User login
Prazosin Outcomes in Older Veterans With Posttraumatic Stress Disorder
Posttraumatic stress disorder (PTSD) is a common psychiatric condition in the veteran population and is associated with significant sleep disturbances and trauma-related nightmares.1 PTSD can present with intrusive symptoms, such as recurrent memories or dreams, which are associated with traumatic events.2 Clinical studies have described an increase in central nervous system (CNS) noradrenergic activity in PTSD; specifically, noradrenergic outflow and/or postsynaptic adrenoreceptor responsiveness is increased.3,4 Targeting a reduction in noradrenergic activity via antagonism of noradrenergic receptors has been a therapeutic treatment strategy in PTSD.
Prazosin crosses the blood-brain barrier and works to antagonize α-1 adrenoreceptors to decrease noradrenergic outflow.5 It has been shown in multiple trials to effectively reduce nightmares and improve sleep quality in the veteran population.6-12 However, a recent negative trial contributed to a downgraded recommendation for prazosin in the treatment of PTSD-related nightmares in the joint PTSD guideline from the US Department of Veterans Affairs (VA) and US Department of Defense (DoD).13,14
The diagnosis of PTSD in veterans aged ≥ 65 years has been increasing due to improved recognition.15 As a result, prazosin may be considered more frequently as a treatment option for those patients who report PTSD-related nightmares. It is important to recognize that the normal physiologic process of aging is associated with increased noradrenergic outflow, which may change the pharmacodynamics of prazosin in geriatric patients.12,16 This may necessitate increased doses to adequately antagonize the α-1 adenoreceptor.17 High doses of prazosin may increase the risk of hypotension in older patients.12 This increased risk is especially concerning for patients who already receive multiple medications or have comorbid conditions that impact blood pressure (BP).
The existing literature has few studies that have reported on outcomes with prazosin use in older veterans.11,12 The few existing reports provide clinically valuable descriptions of tolerability and efficacy with prazosin. For example, Peskind and colleagues showed prazosin to be an effective agent in the treatment of PTSD-related nightmares.12 However, in older veterans prazosin dosing > 4 mg has not been described or reported in the literature.
There appears to be a lack of clinical guidance with regards to dosing of prazosin in older patients. The goal of the current study was to assess the outcomes of older veterans with PTSD under pharmacist management of prazosin at our outpatient Prazosin Titration Clinic (PTC) in order to contribute to the minimal, yet valuable, existing clinical literature.
Methods
This study was approved by the University of Iowa Institutional Review Board and Iowa City Veterans Affairs Health Care System (ICVAHCS) research and development committee. The study was a retrospective chart review of older patients with consultations referred to the ICVAHCS PTC. To be eligible for inclusion, veterans with a PTSD diagnosis must have been evaluated at an initial consult appointment with a mental health clinical pharmacy specialist (MH CPS) from February 1, 2016 to August 31, 2018, and had at least 1 follow-up appointment. Follow-up visits were conducted either by telephone or in a face-to-face clinic visit.
Prazosin Titration Clinics
VA health care systems use pharmacists to manage veterans prescribed prazosin through PTC consultations. PTCs provide a process for close follow-up and assessment of PTSD-related outcomes. Due to the frequency of follow-up, this service may be beneficial for older veterans with more complex comorbidities and medication regimens. Any veteran with PTSD-related nightmares may be referred to the PTC for a consultation by any health care provider. Once referred to the clinic, MH CPSs assume responsibility for the prazosin prescription, including dose adjustments. For example, if a veteran reported no issues with tolerability but continued to have frequent and distressing nightmares, the dose may be increased, typically by 1-mg to 2-mg increments. Once the veteran reaches a stable and tolerable dose of prazosin, they are discharged from the PTC, and the referring health care provider resumes responsibility for the prazosin prescription.
Clinically Measured Outcomes
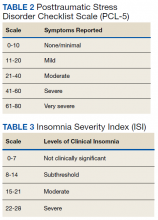
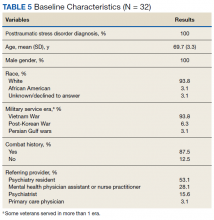
Nightmare frequency and intensity were measured using the Recurrent Distressing Dreams item B2 of the Clinician Administered PTSD Scale (CAPS) (Table 1). The PTSD Checklist (PCL-5), Insomnia Severity Index (ISI), and total sleep hours were used to determine the effect of prazosin on symptom severity (Table 2). The PCL-5 is a 20-item self-report used to monitor and quantify symptom level and change over time. It evaluates the frequency over the past month that a patient was bothered by any of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) PTSD criterion.2 Scores range from 0 (not at all) to 4 (extreme), with a maximum score of 80. The ISI is a 7-item self-report of sleep symptoms, with a total score of 28, where increasing scores indicate increasing severity of insomnia (Table 3).
Clinically measured outcome scales were performed and assessed by MH CPSs. CAPS frequency and intensity were measured at each clinic visit. PCL-5 and ISI scores were assessed at baseline and at the endpoint of study or discharge from clinic (Table 4). Patients who continued in the PTC after the end of the study date or who were lost to follow-up did not complete these measures at time of discharge.
Data Analysis
The primary outcome was change in CAPS nightmare frequency and intensity from time of initial clinic visit to time of discharge or end of study. The secondary outcomes included change in PCL-5, ISI, and sleep hours. Other secondary outcomes included measures of tolerability: BP changes, adverse effects (AEs) reported, and outcome of prazosin therapy when AEs were reported. Change in PTSD symptoms, PCL-5, and ISI were assessed using the Wilcoxon signed rank tests. Findings were considered to be statistically significant at P ≤ .05. Other variables were reported descriptively.
Results
Thirty-two veterans, aged ≥ 65 years, with clinical diagnosis of PTSD at the time of referral to the PTC were reviewed (Table 5). All patients were male and 93.8% were white. Thirty were Vietnam era veterans, 1 served in the Persian Gulf era, and 2 served in the post-Korean War era. Twenty-eight veterans had a combat history. Severe PTSD symptoms were reported as indicated by baseline PCL-5 scores, and moderate severity insomnia symptoms as indicated by baseline ISI scores.
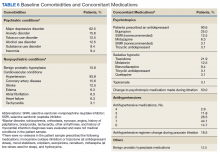
All veterans had at least 1 comorbid medical condition, and the majority had multiple medical comorbidities. All were taking multiple medical and psychiatric medications. More than 80% of veterans were taking antihypertensive agents at baseline (Table 6). Twenty-two of the 32 veterans were prescribed a VA/DoD PTSD guideline-recommended antidepressant.
Primary Outcomes
The baseline, final, and changes in the primary outcomes are included in the Figure. Treatment with prazosin was associated with significant improvement in median scores from baseline to endpoint for CAPS nightmare frequency (-2, P = .0001), CAPS nightmare intensity (-2, P = .001), and total CAPS item score (-4, P < .001).
Secondary Outcomes
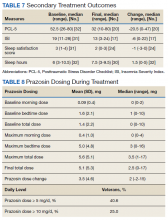
Of the 32 patients included in the study, PCL-5 was obtained from 20 veterans and ISI from 17 veterans at discharge from clinic. Thirty veterans reported final sleep hours, 2 veterans were unable to quantify average sleep hours per night at their final visit. PTSD symptom severity showed significant median change from baseline to endpoint of management in PTC for PCL-5 (-20.5, P = .0002) and ISI (-6.5, P = .002). Total sleep hours also showed significant improvement from baseline to endpoint (1.5, P = .003) (Table 7).
Prazosin Dosing
Maximum prazosin total daily doses were evaluated from the study baseline to the endpoint (Table 8). The mean (SD) maximum total daily dose of prazosin reached was 5.6 (5.1) mg (median, 3.5 mg; range, 1-17 mg). The mean (SD) total daily dose of prazosin at endpoint of study was 5.1 (5.3) mg (median, 2.5 mg; range, 0-17 mg). The average (SD) change of prazosin dose from baseline to endpoint was 3.5 (4.6) mg (median, 2 mg; range, -2 to 15 mg).
Tolerability
The average (SD) baseline systolic BP (SBP) was 135.8 (20.5) mm Hg and diastolic BP (DBP) was 77.2 (11.0) mm Hg. The average SBP and DBP at study endpoint were 131.8 (16.6) mm Hg and 75.9 (13.7) mm Hg, respectively. Endpoint BP values were missing for 6 patients.
Nine of 32 veterans reported AEs during PTC management of prazosin. Dizziness was the most common AE reported. Other AEs noted included orthostatic hypotension, headache, and falls. Of 12 reported AEs, 8 were related to dizziness, 5 of which were transient or tolerable. One veteran had a dose reduction of prazosin due to dizziness, and 3 veterans discontinued prazosin due to orthostasis. Several veterans had changes made to their antihypertensive medication regimen during prazosin titration, including dose reductions and/or decreased number of medications. If indicated, the MH CPS collaborated with the antihypertensive prescriber to make dosing adjustments. Two veterans reported a fall during prazosin titration; 1 veteran had other mobility-related factors thought to precipitate to their fall, and neither veterans were injured because of the falls.
Twenty-eight veterans (87.5%) treated in the PTC continued prazosin therapy after discharge. Six months postdischarge, 70% of veterans had maintained prazosin therapy. Two veterans required a dose increase postdischarge from PTC, and 1 veteran required a dose reduction. About one-third of veterans included in this study continued in the PTC beyond the end of the study period. Common reasons for clinic discharge were symptom resolution (37.5%), adverse reactions (12.5%), lost to follow-up (6.3%), or nonadherence (3.1%).
Discussion
The existing literature reports few outcomes for older veterans prescribed prazosin for PTSD. One report included a 75-year-old otherwise-healthy veteran, who received 2-mg prazosin at bedtime. At this dose, he reported good tolerability and response, as indicated by a reduction in his CAPS nightmare severity score.11 An open-label trial assessed prazosin in 9 geriatric men with chronic PTSD and found low-dose prazosin (average [SD] maximum prazosin dose reported was 2.3 [0.7] mg, range 2-4 mg per day) greatly reduced nightmares and overall PTSD severity in 8 of 9 subjects.12 Despite the veterans in that study having multiple medical comorbid conditions and taking concomitant medications, prazosin was reported to be well tolerated, and changes in BP were determined to be clinically insignificant.12 A recent study of middle-aged veterans (average [SD] age 52 [14] years) reported prazosin did not significantly alleviate PTSD-related nightmares.13 However, we observed prazosin therapy significantly reduced nightmares and sleep disturbances, and significantly improved PTSD severity in our older veteran population.
To our knowledge, the current study is the largest retrospective study that evaluates prazosin therapy for the treatment of PTSD-related nightmares in older veterans. The findings of this study are similar to a previous study in older veterans as well as studies of prazosin in younger and middle-aged adult veterans, with the average age ranging from 30 to 56 years.6-12 Like the previously reported studies, prazosin also was well tolerated in our sample of veterans with multiple comorbidities and concomitant medications. Changes in BP were not clinically significant.
Studies have demonstrated increased noradrenergic activity as a component of the normal aging process.16,17 This may require utilizing caution during prazosin dose titration and frequent patient assessment, due to the concern for risk of hypotension in older patients and in particular those who may require increased doses to achieve efficacy. In our study, favorable outcomes were achieved at an average (SD) total daily dose of 5.1 (5.3) mg (median, 2.5 mg; range 0-17 mg). A previous report showed efficacy of prazosin around an average (SD) maximum dose of 2.3 (0.7) mg, which is lower than the doses reported in the current study.12 In addition, 13 veterans (40.6%) from our sample reached doses of ≥ 5 mg per day, and 8 veterans (25.0%) reached doses of ≥ 10 mg per day.
The doses reached in this study were reflective of a management approach using assessment of patient-reported symptoms at weekly to biweekly follow-up visits. The individualized management approach applied in the PTC by MH CPSs aids in uncovering the most efficacious and tolerated dose of prazosin for each veteran. Evaluation of symptom change during treatment in PTC was facilitated use of objective rating scales, which helped measure nightmare frequency and intensity, sleep satisfaction, and global PTSD severity. Given the variability in dosing of prazosin reported in the literature, further studies may be warranted to provide more definitive clinical guidance as far as dosing prazosin in older patients.
The study by Peskind and colleaguesrationalized that lower doses of prazosin may be used in older patients given pharmacokinetic effects of aging, age-associated changes in PTSD pathophysiology, and effects and interactions of concomitant medications.12 However, our study found that prazosin could be well tolerated at higher doses. The rate of discontinuation due to intolerable AEs was low. AEs reported were consistent with the established AE profile of prazosin, with dizziness, orthostasis, and headache most commonly reported. Similar to the Peskind and colleagues study, BP had a tendency to decrease in this current study; however, the change was not clinically significant.12 That study also reported transient dizziness with prazosin titration, which was shown to be tolerable in the majority of our veterans reporting dizziness.12 Other common AEs with prazosin, such as rash, priapism, sedation, syncope, other cardiac AEs, and sleep disturbance were not reported in our study population.
MH CPS-managed PTCs are one venue that may allow veterans to achieve favorable outcomes through frequent follow-up. As prazosin dosing is specific to each individual patient, frequent follow-up visits are helpful in determining optimal doses that maximize efficacy while minimizing intolerable AEs. The majority of veterans treated in our PTC continued use of prazosin 6 months postdischarge, while 3 veterans required a postdischarge dose change.
The 2017 VA/DoD PTSD guidelines recommend individual, trauma-focused psychotherapy over pharmacologic therapy for the primary treatment of PTSD.14 About half of the veterans in the current study participated in either group or individual psychotherapy during enrollment in the PTC. A systematic review of psychotherapy in older veterans reported mixed results, with 4 studies indicating positive effects of therapy, while the other 3 studies reported no benefit or mixed effects for PTSD symptoms. The review concluded that fewer older adults experience complete remission of symptoms with psychotherapy alone.18 A previous study of older veterans described improvement in PTSD-related symptoms with prazosin without concurrent psychotherapy.12
Limitations and Strengths
While this study is the largest study to evaluate outcomes of prazosin in older patients with PTSD, there are several important limitations. The study population was small and all were male. The results of this study may not be applicable to women. Another limitation was several missing values in our data set, as some secondary outcomes were not collected via telephone follow-up visits. This could potentially contribute a measurement bias in the reported secondary outcomes results, specifically for the PCL-5 and ISI. Additionally, some veterans in this study may have reported symptomatic improvement based on the additional supportive intervention that clinical pharmacists were able to offer, as well as concomitant participation in psychotherapy. This may be reflected in the study results. This study did not have a true placebo group, as we may find a reduction in symptoms with placebo.
Strengths of this study include multiple data points for assessment of prazosin tolerability and a pre- and poststudy design, which allowed for the veterans to serve as their own control. Another strength of this study is that data were complete for primary outcome measures, including the CAPS Recurrent and Distressing Dreams Item, where prazosin showed significant benefit in reduction of PTSD-related nightmares. While the results of this study are reassuring, further randomized, double-blind, placebo-controlled trials are likely needed in order to establish efficacy and tolerability of prazosin in older veterans for PTSD related nightmares.
Conclusion
These results demonstrate prazosin therapy in older veterans can significantly improve PTSD-related nightmares and PTSD severity. Prazosin was well tolerated in this population at doses higher than previously reported in other studies. This study shows that prazosin therapy can be effectively managed and tolerated in older veterans with complex medical and psychiatric comorbidities to provide favorable patient outcomes.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Iowa City VA Health Care System and by the Health Services Research and Development Service, US Department of Veterans Affairs.
1. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697-707.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington VA: American Psychiatric Association; 2013.
3. Southwick SM, Krystal JH, Morgan CA, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50(4):266-274.
4. Geracioti TD Jr, Baker DG, Ekhator NN, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227-1230.
5. Friedman MJ. Posttraumatic and Acute Stress Disorders. 6th ed. New York: Springer Publishing; 2015.
6. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
8. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89-96.
10. Taylor HR, Freeman MK, Cates ME. Prazosin for treatment of nightmares related to posttraumatic stress disorder. Am J Health Syst Pharm. 2008;65(8):716-722.
11. Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
12. Peskind ER, Bonner LT, Hoff DJ, Raskind MA. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171.
13. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
14. The Management of Posttraumatic Stress Disorder Work Group. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0–2017. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal.pdf. Published June 2017. Accessed January 7, 2020.
15. Nichols BL, Czirr R. 24/Post-traumatic stress disorder: hidden syndrome in elders. Clin Gerontol. 1986;5(3-4):417-433.
16. Supiano MA, Linares OA, Smith MJ, Halter JB. Age-related differences in norepinephrine kinetics: effect of posture and sodium-restricted diet. Am J Physiol. 1990;259(3, pt 1):E422-E431.
17. Raskind MA, Peskind ER, Holmes C, Goldstein DS. Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer’s disease. Biol Psychiatry. 1999;46(6):756-765.
18. Dinnen S, Simiola V, Cook JM. Post-traumatic stress disorder in older adults: a systematic review of the psychotherapy treatment literature. Aging Ment Health. 2015;19(2):144-150.
Posttraumatic stress disorder (PTSD) is a common psychiatric condition in the veteran population and is associated with significant sleep disturbances and trauma-related nightmares.1 PTSD can present with intrusive symptoms, such as recurrent memories or dreams, which are associated with traumatic events.2 Clinical studies have described an increase in central nervous system (CNS) noradrenergic activity in PTSD; specifically, noradrenergic outflow and/or postsynaptic adrenoreceptor responsiveness is increased.3,4 Targeting a reduction in noradrenergic activity via antagonism of noradrenergic receptors has been a therapeutic treatment strategy in PTSD.
Prazosin crosses the blood-brain barrier and works to antagonize α-1 adrenoreceptors to decrease noradrenergic outflow.5 It has been shown in multiple trials to effectively reduce nightmares and improve sleep quality in the veteran population.6-12 However, a recent negative trial contributed to a downgraded recommendation for prazosin in the treatment of PTSD-related nightmares in the joint PTSD guideline from the US Department of Veterans Affairs (VA) and US Department of Defense (DoD).13,14
The diagnosis of PTSD in veterans aged ≥ 65 years has been increasing due to improved recognition.15 As a result, prazosin may be considered more frequently as a treatment option for those patients who report PTSD-related nightmares. It is important to recognize that the normal physiologic process of aging is associated with increased noradrenergic outflow, which may change the pharmacodynamics of prazosin in geriatric patients.12,16 This may necessitate increased doses to adequately antagonize the α-1 adenoreceptor.17 High doses of prazosin may increase the risk of hypotension in older patients.12 This increased risk is especially concerning for patients who already receive multiple medications or have comorbid conditions that impact blood pressure (BP).
The existing literature has few studies that have reported on outcomes with prazosin use in older veterans.11,12 The few existing reports provide clinically valuable descriptions of tolerability and efficacy with prazosin. For example, Peskind and colleagues showed prazosin to be an effective agent in the treatment of PTSD-related nightmares.12 However, in older veterans prazosin dosing > 4 mg has not been described or reported in the literature.
There appears to be a lack of clinical guidance with regards to dosing of prazosin in older patients. The goal of the current study was to assess the outcomes of older veterans with PTSD under pharmacist management of prazosin at our outpatient Prazosin Titration Clinic (PTC) in order to contribute to the minimal, yet valuable, existing clinical literature.
Methods
This study was approved by the University of Iowa Institutional Review Board and Iowa City Veterans Affairs Health Care System (ICVAHCS) research and development committee. The study was a retrospective chart review of older patients with consultations referred to the ICVAHCS PTC. To be eligible for inclusion, veterans with a PTSD diagnosis must have been evaluated at an initial consult appointment with a mental health clinical pharmacy specialist (MH CPS) from February 1, 2016 to August 31, 2018, and had at least 1 follow-up appointment. Follow-up visits were conducted either by telephone or in a face-to-face clinic visit.
Prazosin Titration Clinics
VA health care systems use pharmacists to manage veterans prescribed prazosin through PTC consultations. PTCs provide a process for close follow-up and assessment of PTSD-related outcomes. Due to the frequency of follow-up, this service may be beneficial for older veterans with more complex comorbidities and medication regimens. Any veteran with PTSD-related nightmares may be referred to the PTC for a consultation by any health care provider. Once referred to the clinic, MH CPSs assume responsibility for the prazosin prescription, including dose adjustments. For example, if a veteran reported no issues with tolerability but continued to have frequent and distressing nightmares, the dose may be increased, typically by 1-mg to 2-mg increments. Once the veteran reaches a stable and tolerable dose of prazosin, they are discharged from the PTC, and the referring health care provider resumes responsibility for the prazosin prescription.
Clinically Measured Outcomes
Nightmare frequency and intensity were measured using the Recurrent Distressing Dreams item B2 of the Clinician Administered PTSD Scale (CAPS) (Table 1). The PTSD Checklist (PCL-5), Insomnia Severity Index (ISI), and total sleep hours were used to determine the effect of prazosin on symptom severity (Table 2). The PCL-5 is a 20-item self-report used to monitor and quantify symptom level and change over time. It evaluates the frequency over the past month that a patient was bothered by any of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) PTSD criterion.2 Scores range from 0 (not at all) to 4 (extreme), with a maximum score of 80. The ISI is a 7-item self-report of sleep symptoms, with a total score of 28, where increasing scores indicate increasing severity of insomnia (Table 3).
Clinically measured outcome scales were performed and assessed by MH CPSs. CAPS frequency and intensity were measured at each clinic visit. PCL-5 and ISI scores were assessed at baseline and at the endpoint of study or discharge from clinic (Table 4). Patients who continued in the PTC after the end of the study date or who were lost to follow-up did not complete these measures at time of discharge.
Data Analysis
The primary outcome was change in CAPS nightmare frequency and intensity from time of initial clinic visit to time of discharge or end of study. The secondary outcomes included change in PCL-5, ISI, and sleep hours. Other secondary outcomes included measures of tolerability: BP changes, adverse effects (AEs) reported, and outcome of prazosin therapy when AEs were reported. Change in PTSD symptoms, PCL-5, and ISI were assessed using the Wilcoxon signed rank tests. Findings were considered to be statistically significant at P ≤ .05. Other variables were reported descriptively.
Results
Thirty-two veterans, aged ≥ 65 years, with clinical diagnosis of PTSD at the time of referral to the PTC were reviewed (Table 5). All patients were male and 93.8% were white. Thirty were Vietnam era veterans, 1 served in the Persian Gulf era, and 2 served in the post-Korean War era. Twenty-eight veterans had a combat history. Severe PTSD symptoms were reported as indicated by baseline PCL-5 scores, and moderate severity insomnia symptoms as indicated by baseline ISI scores.
All veterans had at least 1 comorbid medical condition, and the majority had multiple medical comorbidities. All were taking multiple medical and psychiatric medications. More than 80% of veterans were taking antihypertensive agents at baseline (Table 6). Twenty-two of the 32 veterans were prescribed a VA/DoD PTSD guideline-recommended antidepressant.
Primary Outcomes
The baseline, final, and changes in the primary outcomes are included in the Figure. Treatment with prazosin was associated with significant improvement in median scores from baseline to endpoint for CAPS nightmare frequency (-2, P = .0001), CAPS nightmare intensity (-2, P = .001), and total CAPS item score (-4, P < .001).
Secondary Outcomes
Of the 32 patients included in the study, PCL-5 was obtained from 20 veterans and ISI from 17 veterans at discharge from clinic. Thirty veterans reported final sleep hours, 2 veterans were unable to quantify average sleep hours per night at their final visit. PTSD symptom severity showed significant median change from baseline to endpoint of management in PTC for PCL-5 (-20.5, P = .0002) and ISI (-6.5, P = .002). Total sleep hours also showed significant improvement from baseline to endpoint (1.5, P = .003) (Table 7).
Prazosin Dosing
Maximum prazosin total daily doses were evaluated from the study baseline to the endpoint (Table 8). The mean (SD) maximum total daily dose of prazosin reached was 5.6 (5.1) mg (median, 3.5 mg; range, 1-17 mg). The mean (SD) total daily dose of prazosin at endpoint of study was 5.1 (5.3) mg (median, 2.5 mg; range, 0-17 mg). The average (SD) change of prazosin dose from baseline to endpoint was 3.5 (4.6) mg (median, 2 mg; range, -2 to 15 mg).
Tolerability
The average (SD) baseline systolic BP (SBP) was 135.8 (20.5) mm Hg and diastolic BP (DBP) was 77.2 (11.0) mm Hg. The average SBP and DBP at study endpoint were 131.8 (16.6) mm Hg and 75.9 (13.7) mm Hg, respectively. Endpoint BP values were missing for 6 patients.
Nine of 32 veterans reported AEs during PTC management of prazosin. Dizziness was the most common AE reported. Other AEs noted included orthostatic hypotension, headache, and falls. Of 12 reported AEs, 8 were related to dizziness, 5 of which were transient or tolerable. One veteran had a dose reduction of prazosin due to dizziness, and 3 veterans discontinued prazosin due to orthostasis. Several veterans had changes made to their antihypertensive medication regimen during prazosin titration, including dose reductions and/or decreased number of medications. If indicated, the MH CPS collaborated with the antihypertensive prescriber to make dosing adjustments. Two veterans reported a fall during prazosin titration; 1 veteran had other mobility-related factors thought to precipitate to their fall, and neither veterans were injured because of the falls.
Twenty-eight veterans (87.5%) treated in the PTC continued prazosin therapy after discharge. Six months postdischarge, 70% of veterans had maintained prazosin therapy. Two veterans required a dose increase postdischarge from PTC, and 1 veteran required a dose reduction. About one-third of veterans included in this study continued in the PTC beyond the end of the study period. Common reasons for clinic discharge were symptom resolution (37.5%), adverse reactions (12.5%), lost to follow-up (6.3%), or nonadherence (3.1%).
Discussion
The existing literature reports few outcomes for older veterans prescribed prazosin for PTSD. One report included a 75-year-old otherwise-healthy veteran, who received 2-mg prazosin at bedtime. At this dose, he reported good tolerability and response, as indicated by a reduction in his CAPS nightmare severity score.11 An open-label trial assessed prazosin in 9 geriatric men with chronic PTSD and found low-dose prazosin (average [SD] maximum prazosin dose reported was 2.3 [0.7] mg, range 2-4 mg per day) greatly reduced nightmares and overall PTSD severity in 8 of 9 subjects.12 Despite the veterans in that study having multiple medical comorbid conditions and taking concomitant medications, prazosin was reported to be well tolerated, and changes in BP were determined to be clinically insignificant.12 A recent study of middle-aged veterans (average [SD] age 52 [14] years) reported prazosin did not significantly alleviate PTSD-related nightmares.13 However, we observed prazosin therapy significantly reduced nightmares and sleep disturbances, and significantly improved PTSD severity in our older veteran population.
To our knowledge, the current study is the largest retrospective study that evaluates prazosin therapy for the treatment of PTSD-related nightmares in older veterans. The findings of this study are similar to a previous study in older veterans as well as studies of prazosin in younger and middle-aged adult veterans, with the average age ranging from 30 to 56 years.6-12 Like the previously reported studies, prazosin also was well tolerated in our sample of veterans with multiple comorbidities and concomitant medications. Changes in BP were not clinically significant.
Studies have demonstrated increased noradrenergic activity as a component of the normal aging process.16,17 This may require utilizing caution during prazosin dose titration and frequent patient assessment, due to the concern for risk of hypotension in older patients and in particular those who may require increased doses to achieve efficacy. In our study, favorable outcomes were achieved at an average (SD) total daily dose of 5.1 (5.3) mg (median, 2.5 mg; range 0-17 mg). A previous report showed efficacy of prazosin around an average (SD) maximum dose of 2.3 (0.7) mg, which is lower than the doses reported in the current study.12 In addition, 13 veterans (40.6%) from our sample reached doses of ≥ 5 mg per day, and 8 veterans (25.0%) reached doses of ≥ 10 mg per day.
The doses reached in this study were reflective of a management approach using assessment of patient-reported symptoms at weekly to biweekly follow-up visits. The individualized management approach applied in the PTC by MH CPSs aids in uncovering the most efficacious and tolerated dose of prazosin for each veteran. Evaluation of symptom change during treatment in PTC was facilitated use of objective rating scales, which helped measure nightmare frequency and intensity, sleep satisfaction, and global PTSD severity. Given the variability in dosing of prazosin reported in the literature, further studies may be warranted to provide more definitive clinical guidance as far as dosing prazosin in older patients.
The study by Peskind and colleaguesrationalized that lower doses of prazosin may be used in older patients given pharmacokinetic effects of aging, age-associated changes in PTSD pathophysiology, and effects and interactions of concomitant medications.12 However, our study found that prazosin could be well tolerated at higher doses. The rate of discontinuation due to intolerable AEs was low. AEs reported were consistent with the established AE profile of prazosin, with dizziness, orthostasis, and headache most commonly reported. Similar to the Peskind and colleagues study, BP had a tendency to decrease in this current study; however, the change was not clinically significant.12 That study also reported transient dizziness with prazosin titration, which was shown to be tolerable in the majority of our veterans reporting dizziness.12 Other common AEs with prazosin, such as rash, priapism, sedation, syncope, other cardiac AEs, and sleep disturbance were not reported in our study population.
MH CPS-managed PTCs are one venue that may allow veterans to achieve favorable outcomes through frequent follow-up. As prazosin dosing is specific to each individual patient, frequent follow-up visits are helpful in determining optimal doses that maximize efficacy while minimizing intolerable AEs. The majority of veterans treated in our PTC continued use of prazosin 6 months postdischarge, while 3 veterans required a postdischarge dose change.
The 2017 VA/DoD PTSD guidelines recommend individual, trauma-focused psychotherapy over pharmacologic therapy for the primary treatment of PTSD.14 About half of the veterans in the current study participated in either group or individual psychotherapy during enrollment in the PTC. A systematic review of psychotherapy in older veterans reported mixed results, with 4 studies indicating positive effects of therapy, while the other 3 studies reported no benefit or mixed effects for PTSD symptoms. The review concluded that fewer older adults experience complete remission of symptoms with psychotherapy alone.18 A previous study of older veterans described improvement in PTSD-related symptoms with prazosin without concurrent psychotherapy.12
Limitations and Strengths
While this study is the largest study to evaluate outcomes of prazosin in older patients with PTSD, there are several important limitations. The study population was small and all were male. The results of this study may not be applicable to women. Another limitation was several missing values in our data set, as some secondary outcomes were not collected via telephone follow-up visits. This could potentially contribute a measurement bias in the reported secondary outcomes results, specifically for the PCL-5 and ISI. Additionally, some veterans in this study may have reported symptomatic improvement based on the additional supportive intervention that clinical pharmacists were able to offer, as well as concomitant participation in psychotherapy. This may be reflected in the study results. This study did not have a true placebo group, as we may find a reduction in symptoms with placebo.
Strengths of this study include multiple data points for assessment of prazosin tolerability and a pre- and poststudy design, which allowed for the veterans to serve as their own control. Another strength of this study is that data were complete for primary outcome measures, including the CAPS Recurrent and Distressing Dreams Item, where prazosin showed significant benefit in reduction of PTSD-related nightmares. While the results of this study are reassuring, further randomized, double-blind, placebo-controlled trials are likely needed in order to establish efficacy and tolerability of prazosin in older veterans for PTSD related nightmares.
Conclusion
These results demonstrate prazosin therapy in older veterans can significantly improve PTSD-related nightmares and PTSD severity. Prazosin was well tolerated in this population at doses higher than previously reported in other studies. This study shows that prazosin therapy can be effectively managed and tolerated in older veterans with complex medical and psychiatric comorbidities to provide favorable patient outcomes.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Iowa City VA Health Care System and by the Health Services Research and Development Service, US Department of Veterans Affairs.
Posttraumatic stress disorder (PTSD) is a common psychiatric condition in the veteran population and is associated with significant sleep disturbances and trauma-related nightmares.1 PTSD can present with intrusive symptoms, such as recurrent memories or dreams, which are associated with traumatic events.2 Clinical studies have described an increase in central nervous system (CNS) noradrenergic activity in PTSD; specifically, noradrenergic outflow and/or postsynaptic adrenoreceptor responsiveness is increased.3,4 Targeting a reduction in noradrenergic activity via antagonism of noradrenergic receptors has been a therapeutic treatment strategy in PTSD.
Prazosin crosses the blood-brain barrier and works to antagonize α-1 adrenoreceptors to decrease noradrenergic outflow.5 It has been shown in multiple trials to effectively reduce nightmares and improve sleep quality in the veteran population.6-12 However, a recent negative trial contributed to a downgraded recommendation for prazosin in the treatment of PTSD-related nightmares in the joint PTSD guideline from the US Department of Veterans Affairs (VA) and US Department of Defense (DoD).13,14
The diagnosis of PTSD in veterans aged ≥ 65 years has been increasing due to improved recognition.15 As a result, prazosin may be considered more frequently as a treatment option for those patients who report PTSD-related nightmares. It is important to recognize that the normal physiologic process of aging is associated with increased noradrenergic outflow, which may change the pharmacodynamics of prazosin in geriatric patients.12,16 This may necessitate increased doses to adequately antagonize the α-1 adenoreceptor.17 High doses of prazosin may increase the risk of hypotension in older patients.12 This increased risk is especially concerning for patients who already receive multiple medications or have comorbid conditions that impact blood pressure (BP).
The existing literature has few studies that have reported on outcomes with prazosin use in older veterans.11,12 The few existing reports provide clinically valuable descriptions of tolerability and efficacy with prazosin. For example, Peskind and colleagues showed prazosin to be an effective agent in the treatment of PTSD-related nightmares.12 However, in older veterans prazosin dosing > 4 mg has not been described or reported in the literature.
There appears to be a lack of clinical guidance with regards to dosing of prazosin in older patients. The goal of the current study was to assess the outcomes of older veterans with PTSD under pharmacist management of prazosin at our outpatient Prazosin Titration Clinic (PTC) in order to contribute to the minimal, yet valuable, existing clinical literature.
Methods
This study was approved by the University of Iowa Institutional Review Board and Iowa City Veterans Affairs Health Care System (ICVAHCS) research and development committee. The study was a retrospective chart review of older patients with consultations referred to the ICVAHCS PTC. To be eligible for inclusion, veterans with a PTSD diagnosis must have been evaluated at an initial consult appointment with a mental health clinical pharmacy specialist (MH CPS) from February 1, 2016 to August 31, 2018, and had at least 1 follow-up appointment. Follow-up visits were conducted either by telephone or in a face-to-face clinic visit.
Prazosin Titration Clinics
VA health care systems use pharmacists to manage veterans prescribed prazosin through PTC consultations. PTCs provide a process for close follow-up and assessment of PTSD-related outcomes. Due to the frequency of follow-up, this service may be beneficial for older veterans with more complex comorbidities and medication regimens. Any veteran with PTSD-related nightmares may be referred to the PTC for a consultation by any health care provider. Once referred to the clinic, MH CPSs assume responsibility for the prazosin prescription, including dose adjustments. For example, if a veteran reported no issues with tolerability but continued to have frequent and distressing nightmares, the dose may be increased, typically by 1-mg to 2-mg increments. Once the veteran reaches a stable and tolerable dose of prazosin, they are discharged from the PTC, and the referring health care provider resumes responsibility for the prazosin prescription.
Clinically Measured Outcomes
Nightmare frequency and intensity were measured using the Recurrent Distressing Dreams item B2 of the Clinician Administered PTSD Scale (CAPS) (Table 1). The PTSD Checklist (PCL-5), Insomnia Severity Index (ISI), and total sleep hours were used to determine the effect of prazosin on symptom severity (Table 2). The PCL-5 is a 20-item self-report used to monitor and quantify symptom level and change over time. It evaluates the frequency over the past month that a patient was bothered by any of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) PTSD criterion.2 Scores range from 0 (not at all) to 4 (extreme), with a maximum score of 80. The ISI is a 7-item self-report of sleep symptoms, with a total score of 28, where increasing scores indicate increasing severity of insomnia (Table 3).
Clinically measured outcome scales were performed and assessed by MH CPSs. CAPS frequency and intensity were measured at each clinic visit. PCL-5 and ISI scores were assessed at baseline and at the endpoint of study or discharge from clinic (Table 4). Patients who continued in the PTC after the end of the study date or who were lost to follow-up did not complete these measures at time of discharge.
Data Analysis
The primary outcome was change in CAPS nightmare frequency and intensity from time of initial clinic visit to time of discharge or end of study. The secondary outcomes included change in PCL-5, ISI, and sleep hours. Other secondary outcomes included measures of tolerability: BP changes, adverse effects (AEs) reported, and outcome of prazosin therapy when AEs were reported. Change in PTSD symptoms, PCL-5, and ISI were assessed using the Wilcoxon signed rank tests. Findings were considered to be statistically significant at P ≤ .05. Other variables were reported descriptively.
Results
Thirty-two veterans, aged ≥ 65 years, with clinical diagnosis of PTSD at the time of referral to the PTC were reviewed (Table 5). All patients were male and 93.8% were white. Thirty were Vietnam era veterans, 1 served in the Persian Gulf era, and 2 served in the post-Korean War era. Twenty-eight veterans had a combat history. Severe PTSD symptoms were reported as indicated by baseline PCL-5 scores, and moderate severity insomnia symptoms as indicated by baseline ISI scores.
All veterans had at least 1 comorbid medical condition, and the majority had multiple medical comorbidities. All were taking multiple medical and psychiatric medications. More than 80% of veterans were taking antihypertensive agents at baseline (Table 6). Twenty-two of the 32 veterans were prescribed a VA/DoD PTSD guideline-recommended antidepressant.
Primary Outcomes
The baseline, final, and changes in the primary outcomes are included in the Figure. Treatment with prazosin was associated with significant improvement in median scores from baseline to endpoint for CAPS nightmare frequency (-2, P = .0001), CAPS nightmare intensity (-2, P = .001), and total CAPS item score (-4, P < .001).
Secondary Outcomes
Of the 32 patients included in the study, PCL-5 was obtained from 20 veterans and ISI from 17 veterans at discharge from clinic. Thirty veterans reported final sleep hours, 2 veterans were unable to quantify average sleep hours per night at their final visit. PTSD symptom severity showed significant median change from baseline to endpoint of management in PTC for PCL-5 (-20.5, P = .0002) and ISI (-6.5, P = .002). Total sleep hours also showed significant improvement from baseline to endpoint (1.5, P = .003) (Table 7).
Prazosin Dosing
Maximum prazosin total daily doses were evaluated from the study baseline to the endpoint (Table 8). The mean (SD) maximum total daily dose of prazosin reached was 5.6 (5.1) mg (median, 3.5 mg; range, 1-17 mg). The mean (SD) total daily dose of prazosin at endpoint of study was 5.1 (5.3) mg (median, 2.5 mg; range, 0-17 mg). The average (SD) change of prazosin dose from baseline to endpoint was 3.5 (4.6) mg (median, 2 mg; range, -2 to 15 mg).
Tolerability
The average (SD) baseline systolic BP (SBP) was 135.8 (20.5) mm Hg and diastolic BP (DBP) was 77.2 (11.0) mm Hg. The average SBP and DBP at study endpoint were 131.8 (16.6) mm Hg and 75.9 (13.7) mm Hg, respectively. Endpoint BP values were missing for 6 patients.
Nine of 32 veterans reported AEs during PTC management of prazosin. Dizziness was the most common AE reported. Other AEs noted included orthostatic hypotension, headache, and falls. Of 12 reported AEs, 8 were related to dizziness, 5 of which were transient or tolerable. One veteran had a dose reduction of prazosin due to dizziness, and 3 veterans discontinued prazosin due to orthostasis. Several veterans had changes made to their antihypertensive medication regimen during prazosin titration, including dose reductions and/or decreased number of medications. If indicated, the MH CPS collaborated with the antihypertensive prescriber to make dosing adjustments. Two veterans reported a fall during prazosin titration; 1 veteran had other mobility-related factors thought to precipitate to their fall, and neither veterans were injured because of the falls.
Twenty-eight veterans (87.5%) treated in the PTC continued prazosin therapy after discharge. Six months postdischarge, 70% of veterans had maintained prazosin therapy. Two veterans required a dose increase postdischarge from PTC, and 1 veteran required a dose reduction. About one-third of veterans included in this study continued in the PTC beyond the end of the study period. Common reasons for clinic discharge were symptom resolution (37.5%), adverse reactions (12.5%), lost to follow-up (6.3%), or nonadherence (3.1%).
Discussion
The existing literature reports few outcomes for older veterans prescribed prazosin for PTSD. One report included a 75-year-old otherwise-healthy veteran, who received 2-mg prazosin at bedtime. At this dose, he reported good tolerability and response, as indicated by a reduction in his CAPS nightmare severity score.11 An open-label trial assessed prazosin in 9 geriatric men with chronic PTSD and found low-dose prazosin (average [SD] maximum prazosin dose reported was 2.3 [0.7] mg, range 2-4 mg per day) greatly reduced nightmares and overall PTSD severity in 8 of 9 subjects.12 Despite the veterans in that study having multiple medical comorbid conditions and taking concomitant medications, prazosin was reported to be well tolerated, and changes in BP were determined to be clinically insignificant.12 A recent study of middle-aged veterans (average [SD] age 52 [14] years) reported prazosin did not significantly alleviate PTSD-related nightmares.13 However, we observed prazosin therapy significantly reduced nightmares and sleep disturbances, and significantly improved PTSD severity in our older veteran population.
To our knowledge, the current study is the largest retrospective study that evaluates prazosin therapy for the treatment of PTSD-related nightmares in older veterans. The findings of this study are similar to a previous study in older veterans as well as studies of prazosin in younger and middle-aged adult veterans, with the average age ranging from 30 to 56 years.6-12 Like the previously reported studies, prazosin also was well tolerated in our sample of veterans with multiple comorbidities and concomitant medications. Changes in BP were not clinically significant.
Studies have demonstrated increased noradrenergic activity as a component of the normal aging process.16,17 This may require utilizing caution during prazosin dose titration and frequent patient assessment, due to the concern for risk of hypotension in older patients and in particular those who may require increased doses to achieve efficacy. In our study, favorable outcomes were achieved at an average (SD) total daily dose of 5.1 (5.3) mg (median, 2.5 mg; range 0-17 mg). A previous report showed efficacy of prazosin around an average (SD) maximum dose of 2.3 (0.7) mg, which is lower than the doses reported in the current study.12 In addition, 13 veterans (40.6%) from our sample reached doses of ≥ 5 mg per day, and 8 veterans (25.0%) reached doses of ≥ 10 mg per day.
The doses reached in this study were reflective of a management approach using assessment of patient-reported symptoms at weekly to biweekly follow-up visits. The individualized management approach applied in the PTC by MH CPSs aids in uncovering the most efficacious and tolerated dose of prazosin for each veteran. Evaluation of symptom change during treatment in PTC was facilitated use of objective rating scales, which helped measure nightmare frequency and intensity, sleep satisfaction, and global PTSD severity. Given the variability in dosing of prazosin reported in the literature, further studies may be warranted to provide more definitive clinical guidance as far as dosing prazosin in older patients.
The study by Peskind and colleaguesrationalized that lower doses of prazosin may be used in older patients given pharmacokinetic effects of aging, age-associated changes in PTSD pathophysiology, and effects and interactions of concomitant medications.12 However, our study found that prazosin could be well tolerated at higher doses. The rate of discontinuation due to intolerable AEs was low. AEs reported were consistent with the established AE profile of prazosin, with dizziness, orthostasis, and headache most commonly reported. Similar to the Peskind and colleagues study, BP had a tendency to decrease in this current study; however, the change was not clinically significant.12 That study also reported transient dizziness with prazosin titration, which was shown to be tolerable in the majority of our veterans reporting dizziness.12 Other common AEs with prazosin, such as rash, priapism, sedation, syncope, other cardiac AEs, and sleep disturbance were not reported in our study population.
MH CPS-managed PTCs are one venue that may allow veterans to achieve favorable outcomes through frequent follow-up. As prazosin dosing is specific to each individual patient, frequent follow-up visits are helpful in determining optimal doses that maximize efficacy while minimizing intolerable AEs. The majority of veterans treated in our PTC continued use of prazosin 6 months postdischarge, while 3 veterans required a postdischarge dose change.
The 2017 VA/DoD PTSD guidelines recommend individual, trauma-focused psychotherapy over pharmacologic therapy for the primary treatment of PTSD.14 About half of the veterans in the current study participated in either group or individual psychotherapy during enrollment in the PTC. A systematic review of psychotherapy in older veterans reported mixed results, with 4 studies indicating positive effects of therapy, while the other 3 studies reported no benefit or mixed effects for PTSD symptoms. The review concluded that fewer older adults experience complete remission of symptoms with psychotherapy alone.18 A previous study of older veterans described improvement in PTSD-related symptoms with prazosin without concurrent psychotherapy.12
Limitations and Strengths
While this study is the largest study to evaluate outcomes of prazosin in older patients with PTSD, there are several important limitations. The study population was small and all were male. The results of this study may not be applicable to women. Another limitation was several missing values in our data set, as some secondary outcomes were not collected via telephone follow-up visits. This could potentially contribute a measurement bias in the reported secondary outcomes results, specifically for the PCL-5 and ISI. Additionally, some veterans in this study may have reported symptomatic improvement based on the additional supportive intervention that clinical pharmacists were able to offer, as well as concomitant participation in psychotherapy. This may be reflected in the study results. This study did not have a true placebo group, as we may find a reduction in symptoms with placebo.
Strengths of this study include multiple data points for assessment of prazosin tolerability and a pre- and poststudy design, which allowed for the veterans to serve as their own control. Another strength of this study is that data were complete for primary outcome measures, including the CAPS Recurrent and Distressing Dreams Item, where prazosin showed significant benefit in reduction of PTSD-related nightmares. While the results of this study are reassuring, further randomized, double-blind, placebo-controlled trials are likely needed in order to establish efficacy and tolerability of prazosin in older veterans for PTSD related nightmares.
Conclusion
These results demonstrate prazosin therapy in older veterans can significantly improve PTSD-related nightmares and PTSD severity. Prazosin was well tolerated in this population at doses higher than previously reported in other studies. This study shows that prazosin therapy can be effectively managed and tolerated in older veterans with complex medical and psychiatric comorbidities to provide favorable patient outcomes.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Iowa City VA Health Care System and by the Health Services Research and Development Service, US Department of Veterans Affairs.
1. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697-707.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington VA: American Psychiatric Association; 2013.
3. Southwick SM, Krystal JH, Morgan CA, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50(4):266-274.
4. Geracioti TD Jr, Baker DG, Ekhator NN, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227-1230.
5. Friedman MJ. Posttraumatic and Acute Stress Disorders. 6th ed. New York: Springer Publishing; 2015.
6. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
8. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89-96.
10. Taylor HR, Freeman MK, Cates ME. Prazosin for treatment of nightmares related to posttraumatic stress disorder. Am J Health Syst Pharm. 2008;65(8):716-722.
11. Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
12. Peskind ER, Bonner LT, Hoff DJ, Raskind MA. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171.
13. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
14. The Management of Posttraumatic Stress Disorder Work Group. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0–2017. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal.pdf. Published June 2017. Accessed January 7, 2020.
15. Nichols BL, Czirr R. 24/Post-traumatic stress disorder: hidden syndrome in elders. Clin Gerontol. 1986;5(3-4):417-433.
16. Supiano MA, Linares OA, Smith MJ, Halter JB. Age-related differences in norepinephrine kinetics: effect of posture and sodium-restricted diet. Am J Physiol. 1990;259(3, pt 1):E422-E431.
17. Raskind MA, Peskind ER, Holmes C, Goldstein DS. Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer’s disease. Biol Psychiatry. 1999;46(6):756-765.
18. Dinnen S, Simiola V, Cook JM. Post-traumatic stress disorder in older adults: a systematic review of the psychotherapy treatment literature. Aging Ment Health. 2015;19(2):144-150.
1. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697-707.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington VA: American Psychiatric Association; 2013.
3. Southwick SM, Krystal JH, Morgan CA, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50(4):266-274.
4. Geracioti TD Jr, Baker DG, Ekhator NN, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227-1230.
5. Friedman MJ. Posttraumatic and Acute Stress Disorders. 6th ed. New York: Springer Publishing; 2015.
6. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
8. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89-96.
10. Taylor HR, Freeman MK, Cates ME. Prazosin for treatment of nightmares related to posttraumatic stress disorder. Am J Health Syst Pharm. 2008;65(8):716-722.
11. Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
12. Peskind ER, Bonner LT, Hoff DJ, Raskind MA. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171.
13. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
14. The Management of Posttraumatic Stress Disorder Work Group. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0–2017. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal.pdf. Published June 2017. Accessed January 7, 2020.
15. Nichols BL, Czirr R. 24/Post-traumatic stress disorder: hidden syndrome in elders. Clin Gerontol. 1986;5(3-4):417-433.
16. Supiano MA, Linares OA, Smith MJ, Halter JB. Age-related differences in norepinephrine kinetics: effect of posture and sodium-restricted diet. Am J Physiol. 1990;259(3, pt 1):E422-E431.
17. Raskind MA, Peskind ER, Holmes C, Goldstein DS. Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer’s disease. Biol Psychiatry. 1999;46(6):756-765.
18. Dinnen S, Simiola V, Cook JM. Post-traumatic stress disorder in older adults: a systematic review of the psychotherapy treatment literature. Aging Ment Health. 2015;19(2):144-150.
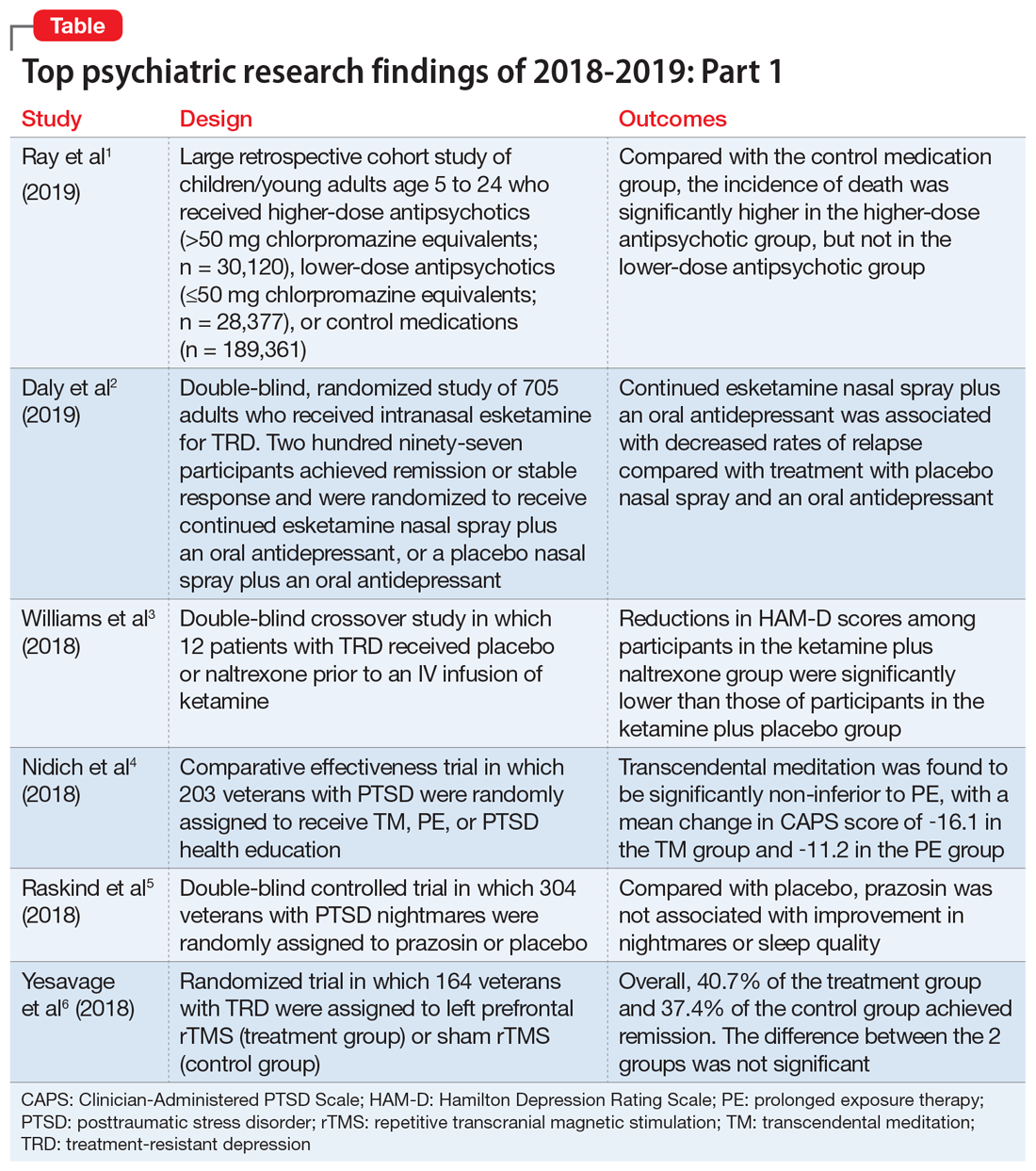
Top research findings of 2018-2019 for clinical practice
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
Aging and Trauma: Post Traumatic Stress Disorder Among Korean War Veterans
The Korean War lasted from June 25, 1950 through July 27, 1953. Although many veterans of the Korean War experienced traumas during extremely stressful combat conditions. However, they would not have been diagnosed with posttraumatic stress disorder (PTSD) at the time because the latter did not exist as a formal diagnosis until the publication of the third edition of the Diagnostic and Statistical Manual (DSM) in 1980.1 Prior to 1980, psychiatric syndromes resulting from war and combat exposure where known by numerous other terms including shell shock, chronic traumatic war neurosis, and combat fatigue/combat exhaustion.2,3 Military psychiatrists attended to combat fatigue during the course of the Korean War, but as was true of World War I and II, the focus was on returning soldiers to duty. Combat fatigue was generally viewed as a transient condition.4-8
Although now octo- and nonagenarians, in 2019 there are 1.2 million living Korean War veterans in the US, representing 6.7% of all current veterans.9 Understanding their war experiences and the nature of their current and past presentation of PTSD is relevant not only in formal mental health settings, but in primary care settings, including home-based primary care, as well as community living centers, skilled nursing facilities and assisted living facilities. Older adults with PTSD often present with somatic concerns rather than spontaneously reporting mental health symptoms.10 Beyond the short-term clinical management of Korean War veterans with PTSD, consideration of their experiences also has long-term relevance for the appropriate treatment of other veteran cohorts as they age in coming decades.
The purpose of this article is to provide a clinically focused overview of PTSD in Korean War veterans, to help promote understanding of this often-forgotten group of veterans, and to foster optimized personalized care. This overview will include a description of the Korean War veteran population and the Korean War itself, the manifestations and identification of PTSD among Korean War veterans, and treatment approaches using evidence-based psychotherapies and pharmacotherapies. Finally, we provide recommendations for future research to address present empirical gaps in the understanding and treatment of Korean War veterans with PTSD.
Causes and Course of the Korean War
When working with Korean War veterans it is important to consider the special nature of that specific conflict. Space considerations limit our ability to do justice to the complex history and numerous battles of the Korean War, but information in the following summary was gleaned from several excellent histories.11-13
The Korean War has been referred to as The Forgotten War, a concern expressed even during the latter parts of the war.14,15 But the war and its veterans warrant remembering. The root and proximal causes of the Korean War are complex and not fully agreed upon by the main participants.16-19 In part this may reflect the fact that there was no clear victor in the Korean War, so that the different protagonists have developed their own versions of the history of the conflict. Also, US involvement and the public reaction to the war must be viewed within the larger historical context of that time. This context included the recent end of 4 years of US involvement in World War II (1941-1945) and the subsequent rapid rise of Cold War tensions between the US and the Soviet Union. The latter also included a worldwide fear of nuclear war and the US fear of the global spread of communism. These fears were fueled by the Soviet-led Berlin Blockade from June 1948 through May 1949, the Soviet Union’s successful atomic bomb test in August 1949, the founding of the People’s Republic of China in October 1949, and the February 1950 Sino-Soviet Treaty of Friendship and Alliance.13
In the closing days of World War II, the US and Soviet Union agreed to a temporary division of Korea along the 38th parallel to facilitate timely and efficient surrender of Japanese troops. But as Cold War tensions rose, the temporary division became permanent, and Soviet- and US-backed governments of the north and south, respectively, were officially established on the Korean peninsula in 1948. Although by 1949 the Soviets and US had withdrawn most troops from the peninsula, tensions between the north and south continued to mount and hostilities increased. To this day the exact causes of the eruption of war remain disputed, although it is clear that ideological as well as economic factors played a role, and both leaders of North and South Korea were pledging to reunite the peninsula under their respective leadership.16-19 The tension culminated on June 25, 1950, when North Korean troops crossed the 38th parallel and invaded South Korea. On June 27, 1950, President Truman ordered US naval and air forces to support South Korea and then ordered the involvement of ground troops on June 30.16,17,19
Although several other member countries of the United Nations (UN) provided troops, 90% of the troops were from the US. About 5.7 million US military personnel served during the war, including about 1.8 million in Korea itself. The US forces experienced approximately 34,000 battle-related deaths, 103,000 were wounded, and 7,000 were prisoners of war (POWs).11,20-22 The nature and events of the Korean War made it particularly stressful and traumatizing for the soldiers, sailors, and marines involved throughout its entire course. These included near defeat in the early months, a widely alternating war front along the north/south axis during the first year, and subsequently, not only intense constant battles on the fronts, but also a demanding and exhausting guerrilla war in the south, which lasted throughout the remainder of the conflict.11,15 The US troops during the initial months of the war have been described as outnumbered and underprepared, as many in the initial phase were reassigned from peace-time occupation duty in Japan.7
The first year of war was characterized by a repeated north-to-south/south-to-north shifts in control of territory. During the first 3 months, the North Korean forces overwhelmed the South and captured control of all but 2 South Korean cities in the far southeastern region (Pusan, now Busan; and Daegu), and US and UN forces were forced to retreat to the perimeter around Pusan. The intense Battle of Pusan Perimeter lasted from August 4, 1950 to September 18, 1950, and resulted in massive causalities as well as a flood of civilian refugees.
The course of the war began to change in early September 1950 with the landing of amphibious US/UN forces at Inchon, behind North Korean lines, which cut off southern supply routes for the North Korean troops.11 US/UN forces soon crossed to the north of the 38th parallel and captured the North Korean capital, Pyongyang, on October 19, 1950. They continued to push north and approached the Yalu River border with China by late November 1950, but then the Chinese introduced their own troops forcing a southward retreat of US/UN troops during which there were again numerous US/UN casualties. Chinese troops retook Seoul in late December 1950/early January 1951. However, the US/UN forces soon recaptured Seoul and advanced back to the 38th parallel. This back-and-forth across the 38th parallel continued until July 1951 when the front line of battle stabilized there. Although the line stabilized, intense battles and casualties continued for 2 more years. During this period US/UN troops also had to deal with guerrilla warfare behind the front lines due to the actions of communist partisans and isolated North Korean troops. This situation continued until the armistice was signed July 27, 1953.
Trauma and Characteristic Stresses of the War
There were many factors that made the Korean War experience different from previous wars, particularly World War II. For example, in contrast to the strong public support during and after World War II, public support for the Korean War in the US was low, particularly during its final year.23 In public opinion polls from October 1952 through April 1953, only 23% to 39% reported feeling that the war was worth fighting.23 A retrospective 1985 survey also found that 70% of World War II veterans, but only 33% of Korean War veterans reported feeling appreciated by the US public on their return from the war.24
Those fighting in the initial months of the war faced a particularly grim situation. According to LTC Philip Smith, who served as Division Psychiatrist on the Masan Front (Pusan Perimeter) during August and September of 1950, “Fighting was almost continuous and all available troops were on the fighting front… For the most part these soldiers were soft from occupation duty, many had not received adequate combat basic training, no refresher combat training in Korea had as yet been instituted,” he reported.7 “The extremes of climate coupled with the generally rugged mountainous terrain in Korea were physical factors of importance…These men were psychologically unprepared for the horrors and isolation of war.” LTC Smith noted that the change in status from civilian or occupation life to the marked deprivation of the war in Korea had been “too abrupt to allow as yet for a reasonable adjustment to the new setting” and that as a result “the highest rate of wounded and neuropsychiatric casualties in the Korean campaign resulted.”7
Even after this initial period, the nature of the shifting war, the challenging terrain, the high military casualty rate, and the high rate of civilian casualties and displacement continued throughout the war.
PTSD in Korean War Veterans
It is clear that Korean War combat veterans were exposed to traumatic events. It is unknown how many developed PTSD. While notions of psychological distress and disability related to combat trauma exposure have existed for centuries, Korean War and World War II veterans are a remaining link to pre-DSM PTSD mental health in the military. Military/forward psychiatry—psychiatric services near the battle zone rather than requiring evacuation of patients—was present in Korea from the early months of the war, but the focus of forward psychiatry was to reduce psychiatric causalities from combat fatigue and maximize rapid return-to-duty.4-6 With no real conception of PTSD, there were limited treatments available, and evidenced-based trauma-focused treatments for PTSD would not be introduced for at least another 4 decades.27-29
Skinner and Kaplick conducted a historical review of case descriptions of trauma-related conditions from World War I through the Vietnam War and noted the consistent inclusion of hyperarousal and intrusive symptoms, although there also was a greater emphasis on somatic conversion or hysteria symptoms in the earlier descriptions.30 By the Korean War, descriptions of combat fatigue included a number of symptoms that overlap with PTSD, including preoccupation with the traumatic stressor, nightmares, irritability/anger, increased startle, and hyperarousal.31 But following the acute phases, attention to any chronic problems associated with these conditions waned. As was acknowledged by a military psychiatrist in a 1954 talk, studies of the long-term adjustment of those who had “broken down in combat” were sorely needed.6 In a small 1965 study reported by Archibald and Tuddenham, persistent symptoms of combat fatigue among Korean War veterans were definitely present, and there was even a suggestion that the symptoms had increased over the decade since the war.32
Given the stoicism that typified cultural expectations for military men during this period, Korean War veterans may also have been reluctant to seek mental health treatment either at the time or later. In short, it is likely that a nontrivial proportion of Korean War veterans with PTSD were underdiagnosed and received suboptimal or no mental health treatment for decades following their war experiences.33 Although the nature of the war, deployment, and public support were distinct in World War II vs the Korean War, the absence of attention to the long-term effects of disorders related to combat trauma and the cultural expectations for stoicism suggest that PTSD among aging World War II veterans may also have gone underrecognized and undertreated.
Apart from the lack of interest in chronic effects of stressors, another problem that has plagued the limited empirical research on Korean War veterans has been the propensity to combine Korean War with World War II veteran samples in studies. Because World War II veterans have outnumbered Korean War veterans until recently, combined samples tended to have relatively few Korean War veterans. Nevertheless, from those studies that have been reported in which 2 groups were compared, important differences have been revealed. Specifically, although precise estimates of the prevalence of PTSD among Korean War combat veterans have varied depending on sampling and method, studies from the 1990s and early 2000s suggested that the prevalence of PTSD and other mental health concerns as well as the severity of symptoms, suicide risk, and psychosocial adjustment difficulties were worse among Korean War combat veterans relative to those among World War II combat veterans; however, both groups had lower prevalence than did Vietnam War combat veterans.21,34-37 Several authors speculated that these differences in outcome were at least partially due to differences in public support for the respective wars.36,37
Although there has been a paucity of research on psychiatric issues and PTSD in Korean War veterans, POWs who were very likely to have been exposed to extreme psychological traumas have received some attention. There have been comparisons of mortality and morbidity among POWs from the Korean War (PWK), World War II Pacific Theater (PWJ), and Europe (PWE).38 Among measures that were administered to the former POWs, the overall pattern seen from survey data in the mid-1960s revealed significantly worse health and functioning among the PWK and PWJ groups relative to the PWE group, with psychiatric difficulties being the most commonly reported impairments among the former 2 groups. This pattern was found most strongly with regards to objective measures, such as hospitalizations for “psychoneuroses,” and US Department of Veterans Affairs (VA) disability records, as well as based on self-reported psychosocial/recreational difficulties measured using the Cornell Medical Index (CMI).38
Gold and colleagues reported a follow-up study of more than 700 former POWs who were reinterviewed between 1989 and 1992.39 Although there was no scale of PTSD symptoms prior to formulation of the diagnosis in 1980, the CMI was a self-reported checklist that included a large range of both medical as well as behavioral and psychiatric symptoms. Thus, using CMI survey responses from 1965, the authors examined the factor structure (ie, the correlational relationships between multiple scale items and subgroupings of items) of the CMI relative to diagnosis of PTSD in 1989 to 1992 based on results from the Structured Clinical Interview for the DSM-III-R (SCID). The intent was to help discern whether the component domains of PTSD were present and intercorrelated in a pattern similar to that of the contemporary diagnosis. The investigators examined the factor structure of 20 psychological items from the CMI that appeared relevant to PTSD criteria using the 1965 data. Three factors (subgroups of highly intercorrelated items) were found: irritability (31% of variance), fearfulness/anxiousness (9% of the variance), and social withdrawal (7% of the variance). Although these did not directly correspond to, or fully cover, DSM PTSD domains or criteria, there does appear to be a thematic resemblance of the CMI findings with PTSD, including alterations in arousal and mood, vigilance, and startle.
Identification and Treatment of PTSD in Older Veterans
Of the 1.2 million living Korean War veterans in the US, 36.3% use VA provided health care.40 There are a number of complicating factors to consider in the current identification and treatment of PTSD in this cohort, including their advanced age; physical, cognitive, and social changes associated with normal aging; the associated medical and cognitive comorbidities; and the specific social-contextual factors in that age cohort. Any combination of these factors may complicate recognition, diagnosis, and treatment. It is also important to be cognizant of the additional stressors that may have been experienced by ethnic minorites and women serving in Korea, which are poorly documented and studied. Racial integration of the US military began during the Korean War, but the general pattern was for African American soldiers to be assigned to all-white units, rather than the reverse.14,41,42 And although the majority of military personnel serving in Korea were male, there were women serving in health care positions at mobile army surgical hospital (MASH) units, medical air evacuation (Medevac) aircraft, and off-shore hospital ships.
The clinical presentation of PTSD in older adults has varied, which may partially relate to the time elapsed since the index trauma. For example, older veterans in general may show less avoidance behavior as a part of PTSD, but in those who experience trauma later in life there may actually be greater avoidance.43,44 There have also been discrepant reports of intrusion or reexperiencing of symptoms, with these also potentially reduced in older veterans.43,44 However, sleep disturbances seem to be very common among elderly combat veterans, and attention should be paid to the possible presence of sleep apnea, which may be more common in veterans with PTSD in general.43,45,46
PTSD symptoms may reemerge after decades of remission or quiescence during retirement and/or with the emergence of neurocognitive impairment, such as Alzheimer disease or dementia. These individuals may have more difficulty engaging in distracting activities and work and spend more time engaging in reminiscence about the past, which can include increased focus on traumatic memories.45,47 Davison and colleagues have suggested a concept they call later-adulthood trauma reengagement (LATR) where later in life combat veterans may “confront and rework their wartime memories in an effort to find meaning and build coherence.”48 This process can be a double-edged sword, leading at times positively to enhanced personal growth or negatively to increased symptoms; preventive interventions may be able foster a more positive outcome.48
There is some evidence supporting the validity of the Clinician Administered PTSD Scale (CAPS) for the evaluation of PTSD in older adults, although this was based on the DSM-III-revised criteria for PTSD and an earlier version of CAPS.49 Bhattarai and colleagues examined responses to the 35-item Mississippi Scale for Combat-Related PTSD (M-PTSD) using VA clinical data collected between 2008 and 2015 on veterans of each combat era from World War II through the post-9/11.50 Strong internal consistency and test-retest reliability of the M-PTSD was observed within each veteran era sample. However, using chart diagnosis of PTSD as the criterion standard, the cut-scores for optimal balance of sensitivity and specificity of the M-PTSD scores were substantially lower for the older cohorts (World War II and Korean War veterans) relative to those for Vietnam and more recent veteran cohorts. The authors concluded that M-PTSD can be validly used to screen for PTSD in veterans within each of these cohorts but recommended using lower than standard cut-scores for Korean War and World War II veterans.50
This is also consistent with reports that suggest the use of lower cut-scores on self-administered PTSD symptom screens.43,44 For the clinician interested in quantifying the severity of PTSD, the most recent tools available are the CAPS-5 and the PCL-5, which have both been created in accordance with the DSM-5. The CAPS-5 is a rater-administered tool, and the PCL-5 is self-administered by the veteran. Although there has been little research using these newer tools in geriatric populations, they can currently serve as a means of tracking the severity of PTSD while we await measures that are better validated in Korean War and other older veterans.
Beyond specific empirical guidance, VA clinicians must presently rely on clinical observations and experience. Patients from the Korean War cohort often present at the insistence of a family member for changes in sleep, mood, behavior, or cognition. When the veterans themselves present, older adults with PTSD often focus more on somatic concerns (including pain, sleep, and gastrointestinal disturbance) than psychiatric problems per se. The latter tendency may in part be due to the salience of such symptoms for them, but perhaps also due to considerable stigma of mental health care that is still largely present in this group.43,44
Psychotherapy
Current VA treatment guidelines recommend trauma-focused therapies, with the strongest evidence base for prolonged exposure (PE), cognitive processing therapy (CPT), and eye movement desensitization and reprocessing (EMDR) therapies.51
There have been several excellent prior reviews discussing treatment of PTSD in older adults generally.10,43,44,52 These reviews have invariably expressed concern about the lack of sufficient empirical studies, but based on evidence from studies and case reports, there seems to be tentative support that trauma-focused therapies are acceptable and efficacious for use with older adults with PTSD. In their recent scoping review, Pless Kaiser and colleagues made several recommendations for trauma-focused therapy with older adults, including slow/careful pacing and use of compensatory aids for cognitive and sensory deficits.44 When cognitive impairment has exacerbated PTSD symptoms, they suggest therapists consider using an adapted form of CPT completed without a trauma narrative. For PE they recommend extending content across sessions and involving spouse or caregivers to assist with in vivo exposure and homework completion.44
Recent studies suggest that PTSD may be a risk factor for the later development of neurodegenerative disorders, and it is often during assessments for dementia that a revelation of PTSD occurs.10,43,47,55 Cognitive impairment may also be of relevance in deciding on the type of psychotherapy to be implemented, as it may have more adverse effects on the effectiveness of CPT than of exposure-based treatments (PE or EMDR). It may be useful to perform a cognitive assessment prior to initiation of a cognitive-based therapy, although extensive cognitive testing may not be practical or may be contraindicated because of fatigue. A brief screening tool such as the Montreal Cognitive Assessment or the Mini-Mental State Examinationmay be helpful.56, 57
Prolonged exposure has been reported by many clinicians to be effective in older adults with PTSD; however, due consideration should be given to the needs of individuals, as many have functioned for decades by suppressing memories.
Apart from the treatment needs for specific PTSD symptoms, the decades-long effects of poor sleep, irritability, hypervigilance, and dissociation also have social consequences for patients, including marital discord and divorce, and social and family isolation that should be addressed in therapy when appropriate. In addition, many Korean War veterans, like all veterans, sought postmilitary employment in professions that are associated with higher rates of exposure to psychological trauma, such as police or fire departments, and this may have an exacerbating effect on PTSD.58
Pharmacotherapy
There is very little empirical evidence guiding pharmacologic approaches to PTSD in older veterans. This population is at increased risk for many comorbidities, and pharmacologic treatments many require dosage adjustments, as is the case for any geriatric patient. Selective serotonin reuptake inhibitor (SSRI) and serotonin norepinephrine reuptake inhibitor (SNRI) medications have been proposed for some cases of PTSD.59,60 Health care providers may consider the SSRIs escitalopram or sertraline preferentially given their decreased potential for drug-drug interactions, anticholinergic effects, or cardiac toxicity compared with that of other drugs in this class.60,61 As venlafaxine can increase blood pressure, especially at higher doses, prescribers may choose duloxetine as an alternative if a SNRI is indicated.60 For veterans when prazosin is being considered for nightmare control, monitoring for hypotension, orthostasis, and the administration of other antihypertensives or prostatic hypertrophy medications is necessary.61 The use of benzodiazepines, while not recommended for PTSD, should be viewed with even greater trepidation in a geriatric population given enhanced risk of falls and confusion in the geriatric veteran population.60,62
Conclusions
Many of the oldest veterans (aged > 80 years) are from the Korean War era. The harsh and unique nature of the war, as well as the differences in context and support from the US public, and the outcome of the war, may have all contributed to and elevation of “combat fatigue” and PTSD among combat veterans from the Korean War. As the “forgotten war” cohort also has been forgotten by researchers, relatively little is known about posttraumatic stress sequelae of these veterans in the decades following the war.
From available evidence, we can readily surmise that problems were underrecognized and suboptimally diagnosed and treated. There is tentative evidence supporting the use of standard interviews and rating scales, such as the CAPS, M-PTSD, and PCL, but lower cut-scores than applied with Vietnam and later veteran cohorts are generally recommended to avoid excessive false negative errors. In terms of psychotherapy treatment, there is again a stark paucity of systematic research, but the limited evidence from studies of PTSD treatment in older adults from the general population tentatively support the acceptability and potential efficacy of recognized evidence-based trauma-focused psychotherapies for PTSD. Research on medication treatment is similarly lacking, but the general recommendations for the use of SSRI or SNRI medications seem to be valid, at least in our clinical experience, and the general rules for geriatric psychopharmacology definitely apply here—start low, go slow.
There are several important avenues for future research. Most pressing among these are establishing the effectiveness of existing treatments, and the modifications that may be needed in the broader context of the above factors, as well as the physical and cognitive changes associated with advanced age. Further research on the phenomenologic aspects of PTSD among Korean War and subsequent cohorts are also needed, as the information obtained will not only guide more effective personalized treatment of the Korean War veterans who remain with us, but also inform future generations of care in terms of the degree and dimensions of variability that may present between cohorts and within cohorts over the life span.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Arlington VA: American Psychiatric Association; 1980.
2. Friedman MJ, Schnurr PP, McDonagh-Coyle A. Posttraumatic stress disorder in the military veteran. Psychiatr Clin North Am. 1994;17(2):265-277.
3. Salmon TW. The Care and Treatment of Mental Diseases and War Neuroses (“Shell Shock”) in the British Army. New York: War Work Committee of the National Committee for Mental Hygiene, Inc; 1917.
4. Jones E, Wessely S. “Forward psychiatry” in the military: its origins and effectiveness. J Trauma Stress. 2003;16(4):411-419.
5. Newman RA. Combat fatigue: a review to the Korean conflict. Mil Med. 1964;129:921-928.
6. Harris FG. Some comments on the differential diagnosis and treatment of psychiatric breakdowns in Korea. https://history.amedd.army.mil/booksdocs/korea/recad2/ch9-2.html. Published April 30, 1954. Accessed November 8, 2019.
7. Smith PB. Psychiatric experiences during the Korean conflict. Am Pract Dig Treat. 1955;6(2):183-189.
8. Koontz AR. Psychiatry in the Korean War. Military Surg.
1950;107(6):444-445.
9. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Population Tables - Table 2L: VETPOP2016 Living Veterans by period of service, gender, 2015-2045. https://www.va.gov/vetdata/docs/Demographics/New_Vetpop_Model/2L_VetPop2016
_POS_National.xlsx. Accessed November 8, 2019.
10. Cook JM, McCarthy E, Thorp SR. Older adults with PTSD: brief state of research and evidence-based psychotherapy case illustration. Am J Geriatr Psychiatry. 2017;25(5):522-530.
11. Millett AR. Korean War: 1950-1953. Encylopaedia Britannica. https://www.britannica.com/event/Korean-War#accordion-article-history. Updated Nov 7, 2019. Accessed November 8, 2019.
12. Stack L. Korean War, a ‘forgotten’ conflict that shaped the modern world. The New York Times. January 2, 2018. https://www.nytimes.com/2018/01/01/world/asia/korean-war-history.html. Accessed November 8, 2019.
13. Westad OA. The Cold War: A World History. New York: Basic Books; 2018.
14. Young C, Conard PL, Armstrong ML, Lacy D. Older military veteran care: many still believe they are forgotten. J Holist Nurs. 2018;36(3):291-300.
15. Huebner AJ. Kilroy is back, 1950-1953. In: The Warrior Image: Soldiers in American Culture From the Second World War to the Vietnam Era. Chapel Hill, NC: The University of North Carolina Press; 2008:97-131.
16. The annexation of Korea (editorial). Japan Times. https://www.japantimes.co.jp/opinion/2010/08/29/editorials/the-annexation-of-korea/#.XPgvJvlKhhE. Published August 29, 2010. Accessed November 8, 2019.
17. Gupta K. How did the Korean war begin? China Q. 1972;52:699-716.
18. Lin L, Zhao Y, Ogawa M, Hoge J, Kim BY. Whose history? An analysis of the Korean War in history textbooks from the United States, South Korea, Japan, and China. Social Studies. 2009;100(5):222-232.
19. Weathersby K. The Korean War revisited. Wilson Q. 1999;23(3):91.
20. US Department of Veterans Affairs, Office of Program and Data Analyses, Assistant Secretary for Planning and Analysis. Data on veterans of the Korean War. https://www.va.gov/vetdata/docs/SpecialReports/KW2000.pdf. Published June 2000. Accessed November 8, 2019.
21. Brooks MS, Fulton L. Evidence of poorer life-course mental health outcomes among veterans of the Korean War cohort. Aging Ment Health. 2010;14(2):177-183.
22. US Department of Veterans Affairs, Office of Public Affairs. America’s wars. https://www.va.gov/opa/publications/factsheets/fs_americas_wars.pdf. Accessed November 8, 2019.
23. Memorandum on recent polls on Korea. https://www.eisenhowerlibrary.gov/sites/default/files/research/online-documents/korean-war/public-opinion-1953-06-02.pdf. Published June 2, 1953. Accessed November 8, 2019.
24. Elder GH Jr, Clipp EC. Combat experience and emotional health: impairment and resilience in later life. J Pers. 1989;57(2):311-341.
25. US Department of Veterans Affairs. Public health: cold injuries. https://www.publichealth.va.gov/PUBLICHEALTH/exposures/cold-injuries/index.asp. Updated July 31, 2019. Accessed November 8, 2019.
26. US Department of Veterans Affairs. Korean War veterans health issues. https://www.va.gov/health-care/health-needs-conditions/health-issues-related-to-service-era
/korean-war/. Updated June 14, 2019. Accessed November 8, 2019.
27. Shapiro F. Efficacy of the eye movement desensitization procedure in the treatment of traumatic memories. J Trauma Stress. 1989;2(2):199-223.
28. Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. J Consul Clin Psychol. 1992;60(5):748-756.
29. Foa EB, Rothbaum BO. Treating Trauma of Rape: Cognitive-Behavioral Therapy for PTSD. New York: Guilford; 2001.
30. Skinner R, Kaplick PM. Cultural shift in mental illness: a comparison of stress responses in World War I and the Vietnam War. JRSM Open. 2017;8(12):2054270417746061.
31. Kardiner A, Spiegel H. War Stress and Neurotic Illness. New York: Hoeber; 1947.
32. Archibald HC, Tuddenham RD. Persistent stress reaction after combat: a 20-year follow-up. Arch Gen Psychiatry. 1965;12:475-481.
33. Cook JM, Simiola V. Trauma and aging. Curr Psychiatry Rep. 2018;20(10):93.
34. Rosenheck R, Fontana A. Long-term sequelae of combat in World War II, Korea and Vietnam: a comparative study. In: McCaughey BG, Fullerton CS, Ursano RJ, eds. Individual
and Community Responses to Trauma and Disaster: The Structure of Human Chaos. New York: Cambridge University Press; 1994:330-359.
35. Blake DD, Keane TM, Wine PR, Mora C, Taylor KL, Lyons JA. Prevalence of PTSD symptoms in combat veterans seeking medical treatment. J Trauma Stress. 1990;3(1):15-27.
36. McCranie EW, Hyer LA. Posttraumatic stress disorder symptoms in Korean conflict and World War II combat veterans seeking outpatient treatment. J Trauma Stress. 2000;13(3):427-439.
37. Fontana A, Rosenheck R. Traumatic war stressors and psychiatric symptoms among World War II, Korean, and Vietnam War veterans. Psychology Aging. 1994;9(1):27-33.
38. Beebe GW. Follow-up studies of World War II and Korean war prisoners. II. Morbidity, disability, and maladjustments. Am J Epidemiol. 1975;101(5):400-422.
39. Gold PB, Engdahl BE, Eberly RE, Blake RJ, Page WF, Frueh BC. Trauma exposure, resilience, social support, and PTSD construct validity among former prisoners of war. Social Psychiatry Psychiatr Epidemiol. 2000;35(1):36-42.
40. US Department of Veterans Affairs. Key statistics by veteran status and period of service. https://www.va.gov/vetdata/docs/SpecialReports/KeyStats.pdf. Accessed November 11, 2019.
41. Bowers WT, Hammond WM, MacGarrigle GL. Black Soldier, White Army. Washington DC: US Army Center of Military History; 1996.
42. Black HK. Three generations, three wars: African American veterans. Gerontologist. 2016;56(1):33-41.
43. Thorp SR, Sones HM, Cook JM. Posttraumatic stress disorder among older adults. In: Sorocco KH, Lauderdale S, eds. Cognitive Behavior Therapy With Older Adults: Innovations Across Care Settings. New York: Springer; 2011:189-217.
44. Pless Kaiser A, Cook JM, Glick DM, Moye J. Posttraumatic stress disorder in older adults: a conceptual review. Clinical Gerontol. 2019;42(4):359-376.
45. Sadavoy J. Survivors. A review of the late-life effects of prior psychological trauma. Am J Geriatr Psychiatry. 1997;5(4):287-301.
46. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636.
47. Mota N, Tsai J, Kirwin PD, et al. Late-life exacerbation of PTSD symptoms in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2016;77(3):348-354.
48. Davison EH, Kaiser AP, Spiro A 3rd, Moye J, King LA, King DW. From Late-onset stress symptomatology to later-adulthood trauma reengagement in aging combat veterans: taking a broader view. Gerontologist. 2016;56(1):14-21.
49. Hyer L, Summers MN, Boyd S, Litaker M, Boudewyns P. Assessment of older combat veterans with the clinician-administered PTSD scale. J Trauma Stress. 1996;9(3):587-593.
50. Bhattarai JJ, Oehlert ME, Weber DK. Psychometric properties of the Mississippi Scale for combat-related posttraumatic stress disorder based on veterans’ period of service. Psychol Serv. 2018. [Epub ahead of print]
51. US Department of Veterans Affairs, US Department of Defense. VA/DOD Clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf.
Updated 2017. Accessed November 11, 2019.
52. Dinnen S, Simiola V, Cook JM. Post-traumatic stress disorder in older adults: a systematic review of the psychotherapy treatment literature. Aging Ment Health. 2015;19(2):144-150.
53. Jakel RJ. Posttraumatic Stress Disorder in the Elderly. Psychiatr Clin North Am. 2018;41(1):165-175.
54. Thorp SR, Glassman LH, Wells SY, et al. A randomized controlled trial of prolonged exposure therapy versus relaxation training for older veterans with military-related PTSD. J Anxiety Disord. 2019;64:45-54.
55. Kang B, Xu H, McConnell ES. Neurocognitive and psychiatric comorbidities of posttraumatic stress disorder among older veterans: a systematic review. Int J Geriatr Psychiatry. 2019;34(4):522-538.
56. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
57. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
58. Paton D. Traumatic Stress in Police Officers a Career-Length Assessment From Recruitment to Retirement. Springfield, IL: Charles C. Thomas; 2009.
59. Alexander W. Pharmacotherapy for post-traumatic stress disorder in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37(1):32-38.
60. Beck JG, Sloan DM, Friedman MJ. Pharmacotherapy for PTSD. In: The Oxford Handbook of Traumatic Stress Disorders. Oxford University Press; 2012.
61. Waltman SH, Shearer D, Moore BA. Management of posttraumatic nightmares: a review of pharmacologic and nonpharmacologic treatments since 2013. Curr Psychiatry Rep. 2018;20(12):108.
62. Díaz-Gutiérrez MJ, Martínez-Cengotitabengoa M, Sáez de Adana E, et al. Relationship between the use of benzodiazepines and falls in older adults: a systematic review. Maturitas. 2017;101:17-22.
The Korean War lasted from June 25, 1950 through July 27, 1953. Although many veterans of the Korean War experienced traumas during extremely stressful combat conditions. However, they would not have been diagnosed with posttraumatic stress disorder (PTSD) at the time because the latter did not exist as a formal diagnosis until the publication of the third edition of the Diagnostic and Statistical Manual (DSM) in 1980.1 Prior to 1980, psychiatric syndromes resulting from war and combat exposure where known by numerous other terms including shell shock, chronic traumatic war neurosis, and combat fatigue/combat exhaustion.2,3 Military psychiatrists attended to combat fatigue during the course of the Korean War, but as was true of World War I and II, the focus was on returning soldiers to duty. Combat fatigue was generally viewed as a transient condition.4-8
Although now octo- and nonagenarians, in 2019 there are 1.2 million living Korean War veterans in the US, representing 6.7% of all current veterans.9 Understanding their war experiences and the nature of their current and past presentation of PTSD is relevant not only in formal mental health settings, but in primary care settings, including home-based primary care, as well as community living centers, skilled nursing facilities and assisted living facilities. Older adults with PTSD often present with somatic concerns rather than spontaneously reporting mental health symptoms.10 Beyond the short-term clinical management of Korean War veterans with PTSD, consideration of their experiences also has long-term relevance for the appropriate treatment of other veteran cohorts as they age in coming decades.
The purpose of this article is to provide a clinically focused overview of PTSD in Korean War veterans, to help promote understanding of this often-forgotten group of veterans, and to foster optimized personalized care. This overview will include a description of the Korean War veteran population and the Korean War itself, the manifestations and identification of PTSD among Korean War veterans, and treatment approaches using evidence-based psychotherapies and pharmacotherapies. Finally, we provide recommendations for future research to address present empirical gaps in the understanding and treatment of Korean War veterans with PTSD.
Causes and Course of the Korean War
When working with Korean War veterans it is important to consider the special nature of that specific conflict. Space considerations limit our ability to do justice to the complex history and numerous battles of the Korean War, but information in the following summary was gleaned from several excellent histories.11-13
The Korean War has been referred to as The Forgotten War, a concern expressed even during the latter parts of the war.14,15 But the war and its veterans warrant remembering. The root and proximal causes of the Korean War are complex and not fully agreed upon by the main participants.16-19 In part this may reflect the fact that there was no clear victor in the Korean War, so that the different protagonists have developed their own versions of the history of the conflict. Also, US involvement and the public reaction to the war must be viewed within the larger historical context of that time. This context included the recent end of 4 years of US involvement in World War II (1941-1945) and the subsequent rapid rise of Cold War tensions between the US and the Soviet Union. The latter also included a worldwide fear of nuclear war and the US fear of the global spread of communism. These fears were fueled by the Soviet-led Berlin Blockade from June 1948 through May 1949, the Soviet Union’s successful atomic bomb test in August 1949, the founding of the People’s Republic of China in October 1949, and the February 1950 Sino-Soviet Treaty of Friendship and Alliance.13
In the closing days of World War II, the US and Soviet Union agreed to a temporary division of Korea along the 38th parallel to facilitate timely and efficient surrender of Japanese troops. But as Cold War tensions rose, the temporary division became permanent, and Soviet- and US-backed governments of the north and south, respectively, were officially established on the Korean peninsula in 1948. Although by 1949 the Soviets and US had withdrawn most troops from the peninsula, tensions between the north and south continued to mount and hostilities increased. To this day the exact causes of the eruption of war remain disputed, although it is clear that ideological as well as economic factors played a role, and both leaders of North and South Korea were pledging to reunite the peninsula under their respective leadership.16-19 The tension culminated on June 25, 1950, when North Korean troops crossed the 38th parallel and invaded South Korea. On June 27, 1950, President Truman ordered US naval and air forces to support South Korea and then ordered the involvement of ground troops on June 30.16,17,19
Although several other member countries of the United Nations (UN) provided troops, 90% of the troops were from the US. About 5.7 million US military personnel served during the war, including about 1.8 million in Korea itself. The US forces experienced approximately 34,000 battle-related deaths, 103,000 were wounded, and 7,000 were prisoners of war (POWs).11,20-22 The nature and events of the Korean War made it particularly stressful and traumatizing for the soldiers, sailors, and marines involved throughout its entire course. These included near defeat in the early months, a widely alternating war front along the north/south axis during the first year, and subsequently, not only intense constant battles on the fronts, but also a demanding and exhausting guerrilla war in the south, which lasted throughout the remainder of the conflict.11,15 The US troops during the initial months of the war have been described as outnumbered and underprepared, as many in the initial phase were reassigned from peace-time occupation duty in Japan.7
The first year of war was characterized by a repeated north-to-south/south-to-north shifts in control of territory. During the first 3 months, the North Korean forces overwhelmed the South and captured control of all but 2 South Korean cities in the far southeastern region (Pusan, now Busan; and Daegu), and US and UN forces were forced to retreat to the perimeter around Pusan. The intense Battle of Pusan Perimeter lasted from August 4, 1950 to September 18, 1950, and resulted in massive causalities as well as a flood of civilian refugees.
The course of the war began to change in early September 1950 with the landing of amphibious US/UN forces at Inchon, behind North Korean lines, which cut off southern supply routes for the North Korean troops.11 US/UN forces soon crossed to the north of the 38th parallel and captured the North Korean capital, Pyongyang, on October 19, 1950. They continued to push north and approached the Yalu River border with China by late November 1950, but then the Chinese introduced their own troops forcing a southward retreat of US/UN troops during which there were again numerous US/UN casualties. Chinese troops retook Seoul in late December 1950/early January 1951. However, the US/UN forces soon recaptured Seoul and advanced back to the 38th parallel. This back-and-forth across the 38th parallel continued until July 1951 when the front line of battle stabilized there. Although the line stabilized, intense battles and casualties continued for 2 more years. During this period US/UN troops also had to deal with guerrilla warfare behind the front lines due to the actions of communist partisans and isolated North Korean troops. This situation continued until the armistice was signed July 27, 1953.
Trauma and Characteristic Stresses of the War
There were many factors that made the Korean War experience different from previous wars, particularly World War II. For example, in contrast to the strong public support during and after World War II, public support for the Korean War in the US was low, particularly during its final year.23 In public opinion polls from October 1952 through April 1953, only 23% to 39% reported feeling that the war was worth fighting.23 A retrospective 1985 survey also found that 70% of World War II veterans, but only 33% of Korean War veterans reported feeling appreciated by the US public on their return from the war.24
Those fighting in the initial months of the war faced a particularly grim situation. According to LTC Philip Smith, who served as Division Psychiatrist on the Masan Front (Pusan Perimeter) during August and September of 1950, “Fighting was almost continuous and all available troops were on the fighting front… For the most part these soldiers were soft from occupation duty, many had not received adequate combat basic training, no refresher combat training in Korea had as yet been instituted,” he reported.7 “The extremes of climate coupled with the generally rugged mountainous terrain in Korea were physical factors of importance…These men were psychologically unprepared for the horrors and isolation of war.” LTC Smith noted that the change in status from civilian or occupation life to the marked deprivation of the war in Korea had been “too abrupt to allow as yet for a reasonable adjustment to the new setting” and that as a result “the highest rate of wounded and neuropsychiatric casualties in the Korean campaign resulted.”7
Even after this initial period, the nature of the shifting war, the challenging terrain, the high military casualty rate, and the high rate of civilian casualties and displacement continued throughout the war.
PTSD in Korean War Veterans
It is clear that Korean War combat veterans were exposed to traumatic events. It is unknown how many developed PTSD. While notions of psychological distress and disability related to combat trauma exposure have existed for centuries, Korean War and World War II veterans are a remaining link to pre-DSM PTSD mental health in the military. Military/forward psychiatry—psychiatric services near the battle zone rather than requiring evacuation of patients—was present in Korea from the early months of the war, but the focus of forward psychiatry was to reduce psychiatric causalities from combat fatigue and maximize rapid return-to-duty.4-6 With no real conception of PTSD, there were limited treatments available, and evidenced-based trauma-focused treatments for PTSD would not be introduced for at least another 4 decades.27-29
Skinner and Kaplick conducted a historical review of case descriptions of trauma-related conditions from World War I through the Vietnam War and noted the consistent inclusion of hyperarousal and intrusive symptoms, although there also was a greater emphasis on somatic conversion or hysteria symptoms in the earlier descriptions.30 By the Korean War, descriptions of combat fatigue included a number of symptoms that overlap with PTSD, including preoccupation with the traumatic stressor, nightmares, irritability/anger, increased startle, and hyperarousal.31 But following the acute phases, attention to any chronic problems associated with these conditions waned. As was acknowledged by a military psychiatrist in a 1954 talk, studies of the long-term adjustment of those who had “broken down in combat” were sorely needed.6 In a small 1965 study reported by Archibald and Tuddenham, persistent symptoms of combat fatigue among Korean War veterans were definitely present, and there was even a suggestion that the symptoms had increased over the decade since the war.32
Given the stoicism that typified cultural expectations for military men during this period, Korean War veterans may also have been reluctant to seek mental health treatment either at the time or later. In short, it is likely that a nontrivial proportion of Korean War veterans with PTSD were underdiagnosed and received suboptimal or no mental health treatment for decades following their war experiences.33 Although the nature of the war, deployment, and public support were distinct in World War II vs the Korean War, the absence of attention to the long-term effects of disorders related to combat trauma and the cultural expectations for stoicism suggest that PTSD among aging World War II veterans may also have gone underrecognized and undertreated.
Apart from the lack of interest in chronic effects of stressors, another problem that has plagued the limited empirical research on Korean War veterans has been the propensity to combine Korean War with World War II veteran samples in studies. Because World War II veterans have outnumbered Korean War veterans until recently, combined samples tended to have relatively few Korean War veterans. Nevertheless, from those studies that have been reported in which 2 groups were compared, important differences have been revealed. Specifically, although precise estimates of the prevalence of PTSD among Korean War combat veterans have varied depending on sampling and method, studies from the 1990s and early 2000s suggested that the prevalence of PTSD and other mental health concerns as well as the severity of symptoms, suicide risk, and psychosocial adjustment difficulties were worse among Korean War combat veterans relative to those among World War II combat veterans; however, both groups had lower prevalence than did Vietnam War combat veterans.21,34-37 Several authors speculated that these differences in outcome were at least partially due to differences in public support for the respective wars.36,37
Although there has been a paucity of research on psychiatric issues and PTSD in Korean War veterans, POWs who were very likely to have been exposed to extreme psychological traumas have received some attention. There have been comparisons of mortality and morbidity among POWs from the Korean War (PWK), World War II Pacific Theater (PWJ), and Europe (PWE).38 Among measures that were administered to the former POWs, the overall pattern seen from survey data in the mid-1960s revealed significantly worse health and functioning among the PWK and PWJ groups relative to the PWE group, with psychiatric difficulties being the most commonly reported impairments among the former 2 groups. This pattern was found most strongly with regards to objective measures, such as hospitalizations for “psychoneuroses,” and US Department of Veterans Affairs (VA) disability records, as well as based on self-reported psychosocial/recreational difficulties measured using the Cornell Medical Index (CMI).38
Gold and colleagues reported a follow-up study of more than 700 former POWs who were reinterviewed between 1989 and 1992.39 Although there was no scale of PTSD symptoms prior to formulation of the diagnosis in 1980, the CMI was a self-reported checklist that included a large range of both medical as well as behavioral and psychiatric symptoms. Thus, using CMI survey responses from 1965, the authors examined the factor structure (ie, the correlational relationships between multiple scale items and subgroupings of items) of the CMI relative to diagnosis of PTSD in 1989 to 1992 based on results from the Structured Clinical Interview for the DSM-III-R (SCID). The intent was to help discern whether the component domains of PTSD were present and intercorrelated in a pattern similar to that of the contemporary diagnosis. The investigators examined the factor structure of 20 psychological items from the CMI that appeared relevant to PTSD criteria using the 1965 data. Three factors (subgroups of highly intercorrelated items) were found: irritability (31% of variance), fearfulness/anxiousness (9% of the variance), and social withdrawal (7% of the variance). Although these did not directly correspond to, or fully cover, DSM PTSD domains or criteria, there does appear to be a thematic resemblance of the CMI findings with PTSD, including alterations in arousal and mood, vigilance, and startle.
Identification and Treatment of PTSD in Older Veterans
Of the 1.2 million living Korean War veterans in the US, 36.3% use VA provided health care.40 There are a number of complicating factors to consider in the current identification and treatment of PTSD in this cohort, including their advanced age; physical, cognitive, and social changes associated with normal aging; the associated medical and cognitive comorbidities; and the specific social-contextual factors in that age cohort. Any combination of these factors may complicate recognition, diagnosis, and treatment. It is also important to be cognizant of the additional stressors that may have been experienced by ethnic minorites and women serving in Korea, which are poorly documented and studied. Racial integration of the US military began during the Korean War, but the general pattern was for African American soldiers to be assigned to all-white units, rather than the reverse.14,41,42 And although the majority of military personnel serving in Korea were male, there were women serving in health care positions at mobile army surgical hospital (MASH) units, medical air evacuation (Medevac) aircraft, and off-shore hospital ships.
The clinical presentation of PTSD in older adults has varied, which may partially relate to the time elapsed since the index trauma. For example, older veterans in general may show less avoidance behavior as a part of PTSD, but in those who experience trauma later in life there may actually be greater avoidance.43,44 There have also been discrepant reports of intrusion or reexperiencing of symptoms, with these also potentially reduced in older veterans.43,44 However, sleep disturbances seem to be very common among elderly combat veterans, and attention should be paid to the possible presence of sleep apnea, which may be more common in veterans with PTSD in general.43,45,46
PTSD symptoms may reemerge after decades of remission or quiescence during retirement and/or with the emergence of neurocognitive impairment, such as Alzheimer disease or dementia. These individuals may have more difficulty engaging in distracting activities and work and spend more time engaging in reminiscence about the past, which can include increased focus on traumatic memories.45,47 Davison and colleagues have suggested a concept they call later-adulthood trauma reengagement (LATR) where later in life combat veterans may “confront and rework their wartime memories in an effort to find meaning and build coherence.”48 This process can be a double-edged sword, leading at times positively to enhanced personal growth or negatively to increased symptoms; preventive interventions may be able foster a more positive outcome.48
There is some evidence supporting the validity of the Clinician Administered PTSD Scale (CAPS) for the evaluation of PTSD in older adults, although this was based on the DSM-III-revised criteria for PTSD and an earlier version of CAPS.49 Bhattarai and colleagues examined responses to the 35-item Mississippi Scale for Combat-Related PTSD (M-PTSD) using VA clinical data collected between 2008 and 2015 on veterans of each combat era from World War II through the post-9/11.50 Strong internal consistency and test-retest reliability of the M-PTSD was observed within each veteran era sample. However, using chart diagnosis of PTSD as the criterion standard, the cut-scores for optimal balance of sensitivity and specificity of the M-PTSD scores were substantially lower for the older cohorts (World War II and Korean War veterans) relative to those for Vietnam and more recent veteran cohorts. The authors concluded that M-PTSD can be validly used to screen for PTSD in veterans within each of these cohorts but recommended using lower than standard cut-scores for Korean War and World War II veterans.50
This is also consistent with reports that suggest the use of lower cut-scores on self-administered PTSD symptom screens.43,44 For the clinician interested in quantifying the severity of PTSD, the most recent tools available are the CAPS-5 and the PCL-5, which have both been created in accordance with the DSM-5. The CAPS-5 is a rater-administered tool, and the PCL-5 is self-administered by the veteran. Although there has been little research using these newer tools in geriatric populations, they can currently serve as a means of tracking the severity of PTSD while we await measures that are better validated in Korean War and other older veterans.
Beyond specific empirical guidance, VA clinicians must presently rely on clinical observations and experience. Patients from the Korean War cohort often present at the insistence of a family member for changes in sleep, mood, behavior, or cognition. When the veterans themselves present, older adults with PTSD often focus more on somatic concerns (including pain, sleep, and gastrointestinal disturbance) than psychiatric problems per se. The latter tendency may in part be due to the salience of such symptoms for them, but perhaps also due to considerable stigma of mental health care that is still largely present in this group.43,44
Psychotherapy
Current VA treatment guidelines recommend trauma-focused therapies, with the strongest evidence base for prolonged exposure (PE), cognitive processing therapy (CPT), and eye movement desensitization and reprocessing (EMDR) therapies.51
There have been several excellent prior reviews discussing treatment of PTSD in older adults generally.10,43,44,52 These reviews have invariably expressed concern about the lack of sufficient empirical studies, but based on evidence from studies and case reports, there seems to be tentative support that trauma-focused therapies are acceptable and efficacious for use with older adults with PTSD. In their recent scoping review, Pless Kaiser and colleagues made several recommendations for trauma-focused therapy with older adults, including slow/careful pacing and use of compensatory aids for cognitive and sensory deficits.44 When cognitive impairment has exacerbated PTSD symptoms, they suggest therapists consider using an adapted form of CPT completed without a trauma narrative. For PE they recommend extending content across sessions and involving spouse or caregivers to assist with in vivo exposure and homework completion.44
Recent studies suggest that PTSD may be a risk factor for the later development of neurodegenerative disorders, and it is often during assessments for dementia that a revelation of PTSD occurs.10,43,47,55 Cognitive impairment may also be of relevance in deciding on the type of psychotherapy to be implemented, as it may have more adverse effects on the effectiveness of CPT than of exposure-based treatments (PE or EMDR). It may be useful to perform a cognitive assessment prior to initiation of a cognitive-based therapy, although extensive cognitive testing may not be practical or may be contraindicated because of fatigue. A brief screening tool such as the Montreal Cognitive Assessment or the Mini-Mental State Examinationmay be helpful.56, 57
Prolonged exposure has been reported by many clinicians to be effective in older adults with PTSD; however, due consideration should be given to the needs of individuals, as many have functioned for decades by suppressing memories.
Apart from the treatment needs for specific PTSD symptoms, the decades-long effects of poor sleep, irritability, hypervigilance, and dissociation also have social consequences for patients, including marital discord and divorce, and social and family isolation that should be addressed in therapy when appropriate. In addition, many Korean War veterans, like all veterans, sought postmilitary employment in professions that are associated with higher rates of exposure to psychological trauma, such as police or fire departments, and this may have an exacerbating effect on PTSD.58
Pharmacotherapy
There is very little empirical evidence guiding pharmacologic approaches to PTSD in older veterans. This population is at increased risk for many comorbidities, and pharmacologic treatments many require dosage adjustments, as is the case for any geriatric patient. Selective serotonin reuptake inhibitor (SSRI) and serotonin norepinephrine reuptake inhibitor (SNRI) medications have been proposed for some cases of PTSD.59,60 Health care providers may consider the SSRIs escitalopram or sertraline preferentially given their decreased potential for drug-drug interactions, anticholinergic effects, or cardiac toxicity compared with that of other drugs in this class.60,61 As venlafaxine can increase blood pressure, especially at higher doses, prescribers may choose duloxetine as an alternative if a SNRI is indicated.60 For veterans when prazosin is being considered for nightmare control, monitoring for hypotension, orthostasis, and the administration of other antihypertensives or prostatic hypertrophy medications is necessary.61 The use of benzodiazepines, while not recommended for PTSD, should be viewed with even greater trepidation in a geriatric population given enhanced risk of falls and confusion in the geriatric veteran population.60,62
Conclusions
Many of the oldest veterans (aged > 80 years) are from the Korean War era. The harsh and unique nature of the war, as well as the differences in context and support from the US public, and the outcome of the war, may have all contributed to and elevation of “combat fatigue” and PTSD among combat veterans from the Korean War. As the “forgotten war” cohort also has been forgotten by researchers, relatively little is known about posttraumatic stress sequelae of these veterans in the decades following the war.
From available evidence, we can readily surmise that problems were underrecognized and suboptimally diagnosed and treated. There is tentative evidence supporting the use of standard interviews and rating scales, such as the CAPS, M-PTSD, and PCL, but lower cut-scores than applied with Vietnam and later veteran cohorts are generally recommended to avoid excessive false negative errors. In terms of psychotherapy treatment, there is again a stark paucity of systematic research, but the limited evidence from studies of PTSD treatment in older adults from the general population tentatively support the acceptability and potential efficacy of recognized evidence-based trauma-focused psychotherapies for PTSD. Research on medication treatment is similarly lacking, but the general recommendations for the use of SSRI or SNRI medications seem to be valid, at least in our clinical experience, and the general rules for geriatric psychopharmacology definitely apply here—start low, go slow.
There are several important avenues for future research. Most pressing among these are establishing the effectiveness of existing treatments, and the modifications that may be needed in the broader context of the above factors, as well as the physical and cognitive changes associated with advanced age. Further research on the phenomenologic aspects of PTSD among Korean War and subsequent cohorts are also needed, as the information obtained will not only guide more effective personalized treatment of the Korean War veterans who remain with us, but also inform future generations of care in terms of the degree and dimensions of variability that may present between cohorts and within cohorts over the life span.
The Korean War lasted from June 25, 1950 through July 27, 1953. Although many veterans of the Korean War experienced traumas during extremely stressful combat conditions. However, they would not have been diagnosed with posttraumatic stress disorder (PTSD) at the time because the latter did not exist as a formal diagnosis until the publication of the third edition of the Diagnostic and Statistical Manual (DSM) in 1980.1 Prior to 1980, psychiatric syndromes resulting from war and combat exposure where known by numerous other terms including shell shock, chronic traumatic war neurosis, and combat fatigue/combat exhaustion.2,3 Military psychiatrists attended to combat fatigue during the course of the Korean War, but as was true of World War I and II, the focus was on returning soldiers to duty. Combat fatigue was generally viewed as a transient condition.4-8
Although now octo- and nonagenarians, in 2019 there are 1.2 million living Korean War veterans in the US, representing 6.7% of all current veterans.9 Understanding their war experiences and the nature of their current and past presentation of PTSD is relevant not only in formal mental health settings, but in primary care settings, including home-based primary care, as well as community living centers, skilled nursing facilities and assisted living facilities. Older adults with PTSD often present with somatic concerns rather than spontaneously reporting mental health symptoms.10 Beyond the short-term clinical management of Korean War veterans with PTSD, consideration of their experiences also has long-term relevance for the appropriate treatment of other veteran cohorts as they age in coming decades.
The purpose of this article is to provide a clinically focused overview of PTSD in Korean War veterans, to help promote understanding of this often-forgotten group of veterans, and to foster optimized personalized care. This overview will include a description of the Korean War veteran population and the Korean War itself, the manifestations and identification of PTSD among Korean War veterans, and treatment approaches using evidence-based psychotherapies and pharmacotherapies. Finally, we provide recommendations for future research to address present empirical gaps in the understanding and treatment of Korean War veterans with PTSD.
Causes and Course of the Korean War
When working with Korean War veterans it is important to consider the special nature of that specific conflict. Space considerations limit our ability to do justice to the complex history and numerous battles of the Korean War, but information in the following summary was gleaned from several excellent histories.11-13
The Korean War has been referred to as The Forgotten War, a concern expressed even during the latter parts of the war.14,15 But the war and its veterans warrant remembering. The root and proximal causes of the Korean War are complex and not fully agreed upon by the main participants.16-19 In part this may reflect the fact that there was no clear victor in the Korean War, so that the different protagonists have developed their own versions of the history of the conflict. Also, US involvement and the public reaction to the war must be viewed within the larger historical context of that time. This context included the recent end of 4 years of US involvement in World War II (1941-1945) and the subsequent rapid rise of Cold War tensions between the US and the Soviet Union. The latter also included a worldwide fear of nuclear war and the US fear of the global spread of communism. These fears were fueled by the Soviet-led Berlin Blockade from June 1948 through May 1949, the Soviet Union’s successful atomic bomb test in August 1949, the founding of the People’s Republic of China in October 1949, and the February 1950 Sino-Soviet Treaty of Friendship and Alliance.13
In the closing days of World War II, the US and Soviet Union agreed to a temporary division of Korea along the 38th parallel to facilitate timely and efficient surrender of Japanese troops. But as Cold War tensions rose, the temporary division became permanent, and Soviet- and US-backed governments of the north and south, respectively, were officially established on the Korean peninsula in 1948. Although by 1949 the Soviets and US had withdrawn most troops from the peninsula, tensions between the north and south continued to mount and hostilities increased. To this day the exact causes of the eruption of war remain disputed, although it is clear that ideological as well as economic factors played a role, and both leaders of North and South Korea were pledging to reunite the peninsula under their respective leadership.16-19 The tension culminated on June 25, 1950, when North Korean troops crossed the 38th parallel and invaded South Korea. On June 27, 1950, President Truman ordered US naval and air forces to support South Korea and then ordered the involvement of ground troops on June 30.16,17,19
Although several other member countries of the United Nations (UN) provided troops, 90% of the troops were from the US. About 5.7 million US military personnel served during the war, including about 1.8 million in Korea itself. The US forces experienced approximately 34,000 battle-related deaths, 103,000 were wounded, and 7,000 were prisoners of war (POWs).11,20-22 The nature and events of the Korean War made it particularly stressful and traumatizing for the soldiers, sailors, and marines involved throughout its entire course. These included near defeat in the early months, a widely alternating war front along the north/south axis during the first year, and subsequently, not only intense constant battles on the fronts, but also a demanding and exhausting guerrilla war in the south, which lasted throughout the remainder of the conflict.11,15 The US troops during the initial months of the war have been described as outnumbered and underprepared, as many in the initial phase were reassigned from peace-time occupation duty in Japan.7
The first year of war was characterized by a repeated north-to-south/south-to-north shifts in control of territory. During the first 3 months, the North Korean forces overwhelmed the South and captured control of all but 2 South Korean cities in the far southeastern region (Pusan, now Busan; and Daegu), and US and UN forces were forced to retreat to the perimeter around Pusan. The intense Battle of Pusan Perimeter lasted from August 4, 1950 to September 18, 1950, and resulted in massive causalities as well as a flood of civilian refugees.
The course of the war began to change in early September 1950 with the landing of amphibious US/UN forces at Inchon, behind North Korean lines, which cut off southern supply routes for the North Korean troops.11 US/UN forces soon crossed to the north of the 38th parallel and captured the North Korean capital, Pyongyang, on October 19, 1950. They continued to push north and approached the Yalu River border with China by late November 1950, but then the Chinese introduced their own troops forcing a southward retreat of US/UN troops during which there were again numerous US/UN casualties. Chinese troops retook Seoul in late December 1950/early January 1951. However, the US/UN forces soon recaptured Seoul and advanced back to the 38th parallel. This back-and-forth across the 38th parallel continued until July 1951 when the front line of battle stabilized there. Although the line stabilized, intense battles and casualties continued for 2 more years. During this period US/UN troops also had to deal with guerrilla warfare behind the front lines due to the actions of communist partisans and isolated North Korean troops. This situation continued until the armistice was signed July 27, 1953.
Trauma and Characteristic Stresses of the War
There were many factors that made the Korean War experience different from previous wars, particularly World War II. For example, in contrast to the strong public support during and after World War II, public support for the Korean War in the US was low, particularly during its final year.23 In public opinion polls from October 1952 through April 1953, only 23% to 39% reported feeling that the war was worth fighting.23 A retrospective 1985 survey also found that 70% of World War II veterans, but only 33% of Korean War veterans reported feeling appreciated by the US public on their return from the war.24
Those fighting in the initial months of the war faced a particularly grim situation. According to LTC Philip Smith, who served as Division Psychiatrist on the Masan Front (Pusan Perimeter) during August and September of 1950, “Fighting was almost continuous and all available troops were on the fighting front… For the most part these soldiers were soft from occupation duty, many had not received adequate combat basic training, no refresher combat training in Korea had as yet been instituted,” he reported.7 “The extremes of climate coupled with the generally rugged mountainous terrain in Korea were physical factors of importance…These men were psychologically unprepared for the horrors and isolation of war.” LTC Smith noted that the change in status from civilian or occupation life to the marked deprivation of the war in Korea had been “too abrupt to allow as yet for a reasonable adjustment to the new setting” and that as a result “the highest rate of wounded and neuropsychiatric casualties in the Korean campaign resulted.”7
Even after this initial period, the nature of the shifting war, the challenging terrain, the high military casualty rate, and the high rate of civilian casualties and displacement continued throughout the war.
PTSD in Korean War Veterans
It is clear that Korean War combat veterans were exposed to traumatic events. It is unknown how many developed PTSD. While notions of psychological distress and disability related to combat trauma exposure have existed for centuries, Korean War and World War II veterans are a remaining link to pre-DSM PTSD mental health in the military. Military/forward psychiatry—psychiatric services near the battle zone rather than requiring evacuation of patients—was present in Korea from the early months of the war, but the focus of forward psychiatry was to reduce psychiatric causalities from combat fatigue and maximize rapid return-to-duty.4-6 With no real conception of PTSD, there were limited treatments available, and evidenced-based trauma-focused treatments for PTSD would not be introduced for at least another 4 decades.27-29
Skinner and Kaplick conducted a historical review of case descriptions of trauma-related conditions from World War I through the Vietnam War and noted the consistent inclusion of hyperarousal and intrusive symptoms, although there also was a greater emphasis on somatic conversion or hysteria symptoms in the earlier descriptions.30 By the Korean War, descriptions of combat fatigue included a number of symptoms that overlap with PTSD, including preoccupation with the traumatic stressor, nightmares, irritability/anger, increased startle, and hyperarousal.31 But following the acute phases, attention to any chronic problems associated with these conditions waned. As was acknowledged by a military psychiatrist in a 1954 talk, studies of the long-term adjustment of those who had “broken down in combat” were sorely needed.6 In a small 1965 study reported by Archibald and Tuddenham, persistent symptoms of combat fatigue among Korean War veterans were definitely present, and there was even a suggestion that the symptoms had increased over the decade since the war.32
Given the stoicism that typified cultural expectations for military men during this period, Korean War veterans may also have been reluctant to seek mental health treatment either at the time or later. In short, it is likely that a nontrivial proportion of Korean War veterans with PTSD were underdiagnosed and received suboptimal or no mental health treatment for decades following their war experiences.33 Although the nature of the war, deployment, and public support were distinct in World War II vs the Korean War, the absence of attention to the long-term effects of disorders related to combat trauma and the cultural expectations for stoicism suggest that PTSD among aging World War II veterans may also have gone underrecognized and undertreated.
Apart from the lack of interest in chronic effects of stressors, another problem that has plagued the limited empirical research on Korean War veterans has been the propensity to combine Korean War with World War II veteran samples in studies. Because World War II veterans have outnumbered Korean War veterans until recently, combined samples tended to have relatively few Korean War veterans. Nevertheless, from those studies that have been reported in which 2 groups were compared, important differences have been revealed. Specifically, although precise estimates of the prevalence of PTSD among Korean War combat veterans have varied depending on sampling and method, studies from the 1990s and early 2000s suggested that the prevalence of PTSD and other mental health concerns as well as the severity of symptoms, suicide risk, and psychosocial adjustment difficulties were worse among Korean War combat veterans relative to those among World War II combat veterans; however, both groups had lower prevalence than did Vietnam War combat veterans.21,34-37 Several authors speculated that these differences in outcome were at least partially due to differences in public support for the respective wars.36,37
Although there has been a paucity of research on psychiatric issues and PTSD in Korean War veterans, POWs who were very likely to have been exposed to extreme psychological traumas have received some attention. There have been comparisons of mortality and morbidity among POWs from the Korean War (PWK), World War II Pacific Theater (PWJ), and Europe (PWE).38 Among measures that were administered to the former POWs, the overall pattern seen from survey data in the mid-1960s revealed significantly worse health and functioning among the PWK and PWJ groups relative to the PWE group, with psychiatric difficulties being the most commonly reported impairments among the former 2 groups. This pattern was found most strongly with regards to objective measures, such as hospitalizations for “psychoneuroses,” and US Department of Veterans Affairs (VA) disability records, as well as based on self-reported psychosocial/recreational difficulties measured using the Cornell Medical Index (CMI).38
Gold and colleagues reported a follow-up study of more than 700 former POWs who were reinterviewed between 1989 and 1992.39 Although there was no scale of PTSD symptoms prior to formulation of the diagnosis in 1980, the CMI was a self-reported checklist that included a large range of both medical as well as behavioral and psychiatric symptoms. Thus, using CMI survey responses from 1965, the authors examined the factor structure (ie, the correlational relationships between multiple scale items and subgroupings of items) of the CMI relative to diagnosis of PTSD in 1989 to 1992 based on results from the Structured Clinical Interview for the DSM-III-R (SCID). The intent was to help discern whether the component domains of PTSD were present and intercorrelated in a pattern similar to that of the contemporary diagnosis. The investigators examined the factor structure of 20 psychological items from the CMI that appeared relevant to PTSD criteria using the 1965 data. Three factors (subgroups of highly intercorrelated items) were found: irritability (31% of variance), fearfulness/anxiousness (9% of the variance), and social withdrawal (7% of the variance). Although these did not directly correspond to, or fully cover, DSM PTSD domains or criteria, there does appear to be a thematic resemblance of the CMI findings with PTSD, including alterations in arousal and mood, vigilance, and startle.
Identification and Treatment of PTSD in Older Veterans
Of the 1.2 million living Korean War veterans in the US, 36.3% use VA provided health care.40 There are a number of complicating factors to consider in the current identification and treatment of PTSD in this cohort, including their advanced age; physical, cognitive, and social changes associated with normal aging; the associated medical and cognitive comorbidities; and the specific social-contextual factors in that age cohort. Any combination of these factors may complicate recognition, diagnosis, and treatment. It is also important to be cognizant of the additional stressors that may have been experienced by ethnic minorites and women serving in Korea, which are poorly documented and studied. Racial integration of the US military began during the Korean War, but the general pattern was for African American soldiers to be assigned to all-white units, rather than the reverse.14,41,42 And although the majority of military personnel serving in Korea were male, there were women serving in health care positions at mobile army surgical hospital (MASH) units, medical air evacuation (Medevac) aircraft, and off-shore hospital ships.
The clinical presentation of PTSD in older adults has varied, which may partially relate to the time elapsed since the index trauma. For example, older veterans in general may show less avoidance behavior as a part of PTSD, but in those who experience trauma later in life there may actually be greater avoidance.43,44 There have also been discrepant reports of intrusion or reexperiencing of symptoms, with these also potentially reduced in older veterans.43,44 However, sleep disturbances seem to be very common among elderly combat veterans, and attention should be paid to the possible presence of sleep apnea, which may be more common in veterans with PTSD in general.43,45,46
PTSD symptoms may reemerge after decades of remission or quiescence during retirement and/or with the emergence of neurocognitive impairment, such as Alzheimer disease or dementia. These individuals may have more difficulty engaging in distracting activities and work and spend more time engaging in reminiscence about the past, which can include increased focus on traumatic memories.45,47 Davison and colleagues have suggested a concept they call later-adulthood trauma reengagement (LATR) where later in life combat veterans may “confront and rework their wartime memories in an effort to find meaning and build coherence.”48 This process can be a double-edged sword, leading at times positively to enhanced personal growth or negatively to increased symptoms; preventive interventions may be able foster a more positive outcome.48
There is some evidence supporting the validity of the Clinician Administered PTSD Scale (CAPS) for the evaluation of PTSD in older adults, although this was based on the DSM-III-revised criteria for PTSD and an earlier version of CAPS.49 Bhattarai and colleagues examined responses to the 35-item Mississippi Scale for Combat-Related PTSD (M-PTSD) using VA clinical data collected between 2008 and 2015 on veterans of each combat era from World War II through the post-9/11.50 Strong internal consistency and test-retest reliability of the M-PTSD was observed within each veteran era sample. However, using chart diagnosis of PTSD as the criterion standard, the cut-scores for optimal balance of sensitivity and specificity of the M-PTSD scores were substantially lower for the older cohorts (World War II and Korean War veterans) relative to those for Vietnam and more recent veteran cohorts. The authors concluded that M-PTSD can be validly used to screen for PTSD in veterans within each of these cohorts but recommended using lower than standard cut-scores for Korean War and World War II veterans.50
This is also consistent with reports that suggest the use of lower cut-scores on self-administered PTSD symptom screens.43,44 For the clinician interested in quantifying the severity of PTSD, the most recent tools available are the CAPS-5 and the PCL-5, which have both been created in accordance with the DSM-5. The CAPS-5 is a rater-administered tool, and the PCL-5 is self-administered by the veteran. Although there has been little research using these newer tools in geriatric populations, they can currently serve as a means of tracking the severity of PTSD while we await measures that are better validated in Korean War and other older veterans.
Beyond specific empirical guidance, VA clinicians must presently rely on clinical observations and experience. Patients from the Korean War cohort often present at the insistence of a family member for changes in sleep, mood, behavior, or cognition. When the veterans themselves present, older adults with PTSD often focus more on somatic concerns (including pain, sleep, and gastrointestinal disturbance) than psychiatric problems per se. The latter tendency may in part be due to the salience of such symptoms for them, but perhaps also due to considerable stigma of mental health care that is still largely present in this group.43,44
Psychotherapy
Current VA treatment guidelines recommend trauma-focused therapies, with the strongest evidence base for prolonged exposure (PE), cognitive processing therapy (CPT), and eye movement desensitization and reprocessing (EMDR) therapies.51
There have been several excellent prior reviews discussing treatment of PTSD in older adults generally.10,43,44,52 These reviews have invariably expressed concern about the lack of sufficient empirical studies, but based on evidence from studies and case reports, there seems to be tentative support that trauma-focused therapies are acceptable and efficacious for use with older adults with PTSD. In their recent scoping review, Pless Kaiser and colleagues made several recommendations for trauma-focused therapy with older adults, including slow/careful pacing and use of compensatory aids for cognitive and sensory deficits.44 When cognitive impairment has exacerbated PTSD symptoms, they suggest therapists consider using an adapted form of CPT completed without a trauma narrative. For PE they recommend extending content across sessions and involving spouse or caregivers to assist with in vivo exposure and homework completion.44
Recent studies suggest that PTSD may be a risk factor for the later development of neurodegenerative disorders, and it is often during assessments for dementia that a revelation of PTSD occurs.10,43,47,55 Cognitive impairment may also be of relevance in deciding on the type of psychotherapy to be implemented, as it may have more adverse effects on the effectiveness of CPT than of exposure-based treatments (PE or EMDR). It may be useful to perform a cognitive assessment prior to initiation of a cognitive-based therapy, although extensive cognitive testing may not be practical or may be contraindicated because of fatigue. A brief screening tool such as the Montreal Cognitive Assessment or the Mini-Mental State Examinationmay be helpful.56, 57
Prolonged exposure has been reported by many clinicians to be effective in older adults with PTSD; however, due consideration should be given to the needs of individuals, as many have functioned for decades by suppressing memories.
Apart from the treatment needs for specific PTSD symptoms, the decades-long effects of poor sleep, irritability, hypervigilance, and dissociation also have social consequences for patients, including marital discord and divorce, and social and family isolation that should be addressed in therapy when appropriate. In addition, many Korean War veterans, like all veterans, sought postmilitary employment in professions that are associated with higher rates of exposure to psychological trauma, such as police or fire departments, and this may have an exacerbating effect on PTSD.58
Pharmacotherapy
There is very little empirical evidence guiding pharmacologic approaches to PTSD in older veterans. This population is at increased risk for many comorbidities, and pharmacologic treatments many require dosage adjustments, as is the case for any geriatric patient. Selective serotonin reuptake inhibitor (SSRI) and serotonin norepinephrine reuptake inhibitor (SNRI) medications have been proposed for some cases of PTSD.59,60 Health care providers may consider the SSRIs escitalopram or sertraline preferentially given their decreased potential for drug-drug interactions, anticholinergic effects, or cardiac toxicity compared with that of other drugs in this class.60,61 As venlafaxine can increase blood pressure, especially at higher doses, prescribers may choose duloxetine as an alternative if a SNRI is indicated.60 For veterans when prazosin is being considered for nightmare control, monitoring for hypotension, orthostasis, and the administration of other antihypertensives or prostatic hypertrophy medications is necessary.61 The use of benzodiazepines, while not recommended for PTSD, should be viewed with even greater trepidation in a geriatric population given enhanced risk of falls and confusion in the geriatric veteran population.60,62
Conclusions
Many of the oldest veterans (aged > 80 years) are from the Korean War era. The harsh and unique nature of the war, as well as the differences in context and support from the US public, and the outcome of the war, may have all contributed to and elevation of “combat fatigue” and PTSD among combat veterans from the Korean War. As the “forgotten war” cohort also has been forgotten by researchers, relatively little is known about posttraumatic stress sequelae of these veterans in the decades following the war.
From available evidence, we can readily surmise that problems were underrecognized and suboptimally diagnosed and treated. There is tentative evidence supporting the use of standard interviews and rating scales, such as the CAPS, M-PTSD, and PCL, but lower cut-scores than applied with Vietnam and later veteran cohorts are generally recommended to avoid excessive false negative errors. In terms of psychotherapy treatment, there is again a stark paucity of systematic research, but the limited evidence from studies of PTSD treatment in older adults from the general population tentatively support the acceptability and potential efficacy of recognized evidence-based trauma-focused psychotherapies for PTSD. Research on medication treatment is similarly lacking, but the general recommendations for the use of SSRI or SNRI medications seem to be valid, at least in our clinical experience, and the general rules for geriatric psychopharmacology definitely apply here—start low, go slow.
There are several important avenues for future research. Most pressing among these are establishing the effectiveness of existing treatments, and the modifications that may be needed in the broader context of the above factors, as well as the physical and cognitive changes associated with advanced age. Further research on the phenomenologic aspects of PTSD among Korean War and subsequent cohorts are also needed, as the information obtained will not only guide more effective personalized treatment of the Korean War veterans who remain with us, but also inform future generations of care in terms of the degree and dimensions of variability that may present between cohorts and within cohorts over the life span.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Arlington VA: American Psychiatric Association; 1980.
2. Friedman MJ, Schnurr PP, McDonagh-Coyle A. Posttraumatic stress disorder in the military veteran. Psychiatr Clin North Am. 1994;17(2):265-277.
3. Salmon TW. The Care and Treatment of Mental Diseases and War Neuroses (“Shell Shock”) in the British Army. New York: War Work Committee of the National Committee for Mental Hygiene, Inc; 1917.
4. Jones E, Wessely S. “Forward psychiatry” in the military: its origins and effectiveness. J Trauma Stress. 2003;16(4):411-419.
5. Newman RA. Combat fatigue: a review to the Korean conflict. Mil Med. 1964;129:921-928.
6. Harris FG. Some comments on the differential diagnosis and treatment of psychiatric breakdowns in Korea. https://history.amedd.army.mil/booksdocs/korea/recad2/ch9-2.html. Published April 30, 1954. Accessed November 8, 2019.
7. Smith PB. Psychiatric experiences during the Korean conflict. Am Pract Dig Treat. 1955;6(2):183-189.
8. Koontz AR. Psychiatry in the Korean War. Military Surg.
1950;107(6):444-445.
9. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Population Tables - Table 2L: VETPOP2016 Living Veterans by period of service, gender, 2015-2045. https://www.va.gov/vetdata/docs/Demographics/New_Vetpop_Model/2L_VetPop2016
_POS_National.xlsx. Accessed November 8, 2019.
10. Cook JM, McCarthy E, Thorp SR. Older adults with PTSD: brief state of research and evidence-based psychotherapy case illustration. Am J Geriatr Psychiatry. 2017;25(5):522-530.
11. Millett AR. Korean War: 1950-1953. Encylopaedia Britannica. https://www.britannica.com/event/Korean-War#accordion-article-history. Updated Nov 7, 2019. Accessed November 8, 2019.
12. Stack L. Korean War, a ‘forgotten’ conflict that shaped the modern world. The New York Times. January 2, 2018. https://www.nytimes.com/2018/01/01/world/asia/korean-war-history.html. Accessed November 8, 2019.
13. Westad OA. The Cold War: A World History. New York: Basic Books; 2018.
14. Young C, Conard PL, Armstrong ML, Lacy D. Older military veteran care: many still believe they are forgotten. J Holist Nurs. 2018;36(3):291-300.
15. Huebner AJ. Kilroy is back, 1950-1953. In: The Warrior Image: Soldiers in American Culture From the Second World War to the Vietnam Era. Chapel Hill, NC: The University of North Carolina Press; 2008:97-131.
16. The annexation of Korea (editorial). Japan Times. https://www.japantimes.co.jp/opinion/2010/08/29/editorials/the-annexation-of-korea/#.XPgvJvlKhhE. Published August 29, 2010. Accessed November 8, 2019.
17. Gupta K. How did the Korean war begin? China Q. 1972;52:699-716.
18. Lin L, Zhao Y, Ogawa M, Hoge J, Kim BY. Whose history? An analysis of the Korean War in history textbooks from the United States, South Korea, Japan, and China. Social Studies. 2009;100(5):222-232.
19. Weathersby K. The Korean War revisited. Wilson Q. 1999;23(3):91.
20. US Department of Veterans Affairs, Office of Program and Data Analyses, Assistant Secretary for Planning and Analysis. Data on veterans of the Korean War. https://www.va.gov/vetdata/docs/SpecialReports/KW2000.pdf. Published June 2000. Accessed November 8, 2019.
21. Brooks MS, Fulton L. Evidence of poorer life-course mental health outcomes among veterans of the Korean War cohort. Aging Ment Health. 2010;14(2):177-183.
22. US Department of Veterans Affairs, Office of Public Affairs. America’s wars. https://www.va.gov/opa/publications/factsheets/fs_americas_wars.pdf. Accessed November 8, 2019.
23. Memorandum on recent polls on Korea. https://www.eisenhowerlibrary.gov/sites/default/files/research/online-documents/korean-war/public-opinion-1953-06-02.pdf. Published June 2, 1953. Accessed November 8, 2019.
24. Elder GH Jr, Clipp EC. Combat experience and emotional health: impairment and resilience in later life. J Pers. 1989;57(2):311-341.
25. US Department of Veterans Affairs. Public health: cold injuries. https://www.publichealth.va.gov/PUBLICHEALTH/exposures/cold-injuries/index.asp. Updated July 31, 2019. Accessed November 8, 2019.
26. US Department of Veterans Affairs. Korean War veterans health issues. https://www.va.gov/health-care/health-needs-conditions/health-issues-related-to-service-era
/korean-war/. Updated June 14, 2019. Accessed November 8, 2019.
27. Shapiro F. Efficacy of the eye movement desensitization procedure in the treatment of traumatic memories. J Trauma Stress. 1989;2(2):199-223.
28. Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. J Consul Clin Psychol. 1992;60(5):748-756.
29. Foa EB, Rothbaum BO. Treating Trauma of Rape: Cognitive-Behavioral Therapy for PTSD. New York: Guilford; 2001.
30. Skinner R, Kaplick PM. Cultural shift in mental illness: a comparison of stress responses in World War I and the Vietnam War. JRSM Open. 2017;8(12):2054270417746061.
31. Kardiner A, Spiegel H. War Stress and Neurotic Illness. New York: Hoeber; 1947.
32. Archibald HC, Tuddenham RD. Persistent stress reaction after combat: a 20-year follow-up. Arch Gen Psychiatry. 1965;12:475-481.
33. Cook JM, Simiola V. Trauma and aging. Curr Psychiatry Rep. 2018;20(10):93.
34. Rosenheck R, Fontana A. Long-term sequelae of combat in World War II, Korea and Vietnam: a comparative study. In: McCaughey BG, Fullerton CS, Ursano RJ, eds. Individual
and Community Responses to Trauma and Disaster: The Structure of Human Chaos. New York: Cambridge University Press; 1994:330-359.
35. Blake DD, Keane TM, Wine PR, Mora C, Taylor KL, Lyons JA. Prevalence of PTSD symptoms in combat veterans seeking medical treatment. J Trauma Stress. 1990;3(1):15-27.
36. McCranie EW, Hyer LA. Posttraumatic stress disorder symptoms in Korean conflict and World War II combat veterans seeking outpatient treatment. J Trauma Stress. 2000;13(3):427-439.
37. Fontana A, Rosenheck R. Traumatic war stressors and psychiatric symptoms among World War II, Korean, and Vietnam War veterans. Psychology Aging. 1994;9(1):27-33.
38. Beebe GW. Follow-up studies of World War II and Korean war prisoners. II. Morbidity, disability, and maladjustments. Am J Epidemiol. 1975;101(5):400-422.
39. Gold PB, Engdahl BE, Eberly RE, Blake RJ, Page WF, Frueh BC. Trauma exposure, resilience, social support, and PTSD construct validity among former prisoners of war. Social Psychiatry Psychiatr Epidemiol. 2000;35(1):36-42.
40. US Department of Veterans Affairs. Key statistics by veteran status and period of service. https://www.va.gov/vetdata/docs/SpecialReports/KeyStats.pdf. Accessed November 11, 2019.
41. Bowers WT, Hammond WM, MacGarrigle GL. Black Soldier, White Army. Washington DC: US Army Center of Military History; 1996.
42. Black HK. Three generations, three wars: African American veterans. Gerontologist. 2016;56(1):33-41.
43. Thorp SR, Sones HM, Cook JM. Posttraumatic stress disorder among older adults. In: Sorocco KH, Lauderdale S, eds. Cognitive Behavior Therapy With Older Adults: Innovations Across Care Settings. New York: Springer; 2011:189-217.
44. Pless Kaiser A, Cook JM, Glick DM, Moye J. Posttraumatic stress disorder in older adults: a conceptual review. Clinical Gerontol. 2019;42(4):359-376.
45. Sadavoy J. Survivors. A review of the late-life effects of prior psychological trauma. Am J Geriatr Psychiatry. 1997;5(4):287-301.
46. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636.
47. Mota N, Tsai J, Kirwin PD, et al. Late-life exacerbation of PTSD symptoms in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2016;77(3):348-354.
48. Davison EH, Kaiser AP, Spiro A 3rd, Moye J, King LA, King DW. From Late-onset stress symptomatology to later-adulthood trauma reengagement in aging combat veterans: taking a broader view. Gerontologist. 2016;56(1):14-21.
49. Hyer L, Summers MN, Boyd S, Litaker M, Boudewyns P. Assessment of older combat veterans with the clinician-administered PTSD scale. J Trauma Stress. 1996;9(3):587-593.
50. Bhattarai JJ, Oehlert ME, Weber DK. Psychometric properties of the Mississippi Scale for combat-related posttraumatic stress disorder based on veterans’ period of service. Psychol Serv. 2018. [Epub ahead of print]
51. US Department of Veterans Affairs, US Department of Defense. VA/DOD Clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf.
Updated 2017. Accessed November 11, 2019.
52. Dinnen S, Simiola V, Cook JM. Post-traumatic stress disorder in older adults: a systematic review of the psychotherapy treatment literature. Aging Ment Health. 2015;19(2):144-150.
53. Jakel RJ. Posttraumatic Stress Disorder in the Elderly. Psychiatr Clin North Am. 2018;41(1):165-175.
54. Thorp SR, Glassman LH, Wells SY, et al. A randomized controlled trial of prolonged exposure therapy versus relaxation training for older veterans with military-related PTSD. J Anxiety Disord. 2019;64:45-54.
55. Kang B, Xu H, McConnell ES. Neurocognitive and psychiatric comorbidities of posttraumatic stress disorder among older veterans: a systematic review. Int J Geriatr Psychiatry. 2019;34(4):522-538.
56. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
57. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
58. Paton D. Traumatic Stress in Police Officers a Career-Length Assessment From Recruitment to Retirement. Springfield, IL: Charles C. Thomas; 2009.
59. Alexander W. Pharmacotherapy for post-traumatic stress disorder in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37(1):32-38.
60. Beck JG, Sloan DM, Friedman MJ. Pharmacotherapy for PTSD. In: The Oxford Handbook of Traumatic Stress Disorders. Oxford University Press; 2012.
61. Waltman SH, Shearer D, Moore BA. Management of posttraumatic nightmares: a review of pharmacologic and nonpharmacologic treatments since 2013. Curr Psychiatry Rep. 2018;20(12):108.
62. Díaz-Gutiérrez MJ, Martínez-Cengotitabengoa M, Sáez de Adana E, et al. Relationship between the use of benzodiazepines and falls in older adults: a systematic review. Maturitas. 2017;101:17-22.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Arlington VA: American Psychiatric Association; 1980.
2. Friedman MJ, Schnurr PP, McDonagh-Coyle A. Posttraumatic stress disorder in the military veteran. Psychiatr Clin North Am. 1994;17(2):265-277.
3. Salmon TW. The Care and Treatment of Mental Diseases and War Neuroses (“Shell Shock”) in the British Army. New York: War Work Committee of the National Committee for Mental Hygiene, Inc; 1917.
4. Jones E, Wessely S. “Forward psychiatry” in the military: its origins and effectiveness. J Trauma Stress. 2003;16(4):411-419.
5. Newman RA. Combat fatigue: a review to the Korean conflict. Mil Med. 1964;129:921-928.
6. Harris FG. Some comments on the differential diagnosis and treatment of psychiatric breakdowns in Korea. https://history.amedd.army.mil/booksdocs/korea/recad2/ch9-2.html. Published April 30, 1954. Accessed November 8, 2019.
7. Smith PB. Psychiatric experiences during the Korean conflict. Am Pract Dig Treat. 1955;6(2):183-189.
8. Koontz AR. Psychiatry in the Korean War. Military Surg.
1950;107(6):444-445.
9. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Population Tables - Table 2L: VETPOP2016 Living Veterans by period of service, gender, 2015-2045. https://www.va.gov/vetdata/docs/Demographics/New_Vetpop_Model/2L_VetPop2016
_POS_National.xlsx. Accessed November 8, 2019.
10. Cook JM, McCarthy E, Thorp SR. Older adults with PTSD: brief state of research and evidence-based psychotherapy case illustration. Am J Geriatr Psychiatry. 2017;25(5):522-530.
11. Millett AR. Korean War: 1950-1953. Encylopaedia Britannica. https://www.britannica.com/event/Korean-War#accordion-article-history. Updated Nov 7, 2019. Accessed November 8, 2019.
12. Stack L. Korean War, a ‘forgotten’ conflict that shaped the modern world. The New York Times. January 2, 2018. https://www.nytimes.com/2018/01/01/world/asia/korean-war-history.html. Accessed November 8, 2019.
13. Westad OA. The Cold War: A World History. New York: Basic Books; 2018.
14. Young C, Conard PL, Armstrong ML, Lacy D. Older military veteran care: many still believe they are forgotten. J Holist Nurs. 2018;36(3):291-300.
15. Huebner AJ. Kilroy is back, 1950-1953. In: The Warrior Image: Soldiers in American Culture From the Second World War to the Vietnam Era. Chapel Hill, NC: The University of North Carolina Press; 2008:97-131.
16. The annexation of Korea (editorial). Japan Times. https://www.japantimes.co.jp/opinion/2010/08/29/editorials/the-annexation-of-korea/#.XPgvJvlKhhE. Published August 29, 2010. Accessed November 8, 2019.
17. Gupta K. How did the Korean war begin? China Q. 1972;52:699-716.
18. Lin L, Zhao Y, Ogawa M, Hoge J, Kim BY. Whose history? An analysis of the Korean War in history textbooks from the United States, South Korea, Japan, and China. Social Studies. 2009;100(5):222-232.
19. Weathersby K. The Korean War revisited. Wilson Q. 1999;23(3):91.
20. US Department of Veterans Affairs, Office of Program and Data Analyses, Assistant Secretary for Planning and Analysis. Data on veterans of the Korean War. https://www.va.gov/vetdata/docs/SpecialReports/KW2000.pdf. Published June 2000. Accessed November 8, 2019.
21. Brooks MS, Fulton L. Evidence of poorer life-course mental health outcomes among veterans of the Korean War cohort. Aging Ment Health. 2010;14(2):177-183.
22. US Department of Veterans Affairs, Office of Public Affairs. America’s wars. https://www.va.gov/opa/publications/factsheets/fs_americas_wars.pdf. Accessed November 8, 2019.
23. Memorandum on recent polls on Korea. https://www.eisenhowerlibrary.gov/sites/default/files/research/online-documents/korean-war/public-opinion-1953-06-02.pdf. Published June 2, 1953. Accessed November 8, 2019.
24. Elder GH Jr, Clipp EC. Combat experience and emotional health: impairment and resilience in later life. J Pers. 1989;57(2):311-341.
25. US Department of Veterans Affairs. Public health: cold injuries. https://www.publichealth.va.gov/PUBLICHEALTH/exposures/cold-injuries/index.asp. Updated July 31, 2019. Accessed November 8, 2019.
26. US Department of Veterans Affairs. Korean War veterans health issues. https://www.va.gov/health-care/health-needs-conditions/health-issues-related-to-service-era
/korean-war/. Updated June 14, 2019. Accessed November 8, 2019.
27. Shapiro F. Efficacy of the eye movement desensitization procedure in the treatment of traumatic memories. J Trauma Stress. 1989;2(2):199-223.
28. Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. J Consul Clin Psychol. 1992;60(5):748-756.
29. Foa EB, Rothbaum BO. Treating Trauma of Rape: Cognitive-Behavioral Therapy for PTSD. New York: Guilford; 2001.
30. Skinner R, Kaplick PM. Cultural shift in mental illness: a comparison of stress responses in World War I and the Vietnam War. JRSM Open. 2017;8(12):2054270417746061.
31. Kardiner A, Spiegel H. War Stress and Neurotic Illness. New York: Hoeber; 1947.
32. Archibald HC, Tuddenham RD. Persistent stress reaction after combat: a 20-year follow-up. Arch Gen Psychiatry. 1965;12:475-481.
33. Cook JM, Simiola V. Trauma and aging. Curr Psychiatry Rep. 2018;20(10):93.
34. Rosenheck R, Fontana A. Long-term sequelae of combat in World War II, Korea and Vietnam: a comparative study. In: McCaughey BG, Fullerton CS, Ursano RJ, eds. Individual
and Community Responses to Trauma and Disaster: The Structure of Human Chaos. New York: Cambridge University Press; 1994:330-359.
35. Blake DD, Keane TM, Wine PR, Mora C, Taylor KL, Lyons JA. Prevalence of PTSD symptoms in combat veterans seeking medical treatment. J Trauma Stress. 1990;3(1):15-27.
36. McCranie EW, Hyer LA. Posttraumatic stress disorder symptoms in Korean conflict and World War II combat veterans seeking outpatient treatment. J Trauma Stress. 2000;13(3):427-439.
37. Fontana A, Rosenheck R. Traumatic war stressors and psychiatric symptoms among World War II, Korean, and Vietnam War veterans. Psychology Aging. 1994;9(1):27-33.
38. Beebe GW. Follow-up studies of World War II and Korean war prisoners. II. Morbidity, disability, and maladjustments. Am J Epidemiol. 1975;101(5):400-422.
39. Gold PB, Engdahl BE, Eberly RE, Blake RJ, Page WF, Frueh BC. Trauma exposure, resilience, social support, and PTSD construct validity among former prisoners of war. Social Psychiatry Psychiatr Epidemiol. 2000;35(1):36-42.
40. US Department of Veterans Affairs. Key statistics by veteran status and period of service. https://www.va.gov/vetdata/docs/SpecialReports/KeyStats.pdf. Accessed November 11, 2019.
41. Bowers WT, Hammond WM, MacGarrigle GL. Black Soldier, White Army. Washington DC: US Army Center of Military History; 1996.
42. Black HK. Three generations, three wars: African American veterans. Gerontologist. 2016;56(1):33-41.
43. Thorp SR, Sones HM, Cook JM. Posttraumatic stress disorder among older adults. In: Sorocco KH, Lauderdale S, eds. Cognitive Behavior Therapy With Older Adults: Innovations Across Care Settings. New York: Springer; 2011:189-217.
44. Pless Kaiser A, Cook JM, Glick DM, Moye J. Posttraumatic stress disorder in older adults: a conceptual review. Clinical Gerontol. 2019;42(4):359-376.
45. Sadavoy J. Survivors. A review of the late-life effects of prior psychological trauma. Am J Geriatr Psychiatry. 1997;5(4):287-301.
46. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636.
47. Mota N, Tsai J, Kirwin PD, et al. Late-life exacerbation of PTSD symptoms in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2016;77(3):348-354.
48. Davison EH, Kaiser AP, Spiro A 3rd, Moye J, King LA, King DW. From Late-onset stress symptomatology to later-adulthood trauma reengagement in aging combat veterans: taking a broader view. Gerontologist. 2016;56(1):14-21.
49. Hyer L, Summers MN, Boyd S, Litaker M, Boudewyns P. Assessment of older combat veterans with the clinician-administered PTSD scale. J Trauma Stress. 1996;9(3):587-593.
50. Bhattarai JJ, Oehlert ME, Weber DK. Psychometric properties of the Mississippi Scale for combat-related posttraumatic stress disorder based on veterans’ period of service. Psychol Serv. 2018. [Epub ahead of print]
51. US Department of Veterans Affairs, US Department of Defense. VA/DOD Clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf.
Updated 2017. Accessed November 11, 2019.
52. Dinnen S, Simiola V, Cook JM. Post-traumatic stress disorder in older adults: a systematic review of the psychotherapy treatment literature. Aging Ment Health. 2015;19(2):144-150.
53. Jakel RJ. Posttraumatic Stress Disorder in the Elderly. Psychiatr Clin North Am. 2018;41(1):165-175.
54. Thorp SR, Glassman LH, Wells SY, et al. A randomized controlled trial of prolonged exposure therapy versus relaxation training for older veterans with military-related PTSD. J Anxiety Disord. 2019;64:45-54.
55. Kang B, Xu H, McConnell ES. Neurocognitive and psychiatric comorbidities of posttraumatic stress disorder among older veterans: a systematic review. Int J Geriatr Psychiatry. 2019;34(4):522-538.
56. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
57. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
58. Paton D. Traumatic Stress in Police Officers a Career-Length Assessment From Recruitment to Retirement. Springfield, IL: Charles C. Thomas; 2009.
59. Alexander W. Pharmacotherapy for post-traumatic stress disorder in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37(1):32-38.
60. Beck JG, Sloan DM, Friedman MJ. Pharmacotherapy for PTSD. In: The Oxford Handbook of Traumatic Stress Disorders. Oxford University Press; 2012.
61. Waltman SH, Shearer D, Moore BA. Management of posttraumatic nightmares: a review of pharmacologic and nonpharmacologic treatments since 2013. Curr Psychiatry Rep. 2018;20(12):108.
62. Díaz-Gutiérrez MJ, Martínez-Cengotitabengoa M, Sáez de Adana E, et al. Relationship between the use of benzodiazepines and falls in older adults: a systematic review. Maturitas. 2017;101:17-22.
VA Ketamine Controversies
To the Editor: We read with interest the editorial on the clinical use of intranasal esketamine in treatment-resistant depression by Editor-in-Chief Cynthia Geppert in the October 2019 issue of Federal Practitioner.1 A recent case report published in your journal illustrated the success of IV ketamine in alleviating refractory chronic pain caused by a rare disease.2 Ketamine has been well established as an appropriate adjuvant as well as an alternative to opioids in attenuating acute postoperative pain and in certain chronic pain syndromes.3 We write out of concern for the rapidity of adoption of intranasal esketamine without considering the merits of IV ketamine.
When adopting new treatments or extending established drugs for newer indications, clinicians must balance beneficence and nonmaleficence. There is an urgent need for better treatment options for depression, suicidality, posttraumatic stress disorder (PTSD), and chronic pain in the veteran population. However, one must proceed with caution before wide adoption of a treatment that lacks real-world data on sustained or long-term benefits.4 Enthusiasm for this drug must also be tempered by the documented adverse effect (AE) of hepatic injury and the lack of data tracking this AE from repeated, long-term use.5 With these considerations in mind, reliable dosing and predictable pharmacokinetics are of great importance.
In addition to outpatient esketamine, outpatient IV administration of racemic ketamine remains an advantageous option with unique benefits compared with esketamine. Pharmacokinetically, IV ketamine is superior to intranasal esketamine. The bioavailability of intranasal esketamine is likely to be variable. A patient with a poor intranasal application or poor absorption might be falsely labeled an esketamine nonresponder. Increasing intranasal esketamine dosage to avoid false nonresponders may place other patients at risk for overdose and undesired AEs, including dysphoria and hallucinations. The variable bioavailability of intranasal ketamine adds complexity to the examination of its clinical effectiveness. IV ketamine should provide a predictable drug level and more reliable data. One might retort that esketamine is not the same as ketamine. True, esketamine is the S-enantiomer of ketamine, whereas ketamine is a racemic mixture of S- and R-ketamine. However, there is no clear evidence of clinically relevant differences between these formulations.5
Psychomimetic effects and cardiovascular changes are the most common short-term AEs resulting from ketamine.5 An IV infusion allows the treating physician to slowly titrate the administered ketamine to reach an effective concentration at the target site. Unlike an all-or-none intranasal administration, an infusion can be stopped at the first appearance of an AE. Psychomimetic effects, such as hallucinations, visual disturbances, and dysphoria are thought to occur in a dose-dependent fashion and remit once a ketamine infusion is stopped.5 Furthermore, cardiovascular AEs, such as hypertension and tachycardia, are commonly in patients with a body mass index > 30, with IV administration on a mg/kg basis. This suggests that calculated ideal body weight is a safer denominator, and reliable dosing is important to mitigating AEs.6
We urge caution with the widespread adoption of intranasal esketamine and suggest the advantages of the IV route, which offers predictability of AEs and titratability of dose. Questions remain regarding the appropriate dose and formulation of ketamine, rate of infusion, and route of administration for chronic pain and psychiatric indications.5,7 It is our responsibility to further study the long-term safety profile of ketamine and determine an appropriate dose of ketamine. The IV route allows many veterans to be helped in a safe and controllable manner.
Eugene Raggi, MD; and Srikantha L. Rao, MD, MS, FAS
1. Geppert CMA. The VA ketamine controversies. Fed Pract. 2019;36(10):446-447.
2. Eliason AH, Seo Y, Murphy D, Beal C. Adiposis dolorosa pain management. Fed Pract. 2019;36(11):530-533.
3. Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241-254.
4. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci. 2019;44(6):384-385.
5. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
6. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
7. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852-e857.
To the Editor: We read with interest the editorial on the clinical use of intranasal esketamine in treatment-resistant depression by Editor-in-Chief Cynthia Geppert in the October 2019 issue of Federal Practitioner.1 A recent case report published in your journal illustrated the success of IV ketamine in alleviating refractory chronic pain caused by a rare disease.2 Ketamine has been well established as an appropriate adjuvant as well as an alternative to opioids in attenuating acute postoperative pain and in certain chronic pain syndromes.3 We write out of concern for the rapidity of adoption of intranasal esketamine without considering the merits of IV ketamine.
When adopting new treatments or extending established drugs for newer indications, clinicians must balance beneficence and nonmaleficence. There is an urgent need for better treatment options for depression, suicidality, posttraumatic stress disorder (PTSD), and chronic pain in the veteran population. However, one must proceed with caution before wide adoption of a treatment that lacks real-world data on sustained or long-term benefits.4 Enthusiasm for this drug must also be tempered by the documented adverse effect (AE) of hepatic injury and the lack of data tracking this AE from repeated, long-term use.5 With these considerations in mind, reliable dosing and predictable pharmacokinetics are of great importance.
In addition to outpatient esketamine, outpatient IV administration of racemic ketamine remains an advantageous option with unique benefits compared with esketamine. Pharmacokinetically, IV ketamine is superior to intranasal esketamine. The bioavailability of intranasal esketamine is likely to be variable. A patient with a poor intranasal application or poor absorption might be falsely labeled an esketamine nonresponder. Increasing intranasal esketamine dosage to avoid false nonresponders may place other patients at risk for overdose and undesired AEs, including dysphoria and hallucinations. The variable bioavailability of intranasal ketamine adds complexity to the examination of its clinical effectiveness. IV ketamine should provide a predictable drug level and more reliable data. One might retort that esketamine is not the same as ketamine. True, esketamine is the S-enantiomer of ketamine, whereas ketamine is a racemic mixture of S- and R-ketamine. However, there is no clear evidence of clinically relevant differences between these formulations.5
Psychomimetic effects and cardiovascular changes are the most common short-term AEs resulting from ketamine.5 An IV infusion allows the treating physician to slowly titrate the administered ketamine to reach an effective concentration at the target site. Unlike an all-or-none intranasal administration, an infusion can be stopped at the first appearance of an AE. Psychomimetic effects, such as hallucinations, visual disturbances, and dysphoria are thought to occur in a dose-dependent fashion and remit once a ketamine infusion is stopped.5 Furthermore, cardiovascular AEs, such as hypertension and tachycardia, are commonly in patients with a body mass index > 30, with IV administration on a mg/kg basis. This suggests that calculated ideal body weight is a safer denominator, and reliable dosing is important to mitigating AEs.6
We urge caution with the widespread adoption of intranasal esketamine and suggest the advantages of the IV route, which offers predictability of AEs and titratability of dose. Questions remain regarding the appropriate dose and formulation of ketamine, rate of infusion, and route of administration for chronic pain and psychiatric indications.5,7 It is our responsibility to further study the long-term safety profile of ketamine and determine an appropriate dose of ketamine. The IV route allows many veterans to be helped in a safe and controllable manner.
Eugene Raggi, MD; and Srikantha L. Rao, MD, MS, FAS
To the Editor: We read with interest the editorial on the clinical use of intranasal esketamine in treatment-resistant depression by Editor-in-Chief Cynthia Geppert in the October 2019 issue of Federal Practitioner.1 A recent case report published in your journal illustrated the success of IV ketamine in alleviating refractory chronic pain caused by a rare disease.2 Ketamine has been well established as an appropriate adjuvant as well as an alternative to opioids in attenuating acute postoperative pain and in certain chronic pain syndromes.3 We write out of concern for the rapidity of adoption of intranasal esketamine without considering the merits of IV ketamine.
When adopting new treatments or extending established drugs for newer indications, clinicians must balance beneficence and nonmaleficence. There is an urgent need for better treatment options for depression, suicidality, posttraumatic stress disorder (PTSD), and chronic pain in the veteran population. However, one must proceed with caution before wide adoption of a treatment that lacks real-world data on sustained or long-term benefits.4 Enthusiasm for this drug must also be tempered by the documented adverse effect (AE) of hepatic injury and the lack of data tracking this AE from repeated, long-term use.5 With these considerations in mind, reliable dosing and predictable pharmacokinetics are of great importance.
In addition to outpatient esketamine, outpatient IV administration of racemic ketamine remains an advantageous option with unique benefits compared with esketamine. Pharmacokinetically, IV ketamine is superior to intranasal esketamine. The bioavailability of intranasal esketamine is likely to be variable. A patient with a poor intranasal application or poor absorption might be falsely labeled an esketamine nonresponder. Increasing intranasal esketamine dosage to avoid false nonresponders may place other patients at risk for overdose and undesired AEs, including dysphoria and hallucinations. The variable bioavailability of intranasal ketamine adds complexity to the examination of its clinical effectiveness. IV ketamine should provide a predictable drug level and more reliable data. One might retort that esketamine is not the same as ketamine. True, esketamine is the S-enantiomer of ketamine, whereas ketamine is a racemic mixture of S- and R-ketamine. However, there is no clear evidence of clinically relevant differences between these formulations.5
Psychomimetic effects and cardiovascular changes are the most common short-term AEs resulting from ketamine.5 An IV infusion allows the treating physician to slowly titrate the administered ketamine to reach an effective concentration at the target site. Unlike an all-or-none intranasal administration, an infusion can be stopped at the first appearance of an AE. Psychomimetic effects, such as hallucinations, visual disturbances, and dysphoria are thought to occur in a dose-dependent fashion and remit once a ketamine infusion is stopped.5 Furthermore, cardiovascular AEs, such as hypertension and tachycardia, are commonly in patients with a body mass index > 30, with IV administration on a mg/kg basis. This suggests that calculated ideal body weight is a safer denominator, and reliable dosing is important to mitigating AEs.6
We urge caution with the widespread adoption of intranasal esketamine and suggest the advantages of the IV route, which offers predictability of AEs and titratability of dose. Questions remain regarding the appropriate dose and formulation of ketamine, rate of infusion, and route of administration for chronic pain and psychiatric indications.5,7 It is our responsibility to further study the long-term safety profile of ketamine and determine an appropriate dose of ketamine. The IV route allows many veterans to be helped in a safe and controllable manner.
Eugene Raggi, MD; and Srikantha L. Rao, MD, MS, FAS
1. Geppert CMA. The VA ketamine controversies. Fed Pract. 2019;36(10):446-447.
2. Eliason AH, Seo Y, Murphy D, Beal C. Adiposis dolorosa pain management. Fed Pract. 2019;36(11):530-533.
3. Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241-254.
4. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci. 2019;44(6):384-385.
5. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
6. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
7. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852-e857.
1. Geppert CMA. The VA ketamine controversies. Fed Pract. 2019;36(10):446-447.
2. Eliason AH, Seo Y, Murphy D, Beal C. Adiposis dolorosa pain management. Fed Pract. 2019;36(11):530-533.
3. Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241-254.
4. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci. 2019;44(6):384-385.
5. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
6. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
7. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852-e857.
Veterans at higher risk of sleep behavior disorders
Veterans may be at higher risk of idiopathic rapid eye movement sleep behavior disorders, particularly if they have traumatic brain injury (TBI) or posttraumatic stress disorder (PTSD) according to a paper published in Sleep.
In a prospective, cross-sectional study, researchers recruited 394 veterans – 94% of whom were male – who underwent in-lab video-polysomnography and questionnaires about REM sleep behavior disorder (RBD), as well as assessment of their trauma status and medical history.
Overall, 9% of subjects had RBD, a figure considerably higher than has been estimated in the general population (prevalence of 0.38%-1.0%). Seven percent had REM sleep without atonia, 31% had other parasomnias such as a history of dream enactment behavior, and 53% were classified as normal.
The majority of subjects determined to have RBD (n = 34) had either PTSD or comorbid TBI+PTSD (n = 19, 56%). The combined overall crude prevalence of RBD in subjects with either PTSD alone or TBI+PTSD was 16.8% (n = 19 out of 113).
The individuals with PTSD had a 2.81-fold greater odds of RBD and 3.13-fold greater odds of other parasomnias compared with those without PTSD.
Those with both traumatic brain injury and PTSD had 3.43-fold greater odds of RBD and 3.22-fold greater odds of other parasomnias compared with individuals without.
“Interestingly, the neuropathology underpinning PTSD shares common features with RBD, raising the question as to whether or not PTSD has a causal role in the development of RBD, or if a single pathophysiologic process generates two clinical entities,” wrote Jonathan E. Elliott, PhD, of the VA Portland Health Care System, and coauthors.
The researchers also looked for evidence of trauma-associated sleep disorder (TASD), a recently proposed phenomenological sleep disorder whose diagnostic criteria overlaps with REM sleep behavior disorder but includes subjects reporting having an inciting traumatic experience and a history of dreaming related to this experience as well as evidence of autonomic hyperarousal.
The researchers found 22 of the subjects with REM behavior disorder had a traumatic brain injury and/or PTSD, and 9 of these subjects reported evidence of altered dream mentation related to that prior traumatic experience. However, none showed evidence of autonomic nervous system hyperarousal that coincided with abnormal REM sleep activity.
The investigators noted that although the sample of 394 subjects with in-lab video-polysomnography is large, the study is underpowered to draw broader conclusions about prevalence of RBD among veterans. In addition, the study did not establish whether or not trauma exposure preceded, and contributed to, the development of parasomnias and this question should be pursued in further studies.
“Given the purported relationships between TBI, PTSD, RBD, and neurodegeneration, we sought to determine the crude prevalence and related associations of RBD following TBI and PTSD among veterans. Our data show that the prevalence of RBD and related parasomnias is significantly higher in veterans with PTSD and TBI+PTSD compared to veterans without a history of neuropsychiatric trauma,” the authors wrote.
No funding or conflicts of interest were declared.
SOURCE: Elliott JE et al. Sleep 2019 Oct 7. doi: 10.1093/sleep/zsz237.
Veterans may be at higher risk of idiopathic rapid eye movement sleep behavior disorders, particularly if they have traumatic brain injury (TBI) or posttraumatic stress disorder (PTSD) according to a paper published in Sleep.
In a prospective, cross-sectional study, researchers recruited 394 veterans – 94% of whom were male – who underwent in-lab video-polysomnography and questionnaires about REM sleep behavior disorder (RBD), as well as assessment of their trauma status and medical history.
Overall, 9% of subjects had RBD, a figure considerably higher than has been estimated in the general population (prevalence of 0.38%-1.0%). Seven percent had REM sleep without atonia, 31% had other parasomnias such as a history of dream enactment behavior, and 53% were classified as normal.
The majority of subjects determined to have RBD (n = 34) had either PTSD or comorbid TBI+PTSD (n = 19, 56%). The combined overall crude prevalence of RBD in subjects with either PTSD alone or TBI+PTSD was 16.8% (n = 19 out of 113).
The individuals with PTSD had a 2.81-fold greater odds of RBD and 3.13-fold greater odds of other parasomnias compared with those without PTSD.
Those with both traumatic brain injury and PTSD had 3.43-fold greater odds of RBD and 3.22-fold greater odds of other parasomnias compared with individuals without.
“Interestingly, the neuropathology underpinning PTSD shares common features with RBD, raising the question as to whether or not PTSD has a causal role in the development of RBD, or if a single pathophysiologic process generates two clinical entities,” wrote Jonathan E. Elliott, PhD, of the VA Portland Health Care System, and coauthors.
The researchers also looked for evidence of trauma-associated sleep disorder (TASD), a recently proposed phenomenological sleep disorder whose diagnostic criteria overlaps with REM sleep behavior disorder but includes subjects reporting having an inciting traumatic experience and a history of dreaming related to this experience as well as evidence of autonomic hyperarousal.
The researchers found 22 of the subjects with REM behavior disorder had a traumatic brain injury and/or PTSD, and 9 of these subjects reported evidence of altered dream mentation related to that prior traumatic experience. However, none showed evidence of autonomic nervous system hyperarousal that coincided with abnormal REM sleep activity.
The investigators noted that although the sample of 394 subjects with in-lab video-polysomnography is large, the study is underpowered to draw broader conclusions about prevalence of RBD among veterans. In addition, the study did not establish whether or not trauma exposure preceded, and contributed to, the development of parasomnias and this question should be pursued in further studies.
“Given the purported relationships between TBI, PTSD, RBD, and neurodegeneration, we sought to determine the crude prevalence and related associations of RBD following TBI and PTSD among veterans. Our data show that the prevalence of RBD and related parasomnias is significantly higher in veterans with PTSD and TBI+PTSD compared to veterans without a history of neuropsychiatric trauma,” the authors wrote.
No funding or conflicts of interest were declared.
SOURCE: Elliott JE et al. Sleep 2019 Oct 7. doi: 10.1093/sleep/zsz237.
Veterans may be at higher risk of idiopathic rapid eye movement sleep behavior disorders, particularly if they have traumatic brain injury (TBI) or posttraumatic stress disorder (PTSD) according to a paper published in Sleep.
In a prospective, cross-sectional study, researchers recruited 394 veterans – 94% of whom were male – who underwent in-lab video-polysomnography and questionnaires about REM sleep behavior disorder (RBD), as well as assessment of their trauma status and medical history.
Overall, 9% of subjects had RBD, a figure considerably higher than has been estimated in the general population (prevalence of 0.38%-1.0%). Seven percent had REM sleep without atonia, 31% had other parasomnias such as a history of dream enactment behavior, and 53% were classified as normal.
The majority of subjects determined to have RBD (n = 34) had either PTSD or comorbid TBI+PTSD (n = 19, 56%). The combined overall crude prevalence of RBD in subjects with either PTSD alone or TBI+PTSD was 16.8% (n = 19 out of 113).
The individuals with PTSD had a 2.81-fold greater odds of RBD and 3.13-fold greater odds of other parasomnias compared with those without PTSD.
Those with both traumatic brain injury and PTSD had 3.43-fold greater odds of RBD and 3.22-fold greater odds of other parasomnias compared with individuals without.
“Interestingly, the neuropathology underpinning PTSD shares common features with RBD, raising the question as to whether or not PTSD has a causal role in the development of RBD, or if a single pathophysiologic process generates two clinical entities,” wrote Jonathan E. Elliott, PhD, of the VA Portland Health Care System, and coauthors.
The researchers also looked for evidence of trauma-associated sleep disorder (TASD), a recently proposed phenomenological sleep disorder whose diagnostic criteria overlaps with REM sleep behavior disorder but includes subjects reporting having an inciting traumatic experience and a history of dreaming related to this experience as well as evidence of autonomic hyperarousal.
The researchers found 22 of the subjects with REM behavior disorder had a traumatic brain injury and/or PTSD, and 9 of these subjects reported evidence of altered dream mentation related to that prior traumatic experience. However, none showed evidence of autonomic nervous system hyperarousal that coincided with abnormal REM sleep activity.
The investigators noted that although the sample of 394 subjects with in-lab video-polysomnography is large, the study is underpowered to draw broader conclusions about prevalence of RBD among veterans. In addition, the study did not establish whether or not trauma exposure preceded, and contributed to, the development of parasomnias and this question should be pursued in further studies.
“Given the purported relationships between TBI, PTSD, RBD, and neurodegeneration, we sought to determine the crude prevalence and related associations of RBD following TBI and PTSD among veterans. Our data show that the prevalence of RBD and related parasomnias is significantly higher in veterans with PTSD and TBI+PTSD compared to veterans without a history of neuropsychiatric trauma,” the authors wrote.
No funding or conflicts of interest were declared.
SOURCE: Elliott JE et al. Sleep 2019 Oct 7. doi: 10.1093/sleep/zsz237.
FROM SLEEP
Psilocybin research is mind expanding
Microdosing tied to enhanced divergent thinking in the short term
COPENHAGEN – A single dose of psilocybin taken in a supportive social setting was associated with demonstrably enhanced empathy, creative thinking, and subjective well-being lasting for up to 7 days in an observational study, Kim P.C. Kuypers, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
The participants in this uncontrolled study were healthy individuals curious to experience psilocybin in a controlled environment, but the observed benefits are of special interest because of psilocybin’s potential role in the treatment of psychiatric disorders, including depression and PTSD, in which preliminary study results have been quite encouraging, observed Dr. Kuypers, a neuropsychologist at Maastricht (the Netherlands) University.
For example, hallmarks of depression include psychological inflexibility, negative thoughts, and disturbed empathy. As this study showed, psilocybin addresses all those issues, she said.
according to Dr. Kuypers.
She and her coinvestigators studied 55 participants at a psilocybin retreat. These are events sprouting up across Europe to accommodate people who are curious about psilocybin and interested in trying it in a supportive setting. The investigators administered structured tests of convergent and divergent thinking, emotional empathy, and satisfaction with life before participants ingested the so-called magic mushroom, the morning after, and again 7 days later. In the Netherlands, the above-ground portion of the plant is an illegal substance, but the underground stem is not. The average dose was 27 mg, slightly higher than is often used in controlled laboratory studies; however, there were no adverse events.
The results showed that divergent thinking was enhanced the morning after taking psilocybin, an effect that was not sustained at the 7-day mark. In contrast, convergent thinking, emotional empathy, and a satisfaction-with-life scores above baseline were maintained 7 days after ingestion.
Psilocybin is a 5-HT2A agonist. It is considered a classic psychedelic, as are LSD, mescaline, and ayahuasca. These agents are often used recreationally to broaden consciousness, for relaxation, and to amplify emotions. Cave paintings indicate that psilocybin has been used medicinally for 11,000 years. But even early hunter-gatherers could not possibly have envisioned the current phenomenon of psilocybin microdosing to enhance work performance as adopted by hard-driving Silicon Valley professionals and similarly motivated strivers elsewhere around the world.
Microdosing for enhanced performance
Microdosing of psychedelics, especially psilocybin and LSD, is the practice of taking a low dose – typically 1/10th of a full recreational dose, which is too small an amount to cause full-blown perceptual alterations – once every several days in order to stimulate productivity. Psychedelic microdosing has garnered considerable mass media attention as an increasingly popular practice among younger professionals in the fields of computer science, engineering, mathematics, and technology. But it hasn’t been subjected to much scientific scrutiny.
Dr. Kuypers and colleagues wanted to find out whether it is actually effective and whether there are negative effects. They conducted an online questionnaire survey during a 5-month period of 2018 that drew 1,116 respondents, 80% of whom were currently microdosing, while the other 20% were former microdosers who had stopped completely.
The most common motivation for microdosing was indeed to stimulate productivity through increased focus, energy, and creativity. Almost half of microdosers indicated that they designed their own dosing schedule – typically once every 2-4 days – and two-thirds of microdosers were oblivious as to the consumed dose.
The most common reasons that respondents stopped microdosing were negative experiences or loss of interest because of lack of efficacy. The negative experiences typically involved acute anxiety or other psychological symptoms limited to when they were under the influence (Int J Neuropsychopharmacol. 2019 Jul 1;22[7]:426-34).
A subset of survey respondents microdosed to alleviate symptoms of a physician-diagnosed mental or physical health disorder. They were the focus of a separate analysis.
Microdosing for mental, physical health problems
Four hundred and ten survey respondents reported microdosing to self-treat a total of 901 physician-diagnosed mental and 161 physiologic conditions. They were asked three efficacy questions: Did it work? Did symptoms disappear? Did your quality of life improve?
The responses were disorder-specific. Individuals with anxiety disorders, ADHD, migraine, and other pain syndromes were the only ones to consistently rate microdosing as more effective than conventional treatment. Microdosing was rated less effective for symptom relief than were full-dose psychedelics only in respondents with depression or anxiety; individuals with other mental disorders or with physiological disorders rated the two dosing strategies similarly (Front Psychiatry. 2019 Sep 13. doi: 10.3389/fpsyt.2019.00672).
Future randomized controlled trials are warranted to more accurately assess efficacy claims for various psychedelics, optimal dosing, disorder specificity, and how they stack up compared with standard therapies, she said.
Dr. Kuypers reported having no financial conflicts of interest regarding her studies, which were conducted free of commercial support.
Microdosing tied to enhanced divergent thinking in the short term
Microdosing tied to enhanced divergent thinking in the short term
COPENHAGEN – A single dose of psilocybin taken in a supportive social setting was associated with demonstrably enhanced empathy, creative thinking, and subjective well-being lasting for up to 7 days in an observational study, Kim P.C. Kuypers, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
The participants in this uncontrolled study were healthy individuals curious to experience psilocybin in a controlled environment, but the observed benefits are of special interest because of psilocybin’s potential role in the treatment of psychiatric disorders, including depression and PTSD, in which preliminary study results have been quite encouraging, observed Dr. Kuypers, a neuropsychologist at Maastricht (the Netherlands) University.
For example, hallmarks of depression include psychological inflexibility, negative thoughts, and disturbed empathy. As this study showed, psilocybin addresses all those issues, she said.
according to Dr. Kuypers.
She and her coinvestigators studied 55 participants at a psilocybin retreat. These are events sprouting up across Europe to accommodate people who are curious about psilocybin and interested in trying it in a supportive setting. The investigators administered structured tests of convergent and divergent thinking, emotional empathy, and satisfaction with life before participants ingested the so-called magic mushroom, the morning after, and again 7 days later. In the Netherlands, the above-ground portion of the plant is an illegal substance, but the underground stem is not. The average dose was 27 mg, slightly higher than is often used in controlled laboratory studies; however, there were no adverse events.
The results showed that divergent thinking was enhanced the morning after taking psilocybin, an effect that was not sustained at the 7-day mark. In contrast, convergent thinking, emotional empathy, and a satisfaction-with-life scores above baseline were maintained 7 days after ingestion.
Psilocybin is a 5-HT2A agonist. It is considered a classic psychedelic, as are LSD, mescaline, and ayahuasca. These agents are often used recreationally to broaden consciousness, for relaxation, and to amplify emotions. Cave paintings indicate that psilocybin has been used medicinally for 11,000 years. But even early hunter-gatherers could not possibly have envisioned the current phenomenon of psilocybin microdosing to enhance work performance as adopted by hard-driving Silicon Valley professionals and similarly motivated strivers elsewhere around the world.
Microdosing for enhanced performance
Microdosing of psychedelics, especially psilocybin and LSD, is the practice of taking a low dose – typically 1/10th of a full recreational dose, which is too small an amount to cause full-blown perceptual alterations – once every several days in order to stimulate productivity. Psychedelic microdosing has garnered considerable mass media attention as an increasingly popular practice among younger professionals in the fields of computer science, engineering, mathematics, and technology. But it hasn’t been subjected to much scientific scrutiny.
Dr. Kuypers and colleagues wanted to find out whether it is actually effective and whether there are negative effects. They conducted an online questionnaire survey during a 5-month period of 2018 that drew 1,116 respondents, 80% of whom were currently microdosing, while the other 20% were former microdosers who had stopped completely.
The most common motivation for microdosing was indeed to stimulate productivity through increased focus, energy, and creativity. Almost half of microdosers indicated that they designed their own dosing schedule – typically once every 2-4 days – and two-thirds of microdosers were oblivious as to the consumed dose.
The most common reasons that respondents stopped microdosing were negative experiences or loss of interest because of lack of efficacy. The negative experiences typically involved acute anxiety or other psychological symptoms limited to when they were under the influence (Int J Neuropsychopharmacol. 2019 Jul 1;22[7]:426-34).
A subset of survey respondents microdosed to alleviate symptoms of a physician-diagnosed mental or physical health disorder. They were the focus of a separate analysis.
Microdosing for mental, physical health problems
Four hundred and ten survey respondents reported microdosing to self-treat a total of 901 physician-diagnosed mental and 161 physiologic conditions. They were asked three efficacy questions: Did it work? Did symptoms disappear? Did your quality of life improve?
The responses were disorder-specific. Individuals with anxiety disorders, ADHD, migraine, and other pain syndromes were the only ones to consistently rate microdosing as more effective than conventional treatment. Microdosing was rated less effective for symptom relief than were full-dose psychedelics only in respondents with depression or anxiety; individuals with other mental disorders or with physiological disorders rated the two dosing strategies similarly (Front Psychiatry. 2019 Sep 13. doi: 10.3389/fpsyt.2019.00672).
Future randomized controlled trials are warranted to more accurately assess efficacy claims for various psychedelics, optimal dosing, disorder specificity, and how they stack up compared with standard therapies, she said.
Dr. Kuypers reported having no financial conflicts of interest regarding her studies, which were conducted free of commercial support.
COPENHAGEN – A single dose of psilocybin taken in a supportive social setting was associated with demonstrably enhanced empathy, creative thinking, and subjective well-being lasting for up to 7 days in an observational study, Kim P.C. Kuypers, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
The participants in this uncontrolled study were healthy individuals curious to experience psilocybin in a controlled environment, but the observed benefits are of special interest because of psilocybin’s potential role in the treatment of psychiatric disorders, including depression and PTSD, in which preliminary study results have been quite encouraging, observed Dr. Kuypers, a neuropsychologist at Maastricht (the Netherlands) University.
For example, hallmarks of depression include psychological inflexibility, negative thoughts, and disturbed empathy. As this study showed, psilocybin addresses all those issues, she said.
according to Dr. Kuypers.
She and her coinvestigators studied 55 participants at a psilocybin retreat. These are events sprouting up across Europe to accommodate people who are curious about psilocybin and interested in trying it in a supportive setting. The investigators administered structured tests of convergent and divergent thinking, emotional empathy, and satisfaction with life before participants ingested the so-called magic mushroom, the morning after, and again 7 days later. In the Netherlands, the above-ground portion of the plant is an illegal substance, but the underground stem is not. The average dose was 27 mg, slightly higher than is often used in controlled laboratory studies; however, there were no adverse events.
The results showed that divergent thinking was enhanced the morning after taking psilocybin, an effect that was not sustained at the 7-day mark. In contrast, convergent thinking, emotional empathy, and a satisfaction-with-life scores above baseline were maintained 7 days after ingestion.
Psilocybin is a 5-HT2A agonist. It is considered a classic psychedelic, as are LSD, mescaline, and ayahuasca. These agents are often used recreationally to broaden consciousness, for relaxation, and to amplify emotions. Cave paintings indicate that psilocybin has been used medicinally for 11,000 years. But even early hunter-gatherers could not possibly have envisioned the current phenomenon of psilocybin microdosing to enhance work performance as adopted by hard-driving Silicon Valley professionals and similarly motivated strivers elsewhere around the world.
Microdosing for enhanced performance
Microdosing of psychedelics, especially psilocybin and LSD, is the practice of taking a low dose – typically 1/10th of a full recreational dose, which is too small an amount to cause full-blown perceptual alterations – once every several days in order to stimulate productivity. Psychedelic microdosing has garnered considerable mass media attention as an increasingly popular practice among younger professionals in the fields of computer science, engineering, mathematics, and technology. But it hasn’t been subjected to much scientific scrutiny.
Dr. Kuypers and colleagues wanted to find out whether it is actually effective and whether there are negative effects. They conducted an online questionnaire survey during a 5-month period of 2018 that drew 1,116 respondents, 80% of whom were currently microdosing, while the other 20% were former microdosers who had stopped completely.
The most common motivation for microdosing was indeed to stimulate productivity through increased focus, energy, and creativity. Almost half of microdosers indicated that they designed their own dosing schedule – typically once every 2-4 days – and two-thirds of microdosers were oblivious as to the consumed dose.
The most common reasons that respondents stopped microdosing were negative experiences or loss of interest because of lack of efficacy. The negative experiences typically involved acute anxiety or other psychological symptoms limited to when they were under the influence (Int J Neuropsychopharmacol. 2019 Jul 1;22[7]:426-34).
A subset of survey respondents microdosed to alleviate symptoms of a physician-diagnosed mental or physical health disorder. They were the focus of a separate analysis.
Microdosing for mental, physical health problems
Four hundred and ten survey respondents reported microdosing to self-treat a total of 901 physician-diagnosed mental and 161 physiologic conditions. They were asked three efficacy questions: Did it work? Did symptoms disappear? Did your quality of life improve?
The responses were disorder-specific. Individuals with anxiety disorders, ADHD, migraine, and other pain syndromes were the only ones to consistently rate microdosing as more effective than conventional treatment. Microdosing was rated less effective for symptom relief than were full-dose psychedelics only in respondents with depression or anxiety; individuals with other mental disorders or with physiological disorders rated the two dosing strategies similarly (Front Psychiatry. 2019 Sep 13. doi: 10.3389/fpsyt.2019.00672).
Future randomized controlled trials are warranted to more accurately assess efficacy claims for various psychedelics, optimal dosing, disorder specificity, and how they stack up compared with standard therapies, she said.
Dr. Kuypers reported having no financial conflicts of interest regarding her studies, which were conducted free of commercial support.
EXPERT ANALYSIS FROM ECNP 2019
The Jewel in the Lotus: A Meditation on Memory for Veterans Day 2019
On the 11th day of the 11th month, we celebrate Veterans Day (no apostrophe because it is not a day that veterans possess or that belongs to any individual veteran).2,3 Interestingly, the US Department of Defense (DoD) and the US Department of Veterans Affairs (VA) have web pages correcting any confusion about the meaning of Memorial Day and Veterans Day so that the public understands the unique purpose of each holiday. Memorial Day commemorates all those who lost their lives in the line of duty to the nation, whereas Veterans Day commemorates all those who have honorably served their country as service members. While Memorial Day is a solemn occasion of remembering and respect for those who have died, Veterans Day is an event of gratitude and appreciation focused on veterans still living. The dual mission of the 2 holidays is to remind the public of the debt of remembrance and reverence we owe all veterans both those who have gone before us and those who remain with us.
Memory is what most intrinsically unites the 2 commemorations. In fact, in Great Britain, Canada, and Australia, November 11 is called Remembrance Day.2 Yet memory is a double-edged sword that can be raised in tribute to service members or can deeply lacerate them. Many of the wounds that cause the most prolonged and deepest suffering are not physical—they are mental. Disturbances of memory are among the criteria for posttraumatic stress disorder (PTSD). Under its section on intrusive cluster, the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM) lists “recurrent, involuntary, and intrusive distressing memories of the traumatic event(s).” The avoidance cluster underscores how the afflicted mind tries to escape itself: “Avoidance of or efforts to avoid distressing memories, thoughts, or feelings about or closely associated with the traumatic event(s).”4
PTSD was first recognized as a psychiatric diagnosis in DSM-III in 1980, and since then VA and DoD have devoted enormous resources to developing effective treatments for the disorder, most notably evidence-based psychotherapies. Sadly ironic, the only psychiatric disorder whose etiology is understood has proved to be the most difficult to treat much less cure. As with most serious mental illnesses, some cases become chronic and refractory to the best of care. These tormented individuals live as if in a twilight zone between the past and the present.
Memory and war have a long history in literature, poetry, and history. Haunting memories of PTSD are found in the ancient epics of Homer. On the long treacherous journey home from sacking Troy, Odysseus and his army arrive in the land of the Lotus-eaters, where native sweet fruit induces a state of timeless forgetfulness in which torment and tragedy dissolve along with motivation and meaning.5 Jonathan Shay, VA psychiatrist and pioneer of the Homer-PTSD connection, suggested the analogy between the land of the Lotus-eaters and addiction: Each is a self-medication of the psychic aftermath of war.6
But what if those devastating memories could be selectively erased or even better blocked before they were formed? Although this solution may seem like science fiction, research into these possibilities is in reality science fact. Over the past decades, the DoD and the VA have sought such a neuroscience jewel in the lotus. Studies in rodents and humans have looked at the ability of a number of medications, most recently β blockers, such as propranolol, to interfere with the consolidation of emotionally traumatic memories (memory erasure) and disrupting their retention once consolidated (memory extinction).7 While researchers cannot yet completely wipe out a selected memory, like in the movie Star Trek, it has been shown that medications at least in study settings do reduce fear and can attenuate the development of PTSD when combined with psychotherapy. Neuroscientists call these more realistic alterations of recall memory dampening. Though these medications are not ready for regular clinical application, the unprecedented pace of neuroscience makes it nearly inevitable that in the not so distant future some significant blunting of traumatic memory will be possible.
Once science answers in the affirmative the question, “Is this intervention something we could conceivably do?” The next question belongs to ethics, “Is this intervention something we should do even if we can?” As early as 2001, the President’s Council on Bioethics answered the latter with “probably not.”
Use of memory-blunters at the time of traumatic events could interfere with the normal psychic work and adaptive value of emotionally charged memory....Thus, by blunting the emotional impact of events, beta-blockers or their successors would concomitantly weaken our recollection of the traumatic events we have just experienced. Yet often it is important in the after of such events that at least someone remember them clearly. For legal reasons, to say nothing of deeper social and personal ones, the wisdom of routinely interfering with the memories of traumatic survivors and witnesses is highly questionable.8
Many neuroscientists and neuroethicists objected to the perspective of the Bioethics Council as being too puritanical and its position overly pessimistic:
Whereas memory dampening has its drawbacks, such may be the price we pay in order to heal immense suffering. In some contexts, there may be steps that ought to be taken to preserve valuable factual or emotional information contained in memory, even when we must delay or otherwise impose limits on access to memory dampening. None of these concerns, however, even if they find empirical support, are strong enough to justify brushed restrictions on memory dampening.9
The proponents of the 2 views propose and oppose the contrarian position on issues both philosophical and practical, such as the function of traumatic experience in personal growth; how the preservation of memory is related to the integrity of the person and authenticity of the life lived; how blunting of memories of especially combat trauma may normalize our reactions to suffering and evil; and most important for this Veterans Day essay, whether remembering is an ethical duty and if so whose is it to discharge, the individual, his family, community, or country.
More pragmatic, there would be a need to refine our understanding of the risk factors for chronic and disabling PTSD; to determine when in the course of the trauma experience to pharmacologically interfere with memory and to what degree and scope; how to protect the autonomy of the service member to consent or to refuse the procedure within the recognized confines of military ethics; and most important for this essay, how to prevent governments, corporations, or any other entity from exploiting neurobiologic discoveries for power or profit.
Elie Wiesel is an important modern prophet of the critical role of memory in the survival of civilization. His prophecy is rooted in the incomprehensible anguish and horror he personally and communally witnessed in the Holocaust. He suggests in this editorial’s epigraph that there are deep and profound issues to be pondered about memory and its inextricable link to suffering. Meditations offer thoughts, not answers, and I encourage readers to spend a few minutes considering the solemn ones presented here this Veterans Day.
1. Wiesel E. Nobel lecture: hope, despair and memory. https://www.nobelprize.org/prizes/peace/1986/wiesel/lecture. Published December 11, 1986. Accessed October 20, 2019.
2. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. Veterans Day frequently asked questions. https://www.va.gov/opa/vetsday/vetday_faq.asp. Accessed October 29, 2019.
3. Lange K. Five facts to know about Veterans Day. https://www.defense.gov/explore/story/article/1675470/5-facts-to-know-about-veterans-day. Published November 5, 2019. Accessed October 29, 2019.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
5. Homer. The Odyssey. Wilson E, trans. New York: Norton; 2018:Bk 9:90 ff.
6. Shay J. Odysseus in America. New York: Scribner’s; 2002:35-41. 7. Giustino TF, Fitzgerald PJ, Maren S. Revisiting propranolol and PTSD: memory erasure or extinction enhancement. Neurobiol Learn Mem. 2016;130:26-33.
8. President’s Council on Bioethics. Beyond therapy: biotechnology and the pursuit of happiness. https://bioethicsarchive.georgetown.edu/pcbe/reports/beyondtherapy/fulldoc.html. Published October 15, 2003. Accessed October 30, 2019.
9. Kobler AJ. Ethical implications of memory dampening. In: Farah MJ, ed. Neuroethics: An Introduction with Readings. Cambridge MA: MIT Press; 2010:112.
On the 11th day of the 11th month, we celebrate Veterans Day (no apostrophe because it is not a day that veterans possess or that belongs to any individual veteran).2,3 Interestingly, the US Department of Defense (DoD) and the US Department of Veterans Affairs (VA) have web pages correcting any confusion about the meaning of Memorial Day and Veterans Day so that the public understands the unique purpose of each holiday. Memorial Day commemorates all those who lost their lives in the line of duty to the nation, whereas Veterans Day commemorates all those who have honorably served their country as service members. While Memorial Day is a solemn occasion of remembering and respect for those who have died, Veterans Day is an event of gratitude and appreciation focused on veterans still living. The dual mission of the 2 holidays is to remind the public of the debt of remembrance and reverence we owe all veterans both those who have gone before us and those who remain with us.
Memory is what most intrinsically unites the 2 commemorations. In fact, in Great Britain, Canada, and Australia, November 11 is called Remembrance Day.2 Yet memory is a double-edged sword that can be raised in tribute to service members or can deeply lacerate them. Many of the wounds that cause the most prolonged and deepest suffering are not physical—they are mental. Disturbances of memory are among the criteria for posttraumatic stress disorder (PTSD). Under its section on intrusive cluster, the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM) lists “recurrent, involuntary, and intrusive distressing memories of the traumatic event(s).” The avoidance cluster underscores how the afflicted mind tries to escape itself: “Avoidance of or efforts to avoid distressing memories, thoughts, or feelings about or closely associated with the traumatic event(s).”4
PTSD was first recognized as a psychiatric diagnosis in DSM-III in 1980, and since then VA and DoD have devoted enormous resources to developing effective treatments for the disorder, most notably evidence-based psychotherapies. Sadly ironic, the only psychiatric disorder whose etiology is understood has proved to be the most difficult to treat much less cure. As with most serious mental illnesses, some cases become chronic and refractory to the best of care. These tormented individuals live as if in a twilight zone between the past and the present.
Memory and war have a long history in literature, poetry, and history. Haunting memories of PTSD are found in the ancient epics of Homer. On the long treacherous journey home from sacking Troy, Odysseus and his army arrive in the land of the Lotus-eaters, where native sweet fruit induces a state of timeless forgetfulness in which torment and tragedy dissolve along with motivation and meaning.5 Jonathan Shay, VA psychiatrist and pioneer of the Homer-PTSD connection, suggested the analogy between the land of the Lotus-eaters and addiction: Each is a self-medication of the psychic aftermath of war.6
But what if those devastating memories could be selectively erased or even better blocked before they were formed? Although this solution may seem like science fiction, research into these possibilities is in reality science fact. Over the past decades, the DoD and the VA have sought such a neuroscience jewel in the lotus. Studies in rodents and humans have looked at the ability of a number of medications, most recently β blockers, such as propranolol, to interfere with the consolidation of emotionally traumatic memories (memory erasure) and disrupting their retention once consolidated (memory extinction).7 While researchers cannot yet completely wipe out a selected memory, like in the movie Star Trek, it has been shown that medications at least in study settings do reduce fear and can attenuate the development of PTSD when combined with psychotherapy. Neuroscientists call these more realistic alterations of recall memory dampening. Though these medications are not ready for regular clinical application, the unprecedented pace of neuroscience makes it nearly inevitable that in the not so distant future some significant blunting of traumatic memory will be possible.
Once science answers in the affirmative the question, “Is this intervention something we could conceivably do?” The next question belongs to ethics, “Is this intervention something we should do even if we can?” As early as 2001, the President’s Council on Bioethics answered the latter with “probably not.”
Use of memory-blunters at the time of traumatic events could interfere with the normal psychic work and adaptive value of emotionally charged memory....Thus, by blunting the emotional impact of events, beta-blockers or their successors would concomitantly weaken our recollection of the traumatic events we have just experienced. Yet often it is important in the after of such events that at least someone remember them clearly. For legal reasons, to say nothing of deeper social and personal ones, the wisdom of routinely interfering with the memories of traumatic survivors and witnesses is highly questionable.8
Many neuroscientists and neuroethicists objected to the perspective of the Bioethics Council as being too puritanical and its position overly pessimistic:
Whereas memory dampening has its drawbacks, such may be the price we pay in order to heal immense suffering. In some contexts, there may be steps that ought to be taken to preserve valuable factual or emotional information contained in memory, even when we must delay or otherwise impose limits on access to memory dampening. None of these concerns, however, even if they find empirical support, are strong enough to justify brushed restrictions on memory dampening.9
The proponents of the 2 views propose and oppose the contrarian position on issues both philosophical and practical, such as the function of traumatic experience in personal growth; how the preservation of memory is related to the integrity of the person and authenticity of the life lived; how blunting of memories of especially combat trauma may normalize our reactions to suffering and evil; and most important for this Veterans Day essay, whether remembering is an ethical duty and if so whose is it to discharge, the individual, his family, community, or country.
More pragmatic, there would be a need to refine our understanding of the risk factors for chronic and disabling PTSD; to determine when in the course of the trauma experience to pharmacologically interfere with memory and to what degree and scope; how to protect the autonomy of the service member to consent or to refuse the procedure within the recognized confines of military ethics; and most important for this essay, how to prevent governments, corporations, or any other entity from exploiting neurobiologic discoveries for power or profit.
Elie Wiesel is an important modern prophet of the critical role of memory in the survival of civilization. His prophecy is rooted in the incomprehensible anguish and horror he personally and communally witnessed in the Holocaust. He suggests in this editorial’s epigraph that there are deep and profound issues to be pondered about memory and its inextricable link to suffering. Meditations offer thoughts, not answers, and I encourage readers to spend a few minutes considering the solemn ones presented here this Veterans Day.
On the 11th day of the 11th month, we celebrate Veterans Day (no apostrophe because it is not a day that veterans possess or that belongs to any individual veteran).2,3 Interestingly, the US Department of Defense (DoD) and the US Department of Veterans Affairs (VA) have web pages correcting any confusion about the meaning of Memorial Day and Veterans Day so that the public understands the unique purpose of each holiday. Memorial Day commemorates all those who lost their lives in the line of duty to the nation, whereas Veterans Day commemorates all those who have honorably served their country as service members. While Memorial Day is a solemn occasion of remembering and respect for those who have died, Veterans Day is an event of gratitude and appreciation focused on veterans still living. The dual mission of the 2 holidays is to remind the public of the debt of remembrance and reverence we owe all veterans both those who have gone before us and those who remain with us.
Memory is what most intrinsically unites the 2 commemorations. In fact, in Great Britain, Canada, and Australia, November 11 is called Remembrance Day.2 Yet memory is a double-edged sword that can be raised in tribute to service members or can deeply lacerate them. Many of the wounds that cause the most prolonged and deepest suffering are not physical—they are mental. Disturbances of memory are among the criteria for posttraumatic stress disorder (PTSD). Under its section on intrusive cluster, the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM) lists “recurrent, involuntary, and intrusive distressing memories of the traumatic event(s).” The avoidance cluster underscores how the afflicted mind tries to escape itself: “Avoidance of or efforts to avoid distressing memories, thoughts, or feelings about or closely associated with the traumatic event(s).”4
PTSD was first recognized as a psychiatric diagnosis in DSM-III in 1980, and since then VA and DoD have devoted enormous resources to developing effective treatments for the disorder, most notably evidence-based psychotherapies. Sadly ironic, the only psychiatric disorder whose etiology is understood has proved to be the most difficult to treat much less cure. As with most serious mental illnesses, some cases become chronic and refractory to the best of care. These tormented individuals live as if in a twilight zone between the past and the present.
Memory and war have a long history in literature, poetry, and history. Haunting memories of PTSD are found in the ancient epics of Homer. On the long treacherous journey home from sacking Troy, Odysseus and his army arrive in the land of the Lotus-eaters, where native sweet fruit induces a state of timeless forgetfulness in which torment and tragedy dissolve along with motivation and meaning.5 Jonathan Shay, VA psychiatrist and pioneer of the Homer-PTSD connection, suggested the analogy between the land of the Lotus-eaters and addiction: Each is a self-medication of the psychic aftermath of war.6
But what if those devastating memories could be selectively erased or even better blocked before they were formed? Although this solution may seem like science fiction, research into these possibilities is in reality science fact. Over the past decades, the DoD and the VA have sought such a neuroscience jewel in the lotus. Studies in rodents and humans have looked at the ability of a number of medications, most recently β blockers, such as propranolol, to interfere with the consolidation of emotionally traumatic memories (memory erasure) and disrupting their retention once consolidated (memory extinction).7 While researchers cannot yet completely wipe out a selected memory, like in the movie Star Trek, it has been shown that medications at least in study settings do reduce fear and can attenuate the development of PTSD when combined with psychotherapy. Neuroscientists call these more realistic alterations of recall memory dampening. Though these medications are not ready for regular clinical application, the unprecedented pace of neuroscience makes it nearly inevitable that in the not so distant future some significant blunting of traumatic memory will be possible.
Once science answers in the affirmative the question, “Is this intervention something we could conceivably do?” The next question belongs to ethics, “Is this intervention something we should do even if we can?” As early as 2001, the President’s Council on Bioethics answered the latter with “probably not.”
Use of memory-blunters at the time of traumatic events could interfere with the normal psychic work and adaptive value of emotionally charged memory....Thus, by blunting the emotional impact of events, beta-blockers or their successors would concomitantly weaken our recollection of the traumatic events we have just experienced. Yet often it is important in the after of such events that at least someone remember them clearly. For legal reasons, to say nothing of deeper social and personal ones, the wisdom of routinely interfering with the memories of traumatic survivors and witnesses is highly questionable.8
Many neuroscientists and neuroethicists objected to the perspective of the Bioethics Council as being too puritanical and its position overly pessimistic:
Whereas memory dampening has its drawbacks, such may be the price we pay in order to heal immense suffering. In some contexts, there may be steps that ought to be taken to preserve valuable factual or emotional information contained in memory, even when we must delay or otherwise impose limits on access to memory dampening. None of these concerns, however, even if they find empirical support, are strong enough to justify brushed restrictions on memory dampening.9
The proponents of the 2 views propose and oppose the contrarian position on issues both philosophical and practical, such as the function of traumatic experience in personal growth; how the preservation of memory is related to the integrity of the person and authenticity of the life lived; how blunting of memories of especially combat trauma may normalize our reactions to suffering and evil; and most important for this Veterans Day essay, whether remembering is an ethical duty and if so whose is it to discharge, the individual, his family, community, or country.
More pragmatic, there would be a need to refine our understanding of the risk factors for chronic and disabling PTSD; to determine when in the course of the trauma experience to pharmacologically interfere with memory and to what degree and scope; how to protect the autonomy of the service member to consent or to refuse the procedure within the recognized confines of military ethics; and most important for this essay, how to prevent governments, corporations, or any other entity from exploiting neurobiologic discoveries for power or profit.
Elie Wiesel is an important modern prophet of the critical role of memory in the survival of civilization. His prophecy is rooted in the incomprehensible anguish and horror he personally and communally witnessed in the Holocaust. He suggests in this editorial’s epigraph that there are deep and profound issues to be pondered about memory and its inextricable link to suffering. Meditations offer thoughts, not answers, and I encourage readers to spend a few minutes considering the solemn ones presented here this Veterans Day.
1. Wiesel E. Nobel lecture: hope, despair and memory. https://www.nobelprize.org/prizes/peace/1986/wiesel/lecture. Published December 11, 1986. Accessed October 20, 2019.
2. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. Veterans Day frequently asked questions. https://www.va.gov/opa/vetsday/vetday_faq.asp. Accessed October 29, 2019.
3. Lange K. Five facts to know about Veterans Day. https://www.defense.gov/explore/story/article/1675470/5-facts-to-know-about-veterans-day. Published November 5, 2019. Accessed October 29, 2019.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
5. Homer. The Odyssey. Wilson E, trans. New York: Norton; 2018:Bk 9:90 ff.
6. Shay J. Odysseus in America. New York: Scribner’s; 2002:35-41. 7. Giustino TF, Fitzgerald PJ, Maren S. Revisiting propranolol and PTSD: memory erasure or extinction enhancement. Neurobiol Learn Mem. 2016;130:26-33.
8. President’s Council on Bioethics. Beyond therapy: biotechnology and the pursuit of happiness. https://bioethicsarchive.georgetown.edu/pcbe/reports/beyondtherapy/fulldoc.html. Published October 15, 2003. Accessed October 30, 2019.
9. Kobler AJ. Ethical implications of memory dampening. In: Farah MJ, ed. Neuroethics: An Introduction with Readings. Cambridge MA: MIT Press; 2010:112.
1. Wiesel E. Nobel lecture: hope, despair and memory. https://www.nobelprize.org/prizes/peace/1986/wiesel/lecture. Published December 11, 1986. Accessed October 20, 2019.
2. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. Veterans Day frequently asked questions. https://www.va.gov/opa/vetsday/vetday_faq.asp. Accessed October 29, 2019.
3. Lange K. Five facts to know about Veterans Day. https://www.defense.gov/explore/story/article/1675470/5-facts-to-know-about-veterans-day. Published November 5, 2019. Accessed October 29, 2019.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
5. Homer. The Odyssey. Wilson E, trans. New York: Norton; 2018:Bk 9:90 ff.
6. Shay J. Odysseus in America. New York: Scribner’s; 2002:35-41. 7. Giustino TF, Fitzgerald PJ, Maren S. Revisiting propranolol and PTSD: memory erasure or extinction enhancement. Neurobiol Learn Mem. 2016;130:26-33.
8. President’s Council on Bioethics. Beyond therapy: biotechnology and the pursuit of happiness. https://bioethicsarchive.georgetown.edu/pcbe/reports/beyondtherapy/fulldoc.html. Published October 15, 2003. Accessed October 30, 2019.
9. Kobler AJ. Ethical implications of memory dampening. In: Farah MJ, ed. Neuroethics: An Introduction with Readings. Cambridge MA: MIT Press; 2010:112.
T3 levels are higher in combatants with PTSD
CHICAGO – Higher levels of triiodothyronine (T3) were seen in combatants with PTSD, compared with patients whose PTSD arose from other adverse experiences, according to findings from a systematic review and meta-analysis.
who had experienced childhood or sexual abuse or were a wartime refugee without PTSD (FT3, 0.36 pg/mL higher, and total T3, 31.6 ng/mL higher, respectively; P = .0004 and P less than .00001).
“We found statistically higher free T3 and total T3 levels in patients with [combat-related] PTSD, compared with controls,” said Freddy J.K. Toloza, MD, in an interview during a poster session of the annual meeting of the American Thyroid Association.
However, he noted that there were no overall differences in thyroid-stimulating hormone, free tetraiodothyronine (T4), and total T4 levels between individuals with PTSD and the non-PTSD control participants. In addition, though free and total T3 levels were significantly higher for the overall PTSD cohort than for control participants, the differences were driven by the studies that included combat-exposed individuals.
Dr. Toloza and colleagues included 10 observational studies in their final review and meta-analysis. Five studies looked at war veterans; the others examined individuals who had experienced child abuse or sexual abuse, who were refugees, or who were from the general population.
For inclusion, the studies had to report both mean values and standard deviations for standard thyroid-hormone test values in patients with PTSD, compared with a non-PTSD control group. These included 373 patients with PTSD and 301 control participants. Just under half (47%) were women. None of the studies, wrote the investigators, “compared rates of overt/subclinical thyroid disease between groups.”
There are known links between many endocrine disorders and psychiatric conditions, said Dr. Toloza, but the interplay between disordered thyroid function and neuropsychiatric problems is still being examined. Looking at PTSD is important because it’s estimated that 6%-9% of the U.S. adult population has experienced PTSD over the course of a lifetime.
Levels of thyroid hormones in the systematic review and meta-analysis were still within normal range for the participants with PTSD, acknowledged Dr. Toloza, a research fellow in the division of endocrinology and metabolism at University of Arkansas for Medical Sciences, Little Rock.
However, even though there was no sign of frank thyroid disease in the PTSD population, the elevated T3 levels seen in the analysis are consistent with other studies showing a correlation between higher T3 levels and more-severe PTSD.
It is not known exactly why significant increases in the levels of total and free T3 were seen only in the combat-exposed PTSD population, Dr. Toloza said. “The type of trauma trigger may influence the adaptive responses to stress and might result in diverse thyroid alterations.”
Elevated catecholamine levels, seen in individuals with PTSD, can increase peripheral conversion of T4 to T3, explained Dr. Toloza. Ongoing catecholamine elevation may account for the isolated elevation in T3 levels in the PTSD population. Beta1-adrenergic blockade is an accepted pharmacologic strategy to help alleviate PTSD symptoms.
Dr. Toloza and coinvestigators did not have access to data that would have allowed them to ascertain what types of injuries were sustained by individuals with combat-related PTSD, but he noted in response to a question, that it would be worthwhile to see whether combatants who were blast exposed had different thyroid hormone values than those who were not, because hypothalamic injury is common in blast. This is a future direction Dr. Toloza wishes to pursue.
“Our findings add to the growing literature suggesting that thyroid function changes may be associated with PTSD,” the investigators wrote, but “further research is needed to ascertain the role of thyroid function alterations in PTSD.”
Dr. Toloza reported no financial disclosures or conflicts of interest.
CHICAGO – Higher levels of triiodothyronine (T3) were seen in combatants with PTSD, compared with patients whose PTSD arose from other adverse experiences, according to findings from a systematic review and meta-analysis.
who had experienced childhood or sexual abuse or were a wartime refugee without PTSD (FT3, 0.36 pg/mL higher, and total T3, 31.6 ng/mL higher, respectively; P = .0004 and P less than .00001).
“We found statistically higher free T3 and total T3 levels in patients with [combat-related] PTSD, compared with controls,” said Freddy J.K. Toloza, MD, in an interview during a poster session of the annual meeting of the American Thyroid Association.
However, he noted that there were no overall differences in thyroid-stimulating hormone, free tetraiodothyronine (T4), and total T4 levels between individuals with PTSD and the non-PTSD control participants. In addition, though free and total T3 levels were significantly higher for the overall PTSD cohort than for control participants, the differences were driven by the studies that included combat-exposed individuals.
Dr. Toloza and colleagues included 10 observational studies in their final review and meta-analysis. Five studies looked at war veterans; the others examined individuals who had experienced child abuse or sexual abuse, who were refugees, or who were from the general population.
For inclusion, the studies had to report both mean values and standard deviations for standard thyroid-hormone test values in patients with PTSD, compared with a non-PTSD control group. These included 373 patients with PTSD and 301 control participants. Just under half (47%) were women. None of the studies, wrote the investigators, “compared rates of overt/subclinical thyroid disease between groups.”
There are known links between many endocrine disorders and psychiatric conditions, said Dr. Toloza, but the interplay between disordered thyroid function and neuropsychiatric problems is still being examined. Looking at PTSD is important because it’s estimated that 6%-9% of the U.S. adult population has experienced PTSD over the course of a lifetime.
Levels of thyroid hormones in the systematic review and meta-analysis were still within normal range for the participants with PTSD, acknowledged Dr. Toloza, a research fellow in the division of endocrinology and metabolism at University of Arkansas for Medical Sciences, Little Rock.
However, even though there was no sign of frank thyroid disease in the PTSD population, the elevated T3 levels seen in the analysis are consistent with other studies showing a correlation between higher T3 levels and more-severe PTSD.
It is not known exactly why significant increases in the levels of total and free T3 were seen only in the combat-exposed PTSD population, Dr. Toloza said. “The type of trauma trigger may influence the adaptive responses to stress and might result in diverse thyroid alterations.”
Elevated catecholamine levels, seen in individuals with PTSD, can increase peripheral conversion of T4 to T3, explained Dr. Toloza. Ongoing catecholamine elevation may account for the isolated elevation in T3 levels in the PTSD population. Beta1-adrenergic blockade is an accepted pharmacologic strategy to help alleviate PTSD symptoms.
Dr. Toloza and coinvestigators did not have access to data that would have allowed them to ascertain what types of injuries were sustained by individuals with combat-related PTSD, but he noted in response to a question, that it would be worthwhile to see whether combatants who were blast exposed had different thyroid hormone values than those who were not, because hypothalamic injury is common in blast. This is a future direction Dr. Toloza wishes to pursue.
“Our findings add to the growing literature suggesting that thyroid function changes may be associated with PTSD,” the investigators wrote, but “further research is needed to ascertain the role of thyroid function alterations in PTSD.”
Dr. Toloza reported no financial disclosures or conflicts of interest.
CHICAGO – Higher levels of triiodothyronine (T3) were seen in combatants with PTSD, compared with patients whose PTSD arose from other adverse experiences, according to findings from a systematic review and meta-analysis.
who had experienced childhood or sexual abuse or were a wartime refugee without PTSD (FT3, 0.36 pg/mL higher, and total T3, 31.6 ng/mL higher, respectively; P = .0004 and P less than .00001).
“We found statistically higher free T3 and total T3 levels in patients with [combat-related] PTSD, compared with controls,” said Freddy J.K. Toloza, MD, in an interview during a poster session of the annual meeting of the American Thyroid Association.
However, he noted that there were no overall differences in thyroid-stimulating hormone, free tetraiodothyronine (T4), and total T4 levels between individuals with PTSD and the non-PTSD control participants. In addition, though free and total T3 levels were significantly higher for the overall PTSD cohort than for control participants, the differences were driven by the studies that included combat-exposed individuals.
Dr. Toloza and colleagues included 10 observational studies in their final review and meta-analysis. Five studies looked at war veterans; the others examined individuals who had experienced child abuse or sexual abuse, who were refugees, or who were from the general population.
For inclusion, the studies had to report both mean values and standard deviations for standard thyroid-hormone test values in patients with PTSD, compared with a non-PTSD control group. These included 373 patients with PTSD and 301 control participants. Just under half (47%) were women. None of the studies, wrote the investigators, “compared rates of overt/subclinical thyroid disease between groups.”
There are known links between many endocrine disorders and psychiatric conditions, said Dr. Toloza, but the interplay between disordered thyroid function and neuropsychiatric problems is still being examined. Looking at PTSD is important because it’s estimated that 6%-9% of the U.S. adult population has experienced PTSD over the course of a lifetime.
Levels of thyroid hormones in the systematic review and meta-analysis were still within normal range for the participants with PTSD, acknowledged Dr. Toloza, a research fellow in the division of endocrinology and metabolism at University of Arkansas for Medical Sciences, Little Rock.
However, even though there was no sign of frank thyroid disease in the PTSD population, the elevated T3 levels seen in the analysis are consistent with other studies showing a correlation between higher T3 levels and more-severe PTSD.
It is not known exactly why significant increases in the levels of total and free T3 were seen only in the combat-exposed PTSD population, Dr. Toloza said. “The type of trauma trigger may influence the adaptive responses to stress and might result in diverse thyroid alterations.”
Elevated catecholamine levels, seen in individuals with PTSD, can increase peripheral conversion of T4 to T3, explained Dr. Toloza. Ongoing catecholamine elevation may account for the isolated elevation in T3 levels in the PTSD population. Beta1-adrenergic blockade is an accepted pharmacologic strategy to help alleviate PTSD symptoms.
Dr. Toloza and coinvestigators did not have access to data that would have allowed them to ascertain what types of injuries were sustained by individuals with combat-related PTSD, but he noted in response to a question, that it would be worthwhile to see whether combatants who were blast exposed had different thyroid hormone values than those who were not, because hypothalamic injury is common in blast. This is a future direction Dr. Toloza wishes to pursue.
“Our findings add to the growing literature suggesting that thyroid function changes may be associated with PTSD,” the investigators wrote, but “further research is needed to ascertain the role of thyroid function alterations in PTSD.”
Dr. Toloza reported no financial disclosures or conflicts of interest.
REPORTING FROM ATA 2019
The Health Impacts of Comorbid PTSD and MDD
It is well established, both in research and everyday real-world experience, that posttraumatic stress disorder (PTSD) and major depressive disorder (MDD) independently can have a huge impact on physical health. There is evidence, for instance, that both are independent “robust risk factors” for the onset of chronic physical illnesses, including musculoskeletal, digestive, and circulatory, say researchers from VA San Diego; University of California, San Diego; VA Center of Excellence for Stress and Mental Health, San Diego; National Center for PTSD, Vermont; VA Connecticut Health Care System, and Yale. However, less is known about how the 2 conditions might synergistically affect physical health and well-being.
In this, the first population-based study of the burden of medical illness associated with PTSD, MDD, and their comorbidity, the researchers examined data from 2,732 participants in the National Health and Resilience in Veterans Study.
Of the participants, 40 had PTSD only, 141 had MDD only, and 60 had both. Among veterans who screened positive for probable PTSD, 47% also screened positive for probable MDD. Among veterans who screened positive for probable MDD, 83% screened positive for probable PTSD.
The participants with PTSD, MDD, or both had substantially greater burden of medical illness compared with that of those participants who had no lifetime history of either condition. Consistent with findings from previous studies, each group had a greater prevalence of a broad range of medical conditions, including cardiovascular, respiratory, neurologic, and chronic pain-related diseases.
However, the study results indicated that comorbid PTSD/MDD was associated with substantially greater medical comorbidity compared with either disorder alone. Veterans with co-occurring PTSD and MDD had higher odds of being diagnosed with migraine, fibromyalgia, and rheumatoid arthritis, for instance, relative to those with MDD alone.
Co-occurring PTSD/MDD was also associated with “markedly worse” cardiovascular health compared with either condition alone. Veterans with PTSD/MDD had more than twice the likelihood of being diagnosed with hypercholesterolemia and hypertension compared with those who had PTSD alone. They had more than double the odds of being diagnosed with heart disease compared with those who had only MDD.
Several factors may account for why PTSD seems to compound risk for pain-related conditions, the researchers say. People with PTSD may have increased attentional bias toward threatening internal stimuli (above and beyond MDD), which may heighten appraisal of pain; they also tend to have higher levels of anxiety sensitivity, which may amplify fear reactivity to pain. Some evidence suggests that the brain region involved in processing the affective component of pain is dysfunctional in PTSD, leading to an exaggerated response.
The associations between PTSD and pain, and PTSD/MDD and cardiovascular risks were noteworthy, the researchers say, because they were found even after “stringently controlling” for relevant covariates, including lifetime trauma exposure; combat veteran status; and alcohol, drug, and nicotine use disorder.
The finding that PTSD/MDD and PTSD were associated with higher levels of somatization is consistent with other research, the researchers note. But they say more research is needed to examine whether somatization increases vulnerability to the development of PTSD and MDD, or whether symptoms arise as a consequence of the disorders.
Further, they underscore the importance of integrating mental health services in primary care settings. Previous research has shown that older veterans tend to report mental health concerns to their primary care provider rather than seek specialty mental health treatment; they also underreport symptoms related to emotional difficulties and overreport somatic complaints.
Perhaps most important from a public health perspective, the researchers say, is that current findings suggest that veterans with co-occurring PTSD/MDD represent a “particularly high-risk group” for cardiovascular problems. The issue, they emphasize, “deserves careful attention” from the VA and other health care systems.
It is well established, both in research and everyday real-world experience, that posttraumatic stress disorder (PTSD) and major depressive disorder (MDD) independently can have a huge impact on physical health. There is evidence, for instance, that both are independent “robust risk factors” for the onset of chronic physical illnesses, including musculoskeletal, digestive, and circulatory, say researchers from VA San Diego; University of California, San Diego; VA Center of Excellence for Stress and Mental Health, San Diego; National Center for PTSD, Vermont; VA Connecticut Health Care System, and Yale. However, less is known about how the 2 conditions might synergistically affect physical health and well-being.
In this, the first population-based study of the burden of medical illness associated with PTSD, MDD, and their comorbidity, the researchers examined data from 2,732 participants in the National Health and Resilience in Veterans Study.
Of the participants, 40 had PTSD only, 141 had MDD only, and 60 had both. Among veterans who screened positive for probable PTSD, 47% also screened positive for probable MDD. Among veterans who screened positive for probable MDD, 83% screened positive for probable PTSD.
The participants with PTSD, MDD, or both had substantially greater burden of medical illness compared with that of those participants who had no lifetime history of either condition. Consistent with findings from previous studies, each group had a greater prevalence of a broad range of medical conditions, including cardiovascular, respiratory, neurologic, and chronic pain-related diseases.
However, the study results indicated that comorbid PTSD/MDD was associated with substantially greater medical comorbidity compared with either disorder alone. Veterans with co-occurring PTSD and MDD had higher odds of being diagnosed with migraine, fibromyalgia, and rheumatoid arthritis, for instance, relative to those with MDD alone.
Co-occurring PTSD/MDD was also associated with “markedly worse” cardiovascular health compared with either condition alone. Veterans with PTSD/MDD had more than twice the likelihood of being diagnosed with hypercholesterolemia and hypertension compared with those who had PTSD alone. They had more than double the odds of being diagnosed with heart disease compared with those who had only MDD.
Several factors may account for why PTSD seems to compound risk for pain-related conditions, the researchers say. People with PTSD may have increased attentional bias toward threatening internal stimuli (above and beyond MDD), which may heighten appraisal of pain; they also tend to have higher levels of anxiety sensitivity, which may amplify fear reactivity to pain. Some evidence suggests that the brain region involved in processing the affective component of pain is dysfunctional in PTSD, leading to an exaggerated response.
The associations between PTSD and pain, and PTSD/MDD and cardiovascular risks were noteworthy, the researchers say, because they were found even after “stringently controlling” for relevant covariates, including lifetime trauma exposure; combat veteran status; and alcohol, drug, and nicotine use disorder.
The finding that PTSD/MDD and PTSD were associated with higher levels of somatization is consistent with other research, the researchers note. But they say more research is needed to examine whether somatization increases vulnerability to the development of PTSD and MDD, or whether symptoms arise as a consequence of the disorders.
Further, they underscore the importance of integrating mental health services in primary care settings. Previous research has shown that older veterans tend to report mental health concerns to their primary care provider rather than seek specialty mental health treatment; they also underreport symptoms related to emotional difficulties and overreport somatic complaints.
Perhaps most important from a public health perspective, the researchers say, is that current findings suggest that veterans with co-occurring PTSD/MDD represent a “particularly high-risk group” for cardiovascular problems. The issue, they emphasize, “deserves careful attention” from the VA and other health care systems.
It is well established, both in research and everyday real-world experience, that posttraumatic stress disorder (PTSD) and major depressive disorder (MDD) independently can have a huge impact on physical health. There is evidence, for instance, that both are independent “robust risk factors” for the onset of chronic physical illnesses, including musculoskeletal, digestive, and circulatory, say researchers from VA San Diego; University of California, San Diego; VA Center of Excellence for Stress and Mental Health, San Diego; National Center for PTSD, Vermont; VA Connecticut Health Care System, and Yale. However, less is known about how the 2 conditions might synergistically affect physical health and well-being.
In this, the first population-based study of the burden of medical illness associated with PTSD, MDD, and their comorbidity, the researchers examined data from 2,732 participants in the National Health and Resilience in Veterans Study.
Of the participants, 40 had PTSD only, 141 had MDD only, and 60 had both. Among veterans who screened positive for probable PTSD, 47% also screened positive for probable MDD. Among veterans who screened positive for probable MDD, 83% screened positive for probable PTSD.
The participants with PTSD, MDD, or both had substantially greater burden of medical illness compared with that of those participants who had no lifetime history of either condition. Consistent with findings from previous studies, each group had a greater prevalence of a broad range of medical conditions, including cardiovascular, respiratory, neurologic, and chronic pain-related diseases.
However, the study results indicated that comorbid PTSD/MDD was associated with substantially greater medical comorbidity compared with either disorder alone. Veterans with co-occurring PTSD and MDD had higher odds of being diagnosed with migraine, fibromyalgia, and rheumatoid arthritis, for instance, relative to those with MDD alone.
Co-occurring PTSD/MDD was also associated with “markedly worse” cardiovascular health compared with either condition alone. Veterans with PTSD/MDD had more than twice the likelihood of being diagnosed with hypercholesterolemia and hypertension compared with those who had PTSD alone. They had more than double the odds of being diagnosed with heart disease compared with those who had only MDD.
Several factors may account for why PTSD seems to compound risk for pain-related conditions, the researchers say. People with PTSD may have increased attentional bias toward threatening internal stimuli (above and beyond MDD), which may heighten appraisal of pain; they also tend to have higher levels of anxiety sensitivity, which may amplify fear reactivity to pain. Some evidence suggests that the brain region involved in processing the affective component of pain is dysfunctional in PTSD, leading to an exaggerated response.
The associations between PTSD and pain, and PTSD/MDD and cardiovascular risks were noteworthy, the researchers say, because they were found even after “stringently controlling” for relevant covariates, including lifetime trauma exposure; combat veteran status; and alcohol, drug, and nicotine use disorder.
The finding that PTSD/MDD and PTSD were associated with higher levels of somatization is consistent with other research, the researchers note. But they say more research is needed to examine whether somatization increases vulnerability to the development of PTSD and MDD, or whether symptoms arise as a consequence of the disorders.
Further, they underscore the importance of integrating mental health services in primary care settings. Previous research has shown that older veterans tend to report mental health concerns to their primary care provider rather than seek specialty mental health treatment; they also underreport symptoms related to emotional difficulties and overreport somatic complaints.
Perhaps most important from a public health perspective, the researchers say, is that current findings suggest that veterans with co-occurring PTSD/MDD represent a “particularly high-risk group” for cardiovascular problems. The issue, they emphasize, “deserves careful attention” from the VA and other health care systems.
Retraining Working Memory to Reduce PTSD Symptoms
Working memory (WM)—the function that allows us to temporarily store information and use it for cognitive tasks like learning, reasoning, and comprehension—is thought to play an important role in managing anxiety and intrusive symptoms of posttraumatic stress disorder (PTSD). Researchers have found inefficient filtering of threatening material from WM and increased storage of task-irrelevant threat distractors are associated with elevated anxiety. Thus, veterans with high levels of anxiety may disproportionately allocate cognitive resources toward threatening stimuli, and have trouble keeping threatening thoughts from entering WM and staying there. People with PTSD also have been shown to have problems using WM in emotional contexts, making it hard for them to deal with stressful situations in work and personal life. Some research has suggested that WM training that incorporates affective stimuli may increase the ability to use WM in emotional contexts. In a pilot study, researchers from Medical College of Wisconsin and University of Wisconsin-Milwaukee expanded on that possibility by testing 2 kinds of WM training in 21 veterans with elevated PTSD symptoms.
Participants completed a pretest, 15 sessions of at-home computerized training, a posttest, and a 1-month follow-up session. The computerized tests included tasks to measure WM capacity, such as solving math questions while trying to remember a set of unrelated numbers. The participants were then assigned to active emotional WM training (n-back) or control emotional WM training (1-back). The n-back training involved constant updating of information stored in WM and shifting between visual and auditory stimuli using 8 different faces and 8 different negative words. The training sessions started at the 1-back level and increased in difficulty level by level with performance with > 95% accuracy, or were scaled back with performance with < 75% accuracy. The control training was the same but consisted only of 1 level.
Overall, the researchers say, contrary to their hypothesis, they did not find a significant difference in results from the 2 groups. But they did find that both trainings had an overall “significant and sizable” impact on PTSD symptoms in both groups: 73% of the n-back group and 60% of the 1-back group had a reduction of ≥ 10 points on the PTSD Checklist total scores, which the researchers say is considered a clinically meaningful change. Moreover, both groups showed a clinically meaningful reduction in symptoms at follow-up: 55% and 40%, respectively.
It was interesting, the researchers say, that both groups showed improvement, especially with the 1-back intervention, which they had used as a “minimally effective” control condition. They also note that, anecdotally, the participants found both interventions “quite challenging.” It may be that in this population even the 1-back intervention is enough to detectably reduce symptoms, the researchers say. They were also encouraged to see that the n-back condition (marginally) outperformed the 1-back condition in improving reexperiencing symptoms, theoretically the most relevant training target of WM-focused intervention. The researchers believe that their study yields useful pilot data, suggesting that n-back training can have a clinical impact primarily by reducing reexperiencing symptoms, which are highly likely to indicate the presence of impaired WM functioning.
Working memory (WM)—the function that allows us to temporarily store information and use it for cognitive tasks like learning, reasoning, and comprehension—is thought to play an important role in managing anxiety and intrusive symptoms of posttraumatic stress disorder (PTSD). Researchers have found inefficient filtering of threatening material from WM and increased storage of task-irrelevant threat distractors are associated with elevated anxiety. Thus, veterans with high levels of anxiety may disproportionately allocate cognitive resources toward threatening stimuli, and have trouble keeping threatening thoughts from entering WM and staying there. People with PTSD also have been shown to have problems using WM in emotional contexts, making it hard for them to deal with stressful situations in work and personal life. Some research has suggested that WM training that incorporates affective stimuli may increase the ability to use WM in emotional contexts. In a pilot study, researchers from Medical College of Wisconsin and University of Wisconsin-Milwaukee expanded on that possibility by testing 2 kinds of WM training in 21 veterans with elevated PTSD symptoms.
Participants completed a pretest, 15 sessions of at-home computerized training, a posttest, and a 1-month follow-up session. The computerized tests included tasks to measure WM capacity, such as solving math questions while trying to remember a set of unrelated numbers. The participants were then assigned to active emotional WM training (n-back) or control emotional WM training (1-back). The n-back training involved constant updating of information stored in WM and shifting between visual and auditory stimuli using 8 different faces and 8 different negative words. The training sessions started at the 1-back level and increased in difficulty level by level with performance with > 95% accuracy, or were scaled back with performance with < 75% accuracy. The control training was the same but consisted only of 1 level.
Overall, the researchers say, contrary to their hypothesis, they did not find a significant difference in results from the 2 groups. But they did find that both trainings had an overall “significant and sizable” impact on PTSD symptoms in both groups: 73% of the n-back group and 60% of the 1-back group had a reduction of ≥ 10 points on the PTSD Checklist total scores, which the researchers say is considered a clinically meaningful change. Moreover, both groups showed a clinically meaningful reduction in symptoms at follow-up: 55% and 40%, respectively.
It was interesting, the researchers say, that both groups showed improvement, especially with the 1-back intervention, which they had used as a “minimally effective” control condition. They also note that, anecdotally, the participants found both interventions “quite challenging.” It may be that in this population even the 1-back intervention is enough to detectably reduce symptoms, the researchers say. They were also encouraged to see that the n-back condition (marginally) outperformed the 1-back condition in improving reexperiencing symptoms, theoretically the most relevant training target of WM-focused intervention. The researchers believe that their study yields useful pilot data, suggesting that n-back training can have a clinical impact primarily by reducing reexperiencing symptoms, which are highly likely to indicate the presence of impaired WM functioning.
Working memory (WM)—the function that allows us to temporarily store information and use it for cognitive tasks like learning, reasoning, and comprehension—is thought to play an important role in managing anxiety and intrusive symptoms of posttraumatic stress disorder (PTSD). Researchers have found inefficient filtering of threatening material from WM and increased storage of task-irrelevant threat distractors are associated with elevated anxiety. Thus, veterans with high levels of anxiety may disproportionately allocate cognitive resources toward threatening stimuli, and have trouble keeping threatening thoughts from entering WM and staying there. People with PTSD also have been shown to have problems using WM in emotional contexts, making it hard for them to deal with stressful situations in work and personal life. Some research has suggested that WM training that incorporates affective stimuli may increase the ability to use WM in emotional contexts. In a pilot study, researchers from Medical College of Wisconsin and University of Wisconsin-Milwaukee expanded on that possibility by testing 2 kinds of WM training in 21 veterans with elevated PTSD symptoms.
Participants completed a pretest, 15 sessions of at-home computerized training, a posttest, and a 1-month follow-up session. The computerized tests included tasks to measure WM capacity, such as solving math questions while trying to remember a set of unrelated numbers. The participants were then assigned to active emotional WM training (n-back) or control emotional WM training (1-back). The n-back training involved constant updating of information stored in WM and shifting between visual and auditory stimuli using 8 different faces and 8 different negative words. The training sessions started at the 1-back level and increased in difficulty level by level with performance with > 95% accuracy, or were scaled back with performance with < 75% accuracy. The control training was the same but consisted only of 1 level.
Overall, the researchers say, contrary to their hypothesis, they did not find a significant difference in results from the 2 groups. But they did find that both trainings had an overall “significant and sizable” impact on PTSD symptoms in both groups: 73% of the n-back group and 60% of the 1-back group had a reduction of ≥ 10 points on the PTSD Checklist total scores, which the researchers say is considered a clinically meaningful change. Moreover, both groups showed a clinically meaningful reduction in symptoms at follow-up: 55% and 40%, respectively.
It was interesting, the researchers say, that both groups showed improvement, especially with the 1-back intervention, which they had used as a “minimally effective” control condition. They also note that, anecdotally, the participants found both interventions “quite challenging.” It may be that in this population even the 1-back intervention is enough to detectably reduce symptoms, the researchers say. They were also encouraged to see that the n-back condition (marginally) outperformed the 1-back condition in improving reexperiencing symptoms, theoretically the most relevant training target of WM-focused intervention. The researchers believe that their study yields useful pilot data, suggesting that n-back training can have a clinical impact primarily by reducing reexperiencing symptoms, which are highly likely to indicate the presence of impaired WM functioning.