User login
FDA Panel Votes for Limits on Gastric, Esophageal Cancer Immunotherapy
During the meeting, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted in favor of restricting the use of these immunotherapy agents to patients with programmed death-ligand 1 (PD-L1) expression of 1% or higher.
The agency usually follows the ODAC’s advice.
The FDA had originally approved the two immune checkpoint inhibitors for both indications in combination with chemotherapy, regardless of patients’ PD-L1 status. The FDA had also approved nivolumab in combination with ipilimumab for esophageal cancer, regardless of PD-L1 expression. The approvals were based on overall benefit in intent-to-treat populations, not on specific PD-L1 expression subgroups.
Since then, additional studies — including the agency’s own pooled analyses of the approval trials — have found that overall survival benefits are limited to patients with PD-L1 expression of 1% or higher.
These findings have raised concerns that patients with no or low PD-L1 expression face the risks associated with immunotherapy, which include death, but minimal to no benefits.
In response, the FDA has considered changing the labeling for these indications to require a PD-L1 cutoff point of 1% or higher. The move would mirror guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network that already recommend use with chemotherapy only at certain PD-L1 cutoffs.
Before the agency acts, however, the FDA wanted the advice of the ODAC. It asked the 14-member panel whether the risk-benefit assessment is “favorable for the use of PD-1 inhibitors in first line” for the two indications among patients with PD-L1 expression below 1%.
In two nearly unanimous decisions for each indication, the panel voted that risk-benefit assessment was not favorable. In other words, the risks do outweigh the benefits for this patient population with no or low PD-L1 expression.
The determination also applies to tislelizumab (Tevimbra), an immune checkpoint inhibitor under review by the FDA for the same indications.
Voting came after hours of testimony from FDA scientists and the three drug companies involved in the decisions.
Merck, maker of pembrolizumab, was against any labeling change. Nivolumab’s maker, Bristol Myers Squibb (BMS), also wanted to stick with the current PD-L1 agnostic indications but said that any indication change should be class-wide to avoid confusion. BeiGene USA, maker of tislelizumab, had no problem with a PD-L1 cutoff of 1% because its approval trial showed benefit only in patients at that level or higher.
In general, Merck and BMS said the drug benefits correspond with higher PD-L1 expression but noted that patients with low or no PD-L1 expression can sometimes benefit from treatment. The companies had several patients testify to the benefits of the agents and noted patients like this would likely lose access. But an ODAC panelist noted that patients who died from immunotherapy complications weren’t there to respond.
The companies also expressed concern about taking treatment decisions out of the hands of oncologists as well as the need for additional biopsies to determine if tumors cross the proposed PD-L1 threshold at some point during treatment. With this potential new restriction, the companies were worried that insurance companies would be even less likely to pay for their checkpoint inhibitors in low or no PD-L1 expressors.
ODAC wasn’t moved. With consistent findings across multiple trials, the strength of the FDA’s data carried the day.
In a pooled analysis of the three companies’ unresectable or metastatic HER2–negative, microsatellite-stable gastric/gastroesophageal adenocarcinoma approval trials across almost 4000 patients, those with PD-L1 levels below 1% did not demonstrate a significant overall survival benefit (hazard ratio [HR], 0.91; 95% CI, 0.75-1.09). The median overall survival with immunotherapy plus chemotherapy was only 1 month more — 13.4 months vs 12.4 months with chemotherapy alone.
FDA’s pooled analysis for unresectable or metastatic esophageal squamous cell carcinoma also showed no overall survival benefit (HR, 1.1; 95% CI, 0.76-1.58), with a trend suggesting harm. Median overall survival with immunotherapy plus chemotherapy was 14.6 months vs 9.8 months with chemotherapy alone.
Despite the vote on esophageal squamous cell carcinoma, panelists had reservations about making decisions based on just over 160 patients with PD-L1 levels below 1% in the three esophageal squamous cell carcinoma trials.
Still, one panelist said, it’s likely “the best dataset we will get.”
The companies all used different methods to test PD-L1 levels, and attendees called for a single standardized PD-L1 test. Richard Pazdur, MD, head of the FDA’s Oncology Center of Excellence, said the agency has been working with companies for years to get them to agree to such a test, with no luck.
If the FDA ultimately decides to restrict immunotherapy use in this patient population based on PD-L1 levels, insurance company coverage may become more limited. Pazdur asked the companies if they would be willing to expand their patient assistance programs to provide free coverage of immune checkpoint blockers to patients with low or no PD-L1 expression.
BeiGene and BMS seemed open to the idea. Merck said, “We’ll have to ... think about it.”
A version of this article first appeared on Medscape.com.
During the meeting, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted in favor of restricting the use of these immunotherapy agents to patients with programmed death-ligand 1 (PD-L1) expression of 1% or higher.
The agency usually follows the ODAC’s advice.
The FDA had originally approved the two immune checkpoint inhibitors for both indications in combination with chemotherapy, regardless of patients’ PD-L1 status. The FDA had also approved nivolumab in combination with ipilimumab for esophageal cancer, regardless of PD-L1 expression. The approvals were based on overall benefit in intent-to-treat populations, not on specific PD-L1 expression subgroups.
Since then, additional studies — including the agency’s own pooled analyses of the approval trials — have found that overall survival benefits are limited to patients with PD-L1 expression of 1% or higher.
These findings have raised concerns that patients with no or low PD-L1 expression face the risks associated with immunotherapy, which include death, but minimal to no benefits.
In response, the FDA has considered changing the labeling for these indications to require a PD-L1 cutoff point of 1% or higher. The move would mirror guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network that already recommend use with chemotherapy only at certain PD-L1 cutoffs.
Before the agency acts, however, the FDA wanted the advice of the ODAC. It asked the 14-member panel whether the risk-benefit assessment is “favorable for the use of PD-1 inhibitors in first line” for the two indications among patients with PD-L1 expression below 1%.
In two nearly unanimous decisions for each indication, the panel voted that risk-benefit assessment was not favorable. In other words, the risks do outweigh the benefits for this patient population with no or low PD-L1 expression.
The determination also applies to tislelizumab (Tevimbra), an immune checkpoint inhibitor under review by the FDA for the same indications.
Voting came after hours of testimony from FDA scientists and the three drug companies involved in the decisions.
Merck, maker of pembrolizumab, was against any labeling change. Nivolumab’s maker, Bristol Myers Squibb (BMS), also wanted to stick with the current PD-L1 agnostic indications but said that any indication change should be class-wide to avoid confusion. BeiGene USA, maker of tislelizumab, had no problem with a PD-L1 cutoff of 1% because its approval trial showed benefit only in patients at that level or higher.
In general, Merck and BMS said the drug benefits correspond with higher PD-L1 expression but noted that patients with low or no PD-L1 expression can sometimes benefit from treatment. The companies had several patients testify to the benefits of the agents and noted patients like this would likely lose access. But an ODAC panelist noted that patients who died from immunotherapy complications weren’t there to respond.
The companies also expressed concern about taking treatment decisions out of the hands of oncologists as well as the need for additional biopsies to determine if tumors cross the proposed PD-L1 threshold at some point during treatment. With this potential new restriction, the companies were worried that insurance companies would be even less likely to pay for their checkpoint inhibitors in low or no PD-L1 expressors.
ODAC wasn’t moved. With consistent findings across multiple trials, the strength of the FDA’s data carried the day.
In a pooled analysis of the three companies’ unresectable or metastatic HER2–negative, microsatellite-stable gastric/gastroesophageal adenocarcinoma approval trials across almost 4000 patients, those with PD-L1 levels below 1% did not demonstrate a significant overall survival benefit (hazard ratio [HR], 0.91; 95% CI, 0.75-1.09). The median overall survival with immunotherapy plus chemotherapy was only 1 month more — 13.4 months vs 12.4 months with chemotherapy alone.
FDA’s pooled analysis for unresectable or metastatic esophageal squamous cell carcinoma also showed no overall survival benefit (HR, 1.1; 95% CI, 0.76-1.58), with a trend suggesting harm. Median overall survival with immunotherapy plus chemotherapy was 14.6 months vs 9.8 months with chemotherapy alone.
Despite the vote on esophageal squamous cell carcinoma, panelists had reservations about making decisions based on just over 160 patients with PD-L1 levels below 1% in the three esophageal squamous cell carcinoma trials.
Still, one panelist said, it’s likely “the best dataset we will get.”
The companies all used different methods to test PD-L1 levels, and attendees called for a single standardized PD-L1 test. Richard Pazdur, MD, head of the FDA’s Oncology Center of Excellence, said the agency has been working with companies for years to get them to agree to such a test, with no luck.
If the FDA ultimately decides to restrict immunotherapy use in this patient population based on PD-L1 levels, insurance company coverage may become more limited. Pazdur asked the companies if they would be willing to expand their patient assistance programs to provide free coverage of immune checkpoint blockers to patients with low or no PD-L1 expression.
BeiGene and BMS seemed open to the idea. Merck said, “We’ll have to ... think about it.”
A version of this article first appeared on Medscape.com.
During the meeting, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted in favor of restricting the use of these immunotherapy agents to patients with programmed death-ligand 1 (PD-L1) expression of 1% or higher.
The agency usually follows the ODAC’s advice.
The FDA had originally approved the two immune checkpoint inhibitors for both indications in combination with chemotherapy, regardless of patients’ PD-L1 status. The FDA had also approved nivolumab in combination with ipilimumab for esophageal cancer, regardless of PD-L1 expression. The approvals were based on overall benefit in intent-to-treat populations, not on specific PD-L1 expression subgroups.
Since then, additional studies — including the agency’s own pooled analyses of the approval trials — have found that overall survival benefits are limited to patients with PD-L1 expression of 1% or higher.
These findings have raised concerns that patients with no or low PD-L1 expression face the risks associated with immunotherapy, which include death, but minimal to no benefits.
In response, the FDA has considered changing the labeling for these indications to require a PD-L1 cutoff point of 1% or higher. The move would mirror guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network that already recommend use with chemotherapy only at certain PD-L1 cutoffs.
Before the agency acts, however, the FDA wanted the advice of the ODAC. It asked the 14-member panel whether the risk-benefit assessment is “favorable for the use of PD-1 inhibitors in first line” for the two indications among patients with PD-L1 expression below 1%.
In two nearly unanimous decisions for each indication, the panel voted that risk-benefit assessment was not favorable. In other words, the risks do outweigh the benefits for this patient population with no or low PD-L1 expression.
The determination also applies to tislelizumab (Tevimbra), an immune checkpoint inhibitor under review by the FDA for the same indications.
Voting came after hours of testimony from FDA scientists and the three drug companies involved in the decisions.
Merck, maker of pembrolizumab, was against any labeling change. Nivolumab’s maker, Bristol Myers Squibb (BMS), also wanted to stick with the current PD-L1 agnostic indications but said that any indication change should be class-wide to avoid confusion. BeiGene USA, maker of tislelizumab, had no problem with a PD-L1 cutoff of 1% because its approval trial showed benefit only in patients at that level or higher.
In general, Merck and BMS said the drug benefits correspond with higher PD-L1 expression but noted that patients with low or no PD-L1 expression can sometimes benefit from treatment. The companies had several patients testify to the benefits of the agents and noted patients like this would likely lose access. But an ODAC panelist noted that patients who died from immunotherapy complications weren’t there to respond.
The companies also expressed concern about taking treatment decisions out of the hands of oncologists as well as the need for additional biopsies to determine if tumors cross the proposed PD-L1 threshold at some point during treatment. With this potential new restriction, the companies were worried that insurance companies would be even less likely to pay for their checkpoint inhibitors in low or no PD-L1 expressors.
ODAC wasn’t moved. With consistent findings across multiple trials, the strength of the FDA’s data carried the day.
In a pooled analysis of the three companies’ unresectable or metastatic HER2–negative, microsatellite-stable gastric/gastroesophageal adenocarcinoma approval trials across almost 4000 patients, those with PD-L1 levels below 1% did not demonstrate a significant overall survival benefit (hazard ratio [HR], 0.91; 95% CI, 0.75-1.09). The median overall survival with immunotherapy plus chemotherapy was only 1 month more — 13.4 months vs 12.4 months with chemotherapy alone.
FDA’s pooled analysis for unresectable or metastatic esophageal squamous cell carcinoma also showed no overall survival benefit (HR, 1.1; 95% CI, 0.76-1.58), with a trend suggesting harm. Median overall survival with immunotherapy plus chemotherapy was 14.6 months vs 9.8 months with chemotherapy alone.
Despite the vote on esophageal squamous cell carcinoma, panelists had reservations about making decisions based on just over 160 patients with PD-L1 levels below 1% in the three esophageal squamous cell carcinoma trials.
Still, one panelist said, it’s likely “the best dataset we will get.”
The companies all used different methods to test PD-L1 levels, and attendees called for a single standardized PD-L1 test. Richard Pazdur, MD, head of the FDA’s Oncology Center of Excellence, said the agency has been working with companies for years to get them to agree to such a test, with no luck.
If the FDA ultimately decides to restrict immunotherapy use in this patient population based on PD-L1 levels, insurance company coverage may become more limited. Pazdur asked the companies if they would be willing to expand their patient assistance programs to provide free coverage of immune checkpoint blockers to patients with low or no PD-L1 expression.
BeiGene and BMS seemed open to the idea. Merck said, “We’ll have to ... think about it.”
A version of this article first appeared on Medscape.com.
Melanoma: Neoadjuvant Immunotherapy Provides Optimal Survival Results
BARCELONA, SPAIN — with immunotherapy or a targeted agent or targeted therapy plus immunotherapy, according to a large-scale pooled analysis from the International Neoadjuvant Melanoma Consortium.
Importantly, the analysis — presented at the annual meeting of the European Society for Medical Oncology — showed that achieving a major pathological response to neoadjuvant therapy is a key indicator of survival outcomes.
After 3 years of follow-up, the results showed that neoadjuvant therapy is not delaying melanoma recurrence, “it’s actually preventing it,” coinvestigator Hussein A. Tawbi, MD, PhD, Department of Melanoma Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, said in an interview. That’s “a big deal.”
Since 2010, the introduction of novel adjuvant and neoadjuvant therapies for high-risk stage III resectable melanoma has led to incremental gains for patients, said Georgina V. Long, MD, PhD, BSc, chair of Melanoma Medical Oncology and Translational Research at the University of Sydney in Australia, who presented the results.
The first pooled analysis of neoadjuvant therapy in 189 patients, published in 2021, indicated that those who achieved a major pathological response — defined as either a pathological complete response (with no remaining vital tumor) or a near-complete pathological response (with vital tumor ≤ 10%) — had the best recurrence-free survival rates.
In the current study, the researchers expanded their cohort to include 818 patients from 18 centers. Patients received at least one dose of neoadjuvant therapy — either combination immunotherapy, combination of targeted and immunotherapy agents, or monotherapy with either an immune checkpoint inhibitor or a targeted agent.
The median age was 59 years, and 38% of patients were women. The median follow-up so far is 38.8 months.
Overall, the 3-year event-free survival was 74% in patients who received any immunotherapy, 72% in those who received immunotherapy plus a targeted BRAF/MEK therapy, and just 37% in those who received targeted therapy alone. Similarly, 3-year recurrence-free survival rates were highest in patients who received immunotherapy at 77% vs 73% in those who received immunotherapy plus a targeted BRAF/MEK therapy and just 37% in those who received targeted therapy alone.
Looking specifically at progressive death 1 (PD-1)–based immunotherapy regimens, combination therapy led to a 3-year event-free survival rate between 77% and 95%, depending on the specific combinations, vs 64% with PD-1 monotherapy and 37% with combination targeted therapy.
Overall, patients who had a major pathological response were more likely to be recurrence free at 3 years. The 3-year recurrence-free survival was 88% in patients with a complete response, 68% in those with a partial pathological response, and 40% in those without a response.
Patients who received immunotherapy were more likely to have major pathological response. The 3-year recurrence-free survival was about 94% in patients who received combination or monotherapy with immune checkpoint inhibition, and about 87% in those who received immunotherapy plus targeted therapy. The recurrence-free survival rate was much lower in patients given only BRAF/MEK inhibitors.
The current overall survival data, which are still immature, suggested a few differences when stratifying the patients by treatment. Almost all patients with a major pathological response were alive at 3 years, compared with 86% of those with a partial pathological response and 70% of those without a pathological response.
Overall, the results showed that immunotherapy — as either combination or monotherapy — is “quite a bit” better than targeted therapy with BRAF/MEK agents, which offers no substantial benefit, said Dr. Twabi.
“When you see the same pattern happening in study after study, in a very clear, robust way, it actually becomes very powerful,” he explained.
Rebecca A. Dent, MD, MSc, chair of the ESMO Scientific Committee who was not involved in the study, told a press conference that the introduction of immunotherapy and combination immunotherapy has dramatically changed outcomes in melanoma.
Commenting on the current study results, Dr. Dent said that “combination immunotherapy is clearly showing exceptional stability in terms of long-term benefits.”
The question now is what are the toxicities and costs that come with combination immunotherapy, said Dr. Dent, from National Cancer Centre Singapore and Duke-NUS Medical School, Singapore.
No funding source was declared. Dr. Long declared relationships with a variety of companies, including AstraZeneca UK Limited, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, and Regeneron. Dr. Twabi declared relationships with Bristol-Myers Squibb, Novartis, Merck, Genentech, GlaxoSmithKline, Eisai, and others. Dr. Dent declared relationships with AstraZeneca, Roche, Eisai, Gilead Sciences, Eli Lilly, Merck, and Pfizer.
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — with immunotherapy or a targeted agent or targeted therapy plus immunotherapy, according to a large-scale pooled analysis from the International Neoadjuvant Melanoma Consortium.
Importantly, the analysis — presented at the annual meeting of the European Society for Medical Oncology — showed that achieving a major pathological response to neoadjuvant therapy is a key indicator of survival outcomes.
After 3 years of follow-up, the results showed that neoadjuvant therapy is not delaying melanoma recurrence, “it’s actually preventing it,” coinvestigator Hussein A. Tawbi, MD, PhD, Department of Melanoma Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, said in an interview. That’s “a big deal.”
Since 2010, the introduction of novel adjuvant and neoadjuvant therapies for high-risk stage III resectable melanoma has led to incremental gains for patients, said Georgina V. Long, MD, PhD, BSc, chair of Melanoma Medical Oncology and Translational Research at the University of Sydney in Australia, who presented the results.
The first pooled analysis of neoadjuvant therapy in 189 patients, published in 2021, indicated that those who achieved a major pathological response — defined as either a pathological complete response (with no remaining vital tumor) or a near-complete pathological response (with vital tumor ≤ 10%) — had the best recurrence-free survival rates.
In the current study, the researchers expanded their cohort to include 818 patients from 18 centers. Patients received at least one dose of neoadjuvant therapy — either combination immunotherapy, combination of targeted and immunotherapy agents, or monotherapy with either an immune checkpoint inhibitor or a targeted agent.
The median age was 59 years, and 38% of patients were women. The median follow-up so far is 38.8 months.
Overall, the 3-year event-free survival was 74% in patients who received any immunotherapy, 72% in those who received immunotherapy plus a targeted BRAF/MEK therapy, and just 37% in those who received targeted therapy alone. Similarly, 3-year recurrence-free survival rates were highest in patients who received immunotherapy at 77% vs 73% in those who received immunotherapy plus a targeted BRAF/MEK therapy and just 37% in those who received targeted therapy alone.
Looking specifically at progressive death 1 (PD-1)–based immunotherapy regimens, combination therapy led to a 3-year event-free survival rate between 77% and 95%, depending on the specific combinations, vs 64% with PD-1 monotherapy and 37% with combination targeted therapy.
Overall, patients who had a major pathological response were more likely to be recurrence free at 3 years. The 3-year recurrence-free survival was 88% in patients with a complete response, 68% in those with a partial pathological response, and 40% in those without a response.
Patients who received immunotherapy were more likely to have major pathological response. The 3-year recurrence-free survival was about 94% in patients who received combination or monotherapy with immune checkpoint inhibition, and about 87% in those who received immunotherapy plus targeted therapy. The recurrence-free survival rate was much lower in patients given only BRAF/MEK inhibitors.
The current overall survival data, which are still immature, suggested a few differences when stratifying the patients by treatment. Almost all patients with a major pathological response were alive at 3 years, compared with 86% of those with a partial pathological response and 70% of those without a pathological response.
Overall, the results showed that immunotherapy — as either combination or monotherapy — is “quite a bit” better than targeted therapy with BRAF/MEK agents, which offers no substantial benefit, said Dr. Twabi.
“When you see the same pattern happening in study after study, in a very clear, robust way, it actually becomes very powerful,” he explained.
Rebecca A. Dent, MD, MSc, chair of the ESMO Scientific Committee who was not involved in the study, told a press conference that the introduction of immunotherapy and combination immunotherapy has dramatically changed outcomes in melanoma.
Commenting on the current study results, Dr. Dent said that “combination immunotherapy is clearly showing exceptional stability in terms of long-term benefits.”
The question now is what are the toxicities and costs that come with combination immunotherapy, said Dr. Dent, from National Cancer Centre Singapore and Duke-NUS Medical School, Singapore.
No funding source was declared. Dr. Long declared relationships with a variety of companies, including AstraZeneca UK Limited, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, and Regeneron. Dr. Twabi declared relationships with Bristol-Myers Squibb, Novartis, Merck, Genentech, GlaxoSmithKline, Eisai, and others. Dr. Dent declared relationships with AstraZeneca, Roche, Eisai, Gilead Sciences, Eli Lilly, Merck, and Pfizer.
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — with immunotherapy or a targeted agent or targeted therapy plus immunotherapy, according to a large-scale pooled analysis from the International Neoadjuvant Melanoma Consortium.
Importantly, the analysis — presented at the annual meeting of the European Society for Medical Oncology — showed that achieving a major pathological response to neoadjuvant therapy is a key indicator of survival outcomes.
After 3 years of follow-up, the results showed that neoadjuvant therapy is not delaying melanoma recurrence, “it’s actually preventing it,” coinvestigator Hussein A. Tawbi, MD, PhD, Department of Melanoma Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, said in an interview. That’s “a big deal.”
Since 2010, the introduction of novel adjuvant and neoadjuvant therapies for high-risk stage III resectable melanoma has led to incremental gains for patients, said Georgina V. Long, MD, PhD, BSc, chair of Melanoma Medical Oncology and Translational Research at the University of Sydney in Australia, who presented the results.
The first pooled analysis of neoadjuvant therapy in 189 patients, published in 2021, indicated that those who achieved a major pathological response — defined as either a pathological complete response (with no remaining vital tumor) or a near-complete pathological response (with vital tumor ≤ 10%) — had the best recurrence-free survival rates.
In the current study, the researchers expanded their cohort to include 818 patients from 18 centers. Patients received at least one dose of neoadjuvant therapy — either combination immunotherapy, combination of targeted and immunotherapy agents, or monotherapy with either an immune checkpoint inhibitor or a targeted agent.
The median age was 59 years, and 38% of patients were women. The median follow-up so far is 38.8 months.
Overall, the 3-year event-free survival was 74% in patients who received any immunotherapy, 72% in those who received immunotherapy plus a targeted BRAF/MEK therapy, and just 37% in those who received targeted therapy alone. Similarly, 3-year recurrence-free survival rates were highest in patients who received immunotherapy at 77% vs 73% in those who received immunotherapy plus a targeted BRAF/MEK therapy and just 37% in those who received targeted therapy alone.
Looking specifically at progressive death 1 (PD-1)–based immunotherapy regimens, combination therapy led to a 3-year event-free survival rate between 77% and 95%, depending on the specific combinations, vs 64% with PD-1 monotherapy and 37% with combination targeted therapy.
Overall, patients who had a major pathological response were more likely to be recurrence free at 3 years. The 3-year recurrence-free survival was 88% in patients with a complete response, 68% in those with a partial pathological response, and 40% in those without a response.
Patients who received immunotherapy were more likely to have major pathological response. The 3-year recurrence-free survival was about 94% in patients who received combination or monotherapy with immune checkpoint inhibition, and about 87% in those who received immunotherapy plus targeted therapy. The recurrence-free survival rate was much lower in patients given only BRAF/MEK inhibitors.
The current overall survival data, which are still immature, suggested a few differences when stratifying the patients by treatment. Almost all patients with a major pathological response were alive at 3 years, compared with 86% of those with a partial pathological response and 70% of those without a pathological response.
Overall, the results showed that immunotherapy — as either combination or monotherapy — is “quite a bit” better than targeted therapy with BRAF/MEK agents, which offers no substantial benefit, said Dr. Twabi.
“When you see the same pattern happening in study after study, in a very clear, robust way, it actually becomes very powerful,” he explained.
Rebecca A. Dent, MD, MSc, chair of the ESMO Scientific Committee who was not involved in the study, told a press conference that the introduction of immunotherapy and combination immunotherapy has dramatically changed outcomes in melanoma.
Commenting on the current study results, Dr. Dent said that “combination immunotherapy is clearly showing exceptional stability in terms of long-term benefits.”
The question now is what are the toxicities and costs that come with combination immunotherapy, said Dr. Dent, from National Cancer Centre Singapore and Duke-NUS Medical School, Singapore.
No funding source was declared. Dr. Long declared relationships with a variety of companies, including AstraZeneca UK Limited, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, and Regeneron. Dr. Twabi declared relationships with Bristol-Myers Squibb, Novartis, Merck, Genentech, GlaxoSmithKline, Eisai, and others. Dr. Dent declared relationships with AstraZeneca, Roche, Eisai, Gilead Sciences, Eli Lilly, Merck, and Pfizer.
A version of this article appeared on Medscape.com.
FROM ESMO 2024
Cancer Treatment 101: A Primer for Non-Oncologists
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
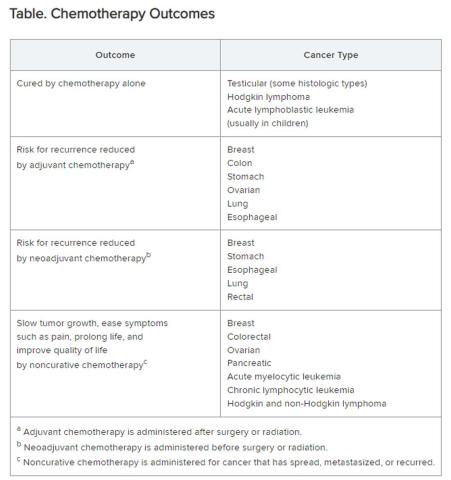
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Diagnosing, Treating Rashes In Patients on Immune Checkpoint Inhibitors
WASHINGTON, DC — and with judicious usage and dosing of prednisone when deemed necessary, Blair Allais, MD, said during a session on supportive oncodermatology at the ElderDerm conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“It’s important when you see these patients to be as specific as possible” based on morphology and histopathology, and to treat the rashes in a similar way as in the non-ICI setting,” said Dr. Allais, a dermato-oncologist at the Inova Schar Cancer Institute, Fairfax, Virginia.
cirAEs are the most frequently reported and most visible adverse effects of checkpoint inhibition — a treatment that has emerged as a standard therapy for many malignancies since the first ICI was approved in 2011 for metastatic melanoma.
And contrary to what the phenomenon of immunosenescence might suggest, older patients are no less prone to cirAEs than younger patients. “You’d think you’d have fewer rashes and side effects as you age, but that’s not true,” said Dr. Allais, who completed a fellowship in cutaneous oncology after her dermatology residency.
A 2021 multicenter international cohort study of over 900 patients aged ≥ 80 years treated with single-agent ICIs for cancer did not find any significant differences in the development of immune-related adverse events among those younger than 85, those aged 85-89 years, and those 90 and older. Neither did the ELDERS study in the United Kingdom; this prospective observational study found similar rates of high-grade and low-grade immune toxicity in its two cohorts of patients ≥ 70 and < 70 years of age.
At the meeting, Dr. Allais, who coauthored a 2023 review of cirAEs from ICIs, reviewed recent developments and provided the following advice:
New diagnostic criteria: “Really exciting” news for more precise diagnosis and optimal therapy of cirAEs, Dr. Allais said, is a position paper published in the Journal for ImmunoTherapy of Cancer that offers consensus-based diagnostic criteria for the 10 most common types of dermatologic immune-related adverse events and an overall diagnostic framework. “Luckily, through the work of a Delphi consensus group, we can now have [more diagnostic specificity],” which is important for both clinical care and research, she said.
Most cirAEs have typically been reported nonspecifically as “rash,” but diagnosing a rash subtype is “critical in tailoring appropriate therapy that it is both effective and the least detrimental to the oncology treatment plan for patients with cancer,” the group’s coauthors wrote.
The 10 core diagnoses include psoriasis, eczematous dermatitis, vitiligo, Grover disease, eruptive atypical squamous proliferation, and bullous pemphigoid. Outside of the core diagnoses are other nonspecific presentations that require evaluation to arrive at a diagnosis, if possible, or to reveal data that can allow for targeted therapy and severity grading, the group explains in its paper.
“To prednisone or not to prednisone”: The development of cirAEs is associated with reduced mortality and improved cancer outcomes, making the use of immunosuppressants such as corticosteroids a therapeutic dilemma. “Patients who get these rashes usually do better with respect to their cancer, so the concern has been, if we affect how they respond to their immunotherapy, we may minimize that improvement in mortality,” said Dr. Allais, also assistant professor at the University of Virginia, Charlottesville, and clinical assistant professor of dermatology at George Washington University.
A widely discussed study published in 2015 reported on 254 patients with melanoma who developed an immune-related adverse event during treatment with ipilimumab — approximately one third of whom required systemic corticosteroids — and concluded that systemic corticosteroids did not affect overall survival or time to (cancer) treatment failure. This study from Memorial Sloan Kettering Cancer Center, New York City, “was the first large study looking at this question,” she said, and the subsequent message for several years in conferences and the literature was that steroids do not affect the efficacy of checkpoint inhibitors.
“But the study was not without limitations,” Dr. Allais said, “because the patients who got prednisone were mainly those with higher-grade toxicities,” while those not treated with corticosteroids had either no toxicities or low-grade toxicities. “If higher-grade toxicities were associated with better (antitumor) response, the steroids may have just [blunted] that benefit.”
The current totality of data available in the literature suggests that corticosteroids may indeed have an impact on the efficacy of ICI therapy. “Subsequent studies have come out in the community that have shown that we should probably think twice about giving prednisone to some patients, particularly within the first 50 days of ICI treatment, and that we should be mindful of the dose,” Dr. Allais said.
The takeaways from these studies — all published in the past few years — are to use prednisone early and liberally for life-threatening toxicity, to use it at the lowest dose and for the shortest course when there is not an appropriate alternative, to avoid it for diagnoses that are not treated with prednisone outside the ICI setting, and to “have a plan” for a steroid-sparing agent to use after prednisone, she said.
Dr. Allais recommends heightened consideration during the first 50 days of ICI treatment based on a multicenter retrospective study that found a significant association between use of high-dose glucocorticoids (≥ 60 mg prednisone equivalent once a day) within 8 weeks of anti–programmed cell death protein 1 (PD-1) monotherapy initiation and poorer progression-free and overall survival. The study covered a cohort of 947 patients with advanced melanoma treated with anti–PD-1 monotherapy between 2009 and 2019, 54% of whom developed immune-related adverse events.
This study and other recent studies addressing the association between steroids and survival outcomes in patients with immune-related adverse events during ICI therapy are described in Dr. Allais’ 2023 review of cirAEs from ICIs.
Approach to morbilliform eruptions: This rash is “super common” in patients on ICIs, occurring generally within 2-3 weeks of starting treatment. “It tends to be self-limited and can recur with future infusions,” Dr. Allais said.
Systemic steroids should be reserved for severe or refractory eruptions. “Usually, I treat the patients with topical steroids, and I manage their expectations (that the rash may recur with subsequent infusions), but I closely follow them up” within 2-3 weeks, she said. It’s important to rule out a severe cutaneous adverse drug eruption, of course, and to start high-dose systemic steroids immediately if necessary. “Antibiotics are a big culprit” and often can be discontinued.
Soak and smear: “I’m obsessed” with this technique of a 20-minute soak in plain water followed by application of steroid ointment, said Dr. Allais, referring to a small study published in 2005 that reported a complete response after 2 weeks in 60% of patients with psoriasis, atopic dermatitis, and other inflammatory skin conditions (none had cancer), who had failed prior systemic therapy. All patients had at least a 75% response.
The method offers a way to “avoid the systemic immunosuppression we’d get with prednisone,” she said. One just needs to make sure the older patient can get in and out of their tub safely.
ICI-induced bullous pemphigoid (BP): BP occurs more frequently in the ICI setting, compared with the general population, with a median time to development of 8.5 months after ICI initiation. It is associated in this setting with improved tumor response, but “many oncologists stop anticancer treatment because of this diagnosis,” she said.
In the supportive oncodermatology space, however, ICI-induced BP exemplifies the value of tailored treatment regimens, she said. A small multi-institutional retrospective cohort study published in 2023 identified 35 cases of ICI-BP among 5636 ICI-treated patients and found that 8 out of 11 patients who received biologic therapy (rituximab, omalizumab, or dupilumab) had a complete response to ICI-BP without flares following subsequent ICI cycles. And while statistical significance was not reached, the study showed that no cancer-related outcomes were worsened.
“If you see someone with ICI-induced BP and they have a lot of involvement, you could start them on steroids and get that steroid-sparing agent initiated for approval. ... And if IgE is elevated, you might reach for omalizumab,” said Dr. Allais, noting that her favored treatment overall is dupilumab.
Risk factors for the development of ICI-induced BP include age > 70, skin cancer, and having an initial response to ICI on first imaging, the latter of which “I find fascinating ... because imaging occurs within the first 12 weeks of treatment, but we don’t see BP popping up until 8.5 months into treatment,” she noted. “So maybe there’s a baseline risk factor that could predispose them.”
Caution with antibiotics: “I try to avoid antibiotics in the ICI setting,” Dr. Allais said, in deference to the “ever-important microbiome.” Studies have demonstrated that the microbiomes of responders to ICI treatment are different from those of nonresponders, she said.
And a “fascinating” study of patients with melanoma undergoing ICI therapy showed not only a higher abundance of Ruminococcaceae bacteria in responders vs nonresponders but a significant impact of dietary fiber. High dietary fiber was associated with significantly improved overall survival in the patients on ICI, with the most pronounced benefit in patients with good fiber intake and no probiotic use. “Even wilder, their T cells changed,” she said. “They had a high expression of genes related to T-cell activation ... so more tumor-infiltrating lymphocytes.”
A retrospective study of 568 patients with stages III and IV melanoma treated with ICI showed that those exposed to antibiotics prior to ICI had significantly worse overall survival than those not exposed to antibiotics. “Think before you give them,” Dr. Allais said. “And try to tell your older patients to eat beans and greens.”
Dr. Allais reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — and with judicious usage and dosing of prednisone when deemed necessary, Blair Allais, MD, said during a session on supportive oncodermatology at the ElderDerm conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“It’s important when you see these patients to be as specific as possible” based on morphology and histopathology, and to treat the rashes in a similar way as in the non-ICI setting,” said Dr. Allais, a dermato-oncologist at the Inova Schar Cancer Institute, Fairfax, Virginia.
cirAEs are the most frequently reported and most visible adverse effects of checkpoint inhibition — a treatment that has emerged as a standard therapy for many malignancies since the first ICI was approved in 2011 for metastatic melanoma.
And contrary to what the phenomenon of immunosenescence might suggest, older patients are no less prone to cirAEs than younger patients. “You’d think you’d have fewer rashes and side effects as you age, but that’s not true,” said Dr. Allais, who completed a fellowship in cutaneous oncology after her dermatology residency.
A 2021 multicenter international cohort study of over 900 patients aged ≥ 80 years treated with single-agent ICIs for cancer did not find any significant differences in the development of immune-related adverse events among those younger than 85, those aged 85-89 years, and those 90 and older. Neither did the ELDERS study in the United Kingdom; this prospective observational study found similar rates of high-grade and low-grade immune toxicity in its two cohorts of patients ≥ 70 and < 70 years of age.
At the meeting, Dr. Allais, who coauthored a 2023 review of cirAEs from ICIs, reviewed recent developments and provided the following advice:
New diagnostic criteria: “Really exciting” news for more precise diagnosis and optimal therapy of cirAEs, Dr. Allais said, is a position paper published in the Journal for ImmunoTherapy of Cancer that offers consensus-based diagnostic criteria for the 10 most common types of dermatologic immune-related adverse events and an overall diagnostic framework. “Luckily, through the work of a Delphi consensus group, we can now have [more diagnostic specificity],” which is important for both clinical care and research, she said.
Most cirAEs have typically been reported nonspecifically as “rash,” but diagnosing a rash subtype is “critical in tailoring appropriate therapy that it is both effective and the least detrimental to the oncology treatment plan for patients with cancer,” the group’s coauthors wrote.
The 10 core diagnoses include psoriasis, eczematous dermatitis, vitiligo, Grover disease, eruptive atypical squamous proliferation, and bullous pemphigoid. Outside of the core diagnoses are other nonspecific presentations that require evaluation to arrive at a diagnosis, if possible, or to reveal data that can allow for targeted therapy and severity grading, the group explains in its paper.
“To prednisone or not to prednisone”: The development of cirAEs is associated with reduced mortality and improved cancer outcomes, making the use of immunosuppressants such as corticosteroids a therapeutic dilemma. “Patients who get these rashes usually do better with respect to their cancer, so the concern has been, if we affect how they respond to their immunotherapy, we may minimize that improvement in mortality,” said Dr. Allais, also assistant professor at the University of Virginia, Charlottesville, and clinical assistant professor of dermatology at George Washington University.
A widely discussed study published in 2015 reported on 254 patients with melanoma who developed an immune-related adverse event during treatment with ipilimumab — approximately one third of whom required systemic corticosteroids — and concluded that systemic corticosteroids did not affect overall survival or time to (cancer) treatment failure. This study from Memorial Sloan Kettering Cancer Center, New York City, “was the first large study looking at this question,” she said, and the subsequent message for several years in conferences and the literature was that steroids do not affect the efficacy of checkpoint inhibitors.
“But the study was not without limitations,” Dr. Allais said, “because the patients who got prednisone were mainly those with higher-grade toxicities,” while those not treated with corticosteroids had either no toxicities or low-grade toxicities. “If higher-grade toxicities were associated with better (antitumor) response, the steroids may have just [blunted] that benefit.”
The current totality of data available in the literature suggests that corticosteroids may indeed have an impact on the efficacy of ICI therapy. “Subsequent studies have come out in the community that have shown that we should probably think twice about giving prednisone to some patients, particularly within the first 50 days of ICI treatment, and that we should be mindful of the dose,” Dr. Allais said.
The takeaways from these studies — all published in the past few years — are to use prednisone early and liberally for life-threatening toxicity, to use it at the lowest dose and for the shortest course when there is not an appropriate alternative, to avoid it for diagnoses that are not treated with prednisone outside the ICI setting, and to “have a plan” for a steroid-sparing agent to use after prednisone, she said.
Dr. Allais recommends heightened consideration during the first 50 days of ICI treatment based on a multicenter retrospective study that found a significant association between use of high-dose glucocorticoids (≥ 60 mg prednisone equivalent once a day) within 8 weeks of anti–programmed cell death protein 1 (PD-1) monotherapy initiation and poorer progression-free and overall survival. The study covered a cohort of 947 patients with advanced melanoma treated with anti–PD-1 monotherapy between 2009 and 2019, 54% of whom developed immune-related adverse events.
This study and other recent studies addressing the association between steroids and survival outcomes in patients with immune-related adverse events during ICI therapy are described in Dr. Allais’ 2023 review of cirAEs from ICIs.
Approach to morbilliform eruptions: This rash is “super common” in patients on ICIs, occurring generally within 2-3 weeks of starting treatment. “It tends to be self-limited and can recur with future infusions,” Dr. Allais said.
Systemic steroids should be reserved for severe or refractory eruptions. “Usually, I treat the patients with topical steroids, and I manage their expectations (that the rash may recur with subsequent infusions), but I closely follow them up” within 2-3 weeks, she said. It’s important to rule out a severe cutaneous adverse drug eruption, of course, and to start high-dose systemic steroids immediately if necessary. “Antibiotics are a big culprit” and often can be discontinued.
Soak and smear: “I’m obsessed” with this technique of a 20-minute soak in plain water followed by application of steroid ointment, said Dr. Allais, referring to a small study published in 2005 that reported a complete response after 2 weeks in 60% of patients with psoriasis, atopic dermatitis, and other inflammatory skin conditions (none had cancer), who had failed prior systemic therapy. All patients had at least a 75% response.
The method offers a way to “avoid the systemic immunosuppression we’d get with prednisone,” she said. One just needs to make sure the older patient can get in and out of their tub safely.
ICI-induced bullous pemphigoid (BP): BP occurs more frequently in the ICI setting, compared with the general population, with a median time to development of 8.5 months after ICI initiation. It is associated in this setting with improved tumor response, but “many oncologists stop anticancer treatment because of this diagnosis,” she said.
In the supportive oncodermatology space, however, ICI-induced BP exemplifies the value of tailored treatment regimens, she said. A small multi-institutional retrospective cohort study published in 2023 identified 35 cases of ICI-BP among 5636 ICI-treated patients and found that 8 out of 11 patients who received biologic therapy (rituximab, omalizumab, or dupilumab) had a complete response to ICI-BP without flares following subsequent ICI cycles. And while statistical significance was not reached, the study showed that no cancer-related outcomes were worsened.
“If you see someone with ICI-induced BP and they have a lot of involvement, you could start them on steroids and get that steroid-sparing agent initiated for approval. ... And if IgE is elevated, you might reach for omalizumab,” said Dr. Allais, noting that her favored treatment overall is dupilumab.
Risk factors for the development of ICI-induced BP include age > 70, skin cancer, and having an initial response to ICI on first imaging, the latter of which “I find fascinating ... because imaging occurs within the first 12 weeks of treatment, but we don’t see BP popping up until 8.5 months into treatment,” she noted. “So maybe there’s a baseline risk factor that could predispose them.”
Caution with antibiotics: “I try to avoid antibiotics in the ICI setting,” Dr. Allais said, in deference to the “ever-important microbiome.” Studies have demonstrated that the microbiomes of responders to ICI treatment are different from those of nonresponders, she said.
And a “fascinating” study of patients with melanoma undergoing ICI therapy showed not only a higher abundance of Ruminococcaceae bacteria in responders vs nonresponders but a significant impact of dietary fiber. High dietary fiber was associated with significantly improved overall survival in the patients on ICI, with the most pronounced benefit in patients with good fiber intake and no probiotic use. “Even wilder, their T cells changed,” she said. “They had a high expression of genes related to T-cell activation ... so more tumor-infiltrating lymphocytes.”
A retrospective study of 568 patients with stages III and IV melanoma treated with ICI showed that those exposed to antibiotics prior to ICI had significantly worse overall survival than those not exposed to antibiotics. “Think before you give them,” Dr. Allais said. “And try to tell your older patients to eat beans and greens.”
Dr. Allais reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — and with judicious usage and dosing of prednisone when deemed necessary, Blair Allais, MD, said during a session on supportive oncodermatology at the ElderDerm conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“It’s important when you see these patients to be as specific as possible” based on morphology and histopathology, and to treat the rashes in a similar way as in the non-ICI setting,” said Dr. Allais, a dermato-oncologist at the Inova Schar Cancer Institute, Fairfax, Virginia.
cirAEs are the most frequently reported and most visible adverse effects of checkpoint inhibition — a treatment that has emerged as a standard therapy for many malignancies since the first ICI was approved in 2011 for metastatic melanoma.
And contrary to what the phenomenon of immunosenescence might suggest, older patients are no less prone to cirAEs than younger patients. “You’d think you’d have fewer rashes and side effects as you age, but that’s not true,” said Dr. Allais, who completed a fellowship in cutaneous oncology after her dermatology residency.
A 2021 multicenter international cohort study of over 900 patients aged ≥ 80 years treated with single-agent ICIs for cancer did not find any significant differences in the development of immune-related adverse events among those younger than 85, those aged 85-89 years, and those 90 and older. Neither did the ELDERS study in the United Kingdom; this prospective observational study found similar rates of high-grade and low-grade immune toxicity in its two cohorts of patients ≥ 70 and < 70 years of age.
At the meeting, Dr. Allais, who coauthored a 2023 review of cirAEs from ICIs, reviewed recent developments and provided the following advice:
New diagnostic criteria: “Really exciting” news for more precise diagnosis and optimal therapy of cirAEs, Dr. Allais said, is a position paper published in the Journal for ImmunoTherapy of Cancer that offers consensus-based diagnostic criteria for the 10 most common types of dermatologic immune-related adverse events and an overall diagnostic framework. “Luckily, through the work of a Delphi consensus group, we can now have [more diagnostic specificity],” which is important for both clinical care and research, she said.
Most cirAEs have typically been reported nonspecifically as “rash,” but diagnosing a rash subtype is “critical in tailoring appropriate therapy that it is both effective and the least detrimental to the oncology treatment plan for patients with cancer,” the group’s coauthors wrote.
The 10 core diagnoses include psoriasis, eczematous dermatitis, vitiligo, Grover disease, eruptive atypical squamous proliferation, and bullous pemphigoid. Outside of the core diagnoses are other nonspecific presentations that require evaluation to arrive at a diagnosis, if possible, or to reveal data that can allow for targeted therapy and severity grading, the group explains in its paper.
“To prednisone or not to prednisone”: The development of cirAEs is associated with reduced mortality and improved cancer outcomes, making the use of immunosuppressants such as corticosteroids a therapeutic dilemma. “Patients who get these rashes usually do better with respect to their cancer, so the concern has been, if we affect how they respond to their immunotherapy, we may minimize that improvement in mortality,” said Dr. Allais, also assistant professor at the University of Virginia, Charlottesville, and clinical assistant professor of dermatology at George Washington University.
A widely discussed study published in 2015 reported on 254 patients with melanoma who developed an immune-related adverse event during treatment with ipilimumab — approximately one third of whom required systemic corticosteroids — and concluded that systemic corticosteroids did not affect overall survival or time to (cancer) treatment failure. This study from Memorial Sloan Kettering Cancer Center, New York City, “was the first large study looking at this question,” she said, and the subsequent message for several years in conferences and the literature was that steroids do not affect the efficacy of checkpoint inhibitors.
“But the study was not without limitations,” Dr. Allais said, “because the patients who got prednisone were mainly those with higher-grade toxicities,” while those not treated with corticosteroids had either no toxicities or low-grade toxicities. “If higher-grade toxicities were associated with better (antitumor) response, the steroids may have just [blunted] that benefit.”
The current totality of data available in the literature suggests that corticosteroids may indeed have an impact on the efficacy of ICI therapy. “Subsequent studies have come out in the community that have shown that we should probably think twice about giving prednisone to some patients, particularly within the first 50 days of ICI treatment, and that we should be mindful of the dose,” Dr. Allais said.
The takeaways from these studies — all published in the past few years — are to use prednisone early and liberally for life-threatening toxicity, to use it at the lowest dose and for the shortest course when there is not an appropriate alternative, to avoid it for diagnoses that are not treated with prednisone outside the ICI setting, and to “have a plan” for a steroid-sparing agent to use after prednisone, she said.
Dr. Allais recommends heightened consideration during the first 50 days of ICI treatment based on a multicenter retrospective study that found a significant association between use of high-dose glucocorticoids (≥ 60 mg prednisone equivalent once a day) within 8 weeks of anti–programmed cell death protein 1 (PD-1) monotherapy initiation and poorer progression-free and overall survival. The study covered a cohort of 947 patients with advanced melanoma treated with anti–PD-1 monotherapy between 2009 and 2019, 54% of whom developed immune-related adverse events.
This study and other recent studies addressing the association between steroids and survival outcomes in patients with immune-related adverse events during ICI therapy are described in Dr. Allais’ 2023 review of cirAEs from ICIs.
Approach to morbilliform eruptions: This rash is “super common” in patients on ICIs, occurring generally within 2-3 weeks of starting treatment. “It tends to be self-limited and can recur with future infusions,” Dr. Allais said.
Systemic steroids should be reserved for severe or refractory eruptions. “Usually, I treat the patients with topical steroids, and I manage their expectations (that the rash may recur with subsequent infusions), but I closely follow them up” within 2-3 weeks, she said. It’s important to rule out a severe cutaneous adverse drug eruption, of course, and to start high-dose systemic steroids immediately if necessary. “Antibiotics are a big culprit” and often can be discontinued.
Soak and smear: “I’m obsessed” with this technique of a 20-minute soak in plain water followed by application of steroid ointment, said Dr. Allais, referring to a small study published in 2005 that reported a complete response after 2 weeks in 60% of patients with psoriasis, atopic dermatitis, and other inflammatory skin conditions (none had cancer), who had failed prior systemic therapy. All patients had at least a 75% response.
The method offers a way to “avoid the systemic immunosuppression we’d get with prednisone,” she said. One just needs to make sure the older patient can get in and out of their tub safely.
ICI-induced bullous pemphigoid (BP): BP occurs more frequently in the ICI setting, compared with the general population, with a median time to development of 8.5 months after ICI initiation. It is associated in this setting with improved tumor response, but “many oncologists stop anticancer treatment because of this diagnosis,” she said.
In the supportive oncodermatology space, however, ICI-induced BP exemplifies the value of tailored treatment regimens, she said. A small multi-institutional retrospective cohort study published in 2023 identified 35 cases of ICI-BP among 5636 ICI-treated patients and found that 8 out of 11 patients who received biologic therapy (rituximab, omalizumab, or dupilumab) had a complete response to ICI-BP without flares following subsequent ICI cycles. And while statistical significance was not reached, the study showed that no cancer-related outcomes were worsened.
“If you see someone with ICI-induced BP and they have a lot of involvement, you could start them on steroids and get that steroid-sparing agent initiated for approval. ... And if IgE is elevated, you might reach for omalizumab,” said Dr. Allais, noting that her favored treatment overall is dupilumab.
Risk factors for the development of ICI-induced BP include age > 70, skin cancer, and having an initial response to ICI on first imaging, the latter of which “I find fascinating ... because imaging occurs within the first 12 weeks of treatment, but we don’t see BP popping up until 8.5 months into treatment,” she noted. “So maybe there’s a baseline risk factor that could predispose them.”
Caution with antibiotics: “I try to avoid antibiotics in the ICI setting,” Dr. Allais said, in deference to the “ever-important microbiome.” Studies have demonstrated that the microbiomes of responders to ICI treatment are different from those of nonresponders, she said.
And a “fascinating” study of patients with melanoma undergoing ICI therapy showed not only a higher abundance of Ruminococcaceae bacteria in responders vs nonresponders but a significant impact of dietary fiber. High dietary fiber was associated with significantly improved overall survival in the patients on ICI, with the most pronounced benefit in patients with good fiber intake and no probiotic use. “Even wilder, their T cells changed,” she said. “They had a high expression of genes related to T-cell activation ... so more tumor-infiltrating lymphocytes.”
A retrospective study of 568 patients with stages III and IV melanoma treated with ICI showed that those exposed to antibiotics prior to ICI had significantly worse overall survival than those not exposed to antibiotics. “Think before you give them,” Dr. Allais said. “And try to tell your older patients to eat beans and greens.”
Dr. Allais reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ELDERDERM 2024
Jeffrey Weber, MD, PhD, Giant of Cancer Care, Dies
Dr. Weber, a melanoma and cancer immunotherapy specialist, served as deputy director of the Laura and Isaac Perlmutter Cancer Center at New York University (NYU) Langone Medical Center in New York City. He also held positions as the Laura and Isaac Perlmutter Professor of Oncology in the Department of Medicine at the NYU Grossman School of Medicine, director of the Experimental Therapeutics Program, and co-leader of the Clinical Melanoma Program Board at NYU Langone Health.
Dr. Weber was a principal investigator on many studies, including pivotal clinical drug trials in melanoma and trials focused on managing autoimmune side effects from immunotherapy. He published more than 150 articles in top peer-reviewed journals.
For many years, Dr. Weber hosted the popular “Weber on Oncology” series of video contributions for Medscape Oncology, sharing updates and insights on noteworthy research and breakthroughs in melanoma.
“The Melanoma Research Alliance mourns the passing of Dr. Jeffrey S. Weber, a true pioneer in the field of cancer immunotherapy and an extraordinary leader in melanoma research. His contributions have forever changed the landscape of melanoma treatment, bringing groundbreaking advances from the lab into clinical practice and offering hope to countless patients,” the Melanoma Research Alliance posted on LinkedIn.
Many X users also shared condolences and memories of Dr. Weber, praising his numerous contributions and accomplishments.
“[Cancer Research Institute] mourns the loss of Dr. Jeffrey S. Weber ... [a]s an accomplished physician scientist, Dr. Weber drove advances in melanoma research, and played an active role in educating patients about the lifesaving power of immunotherapy,” the Cancer Research Institute posted.
A colleague noted that “[h]e was involved in the early days of cytokine and cell therapy and most recently led studies of personalized vaccines for melanoma patients. ... He was a great friend and colleague to many of us in the melanoma and immunotherapy field and we will remember him as a pioneer, thought leader and compassionate physician.”
A version of this article first appeared on Medscape.com.
Dr. Weber, a melanoma and cancer immunotherapy specialist, served as deputy director of the Laura and Isaac Perlmutter Cancer Center at New York University (NYU) Langone Medical Center in New York City. He also held positions as the Laura and Isaac Perlmutter Professor of Oncology in the Department of Medicine at the NYU Grossman School of Medicine, director of the Experimental Therapeutics Program, and co-leader of the Clinical Melanoma Program Board at NYU Langone Health.
Dr. Weber was a principal investigator on many studies, including pivotal clinical drug trials in melanoma and trials focused on managing autoimmune side effects from immunotherapy. He published more than 150 articles in top peer-reviewed journals.
For many years, Dr. Weber hosted the popular “Weber on Oncology” series of video contributions for Medscape Oncology, sharing updates and insights on noteworthy research and breakthroughs in melanoma.
“The Melanoma Research Alliance mourns the passing of Dr. Jeffrey S. Weber, a true pioneer in the field of cancer immunotherapy and an extraordinary leader in melanoma research. His contributions have forever changed the landscape of melanoma treatment, bringing groundbreaking advances from the lab into clinical practice and offering hope to countless patients,” the Melanoma Research Alliance posted on LinkedIn.
Many X users also shared condolences and memories of Dr. Weber, praising his numerous contributions and accomplishments.
“[Cancer Research Institute] mourns the loss of Dr. Jeffrey S. Weber ... [a]s an accomplished physician scientist, Dr. Weber drove advances in melanoma research, and played an active role in educating patients about the lifesaving power of immunotherapy,” the Cancer Research Institute posted.
A colleague noted that “[h]e was involved in the early days of cytokine and cell therapy and most recently led studies of personalized vaccines for melanoma patients. ... He was a great friend and colleague to many of us in the melanoma and immunotherapy field and we will remember him as a pioneer, thought leader and compassionate physician.”
A version of this article first appeared on Medscape.com.
Dr. Weber, a melanoma and cancer immunotherapy specialist, served as deputy director of the Laura and Isaac Perlmutter Cancer Center at New York University (NYU) Langone Medical Center in New York City. He also held positions as the Laura and Isaac Perlmutter Professor of Oncology in the Department of Medicine at the NYU Grossman School of Medicine, director of the Experimental Therapeutics Program, and co-leader of the Clinical Melanoma Program Board at NYU Langone Health.
Dr. Weber was a principal investigator on many studies, including pivotal clinical drug trials in melanoma and trials focused on managing autoimmune side effects from immunotherapy. He published more than 150 articles in top peer-reviewed journals.
For many years, Dr. Weber hosted the popular “Weber on Oncology” series of video contributions for Medscape Oncology, sharing updates and insights on noteworthy research and breakthroughs in melanoma.
“The Melanoma Research Alliance mourns the passing of Dr. Jeffrey S. Weber, a true pioneer in the field of cancer immunotherapy and an extraordinary leader in melanoma research. His contributions have forever changed the landscape of melanoma treatment, bringing groundbreaking advances from the lab into clinical practice and offering hope to countless patients,” the Melanoma Research Alliance posted on LinkedIn.
Many X users also shared condolences and memories of Dr. Weber, praising his numerous contributions and accomplishments.
“[Cancer Research Institute] mourns the loss of Dr. Jeffrey S. Weber ... [a]s an accomplished physician scientist, Dr. Weber drove advances in melanoma research, and played an active role in educating patients about the lifesaving power of immunotherapy,” the Cancer Research Institute posted.
A colleague noted that “[h]e was involved in the early days of cytokine and cell therapy and most recently led studies of personalized vaccines for melanoma patients. ... He was a great friend and colleague to many of us in the melanoma and immunotherapy field and we will remember him as a pioneer, thought leader and compassionate physician.”
A version of this article first appeared on Medscape.com.
Few Severe Toxicities After SBRT in Oligometastatic Cancer
TOPLINE:
according to a large real-world analysis.
METHODOLOGY:
- Advances in cancer imaging have helped identify more patients with oligometastatic disease. Although the standard treatment approach typically involves systemic therapy such as chemotherapy and immunotherapy, SBRT has increasingly become an option for these patients. However, the toxicities associated with SBRT remain less clear.
- OligoCare, a European, prospective, registry-based, single-arm observational study, aims to provide real-world outcomes among patients with oligometastatic cancer who received SBRT. In this analysis, the researchers evaluated early toxicities among 1468 patients with different primary cancers — non–small cell lung cancer (NSCLC; 19.7%), colorectal cancer (20%), breast cancer (15.5%), and prostate cancer (44.8%).
- The primary outcome was acute toxicities, including new malignancies and deaths, within 6 months of initiating SBRT.
- Overall, 527 (35.9%) patients received concomitant systemic treatment and 828 (56%) had de novo oligometastatic disease.
TAKEAWAY:
- Overall, though, only eight patients (0.5%) experienced acute SBRT-related toxicity of grade 3 and above within 6 months; two events, however, were fatal (pneumonitis and cerebral hemorrhage), and both occurred in patients with NSCLC.
- The other six grade 3 events included one instance of each of the following: empyema, pneumonia, radiation pneumonitis, radiation skin injury, decreased appetite, and bone pain. Two of these events occurred in patients with NSCLC, two in patients with breast cancer, one in patients with colorectal cancer, and one in patients with prostate cancer.
- New primary malignancies were reported in 13 (0.9%) patients, which included bladder cancer (n = 3), nonmelanoma skin cancer (n = 3), and leukemia (n = 1).
- Overall, 43 (2.9%) patients died within 6 months, most from their primary cancer (58.1%).
IN PRACTICE:
Low rates of early acute toxicities reported in this real-world study help confirm the safety of SBRT in the treatment of oligometastases, the authors concluded. However, “some anatomical sites might be associated with an increased risk of even severe or fatal toxicities.”
SOURCE:
The study, led by Filippo Alongi, Advanced Radiation Oncology Department, IRCCS Sacro Cuore Don Calabria Hospital, Cancer Care Center, Negrar di Valpolicella, Italy, and University of Brescia, also in Italy, was published online in Radiotherapy & Oncology .
LIMITATIONS:
Some limitations of the study include the nonrandomized design and potential variability in patient selection criteria, treatment doses, and schedules.
DISCLOSURES:
The study did not receive any funding support. Two authors declared receiving speaker or lecture honoraria or consultation fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
according to a large real-world analysis.
METHODOLOGY:
- Advances in cancer imaging have helped identify more patients with oligometastatic disease. Although the standard treatment approach typically involves systemic therapy such as chemotherapy and immunotherapy, SBRT has increasingly become an option for these patients. However, the toxicities associated with SBRT remain less clear.
- OligoCare, a European, prospective, registry-based, single-arm observational study, aims to provide real-world outcomes among patients with oligometastatic cancer who received SBRT. In this analysis, the researchers evaluated early toxicities among 1468 patients with different primary cancers — non–small cell lung cancer (NSCLC; 19.7%), colorectal cancer (20%), breast cancer (15.5%), and prostate cancer (44.8%).
- The primary outcome was acute toxicities, including new malignancies and deaths, within 6 months of initiating SBRT.
- Overall, 527 (35.9%) patients received concomitant systemic treatment and 828 (56%) had de novo oligometastatic disease.
TAKEAWAY:
- Overall, though, only eight patients (0.5%) experienced acute SBRT-related toxicity of grade 3 and above within 6 months; two events, however, were fatal (pneumonitis and cerebral hemorrhage), and both occurred in patients with NSCLC.
- The other six grade 3 events included one instance of each of the following: empyema, pneumonia, radiation pneumonitis, radiation skin injury, decreased appetite, and bone pain. Two of these events occurred in patients with NSCLC, two in patients with breast cancer, one in patients with colorectal cancer, and one in patients with prostate cancer.
- New primary malignancies were reported in 13 (0.9%) patients, which included bladder cancer (n = 3), nonmelanoma skin cancer (n = 3), and leukemia (n = 1).
- Overall, 43 (2.9%) patients died within 6 months, most from their primary cancer (58.1%).
IN PRACTICE:
Low rates of early acute toxicities reported in this real-world study help confirm the safety of SBRT in the treatment of oligometastases, the authors concluded. However, “some anatomical sites might be associated with an increased risk of even severe or fatal toxicities.”
SOURCE:
The study, led by Filippo Alongi, Advanced Radiation Oncology Department, IRCCS Sacro Cuore Don Calabria Hospital, Cancer Care Center, Negrar di Valpolicella, Italy, and University of Brescia, also in Italy, was published online in Radiotherapy & Oncology .
LIMITATIONS:
Some limitations of the study include the nonrandomized design and potential variability in patient selection criteria, treatment doses, and schedules.
DISCLOSURES:
The study did not receive any funding support. Two authors declared receiving speaker or lecture honoraria or consultation fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
according to a large real-world analysis.
METHODOLOGY:
- Advances in cancer imaging have helped identify more patients with oligometastatic disease. Although the standard treatment approach typically involves systemic therapy such as chemotherapy and immunotherapy, SBRT has increasingly become an option for these patients. However, the toxicities associated with SBRT remain less clear.
- OligoCare, a European, prospective, registry-based, single-arm observational study, aims to provide real-world outcomes among patients with oligometastatic cancer who received SBRT. In this analysis, the researchers evaluated early toxicities among 1468 patients with different primary cancers — non–small cell lung cancer (NSCLC; 19.7%), colorectal cancer (20%), breast cancer (15.5%), and prostate cancer (44.8%).
- The primary outcome was acute toxicities, including new malignancies and deaths, within 6 months of initiating SBRT.
- Overall, 527 (35.9%) patients received concomitant systemic treatment and 828 (56%) had de novo oligometastatic disease.
TAKEAWAY:
- Overall, though, only eight patients (0.5%) experienced acute SBRT-related toxicity of grade 3 and above within 6 months; two events, however, were fatal (pneumonitis and cerebral hemorrhage), and both occurred in patients with NSCLC.
- The other six grade 3 events included one instance of each of the following: empyema, pneumonia, radiation pneumonitis, radiation skin injury, decreased appetite, and bone pain. Two of these events occurred in patients with NSCLC, two in patients with breast cancer, one in patients with colorectal cancer, and one in patients with prostate cancer.
- New primary malignancies were reported in 13 (0.9%) patients, which included bladder cancer (n = 3), nonmelanoma skin cancer (n = 3), and leukemia (n = 1).
- Overall, 43 (2.9%) patients died within 6 months, most from their primary cancer (58.1%).
IN PRACTICE:
Low rates of early acute toxicities reported in this real-world study help confirm the safety of SBRT in the treatment of oligometastases, the authors concluded. However, “some anatomical sites might be associated with an increased risk of even severe or fatal toxicities.”
SOURCE:
The study, led by Filippo Alongi, Advanced Radiation Oncology Department, IRCCS Sacro Cuore Don Calabria Hospital, Cancer Care Center, Negrar di Valpolicella, Italy, and University of Brescia, also in Italy, was published online in Radiotherapy & Oncology .
LIMITATIONS:
Some limitations of the study include the nonrandomized design and potential variability in patient selection criteria, treatment doses, and schedules.
DISCLOSURES:
The study did not receive any funding support. Two authors declared receiving speaker or lecture honoraria or consultation fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Immunotherapy and Survival in Advanced NSCLC: Does Obesity Matter?
TOPLINE:
Overall, however, compared with low body mass index (BMI), overweight or obesity was associated with a lower risk for mortality among patients receiving either therapy.
METHODOLOGY:
- The association between BMI and overall survival in patients with cancer who receive immunotherapy or conventional chemotherapy in the frontline remains unclear. Patients with cancer and obesity are generally considered to have a worse prognosis, but some data suggest an obesity paradox, where patients with cancer and a higher BMI demonstrate better overall survival following immunotherapy or chemotherapy.
- To clarify whether (or how) BMI affects overall survival outcomes and the optimal frontline treatment choice, researchers evaluated 31,257 patients with advanced NSCLC from Japan who received immune checkpoint inhibitors (n = 12,816) or conventional chemotherapy (n = 18,441).
- Patient outcomes were assessed according to weight categories and frontline therapy type (immune checkpoint inhibitors or conventional chemotherapy), with overall survival as the primary outcome.
- A BMI < 18.5 was considered underweight, 18.5-24.9 was considered normal weight, 25.0-29.9 was considered overweight, and ≥ 30.0 was considered obese.
TAKEAWAY:
- In the overall population, regardless of weight, patients who received chemotherapy had a higher mortality rate than those who received immunotherapy — 35.9% vs 28.0%, respectively — over a follow-up of 3 years.
- However, overweight or obesity was associated with a lower risk for mortality compared with a lower BMI among patients with advanced NSCLC, regardless of whether they received immune checkpoint inhibitor therapy or conventional chemotherapy.
- Among patients who received immunotherapy, the risk for mortality decreased steadily as BMI increased from 15 to 24 and then increased at higher BMIs, indicating a U-shaped association.
- Immunotherapy was associated with a significant improvement in overall survival compared with conventional chemotherapy among patients with a BMI < 28; however, researchers observed no difference in overall survival between the two therapies in those with a BMI ≥ 28.
IN PRACTICE:
Overall, “these results support the presence of the obesity paradox in patients with [advanced] NSCLC who underwent either therapy,” the authors concluded.
But when focused on patients in the higher BMI group, there was no overall survival benefit with the frontline immunotherapy vs the conventional chemotherapy. “Immunotherapy therapy may not necessarily be the optimal first-line therapy for patients with overweight or obesity,” the authors wrote, adding that “the use of conventional chemotherapy should also be considered.”
SOURCE:
The study, led by Yasutaka Ihara, PharmD, Osaka Metropolitan University, Osaka, Japan, was published online in JAMA Network Open.
LIMITATIONS:
Retrospective design has inherent bias. PD-L1 status was not known, and the inclusion of Japanese population may have limited the generalizability of the findings.
DISCLOSURES:
This study received funding from the Graduate School of Medicine, Osaka Metropolitan University. Several authors reported receiving personal fees from various pharmaceutical sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Overall, however, compared with low body mass index (BMI), overweight or obesity was associated with a lower risk for mortality among patients receiving either therapy.
METHODOLOGY:
- The association between BMI and overall survival in patients with cancer who receive immunotherapy or conventional chemotherapy in the frontline remains unclear. Patients with cancer and obesity are generally considered to have a worse prognosis, but some data suggest an obesity paradox, where patients with cancer and a higher BMI demonstrate better overall survival following immunotherapy or chemotherapy.
- To clarify whether (or how) BMI affects overall survival outcomes and the optimal frontline treatment choice, researchers evaluated 31,257 patients with advanced NSCLC from Japan who received immune checkpoint inhibitors (n = 12,816) or conventional chemotherapy (n = 18,441).
- Patient outcomes were assessed according to weight categories and frontline therapy type (immune checkpoint inhibitors or conventional chemotherapy), with overall survival as the primary outcome.
- A BMI < 18.5 was considered underweight, 18.5-24.9 was considered normal weight, 25.0-29.9 was considered overweight, and ≥ 30.0 was considered obese.
TAKEAWAY:
- In the overall population, regardless of weight, patients who received chemotherapy had a higher mortality rate than those who received immunotherapy — 35.9% vs 28.0%, respectively — over a follow-up of 3 years.
- However, overweight or obesity was associated with a lower risk for mortality compared with a lower BMI among patients with advanced NSCLC, regardless of whether they received immune checkpoint inhibitor therapy or conventional chemotherapy.
- Among patients who received immunotherapy, the risk for mortality decreased steadily as BMI increased from 15 to 24 and then increased at higher BMIs, indicating a U-shaped association.
- Immunotherapy was associated with a significant improvement in overall survival compared with conventional chemotherapy among patients with a BMI < 28; however, researchers observed no difference in overall survival between the two therapies in those with a BMI ≥ 28.
IN PRACTICE:
Overall, “these results support the presence of the obesity paradox in patients with [advanced] NSCLC who underwent either therapy,” the authors concluded.
But when focused on patients in the higher BMI group, there was no overall survival benefit with the frontline immunotherapy vs the conventional chemotherapy. “Immunotherapy therapy may not necessarily be the optimal first-line therapy for patients with overweight or obesity,” the authors wrote, adding that “the use of conventional chemotherapy should also be considered.”
SOURCE:
The study, led by Yasutaka Ihara, PharmD, Osaka Metropolitan University, Osaka, Japan, was published online in JAMA Network Open.
LIMITATIONS:
Retrospective design has inherent bias. PD-L1 status was not known, and the inclusion of Japanese population may have limited the generalizability of the findings.
DISCLOSURES:
This study received funding from the Graduate School of Medicine, Osaka Metropolitan University. Several authors reported receiving personal fees from various pharmaceutical sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Overall, however, compared with low body mass index (BMI), overweight or obesity was associated with a lower risk for mortality among patients receiving either therapy.
METHODOLOGY:
- The association between BMI and overall survival in patients with cancer who receive immunotherapy or conventional chemotherapy in the frontline remains unclear. Patients with cancer and obesity are generally considered to have a worse prognosis, but some data suggest an obesity paradox, where patients with cancer and a higher BMI demonstrate better overall survival following immunotherapy or chemotherapy.
- To clarify whether (or how) BMI affects overall survival outcomes and the optimal frontline treatment choice, researchers evaluated 31,257 patients with advanced NSCLC from Japan who received immune checkpoint inhibitors (n = 12,816) or conventional chemotherapy (n = 18,441).
- Patient outcomes were assessed according to weight categories and frontline therapy type (immune checkpoint inhibitors or conventional chemotherapy), with overall survival as the primary outcome.
- A BMI < 18.5 was considered underweight, 18.5-24.9 was considered normal weight, 25.0-29.9 was considered overweight, and ≥ 30.0 was considered obese.
TAKEAWAY:
- In the overall population, regardless of weight, patients who received chemotherapy had a higher mortality rate than those who received immunotherapy — 35.9% vs 28.0%, respectively — over a follow-up of 3 years.
- However, overweight or obesity was associated with a lower risk for mortality compared with a lower BMI among patients with advanced NSCLC, regardless of whether they received immune checkpoint inhibitor therapy or conventional chemotherapy.
- Among patients who received immunotherapy, the risk for mortality decreased steadily as BMI increased from 15 to 24 and then increased at higher BMIs, indicating a U-shaped association.
- Immunotherapy was associated with a significant improvement in overall survival compared with conventional chemotherapy among patients with a BMI < 28; however, researchers observed no difference in overall survival between the two therapies in those with a BMI ≥ 28.
IN PRACTICE:
Overall, “these results support the presence of the obesity paradox in patients with [advanced] NSCLC who underwent either therapy,” the authors concluded.
But when focused on patients in the higher BMI group, there was no overall survival benefit with the frontline immunotherapy vs the conventional chemotherapy. “Immunotherapy therapy may not necessarily be the optimal first-line therapy for patients with overweight or obesity,” the authors wrote, adding that “the use of conventional chemotherapy should also be considered.”
SOURCE:
The study, led by Yasutaka Ihara, PharmD, Osaka Metropolitan University, Osaka, Japan, was published online in JAMA Network Open.
LIMITATIONS:
Retrospective design has inherent bias. PD-L1 status was not known, and the inclusion of Japanese population may have limited the generalizability of the findings.
DISCLOSURES:
This study received funding from the Graduate School of Medicine, Osaka Metropolitan University. Several authors reported receiving personal fees from various pharmaceutical sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
FDA Approves Lymphir for R/R Cutaneous T-Cell Lymphoma
The immunotherapy is a reformulation of denileukin diftitox (Ontak), initially approved in 1999 for certain patients with persistent or recurrent cutaneous T-cell lymphoma. In 2014, the original formulation was voluntarily withdrawn from the US market. Citius acquired rights to market a reformulated product outside of Asia in 2021.
This is the first indication for Lymphir, which targets interleukin-2 receptors on malignant T cells.
This approval marks “a significant milestone” for patients with cutaneous T-cell lymphoma, a rare cancer, company CEO Leonard Mazur said in a press release announcing the approval. “The introduction of Lymphir, with its potential to rapidly reduce skin disease and control symptomatic itching without cumulative toxicity, is expected to expand the [cutaneous T-cell lymphoma] treatment landscape and grow the overall market, currently estimated to be $300-$400 million.”
Approval was based on the single-arm, open-label 302 study in 69 patients who had a median of four prior anticancer therapies. Patients received 9 mcg/kg daily from day 1 to day 5 of 21-day cycles until disease progression or unacceptable toxicity.
The objective response rate was 36.2%, including complete responses in 8.7% of patients. Responses lasted 6 months or longer in 52% of patients. Over 80% of subjects had a decrease in skin tumor burden, and almost a third had clinically significant improvements in pruritus.
Adverse events occurring in 20% or more of patients include increased transaminases, decreased albumin, decreased hemoglobin, nausea, edema, fatigue, musculoskeletal pain, rash, chills, constipation, pyrexia, and capillary leak syndrome.
Labeling carries a boxed warning of capillary leak syndrome. Other warnings include visual impairment, infusion reactions, hepatotoxicity, and embryo-fetal toxicity. Citius is under a postmarketing requirement to characterize the risk for visual impairment.
The company expects to launch the agent within 5 months.
A version of this article first appeared on Medscape.com.
The immunotherapy is a reformulation of denileukin diftitox (Ontak), initially approved in 1999 for certain patients with persistent or recurrent cutaneous T-cell lymphoma. In 2014, the original formulation was voluntarily withdrawn from the US market. Citius acquired rights to market a reformulated product outside of Asia in 2021.
This is the first indication for Lymphir, which targets interleukin-2 receptors on malignant T cells.
This approval marks “a significant milestone” for patients with cutaneous T-cell lymphoma, a rare cancer, company CEO Leonard Mazur said in a press release announcing the approval. “The introduction of Lymphir, with its potential to rapidly reduce skin disease and control symptomatic itching without cumulative toxicity, is expected to expand the [cutaneous T-cell lymphoma] treatment landscape and grow the overall market, currently estimated to be $300-$400 million.”
Approval was based on the single-arm, open-label 302 study in 69 patients who had a median of four prior anticancer therapies. Patients received 9 mcg/kg daily from day 1 to day 5 of 21-day cycles until disease progression or unacceptable toxicity.
The objective response rate was 36.2%, including complete responses in 8.7% of patients. Responses lasted 6 months or longer in 52% of patients. Over 80% of subjects had a decrease in skin tumor burden, and almost a third had clinically significant improvements in pruritus.
Adverse events occurring in 20% or more of patients include increased transaminases, decreased albumin, decreased hemoglobin, nausea, edema, fatigue, musculoskeletal pain, rash, chills, constipation, pyrexia, and capillary leak syndrome.
Labeling carries a boxed warning of capillary leak syndrome. Other warnings include visual impairment, infusion reactions, hepatotoxicity, and embryo-fetal toxicity. Citius is under a postmarketing requirement to characterize the risk for visual impairment.
The company expects to launch the agent within 5 months.
A version of this article first appeared on Medscape.com.
The immunotherapy is a reformulation of denileukin diftitox (Ontak), initially approved in 1999 for certain patients with persistent or recurrent cutaneous T-cell lymphoma. In 2014, the original formulation was voluntarily withdrawn from the US market. Citius acquired rights to market a reformulated product outside of Asia in 2021.
This is the first indication for Lymphir, which targets interleukin-2 receptors on malignant T cells.
This approval marks “a significant milestone” for patients with cutaneous T-cell lymphoma, a rare cancer, company CEO Leonard Mazur said in a press release announcing the approval. “The introduction of Lymphir, with its potential to rapidly reduce skin disease and control symptomatic itching without cumulative toxicity, is expected to expand the [cutaneous T-cell lymphoma] treatment landscape and grow the overall market, currently estimated to be $300-$400 million.”
Approval was based on the single-arm, open-label 302 study in 69 patients who had a median of four prior anticancer therapies. Patients received 9 mcg/kg daily from day 1 to day 5 of 21-day cycles until disease progression or unacceptable toxicity.
The objective response rate was 36.2%, including complete responses in 8.7% of patients. Responses lasted 6 months or longer in 52% of patients. Over 80% of subjects had a decrease in skin tumor burden, and almost a third had clinically significant improvements in pruritus.
Adverse events occurring in 20% or more of patients include increased transaminases, decreased albumin, decreased hemoglobin, nausea, edema, fatigue, musculoskeletal pain, rash, chills, constipation, pyrexia, and capillary leak syndrome.
Labeling carries a boxed warning of capillary leak syndrome. Other warnings include visual impairment, infusion reactions, hepatotoxicity, and embryo-fetal toxicity. Citius is under a postmarketing requirement to characterize the risk for visual impairment.
The company expects to launch the agent within 5 months.
A version of this article first appeared on Medscape.com.
Immunotherapy May Be Overused in Dying Patients With Cancer
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Modest Gains Shown in Breast Cancer Immunotherapy Trials
TOPLINE:
particularly among single-center studies which are more likely to go unreported, and many phase 2 studies failing to translate into successful phase 3 trials.
METHODOLOGY:
- Few immunotherapy agents — only pembrolizumab in the United States, as of December 2023, and atezolizumab in Europe — have received approvals for use in patients with breast cancer, indicating low returns on the large number of breast cancer immunotherapy trials launched in the early 2010s.
- In this cross-sectional study, researchers evaluated 331 immunotherapy trials, initiated between January 2004 and April 2023, that enrolled 48,844 patients with breast cancer.
- Of these, 47 were phase 1 trials, 242 were phase 2 trials, and 42 were phase 3 trials.
- A trial was considered reported if the results were posted on ClinicalTrial.gov or reported as an abstract or a manuscript.
- Overall, 120 trials met their completion date up to November 2022; of these, 30 (25%) failed to report outcomes, which included two phase 3 trials.
TAKEAWAY:
- Phase 1 trials had the highest rate of nonreporting (31.8%), followed by phase 2 (23.6%) and phase 3 (22.2%) trials.
- Single-center studies were more likely to be unreported than multicenter studies (35.2% vs 15.0%; P = .02).
- Of 90 reported trials, 47 (52.2%) met their primary endpoints and 43 (47.8%) did not.
- The majority, 17 out of 19 (89.5%), of the reported randomized trials had negative results.
IN PRACTICE:
“The findings of this study suggest that the large number of immunotherapy trials being run have yielded modest clinical impact,” the authors wrote. “More selective initiation of phase 2 trials, grounded in preclinical and biomarker observations and with optimal statistical designs for early efficacy assessment, is needed to increase trial efficiency.”
SOURCE:
The study, led by Marco Mariani, MD, Università Vita-Salute San Raffaele, Milan, Italy, was published online in JAMA Network Open.
LIMITATIONS:
The study’s reliance on ClinicalTrials.gov as the primary source of trial data might have resulted in some trials being overlooked. In addition, manual data extraction could cause inaccuracies and potentially introduced biases in the interpretation of trial results. Primary study completion date cutoff of December 2022 could have excluded significant data from more recent trials.
DISCLOSURES:
This study received support via Susan Komen Leadership Grant and the Fondazione AIRC per la Ricerca sul Cancro. Several authors reported receiving grants and personal fees and having other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
particularly among single-center studies which are more likely to go unreported, and many phase 2 studies failing to translate into successful phase 3 trials.
METHODOLOGY:
- Few immunotherapy agents — only pembrolizumab in the United States, as of December 2023, and atezolizumab in Europe — have received approvals for use in patients with breast cancer, indicating low returns on the large number of breast cancer immunotherapy trials launched in the early 2010s.
- In this cross-sectional study, researchers evaluated 331 immunotherapy trials, initiated between January 2004 and April 2023, that enrolled 48,844 patients with breast cancer.
- Of these, 47 were phase 1 trials, 242 were phase 2 trials, and 42 were phase 3 trials.
- A trial was considered reported if the results were posted on ClinicalTrial.gov or reported as an abstract or a manuscript.
- Overall, 120 trials met their completion date up to November 2022; of these, 30 (25%) failed to report outcomes, which included two phase 3 trials.
TAKEAWAY:
- Phase 1 trials had the highest rate of nonreporting (31.8%), followed by phase 2 (23.6%) and phase 3 (22.2%) trials.
- Single-center studies were more likely to be unreported than multicenter studies (35.2% vs 15.0%; P = .02).
- Of 90 reported trials, 47 (52.2%) met their primary endpoints and 43 (47.8%) did not.
- The majority, 17 out of 19 (89.5%), of the reported randomized trials had negative results.
IN PRACTICE:
“The findings of this study suggest that the large number of immunotherapy trials being run have yielded modest clinical impact,” the authors wrote. “More selective initiation of phase 2 trials, grounded in preclinical and biomarker observations and with optimal statistical designs for early efficacy assessment, is needed to increase trial efficiency.”
SOURCE:
The study, led by Marco Mariani, MD, Università Vita-Salute San Raffaele, Milan, Italy, was published online in JAMA Network Open.
LIMITATIONS:
The study’s reliance on ClinicalTrials.gov as the primary source of trial data might have resulted in some trials being overlooked. In addition, manual data extraction could cause inaccuracies and potentially introduced biases in the interpretation of trial results. Primary study completion date cutoff of December 2022 could have excluded significant data from more recent trials.
DISCLOSURES:
This study received support via Susan Komen Leadership Grant and the Fondazione AIRC per la Ricerca sul Cancro. Several authors reported receiving grants and personal fees and having other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
particularly among single-center studies which are more likely to go unreported, and many phase 2 studies failing to translate into successful phase 3 trials.
METHODOLOGY:
- Few immunotherapy agents — only pembrolizumab in the United States, as of December 2023, and atezolizumab in Europe — have received approvals for use in patients with breast cancer, indicating low returns on the large number of breast cancer immunotherapy trials launched in the early 2010s.
- In this cross-sectional study, researchers evaluated 331 immunotherapy trials, initiated between January 2004 and April 2023, that enrolled 48,844 patients with breast cancer.
- Of these, 47 were phase 1 trials, 242 were phase 2 trials, and 42 were phase 3 trials.
- A trial was considered reported if the results were posted on ClinicalTrial.gov or reported as an abstract or a manuscript.
- Overall, 120 trials met their completion date up to November 2022; of these, 30 (25%) failed to report outcomes, which included two phase 3 trials.
TAKEAWAY:
- Phase 1 trials had the highest rate of nonreporting (31.8%), followed by phase 2 (23.6%) and phase 3 (22.2%) trials.
- Single-center studies were more likely to be unreported than multicenter studies (35.2% vs 15.0%; P = .02).
- Of 90 reported trials, 47 (52.2%) met their primary endpoints and 43 (47.8%) did not.
- The majority, 17 out of 19 (89.5%), of the reported randomized trials had negative results.
IN PRACTICE:
“The findings of this study suggest that the large number of immunotherapy trials being run have yielded modest clinical impact,” the authors wrote. “More selective initiation of phase 2 trials, grounded in preclinical and biomarker observations and with optimal statistical designs for early efficacy assessment, is needed to increase trial efficiency.”
SOURCE:
The study, led by Marco Mariani, MD, Università Vita-Salute San Raffaele, Milan, Italy, was published online in JAMA Network Open.
LIMITATIONS:
The study’s reliance on ClinicalTrials.gov as the primary source of trial data might have resulted in some trials being overlooked. In addition, manual data extraction could cause inaccuracies and potentially introduced biases in the interpretation of trial results. Primary study completion date cutoff of December 2022 could have excluded significant data from more recent trials.
DISCLOSURES:
This study received support via Susan Komen Leadership Grant and the Fondazione AIRC per la Ricerca sul Cancro. Several authors reported receiving grants and personal fees and having other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.