User login
Influenza: U.S. activity was low this summer
Influenza activity in the United States was typically low over the summer months, with influenza A(H3N2) viruses predominating, according to the Centers for Disease Control and Prevention.
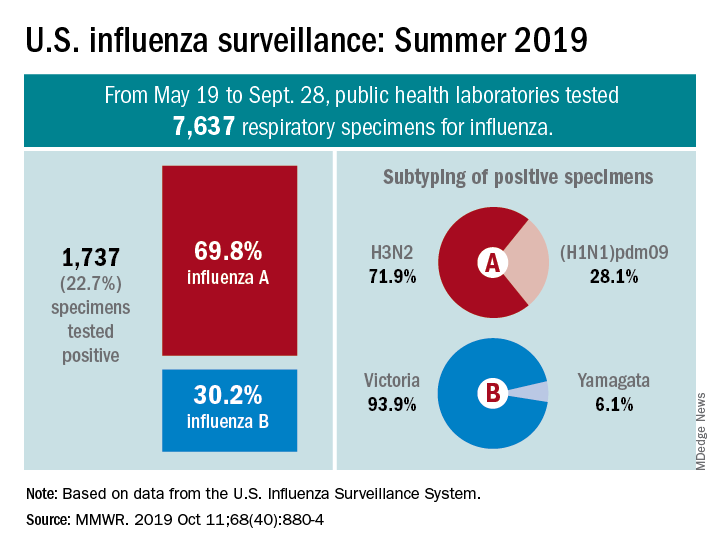
From May 19 to Sept. 28, 2019, weekly flu activity – measured by the percentage of outpatient visits to health care professionals for influenza-like illness (ILI) – was below the national baseline of 2.2%, ranging from 0.7% to 1.4%. Since mid-August, however, when the rate was last 0.7%, it has been climbing slowly but steadily and was up to 1.3% for the week ending Sept. 28, CDC data show.
The various public health laboratories of the U.S. Influenza Surveillance System tested over 7,600 respiratory samples from May 19 to Sept. 28, and 22.7% were positive for influenza viruses, Scott Epperson, DVM, and associates at the CDC’s influenza division said Oct. 10 in the MMWR.
Of the 1,737 samples found to be positive, 69.8% were influenza A and 30.2% were influenza B. The subtype split among specimens positive for Influenza A was 71.9% A(H3N2) and 28.1% A(H1N1)pdm09, while the samples positive for influenza B went 93.9% B/Victoria and 6.1% B/Yamagata, they reported.
Over the same time period in the Southern Hemisphere, “seasonal influenza viruses circulated widely, with influenza A(H3) predominating in many regions; however, influenza A(H1N1)pdm09 and influenza B viruses were predominant in some countries,” the CDC investigators noted.
They also reported the World Health Organization recommendations for the Southern Hemisphere’s 2020 flu vaccines. Components of the egg-based trivalent vaccine are an A/Brisbane/02/2018(H1N1)pdm09-like virus, an A/South Australia/34/2019(H3N2)-like virus, and a B/Washington/02/2019-like virus(B/Victoria lineage). The recommended quadrivalent vaccine adds a B/Phuket/3073/2013-like virus(B/Yamagata lineage), they wrote.
“It is too early in the season to know which viruses will circulate in the United States later this fall and winter or how severe the season might be; however, regardless of what is circulating, the best protection against influenza is an influenza vaccination,” Dr. Epperson and associates wrote.
SOURCE: Epperson S et al. MMWR. 2019 Oct 11;68(40):880-4.
Influenza activity in the United States was typically low over the summer months, with influenza A(H3N2) viruses predominating, according to the Centers for Disease Control and Prevention.

From May 19 to Sept. 28, 2019, weekly flu activity – measured by the percentage of outpatient visits to health care professionals for influenza-like illness (ILI) – was below the national baseline of 2.2%, ranging from 0.7% to 1.4%. Since mid-August, however, when the rate was last 0.7%, it has been climbing slowly but steadily and was up to 1.3% for the week ending Sept. 28, CDC data show.
The various public health laboratories of the U.S. Influenza Surveillance System tested over 7,600 respiratory samples from May 19 to Sept. 28, and 22.7% were positive for influenza viruses, Scott Epperson, DVM, and associates at the CDC’s influenza division said Oct. 10 in the MMWR.
Of the 1,737 samples found to be positive, 69.8% were influenza A and 30.2% were influenza B. The subtype split among specimens positive for Influenza A was 71.9% A(H3N2) and 28.1% A(H1N1)pdm09, while the samples positive for influenza B went 93.9% B/Victoria and 6.1% B/Yamagata, they reported.
Over the same time period in the Southern Hemisphere, “seasonal influenza viruses circulated widely, with influenza A(H3) predominating in many regions; however, influenza A(H1N1)pdm09 and influenza B viruses were predominant in some countries,” the CDC investigators noted.
They also reported the World Health Organization recommendations for the Southern Hemisphere’s 2020 flu vaccines. Components of the egg-based trivalent vaccine are an A/Brisbane/02/2018(H1N1)pdm09-like virus, an A/South Australia/34/2019(H3N2)-like virus, and a B/Washington/02/2019-like virus(B/Victoria lineage). The recommended quadrivalent vaccine adds a B/Phuket/3073/2013-like virus(B/Yamagata lineage), they wrote.
“It is too early in the season to know which viruses will circulate in the United States later this fall and winter or how severe the season might be; however, regardless of what is circulating, the best protection against influenza is an influenza vaccination,” Dr. Epperson and associates wrote.
SOURCE: Epperson S et al. MMWR. 2019 Oct 11;68(40):880-4.
Influenza activity in the United States was typically low over the summer months, with influenza A(H3N2) viruses predominating, according to the Centers for Disease Control and Prevention.

From May 19 to Sept. 28, 2019, weekly flu activity – measured by the percentage of outpatient visits to health care professionals for influenza-like illness (ILI) – was below the national baseline of 2.2%, ranging from 0.7% to 1.4%. Since mid-August, however, when the rate was last 0.7%, it has been climbing slowly but steadily and was up to 1.3% for the week ending Sept. 28, CDC data show.
The various public health laboratories of the U.S. Influenza Surveillance System tested over 7,600 respiratory samples from May 19 to Sept. 28, and 22.7% were positive for influenza viruses, Scott Epperson, DVM, and associates at the CDC’s influenza division said Oct. 10 in the MMWR.
Of the 1,737 samples found to be positive, 69.8% were influenza A and 30.2% were influenza B. The subtype split among specimens positive for Influenza A was 71.9% A(H3N2) and 28.1% A(H1N1)pdm09, while the samples positive for influenza B went 93.9% B/Victoria and 6.1% B/Yamagata, they reported.
Over the same time period in the Southern Hemisphere, “seasonal influenza viruses circulated widely, with influenza A(H3) predominating in many regions; however, influenza A(H1N1)pdm09 and influenza B viruses were predominant in some countries,” the CDC investigators noted.
They also reported the World Health Organization recommendations for the Southern Hemisphere’s 2020 flu vaccines. Components of the egg-based trivalent vaccine are an A/Brisbane/02/2018(H1N1)pdm09-like virus, an A/South Australia/34/2019(H3N2)-like virus, and a B/Washington/02/2019-like virus(B/Victoria lineage). The recommended quadrivalent vaccine adds a B/Phuket/3073/2013-like virus(B/Yamagata lineage), they wrote.
“It is too early in the season to know which viruses will circulate in the United States later this fall and winter or how severe the season might be; however, regardless of what is circulating, the best protection against influenza is an influenza vaccination,” Dr. Epperson and associates wrote.
SOURCE: Epperson S et al. MMWR. 2019 Oct 11;68(40):880-4.
FROM MMWR
Too few pregnant women receive both influenza and Tdap vaccines
according to a Morbidity and Mortality Weekly Report published by the Centers for Disease Control and Prevention.
The CDC recommends that all pregnant women receive the Tdap vaccine, preferably between 27 and 36 weeks’ gestation. The flu vaccine is recommended for all women at any point in pregnancy if the pregnancy falls within flu season. Women do not need a second flu shot if they received the vaccine before pregnancy in the same influenza season. Both vaccines provide protection to infants after birth.
“Clinicians caring for women who are pregnant have a huge role in helping women understand risks and benefits and the value of vaccines,” Anne Schuchat, MD, principal deputy director of the CDC, Atlanta, said in a telebriefing about the new report. “A lot of women are worried about taking any extra medicine or getting shots during pregnancy, and clinicians can let them know about the large data available showing the safety of the vaccine as well as the effectiveness. We also think it’s important to let people know about the risk of not vaccinating.”
Pregnant women are at higher risk for influenza complications and represent a disproportionate number of flu-related hospitalizations. From the 2010-2011 to 2017-2018 influenza seasons, 24%-34% of influenza hospitalizations each season were pregnant women aged 15-44, yet only 9% of women in this age group are pregnant at any point each year, according to the report.
Similarly, infants under 6 months have the greatest risk of hospitalization from influenza, and half of pertussis hospitalizations and 69% of pertussis deaths occur in infants under 2 months old. But a fetus receives protective maternal antibodies from flu and pertussis vaccines about 2 weeks after the mother is vaccinated.
Influenza hospitalization is 40% lower among pregnant women vaccinated against flu and 72% lower in infants under 6 months who received maternal influenza antibodies during gestation. Similarly, Tdap vaccination during the third trimester of pregnancy reduces pertussis infection risk by 78% and pertussis hospitalization by 91% in infants under 2 months.
“Infant protection can motivate pregnant women to receive recommended vaccines, and intention to vaccinate is higher among women who perceive more serious consequences of influenza or pertussis disease for their own or their infant’s health,” Megan C. Lindley, MPH, of the CDC’s Immunization Services Division, and colleagues wrote in the MMWR report.
In March-April 2019, Ms. Lindley and associates conducted an Internet survey about flu and Tdap immunizations among women aged 18-49 who had been pregnant at any point since August 1, 2018. A total of 2,626 women completed the survey of 2,762 invitations (95% response rate).
Among 817 women who knew their Tdap status during pregnancy, 55% received the Tdap vaccine. Among 2,097 women who reported a pregnancy between October 2018 and January 2019, 54% received the flu vaccine before or during pregnancy.
But many women received one vaccine without the other: 65% of women surveyed had not received both vaccines during pregnancy. Higher immunization rates occurred among women whose clinicians recommended the vaccines: 66% received a flu shot and 71% received Tdap.
“We’re learning a lot about improved communication between clinicians and patients. One thing we suggest is to begin the conversations early.” Dr Schuchat said. “If you begin talking early in the pregnancy about the things you’ll be looking forward to and provide information, by the time it is flu season or it is that third trimester, they’re prepared to make a good choice.”
Most women surveyed (75%) said their providers did offer a flu or Tdap vaccine in the office or a referral for one. Yet more than 30% of these women did not get the recommended vaccine.
The most common reason for not getting the Tdap during pregnancy, cited by 38% of women who didn’t receive it, was not knowing about the recommendation. Those who did not receive flu vaccination, however, cited concerns about effectiveness (18%) or safety for the baby (16%). A similar proportion of women cited safety concerns for not getting the Tdap (17%).
Sharing information early and engaging respectfully with patients are key to successful provider recommendations, Dr Schuchat said.
“It’s really important for clinicians to begin by listening to women, asking, ‘Can I answer your questions? What are the concerns that you have?’ ” she said. “We find that, when a clinician validates a patient’s concerns and really shows that they’re listening, they can build trust and respect.”
Providers’ sharing their personal experience can help as well, Dr Schuchat added. Clinicians can let patients know if they themselves, or their partner, received the vaccines during pregnancy.
Rates for turning down vaccines were higher for black women: 47% received the flu vaccine after a recommendation, compared with 69% of white women. Among those receiving a Tdap recommendation, 53% of black women accepted it, compared with 77% of white women and 66% of Latina women. The authors noted a past study showing black adults had a higher distrust of flu vaccination, their doctor, and CDC information than white adults.
“Differential effects of provider vaccination offers or referrals might also be explained by less patient-centered provider communication with black patients,” Ms. Lindley and associates wrote.
SOURCE: Lindley MC. MMWR Morb Mortal Wkly Rep. 2019 Oct 8. doi: 10.15585/mmwr.mm6840e1.
according to a Morbidity and Mortality Weekly Report published by the Centers for Disease Control and Prevention.
The CDC recommends that all pregnant women receive the Tdap vaccine, preferably between 27 and 36 weeks’ gestation. The flu vaccine is recommended for all women at any point in pregnancy if the pregnancy falls within flu season. Women do not need a second flu shot if they received the vaccine before pregnancy in the same influenza season. Both vaccines provide protection to infants after birth.
“Clinicians caring for women who are pregnant have a huge role in helping women understand risks and benefits and the value of vaccines,” Anne Schuchat, MD, principal deputy director of the CDC, Atlanta, said in a telebriefing about the new report. “A lot of women are worried about taking any extra medicine or getting shots during pregnancy, and clinicians can let them know about the large data available showing the safety of the vaccine as well as the effectiveness. We also think it’s important to let people know about the risk of not vaccinating.”
Pregnant women are at higher risk for influenza complications and represent a disproportionate number of flu-related hospitalizations. From the 2010-2011 to 2017-2018 influenza seasons, 24%-34% of influenza hospitalizations each season were pregnant women aged 15-44, yet only 9% of women in this age group are pregnant at any point each year, according to the report.
Similarly, infants under 6 months have the greatest risk of hospitalization from influenza, and half of pertussis hospitalizations and 69% of pertussis deaths occur in infants under 2 months old. But a fetus receives protective maternal antibodies from flu and pertussis vaccines about 2 weeks after the mother is vaccinated.
Influenza hospitalization is 40% lower among pregnant women vaccinated against flu and 72% lower in infants under 6 months who received maternal influenza antibodies during gestation. Similarly, Tdap vaccination during the third trimester of pregnancy reduces pertussis infection risk by 78% and pertussis hospitalization by 91% in infants under 2 months.
“Infant protection can motivate pregnant women to receive recommended vaccines, and intention to vaccinate is higher among women who perceive more serious consequences of influenza or pertussis disease for their own or their infant’s health,” Megan C. Lindley, MPH, of the CDC’s Immunization Services Division, and colleagues wrote in the MMWR report.
In March-April 2019, Ms. Lindley and associates conducted an Internet survey about flu and Tdap immunizations among women aged 18-49 who had been pregnant at any point since August 1, 2018. A total of 2,626 women completed the survey of 2,762 invitations (95% response rate).
Among 817 women who knew their Tdap status during pregnancy, 55% received the Tdap vaccine. Among 2,097 women who reported a pregnancy between October 2018 and January 2019, 54% received the flu vaccine before or during pregnancy.
But many women received one vaccine without the other: 65% of women surveyed had not received both vaccines during pregnancy. Higher immunization rates occurred among women whose clinicians recommended the vaccines: 66% received a flu shot and 71% received Tdap.
“We’re learning a lot about improved communication between clinicians and patients. One thing we suggest is to begin the conversations early.” Dr Schuchat said. “If you begin talking early in the pregnancy about the things you’ll be looking forward to and provide information, by the time it is flu season or it is that third trimester, they’re prepared to make a good choice.”
Most women surveyed (75%) said their providers did offer a flu or Tdap vaccine in the office or a referral for one. Yet more than 30% of these women did not get the recommended vaccine.
The most common reason for not getting the Tdap during pregnancy, cited by 38% of women who didn’t receive it, was not knowing about the recommendation. Those who did not receive flu vaccination, however, cited concerns about effectiveness (18%) or safety for the baby (16%). A similar proportion of women cited safety concerns for not getting the Tdap (17%).
Sharing information early and engaging respectfully with patients are key to successful provider recommendations, Dr Schuchat said.
“It’s really important for clinicians to begin by listening to women, asking, ‘Can I answer your questions? What are the concerns that you have?’ ” she said. “We find that, when a clinician validates a patient’s concerns and really shows that they’re listening, they can build trust and respect.”
Providers’ sharing their personal experience can help as well, Dr Schuchat added. Clinicians can let patients know if they themselves, or their partner, received the vaccines during pregnancy.
Rates for turning down vaccines were higher for black women: 47% received the flu vaccine after a recommendation, compared with 69% of white women. Among those receiving a Tdap recommendation, 53% of black women accepted it, compared with 77% of white women and 66% of Latina women. The authors noted a past study showing black adults had a higher distrust of flu vaccination, their doctor, and CDC information than white adults.
“Differential effects of provider vaccination offers or referrals might also be explained by less patient-centered provider communication with black patients,” Ms. Lindley and associates wrote.
SOURCE: Lindley MC. MMWR Morb Mortal Wkly Rep. 2019 Oct 8. doi: 10.15585/mmwr.mm6840e1.
according to a Morbidity and Mortality Weekly Report published by the Centers for Disease Control and Prevention.
The CDC recommends that all pregnant women receive the Tdap vaccine, preferably between 27 and 36 weeks’ gestation. The flu vaccine is recommended for all women at any point in pregnancy if the pregnancy falls within flu season. Women do not need a second flu shot if they received the vaccine before pregnancy in the same influenza season. Both vaccines provide protection to infants after birth.
“Clinicians caring for women who are pregnant have a huge role in helping women understand risks and benefits and the value of vaccines,” Anne Schuchat, MD, principal deputy director of the CDC, Atlanta, said in a telebriefing about the new report. “A lot of women are worried about taking any extra medicine or getting shots during pregnancy, and clinicians can let them know about the large data available showing the safety of the vaccine as well as the effectiveness. We also think it’s important to let people know about the risk of not vaccinating.”
Pregnant women are at higher risk for influenza complications and represent a disproportionate number of flu-related hospitalizations. From the 2010-2011 to 2017-2018 influenza seasons, 24%-34% of influenza hospitalizations each season were pregnant women aged 15-44, yet only 9% of women in this age group are pregnant at any point each year, according to the report.
Similarly, infants under 6 months have the greatest risk of hospitalization from influenza, and half of pertussis hospitalizations and 69% of pertussis deaths occur in infants under 2 months old. But a fetus receives protective maternal antibodies from flu and pertussis vaccines about 2 weeks after the mother is vaccinated.
Influenza hospitalization is 40% lower among pregnant women vaccinated against flu and 72% lower in infants under 6 months who received maternal influenza antibodies during gestation. Similarly, Tdap vaccination during the third trimester of pregnancy reduces pertussis infection risk by 78% and pertussis hospitalization by 91% in infants under 2 months.
“Infant protection can motivate pregnant women to receive recommended vaccines, and intention to vaccinate is higher among women who perceive more serious consequences of influenza or pertussis disease for their own or their infant’s health,” Megan C. Lindley, MPH, of the CDC’s Immunization Services Division, and colleagues wrote in the MMWR report.
In March-April 2019, Ms. Lindley and associates conducted an Internet survey about flu and Tdap immunizations among women aged 18-49 who had been pregnant at any point since August 1, 2018. A total of 2,626 women completed the survey of 2,762 invitations (95% response rate).
Among 817 women who knew their Tdap status during pregnancy, 55% received the Tdap vaccine. Among 2,097 women who reported a pregnancy between October 2018 and January 2019, 54% received the flu vaccine before or during pregnancy.
But many women received one vaccine without the other: 65% of women surveyed had not received both vaccines during pregnancy. Higher immunization rates occurred among women whose clinicians recommended the vaccines: 66% received a flu shot and 71% received Tdap.
“We’re learning a lot about improved communication between clinicians and patients. One thing we suggest is to begin the conversations early.” Dr Schuchat said. “If you begin talking early in the pregnancy about the things you’ll be looking forward to and provide information, by the time it is flu season or it is that third trimester, they’re prepared to make a good choice.”
Most women surveyed (75%) said their providers did offer a flu or Tdap vaccine in the office or a referral for one. Yet more than 30% of these women did not get the recommended vaccine.
The most common reason for not getting the Tdap during pregnancy, cited by 38% of women who didn’t receive it, was not knowing about the recommendation. Those who did not receive flu vaccination, however, cited concerns about effectiveness (18%) or safety for the baby (16%). A similar proportion of women cited safety concerns for not getting the Tdap (17%).
Sharing information early and engaging respectfully with patients are key to successful provider recommendations, Dr Schuchat said.
“It’s really important for clinicians to begin by listening to women, asking, ‘Can I answer your questions? What are the concerns that you have?’ ” she said. “We find that, when a clinician validates a patient’s concerns and really shows that they’re listening, they can build trust and respect.”
Providers’ sharing their personal experience can help as well, Dr Schuchat added. Clinicians can let patients know if they themselves, or their partner, received the vaccines during pregnancy.
Rates for turning down vaccines were higher for black women: 47% received the flu vaccine after a recommendation, compared with 69% of white women. Among those receiving a Tdap recommendation, 53% of black women accepted it, compared with 77% of white women and 66% of Latina women. The authors noted a past study showing black adults had a higher distrust of flu vaccination, their doctor, and CDC information than white adults.
“Differential effects of provider vaccination offers or referrals might also be explained by less patient-centered provider communication with black patients,” Ms. Lindley and associates wrote.
SOURCE: Lindley MC. MMWR Morb Mortal Wkly Rep. 2019 Oct 8. doi: 10.15585/mmwr.mm6840e1.
FROM MMWR TELEBRIEFING
FUO, pneumonia often distinguishes influenza from RSV in hospitalized young children
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.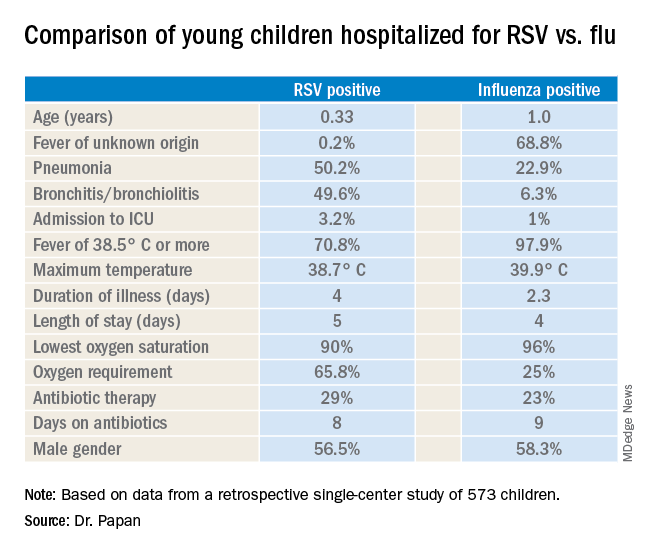
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
REPORTING FROM ESPID 2019
LAIV doesn’t up asthmatic children’s risk of lower respiratory events
, according to an analysis published in Vaccine.
The data corroborate other research indicating that live attenuated influenza vaccine (LAIV) is safe for children with asthma older than 2 years and suggest that the choice of vaccination in this population should be based on effectiveness, according to James D. Nordin, MD, MPH, a clinical researcher at HealthPartners Institute in Minneapolis, and colleagues.
Children and adolescents with asthma have an increased risk of morbidity if they contract influenza. They represent a disproportionate number of pediatric influenza hospitalizations and have been a focus of efforts to vaccinate children against influenza. Since 2003, the inactivated influenza vaccine (IIV) and the LAIV have been available. Research indicates that LAIV is more effective than IIV at preventing culture-confirmed influenza in children. Two studies found an increased risk of wheezing in children who received LAIV, but other studies failed to replicate these findings.
A retrospective cohort study
Dr. Nordin and associates conducted a retrospective observational cohort study to investigate whether use of a guideline recommending LAIV for children aged 2 years and older with asthma increased the risk of lower respiratory events within 21 or 42 days of vaccination, compared with standard guidelines to administer IIV in children with asthma. The investigators drew data from two large medical groups with independent clinical leadership that serve demographically similar populations in Minnesota. One group (the LAIV group) switched its preference for all children from IIV to LAIV in 2010. The control group continued using IIV for children with asthma throughout the study period. Each group operates more than 20 clinics.
The investigators included children and adolescents aged 2-17 years who presented during one or more influenza season from 2007-2008 through 2014-2015. Eligible participants had a diagnosis of asthma or wheezing, received one or more influenza vaccines, had continuous insurance enrollment, and had at least one primary care or asthma related subspecialty encounter. They excluded patients with contraindications for LAIV (e.g., pregnancy, malignancy, and cystic fibrosis) and those with any hospitalization, ED visit, or outpatient encounter for a lower respiratory event in the 42 days before influenza vaccination.
Dr. Nordin and colleagues used a generalized estimating equation regression to estimate the ratio of rate ratios (RORs) comparing events before and after vaccination between the LAIV guideline and control groups. The researchers examined covariates such as age, gender, race or ethnicity, Medicaid insurance for at least 1 month in the previous year, neighborhood poverty, and neighborhood rates of asthma.
No increased risk
The investigators included 4,771 children and 7,851 child-influenza records in their analysis. During the period from 2007 to 2010, there were 2,215 child-influenza records from children and adolescents included from the LAIV group and 735 from the IIV guideline group. From 2010 to 2015, there were 3,767 child-influenza records in children and adolescents from the LAIV group and 1,134 from the IIV guideline group. After the LAIV group adopted the new guideline, the proportion of patients receiving LAIV increased from 23% to 68% in the LAIV group and from 7% to 11% in the control group.
About 88% of lower respiratory events included diagnoses for asthma exacerbations. When the investigators adjusted the data for age, asthma severity, asthma control, race or ethnicity, and Medicaid coverage, they found no increase in lower respiratory events associated with the LAIV guideline. The adjusted ROR was 0.74 for lower respiratory events within 21 days of vaccination and 0.77 for lower respiratory events within 42 days of vaccination. The results were similar when Dr. Nordin and colleagues stratified the data by age group, and including additional covariates did not alter the ROR estimates. In all, 21 hospitalizations occurred within 42 days of influenza vaccination, and the LAIV guideline did not increase the risk for hospitalization.
“Findings from this study are consistent with several recent observational studies of LAIV in children and adolescents with asthma,” said Dr. Nordin and colleagues.
One limitation of the current study was that the data were restricted to the information available in electronic health care or claims records. The researchers therefore were able to observe only medically attended lower respiratory events. Furthermore, the exclusion of asthma management encounters and the classification of asthma severity were based on diagnoses, visits, and medication orders and fills. The estimates thus are prone to misclassification, which may have biased the results. Finally, information on important variables such as daycare attendance, presence of school-age siblings, and exposure to secondhand smoke was not available.
The research was funded by a grant from the National Institute of Allergy and Infectious Diseases. The authors had no relevant financial disclosures.
SOURCE: Nordin JD et al. Vaccine. 2019 Jun 10. doi: 10.1016/j.vaccine.2019.05.081.
, according to an analysis published in Vaccine.
The data corroborate other research indicating that live attenuated influenza vaccine (LAIV) is safe for children with asthma older than 2 years and suggest that the choice of vaccination in this population should be based on effectiveness, according to James D. Nordin, MD, MPH, a clinical researcher at HealthPartners Institute in Minneapolis, and colleagues.
Children and adolescents with asthma have an increased risk of morbidity if they contract influenza. They represent a disproportionate number of pediatric influenza hospitalizations and have been a focus of efforts to vaccinate children against influenza. Since 2003, the inactivated influenza vaccine (IIV) and the LAIV have been available. Research indicates that LAIV is more effective than IIV at preventing culture-confirmed influenza in children. Two studies found an increased risk of wheezing in children who received LAIV, but other studies failed to replicate these findings.
A retrospective cohort study
Dr. Nordin and associates conducted a retrospective observational cohort study to investigate whether use of a guideline recommending LAIV for children aged 2 years and older with asthma increased the risk of lower respiratory events within 21 or 42 days of vaccination, compared with standard guidelines to administer IIV in children with asthma. The investigators drew data from two large medical groups with independent clinical leadership that serve demographically similar populations in Minnesota. One group (the LAIV group) switched its preference for all children from IIV to LAIV in 2010. The control group continued using IIV for children with asthma throughout the study period. Each group operates more than 20 clinics.
The investigators included children and adolescents aged 2-17 years who presented during one or more influenza season from 2007-2008 through 2014-2015. Eligible participants had a diagnosis of asthma or wheezing, received one or more influenza vaccines, had continuous insurance enrollment, and had at least one primary care or asthma related subspecialty encounter. They excluded patients with contraindications for LAIV (e.g., pregnancy, malignancy, and cystic fibrosis) and those with any hospitalization, ED visit, or outpatient encounter for a lower respiratory event in the 42 days before influenza vaccination.
Dr. Nordin and colleagues used a generalized estimating equation regression to estimate the ratio of rate ratios (RORs) comparing events before and after vaccination between the LAIV guideline and control groups. The researchers examined covariates such as age, gender, race or ethnicity, Medicaid insurance for at least 1 month in the previous year, neighborhood poverty, and neighborhood rates of asthma.
No increased risk
The investigators included 4,771 children and 7,851 child-influenza records in their analysis. During the period from 2007 to 2010, there were 2,215 child-influenza records from children and adolescents included from the LAIV group and 735 from the IIV guideline group. From 2010 to 2015, there were 3,767 child-influenza records in children and adolescents from the LAIV group and 1,134 from the IIV guideline group. After the LAIV group adopted the new guideline, the proportion of patients receiving LAIV increased from 23% to 68% in the LAIV group and from 7% to 11% in the control group.
About 88% of lower respiratory events included diagnoses for asthma exacerbations. When the investigators adjusted the data for age, asthma severity, asthma control, race or ethnicity, and Medicaid coverage, they found no increase in lower respiratory events associated with the LAIV guideline. The adjusted ROR was 0.74 for lower respiratory events within 21 days of vaccination and 0.77 for lower respiratory events within 42 days of vaccination. The results were similar when Dr. Nordin and colleagues stratified the data by age group, and including additional covariates did not alter the ROR estimates. In all, 21 hospitalizations occurred within 42 days of influenza vaccination, and the LAIV guideline did not increase the risk for hospitalization.
“Findings from this study are consistent with several recent observational studies of LAIV in children and adolescents with asthma,” said Dr. Nordin and colleagues.
One limitation of the current study was that the data were restricted to the information available in electronic health care or claims records. The researchers therefore were able to observe only medically attended lower respiratory events. Furthermore, the exclusion of asthma management encounters and the classification of asthma severity were based on diagnoses, visits, and medication orders and fills. The estimates thus are prone to misclassification, which may have biased the results. Finally, information on important variables such as daycare attendance, presence of school-age siblings, and exposure to secondhand smoke was not available.
The research was funded by a grant from the National Institute of Allergy and Infectious Diseases. The authors had no relevant financial disclosures.
SOURCE: Nordin JD et al. Vaccine. 2019 Jun 10. doi: 10.1016/j.vaccine.2019.05.081.
, according to an analysis published in Vaccine.
The data corroborate other research indicating that live attenuated influenza vaccine (LAIV) is safe for children with asthma older than 2 years and suggest that the choice of vaccination in this population should be based on effectiveness, according to James D. Nordin, MD, MPH, a clinical researcher at HealthPartners Institute in Minneapolis, and colleagues.
Children and adolescents with asthma have an increased risk of morbidity if they contract influenza. They represent a disproportionate number of pediatric influenza hospitalizations and have been a focus of efforts to vaccinate children against influenza. Since 2003, the inactivated influenza vaccine (IIV) and the LAIV have been available. Research indicates that LAIV is more effective than IIV at preventing culture-confirmed influenza in children. Two studies found an increased risk of wheezing in children who received LAIV, but other studies failed to replicate these findings.
A retrospective cohort study
Dr. Nordin and associates conducted a retrospective observational cohort study to investigate whether use of a guideline recommending LAIV for children aged 2 years and older with asthma increased the risk of lower respiratory events within 21 or 42 days of vaccination, compared with standard guidelines to administer IIV in children with asthma. The investigators drew data from two large medical groups with independent clinical leadership that serve demographically similar populations in Minnesota. One group (the LAIV group) switched its preference for all children from IIV to LAIV in 2010. The control group continued using IIV for children with asthma throughout the study period. Each group operates more than 20 clinics.
The investigators included children and adolescents aged 2-17 years who presented during one or more influenza season from 2007-2008 through 2014-2015. Eligible participants had a diagnosis of asthma or wheezing, received one or more influenza vaccines, had continuous insurance enrollment, and had at least one primary care or asthma related subspecialty encounter. They excluded patients with contraindications for LAIV (e.g., pregnancy, malignancy, and cystic fibrosis) and those with any hospitalization, ED visit, or outpatient encounter for a lower respiratory event in the 42 days before influenza vaccination.
Dr. Nordin and colleagues used a generalized estimating equation regression to estimate the ratio of rate ratios (RORs) comparing events before and after vaccination between the LAIV guideline and control groups. The researchers examined covariates such as age, gender, race or ethnicity, Medicaid insurance for at least 1 month in the previous year, neighborhood poverty, and neighborhood rates of asthma.
No increased risk
The investigators included 4,771 children and 7,851 child-influenza records in their analysis. During the period from 2007 to 2010, there were 2,215 child-influenza records from children and adolescents included from the LAIV group and 735 from the IIV guideline group. From 2010 to 2015, there were 3,767 child-influenza records in children and adolescents from the LAIV group and 1,134 from the IIV guideline group. After the LAIV group adopted the new guideline, the proportion of patients receiving LAIV increased from 23% to 68% in the LAIV group and from 7% to 11% in the control group.
About 88% of lower respiratory events included diagnoses for asthma exacerbations. When the investigators adjusted the data for age, asthma severity, asthma control, race or ethnicity, and Medicaid coverage, they found no increase in lower respiratory events associated with the LAIV guideline. The adjusted ROR was 0.74 for lower respiratory events within 21 days of vaccination and 0.77 for lower respiratory events within 42 days of vaccination. The results were similar when Dr. Nordin and colleagues stratified the data by age group, and including additional covariates did not alter the ROR estimates. In all, 21 hospitalizations occurred within 42 days of influenza vaccination, and the LAIV guideline did not increase the risk for hospitalization.
“Findings from this study are consistent with several recent observational studies of LAIV in children and adolescents with asthma,” said Dr. Nordin and colleagues.
One limitation of the current study was that the data were restricted to the information available in electronic health care or claims records. The researchers therefore were able to observe only medically attended lower respiratory events. Furthermore, the exclusion of asthma management encounters and the classification of asthma severity were based on diagnoses, visits, and medication orders and fills. The estimates thus are prone to misclassification, which may have biased the results. Finally, information on important variables such as daycare attendance, presence of school-age siblings, and exposure to secondhand smoke was not available.
The research was funded by a grant from the National Institute of Allergy and Infectious Diseases. The authors had no relevant financial disclosures.
SOURCE: Nordin JD et al. Vaccine. 2019 Jun 10. doi: 10.1016/j.vaccine.2019.05.081.
FROM VACCINE
ACIP approves flu vaccine recommendations for 2019-2020 season
All individuals aged 6 months and older should receive the influenza vaccine by the end of October next season, according to the Centers for Disease Control and Prevention’s Committee on Immunization Practices. The committee voted unanimously to accept minor updates to the ACIP flu recommendations for the 2019-2020 season, but no major changes were made from recent years.
The past flu season was moderate overall, but notable for two waves of viral infections of similar magnitude, one with H1N1 and another with H3N2, said Lynette Brewer of the CDC’s National Center for Immunization and Respiratory Diseases, who presented data on last year’s flu activity.
Last year’s vaccine likely prevented between 40,000 and 90,000 hospitalizations, but mostly reduced the burden of H1N1 disease and provided no real protection against H3N2, she said.
The recommended H3N2 component for next season is A/Kansas/14/2017–like virus, which is genetically similar to the H3N2 that circulated last year.
Lisa Grohskopf, MD, of the CDC’s influenza division, presented the minor adjustments that included the changes in vaccine composition for next year, some licensure changes, and a new table summarizing dose volumes. Also, language was changed to advise vaccination for all eligible individuals by the end of October, and individuals who need two doses should have the first one as soon as it becomes available, in July or August if possible. The updated language also clarified that 8 year olds who need two doses should receive the second dose, even if they turn 9 between the two doses.
Additional guidance updates approved by the committee included harmonizing language on groups that should be the focus of vaccination in the event of limited supply to be more consistent with the 2011 ACIP Recommendations for the Immunization of Health Care Personnel.
The committee also voted unanimously to accept the proposed influenza vaccine in the Vaccines for Children program; there were no changes in recommended dosing intervals, dosages, contraindications, or precautions, according to Frank Whitlach of the National Center for Immunization and Respiratory Diseases, who presented the Vaccines for Children information.
The ACIP members had no financial conflicts to disclose.
All individuals aged 6 months and older should receive the influenza vaccine by the end of October next season, according to the Centers for Disease Control and Prevention’s Committee on Immunization Practices. The committee voted unanimously to accept minor updates to the ACIP flu recommendations for the 2019-2020 season, but no major changes were made from recent years.
The past flu season was moderate overall, but notable for two waves of viral infections of similar magnitude, one with H1N1 and another with H3N2, said Lynette Brewer of the CDC’s National Center for Immunization and Respiratory Diseases, who presented data on last year’s flu activity.
Last year’s vaccine likely prevented between 40,000 and 90,000 hospitalizations, but mostly reduced the burden of H1N1 disease and provided no real protection against H3N2, she said.
The recommended H3N2 component for next season is A/Kansas/14/2017–like virus, which is genetically similar to the H3N2 that circulated last year.
Lisa Grohskopf, MD, of the CDC’s influenza division, presented the minor adjustments that included the changes in vaccine composition for next year, some licensure changes, and a new table summarizing dose volumes. Also, language was changed to advise vaccination for all eligible individuals by the end of October, and individuals who need two doses should have the first one as soon as it becomes available, in July or August if possible. The updated language also clarified that 8 year olds who need two doses should receive the second dose, even if they turn 9 between the two doses.
Additional guidance updates approved by the committee included harmonizing language on groups that should be the focus of vaccination in the event of limited supply to be more consistent with the 2011 ACIP Recommendations for the Immunization of Health Care Personnel.
The committee also voted unanimously to accept the proposed influenza vaccine in the Vaccines for Children program; there were no changes in recommended dosing intervals, dosages, contraindications, or precautions, according to Frank Whitlach of the National Center for Immunization and Respiratory Diseases, who presented the Vaccines for Children information.
The ACIP members had no financial conflicts to disclose.
All individuals aged 6 months and older should receive the influenza vaccine by the end of October next season, according to the Centers for Disease Control and Prevention’s Committee on Immunization Practices. The committee voted unanimously to accept minor updates to the ACIP flu recommendations for the 2019-2020 season, but no major changes were made from recent years.
The past flu season was moderate overall, but notable for two waves of viral infections of similar magnitude, one with H1N1 and another with H3N2, said Lynette Brewer of the CDC’s National Center for Immunization and Respiratory Diseases, who presented data on last year’s flu activity.
Last year’s vaccine likely prevented between 40,000 and 90,000 hospitalizations, but mostly reduced the burden of H1N1 disease and provided no real protection against H3N2, she said.
The recommended H3N2 component for next season is A/Kansas/14/2017–like virus, which is genetically similar to the H3N2 that circulated last year.
Lisa Grohskopf, MD, of the CDC’s influenza division, presented the minor adjustments that included the changes in vaccine composition for next year, some licensure changes, and a new table summarizing dose volumes. Also, language was changed to advise vaccination for all eligible individuals by the end of October, and individuals who need two doses should have the first one as soon as it becomes available, in July or August if possible. The updated language also clarified that 8 year olds who need two doses should receive the second dose, even if they turn 9 between the two doses.
Additional guidance updates approved by the committee included harmonizing language on groups that should be the focus of vaccination in the event of limited supply to be more consistent with the 2011 ACIP Recommendations for the Immunization of Health Care Personnel.
The committee also voted unanimously to accept the proposed influenza vaccine in the Vaccines for Children program; there were no changes in recommended dosing intervals, dosages, contraindications, or precautions, according to Frank Whitlach of the National Center for Immunization and Respiratory Diseases, who presented the Vaccines for Children information.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
Building better flu vaccines is daunting
LJUBLJANA, SLOVENIA – Don’t hold your breath waiting for a substantially better, more reliably effective influenza vaccine.
That was a key cautionary message provided by vaccine expert Edward A. Belongia, MD, at the annual meeting of the European Society for Paediatric Infectious Diseases.
The effectiveness of seasonal influenza vaccine varies from 10% to 60% year by year, leaving enormous room for improvement. But many obstacles exist to developing a more consistent and reliably effective version of the seasonal influenza vaccine. And the lofty goal of creating a universal vaccine is even more ambitious, although the National Institute of Allergy and Infectious Diseases has declared it to be a top priority and mapped out a strategic plan for getting there (J Infect Dis. 2018 Jul 2;218[3]:347-54).
“Ultimately the Holy Grail is a universal flu vaccine that would provide pan-A and pan-B protection that would last for more than 1 year, with protection against avian and pandemic viruses, and would work for both children and adults. We are nowhere near that. Every 5 years someone says we’re 5 years away, and then 5 years go by and we’re still 5 years away. So I’m not making any predictions on that,” said Dr. Belongia, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wisc.) Clinic Research Institute, which is part of the U.S. Influenza Vaccine Effectiveness Network.
One of the big problems in creating a more effective flu vaccine, particularly for children, is the H3N2 virus subtype. Dr. Belongia was first author of a systematic review and meta-analysis of studies of more than a dozen recent flu seasons showing that although vaccine effectiveness against H3N2 varied widely from year to year, it was consistently lower than against influenza type B and H1N1 (Lancet Infect Dis. 2016 Aug;16[8]:942-51).
And that’s especially true in children and adolescents. Notably, in the 2014-2015 U.S. flu season, vaccine effectiveness against H3N2 in children aged 6 months to 8 years was low at 23%, but shockingly lower at a mere 7% in the 9- to 17-year-olds. Whereas in the 2017-2018 season, vaccine effectiveness against H3N2 in the 9- to 17-year-olds jumped to 46% while remaining low but consistent at 22% in the younger children.
“We see a very different age pattern here for the older children compared to the younger children, and quite frankly we don’t really understand what’s doing this,” said Dr. Belongia.
What is well understood, however, is that the problematic performance of influenza vaccines when it comes to protecting against H3N2 is a complicated matter stemming from three sources: the virus itself; the current egg-based vaccine manufacturing methodology, which is now 7 decades old; and host factors.
That troublesome H3N2 virus
Antigenic evolution of the H3N2 virus occurs at a 5- to 6-fold higher rate than for influenza B virus and roughly 17-fold faster than for H1N1. That high mutation rate makes for a moving target that’s a real problem when trying to keep a vaccine current. Also, the globular head of the virus is prone to glycosylation, which enables the virus to evade immune detection.
Vaccine-related factors
It’s likely that the availability of the flu vaccine for the upcoming 2019-2020 season is going to be delayed because of late selection of the strains for inclusion. The World Health Organization ordinarily selects strains for vaccines for the Northern Hemisphere in February, giving vaccine manufacturers 6-8 months to produce their vaccines and ship them in time for administration from September through November. This year, however, the WHO delayed selection of the H3N2 component until March because of the high level of antigenic and genetic diversity of circulating strains.
“This hasn’t happened since 2003 – it’s a very rare occurrence – but it does increase the potential that there’s going to be a delay in the availability of the vaccine in the fall,” he explained.
Eventually, the WHO selected a new clade 3C.3a virus called A/Kansas/14/2017 for the 2019-2020 vaccine. It should cover the circulating strains of H3N2 “reasonably well,” according to the physician.
Another issue: H3N2 has become adapted to the mammalian environment, so growing the virus in eggs introduces strong selection pressure for mutations leading to reduced vaccine effectiveness. Yet only two flu vaccines licensed in the United States are manufactured without eggs: Flucelvax, marketed by Seqirus for patients aged 4 years and up, and Sanofi’s Flublok, which is licensed for individuals who are 18 years of age or older. Studies are underway looking at the relative effectiveness of egg-based versus cell culture-manufactured flu vaccines in real-world settings.
Host factors
Hemagglutinin imprinting, sometimes referred to as “original antigenic sin,” is a decades-old concept whereby early childhood exposure to influenza viruses shapes future vaccine response.
“It suggests there could be some birth cohort effects in vaccine responsiveness, depending on what was circulating in the first 2-3 years after birth. It would complicate vaccine strategy quite a bit if you had to have different strategies for different birth cohorts,” Dr. Belongia observed.
Another host factor issue is the controversial topic of negative interference stemming from repeated vaccinations. It’s unclear how important this is in the real world, because studies have been inconsistent. Reassuringly, Dr. Belongia and coworkers found no association between prior-season influenza vaccination and diminished vaccine effectiveness in 3,369 U.S. children aged 2-17 years studied during the 2013-14 through 2015-16 flu seasons (JAMA Netw Open. 2018 Oct 5;1[6]:e183742. doi: 10.1001/jamanetworkopen.2018.3742).
“We found no suggestion at all of a problem with being vaccinated two seasons in a row,” according to Dr. Belongia.
How to build a better influenza vaccine for children
“I would say that even before we get to a universal vaccine, the next generation of flu vaccines that are more effective are not going to be manufactured using eggs, although we’re not real close to that. But I think that’s eventually where we’re going,” he said.
“I think it’s going to take a systems biology approach in order to really understand the adaptive immune response to infection and vaccination in early life. That means a much more detailed understanding of what is underlying the imprinting mechanisms and what is the adaptive response to repeated vaccination and infection. I think this is going to take prospective infant cohort studies; the National Institutes of Health is funding some that will begin within the next year,” Dr. Belongia added.
Many investigational approaches to improving influenza virus subtype-level protection are being explored. These include novel adjuvants, nanoparticle vaccines, computationally optimized broadly reactive antigens, and standardization of neuraminidase content.
And as for the much-desired universal flu vaccine?
“I will say that if a universal vaccine is going to work it’s probably going to work first in children. They have a much shorter immune history and their antibody landscape is a lot smaller, so you have a much better opportunity, I think, to generate a broad response to a universal vaccine compared to adults, who have much more complex immune landscapes,” he said.
Dr. Belongia reported having no financial conflicts regarding his presentation.
LJUBLJANA, SLOVENIA – Don’t hold your breath waiting for a substantially better, more reliably effective influenza vaccine.
That was a key cautionary message provided by vaccine expert Edward A. Belongia, MD, at the annual meeting of the European Society for Paediatric Infectious Diseases.
The effectiveness of seasonal influenza vaccine varies from 10% to 60% year by year, leaving enormous room for improvement. But many obstacles exist to developing a more consistent and reliably effective version of the seasonal influenza vaccine. And the lofty goal of creating a universal vaccine is even more ambitious, although the National Institute of Allergy and Infectious Diseases has declared it to be a top priority and mapped out a strategic plan for getting there (J Infect Dis. 2018 Jul 2;218[3]:347-54).
“Ultimately the Holy Grail is a universal flu vaccine that would provide pan-A and pan-B protection that would last for more than 1 year, with protection against avian and pandemic viruses, and would work for both children and adults. We are nowhere near that. Every 5 years someone says we’re 5 years away, and then 5 years go by and we’re still 5 years away. So I’m not making any predictions on that,” said Dr. Belongia, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wisc.) Clinic Research Institute, which is part of the U.S. Influenza Vaccine Effectiveness Network.
One of the big problems in creating a more effective flu vaccine, particularly for children, is the H3N2 virus subtype. Dr. Belongia was first author of a systematic review and meta-analysis of studies of more than a dozen recent flu seasons showing that although vaccine effectiveness against H3N2 varied widely from year to year, it was consistently lower than against influenza type B and H1N1 (Lancet Infect Dis. 2016 Aug;16[8]:942-51).
And that’s especially true in children and adolescents. Notably, in the 2014-2015 U.S. flu season, vaccine effectiveness against H3N2 in children aged 6 months to 8 years was low at 23%, but shockingly lower at a mere 7% in the 9- to 17-year-olds. Whereas in the 2017-2018 season, vaccine effectiveness against H3N2 in the 9- to 17-year-olds jumped to 46% while remaining low but consistent at 22% in the younger children.
“We see a very different age pattern here for the older children compared to the younger children, and quite frankly we don’t really understand what’s doing this,” said Dr. Belongia.
What is well understood, however, is that the problematic performance of influenza vaccines when it comes to protecting against H3N2 is a complicated matter stemming from three sources: the virus itself; the current egg-based vaccine manufacturing methodology, which is now 7 decades old; and host factors.
That troublesome H3N2 virus
Antigenic evolution of the H3N2 virus occurs at a 5- to 6-fold higher rate than for influenza B virus and roughly 17-fold faster than for H1N1. That high mutation rate makes for a moving target that’s a real problem when trying to keep a vaccine current. Also, the globular head of the virus is prone to glycosylation, which enables the virus to evade immune detection.
Vaccine-related factors
It’s likely that the availability of the flu vaccine for the upcoming 2019-2020 season is going to be delayed because of late selection of the strains for inclusion. The World Health Organization ordinarily selects strains for vaccines for the Northern Hemisphere in February, giving vaccine manufacturers 6-8 months to produce their vaccines and ship them in time for administration from September through November. This year, however, the WHO delayed selection of the H3N2 component until March because of the high level of antigenic and genetic diversity of circulating strains.
“This hasn’t happened since 2003 – it’s a very rare occurrence – but it does increase the potential that there’s going to be a delay in the availability of the vaccine in the fall,” he explained.
Eventually, the WHO selected a new clade 3C.3a virus called A/Kansas/14/2017 for the 2019-2020 vaccine. It should cover the circulating strains of H3N2 “reasonably well,” according to the physician.
Another issue: H3N2 has become adapted to the mammalian environment, so growing the virus in eggs introduces strong selection pressure for mutations leading to reduced vaccine effectiveness. Yet only two flu vaccines licensed in the United States are manufactured without eggs: Flucelvax, marketed by Seqirus for patients aged 4 years and up, and Sanofi’s Flublok, which is licensed for individuals who are 18 years of age or older. Studies are underway looking at the relative effectiveness of egg-based versus cell culture-manufactured flu vaccines in real-world settings.
Host factors
Hemagglutinin imprinting, sometimes referred to as “original antigenic sin,” is a decades-old concept whereby early childhood exposure to influenza viruses shapes future vaccine response.
“It suggests there could be some birth cohort effects in vaccine responsiveness, depending on what was circulating in the first 2-3 years after birth. It would complicate vaccine strategy quite a bit if you had to have different strategies for different birth cohorts,” Dr. Belongia observed.
Another host factor issue is the controversial topic of negative interference stemming from repeated vaccinations. It’s unclear how important this is in the real world, because studies have been inconsistent. Reassuringly, Dr. Belongia and coworkers found no association between prior-season influenza vaccination and diminished vaccine effectiveness in 3,369 U.S. children aged 2-17 years studied during the 2013-14 through 2015-16 flu seasons (JAMA Netw Open. 2018 Oct 5;1[6]:e183742. doi: 10.1001/jamanetworkopen.2018.3742).
“We found no suggestion at all of a problem with being vaccinated two seasons in a row,” according to Dr. Belongia.
How to build a better influenza vaccine for children
“I would say that even before we get to a universal vaccine, the next generation of flu vaccines that are more effective are not going to be manufactured using eggs, although we’re not real close to that. But I think that’s eventually where we’re going,” he said.
“I think it’s going to take a systems biology approach in order to really understand the adaptive immune response to infection and vaccination in early life. That means a much more detailed understanding of what is underlying the imprinting mechanisms and what is the adaptive response to repeated vaccination and infection. I think this is going to take prospective infant cohort studies; the National Institutes of Health is funding some that will begin within the next year,” Dr. Belongia added.
Many investigational approaches to improving influenza virus subtype-level protection are being explored. These include novel adjuvants, nanoparticle vaccines, computationally optimized broadly reactive antigens, and standardization of neuraminidase content.
And as for the much-desired universal flu vaccine?
“I will say that if a universal vaccine is going to work it’s probably going to work first in children. They have a much shorter immune history and their antibody landscape is a lot smaller, so you have a much better opportunity, I think, to generate a broad response to a universal vaccine compared to adults, who have much more complex immune landscapes,” he said.
Dr. Belongia reported having no financial conflicts regarding his presentation.
LJUBLJANA, SLOVENIA – Don’t hold your breath waiting for a substantially better, more reliably effective influenza vaccine.
That was a key cautionary message provided by vaccine expert Edward A. Belongia, MD, at the annual meeting of the European Society for Paediatric Infectious Diseases.
The effectiveness of seasonal influenza vaccine varies from 10% to 60% year by year, leaving enormous room for improvement. But many obstacles exist to developing a more consistent and reliably effective version of the seasonal influenza vaccine. And the lofty goal of creating a universal vaccine is even more ambitious, although the National Institute of Allergy and Infectious Diseases has declared it to be a top priority and mapped out a strategic plan for getting there (J Infect Dis. 2018 Jul 2;218[3]:347-54).
“Ultimately the Holy Grail is a universal flu vaccine that would provide pan-A and pan-B protection that would last for more than 1 year, with protection against avian and pandemic viruses, and would work for both children and adults. We are nowhere near that. Every 5 years someone says we’re 5 years away, and then 5 years go by and we’re still 5 years away. So I’m not making any predictions on that,” said Dr. Belongia, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wisc.) Clinic Research Institute, which is part of the U.S. Influenza Vaccine Effectiveness Network.
One of the big problems in creating a more effective flu vaccine, particularly for children, is the H3N2 virus subtype. Dr. Belongia was first author of a systematic review and meta-analysis of studies of more than a dozen recent flu seasons showing that although vaccine effectiveness against H3N2 varied widely from year to year, it was consistently lower than against influenza type B and H1N1 (Lancet Infect Dis. 2016 Aug;16[8]:942-51).
And that’s especially true in children and adolescents. Notably, in the 2014-2015 U.S. flu season, vaccine effectiveness against H3N2 in children aged 6 months to 8 years was low at 23%, but shockingly lower at a mere 7% in the 9- to 17-year-olds. Whereas in the 2017-2018 season, vaccine effectiveness against H3N2 in the 9- to 17-year-olds jumped to 46% while remaining low but consistent at 22% in the younger children.
“We see a very different age pattern here for the older children compared to the younger children, and quite frankly we don’t really understand what’s doing this,” said Dr. Belongia.
What is well understood, however, is that the problematic performance of influenza vaccines when it comes to protecting against H3N2 is a complicated matter stemming from three sources: the virus itself; the current egg-based vaccine manufacturing methodology, which is now 7 decades old; and host factors.
That troublesome H3N2 virus
Antigenic evolution of the H3N2 virus occurs at a 5- to 6-fold higher rate than for influenza B virus and roughly 17-fold faster than for H1N1. That high mutation rate makes for a moving target that’s a real problem when trying to keep a vaccine current. Also, the globular head of the virus is prone to glycosylation, which enables the virus to evade immune detection.
Vaccine-related factors
It’s likely that the availability of the flu vaccine for the upcoming 2019-2020 season is going to be delayed because of late selection of the strains for inclusion. The World Health Organization ordinarily selects strains for vaccines for the Northern Hemisphere in February, giving vaccine manufacturers 6-8 months to produce their vaccines and ship them in time for administration from September through November. This year, however, the WHO delayed selection of the H3N2 component until March because of the high level of antigenic and genetic diversity of circulating strains.
“This hasn’t happened since 2003 – it’s a very rare occurrence – but it does increase the potential that there’s going to be a delay in the availability of the vaccine in the fall,” he explained.
Eventually, the WHO selected a new clade 3C.3a virus called A/Kansas/14/2017 for the 2019-2020 vaccine. It should cover the circulating strains of H3N2 “reasonably well,” according to the physician.
Another issue: H3N2 has become adapted to the mammalian environment, so growing the virus in eggs introduces strong selection pressure for mutations leading to reduced vaccine effectiveness. Yet only two flu vaccines licensed in the United States are manufactured without eggs: Flucelvax, marketed by Seqirus for patients aged 4 years and up, and Sanofi’s Flublok, which is licensed for individuals who are 18 years of age or older. Studies are underway looking at the relative effectiveness of egg-based versus cell culture-manufactured flu vaccines in real-world settings.
Host factors
Hemagglutinin imprinting, sometimes referred to as “original antigenic sin,” is a decades-old concept whereby early childhood exposure to influenza viruses shapes future vaccine response.
“It suggests there could be some birth cohort effects in vaccine responsiveness, depending on what was circulating in the first 2-3 years after birth. It would complicate vaccine strategy quite a bit if you had to have different strategies for different birth cohorts,” Dr. Belongia observed.
Another host factor issue is the controversial topic of negative interference stemming from repeated vaccinations. It’s unclear how important this is in the real world, because studies have been inconsistent. Reassuringly, Dr. Belongia and coworkers found no association between prior-season influenza vaccination and diminished vaccine effectiveness in 3,369 U.S. children aged 2-17 years studied during the 2013-14 through 2015-16 flu seasons (JAMA Netw Open. 2018 Oct 5;1[6]:e183742. doi: 10.1001/jamanetworkopen.2018.3742).
“We found no suggestion at all of a problem with being vaccinated two seasons in a row,” according to Dr. Belongia.
How to build a better influenza vaccine for children
“I would say that even before we get to a universal vaccine, the next generation of flu vaccines that are more effective are not going to be manufactured using eggs, although we’re not real close to that. But I think that’s eventually where we’re going,” he said.
“I think it’s going to take a systems biology approach in order to really understand the adaptive immune response to infection and vaccination in early life. That means a much more detailed understanding of what is underlying the imprinting mechanisms and what is the adaptive response to repeated vaccination and infection. I think this is going to take prospective infant cohort studies; the National Institutes of Health is funding some that will begin within the next year,” Dr. Belongia added.
Many investigational approaches to improving influenza virus subtype-level protection are being explored. These include novel adjuvants, nanoparticle vaccines, computationally optimized broadly reactive antigens, and standardization of neuraminidase content.
And as for the much-desired universal flu vaccine?
“I will say that if a universal vaccine is going to work it’s probably going to work first in children. They have a much shorter immune history and their antibody landscape is a lot smaller, so you have a much better opportunity, I think, to generate a broad response to a universal vaccine compared to adults, who have much more complex immune landscapes,” he said.
Dr. Belongia reported having no financial conflicts regarding his presentation.
REPORTING FROM ESPID 2019
Flu vaccine visits reveal missed opportunities for HPV vaccination
BALTIMORE – according to a study.
“Overall in preventive visits, missed opportunities were much higher for HPV, compared to the other two vaccines” recommended for adolescents, MenACWY (meningococcal conjugate vaccine) and Tdap, Mary Kate Kelly, MPH, of Children’s Hospital of Philadelphia, told attendees at the Pediatric Academic Societies annual meeting. “In order to increase vaccination rates, it’s essential to implement efforts to reduce missed opportunities.”
According to 2018 Centers for Disease Control and Prevention data, Ms. Kelly said, vaccine coverage for the HPV vaccine is approximately 66%, compared with 85% for the MenACWY vaccine and 89% for the Tdap vaccine.
Ms. Kelly and her colleagues investigated how often children or adolescents missed an opportunity to get an HPV vaccine when they received an influenza vaccine during an office visit. This study was part of the larger STOP HPV trial funded by the National Institutes of Health and aimed at implementing evidence-based interventions to reduce missed opportunities for HPV vaccination in primary care.
The researchers retrospectively reviewed EHRs from 2015 to 2018 for 48 pediatric practices across 19 states. All practices were part of the American Academy of Pediatrics’ Pediatric Research in Office Settings (PROS) national pediatric primary care network. The researchers isolated all visits for patients aged 11-17 years who received their flu vaccine and were eligible to receive the HPV vaccine.
The investigators defined a missed opportunity as one in which a patient was due for the HPV vaccine but did not receive one at the visit when they received their flu vaccine.
The study involved 40,129 patients who received the flu vaccine at 52,818 visits when they also were eligible to receive the HPV vaccine. The median age of patients was 12 years old, and 47% were female.
In 68% of visits, the patient could have received an HPV vaccine but did not – even though they were due and eligible for one. The rate was the same for boys and for girls. By contrast, only 38% of visits involved a missed opportunity for the MenACWY vaccines and 39% for the Tdap vaccine.
Rates of missed opportunities for HPV vaccination ranged among individual practices from 22% to 81% of overall visits. Patients were more than twice as likely to miss the opportunity for an HPV vaccine dose if it would have been their first dose – 70% of missed opportunities – versus being a second or third dose, which comprised 30% of missed opportunities (adjusted relative risk, 2.48; P less than .001)).
“However, missed opportunities were also common for subsequent HPV doses when vaccine hesitancy is less likely to be an issue,” Ms. Kelly added.
It also was much more likely that missed opportunities occurred during nurse visits or visits for an acute or chronic condition rather than preventive visits, which made up about half (51%) of all visits analyzed. While 48% of preventive visits involved a missed opportunity, 93% of nurse visits (aRR compared with preventive, 2.18; P less than.001) and 89% of acute or chronic visits (aRR, 2.11; P less than .001) did.
Percentages of missed opportunities were similarly high for the MenACWY and Tdap vaccines at nurse visits and acute/chronic visits, but much lower at preventive visits for the MenACWY (12%) and Tdap (15%) vaccines.
“Increasing simultaneous administration of HPV and other adolescent vaccines with the influenza vaccine may help to improve coverage,” Ms. Kelly concluded.
The study was limited by its use of a convenience sample from practices that were interested in participating and willing to stock the HPV vaccine. Additionally, the researchers could not detect or adjust for EHR errors or inaccurate or incomplete vaccine histories, and they were unable to look at vaccine hesitancy or refusal with the EHRs.
The research was funded by the National Institutes of Health, the U.S. Department of Health & Human Services, and the National Research Network to Improve Children’s Health. The authors reported no relevant financial disclosures.
BALTIMORE – according to a study.
“Overall in preventive visits, missed opportunities were much higher for HPV, compared to the other two vaccines” recommended for adolescents, MenACWY (meningococcal conjugate vaccine) and Tdap, Mary Kate Kelly, MPH, of Children’s Hospital of Philadelphia, told attendees at the Pediatric Academic Societies annual meeting. “In order to increase vaccination rates, it’s essential to implement efforts to reduce missed opportunities.”
According to 2018 Centers for Disease Control and Prevention data, Ms. Kelly said, vaccine coverage for the HPV vaccine is approximately 66%, compared with 85% for the MenACWY vaccine and 89% for the Tdap vaccine.
Ms. Kelly and her colleagues investigated how often children or adolescents missed an opportunity to get an HPV vaccine when they received an influenza vaccine during an office visit. This study was part of the larger STOP HPV trial funded by the National Institutes of Health and aimed at implementing evidence-based interventions to reduce missed opportunities for HPV vaccination in primary care.
The researchers retrospectively reviewed EHRs from 2015 to 2018 for 48 pediatric practices across 19 states. All practices were part of the American Academy of Pediatrics’ Pediatric Research in Office Settings (PROS) national pediatric primary care network. The researchers isolated all visits for patients aged 11-17 years who received their flu vaccine and were eligible to receive the HPV vaccine.
The investigators defined a missed opportunity as one in which a patient was due for the HPV vaccine but did not receive one at the visit when they received their flu vaccine.
The study involved 40,129 patients who received the flu vaccine at 52,818 visits when they also were eligible to receive the HPV vaccine. The median age of patients was 12 years old, and 47% were female.
In 68% of visits, the patient could have received an HPV vaccine but did not – even though they were due and eligible for one. The rate was the same for boys and for girls. By contrast, only 38% of visits involved a missed opportunity for the MenACWY vaccines and 39% for the Tdap vaccine.
Rates of missed opportunities for HPV vaccination ranged among individual practices from 22% to 81% of overall visits. Patients were more than twice as likely to miss the opportunity for an HPV vaccine dose if it would have been their first dose – 70% of missed opportunities – versus being a second or third dose, which comprised 30% of missed opportunities (adjusted relative risk, 2.48; P less than .001)).
“However, missed opportunities were also common for subsequent HPV doses when vaccine hesitancy is less likely to be an issue,” Ms. Kelly added.
It also was much more likely that missed opportunities occurred during nurse visits or visits for an acute or chronic condition rather than preventive visits, which made up about half (51%) of all visits analyzed. While 48% of preventive visits involved a missed opportunity, 93% of nurse visits (aRR compared with preventive, 2.18; P less than.001) and 89% of acute or chronic visits (aRR, 2.11; P less than .001) did.
Percentages of missed opportunities were similarly high for the MenACWY and Tdap vaccines at nurse visits and acute/chronic visits, but much lower at preventive visits for the MenACWY (12%) and Tdap (15%) vaccines.
“Increasing simultaneous administration of HPV and other adolescent vaccines with the influenza vaccine may help to improve coverage,” Ms. Kelly concluded.
The study was limited by its use of a convenience sample from practices that were interested in participating and willing to stock the HPV vaccine. Additionally, the researchers could not detect or adjust for EHR errors or inaccurate or incomplete vaccine histories, and they were unable to look at vaccine hesitancy or refusal with the EHRs.
The research was funded by the National Institutes of Health, the U.S. Department of Health & Human Services, and the National Research Network to Improve Children’s Health. The authors reported no relevant financial disclosures.
BALTIMORE – according to a study.
“Overall in preventive visits, missed opportunities were much higher for HPV, compared to the other two vaccines” recommended for adolescents, MenACWY (meningococcal conjugate vaccine) and Tdap, Mary Kate Kelly, MPH, of Children’s Hospital of Philadelphia, told attendees at the Pediatric Academic Societies annual meeting. “In order to increase vaccination rates, it’s essential to implement efforts to reduce missed opportunities.”
According to 2018 Centers for Disease Control and Prevention data, Ms. Kelly said, vaccine coverage for the HPV vaccine is approximately 66%, compared with 85% for the MenACWY vaccine and 89% for the Tdap vaccine.
Ms. Kelly and her colleagues investigated how often children or adolescents missed an opportunity to get an HPV vaccine when they received an influenza vaccine during an office visit. This study was part of the larger STOP HPV trial funded by the National Institutes of Health and aimed at implementing evidence-based interventions to reduce missed opportunities for HPV vaccination in primary care.
The researchers retrospectively reviewed EHRs from 2015 to 2018 for 48 pediatric practices across 19 states. All practices were part of the American Academy of Pediatrics’ Pediatric Research in Office Settings (PROS) national pediatric primary care network. The researchers isolated all visits for patients aged 11-17 years who received their flu vaccine and were eligible to receive the HPV vaccine.
The investigators defined a missed opportunity as one in which a patient was due for the HPV vaccine but did not receive one at the visit when they received their flu vaccine.
The study involved 40,129 patients who received the flu vaccine at 52,818 visits when they also were eligible to receive the HPV vaccine. The median age of patients was 12 years old, and 47% were female.
In 68% of visits, the patient could have received an HPV vaccine but did not – even though they were due and eligible for one. The rate was the same for boys and for girls. By contrast, only 38% of visits involved a missed opportunity for the MenACWY vaccines and 39% for the Tdap vaccine.
Rates of missed opportunities for HPV vaccination ranged among individual practices from 22% to 81% of overall visits. Patients were more than twice as likely to miss the opportunity for an HPV vaccine dose if it would have been their first dose – 70% of missed opportunities – versus being a second or third dose, which comprised 30% of missed opportunities (adjusted relative risk, 2.48; P less than .001)).
“However, missed opportunities were also common for subsequent HPV doses when vaccine hesitancy is less likely to be an issue,” Ms. Kelly added.
It also was much more likely that missed opportunities occurred during nurse visits or visits for an acute or chronic condition rather than preventive visits, which made up about half (51%) of all visits analyzed. While 48% of preventive visits involved a missed opportunity, 93% of nurse visits (aRR compared with preventive, 2.18; P less than.001) and 89% of acute or chronic visits (aRR, 2.11; P less than .001) did.
Percentages of missed opportunities were similarly high for the MenACWY and Tdap vaccines at nurse visits and acute/chronic visits, but much lower at preventive visits for the MenACWY (12%) and Tdap (15%) vaccines.
“Increasing simultaneous administration of HPV and other adolescent vaccines with the influenza vaccine may help to improve coverage,” Ms. Kelly concluded.
The study was limited by its use of a convenience sample from practices that were interested in participating and willing to stock the HPV vaccine. Additionally, the researchers could not detect or adjust for EHR errors or inaccurate or incomplete vaccine histories, and they were unable to look at vaccine hesitancy or refusal with the EHRs.
The research was funded by the National Institutes of Health, the U.S. Department of Health & Human Services, and the National Research Network to Improve Children’s Health. The authors reported no relevant financial disclosures.
REPORTING FROM PAS 2019
Flu activity falling but still elevated
Measures of influenza activity fell again as the flu season continues to make its later-than-usual departure this year, according to the Centers for Disease Control and Prevention.
On the geographic front, the map of influenza-like illness (ILI) activity for the week ending March 30 shows that only 6 states are at level 10 on the CDC’s 1-10 scale, compared with 11 for the previous week, and that those same 6 states make up the entire membership of the high range of levels 8-10, which is down from 20 states a week ago, data from the CDC’s Outpatient ILI Surveillance Network show.
The proportion of outpatient visits for ILI, now at 3.2%, dropped for the sixth consecutive week after reaching its season high of 5.1% back in mid-February. The outpatient rate has now been at or above the national baseline of 2.2% for 19 weeks this season, the CDC’s influenza division said April 5, noting that the average for the past five seasons is 16 weeks.
Six flu-related pediatric deaths were reported in the week ending March 30, and the total is now 82 for the 2018-2019 season. Five of the six occurred during previous weeks of this season, and one occurred in the 2017-2018 season, the CDC said.
Measures of influenza activity fell again as the flu season continues to make its later-than-usual departure this year, according to the Centers for Disease Control and Prevention.

On the geographic front, the map of influenza-like illness (ILI) activity for the week ending March 30 shows that only 6 states are at level 10 on the CDC’s 1-10 scale, compared with 11 for the previous week, and that those same 6 states make up the entire membership of the high range of levels 8-10, which is down from 20 states a week ago, data from the CDC’s Outpatient ILI Surveillance Network show.
The proportion of outpatient visits for ILI, now at 3.2%, dropped for the sixth consecutive week after reaching its season high of 5.1% back in mid-February. The outpatient rate has now been at or above the national baseline of 2.2% for 19 weeks this season, the CDC’s influenza division said April 5, noting that the average for the past five seasons is 16 weeks.
Six flu-related pediatric deaths were reported in the week ending March 30, and the total is now 82 for the 2018-2019 season. Five of the six occurred during previous weeks of this season, and one occurred in the 2017-2018 season, the CDC said.
Measures of influenza activity fell again as the flu season continues to make its later-than-usual departure this year, according to the Centers for Disease Control and Prevention.

On the geographic front, the map of influenza-like illness (ILI) activity for the week ending March 30 shows that only 6 states are at level 10 on the CDC’s 1-10 scale, compared with 11 for the previous week, and that those same 6 states make up the entire membership of the high range of levels 8-10, which is down from 20 states a week ago, data from the CDC’s Outpatient ILI Surveillance Network show.
The proportion of outpatient visits for ILI, now at 3.2%, dropped for the sixth consecutive week after reaching its season high of 5.1% back in mid-February. The outpatient rate has now been at or above the national baseline of 2.2% for 19 weeks this season, the CDC’s influenza division said April 5, noting that the average for the past five seasons is 16 weeks.
Six flu-related pediatric deaths were reported in the week ending March 30, and the total is now 82 for the 2018-2019 season. Five of the six occurred during previous weeks of this season, and one occurred in the 2017-2018 season, the CDC said.
NIH to undertake first in-human trial of universal influenza vaccine
The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, is launching the first in-human trial of a universal influenza vaccine candidate.
The experimental vaccine, H1ssF_3928, is derived from the stem of an H1N1 virus and has a surface made from hemagglutinin and ferritin. By including only the stem of the virus, which changes less than the head, the vaccine should require fewer updates. A similar vaccine made from the same materials was shown to be safe and well tolerated in humans.
The clinical trial (NCT03814720) will be conducted at the NIH Clinical Center in Bethesda, Md., and will gradually enroll at least 53 healthy adults aged 18-70 years. The first 5 participants will receive one 20-mcg intramuscular injection of the vaccine; the other 48 participants will receive two 60-mcg vaccinations 16 weeks apart. Patients will return for 9-11 follow-ups over a 12- to 15-month period, and will provide blood samples for analysis of anti-influenza antibodies.
“Seasonal influenza is a perpetual public health challenge, and we continually face the possibility of an influenza pandemic resulting from the emergence and spread of novel influenza viruses. This phase 1 clinical trial is a step forward in our efforts to develop a durable and broadly protective universal influenza vaccine,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in the press release.
Find the full press release on the NIH website.
The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, is launching the first in-human trial of a universal influenza vaccine candidate.
The experimental vaccine, H1ssF_3928, is derived from the stem of an H1N1 virus and has a surface made from hemagglutinin and ferritin. By including only the stem of the virus, which changes less than the head, the vaccine should require fewer updates. A similar vaccine made from the same materials was shown to be safe and well tolerated in humans.
The clinical trial (NCT03814720) will be conducted at the NIH Clinical Center in Bethesda, Md., and will gradually enroll at least 53 healthy adults aged 18-70 years. The first 5 participants will receive one 20-mcg intramuscular injection of the vaccine; the other 48 participants will receive two 60-mcg vaccinations 16 weeks apart. Patients will return for 9-11 follow-ups over a 12- to 15-month period, and will provide blood samples for analysis of anti-influenza antibodies.
“Seasonal influenza is a perpetual public health challenge, and we continually face the possibility of an influenza pandemic resulting from the emergence and spread of novel influenza viruses. This phase 1 clinical trial is a step forward in our efforts to develop a durable and broadly protective universal influenza vaccine,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in the press release.
Find the full press release on the NIH website.
The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, is launching the first in-human trial of a universal influenza vaccine candidate.
The experimental vaccine, H1ssF_3928, is derived from the stem of an H1N1 virus and has a surface made from hemagglutinin and ferritin. By including only the stem of the virus, which changes less than the head, the vaccine should require fewer updates. A similar vaccine made from the same materials was shown to be safe and well tolerated in humans.
The clinical trial (NCT03814720) will be conducted at the NIH Clinical Center in Bethesda, Md., and will gradually enroll at least 53 healthy adults aged 18-70 years. The first 5 participants will receive one 20-mcg intramuscular injection of the vaccine; the other 48 participants will receive two 60-mcg vaccinations 16 weeks apart. Patients will return for 9-11 follow-ups over a 12- to 15-month period, and will provide blood samples for analysis of anti-influenza antibodies.
“Seasonal influenza is a perpetual public health challenge, and we continually face the possibility of an influenza pandemic resulting from the emergence and spread of novel influenza viruses. This phase 1 clinical trial is a step forward in our efforts to develop a durable and broadly protective universal influenza vaccine,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in the press release.
Find the full press release on the NIH website.
Flu shot can be given irrespective of the time of last methotrexate dose
Immune response to influenza vaccination in rheumatoid arthritis patients taking methotrexate appears to depend most on stopping the next two weekly doses of the drug rather than any effect from the timing of the last dose, new research concludes.
The new finding, reported in Annals of the Rheumatic Diseases, stems from a post hoc analysis of a randomized, controlled trial that Jin Kyun Park, MD, of Seoul (Korea) National University, and his colleagues had conducted earlier on immune response when patients stopped methotrexate for either 2 or 4 weeks after vaccination. While the main endpoint of that study showed no difference in the improvement in vaccine response with either stopping methotrexate for 2 or 4 weeks and no increase in disease activity with stopping for 2 weeks, it was unclear whether the timing of the last dose mattered when stopping for 2 weeks.
In a bid to identify the optimal time between the last dose of methotrexate and administration of a flu vaccine, Dr. Park and his colleagues conducted a post hoc analysis of the trial, which involved 316 patients with RA receiving methotrexate for 6 weeks or longer to continue (n = 156) or to hold methotrexate (n = 160) for 2 weeks after receiving a quadrivalent influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria.
The study authors defined a positive vaccine response as a fourfold or greater increase in hemagglutination inhibition (HI) antibody titer. A satisfactory vaccine response was a positive response to two or more of four vaccine antigens.
Patients who stopped taking methotrexate were divided into eight subgroups according to the number of days between their last dose and their vaccination.
The research team reported that response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
However, they conceded that “the absence of impact of the number of days between the last methotrexate dose and vaccination could be due to the small patient numbers in eight subgroups.”
Vaccine response also did not differ between patients who received the influenza vaccination within 3 days of the last methotrexate dose (n = 65) and those who received it between 4-7 days of the last methotrexate dose (n = 95).
Furthermore, RA disease activity, seropositivity, or use of conventional or biologic disease-modifying antirheumatic drugs did not have an impact on methotrexate discontinuation.
The authors concluded that vaccinations could be given irrespective of the time of the last methotrexate dose, and patients should be advised to skip two weekly doses following vaccination.
“This supports the notion that the effects of methotrexate on humeral immunity occur rapidly, despite the delayed effects on arthritis; therefore, the absence of methotrexate during the first 2 weeks postvaccination is critical for humoral immunity,” they wrote.
The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
SOURCE: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187.
Immune response to influenza vaccination in rheumatoid arthritis patients taking methotrexate appears to depend most on stopping the next two weekly doses of the drug rather than any effect from the timing of the last dose, new research concludes.
The new finding, reported in Annals of the Rheumatic Diseases, stems from a post hoc analysis of a randomized, controlled trial that Jin Kyun Park, MD, of Seoul (Korea) National University, and his colleagues had conducted earlier on immune response when patients stopped methotrexate for either 2 or 4 weeks after vaccination. While the main endpoint of that study showed no difference in the improvement in vaccine response with either stopping methotrexate for 2 or 4 weeks and no increase in disease activity with stopping for 2 weeks, it was unclear whether the timing of the last dose mattered when stopping for 2 weeks.
In a bid to identify the optimal time between the last dose of methotrexate and administration of a flu vaccine, Dr. Park and his colleagues conducted a post hoc analysis of the trial, which involved 316 patients with RA receiving methotrexate for 6 weeks or longer to continue (n = 156) or to hold methotrexate (n = 160) for 2 weeks after receiving a quadrivalent influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria.
The study authors defined a positive vaccine response as a fourfold or greater increase in hemagglutination inhibition (HI) antibody titer. A satisfactory vaccine response was a positive response to two or more of four vaccine antigens.
Patients who stopped taking methotrexate were divided into eight subgroups according to the number of days between their last dose and their vaccination.
The research team reported that response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
However, they conceded that “the absence of impact of the number of days between the last methotrexate dose and vaccination could be due to the small patient numbers in eight subgroups.”
Vaccine response also did not differ between patients who received the influenza vaccination within 3 days of the last methotrexate dose (n = 65) and those who received it between 4-7 days of the last methotrexate dose (n = 95).
Furthermore, RA disease activity, seropositivity, or use of conventional or biologic disease-modifying antirheumatic drugs did not have an impact on methotrexate discontinuation.
The authors concluded that vaccinations could be given irrespective of the time of the last methotrexate dose, and patients should be advised to skip two weekly doses following vaccination.
“This supports the notion that the effects of methotrexate on humeral immunity occur rapidly, despite the delayed effects on arthritis; therefore, the absence of methotrexate during the first 2 weeks postvaccination is critical for humoral immunity,” they wrote.
The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
SOURCE: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187.
Immune response to influenza vaccination in rheumatoid arthritis patients taking methotrexate appears to depend most on stopping the next two weekly doses of the drug rather than any effect from the timing of the last dose, new research concludes.
The new finding, reported in Annals of the Rheumatic Diseases, stems from a post hoc analysis of a randomized, controlled trial that Jin Kyun Park, MD, of Seoul (Korea) National University, and his colleagues had conducted earlier on immune response when patients stopped methotrexate for either 2 or 4 weeks after vaccination. While the main endpoint of that study showed no difference in the improvement in vaccine response with either stopping methotrexate for 2 or 4 weeks and no increase in disease activity with stopping for 2 weeks, it was unclear whether the timing of the last dose mattered when stopping for 2 weeks.
In a bid to identify the optimal time between the last dose of methotrexate and administration of a flu vaccine, Dr. Park and his colleagues conducted a post hoc analysis of the trial, which involved 316 patients with RA receiving methotrexate for 6 weeks or longer to continue (n = 156) or to hold methotrexate (n = 160) for 2 weeks after receiving a quadrivalent influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria.
The study authors defined a positive vaccine response as a fourfold or greater increase in hemagglutination inhibition (HI) antibody titer. A satisfactory vaccine response was a positive response to two or more of four vaccine antigens.
Patients who stopped taking methotrexate were divided into eight subgroups according to the number of days between their last dose and their vaccination.
The research team reported that response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
However, they conceded that “the absence of impact of the number of days between the last methotrexate dose and vaccination could be due to the small patient numbers in eight subgroups.”
Vaccine response also did not differ between patients who received the influenza vaccination within 3 days of the last methotrexate dose (n = 65) and those who received it between 4-7 days of the last methotrexate dose (n = 95).
Furthermore, RA disease activity, seropositivity, or use of conventional or biologic disease-modifying antirheumatic drugs did not have an impact on methotrexate discontinuation.
The authors concluded that vaccinations could be given irrespective of the time of the last methotrexate dose, and patients should be advised to skip two weekly doses following vaccination.
“This supports the notion that the effects of methotrexate on humeral immunity occur rapidly, despite the delayed effects on arthritis; therefore, the absence of methotrexate during the first 2 weeks postvaccination is critical for humoral immunity,” they wrote.
The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
SOURCE: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
Study details: A post hoc analysis of a randomized, controlled trial involving 316 patients with rheumatoid arthritis who continued or stopped methotrexate for 2 weeks following influenza vaccination.
Disclosures: The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
Source: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187