User login
Do Plant-based Psychedelics Offer a New Option for TBI Treatment?
Oneirogens are substances that produce or enhance dreamlike states of consciousness—could one of those, ibogaine, be key to relieving the sequelae of traumatic brain injury (TBI) in veterans?
An extract from the root bark of Tabernanthe iboga, an African shrub, ibogaine has both pharmacological and psychological effects. Acting on opioid receptors and the serotonin and dopamine systems, it can relieve withdrawal symptoms and reduce drug cravings—reportedly, often, in just a few hours—and reduce the risk of regular use. The results can last for weeks, months, or sometimes longer.
In the US, ibogaine is a Schedule I drug. Few controlled studies of ibogaine are available; most data come from anecdotal reports and case studies. Clinical research into ibogaine stalled due to legal restrictions that come with a Schedule I drug, as well as concerns about possible cardiac consequences. For example, some reports have described QT interval prolongation, with instances of subsequent fatal arrhythmia.
That may change now, with findings from the Magnesium–Ibogaine: the Stanford Traumatic Injury to the CNS protocol (MISTIC), which took place at a treatment center in Mexico. Researchers from Stanford School of Medicine and the Veterans Affairs Palo Alto Health Care System combined prophylactic intravenous magnesium with ibogaine, in hopes of mitigating the cardiac risks. Magnesium supplementation has been shown to protect against QT interval prolongation when coadministered with medications that ordinarily would have such an effect.
The researchers studied 30 male Special Operations Forces veterans (SOVs) who had predominantly mild TBI. Of those, 15 participants met the criteria for major depressive disorder, 14 for an anxiety disorder, and 23 for PTSD; 19 had past suicidal ideation and 7 had attempted suicide.
Special Operations Forces, the researchers note, are “deployed at a greater pace and to higher intensity combat than conventional military, exposing them to greater allostatic load and risk of injury, including from blast exposure.” This, they say, may result in a “unique pattern” of physical, cognitive, behavioral, psychiatric, and endocrine-related problems across several domains.
Participants received a mean (SD) of 12.1 (1.2) mg kg-1 of oral ibogaine. The researchers assessed changes in the World Health Organization Disability Assessment Schedule at baseline, immediately after treatment, and 1 month after treatment. They also assessed changes in posttraumatic stress disorder (PTSD), depression, and anxiety.
The treatment significantly improved functioning both immediately and at 1 month after treatment and PTSD, depression, and anxiety at 1 month after treatment. There were no unexpected or serious treatment-emergent adverse effects, nor were there instances of bradycardia, tachycardia, clinically meaningful QT prolongation, or hemodynamic instability. All participants experienced transient cerebellar signs, such as mild ataxia and intention tremor, that resolved within 24 hours. While experiencing oneirogenic effects, 12 participants were treated for headache, 7 for nausea, 3 for anxiety, 2 for hypertension, and 1 for insomnia.
At 1 month, suicidal ideation had declined from 47% to 7%—a statistically significant change. “Given the alarming rates of suicide in veterans, as well as evidence that military-related TBI increases the risk of suicide,” the researchers say, “the substantial reduction in SI that we observed—which must be interpreted cautiously as an exploratory analysis—is noteworthy.” TBI also is associated with increased impulsivity, a well-known risk factor for suicide, they note. MISTIC resulted in a measurable improvement in cognitive inhibition.
Results of a neuropsychological battery indicated statistically significant improvements in processing speed and executive functioning (including inhibition, cognitive flexibility, problem-solving, phonemic fluency, and working memory), both immediately after treatment and at 1 month. No declines were noted across any performance domain.
Interestingly, mean performances on these tests moved from the average to the high average score range relative to same-age peers and, in all but one instance, phonemic fluency was high average at baseline and improved to the superior range relative to same-age peers at the 1-month follow-up. Learning and memory tests showed a significant improvement in visual memory and verbal memory. Sustained attention showed a significant improvement in accuracy (detection) and a weak but significant slowing of reaction time, consistent with a prioritization of accuracy over speed and reduced impulsivity.
In a Scientific American article, lead researcher Nolan Williams said he suspects the powerful effects of psychedelics have to do with their “profound ability to increase plasticity in the brain” by “bringing it back to a more juvenile state where reorganization can occur.” People often experience a life review that appears in their minds almost like a slideshow. “It somehow drives a particular sort of psychological phenomenon that you don’t achieve through guidance,” Williams said.
The data from the MISTIC trial in Mexico may spur more research in the US. The National Defense Authorization Act, signed by President Joe Biden last December, authorizes service members diagnosed with PTSD or TBI to take part in clinical studies of any “qualified plant-based alternative therapies.”
“It’s all really timely,” Williams said. “From my perspective, we should have some traction to make a strong argument that the risk-benefit is right.”
Oneirogens are substances that produce or enhance dreamlike states of consciousness—could one of those, ibogaine, be key to relieving the sequelae of traumatic brain injury (TBI) in veterans?
An extract from the root bark of Tabernanthe iboga, an African shrub, ibogaine has both pharmacological and psychological effects. Acting on opioid receptors and the serotonin and dopamine systems, it can relieve withdrawal symptoms and reduce drug cravings—reportedly, often, in just a few hours—and reduce the risk of regular use. The results can last for weeks, months, or sometimes longer.
In the US, ibogaine is a Schedule I drug. Few controlled studies of ibogaine are available; most data come from anecdotal reports and case studies. Clinical research into ibogaine stalled due to legal restrictions that come with a Schedule I drug, as well as concerns about possible cardiac consequences. For example, some reports have described QT interval prolongation, with instances of subsequent fatal arrhythmia.
That may change now, with findings from the Magnesium–Ibogaine: the Stanford Traumatic Injury to the CNS protocol (MISTIC), which took place at a treatment center in Mexico. Researchers from Stanford School of Medicine and the Veterans Affairs Palo Alto Health Care System combined prophylactic intravenous magnesium with ibogaine, in hopes of mitigating the cardiac risks. Magnesium supplementation has been shown to protect against QT interval prolongation when coadministered with medications that ordinarily would have such an effect.
The researchers studied 30 male Special Operations Forces veterans (SOVs) who had predominantly mild TBI. Of those, 15 participants met the criteria for major depressive disorder, 14 for an anxiety disorder, and 23 for PTSD; 19 had past suicidal ideation and 7 had attempted suicide.
Special Operations Forces, the researchers note, are “deployed at a greater pace and to higher intensity combat than conventional military, exposing them to greater allostatic load and risk of injury, including from blast exposure.” This, they say, may result in a “unique pattern” of physical, cognitive, behavioral, psychiatric, and endocrine-related problems across several domains.
Participants received a mean (SD) of 12.1 (1.2) mg kg-1 of oral ibogaine. The researchers assessed changes in the World Health Organization Disability Assessment Schedule at baseline, immediately after treatment, and 1 month after treatment. They also assessed changes in posttraumatic stress disorder (PTSD), depression, and anxiety.
The treatment significantly improved functioning both immediately and at 1 month after treatment and PTSD, depression, and anxiety at 1 month after treatment. There were no unexpected or serious treatment-emergent adverse effects, nor were there instances of bradycardia, tachycardia, clinically meaningful QT prolongation, or hemodynamic instability. All participants experienced transient cerebellar signs, such as mild ataxia and intention tremor, that resolved within 24 hours. While experiencing oneirogenic effects, 12 participants were treated for headache, 7 for nausea, 3 for anxiety, 2 for hypertension, and 1 for insomnia.
At 1 month, suicidal ideation had declined from 47% to 7%—a statistically significant change. “Given the alarming rates of suicide in veterans, as well as evidence that military-related TBI increases the risk of suicide,” the researchers say, “the substantial reduction in SI that we observed—which must be interpreted cautiously as an exploratory analysis—is noteworthy.” TBI also is associated with increased impulsivity, a well-known risk factor for suicide, they note. MISTIC resulted in a measurable improvement in cognitive inhibition.
Results of a neuropsychological battery indicated statistically significant improvements in processing speed and executive functioning (including inhibition, cognitive flexibility, problem-solving, phonemic fluency, and working memory), both immediately after treatment and at 1 month. No declines were noted across any performance domain.
Interestingly, mean performances on these tests moved from the average to the high average score range relative to same-age peers and, in all but one instance, phonemic fluency was high average at baseline and improved to the superior range relative to same-age peers at the 1-month follow-up. Learning and memory tests showed a significant improvement in visual memory and verbal memory. Sustained attention showed a significant improvement in accuracy (detection) and a weak but significant slowing of reaction time, consistent with a prioritization of accuracy over speed and reduced impulsivity.
In a Scientific American article, lead researcher Nolan Williams said he suspects the powerful effects of psychedelics have to do with their “profound ability to increase plasticity in the brain” by “bringing it back to a more juvenile state where reorganization can occur.” People often experience a life review that appears in their minds almost like a slideshow. “It somehow drives a particular sort of psychological phenomenon that you don’t achieve through guidance,” Williams said.
The data from the MISTIC trial in Mexico may spur more research in the US. The National Defense Authorization Act, signed by President Joe Biden last December, authorizes service members diagnosed with PTSD or TBI to take part in clinical studies of any “qualified plant-based alternative therapies.”
“It’s all really timely,” Williams said. “From my perspective, we should have some traction to make a strong argument that the risk-benefit is right.”
Oneirogens are substances that produce or enhance dreamlike states of consciousness—could one of those, ibogaine, be key to relieving the sequelae of traumatic brain injury (TBI) in veterans?
An extract from the root bark of Tabernanthe iboga, an African shrub, ibogaine has both pharmacological and psychological effects. Acting on opioid receptors and the serotonin and dopamine systems, it can relieve withdrawal symptoms and reduce drug cravings—reportedly, often, in just a few hours—and reduce the risk of regular use. The results can last for weeks, months, or sometimes longer.
In the US, ibogaine is a Schedule I drug. Few controlled studies of ibogaine are available; most data come from anecdotal reports and case studies. Clinical research into ibogaine stalled due to legal restrictions that come with a Schedule I drug, as well as concerns about possible cardiac consequences. For example, some reports have described QT interval prolongation, with instances of subsequent fatal arrhythmia.
That may change now, with findings from the Magnesium–Ibogaine: the Stanford Traumatic Injury to the CNS protocol (MISTIC), which took place at a treatment center in Mexico. Researchers from Stanford School of Medicine and the Veterans Affairs Palo Alto Health Care System combined prophylactic intravenous magnesium with ibogaine, in hopes of mitigating the cardiac risks. Magnesium supplementation has been shown to protect against QT interval prolongation when coadministered with medications that ordinarily would have such an effect.
The researchers studied 30 male Special Operations Forces veterans (SOVs) who had predominantly mild TBI. Of those, 15 participants met the criteria for major depressive disorder, 14 for an anxiety disorder, and 23 for PTSD; 19 had past suicidal ideation and 7 had attempted suicide.
Special Operations Forces, the researchers note, are “deployed at a greater pace and to higher intensity combat than conventional military, exposing them to greater allostatic load and risk of injury, including from blast exposure.” This, they say, may result in a “unique pattern” of physical, cognitive, behavioral, psychiatric, and endocrine-related problems across several domains.
Participants received a mean (SD) of 12.1 (1.2) mg kg-1 of oral ibogaine. The researchers assessed changes in the World Health Organization Disability Assessment Schedule at baseline, immediately after treatment, and 1 month after treatment. They also assessed changes in posttraumatic stress disorder (PTSD), depression, and anxiety.
The treatment significantly improved functioning both immediately and at 1 month after treatment and PTSD, depression, and anxiety at 1 month after treatment. There were no unexpected or serious treatment-emergent adverse effects, nor were there instances of bradycardia, tachycardia, clinically meaningful QT prolongation, or hemodynamic instability. All participants experienced transient cerebellar signs, such as mild ataxia and intention tremor, that resolved within 24 hours. While experiencing oneirogenic effects, 12 participants were treated for headache, 7 for nausea, 3 for anxiety, 2 for hypertension, and 1 for insomnia.
At 1 month, suicidal ideation had declined from 47% to 7%—a statistically significant change. “Given the alarming rates of suicide in veterans, as well as evidence that military-related TBI increases the risk of suicide,” the researchers say, “the substantial reduction in SI that we observed—which must be interpreted cautiously as an exploratory analysis—is noteworthy.” TBI also is associated with increased impulsivity, a well-known risk factor for suicide, they note. MISTIC resulted in a measurable improvement in cognitive inhibition.
Results of a neuropsychological battery indicated statistically significant improvements in processing speed and executive functioning (including inhibition, cognitive flexibility, problem-solving, phonemic fluency, and working memory), both immediately after treatment and at 1 month. No declines were noted across any performance domain.
Interestingly, mean performances on these tests moved from the average to the high average score range relative to same-age peers and, in all but one instance, phonemic fluency was high average at baseline and improved to the superior range relative to same-age peers at the 1-month follow-up. Learning and memory tests showed a significant improvement in visual memory and verbal memory. Sustained attention showed a significant improvement in accuracy (detection) and a weak but significant slowing of reaction time, consistent with a prioritization of accuracy over speed and reduced impulsivity.
In a Scientific American article, lead researcher Nolan Williams said he suspects the powerful effects of psychedelics have to do with their “profound ability to increase plasticity in the brain” by “bringing it back to a more juvenile state where reorganization can occur.” People often experience a life review that appears in their minds almost like a slideshow. “It somehow drives a particular sort of psychological phenomenon that you don’t achieve through guidance,” Williams said.
The data from the MISTIC trial in Mexico may spur more research in the US. The National Defense Authorization Act, signed by President Joe Biden last December, authorizes service members diagnosed with PTSD or TBI to take part in clinical studies of any “qualified plant-based alternative therapies.”
“It’s all really timely,” Williams said. “From my perspective, we should have some traction to make a strong argument that the risk-benefit is right.”
Lump in breast
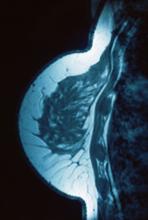
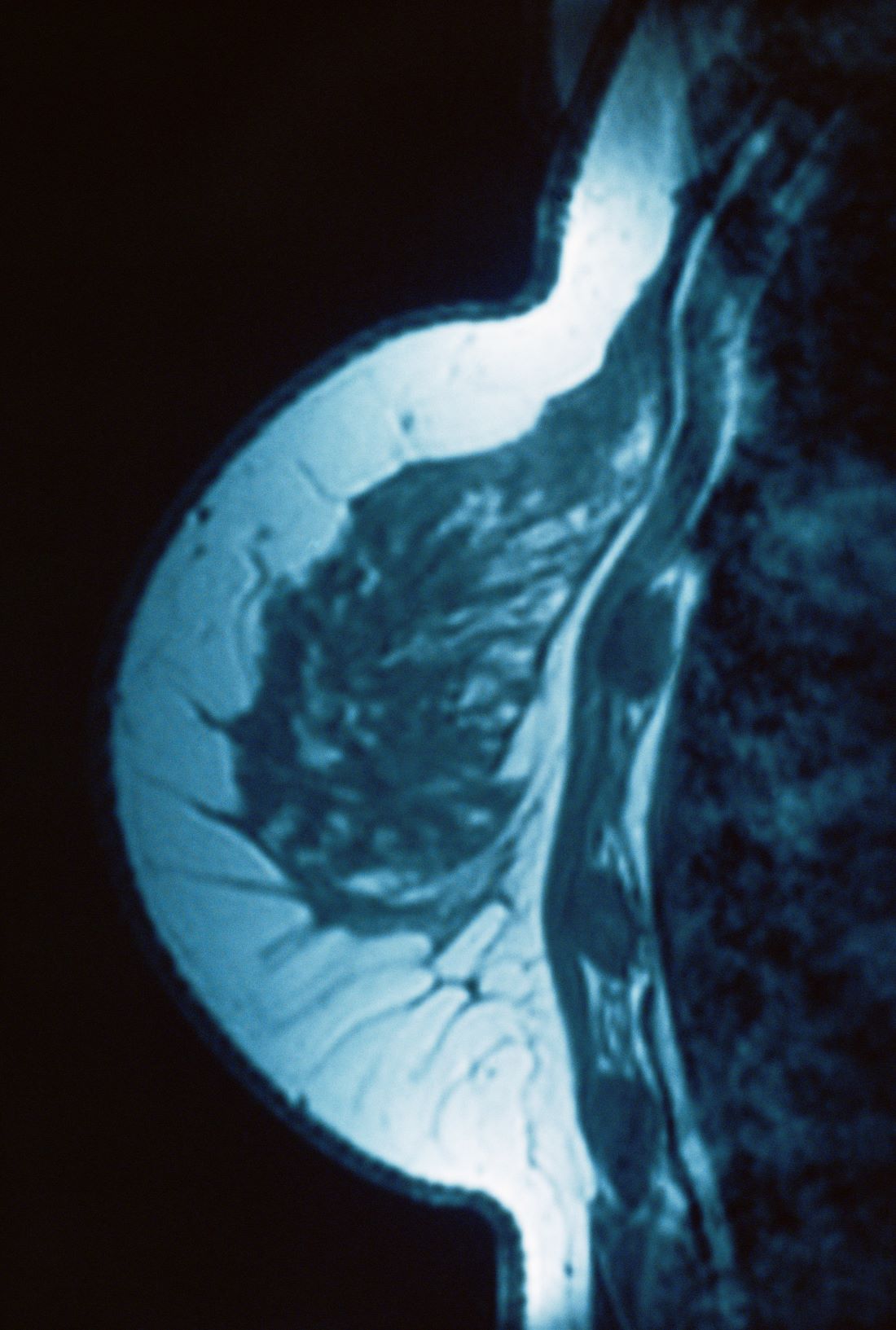
Given clinical and imaging outcomes, as well as results on IHC assay, this patient is diagnosed with triple-negative breast cancer and is referred for further consultation with a multidisciplinary care team.
Triple-negative breast cancer accounts for 15% of all female breast cancer cases. The term "triple-negative" refers to the absence of estrogen receptor (ER) and progesterone receptor (PR) targets and the HER2 target for treatment. Otherwise, triple-negative breast cancer is highly heterogeneous and includes multiple molecular subtypes. Typically, triple-negative breast cancer occurs in younger patients, has a higher grade, a greater tumor size, a higher clinical stage at diagnosis, and a poorer prognosis than non–triple-negative breast cancers.
Biopsy samples from patients with a new primary or newly metastatic breast cancer diagnosis should undergo HR testing, both ER and PR, as well as HER2 receptor testing. Results of ER and PR testing are negative if a validated IHC assay shows 0% to < 1% of nuclei stain, according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HR testing guideline. HER2 results are negative if a validated IHC assay shows weak to moderate complete membrane staining in < 10% of tumor cells, according to ASCO/CAP guidelines. If HER2 results of a primary tumor are negative and specific clinical criteria suggest further testing, the oncologist may choose to order another IHC assay or a validated dual probe in situ hybridization (ISH) assay to aid in clinical decision-making.
Pathologic classification of triple-negative breast cancers includes multiple subgroups determined by therapeutic possibilities: low-risk histologic types (salivary gland–like carcinomas, fibromatosis-like carcinoma, low-grade adenosquamous breast carcinoma), immune activation (tumor-infiltrating lymphocytes, CD8, programmed death–ligand 1, tumor mutational burden), HER2-low status (IHC score 1+/2+ nonamplified), associated germline BRCA mutations, and other targets (trophoblast cell-surface antigen 2, HER3).
Family history is the most common risk factor for developing breast cancer. If a person's mother or sister had breast cancer, the lifetime risk for that individual is four times greater than that of the general population. Risk for breast cancer grows even more if two or more first-degree relatives are diagnosed, or if one first-degree relative was diagnosed with breast cancer at ≤ 50 years of age. About 25% of triple-negative breast cancer cases have accompanying germline BRCA mutations. A recent study by Ahearn and colleagues found that 85 variants were linked to one or more tumor feature of ER-negative disease, and 32 of those variants were associated with triple-negative disease.
For a breast cancer diagnosis, the National Comprehensive Cancer Network recommends multidisciplinary care as well as the development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Because triple-negative breast cancer lacks HR and HER2 therapeutic targets, it can be difficult to treat. Standard therapy for high-risk triple-negative tumors includes chemotherapy with taxanes (paclitaxel or docetaxel) plus anthracycline-based treatment (cyclophosphamide plus doxorubicin). Neoadjuvant chemotherapy is recommended for patients with stage 2 and 3 tumors to reduce locoregional surgical extension and to allow for personalized treatment based on pathologic response. In 2018, two poly ADP ribose polymerase inhibitors, olaparib and talazoparib, were approved for use in select patients with triple-negative breast cancer, significantly changing the treatment paradigm for this disease. Other promising treatments have emerged recently in clinical trials for triple-negative disease, including immune checkpoint inhibitors, PI3K/AKT pathway inhibitors, and cytotoxin-conjugated antibodies.
Daniel S. Schwartz, MD, is Medical Director of Thoracic Oncology, St. Catherine of Siena Medical Center, Catholic Health Services, Smithtown, New York.
Dr. Schwartz serve(d) as a member of the following medical societies:
American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, Western Thoracic Surgical Association.
Disclosure: Daniel S. Schwartz, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given clinical and imaging outcomes, as well as results on IHC assay, this patient is diagnosed with triple-negative breast cancer and is referred for further consultation with a multidisciplinary care team.
Triple-negative breast cancer accounts for 15% of all female breast cancer cases. The term "triple-negative" refers to the absence of estrogen receptor (ER) and progesterone receptor (PR) targets and the HER2 target for treatment. Otherwise, triple-negative breast cancer is highly heterogeneous and includes multiple molecular subtypes. Typically, triple-negative breast cancer occurs in younger patients, has a higher grade, a greater tumor size, a higher clinical stage at diagnosis, and a poorer prognosis than non–triple-negative breast cancers.
Biopsy samples from patients with a new primary or newly metastatic breast cancer diagnosis should undergo HR testing, both ER and PR, as well as HER2 receptor testing. Results of ER and PR testing are negative if a validated IHC assay shows 0% to < 1% of nuclei stain, according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HR testing guideline. HER2 results are negative if a validated IHC assay shows weak to moderate complete membrane staining in < 10% of tumor cells, according to ASCO/CAP guidelines. If HER2 results of a primary tumor are negative and specific clinical criteria suggest further testing, the oncologist may choose to order another IHC assay or a validated dual probe in situ hybridization (ISH) assay to aid in clinical decision-making.
Pathologic classification of triple-negative breast cancers includes multiple subgroups determined by therapeutic possibilities: low-risk histologic types (salivary gland–like carcinomas, fibromatosis-like carcinoma, low-grade adenosquamous breast carcinoma), immune activation (tumor-infiltrating lymphocytes, CD8, programmed death–ligand 1, tumor mutational burden), HER2-low status (IHC score 1+/2+ nonamplified), associated germline BRCA mutations, and other targets (trophoblast cell-surface antigen 2, HER3).
Family history is the most common risk factor for developing breast cancer. If a person's mother or sister had breast cancer, the lifetime risk for that individual is four times greater than that of the general population. Risk for breast cancer grows even more if two or more first-degree relatives are diagnosed, or if one first-degree relative was diagnosed with breast cancer at ≤ 50 years of age. About 25% of triple-negative breast cancer cases have accompanying germline BRCA mutations. A recent study by Ahearn and colleagues found that 85 variants were linked to one or more tumor feature of ER-negative disease, and 32 of those variants were associated with triple-negative disease.
For a breast cancer diagnosis, the National Comprehensive Cancer Network recommends multidisciplinary care as well as the development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Because triple-negative breast cancer lacks HR and HER2 therapeutic targets, it can be difficult to treat. Standard therapy for high-risk triple-negative tumors includes chemotherapy with taxanes (paclitaxel or docetaxel) plus anthracycline-based treatment (cyclophosphamide plus doxorubicin). Neoadjuvant chemotherapy is recommended for patients with stage 2 and 3 tumors to reduce locoregional surgical extension and to allow for personalized treatment based on pathologic response. In 2018, two poly ADP ribose polymerase inhibitors, olaparib and talazoparib, were approved for use in select patients with triple-negative breast cancer, significantly changing the treatment paradigm for this disease. Other promising treatments have emerged recently in clinical trials for triple-negative disease, including immune checkpoint inhibitors, PI3K/AKT pathway inhibitors, and cytotoxin-conjugated antibodies.
Daniel S. Schwartz, MD, is Medical Director of Thoracic Oncology, St. Catherine of Siena Medical Center, Catholic Health Services, Smithtown, New York.
Dr. Schwartz serve(d) as a member of the following medical societies:
American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, Western Thoracic Surgical Association.
Disclosure: Daniel S. Schwartz, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given clinical and imaging outcomes, as well as results on IHC assay, this patient is diagnosed with triple-negative breast cancer and is referred for further consultation with a multidisciplinary care team.
Triple-negative breast cancer accounts for 15% of all female breast cancer cases. The term "triple-negative" refers to the absence of estrogen receptor (ER) and progesterone receptor (PR) targets and the HER2 target for treatment. Otherwise, triple-negative breast cancer is highly heterogeneous and includes multiple molecular subtypes. Typically, triple-negative breast cancer occurs in younger patients, has a higher grade, a greater tumor size, a higher clinical stage at diagnosis, and a poorer prognosis than non–triple-negative breast cancers.
Biopsy samples from patients with a new primary or newly metastatic breast cancer diagnosis should undergo HR testing, both ER and PR, as well as HER2 receptor testing. Results of ER and PR testing are negative if a validated IHC assay shows 0% to < 1% of nuclei stain, according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HR testing guideline. HER2 results are negative if a validated IHC assay shows weak to moderate complete membrane staining in < 10% of tumor cells, according to ASCO/CAP guidelines. If HER2 results of a primary tumor are negative and specific clinical criteria suggest further testing, the oncologist may choose to order another IHC assay or a validated dual probe in situ hybridization (ISH) assay to aid in clinical decision-making.
Pathologic classification of triple-negative breast cancers includes multiple subgroups determined by therapeutic possibilities: low-risk histologic types (salivary gland–like carcinomas, fibromatosis-like carcinoma, low-grade adenosquamous breast carcinoma), immune activation (tumor-infiltrating lymphocytes, CD8, programmed death–ligand 1, tumor mutational burden), HER2-low status (IHC score 1+/2+ nonamplified), associated germline BRCA mutations, and other targets (trophoblast cell-surface antigen 2, HER3).
Family history is the most common risk factor for developing breast cancer. If a person's mother or sister had breast cancer, the lifetime risk for that individual is four times greater than that of the general population. Risk for breast cancer grows even more if two or more first-degree relatives are diagnosed, or if one first-degree relative was diagnosed with breast cancer at ≤ 50 years of age. About 25% of triple-negative breast cancer cases have accompanying germline BRCA mutations. A recent study by Ahearn and colleagues found that 85 variants were linked to one or more tumor feature of ER-negative disease, and 32 of those variants were associated with triple-negative disease.
For a breast cancer diagnosis, the National Comprehensive Cancer Network recommends multidisciplinary care as well as the development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Because triple-negative breast cancer lacks HR and HER2 therapeutic targets, it can be difficult to treat. Standard therapy for high-risk triple-negative tumors includes chemotherapy with taxanes (paclitaxel or docetaxel) plus anthracycline-based treatment (cyclophosphamide plus doxorubicin). Neoadjuvant chemotherapy is recommended for patients with stage 2 and 3 tumors to reduce locoregional surgical extension and to allow for personalized treatment based on pathologic response. In 2018, two poly ADP ribose polymerase inhibitors, olaparib and talazoparib, were approved for use in select patients with triple-negative breast cancer, significantly changing the treatment paradigm for this disease. Other promising treatments have emerged recently in clinical trials for triple-negative disease, including immune checkpoint inhibitors, PI3K/AKT pathway inhibitors, and cytotoxin-conjugated antibodies.
Daniel S. Schwartz, MD, is Medical Director of Thoracic Oncology, St. Catherine of Siena Medical Center, Catholic Health Services, Smithtown, New York.
Dr. Schwartz serve(d) as a member of the following medical societies:
American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, Western Thoracic Surgical Association.
Disclosure: Daniel S. Schwartz, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 34-year-old woman visits her primary care physician after detecting a lump in her right breast. The physician conducts a physical exam and finds a hard, poorly mobile mass in the outer upper quadrant. Ultrasonography reveals an irregular hypoechoic mass of 22.1 x 15.1 mm with several abnormal axillary lymph nodes. MRI of the right breast shows an irregularly shaped and lobulated mass in the outer upper quadrant with enlarged axillary lymph nodes. On further radiologic examination, there is no detectable metastatic spread to the brain, chest, or upper abdomen. The patient undergoes a core needle biopsy of the tumor, which confirms ductal carcinoma and right axillary lymph node metastasis. Immunohistochemistry (IHC) assay of the sample is negative for hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2).
Fall Abstract Las Vegas Dermatology Seminar Compendium; Las Vegas, Nevada; November 2-4, 2023
Men with atopic dermatitis more likely to have poorer cognitive function
Key clinical point: A significant association was observed between atopic dermatitis (AD) and poorer cognitive function in men, and familial characteristics exerted a confounding effect on this association.
Major finding: After effectively controlling for familial environmental confounding factors and addressing genetic influences, AD in men was significantly associated with poorer cognitive function (regression coefficient −0.04; 95% CI −0.07 to −0.003).
Study details: This sibling-comparison study included 1,687,038 men who underwent a military conscription examination at 17-22 years of age, of which 25,995 were diagnosed with AD.
Disclosures: This study was sponsored by grants from the Swedish Research Council for Health, Working Life, and Welfare (Forte) and the UK Economic and Social Research Council. L von Kobyletzki declared being a consultant for and receiving research funding from various organizations. The other authors declared no conflicts of interest.
Source: Smith KA et al. Atopic dermatitis and cognitive function: A sibling comparison study among males in Sweden. Br J Dermatol. 2024 (Jan 3). doi: 10.1093/bjd/ljae004
Key clinical point: A significant association was observed between atopic dermatitis (AD) and poorer cognitive function in men, and familial characteristics exerted a confounding effect on this association.
Major finding: After effectively controlling for familial environmental confounding factors and addressing genetic influences, AD in men was significantly associated with poorer cognitive function (regression coefficient −0.04; 95% CI −0.07 to −0.003).
Study details: This sibling-comparison study included 1,687,038 men who underwent a military conscription examination at 17-22 years of age, of which 25,995 were diagnosed with AD.
Disclosures: This study was sponsored by grants from the Swedish Research Council for Health, Working Life, and Welfare (Forte) and the UK Economic and Social Research Council. L von Kobyletzki declared being a consultant for and receiving research funding from various organizations. The other authors declared no conflicts of interest.
Source: Smith KA et al. Atopic dermatitis and cognitive function: A sibling comparison study among males in Sweden. Br J Dermatol. 2024 (Jan 3). doi: 10.1093/bjd/ljae004
Key clinical point: A significant association was observed between atopic dermatitis (AD) and poorer cognitive function in men, and familial characteristics exerted a confounding effect on this association.
Major finding: After effectively controlling for familial environmental confounding factors and addressing genetic influences, AD in men was significantly associated with poorer cognitive function (regression coefficient −0.04; 95% CI −0.07 to −0.003).
Study details: This sibling-comparison study included 1,687,038 men who underwent a military conscription examination at 17-22 years of age, of which 25,995 were diagnosed with AD.
Disclosures: This study was sponsored by grants from the Swedish Research Council for Health, Working Life, and Welfare (Forte) and the UK Economic and Social Research Council. L von Kobyletzki declared being a consultant for and receiving research funding from various organizations. The other authors declared no conflicts of interest.
Source: Smith KA et al. Atopic dermatitis and cognitive function: A sibling comparison study among males in Sweden. Br J Dermatol. 2024 (Jan 3). doi: 10.1093/bjd/ljae004
Atopic dermatitis is associated with increased prevalence of inflammatory bowel disease
Key clinical point: Patients with atopic dermatitis (AD), especially moderate-to-severe AD, had an increased prevalence of inflammatory bowel disease (IBD).
Major finding: A significant association was observed between IBD and AD (adjusted odds ratio [aOR] 3.89; P = .0169); however, when stratified by AD severity, only moderate-to-severe AD was found to be associated with IBD (aOR 4.45; P = .0102).
Study details: Findings are from a retrospective observational study including 364 patients with AD and 725 matched control individuals without AD.
Disclosures: This study was sponsored by an independent investigator grant from AbbVie. Two authors declared serving as investigators for or receiving honoraria or fees as consultants or advisory board members from various organizations, including AbbVie. The other authors declared no conflicts of interest.
Source: Rom H et al. The association between atopic dermatitis and inflammatory bowel disease in adults: A cross-sectional study in a specialized atopic dermatitis clinic. J Eur Acad Dermatol Venereol. 2023 (Dec 21). doi: 10.1111/jdv.19769
Key clinical point: Patients with atopic dermatitis (AD), especially moderate-to-severe AD, had an increased prevalence of inflammatory bowel disease (IBD).
Major finding: A significant association was observed between IBD and AD (adjusted odds ratio [aOR] 3.89; P = .0169); however, when stratified by AD severity, only moderate-to-severe AD was found to be associated with IBD (aOR 4.45; P = .0102).
Study details: Findings are from a retrospective observational study including 364 patients with AD and 725 matched control individuals without AD.
Disclosures: This study was sponsored by an independent investigator grant from AbbVie. Two authors declared serving as investigators for or receiving honoraria or fees as consultants or advisory board members from various organizations, including AbbVie. The other authors declared no conflicts of interest.
Source: Rom H et al. The association between atopic dermatitis and inflammatory bowel disease in adults: A cross-sectional study in a specialized atopic dermatitis clinic. J Eur Acad Dermatol Venereol. 2023 (Dec 21). doi: 10.1111/jdv.19769
Key clinical point: Patients with atopic dermatitis (AD), especially moderate-to-severe AD, had an increased prevalence of inflammatory bowel disease (IBD).
Major finding: A significant association was observed between IBD and AD (adjusted odds ratio [aOR] 3.89; P = .0169); however, when stratified by AD severity, only moderate-to-severe AD was found to be associated with IBD (aOR 4.45; P = .0102).
Study details: Findings are from a retrospective observational study including 364 patients with AD and 725 matched control individuals without AD.
Disclosures: This study was sponsored by an independent investigator grant from AbbVie. Two authors declared serving as investigators for or receiving honoraria or fees as consultants or advisory board members from various organizations, including AbbVie. The other authors declared no conflicts of interest.
Source: Rom H et al. The association between atopic dermatitis and inflammatory bowel disease in adults: A cross-sectional study in a specialized atopic dermatitis clinic. J Eur Acad Dermatol Venereol. 2023 (Dec 21). doi: 10.1111/jdv.19769
Real-world study confirms the multidimensional efficacy of tralokinumab in atopic dermatitis
Key clinical point: The majority of tralokinumab-treated patients with moderate-to-severe atopic dermatitis (AD) attained physician- and patient-reported outcomes over 32 weeks of observation, highlighting the multidimensional efficacy of tralokinumab in real-world settings.
Major finding: The proportion of patients achieving a ≥75% improvement in the baseline Eczema Area and Severity Index (EASI) score increased significantly from 42% at week 4 to 76% at week 32 (P = .0075). A similar trend was observed for patient-reported outcomes. At week 16, at least one real-world therapeutic endpoint was achieved by 88% of patients treated with tralokinumab.
Study details: Findings are from a multicenter real-world retrospective cohort study including 194 patients with moderate-to-severe AD who were treated with tralokinumab for ≥16 weeks.
Disclosures: This study did not receive any funding. Several authors declared serving as speakers, consultants, or scientific advisors; receiving personal fees, speaker’s honoraria, or travel support, or having other ties with various pharmaceutical companies.
Source: Chiricozzi A et al for the MEDaCoTRA Study Group. Current treatment goals are achieved by the majority of patients with atopic dermatitis treated with tralokinumab: Results from a multicentric, multinational, retrospective, cohort study. Expert Opin Biol Ther. 2023;23(12):1307-1315 (Dec 18). doi: 10.1080/14712598.2023.2292627
Key clinical point: The majority of tralokinumab-treated patients with moderate-to-severe atopic dermatitis (AD) attained physician- and patient-reported outcomes over 32 weeks of observation, highlighting the multidimensional efficacy of tralokinumab in real-world settings.
Major finding: The proportion of patients achieving a ≥75% improvement in the baseline Eczema Area and Severity Index (EASI) score increased significantly from 42% at week 4 to 76% at week 32 (P = .0075). A similar trend was observed for patient-reported outcomes. At week 16, at least one real-world therapeutic endpoint was achieved by 88% of patients treated with tralokinumab.
Study details: Findings are from a multicenter real-world retrospective cohort study including 194 patients with moderate-to-severe AD who were treated with tralokinumab for ≥16 weeks.
Disclosures: This study did not receive any funding. Several authors declared serving as speakers, consultants, or scientific advisors; receiving personal fees, speaker’s honoraria, or travel support, or having other ties with various pharmaceutical companies.
Source: Chiricozzi A et al for the MEDaCoTRA Study Group. Current treatment goals are achieved by the majority of patients with atopic dermatitis treated with tralokinumab: Results from a multicentric, multinational, retrospective, cohort study. Expert Opin Biol Ther. 2023;23(12):1307-1315 (Dec 18). doi: 10.1080/14712598.2023.2292627
Key clinical point: The majority of tralokinumab-treated patients with moderate-to-severe atopic dermatitis (AD) attained physician- and patient-reported outcomes over 32 weeks of observation, highlighting the multidimensional efficacy of tralokinumab in real-world settings.
Major finding: The proportion of patients achieving a ≥75% improvement in the baseline Eczema Area and Severity Index (EASI) score increased significantly from 42% at week 4 to 76% at week 32 (P = .0075). A similar trend was observed for patient-reported outcomes. At week 16, at least one real-world therapeutic endpoint was achieved by 88% of patients treated with tralokinumab.
Study details: Findings are from a multicenter real-world retrospective cohort study including 194 patients with moderate-to-severe AD who were treated with tralokinumab for ≥16 weeks.
Disclosures: This study did not receive any funding. Several authors declared serving as speakers, consultants, or scientific advisors; receiving personal fees, speaker’s honoraria, or travel support, or having other ties with various pharmaceutical companies.
Source: Chiricozzi A et al for the MEDaCoTRA Study Group. Current treatment goals are achieved by the majority of patients with atopic dermatitis treated with tralokinumab: Results from a multicentric, multinational, retrospective, cohort study. Expert Opin Biol Ther. 2023;23(12):1307-1315 (Dec 18). doi: 10.1080/14712598.2023.2292627
Abrocitinib downregulates genes associated with atopic dermatitis pathology
Key clinical point: Abrocitinib treatment over 12 weeks significantly decreased the cutaneous expression of selected genes involved in inflammation, epidermal hyperplasia, and T-helper (Th) 2 and Th22 immune responses in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Compared with placebo, 12-week abrocitinib treatment led to a dose-dependent reduction in the cutaneous expression of genes involved in inflammation (MMP-12), epidermal hyperplasia (KRT16), Th2 (CCL17 and CCL18), and Th22 (S100A8, S100A9, and S100A12) responses (all P < .05).
Study details: Findings are from the phase 2a JADE MOA trial including patients with moderate-to-severe AD who were randomly assigned to receive 100 mg (n = 16) or 200 mg (n = 14) abrocitinib monotherapy or placebo (n = 16) daily for 12 weeks.
Disclosures: This study was sponsored by Pfizer Inc. Several authors declared being on the advisory board of; serving as consultants, advisors, or speakers for; or receiving honoraria or grants from Pfizer or others. Seven authors declared being current or former employees and shareholders of Pfizer.
Source: Guttman-Yassky E et al. Effect of abrocitinib on skin biomarkers in patients with moderate-to-severe atopic dermatitis. Allergy. 2023 (Dec 18). doi: 10.1111/all.15969
Key clinical point: Abrocitinib treatment over 12 weeks significantly decreased the cutaneous expression of selected genes involved in inflammation, epidermal hyperplasia, and T-helper (Th) 2 and Th22 immune responses in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Compared with placebo, 12-week abrocitinib treatment led to a dose-dependent reduction in the cutaneous expression of genes involved in inflammation (MMP-12), epidermal hyperplasia (KRT16), Th2 (CCL17 and CCL18), and Th22 (S100A8, S100A9, and S100A12) responses (all P < .05).
Study details: Findings are from the phase 2a JADE MOA trial including patients with moderate-to-severe AD who were randomly assigned to receive 100 mg (n = 16) or 200 mg (n = 14) abrocitinib monotherapy or placebo (n = 16) daily for 12 weeks.
Disclosures: This study was sponsored by Pfizer Inc. Several authors declared being on the advisory board of; serving as consultants, advisors, or speakers for; or receiving honoraria or grants from Pfizer or others. Seven authors declared being current or former employees and shareholders of Pfizer.
Source: Guttman-Yassky E et al. Effect of abrocitinib on skin biomarkers in patients with moderate-to-severe atopic dermatitis. Allergy. 2023 (Dec 18). doi: 10.1111/all.15969
Key clinical point: Abrocitinib treatment over 12 weeks significantly decreased the cutaneous expression of selected genes involved in inflammation, epidermal hyperplasia, and T-helper (Th) 2 and Th22 immune responses in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Compared with placebo, 12-week abrocitinib treatment led to a dose-dependent reduction in the cutaneous expression of genes involved in inflammation (MMP-12), epidermal hyperplasia (KRT16), Th2 (CCL17 and CCL18), and Th22 (S100A8, S100A9, and S100A12) responses (all P < .05).
Study details: Findings are from the phase 2a JADE MOA trial including patients with moderate-to-severe AD who were randomly assigned to receive 100 mg (n = 16) or 200 mg (n = 14) abrocitinib monotherapy or placebo (n = 16) daily for 12 weeks.
Disclosures: This study was sponsored by Pfizer Inc. Several authors declared being on the advisory board of; serving as consultants, advisors, or speakers for; or receiving honoraria or grants from Pfizer or others. Seven authors declared being current or former employees and shareholders of Pfizer.
Source: Guttman-Yassky E et al. Effect of abrocitinib on skin biomarkers in patients with moderate-to-severe atopic dermatitis. Allergy. 2023 (Dec 18). doi: 10.1111/all.15969
Rapid and sustained improvement in skin pain with abrocitinib in atopic dermatitis
Key clinical point: Abrocitinib as monotherapy or in combination with topical therapy improves skin pain in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Abrocitinib vs placebo led to a significantly greater dose-dependent least squares mean change in Pruritus and Symptoms Assessment for AD (PSAAD) skin pain score from baseline to as early as week 1 that were sustained through week 12 or 16 (nominal P < .05). A greater proportion of patients achieved a stringent threshold of skin pain improvement (PSAAD skin pain score < 2) with abrocitinib vs placebo (nominal P < .05).
Study details: This post hoc analysis of five phase 2/3 trials included 1822 patients with moderate-to-severe AD (age ≥ 12 years) treated with 100 mg or 200 mg abrocitinib as monotherapy or in combination with topical therapy or placebo for 12 or 16 weeks.
Disclosures: This study was funded by Pfizer Inc., USA. Six authors declared being employees and stockholders of Pfizer. The other authors declared receiving research or travel grants or having other ties with various sources, including Pfizer.
Source: Thyssen JP et al. Abrocitinib provides rapid and sustained improvement in skin pain and is associated with improved quality of life outcomes in adult and adolescent patients with moderate-to-severe atopic dermatitis. Dermatology. 2023 (Dec 11). doi: 10.1159/000535285
Key clinical point: Abrocitinib as monotherapy or in combination with topical therapy improves skin pain in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Abrocitinib vs placebo led to a significantly greater dose-dependent least squares mean change in Pruritus and Symptoms Assessment for AD (PSAAD) skin pain score from baseline to as early as week 1 that were sustained through week 12 or 16 (nominal P < .05). A greater proportion of patients achieved a stringent threshold of skin pain improvement (PSAAD skin pain score < 2) with abrocitinib vs placebo (nominal P < .05).
Study details: This post hoc analysis of five phase 2/3 trials included 1822 patients with moderate-to-severe AD (age ≥ 12 years) treated with 100 mg or 200 mg abrocitinib as monotherapy or in combination with topical therapy or placebo for 12 or 16 weeks.
Disclosures: This study was funded by Pfizer Inc., USA. Six authors declared being employees and stockholders of Pfizer. The other authors declared receiving research or travel grants or having other ties with various sources, including Pfizer.
Source: Thyssen JP et al. Abrocitinib provides rapid and sustained improvement in skin pain and is associated with improved quality of life outcomes in adult and adolescent patients with moderate-to-severe atopic dermatitis. Dermatology. 2023 (Dec 11). doi: 10.1159/000535285
Key clinical point: Abrocitinib as monotherapy or in combination with topical therapy improves skin pain in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Abrocitinib vs placebo led to a significantly greater dose-dependent least squares mean change in Pruritus and Symptoms Assessment for AD (PSAAD) skin pain score from baseline to as early as week 1 that were sustained through week 12 or 16 (nominal P < .05). A greater proportion of patients achieved a stringent threshold of skin pain improvement (PSAAD skin pain score < 2) with abrocitinib vs placebo (nominal P < .05).
Study details: This post hoc analysis of five phase 2/3 trials included 1822 patients with moderate-to-severe AD (age ≥ 12 years) treated with 100 mg or 200 mg abrocitinib as monotherapy or in combination with topical therapy or placebo for 12 or 16 weeks.
Disclosures: This study was funded by Pfizer Inc., USA. Six authors declared being employees and stockholders of Pfizer. The other authors declared receiving research or travel grants or having other ties with various sources, including Pfizer.
Source: Thyssen JP et al. Abrocitinib provides rapid and sustained improvement in skin pain and is associated with improved quality of life outcomes in adult and adolescent patients with moderate-to-severe atopic dermatitis. Dermatology. 2023 (Dec 11). doi: 10.1159/000535285
Allergic contact dermatitis a crucial comorbidity in atopic dermatitis
Key clinical point: Allergic contact dermatitis (ACD) is an important comorbidity in patients with atopic dermatitis (AD) and leads to the maintenance and aggravation of their dermatosis, with a high frequency of ACD observed to textile dyes, isothiazolinones, and fragrances.
Major finding: Contact sensitization was significantly associated with facial involvement (P = .04) and a longer duration of AD (P = .005). The most frequent allergen was textile dye mix (24.70%) followed by nickel (20.21%), cobalt (12.70%), and methylchlorisothiazolinone+methylisothiazolinone (8.50%). The avoidance of relevant allergens led to a significant reduction in the Scoring of Atopic Dermatitis (SCORAD) scores at 6 months (P < .001).
Study details: This longitudinal prospective study included 93 patients with AD (age > 2 years) who were patch-tested with the 2019 European baseline series and the corticosteroid series, 60.2% of whom had positive patch test results.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Trimeche K et al. Contact allergy in atopic dermatitis: A prospective study on prevalence, incriminated allergens and clinical insights. Contact Dermatitis. 2023 (Dec 27). doi: 10.1111/cod.14494
Key clinical point: Allergic contact dermatitis (ACD) is an important comorbidity in patients with atopic dermatitis (AD) and leads to the maintenance and aggravation of their dermatosis, with a high frequency of ACD observed to textile dyes, isothiazolinones, and fragrances.
Major finding: Contact sensitization was significantly associated with facial involvement (P = .04) and a longer duration of AD (P = .005). The most frequent allergen was textile dye mix (24.70%) followed by nickel (20.21%), cobalt (12.70%), and methylchlorisothiazolinone+methylisothiazolinone (8.50%). The avoidance of relevant allergens led to a significant reduction in the Scoring of Atopic Dermatitis (SCORAD) scores at 6 months (P < .001).
Study details: This longitudinal prospective study included 93 patients with AD (age > 2 years) who were patch-tested with the 2019 European baseline series and the corticosteroid series, 60.2% of whom had positive patch test results.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Trimeche K et al. Contact allergy in atopic dermatitis: A prospective study on prevalence, incriminated allergens and clinical insights. Contact Dermatitis. 2023 (Dec 27). doi: 10.1111/cod.14494
Key clinical point: Allergic contact dermatitis (ACD) is an important comorbidity in patients with atopic dermatitis (AD) and leads to the maintenance and aggravation of their dermatosis, with a high frequency of ACD observed to textile dyes, isothiazolinones, and fragrances.
Major finding: Contact sensitization was significantly associated with facial involvement (P = .04) and a longer duration of AD (P = .005). The most frequent allergen was textile dye mix (24.70%) followed by nickel (20.21%), cobalt (12.70%), and methylchlorisothiazolinone+methylisothiazolinone (8.50%). The avoidance of relevant allergens led to a significant reduction in the Scoring of Atopic Dermatitis (SCORAD) scores at 6 months (P < .001).
Study details: This longitudinal prospective study included 93 patients with AD (age > 2 years) who were patch-tested with the 2019 European baseline series and the corticosteroid series, 60.2% of whom had positive patch test results.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Trimeche K et al. Contact allergy in atopic dermatitis: A prospective study on prevalence, incriminated allergens and clinical insights. Contact Dermatitis. 2023 (Dec 27). doi: 10.1111/cod.14494
Aggregate response benefit in skin clearance and itch reduction favor upadacitinib over dupilumab in AD
Key clinical point: The overall improvement in skin clearance and itch reduction suggested a preference for 30 mg upadacitinib over dupilumab and that for 15 mg or 30 mg upadacitinib over placebo in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 24, the aggregate response benefit for skin clearance and itch, respectively, was 32.5% and 25.8% higher with 30 mg upadacitinib vs dupilumab. The benefit favored upadacitinib over dupilumab as early as week 4. Moreover, 15 and 30 mg upadacitinib showed similar benefits over placebo.
Study details: This post hoc analysis of the data from phase 3 studies (Heads Up, Measure Up 1, and Measure Up 2) included 2356 patients with moderate-to-severe AD who received upadacitinib, dupilumab, or placebo.
Disclosures: This study was sponsored by AbbVie. Five authors declared being employees of or owning stock or stock options in AbbVie. Several authors declared being consultants, speakers, or advisors of or having other ties with various sources, including AbbVie.
Source: Silverberg JI et al. Aggregate response benefit in skin clearance and itch reduction with upadacitinib or dupilumab in patients with moderate-to-severe atopic dermatitis. Dermatitis. 2023 (Dec 18). doi: 10.1089/derm.2023.0153
Key clinical point: The overall improvement in skin clearance and itch reduction suggested a preference for 30 mg upadacitinib over dupilumab and that for 15 mg or 30 mg upadacitinib over placebo in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 24, the aggregate response benefit for skin clearance and itch, respectively, was 32.5% and 25.8% higher with 30 mg upadacitinib vs dupilumab. The benefit favored upadacitinib over dupilumab as early as week 4. Moreover, 15 and 30 mg upadacitinib showed similar benefits over placebo.
Study details: This post hoc analysis of the data from phase 3 studies (Heads Up, Measure Up 1, and Measure Up 2) included 2356 patients with moderate-to-severe AD who received upadacitinib, dupilumab, or placebo.
Disclosures: This study was sponsored by AbbVie. Five authors declared being employees of or owning stock or stock options in AbbVie. Several authors declared being consultants, speakers, or advisors of or having other ties with various sources, including AbbVie.
Source: Silverberg JI et al. Aggregate response benefit in skin clearance and itch reduction with upadacitinib or dupilumab in patients with moderate-to-severe atopic dermatitis. Dermatitis. 2023 (Dec 18). doi: 10.1089/derm.2023.0153
Key clinical point: The overall improvement in skin clearance and itch reduction suggested a preference for 30 mg upadacitinib over dupilumab and that for 15 mg or 30 mg upadacitinib over placebo in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 24, the aggregate response benefit for skin clearance and itch, respectively, was 32.5% and 25.8% higher with 30 mg upadacitinib vs dupilumab. The benefit favored upadacitinib over dupilumab as early as week 4. Moreover, 15 and 30 mg upadacitinib showed similar benefits over placebo.
Study details: This post hoc analysis of the data from phase 3 studies (Heads Up, Measure Up 1, and Measure Up 2) included 2356 patients with moderate-to-severe AD who received upadacitinib, dupilumab, or placebo.
Disclosures: This study was sponsored by AbbVie. Five authors declared being employees of or owning stock or stock options in AbbVie. Several authors declared being consultants, speakers, or advisors of or having other ties with various sources, including AbbVie.
Source: Silverberg JI et al. Aggregate response benefit in skin clearance and itch reduction with upadacitinib or dupilumab in patients with moderate-to-severe atopic dermatitis. Dermatitis. 2023 (Dec 18). doi: 10.1089/derm.2023.0153