User login
Women with recurrent UTIs express fear, frustration
Fear of antibiotic overuse and frustration with physicians who prescribe them too freely are key sentiments expressed by women with recurrent urinary tract infections (rUTIs), according to findings from a study involving six focus groups.
“Here in our female pelvic medicine reconstructive urology clinic at Cedars-Sinai and at UCLA, we see many women who are referred for evaluation of rUTIs who are very frustrated with their care,” Victoria Scott, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“So with these focus groups, we saw an opportunity to explore why women are so frustrated and to try and improve the care delivered,” she added.
Findings from the study were published online Sept. 1 in The Journal of Urology.
“There is a need for physicians to modify management strategies ... and to devote more research efforts to improving nonantibiotic options for the prevention and treatment of recurrent urinary tract infections, as well as management strategies that better empower patients,” the authors wrote.
Six focus groups
Four or five participants were included in each of the six focus groups – a total of 29 women. All participants reported a history of symptomatic, culture-proven UTI episodes. They had experienced two or more infections in 6 months or three or more infections within 1 year. Women were predominantly White. Most were employed part- or full-time and held a college degree.
From a qualitative analysis of all focus group transcripts, two main themes emerged:
- The negative impact of taking antibiotics for the prevention and treatment of rUTIs.
- Resentment of the medical profession for the way it managed rUTIs.
The researchers found that participants had a good understanding of the deleterious effects from inappropriate antibiotic use, largely gleaned from media sources and the Internet. “Numerous women stated that they had reached such a level of concern about antibiotics that they would resist taking them for prevention or treatment of infections,” Dr. Scott and colleagues pointed out.
These concerns centered around the risk of developing resistance to antibiotics and the ill effects that antibiotics can have on the gastrointestinal and genitourinary microbiomes. Several women reported that they had developed Clostridium difficile infections after taking antibiotics; one of the patients required hospitalization for the infection.
Women also reported concerns that they had been given an antibiotic needlessly for symptoms that might have been caused by a genitourinary condition other than a UTI. They also reported feeling resentful toward practitioners, particularly if they felt the practitioner was overprescribing antibiotics. Some had resorted to consultations with alternative practitioners, such as herbalists. “A second concern discussed by participants was the feeling of being ignored by physicians,” the authors observed.
In this regard, the women felt that their physicians underestimated the burden that rUTIs had on their lives and the detrimental effect that repeated infections had on their relationships, work, and overall quality of life. “These perceptions led to a prevalent mistrust of physicians,” the investigators wrote. This prompted many women to insist that the medical community devote more effort to the development of nonantibiotic options for the prevention and treatment of UTIs.
Improved management strategies
Asked how physicians might improve their management of rUTIs, Dr. Scott shared a number of suggestions. Cardinal rule No. 1: Have the patient undergo a urinalysis to make sure she does have a UTI. “There is a subset of patients among women with rUTIs who come in with a diagnosis of an rUTI but who really have not had documentation of more than one positive urine culture,” Dr. Scott noted. Such a history suggests that they do not have an rUTI.
It’s imperative that physicians rule out commonly misdiagnosed disorders, such as overactive bladder, as a cause of the patient’s symptoms. Symptoms of overactive bladder and rUTIs often overlap. While waiting for results from the urinalysis to confirm or rule out a UTI, young and healthy women may be prescribed a nonsteroidal anti-inflammatory drug (NSAID), such as naproxen, which can help ameliorate symptoms.
Because UTIs are frequently self-limiting, Dr. Scott and others have found that for young, otherwise healthy women, NSAIDs alone can often resolve symptoms of the UTI without use of an antibiotic. For relatively severe symptoms, a urinary analgesic, such as phenazopyridine (Pyridium), may soothe the lining of the urinary tract and relieve pain. Cystex is an over-the-counter urinary analgesic that women can procure themselves, Dr. Scott added.
If an antibiotic is indicated, those most commonly prescribed for a single episode of acute cystitis are nitrofurantoin and sulfamethoxazole plus trimethoprim (Bactrim). For recurrent UTIs, “patients are a bit more complicated,” Dr. Scott admitted. “I think the best practice is to look back at a woman’s prior urine culture and select an antibiotic that showed good sensitivity in the last positive urine test,” she said.
Prevention starts with behavioral strategies, such as voiding after sexual intercourse and wiping from front to back following urination to avoid introducing fecal bacteria into the urethra. Evidence suggests that premenopausal women who drink at least 1.5 L of water a day have significantly fewer UTI episodes, Dr. Scott noted. There is also “pretty good” evidence that cranberry supplements (not juice) can prevent rUTIs. Use of cranberry supplements is supported by the American Urological Association (conditional recommendation; evidence level of grade C).
For peri- and postmenopausal women, vaginal estrogen may be effective. It’s use for UTI prevention is well supported by the literature. Although not as well supported by evidence, some women find that a supplement such as D-mannose may prevent or treat UTIs by causing bacteria to bind to it rather than to the bladder wall. Probiotics are another possibility, she noted. Empathy can’t hurt, she added.
“A common theme among satisfied women was the sentiment that their physicians understood their problems and had a system in place to allow rapid diagnosis and treatment for UTI episodes,” the authors emphasized.
“[Such attitudes] highlight the need to investigate each patient’s experience and perceptions to allow for shared decision making regarding the management of rUTIs,” they wrote.
Further commentary
Asked to comment on the findings, editorialist Michelle Van Kuiken, MD, assistant professor of urology, University of California, San Francisco, acknowledged that there is not a lot of good evidence to support many of the strategies recommended by the American Urological Association to prevent and treat rUTIs, but she often follows these recommendations anyway. “The one statement in the guidelines that is the most supported by evidence is the use of cranberry supplements, and I do routinely recommended daily use of some form of concentrated cranberry supplements for all of my patients with rUTIs,” she said in an interview.
Dr. Van Kuiken said that vaginal estrogen is a very good option for all postmenopausal women who suffer from rUTIs and that there is growing acceptance of its use for this and other indications. There is some evidence to support D-mannose as well, although it’s not that robust, she acknowledged.
She said the evidence supporting the use of probiotics for this indication is very thin. She does not routinely recommend them for rUTIs, although they are not inherently harmful. “I think for a lot of women who have rUTIs, it can be pretty debilitating and upsetting for them – it can impact travel plans, work, and social events,” Dr. Van Kuiken said.
“Until we develop better diagnostic and therapeutic strategies, validating women’s experiences and concerns with rUTI while limiting unnecessary antibiotics remains our best option,” she wrote.
Dr. Scott and Dr. Van Kuiken have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fear of antibiotic overuse and frustration with physicians who prescribe them too freely are key sentiments expressed by women with recurrent urinary tract infections (rUTIs), according to findings from a study involving six focus groups.
“Here in our female pelvic medicine reconstructive urology clinic at Cedars-Sinai and at UCLA, we see many women who are referred for evaluation of rUTIs who are very frustrated with their care,” Victoria Scott, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“So with these focus groups, we saw an opportunity to explore why women are so frustrated and to try and improve the care delivered,” she added.
Findings from the study were published online Sept. 1 in The Journal of Urology.
“There is a need for physicians to modify management strategies ... and to devote more research efforts to improving nonantibiotic options for the prevention and treatment of recurrent urinary tract infections, as well as management strategies that better empower patients,” the authors wrote.
Six focus groups
Four or five participants were included in each of the six focus groups – a total of 29 women. All participants reported a history of symptomatic, culture-proven UTI episodes. They had experienced two or more infections in 6 months or three or more infections within 1 year. Women were predominantly White. Most were employed part- or full-time and held a college degree.
From a qualitative analysis of all focus group transcripts, two main themes emerged:
- The negative impact of taking antibiotics for the prevention and treatment of rUTIs.
- Resentment of the medical profession for the way it managed rUTIs.
The researchers found that participants had a good understanding of the deleterious effects from inappropriate antibiotic use, largely gleaned from media sources and the Internet. “Numerous women stated that they had reached such a level of concern about antibiotics that they would resist taking them for prevention or treatment of infections,” Dr. Scott and colleagues pointed out.
These concerns centered around the risk of developing resistance to antibiotics and the ill effects that antibiotics can have on the gastrointestinal and genitourinary microbiomes. Several women reported that they had developed Clostridium difficile infections after taking antibiotics; one of the patients required hospitalization for the infection.
Women also reported concerns that they had been given an antibiotic needlessly for symptoms that might have been caused by a genitourinary condition other than a UTI. They also reported feeling resentful toward practitioners, particularly if they felt the practitioner was overprescribing antibiotics. Some had resorted to consultations with alternative practitioners, such as herbalists. “A second concern discussed by participants was the feeling of being ignored by physicians,” the authors observed.
In this regard, the women felt that their physicians underestimated the burden that rUTIs had on their lives and the detrimental effect that repeated infections had on their relationships, work, and overall quality of life. “These perceptions led to a prevalent mistrust of physicians,” the investigators wrote. This prompted many women to insist that the medical community devote more effort to the development of nonantibiotic options for the prevention and treatment of UTIs.
Improved management strategies
Asked how physicians might improve their management of rUTIs, Dr. Scott shared a number of suggestions. Cardinal rule No. 1: Have the patient undergo a urinalysis to make sure she does have a UTI. “There is a subset of patients among women with rUTIs who come in with a diagnosis of an rUTI but who really have not had documentation of more than one positive urine culture,” Dr. Scott noted. Such a history suggests that they do not have an rUTI.
It’s imperative that physicians rule out commonly misdiagnosed disorders, such as overactive bladder, as a cause of the patient’s symptoms. Symptoms of overactive bladder and rUTIs often overlap. While waiting for results from the urinalysis to confirm or rule out a UTI, young and healthy women may be prescribed a nonsteroidal anti-inflammatory drug (NSAID), such as naproxen, which can help ameliorate symptoms.
Because UTIs are frequently self-limiting, Dr. Scott and others have found that for young, otherwise healthy women, NSAIDs alone can often resolve symptoms of the UTI without use of an antibiotic. For relatively severe symptoms, a urinary analgesic, such as phenazopyridine (Pyridium), may soothe the lining of the urinary tract and relieve pain. Cystex is an over-the-counter urinary analgesic that women can procure themselves, Dr. Scott added.
If an antibiotic is indicated, those most commonly prescribed for a single episode of acute cystitis are nitrofurantoin and sulfamethoxazole plus trimethoprim (Bactrim). For recurrent UTIs, “patients are a bit more complicated,” Dr. Scott admitted. “I think the best practice is to look back at a woman’s prior urine culture and select an antibiotic that showed good sensitivity in the last positive urine test,” she said.
Prevention starts with behavioral strategies, such as voiding after sexual intercourse and wiping from front to back following urination to avoid introducing fecal bacteria into the urethra. Evidence suggests that premenopausal women who drink at least 1.5 L of water a day have significantly fewer UTI episodes, Dr. Scott noted. There is also “pretty good” evidence that cranberry supplements (not juice) can prevent rUTIs. Use of cranberry supplements is supported by the American Urological Association (conditional recommendation; evidence level of grade C).
For peri- and postmenopausal women, vaginal estrogen may be effective. It’s use for UTI prevention is well supported by the literature. Although not as well supported by evidence, some women find that a supplement such as D-mannose may prevent or treat UTIs by causing bacteria to bind to it rather than to the bladder wall. Probiotics are another possibility, she noted. Empathy can’t hurt, she added.
“A common theme among satisfied women was the sentiment that their physicians understood their problems and had a system in place to allow rapid diagnosis and treatment for UTI episodes,” the authors emphasized.
“[Such attitudes] highlight the need to investigate each patient’s experience and perceptions to allow for shared decision making regarding the management of rUTIs,” they wrote.
Further commentary
Asked to comment on the findings, editorialist Michelle Van Kuiken, MD, assistant professor of urology, University of California, San Francisco, acknowledged that there is not a lot of good evidence to support many of the strategies recommended by the American Urological Association to prevent and treat rUTIs, but she often follows these recommendations anyway. “The one statement in the guidelines that is the most supported by evidence is the use of cranberry supplements, and I do routinely recommended daily use of some form of concentrated cranberry supplements for all of my patients with rUTIs,” she said in an interview.
Dr. Van Kuiken said that vaginal estrogen is a very good option for all postmenopausal women who suffer from rUTIs and that there is growing acceptance of its use for this and other indications. There is some evidence to support D-mannose as well, although it’s not that robust, she acknowledged.
She said the evidence supporting the use of probiotics for this indication is very thin. She does not routinely recommend them for rUTIs, although they are not inherently harmful. “I think for a lot of women who have rUTIs, it can be pretty debilitating and upsetting for them – it can impact travel plans, work, and social events,” Dr. Van Kuiken said.
“Until we develop better diagnostic and therapeutic strategies, validating women’s experiences and concerns with rUTI while limiting unnecessary antibiotics remains our best option,” she wrote.
Dr. Scott and Dr. Van Kuiken have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fear of antibiotic overuse and frustration with physicians who prescribe them too freely are key sentiments expressed by women with recurrent urinary tract infections (rUTIs), according to findings from a study involving six focus groups.
“Here in our female pelvic medicine reconstructive urology clinic at Cedars-Sinai and at UCLA, we see many women who are referred for evaluation of rUTIs who are very frustrated with their care,” Victoria Scott, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“So with these focus groups, we saw an opportunity to explore why women are so frustrated and to try and improve the care delivered,” she added.
Findings from the study were published online Sept. 1 in The Journal of Urology.
“There is a need for physicians to modify management strategies ... and to devote more research efforts to improving nonantibiotic options for the prevention and treatment of recurrent urinary tract infections, as well as management strategies that better empower patients,” the authors wrote.
Six focus groups
Four or five participants were included in each of the six focus groups – a total of 29 women. All participants reported a history of symptomatic, culture-proven UTI episodes. They had experienced two or more infections in 6 months or three or more infections within 1 year. Women were predominantly White. Most were employed part- or full-time and held a college degree.
From a qualitative analysis of all focus group transcripts, two main themes emerged:
- The negative impact of taking antibiotics for the prevention and treatment of rUTIs.
- Resentment of the medical profession for the way it managed rUTIs.
The researchers found that participants had a good understanding of the deleterious effects from inappropriate antibiotic use, largely gleaned from media sources and the Internet. “Numerous women stated that they had reached such a level of concern about antibiotics that they would resist taking them for prevention or treatment of infections,” Dr. Scott and colleagues pointed out.
These concerns centered around the risk of developing resistance to antibiotics and the ill effects that antibiotics can have on the gastrointestinal and genitourinary microbiomes. Several women reported that they had developed Clostridium difficile infections after taking antibiotics; one of the patients required hospitalization for the infection.
Women also reported concerns that they had been given an antibiotic needlessly for symptoms that might have been caused by a genitourinary condition other than a UTI. They also reported feeling resentful toward practitioners, particularly if they felt the practitioner was overprescribing antibiotics. Some had resorted to consultations with alternative practitioners, such as herbalists. “A second concern discussed by participants was the feeling of being ignored by physicians,” the authors observed.
In this regard, the women felt that their physicians underestimated the burden that rUTIs had on their lives and the detrimental effect that repeated infections had on their relationships, work, and overall quality of life. “These perceptions led to a prevalent mistrust of physicians,” the investigators wrote. This prompted many women to insist that the medical community devote more effort to the development of nonantibiotic options for the prevention and treatment of UTIs.
Improved management strategies
Asked how physicians might improve their management of rUTIs, Dr. Scott shared a number of suggestions. Cardinal rule No. 1: Have the patient undergo a urinalysis to make sure she does have a UTI. “There is a subset of patients among women with rUTIs who come in with a diagnosis of an rUTI but who really have not had documentation of more than one positive urine culture,” Dr. Scott noted. Such a history suggests that they do not have an rUTI.
It’s imperative that physicians rule out commonly misdiagnosed disorders, such as overactive bladder, as a cause of the patient’s symptoms. Symptoms of overactive bladder and rUTIs often overlap. While waiting for results from the urinalysis to confirm or rule out a UTI, young and healthy women may be prescribed a nonsteroidal anti-inflammatory drug (NSAID), such as naproxen, which can help ameliorate symptoms.
Because UTIs are frequently self-limiting, Dr. Scott and others have found that for young, otherwise healthy women, NSAIDs alone can often resolve symptoms of the UTI without use of an antibiotic. For relatively severe symptoms, a urinary analgesic, such as phenazopyridine (Pyridium), may soothe the lining of the urinary tract and relieve pain. Cystex is an over-the-counter urinary analgesic that women can procure themselves, Dr. Scott added.
If an antibiotic is indicated, those most commonly prescribed for a single episode of acute cystitis are nitrofurantoin and sulfamethoxazole plus trimethoprim (Bactrim). For recurrent UTIs, “patients are a bit more complicated,” Dr. Scott admitted. “I think the best practice is to look back at a woman’s prior urine culture and select an antibiotic that showed good sensitivity in the last positive urine test,” she said.
Prevention starts with behavioral strategies, such as voiding after sexual intercourse and wiping from front to back following urination to avoid introducing fecal bacteria into the urethra. Evidence suggests that premenopausal women who drink at least 1.5 L of water a day have significantly fewer UTI episodes, Dr. Scott noted. There is also “pretty good” evidence that cranberry supplements (not juice) can prevent rUTIs. Use of cranberry supplements is supported by the American Urological Association (conditional recommendation; evidence level of grade C).
For peri- and postmenopausal women, vaginal estrogen may be effective. It’s use for UTI prevention is well supported by the literature. Although not as well supported by evidence, some women find that a supplement such as D-mannose may prevent or treat UTIs by causing bacteria to bind to it rather than to the bladder wall. Probiotics are another possibility, she noted. Empathy can’t hurt, she added.
“A common theme among satisfied women was the sentiment that their physicians understood their problems and had a system in place to allow rapid diagnosis and treatment for UTI episodes,” the authors emphasized.
“[Such attitudes] highlight the need to investigate each patient’s experience and perceptions to allow for shared decision making regarding the management of rUTIs,” they wrote.
Further commentary
Asked to comment on the findings, editorialist Michelle Van Kuiken, MD, assistant professor of urology, University of California, San Francisco, acknowledged that there is not a lot of good evidence to support many of the strategies recommended by the American Urological Association to prevent and treat rUTIs, but she often follows these recommendations anyway. “The one statement in the guidelines that is the most supported by evidence is the use of cranberry supplements, and I do routinely recommended daily use of some form of concentrated cranberry supplements for all of my patients with rUTIs,” she said in an interview.
Dr. Van Kuiken said that vaginal estrogen is a very good option for all postmenopausal women who suffer from rUTIs and that there is growing acceptance of its use for this and other indications. There is some evidence to support D-mannose as well, although it’s not that robust, she acknowledged.
She said the evidence supporting the use of probiotics for this indication is very thin. She does not routinely recommend them for rUTIs, although they are not inherently harmful. “I think for a lot of women who have rUTIs, it can be pretty debilitating and upsetting for them – it can impact travel plans, work, and social events,” Dr. Van Kuiken said.
“Until we develop better diagnostic and therapeutic strategies, validating women’s experiences and concerns with rUTI while limiting unnecessary antibiotics remains our best option,” she wrote.
Dr. Scott and Dr. Van Kuiken have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pelvic floor dysfunction imaging: New guidelines provide recommendations
New consensus guidelines from a multispecialty working group of the Pelvic Floor Disorders Consortium (PFDC) clear up inconsistencies in the use of magnetic resonance defecography (MRD) and provide universal recommendations on MRD technique, interpretation, reporting, and other factors.
“The consensus language used to describe pelvic floor disorders is critical, so as to allow the various experts who treat these patients [to] communicate and collaborate effectively with each other,” coauthor Liliana Bordeianou, MD, MPH, an associate professor of surgery at Harvard Medical School and chair of the Massachusetts General Hospital Colorectal and Pelvic Floor Centers, told this news organization.
“These diseases do not choose an arbitrary side in the pelvis,” she noted. “Instead, these diseases affect the entire pelvis and require a multidisciplinary and collaborative solution.”
MRD is a key component in that solution, providing dynamic evaluation of pelvic floor function and visualization of the complex interaction in pelvic compartments among patients with defecatory pelvic floor disorders, such as vaginal or uterine prolapse, constipation, incontinence, or other pelvic floor dysfunctions.
However, a key shortcoming has been a lack of consistency in nomenclature and the reporting of MRD findings among institutions and subspecialties.
Clinicians may wind up using different definitions for the same condition and different thresholds for grading severity, resulting in inconsistent communication not only between clinicians across institutions but even within the same institution, the report notes.
To address the situation, radiologists with the Pelvic Floor Dysfunction Disease Focused Panel of the Society of Abdominal Radiology (SAR) published recommendations on MRD protocol and technique in April.
However, even with that guidance, there has been significant variability in the interpretation and utilization of MRD findings among specialties outside of radiology.
The new report was therefore developed to include input from the broad variety of specialists involved in the treatment of patients with pelvic floor disorders, including colorectal surgeons, urogynecologists, urologists, gynecologists, gastroenterologists, radiologists, physiotherapists, and other advanced care practitioners.
“The goal of this effort was to create a universal set of recommendations and language for MRD technique, interpretation, and reporting that can be utilized and carry the same significance across disciplines,” write the authors of the report, published in the American Journal of Roentgenology.
One key area addressed in the report is a recommendation that MRD can be performed in either the upright or supine position, which has been a topic of inconsistency, said Brooke Gurland, MD, medical director of the Pelvic Health Center at Stanford University, California, a co-author on the consensus statement.
“Supine versus upright position was a source of debate, but ultimately there was a consensus that supine position was acceptable,” she told said in an interview.
Regarding positioning, the recommendations conclude that “given the variable results from different studies, consortium members agreed that it is acceptable to perform MRD in the supine position when upright MRD is not available.”
“Importantly, consortium experts stressed that it is very important that this imaging be performed after proper patient education on the purpose of the examination,” they note.
Other recommendations delve into contrast medium considerations, such as the recommendation that MRD does not require the routine use of vaginal contrast medium for adequate imaging of pathology.
And guidance on the technique and grading of relevant pathology include a recommendation to use the pubococcygeal line (PCL) as a point of reference to quantify the prolapse of organs in all compartments of the pelvic floor.
“There is an increasing appreciation that most patients with pelvic organ prolapse experience dual or even triple compartment pathology, making it important to describe the observations in all three compartments to ensure the mobilization of the appropriate team of experts to treat the patient,” the authors note.
The consensus report features an interpretative template providing synopses of the recommendations, which can be adjusted and modified according to additional radiologic information, as well as individualized patient information or clinician preferences.
However, “the suggested verbiage and steps should be advocated as the minimum requirements when performing and interpreting MRD in patients with evacuation disorders of the pelvic floor,” the authors note.
Dr. Gurland added that, in addition to providing benefits in the present utilization of MRD, the clearer guidelines should help advance its use to improve patient care in the future.
“Standardizing imaging techniques, reporting, and language is critical to improving our understanding and then developing therapies for pelvic floor disorders,” she said.
“In the future, correlating MRD with surgical outcomes and identifying modifiable risk factors will improve patient care.”
In addition to being published in the AJR, the report was published concurrently in the journals Diseases of the Colon & Rectum, International Urogynecology Journal, and Female Pelvic Medicine and Reconstructive Surgery.
The authors of the guidelines have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New consensus guidelines from a multispecialty working group of the Pelvic Floor Disorders Consortium (PFDC) clear up inconsistencies in the use of magnetic resonance defecography (MRD) and provide universal recommendations on MRD technique, interpretation, reporting, and other factors.
“The consensus language used to describe pelvic floor disorders is critical, so as to allow the various experts who treat these patients [to] communicate and collaborate effectively with each other,” coauthor Liliana Bordeianou, MD, MPH, an associate professor of surgery at Harvard Medical School and chair of the Massachusetts General Hospital Colorectal and Pelvic Floor Centers, told this news organization.
“These diseases do not choose an arbitrary side in the pelvis,” she noted. “Instead, these diseases affect the entire pelvis and require a multidisciplinary and collaborative solution.”
MRD is a key component in that solution, providing dynamic evaluation of pelvic floor function and visualization of the complex interaction in pelvic compartments among patients with defecatory pelvic floor disorders, such as vaginal or uterine prolapse, constipation, incontinence, or other pelvic floor dysfunctions.
However, a key shortcoming has been a lack of consistency in nomenclature and the reporting of MRD findings among institutions and subspecialties.
Clinicians may wind up using different definitions for the same condition and different thresholds for grading severity, resulting in inconsistent communication not only between clinicians across institutions but even within the same institution, the report notes.
To address the situation, radiologists with the Pelvic Floor Dysfunction Disease Focused Panel of the Society of Abdominal Radiology (SAR) published recommendations on MRD protocol and technique in April.
However, even with that guidance, there has been significant variability in the interpretation and utilization of MRD findings among specialties outside of radiology.
The new report was therefore developed to include input from the broad variety of specialists involved in the treatment of patients with pelvic floor disorders, including colorectal surgeons, urogynecologists, urologists, gynecologists, gastroenterologists, radiologists, physiotherapists, and other advanced care practitioners.
“The goal of this effort was to create a universal set of recommendations and language for MRD technique, interpretation, and reporting that can be utilized and carry the same significance across disciplines,” write the authors of the report, published in the American Journal of Roentgenology.
One key area addressed in the report is a recommendation that MRD can be performed in either the upright or supine position, which has been a topic of inconsistency, said Brooke Gurland, MD, medical director of the Pelvic Health Center at Stanford University, California, a co-author on the consensus statement.
“Supine versus upright position was a source of debate, but ultimately there was a consensus that supine position was acceptable,” she told said in an interview.
Regarding positioning, the recommendations conclude that “given the variable results from different studies, consortium members agreed that it is acceptable to perform MRD in the supine position when upright MRD is not available.”
“Importantly, consortium experts stressed that it is very important that this imaging be performed after proper patient education on the purpose of the examination,” they note.
Other recommendations delve into contrast medium considerations, such as the recommendation that MRD does not require the routine use of vaginal contrast medium for adequate imaging of pathology.
And guidance on the technique and grading of relevant pathology include a recommendation to use the pubococcygeal line (PCL) as a point of reference to quantify the prolapse of organs in all compartments of the pelvic floor.
“There is an increasing appreciation that most patients with pelvic organ prolapse experience dual or even triple compartment pathology, making it important to describe the observations in all three compartments to ensure the mobilization of the appropriate team of experts to treat the patient,” the authors note.
The consensus report features an interpretative template providing synopses of the recommendations, which can be adjusted and modified according to additional radiologic information, as well as individualized patient information or clinician preferences.
However, “the suggested verbiage and steps should be advocated as the minimum requirements when performing and interpreting MRD in patients with evacuation disorders of the pelvic floor,” the authors note.
Dr. Gurland added that, in addition to providing benefits in the present utilization of MRD, the clearer guidelines should help advance its use to improve patient care in the future.
“Standardizing imaging techniques, reporting, and language is critical to improving our understanding and then developing therapies for pelvic floor disorders,” she said.
“In the future, correlating MRD with surgical outcomes and identifying modifiable risk factors will improve patient care.”
In addition to being published in the AJR, the report was published concurrently in the journals Diseases of the Colon & Rectum, International Urogynecology Journal, and Female Pelvic Medicine and Reconstructive Surgery.
The authors of the guidelines have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New consensus guidelines from a multispecialty working group of the Pelvic Floor Disorders Consortium (PFDC) clear up inconsistencies in the use of magnetic resonance defecography (MRD) and provide universal recommendations on MRD technique, interpretation, reporting, and other factors.
“The consensus language used to describe pelvic floor disorders is critical, so as to allow the various experts who treat these patients [to] communicate and collaborate effectively with each other,” coauthor Liliana Bordeianou, MD, MPH, an associate professor of surgery at Harvard Medical School and chair of the Massachusetts General Hospital Colorectal and Pelvic Floor Centers, told this news organization.
“These diseases do not choose an arbitrary side in the pelvis,” she noted. “Instead, these diseases affect the entire pelvis and require a multidisciplinary and collaborative solution.”
MRD is a key component in that solution, providing dynamic evaluation of pelvic floor function and visualization of the complex interaction in pelvic compartments among patients with defecatory pelvic floor disorders, such as vaginal or uterine prolapse, constipation, incontinence, or other pelvic floor dysfunctions.
However, a key shortcoming has been a lack of consistency in nomenclature and the reporting of MRD findings among institutions and subspecialties.
Clinicians may wind up using different definitions for the same condition and different thresholds for grading severity, resulting in inconsistent communication not only between clinicians across institutions but even within the same institution, the report notes.
To address the situation, radiologists with the Pelvic Floor Dysfunction Disease Focused Panel of the Society of Abdominal Radiology (SAR) published recommendations on MRD protocol and technique in April.
However, even with that guidance, there has been significant variability in the interpretation and utilization of MRD findings among specialties outside of radiology.
The new report was therefore developed to include input from the broad variety of specialists involved in the treatment of patients with pelvic floor disorders, including colorectal surgeons, urogynecologists, urologists, gynecologists, gastroenterologists, radiologists, physiotherapists, and other advanced care practitioners.
“The goal of this effort was to create a universal set of recommendations and language for MRD technique, interpretation, and reporting that can be utilized and carry the same significance across disciplines,” write the authors of the report, published in the American Journal of Roentgenology.
One key area addressed in the report is a recommendation that MRD can be performed in either the upright or supine position, which has been a topic of inconsistency, said Brooke Gurland, MD, medical director of the Pelvic Health Center at Stanford University, California, a co-author on the consensus statement.
“Supine versus upright position was a source of debate, but ultimately there was a consensus that supine position was acceptable,” she told said in an interview.
Regarding positioning, the recommendations conclude that “given the variable results from different studies, consortium members agreed that it is acceptable to perform MRD in the supine position when upright MRD is not available.”
“Importantly, consortium experts stressed that it is very important that this imaging be performed after proper patient education on the purpose of the examination,” they note.
Other recommendations delve into contrast medium considerations, such as the recommendation that MRD does not require the routine use of vaginal contrast medium for adequate imaging of pathology.
And guidance on the technique and grading of relevant pathology include a recommendation to use the pubococcygeal line (PCL) as a point of reference to quantify the prolapse of organs in all compartments of the pelvic floor.
“There is an increasing appreciation that most patients with pelvic organ prolapse experience dual or even triple compartment pathology, making it important to describe the observations in all three compartments to ensure the mobilization of the appropriate team of experts to treat the patient,” the authors note.
The consensus report features an interpretative template providing synopses of the recommendations, which can be adjusted and modified according to additional radiologic information, as well as individualized patient information or clinician preferences.
However, “the suggested verbiage and steps should be advocated as the minimum requirements when performing and interpreting MRD in patients with evacuation disorders of the pelvic floor,” the authors note.
Dr. Gurland added that, in addition to providing benefits in the present utilization of MRD, the clearer guidelines should help advance its use to improve patient care in the future.
“Standardizing imaging techniques, reporting, and language is critical to improving our understanding and then developing therapies for pelvic floor disorders,” she said.
“In the future, correlating MRD with surgical outcomes and identifying modifiable risk factors will improve patient care.”
In addition to being published in the AJR, the report was published concurrently in the journals Diseases of the Colon & Rectum, International Urogynecology Journal, and Female Pelvic Medicine and Reconstructive Surgery.
The authors of the guidelines have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
2021 Update on pelvic floor disorders
With the increasing prevalence of pelvic floor disorders among our aging population, women’s health clinicians should be prepared to counsel patients on treatment options and posttreatment expectations. In this Update, we will review recent literature on surgical treatments for pelvic organ prolapse (POP) and stress urinary incontinence (SUI). We also include our review of an award-winning and practice-changing study on office-based pessary care. Lastly, we will finish with a summary of a recent Society of Gynecologic Surgeons collaborative systematic review on sexual function after surgery.
5-year RCT data on hysteropexy vs hysterectomy for POP
Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153. e1-153.e31. doi: 10.1016/j.ajog.2021.03.012.
The Pelvic Floor Disorders Network conducted a multisite randomized superiority trial comparing sacrospinous hysteropexy with mesh graft to vaginal hysterectomy with uterosacral ligament suspension for POP.
Study details
Postmenopausal women who desired surgery for symptomatic uterovaginal prolapse were randomly assigned to sacrospinous hysteropexy with polypropylene mesh graft using the Uphold-LITE device (Boston Scientific) versus vaginal hysterectomy with uterosacral ligament suspension. Participants were masked to treatment allocation and completed study visits at 6-month intervals through 60 months. Quantitative prolapse POP-Q exams were performed and patients completed multiple validated questionnaires regarding the presence; severity; and impact of prolapse, urinary, bowel, and pelvic pain symptoms.
Results
A total of 183 postmenopausal women were randomized, and 156 (81 hysteropexy and 75 hysterectomy) patients completed 5-year follow up with no demographic differences between the 2 intervention groups. Operative time was statistically less in the hysteropexy group (111.5 min vs 156.7 min). There were fewer treatment failures (a composite including retreatment for prolapse, prolapse beyond the hymen, and/or bothersome bulge symptoms) in the hysteropexy than in the hysterectomy group (37% vs 54%, respectively) at 5 years of follow up. However, most patients with treatment failure were classified as an intermittent failure, with only 16% of hysteropexy patients and 22% of hysterectomy patients classified as persistent failures. There were no meaningful differences between patient-reported outcomes. Hysteropexy had an 8% mesh exposure risk, with none requiring surgical management.
This study represents the highest quality randomized trial design and boasts high patient retention rates and 5-year follow up. Findings support further investigation on the use of polypropylene mesh for POP. In April of 2019, the US Food and Drug Administration halted the selling and distribution of vaginal mesh products for prolapse repair given the lack of safety outcomes, concerns about mesh exposure rates, and possible increased rates of pelvic pain and adverse events. This study invites pelvic reconstructive surgeons to revisit the debate of hysteropexy versus hysterectomy and synthetic mesh versus native tissue repairs. The 8% mesh exposure rate represents a challenge for the future design and development of vaginal implant materials, weighing the balancing of improved long-term efficacy with the safety and complication concerns.
Continue to: Preliminary 12-month data for a single-incision sling for surgical management of SUI...
Preliminary 12-month data for a single-incision sling for surgical management of SUI
Erickson T, Roovers JP, Gheiler E, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol. 2021;28:93-99. doi: 10.1016/j.jmig.2020.04.014.
In this industry-sponsored study, researchers compared a novel single-incision sling to currently available midurethral slings for SUI with 12-month outcomes and adverse event details. However, results are primarily descriptive with no statistical testing.
Study details
Patients were eligible for inclusion in this prospective, nonrandomized cohort study if SUI was their primary incontinence symptom, with confirmatory office testing. Exclusion criteria included POP greater than stage 2, prior SUI surgery, plans for future pregnancy, elevated postvoid residuals, or concomitant surgical procedures. The single-incision Altis (Coloplast) sling was compared to all commercially available transobturator and retropubic midurethral slings. The primary outcome of this study was reduction in 24-hour pad weights, and secondary outcomes included negative cough-stress test and subjective patient-reported outcomes via validated questionnaires.
Results
A total of 184 women were enrolled in the Altis group and 171 in the comparator other sling group. Symptom severity was similar between groups, but more patients in the comparator group had mixed urinary incontinence, and more patients in the Altis group had intrinsic sphincter deficiency. The Altis group had a higher proportion of “dry patients,” but otherwise the outcomes were similar between the 2 groups, including negative cough-stress test and patientreported outcomes. Two patients in the Altis group and 7 patients in the comparator group underwent device revisions. Again, statistical analysis was not performed.
Single-incision slings may reduce the risk of groin pain associated with transobturator slings and may be a good option for patients who desire less mesh burden than the traditional retropubic slings or who are not good candidates. This trial suggests that the Altis single-incision sling may be similar in outcomes and adverse events to currently available midurethral slings, but further, more rigorous trials are underway to fully evaluate this—including a US-based multicenter randomized trial of Altis single-incision slings versus retropubic slings (ClinicalTrials.gov Identifier: NCT03520114).
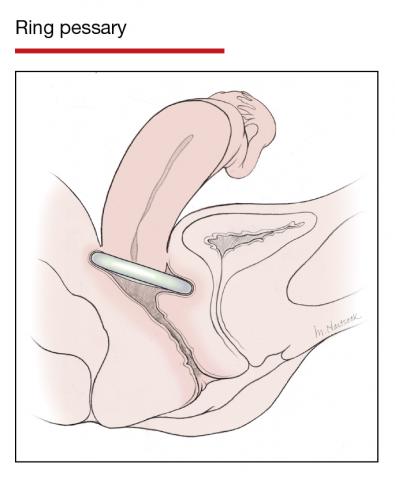
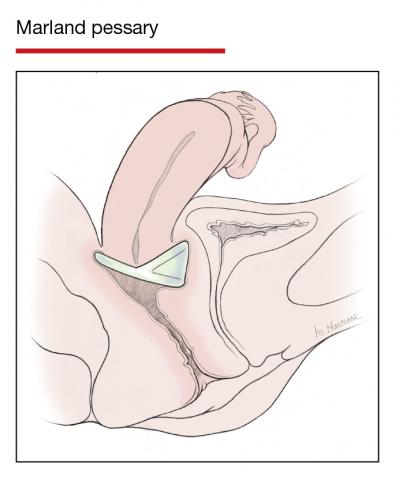
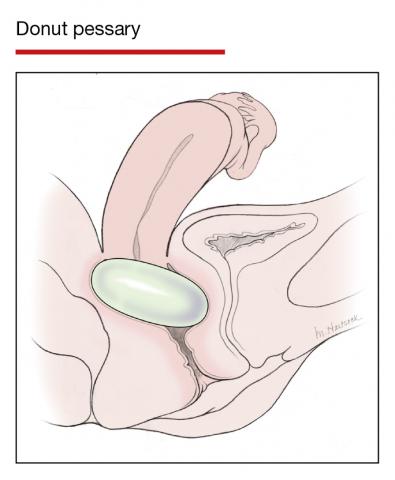
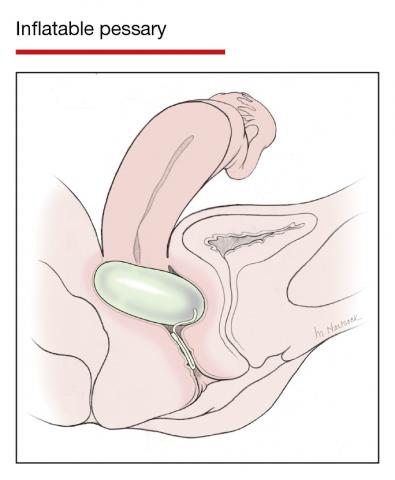
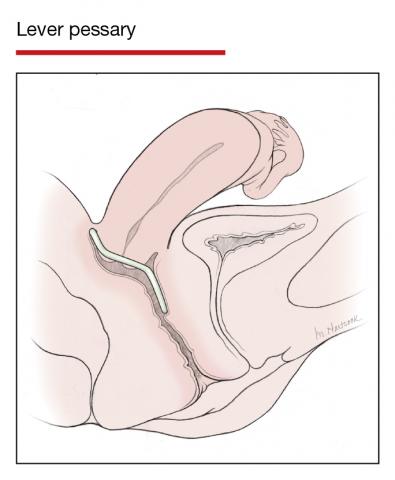
Office-based pessary care can be safely spaced out to 24 weeks without an increase in erosions
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2020;135:100-105. doi: 10.1097 /AOG.0000000000003580.
For women already using a pessary without issues, extending office visits to every 6 months does not increase rates of vaginal epithelial abnormalities, according to results of this randomized controlled trial.
Study details
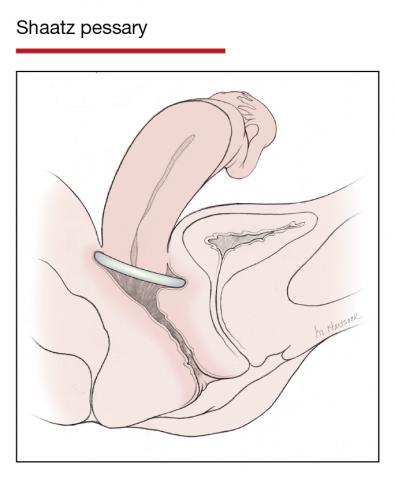
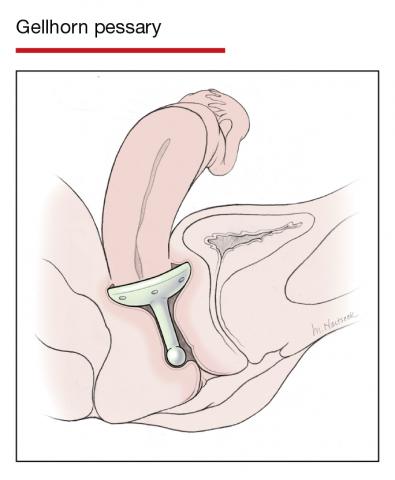
Women already using a Gelhorn, ring, or incontinence dish pessary for POP, SUI, or both were randomized to continue routine care with office evaluation every 12 weeks versus the extended-care cohort (with office evaluation every 24 weeks). Women were excluded if they removed and replaced the pessary themselves or if there was a presence of vaginal epithelial abnormalities, such as erosion or granulation tissue.
Results
The rate of vaginal epithelium erosion was 7.4% in the routine arm and 1.7% in the extended-care arm, meeting criteria for noninferiority of extended care. The majority of patients with office visits every 24 weeks preferred the less frequent examinations, and there was no difference in degree of bother due to vaginal discharge. There was also no difference in the percentage of patients with unscheduled visits. The only factors associated with vaginal epithelium abnormalities were prior abnormalities and lifetime duration of pessary use.
As there are currently no evidenced-based guidelines for pessary care, this study contributes data to support extended office-based care up to 24 weeks, a common practice in the United Kingdom. During the COVID-19 pandemic, with reduced health care access, these findings should be reassuring to clinicians and patients.
Continue to: How can we counsel patients regarding changes in sexual activity and function after surgery for POP?...
How can we counsel patients regarding changes in sexual activity and function after surgery for POP?
Antosh DD, Dieter AA, Balk EM, et al. Sexual function after pelvic organ prolapse surgery: a systematic review comparing different approaches to pelvic floor repair. Am J Obstet Gynecol. 2021;2:S0002-9378(21)00610-4. doi: 10.1016/j.ajog.2021.05.042.
A secondary analysis of a recent systematic review found overall moderate- to high-quality evidence that were no differences in total dyspareunia, de novo dyspareunia, and scores on a validated sexual function questionnaire (PISQ12) when comparing postoperative sexual function outcomes of native tissue repair to sacrocolpopexy, transvaginal mesh, or biologic graft. Rates of postoperative dyspareunia were higher for transvaginal mesh than for sacrocolpopexy.
Study details
The Society of Gynecologic Surgeons Systematic Review Group identified 43 original prospective, comparative studies of reconstructive prolapse surgery that reported sexual function outcomes when comparing 2 different types of POP procedures. Thirty-seven of those studies were randomized controlled trials. Specifically, they looked at data comparing outcomes for native tissue versus sacrocolpopexy, native tissue versus transvaginal mesh, native tissue versus biologic graft, and transvaginal mesh versus sacrocolpopexy.
Results
Overall, the prevalence of postoperative dyspareunia was lower than preoperatively after all surgery types. The only statistical difference in this review demonstrated higher postoperative prevalence of dyspareunia after transvaginal mesh than sacrocolpopexy, based on 2 studies. When comparing native tissue prolapse repair to transvaginal mesh, sacrocolpopexy, or biologic grafts, there were no significant differences in sexual activity, baseline, or postoperative total dyspareunia, de-novo dyspareunia, or sexual function changes as measured by the PISQ12 validated questionnaire. ●
This systematic review further contributes to the growing evidence that, regardless of surgical approach to POP, sexual function generally improves and dyspareunia rates generally decrease postoperatively, with overall low rates of de novo dyspareunia. This will help patients and providers select the best-fit surgical approach without concern for worsened sexual function. It also underscores the need for inclusion of standardized sexual function terminology use and sexual health outcomes in future prolapse surgery research.

With the increasing prevalence of pelvic floor disorders among our aging population, women’s health clinicians should be prepared to counsel patients on treatment options and posttreatment expectations. In this Update, we will review recent literature on surgical treatments for pelvic organ prolapse (POP) and stress urinary incontinence (SUI). We also include our review of an award-winning and practice-changing study on office-based pessary care. Lastly, we will finish with a summary of a recent Society of Gynecologic Surgeons collaborative systematic review on sexual function after surgery.
5-year RCT data on hysteropexy vs hysterectomy for POP
Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153. e1-153.e31. doi: 10.1016/j.ajog.2021.03.012.
The Pelvic Floor Disorders Network conducted a multisite randomized superiority trial comparing sacrospinous hysteropexy with mesh graft to vaginal hysterectomy with uterosacral ligament suspension for POP.
Study details
Postmenopausal women who desired surgery for symptomatic uterovaginal prolapse were randomly assigned to sacrospinous hysteropexy with polypropylene mesh graft using the Uphold-LITE device (Boston Scientific) versus vaginal hysterectomy with uterosacral ligament suspension. Participants were masked to treatment allocation and completed study visits at 6-month intervals through 60 months. Quantitative prolapse POP-Q exams were performed and patients completed multiple validated questionnaires regarding the presence; severity; and impact of prolapse, urinary, bowel, and pelvic pain symptoms.
Results
A total of 183 postmenopausal women were randomized, and 156 (81 hysteropexy and 75 hysterectomy) patients completed 5-year follow up with no demographic differences between the 2 intervention groups. Operative time was statistically less in the hysteropexy group (111.5 min vs 156.7 min). There were fewer treatment failures (a composite including retreatment for prolapse, prolapse beyond the hymen, and/or bothersome bulge symptoms) in the hysteropexy than in the hysterectomy group (37% vs 54%, respectively) at 5 years of follow up. However, most patients with treatment failure were classified as an intermittent failure, with only 16% of hysteropexy patients and 22% of hysterectomy patients classified as persistent failures. There were no meaningful differences between patient-reported outcomes. Hysteropexy had an 8% mesh exposure risk, with none requiring surgical management.
This study represents the highest quality randomized trial design and boasts high patient retention rates and 5-year follow up. Findings support further investigation on the use of polypropylene mesh for POP. In April of 2019, the US Food and Drug Administration halted the selling and distribution of vaginal mesh products for prolapse repair given the lack of safety outcomes, concerns about mesh exposure rates, and possible increased rates of pelvic pain and adverse events. This study invites pelvic reconstructive surgeons to revisit the debate of hysteropexy versus hysterectomy and synthetic mesh versus native tissue repairs. The 8% mesh exposure rate represents a challenge for the future design and development of vaginal implant materials, weighing the balancing of improved long-term efficacy with the safety and complication concerns.
Continue to: Preliminary 12-month data for a single-incision sling for surgical management of SUI...
Preliminary 12-month data for a single-incision sling for surgical management of SUI
Erickson T, Roovers JP, Gheiler E, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol. 2021;28:93-99. doi: 10.1016/j.jmig.2020.04.014.
In this industry-sponsored study, researchers compared a novel single-incision sling to currently available midurethral slings for SUI with 12-month outcomes and adverse event details. However, results are primarily descriptive with no statistical testing.
Study details
Patients were eligible for inclusion in this prospective, nonrandomized cohort study if SUI was their primary incontinence symptom, with confirmatory office testing. Exclusion criteria included POP greater than stage 2, prior SUI surgery, plans for future pregnancy, elevated postvoid residuals, or concomitant surgical procedures. The single-incision Altis (Coloplast) sling was compared to all commercially available transobturator and retropubic midurethral slings. The primary outcome of this study was reduction in 24-hour pad weights, and secondary outcomes included negative cough-stress test and subjective patient-reported outcomes via validated questionnaires.
Results
A total of 184 women were enrolled in the Altis group and 171 in the comparator other sling group. Symptom severity was similar between groups, but more patients in the comparator group had mixed urinary incontinence, and more patients in the Altis group had intrinsic sphincter deficiency. The Altis group had a higher proportion of “dry patients,” but otherwise the outcomes were similar between the 2 groups, including negative cough-stress test and patientreported outcomes. Two patients in the Altis group and 7 patients in the comparator group underwent device revisions. Again, statistical analysis was not performed.
Single-incision slings may reduce the risk of groin pain associated with transobturator slings and may be a good option for patients who desire less mesh burden than the traditional retropubic slings or who are not good candidates. This trial suggests that the Altis single-incision sling may be similar in outcomes and adverse events to currently available midurethral slings, but further, more rigorous trials are underway to fully evaluate this—including a US-based multicenter randomized trial of Altis single-incision slings versus retropubic slings (ClinicalTrials.gov Identifier: NCT03520114).
Office-based pessary care can be safely spaced out to 24 weeks without an increase in erosions
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2020;135:100-105. doi: 10.1097 /AOG.0000000000003580.
For women already using a pessary without issues, extending office visits to every 6 months does not increase rates of vaginal epithelial abnormalities, according to results of this randomized controlled trial.
Study details
Women already using a Gelhorn, ring, or incontinence dish pessary for POP, SUI, or both were randomized to continue routine care with office evaluation every 12 weeks versus the extended-care cohort (with office evaluation every 24 weeks). Women were excluded if they removed and replaced the pessary themselves or if there was a presence of vaginal epithelial abnormalities, such as erosion or granulation tissue.
Results
The rate of vaginal epithelium erosion was 7.4% in the routine arm and 1.7% in the extended-care arm, meeting criteria for noninferiority of extended care. The majority of patients with office visits every 24 weeks preferred the less frequent examinations, and there was no difference in degree of bother due to vaginal discharge. There was also no difference in the percentage of patients with unscheduled visits. The only factors associated with vaginal epithelium abnormalities were prior abnormalities and lifetime duration of pessary use.
As there are currently no evidenced-based guidelines for pessary care, this study contributes data to support extended office-based care up to 24 weeks, a common practice in the United Kingdom. During the COVID-19 pandemic, with reduced health care access, these findings should be reassuring to clinicians and patients.
Continue to: How can we counsel patients regarding changes in sexual activity and function after surgery for POP?...
How can we counsel patients regarding changes in sexual activity and function after surgery for POP?
Antosh DD, Dieter AA, Balk EM, et al. Sexual function after pelvic organ prolapse surgery: a systematic review comparing different approaches to pelvic floor repair. Am J Obstet Gynecol. 2021;2:S0002-9378(21)00610-4. doi: 10.1016/j.ajog.2021.05.042.
A secondary analysis of a recent systematic review found overall moderate- to high-quality evidence that were no differences in total dyspareunia, de novo dyspareunia, and scores on a validated sexual function questionnaire (PISQ12) when comparing postoperative sexual function outcomes of native tissue repair to sacrocolpopexy, transvaginal mesh, or biologic graft. Rates of postoperative dyspareunia were higher for transvaginal mesh than for sacrocolpopexy.
Study details
The Society of Gynecologic Surgeons Systematic Review Group identified 43 original prospective, comparative studies of reconstructive prolapse surgery that reported sexual function outcomes when comparing 2 different types of POP procedures. Thirty-seven of those studies were randomized controlled trials. Specifically, they looked at data comparing outcomes for native tissue versus sacrocolpopexy, native tissue versus transvaginal mesh, native tissue versus biologic graft, and transvaginal mesh versus sacrocolpopexy.
Results
Overall, the prevalence of postoperative dyspareunia was lower than preoperatively after all surgery types. The only statistical difference in this review demonstrated higher postoperative prevalence of dyspareunia after transvaginal mesh than sacrocolpopexy, based on 2 studies. When comparing native tissue prolapse repair to transvaginal mesh, sacrocolpopexy, or biologic grafts, there were no significant differences in sexual activity, baseline, or postoperative total dyspareunia, de-novo dyspareunia, or sexual function changes as measured by the PISQ12 validated questionnaire. ●
This systematic review further contributes to the growing evidence that, regardless of surgical approach to POP, sexual function generally improves and dyspareunia rates generally decrease postoperatively, with overall low rates of de novo dyspareunia. This will help patients and providers select the best-fit surgical approach without concern for worsened sexual function. It also underscores the need for inclusion of standardized sexual function terminology use and sexual health outcomes in future prolapse surgery research.

With the increasing prevalence of pelvic floor disorders among our aging population, women’s health clinicians should be prepared to counsel patients on treatment options and posttreatment expectations. In this Update, we will review recent literature on surgical treatments for pelvic organ prolapse (POP) and stress urinary incontinence (SUI). We also include our review of an award-winning and practice-changing study on office-based pessary care. Lastly, we will finish with a summary of a recent Society of Gynecologic Surgeons collaborative systematic review on sexual function after surgery.
5-year RCT data on hysteropexy vs hysterectomy for POP
Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153. e1-153.e31. doi: 10.1016/j.ajog.2021.03.012.
The Pelvic Floor Disorders Network conducted a multisite randomized superiority trial comparing sacrospinous hysteropexy with mesh graft to vaginal hysterectomy with uterosacral ligament suspension for POP.
Study details
Postmenopausal women who desired surgery for symptomatic uterovaginal prolapse were randomly assigned to sacrospinous hysteropexy with polypropylene mesh graft using the Uphold-LITE device (Boston Scientific) versus vaginal hysterectomy with uterosacral ligament suspension. Participants were masked to treatment allocation and completed study visits at 6-month intervals through 60 months. Quantitative prolapse POP-Q exams were performed and patients completed multiple validated questionnaires regarding the presence; severity; and impact of prolapse, urinary, bowel, and pelvic pain symptoms.
Results
A total of 183 postmenopausal women were randomized, and 156 (81 hysteropexy and 75 hysterectomy) patients completed 5-year follow up with no demographic differences between the 2 intervention groups. Operative time was statistically less in the hysteropexy group (111.5 min vs 156.7 min). There were fewer treatment failures (a composite including retreatment for prolapse, prolapse beyond the hymen, and/or bothersome bulge symptoms) in the hysteropexy than in the hysterectomy group (37% vs 54%, respectively) at 5 years of follow up. However, most patients with treatment failure were classified as an intermittent failure, with only 16% of hysteropexy patients and 22% of hysterectomy patients classified as persistent failures. There were no meaningful differences between patient-reported outcomes. Hysteropexy had an 8% mesh exposure risk, with none requiring surgical management.
This study represents the highest quality randomized trial design and boasts high patient retention rates and 5-year follow up. Findings support further investigation on the use of polypropylene mesh for POP. In April of 2019, the US Food and Drug Administration halted the selling and distribution of vaginal mesh products for prolapse repair given the lack of safety outcomes, concerns about mesh exposure rates, and possible increased rates of pelvic pain and adverse events. This study invites pelvic reconstructive surgeons to revisit the debate of hysteropexy versus hysterectomy and synthetic mesh versus native tissue repairs. The 8% mesh exposure rate represents a challenge for the future design and development of vaginal implant materials, weighing the balancing of improved long-term efficacy with the safety and complication concerns.
Continue to: Preliminary 12-month data for a single-incision sling for surgical management of SUI...
Preliminary 12-month data for a single-incision sling for surgical management of SUI
Erickson T, Roovers JP, Gheiler E, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol. 2021;28:93-99. doi: 10.1016/j.jmig.2020.04.014.
In this industry-sponsored study, researchers compared a novel single-incision sling to currently available midurethral slings for SUI with 12-month outcomes and adverse event details. However, results are primarily descriptive with no statistical testing.
Study details
Patients were eligible for inclusion in this prospective, nonrandomized cohort study if SUI was their primary incontinence symptom, with confirmatory office testing. Exclusion criteria included POP greater than stage 2, prior SUI surgery, plans for future pregnancy, elevated postvoid residuals, or concomitant surgical procedures. The single-incision Altis (Coloplast) sling was compared to all commercially available transobturator and retropubic midurethral slings. The primary outcome of this study was reduction in 24-hour pad weights, and secondary outcomes included negative cough-stress test and subjective patient-reported outcomes via validated questionnaires.
Results
A total of 184 women were enrolled in the Altis group and 171 in the comparator other sling group. Symptom severity was similar between groups, but more patients in the comparator group had mixed urinary incontinence, and more patients in the Altis group had intrinsic sphincter deficiency. The Altis group had a higher proportion of “dry patients,” but otherwise the outcomes were similar between the 2 groups, including negative cough-stress test and patientreported outcomes. Two patients in the Altis group and 7 patients in the comparator group underwent device revisions. Again, statistical analysis was not performed.
Single-incision slings may reduce the risk of groin pain associated with transobturator slings and may be a good option for patients who desire less mesh burden than the traditional retropubic slings or who are not good candidates. This trial suggests that the Altis single-incision sling may be similar in outcomes and adverse events to currently available midurethral slings, but further, more rigorous trials are underway to fully evaluate this—including a US-based multicenter randomized trial of Altis single-incision slings versus retropubic slings (ClinicalTrials.gov Identifier: NCT03520114).
Office-based pessary care can be safely spaced out to 24 weeks without an increase in erosions
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2020;135:100-105. doi: 10.1097 /AOG.0000000000003580.
For women already using a pessary without issues, extending office visits to every 6 months does not increase rates of vaginal epithelial abnormalities, according to results of this randomized controlled trial.
Study details
Women already using a Gelhorn, ring, or incontinence dish pessary for POP, SUI, or both were randomized to continue routine care with office evaluation every 12 weeks versus the extended-care cohort (with office evaluation every 24 weeks). Women were excluded if they removed and replaced the pessary themselves or if there was a presence of vaginal epithelial abnormalities, such as erosion or granulation tissue.
Results
The rate of vaginal epithelium erosion was 7.4% in the routine arm and 1.7% in the extended-care arm, meeting criteria for noninferiority of extended care. The majority of patients with office visits every 24 weeks preferred the less frequent examinations, and there was no difference in degree of bother due to vaginal discharge. There was also no difference in the percentage of patients with unscheduled visits. The only factors associated with vaginal epithelium abnormalities were prior abnormalities and lifetime duration of pessary use.
As there are currently no evidenced-based guidelines for pessary care, this study contributes data to support extended office-based care up to 24 weeks, a common practice in the United Kingdom. During the COVID-19 pandemic, with reduced health care access, these findings should be reassuring to clinicians and patients.
Continue to: How can we counsel patients regarding changes in sexual activity and function after surgery for POP?...
How can we counsel patients regarding changes in sexual activity and function after surgery for POP?
Antosh DD, Dieter AA, Balk EM, et al. Sexual function after pelvic organ prolapse surgery: a systematic review comparing different approaches to pelvic floor repair. Am J Obstet Gynecol. 2021;2:S0002-9378(21)00610-4. doi: 10.1016/j.ajog.2021.05.042.
A secondary analysis of a recent systematic review found overall moderate- to high-quality evidence that were no differences in total dyspareunia, de novo dyspareunia, and scores on a validated sexual function questionnaire (PISQ12) when comparing postoperative sexual function outcomes of native tissue repair to sacrocolpopexy, transvaginal mesh, or biologic graft. Rates of postoperative dyspareunia were higher for transvaginal mesh than for sacrocolpopexy.
Study details
The Society of Gynecologic Surgeons Systematic Review Group identified 43 original prospective, comparative studies of reconstructive prolapse surgery that reported sexual function outcomes when comparing 2 different types of POP procedures. Thirty-seven of those studies were randomized controlled trials. Specifically, they looked at data comparing outcomes for native tissue versus sacrocolpopexy, native tissue versus transvaginal mesh, native tissue versus biologic graft, and transvaginal mesh versus sacrocolpopexy.
Results
Overall, the prevalence of postoperative dyspareunia was lower than preoperatively after all surgery types. The only statistical difference in this review demonstrated higher postoperative prevalence of dyspareunia after transvaginal mesh than sacrocolpopexy, based on 2 studies. When comparing native tissue prolapse repair to transvaginal mesh, sacrocolpopexy, or biologic grafts, there were no significant differences in sexual activity, baseline, or postoperative total dyspareunia, de-novo dyspareunia, or sexual function changes as measured by the PISQ12 validated questionnaire. ●
This systematic review further contributes to the growing evidence that, regardless of surgical approach to POP, sexual function generally improves and dyspareunia rates generally decrease postoperatively, with overall low rates of de novo dyspareunia. This will help patients and providers select the best-fit surgical approach without concern for worsened sexual function. It also underscores the need for inclusion of standardized sexual function terminology use and sexual health outcomes in future prolapse surgery research.
A multidisciplinary approach to gyn care: A single center’s experience
In her book The Silo Effect: The Peril of Expertise and the Promise of Breaking Down Barriers, Gillian Tett wrote that “the word ‘silo’ does not just refer to a physical structure or organization (such as a department). It can also be a state of mind. Silos exist in structures. But they exist in our minds and social groups too. Silos breed tribalism. But they can also go hand in hand with tunnel vision.”
Tertiary care referral centers seem to be trending toward being more and more “un-siloed” and collaborative within their own departments and between departments in order to care for patients. The terms multidisciplinary and intradisciplinary have become popular in medicine, and teams are joining forces to create care paths for patients that are intended to improve the efficiency of and the quality of care that is rendered. There is no better example of the move to improve collaboration in medicine than the theme of the 2021 Society of Gynecologic Surgeons annual meeting, “Working Together: How Collaboration Enables Us to Better Help Our Patients.”
In this article, we provide examples of how collaborating with other specialties—within and outside of an ObGyn department—should become the standard of care. We discuss how to make this team approach easier and provide evidence that patients experience favorable outcomes. While data on combined care remain sparse, the existing literature on this topic helps us to guide and counsel patients about what to expect when a combined approach is taken.
Addressing pelvic floor disorders in women with gynecologic malignancy
In 2018, authors of a systematic review that looked at concurrent pelvic floor disorders in gynecologic oncologic survivors found that the prevalence of these disorders was high enough to warrant evaluation and management of these conditions to help improve quality of life for patients.1 Furthermore, it is possible that the prevalence of urinary incontinence is higher in patients who have undergone surgery for a gynecologic malignancy compared with controls, which has been reported in previous studies.2,3 At Cleveland Clinic, we recognize the need to evaluate our patients receiving oncologic care for urinary, fecal, and pelvic organ prolapse symptoms. Our oncologists routinely inquire about these symptoms once their patients have undergone surgery with them, and they make referrals for all their symptomatic patients. They have even learned about our own counseling, and they pre-emptively let patients know what our counseling may encompass.
For instance, many patients who received radiation therapy have stress urinary incontinence that is likely related to a hypomobile urethra, and they may benefit more from transurethral bulking than an anti-incontinence procedure in the operating room. Reassuring patients ahead of time that they do not need major interventions for their symptoms is helpful, as these patients are already experiencing tremendous burden from their oncologic conditions. We have made our referral patterns easy for these patients, and most patients are seen within days to weeks of the referral placed, depending on the urgency of the consult and the need to proceed with their oncologic treatment plan.
Gynecologic oncology patients who present with preoperative stress urinary incontinence and pelvic organ prolapse also are referred to a urogynecology specialist for concurrent care. Care paths have been created to help inform both the urogynecologists and the oncologists about options for patients depending on their respective conditions, as both their malignancy and their pelvic floor disorder(s) are considered in treatment planning. There is agreement in this planning that the oncologic surgery takes priority, and the urogynecologic approach is based on the oncologic plan.
Our urogynecologists routinely ask if future radiation is in the treatment plan, as this usually precludes us from placing a midurethral sling at the time of any surgery. Surgical approach (vaginal versus abdominal; open or minimally invasive) also is determined by the oncologic team. At the time of surgery, patient positioning is considered to optimize access for all of the surgeons. For instance, having the oncologist know that the patient needs to be far down on the bed as their steep Trendelenburg positioning during laparoscopy or robotic surgery may cause the patient to slide cephalad during the case may make a vaginal repair or sling placement at the end of the case challenging. All these small nuances are important, and a collaborative team develops the right plan for each patient in advance.
Data on the outcomes of combined surgery are sparse. In a retrospective matched cohort study, our group compared outcomes in women who underwent concurrent surgery with those who underwent urogynecologic surgery alone.4 We found that concurrent surgeries had an increased incidence of minor but not serious perioperative adverse events. Importantly, we determined that 1 in 10 planned urogynecologic procedures needed to be either modified or abandoned as a result of the oncologic plan. These data help guide our counseling, and both the oncologist and urogynecologist contributing to the combined case counsel patients according to these data.
Continue to: Concurrent colorectal and gynecologic surgery...
Concurrent colorectal and gynecologic surgery
Many women have pelvic floor disorders. As gynecologists, we often compartmentalize these conditions as gynecologic problems; frequently, however, colorectal conditions are at play as well and should be addressed concurrently. For instance, a high incidence of anorectal dysfunction occurs in women who present with pelvic organ prolapse.5 Furthermore, outlet defecation disorders are not always a result of a straightforward rectocele that can be fixed vaginally. Sometimes, a more thorough evaluation is warranted depending on the patient’s concurrent symptoms and history. Outlet symptoms may be attributed to large enteroceles, sigmoidoceles, perineal descent, rectal intussusception, and rectal prolapse.6
As a result, a combined approach to caring for patients with complex pelvic floor disorders is optimal. Several studies describe this type of combined and coordinated patient care.7,8 Ideally, patients are seen by both surgeons in the office so that the surgeons may make a combined plan for their care, especially if the decision is made to proceed with surgery. Urogynecology specialists and colorectal surgeons must decide together whether to approach combined prolapse procedures via a perineal and vaginal approach versus an abdominal approach. Several factors can determine this, including surgeon experience and preference, which is why it is important for surgeons working together to have either well-designed care paths or simply open communication and experience working together for the conditions they are treating.
In an ideal coordinated care approach, both surgeons review the patient records in advance. Any needed imaging or testing is done before the official patient consult; the patient is then seen by both clinicians in the same visit and counseled about the options. This is the most efficient and effective way to see patients, and we have had significant success using this approach.
Complications of combined surgery
The safety of combining procedures such as laparoscopic sacrocolpopexy and concurrent rectopexy has been studied, and intraoperative complications have been reported to be low.9,10 In a cohort study, Wallace and colleagues looked at postoperative outcomes and complications following combined surgery and reported that reoperation for the rectal prolapse component of the surgery was more common than the pelvic organ prolapse component, and that 1 in 5 of their patients experienced a surgical complication within 30 days of their surgery.11 This incidence is higher than that seen with isolated pelvic organ prolapse surgery. These data help us understand that a combined approach requires good patient counseling in the office about both the need for repeat surgery in certain circumstances and the increased risk of complications. Further, combined perineal and vaginal approaches have been compared with abdominal approaches and also have shown no age-adjusted differences in outcomes and complications.12
These data point to the need for surgeons to choose the approach to surgery that best fits their own experiences and to discuss this together before counseling the patient in the office, thus streamlining the effort so that the patient feels comfortable under the care of 2 surgeons.
Patients presenting with urogynecologic and gynecologic conditions also report symptomatic hemorrhoids, and colorectal referral is often made by the gynecologist. Sparse data are available regarding combined approaches to managing hemorrhoids and gynecologic conditions. Our group was the first to publish on outcomes and complications in patients undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery.13 In that retrospective cohort, we found that minor complications, such as postoperative urinary tract infection and transient voiding dysfunction, was more common in patients who underwent combined surgery. From this, we gathered that there is a need to counsel patients appropriately about the risk of combined surgery. That said, for some patients, coordinated care is desirable, and surgeons should make the effort to work together in combining their procedures.
Continue to: Integrating plastic and reconstructive surgery in gynecology...
Integrating plastic and reconstructive surgery in gynecology
Reconstructive gynecologic procedures often require a multidisciplinary approach to what can be very complex reconstructive surgery. The intended goal usually is to achieve a good cosmetic result in the genital area, as well as to restore sexual, defecatory, and/or genitourinary functionality. As a result, surgeons must work together to develop a feasible reconstructive plan for these patients.
Women experience vaginal stenosis or foreshortening for a number of reasons. Women with congenital anomalies often are cared for by specialists in pediatric and adolescent gynecology. Other women, such as those who have undergone vaginectomy and/or pelvic or vaginal radiation for cancer treatment, complications from vaginal mesh placement, and severe vaginal scarring from dermatologic conditions like lichen planus, are cared for by other gynecologic specialists, often general gynecologists or urogynecologists. In some of these cases, a gynecologic surgeon can perform vaginal adhesiolysis followed by vaginal estrogen treatment (when appropriate) and aggressive postoperative vaginal dilation with adjunctive pelvic floor physical therapy as well as sex therapy or counseling. A simple reconstructive approach may be necessary if lysis of adhesions alone is not sufficient. Sometimes, the vaginal apex must be opened vaginally or abdominally, or releasing incisions need to be made to improve the caliber of the vagina in addition to its length. Under these circumstances, the use of additional local skin grafts, local peritoneal flaps, or biologic grafts or xenografts can help achieve a satisfying result. While not all gynecologists are trained to perform these procedures, some are, and certainly gynecologic subspecialists have the skill sets to care for these patients.
Under other circumstances, when the vagina is truly foreshortened, more aggressive reconstructive surgery is necessary and consultation and collaboration with plastic surgery specialists often is helpful. At our center, these patients’ care is initially managed by gynecologists and, when simple approaches to their reconstructive needs are exhausted, collaboration is warranted. As with the other team approaches discussed in this article, the recommendation is for a consistent referral team that has established care paths for patients. Not all plastic surgeons are familiar with neovaginal reconstruction and understand the functional aspects that gynecologists are hoping to achieve for their patients. Therefore, it is important to form cohesive teams that have the same goals for the patient.
The literature on neovaginal reconstruction is sparse. There are no true agreed on approaches or techniques for vaginal reconstruction because there is no “one size fits all” for these repairs. Defects also vary depending on whether they are due to resections or radiation for oncologic treatment, reconstruction as part of the repair of a genitourinary or rectovaginal fistula, or stenosis from other etiologies.
In 2002, Cordeiro and colleagues published a classification system and reconstructive algorithm for acquired vaginal defects.14 Not all reconstructive surgeons subscribe to this algorithm, but it is the only rubric that currently exists. The authors differentiate between “partial” and “circumferential” defects and recommend different types of fasciocutaneous and myocutaneous flaps for reconstruction.
In our experience at our center, we believe that the choice of flap should also depend on whether or not perineal reconstruction is needed. This decision is made by both the gynecologic specialist and the plastic surgeon. Common flap choices include the Singapore flap, a fasciocutaneous flap based on perforators from the pudendal vessels; the gracilis flap, a myocutaneous flap based off the medial circumflex femoral vessels; and the rectus abdominis flap (transverse or vertical), which is also a myocutaneous flap that relies on the blood supply from the deep inferior epigastric vessels.
One of the most important parts of the coordinated effort of neovaginal surgery is postoperative care. Plastic surgeons play a key role in ensuring that the flap survives in the immediate postoperative period. The gynecology team should be responsible for postoperative vaginal dilation teaching and follow-up to ensure that the patient dilates properly and upsizes her dilator appropriately over the postoperative period. In our practice, our advanced practice clinicians often care for these patients and are responsible for continuity and dilation teaching. Patients have easy access to these clinicians, and this enhances the postoperative experience. Referral to a pelvic floor physical therapist knowledgeable about neovaginal surgery also helps to ensure that the dilation process goes successfully. It also helps to have office days on the same days as the plastic surgery team that is following the patient. This way, the patient may be seen by both teams on the same day. This allows for good patient communication with regard to aftercare, as well as a combined approach to teaching the trainees involved in the case. Coordination with pelvic floor physical therapists on those days also enhances the patient experience and is highly recommended.
Continue to: Combining gyn and urogyn procedures with plastic surgery...
Combining gyn and urogyn procedures with plastic surgery
While there are no data on combining gynecologic and urogynecologic procedures with plastic reconstructive surgeries, a team approach to combining surgeries is possible. At our center, we have performed tubal ligation, ovarian surgery, hysterectomy, and sling and prolapse surgery in patients who were undergoing cosmetic procedures, such as breast augmentation and abdominoplasty.
Gender affirmation surgery also can be performed through a combined approach between gynecologists and plastic surgeons. Our gynecologists perform hysterectomy for transmasculine men, and this procedure is sometimes safely and effectively performed in combination with masculinizing chest surgery (mastectomy) performed by our plastic surgeons. Vaginoplasty surgery (feminizing genital surgery) also is performed by urogynecology specialists at our center, and it is sometimes done concurrently at the time of breast augmentation and/or facial feminization surgery.
Case order. Some plastic surgeons vocalize concerns about combining clean procedures with clean contaminated cases, especially in situations in which implants are being placed in the body. During these cases, communication and organization between surgeons is important. For instance, there should be a discussion about case order. In general, the clean procedures should be performed first. In addition, separate operating tables and instruments should be used. Simultaneous operating also should be avoided. Fresh incisions should be dressed and covered before subsequent procedures are performed.
Incision placement. Last, planning around incision placement should be discussed before each case. Laparoscopic and abdominal incisions may interfere with plastic surgery procedures and alter the end cosmesis. These incisions often can be incorporated into the reconstructive procedure. The most important part of the coordinated surgical effort is ensuring that both surgical teams understand each other’s respective surgeries and the approach needed to complete them. When this is achieved, the cases are usually very successful.
Creating collaboration between obstetricians and gynecologic specialists
The impacts of pregnancy and vaginal delivery on the pelvic floor are well established. Urinary and fecal incontinence, pelvic organ prolapse, perineal pain, and dyspareunia are not uncommon in the postpartum period and may persist long term. The effects of obstetric anal sphincter injury (OASI) are significant, with up to 25% of women experiencing wound complications and 17% experiencing fecal incontinence at 6 months postpartum.15,16 Care of women with peripartum pelvic floor disorders and OASIs present an ideal opportunity for collaboration between urogynecologists and obstetricians. The Cleveland Clinic has a multidisciplinary Postpartum Care Clinic (PPCC) where we provide specialized, collaborative care for women with peripartum pelvic floor disorders and complex obstetric lacerations.
Our PPCC accepts referrals up to 1 year postpartum for women who experience OASI, urinary or fecal incontinence, perineal pain or dyspareunia, voiding dysfunction or urinary retention, and wound healing complications. When a woman is diagnosed with an OASI at the time of delivery, a “best practice alert” is released in the medical record recommending a referral to the PPCC to encourage referral of all women with OASI. We strive to see all referrals within 2 weeks of delivery.
At the time of the initial consultation, we collect validated questionnaires on bowel and bladder function, assess pain and healing, and discuss future delivery planning. The success of the PPCC is rooted in communication. When the clinic first opened, we provided education to our obstetrics colleagues on the purpose of the clinic, when and how to refer, and what to expect from our consultations. Open communication between referring obstetric clinicians and the urogynecologists that run the PPCC is key in providing collaborative care where patients know that their clinicians are working as a team. All recommendations are communicated to referring clinicians, and all women are ultimately referred back to their primary clinician for long-term care. Evidence demonstrates that this type of clinic leads to high obstetric clinician satisfaction and increased awareness of OASIs and their impact on maternal health.17
Combined team approach fosters innovation in patient care
A combined approach to the care of the patient who presents with gynecologic conditions is optimal. In this article, we presented examples of care that integrates gynecology, urogynecology, gynecologic oncology, colorectal surgery, plastic surgery, and obstetrics. There are, however, many more existing examples as well as opportunities to create teams that really make a difference in the way patients receive—and perceive—their care. This is a good starting point, and we should strive to use this model to continue to innovate our approach to patient care.
- Ramaseshan AS, Felton J, Roque D, et al. Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J. 2018;29:459-476.
- Nakayama N, Tsuji T, Aoyama M, et al. Quality of life and the prevalence of urinary incontinence after surgical treatment for gynecologic cancer: a questionnaire survey. BMC Womens Health. 2020;20:148-157.
- Cascales-Campos PA, Gonzalez-Gil A, Fernandez-Luna E, et al. Urinary and fecal incontinence in patients with advanced ovarian cancer treated with CRS + HIPEC. Surg Oncol. 2021;36:115-119.
- Davidson ER, Woodburn K, AlHilli M, et al. Perioperative adverse events in women undergoing concurrent urogynecologic and gynecologic oncology surgeries for suspected malignancy. Int Urogynecol J. 2019;30:1195-1201.
- Spence-Jones C, Kamm MA, Henry MM, et al. Bowel dysfunction: a pathogenic factor in uterovaginal prolapse and stress urinary incontinence. Br J Obstet Gynaecol. 1994;101:147-152.
- Thompson JR, Chen AH, Pettit PD, et al. Incidence of occult rectal prolapse in patients with clinical rectoceles and defecatory dysfunction. Am J Obstet Gynecol. 2002;187:1494-1500.
- Jallad K, Gurland B. Multidisciplinary approach to the treatment of concomitant rectal and vaginal prolapse. Clin Colon Rectal Surg. 2016;29:101-105.
- Kapoor DS, Sultan AH, Thakar R, et al. Management of complex pelvic floor disorders in a multidisciplinary pelvic floor clinic. Colorectal Dis. 2008;10:118-123.
- Weinberg D, Qeadan F, McKee R, et al. Safety of laparoscopic sacrocolpopexy with concurrent rectopexy: peri-operative morbidity in a nationwide cohort. Int Urogynecol J. 2019;30:385-392.
- Geltzeiler CB, Birnbaum EH, Silviera ML, et al. Combined rectopexy and sacrocolpopexy is safe for correction of pelvic organ prolapse. Int J Colorectal Dis. 2018;33:1453-1459.
- Wallace SL, Syan R, Enemchukwu EA, et al. Surgical approach, complications, and reoperation rates of combined rectal and pelvic organ prolapse surgery. Int Urogynecol J. 2020;31:2101-2108.
- Smith PE, Hade EM, Pandya LK, et al. Perioperative outcomes for combined ventral rectopexy with sacrocolpopexy compared to perineal rectopexy with vaginal apical suspension. Female Pelvic Med Reconstr Surg. 2020;26:376-381.
- Casas-Puig V, Bretschneider CE, Ferrando CA. Perioperative adverse events in women undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery. Female Pelvic Med Reconstr Surg. 2019;25:88-92.
- Cordeiro PG, Pusic AL, Disa JJ. A classification system and reconstructive algorithm for acquired vaginal defects. Plast Reconstr Surg. 2002;110:1058-1065.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetric anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Borello-France D, Burgio KL, Richter HE, et al; Pelvic Floor Disorders Network. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006;108:863-872.
- Propst K, Hickman LC. Peripartum pelvic floor disorder clinics inform obstetric provider practices. Int Urogynecol J. 2021;32:1793-1799.
In her book The Silo Effect: The Peril of Expertise and the Promise of Breaking Down Barriers, Gillian Tett wrote that “the word ‘silo’ does not just refer to a physical structure or organization (such as a department). It can also be a state of mind. Silos exist in structures. But they exist in our minds and social groups too. Silos breed tribalism. But they can also go hand in hand with tunnel vision.”
Tertiary care referral centers seem to be trending toward being more and more “un-siloed” and collaborative within their own departments and between departments in order to care for patients. The terms multidisciplinary and intradisciplinary have become popular in medicine, and teams are joining forces to create care paths for patients that are intended to improve the efficiency of and the quality of care that is rendered. There is no better example of the move to improve collaboration in medicine than the theme of the 2021 Society of Gynecologic Surgeons annual meeting, “Working Together: How Collaboration Enables Us to Better Help Our Patients.”
In this article, we provide examples of how collaborating with other specialties—within and outside of an ObGyn department—should become the standard of care. We discuss how to make this team approach easier and provide evidence that patients experience favorable outcomes. While data on combined care remain sparse, the existing literature on this topic helps us to guide and counsel patients about what to expect when a combined approach is taken.
Addressing pelvic floor disorders in women with gynecologic malignancy
In 2018, authors of a systematic review that looked at concurrent pelvic floor disorders in gynecologic oncologic survivors found that the prevalence of these disorders was high enough to warrant evaluation and management of these conditions to help improve quality of life for patients.1 Furthermore, it is possible that the prevalence of urinary incontinence is higher in patients who have undergone surgery for a gynecologic malignancy compared with controls, which has been reported in previous studies.2,3 At Cleveland Clinic, we recognize the need to evaluate our patients receiving oncologic care for urinary, fecal, and pelvic organ prolapse symptoms. Our oncologists routinely inquire about these symptoms once their patients have undergone surgery with them, and they make referrals for all their symptomatic patients. They have even learned about our own counseling, and they pre-emptively let patients know what our counseling may encompass.
For instance, many patients who received radiation therapy have stress urinary incontinence that is likely related to a hypomobile urethra, and they may benefit more from transurethral bulking than an anti-incontinence procedure in the operating room. Reassuring patients ahead of time that they do not need major interventions for their symptoms is helpful, as these patients are already experiencing tremendous burden from their oncologic conditions. We have made our referral patterns easy for these patients, and most patients are seen within days to weeks of the referral placed, depending on the urgency of the consult and the need to proceed with their oncologic treatment plan.
Gynecologic oncology patients who present with preoperative stress urinary incontinence and pelvic organ prolapse also are referred to a urogynecology specialist for concurrent care. Care paths have been created to help inform both the urogynecologists and the oncologists about options for patients depending on their respective conditions, as both their malignancy and their pelvic floor disorder(s) are considered in treatment planning. There is agreement in this planning that the oncologic surgery takes priority, and the urogynecologic approach is based on the oncologic plan.
Our urogynecologists routinely ask if future radiation is in the treatment plan, as this usually precludes us from placing a midurethral sling at the time of any surgery. Surgical approach (vaginal versus abdominal; open or minimally invasive) also is determined by the oncologic team. At the time of surgery, patient positioning is considered to optimize access for all of the surgeons. For instance, having the oncologist know that the patient needs to be far down on the bed as their steep Trendelenburg positioning during laparoscopy or robotic surgery may cause the patient to slide cephalad during the case may make a vaginal repair or sling placement at the end of the case challenging. All these small nuances are important, and a collaborative team develops the right plan for each patient in advance.
Data on the outcomes of combined surgery are sparse. In a retrospective matched cohort study, our group compared outcomes in women who underwent concurrent surgery with those who underwent urogynecologic surgery alone.4 We found that concurrent surgeries had an increased incidence of minor but not serious perioperative adverse events. Importantly, we determined that 1 in 10 planned urogynecologic procedures needed to be either modified or abandoned as a result of the oncologic plan. These data help guide our counseling, and both the oncologist and urogynecologist contributing to the combined case counsel patients according to these data.
Continue to: Concurrent colorectal and gynecologic surgery...
Concurrent colorectal and gynecologic surgery
Many women have pelvic floor disorders. As gynecologists, we often compartmentalize these conditions as gynecologic problems; frequently, however, colorectal conditions are at play as well and should be addressed concurrently. For instance, a high incidence of anorectal dysfunction occurs in women who present with pelvic organ prolapse.5 Furthermore, outlet defecation disorders are not always a result of a straightforward rectocele that can be fixed vaginally. Sometimes, a more thorough evaluation is warranted depending on the patient’s concurrent symptoms and history. Outlet symptoms may be attributed to large enteroceles, sigmoidoceles, perineal descent, rectal intussusception, and rectal prolapse.6
As a result, a combined approach to caring for patients with complex pelvic floor disorders is optimal. Several studies describe this type of combined and coordinated patient care.7,8 Ideally, patients are seen by both surgeons in the office so that the surgeons may make a combined plan for their care, especially if the decision is made to proceed with surgery. Urogynecology specialists and colorectal surgeons must decide together whether to approach combined prolapse procedures via a perineal and vaginal approach versus an abdominal approach. Several factors can determine this, including surgeon experience and preference, which is why it is important for surgeons working together to have either well-designed care paths or simply open communication and experience working together for the conditions they are treating.
In an ideal coordinated care approach, both surgeons review the patient records in advance. Any needed imaging or testing is done before the official patient consult; the patient is then seen by both clinicians in the same visit and counseled about the options. This is the most efficient and effective way to see patients, and we have had significant success using this approach.
Complications of combined surgery
The safety of combining procedures such as laparoscopic sacrocolpopexy and concurrent rectopexy has been studied, and intraoperative complications have been reported to be low.9,10 In a cohort study, Wallace and colleagues looked at postoperative outcomes and complications following combined surgery and reported that reoperation for the rectal prolapse component of the surgery was more common than the pelvic organ prolapse component, and that 1 in 5 of their patients experienced a surgical complication within 30 days of their surgery.11 This incidence is higher than that seen with isolated pelvic organ prolapse surgery. These data help us understand that a combined approach requires good patient counseling in the office about both the need for repeat surgery in certain circumstances and the increased risk of complications. Further, combined perineal and vaginal approaches have been compared with abdominal approaches and also have shown no age-adjusted differences in outcomes and complications.12
These data point to the need for surgeons to choose the approach to surgery that best fits their own experiences and to discuss this together before counseling the patient in the office, thus streamlining the effort so that the patient feels comfortable under the care of 2 surgeons.
Patients presenting with urogynecologic and gynecologic conditions also report symptomatic hemorrhoids, and colorectal referral is often made by the gynecologist. Sparse data are available regarding combined approaches to managing hemorrhoids and gynecologic conditions. Our group was the first to publish on outcomes and complications in patients undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery.13 In that retrospective cohort, we found that minor complications, such as postoperative urinary tract infection and transient voiding dysfunction, was more common in patients who underwent combined surgery. From this, we gathered that there is a need to counsel patients appropriately about the risk of combined surgery. That said, for some patients, coordinated care is desirable, and surgeons should make the effort to work together in combining their procedures.
Continue to: Integrating plastic and reconstructive surgery in gynecology...
Integrating plastic and reconstructive surgery in gynecology
Reconstructive gynecologic procedures often require a multidisciplinary approach to what can be very complex reconstructive surgery. The intended goal usually is to achieve a good cosmetic result in the genital area, as well as to restore sexual, defecatory, and/or genitourinary functionality. As a result, surgeons must work together to develop a feasible reconstructive plan for these patients.
Women experience vaginal stenosis or foreshortening for a number of reasons. Women with congenital anomalies often are cared for by specialists in pediatric and adolescent gynecology. Other women, such as those who have undergone vaginectomy and/or pelvic or vaginal radiation for cancer treatment, complications from vaginal mesh placement, and severe vaginal scarring from dermatologic conditions like lichen planus, are cared for by other gynecologic specialists, often general gynecologists or urogynecologists. In some of these cases, a gynecologic surgeon can perform vaginal adhesiolysis followed by vaginal estrogen treatment (when appropriate) and aggressive postoperative vaginal dilation with adjunctive pelvic floor physical therapy as well as sex therapy or counseling. A simple reconstructive approach may be necessary if lysis of adhesions alone is not sufficient. Sometimes, the vaginal apex must be opened vaginally or abdominally, or releasing incisions need to be made to improve the caliber of the vagina in addition to its length. Under these circumstances, the use of additional local skin grafts, local peritoneal flaps, or biologic grafts or xenografts can help achieve a satisfying result. While not all gynecologists are trained to perform these procedures, some are, and certainly gynecologic subspecialists have the skill sets to care for these patients.
Under other circumstances, when the vagina is truly foreshortened, more aggressive reconstructive surgery is necessary and consultation and collaboration with plastic surgery specialists often is helpful. At our center, these patients’ care is initially managed by gynecologists and, when simple approaches to their reconstructive needs are exhausted, collaboration is warranted. As with the other team approaches discussed in this article, the recommendation is for a consistent referral team that has established care paths for patients. Not all plastic surgeons are familiar with neovaginal reconstruction and understand the functional aspects that gynecologists are hoping to achieve for their patients. Therefore, it is important to form cohesive teams that have the same goals for the patient.
The literature on neovaginal reconstruction is sparse. There are no true agreed on approaches or techniques for vaginal reconstruction because there is no “one size fits all” for these repairs. Defects also vary depending on whether they are due to resections or radiation for oncologic treatment, reconstruction as part of the repair of a genitourinary or rectovaginal fistula, or stenosis from other etiologies.
In 2002, Cordeiro and colleagues published a classification system and reconstructive algorithm for acquired vaginal defects.14 Not all reconstructive surgeons subscribe to this algorithm, but it is the only rubric that currently exists. The authors differentiate between “partial” and “circumferential” defects and recommend different types of fasciocutaneous and myocutaneous flaps for reconstruction.
In our experience at our center, we believe that the choice of flap should also depend on whether or not perineal reconstruction is needed. This decision is made by both the gynecologic specialist and the plastic surgeon. Common flap choices include the Singapore flap, a fasciocutaneous flap based on perforators from the pudendal vessels; the gracilis flap, a myocutaneous flap based off the medial circumflex femoral vessels; and the rectus abdominis flap (transverse or vertical), which is also a myocutaneous flap that relies on the blood supply from the deep inferior epigastric vessels.
One of the most important parts of the coordinated effort of neovaginal surgery is postoperative care. Plastic surgeons play a key role in ensuring that the flap survives in the immediate postoperative period. The gynecology team should be responsible for postoperative vaginal dilation teaching and follow-up to ensure that the patient dilates properly and upsizes her dilator appropriately over the postoperative period. In our practice, our advanced practice clinicians often care for these patients and are responsible for continuity and dilation teaching. Patients have easy access to these clinicians, and this enhances the postoperative experience. Referral to a pelvic floor physical therapist knowledgeable about neovaginal surgery also helps to ensure that the dilation process goes successfully. It also helps to have office days on the same days as the plastic surgery team that is following the patient. This way, the patient may be seen by both teams on the same day. This allows for good patient communication with regard to aftercare, as well as a combined approach to teaching the trainees involved in the case. Coordination with pelvic floor physical therapists on those days also enhances the patient experience and is highly recommended.
Continue to: Combining gyn and urogyn procedures with plastic surgery...
Combining gyn and urogyn procedures with plastic surgery
While there are no data on combining gynecologic and urogynecologic procedures with plastic reconstructive surgeries, a team approach to combining surgeries is possible. At our center, we have performed tubal ligation, ovarian surgery, hysterectomy, and sling and prolapse surgery in patients who were undergoing cosmetic procedures, such as breast augmentation and abdominoplasty.
Gender affirmation surgery also can be performed through a combined approach between gynecologists and plastic surgeons. Our gynecologists perform hysterectomy for transmasculine men, and this procedure is sometimes safely and effectively performed in combination with masculinizing chest surgery (mastectomy) performed by our plastic surgeons. Vaginoplasty surgery (feminizing genital surgery) also is performed by urogynecology specialists at our center, and it is sometimes done concurrently at the time of breast augmentation and/or facial feminization surgery.
Case order. Some plastic surgeons vocalize concerns about combining clean procedures with clean contaminated cases, especially in situations in which implants are being placed in the body. During these cases, communication and organization between surgeons is important. For instance, there should be a discussion about case order. In general, the clean procedures should be performed first. In addition, separate operating tables and instruments should be used. Simultaneous operating also should be avoided. Fresh incisions should be dressed and covered before subsequent procedures are performed.
Incision placement. Last, planning around incision placement should be discussed before each case. Laparoscopic and abdominal incisions may interfere with plastic surgery procedures and alter the end cosmesis. These incisions often can be incorporated into the reconstructive procedure. The most important part of the coordinated surgical effort is ensuring that both surgical teams understand each other’s respective surgeries and the approach needed to complete them. When this is achieved, the cases are usually very successful.
Creating collaboration between obstetricians and gynecologic specialists
The impacts of pregnancy and vaginal delivery on the pelvic floor are well established. Urinary and fecal incontinence, pelvic organ prolapse, perineal pain, and dyspareunia are not uncommon in the postpartum period and may persist long term. The effects of obstetric anal sphincter injury (OASI) are significant, with up to 25% of women experiencing wound complications and 17% experiencing fecal incontinence at 6 months postpartum.15,16 Care of women with peripartum pelvic floor disorders and OASIs present an ideal opportunity for collaboration between urogynecologists and obstetricians. The Cleveland Clinic has a multidisciplinary Postpartum Care Clinic (PPCC) where we provide specialized, collaborative care for women with peripartum pelvic floor disorders and complex obstetric lacerations.
Our PPCC accepts referrals up to 1 year postpartum for women who experience OASI, urinary or fecal incontinence, perineal pain or dyspareunia, voiding dysfunction or urinary retention, and wound healing complications. When a woman is diagnosed with an OASI at the time of delivery, a “best practice alert” is released in the medical record recommending a referral to the PPCC to encourage referral of all women with OASI. We strive to see all referrals within 2 weeks of delivery.
At the time of the initial consultation, we collect validated questionnaires on bowel and bladder function, assess pain and healing, and discuss future delivery planning. The success of the PPCC is rooted in communication. When the clinic first opened, we provided education to our obstetrics colleagues on the purpose of the clinic, when and how to refer, and what to expect from our consultations. Open communication between referring obstetric clinicians and the urogynecologists that run the PPCC is key in providing collaborative care where patients know that their clinicians are working as a team. All recommendations are communicated to referring clinicians, and all women are ultimately referred back to their primary clinician for long-term care. Evidence demonstrates that this type of clinic leads to high obstetric clinician satisfaction and increased awareness of OASIs and their impact on maternal health.17
Combined team approach fosters innovation in patient care
A combined approach to the care of the patient who presents with gynecologic conditions is optimal. In this article, we presented examples of care that integrates gynecology, urogynecology, gynecologic oncology, colorectal surgery, plastic surgery, and obstetrics. There are, however, many more existing examples as well as opportunities to create teams that really make a difference in the way patients receive—and perceive—their care. This is a good starting point, and we should strive to use this model to continue to innovate our approach to patient care.
In her book The Silo Effect: The Peril of Expertise and the Promise of Breaking Down Barriers, Gillian Tett wrote that “the word ‘silo’ does not just refer to a physical structure or organization (such as a department). It can also be a state of mind. Silos exist in structures. But they exist in our minds and social groups too. Silos breed tribalism. But they can also go hand in hand with tunnel vision.”
Tertiary care referral centers seem to be trending toward being more and more “un-siloed” and collaborative within their own departments and between departments in order to care for patients. The terms multidisciplinary and intradisciplinary have become popular in medicine, and teams are joining forces to create care paths for patients that are intended to improve the efficiency of and the quality of care that is rendered. There is no better example of the move to improve collaboration in medicine than the theme of the 2021 Society of Gynecologic Surgeons annual meeting, “Working Together: How Collaboration Enables Us to Better Help Our Patients.”
In this article, we provide examples of how collaborating with other specialties—within and outside of an ObGyn department—should become the standard of care. We discuss how to make this team approach easier and provide evidence that patients experience favorable outcomes. While data on combined care remain sparse, the existing literature on this topic helps us to guide and counsel patients about what to expect when a combined approach is taken.
Addressing pelvic floor disorders in women with gynecologic malignancy
In 2018, authors of a systematic review that looked at concurrent pelvic floor disorders in gynecologic oncologic survivors found that the prevalence of these disorders was high enough to warrant evaluation and management of these conditions to help improve quality of life for patients.1 Furthermore, it is possible that the prevalence of urinary incontinence is higher in patients who have undergone surgery for a gynecologic malignancy compared with controls, which has been reported in previous studies.2,3 At Cleveland Clinic, we recognize the need to evaluate our patients receiving oncologic care for urinary, fecal, and pelvic organ prolapse symptoms. Our oncologists routinely inquire about these symptoms once their patients have undergone surgery with them, and they make referrals for all their symptomatic patients. They have even learned about our own counseling, and they pre-emptively let patients know what our counseling may encompass.
For instance, many patients who received radiation therapy have stress urinary incontinence that is likely related to a hypomobile urethra, and they may benefit more from transurethral bulking than an anti-incontinence procedure in the operating room. Reassuring patients ahead of time that they do not need major interventions for their symptoms is helpful, as these patients are already experiencing tremendous burden from their oncologic conditions. We have made our referral patterns easy for these patients, and most patients are seen within days to weeks of the referral placed, depending on the urgency of the consult and the need to proceed with their oncologic treatment plan.
Gynecologic oncology patients who present with preoperative stress urinary incontinence and pelvic organ prolapse also are referred to a urogynecology specialist for concurrent care. Care paths have been created to help inform both the urogynecologists and the oncologists about options for patients depending on their respective conditions, as both their malignancy and their pelvic floor disorder(s) are considered in treatment planning. There is agreement in this planning that the oncologic surgery takes priority, and the urogynecologic approach is based on the oncologic plan.
Our urogynecologists routinely ask if future radiation is in the treatment plan, as this usually precludes us from placing a midurethral sling at the time of any surgery. Surgical approach (vaginal versus abdominal; open or minimally invasive) also is determined by the oncologic team. At the time of surgery, patient positioning is considered to optimize access for all of the surgeons. For instance, having the oncologist know that the patient needs to be far down on the bed as their steep Trendelenburg positioning during laparoscopy or robotic surgery may cause the patient to slide cephalad during the case may make a vaginal repair or sling placement at the end of the case challenging. All these small nuances are important, and a collaborative team develops the right plan for each patient in advance.
Data on the outcomes of combined surgery are sparse. In a retrospective matched cohort study, our group compared outcomes in women who underwent concurrent surgery with those who underwent urogynecologic surgery alone.4 We found that concurrent surgeries had an increased incidence of minor but not serious perioperative adverse events. Importantly, we determined that 1 in 10 planned urogynecologic procedures needed to be either modified or abandoned as a result of the oncologic plan. These data help guide our counseling, and both the oncologist and urogynecologist contributing to the combined case counsel patients according to these data.
Continue to: Concurrent colorectal and gynecologic surgery...
Concurrent colorectal and gynecologic surgery
Many women have pelvic floor disorders. As gynecologists, we often compartmentalize these conditions as gynecologic problems; frequently, however, colorectal conditions are at play as well and should be addressed concurrently. For instance, a high incidence of anorectal dysfunction occurs in women who present with pelvic organ prolapse.5 Furthermore, outlet defecation disorders are not always a result of a straightforward rectocele that can be fixed vaginally. Sometimes, a more thorough evaluation is warranted depending on the patient’s concurrent symptoms and history. Outlet symptoms may be attributed to large enteroceles, sigmoidoceles, perineal descent, rectal intussusception, and rectal prolapse.6
As a result, a combined approach to caring for patients with complex pelvic floor disorders is optimal. Several studies describe this type of combined and coordinated patient care.7,8 Ideally, patients are seen by both surgeons in the office so that the surgeons may make a combined plan for their care, especially if the decision is made to proceed with surgery. Urogynecology specialists and colorectal surgeons must decide together whether to approach combined prolapse procedures via a perineal and vaginal approach versus an abdominal approach. Several factors can determine this, including surgeon experience and preference, which is why it is important for surgeons working together to have either well-designed care paths or simply open communication and experience working together for the conditions they are treating.
In an ideal coordinated care approach, both surgeons review the patient records in advance. Any needed imaging or testing is done before the official patient consult; the patient is then seen by both clinicians in the same visit and counseled about the options. This is the most efficient and effective way to see patients, and we have had significant success using this approach.
Complications of combined surgery
The safety of combining procedures such as laparoscopic sacrocolpopexy and concurrent rectopexy has been studied, and intraoperative complications have been reported to be low.9,10 In a cohort study, Wallace and colleagues looked at postoperative outcomes and complications following combined surgery and reported that reoperation for the rectal prolapse component of the surgery was more common than the pelvic organ prolapse component, and that 1 in 5 of their patients experienced a surgical complication within 30 days of their surgery.11 This incidence is higher than that seen with isolated pelvic organ prolapse surgery. These data help us understand that a combined approach requires good patient counseling in the office about both the need for repeat surgery in certain circumstances and the increased risk of complications. Further, combined perineal and vaginal approaches have been compared with abdominal approaches and also have shown no age-adjusted differences in outcomes and complications.12
These data point to the need for surgeons to choose the approach to surgery that best fits their own experiences and to discuss this together before counseling the patient in the office, thus streamlining the effort so that the patient feels comfortable under the care of 2 surgeons.
Patients presenting with urogynecologic and gynecologic conditions also report symptomatic hemorrhoids, and colorectal referral is often made by the gynecologist. Sparse data are available regarding combined approaches to managing hemorrhoids and gynecologic conditions. Our group was the first to publish on outcomes and complications in patients undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery.13 In that retrospective cohort, we found that minor complications, such as postoperative urinary tract infection and transient voiding dysfunction, was more common in patients who underwent combined surgery. From this, we gathered that there is a need to counsel patients appropriately about the risk of combined surgery. That said, for some patients, coordinated care is desirable, and surgeons should make the effort to work together in combining their procedures.
Continue to: Integrating plastic and reconstructive surgery in gynecology...
Integrating plastic and reconstructive surgery in gynecology
Reconstructive gynecologic procedures often require a multidisciplinary approach to what can be very complex reconstructive surgery. The intended goal usually is to achieve a good cosmetic result in the genital area, as well as to restore sexual, defecatory, and/or genitourinary functionality. As a result, surgeons must work together to develop a feasible reconstructive plan for these patients.
Women experience vaginal stenosis or foreshortening for a number of reasons. Women with congenital anomalies often are cared for by specialists in pediatric and adolescent gynecology. Other women, such as those who have undergone vaginectomy and/or pelvic or vaginal radiation for cancer treatment, complications from vaginal mesh placement, and severe vaginal scarring from dermatologic conditions like lichen planus, are cared for by other gynecologic specialists, often general gynecologists or urogynecologists. In some of these cases, a gynecologic surgeon can perform vaginal adhesiolysis followed by vaginal estrogen treatment (when appropriate) and aggressive postoperative vaginal dilation with adjunctive pelvic floor physical therapy as well as sex therapy or counseling. A simple reconstructive approach may be necessary if lysis of adhesions alone is not sufficient. Sometimes, the vaginal apex must be opened vaginally or abdominally, or releasing incisions need to be made to improve the caliber of the vagina in addition to its length. Under these circumstances, the use of additional local skin grafts, local peritoneal flaps, or biologic grafts or xenografts can help achieve a satisfying result. While not all gynecologists are trained to perform these procedures, some are, and certainly gynecologic subspecialists have the skill sets to care for these patients.
Under other circumstances, when the vagina is truly foreshortened, more aggressive reconstructive surgery is necessary and consultation and collaboration with plastic surgery specialists often is helpful. At our center, these patients’ care is initially managed by gynecologists and, when simple approaches to their reconstructive needs are exhausted, collaboration is warranted. As with the other team approaches discussed in this article, the recommendation is for a consistent referral team that has established care paths for patients. Not all plastic surgeons are familiar with neovaginal reconstruction and understand the functional aspects that gynecologists are hoping to achieve for their patients. Therefore, it is important to form cohesive teams that have the same goals for the patient.
The literature on neovaginal reconstruction is sparse. There are no true agreed on approaches or techniques for vaginal reconstruction because there is no “one size fits all” for these repairs. Defects also vary depending on whether they are due to resections or radiation for oncologic treatment, reconstruction as part of the repair of a genitourinary or rectovaginal fistula, or stenosis from other etiologies.
In 2002, Cordeiro and colleagues published a classification system and reconstructive algorithm for acquired vaginal defects.14 Not all reconstructive surgeons subscribe to this algorithm, but it is the only rubric that currently exists. The authors differentiate between “partial” and “circumferential” defects and recommend different types of fasciocutaneous and myocutaneous flaps for reconstruction.
In our experience at our center, we believe that the choice of flap should also depend on whether or not perineal reconstruction is needed. This decision is made by both the gynecologic specialist and the plastic surgeon. Common flap choices include the Singapore flap, a fasciocutaneous flap based on perforators from the pudendal vessels; the gracilis flap, a myocutaneous flap based off the medial circumflex femoral vessels; and the rectus abdominis flap (transverse or vertical), which is also a myocutaneous flap that relies on the blood supply from the deep inferior epigastric vessels.
One of the most important parts of the coordinated effort of neovaginal surgery is postoperative care. Plastic surgeons play a key role in ensuring that the flap survives in the immediate postoperative period. The gynecology team should be responsible for postoperative vaginal dilation teaching and follow-up to ensure that the patient dilates properly and upsizes her dilator appropriately over the postoperative period. In our practice, our advanced practice clinicians often care for these patients and are responsible for continuity and dilation teaching. Patients have easy access to these clinicians, and this enhances the postoperative experience. Referral to a pelvic floor physical therapist knowledgeable about neovaginal surgery also helps to ensure that the dilation process goes successfully. It also helps to have office days on the same days as the plastic surgery team that is following the patient. This way, the patient may be seen by both teams on the same day. This allows for good patient communication with regard to aftercare, as well as a combined approach to teaching the trainees involved in the case. Coordination with pelvic floor physical therapists on those days also enhances the patient experience and is highly recommended.
Continue to: Combining gyn and urogyn procedures with plastic surgery...
Combining gyn and urogyn procedures with plastic surgery
While there are no data on combining gynecologic and urogynecologic procedures with plastic reconstructive surgeries, a team approach to combining surgeries is possible. At our center, we have performed tubal ligation, ovarian surgery, hysterectomy, and sling and prolapse surgery in patients who were undergoing cosmetic procedures, such as breast augmentation and abdominoplasty.
Gender affirmation surgery also can be performed through a combined approach between gynecologists and plastic surgeons. Our gynecologists perform hysterectomy for transmasculine men, and this procedure is sometimes safely and effectively performed in combination with masculinizing chest surgery (mastectomy) performed by our plastic surgeons. Vaginoplasty surgery (feminizing genital surgery) also is performed by urogynecology specialists at our center, and it is sometimes done concurrently at the time of breast augmentation and/or facial feminization surgery.
Case order. Some plastic surgeons vocalize concerns about combining clean procedures with clean contaminated cases, especially in situations in which implants are being placed in the body. During these cases, communication and organization between surgeons is important. For instance, there should be a discussion about case order. In general, the clean procedures should be performed first. In addition, separate operating tables and instruments should be used. Simultaneous operating also should be avoided. Fresh incisions should be dressed and covered before subsequent procedures are performed.
Incision placement. Last, planning around incision placement should be discussed before each case. Laparoscopic and abdominal incisions may interfere with plastic surgery procedures and alter the end cosmesis. These incisions often can be incorporated into the reconstructive procedure. The most important part of the coordinated surgical effort is ensuring that both surgical teams understand each other’s respective surgeries and the approach needed to complete them. When this is achieved, the cases are usually very successful.
Creating collaboration between obstetricians and gynecologic specialists
The impacts of pregnancy and vaginal delivery on the pelvic floor are well established. Urinary and fecal incontinence, pelvic organ prolapse, perineal pain, and dyspareunia are not uncommon in the postpartum period and may persist long term. The effects of obstetric anal sphincter injury (OASI) are significant, with up to 25% of women experiencing wound complications and 17% experiencing fecal incontinence at 6 months postpartum.15,16 Care of women with peripartum pelvic floor disorders and OASIs present an ideal opportunity for collaboration between urogynecologists and obstetricians. The Cleveland Clinic has a multidisciplinary Postpartum Care Clinic (PPCC) where we provide specialized, collaborative care for women with peripartum pelvic floor disorders and complex obstetric lacerations.
Our PPCC accepts referrals up to 1 year postpartum for women who experience OASI, urinary or fecal incontinence, perineal pain or dyspareunia, voiding dysfunction or urinary retention, and wound healing complications. When a woman is diagnosed with an OASI at the time of delivery, a “best practice alert” is released in the medical record recommending a referral to the PPCC to encourage referral of all women with OASI. We strive to see all referrals within 2 weeks of delivery.
At the time of the initial consultation, we collect validated questionnaires on bowel and bladder function, assess pain and healing, and discuss future delivery planning. The success of the PPCC is rooted in communication. When the clinic first opened, we provided education to our obstetrics colleagues on the purpose of the clinic, when and how to refer, and what to expect from our consultations. Open communication between referring obstetric clinicians and the urogynecologists that run the PPCC is key in providing collaborative care where patients know that their clinicians are working as a team. All recommendations are communicated to referring clinicians, and all women are ultimately referred back to their primary clinician for long-term care. Evidence demonstrates that this type of clinic leads to high obstetric clinician satisfaction and increased awareness of OASIs and their impact on maternal health.17
Combined team approach fosters innovation in patient care
A combined approach to the care of the patient who presents with gynecologic conditions is optimal. In this article, we presented examples of care that integrates gynecology, urogynecology, gynecologic oncology, colorectal surgery, plastic surgery, and obstetrics. There are, however, many more existing examples as well as opportunities to create teams that really make a difference in the way patients receive—and perceive—their care. This is a good starting point, and we should strive to use this model to continue to innovate our approach to patient care.
- Ramaseshan AS, Felton J, Roque D, et al. Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J. 2018;29:459-476.
- Nakayama N, Tsuji T, Aoyama M, et al. Quality of life and the prevalence of urinary incontinence after surgical treatment for gynecologic cancer: a questionnaire survey. BMC Womens Health. 2020;20:148-157.
- Cascales-Campos PA, Gonzalez-Gil A, Fernandez-Luna E, et al. Urinary and fecal incontinence in patients with advanced ovarian cancer treated with CRS + HIPEC. Surg Oncol. 2021;36:115-119.
- Davidson ER, Woodburn K, AlHilli M, et al. Perioperative adverse events in women undergoing concurrent urogynecologic and gynecologic oncology surgeries for suspected malignancy. Int Urogynecol J. 2019;30:1195-1201.
- Spence-Jones C, Kamm MA, Henry MM, et al. Bowel dysfunction: a pathogenic factor in uterovaginal prolapse and stress urinary incontinence. Br J Obstet Gynaecol. 1994;101:147-152.
- Thompson JR, Chen AH, Pettit PD, et al. Incidence of occult rectal prolapse in patients with clinical rectoceles and defecatory dysfunction. Am J Obstet Gynecol. 2002;187:1494-1500.
- Jallad K, Gurland B. Multidisciplinary approach to the treatment of concomitant rectal and vaginal prolapse. Clin Colon Rectal Surg. 2016;29:101-105.
- Kapoor DS, Sultan AH, Thakar R, et al. Management of complex pelvic floor disorders in a multidisciplinary pelvic floor clinic. Colorectal Dis. 2008;10:118-123.
- Weinberg D, Qeadan F, McKee R, et al. Safety of laparoscopic sacrocolpopexy with concurrent rectopexy: peri-operative morbidity in a nationwide cohort. Int Urogynecol J. 2019;30:385-392.
- Geltzeiler CB, Birnbaum EH, Silviera ML, et al. Combined rectopexy and sacrocolpopexy is safe for correction of pelvic organ prolapse. Int J Colorectal Dis. 2018;33:1453-1459.
- Wallace SL, Syan R, Enemchukwu EA, et al. Surgical approach, complications, and reoperation rates of combined rectal and pelvic organ prolapse surgery. Int Urogynecol J. 2020;31:2101-2108.
- Smith PE, Hade EM, Pandya LK, et al. Perioperative outcomes for combined ventral rectopexy with sacrocolpopexy compared to perineal rectopexy with vaginal apical suspension. Female Pelvic Med Reconstr Surg. 2020;26:376-381.
- Casas-Puig V, Bretschneider CE, Ferrando CA. Perioperative adverse events in women undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery. Female Pelvic Med Reconstr Surg. 2019;25:88-92.
- Cordeiro PG, Pusic AL, Disa JJ. A classification system and reconstructive algorithm for acquired vaginal defects. Plast Reconstr Surg. 2002;110:1058-1065.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetric anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Borello-France D, Burgio KL, Richter HE, et al; Pelvic Floor Disorders Network. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006;108:863-872.
- Propst K, Hickman LC. Peripartum pelvic floor disorder clinics inform obstetric provider practices. Int Urogynecol J. 2021;32:1793-1799.
- Ramaseshan AS, Felton J, Roque D, et al. Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J. 2018;29:459-476.
- Nakayama N, Tsuji T, Aoyama M, et al. Quality of life and the prevalence of urinary incontinence after surgical treatment for gynecologic cancer: a questionnaire survey. BMC Womens Health. 2020;20:148-157.
- Cascales-Campos PA, Gonzalez-Gil A, Fernandez-Luna E, et al. Urinary and fecal incontinence in patients with advanced ovarian cancer treated with CRS + HIPEC. Surg Oncol. 2021;36:115-119.
- Davidson ER, Woodburn K, AlHilli M, et al. Perioperative adverse events in women undergoing concurrent urogynecologic and gynecologic oncology surgeries for suspected malignancy. Int Urogynecol J. 2019;30:1195-1201.
- Spence-Jones C, Kamm MA, Henry MM, et al. Bowel dysfunction: a pathogenic factor in uterovaginal prolapse and stress urinary incontinence. Br J Obstet Gynaecol. 1994;101:147-152.
- Thompson JR, Chen AH, Pettit PD, et al. Incidence of occult rectal prolapse in patients with clinical rectoceles and defecatory dysfunction. Am J Obstet Gynecol. 2002;187:1494-1500.
- Jallad K, Gurland B. Multidisciplinary approach to the treatment of concomitant rectal and vaginal prolapse. Clin Colon Rectal Surg. 2016;29:101-105.
- Kapoor DS, Sultan AH, Thakar R, et al. Management of complex pelvic floor disorders in a multidisciplinary pelvic floor clinic. Colorectal Dis. 2008;10:118-123.
- Weinberg D, Qeadan F, McKee R, et al. Safety of laparoscopic sacrocolpopexy with concurrent rectopexy: peri-operative morbidity in a nationwide cohort. Int Urogynecol J. 2019;30:385-392.
- Geltzeiler CB, Birnbaum EH, Silviera ML, et al. Combined rectopexy and sacrocolpopexy is safe for correction of pelvic organ prolapse. Int J Colorectal Dis. 2018;33:1453-1459.
- Wallace SL, Syan R, Enemchukwu EA, et al. Surgical approach, complications, and reoperation rates of combined rectal and pelvic organ prolapse surgery. Int Urogynecol J. 2020;31:2101-2108.
- Smith PE, Hade EM, Pandya LK, et al. Perioperative outcomes for combined ventral rectopexy with sacrocolpopexy compared to perineal rectopexy with vaginal apical suspension. Female Pelvic Med Reconstr Surg. 2020;26:376-381.
- Casas-Puig V, Bretschneider CE, Ferrando CA. Perioperative adverse events in women undergoing concurrent hemorrhoidectomy at the time of urogynecologic surgery. Female Pelvic Med Reconstr Surg. 2019;25:88-92.
- Cordeiro PG, Pusic AL, Disa JJ. A classification system and reconstructive algorithm for acquired vaginal defects. Plast Reconstr Surg. 2002;110:1058-1065.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetric anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Borello-France D, Burgio KL, Richter HE, et al; Pelvic Floor Disorders Network. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006;108:863-872.
- Propst K, Hickman LC. Peripartum pelvic floor disorder clinics inform obstetric provider practices. Int Urogynecol J. 2021;32:1793-1799.
Closing the racial gap in minimally invasive gyn hysterectomy and myomectomy
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
HIFEM procedure helped to improve UI and female sexual function
Using high-intensity focused electromagnetic (HIFEM) technology to strengthen pelvic floor muscles for the improvement of urinary incontinence (UI) and female sexual function was safe and effective at 9 months follow-up, results from a multicenter study showed.
“The pelvic floor consists of three pairs of muscles: the pubococcygeus, the iliococcygeus, and the puborectalis,” lead study author Joseph Berenholz, MD, and a diplomate of the American Board of Obstetics & Gynecology, said during the annual conference of the American Society for Laser Medicine and Surgery. “They control continence through support of pelvic organs. The urethra, the vagina, and the rectum pass through that diaphragm. It also contributes to sexual sensation and arousal. A deconditioning of the pelvic floor is usually the result of child-bearing years or aging, which usually results in urinary incontinence and impairment of sexual function. The noninvasive strengthening of the pelvic floor muscles helps to regain muscle tone and strength.”
In a prospective, open-label, single-arm study conducted at four sites, Dr. Berenholz, medical director of the Michigan Center for Women’s Health in Farmington Hills, and colleagues investigated the long-term effectiveness of HIFEM-induced pelvic floor muscle (PFM) strengthening for improvement of UI and sexual function. HIFEM selectively targets neuromuscular tissue and induces supramaximal PFM contractions that cannot be achieved voluntarily, he said, causing muscle strengthening due to muscle fiber hypertrophy, which helps patients to better isolate and command their muscles.
The study population consisted of 33 females with a mean age of 49 years who had UI and UI-related problems in sexual life. They received six 28-minute HIFEM treatments of the pelvic floor with the BTL Emsella, which is FDA cleared for both stress and urge incontinence. The frequency of visits was two treatments per week and the intensity of HIFEM was adjusted between 0% and 100% based on the patient’s tolerance threshold. Evaluations were conducted at baseline, after the last treatment, at 1, 3, 6, and 9 months. The primary outcomes were change in urine leakage based on the International Consultation on Incontinence Questionnaire–Short Form (ICIQ-UI-SF) and change in sexual function based on the Female Sexual Function Index (FSFI) and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Secondary endpoints were adverse events and the comfort of therapy based on a 7-point Likert scale.
Dr. Berenholz reported that from baseline the severity of UI based on the ICIQ-SF significantly decreased 60% by a mean of 8.1 points between baseline and 9 months (P < .001). At 1 month, the FSFI score improved 32% by a mean of 7.1 points (P < .001) and was sustained throughout the study. The most prominent changes were seen in the subdomains of desire, arousal, lubrication, and orgasm response.
The PISQ-12 score incrementally increased 25% to a mean improvement of 8.2 points at 9 months (P < .001). Subjects improved most in the emotive subdomain, reporting more frequent orgasms, increased desire, and sexual excitement. The minimal important difference was 6 points.
“This is a true paradigm shift in the treatment of incontinence and sexual dysfunction,” Dr. Berenholz said. “The therapy was safe, comfortable, no adverse events emerged, and 31 subjects (94%) described the therapy as comfortable. Interim data suggest that treatment effect was maintained for 9 months, and there were no significant declines in scores in the long term. The upcoming 12-month follow-up data will let us know if more maintenance therapy is needed.”
During a question-and-answer session, one of the abstract section chairs, Albert Wolkerstorfer, MD, PhD, wondered about the potential for combination treatments in this patient population. “I can imagine that something that is working on the muscle tone has a totally different mechanism than something that is working on the mucosa and the underlying tissue without really affecting the muscle,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam. “Would a combination be the way to go?”
Dr. Berenholz said that he sometimes combines HIFEM with the ULTRA Femme 360, a radiofrequency thermal energy device. “We thought this addresses two issues,” he said. “One is fascial muscle, which is the underlying structural issue for incontinence. The other is thermal energy to aid in incontinence prevention by inducing production of elastin and collagen in the midurethra, but also to promote lubrication and heightened sensitivity in the patient who’s either menopausal or has undergone chemotherapy for breast cancer.”
Dr. Berenholz reported having no financial disclosures. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
Using high-intensity focused electromagnetic (HIFEM) technology to strengthen pelvic floor muscles for the improvement of urinary incontinence (UI) and female sexual function was safe and effective at 9 months follow-up, results from a multicenter study showed.
“The pelvic floor consists of three pairs of muscles: the pubococcygeus, the iliococcygeus, and the puborectalis,” lead study author Joseph Berenholz, MD, and a diplomate of the American Board of Obstetics & Gynecology, said during the annual conference of the American Society for Laser Medicine and Surgery. “They control continence through support of pelvic organs. The urethra, the vagina, and the rectum pass through that diaphragm. It also contributes to sexual sensation and arousal. A deconditioning of the pelvic floor is usually the result of child-bearing years or aging, which usually results in urinary incontinence and impairment of sexual function. The noninvasive strengthening of the pelvic floor muscles helps to regain muscle tone and strength.”
In a prospective, open-label, single-arm study conducted at four sites, Dr. Berenholz, medical director of the Michigan Center for Women’s Health in Farmington Hills, and colleagues investigated the long-term effectiveness of HIFEM-induced pelvic floor muscle (PFM) strengthening for improvement of UI and sexual function. HIFEM selectively targets neuromuscular tissue and induces supramaximal PFM contractions that cannot be achieved voluntarily, he said, causing muscle strengthening due to muscle fiber hypertrophy, which helps patients to better isolate and command their muscles.
The study population consisted of 33 females with a mean age of 49 years who had UI and UI-related problems in sexual life. They received six 28-minute HIFEM treatments of the pelvic floor with the BTL Emsella, which is FDA cleared for both stress and urge incontinence. The frequency of visits was two treatments per week and the intensity of HIFEM was adjusted between 0% and 100% based on the patient’s tolerance threshold. Evaluations were conducted at baseline, after the last treatment, at 1, 3, 6, and 9 months. The primary outcomes were change in urine leakage based on the International Consultation on Incontinence Questionnaire–Short Form (ICIQ-UI-SF) and change in sexual function based on the Female Sexual Function Index (FSFI) and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Secondary endpoints were adverse events and the comfort of therapy based on a 7-point Likert scale.
Dr. Berenholz reported that from baseline the severity of UI based on the ICIQ-SF significantly decreased 60% by a mean of 8.1 points between baseline and 9 months (P < .001). At 1 month, the FSFI score improved 32% by a mean of 7.1 points (P < .001) and was sustained throughout the study. The most prominent changes were seen in the subdomains of desire, arousal, lubrication, and orgasm response.
The PISQ-12 score incrementally increased 25% to a mean improvement of 8.2 points at 9 months (P < .001). Subjects improved most in the emotive subdomain, reporting more frequent orgasms, increased desire, and sexual excitement. The minimal important difference was 6 points.
“This is a true paradigm shift in the treatment of incontinence and sexual dysfunction,” Dr. Berenholz said. “The therapy was safe, comfortable, no adverse events emerged, and 31 subjects (94%) described the therapy as comfortable. Interim data suggest that treatment effect was maintained for 9 months, and there were no significant declines in scores in the long term. The upcoming 12-month follow-up data will let us know if more maintenance therapy is needed.”
During a question-and-answer session, one of the abstract section chairs, Albert Wolkerstorfer, MD, PhD, wondered about the potential for combination treatments in this patient population. “I can imagine that something that is working on the muscle tone has a totally different mechanism than something that is working on the mucosa and the underlying tissue without really affecting the muscle,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam. “Would a combination be the way to go?”
Dr. Berenholz said that he sometimes combines HIFEM with the ULTRA Femme 360, a radiofrequency thermal energy device. “We thought this addresses two issues,” he said. “One is fascial muscle, which is the underlying structural issue for incontinence. The other is thermal energy to aid in incontinence prevention by inducing production of elastin and collagen in the midurethra, but also to promote lubrication and heightened sensitivity in the patient who’s either menopausal or has undergone chemotherapy for breast cancer.”
Dr. Berenholz reported having no financial disclosures. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
Using high-intensity focused electromagnetic (HIFEM) technology to strengthen pelvic floor muscles for the improvement of urinary incontinence (UI) and female sexual function was safe and effective at 9 months follow-up, results from a multicenter study showed.
“The pelvic floor consists of three pairs of muscles: the pubococcygeus, the iliococcygeus, and the puborectalis,” lead study author Joseph Berenholz, MD, and a diplomate of the American Board of Obstetics & Gynecology, said during the annual conference of the American Society for Laser Medicine and Surgery. “They control continence through support of pelvic organs. The urethra, the vagina, and the rectum pass through that diaphragm. It also contributes to sexual sensation and arousal. A deconditioning of the pelvic floor is usually the result of child-bearing years or aging, which usually results in urinary incontinence and impairment of sexual function. The noninvasive strengthening of the pelvic floor muscles helps to regain muscle tone and strength.”
In a prospective, open-label, single-arm study conducted at four sites, Dr. Berenholz, medical director of the Michigan Center for Women’s Health in Farmington Hills, and colleagues investigated the long-term effectiveness of HIFEM-induced pelvic floor muscle (PFM) strengthening for improvement of UI and sexual function. HIFEM selectively targets neuromuscular tissue and induces supramaximal PFM contractions that cannot be achieved voluntarily, he said, causing muscle strengthening due to muscle fiber hypertrophy, which helps patients to better isolate and command their muscles.
The study population consisted of 33 females with a mean age of 49 years who had UI and UI-related problems in sexual life. They received six 28-minute HIFEM treatments of the pelvic floor with the BTL Emsella, which is FDA cleared for both stress and urge incontinence. The frequency of visits was two treatments per week and the intensity of HIFEM was adjusted between 0% and 100% based on the patient’s tolerance threshold. Evaluations were conducted at baseline, after the last treatment, at 1, 3, 6, and 9 months. The primary outcomes were change in urine leakage based on the International Consultation on Incontinence Questionnaire–Short Form (ICIQ-UI-SF) and change in sexual function based on the Female Sexual Function Index (FSFI) and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Secondary endpoints were adverse events and the comfort of therapy based on a 7-point Likert scale.
Dr. Berenholz reported that from baseline the severity of UI based on the ICIQ-SF significantly decreased 60% by a mean of 8.1 points between baseline and 9 months (P < .001). At 1 month, the FSFI score improved 32% by a mean of 7.1 points (P < .001) and was sustained throughout the study. The most prominent changes were seen in the subdomains of desire, arousal, lubrication, and orgasm response.
The PISQ-12 score incrementally increased 25% to a mean improvement of 8.2 points at 9 months (P < .001). Subjects improved most in the emotive subdomain, reporting more frequent orgasms, increased desire, and sexual excitement. The minimal important difference was 6 points.
“This is a true paradigm shift in the treatment of incontinence and sexual dysfunction,” Dr. Berenholz said. “The therapy was safe, comfortable, no adverse events emerged, and 31 subjects (94%) described the therapy as comfortable. Interim data suggest that treatment effect was maintained for 9 months, and there were no significant declines in scores in the long term. The upcoming 12-month follow-up data will let us know if more maintenance therapy is needed.”
During a question-and-answer session, one of the abstract section chairs, Albert Wolkerstorfer, MD, PhD, wondered about the potential for combination treatments in this patient population. “I can imagine that something that is working on the muscle tone has a totally different mechanism than something that is working on the mucosa and the underlying tissue without really affecting the muscle,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam. “Would a combination be the way to go?”
Dr. Berenholz said that he sometimes combines HIFEM with the ULTRA Femme 360, a radiofrequency thermal energy device. “We thought this addresses two issues,” he said. “One is fascial muscle, which is the underlying structural issue for incontinence. The other is thermal energy to aid in incontinence prevention by inducing production of elastin and collagen in the midurethra, but also to promote lubrication and heightened sensitivity in the patient who’s either menopausal or has undergone chemotherapy for breast cancer.”
Dr. Berenholz reported having no financial disclosures. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
FROM ASLMS 2021
Obstetric anal sphincter injury: Prevention and repair
The rate of obstetric anal sphincter injury (OASIS) is approximately 4.4% of vaginal deliveries, with 3.3% 3rd-degree tears and 1.1% 4th-degree tears.1 In the United States in 2019 there were 3,745,540 births—a 31.7% rate of cesarean delivery (CD) and a 68.3% rate of vaginal delivery—resulting in approximately 112,600 births with OASIS.2 A meta-analysis reported that, among 716,031 vaginal births, the risk factors for OASIS included: forceps delivery (relative risk [RR], 3.15), midline episiotomy (RR, 2.88), occiput posterior fetal position (RR, 2.73), vacuum delivery (RR, 2.60), Asian race (RR, 1.87), primiparity (RR, 1.59), mediolateral episiotomy (RR, 1.55), augmentation of labor (RR, 1.46), and epidural anesthesia (RR, 1.21).3 OASIS is associated with an increased risk for developing postpartum perineal pain, anal incontinence, dyspareunia, and wound breakdown.4 Complications following OASIS repair can trigger many follow-up appointments to assess wound healing and provide physical therapy.
This editorial review focuses on evolving recommendations for preventing and repairing OASIS.
The optimal cutting angle for a mediolateral episiotomy is 60 degrees from the midline
For spontaneous vaginal delivery, a policy of restricted episiotomy reduces the risk of OASIS by approximately 30%.5 With an operative vaginal delivery, especially forceps delivery of a large fetus in the occiput posterior position, a mediolateral episiotomy may help to reduce the risk of OASIS, although there are minimal data from clinical trials to support this practice. In one clinical trial, 407 women were randomly assigned to either a mediolateral or midline episiotomy.6 Approximately 25% of the births in both groups were operative deliveries. The mediolateral episiotomy began in the posterior midline of the vaginal introitus and was carried to the right side of the anal sphincter for 3 cm to 4 cm. The midline episiotomy began in the posterior midline of the vagina and was carried 2 cm to 3 cm into the midline perineal tissue. In the women having a midline or mediolateral episiotomy, a 4th-degree tear occurred in 5.5% and 0.4% of births, respectively. For the midline or mediolateral episiotomy, a third-degree tear occurred in 18.4% and 8.6%, respectively. In a prospective cohort study of 1,302 women with an episiotomy and vaginal birth, the rate of OASIS associated with midline or mediolateral episiotomy was 14.8% and 7%, respectively (P<.05).7 In this study, the operative vaginal delivery rate was 11.6% and 15.2% for the women in the midline and mediolateral groups, respectively.
The angle of the mediolateral episiotomy may influence the rate of OASIS and persistent postpartum perineal pain. In one study, 330 nulliparous women who were assessed to need a mediolateral episiotomy at delivery were randomized to an incision with a 40- or 60-degree angle from the midline.8 Prior to incision, a line was drawn on the skin to mark the course of the incision and then infiltrated with 10 mL of lignocaine. The fetal head was delivered with a Ritgen maneuver. The length of the episiotomy averaged 4 cm in both groups. After delivery, the angle of the episiotomy incision was reassessed. The episiotomy incision cut 60 degrees from the midline was measured on average to be 44 degrees from the midline after delivery of the newborn. Similarly, the incision cut at a 40-degree angle was measured to be 24 degrees from the midline after delivery. The rates of OASIS in the women who had a 40- and 60-degree angle incision were 5.5% and 2.4%, respectively (P = .16).
Continue to: Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair...
Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair
Many experts recommend one dose of a prophylactic antibiotic prior to, or during, OASIS repair in order to reduce the risk of wound complications. In a trial 147 women with OASIS were randomly assigned to receive one dose of a second-generation cephalosporin (cefotetan or cefoxitin) with extended anaerobic coverage or a placebo just before repair of the laceration.9 At 2 weeks postpartum, perineal wound complications were significantly lower in women receiving one dose of prophylactic antibiotic with extended anaerobe coverage compared with placebo—8.2% and 24.1%, respectively (P = .037). Additionally, at 2 weeks postpartum, purulent wound discharge was significantly lower in women receiving antibiotic versus placebo, 4% and 17%, respectively (P = .036). Experts writing for the Society of Obstetricians and Gynaecologists of Canada also recommend one dose of cefotetan or cefoxitin.10 Extended anaerobic coverage also can be achieved by administering a single dose of BOTH cefazolin 2 g by intravenous (IV) infusion PLUS metronidazole 500 mg by IV infusion or oral medication.11 For women with severe penicillin allergy, a recommended regimen is gentamicin 5 mg/kg plus clindamycin 900 mg by IV infusion.11 There is evidence that for colorectal or hysterectomy surgery, expanding prophylactic antibiotic coverage of anaerobes with cefazolin PLUS metronidazole significantly reduces postoperative surgical site infection.12,13 Following an OASIS repair, wound breakdown is a catastrophic problem that may take many months to resolve. Administration of a prophylactic antibiotic with extended coverage of anaerobes may help to prevent wound breakdown.
Prioritize identifying and separately repairing the internal anal sphincter
The internal anal sphincter is a smooth muscle that runs along the outside of the rectal wall and thickens into a sphincter toward the anal canal. The internal anal sphincter is thin and grey-white in appearance, like a veil. By contrast, the external anal sphincter is a thick band of red striated muscle tissue. In one study of 3,333 primiparous women with OASIS, an internal anal sphincter injury was detected in 33% of cases.14 In this large cohort, the rate of internal anal sphincter injury with a 3A tear, a 3B tear, a complete tear of the external sphincter and a 4th-degree perineal tear was 22%, 23%, 42%, and 71%, respectively. The internal anal sphincter is important for maintaining rectal continence and is estimated to contribute 50% to 85% of resting anal tone.15 If injury to the internal anal sphincter is detected at a birth with an OASIS, it is important to separately repair the internal anal sphincter to reduce the risk of postpartum rectal incontinence.16
Polyglactin 910 vs Polydioxanone (PDS) Suture—Is PDS the winner?
Polyglactin 910 (Vicryl) is a braided suture that is absorbed within 56 to 70 days. Polydioxanone suture is a long-lasting monofilament suture that is absorbed within 200 days. Many colorectal surgeons and urogynecologists prefer PDS suture for the repair of both the internal and external anal sphincters.16 Authors of one randomized trial of OASIS repair with Vicryl or PDS suture did not report significant differences in most clinical outcomes.17 However, in this study, anal endosonographic imaging of the internal and external anal sphincter demonstrated more internal sphincter defects but not external sphincter defects when the repair was performed with Vicryl rather than PDS. The investigators concluded that comprehensive training of the surgeon, not choice of suture, is probably the most important factor in achieving a good OASIS repair. However, because many subspecialists favor PDS suture for sphincter repair, specialists in obstetrics and gynecology should consider this option.
Continue to: Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
The breakdown of an OASIS repair is an obstetric catastrophe with complications that can last many months and sometimes stretch into years. The best approach to a perineal laceration wound breakdown remains controversial. It is optimal if all patients with a wound breakdown can be offered an early secondary repair or healing by secondary intention, permitting the patient to select the best approach for their specific situation.
As noted by the pioneers of early repair of episiotomy dehiscence, Drs. Hankins, Haugh, Gilstrap, Ramin, and others,18-20 conventional doctrine is that an episiotomy repair dehiscence should be managed expectantly, allowing healing by secondary intention and delaying repair of the sphincters for a minimum of 3 to 4 months.21 However, many small case-series report that early secondary repair of a perineal laceration wound breakdown is possible following multiple days of wound preparation prior to the repair, good surgical technique and diligent postoperative follow-up care. One large case series reported on 72 women with complete perineal wound dehiscence who had early secondary repair.22 The median time to complete wound healing following early repair was 28 days. About 36% of the patients had one or more complications, including skin dehiscence, granuloma formation, perineal pain, and sinus formation. A pilot randomized trial reported that, compared with expectant management of a wound breakdown, early repair resulted in a shorter time to wound healing.23
Early repair of perineal wound dehiscence often involves a course of care that extends over multiple weeks. As an example, following a vaginal birth with OASIS and immediate repair, the patient is often discharged from the hospital to home on postpartum day 3. The wound breakdown often is detected between postpartum days 6 to 10. If early secondary repair is selected as the best treatment, 1 to 6 days of daily debridement of the wound is needed to prepare the wound for early secondary repair. The daily debridement required to prepare the wound for early repair is often performed in the hospital, potentially disrupting early mother-newborn bonding. Following the repair, the patient is observed in the hospital for 1 to 3 days and then discharged home with daily wound care and multiple follow-up visits to monitor wound healing. Pelvic floor physical therapy may be initiated when the wound is healed. The prolonged process required for early secondary repair may be best undertaken by a subspecialty practice.24
The surgical repair and postpartum care of OASIS continues to evolve. In your practice you should consider:
- performing a mediolateral episiotomy at a 60-degree angle to reduce the risk of OASIS in situations where there is a high risk of anal sphincter injury, such as in forceps delivery
- using one dose of a prophylactic antibiotic with extended anaerobic coverage, such as cefotetan or cefoxitin
- focus on identifying and separately repairing an internal anal sphincter injury
- using a long-lasting absorbable suture, such as PDS, to repair the internal and external anal sphincters
- ensuring that the patient with a dehiscence following an episiotomy or anal sphincter injury has access to early secondary repair. Standardizing your approach to the prevention and repair of anal sphincter injury will benefit the approximately 112,600 US women who experience OASIS each year. ●
A Cochrane Database Systematic Review reported that moderate-quality evidence showed a decrease in OASIS with the use of intrapartum warm compresses to the perineum and perineal massage.1 Compared with control, intrapartum warm compresses to the perineum did not result in a reduction in first- or second-degree tears, suturing of perineal tears, or use of episiotomy. However, compared with control, intrapartum warm compresses to the perineum was associated with a reduction in OASIS (relative risk [RR], 0.46; 95% confidence interval [CI], 0.27–0.79; 1,799 women; 4 studies; moderate quality evidence; substantial heterogeneity among studies). In addition to a possible reduction in OASIS, warm compresses also may provide the laboring woman, especially those having a natural childbirth, a positive sensory experience and reinforce her perception of the thoughtfulness and caring of her clinicians.
Compared with control, perineal massage was associated with an increase in the rate of an intact perineum (RR, 1.74; 95% CI, 1.11–2.73; 6 studies; 2,618 women; low-quality evidence; substantial heterogeneity among studies) and a decrease in OASIS (RR, 0.49; 95% CI, 0.25–0.94; 5 studies; 2,477 women; moderate quality evidence). Compared with control, perineal massage did not significantly reduce first- or second-degree tears, perineal tears requiring suturing, or the use of episiotomy (very low-quality evidence). Although perineal massage may have benefit, excessive perineal massage likely can contribute to tissue edema and epithelial trauma.
Reference
1. Aasheim V, Nilsen ABC, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;CD006672.
- Friedman AM, Ananth CV, Prendergast E, et al. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125:927-937.
- Hamilton BE, Martin JA, Osterman MK. Births: Provisional data for 2019. Vital Statistics Rapid Release; No. 8. Hyattsville MD: National Center for Health Statistics; May 2020. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf
- Pergialitotis V, Bellos I, Fanaki M, et al. Risk factors for severe perineal trauma during childbirth: an updated meta-analysis. European J Obstet Gynecol Repro Biol. 2020;247:94-100.
- Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and anal sphincter trauma. 1st ed. London, England: Springer-Verlag; 2009:33-51.
- Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev. 2017;CD000081.
- Coats PM, Chan KK, Wilkins M, et al. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
- Sooklim R, Thinkhamrop J, Lumbiganon P, et al. The outcomes of midline versus medio-lateral episiotomy. Reprod Health. 2007;4:10.
- El-Din AS, Kamal MM, Amin MA. Comparison between two incision angles of mediolateral episiotomy in primiparous women: a randomized controlled trial. J Obstet Gynaecol Res. 2014;40:1877-1882.
- Duggal N, Mercado C, Daniels K, et al. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111:1268-1273.
- Harvey MA, Pierce M. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynecol Can. 2015;37:1131-1148.
- Cox CK, Bugosh MD, Fenner DE, et al. Antibiotic use during repair of obstetrical anal sphincter injury: a qualitative improvement initiative. Int J Gynaecol Obstet. 2021; Epub January 28.
- Deierhoi RJ, Dawes LG, Vick C, et al. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763-769.
- Till Sr, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217:187.e1-e11.
- Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period: prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. European J Obst Gynecol Repro Biol. 2020;245:1-6.
- Fornell EU, Matthiesen L, Sjodahl R, et al. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312-316.
- Sultan AH, Monga AK, Kumar D, et al. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
- Williams A, Adams EJ, Tincello DG, et al. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
- Hauth JC, Gilstrap LC, Ward SC, et al. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67:806-809.
- Hankins GD, Hauth JC, Gilstrap LC, et al. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75:48-51.
- Ramin SR, Ramus RM, Little BB, et al. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167:1104-1107.
- Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. Norwalk Connecticut: Appleton-Century-Crofts; 1985:349-350.
- Okeahialam NA, Thakar R, Kleprlikova H, et al. Early re-suturing of dehisced obstetric perineal woulds: a 13-year experience. Eur J Obstet Gynecol Repro Biol. 2020;254:69-73.
- Dudley L, Kettle C, Thomas PW, et al. Perineal resuturing versus expectant management following vaginal delivery complicated by a dehisced wound (PREVIEW): a pilot and feasibility randomised controlled trial. BMJ Open. 2017;7:e012766.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetrical anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
The rate of obstetric anal sphincter injury (OASIS) is approximately 4.4% of vaginal deliveries, with 3.3% 3rd-degree tears and 1.1% 4th-degree tears.1 In the United States in 2019 there were 3,745,540 births—a 31.7% rate of cesarean delivery (CD) and a 68.3% rate of vaginal delivery—resulting in approximately 112,600 births with OASIS.2 A meta-analysis reported that, among 716,031 vaginal births, the risk factors for OASIS included: forceps delivery (relative risk [RR], 3.15), midline episiotomy (RR, 2.88), occiput posterior fetal position (RR, 2.73), vacuum delivery (RR, 2.60), Asian race (RR, 1.87), primiparity (RR, 1.59), mediolateral episiotomy (RR, 1.55), augmentation of labor (RR, 1.46), and epidural anesthesia (RR, 1.21).3 OASIS is associated with an increased risk for developing postpartum perineal pain, anal incontinence, dyspareunia, and wound breakdown.4 Complications following OASIS repair can trigger many follow-up appointments to assess wound healing and provide physical therapy.
This editorial review focuses on evolving recommendations for preventing and repairing OASIS.
The optimal cutting angle for a mediolateral episiotomy is 60 degrees from the midline
For spontaneous vaginal delivery, a policy of restricted episiotomy reduces the risk of OASIS by approximately 30%.5 With an operative vaginal delivery, especially forceps delivery of a large fetus in the occiput posterior position, a mediolateral episiotomy may help to reduce the risk of OASIS, although there are minimal data from clinical trials to support this practice. In one clinical trial, 407 women were randomly assigned to either a mediolateral or midline episiotomy.6 Approximately 25% of the births in both groups were operative deliveries. The mediolateral episiotomy began in the posterior midline of the vaginal introitus and was carried to the right side of the anal sphincter for 3 cm to 4 cm. The midline episiotomy began in the posterior midline of the vagina and was carried 2 cm to 3 cm into the midline perineal tissue. In the women having a midline or mediolateral episiotomy, a 4th-degree tear occurred in 5.5% and 0.4% of births, respectively. For the midline or mediolateral episiotomy, a third-degree tear occurred in 18.4% and 8.6%, respectively. In a prospective cohort study of 1,302 women with an episiotomy and vaginal birth, the rate of OASIS associated with midline or mediolateral episiotomy was 14.8% and 7%, respectively (P<.05).7 In this study, the operative vaginal delivery rate was 11.6% and 15.2% for the women in the midline and mediolateral groups, respectively.
The angle of the mediolateral episiotomy may influence the rate of OASIS and persistent postpartum perineal pain. In one study, 330 nulliparous women who were assessed to need a mediolateral episiotomy at delivery were randomized to an incision with a 40- or 60-degree angle from the midline.8 Prior to incision, a line was drawn on the skin to mark the course of the incision and then infiltrated with 10 mL of lignocaine. The fetal head was delivered with a Ritgen maneuver. The length of the episiotomy averaged 4 cm in both groups. After delivery, the angle of the episiotomy incision was reassessed. The episiotomy incision cut 60 degrees from the midline was measured on average to be 44 degrees from the midline after delivery of the newborn. Similarly, the incision cut at a 40-degree angle was measured to be 24 degrees from the midline after delivery. The rates of OASIS in the women who had a 40- and 60-degree angle incision were 5.5% and 2.4%, respectively (P = .16).
Continue to: Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair...
Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair
Many experts recommend one dose of a prophylactic antibiotic prior to, or during, OASIS repair in order to reduce the risk of wound complications. In a trial 147 women with OASIS were randomly assigned to receive one dose of a second-generation cephalosporin (cefotetan or cefoxitin) with extended anaerobic coverage or a placebo just before repair of the laceration.9 At 2 weeks postpartum, perineal wound complications were significantly lower in women receiving one dose of prophylactic antibiotic with extended anaerobe coverage compared with placebo—8.2% and 24.1%, respectively (P = .037). Additionally, at 2 weeks postpartum, purulent wound discharge was significantly lower in women receiving antibiotic versus placebo, 4% and 17%, respectively (P = .036). Experts writing for the Society of Obstetricians and Gynaecologists of Canada also recommend one dose of cefotetan or cefoxitin.10 Extended anaerobic coverage also can be achieved by administering a single dose of BOTH cefazolin 2 g by intravenous (IV) infusion PLUS metronidazole 500 mg by IV infusion or oral medication.11 For women with severe penicillin allergy, a recommended regimen is gentamicin 5 mg/kg plus clindamycin 900 mg by IV infusion.11 There is evidence that for colorectal or hysterectomy surgery, expanding prophylactic antibiotic coverage of anaerobes with cefazolin PLUS metronidazole significantly reduces postoperative surgical site infection.12,13 Following an OASIS repair, wound breakdown is a catastrophic problem that may take many months to resolve. Administration of a prophylactic antibiotic with extended coverage of anaerobes may help to prevent wound breakdown.
Prioritize identifying and separately repairing the internal anal sphincter
The internal anal sphincter is a smooth muscle that runs along the outside of the rectal wall and thickens into a sphincter toward the anal canal. The internal anal sphincter is thin and grey-white in appearance, like a veil. By contrast, the external anal sphincter is a thick band of red striated muscle tissue. In one study of 3,333 primiparous women with OASIS, an internal anal sphincter injury was detected in 33% of cases.14 In this large cohort, the rate of internal anal sphincter injury with a 3A tear, a 3B tear, a complete tear of the external sphincter and a 4th-degree perineal tear was 22%, 23%, 42%, and 71%, respectively. The internal anal sphincter is important for maintaining rectal continence and is estimated to contribute 50% to 85% of resting anal tone.15 If injury to the internal anal sphincter is detected at a birth with an OASIS, it is important to separately repair the internal anal sphincter to reduce the risk of postpartum rectal incontinence.16
Polyglactin 910 vs Polydioxanone (PDS) Suture—Is PDS the winner?
Polyglactin 910 (Vicryl) is a braided suture that is absorbed within 56 to 70 days. Polydioxanone suture is a long-lasting monofilament suture that is absorbed within 200 days. Many colorectal surgeons and urogynecologists prefer PDS suture for the repair of both the internal and external anal sphincters.16 Authors of one randomized trial of OASIS repair with Vicryl or PDS suture did not report significant differences in most clinical outcomes.17 However, in this study, anal endosonographic imaging of the internal and external anal sphincter demonstrated more internal sphincter defects but not external sphincter defects when the repair was performed with Vicryl rather than PDS. The investigators concluded that comprehensive training of the surgeon, not choice of suture, is probably the most important factor in achieving a good OASIS repair. However, because many subspecialists favor PDS suture for sphincter repair, specialists in obstetrics and gynecology should consider this option.
Continue to: Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
The breakdown of an OASIS repair is an obstetric catastrophe with complications that can last many months and sometimes stretch into years. The best approach to a perineal laceration wound breakdown remains controversial. It is optimal if all patients with a wound breakdown can be offered an early secondary repair or healing by secondary intention, permitting the patient to select the best approach for their specific situation.
As noted by the pioneers of early repair of episiotomy dehiscence, Drs. Hankins, Haugh, Gilstrap, Ramin, and others,18-20 conventional doctrine is that an episiotomy repair dehiscence should be managed expectantly, allowing healing by secondary intention and delaying repair of the sphincters for a minimum of 3 to 4 months.21 However, many small case-series report that early secondary repair of a perineal laceration wound breakdown is possible following multiple days of wound preparation prior to the repair, good surgical technique and diligent postoperative follow-up care. One large case series reported on 72 women with complete perineal wound dehiscence who had early secondary repair.22 The median time to complete wound healing following early repair was 28 days. About 36% of the patients had one or more complications, including skin dehiscence, granuloma formation, perineal pain, and sinus formation. A pilot randomized trial reported that, compared with expectant management of a wound breakdown, early repair resulted in a shorter time to wound healing.23
Early repair of perineal wound dehiscence often involves a course of care that extends over multiple weeks. As an example, following a vaginal birth with OASIS and immediate repair, the patient is often discharged from the hospital to home on postpartum day 3. The wound breakdown often is detected between postpartum days 6 to 10. If early secondary repair is selected as the best treatment, 1 to 6 days of daily debridement of the wound is needed to prepare the wound for early secondary repair. The daily debridement required to prepare the wound for early repair is often performed in the hospital, potentially disrupting early mother-newborn bonding. Following the repair, the patient is observed in the hospital for 1 to 3 days and then discharged home with daily wound care and multiple follow-up visits to monitor wound healing. Pelvic floor physical therapy may be initiated when the wound is healed. The prolonged process required for early secondary repair may be best undertaken by a subspecialty practice.24
The surgical repair and postpartum care of OASIS continues to evolve. In your practice you should consider:
- performing a mediolateral episiotomy at a 60-degree angle to reduce the risk of OASIS in situations where there is a high risk of anal sphincter injury, such as in forceps delivery
- using one dose of a prophylactic antibiotic with extended anaerobic coverage, such as cefotetan or cefoxitin
- focus on identifying and separately repairing an internal anal sphincter injury
- using a long-lasting absorbable suture, such as PDS, to repair the internal and external anal sphincters
- ensuring that the patient with a dehiscence following an episiotomy or anal sphincter injury has access to early secondary repair. Standardizing your approach to the prevention and repair of anal sphincter injury will benefit the approximately 112,600 US women who experience OASIS each year. ●
A Cochrane Database Systematic Review reported that moderate-quality evidence showed a decrease in OASIS with the use of intrapartum warm compresses to the perineum and perineal massage.1 Compared with control, intrapartum warm compresses to the perineum did not result in a reduction in first- or second-degree tears, suturing of perineal tears, or use of episiotomy. However, compared with control, intrapartum warm compresses to the perineum was associated with a reduction in OASIS (relative risk [RR], 0.46; 95% confidence interval [CI], 0.27–0.79; 1,799 women; 4 studies; moderate quality evidence; substantial heterogeneity among studies). In addition to a possible reduction in OASIS, warm compresses also may provide the laboring woman, especially those having a natural childbirth, a positive sensory experience and reinforce her perception of the thoughtfulness and caring of her clinicians.
Compared with control, perineal massage was associated with an increase in the rate of an intact perineum (RR, 1.74; 95% CI, 1.11–2.73; 6 studies; 2,618 women; low-quality evidence; substantial heterogeneity among studies) and a decrease in OASIS (RR, 0.49; 95% CI, 0.25–0.94; 5 studies; 2,477 women; moderate quality evidence). Compared with control, perineal massage did not significantly reduce first- or second-degree tears, perineal tears requiring suturing, or the use of episiotomy (very low-quality evidence). Although perineal massage may have benefit, excessive perineal massage likely can contribute to tissue edema and epithelial trauma.
Reference
1. Aasheim V, Nilsen ABC, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;CD006672.
The rate of obstetric anal sphincter injury (OASIS) is approximately 4.4% of vaginal deliveries, with 3.3% 3rd-degree tears and 1.1% 4th-degree tears.1 In the United States in 2019 there were 3,745,540 births—a 31.7% rate of cesarean delivery (CD) and a 68.3% rate of vaginal delivery—resulting in approximately 112,600 births with OASIS.2 A meta-analysis reported that, among 716,031 vaginal births, the risk factors for OASIS included: forceps delivery (relative risk [RR], 3.15), midline episiotomy (RR, 2.88), occiput posterior fetal position (RR, 2.73), vacuum delivery (RR, 2.60), Asian race (RR, 1.87), primiparity (RR, 1.59), mediolateral episiotomy (RR, 1.55), augmentation of labor (RR, 1.46), and epidural anesthesia (RR, 1.21).3 OASIS is associated with an increased risk for developing postpartum perineal pain, anal incontinence, dyspareunia, and wound breakdown.4 Complications following OASIS repair can trigger many follow-up appointments to assess wound healing and provide physical therapy.
This editorial review focuses on evolving recommendations for preventing and repairing OASIS.
The optimal cutting angle for a mediolateral episiotomy is 60 degrees from the midline
For spontaneous vaginal delivery, a policy of restricted episiotomy reduces the risk of OASIS by approximately 30%.5 With an operative vaginal delivery, especially forceps delivery of a large fetus in the occiput posterior position, a mediolateral episiotomy may help to reduce the risk of OASIS, although there are minimal data from clinical trials to support this practice. In one clinical trial, 407 women were randomly assigned to either a mediolateral or midline episiotomy.6 Approximately 25% of the births in both groups were operative deliveries. The mediolateral episiotomy began in the posterior midline of the vaginal introitus and was carried to the right side of the anal sphincter for 3 cm to 4 cm. The midline episiotomy began in the posterior midline of the vagina and was carried 2 cm to 3 cm into the midline perineal tissue. In the women having a midline or mediolateral episiotomy, a 4th-degree tear occurred in 5.5% and 0.4% of births, respectively. For the midline or mediolateral episiotomy, a third-degree tear occurred in 18.4% and 8.6%, respectively. In a prospective cohort study of 1,302 women with an episiotomy and vaginal birth, the rate of OASIS associated with midline or mediolateral episiotomy was 14.8% and 7%, respectively (P<.05).7 In this study, the operative vaginal delivery rate was 11.6% and 15.2% for the women in the midline and mediolateral groups, respectively.
The angle of the mediolateral episiotomy may influence the rate of OASIS and persistent postpartum perineal pain. In one study, 330 nulliparous women who were assessed to need a mediolateral episiotomy at delivery were randomized to an incision with a 40- or 60-degree angle from the midline.8 Prior to incision, a line was drawn on the skin to mark the course of the incision and then infiltrated with 10 mL of lignocaine. The fetal head was delivered with a Ritgen maneuver. The length of the episiotomy averaged 4 cm in both groups. After delivery, the angle of the episiotomy incision was reassessed. The episiotomy incision cut 60 degrees from the midline was measured on average to be 44 degrees from the midline after delivery of the newborn. Similarly, the incision cut at a 40-degree angle was measured to be 24 degrees from the midline after delivery. The rates of OASIS in the women who had a 40- and 60-degree angle incision were 5.5% and 2.4%, respectively (P = .16).
Continue to: Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair...
Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair
Many experts recommend one dose of a prophylactic antibiotic prior to, or during, OASIS repair in order to reduce the risk of wound complications. In a trial 147 women with OASIS were randomly assigned to receive one dose of a second-generation cephalosporin (cefotetan or cefoxitin) with extended anaerobic coverage or a placebo just before repair of the laceration.9 At 2 weeks postpartum, perineal wound complications were significantly lower in women receiving one dose of prophylactic antibiotic with extended anaerobe coverage compared with placebo—8.2% and 24.1%, respectively (P = .037). Additionally, at 2 weeks postpartum, purulent wound discharge was significantly lower in women receiving antibiotic versus placebo, 4% and 17%, respectively (P = .036). Experts writing for the Society of Obstetricians and Gynaecologists of Canada also recommend one dose of cefotetan or cefoxitin.10 Extended anaerobic coverage also can be achieved by administering a single dose of BOTH cefazolin 2 g by intravenous (IV) infusion PLUS metronidazole 500 mg by IV infusion or oral medication.11 For women with severe penicillin allergy, a recommended regimen is gentamicin 5 mg/kg plus clindamycin 900 mg by IV infusion.11 There is evidence that for colorectal or hysterectomy surgery, expanding prophylactic antibiotic coverage of anaerobes with cefazolin PLUS metronidazole significantly reduces postoperative surgical site infection.12,13 Following an OASIS repair, wound breakdown is a catastrophic problem that may take many months to resolve. Administration of a prophylactic antibiotic with extended coverage of anaerobes may help to prevent wound breakdown.
Prioritize identifying and separately repairing the internal anal sphincter
The internal anal sphincter is a smooth muscle that runs along the outside of the rectal wall and thickens into a sphincter toward the anal canal. The internal anal sphincter is thin and grey-white in appearance, like a veil. By contrast, the external anal sphincter is a thick band of red striated muscle tissue. In one study of 3,333 primiparous women with OASIS, an internal anal sphincter injury was detected in 33% of cases.14 In this large cohort, the rate of internal anal sphincter injury with a 3A tear, a 3B tear, a complete tear of the external sphincter and a 4th-degree perineal tear was 22%, 23%, 42%, and 71%, respectively. The internal anal sphincter is important for maintaining rectal continence and is estimated to contribute 50% to 85% of resting anal tone.15 If injury to the internal anal sphincter is detected at a birth with an OASIS, it is important to separately repair the internal anal sphincter to reduce the risk of postpartum rectal incontinence.16
Polyglactin 910 vs Polydioxanone (PDS) Suture—Is PDS the winner?
Polyglactin 910 (Vicryl) is a braided suture that is absorbed within 56 to 70 days. Polydioxanone suture is a long-lasting monofilament suture that is absorbed within 200 days. Many colorectal surgeons and urogynecologists prefer PDS suture for the repair of both the internal and external anal sphincters.16 Authors of one randomized trial of OASIS repair with Vicryl or PDS suture did not report significant differences in most clinical outcomes.17 However, in this study, anal endosonographic imaging of the internal and external anal sphincter demonstrated more internal sphincter defects but not external sphincter defects when the repair was performed with Vicryl rather than PDS. The investigators concluded that comprehensive training of the surgeon, not choice of suture, is probably the most important factor in achieving a good OASIS repair. However, because many subspecialists favor PDS suture for sphincter repair, specialists in obstetrics and gynecology should consider this option.
Continue to: Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
The breakdown of an OASIS repair is an obstetric catastrophe with complications that can last many months and sometimes stretch into years. The best approach to a perineal laceration wound breakdown remains controversial. It is optimal if all patients with a wound breakdown can be offered an early secondary repair or healing by secondary intention, permitting the patient to select the best approach for their specific situation.
As noted by the pioneers of early repair of episiotomy dehiscence, Drs. Hankins, Haugh, Gilstrap, Ramin, and others,18-20 conventional doctrine is that an episiotomy repair dehiscence should be managed expectantly, allowing healing by secondary intention and delaying repair of the sphincters for a minimum of 3 to 4 months.21 However, many small case-series report that early secondary repair of a perineal laceration wound breakdown is possible following multiple days of wound preparation prior to the repair, good surgical technique and diligent postoperative follow-up care. One large case series reported on 72 women with complete perineal wound dehiscence who had early secondary repair.22 The median time to complete wound healing following early repair was 28 days. About 36% of the patients had one or more complications, including skin dehiscence, granuloma formation, perineal pain, and sinus formation. A pilot randomized trial reported that, compared with expectant management of a wound breakdown, early repair resulted in a shorter time to wound healing.23
Early repair of perineal wound dehiscence often involves a course of care that extends over multiple weeks. As an example, following a vaginal birth with OASIS and immediate repair, the patient is often discharged from the hospital to home on postpartum day 3. The wound breakdown often is detected between postpartum days 6 to 10. If early secondary repair is selected as the best treatment, 1 to 6 days of daily debridement of the wound is needed to prepare the wound for early secondary repair. The daily debridement required to prepare the wound for early repair is often performed in the hospital, potentially disrupting early mother-newborn bonding. Following the repair, the patient is observed in the hospital for 1 to 3 days and then discharged home with daily wound care and multiple follow-up visits to monitor wound healing. Pelvic floor physical therapy may be initiated when the wound is healed. The prolonged process required for early secondary repair may be best undertaken by a subspecialty practice.24
The surgical repair and postpartum care of OASIS continues to evolve. In your practice you should consider:
- performing a mediolateral episiotomy at a 60-degree angle to reduce the risk of OASIS in situations where there is a high risk of anal sphincter injury, such as in forceps delivery
- using one dose of a prophylactic antibiotic with extended anaerobic coverage, such as cefotetan or cefoxitin
- focus on identifying and separately repairing an internal anal sphincter injury
- using a long-lasting absorbable suture, such as PDS, to repair the internal and external anal sphincters
- ensuring that the patient with a dehiscence following an episiotomy or anal sphincter injury has access to early secondary repair. Standardizing your approach to the prevention and repair of anal sphincter injury will benefit the approximately 112,600 US women who experience OASIS each year. ●
A Cochrane Database Systematic Review reported that moderate-quality evidence showed a decrease in OASIS with the use of intrapartum warm compresses to the perineum and perineal massage.1 Compared with control, intrapartum warm compresses to the perineum did not result in a reduction in first- or second-degree tears, suturing of perineal tears, or use of episiotomy. However, compared with control, intrapartum warm compresses to the perineum was associated with a reduction in OASIS (relative risk [RR], 0.46; 95% confidence interval [CI], 0.27–0.79; 1,799 women; 4 studies; moderate quality evidence; substantial heterogeneity among studies). In addition to a possible reduction in OASIS, warm compresses also may provide the laboring woman, especially those having a natural childbirth, a positive sensory experience and reinforce her perception of the thoughtfulness and caring of her clinicians.
Compared with control, perineal massage was associated with an increase in the rate of an intact perineum (RR, 1.74; 95% CI, 1.11–2.73; 6 studies; 2,618 women; low-quality evidence; substantial heterogeneity among studies) and a decrease in OASIS (RR, 0.49; 95% CI, 0.25–0.94; 5 studies; 2,477 women; moderate quality evidence). Compared with control, perineal massage did not significantly reduce first- or second-degree tears, perineal tears requiring suturing, or the use of episiotomy (very low-quality evidence). Although perineal massage may have benefit, excessive perineal massage likely can contribute to tissue edema and epithelial trauma.
Reference
1. Aasheim V, Nilsen ABC, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;CD006672.
- Friedman AM, Ananth CV, Prendergast E, et al. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125:927-937.
- Hamilton BE, Martin JA, Osterman MK. Births: Provisional data for 2019. Vital Statistics Rapid Release; No. 8. Hyattsville MD: National Center for Health Statistics; May 2020. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf
- Pergialitotis V, Bellos I, Fanaki M, et al. Risk factors for severe perineal trauma during childbirth: an updated meta-analysis. European J Obstet Gynecol Repro Biol. 2020;247:94-100.
- Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and anal sphincter trauma. 1st ed. London, England: Springer-Verlag; 2009:33-51.
- Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev. 2017;CD000081.
- Coats PM, Chan KK, Wilkins M, et al. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
- Sooklim R, Thinkhamrop J, Lumbiganon P, et al. The outcomes of midline versus medio-lateral episiotomy. Reprod Health. 2007;4:10.
- El-Din AS, Kamal MM, Amin MA. Comparison between two incision angles of mediolateral episiotomy in primiparous women: a randomized controlled trial. J Obstet Gynaecol Res. 2014;40:1877-1882.
- Duggal N, Mercado C, Daniels K, et al. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111:1268-1273.
- Harvey MA, Pierce M. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynecol Can. 2015;37:1131-1148.
- Cox CK, Bugosh MD, Fenner DE, et al. Antibiotic use during repair of obstetrical anal sphincter injury: a qualitative improvement initiative. Int J Gynaecol Obstet. 2021; Epub January 28.
- Deierhoi RJ, Dawes LG, Vick C, et al. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763-769.
- Till Sr, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217:187.e1-e11.
- Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period: prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. European J Obst Gynecol Repro Biol. 2020;245:1-6.
- Fornell EU, Matthiesen L, Sjodahl R, et al. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312-316.
- Sultan AH, Monga AK, Kumar D, et al. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
- Williams A, Adams EJ, Tincello DG, et al. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
- Hauth JC, Gilstrap LC, Ward SC, et al. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67:806-809.
- Hankins GD, Hauth JC, Gilstrap LC, et al. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75:48-51.
- Ramin SR, Ramus RM, Little BB, et al. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167:1104-1107.
- Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. Norwalk Connecticut: Appleton-Century-Crofts; 1985:349-350.
- Okeahialam NA, Thakar R, Kleprlikova H, et al. Early re-suturing of dehisced obstetric perineal woulds: a 13-year experience. Eur J Obstet Gynecol Repro Biol. 2020;254:69-73.
- Dudley L, Kettle C, Thomas PW, et al. Perineal resuturing versus expectant management following vaginal delivery complicated by a dehisced wound (PREVIEW): a pilot and feasibility randomised controlled trial. BMJ Open. 2017;7:e012766.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetrical anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Friedman AM, Ananth CV, Prendergast E, et al. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125:927-937.
- Hamilton BE, Martin JA, Osterman MK. Births: Provisional data for 2019. Vital Statistics Rapid Release; No. 8. Hyattsville MD: National Center for Health Statistics; May 2020. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf
- Pergialitotis V, Bellos I, Fanaki M, et al. Risk factors for severe perineal trauma during childbirth: an updated meta-analysis. European J Obstet Gynecol Repro Biol. 2020;247:94-100.
- Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and anal sphincter trauma. 1st ed. London, England: Springer-Verlag; 2009:33-51.
- Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev. 2017;CD000081.
- Coats PM, Chan KK, Wilkins M, et al. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
- Sooklim R, Thinkhamrop J, Lumbiganon P, et al. The outcomes of midline versus medio-lateral episiotomy. Reprod Health. 2007;4:10.
- El-Din AS, Kamal MM, Amin MA. Comparison between two incision angles of mediolateral episiotomy in primiparous women: a randomized controlled trial. J Obstet Gynaecol Res. 2014;40:1877-1882.
- Duggal N, Mercado C, Daniels K, et al. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111:1268-1273.
- Harvey MA, Pierce M. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynecol Can. 2015;37:1131-1148.
- Cox CK, Bugosh MD, Fenner DE, et al. Antibiotic use during repair of obstetrical anal sphincter injury: a qualitative improvement initiative. Int J Gynaecol Obstet. 2021; Epub January 28.
- Deierhoi RJ, Dawes LG, Vick C, et al. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763-769.
- Till Sr, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217:187.e1-e11.
- Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period: prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. European J Obst Gynecol Repro Biol. 2020;245:1-6.
- Fornell EU, Matthiesen L, Sjodahl R, et al. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312-316.
- Sultan AH, Monga AK, Kumar D, et al. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
- Williams A, Adams EJ, Tincello DG, et al. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
- Hauth JC, Gilstrap LC, Ward SC, et al. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67:806-809.
- Hankins GD, Hauth JC, Gilstrap LC, et al. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75:48-51.
- Ramin SR, Ramus RM, Little BB, et al. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167:1104-1107.
- Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. Norwalk Connecticut: Appleton-Century-Crofts; 1985:349-350.
- Okeahialam NA, Thakar R, Kleprlikova H, et al. Early re-suturing of dehisced obstetric perineal woulds: a 13-year experience. Eur J Obstet Gynecol Repro Biol. 2020;254:69-73.
- Dudley L, Kettle C, Thomas PW, et al. Perineal resuturing versus expectant management following vaginal delivery complicated by a dehisced wound (PREVIEW): a pilot and feasibility randomised controlled trial. BMJ Open. 2017;7:e012766.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetrical anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
FDA and power morcellation, gel for vaginal odor, and an intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
Caring for women with pelvic floor disorders during pregnancy and postpartum: Expert guidance
Pelvic floor disorders (PFDs) affect many pregnant and newly postpartum women. These conditions, including urinary incontinence, anal incontinence, and pelvic organ prolapse (POP), can be overshadowed by common pregnancy and postpartum concerns (TABLE 1).1 With the use of a few quick screening questions, however, PFDs easily can be identified in this at-risk population. Active management need not be delayed until after delivery for women experiencing bother, as options exist for women with PFDs during pregnancy as well as postpartum.
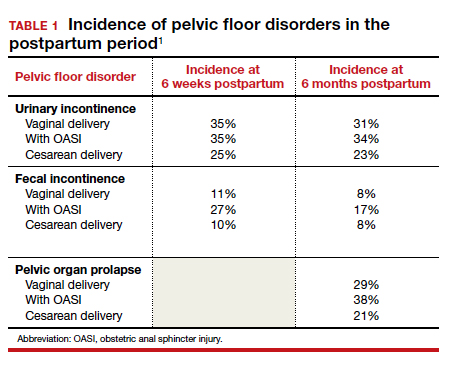
In this article, we discuss the common PFDs that obstetric clinicians face in the context of case scenarios and review how you can be better equipped to care for affected individuals.
CASE 1 Screening
A 30-year-old woman (G1P1) presents for her routine postpartum visit after an operative vaginal delivery with a second-degree laceration.
How would you screen this patient for PFDs?
Why screening for PFDs matters
While there are no validated PFD screening tools for this patient population, clinicians can ask a series of brief open-ended questions as part of the review of systems to efficiently evaluate for the common PFDs in peripartum patients (see “Screening questions to evaluate patients for peripartum pelvic floor disorders” below).
Pelvic floor disorders in the peripartum period can have a significant negative impact. In pregnancy, nearly half of women report psychological strain due to the presence of bowel, bladder, prolapse, or sexual dysfunction symptoms.2 Postpartum, PFDs have negative effects on overall health, well-being, and self-esteem, with significantly increased rates of postpartum depression in women who experience urinary incontinence.3,4 Proactively inquiring about PFD symptoms, providing anticipatory guidance, and recommending treatment options can positively impact a patient in multiple domains.
Sometimes during pregnancy or after having a baby, a woman experiences pelvic floor symptoms. Do you have any of the following?
- leakage with coughing, laughing, sneezing, or physical activity
- urgency to urinate or leakage due to urgency
- bulging or pressure within the vagina
- pain with intercourse
- accidental bowel leakage of stool or flatus
CASE 2 Stress urinary incontinence
A 27-year-old woman (G1P1) presents 2 months following spontaneous vaginal delivery with symptoms of urine leakage with laughing and running. Her urinary incontinence has been improving since delivery, but it continues to be bothersome.
What would you recommend for this patient?
Conservative SUI management strategies in pregnancy
Urinary tract symptoms are common in pregnancy, with up to 41.8% of women reporting urinary symptom distress in the third trimester.5 During pregnancy, estrogen and progesterone decrease urethral pressure that, together with increased intra-abdominal pressure from the gravid uterus, can cause or worsen stress urinary incontinence (SUI).6
During pregnancy, women should be offered conservative therapies for SUI. For women who can perform a pelvic floor contraction (a Kegel exercise), self-guided pelvic floor muscle exercises (PFMEs) may be helpful (see “Pelvic floor muscle exercises” below). We recommend that women start with 1 to 2 sets of 10 Kegel exercises per day and that they hold the squeeze for 2 to 3 seconds, working up to holding for 10 seconds. The goal is to strengthen and improve muscle control so that the Kegel squeeze can be paired with activities that cause SUI.
For women who are unable to perform a Kegel exercise or are not improving with a home PFME regimen, referral to pelvic floor physical therapy (PFPT) can be considered. While data support the efficacy of PFPT for SUI treatment in nonpregnant women,7 data are lacking on PFME in pregnancy.
In women without urinary incontinence, PFME in early pregnancy can prevent the onset of incontinence in late pregnancy and the postpartum period.8 By contrast, the same 2020 Cochrane Review found no evidence that antenatal pelvic floor muscle therapy in incontinent women decreases incontinence in mid- or late-pregnancy or in the postpartum period.8 As the quality of this evidence is very low and there is no evidence of harm with PFME, we continue to recommend it for women with bothersome SUI.
Incontinence pessaries or vaginal inserts (such as Poise Impressa bladder supports) can be helpful for SUI treatment. An incontinence pessary can be fitted in the office, and fitting kits are available for both. Pessaries can safely be used in pregnancy, but there are no data on the efficacy of pessaries for treating SUI in pregnancy. In nonpregnant women, evidence demonstrates 63% satisfaction 3 months post–pessary placement for SUI.7
We do not recommend invasive procedures for the treatment of SUI during pregnancy or in the first 6 months following delivery. There is no evidence that elective cesarean delivery prevents persistent SUI postpartum.9
To identify and engage the proper pelvic floor muscles:
- Insert a finger in the vagina and squeeze the vaginal muscles around your finger.
- Imagine you are sitting on a marble and have to pick it up with the vaginal muscles.
- Squeeze the muscles you would use to stop the flow of urine or hold back flatulence.
Perform sets of 10, 2 to 3 times per day as follows:
- Squeeze: Engage the pelvic floor muscles as described above; avoid performing Kegels while voiding.
- Hold: For 2 to 10 seconds; increase the duration to 10 seconds as able.
- Relax: Completely relax muscles before initating the next squeeze.
Reference
1. UpToDate. Patient education: pelvic muscle (Kegel) exercises (the basics). 2018. https://uptodatefree.ir/topic.htm?path=pelvic-muscle-kegel-exercises-the-basics. Accessed February 24, 2021.
Continue to: Managing SUI in the postpartum period...
Managing SUI in the postpartum period
After the first 6 months postpartum and exhaustion of conservative measures, we offer surgical interventions for women with persistent, bothersome incontinence. Surgery for SUI typically is not recommended until childbearing is complete, but it can be considered if the patient’s bother is significant.
For women with bothersome SUI who still desire future pregnancy, management options include periurethral bulking, a retropubic urethropexy (Burch procedure), or a midurethral sling procedure. Women who undergo an anti-incontinence procedure have an increased risk for urinary retention during a subsequent pregnancy.10 Most women with a midurethral sling will continue to be continent following an obstetric delivery.
Anticipatory guidance
At 3 months postpartum, the incidence of urinary incontinence is 6% to 40%, depending on parity and delivery type. Postpartum urinary incontinence is most common after instrumented vaginal delivery (32%) followed by spontaneous vaginal delivery (28%) and cesarean delivery (15%). The mean prevalence of any type of urinary incontinence is 33% at 3 months postpartum, and only small changes in the rate of urinary incontinence occur over the first postpartum year.11 While urinary incontinence is common postpartum, it should not be considered normal. We counsel that symptoms may improve spontaneously, but treatment can be initiated if the patient experiences significant bother.
A longitudinal cohort study that followed women from 3 months to 12 years postpartum found that, of women with urinary incontinence at 3 months postpartum, 76% continued to report incontinence at 12 years postpartum.12 We recommend that women be counseled that, even when symptoms resolve, they remain at increased risk for urinary incontinence in the future. Invasive therapies should be used to treat bothersome urinary incontinence, not to prevent future incontinence.
CASE 3 Fecal incontinence
A 24-year-old woman (G1P1) presents 3 weeks postpartum following a forceps-assisted vaginal delivery complicated by a 3c laceration. She reports fecal urgency, inability to control flatus, and once-daily fecal incontinence.
How would you evaluate these symptoms?
Steps in evaluation
The initial evaluation should include an inquiry regarding the patient’s stool consistency and bowel regimen. The Bristol stool form scale can be used to help patients describe their typical bowel movements (TABLE 2).13 During healing, the goal is to achieve a Bristol type 4 stool, both to avoid straining and to improve continence, as loose stool is the most difficult to control.
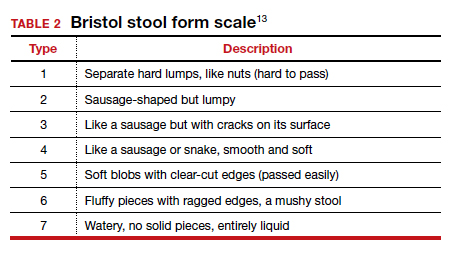
A physical examination can evaluate healing and sphincter integrity; it should include inspection of the distal vagina and perineal body and a digital rectal exam. Anal canal resting tone and squeeze strength should be evaluated, and the digital rectal examination scoring system (DRESS) can be useful for quantification (TABLE 3).14 Lack of tone at rest in the anterolateral portion of the sphincter complex can indicate an internal anal sphincter defect, as 80% of the resting tone comes from this muscle (FIGURE).15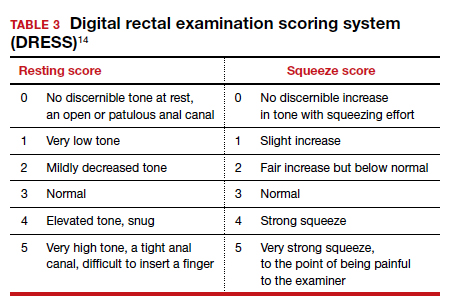
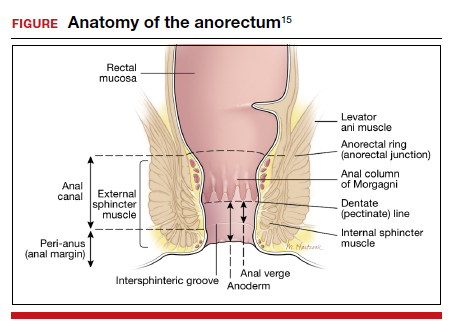
The rectovaginal septum should be assessed given the increased risk of rectovaginal fistula in women with obstetric anal sphincter injury (OASI). The patient should be instructed to contract the anal sphincter, allowing evaluation of muscular contraction. Lack of contraction anteriolaterally may indicate external anal sphincter separation.
Continue to: Conservative options for improving fecal incontinence symptoms...
Conservative options for improving fecal incontinence symptoms
The patient can be counseled regarding stool bulking, first with insoluble fiber supplementation and cessation of stool softeners if she is incontinent of liquid stool. If these measures are not effective, use of a constipating agent, such as loperamide, can improve stool consistency and thereby decrease incontinence episodes. PFPT with biofeedback can be offered as well. While typically we do not recommend initiating PFPT before 6 weeks postpartum, so the initial phases of healing can occur, early referral enables the patient to avoid a delay in access to care.
The patient also can be counseled about a referral to a pelvic floor specialist for further evaluation. A variety of peripartum pelvic floor disorder clinics are being established by Female Pelvic Medicine and Reconstructive Surgery (FPMRS) physicians. These clinics provide the benefit of comprehensive care for pelvic floor disorders in this unique population.
When conservative measures fail. If a patient has persistent bowel control issues despite conservative measures, a referral to an FPMRS physician should be initiated.
Delivery route in future pregnancies
The risk of a subsequent OASI is low. While this means that many women can safely pursue a future vaginal delivery, a scheduled cesarean delivery is indicated for women with persistent bowel control issues, wound healing complications, and those who experienced psychological trauma from their delivery.16 We recommend a shared-decision making approach, reviewing modifiable and nonmodifiable risk factors to help determine whether or not a future vaginal birth is appropriate. It is important to highlight that a cesarean delivery does not protect against fecal incontinence in women with a history of OASI; however, there is benefit in preventing worsening of anal incontinence, if present.17
CASE 4 Uterovaginal prolapse
A 36-year-old woman (G3P3) presents for her routine postpartum visit at 6 weeks after a spontaneous vaginal delivery without lacerations. She reports a persistent feeling of vaginal pressure and fullness. She thinks she felt a bulge with wiping after a bowel movement.
What options are available for this patient?
Prolapse in the peripartum population
Previous studies have revealed an increased prevalence of POP in pregnant women on examination compared with their nulligravid counterparts (47.6% vs 0%).18 With the changes in the hormonal milieu in pregnancy, as well as the weight of the gravid uterus on the pelvic floor, it is not surprising that pregnancy may be the inciting event to expose even transient defects in pelvic organ support.19
It is well established that increasing parity and, to a lesser extent, larger babies are associated with increased risk for future POP and surgery for prolapse. In the first year postpartum, nearly one-third of women have stage 2 or greater prolapse on exam, with studies demonstrating an increased prevalence of postpartum POP in women who delivered vaginally compared with those who delivered by cesarean.20,21
Initial evaluation
Diagnosis can be made during a routine pelvic exam by having the patient perform a Valsalva maneuver while in the lithotomy position. Using half of a speculum permits evaluation of the anterior and posterior vaginal walls separately, and Valsalva during a bimanual exam can aid in evaluating descensus of the uterus and cervix.
Excellent free patient education resources available online through the American Urogynecologic Society and the International Urogynecological Association can be used to direct counseling.
Continue to: Treatments you can offer for POP...
Treatments you can offer for POP
For pregnant or postpartum patients with bothersome prolapse, initial management options include pessary fitting and/or PFPT referral. In pregnancy, women often can be successfully fitted with a pessary for POP; however, as expulsion is a common issue, selection of a stiffer or space-occupying device may be more efficacious.
Often, early onset POP in pregnancy resolves as the gravid uterus lifts out of the pelvis in the second trimester, at which time the pessary can be discontinued. In the postpartum period, a pessary fitting can be undertaken similarly to that in nonpregnant patients. While data are lacking in the peripartum population, evidence supports the positive impact of PFPT on improving POP symptom bother.22 Additionally, for postpartum women who experience OASI, PFPT can produce significant improvement in subjective POP and associated bother.23
Impact of future childbearing wishes on treatment
The desire for future childbearing does not preclude treatment of patients experiencing bother from POP after conservative management options have failed. Both vaginal native tissue and mesh-augmented uterine-sparing repairs are performed by many FPMRS specialists and are associated with good outcomes. As with SUI, we do not recommend invasive treatment for POP during pregnancy or before 6 months postpartum.
In conclusion
Obstetric specialists play an essential role in caring for women with PFDs in the peripartum period. Basic evaluation, counseling, and management can be initiated using many of the resources already available in an obstetric ambulatory practice. Important adjunctive resources include those available for both providers and patients through the American Urogynecologic Society and the International Urogynecological Association. In addition, clinicians can partner with pelvic floor specialists through the growing number of FPMRS-run peripartum pelvic floor disorder clinics across the country and pelvic floor physical therapists.
If these specialty clinics and therapists are not available in your area, FPMRS specialists, urologists, gastroenterologists, and/or colorectal surgeons can aid in patient diagnosis and management to reach the ultimate goal of improving PFDs in this at-risk population. ●
- Madsen AM, Hickman LC, Propst K. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. Forthcoming 2021.
- Bodner-Adler B, Kimberger O, Laml T, et al. Prevalence and risk factors for pelvic floor disorders during early and late pregnancy in a cohort of Austrian women. Arch Gynecol Obstet. 2019;300:1325-1330.
- Swenson CW, DePorre JA, Haefner JK, et al. Postpartum depression screening and pelvic floor symptoms among women referred to a specialty postpartum perineal clinic. Am J Obstet Gynecol. 2018;218:335.e1-335.e6.
- Skinner EM, Dietz HP. Psychological and somatic sequelae of traumatic vaginal delivery: a literature review. Aust N Z J Obstet Gynaecol. 2015;55:309-314.
- Yohay D, Weintraub AY, Mauer-Perry N, et al. Prevalence and trends of pelvic floor disorders in late pregnancy and after delivery in a cohort of Israeli women using the PFDI-20. Eur J Obstet Gynecol Reprod Biol. 2016;200:35-39.
- Gregory WT, Sibai BM. Obstetrics and pelvic floor disorders. In: Walters M, Karram M, eds. Urogynecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Saunders; 2015:224-237.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Woodley SJ, Lawrenson P, Boyle R, et al. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;6:CD007471.
- Foldspang A, Hvidman L, Mommsen S, et al. Risk of postpartum urinary incontinence associated with pregnancy and mode of delivery. Acta Obstet Gynecol Scand. 2004;83:923-927.
- Wieslander CK, Weinstein MM, Handa V, et al. Pregnancy in women with prior treatments for pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2020;26:299-305.
- Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89:1511-1522.
- MacArthur C, Wilson D, Herbison P, et al; Prolong Study Group. Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG. 2016;123:1022-1029.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693-703
- Orkin BA, Sinykin SB, Lloyd PC. The digital rectal examination scoring system (DRESS). Dis Colon Rectum. 2010;53:1656-1660.
- UpToDate. Repair of episiotomy and perineal lacerations associated with childbirth. 2020. https://www-uptodate-com .ccmain.ohionet.org/contents/repair-of-perineal-and-other -lacerations-associated-with-childbirth?search=repair%20 episiotomy&source=search_result&selectedTitle=1~150&usa ge_type=default&display_rank=1. Accessed February 28, 2021.
- Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 198: prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2018;132:e87-e102.
- Jangö H, Langhoff-Roos J, Rosthøj S, et al. Long-term anal incontinence after obstetric anal sphincter injury—does grade of tear matter? Am J Obstet Gynecol. 2018;218:232.e1-232.e10.
- O’Boyle AL, Woodman PJ, O’Boyle JD, et al. Pelvic organ support in nulliparous pregnant and nonpregnant women: a case control study. Am J Obstet Gynecol. 2002;187:99-102.
- Handa VL, Blomquist JL, McDermott KC, et al. Pelvic floor disorders after vaginal birth. Obstet Gynecol. 2012;119 (2, pt 1):233-239.
- Handa VL, Nygaard I, Kenton K, et al; Pelvic Floor Disorders Network. Pelvic organ support among primiparous women in the first year after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1407-1411.
- O’Boyle AL, O’Boyle JD, Calhoun B, et al. Pelvic organ support in pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:69-72.
- Hagen S, Stark D, Glazener C, et al; POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet. 2014;383:796-806.
- Von Bargen E, Haviland MJ, Chang OH, et al. Evaluation of postpartum pelvic floor physical therapy on obstetrical anal sphincter injury: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020. doi: 10.1097/SPV.0000000000000849.
Pelvic floor disorders (PFDs) affect many pregnant and newly postpartum women. These conditions, including urinary incontinence, anal incontinence, and pelvic organ prolapse (POP), can be overshadowed by common pregnancy and postpartum concerns (TABLE 1).1 With the use of a few quick screening questions, however, PFDs easily can be identified in this at-risk population. Active management need not be delayed until after delivery for women experiencing bother, as options exist for women with PFDs during pregnancy as well as postpartum.

In this article, we discuss the common PFDs that obstetric clinicians face in the context of case scenarios and review how you can be better equipped to care for affected individuals.
CASE 1 Screening
A 30-year-old woman (G1P1) presents for her routine postpartum visit after an operative vaginal delivery with a second-degree laceration.
How would you screen this patient for PFDs?
Why screening for PFDs matters
While there are no validated PFD screening tools for this patient population, clinicians can ask a series of brief open-ended questions as part of the review of systems to efficiently evaluate for the common PFDs in peripartum patients (see “Screening questions to evaluate patients for peripartum pelvic floor disorders” below).
Pelvic floor disorders in the peripartum period can have a significant negative impact. In pregnancy, nearly half of women report psychological strain due to the presence of bowel, bladder, prolapse, or sexual dysfunction symptoms.2 Postpartum, PFDs have negative effects on overall health, well-being, and self-esteem, with significantly increased rates of postpartum depression in women who experience urinary incontinence.3,4 Proactively inquiring about PFD symptoms, providing anticipatory guidance, and recommending treatment options can positively impact a patient in multiple domains.
Sometimes during pregnancy or after having a baby, a woman experiences pelvic floor symptoms. Do you have any of the following?
- leakage with coughing, laughing, sneezing, or physical activity
- urgency to urinate or leakage due to urgency
- bulging or pressure within the vagina
- pain with intercourse
- accidental bowel leakage of stool or flatus
CASE 2 Stress urinary incontinence
A 27-year-old woman (G1P1) presents 2 months following spontaneous vaginal delivery with symptoms of urine leakage with laughing and running. Her urinary incontinence has been improving since delivery, but it continues to be bothersome.
What would you recommend for this patient?
Conservative SUI management strategies in pregnancy
Urinary tract symptoms are common in pregnancy, with up to 41.8% of women reporting urinary symptom distress in the third trimester.5 During pregnancy, estrogen and progesterone decrease urethral pressure that, together with increased intra-abdominal pressure from the gravid uterus, can cause or worsen stress urinary incontinence (SUI).6
During pregnancy, women should be offered conservative therapies for SUI. For women who can perform a pelvic floor contraction (a Kegel exercise), self-guided pelvic floor muscle exercises (PFMEs) may be helpful (see “Pelvic floor muscle exercises” below). We recommend that women start with 1 to 2 sets of 10 Kegel exercises per day and that they hold the squeeze for 2 to 3 seconds, working up to holding for 10 seconds. The goal is to strengthen and improve muscle control so that the Kegel squeeze can be paired with activities that cause SUI.
For women who are unable to perform a Kegel exercise or are not improving with a home PFME regimen, referral to pelvic floor physical therapy (PFPT) can be considered. While data support the efficacy of PFPT for SUI treatment in nonpregnant women,7 data are lacking on PFME in pregnancy.
In women without urinary incontinence, PFME in early pregnancy can prevent the onset of incontinence in late pregnancy and the postpartum period.8 By contrast, the same 2020 Cochrane Review found no evidence that antenatal pelvic floor muscle therapy in incontinent women decreases incontinence in mid- or late-pregnancy or in the postpartum period.8 As the quality of this evidence is very low and there is no evidence of harm with PFME, we continue to recommend it for women with bothersome SUI.
Incontinence pessaries or vaginal inserts (such as Poise Impressa bladder supports) can be helpful for SUI treatment. An incontinence pessary can be fitted in the office, and fitting kits are available for both. Pessaries can safely be used in pregnancy, but there are no data on the efficacy of pessaries for treating SUI in pregnancy. In nonpregnant women, evidence demonstrates 63% satisfaction 3 months post–pessary placement for SUI.7
We do not recommend invasive procedures for the treatment of SUI during pregnancy or in the first 6 months following delivery. There is no evidence that elective cesarean delivery prevents persistent SUI postpartum.9
To identify and engage the proper pelvic floor muscles:
- Insert a finger in the vagina and squeeze the vaginal muscles around your finger.
- Imagine you are sitting on a marble and have to pick it up with the vaginal muscles.
- Squeeze the muscles you would use to stop the flow of urine or hold back flatulence.
Perform sets of 10, 2 to 3 times per day as follows:
- Squeeze: Engage the pelvic floor muscles as described above; avoid performing Kegels while voiding.
- Hold: For 2 to 10 seconds; increase the duration to 10 seconds as able.
- Relax: Completely relax muscles before initating the next squeeze.
Reference
1. UpToDate. Patient education: pelvic muscle (Kegel) exercises (the basics). 2018. https://uptodatefree.ir/topic.htm?path=pelvic-muscle-kegel-exercises-the-basics. Accessed February 24, 2021.
Continue to: Managing SUI in the postpartum period...
Managing SUI in the postpartum period
After the first 6 months postpartum and exhaustion of conservative measures, we offer surgical interventions for women with persistent, bothersome incontinence. Surgery for SUI typically is not recommended until childbearing is complete, but it can be considered if the patient’s bother is significant.
For women with bothersome SUI who still desire future pregnancy, management options include periurethral bulking, a retropubic urethropexy (Burch procedure), or a midurethral sling procedure. Women who undergo an anti-incontinence procedure have an increased risk for urinary retention during a subsequent pregnancy.10 Most women with a midurethral sling will continue to be continent following an obstetric delivery.
Anticipatory guidance
At 3 months postpartum, the incidence of urinary incontinence is 6% to 40%, depending on parity and delivery type. Postpartum urinary incontinence is most common after instrumented vaginal delivery (32%) followed by spontaneous vaginal delivery (28%) and cesarean delivery (15%). The mean prevalence of any type of urinary incontinence is 33% at 3 months postpartum, and only small changes in the rate of urinary incontinence occur over the first postpartum year.11 While urinary incontinence is common postpartum, it should not be considered normal. We counsel that symptoms may improve spontaneously, but treatment can be initiated if the patient experiences significant bother.
A longitudinal cohort study that followed women from 3 months to 12 years postpartum found that, of women with urinary incontinence at 3 months postpartum, 76% continued to report incontinence at 12 years postpartum.12 We recommend that women be counseled that, even when symptoms resolve, they remain at increased risk for urinary incontinence in the future. Invasive therapies should be used to treat bothersome urinary incontinence, not to prevent future incontinence.
CASE 3 Fecal incontinence
A 24-year-old woman (G1P1) presents 3 weeks postpartum following a forceps-assisted vaginal delivery complicated by a 3c laceration. She reports fecal urgency, inability to control flatus, and once-daily fecal incontinence.
How would you evaluate these symptoms?
Steps in evaluation
The initial evaluation should include an inquiry regarding the patient’s stool consistency and bowel regimen. The Bristol stool form scale can be used to help patients describe their typical bowel movements (TABLE 2).13 During healing, the goal is to achieve a Bristol type 4 stool, both to avoid straining and to improve continence, as loose stool is the most difficult to control.

A physical examination can evaluate healing and sphincter integrity; it should include inspection of the distal vagina and perineal body and a digital rectal exam. Anal canal resting tone and squeeze strength should be evaluated, and the digital rectal examination scoring system (DRESS) can be useful for quantification (TABLE 3).14 Lack of tone at rest in the anterolateral portion of the sphincter complex can indicate an internal anal sphincter defect, as 80% of the resting tone comes from this muscle (FIGURE).15

The rectovaginal septum should be assessed given the increased risk of rectovaginal fistula in women with obstetric anal sphincter injury (OASI). The patient should be instructed to contract the anal sphincter, allowing evaluation of muscular contraction. Lack of contraction anteriolaterally may indicate external anal sphincter separation.
Continue to: Conservative options for improving fecal incontinence symptoms...
Conservative options for improving fecal incontinence symptoms
The patient can be counseled regarding stool bulking, first with insoluble fiber supplementation and cessation of stool softeners if she is incontinent of liquid stool. If these measures are not effective, use of a constipating agent, such as loperamide, can improve stool consistency and thereby decrease incontinence episodes. PFPT with biofeedback can be offered as well. While typically we do not recommend initiating PFPT before 6 weeks postpartum, so the initial phases of healing can occur, early referral enables the patient to avoid a delay in access to care.
The patient also can be counseled about a referral to a pelvic floor specialist for further evaluation. A variety of peripartum pelvic floor disorder clinics are being established by Female Pelvic Medicine and Reconstructive Surgery (FPMRS) physicians. These clinics provide the benefit of comprehensive care for pelvic floor disorders in this unique population.
When conservative measures fail. If a patient has persistent bowel control issues despite conservative measures, a referral to an FPMRS physician should be initiated.
Delivery route in future pregnancies
The risk of a subsequent OASI is low. While this means that many women can safely pursue a future vaginal delivery, a scheduled cesarean delivery is indicated for women with persistent bowel control issues, wound healing complications, and those who experienced psychological trauma from their delivery.16 We recommend a shared-decision making approach, reviewing modifiable and nonmodifiable risk factors to help determine whether or not a future vaginal birth is appropriate. It is important to highlight that a cesarean delivery does not protect against fecal incontinence in women with a history of OASI; however, there is benefit in preventing worsening of anal incontinence, if present.17
CASE 4 Uterovaginal prolapse
A 36-year-old woman (G3P3) presents for her routine postpartum visit at 6 weeks after a spontaneous vaginal delivery without lacerations. She reports a persistent feeling of vaginal pressure and fullness. She thinks she felt a bulge with wiping after a bowel movement.
What options are available for this patient?
Prolapse in the peripartum population
Previous studies have revealed an increased prevalence of POP in pregnant women on examination compared with their nulligravid counterparts (47.6% vs 0%).18 With the changes in the hormonal milieu in pregnancy, as well as the weight of the gravid uterus on the pelvic floor, it is not surprising that pregnancy may be the inciting event to expose even transient defects in pelvic organ support.19
It is well established that increasing parity and, to a lesser extent, larger babies are associated with increased risk for future POP and surgery for prolapse. In the first year postpartum, nearly one-third of women have stage 2 or greater prolapse on exam, with studies demonstrating an increased prevalence of postpartum POP in women who delivered vaginally compared with those who delivered by cesarean.20,21
Initial evaluation
Diagnosis can be made during a routine pelvic exam by having the patient perform a Valsalva maneuver while in the lithotomy position. Using half of a speculum permits evaluation of the anterior and posterior vaginal walls separately, and Valsalva during a bimanual exam can aid in evaluating descensus of the uterus and cervix.
Excellent free patient education resources available online through the American Urogynecologic Society and the International Urogynecological Association can be used to direct counseling.
Continue to: Treatments you can offer for POP...
Treatments you can offer for POP
For pregnant or postpartum patients with bothersome prolapse, initial management options include pessary fitting and/or PFPT referral. In pregnancy, women often can be successfully fitted with a pessary for POP; however, as expulsion is a common issue, selection of a stiffer or space-occupying device may be more efficacious.
Often, early onset POP in pregnancy resolves as the gravid uterus lifts out of the pelvis in the second trimester, at which time the pessary can be discontinued. In the postpartum period, a pessary fitting can be undertaken similarly to that in nonpregnant patients. While data are lacking in the peripartum population, evidence supports the positive impact of PFPT on improving POP symptom bother.22 Additionally, for postpartum women who experience OASI, PFPT can produce significant improvement in subjective POP and associated bother.23
Impact of future childbearing wishes on treatment
The desire for future childbearing does not preclude treatment of patients experiencing bother from POP after conservative management options have failed. Both vaginal native tissue and mesh-augmented uterine-sparing repairs are performed by many FPMRS specialists and are associated with good outcomes. As with SUI, we do not recommend invasive treatment for POP during pregnancy or before 6 months postpartum.
In conclusion
Obstetric specialists play an essential role in caring for women with PFDs in the peripartum period. Basic evaluation, counseling, and management can be initiated using many of the resources already available in an obstetric ambulatory practice. Important adjunctive resources include those available for both providers and patients through the American Urogynecologic Society and the International Urogynecological Association. In addition, clinicians can partner with pelvic floor specialists through the growing number of FPMRS-run peripartum pelvic floor disorder clinics across the country and pelvic floor physical therapists.
If these specialty clinics and therapists are not available in your area, FPMRS specialists, urologists, gastroenterologists, and/or colorectal surgeons can aid in patient diagnosis and management to reach the ultimate goal of improving PFDs in this at-risk population. ●
Pelvic floor disorders (PFDs) affect many pregnant and newly postpartum women. These conditions, including urinary incontinence, anal incontinence, and pelvic organ prolapse (POP), can be overshadowed by common pregnancy and postpartum concerns (TABLE 1).1 With the use of a few quick screening questions, however, PFDs easily can be identified in this at-risk population. Active management need not be delayed until after delivery for women experiencing bother, as options exist for women with PFDs during pregnancy as well as postpartum.

In this article, we discuss the common PFDs that obstetric clinicians face in the context of case scenarios and review how you can be better equipped to care for affected individuals.
CASE 1 Screening
A 30-year-old woman (G1P1) presents for her routine postpartum visit after an operative vaginal delivery with a second-degree laceration.
How would you screen this patient for PFDs?
Why screening for PFDs matters
While there are no validated PFD screening tools for this patient population, clinicians can ask a series of brief open-ended questions as part of the review of systems to efficiently evaluate for the common PFDs in peripartum patients (see “Screening questions to evaluate patients for peripartum pelvic floor disorders” below).
Pelvic floor disorders in the peripartum period can have a significant negative impact. In pregnancy, nearly half of women report psychological strain due to the presence of bowel, bladder, prolapse, or sexual dysfunction symptoms.2 Postpartum, PFDs have negative effects on overall health, well-being, and self-esteem, with significantly increased rates of postpartum depression in women who experience urinary incontinence.3,4 Proactively inquiring about PFD symptoms, providing anticipatory guidance, and recommending treatment options can positively impact a patient in multiple domains.
Sometimes during pregnancy or after having a baby, a woman experiences pelvic floor symptoms. Do you have any of the following?
- leakage with coughing, laughing, sneezing, or physical activity
- urgency to urinate or leakage due to urgency
- bulging or pressure within the vagina
- pain with intercourse
- accidental bowel leakage of stool or flatus
CASE 2 Stress urinary incontinence
A 27-year-old woman (G1P1) presents 2 months following spontaneous vaginal delivery with symptoms of urine leakage with laughing and running. Her urinary incontinence has been improving since delivery, but it continues to be bothersome.
What would you recommend for this patient?
Conservative SUI management strategies in pregnancy
Urinary tract symptoms are common in pregnancy, with up to 41.8% of women reporting urinary symptom distress in the third trimester.5 During pregnancy, estrogen and progesterone decrease urethral pressure that, together with increased intra-abdominal pressure from the gravid uterus, can cause or worsen stress urinary incontinence (SUI).6
During pregnancy, women should be offered conservative therapies for SUI. For women who can perform a pelvic floor contraction (a Kegel exercise), self-guided pelvic floor muscle exercises (PFMEs) may be helpful (see “Pelvic floor muscle exercises” below). We recommend that women start with 1 to 2 sets of 10 Kegel exercises per day and that they hold the squeeze for 2 to 3 seconds, working up to holding for 10 seconds. The goal is to strengthen and improve muscle control so that the Kegel squeeze can be paired with activities that cause SUI.
For women who are unable to perform a Kegel exercise or are not improving with a home PFME regimen, referral to pelvic floor physical therapy (PFPT) can be considered. While data support the efficacy of PFPT for SUI treatment in nonpregnant women,7 data are lacking on PFME in pregnancy.
In women without urinary incontinence, PFME in early pregnancy can prevent the onset of incontinence in late pregnancy and the postpartum period.8 By contrast, the same 2020 Cochrane Review found no evidence that antenatal pelvic floor muscle therapy in incontinent women decreases incontinence in mid- or late-pregnancy or in the postpartum period.8 As the quality of this evidence is very low and there is no evidence of harm with PFME, we continue to recommend it for women with bothersome SUI.
Incontinence pessaries or vaginal inserts (such as Poise Impressa bladder supports) can be helpful for SUI treatment. An incontinence pessary can be fitted in the office, and fitting kits are available for both. Pessaries can safely be used in pregnancy, but there are no data on the efficacy of pessaries for treating SUI in pregnancy. In nonpregnant women, evidence demonstrates 63% satisfaction 3 months post–pessary placement for SUI.7
We do not recommend invasive procedures for the treatment of SUI during pregnancy or in the first 6 months following delivery. There is no evidence that elective cesarean delivery prevents persistent SUI postpartum.9
To identify and engage the proper pelvic floor muscles:
- Insert a finger in the vagina and squeeze the vaginal muscles around your finger.
- Imagine you are sitting on a marble and have to pick it up with the vaginal muscles.
- Squeeze the muscles you would use to stop the flow of urine or hold back flatulence.
Perform sets of 10, 2 to 3 times per day as follows:
- Squeeze: Engage the pelvic floor muscles as described above; avoid performing Kegels while voiding.
- Hold: For 2 to 10 seconds; increase the duration to 10 seconds as able.
- Relax: Completely relax muscles before initating the next squeeze.
Reference
1. UpToDate. Patient education: pelvic muscle (Kegel) exercises (the basics). 2018. https://uptodatefree.ir/topic.htm?path=pelvic-muscle-kegel-exercises-the-basics. Accessed February 24, 2021.
Continue to: Managing SUI in the postpartum period...
Managing SUI in the postpartum period
After the first 6 months postpartum and exhaustion of conservative measures, we offer surgical interventions for women with persistent, bothersome incontinence. Surgery for SUI typically is not recommended until childbearing is complete, but it can be considered if the patient’s bother is significant.
For women with bothersome SUI who still desire future pregnancy, management options include periurethral bulking, a retropubic urethropexy (Burch procedure), or a midurethral sling procedure. Women who undergo an anti-incontinence procedure have an increased risk for urinary retention during a subsequent pregnancy.10 Most women with a midurethral sling will continue to be continent following an obstetric delivery.
Anticipatory guidance
At 3 months postpartum, the incidence of urinary incontinence is 6% to 40%, depending on parity and delivery type. Postpartum urinary incontinence is most common after instrumented vaginal delivery (32%) followed by spontaneous vaginal delivery (28%) and cesarean delivery (15%). The mean prevalence of any type of urinary incontinence is 33% at 3 months postpartum, and only small changes in the rate of urinary incontinence occur over the first postpartum year.11 While urinary incontinence is common postpartum, it should not be considered normal. We counsel that symptoms may improve spontaneously, but treatment can be initiated if the patient experiences significant bother.
A longitudinal cohort study that followed women from 3 months to 12 years postpartum found that, of women with urinary incontinence at 3 months postpartum, 76% continued to report incontinence at 12 years postpartum.12 We recommend that women be counseled that, even when symptoms resolve, they remain at increased risk for urinary incontinence in the future. Invasive therapies should be used to treat bothersome urinary incontinence, not to prevent future incontinence.
CASE 3 Fecal incontinence
A 24-year-old woman (G1P1) presents 3 weeks postpartum following a forceps-assisted vaginal delivery complicated by a 3c laceration. She reports fecal urgency, inability to control flatus, and once-daily fecal incontinence.
How would you evaluate these symptoms?
Steps in evaluation
The initial evaluation should include an inquiry regarding the patient’s stool consistency and bowel regimen. The Bristol stool form scale can be used to help patients describe their typical bowel movements (TABLE 2).13 During healing, the goal is to achieve a Bristol type 4 stool, both to avoid straining and to improve continence, as loose stool is the most difficult to control.

A physical examination can evaluate healing and sphincter integrity; it should include inspection of the distal vagina and perineal body and a digital rectal exam. Anal canal resting tone and squeeze strength should be evaluated, and the digital rectal examination scoring system (DRESS) can be useful for quantification (TABLE 3).14 Lack of tone at rest in the anterolateral portion of the sphincter complex can indicate an internal anal sphincter defect, as 80% of the resting tone comes from this muscle (FIGURE).15

The rectovaginal septum should be assessed given the increased risk of rectovaginal fistula in women with obstetric anal sphincter injury (OASI). The patient should be instructed to contract the anal sphincter, allowing evaluation of muscular contraction. Lack of contraction anteriolaterally may indicate external anal sphincter separation.
Continue to: Conservative options for improving fecal incontinence symptoms...
Conservative options for improving fecal incontinence symptoms
The patient can be counseled regarding stool bulking, first with insoluble fiber supplementation and cessation of stool softeners if she is incontinent of liquid stool. If these measures are not effective, use of a constipating agent, such as loperamide, can improve stool consistency and thereby decrease incontinence episodes. PFPT with biofeedback can be offered as well. While typically we do not recommend initiating PFPT before 6 weeks postpartum, so the initial phases of healing can occur, early referral enables the patient to avoid a delay in access to care.
The patient also can be counseled about a referral to a pelvic floor specialist for further evaluation. A variety of peripartum pelvic floor disorder clinics are being established by Female Pelvic Medicine and Reconstructive Surgery (FPMRS) physicians. These clinics provide the benefit of comprehensive care for pelvic floor disorders in this unique population.
When conservative measures fail. If a patient has persistent bowel control issues despite conservative measures, a referral to an FPMRS physician should be initiated.
Delivery route in future pregnancies
The risk of a subsequent OASI is low. While this means that many women can safely pursue a future vaginal delivery, a scheduled cesarean delivery is indicated for women with persistent bowel control issues, wound healing complications, and those who experienced psychological trauma from their delivery.16 We recommend a shared-decision making approach, reviewing modifiable and nonmodifiable risk factors to help determine whether or not a future vaginal birth is appropriate. It is important to highlight that a cesarean delivery does not protect against fecal incontinence in women with a history of OASI; however, there is benefit in preventing worsening of anal incontinence, if present.17
CASE 4 Uterovaginal prolapse
A 36-year-old woman (G3P3) presents for her routine postpartum visit at 6 weeks after a spontaneous vaginal delivery without lacerations. She reports a persistent feeling of vaginal pressure and fullness. She thinks she felt a bulge with wiping after a bowel movement.
What options are available for this patient?
Prolapse in the peripartum population
Previous studies have revealed an increased prevalence of POP in pregnant women on examination compared with their nulligravid counterparts (47.6% vs 0%).18 With the changes in the hormonal milieu in pregnancy, as well as the weight of the gravid uterus on the pelvic floor, it is not surprising that pregnancy may be the inciting event to expose even transient defects in pelvic organ support.19
It is well established that increasing parity and, to a lesser extent, larger babies are associated with increased risk for future POP and surgery for prolapse. In the first year postpartum, nearly one-third of women have stage 2 or greater prolapse on exam, with studies demonstrating an increased prevalence of postpartum POP in women who delivered vaginally compared with those who delivered by cesarean.20,21
Initial evaluation
Diagnosis can be made during a routine pelvic exam by having the patient perform a Valsalva maneuver while in the lithotomy position. Using half of a speculum permits evaluation of the anterior and posterior vaginal walls separately, and Valsalva during a bimanual exam can aid in evaluating descensus of the uterus and cervix.
Excellent free patient education resources available online through the American Urogynecologic Society and the International Urogynecological Association can be used to direct counseling.
Continue to: Treatments you can offer for POP...
Treatments you can offer for POP
For pregnant or postpartum patients with bothersome prolapse, initial management options include pessary fitting and/or PFPT referral. In pregnancy, women often can be successfully fitted with a pessary for POP; however, as expulsion is a common issue, selection of a stiffer or space-occupying device may be more efficacious.
Often, early onset POP in pregnancy resolves as the gravid uterus lifts out of the pelvis in the second trimester, at which time the pessary can be discontinued. In the postpartum period, a pessary fitting can be undertaken similarly to that in nonpregnant patients. While data are lacking in the peripartum population, evidence supports the positive impact of PFPT on improving POP symptom bother.22 Additionally, for postpartum women who experience OASI, PFPT can produce significant improvement in subjective POP and associated bother.23
Impact of future childbearing wishes on treatment
The desire for future childbearing does not preclude treatment of patients experiencing bother from POP after conservative management options have failed. Both vaginal native tissue and mesh-augmented uterine-sparing repairs are performed by many FPMRS specialists and are associated with good outcomes. As with SUI, we do not recommend invasive treatment for POP during pregnancy or before 6 months postpartum.
In conclusion
Obstetric specialists play an essential role in caring for women with PFDs in the peripartum period. Basic evaluation, counseling, and management can be initiated using many of the resources already available in an obstetric ambulatory practice. Important adjunctive resources include those available for both providers and patients through the American Urogynecologic Society and the International Urogynecological Association. In addition, clinicians can partner with pelvic floor specialists through the growing number of FPMRS-run peripartum pelvic floor disorder clinics across the country and pelvic floor physical therapists.
If these specialty clinics and therapists are not available in your area, FPMRS specialists, urologists, gastroenterologists, and/or colorectal surgeons can aid in patient diagnosis and management to reach the ultimate goal of improving PFDs in this at-risk population. ●
- Madsen AM, Hickman LC, Propst K. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. Forthcoming 2021.
- Bodner-Adler B, Kimberger O, Laml T, et al. Prevalence and risk factors for pelvic floor disorders during early and late pregnancy in a cohort of Austrian women. Arch Gynecol Obstet. 2019;300:1325-1330.
- Swenson CW, DePorre JA, Haefner JK, et al. Postpartum depression screening and pelvic floor symptoms among women referred to a specialty postpartum perineal clinic. Am J Obstet Gynecol. 2018;218:335.e1-335.e6.
- Skinner EM, Dietz HP. Psychological and somatic sequelae of traumatic vaginal delivery: a literature review. Aust N Z J Obstet Gynaecol. 2015;55:309-314.
- Yohay D, Weintraub AY, Mauer-Perry N, et al. Prevalence and trends of pelvic floor disorders in late pregnancy and after delivery in a cohort of Israeli women using the PFDI-20. Eur J Obstet Gynecol Reprod Biol. 2016;200:35-39.
- Gregory WT, Sibai BM. Obstetrics and pelvic floor disorders. In: Walters M, Karram M, eds. Urogynecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Saunders; 2015:224-237.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Woodley SJ, Lawrenson P, Boyle R, et al. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;6:CD007471.
- Foldspang A, Hvidman L, Mommsen S, et al. Risk of postpartum urinary incontinence associated with pregnancy and mode of delivery. Acta Obstet Gynecol Scand. 2004;83:923-927.
- Wieslander CK, Weinstein MM, Handa V, et al. Pregnancy in women with prior treatments for pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2020;26:299-305.
- Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89:1511-1522.
- MacArthur C, Wilson D, Herbison P, et al; Prolong Study Group. Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG. 2016;123:1022-1029.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693-703
- Orkin BA, Sinykin SB, Lloyd PC. The digital rectal examination scoring system (DRESS). Dis Colon Rectum. 2010;53:1656-1660.
- UpToDate. Repair of episiotomy and perineal lacerations associated with childbirth. 2020. https://www-uptodate-com .ccmain.ohionet.org/contents/repair-of-perineal-and-other -lacerations-associated-with-childbirth?search=repair%20 episiotomy&source=search_result&selectedTitle=1~150&usa ge_type=default&display_rank=1. Accessed February 28, 2021.
- Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 198: prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2018;132:e87-e102.
- Jangö H, Langhoff-Roos J, Rosthøj S, et al. Long-term anal incontinence after obstetric anal sphincter injury—does grade of tear matter? Am J Obstet Gynecol. 2018;218:232.e1-232.e10.
- O’Boyle AL, Woodman PJ, O’Boyle JD, et al. Pelvic organ support in nulliparous pregnant and nonpregnant women: a case control study. Am J Obstet Gynecol. 2002;187:99-102.
- Handa VL, Blomquist JL, McDermott KC, et al. Pelvic floor disorders after vaginal birth. Obstet Gynecol. 2012;119 (2, pt 1):233-239.
- Handa VL, Nygaard I, Kenton K, et al; Pelvic Floor Disorders Network. Pelvic organ support among primiparous women in the first year after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1407-1411.
- O’Boyle AL, O’Boyle JD, Calhoun B, et al. Pelvic organ support in pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:69-72.
- Hagen S, Stark D, Glazener C, et al; POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet. 2014;383:796-806.
- Von Bargen E, Haviland MJ, Chang OH, et al. Evaluation of postpartum pelvic floor physical therapy on obstetrical anal sphincter injury: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020. doi: 10.1097/SPV.0000000000000849.
- Madsen AM, Hickman LC, Propst K. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. Forthcoming 2021.
- Bodner-Adler B, Kimberger O, Laml T, et al. Prevalence and risk factors for pelvic floor disorders during early and late pregnancy in a cohort of Austrian women. Arch Gynecol Obstet. 2019;300:1325-1330.
- Swenson CW, DePorre JA, Haefner JK, et al. Postpartum depression screening and pelvic floor symptoms among women referred to a specialty postpartum perineal clinic. Am J Obstet Gynecol. 2018;218:335.e1-335.e6.
- Skinner EM, Dietz HP. Psychological and somatic sequelae of traumatic vaginal delivery: a literature review. Aust N Z J Obstet Gynaecol. 2015;55:309-314.
- Yohay D, Weintraub AY, Mauer-Perry N, et al. Prevalence and trends of pelvic floor disorders in late pregnancy and after delivery in a cohort of Israeli women using the PFDI-20. Eur J Obstet Gynecol Reprod Biol. 2016;200:35-39.
- Gregory WT, Sibai BM. Obstetrics and pelvic floor disorders. In: Walters M, Karram M, eds. Urogynecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Saunders; 2015:224-237.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Woodley SJ, Lawrenson P, Boyle R, et al. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;6:CD007471.
- Foldspang A, Hvidman L, Mommsen S, et al. Risk of postpartum urinary incontinence associated with pregnancy and mode of delivery. Acta Obstet Gynecol Scand. 2004;83:923-927.
- Wieslander CK, Weinstein MM, Handa V, et al. Pregnancy in women with prior treatments for pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2020;26:299-305.
- Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89:1511-1522.
- MacArthur C, Wilson D, Herbison P, et al; Prolong Study Group. Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG. 2016;123:1022-1029.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693-703
- Orkin BA, Sinykin SB, Lloyd PC. The digital rectal examination scoring system (DRESS). Dis Colon Rectum. 2010;53:1656-1660.
- UpToDate. Repair of episiotomy and perineal lacerations associated with childbirth. 2020. https://www-uptodate-com .ccmain.ohionet.org/contents/repair-of-perineal-and-other -lacerations-associated-with-childbirth?search=repair%20 episiotomy&source=search_result&selectedTitle=1~150&usa ge_type=default&display_rank=1. Accessed February 28, 2021.
- Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 198: prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2018;132:e87-e102.
- Jangö H, Langhoff-Roos J, Rosthøj S, et al. Long-term anal incontinence after obstetric anal sphincter injury—does grade of tear matter? Am J Obstet Gynecol. 2018;218:232.e1-232.e10.
- O’Boyle AL, Woodman PJ, O’Boyle JD, et al. Pelvic organ support in nulliparous pregnant and nonpregnant women: a case control study. Am J Obstet Gynecol. 2002;187:99-102.
- Handa VL, Blomquist JL, McDermott KC, et al. Pelvic floor disorders after vaginal birth. Obstet Gynecol. 2012;119 (2, pt 1):233-239.
- Handa VL, Nygaard I, Kenton K, et al; Pelvic Floor Disorders Network. Pelvic organ support among primiparous women in the first year after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1407-1411.
- O’Boyle AL, O’Boyle JD, Calhoun B, et al. Pelvic organ support in pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:69-72.
- Hagen S, Stark D, Glazener C, et al; POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet. 2014;383:796-806.
- Von Bargen E, Haviland MJ, Chang OH, et al. Evaluation of postpartum pelvic floor physical therapy on obstetrical anal sphincter injury: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020. doi: 10.1097/SPV.0000000000000849.
Pessaries for POP and SUI: Their fitting, care, and effectiveness in various disorders
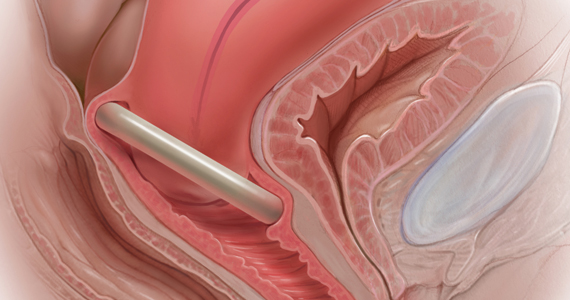
In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13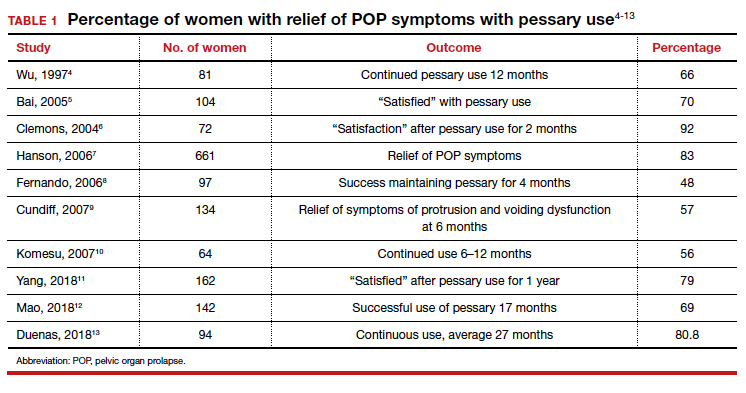
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17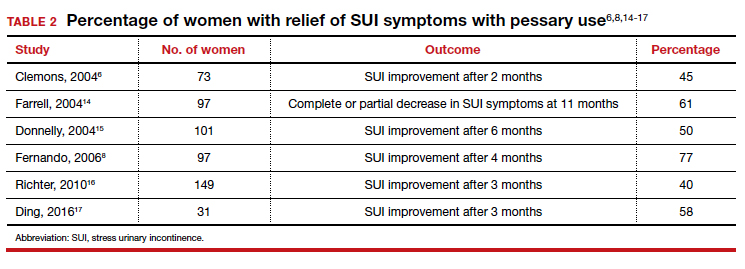
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33
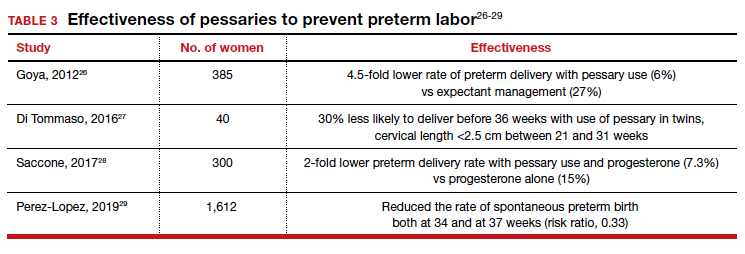
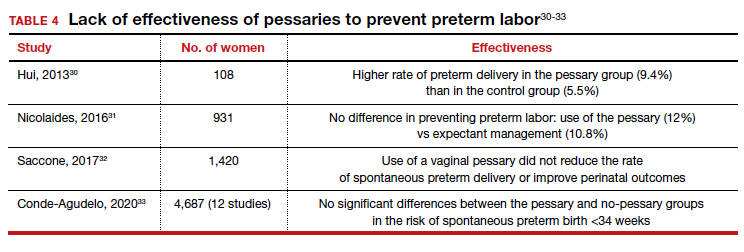
From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42
A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.

In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33


From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42



A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●

In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33


From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42



A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.