User login
How to identify, evaluate, and treat patients with ‘Percocet use disorder’
In recent years, Percocet (oxycodone/paracetamol) has experienced a meteoric rise to prominence because of the presence of conspicuous references in pop culture and the ever-evolving hip-hop scene,1 so much so that even propafenone is being mislabeled as the agent.2 It is of utmost importance for clinicians to be made aware of the adverse effects and the treatment protocols associated with Percocet as well as propafenone.
Propafenone is identified as a class 1C antiarrhythmic with adverse effects associated with that particular class of drugs (e.g., generalized tonic-clonic seizures coupled with widened QRS complex), however, Percocet’s toxidrome is the product of the opioid/nonopioid (in the form of oxycodone/acetaminophen) components found within the formulation. Percocet is often recreationally used with MDMA (“molly”) or ecstasy as popularized by the lyrics of “Mask Off” by Future (“Percocets, Molly, Percocets”).3,4
Addressing the challenge of imitation Percocet pills
Differentiating the untoward effects of Percocet and propafenone isn’t too challenging because the agents belong to separate classes – the problem is the use of deceitful labels on propafenone with both medications sporting the “512 imprint” on their respective pills. Initial symptoms of propafenone ingestion may include weakness and dizziness followed by seizures.5As an emergent situation, the patient should be immediately treated with a sodium bicarbonate infusion to effectively reverse the sodium channel blockade associated with the widened QRS.
However, a more likely scenario is that of Percocet counterfeit pills designed to illicitly emulate the properties of officially marketed Percocet. As expected, Percocet overdose management will require that the clinician be familiar with treating general opioid toxicity (in this case, derived from the oxycodone component), in particular respiratory or CNS depression. Symptoms of opioid overdose also include the loss of consciousness with pupillary miosis. Therapy entails the use of naloxone and/or mechanical ventilation for respiratory support. The patient can also exhibit cardiovascular compromise. If further information is elicited during a patient interview, it may reveal a history of drug procurement from the streets.
Epidemiologists from Georgia collaborated with the state’s department of public health’s office of emergency services, forensic experts, and drug enforcement professionals to evaluate almost 40 cases of counterfeit Percocet overdoses during the period spanning the second week of June 2017. Of these cases, a cluster triad was identified consisting of general opioid toxicity symptoms (for example, CNS or respiratory depression with concomitant pupillary constriction, a history of drug procurement, and a history of ingesting only one or two pills with rapid deterioration.6 Unfortunately, the screening process is often hindered by the fact that synthetic opioids such as Percocet are not readily identified on urine drug screens (UDS).
Despite shortcomings in assessment procedures, a UDS will yield positive results for multiple drugs, a feature that is common to seasoned opioid users and serves as an instrumental diagnostic clue in the investigative process. To address the crisis and prevent further spread, numerous Georgia agencies (e.g., drug trafficking and legal authorities) worked with the health care community to expediently identify cases of interest and bring forth public awareness concerning the ongoing perils of counterfeit drug intake. Future investigations might benefit from the implementation of DNA-verified UDS, because those screens are versatile enough to detect the presence of synthetic urine substitutes within the context of opioid use.7,8 Moreover, an expanded panel could be tailored to provide coverage for semisynthetics, including hydrocodone, oxycodone, hydromorphone, and oxymorphone.9
As a well-received painkiller from the opioid family, Percocet derives its analgesic properties from the fast-acting oxycodone; hepatic failure is also possible from Percocet (because of the acetaminophen component) or counterfeit Percocet overdose but is less common unless the Tylenol content approaches 4 grams. By binding to the brain’s opiate receptors, Percocet modulates pain pathways leading to a dulling of pain sensation along with euphoria, which is particularly attractive to drug seekers. Chronic Percocet use corresponds with a myriad of psychological and physical consequences, and the Drug Enforcement Administration recognizes oxycodone as a Schedule II drug.
A chronic Percocet user may try to disrupt the cycle of symptoms by abruptly ceasing use of the offending agent. This can precipitate the development of classical opioid-based withdrawal symptoms, including but not limited to nausea, vomiting, irritability, tachycardia, body aches, and episodes of cold sweats. Physicians have noted that misuse (i.e., deviations from intended prescribed) might include crushing and snorting as well as “doctor-shopping” behaviors for a continuous supply of Percocet.
Treatment recommendations
According to Sarah Wakeman, MD, medical director of the substance use disorders initiative at Massachusetts General Hospital in Boston, there are apparently two clinical manifestations of Percocet use. The primary consequence is derived from the oxycodone component of Percocet; as an opioid, oxycodone toxicity leads to disrupted breathing and oxygenation, negatively impacting vital organs such as the brain or the heart. Patients experiencing a lack of oxygen will often display cyanosis and may not respond appropriately to stimuli. For individuals suspected of succumbing to overdose, Dr. Wakeman reportedly advised that the clinician or trained professional rub his or her knuckles along the breastbone of the potential user – a drug overdose patient will fail to wake up. On the other hand, a Percocet user may exhibit the symptoms of liver failure depending on the overall level of acetaminophen in the formulation. To prevent relapses, Percocet use disorder is best managed in a professional setting under the direction of trained clinicians; users are provided medications to address ongoing cravings and symptoms associated with the withdrawal process. A detoxification center can tailor the treatment with opioid-based medications such as methadone, buprenorphine, and naltrexone to help patients be weaned off Percocet.
Clinicians may further improve the efficacy of a therapeutic regimen by incorporating a personalized plan with a comprehensive substance UDS panel for monitoring and treatment purposes. This may prove to be beneficial in the event of suspected polysubstance use, as is the case with patients who dabble with Percocet and “molly.” Preparations can also be instituted at the outset of therapy with genetic testing implemented in high-risk patients who exhibit an inclination for opioid use disorder.10 Genetic polymorphisms provide robust clinical assets for evaluating patients most at risk for relapse. For individuals with biological susceptibility, arrangements can be made to incorporate nonopioid treatment alternatives.
References
1. Thomas BB. The death of Lil Peep: How the U.S. prescription drug epidemic is changing hip-hop. The Guardian. 2017 Nov 16.
2. D’Orazio JL and Curtis JA. J Emer Med. 2011 Aug 1;41(2):172-5.
3. Levy L. These are the drugs influencing pop culture now. Vulture. 2018 Feb 6.
4. Kounang N and Bender M. “What is Percocet? Drug facts, side effects, abuse and more.” CNN. 2018 Jul 12.
5. The dangers of Percocet use and overdose. American Addiction Centers. Last updated 2020 Feb 3. https://americanaddictioncenters.org/percocet-treatment/dangers-of-use-and-overdose.
6. Edison L et al. MMWR. 2017 Oct 20;66(41):1119-20.
7. Choudhry Z et al. J Psychiatry. 2015. doi: 10.4172/2378-5756.10000319.
8. Islam F and Choudhry Z. Current Psychiatry. 2018 Dec;17(12):43-4.
9. Jupe N. Ask the Experts: DOT 5-panel drug test regimen. Quest Diagnostics. 2018 Mar 21. https://blog.employersolutions.com/ask-experts-dot-5-panel-drug-test-regimen/.
10. Ahmed S et al. Pharmacogenomics. 2019 Jun 28;20(9):685-703.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam reported no relevant disclosures. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He reported no relevant disclosures.
In recent years, Percocet (oxycodone/paracetamol) has experienced a meteoric rise to prominence because of the presence of conspicuous references in pop culture and the ever-evolving hip-hop scene,1 so much so that even propafenone is being mislabeled as the agent.2 It is of utmost importance for clinicians to be made aware of the adverse effects and the treatment protocols associated with Percocet as well as propafenone.
Propafenone is identified as a class 1C antiarrhythmic with adverse effects associated with that particular class of drugs (e.g., generalized tonic-clonic seizures coupled with widened QRS complex), however, Percocet’s toxidrome is the product of the opioid/nonopioid (in the form of oxycodone/acetaminophen) components found within the formulation. Percocet is often recreationally used with MDMA (“molly”) or ecstasy as popularized by the lyrics of “Mask Off” by Future (“Percocets, Molly, Percocets”).3,4
Addressing the challenge of imitation Percocet pills
Differentiating the untoward effects of Percocet and propafenone isn’t too challenging because the agents belong to separate classes – the problem is the use of deceitful labels on propafenone with both medications sporting the “512 imprint” on their respective pills. Initial symptoms of propafenone ingestion may include weakness and dizziness followed by seizures.5As an emergent situation, the patient should be immediately treated with a sodium bicarbonate infusion to effectively reverse the sodium channel blockade associated with the widened QRS.
However, a more likely scenario is that of Percocet counterfeit pills designed to illicitly emulate the properties of officially marketed Percocet. As expected, Percocet overdose management will require that the clinician be familiar with treating general opioid toxicity (in this case, derived from the oxycodone component), in particular respiratory or CNS depression. Symptoms of opioid overdose also include the loss of consciousness with pupillary miosis. Therapy entails the use of naloxone and/or mechanical ventilation for respiratory support. The patient can also exhibit cardiovascular compromise. If further information is elicited during a patient interview, it may reveal a history of drug procurement from the streets.
Epidemiologists from Georgia collaborated with the state’s department of public health’s office of emergency services, forensic experts, and drug enforcement professionals to evaluate almost 40 cases of counterfeit Percocet overdoses during the period spanning the second week of June 2017. Of these cases, a cluster triad was identified consisting of general opioid toxicity symptoms (for example, CNS or respiratory depression with concomitant pupillary constriction, a history of drug procurement, and a history of ingesting only one or two pills with rapid deterioration.6 Unfortunately, the screening process is often hindered by the fact that synthetic opioids such as Percocet are not readily identified on urine drug screens (UDS).
Despite shortcomings in assessment procedures, a UDS will yield positive results for multiple drugs, a feature that is common to seasoned opioid users and serves as an instrumental diagnostic clue in the investigative process. To address the crisis and prevent further spread, numerous Georgia agencies (e.g., drug trafficking and legal authorities) worked with the health care community to expediently identify cases of interest and bring forth public awareness concerning the ongoing perils of counterfeit drug intake. Future investigations might benefit from the implementation of DNA-verified UDS, because those screens are versatile enough to detect the presence of synthetic urine substitutes within the context of opioid use.7,8 Moreover, an expanded panel could be tailored to provide coverage for semisynthetics, including hydrocodone, oxycodone, hydromorphone, and oxymorphone.9
As a well-received painkiller from the opioid family, Percocet derives its analgesic properties from the fast-acting oxycodone; hepatic failure is also possible from Percocet (because of the acetaminophen component) or counterfeit Percocet overdose but is less common unless the Tylenol content approaches 4 grams. By binding to the brain’s opiate receptors, Percocet modulates pain pathways leading to a dulling of pain sensation along with euphoria, which is particularly attractive to drug seekers. Chronic Percocet use corresponds with a myriad of psychological and physical consequences, and the Drug Enforcement Administration recognizes oxycodone as a Schedule II drug.
A chronic Percocet user may try to disrupt the cycle of symptoms by abruptly ceasing use of the offending agent. This can precipitate the development of classical opioid-based withdrawal symptoms, including but not limited to nausea, vomiting, irritability, tachycardia, body aches, and episodes of cold sweats. Physicians have noted that misuse (i.e., deviations from intended prescribed) might include crushing and snorting as well as “doctor-shopping” behaviors for a continuous supply of Percocet.
Treatment recommendations
According to Sarah Wakeman, MD, medical director of the substance use disorders initiative at Massachusetts General Hospital in Boston, there are apparently two clinical manifestations of Percocet use. The primary consequence is derived from the oxycodone component of Percocet; as an opioid, oxycodone toxicity leads to disrupted breathing and oxygenation, negatively impacting vital organs such as the brain or the heart. Patients experiencing a lack of oxygen will often display cyanosis and may not respond appropriately to stimuli. For individuals suspected of succumbing to overdose, Dr. Wakeman reportedly advised that the clinician or trained professional rub his or her knuckles along the breastbone of the potential user – a drug overdose patient will fail to wake up. On the other hand, a Percocet user may exhibit the symptoms of liver failure depending on the overall level of acetaminophen in the formulation. To prevent relapses, Percocet use disorder is best managed in a professional setting under the direction of trained clinicians; users are provided medications to address ongoing cravings and symptoms associated with the withdrawal process. A detoxification center can tailor the treatment with opioid-based medications such as methadone, buprenorphine, and naltrexone to help patients be weaned off Percocet.
Clinicians may further improve the efficacy of a therapeutic regimen by incorporating a personalized plan with a comprehensive substance UDS panel for monitoring and treatment purposes. This may prove to be beneficial in the event of suspected polysubstance use, as is the case with patients who dabble with Percocet and “molly.” Preparations can also be instituted at the outset of therapy with genetic testing implemented in high-risk patients who exhibit an inclination for opioid use disorder.10 Genetic polymorphisms provide robust clinical assets for evaluating patients most at risk for relapse. For individuals with biological susceptibility, arrangements can be made to incorporate nonopioid treatment alternatives.
References
1. Thomas BB. The death of Lil Peep: How the U.S. prescription drug epidemic is changing hip-hop. The Guardian. 2017 Nov 16.
2. D’Orazio JL and Curtis JA. J Emer Med. 2011 Aug 1;41(2):172-5.
3. Levy L. These are the drugs influencing pop culture now. Vulture. 2018 Feb 6.
4. Kounang N and Bender M. “What is Percocet? Drug facts, side effects, abuse and more.” CNN. 2018 Jul 12.
5. The dangers of Percocet use and overdose. American Addiction Centers. Last updated 2020 Feb 3. https://americanaddictioncenters.org/percocet-treatment/dangers-of-use-and-overdose.
6. Edison L et al. MMWR. 2017 Oct 20;66(41):1119-20.
7. Choudhry Z et al. J Psychiatry. 2015. doi: 10.4172/2378-5756.10000319.
8. Islam F and Choudhry Z. Current Psychiatry. 2018 Dec;17(12):43-4.
9. Jupe N. Ask the Experts: DOT 5-panel drug test regimen. Quest Diagnostics. 2018 Mar 21. https://blog.employersolutions.com/ask-experts-dot-5-panel-drug-test-regimen/.
10. Ahmed S et al. Pharmacogenomics. 2019 Jun 28;20(9):685-703.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam reported no relevant disclosures. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He reported no relevant disclosures.
In recent years, Percocet (oxycodone/paracetamol) has experienced a meteoric rise to prominence because of the presence of conspicuous references in pop culture and the ever-evolving hip-hop scene,1 so much so that even propafenone is being mislabeled as the agent.2 It is of utmost importance for clinicians to be made aware of the adverse effects and the treatment protocols associated with Percocet as well as propafenone.
Propafenone is identified as a class 1C antiarrhythmic with adverse effects associated with that particular class of drugs (e.g., generalized tonic-clonic seizures coupled with widened QRS complex), however, Percocet’s toxidrome is the product of the opioid/nonopioid (in the form of oxycodone/acetaminophen) components found within the formulation. Percocet is often recreationally used with MDMA (“molly”) or ecstasy as popularized by the lyrics of “Mask Off” by Future (“Percocets, Molly, Percocets”).3,4
Addressing the challenge of imitation Percocet pills
Differentiating the untoward effects of Percocet and propafenone isn’t too challenging because the agents belong to separate classes – the problem is the use of deceitful labels on propafenone with both medications sporting the “512 imprint” on their respective pills. Initial symptoms of propafenone ingestion may include weakness and dizziness followed by seizures.5As an emergent situation, the patient should be immediately treated with a sodium bicarbonate infusion to effectively reverse the sodium channel blockade associated with the widened QRS.
However, a more likely scenario is that of Percocet counterfeit pills designed to illicitly emulate the properties of officially marketed Percocet. As expected, Percocet overdose management will require that the clinician be familiar with treating general opioid toxicity (in this case, derived from the oxycodone component), in particular respiratory or CNS depression. Symptoms of opioid overdose also include the loss of consciousness with pupillary miosis. Therapy entails the use of naloxone and/or mechanical ventilation for respiratory support. The patient can also exhibit cardiovascular compromise. If further information is elicited during a patient interview, it may reveal a history of drug procurement from the streets.
Epidemiologists from Georgia collaborated with the state’s department of public health’s office of emergency services, forensic experts, and drug enforcement professionals to evaluate almost 40 cases of counterfeit Percocet overdoses during the period spanning the second week of June 2017. Of these cases, a cluster triad was identified consisting of general opioid toxicity symptoms (for example, CNS or respiratory depression with concomitant pupillary constriction, a history of drug procurement, and a history of ingesting only one or two pills with rapid deterioration.6 Unfortunately, the screening process is often hindered by the fact that synthetic opioids such as Percocet are not readily identified on urine drug screens (UDS).
Despite shortcomings in assessment procedures, a UDS will yield positive results for multiple drugs, a feature that is common to seasoned opioid users and serves as an instrumental diagnostic clue in the investigative process. To address the crisis and prevent further spread, numerous Georgia agencies (e.g., drug trafficking and legal authorities) worked with the health care community to expediently identify cases of interest and bring forth public awareness concerning the ongoing perils of counterfeit drug intake. Future investigations might benefit from the implementation of DNA-verified UDS, because those screens are versatile enough to detect the presence of synthetic urine substitutes within the context of opioid use.7,8 Moreover, an expanded panel could be tailored to provide coverage for semisynthetics, including hydrocodone, oxycodone, hydromorphone, and oxymorphone.9
As a well-received painkiller from the opioid family, Percocet derives its analgesic properties from the fast-acting oxycodone; hepatic failure is also possible from Percocet (because of the acetaminophen component) or counterfeit Percocet overdose but is less common unless the Tylenol content approaches 4 grams. By binding to the brain’s opiate receptors, Percocet modulates pain pathways leading to a dulling of pain sensation along with euphoria, which is particularly attractive to drug seekers. Chronic Percocet use corresponds with a myriad of psychological and physical consequences, and the Drug Enforcement Administration recognizes oxycodone as a Schedule II drug.
A chronic Percocet user may try to disrupt the cycle of symptoms by abruptly ceasing use of the offending agent. This can precipitate the development of classical opioid-based withdrawal symptoms, including but not limited to nausea, vomiting, irritability, tachycardia, body aches, and episodes of cold sweats. Physicians have noted that misuse (i.e., deviations from intended prescribed) might include crushing and snorting as well as “doctor-shopping” behaviors for a continuous supply of Percocet.
Treatment recommendations
According to Sarah Wakeman, MD, medical director of the substance use disorders initiative at Massachusetts General Hospital in Boston, there are apparently two clinical manifestations of Percocet use. The primary consequence is derived from the oxycodone component of Percocet; as an opioid, oxycodone toxicity leads to disrupted breathing and oxygenation, negatively impacting vital organs such as the brain or the heart. Patients experiencing a lack of oxygen will often display cyanosis and may not respond appropriately to stimuli. For individuals suspected of succumbing to overdose, Dr. Wakeman reportedly advised that the clinician or trained professional rub his or her knuckles along the breastbone of the potential user – a drug overdose patient will fail to wake up. On the other hand, a Percocet user may exhibit the symptoms of liver failure depending on the overall level of acetaminophen in the formulation. To prevent relapses, Percocet use disorder is best managed in a professional setting under the direction of trained clinicians; users are provided medications to address ongoing cravings and symptoms associated with the withdrawal process. A detoxification center can tailor the treatment with opioid-based medications such as methadone, buprenorphine, and naltrexone to help patients be weaned off Percocet.
Clinicians may further improve the efficacy of a therapeutic regimen by incorporating a personalized plan with a comprehensive substance UDS panel for monitoring and treatment purposes. This may prove to be beneficial in the event of suspected polysubstance use, as is the case with patients who dabble with Percocet and “molly.” Preparations can also be instituted at the outset of therapy with genetic testing implemented in high-risk patients who exhibit an inclination for opioid use disorder.10 Genetic polymorphisms provide robust clinical assets for evaluating patients most at risk for relapse. For individuals with biological susceptibility, arrangements can be made to incorporate nonopioid treatment alternatives.
References
1. Thomas BB. The death of Lil Peep: How the U.S. prescription drug epidemic is changing hip-hop. The Guardian. 2017 Nov 16.
2. D’Orazio JL and Curtis JA. J Emer Med. 2011 Aug 1;41(2):172-5.
3. Levy L. These are the drugs influencing pop culture now. Vulture. 2018 Feb 6.
4. Kounang N and Bender M. “What is Percocet? Drug facts, side effects, abuse and more.” CNN. 2018 Jul 12.
5. The dangers of Percocet use and overdose. American Addiction Centers. Last updated 2020 Feb 3. https://americanaddictioncenters.org/percocet-treatment/dangers-of-use-and-overdose.
6. Edison L et al. MMWR. 2017 Oct 20;66(41):1119-20.
7. Choudhry Z et al. J Psychiatry. 2015. doi: 10.4172/2378-5756.10000319.
8. Islam F and Choudhry Z. Current Psychiatry. 2018 Dec;17(12):43-4.
9. Jupe N. Ask the Experts: DOT 5-panel drug test regimen. Quest Diagnostics. 2018 Mar 21. https://blog.employersolutions.com/ask-experts-dot-5-panel-drug-test-regimen/.
10. Ahmed S et al. Pharmacogenomics. 2019 Jun 28;20(9):685-703.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam reported no relevant disclosures. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He reported no relevant disclosures.
Rapid relief of opioid-induced constipation with MNTX
Subcutaneously administered methylnaltrexone (MNTX) (Relistor), a peripherally acting mu-opioid receptor antagonist, relieves opioid-induced constipation (OID) in both chronic, noncancer-related illness and cancer-related illness, a new analysis concludes.
“While these are two very different patient groups, the ability to have something to treat OIC in noncancer patients who stay on opioids for whatever reason helps, because [otherwise] these patients are not doing well,” said lead author Eric Shah, MD, motility director for the Dartmouth program at Dartmouth Hitchcock Health, Lebanon, N.H.
Importantly, peripherally acting mu-opioid receptor antagonists such as MNTX do not affect overall pain control to any significant extent, which is “reassuring,” he said in an interview.
These drugs decrease the constipating effects of opioids without reversing CNS-mediated opioid effects, he explained.
“Methylnaltrexone has already been approved for the treatment of OIC in adults with chronic noncancer pain as well as for OIC in adults with advanced illness who are receiving palliative care, which is often the case in patients with cancer-related pain,” he noted.
Dr. Shah discussed the new analysis during PAINWeek 2020, the American Society of Regional Anesthesia and Pain Medicine 19th Annual Pain Medicine Meeting.
The analysis was based on a review of data collected in two previously reported randomized, placebo-controlled studies (study 302 and 4000), which were used to gain approval.
The new analysis shows that “the drug works up front, and the effect is able to be maintained. I think the studies are clinically relevant in that patients are able to have a bowel movement quickly after you give them an injectable formulation when they are vomiting or otherwise can’t tolerate a pill and they are feeling miserable,” Dr. Shah commented. Many patients with OIC are constipated for reasons other than from opioid use. They often have other side effects from opioids, including bloating, nausea, and vomiting.
“When patients go to the emergency room, it’s not just that they are not able to have a bowel movement; they are often also vomiting, so it’s important to have agents that can be given in a manner that avoids the need for oral medication,” Dr. Shah said. MNTX is the only peripherally acting opioid antagonist available in a subcutaneous formulation.
Moreover, if patients are able to control these symptoms at home with an injectable formulation, they may not need to go to the ED for treatment of their gastrointestinal distress, he added.
Viable product
In a comment, Darren Brenner, MD, associate professor of medicine and surgery, Northwestern University, Chicago, who has worked with this subcutaneous formulation, said it is “definitely a viable product.
“The data presented here were in patients with advanced illness receiving palliative care when other laxatives have failed, and the difference and the potential benefit for MNTX is that it is the only peripherally acting mu-opioid receptor antagonist that is approved for advanced cancer,” he added. The other products that are currently approved, naloxegol (Movantik) and naldemedine (Symproic), are both indicated for chronic, noncancer pain.
The other potential benefit of subcutaneous MNTX is that it can work very rapidly for the patients who respond to it. “One of the things investigators did not mention in these two trials but which has been shown in previous studies is that almost half of patients who respond to this drug respond within the first 30 minutes of receiving the injection,” Dr. Brenner said in an interview.
This can be very beneficial in an emergency setting, because it may avoid having patients admitted to hospital. They can be discharged and sent home with enough drug to use on demand, Dr. Brenner suggested.
New analysis of data from studies 302 and 4000
Both studies were carried out in adults with advanced illness and OIC whose conditions were refractory to laxative use. Both of the studies were placebo controlled.
Study 302 involved 78 patients with cancer and 56 patients with noncancer-related OIC. MNTX was given at a dose of 0.15 mg/kg subcutaneously every other day for 2 weeks.
Study 4000 included 152 patients with cancer and OIC and 78 patients with noncancer-related OIC. In this study, the dose of MNTX was based on body weight. Seven or fewer doses of either 8 mg or 12 mg were given subcutaneously for 2 weeks.
The main endpoints of both studies was the proportion of patients who achieved a rescue-free laxation (RFL) response within 4 hours after the first dose and the proportion of patients with an RFL response within 4 hours for two or more of the first four doses within 24 hours.
Dr. Shah explained that RFL is a meaningful clinical endpoint. Patients could achieve a bowel movement with the two prespecified time endpoints in both studies.
Not all patients were hospitalized for OIC, Dr. Shah noted. Entry criteria were strict and included having fewer than three bowel movements during the previous week and no clinically significant laxation (defecation) within 48 hours of receiving the first dose of study drug.
“In both studies, a significantly greater proportion of patients treated with MNTX versus placebo achieved an RFL within 4 hours after the first dose among both cancer and noncancer patients,” the investigators reported.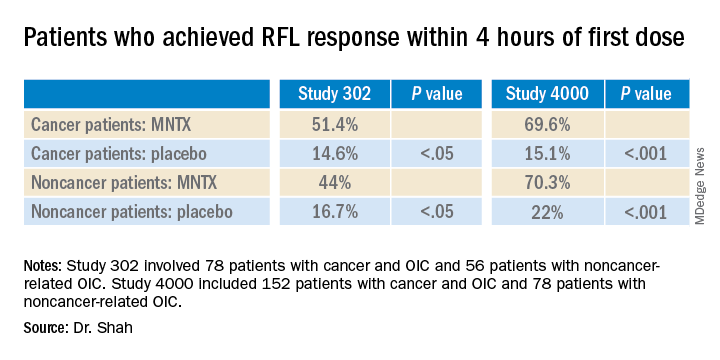
Results were relatively comparable between cancer and noncancer patients who were treated for OIC in study 4000, the investigators noted.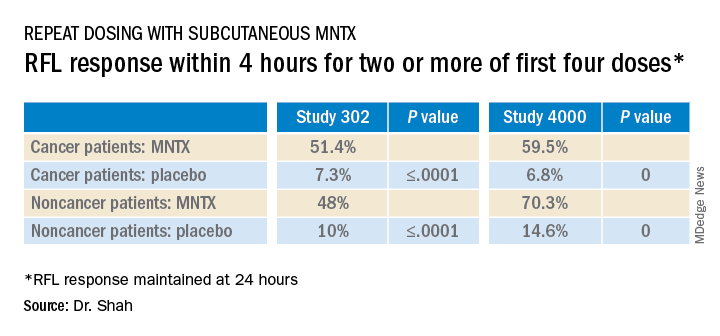
Both studies were sponsored by Salix Pharmaceuticals. Dr. Shah has received travel fees from Salix Pharmaceuticals. Dr. Brenner has served as a consultant for Salix Pharmaceuticals, AstraZeneca, and Purdue Pharma. AstraZeneca developed naloxegol.
This article first appeared on Medscape.com.
Subcutaneously administered methylnaltrexone (MNTX) (Relistor), a peripherally acting mu-opioid receptor antagonist, relieves opioid-induced constipation (OID) in both chronic, noncancer-related illness and cancer-related illness, a new analysis concludes.
“While these are two very different patient groups, the ability to have something to treat OIC in noncancer patients who stay on opioids for whatever reason helps, because [otherwise] these patients are not doing well,” said lead author Eric Shah, MD, motility director for the Dartmouth program at Dartmouth Hitchcock Health, Lebanon, N.H.
Importantly, peripherally acting mu-opioid receptor antagonists such as MNTX do not affect overall pain control to any significant extent, which is “reassuring,” he said in an interview.
These drugs decrease the constipating effects of opioids without reversing CNS-mediated opioid effects, he explained.
“Methylnaltrexone has already been approved for the treatment of OIC in adults with chronic noncancer pain as well as for OIC in adults with advanced illness who are receiving palliative care, which is often the case in patients with cancer-related pain,” he noted.
Dr. Shah discussed the new analysis during PAINWeek 2020, the American Society of Regional Anesthesia and Pain Medicine 19th Annual Pain Medicine Meeting.
The analysis was based on a review of data collected in two previously reported randomized, placebo-controlled studies (study 302 and 4000), which were used to gain approval.
The new analysis shows that “the drug works up front, and the effect is able to be maintained. I think the studies are clinically relevant in that patients are able to have a bowel movement quickly after you give them an injectable formulation when they are vomiting or otherwise can’t tolerate a pill and they are feeling miserable,” Dr. Shah commented. Many patients with OIC are constipated for reasons other than from opioid use. They often have other side effects from opioids, including bloating, nausea, and vomiting.
“When patients go to the emergency room, it’s not just that they are not able to have a bowel movement; they are often also vomiting, so it’s important to have agents that can be given in a manner that avoids the need for oral medication,” Dr. Shah said. MNTX is the only peripherally acting opioid antagonist available in a subcutaneous formulation.
Moreover, if patients are able to control these symptoms at home with an injectable formulation, they may not need to go to the ED for treatment of their gastrointestinal distress, he added.
Viable product
In a comment, Darren Brenner, MD, associate professor of medicine and surgery, Northwestern University, Chicago, who has worked with this subcutaneous formulation, said it is “definitely a viable product.
“The data presented here were in patients with advanced illness receiving palliative care when other laxatives have failed, and the difference and the potential benefit for MNTX is that it is the only peripherally acting mu-opioid receptor antagonist that is approved for advanced cancer,” he added. The other products that are currently approved, naloxegol (Movantik) and naldemedine (Symproic), are both indicated for chronic, noncancer pain.
The other potential benefit of subcutaneous MNTX is that it can work very rapidly for the patients who respond to it. “One of the things investigators did not mention in these two trials but which has been shown in previous studies is that almost half of patients who respond to this drug respond within the first 30 minutes of receiving the injection,” Dr. Brenner said in an interview.
This can be very beneficial in an emergency setting, because it may avoid having patients admitted to hospital. They can be discharged and sent home with enough drug to use on demand, Dr. Brenner suggested.
New analysis of data from studies 302 and 4000
Both studies were carried out in adults with advanced illness and OIC whose conditions were refractory to laxative use. Both of the studies were placebo controlled.
Study 302 involved 78 patients with cancer and 56 patients with noncancer-related OIC. MNTX was given at a dose of 0.15 mg/kg subcutaneously every other day for 2 weeks.
Study 4000 included 152 patients with cancer and OIC and 78 patients with noncancer-related OIC. In this study, the dose of MNTX was based on body weight. Seven or fewer doses of either 8 mg or 12 mg were given subcutaneously for 2 weeks.
The main endpoints of both studies was the proportion of patients who achieved a rescue-free laxation (RFL) response within 4 hours after the first dose and the proportion of patients with an RFL response within 4 hours for two or more of the first four doses within 24 hours.
Dr. Shah explained that RFL is a meaningful clinical endpoint. Patients could achieve a bowel movement with the two prespecified time endpoints in both studies.
Not all patients were hospitalized for OIC, Dr. Shah noted. Entry criteria were strict and included having fewer than three bowel movements during the previous week and no clinically significant laxation (defecation) within 48 hours of receiving the first dose of study drug.
“In both studies, a significantly greater proportion of patients treated with MNTX versus placebo achieved an RFL within 4 hours after the first dose among both cancer and noncancer patients,” the investigators reported.
Results were relatively comparable between cancer and noncancer patients who were treated for OIC in study 4000, the investigators noted.
Both studies were sponsored by Salix Pharmaceuticals. Dr. Shah has received travel fees from Salix Pharmaceuticals. Dr. Brenner has served as a consultant for Salix Pharmaceuticals, AstraZeneca, and Purdue Pharma. AstraZeneca developed naloxegol.
This article first appeared on Medscape.com.
Subcutaneously administered methylnaltrexone (MNTX) (Relistor), a peripherally acting mu-opioid receptor antagonist, relieves opioid-induced constipation (OID) in both chronic, noncancer-related illness and cancer-related illness, a new analysis concludes.
“While these are two very different patient groups, the ability to have something to treat OIC in noncancer patients who stay on opioids for whatever reason helps, because [otherwise] these patients are not doing well,” said lead author Eric Shah, MD, motility director for the Dartmouth program at Dartmouth Hitchcock Health, Lebanon, N.H.
Importantly, peripherally acting mu-opioid receptor antagonists such as MNTX do not affect overall pain control to any significant extent, which is “reassuring,” he said in an interview.
These drugs decrease the constipating effects of opioids without reversing CNS-mediated opioid effects, he explained.
“Methylnaltrexone has already been approved for the treatment of OIC in adults with chronic noncancer pain as well as for OIC in adults with advanced illness who are receiving palliative care, which is often the case in patients with cancer-related pain,” he noted.
Dr. Shah discussed the new analysis during PAINWeek 2020, the American Society of Regional Anesthesia and Pain Medicine 19th Annual Pain Medicine Meeting.
The analysis was based on a review of data collected in two previously reported randomized, placebo-controlled studies (study 302 and 4000), which were used to gain approval.
The new analysis shows that “the drug works up front, and the effect is able to be maintained. I think the studies are clinically relevant in that patients are able to have a bowel movement quickly after you give them an injectable formulation when they are vomiting or otherwise can’t tolerate a pill and they are feeling miserable,” Dr. Shah commented. Many patients with OIC are constipated for reasons other than from opioid use. They often have other side effects from opioids, including bloating, nausea, and vomiting.
“When patients go to the emergency room, it’s not just that they are not able to have a bowel movement; they are often also vomiting, so it’s important to have agents that can be given in a manner that avoids the need for oral medication,” Dr. Shah said. MNTX is the only peripherally acting opioid antagonist available in a subcutaneous formulation.
Moreover, if patients are able to control these symptoms at home with an injectable formulation, they may not need to go to the ED for treatment of their gastrointestinal distress, he added.
Viable product
In a comment, Darren Brenner, MD, associate professor of medicine and surgery, Northwestern University, Chicago, who has worked with this subcutaneous formulation, said it is “definitely a viable product.
“The data presented here were in patients with advanced illness receiving palliative care when other laxatives have failed, and the difference and the potential benefit for MNTX is that it is the only peripherally acting mu-opioid receptor antagonist that is approved for advanced cancer,” he added. The other products that are currently approved, naloxegol (Movantik) and naldemedine (Symproic), are both indicated for chronic, noncancer pain.
The other potential benefit of subcutaneous MNTX is that it can work very rapidly for the patients who respond to it. “One of the things investigators did not mention in these two trials but which has been shown in previous studies is that almost half of patients who respond to this drug respond within the first 30 minutes of receiving the injection,” Dr. Brenner said in an interview.
This can be very beneficial in an emergency setting, because it may avoid having patients admitted to hospital. They can be discharged and sent home with enough drug to use on demand, Dr. Brenner suggested.
New analysis of data from studies 302 and 4000
Both studies were carried out in adults with advanced illness and OIC whose conditions were refractory to laxative use. Both of the studies were placebo controlled.
Study 302 involved 78 patients with cancer and 56 patients with noncancer-related OIC. MNTX was given at a dose of 0.15 mg/kg subcutaneously every other day for 2 weeks.
Study 4000 included 152 patients with cancer and OIC and 78 patients with noncancer-related OIC. In this study, the dose of MNTX was based on body weight. Seven or fewer doses of either 8 mg or 12 mg were given subcutaneously for 2 weeks.
The main endpoints of both studies was the proportion of patients who achieved a rescue-free laxation (RFL) response within 4 hours after the first dose and the proportion of patients with an RFL response within 4 hours for two or more of the first four doses within 24 hours.
Dr. Shah explained that RFL is a meaningful clinical endpoint. Patients could achieve a bowel movement with the two prespecified time endpoints in both studies.
Not all patients were hospitalized for OIC, Dr. Shah noted. Entry criteria were strict and included having fewer than three bowel movements during the previous week and no clinically significant laxation (defecation) within 48 hours of receiving the first dose of study drug.
“In both studies, a significantly greater proportion of patients treated with MNTX versus placebo achieved an RFL within 4 hours after the first dose among both cancer and noncancer patients,” the investigators reported.
Results were relatively comparable between cancer and noncancer patients who were treated for OIC in study 4000, the investigators noted.
Both studies were sponsored by Salix Pharmaceuticals. Dr. Shah has received travel fees from Salix Pharmaceuticals. Dr. Brenner has served as a consultant for Salix Pharmaceuticals, AstraZeneca, and Purdue Pharma. AstraZeneca developed naloxegol.
This article first appeared on Medscape.com.
How mental health care would look under a Trump vs. Biden administration
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
Children’s opioid harms vary by race, location
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.
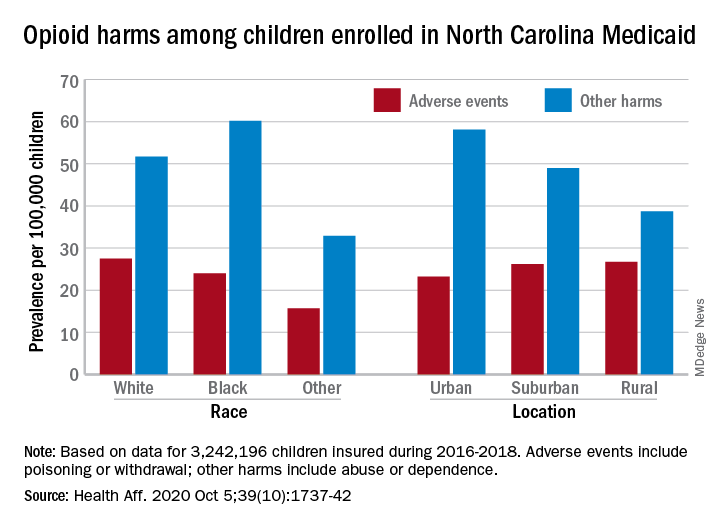
Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.

Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.

Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
FROM HEALTH AFFAIRS
FDA orders stronger warnings on benzodiazepines
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
New acute pain guidelines from the ACP and AAFP have limitations
The American College of Physicians and the American Academy of Family Physicians recently authored a guideline regarding the treatment of acute, non–low back, musculoskeletal injuries in adults in the outpatient setting.
According to the authors, musculoskeletal injuries result in more than 65 million medical visits a year with an annual estimated cost of $176.1 billion in 2010.
In summary, the guideline, which was published in the Annals of Internal Medicine, is based on a review of the best available evidence. The research reviewed by the guideline authors showed favorable results with topical NSAIDs, oral NSAIDs, oral acetaminophen, acupressure, and transcutaneous electrical nerve stimulation in reducing pain and/or improving function. The guideline authors “recommend that clinicians treat patients with acute pain from non–low back, musculoskeletal injuries with topical [NSAIDs] with or without gel as first-line therapy to reduce or relieve symptoms, including pain; improve physical function; and improve the patient’s treatment satisfaction (Grade: strong recommendation; moderate-certainty evidence).” Additionally, the guideline recommends against treating acute pain from non–low back, musculoskeletal injuries with opioids, including tramadol (Grade: conditional recommendation; low-certainty evidence).
The guideline also mentions improving function in relation to decreasing pain, which can be multifactorial.
Treating pain requires a multipronged approach. Many patients require more than one therapy to treat their pain, such as NSAIDs plus physical therapy. The ACP and AAFP did not make any recommendations for combination therapies in this guideline.
When physical therapy is needed
Nonopioid pain medications can do a great job of reducing a patient’s physical discomfort, which the evidence for these guideline demonstrates. However, much of the dysfunction caused by musculoskeletal injuries will not improve by reducing the pain alone. Physical therapy, exercise, and mobilization did not show a significant benefit in reducing symptoms in the systematic review and meta-analysis of randomized trials that appeared alongside the guideline. The type of pain, however, was not evaluated in relation to the effectiveness of these treatments. A fractured bone, for example, may heal just fine with casting and pain management, without the need for additional therapies. However, the muscles surrounding that bone can atrophy and become weak from not being used. Physical therapy may be needed to restrengthen those muscles. Therefore, a multifaceted approach is often needed, even for uncomplicated conditions.
Mental pain often comes with physical pain, and this is an aspect of care that is often neglected. It can be quite devastating for patients to not be able to do the things they were previously able to do. While this is easily recognized in professional athletes when they can no longer play, it is not so readily apparent with a mother who is just trying to take care of her kids. As doctors, especially those of us in family medicine, we should be addressing more than just physical pain.
Patients can also do activities that exacerbate their pain. As doctors, we need to be asking questions that help us determine whether a patient’s pain is caused by a particular action. Maybe that increase in shoulder pain is due to nothing more than lifting something heavy rather than a failure in a prescribed medication. Pain diaries are helpful, and clinicians don’t use them often enough.
How pain affects mental health
Acute injuries can also lead to disability. Many patients become quite distressed about being unable to work. They often need Famiy & Medical Leave Act forms filled out, and this task usually falls to the primary care doctor. In addition to assessing the pain, we need to be evaluating, at each visit, a patient’s level of functioning and their ability to do their job.
Every patient responds to pain differently, and it is important to evaluate patients’ mindsets regarding theirs. A patient may be in severe pain and may try to ignore it for a variety of reasons. A patient may “catastrophize” their pain, believing only the worst outcome will happen to them. Helping patients set appropriate expectations and having a positive mindset can help.
Overall, the new recommendations are a great tool as a guideline, but they are not complete enough to be the only ones used in managing acute, non–low back, musculoskeletal pain in adults.
They are very important for clinicians who may be prescribing opioid medications for patients with this type of pain. Amid an opioid crisis, it is the responsibility of every doctor to prescribe these medications appropriately. The evidence clearly shows they provide little benefit and place patients at risk of addiction.
We should all be following these recommendations as the baseline of care for acute pain. However, we need to delve deeper and manage all the components involved. We would be ignoring very real suffering in our patients if we limited our focus to only the physical discomfort.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Rutgers RWJ Medical School.
SOURCE: Ann Intern Med. 2020 Aug 18. doi: 10.7326/M19-3602.
The American College of Physicians and the American Academy of Family Physicians recently authored a guideline regarding the treatment of acute, non–low back, musculoskeletal injuries in adults in the outpatient setting.
According to the authors, musculoskeletal injuries result in more than 65 million medical visits a year with an annual estimated cost of $176.1 billion in 2010.
In summary, the guideline, which was published in the Annals of Internal Medicine, is based on a review of the best available evidence. The research reviewed by the guideline authors showed favorable results with topical NSAIDs, oral NSAIDs, oral acetaminophen, acupressure, and transcutaneous electrical nerve stimulation in reducing pain and/or improving function. The guideline authors “recommend that clinicians treat patients with acute pain from non–low back, musculoskeletal injuries with topical [NSAIDs] with or without gel as first-line therapy to reduce or relieve symptoms, including pain; improve physical function; and improve the patient’s treatment satisfaction (Grade: strong recommendation; moderate-certainty evidence).” Additionally, the guideline recommends against treating acute pain from non–low back, musculoskeletal injuries with opioids, including tramadol (Grade: conditional recommendation; low-certainty evidence).
The guideline also mentions improving function in relation to decreasing pain, which can be multifactorial.
Treating pain requires a multipronged approach. Many patients require more than one therapy to treat their pain, such as NSAIDs plus physical therapy. The ACP and AAFP did not make any recommendations for combination therapies in this guideline.
When physical therapy is needed
Nonopioid pain medications can do a great job of reducing a patient’s physical discomfort, which the evidence for these guideline demonstrates. However, much of the dysfunction caused by musculoskeletal injuries will not improve by reducing the pain alone. Physical therapy, exercise, and mobilization did not show a significant benefit in reducing symptoms in the systematic review and meta-analysis of randomized trials that appeared alongside the guideline. The type of pain, however, was not evaluated in relation to the effectiveness of these treatments. A fractured bone, for example, may heal just fine with casting and pain management, without the need for additional therapies. However, the muscles surrounding that bone can atrophy and become weak from not being used. Physical therapy may be needed to restrengthen those muscles. Therefore, a multifaceted approach is often needed, even for uncomplicated conditions.
Mental pain often comes with physical pain, and this is an aspect of care that is often neglected. It can be quite devastating for patients to not be able to do the things they were previously able to do. While this is easily recognized in professional athletes when they can no longer play, it is not so readily apparent with a mother who is just trying to take care of her kids. As doctors, especially those of us in family medicine, we should be addressing more than just physical pain.
Patients can also do activities that exacerbate their pain. As doctors, we need to be asking questions that help us determine whether a patient’s pain is caused by a particular action. Maybe that increase in shoulder pain is due to nothing more than lifting something heavy rather than a failure in a prescribed medication. Pain diaries are helpful, and clinicians don’t use them often enough.
How pain affects mental health
Acute injuries can also lead to disability. Many patients become quite distressed about being unable to work. They often need Famiy & Medical Leave Act forms filled out, and this task usually falls to the primary care doctor. In addition to assessing the pain, we need to be evaluating, at each visit, a patient’s level of functioning and their ability to do their job.
Every patient responds to pain differently, and it is important to evaluate patients’ mindsets regarding theirs. A patient may be in severe pain and may try to ignore it for a variety of reasons. A patient may “catastrophize” their pain, believing only the worst outcome will happen to them. Helping patients set appropriate expectations and having a positive mindset can help.
Overall, the new recommendations are a great tool as a guideline, but they are not complete enough to be the only ones used in managing acute, non–low back, musculoskeletal pain in adults.
They are very important for clinicians who may be prescribing opioid medications for patients with this type of pain. Amid an opioid crisis, it is the responsibility of every doctor to prescribe these medications appropriately. The evidence clearly shows they provide little benefit and place patients at risk of addiction.
We should all be following these recommendations as the baseline of care for acute pain. However, we need to delve deeper and manage all the components involved. We would be ignoring very real suffering in our patients if we limited our focus to only the physical discomfort.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Rutgers RWJ Medical School.
SOURCE: Ann Intern Med. 2020 Aug 18. doi: 10.7326/M19-3602.
The American College of Physicians and the American Academy of Family Physicians recently authored a guideline regarding the treatment of acute, non–low back, musculoskeletal injuries in adults in the outpatient setting.
According to the authors, musculoskeletal injuries result in more than 65 million medical visits a year with an annual estimated cost of $176.1 billion in 2010.
In summary, the guideline, which was published in the Annals of Internal Medicine, is based on a review of the best available evidence. The research reviewed by the guideline authors showed favorable results with topical NSAIDs, oral NSAIDs, oral acetaminophen, acupressure, and transcutaneous electrical nerve stimulation in reducing pain and/or improving function. The guideline authors “recommend that clinicians treat patients with acute pain from non–low back, musculoskeletal injuries with topical [NSAIDs] with or without gel as first-line therapy to reduce or relieve symptoms, including pain; improve physical function; and improve the patient’s treatment satisfaction (Grade: strong recommendation; moderate-certainty evidence).” Additionally, the guideline recommends against treating acute pain from non–low back, musculoskeletal injuries with opioids, including tramadol (Grade: conditional recommendation; low-certainty evidence).
The guideline also mentions improving function in relation to decreasing pain, which can be multifactorial.
Treating pain requires a multipronged approach. Many patients require more than one therapy to treat their pain, such as NSAIDs plus physical therapy. The ACP and AAFP did not make any recommendations for combination therapies in this guideline.
When physical therapy is needed
Nonopioid pain medications can do a great job of reducing a patient’s physical discomfort, which the evidence for these guideline demonstrates. However, much of the dysfunction caused by musculoskeletal injuries will not improve by reducing the pain alone. Physical therapy, exercise, and mobilization did not show a significant benefit in reducing symptoms in the systematic review and meta-analysis of randomized trials that appeared alongside the guideline. The type of pain, however, was not evaluated in relation to the effectiveness of these treatments. A fractured bone, for example, may heal just fine with casting and pain management, without the need for additional therapies. However, the muscles surrounding that bone can atrophy and become weak from not being used. Physical therapy may be needed to restrengthen those muscles. Therefore, a multifaceted approach is often needed, even for uncomplicated conditions.
Mental pain often comes with physical pain, and this is an aspect of care that is often neglected. It can be quite devastating for patients to not be able to do the things they were previously able to do. While this is easily recognized in professional athletes when they can no longer play, it is not so readily apparent with a mother who is just trying to take care of her kids. As doctors, especially those of us in family medicine, we should be addressing more than just physical pain.
Patients can also do activities that exacerbate their pain. As doctors, we need to be asking questions that help us determine whether a patient’s pain is caused by a particular action. Maybe that increase in shoulder pain is due to nothing more than lifting something heavy rather than a failure in a prescribed medication. Pain diaries are helpful, and clinicians don’t use them often enough.
How pain affects mental health
Acute injuries can also lead to disability. Many patients become quite distressed about being unable to work. They often need Famiy & Medical Leave Act forms filled out, and this task usually falls to the primary care doctor. In addition to assessing the pain, we need to be evaluating, at each visit, a patient’s level of functioning and their ability to do their job.
Every patient responds to pain differently, and it is important to evaluate patients’ mindsets regarding theirs. A patient may be in severe pain and may try to ignore it for a variety of reasons. A patient may “catastrophize” their pain, believing only the worst outcome will happen to them. Helping patients set appropriate expectations and having a positive mindset can help.
Overall, the new recommendations are a great tool as a guideline, but they are not complete enough to be the only ones used in managing acute, non–low back, musculoskeletal pain in adults.
They are very important for clinicians who may be prescribing opioid medications for patients with this type of pain. Amid an opioid crisis, it is the responsibility of every doctor to prescribe these medications appropriately. The evidence clearly shows they provide little benefit and place patients at risk of addiction.
We should all be following these recommendations as the baseline of care for acute pain. However, we need to delve deeper and manage all the components involved. We would be ignoring very real suffering in our patients if we limited our focus to only the physical discomfort.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Rutgers RWJ Medical School.
SOURCE: Ann Intern Med. 2020 Aug 18. doi: 10.7326/M19-3602.
Reworked OxyContin fails to cut overall opioid abuse, FDA panel says
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
One in seven high schoolers is misusing opioids
according to an analysis from the Centers for Disease Control and Prevention.
That type of opioid use/misuse, reported by 14.3% of respondents to the 2019 Youth Risk Behavior Survey, was more common among females (16.1%) than males (12.4%) and even more prevalent among nonheterosexuals and those who are unsure about their sexual identity, Christopher M. Jones, PharmD, DrPH, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
The YRBS data show that 18.5% of gay or lesbian students had, at some point in their lives, used a prescription opioid differently than a physician had told them to or taken one without a prescription. That figure was slightly higher (19.1%) for those unsure of their sexual identity, considerably higher (25.4%) for bisexuals, and lower for heterosexuals (12.7%), they reported.
The pattern for current use/misuse of opioids, defined as use one or more times in the 30 days before the survey, was similar to ever use but somewhat less pronounced in 2019. Prevalence was 7.2% for all students in grades 9-12, 8.3% for females, and 6.1% for males. By sexual identity, prevalence was 6.4% for heterosexuals, 7.6% for gays or lesbians, 11.5% for those unsure about their sexual identity, and 13.1% for bisexuals, based on the YRBS data.
This increased misuse of opioids among sexual minority youths, “even after controlling for other demographic and substance use characteristics ... emphasizes the importance of identifying tailored prevention strategies to address disparities among this vulnerable population,” the CDC researchers wrote.
SOURCE: Jones CM et al. MMWR Suppl. 2020 Aug 21;69(1):38-46.
according to an analysis from the Centers for Disease Control and Prevention.

That type of opioid use/misuse, reported by 14.3% of respondents to the 2019 Youth Risk Behavior Survey, was more common among females (16.1%) than males (12.4%) and even more prevalent among nonheterosexuals and those who are unsure about their sexual identity, Christopher M. Jones, PharmD, DrPH, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
The YRBS data show that 18.5% of gay or lesbian students had, at some point in their lives, used a prescription opioid differently than a physician had told them to or taken one without a prescription. That figure was slightly higher (19.1%) for those unsure of their sexual identity, considerably higher (25.4%) for bisexuals, and lower for heterosexuals (12.7%), they reported.
The pattern for current use/misuse of opioids, defined as use one or more times in the 30 days before the survey, was similar to ever use but somewhat less pronounced in 2019. Prevalence was 7.2% for all students in grades 9-12, 8.3% for females, and 6.1% for males. By sexual identity, prevalence was 6.4% for heterosexuals, 7.6% for gays or lesbians, 11.5% for those unsure about their sexual identity, and 13.1% for bisexuals, based on the YRBS data.
This increased misuse of opioids among sexual minority youths, “even after controlling for other demographic and substance use characteristics ... emphasizes the importance of identifying tailored prevention strategies to address disparities among this vulnerable population,” the CDC researchers wrote.
SOURCE: Jones CM et al. MMWR Suppl. 2020 Aug 21;69(1):38-46.
according to an analysis from the Centers for Disease Control and Prevention.

That type of opioid use/misuse, reported by 14.3% of respondents to the 2019 Youth Risk Behavior Survey, was more common among females (16.1%) than males (12.4%) and even more prevalent among nonheterosexuals and those who are unsure about their sexual identity, Christopher M. Jones, PharmD, DrPH, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
The YRBS data show that 18.5% of gay or lesbian students had, at some point in their lives, used a prescription opioid differently than a physician had told them to or taken one without a prescription. That figure was slightly higher (19.1%) for those unsure of their sexual identity, considerably higher (25.4%) for bisexuals, and lower for heterosexuals (12.7%), they reported.
The pattern for current use/misuse of opioids, defined as use one or more times in the 30 days before the survey, was similar to ever use but somewhat less pronounced in 2019. Prevalence was 7.2% for all students in grades 9-12, 8.3% for females, and 6.1% for males. By sexual identity, prevalence was 6.4% for heterosexuals, 7.6% for gays or lesbians, 11.5% for those unsure about their sexual identity, and 13.1% for bisexuals, based on the YRBS data.
This increased misuse of opioids among sexual minority youths, “even after controlling for other demographic and substance use characteristics ... emphasizes the importance of identifying tailored prevention strategies to address disparities among this vulnerable population,” the CDC researchers wrote.
SOURCE: Jones CM et al. MMWR Suppl. 2020 Aug 21;69(1):38-46.
FROM MMWR
Deaths, despair tied to drug dependence are accelerating amid COVID-19
Patients with OUDs need assistance now more than ever.
The Centers for Disease Control and Prevention reported recently that opioid overdose deaths will increase to a new U.S. record, and more are expected as pandemic-related overdose deaths are yet to be counted.1
Specifically, according to the CDC, 70,980 people died from fatal overdoses in 2019,2 which is record high. Experts such as Bruce A. Goldberger, PhD, fear that the 2020 numbers could rise even higher, exacerbated by the coronavirus pandemic.
Deaths from drug overdoses remain higher than the peak yearly death totals ever recorded for car accidents, guns, or AIDS. Overdose deaths have accelerated further – pushing down overall life expectancy in the United States.3 Headlines purporting to identify good news in drug death figures don’t always get below top-level data. Deaths and despair tied to drug dependence are indeed accelerating. I am concerned about these alarmingly dangerous trends.
Synthetic opioids such as fentanyl accounted for about 3,000 deaths in 2013. By 2019, they accounted for more than 37,137.4 In addition, 16,539 deaths involved stimulants such as methamphetamine, and 16,196 deaths involved cocaine, the most recent CDC reporting shows. Opioids continue to play a role in U.S. “deaths of despair,” or rising fatalities from drugs, suicides, and alcohol among Americans without employment, hope of job opportunities, or college degrees.5 As the American Medical Association has warned,6 more people are dying from overdoses amid the COVID-19 pandemic. Clinicians need to be aware of trends so that we can help our patients navigate these challenges.
Fentanyl presents dangers
Experts had predicted that the pandemic, by limiting access to treatment, rescue, or overdose services, and increasing time at home and in the neighborhood, would result in more tragedy. In addition, the shift from prescription opioids to heroin and now to fentanyl has made deaths more common.
Fentanyls – synthetic opioids – are involved in more than half of overdose deaths, and in many of the cocaine and methamphetamine-related deaths, which also are on the rise. Fentanyl is about 100 times more potent than morphine and 50 times more potent than heroin. Breathing can stop after use of just 2 mg of fentanyl, which is about as much as trace amounts of table salt. Fentanyl has replaced heroin in many cities as the pandemic changed the relative ease of importing raw drugs such as heroin.
Another important trend is that fentanyl production and distribution throughout the United States have expanded. The ease of manufacture in unregulated sectors of the Chinese and Mexican economies is difficult for U.S. authorities to curb or eliminate. The Internet promotes novel strategies for synthesizing the substance, spreading its production across many labs; suppliers use the U.S. Postal Service for distribution, and e-commerce helps to get the drug from manufacturers to U.S. consumers for fentanyl transactions.
A recent RAND report observes that, for only $10 through the postal service, suppliers can ship a 1-kg parcel from China to the United States, and private shipments cost about $100.7 And with large volumes of legal trade between the two countries making rigorous scrutiny of products difficult, especially given the light weight of fentanyl, suppliers find it relatively easy to hide illicit substances in licit shipments. Opioid users have made the switch to fentanyl, and have seen fentanyl added to cocaine and methamphetamine they buy on the streets.
OUD and buprenorphine
Fentanyl is one part of the overdose crisis. Opioid use disorder (OUD) is the other. Both need to be addressed if we are to make any progress in this epidemic of death and dependency.
The OUD crisis continues amid the pandemic – and isn’t going away.8 Slips, relapses, and overdoses are all too common. Medication-assisted treatment (MAT) and OUD treatment programs are essential parts of our response to overdose initiatives. After naloxone rescue, the best anti-overdose response is to get the OUD patient into treatment with MATs. Patients with OUD have continuously high risks of overdose. The best outcomes appear to be related to treatment duration of greater than 2 years. But it is common to see patients with OUDs who have been in treatment multiple times, taking MATs, dropping out, overdosing, and dying. Some have been described as treatment resistant.9 It is clear that treatment can work, but also that even evidence-based treatments often fail.10
A recent study compared OUD patients who continued treatment for 6-9 months to those patients who had continued MAT treatment for 15-18 months. The longer the treatment, the fewer emergencies, prescriptions, or hospitalizations.11
But this study reminds us that all OUD patients, whether they are currently buprenorphine treated or not, experience overdoses and emergency department interventions. Short and longer treatment groups have a similar nonfatal overdose rate, about 6%, and went to the emergency department at a high rate, above 40%. Discontinuation of buprenorphine treatment is a major risk factor in opioid relapse, emergency department visits, and overdose. Cures are not common. Whether an OUD patient is being treated or has been treated in the past, carrying naloxone (brand name Narcan), makes sense and can save lives.
Methadone still considered most effective
Methadone is a synthetic opioid first studied as a treatment for OUD at Rockefeller University in New York City in the 1960s. Methadone may be the most effective treatment for OUD in promoting treatment retention for years, decreasing intravenous drug use, and decreasing deaths.12 It has been studied and safely used in treatment programs for decades. Methadone is typically administered in a clinic, daily, and with observation. In addition, methadone patients periodically take urine drug tests, which can distinguish methadone from substances of abuse. They also receive counseling. But methadone can be prescribed and administered only in methadone clinics in the United States. It is available for prescription in primary care clinics in Great Britain, Canada, and Australia.13 Numerous experts have suggested passing new legislation aimed at changing how methadone can be prescribed. Allowing primary care to administer methadone, just like buprenorphine, can improve access and benefit OUD patients.12
Availability of Narcan is critical
A comprehensive treatment model for OUDs includes prescribing naloxone, encouraging those patients with an OUD and their loved ones to have naloxone with them, and providing MATs and appropriate therapies, such as counseling.
As described by Allison L. Pitt and colleagues at Stanford (Calif.) University,14 the United States might be on track to have up to 500,000 deaths tied to opioid overdoses that might occur over the next 5 years. They modeled the effect on overdose of a long list of interventions, but only a few had an impact. At the top of the list was naloxone availability. We need to focus on saving lives by increasing naloxone availability, improving initiation, and expanding access to MAT, and increasing psychosocial treatment to improve outcomes, increase life-years and quality-adjusted life-years, and reduce opioid-related deaths. When Ms. Pitt and colleagues looked at what would make the most impact in reducing OUD deaths, it was naloxone. Pain patients on higher doses of opioids, nonprescription opioid users, OUD patients should be given naloxone prescriptions. While many can give a Heimlich to a choking person or CPR, few have naloxone to rescue a person who has overdosed on opioids. If an overdose is suspected, it should be administered by anyone who has it, as soon as possible. Then, the person who is intervening should call 911.
What we can do today
At this moment, clinicians can follow the Surgeon General’s advice,15 and prescribe naloxone.
We should give naloxone to OUD patients and their families, to pain patients at dosages of greater than or equal to 50 MME. Our top priorities should be patients with comorbid pain syndromes, those being treated with benzodiazepines and sleeping medications, and patients with alcohol use disorders. This is also an important intervention for those who binge drink, and have sleep apnea, and heart and respiratory diseases.
Naloxone is available without a prescription in at least 43 states. Naloxone is available in harm reduction programs and in hospitals, and is carried by emergency medical staff, law enforcement, and EMTs. It also is available on the streets, though it does not appear to have a dollar value like opioids or even buprenorphine. Also, the availability of naloxone in pharmacies has made it easier for family members and caregivers of pain patients or those with OUD to have it to administer in an emergency.
An excellent place for MDs to start is to do more to encourage all patients with OUD to carry naloxone, for their loved ones to carry naloxone, and for their homes to have naloxone nearby in the bedroom or bathroom. It is not logical to expect a person with an OUD to rescue themselves. Current and past OUD patients, as well as their loved ones, are at high risk – and should have naloxone nearby at all times.
Naloxone reverses an opioid overdose, but it should be thought about like cardioversion or CPR rather than a treatment for an underlying disease. Increasing access to buprenorphine, buprenorphine + naloxone, and naltrexone treatment for OUDs is an important organizing principle. Initiation of MAT treatment in the emergency setting or most anywhere and any place a patient with an OUD can begin treatment is necessary. Treatment with buprenorphine or methadone reduces opioid overdose and opioid-related acute care use.16
Reducing racial disparities in OUD treatment is necessary, because buprenorphine treatment is concentrated among White patients who either use private insurance or are self-pay.17 Reducing barriers to methadone program licenses, expanding sites for distribution,18 prescribing methadone in an office setting might help. Clinicians can do a better job of explaining the risks associated with opioid prescriptions, including diversion and overdose, and the benefits of OUD treatment. So, To reduce opioid overdoses, we must increase physician competencies in addiction medicine.
Dr. Gold is professor of psychiatry (adjunct) at Washington University, St. Louis. He is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville. For more than 40 years, Dr. Gold has worked on developing models for understanding the effects of opioid, tobacco, cocaine, and other drugs, as well as food, on the brain and behavior. He disclosed financial ties with ADAPT Pharma and Magstim Ltd.
References
1. Kamp J. Overdose deaths rise, may reach record level, federal data show. Wall Street Journal. 2020 Jul 15.
2. 12 month–ending provisional number of drug overdose drugs. Centers for Disease Control and Prevention. 2020 Jul 5.
3. Katz J et al. In shadow of pandemic, U.S. drug overdose deaths resurge to record. New York Times. 2020 Jul 15.
4. Gold MS. The fentanyl crisis is only getting worse. Addiction Policy Forum. Updated 2020 Mar 12.
5. Gold MS. Mo Med. 2020-Mar-Apr;117(2):99-101.
6. Reports of increases in opioid-related overdoses and other concerns during the COVID-19 pandemic. American Medical Association. Issue brief. Updated 2020 Jul 20.
7. Pardo B et al. The future of fentanyl and other synthetic opioids. RAND report.
8. Gold MS. New challenges in the opioid epidemic. Addiction Policy Forum. 2020 Jun 4.
9. Patterson Silver Wolf DA and Gold MS. J Neurol Sci. 2020;411:116718.
10. Oesterle TS et al. Mayo Clin Proc. 2019;94(10):2072-86.
11. Connery HS and Weiss RD. Am J Psychiatry. 2020;177(2):104-6.
12. Kleber HD. JAMA. 2008;300(19):2303-5.
13. Samet JH et al. N Engl J Med. 2018;379(1):7-8.
14. Pitt AL et al. Am J Public Health. 2018;108(10):1394-1400.
15. U.S. Surgeon General’s Advisory on Naloxone and Opioid Overdose. hhs.gov.
16. Wakeman SE et al. JAMA Netw Open. 2020;3(2):e1920622.
17. Lagisetty PA et al. JAMA Psychiatry. 2019;76(9):979-81.
18. Kleinman RA. JAMA Psychiatry. 2020 Jul 15. doi: 10.1001/jamapsychiatry.2020.1624.
Patients with OUDs need assistance now more than ever.
Patients with OUDs need assistance now more than ever.
The Centers for Disease Control and Prevention reported recently that opioid overdose deaths will increase to a new U.S. record, and more are expected as pandemic-related overdose deaths are yet to be counted.1
Specifically, according to the CDC, 70,980 people died from fatal overdoses in 2019,2 which is record high. Experts such as Bruce A. Goldberger, PhD, fear that the 2020 numbers could rise even higher, exacerbated by the coronavirus pandemic.
Deaths from drug overdoses remain higher than the peak yearly death totals ever recorded for car accidents, guns, or AIDS. Overdose deaths have accelerated further – pushing down overall life expectancy in the United States.3 Headlines purporting to identify good news in drug death figures don’t always get below top-level data. Deaths and despair tied to drug dependence are indeed accelerating. I am concerned about these alarmingly dangerous trends.
Synthetic opioids such as fentanyl accounted for about 3,000 deaths in 2013. By 2019, they accounted for more than 37,137.4 In addition, 16,539 deaths involved stimulants such as methamphetamine, and 16,196 deaths involved cocaine, the most recent CDC reporting shows. Opioids continue to play a role in U.S. “deaths of despair,” or rising fatalities from drugs, suicides, and alcohol among Americans without employment, hope of job opportunities, or college degrees.5 As the American Medical Association has warned,6 more people are dying from overdoses amid the COVID-19 pandemic. Clinicians need to be aware of trends so that we can help our patients navigate these challenges.
Fentanyl presents dangers
Experts had predicted that the pandemic, by limiting access to treatment, rescue, or overdose services, and increasing time at home and in the neighborhood, would result in more tragedy. In addition, the shift from prescription opioids to heroin and now to fentanyl has made deaths more common.
Fentanyls – synthetic opioids – are involved in more than half of overdose deaths, and in many of the cocaine and methamphetamine-related deaths, which also are on the rise. Fentanyl is about 100 times more potent than morphine and 50 times more potent than heroin. Breathing can stop after use of just 2 mg of fentanyl, which is about as much as trace amounts of table salt. Fentanyl has replaced heroin in many cities as the pandemic changed the relative ease of importing raw drugs such as heroin.
Another important trend is that fentanyl production and distribution throughout the United States have expanded. The ease of manufacture in unregulated sectors of the Chinese and Mexican economies is difficult for U.S. authorities to curb or eliminate. The Internet promotes novel strategies for synthesizing the substance, spreading its production across many labs; suppliers use the U.S. Postal Service for distribution, and e-commerce helps to get the drug from manufacturers to U.S. consumers for fentanyl transactions.
A recent RAND report observes that, for only $10 through the postal service, suppliers can ship a 1-kg parcel from China to the United States, and private shipments cost about $100.7 And with large volumes of legal trade between the two countries making rigorous scrutiny of products difficult, especially given the light weight of fentanyl, suppliers find it relatively easy to hide illicit substances in licit shipments. Opioid users have made the switch to fentanyl, and have seen fentanyl added to cocaine and methamphetamine they buy on the streets.
OUD and buprenorphine
Fentanyl is one part of the overdose crisis. Opioid use disorder (OUD) is the other. Both need to be addressed if we are to make any progress in this epidemic of death and dependency.
The OUD crisis continues amid the pandemic – and isn’t going away.8 Slips, relapses, and overdoses are all too common. Medication-assisted treatment (MAT) and OUD treatment programs are essential parts of our response to overdose initiatives. After naloxone rescue, the best anti-overdose response is to get the OUD patient into treatment with MATs. Patients with OUD have continuously high risks of overdose. The best outcomes appear to be related to treatment duration of greater than 2 years. But it is common to see patients with OUDs who have been in treatment multiple times, taking MATs, dropping out, overdosing, and dying. Some have been described as treatment resistant.9 It is clear that treatment can work, but also that even evidence-based treatments often fail.10
A recent study compared OUD patients who continued treatment for 6-9 months to those patients who had continued MAT treatment for 15-18 months. The longer the treatment, the fewer emergencies, prescriptions, or hospitalizations.11
But this study reminds us that all OUD patients, whether they are currently buprenorphine treated or not, experience overdoses and emergency department interventions. Short and longer treatment groups have a similar nonfatal overdose rate, about 6%, and went to the emergency department at a high rate, above 40%. Discontinuation of buprenorphine treatment is a major risk factor in opioid relapse, emergency department visits, and overdose. Cures are not common. Whether an OUD patient is being treated or has been treated in the past, carrying naloxone (brand name Narcan), makes sense and can save lives.
Methadone still considered most effective
Methadone is a synthetic opioid first studied as a treatment for OUD at Rockefeller University in New York City in the 1960s. Methadone may be the most effective treatment for OUD in promoting treatment retention for years, decreasing intravenous drug use, and decreasing deaths.12 It has been studied and safely used in treatment programs for decades. Methadone is typically administered in a clinic, daily, and with observation. In addition, methadone patients periodically take urine drug tests, which can distinguish methadone from substances of abuse. They also receive counseling. But methadone can be prescribed and administered only in methadone clinics in the United States. It is available for prescription in primary care clinics in Great Britain, Canada, and Australia.13 Numerous experts have suggested passing new legislation aimed at changing how methadone can be prescribed. Allowing primary care to administer methadone, just like buprenorphine, can improve access and benefit OUD patients.12
Availability of Narcan is critical
A comprehensive treatment model for OUDs includes prescribing naloxone, encouraging those patients with an OUD and their loved ones to have naloxone with them, and providing MATs and appropriate therapies, such as counseling.
As described by Allison L. Pitt and colleagues at Stanford (Calif.) University,14 the United States might be on track to have up to 500,000 deaths tied to opioid overdoses that might occur over the next 5 years. They modeled the effect on overdose of a long list of interventions, but only a few had an impact. At the top of the list was naloxone availability. We need to focus on saving lives by increasing naloxone availability, improving initiation, and expanding access to MAT, and increasing psychosocial treatment to improve outcomes, increase life-years and quality-adjusted life-years, and reduce opioid-related deaths. When Ms. Pitt and colleagues looked at what would make the most impact in reducing OUD deaths, it was naloxone. Pain patients on higher doses of opioids, nonprescription opioid users, OUD patients should be given naloxone prescriptions. While many can give a Heimlich to a choking person or CPR, few have naloxone to rescue a person who has overdosed on opioids. If an overdose is suspected, it should be administered by anyone who has it, as soon as possible. Then, the person who is intervening should call 911.
What we can do today
At this moment, clinicians can follow the Surgeon General’s advice,15 and prescribe naloxone.
We should give naloxone to OUD patients and their families, to pain patients at dosages of greater than or equal to 50 MME. Our top priorities should be patients with comorbid pain syndromes, those being treated with benzodiazepines and sleeping medications, and patients with alcohol use disorders. This is also an important intervention for those who binge drink, and have sleep apnea, and heart and respiratory diseases.
Naloxone is available without a prescription in at least 43 states. Naloxone is available in harm reduction programs and in hospitals, and is carried by emergency medical staff, law enforcement, and EMTs. It also is available on the streets, though it does not appear to have a dollar value like opioids or even buprenorphine. Also, the availability of naloxone in pharmacies has made it easier for family members and caregivers of pain patients or those with OUD to have it to administer in an emergency.
An excellent place for MDs to start is to do more to encourage all patients with OUD to carry naloxone, for their loved ones to carry naloxone, and for their homes to have naloxone nearby in the bedroom or bathroom. It is not logical to expect a person with an OUD to rescue themselves. Current and past OUD patients, as well as their loved ones, are at high risk – and should have naloxone nearby at all times.
Naloxone reverses an opioid overdose, but it should be thought about like cardioversion or CPR rather than a treatment for an underlying disease. Increasing access to buprenorphine, buprenorphine + naloxone, and naltrexone treatment for OUDs is an important organizing principle. Initiation of MAT treatment in the emergency setting or most anywhere and any place a patient with an OUD can begin treatment is necessary. Treatment with buprenorphine or methadone reduces opioid overdose and opioid-related acute care use.16
Reducing racial disparities in OUD treatment is necessary, because buprenorphine treatment is concentrated among White patients who either use private insurance or are self-pay.17 Reducing barriers to methadone program licenses, expanding sites for distribution,18 prescribing methadone in an office setting might help. Clinicians can do a better job of explaining the risks associated with opioid prescriptions, including diversion and overdose, and the benefits of OUD treatment. So, To reduce opioid overdoses, we must increase physician competencies in addiction medicine.
Dr. Gold is professor of psychiatry (adjunct) at Washington University, St. Louis. He is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville. For more than 40 years, Dr. Gold has worked on developing models for understanding the effects of opioid, tobacco, cocaine, and other drugs, as well as food, on the brain and behavior. He disclosed financial ties with ADAPT Pharma and Magstim Ltd.
References
1. Kamp J. Overdose deaths rise, may reach record level, federal data show. Wall Street Journal. 2020 Jul 15.
2. 12 month–ending provisional number of drug overdose drugs. Centers for Disease Control and Prevention. 2020 Jul 5.
3. Katz J et al. In shadow of pandemic, U.S. drug overdose deaths resurge to record. New York Times. 2020 Jul 15.
4. Gold MS. The fentanyl crisis is only getting worse. Addiction Policy Forum. Updated 2020 Mar 12.
5. Gold MS. Mo Med. 2020-Mar-Apr;117(2):99-101.
6. Reports of increases in opioid-related overdoses and other concerns during the COVID-19 pandemic. American Medical Association. Issue brief. Updated 2020 Jul 20.
7. Pardo B et al. The future of fentanyl and other synthetic opioids. RAND report.
8. Gold MS. New challenges in the opioid epidemic. Addiction Policy Forum. 2020 Jun 4.
9. Patterson Silver Wolf DA and Gold MS. J Neurol Sci. 2020;411:116718.
10. Oesterle TS et al. Mayo Clin Proc. 2019;94(10):2072-86.
11. Connery HS and Weiss RD. Am J Psychiatry. 2020;177(2):104-6.
12. Kleber HD. JAMA. 2008;300(19):2303-5.
13. Samet JH et al. N Engl J Med. 2018;379(1):7-8.
14. Pitt AL et al. Am J Public Health. 2018;108(10):1394-1400.
15. U.S. Surgeon General’s Advisory on Naloxone and Opioid Overdose. hhs.gov.
16. Wakeman SE et al. JAMA Netw Open. 2020;3(2):e1920622.
17. Lagisetty PA et al. JAMA Psychiatry. 2019;76(9):979-81.
18. Kleinman RA. JAMA Psychiatry. 2020 Jul 15. doi: 10.1001/jamapsychiatry.2020.1624.
The Centers for Disease Control and Prevention reported recently that opioid overdose deaths will increase to a new U.S. record, and more are expected as pandemic-related overdose deaths are yet to be counted.1
Specifically, according to the CDC, 70,980 people died from fatal overdoses in 2019,2 which is record high. Experts such as Bruce A. Goldberger, PhD, fear that the 2020 numbers could rise even higher, exacerbated by the coronavirus pandemic.
Deaths from drug overdoses remain higher than the peak yearly death totals ever recorded for car accidents, guns, or AIDS. Overdose deaths have accelerated further – pushing down overall life expectancy in the United States.3 Headlines purporting to identify good news in drug death figures don’t always get below top-level data. Deaths and despair tied to drug dependence are indeed accelerating. I am concerned about these alarmingly dangerous trends.
Synthetic opioids such as fentanyl accounted for about 3,000 deaths in 2013. By 2019, they accounted for more than 37,137.4 In addition, 16,539 deaths involved stimulants such as methamphetamine, and 16,196 deaths involved cocaine, the most recent CDC reporting shows. Opioids continue to play a role in U.S. “deaths of despair,” or rising fatalities from drugs, suicides, and alcohol among Americans without employment, hope of job opportunities, or college degrees.5 As the American Medical Association has warned,6 more people are dying from overdoses amid the COVID-19 pandemic. Clinicians need to be aware of trends so that we can help our patients navigate these challenges.
Fentanyl presents dangers
Experts had predicted that the pandemic, by limiting access to treatment, rescue, or overdose services, and increasing time at home and in the neighborhood, would result in more tragedy. In addition, the shift from prescription opioids to heroin and now to fentanyl has made deaths more common.
Fentanyls – synthetic opioids – are involved in more than half of overdose deaths, and in many of the cocaine and methamphetamine-related deaths, which also are on the rise. Fentanyl is about 100 times more potent than morphine and 50 times more potent than heroin. Breathing can stop after use of just 2 mg of fentanyl, which is about as much as trace amounts of table salt. Fentanyl has replaced heroin in many cities as the pandemic changed the relative ease of importing raw drugs such as heroin.
Another important trend is that fentanyl production and distribution throughout the United States have expanded. The ease of manufacture in unregulated sectors of the Chinese and Mexican economies is difficult for U.S. authorities to curb or eliminate. The Internet promotes novel strategies for synthesizing the substance, spreading its production across many labs; suppliers use the U.S. Postal Service for distribution, and e-commerce helps to get the drug from manufacturers to U.S. consumers for fentanyl transactions.
A recent RAND report observes that, for only $10 through the postal service, suppliers can ship a 1-kg parcel from China to the United States, and private shipments cost about $100.7 And with large volumes of legal trade between the two countries making rigorous scrutiny of products difficult, especially given the light weight of fentanyl, suppliers find it relatively easy to hide illicit substances in licit shipments. Opioid users have made the switch to fentanyl, and have seen fentanyl added to cocaine and methamphetamine they buy on the streets.
OUD and buprenorphine
Fentanyl is one part of the overdose crisis. Opioid use disorder (OUD) is the other. Both need to be addressed if we are to make any progress in this epidemic of death and dependency.
The OUD crisis continues amid the pandemic – and isn’t going away.8 Slips, relapses, and overdoses are all too common. Medication-assisted treatment (MAT) and OUD treatment programs are essential parts of our response to overdose initiatives. After naloxone rescue, the best anti-overdose response is to get the OUD patient into treatment with MATs. Patients with OUD have continuously high risks of overdose. The best outcomes appear to be related to treatment duration of greater than 2 years. But it is common to see patients with OUDs who have been in treatment multiple times, taking MATs, dropping out, overdosing, and dying. Some have been described as treatment resistant.9 It is clear that treatment can work, but also that even evidence-based treatments often fail.10
A recent study compared OUD patients who continued treatment for 6-9 months to those patients who had continued MAT treatment for 15-18 months. The longer the treatment, the fewer emergencies, prescriptions, or hospitalizations.11
But this study reminds us that all OUD patients, whether they are currently buprenorphine treated or not, experience overdoses and emergency department interventions. Short and longer treatment groups have a similar nonfatal overdose rate, about 6%, and went to the emergency department at a high rate, above 40%. Discontinuation of buprenorphine treatment is a major risk factor in opioid relapse, emergency department visits, and overdose. Cures are not common. Whether an OUD patient is being treated or has been treated in the past, carrying naloxone (brand name Narcan), makes sense and can save lives.
Methadone still considered most effective
Methadone is a synthetic opioid first studied as a treatment for OUD at Rockefeller University in New York City in the 1960s. Methadone may be the most effective treatment for OUD in promoting treatment retention for years, decreasing intravenous drug use, and decreasing deaths.12 It has been studied and safely used in treatment programs for decades. Methadone is typically administered in a clinic, daily, and with observation. In addition, methadone patients periodically take urine drug tests, which can distinguish methadone from substances of abuse. They also receive counseling. But methadone can be prescribed and administered only in methadone clinics in the United States. It is available for prescription in primary care clinics in Great Britain, Canada, and Australia.13 Numerous experts have suggested passing new legislation aimed at changing how methadone can be prescribed. Allowing primary care to administer methadone, just like buprenorphine, can improve access and benefit OUD patients.12
Availability of Narcan is critical
A comprehensive treatment model for OUDs includes prescribing naloxone, encouraging those patients with an OUD and their loved ones to have naloxone with them, and providing MATs and appropriate therapies, such as counseling.
As described by Allison L. Pitt and colleagues at Stanford (Calif.) University,14 the United States might be on track to have up to 500,000 deaths tied to opioid overdoses that might occur over the next 5 years. They modeled the effect on overdose of a long list of interventions, but only a few had an impact. At the top of the list was naloxone availability. We need to focus on saving lives by increasing naloxone availability, improving initiation, and expanding access to MAT, and increasing psychosocial treatment to improve outcomes, increase life-years and quality-adjusted life-years, and reduce opioid-related deaths. When Ms. Pitt and colleagues looked at what would make the most impact in reducing OUD deaths, it was naloxone. Pain patients on higher doses of opioids, nonprescription opioid users, OUD patients should be given naloxone prescriptions. While many can give a Heimlich to a choking person or CPR, few have naloxone to rescue a person who has overdosed on opioids. If an overdose is suspected, it should be administered by anyone who has it, as soon as possible. Then, the person who is intervening should call 911.
What we can do today
At this moment, clinicians can follow the Surgeon General’s advice,15 and prescribe naloxone.
We should give naloxone to OUD patients and their families, to pain patients at dosages of greater than or equal to 50 MME. Our top priorities should be patients with comorbid pain syndromes, those being treated with benzodiazepines and sleeping medications, and patients with alcohol use disorders. This is also an important intervention for those who binge drink, and have sleep apnea, and heart and respiratory diseases.
Naloxone is available without a prescription in at least 43 states. Naloxone is available in harm reduction programs and in hospitals, and is carried by emergency medical staff, law enforcement, and EMTs. It also is available on the streets, though it does not appear to have a dollar value like opioids or even buprenorphine. Also, the availability of naloxone in pharmacies has made it easier for family members and caregivers of pain patients or those with OUD to have it to administer in an emergency.
An excellent place for MDs to start is to do more to encourage all patients with OUD to carry naloxone, for their loved ones to carry naloxone, and for their homes to have naloxone nearby in the bedroom or bathroom. It is not logical to expect a person with an OUD to rescue themselves. Current and past OUD patients, as well as their loved ones, are at high risk – and should have naloxone nearby at all times.
Naloxone reverses an opioid overdose, but it should be thought about like cardioversion or CPR rather than a treatment for an underlying disease. Increasing access to buprenorphine, buprenorphine + naloxone, and naltrexone treatment for OUDs is an important organizing principle. Initiation of MAT treatment in the emergency setting or most anywhere and any place a patient with an OUD can begin treatment is necessary. Treatment with buprenorphine or methadone reduces opioid overdose and opioid-related acute care use.16
Reducing racial disparities in OUD treatment is necessary, because buprenorphine treatment is concentrated among White patients who either use private insurance or are self-pay.17 Reducing barriers to methadone program licenses, expanding sites for distribution,18 prescribing methadone in an office setting might help. Clinicians can do a better job of explaining the risks associated with opioid prescriptions, including diversion and overdose, and the benefits of OUD treatment. So, To reduce opioid overdoses, we must increase physician competencies in addiction medicine.
Dr. Gold is professor of psychiatry (adjunct) at Washington University, St. Louis. He is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville. For more than 40 years, Dr. Gold has worked on developing models for understanding the effects of opioid, tobacco, cocaine, and other drugs, as well as food, on the brain and behavior. He disclosed financial ties with ADAPT Pharma and Magstim Ltd.
References
1. Kamp J. Overdose deaths rise, may reach record level, federal data show. Wall Street Journal. 2020 Jul 15.
2. 12 month–ending provisional number of drug overdose drugs. Centers for Disease Control and Prevention. 2020 Jul 5.
3. Katz J et al. In shadow of pandemic, U.S. drug overdose deaths resurge to record. New York Times. 2020 Jul 15.
4. Gold MS. The fentanyl crisis is only getting worse. Addiction Policy Forum. Updated 2020 Mar 12.
5. Gold MS. Mo Med. 2020-Mar-Apr;117(2):99-101.
6. Reports of increases in opioid-related overdoses and other concerns during the COVID-19 pandemic. American Medical Association. Issue brief. Updated 2020 Jul 20.
7. Pardo B et al. The future of fentanyl and other synthetic opioids. RAND report.
8. Gold MS. New challenges in the opioid epidemic. Addiction Policy Forum. 2020 Jun 4.
9. Patterson Silver Wolf DA and Gold MS. J Neurol Sci. 2020;411:116718.
10. Oesterle TS et al. Mayo Clin Proc. 2019;94(10):2072-86.
11. Connery HS and Weiss RD. Am J Psychiatry. 2020;177(2):104-6.
12. Kleber HD. JAMA. 2008;300(19):2303-5.
13. Samet JH et al. N Engl J Med. 2018;379(1):7-8.
14. Pitt AL et al. Am J Public Health. 2018;108(10):1394-1400.
15. U.S. Surgeon General’s Advisory on Naloxone and Opioid Overdose. hhs.gov.
16. Wakeman SE et al. JAMA Netw Open. 2020;3(2):e1920622.
17. Lagisetty PA et al. JAMA Psychiatry. 2019;76(9):979-81.
18. Kleinman RA. JAMA Psychiatry. 2020 Jul 15. doi: 10.1001/jamapsychiatry.2020.1624.
AMA urges change after dramatic increase in illicit opioid fatalities
In the past 5 years, there has been a significant drop in the use of prescription opioids and in deaths associated with such use; but at the same time there’s been a dramatic increase in fatalities involving illicit opioids and stimulants, a new report from the American Medical Association (AMA) Opioid Task Force shows.
Although the medical community has made some important progress against the opioid epidemic, with a 37% reduction in opioid prescribing since 2013, illicit drugs are now the dominant reason why drug overdoses kill more than 70,000 people each year, the report says.
In an effort to improve the situation, the AMA Opioid Task Force is urging the removal of barriers to evidence-based care for patients who have pain and for those who have substance use disorders (SUDs). The report notes that “red tape and misguided policies are grave dangers” to these patients.
“It is critically important as we see drug overdoses increasing that we work towards reducing barriers of care for substance use abusers,” Task Force Chair Patrice A. Harris, MD, said in an interview.
“At present, the status quo is killing far too many of our loved ones and wreaking havoc in our communities,” she said.
Dr. Harris noted that “a more coordinated/integrated approach” is needed to help individuals with SUDs.
“It is vitally important that these individuals can get access to treatment. Everyone deserves the opportunity for care,” she added.
Dramatic increases
The report cites figures from the Centers for Disease Control and Prevention that indicate the following regarding the period from the beginning of 2015 to the end of 2019:
- Deaths involving illicitly manufactured and fentanyl analogues increased from 5,766 to 36,509.
- Deaths involving stimulants such as increased from 4,402 to 16,279.
- Deaths involving cocaine increased from 5,496 to 15,974.
- Deaths involving heroin increased from 10,788 to 14,079.
- Deaths involving prescription opioids decreased from 12,269 to 11,904.
The report notes that deaths involving prescription opioids peaked in July 2017 at 15,003.
Some good news
In addition to the 37% reduction in opioid prescribing in recent years, the AMA lists other points of progress, such as a large increase in prescription drug monitoring program registrations. More than 1.8 million physicians and other healthcare professionals now participate in these programs.
Also, more physicians are now certified to treat opioid use disorder. More than 85,000 physicians, as well as a growing number of nurse practitioners and physician assistants, are now certified to treat patients in the office with buprenorphine. This represents an increase of more than 50,000 from 2017.
Access to naloxone is also increasing. More than 1 million naloxone prescriptions were dispensed in 2019 – nearly double the amount in 2018. This represents a 649% increase from 2017.
“We have made some good progress, but we can’t declare victory, and there are far too many barriers to getting treatment for substance use disorder,” Dr. Harris said.
“Policymakers, public health officials, and insurance companies need to come together to create a system where there are no barriers to care for people with substance use disorder and for those needing pain medications,” she added.
At present, prior authorization is often needed before these patients can receive medication. “This involves quite a bit of administration, filling in forms, making phone calls, and this is stopping people getting the care they need,” said Dr. Harris.
“This is a highly regulated environment. There are also regulations on the amount of methadone that can be prescribed and for the prescription of buprenorphine, which has to be initiated in person,” she said.
Will COVID-19 bring change?
Dr. Harris noted that some of these regulations have been relaxed during the COVID-19 crisis so that physicians could ensure that patients have continued access to medication, and she suggested that this may pave the way for the future.
“We need now to look at this carefully and have a conversation about whether these relaxations can be continued. But this would have to be evidence based. Perhaps we can use experience from the COVID-19 period to guide future policy on this,” she said.
The report highlights that despite medical society and patient advocacy, only 21 states and the District of Columbia have enacted laws that limit public and private insurers from imposing prior authorization requirements on SUD services or medications.
The Task Force urges removal of remaining prior authorizations, step therapy, and other inappropriate administrative burdens that delay or deny care for Food and Drug Administration–approved medications used as part of medication-assisted treatment for opioid use disorder.
The organization is also calling for better implementation of mental health and substance use disorder parity laws that require health insurers to provide the same level of benefits for mental health and SUD treatment and services that they do for medical/surgical care.
At present, only a few states have taken meaningful action to enact or enforce those laws, the report notes.
The Task Force also recommends the implementation of systems to track overdose and mortality trends to provide equitable public health interventions. These measures would include comprehensive, disaggregated racial and ethnic data collection related to testing, hospitalization, and mortality associated with opioids and other substances.
“We know that ending the drug overdose epidemic will not be easy, but if policymakers allow the status quo to continue, it will be impossible,” Dr. Harris said.
“ Physicians will continue to do our part. We urge policymakers to do theirs,” she added.
A version of this article originally appeared on Medscape.com.
In the past 5 years, there has been a significant drop in the use of prescription opioids and in deaths associated with such use; but at the same time there’s been a dramatic increase in fatalities involving illicit opioids and stimulants, a new report from the American Medical Association (AMA) Opioid Task Force shows.
Although the medical community has made some important progress against the opioid epidemic, with a 37% reduction in opioid prescribing since 2013, illicit drugs are now the dominant reason why drug overdoses kill more than 70,000 people each year, the report says.
In an effort to improve the situation, the AMA Opioid Task Force is urging the removal of barriers to evidence-based care for patients who have pain and for those who have substance use disorders (SUDs). The report notes that “red tape and misguided policies are grave dangers” to these patients.
“It is critically important as we see drug overdoses increasing that we work towards reducing barriers of care for substance use abusers,” Task Force Chair Patrice A. Harris, MD, said in an interview.
“At present, the status quo is killing far too many of our loved ones and wreaking havoc in our communities,” she said.
Dr. Harris noted that “a more coordinated/integrated approach” is needed to help individuals with SUDs.
“It is vitally important that these individuals can get access to treatment. Everyone deserves the opportunity for care,” she added.
Dramatic increases
The report cites figures from the Centers for Disease Control and Prevention that indicate the following regarding the period from the beginning of 2015 to the end of 2019:
- Deaths involving illicitly manufactured and fentanyl analogues increased from 5,766 to 36,509.
- Deaths involving stimulants such as increased from 4,402 to 16,279.
- Deaths involving cocaine increased from 5,496 to 15,974.
- Deaths involving heroin increased from 10,788 to 14,079.
- Deaths involving prescription opioids decreased from 12,269 to 11,904.
The report notes that deaths involving prescription opioids peaked in July 2017 at 15,003.
Some good news
In addition to the 37% reduction in opioid prescribing in recent years, the AMA lists other points of progress, such as a large increase in prescription drug monitoring program registrations. More than 1.8 million physicians and other healthcare professionals now participate in these programs.
Also, more physicians are now certified to treat opioid use disorder. More than 85,000 physicians, as well as a growing number of nurse practitioners and physician assistants, are now certified to treat patients in the office with buprenorphine. This represents an increase of more than 50,000 from 2017.
Access to naloxone is also increasing. More than 1 million naloxone prescriptions were dispensed in 2019 – nearly double the amount in 2018. This represents a 649% increase from 2017.
“We have made some good progress, but we can’t declare victory, and there are far too many barriers to getting treatment for substance use disorder,” Dr. Harris said.
“Policymakers, public health officials, and insurance companies need to come together to create a system where there are no barriers to care for people with substance use disorder and for those needing pain medications,” she added.
At present, prior authorization is often needed before these patients can receive medication. “This involves quite a bit of administration, filling in forms, making phone calls, and this is stopping people getting the care they need,” said Dr. Harris.
“This is a highly regulated environment. There are also regulations on the amount of methadone that can be prescribed and for the prescription of buprenorphine, which has to be initiated in person,” she said.
Will COVID-19 bring change?
Dr. Harris noted that some of these regulations have been relaxed during the COVID-19 crisis so that physicians could ensure that patients have continued access to medication, and she suggested that this may pave the way for the future.
“We need now to look at this carefully and have a conversation about whether these relaxations can be continued. But this would have to be evidence based. Perhaps we can use experience from the COVID-19 period to guide future policy on this,” she said.
The report highlights that despite medical society and patient advocacy, only 21 states and the District of Columbia have enacted laws that limit public and private insurers from imposing prior authorization requirements on SUD services or medications.
The Task Force urges removal of remaining prior authorizations, step therapy, and other inappropriate administrative burdens that delay or deny care for Food and Drug Administration–approved medications used as part of medication-assisted treatment for opioid use disorder.
The organization is also calling for better implementation of mental health and substance use disorder parity laws that require health insurers to provide the same level of benefits for mental health and SUD treatment and services that they do for medical/surgical care.
At present, only a few states have taken meaningful action to enact or enforce those laws, the report notes.
The Task Force also recommends the implementation of systems to track overdose and mortality trends to provide equitable public health interventions. These measures would include comprehensive, disaggregated racial and ethnic data collection related to testing, hospitalization, and mortality associated with opioids and other substances.
“We know that ending the drug overdose epidemic will not be easy, but if policymakers allow the status quo to continue, it will be impossible,” Dr. Harris said.
“ Physicians will continue to do our part. We urge policymakers to do theirs,” she added.
A version of this article originally appeared on Medscape.com.
In the past 5 years, there has been a significant drop in the use of prescription opioids and in deaths associated with such use; but at the same time there’s been a dramatic increase in fatalities involving illicit opioids and stimulants, a new report from the American Medical Association (AMA) Opioid Task Force shows.
Although the medical community has made some important progress against the opioid epidemic, with a 37% reduction in opioid prescribing since 2013, illicit drugs are now the dominant reason why drug overdoses kill more than 70,000 people each year, the report says.
In an effort to improve the situation, the AMA Opioid Task Force is urging the removal of barriers to evidence-based care for patients who have pain and for those who have substance use disorders (SUDs). The report notes that “red tape and misguided policies are grave dangers” to these patients.
“It is critically important as we see drug overdoses increasing that we work towards reducing barriers of care for substance use abusers,” Task Force Chair Patrice A. Harris, MD, said in an interview.
“At present, the status quo is killing far too many of our loved ones and wreaking havoc in our communities,” she said.
Dr. Harris noted that “a more coordinated/integrated approach” is needed to help individuals with SUDs.
“It is vitally important that these individuals can get access to treatment. Everyone deserves the opportunity for care,” she added.
Dramatic increases
The report cites figures from the Centers for Disease Control and Prevention that indicate the following regarding the period from the beginning of 2015 to the end of 2019:
- Deaths involving illicitly manufactured and fentanyl analogues increased from 5,766 to 36,509.
- Deaths involving stimulants such as increased from 4,402 to 16,279.
- Deaths involving cocaine increased from 5,496 to 15,974.
- Deaths involving heroin increased from 10,788 to 14,079.
- Deaths involving prescription opioids decreased from 12,269 to 11,904.
The report notes that deaths involving prescription opioids peaked in July 2017 at 15,003.
Some good news
In addition to the 37% reduction in opioid prescribing in recent years, the AMA lists other points of progress, such as a large increase in prescription drug monitoring program registrations. More than 1.8 million physicians and other healthcare professionals now participate in these programs.
Also, more physicians are now certified to treat opioid use disorder. More than 85,000 physicians, as well as a growing number of nurse practitioners and physician assistants, are now certified to treat patients in the office with buprenorphine. This represents an increase of more than 50,000 from 2017.
Access to naloxone is also increasing. More than 1 million naloxone prescriptions were dispensed in 2019 – nearly double the amount in 2018. This represents a 649% increase from 2017.
“We have made some good progress, but we can’t declare victory, and there are far too many barriers to getting treatment for substance use disorder,” Dr. Harris said.
“Policymakers, public health officials, and insurance companies need to come together to create a system where there are no barriers to care for people with substance use disorder and for those needing pain medications,” she added.
At present, prior authorization is often needed before these patients can receive medication. “This involves quite a bit of administration, filling in forms, making phone calls, and this is stopping people getting the care they need,” said Dr. Harris.
“This is a highly regulated environment. There are also regulations on the amount of methadone that can be prescribed and for the prescription of buprenorphine, which has to be initiated in person,” she said.
Will COVID-19 bring change?
Dr. Harris noted that some of these regulations have been relaxed during the COVID-19 crisis so that physicians could ensure that patients have continued access to medication, and she suggested that this may pave the way for the future.
“We need now to look at this carefully and have a conversation about whether these relaxations can be continued. But this would have to be evidence based. Perhaps we can use experience from the COVID-19 period to guide future policy on this,” she said.
The report highlights that despite medical society and patient advocacy, only 21 states and the District of Columbia have enacted laws that limit public and private insurers from imposing prior authorization requirements on SUD services or medications.
The Task Force urges removal of remaining prior authorizations, step therapy, and other inappropriate administrative burdens that delay or deny care for Food and Drug Administration–approved medications used as part of medication-assisted treatment for opioid use disorder.
The organization is also calling for better implementation of mental health and substance use disorder parity laws that require health insurers to provide the same level of benefits for mental health and SUD treatment and services that they do for medical/surgical care.
At present, only a few states have taken meaningful action to enact or enforce those laws, the report notes.
The Task Force also recommends the implementation of systems to track overdose and mortality trends to provide equitable public health interventions. These measures would include comprehensive, disaggregated racial and ethnic data collection related to testing, hospitalization, and mortality associated with opioids and other substances.
“We know that ending the drug overdose epidemic will not be easy, but if policymakers allow the status quo to continue, it will be impossible,” Dr. Harris said.
“ Physicians will continue to do our part. We urge policymakers to do theirs,” she added.
A version of this article originally appeared on Medscape.com.
















