User login
Movement-based yoga ‘viable’ for depression in many mental disorders
Movement-based yoga appears to ease depressive symptoms in a wide range of mental health disorders, a new systematic review and meta-analysis suggest.
Results of the research, which included 19 studies and more than 1,000 patients with a variety of mental health diagnoses, showed that those who practiced yoga experienced greater reductions in depressive symptoms versus those undergoing no treatment, usual treatment, or attention-control exercises. In addition, there was a dose-dependent effect such that more weekly yoga sessions were associated with the greatest reduction in depressive symptoms.
“Once we reviewed all the existing science about the mental health benefits of movement-based yoga, we found that movement-based yoga – which is the same thing as postural yoga or asana – helped reduce symptoms of depression,” study investigator Jacinta Brinsley, BClinExPhys, of the University of South Australia, Adelaide, said in an interview.
“We also found those who practiced more frequently had bigger reductions. However, it didn’t matter how long the individual sessions were; what mattered was how many times per week people practiced,” she added.
The researchers noted that the study is the first to focus specifically on movement-based yoga.
“We excluded meditative forms of yoga, which have often been included in previous reviews, yielding mixed findings. The other thing we’ve done a bit differently is pool all the different diagnoses together and then look at depressive symptoms across them,” said Ms. Brinsley.
The study was published online May 18 in the British Journal of Sports Medicine.
Getting clarity
Depressive disorders are currently the world’s leading cause of disability, affecting more than 340 million people.
Most individuals who suffer from depressive disorders also experience a host of physical comorbidities including obesity, type 2 diabetes, metabolic syndrome, and cardiovascular disease.
Perhaps not surprisingly, physical inactivity is also associated with higher levels of depressive symptoms, which may be the reason some international organizations now recommend that physical activity be included as part of routine psychiatric care.
One potential form of exercise is yoga, which has become popular in Western culture, including among psychiatric patients. Although previous systematic reviews and meta-analyses have examined the effects of various yoga interventions on mental health, none has investigated the benefits of yoga across a range of psychiatric diagnoses.
What’s more, the authors of these reviews all urge caution when interpreting their results because of potential heterogeneity of the various yoga interventions, as well as poor methodological reporting.
“As an exercise physiologist, I prescribe evidence-based treatment,” said Ms. Brinsley. “I was interested in seeing if there’s evidence to support movement-based yoga in people who were struggling with mental health or who had a diagnosed mental illness.
“The [previous] findings are quite contradictory and there’s not a clear outcome in terms of intervention results, so we pooled the data and ran the meta-analysis, thinking it would be a great way to add some important evidence to the science,” she added.
To allow for a more comprehensive assessment of yoga’s potential mental health benefits, the investigators included a range of mental health diagnoses.
Dose-dependent effect
Studies were only included in the analysis if they were randomized, controlled trials with a yoga intervention that had a minimum of 50% physical activity during each session in adults with a recognized diagnosed mental disorder. Control conditions were defined as treatment as usual, wait list, or attention controls.
Two investigators independently scanned article titles and abstracts, and a final list of articles for the study was decided by consensus. Study quality was reported using the PEDro checklist; a random-effects meta-analysis was conducted using Comprehensive Meta-Analysis software.
A total of 3,880 records were identified and screened. The investigators assessed full-text versions of 80 articles, 19 of which (1,080 patients) were eligible for inclusion in the review.
Of these, nine studies included patients with a depressive disorder; five trials were in patients with a diagnosis of schizophrenia, three studies included patients with a diagnosis of PTSD, one study included patients diagnosed with alcohol dependence, and one study included patients with a range of psychiatric disorders.
Of the 1,080 patients included in the review, 578 were assigned to yoga and 502 to control conditions. Yoga practice involved a mixture of movement, breathing exercises, and/or mindfulness, but the movement component took up more than half of each session.
The yoga interventions lasted an average of 2.4 months (range, 1.5-2.5 months), with an average of 1.6 sessions per week (range, 1-3 sessions) that lasted an average of 60 minutes (range, 20-90 minutes).
Of the 19 studies (632 patients), 13 reported changes in depressive symptoms and were therefore included in the meta-analysis. The six studies excluded from the quantitative analysis did not report depression symptom scores.
With respect to primary outcomes, individuals who performed yoga showed a greater reduction in depressive symptoms, compared with the three control groups (standardized mean difference, –0.41; 95% CI, –0.65 to –0.17; P < .001).
Specific subgroup analyses showed a moderate effect of yoga on depressive symptoms, compared with wait-list controls (SMD, –0.58; P < .05), treatment as usual (SMD, –0.39; P = .31), and attention controls (SMD, –0.21; P = .22).
Subgroup analyses were also performed with respect to diagnostic category. These data showed a moderate effect of yoga on depressive symptoms in depressive disorders (SMD, –0.40; P < .01), no effect in PTSD (SMD, –0.01; P = .95), a nominal effect in alcohol use disorders (SMD, –0.24; P = .69), and a marked effect in schizophrenia (SMD, –0.90; P < .01).
Movement may be key
Researchers also performed a series of meta-regression analyses, which showed that the number of yoga sessions performed each week had a significant effect on depressive symptoms. Indeed, individuals with higher session frequencies demonstrated a greater improvement in symptoms (beta, –0.44; P < .001).
These findings, said Ms. Brinsley, suggest yoga may be a viable intervention for managing depressive symptoms in patients with a variety of mental disorders.
Based on these findings, along with other conventional forms of exercise.
Equally important was the finding that the number of weekly yoga sessions moderated the effect of depressive symptoms, as it may inform the future design of yoga interventions in patients with mental disorders.
With this in mind, the researchers recommended that such interventions should aim to increase the frequency or weekly sessions rather than the duration of each individual session or the overall duration of the intervention.
However, said Ms. Brinsley, these findings suggest it is the physical aspect of the yoga practice that may be key.
“Yoga comprises several different components, including the movement postures, the breathing component, and the mindfulness or meditative component, but in this meta-analysis we looked specifically at yoga that was at least 50% movement based. So it might have also included mindfulness and breathing, but it had to have the movement,” she said.
Don’t discount meditation
Commenting on the findings, Holger Cramer, MSc, PhD, DSc, who was not involved in the study, noted that the systematic review and meta-analysis builds on a number of previous reviews regarding the benefits of yoga for mental disorders.
“Surprisingly, the largest effect in this analysis was found in schizophrenia, even higher than in patients with depressive disorders,” said Dr. Cramer of the University of Duisburg-Essen (Germany). “This is in strong contradiction to what would otherwise be expected. As the authors point out, only about a quarter of all schizophrenia patients suffer from depression, so there should not be so much room for improvement.”
Dr. Cramer also advised against reducing yoga to simply a physical undertaking. “We have shown in our meta-analysis that those interventions focusing on meditation and/or breathing techniques are the most effective ones,” he added.
As such, he urged that breathing techniques be a part of yoga for treating depression in psychiatric disorders, though care should be taken in patients with PTSD, “since breath control might be perceived as unpleasant.”
For Ms. Brinsley, the findings help solidify yoga’s potential as a genuine treatment option for a variety of mental health patients suffering depressive symptoms.
“It’s about acknowledging that yoga can be a helpful part of treatment and can have a significant effect on mental health,” she noted.
At the same time, practitioners also need to acknowledge that patients suffering from mental health disorders may struggle with motivation when it comes to activities such as yoga.
“Engaging in a new activity can be particularly challenging if you’re struggling with mental health. Nevertheless, it’s important for people to have a choice and do something they enjoy. And yoga can be another tool in their toolbox for managing their mental health,” she said.
The study was funded by the U.K. National Institute for Health Research and Health Education England. Ms. Brinsley and Dr. Cramer have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Movement-based yoga appears to ease depressive symptoms in a wide range of mental health disorders, a new systematic review and meta-analysis suggest.
Results of the research, which included 19 studies and more than 1,000 patients with a variety of mental health diagnoses, showed that those who practiced yoga experienced greater reductions in depressive symptoms versus those undergoing no treatment, usual treatment, or attention-control exercises. In addition, there was a dose-dependent effect such that more weekly yoga sessions were associated with the greatest reduction in depressive symptoms.
“Once we reviewed all the existing science about the mental health benefits of movement-based yoga, we found that movement-based yoga – which is the same thing as postural yoga or asana – helped reduce symptoms of depression,” study investigator Jacinta Brinsley, BClinExPhys, of the University of South Australia, Adelaide, said in an interview.
“We also found those who practiced more frequently had bigger reductions. However, it didn’t matter how long the individual sessions were; what mattered was how many times per week people practiced,” she added.
The researchers noted that the study is the first to focus specifically on movement-based yoga.
“We excluded meditative forms of yoga, which have often been included in previous reviews, yielding mixed findings. The other thing we’ve done a bit differently is pool all the different diagnoses together and then look at depressive symptoms across them,” said Ms. Brinsley.
The study was published online May 18 in the British Journal of Sports Medicine.
Getting clarity
Depressive disorders are currently the world’s leading cause of disability, affecting more than 340 million people.
Most individuals who suffer from depressive disorders also experience a host of physical comorbidities including obesity, type 2 diabetes, metabolic syndrome, and cardiovascular disease.
Perhaps not surprisingly, physical inactivity is also associated with higher levels of depressive symptoms, which may be the reason some international organizations now recommend that physical activity be included as part of routine psychiatric care.
One potential form of exercise is yoga, which has become popular in Western culture, including among psychiatric patients. Although previous systematic reviews and meta-analyses have examined the effects of various yoga interventions on mental health, none has investigated the benefits of yoga across a range of psychiatric diagnoses.
What’s more, the authors of these reviews all urge caution when interpreting their results because of potential heterogeneity of the various yoga interventions, as well as poor methodological reporting.
“As an exercise physiologist, I prescribe evidence-based treatment,” said Ms. Brinsley. “I was interested in seeing if there’s evidence to support movement-based yoga in people who were struggling with mental health or who had a diagnosed mental illness.
“The [previous] findings are quite contradictory and there’s not a clear outcome in terms of intervention results, so we pooled the data and ran the meta-analysis, thinking it would be a great way to add some important evidence to the science,” she added.
To allow for a more comprehensive assessment of yoga’s potential mental health benefits, the investigators included a range of mental health diagnoses.
Dose-dependent effect
Studies were only included in the analysis if they were randomized, controlled trials with a yoga intervention that had a minimum of 50% physical activity during each session in adults with a recognized diagnosed mental disorder. Control conditions were defined as treatment as usual, wait list, or attention controls.
Two investigators independently scanned article titles and abstracts, and a final list of articles for the study was decided by consensus. Study quality was reported using the PEDro checklist; a random-effects meta-analysis was conducted using Comprehensive Meta-Analysis software.
A total of 3,880 records were identified and screened. The investigators assessed full-text versions of 80 articles, 19 of which (1,080 patients) were eligible for inclusion in the review.
Of these, nine studies included patients with a depressive disorder; five trials were in patients with a diagnosis of schizophrenia, three studies included patients with a diagnosis of PTSD, one study included patients diagnosed with alcohol dependence, and one study included patients with a range of psychiatric disorders.
Of the 1,080 patients included in the review, 578 were assigned to yoga and 502 to control conditions. Yoga practice involved a mixture of movement, breathing exercises, and/or mindfulness, but the movement component took up more than half of each session.
The yoga interventions lasted an average of 2.4 months (range, 1.5-2.5 months), with an average of 1.6 sessions per week (range, 1-3 sessions) that lasted an average of 60 minutes (range, 20-90 minutes).
Of the 19 studies (632 patients), 13 reported changes in depressive symptoms and were therefore included in the meta-analysis. The six studies excluded from the quantitative analysis did not report depression symptom scores.
With respect to primary outcomes, individuals who performed yoga showed a greater reduction in depressive symptoms, compared with the three control groups (standardized mean difference, –0.41; 95% CI, –0.65 to –0.17; P < .001).
Specific subgroup analyses showed a moderate effect of yoga on depressive symptoms, compared with wait-list controls (SMD, –0.58; P < .05), treatment as usual (SMD, –0.39; P = .31), and attention controls (SMD, –0.21; P = .22).
Subgroup analyses were also performed with respect to diagnostic category. These data showed a moderate effect of yoga on depressive symptoms in depressive disorders (SMD, –0.40; P < .01), no effect in PTSD (SMD, –0.01; P = .95), a nominal effect in alcohol use disorders (SMD, –0.24; P = .69), and a marked effect in schizophrenia (SMD, –0.90; P < .01).
Movement may be key
Researchers also performed a series of meta-regression analyses, which showed that the number of yoga sessions performed each week had a significant effect on depressive symptoms. Indeed, individuals with higher session frequencies demonstrated a greater improvement in symptoms (beta, –0.44; P < .001).
These findings, said Ms. Brinsley, suggest yoga may be a viable intervention for managing depressive symptoms in patients with a variety of mental disorders.
Based on these findings, along with other conventional forms of exercise.
Equally important was the finding that the number of weekly yoga sessions moderated the effect of depressive symptoms, as it may inform the future design of yoga interventions in patients with mental disorders.
With this in mind, the researchers recommended that such interventions should aim to increase the frequency or weekly sessions rather than the duration of each individual session or the overall duration of the intervention.
However, said Ms. Brinsley, these findings suggest it is the physical aspect of the yoga practice that may be key.
“Yoga comprises several different components, including the movement postures, the breathing component, and the mindfulness or meditative component, but in this meta-analysis we looked specifically at yoga that was at least 50% movement based. So it might have also included mindfulness and breathing, but it had to have the movement,” she said.
Don’t discount meditation
Commenting on the findings, Holger Cramer, MSc, PhD, DSc, who was not involved in the study, noted that the systematic review and meta-analysis builds on a number of previous reviews regarding the benefits of yoga for mental disorders.
“Surprisingly, the largest effect in this analysis was found in schizophrenia, even higher than in patients with depressive disorders,” said Dr. Cramer of the University of Duisburg-Essen (Germany). “This is in strong contradiction to what would otherwise be expected. As the authors point out, only about a quarter of all schizophrenia patients suffer from depression, so there should not be so much room for improvement.”
Dr. Cramer also advised against reducing yoga to simply a physical undertaking. “We have shown in our meta-analysis that those interventions focusing on meditation and/or breathing techniques are the most effective ones,” he added.
As such, he urged that breathing techniques be a part of yoga for treating depression in psychiatric disorders, though care should be taken in patients with PTSD, “since breath control might be perceived as unpleasant.”
For Ms. Brinsley, the findings help solidify yoga’s potential as a genuine treatment option for a variety of mental health patients suffering depressive symptoms.
“It’s about acknowledging that yoga can be a helpful part of treatment and can have a significant effect on mental health,” she noted.
At the same time, practitioners also need to acknowledge that patients suffering from mental health disorders may struggle with motivation when it comes to activities such as yoga.
“Engaging in a new activity can be particularly challenging if you’re struggling with mental health. Nevertheless, it’s important for people to have a choice and do something they enjoy. And yoga can be another tool in their toolbox for managing their mental health,” she said.
The study was funded by the U.K. National Institute for Health Research and Health Education England. Ms. Brinsley and Dr. Cramer have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Movement-based yoga appears to ease depressive symptoms in a wide range of mental health disorders, a new systematic review and meta-analysis suggest.
Results of the research, which included 19 studies and more than 1,000 patients with a variety of mental health diagnoses, showed that those who practiced yoga experienced greater reductions in depressive symptoms versus those undergoing no treatment, usual treatment, or attention-control exercises. In addition, there was a dose-dependent effect such that more weekly yoga sessions were associated with the greatest reduction in depressive symptoms.
“Once we reviewed all the existing science about the mental health benefits of movement-based yoga, we found that movement-based yoga – which is the same thing as postural yoga or asana – helped reduce symptoms of depression,” study investigator Jacinta Brinsley, BClinExPhys, of the University of South Australia, Adelaide, said in an interview.
“We also found those who practiced more frequently had bigger reductions. However, it didn’t matter how long the individual sessions were; what mattered was how many times per week people practiced,” she added.
The researchers noted that the study is the first to focus specifically on movement-based yoga.
“We excluded meditative forms of yoga, which have often been included in previous reviews, yielding mixed findings. The other thing we’ve done a bit differently is pool all the different diagnoses together and then look at depressive symptoms across them,” said Ms. Brinsley.
The study was published online May 18 in the British Journal of Sports Medicine.
Getting clarity
Depressive disorders are currently the world’s leading cause of disability, affecting more than 340 million people.
Most individuals who suffer from depressive disorders also experience a host of physical comorbidities including obesity, type 2 diabetes, metabolic syndrome, and cardiovascular disease.
Perhaps not surprisingly, physical inactivity is also associated with higher levels of depressive symptoms, which may be the reason some international organizations now recommend that physical activity be included as part of routine psychiatric care.
One potential form of exercise is yoga, which has become popular in Western culture, including among psychiatric patients. Although previous systematic reviews and meta-analyses have examined the effects of various yoga interventions on mental health, none has investigated the benefits of yoga across a range of psychiatric diagnoses.
What’s more, the authors of these reviews all urge caution when interpreting their results because of potential heterogeneity of the various yoga interventions, as well as poor methodological reporting.
“As an exercise physiologist, I prescribe evidence-based treatment,” said Ms. Brinsley. “I was interested in seeing if there’s evidence to support movement-based yoga in people who were struggling with mental health or who had a diagnosed mental illness.
“The [previous] findings are quite contradictory and there’s not a clear outcome in terms of intervention results, so we pooled the data and ran the meta-analysis, thinking it would be a great way to add some important evidence to the science,” she added.
To allow for a more comprehensive assessment of yoga’s potential mental health benefits, the investigators included a range of mental health diagnoses.
Dose-dependent effect
Studies were only included in the analysis if they were randomized, controlled trials with a yoga intervention that had a minimum of 50% physical activity during each session in adults with a recognized diagnosed mental disorder. Control conditions were defined as treatment as usual, wait list, or attention controls.
Two investigators independently scanned article titles and abstracts, and a final list of articles for the study was decided by consensus. Study quality was reported using the PEDro checklist; a random-effects meta-analysis was conducted using Comprehensive Meta-Analysis software.
A total of 3,880 records were identified and screened. The investigators assessed full-text versions of 80 articles, 19 of which (1,080 patients) were eligible for inclusion in the review.
Of these, nine studies included patients with a depressive disorder; five trials were in patients with a diagnosis of schizophrenia, three studies included patients with a diagnosis of PTSD, one study included patients diagnosed with alcohol dependence, and one study included patients with a range of psychiatric disorders.
Of the 1,080 patients included in the review, 578 were assigned to yoga and 502 to control conditions. Yoga practice involved a mixture of movement, breathing exercises, and/or mindfulness, but the movement component took up more than half of each session.
The yoga interventions lasted an average of 2.4 months (range, 1.5-2.5 months), with an average of 1.6 sessions per week (range, 1-3 sessions) that lasted an average of 60 minutes (range, 20-90 minutes).
Of the 19 studies (632 patients), 13 reported changes in depressive symptoms and were therefore included in the meta-analysis. The six studies excluded from the quantitative analysis did not report depression symptom scores.
With respect to primary outcomes, individuals who performed yoga showed a greater reduction in depressive symptoms, compared with the three control groups (standardized mean difference, –0.41; 95% CI, –0.65 to –0.17; P < .001).
Specific subgroup analyses showed a moderate effect of yoga on depressive symptoms, compared with wait-list controls (SMD, –0.58; P < .05), treatment as usual (SMD, –0.39; P = .31), and attention controls (SMD, –0.21; P = .22).
Subgroup analyses were also performed with respect to diagnostic category. These data showed a moderate effect of yoga on depressive symptoms in depressive disorders (SMD, –0.40; P < .01), no effect in PTSD (SMD, –0.01; P = .95), a nominal effect in alcohol use disorders (SMD, –0.24; P = .69), and a marked effect in schizophrenia (SMD, –0.90; P < .01).
Movement may be key
Researchers also performed a series of meta-regression analyses, which showed that the number of yoga sessions performed each week had a significant effect on depressive symptoms. Indeed, individuals with higher session frequencies demonstrated a greater improvement in symptoms (beta, –0.44; P < .001).
These findings, said Ms. Brinsley, suggest yoga may be a viable intervention for managing depressive symptoms in patients with a variety of mental disorders.
Based on these findings, along with other conventional forms of exercise.
Equally important was the finding that the number of weekly yoga sessions moderated the effect of depressive symptoms, as it may inform the future design of yoga interventions in patients with mental disorders.
With this in mind, the researchers recommended that such interventions should aim to increase the frequency or weekly sessions rather than the duration of each individual session or the overall duration of the intervention.
However, said Ms. Brinsley, these findings suggest it is the physical aspect of the yoga practice that may be key.
“Yoga comprises several different components, including the movement postures, the breathing component, and the mindfulness or meditative component, but in this meta-analysis we looked specifically at yoga that was at least 50% movement based. So it might have also included mindfulness and breathing, but it had to have the movement,” she said.
Don’t discount meditation
Commenting on the findings, Holger Cramer, MSc, PhD, DSc, who was not involved in the study, noted that the systematic review and meta-analysis builds on a number of previous reviews regarding the benefits of yoga for mental disorders.
“Surprisingly, the largest effect in this analysis was found in schizophrenia, even higher than in patients with depressive disorders,” said Dr. Cramer of the University of Duisburg-Essen (Germany). “This is in strong contradiction to what would otherwise be expected. As the authors point out, only about a quarter of all schizophrenia patients suffer from depression, so there should not be so much room for improvement.”
Dr. Cramer also advised against reducing yoga to simply a physical undertaking. “We have shown in our meta-analysis that those interventions focusing on meditation and/or breathing techniques are the most effective ones,” he added.
As such, he urged that breathing techniques be a part of yoga for treating depression in psychiatric disorders, though care should be taken in patients with PTSD, “since breath control might be perceived as unpleasant.”
For Ms. Brinsley, the findings help solidify yoga’s potential as a genuine treatment option for a variety of mental health patients suffering depressive symptoms.
“It’s about acknowledging that yoga can be a helpful part of treatment and can have a significant effect on mental health,” she noted.
At the same time, practitioners also need to acknowledge that patients suffering from mental health disorders may struggle with motivation when it comes to activities such as yoga.
“Engaging in a new activity can be particularly challenging if you’re struggling with mental health. Nevertheless, it’s important for people to have a choice and do something they enjoy. And yoga can be another tool in their toolbox for managing their mental health,” she said.
The study was funded by the U.K. National Institute for Health Research and Health Education England. Ms. Brinsley and Dr. Cramer have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A surge in PTSD may be the ‘new normal’
The prolonged and unique stresses imparted by the COVID-19 pandemic has many predicting a significant rise in mental health issues in the weeks, months, and years ahead.
To understand how health care workers can best get ahead of this emerging crisis within a crisis, Medscape Psychiatry editorial director Bret Stetka, MD, spoke with Sheila Rauch, PhD, who’s with the Department of Psychiatry and Behavioral Sciences at the Emory University, Atlanta. The director of Mental Health Research and Program Evaluation at the Atlanta VA Medical Center, Dr. Rauch has studied the effects of and best treatments for posttraumatic stress disorder (PTSD) and anxiety disorders over the past 20 years.
Are we going to see a PTSD or anxiety epidemic as a result of the pandemic?
First, I think it’s really important that we prepare for the worst but hope for the best. But I would expect that, given the high levels of stress, the impact on resources, and other factors, we are going to see a pretty significant mental health impact over time. This could be the new normal for a while. Some of that will be PTSD, but there will also be other things. I would suspect that the resulting increase in rates of depression, traumatic grief, and loss is probably going to be a significant issue for years to come.
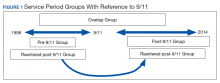
What will the anxiety we see as a result of COVID-19 look like compared with that seen in past disasters, like 9/11?
Most disasters in recent history, like 9/11, are single incidents. Something horrible happened, it impacted people at different levels, and we were able to start putting the pieces back together right away. The prolonged nature of this pandemic makes it even more variable given that the impact is going to be extended over time.
We’re also going to see a lot more people with compound impact – people who’ve lost their jobs, loved ones, maybe even their homes. All of those financial and resource losses put people in a higher risk category for negative mental health outcomes.
Is this analogous to the prolonged trauma that can occur with military service during war?
There is some similarity there. Combat is kind of an overarching context in which people experience trauma and, much like this pandemic, may or may not have traumatic exposures during it.
We’re asking health care workers to actually be in a role similar to what we ask of our military: going into danger, sometimes even without proper protective equipment, in order to save the lives of others. That’s also something we need to be factoring in as we plan to support those people and their families.
This is an ongoing incident, but is there a time window we need to be particularly worried about for seeing spikes in anxiety and PTSD?
I think we’re going to see variability on that. PTSD is a disorder that’s related to a specific incident or a couple of incidents that are similar. It’s a memory that’s haunting you.
For instance, typically if you have a combat veteran who has PTSD, they’ve been exposed to the overarching context of combat but then they have specific memories that are stuck. If they don’t have PTSD about 3-6 months after those incidents happen, then we would expect that they will not develop it, or it’s much less common that they would.
Depression has a very different course. It’s more prolonged and tends to grow with time.
Are you already seeing increased symptoms in your patients?
This is pretty similar to what we see in combat veterans. They’ll often be unhappy with the leadership decisions that were made as they were being deployed.
We’re also seeing lots more anger, sadness, and isolation now. Especially over the past couple of weeks, we’ve seen a rise in things like people reaching out for help in our intakes because we’re still open and doing phone assessments and telehealth with veterans and the veterans program.
In terms of interventions for this, what should psychiatrists, psychologists, and other clinicians be thinking about?
Right now, the best thing that we can do as mental health providers for people affected by the trauma is provide crisis intervention for those saying they are a danger to themselves and others. That means providing coping strategies and support. It also means making sure people are taking breaks and taking care of themselves, taking that little bit of time off so that they can go back, fully recharged, to their jobs and really stay there.
As we move forward, it will be clearer whether people are going to naturally recover, which most people will. For those who are going to have ongoing problems with time, we need to be getting ready as a system and as a country for those long-term mental health issues that are going to be coming up. And when I say long-term, it means the next 1-3 months. We want to be providing preventive interventions, versions of prolonged exposure, and other things that have shown some help in preventing PTSD. Psychological first aid is helpful.
There’s also an app called COVID Coach that the National Center for PTSD has created. That features a lot of positive coping resources together in one source.
Then when we get to the middle of that point and beyond it, we need to be ready to provide those evidence-based interventions for PTSD, depression, panic disorder, and other issues that are going to come out of this current situation.
But we were already short-staffed as far as mental health resources in general across the country, and especially in rural areas. So that means finding ways to efficiently use what we have through potentially briefer versions of interventions, through primary care, mental health, and other staff.
In what ways can primary care providers help?
There are versions of prolonged exposure therapy for primary care. That’s one of my big areas of research – increasing access. That would be something that we need to be building, by training and embedding mental health providers in primary care settings so that they can help to accommodate the increased need for access that’s going to be showing up for the next, I would suspect, several years with the pandemic.
Is there evidence that a prior episode of PTSD or traumatic experience like combat influences a subsequent reaction to a trauma like this?
It depends on how they manage. Research suggests that veterans or other people who have experienced trauma and naturally recovered, or who have gotten good treatment and remitted from that issue, are probably at no higher risk. But people who have subsyndromal PTSD or depression, or who are still experiencing symptoms from a history of trauma exposure, are maybe at a higher risk of having problems over time.
Do you have any guidance for healthcare providers on how to approach the pandemic with their patients, and also on how they can look after their own mental health?
In talking to patients, make sure that they have what they need. Ask if they’ve thought through how they’re going to cope if things get harder for them.
For people who have preexisting mental health issues, I’m talking with them about whether things have gotten worse. If they’re at high risk for suicide, I’m checking in to make sure that they’ve got new plans and ways to connect with people to reduce isolation, keeping in mind the social distancing that we’re asked to engage in so that they can do that safely.
It’s important to check and see if they have had any losses, whether it’s a financial loss or a personal loss of people that they care about. Also have them think through ways to stay entertained, which tends to help manage their own anxiety.
Every coping strategy we outline for patients also applies to mental health professionals. However, you would add to it the real need to take time to recharge, to take breaks, time off. It can feel overwhelming and like you need to just keep going. But the more that you get stuck in that mode of overdoing it, the less effective you’re going to be in helping people and also the more likely that you’ll be at risk of perhaps being one of the people that needs help.
It’s also important to make sure you’re staying connected with family and friends virtually, in whatever ways you can safely do that with social distancing.
So take a break to watch some Netflix now and then?
Yes!
A version of this article originally appeared on Medscape.com.
The prolonged and unique stresses imparted by the COVID-19 pandemic has many predicting a significant rise in mental health issues in the weeks, months, and years ahead.
To understand how health care workers can best get ahead of this emerging crisis within a crisis, Medscape Psychiatry editorial director Bret Stetka, MD, spoke with Sheila Rauch, PhD, who’s with the Department of Psychiatry and Behavioral Sciences at the Emory University, Atlanta. The director of Mental Health Research and Program Evaluation at the Atlanta VA Medical Center, Dr. Rauch has studied the effects of and best treatments for posttraumatic stress disorder (PTSD) and anxiety disorders over the past 20 years.
Are we going to see a PTSD or anxiety epidemic as a result of the pandemic?
First, I think it’s really important that we prepare for the worst but hope for the best. But I would expect that, given the high levels of stress, the impact on resources, and other factors, we are going to see a pretty significant mental health impact over time. This could be the new normal for a while. Some of that will be PTSD, but there will also be other things. I would suspect that the resulting increase in rates of depression, traumatic grief, and loss is probably going to be a significant issue for years to come.
What will the anxiety we see as a result of COVID-19 look like compared with that seen in past disasters, like 9/11?
Most disasters in recent history, like 9/11, are single incidents. Something horrible happened, it impacted people at different levels, and we were able to start putting the pieces back together right away. The prolonged nature of this pandemic makes it even more variable given that the impact is going to be extended over time.
We’re also going to see a lot more people with compound impact – people who’ve lost their jobs, loved ones, maybe even their homes. All of those financial and resource losses put people in a higher risk category for negative mental health outcomes.
Is this analogous to the prolonged trauma that can occur with military service during war?
There is some similarity there. Combat is kind of an overarching context in which people experience trauma and, much like this pandemic, may or may not have traumatic exposures during it.
We’re asking health care workers to actually be in a role similar to what we ask of our military: going into danger, sometimes even without proper protective equipment, in order to save the lives of others. That’s also something we need to be factoring in as we plan to support those people and their families.
This is an ongoing incident, but is there a time window we need to be particularly worried about for seeing spikes in anxiety and PTSD?
I think we’re going to see variability on that. PTSD is a disorder that’s related to a specific incident or a couple of incidents that are similar. It’s a memory that’s haunting you.
For instance, typically if you have a combat veteran who has PTSD, they’ve been exposed to the overarching context of combat but then they have specific memories that are stuck. If they don’t have PTSD about 3-6 months after those incidents happen, then we would expect that they will not develop it, or it’s much less common that they would.
Depression has a very different course. It’s more prolonged and tends to grow with time.
Are you already seeing increased symptoms in your patients?
This is pretty similar to what we see in combat veterans. They’ll often be unhappy with the leadership decisions that were made as they were being deployed.
We’re also seeing lots more anger, sadness, and isolation now. Especially over the past couple of weeks, we’ve seen a rise in things like people reaching out for help in our intakes because we’re still open and doing phone assessments and telehealth with veterans and the veterans program.
In terms of interventions for this, what should psychiatrists, psychologists, and other clinicians be thinking about?
Right now, the best thing that we can do as mental health providers for people affected by the trauma is provide crisis intervention for those saying they are a danger to themselves and others. That means providing coping strategies and support. It also means making sure people are taking breaks and taking care of themselves, taking that little bit of time off so that they can go back, fully recharged, to their jobs and really stay there.
As we move forward, it will be clearer whether people are going to naturally recover, which most people will. For those who are going to have ongoing problems with time, we need to be getting ready as a system and as a country for those long-term mental health issues that are going to be coming up. And when I say long-term, it means the next 1-3 months. We want to be providing preventive interventions, versions of prolonged exposure, and other things that have shown some help in preventing PTSD. Psychological first aid is helpful.
There’s also an app called COVID Coach that the National Center for PTSD has created. That features a lot of positive coping resources together in one source.
Then when we get to the middle of that point and beyond it, we need to be ready to provide those evidence-based interventions for PTSD, depression, panic disorder, and other issues that are going to come out of this current situation.
But we were already short-staffed as far as mental health resources in general across the country, and especially in rural areas. So that means finding ways to efficiently use what we have through potentially briefer versions of interventions, through primary care, mental health, and other staff.
In what ways can primary care providers help?
There are versions of prolonged exposure therapy for primary care. That’s one of my big areas of research – increasing access. That would be something that we need to be building, by training and embedding mental health providers in primary care settings so that they can help to accommodate the increased need for access that’s going to be showing up for the next, I would suspect, several years with the pandemic.
Is there evidence that a prior episode of PTSD or traumatic experience like combat influences a subsequent reaction to a trauma like this?
It depends on how they manage. Research suggests that veterans or other people who have experienced trauma and naturally recovered, or who have gotten good treatment and remitted from that issue, are probably at no higher risk. But people who have subsyndromal PTSD or depression, or who are still experiencing symptoms from a history of trauma exposure, are maybe at a higher risk of having problems over time.
Do you have any guidance for healthcare providers on how to approach the pandemic with their patients, and also on how they can look after their own mental health?
In talking to patients, make sure that they have what they need. Ask if they’ve thought through how they’re going to cope if things get harder for them.
For people who have preexisting mental health issues, I’m talking with them about whether things have gotten worse. If they’re at high risk for suicide, I’m checking in to make sure that they’ve got new plans and ways to connect with people to reduce isolation, keeping in mind the social distancing that we’re asked to engage in so that they can do that safely.
It’s important to check and see if they have had any losses, whether it’s a financial loss or a personal loss of people that they care about. Also have them think through ways to stay entertained, which tends to help manage their own anxiety.
Every coping strategy we outline for patients also applies to mental health professionals. However, you would add to it the real need to take time to recharge, to take breaks, time off. It can feel overwhelming and like you need to just keep going. But the more that you get stuck in that mode of overdoing it, the less effective you’re going to be in helping people and also the more likely that you’ll be at risk of perhaps being one of the people that needs help.
It’s also important to make sure you’re staying connected with family and friends virtually, in whatever ways you can safely do that with social distancing.
So take a break to watch some Netflix now and then?
Yes!
A version of this article originally appeared on Medscape.com.
The prolonged and unique stresses imparted by the COVID-19 pandemic has many predicting a significant rise in mental health issues in the weeks, months, and years ahead.
To understand how health care workers can best get ahead of this emerging crisis within a crisis, Medscape Psychiatry editorial director Bret Stetka, MD, spoke with Sheila Rauch, PhD, who’s with the Department of Psychiatry and Behavioral Sciences at the Emory University, Atlanta. The director of Mental Health Research and Program Evaluation at the Atlanta VA Medical Center, Dr. Rauch has studied the effects of and best treatments for posttraumatic stress disorder (PTSD) and anxiety disorders over the past 20 years.
Are we going to see a PTSD or anxiety epidemic as a result of the pandemic?
First, I think it’s really important that we prepare for the worst but hope for the best. But I would expect that, given the high levels of stress, the impact on resources, and other factors, we are going to see a pretty significant mental health impact over time. This could be the new normal for a while. Some of that will be PTSD, but there will also be other things. I would suspect that the resulting increase in rates of depression, traumatic grief, and loss is probably going to be a significant issue for years to come.
What will the anxiety we see as a result of COVID-19 look like compared with that seen in past disasters, like 9/11?
Most disasters in recent history, like 9/11, are single incidents. Something horrible happened, it impacted people at different levels, and we were able to start putting the pieces back together right away. The prolonged nature of this pandemic makes it even more variable given that the impact is going to be extended over time.
We’re also going to see a lot more people with compound impact – people who’ve lost their jobs, loved ones, maybe even their homes. All of those financial and resource losses put people in a higher risk category for negative mental health outcomes.
Is this analogous to the prolonged trauma that can occur with military service during war?
There is some similarity there. Combat is kind of an overarching context in which people experience trauma and, much like this pandemic, may or may not have traumatic exposures during it.
We’re asking health care workers to actually be in a role similar to what we ask of our military: going into danger, sometimes even without proper protective equipment, in order to save the lives of others. That’s also something we need to be factoring in as we plan to support those people and their families.
This is an ongoing incident, but is there a time window we need to be particularly worried about for seeing spikes in anxiety and PTSD?
I think we’re going to see variability on that. PTSD is a disorder that’s related to a specific incident or a couple of incidents that are similar. It’s a memory that’s haunting you.
For instance, typically if you have a combat veteran who has PTSD, they’ve been exposed to the overarching context of combat but then they have specific memories that are stuck. If they don’t have PTSD about 3-6 months after those incidents happen, then we would expect that they will not develop it, or it’s much less common that they would.
Depression has a very different course. It’s more prolonged and tends to grow with time.
Are you already seeing increased symptoms in your patients?
This is pretty similar to what we see in combat veterans. They’ll often be unhappy with the leadership decisions that were made as they were being deployed.
We’re also seeing lots more anger, sadness, and isolation now. Especially over the past couple of weeks, we’ve seen a rise in things like people reaching out for help in our intakes because we’re still open and doing phone assessments and telehealth with veterans and the veterans program.
In terms of interventions for this, what should psychiatrists, psychologists, and other clinicians be thinking about?
Right now, the best thing that we can do as mental health providers for people affected by the trauma is provide crisis intervention for those saying they are a danger to themselves and others. That means providing coping strategies and support. It also means making sure people are taking breaks and taking care of themselves, taking that little bit of time off so that they can go back, fully recharged, to their jobs and really stay there.
As we move forward, it will be clearer whether people are going to naturally recover, which most people will. For those who are going to have ongoing problems with time, we need to be getting ready as a system and as a country for those long-term mental health issues that are going to be coming up. And when I say long-term, it means the next 1-3 months. We want to be providing preventive interventions, versions of prolonged exposure, and other things that have shown some help in preventing PTSD. Psychological first aid is helpful.
There’s also an app called COVID Coach that the National Center for PTSD has created. That features a lot of positive coping resources together in one source.
Then when we get to the middle of that point and beyond it, we need to be ready to provide those evidence-based interventions for PTSD, depression, panic disorder, and other issues that are going to come out of this current situation.
But we were already short-staffed as far as mental health resources in general across the country, and especially in rural areas. So that means finding ways to efficiently use what we have through potentially briefer versions of interventions, through primary care, mental health, and other staff.
In what ways can primary care providers help?
There are versions of prolonged exposure therapy for primary care. That’s one of my big areas of research – increasing access. That would be something that we need to be building, by training and embedding mental health providers in primary care settings so that they can help to accommodate the increased need for access that’s going to be showing up for the next, I would suspect, several years with the pandemic.
Is there evidence that a prior episode of PTSD or traumatic experience like combat influences a subsequent reaction to a trauma like this?
It depends on how they manage. Research suggests that veterans or other people who have experienced trauma and naturally recovered, or who have gotten good treatment and remitted from that issue, are probably at no higher risk. But people who have subsyndromal PTSD or depression, or who are still experiencing symptoms from a history of trauma exposure, are maybe at a higher risk of having problems over time.
Do you have any guidance for healthcare providers on how to approach the pandemic with their patients, and also on how they can look after their own mental health?
In talking to patients, make sure that they have what they need. Ask if they’ve thought through how they’re going to cope if things get harder for them.
For people who have preexisting mental health issues, I’m talking with them about whether things have gotten worse. If they’re at high risk for suicide, I’m checking in to make sure that they’ve got new plans and ways to connect with people to reduce isolation, keeping in mind the social distancing that we’re asked to engage in so that they can do that safely.
It’s important to check and see if they have had any losses, whether it’s a financial loss or a personal loss of people that they care about. Also have them think through ways to stay entertained, which tends to help manage their own anxiety.
Every coping strategy we outline for patients also applies to mental health professionals. However, you would add to it the real need to take time to recharge, to take breaks, time off. It can feel overwhelming and like you need to just keep going. But the more that you get stuck in that mode of overdoing it, the less effective you’re going to be in helping people and also the more likely that you’ll be at risk of perhaps being one of the people that needs help.
It’s also important to make sure you’re staying connected with family and friends virtually, in whatever ways you can safely do that with social distancing.
So take a break to watch some Netflix now and then?
Yes!
A version of this article originally appeared on Medscape.com.
Plan now to address the COVID-19 mental health fallout
COVID-19 affects the physical, psychological, and social health of people around the world. In the United States, newly reported cases are rising at alarming rates.
As of early May, more than 1.3 million people were confirmed to be COVID-19 infected in the United States and more than 4 million cases were reported globally.1
According to new internal projections from the Centers for Disease Control and Prevention, by June 1, the number of daily deaths could reach about 3,000. By the end of June, a draft CDC report projects that the United States will see 200,000 new cases each day.2
COVID-19 undeniably harms mental health. It gravely instills uncertainty and anxiety, sometimes compounded by the grief of losing loved ones and not being able to mourn those losses in traditional ways. The pandemic also has led to occupational and/or financial losses. Physical distancing and shelter-in-place practices make it even harder to cope with those stresses, although those practices mitigate the dangers. The fears tied to those practices are thought to be keeping some patients with health problems from seeking needed care from hospital EDs.3 In light of the mental health crisis emerging because of the profound impact of this pandemic on all aspects of life, clinicians should start working with public health and political leaders to develop plans to address these issues now.
Known impact of previous outbreaks
Previous disease outbreaks evidence a similar pattern of heightened anxiety as the patterns seen with COVID-19. For example, during the 2009 swine flu outbreak, 36 surveys of more than 3,000 participants in the United Kingdom found that 9.6%-32.9% of the participants were “very” or “fairly” worried about the possibility of contracting swine flu.4 The 1995 Ebola outbreak in the Democratic Republic of the Congo produced stigmatization tied to the illness. That outbreak provided many lessons for physicians.5
The metaphors ascribed to different diseases affect communities’ responses to it. The SARS virus has been particularly insidious and has been thought of as a “plague.”6 Epidemics of all kinds cause fears, not only of contracting the disease and dying, but also of social exclusion.7 The emotional responses to COVID-19 can precipitate anxiety, depression, insomnia, and somatic symptoms.
Repeated exposure to news media about the disease adds to theses stresss.10 Constant news consumption can result in panicky hoarding of resources, such as masks; gloves; first-aid kits; alcohol hand rubs; and daily necessities such as food, water, and toilet paper.
Who is most affected by outbreaks?
Those most affected after a disease outbreak are patients, their families, and medical personnel. In one study, researchers who conducted an online survey of 1,210 respondents in 194 cities in China during the early phase of the outbreak found that the psychological effects were worst among women, students, and vulnerable populations.11
Meanwhile, a 2003 cross-sectional survey of 1,115 ethnic Chinese adults in Hong Kong who responded to the SARS outbreak found that the respondents most likely to heed precautionary measures against the infection were “older, female, more educated people as well as those with a positive contact history and SARS-like symptoms.”12
Negative mental health consequences of a disease outbreak might persist long after the infection has dissipated. An increased association has been found between people with mental illness and posttraumatic stress following many disasters.13,14,15
Political and health care leaders should develop plans aimed at helping people copewith pandemics.16 Such strategies should include prioritizing treatment of the physical and mental health needs of patients infected with COVID-19 and of the general population. Screening for anxiety, depression, and suicidal thoughts ought to be implemented, and specialized psychiatric care teams should be assigned.17 We know that psychiatrists and other physicians turned to telemedicine to provide support, psychotherapy, and medical attention to patients soon after physical distancing measures were put into place. Those kinds of quick responses are important for our patients.
Fear of contagious diseases often creates social divisions. Governments should offer accurate information to reduce the detrimental effect of rumors and false propaganda.18 “Social distancing” is a misleading term; these practices should be referred to as “physical distancing.” We should encourage patients to maintain interpersonal contacts – albeit at a distance – to reach out to those in need, and to support one another during these troubled times.19
References
1. World Health Organization. Situation Report–107. 2020 May 6.
2. Centers for Disease Control and Prevention. Situation Update. 2020 Apr 30.
3. O’Brien M. “Are Americans in medical crisis avoiding the ER due to coronavirus?” PBS Newshour. 2020 May 6.
4. Rubin G et al. Health Technol Assess. 2010 Jul;14(340):183-266.
5. Hall R et al. Gen Hosp Psychiatry. 2008 Sep-Oct;30(5):466-52.
6. Verghese A. Clin Infect Dis. 2004;38:932-3.
7. Interagency Standing Committee. Briefing note on addressing health and psychosocial aspects of COVID-19 Outbreak – Version 11. 2020 Feb.
8. Sim K et al. J Psychosom Res. 2010;68:195-202.
9. Shigemura J et al. Psychiatry Clin Neurosci. 2020;74:281-2.
10. Garfin DR et al. Health Psychol. 2020 May;39(5):355-7.
11. Wang C et al. Int J Environ Res Public Health. 2020 Mar 6. doi: 10.3390/ijerph1751729.
12. Leung GM et al. J Epidemiol Community Health. 2003 Nov;57(1):857-63.
13. Xiang Y et al. Int J Biol Sci. 2020;16:1741-4.
14. Alvarez J, Hunt M. J Trauma Stress. 2005 Oct 18(5);18:497-505.
15. Cukor J et al. Depress Anxiety. 2011 Mar;28(3):210-7.
16. Horton R. Lancet. 2020 Feb;395(10222):400.
17. Xiang Y-T et al. Lancet Psychiatry. 2020 Feb 4;7:228-9.
18. World Health Organization. “Rational use of personal protective equipment (PPE) for coronavirus (COVID-19).” Interim Guidance. 2020 Mar.
19. Brooks S et al. Lancet 2020 Mar 14;395:912-20.
Dr. Doppalapudi is affiliated with Griffin Memorial Hospital in Norman, Okla. Dr. Lippmann is emeritus professor of psychiatry and also in family medicine at the University of Louisville (Ky.) Dr. Doppalapudi and Dr. Lippmann disclosed no conflicts of interest.
COVID-19 affects the physical, psychological, and social health of people around the world. In the United States, newly reported cases are rising at alarming rates.
As of early May, more than 1.3 million people were confirmed to be COVID-19 infected in the United States and more than 4 million cases were reported globally.1
According to new internal projections from the Centers for Disease Control and Prevention, by June 1, the number of daily deaths could reach about 3,000. By the end of June, a draft CDC report projects that the United States will see 200,000 new cases each day.2
COVID-19 undeniably harms mental health. It gravely instills uncertainty and anxiety, sometimes compounded by the grief of losing loved ones and not being able to mourn those losses in traditional ways. The pandemic also has led to occupational and/or financial losses. Physical distancing and shelter-in-place practices make it even harder to cope with those stresses, although those practices mitigate the dangers. The fears tied to those practices are thought to be keeping some patients with health problems from seeking needed care from hospital EDs.3 In light of the mental health crisis emerging because of the profound impact of this pandemic on all aspects of life, clinicians should start working with public health and political leaders to develop plans to address these issues now.
Known impact of previous outbreaks
Previous disease outbreaks evidence a similar pattern of heightened anxiety as the patterns seen with COVID-19. For example, during the 2009 swine flu outbreak, 36 surveys of more than 3,000 participants in the United Kingdom found that 9.6%-32.9% of the participants were “very” or “fairly” worried about the possibility of contracting swine flu.4 The 1995 Ebola outbreak in the Democratic Republic of the Congo produced stigmatization tied to the illness. That outbreak provided many lessons for physicians.5
The metaphors ascribed to different diseases affect communities’ responses to it. The SARS virus has been particularly insidious and has been thought of as a “plague.”6 Epidemics of all kinds cause fears, not only of contracting the disease and dying, but also of social exclusion.7 The emotional responses to COVID-19 can precipitate anxiety, depression, insomnia, and somatic symptoms.
Repeated exposure to news media about the disease adds to theses stresss.10 Constant news consumption can result in panicky hoarding of resources, such as masks; gloves; first-aid kits; alcohol hand rubs; and daily necessities such as food, water, and toilet paper.
Who is most affected by outbreaks?
Those most affected after a disease outbreak are patients, their families, and medical personnel. In one study, researchers who conducted an online survey of 1,210 respondents in 194 cities in China during the early phase of the outbreak found that the psychological effects were worst among women, students, and vulnerable populations.11
Meanwhile, a 2003 cross-sectional survey of 1,115 ethnic Chinese adults in Hong Kong who responded to the SARS outbreak found that the respondents most likely to heed precautionary measures against the infection were “older, female, more educated people as well as those with a positive contact history and SARS-like symptoms.”12
Negative mental health consequences of a disease outbreak might persist long after the infection has dissipated. An increased association has been found between people with mental illness and posttraumatic stress following many disasters.13,14,15
Political and health care leaders should develop plans aimed at helping people copewith pandemics.16 Such strategies should include prioritizing treatment of the physical and mental health needs of patients infected with COVID-19 and of the general population. Screening for anxiety, depression, and suicidal thoughts ought to be implemented, and specialized psychiatric care teams should be assigned.17 We know that psychiatrists and other physicians turned to telemedicine to provide support, psychotherapy, and medical attention to patients soon after physical distancing measures were put into place. Those kinds of quick responses are important for our patients.
Fear of contagious diseases often creates social divisions. Governments should offer accurate information to reduce the detrimental effect of rumors and false propaganda.18 “Social distancing” is a misleading term; these practices should be referred to as “physical distancing.” We should encourage patients to maintain interpersonal contacts – albeit at a distance – to reach out to those in need, and to support one another during these troubled times.19
References
1. World Health Organization. Situation Report–107. 2020 May 6.
2. Centers for Disease Control and Prevention. Situation Update. 2020 Apr 30.
3. O’Brien M. “Are Americans in medical crisis avoiding the ER due to coronavirus?” PBS Newshour. 2020 May 6.
4. Rubin G et al. Health Technol Assess. 2010 Jul;14(340):183-266.
5. Hall R et al. Gen Hosp Psychiatry. 2008 Sep-Oct;30(5):466-52.
6. Verghese A. Clin Infect Dis. 2004;38:932-3.
7. Interagency Standing Committee. Briefing note on addressing health and psychosocial aspects of COVID-19 Outbreak – Version 11. 2020 Feb.
8. Sim K et al. J Psychosom Res. 2010;68:195-202.
9. Shigemura J et al. Psychiatry Clin Neurosci. 2020;74:281-2.
10. Garfin DR et al. Health Psychol. 2020 May;39(5):355-7.
11. Wang C et al. Int J Environ Res Public Health. 2020 Mar 6. doi: 10.3390/ijerph1751729.
12. Leung GM et al. J Epidemiol Community Health. 2003 Nov;57(1):857-63.
13. Xiang Y et al. Int J Biol Sci. 2020;16:1741-4.
14. Alvarez J, Hunt M. J Trauma Stress. 2005 Oct 18(5);18:497-505.
15. Cukor J et al. Depress Anxiety. 2011 Mar;28(3):210-7.
16. Horton R. Lancet. 2020 Feb;395(10222):400.
17. Xiang Y-T et al. Lancet Psychiatry. 2020 Feb 4;7:228-9.
18. World Health Organization. “Rational use of personal protective equipment (PPE) for coronavirus (COVID-19).” Interim Guidance. 2020 Mar.
19. Brooks S et al. Lancet 2020 Mar 14;395:912-20.
Dr. Doppalapudi is affiliated with Griffin Memorial Hospital in Norman, Okla. Dr. Lippmann is emeritus professor of psychiatry and also in family medicine at the University of Louisville (Ky.) Dr. Doppalapudi and Dr. Lippmann disclosed no conflicts of interest.
COVID-19 affects the physical, psychological, and social health of people around the world. In the United States, newly reported cases are rising at alarming rates.
As of early May, more than 1.3 million people were confirmed to be COVID-19 infected in the United States and more than 4 million cases were reported globally.1
According to new internal projections from the Centers for Disease Control and Prevention, by June 1, the number of daily deaths could reach about 3,000. By the end of June, a draft CDC report projects that the United States will see 200,000 new cases each day.2
COVID-19 undeniably harms mental health. It gravely instills uncertainty and anxiety, sometimes compounded by the grief of losing loved ones and not being able to mourn those losses in traditional ways. The pandemic also has led to occupational and/or financial losses. Physical distancing and shelter-in-place practices make it even harder to cope with those stresses, although those practices mitigate the dangers. The fears tied to those practices are thought to be keeping some patients with health problems from seeking needed care from hospital EDs.3 In light of the mental health crisis emerging because of the profound impact of this pandemic on all aspects of life, clinicians should start working with public health and political leaders to develop plans to address these issues now.
Known impact of previous outbreaks
Previous disease outbreaks evidence a similar pattern of heightened anxiety as the patterns seen with COVID-19. For example, during the 2009 swine flu outbreak, 36 surveys of more than 3,000 participants in the United Kingdom found that 9.6%-32.9% of the participants were “very” or “fairly” worried about the possibility of contracting swine flu.4 The 1995 Ebola outbreak in the Democratic Republic of the Congo produced stigmatization tied to the illness. That outbreak provided many lessons for physicians.5
The metaphors ascribed to different diseases affect communities’ responses to it. The SARS virus has been particularly insidious and has been thought of as a “plague.”6 Epidemics of all kinds cause fears, not only of contracting the disease and dying, but also of social exclusion.7 The emotional responses to COVID-19 can precipitate anxiety, depression, insomnia, and somatic symptoms.
Repeated exposure to news media about the disease adds to theses stresss.10 Constant news consumption can result in panicky hoarding of resources, such as masks; gloves; first-aid kits; alcohol hand rubs; and daily necessities such as food, water, and toilet paper.
Who is most affected by outbreaks?
Those most affected after a disease outbreak are patients, their families, and medical personnel. In one study, researchers who conducted an online survey of 1,210 respondents in 194 cities in China during the early phase of the outbreak found that the psychological effects were worst among women, students, and vulnerable populations.11
Meanwhile, a 2003 cross-sectional survey of 1,115 ethnic Chinese adults in Hong Kong who responded to the SARS outbreak found that the respondents most likely to heed precautionary measures against the infection were “older, female, more educated people as well as those with a positive contact history and SARS-like symptoms.”12
Negative mental health consequences of a disease outbreak might persist long after the infection has dissipated. An increased association has been found between people with mental illness and posttraumatic stress following many disasters.13,14,15
Political and health care leaders should develop plans aimed at helping people copewith pandemics.16 Such strategies should include prioritizing treatment of the physical and mental health needs of patients infected with COVID-19 and of the general population. Screening for anxiety, depression, and suicidal thoughts ought to be implemented, and specialized psychiatric care teams should be assigned.17 We know that psychiatrists and other physicians turned to telemedicine to provide support, psychotherapy, and medical attention to patients soon after physical distancing measures were put into place. Those kinds of quick responses are important for our patients.
Fear of contagious diseases often creates social divisions. Governments should offer accurate information to reduce the detrimental effect of rumors and false propaganda.18 “Social distancing” is a misleading term; these practices should be referred to as “physical distancing.” We should encourage patients to maintain interpersonal contacts – albeit at a distance – to reach out to those in need, and to support one another during these troubled times.19
References
1. World Health Organization. Situation Report–107. 2020 May 6.
2. Centers for Disease Control and Prevention. Situation Update. 2020 Apr 30.
3. O’Brien M. “Are Americans in medical crisis avoiding the ER due to coronavirus?” PBS Newshour. 2020 May 6.
4. Rubin G et al. Health Technol Assess. 2010 Jul;14(340):183-266.
5. Hall R et al. Gen Hosp Psychiatry. 2008 Sep-Oct;30(5):466-52.
6. Verghese A. Clin Infect Dis. 2004;38:932-3.
7. Interagency Standing Committee. Briefing note on addressing health and psychosocial aspects of COVID-19 Outbreak – Version 11. 2020 Feb.
8. Sim K et al. J Psychosom Res. 2010;68:195-202.
9. Shigemura J et al. Psychiatry Clin Neurosci. 2020;74:281-2.
10. Garfin DR et al. Health Psychol. 2020 May;39(5):355-7.
11. Wang C et al. Int J Environ Res Public Health. 2020 Mar 6. doi: 10.3390/ijerph1751729.
12. Leung GM et al. J Epidemiol Community Health. 2003 Nov;57(1):857-63.
13. Xiang Y et al. Int J Biol Sci. 2020;16:1741-4.
14. Alvarez J, Hunt M. J Trauma Stress. 2005 Oct 18(5);18:497-505.
15. Cukor J et al. Depress Anxiety. 2011 Mar;28(3):210-7.
16. Horton R. Lancet. 2020 Feb;395(10222):400.
17. Xiang Y-T et al. Lancet Psychiatry. 2020 Feb 4;7:228-9.
18. World Health Organization. “Rational use of personal protective equipment (PPE) for coronavirus (COVID-19).” Interim Guidance. 2020 Mar.
19. Brooks S et al. Lancet 2020 Mar 14;395:912-20.
Dr. Doppalapudi is affiliated with Griffin Memorial Hospital in Norman, Okla. Dr. Lippmann is emeritus professor of psychiatry and also in family medicine at the University of Louisville (Ky.) Dr. Doppalapudi and Dr. Lippmann disclosed no conflicts of interest.
COVID-19: Addressing the mental health needs of clinicians
SARS-CoV-2 and the disease it causes, COVID-19, continues to spread around the world with a devastating social and economic impact. Undoubtedly, health care workers are essential to overcoming this crisis. If these issues are left unaddressed, low morale, burnout, or absenteeism could lead to the collapse of health care systems.
Historically, the health care industry has been one of the most hazardous environments in which to work. Employees in this industry are constantly exposed to a complex variety of health and safety hazards.
Particularly, risks from biological exposure to diseases such as tuberculosis, HIV, and currently COVID-19 are taking a considerable toll on health care workers’ health and well-being. Health care workers are leaving their families to work extra shifts, dealing with limited resources, and navigating the chaos. On top of all that, they are sacrificing their lives through these uncertain times.
Despite their resilience, health care workers – like the general population – can have strong psychological reactions of anxiety and fear during a pandemic. Still, they are required to continue their work amid uncertainty and danger.
Current research studies on COVID-19
Several studies have identified the impact of working in this type of environment during previous pandemics and disasters. In a study of hospital employees in China during the SARS epidemic (2002-2003), Ping Wu, PhD, and colleagues found that 10% of the participants experienced high levels of posttraumatic stress.1 In a similar study in Taiwan, researchers found that 17.3% of employees had developed significant mental health symptoms during the SARS outbreak.2
The impact of COVID-19 on health care workers seems to be much worse. A recent study from China indicates that 50.4% of hospital employees showed signs of depression, 44.6% had anxiety, and 34% had insomnia.3
Another recent cross-sectional study conducted by Lijun Kang, PhD, and associates evaluated the impact on mental health among health care workers in Wuhan, China, during the COVID-19 outbreak. This was the first study on the mental health of health care workers. This study recruited health care workers in Wuhan to participate in the survey from Jan. 29 to Feb. 4, 2020. The data were collected online with an anonymous, self-rated questionnaire that was distributed to all workstations. All subjects provided informed consent electronically prior to participating in the survey.
The survey questionnaire was made up of six components: primary demographic data, mental health assessment, risks of direct and indirect exposure to COVID-19, mental health care services accessed, psychological needs, and self-perceived health status, compared with that before the COVID-19 outbreak. A total of 994 health care workers responded to this survey, and the results are fascinating: 36.9% had subthreshold mental health distress (mean Patient Health Questionnaire–9 score, 2.4), 34.4% reported mild disturbances (mean PHQ-9, 5.4), 22.4% had moderate (mean PHQ-9, 9.0), and 6.2% reported severe disturbance (mean PHQ-9, 15.1). In this study, young women experienced more significant psychological distress. Regarding access to mental health services, 36.3% reported access to psychological materials, such as books on mental health; 50.4% used psychological resources available through media, such as online self-help coping methods; and 17.5% participated in counseling or psychotherapy.4
These findings emphasize the importance of being equipped to ensure the health and safety of health care workers through mental health interventions, both at work and in the community during this time of anxiety and uncertainty.
Future studies will become more critical in addressing this issue.
Risks to clinicians, families prevail
According to a recent report released by the Centers for Disease Control and Prevention, more than 9,000 health care workers across the United States had contracted COVID-19 as of mid-April, and 27 had died since the start of the pandemic.5
Health care workers are at risk around the globe, not only by the nature of their jobs but also by the shortage of personal protective equipment (PPE). In addition, the scarcity of N95 masks, respirators, and COVID-19 testing programs is causing the virus to spread among health care workers all over the world.
A study published recently by Celso Arango, MD, PhD, reported that 18% of staff at a hospital in Madrid had been infected with COVID-19. Dr. Arango speculated that transmission might be attributable to interactions with colleagues rather than with patients.6 We know, for example, that large proportions of people in China reportedly carried the virus while being asymptomatic.7 Those findings might not be generalizable, but they do suggest that an asymptomatic person could be a cause of contagion among professionals. Therefore, early screening and testing are critical – and should be priorities in health care settings.
Another problem clinicians can encounter is that, when they are called on to deal with very agitated patients, they might not get enough time to put on PPE. In addition, PPE can easily break and tear during the physical restraint process.
Working long hours is also putting a significant strain on health care workers and exposes them to the risk of infection. Also, health care workers not only worry about their safety but also fear bringing the virus to their families. They can also feel guilty about their conflicting feelings about exposing themselves and their families to risk. It is quite possible that, during this COVID-19 pandemic, health care workers will face a “care paradox,” in which they must choose between patients’ safety and their own. This care paradox can significantly contribute to a feeling of burnout, stress, and anxiety. Ultimately, this pandemic could lead to attrition from the field at a time when we most need all hands on deck.8
Further, according to a World Health Organization report on mental health and psychosocial consideration during the COVID-19 outbreak, some health care workers, unfortunately, experience avoidance by their family members or communities because of stigma, fear, and anxiety. This avoidance threatens to make an already challenging situation far worse for health care workers by increasing isolation.
Even after acute outbreak are over, the effects on health care workers can persist for years. In a follow-up study 13-26 months after the SARS outbreak, Robert G. Maunder, MD, and associates found that Toronto-area health care workers reported significantly higher levels of burnout, psychological distress, and posttraumatic stress. They were more likely to have reduced patient contact and work hours, and to have avoided behavioral consequences of stress.9 Exposure to stressful work conditions during a pandemic also might put hospital employees at a much higher risk of alcohol and substance use disorders.10
Potential solutions for improving care
COVID-19 has had a massive impact on the mental health of health care workers around the globe. Fortunately, there are evidence-based strategies aimed at mitigating the effects of this pandemic on health care workers. Fostering self-efficacy and optimism has been shown to improve coping and efficiency during disasters.9 Higher perceived workplace safety is associated with a lower risk of anxiety, depression, and posttraumatic stress among health care workers, while a lack of social support has been linked to adverse behavioral outcomes.10
A recent study found that, among Chinese physicians who cared for COVID-19 victims, more significant social support was associated with better sleep quality, greater self-effectiveness, and less psychological distress.11 Positive leadership and a professional culture of trust, and openness with unambiguous communication have been shown to improve the engagement of the medical workforce.12,13 Psychiatrists must advocate for the adoption of these practices in the workplace. Assessing and addressing mental health needs, in addition to the physical health of the health care workforce, is of utmost importance.
We can accomplish this in many ways, but we have to access our health care workers. Similar to our patient population, health care workers also experience stigma and anxiety tied to the disclosure of mental health challenges. This was reported in a study conducted in China, in which a specific psychological intervention using a hotline program was used for the medical team.14 This program provided psychological interventions/group activities aimed at releasing stress and anxiety. However, initially, the implementation of psychological interventions encountered obstacles.
For example, some members of the medical staff declined to participate in group or individual psychological interventions. Moreover, nurses showed irritability, unwillingness to join, and some staff refused, stating that “they did not have any problems.” Finally, psychological counselors regularly visited the facility to listen to difficulties or stories encountered by staff at work and provide support accordingly. More than 100 frontline medical staff participated and reported feeling better.15
Currently, several U.S. universities/institutes have implemented programs aimed at protecting the health and well-being of their staff during the COVID-19 pandemic. For instance, the department of psychiatry and behavioral health at Hackensack Meridian Health has put comprehensive system programs in place for at 16 affiliated medical centers and other patient care facilities to provide support during the COVID-19 crisis. A 24/7 team member support hotline connecting team members with a behavioral health specialist has become available when needed. This hotline is backed up by social workers, who provide mental health resources. In addition, another service called “Coping with COVID Talks” is available. This service is a virtual psychoeducational group facilitated by psychologists focusing on building coping skills and resilience.
Also, the consultation-liaison psychiatrists in the medical centers provide daily support to clinicians working in ICUs. These efforts have led to paradoxical benefits for employers, further leading to less commuting, more safety, and enhanced productivity for the clinician, according to Ramon Solhkhah, MD, MBA, chairman of the psychiatry department.16
Some universities, such as the University of North Carolina at Chapel Hill, have created mental health/telehealth support for health care workers, where they are conducting webinars on coping with uncertainty tied to COVID-19.17 The University of California, San Francisco, also has been a leader in this effort. That institution has employed its psychiatric workforce as volunteers – encouraging health care workers to use digital health apps and referral resources. Also, these volunteers provide peer counseling, phone support, and spiritual counseling to their health care workers.18
These approaches are crucial in this uncertain, challenging time. Our mental health system is deeply flawed, understaffed, and not well prepared to manage the mental health issues among health care workers. Psychiatric institutes/facilities should follow comprehensive and multifaceted approaches to combat the COVID-19 crisis. Several preventive measures can be considered in coping with this pandemic, such as stress reduction, mindfulness, and disseminating educational materials. Also, increased use of technology, such as in-the-moment measures, development of hotlines, crisis support, and treatment telepsychiatry for therapy and medication, should play a pivotal role in addressing the mental health needs of health care workers.
In addition, it is expected that, as a nation, we will see a surge of mental health needs for illnesses such as depression and PTSD, just as we do after “natural disasters” caused by a variety of reasons, including economic downturns. After the SARS outbreak in 2003, for example, health care workers showed symptoms of PTSD. The COVID-19 pandemic could have a similar impact.
The severity of mental health challenges among clinicians cannot be predicted at this time, but we can speculate that the traumatic impact of COVID-19 will prove long lasting, particularly among clinicians who served vulnerable populations and witnessed suffering, misery, and deaths. The long-term consequences might range from stress and anxiety to fear, depression, and PTSD. Implementation of mental health programs/psychological interventions/support will reduce the impact of mental health issues among these clinicians.
We must think about the best ways to optimize mental health among health care workers while also come up with innovative ways to target this at-risk group. The mental health of people who are saving lives – our frontline heroes – should be taken into consideration seriously around the globe. We also must prioritize the mental health of these workers during this unprecedented, challenging, and anxiety-provoking time.
Dr. Malik and Mr. Van Wert are affiliated with Johns Hopkins University, Baltimore. Dr. Kumari, Dr. Afzal, Dr. Doumas, and Dr. Solhkhah are affiliated with Hackensack Meridian Health at Ocean Medical Center, Brick, N.J. All six authors disclosed having no conflicts of interest. The authors would like to thank Vinay Kumar for his assistance with the literature review and for proofreading and editing this article.
References
1. Wu P et al. Can J Psychiatry. 2009;54(5):302-11.
2. Lu YC et al. Psychother Psychosom. 2006;75(6):370-5.
3. Lai J et al. JAMA Netw Open. 2020;3(3):e203976.
4. Kang L et al. Brain Behav Immun. 2020 Mar 30. doi: 10.1016/j.bbi.2020.03.028.
5. Centers for Disease Control and Prevention COVID-19 Response Team. MMWR. 2020 Apr 17;69(15):477-81.
6. Arango C. Biol Psychiatry. 2020 Apr 8. doi: 10.1016/j.biopsych.2020.04.003.
7. Day M. BMJ. 2020 Apr 2. doi: 10.1136/bmj.m1375.
8. Kirsch T. “Coronavirus, COVID-19: What happens if health care workers stop showing up?” The Atlantic. 2020 Mar 24.
9. Maunder RG et al. Emerg Infect Dis. 2006;12(12):1924-32.
10. Wu P et al. Alcohol Alcohol. 2008;43(6):706-12.
11. Brooks SK et al. BMC Psychol. 2016 Apr 26;4:18.
12. Smith BW et al. Am J Infect Control. 2009; 37:371-80.
13. Chen Q et al. Lancet Psychiatry. 2020 Apr 1;7(14):PE15-6.
14. Xiao H et al. Med Sci Monit. 2020;26:e923549.
15. Bergus GR et al. Acad Med. 2001;76:1148-52.
16. Bergeron T. “Working from home will be stressful. Here’s how employees (and employers) can handle it.” roi-nj.com. 2020 Mar 23.
17. UNChealthcare.org. “Mental Health/Emotional Support Resources for Coworkers and Providers Coping with COVID-19.”
18. Psych.ucsf.edu/coronoavirus. “Resources to Support Your Mental Health During the COVID-19 Outbreak.”
SARS-CoV-2 and the disease it causes, COVID-19, continues to spread around the world with a devastating social and economic impact. Undoubtedly, health care workers are essential to overcoming this crisis. If these issues are left unaddressed, low morale, burnout, or absenteeism could lead to the collapse of health care systems.
Historically, the health care industry has been one of the most hazardous environments in which to work. Employees in this industry are constantly exposed to a complex variety of health and safety hazards.
Particularly, risks from biological exposure to diseases such as tuberculosis, HIV, and currently COVID-19 are taking a considerable toll on health care workers’ health and well-being. Health care workers are leaving their families to work extra shifts, dealing with limited resources, and navigating the chaos. On top of all that, they are sacrificing their lives through these uncertain times.
Despite their resilience, health care workers – like the general population – can have strong psychological reactions of anxiety and fear during a pandemic. Still, they are required to continue their work amid uncertainty and danger.
Current research studies on COVID-19
Several studies have identified the impact of working in this type of environment during previous pandemics and disasters. In a study of hospital employees in China during the SARS epidemic (2002-2003), Ping Wu, PhD, and colleagues found that 10% of the participants experienced high levels of posttraumatic stress.1 In a similar study in Taiwan, researchers found that 17.3% of employees had developed significant mental health symptoms during the SARS outbreak.2
The impact of COVID-19 on health care workers seems to be much worse. A recent study from China indicates that 50.4% of hospital employees showed signs of depression, 44.6% had anxiety, and 34% had insomnia.3
Another recent cross-sectional study conducted by Lijun Kang, PhD, and associates evaluated the impact on mental health among health care workers in Wuhan, China, during the COVID-19 outbreak. This was the first study on the mental health of health care workers. This study recruited health care workers in Wuhan to participate in the survey from Jan. 29 to Feb. 4, 2020. The data were collected online with an anonymous, self-rated questionnaire that was distributed to all workstations. All subjects provided informed consent electronically prior to participating in the survey.
The survey questionnaire was made up of six components: primary demographic data, mental health assessment, risks of direct and indirect exposure to COVID-19, mental health care services accessed, psychological needs, and self-perceived health status, compared with that before the COVID-19 outbreak. A total of 994 health care workers responded to this survey, and the results are fascinating: 36.9% had subthreshold mental health distress (mean Patient Health Questionnaire–9 score, 2.4), 34.4% reported mild disturbances (mean PHQ-9, 5.4), 22.4% had moderate (mean PHQ-9, 9.0), and 6.2% reported severe disturbance (mean PHQ-9, 15.1). In this study, young women experienced more significant psychological distress. Regarding access to mental health services, 36.3% reported access to psychological materials, such as books on mental health; 50.4% used psychological resources available through media, such as online self-help coping methods; and 17.5% participated in counseling or psychotherapy.4
These findings emphasize the importance of being equipped to ensure the health and safety of health care workers through mental health interventions, both at work and in the community during this time of anxiety and uncertainty.
Future studies will become more critical in addressing this issue.
Risks to clinicians, families prevail
According to a recent report released by the Centers for Disease Control and Prevention, more than 9,000 health care workers across the United States had contracted COVID-19 as of mid-April, and 27 had died since the start of the pandemic.5
Health care workers are at risk around the globe, not only by the nature of their jobs but also by the shortage of personal protective equipment (PPE). In addition, the scarcity of N95 masks, respirators, and COVID-19 testing programs is causing the virus to spread among health care workers all over the world.
A study published recently by Celso Arango, MD, PhD, reported that 18% of staff at a hospital in Madrid had been infected with COVID-19. Dr. Arango speculated that transmission might be attributable to interactions with colleagues rather than with patients.6 We know, for example, that large proportions of people in China reportedly carried the virus while being asymptomatic.7 Those findings might not be generalizable, but they do suggest that an asymptomatic person could be a cause of contagion among professionals. Therefore, early screening and testing are critical – and should be priorities in health care settings.
Another problem clinicians can encounter is that, when they are called on to deal with very agitated patients, they might not get enough time to put on PPE. In addition, PPE can easily break and tear during the physical restraint process.
Working long hours is also putting a significant strain on health care workers and exposes them to the risk of infection. Also, health care workers not only worry about their safety but also fear bringing the virus to their families. They can also feel guilty about their conflicting feelings about exposing themselves and their families to risk. It is quite possible that, during this COVID-19 pandemic, health care workers will face a “care paradox,” in which they must choose between patients’ safety and their own. This care paradox can significantly contribute to a feeling of burnout, stress, and anxiety. Ultimately, this pandemic could lead to attrition from the field at a time when we most need all hands on deck.8
Further, according to a World Health Organization report on mental health and psychosocial consideration during the COVID-19 outbreak, some health care workers, unfortunately, experience avoidance by their family members or communities because of stigma, fear, and anxiety. This avoidance threatens to make an already challenging situation far worse for health care workers by increasing isolation.
Even after acute outbreak are over, the effects on health care workers can persist for years. In a follow-up study 13-26 months after the SARS outbreak, Robert G. Maunder, MD, and associates found that Toronto-area health care workers reported significantly higher levels of burnout, psychological distress, and posttraumatic stress. They were more likely to have reduced patient contact and work hours, and to have avoided behavioral consequences of stress.9 Exposure to stressful work conditions during a pandemic also might put hospital employees at a much higher risk of alcohol and substance use disorders.10
Potential solutions for improving care
COVID-19 has had a massive impact on the mental health of health care workers around the globe. Fortunately, there are evidence-based strategies aimed at mitigating the effects of this pandemic on health care workers. Fostering self-efficacy and optimism has been shown to improve coping and efficiency during disasters.9 Higher perceived workplace safety is associated with a lower risk of anxiety, depression, and posttraumatic stress among health care workers, while a lack of social support has been linked to adverse behavioral outcomes.10
A recent study found that, among Chinese physicians who cared for COVID-19 victims, more significant social support was associated with better sleep quality, greater self-effectiveness, and less psychological distress.11 Positive leadership and a professional culture of trust, and openness with unambiguous communication have been shown to improve the engagement of the medical workforce.12,13 Psychiatrists must advocate for the adoption of these practices in the workplace. Assessing and addressing mental health needs, in addition to the physical health of the health care workforce, is of utmost importance.
We can accomplish this in many ways, but we have to access our health care workers. Similar to our patient population, health care workers also experience stigma and anxiety tied to the disclosure of mental health challenges. This was reported in a study conducted in China, in which a specific psychological intervention using a hotline program was used for the medical team.14 This program provided psychological interventions/group activities aimed at releasing stress and anxiety. However, initially, the implementation of psychological interventions encountered obstacles.
For example, some members of the medical staff declined to participate in group or individual psychological interventions. Moreover, nurses showed irritability, unwillingness to join, and some staff refused, stating that “they did not have any problems.” Finally, psychological counselors regularly visited the facility to listen to difficulties or stories encountered by staff at work and provide support accordingly. More than 100 frontline medical staff participated and reported feeling better.15
Currently, several U.S. universities/institutes have implemented programs aimed at protecting the health and well-being of their staff during the COVID-19 pandemic. For instance, the department of psychiatry and behavioral health at Hackensack Meridian Health has put comprehensive system programs in place for at 16 affiliated medical centers and other patient care facilities to provide support during the COVID-19 crisis. A 24/7 team member support hotline connecting team members with a behavioral health specialist has become available when needed. This hotline is backed up by social workers, who provide mental health resources. In addition, another service called “Coping with COVID Talks” is available. This service is a virtual psychoeducational group facilitated by psychologists focusing on building coping skills and resilience.
Also, the consultation-liaison psychiatrists in the medical centers provide daily support to clinicians working in ICUs. These efforts have led to paradoxical benefits for employers, further leading to less commuting, more safety, and enhanced productivity for the clinician, according to Ramon Solhkhah, MD, MBA, chairman of the psychiatry department.16
Some universities, such as the University of North Carolina at Chapel Hill, have created mental health/telehealth support for health care workers, where they are conducting webinars on coping with uncertainty tied to COVID-19.17 The University of California, San Francisco, also has been a leader in this effort. That institution has employed its psychiatric workforce as volunteers – encouraging health care workers to use digital health apps and referral resources. Also, these volunteers provide peer counseling, phone support, and spiritual counseling to their health care workers.18
These approaches are crucial in this uncertain, challenging time. Our mental health system is deeply flawed, understaffed, and not well prepared to manage the mental health issues among health care workers. Psychiatric institutes/facilities should follow comprehensive and multifaceted approaches to combat the COVID-19 crisis. Several preventive measures can be considered in coping with this pandemic, such as stress reduction, mindfulness, and disseminating educational materials. Also, increased use of technology, such as in-the-moment measures, development of hotlines, crisis support, and treatment telepsychiatry for therapy and medication, should play a pivotal role in addressing the mental health needs of health care workers.
In addition, it is expected that, as a nation, we will see a surge of mental health needs for illnesses such as depression and PTSD, just as we do after “natural disasters” caused by a variety of reasons, including economic downturns. After the SARS outbreak in 2003, for example, health care workers showed symptoms of PTSD. The COVID-19 pandemic could have a similar impact.
The severity of mental health challenges among clinicians cannot be predicted at this time, but we can speculate that the traumatic impact of COVID-19 will prove long lasting, particularly among clinicians who served vulnerable populations and witnessed suffering, misery, and deaths. The long-term consequences might range from stress and anxiety to fear, depression, and PTSD. Implementation of mental health programs/psychological interventions/support will reduce the impact of mental health issues among these clinicians.
We must think about the best ways to optimize mental health among health care workers while also come up with innovative ways to target this at-risk group. The mental health of people who are saving lives – our frontline heroes – should be taken into consideration seriously around the globe. We also must prioritize the mental health of these workers during this unprecedented, challenging, and anxiety-provoking time.
Dr. Malik and Mr. Van Wert are affiliated with Johns Hopkins University, Baltimore. Dr. Kumari, Dr. Afzal, Dr. Doumas, and Dr. Solhkhah are affiliated with Hackensack Meridian Health at Ocean Medical Center, Brick, N.J. All six authors disclosed having no conflicts of interest. The authors would like to thank Vinay Kumar for his assistance with the literature review and for proofreading and editing this article.
References
1. Wu P et al. Can J Psychiatry. 2009;54(5):302-11.
2. Lu YC et al. Psychother Psychosom. 2006;75(6):370-5.
3. Lai J et al. JAMA Netw Open. 2020;3(3):e203976.
4. Kang L et al. Brain Behav Immun. 2020 Mar 30. doi: 10.1016/j.bbi.2020.03.028.
5. Centers for Disease Control and Prevention COVID-19 Response Team. MMWR. 2020 Apr 17;69(15):477-81.
6. Arango C. Biol Psychiatry. 2020 Apr 8. doi: 10.1016/j.biopsych.2020.04.003.
7. Day M. BMJ. 2020 Apr 2. doi: 10.1136/bmj.m1375.
8. Kirsch T. “Coronavirus, COVID-19: What happens if health care workers stop showing up?” The Atlantic. 2020 Mar 24.
9. Maunder RG et al. Emerg Infect Dis. 2006;12(12):1924-32.
10. Wu P et al. Alcohol Alcohol. 2008;43(6):706-12.
11. Brooks SK et al. BMC Psychol. 2016 Apr 26;4:18.
12. Smith BW et al. Am J Infect Control. 2009; 37:371-80.
13. Chen Q et al. Lancet Psychiatry. 2020 Apr 1;7(14):PE15-6.
14. Xiao H et al. Med Sci Monit. 2020;26:e923549.
15. Bergus GR et al. Acad Med. 2001;76:1148-52.
16. Bergeron T. “Working from home will be stressful. Here’s how employees (and employers) can handle it.” roi-nj.com. 2020 Mar 23.
17. UNChealthcare.org. “Mental Health/Emotional Support Resources for Coworkers and Providers Coping with COVID-19.”
18. Psych.ucsf.edu/coronoavirus. “Resources to Support Your Mental Health During the COVID-19 Outbreak.”
SARS-CoV-2 and the disease it causes, COVID-19, continues to spread around the world with a devastating social and economic impact. Undoubtedly, health care workers are essential to overcoming this crisis. If these issues are left unaddressed, low morale, burnout, or absenteeism could lead to the collapse of health care systems.
Historically, the health care industry has been one of the most hazardous environments in which to work. Employees in this industry are constantly exposed to a complex variety of health and safety hazards.
Particularly, risks from biological exposure to diseases such as tuberculosis, HIV, and currently COVID-19 are taking a considerable toll on health care workers’ health and well-being. Health care workers are leaving their families to work extra shifts, dealing with limited resources, and navigating the chaos. On top of all that, they are sacrificing their lives through these uncertain times.
Despite their resilience, health care workers – like the general population – can have strong psychological reactions of anxiety and fear during a pandemic. Still, they are required to continue their work amid uncertainty and danger.
Current research studies on COVID-19
Several studies have identified the impact of working in this type of environment during previous pandemics and disasters. In a study of hospital employees in China during the SARS epidemic (2002-2003), Ping Wu, PhD, and colleagues found that 10% of the participants experienced high levels of posttraumatic stress.1 In a similar study in Taiwan, researchers found that 17.3% of employees had developed significant mental health symptoms during the SARS outbreak.2
The impact of COVID-19 on health care workers seems to be much worse. A recent study from China indicates that 50.4% of hospital employees showed signs of depression, 44.6% had anxiety, and 34% had insomnia.3
Another recent cross-sectional study conducted by Lijun Kang, PhD, and associates evaluated the impact on mental health among health care workers in Wuhan, China, during the COVID-19 outbreak. This was the first study on the mental health of health care workers. This study recruited health care workers in Wuhan to participate in the survey from Jan. 29 to Feb. 4, 2020. The data were collected online with an anonymous, self-rated questionnaire that was distributed to all workstations. All subjects provided informed consent electronically prior to participating in the survey.
The survey questionnaire was made up of six components: primary demographic data, mental health assessment, risks of direct and indirect exposure to COVID-19, mental health care services accessed, psychological needs, and self-perceived health status, compared with that before the COVID-19 outbreak. A total of 994 health care workers responded to this survey, and the results are fascinating: 36.9% had subthreshold mental health distress (mean Patient Health Questionnaire–9 score, 2.4), 34.4% reported mild disturbances (mean PHQ-9, 5.4), 22.4% had moderate (mean PHQ-9, 9.0), and 6.2% reported severe disturbance (mean PHQ-9, 15.1). In this study, young women experienced more significant psychological distress. Regarding access to mental health services, 36.3% reported access to psychological materials, such as books on mental health; 50.4% used psychological resources available through media, such as online self-help coping methods; and 17.5% participated in counseling or psychotherapy.4
These findings emphasize the importance of being equipped to ensure the health and safety of health care workers through mental health interventions, both at work and in the community during this time of anxiety and uncertainty.
Future studies will become more critical in addressing this issue.
Risks to clinicians, families prevail
According to a recent report released by the Centers for Disease Control and Prevention, more than 9,000 health care workers across the United States had contracted COVID-19 as of mid-April, and 27 had died since the start of the pandemic.5
Health care workers are at risk around the globe, not only by the nature of their jobs but also by the shortage of personal protective equipment (PPE). In addition, the scarcity of N95 masks, respirators, and COVID-19 testing programs is causing the virus to spread among health care workers all over the world.
A study published recently by Celso Arango, MD, PhD, reported that 18% of staff at a hospital in Madrid had been infected with COVID-19. Dr. Arango speculated that transmission might be attributable to interactions with colleagues rather than with patients.6 We know, for example, that large proportions of people in China reportedly carried the virus while being asymptomatic.7 Those findings might not be generalizable, but they do suggest that an asymptomatic person could be a cause of contagion among professionals. Therefore, early screening and testing are critical – and should be priorities in health care settings.
Another problem clinicians can encounter is that, when they are called on to deal with very agitated patients, they might not get enough time to put on PPE. In addition, PPE can easily break and tear during the physical restraint process.
Working long hours is also putting a significant strain on health care workers and exposes them to the risk of infection. Also, health care workers not only worry about their safety but also fear bringing the virus to their families. They can also feel guilty about their conflicting feelings about exposing themselves and their families to risk. It is quite possible that, during this COVID-19 pandemic, health care workers will face a “care paradox,” in which they must choose between patients’ safety and their own. This care paradox can significantly contribute to a feeling of burnout, stress, and anxiety. Ultimately, this pandemic could lead to attrition from the field at a time when we most need all hands on deck.8
Further, according to a World Health Organization report on mental health and psychosocial consideration during the COVID-19 outbreak, some health care workers, unfortunately, experience avoidance by their family members or communities because of stigma, fear, and anxiety. This avoidance threatens to make an already challenging situation far worse for health care workers by increasing isolation.
Even after acute outbreak are over, the effects on health care workers can persist for years. In a follow-up study 13-26 months after the SARS outbreak, Robert G. Maunder, MD, and associates found that Toronto-area health care workers reported significantly higher levels of burnout, psychological distress, and posttraumatic stress. They were more likely to have reduced patient contact and work hours, and to have avoided behavioral consequences of stress.9 Exposure to stressful work conditions during a pandemic also might put hospital employees at a much higher risk of alcohol and substance use disorders.10
Potential solutions for improving care
COVID-19 has had a massive impact on the mental health of health care workers around the globe. Fortunately, there are evidence-based strategies aimed at mitigating the effects of this pandemic on health care workers. Fostering self-efficacy and optimism has been shown to improve coping and efficiency during disasters.9 Higher perceived workplace safety is associated with a lower risk of anxiety, depression, and posttraumatic stress among health care workers, while a lack of social support has been linked to adverse behavioral outcomes.10
A recent study found that, among Chinese physicians who cared for COVID-19 victims, more significant social support was associated with better sleep quality, greater self-effectiveness, and less psychological distress.11 Positive leadership and a professional culture of trust, and openness with unambiguous communication have been shown to improve the engagement of the medical workforce.12,13 Psychiatrists must advocate for the adoption of these practices in the workplace. Assessing and addressing mental health needs, in addition to the physical health of the health care workforce, is of utmost importance.
We can accomplish this in many ways, but we have to access our health care workers. Similar to our patient population, health care workers also experience stigma and anxiety tied to the disclosure of mental health challenges. This was reported in a study conducted in China, in which a specific psychological intervention using a hotline program was used for the medical team.14 This program provided psychological interventions/group activities aimed at releasing stress and anxiety. However, initially, the implementation of psychological interventions encountered obstacles.
For example, some members of the medical staff declined to participate in group or individual psychological interventions. Moreover, nurses showed irritability, unwillingness to join, and some staff refused, stating that “they did not have any problems.” Finally, psychological counselors regularly visited the facility to listen to difficulties or stories encountered by staff at work and provide support accordingly. More than 100 frontline medical staff participated and reported feeling better.15
Currently, several U.S. universities/institutes have implemented programs aimed at protecting the health and well-being of their staff during the COVID-19 pandemic. For instance, the department of psychiatry and behavioral health at Hackensack Meridian Health has put comprehensive system programs in place for at 16 affiliated medical centers and other patient care facilities to provide support during the COVID-19 crisis. A 24/7 team member support hotline connecting team members with a behavioral health specialist has become available when needed. This hotline is backed up by social workers, who provide mental health resources. In addition, another service called “Coping with COVID Talks” is available. This service is a virtual psychoeducational group facilitated by psychologists focusing on building coping skills and resilience.
Also, the consultation-liaison psychiatrists in the medical centers provide daily support to clinicians working in ICUs. These efforts have led to paradoxical benefits for employers, further leading to less commuting, more safety, and enhanced productivity for the clinician, according to Ramon Solhkhah, MD, MBA, chairman of the psychiatry department.16
Some universities, such as the University of North Carolina at Chapel Hill, have created mental health/telehealth support for health care workers, where they are conducting webinars on coping with uncertainty tied to COVID-19.17 The University of California, San Francisco, also has been a leader in this effort. That institution has employed its psychiatric workforce as volunteers – encouraging health care workers to use digital health apps and referral resources. Also, these volunteers provide peer counseling, phone support, and spiritual counseling to their health care workers.18
These approaches are crucial in this uncertain, challenging time. Our mental health system is deeply flawed, understaffed, and not well prepared to manage the mental health issues among health care workers. Psychiatric institutes/facilities should follow comprehensive and multifaceted approaches to combat the COVID-19 crisis. Several preventive measures can be considered in coping with this pandemic, such as stress reduction, mindfulness, and disseminating educational materials. Also, increased use of technology, such as in-the-moment measures, development of hotlines, crisis support, and treatment telepsychiatry for therapy and medication, should play a pivotal role in addressing the mental health needs of health care workers.
In addition, it is expected that, as a nation, we will see a surge of mental health needs for illnesses such as depression and PTSD, just as we do after “natural disasters” caused by a variety of reasons, including economic downturns. After the SARS outbreak in 2003, for example, health care workers showed symptoms of PTSD. The COVID-19 pandemic could have a similar impact.
The severity of mental health challenges among clinicians cannot be predicted at this time, but we can speculate that the traumatic impact of COVID-19 will prove long lasting, particularly among clinicians who served vulnerable populations and witnessed suffering, misery, and deaths. The long-term consequences might range from stress and anxiety to fear, depression, and PTSD. Implementation of mental health programs/psychological interventions/support will reduce the impact of mental health issues among these clinicians.
We must think about the best ways to optimize mental health among health care workers while also come up with innovative ways to target this at-risk group. The mental health of people who are saving lives – our frontline heroes – should be taken into consideration seriously around the globe. We also must prioritize the mental health of these workers during this unprecedented, challenging, and anxiety-provoking time.
Dr. Malik and Mr. Van Wert are affiliated with Johns Hopkins University, Baltimore. Dr. Kumari, Dr. Afzal, Dr. Doumas, and Dr. Solhkhah are affiliated with Hackensack Meridian Health at Ocean Medical Center, Brick, N.J. All six authors disclosed having no conflicts of interest. The authors would like to thank Vinay Kumar for his assistance with the literature review and for proofreading and editing this article.
References
1. Wu P et al. Can J Psychiatry. 2009;54(5):302-11.
2. Lu YC et al. Psychother Psychosom. 2006;75(6):370-5.
3. Lai J et al. JAMA Netw Open. 2020;3(3):e203976.
4. Kang L et al. Brain Behav Immun. 2020 Mar 30. doi: 10.1016/j.bbi.2020.03.028.
5. Centers for Disease Control and Prevention COVID-19 Response Team. MMWR. 2020 Apr 17;69(15):477-81.
6. Arango C. Biol Psychiatry. 2020 Apr 8. doi: 10.1016/j.biopsych.2020.04.003.
7. Day M. BMJ. 2020 Apr 2. doi: 10.1136/bmj.m1375.
8. Kirsch T. “Coronavirus, COVID-19: What happens if health care workers stop showing up?” The Atlantic. 2020 Mar 24.
9. Maunder RG et al. Emerg Infect Dis. 2006;12(12):1924-32.
10. Wu P et al. Alcohol Alcohol. 2008;43(6):706-12.
11. Brooks SK et al. BMC Psychol. 2016 Apr 26;4:18.
12. Smith BW et al. Am J Infect Control. 2009; 37:371-80.
13. Chen Q et al. Lancet Psychiatry. 2020 Apr 1;7(14):PE15-6.
14. Xiao H et al. Med Sci Monit. 2020;26:e923549.
15. Bergus GR et al. Acad Med. 2001;76:1148-52.
16. Bergeron T. “Working from home will be stressful. Here’s how employees (and employers) can handle it.” roi-nj.com. 2020 Mar 23.
17. UNChealthcare.org. “Mental Health/Emotional Support Resources for Coworkers and Providers Coping with COVID-19.”
18. Psych.ucsf.edu/coronoavirus. “Resources to Support Your Mental Health During the COVID-19 Outbreak.”
Posttraumatic stress disorder: From pathophysiology to pharmacology
Posttraumatic stress disorder (PTSD) occurs acutely and chronically in the aftermath of severe and potentially life-threatening trauma.1 The prevalence of PTSD varies significantly across countries and by type of trauma (Box1-7).
Box
In the general population, the prevalence of posttraumatic stress disorder (PTSD) varies from as low as 0.3% in China to as high as 6.1% in New Zealand1 and 6.8% in the United States.2 These rates are actually much lower than expected when one considers that severe trauma is experienced by 60.7% of men and 51.2% of women.3,4 Although the majority of individuals exposed to trauma experience emotional distress immediately following a traumatic event, most of them do not develop PTSD.5
It appears that the context of trauma is important: 12% to 15% of veterans experience PTSD, compared with 19% to 75% of crime victims and 80% of rape victims.1 The lifetime risk for PTSD is twice as high in women as it is in men,6 and genetic vulnerability may play a role. For example, twin studies showed that approximately 30% of the risk for PTSD may be mediated by genetic predisposition.7
Individuals who develop PTSD experience a wide range of symptoms.8 These can be categorized as PTSD-specific symptoms, or nonspecific symptoms. PTSD-specific symptoms include nightmares, flashbacks, dissociative reactions, hyperreactivity or hyperarousal, distress with reminders of trauma, and avoidance of trauma-related physical reminders and thoughts/feelings (Table8). Nonspecific symptoms include depressive and anxiety symptoms and significant problems in social, relationship, or work situations.8
While successful treatment necessitates taking all of these symptoms into account, understanding the pathophysiology of PTSD can inform a more focused and rational treatment approach. In this article, we describe some key pathophysiologic PTSD studies, and focus on PTSD-specific psychopathology to inform treatment.
Brain systems implicated in PTSD
Neuropeptide Y (NPY) is an anxiolytic endogenous peptide that has connections to the hypothalamic-pituitary-adrenal (HPA) axis. Its levels can be modulated by stress.9 Preclinical and clinical studies strongly support a potential role of NPY dysfunction in the pathophysiology of PTSD. Lower concentrations of NPY increase susceptibility to PTSD in combat veterans10 and in animal models.11 Three single-nucleotide polymorphisms (SNPs) appear to mediate this effect.12 These findings strongly support pharmaceutical targeting this system as a useful therapeutic approach.13,14 Indeed, intranasal NPY administered as a single dose reduces anxiety in animal models15 and in humans,16 but this work has not yet translated into clinical tools.
Corticotropin-releasing hormone receptor (CRHR1) gene. Corticotropin-releasing hormone has been implicated in PTSD.17 Corticotropin-releasing hormone receptors (CRHR) are important mediators in response to stress.18,19 They bind corticotropin-releasing hormone and contribute to the integration of autonomic, behavioral, and immune responses to stress.20 Single-nucleotide polymorphisms in the regulatory portion of the CRHR1 gene are associated with an increased risk for depression in adults who have a history of child abuse.21
The CRHR1 receptor antagonist GSK561679 is an investigational agent for the treatment of mood and anxiety disorders.22 In exploratory studies,23,24 GSK561679 was found to inhibit fear-potentiated startle in patients with PTSD, but not overall PTSD symptoms, although a subset of women with a specific genetic variant of the CRHR1 gene (rs110402) experienced significant benefit.25,26 This suggests that we must learn more about this system before we proceed.27
Brain-derived neurotrophic factor (BDNF). The synthesis of BDNF is influenced by neuronal activity in the brain and plays a role in synaptic transmission and plasticity.28 Brain-derived neurotrophic factor is encoded by the BDNF gene, which has been implicated in stress vulnerability.29 A common SNP in the pro-region of the human BDNF gene results in a valine-to-methionine substitution at the 66th amino acid (Val66Met). The functional Val66Met polymorphism may have a role in the risk of developing PTSD. However, not all studies support this finding. One study found that an SNP with a resulting Val66Met polymorphism is associated with adult PTSD symptoms after childhood abuse, while a meta-analysis of 7 studies did not confirm this.30,31 We need to learn more about BDNF before we proceed.32
Continue to: Serotonin transporter (5-HTT) gene
Serotonin transporter (5-HTT) gene. Serotonin transporter is a monoamine transporter protein that terminates the neurotransmitter signal by transporting serotonin from the synaptic cleft back into the presynaptic neuron. It is encoded by the SLC6A4 gene, which resides on the long arm of chromosome 17(17q11.1-q12). It is a large gene with 31 kilo bases and 14 separate exons (transcribed regions).33,34
This gene has several variants. The best-studied is a variation in the promoter region. A 44-bp insertion or deletion yields the “long” and “short” alleles, respectively. The proteins produced by the 2 alleles are identical, but the amount of expressed protein is different. The short allele (“S”) is associated with a nearly 50% reduction in 5-HTT expression in both homozygotes and heterozygotes.35 A greater incidence of serotonin transporter promoter region (5-HTTLPR) S has been found in individuals with PTSD compared with those without PTSD,36-38 and 5-HTTLPR S increases the risk of PTSD in individuals with low social support39 or after very few traumatic events.40 The short allele variant is also associated with depression in individuals who face adversity.35,41
The overrepresentation of the short form of 5-HTTLPR in individuals who develop PTSD may represent a potential problem with current treatment paradigms, in which an antidepressant is the first-line treatment, because this allele is associated with reduced response to antidepressants.42,43 More distressing is the possible association of this allele with increased suicide risk, particularly violent suicide44 or repeated suicide attempts.45
Furthermore, a functional MRI study of patients who were anxious revealed that in individuals with the short allele, administration of
Catechol-o-methyltransferase (COMT) is one of the enzymes that degrades catecholamines such as dopamine, epinephrine, and norepinephrine (NE).47 In humans, COMT protein is encoded by the COMT gene. This gene is associated with allelic variants; the best-studied of these is Val158Met. COMT Val158Met polymorphism (rs4860) has been linked to deficits in stress response and emotional resilience.48,49 Val158Met is associated with a 40% reduction in enzyme activity and slower catalysis of catecholamines, resulting in increases in catecholamines levels in the brain, which may increase the risk of developing PTSD.50 Individuals homozygous for this SNP (Met/Met) are highly susceptible to develop PTSD independently of the severity of the trauma they experienced.51 The Val158Met polymorphism may be associated with other abnormalities, such as cognitive problems with specific frontal cortical activity, and also with improved antidepressant response (valine homozygotes less responsive than methionine homozygotes).52 This gene is available on gene testing profiles.
Continue to: The role of norepinephrine in PTSD
The role of norepinephrine in PTSD
Perhaps the greatest advance in the understanding of the pathophysiology of PTSD relates to changes in brain NE. The HPA axis is responsible for coordinating the hormonal response to stress. Dysregulation of this axis and increased activity of the central and peripheral noradrenergic systems are usually observed in patients with PTSD.53 Several monoamine neurotransmitters are important in the regulation and function of the HPA axis. Norepinephrine plays a major role in stress.
The clinical PTSD-specific criteria are all descriptions of excessive noradrenergic tone.54 For example, hypervigilance and hyperstartle are clearly anticipated as evidence of NE stimulation. Flashbacks, particularly those that might be precipitated by environmental cues, also can be a manifestation of the vigilance induced by NE. Sleep disturbances (insomnia and nightmares) are present; insomnia is reported more often than nightmares.55 Increased catecholamine levels, particularly NE, are a feature of sleep disturbances associated with middle insomnia. Dreams can be remembered only if you wake up during dreaming. Catecholamines do not change the content of dreams, just recall.56
In a study of central noradrenergic tone in patients with PTSD, 6 hourly CSF samples were collected from 11 male combat veterans with PTSD and 8 healthy controls.57 Participants with PTSD had significantly higher CSF NE concentrations (0.55 ± 0.17 pmol/ml vs 0.39 ± 0.16 pmol/mL in the PTSD and control groups, respectively; F = 4.49, P < .05).57 Overall PTSD symptoms correlated significantly with CSF NE levels (r = 0.82, P <.005), and PTSD-specific symptoms such as avoidance (r = 0.79, P = .004). Intrusive thoughts (r = 0.57, P = .07) and hyperarousal (r = 0.54, P = .09) were also related.57 This relationship is unique; patients with PTSD with predominant depressive symptoms do not have elevated plasma NE levels.58
In the human brain, there are 3 main groups of NE receptors: alpha-1 receptors, alpha-2 receptors, and beta receptors.59 Alpha-1 receptors (alpha-1A, alpha-1B, and alpha-1D) are postsynaptic and mediate increase in inositol trisphosphate (IP3) and intracellular calcium (Ca2+). Alpha-2 receptors (alpha-2A, alpha-2B, alpha-2C) in the CNS are presynaptic autoreceptors and serve to reduce NE release. Beta receptors (beta-1, beta-2, beta-3) inhibit cyclic adenosine monophosphate (cAMP) production.59 The effects of inhibition of alpha or beta receptors are different. Inhibition of beta receptors is associated with depressive symptoms and depressive syndrome, inhibition of peripheral beta receptors is associated with reductions in anxiety (generally reduction of pulse, sweating, tremor),60 and inhibition of central alpha-1 receptors is associated with reduced PTSD symptoms.61
Choice of agents for PTSD-specific symptoms
As outlined in the Table,8 PTSD is characterized by 3 types of symptoms that are specific for PTSD. Trauma-focused psychotherapy62,63 and selective serotonin reuptake inhibitors (SSRIs)64 are considered first-line therapy for PTSD. Only
Continue to: Serotonin transporter promoter...
Serotonin transporter promoter region gene short-type variants, which possibly increase an individual’s predisposition to developing PTSD, may explain the abundance of depressive symptoms in this condition and the subdued response to antidepressants. Specifically, an anticipated preponderance of these alleles may be associated with poorer outcomes. Non-SSRI treatments, such as low-dose
On the other hand, animal models support antagonism of the postsynaptic alpha-1 adrenergic receptor of the CNS as a target for PTSD treatment.71 Although
Quetiapine might be another non-SSRI option for treating patients with PTSD. It is an antagonist with high affinity tothehistamine-1 receptor at low doses. Norquetiapine is an alpha-2 antagonist that increases brain NE levels. Both quetiapine and norquetiapine are alpha-1 antagonists. There is no beta blockade and no SSRI effect, but some 5HT2A blockade, which may be anxiolytic. Compared with placebo, an average quetiapine dose of 258 mg/d resulted in significantly greater reductions in Clinician-Administered PTSD Scale total score, re-experiencing score, and hyperarousal score.73
Unfortunately, none of the non-SSRI options have been adequately evaluated. For now, clinicians need to continue to use SSRIs, and researchers need to continue to explore mechanism-guided alternatives.
Bottom Line
Understanding the mechanisms of the pathophysiology of posttraumatic stress disorder (PTSD) may allow clinicians to “jump ahead” of clinical studies and FDA indications. Clinicians may reasonably use alpha-1 antagonists (eg, prazosin, quetiapine) for general clinical improvement of patients with PTSD, particularly for PTSD-specific symptoms. Using antihistamines to reduce anxiety (especially in patients who have the COMT Val158Met polymorphism) may also be reasonable.
Related Resources
- North CS, Hong BA, Downs DL. PTSD: a systematic approach to diagnosis and treatment. Current Psychiatry. 2018;17(4):35-43.
- Zhang Y, Ren R, Sanford LD, et al. The effects of prazosin on sleep disturbances in post-traumatic stress disorder: a systematic review and meta-analysis. Sleep Med. 2019; 67:225-231.
Drug Brand Names
Aripiprazole • Abilify
Citalopram • Celexa
Paroxetine • Paxil
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
1. Javidi H, Yadollahie M. Post-traumatic stress disorder. Int J Occup Environ Med. 2012;3(1):2-9.
2. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
3. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
4. Breslau N, Kessler RC, Chilcoat HD, et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626-632.
5. Cerda M, Sagdeo A, Johnson J, et al. Genetic and environmental influences on psychiatric comorbidity: a systematic review. J Affect Disord. 2010;126(1-2):14-38.
6. Yehuda R, Hoge CW, McFarlane AC, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057. doi: 10.1038/nrdp.2015.57.
7. True WR, Rice J, Eisen SA, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257-264.
8. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:271-280.
9. Reichmann F, Holzer P. Neuropeptide Y: a stressful review. Neuropeptides. 2016;55:99-109.
10. Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59(7):660-663.
11. Cohen H, Liu T, Kozlovsky N, et al. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37(2):350-363.
12. Donner J, Sipilä T, Ripatti S, et al. Support for involvement of glutamate decarboxylase 1 and neuropeptide Y in anxiety susceptibility. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(3):316-327.
13. Schmeltzer SN, Herman JP, Sah R. Neuropeptide Y (NPY) and posttraumatic stress disorder (PTSD): a translational update. Exp Neurol. 2016;284(pt B):196-210.
14. Kautz M, Charney DS, Murrough JW. Neuropeptide Y, resilience, and PTSD therapeutics. Neurosci Lett. 2017;649:164-169.
15. Serova LI, Laukova M, Alaluf LG, et al. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur Neuropsychopharmacol. 2014;24(1):142-147.
16. Sayed S, Van Dam NT, Horn SR, et al. A randomized dose-ranging study of neuropeptide Y in patients with posttraumatic stress disorder. Int J Neuropsychopharmacol. 2018;21(1):3-11.
17. Toth M, Flandreau EI, Deslauriers J, et al. Overexpression of forebrain CRH during early life increases trauma susceptibility in adulthood. Neuropsychopharmacology. 2016;41(6):1681-1690.
18. White S, Acierno R, Ruggiero KJ, et al. Association of CRHR1 variants and posttraumatic stress symptoms in hurricane exposed adults. J Anxiety Disord. 2013;27(7):678-683.
19. Wolf EJ, Mitchell KS, Logue MW, et al. Corticotropin releasing hormone receptor 2 (CRHR-2) gene is associated with decreased risk and severity of posttraumatic stress disorder in women. Depress Anxiety. 2013;30(12):1161-1169.
20. Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40(5):573-629.
21. Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190-200.
22. Tellew JE, Lanier M, Moorjani M, et al. Discovery of NBI-77860/GSK561679, a potent corticotropin-releasing factor (CRF1) receptor antagonist with improved pharmacokinetic properties. Bioorg Med Chem Lett. 2010;20(24):7259-7264.
23. Dunlop BW, Rothbaum BO, Binder EB, et al. Evaluation of a corticotropin releasing hormone type 1 receptor antagonist in women with posttraumatic stress disorder: study protocol for a randomized controlled trial. Trials. 2014;15:240. doi: 10.1186/1745-6215-15-240.
24. Jovanovic T, Duncan EJ, Kaye J, et al. Psychophysiological treatment outcomes: Corticotropin-releasing factor type 1 receptor antagonist increases inhibition of fear-potentiated startle in PTSD patients. Psychophysiology. 2019:e13356. doi: 10.1111/psyp.13356.
25. Dunlop BW, Binder EB, Iosifescu D, et al. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol Psychiatry. 2017;82(12):866-874.
26. Pape JC, Carrillo-Roa T, Rothbaum BO, et al. DNA methylation levels are associated with CRF1 receptor antagonist treatment outcome in women with post-traumatic stress disorder. Clin Epigenetics. 2018;10(1):136. doi: 10.1186/s13148-018-0569-x.
27. Murrough JW, Charney DS. Corticotropin-releasing factor type 1 receptor antagonists for stress-related disorders: time to call it quits? Biol Psychiatry. 2017;82(12):858-860.
28. Leal G, Bramham CR, Duarte CB. BDNF and hippocampal synaptic plasticity. Vitam Horm. 2017;104:153-195.
29. Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12:1079-1088.
30. Frustaci A, Pozzi G, Gianfagna F, et al. Meta-analysis of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. Neuropsychobiology. 2008;58(3-4):163-170.
31. Gatt JM, Nemeroff CB, Dobson-Stone C, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14(7):681-695.
32. Ragen BJ, Seidel J, Chollak C, et al. Investigational drugs under development for the treatment of PTSD. Expert Opin Investig Drugs. 2015;24(5):659-672.
33. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386-389.
34. Murphy DL, Fox MA, Timpano KR, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55(6):932-960.
35. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Compan J Clin Psychiatry. 2009;11:(3):93-102.
36. Lee HJ, Lee MS, Kang RH, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21(3):135-139.
37. Liu Y, Garrett ME, Dennis MF, et al. An examination of the association between 5-HTTLPR, combat exposure, and PTSD diagnosis among U.S. veterans. PLoS One. 2015;10(3):e0119998. doi: 10.1371/journal.pone.0119998.
38. Mehta D, Voisey J, Bruenig D, et al. Transcriptome analysis reveals novel genes and immune networks dysregulated in veterans with PTSD. Brain Behav Immun. 2018;74:133-142. doi: 10.1016/j.bbi.2018.08.014.
39. Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164(11):1693-1699.
40. Kolassa IT, Ertl V, Eckart C, et al. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. J Clin Psychiatry. 2010;71(5):543-547.
41. Bryant RA, Felmingham KL, Falconer EM, et al. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biol Psychiatry. 2010;67(12):1217-1219.
42. Mrazek DA, Rush AJ, Biernacka JM, et al. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):341-351.
43. Shiroma PR, Drews MS, Geske JR, et al. SLC6A4 polymorphisms and age of onset in late-life depression on treatment outcomes with citalopram: a Sequenced Treatment Alternatives to Relieve Depression (STAR*D) report. Am J Geriatr Psychiatry. 2014;22(11):1140-1148.
44. Fanelli G, Serretti A. The influence of the serotonin transporter gene 5-HTTLPR polymorphism on suicidal behaviors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:375-387.
45. Courtet P, Picot MC, Bellivier F, et al. Serotonin transporter gene may be involved in short-term risk of subsequent suicide attempts. Biol Psychiatry. 2003;55(1):46-51.
46. Outhred T, Das P, Dobson-Stone C, et al. The impact of 5-HTTLPR on acute serotonin transporter blockade by escitalopram on emotion processing: Preliminary findings from a randomised, crossover fMRI study. Aust NZ J Psychiatry. 2014;48(12):1115-1125.
47. Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243-250.
48. Valente NL, Vallada H, Cordeiro Q, et al. Catechol-O-methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. J Mol Neurosci. 2011;43(3):516-523.
49. van Rooij SJ, Stevens JS, Ely TD, et al. Childhood trauma and COMT genotype interact to increase hippocampal activation in resilient individuals. Front Psychiatry. 2016;7:156. doi: 10.3389/fpsyt.2016.00156.
50. Wu G, Feder A, Cohen H, et al. Understanding resilience. Front Behav Neuroscience. 2013;7:10. doi: 10.3389/fnbeh.2013.00010.
51. Kolassa I, Kolassa S, Ertl V, et al. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 2010;67(4):304-308.
52. Bruder GE, Keilp JG, Xu H, et al. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry. 2005;58(11):901-907.
53. Strawn JR, Geracioti TD Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
54. Hendrickson RC, Raskind MA. Noradrenergic dysregulation in the pathophysiology of PTSD. Exp Neurol. 2016;284(pt B):181-195.
55. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929-933.
56. Roehrs TA, Roth T. Hyperarousal in insomnia and hypnotic dose escalation. Sleep Med. 2016;23:16-20.
57. Geracioti TD Jr, Baker DG, Ekhator NN, et al. CSF Norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227-1230.
58. Yehuda R, Siever LJ, Teicher MH, et al. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 1998;44(1):56-63.
59. Molinoff PB. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs. 1984;28(suppl 2):1-15.
60. El-Mallakh RS. The use of beta-blockers in psychiatry. Res Staff Phys. 1989;35:49-52,59,62.
61. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
62. Bisson JI, Roberts NP, Andrew M, et al. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev. 2013;(12):CD003388.
63. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
64. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
65. Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286.
66. Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283(14):1837-1844.
67. Davidson JRT, Landerman LR, Farfel GM, et al. Characterizing the effects of sertraline in post-traumatic stress disorder. Psychol Med. 2002;32(4):661-670.
68. Hertzberg MA, Feldman ME, Beckham JC, et al. Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Ann Clin Psychiatry. 2000;12(2):101-105.
69. Friedman MJ, Marmar CR, Baker DG, et al. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68(5):711-720.
70. Mello MF, Costa MCP, Schoedl AF, et al. Aripiprazole in the treatment of posttraumatic stress disorder: an open-label trial. Rev Bras Psiquiatr. 2008;30(4):358-361.
71. Birnbaum S, Gobeske KT, Auerbach J, et al. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46(9):1266-1274.
72. Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014;69(4):235-242.
73. Villarreal G, Hamner MB, Cañive JM, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205-1212.
Posttraumatic stress disorder (PTSD) occurs acutely and chronically in the aftermath of severe and potentially life-threatening trauma.1 The prevalence of PTSD varies significantly across countries and by type of trauma (Box1-7).
Box
In the general population, the prevalence of posttraumatic stress disorder (PTSD) varies from as low as 0.3% in China to as high as 6.1% in New Zealand1 and 6.8% in the United States.2 These rates are actually much lower than expected when one considers that severe trauma is experienced by 60.7% of men and 51.2% of women.3,4 Although the majority of individuals exposed to trauma experience emotional distress immediately following a traumatic event, most of them do not develop PTSD.5
It appears that the context of trauma is important: 12% to 15% of veterans experience PTSD, compared with 19% to 75% of crime victims and 80% of rape victims.1 The lifetime risk for PTSD is twice as high in women as it is in men,6 and genetic vulnerability may play a role. For example, twin studies showed that approximately 30% of the risk for PTSD may be mediated by genetic predisposition.7
Individuals who develop PTSD experience a wide range of symptoms.8 These can be categorized as PTSD-specific symptoms, or nonspecific symptoms. PTSD-specific symptoms include nightmares, flashbacks, dissociative reactions, hyperreactivity or hyperarousal, distress with reminders of trauma, and avoidance of trauma-related physical reminders and thoughts/feelings (Table8). Nonspecific symptoms include depressive and anxiety symptoms and significant problems in social, relationship, or work situations.8
While successful treatment necessitates taking all of these symptoms into account, understanding the pathophysiology of PTSD can inform a more focused and rational treatment approach. In this article, we describe some key pathophysiologic PTSD studies, and focus on PTSD-specific psychopathology to inform treatment.
Brain systems implicated in PTSD
Neuropeptide Y (NPY) is an anxiolytic endogenous peptide that has connections to the hypothalamic-pituitary-adrenal (HPA) axis. Its levels can be modulated by stress.9 Preclinical and clinical studies strongly support a potential role of NPY dysfunction in the pathophysiology of PTSD. Lower concentrations of NPY increase susceptibility to PTSD in combat veterans10 and in animal models.11 Three single-nucleotide polymorphisms (SNPs) appear to mediate this effect.12 These findings strongly support pharmaceutical targeting this system as a useful therapeutic approach.13,14 Indeed, intranasal NPY administered as a single dose reduces anxiety in animal models15 and in humans,16 but this work has not yet translated into clinical tools.
Corticotropin-releasing hormone receptor (CRHR1) gene. Corticotropin-releasing hormone has been implicated in PTSD.17 Corticotropin-releasing hormone receptors (CRHR) are important mediators in response to stress.18,19 They bind corticotropin-releasing hormone and contribute to the integration of autonomic, behavioral, and immune responses to stress.20 Single-nucleotide polymorphisms in the regulatory portion of the CRHR1 gene are associated with an increased risk for depression in adults who have a history of child abuse.21
The CRHR1 receptor antagonist GSK561679 is an investigational agent for the treatment of mood and anxiety disorders.22 In exploratory studies,23,24 GSK561679 was found to inhibit fear-potentiated startle in patients with PTSD, but not overall PTSD symptoms, although a subset of women with a specific genetic variant of the CRHR1 gene (rs110402) experienced significant benefit.25,26 This suggests that we must learn more about this system before we proceed.27
Brain-derived neurotrophic factor (BDNF). The synthesis of BDNF is influenced by neuronal activity in the brain and plays a role in synaptic transmission and plasticity.28 Brain-derived neurotrophic factor is encoded by the BDNF gene, which has been implicated in stress vulnerability.29 A common SNP in the pro-region of the human BDNF gene results in a valine-to-methionine substitution at the 66th amino acid (Val66Met). The functional Val66Met polymorphism may have a role in the risk of developing PTSD. However, not all studies support this finding. One study found that an SNP with a resulting Val66Met polymorphism is associated with adult PTSD symptoms after childhood abuse, while a meta-analysis of 7 studies did not confirm this.30,31 We need to learn more about BDNF before we proceed.32
Continue to: Serotonin transporter (5-HTT) gene
Serotonin transporter (5-HTT) gene. Serotonin transporter is a monoamine transporter protein that terminates the neurotransmitter signal by transporting serotonin from the synaptic cleft back into the presynaptic neuron. It is encoded by the SLC6A4 gene, which resides on the long arm of chromosome 17(17q11.1-q12). It is a large gene with 31 kilo bases and 14 separate exons (transcribed regions).33,34
This gene has several variants. The best-studied is a variation in the promoter region. A 44-bp insertion or deletion yields the “long” and “short” alleles, respectively. The proteins produced by the 2 alleles are identical, but the amount of expressed protein is different. The short allele (“S”) is associated with a nearly 50% reduction in 5-HTT expression in both homozygotes and heterozygotes.35 A greater incidence of serotonin transporter promoter region (5-HTTLPR) S has been found in individuals with PTSD compared with those without PTSD,36-38 and 5-HTTLPR S increases the risk of PTSD in individuals with low social support39 or after very few traumatic events.40 The short allele variant is also associated with depression in individuals who face adversity.35,41
The overrepresentation of the short form of 5-HTTLPR in individuals who develop PTSD may represent a potential problem with current treatment paradigms, in which an antidepressant is the first-line treatment, because this allele is associated with reduced response to antidepressants.42,43 More distressing is the possible association of this allele with increased suicide risk, particularly violent suicide44 or repeated suicide attempts.45
Furthermore, a functional MRI study of patients who were anxious revealed that in individuals with the short allele, administration of
Catechol-o-methyltransferase (COMT) is one of the enzymes that degrades catecholamines such as dopamine, epinephrine, and norepinephrine (NE).47 In humans, COMT protein is encoded by the COMT gene. This gene is associated with allelic variants; the best-studied of these is Val158Met. COMT Val158Met polymorphism (rs4860) has been linked to deficits in stress response and emotional resilience.48,49 Val158Met is associated with a 40% reduction in enzyme activity and slower catalysis of catecholamines, resulting in increases in catecholamines levels in the brain, which may increase the risk of developing PTSD.50 Individuals homozygous for this SNP (Met/Met) are highly susceptible to develop PTSD independently of the severity of the trauma they experienced.51 The Val158Met polymorphism may be associated with other abnormalities, such as cognitive problems with specific frontal cortical activity, and also with improved antidepressant response (valine homozygotes less responsive than methionine homozygotes).52 This gene is available on gene testing profiles.
Continue to: The role of norepinephrine in PTSD
The role of norepinephrine in PTSD
Perhaps the greatest advance in the understanding of the pathophysiology of PTSD relates to changes in brain NE. The HPA axis is responsible for coordinating the hormonal response to stress. Dysregulation of this axis and increased activity of the central and peripheral noradrenergic systems are usually observed in patients with PTSD.53 Several monoamine neurotransmitters are important in the regulation and function of the HPA axis. Norepinephrine plays a major role in stress.
The clinical PTSD-specific criteria are all descriptions of excessive noradrenergic tone.54 For example, hypervigilance and hyperstartle are clearly anticipated as evidence of NE stimulation. Flashbacks, particularly those that might be precipitated by environmental cues, also can be a manifestation of the vigilance induced by NE. Sleep disturbances (insomnia and nightmares) are present; insomnia is reported more often than nightmares.55 Increased catecholamine levels, particularly NE, are a feature of sleep disturbances associated with middle insomnia. Dreams can be remembered only if you wake up during dreaming. Catecholamines do not change the content of dreams, just recall.56
In a study of central noradrenergic tone in patients with PTSD, 6 hourly CSF samples were collected from 11 male combat veterans with PTSD and 8 healthy controls.57 Participants with PTSD had significantly higher CSF NE concentrations (0.55 ± 0.17 pmol/ml vs 0.39 ± 0.16 pmol/mL in the PTSD and control groups, respectively; F = 4.49, P < .05).57 Overall PTSD symptoms correlated significantly with CSF NE levels (r = 0.82, P <.005), and PTSD-specific symptoms such as avoidance (r = 0.79, P = .004). Intrusive thoughts (r = 0.57, P = .07) and hyperarousal (r = 0.54, P = .09) were also related.57 This relationship is unique; patients with PTSD with predominant depressive symptoms do not have elevated plasma NE levels.58
In the human brain, there are 3 main groups of NE receptors: alpha-1 receptors, alpha-2 receptors, and beta receptors.59 Alpha-1 receptors (alpha-1A, alpha-1B, and alpha-1D) are postsynaptic and mediate increase in inositol trisphosphate (IP3) and intracellular calcium (Ca2+). Alpha-2 receptors (alpha-2A, alpha-2B, alpha-2C) in the CNS are presynaptic autoreceptors and serve to reduce NE release. Beta receptors (beta-1, beta-2, beta-3) inhibit cyclic adenosine monophosphate (cAMP) production.59 The effects of inhibition of alpha or beta receptors are different. Inhibition of beta receptors is associated with depressive symptoms and depressive syndrome, inhibition of peripheral beta receptors is associated with reductions in anxiety (generally reduction of pulse, sweating, tremor),60 and inhibition of central alpha-1 receptors is associated with reduced PTSD symptoms.61
Choice of agents for PTSD-specific symptoms
As outlined in the Table,8 PTSD is characterized by 3 types of symptoms that are specific for PTSD. Trauma-focused psychotherapy62,63 and selective serotonin reuptake inhibitors (SSRIs)64 are considered first-line therapy for PTSD. Only
Continue to: Serotonin transporter promoter...
Serotonin transporter promoter region gene short-type variants, which possibly increase an individual’s predisposition to developing PTSD, may explain the abundance of depressive symptoms in this condition and the subdued response to antidepressants. Specifically, an anticipated preponderance of these alleles may be associated with poorer outcomes. Non-SSRI treatments, such as low-dose
On the other hand, animal models support antagonism of the postsynaptic alpha-1 adrenergic receptor of the CNS as a target for PTSD treatment.71 Although
Quetiapine might be another non-SSRI option for treating patients with PTSD. It is an antagonist with high affinity tothehistamine-1 receptor at low doses. Norquetiapine is an alpha-2 antagonist that increases brain NE levels. Both quetiapine and norquetiapine are alpha-1 antagonists. There is no beta blockade and no SSRI effect, but some 5HT2A blockade, which may be anxiolytic. Compared with placebo, an average quetiapine dose of 258 mg/d resulted in significantly greater reductions in Clinician-Administered PTSD Scale total score, re-experiencing score, and hyperarousal score.73
Unfortunately, none of the non-SSRI options have been adequately evaluated. For now, clinicians need to continue to use SSRIs, and researchers need to continue to explore mechanism-guided alternatives.
Bottom Line
Understanding the mechanisms of the pathophysiology of posttraumatic stress disorder (PTSD) may allow clinicians to “jump ahead” of clinical studies and FDA indications. Clinicians may reasonably use alpha-1 antagonists (eg, prazosin, quetiapine) for general clinical improvement of patients with PTSD, particularly for PTSD-specific symptoms. Using antihistamines to reduce anxiety (especially in patients who have the COMT Val158Met polymorphism) may also be reasonable.
Related Resources
- North CS, Hong BA, Downs DL. PTSD: a systematic approach to diagnosis and treatment. Current Psychiatry. 2018;17(4):35-43.
- Zhang Y, Ren R, Sanford LD, et al. The effects of prazosin on sleep disturbances in post-traumatic stress disorder: a systematic review and meta-analysis. Sleep Med. 2019; 67:225-231.
Drug Brand Names
Aripiprazole • Abilify
Citalopram • Celexa
Paroxetine • Paxil
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Posttraumatic stress disorder (PTSD) occurs acutely and chronically in the aftermath of severe and potentially life-threatening trauma.1 The prevalence of PTSD varies significantly across countries and by type of trauma (Box1-7).
Box
In the general population, the prevalence of posttraumatic stress disorder (PTSD) varies from as low as 0.3% in China to as high as 6.1% in New Zealand1 and 6.8% in the United States.2 These rates are actually much lower than expected when one considers that severe trauma is experienced by 60.7% of men and 51.2% of women.3,4 Although the majority of individuals exposed to trauma experience emotional distress immediately following a traumatic event, most of them do not develop PTSD.5
It appears that the context of trauma is important: 12% to 15% of veterans experience PTSD, compared with 19% to 75% of crime victims and 80% of rape victims.1 The lifetime risk for PTSD is twice as high in women as it is in men,6 and genetic vulnerability may play a role. For example, twin studies showed that approximately 30% of the risk for PTSD may be mediated by genetic predisposition.7
Individuals who develop PTSD experience a wide range of symptoms.8 These can be categorized as PTSD-specific symptoms, or nonspecific symptoms. PTSD-specific symptoms include nightmares, flashbacks, dissociative reactions, hyperreactivity or hyperarousal, distress with reminders of trauma, and avoidance of trauma-related physical reminders and thoughts/feelings (Table8). Nonspecific symptoms include depressive and anxiety symptoms and significant problems in social, relationship, or work situations.8
While successful treatment necessitates taking all of these symptoms into account, understanding the pathophysiology of PTSD can inform a more focused and rational treatment approach. In this article, we describe some key pathophysiologic PTSD studies, and focus on PTSD-specific psychopathology to inform treatment.
Brain systems implicated in PTSD
Neuropeptide Y (NPY) is an anxiolytic endogenous peptide that has connections to the hypothalamic-pituitary-adrenal (HPA) axis. Its levels can be modulated by stress.9 Preclinical and clinical studies strongly support a potential role of NPY dysfunction in the pathophysiology of PTSD. Lower concentrations of NPY increase susceptibility to PTSD in combat veterans10 and in animal models.11 Three single-nucleotide polymorphisms (SNPs) appear to mediate this effect.12 These findings strongly support pharmaceutical targeting this system as a useful therapeutic approach.13,14 Indeed, intranasal NPY administered as a single dose reduces anxiety in animal models15 and in humans,16 but this work has not yet translated into clinical tools.
Corticotropin-releasing hormone receptor (CRHR1) gene. Corticotropin-releasing hormone has been implicated in PTSD.17 Corticotropin-releasing hormone receptors (CRHR) are important mediators in response to stress.18,19 They bind corticotropin-releasing hormone and contribute to the integration of autonomic, behavioral, and immune responses to stress.20 Single-nucleotide polymorphisms in the regulatory portion of the CRHR1 gene are associated with an increased risk for depression in adults who have a history of child abuse.21
The CRHR1 receptor antagonist GSK561679 is an investigational agent for the treatment of mood and anxiety disorders.22 In exploratory studies,23,24 GSK561679 was found to inhibit fear-potentiated startle in patients with PTSD, but not overall PTSD symptoms, although a subset of women with a specific genetic variant of the CRHR1 gene (rs110402) experienced significant benefit.25,26 This suggests that we must learn more about this system before we proceed.27
Brain-derived neurotrophic factor (BDNF). The synthesis of BDNF is influenced by neuronal activity in the brain and plays a role in synaptic transmission and plasticity.28 Brain-derived neurotrophic factor is encoded by the BDNF gene, which has been implicated in stress vulnerability.29 A common SNP in the pro-region of the human BDNF gene results in a valine-to-methionine substitution at the 66th amino acid (Val66Met). The functional Val66Met polymorphism may have a role in the risk of developing PTSD. However, not all studies support this finding. One study found that an SNP with a resulting Val66Met polymorphism is associated with adult PTSD symptoms after childhood abuse, while a meta-analysis of 7 studies did not confirm this.30,31 We need to learn more about BDNF before we proceed.32
Continue to: Serotonin transporter (5-HTT) gene
Serotonin transporter (5-HTT) gene. Serotonin transporter is a monoamine transporter protein that terminates the neurotransmitter signal by transporting serotonin from the synaptic cleft back into the presynaptic neuron. It is encoded by the SLC6A4 gene, which resides on the long arm of chromosome 17(17q11.1-q12). It is a large gene with 31 kilo bases and 14 separate exons (transcribed regions).33,34
This gene has several variants. The best-studied is a variation in the promoter region. A 44-bp insertion or deletion yields the “long” and “short” alleles, respectively. The proteins produced by the 2 alleles are identical, but the amount of expressed protein is different. The short allele (“S”) is associated with a nearly 50% reduction in 5-HTT expression in both homozygotes and heterozygotes.35 A greater incidence of serotonin transporter promoter region (5-HTTLPR) S has been found in individuals with PTSD compared with those without PTSD,36-38 and 5-HTTLPR S increases the risk of PTSD in individuals with low social support39 or after very few traumatic events.40 The short allele variant is also associated with depression in individuals who face adversity.35,41
The overrepresentation of the short form of 5-HTTLPR in individuals who develop PTSD may represent a potential problem with current treatment paradigms, in which an antidepressant is the first-line treatment, because this allele is associated with reduced response to antidepressants.42,43 More distressing is the possible association of this allele with increased suicide risk, particularly violent suicide44 or repeated suicide attempts.45
Furthermore, a functional MRI study of patients who were anxious revealed that in individuals with the short allele, administration of
Catechol-o-methyltransferase (COMT) is one of the enzymes that degrades catecholamines such as dopamine, epinephrine, and norepinephrine (NE).47 In humans, COMT protein is encoded by the COMT gene. This gene is associated with allelic variants; the best-studied of these is Val158Met. COMT Val158Met polymorphism (rs4860) has been linked to deficits in stress response and emotional resilience.48,49 Val158Met is associated with a 40% reduction in enzyme activity and slower catalysis of catecholamines, resulting in increases in catecholamines levels in the brain, which may increase the risk of developing PTSD.50 Individuals homozygous for this SNP (Met/Met) are highly susceptible to develop PTSD independently of the severity of the trauma they experienced.51 The Val158Met polymorphism may be associated with other abnormalities, such as cognitive problems with specific frontal cortical activity, and also with improved antidepressant response (valine homozygotes less responsive than methionine homozygotes).52 This gene is available on gene testing profiles.
Continue to: The role of norepinephrine in PTSD
The role of norepinephrine in PTSD
Perhaps the greatest advance in the understanding of the pathophysiology of PTSD relates to changes in brain NE. The HPA axis is responsible for coordinating the hormonal response to stress. Dysregulation of this axis and increased activity of the central and peripheral noradrenergic systems are usually observed in patients with PTSD.53 Several monoamine neurotransmitters are important in the regulation and function of the HPA axis. Norepinephrine plays a major role in stress.
The clinical PTSD-specific criteria are all descriptions of excessive noradrenergic tone.54 For example, hypervigilance and hyperstartle are clearly anticipated as evidence of NE stimulation. Flashbacks, particularly those that might be precipitated by environmental cues, also can be a manifestation of the vigilance induced by NE. Sleep disturbances (insomnia and nightmares) are present; insomnia is reported more often than nightmares.55 Increased catecholamine levels, particularly NE, are a feature of sleep disturbances associated with middle insomnia. Dreams can be remembered only if you wake up during dreaming. Catecholamines do not change the content of dreams, just recall.56
In a study of central noradrenergic tone in patients with PTSD, 6 hourly CSF samples were collected from 11 male combat veterans with PTSD and 8 healthy controls.57 Participants with PTSD had significantly higher CSF NE concentrations (0.55 ± 0.17 pmol/ml vs 0.39 ± 0.16 pmol/mL in the PTSD and control groups, respectively; F = 4.49, P < .05).57 Overall PTSD symptoms correlated significantly with CSF NE levels (r = 0.82, P <.005), and PTSD-specific symptoms such as avoidance (r = 0.79, P = .004). Intrusive thoughts (r = 0.57, P = .07) and hyperarousal (r = 0.54, P = .09) were also related.57 This relationship is unique; patients with PTSD with predominant depressive symptoms do not have elevated plasma NE levels.58
In the human brain, there are 3 main groups of NE receptors: alpha-1 receptors, alpha-2 receptors, and beta receptors.59 Alpha-1 receptors (alpha-1A, alpha-1B, and alpha-1D) are postsynaptic and mediate increase in inositol trisphosphate (IP3) and intracellular calcium (Ca2+). Alpha-2 receptors (alpha-2A, alpha-2B, alpha-2C) in the CNS are presynaptic autoreceptors and serve to reduce NE release. Beta receptors (beta-1, beta-2, beta-3) inhibit cyclic adenosine monophosphate (cAMP) production.59 The effects of inhibition of alpha or beta receptors are different. Inhibition of beta receptors is associated with depressive symptoms and depressive syndrome, inhibition of peripheral beta receptors is associated with reductions in anxiety (generally reduction of pulse, sweating, tremor),60 and inhibition of central alpha-1 receptors is associated with reduced PTSD symptoms.61
Choice of agents for PTSD-specific symptoms
As outlined in the Table,8 PTSD is characterized by 3 types of symptoms that are specific for PTSD. Trauma-focused psychotherapy62,63 and selective serotonin reuptake inhibitors (SSRIs)64 are considered first-line therapy for PTSD. Only
Continue to: Serotonin transporter promoter...
Serotonin transporter promoter region gene short-type variants, which possibly increase an individual’s predisposition to developing PTSD, may explain the abundance of depressive symptoms in this condition and the subdued response to antidepressants. Specifically, an anticipated preponderance of these alleles may be associated with poorer outcomes. Non-SSRI treatments, such as low-dose
On the other hand, animal models support antagonism of the postsynaptic alpha-1 adrenergic receptor of the CNS as a target for PTSD treatment.71 Although
Quetiapine might be another non-SSRI option for treating patients with PTSD. It is an antagonist with high affinity tothehistamine-1 receptor at low doses. Norquetiapine is an alpha-2 antagonist that increases brain NE levels. Both quetiapine and norquetiapine are alpha-1 antagonists. There is no beta blockade and no SSRI effect, but some 5HT2A blockade, which may be anxiolytic. Compared with placebo, an average quetiapine dose of 258 mg/d resulted in significantly greater reductions in Clinician-Administered PTSD Scale total score, re-experiencing score, and hyperarousal score.73
Unfortunately, none of the non-SSRI options have been adequately evaluated. For now, clinicians need to continue to use SSRIs, and researchers need to continue to explore mechanism-guided alternatives.
Bottom Line
Understanding the mechanisms of the pathophysiology of posttraumatic stress disorder (PTSD) may allow clinicians to “jump ahead” of clinical studies and FDA indications. Clinicians may reasonably use alpha-1 antagonists (eg, prazosin, quetiapine) for general clinical improvement of patients with PTSD, particularly for PTSD-specific symptoms. Using antihistamines to reduce anxiety (especially in patients who have the COMT Val158Met polymorphism) may also be reasonable.
Related Resources
- North CS, Hong BA, Downs DL. PTSD: a systematic approach to diagnosis and treatment. Current Psychiatry. 2018;17(4):35-43.
- Zhang Y, Ren R, Sanford LD, et al. The effects of prazosin on sleep disturbances in post-traumatic stress disorder: a systematic review and meta-analysis. Sleep Med. 2019; 67:225-231.
Drug Brand Names
Aripiprazole • Abilify
Citalopram • Celexa
Paroxetine • Paxil
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
1. Javidi H, Yadollahie M. Post-traumatic stress disorder. Int J Occup Environ Med. 2012;3(1):2-9.
2. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
3. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
4. Breslau N, Kessler RC, Chilcoat HD, et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626-632.
5. Cerda M, Sagdeo A, Johnson J, et al. Genetic and environmental influences on psychiatric comorbidity: a systematic review. J Affect Disord. 2010;126(1-2):14-38.
6. Yehuda R, Hoge CW, McFarlane AC, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057. doi: 10.1038/nrdp.2015.57.
7. True WR, Rice J, Eisen SA, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257-264.
8. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:271-280.
9. Reichmann F, Holzer P. Neuropeptide Y: a stressful review. Neuropeptides. 2016;55:99-109.
10. Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59(7):660-663.
11. Cohen H, Liu T, Kozlovsky N, et al. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37(2):350-363.
12. Donner J, Sipilä T, Ripatti S, et al. Support for involvement of glutamate decarboxylase 1 and neuropeptide Y in anxiety susceptibility. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(3):316-327.
13. Schmeltzer SN, Herman JP, Sah R. Neuropeptide Y (NPY) and posttraumatic stress disorder (PTSD): a translational update. Exp Neurol. 2016;284(pt B):196-210.
14. Kautz M, Charney DS, Murrough JW. Neuropeptide Y, resilience, and PTSD therapeutics. Neurosci Lett. 2017;649:164-169.
15. Serova LI, Laukova M, Alaluf LG, et al. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur Neuropsychopharmacol. 2014;24(1):142-147.
16. Sayed S, Van Dam NT, Horn SR, et al. A randomized dose-ranging study of neuropeptide Y in patients with posttraumatic stress disorder. Int J Neuropsychopharmacol. 2018;21(1):3-11.
17. Toth M, Flandreau EI, Deslauriers J, et al. Overexpression of forebrain CRH during early life increases trauma susceptibility in adulthood. Neuropsychopharmacology. 2016;41(6):1681-1690.
18. White S, Acierno R, Ruggiero KJ, et al. Association of CRHR1 variants and posttraumatic stress symptoms in hurricane exposed adults. J Anxiety Disord. 2013;27(7):678-683.
19. Wolf EJ, Mitchell KS, Logue MW, et al. Corticotropin releasing hormone receptor 2 (CRHR-2) gene is associated with decreased risk and severity of posttraumatic stress disorder in women. Depress Anxiety. 2013;30(12):1161-1169.
20. Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40(5):573-629.
21. Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190-200.
22. Tellew JE, Lanier M, Moorjani M, et al. Discovery of NBI-77860/GSK561679, a potent corticotropin-releasing factor (CRF1) receptor antagonist with improved pharmacokinetic properties. Bioorg Med Chem Lett. 2010;20(24):7259-7264.
23. Dunlop BW, Rothbaum BO, Binder EB, et al. Evaluation of a corticotropin releasing hormone type 1 receptor antagonist in women with posttraumatic stress disorder: study protocol for a randomized controlled trial. Trials. 2014;15:240. doi: 10.1186/1745-6215-15-240.
24. Jovanovic T, Duncan EJ, Kaye J, et al. Psychophysiological treatment outcomes: Corticotropin-releasing factor type 1 receptor antagonist increases inhibition of fear-potentiated startle in PTSD patients. Psychophysiology. 2019:e13356. doi: 10.1111/psyp.13356.
25. Dunlop BW, Binder EB, Iosifescu D, et al. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol Psychiatry. 2017;82(12):866-874.
26. Pape JC, Carrillo-Roa T, Rothbaum BO, et al. DNA methylation levels are associated with CRF1 receptor antagonist treatment outcome in women with post-traumatic stress disorder. Clin Epigenetics. 2018;10(1):136. doi: 10.1186/s13148-018-0569-x.
27. Murrough JW, Charney DS. Corticotropin-releasing factor type 1 receptor antagonists for stress-related disorders: time to call it quits? Biol Psychiatry. 2017;82(12):858-860.
28. Leal G, Bramham CR, Duarte CB. BDNF and hippocampal synaptic plasticity. Vitam Horm. 2017;104:153-195.
29. Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12:1079-1088.
30. Frustaci A, Pozzi G, Gianfagna F, et al. Meta-analysis of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. Neuropsychobiology. 2008;58(3-4):163-170.
31. Gatt JM, Nemeroff CB, Dobson-Stone C, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14(7):681-695.
32. Ragen BJ, Seidel J, Chollak C, et al. Investigational drugs under development for the treatment of PTSD. Expert Opin Investig Drugs. 2015;24(5):659-672.
33. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386-389.
34. Murphy DL, Fox MA, Timpano KR, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55(6):932-960.
35. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Compan J Clin Psychiatry. 2009;11:(3):93-102.
36. Lee HJ, Lee MS, Kang RH, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21(3):135-139.
37. Liu Y, Garrett ME, Dennis MF, et al. An examination of the association between 5-HTTLPR, combat exposure, and PTSD diagnosis among U.S. veterans. PLoS One. 2015;10(3):e0119998. doi: 10.1371/journal.pone.0119998.
38. Mehta D, Voisey J, Bruenig D, et al. Transcriptome analysis reveals novel genes and immune networks dysregulated in veterans with PTSD. Brain Behav Immun. 2018;74:133-142. doi: 10.1016/j.bbi.2018.08.014.
39. Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164(11):1693-1699.
40. Kolassa IT, Ertl V, Eckart C, et al. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. J Clin Psychiatry. 2010;71(5):543-547.
41. Bryant RA, Felmingham KL, Falconer EM, et al. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biol Psychiatry. 2010;67(12):1217-1219.
42. Mrazek DA, Rush AJ, Biernacka JM, et al. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):341-351.
43. Shiroma PR, Drews MS, Geske JR, et al. SLC6A4 polymorphisms and age of onset in late-life depression on treatment outcomes with citalopram: a Sequenced Treatment Alternatives to Relieve Depression (STAR*D) report. Am J Geriatr Psychiatry. 2014;22(11):1140-1148.
44. Fanelli G, Serretti A. The influence of the serotonin transporter gene 5-HTTLPR polymorphism on suicidal behaviors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:375-387.
45. Courtet P, Picot MC, Bellivier F, et al. Serotonin transporter gene may be involved in short-term risk of subsequent suicide attempts. Biol Psychiatry. 2003;55(1):46-51.
46. Outhred T, Das P, Dobson-Stone C, et al. The impact of 5-HTTLPR on acute serotonin transporter blockade by escitalopram on emotion processing: Preliminary findings from a randomised, crossover fMRI study. Aust NZ J Psychiatry. 2014;48(12):1115-1125.
47. Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243-250.
48. Valente NL, Vallada H, Cordeiro Q, et al. Catechol-O-methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. J Mol Neurosci. 2011;43(3):516-523.
49. van Rooij SJ, Stevens JS, Ely TD, et al. Childhood trauma and COMT genotype interact to increase hippocampal activation in resilient individuals. Front Psychiatry. 2016;7:156. doi: 10.3389/fpsyt.2016.00156.
50. Wu G, Feder A, Cohen H, et al. Understanding resilience. Front Behav Neuroscience. 2013;7:10. doi: 10.3389/fnbeh.2013.00010.
51. Kolassa I, Kolassa S, Ertl V, et al. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 2010;67(4):304-308.
52. Bruder GE, Keilp JG, Xu H, et al. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry. 2005;58(11):901-907.
53. Strawn JR, Geracioti TD Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
54. Hendrickson RC, Raskind MA. Noradrenergic dysregulation in the pathophysiology of PTSD. Exp Neurol. 2016;284(pt B):181-195.
55. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929-933.
56. Roehrs TA, Roth T. Hyperarousal in insomnia and hypnotic dose escalation. Sleep Med. 2016;23:16-20.
57. Geracioti TD Jr, Baker DG, Ekhator NN, et al. CSF Norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227-1230.
58. Yehuda R, Siever LJ, Teicher MH, et al. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 1998;44(1):56-63.
59. Molinoff PB. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs. 1984;28(suppl 2):1-15.
60. El-Mallakh RS. The use of beta-blockers in psychiatry. Res Staff Phys. 1989;35:49-52,59,62.
61. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
62. Bisson JI, Roberts NP, Andrew M, et al. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev. 2013;(12):CD003388.
63. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
64. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
65. Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286.
66. Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283(14):1837-1844.
67. Davidson JRT, Landerman LR, Farfel GM, et al. Characterizing the effects of sertraline in post-traumatic stress disorder. Psychol Med. 2002;32(4):661-670.
68. Hertzberg MA, Feldman ME, Beckham JC, et al. Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Ann Clin Psychiatry. 2000;12(2):101-105.
69. Friedman MJ, Marmar CR, Baker DG, et al. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68(5):711-720.
70. Mello MF, Costa MCP, Schoedl AF, et al. Aripiprazole in the treatment of posttraumatic stress disorder: an open-label trial. Rev Bras Psiquiatr. 2008;30(4):358-361.
71. Birnbaum S, Gobeske KT, Auerbach J, et al. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46(9):1266-1274.
72. Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014;69(4):235-242.
73. Villarreal G, Hamner MB, Cañive JM, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205-1212.
1. Javidi H, Yadollahie M. Post-traumatic stress disorder. Int J Occup Environ Med. 2012;3(1):2-9.
2. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
3. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
4. Breslau N, Kessler RC, Chilcoat HD, et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626-632.
5. Cerda M, Sagdeo A, Johnson J, et al. Genetic and environmental influences on psychiatric comorbidity: a systematic review. J Affect Disord. 2010;126(1-2):14-38.
6. Yehuda R, Hoge CW, McFarlane AC, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057. doi: 10.1038/nrdp.2015.57.
7. True WR, Rice J, Eisen SA, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257-264.
8. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:271-280.
9. Reichmann F, Holzer P. Neuropeptide Y: a stressful review. Neuropeptides. 2016;55:99-109.
10. Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59(7):660-663.
11. Cohen H, Liu T, Kozlovsky N, et al. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37(2):350-363.
12. Donner J, Sipilä T, Ripatti S, et al. Support for involvement of glutamate decarboxylase 1 and neuropeptide Y in anxiety susceptibility. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(3):316-327.
13. Schmeltzer SN, Herman JP, Sah R. Neuropeptide Y (NPY) and posttraumatic stress disorder (PTSD): a translational update. Exp Neurol. 2016;284(pt B):196-210.
14. Kautz M, Charney DS, Murrough JW. Neuropeptide Y, resilience, and PTSD therapeutics. Neurosci Lett. 2017;649:164-169.
15. Serova LI, Laukova M, Alaluf LG, et al. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur Neuropsychopharmacol. 2014;24(1):142-147.
16. Sayed S, Van Dam NT, Horn SR, et al. A randomized dose-ranging study of neuropeptide Y in patients with posttraumatic stress disorder. Int J Neuropsychopharmacol. 2018;21(1):3-11.
17. Toth M, Flandreau EI, Deslauriers J, et al. Overexpression of forebrain CRH during early life increases trauma susceptibility in adulthood. Neuropsychopharmacology. 2016;41(6):1681-1690.
18. White S, Acierno R, Ruggiero KJ, et al. Association of CRHR1 variants and posttraumatic stress symptoms in hurricane exposed adults. J Anxiety Disord. 2013;27(7):678-683.
19. Wolf EJ, Mitchell KS, Logue MW, et al. Corticotropin releasing hormone receptor 2 (CRHR-2) gene is associated with decreased risk and severity of posttraumatic stress disorder in women. Depress Anxiety. 2013;30(12):1161-1169.
20. Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40(5):573-629.
21. Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190-200.
22. Tellew JE, Lanier M, Moorjani M, et al. Discovery of NBI-77860/GSK561679, a potent corticotropin-releasing factor (CRF1) receptor antagonist with improved pharmacokinetic properties. Bioorg Med Chem Lett. 2010;20(24):7259-7264.
23. Dunlop BW, Rothbaum BO, Binder EB, et al. Evaluation of a corticotropin releasing hormone type 1 receptor antagonist in women with posttraumatic stress disorder: study protocol for a randomized controlled trial. Trials. 2014;15:240. doi: 10.1186/1745-6215-15-240.
24. Jovanovic T, Duncan EJ, Kaye J, et al. Psychophysiological treatment outcomes: Corticotropin-releasing factor type 1 receptor antagonist increases inhibition of fear-potentiated startle in PTSD patients. Psychophysiology. 2019:e13356. doi: 10.1111/psyp.13356.
25. Dunlop BW, Binder EB, Iosifescu D, et al. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol Psychiatry. 2017;82(12):866-874.
26. Pape JC, Carrillo-Roa T, Rothbaum BO, et al. DNA methylation levels are associated with CRF1 receptor antagonist treatment outcome in women with post-traumatic stress disorder. Clin Epigenetics. 2018;10(1):136. doi: 10.1186/s13148-018-0569-x.
27. Murrough JW, Charney DS. Corticotropin-releasing factor type 1 receptor antagonists for stress-related disorders: time to call it quits? Biol Psychiatry. 2017;82(12):858-860.
28. Leal G, Bramham CR, Duarte CB. BDNF and hippocampal synaptic plasticity. Vitam Horm. 2017;104:153-195.
29. Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12:1079-1088.
30. Frustaci A, Pozzi G, Gianfagna F, et al. Meta-analysis of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. Neuropsychobiology. 2008;58(3-4):163-170.
31. Gatt JM, Nemeroff CB, Dobson-Stone C, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14(7):681-695.
32. Ragen BJ, Seidel J, Chollak C, et al. Investigational drugs under development for the treatment of PTSD. Expert Opin Investig Drugs. 2015;24(5):659-672.
33. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386-389.
34. Murphy DL, Fox MA, Timpano KR, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55(6):932-960.
35. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Compan J Clin Psychiatry. 2009;11:(3):93-102.
36. Lee HJ, Lee MS, Kang RH, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21(3):135-139.
37. Liu Y, Garrett ME, Dennis MF, et al. An examination of the association between 5-HTTLPR, combat exposure, and PTSD diagnosis among U.S. veterans. PLoS One. 2015;10(3):e0119998. doi: 10.1371/journal.pone.0119998.
38. Mehta D, Voisey J, Bruenig D, et al. Transcriptome analysis reveals novel genes and immune networks dysregulated in veterans with PTSD. Brain Behav Immun. 2018;74:133-142. doi: 10.1016/j.bbi.2018.08.014.
39. Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164(11):1693-1699.
40. Kolassa IT, Ertl V, Eckart C, et al. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. J Clin Psychiatry. 2010;71(5):543-547.
41. Bryant RA, Felmingham KL, Falconer EM, et al. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biol Psychiatry. 2010;67(12):1217-1219.
42. Mrazek DA, Rush AJ, Biernacka JM, et al. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):341-351.
43. Shiroma PR, Drews MS, Geske JR, et al. SLC6A4 polymorphisms and age of onset in late-life depression on treatment outcomes with citalopram: a Sequenced Treatment Alternatives to Relieve Depression (STAR*D) report. Am J Geriatr Psychiatry. 2014;22(11):1140-1148.
44. Fanelli G, Serretti A. The influence of the serotonin transporter gene 5-HTTLPR polymorphism on suicidal behaviors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:375-387.
45. Courtet P, Picot MC, Bellivier F, et al. Serotonin transporter gene may be involved in short-term risk of subsequent suicide attempts. Biol Psychiatry. 2003;55(1):46-51.
46. Outhred T, Das P, Dobson-Stone C, et al. The impact of 5-HTTLPR on acute serotonin transporter blockade by escitalopram on emotion processing: Preliminary findings from a randomised, crossover fMRI study. Aust NZ J Psychiatry. 2014;48(12):1115-1125.
47. Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243-250.
48. Valente NL, Vallada H, Cordeiro Q, et al. Catechol-O-methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. J Mol Neurosci. 2011;43(3):516-523.
49. van Rooij SJ, Stevens JS, Ely TD, et al. Childhood trauma and COMT genotype interact to increase hippocampal activation in resilient individuals. Front Psychiatry. 2016;7:156. doi: 10.3389/fpsyt.2016.00156.
50. Wu G, Feder A, Cohen H, et al. Understanding resilience. Front Behav Neuroscience. 2013;7:10. doi: 10.3389/fnbeh.2013.00010.
51. Kolassa I, Kolassa S, Ertl V, et al. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 2010;67(4):304-308.
52. Bruder GE, Keilp JG, Xu H, et al. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry. 2005;58(11):901-907.
53. Strawn JR, Geracioti TD Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
54. Hendrickson RC, Raskind MA. Noradrenergic dysregulation in the pathophysiology of PTSD. Exp Neurol. 2016;284(pt B):181-195.
55. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929-933.
56. Roehrs TA, Roth T. Hyperarousal in insomnia and hypnotic dose escalation. Sleep Med. 2016;23:16-20.
57. Geracioti TD Jr, Baker DG, Ekhator NN, et al. CSF Norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227-1230.
58. Yehuda R, Siever LJ, Teicher MH, et al. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 1998;44(1):56-63.
59. Molinoff PB. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs. 1984;28(suppl 2):1-15.
60. El-Mallakh RS. The use of beta-blockers in psychiatry. Res Staff Phys. 1989;35:49-52,59,62.
61. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
62. Bisson JI, Roberts NP, Andrew M, et al. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev. 2013;(12):CD003388.
63. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
64. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
65. Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286.
66. Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283(14):1837-1844.
67. Davidson JRT, Landerman LR, Farfel GM, et al. Characterizing the effects of sertraline in post-traumatic stress disorder. Psychol Med. 2002;32(4):661-670.
68. Hertzberg MA, Feldman ME, Beckham JC, et al. Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Ann Clin Psychiatry. 2000;12(2):101-105.
69. Friedman MJ, Marmar CR, Baker DG, et al. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68(5):711-720.
70. Mello MF, Costa MCP, Schoedl AF, et al. Aripiprazole in the treatment of posttraumatic stress disorder: an open-label trial. Rev Bras Psiquiatr. 2008;30(4):358-361.
71. Birnbaum S, Gobeske KT, Auerbach J, et al. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46(9):1266-1274.
72. Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014;69(4):235-242.
73. Villarreal G, Hamner MB, Cañive JM, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205-1212.
Climate changes are leading to ‘eco-anxiety,’ trauma
It is difficult right now to contemplate issues other than battling COVID-19. However, we must not lose sight of another worldwide crisis that, unless we confront it head-on, will be with us long after the pandemic is behind us. That crisis is climate change. Increased susceptibility to pandemics is likely to be a consequence of it. Unlike pandemics, climate change poses an even more long-term and pervasive existential threat to both our mental and physical health, and our existences. Many more of us who live in Australia now fear that climate change is upon us and here to stay.
Droughts, no stranger to Australians, often are punctuated by dramatic floods, and we are now dealing with extended summer seasons filled with bushfires. We are experienced in managing them. These fires are usually limited to a few different states, so fire crews typically help one another out as they are controlled and extinguished. Australians pull together with great community spirit and resilience under these circumstances.
But the last two fire seasons have been different. They have become unseasonably long, more severe, and often uncontrollable and overwhelming. We have experienced two uncharacteristically prolonged droughts, more recently creeping across most of our continent. Last spring, wild fires took hold very early and were ubiquitous, increasing during the unusually high summer heat. Climate change already had worsened our accustomed pattern of droughts, fires, and floods.
Meanwhile, the Australian federal government repeatedly ignored advice from highly respected meteorological, environmental, scientific, and economic experts.1
Warnings from experts
The state fire commissioners had formally warned our government of increasing vulnerability via climate change to bushfires. This occurred in the context of government inaction, lack of national investment (for example, insufficient water bombing equipment), and the absence of national preparation for the predicted catastrophic fire season. Prime Minister Scott Morrison declined to meet with them, minimizing the role of climate change. He provided no extra resources, emphatically leaving the responsibility to state governments.2
Distinguished economist Ross Garnaut concluded that Australia could lead the world in renewable energy production and harness it for industries and employment, if only the government chose to invest in our ample renewable sources. Sadly, our conservative government and its corporate sponsors maintain an addiction to fossil fuels, arguing that they protect employment. Meanwhile, the economic “trickle-down” benefit from massive coal and gas exports has been illusory. Socioeconomic inequities have widened, with profits favoring the mega-rich, while mining automation takes jobs.
With the fire emergency crisis at its height, Mr. Morrison sent his energy minister to the U.N. Madrid Climate Change Conference with the goal of preventing meaningful CO2 reductions, in collaboration with Brazil, Saudi Arabia, and the United States.
The sustained drought and desiccated vegetation, the escalating fuel load growth, and early hot weather led to super-hot fires, with catapulted ember attacks and fireballs falling from the sky, which burned down thousands of homes and incinerated livestock. The fires led to numerous human fatalities and overloaded hospital burn units. The unprecedented fire season duration and uncontrollable fires exhausted voluntary fire crews. There have even been fires in cool damp rain forests – the usual refuge/reservoir of endangered flora and fauna species.
The simultaneous droughts, unusual heat, and pervasive smoke also badly affect major cities, and intense fires terrorized the entire nation. Consequently, regional firefighting teams were unable to help other regions. Huge, unquenchable fires created spiraling micro-weather systems, with thunderstorms spitting dry lightning, sparking new fires and twisters, tornadoes, and updrafts hurtling heavy fire trucks into the air, which caused terrible injuries and death to fire crews. Ultimately, the federal government had to supply large-scale sea and air evacuations, and call up military reservists for civic duties.
Mental health implications
In 2007, Australian Glenn Albrecht defined “solastalgia” as the emotional pain, existential distress, loss, and grieving derived from rapid and severe changes in one’s geophysical environment or familiar habitat.3 Studies now support its existence worldwide in communities suffering great environmental change, indicating its contribution to climate change’s psychosocial impacts.4 Mental health studies also recognize the reality of “eco-anxiety,” defined as “a chronic fear of ecological doom” for self, family, community, future generations, and our planet.5
Other climate-derived psychiatric consequences include trauma, which leads to lifelong consequences for survivors of fires; grief associated with lost lives, homes, and livelihoods; posttraumatic hyperarousal; hypervigilance, re-experiencing, and rekindling; anxiety; depression; substance misuse; and long-term cognitive impacts of poor air quality. These effects are all borne from anticipated and actual loss, uncertainty about the future, and distrust in the capacity of leadership to aid recovery or prevent future recurrences. The Australian government has announced commendable, but long overdue, funds for psychological first aid, counseling, telepsychiatry, and support for developing community cohesion and resilience for first responders, young people, and badly affected rural families and communities. However, those efforts do nothing to prevent the ongoing shift of resources away from rural community mental health services, which results in severe depletion of community mental health teams, often in the very locations and communities that are suffering most from bushfires. This forces affected communities to rely on less reliable and time-limited telehealth assessments and other online services conducted by strangers, rather than more familiar and engaging in-person services – thus betraying community expectations of continuity of care and support.
While we observe our country’s path to a fateful rendezvous with an rapidly accelerating climate emergency, we can only hope that Australia and the world beyond can awaken to its reality, immediacy, extremity, and persistence and to the compelling need for serious constructive responses. It is finally dawning on the easy-going and complacent Australian public that climate change is here to stay, fully formed, as a runaway, spiraling vicious cycle – unpredictable and uncontrolled. This is not “the new normal”: It can only get worse, unless and until the nations of the world move collaboratively beyond their denial to ensure the survival of the planet and our species.
So, rather than just exemplifying a tragic casualty of rampant climate change for the world, maybe we can transform this catastrophe into an opportunity to collectively wake us up. Only then, can Australia ultimately become a positive example of developing a full national awareness of the reality and severity of the threat. Hopefully, we Australians will then commit ourselves to a full share of the global effort needed to effectively address our climate’s dire last-ditch warnings to us all.
References
1. Easton S. “ ‘Ignored and trivialized’: Experts warned Australian government before catastrophic blazes.” NBCnews.com. 2020 Feb 9.
2. Rouse A. “Scott Morrison defends why he refused to meet former fire chiefs who warned him about horror season – as he defends his handling of bushfire crisis.” Daily Mail Australia. 2020 Jan 3.
3. Albrecht G et al. Australas Psychiatry. 2007;15 Supp1:S95-8.
4. Prescott SL et al. Int J Environ Res Public Health. 2019 Nov 5;16(21).
5. Usher K et al. Int J Ment Health Nurs. 2019. Dec;28(6):1233-4.
Dr. Rosen, an officer of the Order of Australia and a Fellow of the Royal Australian and New Zealand College of Psychiatrists, is affiliated with the Brain & Mind Centre, University of Sydney, and the Institute of Mental Health at the University of Wollongong, Australia. He also is a community psychiatrist in a remote region of New South Wales, Australia. Dr. Rosen has no conflicts of interest. In Part 2, he discusses the impact of the fires on Australia’s indigenous population.
It is difficult right now to contemplate issues other than battling COVID-19. However, we must not lose sight of another worldwide crisis that, unless we confront it head-on, will be with us long after the pandemic is behind us. That crisis is climate change. Increased susceptibility to pandemics is likely to be a consequence of it. Unlike pandemics, climate change poses an even more long-term and pervasive existential threat to both our mental and physical health, and our existences. Many more of us who live in Australia now fear that climate change is upon us and here to stay.
Droughts, no stranger to Australians, often are punctuated by dramatic floods, and we are now dealing with extended summer seasons filled with bushfires. We are experienced in managing them. These fires are usually limited to a few different states, so fire crews typically help one another out as they are controlled and extinguished. Australians pull together with great community spirit and resilience under these circumstances.
But the last two fire seasons have been different. They have become unseasonably long, more severe, and often uncontrollable and overwhelming. We have experienced two uncharacteristically prolonged droughts, more recently creeping across most of our continent. Last spring, wild fires took hold very early and were ubiquitous, increasing during the unusually high summer heat. Climate change already had worsened our accustomed pattern of droughts, fires, and floods.
Meanwhile, the Australian federal government repeatedly ignored advice from highly respected meteorological, environmental, scientific, and economic experts.1
Warnings from experts
The state fire commissioners had formally warned our government of increasing vulnerability via climate change to bushfires. This occurred in the context of government inaction, lack of national investment (for example, insufficient water bombing equipment), and the absence of national preparation for the predicted catastrophic fire season. Prime Minister Scott Morrison declined to meet with them, minimizing the role of climate change. He provided no extra resources, emphatically leaving the responsibility to state governments.2
Distinguished economist Ross Garnaut concluded that Australia could lead the world in renewable energy production and harness it for industries and employment, if only the government chose to invest in our ample renewable sources. Sadly, our conservative government and its corporate sponsors maintain an addiction to fossil fuels, arguing that they protect employment. Meanwhile, the economic “trickle-down” benefit from massive coal and gas exports has been illusory. Socioeconomic inequities have widened, with profits favoring the mega-rich, while mining automation takes jobs.
With the fire emergency crisis at its height, Mr. Morrison sent his energy minister to the U.N. Madrid Climate Change Conference with the goal of preventing meaningful CO2 reductions, in collaboration with Brazil, Saudi Arabia, and the United States.
The sustained drought and desiccated vegetation, the escalating fuel load growth, and early hot weather led to super-hot fires, with catapulted ember attacks and fireballs falling from the sky, which burned down thousands of homes and incinerated livestock. The fires led to numerous human fatalities and overloaded hospital burn units. The unprecedented fire season duration and uncontrollable fires exhausted voluntary fire crews. There have even been fires in cool damp rain forests – the usual refuge/reservoir of endangered flora and fauna species.
The simultaneous droughts, unusual heat, and pervasive smoke also badly affect major cities, and intense fires terrorized the entire nation. Consequently, regional firefighting teams were unable to help other regions. Huge, unquenchable fires created spiraling micro-weather systems, with thunderstorms spitting dry lightning, sparking new fires and twisters, tornadoes, and updrafts hurtling heavy fire trucks into the air, which caused terrible injuries and death to fire crews. Ultimately, the federal government had to supply large-scale sea and air evacuations, and call up military reservists for civic duties.
Mental health implications
In 2007, Australian Glenn Albrecht defined “solastalgia” as the emotional pain, existential distress, loss, and grieving derived from rapid and severe changes in one’s geophysical environment or familiar habitat.3 Studies now support its existence worldwide in communities suffering great environmental change, indicating its contribution to climate change’s psychosocial impacts.4 Mental health studies also recognize the reality of “eco-anxiety,” defined as “a chronic fear of ecological doom” for self, family, community, future generations, and our planet.5
Other climate-derived psychiatric consequences include trauma, which leads to lifelong consequences for survivors of fires; grief associated with lost lives, homes, and livelihoods; posttraumatic hyperarousal; hypervigilance, re-experiencing, and rekindling; anxiety; depression; substance misuse; and long-term cognitive impacts of poor air quality. These effects are all borne from anticipated and actual loss, uncertainty about the future, and distrust in the capacity of leadership to aid recovery or prevent future recurrences. The Australian government has announced commendable, but long overdue, funds for psychological first aid, counseling, telepsychiatry, and support for developing community cohesion and resilience for first responders, young people, and badly affected rural families and communities. However, those efforts do nothing to prevent the ongoing shift of resources away from rural community mental health services, which results in severe depletion of community mental health teams, often in the very locations and communities that are suffering most from bushfires. This forces affected communities to rely on less reliable and time-limited telehealth assessments and other online services conducted by strangers, rather than more familiar and engaging in-person services – thus betraying community expectations of continuity of care and support.
While we observe our country’s path to a fateful rendezvous with an rapidly accelerating climate emergency, we can only hope that Australia and the world beyond can awaken to its reality, immediacy, extremity, and persistence and to the compelling need for serious constructive responses. It is finally dawning on the easy-going and complacent Australian public that climate change is here to stay, fully formed, as a runaway, spiraling vicious cycle – unpredictable and uncontrolled. This is not “the new normal”: It can only get worse, unless and until the nations of the world move collaboratively beyond their denial to ensure the survival of the planet and our species.
So, rather than just exemplifying a tragic casualty of rampant climate change for the world, maybe we can transform this catastrophe into an opportunity to collectively wake us up. Only then, can Australia ultimately become a positive example of developing a full national awareness of the reality and severity of the threat. Hopefully, we Australians will then commit ourselves to a full share of the global effort needed to effectively address our climate’s dire last-ditch warnings to us all.
References
1. Easton S. “ ‘Ignored and trivialized’: Experts warned Australian government before catastrophic blazes.” NBCnews.com. 2020 Feb 9.
2. Rouse A. “Scott Morrison defends why he refused to meet former fire chiefs who warned him about horror season – as he defends his handling of bushfire crisis.” Daily Mail Australia. 2020 Jan 3.
3. Albrecht G et al. Australas Psychiatry. 2007;15 Supp1:S95-8.
4. Prescott SL et al. Int J Environ Res Public Health. 2019 Nov 5;16(21).
5. Usher K et al. Int J Ment Health Nurs. 2019. Dec;28(6):1233-4.
Dr. Rosen, an officer of the Order of Australia and a Fellow of the Royal Australian and New Zealand College of Psychiatrists, is affiliated with the Brain & Mind Centre, University of Sydney, and the Institute of Mental Health at the University of Wollongong, Australia. He also is a community psychiatrist in a remote region of New South Wales, Australia. Dr. Rosen has no conflicts of interest. In Part 2, he discusses the impact of the fires on Australia’s indigenous population.
It is difficult right now to contemplate issues other than battling COVID-19. However, we must not lose sight of another worldwide crisis that, unless we confront it head-on, will be with us long after the pandemic is behind us. That crisis is climate change. Increased susceptibility to pandemics is likely to be a consequence of it. Unlike pandemics, climate change poses an even more long-term and pervasive existential threat to both our mental and physical health, and our existences. Many more of us who live in Australia now fear that climate change is upon us and here to stay.
Droughts, no stranger to Australians, often are punctuated by dramatic floods, and we are now dealing with extended summer seasons filled with bushfires. We are experienced in managing them. These fires are usually limited to a few different states, so fire crews typically help one another out as they are controlled and extinguished. Australians pull together with great community spirit and resilience under these circumstances.
But the last two fire seasons have been different. They have become unseasonably long, more severe, and often uncontrollable and overwhelming. We have experienced two uncharacteristically prolonged droughts, more recently creeping across most of our continent. Last spring, wild fires took hold very early and were ubiquitous, increasing during the unusually high summer heat. Climate change already had worsened our accustomed pattern of droughts, fires, and floods.
Meanwhile, the Australian federal government repeatedly ignored advice from highly respected meteorological, environmental, scientific, and economic experts.1
Warnings from experts
The state fire commissioners had formally warned our government of increasing vulnerability via climate change to bushfires. This occurred in the context of government inaction, lack of national investment (for example, insufficient water bombing equipment), and the absence of national preparation for the predicted catastrophic fire season. Prime Minister Scott Morrison declined to meet with them, minimizing the role of climate change. He provided no extra resources, emphatically leaving the responsibility to state governments.2
Distinguished economist Ross Garnaut concluded that Australia could lead the world in renewable energy production and harness it for industries and employment, if only the government chose to invest in our ample renewable sources. Sadly, our conservative government and its corporate sponsors maintain an addiction to fossil fuels, arguing that they protect employment. Meanwhile, the economic “trickle-down” benefit from massive coal and gas exports has been illusory. Socioeconomic inequities have widened, with profits favoring the mega-rich, while mining automation takes jobs.
With the fire emergency crisis at its height, Mr. Morrison sent his energy minister to the U.N. Madrid Climate Change Conference with the goal of preventing meaningful CO2 reductions, in collaboration with Brazil, Saudi Arabia, and the United States.
The sustained drought and desiccated vegetation, the escalating fuel load growth, and early hot weather led to super-hot fires, with catapulted ember attacks and fireballs falling from the sky, which burned down thousands of homes and incinerated livestock. The fires led to numerous human fatalities and overloaded hospital burn units. The unprecedented fire season duration and uncontrollable fires exhausted voluntary fire crews. There have even been fires in cool damp rain forests – the usual refuge/reservoir of endangered flora and fauna species.
The simultaneous droughts, unusual heat, and pervasive smoke also badly affect major cities, and intense fires terrorized the entire nation. Consequently, regional firefighting teams were unable to help other regions. Huge, unquenchable fires created spiraling micro-weather systems, with thunderstorms spitting dry lightning, sparking new fires and twisters, tornadoes, and updrafts hurtling heavy fire trucks into the air, which caused terrible injuries and death to fire crews. Ultimately, the federal government had to supply large-scale sea and air evacuations, and call up military reservists for civic duties.
Mental health implications
In 2007, Australian Glenn Albrecht defined “solastalgia” as the emotional pain, existential distress, loss, and grieving derived from rapid and severe changes in one’s geophysical environment or familiar habitat.3 Studies now support its existence worldwide in communities suffering great environmental change, indicating its contribution to climate change’s psychosocial impacts.4 Mental health studies also recognize the reality of “eco-anxiety,” defined as “a chronic fear of ecological doom” for self, family, community, future generations, and our planet.5
Other climate-derived psychiatric consequences include trauma, which leads to lifelong consequences for survivors of fires; grief associated with lost lives, homes, and livelihoods; posttraumatic hyperarousal; hypervigilance, re-experiencing, and rekindling; anxiety; depression; substance misuse; and long-term cognitive impacts of poor air quality. These effects are all borne from anticipated and actual loss, uncertainty about the future, and distrust in the capacity of leadership to aid recovery or prevent future recurrences. The Australian government has announced commendable, but long overdue, funds for psychological first aid, counseling, telepsychiatry, and support for developing community cohesion and resilience for first responders, young people, and badly affected rural families and communities. However, those efforts do nothing to prevent the ongoing shift of resources away from rural community mental health services, which results in severe depletion of community mental health teams, often in the very locations and communities that are suffering most from bushfires. This forces affected communities to rely on less reliable and time-limited telehealth assessments and other online services conducted by strangers, rather than more familiar and engaging in-person services – thus betraying community expectations of continuity of care and support.
While we observe our country’s path to a fateful rendezvous with an rapidly accelerating climate emergency, we can only hope that Australia and the world beyond can awaken to its reality, immediacy, extremity, and persistence and to the compelling need for serious constructive responses. It is finally dawning on the easy-going and complacent Australian public that climate change is here to stay, fully formed, as a runaway, spiraling vicious cycle – unpredictable and uncontrolled. This is not “the new normal”: It can only get worse, unless and until the nations of the world move collaboratively beyond their denial to ensure the survival of the planet and our species.
So, rather than just exemplifying a tragic casualty of rampant climate change for the world, maybe we can transform this catastrophe into an opportunity to collectively wake us up. Only then, can Australia ultimately become a positive example of developing a full national awareness of the reality and severity of the threat. Hopefully, we Australians will then commit ourselves to a full share of the global effort needed to effectively address our climate’s dire last-ditch warnings to us all.
References
1. Easton S. “ ‘Ignored and trivialized’: Experts warned Australian government before catastrophic blazes.” NBCnews.com. 2020 Feb 9.
2. Rouse A. “Scott Morrison defends why he refused to meet former fire chiefs who warned him about horror season – as he defends his handling of bushfire crisis.” Daily Mail Australia. 2020 Jan 3.
3. Albrecht G et al. Australas Psychiatry. 2007;15 Supp1:S95-8.
4. Prescott SL et al. Int J Environ Res Public Health. 2019 Nov 5;16(21).
5. Usher K et al. Int J Ment Health Nurs. 2019. Dec;28(6):1233-4.
Dr. Rosen, an officer of the Order of Australia and a Fellow of the Royal Australian and New Zealand College of Psychiatrists, is affiliated with the Brain & Mind Centre, University of Sydney, and the Institute of Mental Health at the University of Wollongong, Australia. He also is a community psychiatrist in a remote region of New South Wales, Australia. Dr. Rosen has no conflicts of interest. In Part 2, he discusses the impact of the fires on Australia’s indigenous population.
Rapid response to PTSD therapy may predict long-term improvement
Patients who experience a rapid response to cognitive processing therapy (CPT) for posttraumatic stress disorder have a greater likelihood of sustained improvement, new research suggests.
A study of 136 veterans with PTSD showed that those who responded quickly to a 3-week CPT program were significantly more likely to report lower symptom scores 3 months post treatment, compared with those participants who responded more slowly.
The results add to previous evidence that intensified, short-term treatment programs can accomplish long-term benefits, noted the investigators, led by Jenna Bagley, Rush University Medical Center, Chicago.
“These findings show promise for the success of condensed evidence-based, trauma-focused interventions,” they added.
Ms. Bagley was scheduled to present the study in March at the Anxiety and Depression Association of America (ADAA) Conference 2020. That conference was canceled because of the coronavirus pandemic.
Reducing high dropout rates
PTSD treatments such as CPT and prolonged exposure have been shown to have high efficacy, but they also have been shown to have a nearly 40% dropout rate, the researchers note.
This problem has prompted a focus on shorter-term interventions that can deliver intensified treatment before participants drop out. However, evidence has been lacking as to the sustainability of symptom improvements that occur in a short period.
The researchers evaluated data on 136 veterans (66% men; mean age, 41 years) with PTSD who completed a non–Veterans Affairs, 3-week CPT-based intensive treatment program. Follow-up assessments were carried out at 3 months.
Symptom reduction rates represented the number of days from intake in the program to the first day a reduction was reported of at least 15 points on the PTSD Checklist for DSM-5 (PCL-5), which was indicative of a clinically meaningful improvement.
Results showed representing greater ongoing symptoms (P = .04).
The amount of time to reach the 15-point reduction also predicted symptom reductions at the 3-month follow-up, even when controlling for the total change in PCL-5 score during the program (P = .03) and when controlling for type of trauma, such as combat or military sexual trauma.
Another puzzle piece?
Commenting on the study, David C. Rozek, PhD, assistant professor at the University of Central Florida, Orlando, said the findings are encouraging, particularly in terms of improvements seen with shorter treatment programs.
“Sudden gains are important to look at in all treatments,” said Dr. Rozek, who was not involved with the research.
“Seeing that these sudden gains occur in intensive treatment and predict long-term outcome provides another piece of the puzzle and provides additional support to intensive treatments,” he added.
Dr. Rozek noted that he has also observed this with patients. However, they, along with mental health practitioners, often question whether short-term improvements will last.
“There is some concern that a rapid drop in a patient’s symptoms could be an increased risk for a rebound,” he said.
“However, that is when the skills learned in therapy can kick in and provide [patients] tools to use in their everyday life and to help continue recovering,” he added.
Dr. Rozek was scheduled to report results from a pilot study on his own experience at the canceled ADAA meeting. The study was about an even shorter, 7-day intensive CPT program (CPT-7) conducted through the National Center for Veterans Studies.
The program involved one daily individual CPT-7 session with a mental health provider in the morning followed by optional group recreational activities in the afternoon. Twelve military personnel in two cohorts with either PTSD or subthreshold PTSD, defined as having all but one symptom cluster present, were included in the study.
Keep patients engaged
Preliminary results showed reductions in PCL-5 scores from pre- to posttreatment of approximately 40% (P < .001).
“Just over 50% of patients left [the program] with symptoms below probable PTSD diagnosis on a self-report measure,” Dr. Rozek said.
Importantly, none of the participants dropped out of the treatment, but Dr. Rozek said that this was not necessarily surprising because of the nature of the program.
“These patients are brought in as a cohort and form some bonds, as they all have experienced traumatic events, although often [they have had] very different traumas,” he said.
“We’ve found that by doing daily treatment, it is more accessible and removes barriers, as it is often easier to take a week or a few weeks off at a time and participate in treatment than the logistics of weekly treatment,” he added.
Dr. Rozek said he suspects two key factors may predict treatment response in such programs – cognitive flexibility and emotion regulation.
“Patients who come into treatment and are extremely rigid in their thinking and are unable to manage their emotions may be slower to respond to treatment,” he noted.
“That being said, there are a variety of treatments that target these factors in different ways. Now we need to do the work to determine which treatments work for whom.”
The findings on longer-term durability of rapid improvement bode well for the program. “Although the work in treatment is hard, they patients really start to see the gains quickly, within a week or weeks instead of months,” Dr. Rozek said.
“This is rewarding in itself, and I would say is a strong factor for keeping patients engaged,” he concluded.
Dr. Rozek has received research funding from the National Institutes of Health, the Department of Defense, the Bob Woodruff Foundation, and the Boeing Corporation.
A version of this article originally appeared on Medscape.com.
Patients who experience a rapid response to cognitive processing therapy (CPT) for posttraumatic stress disorder have a greater likelihood of sustained improvement, new research suggests.
A study of 136 veterans with PTSD showed that those who responded quickly to a 3-week CPT program were significantly more likely to report lower symptom scores 3 months post treatment, compared with those participants who responded more slowly.
The results add to previous evidence that intensified, short-term treatment programs can accomplish long-term benefits, noted the investigators, led by Jenna Bagley, Rush University Medical Center, Chicago.
“These findings show promise for the success of condensed evidence-based, trauma-focused interventions,” they added.
Ms. Bagley was scheduled to present the study in March at the Anxiety and Depression Association of America (ADAA) Conference 2020. That conference was canceled because of the coronavirus pandemic.
Reducing high dropout rates
PTSD treatments such as CPT and prolonged exposure have been shown to have high efficacy, but they also have been shown to have a nearly 40% dropout rate, the researchers note.
This problem has prompted a focus on shorter-term interventions that can deliver intensified treatment before participants drop out. However, evidence has been lacking as to the sustainability of symptom improvements that occur in a short period.
The researchers evaluated data on 136 veterans (66% men; mean age, 41 years) with PTSD who completed a non–Veterans Affairs, 3-week CPT-based intensive treatment program. Follow-up assessments were carried out at 3 months.
Symptom reduction rates represented the number of days from intake in the program to the first day a reduction was reported of at least 15 points on the PTSD Checklist for DSM-5 (PCL-5), which was indicative of a clinically meaningful improvement.
Results showed representing greater ongoing symptoms (P = .04).
The amount of time to reach the 15-point reduction also predicted symptom reductions at the 3-month follow-up, even when controlling for the total change in PCL-5 score during the program (P = .03) and when controlling for type of trauma, such as combat or military sexual trauma.
Another puzzle piece?
Commenting on the study, David C. Rozek, PhD, assistant professor at the University of Central Florida, Orlando, said the findings are encouraging, particularly in terms of improvements seen with shorter treatment programs.
“Sudden gains are important to look at in all treatments,” said Dr. Rozek, who was not involved with the research.
“Seeing that these sudden gains occur in intensive treatment and predict long-term outcome provides another piece of the puzzle and provides additional support to intensive treatments,” he added.
Dr. Rozek noted that he has also observed this with patients. However, they, along with mental health practitioners, often question whether short-term improvements will last.
“There is some concern that a rapid drop in a patient’s symptoms could be an increased risk for a rebound,” he said.
“However, that is when the skills learned in therapy can kick in and provide [patients] tools to use in their everyday life and to help continue recovering,” he added.
Dr. Rozek was scheduled to report results from a pilot study on his own experience at the canceled ADAA meeting. The study was about an even shorter, 7-day intensive CPT program (CPT-7) conducted through the National Center for Veterans Studies.
The program involved one daily individual CPT-7 session with a mental health provider in the morning followed by optional group recreational activities in the afternoon. Twelve military personnel in two cohorts with either PTSD or subthreshold PTSD, defined as having all but one symptom cluster present, were included in the study.
Keep patients engaged
Preliminary results showed reductions in PCL-5 scores from pre- to posttreatment of approximately 40% (P < .001).
“Just over 50% of patients left [the program] with symptoms below probable PTSD diagnosis on a self-report measure,” Dr. Rozek said.
Importantly, none of the participants dropped out of the treatment, but Dr. Rozek said that this was not necessarily surprising because of the nature of the program.
“These patients are brought in as a cohort and form some bonds, as they all have experienced traumatic events, although often [they have had] very different traumas,” he said.
“We’ve found that by doing daily treatment, it is more accessible and removes barriers, as it is often easier to take a week or a few weeks off at a time and participate in treatment than the logistics of weekly treatment,” he added.
Dr. Rozek said he suspects two key factors may predict treatment response in such programs – cognitive flexibility and emotion regulation.
“Patients who come into treatment and are extremely rigid in their thinking and are unable to manage their emotions may be slower to respond to treatment,” he noted.
“That being said, there are a variety of treatments that target these factors in different ways. Now we need to do the work to determine which treatments work for whom.”
The findings on longer-term durability of rapid improvement bode well for the program. “Although the work in treatment is hard, they patients really start to see the gains quickly, within a week or weeks instead of months,” Dr. Rozek said.
“This is rewarding in itself, and I would say is a strong factor for keeping patients engaged,” he concluded.
Dr. Rozek has received research funding from the National Institutes of Health, the Department of Defense, the Bob Woodruff Foundation, and the Boeing Corporation.
A version of this article originally appeared on Medscape.com.
Patients who experience a rapid response to cognitive processing therapy (CPT) for posttraumatic stress disorder have a greater likelihood of sustained improvement, new research suggests.
A study of 136 veterans with PTSD showed that those who responded quickly to a 3-week CPT program were significantly more likely to report lower symptom scores 3 months post treatment, compared with those participants who responded more slowly.
The results add to previous evidence that intensified, short-term treatment programs can accomplish long-term benefits, noted the investigators, led by Jenna Bagley, Rush University Medical Center, Chicago.
“These findings show promise for the success of condensed evidence-based, trauma-focused interventions,” they added.
Ms. Bagley was scheduled to present the study in March at the Anxiety and Depression Association of America (ADAA) Conference 2020. That conference was canceled because of the coronavirus pandemic.
Reducing high dropout rates
PTSD treatments such as CPT and prolonged exposure have been shown to have high efficacy, but they also have been shown to have a nearly 40% dropout rate, the researchers note.
This problem has prompted a focus on shorter-term interventions that can deliver intensified treatment before participants drop out. However, evidence has been lacking as to the sustainability of symptom improvements that occur in a short period.
The researchers evaluated data on 136 veterans (66% men; mean age, 41 years) with PTSD who completed a non–Veterans Affairs, 3-week CPT-based intensive treatment program. Follow-up assessments were carried out at 3 months.
Symptom reduction rates represented the number of days from intake in the program to the first day a reduction was reported of at least 15 points on the PTSD Checklist for DSM-5 (PCL-5), which was indicative of a clinically meaningful improvement.
Results showed representing greater ongoing symptoms (P = .04).
The amount of time to reach the 15-point reduction also predicted symptom reductions at the 3-month follow-up, even when controlling for the total change in PCL-5 score during the program (P = .03) and when controlling for type of trauma, such as combat or military sexual trauma.
Another puzzle piece?
Commenting on the study, David C. Rozek, PhD, assistant professor at the University of Central Florida, Orlando, said the findings are encouraging, particularly in terms of improvements seen with shorter treatment programs.
“Sudden gains are important to look at in all treatments,” said Dr. Rozek, who was not involved with the research.
“Seeing that these sudden gains occur in intensive treatment and predict long-term outcome provides another piece of the puzzle and provides additional support to intensive treatments,” he added.
Dr. Rozek noted that he has also observed this with patients. However, they, along with mental health practitioners, often question whether short-term improvements will last.
“There is some concern that a rapid drop in a patient’s symptoms could be an increased risk for a rebound,” he said.
“However, that is when the skills learned in therapy can kick in and provide [patients] tools to use in their everyday life and to help continue recovering,” he added.
Dr. Rozek was scheduled to report results from a pilot study on his own experience at the canceled ADAA meeting. The study was about an even shorter, 7-day intensive CPT program (CPT-7) conducted through the National Center for Veterans Studies.
The program involved one daily individual CPT-7 session with a mental health provider in the morning followed by optional group recreational activities in the afternoon. Twelve military personnel in two cohorts with either PTSD or subthreshold PTSD, defined as having all but one symptom cluster present, were included in the study.
Keep patients engaged
Preliminary results showed reductions in PCL-5 scores from pre- to posttreatment of approximately 40% (P < .001).
“Just over 50% of patients left [the program] with symptoms below probable PTSD diagnosis on a self-report measure,” Dr. Rozek said.
Importantly, none of the participants dropped out of the treatment, but Dr. Rozek said that this was not necessarily surprising because of the nature of the program.
“These patients are brought in as a cohort and form some bonds, as they all have experienced traumatic events, although often [they have had] very different traumas,” he said.
“We’ve found that by doing daily treatment, it is more accessible and removes barriers, as it is often easier to take a week or a few weeks off at a time and participate in treatment than the logistics of weekly treatment,” he added.
Dr. Rozek said he suspects two key factors may predict treatment response in such programs – cognitive flexibility and emotion regulation.
“Patients who come into treatment and are extremely rigid in their thinking and are unable to manage their emotions may be slower to respond to treatment,” he noted.
“That being said, there are a variety of treatments that target these factors in different ways. Now we need to do the work to determine which treatments work for whom.”
The findings on longer-term durability of rapid improvement bode well for the program. “Although the work in treatment is hard, they patients really start to see the gains quickly, within a week or weeks instead of months,” Dr. Rozek said.
“This is rewarding in itself, and I would say is a strong factor for keeping patients engaged,” he concluded.
Dr. Rozek has received research funding from the National Institutes of Health, the Department of Defense, the Bob Woodruff Foundation, and the Boeing Corporation.
A version of this article originally appeared on Medscape.com.
Demographic Profile and Service-Connection Trends of Posttraumatic Stress Disorder and Traumatic Brain Injury in US Veterans Pre- and Post-9/11
The nature of combat and associated injuries in Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), Operation New Dawn (OND), and Afghanistan War is different from previous conflicts. Multiple protracted deployments with infrequent breaks after September 11, 2001 (9/11) have further compounded the problem.
Posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) are the signature wounds of recent wars, with a higher incidence among the veterans of OEF and OIF compared with those from previous conflicts.1,2 More than 2.7 million who served in Iraq and Afghanistan suffer from PTSD.3,4 Symptoms of PTSD may appear within the first 3 months after exposure to a traumatic event or after many months and, in some cases, after a delay of many years and continue for life.5 Although delayed onset of PTSD in the absence of prior symptoms is rare,6,7 its incidence rises with increasing frequency of exposure to traumatic events8,9 and over time.10
According to the Brain Injury Association of America, TBI is “an alteration in brain function, or other evidence of brain pathology, caused by an external force.”8 TBI is often associated with increased risk of PTSD, depression, and posttraumatic headache,11-13 which may lead to broader cognitive, somatic, neurobiological, and psychosocial dysfunctions.14-17 According to Veterans Health Administration (VHA) data, 201,435 veterans from all eras enrolled with the US Department of Veterans Affairs (VA) have a diagnosis associated with TBI and 56,695 OEF/OIF veterans have been evaluated for a TBI-related condition.2 According to the Defense and Veterans Brain Injury Center (DVBIC), > 361,000 veterans have been diagnosed with TBI, with a peak of 32,000 cases in 2011.1,18 Moreover, the reported incidence and prevalence of PTSD and TBI among US veterans are not consistent. The incidence of PTSD has been estimated at 15% to 20% in recent wars3,19 compared with 10% to 30% in previous wars.3,19,20
When PTSD or TBI is deemed “related” to military service, the veteran may receive a service-connected disability rating ranging from 0% (no life-interfering symptoms due to injury) to 100% (totally disabling injury). The percentage of service connection associated with an injury is a quantifiable measure of the debilitating effect of injury on the individual. A significant majority (94%) of those who seek mental health services and treatment at VHA clinics apply for PTSD-related disability benefits.21 The estimated cost related to PTSD/TBI service-connected pensions is $20.28 billion per year and approximately $514 billion over 50 years.22 The cost of VA and Social Security disability payments combined with health care costs and treatment of PTSD is estimated to exceed $1 trillion over the next 30 years.22
The National Vietnam Veterans Readjustment Study (NVVRS) provided valuable information on prevalence rates of PTSD and other postwar psychological problems.23 Meanwhile, there have been no recent large-scale studies to compare the demographics of veterans diagnosed with PTSD and TBI who served prior to and after 9/11. A better understanding of demographic changes is considered essential for designing and tailoring therapeutic interventions to manage the rising cost.22
The present study focused on identifying changing trends in the demographics of veterans who served prior to and after 9/11 and who received a VA inpatient or outpatient diagnosis of PTSD and/or TBI. Specifically, this study addressed the changes in demographics of veterans with PTSD, TBI, or PTSD+TBI seen at the VHA clinics between December 1,1998 and May 31, 2014 (before and after September 11, 2001) for diagnosis, treatment and health care policy issues.
Methods
This study was approved by the Kansas City VA Medical Center Institutional Review Board. VHA data from the Corporate Data Warehouse (CDW) and the National Patient Care Database were extracted using the VA Informatics and Computing Infrastructure (VINCI) workspace. CDW uses a unique identifier to identify veterans across treatment episodes at more than 1,400 VHA centers organized under 21 Veterans Integrated Service Networks (VISNs). These sources of VA data are widely used for retrospective longitudinal studies.
Study Population
The study population consisted of 1,339,937 veterans with a VA inpatient/outpatient diagnosis of PTSD or TBI using International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) codes between December 1, 1998 and May 31, 2014. Demographic (gender classification, race, ethnicity, marital status, age at date of data extraction, and date of death if indicated), service-connection disability rating, and geographic distribution within VISN data on each veteran were then extracted.
Veterans in the cohort were assigned to 1 of 4 US military services period groups. The pre-9/11 group included veterans who entered and left the military prior to September 11, 2001. This group mostly included veterans from World War II, Korean War, Vietnam War, and the first Gulf War (1990-1991). The post-9/11 group included veterans who first entered military services after September 11, 2001. The overlap group included veterans who entered military services prior to 9/11, remained in service and left after September 11, 2001. The reentered group included veterans who entered and left service prior to September 11, 2001, and then reentered military service after September 11, 2001 (Figure 1). Using ICD-9 codes, veterans also were placed into the following categories: PTSD alone (ICD-9 309.81 only), TBI alone (ICD-9 850.0-859.9, V15.52), and PTSD+TBI (any combination of ICD-9 codes from the other categories).
Statistical Analysis
Descriptive statistics were applied using proportions and means. Relationships between variables were examined using χ2 tests, t tests, analysis of variance, and nonparametric tests. All hypotheses were 2-sided at 95% CI. Results are presented as absolute numbers.
Results
PTSD only (n = 1,132,356, 85%) was the predominant diagnosis category followed by PTSD+TBI (n = 106,792, 8%) and TBI only (n = 100,789, 7%) (Figure 2). Most of the veterans in the study served pre-9/11 (77%), followed by post-9/11 (15%); 7% were in the overlap group, and 1% in the reentered group (Table 1). It is notable that the proportion of veterans diagnosed with PTSD decreased from pre-9/11 (88%) to post-9/11 (71%), overlap (77%), and reentered (74%) service periods. Increases were noted in those with PTSD+TBI diagnosis category from pre-9/11 (4%) to post-9/11 (23%), overlap (17%), and reentered (22%) service periods (Figure 3). In general, the relative distribution of diagnostic categories in all the service periods showed a similar trend, with the majority of veterans diagnosed with PTSD only. Across all service periods, significantly smaller proportions of veterans were diagnosed with TBI only (P < .001).
Distribution by Gender and Age
The cohort was 92% male (n = 1,239,295), but there was a marked increase in the percentage of nonmale veterans in post-9/11 groups. Study population ages ranged from 18 to 99 years based on date of birth to the date data were obtained; or date of birth to date of death, for those who were reported deceased at the time the data were obtained. The average (SD) ages for veterans in the pre-9/11 group were significantly older (66.3 [11.2] years) compared with the ages of veterans in the post-9/11 group (36.1 [8.7] years), the overlap group (41.4 [8.2] years), and the reentered group (46.9 [9.2] years), respectively.
Distribution by Race and Marital Status
The cohort identified as 65.7% white and 18.2% African American with much smaller percentages of Asians, American Indian/Alaska Natives (AI/AN) and Native Hawaiian/Pacific Islanders (Table 2). The relative proportion of AI/AN and Native Hawaiian/Pacific Islanders remained constant across all groups, whereas the number of Asians diagnosed with PTSD, TBI, or PTSD+TBI increased in the post-9/11 group. The number of African Americans diagnosed with PTSD, TBI, or both markedly increased in the overlap and reentered groups when compared with the pre-9/11 group, yet it went down in the post-9/11/group.
Half the cohort identified themselves as married (n = 675,145) (Table 3). A slightly larger proportion of those diagnosed with PTSD alone were married (51.7%), compared with those diagnosed with TBI only (40.3%), or PTSD+TBI (45.8%). Veterans in the post-9/11 group were less likely to identify as married (45.2%) compared with the pre-9/11 (51.2%), overlap (52.6%), or reentered (53.2%) groups. Divorce rates among pre-9/11 group, overlap group, and reentered group were higher compared with that of the post-9/11 group in all diagnosis categories.
Geographic Distribution
Veterans diagnosed with PTSD, TBI, or both were not evenly distributed across the VISNs VISNs 7, 8, 10, and 22 treated the most veterans, whereas VISN 9 and 15 treated the fewest. Taken together, the top 3 VISNs accounted for 27% to 28% of the total while lowest 3 accounted for 8% to 9% of the total cohort.
Service-Connected Disability
Of 1,339,937 veterans in the cohort, 1,067,691 had a service-connected disability rating for PTSD and/or TBI. Most were diagnosed with PTSD (n = 923,523, 86.5%) followed by both PTSD+TBI (n = 94,051, 8.8%). Three-quarters of the veterans with a service-connected disability were in the pre-9/11 group. Nearly 80% of veterans with a service-connected disability rating had a rating of > 50%. The average (SD) age of veterans with PTSD+TBI and a > 50% service-connected disability was 66.3 (11.2) years in the pre-9/11 group compared with 36.1 (8.7) years in the post-9/11 group.
Discussion
The demographic profile of veterans diagnosed with PTSD+TBI has changed across the service periods covered in this study. Compared with pre-9/11 veterans, the post-9/11 cohort: (1) higher percentage were diagnosed with PTSD+TBI; (2) higher proportion were nonmale veterans; (3) included more young veterans with > 50% service-connected disability; (4) were more racially diverse; and (5) were less likely to be married and divorced and more likely to be self-identified as single. Additionally, data revealed that veterans tended to locate more to some geographic regions than to others.
The nature of the warfare has changed remarkably over the past few decades. Gunshot wounds accounted for 65% of all injuries in World War I, 35% during Vietnam War, and 16% to 23% in the First Gulf War.24 In post-9/11 military conflicts, 81% of injuries were explosion related.24,25 Although improvements in personal protective gear and battlefield trauma care led to increased survival, several factors may have contributed to increased reporting of TBI, which peaked in 2011 at 32,000 cases.24-26
Increases in PTSD Diagnosis
Increasing media awareness, mandatory battlefield concussion screening programs instituted by the US Department of Defense (DoD), and stressful conditions that exacerbate mild TBI (mTBI) may have all contributed to the increase in numbers of veterans seeking evaluations and being diagnosed with PTSD and/or TBI in the post-9/11 groups. Additionally, the 2007 National Defense Authorization Act requested the Secretary of Defense to develop a comprehensive, systematic approach for the identification, treatment, disposition, and documentation of TBI in combat and peacetime. By a conservative estimate, significant numbers of veterans will continue to be seen for mTBI at about 20,000 new cases per year.25-27
More frequent diagnosis of mTBI may have contributed to the increase in veterans diagnosed with PTSD+TBI in the post-9/11 groups. A recent study found that almost 44% of US Army infantry soldiers in Iraq did not lose consciousness but reported symptoms consistent with TBI.14 Compared with veterans of previous wars, veterans of the post-9/11 conflicts (OIF, OED, and OND) have experienced multiple, protracted deployments with infrequent breaks that can have a cumulative effect on the development of PTSD.8-10
The findings from the NVVRS study led to creation of specialized PTSD programs in the late 1980s. Since then, there has been an explosion of knowledge and awareness about PTSD, TBI, and the associated service-connected disability ratings and benefits, leading to an increased number of veterans seeking care for PTSD. For example, media coverage of the 50th anniversary of the D-day celebrations resulted in a surge of World War II veterans seeking treatment for PTSD and a surge of Vietnam veterans sought treatment for PTSD during the wars in Iraq and Afghanistan.28 An increased number of veterans reporting PTSD symptoms prompted the DoD to increase screening for PTSD, and to encourage service members to seek treatment when appropriate.
The VA has instituted training programs for clinicians and psychologists to screen and provide care for PTSD. Beginning in 2007, the VA implemented mandatory TBI screening for all veterans who served in combat operations and separated from active-duty service after September 11, 2001. The 4-question screen identifies veterans who are at increased risk of TBI and who experience symptoms that may be related to specific event(s).29 A positive screen does not diagnose TBI but rather indicates a need for further evaluation, which may or may not be responsible for inflated reporting of TBI. Renewed research also has led providers to recognize and study PTSD resulting from noncombat trauma and moral injury. The possibility of delayed onset also drives up the number of veterans diagnosed with PTSD.5-7
Prevalence
A wide variability exists in the reported prevalence of PTSD among US war veterans with estimates ranging from 15% to 20% of veterans from recent conflicts3,20 and 10% to 30% of veterans from previous wars.3,19 These rates are higher than estimates from allied forces from other countries.19 Meta-analyses suggest that the prevalence of PTSD is 2% to 15% among Vietnam War veterans, 1% to 13% among first (pre-9/11) Gulf War veterans, 4% to 17% among OEF/OIF/OND veterans; these veterans have a lifetime prevalence of 6% to 31%.3,11,19,30-38 The prevalence of PTSD is 2 to 4 times higher among the US veterans19,39 when compared with that of civilians.40,41 According to one study, concomitant PTSD and TBI appears to be much higher in US war veterans (4%-17%) compared with United Kingdom Iraq War veterans (3%-6%).19
This study’s finding of an increase in nonmale soldiers with PTSD and/or TBI was not surprising. There is a paucity of data on the effect of war zone exposure on women veterans. Recently, women have been more actively involved in combat roles with 41,000 women deployed to a combat zone. Results of this study indicate a 2- to 3-fold increase in veterans identifying themselves as nonmale in post-9/11 groups with a higher proportion diagnosed with either PTSD alone or PTSD and TBI. Women are at a higher risk for PTSD than are men due in part to exposure to abuse/trauma prior to deployment, experience of higher rates of discrimination, and/or sexual assault.31-33 One study involving First Gulf War female veterans reported higher precombat psychiatric histories as well as higher rates of physical and sexual abuse when compared with that of men.31
In this study, the average age of veterans adjudicated and compensated for PTSD and/or TBI pre-9/11, was 66 years compared with 36 years for post-9/11 veterans. Sixty-six percent of veterans from the post-9/11 group had ≥ 50% service-connected disability at age 36 years; 75% of veterans from the overlap group had ≥ 50% service-connected disability at age 41 years; and 76% veterans from the reentered group had ≥ 50% service-connected disability at age 46 years. Younger age at diagnosis and higher rates of disability not only pose unique challenges for veterans and family members, but also suggest implications for career prospects, family earnings, loss of productivity, and disease-adjusted life years. Also noted in the results, this younger cohort has a higher percentage of single/unmarried veterans, suggesting familial support systems may be more parental than spousal. Treatment for this younger cohort will likely need to focus on early and sustained rehabilitation that can be integrated with career plans.
For treatment to be effective, there must be evidence for veterans enrolling, remaining, and reporting benefits from the treatment. Limited research has shown currently advocated evidence-based therapies to have low enrollment rates, high drop-out rates, and mixed outcomes.42
Results showing a gradual increase in the proportion of nonwhite, non-African American veterans diagnosed with PTSD alone, TBI alone, or both, likely reflect the changing demographic profile of the US as well as the Army. However, the reason that more African Americans were diagnosed with PTSD and/or TBI in the overlap and reentered groups when compared with the pre-9/11 group could not be ascertained. It is possible that more veterans identified themselves as African Americans as evident from a decrease in the number of veterans in the unknown category post-9/11 when compared with the pre-9/11 group. In 2016, the American Community Survey showed that Hispanic and African American veterans were more likely to use VA health care and other benefits than were any other racial group.40 Improved screening for PTSD and TBI diagnoses, increased awareness, and education about the availability of VA services and benefits may have contributed to the increased use of VA benefits in these groups.
Data from this study are concordant with data from the National Center for Veterans Analysis and Statistics reporting on the younger age of diagnosis and higher rates of initial service-connected disability in veterans with PTSD and PTSD+TBI.43 One study analyzing records from 1999 to 2004 showed that the number of PTSD cases grew by 79.5%, resulting in 148.7% increase in benefits payment from $1.7 billion to $4.3 billion per year.44 In contrast, the compensation cost for all other disability categories increased by only 41.7% over this period. This study also revealed that while veterans with PTSD represented only 8.7% of compensation recipients, they received 20.5% of all compensation payments, driven in large part by an increase in > 50% service-connected disability ratings.44
Thus, from financial as well as treatment points of view, the change in the demographic profile of the veteran must be considered when developing PTSD treatment strategies. While treatment in the past focused solely on addressing trauma-associated psychiatric issues, TBI and PTSD association will likely shift the focus to concurrent psychiatric and physical symptomology. Similarly, PTSD/TBI treatment modalities must consider that the profile of post-9/11 service members includes more women, younger age, and a greater racial diversity. For instance, younger age for a disabled veteran brings additional challenges, including reliance on parental or buddy support systems vs a spousal support system, integrating career with treatment, selecting geographic locations that can support both career and treatment, sustaining rehabilitation over time. The treatment needs of a 35-year-old soldier with PTSD and/or TBI, whether male or female, Asian or African American are likely to be very different from the treatment needs of a 65-year-old white male. Newer treatment approaches will have to address the needs of all soldiers.
Limitations
Our study may underestimate the actual PTSD and/or TBI disease burden because of the social stigma associated with diagnosis, military culture, limitations in data collection.45-50 In addition, in this retrospective database cohort study, we considered and tried to minimize the impact of any of the usual potential limitations, including (1) accuracy of data quality and linkage; (2) identifying cohort appropriately (study groups); (3) defining endpoints clearly to avoid misclassifications; and (4) incorporating all important confounders. We identified veterans utilizing medical services at VA hospitals during a defined period and diagnosed with PTSD and TBI using ICD-9 codes and divided in 4 well-defined groups. In addition, another limitation of our study is to not accurately capture the veterans who have alternative health coverage and may choose not to enroll and/or participate in VA health care. In addition, some service members leaving war zones may not disclose or downplay the mental health symptoms to avoid any delay in their return home.
Conclusions
This study highlights the changing profile of the soldier diagnosed with PTSD and/or TBI who served pre-9/11 compared with that of those who served post-9/11. Treatment modalities must address the changes in warfare and demographics of US service members. Future treatment will need to focus more on concurrent PTSD/TBI therapies, the needs of younger soldiers, the needs of women injured in combat, and the needs of a more racially and ethnically diverse population. Severe injuries at a younger age will require early detection and rehabilitation for return to optimum functioning over a lifetime. The current study underscores a need for identifying the gaps in ongoing programs and services, developing alternatives, and implementing improved systems of care. More studies are needed to identify the cost implications and the effectiveness of current therapies for PTSD and/or TBI.
Acknowledgments
This study was supported by VA Medical Center and Midwest BioMedical Research Foundation (MBRF), Kansas City, Missouri. The manuscript received support, in part, from NIH-RO1 DK107490. These agencies did not participate in the design/conduct of the study or, in the interpretation of the data.
1. Bagalman E. Traumatic brain injury among veterans. http://www.ncsl.org/documents/statefed/health/TBI_Vets2013.pdf. Published January 4, 2013. Accessed February 3, 2020.
2. Veterans Health Administration, Support Service Center. Workload files fiscal year 2008-fiscal year 2012. [Source not verified.]
3. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
4. Bagalman E. Health care for veterans: traumatic brain injury. https://fas.org/sgp/crs/misc/R40941.pdf. Published March 9, 2015. Accessed February 4, 2020.
5. Ikin JF, Sim MR, McKenzie DP, et al. Anxiety, post-traumatic stress disorder and depression in Korean War veterans 50 years after the war. Br J Psychiatry. 2007;190(6):475-483.
6. Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164(9):1319-1326.
7. Frueh BC, Grubaugh AL, Yeager DE, Magruder KM. Delayed-onset post-traumatic stress disorder among war veterans in primary care clinics. Br J Psychiatry. 2009;194(6):515-520.
8. McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13(3):287-300.
9. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of posttraumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
10. Friedman MJ, Resick PA, Bryant RA, Strain J, Horowitz M, Spiegel D. Classification of trauma and stressor-related disorders in DSM-5. Depress Anxiety. 2011;28(9):737-749.
11. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702.
12. Carlson K, Kehle S, Meis L, et al. The Assessment and Treatment of Individuals with History of Traumatic Brain Injury and Post-Traumatic Stress Disorder: A Systematic Review of the Evidence. Washington, DC: US Department of Veterans Affairs; 2009.
13. Gironda RJ, Clark ME, Ruff RL, et al. Traumatic brain injury, polytrauma, and pain: challenges and treatment strategies for the polytrauma rehabilitation. Rehabil Psychol. 2009;54(3):247-258.
14. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358(5):453-463.
15. Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):439-451.
16. Peskind ER, Brody D, Cernak I, McKee A, Ruff RL. Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J Clin Psychiatry. 2013;74(2):180-188.
17. Riggio S. Traumatic brain injury and its neurobehavioral sequelae. Neurol Clin. 2011;29(1):35-47, vii.
18. Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav. 2015;9(3):358-366.
19. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19.
20. Thompson WW, Gottesman II, Zalewski C. Reconciling disparate prevalence rates of PTSD in large samples of US male Vietnam veterans and their controls. BMC Psychiatry. 2006;6:19.
21. Frueh BC, Elhai JD, Gold PB, et al Disability compensation seeking among veterans evaluated for posttraumatic stress disorder. Psychiatr Serv. 2003;54(1):84-91.
22. Thakur H, Oni O, Singh V, et al. Increases in the service connection disability and treatment costs associated with posttraumatic stress disorder and/or traumatic brain injury in United States veterans pre- and post-9/11: the strong need for a novel therapeutic approach. Epidemiology (Sunnyvale). 2018;8(4):353.
23. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of post-traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
24. Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2-7.
25. Owens BD, Kragh JG Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2008;64(2):295-299.
26. Defense Health Agency, Defense and Veterans Brain Injury Center. DOD worldwide numbers for TBI since 2000. https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated February 14, 2020. Accessed February 14, 2020.
27. Armed Forces Health Surveillance Center. Deployment-related conditions of special surveillance interest, U.S. armed forces, by month and service, January 2003-December 2012 (data as of 22 January 2013). MSMR. 2013;20(1):16-19.
28. Harvey JH, Stein SK, Scott PK. Fifty years of grief: accounts and reported psychological reactions of Normandy invasion veterans. J Narrative Life History. 1995;5(4):321-332.
29. US Department of Veterans Affairs. Polytrauma/TBI system of care. https://www.polytrauma.va.gov/system-of-care/index.asp. Updated June 3, 2015. Accessed February 4, 2020.
30. Wolfe J, Erickson DJ, Sharkansky EJ, King DW, King LA. Course and predictors of posttraumatic stress disorder among Gulf War veterans: a prospective analysis. J Consult Clin Psychol. 1999;67(4):520-528.
31. Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54(1):81-87.
32. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
33. Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson J, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York: Guilford; 2004:192-238.
34. Engel CC Jr, Engel AL, Campbell SJ, McFall ME, Russo J, Katon W. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis. 1993;181(11):683-688.
35. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2016 data from the American Community Survey. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf. Published February 2018. Accessed February 4, 2020.
36. US Department of Commerce Economics and Statistics Administration, US Census Bureau, Geography Division. 2010 population distribution in the United States and Puerto Rico. https://www2.census.gov/geo/maps/dc10_thematic/2010_Nighttime_PopDist/2010_Nighttime_PopDist_Page_Map.pdf. Accessed February 4, 2020.
37. Cifu DX, Taylor BC, Carne WF, et al. Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND veterans. J Rehabil Res Dev. 2013;50(9):1169-1176.
38. Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
39. Magruder KM, Frueh BC, Knapp RG, et al. Prevalence of posttraumatic stress disorder in Veterans Affairs primary care clinics. Gen Hosp Psychiatry. 2005;27(3):169-179.
40. Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60(3):409-418.
41. Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984-991.
42. Najavits LM. The problem of dropout from “gold standard” PTSD therapies. F1000Prime Rep. 2015;7:43.
43. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Trends in veterans with a service-connected disability: 1985 to 2014. https://www.va.gov/vetdata/docs/QuickFacts/SCD_trends_FINAL_2014.PDF. Published June 2015. Accessed February 4, 2020.
44. US Department of Veterans Affairs, Office of Inspector General. Review of state variances in VA disability compensation payments. Report 05-00765-137. https://www.va.gov/oig/52/reports/2005/VAOIG-05-00765-137.pdf. Published May 19, 2015. Accessed February 4, 2020.
45. McNally RJ. Progress and controversy in the study of posttraumatic stress disorder. Annu Rev Psychol. 2003;54:229-252.
46. Freeman T, Powell M, Kimbrell T. Measuring symptom exaggeration in veterans with chronic posttraumatic stress disorder. Psychiatry Res. 2008;158(3):374-380.
47. Frueh BC, Elhai JD, Grubaugh AL, et al. Documented combat exposure of US veterans seeking treatment for combat-related post-traumatic stress disorder. Br J Psychiatry. 2005;186(6):467-475.
48. Frueh BC, Hamner MB, Cahill SP, Gold PB, Hamlin KL. Apparent symptom overreporting in combat veterans evaluated for PTSD. Clin Psychol Rev. 2000;20(7):853-885.
49. Sparr L, Pankratz LD. Factitious posttraumatic stress disorder. Am J Psychiatry. 1983;140(8):1016-1019.
50. Baggaley M. ‘Military Munchausen’s’: assessment of factitious claims of military service in psychiatric patients. Psychiatr Bull. 1998;22(3):153-154.
The nature of combat and associated injuries in Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), Operation New Dawn (OND), and Afghanistan War is different from previous conflicts. Multiple protracted deployments with infrequent breaks after September 11, 2001 (9/11) have further compounded the problem.
Posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) are the signature wounds of recent wars, with a higher incidence among the veterans of OEF and OIF compared with those from previous conflicts.1,2 More than 2.7 million who served in Iraq and Afghanistan suffer from PTSD.3,4 Symptoms of PTSD may appear within the first 3 months after exposure to a traumatic event or after many months and, in some cases, after a delay of many years and continue for life.5 Although delayed onset of PTSD in the absence of prior symptoms is rare,6,7 its incidence rises with increasing frequency of exposure to traumatic events8,9 and over time.10
According to the Brain Injury Association of America, TBI is “an alteration in brain function, or other evidence of brain pathology, caused by an external force.”8 TBI is often associated with increased risk of PTSD, depression, and posttraumatic headache,11-13 which may lead to broader cognitive, somatic, neurobiological, and psychosocial dysfunctions.14-17 According to Veterans Health Administration (VHA) data, 201,435 veterans from all eras enrolled with the US Department of Veterans Affairs (VA) have a diagnosis associated with TBI and 56,695 OEF/OIF veterans have been evaluated for a TBI-related condition.2 According to the Defense and Veterans Brain Injury Center (DVBIC), > 361,000 veterans have been diagnosed with TBI, with a peak of 32,000 cases in 2011.1,18 Moreover, the reported incidence and prevalence of PTSD and TBI among US veterans are not consistent. The incidence of PTSD has been estimated at 15% to 20% in recent wars3,19 compared with 10% to 30% in previous wars.3,19,20
When PTSD or TBI is deemed “related” to military service, the veteran may receive a service-connected disability rating ranging from 0% (no life-interfering symptoms due to injury) to 100% (totally disabling injury). The percentage of service connection associated with an injury is a quantifiable measure of the debilitating effect of injury on the individual. A significant majority (94%) of those who seek mental health services and treatment at VHA clinics apply for PTSD-related disability benefits.21 The estimated cost related to PTSD/TBI service-connected pensions is $20.28 billion per year and approximately $514 billion over 50 years.22 The cost of VA and Social Security disability payments combined with health care costs and treatment of PTSD is estimated to exceed $1 trillion over the next 30 years.22
The National Vietnam Veterans Readjustment Study (NVVRS) provided valuable information on prevalence rates of PTSD and other postwar psychological problems.23 Meanwhile, there have been no recent large-scale studies to compare the demographics of veterans diagnosed with PTSD and TBI who served prior to and after 9/11. A better understanding of demographic changes is considered essential for designing and tailoring therapeutic interventions to manage the rising cost.22
The present study focused on identifying changing trends in the demographics of veterans who served prior to and after 9/11 and who received a VA inpatient or outpatient diagnosis of PTSD and/or TBI. Specifically, this study addressed the changes in demographics of veterans with PTSD, TBI, or PTSD+TBI seen at the VHA clinics between December 1,1998 and May 31, 2014 (before and after September 11, 2001) for diagnosis, treatment and health care policy issues.
Methods
This study was approved by the Kansas City VA Medical Center Institutional Review Board. VHA data from the Corporate Data Warehouse (CDW) and the National Patient Care Database were extracted using the VA Informatics and Computing Infrastructure (VINCI) workspace. CDW uses a unique identifier to identify veterans across treatment episodes at more than 1,400 VHA centers organized under 21 Veterans Integrated Service Networks (VISNs). These sources of VA data are widely used for retrospective longitudinal studies.
Study Population
The study population consisted of 1,339,937 veterans with a VA inpatient/outpatient diagnosis of PTSD or TBI using International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) codes between December 1, 1998 and May 31, 2014. Demographic (gender classification, race, ethnicity, marital status, age at date of data extraction, and date of death if indicated), service-connection disability rating, and geographic distribution within VISN data on each veteran were then extracted.
Veterans in the cohort were assigned to 1 of 4 US military services period groups. The pre-9/11 group included veterans who entered and left the military prior to September 11, 2001. This group mostly included veterans from World War II, Korean War, Vietnam War, and the first Gulf War (1990-1991). The post-9/11 group included veterans who first entered military services after September 11, 2001. The overlap group included veterans who entered military services prior to 9/11, remained in service and left after September 11, 2001. The reentered group included veterans who entered and left service prior to September 11, 2001, and then reentered military service after September 11, 2001 (Figure 1). Using ICD-9 codes, veterans also were placed into the following categories: PTSD alone (ICD-9 309.81 only), TBI alone (ICD-9 850.0-859.9, V15.52), and PTSD+TBI (any combination of ICD-9 codes from the other categories).
Statistical Analysis
Descriptive statistics were applied using proportions and means. Relationships between variables were examined using χ2 tests, t tests, analysis of variance, and nonparametric tests. All hypotheses were 2-sided at 95% CI. Results are presented as absolute numbers.
Results
PTSD only (n = 1,132,356, 85%) was the predominant diagnosis category followed by PTSD+TBI (n = 106,792, 8%) and TBI only (n = 100,789, 7%) (Figure 2). Most of the veterans in the study served pre-9/11 (77%), followed by post-9/11 (15%); 7% were in the overlap group, and 1% in the reentered group (Table 1). It is notable that the proportion of veterans diagnosed with PTSD decreased from pre-9/11 (88%) to post-9/11 (71%), overlap (77%), and reentered (74%) service periods. Increases were noted in those with PTSD+TBI diagnosis category from pre-9/11 (4%) to post-9/11 (23%), overlap (17%), and reentered (22%) service periods (Figure 3). In general, the relative distribution of diagnostic categories in all the service periods showed a similar trend, with the majority of veterans diagnosed with PTSD only. Across all service periods, significantly smaller proportions of veterans were diagnosed with TBI only (P < .001).
Distribution by Gender and Age
The cohort was 92% male (n = 1,239,295), but there was a marked increase in the percentage of nonmale veterans in post-9/11 groups. Study population ages ranged from 18 to 99 years based on date of birth to the date data were obtained; or date of birth to date of death, for those who were reported deceased at the time the data were obtained. The average (SD) ages for veterans in the pre-9/11 group were significantly older (66.3 [11.2] years) compared with the ages of veterans in the post-9/11 group (36.1 [8.7] years), the overlap group (41.4 [8.2] years), and the reentered group (46.9 [9.2] years), respectively.
Distribution by Race and Marital Status
The cohort identified as 65.7% white and 18.2% African American with much smaller percentages of Asians, American Indian/Alaska Natives (AI/AN) and Native Hawaiian/Pacific Islanders (Table 2). The relative proportion of AI/AN and Native Hawaiian/Pacific Islanders remained constant across all groups, whereas the number of Asians diagnosed with PTSD, TBI, or PTSD+TBI increased in the post-9/11 group. The number of African Americans diagnosed with PTSD, TBI, or both markedly increased in the overlap and reentered groups when compared with the pre-9/11 group, yet it went down in the post-9/11/group.
Half the cohort identified themselves as married (n = 675,145) (Table 3). A slightly larger proportion of those diagnosed with PTSD alone were married (51.7%), compared with those diagnosed with TBI only (40.3%), or PTSD+TBI (45.8%). Veterans in the post-9/11 group were less likely to identify as married (45.2%) compared with the pre-9/11 (51.2%), overlap (52.6%), or reentered (53.2%) groups. Divorce rates among pre-9/11 group, overlap group, and reentered group were higher compared with that of the post-9/11 group in all diagnosis categories.
Geographic Distribution
Veterans diagnosed with PTSD, TBI, or both were not evenly distributed across the VISNs VISNs 7, 8, 10, and 22 treated the most veterans, whereas VISN 9 and 15 treated the fewest. Taken together, the top 3 VISNs accounted for 27% to 28% of the total while lowest 3 accounted for 8% to 9% of the total cohort.
Service-Connected Disability
Of 1,339,937 veterans in the cohort, 1,067,691 had a service-connected disability rating for PTSD and/or TBI. Most were diagnosed with PTSD (n = 923,523, 86.5%) followed by both PTSD+TBI (n = 94,051, 8.8%). Three-quarters of the veterans with a service-connected disability were in the pre-9/11 group. Nearly 80% of veterans with a service-connected disability rating had a rating of > 50%. The average (SD) age of veterans with PTSD+TBI and a > 50% service-connected disability was 66.3 (11.2) years in the pre-9/11 group compared with 36.1 (8.7) years in the post-9/11 group.
Discussion
The demographic profile of veterans diagnosed with PTSD+TBI has changed across the service periods covered in this study. Compared with pre-9/11 veterans, the post-9/11 cohort: (1) higher percentage were diagnosed with PTSD+TBI; (2) higher proportion were nonmale veterans; (3) included more young veterans with > 50% service-connected disability; (4) were more racially diverse; and (5) were less likely to be married and divorced and more likely to be self-identified as single. Additionally, data revealed that veterans tended to locate more to some geographic regions than to others.
The nature of the warfare has changed remarkably over the past few decades. Gunshot wounds accounted for 65% of all injuries in World War I, 35% during Vietnam War, and 16% to 23% in the First Gulf War.24 In post-9/11 military conflicts, 81% of injuries were explosion related.24,25 Although improvements in personal protective gear and battlefield trauma care led to increased survival, several factors may have contributed to increased reporting of TBI, which peaked in 2011 at 32,000 cases.24-26
Increases in PTSD Diagnosis
Increasing media awareness, mandatory battlefield concussion screening programs instituted by the US Department of Defense (DoD), and stressful conditions that exacerbate mild TBI (mTBI) may have all contributed to the increase in numbers of veterans seeking evaluations and being diagnosed with PTSD and/or TBI in the post-9/11 groups. Additionally, the 2007 National Defense Authorization Act requested the Secretary of Defense to develop a comprehensive, systematic approach for the identification, treatment, disposition, and documentation of TBI in combat and peacetime. By a conservative estimate, significant numbers of veterans will continue to be seen for mTBI at about 20,000 new cases per year.25-27
More frequent diagnosis of mTBI may have contributed to the increase in veterans diagnosed with PTSD+TBI in the post-9/11 groups. A recent study found that almost 44% of US Army infantry soldiers in Iraq did not lose consciousness but reported symptoms consistent with TBI.14 Compared with veterans of previous wars, veterans of the post-9/11 conflicts (OIF, OED, and OND) have experienced multiple, protracted deployments with infrequent breaks that can have a cumulative effect on the development of PTSD.8-10
The findings from the NVVRS study led to creation of specialized PTSD programs in the late 1980s. Since then, there has been an explosion of knowledge and awareness about PTSD, TBI, and the associated service-connected disability ratings and benefits, leading to an increased number of veterans seeking care for PTSD. For example, media coverage of the 50th anniversary of the D-day celebrations resulted in a surge of World War II veterans seeking treatment for PTSD and a surge of Vietnam veterans sought treatment for PTSD during the wars in Iraq and Afghanistan.28 An increased number of veterans reporting PTSD symptoms prompted the DoD to increase screening for PTSD, and to encourage service members to seek treatment when appropriate.
The VA has instituted training programs for clinicians and psychologists to screen and provide care for PTSD. Beginning in 2007, the VA implemented mandatory TBI screening for all veterans who served in combat operations and separated from active-duty service after September 11, 2001. The 4-question screen identifies veterans who are at increased risk of TBI and who experience symptoms that may be related to specific event(s).29 A positive screen does not diagnose TBI but rather indicates a need for further evaluation, which may or may not be responsible for inflated reporting of TBI. Renewed research also has led providers to recognize and study PTSD resulting from noncombat trauma and moral injury. The possibility of delayed onset also drives up the number of veterans diagnosed with PTSD.5-7
Prevalence
A wide variability exists in the reported prevalence of PTSD among US war veterans with estimates ranging from 15% to 20% of veterans from recent conflicts3,20 and 10% to 30% of veterans from previous wars.3,19 These rates are higher than estimates from allied forces from other countries.19 Meta-analyses suggest that the prevalence of PTSD is 2% to 15% among Vietnam War veterans, 1% to 13% among first (pre-9/11) Gulf War veterans, 4% to 17% among OEF/OIF/OND veterans; these veterans have a lifetime prevalence of 6% to 31%.3,11,19,30-38 The prevalence of PTSD is 2 to 4 times higher among the US veterans19,39 when compared with that of civilians.40,41 According to one study, concomitant PTSD and TBI appears to be much higher in US war veterans (4%-17%) compared with United Kingdom Iraq War veterans (3%-6%).19
This study’s finding of an increase in nonmale soldiers with PTSD and/or TBI was not surprising. There is a paucity of data on the effect of war zone exposure on women veterans. Recently, women have been more actively involved in combat roles with 41,000 women deployed to a combat zone. Results of this study indicate a 2- to 3-fold increase in veterans identifying themselves as nonmale in post-9/11 groups with a higher proportion diagnosed with either PTSD alone or PTSD and TBI. Women are at a higher risk for PTSD than are men due in part to exposure to abuse/trauma prior to deployment, experience of higher rates of discrimination, and/or sexual assault.31-33 One study involving First Gulf War female veterans reported higher precombat psychiatric histories as well as higher rates of physical and sexual abuse when compared with that of men.31
In this study, the average age of veterans adjudicated and compensated for PTSD and/or TBI pre-9/11, was 66 years compared with 36 years for post-9/11 veterans. Sixty-six percent of veterans from the post-9/11 group had ≥ 50% service-connected disability at age 36 years; 75% of veterans from the overlap group had ≥ 50% service-connected disability at age 41 years; and 76% veterans from the reentered group had ≥ 50% service-connected disability at age 46 years. Younger age at diagnosis and higher rates of disability not only pose unique challenges for veterans and family members, but also suggest implications for career prospects, family earnings, loss of productivity, and disease-adjusted life years. Also noted in the results, this younger cohort has a higher percentage of single/unmarried veterans, suggesting familial support systems may be more parental than spousal. Treatment for this younger cohort will likely need to focus on early and sustained rehabilitation that can be integrated with career plans.
For treatment to be effective, there must be evidence for veterans enrolling, remaining, and reporting benefits from the treatment. Limited research has shown currently advocated evidence-based therapies to have low enrollment rates, high drop-out rates, and mixed outcomes.42
Results showing a gradual increase in the proportion of nonwhite, non-African American veterans diagnosed with PTSD alone, TBI alone, or both, likely reflect the changing demographic profile of the US as well as the Army. However, the reason that more African Americans were diagnosed with PTSD and/or TBI in the overlap and reentered groups when compared with the pre-9/11 group could not be ascertained. It is possible that more veterans identified themselves as African Americans as evident from a decrease in the number of veterans in the unknown category post-9/11 when compared with the pre-9/11 group. In 2016, the American Community Survey showed that Hispanic and African American veterans were more likely to use VA health care and other benefits than were any other racial group.40 Improved screening for PTSD and TBI diagnoses, increased awareness, and education about the availability of VA services and benefits may have contributed to the increased use of VA benefits in these groups.
Data from this study are concordant with data from the National Center for Veterans Analysis and Statistics reporting on the younger age of diagnosis and higher rates of initial service-connected disability in veterans with PTSD and PTSD+TBI.43 One study analyzing records from 1999 to 2004 showed that the number of PTSD cases grew by 79.5%, resulting in 148.7% increase in benefits payment from $1.7 billion to $4.3 billion per year.44 In contrast, the compensation cost for all other disability categories increased by only 41.7% over this period. This study also revealed that while veterans with PTSD represented only 8.7% of compensation recipients, they received 20.5% of all compensation payments, driven in large part by an increase in > 50% service-connected disability ratings.44
Thus, from financial as well as treatment points of view, the change in the demographic profile of the veteran must be considered when developing PTSD treatment strategies. While treatment in the past focused solely on addressing trauma-associated psychiatric issues, TBI and PTSD association will likely shift the focus to concurrent psychiatric and physical symptomology. Similarly, PTSD/TBI treatment modalities must consider that the profile of post-9/11 service members includes more women, younger age, and a greater racial diversity. For instance, younger age for a disabled veteran brings additional challenges, including reliance on parental or buddy support systems vs a spousal support system, integrating career with treatment, selecting geographic locations that can support both career and treatment, sustaining rehabilitation over time. The treatment needs of a 35-year-old soldier with PTSD and/or TBI, whether male or female, Asian or African American are likely to be very different from the treatment needs of a 65-year-old white male. Newer treatment approaches will have to address the needs of all soldiers.
Limitations
Our study may underestimate the actual PTSD and/or TBI disease burden because of the social stigma associated with diagnosis, military culture, limitations in data collection.45-50 In addition, in this retrospective database cohort study, we considered and tried to minimize the impact of any of the usual potential limitations, including (1) accuracy of data quality and linkage; (2) identifying cohort appropriately (study groups); (3) defining endpoints clearly to avoid misclassifications; and (4) incorporating all important confounders. We identified veterans utilizing medical services at VA hospitals during a defined period and diagnosed with PTSD and TBI using ICD-9 codes and divided in 4 well-defined groups. In addition, another limitation of our study is to not accurately capture the veterans who have alternative health coverage and may choose not to enroll and/or participate in VA health care. In addition, some service members leaving war zones may not disclose or downplay the mental health symptoms to avoid any delay in their return home.
Conclusions
This study highlights the changing profile of the soldier diagnosed with PTSD and/or TBI who served pre-9/11 compared with that of those who served post-9/11. Treatment modalities must address the changes in warfare and demographics of US service members. Future treatment will need to focus more on concurrent PTSD/TBI therapies, the needs of younger soldiers, the needs of women injured in combat, and the needs of a more racially and ethnically diverse population. Severe injuries at a younger age will require early detection and rehabilitation for return to optimum functioning over a lifetime. The current study underscores a need for identifying the gaps in ongoing programs and services, developing alternatives, and implementing improved systems of care. More studies are needed to identify the cost implications and the effectiveness of current therapies for PTSD and/or TBI.
Acknowledgments
This study was supported by VA Medical Center and Midwest BioMedical Research Foundation (MBRF), Kansas City, Missouri. The manuscript received support, in part, from NIH-RO1 DK107490. These agencies did not participate in the design/conduct of the study or, in the interpretation of the data.
The nature of combat and associated injuries in Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), Operation New Dawn (OND), and Afghanistan War is different from previous conflicts. Multiple protracted deployments with infrequent breaks after September 11, 2001 (9/11) have further compounded the problem.
Posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) are the signature wounds of recent wars, with a higher incidence among the veterans of OEF and OIF compared with those from previous conflicts.1,2 More than 2.7 million who served in Iraq and Afghanistan suffer from PTSD.3,4 Symptoms of PTSD may appear within the first 3 months after exposure to a traumatic event or after many months and, in some cases, after a delay of many years and continue for life.5 Although delayed onset of PTSD in the absence of prior symptoms is rare,6,7 its incidence rises with increasing frequency of exposure to traumatic events8,9 and over time.10
According to the Brain Injury Association of America, TBI is “an alteration in brain function, or other evidence of brain pathology, caused by an external force.”8 TBI is often associated with increased risk of PTSD, depression, and posttraumatic headache,11-13 which may lead to broader cognitive, somatic, neurobiological, and psychosocial dysfunctions.14-17 According to Veterans Health Administration (VHA) data, 201,435 veterans from all eras enrolled with the US Department of Veterans Affairs (VA) have a diagnosis associated with TBI and 56,695 OEF/OIF veterans have been evaluated for a TBI-related condition.2 According to the Defense and Veterans Brain Injury Center (DVBIC), > 361,000 veterans have been diagnosed with TBI, with a peak of 32,000 cases in 2011.1,18 Moreover, the reported incidence and prevalence of PTSD and TBI among US veterans are not consistent. The incidence of PTSD has been estimated at 15% to 20% in recent wars3,19 compared with 10% to 30% in previous wars.3,19,20
When PTSD or TBI is deemed “related” to military service, the veteran may receive a service-connected disability rating ranging from 0% (no life-interfering symptoms due to injury) to 100% (totally disabling injury). The percentage of service connection associated with an injury is a quantifiable measure of the debilitating effect of injury on the individual. A significant majority (94%) of those who seek mental health services and treatment at VHA clinics apply for PTSD-related disability benefits.21 The estimated cost related to PTSD/TBI service-connected pensions is $20.28 billion per year and approximately $514 billion over 50 years.22 The cost of VA and Social Security disability payments combined with health care costs and treatment of PTSD is estimated to exceed $1 trillion over the next 30 years.22
The National Vietnam Veterans Readjustment Study (NVVRS) provided valuable information on prevalence rates of PTSD and other postwar psychological problems.23 Meanwhile, there have been no recent large-scale studies to compare the demographics of veterans diagnosed with PTSD and TBI who served prior to and after 9/11. A better understanding of demographic changes is considered essential for designing and tailoring therapeutic interventions to manage the rising cost.22
The present study focused on identifying changing trends in the demographics of veterans who served prior to and after 9/11 and who received a VA inpatient or outpatient diagnosis of PTSD and/or TBI. Specifically, this study addressed the changes in demographics of veterans with PTSD, TBI, or PTSD+TBI seen at the VHA clinics between December 1,1998 and May 31, 2014 (before and after September 11, 2001) for diagnosis, treatment and health care policy issues.
Methods
This study was approved by the Kansas City VA Medical Center Institutional Review Board. VHA data from the Corporate Data Warehouse (CDW) and the National Patient Care Database were extracted using the VA Informatics and Computing Infrastructure (VINCI) workspace. CDW uses a unique identifier to identify veterans across treatment episodes at more than 1,400 VHA centers organized under 21 Veterans Integrated Service Networks (VISNs). These sources of VA data are widely used for retrospective longitudinal studies.
Study Population
The study population consisted of 1,339,937 veterans with a VA inpatient/outpatient diagnosis of PTSD or TBI using International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) codes between December 1, 1998 and May 31, 2014. Demographic (gender classification, race, ethnicity, marital status, age at date of data extraction, and date of death if indicated), service-connection disability rating, and geographic distribution within VISN data on each veteran were then extracted.
Veterans in the cohort were assigned to 1 of 4 US military services period groups. The pre-9/11 group included veterans who entered and left the military prior to September 11, 2001. This group mostly included veterans from World War II, Korean War, Vietnam War, and the first Gulf War (1990-1991). The post-9/11 group included veterans who first entered military services after September 11, 2001. The overlap group included veterans who entered military services prior to 9/11, remained in service and left after September 11, 2001. The reentered group included veterans who entered and left service prior to September 11, 2001, and then reentered military service after September 11, 2001 (Figure 1). Using ICD-9 codes, veterans also were placed into the following categories: PTSD alone (ICD-9 309.81 only), TBI alone (ICD-9 850.0-859.9, V15.52), and PTSD+TBI (any combination of ICD-9 codes from the other categories).
Statistical Analysis
Descriptive statistics were applied using proportions and means. Relationships between variables were examined using χ2 tests, t tests, analysis of variance, and nonparametric tests. All hypotheses were 2-sided at 95% CI. Results are presented as absolute numbers.
Results
PTSD only (n = 1,132,356, 85%) was the predominant diagnosis category followed by PTSD+TBI (n = 106,792, 8%) and TBI only (n = 100,789, 7%) (Figure 2). Most of the veterans in the study served pre-9/11 (77%), followed by post-9/11 (15%); 7% were in the overlap group, and 1% in the reentered group (Table 1). It is notable that the proportion of veterans diagnosed with PTSD decreased from pre-9/11 (88%) to post-9/11 (71%), overlap (77%), and reentered (74%) service periods. Increases were noted in those with PTSD+TBI diagnosis category from pre-9/11 (4%) to post-9/11 (23%), overlap (17%), and reentered (22%) service periods (Figure 3). In general, the relative distribution of diagnostic categories in all the service periods showed a similar trend, with the majority of veterans diagnosed with PTSD only. Across all service periods, significantly smaller proportions of veterans were diagnosed with TBI only (P < .001).
Distribution by Gender and Age
The cohort was 92% male (n = 1,239,295), but there was a marked increase in the percentage of nonmale veterans in post-9/11 groups. Study population ages ranged from 18 to 99 years based on date of birth to the date data were obtained; or date of birth to date of death, for those who were reported deceased at the time the data were obtained. The average (SD) ages for veterans in the pre-9/11 group were significantly older (66.3 [11.2] years) compared with the ages of veterans in the post-9/11 group (36.1 [8.7] years), the overlap group (41.4 [8.2] years), and the reentered group (46.9 [9.2] years), respectively.
Distribution by Race and Marital Status
The cohort identified as 65.7% white and 18.2% African American with much smaller percentages of Asians, American Indian/Alaska Natives (AI/AN) and Native Hawaiian/Pacific Islanders (Table 2). The relative proportion of AI/AN and Native Hawaiian/Pacific Islanders remained constant across all groups, whereas the number of Asians diagnosed with PTSD, TBI, or PTSD+TBI increased in the post-9/11 group. The number of African Americans diagnosed with PTSD, TBI, or both markedly increased in the overlap and reentered groups when compared with the pre-9/11 group, yet it went down in the post-9/11/group.
Half the cohort identified themselves as married (n = 675,145) (Table 3). A slightly larger proportion of those diagnosed with PTSD alone were married (51.7%), compared with those diagnosed with TBI only (40.3%), or PTSD+TBI (45.8%). Veterans in the post-9/11 group were less likely to identify as married (45.2%) compared with the pre-9/11 (51.2%), overlap (52.6%), or reentered (53.2%) groups. Divorce rates among pre-9/11 group, overlap group, and reentered group were higher compared with that of the post-9/11 group in all diagnosis categories.
Geographic Distribution
Veterans diagnosed with PTSD, TBI, or both were not evenly distributed across the VISNs VISNs 7, 8, 10, and 22 treated the most veterans, whereas VISN 9 and 15 treated the fewest. Taken together, the top 3 VISNs accounted for 27% to 28% of the total while lowest 3 accounted for 8% to 9% of the total cohort.
Service-Connected Disability
Of 1,339,937 veterans in the cohort, 1,067,691 had a service-connected disability rating for PTSD and/or TBI. Most were diagnosed with PTSD (n = 923,523, 86.5%) followed by both PTSD+TBI (n = 94,051, 8.8%). Three-quarters of the veterans with a service-connected disability were in the pre-9/11 group. Nearly 80% of veterans with a service-connected disability rating had a rating of > 50%. The average (SD) age of veterans with PTSD+TBI and a > 50% service-connected disability was 66.3 (11.2) years in the pre-9/11 group compared with 36.1 (8.7) years in the post-9/11 group.
Discussion
The demographic profile of veterans diagnosed with PTSD+TBI has changed across the service periods covered in this study. Compared with pre-9/11 veterans, the post-9/11 cohort: (1) higher percentage were diagnosed with PTSD+TBI; (2) higher proportion were nonmale veterans; (3) included more young veterans with > 50% service-connected disability; (4) were more racially diverse; and (5) were less likely to be married and divorced and more likely to be self-identified as single. Additionally, data revealed that veterans tended to locate more to some geographic regions than to others.
The nature of the warfare has changed remarkably over the past few decades. Gunshot wounds accounted for 65% of all injuries in World War I, 35% during Vietnam War, and 16% to 23% in the First Gulf War.24 In post-9/11 military conflicts, 81% of injuries were explosion related.24,25 Although improvements in personal protective gear and battlefield trauma care led to increased survival, several factors may have contributed to increased reporting of TBI, which peaked in 2011 at 32,000 cases.24-26
Increases in PTSD Diagnosis
Increasing media awareness, mandatory battlefield concussion screening programs instituted by the US Department of Defense (DoD), and stressful conditions that exacerbate mild TBI (mTBI) may have all contributed to the increase in numbers of veterans seeking evaluations and being diagnosed with PTSD and/or TBI in the post-9/11 groups. Additionally, the 2007 National Defense Authorization Act requested the Secretary of Defense to develop a comprehensive, systematic approach for the identification, treatment, disposition, and documentation of TBI in combat and peacetime. By a conservative estimate, significant numbers of veterans will continue to be seen for mTBI at about 20,000 new cases per year.25-27
More frequent diagnosis of mTBI may have contributed to the increase in veterans diagnosed with PTSD+TBI in the post-9/11 groups. A recent study found that almost 44% of US Army infantry soldiers in Iraq did not lose consciousness but reported symptoms consistent with TBI.14 Compared with veterans of previous wars, veterans of the post-9/11 conflicts (OIF, OED, and OND) have experienced multiple, protracted deployments with infrequent breaks that can have a cumulative effect on the development of PTSD.8-10
The findings from the NVVRS study led to creation of specialized PTSD programs in the late 1980s. Since then, there has been an explosion of knowledge and awareness about PTSD, TBI, and the associated service-connected disability ratings and benefits, leading to an increased number of veterans seeking care for PTSD. For example, media coverage of the 50th anniversary of the D-day celebrations resulted in a surge of World War II veterans seeking treatment for PTSD and a surge of Vietnam veterans sought treatment for PTSD during the wars in Iraq and Afghanistan.28 An increased number of veterans reporting PTSD symptoms prompted the DoD to increase screening for PTSD, and to encourage service members to seek treatment when appropriate.
The VA has instituted training programs for clinicians and psychologists to screen and provide care for PTSD. Beginning in 2007, the VA implemented mandatory TBI screening for all veterans who served in combat operations and separated from active-duty service after September 11, 2001. The 4-question screen identifies veterans who are at increased risk of TBI and who experience symptoms that may be related to specific event(s).29 A positive screen does not diagnose TBI but rather indicates a need for further evaluation, which may or may not be responsible for inflated reporting of TBI. Renewed research also has led providers to recognize and study PTSD resulting from noncombat trauma and moral injury. The possibility of delayed onset also drives up the number of veterans diagnosed with PTSD.5-7
Prevalence
A wide variability exists in the reported prevalence of PTSD among US war veterans with estimates ranging from 15% to 20% of veterans from recent conflicts3,20 and 10% to 30% of veterans from previous wars.3,19 These rates are higher than estimates from allied forces from other countries.19 Meta-analyses suggest that the prevalence of PTSD is 2% to 15% among Vietnam War veterans, 1% to 13% among first (pre-9/11) Gulf War veterans, 4% to 17% among OEF/OIF/OND veterans; these veterans have a lifetime prevalence of 6% to 31%.3,11,19,30-38 The prevalence of PTSD is 2 to 4 times higher among the US veterans19,39 when compared with that of civilians.40,41 According to one study, concomitant PTSD and TBI appears to be much higher in US war veterans (4%-17%) compared with United Kingdom Iraq War veterans (3%-6%).19
This study’s finding of an increase in nonmale soldiers with PTSD and/or TBI was not surprising. There is a paucity of data on the effect of war zone exposure on women veterans. Recently, women have been more actively involved in combat roles with 41,000 women deployed to a combat zone. Results of this study indicate a 2- to 3-fold increase in veterans identifying themselves as nonmale in post-9/11 groups with a higher proportion diagnosed with either PTSD alone or PTSD and TBI. Women are at a higher risk for PTSD than are men due in part to exposure to abuse/trauma prior to deployment, experience of higher rates of discrimination, and/or sexual assault.31-33 One study involving First Gulf War female veterans reported higher precombat psychiatric histories as well as higher rates of physical and sexual abuse when compared with that of men.31
In this study, the average age of veterans adjudicated and compensated for PTSD and/or TBI pre-9/11, was 66 years compared with 36 years for post-9/11 veterans. Sixty-six percent of veterans from the post-9/11 group had ≥ 50% service-connected disability at age 36 years; 75% of veterans from the overlap group had ≥ 50% service-connected disability at age 41 years; and 76% veterans from the reentered group had ≥ 50% service-connected disability at age 46 years. Younger age at diagnosis and higher rates of disability not only pose unique challenges for veterans and family members, but also suggest implications for career prospects, family earnings, loss of productivity, and disease-adjusted life years. Also noted in the results, this younger cohort has a higher percentage of single/unmarried veterans, suggesting familial support systems may be more parental than spousal. Treatment for this younger cohort will likely need to focus on early and sustained rehabilitation that can be integrated with career plans.
For treatment to be effective, there must be evidence for veterans enrolling, remaining, and reporting benefits from the treatment. Limited research has shown currently advocated evidence-based therapies to have low enrollment rates, high drop-out rates, and mixed outcomes.42
Results showing a gradual increase in the proportion of nonwhite, non-African American veterans diagnosed with PTSD alone, TBI alone, or both, likely reflect the changing demographic profile of the US as well as the Army. However, the reason that more African Americans were diagnosed with PTSD and/or TBI in the overlap and reentered groups when compared with the pre-9/11 group could not be ascertained. It is possible that more veterans identified themselves as African Americans as evident from a decrease in the number of veterans in the unknown category post-9/11 when compared with the pre-9/11 group. In 2016, the American Community Survey showed that Hispanic and African American veterans were more likely to use VA health care and other benefits than were any other racial group.40 Improved screening for PTSD and TBI diagnoses, increased awareness, and education about the availability of VA services and benefits may have contributed to the increased use of VA benefits in these groups.
Data from this study are concordant with data from the National Center for Veterans Analysis and Statistics reporting on the younger age of diagnosis and higher rates of initial service-connected disability in veterans with PTSD and PTSD+TBI.43 One study analyzing records from 1999 to 2004 showed that the number of PTSD cases grew by 79.5%, resulting in 148.7% increase in benefits payment from $1.7 billion to $4.3 billion per year.44 In contrast, the compensation cost for all other disability categories increased by only 41.7% over this period. This study also revealed that while veterans with PTSD represented only 8.7% of compensation recipients, they received 20.5% of all compensation payments, driven in large part by an increase in > 50% service-connected disability ratings.44
Thus, from financial as well as treatment points of view, the change in the demographic profile of the veteran must be considered when developing PTSD treatment strategies. While treatment in the past focused solely on addressing trauma-associated psychiatric issues, TBI and PTSD association will likely shift the focus to concurrent psychiatric and physical symptomology. Similarly, PTSD/TBI treatment modalities must consider that the profile of post-9/11 service members includes more women, younger age, and a greater racial diversity. For instance, younger age for a disabled veteran brings additional challenges, including reliance on parental or buddy support systems vs a spousal support system, integrating career with treatment, selecting geographic locations that can support both career and treatment, sustaining rehabilitation over time. The treatment needs of a 35-year-old soldier with PTSD and/or TBI, whether male or female, Asian or African American are likely to be very different from the treatment needs of a 65-year-old white male. Newer treatment approaches will have to address the needs of all soldiers.
Limitations
Our study may underestimate the actual PTSD and/or TBI disease burden because of the social stigma associated with diagnosis, military culture, limitations in data collection.45-50 In addition, in this retrospective database cohort study, we considered and tried to minimize the impact of any of the usual potential limitations, including (1) accuracy of data quality and linkage; (2) identifying cohort appropriately (study groups); (3) defining endpoints clearly to avoid misclassifications; and (4) incorporating all important confounders. We identified veterans utilizing medical services at VA hospitals during a defined period and diagnosed with PTSD and TBI using ICD-9 codes and divided in 4 well-defined groups. In addition, another limitation of our study is to not accurately capture the veterans who have alternative health coverage and may choose not to enroll and/or participate in VA health care. In addition, some service members leaving war zones may not disclose or downplay the mental health symptoms to avoid any delay in their return home.
Conclusions
This study highlights the changing profile of the soldier diagnosed with PTSD and/or TBI who served pre-9/11 compared with that of those who served post-9/11. Treatment modalities must address the changes in warfare and demographics of US service members. Future treatment will need to focus more on concurrent PTSD/TBI therapies, the needs of younger soldiers, the needs of women injured in combat, and the needs of a more racially and ethnically diverse population. Severe injuries at a younger age will require early detection and rehabilitation for return to optimum functioning over a lifetime. The current study underscores a need for identifying the gaps in ongoing programs and services, developing alternatives, and implementing improved systems of care. More studies are needed to identify the cost implications and the effectiveness of current therapies for PTSD and/or TBI.
Acknowledgments
This study was supported by VA Medical Center and Midwest BioMedical Research Foundation (MBRF), Kansas City, Missouri. The manuscript received support, in part, from NIH-RO1 DK107490. These agencies did not participate in the design/conduct of the study or, in the interpretation of the data.
1. Bagalman E. Traumatic brain injury among veterans. http://www.ncsl.org/documents/statefed/health/TBI_Vets2013.pdf. Published January 4, 2013. Accessed February 3, 2020.
2. Veterans Health Administration, Support Service Center. Workload files fiscal year 2008-fiscal year 2012. [Source not verified.]
3. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
4. Bagalman E. Health care for veterans: traumatic brain injury. https://fas.org/sgp/crs/misc/R40941.pdf. Published March 9, 2015. Accessed February 4, 2020.
5. Ikin JF, Sim MR, McKenzie DP, et al. Anxiety, post-traumatic stress disorder and depression in Korean War veterans 50 years after the war. Br J Psychiatry. 2007;190(6):475-483.
6. Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164(9):1319-1326.
7. Frueh BC, Grubaugh AL, Yeager DE, Magruder KM. Delayed-onset post-traumatic stress disorder among war veterans in primary care clinics. Br J Psychiatry. 2009;194(6):515-520.
8. McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13(3):287-300.
9. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of posttraumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
10. Friedman MJ, Resick PA, Bryant RA, Strain J, Horowitz M, Spiegel D. Classification of trauma and stressor-related disorders in DSM-5. Depress Anxiety. 2011;28(9):737-749.
11. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702.
12. Carlson K, Kehle S, Meis L, et al. The Assessment and Treatment of Individuals with History of Traumatic Brain Injury and Post-Traumatic Stress Disorder: A Systematic Review of the Evidence. Washington, DC: US Department of Veterans Affairs; 2009.
13. Gironda RJ, Clark ME, Ruff RL, et al. Traumatic brain injury, polytrauma, and pain: challenges and treatment strategies for the polytrauma rehabilitation. Rehabil Psychol. 2009;54(3):247-258.
14. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358(5):453-463.
15. Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):439-451.
16. Peskind ER, Brody D, Cernak I, McKee A, Ruff RL. Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J Clin Psychiatry. 2013;74(2):180-188.
17. Riggio S. Traumatic brain injury and its neurobehavioral sequelae. Neurol Clin. 2011;29(1):35-47, vii.
18. Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav. 2015;9(3):358-366.
19. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19.
20. Thompson WW, Gottesman II, Zalewski C. Reconciling disparate prevalence rates of PTSD in large samples of US male Vietnam veterans and their controls. BMC Psychiatry. 2006;6:19.
21. Frueh BC, Elhai JD, Gold PB, et al Disability compensation seeking among veterans evaluated for posttraumatic stress disorder. Psychiatr Serv. 2003;54(1):84-91.
22. Thakur H, Oni O, Singh V, et al. Increases in the service connection disability and treatment costs associated with posttraumatic stress disorder and/or traumatic brain injury in United States veterans pre- and post-9/11: the strong need for a novel therapeutic approach. Epidemiology (Sunnyvale). 2018;8(4):353.
23. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of post-traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
24. Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2-7.
25. Owens BD, Kragh JG Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2008;64(2):295-299.
26. Defense Health Agency, Defense and Veterans Brain Injury Center. DOD worldwide numbers for TBI since 2000. https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated February 14, 2020. Accessed February 14, 2020.
27. Armed Forces Health Surveillance Center. Deployment-related conditions of special surveillance interest, U.S. armed forces, by month and service, January 2003-December 2012 (data as of 22 January 2013). MSMR. 2013;20(1):16-19.
28. Harvey JH, Stein SK, Scott PK. Fifty years of grief: accounts and reported psychological reactions of Normandy invasion veterans. J Narrative Life History. 1995;5(4):321-332.
29. US Department of Veterans Affairs. Polytrauma/TBI system of care. https://www.polytrauma.va.gov/system-of-care/index.asp. Updated June 3, 2015. Accessed February 4, 2020.
30. Wolfe J, Erickson DJ, Sharkansky EJ, King DW, King LA. Course and predictors of posttraumatic stress disorder among Gulf War veterans: a prospective analysis. J Consult Clin Psychol. 1999;67(4):520-528.
31. Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54(1):81-87.
32. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
33. Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson J, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York: Guilford; 2004:192-238.
34. Engel CC Jr, Engel AL, Campbell SJ, McFall ME, Russo J, Katon W. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis. 1993;181(11):683-688.
35. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2016 data from the American Community Survey. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf. Published February 2018. Accessed February 4, 2020.
36. US Department of Commerce Economics and Statistics Administration, US Census Bureau, Geography Division. 2010 population distribution in the United States and Puerto Rico. https://www2.census.gov/geo/maps/dc10_thematic/2010_Nighttime_PopDist/2010_Nighttime_PopDist_Page_Map.pdf. Accessed February 4, 2020.
37. Cifu DX, Taylor BC, Carne WF, et al. Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND veterans. J Rehabil Res Dev. 2013;50(9):1169-1176.
38. Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
39. Magruder KM, Frueh BC, Knapp RG, et al. Prevalence of posttraumatic stress disorder in Veterans Affairs primary care clinics. Gen Hosp Psychiatry. 2005;27(3):169-179.
40. Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60(3):409-418.
41. Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984-991.
42. Najavits LM. The problem of dropout from “gold standard” PTSD therapies. F1000Prime Rep. 2015;7:43.
43. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Trends in veterans with a service-connected disability: 1985 to 2014. https://www.va.gov/vetdata/docs/QuickFacts/SCD_trends_FINAL_2014.PDF. Published June 2015. Accessed February 4, 2020.
44. US Department of Veterans Affairs, Office of Inspector General. Review of state variances in VA disability compensation payments. Report 05-00765-137. https://www.va.gov/oig/52/reports/2005/VAOIG-05-00765-137.pdf. Published May 19, 2015. Accessed February 4, 2020.
45. McNally RJ. Progress and controversy in the study of posttraumatic stress disorder. Annu Rev Psychol. 2003;54:229-252.
46. Freeman T, Powell M, Kimbrell T. Measuring symptom exaggeration in veterans with chronic posttraumatic stress disorder. Psychiatry Res. 2008;158(3):374-380.
47. Frueh BC, Elhai JD, Grubaugh AL, et al. Documented combat exposure of US veterans seeking treatment for combat-related post-traumatic stress disorder. Br J Psychiatry. 2005;186(6):467-475.
48. Frueh BC, Hamner MB, Cahill SP, Gold PB, Hamlin KL. Apparent symptom overreporting in combat veterans evaluated for PTSD. Clin Psychol Rev. 2000;20(7):853-885.
49. Sparr L, Pankratz LD. Factitious posttraumatic stress disorder. Am J Psychiatry. 1983;140(8):1016-1019.
50. Baggaley M. ‘Military Munchausen’s’: assessment of factitious claims of military service in psychiatric patients. Psychiatr Bull. 1998;22(3):153-154.
1. Bagalman E. Traumatic brain injury among veterans. http://www.ncsl.org/documents/statefed/health/TBI_Vets2013.pdf. Published January 4, 2013. Accessed February 3, 2020.
2. Veterans Health Administration, Support Service Center. Workload files fiscal year 2008-fiscal year 2012. [Source not verified.]
3. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
4. Bagalman E. Health care for veterans: traumatic brain injury. https://fas.org/sgp/crs/misc/R40941.pdf. Published March 9, 2015. Accessed February 4, 2020.
5. Ikin JF, Sim MR, McKenzie DP, et al. Anxiety, post-traumatic stress disorder and depression in Korean War veterans 50 years after the war. Br J Psychiatry. 2007;190(6):475-483.
6. Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164(9):1319-1326.
7. Frueh BC, Grubaugh AL, Yeager DE, Magruder KM. Delayed-onset post-traumatic stress disorder among war veterans in primary care clinics. Br J Psychiatry. 2009;194(6):515-520.
8. McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13(3):287-300.
9. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of posttraumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
10. Friedman MJ, Resick PA, Bryant RA, Strain J, Horowitz M, Spiegel D. Classification of trauma and stressor-related disorders in DSM-5. Depress Anxiety. 2011;28(9):737-749.
11. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702.
12. Carlson K, Kehle S, Meis L, et al. The Assessment and Treatment of Individuals with History of Traumatic Brain Injury and Post-Traumatic Stress Disorder: A Systematic Review of the Evidence. Washington, DC: US Department of Veterans Affairs; 2009.
13. Gironda RJ, Clark ME, Ruff RL, et al. Traumatic brain injury, polytrauma, and pain: challenges and treatment strategies for the polytrauma rehabilitation. Rehabil Psychol. 2009;54(3):247-258.
14. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358(5):453-463.
15. Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):439-451.
16. Peskind ER, Brody D, Cernak I, McKee A, Ruff RL. Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J Clin Psychiatry. 2013;74(2):180-188.
17. Riggio S. Traumatic brain injury and its neurobehavioral sequelae. Neurol Clin. 2011;29(1):35-47, vii.
18. Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav. 2015;9(3):358-366.
19. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19.
20. Thompson WW, Gottesman II, Zalewski C. Reconciling disparate prevalence rates of PTSD in large samples of US male Vietnam veterans and their controls. BMC Psychiatry. 2006;6:19.
21. Frueh BC, Elhai JD, Gold PB, et al Disability compensation seeking among veterans evaluated for posttraumatic stress disorder. Psychiatr Serv. 2003;54(1):84-91.
22. Thakur H, Oni O, Singh V, et al. Increases in the service connection disability and treatment costs associated with posttraumatic stress disorder and/or traumatic brain injury in United States veterans pre- and post-9/11: the strong need for a novel therapeutic approach. Epidemiology (Sunnyvale). 2018;8(4):353.
23. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of post-traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
24. Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2-7.
25. Owens BD, Kragh JG Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2008;64(2):295-299.
26. Defense Health Agency, Defense and Veterans Brain Injury Center. DOD worldwide numbers for TBI since 2000. https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated February 14, 2020. Accessed February 14, 2020.
27. Armed Forces Health Surveillance Center. Deployment-related conditions of special surveillance interest, U.S. armed forces, by month and service, January 2003-December 2012 (data as of 22 January 2013). MSMR. 2013;20(1):16-19.
28. Harvey JH, Stein SK, Scott PK. Fifty years of grief: accounts and reported psychological reactions of Normandy invasion veterans. J Narrative Life History. 1995;5(4):321-332.
29. US Department of Veterans Affairs. Polytrauma/TBI system of care. https://www.polytrauma.va.gov/system-of-care/index.asp. Updated June 3, 2015. Accessed February 4, 2020.
30. Wolfe J, Erickson DJ, Sharkansky EJ, King DW, King LA. Course and predictors of posttraumatic stress disorder among Gulf War veterans: a prospective analysis. J Consult Clin Psychol. 1999;67(4):520-528.
31. Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54(1):81-87.
32. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
33. Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson J, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York: Guilford; 2004:192-238.
34. Engel CC Jr, Engel AL, Campbell SJ, McFall ME, Russo J, Katon W. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis. 1993;181(11):683-688.
35. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2016 data from the American Community Survey. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf. Published February 2018. Accessed February 4, 2020.
36. US Department of Commerce Economics and Statistics Administration, US Census Bureau, Geography Division. 2010 population distribution in the United States and Puerto Rico. https://www2.census.gov/geo/maps/dc10_thematic/2010_Nighttime_PopDist/2010_Nighttime_PopDist_Page_Map.pdf. Accessed February 4, 2020.
37. Cifu DX, Taylor BC, Carne WF, et al. Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND veterans. J Rehabil Res Dev. 2013;50(9):1169-1176.
38. Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
39. Magruder KM, Frueh BC, Knapp RG, et al. Prevalence of posttraumatic stress disorder in Veterans Affairs primary care clinics. Gen Hosp Psychiatry. 2005;27(3):169-179.
40. Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60(3):409-418.
41. Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984-991.
42. Najavits LM. The problem of dropout from “gold standard” PTSD therapies. F1000Prime Rep. 2015;7:43.
43. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Trends in veterans with a service-connected disability: 1985 to 2014. https://www.va.gov/vetdata/docs/QuickFacts/SCD_trends_FINAL_2014.PDF. Published June 2015. Accessed February 4, 2020.
44. US Department of Veterans Affairs, Office of Inspector General. Review of state variances in VA disability compensation payments. Report 05-00765-137. https://www.va.gov/oig/52/reports/2005/VAOIG-05-00765-137.pdf. Published May 19, 2015. Accessed February 4, 2020.
45. McNally RJ. Progress and controversy in the study of posttraumatic stress disorder. Annu Rev Psychol. 2003;54:229-252.
46. Freeman T, Powell M, Kimbrell T. Measuring symptom exaggeration in veterans with chronic posttraumatic stress disorder. Psychiatry Res. 2008;158(3):374-380.
47. Frueh BC, Elhai JD, Grubaugh AL, et al. Documented combat exposure of US veterans seeking treatment for combat-related post-traumatic stress disorder. Br J Psychiatry. 2005;186(6):467-475.
48. Frueh BC, Hamner MB, Cahill SP, Gold PB, Hamlin KL. Apparent symptom overreporting in combat veterans evaluated for PTSD. Clin Psychol Rev. 2000;20(7):853-885.
49. Sparr L, Pankratz LD. Factitious posttraumatic stress disorder. Am J Psychiatry. 1983;140(8):1016-1019.
50. Baggaley M. ‘Military Munchausen’s’: assessment of factitious claims of military service in psychiatric patients. Psychiatr Bull. 1998;22(3):153-154.
Posttraumatic stress may persist up to 9 months after pregnancy loss
new research suggests.
The outcomes of a prospective cohort study involving 737 women who had experienced miscarriage or ectopic pregnancy and 171 controls with healthy pregnancies were presented in a report in the American Journal of Obstetrics & Gynecology.
One month after their pregnancy loss, 29% of these women met the criteria for posttraumatic stress, 24% reported moderate to severe anxiety, and 11% reported moderate to severe depression. In comparison, just 13% of women in the control group met the criteria for anxiety, and 2% met the criteria for depression, which meant women who had experienced early pregnancy loss had a greater than twofold odds of anxiety and nearly fourfold (odds ratio, 3.88) greater odds of depression, reported Jessica Farren, PhD, of the Queen Charlotte’s and Chelsea Hospital, London, and coauthors.
The most common posttraumatic symptom, experienced by 91% of respondents with posttraumatic stress at 1 month after the pregnancy, was reexperiencing symptoms, while 60% experienced avoidance and hyperarousal symptoms. At 3 months after the loss, 50% of those with posttraumatic stress reported an interruption of their general satisfaction with life.
While the incidence of posttraumatic stress, anxiety, and depression decreased over time in the women who had early pregnancy loss, by the third month 21% still met the criteria for posttraumatic stress, and by 9 months, 18% still were experiencing posttraumatic stress. Similarly, moderate to severe anxiety was still present in 23% of women at 3 months and 17% at 9 months, and moderate to severe depression was still experienced by 8% of women at 3 months and 6% of women at 9 months.
Dr. Farren and coauthors wrote that, given the incidence of miscarriage and ectopic pregnancy in the population, the high proportion of women still experiencing posttraumatic stress, anxiety, and depression at 9 months pointed to a significant public health issue. “It is recognized that PTSD in other contexts can have a significant impact on work, social interaction, health care utilization, and risks in future pregnancies,” they wrote. “Work is needed to evaluate strategies to effectively identify and treat affected women with these specific psychopathologies.”
The investigators also looked at the differences in outcomes in women who experienced miscarriage, compared with those who experienced ectopic pregnancy.
Of the 363 women who had a miscarriage, 30% met criteria for posttraumatic stress at 1 month, 20% at 3 months, and 17% at 9 months. Moderate to severe anxiety was reported by 25% women at 1 month, 22% at 3 months, and 17% at 9 months. Moderate to severe depression was reported by 12% at 1 month, 7% at 3 months, and 5% at 9 months.
Of the 74 women who had an ectopic pregnancy, 23% met criteria for posttraumatic stress at 1 month, 28% at 3 months, and 21% at 9 months. Moderate to severe anxiety was reported by 21% at 1 month, 30% at 3 months, and 23% at 9 months. Moderate to severe depression was reported by 7% at 1 month, 12% at 3 months, and 11% at 9 months.
The authors noted that the incidence of posttraumatic stress, anxiety, and depression decreased more strongly over time in women who had experienced miscarriage, compared with those who experienced ectopic pregnancy, although they commented that the confidence intervals were wide.
One coauthor was supported by an Imperial Health Charity grant and another by the National Institute for Health Research Biomedical Research Centre. No conflicts of interest were declared.
SOURCE: Farren J et al. Amer J Obstet Gynecol. 2019 Dec 13. doi: 10.1016/j.ajog.2019.10.102.
new research suggests.
The outcomes of a prospective cohort study involving 737 women who had experienced miscarriage or ectopic pregnancy and 171 controls with healthy pregnancies were presented in a report in the American Journal of Obstetrics & Gynecology.
One month after their pregnancy loss, 29% of these women met the criteria for posttraumatic stress, 24% reported moderate to severe anxiety, and 11% reported moderate to severe depression. In comparison, just 13% of women in the control group met the criteria for anxiety, and 2% met the criteria for depression, which meant women who had experienced early pregnancy loss had a greater than twofold odds of anxiety and nearly fourfold (odds ratio, 3.88) greater odds of depression, reported Jessica Farren, PhD, of the Queen Charlotte’s and Chelsea Hospital, London, and coauthors.
The most common posttraumatic symptom, experienced by 91% of respondents with posttraumatic stress at 1 month after the pregnancy, was reexperiencing symptoms, while 60% experienced avoidance and hyperarousal symptoms. At 3 months after the loss, 50% of those with posttraumatic stress reported an interruption of their general satisfaction with life.
While the incidence of posttraumatic stress, anxiety, and depression decreased over time in the women who had early pregnancy loss, by the third month 21% still met the criteria for posttraumatic stress, and by 9 months, 18% still were experiencing posttraumatic stress. Similarly, moderate to severe anxiety was still present in 23% of women at 3 months and 17% at 9 months, and moderate to severe depression was still experienced by 8% of women at 3 months and 6% of women at 9 months.
Dr. Farren and coauthors wrote that, given the incidence of miscarriage and ectopic pregnancy in the population, the high proportion of women still experiencing posttraumatic stress, anxiety, and depression at 9 months pointed to a significant public health issue. “It is recognized that PTSD in other contexts can have a significant impact on work, social interaction, health care utilization, and risks in future pregnancies,” they wrote. “Work is needed to evaluate strategies to effectively identify and treat affected women with these specific psychopathologies.”
The investigators also looked at the differences in outcomes in women who experienced miscarriage, compared with those who experienced ectopic pregnancy.
Of the 363 women who had a miscarriage, 30% met criteria for posttraumatic stress at 1 month, 20% at 3 months, and 17% at 9 months. Moderate to severe anxiety was reported by 25% women at 1 month, 22% at 3 months, and 17% at 9 months. Moderate to severe depression was reported by 12% at 1 month, 7% at 3 months, and 5% at 9 months.
Of the 74 women who had an ectopic pregnancy, 23% met criteria for posttraumatic stress at 1 month, 28% at 3 months, and 21% at 9 months. Moderate to severe anxiety was reported by 21% at 1 month, 30% at 3 months, and 23% at 9 months. Moderate to severe depression was reported by 7% at 1 month, 12% at 3 months, and 11% at 9 months.
The authors noted that the incidence of posttraumatic stress, anxiety, and depression decreased more strongly over time in women who had experienced miscarriage, compared with those who experienced ectopic pregnancy, although they commented that the confidence intervals were wide.
One coauthor was supported by an Imperial Health Charity grant and another by the National Institute for Health Research Biomedical Research Centre. No conflicts of interest were declared.
SOURCE: Farren J et al. Amer J Obstet Gynecol. 2019 Dec 13. doi: 10.1016/j.ajog.2019.10.102.
new research suggests.
The outcomes of a prospective cohort study involving 737 women who had experienced miscarriage or ectopic pregnancy and 171 controls with healthy pregnancies were presented in a report in the American Journal of Obstetrics & Gynecology.
One month after their pregnancy loss, 29% of these women met the criteria for posttraumatic stress, 24% reported moderate to severe anxiety, and 11% reported moderate to severe depression. In comparison, just 13% of women in the control group met the criteria for anxiety, and 2% met the criteria for depression, which meant women who had experienced early pregnancy loss had a greater than twofold odds of anxiety and nearly fourfold (odds ratio, 3.88) greater odds of depression, reported Jessica Farren, PhD, of the Queen Charlotte’s and Chelsea Hospital, London, and coauthors.
The most common posttraumatic symptom, experienced by 91% of respondents with posttraumatic stress at 1 month after the pregnancy, was reexperiencing symptoms, while 60% experienced avoidance and hyperarousal symptoms. At 3 months after the loss, 50% of those with posttraumatic stress reported an interruption of their general satisfaction with life.
While the incidence of posttraumatic stress, anxiety, and depression decreased over time in the women who had early pregnancy loss, by the third month 21% still met the criteria for posttraumatic stress, and by 9 months, 18% still were experiencing posttraumatic stress. Similarly, moderate to severe anxiety was still present in 23% of women at 3 months and 17% at 9 months, and moderate to severe depression was still experienced by 8% of women at 3 months and 6% of women at 9 months.
Dr. Farren and coauthors wrote that, given the incidence of miscarriage and ectopic pregnancy in the population, the high proportion of women still experiencing posttraumatic stress, anxiety, and depression at 9 months pointed to a significant public health issue. “It is recognized that PTSD in other contexts can have a significant impact on work, social interaction, health care utilization, and risks in future pregnancies,” they wrote. “Work is needed to evaluate strategies to effectively identify and treat affected women with these specific psychopathologies.”
The investigators also looked at the differences in outcomes in women who experienced miscarriage, compared with those who experienced ectopic pregnancy.
Of the 363 women who had a miscarriage, 30% met criteria for posttraumatic stress at 1 month, 20% at 3 months, and 17% at 9 months. Moderate to severe anxiety was reported by 25% women at 1 month, 22% at 3 months, and 17% at 9 months. Moderate to severe depression was reported by 12% at 1 month, 7% at 3 months, and 5% at 9 months.
Of the 74 women who had an ectopic pregnancy, 23% met criteria for posttraumatic stress at 1 month, 28% at 3 months, and 21% at 9 months. Moderate to severe anxiety was reported by 21% at 1 month, 30% at 3 months, and 23% at 9 months. Moderate to severe depression was reported by 7% at 1 month, 12% at 3 months, and 11% at 9 months.
The authors noted that the incidence of posttraumatic stress, anxiety, and depression decreased more strongly over time in women who had experienced miscarriage, compared with those who experienced ectopic pregnancy, although they commented that the confidence intervals were wide.
One coauthor was supported by an Imperial Health Charity grant and another by the National Institute for Health Research Biomedical Research Centre. No conflicts of interest were declared.
SOURCE: Farren J et al. Amer J Obstet Gynecol. 2019 Dec 13. doi: 10.1016/j.ajog.2019.10.102.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Top research findings of 2018-2019 for clinical practice
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.