User login
Navigating Hair Loss in Medical School: Experiences of 2 Young Black Women
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
Practice Points
- Hair loss is a common dermatologic concern among Black women and can represent a diagnostic challenge to dermatologists who may not be familiar with textured hair.
- Dermatologists should practice cultural sensitivity and provide relevant recommendations to Black patients dealing with hair loss.
Thalidomide Analogue Drug Eruption Along the Lines of Blaschko
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
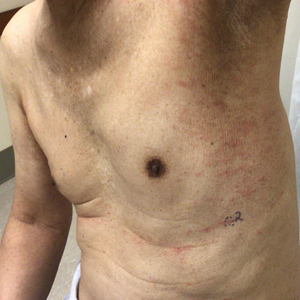
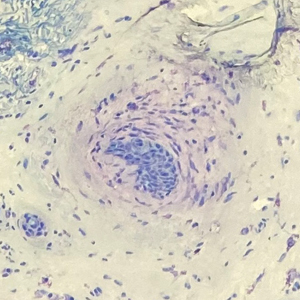
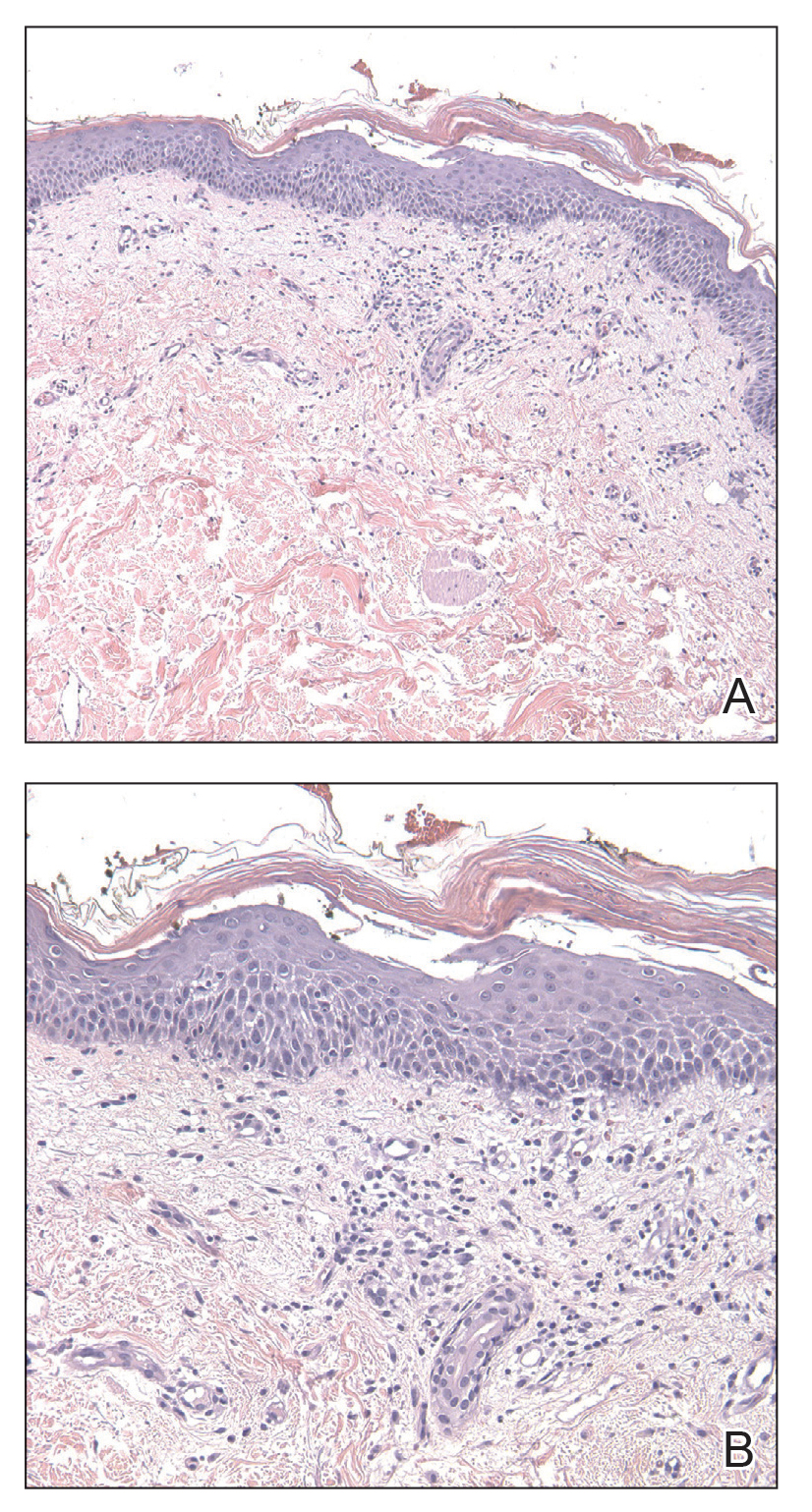
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.
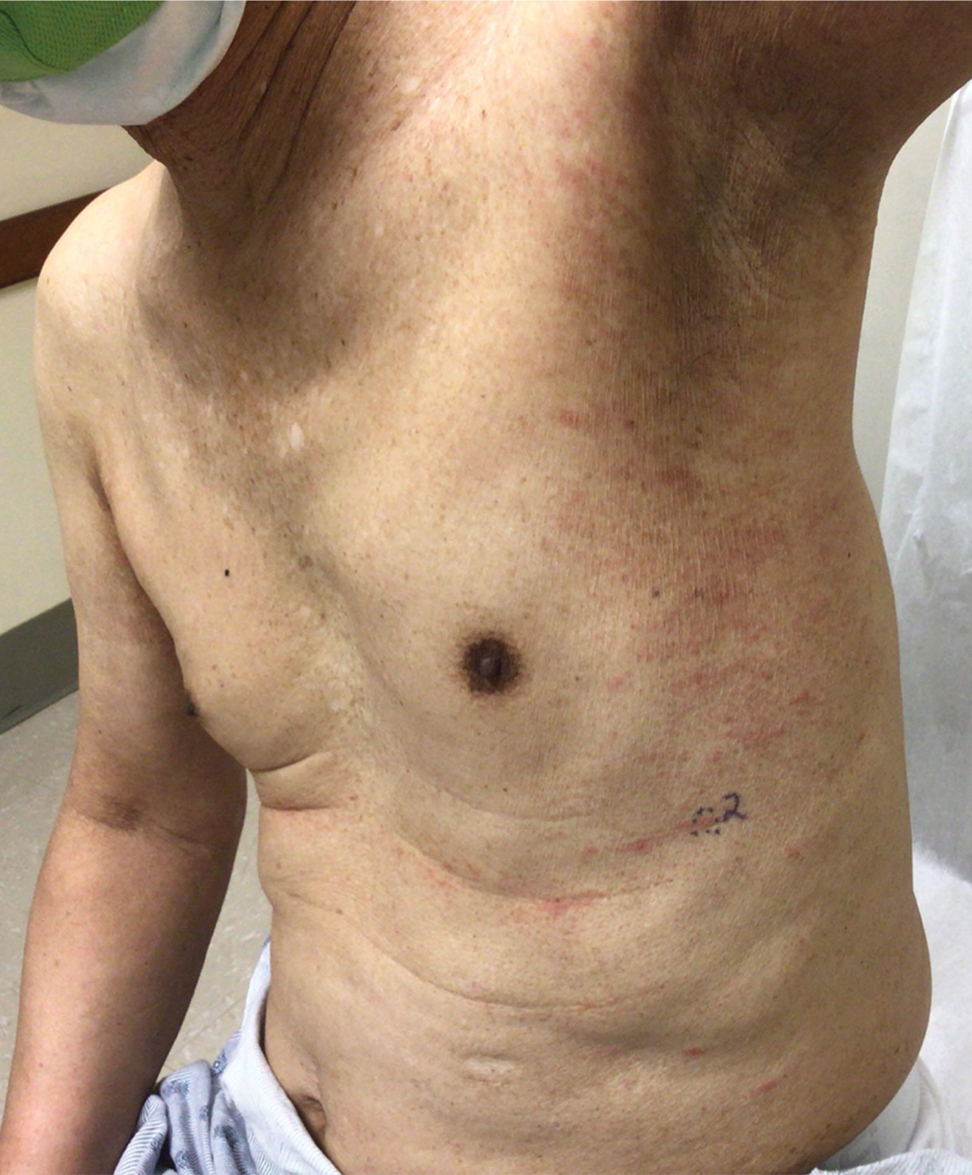
After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.
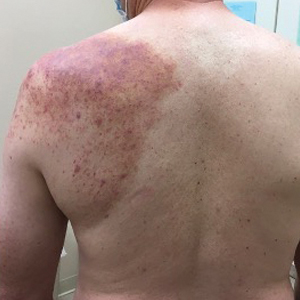
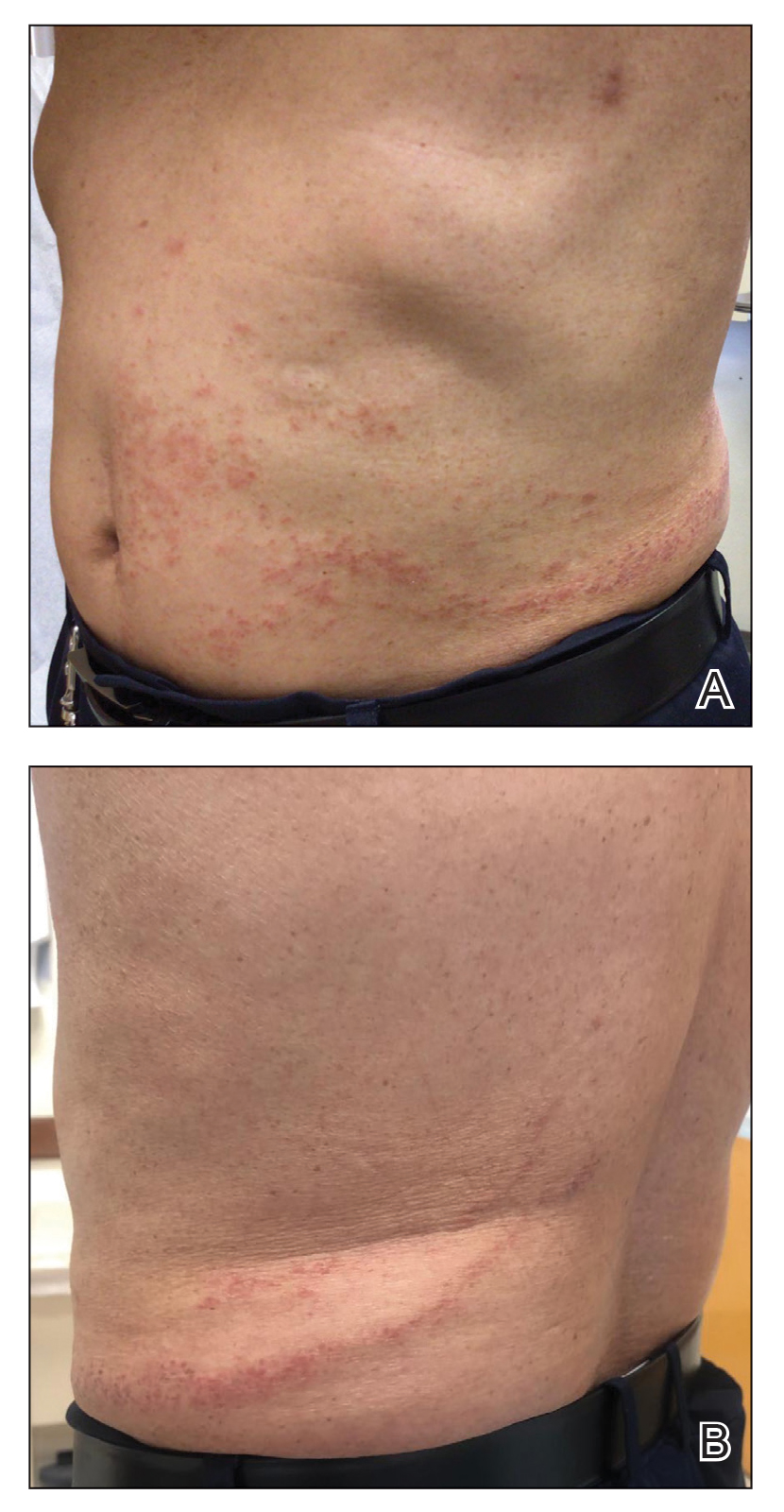
At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).
We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.

After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.

At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).

We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.

After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.

At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).

We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
Practice Points
- Dermatologists should be aware of the variety of cutaneous adverse events that can arise from the use of immunotherapeutic agents for hematologic malignancies.
- Some cutaneous reactions to immunotherapeutic medications, such as pityriasiform eruption and blaschkitis, generally are benign and may not necessitate halting an important therapy.
The Role of Toluidine Blue in Mohs Micrographic Surgery: A Systematic Review
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.
Results
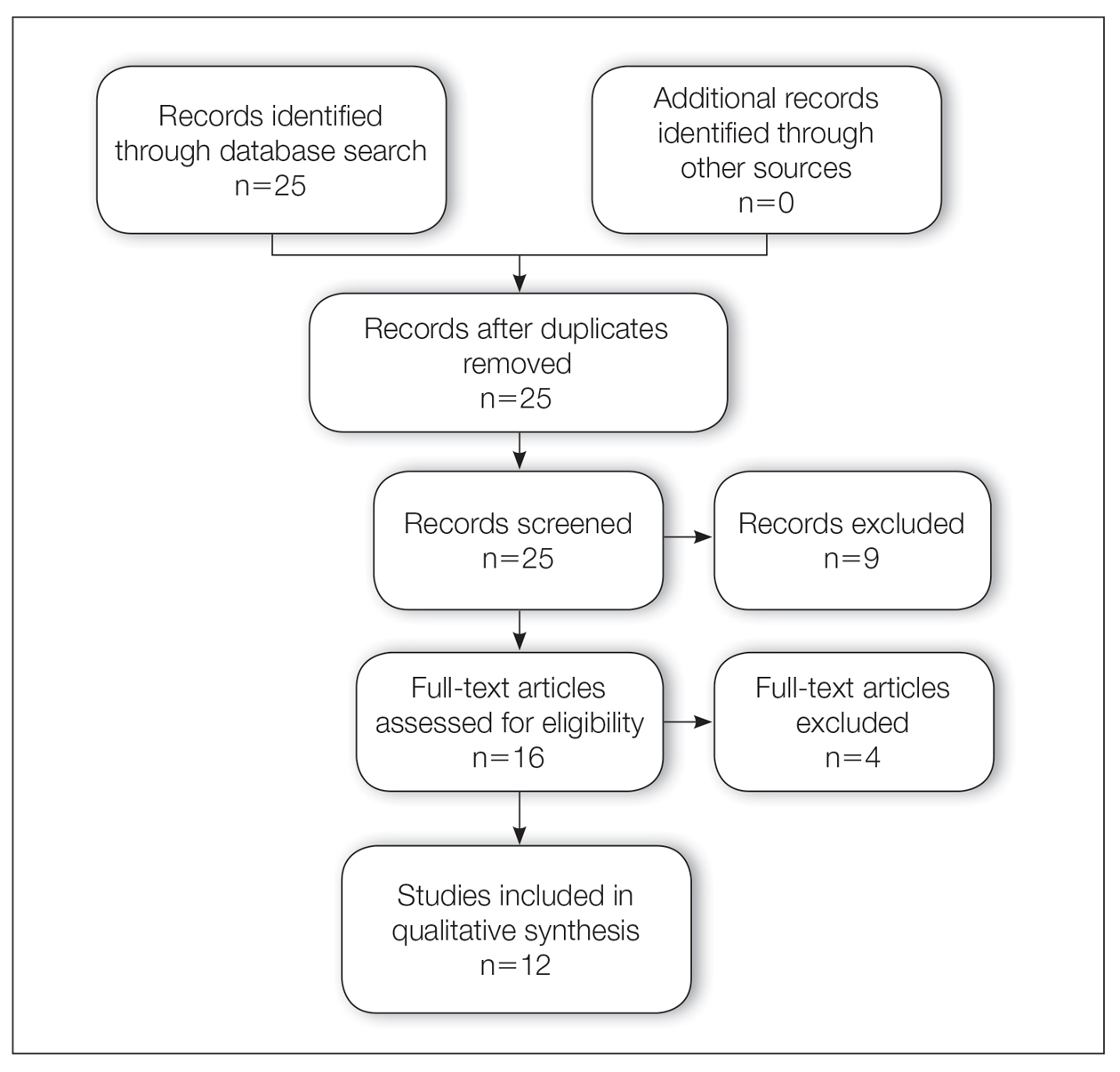
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).
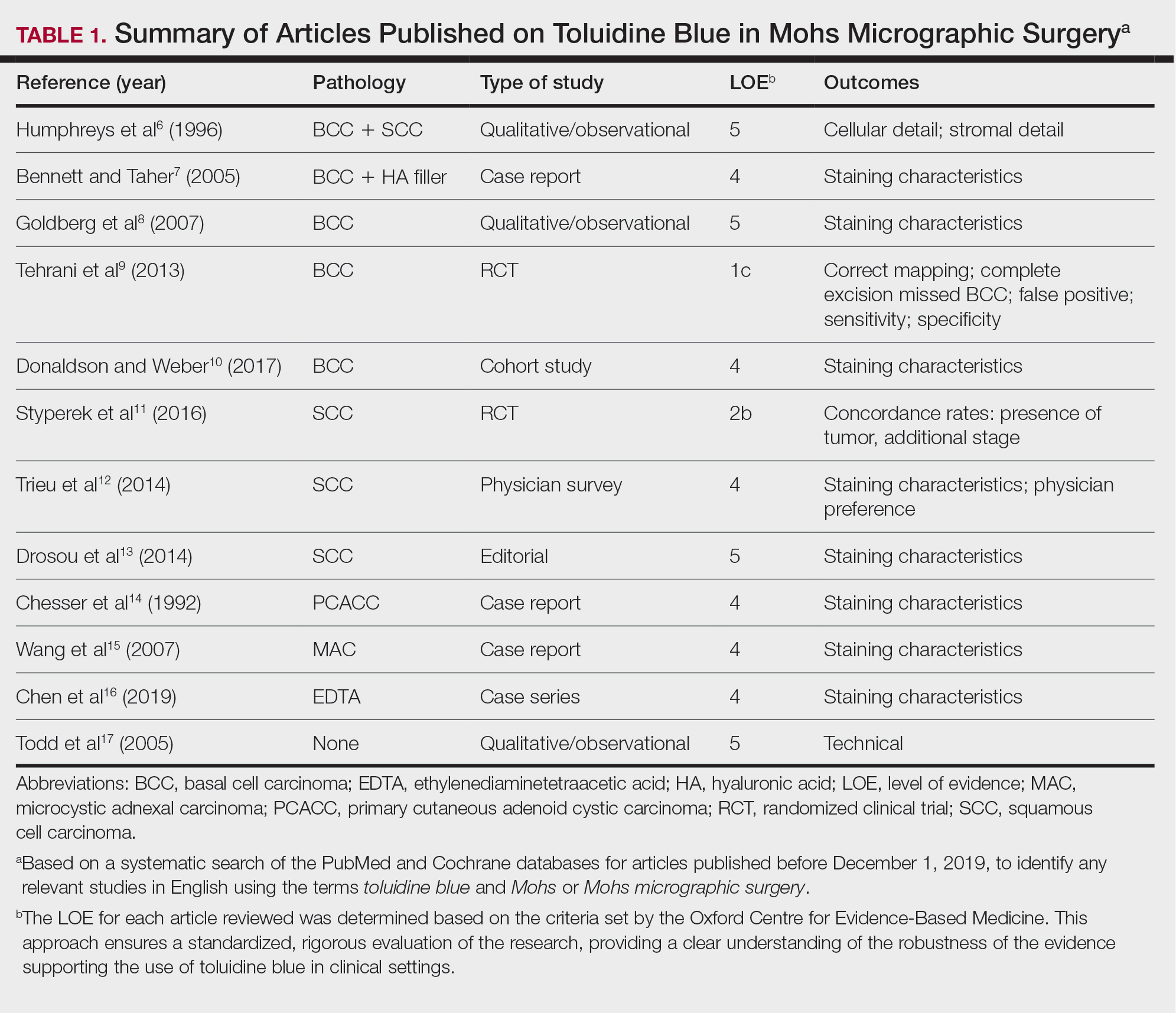
A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.
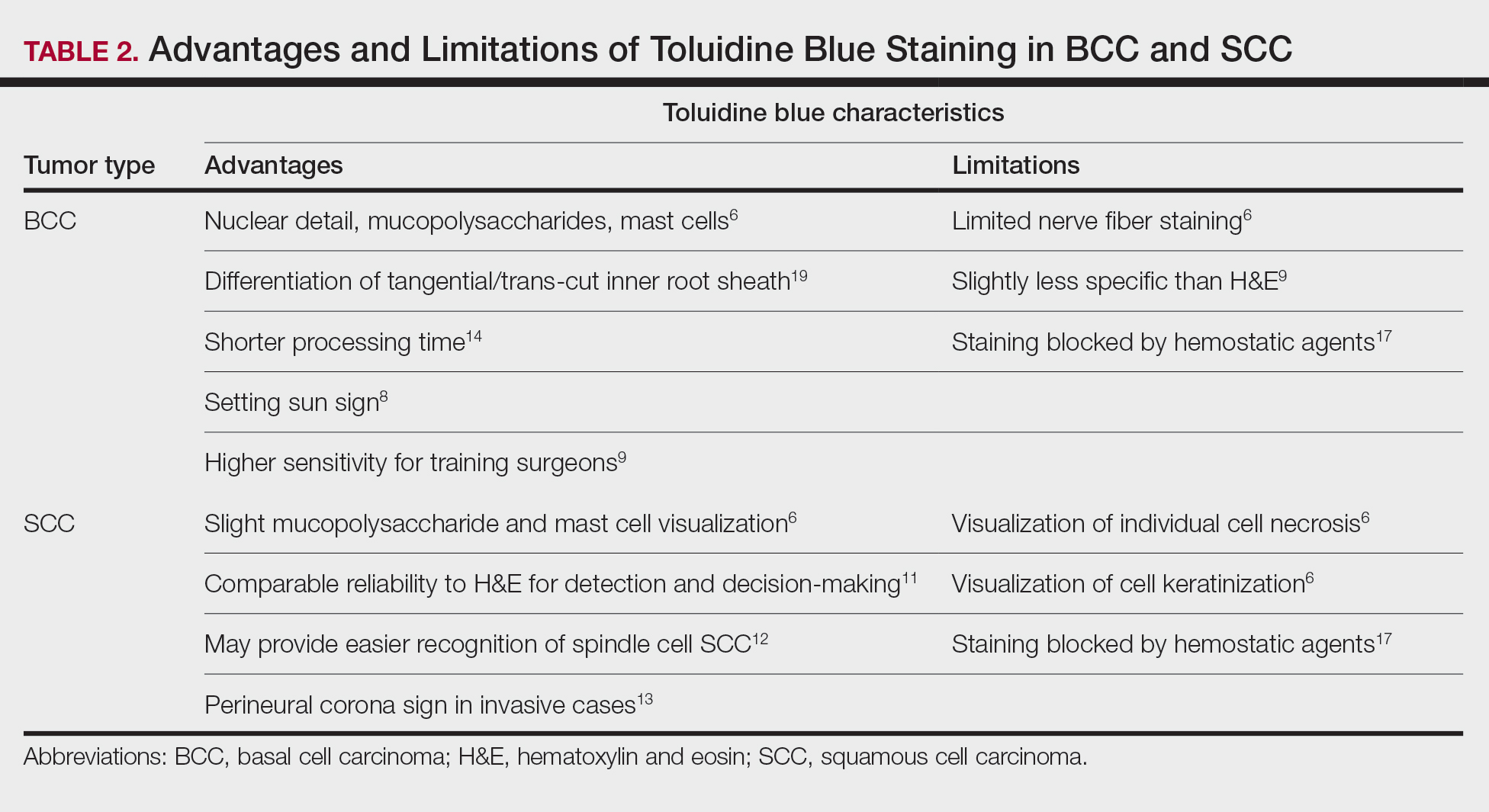
Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
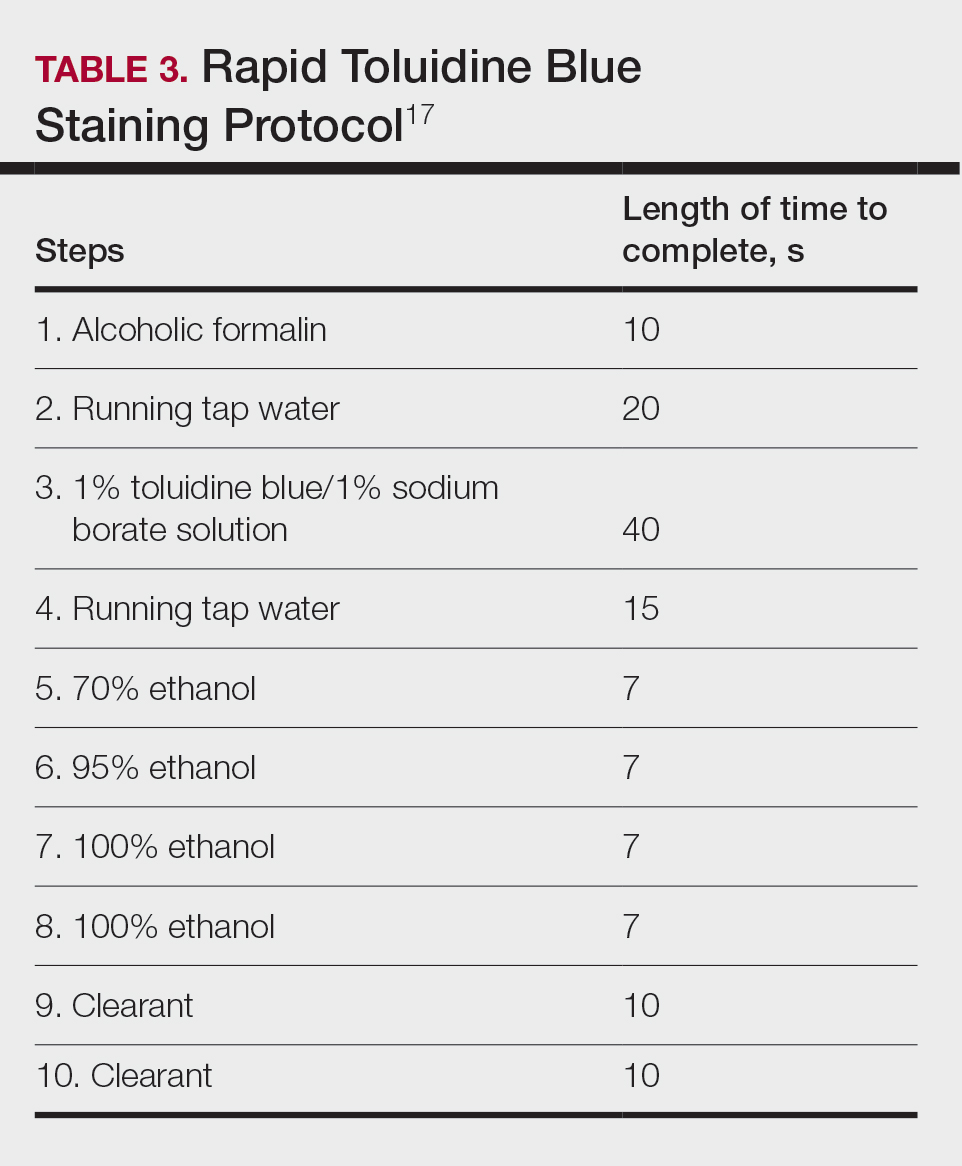
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6
Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.

Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).

A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.

Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6

Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
Toluidine blue (TB), a dye with metachromatic staining properties, was developed in 1856 by William Henry Perkin.1 Metachromasia is a perceptible change in the color of staining of living tissue due to the electrochemical properties of the tissue. Tissues that contain high concentrations of ionized sulfate and phosphate groups (high concentrations of free electronegative groups) form polymeric aggregates of the basic dye solution that alter the absorbed wavelengths of light.2 The function of this characteristic is to use a single dye to highlight different structures in tissue based on their relative chemical differences.3
Toluidine blue primarily was used within the dye industry until the 1960s, when it was first used in vital staining of the oral mucosa.2 Because of the tissue absorption potential, this technique was used to detect the location of oral malignancies.4 Since then, TB has progressively been used for staining fresh frozen sections in Mohs micrographic surgery (MMS). In a 2003 survey study (N=310), 16.8% of surgeons performing MMS reported using TB in their laboratory.5 We sought to systematically review the published literature describing the uses of TB in the setting of fresh frozen sections and MMS.
Methods
We conducted a systematic search of the PubMed and Cochrane databases for articles published before December 1, 2019, to identify any relevant studies in English. Electronic searches were performed using the terms toluidine blue and Mohs or Mohs micrographic surgery. We manually checked the bibliographies of the identified articles to further identify eligible studies.
Eligibility Criteria—The inclusion criteria were articles that (1) considered TB in the context of MMS, (2) were published in peer-reviewed journals, (3) were published in English, and (4) were available as full text. Systematic reviews were excluded.
Data Extraction and Outcomes—All relevant information regarding the study characteristics, including design, level of evidence, methodologic quality of evidence, pathology examined, and outcome measures, were collected by 2 independent reviewers (T.L. and A.D.) using a predetermined data sheet. The same 2 reviewers were used for all steps of the review process, data were independently obtained, and any discrepancy was introduced for a third opinion (D.H.) and agreed upon by the majority.
Quality Assessment—The level of evidence was evaluated based on the criteria of the Oxford Centre for Evidence-Based Medicine. Two reviewers (T.L. and A.D.) graded each article included in the review.

Results
A total of 25 articles were reviewed. After the titles and abstracts were screened for relevance, 12 articles remained (Figure 1). Of these, 1 compared basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), 4 were related to BCC, 3 were related to SCC, 1 was related to microcystic adnexal carcinoma (MAC), 1 was related to primary cutaneous adenoid cystic carcinoma (PCACC), and 2 were related to technical aspects of the staining process (Table 1).

A majority of the articles included in this review were qualitative and observational in nature, describing the staining characteristics of TB. Study characteristics are summarized in Table 1.
Comment
Basal Cell Carcinoma—Toluidine blue staining characteristics help to identify BCC nests by differentiating them from hair follicles in frozen sections. The metachromatic characteristic of TB stains the inner root sheath deep blue and highlights the surrounding stromal mucin of BCC a magenta color.18,19 In hematoxylin and eosin (H&E) stains, these 2 distinct structures can be differentiated by cleft formation around tumor nests, mitotic figures, and the lack of a fibrous sheath present in BCC tumors.20 The advantages and limitations of TB staining of BCC are presented in Table 2.

Humphreys et al6 suggested a noticeable difference between H&E and TB in the staining of cellular and stromal components. The nuclear detail of tumor cells was subjectively sharper and clearer with TB staining. The staining of stromal components may provide the most assistance in locating BCC islands. Mucopolysaccharide staining may be absent in H&E but stain a deep magenta with TB. Although the presence of mucopolysaccharides does not specifically indicate a tumor, it may prompt further attention and provide an indicator for sparse and infiltrative tumor cells.6 The metachromatic stromal change may indicate a narrow tumor-free margin where additional deeper sections often reveal tumor that may warrant additional resection margin in more aggressive malignancies. In particular, sclerosing/morpheaform BCCs have been shown to induce glycosaminoglycan synthesis and are highlighted more readily with TB than with H&E when compared to surrounding tissue.21 This differentiation in staining has remained a popular reason to routinely incorporate TB into the staining of infiltrative and morpheaform variants of BCC. Additionally, stromal mast cells are believed to be more abundant in the stroma of BCC and are more readily visualized in tissue specimens stained with TB, appearing as bright purple metachromatic granules. These granules are larger than normal and are increased in number.6
The margin behavior of BCC stained with TB was further characterized by Goldberg et al,8 who coined the term setting sun sign, which may be present in sequential sections of a disappearing nodule of a BCC tumor. Stroma, inflammatory infiltrate, and mast cells produce a magenta glow surrounding BCC tumors that is reminiscent of a setting sun (Figure 2). Invasive BCC is considered variable in this presentation, primarily because of zones of cell-free fluid and edema or the second area of inflammatory cells. This unique sign may benefit the inspecting Mohs surgeon by providing a clue to an underlying process that may have residual BCC tumors. The setting sun sign also may assist in identifying exact surgical margins.8

The nasal surface has a predilection for BCC.22 The skin of the nose has numerous look-alike structures to consider for complete tumor removal and avoidance of unnecessary removal. One challenge is distinguishing follicular basaloid proliferations (FBP) from BCC, a scenario that is more common on the nose.22 When TB staining was used, the sensitivity for detecting FBP reached 100% in 34 cases reviewed by Donaldson and Weber.10 None of the cases examined showed TB metachromasia surrounding FBP, thus indicating that TB can dependably identify this benign entity. Conversely, 5% (N=279) of BCCs confirmed on H&E did not exhibit surrounding TB metachromasia. This finding is concerning regarding the specificity of TB staining for BCC, but the authors of this study suggested the possibility that these exceptions were benign “simulants” (ie, trichoepithelioma) of BCC.10
The use of TB also has been shown to be statistically beneficial in Mohs training. In a single-center, single-fellow experiment, the sensitivity and specificity of using TB for BCC were extrapolated.9 Using TB as an adjunct in deep sections showed superior sensitivity to H&E alone in identifying BCC, increasing sensitivity from 96.3% to 99.7%. In a cohort of 352 BCC excisions and frozen sections, only 1 BCC was not completely excised. If H&E only had been performed, the fellow would have missed 13 residual BCC tumors.9
Bennett and Taher7 described a case in which hyaluronic acid (HA) from a filler injection was confused with the HA surrounding BCC tumor nests. They found that when TB is used as an adjunct, the HA filler is easier to differentiate from the HA surrounding the BCC tumor nests. In frozen sections stained with TB, the HA filler appeared as an amorphous, metachromatic, reddish-purple, whereas the HA surrounding the BCC tumor nests appeared as a well-defined red. These findings were less obvious in the same sections stained with H&E alone.7
Squamous Cell Carcinoma—In early investigations, the utility of TB in identifying SCC in frozen sections was thought to be limited. The description by Humphreys and colleagues6 of staining characteristics in SCC suggested that the nuclear detail that H&E provides is more easily recognized. The deep aqua nuclear staining produced with TB was considered more difficult to observe than the cytoplasmic eosinophilia of pyknotic and keratinizing cells in H&E.6
Toluidine blue may be beneficial in providing unique staining characteristics to further detail tumors that are difficult to interpret, such as spindle cell SCC and perineural invasion of aggressive SCC. In H&E, squamous cells of spindle cell SCC (scSCC) blend into the background of inflammatory cells and can be perceptibly difficult to locate. A small cohort of 3 Mohs surgeons who routinely use H&E were surveyed on their ability to detect a proven scSCC in H&E or TB by photograph.12 All 3 were able to detect the scSCC in the TB photographs, but only 2 of 3 were able to detect it in H&E photographs. All 3 surgeons agreed that TB was preferable to H&E for this tumor type. These findings suggested that TB may be superior and preferred over H&E for visualizing tumor cells of scSCC.12 The TB staining characteristics of perineural invasion of aggressive SCC have been referred to as the perineural corona sign because of the bright magenta stain that forms around affected nerves.13 Drosou et al13 suggested that TB may enhance the diagnostic accuracy for perineural SCC.
Rare Tumors—The adjunctive use of TB with H&E has been examined in rare tumors. Published reports have highlighted its use in MMS for treating MAC and PCACC. Toluidine blue exhibits staining advantages for these tumors. It may render isolated nests and perineural invasion of MAC more easily visible on frozen section.15
Although PCACC is rare, the recurrence rate is high.23 Toluidine blue has been used with MMS to ensure complete removal and higher cure rates. The metachromatic nature of TB is advantageous in staining the HA present in these tumors. Those who have reported the use of TB for PCACC prefer it to H&E for frozen sections.14
Technical Aspects—The staining time for TB-treated slides is reduced compared to H&E staining; staining can be efficiently done in frozen sections in less than 2.5 minutes using the method shown in Table 3.17 In comparison, typical H&E staining takes 9 minutes, and older TB techniques take 7 minutes.6

Conclusion
Toluidine blue may play an important and helpful role in the successful diagnosis and treatment of particular cutaneous tumors by providing additional diagnostic information. Although surgeons performing MMS will continue using the staining protocols with which they are most comfortable, adjunctive use of TB over time may provide an additional benefit at low risk for disrupting practice efficiency or workflow. Many Mohs surgeons are accustomed to using this stain, even preferring to interpret only TB-stained slides for cutaneous malignancy. Most published studies on this topic have been observational in nature, and additional controlled trials may be warranted to determine the effects on outcomes in real-world practice.
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
- Culling CF, Allison TR. Cellular Pathology Technique. 4th ed. Butterworths; 1985.
- Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4:433-457. doi:10.1083/jcb.4.4.433
- Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160-163. doi:10.1111/j.1600-0714.1992.tb00094.x
- Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97-103. doi:10.1111/j.1600-0714.1996.tb00201.x
- Silapunt S, Peterson SR, Alcalay J, et al. Mohs tissue mapping and processing: a survey study. Dermatol Surg. 2003;29:1109-1112; discussion 1112.
- Humphreys TR, Nemeth A, McCrevey S, et al. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatol Surg. 1996;22:693-697. doi:10.1111/j.1524-4725.1996.tb00619.x
- Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366-1369. doi:10.1111/j.1524-4725.2005.31223
- Goldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatol Surg. 2007;33:761-763. doi:10.1111/j.1524-4725.2007.33158.x
- Tehrani H, May K, Morris A, et al. Does the dual use of toluidine blue and hematoxylin and eosin staining improve basal cell carcinoma detection by Mohs surgery trainees? Dermatol Surg. 2013;39:995-1000. doi:10.1111/dsu.12180
- Donaldson MR, Weber LA. Toluidine blue supports differentiation of folliculocentric basaloid proliferation from basal cell carcinoma on frozen sections in a small single-practice cohort. Dermatol Surg. 2017;43:1303-1306. doi:10.1097/DSS.0000000000001107
- Styperek AR, Goldberg LH, Goldschmidt LE, et al. Toluidine blue and hematoxylin and eosin stains are comparable in evaluating squamous cell carcinoma during Mohs. Dermatol Surg. 2016;42:1279-1284. doi:10.1097/DSS.0000000000000872
- Trieu D, Drosou A, Goldberg LH, et al. Detecting spindle cell squamous cell carcinomas with toluidine blue on frozen sections. Dermatol Surg. 2014;40:1259-1260. doi:10.1097/DSS.0000000000000147
- Drosou A, Trieu D, Goldberg LH, et al. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. J Am Acad Dermatol. 2014;71:826-827. doi:10.1016/j.jaad.2014.04.076
- Chesser RS, Bertler DE, Fitzpatrick JE, et al. Primary cutaneous adenoid cystic carcinoma treated with Mohs micrographic surgery toluidine blue technique. J Dermatol Surg Oncol. 1992;18:175-176. doi:10.1111/j.1524-4725.1992.tb02794.x
- Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. J Am Acad Dermatol. 2007;56:1067-1069. doi:10.1016/j.jaad.2007.01.008
- Chen CL, Wilson S, Afzalneia R, et al. Topical aluminum chloride and Monsel’s solution block toluidine blue staining in Mohs frozen sections: mechanism and solution. Dermatol Surg. 2019;45:1019-1025. doi:10.1097/DSS.0000000000001761
- Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs’ micrographic surgery. Dermatol Surg. 2005;31:244-245. doi:10.1111/j.1524-4725.2005.31053
- Picoto AM, Picoto A. Technical procedures for Mohs fresh tissue surgery. J Derm Surg Oncol. 1986;12:134-138. doi:10.1111/j.1524-4725.1986.tb01442.x
- Sperling LC, Winton GB. The transverse anatomy of androgenic alopecia. J Derm Surg Oncol. 1990;16:1127-1133. doi:10.1111/j.1524 -4725.1990.tb00024.x
- Smith-Zagone MJ, Schwartz MR. Frozen section of skin specimens. Arch Pathol Lab Med. 2005;129:1536-1543. doi:10.5858/2005-129-1536-FSOSS
- Moy RL, Potter TS, Uitto J. Increased glycosaminoglycans production in sclerosing basal cell carcinoma–derived fibroblasts and stimulation of normal skin fibroblast glycosaminoglycans production by a cytokine-derived from sclerosing basal cell carcinoma. Dermatol Surg. 2000;26:1029-1036. doi:10.1046/j.1524-4725.2000.0260111029.x
- Leshin B, White WL. Folliculocentric basaloid proliferation. The bulge (der Wulst) revisited. Arch Dermatol. 1990;126:900-906. doi:10.1001/archderm.126.7.900
- Seab JA, Graham JH. Primary cutaneous adenoid cystic carcinoma.J Am Acad Dermatol. 1987;17:113-118. doi:10.1016/s0190 -9622(87)70182-0
Practice Points
- Toluidine blue (TB) staining can be integrated into Mohs micrographic surgery (MMS) for enhanced diagnosis of cutaneous tumors. Its metachromatic properties can aid in differentiating tumor cells from surrounding tissues, especially in basal cell carcinomas and squamous cell carcinomas.
- It is important to develop expertise in interpreting TB-stained sections, as it may offer clearer visualization of nuclear details and stromal components, potentially leading to more accurate diagnosis and effective tumor margin identification.
- Toluidine blue staining can be incorporated into routine MMS practice considering its quick staining process and low disruption to workflow. This can potentially improve diagnostic efficiency without significantly lengthening surgery time.
Reactive Angioendotheliomatosis Following Ad26.COV2.S Vaccination
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.
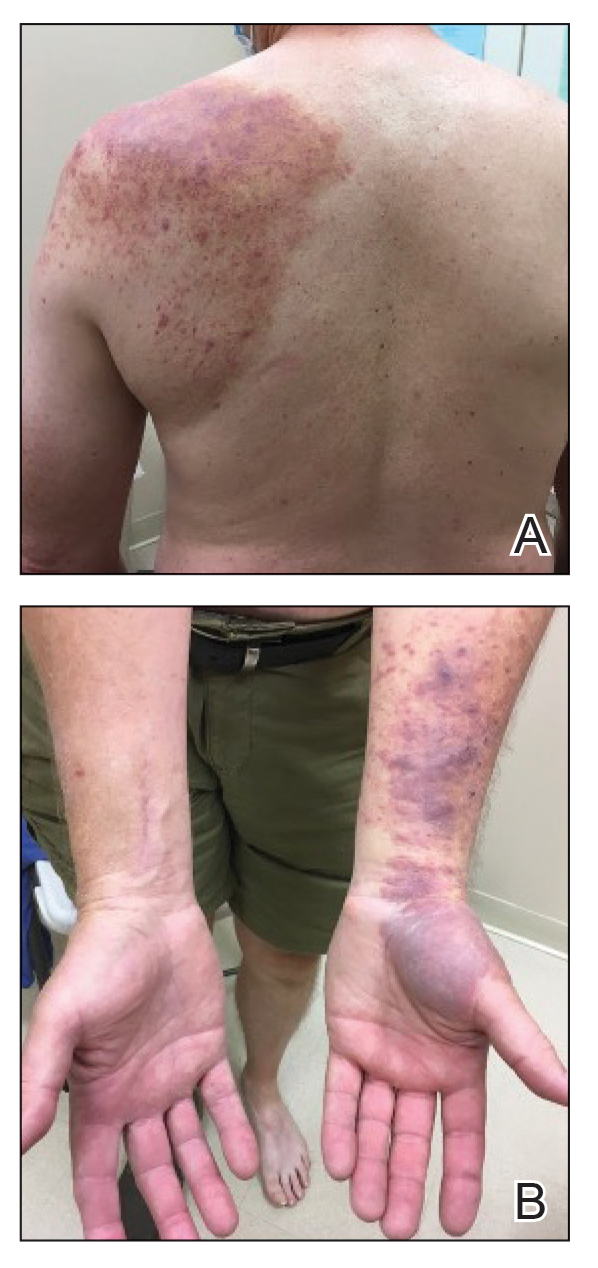
Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.
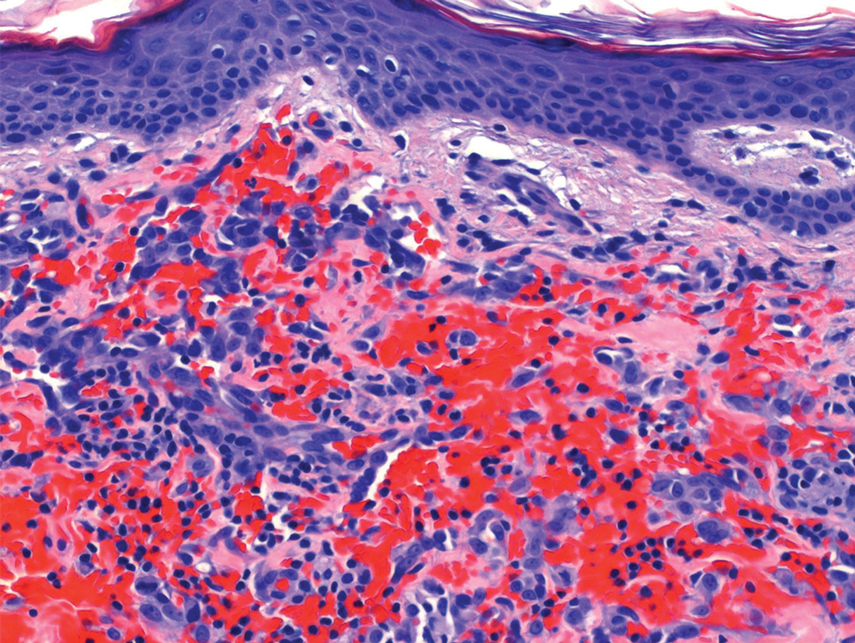
Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.
A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.
Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.

Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.

Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.

A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.

Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.

Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.

Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.

A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.

Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
Practice points
- Reactive angioendotheliomatosis (RAE) is a rare benign vascular proliferation of endothelial cells lining blood vessels that clinically appears similar to Kaposi sarcoma and must be differentiated by microscopic evaluation.
- An increasing number of reports link SARS-CoV-2 viral infection or vaccination against this virus with various cutaneous manifestations. Our case offers a link between RAE and Ad26.COV2.S vaccination.
Genetics of Migraine
The Evolving Treatment Paradigm for Diffuse Large B-Cell Lymphoma
Non-Hodgkin lymphomas (NHLs) are cancers that arise in a type of white blood cell called the lymphocyte. NHLs are divided into B- and T-cell subtypes, as well as aggressive and indolent forms. Management varies widely depending on the disease type. We will focus on the most common type of NHL, diffuse large B-cell lymphoma (DLBCL), for which there have been significant treatment advances in recent years.
DLBCL is curable in about two-thirds of patients using chemoimmunotherapy. The longstanding frontline treatment for this disease has been R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). In 2023, an antibody-drug conjugate against the B-cell surface protein CD79b, polatuzumab vedotin, was approved by the US Food and Drug Administration (FDA) in combination with R-CHP (rituximab, cyclophosphamide, doxorubicin, prednisone) for newly diagnosed DLBCL based on an improvement in progression-free survival at 2 years in patients with high-risk disease features enrolled in the POLARIX study.
For patients who do not respond to the initial treatment or in whom the disease recurs, the historical standard of care treatment strategy was high-dose chemotherapy followed by autologous stem cell transplant (ASCT). Unfortunately, this approach is not feasible or not successful in a significant percentage of patients with relapsed or refractory DLBCL.
A newer strategy for DLBCL is chimeric antigen receptor (CAR) T-cell therapy. In this treatment, T cells are collected from a patient and genetically modified to target a protein on the lymphoma cells called CD19. This type of treatment was initially approved in the third-line setting for DLBCL based on the ZUMA-1 (axi-cel), JULIET (tisa-cel), and TRANSCEND (liso-cel) clinical trials. More recently, in 2022, 2 of these agents received approval in the second-line setting in patients who relapse or are refractory to initial treatment within 1 year; axi-cel was approved based on the ZUMA-7 trial and liso-cel was approved based on the TRANSFORM trial.
Unfortunately, not all patients are eligible for ASCT and CAR T-cell therapy due to factors including age, comorbidities, and disease characteristics. Some patients prefer alternative therapies based on the potential side effects of CAR T-cell therapy and ASCT. Toxicities associated with CAR T-cell therapy include an inflammatory response called cytokine release syndrome and neurologic events.
For patients who are not eligible for or who relapse after ASCT or CAR T-cell therapy, several alternative treatment options are FDA approved. Novel strategies include polatuzumab vedotin with bendamustine and rituximab and tafasitamab plus lenalidomide. Tafasitamab is a monoclonal antibody against CD19 and lenalidomide is an oral anticancer agent originally approved for use in multiple myeloma. Lenalidomide is also effective and commonly used in other NHL subtypes.
In 2023, a new category of treatment called bispecific antibodies was approved in patients with DLBCL in whom the disease recurs after 2 lines of therapy. These drugs (epcoritamab and glofitamab) are a form of immunotherapy that connects B cells with T cells to enable a person’s own immune system to better fight the lymphoma. While these drugs can have similar toxicities as CAR T-cell therapy, the severity and incidence are much lower. In contrast to CAR T-cell therapy, which requires only 1 infusion, these drugs are given regularly in either subcutaneous or intravenous form for several months.
Two other FDA-approved treatment options for relapsed and refractory DLBCL are loncastuximab tesirine, an antibody-drug conjugate targeting CD19 with approval based on the results of the LOTIS-2 trial, and the oral selective inhibitor of nuclear export called selinexor, based on the results from the SADAL trial. Selinexor is a fully synthetic small-molecule compound, developed by means of a structure-based drug design process known as induced-fit docking. It binds to a cysteine residue in the nuclear export signal groove of exportin 1. Selinexor is approved for use in adults with relapsed or refractory DLBCL who have received at least 2 types of systemic therapy. Trials investigating these agents in combination with other novel treatments are ongoing.
The treatment landscape for DLBCL has changed markedly over the past several years. Therapies can be tailored for individual patients based on their disease status and characteristics, comorbidities, and treatment preferences. Research with novel strategies continues with the goal of a cure for all patients diagnosed with DLBCL.
Non-Hodgkin lymphomas (NHLs) are cancers that arise in a type of white blood cell called the lymphocyte. NHLs are divided into B- and T-cell subtypes, as well as aggressive and indolent forms. Management varies widely depending on the disease type. We will focus on the most common type of NHL, diffuse large B-cell lymphoma (DLBCL), for which there have been significant treatment advances in recent years.
DLBCL is curable in about two-thirds of patients using chemoimmunotherapy. The longstanding frontline treatment for this disease has been R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). In 2023, an antibody-drug conjugate against the B-cell surface protein CD79b, polatuzumab vedotin, was approved by the US Food and Drug Administration (FDA) in combination with R-CHP (rituximab, cyclophosphamide, doxorubicin, prednisone) for newly diagnosed DLBCL based on an improvement in progression-free survival at 2 years in patients with high-risk disease features enrolled in the POLARIX study.
For patients who do not respond to the initial treatment or in whom the disease recurs, the historical standard of care treatment strategy was high-dose chemotherapy followed by autologous stem cell transplant (ASCT). Unfortunately, this approach is not feasible or not successful in a significant percentage of patients with relapsed or refractory DLBCL.
A newer strategy for DLBCL is chimeric antigen receptor (CAR) T-cell therapy. In this treatment, T cells are collected from a patient and genetically modified to target a protein on the lymphoma cells called CD19. This type of treatment was initially approved in the third-line setting for DLBCL based on the ZUMA-1 (axi-cel), JULIET (tisa-cel), and TRANSCEND (liso-cel) clinical trials. More recently, in 2022, 2 of these agents received approval in the second-line setting in patients who relapse or are refractory to initial treatment within 1 year; axi-cel was approved based on the ZUMA-7 trial and liso-cel was approved based on the TRANSFORM trial.
Unfortunately, not all patients are eligible for ASCT and CAR T-cell therapy due to factors including age, comorbidities, and disease characteristics. Some patients prefer alternative therapies based on the potential side effects of CAR T-cell therapy and ASCT. Toxicities associated with CAR T-cell therapy include an inflammatory response called cytokine release syndrome and neurologic events.
For patients who are not eligible for or who relapse after ASCT or CAR T-cell therapy, several alternative treatment options are FDA approved. Novel strategies include polatuzumab vedotin with bendamustine and rituximab and tafasitamab plus lenalidomide. Tafasitamab is a monoclonal antibody against CD19 and lenalidomide is an oral anticancer agent originally approved for use in multiple myeloma. Lenalidomide is also effective and commonly used in other NHL subtypes.
In 2023, a new category of treatment called bispecific antibodies was approved in patients with DLBCL in whom the disease recurs after 2 lines of therapy. These drugs (epcoritamab and glofitamab) are a form of immunotherapy that connects B cells with T cells to enable a person’s own immune system to better fight the lymphoma. While these drugs can have similar toxicities as CAR T-cell therapy, the severity and incidence are much lower. In contrast to CAR T-cell therapy, which requires only 1 infusion, these drugs are given regularly in either subcutaneous or intravenous form for several months.
Two other FDA-approved treatment options for relapsed and refractory DLBCL are loncastuximab tesirine, an antibody-drug conjugate targeting CD19 with approval based on the results of the LOTIS-2 trial, and the oral selective inhibitor of nuclear export called selinexor, based on the results from the SADAL trial. Selinexor is a fully synthetic small-molecule compound, developed by means of a structure-based drug design process known as induced-fit docking. It binds to a cysteine residue in the nuclear export signal groove of exportin 1. Selinexor is approved for use in adults with relapsed or refractory DLBCL who have received at least 2 types of systemic therapy. Trials investigating these agents in combination with other novel treatments are ongoing.
The treatment landscape for DLBCL has changed markedly over the past several years. Therapies can be tailored for individual patients based on their disease status and characteristics, comorbidities, and treatment preferences. Research with novel strategies continues with the goal of a cure for all patients diagnosed with DLBCL.
Non-Hodgkin lymphomas (NHLs) are cancers that arise in a type of white blood cell called the lymphocyte. NHLs are divided into B- and T-cell subtypes, as well as aggressive and indolent forms. Management varies widely depending on the disease type. We will focus on the most common type of NHL, diffuse large B-cell lymphoma (DLBCL), for which there have been significant treatment advances in recent years.
DLBCL is curable in about two-thirds of patients using chemoimmunotherapy. The longstanding frontline treatment for this disease has been R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). In 2023, an antibody-drug conjugate against the B-cell surface protein CD79b, polatuzumab vedotin, was approved by the US Food and Drug Administration (FDA) in combination with R-CHP (rituximab, cyclophosphamide, doxorubicin, prednisone) for newly diagnosed DLBCL based on an improvement in progression-free survival at 2 years in patients with high-risk disease features enrolled in the POLARIX study.
For patients who do not respond to the initial treatment or in whom the disease recurs, the historical standard of care treatment strategy was high-dose chemotherapy followed by autologous stem cell transplant (ASCT). Unfortunately, this approach is not feasible or not successful in a significant percentage of patients with relapsed or refractory DLBCL.
A newer strategy for DLBCL is chimeric antigen receptor (CAR) T-cell therapy. In this treatment, T cells are collected from a patient and genetically modified to target a protein on the lymphoma cells called CD19. This type of treatment was initially approved in the third-line setting for DLBCL based on the ZUMA-1 (axi-cel), JULIET (tisa-cel), and TRANSCEND (liso-cel) clinical trials. More recently, in 2022, 2 of these agents received approval in the second-line setting in patients who relapse or are refractory to initial treatment within 1 year; axi-cel was approved based on the ZUMA-7 trial and liso-cel was approved based on the TRANSFORM trial.
Unfortunately, not all patients are eligible for ASCT and CAR T-cell therapy due to factors including age, comorbidities, and disease characteristics. Some patients prefer alternative therapies based on the potential side effects of CAR T-cell therapy and ASCT. Toxicities associated with CAR T-cell therapy include an inflammatory response called cytokine release syndrome and neurologic events.
For patients who are not eligible for or who relapse after ASCT or CAR T-cell therapy, several alternative treatment options are FDA approved. Novel strategies include polatuzumab vedotin with bendamustine and rituximab and tafasitamab plus lenalidomide. Tafasitamab is a monoclonal antibody against CD19 and lenalidomide is an oral anticancer agent originally approved for use in multiple myeloma. Lenalidomide is also effective and commonly used in other NHL subtypes.
In 2023, a new category of treatment called bispecific antibodies was approved in patients with DLBCL in whom the disease recurs after 2 lines of therapy. These drugs (epcoritamab and glofitamab) are a form of immunotherapy that connects B cells with T cells to enable a person’s own immune system to better fight the lymphoma. While these drugs can have similar toxicities as CAR T-cell therapy, the severity and incidence are much lower. In contrast to CAR T-cell therapy, which requires only 1 infusion, these drugs are given regularly in either subcutaneous or intravenous form for several months.
Two other FDA-approved treatment options for relapsed and refractory DLBCL are loncastuximab tesirine, an antibody-drug conjugate targeting CD19 with approval based on the results of the LOTIS-2 trial, and the oral selective inhibitor of nuclear export called selinexor, based on the results from the SADAL trial. Selinexor is a fully synthetic small-molecule compound, developed by means of a structure-based drug design process known as induced-fit docking. It binds to a cysteine residue in the nuclear export signal groove of exportin 1. Selinexor is approved for use in adults with relapsed or refractory DLBCL who have received at least 2 types of systemic therapy. Trials investigating these agents in combination with other novel treatments are ongoing.
The treatment landscape for DLBCL has changed markedly over the past several years. Therapies can be tailored for individual patients based on their disease status and characteristics, comorbidities, and treatment preferences. Research with novel strategies continues with the goal of a cure for all patients diagnosed with DLBCL.
Does residential proximity to swine farms increase odds of developing eosinophilic esophagitis?
Key clinical point: People from a tertiary care center database were at a significantly increased risk of getting eosinophilic esophagitis (EoE) when they lived close (<1 mile) to a commercial swine farm or in an area with a high density of swine farm operations.
Major finding: Odds of EoE were ~2.5 times higher in participants who had undergone upper endoscopy and lived in an area with <1 mile proximity to a permitted swine facility (adjusted odds ratio [aOR] 2.56; 95% CI 1.33-4.95) or where the density of swine farms was >10 farms per census tract (aOR 2.76; 95% CI 1.30-5.84).
Study details: This case-control study including 401 patients with EoE and 1852 control individuals who had undergone endoscopy but did not show any esophageal pathology from a tertiary care center and 904 patients with EoE and 4074 endoscopy-based control participants from a pathology database.
Disclosures: This study was partly funded by a grant from the US National Institutes of Health. The authors declared no conflicts of interest.
Source: Cotton CC et al. Proximity to swine farming operations as a risk factor for eosinophilic esophagitis. JPGN Rep. 2023;4(4):e391 (Nov 8). doi: 10.1097/PG9.0000000000000391
Key clinical point: People from a tertiary care center database were at a significantly increased risk of getting eosinophilic esophagitis (EoE) when they lived close (<1 mile) to a commercial swine farm or in an area with a high density of swine farm operations.
Major finding: Odds of EoE were ~2.5 times higher in participants who had undergone upper endoscopy and lived in an area with <1 mile proximity to a permitted swine facility (adjusted odds ratio [aOR] 2.56; 95% CI 1.33-4.95) or where the density of swine farms was >10 farms per census tract (aOR 2.76; 95% CI 1.30-5.84).
Study details: This case-control study including 401 patients with EoE and 1852 control individuals who had undergone endoscopy but did not show any esophageal pathology from a tertiary care center and 904 patients with EoE and 4074 endoscopy-based control participants from a pathology database.
Disclosures: This study was partly funded by a grant from the US National Institutes of Health. The authors declared no conflicts of interest.
Source: Cotton CC et al. Proximity to swine farming operations as a risk factor for eosinophilic esophagitis. JPGN Rep. 2023;4(4):e391 (Nov 8). doi: 10.1097/PG9.0000000000000391
Key clinical point: People from a tertiary care center database were at a significantly increased risk of getting eosinophilic esophagitis (EoE) when they lived close (<1 mile) to a commercial swine farm or in an area with a high density of swine farm operations.
Major finding: Odds of EoE were ~2.5 times higher in participants who had undergone upper endoscopy and lived in an area with <1 mile proximity to a permitted swine facility (adjusted odds ratio [aOR] 2.56; 95% CI 1.33-4.95) or where the density of swine farms was >10 farms per census tract (aOR 2.76; 95% CI 1.30-5.84).
Study details: This case-control study including 401 patients with EoE and 1852 control individuals who had undergone endoscopy but did not show any esophageal pathology from a tertiary care center and 904 patients with EoE and 4074 endoscopy-based control participants from a pathology database.
Disclosures: This study was partly funded by a grant from the US National Institutes of Health. The authors declared no conflicts of interest.
Source: Cotton CC et al. Proximity to swine farming operations as a risk factor for eosinophilic esophagitis. JPGN Rep. 2023;4(4):e391 (Nov 8). doi: 10.1097/PG9.0000000000000391
Eosinophilic esophagitis histology scoring system predicts response to PPI therapy in pediatric EoE
Key clinical point: The eosinophilic esophagitis (EoE) histology scoring system (HSS) can predict the response to proton pump inhibitor (PPI) therapy in pediatric patients with EoE.
Major finding: The final EoEHSS grade score for the middle segment of the esophagus was significantly lower in patients who responded (peak eosinophil count < 15/high-power field on esophageal biopsy) vs did not respond to PPI therapy (0.3 vs 0.5; P = .037). Responders vs nonresponders had significantly lower grade values for the individual EoEHSS parameters eosinophilic abscesses (P = .044) and eosinophil surface layering (P = .046) for the middle esophagus.
Study details: Findings are from a cross-sectional study including 89 pediatric patients with EoE (age 0-18 years) who received PPI daily and were either responsive (n = 17) or nonresponsive (n = 72) at 3 months of therapy.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Jevtić J et al. The usefulness of the eosinophilic esophagitis histology scoring system in predicting response to proton pump inhibitor monotherapy in children with eosinophilic esophagitis. Diagnostics (Basel). 2023; 13(22):3445 (Nov 15). doi: 10.3390/diagnostics13223445
Key clinical point: The eosinophilic esophagitis (EoE) histology scoring system (HSS) can predict the response to proton pump inhibitor (PPI) therapy in pediatric patients with EoE.
Major finding: The final EoEHSS grade score for the middle segment of the esophagus was significantly lower in patients who responded (peak eosinophil count < 15/high-power field on esophageal biopsy) vs did not respond to PPI therapy (0.3 vs 0.5; P = .037). Responders vs nonresponders had significantly lower grade values for the individual EoEHSS parameters eosinophilic abscesses (P = .044) and eosinophil surface layering (P = .046) for the middle esophagus.
Study details: Findings are from a cross-sectional study including 89 pediatric patients with EoE (age 0-18 years) who received PPI daily and were either responsive (n = 17) or nonresponsive (n = 72) at 3 months of therapy.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Jevtić J et al. The usefulness of the eosinophilic esophagitis histology scoring system in predicting response to proton pump inhibitor monotherapy in children with eosinophilic esophagitis. Diagnostics (Basel). 2023; 13(22):3445 (Nov 15). doi: 10.3390/diagnostics13223445
Key clinical point: The eosinophilic esophagitis (EoE) histology scoring system (HSS) can predict the response to proton pump inhibitor (PPI) therapy in pediatric patients with EoE.
Major finding: The final EoEHSS grade score for the middle segment of the esophagus was significantly lower in patients who responded (peak eosinophil count < 15/high-power field on esophageal biopsy) vs did not respond to PPI therapy (0.3 vs 0.5; P = .037). Responders vs nonresponders had significantly lower grade values for the individual EoEHSS parameters eosinophilic abscesses (P = .044) and eosinophil surface layering (P = .046) for the middle esophagus.
Study details: Findings are from a cross-sectional study including 89 pediatric patients with EoE (age 0-18 years) who received PPI daily and were either responsive (n = 17) or nonresponsive (n = 72) at 3 months of therapy.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Jevtić J et al. The usefulness of the eosinophilic esophagitis histology scoring system in predicting response to proton pump inhibitor monotherapy in children with eosinophilic esophagitis. Diagnostics (Basel). 2023; 13(22):3445 (Nov 15). doi: 10.3390/diagnostics13223445
Age predicts the probability of esophageal biopsies in patients with EoE symptoms
Key clinical point: Elderly patients with eosinophilic esophagitis (EoE) symptoms had <50% the odds of undergoing esophageal biopsies and longer time to diagnosis after the onset of symptoms compared with non-elderly patients.
Major finding: Elderly vs non-elderly patients were 56% less likely to undergo esophageal biopsies (adjusted odds ratio [aOR] 0.44; 95% CI 0.21-0.92) and had significantly longer time to diagnosis following symptom onset (aOR per year of symptoms preceding diagnosis 1.08; 95% CI 1.04-1.11).
Study details: The data come from a retrospective cohort study including elderly (age ≥ 65 years; n = 91) or non-elderly (age < 65 years; n = 102) patients with newly diagnosed EoE who presented with symptoms including dysphagia, chest pain, and heartburn.
Disclosures: This study was supported by a grant presented by the University of North Carolina Medical Student Summer Research Program. ES Dellon declared serving as a consultant for and receiving research funding and educational grants from various sources. The other authors declared no conflicts of interest.
Source: Kiran A et al. Retrospective cohort study: Effect of age as a barrier to diagnosis of eosinophilic oesophagitis. Aliment Pharmacol Ther. 2023 (Oct 25). doi: 10.1111/apt.17781
Key clinical point: Elderly patients with eosinophilic esophagitis (EoE) symptoms had <50% the odds of undergoing esophageal biopsies and longer time to diagnosis after the onset of symptoms compared with non-elderly patients.
Major finding: Elderly vs non-elderly patients were 56% less likely to undergo esophageal biopsies (adjusted odds ratio [aOR] 0.44; 95% CI 0.21-0.92) and had significantly longer time to diagnosis following symptom onset (aOR per year of symptoms preceding diagnosis 1.08; 95% CI 1.04-1.11).
Study details: The data come from a retrospective cohort study including elderly (age ≥ 65 years; n = 91) or non-elderly (age < 65 years; n = 102) patients with newly diagnosed EoE who presented with symptoms including dysphagia, chest pain, and heartburn.
Disclosures: This study was supported by a grant presented by the University of North Carolina Medical Student Summer Research Program. ES Dellon declared serving as a consultant for and receiving research funding and educational grants from various sources. The other authors declared no conflicts of interest.
Source: Kiran A et al. Retrospective cohort study: Effect of age as a barrier to diagnosis of eosinophilic oesophagitis. Aliment Pharmacol Ther. 2023 (Oct 25). doi: 10.1111/apt.17781
Key clinical point: Elderly patients with eosinophilic esophagitis (EoE) symptoms had <50% the odds of undergoing esophageal biopsies and longer time to diagnosis after the onset of symptoms compared with non-elderly patients.
Major finding: Elderly vs non-elderly patients were 56% less likely to undergo esophageal biopsies (adjusted odds ratio [aOR] 0.44; 95% CI 0.21-0.92) and had significantly longer time to diagnosis following symptom onset (aOR per year of symptoms preceding diagnosis 1.08; 95% CI 1.04-1.11).
Study details: The data come from a retrospective cohort study including elderly (age ≥ 65 years; n = 91) or non-elderly (age < 65 years; n = 102) patients with newly diagnosed EoE who presented with symptoms including dysphagia, chest pain, and heartburn.
Disclosures: This study was supported by a grant presented by the University of North Carolina Medical Student Summer Research Program. ES Dellon declared serving as a consultant for and receiving research funding and educational grants from various sources. The other authors declared no conflicts of interest.
Source: Kiran A et al. Retrospective cohort study: Effect of age as a barrier to diagnosis of eosinophilic oesophagitis. Aliment Pharmacol Ther. 2023 (Oct 25). doi: 10.1111/apt.17781
Evolving phenotypes and increasing age at diagnosis of eosinophilic esophagitis over the last 20 years
Key clinical point: The phenotypes of eosinophilic esophagitis (EoE) have evolved over the past two decades, with an increase in both age at diagnosis and age at onset of symptoms.
Major finding: The interval 2017-2021 vs 2002-2006 was associated with a significant increase in age at diagnosis (31.8 vs 22.0 years), frequency of dysphagia (92% vs 67%), proportion of patients with mixed presentation of inflammatory and fibrostenotic findings (68% vs 26%), and proportion of patients (age groups ≥18 years and ≥12 years) having later EoE symptom onset (all P < .001). The increase in the mixed phenotype rate persisted for the intervals after multivariate adjustment (adjusted odds ratio 1.51/interval; 95% CI 1.31-1.73).
Study details: This retrospective cohort study included 1187 adults or children (age < 18 years) with newly diagnosed EoE.
Disclosures: This study was supported by grants from the US National Institutes of Health. The authors declared no conflicts of interest.
Source: Kiran A et al. Increasing age at the time of diagnosis and evolving phenotypes of eosinophilic esophagitis over 20 years. Dig Dis Sci. 2023 (Nov 15). doi: 10.1007/s10620-023-08165-z
Key clinical point: The phenotypes of eosinophilic esophagitis (EoE) have evolved over the past two decades, with an increase in both age at diagnosis and age at onset of symptoms.
Major finding: The interval 2017-2021 vs 2002-2006 was associated with a significant increase in age at diagnosis (31.8 vs 22.0 years), frequency of dysphagia (92% vs 67%), proportion of patients with mixed presentation of inflammatory and fibrostenotic findings (68% vs 26%), and proportion of patients (age groups ≥18 years and ≥12 years) having later EoE symptom onset (all P < .001). The increase in the mixed phenotype rate persisted for the intervals after multivariate adjustment (adjusted odds ratio 1.51/interval; 95% CI 1.31-1.73).
Study details: This retrospective cohort study included 1187 adults or children (age < 18 years) with newly diagnosed EoE.
Disclosures: This study was supported by grants from the US National Institutes of Health. The authors declared no conflicts of interest.
Source: Kiran A et al. Increasing age at the time of diagnosis and evolving phenotypes of eosinophilic esophagitis over 20 years. Dig Dis Sci. 2023 (Nov 15). doi: 10.1007/s10620-023-08165-z
Key clinical point: The phenotypes of eosinophilic esophagitis (EoE) have evolved over the past two decades, with an increase in both age at diagnosis and age at onset of symptoms.
Major finding: The interval 2017-2021 vs 2002-2006 was associated with a significant increase in age at diagnosis (31.8 vs 22.0 years), frequency of dysphagia (92% vs 67%), proportion of patients with mixed presentation of inflammatory and fibrostenotic findings (68% vs 26%), and proportion of patients (age groups ≥18 years and ≥12 years) having later EoE symptom onset (all P < .001). The increase in the mixed phenotype rate persisted for the intervals after multivariate adjustment (adjusted odds ratio 1.51/interval; 95% CI 1.31-1.73).
Study details: This retrospective cohort study included 1187 adults or children (age < 18 years) with newly diagnosed EoE.
Disclosures: This study was supported by grants from the US National Institutes of Health. The authors declared no conflicts of interest.
Source: Kiran A et al. Increasing age at the time of diagnosis and evolving phenotypes of eosinophilic esophagitis over 20 years. Dig Dis Sci. 2023 (Nov 15). doi: 10.1007/s10620-023-08165-z