User login
Fluarix Quadrivalent effective in very young, simplifies flu shots for all ages
Fluarix Quadrivalent is highly effective against moderate and severe flu strains in children aged 6-35 months, and has the potential to simplify influenza vaccinations for all ages, according the results of a phase 3 clinical trial presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“Fluarix Quadrivalent, at the 0.5-mL dose in young children 6 to 35 months of age, demonstrated efficacy of 63.2% against moderate to severe influenza and 49.8% against any severity influenza disease” stated Leonard Friedland, MD, director of scientific affairs and public health, Vaccines North America, GlaxoSmithKline. Dr. Friedland, a pediatrician in Pennsylvania, said that a standard 0.5-mL dose of Fluarix Quadrivalent has practice-changing implications for physicians. “The use of a 0.5-mL dose (15 mcg per strain) for all persons aged 6 months and older potentially simplifies influenza vaccination by allowing the same vaccine dose to be used for all eligible individuals.”
The high efficacy of Fluarix against almost half of all influenza strains, regardless of severity, and in preventing moderate to severe influenza, correlated with a reduction in health care utilization by pediatric influenza patients, he said. Visits to general practitioners and emergency departments decreased by 47% and 79%, respectively, in children aged 6-35 months. Influenza-associated antibiotic use in these pediatric influenza patients also decreased by 50%.
These findings were the result of D-QIV-004, a phase 3, observer-blinded, randomized trial of 12,018 children aged 6-35 months. These children were split into five cohorts, each in a different influenza season. The study spanned 13 countries and ran from October 2011 to December 2014. To determine the safety of Fluarix, the study utilized noninfluenza vaccine comparator vaccines that were age appropriate, including Prevnar 13, Havrix, and Varivax.
A majority of the children in the study (98%) were vaccine unprimed (had never received two doses of seasonal influenza vaccine) and received two doses of Fluarix. The remaining children received one dose.
On Jan. 11, 2018, the Food and Drug Administration expanded the indication of Fluarix Quadrivalent to include use in persons 6 months and older. Previously, it was approved only for persons 3 years and older.
“These study results support universal vaccination of all individuals from 6 months of age [with Fluarix] to prevent influenza.” Dr. Friedland concluded.
For live updates and information concerning influenza, visit the CDC website.
ilacy@frontlinemedcom.com
SOURCE: D-QIV-004.
Fluarix Quadrivalent is highly effective against moderate and severe flu strains in children aged 6-35 months, and has the potential to simplify influenza vaccinations for all ages, according the results of a phase 3 clinical trial presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“Fluarix Quadrivalent, at the 0.5-mL dose in young children 6 to 35 months of age, demonstrated efficacy of 63.2% against moderate to severe influenza and 49.8% against any severity influenza disease” stated Leonard Friedland, MD, director of scientific affairs and public health, Vaccines North America, GlaxoSmithKline. Dr. Friedland, a pediatrician in Pennsylvania, said that a standard 0.5-mL dose of Fluarix Quadrivalent has practice-changing implications for physicians. “The use of a 0.5-mL dose (15 mcg per strain) for all persons aged 6 months and older potentially simplifies influenza vaccination by allowing the same vaccine dose to be used for all eligible individuals.”
The high efficacy of Fluarix against almost half of all influenza strains, regardless of severity, and in preventing moderate to severe influenza, correlated with a reduction in health care utilization by pediatric influenza patients, he said. Visits to general practitioners and emergency departments decreased by 47% and 79%, respectively, in children aged 6-35 months. Influenza-associated antibiotic use in these pediatric influenza patients also decreased by 50%.
These findings were the result of D-QIV-004, a phase 3, observer-blinded, randomized trial of 12,018 children aged 6-35 months. These children were split into five cohorts, each in a different influenza season. The study spanned 13 countries and ran from October 2011 to December 2014. To determine the safety of Fluarix, the study utilized noninfluenza vaccine comparator vaccines that were age appropriate, including Prevnar 13, Havrix, and Varivax.
A majority of the children in the study (98%) were vaccine unprimed (had never received two doses of seasonal influenza vaccine) and received two doses of Fluarix. The remaining children received one dose.
On Jan. 11, 2018, the Food and Drug Administration expanded the indication of Fluarix Quadrivalent to include use in persons 6 months and older. Previously, it was approved only for persons 3 years and older.
“These study results support universal vaccination of all individuals from 6 months of age [with Fluarix] to prevent influenza.” Dr. Friedland concluded.
For live updates and information concerning influenza, visit the CDC website.
ilacy@frontlinemedcom.com
SOURCE: D-QIV-004.
Fluarix Quadrivalent is highly effective against moderate and severe flu strains in children aged 6-35 months, and has the potential to simplify influenza vaccinations for all ages, according the results of a phase 3 clinical trial presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“Fluarix Quadrivalent, at the 0.5-mL dose in young children 6 to 35 months of age, demonstrated efficacy of 63.2% against moderate to severe influenza and 49.8% against any severity influenza disease” stated Leonard Friedland, MD, director of scientific affairs and public health, Vaccines North America, GlaxoSmithKline. Dr. Friedland, a pediatrician in Pennsylvania, said that a standard 0.5-mL dose of Fluarix Quadrivalent has practice-changing implications for physicians. “The use of a 0.5-mL dose (15 mcg per strain) for all persons aged 6 months and older potentially simplifies influenza vaccination by allowing the same vaccine dose to be used for all eligible individuals.”
The high efficacy of Fluarix against almost half of all influenza strains, regardless of severity, and in preventing moderate to severe influenza, correlated with a reduction in health care utilization by pediatric influenza patients, he said. Visits to general practitioners and emergency departments decreased by 47% and 79%, respectively, in children aged 6-35 months. Influenza-associated antibiotic use in these pediatric influenza patients also decreased by 50%.
These findings were the result of D-QIV-004, a phase 3, observer-blinded, randomized trial of 12,018 children aged 6-35 months. These children were split into five cohorts, each in a different influenza season. The study spanned 13 countries and ran from October 2011 to December 2014. To determine the safety of Fluarix, the study utilized noninfluenza vaccine comparator vaccines that were age appropriate, including Prevnar 13, Havrix, and Varivax.
A majority of the children in the study (98%) were vaccine unprimed (had never received two doses of seasonal influenza vaccine) and received two doses of Fluarix. The remaining children received one dose.
On Jan. 11, 2018, the Food and Drug Administration expanded the indication of Fluarix Quadrivalent to include use in persons 6 months and older. Previously, it was approved only for persons 3 years and older.
“These study results support universal vaccination of all individuals from 6 months of age [with Fluarix] to prevent influenza.” Dr. Friedland concluded.
For live updates and information concerning influenza, visit the CDC website.
ilacy@frontlinemedcom.com
SOURCE: D-QIV-004.
FROM AN ACIP MEETING
Key clinical point: The high efficacy of Fluarix against almost half of all influenza strains, regardless of severity, as well as preventing moderate to severe influenza, reduced health care utilization by pediatric influenza patients.
Major finding: Fluarix Quadrivalent was effective against moderate to severe influenza in 63.2% and against any severity of influenza in 49.8% of children aged 6-35 months.
Study details: A phase 3, observer-blinded, randomized trial of 12,018 children aged 6-35 months, in which the children were split into five cohorts, each in a different influenza season from October 2011 to December 2014.
Disclosures: No disclosures were reported.
Source: The D-QIV-004 study.
Flu season shows signs of slowing
Flu-related outpatient activity dropped for the second week in a row as the cumulative hospitalization rate continues to rise, according to data from the Centers for Disease Control and Prevention.
For the week ending Feb. 17, the proportion of outpatient visits for influenza-like illness (ILI) was 6.4%, which was down from 7.4% the previous week (Feb. 10) and down from the seasonal high of 7.5% set 2 weeks earlier, the CDC said in its weekly flu surveillance report. The rate for the week ending Feb. 10 was reported last week as 7.5%, but it has been revised downward.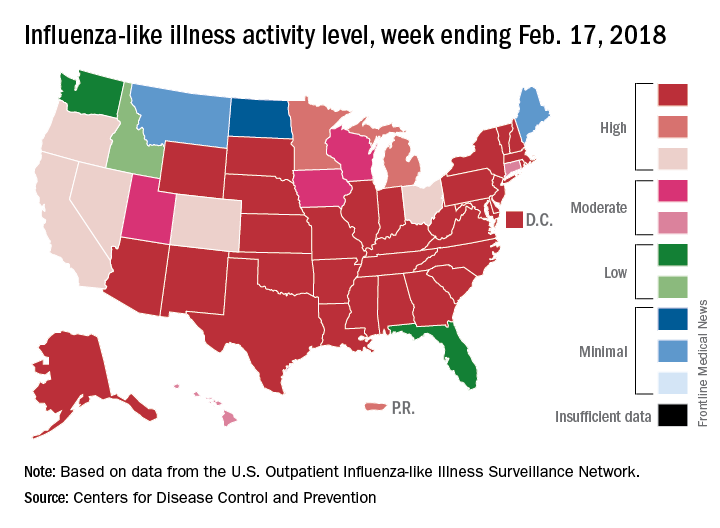
State reports of ILI activity support the decreases seen in the national outpatient rate. There were 33 states at level 10 on the CDC’s 1-10 scale for the week ending Feb. 17 – down from 39 the week before – and a total of 41 states in the “high” range from levels 8-10, compared with 45 the previous week, CDC’s FluView website shows.
Reports of flu-related pediatric deaths continued: 13 deaths were reported during the week, although 9 occurred in previous weeks. The total for the 2017-2018 season is now 97. There were 110 pediatric deaths in the entire 2016-2017 season, 93 during the 2015-2016 season, and 149 in 2014-2015, the CDC said.
Flu-related outpatient activity dropped for the second week in a row as the cumulative hospitalization rate continues to rise, according to data from the Centers for Disease Control and Prevention.
For the week ending Feb. 17, the proportion of outpatient visits for influenza-like illness (ILI) was 6.4%, which was down from 7.4% the previous week (Feb. 10) and down from the seasonal high of 7.5% set 2 weeks earlier, the CDC said in its weekly flu surveillance report. The rate for the week ending Feb. 10 was reported last week as 7.5%, but it has been revised downward.
State reports of ILI activity support the decreases seen in the national outpatient rate. There were 33 states at level 10 on the CDC’s 1-10 scale for the week ending Feb. 17 – down from 39 the week before – and a total of 41 states in the “high” range from levels 8-10, compared with 45 the previous week, CDC’s FluView website shows.
Reports of flu-related pediatric deaths continued: 13 deaths were reported during the week, although 9 occurred in previous weeks. The total for the 2017-2018 season is now 97. There were 110 pediatric deaths in the entire 2016-2017 season, 93 during the 2015-2016 season, and 149 in 2014-2015, the CDC said.
Flu-related outpatient activity dropped for the second week in a row as the cumulative hospitalization rate continues to rise, according to data from the Centers for Disease Control and Prevention.
For the week ending Feb. 17, the proportion of outpatient visits for influenza-like illness (ILI) was 6.4%, which was down from 7.4% the previous week (Feb. 10) and down from the seasonal high of 7.5% set 2 weeks earlier, the CDC said in its weekly flu surveillance report. The rate for the week ending Feb. 10 was reported last week as 7.5%, but it has been revised downward.
State reports of ILI activity support the decreases seen in the national outpatient rate. There were 33 states at level 10 on the CDC’s 1-10 scale for the week ending Feb. 17 – down from 39 the week before – and a total of 41 states in the “high” range from levels 8-10, compared with 45 the previous week, CDC’s FluView website shows.
Reports of flu-related pediatric deaths continued: 13 deaths were reported during the week, although 9 occurred in previous weeks. The total for the 2017-2018 season is now 97. There were 110 pediatric deaths in the entire 2016-2017 season, 93 during the 2015-2016 season, and 149 in 2014-2015, the CDC said.
Flu increase may be slowing
A bit of revisionist history has outpatient influenza activity at a lower level than was reported last week, even though it hasn’t dropped.
The proportion of outpatient visits for influenza-like illness (ILI) for the week ending Feb. 10 was 7.5%, according to the Centers for Disease Control. That is lower than the 7.7% previously reported for the week ending Feb. 3, which would seem to be a drop, but the CDC also has revised that earlier number to 7.5%, so there is no change. (This is not the first time an earlier ILI level has been retroactively lowered: The figure reported for the week ending Jan. 13 was revised in the following report from 6.3% down to 6.0%.)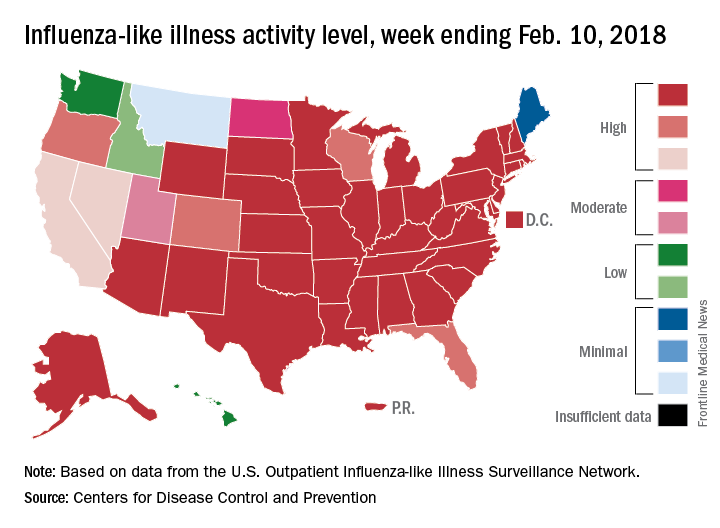
Hospital visits, however, continue to rise at record levels. The cumulative rate for the week ending Feb. 10 was 67.9 visits per 100,000 population, which is higher than the same week for the 2014-2015 (52.9 per 100,000) when flu hospitalizations for the season hit a high of 710,000. Flu-related pediatric deaths also went up, with 22 new reports; this brings the total to 84 for the 2017-2018 season.
A bit of revisionist history has outpatient influenza activity at a lower level than was reported last week, even though it hasn’t dropped.
The proportion of outpatient visits for influenza-like illness (ILI) for the week ending Feb. 10 was 7.5%, according to the Centers for Disease Control. That is lower than the 7.7% previously reported for the week ending Feb. 3, which would seem to be a drop, but the CDC also has revised that earlier number to 7.5%, so there is no change. (This is not the first time an earlier ILI level has been retroactively lowered: The figure reported for the week ending Jan. 13 was revised in the following report from 6.3% down to 6.0%.)
Hospital visits, however, continue to rise at record levels. The cumulative rate for the week ending Feb. 10 was 67.9 visits per 100,000 population, which is higher than the same week for the 2014-2015 (52.9 per 100,000) when flu hospitalizations for the season hit a high of 710,000. Flu-related pediatric deaths also went up, with 22 new reports; this brings the total to 84 for the 2017-2018 season.
A bit of revisionist history has outpatient influenza activity at a lower level than was reported last week, even though it hasn’t dropped.
The proportion of outpatient visits for influenza-like illness (ILI) for the week ending Feb. 10 was 7.5%, according to the Centers for Disease Control. That is lower than the 7.7% previously reported for the week ending Feb. 3, which would seem to be a drop, but the CDC also has revised that earlier number to 7.5%, so there is no change. (This is not the first time an earlier ILI level has been retroactively lowered: The figure reported for the week ending Jan. 13 was revised in the following report from 6.3% down to 6.0%.)
Hospital visits, however, continue to rise at record levels. The cumulative rate for the week ending Feb. 10 was 67.9 visits per 100,000 population, which is higher than the same week for the 2014-2015 (52.9 per 100,000) when flu hospitalizations for the season hit a high of 710,000. Flu-related pediatric deaths also went up, with 22 new reports; this brings the total to 84 for the 2017-2018 season.
FROM THE CDC WEEKLY U.S. INFLUENZA SURVEILLANCE REPORT
MMWR: Current flu vaccine does not protect elderly
, according to the Feb. 16 issue of Morbidity and Mortality Weekly Report.
The elderly are not among them. Although the vaccine was somewhat protective in children and adults up to 49 years old, “no statistically significant protection was observed in other age groups,” including people 65 years and older, reported investigators led by Brendan Flannery, PhD, of the Centers for Disease Control and Prevention influenza division.
They also reported that the cumulative hospitalization rate attributed to laboratory-confirmed influenza for the week ending Feb. 3, 2018 (59.9/100,000), exceeded the rate for the same week in 2014-2015 (50.9/100,000), an A(H3N2) virus–predominant season, and is the highest rate observed for this week since the system expanded to include adults during the 2005-2006 season.
This year’s overall effectiveness rating was in contrast to the 2016-2017 seasonal effectiveness of 48% (MMWR. 2017 Feb 17;66[6];167-71).
The CDC noted that influenza is going to be active for several more weeks, so “vaccination is still recommended,” but “treatment with influenza antiviral medications, where appropriate, is especially important this season.” Meanwhile, “influenza vaccines with improved effectiveness are needed,” the CDC said.
The estimates are based on 4,562 patients 6 months to over 65 years old presenting with acute respiratory illness in 2018 from Nov. 2 to Feb. 3 at five outpatient medical clinics scattered across the United States. Nasal and oropharyngeal swabs were tested with reverse transcription polymerase chain reaction for the presence of influenza viruses; 413 subjects were 65 years or older.
Vaccine effectiveness against the less common virus A(H1N1)pdm09 was 67%, and 42% against the even rarer influenza B viruses. Estimates were adjusted for a range of confounders, including study site, age, general health, and week of illness. Vaccination rates ranged from 45% to 59% across the study sites; 38% of the subjects tested positive for influenza, most for type A viruses. The shot didn’t work too well: 43% of the influenza cases had gotten it.
The 25% effectiveness against A(H3N2) is a bit higher than recent reports of 17% from Canada and 10% from Australia, but similar to the 32% efficacy reported in the United States for the 2016-2017 season.
“These interim estimates reflect ongoing challenges with the A(H3N2) vaccine component since the 2011-12 season,” the investigators wrote. “Multiple factors might be contributing to the reported [vaccine effectiveness] against A(H3N2) viruses this season. … Genetic changes in the vaccine virus hemagglutinin protein that arise during passage in eggs might result in a vaccine immune response that is less effective against circulating viruses.”
On a related note, on Feb. 18, Senators Edward J. Markey (D-Mass.), Richard Blumenthal (D-Conn.), and Amy Klobuchar (D-Minn.) held a press conference to announce they were introducing the Flu Vaccine Bill to dedicate $1 billion over a 5-year period in order to develop a flu vaccine that could provide lifetime protection.
The investigators had no conflicts of interest.
SOURCE: Flannery B. et al. MMWR. 2018 Feb 16;67(6):180-5; Budd A. et al. MMWR. 2018 Feb 16;67(6):169-79.
, according to the Feb. 16 issue of Morbidity and Mortality Weekly Report.
The elderly are not among them. Although the vaccine was somewhat protective in children and adults up to 49 years old, “no statistically significant protection was observed in other age groups,” including people 65 years and older, reported investigators led by Brendan Flannery, PhD, of the Centers for Disease Control and Prevention influenza division.
They also reported that the cumulative hospitalization rate attributed to laboratory-confirmed influenza for the week ending Feb. 3, 2018 (59.9/100,000), exceeded the rate for the same week in 2014-2015 (50.9/100,000), an A(H3N2) virus–predominant season, and is the highest rate observed for this week since the system expanded to include adults during the 2005-2006 season.
This year’s overall effectiveness rating was in contrast to the 2016-2017 seasonal effectiveness of 48% (MMWR. 2017 Feb 17;66[6];167-71).
The CDC noted that influenza is going to be active for several more weeks, so “vaccination is still recommended,” but “treatment with influenza antiviral medications, where appropriate, is especially important this season.” Meanwhile, “influenza vaccines with improved effectiveness are needed,” the CDC said.
The estimates are based on 4,562 patients 6 months to over 65 years old presenting with acute respiratory illness in 2018 from Nov. 2 to Feb. 3 at five outpatient medical clinics scattered across the United States. Nasal and oropharyngeal swabs were tested with reverse transcription polymerase chain reaction for the presence of influenza viruses; 413 subjects were 65 years or older.
Vaccine effectiveness against the less common virus A(H1N1)pdm09 was 67%, and 42% against the even rarer influenza B viruses. Estimates were adjusted for a range of confounders, including study site, age, general health, and week of illness. Vaccination rates ranged from 45% to 59% across the study sites; 38% of the subjects tested positive for influenza, most for type A viruses. The shot didn’t work too well: 43% of the influenza cases had gotten it.
The 25% effectiveness against A(H3N2) is a bit higher than recent reports of 17% from Canada and 10% from Australia, but similar to the 32% efficacy reported in the United States for the 2016-2017 season.
“These interim estimates reflect ongoing challenges with the A(H3N2) vaccine component since the 2011-12 season,” the investigators wrote. “Multiple factors might be contributing to the reported [vaccine effectiveness] against A(H3N2) viruses this season. … Genetic changes in the vaccine virus hemagglutinin protein that arise during passage in eggs might result in a vaccine immune response that is less effective against circulating viruses.”
On a related note, on Feb. 18, Senators Edward J. Markey (D-Mass.), Richard Blumenthal (D-Conn.), and Amy Klobuchar (D-Minn.) held a press conference to announce they were introducing the Flu Vaccine Bill to dedicate $1 billion over a 5-year period in order to develop a flu vaccine that could provide lifetime protection.
The investigators had no conflicts of interest.
SOURCE: Flannery B. et al. MMWR. 2018 Feb 16;67(6):180-5; Budd A. et al. MMWR. 2018 Feb 16;67(6):169-79.
, according to the Feb. 16 issue of Morbidity and Mortality Weekly Report.
The elderly are not among them. Although the vaccine was somewhat protective in children and adults up to 49 years old, “no statistically significant protection was observed in other age groups,” including people 65 years and older, reported investigators led by Brendan Flannery, PhD, of the Centers for Disease Control and Prevention influenza division.
They also reported that the cumulative hospitalization rate attributed to laboratory-confirmed influenza for the week ending Feb. 3, 2018 (59.9/100,000), exceeded the rate for the same week in 2014-2015 (50.9/100,000), an A(H3N2) virus–predominant season, and is the highest rate observed for this week since the system expanded to include adults during the 2005-2006 season.
This year’s overall effectiveness rating was in contrast to the 2016-2017 seasonal effectiveness of 48% (MMWR. 2017 Feb 17;66[6];167-71).
The CDC noted that influenza is going to be active for several more weeks, so “vaccination is still recommended,” but “treatment with influenza antiviral medications, where appropriate, is especially important this season.” Meanwhile, “influenza vaccines with improved effectiveness are needed,” the CDC said.
The estimates are based on 4,562 patients 6 months to over 65 years old presenting with acute respiratory illness in 2018 from Nov. 2 to Feb. 3 at five outpatient medical clinics scattered across the United States. Nasal and oropharyngeal swabs were tested with reverse transcription polymerase chain reaction for the presence of influenza viruses; 413 subjects were 65 years or older.
Vaccine effectiveness against the less common virus A(H1N1)pdm09 was 67%, and 42% against the even rarer influenza B viruses. Estimates were adjusted for a range of confounders, including study site, age, general health, and week of illness. Vaccination rates ranged from 45% to 59% across the study sites; 38% of the subjects tested positive for influenza, most for type A viruses. The shot didn’t work too well: 43% of the influenza cases had gotten it.
The 25% effectiveness against A(H3N2) is a bit higher than recent reports of 17% from Canada and 10% from Australia, but similar to the 32% efficacy reported in the United States for the 2016-2017 season.
“These interim estimates reflect ongoing challenges with the A(H3N2) vaccine component since the 2011-12 season,” the investigators wrote. “Multiple factors might be contributing to the reported [vaccine effectiveness] against A(H3N2) viruses this season. … Genetic changes in the vaccine virus hemagglutinin protein that arise during passage in eggs might result in a vaccine immune response that is less effective against circulating viruses.”
On a related note, on Feb. 18, Senators Edward J. Markey (D-Mass.), Richard Blumenthal (D-Conn.), and Amy Klobuchar (D-Minn.) held a press conference to announce they were introducing the Flu Vaccine Bill to dedicate $1 billion over a 5-year period in order to develop a flu vaccine that could provide lifetime protection.
The investigators had no conflicts of interest.
SOURCE: Flannery B. et al. MMWR. 2018 Feb 16;67(6):180-5; Budd A. et al. MMWR. 2018 Feb 16;67(6):169-79.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
This is what a flu pandemic looks like
For the week ending Feb. 3, 2018, the proportion of outpatient visits for influenza-like illness (ILI) was 7.7%, which would appear to equal the mark of 7.7% set in October of 2009. The earlier 7.7%, however, is rounded down from 7.715%, while the current mark is rounded up from 7.653%, data from the CDC’s Fluview website show.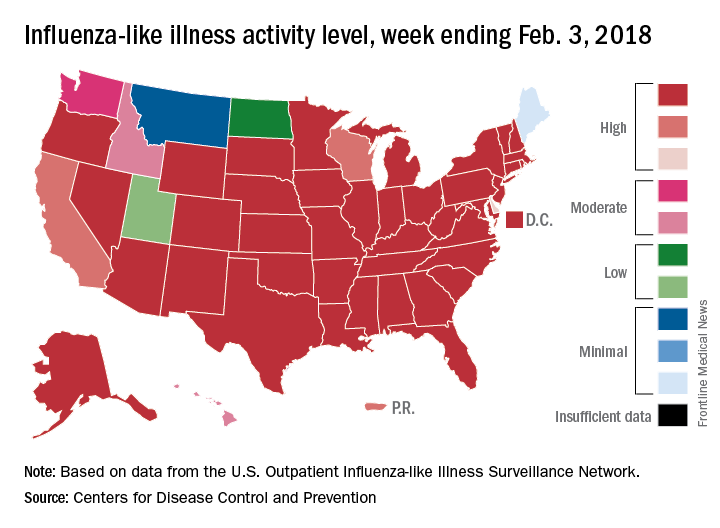
Deaths attributed to pneumonia and influenza were above the epidemic threshold set by the National Center for Health Statistics Mortality Surveillance system, acting CDC director Anne Schuchat, MD, said in a teleconference sponsored by the agency.
ILI activity was at level 10 on the CDC’s 1-10 scale in 41 states, compared with 34 the week before, and was categorized in the “high” range (levels 8-10) in another 3 states and Puerto Rico, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network. In California, which was noted as a possible bright spot last week by Dr. Schuchat because activity there had been decreasing, the ILI level went back up to level 9 after being at 7 the week before.
Flu-related hospitalizations are continuing to rise at a record clip, with the cumulative rate for the week of Feb. 3 at 59.9 per 100,000 population, the CDC reported. A total of 1 in 10 hospital-based deaths last week were related to influenza. At this point in the 2014-2015 flu season – which has the highest number of hospitalizations at 710,000 – the hospitalization rate was only 50.9 per 100,000 population.
There were 10 pediatric deaths reported for the week ending Feb. 3, although 9 occurred in previous weeks. There have been 63 flu-related deaths among children so far during the 2017-2018 season.
Dr. Schuchat continued to recommend members of the public to get a flu shot and to stay home if they are feeling sick.
“What could be mild symptoms for you could be deadly for someone else,” Dr. Schuchat said, adding that antiviral medications remain important. “Physicians do not have to wait for confirmatory flu testing. They should begin treatment with antiviral drugs immediately in they suspect they have a severely ill or a high risk patient.”
“Flu vaccines often have lower effectiveness against H3N1 viruses. However, some protection is better than none. The vaccine’s effectiveness against other flu viruses, like B and H1N1, is better. Because of the ongoing intensity of the flu season and the increasing circulation of influenza B and h1n1, we do continue to recommend vaccination even this late in the season.”
Dr. Schuchat stressed the importance of the pneumococcal pneumonia vaccine. “Flu can make people more vulnerable to secondary infections like bacterial pneumonia. We recommend people aged 65 and over get a pneumococcal pneumonia vaccine,” she said.
For the week ending Feb. 3, 2018, the proportion of outpatient visits for influenza-like illness (ILI) was 7.7%, which would appear to equal the mark of 7.7% set in October of 2009. The earlier 7.7%, however, is rounded down from 7.715%, while the current mark is rounded up from 7.653%, data from the CDC’s Fluview website show.
Deaths attributed to pneumonia and influenza were above the epidemic threshold set by the National Center for Health Statistics Mortality Surveillance system, acting CDC director Anne Schuchat, MD, said in a teleconference sponsored by the agency.
ILI activity was at level 10 on the CDC’s 1-10 scale in 41 states, compared with 34 the week before, and was categorized in the “high” range (levels 8-10) in another 3 states and Puerto Rico, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network. In California, which was noted as a possible bright spot last week by Dr. Schuchat because activity there had been decreasing, the ILI level went back up to level 9 after being at 7 the week before.
Flu-related hospitalizations are continuing to rise at a record clip, with the cumulative rate for the week of Feb. 3 at 59.9 per 100,000 population, the CDC reported. A total of 1 in 10 hospital-based deaths last week were related to influenza. At this point in the 2014-2015 flu season – which has the highest number of hospitalizations at 710,000 – the hospitalization rate was only 50.9 per 100,000 population.
There were 10 pediatric deaths reported for the week ending Feb. 3, although 9 occurred in previous weeks. There have been 63 flu-related deaths among children so far during the 2017-2018 season.
Dr. Schuchat continued to recommend members of the public to get a flu shot and to stay home if they are feeling sick.
“What could be mild symptoms for you could be deadly for someone else,” Dr. Schuchat said, adding that antiviral medications remain important. “Physicians do not have to wait for confirmatory flu testing. They should begin treatment with antiviral drugs immediately in they suspect they have a severely ill or a high risk patient.”
“Flu vaccines often have lower effectiveness against H3N1 viruses. However, some protection is better than none. The vaccine’s effectiveness against other flu viruses, like B and H1N1, is better. Because of the ongoing intensity of the flu season and the increasing circulation of influenza B and h1n1, we do continue to recommend vaccination even this late in the season.”
Dr. Schuchat stressed the importance of the pneumococcal pneumonia vaccine. “Flu can make people more vulnerable to secondary infections like bacterial pneumonia. We recommend people aged 65 and over get a pneumococcal pneumonia vaccine,” she said.
For the week ending Feb. 3, 2018, the proportion of outpatient visits for influenza-like illness (ILI) was 7.7%, which would appear to equal the mark of 7.7% set in October of 2009. The earlier 7.7%, however, is rounded down from 7.715%, while the current mark is rounded up from 7.653%, data from the CDC’s Fluview website show.
Deaths attributed to pneumonia and influenza were above the epidemic threshold set by the National Center for Health Statistics Mortality Surveillance system, acting CDC director Anne Schuchat, MD, said in a teleconference sponsored by the agency.
ILI activity was at level 10 on the CDC’s 1-10 scale in 41 states, compared with 34 the week before, and was categorized in the “high” range (levels 8-10) in another 3 states and Puerto Rico, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network. In California, which was noted as a possible bright spot last week by Dr. Schuchat because activity there had been decreasing, the ILI level went back up to level 9 after being at 7 the week before.
Flu-related hospitalizations are continuing to rise at a record clip, with the cumulative rate for the week of Feb. 3 at 59.9 per 100,000 population, the CDC reported. A total of 1 in 10 hospital-based deaths last week were related to influenza. At this point in the 2014-2015 flu season – which has the highest number of hospitalizations at 710,000 – the hospitalization rate was only 50.9 per 100,000 population.
There were 10 pediatric deaths reported for the week ending Feb. 3, although 9 occurred in previous weeks. There have been 63 flu-related deaths among children so far during the 2017-2018 season.
Dr. Schuchat continued to recommend members of the public to get a flu shot and to stay home if they are feeling sick.
“What could be mild symptoms for you could be deadly for someone else,” Dr. Schuchat said, adding that antiviral medications remain important. “Physicians do not have to wait for confirmatory flu testing. They should begin treatment with antiviral drugs immediately in they suspect they have a severely ill or a high risk patient.”
“Flu vaccines often have lower effectiveness against H3N1 viruses. However, some protection is better than none. The vaccine’s effectiveness against other flu viruses, like B and H1N1, is better. Because of the ongoing intensity of the flu season and the increasing circulation of influenza B and h1n1, we do continue to recommend vaccination even this late in the season.”
Dr. Schuchat stressed the importance of the pneumococcal pneumonia vaccine. “Flu can make people more vulnerable to secondary infections like bacterial pneumonia. We recommend people aged 65 and over get a pneumococcal pneumonia vaccine,” she said.
FROM A CDC TELECONFERENCE
Hospitals filling as flu season worsens
Through the last full week of January, the cumulative “hospitalization rate is the highest we’ve seen,” acting Centers for Disease Control and Prevention director Anne Schuchat, MD, said. For the current season so far, the hospitalization rate stands at 51.4 per 100,000 population, putting it on pace to top the total of 710,000 flu-related admissions that occurred during the 2014-2015 season, she said in a weekly briefing Feb. 2.
Flu-related pediatric deaths also took a big jump for the week as another 16 were reported, which brings the total for the season to 53. Of the children who have died so far, only 20% were vaccinated, said Dan Jernigan, MD, MPH, director of the influenza division at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta. He also noted that half of the children who have been hospitalized did not had an underlying condition.
The one bit of good news for the week was that activity in the West seems to be easing up, Dr. Schuchat said. The geographic spread of ILI was reported as widespread in 48 states, which is down from 49 the previous week because Oregon dropped off the list. To go along with that, the ILI activity level in California has dropped 2 weeks in a row and now stands at level 7, the CDC data show.
Through the last full week of January, the cumulative “hospitalization rate is the highest we’ve seen,” acting Centers for Disease Control and Prevention director Anne Schuchat, MD, said. For the current season so far, the hospitalization rate stands at 51.4 per 100,000 population, putting it on pace to top the total of 710,000 flu-related admissions that occurred during the 2014-2015 season, she said in a weekly briefing Feb. 2.

Flu-related pediatric deaths also took a big jump for the week as another 16 were reported, which brings the total for the season to 53. Of the children who have died so far, only 20% were vaccinated, said Dan Jernigan, MD, MPH, director of the influenza division at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta. He also noted that half of the children who have been hospitalized did not had an underlying condition.
The one bit of good news for the week was that activity in the West seems to be easing up, Dr. Schuchat said. The geographic spread of ILI was reported as widespread in 48 states, which is down from 49 the previous week because Oregon dropped off the list. To go along with that, the ILI activity level in California has dropped 2 weeks in a row and now stands at level 7, the CDC data show.
Through the last full week of January, the cumulative “hospitalization rate is the highest we’ve seen,” acting Centers for Disease Control and Prevention director Anne Schuchat, MD, said. For the current season so far, the hospitalization rate stands at 51.4 per 100,000 population, putting it on pace to top the total of 710,000 flu-related admissions that occurred during the 2014-2015 season, she said in a weekly briefing Feb. 2.

Flu-related pediatric deaths also took a big jump for the week as another 16 were reported, which brings the total for the season to 53. Of the children who have died so far, only 20% were vaccinated, said Dan Jernigan, MD, MPH, director of the influenza division at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta. He also noted that half of the children who have been hospitalized did not had an underlying condition.
The one bit of good news for the week was that activity in the West seems to be easing up, Dr. Schuchat said. The geographic spread of ILI was reported as widespread in 48 states, which is down from 49 the previous week because Oregon dropped off the list. To go along with that, the ILI activity level in California has dropped 2 weeks in a row and now stands at level 7, the CDC data show.
Birth cohort affected 2015-2016 flu vaccine effectiveness
The influenza vaccine introduced in 2009 showed reduced effectiveness during the 2015-2016 influenza season, but only in adults born between 1958 and 1979, according to an analysis published online in the Journal of Infectious Diseases.
Using the Influenza Vaccine Effectiveness Network, researchers analyzed data from 2,115 patients with medically attended acute respiratory illness who tested positive for A(H1N1)pdm09 influenza virus, and 14,696 patients who tested negative for the influenza virus, from 2010-2011 to 2015-2016 (excluding the 2014-2015 influenza season).
Overall, 48% of the influenza virus–negative patients and 28% of the virus-positive patients had received at least one dose of the seasonal inactivated influenza vaccine more than 2 weeks before they fell ill.
However, the vaccine, which was based on the A/California/07/2009 strain of the A(H1N1)pdm09 virus, was only 47% effective during the 2015-2016 season, compared with 61% effectiveness during the 2010-2011 season through to the 2013-2014 season.
When researchers looked at vaccine effectiveness by birth cohort, they found that one particular cohort – individuals born between 1958 and 1979 – showed a significantly reduced vaccine effectiveness (22%) during the 2015-2016 season. By comparison, vaccine effectiveness in this cohort was 61% during the 2010-2013 seasons, and 56% during the 2013-2014 season.
When this birth cohort was excluded from analysis of the 2015-2016 season, the overall vaccine effectiveness for that season was 61%.
While the vaccine was based on an early reference strain of A(H1N1)pdm09, the virus itself later acquired mutations in the hemagglutinin gene, leading to the emergence of new genetic clades, including 6B, which dominated in the 2013-2014 influenza season, and 6B.1, which dominated in 2015-2016.
“Limited serologic data suggest that some adults born during 1958-1979 (age range in 2015-2016, 36-57 years) have decreased antibody titers against A(H1N1)pdm09 group 6B and 6B.1 viruses,” wrote Brendan Flannery, PhD, from the Centers for Disease Control and Prevention, and his coauthors.
They suggested that individuals in this cohort may have been immunologically primed with A/USSR/90/1977-like viruses, which were the first group of A(H1N1) viruses that this cohort would have been exposed to. A(H1N1) strains didn’t circulate between 1958 and 1977. Vaccination with A(H1N1)pdm09 viruses may have induced antibodies against shared antigenic components found on early versions of A(H1N1)pdm09.
If these shared antigenic epitopes were then altered in the later 6B and 6B.1 viruses, that might account for decreased antibody titers in this age group.
“Replacement of the A/California/07/2009(H1N1)pdm09 vaccine reference strain with A/Michigan/45/2015 (group 6B.1) should lead to improved [vaccine effectiveness] against circulating A(H1N1)pdm09 viruses,” the investigators noted.
The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, and the National Center for Advancing Translational Sciences. Eight authors declared funding, grants, and consultancies with the pharmaceutical industry, with five also declaring funding from the CDC.
SOURCE: Flannery B et al. J Infect Dis. 2018 Jan 18. doi: 10.1093/infdis/jix634.
This study proposes that influenza virus strains encountered early in life focus the immune response to later infection or vaccination on shared epitopes between the early and later strains. Supporting this hypothesis is evidence from other studies showing that 60% of the serological response to inactivated influenza vaccines is the result of boosting pre-existing antibodies, rather than the creation of new, vaccine-induced antibodies.
However there are also some flaws to this argument, and we should be careful to avoid confirmation bias. For example, the reduction in effectiveness of vaccines against A(H1N1) has been observed in North America, where this study is located, but to a lesser extent in studies conducted in other regions. Reductions in vaccine effectiveness have also been observed in other birth cohorts and during other influenza seasons.
That aside, accumulating evidence suggests that the vaccine strain be updated from A/California/7/2009 to A/Michigan/45/2015 (a clade 6B.1 strain) for the 2016-2017 influenza seasons.
Allen C. Cheng, PhD, is from the School of Public Health and Preventive Medicine at Monash University, Melbourne, and Kanta Subbarao, MBBS, is from the World Health Organization Collaborating Centre for Reference and Research on Influenza and the Peter Doherty Institute for Infection and Immunity, Australia. These comments are taken from an accompanying editorial (J Infect Dis. 2018, Jan 18. doi: 10.1093/infdis/jix635). The authors declared support from the Australian Department of Health and the Australian National Health and Medical Research Council. No conflicts of interest were declared.
This study proposes that influenza virus strains encountered early in life focus the immune response to later infection or vaccination on shared epitopes between the early and later strains. Supporting this hypothesis is evidence from other studies showing that 60% of the serological response to inactivated influenza vaccines is the result of boosting pre-existing antibodies, rather than the creation of new, vaccine-induced antibodies.
However there are also some flaws to this argument, and we should be careful to avoid confirmation bias. For example, the reduction in effectiveness of vaccines against A(H1N1) has been observed in North America, where this study is located, but to a lesser extent in studies conducted in other regions. Reductions in vaccine effectiveness have also been observed in other birth cohorts and during other influenza seasons.
That aside, accumulating evidence suggests that the vaccine strain be updated from A/California/7/2009 to A/Michigan/45/2015 (a clade 6B.1 strain) for the 2016-2017 influenza seasons.
Allen C. Cheng, PhD, is from the School of Public Health and Preventive Medicine at Monash University, Melbourne, and Kanta Subbarao, MBBS, is from the World Health Organization Collaborating Centre for Reference and Research on Influenza and the Peter Doherty Institute for Infection and Immunity, Australia. These comments are taken from an accompanying editorial (J Infect Dis. 2018, Jan 18. doi: 10.1093/infdis/jix635). The authors declared support from the Australian Department of Health and the Australian National Health and Medical Research Council. No conflicts of interest were declared.
This study proposes that influenza virus strains encountered early in life focus the immune response to later infection or vaccination on shared epitopes between the early and later strains. Supporting this hypothesis is evidence from other studies showing that 60% of the serological response to inactivated influenza vaccines is the result of boosting pre-existing antibodies, rather than the creation of new, vaccine-induced antibodies.
However there are also some flaws to this argument, and we should be careful to avoid confirmation bias. For example, the reduction in effectiveness of vaccines against A(H1N1) has been observed in North America, where this study is located, but to a lesser extent in studies conducted in other regions. Reductions in vaccine effectiveness have also been observed in other birth cohorts and during other influenza seasons.
That aside, accumulating evidence suggests that the vaccine strain be updated from A/California/7/2009 to A/Michigan/45/2015 (a clade 6B.1 strain) for the 2016-2017 influenza seasons.
Allen C. Cheng, PhD, is from the School of Public Health and Preventive Medicine at Monash University, Melbourne, and Kanta Subbarao, MBBS, is from the World Health Organization Collaborating Centre for Reference and Research on Influenza and the Peter Doherty Institute for Infection and Immunity, Australia. These comments are taken from an accompanying editorial (J Infect Dis. 2018, Jan 18. doi: 10.1093/infdis/jix635). The authors declared support from the Australian Department of Health and the Australian National Health and Medical Research Council. No conflicts of interest were declared.
The influenza vaccine introduced in 2009 showed reduced effectiveness during the 2015-2016 influenza season, but only in adults born between 1958 and 1979, according to an analysis published online in the Journal of Infectious Diseases.
Using the Influenza Vaccine Effectiveness Network, researchers analyzed data from 2,115 patients with medically attended acute respiratory illness who tested positive for A(H1N1)pdm09 influenza virus, and 14,696 patients who tested negative for the influenza virus, from 2010-2011 to 2015-2016 (excluding the 2014-2015 influenza season).
Overall, 48% of the influenza virus–negative patients and 28% of the virus-positive patients had received at least one dose of the seasonal inactivated influenza vaccine more than 2 weeks before they fell ill.
However, the vaccine, which was based on the A/California/07/2009 strain of the A(H1N1)pdm09 virus, was only 47% effective during the 2015-2016 season, compared with 61% effectiveness during the 2010-2011 season through to the 2013-2014 season.
When researchers looked at vaccine effectiveness by birth cohort, they found that one particular cohort – individuals born between 1958 and 1979 – showed a significantly reduced vaccine effectiveness (22%) during the 2015-2016 season. By comparison, vaccine effectiveness in this cohort was 61% during the 2010-2013 seasons, and 56% during the 2013-2014 season.
When this birth cohort was excluded from analysis of the 2015-2016 season, the overall vaccine effectiveness for that season was 61%.
While the vaccine was based on an early reference strain of A(H1N1)pdm09, the virus itself later acquired mutations in the hemagglutinin gene, leading to the emergence of new genetic clades, including 6B, which dominated in the 2013-2014 influenza season, and 6B.1, which dominated in 2015-2016.
“Limited serologic data suggest that some adults born during 1958-1979 (age range in 2015-2016, 36-57 years) have decreased antibody titers against A(H1N1)pdm09 group 6B and 6B.1 viruses,” wrote Brendan Flannery, PhD, from the Centers for Disease Control and Prevention, and his coauthors.
They suggested that individuals in this cohort may have been immunologically primed with A/USSR/90/1977-like viruses, which were the first group of A(H1N1) viruses that this cohort would have been exposed to. A(H1N1) strains didn’t circulate between 1958 and 1977. Vaccination with A(H1N1)pdm09 viruses may have induced antibodies against shared antigenic components found on early versions of A(H1N1)pdm09.
If these shared antigenic epitopes were then altered in the later 6B and 6B.1 viruses, that might account for decreased antibody titers in this age group.
“Replacement of the A/California/07/2009(H1N1)pdm09 vaccine reference strain with A/Michigan/45/2015 (group 6B.1) should lead to improved [vaccine effectiveness] against circulating A(H1N1)pdm09 viruses,” the investigators noted.
The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, and the National Center for Advancing Translational Sciences. Eight authors declared funding, grants, and consultancies with the pharmaceutical industry, with five also declaring funding from the CDC.
SOURCE: Flannery B et al. J Infect Dis. 2018 Jan 18. doi: 10.1093/infdis/jix634.
The influenza vaccine introduced in 2009 showed reduced effectiveness during the 2015-2016 influenza season, but only in adults born between 1958 and 1979, according to an analysis published online in the Journal of Infectious Diseases.
Using the Influenza Vaccine Effectiveness Network, researchers analyzed data from 2,115 patients with medically attended acute respiratory illness who tested positive for A(H1N1)pdm09 influenza virus, and 14,696 patients who tested negative for the influenza virus, from 2010-2011 to 2015-2016 (excluding the 2014-2015 influenza season).
Overall, 48% of the influenza virus–negative patients and 28% of the virus-positive patients had received at least one dose of the seasonal inactivated influenza vaccine more than 2 weeks before they fell ill.
However, the vaccine, which was based on the A/California/07/2009 strain of the A(H1N1)pdm09 virus, was only 47% effective during the 2015-2016 season, compared with 61% effectiveness during the 2010-2011 season through to the 2013-2014 season.
When researchers looked at vaccine effectiveness by birth cohort, they found that one particular cohort – individuals born between 1958 and 1979 – showed a significantly reduced vaccine effectiveness (22%) during the 2015-2016 season. By comparison, vaccine effectiveness in this cohort was 61% during the 2010-2013 seasons, and 56% during the 2013-2014 season.
When this birth cohort was excluded from analysis of the 2015-2016 season, the overall vaccine effectiveness for that season was 61%.
While the vaccine was based on an early reference strain of A(H1N1)pdm09, the virus itself later acquired mutations in the hemagglutinin gene, leading to the emergence of new genetic clades, including 6B, which dominated in the 2013-2014 influenza season, and 6B.1, which dominated in 2015-2016.
“Limited serologic data suggest that some adults born during 1958-1979 (age range in 2015-2016, 36-57 years) have decreased antibody titers against A(H1N1)pdm09 group 6B and 6B.1 viruses,” wrote Brendan Flannery, PhD, from the Centers for Disease Control and Prevention, and his coauthors.
They suggested that individuals in this cohort may have been immunologically primed with A/USSR/90/1977-like viruses, which were the first group of A(H1N1) viruses that this cohort would have been exposed to. A(H1N1) strains didn’t circulate between 1958 and 1977. Vaccination with A(H1N1)pdm09 viruses may have induced antibodies against shared antigenic components found on early versions of A(H1N1)pdm09.
If these shared antigenic epitopes were then altered in the later 6B and 6B.1 viruses, that might account for decreased antibody titers in this age group.
“Replacement of the A/California/07/2009(H1N1)pdm09 vaccine reference strain with A/Michigan/45/2015 (group 6B.1) should lead to improved [vaccine effectiveness] against circulating A(H1N1)pdm09 viruses,” the investigators noted.
The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, and the National Center for Advancing Translational Sciences. Eight authors declared funding, grants, and consultancies with the pharmaceutical industry, with five also declaring funding from the CDC.
SOURCE: Flannery B et al. J Infect Dis. 2018 Jan 18. doi: 10.1093/infdis/jix634.
FROM THE JOURNAL OF INFECTIOUS DISEASES
Key clinical point:
Major finding: The influenza vaccine effectiveness during the 2015-2016 season was just 22% in individuals born between 1958 and 1979.
Data source: A retrospective case-control study of 2,115 patients who tested positive for A(H1N1)pdm09 influenza virus, and 14,696 negative controls.
Disclosures: The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, and the National Center for Advancing Translational Sciences. Eight authors declared funding, grants, and consultancies with the pharmaceutical industry, with five also declaring funding from the CDC.
Source: Flannery B et al. J Infect Dis. 2018 Jan 18. doi: 10.1093/infdis/jix634.
CDC: Flu levels highest since pandemic year 2009
according to data from the Centers for Disease Control and Prevention.
That season was dominated by influenza A (H3N2), and the 2017-2018 season seems to be going down that same path. For the week ending Jan. 20, the proportion of outpatient visits for influenza-like illness increased to 6.6%, which is, for the second consecutive week, the highest level reported since October of – you guessed it – 2009, when it hit 7.7%, the CDC said in its weekly flu surveillance report.
The level reported last week, 6.3%, has been revised downward and now stands at an even 6%.
It turns out that 2018 is something of a milestone for the H3N2 virus. The virus first emerged in 1968, so it has reached its 50th anniversary, Dan Jernigan, MD, director of the influenza division at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta, said on Jan. 26 in a weekly briefing.
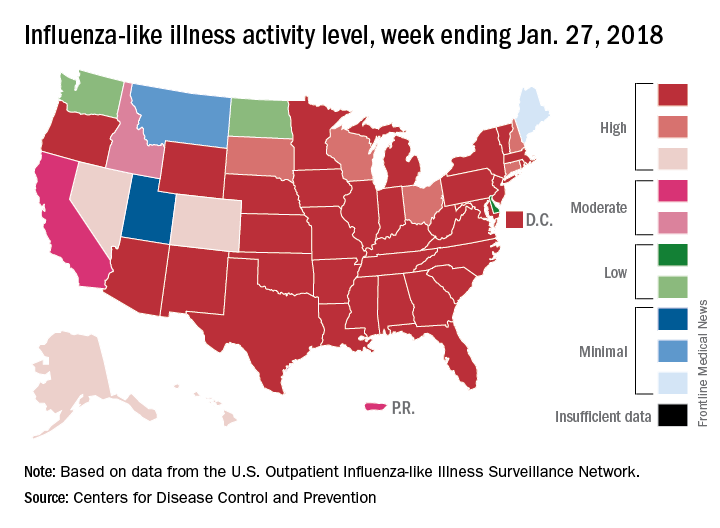
H3N2 must not be happy about hitting the big 5-0, however, because the map of influenza-like illness activity looks pretty red and angry. For the week ending Jan. 20, there were 30 states at the highest level of flu activity on the CDC’s 1-10 scale, with another nine in the “high” range at levels 8 and 9.
Dr. Jernigan did suggest that activity may have peaked in some areas of the country, with California among them.
There were seven pediatric deaths reported for the week ending Jan. 20, although six occurred in previous weeks. There have been 37 flu-related deaths among children so far during the 2017-2018 season, the CDC said.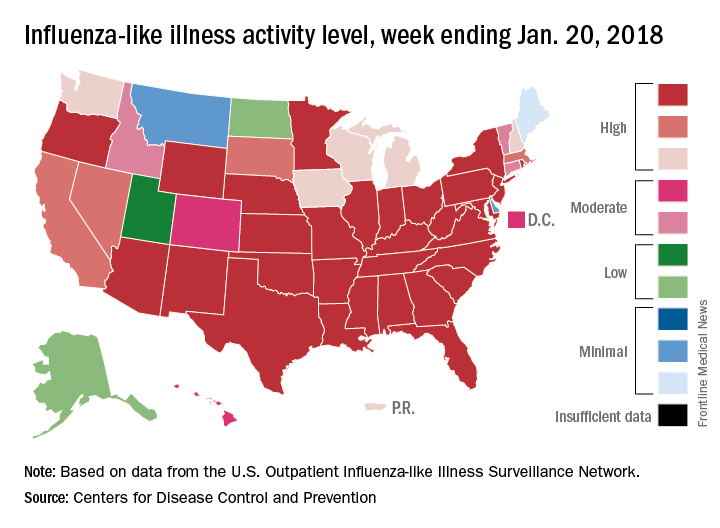
according to data from the Centers for Disease Control and Prevention.
That season was dominated by influenza A (H3N2), and the 2017-2018 season seems to be going down that same path. For the week ending Jan. 20, the proportion of outpatient visits for influenza-like illness increased to 6.6%, which is, for the second consecutive week, the highest level reported since October of – you guessed it – 2009, when it hit 7.7%, the CDC said in its weekly flu surveillance report.
The level reported last week, 6.3%, has been revised downward and now stands at an even 6%.
It turns out that 2018 is something of a milestone for the H3N2 virus. The virus first emerged in 1968, so it has reached its 50th anniversary, Dan Jernigan, MD, director of the influenza division at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta, said on Jan. 26 in a weekly briefing.
H3N2 must not be happy about hitting the big 5-0, however, because the map of influenza-like illness activity looks pretty red and angry. For the week ending Jan. 20, there were 30 states at the highest level of flu activity on the CDC’s 1-10 scale, with another nine in the “high” range at levels 8 and 9.
Dr. Jernigan did suggest that activity may have peaked in some areas of the country, with California among them.
There were seven pediatric deaths reported for the week ending Jan. 20, although six occurred in previous weeks. There have been 37 flu-related deaths among children so far during the 2017-2018 season, the CDC said.
according to data from the Centers for Disease Control and Prevention.
That season was dominated by influenza A (H3N2), and the 2017-2018 season seems to be going down that same path. For the week ending Jan. 20, the proportion of outpatient visits for influenza-like illness increased to 6.6%, which is, for the second consecutive week, the highest level reported since October of – you guessed it – 2009, when it hit 7.7%, the CDC said in its weekly flu surveillance report.
The level reported last week, 6.3%, has been revised downward and now stands at an even 6%.
It turns out that 2018 is something of a milestone for the H3N2 virus. The virus first emerged in 1968, so it has reached its 50th anniversary, Dan Jernigan, MD, director of the influenza division at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta, said on Jan. 26 in a weekly briefing.
H3N2 must not be happy about hitting the big 5-0, however, because the map of influenza-like illness activity looks pretty red and angry. For the week ending Jan. 20, there were 30 states at the highest level of flu activity on the CDC’s 1-10 scale, with another nine in the “high” range at levels 8 and 9.
Dr. Jernigan did suggest that activity may have peaked in some areas of the country, with California among them.
There were seven pediatric deaths reported for the week ending Jan. 20, although six occurred in previous weeks. There have been 37 flu-related deaths among children so far during the 2017-2018 season, the CDC said.
Influenza: All that and MI too
Myocardial infarction admissions were six times more likely to occur in the week after a positive test for influenza than in the year before or the 51 weeks after the infection, according to analysis of a Canadian cohort that links laboratories with administrative databases.
The investigators used this cohort data to define definitions of “risk interval” – the first 7 days after flu detection – and a combined “control interval” – 52 weeks before the flu detection and 51 weeks after the end of the risk interval.
Among the total of 364 hospital admissions for MI in patients with confirmed influenza, 20 occurred during the defined 1-week risk interval (20 admissions/week) and 344 occurred during the control interval (3.3 admissions/week), giving an incidence ratio (IR) of 6.05, Jeffrey C. Kwong, MD, of the University of Toronto and his associates reported in the New England Journal of Medicine.
There was little difference between days 1 and 3 after flu confirmation (IR, 6.3) and days 4-7 (IR, 5.8), but risk dropped off quickly after that, with IRs of 0.6 at days 8-14 and 0.75 at days 15-28. Risk was increased for older adults, those with influenza B infection, and those who had their first MI, the investigators said.
MI incidence also was elevated after infection with noninfluenza respiratory viruses, although to a lesser extent than with influenza, which suggests that “influenza is illustrative of the role that acute respiratory infections have in precipitating acute myocardial infarction,” Dr. Kwong and his associates wrote.
The study was supported by the Canadian Institutes of Health Research, by Public Health Ontario, and by the Institute for Clinical Evaluative Sciences. Dr. Kwong reported grants from Canadian Institutes of Health Research during the conduct of the study, as well as grants from Canadian Institutes of Health Research and University of Toronto.
SOURCE: Kwong JC et al. N Engl J Med. 2018. 378(4):345-53. doi: 10.1056/NEJMoa1702090.
Myocardial infarction admissions were six times more likely to occur in the week after a positive test for influenza than in the year before or the 51 weeks after the infection, according to analysis of a Canadian cohort that links laboratories with administrative databases.
The investigators used this cohort data to define definitions of “risk interval” – the first 7 days after flu detection – and a combined “control interval” – 52 weeks before the flu detection and 51 weeks after the end of the risk interval.
Among the total of 364 hospital admissions for MI in patients with confirmed influenza, 20 occurred during the defined 1-week risk interval (20 admissions/week) and 344 occurred during the control interval (3.3 admissions/week), giving an incidence ratio (IR) of 6.05, Jeffrey C. Kwong, MD, of the University of Toronto and his associates reported in the New England Journal of Medicine.
There was little difference between days 1 and 3 after flu confirmation (IR, 6.3) and days 4-7 (IR, 5.8), but risk dropped off quickly after that, with IRs of 0.6 at days 8-14 and 0.75 at days 15-28. Risk was increased for older adults, those with influenza B infection, and those who had their first MI, the investigators said.
MI incidence also was elevated after infection with noninfluenza respiratory viruses, although to a lesser extent than with influenza, which suggests that “influenza is illustrative of the role that acute respiratory infections have in precipitating acute myocardial infarction,” Dr. Kwong and his associates wrote.
The study was supported by the Canadian Institutes of Health Research, by Public Health Ontario, and by the Institute for Clinical Evaluative Sciences. Dr. Kwong reported grants from Canadian Institutes of Health Research during the conduct of the study, as well as grants from Canadian Institutes of Health Research and University of Toronto.
SOURCE: Kwong JC et al. N Engl J Med. 2018. 378(4):345-53. doi: 10.1056/NEJMoa1702090.
Myocardial infarction admissions were six times more likely to occur in the week after a positive test for influenza than in the year before or the 51 weeks after the infection, according to analysis of a Canadian cohort that links laboratories with administrative databases.
The investigators used this cohort data to define definitions of “risk interval” – the first 7 days after flu detection – and a combined “control interval” – 52 weeks before the flu detection and 51 weeks after the end of the risk interval.
Among the total of 364 hospital admissions for MI in patients with confirmed influenza, 20 occurred during the defined 1-week risk interval (20 admissions/week) and 344 occurred during the control interval (3.3 admissions/week), giving an incidence ratio (IR) of 6.05, Jeffrey C. Kwong, MD, of the University of Toronto and his associates reported in the New England Journal of Medicine.
There was little difference between days 1 and 3 after flu confirmation (IR, 6.3) and days 4-7 (IR, 5.8), but risk dropped off quickly after that, with IRs of 0.6 at days 8-14 and 0.75 at days 15-28. Risk was increased for older adults, those with influenza B infection, and those who had their first MI, the investigators said.
MI incidence also was elevated after infection with noninfluenza respiratory viruses, although to a lesser extent than with influenza, which suggests that “influenza is illustrative of the role that acute respiratory infections have in precipitating acute myocardial infarction,” Dr. Kwong and his associates wrote.
The study was supported by the Canadian Institutes of Health Research, by Public Health Ontario, and by the Institute for Clinical Evaluative Sciences. Dr. Kwong reported grants from Canadian Institutes of Health Research during the conduct of the study, as well as grants from Canadian Institutes of Health Research and University of Toronto.
SOURCE: Kwong JC et al. N Engl J Med. 2018. 378(4):345-53. doi: 10.1056/NEJMoa1702090.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Flu season takes another turn for the worse
By one measure at least – the proportion of outpatient visits for influenza-like illness (ILI) – this flu season is now the worst in almost a decade, according to data from the Centers for Disease Control and Prevention.
which hit an early peak of 7.7% in October of 2009. The slight pause that occurred in the first week of January as the rate only rose from 5.7% to 5.8% now looks more like the earlier trend from December, when the level of outpatient visits more than doubled over a 3-week period, data from the CDC FluView website show.
“The geographic spread of influenza in Puerto Rico and 49 states was reported as widespread” for the week ending Jan. 13, and 24 states had the highest level of ILI activity on the CDC’s 1-10 scale, the CDC influenza division reported Jan 19.
There were 10 flu-related pediatric deaths reported during the week, with two occurring in the week ending Jan. 13. A total of 30 deaths in children have been associated with influenza so far for the 2017-2018 season, the CDC said.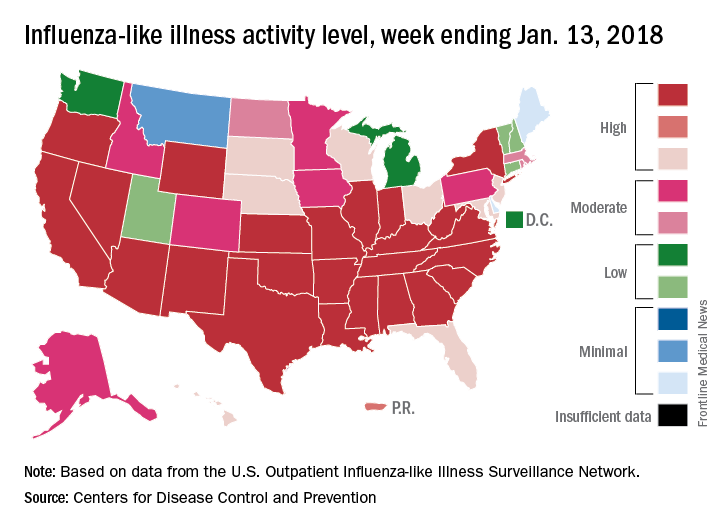
By one measure at least – the proportion of outpatient visits for influenza-like illness (ILI) – this flu season is now the worst in almost a decade, according to data from the Centers for Disease Control and Prevention.
which hit an early peak of 7.7% in October of 2009. The slight pause that occurred in the first week of January as the rate only rose from 5.7% to 5.8% now looks more like the earlier trend from December, when the level of outpatient visits more than doubled over a 3-week period, data from the CDC FluView website show.
“The geographic spread of influenza in Puerto Rico and 49 states was reported as widespread” for the week ending Jan. 13, and 24 states had the highest level of ILI activity on the CDC’s 1-10 scale, the CDC influenza division reported Jan 19.
There were 10 flu-related pediatric deaths reported during the week, with two occurring in the week ending Jan. 13. A total of 30 deaths in children have been associated with influenza so far for the 2017-2018 season, the CDC said.
By one measure at least – the proportion of outpatient visits for influenza-like illness (ILI) – this flu season is now the worst in almost a decade, according to data from the Centers for Disease Control and Prevention.
which hit an early peak of 7.7% in October of 2009. The slight pause that occurred in the first week of January as the rate only rose from 5.7% to 5.8% now looks more like the earlier trend from December, when the level of outpatient visits more than doubled over a 3-week period, data from the CDC FluView website show.
“The geographic spread of influenza in Puerto Rico and 49 states was reported as widespread” for the week ending Jan. 13, and 24 states had the highest level of ILI activity on the CDC’s 1-10 scale, the CDC influenza division reported Jan 19.
There were 10 flu-related pediatric deaths reported during the week, with two occurring in the week ending Jan. 13. A total of 30 deaths in children have been associated with influenza so far for the 2017-2018 season, the CDC said.