User login
Tender Subcutaneous Nodule in a Prepubescent Boy
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
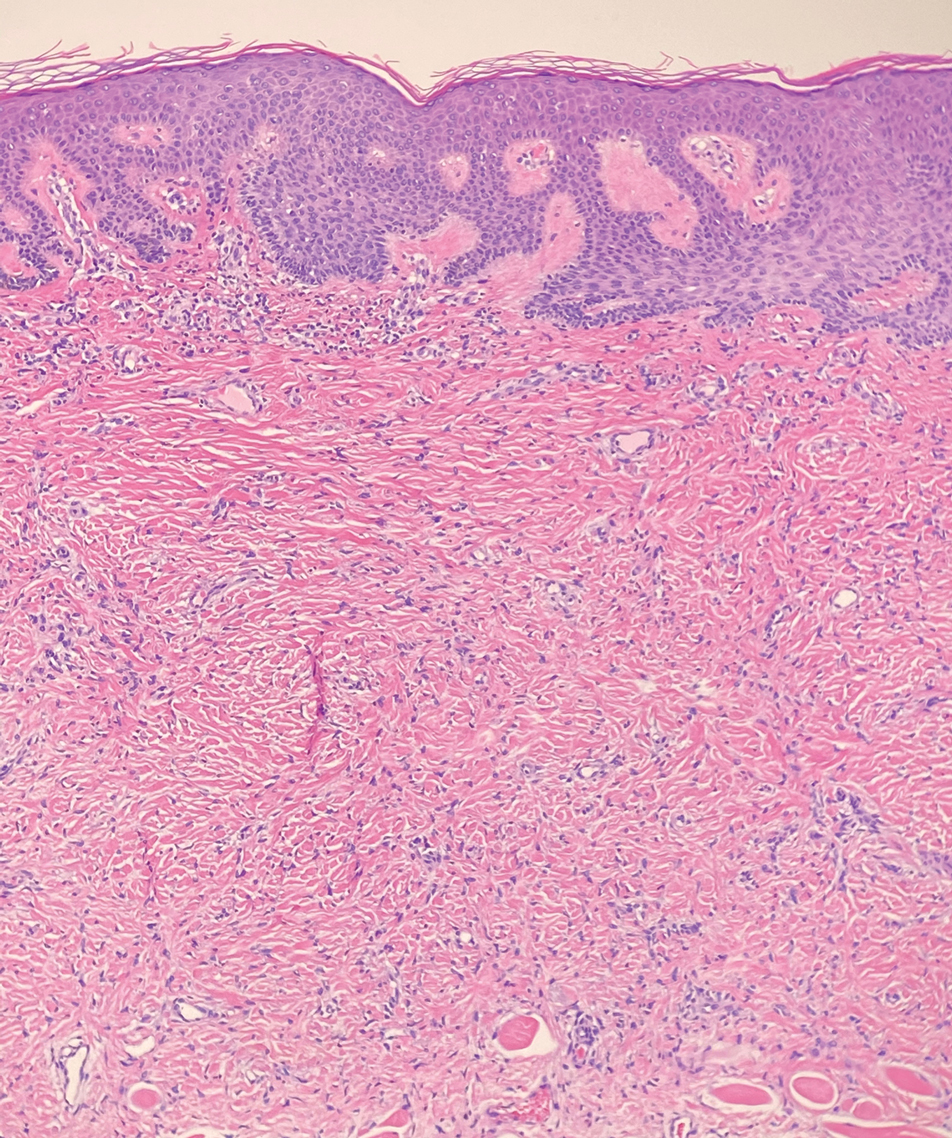
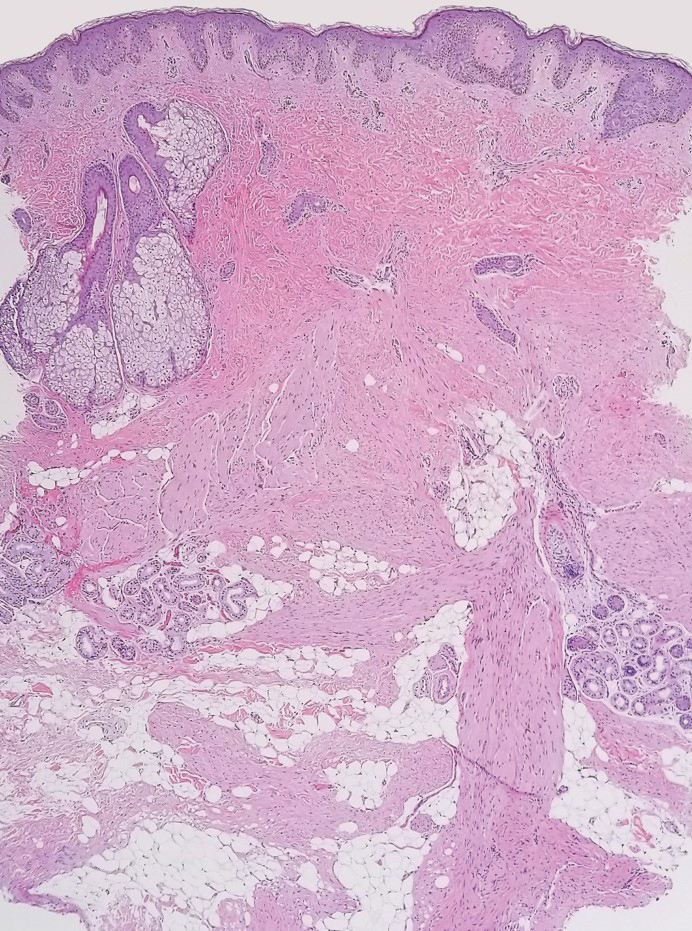
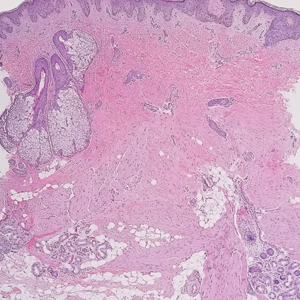
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6
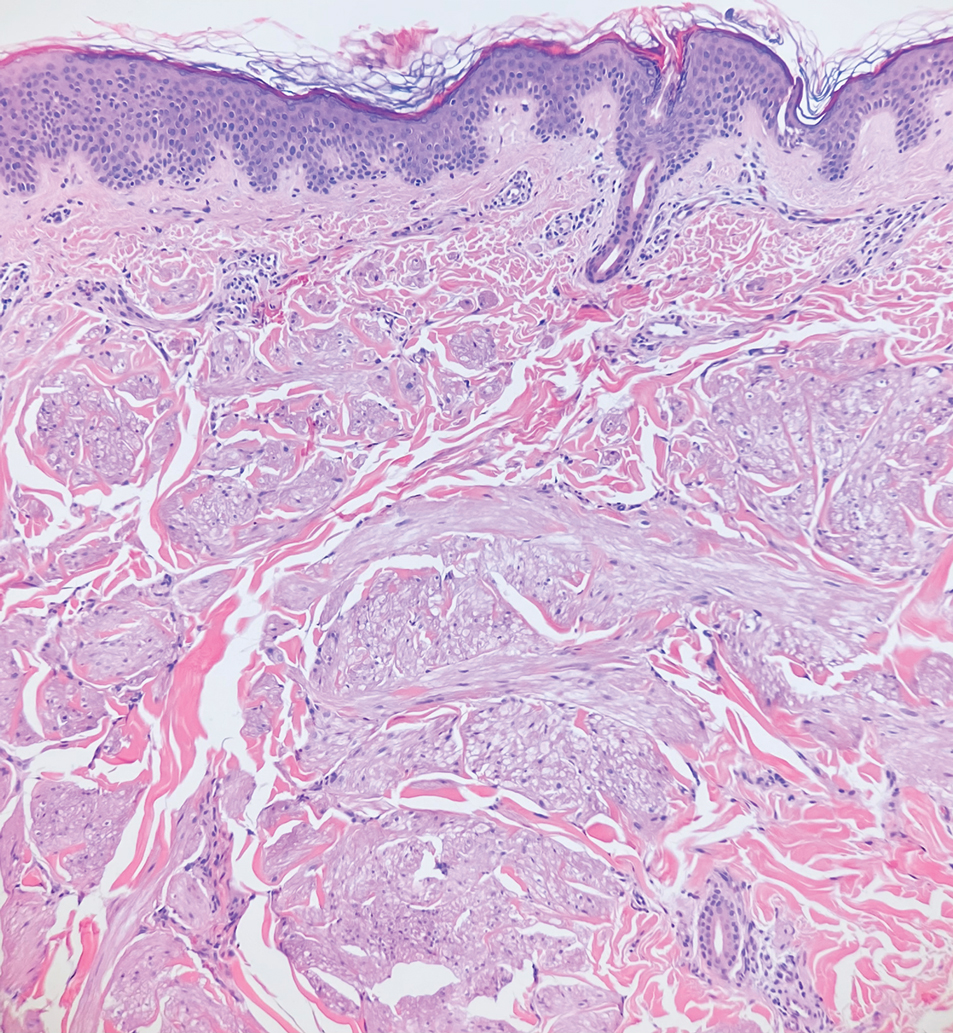
Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7
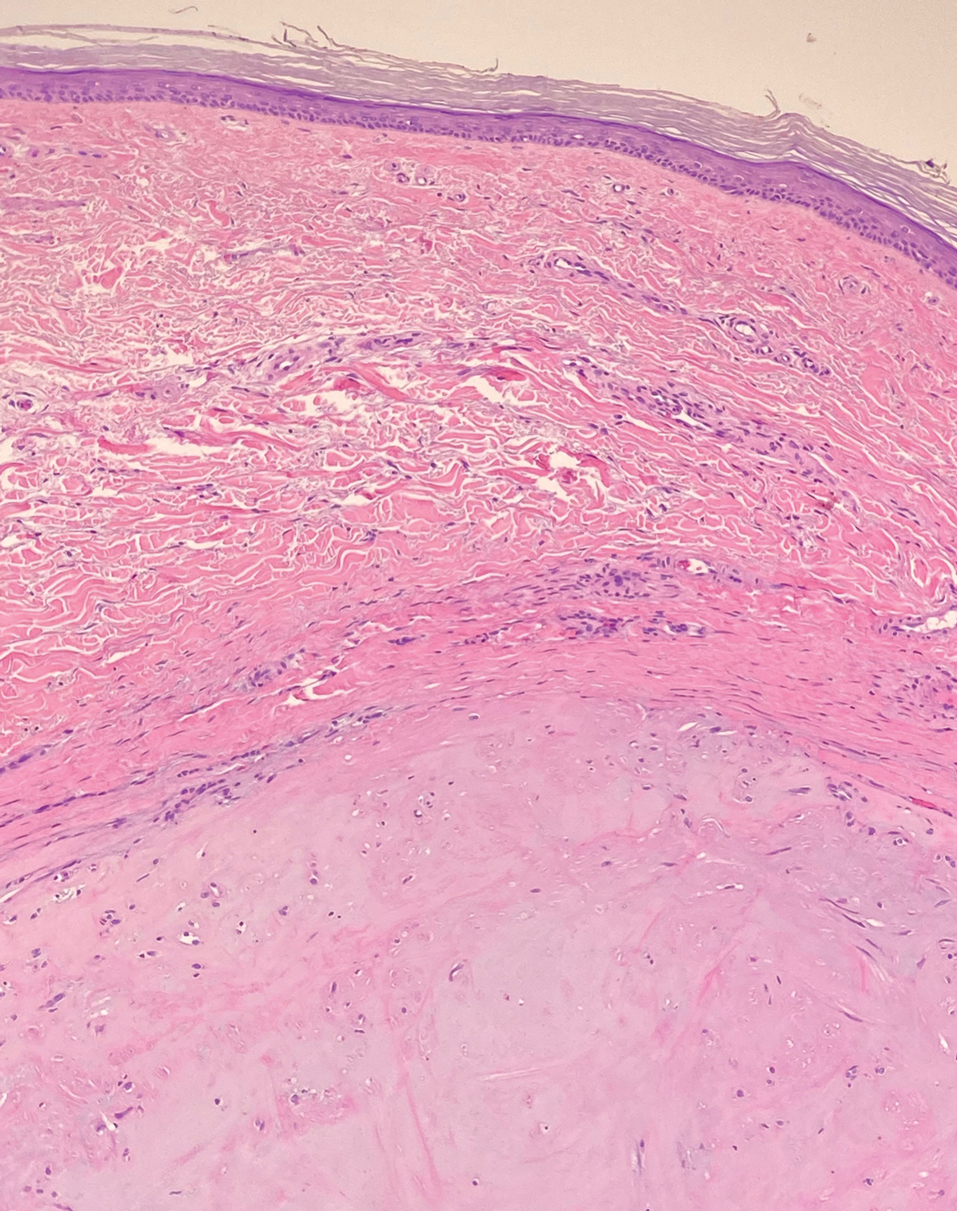
Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9
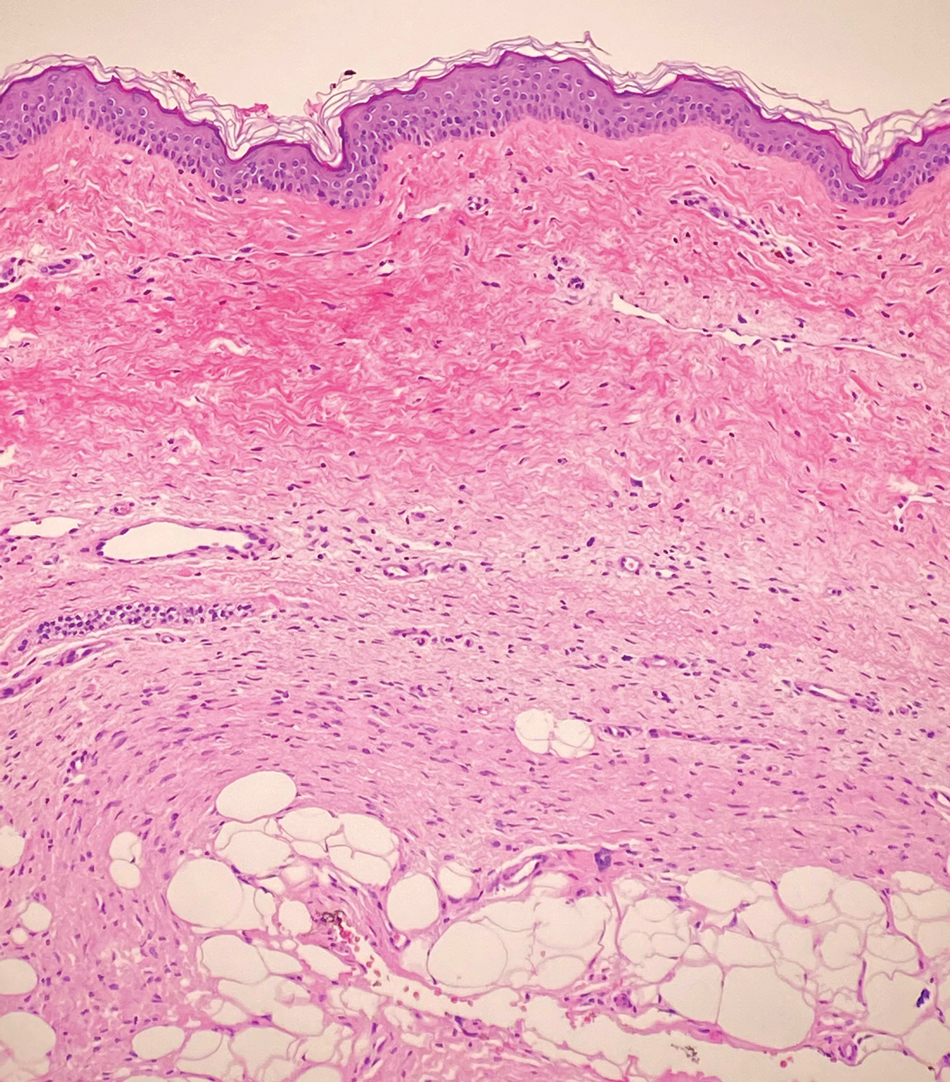
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6
Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7
Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6
Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7
Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
A 12-year-old boy with olive skin presented with a tender subcutaneous nodule on the back of 6 months’ duration. He reported the lesion initially grew rapidly with increasing pain for approximately 3 months with subsequent stabilization in size and modest resolution of his symptoms. Physical examination revealed a solitary, 15-mm, ill-defined, indurated, tender, subcutaneous nodule with subtle overlying hyperpigmentation on the left side of the upper back. Hematoxylin and eosin staining of a 4-mm punch biopsy revealed a nonencapsulated mass of monomorphic eosinophilic spindle cells organized into fascicles arranged predominantly parallel to the skin surface. The mass extended from the mid reticular dermis to the upper subcutis, sparing adnexal structures.
Rapid Screening of Invasive Fungal Infections in the Hospital Setting Using the (1,3)-β-D-glucan Assay
Practice Gap
Invasive fungal infections are a leading cause of morbidity and mortality among neutropenic, immunocompromised, and critically ill patients. Candida species are the most common cause of fungemia, with portals of entry into the bloodstream including the gastrointestinal tract, contaminated intravascular catheters, and localized foci of infection.1 Diagnosis of invasive candidiasis remains challenging due to an absence of specific clinical signs and symptoms, varying from a mild fever that is unresponsive to antibiotics to florid sepsis. When present, clinical clues may include chorioretinitis; muscle abscesses; and skin eruptions, characteristically with Candida tropicalis. Cutaneous manifestations of disseminated Candida infections appear in only 13% of affected patients.1 The lesions typically present as 5- to 10-mm pink dermal papules or painless pustules on an erythematous base and may be singular, localized, or diffuse in distribution. Body regions normally involved are the trunk, arms, and legs, rarely the head and neck.1 Cutaneous lesions often develop at a time when patients are febrile, are not responding to antibiotics, and are clinically deteriorating.
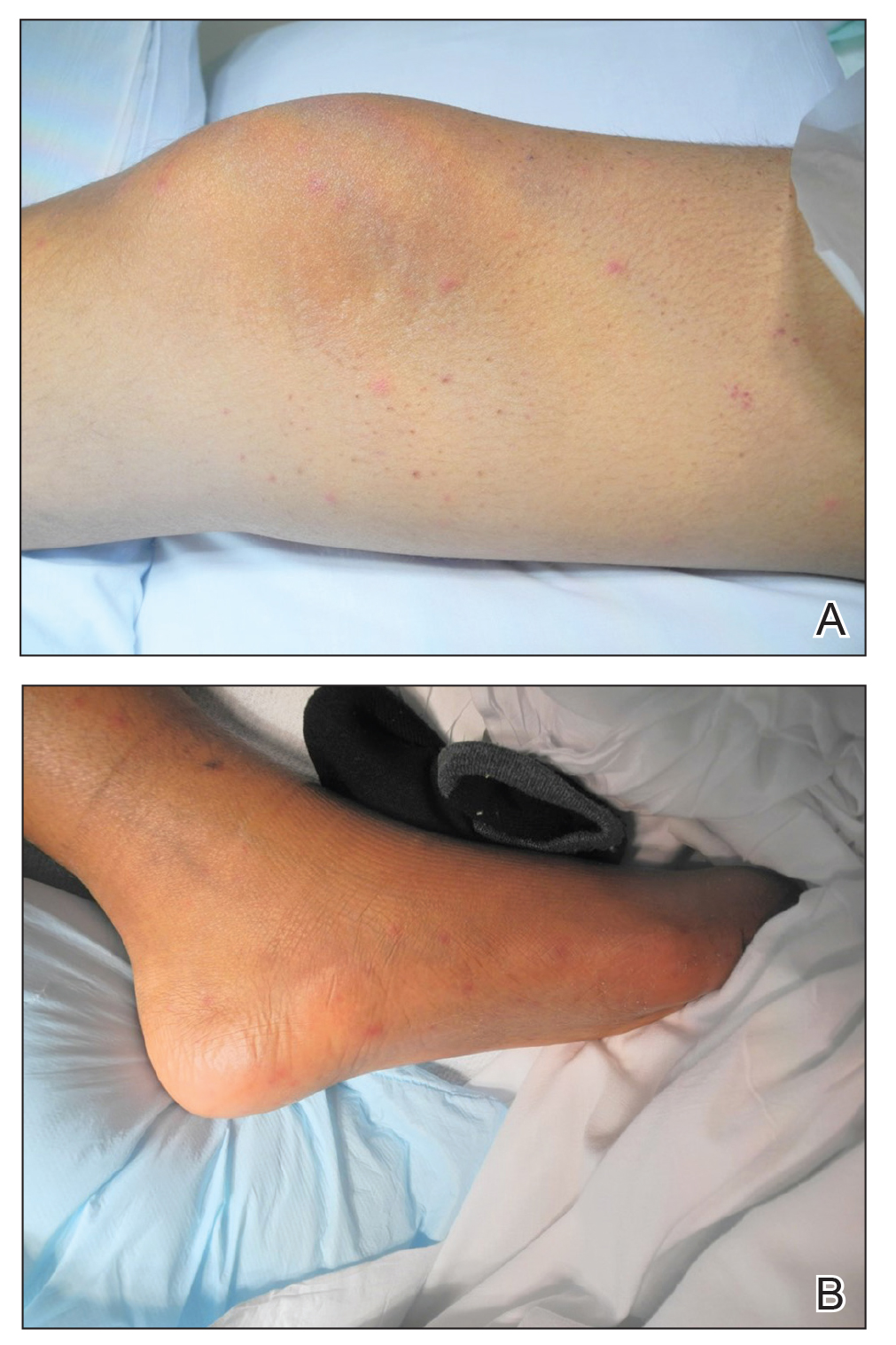
A 15-year-old adolescent boy with pre–B-cell acute lymphoblastic leukemia was admitted with febrile neutropenia for presumed septic shock secondary to an unknown infectious etiology. The patient was started on broad-spectrum intravenous antibiotics, and blood cultures were obtained. On the second day of hospitalization, he developed approximately 10 to 15 discrete, 3- to 6-mm, pink to violaceous papules scattered on the chest and arms (Figure 1). Over several hours, the number of lesions increased to more than 50 with involvement of the legs (Figure 2). A punch biopsy of lesional skin from the left dorsal wrist demonstrated a circumscribed abscess of yeast in the papillary dermis, which was highlighted by periodic acid–Schiff staining with minimal associated inflammation (Figure 3). Blood and tissue cultures persistently grew C tropicalis. The patient was started on intravenous liposomal amphotericin B but died on day 5 of hospitalization after developing endocarditis.
Early and reliable diagnosis of Candida species fungemia is of critical importance to successful treatment, particularly with the emergence of multidrug-resistant strains such as Candida auris.2 In patients with apparent cutaneous manifestations, a lesional punch biopsy for culture and histopathologic evaluation is recommended in addition to blood culture; however, organisms may or may not be present in large numbers, and they may be difficult to identify on routine hematoxylin and eosin–stained tissue sections. To enhance the likelihood of highlighting the fungus within the sample, the pathologist must be made aware of the presumptive diagnosis of disseminated candidiasis so that special techniques can be utilized, such as periodic acid–Schiff stain.
Although positive blood culture is the gold standard for candidemia diagnosis, only 30% to 50% of patients with disseminated candidiasis had positive blood cultures at autopsy.1 Another study showed the sensitivity of blood culture for the detection of invasive fungal infection to be as low as 8.3%.3 In cases with positive blood cultures, the median time to positivity is 2 to 3 days, but it can take as long as 8 days, thus limiting its clinical utility in acutely ill patients.4 Given the low sensitivity and prolonged time required for culture growth of most fungal organisms, novel assays for rapid, non–culture-based diagnosis of systemic fungal infections hold substantial clinical promise moving forward.
The Technique
One of the more promising non–culture-based fungal diagnostic methodologies is an antigen assay based on the detection of serum (1,3)-β-D-glucan (BDG), a major cell wall constituent of most pathogenic fungi. This assay is not specific for Candida species and can be positive for Aspergillosis species, Fusarium species, Coccidioides immitis, Histoplasma capsulatum, and Pneumocystis jirovecii pneumonia, among others; therefore, it functions as a general biomarker for fungi in the bloodstream.4,5 (1,3)-β-D-glucan assay can be useful as an adjunct for blood cultures and punch biopsy, especially when cultures are negative or the results remain outstanding. The results of the BDG assay are available in less than 24 hours at minimal cost, and the test is approved by the US Food and Drug Administration for use as an aid in invasive fungal disease diagnosis. In a meta-analysis of 11 studies, BDG sensitivity was 75%.4 In a study based on autopsy cases from 6 years, BDG specificity was 98.4% with positive and negative predictive values of 86.7% and 97.1%, respectively.3 Optimal results were achieved when 2 consecutive tests were positive.4 The serum assay output is based on spectrophotometer readings, which are converted to BDG concentrations (negative, <60 pg/mL; indeterminate, 60–79 pg/mL; positive ≥80 pg/mL).5 Although we cannot be certain, utilizing the BDG assay in our patient may have led to earlier treatment and a better outcome.
A disadvantage of the BDG assay is the potential for false-positive results, which have been reported in lung transplant recipients with respiratory mold colonization and patients with other systemic bacterial infections.4 False-positive results also have been associated with use of ampicillin-clavulanate and piperacillin-tazobactam antibiotics and human blood products, hemodialysis, and severe mucositis, thus reaffirming the importance of judicious interpretation of BDG assay results by the clinician.4,6 There also is a potential for false-negative results, as the BDG assay does not detect certain fungal species such as Cryptococcus species and Blastomyces dermatitidis, which produce very low levels of BDG, or zygomycetes (Absidia, Mucor, and Rizopus species), which are not known to produce BDG.6
Practice Implications
In the setting of invasive fungal infections, a high degree of clinical suspicion is paramount due to the often subtle nature of cutaneous manifestations. A positive BDG assay can be used to identify high-risk patients for empiric antifungal therapy, prompting early intervention and improved outcomes in these acutely ill patients. The BDG assay’s excellent negative predictive value is useful in ruling out invasive Candida infections and may justify stopping unnecessary empiric antifungal therapy.4 For the dermatology hospitalist, incorporation of the BDG assay as a noninvasive screening tool may allow for more rapid initiation of appropriate antifungal therapy while awaiting confirmatory skin biopsy or culture results in disseminated candidemia and other invasive fungal infections.
- Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31-43.
- Candida auris. Centers for Disease Control and Prevention website. https://www.cdc.gov/fungal/candida-auris/. Updated May 15, 2020. Accessed July 10, 2020.
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1,3)-β-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864-1870.
- Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284-1292.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational development and application of (1→3)-β-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses [published online May 24, 2017]. Int J Mol Sci. doi:10.3390/ijms18061124.
- Beta-D glucan assay. MiraVista Diagnostics website. https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/beta-d-glucan-test/. Accessed June 5, 2020.
Practice Gap
Invasive fungal infections are a leading cause of morbidity and mortality among neutropenic, immunocompromised, and critically ill patients. Candida species are the most common cause of fungemia, with portals of entry into the bloodstream including the gastrointestinal tract, contaminated intravascular catheters, and localized foci of infection.1 Diagnosis of invasive candidiasis remains challenging due to an absence of specific clinical signs and symptoms, varying from a mild fever that is unresponsive to antibiotics to florid sepsis. When present, clinical clues may include chorioretinitis; muscle abscesses; and skin eruptions, characteristically with Candida tropicalis. Cutaneous manifestations of disseminated Candida infections appear in only 13% of affected patients.1 The lesions typically present as 5- to 10-mm pink dermal papules or painless pustules on an erythematous base and may be singular, localized, or diffuse in distribution. Body regions normally involved are the trunk, arms, and legs, rarely the head and neck.1 Cutaneous lesions often develop at a time when patients are febrile, are not responding to antibiotics, and are clinically deteriorating.
A 15-year-old adolescent boy with pre–B-cell acute lymphoblastic leukemia was admitted with febrile neutropenia for presumed septic shock secondary to an unknown infectious etiology. The patient was started on broad-spectrum intravenous antibiotics, and blood cultures were obtained. On the second day of hospitalization, he developed approximately 10 to 15 discrete, 3- to 6-mm, pink to violaceous papules scattered on the chest and arms (Figure 1). Over several hours, the number of lesions increased to more than 50 with involvement of the legs (Figure 2). A punch biopsy of lesional skin from the left dorsal wrist demonstrated a circumscribed abscess of yeast in the papillary dermis, which was highlighted by periodic acid–Schiff staining with minimal associated inflammation (Figure 3). Blood and tissue cultures persistently grew C tropicalis. The patient was started on intravenous liposomal amphotericin B but died on day 5 of hospitalization after developing endocarditis.



Early and reliable diagnosis of Candida species fungemia is of critical importance to successful treatment, particularly with the emergence of multidrug-resistant strains such as Candida auris.2 In patients with apparent cutaneous manifestations, a lesional punch biopsy for culture and histopathologic evaluation is recommended in addition to blood culture; however, organisms may or may not be present in large numbers, and they may be difficult to identify on routine hematoxylin and eosin–stained tissue sections. To enhance the likelihood of highlighting the fungus within the sample, the pathologist must be made aware of the presumptive diagnosis of disseminated candidiasis so that special techniques can be utilized, such as periodic acid–Schiff stain.
Although positive blood culture is the gold standard for candidemia diagnosis, only 30% to 50% of patients with disseminated candidiasis had positive blood cultures at autopsy.1 Another study showed the sensitivity of blood culture for the detection of invasive fungal infection to be as low as 8.3%.3 In cases with positive blood cultures, the median time to positivity is 2 to 3 days, but it can take as long as 8 days, thus limiting its clinical utility in acutely ill patients.4 Given the low sensitivity and prolonged time required for culture growth of most fungal organisms, novel assays for rapid, non–culture-based diagnosis of systemic fungal infections hold substantial clinical promise moving forward.
The Technique
One of the more promising non–culture-based fungal diagnostic methodologies is an antigen assay based on the detection of serum (1,3)-β-D-glucan (BDG), a major cell wall constituent of most pathogenic fungi. This assay is not specific for Candida species and can be positive for Aspergillosis species, Fusarium species, Coccidioides immitis, Histoplasma capsulatum, and Pneumocystis jirovecii pneumonia, among others; therefore, it functions as a general biomarker for fungi in the bloodstream.4,5 (1,3)-β-D-glucan assay can be useful as an adjunct for blood cultures and punch biopsy, especially when cultures are negative or the results remain outstanding. The results of the BDG assay are available in less than 24 hours at minimal cost, and the test is approved by the US Food and Drug Administration for use as an aid in invasive fungal disease diagnosis. In a meta-analysis of 11 studies, BDG sensitivity was 75%.4 In a study based on autopsy cases from 6 years, BDG specificity was 98.4% with positive and negative predictive values of 86.7% and 97.1%, respectively.3 Optimal results were achieved when 2 consecutive tests were positive.4 The serum assay output is based on spectrophotometer readings, which are converted to BDG concentrations (negative, <60 pg/mL; indeterminate, 60–79 pg/mL; positive ≥80 pg/mL).5 Although we cannot be certain, utilizing the BDG assay in our patient may have led to earlier treatment and a better outcome.
A disadvantage of the BDG assay is the potential for false-positive results, which have been reported in lung transplant recipients with respiratory mold colonization and patients with other systemic bacterial infections.4 False-positive results also have been associated with use of ampicillin-clavulanate and piperacillin-tazobactam antibiotics and human blood products, hemodialysis, and severe mucositis, thus reaffirming the importance of judicious interpretation of BDG assay results by the clinician.4,6 There also is a potential for false-negative results, as the BDG assay does not detect certain fungal species such as Cryptococcus species and Blastomyces dermatitidis, which produce very low levels of BDG, or zygomycetes (Absidia, Mucor, and Rizopus species), which are not known to produce BDG.6
Practice Implications
In the setting of invasive fungal infections, a high degree of clinical suspicion is paramount due to the often subtle nature of cutaneous manifestations. A positive BDG assay can be used to identify high-risk patients for empiric antifungal therapy, prompting early intervention and improved outcomes in these acutely ill patients. The BDG assay’s excellent negative predictive value is useful in ruling out invasive Candida infections and may justify stopping unnecessary empiric antifungal therapy.4 For the dermatology hospitalist, incorporation of the BDG assay as a noninvasive screening tool may allow for more rapid initiation of appropriate antifungal therapy while awaiting confirmatory skin biopsy or culture results in disseminated candidemia and other invasive fungal infections.
Practice Gap
Invasive fungal infections are a leading cause of morbidity and mortality among neutropenic, immunocompromised, and critically ill patients. Candida species are the most common cause of fungemia, with portals of entry into the bloodstream including the gastrointestinal tract, contaminated intravascular catheters, and localized foci of infection.1 Diagnosis of invasive candidiasis remains challenging due to an absence of specific clinical signs and symptoms, varying from a mild fever that is unresponsive to antibiotics to florid sepsis. When present, clinical clues may include chorioretinitis; muscle abscesses; and skin eruptions, characteristically with Candida tropicalis. Cutaneous manifestations of disseminated Candida infections appear in only 13% of affected patients.1 The lesions typically present as 5- to 10-mm pink dermal papules or painless pustules on an erythematous base and may be singular, localized, or diffuse in distribution. Body regions normally involved are the trunk, arms, and legs, rarely the head and neck.1 Cutaneous lesions often develop at a time when patients are febrile, are not responding to antibiotics, and are clinically deteriorating.
A 15-year-old adolescent boy with pre–B-cell acute lymphoblastic leukemia was admitted with febrile neutropenia for presumed septic shock secondary to an unknown infectious etiology. The patient was started on broad-spectrum intravenous antibiotics, and blood cultures were obtained. On the second day of hospitalization, he developed approximately 10 to 15 discrete, 3- to 6-mm, pink to violaceous papules scattered on the chest and arms (Figure 1). Over several hours, the number of lesions increased to more than 50 with involvement of the legs (Figure 2). A punch biopsy of lesional skin from the left dorsal wrist demonstrated a circumscribed abscess of yeast in the papillary dermis, which was highlighted by periodic acid–Schiff staining with minimal associated inflammation (Figure 3). Blood and tissue cultures persistently grew C tropicalis. The patient was started on intravenous liposomal amphotericin B but died on day 5 of hospitalization after developing endocarditis.



Early and reliable diagnosis of Candida species fungemia is of critical importance to successful treatment, particularly with the emergence of multidrug-resistant strains such as Candida auris.2 In patients with apparent cutaneous manifestations, a lesional punch biopsy for culture and histopathologic evaluation is recommended in addition to blood culture; however, organisms may or may not be present in large numbers, and they may be difficult to identify on routine hematoxylin and eosin–stained tissue sections. To enhance the likelihood of highlighting the fungus within the sample, the pathologist must be made aware of the presumptive diagnosis of disseminated candidiasis so that special techniques can be utilized, such as periodic acid–Schiff stain.
Although positive blood culture is the gold standard for candidemia diagnosis, only 30% to 50% of patients with disseminated candidiasis had positive blood cultures at autopsy.1 Another study showed the sensitivity of blood culture for the detection of invasive fungal infection to be as low as 8.3%.3 In cases with positive blood cultures, the median time to positivity is 2 to 3 days, but it can take as long as 8 days, thus limiting its clinical utility in acutely ill patients.4 Given the low sensitivity and prolonged time required for culture growth of most fungal organisms, novel assays for rapid, non–culture-based diagnosis of systemic fungal infections hold substantial clinical promise moving forward.
The Technique
One of the more promising non–culture-based fungal diagnostic methodologies is an antigen assay based on the detection of serum (1,3)-β-D-glucan (BDG), a major cell wall constituent of most pathogenic fungi. This assay is not specific for Candida species and can be positive for Aspergillosis species, Fusarium species, Coccidioides immitis, Histoplasma capsulatum, and Pneumocystis jirovecii pneumonia, among others; therefore, it functions as a general biomarker for fungi in the bloodstream.4,5 (1,3)-β-D-glucan assay can be useful as an adjunct for blood cultures and punch biopsy, especially when cultures are negative or the results remain outstanding. The results of the BDG assay are available in less than 24 hours at minimal cost, and the test is approved by the US Food and Drug Administration for use as an aid in invasive fungal disease diagnosis. In a meta-analysis of 11 studies, BDG sensitivity was 75%.4 In a study based on autopsy cases from 6 years, BDG specificity was 98.4% with positive and negative predictive values of 86.7% and 97.1%, respectively.3 Optimal results were achieved when 2 consecutive tests were positive.4 The serum assay output is based on spectrophotometer readings, which are converted to BDG concentrations (negative, <60 pg/mL; indeterminate, 60–79 pg/mL; positive ≥80 pg/mL).5 Although we cannot be certain, utilizing the BDG assay in our patient may have led to earlier treatment and a better outcome.
A disadvantage of the BDG assay is the potential for false-positive results, which have been reported in lung transplant recipients with respiratory mold colonization and patients with other systemic bacterial infections.4 False-positive results also have been associated with use of ampicillin-clavulanate and piperacillin-tazobactam antibiotics and human blood products, hemodialysis, and severe mucositis, thus reaffirming the importance of judicious interpretation of BDG assay results by the clinician.4,6 There also is a potential for false-negative results, as the BDG assay does not detect certain fungal species such as Cryptococcus species and Blastomyces dermatitidis, which produce very low levels of BDG, or zygomycetes (Absidia, Mucor, and Rizopus species), which are not known to produce BDG.6
Practice Implications
In the setting of invasive fungal infections, a high degree of clinical suspicion is paramount due to the often subtle nature of cutaneous manifestations. A positive BDG assay can be used to identify high-risk patients for empiric antifungal therapy, prompting early intervention and improved outcomes in these acutely ill patients. The BDG assay’s excellent negative predictive value is useful in ruling out invasive Candida infections and may justify stopping unnecessary empiric antifungal therapy.4 For the dermatology hospitalist, incorporation of the BDG assay as a noninvasive screening tool may allow for more rapid initiation of appropriate antifungal therapy while awaiting confirmatory skin biopsy or culture results in disseminated candidemia and other invasive fungal infections.
- Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31-43.
- Candida auris. Centers for Disease Control and Prevention website. https://www.cdc.gov/fungal/candida-auris/. Updated May 15, 2020. Accessed July 10, 2020.
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1,3)-β-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864-1870.
- Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284-1292.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational development and application of (1→3)-β-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses [published online May 24, 2017]. Int J Mol Sci. doi:10.3390/ijms18061124.
- Beta-D glucan assay. MiraVista Diagnostics website. https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/beta-d-glucan-test/. Accessed June 5, 2020.
- Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31-43.
- Candida auris. Centers for Disease Control and Prevention website. https://www.cdc.gov/fungal/candida-auris/. Updated May 15, 2020. Accessed July 10, 2020.
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1,3)-β-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864-1870.
- Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284-1292.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational development and application of (1→3)-β-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses [published online May 24, 2017]. Int J Mol Sci. doi:10.3390/ijms18061124.
- Beta-D glucan assay. MiraVista Diagnostics website. https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/beta-d-glucan-test/. Accessed June 5, 2020.
Streaked Discoloration on the Upper Body
The Diagnosis: Bleomycin-Induced Flagellate Hyperpigmentation
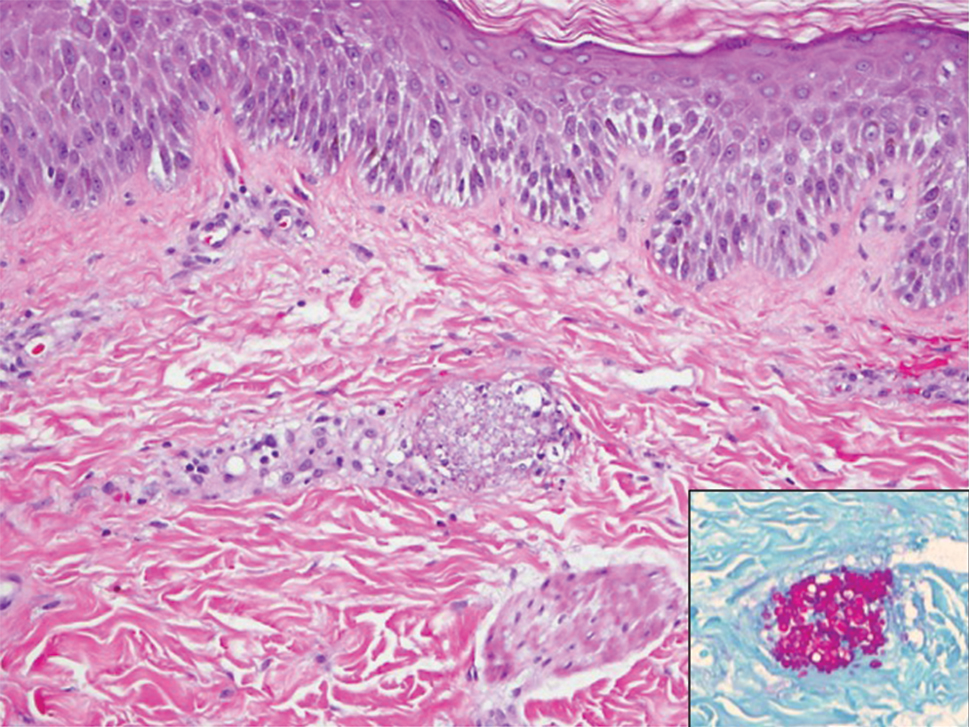
Histopathology of the affected skin demonstrated a slight increase in collagen bundle thickness, a chronic dermal perivascular inflammation, and associated pigment incontinence with dermal melanophages compared to unaffected skin (Figure). CD34 was faintly decreased, and dermal mucin increased in affected skin. This postinflammatory pigmentary alteration with subtle dermal sclerosis had persisted unchanged for more than 5 years after cessation of bleomycin therapy. Topical hydroquinone, physical blocker photoprotection, and laser modalities such as the Q-switched alexandrite (755-nm)/Nd:YAG (1064-nm) and ablative CO2 resurfacing lasers were attempted with minimal overall impact on cosmesis.
Bleomycin is a chemotherapeutic antibiotic that has been commonly used to treat Hodgkin lymphoma, germ cell tumors, and recurrent malignant pleural effusions.1 The drug is inactivated in most tissues by the enzyme bleomycin hydrolase. This enzyme is not present in skin and lung tissue; as a result, these organs are the most common sites of bleomycin toxicity.1 There are a variety of cutaneous effects associated with bleomycin including alopecia, hyperpigmentation, acral erythema, Raynaud phenomenon, and nail dystrophy.2 Flagellate hyperpigmentation is a less common cutaneous toxicity. It is an unusual eruption that appears as whiplike linear streaks on the upper chest and back, limbs, and flanks.3 This cutaneous manifestation was once thought to be specific to bleomycin use; however, it also has been described in dermatomyositis, adult-onset Still disease, and after the ingestion of uncooked or undercooked shiitake mushrooms.4 Flagellate hyperpigmentation also was once thought to be dose dependent; however, it has been described in even very small doses.5 The eruption has been described as independent of the route of drug administration, appearing with intravenous, subcutaneous, and intramuscular bleomycin.2 The association of bleomycin and flagellate hyperpigmentation has been reported since 1970; however, it is less commonly seen in clinical practice with the declining use of bleomycin.1
The exact mechanism for the hyperpigmentation is unknown. It has been proposed that the linear lesions are related to areas of pruritus and subsequent excoriations.1 Dermatographism may be present to a limited extent, but it is unlikely to be a chief cause of flagellate hyperpigmentation, as linear streaks have been reported in the absence of trauma. It also has been proposed that bleomycin has a direct toxic effect on the melanocytes, which stimulates increased melanin secretion.2 The hyperpigmentation also may be due to pigmentary incontinence secondary to inflammation.5 Histopathologic findings usually are varied and nonspecific.2 There may be a deep perivascular lymphocytic infiltrate, which is nonspecific but can be associated with drug-induced pathology.4 Bleomycin also is used to induce localized scleroderma in mouse-model research6 and has been reported to cause localized scleroderma at an infusion site or after an intralesional injection,7,8 which is not typically reported in flagellate erythema, but bleomycin's sclerosing effects may have played a role in the visible and sclerosing atrophy noted in our patient. Yamamoto et al9 reported a similar case of dermal sclerosis induced by bleomycin.
Flagellate hyperpigmentation typically lasts for up to 6 months.3 Patients with cutaneous manifestations from bleomycin therapy usually respond to steroid therapy and discontinuation of the drug. Bleomycin re-exposure should be avoided, as it may cause extension or widespread recurrence of flagellate hyperpigmentation.3 Postinflammatory pigment alteration may persist in patients with darker skin types and in patients with dramatic inciting inflammation.
Atrophoderma of Pasini and Pierini is a form of dermal atrophy that presents with 1 or more sharply demarcated depressed patches. There is some debate whether it is a distinct entity or a primary atrophic morphea.10 Linear atrophoderma of Moulin has a similar morphology with hyperpigmented depressions and "cliff-drop" borders, but these lesions follow the lines of Blaschko.11 Linear morphea initially can present as a linear erythematous streak but more commonly appears as a plaque-type morphea lesion that forms a scarlike band.12 Erythema dyschromicum perstans is an ashy dermatosis characterized by gray or blue-brown macules seen in Fitzpatrick skin types III through V and typically is chronic and progressive.13
- Lee HY, Lim KH, Ryu Y, et al. Bleomycininduced flagellate erythema: a case report and review of the literature. Oncol Lett. 2014;8:933-935.
- Simpson RC, Da Forno P, Nagarajan C, et al. A pruritic rash in a patient with Hodgkin lymphoma. Clin Exp Dermatol. 2011;36:680-682.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213-215.
- Lu CC, Lu YY, Wang QR, et al. Bleomycin-induced flagellate erythema. Balkan Med J. 2014;31:189-190.
- Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337-339.
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333-344.
- Kim KH, Yoon TJ, Oh CW, et al. A case of bleomycin-induced scleroderma. J Korean Med Sci. 1996;11:454-456.
- Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992;19:294-296.
- Yamamoto T, Yokozeki H, Nishioka K. Dermal sclerosis in the lesional skin of 'flagellate' erythema (scratch dermatitis) induced by bleomycin. Dermatology. 1998;197:399-400.
- Kencka D, Blaszczyk M, Jablońska S. Atrophoderma Pasini-Pierini is a primary atrophic abortive morphea. Dermatology. 1995;190:203-206.
- Moulin G, Hill MP, Guillaud V, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol. 1992;119:729-736.
- Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
- Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatosis--a critical review of literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542-544.
The Diagnosis: Bleomycin-Induced Flagellate Hyperpigmentation
Histopathology of the affected skin demonstrated a slight increase in collagen bundle thickness, a chronic dermal perivascular inflammation, and associated pigment incontinence with dermal melanophages compared to unaffected skin (Figure). CD34 was faintly decreased, and dermal mucin increased in affected skin. This postinflammatory pigmentary alteration with subtle dermal sclerosis had persisted unchanged for more than 5 years after cessation of bleomycin therapy. Topical hydroquinone, physical blocker photoprotection, and laser modalities such as the Q-switched alexandrite (755-nm)/Nd:YAG (1064-nm) and ablative CO2 resurfacing lasers were attempted with minimal overall impact on cosmesis.
Bleomycin is a chemotherapeutic antibiotic that has been commonly used to treat Hodgkin lymphoma, germ cell tumors, and recurrent malignant pleural effusions.1 The drug is inactivated in most tissues by the enzyme bleomycin hydrolase. This enzyme is not present in skin and lung tissue; as a result, these organs are the most common sites of bleomycin toxicity.1 There are a variety of cutaneous effects associated with bleomycin including alopecia, hyperpigmentation, acral erythema, Raynaud phenomenon, and nail dystrophy.2 Flagellate hyperpigmentation is a less common cutaneous toxicity. It is an unusual eruption that appears as whiplike linear streaks on the upper chest and back, limbs, and flanks.3 This cutaneous manifestation was once thought to be specific to bleomycin use; however, it also has been described in dermatomyositis, adult-onset Still disease, and after the ingestion of uncooked or undercooked shiitake mushrooms.4 Flagellate hyperpigmentation also was once thought to be dose dependent; however, it has been described in even very small doses.5 The eruption has been described as independent of the route of drug administration, appearing with intravenous, subcutaneous, and intramuscular bleomycin.2 The association of bleomycin and flagellate hyperpigmentation has been reported since 1970; however, it is less commonly seen in clinical practice with the declining use of bleomycin.1
The exact mechanism for the hyperpigmentation is unknown. It has been proposed that the linear lesions are related to areas of pruritus and subsequent excoriations.1 Dermatographism may be present to a limited extent, but it is unlikely to be a chief cause of flagellate hyperpigmentation, as linear streaks have been reported in the absence of trauma. It also has been proposed that bleomycin has a direct toxic effect on the melanocytes, which stimulates increased melanin secretion.2 The hyperpigmentation also may be due to pigmentary incontinence secondary to inflammation.5 Histopathologic findings usually are varied and nonspecific.2 There may be a deep perivascular lymphocytic infiltrate, which is nonspecific but can be associated with drug-induced pathology.4 Bleomycin also is used to induce localized scleroderma in mouse-model research6 and has been reported to cause localized scleroderma at an infusion site or after an intralesional injection,7,8 which is not typically reported in flagellate erythema, but bleomycin's sclerosing effects may have played a role in the visible and sclerosing atrophy noted in our patient. Yamamoto et al9 reported a similar case of dermal sclerosis induced by bleomycin.
Flagellate hyperpigmentation typically lasts for up to 6 months.3 Patients with cutaneous manifestations from bleomycin therapy usually respond to steroid therapy and discontinuation of the drug. Bleomycin re-exposure should be avoided, as it may cause extension or widespread recurrence of flagellate hyperpigmentation.3 Postinflammatory pigment alteration may persist in patients with darker skin types and in patients with dramatic inciting inflammation.
Atrophoderma of Pasini and Pierini is a form of dermal atrophy that presents with 1 or more sharply demarcated depressed patches. There is some debate whether it is a distinct entity or a primary atrophic morphea.10 Linear atrophoderma of Moulin has a similar morphology with hyperpigmented depressions and "cliff-drop" borders, but these lesions follow the lines of Blaschko.11 Linear morphea initially can present as a linear erythematous streak but more commonly appears as a plaque-type morphea lesion that forms a scarlike band.12 Erythema dyschromicum perstans is an ashy dermatosis characterized by gray or blue-brown macules seen in Fitzpatrick skin types III through V and typically is chronic and progressive.13
The Diagnosis: Bleomycin-Induced Flagellate Hyperpigmentation
Histopathology of the affected skin demonstrated a slight increase in collagen bundle thickness, a chronic dermal perivascular inflammation, and associated pigment incontinence with dermal melanophages compared to unaffected skin (Figure). CD34 was faintly decreased, and dermal mucin increased in affected skin. This postinflammatory pigmentary alteration with subtle dermal sclerosis had persisted unchanged for more than 5 years after cessation of bleomycin therapy. Topical hydroquinone, physical blocker photoprotection, and laser modalities such as the Q-switched alexandrite (755-nm)/Nd:YAG (1064-nm) and ablative CO2 resurfacing lasers were attempted with minimal overall impact on cosmesis.
Bleomycin is a chemotherapeutic antibiotic that has been commonly used to treat Hodgkin lymphoma, germ cell tumors, and recurrent malignant pleural effusions.1 The drug is inactivated in most tissues by the enzyme bleomycin hydrolase. This enzyme is not present in skin and lung tissue; as a result, these organs are the most common sites of bleomycin toxicity.1 There are a variety of cutaneous effects associated with bleomycin including alopecia, hyperpigmentation, acral erythema, Raynaud phenomenon, and nail dystrophy.2 Flagellate hyperpigmentation is a less common cutaneous toxicity. It is an unusual eruption that appears as whiplike linear streaks on the upper chest and back, limbs, and flanks.3 This cutaneous manifestation was once thought to be specific to bleomycin use; however, it also has been described in dermatomyositis, adult-onset Still disease, and after the ingestion of uncooked or undercooked shiitake mushrooms.4 Flagellate hyperpigmentation also was once thought to be dose dependent; however, it has been described in even very small doses.5 The eruption has been described as independent of the route of drug administration, appearing with intravenous, subcutaneous, and intramuscular bleomycin.2 The association of bleomycin and flagellate hyperpigmentation has been reported since 1970; however, it is less commonly seen in clinical practice with the declining use of bleomycin.1
The exact mechanism for the hyperpigmentation is unknown. It has been proposed that the linear lesions are related to areas of pruritus and subsequent excoriations.1 Dermatographism may be present to a limited extent, but it is unlikely to be a chief cause of flagellate hyperpigmentation, as linear streaks have been reported in the absence of trauma. It also has been proposed that bleomycin has a direct toxic effect on the melanocytes, which stimulates increased melanin secretion.2 The hyperpigmentation also may be due to pigmentary incontinence secondary to inflammation.5 Histopathologic findings usually are varied and nonspecific.2 There may be a deep perivascular lymphocytic infiltrate, which is nonspecific but can be associated with drug-induced pathology.4 Bleomycin also is used to induce localized scleroderma in mouse-model research6 and has been reported to cause localized scleroderma at an infusion site or after an intralesional injection,7,8 which is not typically reported in flagellate erythema, but bleomycin's sclerosing effects may have played a role in the visible and sclerosing atrophy noted in our patient. Yamamoto et al9 reported a similar case of dermal sclerosis induced by bleomycin.
Flagellate hyperpigmentation typically lasts for up to 6 months.3 Patients with cutaneous manifestations from bleomycin therapy usually respond to steroid therapy and discontinuation of the drug. Bleomycin re-exposure should be avoided, as it may cause extension or widespread recurrence of flagellate hyperpigmentation.3 Postinflammatory pigment alteration may persist in patients with darker skin types and in patients with dramatic inciting inflammation.
Atrophoderma of Pasini and Pierini is a form of dermal atrophy that presents with 1 or more sharply demarcated depressed patches. There is some debate whether it is a distinct entity or a primary atrophic morphea.10 Linear atrophoderma of Moulin has a similar morphology with hyperpigmented depressions and "cliff-drop" borders, but these lesions follow the lines of Blaschko.11 Linear morphea initially can present as a linear erythematous streak but more commonly appears as a plaque-type morphea lesion that forms a scarlike band.12 Erythema dyschromicum perstans is an ashy dermatosis characterized by gray or blue-brown macules seen in Fitzpatrick skin types III through V and typically is chronic and progressive.13
- Lee HY, Lim KH, Ryu Y, et al. Bleomycininduced flagellate erythema: a case report and review of the literature. Oncol Lett. 2014;8:933-935.
- Simpson RC, Da Forno P, Nagarajan C, et al. A pruritic rash in a patient with Hodgkin lymphoma. Clin Exp Dermatol. 2011;36:680-682.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213-215.
- Lu CC, Lu YY, Wang QR, et al. Bleomycin-induced flagellate erythema. Balkan Med J. 2014;31:189-190.
- Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337-339.
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333-344.
- Kim KH, Yoon TJ, Oh CW, et al. A case of bleomycin-induced scleroderma. J Korean Med Sci. 1996;11:454-456.
- Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992;19:294-296.
- Yamamoto T, Yokozeki H, Nishioka K. Dermal sclerosis in the lesional skin of 'flagellate' erythema (scratch dermatitis) induced by bleomycin. Dermatology. 1998;197:399-400.
- Kencka D, Blaszczyk M, Jablońska S. Atrophoderma Pasini-Pierini is a primary atrophic abortive morphea. Dermatology. 1995;190:203-206.
- Moulin G, Hill MP, Guillaud V, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol. 1992;119:729-736.
- Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
- Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatosis--a critical review of literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542-544.
- Lee HY, Lim KH, Ryu Y, et al. Bleomycininduced flagellate erythema: a case report and review of the literature. Oncol Lett. 2014;8:933-935.
- Simpson RC, Da Forno P, Nagarajan C, et al. A pruritic rash in a patient with Hodgkin lymphoma. Clin Exp Dermatol. 2011;36:680-682.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213-215.
- Lu CC, Lu YY, Wang QR, et al. Bleomycin-induced flagellate erythema. Balkan Med J. 2014;31:189-190.
- Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337-339.
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333-344.
- Kim KH, Yoon TJ, Oh CW, et al. A case of bleomycin-induced scleroderma. J Korean Med Sci. 1996;11:454-456.
- Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992;19:294-296.
- Yamamoto T, Yokozeki H, Nishioka K. Dermal sclerosis in the lesional skin of 'flagellate' erythema (scratch dermatitis) induced by bleomycin. Dermatology. 1998;197:399-400.
- Kencka D, Blaszczyk M, Jablońska S. Atrophoderma Pasini-Pierini is a primary atrophic abortive morphea. Dermatology. 1995;190:203-206.
- Moulin G, Hill MP, Guillaud V, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol. 1992;119:729-736.
- Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
- Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatosis--a critical review of literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542-544.
An 18-year-old woman presented to our dermatology clinic with persistent diffuse discoloration on the upper body of more than 5 years’ duration. Her medical history was notable for primary mediastinal classical Hodgkin lymphoma treated with ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide) chemotherapy and 22 Gy radiation therapy to the chest 5 years prior. She reported the initial onset of diffuse pruritus with associated scratching and persistent skin discoloration while receiving a course of chemotherapy. Physical examination revealed numerous thin, flagellate, faintly hyperpigmented streaks with subtle atrophy in a parallel configuration on the bilateral shoulders (top), upper back (bottom), and abdomen. Punch biopsies (5 mm) of both affected and unaffected skin on the left side of the lateral upper back were performed.
Painful Nail with Longitudinal Erythronychia
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
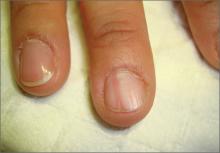
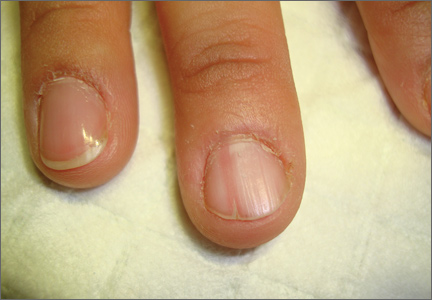
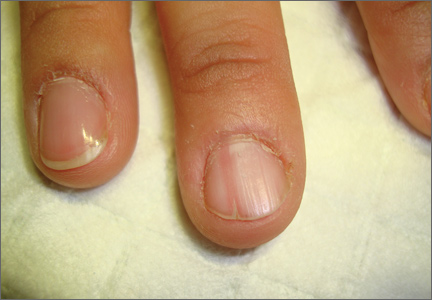
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
Patients report relief of symptoms following excision, although it may take several weeks for the pain to resolve completely.1 The rate of recurrence following excision is estimated at 10% to 20%.1 This may be due to incomplete excision or the development of a new lesion. Therefore, patients should be re-evaluated and considered for possible re-exploration if symptoms return or persist for more than 3 months after the excision.13
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; thomas.beachkofsky@us.af.mil
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
Patients report relief of symptoms following excision, although it may take several weeks for the pain to resolve completely.1 The rate of recurrence following excision is estimated at 10% to 20%.1 This may be due to incomplete excision or the development of a new lesion. Therefore, patients should be re-evaluated and considered for possible re-exploration if symptoms return or persist for more than 3 months after the excision.13
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; thomas.beachkofsky@us.af.mil
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
Patients report relief of symptoms following excision, although it may take several weeks for the pain to resolve completely.1 The rate of recurrence following excision is estimated at 10% to 20%.1 This may be due to incomplete excision or the development of a new lesion. Therefore, patients should be re-evaluated and considered for possible re-exploration if symptoms return or persist for more than 3 months after the excision.13
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; thomas.beachkofsky@us.af.mil
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
Painful nail with longitudinal erythronychia
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting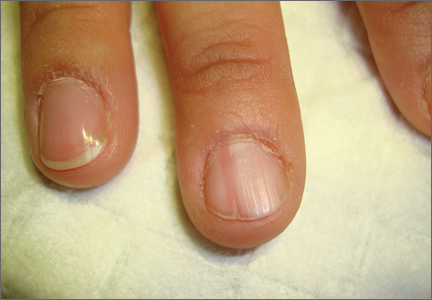
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; thomas.beachkofsky@us.af.mil
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; thomas.beachkofsky@us.af.mil
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; thomas.beachkofsky@us.af.mil
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.