User login
Data Trends 2023: Limb Loss and Prostheses
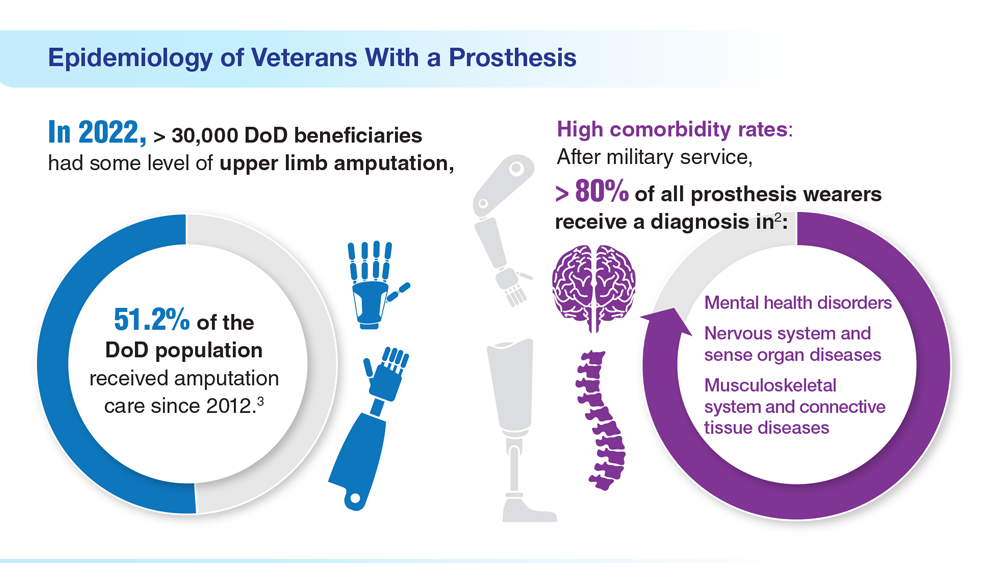
- US Department of Veterans Affairs. Amputation system of care [fact sheet]. Published December 2022. Accessed April 21, 2023. https://www.prosthetics.va.gov/factsheet/ASoC-FactSheet.pdf
- US Department of Veterans Affairs, Office of the Inspector General. Veteran Affairs Inspector General healthcare inspection: prosthetic limb care in VA facilities. Published March 8, 2012. Accessed April 21, 2023. https://www.va.gov/oig/pubs/VAOIG-11-02138-116.pdf
- Department of Veterans Affairs; Department of Defense. Clinical Practice Guideline for the Management of Upper Limb Amputation Rehabilitation. Patient Summary. Published March 2022. Accessed April 10, 2023. https://www.healthquality.va.gov/guidelines/Rehab/ULA/VADoDULACPG_PatientSummary_Final_508.pdf
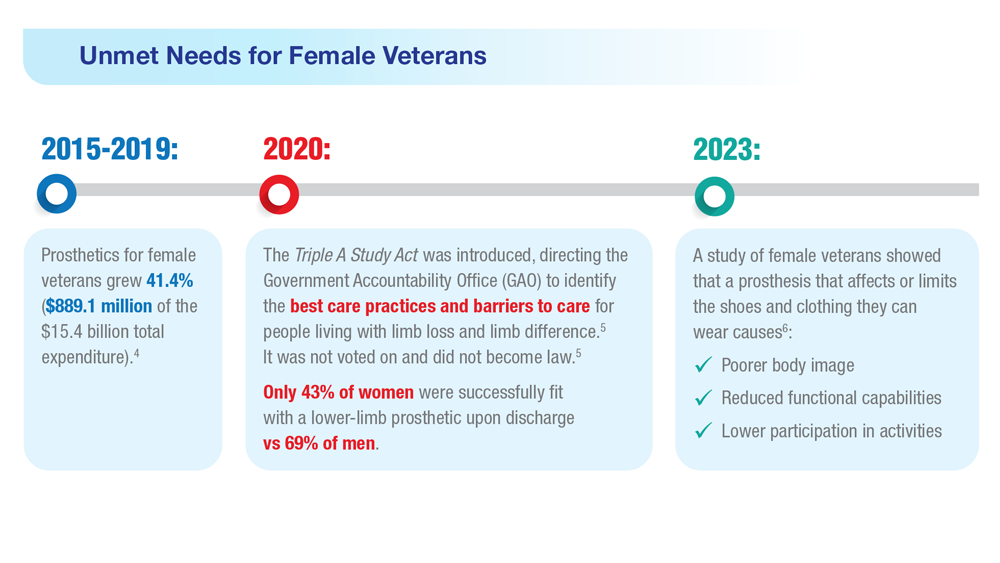
- US Government Accountability Office, Report to Congressional Committees. Veterans Health Care: Agency efforts to provide and study prosthetics for small but growing female veteran population. Published November 2020. Accessed April 21, 2023. https://www.gao.gov/assets/gao-21-60.pdf
- 117th Congress. Access to Assistive Technology and Devices for Americans Study Act or the Triple A Study Act (H.R.2461). April 13, 2021. Accessed April 21, 2023. https://www.congress.gov/bill/117th-congress/housebill/2461
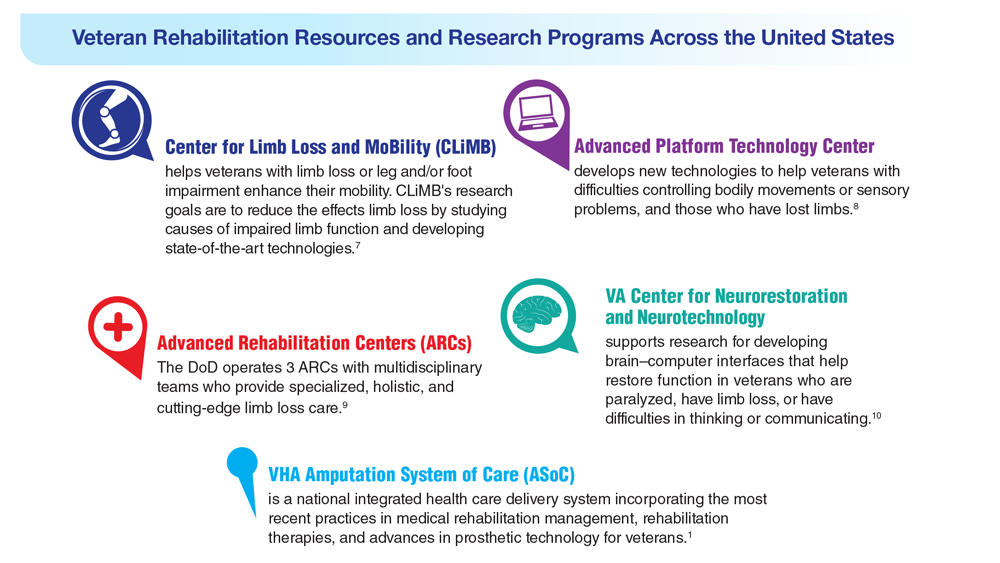
- Russell Esposito E, et al. Prosthet Orthot Int. 2023 Jan 2023. Online ahead of print. doi:10.1097/PXR.0000000000000192
- US Department of Veterans Affairs. Center for Limb Loss and MoBility. Updated January 27, 2022. Accessed April 21, 2023. https://www.amputation.research.va.gov/
- US Department of Veterans Affairs. Advanced Platform Technology Center. Accessed April 21, 2023. https://www.aptcenter.research.va.gov
- Sanchez-Bustamante C. Limb loss: DHA's three advanced rehab centers provide holistic care. Medicine and the Military. Published May 3, 2022. Accessed April 21, 2023. https://health.mil/News/Articles/2022/05/04/Limb-Loss-DHAs-Three-Advanced-Rehab-Centers-Provide-Holistic-Care
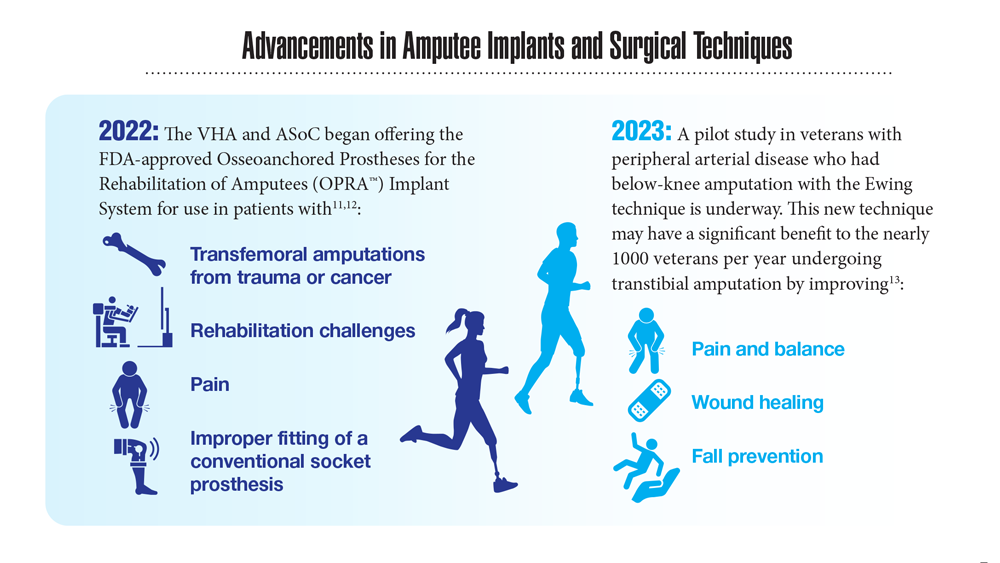
- Center for Neurorestoration and Neurotechnology. VA Providence Healthcare System. Accessed April 21, 2023. https://centerforneuro.org
- Webster JB. OPRA™ patient information sheet. US Department of Veteran Affairs, Rehabilitation and Prosthetic Services. Accessed April 21, 2023. https://www.rehab.va.gov/PROSTHETICS/asoc/resources/OPRA-PatientInformation.pdf
- Hoyt BW, et al. Expert Rev Med Devices. 2020;17(1):17-25. doi:10.1080/17434440.2020.1704623
- Ewing amputation in veterans with PAD undergoing BKA. ClinicalTrials.gov. Updated October 31, 2022. Accessed April 21, 2023. https://www.clinicaltrials.gov/ct2/show/NCT05437562
- US Department of Veterans Affairs. Amputation system of care [fact sheet]. Published December 2022. Accessed April 21, 2023. https://www.prosthetics.va.gov/factsheet/ASoC-FactSheet.pdf
- US Department of Veterans Affairs, Office of the Inspector General. Veteran Affairs Inspector General healthcare inspection: prosthetic limb care in VA facilities. Published March 8, 2012. Accessed April 21, 2023. https://www.va.gov/oig/pubs/VAOIG-11-02138-116.pdf
- Department of Veterans Affairs; Department of Defense. Clinical Practice Guideline for the Management of Upper Limb Amputation Rehabilitation. Patient Summary. Published March 2022. Accessed April 10, 2023. https://www.healthquality.va.gov/guidelines/Rehab/ULA/VADoDULACPG_PatientSummary_Final_508.pdf
- US Government Accountability Office, Report to Congressional Committees. Veterans Health Care: Agency efforts to provide and study prosthetics for small but growing female veteran population. Published November 2020. Accessed April 21, 2023. https://www.gao.gov/assets/gao-21-60.pdf
- 117th Congress. Access to Assistive Technology and Devices for Americans Study Act or the Triple A Study Act (H.R.2461). April 13, 2021. Accessed April 21, 2023. https://www.congress.gov/bill/117th-congress/housebill/2461
- Russell Esposito E, et al. Prosthet Orthot Int. 2023 Jan 2023. Online ahead of print. doi:10.1097/PXR.0000000000000192
- US Department of Veterans Affairs. Center for Limb Loss and MoBility. Updated January 27, 2022. Accessed April 21, 2023. https://www.amputation.research.va.gov/
- US Department of Veterans Affairs. Advanced Platform Technology Center. Accessed April 21, 2023. https://www.aptcenter.research.va.gov
- Sanchez-Bustamante C. Limb loss: DHA's three advanced rehab centers provide holistic care. Medicine and the Military. Published May 3, 2022. Accessed April 21, 2023. https://health.mil/News/Articles/2022/05/04/Limb-Loss-DHAs-Three-Advanced-Rehab-Centers-Provide-Holistic-Care
- Center for Neurorestoration and Neurotechnology. VA Providence Healthcare System. Accessed April 21, 2023. https://centerforneuro.org
- Webster JB. OPRA™ patient information sheet. US Department of Veteran Affairs, Rehabilitation and Prosthetic Services. Accessed April 21, 2023. https://www.rehab.va.gov/PROSTHETICS/asoc/resources/OPRA-PatientInformation.pdf
- Hoyt BW, et al. Expert Rev Med Devices. 2020;17(1):17-25. doi:10.1080/17434440.2020.1704623
- Ewing amputation in veterans with PAD undergoing BKA. ClinicalTrials.gov. Updated October 31, 2022. Accessed April 21, 2023. https://www.clinicaltrials.gov/ct2/show/NCT05437562
- US Department of Veterans Affairs. Amputation system of care [fact sheet]. Published December 2022. Accessed April 21, 2023. https://www.prosthetics.va.gov/factsheet/ASoC-FactSheet.pdf
- US Department of Veterans Affairs, Office of the Inspector General. Veteran Affairs Inspector General healthcare inspection: prosthetic limb care in VA facilities. Published March 8, 2012. Accessed April 21, 2023. https://www.va.gov/oig/pubs/VAOIG-11-02138-116.pdf
- Department of Veterans Affairs; Department of Defense. Clinical Practice Guideline for the Management of Upper Limb Amputation Rehabilitation. Patient Summary. Published March 2022. Accessed April 10, 2023. https://www.healthquality.va.gov/guidelines/Rehab/ULA/VADoDULACPG_PatientSummary_Final_508.pdf
- US Government Accountability Office, Report to Congressional Committees. Veterans Health Care: Agency efforts to provide and study prosthetics for small but growing female veteran population. Published November 2020. Accessed April 21, 2023. https://www.gao.gov/assets/gao-21-60.pdf
- 117th Congress. Access to Assistive Technology and Devices for Americans Study Act or the Triple A Study Act (H.R.2461). April 13, 2021. Accessed April 21, 2023. https://www.congress.gov/bill/117th-congress/housebill/2461
- Russell Esposito E, et al. Prosthet Orthot Int. 2023 Jan 2023. Online ahead of print. doi:10.1097/PXR.0000000000000192
- US Department of Veterans Affairs. Center for Limb Loss and MoBility. Updated January 27, 2022. Accessed April 21, 2023. https://www.amputation.research.va.gov/
- US Department of Veterans Affairs. Advanced Platform Technology Center. Accessed April 21, 2023. https://www.aptcenter.research.va.gov
- Sanchez-Bustamante C. Limb loss: DHA's three advanced rehab centers provide holistic care. Medicine and the Military. Published May 3, 2022. Accessed April 21, 2023. https://health.mil/News/Articles/2022/05/04/Limb-Loss-DHAs-Three-Advanced-Rehab-Centers-Provide-Holistic-Care
- Center for Neurorestoration and Neurotechnology. VA Providence Healthcare System. Accessed April 21, 2023. https://centerforneuro.org
- Webster JB. OPRA™ patient information sheet. US Department of Veteran Affairs, Rehabilitation and Prosthetic Services. Accessed April 21, 2023. https://www.rehab.va.gov/PROSTHETICS/asoc/resources/OPRA-PatientInformation.pdf
- Hoyt BW, et al. Expert Rev Med Devices. 2020;17(1):17-25. doi:10.1080/17434440.2020.1704623
- Ewing amputation in veterans with PAD undergoing BKA. ClinicalTrials.gov. Updated October 31, 2022. Accessed April 21, 2023. https://www.clinicaltrials.gov/ct2/show/NCT05437562
Federal Health Care Data Trends 2023

In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers

In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers

In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Lack of medical device tracking leaves patients vulnerable
.
As a result of this siloing of information, patients are not getting the expected benefits of a regulation finalized over a decade ago by the U.S. Food and Drug Administration.
In 2013, the agency ordered companies to include unique device identifiers (UDIs) in plain-text and barcode format on some device labels, starting with implanted devices that are considered life-sustaining. The FDA said that tracking of UDI information would speed detection of complications linked to devices.
But identifiers are rarely on devices. At the time of the regulation creation, the FDA also said it expected this data would be integrated into EHRs. But only a few pioneer organizations such as Duke University and Mercy Health have so far attempted to track any UDI data in an organized way, researchers say.
Richard J. Kovacs, MD, the chief medical officer of the American College of Cardiology, contrasted the lack of useful implementation of UDI data with the speedy transfers of information that happen routinely in other industries. For example, employees of car rental agencies use handheld devices to gather detailed information about the vehicles being returned.
“But if you go to an emergency room with a medical device in your body, no one knows what it is or where it came from or anything about it,” Dr. Kovacs said in an interview.
Many physicians with expertise in device research have pushed for years to have insurers like Medicare require identification information on medical claims.
Even researchers face multiple obstacles in trying to investigate how well UDIs have been incorporated into EHRs and outcomes tied to certain devices.
In August, a Harvard team published a study in JAMA Internal Medicine, attempting to analyze the risks of endovascular aortic repair (EVAR) devices. They reported an 11.6% risk for serious blood leaks with AFX Endovascular AAA System aneurysm devices, more than double the 5.7% risk estimated for competing products. The team selected EVAR devices for the study due in part to their known safety concerns. Endologix, the maker of the devices, declined to comment for this story.
The Harvard team used data from the Veterans Affairs health system, which is considered more well organized than most other health systems. But UDI information was found for only 19 of the 13,941 patients whose records were studied. In those cases, only partial information was included.
The researchers developed natural language processing tools, which they used to scrounge clinical notes for information about which devices patients received.
Using this method isn’t feasible for most clinicians, given that records from independent hospitals might not provide this kind of data and descriptions to search, according to the authors of an editorial accompanying the paper. Those researchers urged Congress to pass a law mandating inclusion of UDIs for all devices on claims forms as a condition for reimbursement by federal health care programs.
Setback for advocates
The movement toward UDI suffered a setback in June.
An influential, but little known federal advisory panel, the National Committee on Vital Health Statistics (NCVHS), opted to not recommend use of this information in claims, saying the FDA should consider the matter further.
Gaining an NCVHS recommendation would have been a win, said Sen. Elizabeth Warren (D-MA), Sen. Charles E. Grassley (R-IA), and Rep. Bill Pascrell Jr. (D-NJ), in a December 2022 letter to the panel.
Including UDI data would let researchers track patients’ interactions with a health system and could be used to establish population-level correlations between a particular device and a long-term outcome or side effect, the lawmakers said.
That view had the support of at least one major maker of devices, Cook Group, which sells products for a variety of specialties, including cardiology.
In a comment to NCVHS, Cook urged for the inclusion identifiers in Medicare claims.
“While some have argued that the UDI is better suited for inclusion in the electronic health records, Cook believes this argument sets up a false choice between the two,” wrote Stephen L. Ferguson, JD, the chairman of Cook’s board. “Inclusion of the UDI in both electronic health records and claims forms will lead to a more robust system of real-world data.”
In contrast, AdvaMed, the trade group for device makers, told the NCVHS that it did not support adding the information to payment claims submissions, instead just supporting the inclusion in EHRs.
Dr. Kovacs of the ACC said one potential drawback to more transparency could be challenges in interpreting reports of complications in certain cases, at least initially. Reports about a flaw or even a suspected flaw in a device might lead patients to become concerned about their implanted devices, potentially registering unfounded complaints.
But this concern can be addressed through using “scientific rigor and safeguards” and is outweighed by the potential safety benefits for patients, Dr. Kovacs said.
Patients should ask health care systems to track and share information about their implanted devices, Dr. Kovacs suggested.
“I feel it would be my right to demand that that device information follows my electronic medical record, so that it’s readily available to anyone who’s taking care of me,” Dr. Kovacs said. “They would know what it is that’s in me, whether it’s a lens in my eye or a prosthesis in my hip or a highly complicated implantable cardiac electronic device.”
The Harvard study was supported by the FDA and National Institutes of Health. Authors of the study reported receiving fees from the FDA, Burroughs Wellcome Fund, and Harvard-MIT Center for Regulatory Science outside the submitted work. No other disclosures were reported. Authors of the editorial reported past and present connections with F-Prime Capital, FDA, Johnson & Johnson, the Medical Devices Innovation Consortium; the Agency for Healthcare Research and Quality; the National Heart, Lung, and Blood Institute; and Arnold Ventures, as well being an expert witness at in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen. Authors of the Viewpoint reported past and present connections with the National Evaluation System for Health Technology Coordinating Center (NESTcc), which is part of the Medical Device Innovation Consortium (MDIC); AIM North America UDI Advisory Committee, Mass General Brigham, Arnold Ventures; the Institute for Clinical and Economic Review California Technology Assessment Forum; Yale University, Johnson & Johnson, FD, Agency for Healthcare Research and Quality; the National Heart, Lung, and Blood Institute of the National Institutes of Health; as well as having been an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against.
A version of this article first appeared on Medscape.com.
.
As a result of this siloing of information, patients are not getting the expected benefits of a regulation finalized over a decade ago by the U.S. Food and Drug Administration.
In 2013, the agency ordered companies to include unique device identifiers (UDIs) in plain-text and barcode format on some device labels, starting with implanted devices that are considered life-sustaining. The FDA said that tracking of UDI information would speed detection of complications linked to devices.
But identifiers are rarely on devices. At the time of the regulation creation, the FDA also said it expected this data would be integrated into EHRs. But only a few pioneer organizations such as Duke University and Mercy Health have so far attempted to track any UDI data in an organized way, researchers say.
Richard J. Kovacs, MD, the chief medical officer of the American College of Cardiology, contrasted the lack of useful implementation of UDI data with the speedy transfers of information that happen routinely in other industries. For example, employees of car rental agencies use handheld devices to gather detailed information about the vehicles being returned.
“But if you go to an emergency room with a medical device in your body, no one knows what it is or where it came from or anything about it,” Dr. Kovacs said in an interview.
Many physicians with expertise in device research have pushed for years to have insurers like Medicare require identification information on medical claims.
Even researchers face multiple obstacles in trying to investigate how well UDIs have been incorporated into EHRs and outcomes tied to certain devices.
In August, a Harvard team published a study in JAMA Internal Medicine, attempting to analyze the risks of endovascular aortic repair (EVAR) devices. They reported an 11.6% risk for serious blood leaks with AFX Endovascular AAA System aneurysm devices, more than double the 5.7% risk estimated for competing products. The team selected EVAR devices for the study due in part to their known safety concerns. Endologix, the maker of the devices, declined to comment for this story.
The Harvard team used data from the Veterans Affairs health system, which is considered more well organized than most other health systems. But UDI information was found for only 19 of the 13,941 patients whose records were studied. In those cases, only partial information was included.
The researchers developed natural language processing tools, which they used to scrounge clinical notes for information about which devices patients received.
Using this method isn’t feasible for most clinicians, given that records from independent hospitals might not provide this kind of data and descriptions to search, according to the authors of an editorial accompanying the paper. Those researchers urged Congress to pass a law mandating inclusion of UDIs for all devices on claims forms as a condition for reimbursement by federal health care programs.
Setback for advocates
The movement toward UDI suffered a setback in June.
An influential, but little known federal advisory panel, the National Committee on Vital Health Statistics (NCVHS), opted to not recommend use of this information in claims, saying the FDA should consider the matter further.
Gaining an NCVHS recommendation would have been a win, said Sen. Elizabeth Warren (D-MA), Sen. Charles E. Grassley (R-IA), and Rep. Bill Pascrell Jr. (D-NJ), in a December 2022 letter to the panel.
Including UDI data would let researchers track patients’ interactions with a health system and could be used to establish population-level correlations between a particular device and a long-term outcome or side effect, the lawmakers said.
That view had the support of at least one major maker of devices, Cook Group, which sells products for a variety of specialties, including cardiology.
In a comment to NCVHS, Cook urged for the inclusion identifiers in Medicare claims.
“While some have argued that the UDI is better suited for inclusion in the electronic health records, Cook believes this argument sets up a false choice between the two,” wrote Stephen L. Ferguson, JD, the chairman of Cook’s board. “Inclusion of the UDI in both electronic health records and claims forms will lead to a more robust system of real-world data.”
In contrast, AdvaMed, the trade group for device makers, told the NCVHS that it did not support adding the information to payment claims submissions, instead just supporting the inclusion in EHRs.
Dr. Kovacs of the ACC said one potential drawback to more transparency could be challenges in interpreting reports of complications in certain cases, at least initially. Reports about a flaw or even a suspected flaw in a device might lead patients to become concerned about their implanted devices, potentially registering unfounded complaints.
But this concern can be addressed through using “scientific rigor and safeguards” and is outweighed by the potential safety benefits for patients, Dr. Kovacs said.
Patients should ask health care systems to track and share information about their implanted devices, Dr. Kovacs suggested.
“I feel it would be my right to demand that that device information follows my electronic medical record, so that it’s readily available to anyone who’s taking care of me,” Dr. Kovacs said. “They would know what it is that’s in me, whether it’s a lens in my eye or a prosthesis in my hip or a highly complicated implantable cardiac electronic device.”
The Harvard study was supported by the FDA and National Institutes of Health. Authors of the study reported receiving fees from the FDA, Burroughs Wellcome Fund, and Harvard-MIT Center for Regulatory Science outside the submitted work. No other disclosures were reported. Authors of the editorial reported past and present connections with F-Prime Capital, FDA, Johnson & Johnson, the Medical Devices Innovation Consortium; the Agency for Healthcare Research and Quality; the National Heart, Lung, and Blood Institute; and Arnold Ventures, as well being an expert witness at in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen. Authors of the Viewpoint reported past and present connections with the National Evaluation System for Health Technology Coordinating Center (NESTcc), which is part of the Medical Device Innovation Consortium (MDIC); AIM North America UDI Advisory Committee, Mass General Brigham, Arnold Ventures; the Institute for Clinical and Economic Review California Technology Assessment Forum; Yale University, Johnson & Johnson, FD, Agency for Healthcare Research and Quality; the National Heart, Lung, and Blood Institute of the National Institutes of Health; as well as having been an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against.
A version of this article first appeared on Medscape.com.
.
As a result of this siloing of information, patients are not getting the expected benefits of a regulation finalized over a decade ago by the U.S. Food and Drug Administration.
In 2013, the agency ordered companies to include unique device identifiers (UDIs) in plain-text and barcode format on some device labels, starting with implanted devices that are considered life-sustaining. The FDA said that tracking of UDI information would speed detection of complications linked to devices.
But identifiers are rarely on devices. At the time of the regulation creation, the FDA also said it expected this data would be integrated into EHRs. But only a few pioneer organizations such as Duke University and Mercy Health have so far attempted to track any UDI data in an organized way, researchers say.
Richard J. Kovacs, MD, the chief medical officer of the American College of Cardiology, contrasted the lack of useful implementation of UDI data with the speedy transfers of information that happen routinely in other industries. For example, employees of car rental agencies use handheld devices to gather detailed information about the vehicles being returned.
“But if you go to an emergency room with a medical device in your body, no one knows what it is or where it came from or anything about it,” Dr. Kovacs said in an interview.
Many physicians with expertise in device research have pushed for years to have insurers like Medicare require identification information on medical claims.
Even researchers face multiple obstacles in trying to investigate how well UDIs have been incorporated into EHRs and outcomes tied to certain devices.
In August, a Harvard team published a study in JAMA Internal Medicine, attempting to analyze the risks of endovascular aortic repair (EVAR) devices. They reported an 11.6% risk for serious blood leaks with AFX Endovascular AAA System aneurysm devices, more than double the 5.7% risk estimated for competing products. The team selected EVAR devices for the study due in part to their known safety concerns. Endologix, the maker of the devices, declined to comment for this story.
The Harvard team used data from the Veterans Affairs health system, which is considered more well organized than most other health systems. But UDI information was found for only 19 of the 13,941 patients whose records were studied. In those cases, only partial information was included.
The researchers developed natural language processing tools, which they used to scrounge clinical notes for information about which devices patients received.
Using this method isn’t feasible for most clinicians, given that records from independent hospitals might not provide this kind of data and descriptions to search, according to the authors of an editorial accompanying the paper. Those researchers urged Congress to pass a law mandating inclusion of UDIs for all devices on claims forms as a condition for reimbursement by federal health care programs.
Setback for advocates
The movement toward UDI suffered a setback in June.
An influential, but little known federal advisory panel, the National Committee on Vital Health Statistics (NCVHS), opted to not recommend use of this information in claims, saying the FDA should consider the matter further.
Gaining an NCVHS recommendation would have been a win, said Sen. Elizabeth Warren (D-MA), Sen. Charles E. Grassley (R-IA), and Rep. Bill Pascrell Jr. (D-NJ), in a December 2022 letter to the panel.
Including UDI data would let researchers track patients’ interactions with a health system and could be used to establish population-level correlations between a particular device and a long-term outcome or side effect, the lawmakers said.
That view had the support of at least one major maker of devices, Cook Group, which sells products for a variety of specialties, including cardiology.
In a comment to NCVHS, Cook urged for the inclusion identifiers in Medicare claims.
“While some have argued that the UDI is better suited for inclusion in the electronic health records, Cook believes this argument sets up a false choice between the two,” wrote Stephen L. Ferguson, JD, the chairman of Cook’s board. “Inclusion of the UDI in both electronic health records and claims forms will lead to a more robust system of real-world data.”
In contrast, AdvaMed, the trade group for device makers, told the NCVHS that it did not support adding the information to payment claims submissions, instead just supporting the inclusion in EHRs.
Dr. Kovacs of the ACC said one potential drawback to more transparency could be challenges in interpreting reports of complications in certain cases, at least initially. Reports about a flaw or even a suspected flaw in a device might lead patients to become concerned about their implanted devices, potentially registering unfounded complaints.
But this concern can be addressed through using “scientific rigor and safeguards” and is outweighed by the potential safety benefits for patients, Dr. Kovacs said.
Patients should ask health care systems to track and share information about their implanted devices, Dr. Kovacs suggested.
“I feel it would be my right to demand that that device information follows my electronic medical record, so that it’s readily available to anyone who’s taking care of me,” Dr. Kovacs said. “They would know what it is that’s in me, whether it’s a lens in my eye or a prosthesis in my hip or a highly complicated implantable cardiac electronic device.”
The Harvard study was supported by the FDA and National Institutes of Health. Authors of the study reported receiving fees from the FDA, Burroughs Wellcome Fund, and Harvard-MIT Center for Regulatory Science outside the submitted work. No other disclosures were reported. Authors of the editorial reported past and present connections with F-Prime Capital, FDA, Johnson & Johnson, the Medical Devices Innovation Consortium; the Agency for Healthcare Research and Quality; the National Heart, Lung, and Blood Institute; and Arnold Ventures, as well being an expert witness at in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen. Authors of the Viewpoint reported past and present connections with the National Evaluation System for Health Technology Coordinating Center (NESTcc), which is part of the Medical Device Innovation Consortium (MDIC); AIM North America UDI Advisory Committee, Mass General Brigham, Arnold Ventures; the Institute for Clinical and Economic Review California Technology Assessment Forum; Yale University, Johnson & Johnson, FD, Agency for Healthcare Research and Quality; the National Heart, Lung, and Blood Institute of the National Institutes of Health; as well as having been an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against.
A version of this article first appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>165281</fileName> <TBEID>0C04C6BA.SIG</TBEID> <TBUniqueIdentifier>MD_0C04C6BA</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20230928T125300</QCDate> <firstPublished>20230928T134929</firstPublished> <LastPublished>20230928T134929</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20230928T134929</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Kerry Dooley Young</byline> <bylineText>KERRY DOOLEY YOUNG</bylineText> <bylineFull>KERRY DOOLEY YOUNG</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType/> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Some physicians are frustrated that key information about implantable medical devices rarely makes it into electronic health records, despite a 10-year mandate </metaDescription> <articlePDF/> <teaserImage/> <teaser>As a result of this siloing of information, patients are not getting the expected benefits of a regulation finalized over a decade ago by the FDA.</teaser> <title>Lack of medical device tracking leaves patients vulnerable</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>chph</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>rn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">6</term> <term>26</term> <term>15</term> </publications> <sections> <term canonical="true">39313</term> </sections> <topics> <term>264</term> <term>194</term> <term canonical="true">27442</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Lack of medical device tracking leaves patients vulnerable</title> <deck/> </itemMeta> <itemContent> <p><span class="tag metaDescription">Some physicians are frustrated that key information about implantable medical devices rarely makes it into electronic health records, despite a 10-year mandate on manufacturers to label these products with identifiers</span>.</p> <p>As a result of this siloing of information, patients are not getting the expected benefits of a regulation finalized over a decade ago by the U.S. Food and Drug Administration.<br/><br/>In 2013, the agency ordered companies to include unique device identifiers (UDIs) in plain-text and barcode format on some device labels, starting with implanted devices that are considered life-sustaining. The FDA said that tracking of UDI information would speed detection of complications linked to devices.<br/><br/>But identifiers are rarely on devices. At the time of the regulation creation, the FDA also said it expected this data would be integrated into EHRs. But only a few pioneer organizations such as <a href="https://nestcc.org/wp-content/uploads/NESTcc-UDI-Playbook_11-15-2022.pdf">Duke University and Mercy Health</a> have so far attempted to track any UDI data in an organized way, <a href="https://www.healthaffairs.org/content/forefront/unique-device-identifier-unique-opportunity">researchers say</a>.<br/><br/>Richard J. Kovacs, MD, the chief medical officer of the American College of Cardiology, contrasted the lack of useful implementation of UDI data with the speedy transfers of information that happen routinely in other industries. For example, employees of car rental agencies use handheld devices to gather detailed information about the vehicles being returned.<br/><br/>“But if you go to an emergency room with a medical device in your body, no one knows what it is or where it came from or anything about it,” Dr. Kovacs said in an interview.<br/><br/>Many physicians with expertise in device research <a href="https://www.acc.org/Latest-in-Cardiology/Articles/2017/11/16/14/47/ACC-Joins-Letter-of-Support-for-Adding-UDIs-to-Claims-Forms">have pushed for years</a> to have insurers like Medicare require identification information on medical claims.<br/><br/>Even researchers face multiple obstacles in trying to investigate how well UDIs have been incorporated into EHRs and outcomes tied to certain devices.<br/><br/>In August, a Harvard team published <a href="https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/2808239">a study</a> in JAMA Internal Medicine, attempting to analyze the risks of endovascular aortic repair (EVAR) devices. They reported an 11.6% risk for serious blood leaks with AFX Endovascular AAA System aneurysm devices, more than double the 5.7% risk estimated for competing products. The team selected EVAR devices for the study due in part to their <a href="https://www.medscape.com/viewarticle/969402">known safety concerns</a>. Endologix, the maker of the devices, declined to comment for this story.<br/><br/>The Harvard team used data from the Veterans Affairs health system, which is considered more well organized than most other health systems. But UDI information was found for only 19 of the 13,941 patients whose records were studied. In those cases, only partial information was included.<br/><br/>The researchers developed natural language processing tools, which they used to scrounge clinical notes for information about which devices patients received.<br/><br/>Using this method isn’t feasible for most clinicians, given that records from independent hospitals might not provide this kind of data and descriptions to search, according to the authors of <a href="https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2808242">an editorial</a> accompanying the paper. Those researchers urged Congress to pass a law mandating inclusion of UDIs for all devices on claims forms as a condition for reimbursement by federal health care programs.<br/><br/></p> <h2>Setback for advocates</h2> <p>The movement toward UDI suffered a setback in June.</p> <p>An influential, but little known federal advisory panel, the National Committee on Vital Health Statistics (NCVHS), opted to not recommend use of this information in claims, saying the FDA should consider the matter further.<br/><br/>Gaining an NCVHS recommendation would have been a win, said Sen. Elizabeth Warren (D-MA), Sen. Charles E. Grassley (R-IA), and Rep. Bill Pascrell Jr. (D-NJ), in a December 2022 letter to the panel.<br/><br/>Including UDI data would let researchers track patients’ interactions with a health system and could be used to establish population-level correlations between a particular device and a long-term outcome or side effect, the lawmakers said.<br/><br/>That view had the support of at least one major maker of devices, Cook Group, which sells products for a variety of specialties, including cardiology.<br/><br/>In a comment to NCVHS, Cook urged for the inclusion identifiers in Medicare claims.<br/><br/>“While some have argued that the UDI is better suited for inclusion in the electronic health records, Cook believes this argument sets up a false choice between the two,” wrote Stephen L. Ferguson, JD, the chairman of Cook’s board. “Inclusion of the UDI in both electronic health records and claims forms will lead to a more robust system of real-world data.”<br/><br/>In contrast, AdvaMed, the trade group for device makers, told the NCVHS that it did not support adding the information to payment claims submissions, instead just supporting the inclusion in EHRs.<br/><br/>Dr. Kovacs of the ACC said one potential drawback to more transparency could be challenges in interpreting reports of complications in certain cases, at least initially. Reports about a flaw or even a suspected flaw in a device might lead patients to become concerned about their implanted devices, potentially registering unfounded complaints.<br/><br/>But this concern can be addressed through using “scientific rigor and safeguards” and is outweighed by the potential safety benefits for patients, Dr. Kovacs said.<br/><br/>Patients should ask health care systems to track and share information about their implanted devices, Dr. Kovacs suggested.<br/><br/>“I feel it would be my right to demand that that device information follows my electronic medical record, so that it’s readily available to anyone who’s taking care of me,” Dr. Kovacs said. “They would know what it is that’s in me, whether it’s a lens in my eye or a prosthesis in my hip or a highly complicated implantable cardiac electronic device.”<br/><br/>The Harvard study was supported by the FDA and National Institutes of Health. Authors of the study reported receiving fees from the FDA, Burroughs Wellcome Fund, and Harvard-MIT Center for Regulatory Science outside the submitted work. No other disclosures were reported. Authors of the editorial reported past and present connections with F-Prime Capital, FDA, Johnson & Johnson, the Medical Devices Innovation Consortium; the Agency for Healthcare Research and Quality; the National Heart, Lung, and Blood Institute; and Arnold Ventures, as well being an expert witness at in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen. Authors of the Viewpoint reported past and present connections with the National Evaluation System for Health Technology Coordinating Center (NESTcc), which is part of the Medical Device Innovation Consortium (MDIC); AIM North America UDI Advisory Committee, Mass General Brigham, Arnold Ventures; the Institute for Clinical and Economic Review California Technology Assessment Forum; Yale University, Johnson & Johnson, FD, Agency for Healthcare Research and Quality; the National Heart, Lung, and Blood Institute of the National Institutes of Health; as well as having been an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against.<span class="end"/></p> <p> <em>A version of this article first appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/996887">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Rifampin for Prosthetic Joint Infections: Lessons Learned Over 20 Years at a VA Medical Center
Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.1 While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.2 Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.3
The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.4
In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.5 This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.4 Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.6 Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.3,7
Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.8 The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement.
METHODS
We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.
All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection.
Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.6
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.

Forty-two participants (54%) had Staphylococcus aureus and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. Cutibacterium acnes and Streptococcus agalactiae were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). Candida albicans was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%).
Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, P = .24) and a higher proportion of male participants (96% vs 80%, P = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (P = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (P < .001).
The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (P = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had Staphylococcus aureus or coagulase-negative staphylococci isolated.
The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.
Clinical Outcome
Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (P = .02).
Secondary Outcomes
The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.
DISCUSSION
Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.5,7 In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).
When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.5,10 Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.
Limitations
Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities.
Conclusions
DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.
Acknowledgments
We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141
2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0
3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952
4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-430. doi:10.1086/318502
5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537
6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x
Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.1 While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.2 Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.3
The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.4
In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.5 This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.4 Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.6 Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.3,7
Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.8 The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement.
METHODS
We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.
All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection.
Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.6
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.

Forty-two participants (54%) had Staphylococcus aureus and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. Cutibacterium acnes and Streptococcus agalactiae were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). Candida albicans was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%).
Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, P = .24) and a higher proportion of male participants (96% vs 80%, P = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (P = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (P < .001).
The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (P = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had Staphylococcus aureus or coagulase-negative staphylococci isolated.
The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.
Clinical Outcome
Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (P = .02).
Secondary Outcomes
The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.
DISCUSSION
Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.5,7 In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).
When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.5,10 Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.
Limitations
Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities.
Conclusions
DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.
Acknowledgments
We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.
Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.1 While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.2 Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.3
The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.4
In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.5 This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.4 Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.6 Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.3,7
Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.8 The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement.
METHODS
We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.
All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection.
Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.6
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.

Forty-two participants (54%) had Staphylococcus aureus and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. Cutibacterium acnes and Streptococcus agalactiae were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). Candida albicans was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%).
Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, P = .24) and a higher proportion of male participants (96% vs 80%, P = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (P = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (P < .001).
The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (P = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had Staphylococcus aureus or coagulase-negative staphylococci isolated.
The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.
Clinical Outcome
Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (P = .02).
Secondary Outcomes
The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.
DISCUSSION
Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.5,7 In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).
When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.5,10 Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.
Limitations
Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities.
Conclusions
DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.
Acknowledgments
We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141
2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0
3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952
4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-430. doi:10.1086/318502
5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537
6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141
2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0
3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952
4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-430. doi:10.1086/318502
5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537
6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>0923 FED Rifampin</fileName> <TBEID>0C02DE4D.SIG</TBEID> <TBUniqueIdentifier>NJ_0C02DE4D</TBUniqueIdentifier> <newsOrJournal>Journal</newsOrJournal> <publisherName>Frontline Medical Communications Inc.</publisherName> <storyname>0923 FED Rifampin</storyname> <articleType>1</articleType> <TBLocation>Copyfitting-FED</TBLocation> <QCDate/> <firstPublished>20230915T133059</firstPublished> <LastPublished>20230915T133059</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20230915T133059</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline/> <bylineText>Solana Cushinga,b; Dimitri Drekonja, MD, MSb,c</bylineText> <bylineFull/> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType/> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:"> <name/> <rightsInfo> <copyrightHolder> <name/> </copyrightHolder> <copyrightNotice/> </rightsInfo> </provider> <abstract/> <metaDescription>Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA)</metaDescription> <articlePDF/> <teaserImage/> <title>Rifampin for Prosthetic Joint Infections: Lessons Learned Over 20 Years at a VA Medical Center</title> <deck/> <eyebrow>Original Research</eyebrow> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear>2023</pubPubdateYear> <pubPubdateMonth>September</pubPubdateMonth> <pubPubdateDay/> <pubVolume>40</pubVolume> <pubNumber>9</pubNumber> <wireChannels/> <primaryCMSID/> <CMSIDs> <CMSID>2951</CMSID> <CMSID>2881</CMSID> <CMSID>3639</CMSID> </CMSIDs> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>FED</publicationCode> <pubIssueName>September 2022</pubIssueName> <pubArticleType>Feature Articles | 3639</pubArticleType> <pubTopics> <pubTopic>Orthopedics | 2881</pubTopic> </pubTopics> <pubCategories/> <pubSections> <pubSection>Feature | 2951<pubSubsection/></pubSection> </pubSections> <journalTitle>Fed Pract</journalTitle> <journalFullTitle>Federal Practitioner</journalFullTitle> <copyrightStatement>Copyright 2017 Frontline Medical Communications Inc., Parsippany, NJ, USA. All rights reserved.</copyrightStatement> </publicationData> </publications_g> <publications> <term canonical="true">16</term> </publications> <sections> <term canonical="true">104</term> </sections> <topics> <term canonical="true">264</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Rifampin for Prosthetic Joint Infections: Lessons Learned Over 20 Years at a VA Medical Center</title> <deck/> </itemMeta> <itemContent> <p class="Normal"> <b>Background: </b> The Minneapolis Veterans Affairs Health Care System uses debridement and implant retention (DAIR) combined with oral rifampin and a second antibiotic to treat orthopedic implant infections. However, the success rate of this approach in a veteran population is unknown. <br/><br/> <b>Methods: </b> We performed a retrospective analysis of patients who underwent DAIR with a rifampin-containing regimen for an orthopedic implant infection over the past 20 years at the Minneapolis Veterans Affairs Health Care System. The primary outcome was treatment success among participants who were treated with curative intent, defined as planned device retention without ongoing antibiotic use. Secondary outcomes were treatment harms and therapy duration. Treatment success was defined as the absence of recurrent infection or further measures to suppress infection within 1 year of completing antimicrobial therapy. <br/><br/> <b>Results:</b> A total of 78 patients (88% male) were included (median age, 65.5 years), with 50 treated with curative intent (primary analysis group). Forty-one participants (82%) in the curative intent group experienced treatment success. The success rate was higher among participants whose implant was < 2 months old vs those whose implant was ≥ 2 months old (93% vs 65%, respectively; <i>P</i> = .02). The 28 participants treated without curative intent had more comorbidities, higher rates of chronic infection, and older implants than those treated with curative intent.<br/><br/> <b>Conclusions:</b> Veterans with orthopedic implant infections can be successfully treated with DAIR combined with a rifampin-containing antimicrobial regimen. Success is highest for patients with a recent implant. Debridement and implant retention using regimens that include rifampin is an evidence-based strategy for managing patients with infected prosthetic hardware. Here we report that this approach is feasible in a veteran population, especially with recently implanted prosthetic material. </p> <p><span class="dropcap">O</span>rthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.<sup>1</sup> While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.<sup>2</sup> Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.<sup>3</sup> </p> <p>The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.<sup>4 <br/><br/></sup>In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.<sup>5</sup> This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.<sup>4</sup> Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.<sup>6</sup> Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.<sup>3,7<br/><br/></sup>Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.<sup>8</sup> The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement. </p> <h2>METHODS</h2> <p>We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.</p> <p>All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection. <br/><br/>Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.<sup>6</sup></p> <h3>Treatment Outcomes</h3> <p>The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.</p> <p>Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.</p> <h3>Statistical Analysis</h3> <p>Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.<sup>9</sup> Fisher exact test was used to assess differences between categorical variables, and an independent <i>t</i> test was used to assess differences in continuous variables. <i>P</i> < .05 indicated statistical significance.</p> <p>To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.</p> <h2>RESULTS</h2> <p>A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021. No orthopedic device infection was present in 400 patients, leaving 138 potential participants. Of these, 60 were excluded, leaving 78 patients with a diagnosed orthopedic implant infection treated with DAIR and a rifampin-containing antimicrobial regimen who were included in the study (Figure). Most were male (n = 69; 88%) with a median age of 65 years (Table). The mean (SD) Charlson Comorbidity Index was 2.2 (1.4); diabetes was the most common comorbidity (n = 29; 37%). Thirty-eight participants (49%) had an infected knee prosthesis and 29 (37%) had an infected hip prosthesis, accounting for 86% of all infections, while 8 participants (10%) had infected bone fixation devices and the remaining 3 (4%) had infected elbow or ankle implants. The debridement procedure was open for 73 patients (94%) vs arthroscopic for 5 (6%) (all osteosynthesis infections). Rifampin was initiated after debridement in all cases. The median (IQR) implant age was 1.3 months (0.6-30 months). Thirty participants (38%) had a chronic infection. The mean (SD) duration of infection-related symptoms before surgery was 7.6 (6.1) days. </p> <p>Forty-two participants (54%) had <i>Staphylococcus aureus</i> and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. <i>Cutibacterium acnes</i> and <i>Streptococcus agalactiae</i> were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). <i>Candida albicans</i> was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%). <br/><br/>Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, <i>P</i> = .24) and a higher proportion of male participants (96% vs 80%, <i>P</i> = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (<i>P</i> = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (<i>P</i> < .001). <br/><br/>The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (<i>P</i> = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had <i>Staphylococcus aureus</i> or coagulase-negative staphylococci isolated.<br/><br/>The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.</p> <h3>Clinical Outcome</h3> <p>Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (<i>P</i> = .02).</p> <h3>Secondary Outcomes</h3> <p>The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.</p> <h2>DISCUSSION</h2> <p>Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.<sup>5,7</sup> In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).</p> <p>When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.<sup>5,10</sup> Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.</p> <h3>Limitations</h3> <p>Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities. </p> <h2>Conclusions</h2> <p>DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.</p> <h3> Acknowledgments </h3> <p> <em>We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.</em> </p> <h3> Author affiliations </h3> <p> <em><sup>a</sup>Macalester College, St. Paul, Minnesota<br/><br/><sup>b</sup>Minneapolis Veterans Affairs Health Care System, Minnesota <br/><br/><sup>c</sup>University of Minnesota Medical School, Minneapolis</em> </p> <h3> <br/><br/>Author disclosures </h3> <p> <em>The authors report no actual or potential conflicts of interest or outside sources of funding with regard to this article.</em> </p> <h3> Disclaimer </h3> <p> <em>The opinions expressed herein are those of the authors and do not necessarily reflect those of <i>Federal Practitioner</i>, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.</em> </p> <h3> Ethics and consent </h3> <p> <em>This project was reviewed by the Minneapolis VA Healthcare System (MVAHCS) Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review from the Institutional Review Board. It was reviewed and approved by the MVAHCS Research and Development Committee.</em> </p> <h3> References </h3> <p class="reference"> 1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. <i>J Bone Joint Surg Am</i>. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141<br/><br/> 2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. <i>Lancet</i>. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0<br/><br/> 3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. <i>J Bone Joint Surg Am</i>. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952<br/><br/> 4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. <i>Clin Infect Dis</i>. 2001;32(3):419-430. doi:10.1086/318502<br/><br/> 5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. <i>JAMA</i>. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537<br/><br/> 6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. <i>Clin Infect Dis</i>. 2013;56(1):e1-e25. doi:10.1093/cid/cis803<br/><br/> 7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. <i>Int J Antimicrob Agents</i>. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021<br/><br/> 8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. <i>Arch Intern Med</i>. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252<br/><br/> 9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. <i>J Chronic Dis</i>. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8<br/><br/>10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. <i>Clin Microbiol Infect</i>. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x</p> </itemContent> </newsItem> </itemSet></root>
‘Decapitated’ boy saved by surgery team
This transcript has been edited for clarity.
F. Perry Wilson, MD, MSCE: I am joined today by Dr. Ohad Einav. He’s a staff surgeon in orthopedics at Hadassah Medical Center in Jerusalem. He’s with me to talk about an absolutely incredible surgical case, something that is terrifying to most non–orthopedic surgeons and I imagine is fairly scary for spine surgeons like him as well. But what we don’t have is information about how this works from a medical perspective. So, first of all, Dr. Einav, thank you for taking time to speak with me today.
Ohad Einav, MD: Thank you for having me.
Dr. Wilson: Can you tell us about Suleiman Hassan and what happened to him before he came into your care?
Dr. Einav: Hassan is a 12-year-old child who was riding his bicycle on the West Bank, about 40 minutes from here. Unfortunately, he was involved in a motor vehicle accident and he suffered injuries to his abdomen and cervical spine. He was transported to our service by helicopter from the scene of the accident.
Dr. Wilson: “Injury to the cervical spine” might be something of an understatement. He had what’s called atlanto-occipital dislocation, colloquially often referred to as internal decapitation. Can you tell us what that means? It sounds terrifying.
Dr. Einav: It’s an injury to the ligaments between the occiput and the upper cervical spine, with or without bony fracture. The atlanto-occipital joint is formed by the superior articular facet of the atlas and the occipital condyle, stabilized by an articular capsule between the head and neck, and is supported by various ligaments around it that stabilize the joint and allow joint movements, including flexion, extension, and some rotation in the lower levels.
Dr. Wilson: This joint has several degrees of freedom, which means it needs a lot of support. With this type of injury, where essentially you have severing of the ligaments, is it usually survivable? How dangerous is this?
Dr. Einav: The mortality rate is 50%-60%, depending on the primary impact, the injury, transportation later on, and then the surgery and surgical management.
Dr. Wilson: Tell us a bit about this patient’s status when he came to your medical center. I assume he was in bad shape.
Dr. Einav: Hassan arrived at our medical center with a Glasgow Coma Scale score of 15. He was fully conscious. He was hemodynamically stable except for a bad laceration on his abdomen. He had a Philadelphia collar around his neck. He was transported by chopper because the paramedics suspected that he had a cervical spine injury and decided to bring him to a Level 1 trauma center.
He was monitored and we treated him according to the ATLS [advanced trauma life support] protocol. He didn’t have any gross sensory deficits, but he was a little confused about the whole situation and the accident. Therefore, we could do a general examination but we couldn’t rely on that regarding any sensory deficit that he may or may not have. We decided as a team that it would be better to slow down and control the situation. We decided not to operate on him immediately. We basically stabilized him and made sure that he didn’t have any traumatic internal organ damage. Later on we took him to the OR and performed surgery.
Dr. Wilson: It’s amazing that he had intact motor function, considering the extent of his injury. The spinal cord was spared somewhat during the injury. There must have been a moment when you realized that this kid, who was conscious and could move all four extremities, had a very severe neck injury. Was that due to a CT scan or physical exam? And what was your feeling when you saw that he had atlanto-occipital dislocation?
Dr. Einav: As a surgeon, you have a gut feeling in regard to the general examination of the patient. But I never rely on gut feelings. On the CT, I understood exactly what he had, what we needed to do, and the time frame.
Dr. Wilson: You’ve done these types of surgeries before, right? Obviously, no one has done a lot of them because this isn’t very common. But you knew what to do. Did you have a plan? Where does your experience come into play in a situation like this?
Dr. Einav: I graduated from the spine program of Toronto University, where I did a fellowship in trauma of the spine and complex spine surgery. I had very good teachers, and during my fellowship I treated a few cases in older patients that were similar but not the same. Therefore, I knew exactly what needed to be done.
Dr. Wilson: For those of us who aren’t surgeons, take us into the OR with you. This is obviously an incredibly delicate procedure. You are high up in the spinal cord at the base of the brain. The slightest mistake could have devastating consequences. What are the key elements of this procedure? What can go wrong here? What is the number-one thing you have to look out for when you’re trying to fix an internal decapitation?
Dr. Einav: The key element in surgeries of the cervical spine – trauma and complex spine surgery – is planning. I never go to the OR without knowing what I’m going to do. I have a few plans – plan A, plan B, plan C – in case something fails. So, I definitely know what the next step will be. I always think about the surgery a few hours before, if I have time to prepare.
The second thing that is very important is teamwork. The team needs to be coordinated. Everybody needs to know what their job is. With these types of injuries, it’s not the time for rookies. If you are new, please stand back and let the more experienced people do that job. I’m talking about surgeons, nurses, anesthesiologists – everyone.
Another important thing in planning is choosing the right hardware. For example, in this case we had a problem because most of the hardware is designed for adults, and we had to improvise because there isn’t a lot of hardware on the market for the pediatric population. The adult plates and screws are too big, so we had to improvise.
Dr. Wilson: Tell us more about that. How do you improvise spinal hardware for a 12-year-old?
Dr. Einav: In this case, I chose to use hardware from one of the companies that works with us.
You can see in this model the area of the injury, and the area that we worked on. To perform the surgery, I had to use some plates and rods from a different company. This company’s (NuVasive) hardware has a small attachment to the skull, which was helpful for affixing the skull to the cervical spine, instead of using a big plate that would sit at the base of the skull and would not be very good for him. Most of the hardware is made for adults and not for kids.
Dr. Wilson: Will that hardware preserve the motor function of his neck? Will he be able to turn his head and extend and flex it?
Dr. Einav: The injury leads to instability and destruction of both articulations between the head and neck. Therefore, those articulations won’t be able to function the same way in the future. There is a decrease of something like 50% of the flexion and extension of Hassan’s cervical spine. Therefore, I decided that in this case there would be no chance of saving Hassan’s motor function unless we performed a fusion between the head and the neck, and therefore I decided that this would be the best procedure with the best survival rate. So, in the future, he will have some diminished flexion, extension, and rotation of his head.
Dr. Wilson: How long did his surgery take?
Dr. Einav: To be honest, I don’t remember. But I can tell you that it took us time. It was very challenging to coordinate with everyone. The most problematic part of the surgery to perform is what we call “flip-over.”
The anesthesiologist intubated the patient when he was supine, and later on, we flipped him prone to operate on the spine. This maneuver can actually lead to injury by itself, and injury at this level is fatal. So, we took our time and got Hassan into the OR. The anesthesiologist did a great job with the GlideScope – inserting the endotracheal tube. Later on, we neuromonitored him. Basically, we connected Hassan’s peripheral nerves to a computer and monitored his motor function. Gently we flipped him over, and after that we saw a little change in his motor function, so we had to modify his position so we could preserve his motor function. We then started the procedure, which took a few hours. I don’t know exactly how many.
Dr. Wilson: That just speaks to how delicate this is for everything from the intubation, where typically you’re manipulating the head, to the repositioning. Clearly this requires a lot of teamwork.
What happened after the operation? How is he doing?
Dr. Einav: After the operation, Hassan had a great recovery. He’s doing well. He doesn’t have any motor or sensory deficits. He’s able to ambulate without any aid. He had no signs of infection, which can happen after a car accident, neither from his abdominal wound nor from the occipital cervical surgery. He feels well. We saw him in the clinic. We removed his collar. We monitored him at the clinic. He looked amazing.
Dr. Wilson: That’s incredible. Are there long-term risks for him that you need to be looking out for?
Dr. Einav: Yes, and that’s the reason that we are monitoring him post surgery. While he was in the hospital, we monitored his motor and sensory functions, as well as his wound healing. Later on, in the clinic, for a few weeks after surgery we monitored for any failure of the hardware and bone graft. We check for healing of the bone graft and bone substitutes we put in to heal those bones.
Dr. Wilson: He will grow, right? He’s only 12, so he still has some years of growth in him. Is he going to need more surgery or any kind of hardware upgrade?
Dr. Einav: I hope not. In my surgeries, I never rely on the hardware for long durations. If I decide to do, for example, fusion, I rely on the hardware for a certain amount of time. And then I plan that the biology will do the work. If I plan for fusion, I put bone grafts in the preferred area for a fusion. Then if the hardware fails, I wouldn’t need to take out the hardware, and there would be no change in the condition of the patient.
Dr. Wilson: What an incredible story. It’s clear that you and your team kept your cool despite a very high-acuity situation with a ton of risk. What a tremendous outcome that this boy is not only alive but fully functional. So, congratulations to you and your team. That was very strong work.
Dr. Einav: Thank you very much. I would like to thank our team. We have to remember that the surgeon is not standing alone in the war. Hassan’s story is a success story of a very big group of people from various backgrounds and religions. They work day and night to help people and save lives. To the paramedics, the physiologists, the traumatologists, the pediatricians, the nurses, the physiotherapists, and obviously the surgeons, a big thank you. His story is our success story.
Dr. Wilson: It’s inspiring to see so many people come together to do what we all are here for, which is to fight against suffering, disease, and death. Thank you for keeping up that fight. And thank you for joining me here.
Dr. Einav: Thank you very much.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
F. Perry Wilson, MD, MSCE: I am joined today by Dr. Ohad Einav. He’s a staff surgeon in orthopedics at Hadassah Medical Center in Jerusalem. He’s with me to talk about an absolutely incredible surgical case, something that is terrifying to most non–orthopedic surgeons and I imagine is fairly scary for spine surgeons like him as well. But what we don’t have is information about how this works from a medical perspective. So, first of all, Dr. Einav, thank you for taking time to speak with me today.
Ohad Einav, MD: Thank you for having me.
Dr. Wilson: Can you tell us about Suleiman Hassan and what happened to him before he came into your care?
Dr. Einav: Hassan is a 12-year-old child who was riding his bicycle on the West Bank, about 40 minutes from here. Unfortunately, he was involved in a motor vehicle accident and he suffered injuries to his abdomen and cervical spine. He was transported to our service by helicopter from the scene of the accident.
Dr. Wilson: “Injury to the cervical spine” might be something of an understatement. He had what’s called atlanto-occipital dislocation, colloquially often referred to as internal decapitation. Can you tell us what that means? It sounds terrifying.
Dr. Einav: It’s an injury to the ligaments between the occiput and the upper cervical spine, with or without bony fracture. The atlanto-occipital joint is formed by the superior articular facet of the atlas and the occipital condyle, stabilized by an articular capsule between the head and neck, and is supported by various ligaments around it that stabilize the joint and allow joint movements, including flexion, extension, and some rotation in the lower levels.
Dr. Wilson: This joint has several degrees of freedom, which means it needs a lot of support. With this type of injury, where essentially you have severing of the ligaments, is it usually survivable? How dangerous is this?
Dr. Einav: The mortality rate is 50%-60%, depending on the primary impact, the injury, transportation later on, and then the surgery and surgical management.
Dr. Wilson: Tell us a bit about this patient’s status when he came to your medical center. I assume he was in bad shape.
Dr. Einav: Hassan arrived at our medical center with a Glasgow Coma Scale score of 15. He was fully conscious. He was hemodynamically stable except for a bad laceration on his abdomen. He had a Philadelphia collar around his neck. He was transported by chopper because the paramedics suspected that he had a cervical spine injury and decided to bring him to a Level 1 trauma center.
He was monitored and we treated him according to the ATLS [advanced trauma life support] protocol. He didn’t have any gross sensory deficits, but he was a little confused about the whole situation and the accident. Therefore, we could do a general examination but we couldn’t rely on that regarding any sensory deficit that he may or may not have. We decided as a team that it would be better to slow down and control the situation. We decided not to operate on him immediately. We basically stabilized him and made sure that he didn’t have any traumatic internal organ damage. Later on we took him to the OR and performed surgery.
Dr. Wilson: It’s amazing that he had intact motor function, considering the extent of his injury. The spinal cord was spared somewhat during the injury. There must have been a moment when you realized that this kid, who was conscious and could move all four extremities, had a very severe neck injury. Was that due to a CT scan or physical exam? And what was your feeling when you saw that he had atlanto-occipital dislocation?
Dr. Einav: As a surgeon, you have a gut feeling in regard to the general examination of the patient. But I never rely on gut feelings. On the CT, I understood exactly what he had, what we needed to do, and the time frame.
Dr. Wilson: You’ve done these types of surgeries before, right? Obviously, no one has done a lot of them because this isn’t very common. But you knew what to do. Did you have a plan? Where does your experience come into play in a situation like this?
Dr. Einav: I graduated from the spine program of Toronto University, where I did a fellowship in trauma of the spine and complex spine surgery. I had very good teachers, and during my fellowship I treated a few cases in older patients that were similar but not the same. Therefore, I knew exactly what needed to be done.
Dr. Wilson: For those of us who aren’t surgeons, take us into the OR with you. This is obviously an incredibly delicate procedure. You are high up in the spinal cord at the base of the brain. The slightest mistake could have devastating consequences. What are the key elements of this procedure? What can go wrong here? What is the number-one thing you have to look out for when you’re trying to fix an internal decapitation?
Dr. Einav: The key element in surgeries of the cervical spine – trauma and complex spine surgery – is planning. I never go to the OR without knowing what I’m going to do. I have a few plans – plan A, plan B, plan C – in case something fails. So, I definitely know what the next step will be. I always think about the surgery a few hours before, if I have time to prepare.
The second thing that is very important is teamwork. The team needs to be coordinated. Everybody needs to know what their job is. With these types of injuries, it’s not the time for rookies. If you are new, please stand back and let the more experienced people do that job. I’m talking about surgeons, nurses, anesthesiologists – everyone.
Another important thing in planning is choosing the right hardware. For example, in this case we had a problem because most of the hardware is designed for adults, and we had to improvise because there isn’t a lot of hardware on the market for the pediatric population. The adult plates and screws are too big, so we had to improvise.
Dr. Wilson: Tell us more about that. How do you improvise spinal hardware for a 12-year-old?
Dr. Einav: In this case, I chose to use hardware from one of the companies that works with us.
You can see in this model the area of the injury, and the area that we worked on. To perform the surgery, I had to use some plates and rods from a different company. This company’s (NuVasive) hardware has a small attachment to the skull, which was helpful for affixing the skull to the cervical spine, instead of using a big plate that would sit at the base of the skull and would not be very good for him. Most of the hardware is made for adults and not for kids.
Dr. Wilson: Will that hardware preserve the motor function of his neck? Will he be able to turn his head and extend and flex it?
Dr. Einav: The injury leads to instability and destruction of both articulations between the head and neck. Therefore, those articulations won’t be able to function the same way in the future. There is a decrease of something like 50% of the flexion and extension of Hassan’s cervical spine. Therefore, I decided that in this case there would be no chance of saving Hassan’s motor function unless we performed a fusion between the head and the neck, and therefore I decided that this would be the best procedure with the best survival rate. So, in the future, he will have some diminished flexion, extension, and rotation of his head.
Dr. Wilson: How long did his surgery take?
Dr. Einav: To be honest, I don’t remember. But I can tell you that it took us time. It was very challenging to coordinate with everyone. The most problematic part of the surgery to perform is what we call “flip-over.”
The anesthesiologist intubated the patient when he was supine, and later on, we flipped him prone to operate on the spine. This maneuver can actually lead to injury by itself, and injury at this level is fatal. So, we took our time and got Hassan into the OR. The anesthesiologist did a great job with the GlideScope – inserting the endotracheal tube. Later on, we neuromonitored him. Basically, we connected Hassan’s peripheral nerves to a computer and monitored his motor function. Gently we flipped him over, and after that we saw a little change in his motor function, so we had to modify his position so we could preserve his motor function. We then started the procedure, which took a few hours. I don’t know exactly how many.
Dr. Wilson: That just speaks to how delicate this is for everything from the intubation, where typically you’re manipulating the head, to the repositioning. Clearly this requires a lot of teamwork.
What happened after the operation? How is he doing?
Dr. Einav: After the operation, Hassan had a great recovery. He’s doing well. He doesn’t have any motor or sensory deficits. He’s able to ambulate without any aid. He had no signs of infection, which can happen after a car accident, neither from his abdominal wound nor from the occipital cervical surgery. He feels well. We saw him in the clinic. We removed his collar. We monitored him at the clinic. He looked amazing.
Dr. Wilson: That’s incredible. Are there long-term risks for him that you need to be looking out for?
Dr. Einav: Yes, and that’s the reason that we are monitoring him post surgery. While he was in the hospital, we monitored his motor and sensory functions, as well as his wound healing. Later on, in the clinic, for a few weeks after surgery we monitored for any failure of the hardware and bone graft. We check for healing of the bone graft and bone substitutes we put in to heal those bones.
Dr. Wilson: He will grow, right? He’s only 12, so he still has some years of growth in him. Is he going to need more surgery or any kind of hardware upgrade?
Dr. Einav: I hope not. In my surgeries, I never rely on the hardware for long durations. If I decide to do, for example, fusion, I rely on the hardware for a certain amount of time. And then I plan that the biology will do the work. If I plan for fusion, I put bone grafts in the preferred area for a fusion. Then if the hardware fails, I wouldn’t need to take out the hardware, and there would be no change in the condition of the patient.
Dr. Wilson: What an incredible story. It’s clear that you and your team kept your cool despite a very high-acuity situation with a ton of risk. What a tremendous outcome that this boy is not only alive but fully functional. So, congratulations to you and your team. That was very strong work.
Dr. Einav: Thank you very much. I would like to thank our team. We have to remember that the surgeon is not standing alone in the war. Hassan’s story is a success story of a very big group of people from various backgrounds and religions. They work day and night to help people and save lives. To the paramedics, the physiologists, the traumatologists, the pediatricians, the nurses, the physiotherapists, and obviously the surgeons, a big thank you. His story is our success story.
Dr. Wilson: It’s inspiring to see so many people come together to do what we all are here for, which is to fight against suffering, disease, and death. Thank you for keeping up that fight. And thank you for joining me here.
Dr. Einav: Thank you very much.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
F. Perry Wilson, MD, MSCE: I am joined today by Dr. Ohad Einav. He’s a staff surgeon in orthopedics at Hadassah Medical Center in Jerusalem. He’s with me to talk about an absolutely incredible surgical case, something that is terrifying to most non–orthopedic surgeons and I imagine is fairly scary for spine surgeons like him as well. But what we don’t have is information about how this works from a medical perspective. So, first of all, Dr. Einav, thank you for taking time to speak with me today.
Ohad Einav, MD: Thank you for having me.
Dr. Wilson: Can you tell us about Suleiman Hassan and what happened to him before he came into your care?
Dr. Einav: Hassan is a 12-year-old child who was riding his bicycle on the West Bank, about 40 minutes from here. Unfortunately, he was involved in a motor vehicle accident and he suffered injuries to his abdomen and cervical spine. He was transported to our service by helicopter from the scene of the accident.
Dr. Wilson: “Injury to the cervical spine” might be something of an understatement. He had what’s called atlanto-occipital dislocation, colloquially often referred to as internal decapitation. Can you tell us what that means? It sounds terrifying.
Dr. Einav: It’s an injury to the ligaments between the occiput and the upper cervical spine, with or without bony fracture. The atlanto-occipital joint is formed by the superior articular facet of the atlas and the occipital condyle, stabilized by an articular capsule between the head and neck, and is supported by various ligaments around it that stabilize the joint and allow joint movements, including flexion, extension, and some rotation in the lower levels.
Dr. Wilson: This joint has several degrees of freedom, which means it needs a lot of support. With this type of injury, where essentially you have severing of the ligaments, is it usually survivable? How dangerous is this?
Dr. Einav: The mortality rate is 50%-60%, depending on the primary impact, the injury, transportation later on, and then the surgery and surgical management.
Dr. Wilson: Tell us a bit about this patient’s status when he came to your medical center. I assume he was in bad shape.
Dr. Einav: Hassan arrived at our medical center with a Glasgow Coma Scale score of 15. He was fully conscious. He was hemodynamically stable except for a bad laceration on his abdomen. He had a Philadelphia collar around his neck. He was transported by chopper because the paramedics suspected that he had a cervical spine injury and decided to bring him to a Level 1 trauma center.
He was monitored and we treated him according to the ATLS [advanced trauma life support] protocol. He didn’t have any gross sensory deficits, but he was a little confused about the whole situation and the accident. Therefore, we could do a general examination but we couldn’t rely on that regarding any sensory deficit that he may or may not have. We decided as a team that it would be better to slow down and control the situation. We decided not to operate on him immediately. We basically stabilized him and made sure that he didn’t have any traumatic internal organ damage. Later on we took him to the OR and performed surgery.
Dr. Wilson: It’s amazing that he had intact motor function, considering the extent of his injury. The spinal cord was spared somewhat during the injury. There must have been a moment when you realized that this kid, who was conscious and could move all four extremities, had a very severe neck injury. Was that due to a CT scan or physical exam? And what was your feeling when you saw that he had atlanto-occipital dislocation?
Dr. Einav: As a surgeon, you have a gut feeling in regard to the general examination of the patient. But I never rely on gut feelings. On the CT, I understood exactly what he had, what we needed to do, and the time frame.
Dr. Wilson: You’ve done these types of surgeries before, right? Obviously, no one has done a lot of them because this isn’t very common. But you knew what to do. Did you have a plan? Where does your experience come into play in a situation like this?
Dr. Einav: I graduated from the spine program of Toronto University, where I did a fellowship in trauma of the spine and complex spine surgery. I had very good teachers, and during my fellowship I treated a few cases in older patients that were similar but not the same. Therefore, I knew exactly what needed to be done.
Dr. Wilson: For those of us who aren’t surgeons, take us into the OR with you. This is obviously an incredibly delicate procedure. You are high up in the spinal cord at the base of the brain. The slightest mistake could have devastating consequences. What are the key elements of this procedure? What can go wrong here? What is the number-one thing you have to look out for when you’re trying to fix an internal decapitation?
Dr. Einav: The key element in surgeries of the cervical spine – trauma and complex spine surgery – is planning. I never go to the OR without knowing what I’m going to do. I have a few plans – plan A, plan B, plan C – in case something fails. So, I definitely know what the next step will be. I always think about the surgery a few hours before, if I have time to prepare.
The second thing that is very important is teamwork. The team needs to be coordinated. Everybody needs to know what their job is. With these types of injuries, it’s not the time for rookies. If you are new, please stand back and let the more experienced people do that job. I’m talking about surgeons, nurses, anesthesiologists – everyone.
Another important thing in planning is choosing the right hardware. For example, in this case we had a problem because most of the hardware is designed for adults, and we had to improvise because there isn’t a lot of hardware on the market for the pediatric population. The adult plates and screws are too big, so we had to improvise.
Dr. Wilson: Tell us more about that. How do you improvise spinal hardware for a 12-year-old?
Dr. Einav: In this case, I chose to use hardware from one of the companies that works with us.
You can see in this model the area of the injury, and the area that we worked on. To perform the surgery, I had to use some plates and rods from a different company. This company’s (NuVasive) hardware has a small attachment to the skull, which was helpful for affixing the skull to the cervical spine, instead of using a big plate that would sit at the base of the skull and would not be very good for him. Most of the hardware is made for adults and not for kids.
Dr. Wilson: Will that hardware preserve the motor function of his neck? Will he be able to turn his head and extend and flex it?
Dr. Einav: The injury leads to instability and destruction of both articulations between the head and neck. Therefore, those articulations won’t be able to function the same way in the future. There is a decrease of something like 50% of the flexion and extension of Hassan’s cervical spine. Therefore, I decided that in this case there would be no chance of saving Hassan’s motor function unless we performed a fusion between the head and the neck, and therefore I decided that this would be the best procedure with the best survival rate. So, in the future, he will have some diminished flexion, extension, and rotation of his head.
Dr. Wilson: How long did his surgery take?
Dr. Einav: To be honest, I don’t remember. But I can tell you that it took us time. It was very challenging to coordinate with everyone. The most problematic part of the surgery to perform is what we call “flip-over.”
The anesthesiologist intubated the patient when he was supine, and later on, we flipped him prone to operate on the spine. This maneuver can actually lead to injury by itself, and injury at this level is fatal. So, we took our time and got Hassan into the OR. The anesthesiologist did a great job with the GlideScope – inserting the endotracheal tube. Later on, we neuromonitored him. Basically, we connected Hassan’s peripheral nerves to a computer and monitored his motor function. Gently we flipped him over, and after that we saw a little change in his motor function, so we had to modify his position so we could preserve his motor function. We then started the procedure, which took a few hours. I don’t know exactly how many.
Dr. Wilson: That just speaks to how delicate this is for everything from the intubation, where typically you’re manipulating the head, to the repositioning. Clearly this requires a lot of teamwork.
What happened after the operation? How is he doing?
Dr. Einav: After the operation, Hassan had a great recovery. He’s doing well. He doesn’t have any motor or sensory deficits. He’s able to ambulate without any aid. He had no signs of infection, which can happen after a car accident, neither from his abdominal wound nor from the occipital cervical surgery. He feels well. We saw him in the clinic. We removed his collar. We monitored him at the clinic. He looked amazing.
Dr. Wilson: That’s incredible. Are there long-term risks for him that you need to be looking out for?
Dr. Einav: Yes, and that’s the reason that we are monitoring him post surgery. While he was in the hospital, we monitored his motor and sensory functions, as well as his wound healing. Later on, in the clinic, for a few weeks after surgery we monitored for any failure of the hardware and bone graft. We check for healing of the bone graft and bone substitutes we put in to heal those bones.
Dr. Wilson: He will grow, right? He’s only 12, so he still has some years of growth in him. Is he going to need more surgery or any kind of hardware upgrade?
Dr. Einav: I hope not. In my surgeries, I never rely on the hardware for long durations. If I decide to do, for example, fusion, I rely on the hardware for a certain amount of time. And then I plan that the biology will do the work. If I plan for fusion, I put bone grafts in the preferred area for a fusion. Then if the hardware fails, I wouldn’t need to take out the hardware, and there would be no change in the condition of the patient.
Dr. Wilson: What an incredible story. It’s clear that you and your team kept your cool despite a very high-acuity situation with a ton of risk. What a tremendous outcome that this boy is not only alive but fully functional. So, congratulations to you and your team. That was very strong work.
Dr. Einav: Thank you very much. I would like to thank our team. We have to remember that the surgeon is not standing alone in the war. Hassan’s story is a success story of a very big group of people from various backgrounds and religions. They work day and night to help people and save lives. To the paramedics, the physiologists, the traumatologists, the pediatricians, the nurses, the physiotherapists, and obviously the surgeons, a big thank you. His story is our success story.
Dr. Wilson: It’s inspiring to see so many people come together to do what we all are here for, which is to fight against suffering, disease, and death. Thank you for keeping up that fight. And thank you for joining me here.
Dr. Einav: Thank you very much.
A version of this article first appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>164959</fileName> <TBEID>0C04C03E.SIG</TBEID> <TBUniqueIdentifier>MD_0C04C03E</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>353</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20230905T115703</QCDate> <firstPublished>20230905T120816</firstPublished> <LastPublished>20230905T120816</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20230905T120816</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>F Perry Wilson</byline> <bylineText>F. PERRY WILSON, MD, MSCE AND OHAD EINAV, MD</bylineText> <bylineFull>F. PERRY WILSON, MD, MSCE AND OHAD EINAV, MD</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>Opinion</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>It’s a case of internal decapitation that has generated a lot of news around the world because it happened to a young boy.</metaDescription> <articlePDF/> <teaserImage>297371</teaserImage> <teaser>The key element in surgeries of the cervical spine – trauma and complex spine surgery – is planning.</teaser> <title>‘Decapitated’ boy saved by surgery team</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>pn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdsurg</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">25</term> <term>52226</term> <term>15</term> </publications> <sections> <term canonical="true">52</term> </sections> <topics> <term canonical="true">235</term> <term>264</term> <term>271</term> </topics> <links> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/24012196.jpg</altRep> <description role="drol:caption"/> <description role="drol:credit">Hadassah Medical Center</description> </link> <link> <itemClass qcode="ninat:picture"/> <altRep contenttype="image/jpeg">images/24012197.jpg</altRep> <description role="drol:caption"/> <description role="drol:credit">Hadassah Medical Center</description> </link> </links> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>‘Decapitated’ boy saved by surgery team</title> <deck/> </itemMeta> <itemContent> <p> <em>This transcript has been edited for clarity.<br/><br/></em> </p> <p><strong>F. Perry Wilson, MD, MSCE:</strong> I am joined today by Dr. Ohad Einav. He’s a staff surgeon in orthopedics at Hadassah Medical Center in Jerusalem. He’s with me to talk about an absolutely incredible surgical case, something that is terrifying to most non–orthopedic surgeons and I imagine is fairly scary for spine surgeons like him as well. <span class="tag metaDescription">It’s a case of internal decapitation that has generated a lot of news around the world because it happened to a young boy.</span> But what we don’t have is information about how this works from a medical perspective. So, first of all, Dr. Einav, thank you for taking time to speak with me today.<br/><br/><strong>Ohad Einav, MD:</strong> Thank you for having me.<br/><br/><strong>Dr. Wilson:</strong> Can you tell us about Suleiman Hassan and what happened to him before he came into your care?<br/><br/><strong>Dr. Einav:</strong> Hassan is a 12-year-old child who was riding his bicycle on the West Bank, about 40 minutes from here. Unfortunately, he was involved in a motor vehicle accident and he suffered injuries to his abdomen and cervical spine. He was transported to our service by helicopter from the scene of the accident.<br/><br/><strong>[[{"fid":"297371","view_mode":"medstat_image_full_text","fields":{"format":"medstat_image_full_text","field_file_image_alt_text[und][0][value]":"Diagram of atlanto-occipital dislocation","field_file_image_credit[und][0][value]":"Hadassah Medical Center","field_file_image_caption[und][0][value]":""},"type":"media","attributes":{"class":"media-element file-medstat_image_full_text"}}]]<br/><br/>Dr. Wilson:</strong> “Injury to the cervical spine” might be something of an understatement. He had what’s called atlanto-occipital dislocation, colloquially often referred to as internal decapitation. Can you tell us what that means? It sounds terrifying.<br/><br/><strong>Dr. Einav:</strong> It’s an injury to the ligaments between the occiput and the upper cervical spine, with or without bony fracture. The atlanto-occipital joint is formed by the superior articular facet of the atlas and the occipital condyle, stabilized by an articular capsule between the head and neck, and is supported by various ligaments around it that stabilize the joint and allow joint movements, including flexion, extension, and some rotation in the lower levels.<br/><br/><strong>Dr. Wilson:</strong> This joint has several degrees of freedom, which means it needs a lot of support. With this type of injury, where essentially you have severing of the ligaments, is it usually survivable? How dangerous is this?<br/><br/><strong>Dr. Einav:</strong> The mortality rate is 50%-60%, depending on the primary impact, the injury, transportation later on, and then the surgery and surgical management.<br/><br/><strong>Dr. Wilson:</strong> Tell us a bit about this patient’s status when he came to your medical center. I assume he was in bad shape.<br/><br/><strong>Dr. Einav:</strong> Hassan arrived at our medical center with a <a href="https://emedicine.medscape.com/article/2172603-overview">Glasgow Coma Scale</a> score of 15. He was fully conscious. He was hemodynamically stable except for a bad laceration on his abdomen. He had a Philadelphia collar around his neck. He was transported by chopper because the paramedics suspected that he had a cervical spine injury and decided to bring him to a Level 1 trauma center.<br/><br/>He was monitored and we treated him according to the ACLS [advanced cardiac life support] protocol. He didn’t have any gross sensory deficits, but he was a little confused about the whole situation and the accident. Therefore, we could do a general examination but we couldn’t rely on that regarding any sensory deficit that he may or may not have. We decided as a team that it would be better to slow down and control the situation. We decided not to operate on him immediately. We basically stabilized him and made sure that he didn’t have any traumatic internal organ damage. Later on we took him to the OR and performed surgery.<br/><br/><strong>Dr. Wilson: </strong>It’s amazing that he had intact motor function, considering the extent of his injury. The spinal cord was spared somewhat during the injury. There must have been a moment when you realized that this kid, who was conscious and could move all four extremities, had a very severe neck injury. Was that due to a CT scan or physical exam? And what was your feeling when you saw that he had atlanto-occipital dislocation?<br/><br/><strong>Dr. Einav:</strong> As a surgeon, you have a gut feeling in regard to the general examination of the patient. But I never rely on gut feelings. On the CT, I understood exactly what he had, what we needed to do, and the time frame.<br/><br/><strong>Dr. Wilson:</strong> You’ve done these types of surgeries before, right? Obviously, no one has done a lot of them because this isn’t very common. But you knew what to do. Did you have a plan? Where does your experience come into play in a situation like this?<br/><br/><strong>Dr. Einav:</strong> I graduated from the spine program of Toronto University, where I did a fellowship in trauma of the spine and complex spine surgery. I had very good teachers, and during my fellowship I treated a few cases in older patients that were similar but not the same. Therefore, I knew exactly what needed to be done.<br/><br/><strong>Dr. Wilson:</strong> For those of us who aren’t surgeons, take us into the OR with you. This is obviously an incredibly delicate procedure. You are high up in the spinal cord at the base of the brain. The slightest mistake could have devastating consequences. What are the key elements of this procedure? What can go wrong here? What is the number-one thing you have to look out for when you’re trying to fix an internal decapitation?<br/><br/><strong>Dr. Einav:</strong> The key element in surgeries of the cervical spine – trauma and complex spine surgery – is planning. I never go to the OR without knowing what I’m going to do. I have a few plans – plan A, plan B, plan C – in case something fails. So, I definitely know what the next step will be. I always think about the surgery a few hours before, if I have time to prepare.<br/><br/>The second thing that is very important is teamwork. The team needs to be coordinated. Everybody needs to know what their job is. With these types of injuries, it’s not the time for rookies. If you are new, please stand back and let the more experienced people do that job. I’m talking about surgeons, nurses, anesthesiologists – everyone.<br/><br/>Another important thing in planning is choosing the right hardware. For example, in this case we had a problem because most of the hardware is designed for adults, and we had to improvise because there isn’t a lot of hardware on the market for the pediatric population. The adult plates and screws are too big, so we had to improvise.<br/><br/><strong>Dr. Wilson:</strong> Tell us more about that. How do you improvise spinal hardware for a 12-year-old?<br/><br/></p> <p><strong>[[{"fid":"297372","view_mode":"medstat_image_flush_left","fields":{"format":"medstat_image_flush_left","field_file_image_alt_text[und][0][value]":"Hardware for atlanto-occipital dislocation repair","field_file_image_credit[und][0][value]":"Hadassah Medical Center","field_file_image_caption[und][0][value]":""},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_left"}}]]<br/><br/>Dr. Einav:</strong> In this case, I chose to use hardware from one of the companies that works with us. <br/><br/>You can see in this model the area of the injury, and the area that we worked on. To perform the surgery, I had to use some plates and rods from a different company. This company’s (NuVasive) hardware has a small attachment to the skull, which was helpful for affixing the skull to the cervical spine, instead of using a big plate that would sit at the base of the skull and would not be very good for him. Most of the hardware is made for adults and not for kids.<br/><br/><strong>Dr. Wilson:</strong> Will that hardware preserve the motor function of his neck? Will he be able to turn his head and extend and flex it?<br/><br/><strong>Dr. Einav:</strong> The injury leads to instability and destruction of both articulations between the head and neck. Therefore, those articulations won’t be able to function the same way in the future. There is a decrease of something like 50% of the flexion and extension of Hassan’s cervical spine. Therefore, I decided that in this case there would be no chance of saving Hassan’s motor function unless we performed a fusion between the head and the neck, and therefore I decided that this would be the best procedure with the best survival rate. So, in the future, he will have some diminished flexion, extension, and rotation of his head.<br/><br/><strong>Dr. Wilson:</strong> How long did his surgery take?<br/><br/><strong>Dr. Einav:</strong> To be honest, I don’t remember. But I can tell you that it took us time. It was very challenging to coordinate with everyone. The most problematic part of the surgery to perform is what we call “flip-over.”<br/><br/>The anesthesiologist intubated the patient when he was supine, and later on, we flipped him prone to operate on the spine. This maneuver can actually lead to injury by itself, and injury at this level is fatal. So, we took our time and got Hassan into the OR. The anesthesiologist did a great job with the GlideScope – inserting the endotracheal tube. Later on, we neuromonitored him. Basically, we connected Hassan’s peripheral nerves to a computer and monitored his motor function. Gently we flipped him over, and after that we saw a little change in his motor function, so we had to modify his position so we could preserve his motor function. We then started the procedure, which took a few hours. I don’t know exactly how many.<br/><br/><strong>Dr. Wilson:</strong> That just speaks to how delicate this is for everything from the intubation, where typically you’re manipulating the head, to the repositioning. Clearly this requires a lot of teamwork.<br/><br/>What happened after the operation? How is he doing?<br/><br/><strong>Dr. Einav:</strong> After the operation, Hassan had a great recovery. He’s doing well. He doesn’t have any motor or sensory deficits. He’s able to ambulate without any aid. He had no signs of infection, which can happen after a car accident, neither from his abdominal wound nor from the occipital cervical surgery. He feels well. We saw him in the clinic. We removed his collar. We monitored him at the clinic. He looked amazing.<br/><br/><strong>Dr. Wilson:</strong> That’s incredible. Are there long-term risks for him that you need to be looking out for?<br/><br/><strong>Dr. Einav:</strong> Yes, and that’s the reason that we are monitoring him post surgery. While he was in the hospital, we monitored his motor and sensory functions, as well as his <a href="https://emedicine.medscape.com/article/1298129-overview">wound healing</a>. Later on, in the clinic, for a few weeks after surgery we monitored for any failure of the hardware and bone graft. We check for healing of the bone graft and bone substitutes we put in to heal those bones.<br/><br/><strong>Dr. Wilson</strong>: He will grow, right? He’s only 12, so he still has some years of growth in him. Is he going to need more surgery or any kind of hardware upgrade?<br/><br/><strong>Dr. Einav:</strong> I hope not. In my surgeries, I never rely on the hardware for long durations. If I decide to do, for example, fusion, I rely on the hardware for a certain amount of time. And then I plan that the biology will do the work. If I plan for fusion, I put bone grafts in the preferred area for a fusion. Then if the hardware fails, I wouldn’t need to take out the hardware, and there would be no change in the condition of the patient.<br/><br/><strong>Dr. Wilson:</strong> What an incredible story. It’s clear that you and your team kept your cool despite a very high-acuity situation with a ton of risk. What a tremendous outcome that this boy is not only alive but fully functional. So, congratulations to you and your team. That was very strong work.<br/><br/><strong>Dr. Einav:</strong> Thank you very much. I would like to thank our team. We have to remember that the surgeon is not standing alone in the war. Hassan’s story is a success story of a very big group of people from various backgrounds and religions. They work day and night to help people and save lives. To the paramedics, the physiologists, the traumatologists, the pediatricians, the nurses, the physiotherapists, and obviously the surgeons, a big thank you. His story is our success story.<br/><br/><strong>Dr. Wilson:</strong> It’s inspiring to see so many people come together to do what we all are here for, which is to fight against suffering, disease, and death. Thank you for keeping up that fight. And thank you for joining me here.<br/><br/><strong>Dr. Einav:</strong> Thank you very much.</p> <p> <em>A version of this article first appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/995427">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Bone-bashing effects of air pollution becoming clearer
We have long recognized that our environment has a significant impact on our general health. Air pollution is known to contribute to respiratory conditions, poor cardiovascular outcomes, and certain kinds of cancer.
It’s increasingly important to identify factors that might contribute to suboptimal bone density and associated fracture risk in the population as a whole, and particularly in older adults. Aging is associated with a higher risk for osteoporosis and fractures, with their attendant morbidity, but individuals differ in their extent of bone loss and risk for fractures.
Known factors affecting bone health include genetics, age, sex, nutrition, physical activity, and hormonal factors. Certain medications, diseases, and lifestyle choices – such as smoking and alcohol intake – can also have deleterious effects on bone.
More recently, researchers have started examining the impact of air pollution on bone health.
As we know, the degree of pollution varies greatly from one region to another and can potentially significantly affect life in many parts of the world. In fact, the World Health Organization indicates that 99% of the world’s population breathes air exceeding the WHO guideline limits for pollutants.
Air pollutants include particulate matter (PM) as well as gases, such as nitric oxide, nitrogen dioxide, ammonia, carbon monoxide, sulfur dioxide, ozone, and certain volatile organic compounds. Particulate pollutants include a variety of substances produced from mostly human activities (such as vehicle emissions, biofuel combustion, mining, agriculture, and manufacturing, and also forest fires). They are classified not by their composition, but by their size (for example, PM1.0, PM2.5, and PM10 indicate PM with a diameter < 1.0, 2.5, and 10 microns, respectively). The finer the particle, the more likely it is to cross into the systemic circulation from the respiratory tract, with the potential to induce oxidative, inflammatory, and other changes in the body.
Many studies report that air pollution is a risk factor for osteoporosis. Some have found associations of lower bone density, osteoporosis, and fracture risk with higher concentrations of PM1.0, PM2.5, or PM10, even after controlling for other factors that could affect bone health. Some researchers have reported that although they didn’t find a significant association between PM and bone health, they did find an association between distance from the freeway and bone health – thus, exposure to polycyclic aromatic hydrocarbons and black carbon from vehicle emissions needs to be studied as a contributor to fracture risk.
Importantly, a prospective, observational study from the Women’s Health Initiative (which included more than 9,000 ethnically diverse women from three sites in the United States) reported a significant negative impact of PM10, nitric oxide, nitrogen dioxide, and sulfur dioxide over 1, 3, and 5 years on bone density at multiple sites, and particularly at the lumbar spine, in both cross-sectional and longitudinal analyses after controlling for demographic and socioeconomic factors. This study reported that nitrogen dioxide exposure may be a key determinant of bone density at the lumbar spine and in the whole body. Similarly, other studies have reported associations between atmospheric nitrogen dioxide or sulfur dioxide and risk for osteoporotic fractures.
Why the impact on bones?
The potential negative impact of pollution on bone has been attributed to many factors. PM induces systemic inflammation and an increase in cytokines that stimulate bone cells (osteoclasts) that cause bone loss. Other pollutants (gases and metal compounds) can cause oxidative damage to bone cells, whereas others act as endocrine disrupters and affect the functioning of these cells.
Pollution might also affect the synthesis and metabolism of vitamin D, which is necessary for absorption of calcium from the gut. High rates of pollution can reduce the amount of ultraviolet radiation reaching the earth which is important because certain wavelengths of ultraviolet radiation are necessary for vitamin D synthesis in our skin. Reduced vitamin D synthesis in skin can lead to poorly mineralized bone unless there is sufficient intake of vitamin D in diet or as supplements. Also, the conversion of vitamin D to its active form happens in the kidneys, and PM can be harmful to renal function. PM is also believed to cause increased breakdown of vitamin D into its inactive form.
Conversely, some studies have reported no association between pollution and bone density or osteoporosis risk, and two meta-analyses indicated that the association between the two is inconsistent. Some factors explaining variances in results include the number of individuals included in the study (larger studies are generally considered to be more reproducible), the fact that most studies are cross-sectional and not prospective, many do not control for other factors that might be deleterious to bone, and prediction models for the extent of PM or other exposure may not be completely accurate.
However, another recent meta-analysis reported an increased risk for lower total-body bone density and hip fracture after exposure to air pollution, particularly PM2.5 and nitrogen dioxide, but not to PM10, nitric oxide, or ozone. More studies are needed to confirm, or refute, the association between air pollution and impaired bone health. But accumulating evidence suggests that air pollution very likely has a deleterious effect on bone.
When feasible, it’s important to avoid living or working in areas with poor air quality and high pollution rates. However, this isn’t always possible based on one’s occupation, geography, circumstances, or economic status. Therefore, attention to a cleaner environment is critical at both the individual and the macro level.
As an example of the latter, the city of London extended its ultralow emission zone (ULEZ) farther out of the city in October 2021, and a further expansion is planned to include all of the city’s boroughs in August 2023.
We can do our bit by driving less and walking, biking, or using public transportation more often. We can also turn off the car engine when it’s not running, maintain our vehicles, switch to electric or hand-powered yard equipment, and not burn household garbage and limit backyard fires. We can also switch from gas to solar energy or wind, use efficient appliances and heating, and avoid unnecessary energy use. And we can choose sustainable products when possible.
For optimal bone health, we should remind patients to eat a healthy diet with the requisite amount of protein, calcium, and vitamin D. Vitamin D and calcium supplementation may be necessary for people whose intake of dairy and dairy products is low. Other important strategies to optimize bone health include engaging in healthy physical activity; avoiding smoking or excessive alcohol intake; and treating underlying gastrointestinal, endocrine, or other conditions that can reduce bone density.
Madhusmita Misra, MD, MPH, is the chief of the division of pediatric endocrinology, Mass General for Children; the associate director of the Harvard Catalyst Translation and Clinical Research Center; and the director of the Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital, Boston.
A version of this article first appeared on Medscape.com.
We have long recognized that our environment has a significant impact on our general health. Air pollution is known to contribute to respiratory conditions, poor cardiovascular outcomes, and certain kinds of cancer.
It’s increasingly important to identify factors that might contribute to suboptimal bone density and associated fracture risk in the population as a whole, and particularly in older adults. Aging is associated with a higher risk for osteoporosis and fractures, with their attendant morbidity, but individuals differ in their extent of bone loss and risk for fractures.
Known factors affecting bone health include genetics, age, sex, nutrition, physical activity, and hormonal factors. Certain medications, diseases, and lifestyle choices – such as smoking and alcohol intake – can also have deleterious effects on bone.
More recently, researchers have started examining the impact of air pollution on bone health.
As we know, the degree of pollution varies greatly from one region to another and can potentially significantly affect life in many parts of the world. In fact, the World Health Organization indicates that 99% of the world’s population breathes air exceeding the WHO guideline limits for pollutants.
Air pollutants include particulate matter (PM) as well as gases, such as nitric oxide, nitrogen dioxide, ammonia, carbon monoxide, sulfur dioxide, ozone, and certain volatile organic compounds. Particulate pollutants include a variety of substances produced from mostly human activities (such as vehicle emissions, biofuel combustion, mining, agriculture, and manufacturing, and also forest fires). They are classified not by their composition, but by their size (for example, PM1.0, PM2.5, and PM10 indicate PM with a diameter < 1.0, 2.5, and 10 microns, respectively). The finer the particle, the more likely it is to cross into the systemic circulation from the respiratory tract, with the potential to induce oxidative, inflammatory, and other changes in the body.
Many studies report that air pollution is a risk factor for osteoporosis. Some have found associations of lower bone density, osteoporosis, and fracture risk with higher concentrations of PM1.0, PM2.5, or PM10, even after controlling for other factors that could affect bone health. Some researchers have reported that although they didn’t find a significant association between PM and bone health, they did find an association between distance from the freeway and bone health – thus, exposure to polycyclic aromatic hydrocarbons and black carbon from vehicle emissions needs to be studied as a contributor to fracture risk.
Importantly, a prospective, observational study from the Women’s Health Initiative (which included more than 9,000 ethnically diverse women from three sites in the United States) reported a significant negative impact of PM10, nitric oxide, nitrogen dioxide, and sulfur dioxide over 1, 3, and 5 years on bone density at multiple sites, and particularly at the lumbar spine, in both cross-sectional and longitudinal analyses after controlling for demographic and socioeconomic factors. This study reported that nitrogen dioxide exposure may be a key determinant of bone density at the lumbar spine and in the whole body. Similarly, other studies have reported associations between atmospheric nitrogen dioxide or sulfur dioxide and risk for osteoporotic fractures.
Why the impact on bones?
The potential negative impact of pollution on bone has been attributed to many factors. PM induces systemic inflammation and an increase in cytokines that stimulate bone cells (osteoclasts) that cause bone loss. Other pollutants (gases and metal compounds) can cause oxidative damage to bone cells, whereas others act as endocrine disrupters and affect the functioning of these cells.
Pollution might also affect the synthesis and metabolism of vitamin D, which is necessary for absorption of calcium from the gut. High rates of pollution can reduce the amount of ultraviolet radiation reaching the earth which is important because certain wavelengths of ultraviolet radiation are necessary for vitamin D synthesis in our skin. Reduced vitamin D synthesis in skin can lead to poorly mineralized bone unless there is sufficient intake of vitamin D in diet or as supplements. Also, the conversion of vitamin D to its active form happens in the kidneys, and PM can be harmful to renal function. PM is also believed to cause increased breakdown of vitamin D into its inactive form.
Conversely, some studies have reported no association between pollution and bone density or osteoporosis risk, and two meta-analyses indicated that the association between the two is inconsistent. Some factors explaining variances in results include the number of individuals included in the study (larger studies are generally considered to be more reproducible), the fact that most studies are cross-sectional and not prospective, many do not control for other factors that might be deleterious to bone, and prediction models for the extent of PM or other exposure may not be completely accurate.
However, another recent meta-analysis reported an increased risk for lower total-body bone density and hip fracture after exposure to air pollution, particularly PM2.5 and nitrogen dioxide, but not to PM10, nitric oxide, or ozone. More studies are needed to confirm, or refute, the association between air pollution and impaired bone health. But accumulating evidence suggests that air pollution very likely has a deleterious effect on bone.
When feasible, it’s important to avoid living or working in areas with poor air quality and high pollution rates. However, this isn’t always possible based on one’s occupation, geography, circumstances, or economic status. Therefore, attention to a cleaner environment is critical at both the individual and the macro level.
As an example of the latter, the city of London extended its ultralow emission zone (ULEZ) farther out of the city in October 2021, and a further expansion is planned to include all of the city’s boroughs in August 2023.
We can do our bit by driving less and walking, biking, or using public transportation more often. We can also turn off the car engine when it’s not running, maintain our vehicles, switch to electric or hand-powered yard equipment, and not burn household garbage and limit backyard fires. We can also switch from gas to solar energy or wind, use efficient appliances and heating, and avoid unnecessary energy use. And we can choose sustainable products when possible.
For optimal bone health, we should remind patients to eat a healthy diet with the requisite amount of protein, calcium, and vitamin D. Vitamin D and calcium supplementation may be necessary for people whose intake of dairy and dairy products is low. Other important strategies to optimize bone health include engaging in healthy physical activity; avoiding smoking or excessive alcohol intake; and treating underlying gastrointestinal, endocrine, or other conditions that can reduce bone density.
Madhusmita Misra, MD, MPH, is the chief of the division of pediatric endocrinology, Mass General for Children; the associate director of the Harvard Catalyst Translation and Clinical Research Center; and the director of the Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital, Boston.
A version of this article first appeared on Medscape.com.
We have long recognized that our environment has a significant impact on our general health. Air pollution is known to contribute to respiratory conditions, poor cardiovascular outcomes, and certain kinds of cancer.
It’s increasingly important to identify factors that might contribute to suboptimal bone density and associated fracture risk in the population as a whole, and particularly in older adults. Aging is associated with a higher risk for osteoporosis and fractures, with their attendant morbidity, but individuals differ in their extent of bone loss and risk for fractures.
Known factors affecting bone health include genetics, age, sex, nutrition, physical activity, and hormonal factors. Certain medications, diseases, and lifestyle choices – such as smoking and alcohol intake – can also have deleterious effects on bone.
More recently, researchers have started examining the impact of air pollution on bone health.
As we know, the degree of pollution varies greatly from one region to another and can potentially significantly affect life in many parts of the world. In fact, the World Health Organization indicates that 99% of the world’s population breathes air exceeding the WHO guideline limits for pollutants.
Air pollutants include particulate matter (PM) as well as gases, such as nitric oxide, nitrogen dioxide, ammonia, carbon monoxide, sulfur dioxide, ozone, and certain volatile organic compounds. Particulate pollutants include a variety of substances produced from mostly human activities (such as vehicle emissions, biofuel combustion, mining, agriculture, and manufacturing, and also forest fires). They are classified not by their composition, but by their size (for example, PM1.0, PM2.5, and PM10 indicate PM with a diameter < 1.0, 2.5, and 10 microns, respectively). The finer the particle, the more likely it is to cross into the systemic circulation from the respiratory tract, with the potential to induce oxidative, inflammatory, and other changes in the body.
Many studies report that air pollution is a risk factor for osteoporosis. Some have found associations of lower bone density, osteoporosis, and fracture risk with higher concentrations of PM1.0, PM2.5, or PM10, even after controlling for other factors that could affect bone health. Some researchers have reported that although they didn’t find a significant association between PM and bone health, they did find an association between distance from the freeway and bone health – thus, exposure to polycyclic aromatic hydrocarbons and black carbon from vehicle emissions needs to be studied as a contributor to fracture risk.
Importantly, a prospective, observational study from the Women’s Health Initiative (which included more than 9,000 ethnically diverse women from three sites in the United States) reported a significant negative impact of PM10, nitric oxide, nitrogen dioxide, and sulfur dioxide over 1, 3, and 5 years on bone density at multiple sites, and particularly at the lumbar spine, in both cross-sectional and longitudinal analyses after controlling for demographic and socioeconomic factors. This study reported that nitrogen dioxide exposure may be a key determinant of bone density at the lumbar spine and in the whole body. Similarly, other studies have reported associations between atmospheric nitrogen dioxide or sulfur dioxide and risk for osteoporotic fractures.
Why the impact on bones?
The potential negative impact of pollution on bone has been attributed to many factors. PM induces systemic inflammation and an increase in cytokines that stimulate bone cells (osteoclasts) that cause bone loss. Other pollutants (gases and metal compounds) can cause oxidative damage to bone cells, whereas others act as endocrine disrupters and affect the functioning of these cells.
Pollution might also affect the synthesis and metabolism of vitamin D, which is necessary for absorption of calcium from the gut. High rates of pollution can reduce the amount of ultraviolet radiation reaching the earth which is important because certain wavelengths of ultraviolet radiation are necessary for vitamin D synthesis in our skin. Reduced vitamin D synthesis in skin can lead to poorly mineralized bone unless there is sufficient intake of vitamin D in diet or as supplements. Also, the conversion of vitamin D to its active form happens in the kidneys, and PM can be harmful to renal function. PM is also believed to cause increased breakdown of vitamin D into its inactive form.
Conversely, some studies have reported no association between pollution and bone density or osteoporosis risk, and two meta-analyses indicated that the association between the two is inconsistent. Some factors explaining variances in results include the number of individuals included in the study (larger studies are generally considered to be more reproducible), the fact that most studies are cross-sectional and not prospective, many do not control for other factors that might be deleterious to bone, and prediction models for the extent of PM or other exposure may not be completely accurate.
However, another recent meta-analysis reported an increased risk for lower total-body bone density and hip fracture after exposure to air pollution, particularly PM2.5 and nitrogen dioxide, but not to PM10, nitric oxide, or ozone. More studies are needed to confirm, or refute, the association between air pollution and impaired bone health. But accumulating evidence suggests that air pollution very likely has a deleterious effect on bone.
When feasible, it’s important to avoid living or working in areas with poor air quality and high pollution rates. However, this isn’t always possible based on one’s occupation, geography, circumstances, or economic status. Therefore, attention to a cleaner environment is critical at both the individual and the macro level.
As an example of the latter, the city of London extended its ultralow emission zone (ULEZ) farther out of the city in October 2021, and a further expansion is planned to include all of the city’s boroughs in August 2023.
We can do our bit by driving less and walking, biking, or using public transportation more often. We can also turn off the car engine when it’s not running, maintain our vehicles, switch to electric or hand-powered yard equipment, and not burn household garbage and limit backyard fires. We can also switch from gas to solar energy or wind, use efficient appliances and heating, and avoid unnecessary energy use. And we can choose sustainable products when possible.
For optimal bone health, we should remind patients to eat a healthy diet with the requisite amount of protein, calcium, and vitamin D. Vitamin D and calcium supplementation may be necessary for people whose intake of dairy and dairy products is low. Other important strategies to optimize bone health include engaging in healthy physical activity; avoiding smoking or excessive alcohol intake; and treating underlying gastrointestinal, endocrine, or other conditions that can reduce bone density.
Madhusmita Misra, MD, MPH, is the chief of the division of pediatric endocrinology, Mass General for Children; the associate director of the Harvard Catalyst Translation and Clinical Research Center; and the director of the Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital, Boston.
A version of this article first appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>163133</fileName> <TBEID>0C049AFB.SIG</TBEID> <TBUniqueIdentifier>MD_0C049AFB</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>353</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20230420T142915</QCDate> <firstPublished>20230420T150102</firstPublished> <LastPublished>20230420T150102</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20230420T150102</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Madhusmita Misra</byline> <bylineText>MADHUSMITA MISRA, MD, MPH</bylineText> <bylineFull>MADHUSMITA MISRA, MD, MPH</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType/> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Less well-known (or studied) is the potential impact of such fumes on bone health.</metaDescription> <articlePDF/> <teaserImage/> <teaser>Particulate matter induces systemic inflammation and an increase in cytokines that stimulate bone cells (osteoclasts) that cause bone loss.</teaser> <title>Bone-bashing effects of air pollution becoming clearer</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>endo</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>rn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">34</term> <term>26</term> </publications> <sections> <term canonical="true">52</term> </sections> <topics> <term canonical="true">266</term> <term>264</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Bone-bashing effects of air pollution becoming clearer</title> <deck/> </itemMeta> <itemContent> <p>We have long recognized that our environment has a significant impact on our general health. Air pollution is known to contribute to respiratory conditions, poor cardiovascular outcomes, and certain kinds of cancer. <span class="tag metaDescription">Less well-known (or studied) is the potential impact of such fumes on bone health.</span><br/><br/>It’s increasingly important to identify factors that might contribute to suboptimal bone density and associated fracture risk in the population as a whole, and particularly in older adults. Aging is associated with a higher risk for osteoporosis and fractures, with their attendant morbidity, but individuals differ in their extent of bone loss and risk for fractures.<br/><br/>Known factors affecting bone health include genetics, age, sex, nutrition, physical activity, and hormonal factors. Certain medications, diseases, and lifestyle choices – such as smoking and alcohol intake – can also have deleterious effects on bone.</p> <p>More recently, researchers have started examining the impact of air pollution on bone health.<br/><br/>As we know, the degree of pollution varies greatly from one region to another and can potentially significantly affect life in many parts of the world. In fact, the World Health Organization indicates that 99% of the world’s population breathes air exceeding the WHO guideline limits for pollutants.<br/><br/>Air pollutants include particulate matter (PM) as well as gases, such as nitric oxide, nitrogen dioxide, ammonia, carbon monoxide, sulfur dioxide, ozone, and certain volatile organic compounds. <a href="https://www.dovepress.com/particulate-air-pollution-and-osteoporosis-a-systematic-review-peer-reviewed-fulltext-article-RMHP">Particulate pollutants</a> include a variety of substances produced from mostly human activities (such as vehicle emissions, biofuel combustion, mining, agriculture, and manufacturing, and also forest fires). They are classified not by their composition, but by their size (for example, PM1.0, PM2.5, and PM10 indicate PM with a diameter < 1.0, 2.5, and 10 microns, respectively). The finer the particle, the more likely it is to cross into the systemic circulation from the respiratory tract, with the potential to induce oxidative, inflammatory, and other changes in the body.<br/><br/>Many studies report that air pollution is a risk factor for osteoporosis. Some have found associations of lower bone density, osteoporosis, and fracture risk with higher concentrations of PM1.0, PM2.5, or PM10, even after controlling for other factors that could affect bone health. Some researchers have reported that although they didn’t find a significant association between PM and bone health, they did find an association between distance from the freeway and bone health – thus, exposure to polycyclic aromatic hydrocarbons and black carbon from vehicle emissions needs to be studied as a contributor to fracture risk.<br/><br/>Importantly, a prospective, observational study from the Women’s Health Initiative (which included more than 9,000 ethnically diverse women from three sites in the United States) reported a significant negative impact of PM10, nitric oxide, nitrogen dioxide, and sulfur dioxide over 1, 3, and 5 years on bone density at multiple sites, and particularly at the lumbar spine, in both cross-sectional and longitudinal analyses after controlling for demographic and socioeconomic factors. This study reported that nitrogen dioxide exposure may be a key determinant of bone density at the lumbar spine and in the whole body. Similarly, other studies have reported associations between atmospheric nitrogen dioxide or sulfur dioxide and risk for osteoporotic fractures.<br/><br/></p> <h2>Why the impact on bones?</h2> <p>The potential negative impact of pollution on bone has been attributed to many factors. PM induces systemic inflammation and an increase in cytokines that stimulate bone cells (osteoclasts) that cause bone loss. Other pollutants (gases and metal compounds) can cause oxidative damage to bone cells, whereas others act as endocrine disrupters and affect the functioning of these cells.</p> <p>Pollution might also affect the synthesis and metabolism of vitamin D, which is necessary for absorption of calcium from the gut. High rates of pollution can reduce the amount of ultraviolet radiation reaching the earth which is important because certain wavelengths of ultraviolet radiation are necessary for vitamin D synthesis in our skin. Reduced vitamin D synthesis in skin can lead to poorly mineralized bone unless there is sufficient intake of vitamin D in diet or as supplements. Also, the conversion of vitamin D to its active form happens in the kidneys, and PM can be harmful to renal function. PM is also believed to cause increased breakdown of vitamin D into its inactive form.<br/><br/>Conversely, some studies have reported no association between pollution and bone density or osteoporosis risk, and two meta-analyses indicated that the association between the two is inconsistent. Some factors explaining variances in results include the number of individuals included in the study (larger studies are generally considered to be more reproducible), the fact that most studies are cross-sectional and not prospective, many do not control for other factors that might be deleterious to bone, and prediction models for the extent of PM or other exposure may not be completely accurate.<br/><br/>However, another recent meta-analysis reported an increased risk for lower total-body bone density and hip fracture after exposure to air pollution, particularly PM2.5 and nitrogen dioxide, but not to PM10, nitric oxide, or ozone. More studies are needed to confirm, or refute, the association between air pollution and impaired bone health. But accumulating evidence suggests that air pollution very likely has a deleterious effect on bone.<br/><br/>When feasible, it’s important to avoid living or working in areas with poor air quality and high pollution rates. However, this isn’t always possible based on one’s occupation, geography, circumstances, or economic status. Therefore, attention to a cleaner environment is critical at both the individual and the macro level.<br/><br/>As an example of the latter, the city of London extended its <a href="https://tfl.gov.uk/modes/driving/ultra-low-emission-zone/ulez-expansion-2023">ultralow emission zone</a> (ULEZ) farther out of the city in October 2021, and a further expansion is planned to include all of the city’s boroughs in August 2023.<br/><br/>We can do our bit by driving less and walking, biking, or using public transportation more often. We can also turn off the car engine when it’s not running, maintain our vehicles, switch to electric or hand-powered yard equipment, and not burn household garbage and limit backyard fires. We can also switch from gas to solar energy or wind, use efficient appliances and heating, and avoid unnecessary energy use. And we can choose sustainable products when possible.<br/><br/>For optimal bone health, we should remind patients to eat a healthy diet with the requisite amount of protein, calcium, and vitamin D. Vitamin D and calcium supplementation may be necessary for people whose intake of dairy and dairy products is low. Other important strategies to optimize bone health include engaging in healthy physical activity; avoiding smoking or excessive alcohol intake; and treating underlying gastrointestinal, endocrine, or other conditions that can reduce bone density.</p> <p> <em>Madhusmita Misra, MD, MPH, is the chief of the division of pediatric endocrinology, Mass General for Children; the associate director of the Harvard Catalyst Translation and Clinical Research Center; and the director of the Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital, Boston.<span class="end"/></em> </p> <p> <em>A version of this article first appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/990840">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
Surgery for early breast cancer can worsen frailty in older women
according to a new study.
About 1 in 5 experienced clinically significant deterioration in frailty status after treatment, the study team found. Women at highest risk for declines in frailty following treatment had “robust” baseline frailty status at diagnosis and underwent more invasive mastectomy compared with lumpectomy.
The fact that “robust” older women were more likely to become frail after locoregional therapy suggests that “thoughtful treatment decisions should be undertaken in all older women, not simply those who have frailty at diagnosis,” said the investigators, led by Christina Minami, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston.
The study findings emphasize that there is no one-size-fits-all approach to breast cancer treatment in the elderly, said Sarah P. Cate, MD, director, Breast Surgery Quality Program, Mount Sinai Health System, New York, who wasn’t involved in the research. “Some patients will sail through a surgery, and others are severely affected by it.”
The study was published online in JAMA Surgery.
Given the growing number of older adults with breast cancer, understanding how age-related syndromes, such as frailty, may alter cancer outcomes and how cancer treatments change aging trajectories remains important.
To investigate, Dr. Minami and colleagues used Surveillance, Epidemiology, and End Results Medicare data to identify 31,084 women (mean age, 73) who had been diagnosed with ductal carcinoma in situ (DCIS) or stage I HR-positive, ERBB2-positive breast cancer and who underwent surgery (23% mastectomy, 77% lumpectomy) and radiation therapy.
Worsening frailty status was defined as a decline of 0.03 or greater in a validated frailty index from the time of diagnosis to 1 year. This level of change has been linked to greater mortality risk and greater cost of care.
Frailty status at diagnosis was “robust” in 56% of the women, prefrail in 40%, mildly frail in 4%, and moderately to severely frail in 0.3%.
According to the researchers, 21.4% of the women experienced clinically significant declines in their frailty status after treatment. These declines occurred in 25% of women who underwent mastectomy and 20% of those who underwent lumpectomy.
After adjusting for covariates, there was a higher likelihood of worsening frailty among women who were robustly frail at baseline, in comparison with those who were moderately to severely frail at baseline (odds ratio, 6.12), and in those who underwent mastectomy vs. lumpectomy (OR, 1.31).
Older age and race were also linked to worsening frailty status following treatment. Compared with younger women (aged 65-74 years), older women were more likely to experience worsening frailty (OR, 1.21 for women aged 75-79; OR, 1.53 for those aged 80-84; OR, 1.94 for those aged 85 and older). In addition, Black women were more likely than non-Hispanic White women to experience worsening frailty after treatment (OR, 1.12).
“Previous studies have documented lasting declines in functional status after surgery in older patients with breast cancer, but breast cancer treatment has not been implicated in worsening frailty to date,” Dr. Minami and colleagues explain. But “given the substantial proportion of women experiencing worsening frailty and the significant difference by breast surgery type, frailty status as a cancer therapy outcome should be further explored.” In addition, “tailoring locoregional therapy intensity in this population is important,” they write.
Dr. Cate explained that randomized clinical trials such as COMET and LORIS, which explore the monitoring of patients with DCIS in lieu of active treatment, “will likely make a big impact on this population, as we currently do not have randomized controlled data for observation of breast cancer.”
Dr. Cate added as well that assessing a patient’s ECOG [Eastern Cooperative Oncology Group] performance status is vital “to determine who can really tolerate a breast cancer surgery” and that opting for antiestrogens, such as aromatase inhibitors, which can keep cancer at bay for years, “may be preferable for many older patients.”
The study was funded by Brigham and Women’s Hospital’s Department of Surgery’s Beal Fellowship. Dr. Minami and Dr. Cate have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
About 1 in 5 experienced clinically significant deterioration in frailty status after treatment, the study team found. Women at highest risk for declines in frailty following treatment had “robust” baseline frailty status at diagnosis and underwent more invasive mastectomy compared with lumpectomy.
The fact that “robust” older women were more likely to become frail after locoregional therapy suggests that “thoughtful treatment decisions should be undertaken in all older women, not simply those who have frailty at diagnosis,” said the investigators, led by Christina Minami, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston.
The study findings emphasize that there is no one-size-fits-all approach to breast cancer treatment in the elderly, said Sarah P. Cate, MD, director, Breast Surgery Quality Program, Mount Sinai Health System, New York, who wasn’t involved in the research. “Some patients will sail through a surgery, and others are severely affected by it.”
The study was published online in JAMA Surgery.
Given the growing number of older adults with breast cancer, understanding how age-related syndromes, such as frailty, may alter cancer outcomes and how cancer treatments change aging trajectories remains important.
To investigate, Dr. Minami and colleagues used Surveillance, Epidemiology, and End Results Medicare data to identify 31,084 women (mean age, 73) who had been diagnosed with ductal carcinoma in situ (DCIS) or stage I HR-positive, ERBB2-positive breast cancer and who underwent surgery (23% mastectomy, 77% lumpectomy) and radiation therapy.
Worsening frailty status was defined as a decline of 0.03 or greater in a validated frailty index from the time of diagnosis to 1 year. This level of change has been linked to greater mortality risk and greater cost of care.
Frailty status at diagnosis was “robust” in 56% of the women, prefrail in 40%, mildly frail in 4%, and moderately to severely frail in 0.3%.
According to the researchers, 21.4% of the women experienced clinically significant declines in their frailty status after treatment. These declines occurred in 25% of women who underwent mastectomy and 20% of those who underwent lumpectomy.
After adjusting for covariates, there was a higher likelihood of worsening frailty among women who were robustly frail at baseline, in comparison with those who were moderately to severely frail at baseline (odds ratio, 6.12), and in those who underwent mastectomy vs. lumpectomy (OR, 1.31).
Older age and race were also linked to worsening frailty status following treatment. Compared with younger women (aged 65-74 years), older women were more likely to experience worsening frailty (OR, 1.21 for women aged 75-79; OR, 1.53 for those aged 80-84; OR, 1.94 for those aged 85 and older). In addition, Black women were more likely than non-Hispanic White women to experience worsening frailty after treatment (OR, 1.12).
“Previous studies have documented lasting declines in functional status after surgery in older patients with breast cancer, but breast cancer treatment has not been implicated in worsening frailty to date,” Dr. Minami and colleagues explain. But “given the substantial proportion of women experiencing worsening frailty and the significant difference by breast surgery type, frailty status as a cancer therapy outcome should be further explored.” In addition, “tailoring locoregional therapy intensity in this population is important,” they write.
Dr. Cate explained that randomized clinical trials such as COMET and LORIS, which explore the monitoring of patients with DCIS in lieu of active treatment, “will likely make a big impact on this population, as we currently do not have randomized controlled data for observation of breast cancer.”
Dr. Cate added as well that assessing a patient’s ECOG [Eastern Cooperative Oncology Group] performance status is vital “to determine who can really tolerate a breast cancer surgery” and that opting for antiestrogens, such as aromatase inhibitors, which can keep cancer at bay for years, “may be preferable for many older patients.”
The study was funded by Brigham and Women’s Hospital’s Department of Surgery’s Beal Fellowship. Dr. Minami and Dr. Cate have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
About 1 in 5 experienced clinically significant deterioration in frailty status after treatment, the study team found. Women at highest risk for declines in frailty following treatment had “robust” baseline frailty status at diagnosis and underwent more invasive mastectomy compared with lumpectomy.
The fact that “robust” older women were more likely to become frail after locoregional therapy suggests that “thoughtful treatment decisions should be undertaken in all older women, not simply those who have frailty at diagnosis,” said the investigators, led by Christina Minami, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston.
The study findings emphasize that there is no one-size-fits-all approach to breast cancer treatment in the elderly, said Sarah P. Cate, MD, director, Breast Surgery Quality Program, Mount Sinai Health System, New York, who wasn’t involved in the research. “Some patients will sail through a surgery, and others are severely affected by it.”
The study was published online in JAMA Surgery.
Given the growing number of older adults with breast cancer, understanding how age-related syndromes, such as frailty, may alter cancer outcomes and how cancer treatments change aging trajectories remains important.
To investigate, Dr. Minami and colleagues used Surveillance, Epidemiology, and End Results Medicare data to identify 31,084 women (mean age, 73) who had been diagnosed with ductal carcinoma in situ (DCIS) or stage I HR-positive, ERBB2-positive breast cancer and who underwent surgery (23% mastectomy, 77% lumpectomy) and radiation therapy.
Worsening frailty status was defined as a decline of 0.03 or greater in a validated frailty index from the time of diagnosis to 1 year. This level of change has been linked to greater mortality risk and greater cost of care.
Frailty status at diagnosis was “robust” in 56% of the women, prefrail in 40%, mildly frail in 4%, and moderately to severely frail in 0.3%.
According to the researchers, 21.4% of the women experienced clinically significant declines in their frailty status after treatment. These declines occurred in 25% of women who underwent mastectomy and 20% of those who underwent lumpectomy.
After adjusting for covariates, there was a higher likelihood of worsening frailty among women who were robustly frail at baseline, in comparison with those who were moderately to severely frail at baseline (odds ratio, 6.12), and in those who underwent mastectomy vs. lumpectomy (OR, 1.31).
Older age and race were also linked to worsening frailty status following treatment. Compared with younger women (aged 65-74 years), older women were more likely to experience worsening frailty (OR, 1.21 for women aged 75-79; OR, 1.53 for those aged 80-84; OR, 1.94 for those aged 85 and older). In addition, Black women were more likely than non-Hispanic White women to experience worsening frailty after treatment (OR, 1.12).
“Previous studies have documented lasting declines in functional status after surgery in older patients with breast cancer, but breast cancer treatment has not been implicated in worsening frailty to date,” Dr. Minami and colleagues explain. But “given the substantial proportion of women experiencing worsening frailty and the significant difference by breast surgery type, frailty status as a cancer therapy outcome should be further explored.” In addition, “tailoring locoregional therapy intensity in this population is important,” they write.
Dr. Cate explained that randomized clinical trials such as COMET and LORIS, which explore the monitoring of patients with DCIS in lieu of active treatment, “will likely make a big impact on this population, as we currently do not have randomized controlled data for observation of breast cancer.”
Dr. Cate added as well that assessing a patient’s ECOG [Eastern Cooperative Oncology Group] performance status is vital “to determine who can really tolerate a breast cancer surgery” and that opting for antiestrogens, such as aromatase inhibitors, which can keep cancer at bay for years, “may be preferable for many older patients.”
The study was funded by Brigham and Women’s Hospital’s Department of Surgery’s Beal Fellowship. Dr. Minami and Dr. Cate have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>162765</fileName> <TBEID>0C0492B6.SIG</TBEID> <TBUniqueIdentifier>MD_0C0492B6</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20230322T142340</QCDate> <firstPublished>20230322T143729</firstPublished> <LastPublished>20230322T143729</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20230322T143729</CMSDate> <articleSource>FROM JAMA SURGERY</articleSource> <facebookInfo/> <meetingNumber/> <byline>Megan Brooks</byline> <bylineText>MEGAN BROOKS</bylineText> <bylineFull>MEGAN BROOKS</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>A substantial number of older women may experience worsening frailty after undergoing surgery and radiation therapy for early-stage breast cancer,</metaDescription> <articlePDF/> <teaserImage/> <teaser>About 1 in 5 experienced clinically significant deterioration in frailty status after treatment for breast cancer.</teaser> <title>Surgery for early breast cancer can worsen frailty in older women</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>mdsurg</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>oncr</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term>52226</term> <term>15</term> <term canonical="true">31</term> <term>21</term> </publications> <sections> <term>27970</term> <term canonical="true">39313</term> </sections> <topics> <term>252</term> <term>340</term> <term>263</term> <term>264</term> <term>322</term> <term>215</term> <term canonical="true">192</term> <term>270</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Surgery for early breast cancer can worsen frailty in older women</title> <deck/> </itemMeta> <itemContent> <p><span class="tag metaDescription">A substantial number of older women may experience worsening frailty after undergoing surgery and radiation therapy for early-stage breast cancer,</span> according to a new study.</p> <p>About 1 in 5 experienced clinically significant deterioration in frailty status after treatment, the study team found. Women at highest risk for declines in frailty following treatment had “robust” baseline frailty status at diagnosis and underwent more invasive mastectomy compared with lumpectomy.<br/><br/>The fact that “robust” older women were more likely to become frail after locoregional therapy suggests that “thoughtful treatment decisions should be undertaken in all older women, not simply those who have frailty at diagnosis,” said the investigators, led by Christina Minami, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston.<br/><br/>The study findings emphasize that there is no one-size-fits-all approach to breast cancer treatment in the elderly, said Sarah P. Cate, MD, director, Breast Surgery Quality Program, Mount Sinai Health System, New York, who wasn’t involved in the research. “Some patients will sail through a surgery, and others are severely affected by it.”<br/><br/>The study was <a href="https://jamanetwork.com/journals/jamasurgery/fullarticle/2802657">published online</a> in JAMA Surgery.<br/><br/>Given the growing number of older adults with breast cancer, understanding how age-related syndromes, such as frailty, may alter cancer outcomes and how cancer treatments change aging trajectories remains important.<br/><br/>To investigate, Dr. Minami and colleagues used Surveillance, Epidemiology, and End Results Medicare data to identify 31,084 women (mean age, 73) who had been diagnosed with ductal carcinoma in situ (DCIS) or stage I HR-positive, ERBB2-positive breast cancer and who underwent surgery (23% mastectomy, 77% lumpectomy) and radiation therapy.<br/><br/>Worsening frailty status was defined as a decline of 0.03 or greater in a validated frailty index from the time of diagnosis to 1 year. This level of change has been linked to greater mortality risk and greater cost of care.<br/><br/>Frailty status at diagnosis was “robust” in 56% of the women, prefrail in 40%, mildly frail in 4%, and moderately to severely frail in 0.3%.<br/><br/>According to the researchers, 21.4% of the women experienced clinically significant declines in their frailty status after treatment. These declines occurred in 25% of women who underwent mastectomy and 20% of those who underwent lumpectomy.<br/><br/>After adjusting for covariates, there was a higher likelihood of worsening frailty among women who were robustly frail at baseline, in comparison with those who were moderately to severely frail at baseline (odds ratio, 6.12), and in those who underwent mastectomy vs. lumpectomy (OR, 1.31).<br/><br/>Older age and race were also linked to worsening frailty status following treatment. Compared with younger women (aged 65-74 years), older women were more likely to experience worsening frailty (OR, 1.21 for women aged 75-79; OR, 1.53 for those aged 80-84; OR, 1.94 for those aged 85 and older). In addition, Black women were more likely than non-Hispanic White women to experience worsening frailty after treatment (OR, 1.12).<br/><br/>“Previous studies have documented lasting declines in functional status after surgery in older patients with breast cancer, but breast cancer treatment has not been implicated in worsening frailty to date,” Dr. Minami and colleagues explain. But “given the substantial proportion of women experiencing worsening frailty and the significant difference by breast surgery type, frailty status as a cancer therapy outcome should be further explored.” In addition, “tailoring locoregional therapy intensity in this population is important,” they write.<br/><br/>Dr. Cate explained that randomized clinical trials such as COMET and LORIS, which explore the monitoring of patients with DCIS in lieu of active treatment, “will likely make a big impact on this population, as we currently do not have randomized controlled data for observation of breast cancer.”<br/><br/>Dr. Cate added as well that assessing a patient’s ECOG [Eastern Cooperative Oncology Group] performance status is vital “to determine who can really tolerate a breast cancer surgery” and that opting for antiestrogens, such as aromatase inhibitors, which can keep cancer at bay for years, “may be preferable for many older patients.”<br/><br/>The study was funded by Brigham and Women’s Hospital’s Department of Surgery’s Beal Fellowship. Dr. Minami and Dr. Cate have disclosed no relevant financial relationships.<span class="end"/> <br/><br/></p> <p> <em>A version of this article first appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/989973">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
FROM JAMA SURGERY
Presurgical expectations may influence patients’ attitudes, experiences after knee replacement
DENVER – People with lower expectations of how they would be able to use their knees during work activities after a total knee arthroplasty were more dissatisfied with their knee abilities 6 months after their surgery, according to a study presented at the OARSI 2023 World Congress.
Two out of 10 patients are dissatisfied after total knee arthroplasty, which is increasingly performed in younger and working patients who may have higher demands, presenter Yvonne van Zaanen, a physiotherapist in occupational health and ergonomics and a PhD candidate at Amsterdam University Medical Center, told attendees.
The findings suggest a correlation between patients’ low presurgical expectations of their ability to use their knees and having more difficulty with their knees postoperatively, she said. “We should take better care of working patients with low expectations by managing their preoperative expectations and improving their ability to perform work-related knee-straining activities in rehabilitation,” Ms. van Zaanen told attendees.
The researchers conducted a multicenter, prospective cohort study involving seven hospitals. They surveyed 175 employed individuals aged 18-65 years who were scheduled for a total knee arthroplasty and intended to return to work after their surgery. The first survey occurred before the operation, and the follow-up occurred 6 months after the surgery.
Just over half the participants were women (53%), and the average participant age was 59. Respondents had a mean body mass index (BMI) of 29 kg/m2, and had a Knee injury and Osteoarthritis Outcome Score (KOOS) pain score of 42 (on a 0-to-100 scale in which lower scores are worse). About half the respondents (51%) had a job that involved knee-straining activities.
The researchers assessed participants’ ability to perform work-related, knee-straining activities using the Work, Osteoarthritis, or joint-Replacement Questionnaire (WORQ) tool, which considers the following activities: kneeling, crouching, clambering, taking the stairs, walking on rough terrain, working with hands below knee height, standing, lifting or carrying, pushing or pulling, walking on ground level, operating a vehicle, operating foot pedals, and sitting. The 0-to-100 scale rates the difficulty of using knees for each particular activity, with higher scores indicating greater ease and less pain in doing that activity.
Among the 107 patients who expected to be satisfied after their surgery, half (n = 53) were satisfied, compared with 12% (n = 13) who were unsatisfied; the remaining participants (n = 41, 38%) were neither satisfied nor dissatisfied. Among the 24 patients who expected to be dissatisfied after their surgery, one-third (n = 8) were satisfied and 42% (n = 10) were dissatisfied. The remaining 44 patients didn’t expect to be satisfied or dissatisfied before their surgery, and 41% of them were satisfied while 23% were dissatisfied.
The researchers found that patients’ expectation of their satisfaction level going into the surgery was the only preoperative factor to be prognostic for dissatisfaction 6 months after surgery, based on their WORQ score. That is, patients who expected to be dissatisfied before their surgery had approximately five times greater odds of being dissatisfied after their surgery than did those who expected to be satisfied with their ability to do knee-straining activities at work (odds ratio, 5.1; 95% confidence interval, 1.7-15.5). Among those with a WORQ score of 40, indicating a greater expectation of difficulty using their knees postoperatively, 55% were dissatisfied after their surgery, compared with 19% of those with a WORQ score of 85, who expected greater knee ability after their surgery.
[embed:render:related:node:261878]
The other factors that the researchers examined, which had no effect on WORQ scores, included age, sex, BMI, education, comorbidities, KOOS pain subscale, having a knee-straining job, having needed surgery because of work, or having preoperative sick leave.
One discussion prompted by the presentation focused specifically on individuals’ ability to kneel without much difficulty after their surgery, an activity that’s not typically considered likely, Ms. van Zaanen noted. One audience member, Gillian Hawker, MD, MSc, a professor of medicine in the division of rheumatology at the University of Toronto, questioned whether the field should accept that current reality from surgical intervention. Dr. Hawker described a cohort she had analyzed in which two-thirds of the participants had expected they would be able to kneel after their surgery, regardless of whether it was related to work or other activities.
“Kneeling is important, not just for work; it’s important for culture and religion and lots of other things,” Dr. Hawker said. “How will you help these people to kneel after knee replacement when the surgery isn’t really performed to enable people to do that?” In response, Ms. van Zaanen noted it might not be achievable, as the research literature demonstrates, but Dr. Hawker suggested that is itself problematic.
“I guess what I’m asking is, why are we settling for that? If it’s important to so many people, and an expectation of so many people, why don’t we technologically improve such that, post arthroplasty, people can kneel?”
Another commenter suggested that the study’s findings may not indicate a need to manage patients’ expectations prior to surgery so much as showing that some patients simply have realistic expectations of what they will and will not be able to do after knee replacement.
“Is it possible that people who had low expectations – those who expected to be dissatisfied afterwards – were appropriately understanding that they were likely to be dissatisfied afterwards, in which case, managing their expectations might do nothing for their dissatisfaction afterwards?” the commenter asked. It is likely necessary to conduct additional research about expectations before surgery and experiences after surgery to address that question, Ms. van Zaanen suggested.
Ms. van Zaanen and Dr. Hawker reported having no relevant financial relationships. The presentation did not note any external funding. The Congress was sponsored by the Osteoarthritis Research Society International.
DENVER – People with lower expectations of how they would be able to use their knees during work activities after a total knee arthroplasty were more dissatisfied with their knee abilities 6 months after their surgery, according to a study presented at the OARSI 2023 World Congress.
Two out of 10 patients are dissatisfied after total knee arthroplasty, which is increasingly performed in younger and working patients who may have higher demands, presenter Yvonne van Zaanen, a physiotherapist in occupational health and ergonomics and a PhD candidate at Amsterdam University Medical Center, told attendees.
The findings suggest a correlation between patients’ low presurgical expectations of their ability to use their knees and having more difficulty with their knees postoperatively, she said. “We should take better care of working patients with low expectations by managing their preoperative expectations and improving their ability to perform work-related knee-straining activities in rehabilitation,” Ms. van Zaanen told attendees.
The researchers conducted a multicenter, prospective cohort study involving seven hospitals. They surveyed 175 employed individuals aged 18-65 years who were scheduled for a total knee arthroplasty and intended to return to work after their surgery. The first survey occurred before the operation, and the follow-up occurred 6 months after the surgery.
Just over half the participants were women (53%), and the average participant age was 59. Respondents had a mean body mass index (BMI) of 29 kg/m2, and had a Knee injury and Osteoarthritis Outcome Score (KOOS) pain score of 42 (on a 0-to-100 scale in which lower scores are worse). About half the respondents (51%) had a job that involved knee-straining activities.
The researchers assessed participants’ ability to perform work-related, knee-straining activities using the Work, Osteoarthritis, or joint-Replacement Questionnaire (WORQ) tool, which considers the following activities: kneeling, crouching, clambering, taking the stairs, walking on rough terrain, working with hands below knee height, standing, lifting or carrying, pushing or pulling, walking on ground level, operating a vehicle, operating foot pedals, and sitting. The 0-to-100 scale rates the difficulty of using knees for each particular activity, with higher scores indicating greater ease and less pain in doing that activity.
Among the 107 patients who expected to be satisfied after their surgery, half (n = 53) were satisfied, compared with 12% (n = 13) who were unsatisfied; the remaining participants (n = 41, 38%) were neither satisfied nor dissatisfied. Among the 24 patients who expected to be dissatisfied after their surgery, one-third (n = 8) were satisfied and 42% (n = 10) were dissatisfied. The remaining 44 patients didn’t expect to be satisfied or dissatisfied before their surgery, and 41% of them were satisfied while 23% were dissatisfied.
The researchers found that patients’ expectation of their satisfaction level going into the surgery was the only preoperative factor to be prognostic for dissatisfaction 6 months after surgery, based on their WORQ score. That is, patients who expected to be dissatisfied before their surgery had approximately five times greater odds of being dissatisfied after their surgery than did those who expected to be satisfied with their ability to do knee-straining activities at work (odds ratio, 5.1; 95% confidence interval, 1.7-15.5). Among those with a WORQ score of 40, indicating a greater expectation of difficulty using their knees postoperatively, 55% were dissatisfied after their surgery, compared with 19% of those with a WORQ score of 85, who expected greater knee ability after their surgery.
[embed:render:related:node:261878]
The other factors that the researchers examined, which had no effect on WORQ scores, included age, sex, BMI, education, comorbidities, KOOS pain subscale, having a knee-straining job, having needed surgery because of work, or having preoperative sick leave.
One discussion prompted by the presentation focused specifically on individuals’ ability to kneel without much difficulty after their surgery, an activity that’s not typically considered likely, Ms. van Zaanen noted. One audience member, Gillian Hawker, MD, MSc, a professor of medicine in the division of rheumatology at the University of Toronto, questioned whether the field should accept that current reality from surgical intervention. Dr. Hawker described a cohort she had analyzed in which two-thirds of the participants had expected they would be able to kneel after their surgery, regardless of whether it was related to work or other activities.
“Kneeling is important, not just for work; it’s important for culture and religion and lots of other things,” Dr. Hawker said. “How will you help these people to kneel after knee replacement when the surgery isn’t really performed to enable people to do that?” In response, Ms. van Zaanen noted it might not be achievable, as the research literature demonstrates, but Dr. Hawker suggested that is itself problematic.
“I guess what I’m asking is, why are we settling for that? If it’s important to so many people, and an expectation of so many people, why don’t we technologically improve such that, post arthroplasty, people can kneel?”
Another commenter suggested that the study’s findings may not indicate a need to manage patients’ expectations prior to surgery so much as showing that some patients simply have realistic expectations of what they will and will not be able to do after knee replacement.
“Is it possible that people who had low expectations – those who expected to be dissatisfied afterwards – were appropriately understanding that they were likely to be dissatisfied afterwards, in which case, managing their expectations might do nothing for their dissatisfaction afterwards?” the commenter asked. It is likely necessary to conduct additional research about expectations before surgery and experiences after surgery to address that question, Ms. van Zaanen suggested.
Ms. van Zaanen and Dr. Hawker reported having no relevant financial relationships. The presentation did not note any external funding. The Congress was sponsored by the Osteoarthritis Research Society International.
DENVER – People with lower expectations of how they would be able to use their knees during work activities after a total knee arthroplasty were more dissatisfied with their knee abilities 6 months after their surgery, according to a study presented at the OARSI 2023 World Congress.
Two out of 10 patients are dissatisfied after total knee arthroplasty, which is increasingly performed in younger and working patients who may have higher demands, presenter Yvonne van Zaanen, a physiotherapist in occupational health and ergonomics and a PhD candidate at Amsterdam University Medical Center, told attendees.
The findings suggest a correlation between patients’ low presurgical expectations of their ability to use their knees and having more difficulty with their knees postoperatively, she said. “We should take better care of working patients with low expectations by managing their preoperative expectations and improving their ability to perform work-related knee-straining activities in rehabilitation,” Ms. van Zaanen told attendees.
The researchers conducted a multicenter, prospective cohort study involving seven hospitals. They surveyed 175 employed individuals aged 18-65 years who were scheduled for a total knee arthroplasty and intended to return to work after their surgery. The first survey occurred before the operation, and the follow-up occurred 6 months after the surgery.
Just over half the participants were women (53%), and the average participant age was 59. Respondents had a mean body mass index (BMI) of 29 kg/m2, and had a Knee injury and Osteoarthritis Outcome Score (KOOS) pain score of 42 (on a 0-to-100 scale in which lower scores are worse). About half the respondents (51%) had a job that involved knee-straining activities.
The researchers assessed participants’ ability to perform work-related, knee-straining activities using the Work, Osteoarthritis, or joint-Replacement Questionnaire (WORQ) tool, which considers the following activities: kneeling, crouching, clambering, taking the stairs, walking on rough terrain, working with hands below knee height, standing, lifting or carrying, pushing or pulling, walking on ground level, operating a vehicle, operating foot pedals, and sitting. The 0-to-100 scale rates the difficulty of using knees for each particular activity, with higher scores indicating greater ease and less pain in doing that activity.
Among the 107 patients who expected to be satisfied after their surgery, half (n = 53) were satisfied, compared with 12% (n = 13) who were unsatisfied; the remaining participants (n = 41, 38%) were neither satisfied nor dissatisfied. Among the 24 patients who expected to be dissatisfied after their surgery, one-third (n = 8) were satisfied and 42% (n = 10) were dissatisfied. The remaining 44 patients didn’t expect to be satisfied or dissatisfied before their surgery, and 41% of them were satisfied while 23% were dissatisfied.
The researchers found that patients’ expectation of their satisfaction level going into the surgery was the only preoperative factor to be prognostic for dissatisfaction 6 months after surgery, based on their WORQ score. That is, patients who expected to be dissatisfied before their surgery had approximately five times greater odds of being dissatisfied after their surgery than did those who expected to be satisfied with their ability to do knee-straining activities at work (odds ratio, 5.1; 95% confidence interval, 1.7-15.5). Among those with a WORQ score of 40, indicating a greater expectation of difficulty using their knees postoperatively, 55% were dissatisfied after their surgery, compared with 19% of those with a WORQ score of 85, who expected greater knee ability after their surgery.
[embed:render:related:node:261878]
The other factors that the researchers examined, which had no effect on WORQ scores, included age, sex, BMI, education, comorbidities, KOOS pain subscale, having a knee-straining job, having needed surgery because of work, or having preoperative sick leave.
One discussion prompted by the presentation focused specifically on individuals’ ability to kneel without much difficulty after their surgery, an activity that’s not typically considered likely, Ms. van Zaanen noted. One audience member, Gillian Hawker, MD, MSc, a professor of medicine in the division of rheumatology at the University of Toronto, questioned whether the field should accept that current reality from surgical intervention. Dr. Hawker described a cohort she had analyzed in which two-thirds of the participants had expected they would be able to kneel after their surgery, regardless of whether it was related to work or other activities.
“Kneeling is important, not just for work; it’s important for culture and religion and lots of other things,” Dr. Hawker said. “How will you help these people to kneel after knee replacement when the surgery isn’t really performed to enable people to do that?” In response, Ms. van Zaanen noted it might not be achievable, as the research literature demonstrates, but Dr. Hawker suggested that is itself problematic.
“I guess what I’m asking is, why are we settling for that? If it’s important to so many people, and an expectation of so many people, why don’t we technologically improve such that, post arthroplasty, people can kneel?”
Another commenter suggested that the study’s findings may not indicate a need to manage patients’ expectations prior to surgery so much as showing that some patients simply have realistic expectations of what they will and will not be able to do after knee replacement.
“Is it possible that people who had low expectations – those who expected to be dissatisfied afterwards – were appropriately understanding that they were likely to be dissatisfied afterwards, in which case, managing their expectations might do nothing for their dissatisfaction afterwards?” the commenter asked. It is likely necessary to conduct additional research about expectations before surgery and experiences after surgery to address that question, Ms. van Zaanen suggested.
Ms. van Zaanen and Dr. Hawker reported having no relevant financial relationships. The presentation did not note any external funding. The Congress was sponsored by the Osteoarthritis Research Society International.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>162718</fileName> <TBEID>0C049139.SIG</TBEID> <TBUniqueIdentifier>MD_0C049139</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname>TKAexpecs_OARSI2023_Haelle</storyname> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20230321T131602</QCDate> <firstPublished>20230321T135053</firstPublished> <LastPublished>20230321T135053</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20230321T135053</CMSDate> <articleSource>AT OARSI 2023</articleSource> <facebookInfo/> <meetingNumber>3588-23</meetingNumber> <byline>Tara Haelle</byline> <bylineText>TARA HAELLE</bylineText> <bylineFull>TARA HAELLE</bylineFull> <bylineTitleText>MDedge News</bylineTitleText> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>DENVER – People with lower expectations of how they would be able to use their knees during work activities after a total knee arthroplasty were more dissatisfi</metaDescription> <articlePDF/> <teaserImage/> <teaser>It may be worthwhile to discuss patients’ expectations about what they will be able to do with their knees postoperatively, but it’s unclear whether some patients’ expectations are too low or simply realistic before surgery. </teaser> <title>Presurgical expectations may influence patients’ attitudes, experiences after knee replacement</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>rn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdsurg</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> </publications_g> <publications> <term canonical="true">26</term> <term>52226</term> <term>21</term> <term>15</term> </publications> <sections> <term canonical="true">53</term> <term>39313</term> </sections> <topics> <term canonical="true">264</term> <term>265</term> <term>237</term> <term>290</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Presurgical expectations may influence patients’ attitudes, experiences after knee replacement</title> <deck/> </itemMeta> <itemContent> <p><span class="dateline">DENVER </span>– People with lower expectations of how they would be able to use their knees during work activities after a total knee arthroplasty were more dissatisfied with their knee abilities 6 months after their surgery, according to a study presented at the OARSI 2023 World Congress. </p> <p>Two out of 10 patients are dissatisfied after total knee arthroplasty, which is increasingly performed in younger and working patients who may have higher demands, presenter <span class="Hyperlink">Yvonne van Zaanen</span>, a physiotherapist in occupational health and ergonomics and a PhD candidate at Amsterdam University Medical Center, told attendees. <br/><br/>The findings suggest a correlation between patients’ low presurgical expectations of their ability to use their knees and having more difficulty with their knees postoperatively, she said. “We should take better care of working patients with low expectations by managing their preoperative expectations and improving their ability to perform work-related knee-straining activities in rehabilitation,” Ms. van Zaanen told attendees. <br/><br/>The researchers conducted a <span class="Hyperlink"><a href="https://www.oarsijournal.com/article/S1063-4584(23)00540-X/fulltext">multicenter, prospective cohort study</a></span> involving seven hospitals. They surveyed 175 employed individuals aged 18-65 years who were scheduled for a total knee arthroplasty and intended to return to work after their surgery. The first survey occurred before the operation, and the follow-up occurred 6 months after the surgery.<br/><br/>Just over half the participants were women (53%), and the average participant age was 59. Respondents had a mean body mass index (BMI) of 29 kg/m<sup>2</sup>, and had a <span class="Hyperlink"><a href="https://hqlo.biomedcentral.com/articles/10.1186/1477-7525-1-64">Knee injury and Osteoarthritis Outcome Score (KOOS)</a></span> pain score of 42 (on a 0-to-100 scale in which lower scores are worse). About half the respondents (51%) had a job that involved knee-straining activities. <br/><br/>The researchers assessed participants’ ability to perform work-related, knee-straining activities using the <span class="Hyperlink"><a href="https://www.arthroplastyjournal.org/article/S0883-5403(14)00038-2/fulltext">Work, Osteoarthritis, or joint-Replacement Questionnaire (WORQ) tool</a></span>, which considers the following activities: kneeling, crouching, clambering, taking the stairs, walking on rough terrain, working with hands below knee height, standing, lifting or carrying, pushing or pulling, walking on ground level, operating a vehicle, operating foot pedals, and sitting. The 0-to-100 scale rates the difficulty of using knees for each particular activity, with higher scores indicating greater ease and less pain in doing that activity.<br/><br/>Among the 107 patients who expected to be satisfied after their surgery, half (n = 53) were satisfied, compared with 12% (n = 13) who were unsatisfied; the remaining participants (n = 41, 38%) were neither satisfied nor dissatisfied. Among the 24 patients who expected to be dissatisfied after their surgery, one-third (n = 8) were satisfied and 42% (n = 10) were dissatisfied. The remaining 44 patients didn’t expect to be satisfied or dissatisfied before their surgery, and 41% of them were satisfied while 23% were dissatisfied.<br/><br/>The researchers found that patients’ expectation of their satisfaction level going into the surgery was the only preoperative factor to be prognostic for dissatisfaction 6 months after surgery, based on their WORQ score. That is, patients who expected to be dissatisfied before their surgery had approximately five times greater odds of being dissatisfied after their surgery than did those who expected to be satisfied with their ability to do knee-straining activities at work (odds ratio, 5.1; 95% confidence interval, 1.7-15.5). Among those with a WORQ score of 40, indicating a greater expectation of difficulty using their knees postoperatively, 55% were dissatisfied after their surgery, compared with 19% of those with a WORQ score of 85, who expected greater knee ability after their surgery.<br/><br/>The other factors that the researchers examined, which had no effect on WORQ scores, included age, sex, BMI, education, comorbidities, KOOS pain subscale, having a knee-straining job, having needed surgery because of work, or having preoperative sick leave.<br/><br/>One discussion prompted by the presentation focused specifically on individuals’ ability to kneel without much difficulty after their surgery, an activity that’s not typically considered likely, Ms. van Zaanen noted. One audience member, <span class="Hyperlink">Gillian Hawker</span>, MD, MSc, a professor of medicine in the division of rheumatology at the University of Toronto, questioned whether the field should accept that current reality from surgical intervention. Dr. Hawker described a cohort she had analyzed in which two-thirds of the participants had expected they would be able to kneel after their surgery, regardless of whether it was related to work or other activities. <br/><br/>“Kneeling is important, not just for work; it’s important for culture and religion and lots of other things,” Dr. Hawker said. “How will you help these people to kneel after knee replacement when the surgery isn’t really performed to enable people to do that?” In response, Ms. van Zaanen noted it might not be achievable, as the research literature demonstrates, but Dr. Hawker suggested that is itself problematic.<br/><br/>“I guess what I’m asking is, why are we settling for that? If it’s important to so many people, and an expectation of so many people, why don’t we technologically improve such that, post arthroplasty, people can kneel?” <br/><br/>Another commenter suggested that the study’s findings may not indicate a need to manage patients’ expectations prior to surgery so much as showing that some patients simply have realistic expectations of what they will and will not be able to do after knee replacement. <br/><br/>“Is it possible that people who had low expectations – those who expected to be dissatisfied afterwards – were appropriately understanding that they were likely to be dissatisfied afterwards, in which case, managing their expectations might do nothing for their dissatisfaction afterwards?” the commenter asked. It is likely necessary to conduct additional research about expectations before surgery and experiences after surgery to address that question, Ms. van Zaanen suggested.<br/><br/>Ms. van Zaanen and Dr. Hawker reported having no relevant financial relationships. The presentation did not note any external funding. The Congress was sponsored by the Osteoarthritis Research Society International.<span class="end"/></p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
AT OARSI 2023
Marathon running does not increase arthritis risk: Survey
Runners who had undergone knee or hip surgery or had a previous hip or knee injury that prevented running were most likely to have arthritis, researchers found. Family history of arthritis, higher body mass index (BMI), and older age were also associated with increased risk of the condition.
The study was presented at the American Academy of Orthopaedic Surgeons 2023 Annual Meeting.
It has generally been thought that running may increase risk of osteoarthritis because it puts more load on joints than walking or standing, noted Grace Hsiao-Wei Lo, MD, an assistant professor of immunology, allergy, and rheumatology at the Baylor College of Medicine, Houston, who was not involved with the work. Research in this area has yielded mixed results: A 2017 analysis of multiple studies found that competitive runners did have higher rates of arthritis than recreational runners, while another study conducted by Dr. Lo found that runners did not have an increased risk of knee osteoarthritis, compared with nonrunners. A 2018 study showed that marathon runners had lower instances of arthritis, compared with the general population.
In this new study, researchers surveyed 3,804 runners who participated in the 2019 or 2021 Chicago Marathon about their running history, average mileage per week, and average running pace. The survey also asked about known risk factors for osteoarthritis, including BMI, family history of arthritis, and past knee and hip injuries that prevented running.
Runners, on average, were about 44 years old and ran 27.9 miles per week. The largest proportion of respondents had completed 2-5 marathons (37.3%), around 21% of respondents had finished 6-10 marathons, and 17% were running their first marathon. Study participants had an average of 15 years of running experience, 1,892 reported a previous hip or knee injury, and 413 had undergone knee or hip surgery. Overall, 36.4% reported experiencing hip or knee pain in the past year, and 7.3% had been diagnosed with arthritis.
Researchers found that there was no association between the risk of osteoarthritis and weekly mileage, years spent running, number of marathons completed, or running pace. Respondents who had undergone knee or hip surgery had the highest risk of osteoarthritis (odds ratio, 5.85; P < .0001), followed by those with a history of knee or hip injuries that prevented running (OR, 5.04; P < .0001). Other identified risk factors were family history of arthritis (OR, 3.47; P < .0001), BMI (OR, 1.10; P < .0001), and older age (OR, 1.08; P < .0001).
The news should be encouraging for runners, said Matthew Hartwell, MD, an orthopedic surgeon at the University of California, San Francisco, who led the research. If someone does not have injuries or surgeries that keep them from running, “you can still continue to run,” he said. “There may not necessarily be this dose-response relationship where the more you run, the more you break down your knee or your hip.”
Still, 24.2% of runners reported that their physician had advised them to reduce their mileage or stop running altogether. Most runners (94.2%) said they planned to run another marathon.
“The results of this study are consistent with the experiences of many lifelong runners and observations of sports medicine professionals that osteoarthritis is not an inevitable consequence of distance running,” said Brett Toresdahl, MD, a sports medicine physician at the Hospital for Special Surgery in New York, who was not involved with the study.
Still, he emphasized that more research is necessary to understand whether running contributes to the risk of developing osteoarthritis. The participants in the study were current marathoners, he noted, so it is likely they have healthy joints that can tolerate running longer distances. “If there is a subset of people who have joints that are negatively affected by running, they wouldn’t likely be registering for a marathon,” he said in an email interview.
Dr. Lo added that comparing these marathoners to a group who did not run would help assess whether running can be harmful to joints. “To be fair, this is a challenging subject to study,” she said. “Osteoarthritis has a long natural history, and so it is difficult to evaluate this kind of question over many years of running and many years of evaluation of arthritis.”
While the research does not answer the question of whether running can lead to osteoarthritis, it helps show the need for long-term research on how running affects joints over time as well as one’s general health, Dr. Toresdahl noted. “I would not be surprised if future longitudinal research will come to the same conclusion that running for the majority of patients is a net benefit for overall health and at least net neutral for joint health when done in moderation,” he said.
Dr. Hartwell, Dr. Lo, and Dr. Toresdahl report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Runners who had undergone knee or hip surgery or had a previous hip or knee injury that prevented running were most likely to have arthritis, researchers found. Family history of arthritis, higher body mass index (BMI), and older age were also associated with increased risk of the condition.
The study was presented at the American Academy of Orthopaedic Surgeons 2023 Annual Meeting.
It has generally been thought that running may increase risk of osteoarthritis because it puts more load on joints than walking or standing, noted Grace Hsiao-Wei Lo, MD, an assistant professor of immunology, allergy, and rheumatology at the Baylor College of Medicine, Houston, who was not involved with the work. Research in this area has yielded mixed results: A 2017 analysis of multiple studies found that competitive runners did have higher rates of arthritis than recreational runners, while another study conducted by Dr. Lo found that runners did not have an increased risk of knee osteoarthritis, compared with nonrunners. A 2018 study showed that marathon runners had lower instances of arthritis, compared with the general population.
In this new study, researchers surveyed 3,804 runners who participated in the 2019 or 2021 Chicago Marathon about their running history, average mileage per week, and average running pace. The survey also asked about known risk factors for osteoarthritis, including BMI, family history of arthritis, and past knee and hip injuries that prevented running.
Runners, on average, were about 44 years old and ran 27.9 miles per week. The largest proportion of respondents had completed 2-5 marathons (37.3%), around 21% of respondents had finished 6-10 marathons, and 17% were running their first marathon. Study participants had an average of 15 years of running experience, 1,892 reported a previous hip or knee injury, and 413 had undergone knee or hip surgery. Overall, 36.4% reported experiencing hip or knee pain in the past year, and 7.3% had been diagnosed with arthritis.
Researchers found that there was no association between the risk of osteoarthritis and weekly mileage, years spent running, number of marathons completed, or running pace. Respondents who had undergone knee or hip surgery had the highest risk of osteoarthritis (odds ratio, 5.85; P < .0001), followed by those with a history of knee or hip injuries that prevented running (OR, 5.04; P < .0001). Other identified risk factors were family history of arthritis (OR, 3.47; P < .0001), BMI (OR, 1.10; P < .0001), and older age (OR, 1.08; P < .0001).
The news should be encouraging for runners, said Matthew Hartwell, MD, an orthopedic surgeon at the University of California, San Francisco, who led the research. If someone does not have injuries or surgeries that keep them from running, “you can still continue to run,” he said. “There may not necessarily be this dose-response relationship where the more you run, the more you break down your knee or your hip.”
Still, 24.2% of runners reported that their physician had advised them to reduce their mileage or stop running altogether. Most runners (94.2%) said they planned to run another marathon.
“The results of this study are consistent with the experiences of many lifelong runners and observations of sports medicine professionals that osteoarthritis is not an inevitable consequence of distance running,” said Brett Toresdahl, MD, a sports medicine physician at the Hospital for Special Surgery in New York, who was not involved with the study.
Still, he emphasized that more research is necessary to understand whether running contributes to the risk of developing osteoarthritis. The participants in the study were current marathoners, he noted, so it is likely they have healthy joints that can tolerate running longer distances. “If there is a subset of people who have joints that are negatively affected by running, they wouldn’t likely be registering for a marathon,” he said in an email interview.
Dr. Lo added that comparing these marathoners to a group who did not run would help assess whether running can be harmful to joints. “To be fair, this is a challenging subject to study,” she said. “Osteoarthritis has a long natural history, and so it is difficult to evaluate this kind of question over many years of running and many years of evaluation of arthritis.”
While the research does not answer the question of whether running can lead to osteoarthritis, it helps show the need for long-term research on how running affects joints over time as well as one’s general health, Dr. Toresdahl noted. “I would not be surprised if future longitudinal research will come to the same conclusion that running for the majority of patients is a net benefit for overall health and at least net neutral for joint health when done in moderation,” he said.
Dr. Hartwell, Dr. Lo, and Dr. Toresdahl report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Runners who had undergone knee or hip surgery or had a previous hip or knee injury that prevented running were most likely to have arthritis, researchers found. Family history of arthritis, higher body mass index (BMI), and older age were also associated with increased risk of the condition.
The study was presented at the American Academy of Orthopaedic Surgeons 2023 Annual Meeting.
It has generally been thought that running may increase risk of osteoarthritis because it puts more load on joints than walking or standing, noted Grace Hsiao-Wei Lo, MD, an assistant professor of immunology, allergy, and rheumatology at the Baylor College of Medicine, Houston, who was not involved with the work. Research in this area has yielded mixed results: A 2017 analysis of multiple studies found that competitive runners did have higher rates of arthritis than recreational runners, while another study conducted by Dr. Lo found that runners did not have an increased risk of knee osteoarthritis, compared with nonrunners. A 2018 study showed that marathon runners had lower instances of arthritis, compared with the general population.
In this new study, researchers surveyed 3,804 runners who participated in the 2019 or 2021 Chicago Marathon about their running history, average mileage per week, and average running pace. The survey also asked about known risk factors for osteoarthritis, including BMI, family history of arthritis, and past knee and hip injuries that prevented running.
Runners, on average, were about 44 years old and ran 27.9 miles per week. The largest proportion of respondents had completed 2-5 marathons (37.3%), around 21% of respondents had finished 6-10 marathons, and 17% were running their first marathon. Study participants had an average of 15 years of running experience, 1,892 reported a previous hip or knee injury, and 413 had undergone knee or hip surgery. Overall, 36.4% reported experiencing hip or knee pain in the past year, and 7.3% had been diagnosed with arthritis.
Researchers found that there was no association between the risk of osteoarthritis and weekly mileage, years spent running, number of marathons completed, or running pace. Respondents who had undergone knee or hip surgery had the highest risk of osteoarthritis (odds ratio, 5.85; P < .0001), followed by those with a history of knee or hip injuries that prevented running (OR, 5.04; P < .0001). Other identified risk factors were family history of arthritis (OR, 3.47; P < .0001), BMI (OR, 1.10; P < .0001), and older age (OR, 1.08; P < .0001).
The news should be encouraging for runners, said Matthew Hartwell, MD, an orthopedic surgeon at the University of California, San Francisco, who led the research. If someone does not have injuries or surgeries that keep them from running, “you can still continue to run,” he said. “There may not necessarily be this dose-response relationship where the more you run, the more you break down your knee or your hip.”
Still, 24.2% of runners reported that their physician had advised them to reduce their mileage or stop running altogether. Most runners (94.2%) said they planned to run another marathon.
“The results of this study are consistent with the experiences of many lifelong runners and observations of sports medicine professionals that osteoarthritis is not an inevitable consequence of distance running,” said Brett Toresdahl, MD, a sports medicine physician at the Hospital for Special Surgery in New York, who was not involved with the study.
Still, he emphasized that more research is necessary to understand whether running contributes to the risk of developing osteoarthritis. The participants in the study were current marathoners, he noted, so it is likely they have healthy joints that can tolerate running longer distances. “If there is a subset of people who have joints that are negatively affected by running, they wouldn’t likely be registering for a marathon,” he said in an email interview.
Dr. Lo added that comparing these marathoners to a group who did not run would help assess whether running can be harmful to joints. “To be fair, this is a challenging subject to study,” she said. “Osteoarthritis has a long natural history, and so it is difficult to evaluate this kind of question over many years of running and many years of evaluation of arthritis.”
While the research does not answer the question of whether running can lead to osteoarthritis, it helps show the need for long-term research on how running affects joints over time as well as one’s general health, Dr. Toresdahl noted. “I would not be surprised if future longitudinal research will come to the same conclusion that running for the majority of patients is a net benefit for overall health and at least net neutral for joint health when done in moderation,” he said.
Dr. Hartwell, Dr. Lo, and Dr. Toresdahl report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>162723</fileName> <TBEID>0C049144.SIG</TBEID> <TBUniqueIdentifier>MD_0C049144</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20230320T130256</QCDate> <firstPublished>20230320T132037</firstPublished> <LastPublished>20230320T132037</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20230320T132037</CMSDate> <articleSource>FROM AAOS 2023</articleSource> <facebookInfo/> <meetingNumber>2896-23</meetingNumber> <byline>Lucy Hicks</byline> <bylineText>LUCY HICKS</bylineText> <bylineFull>LUCY HICKS</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType/> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Long-distance runners are often warned that they are wearing out their joints, but a new study found that running mileage, frequency, and pace were not associat</metaDescription> <articlePDF/> <teaserImage/> <teaser>For those runners who had undergone knee or hip surgery or had a previous hip or knee injury that prevented running, the likelihood of having arthritis was higher.</teaser> <title>Marathon running does not increase arthritis risk: Survey</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>rn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdsurg</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> </publications_g> <publications> <term>26</term> <term canonical="true">21</term> <term>15</term> <term>52226</term> </publications> <sections> <term>39313</term> <term canonical="true">53</term> </sections> <topics> <term canonical="true">265</term> <term>280</term> <term>268</term> <term>252</term> <term>264</term> <term>227</term> <term>237</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Marathon running does not increase arthritis risk: Survey</title> <deck/> </itemMeta> <itemContent> <p> <span class="tag metaDescription">Long-distance runners are often warned that they are wearing out their joints, but a new study found that running mileage, frequency, and pace were not associated with an increased risk of osteoarthritis.</span> </p> <p>Runners who had undergone knee or hip surgery or had a previous hip or knee injury that prevented running were most likely to have arthritis, researchers found. Family history of arthritis, higher body mass index (BMI), and older age were also associated with increased risk of the condition.<br/><br/>The study was presented at the American Academy of Orthopaedic Surgeons 2023 Annual Meeting.<br/><br/>It has generally been thought that running may increase risk of osteoarthritis because it puts more load on joints than walking or standing, noted Grace Hsiao-Wei Lo, MD, an assistant professor of immunology, allergy, and rheumatology at the Baylor College of Medicine, Houston, who was not involved with the work. Research in this area has yielded mixed results: A 2017 analysis of multiple studies found that <span class="Hyperlink"><a href="https://pubmed.ncbi.nlm.nih.gov/28504066/">competitive runners did have higher rates</a></span> of arthritis than recreational runners, while another study conducted by Dr. Lo found that runners <span class="Hyperlink"><a href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5179322/">did not have an increased risk of knee osteoarthritis</a></span>, compared with nonrunners. A 2018 study showed that marathon runners had <span class="Hyperlink"><a href="https://pubmed.ncbi.nlm.nih.gov/29342063/">lower instances of arthritis</a></span>, compared with the general population.<br/><br/>In this new study, researchers surveyed 3,804 runners who participated in the 2019 or 2021 Chicago Marathon about their running history, average mileage per week, and average running pace. The survey also asked about known risk factors for osteoarthritis, including BMI, family history of arthritis, and past knee and hip injuries that prevented running.<br/><br/>Runners, on average, were about 44 years old and ran 27.9 miles per week. The largest proportion of respondents had completed 2-5 marathons (37.3%), around 21% of respondents had finished 6-10 marathons, and 17% were running their first marathon. Study participants had an average of 15 years of running experience, 1,892 reported a previous hip or knee injury, and 413 had undergone knee or hip surgery. Overall, 36.4% reported experiencing hip or knee pain in the past year, and 7.3% had been diagnosed with arthritis.<br/><br/>Researchers found that there was no association between the risk of osteoarthritis and weekly mileage, years spent running, number of marathons completed, or running pace. Respondents who had undergone knee or hip surgery had the highest risk of osteoarthritis (odds ratio, 5.85; <em>P</em> < .0001), followed by those with a history of knee or hip injuries that prevented running (OR, 5.04; <em>P</em> < .0001). Other identified risk factors were family history of arthritis (OR, 3.47; <em>P</em> < .0001), BMI (OR, 1.10; <em>P</em> < .0001), and older age (OR, 1.08; <em>P</em> < .0001).<br/><br/>The news should be encouraging for runners, said Matthew Hartwell, MD, an orthopedic surgeon at the University of California, San Francisco, who led the research. If someone does not have injuries or surgeries that keep them from running, “you can still continue to run,” he said. “There may not necessarily be this dose-response relationship where the more you run, the more you break down your knee or your hip.”<br/><br/>Still, 24.2% of runners reported that their physician had advised them to reduce their mileage or stop running altogether. Most runners (94.2%) said they planned to run another marathon.<br/><br/>“The results of this study are consistent with the experiences of many lifelong runners and observations of sports medicine professionals that osteoarthritis is not an inevitable consequence of distance running,” said Brett Toresdahl, MD, a sports medicine physician at the Hospital for Special Surgery in New York, who was not involved with the study.<br/><br/>Still, he emphasized that more research is necessary to understand whether running contributes to the risk of developing osteoarthritis. The participants in the study were current marathoners, he noted, so it is likely they have healthy joints that can tolerate running longer distances. “If there is a subset of people who have joints that are negatively affected by running, they wouldn’t likely be registering for a marathon,” he said in an email interview.<br/><br/>Dr. Lo added that comparing these marathoners to a group who did not run would help assess whether running can be harmful to joints. “To be fair, this is a challenging subject to study,” she said. “Osteoarthritis has a long natural history, and so it is difficult to evaluate this kind of question over many years of running and many years of evaluation of arthritis.”<br/><br/>While the research does not answer the question of whether running can lead to osteoarthritis, it helps show the need for long-term research on how running affects joints over time as well as one’s general health, Dr. Toresdahl noted. “I would not be surprised if future longitudinal research will come to the same conclusion that running for the majority of patients is a net benefit for overall health and at least net neutral for joint health when done in moderation,” he said.<br/><br/>Dr. Hartwell, Dr. Lo, and Dr. Toresdahl report no relevant financial relationships.<span class="end"/></p> <p> <em>A version of this article originally appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/989806">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>
FROM AAOS 2023
Guidelines: Don’t delay total joint arthroplasty for additional nonoperative therapies
Patients with moderate to severe osteoarthritis (OA) or osteonecrosis (ON) eligible for total joint arthroplasty (TJA) who have failed one or more nonoperative therapies should proceed directly to surgery, according to new guidelines from the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“One of the reasons for creating this guideline was that many patients have been subjected to delays for surgery after completing nonoperative therapy, despite persistent moderate to severe pain, loss of function, and moderate to severe radiographic OA or ON,” said coauthors Susan M. Goodman, MD, a rheumatologist at Hospital for Special Surgery in New York, and Charles Hannon, MD, an orthopedic surgeon at Washington University in St. Louis, in an email interview with this news organization. “This guideline supports surgery being performed in an expeditious fashion after the decision has been made to proceed with surgery by both the physician and patient through a shared decision-making process,” they said.
The guidelines also state that obesity by itself should not be a reason to delay TJA. “We could not find a rationale for a strict cut off for weight/body mass index (BMI). Our literature review revealed that though many adverse events were, in fact, increased in patients with morbid obesity, there is also an increase in adverse events for those who had bariatric surgery prior to their arthroplasty,” they added, noting that patients need to be made aware of the increased risk for adverse events in patients with obesity. Though the guidelines do not pose any BMI cutoffs, they state that weight loss should be “strongly encouraged.” These new recommendations are conditional, and all had a “low” to “very low” certainty of evidence; however, there was high consensus on the recommendations from the expert panel.
[embed:render:related:node:259937]
The guidelines also recommended:
- Delaying TJA to achieve smoking and nicotine cessation or reduction.
- Delaying TJA to improve glycemic control in patients with diabetes, although the group did not recommend any specific measure or threshold.
- Not delaying TJA in patients with a severe deformity, bone loss, or a neuropathic joint.
The new guidelines formalize what many surgeons have already been doing for the past few years, said Arjun Saxena, MD, MBA, an orthopedic surgeon in Philadelphia who was not involved with the guidelines. “A lot of total joint programs have really focused on patient optimization, including smoking cessation, glycemic control, and weight loss prior to surgery,” he said.
Most importantly, the guidelines put an emphasis on how the decision to proceed with TJA should be a shared decision between a physician and patient, he added. Some insurance companies with prior authorization policies may require a patient to try additional nonoperative therapies before approving surgery, creating barriers to care, he said. “Hopefully [these new recommendations] will help third parties understand that joint replacement is a big decision – most doctors aren’t going to recommend that unless it’s necessary or something that is going to help patients,” he said. “I understand that there is a certain need for preauthorization, but just having strict guidelines isn’t appropriate. You really need to look at the whole picture,” he added.
The full manuscript has been submitted for review and is expected to be jointly published in American College of Rheumatology and the American Association of Hip and Knee Surgeons journals later this year.
Dr. Saxena consults for the orthopedic implant company Corin.
A version of this article originally appeared on Medscape.com.
Patients with moderate to severe osteoarthritis (OA) or osteonecrosis (ON) eligible for total joint arthroplasty (TJA) who have failed one or more nonoperative therapies should proceed directly to surgery, according to new guidelines from the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“One of the reasons for creating this guideline was that many patients have been subjected to delays for surgery after completing nonoperative therapy, despite persistent moderate to severe pain, loss of function, and moderate to severe radiographic OA or ON,” said coauthors Susan M. Goodman, MD, a rheumatologist at Hospital for Special Surgery in New York, and Charles Hannon, MD, an orthopedic surgeon at Washington University in St. Louis, in an email interview with this news organization. “This guideline supports surgery being performed in an expeditious fashion after the decision has been made to proceed with surgery by both the physician and patient through a shared decision-making process,” they said.
The guidelines also state that obesity by itself should not be a reason to delay TJA. “We could not find a rationale for a strict cut off for weight/body mass index (BMI). Our literature review revealed that though many adverse events were, in fact, increased in patients with morbid obesity, there is also an increase in adverse events for those who had bariatric surgery prior to their arthroplasty,” they added, noting that patients need to be made aware of the increased risk for adverse events in patients with obesity. Though the guidelines do not pose any BMI cutoffs, they state that weight loss should be “strongly encouraged.” These new recommendations are conditional, and all had a “low” to “very low” certainty of evidence; however, there was high consensus on the recommendations from the expert panel.
[embed:render:related:node:259937]
The guidelines also recommended:
- Delaying TJA to achieve smoking and nicotine cessation or reduction.
- Delaying TJA to improve glycemic control in patients with diabetes, although the group did not recommend any specific measure or threshold.
- Not delaying TJA in patients with a severe deformity, bone loss, or a neuropathic joint.
The new guidelines formalize what many surgeons have already been doing for the past few years, said Arjun Saxena, MD, MBA, an orthopedic surgeon in Philadelphia who was not involved with the guidelines. “A lot of total joint programs have really focused on patient optimization, including smoking cessation, glycemic control, and weight loss prior to surgery,” he said.
Most importantly, the guidelines put an emphasis on how the decision to proceed with TJA should be a shared decision between a physician and patient, he added. Some insurance companies with prior authorization policies may require a patient to try additional nonoperative therapies before approving surgery, creating barriers to care, he said. “Hopefully [these new recommendations] will help third parties understand that joint replacement is a big decision – most doctors aren’t going to recommend that unless it’s necessary or something that is going to help patients,” he said. “I understand that there is a certain need for preauthorization, but just having strict guidelines isn’t appropriate. You really need to look at the whole picture,” he added.
The full manuscript has been submitted for review and is expected to be jointly published in American College of Rheumatology and the American Association of Hip and Knee Surgeons journals later this year.
Dr. Saxena consults for the orthopedic implant company Corin.
A version of this article originally appeared on Medscape.com.
Patients with moderate to severe osteoarthritis (OA) or osteonecrosis (ON) eligible for total joint arthroplasty (TJA) who have failed one or more nonoperative therapies should proceed directly to surgery, according to new guidelines from the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“One of the reasons for creating this guideline was that many patients have been subjected to delays for surgery after completing nonoperative therapy, despite persistent moderate to severe pain, loss of function, and moderate to severe radiographic OA or ON,” said coauthors Susan M. Goodman, MD, a rheumatologist at Hospital for Special Surgery in New York, and Charles Hannon, MD, an orthopedic surgeon at Washington University in St. Louis, in an email interview with this news organization. “This guideline supports surgery being performed in an expeditious fashion after the decision has been made to proceed with surgery by both the physician and patient through a shared decision-making process,” they said.
The guidelines also state that obesity by itself should not be a reason to delay TJA. “We could not find a rationale for a strict cut off for weight/body mass index (BMI). Our literature review revealed that though many adverse events were, in fact, increased in patients with morbid obesity, there is also an increase in adverse events for those who had bariatric surgery prior to their arthroplasty,” they added, noting that patients need to be made aware of the increased risk for adverse events in patients with obesity. Though the guidelines do not pose any BMI cutoffs, they state that weight loss should be “strongly encouraged.” These new recommendations are conditional, and all had a “low” to “very low” certainty of evidence; however, there was high consensus on the recommendations from the expert panel.
[embed:render:related:node:259937]
The guidelines also recommended:
- Delaying TJA to achieve smoking and nicotine cessation or reduction.
- Delaying TJA to improve glycemic control in patients with diabetes, although the group did not recommend any specific measure or threshold.
- Not delaying TJA in patients with a severe deformity, bone loss, or a neuropathic joint.
The new guidelines formalize what many surgeons have already been doing for the past few years, said Arjun Saxena, MD, MBA, an orthopedic surgeon in Philadelphia who was not involved with the guidelines. “A lot of total joint programs have really focused on patient optimization, including smoking cessation, glycemic control, and weight loss prior to surgery,” he said.
Most importantly, the guidelines put an emphasis on how the decision to proceed with TJA should be a shared decision between a physician and patient, he added. Some insurance companies with prior authorization policies may require a patient to try additional nonoperative therapies before approving surgery, creating barriers to care, he said. “Hopefully [these new recommendations] will help third parties understand that joint replacement is a big decision – most doctors aren’t going to recommend that unless it’s necessary or something that is going to help patients,” he said. “I understand that there is a certain need for preauthorization, but just having strict guidelines isn’t appropriate. You really need to look at the whole picture,” he added.
The full manuscript has been submitted for review and is expected to be jointly published in American College of Rheumatology and the American Association of Hip and Knee Surgeons journals later this year.
Dr. Saxena consults for the orthopedic implant company Corin.
A version of this article originally appeared on Medscape.com.
<!--$RCSfile: InCopy_agile.xsl,v $ $Revision: 1.35 $-->
<!--$RCSfile: drupal.xsl,v $ $Revision: 1.7 $-->
<root generator="drupal.xsl" gversion="1.7"> <header> <fileName>162720</fileName> <TBEID>0C04913E.SIG</TBEID> <TBUniqueIdentifier>MD_0C04913E</TBUniqueIdentifier> <newsOrJournal>News</newsOrJournal> <publisherName>Frontline Medical Communications</publisherName> <storyname/> <articleType>2</articleType> <TBLocation>QC Done-All Pubs</TBLocation> <QCDate>20230320T114500</QCDate> <firstPublished>20230320T120741</firstPublished> <LastPublished>20230320T120741</LastPublished> <pubStatus qcode="stat:"/> <embargoDate/> <killDate/> <CMSDate>20230320T120741</CMSDate> <articleSource/> <facebookInfo/> <meetingNumber/> <byline>Lucy Hicks</byline> <bylineText>LUCY HICKS</bylineText> <bylineFull>LUCY HICKS</bylineFull> <bylineTitleText/> <USOrGlobal/> <wireDocType/> <newsDocType>News</newsDocType> <journalDocType/> <linkLabel/> <pageRange/> <citation/> <quizID/> <indexIssueDate/> <itemClass qcode="ninat:text"/> <provider qcode="provider:imng"> <name>IMNG Medical Media</name> <rightsInfo> <copyrightHolder> <name>Frontline Medical News</name> </copyrightHolder> <copyrightNotice>Copyright (c) 2015 Frontline Medical News, a Frontline Medical Communications Inc. company. All rights reserved. This material may not be published, broadcast, copied, or otherwise reproduced or distributed without the prior written permission of Frontline Medical Communications Inc.</copyrightNotice> </rightsInfo> </provider> <abstract/> <metaDescription>Patients with moderate to severe osteoarthritis (OA) or osteonecrosis (ON) eligible for total joint arthroplasty (TJA) who have failed one or more nonoperative </metaDescription> <articlePDF/> <teaserImage/> <teaser>The guidelines also state that obesity alone should not be a reason to delay surgery. </teaser> <title>Guidelines: Don’t delay total joint arthroplasty for additional nonoperative therapies</title> <deck/> <disclaimer/> <AuthorList/> <articleURL/> <doi/> <pubMedID/> <publishXMLStatus/> <publishXMLVersion>1</publishXMLVersion> <useEISSN>0</useEISSN> <urgency/> <pubPubdateYear/> <pubPubdateMonth/> <pubPubdateDay/> <pubVolume/> <pubNumber/> <wireChannels/> <primaryCMSID/> <CMSIDs/> <keywords/> <seeAlsos/> <publications_g> <publicationData> <publicationCode>rn</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>im</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>fp</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> </publicationData> <publicationData> <publicationCode>mdsurg</publicationCode> <pubIssueName/> <pubArticleType/> <pubTopics/> <pubCategories/> <pubSections/> <journalTitle/> <journalFullTitle/> <copyrightStatement>2018 Frontline Medical Communications Inc.,</copyrightStatement> </publicationData> </publications_g> <publications> <term canonical="true">26</term> <term>21</term> <term>15</term> <term>52226</term> </publications> <sections> <term canonical="true">75</term> <term>39313</term> </sections> <topics> <term canonical="true">264</term> <term>265</term> <term>290</term> <term>237</term> <term>227</term> </topics> <links/> </header> <itemSet> <newsItem> <itemMeta> <itemRole>Main</itemRole> <itemClass>text</itemClass> <title>Guidelines: Don’t delay total joint arthroplasty for additional nonoperative therapies</title> <deck/> </itemMeta> <itemContent> <p>Patients with moderate to severe osteoarthritis (OA) or osteonecrosis (ON) eligible for total joint arthroplasty (TJA) who have failed one or more nonoperative therapies should proceed directly to surgery, according to <span class="Hyperlink"><a href="https://www.rheumatology.org/About-Us/Newsroom/Press-Releases/ID/1259">new guidelines</a></span> from the American College of Rheumatology and the American Association of Hip and Knee Surgeons.</p> <p>“One of the reasons for creating this guideline was that many patients have been subjected to delays for surgery after completing nonoperative therapy, despite persistent moderate to severe pain, loss of function, and moderate to severe radiographic OA or ON,” said coauthors Susan M. Goodman, MD, a rheumatologist at Hospital for Special Surgery in New York, and Charles Hannon, MD, an orthopedic surgeon at Washington University in St. Louis, in an email interview with this news organization. “This guideline supports surgery being performed in an expeditious fashion after the decision has been made to proceed with surgery by both the physician and patient through a shared decision-making process,” they said.<br/><br/>The guidelines also state that obesity by itself should not be a reason to delay TJA. “We could not find a rationale for a strict cut off for weight/body mass index (BMI). Our literature review revealed that though many adverse events were, in fact, increased in patients with morbid obesity, there is also an increase in adverse events for those who had bariatric surgery prior to their arthroplasty,” they added, noting that patients need to be made aware of the increased risk for adverse events in patients with obesity. Though the guidelines do not pose any BMI cutoffs, they state that weight loss should be “strongly encouraged.” These new recommendations are conditional, and all had a “low” to “very low” certainty of evidence; however, there was high consensus on the recommendations from the expert panel.<br/><br/>The guidelines also recommended:</p> <ul class="body"> <li>Delaying TJA to achieve smoking and nicotine cessation or reduction.</li> <li>Delaying TJA to improve glycemic control in patients with diabetes, although the group did not recommend any specific measure or threshold.</li> <li>Not delaying TJA in patients with a severe deformity, bone loss, or a neuropathic joint.</li> </ul> <p>The new guidelines formalize what many surgeons have already been doing for the past few years, said <span class="Hyperlink">Arjun Saxena, MD, MBA</span>, an orthopedic surgeon in Philadelphia who was not involved with the guidelines. “A lot of total joint programs have really focused on patient optimization, including smoking cessation, glycemic control, and weight loss prior to surgery,” he said.<br/><br/>Most importantly, the guidelines put an emphasis on how the decision to proceed with TJA should be a shared decision between a physician and patient, he added. Some insurance companies with prior authorization policies may require a patient to try additional nonoperative therapies before approving surgery, creating barriers to care, he said. “Hopefully [these new recommendations] will help third parties understand that joint replacement is a big decision – most doctors aren’t going to recommend that unless it’s necessary or something that is going to help patients,” he said. “I understand that there is a certain need for preauthorization, but just having strict guidelines isn’t appropriate. You really need to look at the whole picture,” he added.<br/><br/>The full manuscript has been submitted for review and is expected to be jointly published in American College of Rheumatology and the American Association of Hip and Knee Surgeons journals later this year.<br/><br/>Dr. Saxena consults for the orthopedic implant company Corin.<span class="end"/></p> <p> <em>A version of this article originally appeared on <span class="Hyperlink"><a href="https://www.medscape.com/viewarticle/989828">Medscape.com</a></span>.</em> </p> </itemContent> </newsItem> <newsItem> <itemMeta> <itemRole>teaser</itemRole> <itemClass>text</itemClass> <title/> <deck/> </itemMeta> <itemContent> </itemContent> </newsItem> </itemSet></root>