User login
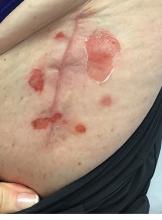
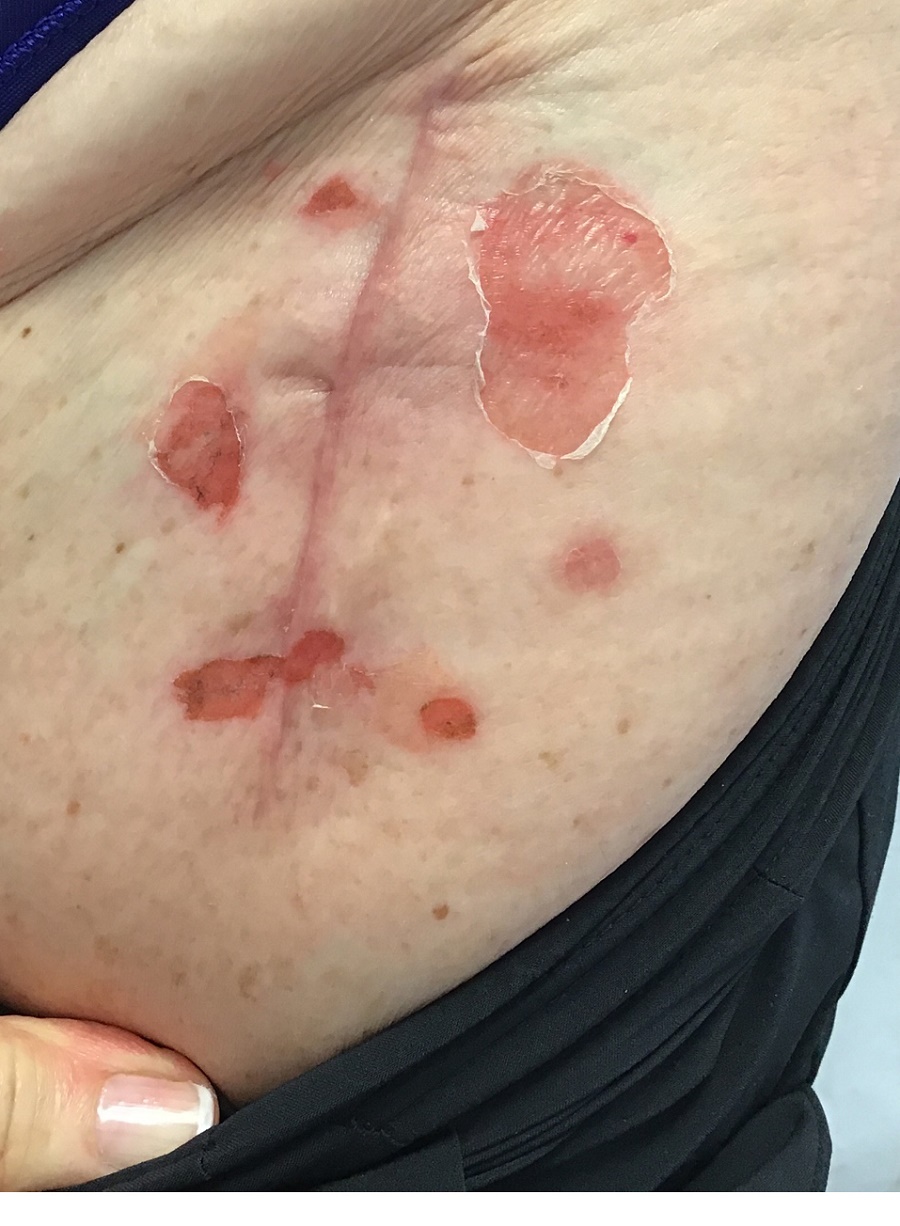
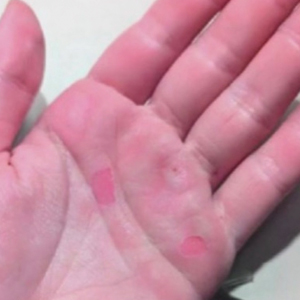
A 71-year-old White female developed erosions after hip replacement surgery 2 months prior to presentation
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
Hypnosis May Offer Relief During Sharp Debridement of Skin Ulcers
TOPLINE:
Hypnosis reduces pain during sharp debridement of skin ulcers in patients with immune-mediated inflammatory diseases, with most patients reporting decreased pain awareness and lasting pain relief for 2-3 days after the procedure.
METHODOLOGY:
- Researchers reported their experience with the anecdotal use of hypnosis for pain management in debridement of skin ulcers in immune-mediated inflammatory diseases.
- They studied 16 participants (14 women; mean age, 56 years; 14 with systemic sclerosis or morphea) with recurrent skin ulcerations requiring sharp debridement, who presented to a wound care clinic at the Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom. The participants had negative experiences with pharmacologic pain management.
- Participants consented to hypnosis during debridement as the only mode of analgesia, conducted by the same hypnosis-trained, experienced healthcare professional in charge of their ulcer care.
- Ulcer pain scores were recorded using a numerical rating pain scale before and immediately after debridement, with a score of 0 indicating no pain and 10 indicating worst pain.
TAKEAWAY:
- Hypnosis reduced the median pre-debridement ulcer pain score from 8 (interquartile range [IQR], 7-10) to 0.5 (IQR, 0-2) immediately after the procedure.
- Of 16 participants, 14 reported being aware of the procedure but not feeling the pain, with only two participants experiencing a brief spike in pain.
- The other two participants reported experiencing reduced awareness and being pain-free during the procedure.
- Five participants reported a lasting decrease in pain perception for 2-3 days after the procedure.
IN PRACTICE:
“These preliminary data underscore the potential for the integration of hypnosis into the management of intervention-related pain in clinical care,” the authors wrote.
SOURCE:
The study was led by Begonya Alcacer-Pitarch, PhD, Leeds Institute of Rheumatic and Musculoskeletal Medicine, the University of Leeds, and Chapel Allerton Hospital in Leeds, United Kingdom. It was published as a correspondence on September 10, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The small sample size may limit the generalizability of the findings. The methods used for data collection were not standardized, and the individuals included in the study may have introduced selection bias.
DISCLOSURES:
The study did not have a funding source. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Hypnosis reduces pain during sharp debridement of skin ulcers in patients with immune-mediated inflammatory diseases, with most patients reporting decreased pain awareness and lasting pain relief for 2-3 days after the procedure.
METHODOLOGY:
- Researchers reported their experience with the anecdotal use of hypnosis for pain management in debridement of skin ulcers in immune-mediated inflammatory diseases.
- They studied 16 participants (14 women; mean age, 56 years; 14 with systemic sclerosis or morphea) with recurrent skin ulcerations requiring sharp debridement, who presented to a wound care clinic at the Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom. The participants had negative experiences with pharmacologic pain management.
- Participants consented to hypnosis during debridement as the only mode of analgesia, conducted by the same hypnosis-trained, experienced healthcare professional in charge of their ulcer care.
- Ulcer pain scores were recorded using a numerical rating pain scale before and immediately after debridement, with a score of 0 indicating no pain and 10 indicating worst pain.
TAKEAWAY:
- Hypnosis reduced the median pre-debridement ulcer pain score from 8 (interquartile range [IQR], 7-10) to 0.5 (IQR, 0-2) immediately after the procedure.
- Of 16 participants, 14 reported being aware of the procedure but not feeling the pain, with only two participants experiencing a brief spike in pain.
- The other two participants reported experiencing reduced awareness and being pain-free during the procedure.
- Five participants reported a lasting decrease in pain perception for 2-3 days after the procedure.
IN PRACTICE:
“These preliminary data underscore the potential for the integration of hypnosis into the management of intervention-related pain in clinical care,” the authors wrote.
SOURCE:
The study was led by Begonya Alcacer-Pitarch, PhD, Leeds Institute of Rheumatic and Musculoskeletal Medicine, the University of Leeds, and Chapel Allerton Hospital in Leeds, United Kingdom. It was published as a correspondence on September 10, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The small sample size may limit the generalizability of the findings. The methods used for data collection were not standardized, and the individuals included in the study may have introduced selection bias.
DISCLOSURES:
The study did not have a funding source. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Hypnosis reduces pain during sharp debridement of skin ulcers in patients with immune-mediated inflammatory diseases, with most patients reporting decreased pain awareness and lasting pain relief for 2-3 days after the procedure.
METHODOLOGY:
- Researchers reported their experience with the anecdotal use of hypnosis for pain management in debridement of skin ulcers in immune-mediated inflammatory diseases.
- They studied 16 participants (14 women; mean age, 56 years; 14 with systemic sclerosis or morphea) with recurrent skin ulcerations requiring sharp debridement, who presented to a wound care clinic at the Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom. The participants had negative experiences with pharmacologic pain management.
- Participants consented to hypnosis during debridement as the only mode of analgesia, conducted by the same hypnosis-trained, experienced healthcare professional in charge of their ulcer care.
- Ulcer pain scores were recorded using a numerical rating pain scale before and immediately after debridement, with a score of 0 indicating no pain and 10 indicating worst pain.
TAKEAWAY:
- Hypnosis reduced the median pre-debridement ulcer pain score from 8 (interquartile range [IQR], 7-10) to 0.5 (IQR, 0-2) immediately after the procedure.
- Of 16 participants, 14 reported being aware of the procedure but not feeling the pain, with only two participants experiencing a brief spike in pain.
- The other two participants reported experiencing reduced awareness and being pain-free during the procedure.
- Five participants reported a lasting decrease in pain perception for 2-3 days after the procedure.
IN PRACTICE:
“These preliminary data underscore the potential for the integration of hypnosis into the management of intervention-related pain in clinical care,” the authors wrote.
SOURCE:
The study was led by Begonya Alcacer-Pitarch, PhD, Leeds Institute of Rheumatic and Musculoskeletal Medicine, the University of Leeds, and Chapel Allerton Hospital in Leeds, United Kingdom. It was published as a correspondence on September 10, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The small sample size may limit the generalizability of the findings. The methods used for data collection were not standardized, and the individuals included in the study may have introduced selection bias.
DISCLOSURES:
The study did not have a funding source. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
First Combined Face and Eye Transplant Performed
In a groundbreaking procedure, a team of surgeons from New York University Langone Health successfully performed the first combined face and eye transplant on a patient with extensive craniofacial tissue loss after an electrical accident.
The highly complex surgery lasted for 21 hours and involved more than 140 surgeons, nurses, and other healthcare professionals under the leadership of Eduardo D. Rodriguez. MD. It not only restored the patient’s facial features, but also integrated a functional eyeball, potentially setting a new standard for future treatments in similar cases.
The transplant took place in May 2023, and the case report was published on September 5 this year in JAMA.
The 46-year-old man lost a large part of his craniofacial tissue and his left eyeball. The approach was highly specialized. Advanced microsurgical techniques such as anastomoses of microscopic vessels and delicate suturing techniques were crucial for the transplant’s success.
Moreover, customized surgical devices, specific implants, and tissue manipulation tools were developed specifically for this case, thus ensuring the viability of the transplant and adequate perfusion of the transplanted ocular tissue.
The initial results are encouraging. Retinal arterial perfusion has been maintained, and retinal architecture has been preserved, as demonstrated by optical coherence tomography. Electroretinography confirmed retinal responses to light, suggesting that the transplanted eye may eventually contribute to the patient’s visual perception. These results are comparable to those of previous facial tissue transplants, but with the significant addition of ocular functionality, which is a notable advance.
“The successful revascularization of the transplanted eye achieved in this study may serve as a step toward the goal of globe transplant for restoration of vision,” wrote the authors.
The complexity of the combined transplant required a deep understanding of facial and ocular anatomy, as well as tissue preservation techniques. The surgical team reported significant challenges, including the need to align delicate anatomical structures and ensure immunological compatibility between the donor and recipient. Meticulous planning from donor selection to postoperative follow-up was considered essential to maximize the likelihood of success and minimize the risk for allograft rejection.
The patient will now be continuously monitored and receive treatment with immunosuppressants such as tacrolimus and prednisone, adjusted according to his response to the transplant. According to the researchers, further studies will be needed to assess the long-term functionality of the transplanted eye and its integration with the central nervous system.
Despite being the fifth facial transplant surgery performed under Dr. Rodriguez’s leadership, this is the first record of a whole-eye transplant. “The mere fact that we have successfully performed the first whole-eye transplant along with a face transplant is a tremendous achievement that many believed to be impossible,” the doctor said in a statement. “We have taken a giant step forward and paved the way for the next chapter in vision restoration.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In a groundbreaking procedure, a team of surgeons from New York University Langone Health successfully performed the first combined face and eye transplant on a patient with extensive craniofacial tissue loss after an electrical accident.
The highly complex surgery lasted for 21 hours and involved more than 140 surgeons, nurses, and other healthcare professionals under the leadership of Eduardo D. Rodriguez. MD. It not only restored the patient’s facial features, but also integrated a functional eyeball, potentially setting a new standard for future treatments in similar cases.
The transplant took place in May 2023, and the case report was published on September 5 this year in JAMA.
The 46-year-old man lost a large part of his craniofacial tissue and his left eyeball. The approach was highly specialized. Advanced microsurgical techniques such as anastomoses of microscopic vessels and delicate suturing techniques were crucial for the transplant’s success.
Moreover, customized surgical devices, specific implants, and tissue manipulation tools were developed specifically for this case, thus ensuring the viability of the transplant and adequate perfusion of the transplanted ocular tissue.
The initial results are encouraging. Retinal arterial perfusion has been maintained, and retinal architecture has been preserved, as demonstrated by optical coherence tomography. Electroretinography confirmed retinal responses to light, suggesting that the transplanted eye may eventually contribute to the patient’s visual perception. These results are comparable to those of previous facial tissue transplants, but with the significant addition of ocular functionality, which is a notable advance.
“The successful revascularization of the transplanted eye achieved in this study may serve as a step toward the goal of globe transplant for restoration of vision,” wrote the authors.
The complexity of the combined transplant required a deep understanding of facial and ocular anatomy, as well as tissue preservation techniques. The surgical team reported significant challenges, including the need to align delicate anatomical structures and ensure immunological compatibility between the donor and recipient. Meticulous planning from donor selection to postoperative follow-up was considered essential to maximize the likelihood of success and minimize the risk for allograft rejection.
The patient will now be continuously monitored and receive treatment with immunosuppressants such as tacrolimus and prednisone, adjusted according to his response to the transplant. According to the researchers, further studies will be needed to assess the long-term functionality of the transplanted eye and its integration with the central nervous system.
Despite being the fifth facial transplant surgery performed under Dr. Rodriguez’s leadership, this is the first record of a whole-eye transplant. “The mere fact that we have successfully performed the first whole-eye transplant along with a face transplant is a tremendous achievement that many believed to be impossible,” the doctor said in a statement. “We have taken a giant step forward and paved the way for the next chapter in vision restoration.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In a groundbreaking procedure, a team of surgeons from New York University Langone Health successfully performed the first combined face and eye transplant on a patient with extensive craniofacial tissue loss after an electrical accident.
The highly complex surgery lasted for 21 hours and involved more than 140 surgeons, nurses, and other healthcare professionals under the leadership of Eduardo D. Rodriguez. MD. It not only restored the patient’s facial features, but also integrated a functional eyeball, potentially setting a new standard for future treatments in similar cases.
The transplant took place in May 2023, and the case report was published on September 5 this year in JAMA.
The 46-year-old man lost a large part of his craniofacial tissue and his left eyeball. The approach was highly specialized. Advanced microsurgical techniques such as anastomoses of microscopic vessels and delicate suturing techniques were crucial for the transplant’s success.
Moreover, customized surgical devices, specific implants, and tissue manipulation tools were developed specifically for this case, thus ensuring the viability of the transplant and adequate perfusion of the transplanted ocular tissue.
The initial results are encouraging. Retinal arterial perfusion has been maintained, and retinal architecture has been preserved, as demonstrated by optical coherence tomography. Electroretinography confirmed retinal responses to light, suggesting that the transplanted eye may eventually contribute to the patient’s visual perception. These results are comparable to those of previous facial tissue transplants, but with the significant addition of ocular functionality, which is a notable advance.
“The successful revascularization of the transplanted eye achieved in this study may serve as a step toward the goal of globe transplant for restoration of vision,” wrote the authors.
The complexity of the combined transplant required a deep understanding of facial and ocular anatomy, as well as tissue preservation techniques. The surgical team reported significant challenges, including the need to align delicate anatomical structures and ensure immunological compatibility between the donor and recipient. Meticulous planning from donor selection to postoperative follow-up was considered essential to maximize the likelihood of success and minimize the risk for allograft rejection.
The patient will now be continuously monitored and receive treatment with immunosuppressants such as tacrolimus and prednisone, adjusted according to his response to the transplant. According to the researchers, further studies will be needed to assess the long-term functionality of the transplanted eye and its integration with the central nervous system.
Despite being the fifth facial transplant surgery performed under Dr. Rodriguez’s leadership, this is the first record of a whole-eye transplant. “The mere fact that we have successfully performed the first whole-eye transplant along with a face transplant is a tremendous achievement that many believed to be impossible,” the doctor said in a statement. “We have taken a giant step forward and paved the way for the next chapter in vision restoration.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Study Identifies Oral Antibiotics Linked to Severe Cutaneous Reactions
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA
Epidermal Tumors Arising on Donor Sites From Autologous Skin Grafts: A Systematic Review
Skin grafting is a surgical technique used to cover skin defects resulting from the removal of skin tumors, ulcers, or burn injuries.1-3 Complications can occur at both donor and recipient sites and may include bleeding, hematoma/seroma formation, postoperative pain, infection, scarring, paresthesia, skin pigmentation, graft contracture, and graft failure.1,2,4,5 The development of epidermal tumors is not commonly reported among the complications of skin grafting; however, cases of epidermal tumor development on skin graft donor sites during the postoperative period have been reported.6-12
We performed a systematic review of the literature for cases of epidermal tumor development on skin graft donor sites in patients undergoing autologous skin graft surgery. We present the clinical characteristics of these cases and discuss the nature of these tumors.
Methods
Search Strategy and Study Selection—A literature search was conducted by 2 independent researchers (Z.P. and V.P.) for articles published before December 2022 in the following databases: MEDLINE/PubMed, Web of Science, Scopus, Cochrane Library, OpenGrey, Google Scholar, and WorldCat. Search terms included all possible combinations of the following: keratoacanthoma, molluscum sebaceum, basal cell carcinoma, squamous cell carcinoma, acanthoma, wart, Merkel cell carcinoma, verruca, Bowen disease, keratosis, skin cancer, cutaneous cancer, skin neoplasia, cutaneous neoplasia, and skin tumor. The literature search terms were selected based on the World Health Organization classification of skin tumors.13 Manual bibliography checks were performed on all eligible search results for possible relevant studies. Discrepancies were resolved through discussion and, if needed, mediation by a third researcher (N.C.). To be included, a study had to report a case(s) of epidermal tumor(s) that was confirmed by histopathology and arose on a graft donor site in a patient receiving autologous skin grafts for any reason. No language, geographic, or report date restrictions were set.
Data Extraction, Quality Assessment, and Statistical Analysis—We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 Two independent researchers (Z.P. and V.P.) retrieved the data from the included studies. We have used the terms case and patient interchangeably, and 1 month was measured as 4 weeks for simplicity. Disagreements were resolved by discussion and mediation by a third researcher (N.C.). The quality of the included studies was assessed by 2 researchers (M.P. and V.P.) using the tool proposed by Murad et al.15
We used descriptive statistical analysis to analyze clinical characteristics of the included cases. We performed separate descriptive analyses based on the most frequently reported types of epidermal tumors and compared the differences between different groups using the Mann-Whitney U test, χ2 test, and Fisher exact test. The level of significance was set at P<.05. All statistical analyses were conducted using SPSS (version 29).
Results
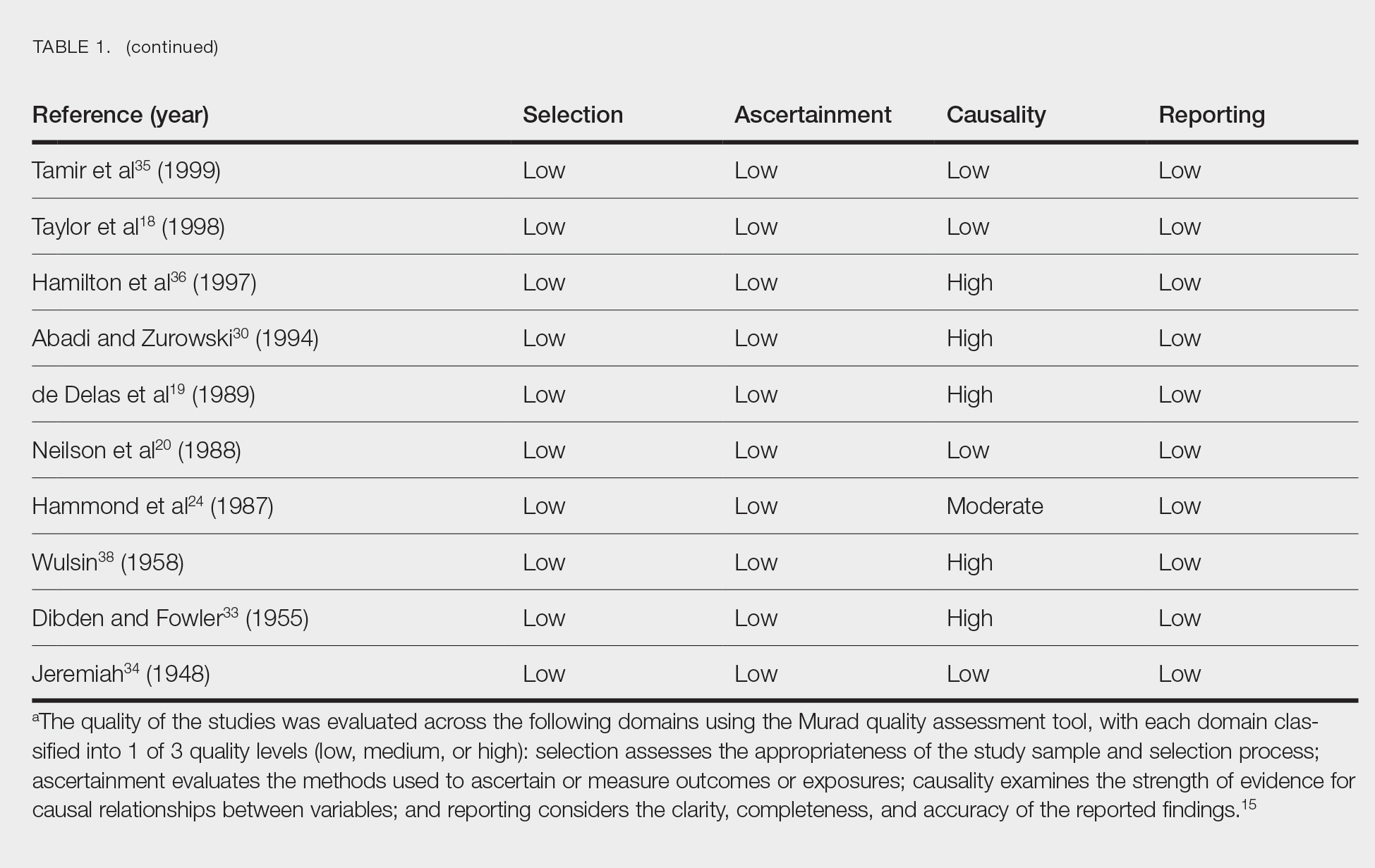
Literature Search and Characteristics of Included Studies—The initial literature search identified 1378 studies, which were screened based on title and abstract. After removing duplicate and irrelevant studies and evaluating the full text of eligible studies, 31 studies (4 case series and 27 case reports) were included in the systematic review (Figure).6-12,16-39 Quality assessment of the included studies is presented in Table 1.
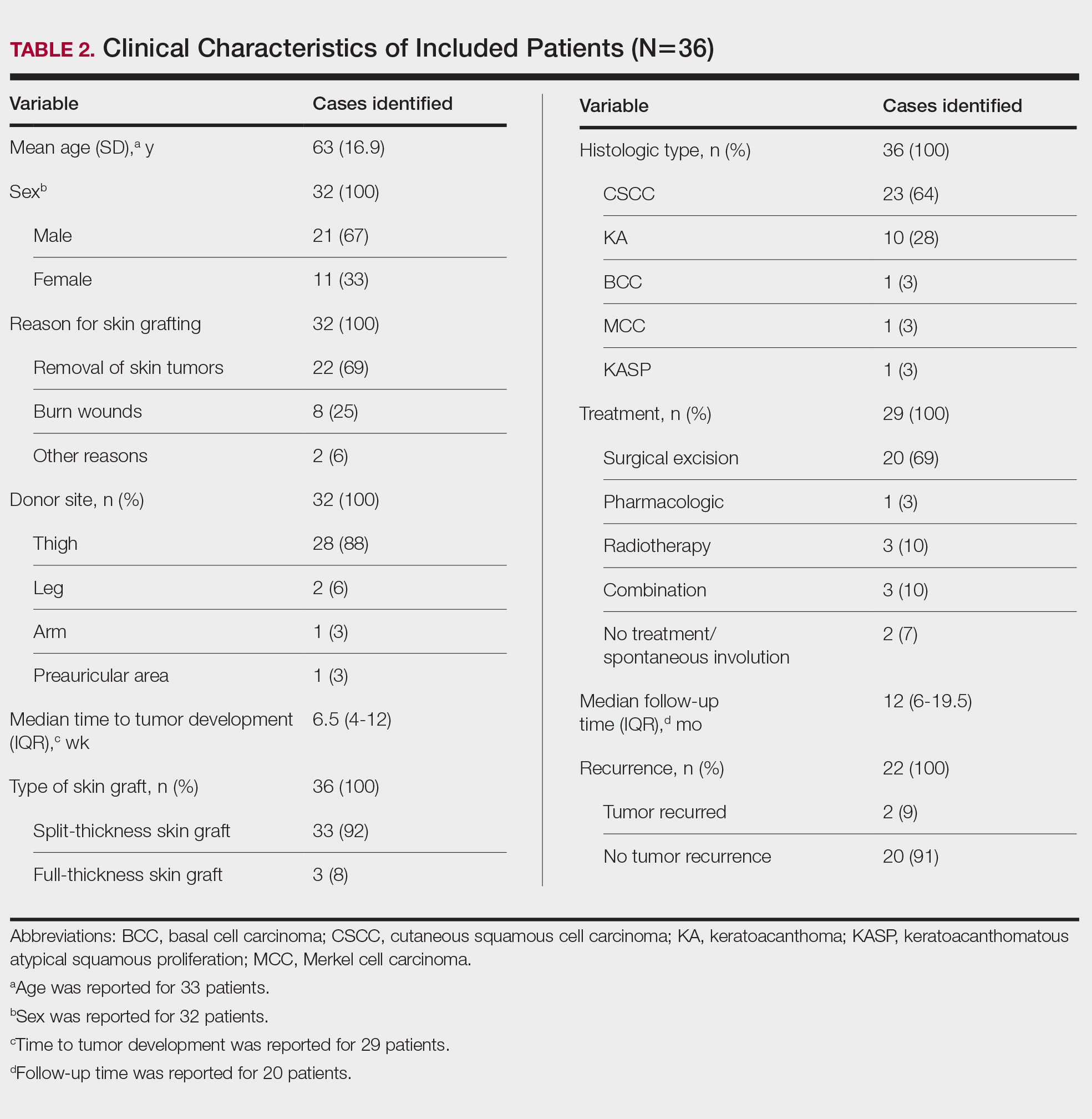
Clinical Characteristics of Included Patients—Our systematic review included 36 patients with a mean age of 63 years and a male to female ratio of 2:1. The 2 most common causes for skin grafting were burn wounds and surgical excision of skin tumors. Most grafts were harvested from the thighs. The development of a solitary lesion on the donor area was reported in two-thirds of the patients, while more than 1 lesion developed in the remaining one-third of patients. The median time to tumor development was 6.5 weeks. In most cases, a split-thickness skin graft was used.
Cutaneous squamous cell carcinomas (CSCCs) were found in 23 patients, with well-differentiated CSCCs in 19 of these cases. Additionally, keratoacanthomas (KAs) were found in 10 patients. The majority of patients underwent surgical excision of the tumor. The median follow-up time was 12 months, during which recurrences were noted in a small percentage of cases. Clinical characteristics of included patients are presented in Table 2.
Comment
Reasons for Tumor Development on Skin Graft Donor Sites—The etiology behind epidermal tumor development on graft donor sites is unclear. According to one theory, iatrogenic contamination of the donor site during the removal of a primary epidermal tumor could be responsible. However, contemporary surgical procedures dictate the use of different sets of instruments for separate surgical sites. Moreover, this theory cannot explain the occurrence of epidermal tumors on donor sites in patients who have undergone skin grafting for the repair of burn wounds.37
Another theory suggests that hematogenous and/or lymphatic spread can occur from the site of the primary epidermal tumor to the donor site, which has increased vascularization.16,37 However, this theory also fails to provide an explanation for the development of epidermal tumors in patients who receive skin grafts for burn wounds.
A third theory states that the microenvironment of the donor site is key to tumor development. The donor site undergoes acute inflammation due to the trauma from harvesting the skin graft. According to this theory, acute inflammation could promote neoplastic growth and thus explain the development of epidermal tumors on the donor site.8,26 However, the relationship between acute inflammation and carcinogenesis remains unclear. What is known to date is that the development of CSCC has been documented primarily in chronically inflamed tissues, whereas the development of KA—a variant of CSCC with distinctive and more benign clinical characteristics—can be expected in the setting of acute trauma-related inflammation.13,40,41
Based on our systematic review, we propose that well-differentiated CSCC on graft donor sites might actually be misdiagnosed KA, given that the histopathologic differential diagnosis between CSCC and KA is extremely challenging.42 This hypothesis could explain the development of well-differentiated CSCC and KA on graft donor sites.
Conclusion
Development of CSCC and KA on graft donor sites can be listed among the postoperative complications of autologous skin grafting. Patients and physicians should be aware of this potential complication, and donor sites should be monitored for the occurrence of epidermal tumors.
- Adams DC, Ramsey ML. Grafts in dermatologic surgery: review and update on full- and split-thickness skin grafts, free cartilage grafts, and composite grafts. Dermatologic Surg. 2005;31(8, pt 2):1055-1067. doi:10.1111/j.1524-4725.2005.31831
- Shimizu R, Kishi K. Skin graft. Plast Surg Int. 2012;2012:563493. doi:10.1155/2012/563493
- Reddy S, El-Haddawi F, Fancourt M, et al. The incidence and risk factors for lower limb skin graft failure. Dermatol Res Pract. 2014;2014:582080. doi:10.1155/2014/582080
- Coughlin MJ, Dockery GD, Crawford ME, et al. Lower Extremity Soft Tissue & Cutaneous Plastic Surgery. 2nd ed. Saunders Ltd; 2012.
- Herskovitz I, Hughes OB, Macquhae F, et al. Epidermal skin grafting. Int Wound J. 2016;13(suppl 3):52-56. doi:10.1111/iwj.12631
- Wright H, McKinnell TH, Dunkin C. Recurrence of cutaneous squamous cell carcinoma at remote limb donor site. J Plast Reconstr Aesthet Surg. 2012;65:1265-1266. doi:10.1016/j.bjps.2012.01.022
- Thomas W, Rezzadeh K, Rossi K, et al. Squamous cell carcinoma arising at a skin graft donor site: case report and review of the literature. Plast Surg Case Stud. 2021;7:2513826X211008425. doi:10.1177/2513826X211008425
- Ponnuvelu G, Ng MFY, Connolly CM, et al. Inflammation to skin malignancy, time to rethink the link: SCC in skin graft donor sites. Surgeon. 2011;9:168-169. doi:10.1016/j.surge.2010.08.006
- Noori VJ, Trehan K, Savetamal A, et al. New onset squamous cell carcinoma in previous split-thickness skin graft donor site. Int J Surg. 2018;52:16-19. doi:10.1016/j.ijsu.2018.01.047
- Morritt DG, Khandwala AR. The development of squamous cell carcinomas in split-thickness skin graft donor sites. Eur J Plast Surg. 2013;36:377-380.
- McCormick M, Miotke S. Squamous cell carcinoma at split thickness skin graft donor site: a case report and review of the literature. J Burn Care Res. 2023;44:210-213. doi:10.1093/jbcr/irac137
- Haik J, Georgiou I, Farber N, et al. Squamous cell carcinoma arising in a split-thickness skin graft donor site. Burns. 2008;34:891-893. doi:10.1016/j.burns.2007.06.006
- Elder DE, Massi D, Scolyer RA WR. WHO Classification of Skin Tumours. 4th ed. IARC Press; 2018.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. doi:10.7326/0003-4819-151-4-200908180-00135
- Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ. 2018;23:60-63. doi:10.1136/bmjebm-2017-110853
- de Moraes LPB, Burchett I, Nicholls S, et al. Large solitary distant metastasis of cutaneous squamous cell carcinoma to skin graft site with complete response following definitive radiotherapy. Int J Bioautomation. 2017;21:103-108.
- Nagase K, Suzuki Y, Misago N, et al. Acute development of keratoacanthoma at a full-thickness skin graft donor site shortly after surgery. J Dermatol. 2016;43:1232-1233. doi:10.1111/1346-8138.13368
- Taylor CD, Snelling CF, Nickerson D, et al. Acute development of invasive squamous cell carcinoma in a split-thickness skin graft donor site. J Burn Care Rehabil. 1998;19:382-385. doi:10.1097/00004630-199809000-00004
- de Delas J, Leache A, Vazquez Doval J, et al. Keratoacanthoma over the donor site of a laminar skin graft. Med Cutan Ibero Lat Am. 1989;17:225-228.
- Neilson D, Emerson DJ, Dunn L. Squamous cell carcinoma of skin developing in a skin graft donor site. Br J Plast Surg. 1988;41:417-419. doi:10.1016/0007-1226(88)90086-0
- May JT, Patil YJ. Keratoacanthoma-type squamous cell carcinoma developing in a skin graft donor site after tumor extirpation at a distant site. Ear Nose Throat J. 2010;89:E11-E13.
- Imbernón-Moya A, Vargas-Laguna E, Lobato-Berezo A, et al. Simultaneous onset of basal cell carcinoma over skin graft and donor site. JAAD Case Rep. 2015;1:244-246. doi:10.1016/j.jdcr.2015.05.004
- Lee S, Coutts I, Ryan A, et al. Keratoacanthoma formation after skin grafting: a brief report and pathophysiological hypothesis. Australas J Dermatol. 2017;58:e117-e119. doi:10.1111/ajd.12501
- Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising acutelyin a skin graft donor site. J Trauma. 1987;27:681-683. doi:10.1097/00005373-198706000-00017
- Herard C, Arnaud D, Goga D, et al. Rapid onset of squamous cell carcinoma in a thin skin graft donor site. Ann Dermatol Venereol. 2016;143:457-461. doi:10.1016/j.annder.2015.03.027
- Ibrahim A, Moisidis E. Case series: rapidly growing squamous cell carcinoma after cutaneous surgical intervention. JPRAS Open. 2017;14:27-32. doi:10.1016/j.jpra.2017.08.004
- Kearney L, Dolan RT, Parfrey NA, et al. Squamous cell carcinoma arising in a skin graft donor site following melanoma extirpation at a distant site: a case report and review of the literature. JPRAS Open. 2015;3:35-38. doi:10.1016/j.jpra.2015.02.002
- Clark MA, Guitart J, Gerami P, et al. Eruptive keratoacanthomatous atypical squamous proliferations (KASPs) arising in skin graft sites. JAAD Case Rep. 2015;1:274-276. doi:10.1016/j.jdcr.2015.06.009
- Aloraifi F, Mulgrew S, James NK. Secondary Merkel cell carcinoma arising from a graft donor site. J Cutan Med Surg. 2017;21:167-169. doi:10.1177/1203475416676805
- Abadir R, Zurowski S. Case report: squamous cell carcinoma of the skin in both palms, axillary node, donor skin graft site and both soles—associated hyperkeratosis and porokeratosis. Br J Radiol. 1994;67:507-510. doi:10.1259/0007-1285-67-797-507
- Griffiths RW. Keratoacanthoma observed. Br J Plast Surg. 2004;57:485-501. doi:10.1016/j.bjps.2004.05.007
- Marous M, Brady K. Cutaneous squamous cell carcinoma arising in a split thickness skin graft donor site in a patient with systemic lupus erythematosus. Dermatologic Surg. 2021;47:1106-1107. doi:10.1097/DSS.0000000000002955
- Dibden FA, Fowler M. The multiple growth of molluscum sebaceum in donor and recipient sites of skin graft. Aust N Z J Surg. 1955;25:157-159. doi:10.1111/j.1445-2197.1955.tb05122.x
- Jeremiah BS. Squamous cell carcinoma development on donor area following removal of a split thickness skin graft. Plast Reconstr Surg. 1948;3:718-721.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;40(5, pt 2):870-871. doi:10.1053/jd.1999.v40.a94419
- Hamilton SA, Dickson WA, O’Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561. doi:10.1016/s0007-1226(97)91308-4
- Hussain A, Ekwobi C, Watson S. Metastatic implantation squamous cell carcinoma in a split-thickness skin graft donor site. J Plast Reconstr Aesthet Surg. 2011;64:690-692. doi:10.1016/j.bjps.2010.06.004
- Wulsin JH. Keratoacanthoma: a benign cutaneous tumors arising in a skin graft donor site. Am Surg. 1958;24:689-692.
- Davis L, Butler D. Acute development of squamous cell carcinoma in a split-thickness skin graft donor site [abstract]. J Am Acad Dermatol. 2012;66:AB208. doi:10.1016/j.jaad.2011.11.874
- Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002;16:217-226, 229; discussion 230-232.
- Piotrowski I, Kulcenty K, Suchorska W. Interplay between inflammation and cancer. Reports Pract Oncol Radiother. 2020;25:422-427. doi:10.1016/j.rpor.2020.04.004
- Carr RA, Houghton JP. Histopathologists’ approach to keratoacanthoma: a multisite survey of regional variation in Great Britain and Ireland. J Clin Pathol. 2014;67:637-638. doi:10.1136/jclinpath-2014-202255
Skin grafting is a surgical technique used to cover skin defects resulting from the removal of skin tumors, ulcers, or burn injuries.1-3 Complications can occur at both donor and recipient sites and may include bleeding, hematoma/seroma formation, postoperative pain, infection, scarring, paresthesia, skin pigmentation, graft contracture, and graft failure.1,2,4,5 The development of epidermal tumors is not commonly reported among the complications of skin grafting; however, cases of epidermal tumor development on skin graft donor sites during the postoperative period have been reported.6-12
We performed a systematic review of the literature for cases of epidermal tumor development on skin graft donor sites in patients undergoing autologous skin graft surgery. We present the clinical characteristics of these cases and discuss the nature of these tumors.
Methods
Search Strategy and Study Selection—A literature search was conducted by 2 independent researchers (Z.P. and V.P.) for articles published before December 2022 in the following databases: MEDLINE/PubMed, Web of Science, Scopus, Cochrane Library, OpenGrey, Google Scholar, and WorldCat. Search terms included all possible combinations of the following: keratoacanthoma, molluscum sebaceum, basal cell carcinoma, squamous cell carcinoma, acanthoma, wart, Merkel cell carcinoma, verruca, Bowen disease, keratosis, skin cancer, cutaneous cancer, skin neoplasia, cutaneous neoplasia, and skin tumor. The literature search terms were selected based on the World Health Organization classification of skin tumors.13 Manual bibliography checks were performed on all eligible search results for possible relevant studies. Discrepancies were resolved through discussion and, if needed, mediation by a third researcher (N.C.). To be included, a study had to report a case(s) of epidermal tumor(s) that was confirmed by histopathology and arose on a graft donor site in a patient receiving autologous skin grafts for any reason. No language, geographic, or report date restrictions were set.
Data Extraction, Quality Assessment, and Statistical Analysis—We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 Two independent researchers (Z.P. and V.P.) retrieved the data from the included studies. We have used the terms case and patient interchangeably, and 1 month was measured as 4 weeks for simplicity. Disagreements were resolved by discussion and mediation by a third researcher (N.C.). The quality of the included studies was assessed by 2 researchers (M.P. and V.P.) using the tool proposed by Murad et al.15
We used descriptive statistical analysis to analyze clinical characteristics of the included cases. We performed separate descriptive analyses based on the most frequently reported types of epidermal tumors and compared the differences between different groups using the Mann-Whitney U test, χ2 test, and Fisher exact test. The level of significance was set at P<.05. All statistical analyses were conducted using SPSS (version 29).
Results
Literature Search and Characteristics of Included Studies—The initial literature search identified 1378 studies, which were screened based on title and abstract. After removing duplicate and irrelevant studies and evaluating the full text of eligible studies, 31 studies (4 case series and 27 case reports) were included in the systematic review (Figure).6-12,16-39 Quality assessment of the included studies is presented in Table 1.


Clinical Characteristics of Included Patients—Our systematic review included 36 patients with a mean age of 63 years and a male to female ratio of 2:1. The 2 most common causes for skin grafting were burn wounds and surgical excision of skin tumors. Most grafts were harvested from the thighs. The development of a solitary lesion on the donor area was reported in two-thirds of the patients, while more than 1 lesion developed in the remaining one-third of patients. The median time to tumor development was 6.5 weeks. In most cases, a split-thickness skin graft was used.
Cutaneous squamous cell carcinomas (CSCCs) were found in 23 patients, with well-differentiated CSCCs in 19 of these cases. Additionally, keratoacanthomas (KAs) were found in 10 patients. The majority of patients underwent surgical excision of the tumor. The median follow-up time was 12 months, during which recurrences were noted in a small percentage of cases. Clinical characteristics of included patients are presented in Table 2.


Comment
Reasons for Tumor Development on Skin Graft Donor Sites—The etiology behind epidermal tumor development on graft donor sites is unclear. According to one theory, iatrogenic contamination of the donor site during the removal of a primary epidermal tumor could be responsible. However, contemporary surgical procedures dictate the use of different sets of instruments for separate surgical sites. Moreover, this theory cannot explain the occurrence of epidermal tumors on donor sites in patients who have undergone skin grafting for the repair of burn wounds.37
Another theory suggests that hematogenous and/or lymphatic spread can occur from the site of the primary epidermal tumor to the donor site, which has increased vascularization.16,37 However, this theory also fails to provide an explanation for the development of epidermal tumors in patients who receive skin grafts for burn wounds.
A third theory states that the microenvironment of the donor site is key to tumor development. The donor site undergoes acute inflammation due to the trauma from harvesting the skin graft. According to this theory, acute inflammation could promote neoplastic growth and thus explain the development of epidermal tumors on the donor site.8,26 However, the relationship between acute inflammation and carcinogenesis remains unclear. What is known to date is that the development of CSCC has been documented primarily in chronically inflamed tissues, whereas the development of KA—a variant of CSCC with distinctive and more benign clinical characteristics—can be expected in the setting of acute trauma-related inflammation.13,40,41
Based on our systematic review, we propose that well-differentiated CSCC on graft donor sites might actually be misdiagnosed KA, given that the histopathologic differential diagnosis between CSCC and KA is extremely challenging.42 This hypothesis could explain the development of well-differentiated CSCC and KA on graft donor sites.
Conclusion
Development of CSCC and KA on graft donor sites can be listed among the postoperative complications of autologous skin grafting. Patients and physicians should be aware of this potential complication, and donor sites should be monitored for the occurrence of epidermal tumors.
Skin grafting is a surgical technique used to cover skin defects resulting from the removal of skin tumors, ulcers, or burn injuries.1-3 Complications can occur at both donor and recipient sites and may include bleeding, hematoma/seroma formation, postoperative pain, infection, scarring, paresthesia, skin pigmentation, graft contracture, and graft failure.1,2,4,5 The development of epidermal tumors is not commonly reported among the complications of skin grafting; however, cases of epidermal tumor development on skin graft donor sites during the postoperative period have been reported.6-12
We performed a systematic review of the literature for cases of epidermal tumor development on skin graft donor sites in patients undergoing autologous skin graft surgery. We present the clinical characteristics of these cases and discuss the nature of these tumors.
Methods
Search Strategy and Study Selection—A literature search was conducted by 2 independent researchers (Z.P. and V.P.) for articles published before December 2022 in the following databases: MEDLINE/PubMed, Web of Science, Scopus, Cochrane Library, OpenGrey, Google Scholar, and WorldCat. Search terms included all possible combinations of the following: keratoacanthoma, molluscum sebaceum, basal cell carcinoma, squamous cell carcinoma, acanthoma, wart, Merkel cell carcinoma, verruca, Bowen disease, keratosis, skin cancer, cutaneous cancer, skin neoplasia, cutaneous neoplasia, and skin tumor. The literature search terms were selected based on the World Health Organization classification of skin tumors.13 Manual bibliography checks were performed on all eligible search results for possible relevant studies. Discrepancies were resolved through discussion and, if needed, mediation by a third researcher (N.C.). To be included, a study had to report a case(s) of epidermal tumor(s) that was confirmed by histopathology and arose on a graft donor site in a patient receiving autologous skin grafts for any reason. No language, geographic, or report date restrictions were set.
Data Extraction, Quality Assessment, and Statistical Analysis—We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 Two independent researchers (Z.P. and V.P.) retrieved the data from the included studies. We have used the terms case and patient interchangeably, and 1 month was measured as 4 weeks for simplicity. Disagreements were resolved by discussion and mediation by a third researcher (N.C.). The quality of the included studies was assessed by 2 researchers (M.P. and V.P.) using the tool proposed by Murad et al.15
We used descriptive statistical analysis to analyze clinical characteristics of the included cases. We performed separate descriptive analyses based on the most frequently reported types of epidermal tumors and compared the differences between different groups using the Mann-Whitney U test, χ2 test, and Fisher exact test. The level of significance was set at P<.05. All statistical analyses were conducted using SPSS (version 29).
Results
Literature Search and Characteristics of Included Studies—The initial literature search identified 1378 studies, which were screened based on title and abstract. After removing duplicate and irrelevant studies and evaluating the full text of eligible studies, 31 studies (4 case series and 27 case reports) were included in the systematic review (Figure).6-12,16-39 Quality assessment of the included studies is presented in Table 1.


Clinical Characteristics of Included Patients—Our systematic review included 36 patients with a mean age of 63 years and a male to female ratio of 2:1. The 2 most common causes for skin grafting were burn wounds and surgical excision of skin tumors. Most grafts were harvested from the thighs. The development of a solitary lesion on the donor area was reported in two-thirds of the patients, while more than 1 lesion developed in the remaining one-third of patients. The median time to tumor development was 6.5 weeks. In most cases, a split-thickness skin graft was used.
Cutaneous squamous cell carcinomas (CSCCs) were found in 23 patients, with well-differentiated CSCCs in 19 of these cases. Additionally, keratoacanthomas (KAs) were found in 10 patients. The majority of patients underwent surgical excision of the tumor. The median follow-up time was 12 months, during which recurrences were noted in a small percentage of cases. Clinical characteristics of included patients are presented in Table 2.


Comment
Reasons for Tumor Development on Skin Graft Donor Sites—The etiology behind epidermal tumor development on graft donor sites is unclear. According to one theory, iatrogenic contamination of the donor site during the removal of a primary epidermal tumor could be responsible. However, contemporary surgical procedures dictate the use of different sets of instruments for separate surgical sites. Moreover, this theory cannot explain the occurrence of epidermal tumors on donor sites in patients who have undergone skin grafting for the repair of burn wounds.37
Another theory suggests that hematogenous and/or lymphatic spread can occur from the site of the primary epidermal tumor to the donor site, which has increased vascularization.16,37 However, this theory also fails to provide an explanation for the development of epidermal tumors in patients who receive skin grafts for burn wounds.
A third theory states that the microenvironment of the donor site is key to tumor development. The donor site undergoes acute inflammation due to the trauma from harvesting the skin graft. According to this theory, acute inflammation could promote neoplastic growth and thus explain the development of epidermal tumors on the donor site.8,26 However, the relationship between acute inflammation and carcinogenesis remains unclear. What is known to date is that the development of CSCC has been documented primarily in chronically inflamed tissues, whereas the development of KA—a variant of CSCC with distinctive and more benign clinical characteristics—can be expected in the setting of acute trauma-related inflammation.13,40,41
Based on our systematic review, we propose that well-differentiated CSCC on graft donor sites might actually be misdiagnosed KA, given that the histopathologic differential diagnosis between CSCC and KA is extremely challenging.42 This hypothesis could explain the development of well-differentiated CSCC and KA on graft donor sites.
Conclusion
Development of CSCC and KA on graft donor sites can be listed among the postoperative complications of autologous skin grafting. Patients and physicians should be aware of this potential complication, and donor sites should be monitored for the occurrence of epidermal tumors.
- Adams DC, Ramsey ML. Grafts in dermatologic surgery: review and update on full- and split-thickness skin grafts, free cartilage grafts, and composite grafts. Dermatologic Surg. 2005;31(8, pt 2):1055-1067. doi:10.1111/j.1524-4725.2005.31831
- Shimizu R, Kishi K. Skin graft. Plast Surg Int. 2012;2012:563493. doi:10.1155/2012/563493
- Reddy S, El-Haddawi F, Fancourt M, et al. The incidence and risk factors for lower limb skin graft failure. Dermatol Res Pract. 2014;2014:582080. doi:10.1155/2014/582080
- Coughlin MJ, Dockery GD, Crawford ME, et al. Lower Extremity Soft Tissue & Cutaneous Plastic Surgery. 2nd ed. Saunders Ltd; 2012.
- Herskovitz I, Hughes OB, Macquhae F, et al. Epidermal skin grafting. Int Wound J. 2016;13(suppl 3):52-56. doi:10.1111/iwj.12631
- Wright H, McKinnell TH, Dunkin C. Recurrence of cutaneous squamous cell carcinoma at remote limb donor site. J Plast Reconstr Aesthet Surg. 2012;65:1265-1266. doi:10.1016/j.bjps.2012.01.022
- Thomas W, Rezzadeh K, Rossi K, et al. Squamous cell carcinoma arising at a skin graft donor site: case report and review of the literature. Plast Surg Case Stud. 2021;7:2513826X211008425. doi:10.1177/2513826X211008425
- Ponnuvelu G, Ng MFY, Connolly CM, et al. Inflammation to skin malignancy, time to rethink the link: SCC in skin graft donor sites. Surgeon. 2011;9:168-169. doi:10.1016/j.surge.2010.08.006
- Noori VJ, Trehan K, Savetamal A, et al. New onset squamous cell carcinoma in previous split-thickness skin graft donor site. Int J Surg. 2018;52:16-19. doi:10.1016/j.ijsu.2018.01.047
- Morritt DG, Khandwala AR. The development of squamous cell carcinomas in split-thickness skin graft donor sites. Eur J Plast Surg. 2013;36:377-380.
- McCormick M, Miotke S. Squamous cell carcinoma at split thickness skin graft donor site: a case report and review of the literature. J Burn Care Res. 2023;44:210-213. doi:10.1093/jbcr/irac137
- Haik J, Georgiou I, Farber N, et al. Squamous cell carcinoma arising in a split-thickness skin graft donor site. Burns. 2008;34:891-893. doi:10.1016/j.burns.2007.06.006
- Elder DE, Massi D, Scolyer RA WR. WHO Classification of Skin Tumours. 4th ed. IARC Press; 2018.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. doi:10.7326/0003-4819-151-4-200908180-00135
- Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ. 2018;23:60-63. doi:10.1136/bmjebm-2017-110853
- de Moraes LPB, Burchett I, Nicholls S, et al. Large solitary distant metastasis of cutaneous squamous cell carcinoma to skin graft site with complete response following definitive radiotherapy. Int J Bioautomation. 2017;21:103-108.
- Nagase K, Suzuki Y, Misago N, et al. Acute development of keratoacanthoma at a full-thickness skin graft donor site shortly after surgery. J Dermatol. 2016;43:1232-1233. doi:10.1111/1346-8138.13368
- Taylor CD, Snelling CF, Nickerson D, et al. Acute development of invasive squamous cell carcinoma in a split-thickness skin graft donor site. J Burn Care Rehabil. 1998;19:382-385. doi:10.1097/00004630-199809000-00004
- de Delas J, Leache A, Vazquez Doval J, et al. Keratoacanthoma over the donor site of a laminar skin graft. Med Cutan Ibero Lat Am. 1989;17:225-228.
- Neilson D, Emerson DJ, Dunn L. Squamous cell carcinoma of skin developing in a skin graft donor site. Br J Plast Surg. 1988;41:417-419. doi:10.1016/0007-1226(88)90086-0
- May JT, Patil YJ. Keratoacanthoma-type squamous cell carcinoma developing in a skin graft donor site after tumor extirpation at a distant site. Ear Nose Throat J. 2010;89:E11-E13.
- Imbernón-Moya A, Vargas-Laguna E, Lobato-Berezo A, et al. Simultaneous onset of basal cell carcinoma over skin graft and donor site. JAAD Case Rep. 2015;1:244-246. doi:10.1016/j.jdcr.2015.05.004
- Lee S, Coutts I, Ryan A, et al. Keratoacanthoma formation after skin grafting: a brief report and pathophysiological hypothesis. Australas J Dermatol. 2017;58:e117-e119. doi:10.1111/ajd.12501
- Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising acutelyin a skin graft donor site. J Trauma. 1987;27:681-683. doi:10.1097/00005373-198706000-00017
- Herard C, Arnaud D, Goga D, et al. Rapid onset of squamous cell carcinoma in a thin skin graft donor site. Ann Dermatol Venereol. 2016;143:457-461. doi:10.1016/j.annder.2015.03.027
- Ibrahim A, Moisidis E. Case series: rapidly growing squamous cell carcinoma after cutaneous surgical intervention. JPRAS Open. 2017;14:27-32. doi:10.1016/j.jpra.2017.08.004
- Kearney L, Dolan RT, Parfrey NA, et al. Squamous cell carcinoma arising in a skin graft donor site following melanoma extirpation at a distant site: a case report and review of the literature. JPRAS Open. 2015;3:35-38. doi:10.1016/j.jpra.2015.02.002
- Clark MA, Guitart J, Gerami P, et al. Eruptive keratoacanthomatous atypical squamous proliferations (KASPs) arising in skin graft sites. JAAD Case Rep. 2015;1:274-276. doi:10.1016/j.jdcr.2015.06.009
- Aloraifi F, Mulgrew S, James NK. Secondary Merkel cell carcinoma arising from a graft donor site. J Cutan Med Surg. 2017;21:167-169. doi:10.1177/1203475416676805
- Abadir R, Zurowski S. Case report: squamous cell carcinoma of the skin in both palms, axillary node, donor skin graft site and both soles—associated hyperkeratosis and porokeratosis. Br J Radiol. 1994;67:507-510. doi:10.1259/0007-1285-67-797-507
- Griffiths RW. Keratoacanthoma observed. Br J Plast Surg. 2004;57:485-501. doi:10.1016/j.bjps.2004.05.007
- Marous M, Brady K. Cutaneous squamous cell carcinoma arising in a split thickness skin graft donor site in a patient with systemic lupus erythematosus. Dermatologic Surg. 2021;47:1106-1107. doi:10.1097/DSS.0000000000002955
- Dibden FA, Fowler M. The multiple growth of molluscum sebaceum in donor and recipient sites of skin graft. Aust N Z J Surg. 1955;25:157-159. doi:10.1111/j.1445-2197.1955.tb05122.x
- Jeremiah BS. Squamous cell carcinoma development on donor area following removal of a split thickness skin graft. Plast Reconstr Surg. 1948;3:718-721.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;40(5, pt 2):870-871. doi:10.1053/jd.1999.v40.a94419
- Hamilton SA, Dickson WA, O’Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561. doi:10.1016/s0007-1226(97)91308-4
- Hussain A, Ekwobi C, Watson S. Metastatic implantation squamous cell carcinoma in a split-thickness skin graft donor site. J Plast Reconstr Aesthet Surg. 2011;64:690-692. doi:10.1016/j.bjps.2010.06.004
- Wulsin JH. Keratoacanthoma: a benign cutaneous tumors arising in a skin graft donor site. Am Surg. 1958;24:689-692.
- Davis L, Butler D. Acute development of squamous cell carcinoma in a split-thickness skin graft donor site [abstract]. J Am Acad Dermatol. 2012;66:AB208. doi:10.1016/j.jaad.2011.11.874
- Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002;16:217-226, 229; discussion 230-232.
- Piotrowski I, Kulcenty K, Suchorska W. Interplay between inflammation and cancer. Reports Pract Oncol Radiother. 2020;25:422-427. doi:10.1016/j.rpor.2020.04.004
- Carr RA, Houghton JP. Histopathologists’ approach to keratoacanthoma: a multisite survey of regional variation in Great Britain and Ireland. J Clin Pathol. 2014;67:637-638. doi:10.1136/jclinpath-2014-202255
- Adams DC, Ramsey ML. Grafts in dermatologic surgery: review and update on full- and split-thickness skin grafts, free cartilage grafts, and composite grafts. Dermatologic Surg. 2005;31(8, pt 2):1055-1067. doi:10.1111/j.1524-4725.2005.31831
- Shimizu R, Kishi K. Skin graft. Plast Surg Int. 2012;2012:563493. doi:10.1155/2012/563493
- Reddy S, El-Haddawi F, Fancourt M, et al. The incidence and risk factors for lower limb skin graft failure. Dermatol Res Pract. 2014;2014:582080. doi:10.1155/2014/582080
- Coughlin MJ, Dockery GD, Crawford ME, et al. Lower Extremity Soft Tissue & Cutaneous Plastic Surgery. 2nd ed. Saunders Ltd; 2012.
- Herskovitz I, Hughes OB, Macquhae F, et al. Epidermal skin grafting. Int Wound J. 2016;13(suppl 3):52-56. doi:10.1111/iwj.12631
- Wright H, McKinnell TH, Dunkin C. Recurrence of cutaneous squamous cell carcinoma at remote limb donor site. J Plast Reconstr Aesthet Surg. 2012;65:1265-1266. doi:10.1016/j.bjps.2012.01.022
- Thomas W, Rezzadeh K, Rossi K, et al. Squamous cell carcinoma arising at a skin graft donor site: case report and review of the literature. Plast Surg Case Stud. 2021;7:2513826X211008425. doi:10.1177/2513826X211008425
- Ponnuvelu G, Ng MFY, Connolly CM, et al. Inflammation to skin malignancy, time to rethink the link: SCC in skin graft donor sites. Surgeon. 2011;9:168-169. doi:10.1016/j.surge.2010.08.006
- Noori VJ, Trehan K, Savetamal A, et al. New onset squamous cell carcinoma in previous split-thickness skin graft donor site. Int J Surg. 2018;52:16-19. doi:10.1016/j.ijsu.2018.01.047
- Morritt DG, Khandwala AR. The development of squamous cell carcinomas in split-thickness skin graft donor sites. Eur J Plast Surg. 2013;36:377-380.
- McCormick M, Miotke S. Squamous cell carcinoma at split thickness skin graft donor site: a case report and review of the literature. J Burn Care Res. 2023;44:210-213. doi:10.1093/jbcr/irac137
- Haik J, Georgiou I, Farber N, et al. Squamous cell carcinoma arising in a split-thickness skin graft donor site. Burns. 2008;34:891-893. doi:10.1016/j.burns.2007.06.006
- Elder DE, Massi D, Scolyer RA WR. WHO Classification of Skin Tumours. 4th ed. IARC Press; 2018.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. doi:10.7326/0003-4819-151-4-200908180-00135
- Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ. 2018;23:60-63. doi:10.1136/bmjebm-2017-110853
- de Moraes LPB, Burchett I, Nicholls S, et al. Large solitary distant metastasis of cutaneous squamous cell carcinoma to skin graft site with complete response following definitive radiotherapy. Int J Bioautomation. 2017;21:103-108.
- Nagase K, Suzuki Y, Misago N, et al. Acute development of keratoacanthoma at a full-thickness skin graft donor site shortly after surgery. J Dermatol. 2016;43:1232-1233. doi:10.1111/1346-8138.13368
- Taylor CD, Snelling CF, Nickerson D, et al. Acute development of invasive squamous cell carcinoma in a split-thickness skin graft donor site. J Burn Care Rehabil. 1998;19:382-385. doi:10.1097/00004630-199809000-00004
- de Delas J, Leache A, Vazquez Doval J, et al. Keratoacanthoma over the donor site of a laminar skin graft. Med Cutan Ibero Lat Am. 1989;17:225-228.
- Neilson D, Emerson DJ, Dunn L. Squamous cell carcinoma of skin developing in a skin graft donor site. Br J Plast Surg. 1988;41:417-419. doi:10.1016/0007-1226(88)90086-0
- May JT, Patil YJ. Keratoacanthoma-type squamous cell carcinoma developing in a skin graft donor site after tumor extirpation at a distant site. Ear Nose Throat J. 2010;89:E11-E13.
- Imbernón-Moya A, Vargas-Laguna E, Lobato-Berezo A, et al. Simultaneous onset of basal cell carcinoma over skin graft and donor site. JAAD Case Rep. 2015;1:244-246. doi:10.1016/j.jdcr.2015.05.004
- Lee S, Coutts I, Ryan A, et al. Keratoacanthoma formation after skin grafting: a brief report and pathophysiological hypothesis. Australas J Dermatol. 2017;58:e117-e119. doi:10.1111/ajd.12501
- Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising acutelyin a skin graft donor site. J Trauma. 1987;27:681-683. doi:10.1097/00005373-198706000-00017
- Herard C, Arnaud D, Goga D, et al. Rapid onset of squamous cell carcinoma in a thin skin graft donor site. Ann Dermatol Venereol. 2016;143:457-461. doi:10.1016/j.annder.2015.03.027
- Ibrahim A, Moisidis E. Case series: rapidly growing squamous cell carcinoma after cutaneous surgical intervention. JPRAS Open. 2017;14:27-32. doi:10.1016/j.jpra.2017.08.004
- Kearney L, Dolan RT, Parfrey NA, et al. Squamous cell carcinoma arising in a skin graft donor site following melanoma extirpation at a distant site: a case report and review of the literature. JPRAS Open. 2015;3:35-38. doi:10.1016/j.jpra.2015.02.002
- Clark MA, Guitart J, Gerami P, et al. Eruptive keratoacanthomatous atypical squamous proliferations (KASPs) arising in skin graft sites. JAAD Case Rep. 2015;1:274-276. doi:10.1016/j.jdcr.2015.06.009
- Aloraifi F, Mulgrew S, James NK. Secondary Merkel cell carcinoma arising from a graft donor site. J Cutan Med Surg. 2017;21:167-169. doi:10.1177/1203475416676805
- Abadir R, Zurowski S. Case report: squamous cell carcinoma of the skin in both palms, axillary node, donor skin graft site and both soles—associated hyperkeratosis and porokeratosis. Br J Radiol. 1994;67:507-510. doi:10.1259/0007-1285-67-797-507
- Griffiths RW. Keratoacanthoma observed. Br J Plast Surg. 2004;57:485-501. doi:10.1016/j.bjps.2004.05.007
- Marous M, Brady K. Cutaneous squamous cell carcinoma arising in a split thickness skin graft donor site in a patient with systemic lupus erythematosus. Dermatologic Surg. 2021;47:1106-1107. doi:10.1097/DSS.0000000000002955
- Dibden FA, Fowler M. The multiple growth of molluscum sebaceum in donor and recipient sites of skin graft. Aust N Z J Surg. 1955;25:157-159. doi:10.1111/j.1445-2197.1955.tb05122.x
- Jeremiah BS. Squamous cell carcinoma development on donor area following removal of a split thickness skin graft. Plast Reconstr Surg. 1948;3:718-721.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;40(5, pt 2):870-871. doi:10.1053/jd.1999.v40.a94419
- Hamilton SA, Dickson WA, O’Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561. doi:10.1016/s0007-1226(97)91308-4
- Hussain A, Ekwobi C, Watson S. Metastatic implantation squamous cell carcinoma in a split-thickness skin graft donor site. J Plast Reconstr Aesthet Surg. 2011;64:690-692. doi:10.1016/j.bjps.2010.06.004
- Wulsin JH. Keratoacanthoma: a benign cutaneous tumors arising in a skin graft donor site. Am Surg. 1958;24:689-692.
- Davis L, Butler D. Acute development of squamous cell carcinoma in a split-thickness skin graft donor site [abstract]. J Am Acad Dermatol. 2012;66:AB208. doi:10.1016/j.jaad.2011.11.874
- Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002;16:217-226, 229; discussion 230-232.
- Piotrowski I, Kulcenty K, Suchorska W. Interplay between inflammation and cancer. Reports Pract Oncol Radiother. 2020;25:422-427. doi:10.1016/j.rpor.2020.04.004
- Carr RA, Houghton JP. Histopathologists’ approach to keratoacanthoma: a multisite survey of regional variation in Great Britain and Ireland. J Clin Pathol. 2014;67:637-638. doi:10.1136/jclinpath-2014-202255
Practice Points
- Donor site cutaneous squamous cell carcinoma (CSCC) and keratoacanthoma (KA) can be postoperative complications of autologous skin grafting.
- Surgical excision of donor site CSCC and KA typically is curative.
Study Finds Gout Drug Effective for Aphthous Ulcers in Children
“Complex aphthous stomatitis in children is typically treated with topical supportive care, which is often not effective,” one of the study investigators, Ananya Shah, a third-year medical student at the University of Rochester School of Medicine & Dentistry, Rochester, New York, told this news organization following the Society for Pediatric Dermatology annual meeting, where the study was presented during a poster session. “There is limited research on CAS and its treatment in children. Colchicine is often used for treatment of CAS in adults, but its use in children has not been studied.”
Ms. Shah, in collaboration with Hilary Kunkel, MD, Nessa Aghazadeh, MD, and Megha Tollefson, MD, of the Department of Dermatology, Mayo Clinic, Rochester, Minnesota, retrospectively reviewed the charts of 20 children diagnosed with CAS who were treated with colchicine, an anti-inflammatory drug often used to treat gout, at the clinic between 2000 and 2023. Treatment responses were defined as no response, partial response, and complete response. Half of the patients were girls, and their median age at presentation was 5 years.
Ulcers were most commonly located in the buccal mucosa (80%), followed by the gingiva (50%), the mucosal lip (50%), and the palate (40%). Nearly all patients (95%) reported that the CAS caused difficulties with eating or drinking. Other effects on their quality of life included weight loss (35%), bleeding (30%), and difficulty brushing teeth (25%). “I was surprised by how much CAS impacts pediatric patients’ quality of life,” Ms. Shah said. “Almost all of the patients experienced trouble with basic activities of daily living, including eating and drinking. In addition, CAS negatively impacted mental health and led to missed school for patients.”
The researchers had follow-up data on responses to colchicine for 14 of the 20 patients. Of these, 12 (86%) had symptom improvement, 5 (36%) had a complete response, 8 (57%) had a partial response, and 1 (7%) did not respond. Nine patients (64%) experienced side effects. Of these, six had diarrhea, two had nausea, and one had constipation.
“Colchicine should be considered as a treatment in pediatric patients who have refractory complex aphthous stomatitis as it is generally well tolerated with minimal side effects,” Ms. Shah said. She acknowledged certain limitations of the study, including its single-center, retrospective design.
The researchers reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Complex aphthous stomatitis in children is typically treated with topical supportive care, which is often not effective,” one of the study investigators, Ananya Shah, a third-year medical student at the University of Rochester School of Medicine & Dentistry, Rochester, New York, told this news organization following the Society for Pediatric Dermatology annual meeting, where the study was presented during a poster session. “There is limited research on CAS and its treatment in children. Colchicine is often used for treatment of CAS in adults, but its use in children has not been studied.”
Ms. Shah, in collaboration with Hilary Kunkel, MD, Nessa Aghazadeh, MD, and Megha Tollefson, MD, of the Department of Dermatology, Mayo Clinic, Rochester, Minnesota, retrospectively reviewed the charts of 20 children diagnosed with CAS who were treated with colchicine, an anti-inflammatory drug often used to treat gout, at the clinic between 2000 and 2023. Treatment responses were defined as no response, partial response, and complete response. Half of the patients were girls, and their median age at presentation was 5 years.
Ulcers were most commonly located in the buccal mucosa (80%), followed by the gingiva (50%), the mucosal lip (50%), and the palate (40%). Nearly all patients (95%) reported that the CAS caused difficulties with eating or drinking. Other effects on their quality of life included weight loss (35%), bleeding (30%), and difficulty brushing teeth (25%). “I was surprised by how much CAS impacts pediatric patients’ quality of life,” Ms. Shah said. “Almost all of the patients experienced trouble with basic activities of daily living, including eating and drinking. In addition, CAS negatively impacted mental health and led to missed school for patients.”
The researchers had follow-up data on responses to colchicine for 14 of the 20 patients. Of these, 12 (86%) had symptom improvement, 5 (36%) had a complete response, 8 (57%) had a partial response, and 1 (7%) did not respond. Nine patients (64%) experienced side effects. Of these, six had diarrhea, two had nausea, and one had constipation.
“Colchicine should be considered as a treatment in pediatric patients who have refractory complex aphthous stomatitis as it is generally well tolerated with minimal side effects,” Ms. Shah said. She acknowledged certain limitations of the study, including its single-center, retrospective design.
The researchers reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Complex aphthous stomatitis in children is typically treated with topical supportive care, which is often not effective,” one of the study investigators, Ananya Shah, a third-year medical student at the University of Rochester School of Medicine & Dentistry, Rochester, New York, told this news organization following the Society for Pediatric Dermatology annual meeting, where the study was presented during a poster session. “There is limited research on CAS and its treatment in children. Colchicine is often used for treatment of CAS in adults, but its use in children has not been studied.”
Ms. Shah, in collaboration with Hilary Kunkel, MD, Nessa Aghazadeh, MD, and Megha Tollefson, MD, of the Department of Dermatology, Mayo Clinic, Rochester, Minnesota, retrospectively reviewed the charts of 20 children diagnosed with CAS who were treated with colchicine, an anti-inflammatory drug often used to treat gout, at the clinic between 2000 and 2023. Treatment responses were defined as no response, partial response, and complete response. Half of the patients were girls, and their median age at presentation was 5 years.
Ulcers were most commonly located in the buccal mucosa (80%), followed by the gingiva (50%), the mucosal lip (50%), and the palate (40%). Nearly all patients (95%) reported that the CAS caused difficulties with eating or drinking. Other effects on their quality of life included weight loss (35%), bleeding (30%), and difficulty brushing teeth (25%). “I was surprised by how much CAS impacts pediatric patients’ quality of life,” Ms. Shah said. “Almost all of the patients experienced trouble with basic activities of daily living, including eating and drinking. In addition, CAS negatively impacted mental health and led to missed school for patients.”
The researchers had follow-up data on responses to colchicine for 14 of the 20 patients. Of these, 12 (86%) had symptom improvement, 5 (36%) had a complete response, 8 (57%) had a partial response, and 1 (7%) did not respond. Nine patients (64%) experienced side effects. Of these, six had diarrhea, two had nausea, and one had constipation.
“Colchicine should be considered as a treatment in pediatric patients who have refractory complex aphthous stomatitis as it is generally well tolerated with minimal side effects,” Ms. Shah said. She acknowledged certain limitations of the study, including its single-center, retrospective design.
The researchers reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
‘Emerging Threat’ Xylazine Use Continues to Spread Across the United States
Illicit use of the veterinary tranquilizer xylazine continues to spread across the United States. The drug, which is increasingly mixed with fentanyl, often fails to respond to the opioid overdose reversal medication naloxone and can cause severe necrotic lesions.
A report released by Millennium Health, a specialty lab that provides medication monitoring for pain management, drug treatment, and behavioral and substance use disorder treatment centers across the country, showed the number of urine specimens collected and tested at the US drug treatment centers were positive for xylazine in the most recent 6 months.
As previously reported by this news organization, in late 2022, the US Food and Drug Administration (FDA) issued a communication alerting clinicians about the special management required for opioid overdoses tainted with xylazine, which is also known as “tranq” or “tranq dope.”
Subsequently, in early 2023, The White House Office of National Drug Control Policy designated xylazine combined with fentanyl as an emerging threat to the United States.
Both the FDA and the Drug Enforcement Administration have taken steps to try to stop trafficking of the combination. However, despite these efforts, xylazine use has continued to spread.
The Millennium Health Signals report showed that the greatest increase in xylazine use was largely in the western United States. In the first 6 months of 2023, 3% of urine drug tests (UDTs) in Washington, Oregon, California, Hawaii, and Alaska were positive for xylazine. From November 2023 to April 2024, this rose to 8%, a 147% increase. In the Mountain West, xylazine-positive UDTs increased from 2% in 2023 to 4% in 2024, an increase of 94%. In addition to growth in the West, the report showed that xylazine use increased by more than 100% in New England — from 14% in 2023 to 28% in 2024.
Nationally, 16% of all urine specimens were positive for xylazine from late 2023 to April 2024, up slightly from 14% from April to October 2023.
Xylazine use was highest in the East and in the mid-Atlantic United States. Still, positivity rates in the mid-Atlantic dropped from 44% to 33%. The states included in that group were New York, Pennsylvania, Delaware, and New Jersey. East North Central states (Ohio, Michigan, Wisconsin, Indiana, and Illinois) also experienced a decline in positive tests from 32% to 30%.
The South Atlantic states, which include Maryland, Virginia, West Virginia, North and South Carolina, Georgia, and Florida, had a 17% increase in positivity — from 22% to 26%.
From April 2023 to April 2024 state-level UDT positivity rates were 40% in Pennsylvania, 37% in New York, and 35% in Ohio. But rates vary by locality. In Clermont and Hamilton counties in Ohio — both in the Cincinnati area — about 70% of specimens were positive for xylazine.
About one third of specimens in Maryland and South Carolina contained xylazine.
“Because xylazine exposure remains a significant challenge in the East and is a growing concern in the West, clinicians across the US need to be prepared to recognize and address the consequences of xylazine use — like diminished responses to naloxone and severe skin wounds that may lead to amputation — among people who use fentanyl,” Millennium Health Chief Clinical Officer Angela Huskey, PharmD, said in a press release.
The Health Signals Alert analyzed more than 50,000 fentanyl-positive UDT specimens collected between April 12, 2023, and April 11, 2024. Millennium Health researchers analyzed xylazine positivity rates in fentanyl-positive UDT specimens by the US Census Division and state.
A version of this article first appeared on Medscape.com.
Illicit use of the veterinary tranquilizer xylazine continues to spread across the United States. The drug, which is increasingly mixed with fentanyl, often fails to respond to the opioid overdose reversal medication naloxone and can cause severe necrotic lesions.
A report released by Millennium Health, a specialty lab that provides medication monitoring for pain management, drug treatment, and behavioral and substance use disorder treatment centers across the country, showed the number of urine specimens collected and tested at the US drug treatment centers were positive for xylazine in the most recent 6 months.
As previously reported by this news organization, in late 2022, the US Food and Drug Administration (FDA) issued a communication alerting clinicians about the special management required for opioid overdoses tainted with xylazine, which is also known as “tranq” or “tranq dope.”
Subsequently, in early 2023, The White House Office of National Drug Control Policy designated xylazine combined with fentanyl as an emerging threat to the United States.
Both the FDA and the Drug Enforcement Administration have taken steps to try to stop trafficking of the combination. However, despite these efforts, xylazine use has continued to spread.
The Millennium Health Signals report showed that the greatest increase in xylazine use was largely in the western United States. In the first 6 months of 2023, 3% of urine drug tests (UDTs) in Washington, Oregon, California, Hawaii, and Alaska were positive for xylazine. From November 2023 to April 2024, this rose to 8%, a 147% increase. In the Mountain West, xylazine-positive UDTs increased from 2% in 2023 to 4% in 2024, an increase of 94%. In addition to growth in the West, the report showed that xylazine use increased by more than 100% in New England — from 14% in 2023 to 28% in 2024.
Nationally, 16% of all urine specimens were positive for xylazine from late 2023 to April 2024, up slightly from 14% from April to October 2023.
Xylazine use was highest in the East and in the mid-Atlantic United States. Still, positivity rates in the mid-Atlantic dropped from 44% to 33%. The states included in that group were New York, Pennsylvania, Delaware, and New Jersey. East North Central states (Ohio, Michigan, Wisconsin, Indiana, and Illinois) also experienced a decline in positive tests from 32% to 30%.
The South Atlantic states, which include Maryland, Virginia, West Virginia, North and South Carolina, Georgia, and Florida, had a 17% increase in positivity — from 22% to 26%.
From April 2023 to April 2024 state-level UDT positivity rates were 40% in Pennsylvania, 37% in New York, and 35% in Ohio. But rates vary by locality. In Clermont and Hamilton counties in Ohio — both in the Cincinnati area — about 70% of specimens were positive for xylazine.
About one third of specimens in Maryland and South Carolina contained xylazine.
“Because xylazine exposure remains a significant challenge in the East and is a growing concern in the West, clinicians across the US need to be prepared to recognize and address the consequences of xylazine use — like diminished responses to naloxone and severe skin wounds that may lead to amputation — among people who use fentanyl,” Millennium Health Chief Clinical Officer Angela Huskey, PharmD, said in a press release.
The Health Signals Alert analyzed more than 50,000 fentanyl-positive UDT specimens collected between April 12, 2023, and April 11, 2024. Millennium Health researchers analyzed xylazine positivity rates in fentanyl-positive UDT specimens by the US Census Division and state.
A version of this article first appeared on Medscape.com.
Illicit use of the veterinary tranquilizer xylazine continues to spread across the United States. The drug, which is increasingly mixed with fentanyl, often fails to respond to the opioid overdose reversal medication naloxone and can cause severe necrotic lesions.
A report released by Millennium Health, a specialty lab that provides medication monitoring for pain management, drug treatment, and behavioral and substance use disorder treatment centers across the country, showed the number of urine specimens collected and tested at the US drug treatment centers were positive for xylazine in the most recent 6 months.
As previously reported by this news organization, in late 2022, the US Food and Drug Administration (FDA) issued a communication alerting clinicians about the special management required for opioid overdoses tainted with xylazine, which is also known as “tranq” or “tranq dope.”
Subsequently, in early 2023, The White House Office of National Drug Control Policy designated xylazine combined with fentanyl as an emerging threat to the United States.
Both the FDA and the Drug Enforcement Administration have taken steps to try to stop trafficking of the combination. However, despite these efforts, xylazine use has continued to spread.
The Millennium Health Signals report showed that the greatest increase in xylazine use was largely in the western United States. In the first 6 months of 2023, 3% of urine drug tests (UDTs) in Washington, Oregon, California, Hawaii, and Alaska were positive for xylazine. From November 2023 to April 2024, this rose to 8%, a 147% increase. In the Mountain West, xylazine-positive UDTs increased from 2% in 2023 to 4% in 2024, an increase of 94%. In addition to growth in the West, the report showed that xylazine use increased by more than 100% in New England — from 14% in 2023 to 28% in 2024.
Nationally, 16% of all urine specimens were positive for xylazine from late 2023 to April 2024, up slightly from 14% from April to October 2023.
Xylazine use was highest in the East and in the mid-Atlantic United States. Still, positivity rates in the mid-Atlantic dropped from 44% to 33%. The states included in that group were New York, Pennsylvania, Delaware, and New Jersey. East North Central states (Ohio, Michigan, Wisconsin, Indiana, and Illinois) also experienced a decline in positive tests from 32% to 30%.
The South Atlantic states, which include Maryland, Virginia, West Virginia, North and South Carolina, Georgia, and Florida, had a 17% increase in positivity — from 22% to 26%.
From April 2023 to April 2024 state-level UDT positivity rates were 40% in Pennsylvania, 37% in New York, and 35% in Ohio. But rates vary by locality. In Clermont and Hamilton counties in Ohio — both in the Cincinnati area — about 70% of specimens were positive for xylazine.
About one third of specimens in Maryland and South Carolina contained xylazine.
“Because xylazine exposure remains a significant challenge in the East and is a growing concern in the West, clinicians across the US need to be prepared to recognize and address the consequences of xylazine use — like diminished responses to naloxone and severe skin wounds that may lead to amputation — among people who use fentanyl,” Millennium Health Chief Clinical Officer Angela Huskey, PharmD, said in a press release.
The Health Signals Alert analyzed more than 50,000 fentanyl-positive UDT specimens collected between April 12, 2023, and April 11, 2024. Millennium Health researchers analyzed xylazine positivity rates in fentanyl-positive UDT specimens by the US Census Division and state.
A version of this article first appeared on Medscape.com.
Isotretinoin-Induced Skin Fragility in an Aerialist
Isotretinoin was introduced more than 3 decades ago and marked a major advancement in the treatment of severe refractory cystic acne. The most common adverse effects linked to isotretinoin usage are mucocutaneous in nature, manifesting as xerosis and cheilitis.1 Skin fragility and poor wound healing also have been reported.2-6 Current recommendations for avoiding these adverse effects include refraining from waxing, laser procedures, and other elective cutaneous procedures for at least 6 months.7 We present a case of isotretinoin-induced cutaneous fragility resulting in blistering and erosions on the palms of a competitive aerial trapeze artist.
Case Report
A 25-year-old woman presented for follow-up during week 12 of isotretinoin therapy (40 mg twice daily) prescribed for acne. She reported peeling of the skin on the palms following intense aerial acrobatic workouts. She had been a performing aerialist for many years and had never sustained a similar injury. The wounds were painful and led to decreased activity. She had no notable medical history. Physical examination of the palms revealed erosions in a distribution that corresponded to horizontal bar contact and friction (Figure). The patient was advised on proper wound care, application of emollients, and minimizing friction. She completed the course of isotretinoin and has continued aerialist activity without recurrence of skin fragility.
Comment
Skin fragility is a well-known adverse effect of isotretinoin therapy.8 Pavlis and Lieblich9 reported skin fragility in a young wrestler who experienced similar skin erosions due to isotretinoin therapy. The proposed mechanism of isotretinoin-induced skin fragility is multifactorial. It involves an apoptotic effect on sebocytes,5 which results in reduced stratum corneum hydration and an associated increase in transepidermal water loss.6,10,11 Retinoids also are known to cause thinning of the skin, likely due to the disadhesion of both the epidermis and the stratum corneum, which was demonstrated by the easy removal of cornified cells through tape stripping in hairless mice treated with isotretinoin.12 In further investigations, human patients and hairless mice treated with isotretinoin readily developed friction blisters through pencil eraser abrasion.13 Examination of the friction blisters using light and electron microscopy revealed fraying or loss of the stratum corneum and viable epidermis as well as loss of desmosomes and tonofilaments. Additionally, intracellular and intercellular deposits of an unidentified amorphous material were noted.13
Overall, the origin of skin fragility induced by isotretinoin is supported by its effect on sebocytes, increased transepidermal water loss, and profound disruption of the integrity of the epidermis, resulting in an elevated risk for inadvertent skin damage. Patients were encouraged to avoid cosmetic procedures in prior case reports,14-16 and because our case demonstrates the risk for cutaneous injury in athletes due to isotretinoin-induced skin fragility, we propose an extension of these warnings to encompass athletes receiving isotretinoin treatment. Offering early guidance on wound prevention is of paramount importance in maintaining athletic performance and minimizing painful injuries.
- Rajput I, Anjankar VP. Side effects of treating acne vulgaris with isotretinoin: a systematic review. Cureus. 2024;16:E55946. doi:10.7759/cureus.55946
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- McDonald KA, Shelley AJ, Alavi A. A systematic review on oral isotretinoin therapy and clinically observable wound healing in acne patients. J Cutan Med Surg. 2017;21:325-333. doi:10.1177/1203475417701419
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169. doi:10.4161/derm.1.3.9364
- Zouboulis CC. Isotretinoin revisited: pluripotent effects on human sebaceous gland cells. J Invest Dermatol. 2006;126:2154-2156. doi:10.1038/sj.jid.5700418
- Kmiec´ ML, Pajor A, Broniarczyk-Dyła G. Evaluation of biophysical skin parameters and assessment of hair growth in patients with acne treated with isotretinoin. Postepy Dermatol Alergol. 2013;30:343-349. doi:10.5114/pdia.2013.39432
- Waldman A, Bolotin D, Arndt KA, et al. ASDS Guidelines Task Force: Consensus recommendations regarding the safety of lasers, dermabrasion, chemical peels, energy devices, and skin surgery during and after isotretinoin use. Dermatolog Surg. 2017;43:1249-1262. doi:10.1097/DSS.0000000000001166
- Aksoy H, Aksoy B, Calikoglu E. Systemic retinoids and scar dehiscence. Indian J Dermatol. 2019;64:68. doi:10.4103/ijd.IJD_148_18
- Pavlis MB, Lieblich L. Isotretinoin-induced skin fragility in a teenaged athlete: a case report. Cutis. 2013;92:33-34.
- Herane MI, Fuenzalida H, Zegpi E, et al. Specific gel-cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase—a double-blind, randomized, placebo-controlled study. J Cosmet Dermatol. 2009;8:181-185. doi:10.1111/j.1473-2165.2009.00455.x
- Park KY, Ko EJ, Kim IS, et al. The effect of evening primrose oil for the prevention of xerotic cheilitis in acne patients being treated with isotretinoin: a pilot study. Ann Dermatol. 2014;26:706-712. doi:10.5021/ad.2014.26.6.706
- Elias PM, Fritsch PO, Lampe M, et al. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981;44:531-540.
- Williams ML, Elias PM. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981;117:611-619.
- Rubenstein R, Roenigk HH, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15:280-285. doi:10.1016/S0190-9622(86)70167-9
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706. doi:10.1111/j.1365-2133.1988.tb02574.x
- Katz BE, Mac Farlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853. doi:10.1016/S0190-9622(94)70096-6
Isotretinoin was introduced more than 3 decades ago and marked a major advancement in the treatment of severe refractory cystic acne. The most common adverse effects linked to isotretinoin usage are mucocutaneous in nature, manifesting as xerosis and cheilitis.1 Skin fragility and poor wound healing also have been reported.2-6 Current recommendations for avoiding these adverse effects include refraining from waxing, laser procedures, and other elective cutaneous procedures for at least 6 months.7 We present a case of isotretinoin-induced cutaneous fragility resulting in blistering and erosions on the palms of a competitive aerial trapeze artist.
Case Report
A 25-year-old woman presented for follow-up during week 12 of isotretinoin therapy (40 mg twice daily) prescribed for acne. She reported peeling of the skin on the palms following intense aerial acrobatic workouts. She had been a performing aerialist for many years and had never sustained a similar injury. The wounds were painful and led to decreased activity. She had no notable medical history. Physical examination of the palms revealed erosions in a distribution that corresponded to horizontal bar contact and friction (Figure). The patient was advised on proper wound care, application of emollients, and minimizing friction. She completed the course of isotretinoin and has continued aerialist activity without recurrence of skin fragility.
Comment
Skin fragility is a well-known adverse effect of isotretinoin therapy.8 Pavlis and Lieblich9 reported skin fragility in a young wrestler who experienced similar skin erosions due to isotretinoin therapy. The proposed mechanism of isotretinoin-induced skin fragility is multifactorial. It involves an apoptotic effect on sebocytes,5 which results in reduced stratum corneum hydration and an associated increase in transepidermal water loss.6,10,11 Retinoids also are known to cause thinning of the skin, likely due to the disadhesion of both the epidermis and the stratum corneum, which was demonstrated by the easy removal of cornified cells through tape stripping in hairless mice treated with isotretinoin.12 In further investigations, human patients and hairless mice treated with isotretinoin readily developed friction blisters through pencil eraser abrasion.13 Examination of the friction blisters using light and electron microscopy revealed fraying or loss of the stratum corneum and viable epidermis as well as loss of desmosomes and tonofilaments. Additionally, intracellular and intercellular deposits of an unidentified amorphous material were noted.13

Overall, the origin of skin fragility induced by isotretinoin is supported by its effect on sebocytes, increased transepidermal water loss, and profound disruption of the integrity of the epidermis, resulting in an elevated risk for inadvertent skin damage. Patients were encouraged to avoid cosmetic procedures in prior case reports,14-16 and because our case demonstrates the risk for cutaneous injury in athletes due to isotretinoin-induced skin fragility, we propose an extension of these warnings to encompass athletes receiving isotretinoin treatment. Offering early guidance on wound prevention is of paramount importance in maintaining athletic performance and minimizing painful injuries.
Isotretinoin was introduced more than 3 decades ago and marked a major advancement in the treatment of severe refractory cystic acne. The most common adverse effects linked to isotretinoin usage are mucocutaneous in nature, manifesting as xerosis and cheilitis.1 Skin fragility and poor wound healing also have been reported.2-6 Current recommendations for avoiding these adverse effects include refraining from waxing, laser procedures, and other elective cutaneous procedures for at least 6 months.7 We present a case of isotretinoin-induced cutaneous fragility resulting in blistering and erosions on the palms of a competitive aerial trapeze artist.
Case Report
A 25-year-old woman presented for follow-up during week 12 of isotretinoin therapy (40 mg twice daily) prescribed for acne. She reported peeling of the skin on the palms following intense aerial acrobatic workouts. She had been a performing aerialist for many years and had never sustained a similar injury. The wounds were painful and led to decreased activity. She had no notable medical history. Physical examination of the palms revealed erosions in a distribution that corresponded to horizontal bar contact and friction (Figure). The patient was advised on proper wound care, application of emollients, and minimizing friction. She completed the course of isotretinoin and has continued aerialist activity without recurrence of skin fragility.
Comment
Skin fragility is a well-known adverse effect of isotretinoin therapy.8 Pavlis and Lieblich9 reported skin fragility in a young wrestler who experienced similar skin erosions due to isotretinoin therapy. The proposed mechanism of isotretinoin-induced skin fragility is multifactorial. It involves an apoptotic effect on sebocytes,5 which results in reduced stratum corneum hydration and an associated increase in transepidermal water loss.6,10,11 Retinoids also are known to cause thinning of the skin, likely due to the disadhesion of both the epidermis and the stratum corneum, which was demonstrated by the easy removal of cornified cells through tape stripping in hairless mice treated with isotretinoin.12 In further investigations, human patients and hairless mice treated with isotretinoin readily developed friction blisters through pencil eraser abrasion.13 Examination of the friction blisters using light and electron microscopy revealed fraying or loss of the stratum corneum and viable epidermis as well as loss of desmosomes and tonofilaments. Additionally, intracellular and intercellular deposits of an unidentified amorphous material were noted.13

Overall, the origin of skin fragility induced by isotretinoin is supported by its effect on sebocytes, increased transepidermal water loss, and profound disruption of the integrity of the epidermis, resulting in an elevated risk for inadvertent skin damage. Patients were encouraged to avoid cosmetic procedures in prior case reports,14-16 and because our case demonstrates the risk for cutaneous injury in athletes due to isotretinoin-induced skin fragility, we propose an extension of these warnings to encompass athletes receiving isotretinoin treatment. Offering early guidance on wound prevention is of paramount importance in maintaining athletic performance and minimizing painful injuries.
- Rajput I, Anjankar VP. Side effects of treating acne vulgaris with isotretinoin: a systematic review. Cureus. 2024;16:E55946. doi:10.7759/cureus.55946
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- McDonald KA, Shelley AJ, Alavi A. A systematic review on oral isotretinoin therapy and clinically observable wound healing in acne patients. J Cutan Med Surg. 2017;21:325-333. doi:10.1177/1203475417701419
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169. doi:10.4161/derm.1.3.9364
- Zouboulis CC. Isotretinoin revisited: pluripotent effects on human sebaceous gland cells. J Invest Dermatol. 2006;126:2154-2156. doi:10.1038/sj.jid.5700418
- Kmiec´ ML, Pajor A, Broniarczyk-Dyła G. Evaluation of biophysical skin parameters and assessment of hair growth in patients with acne treated with isotretinoin. Postepy Dermatol Alergol. 2013;30:343-349. doi:10.5114/pdia.2013.39432
- Waldman A, Bolotin D, Arndt KA, et al. ASDS Guidelines Task Force: Consensus recommendations regarding the safety of lasers, dermabrasion, chemical peels, energy devices, and skin surgery during and after isotretinoin use. Dermatolog Surg. 2017;43:1249-1262. doi:10.1097/DSS.0000000000001166
- Aksoy H, Aksoy B, Calikoglu E. Systemic retinoids and scar dehiscence. Indian J Dermatol. 2019;64:68. doi:10.4103/ijd.IJD_148_18
- Pavlis MB, Lieblich L. Isotretinoin-induced skin fragility in a teenaged athlete: a case report. Cutis. 2013;92:33-34.
- Herane MI, Fuenzalida H, Zegpi E, et al. Specific gel-cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase—a double-blind, randomized, placebo-controlled study. J Cosmet Dermatol. 2009;8:181-185. doi:10.1111/j.1473-2165.2009.00455.x
- Park KY, Ko EJ, Kim IS, et al. The effect of evening primrose oil for the prevention of xerotic cheilitis in acne patients being treated with isotretinoin: a pilot study. Ann Dermatol. 2014;26:706-712. doi:10.5021/ad.2014.26.6.706
- Elias PM, Fritsch PO, Lampe M, et al. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981;44:531-540.
- Williams ML, Elias PM. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981;117:611-619.
- Rubenstein R, Roenigk HH, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15:280-285. doi:10.1016/S0190-9622(86)70167-9
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706. doi:10.1111/j.1365-2133.1988.tb02574.x
- Katz BE, Mac Farlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853. doi:10.1016/S0190-9622(94)70096-6
- Rajput I, Anjankar VP. Side effects of treating acne vulgaris with isotretinoin: a systematic review. Cureus. 2024;16:E55946. doi:10.7759/cureus.55946
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- McDonald KA, Shelley AJ, Alavi A. A systematic review on oral isotretinoin therapy and clinically observable wound healing in acne patients. J Cutan Med Surg. 2017;21:325-333. doi:10.1177/1203475417701419
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169. doi:10.4161/derm.1.3.9364
- Zouboulis CC. Isotretinoin revisited: pluripotent effects on human sebaceous gland cells. J Invest Dermatol. 2006;126:2154-2156. doi:10.1038/sj.jid.5700418
- Kmiec´ ML, Pajor A, Broniarczyk-Dyła G. Evaluation of biophysical skin parameters and assessment of hair growth in patients with acne treated with isotretinoin. Postepy Dermatol Alergol. 2013;30:343-349. doi:10.5114/pdia.2013.39432
- Waldman A, Bolotin D, Arndt KA, et al. ASDS Guidelines Task Force: Consensus recommendations regarding the safety of lasers, dermabrasion, chemical peels, energy devices, and skin surgery during and after isotretinoin use. Dermatolog Surg. 2017;43:1249-1262. doi:10.1097/DSS.0000000000001166
- Aksoy H, Aksoy B, Calikoglu E. Systemic retinoids and scar dehiscence. Indian J Dermatol. 2019;64:68. doi:10.4103/ijd.IJD_148_18
- Pavlis MB, Lieblich L. Isotretinoin-induced skin fragility in a teenaged athlete: a case report. Cutis. 2013;92:33-34.
- Herane MI, Fuenzalida H, Zegpi E, et al. Specific gel-cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase—a double-blind, randomized, placebo-controlled study. J Cosmet Dermatol. 2009;8:181-185. doi:10.1111/j.1473-2165.2009.00455.x
- Park KY, Ko EJ, Kim IS, et al. The effect of evening primrose oil for the prevention of xerotic cheilitis in acne patients being treated with isotretinoin: a pilot study. Ann Dermatol. 2014;26:706-712. doi:10.5021/ad.2014.26.6.706
- Elias PM, Fritsch PO, Lampe M, et al. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981;44:531-540.
- Williams ML, Elias PM. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981;117:611-619.
- Rubenstein R, Roenigk HH, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15:280-285. doi:10.1016/S0190-9622(86)70167-9
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706. doi:10.1111/j.1365-2133.1988.tb02574.x
- Katz BE, Mac Farlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853. doi:10.1016/S0190-9622(94)70096-6
Practice Points
- Isotretinoin is used to treat severe nodulocystic acne but can cause adverse effects such as skin fragility, xerosis, and poor wound healing.
- Dermatologists should inform athletes of heightened skin vulnerability while undergoing isotretinoin treatment.
- Isotretinoin-induced skin fragility involves the effects of isotretinoin on sebocytes, transepidermal water loss, and disruption of the integrity of the epidermis.
Olive Oil Shows Promise for Wound Healing of Ulcers
Olive oil is obtained by mechanical extraction from the fruit of the Olea europaea tree, which is believed to have originated from ancient Iran and Turkestan, later spreading to Anatolia, Syria, Palestine, and Israel. Mechanical extraction of the oil from the olive fruit involves pressure processing, centrifugation, and adhesion filtering.1 Refining of olive oil is done via alkali refining or physical refining, with physical refining being useful in removing oxidation by-products and pro-oxidant metals. Olive oil is composed mainly of triacylglycerols, which are glycerol esters attached to various fatty acids, with the most common fatty acid being the monounsaturated oleic acid. Additional fatty acids include palmitic acid, linoleic acid, stearic acid, and palmitoleic acid.2 Olive oil contains phenolic compounds, the main ones being oleuropein, hydroxytyrosol, and tyrosol. These phenolic compounds are proposed to be strong antioxidants and radical scavengers.3
Mediterranean countries are responsible for approximately 97% of the world’s olive cultivation.4 Olive oil historically was used as lamp fuel, lubricant, body ointment, and later as a source of edible oil.1 Recently, its potential uses in medicine have called for further exploration into other uses for olive oil.
The skin is the largest organ of the body and serves as a protective barrier against pathogens and harmful substances. Skin damage results in 3 main phases to aid in wound healing: inflammation, proliferation, and maturation. In proper skin healing, inflammation will stop once the harmful microbes are removed. However, an excess and prolongation of inflammation can result in delayed healing. Thus, interventions that can limit the amount of inflammation can help promote wound healing. Olive oil contains several anti-inflammatory molecules (compounds or chemicals), including phenolic compounds and omega-3 fatty acids.5 Studies also have shown that olive oil can promote re-epithelialization in tissues.6 Thus, use of olive oil in wound therapy has been of great interest.
This article will review studies that have investigated the use of olive oil for wound healing of diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers. To conduct a comprehensive scoping review of the literature on the effects of olive oil in wound healing, we utilized the resources of the Galter Health Sciences Library & Learning Center (Chicago, Illinois). Our search strategy was structured to encompass a range of relevant databases accessible through the library, including PubMed, Embase, and Web of Science. We formulated our search terms to be broad yet specific to our topic, combining keywords such as olive oil, wound healing, skin repair, and dermal therapy. The inclusion criteria were set to filter studies conducted from January 2000 to December 2019, focusing on clinical trials, observational studies, and review articles. We limited our search to articles published in English, which yielded a preliminary set of articles that were then screened based on their titles and abstracts. Full-text versions of potentially relevant studies were retrieved and assessed for eligibility. We included studies that specifically evaluated the effects of olive oil in wound healing, excluding those that did not directly relate to our research question or had insufficient data. The data extraction from these studies was conducted using a standardized form, capturing study design, population, intervention details, outcomes, and key findings. The synthesis of these data provided a comprehensive overview of the current evidence on the topic, aiding in the identification of gaps in knowledge and directions for future research.
Diabetic Foot Ulcers
Foot ulcers are common in patients with diabetes mellitus and are associated with notable morbidity and mortality. Foot ulcers can clinically manifest in various forms but are classically described as lesions with a deep sinus in the feet. Patients with diabetic foot ulcers are at risk for infection, and severe forms of the ulcers require amputation.7,8 Routine care of foot ulcers involves irrigation of the ulcer and surrounding area with normal saline solution daily, followed by a dressing with sterile gauze. Studies investigating the effect of olive oil on foot ulcers suggest that olive oil use for care and healing of foot ulcers is an area of interest.
A double-blind, randomized clinical trial investigated the effects of topical olive oil on diabetic foot ulcers.9 A total of 34 patients with foot ulcers of Wagner grades 1 (superficial ulcers that involved the skin but not underlying tissue) or 2 (deeper ulcers penetrating to the ligaments and muscles but not the bone) that had remained open and did not improve for more than 3 months were recruited. The patients were randomly assigned to receive topical olive oil and routine care (intervention group) or to receive routine care (control group). Patients who received olive oil had oil poured on their ulcers with gauze wrapped around the ulcer that was soaked with olive oil. The clinical characteristics of the diabetic ulcer (eg, site, grade, size, status of healing) were assessed. The study revealed that after 4 weeks, olive oil significantly decreased ulcer area (P=.01) and ulcer depth (P=.02) compared with the control. Furthermore, there was a significant difference (P=.003) in complete ulcer healing between the olive oil and control groups: 73.3% (11/15) of patients in the olive oil group had complete ulcer healing, whereas 13.3% (2/15) of patients in the control group had complete ulcer healing.9 The positive effect of olive oil on the healing of diabetic foot ulcers encourages further investigation as a possible therapy for foot ulcers.
Another randomized controlled trial of 45 patients with diabetic foot ulcers of Wagner grades 1 or 2 investigated the effect of olive oil.10 Patients were randomly assigned to 1 of 3 groups for 1 month: the olive oil group, the honey group, or the control group. Patients in the olive oil group had their wounds dressed using gauze with olive oil daily, the patients in the honey group had their wounds dressed using gauze with honey daily, and the control group had routine care consisting of irrigation with saline solution and dressing with a sterile gauze. This study calculated a wound healing score based on a predefined checklist for diabetic foot ulcers through 4 variables: wound grading, color, surrounding tissue status, and drainage. Each variable had a maximum score of 100, contributing to a total possible score of 400, which indicated complete healing. A score of 50 signified deterioration. Wound healing was categorized as follows: (1) complete healing is indicated by a total score of 400; (2) partial healing was indicated by an increase of at least 30 points from the initial score; (3) lack of healing occurred when there was no change or less than a 30-point increase from the initial score; and (4) aggravation was noted when the score decreased by at least 10 points from the initial assessment. The study revealed that olive oil and honey treatments resulted in an increase in mean score, which indicated better wound healing. Patients in the olive oil group had a mean score of 253.0 before the intervention and 330.5 after the intervention (P<.0001); patients in the honey group had a mean score of 267.5 before the intervention and 371.5 after the intervention (P<.0001).10
There also have been case reports on combined olive oil and honey in diabetic foot ulcer management. Haghighian et al11 presented a case of a diabetic foot wound that healed completely within 2 weeks after the combined use of olive oil and honey wax. Zahmatkesh and Rashidi12 observed the healing of a diabetic foot wound over a month with daily dressings of a mixture of heated honey and olive oil, resulting in granulation tissue formation within 5 days. Microvascular changes, such as capillary basement membrane thickening, pericyte degeneration, and impairment of vasodilation and constriction, may contribute to inflammation in blood vessels, which can delay the healing of diabetic foot ulcers.7 Because olive oil and honey contain compounds that have antioxidative, antimicrobial, and anti-inflammatory properties, both may play a role in notably reducing inflammation and promoting the healing of foot ulcers.13
Pressure Ulcers
A pressure ulcer is a superficial skin injury that is caused by a prolonged period of pressure on the skin, in which the skin becomes red but there is no rupture. Prolonged periods of immobility resulting in a reduction or pause of blood supply are common causes of pressure ulcers.14 Studies have suggested that topical olive oil may be effective in prevention of pressure ulcers and should be incorporated as part of standard-of-care measures.
In a randomized, single-blind trial, 72 patients with the first stage of bedsore—which is a pressure ulcer—in the sacral, shoulder, heel, or other areas were randomly assigned to either the intervention or control group.14 Patients in the intervention group had 15 mL of olive oil rubbed on the wound for 20 minutes daily and then washed with tepid water. The Pressure Ulcer Scale for Healing tool was utilized to assess the healing status of the pressure ulcer. This tool considers wound surface size, exudate rate, and tissue type to provide a score of 0 to 17 (0=healed ulcer; 17=progression of ulcer). The mean score (SD) was lower in the olive oil group at days 4 and 7 compared with the control group (day 4: 7.50 [2.823] vs 9.50 [1.732]; day 7: 5.44 [3.806] vs 8.83 [2.864])(P<.001). Furthermore, between days 1 and 7, there was significant improvement in the olive oil group (mean difference, 3.56; P<.001) but no significant change in the control group (mean difference, 0.75; P=.052).14 The results indicate that patients in the olive oil group had a better ulcer healing status compared with patients in the control group.
In a noninferiority, randomized, double-blind clinical trial, olive oil was compared to a recommended skin care measure of hyperoxygenated fatty acids (HOFAs) for the prevention of pressure ulcers.15 The study consisted of 571 residents from several nursing homes who were at risk for pressure ulcers. Either olive oil or HOFA was applied to areas at risk for pressure ulcers, with 2 sprays of 0.2 mL per spray to each area every 12 hours. The participants were followed up for 30 days or until a pressure ulcer developed. Researchers performed skin assessments; the Braden Scale was used to assess the risk for pressure ulcers. The incidence difference of pressure ulcers in the olive oil group and HOFA group did not exceed in the noninferiority margin of 7%. Furthermore, Kaplan-Meier survival curves for the time until pressure ulcer onset showed a nonsignificant difference between the 2 groups.15 These findings suggest that olive oil is as effective as HOFA for the prevention of pressure ulcers. Although the mechanism of olive oil on prevention of pressure ulcers has not yet been determined, it has been suggested that anti-inflammatory compounds in olive oil, such as polyphenol and oleocanthal compounds, play an anti-inflammatory role.
Perineal Ulcers
Episiotomy is a surgical incision that is made to open the vagina during birth to aid in delivery of the baby. In contrast to spontaneous vaginal tears, an episiotomy allows for easier repair and healing of the laceration.16 Studies were conducted to investigate the effect of olive oil on women with lacerations after an episiotomy.
A total of 90 primigravid women who had undergone episiotomy were recruited and randomly assigned to 1 of 2 interventions: cold compression with gel packs for 20 minutes within 12 hours after delivery for up to 10 days, if necessary, or topical olive oil twice daily within 12 hours after delivery for up to 10 days.17 Although there was no significant difference in the structural features of the wound, there was a significant difference in the redness severity. After 10 days, the mean REEDA (redness, edema, ecchymosis, discharge, and apposition) score (SD), which assesses tissue healing, was 0.47 (0.96) in patients who received cold compression with gel packs and 0.20 (0.50) in patients who received topical olive oil (P=.04).17 This study suggests that there is the potential for olive oil to be used for wound healing after episiotomy.
A double-blind trial consisted of 60 women who had mediolateral episiotomy or perineal tear grades 1 and 2 who were randomly assigned to 1 of 2 groups for 10 days: olive oil sitz bath or distilled water sitz bath (control group). The results showed a significant difference in pain severity after 5 and 10 days (P<.05), wound redness after 5 days (P<.0001), and redness (P<.000) and edema (P<.05) 10 days after delivery.18 This study encourages further investigation of the benefits of olive oil for care after an episiotomy.
Chronic Ulcers
Chronic ulcers are other persistent wounds that do not respond to standard treatments and pose a notable health burden. Their development is influenced by factors such as oxidative stress, microbial infections, and the body’s immune response. A case series was conducted to investigate the wound healing effects of olive oil on chronic ulcers.19 Fourteen patients who were diagnosed with 1 or more chronic skin ulcers that had not healed with conventional treatment, such as cleansing, debridement, or infection control, were recruited. The mean (SD) of the patients’
Final Thoughts
This review illuminated several key aspects of research on the role of olive oil in wound healing. Although the studies included in this review offer valuable insights, it is essential to acknowledge the variability in the quality of data presented. Several studies demonstrated robust methodology with clear definitions of outcomes and controlled conditions, providing high-quality evidence. However, other studies exhibited limitations, including small sample sizes and potential biases, which may affect the generalizability of the findings. Despite these limitations, the collective evidence suggests potential for olive oil in wound healing, warranting further investigation. Future research should aim for more standardized methodologies and larger, more diverse patient cohorts to validate these findings and explore the mechanisms underlying the therapeutic effects of olive oil.
- Emmons EW, Fedeli E, Firestone D. Olive oil introduction and history. In: Hui YH, ed. Bailey’s Industrial Oil & Fat Products, Vol. 2. Edible Oil and Fat Products: Edible Oils. 5th ed. John Wiley & Sons, Ltd; 241-269.
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/IJMS19030686
- Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636-644. doi:10.1016/S0955-2863(02)00229-2
- Rabiei Z, Enferadi ST. Traceability of origin and authenticity of olive oil. In: Boskou D, ed. Olive Oil: Constituents, Quality, Health Properties and Bioconversions. InTech; 2012.
- Wardhana, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138-143.
- Aboui MM, Eidi A, Mortazavi P. Study of effect of olive oil on re-epithelialization of epithelial tissue in excision wound healing model in rats. J Comp Pathobiol. 2016;13:1875-1884.
- Aldana PC, Cartron AM, Khachemoune A. Reappraising diabetic foot ulcers: a focus on mechanisms of ulceration and clinical evaluation.Int J Low Extrem Wounds. 2022;21:294-302. doi:10.1177/1534734620944514
- Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255-264. doi:10.1007/s40257-019-00495-x
- Nasiri M, Fayazi S, Jahani S, et al. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. doi:10.1186/S40200-015-0167-9
- Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347-354. doi:10.2147/CCID.S198577
- Haghighian HK, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowl Health. 2012;6:35-38.
- Zahmatkesh M, Rashidi M. Case report of diabetic foot ulcer with topical honey and olive oil. J Med Plants. 2008;8:36-41.
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129-135. doi:10.1016/J.COPBIO.2011.09.006
- Miraj S, Pourafzali S, Ahmadabadi ZV, et al. Effect of olive oil in preventing the development of pressure ulcer grade one in intensive care unit patients. Int J Prev Med. 2020;11:23. doi:10.4103/IJPVM.IJPVM_545_18
- Díaz‐Valenzuela A, García‐Fernández FP, Carmona Fernández P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: multicentre, controlled, randomised, and double‐blinded clinical trial. Int Wound J. 2019;16:1314-1322. doi:10.1111/IWJ.13191
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;CD000081. doi:10.1002/14651858.CD000081.PUB2
- Amani R, Kariman N, Mojab F, et al. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. J Babol Univ Med Sci. 2015;17:7-12. doi:10.22088/JBUMS.17.11.7
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, et al. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh. 2013;14:309-315.
- Vitsos A, Tsagarousianos C, Vergos O, et al. Efficacy of a Ceratothoa oestroides olive oil extract in patients with chronic ulcers: a pilot study. Int J Low Extrem Wounds. 2019;18:309-316. doi:10.1177/1534734619856143
Olive oil is obtained by mechanical extraction from the fruit of the Olea europaea tree, which is believed to have originated from ancient Iran and Turkestan, later spreading to Anatolia, Syria, Palestine, and Israel. Mechanical extraction of the oil from the olive fruit involves pressure processing, centrifugation, and adhesion filtering.1 Refining of olive oil is done via alkali refining or physical refining, with physical refining being useful in removing oxidation by-products and pro-oxidant metals. Olive oil is composed mainly of triacylglycerols, which are glycerol esters attached to various fatty acids, with the most common fatty acid being the monounsaturated oleic acid. Additional fatty acids include palmitic acid, linoleic acid, stearic acid, and palmitoleic acid.2 Olive oil contains phenolic compounds, the main ones being oleuropein, hydroxytyrosol, and tyrosol. These phenolic compounds are proposed to be strong antioxidants and radical scavengers.3
Mediterranean countries are responsible for approximately 97% of the world’s olive cultivation.4 Olive oil historically was used as lamp fuel, lubricant, body ointment, and later as a source of edible oil.1 Recently, its potential uses in medicine have called for further exploration into other uses for olive oil.
The skin is the largest organ of the body and serves as a protective barrier against pathogens and harmful substances. Skin damage results in 3 main phases to aid in wound healing: inflammation, proliferation, and maturation. In proper skin healing, inflammation will stop once the harmful microbes are removed. However, an excess and prolongation of inflammation can result in delayed healing. Thus, interventions that can limit the amount of inflammation can help promote wound healing. Olive oil contains several anti-inflammatory molecules (compounds or chemicals), including phenolic compounds and omega-3 fatty acids.5 Studies also have shown that olive oil can promote re-epithelialization in tissues.6 Thus, use of olive oil in wound therapy has been of great interest.
This article will review studies that have investigated the use of olive oil for wound healing of diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers. To conduct a comprehensive scoping review of the literature on the effects of olive oil in wound healing, we utilized the resources of the Galter Health Sciences Library & Learning Center (Chicago, Illinois). Our search strategy was structured to encompass a range of relevant databases accessible through the library, including PubMed, Embase, and Web of Science. We formulated our search terms to be broad yet specific to our topic, combining keywords such as olive oil, wound healing, skin repair, and dermal therapy. The inclusion criteria were set to filter studies conducted from January 2000 to December 2019, focusing on clinical trials, observational studies, and review articles. We limited our search to articles published in English, which yielded a preliminary set of articles that were then screened based on their titles and abstracts. Full-text versions of potentially relevant studies were retrieved and assessed for eligibility. We included studies that specifically evaluated the effects of olive oil in wound healing, excluding those that did not directly relate to our research question or had insufficient data. The data extraction from these studies was conducted using a standardized form, capturing study design, population, intervention details, outcomes, and key findings. The synthesis of these data provided a comprehensive overview of the current evidence on the topic, aiding in the identification of gaps in knowledge and directions for future research.
Diabetic Foot Ulcers
Foot ulcers are common in patients with diabetes mellitus and are associated with notable morbidity and mortality. Foot ulcers can clinically manifest in various forms but are classically described as lesions with a deep sinus in the feet. Patients with diabetic foot ulcers are at risk for infection, and severe forms of the ulcers require amputation.7,8 Routine care of foot ulcers involves irrigation of the ulcer and surrounding area with normal saline solution daily, followed by a dressing with sterile gauze. Studies investigating the effect of olive oil on foot ulcers suggest that olive oil use for care and healing of foot ulcers is an area of interest.
A double-blind, randomized clinical trial investigated the effects of topical olive oil on diabetic foot ulcers.9 A total of 34 patients with foot ulcers of Wagner grades 1 (superficial ulcers that involved the skin but not underlying tissue) or 2 (deeper ulcers penetrating to the ligaments and muscles but not the bone) that had remained open and did not improve for more than 3 months were recruited. The patients were randomly assigned to receive topical olive oil and routine care (intervention group) or to receive routine care (control group). Patients who received olive oil had oil poured on their ulcers with gauze wrapped around the ulcer that was soaked with olive oil. The clinical characteristics of the diabetic ulcer (eg, site, grade, size, status of healing) were assessed. The study revealed that after 4 weeks, olive oil significantly decreased ulcer area (P=.01) and ulcer depth (P=.02) compared with the control. Furthermore, there was a significant difference (P=.003) in complete ulcer healing between the olive oil and control groups: 73.3% (11/15) of patients in the olive oil group had complete ulcer healing, whereas 13.3% (2/15) of patients in the control group had complete ulcer healing.9 The positive effect of olive oil on the healing of diabetic foot ulcers encourages further investigation as a possible therapy for foot ulcers.
Another randomized controlled trial of 45 patients with diabetic foot ulcers of Wagner grades 1 or 2 investigated the effect of olive oil.10 Patients were randomly assigned to 1 of 3 groups for 1 month: the olive oil group, the honey group, or the control group. Patients in the olive oil group had their wounds dressed using gauze with olive oil daily, the patients in the honey group had their wounds dressed using gauze with honey daily, and the control group had routine care consisting of irrigation with saline solution and dressing with a sterile gauze. This study calculated a wound healing score based on a predefined checklist for diabetic foot ulcers through 4 variables: wound grading, color, surrounding tissue status, and drainage. Each variable had a maximum score of 100, contributing to a total possible score of 400, which indicated complete healing. A score of 50 signified deterioration. Wound healing was categorized as follows: (1) complete healing is indicated by a total score of 400; (2) partial healing was indicated by an increase of at least 30 points from the initial score; (3) lack of healing occurred when there was no change or less than a 30-point increase from the initial score; and (4) aggravation was noted when the score decreased by at least 10 points from the initial assessment. The study revealed that olive oil and honey treatments resulted in an increase in mean score, which indicated better wound healing. Patients in the olive oil group had a mean score of 253.0 before the intervention and 330.5 after the intervention (P<.0001); patients in the honey group had a mean score of 267.5 before the intervention and 371.5 after the intervention (P<.0001).10
There also have been case reports on combined olive oil and honey in diabetic foot ulcer management. Haghighian et al11 presented a case of a diabetic foot wound that healed completely within 2 weeks after the combined use of olive oil and honey wax. Zahmatkesh and Rashidi12 observed the healing of a diabetic foot wound over a month with daily dressings of a mixture of heated honey and olive oil, resulting in granulation tissue formation within 5 days. Microvascular changes, such as capillary basement membrane thickening, pericyte degeneration, and impairment of vasodilation and constriction, may contribute to inflammation in blood vessels, which can delay the healing of diabetic foot ulcers.7 Because olive oil and honey contain compounds that have antioxidative, antimicrobial, and anti-inflammatory properties, both may play a role in notably reducing inflammation and promoting the healing of foot ulcers.13
Pressure Ulcers
A pressure ulcer is a superficial skin injury that is caused by a prolonged period of pressure on the skin, in which the skin becomes red but there is no rupture. Prolonged periods of immobility resulting in a reduction or pause of blood supply are common causes of pressure ulcers.14 Studies have suggested that topical olive oil may be effective in prevention of pressure ulcers and should be incorporated as part of standard-of-care measures.
In a randomized, single-blind trial, 72 patients with the first stage of bedsore—which is a pressure ulcer—in the sacral, shoulder, heel, or other areas were randomly assigned to either the intervention or control group.14 Patients in the intervention group had 15 mL of olive oil rubbed on the wound for 20 minutes daily and then washed with tepid water. The Pressure Ulcer Scale for Healing tool was utilized to assess the healing status of the pressure ulcer. This tool considers wound surface size, exudate rate, and tissue type to provide a score of 0 to 17 (0=healed ulcer; 17=progression of ulcer). The mean score (SD) was lower in the olive oil group at days 4 and 7 compared with the control group (day 4: 7.50 [2.823] vs 9.50 [1.732]; day 7: 5.44 [3.806] vs 8.83 [2.864])(P<.001). Furthermore, between days 1 and 7, there was significant improvement in the olive oil group (mean difference, 3.56; P<.001) but no significant change in the control group (mean difference, 0.75; P=.052).14 The results indicate that patients in the olive oil group had a better ulcer healing status compared with patients in the control group.
In a noninferiority, randomized, double-blind clinical trial, olive oil was compared to a recommended skin care measure of hyperoxygenated fatty acids (HOFAs) for the prevention of pressure ulcers.15 The study consisted of 571 residents from several nursing homes who were at risk for pressure ulcers. Either olive oil or HOFA was applied to areas at risk for pressure ulcers, with 2 sprays of 0.2 mL per spray to each area every 12 hours. The participants were followed up for 30 days or until a pressure ulcer developed. Researchers performed skin assessments; the Braden Scale was used to assess the risk for pressure ulcers. The incidence difference of pressure ulcers in the olive oil group and HOFA group did not exceed in the noninferiority margin of 7%. Furthermore, Kaplan-Meier survival curves for the time until pressure ulcer onset showed a nonsignificant difference between the 2 groups.15 These findings suggest that olive oil is as effective as HOFA for the prevention of pressure ulcers. Although the mechanism of olive oil on prevention of pressure ulcers has not yet been determined, it has been suggested that anti-inflammatory compounds in olive oil, such as polyphenol and oleocanthal compounds, play an anti-inflammatory role.
Perineal Ulcers
Episiotomy is a surgical incision that is made to open the vagina during birth to aid in delivery of the baby. In contrast to spontaneous vaginal tears, an episiotomy allows for easier repair and healing of the laceration.16 Studies were conducted to investigate the effect of olive oil on women with lacerations after an episiotomy.
A total of 90 primigravid women who had undergone episiotomy were recruited and randomly assigned to 1 of 2 interventions: cold compression with gel packs for 20 minutes within 12 hours after delivery for up to 10 days, if necessary, or topical olive oil twice daily within 12 hours after delivery for up to 10 days.17 Although there was no significant difference in the structural features of the wound, there was a significant difference in the redness severity. After 10 days, the mean REEDA (redness, edema, ecchymosis, discharge, and apposition) score (SD), which assesses tissue healing, was 0.47 (0.96) in patients who received cold compression with gel packs and 0.20 (0.50) in patients who received topical olive oil (P=.04).17 This study suggests that there is the potential for olive oil to be used for wound healing after episiotomy.
A double-blind trial consisted of 60 women who had mediolateral episiotomy or perineal tear grades 1 and 2 who were randomly assigned to 1 of 2 groups for 10 days: olive oil sitz bath or distilled water sitz bath (control group). The results showed a significant difference in pain severity after 5 and 10 days (P<.05), wound redness after 5 days (P<.0001), and redness (P<.000) and edema (P<.05) 10 days after delivery.18 This study encourages further investigation of the benefits of olive oil for care after an episiotomy.
Chronic Ulcers
Chronic ulcers are other persistent wounds that do not respond to standard treatments and pose a notable health burden. Their development is influenced by factors such as oxidative stress, microbial infections, and the body’s immune response. A case series was conducted to investigate the wound healing effects of olive oil on chronic ulcers.19 Fourteen patients who were diagnosed with 1 or more chronic skin ulcers that had not healed with conventional treatment, such as cleansing, debridement, or infection control, were recruited. The mean (SD) of the patients’
Final Thoughts
This review illuminated several key aspects of research on the role of olive oil in wound healing. Although the studies included in this review offer valuable insights, it is essential to acknowledge the variability in the quality of data presented. Several studies demonstrated robust methodology with clear definitions of outcomes and controlled conditions, providing high-quality evidence. However, other studies exhibited limitations, including small sample sizes and potential biases, which may affect the generalizability of the findings. Despite these limitations, the collective evidence suggests potential for olive oil in wound healing, warranting further investigation. Future research should aim for more standardized methodologies and larger, more diverse patient cohorts to validate these findings and explore the mechanisms underlying the therapeutic effects of olive oil.
Olive oil is obtained by mechanical extraction from the fruit of the Olea europaea tree, which is believed to have originated from ancient Iran and Turkestan, later spreading to Anatolia, Syria, Palestine, and Israel. Mechanical extraction of the oil from the olive fruit involves pressure processing, centrifugation, and adhesion filtering.1 Refining of olive oil is done via alkali refining or physical refining, with physical refining being useful in removing oxidation by-products and pro-oxidant metals. Olive oil is composed mainly of triacylglycerols, which are glycerol esters attached to various fatty acids, with the most common fatty acid being the monounsaturated oleic acid. Additional fatty acids include palmitic acid, linoleic acid, stearic acid, and palmitoleic acid.2 Olive oil contains phenolic compounds, the main ones being oleuropein, hydroxytyrosol, and tyrosol. These phenolic compounds are proposed to be strong antioxidants and radical scavengers.3
Mediterranean countries are responsible for approximately 97% of the world’s olive cultivation.4 Olive oil historically was used as lamp fuel, lubricant, body ointment, and later as a source of edible oil.1 Recently, its potential uses in medicine have called for further exploration into other uses for olive oil.
The skin is the largest organ of the body and serves as a protective barrier against pathogens and harmful substances. Skin damage results in 3 main phases to aid in wound healing: inflammation, proliferation, and maturation. In proper skin healing, inflammation will stop once the harmful microbes are removed. However, an excess and prolongation of inflammation can result in delayed healing. Thus, interventions that can limit the amount of inflammation can help promote wound healing. Olive oil contains several anti-inflammatory molecules (compounds or chemicals), including phenolic compounds and omega-3 fatty acids.5 Studies also have shown that olive oil can promote re-epithelialization in tissues.6 Thus, use of olive oil in wound therapy has been of great interest.
This article will review studies that have investigated the use of olive oil for wound healing of diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers. To conduct a comprehensive scoping review of the literature on the effects of olive oil in wound healing, we utilized the resources of the Galter Health Sciences Library & Learning Center (Chicago, Illinois). Our search strategy was structured to encompass a range of relevant databases accessible through the library, including PubMed, Embase, and Web of Science. We formulated our search terms to be broad yet specific to our topic, combining keywords such as olive oil, wound healing, skin repair, and dermal therapy. The inclusion criteria were set to filter studies conducted from January 2000 to December 2019, focusing on clinical trials, observational studies, and review articles. We limited our search to articles published in English, which yielded a preliminary set of articles that were then screened based on their titles and abstracts. Full-text versions of potentially relevant studies were retrieved and assessed for eligibility. We included studies that specifically evaluated the effects of olive oil in wound healing, excluding those that did not directly relate to our research question or had insufficient data. The data extraction from these studies was conducted using a standardized form, capturing study design, population, intervention details, outcomes, and key findings. The synthesis of these data provided a comprehensive overview of the current evidence on the topic, aiding in the identification of gaps in knowledge and directions for future research.
Diabetic Foot Ulcers
Foot ulcers are common in patients with diabetes mellitus and are associated with notable morbidity and mortality. Foot ulcers can clinically manifest in various forms but are classically described as lesions with a deep sinus in the feet. Patients with diabetic foot ulcers are at risk for infection, and severe forms of the ulcers require amputation.7,8 Routine care of foot ulcers involves irrigation of the ulcer and surrounding area with normal saline solution daily, followed by a dressing with sterile gauze. Studies investigating the effect of olive oil on foot ulcers suggest that olive oil use for care and healing of foot ulcers is an area of interest.
A double-blind, randomized clinical trial investigated the effects of topical olive oil on diabetic foot ulcers.9 A total of 34 patients with foot ulcers of Wagner grades 1 (superficial ulcers that involved the skin but not underlying tissue) or 2 (deeper ulcers penetrating to the ligaments and muscles but not the bone) that had remained open and did not improve for more than 3 months were recruited. The patients were randomly assigned to receive topical olive oil and routine care (intervention group) or to receive routine care (control group). Patients who received olive oil had oil poured on their ulcers with gauze wrapped around the ulcer that was soaked with olive oil. The clinical characteristics of the diabetic ulcer (eg, site, grade, size, status of healing) were assessed. The study revealed that after 4 weeks, olive oil significantly decreased ulcer area (P=.01) and ulcer depth (P=.02) compared with the control. Furthermore, there was a significant difference (P=.003) in complete ulcer healing between the olive oil and control groups: 73.3% (11/15) of patients in the olive oil group had complete ulcer healing, whereas 13.3% (2/15) of patients in the control group had complete ulcer healing.9 The positive effect of olive oil on the healing of diabetic foot ulcers encourages further investigation as a possible therapy for foot ulcers.
Another randomized controlled trial of 45 patients with diabetic foot ulcers of Wagner grades 1 or 2 investigated the effect of olive oil.10 Patients were randomly assigned to 1 of 3 groups for 1 month: the olive oil group, the honey group, or the control group. Patients in the olive oil group had their wounds dressed using gauze with olive oil daily, the patients in the honey group had their wounds dressed using gauze with honey daily, and the control group had routine care consisting of irrigation with saline solution and dressing with a sterile gauze. This study calculated a wound healing score based on a predefined checklist for diabetic foot ulcers through 4 variables: wound grading, color, surrounding tissue status, and drainage. Each variable had a maximum score of 100, contributing to a total possible score of 400, which indicated complete healing. A score of 50 signified deterioration. Wound healing was categorized as follows: (1) complete healing is indicated by a total score of 400; (2) partial healing was indicated by an increase of at least 30 points from the initial score; (3) lack of healing occurred when there was no change or less than a 30-point increase from the initial score; and (4) aggravation was noted when the score decreased by at least 10 points from the initial assessment. The study revealed that olive oil and honey treatments resulted in an increase in mean score, which indicated better wound healing. Patients in the olive oil group had a mean score of 253.0 before the intervention and 330.5 after the intervention (P<.0001); patients in the honey group had a mean score of 267.5 before the intervention and 371.5 after the intervention (P<.0001).10
There also have been case reports on combined olive oil and honey in diabetic foot ulcer management. Haghighian et al11 presented a case of a diabetic foot wound that healed completely within 2 weeks after the combined use of olive oil and honey wax. Zahmatkesh and Rashidi12 observed the healing of a diabetic foot wound over a month with daily dressings of a mixture of heated honey and olive oil, resulting in granulation tissue formation within 5 days. Microvascular changes, such as capillary basement membrane thickening, pericyte degeneration, and impairment of vasodilation and constriction, may contribute to inflammation in blood vessels, which can delay the healing of diabetic foot ulcers.7 Because olive oil and honey contain compounds that have antioxidative, antimicrobial, and anti-inflammatory properties, both may play a role in notably reducing inflammation and promoting the healing of foot ulcers.13
Pressure Ulcers
A pressure ulcer is a superficial skin injury that is caused by a prolonged period of pressure on the skin, in which the skin becomes red but there is no rupture. Prolonged periods of immobility resulting in a reduction or pause of blood supply are common causes of pressure ulcers.14 Studies have suggested that topical olive oil may be effective in prevention of pressure ulcers and should be incorporated as part of standard-of-care measures.
In a randomized, single-blind trial, 72 patients with the first stage of bedsore—which is a pressure ulcer—in the sacral, shoulder, heel, or other areas were randomly assigned to either the intervention or control group.14 Patients in the intervention group had 15 mL of olive oil rubbed on the wound for 20 minutes daily and then washed with tepid water. The Pressure Ulcer Scale for Healing tool was utilized to assess the healing status of the pressure ulcer. This tool considers wound surface size, exudate rate, and tissue type to provide a score of 0 to 17 (0=healed ulcer; 17=progression of ulcer). The mean score (SD) was lower in the olive oil group at days 4 and 7 compared with the control group (day 4: 7.50 [2.823] vs 9.50 [1.732]; day 7: 5.44 [3.806] vs 8.83 [2.864])(P<.001). Furthermore, between days 1 and 7, there was significant improvement in the olive oil group (mean difference, 3.56; P<.001) but no significant change in the control group (mean difference, 0.75; P=.052).14 The results indicate that patients in the olive oil group had a better ulcer healing status compared with patients in the control group.
In a noninferiority, randomized, double-blind clinical trial, olive oil was compared to a recommended skin care measure of hyperoxygenated fatty acids (HOFAs) for the prevention of pressure ulcers.15 The study consisted of 571 residents from several nursing homes who were at risk for pressure ulcers. Either olive oil or HOFA was applied to areas at risk for pressure ulcers, with 2 sprays of 0.2 mL per spray to each area every 12 hours. The participants were followed up for 30 days or until a pressure ulcer developed. Researchers performed skin assessments; the Braden Scale was used to assess the risk for pressure ulcers. The incidence difference of pressure ulcers in the olive oil group and HOFA group did not exceed in the noninferiority margin of 7%. Furthermore, Kaplan-Meier survival curves for the time until pressure ulcer onset showed a nonsignificant difference between the 2 groups.15 These findings suggest that olive oil is as effective as HOFA for the prevention of pressure ulcers. Although the mechanism of olive oil on prevention of pressure ulcers has not yet been determined, it has been suggested that anti-inflammatory compounds in olive oil, such as polyphenol and oleocanthal compounds, play an anti-inflammatory role.
Perineal Ulcers
Episiotomy is a surgical incision that is made to open the vagina during birth to aid in delivery of the baby. In contrast to spontaneous vaginal tears, an episiotomy allows for easier repair and healing of the laceration.16 Studies were conducted to investigate the effect of olive oil on women with lacerations after an episiotomy.
A total of 90 primigravid women who had undergone episiotomy were recruited and randomly assigned to 1 of 2 interventions: cold compression with gel packs for 20 minutes within 12 hours after delivery for up to 10 days, if necessary, or topical olive oil twice daily within 12 hours after delivery for up to 10 days.17 Although there was no significant difference in the structural features of the wound, there was a significant difference in the redness severity. After 10 days, the mean REEDA (redness, edema, ecchymosis, discharge, and apposition) score (SD), which assesses tissue healing, was 0.47 (0.96) in patients who received cold compression with gel packs and 0.20 (0.50) in patients who received topical olive oil (P=.04).17 This study suggests that there is the potential for olive oil to be used for wound healing after episiotomy.
A double-blind trial consisted of 60 women who had mediolateral episiotomy or perineal tear grades 1 and 2 who were randomly assigned to 1 of 2 groups for 10 days: olive oil sitz bath or distilled water sitz bath (control group). The results showed a significant difference in pain severity after 5 and 10 days (P<.05), wound redness after 5 days (P<.0001), and redness (P<.000) and edema (P<.05) 10 days after delivery.18 This study encourages further investigation of the benefits of olive oil for care after an episiotomy.
Chronic Ulcers
Chronic ulcers are other persistent wounds that do not respond to standard treatments and pose a notable health burden. Their development is influenced by factors such as oxidative stress, microbial infections, and the body’s immune response. A case series was conducted to investigate the wound healing effects of olive oil on chronic ulcers.19 Fourteen patients who were diagnosed with 1 or more chronic skin ulcers that had not healed with conventional treatment, such as cleansing, debridement, or infection control, were recruited. The mean (SD) of the patients’
Final Thoughts
This review illuminated several key aspects of research on the role of olive oil in wound healing. Although the studies included in this review offer valuable insights, it is essential to acknowledge the variability in the quality of data presented. Several studies demonstrated robust methodology with clear definitions of outcomes and controlled conditions, providing high-quality evidence. However, other studies exhibited limitations, including small sample sizes and potential biases, which may affect the generalizability of the findings. Despite these limitations, the collective evidence suggests potential for olive oil in wound healing, warranting further investigation. Future research should aim for more standardized methodologies and larger, more diverse patient cohorts to validate these findings and explore the mechanisms underlying the therapeutic effects of olive oil.
- Emmons EW, Fedeli E, Firestone D. Olive oil introduction and history. In: Hui YH, ed. Bailey’s Industrial Oil & Fat Products, Vol. 2. Edible Oil and Fat Products: Edible Oils. 5th ed. John Wiley & Sons, Ltd; 241-269.
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/IJMS19030686
- Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636-644. doi:10.1016/S0955-2863(02)00229-2
- Rabiei Z, Enferadi ST. Traceability of origin and authenticity of olive oil. In: Boskou D, ed. Olive Oil: Constituents, Quality, Health Properties and Bioconversions. InTech; 2012.
- Wardhana, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138-143.
- Aboui MM, Eidi A, Mortazavi P. Study of effect of olive oil on re-epithelialization of epithelial tissue in excision wound healing model in rats. J Comp Pathobiol. 2016;13:1875-1884.
- Aldana PC, Cartron AM, Khachemoune A. Reappraising diabetic foot ulcers: a focus on mechanisms of ulceration and clinical evaluation.Int J Low Extrem Wounds. 2022;21:294-302. doi:10.1177/1534734620944514
- Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255-264. doi:10.1007/s40257-019-00495-x
- Nasiri M, Fayazi S, Jahani S, et al. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. doi:10.1186/S40200-015-0167-9
- Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347-354. doi:10.2147/CCID.S198577
- Haghighian HK, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowl Health. 2012;6:35-38.
- Zahmatkesh M, Rashidi M. Case report of diabetic foot ulcer with topical honey and olive oil. J Med Plants. 2008;8:36-41.
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129-135. doi:10.1016/J.COPBIO.2011.09.006
- Miraj S, Pourafzali S, Ahmadabadi ZV, et al. Effect of olive oil in preventing the development of pressure ulcer grade one in intensive care unit patients. Int J Prev Med. 2020;11:23. doi:10.4103/IJPVM.IJPVM_545_18
- Díaz‐Valenzuela A, García‐Fernández FP, Carmona Fernández P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: multicentre, controlled, randomised, and double‐blinded clinical trial. Int Wound J. 2019;16:1314-1322. doi:10.1111/IWJ.13191
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;CD000081. doi:10.1002/14651858.CD000081.PUB2
- Amani R, Kariman N, Mojab F, et al. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. J Babol Univ Med Sci. 2015;17:7-12. doi:10.22088/JBUMS.17.11.7
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, et al. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh. 2013;14:309-315.
- Vitsos A, Tsagarousianos C, Vergos O, et al. Efficacy of a Ceratothoa oestroides olive oil extract in patients with chronic ulcers: a pilot study. Int J Low Extrem Wounds. 2019;18:309-316. doi:10.1177/1534734619856143
- Emmons EW, Fedeli E, Firestone D. Olive oil introduction and history. In: Hui YH, ed. Bailey’s Industrial Oil & Fat Products, Vol. 2. Edible Oil and Fat Products: Edible Oils. 5th ed. John Wiley & Sons, Ltd; 241-269.
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/IJMS19030686
- Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636-644. doi:10.1016/S0955-2863(02)00229-2
- Rabiei Z, Enferadi ST. Traceability of origin and authenticity of olive oil. In: Boskou D, ed. Olive Oil: Constituents, Quality, Health Properties and Bioconversions. InTech; 2012.
- Wardhana, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138-143.
- Aboui MM, Eidi A, Mortazavi P. Study of effect of olive oil on re-epithelialization of epithelial tissue in excision wound healing model in rats. J Comp Pathobiol. 2016;13:1875-1884.
- Aldana PC, Cartron AM, Khachemoune A. Reappraising diabetic foot ulcers: a focus on mechanisms of ulceration and clinical evaluation.Int J Low Extrem Wounds. 2022;21:294-302. doi:10.1177/1534734620944514
- Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255-264. doi:10.1007/s40257-019-00495-x
- Nasiri M, Fayazi S, Jahani S, et al. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. doi:10.1186/S40200-015-0167-9
- Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347-354. doi:10.2147/CCID.S198577
- Haghighian HK, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowl Health. 2012;6:35-38.
- Zahmatkesh M, Rashidi M. Case report of diabetic foot ulcer with topical honey and olive oil. J Med Plants. 2008;8:36-41.
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129-135. doi:10.1016/J.COPBIO.2011.09.006
- Miraj S, Pourafzali S, Ahmadabadi ZV, et al. Effect of olive oil in preventing the development of pressure ulcer grade one in intensive care unit patients. Int J Prev Med. 2020;11:23. doi:10.4103/IJPVM.IJPVM_545_18
- Díaz‐Valenzuela A, García‐Fernández FP, Carmona Fernández P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: multicentre, controlled, randomised, and double‐blinded clinical trial. Int Wound J. 2019;16:1314-1322. doi:10.1111/IWJ.13191
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;CD000081. doi:10.1002/14651858.CD000081.PUB2
- Amani R, Kariman N, Mojab F, et al. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. J Babol Univ Med Sci. 2015;17:7-12. doi:10.22088/JBUMS.17.11.7
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, et al. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh. 2013;14:309-315.
- Vitsos A, Tsagarousianos C, Vergos O, et al. Efficacy of a Ceratothoa oestroides olive oil extract in patients with chronic ulcers: a pilot study. Int J Low Extrem Wounds. 2019;18:309-316. doi:10.1177/1534734619856143
Practice Points
- Interventions that effectively reduce excessive and prolonged inflammation can help promote timely wound healing. Consider integrating anti-inflammatory treatments into wound care protocols to enhance healing outcomes.
- Utilization of olive oil in wound therapy, particularly for conditions such as diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers, has shown promise for promoting healing.
- Regularly review and incorporate findings from recent studies on the use of olive oil and other novel interventions in wound therapy to ensure the application of the most current and effective treatment strategies.
Analysis Finds Minority of Chronic Wounds Treated by Dermatologists
. However, fewer than 8% of chronic wounds were managed by dermatologists during this time.
Those are among key findings from an analysis of National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2019 presented as a late-breaking abstract at the annual meeting of the Society for Investigative Dermatology. “Cutaneous wounds were estimated to account for 28.1 to 96.1 billion dollars in US health care costs in 2014,” one of the study authors, Rithi Chandy, MD, MS, a research fellow at the Center for Dermatology Research at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said in an interview following the meeting. “By examining national trends in patient visits and treatment, we may be able to better inform health care utilization for cutaneous wounds.”
Dr. Chandy and colleagues analyzed de-identified patient data from the 2011 to 2019 NAMCS for acute and chronic wound diagnoses, medications prescribed, and physician specialty categories. During the time studied, 5.76 billion patient visits were made, including 45.1 million visits for cutaneous wounds. Of these, the most common diagnoses were open wounds of the thumb without nail damage (7.96%), the lower leg (5.75%), nonpressure chronic ulcers of other parts of the foot (5.08%), and open wounds of the ear (5%).
Among all visits for cutaneous wounds, about one third were chronic cutaneous wounds, with the following descriptions: “Nonpressure chronic ulcer of other part of foot” (17.8%); “nonpressure chronic ulcer of skin, not elsewhere classified” (9.38%); and “ulcer of lower limbs, excluding decubitus, unspecified” (8.72%). “The frequency of patient visits per year during the study period remained stable for both acute and chronic wounds,” Dr. Chandy said. The number of visits for which antimicrobials were used was stable over time for both acute and chronic cutaneous wounds, with the exception of increased use of antivirals for chronic cutaneous wounds, he added.
Specifically, prescriptions were issued in 156 million visits over the time studied, most commonly cephalexin (4.22%), topical silver sulfadiazine (1.59%), topical mupirocin (1.12%), and miscellaneous antibiotics (1.18%).
“Our data shows that topical mupirocin is the most commonly used topical antimicrobial for cutaneous wounds,” Dr. Chandy said. “However, there are reports of emerging bacterial resistance to mupirocin. Our data can inform ongoing efforts to promote antimicrobial stewardship and drug development to provide alternative options that are less likely to induce antimicrobial resistance.”
In findings limited to specialty-specific NAMCS data available from 2011 and from 2013 to 2016, dermatologists managed 3.85% of overall cutaneous wounds, 2.35% of acute wounds, and 7.39% of chronic wounds. By contrast, Dr. Chandy said, 21.1% of chronic wounds were managed by general/family practice physicians, 20.7% by internists, 6.84% by general surgeons, and 5.65% by orthopedic surgeons.
“As dermatologists are experts in the structure and function of the skin and are trained to manage cutaneous disorders including wound healing, we [believe that] dermatologists are equipped with the skill set” for managing wounds, especially for chronic ulcers, he said. The decline in dermatologists who specialize in wound care, he added, “underscores the need for structured dermatology fellowship programs to prepare next-generation dermatologists to address this shortage and ensure dermatology leadership in cutaneous wound healing.”
Dr. Chandy acknowledged certain limitations of the study, including the potential for misclassification of diagnoses or medications prescribed and the fact that the NAMCS database is unable to provide insight into individual patient experiences such as continual cutaneous wound management for the same patient over time.
In the opinion of Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the Nail Division at Weill Cornell Medicine, New York, who was asked to comment on the study, the most interesting finding was that dermatologists cared for a small minority of patients with cutaneous wounds. “It would be interesting to know whether this is due to dermatologist shortages or knowledge gaps on the part of primary care physicians or patients that dermatologists are trained to care for wounds,” Dr. Lipner told this news organization. Other unanswered questions, she noted, “are patient demographics, geographic locations, and comorbidities.”
One of the study authors, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, disclosed that he has received research, speaking and/or consulting support from numerous pharmaceutical companies. No other authors reported having relevant disclosures. Dr. Lipner reported having no disclosures.
A version of this article appeared on Medscape.com .
. However, fewer than 8% of chronic wounds were managed by dermatologists during this time.
Those are among key findings from an analysis of National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2019 presented as a late-breaking abstract at the annual meeting of the Society for Investigative Dermatology. “Cutaneous wounds were estimated to account for 28.1 to 96.1 billion dollars in US health care costs in 2014,” one of the study authors, Rithi Chandy, MD, MS, a research fellow at the Center for Dermatology Research at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said in an interview following the meeting. “By examining national trends in patient visits and treatment, we may be able to better inform health care utilization for cutaneous wounds.”
Dr. Chandy and colleagues analyzed de-identified patient data from the 2011 to 2019 NAMCS for acute and chronic wound diagnoses, medications prescribed, and physician specialty categories. During the time studied, 5.76 billion patient visits were made, including 45.1 million visits for cutaneous wounds. Of these, the most common diagnoses were open wounds of the thumb without nail damage (7.96%), the lower leg (5.75%), nonpressure chronic ulcers of other parts of the foot (5.08%), and open wounds of the ear (5%).
Among all visits for cutaneous wounds, about one third were chronic cutaneous wounds, with the following descriptions: “Nonpressure chronic ulcer of other part of foot” (17.8%); “nonpressure chronic ulcer of skin, not elsewhere classified” (9.38%); and “ulcer of lower limbs, excluding decubitus, unspecified” (8.72%). “The frequency of patient visits per year during the study period remained stable for both acute and chronic wounds,” Dr. Chandy said. The number of visits for which antimicrobials were used was stable over time for both acute and chronic cutaneous wounds, with the exception of increased use of antivirals for chronic cutaneous wounds, he added.
Specifically, prescriptions were issued in 156 million visits over the time studied, most commonly cephalexin (4.22%), topical silver sulfadiazine (1.59%), topical mupirocin (1.12%), and miscellaneous antibiotics (1.18%).
“Our data shows that topical mupirocin is the most commonly used topical antimicrobial for cutaneous wounds,” Dr. Chandy said. “However, there are reports of emerging bacterial resistance to mupirocin. Our data can inform ongoing efforts to promote antimicrobial stewardship and drug development to provide alternative options that are less likely to induce antimicrobial resistance.”
In findings limited to specialty-specific NAMCS data available from 2011 and from 2013 to 2016, dermatologists managed 3.85% of overall cutaneous wounds, 2.35% of acute wounds, and 7.39% of chronic wounds. By contrast, Dr. Chandy said, 21.1% of chronic wounds were managed by general/family practice physicians, 20.7% by internists, 6.84% by general surgeons, and 5.65% by orthopedic surgeons.
“As dermatologists are experts in the structure and function of the skin and are trained to manage cutaneous disorders including wound healing, we [believe that] dermatologists are equipped with the skill set” for managing wounds, especially for chronic ulcers, he said. The decline in dermatologists who specialize in wound care, he added, “underscores the need for structured dermatology fellowship programs to prepare next-generation dermatologists to address this shortage and ensure dermatology leadership in cutaneous wound healing.”
Dr. Chandy acknowledged certain limitations of the study, including the potential for misclassification of diagnoses or medications prescribed and the fact that the NAMCS database is unable to provide insight into individual patient experiences such as continual cutaneous wound management for the same patient over time.
In the opinion of Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the Nail Division at Weill Cornell Medicine, New York, who was asked to comment on the study, the most interesting finding was that dermatologists cared for a small minority of patients with cutaneous wounds. “It would be interesting to know whether this is due to dermatologist shortages or knowledge gaps on the part of primary care physicians or patients that dermatologists are trained to care for wounds,” Dr. Lipner told this news organization. Other unanswered questions, she noted, “are patient demographics, geographic locations, and comorbidities.”
One of the study authors, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, disclosed that he has received research, speaking and/or consulting support from numerous pharmaceutical companies. No other authors reported having relevant disclosures. Dr. Lipner reported having no disclosures.
A version of this article appeared on Medscape.com .
. However, fewer than 8% of chronic wounds were managed by dermatologists during this time.
Those are among key findings from an analysis of National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2019 presented as a late-breaking abstract at the annual meeting of the Society for Investigative Dermatology. “Cutaneous wounds were estimated to account for 28.1 to 96.1 billion dollars in US health care costs in 2014,” one of the study authors, Rithi Chandy, MD, MS, a research fellow at the Center for Dermatology Research at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said in an interview following the meeting. “By examining national trends in patient visits and treatment, we may be able to better inform health care utilization for cutaneous wounds.”
Dr. Chandy and colleagues analyzed de-identified patient data from the 2011 to 2019 NAMCS for acute and chronic wound diagnoses, medications prescribed, and physician specialty categories. During the time studied, 5.76 billion patient visits were made, including 45.1 million visits for cutaneous wounds. Of these, the most common diagnoses were open wounds of the thumb without nail damage (7.96%), the lower leg (5.75%), nonpressure chronic ulcers of other parts of the foot (5.08%), and open wounds of the ear (5%).
Among all visits for cutaneous wounds, about one third were chronic cutaneous wounds, with the following descriptions: “Nonpressure chronic ulcer of other part of foot” (17.8%); “nonpressure chronic ulcer of skin, not elsewhere classified” (9.38%); and “ulcer of lower limbs, excluding decubitus, unspecified” (8.72%). “The frequency of patient visits per year during the study period remained stable for both acute and chronic wounds,” Dr. Chandy said. The number of visits for which antimicrobials were used was stable over time for both acute and chronic cutaneous wounds, with the exception of increased use of antivirals for chronic cutaneous wounds, he added.
Specifically, prescriptions were issued in 156 million visits over the time studied, most commonly cephalexin (4.22%), topical silver sulfadiazine (1.59%), topical mupirocin (1.12%), and miscellaneous antibiotics (1.18%).
“Our data shows that topical mupirocin is the most commonly used topical antimicrobial for cutaneous wounds,” Dr. Chandy said. “However, there are reports of emerging bacterial resistance to mupirocin. Our data can inform ongoing efforts to promote antimicrobial stewardship and drug development to provide alternative options that are less likely to induce antimicrobial resistance.”
In findings limited to specialty-specific NAMCS data available from 2011 and from 2013 to 2016, dermatologists managed 3.85% of overall cutaneous wounds, 2.35% of acute wounds, and 7.39% of chronic wounds. By contrast, Dr. Chandy said, 21.1% of chronic wounds were managed by general/family practice physicians, 20.7% by internists, 6.84% by general surgeons, and 5.65% by orthopedic surgeons.
“As dermatologists are experts in the structure and function of the skin and are trained to manage cutaneous disorders including wound healing, we [believe that] dermatologists are equipped with the skill set” for managing wounds, especially for chronic ulcers, he said. The decline in dermatologists who specialize in wound care, he added, “underscores the need for structured dermatology fellowship programs to prepare next-generation dermatologists to address this shortage and ensure dermatology leadership in cutaneous wound healing.”
Dr. Chandy acknowledged certain limitations of the study, including the potential for misclassification of diagnoses or medications prescribed and the fact that the NAMCS database is unable to provide insight into individual patient experiences such as continual cutaneous wound management for the same patient over time.
In the opinion of Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the Nail Division at Weill Cornell Medicine, New York, who was asked to comment on the study, the most interesting finding was that dermatologists cared for a small minority of patients with cutaneous wounds. “It would be interesting to know whether this is due to dermatologist shortages or knowledge gaps on the part of primary care physicians or patients that dermatologists are trained to care for wounds,” Dr. Lipner told this news organization. Other unanswered questions, she noted, “are patient demographics, geographic locations, and comorbidities.”
One of the study authors, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, disclosed that he has received research, speaking and/or consulting support from numerous pharmaceutical companies. No other authors reported having relevant disclosures. Dr. Lipner reported having no disclosures.
A version of this article appeared on Medscape.com .
FROM SID 2024