User login
Researchers examine factors associated with opioid use among migraineurs
Among patients with migraine who use prescription medications, the increasing use of prescription opioids is associated with chronic migraine, more severe disability, and anxiety and depression, according to an analysis published in the January issue of Headache . The use of prescription opioids also is associated with treatment-related variables such as poor acute treatment optimization and treatment in a pain clinic. The results indicate the continued need to educate patients and clinicians about the potential risks of opioids for migraineurs, according to the researchers.
In the Migraine in America Symptoms and Treatment (MAST) study, which the researchers analyzed for their investigation, one-third of migraineurs who use acute prescriptions reported using opioids. Among opioid users, 42% took opioids on 4 or more days per month. “These findings are like [those of] a previous report from the American Migraine Prevalence and Prevention study and more recent findings from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study,” said Richard Lipton, MD, Edwin S. Lowe professor and vice chair of neurology at Albert Einstein College of Medicine in the Bronx, New York. “High rates of opioid use are problematic because opioid use is associated with worsening of migraine over time.”
Opioids remain in widespread use for migraine, even though guidelines recommend against this treatment. Among migraineurs, opioid use is associated with more severe headache-related disability and greater use of health care resources. Opioid use also increases the risk of progressing from episodic migraine to chronic migraine.
A review of MAST data
Dr. Lipton and colleagues set out to identify the variables associated with the frequency of opioid use in people with migraine. Among the variables that they sought to examine were demographic characteristics, comorbidities, headache characteristics, medication use, and patterns of health care use. Dr. Lipton’s group hypothesized that migraine-related severity and burden would increase with increasing frequency of opioid use.
To conduct their research, the investigators examined data from the MAST study, a nationwide sample of American adults with migraine. They focused specifically on participants who reported receiving prescription acute medications. Participants eligible for this analysis reported 3 or more headache days in the previous 3 months and at least 1 monthly headache day in the previous month. In all, 15,133 participants met these criteria.
Dr. Lipton and colleagues categorized participants into four groups based on their frequency of opioid use. The groups had no opioid use, 3 or fewer monthly days of opioid use, 4 to 9 monthly days of opioid use, and 10 or more days of monthly opioid use. The last category is consistent with the International Classification of Headache Disorders-3 criteria for overuse of opioids in migraine.
At baseline, MAST participants provided information about variables such as gender, age, marital status, smoking status, education, and income. Participants also reported how many times in the previous 6 months they had visited a primary care doctor, a neurologist, a headache specialist, or a pain specialist. Dr. Lipton’s group calculated monthly headache days using the number of days during the previous 3 months affected by headache. The Migraine Disability Assessment (MIDAS) questionnaire was used to measure headache-related disability. The four-item Patient Health Questionnaire (PHQ-4) was used to screen for anxiety and depression, and the Migraine Treatment Optimization Questionnaire (mTOQ-4) evaluated participants’ treatment optimization.
Men predominated among opioid users
The investigators included 4,701 MAST participants in their analysis. The population’s mean age was 45 years, and 71.6% of participants were women. Of the entire sample, 67.5% reported no opioid use, and 32.5% reported opioid use. Of the total study population, 18.7% of patients took opioids 3 or fewer days per month, 6.5% took opioids 4 to 9 days per month, and 7.3% took opioids on 10 or more days per month.
Opioid users did not differ from nonusers on race or marital status. Men were overrepresented among all groups of opioid users, however. In addition, opioid use was more prevalent among participants with fewer than 4 years of college education (34.9%) than among participants with 4 or more years of college (30.8%). The proportion of participants with fewer than 4 years of college increased with increasing monthly opioid use. Furthermore, opioid use increased with decreasing household income. As opioid use increased, rates of employment decreased. Approximately 33% of the entire sample were obese, and the proportion of obese participants increased with increasing days per month of opioid use.
The most frequent setting during the previous 6 months for participants seeking care was primary care (49.7%). The next most frequent setting was neurology units (20.9%), pain clinics (8.3%), and headache clinics (7.7%). The prevalence of opioid use was 37.5% among participants with primary care visits, 37.3% among participants with neurologist visits, 43.0% among participants with headache clinic visits, and 53.5% with pain clinic visits.
About 15% of the population had chronic migraine. The prevalence of chronic migraine increased with increasing frequency of opioid use. About 49% of the sample had allodynia, and the prevalence of allodynia increased with increasing frequency of opioid use. Overall, disability was moderate to severe in 57.3% of participants. Participants who used opioids on 3 or fewer days per month had the lowest prevalence of moderate to severe disability (50.2%), and participants who used opioids on 10 or more days per month had the highest prevalence of moderate to severe disability (83.8%).
Approximately 21% of participants had anxiety or depression. The lowest prevalence of anxiety or depression was among participants who took opioids on 3 or fewer days per month (17.4%), and the highest prevalence was among participants who took opioids on 10 or more days per month (43.2%). About 39% of the population had very poor to poor treatment optimization. Among opioid nonusers, 35.6% had very poor to poor treatment optimization, and 59.4% of participants who used opioids on 10 or more days per month had very poor to poor treatment optimization.
Dr. Lipton and colleagues also examined the study population’s use of triptans. Overall, 51.5% of participants reported taking triptans. The prevalence of triptan use was highest among participants who did not use opioids (64.1%) and lowest among participants who used opioids on 3 or fewer days per month (20.5%). Triptan use increased as monthly days of opioid use increased.
Pain clinics and opioid prescription
“In the general population, women are more likely to receive opioids than men,” said Dr. Lipton. “This [finding] could reflect, in part, that women have more pain disorders than men and are more likely to seek medical care for pain than men.” In the current study, however, men with migraine were more likely to receive opioid prescriptions than were women with migraine. One potential explanation for this finding is that men with migraine are less likely to receive a migraine diagnosis, which might attenuate opioid prescribing, than women with migraine. “It may be that opioids are perceived to be serious drugs for serious pain, and that some physicians may be more likely to prescribe opioids to men because the disorder is taken more seriously in men than women,” said Dr. Lipton.
The observation that opioids were more likely to be prescribed for people treated in pain clinics “is consistent with my understanding of practice patterns,” he added. “Generally, neurologists strive to find effective acute treatment alternatives to opioids. The emergence of [drug classes known as] gepants and ditans provides a helpful set of alternatives to tritpans.”
Dr. Lipton and his colleagues plan further research into the treatment of migraineurs. “In a claims analysis, we showed that when people with migraine fail a triptan, they are most likely to get an opioid as their next drug,” he said. “Reasonable [clinicians] might disagree on the next step. The next step, in the absence of contraindications, could be a different oral triptan, a nonoral triptan, or a gepant or ditan. We are planning a randomized trial to probe this question.”
Why are opioids still being used?
The study’s reliance on patients’ self-report and its retrospective design are two of its weaknesses, said Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and editor-in-chief of Neurology Reviews. One strength, however, is that the stratified sampling methodology produced a study population that accurately reflects the demographic characteristics of the U.S. adult population, he added. Another strength is the investigators’ examination of opioid use by patient characteristics such as marital status, education, income, obesity, and smoking.
Given the harmful effects of opioids in migraine, it is hard to understand why as much as one-third of study participants using acute care medication for migraine were using opioids, said Dr. Rapoport. Using opioids for the acute treatment of migraine attacks often indicates inadequate treatment optimization, which leads to ongoing headache. As a consequence, patients may take more medication, which can increase headache frequency and lead to diagnoses of chronic migraine and medication overuse headache. Although the study found an association between the increased use of opioids and decreased household income and increased unemployment, smoking, and obesity, “it is not possible to assign causality to any of these associations, even though some would argue that decreased socioeconomic status was somehow related to more headache, disability, obesity, smoking, and unemployment,” he added.
“The paper suggests that future research should look at the risk factors for use of opioids and should determine if depression is a risk factor for or a consequence of opioid use,” said Dr. Rapoport. “Interventional studies designed to improve the acute care of migraine attacks might be able to reduce the use of opioids. I have not used opioids or butalbital-containing medication in my office for many years.”
This study was funded and sponsored by Dr. Reddy’s Laboratories group of companies, Princeton, N.J. Dr. Lipton has received grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund. He serves as a consultant, serves as an advisory board member, or has received honoraria from Alder, Allergan, American Headache Society, Autonomic Technologies, Biohaven, Dr. Reddy’s Laboratories, Eli Lilly, eNeura Therapeutics, Merck, Novartis, Pfizer, and Teva, Inc. He receives royalties from Wolff’s Headache, 8th Edition (New York: Oxford University Press, 2009) and holds stock options in eNeura Therapeutics and Biohaven.
SOURCE: Lipton RB, et al. Headache. https://doi.org/10.1111/head.14018. 2020;61(1):103-16.
Among patients with migraine who use prescription medications, the increasing use of prescription opioids is associated with chronic migraine, more severe disability, and anxiety and depression, according to an analysis published in the January issue of Headache . The use of prescription opioids also is associated with treatment-related variables such as poor acute treatment optimization and treatment in a pain clinic. The results indicate the continued need to educate patients and clinicians about the potential risks of opioids for migraineurs, according to the researchers.
In the Migraine in America Symptoms and Treatment (MAST) study, which the researchers analyzed for their investigation, one-third of migraineurs who use acute prescriptions reported using opioids. Among opioid users, 42% took opioids on 4 or more days per month. “These findings are like [those of] a previous report from the American Migraine Prevalence and Prevention study and more recent findings from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study,” said Richard Lipton, MD, Edwin S. Lowe professor and vice chair of neurology at Albert Einstein College of Medicine in the Bronx, New York. “High rates of opioid use are problematic because opioid use is associated with worsening of migraine over time.”
Opioids remain in widespread use for migraine, even though guidelines recommend against this treatment. Among migraineurs, opioid use is associated with more severe headache-related disability and greater use of health care resources. Opioid use also increases the risk of progressing from episodic migraine to chronic migraine.
A review of MAST data
Dr. Lipton and colleagues set out to identify the variables associated with the frequency of opioid use in people with migraine. Among the variables that they sought to examine were demographic characteristics, comorbidities, headache characteristics, medication use, and patterns of health care use. Dr. Lipton’s group hypothesized that migraine-related severity and burden would increase with increasing frequency of opioid use.
To conduct their research, the investigators examined data from the MAST study, a nationwide sample of American adults with migraine. They focused specifically on participants who reported receiving prescription acute medications. Participants eligible for this analysis reported 3 or more headache days in the previous 3 months and at least 1 monthly headache day in the previous month. In all, 15,133 participants met these criteria.
Dr. Lipton and colleagues categorized participants into four groups based on their frequency of opioid use. The groups had no opioid use, 3 or fewer monthly days of opioid use, 4 to 9 monthly days of opioid use, and 10 or more days of monthly opioid use. The last category is consistent with the International Classification of Headache Disorders-3 criteria for overuse of opioids in migraine.
At baseline, MAST participants provided information about variables such as gender, age, marital status, smoking status, education, and income. Participants also reported how many times in the previous 6 months they had visited a primary care doctor, a neurologist, a headache specialist, or a pain specialist. Dr. Lipton’s group calculated monthly headache days using the number of days during the previous 3 months affected by headache. The Migraine Disability Assessment (MIDAS) questionnaire was used to measure headache-related disability. The four-item Patient Health Questionnaire (PHQ-4) was used to screen for anxiety and depression, and the Migraine Treatment Optimization Questionnaire (mTOQ-4) evaluated participants’ treatment optimization.
Men predominated among opioid users
The investigators included 4,701 MAST participants in their analysis. The population’s mean age was 45 years, and 71.6% of participants were women. Of the entire sample, 67.5% reported no opioid use, and 32.5% reported opioid use. Of the total study population, 18.7% of patients took opioids 3 or fewer days per month, 6.5% took opioids 4 to 9 days per month, and 7.3% took opioids on 10 or more days per month.
Opioid users did not differ from nonusers on race or marital status. Men were overrepresented among all groups of opioid users, however. In addition, opioid use was more prevalent among participants with fewer than 4 years of college education (34.9%) than among participants with 4 or more years of college (30.8%). The proportion of participants with fewer than 4 years of college increased with increasing monthly opioid use. Furthermore, opioid use increased with decreasing household income. As opioid use increased, rates of employment decreased. Approximately 33% of the entire sample were obese, and the proportion of obese participants increased with increasing days per month of opioid use.
The most frequent setting during the previous 6 months for participants seeking care was primary care (49.7%). The next most frequent setting was neurology units (20.9%), pain clinics (8.3%), and headache clinics (7.7%). The prevalence of opioid use was 37.5% among participants with primary care visits, 37.3% among participants with neurologist visits, 43.0% among participants with headache clinic visits, and 53.5% with pain clinic visits.
About 15% of the population had chronic migraine. The prevalence of chronic migraine increased with increasing frequency of opioid use. About 49% of the sample had allodynia, and the prevalence of allodynia increased with increasing frequency of opioid use. Overall, disability was moderate to severe in 57.3% of participants. Participants who used opioids on 3 or fewer days per month had the lowest prevalence of moderate to severe disability (50.2%), and participants who used opioids on 10 or more days per month had the highest prevalence of moderate to severe disability (83.8%).
Approximately 21% of participants had anxiety or depression. The lowest prevalence of anxiety or depression was among participants who took opioids on 3 or fewer days per month (17.4%), and the highest prevalence was among participants who took opioids on 10 or more days per month (43.2%). About 39% of the population had very poor to poor treatment optimization. Among opioid nonusers, 35.6% had very poor to poor treatment optimization, and 59.4% of participants who used opioids on 10 or more days per month had very poor to poor treatment optimization.
Dr. Lipton and colleagues also examined the study population’s use of triptans. Overall, 51.5% of participants reported taking triptans. The prevalence of triptan use was highest among participants who did not use opioids (64.1%) and lowest among participants who used opioids on 3 or fewer days per month (20.5%). Triptan use increased as monthly days of opioid use increased.
Pain clinics and opioid prescription
“In the general population, women are more likely to receive opioids than men,” said Dr. Lipton. “This [finding] could reflect, in part, that women have more pain disorders than men and are more likely to seek medical care for pain than men.” In the current study, however, men with migraine were more likely to receive opioid prescriptions than were women with migraine. One potential explanation for this finding is that men with migraine are less likely to receive a migraine diagnosis, which might attenuate opioid prescribing, than women with migraine. “It may be that opioids are perceived to be serious drugs for serious pain, and that some physicians may be more likely to prescribe opioids to men because the disorder is taken more seriously in men than women,” said Dr. Lipton.
The observation that opioids were more likely to be prescribed for people treated in pain clinics “is consistent with my understanding of practice patterns,” he added. “Generally, neurologists strive to find effective acute treatment alternatives to opioids. The emergence of [drug classes known as] gepants and ditans provides a helpful set of alternatives to tritpans.”
Dr. Lipton and his colleagues plan further research into the treatment of migraineurs. “In a claims analysis, we showed that when people with migraine fail a triptan, they are most likely to get an opioid as their next drug,” he said. “Reasonable [clinicians] might disagree on the next step. The next step, in the absence of contraindications, could be a different oral triptan, a nonoral triptan, or a gepant or ditan. We are planning a randomized trial to probe this question.”
Why are opioids still being used?
The study’s reliance on patients’ self-report and its retrospective design are two of its weaknesses, said Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and editor-in-chief of Neurology Reviews. One strength, however, is that the stratified sampling methodology produced a study population that accurately reflects the demographic characteristics of the U.S. adult population, he added. Another strength is the investigators’ examination of opioid use by patient characteristics such as marital status, education, income, obesity, and smoking.
Given the harmful effects of opioids in migraine, it is hard to understand why as much as one-third of study participants using acute care medication for migraine were using opioids, said Dr. Rapoport. Using opioids for the acute treatment of migraine attacks often indicates inadequate treatment optimization, which leads to ongoing headache. As a consequence, patients may take more medication, which can increase headache frequency and lead to diagnoses of chronic migraine and medication overuse headache. Although the study found an association between the increased use of opioids and decreased household income and increased unemployment, smoking, and obesity, “it is not possible to assign causality to any of these associations, even though some would argue that decreased socioeconomic status was somehow related to more headache, disability, obesity, smoking, and unemployment,” he added.
“The paper suggests that future research should look at the risk factors for use of opioids and should determine if depression is a risk factor for or a consequence of opioid use,” said Dr. Rapoport. “Interventional studies designed to improve the acute care of migraine attacks might be able to reduce the use of opioids. I have not used opioids or butalbital-containing medication in my office for many years.”
This study was funded and sponsored by Dr. Reddy’s Laboratories group of companies, Princeton, N.J. Dr. Lipton has received grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund. He serves as a consultant, serves as an advisory board member, or has received honoraria from Alder, Allergan, American Headache Society, Autonomic Technologies, Biohaven, Dr. Reddy’s Laboratories, Eli Lilly, eNeura Therapeutics, Merck, Novartis, Pfizer, and Teva, Inc. He receives royalties from Wolff’s Headache, 8th Edition (New York: Oxford University Press, 2009) and holds stock options in eNeura Therapeutics and Biohaven.
SOURCE: Lipton RB, et al. Headache. https://doi.org/10.1111/head.14018. 2020;61(1):103-16.
Among patients with migraine who use prescription medications, the increasing use of prescription opioids is associated with chronic migraine, more severe disability, and anxiety and depression, according to an analysis published in the January issue of Headache . The use of prescription opioids also is associated with treatment-related variables such as poor acute treatment optimization and treatment in a pain clinic. The results indicate the continued need to educate patients and clinicians about the potential risks of opioids for migraineurs, according to the researchers.
In the Migraine in America Symptoms and Treatment (MAST) study, which the researchers analyzed for their investigation, one-third of migraineurs who use acute prescriptions reported using opioids. Among opioid users, 42% took opioids on 4 or more days per month. “These findings are like [those of] a previous report from the American Migraine Prevalence and Prevention study and more recent findings from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study,” said Richard Lipton, MD, Edwin S. Lowe professor and vice chair of neurology at Albert Einstein College of Medicine in the Bronx, New York. “High rates of opioid use are problematic because opioid use is associated with worsening of migraine over time.”
Opioids remain in widespread use for migraine, even though guidelines recommend against this treatment. Among migraineurs, opioid use is associated with more severe headache-related disability and greater use of health care resources. Opioid use also increases the risk of progressing from episodic migraine to chronic migraine.
A review of MAST data
Dr. Lipton and colleagues set out to identify the variables associated with the frequency of opioid use in people with migraine. Among the variables that they sought to examine were demographic characteristics, comorbidities, headache characteristics, medication use, and patterns of health care use. Dr. Lipton’s group hypothesized that migraine-related severity and burden would increase with increasing frequency of opioid use.
To conduct their research, the investigators examined data from the MAST study, a nationwide sample of American adults with migraine. They focused specifically on participants who reported receiving prescription acute medications. Participants eligible for this analysis reported 3 or more headache days in the previous 3 months and at least 1 monthly headache day in the previous month. In all, 15,133 participants met these criteria.
Dr. Lipton and colleagues categorized participants into four groups based on their frequency of opioid use. The groups had no opioid use, 3 or fewer monthly days of opioid use, 4 to 9 monthly days of opioid use, and 10 or more days of monthly opioid use. The last category is consistent with the International Classification of Headache Disorders-3 criteria for overuse of opioids in migraine.
At baseline, MAST participants provided information about variables such as gender, age, marital status, smoking status, education, and income. Participants also reported how many times in the previous 6 months they had visited a primary care doctor, a neurologist, a headache specialist, or a pain specialist. Dr. Lipton’s group calculated monthly headache days using the number of days during the previous 3 months affected by headache. The Migraine Disability Assessment (MIDAS) questionnaire was used to measure headache-related disability. The four-item Patient Health Questionnaire (PHQ-4) was used to screen for anxiety and depression, and the Migraine Treatment Optimization Questionnaire (mTOQ-4) evaluated participants’ treatment optimization.
Men predominated among opioid users
The investigators included 4,701 MAST participants in their analysis. The population’s mean age was 45 years, and 71.6% of participants were women. Of the entire sample, 67.5% reported no opioid use, and 32.5% reported opioid use. Of the total study population, 18.7% of patients took opioids 3 or fewer days per month, 6.5% took opioids 4 to 9 days per month, and 7.3% took opioids on 10 or more days per month.
Opioid users did not differ from nonusers on race or marital status. Men were overrepresented among all groups of opioid users, however. In addition, opioid use was more prevalent among participants with fewer than 4 years of college education (34.9%) than among participants with 4 or more years of college (30.8%). The proportion of participants with fewer than 4 years of college increased with increasing monthly opioid use. Furthermore, opioid use increased with decreasing household income. As opioid use increased, rates of employment decreased. Approximately 33% of the entire sample were obese, and the proportion of obese participants increased with increasing days per month of opioid use.
The most frequent setting during the previous 6 months for participants seeking care was primary care (49.7%). The next most frequent setting was neurology units (20.9%), pain clinics (8.3%), and headache clinics (7.7%). The prevalence of opioid use was 37.5% among participants with primary care visits, 37.3% among participants with neurologist visits, 43.0% among participants with headache clinic visits, and 53.5% with pain clinic visits.
About 15% of the population had chronic migraine. The prevalence of chronic migraine increased with increasing frequency of opioid use. About 49% of the sample had allodynia, and the prevalence of allodynia increased with increasing frequency of opioid use. Overall, disability was moderate to severe in 57.3% of participants. Participants who used opioids on 3 or fewer days per month had the lowest prevalence of moderate to severe disability (50.2%), and participants who used opioids on 10 or more days per month had the highest prevalence of moderate to severe disability (83.8%).
Approximately 21% of participants had anxiety or depression. The lowest prevalence of anxiety or depression was among participants who took opioids on 3 or fewer days per month (17.4%), and the highest prevalence was among participants who took opioids on 10 or more days per month (43.2%). About 39% of the population had very poor to poor treatment optimization. Among opioid nonusers, 35.6% had very poor to poor treatment optimization, and 59.4% of participants who used opioids on 10 or more days per month had very poor to poor treatment optimization.
Dr. Lipton and colleagues also examined the study population’s use of triptans. Overall, 51.5% of participants reported taking triptans. The prevalence of triptan use was highest among participants who did not use opioids (64.1%) and lowest among participants who used opioids on 3 or fewer days per month (20.5%). Triptan use increased as monthly days of opioid use increased.
Pain clinics and opioid prescription
“In the general population, women are more likely to receive opioids than men,” said Dr. Lipton. “This [finding] could reflect, in part, that women have more pain disorders than men and are more likely to seek medical care for pain than men.” In the current study, however, men with migraine were more likely to receive opioid prescriptions than were women with migraine. One potential explanation for this finding is that men with migraine are less likely to receive a migraine diagnosis, which might attenuate opioid prescribing, than women with migraine. “It may be that opioids are perceived to be serious drugs for serious pain, and that some physicians may be more likely to prescribe opioids to men because the disorder is taken more seriously in men than women,” said Dr. Lipton.
The observation that opioids were more likely to be prescribed for people treated in pain clinics “is consistent with my understanding of practice patterns,” he added. “Generally, neurologists strive to find effective acute treatment alternatives to opioids. The emergence of [drug classes known as] gepants and ditans provides a helpful set of alternatives to tritpans.”
Dr. Lipton and his colleagues plan further research into the treatment of migraineurs. “In a claims analysis, we showed that when people with migraine fail a triptan, they are most likely to get an opioid as their next drug,” he said. “Reasonable [clinicians] might disagree on the next step. The next step, in the absence of contraindications, could be a different oral triptan, a nonoral triptan, or a gepant or ditan. We are planning a randomized trial to probe this question.”
Why are opioids still being used?
The study’s reliance on patients’ self-report and its retrospective design are two of its weaknesses, said Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and editor-in-chief of Neurology Reviews. One strength, however, is that the stratified sampling methodology produced a study population that accurately reflects the demographic characteristics of the U.S. adult population, he added. Another strength is the investigators’ examination of opioid use by patient characteristics such as marital status, education, income, obesity, and smoking.
Given the harmful effects of opioids in migraine, it is hard to understand why as much as one-third of study participants using acute care medication for migraine were using opioids, said Dr. Rapoport. Using opioids for the acute treatment of migraine attacks often indicates inadequate treatment optimization, which leads to ongoing headache. As a consequence, patients may take more medication, which can increase headache frequency and lead to diagnoses of chronic migraine and medication overuse headache. Although the study found an association between the increased use of opioids and decreased household income and increased unemployment, smoking, and obesity, “it is not possible to assign causality to any of these associations, even though some would argue that decreased socioeconomic status was somehow related to more headache, disability, obesity, smoking, and unemployment,” he added.
“The paper suggests that future research should look at the risk factors for use of opioids and should determine if depression is a risk factor for or a consequence of opioid use,” said Dr. Rapoport. “Interventional studies designed to improve the acute care of migraine attacks might be able to reduce the use of opioids. I have not used opioids or butalbital-containing medication in my office for many years.”
This study was funded and sponsored by Dr. Reddy’s Laboratories group of companies, Princeton, N.J. Dr. Lipton has received grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund. He serves as a consultant, serves as an advisory board member, or has received honoraria from Alder, Allergan, American Headache Society, Autonomic Technologies, Biohaven, Dr. Reddy’s Laboratories, Eli Lilly, eNeura Therapeutics, Merck, Novartis, Pfizer, and Teva, Inc. He receives royalties from Wolff’s Headache, 8th Edition (New York: Oxford University Press, 2009) and holds stock options in eNeura Therapeutics and Biohaven.
SOURCE: Lipton RB, et al. Headache. https://doi.org/10.1111/head.14018. 2020;61(1):103-16.
FROM HEADACHE
Opioid-related deaths lower in counties with active cannabis dispensaries
Areas with active cannabis dispensaries have seen a decrease in opioid-related mortalities, recent research has shown.
“Our findings suggest that higher storefront cannabis dispensary counts are associated with reduced opioid related mortality rates at the county level,” wrote Greta Hsu, PhD, professor of management, University of California, Davis, and Balázs Kovács, PhD, associate professor of organizational behavior, Yale University, New Haven, Conn. “, which include the highly potent synthetic opioid fentanyl and its analogs.”
In the study, published in BMJ, the researchers evaluated the prevalence of medical and recreational cannabis dispensaries in 812 U.S. counties within 23 states with some degree of cannabis legalization between 2014 and 2018. Overall, dispensaries located in counties in eight U.S. states and the District of Columbia that sold cannabis recreationally and an additional 15 states that contained medical cannabis dispensaries were included.
Dr. Hsu and Dr. Kovács performed their analysis by examining dispensaries that were operating storefronts by the end of 2017 at the county level using panel-regression methods, combining data obtained from the consumer-facing website Weedmaps.com, Centers for Disease Control and Prevention U.S. mortality data, and data from the U.S. Census Bureau.
To measure opioid-related mortality, the researchers measured ICD-10 codes specific to natural opioid analgesics and semisynthetic opioids, methadone, heroin, nonmethadone synthetic opioid analgesics, and fentanyl-related deaths.
The analysis showed a negative association between the number of cannabis dispensaries at the county level and overall opioid-related mortality rates (95% confidence interval, −0.23 to −0.11), with an increase from one to two dispensaries in a county resulting in a 17% decrease in opioid-related mortality rates and an increase from two to three dispensaries resulting in another decrease in opioid-related mortality of 8.5%.
When evaluating mortality by specific opioid type, the researchers found a negative association between the number of dispensaries and synthetic nonmethadone opioids, with an increase from one to two dispensaries resulting in a 21% decrease in mortality attributable to synthetic nonmethadone opioids (95% CI, −0.27 to −0.14; P = .002). There were also negative associations between the number of dispensaries and prescription opioid-related mortality rates (95% CI, −0.13 to −0.03) and heroin-related mortality rates (95% CI, −0.13 to −0.02). The negative association was similar in comparisons between synthetic nonmethadone opioid-related mortality and the number of dispensaries for medical cannabis (95% CI, −0.21 to −0.09; P = .002) and recreational cannabis (95% CI, −0.17 to −0.04; P = .01).
Evidence of a negative association between legalization of medical or recreational cannabis and opioid-related mortality has been mixed in the literature, with some studies also showing a “spurious or nonsignificant” association, according to Dr. Hsu and Dr. Kovács.
While previous studies have looked at the legalization of cannabis for medical or recreational use, legalization on its own is an “incomplete picture,” they said, which might offer one explanation for these mixed findings. Some states that legalize medical cannabis, for example, might not allow dispensaries to legally sell cannabis, and there may be a delay of 1-2 years between the time a state legalizes cannabis for recreational use and when dispensaries are open and available to the public.
“These results were obtained after controlling for county level population characteristics, yearly effects, whether recreational dispensaries were legal or not in the focal county’s state, and opioid-related state policies,” the authors wrote.
Results ‘may be even stronger’ than reported
Christopher G. Fichtner, MD, clinical professor of psychiatry and neuroscience at the University of California, Riverside, said in an interview that the evidence for using cannabis as an opioid substitution for pain management has not been balanced, but noted “the bulk of it suggests that there is some harm reduction benefit by having liberalized access to cannabis.”
One strength of the study by Dr. Hsu and Dr. Kovács was how they were able to examine implementation of legalization of medical or recreational cannabis, rather than simply a change in the law, he said.
“By looking at dispensary count, it’s actually looking at a better measure of on-the-ground implementation than just change in policy,” Dr. Fichtner explained. “You’re looking at what was actually accomplished in terms of making cannabis legally available.”
The choice to evaluate storefront dispensaries only and not include delivery services in their data, “probably makes it a relatively conservative estimate. I think that would be a strength, that their findings may be even stronger than what it is they’re reporting,” Dr. Fichtner said.
“I do think, if anything, the paper is relatively tentative about advancing its conclusions, which I think is a weakness in a lot of these studies,” he added. In 2017, the National Academy of Sciences released a report that found evidence cannabis or cannabinoids can significantly reduce pain symptoms. In that report, “one of their strongest conclusions is that there’s conclusive or substantial evidence that cannabis or cannabinoids are effective management of chronic pain,” Dr. Fichtner said.
He said that digging deeper into what kinds of pain cannabis can treat is one area for future research. “Certainly, it seems that it’s unlikely that cannabis is going to be good for every kind of pain,” he said. “What kinds of pain is it better for than others? Is it some benefit for many kinds of pain, or only a few types of pain?”
The authors reported no relevant financial disclosures. Dr. Fichtner is the author of a book on cannabis policy in the United States, but reported no other financial disclosures.
Areas with active cannabis dispensaries have seen a decrease in opioid-related mortalities, recent research has shown.
“Our findings suggest that higher storefront cannabis dispensary counts are associated with reduced opioid related mortality rates at the county level,” wrote Greta Hsu, PhD, professor of management, University of California, Davis, and Balázs Kovács, PhD, associate professor of organizational behavior, Yale University, New Haven, Conn. “, which include the highly potent synthetic opioid fentanyl and its analogs.”
In the study, published in BMJ, the researchers evaluated the prevalence of medical and recreational cannabis dispensaries in 812 U.S. counties within 23 states with some degree of cannabis legalization between 2014 and 2018. Overall, dispensaries located in counties in eight U.S. states and the District of Columbia that sold cannabis recreationally and an additional 15 states that contained medical cannabis dispensaries were included.
Dr. Hsu and Dr. Kovács performed their analysis by examining dispensaries that were operating storefronts by the end of 2017 at the county level using panel-regression methods, combining data obtained from the consumer-facing website Weedmaps.com, Centers for Disease Control and Prevention U.S. mortality data, and data from the U.S. Census Bureau.
To measure opioid-related mortality, the researchers measured ICD-10 codes specific to natural opioid analgesics and semisynthetic opioids, methadone, heroin, nonmethadone synthetic opioid analgesics, and fentanyl-related deaths.
The analysis showed a negative association between the number of cannabis dispensaries at the county level and overall opioid-related mortality rates (95% confidence interval, −0.23 to −0.11), with an increase from one to two dispensaries in a county resulting in a 17% decrease in opioid-related mortality rates and an increase from two to three dispensaries resulting in another decrease in opioid-related mortality of 8.5%.
When evaluating mortality by specific opioid type, the researchers found a negative association between the number of dispensaries and synthetic nonmethadone opioids, with an increase from one to two dispensaries resulting in a 21% decrease in mortality attributable to synthetic nonmethadone opioids (95% CI, −0.27 to −0.14; P = .002). There were also negative associations between the number of dispensaries and prescription opioid-related mortality rates (95% CI, −0.13 to −0.03) and heroin-related mortality rates (95% CI, −0.13 to −0.02). The negative association was similar in comparisons between synthetic nonmethadone opioid-related mortality and the number of dispensaries for medical cannabis (95% CI, −0.21 to −0.09; P = .002) and recreational cannabis (95% CI, −0.17 to −0.04; P = .01).
Evidence of a negative association between legalization of medical or recreational cannabis and opioid-related mortality has been mixed in the literature, with some studies also showing a “spurious or nonsignificant” association, according to Dr. Hsu and Dr. Kovács.
While previous studies have looked at the legalization of cannabis for medical or recreational use, legalization on its own is an “incomplete picture,” they said, which might offer one explanation for these mixed findings. Some states that legalize medical cannabis, for example, might not allow dispensaries to legally sell cannabis, and there may be a delay of 1-2 years between the time a state legalizes cannabis for recreational use and when dispensaries are open and available to the public.
“These results were obtained after controlling for county level population characteristics, yearly effects, whether recreational dispensaries were legal or not in the focal county’s state, and opioid-related state policies,” the authors wrote.
Results ‘may be even stronger’ than reported
Christopher G. Fichtner, MD, clinical professor of psychiatry and neuroscience at the University of California, Riverside, said in an interview that the evidence for using cannabis as an opioid substitution for pain management has not been balanced, but noted “the bulk of it suggests that there is some harm reduction benefit by having liberalized access to cannabis.”
One strength of the study by Dr. Hsu and Dr. Kovács was how they were able to examine implementation of legalization of medical or recreational cannabis, rather than simply a change in the law, he said.
“By looking at dispensary count, it’s actually looking at a better measure of on-the-ground implementation than just change in policy,” Dr. Fichtner explained. “You’re looking at what was actually accomplished in terms of making cannabis legally available.”
The choice to evaluate storefront dispensaries only and not include delivery services in their data, “probably makes it a relatively conservative estimate. I think that would be a strength, that their findings may be even stronger than what it is they’re reporting,” Dr. Fichtner said.
“I do think, if anything, the paper is relatively tentative about advancing its conclusions, which I think is a weakness in a lot of these studies,” he added. In 2017, the National Academy of Sciences released a report that found evidence cannabis or cannabinoids can significantly reduce pain symptoms. In that report, “one of their strongest conclusions is that there’s conclusive or substantial evidence that cannabis or cannabinoids are effective management of chronic pain,” Dr. Fichtner said.
He said that digging deeper into what kinds of pain cannabis can treat is one area for future research. “Certainly, it seems that it’s unlikely that cannabis is going to be good for every kind of pain,” he said. “What kinds of pain is it better for than others? Is it some benefit for many kinds of pain, or only a few types of pain?”
The authors reported no relevant financial disclosures. Dr. Fichtner is the author of a book on cannabis policy in the United States, but reported no other financial disclosures.
Areas with active cannabis dispensaries have seen a decrease in opioid-related mortalities, recent research has shown.
“Our findings suggest that higher storefront cannabis dispensary counts are associated with reduced opioid related mortality rates at the county level,” wrote Greta Hsu, PhD, professor of management, University of California, Davis, and Balázs Kovács, PhD, associate professor of organizational behavior, Yale University, New Haven, Conn. “, which include the highly potent synthetic opioid fentanyl and its analogs.”
In the study, published in BMJ, the researchers evaluated the prevalence of medical and recreational cannabis dispensaries in 812 U.S. counties within 23 states with some degree of cannabis legalization between 2014 and 2018. Overall, dispensaries located in counties in eight U.S. states and the District of Columbia that sold cannabis recreationally and an additional 15 states that contained medical cannabis dispensaries were included.
Dr. Hsu and Dr. Kovács performed their analysis by examining dispensaries that were operating storefronts by the end of 2017 at the county level using panel-regression methods, combining data obtained from the consumer-facing website Weedmaps.com, Centers for Disease Control and Prevention U.S. mortality data, and data from the U.S. Census Bureau.
To measure opioid-related mortality, the researchers measured ICD-10 codes specific to natural opioid analgesics and semisynthetic opioids, methadone, heroin, nonmethadone synthetic opioid analgesics, and fentanyl-related deaths.
The analysis showed a negative association between the number of cannabis dispensaries at the county level and overall opioid-related mortality rates (95% confidence interval, −0.23 to −0.11), with an increase from one to two dispensaries in a county resulting in a 17% decrease in opioid-related mortality rates and an increase from two to three dispensaries resulting in another decrease in opioid-related mortality of 8.5%.
When evaluating mortality by specific opioid type, the researchers found a negative association between the number of dispensaries and synthetic nonmethadone opioids, with an increase from one to two dispensaries resulting in a 21% decrease in mortality attributable to synthetic nonmethadone opioids (95% CI, −0.27 to −0.14; P = .002). There were also negative associations between the number of dispensaries and prescription opioid-related mortality rates (95% CI, −0.13 to −0.03) and heroin-related mortality rates (95% CI, −0.13 to −0.02). The negative association was similar in comparisons between synthetic nonmethadone opioid-related mortality and the number of dispensaries for medical cannabis (95% CI, −0.21 to −0.09; P = .002) and recreational cannabis (95% CI, −0.17 to −0.04; P = .01).
Evidence of a negative association between legalization of medical or recreational cannabis and opioid-related mortality has been mixed in the literature, with some studies also showing a “spurious or nonsignificant” association, according to Dr. Hsu and Dr. Kovács.
While previous studies have looked at the legalization of cannabis for medical or recreational use, legalization on its own is an “incomplete picture,” they said, which might offer one explanation for these mixed findings. Some states that legalize medical cannabis, for example, might not allow dispensaries to legally sell cannabis, and there may be a delay of 1-2 years between the time a state legalizes cannabis for recreational use and when dispensaries are open and available to the public.
“These results were obtained after controlling for county level population characteristics, yearly effects, whether recreational dispensaries were legal or not in the focal county’s state, and opioid-related state policies,” the authors wrote.
Results ‘may be even stronger’ than reported
Christopher G. Fichtner, MD, clinical professor of psychiatry and neuroscience at the University of California, Riverside, said in an interview that the evidence for using cannabis as an opioid substitution for pain management has not been balanced, but noted “the bulk of it suggests that there is some harm reduction benefit by having liberalized access to cannabis.”
One strength of the study by Dr. Hsu and Dr. Kovács was how they were able to examine implementation of legalization of medical or recreational cannabis, rather than simply a change in the law, he said.
“By looking at dispensary count, it’s actually looking at a better measure of on-the-ground implementation than just change in policy,” Dr. Fichtner explained. “You’re looking at what was actually accomplished in terms of making cannabis legally available.”
The choice to evaluate storefront dispensaries only and not include delivery services in their data, “probably makes it a relatively conservative estimate. I think that would be a strength, that their findings may be even stronger than what it is they’re reporting,” Dr. Fichtner said.
“I do think, if anything, the paper is relatively tentative about advancing its conclusions, which I think is a weakness in a lot of these studies,” he added. In 2017, the National Academy of Sciences released a report that found evidence cannabis or cannabinoids can significantly reduce pain symptoms. In that report, “one of their strongest conclusions is that there’s conclusive or substantial evidence that cannabis or cannabinoids are effective management of chronic pain,” Dr. Fichtner said.
He said that digging deeper into what kinds of pain cannabis can treat is one area for future research. “Certainly, it seems that it’s unlikely that cannabis is going to be good for every kind of pain,” he said. “What kinds of pain is it better for than others? Is it some benefit for many kinds of pain, or only a few types of pain?”
The authors reported no relevant financial disclosures. Dr. Fichtner is the author of a book on cannabis policy in the United States, but reported no other financial disclosures.
FROM BMJ
Biden administration nixes buprenorphine waiver, docs disappointed
The Biden administration has halted a Trump administration initiative that would have allowed more physicians to prescribe buprenorphine for opioid use disorder (OUD).
Under the Trump administration’s plan, many doctors would be exempt from taking a day’s training before they could prescribe buprenorphine for OUD.
On Jan. 25, 2021, citing anonymous sources, the Washington Post reported that this action by the Biden administration was likely. At the time, there were concerns about whether the Department of Health & Human Services had the legal authority to make this policy change, the Post reported. The Substance Abuse and Mental Health Services Administration subsequently announced the derailment of the buprenorphine proposal on its website.
In SAMHSA’s view, the proposal was made “prematurely.” The SAMHSA statement did not detail the reasons for abandoning the Jan. 14 proposal. It had been scheduled to take effect upon publication in the Federal Register.
Instead of finalizing it in this way, the HHS said it would work with other federal agencies to “increase access to buprenorphine, reduce overdose rates and save lives.”
The HHS decision to scupper the proposal disappointed many physician groups. In a letter dated Jan. 27, several physician groups called on the Biden administration to proceed with the Trump proposal.
Under current federal law, physicians who wish to prescribe buprenorphine outside of opioid treatment programs must take an 8-hour course and receive a waiver from the Drug Enforcement Administration, the letter noted. It was signed by the American College of Emergency Physicians, the American Medical Association, and other organizations.
Treatment barrier
After taking the training course, it can take 60-90 days for physicians to receive the waiver. The license application can then be submitted. Physician groups argue that this so-called X-waiver requirement creates a barrier to providing medication-assisted treatment.
“Due to the stigma, some clinicians are not willing to pursue this DEA license or even engage in treatment of patients with [OUD],” the letter said.
The Trump administration’s proposal would have limited most physicians to treating no more than 30 patients with buprenorphine for OUD at any one time. This cap would not have applied to hospital-based physicians, such as those practicing emergency medicine, the HHS noted in a statement. The policy would have applied to only physicians who already have registered with the DEA.
Patrice A. Harris, MD, the immediate past president of the AMA and chair of the organization’s Opioid Task Force, was among the many physicians who supported the Trump administration proposal.
“It is estimated that more than 2 million Americans need treatment for opioid use disorder, but only a small percentage actually receive treatment,” Dr. Harris said in statement. Dr. Harris also noted that overdose deaths have reportedly accelerated during the COVID-19 pandemic.
Centers for Disease Control and Prevention data show there were more than 83,000 drug overdose deaths in the United States in the 12 months ending in June 2020. That is the highest number of overdose deaths ever recorded in a 12-month period and is an increase of more than 21%, compared with the previous year.
A ‘disappointment’
On Jan. 28, Dr. Harris said the decision to drop the plan was a disappointment.
“We encourage the current administration to quickly develop a path forward that removes the burdensome waiver requirement, thus allowing more physicians to prescribe this lifesaving medication,” she said in a statement sent to this news organization.
In a Jan. 26 statement, the American Society of Addiction Medicine urged Congress to eliminate the X-waiver and called for more education and training in the treatment of patients who struggle with opioids.
In the 116th session of Congress, which ended on Jan. 3, there was bipartisan support for proposed legislation to ease requirements for buprenorphine prescribing. A House bill had more than 90 Democratic and 21 Republican sponsors. A companion Senate bill had three Democratic and three Republican Sponsors, including Sen. Maggie Hassan (D-N.H.). On Jan. 25, Dr. Hassan tweeted that she would be seeking an explanation from the Biden administration if it halted the plan to ease the waiver restriction.
“Medication-assisted treatment can save lives, and the buprenorphine waiver requirement should be eliminated so that physicians can more easily prescribe it to those who need it,” she said.
Many clinicians and policy experts turned to Twitter to urge an easing of buprenorphine prescribing, using the hashtag “Xthexwaiver.”
Among them was the official who put forward the Jan. 14 proposal, Brett Giroir, MD. He served as assistant secretary for health during the Trump administration.
Objections
In its Jan. 25 article, the Washington Post referred to an article in Alcoholism and Drug Abuse Weekly in which a top federal official in the Trump administration objected to Dr. Giroir’s plan.
Elinore F. McCance-Katz, MD, PhD, who served as the assistant secretary of HHS for SAMHSA, had earlier proposed raising the cap for addiction experts. Alcoholism and Drug Abuse Weekly quotes Dr. McCance-Katz as saying the Trump buprenorphine proposal was “unfair to the incoming administration.”
“The Biden administration has so much work to do to get their programs and policies into place, and to do something like this at the 11th hour that could get doctors into trouble – it’s heinous,” she said in the article.
Dr. McCance-Katz had resigned before the Trump administration proposal was unveiled. On Jan. 7, she issued a public notice announcing she would resign, citing concerns about the previous day’s attack on the U.S. Capitol.
“It had been my plan to stay until the change in administration occurred, but my plans abruptly changed last evening when, on my way back from visiting an excellent residential treatment program in New York, I saw the violent takeover of the Capitol building,” she said.
On Twitter, Roland Flores, MD, an anesthesiologist and pain specialist, urged his colleagues to consider the need for more education among clinicians who treat OUD. He jousted a bit with those favoring a swift drive to “XtheXwaiver” and questioned their arguments about the burden of the current rules.
“I think ‘all this red tape’ is a little bit of an exaggeration – it’s an 8-hour online course, and an application,” Dr. Flores tweeted in one exchange. “But #XtheXwaiver is fine – it’s probably rooted in stigma. It’s unlikely to make much difference tho. The waiver wasn’t the thing keeping docs from prescribing.”
A version of this article first appeared on Medscape.com.
The Biden administration has halted a Trump administration initiative that would have allowed more physicians to prescribe buprenorphine for opioid use disorder (OUD).
Under the Trump administration’s plan, many doctors would be exempt from taking a day’s training before they could prescribe buprenorphine for OUD.
On Jan. 25, 2021, citing anonymous sources, the Washington Post reported that this action by the Biden administration was likely. At the time, there were concerns about whether the Department of Health & Human Services had the legal authority to make this policy change, the Post reported. The Substance Abuse and Mental Health Services Administration subsequently announced the derailment of the buprenorphine proposal on its website.
In SAMHSA’s view, the proposal was made “prematurely.” The SAMHSA statement did not detail the reasons for abandoning the Jan. 14 proposal. It had been scheduled to take effect upon publication in the Federal Register.
Instead of finalizing it in this way, the HHS said it would work with other federal agencies to “increase access to buprenorphine, reduce overdose rates and save lives.”
The HHS decision to scupper the proposal disappointed many physician groups. In a letter dated Jan. 27, several physician groups called on the Biden administration to proceed with the Trump proposal.
Under current federal law, physicians who wish to prescribe buprenorphine outside of opioid treatment programs must take an 8-hour course and receive a waiver from the Drug Enforcement Administration, the letter noted. It was signed by the American College of Emergency Physicians, the American Medical Association, and other organizations.
Treatment barrier
After taking the training course, it can take 60-90 days for physicians to receive the waiver. The license application can then be submitted. Physician groups argue that this so-called X-waiver requirement creates a barrier to providing medication-assisted treatment.
“Due to the stigma, some clinicians are not willing to pursue this DEA license or even engage in treatment of patients with [OUD],” the letter said.
The Trump administration’s proposal would have limited most physicians to treating no more than 30 patients with buprenorphine for OUD at any one time. This cap would not have applied to hospital-based physicians, such as those practicing emergency medicine, the HHS noted in a statement. The policy would have applied to only physicians who already have registered with the DEA.
Patrice A. Harris, MD, the immediate past president of the AMA and chair of the organization’s Opioid Task Force, was among the many physicians who supported the Trump administration proposal.
“It is estimated that more than 2 million Americans need treatment for opioid use disorder, but only a small percentage actually receive treatment,” Dr. Harris said in statement. Dr. Harris also noted that overdose deaths have reportedly accelerated during the COVID-19 pandemic.
Centers for Disease Control and Prevention data show there were more than 83,000 drug overdose deaths in the United States in the 12 months ending in June 2020. That is the highest number of overdose deaths ever recorded in a 12-month period and is an increase of more than 21%, compared with the previous year.
A ‘disappointment’
On Jan. 28, Dr. Harris said the decision to drop the plan was a disappointment.
“We encourage the current administration to quickly develop a path forward that removes the burdensome waiver requirement, thus allowing more physicians to prescribe this lifesaving medication,” she said in a statement sent to this news organization.
In a Jan. 26 statement, the American Society of Addiction Medicine urged Congress to eliminate the X-waiver and called for more education and training in the treatment of patients who struggle with opioids.
In the 116th session of Congress, which ended on Jan. 3, there was bipartisan support for proposed legislation to ease requirements for buprenorphine prescribing. A House bill had more than 90 Democratic and 21 Republican sponsors. A companion Senate bill had three Democratic and three Republican Sponsors, including Sen. Maggie Hassan (D-N.H.). On Jan. 25, Dr. Hassan tweeted that she would be seeking an explanation from the Biden administration if it halted the plan to ease the waiver restriction.
“Medication-assisted treatment can save lives, and the buprenorphine waiver requirement should be eliminated so that physicians can more easily prescribe it to those who need it,” she said.
Many clinicians and policy experts turned to Twitter to urge an easing of buprenorphine prescribing, using the hashtag “Xthexwaiver.”
Among them was the official who put forward the Jan. 14 proposal, Brett Giroir, MD. He served as assistant secretary for health during the Trump administration.
Objections
In its Jan. 25 article, the Washington Post referred to an article in Alcoholism and Drug Abuse Weekly in which a top federal official in the Trump administration objected to Dr. Giroir’s plan.
Elinore F. McCance-Katz, MD, PhD, who served as the assistant secretary of HHS for SAMHSA, had earlier proposed raising the cap for addiction experts. Alcoholism and Drug Abuse Weekly quotes Dr. McCance-Katz as saying the Trump buprenorphine proposal was “unfair to the incoming administration.”
“The Biden administration has so much work to do to get their programs and policies into place, and to do something like this at the 11th hour that could get doctors into trouble – it’s heinous,” she said in the article.
Dr. McCance-Katz had resigned before the Trump administration proposal was unveiled. On Jan. 7, she issued a public notice announcing she would resign, citing concerns about the previous day’s attack on the U.S. Capitol.
“It had been my plan to stay until the change in administration occurred, but my plans abruptly changed last evening when, on my way back from visiting an excellent residential treatment program in New York, I saw the violent takeover of the Capitol building,” she said.
On Twitter, Roland Flores, MD, an anesthesiologist and pain specialist, urged his colleagues to consider the need for more education among clinicians who treat OUD. He jousted a bit with those favoring a swift drive to “XtheXwaiver” and questioned their arguments about the burden of the current rules.
“I think ‘all this red tape’ is a little bit of an exaggeration – it’s an 8-hour online course, and an application,” Dr. Flores tweeted in one exchange. “But #XtheXwaiver is fine – it’s probably rooted in stigma. It’s unlikely to make much difference tho. The waiver wasn’t the thing keeping docs from prescribing.”
A version of this article first appeared on Medscape.com.
The Biden administration has halted a Trump administration initiative that would have allowed more physicians to prescribe buprenorphine for opioid use disorder (OUD).
Under the Trump administration’s plan, many doctors would be exempt from taking a day’s training before they could prescribe buprenorphine for OUD.
On Jan. 25, 2021, citing anonymous sources, the Washington Post reported that this action by the Biden administration was likely. At the time, there were concerns about whether the Department of Health & Human Services had the legal authority to make this policy change, the Post reported. The Substance Abuse and Mental Health Services Administration subsequently announced the derailment of the buprenorphine proposal on its website.
In SAMHSA’s view, the proposal was made “prematurely.” The SAMHSA statement did not detail the reasons for abandoning the Jan. 14 proposal. It had been scheduled to take effect upon publication in the Federal Register.
Instead of finalizing it in this way, the HHS said it would work with other federal agencies to “increase access to buprenorphine, reduce overdose rates and save lives.”
The HHS decision to scupper the proposal disappointed many physician groups. In a letter dated Jan. 27, several physician groups called on the Biden administration to proceed with the Trump proposal.
Under current federal law, physicians who wish to prescribe buprenorphine outside of opioid treatment programs must take an 8-hour course and receive a waiver from the Drug Enforcement Administration, the letter noted. It was signed by the American College of Emergency Physicians, the American Medical Association, and other organizations.
Treatment barrier
After taking the training course, it can take 60-90 days for physicians to receive the waiver. The license application can then be submitted. Physician groups argue that this so-called X-waiver requirement creates a barrier to providing medication-assisted treatment.
“Due to the stigma, some clinicians are not willing to pursue this DEA license or even engage in treatment of patients with [OUD],” the letter said.
The Trump administration’s proposal would have limited most physicians to treating no more than 30 patients with buprenorphine for OUD at any one time. This cap would not have applied to hospital-based physicians, such as those practicing emergency medicine, the HHS noted in a statement. The policy would have applied to only physicians who already have registered with the DEA.
Patrice A. Harris, MD, the immediate past president of the AMA and chair of the organization’s Opioid Task Force, was among the many physicians who supported the Trump administration proposal.
“It is estimated that more than 2 million Americans need treatment for opioid use disorder, but only a small percentage actually receive treatment,” Dr. Harris said in statement. Dr. Harris also noted that overdose deaths have reportedly accelerated during the COVID-19 pandemic.
Centers for Disease Control and Prevention data show there were more than 83,000 drug overdose deaths in the United States in the 12 months ending in June 2020. That is the highest number of overdose deaths ever recorded in a 12-month period and is an increase of more than 21%, compared with the previous year.
A ‘disappointment’
On Jan. 28, Dr. Harris said the decision to drop the plan was a disappointment.
“We encourage the current administration to quickly develop a path forward that removes the burdensome waiver requirement, thus allowing more physicians to prescribe this lifesaving medication,” she said in a statement sent to this news organization.
In a Jan. 26 statement, the American Society of Addiction Medicine urged Congress to eliminate the X-waiver and called for more education and training in the treatment of patients who struggle with opioids.
In the 116th session of Congress, which ended on Jan. 3, there was bipartisan support for proposed legislation to ease requirements for buprenorphine prescribing. A House bill had more than 90 Democratic and 21 Republican sponsors. A companion Senate bill had three Democratic and three Republican Sponsors, including Sen. Maggie Hassan (D-N.H.). On Jan. 25, Dr. Hassan tweeted that she would be seeking an explanation from the Biden administration if it halted the plan to ease the waiver restriction.
“Medication-assisted treatment can save lives, and the buprenorphine waiver requirement should be eliminated so that physicians can more easily prescribe it to those who need it,” she said.
Many clinicians and policy experts turned to Twitter to urge an easing of buprenorphine prescribing, using the hashtag “Xthexwaiver.”
Among them was the official who put forward the Jan. 14 proposal, Brett Giroir, MD. He served as assistant secretary for health during the Trump administration.
Objections
In its Jan. 25 article, the Washington Post referred to an article in Alcoholism and Drug Abuse Weekly in which a top federal official in the Trump administration objected to Dr. Giroir’s plan.
Elinore F. McCance-Katz, MD, PhD, who served as the assistant secretary of HHS for SAMHSA, had earlier proposed raising the cap for addiction experts. Alcoholism and Drug Abuse Weekly quotes Dr. McCance-Katz as saying the Trump buprenorphine proposal was “unfair to the incoming administration.”
“The Biden administration has so much work to do to get their programs and policies into place, and to do something like this at the 11th hour that could get doctors into trouble – it’s heinous,” she said in the article.
Dr. McCance-Katz had resigned before the Trump administration proposal was unveiled. On Jan. 7, she issued a public notice announcing she would resign, citing concerns about the previous day’s attack on the U.S. Capitol.
“It had been my plan to stay until the change in administration occurred, but my plans abruptly changed last evening when, on my way back from visiting an excellent residential treatment program in New York, I saw the violent takeover of the Capitol building,” she said.
On Twitter, Roland Flores, MD, an anesthesiologist and pain specialist, urged his colleagues to consider the need for more education among clinicians who treat OUD. He jousted a bit with those favoring a swift drive to “XtheXwaiver” and questioned their arguments about the burden of the current rules.
“I think ‘all this red tape’ is a little bit of an exaggeration – it’s an 8-hour online course, and an application,” Dr. Flores tweeted in one exchange. “But #XtheXwaiver is fine – it’s probably rooted in stigma. It’s unlikely to make much difference tho. The waiver wasn’t the thing keeping docs from prescribing.”
A version of this article first appeared on Medscape.com.
HHS will drop buprenorphine waiver rule for most physicians
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Childhood smoking and depression contribute to young adult opioid use
Depression and tobacco use in childhood significantly increased the risk for opioid use in young adults, according to data from a prospective study of approximately 1,000 individuals.
Previous research, including the annual Monitoring the Future study, documents opioid use among adolescents in the United States, but childhood risk factors for opioid use in young adults have not been well studied, wrote Lilly Shanahan, PhD, of the University of Zürich, and colleagues.
In a prospective cohort study published in JAMA Pediatrics, the researchers identified 1,252 non-Hispanic White and American Indian opioid-naive individuals aged 9-16 years in rural North Carolina. They interviewed participants and parents up to 7 times between January 1993 and December 2000, and interviewed participants only at ages 19, 21, 25, and 30 years between January 1999 and December 2015.
Overall, 24.2% of study participants had used a nonheroin opioid by age 30 years, and both chronic depression and dysthymia were significantly associated with this use (odds ratios 5.43 and 7.13, respectively).
In addition, 155 participants (8.8%) reported weekly use of a nonheroin opioid, and 95 (6.6%) reported weekly heroin use by age 30 years. Chronic depression and dysthymia also were strongly associated with weekly nonheroin opioid use (OR 8.89 and 11.51, respectively).
In a multivariate analysis, depression, tobacco use, and cannabis use at ages 9-16 years were strongly associated with overall opioid use at ages 19-30 years.
“One possible reason childhood chronic depression increases the risk of later opioid use is self-medication, including the use of psychoactive substances, to alleviate depression,” the researchers noted. In addition, the mood-altering properties of opioids may increase their appeal to depressed youth as a way to relieve impaired reward system function, they said.
Potential mechanisms for the association between early tobacco use and later opioid use include the alterations to neurodevelopment caused by nicotine exposure in adolescence, as well as increased risk for depression, reduced pain thresholds, and use of nicotine as a gateway to harder drugs, the researchers added.
Several childhood risk factors were not associated with young adult opioid use in multivariate analysis in this study, including alcohol use, sociodemographic status, maltreatment, family dysfunction, and anxiety, the researchers wrote. “Previous studies typically measured these risk factors retrospectively or in late adolescence and young adulthood, and most did not consider depressive disorders, which may mediate associations between select childhood risk factors and later opioid use,” they said.
The study findings were limited by several factors, including the inability to distinguish between medical and nonmedical opioid use, the incomplete list of available opioids, and the exclusion of Black participants because of low sample size, the researchers noted. However, the results were strengthened by the longitudinal, community-representative design and the inclusion of up to 11 assessments of opioid use, they said.
“Our findings suggest strong opportunities for early prevention and intervention, including in primary care settings,” using known evidence-based strategies, they concluded.
More screening is needed
“Children in the United States are at high risk of serious adult health issues as a result of childhood factors such as ACEs (adverse childhood experiences),” said Suzanne C. Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H. “This study looks prospectively at other factors in childhood over a long period of time leading to opioid usage, with its serious risks and health consequences including overdose death,” she said. “It is unclear what the effects of COVID-19 will be on the population of children growing up now and how opioid usage might change as a result,” she noted.
“Some of the links to adult usage are predictable, such as depression, tobacco use, and cannabis use in early adolescence,” said Dr. Boulter. “Surprising was the lack of correlation between anxiety, early alcohol use, child mistreatment, and sociodemographic factors with future opioid use,” she said.
The take-home message for clinicians is to screen children and adolescents for factors leading to opioid usage in young adults “with preventive strategies including avoidance of pain medication prescriptions and early referral and treatment for depression and use of cannabis and tobacco products using tools like SBIRT (Screening, Brief Intervention, and Referral to Treatment),” Dr. Boulter emphasized.
As for additional research, “It would be interesting to study e-cigarette usage and see if the correlation with future opioid usage is similar to older tobacco products,” she said. “Also helpful would be to delve deeper into connections between medical or dental diagnoses when opioids were first prescribed and later usage of those products,” Dr. Boulter noted.
The study was supported in part by the by the National Institute of Mental Health and the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Boulter had no disclosures but serves on the Pediatric News Editorial Advisory Board.
Depression and tobacco use in childhood significantly increased the risk for opioid use in young adults, according to data from a prospective study of approximately 1,000 individuals.
Previous research, including the annual Monitoring the Future study, documents opioid use among adolescents in the United States, but childhood risk factors for opioid use in young adults have not been well studied, wrote Lilly Shanahan, PhD, of the University of Zürich, and colleagues.
In a prospective cohort study published in JAMA Pediatrics, the researchers identified 1,252 non-Hispanic White and American Indian opioid-naive individuals aged 9-16 years in rural North Carolina. They interviewed participants and parents up to 7 times between January 1993 and December 2000, and interviewed participants only at ages 19, 21, 25, and 30 years between January 1999 and December 2015.
Overall, 24.2% of study participants had used a nonheroin opioid by age 30 years, and both chronic depression and dysthymia were significantly associated with this use (odds ratios 5.43 and 7.13, respectively).
In addition, 155 participants (8.8%) reported weekly use of a nonheroin opioid, and 95 (6.6%) reported weekly heroin use by age 30 years. Chronic depression and dysthymia also were strongly associated with weekly nonheroin opioid use (OR 8.89 and 11.51, respectively).
In a multivariate analysis, depression, tobacco use, and cannabis use at ages 9-16 years were strongly associated with overall opioid use at ages 19-30 years.
“One possible reason childhood chronic depression increases the risk of later opioid use is self-medication, including the use of psychoactive substances, to alleviate depression,” the researchers noted. In addition, the mood-altering properties of opioids may increase their appeal to depressed youth as a way to relieve impaired reward system function, they said.
Potential mechanisms for the association between early tobacco use and later opioid use include the alterations to neurodevelopment caused by nicotine exposure in adolescence, as well as increased risk for depression, reduced pain thresholds, and use of nicotine as a gateway to harder drugs, the researchers added.
Several childhood risk factors were not associated with young adult opioid use in multivariate analysis in this study, including alcohol use, sociodemographic status, maltreatment, family dysfunction, and anxiety, the researchers wrote. “Previous studies typically measured these risk factors retrospectively or in late adolescence and young adulthood, and most did not consider depressive disorders, which may mediate associations between select childhood risk factors and later opioid use,” they said.
The study findings were limited by several factors, including the inability to distinguish between medical and nonmedical opioid use, the incomplete list of available opioids, and the exclusion of Black participants because of low sample size, the researchers noted. However, the results were strengthened by the longitudinal, community-representative design and the inclusion of up to 11 assessments of opioid use, they said.
“Our findings suggest strong opportunities for early prevention and intervention, including in primary care settings,” using known evidence-based strategies, they concluded.
More screening is needed
“Children in the United States are at high risk of serious adult health issues as a result of childhood factors such as ACEs (adverse childhood experiences),” said Suzanne C. Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H. “This study looks prospectively at other factors in childhood over a long period of time leading to opioid usage, with its serious risks and health consequences including overdose death,” she said. “It is unclear what the effects of COVID-19 will be on the population of children growing up now and how opioid usage might change as a result,” she noted.
“Some of the links to adult usage are predictable, such as depression, tobacco use, and cannabis use in early adolescence,” said Dr. Boulter. “Surprising was the lack of correlation between anxiety, early alcohol use, child mistreatment, and sociodemographic factors with future opioid use,” she said.
The take-home message for clinicians is to screen children and adolescents for factors leading to opioid usage in young adults “with preventive strategies including avoidance of pain medication prescriptions and early referral and treatment for depression and use of cannabis and tobacco products using tools like SBIRT (Screening, Brief Intervention, and Referral to Treatment),” Dr. Boulter emphasized.
As for additional research, “It would be interesting to study e-cigarette usage and see if the correlation with future opioid usage is similar to older tobacco products,” she said. “Also helpful would be to delve deeper into connections between medical or dental diagnoses when opioids were first prescribed and later usage of those products,” Dr. Boulter noted.
The study was supported in part by the by the National Institute of Mental Health and the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Boulter had no disclosures but serves on the Pediatric News Editorial Advisory Board.
Depression and tobacco use in childhood significantly increased the risk for opioid use in young adults, according to data from a prospective study of approximately 1,000 individuals.
Previous research, including the annual Monitoring the Future study, documents opioid use among adolescents in the United States, but childhood risk factors for opioid use in young adults have not been well studied, wrote Lilly Shanahan, PhD, of the University of Zürich, and colleagues.
In a prospective cohort study published in JAMA Pediatrics, the researchers identified 1,252 non-Hispanic White and American Indian opioid-naive individuals aged 9-16 years in rural North Carolina. They interviewed participants and parents up to 7 times between January 1993 and December 2000, and interviewed participants only at ages 19, 21, 25, and 30 years between January 1999 and December 2015.
Overall, 24.2% of study participants had used a nonheroin opioid by age 30 years, and both chronic depression and dysthymia were significantly associated with this use (odds ratios 5.43 and 7.13, respectively).
In addition, 155 participants (8.8%) reported weekly use of a nonheroin opioid, and 95 (6.6%) reported weekly heroin use by age 30 years. Chronic depression and dysthymia also were strongly associated with weekly nonheroin opioid use (OR 8.89 and 11.51, respectively).
In a multivariate analysis, depression, tobacco use, and cannabis use at ages 9-16 years were strongly associated with overall opioid use at ages 19-30 years.
“One possible reason childhood chronic depression increases the risk of later opioid use is self-medication, including the use of psychoactive substances, to alleviate depression,” the researchers noted. In addition, the mood-altering properties of opioids may increase their appeal to depressed youth as a way to relieve impaired reward system function, they said.
Potential mechanisms for the association between early tobacco use and later opioid use include the alterations to neurodevelopment caused by nicotine exposure in adolescence, as well as increased risk for depression, reduced pain thresholds, and use of nicotine as a gateway to harder drugs, the researchers added.
Several childhood risk factors were not associated with young adult opioid use in multivariate analysis in this study, including alcohol use, sociodemographic status, maltreatment, family dysfunction, and anxiety, the researchers wrote. “Previous studies typically measured these risk factors retrospectively or in late adolescence and young adulthood, and most did not consider depressive disorders, which may mediate associations between select childhood risk factors and later opioid use,” they said.
The study findings were limited by several factors, including the inability to distinguish between medical and nonmedical opioid use, the incomplete list of available opioids, and the exclusion of Black participants because of low sample size, the researchers noted. However, the results were strengthened by the longitudinal, community-representative design and the inclusion of up to 11 assessments of opioid use, they said.
“Our findings suggest strong opportunities for early prevention and intervention, including in primary care settings,” using known evidence-based strategies, they concluded.
More screening is needed
“Children in the United States are at high risk of serious adult health issues as a result of childhood factors such as ACEs (adverse childhood experiences),” said Suzanne C. Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H. “This study looks prospectively at other factors in childhood over a long period of time leading to opioid usage, with its serious risks and health consequences including overdose death,” she said. “It is unclear what the effects of COVID-19 will be on the population of children growing up now and how opioid usage might change as a result,” she noted.
“Some of the links to adult usage are predictable, such as depression, tobacco use, and cannabis use in early adolescence,” said Dr. Boulter. “Surprising was the lack of correlation between anxiety, early alcohol use, child mistreatment, and sociodemographic factors with future opioid use,” she said.
The take-home message for clinicians is to screen children and adolescents for factors leading to opioid usage in young adults “with preventive strategies including avoidance of pain medication prescriptions and early referral and treatment for depression and use of cannabis and tobacco products using tools like SBIRT (Screening, Brief Intervention, and Referral to Treatment),” Dr. Boulter emphasized.
As for additional research, “It would be interesting to study e-cigarette usage and see if the correlation with future opioid usage is similar to older tobacco products,” she said. “Also helpful would be to delve deeper into connections between medical or dental diagnoses when opioids were first prescribed and later usage of those products,” Dr. Boulter noted.
The study was supported in part by the by the National Institute of Mental Health and the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Boulter had no disclosures but serves on the Pediatric News Editorial Advisory Board.
FROM JAMA PEDIATRICS
Reducing COVID-19 opioid deaths
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.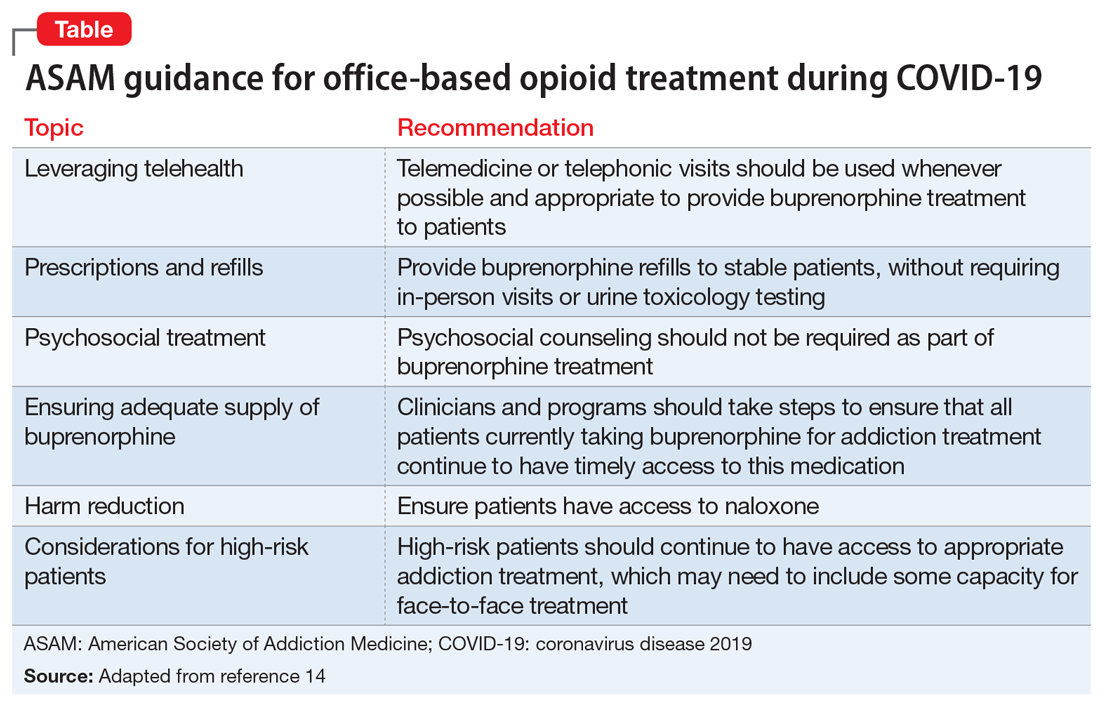
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
Intense intervention may boost addiction program retention
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
Shared medical appointments may bridge the opioid treatment gap
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New coalition demands urgent action on COVID-19 mental health crisis
Fourteen mental health organizations have formed a coalition to press federal and state officials to tackle the ongoing and growing mental health crisis that is accompanying the COVID-19 pandemic.
The coalition is offering a road map, A Unified Vision for Transforming Mental Health and Substance Abuse Care, which spells out “immediate and long-term changes that will lead to a mental health care system capable of saving our nation,” they said in a statement.
The group includes CEOs from the American Psychiatric Association, the American Psychological Association, the Massachusetts Association for Mental Health, Meadows Mental Health Policy Institute, Mental Health America, the National Association for Behavioral Healthcare, the National Alliance on Mental Illness, the National Council for Behavioral Health, One Mind, Peg’s Foundation, the Steinberg Institute, The Kennedy Forum, the Treatment Advocacy Center, and the Well Being Trust.
They have been meeting in weekly sessions since the beginning of the pandemic. The groups have come together in the spirit of previous efforts to address major health crises, including the 1970s war on cancer and the campaign to curtail the HIV/AIDS epidemic in the 1980s, they report.
The coalition reported that since the pandemic began the prevalence of depression symptoms has jumped threefold, overdose deaths have increased in 40 states, and 25% of young adults have had suicidal ideation.
“It requires immediate action by the new administration, as well as state and local governments in all 50 states, and an acknowledged, consistent commitment to fix what’s broken in our system of care,” Daniel H. Gillison Jr, CEO of the National Alliance on Mental Illness, said in a statement.
SAMHSA chief ‘grateful’
Elinore McCance-Katz, MD, PhD, who is the assistant secretary for mental health and substance use and leads the Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services, applauded the coalition.
“I am very grateful that these organizations are stepping up and putting out a report like this,” Dr. McCance-Katz told this news organization. “I hope that they will continue this kind of advocacy and leadership on these issues going forward,” she said, adding that the need for mental health care and substance use disorders will be much greater going forward because of the pandemic.
Seven policy areas
The group’s 17-page strategic plan emphasizes interventions and methods that have already been tried and tested, focusing on seven policy areas:
- Early identification and prevention, especially for families and young people, by, for instance, bringing telehealth into schools and community centers.
- Rapid deployment of emergency crisis response and prevention, including speeding up the implementation of the new 988 number for the National Suicide Prevention Lifeline.
- Leveling inequities in access to care by addressing social and political constructs and historical systemic injustices such as racism.
- Integrating physical and mental health care and substance use services to ensure “whole-person” well-being.
- Achieving parity in payment by health plans for mental health and substance-use coverage.
- Assuring evidence-based standards of treatments and care.
- Increasing the number and diversity of the mental health care workforce, peer support, and community-based programs.
and will be even more so in the near future as the effects of the pandemic continue to ripple out.
SAMHSA received $425 million in the first COVID-19 relief package signed into law in March – the CARES Act. The money was distributed to states and used for direct care for people with serious mental illness and substance-use disorders who could not otherwise get care because of virus-related restrictions, and for boosting support for mental health support lines, said Dr. McCance-Katz.
A senior SAMHSA spokesperson said the agency is “hopeful that we will see additional resources in the upcoming stimulus for mental health and substance abuse” that Congress is still working on.
“We need bold steps from our government and the business community alike,” former Rep. Patrick J. Kennedy, founder of The Kennedy Forum, said in the statement from the new coalition. “We encourage all state governments to engage with mental health leaders, bring them into pandemic-related responses, and actively facilitate their communication with communities across the country,” said Mr. Kennedy, who is a part of the new coalition.
Mr. Kennedy is also cochair of the Action Alliance’s Mental Health and Suicide Prevention National Response to COVID-19, which unveiled its own six-priority Action Plan earlier in December.
A version of this article first appeared on Medscape.com.
Fourteen mental health organizations have formed a coalition to press federal and state officials to tackle the ongoing and growing mental health crisis that is accompanying the COVID-19 pandemic.
The coalition is offering a road map, A Unified Vision for Transforming Mental Health and Substance Abuse Care, which spells out “immediate and long-term changes that will lead to a mental health care system capable of saving our nation,” they said in a statement.
The group includes CEOs from the American Psychiatric Association, the American Psychological Association, the Massachusetts Association for Mental Health, Meadows Mental Health Policy Institute, Mental Health America, the National Association for Behavioral Healthcare, the National Alliance on Mental Illness, the National Council for Behavioral Health, One Mind, Peg’s Foundation, the Steinberg Institute, The Kennedy Forum, the Treatment Advocacy Center, and the Well Being Trust.
They have been meeting in weekly sessions since the beginning of the pandemic. The groups have come together in the spirit of previous efforts to address major health crises, including the 1970s war on cancer and the campaign to curtail the HIV/AIDS epidemic in the 1980s, they report.
The coalition reported that since the pandemic began the prevalence of depression symptoms has jumped threefold, overdose deaths have increased in 40 states, and 25% of young adults have had suicidal ideation.
“It requires immediate action by the new administration, as well as state and local governments in all 50 states, and an acknowledged, consistent commitment to fix what’s broken in our system of care,” Daniel H. Gillison Jr, CEO of the National Alliance on Mental Illness, said in a statement.
SAMHSA chief ‘grateful’
Elinore McCance-Katz, MD, PhD, who is the assistant secretary for mental health and substance use and leads the Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services, applauded the coalition.
“I am very grateful that these organizations are stepping up and putting out a report like this,” Dr. McCance-Katz told this news organization. “I hope that they will continue this kind of advocacy and leadership on these issues going forward,” she said, adding that the need for mental health care and substance use disorders will be much greater going forward because of the pandemic.
Seven policy areas
The group’s 17-page strategic plan emphasizes interventions and methods that have already been tried and tested, focusing on seven policy areas:
- Early identification and prevention, especially for families and young people, by, for instance, bringing telehealth into schools and community centers.
- Rapid deployment of emergency crisis response and prevention, including speeding up the implementation of the new 988 number for the National Suicide Prevention Lifeline.
- Leveling inequities in access to care by addressing social and political constructs and historical systemic injustices such as racism.
- Integrating physical and mental health care and substance use services to ensure “whole-person” well-being.
- Achieving parity in payment by health plans for mental health and substance-use coverage.
- Assuring evidence-based standards of treatments and care.
- Increasing the number and diversity of the mental health care workforce, peer support, and community-based programs.
and will be even more so in the near future as the effects of the pandemic continue to ripple out.
SAMHSA received $425 million in the first COVID-19 relief package signed into law in March – the CARES Act. The money was distributed to states and used for direct care for people with serious mental illness and substance-use disorders who could not otherwise get care because of virus-related restrictions, and for boosting support for mental health support lines, said Dr. McCance-Katz.
A senior SAMHSA spokesperson said the agency is “hopeful that we will see additional resources in the upcoming stimulus for mental health and substance abuse” that Congress is still working on.
“We need bold steps from our government and the business community alike,” former Rep. Patrick J. Kennedy, founder of The Kennedy Forum, said in the statement from the new coalition. “We encourage all state governments to engage with mental health leaders, bring them into pandemic-related responses, and actively facilitate their communication with communities across the country,” said Mr. Kennedy, who is a part of the new coalition.
Mr. Kennedy is also cochair of the Action Alliance’s Mental Health and Suicide Prevention National Response to COVID-19, which unveiled its own six-priority Action Plan earlier in December.
A version of this article first appeared on Medscape.com.
Fourteen mental health organizations have formed a coalition to press federal and state officials to tackle the ongoing and growing mental health crisis that is accompanying the COVID-19 pandemic.
The coalition is offering a road map, A Unified Vision for Transforming Mental Health and Substance Abuse Care, which spells out “immediate and long-term changes that will lead to a mental health care system capable of saving our nation,” they said in a statement.
The group includes CEOs from the American Psychiatric Association, the American Psychological Association, the Massachusetts Association for Mental Health, Meadows Mental Health Policy Institute, Mental Health America, the National Association for Behavioral Healthcare, the National Alliance on Mental Illness, the National Council for Behavioral Health, One Mind, Peg’s Foundation, the Steinberg Institute, The Kennedy Forum, the Treatment Advocacy Center, and the Well Being Trust.
They have been meeting in weekly sessions since the beginning of the pandemic. The groups have come together in the spirit of previous efforts to address major health crises, including the 1970s war on cancer and the campaign to curtail the HIV/AIDS epidemic in the 1980s, they report.
The coalition reported that since the pandemic began the prevalence of depression symptoms has jumped threefold, overdose deaths have increased in 40 states, and 25% of young adults have had suicidal ideation.
“It requires immediate action by the new administration, as well as state and local governments in all 50 states, and an acknowledged, consistent commitment to fix what’s broken in our system of care,” Daniel H. Gillison Jr, CEO of the National Alliance on Mental Illness, said in a statement.
SAMHSA chief ‘grateful’
Elinore McCance-Katz, MD, PhD, who is the assistant secretary for mental health and substance use and leads the Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services, applauded the coalition.
“I am very grateful that these organizations are stepping up and putting out a report like this,” Dr. McCance-Katz told this news organization. “I hope that they will continue this kind of advocacy and leadership on these issues going forward,” she said, adding that the need for mental health care and substance use disorders will be much greater going forward because of the pandemic.
Seven policy areas
The group’s 17-page strategic plan emphasizes interventions and methods that have already been tried and tested, focusing on seven policy areas:
- Early identification and prevention, especially for families and young people, by, for instance, bringing telehealth into schools and community centers.
- Rapid deployment of emergency crisis response and prevention, including speeding up the implementation of the new 988 number for the National Suicide Prevention Lifeline.
- Leveling inequities in access to care by addressing social and political constructs and historical systemic injustices such as racism.
- Integrating physical and mental health care and substance use services to ensure “whole-person” well-being.
- Achieving parity in payment by health plans for mental health and substance-use coverage.
- Assuring evidence-based standards of treatments and care.
- Increasing the number and diversity of the mental health care workforce, peer support, and community-based programs.
and will be even more so in the near future as the effects of the pandemic continue to ripple out.
SAMHSA received $425 million in the first COVID-19 relief package signed into law in March – the CARES Act. The money was distributed to states and used for direct care for people with serious mental illness and substance-use disorders who could not otherwise get care because of virus-related restrictions, and for boosting support for mental health support lines, said Dr. McCance-Katz.
A senior SAMHSA spokesperson said the agency is “hopeful that we will see additional resources in the upcoming stimulus for mental health and substance abuse” that Congress is still working on.
“We need bold steps from our government and the business community alike,” former Rep. Patrick J. Kennedy, founder of The Kennedy Forum, said in the statement from the new coalition. “We encourage all state governments to engage with mental health leaders, bring them into pandemic-related responses, and actively facilitate their communication with communities across the country,” said Mr. Kennedy, who is a part of the new coalition.
Mr. Kennedy is also cochair of the Action Alliance’s Mental Health and Suicide Prevention National Response to COVID-19, which unveiled its own six-priority Action Plan earlier in December.
A version of this article first appeared on Medscape.com.
COVID-19 fuels surge in overdose-related cardiac arrests
There has been a sharp increase in overdose-related cardiac arrests in the United States during the COVID-19 pandemic, a new analysis shows.
Overall rates in 2020 were elevated above the baseline from 2018 and 2019 by about 50%, the data show.
and efforts to combat the COVID-19 pandemic have not been effective at reducing overdoses,” Joseph Friedman, MPH, MD/PhD student, medical scientist training program, University of California, Los Angeles, said in an interview.
“We need to invest heavily in substance use treatment, harm reduction, and the structural drivers of overdose as core elements of the COVID-19 response,” said Mr. Friedman, who coauthored the study with UCLA colleague David Schriger, MD, MPH, and Leo Beletsky, JD, MPH, Northeastern University, Boston.
The study was published as a research letter Dec. 3 in JAMA Psychiatry.
Social isolation a key driver
Emergency medical services (EMS) data are available in near real time, providing a novel source of up-to-date information to monitor epidemiological shifts during the COVID-19 pandemic.
For the study, the researchers leveraged data from the National EMS Information System, a large registry of more than 10,000 EMS agencies in 47 states that represent over 80% of all EMS calls nationally in 2020. They used the data to track shifts in overdose-related cardiac arrests observed by EMS.
They found clear evidence of a large-scale uptick in overdose-related deaths during the COVID-19 pandemic.
The overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends observed during 2018 and 2019, including a maximum peak of 123% above baseline reached in early May.
All overdose-related incidents (fatal and nonfatal) were elevated in 2020, by about 17% above baseline. However, there were larger increases in fatal overdose-related incidents, compared to all incidents, which may suggest a rising case fatality rate, the authors noted.
The observed trends line up in time with reductions in mobility (a metric of social interaction), as measured using cell phone data, they wrote.
“Many of the trends predicted by experts at the beginning of the pandemic could cause these shifts. Increases in social isolation likely play an important role, as people using [drugs] alone are less likely to receive help when they need it. Also shifts in the drug supply, and reduced access to healthcare and treatment,” said Mr. Friedman.
“We need to undertake short- and long-term strategies to combat the rising tide of overdose mortality in the United States,” he added.
In the short term, Mr. Friedman suggested reducing financial and logistical barriers for accessing a safe opioid supply. Such measures include allowing pharmacies to dispense methadone, allowing all physicians to prescribe buprenorphine without a special waiver, and releasing emergency funds to make these medications universally affordable.
“In the longer term, we should acknowledge that overdose is a symptom of structural problems in the U.S. We need to invest in making employment, housing, education, and health care accessible to all to address the upstream drivers of overdose,” he added.
The study had no commercial funding. The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
There has been a sharp increase in overdose-related cardiac arrests in the United States during the COVID-19 pandemic, a new analysis shows.
Overall rates in 2020 were elevated above the baseline from 2018 and 2019 by about 50%, the data show.
and efforts to combat the COVID-19 pandemic have not been effective at reducing overdoses,” Joseph Friedman, MPH, MD/PhD student, medical scientist training program, University of California, Los Angeles, said in an interview.
“We need to invest heavily in substance use treatment, harm reduction, and the structural drivers of overdose as core elements of the COVID-19 response,” said Mr. Friedman, who coauthored the study with UCLA colleague David Schriger, MD, MPH, and Leo Beletsky, JD, MPH, Northeastern University, Boston.
The study was published as a research letter Dec. 3 in JAMA Psychiatry.
Social isolation a key driver
Emergency medical services (EMS) data are available in near real time, providing a novel source of up-to-date information to monitor epidemiological shifts during the COVID-19 pandemic.
For the study, the researchers leveraged data from the National EMS Information System, a large registry of more than 10,000 EMS agencies in 47 states that represent over 80% of all EMS calls nationally in 2020. They used the data to track shifts in overdose-related cardiac arrests observed by EMS.
They found clear evidence of a large-scale uptick in overdose-related deaths during the COVID-19 pandemic.
The overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends observed during 2018 and 2019, including a maximum peak of 123% above baseline reached in early May.
All overdose-related incidents (fatal and nonfatal) were elevated in 2020, by about 17% above baseline. However, there were larger increases in fatal overdose-related incidents, compared to all incidents, which may suggest a rising case fatality rate, the authors noted.
The observed trends line up in time with reductions in mobility (a metric of social interaction), as measured using cell phone data, they wrote.
“Many of the trends predicted by experts at the beginning of the pandemic could cause these shifts. Increases in social isolation likely play an important role, as people using [drugs] alone are less likely to receive help when they need it. Also shifts in the drug supply, and reduced access to healthcare and treatment,” said Mr. Friedman.
“We need to undertake short- and long-term strategies to combat the rising tide of overdose mortality in the United States,” he added.
In the short term, Mr. Friedman suggested reducing financial and logistical barriers for accessing a safe opioid supply. Such measures include allowing pharmacies to dispense methadone, allowing all physicians to prescribe buprenorphine without a special waiver, and releasing emergency funds to make these medications universally affordable.
“In the longer term, we should acknowledge that overdose is a symptom of structural problems in the U.S. We need to invest in making employment, housing, education, and health care accessible to all to address the upstream drivers of overdose,” he added.
The study had no commercial funding. The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
There has been a sharp increase in overdose-related cardiac arrests in the United States during the COVID-19 pandemic, a new analysis shows.
Overall rates in 2020 were elevated above the baseline from 2018 and 2019 by about 50%, the data show.
and efforts to combat the COVID-19 pandemic have not been effective at reducing overdoses,” Joseph Friedman, MPH, MD/PhD student, medical scientist training program, University of California, Los Angeles, said in an interview.
“We need to invest heavily in substance use treatment, harm reduction, and the structural drivers of overdose as core elements of the COVID-19 response,” said Mr. Friedman, who coauthored the study with UCLA colleague David Schriger, MD, MPH, and Leo Beletsky, JD, MPH, Northeastern University, Boston.
The study was published as a research letter Dec. 3 in JAMA Psychiatry.
Social isolation a key driver
Emergency medical services (EMS) data are available in near real time, providing a novel source of up-to-date information to monitor epidemiological shifts during the COVID-19 pandemic.
For the study, the researchers leveraged data from the National EMS Information System, a large registry of more than 10,000 EMS agencies in 47 states that represent over 80% of all EMS calls nationally in 2020. They used the data to track shifts in overdose-related cardiac arrests observed by EMS.
They found clear evidence of a large-scale uptick in overdose-related deaths during the COVID-19 pandemic.
The overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends observed during 2018 and 2019, including a maximum peak of 123% above baseline reached in early May.
All overdose-related incidents (fatal and nonfatal) were elevated in 2020, by about 17% above baseline. However, there were larger increases in fatal overdose-related incidents, compared to all incidents, which may suggest a rising case fatality rate, the authors noted.
The observed trends line up in time with reductions in mobility (a metric of social interaction), as measured using cell phone data, they wrote.
“Many of the trends predicted by experts at the beginning of the pandemic could cause these shifts. Increases in social isolation likely play an important role, as people using [drugs] alone are less likely to receive help when they need it. Also shifts in the drug supply, and reduced access to healthcare and treatment,” said Mr. Friedman.
“We need to undertake short- and long-term strategies to combat the rising tide of overdose mortality in the United States,” he added.
In the short term, Mr. Friedman suggested reducing financial and logistical barriers for accessing a safe opioid supply. Such measures include allowing pharmacies to dispense methadone, allowing all physicians to prescribe buprenorphine without a special waiver, and releasing emergency funds to make these medications universally affordable.
“In the longer term, we should acknowledge that overdose is a symptom of structural problems in the U.S. We need to invest in making employment, housing, education, and health care accessible to all to address the upstream drivers of overdose,” he added.
The study had no commercial funding. The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.








