.5%-10% risk (C grade). The task force recommends using the online American College of Cardiology/American Heart Association risk calculator.
A B grade denotes that USPSTF recommends the service as one where there is “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.” With a C grade, the task force recommends that the service be provided subject to professional judgment and patient preferences.
The new advices from USPSTF is consistent with the task force’s 2015 draft proposals, and is based on a pooled analysis of 19 randomized trials in 71,344 adults at risk for CVD but without prior events; 17 of the studies were sponsored at least in part by the companies that make statins. The median duration of follow-up was 3 years. Trials included atorvastatin (Lipitor) and six other statins from 1994-2016.
Few of the 19 trials enrolled patients older than 75 years, so “the balance of benefits and harms of initiating statin use for” primary prevention in elderly adults “cannot be determined.” The task force did not make a recommendation either way, and called for further investigation.
For younger patients, the scales tipped toward benefit when the group found no overall increased risk of cancer, liver damage, diabetes, or cognitive problems with statins for primary prevention. “Although muscle pain, soreness, or weakness are commonly reported with statin use, there were no statistically significant differences between the intervention and control groups for myalgia, myopathy, or rhabdomyolysis,” according to the task force.
Across the 19 trials, statin therapy was associated with a statistically significant 14% reduction in all-cause mortality; 31% reduction in cardiovascular mortality; 29% reduction in stroke; and a 36% reduction in myocardial infarction; 250 patients needed to be treated to prevent one death from any cause after 1-6 years, and 233 to prevent one cardiovascular death after 2-6 years.
The recommendations call for low to moderate doses because that’s what most of the studies used, and there were no clear benefits when trials stratified patients by dose.
USPSTF cautioned that “reliance on a risk calculator ... alone as a basis for prevention may be problematic, given its possible overestimation of risk in some populations” and noted that the benefits “of statin use may be linear according to a patient’s absolute risk level, and any cut points used are only population estimates of benefits.”
The recommendations do not pertain to adults with very high CVD risk, such as those with familial hypercholesterolemia or an LDL level greater than 190mg/dL, since they were excluded from primary prevention trials.
While the USPSTF was careful in its evidence review, “ the limitations of the evidence were not considered sufficiently, given the serious concerns about the harms of statins for primary prevention,” Rita Redberg, MD, and Mitchell Katz, MD, wrote in an editorial accompanying the recommendations.
“USPSTF also did not have access to patient-level data; they had to rely on peer-reviewed published reports,” according to the editorialists. “The actual trial data are largely held by the Cholesterol Treatment Trialists’ Collaboration on behalf of industry sponsors and have not been made available to other researchers, despite multiple requests over many years.” Further, many of the trials were industry sponsored.
Using the USPSTF data, only 2% of patients who take statins for 5 years will avoid a myocardial infarction; virtually all (98%) will not experience any benefit. At the same time, 5%-20% will experience side effects including rhabdomyolysis, cognitive dysfunction, and increased risk of diabetes (JAMA. 2016 Nov;316[19]:1979-81).
There are unintended consequences of widespread statin use in healthy persons, Dr. Redberg and Dr. Katz wrote. People taking statins are more likely to become obese and more sedentary over time, likely because they mistakenly think they do not need to eat a healthy diet and exercise.
The USPSTF analysis was funded by the Agency for Healthcare Research and Quality. Task force lead Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco, has advised the Institute for Clinical and Economic Review, which is partly funded by industry, on the cost-effectiveness of lipid-lowering drugs. Another member reported comparing how well they worked. The other 15 task force members had no disclosures.
Use of statins for the primary prevention of cardiovascular disease should be based on overall cardiovascular disease risk, not necessarily blood lipid levels, according to new recommendations from the United States Preventive Services Task Force.
Despite widespread use in the elderly, USPSTF also said there’s insufficient evidence to recommend starting statins for cardiovascular disease (CVD) prevention in patients 76 years or older (JAMA. 2016 Nov;316[19]:1997-2007).
USPSTF now recommends low- to moderate-dose statins for primary prevention in adults 40-75 years old who have at least one CVD risk factor – dyslipidemia, diabetes, hypertension, or smoking – and a 10-year CVD event risk of at least 10% (B grade). It also recommended “selectively” offering low- to moderate-dose statins if patients have a 7.5%-10% risk (C grade). The task force recommends using the online American College of Cardiology/American Heart Association risk calculator.
A B grade denotes that USPSTF recommends the service as one where there is “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.” With a C grade, the task force recommends that the service be provided subject to professional judgment and patient preferences.
The new advices from USPSTF is consistent with the task force’s 2015 draft proposals, and is based on a pooled analysis of 19 randomized trials in 71,344 adults at risk for CVD but without prior events; 17 of the studies were sponsored at least in part by the companies that make statins. The median duration of follow-up was 3 years. Trials included atorvastatin (Lipitor) and six other statins from 1994-2016.
Few of the 19 trials enrolled patients older than 75 years, so “the balance of benefits and harms of initiating statin use for” primary prevention in elderly adults “cannot be determined.” The task force did not make a recommendation either way, and called for further investigation.
For younger patients, the scales tipped toward benefit when the group found no overall increased risk of cancer, liver damage, diabetes, or cognitive problems with statins for primary prevention. “Although muscle pain, soreness, or weakness are commonly reported with statin use, there were no statistically significant differences between the intervention and control groups for myalgia, myopathy, or rhabdomyolysis,” according to the task force.
Across the 19 trials, statin therapy was associated with a statistically significant 14% reduction in all-cause mortality; 31% reduction in cardiovascular mortality; 29% reduction in stroke; and a 36% reduction in myocardial infarction; 250 patients needed to be treated to prevent one death from any cause after 1-6 years, and 233 to prevent one cardiovascular death after 2-6 years.
The recommendations call for low to moderate doses because that’s what most of the studies used, and there were no clear benefits when trials stratified patients by dose.
USPSTF cautioned that “reliance on a risk calculator ... alone as a basis for prevention may be problematic, given its possible overestimation of risk in some populations” and noted that the benefits “of statin use may be linear according to a patient’s absolute risk level, and any cut points used are only population estimates of benefits.”
The recommendations do not pertain to adults with very high CVD risk, such as those with familial hypercholesterolemia or an LDL level greater than 190mg/dL, since they were excluded from primary prevention trials.
While the USPSTF was careful in its evidence review, “ the limitations of the evidence were not considered sufficiently, given the serious concerns about the harms of statins for primary prevention,” Rita Redberg, MD, and Mitchell Katz, MD, wrote in an editorial accompanying the recommendations.
“USPSTF also did not have access to patient-level data; they had to rely on peer-reviewed published reports,” according to the editorialists. “The actual trial data are largely held by the Cholesterol Treatment Trialists’ Collaboration on behalf of industry sponsors and have not been made available to other researchers, despite multiple requests over many years.” Further, many of the trials were industry sponsored.
Using the USPSTF data, only 2% of patients who take statins for 5 years will avoid a myocardial infarction; virtually all (98%) will not experience any benefit. At the same time, 5%-20% will experience side effects including rhabdomyolysis, cognitive dysfunction, and increased risk of diabetes (JAMA. 2016 Nov;316[19]:1979-81).
There are unintended consequences of widespread statin use in healthy persons, Dr. Redberg and Dr. Katz wrote. People taking statins are more likely to become obese and more sedentary over time, likely because they mistakenly think they do not need to eat a healthy diet and exercise.
The USPSTF analysis was funded by the Agency for Healthcare Research and Quality. Task force lead Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco, has advised the Institute for Clinical and Economic Review, which is partly funded by industry, on the cost-effectiveness of lipid-lowering drugs. Another member reported comparing how well they worked. The other 15 task force members had no disclosures.