User login
Establishing the Diagnosis of Rosacea in Skin of Color Patients
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
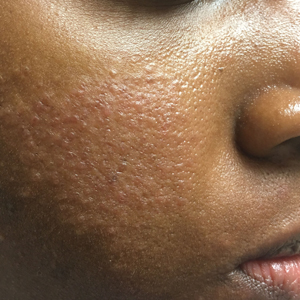
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.
Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
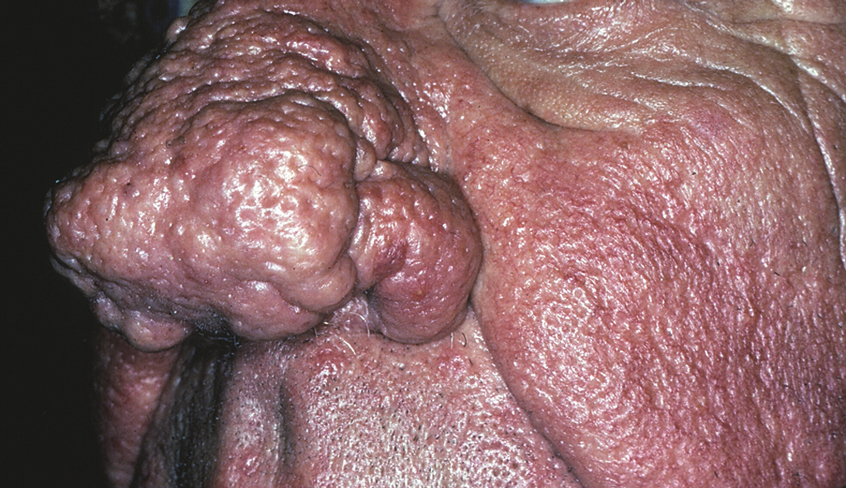
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.
Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.
Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.
Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.
Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.
Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
Practice Points
- The clinical signs of rosacea may be similar in all skin types; however, dermatologists must have a high clinical index of suspicion for rosacea in patients with skin of color (SOC).
- Dermatologists should consider a wide differential diagnosis when presented with an SOC patient with facial erythema and/or papules and pustules.
Update on Rosacea Classification and Its Controversies
Rosacea is an inflammatory skin condition that affects approximately 5% of the adult population, with the highest prevalence in Europe and North America.1 Despite its prevalence, rosacea remains poorly understood from a pathophysiologic perspective, with no diagnostic laboratory markers.2 Because diagnosis relies on clinical judgment, the nomenclature for describing and characterizing rosacea becomes paramount in ensuring that patients are given an accurate diagnosis and subsequent treatment. We review the shortfalls in the recent history of rosacea classification and discuss their implications.
Subtype to Phenotype Classification
In 2002, the National Rosacea Society (NRS) Expert Committee published a standardized classification schema for rosacea (Table).3 The authors described primary and secondary diagnostic criteria. The presence of 1 or more primary features in a central facial distribution was indicative of rosacea. Primary characteristics included flushing (transient erythema), nontransient erythema, papules and pustules, and telangiectasia. Secondary features, which could occur with or independently of primary features, included burning or stinging of the face, dry appearance, facial edema, ocular manifestations, peripheral (nonfacial) occurrence, phymatous changes, and red facial plaques. Whereas these features often present simultaneously in a characteristic pattern, they were grouped into 4 main subtypes—erythematotelangiectatic (ETR), papulopustular, phymatous, and ocular—and 1 variant, granulomatous rosacea.3
To enhance clinical and research applications of this categorization system as well as offer further standardization, the NRS released a supplementary clinical grading scorecard in 2004 in which each of the primary and secondary characteristics could be assigned a subjective severity score of absent, mild, moderate, or severe. The goal was that the subtype classification and clinical grading system, when used in conjunction with each other, would establish a common language for patients, clinicians, and researchers to describe and further investigate rosacea.4
The 2002 categorization system was certainly an impactful first step in the organization of rosacea. It was not without its critics, however, namely rosacea-oriented dermatologists who were concerned about its lack of specificity.5-7 For instance, the NRS Expert Committee did not address the time frame for flushing, which typically has a long duration in rosacea patients, or for the nontransient erythema; telangiectasia secondary to heliodermatitis; or the often-observed periocular sparing. Additionally, the schema did not account for conditions such as gram-negative folliculitis (pustules characteristically located on the central face) or discuss the need to rule out carcinoid, mastocytosis, or connective-tissue disease, which can lead to nontransient facial erythema. Without strict definitions and exclusions, nonrosacea disorders could be incorrectly labeled as rosacea.
Beyond the lack of specificity, there was additional concern if a subtype system was the ideal way to capture disease presentation and severity. By subtyping, there was unnecessary division of interrelated disease into individual disorders; an individual’s clinical presentation might fall along a spectrum rather than within a discrete box.8
Furthermore, from a research standpoint, subtyping rosacea could hinder or confuse epidemiologic studies. For instance, if patients present with phenotypes from different subtypes, into which subtype would they fall?8-10
The global ROSacea COnsensus (ROSCO) panel, comprising 17 international dermatologists and ophthalmologists, convened in 2016 to address this matter. The panel proposed a new system (published in 2017) based on individual phenotypes.9 In this new system, diagnostic features include persistent centrofacial erythema with periods of increased intensity and phymatous changes. Major features, which are diagnostic when there are at least 2, include flushing (transient erythema), inflammatory papules and pustules, centrofacial telangiectasia, and ocular manifestations. Each feature could then be graded on a severity spectrum independent of concurrent phenotypes (Table).8
The panel concluded that this system would provide a stronger foundation for standardization as new knowledge of rosacea continues to be elucidated.8 In support of their argument, ROSCO also released a treatment algorithm that depended on a phenotype scheme.11 The panel emphasized that by focusing on individual lesions rather than a subtype encompassing many characteristics, treatment could be tailored to the patient. Using this à-la-carte therapy option, physicians could choose those rosacea aspects that are particularly concerning to the patient and manage only those aspects or overlap treatments to improve multiple aspects.11
In 2017, 15 years after the original classification system was proposed, the NRS updated their classification system (published in 2018), taking into consideration some of the criticisms as well as new scientific data on rosacea. Similar to the schema proposed by ROSCO, this system was based on phenotype. Inclusion and exclusion criteria were more robust in this update compared to the original classification in 2002. The criteria provide a timeline for transient flushing—it must occur within seconds or minutes in response to a neurovascular stimulant—and state that it is characteristically prolonged (Table).12
However, the Expert Committee still did not define either the length of time of flushing or nontransient erythema. It also did not specify convex surfaces of the face with periocular sparing as the characteristic pattern or provide additional information on how photoaging fits into the definition. The updated classification stated that centrofacial erythema must not be from cutaneous lupus or seborrheic eczema, and steroid-induced rosacea was still excluded.12 However, there is still the need to exclude other systemic conditions, such as mastocytosis, carcinoid, polycythemia vera, and dermatomyositis. Therefore, the potential for subjective error and inclusion of nonrosacea diseases persists.
A critical change was elimination of the granulomatous rosacea variant. In 2002, this variant was defined by monomorphic, yellow-brown to red papules and nodules that led to scarring. This variant, however, did not share the commonalities of the other subtypes, including persistent facial erythema, limitation to convex surfaces, periocular sparing, and transient flushing.3,13 At the time, Crawford et al6 proposed that the variant be recategorized as granulomatous facial dermatitis. In the updated NRS classification, this variant and phenotypic description was eliminated from the schema.12 It is unclear if it was removed because of these discrepancies or if the NRS panel felt it had a distinct pathogenesis from the proposed rosacea pathophysiology; however, we applaud this change.
Subtype Progression
Both the ROSCO and NRS classification schemes mention progression between the various phenotypes,10,12 suggesting that rosacea phenotypes exist along a continuum, progressing and regressing with disease severity. The main study addressing this point was based on the self-reported retrospective patient memory of disease features in rosacea patients. The authors used a modified criterion of centrofacial erythema alone to define ETR; therefore, a person who began their disease with this finding but then acquired inflammatory lesions or phymas was defined as progressing along a spectrum.14 Given that persistent erythema of convex surfaces of the face is common in all subtypes, we do not find it surprising that the authors found (using their modified criteria) that ETR appeared to progress to papulopustular and phymatous subtypes in a small number of patients. We strongly disagree with their interpretation and conclusion.
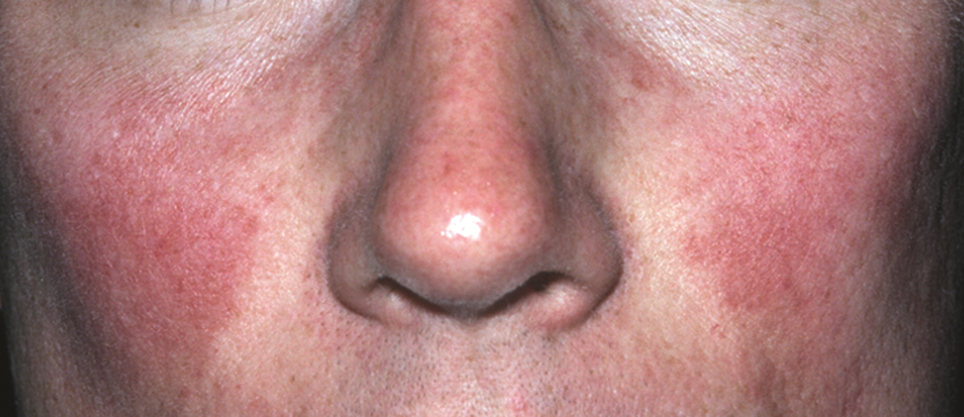
In our experience, ETR patients have fine textured skin without sebaceous quality or a history of extensive acne (Figure 1). Flushing is common and usually lasts 10 minutes to 1 hour. There might be concurrent burning or stinging; however, there is no associated sweating, lightheadedness, palpitations, or diagnostic laboratory findings, which distinguishes ETR from other common causes of flushing. The persistent centrofacial erythema involves convex surfaces, spares periocular skin, and can be best defined as present for longer than 3 months.
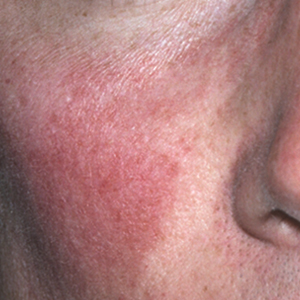
In contrast, phymas occur commonly in patients with thick and sebaceous (glandular) skin (Figure 2).6,15-17 Men are most often affected and usually have a history of moderate to severe acne. It is not uncommon to observe nodules, cysts, and scarring in addition to papules and pustules. These eruptions primarily cluster on the central face and present in areas of nontransient erythema. Flushing, although less prominent than in other phenotypes, also can be seen.
Taken together, we find no convincing evidence from published studies or extensive experience caring for rosacea patients that classic ETR progresses to phymatous rosacea, or the other way around, as displayed in the ROSCO panel report.8 The type of skin seen in Figure 1 will not “progress” to the type seen in Figure 2. Furthermore, treatment will not “reverse” the phymatous skin into thin, ETR-type skin. The implications are important: If a female patient is given a diagnosis of ETR, she will not develop an enlarged phymatous nose. Patients with thick sebaceous skin, as in Figure 2, usually tolerate treatments such as benzoyl peroxide that other rosacea patients do not and frequently respond well to such intervention.
Implications and Future Directions
We present an overview of 2 rosacea classification systems, hoping to stimulate further refinement. Looking forward, there are many directions for further investigation into the pathophysiology of rosacea. From a genetic standpoint, there needs to be continued molecular and epidemiologic data to determine the underlying genetic contributions to disease.
There has been some progress in the realm of understanding the mechanisms of inflammation; we urge further investigation to elucidate how “subclinical neuroinflammation” might lead to glandular hyperplasia.12 We also see value in examining the genetic and hormonal contributions to phymas, as they may be different than those seen in the ETR-type patients. Last, more studies focusing on comorbidities that contribute to or arise from rosacea are welcomed.
The ultimate goal is to develop a classification system that integrates clinical descriptions, pathophysiologic mechanisms, and benchmark indicators of disease. Only then can we have a true gold standard for the diagnosis of rosacea, one that allows for improved personalized treatment and better outcomes.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282-289.
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Saleem MD. Revisiting rosacea criteria: where have we been, where are we going, and how will we get there? Dermatol Clin. 2018;36:161-165.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Tan J, Almeida LMC, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438.
- Wilkin J. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes. Br J Dermatol. 2017;177:597-598.
- Tan J; ROSCO coauthors. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes: reply from the author. Br J Dermatol. 2017;177:598-599.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33:447-455.
- Tan J, Blume‐Peytavi U, Ortonne JP, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169:555-562.
- James WD, Elston D, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. New York, NY: Elsevier; 2019.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea - S1 guideline. J Dtsch Dermatol Ges. 2013;11:768-780.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom‐oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
Rosacea is an inflammatory skin condition that affects approximately 5% of the adult population, with the highest prevalence in Europe and North America.1 Despite its prevalence, rosacea remains poorly understood from a pathophysiologic perspective, with no diagnostic laboratory markers.2 Because diagnosis relies on clinical judgment, the nomenclature for describing and characterizing rosacea becomes paramount in ensuring that patients are given an accurate diagnosis and subsequent treatment. We review the shortfalls in the recent history of rosacea classification and discuss their implications.
Subtype to Phenotype Classification
In 2002, the National Rosacea Society (NRS) Expert Committee published a standardized classification schema for rosacea (Table).3 The authors described primary and secondary diagnostic criteria. The presence of 1 or more primary features in a central facial distribution was indicative of rosacea. Primary characteristics included flushing (transient erythema), nontransient erythema, papules and pustules, and telangiectasia. Secondary features, which could occur with or independently of primary features, included burning or stinging of the face, dry appearance, facial edema, ocular manifestations, peripheral (nonfacial) occurrence, phymatous changes, and red facial plaques. Whereas these features often present simultaneously in a characteristic pattern, they were grouped into 4 main subtypes—erythematotelangiectatic (ETR), papulopustular, phymatous, and ocular—and 1 variant, granulomatous rosacea.3
To enhance clinical and research applications of this categorization system as well as offer further standardization, the NRS released a supplementary clinical grading scorecard in 2004 in which each of the primary and secondary characteristics could be assigned a subjective severity score of absent, mild, moderate, or severe. The goal was that the subtype classification and clinical grading system, when used in conjunction with each other, would establish a common language for patients, clinicians, and researchers to describe and further investigate rosacea.4
The 2002 categorization system was certainly an impactful first step in the organization of rosacea. It was not without its critics, however, namely rosacea-oriented dermatologists who were concerned about its lack of specificity.5-7 For instance, the NRS Expert Committee did not address the time frame for flushing, which typically has a long duration in rosacea patients, or for the nontransient erythema; telangiectasia secondary to heliodermatitis; or the often-observed periocular sparing. Additionally, the schema did not account for conditions such as gram-negative folliculitis (pustules characteristically located on the central face) or discuss the need to rule out carcinoid, mastocytosis, or connective-tissue disease, which can lead to nontransient facial erythema. Without strict definitions and exclusions, nonrosacea disorders could be incorrectly labeled as rosacea.
Beyond the lack of specificity, there was additional concern if a subtype system was the ideal way to capture disease presentation and severity. By subtyping, there was unnecessary division of interrelated disease into individual disorders; an individual’s clinical presentation might fall along a spectrum rather than within a discrete box.8
Furthermore, from a research standpoint, subtyping rosacea could hinder or confuse epidemiologic studies. For instance, if patients present with phenotypes from different subtypes, into which subtype would they fall?8-10
The global ROSacea COnsensus (ROSCO) panel, comprising 17 international dermatologists and ophthalmologists, convened in 2016 to address this matter. The panel proposed a new system (published in 2017) based on individual phenotypes.9 In this new system, diagnostic features include persistent centrofacial erythema with periods of increased intensity and phymatous changes. Major features, which are diagnostic when there are at least 2, include flushing (transient erythema), inflammatory papules and pustules, centrofacial telangiectasia, and ocular manifestations. Each feature could then be graded on a severity spectrum independent of concurrent phenotypes (Table).8
The panel concluded that this system would provide a stronger foundation for standardization as new knowledge of rosacea continues to be elucidated.8 In support of their argument, ROSCO also released a treatment algorithm that depended on a phenotype scheme.11 The panel emphasized that by focusing on individual lesions rather than a subtype encompassing many characteristics, treatment could be tailored to the patient. Using this à-la-carte therapy option, physicians could choose those rosacea aspects that are particularly concerning to the patient and manage only those aspects or overlap treatments to improve multiple aspects.11
In 2017, 15 years after the original classification system was proposed, the NRS updated their classification system (published in 2018), taking into consideration some of the criticisms as well as new scientific data on rosacea. Similar to the schema proposed by ROSCO, this system was based on phenotype. Inclusion and exclusion criteria were more robust in this update compared to the original classification in 2002. The criteria provide a timeline for transient flushing—it must occur within seconds or minutes in response to a neurovascular stimulant—and state that it is characteristically prolonged (Table).12
However, the Expert Committee still did not define either the length of time of flushing or nontransient erythema. It also did not specify convex surfaces of the face with periocular sparing as the characteristic pattern or provide additional information on how photoaging fits into the definition. The updated classification stated that centrofacial erythema must not be from cutaneous lupus or seborrheic eczema, and steroid-induced rosacea was still excluded.12 However, there is still the need to exclude other systemic conditions, such as mastocytosis, carcinoid, polycythemia vera, and dermatomyositis. Therefore, the potential for subjective error and inclusion of nonrosacea diseases persists.
A critical change was elimination of the granulomatous rosacea variant. In 2002, this variant was defined by monomorphic, yellow-brown to red papules and nodules that led to scarring. This variant, however, did not share the commonalities of the other subtypes, including persistent facial erythema, limitation to convex surfaces, periocular sparing, and transient flushing.3,13 At the time, Crawford et al6 proposed that the variant be recategorized as granulomatous facial dermatitis. In the updated NRS classification, this variant and phenotypic description was eliminated from the schema.12 It is unclear if it was removed because of these discrepancies or if the NRS panel felt it had a distinct pathogenesis from the proposed rosacea pathophysiology; however, we applaud this change.
Subtype Progression
Both the ROSCO and NRS classification schemes mention progression between the various phenotypes,10,12 suggesting that rosacea phenotypes exist along a continuum, progressing and regressing with disease severity. The main study addressing this point was based on the self-reported retrospective patient memory of disease features in rosacea patients. The authors used a modified criterion of centrofacial erythema alone to define ETR; therefore, a person who began their disease with this finding but then acquired inflammatory lesions or phymas was defined as progressing along a spectrum.14 Given that persistent erythema of convex surfaces of the face is common in all subtypes, we do not find it surprising that the authors found (using their modified criteria) that ETR appeared to progress to papulopustular and phymatous subtypes in a small number of patients. We strongly disagree with their interpretation and conclusion.
In our experience, ETR patients have fine textured skin without sebaceous quality or a history of extensive acne (Figure 1). Flushing is common and usually lasts 10 minutes to 1 hour. There might be concurrent burning or stinging; however, there is no associated sweating, lightheadedness, palpitations, or diagnostic laboratory findings, which distinguishes ETR from other common causes of flushing. The persistent centrofacial erythema involves convex surfaces, spares periocular skin, and can be best defined as present for longer than 3 months.
In contrast, phymas occur commonly in patients with thick and sebaceous (glandular) skin (Figure 2).6,15-17 Men are most often affected and usually have a history of moderate to severe acne. It is not uncommon to observe nodules, cysts, and scarring in addition to papules and pustules. These eruptions primarily cluster on the central face and present in areas of nontransient erythema. Flushing, although less prominent than in other phenotypes, also can be seen.
Taken together, we find no convincing evidence from published studies or extensive experience caring for rosacea patients that classic ETR progresses to phymatous rosacea, or the other way around, as displayed in the ROSCO panel report.8 The type of skin seen in Figure 1 will not “progress” to the type seen in Figure 2. Furthermore, treatment will not “reverse” the phymatous skin into thin, ETR-type skin. The implications are important: If a female patient is given a diagnosis of ETR, she will not develop an enlarged phymatous nose. Patients with thick sebaceous skin, as in Figure 2, usually tolerate treatments such as benzoyl peroxide that other rosacea patients do not and frequently respond well to such intervention.
Implications and Future Directions
We present an overview of 2 rosacea classification systems, hoping to stimulate further refinement. Looking forward, there are many directions for further investigation into the pathophysiology of rosacea. From a genetic standpoint, there needs to be continued molecular and epidemiologic data to determine the underlying genetic contributions to disease.
There has been some progress in the realm of understanding the mechanisms of inflammation; we urge further investigation to elucidate how “subclinical neuroinflammation” might lead to glandular hyperplasia.12 We also see value in examining the genetic and hormonal contributions to phymas, as they may be different than those seen in the ETR-type patients. Last, more studies focusing on comorbidities that contribute to or arise from rosacea are welcomed.
The ultimate goal is to develop a classification system that integrates clinical descriptions, pathophysiologic mechanisms, and benchmark indicators of disease. Only then can we have a true gold standard for the diagnosis of rosacea, one that allows for improved personalized treatment and better outcomes.
Rosacea is an inflammatory skin condition that affects approximately 5% of the adult population, with the highest prevalence in Europe and North America.1 Despite its prevalence, rosacea remains poorly understood from a pathophysiologic perspective, with no diagnostic laboratory markers.2 Because diagnosis relies on clinical judgment, the nomenclature for describing and characterizing rosacea becomes paramount in ensuring that patients are given an accurate diagnosis and subsequent treatment. We review the shortfalls in the recent history of rosacea classification and discuss their implications.
Subtype to Phenotype Classification
In 2002, the National Rosacea Society (NRS) Expert Committee published a standardized classification schema for rosacea (Table).3 The authors described primary and secondary diagnostic criteria. The presence of 1 or more primary features in a central facial distribution was indicative of rosacea. Primary characteristics included flushing (transient erythema), nontransient erythema, papules and pustules, and telangiectasia. Secondary features, which could occur with or independently of primary features, included burning or stinging of the face, dry appearance, facial edema, ocular manifestations, peripheral (nonfacial) occurrence, phymatous changes, and red facial plaques. Whereas these features often present simultaneously in a characteristic pattern, they were grouped into 4 main subtypes—erythematotelangiectatic (ETR), papulopustular, phymatous, and ocular—and 1 variant, granulomatous rosacea.3
To enhance clinical and research applications of this categorization system as well as offer further standardization, the NRS released a supplementary clinical grading scorecard in 2004 in which each of the primary and secondary characteristics could be assigned a subjective severity score of absent, mild, moderate, or severe. The goal was that the subtype classification and clinical grading system, when used in conjunction with each other, would establish a common language for patients, clinicians, and researchers to describe and further investigate rosacea.4
The 2002 categorization system was certainly an impactful first step in the organization of rosacea. It was not without its critics, however, namely rosacea-oriented dermatologists who were concerned about its lack of specificity.5-7 For instance, the NRS Expert Committee did not address the time frame for flushing, which typically has a long duration in rosacea patients, or for the nontransient erythema; telangiectasia secondary to heliodermatitis; or the often-observed periocular sparing. Additionally, the schema did not account for conditions such as gram-negative folliculitis (pustules characteristically located on the central face) or discuss the need to rule out carcinoid, mastocytosis, or connective-tissue disease, which can lead to nontransient facial erythema. Without strict definitions and exclusions, nonrosacea disorders could be incorrectly labeled as rosacea.
Beyond the lack of specificity, there was additional concern if a subtype system was the ideal way to capture disease presentation and severity. By subtyping, there was unnecessary division of interrelated disease into individual disorders; an individual’s clinical presentation might fall along a spectrum rather than within a discrete box.8
Furthermore, from a research standpoint, subtyping rosacea could hinder or confuse epidemiologic studies. For instance, if patients present with phenotypes from different subtypes, into which subtype would they fall?8-10
The global ROSacea COnsensus (ROSCO) panel, comprising 17 international dermatologists and ophthalmologists, convened in 2016 to address this matter. The panel proposed a new system (published in 2017) based on individual phenotypes.9 In this new system, diagnostic features include persistent centrofacial erythema with periods of increased intensity and phymatous changes. Major features, which are diagnostic when there are at least 2, include flushing (transient erythema), inflammatory papules and pustules, centrofacial telangiectasia, and ocular manifestations. Each feature could then be graded on a severity spectrum independent of concurrent phenotypes (Table).8
The panel concluded that this system would provide a stronger foundation for standardization as new knowledge of rosacea continues to be elucidated.8 In support of their argument, ROSCO also released a treatment algorithm that depended on a phenotype scheme.11 The panel emphasized that by focusing on individual lesions rather than a subtype encompassing many characteristics, treatment could be tailored to the patient. Using this à-la-carte therapy option, physicians could choose those rosacea aspects that are particularly concerning to the patient and manage only those aspects or overlap treatments to improve multiple aspects.11
In 2017, 15 years after the original classification system was proposed, the NRS updated their classification system (published in 2018), taking into consideration some of the criticisms as well as new scientific data on rosacea. Similar to the schema proposed by ROSCO, this system was based on phenotype. Inclusion and exclusion criteria were more robust in this update compared to the original classification in 2002. The criteria provide a timeline for transient flushing—it must occur within seconds or minutes in response to a neurovascular stimulant—and state that it is characteristically prolonged (Table).12
However, the Expert Committee still did not define either the length of time of flushing or nontransient erythema. It also did not specify convex surfaces of the face with periocular sparing as the characteristic pattern or provide additional information on how photoaging fits into the definition. The updated classification stated that centrofacial erythema must not be from cutaneous lupus or seborrheic eczema, and steroid-induced rosacea was still excluded.12 However, there is still the need to exclude other systemic conditions, such as mastocytosis, carcinoid, polycythemia vera, and dermatomyositis. Therefore, the potential for subjective error and inclusion of nonrosacea diseases persists.
A critical change was elimination of the granulomatous rosacea variant. In 2002, this variant was defined by monomorphic, yellow-brown to red papules and nodules that led to scarring. This variant, however, did not share the commonalities of the other subtypes, including persistent facial erythema, limitation to convex surfaces, periocular sparing, and transient flushing.3,13 At the time, Crawford et al6 proposed that the variant be recategorized as granulomatous facial dermatitis. In the updated NRS classification, this variant and phenotypic description was eliminated from the schema.12 It is unclear if it was removed because of these discrepancies or if the NRS panel felt it had a distinct pathogenesis from the proposed rosacea pathophysiology; however, we applaud this change.
Subtype Progression
Both the ROSCO and NRS classification schemes mention progression between the various phenotypes,10,12 suggesting that rosacea phenotypes exist along a continuum, progressing and regressing with disease severity. The main study addressing this point was based on the self-reported retrospective patient memory of disease features in rosacea patients. The authors used a modified criterion of centrofacial erythema alone to define ETR; therefore, a person who began their disease with this finding but then acquired inflammatory lesions or phymas was defined as progressing along a spectrum.14 Given that persistent erythema of convex surfaces of the face is common in all subtypes, we do not find it surprising that the authors found (using their modified criteria) that ETR appeared to progress to papulopustular and phymatous subtypes in a small number of patients. We strongly disagree with their interpretation and conclusion.
In our experience, ETR patients have fine textured skin without sebaceous quality or a history of extensive acne (Figure 1). Flushing is common and usually lasts 10 minutes to 1 hour. There might be concurrent burning or stinging; however, there is no associated sweating, lightheadedness, palpitations, or diagnostic laboratory findings, which distinguishes ETR from other common causes of flushing. The persistent centrofacial erythema involves convex surfaces, spares periocular skin, and can be best defined as present for longer than 3 months.
In contrast, phymas occur commonly in patients with thick and sebaceous (glandular) skin (Figure 2).6,15-17 Men are most often affected and usually have a history of moderate to severe acne. It is not uncommon to observe nodules, cysts, and scarring in addition to papules and pustules. These eruptions primarily cluster on the central face and present in areas of nontransient erythema. Flushing, although less prominent than in other phenotypes, also can be seen.
Taken together, we find no convincing evidence from published studies or extensive experience caring for rosacea patients that classic ETR progresses to phymatous rosacea, or the other way around, as displayed in the ROSCO panel report.8 The type of skin seen in Figure 1 will not “progress” to the type seen in Figure 2. Furthermore, treatment will not “reverse” the phymatous skin into thin, ETR-type skin. The implications are important: If a female patient is given a diagnosis of ETR, she will not develop an enlarged phymatous nose. Patients with thick sebaceous skin, as in Figure 2, usually tolerate treatments such as benzoyl peroxide that other rosacea patients do not and frequently respond well to such intervention.
Implications and Future Directions
We present an overview of 2 rosacea classification systems, hoping to stimulate further refinement. Looking forward, there are many directions for further investigation into the pathophysiology of rosacea. From a genetic standpoint, there needs to be continued molecular and epidemiologic data to determine the underlying genetic contributions to disease.
There has been some progress in the realm of understanding the mechanisms of inflammation; we urge further investigation to elucidate how “subclinical neuroinflammation” might lead to glandular hyperplasia.12 We also see value in examining the genetic and hormonal contributions to phymas, as they may be different than those seen in the ETR-type patients. Last, more studies focusing on comorbidities that contribute to or arise from rosacea are welcomed.
The ultimate goal is to develop a classification system that integrates clinical descriptions, pathophysiologic mechanisms, and benchmark indicators of disease. Only then can we have a true gold standard for the diagnosis of rosacea, one that allows for improved personalized treatment and better outcomes.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282-289.
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Saleem MD. Revisiting rosacea criteria: where have we been, where are we going, and how will we get there? Dermatol Clin. 2018;36:161-165.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Tan J, Almeida LMC, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438.
- Wilkin J. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes. Br J Dermatol. 2017;177:597-598.
- Tan J; ROSCO coauthors. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes: reply from the author. Br J Dermatol. 2017;177:598-599.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33:447-455.
- Tan J, Blume‐Peytavi U, Ortonne JP, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169:555-562.
- James WD, Elston D, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. New York, NY: Elsevier; 2019.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea - S1 guideline. J Dtsch Dermatol Ges. 2013;11:768-780.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom‐oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282-289.
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Saleem MD. Revisiting rosacea criteria: where have we been, where are we going, and how will we get there? Dermatol Clin. 2018;36:161-165.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Tan J, Almeida LMC, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438.
- Wilkin J. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes. Br J Dermatol. 2017;177:597-598.
- Tan J; ROSCO coauthors. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes: reply from the author. Br J Dermatol. 2017;177:598-599.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33:447-455.
- Tan J, Blume‐Peytavi U, Ortonne JP, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169:555-562.
- James WD, Elston D, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. New York, NY: Elsevier; 2019.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea - S1 guideline. J Dtsch Dermatol Ges. 2013;11:768-780.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom‐oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
Practice Points
- Rosacea therapy is based on a phenotype classification system, in which patients can have major and minor features across all previously denoted subtypes. This system allows for greater flexibility in treatment regimens.
- Despite mention of progression between subtypes, there has not been convincing evidence that patients can progress or regress from one end of the rosacea spectrum (erythematotelangiectatic) to the other (phymatous).
Rosacea: Quality of life improves with treatment success
according to a survey from the National Rosacea Society.
The survey was conducted among 1,044 patients with rosacea, and 76% saw at least some improvement in their skin following treatment. Of all patients who responded to treatment, 40% reported improvement in psychological well-being, 35% reported improvement in social well-being, and 31% reported improvement in occupational well-being.
The percentages of patients reporting improvements were higher or lower depending on the degree of skin clearance. Among patients whose signs and symptoms of rosacea were virtually eliminated, 81% said psychological well-being improved, 71% said social well-being did so, and 62% said occupational well-being improved. Among those whose rosacea improved only slightly or moderately, the percentages of well-being improvement were only 24%, 21%, and 19%, respectively.
The National Rosacea Society serves as a resource to provide information on the disorder, as well as increase awareness and support research.
according to a survey from the National Rosacea Society.
The survey was conducted among 1,044 patients with rosacea, and 76% saw at least some improvement in their skin following treatment. Of all patients who responded to treatment, 40% reported improvement in psychological well-being, 35% reported improvement in social well-being, and 31% reported improvement in occupational well-being.
The percentages of patients reporting improvements were higher or lower depending on the degree of skin clearance. Among patients whose signs and symptoms of rosacea were virtually eliminated, 81% said psychological well-being improved, 71% said social well-being did so, and 62% said occupational well-being improved. Among those whose rosacea improved only slightly or moderately, the percentages of well-being improvement were only 24%, 21%, and 19%, respectively.
The National Rosacea Society serves as a resource to provide information on the disorder, as well as increase awareness and support research.
according to a survey from the National Rosacea Society.
The survey was conducted among 1,044 patients with rosacea, and 76% saw at least some improvement in their skin following treatment. Of all patients who responded to treatment, 40% reported improvement in psychological well-being, 35% reported improvement in social well-being, and 31% reported improvement in occupational well-being.
The percentages of patients reporting improvements were higher or lower depending on the degree of skin clearance. Among patients whose signs and symptoms of rosacea were virtually eliminated, 81% said psychological well-being improved, 71% said social well-being did so, and 62% said occupational well-being improved. Among those whose rosacea improved only slightly or moderately, the percentages of well-being improvement were only 24%, 21%, and 19%, respectively.
The National Rosacea Society serves as a resource to provide information on the disorder, as well as increase awareness and support research.
Combined laser-topical therapy improves erythema associated with rosacea
DENVER – Among patients with facial erythema associated with erythrotelangiectatic rosacea, combining a long-pulsed 532 nm laser with daily application of a topical skin care regimen achieved equivalent to superior results in fewer treatments, compared with long-pulsed laser treatment alone.
The findings come from a pilot trial that Brian S. Biesman, MD, presented at the annual conference of the American Society for Laser Medicine and Surgery.
“Vascular laser therapy is the standard of care for reduction of facial erythema associated with erythrotelangiectatic rosacea,” said Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn. “The question was, if we combine topicals plus laser, can we get an enhanced outcome relative to laser treatment alone?”
To find out, he and his colleagues conducted a blinded, controlled prospective study of 30 subjects with mild to moderate erythrotelangiectatic rosacea who were evenly split into two groups. Those in group 1 received three treatments with the Excel V 532 nm long-pulsed laser by Cutera. Those in group 2 received two laser treatments with the Excel V long-pulsed 532 nm long-pulsed laser plus concurrent daily use of the topical Jan Marini Skin Care Management System, which included a glycolic acid cleanser, vitamin C serum, active containing glycolic, salicylic and azelaic acids, peptide, and growth factor moisturizer and a broad-spectrum sunscreen. It also contained RosaLieve, a proprietary redness-reducing complex.
The researchers performed laser treatments at 4-week intervals and evaluated subjects at baseline, 4, 8, and 12 weeks by physician and subject self-assessment using 5-point (0-4) standardized scales: the Clinician Erythema Assessment (CEA) and patient self-assessment as well as a dermatology Quality of Life Assessment. In both treatment groups, reduction in facial erythema as assessed by CEA and patient self-assessment showed statistically significant improvement at all measured intervals. Specifically, average CEA scores improved from 3.00 to 1.87 among patients in group 1, and from 3.07 to 1.64 among those in group 2. “These were both statistically significant from baseline,” Dr. Biesman said. “What does it really say? The laser plus topical was superior to the laser-only treatment at all measured intervals. I didn’t expect to see that. There was continued improvement noted from week 8 to week 12. That was more of a trend; it was not statistically significant. There were no complications or adverse reactions in either group. The study data indicate that best results may be achieved with a combination of laser and home care.”
He acknowledged certain limitations of the study, including its small sample size and relatively short course of follow-up. “We didn’t have standardization of topical therapy in the laser-only group,” Dr. Biesman said. “Those patients were told to use their usual topical regimen. They were not allowed to use retinoids. We also didn’t have a control arm.”
He disclosed that he has received grant funding from Jan Marini Skin Research and Cutera.
DENVER – Among patients with facial erythema associated with erythrotelangiectatic rosacea, combining a long-pulsed 532 nm laser with daily application of a topical skin care regimen achieved equivalent to superior results in fewer treatments, compared with long-pulsed laser treatment alone.
The findings come from a pilot trial that Brian S. Biesman, MD, presented at the annual conference of the American Society for Laser Medicine and Surgery.
“Vascular laser therapy is the standard of care for reduction of facial erythema associated with erythrotelangiectatic rosacea,” said Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn. “The question was, if we combine topicals plus laser, can we get an enhanced outcome relative to laser treatment alone?”
To find out, he and his colleagues conducted a blinded, controlled prospective study of 30 subjects with mild to moderate erythrotelangiectatic rosacea who were evenly split into two groups. Those in group 1 received three treatments with the Excel V 532 nm long-pulsed laser by Cutera. Those in group 2 received two laser treatments with the Excel V long-pulsed 532 nm long-pulsed laser plus concurrent daily use of the topical Jan Marini Skin Care Management System, which included a glycolic acid cleanser, vitamin C serum, active containing glycolic, salicylic and azelaic acids, peptide, and growth factor moisturizer and a broad-spectrum sunscreen. It also contained RosaLieve, a proprietary redness-reducing complex.
The researchers performed laser treatments at 4-week intervals and evaluated subjects at baseline, 4, 8, and 12 weeks by physician and subject self-assessment using 5-point (0-4) standardized scales: the Clinician Erythema Assessment (CEA) and patient self-assessment as well as a dermatology Quality of Life Assessment. In both treatment groups, reduction in facial erythema as assessed by CEA and patient self-assessment showed statistically significant improvement at all measured intervals. Specifically, average CEA scores improved from 3.00 to 1.87 among patients in group 1, and from 3.07 to 1.64 among those in group 2. “These were both statistically significant from baseline,” Dr. Biesman said. “What does it really say? The laser plus topical was superior to the laser-only treatment at all measured intervals. I didn’t expect to see that. There was continued improvement noted from week 8 to week 12. That was more of a trend; it was not statistically significant. There were no complications or adverse reactions in either group. The study data indicate that best results may be achieved with a combination of laser and home care.”
He acknowledged certain limitations of the study, including its small sample size and relatively short course of follow-up. “We didn’t have standardization of topical therapy in the laser-only group,” Dr. Biesman said. “Those patients were told to use their usual topical regimen. They were not allowed to use retinoids. We also didn’t have a control arm.”
He disclosed that he has received grant funding from Jan Marini Skin Research and Cutera.
DENVER – Among patients with facial erythema associated with erythrotelangiectatic rosacea, combining a long-pulsed 532 nm laser with daily application of a topical skin care regimen achieved equivalent to superior results in fewer treatments, compared with long-pulsed laser treatment alone.
The findings come from a pilot trial that Brian S. Biesman, MD, presented at the annual conference of the American Society for Laser Medicine and Surgery.
“Vascular laser therapy is the standard of care for reduction of facial erythema associated with erythrotelangiectatic rosacea,” said Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn. “The question was, if we combine topicals plus laser, can we get an enhanced outcome relative to laser treatment alone?”
To find out, he and his colleagues conducted a blinded, controlled prospective study of 30 subjects with mild to moderate erythrotelangiectatic rosacea who were evenly split into two groups. Those in group 1 received three treatments with the Excel V 532 nm long-pulsed laser by Cutera. Those in group 2 received two laser treatments with the Excel V long-pulsed 532 nm long-pulsed laser plus concurrent daily use of the topical Jan Marini Skin Care Management System, which included a glycolic acid cleanser, vitamin C serum, active containing glycolic, salicylic and azelaic acids, peptide, and growth factor moisturizer and a broad-spectrum sunscreen. It also contained RosaLieve, a proprietary redness-reducing complex.
The researchers performed laser treatments at 4-week intervals and evaluated subjects at baseline, 4, 8, and 12 weeks by physician and subject self-assessment using 5-point (0-4) standardized scales: the Clinician Erythema Assessment (CEA) and patient self-assessment as well as a dermatology Quality of Life Assessment. In both treatment groups, reduction in facial erythema as assessed by CEA and patient self-assessment showed statistically significant improvement at all measured intervals. Specifically, average CEA scores improved from 3.00 to 1.87 among patients in group 1, and from 3.07 to 1.64 among those in group 2. “These were both statistically significant from baseline,” Dr. Biesman said. “What does it really say? The laser plus topical was superior to the laser-only treatment at all measured intervals. I didn’t expect to see that. There was continued improvement noted from week 8 to week 12. That was more of a trend; it was not statistically significant. There were no complications or adverse reactions in either group. The study data indicate that best results may be achieved with a combination of laser and home care.”
He acknowledged certain limitations of the study, including its small sample size and relatively short course of follow-up. “We didn’t have standardization of topical therapy in the laser-only group,” Dr. Biesman said. “Those patients were told to use their usual topical regimen. They were not allowed to use retinoids. We also didn’t have a control arm.”
He disclosed that he has received grant funding from Jan Marini Skin Research and Cutera.
REPORTING FROM ASLMS 2019
Low-dose isotretinoin plus pulsed dye laser found effective for papulopustular rosacea
DENVER – Oral low-dose isotretinoin in combination with pulsed dye laser achieves high percentage of papulopustular rosacea clearance with a low risk of side effects, results from a single-center study found.
“Rosacea has been classically treated with topical and oral antibiotics, retinoids or ivermectin, and it is really important to enhance laser contribution for rosacea clearance,” lead study author Natalia Jiménez Gómez, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “Our work points to the high efficacy of a scarcely published combination treatment: low-dose oral isotretinoin and pulsed dye laser. Moreover, this combined treatment is employed in papulopustular rosacea, a clinical form that is usually treated without employing physical methods.”
For the study, Dr. Gómez and her colleagues retrospectively analyzed 40 patients with moderate or severe papulopustular rosacea who underwent concomitant treatment of pulsed dye laser and low-dose oral isotretinoin (5-10 mg/day). Pulsed dye laser sessions were performed with a 10-mm spot size at a pulse duration of 0.5 milliseconds delivered at a fluence of 8 J/cm2 every 3-4 weeks in combination with low-dose oral isotretinoin during 4- to 6-month periods. The treatment endpoint was purpura. Five patients were withdrawn from the analysis because of loss to follow-up or to a lack of clinical images.
All 35 patients achieved a complete clearance of papulopustular lesions of rosacea within 7-10 days, said Dr. Gómez, who practices at the Madrid-based Hospital Universitario Ramón y Cajal. The researchers did not observe any permanent adverse events such as scarring, hyperpigmentation, or hypopigmentation.
“The most surprising finding was the high percentage of rosacea clearance obtained with the combined treatment, considering that all the patients were previously nonresponsive to oral low-dose isotretinoin monotherapy,” Dr. Gómez said. “This finding highlights the important role of pulsed dye laser.”
She acknowledged certain limitations of the study, including its retrospective design and small sample size. “It would also be interesting to compare pulsed dye laser with intense pulsed light, so that we can conclude if one of them is more effective than the other when we combine it with low dose oral isotretinoin,” she said.
Dr. Gómez reported having no financial disclosures.
DENVER – Oral low-dose isotretinoin in combination with pulsed dye laser achieves high percentage of papulopustular rosacea clearance with a low risk of side effects, results from a single-center study found.
“Rosacea has been classically treated with topical and oral antibiotics, retinoids or ivermectin, and it is really important to enhance laser contribution for rosacea clearance,” lead study author Natalia Jiménez Gómez, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “Our work points to the high efficacy of a scarcely published combination treatment: low-dose oral isotretinoin and pulsed dye laser. Moreover, this combined treatment is employed in papulopustular rosacea, a clinical form that is usually treated without employing physical methods.”
For the study, Dr. Gómez and her colleagues retrospectively analyzed 40 patients with moderate or severe papulopustular rosacea who underwent concomitant treatment of pulsed dye laser and low-dose oral isotretinoin (5-10 mg/day). Pulsed dye laser sessions were performed with a 10-mm spot size at a pulse duration of 0.5 milliseconds delivered at a fluence of 8 J/cm2 every 3-4 weeks in combination with low-dose oral isotretinoin during 4- to 6-month periods. The treatment endpoint was purpura. Five patients were withdrawn from the analysis because of loss to follow-up or to a lack of clinical images.
All 35 patients achieved a complete clearance of papulopustular lesions of rosacea within 7-10 days, said Dr. Gómez, who practices at the Madrid-based Hospital Universitario Ramón y Cajal. The researchers did not observe any permanent adverse events such as scarring, hyperpigmentation, or hypopigmentation.
“The most surprising finding was the high percentage of rosacea clearance obtained with the combined treatment, considering that all the patients were previously nonresponsive to oral low-dose isotretinoin monotherapy,” Dr. Gómez said. “This finding highlights the important role of pulsed dye laser.”
She acknowledged certain limitations of the study, including its retrospective design and small sample size. “It would also be interesting to compare pulsed dye laser with intense pulsed light, so that we can conclude if one of them is more effective than the other when we combine it with low dose oral isotretinoin,” she said.
Dr. Gómez reported having no financial disclosures.
DENVER – Oral low-dose isotretinoin in combination with pulsed dye laser achieves high percentage of papulopustular rosacea clearance with a low risk of side effects, results from a single-center study found.
“Rosacea has been classically treated with topical and oral antibiotics, retinoids or ivermectin, and it is really important to enhance laser contribution for rosacea clearance,” lead study author Natalia Jiménez Gómez, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “Our work points to the high efficacy of a scarcely published combination treatment: low-dose oral isotretinoin and pulsed dye laser. Moreover, this combined treatment is employed in papulopustular rosacea, a clinical form that is usually treated without employing physical methods.”
For the study, Dr. Gómez and her colleagues retrospectively analyzed 40 patients with moderate or severe papulopustular rosacea who underwent concomitant treatment of pulsed dye laser and low-dose oral isotretinoin (5-10 mg/day). Pulsed dye laser sessions were performed with a 10-mm spot size at a pulse duration of 0.5 milliseconds delivered at a fluence of 8 J/cm2 every 3-4 weeks in combination with low-dose oral isotretinoin during 4- to 6-month periods. The treatment endpoint was purpura. Five patients were withdrawn from the analysis because of loss to follow-up or to a lack of clinical images.
All 35 patients achieved a complete clearance of papulopustular lesions of rosacea within 7-10 days, said Dr. Gómez, who practices at the Madrid-based Hospital Universitario Ramón y Cajal. The researchers did not observe any permanent adverse events such as scarring, hyperpigmentation, or hypopigmentation.
“The most surprising finding was the high percentage of rosacea clearance obtained with the combined treatment, considering that all the patients were previously nonresponsive to oral low-dose isotretinoin monotherapy,” Dr. Gómez said. “This finding highlights the important role of pulsed dye laser.”
She acknowledged certain limitations of the study, including its retrospective design and small sample size. “It would also be interesting to compare pulsed dye laser with intense pulsed light, so that we can conclude if one of them is more effective than the other when we combine it with low dose oral isotretinoin,” she said.
Dr. Gómez reported having no financial disclosures.
REPORTING FROM ASLMS 2019
Key clinical point: Combining pulsed dye laser and low-dose oral isotretinoin represents an option for moderate and severe forms of papulopustular rosacea.
Major finding: All patients achieved complete clearance of papulopustular lesions of rosacea within 7-10 days.
Study details: A retrospective study of 35 patients who received concomitant treatment of pulsed dye laser and low-dose oral isotretinoin.
Disclosures: Dr. Gómez reported having no financial disclosures.
Rhymin’ pediatric dermatologist provides Demodex tips
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
How to surf the rosacea treatment algorithm
WAIKOLOA, HAWAII – according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.

Papules and pustules need an oral or topical anti-inflammatory drug. Background erythema requires an alpha adrenergic agonist. Telangiectasia is best handled by a laser device, and if a patient has a phyma, “you’ve got to use a surgical approach,” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. It sounds simple, but there are decisions to be made about what drugs and formulations to use, and when, and when to combine them.
In an interview, Dr. Stein Gold shared her approach to treatment, along with the latest on using ivermectin and brimonidine together, plus her thoughts on new medications under development and the role of the Demodex mite in rosacea.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.

Papules and pustules need an oral or topical anti-inflammatory drug. Background erythema requires an alpha adrenergic agonist. Telangiectasia is best handled by a laser device, and if a patient has a phyma, “you’ve got to use a surgical approach,” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. It sounds simple, but there are decisions to be made about what drugs and formulations to use, and when, and when to combine them.
In an interview, Dr. Stein Gold shared her approach to treatment, along with the latest on using ivermectin and brimonidine together, plus her thoughts on new medications under development and the role of the Demodex mite in rosacea.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.

Papules and pustules need an oral or topical anti-inflammatory drug. Background erythema requires an alpha adrenergic agonist. Telangiectasia is best handled by a laser device, and if a patient has a phyma, “you’ve got to use a surgical approach,” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. It sounds simple, but there are decisions to be made about what drugs and formulations to use, and when, and when to combine them.
In an interview, Dr. Stein Gold shared her approach to treatment, along with the latest on using ivermectin and brimonidine together, plus her thoughts on new medications under development and the role of the Demodex mite in rosacea.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Antibiotic use in dermatology declining, with one exception
Dermatologists are prescribing fewer antibiotics for acne and rosacea, but prescribing after dermatologic surgery has increased in the past decade.
In a study published online Jan. 16 in JAMA Dermatology, researchers report the results of a cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists between 2008 and 2016, using commercial claims data.
The analysis showed that, over this period of time, the overall rate of antibiotic prescribing by dermatologists decreased by 36.6%, from 3.36 courses per 100 dermatologist visits to 2.13 courses. In particular, antibiotic prescribing for acne decreased by 28.1%, from 11.76 courses per 100 visits to 8.45 courses, and for rosacea it decreased by 18.1%, from 10.89 courses per 100 visits to 8.92 courses.
John S. Barbieri, MD, of the department of dermatology, University of Pennsylvania, and his coauthors described the overall decline in antibiotic prescribing as “encouraging,” considering that in 2013 dermatologists were identified as the “most frequent prescribers of oral antibiotics per clinician.” The decline resulted in an estimated 480,000 fewer antibiotic courses a year, they noted.
“Much of the decrease in extended courses of antibiotic therapy is associated with visits for acne and rosacea,” they wrote. “Although recent guidelines suggest limiting the duration of therapy in this patient population, course duration has remained stable over time, suggesting that this decrease may be due to fewer patients being treated with antibiotics rather than patients being treated for a shorter duration.”
However, the rate of oral antibiotic prescriptions associated with surgical visits increased by 69.6%, from 3.92 courses per 100 visits to 6.65. This increase was concerning, given the risk of surgical-site infections was low, the authors pointed out. “In addition, a 2008 advisory statement on antibiotic prophylaxis recommends single-dose perioperative antibiotics for patients at increased risk of surgical-site infection,” they added.
The study also noted a 35.3% increase in antibiotic prescribing for cysts and a 3.2% increase for hidradenitis suppurativa.
Over the entire study period, nearly 1 million courses of oral antibiotics were prescribed. Doxycycline hyclate accounted for around one quarter of prescriptions, as did minocycline, while 19.9% of prescriptions were for cephalexin.
“Given the low rate of infectious complications, even for Mohs surgery, and the lack of evidence to support the use of prolonged rather than single-dose perioperative regimens, the postoperative courses of antibiotics identified in this study may increase risks to patients without substantial benefits,” they added.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. No conflicts of interest were declared.
SOURCE: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
Reducing antibiotic prescribing in dermatology – as in so many other areas of medical practice – is a challenge, but there are a number of strategies that can help.
The first is to take a wait-and-see approach, which has been shown to be effective for childhood otitis media. Communication training for physicians can also help them to manage patient requests for antibiotics by working out the patient’s level of understanding of their condition and treatment options, and their expectations, and getting them to agree to keep antibiotics as a contingency plan. There are clinical decision support tools available to help physicians identify high-risk surgical patients who may require postoperative antibiotics.
It will help to have alternative treatment options for conditions such as acne and rosacea, such as better topical therapies, and an increase in clinical trials for these therapies will hopefully provide more options for patients.
Joslyn S. Kirby, MD, and Jordan S. Lim, MB, are in the department of dermatology, Penn State University, Hershey. These comments are taken from an accompanying editorial (JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4877). They had no disclosures.
Reducing antibiotic prescribing in dermatology – as in so many other areas of medical practice – is a challenge, but there are a number of strategies that can help.
The first is to take a wait-and-see approach, which has been shown to be effective for childhood otitis media. Communication training for physicians can also help them to manage patient requests for antibiotics by working out the patient’s level of understanding of their condition and treatment options, and their expectations, and getting them to agree to keep antibiotics as a contingency plan. There are clinical decision support tools available to help physicians identify high-risk surgical patients who may require postoperative antibiotics.
It will help to have alternative treatment options for conditions such as acne and rosacea, such as better topical therapies, and an increase in clinical trials for these therapies will hopefully provide more options for patients.
Joslyn S. Kirby, MD, and Jordan S. Lim, MB, are in the department of dermatology, Penn State University, Hershey. These comments are taken from an accompanying editorial (JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4877). They had no disclosures.
Reducing antibiotic prescribing in dermatology – as in so many other areas of medical practice – is a challenge, but there are a number of strategies that can help.
The first is to take a wait-and-see approach, which has been shown to be effective for childhood otitis media. Communication training for physicians can also help them to manage patient requests for antibiotics by working out the patient’s level of understanding of their condition and treatment options, and their expectations, and getting them to agree to keep antibiotics as a contingency plan. There are clinical decision support tools available to help physicians identify high-risk surgical patients who may require postoperative antibiotics.
It will help to have alternative treatment options for conditions such as acne and rosacea, such as better topical therapies, and an increase in clinical trials for these therapies will hopefully provide more options for patients.
Joslyn S. Kirby, MD, and Jordan S. Lim, MB, are in the department of dermatology, Penn State University, Hershey. These comments are taken from an accompanying editorial (JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4877). They had no disclosures.
Dermatologists are prescribing fewer antibiotics for acne and rosacea, but prescribing after dermatologic surgery has increased in the past decade.
In a study published online Jan. 16 in JAMA Dermatology, researchers report the results of a cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists between 2008 and 2016, using commercial claims data.
The analysis showed that, over this period of time, the overall rate of antibiotic prescribing by dermatologists decreased by 36.6%, from 3.36 courses per 100 dermatologist visits to 2.13 courses. In particular, antibiotic prescribing for acne decreased by 28.1%, from 11.76 courses per 100 visits to 8.45 courses, and for rosacea it decreased by 18.1%, from 10.89 courses per 100 visits to 8.92 courses.
John S. Barbieri, MD, of the department of dermatology, University of Pennsylvania, and his coauthors described the overall decline in antibiotic prescribing as “encouraging,” considering that in 2013 dermatologists were identified as the “most frequent prescribers of oral antibiotics per clinician.” The decline resulted in an estimated 480,000 fewer antibiotic courses a year, they noted.
“Much of the decrease in extended courses of antibiotic therapy is associated with visits for acne and rosacea,” they wrote. “Although recent guidelines suggest limiting the duration of therapy in this patient population, course duration has remained stable over time, suggesting that this decrease may be due to fewer patients being treated with antibiotics rather than patients being treated for a shorter duration.”
However, the rate of oral antibiotic prescriptions associated with surgical visits increased by 69.6%, from 3.92 courses per 100 visits to 6.65. This increase was concerning, given the risk of surgical-site infections was low, the authors pointed out. “In addition, a 2008 advisory statement on antibiotic prophylaxis recommends single-dose perioperative antibiotics for patients at increased risk of surgical-site infection,” they added.
The study also noted a 35.3% increase in antibiotic prescribing for cysts and a 3.2% increase for hidradenitis suppurativa.
Over the entire study period, nearly 1 million courses of oral antibiotics were prescribed. Doxycycline hyclate accounted for around one quarter of prescriptions, as did minocycline, while 19.9% of prescriptions were for cephalexin.
“Given the low rate of infectious complications, even for Mohs surgery, and the lack of evidence to support the use of prolonged rather than single-dose perioperative regimens, the postoperative courses of antibiotics identified in this study may increase risks to patients without substantial benefits,” they added.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. No conflicts of interest were declared.
SOURCE: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
Dermatologists are prescribing fewer antibiotics for acne and rosacea, but prescribing after dermatologic surgery has increased in the past decade.
In a study published online Jan. 16 in JAMA Dermatology, researchers report the results of a cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists between 2008 and 2016, using commercial claims data.
The analysis showed that, over this period of time, the overall rate of antibiotic prescribing by dermatologists decreased by 36.6%, from 3.36 courses per 100 dermatologist visits to 2.13 courses. In particular, antibiotic prescribing for acne decreased by 28.1%, from 11.76 courses per 100 visits to 8.45 courses, and for rosacea it decreased by 18.1%, from 10.89 courses per 100 visits to 8.92 courses.
John S. Barbieri, MD, of the department of dermatology, University of Pennsylvania, and his coauthors described the overall decline in antibiotic prescribing as “encouraging,” considering that in 2013 dermatologists were identified as the “most frequent prescribers of oral antibiotics per clinician.” The decline resulted in an estimated 480,000 fewer antibiotic courses a year, they noted.
“Much of the decrease in extended courses of antibiotic therapy is associated with visits for acne and rosacea,” they wrote. “Although recent guidelines suggest limiting the duration of therapy in this patient population, course duration has remained stable over time, suggesting that this decrease may be due to fewer patients being treated with antibiotics rather than patients being treated for a shorter duration.”
However, the rate of oral antibiotic prescriptions associated with surgical visits increased by 69.6%, from 3.92 courses per 100 visits to 6.65. This increase was concerning, given the risk of surgical-site infections was low, the authors pointed out. “In addition, a 2008 advisory statement on antibiotic prophylaxis recommends single-dose perioperative antibiotics for patients at increased risk of surgical-site infection,” they added.
The study also noted a 35.3% increase in antibiotic prescribing for cysts and a 3.2% increase for hidradenitis suppurativa.
Over the entire study period, nearly 1 million courses of oral antibiotics were prescribed. Doxycycline hyclate accounted for around one quarter of prescriptions, as did minocycline, while 19.9% of prescriptions were for cephalexin.
“Given the low rate of infectious complications, even for Mohs surgery, and the lack of evidence to support the use of prolonged rather than single-dose perioperative regimens, the postoperative courses of antibiotics identified in this study may increase risks to patients without substantial benefits,” they added.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. No conflicts of interest were declared.
SOURCE: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
FROM JAMA DERMATOLOGY
Key clinical point: Antibiotic prescriptions by dermatologists have decreased since 2008.
Major finding: Between 2008 and 2016, antibiotic prescriptions by dermatologists dropped by 36.6%.
Study details: Cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists from 2008 to 2016.
Disclosures: The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. The authors had no disclosures.
Source: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
A theory of relativity for rosacea patients
Rosacea has several known comorbidities, but the widespread use of their relative, rather than absolute, risks “may result in overestimation of the clinical importance of exposures,” Leonardo A. Tjahjono and his associates wrote.
For a patient with rosacea, the relative risk of hepatic cancer is 1.42, but the attributable risk and the number needed to harm (NNH), which provide “a clearer, absolute picture regarding the association” with rosacea, are 0.46 per 10,000 patient-years and 21,645, respectively. The relative risk of comorbid breast cancer is 1.25, compared with an attributable risk of 6.23 per 10,000 patient-years and an NNH of 1,606, Mr. Tjahjono of Wake Forest University in Winston-Salem, N.C., and his associates reported in the Journal of the American Academy of Dermatology.
Physician misconceptions based on patients’ relative risks of comorbidities may lead to increased cancer screenings, which “can provide great benefit in a proper context; however, they are not without consequences. For example, 0.7 % of liver biopsies result in severe intraperitoneal hematoma,” the investigators said.
The absolute risks – calculated by the investigators using cohort studies that were conducted from June 1, 2008, to June 1, 2018 – present “a better understanding regarding rosacea’s impact on public health and clinical settings, “ they wrote.
SOURCE: Tjahjono LA et al. J Am Acad Dermatol. 2019 Jan 14. doi: 10.1016/j.jaad.2019.01.013.
Rosacea has several known comorbidities, but the widespread use of their relative, rather than absolute, risks “may result in overestimation of the clinical importance of exposures,” Leonardo A. Tjahjono and his associates wrote.

For a patient with rosacea, the relative risk of hepatic cancer is 1.42, but the attributable risk and the number needed to harm (NNH), which provide “a clearer, absolute picture regarding the association” with rosacea, are 0.46 per 10,000 patient-years and 21,645, respectively. The relative risk of comorbid breast cancer is 1.25, compared with an attributable risk of 6.23 per 10,000 patient-years and an NNH of 1,606, Mr. Tjahjono of Wake Forest University in Winston-Salem, N.C., and his associates reported in the Journal of the American Academy of Dermatology.
Physician misconceptions based on patients’ relative risks of comorbidities may lead to increased cancer screenings, which “can provide great benefit in a proper context; however, they are not without consequences. For example, 0.7 % of liver biopsies result in severe intraperitoneal hematoma,” the investigators said.
The absolute risks – calculated by the investigators using cohort studies that were conducted from June 1, 2008, to June 1, 2018 – present “a better understanding regarding rosacea’s impact on public health and clinical settings, “ they wrote.
SOURCE: Tjahjono LA et al. J Am Acad Dermatol. 2019 Jan 14. doi: 10.1016/j.jaad.2019.01.013.
Rosacea has several known comorbidities, but the widespread use of their relative, rather than absolute, risks “may result in overestimation of the clinical importance of exposures,” Leonardo A. Tjahjono and his associates wrote.

For a patient with rosacea, the relative risk of hepatic cancer is 1.42, but the attributable risk and the number needed to harm (NNH), which provide “a clearer, absolute picture regarding the association” with rosacea, are 0.46 per 10,000 patient-years and 21,645, respectively. The relative risk of comorbid breast cancer is 1.25, compared with an attributable risk of 6.23 per 10,000 patient-years and an NNH of 1,606, Mr. Tjahjono of Wake Forest University in Winston-Salem, N.C., and his associates reported in the Journal of the American Academy of Dermatology.
Physician misconceptions based on patients’ relative risks of comorbidities may lead to increased cancer screenings, which “can provide great benefit in a proper context; however, they are not without consequences. For example, 0.7 % of liver biopsies result in severe intraperitoneal hematoma,” the investigators said.
The absolute risks – calculated by the investigators using cohort studies that were conducted from June 1, 2008, to June 1, 2018 – present “a better understanding regarding rosacea’s impact on public health and clinical settings, “ they wrote.
SOURCE: Tjahjono LA et al. J Am Acad Dermatol. 2019 Jan 14. doi: 10.1016/j.jaad.2019.01.013.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Annual cost of branded topical rosacea therapy is twice that of generics
according to a retrospective analysis of claims data published in the Journal of the American Academy of Dermatology.
“We found the mean annual cost of topical therapy for branded medications per person was nearly twice the cost of generics, despite the rise in generic drug costs,” Hadar Lev-Tov, MD, from the department of dermatology and cutaneous surgery at the University of Miami and his colleagues wrote in their study, published as a letter to the editor. “Thus, there is an opportunity to save healthcare costs, nearly $7.5 million annually, for this cohort.”
Dr. Lev-Tov and his colleagues performed an analysis of the MarketScan Commercial Claims and Encounters database to determine real-world costs and usage of rosacea medication and identified 72,173 adults with two or more claims for rosacea who visited a primary care provider, dermatologist, or ophthalmologist over 18 months, from January 2005 through December 2014. The majority of these patients – 62,074 (86%) – received topical medication therapy, while 4,463 (6%) of patients received oral therapy only. Of the patients who received topical therapy, 47,035 (75.8%) received single agent topical therapy and 15,039 (24.2%) patients used combination topical therapy. Metronidazole and azelaic acid were the most common combination used.
The researchers noted that this was “an important proportion” of patients who used combination topical therapy, despite it not being discussed in most guidelines. In addition, they added, “these medications are thought to work by similar mechanisms (anti-inflammatory, antioxidant, and KLK5 modulation) and to our knowledge have not been studied together.”
More patients were treated with branded topical medications (50,334) than with generics (39,621). With regard to price (calculated as the sum of insurance payments, copay, and deductible for each medication over 1 year), the mean annual cost of the branded topical medication was $308.02, compared with $160.37 for generic medications (P less than .0001). The researchers noted switching to a generic medication for treatment of rosacea would potentially save $147.65 per patient a year. (Costs were reported in 2015 US dollars.)
The researchers said their study was limited by lack of Medicare and Medicaid claims data, the retrospective study design, and dependence on an ICD-9 code only for diagnosis of rosacea, but they noted that 92% of patients received a diagnosis from a dermatologist. In addition, they recommended more studies be conducted on the cost-effectiveness of rosacea therapy when comparing systemic medications and topical therapies.
This study was funded in part by a grant from the American Acne and Rosacea Society. The authors report no relevant conflicts of interest.
SOURCE: Lev-Tov H et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.09.039.
according to a retrospective analysis of claims data published in the Journal of the American Academy of Dermatology.
“We found the mean annual cost of topical therapy for branded medications per person was nearly twice the cost of generics, despite the rise in generic drug costs,” Hadar Lev-Tov, MD, from the department of dermatology and cutaneous surgery at the University of Miami and his colleagues wrote in their study, published as a letter to the editor. “Thus, there is an opportunity to save healthcare costs, nearly $7.5 million annually, for this cohort.”
Dr. Lev-Tov and his colleagues performed an analysis of the MarketScan Commercial Claims and Encounters database to determine real-world costs and usage of rosacea medication and identified 72,173 adults with two or more claims for rosacea who visited a primary care provider, dermatologist, or ophthalmologist over 18 months, from January 2005 through December 2014. The majority of these patients – 62,074 (86%) – received topical medication therapy, while 4,463 (6%) of patients received oral therapy only. Of the patients who received topical therapy, 47,035 (75.8%) received single agent topical therapy and 15,039 (24.2%) patients used combination topical therapy. Metronidazole and azelaic acid were the most common combination used.
The researchers noted that this was “an important proportion” of patients who used combination topical therapy, despite it not being discussed in most guidelines. In addition, they added, “these medications are thought to work by similar mechanisms (anti-inflammatory, antioxidant, and KLK5 modulation) and to our knowledge have not been studied together.”
More patients were treated with branded topical medications (50,334) than with generics (39,621). With regard to price (calculated as the sum of insurance payments, copay, and deductible for each medication over 1 year), the mean annual cost of the branded topical medication was $308.02, compared with $160.37 for generic medications (P less than .0001). The researchers noted switching to a generic medication for treatment of rosacea would potentially save $147.65 per patient a year. (Costs were reported in 2015 US dollars.)
The researchers said their study was limited by lack of Medicare and Medicaid claims data, the retrospective study design, and dependence on an ICD-9 code only for diagnosis of rosacea, but they noted that 92% of patients received a diagnosis from a dermatologist. In addition, they recommended more studies be conducted on the cost-effectiveness of rosacea therapy when comparing systemic medications and topical therapies.
This study was funded in part by a grant from the American Acne and Rosacea Society. The authors report no relevant conflicts of interest.
SOURCE: Lev-Tov H et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.09.039.
according to a retrospective analysis of claims data published in the Journal of the American Academy of Dermatology.
“We found the mean annual cost of topical therapy for branded medications per person was nearly twice the cost of generics, despite the rise in generic drug costs,” Hadar Lev-Tov, MD, from the department of dermatology and cutaneous surgery at the University of Miami and his colleagues wrote in their study, published as a letter to the editor. “Thus, there is an opportunity to save healthcare costs, nearly $7.5 million annually, for this cohort.”
Dr. Lev-Tov and his colleagues performed an analysis of the MarketScan Commercial Claims and Encounters database to determine real-world costs and usage of rosacea medication and identified 72,173 adults with two or more claims for rosacea who visited a primary care provider, dermatologist, or ophthalmologist over 18 months, from January 2005 through December 2014. The majority of these patients – 62,074 (86%) – received topical medication therapy, while 4,463 (6%) of patients received oral therapy only. Of the patients who received topical therapy, 47,035 (75.8%) received single agent topical therapy and 15,039 (24.2%) patients used combination topical therapy. Metronidazole and azelaic acid were the most common combination used.
The researchers noted that this was “an important proportion” of patients who used combination topical therapy, despite it not being discussed in most guidelines. In addition, they added, “these medications are thought to work by similar mechanisms (anti-inflammatory, antioxidant, and KLK5 modulation) and to our knowledge have not been studied together.”
More patients were treated with branded topical medications (50,334) than with generics (39,621). With regard to price (calculated as the sum of insurance payments, copay, and deductible for each medication over 1 year), the mean annual cost of the branded topical medication was $308.02, compared with $160.37 for generic medications (P less than .0001). The researchers noted switching to a generic medication for treatment of rosacea would potentially save $147.65 per patient a year. (Costs were reported in 2015 US dollars.)
The researchers said their study was limited by lack of Medicare and Medicaid claims data, the retrospective study design, and dependence on an ICD-9 code only for diagnosis of rosacea, but they noted that 92% of patients received a diagnosis from a dermatologist. In addition, they recommended more studies be conducted on the cost-effectiveness of rosacea therapy when comparing systemic medications and topical therapies.
This study was funded in part by a grant from the American Acne and Rosacea Society. The authors report no relevant conflicts of interest.
SOURCE: Lev-Tov H et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.09.039.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Patients used branded topical medications more often than generic medications for treatment of rosacea.
Major finding: About 50,000 patients were treated with branded topical medications than with generics (almost 40,000), at a mean annual cost of $308.02 and $160.37, respectively.
Study details: A retrospective cohort analysis of 72,173 adults with rosacea treated with topical or oral therapy from a commercial claims database between January 2005 and 2014.
Disclosures: This study was funded in part by a grant from the American Acne and Rosacea Society. The authors report no relevant conflicts of interest.
Source: Lev-Tov H et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.09.039.