User login
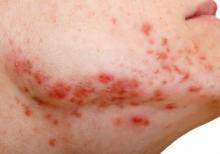
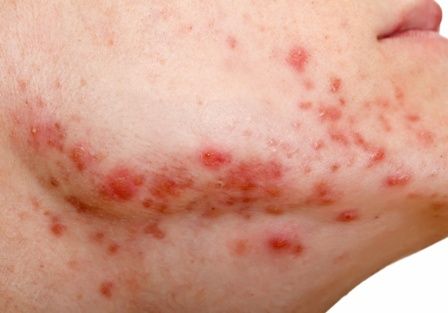
Study documents link between preadolescent acne and elevated BMI
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
FROM PEDIATRIC DERMATOLOGY
Large cohort study finds isotretinoin not associated with IBD
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.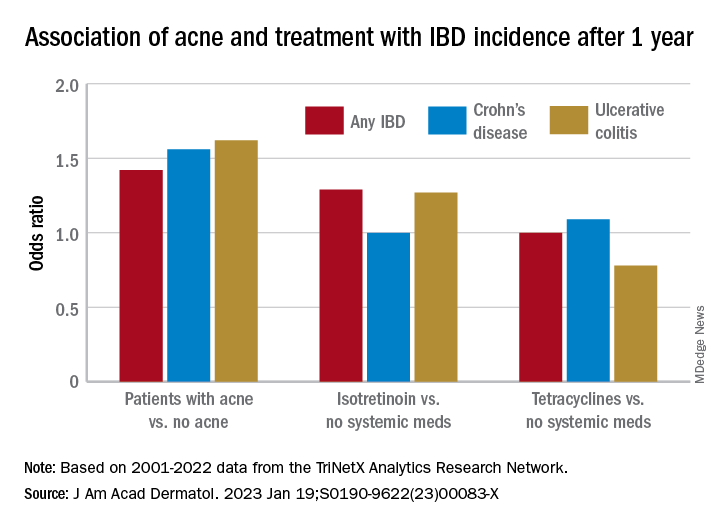
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Small study finds high dose vitamin D relieved toxic erythema of chemotherapy
seen on an inpatient dermatology consultative service.
Currently, chemotherapy cessation, delay, or dose modification are the “only reliable methods of resolving TEC,” and supportive agents such as topical corticosteroids, topical keratolytics, and pain control are associated with variable and “relatively slow improvement involving 2 to 4 weeks of recovery after chemotherapy interruption,” Cuong V. Nguyen, MD, of the department of dermatology at Northwestern University, Chicago, and colleagues, wrote in a research letter.
Onset of TEC in the six patients occurred a mean of 8.5 days after chemotherapy. Vitamin D – 50,000 IU for one patient and 100,000 IU for the others – was administered a mean of 4.3 days from rash onset and again in 7 days. Triamcinolone, 0.1%, or clobetasol, 0.05%, ointments were also prescribed.
All patients experienced symptomatic improvement in pain, pruritus, or swelling within a day of the first vitamin D treatment, and improvement in redness within 1 to 4 days, the authors said. The second treatment was administered for residual symptoms.
Adam Friedman, MD, professor and chair of dermatology and director of the supportive oncodermatology clinic at George Washington University, Washington, said that supporting patients through the “expected, disabling and often treatment-limiting side effects of oncologic therapies” is an area that is “in its infancy” and is characterized by limited evidence-based approaches.
“Creativity is therefore a must,” he said, commenting on the research letter. “Practice starts with anecdote, and this is certainly an exciting finding ... I look forward to trialing this with our patients at GW.”
Five of the six patients had a hematologic condition that required induction chemotherapy before hematopoietic stem cell transplant, and one was receiving regorafenib for treatment of glioblastoma multiforme. Diagnosis of TEC was established by clinical presentation, and five of the six patients underwent a biopsy. Biopsy findings were consistent with a TEC diagnosis in three patients, and showed nonspecific perivascular dermatitis in two, the investigators reported.
Further research is needed to determine optimal dosing, “delineate safety concerns and potential role in cancer treatment, and establish whether a durable response in patients with continuous chemotherapy, such as in an outpatient setting, is possible,” they said.
Dr. Nguyen and his coauthors reported no conflict of interest disclosures.
seen on an inpatient dermatology consultative service.
Currently, chemotherapy cessation, delay, or dose modification are the “only reliable methods of resolving TEC,” and supportive agents such as topical corticosteroids, topical keratolytics, and pain control are associated with variable and “relatively slow improvement involving 2 to 4 weeks of recovery after chemotherapy interruption,” Cuong V. Nguyen, MD, of the department of dermatology at Northwestern University, Chicago, and colleagues, wrote in a research letter.
Onset of TEC in the six patients occurred a mean of 8.5 days after chemotherapy. Vitamin D – 50,000 IU for one patient and 100,000 IU for the others – was administered a mean of 4.3 days from rash onset and again in 7 days. Triamcinolone, 0.1%, or clobetasol, 0.05%, ointments were also prescribed.
All patients experienced symptomatic improvement in pain, pruritus, or swelling within a day of the first vitamin D treatment, and improvement in redness within 1 to 4 days, the authors said. The second treatment was administered for residual symptoms.
Adam Friedman, MD, professor and chair of dermatology and director of the supportive oncodermatology clinic at George Washington University, Washington, said that supporting patients through the “expected, disabling and often treatment-limiting side effects of oncologic therapies” is an area that is “in its infancy” and is characterized by limited evidence-based approaches.
“Creativity is therefore a must,” he said, commenting on the research letter. “Practice starts with anecdote, and this is certainly an exciting finding ... I look forward to trialing this with our patients at GW.”
Five of the six patients had a hematologic condition that required induction chemotherapy before hematopoietic stem cell transplant, and one was receiving regorafenib for treatment of glioblastoma multiforme. Diagnosis of TEC was established by clinical presentation, and five of the six patients underwent a biopsy. Biopsy findings were consistent with a TEC diagnosis in three patients, and showed nonspecific perivascular dermatitis in two, the investigators reported.
Further research is needed to determine optimal dosing, “delineate safety concerns and potential role in cancer treatment, and establish whether a durable response in patients with continuous chemotherapy, such as in an outpatient setting, is possible,” they said.
Dr. Nguyen and his coauthors reported no conflict of interest disclosures.
seen on an inpatient dermatology consultative service.
Currently, chemotherapy cessation, delay, or dose modification are the “only reliable methods of resolving TEC,” and supportive agents such as topical corticosteroids, topical keratolytics, and pain control are associated with variable and “relatively slow improvement involving 2 to 4 weeks of recovery after chemotherapy interruption,” Cuong V. Nguyen, MD, of the department of dermatology at Northwestern University, Chicago, and colleagues, wrote in a research letter.
Onset of TEC in the six patients occurred a mean of 8.5 days after chemotherapy. Vitamin D – 50,000 IU for one patient and 100,000 IU for the others – was administered a mean of 4.3 days from rash onset and again in 7 days. Triamcinolone, 0.1%, or clobetasol, 0.05%, ointments were also prescribed.
All patients experienced symptomatic improvement in pain, pruritus, or swelling within a day of the first vitamin D treatment, and improvement in redness within 1 to 4 days, the authors said. The second treatment was administered for residual symptoms.
Adam Friedman, MD, professor and chair of dermatology and director of the supportive oncodermatology clinic at George Washington University, Washington, said that supporting patients through the “expected, disabling and often treatment-limiting side effects of oncologic therapies” is an area that is “in its infancy” and is characterized by limited evidence-based approaches.
“Creativity is therefore a must,” he said, commenting on the research letter. “Practice starts with anecdote, and this is certainly an exciting finding ... I look forward to trialing this with our patients at GW.”
Five of the six patients had a hematologic condition that required induction chemotherapy before hematopoietic stem cell transplant, and one was receiving regorafenib for treatment of glioblastoma multiforme. Diagnosis of TEC was established by clinical presentation, and five of the six patients underwent a biopsy. Biopsy findings were consistent with a TEC diagnosis in three patients, and showed nonspecific perivascular dermatitis in two, the investigators reported.
Further research is needed to determine optimal dosing, “delineate safety concerns and potential role in cancer treatment, and establish whether a durable response in patients with continuous chemotherapy, such as in an outpatient setting, is possible,” they said.
Dr. Nguyen and his coauthors reported no conflict of interest disclosures.
FROM JAMA DERMATOLOGY
New PDT therapy for CTCL to be reviewed by FDA
based on phase 3 findings published in JAMA Dermatology.
The treatment employs an ointment formulation of synthetic hypericin (HyBryte), a photosensitizer, that is preferentially absorbed into malignant cells and activated with visible light – rather than ultraviolet light – approximately 24 hours later. Investigators saw significant clinical responses in both patch and plaque type lesions and across races during the 24-week placebo-controlled, double-blinded, phase 3, randomized clinical trial.
“Traditional phototherapy, ultraviolet B phototherapy, has a limited depth of penetration, so patients with thicker plaque lesions don’t respond as well ... and UVB phototherapy typically is less effective in penetrating pigmented skin,” Ellen J. Kim, MD, lead author of the FLASH phase 3 trial, said in an interview.
Visible light in the yellow-red spectrum (500-650 nm) “penetrates deeper into the skin” and is nonmutagenic in vitro, so “theoretically it should have a much more favorable long-term safety profile,” said Dr. Kim, a dermatologist at the University of Pennsylvania, Philadelphia.
Currently, she said, the risk of secondary malignancies inherent with UV PDT, including melanoma, is a deterrent for some patients, especially “patients with really fair skin and a history of skin cancer.”
Hypericin PDT also seems well suited for use with an at-home light unit. “In our field, it’s not about which therapy is [universally] better or best, but a matter of what works best for each patient at that moment in time, depending on the side-effect profile and other issues such as access,” Dr. Kim said. “It will be great to have another option for an incurable disease that requires chronic management.”
Mycosis fungoides (MF)/CTCL is considered an orphan disease, and the treatment has received orphan drug and fast track designations from the FDA, and orphan designation from the European Medicines Agency, according to a press release from its developer, Soligenix. The company is anticipating potential approval in the second half of 2023 and is targeting early 2024 for a U.S. launch, the statement said.
Phase 3 results
The pivotal trial involved 169 patients at 39 academic and community-based U.S. medical centers and consisted of several 6-week cycles of twice-weekly treatment punctuated by 2-week breaks. In cycle 1, patients were randomized 2:1 to receive hypericin or placebo treatment of three index lesions. Cycle 2 involved the crossover of placebo patients to active treatment of index lesions, and cycle 3 (optional) involved open-label treatment of all desired lesions (index and nonindex).
The trial defined the primary endpoint in phase 1 as 50% or greater improvement in the modified Composite Assessment of Index Lesion Severity score – a tool that’s endorsed by U.S. and international MF/CTCL specialty group consensus guidelines. For cycles 2 and 3, open-label response rates were secondary endpoints. Responses were assessed after 2-week rest periods to allow for treatment-induced skin reactions to subside.
After one cycle of treatment, topical hypericin PDT was more effective than placebo (an index lesion response rate of 16% vs. 4%; P =.04). The index lesion response rate with treatment increased to 40% after two cycles and 49% after three cycles. All were statistically significant changes.
Response rates were similar in patch and plaque-type lesions and regardless of age, sex, race, stage IA versus IB, time since diagnosis, and number of prior therapies. Adverse events were primarily mild application-site skin reactions. No serious drug-related adverse events occurred, Dr. Kim said, and “we had a low drop-out rate overall.”
Into the real world
The 24-week phase 3 trial duration is short, considering that “typically, phototherapy takes between 4 to 24 months [to achieve] full responses in CTCL,” Dr. Kim said in the interview.
So with real-world application, she said, “we’ll want to see where the overall response peaks with longer treatment, what the effects are of continuous treatment without any built-in breaks, and whether we will indeed see less skin cancer development in patients who are at higher risk of developing skin cancers from light treatment.”
Such questions will be explored as part of a new 4-year, 50-patient, open-label, multicenter study with the primary aim of investigating home-based hypericin PDT therapy in a supervised setting, said Dr. Kim, principal investigator of this study. Patients who are doing well after 6 weeks of twice-weekly therapy will be given at-home light units to continue therapy and achieve 1 year of treatment with no breaks. They will be monitored with video-based telemedicine.
“Long term, having a home unit should really improve patient access and compliance and hopefully effectiveness,” Dr. Kim said. Based on the phase 3 experience, “we think that continuous treatment will be well tolerated and that we may see greater responses.”
On Dec. 19, Soligenix announced that enrollment had begun in a phase 2a study of synthetic hypericin for treating patients with mild to moderate psoriasis.
Dr. Kim reported to JAMA Dermatology grants from Innate Pharma and Galderma; consulting/advisory fees from Almirall, Galderma, and Helsinn; and honoraria from Ology and UptoDate.
based on phase 3 findings published in JAMA Dermatology.
The treatment employs an ointment formulation of synthetic hypericin (HyBryte), a photosensitizer, that is preferentially absorbed into malignant cells and activated with visible light – rather than ultraviolet light – approximately 24 hours later. Investigators saw significant clinical responses in both patch and plaque type lesions and across races during the 24-week placebo-controlled, double-blinded, phase 3, randomized clinical trial.
“Traditional phototherapy, ultraviolet B phototherapy, has a limited depth of penetration, so patients with thicker plaque lesions don’t respond as well ... and UVB phototherapy typically is less effective in penetrating pigmented skin,” Ellen J. Kim, MD, lead author of the FLASH phase 3 trial, said in an interview.
Visible light in the yellow-red spectrum (500-650 nm) “penetrates deeper into the skin” and is nonmutagenic in vitro, so “theoretically it should have a much more favorable long-term safety profile,” said Dr. Kim, a dermatologist at the University of Pennsylvania, Philadelphia.
Currently, she said, the risk of secondary malignancies inherent with UV PDT, including melanoma, is a deterrent for some patients, especially “patients with really fair skin and a history of skin cancer.”
Hypericin PDT also seems well suited for use with an at-home light unit. “In our field, it’s not about which therapy is [universally] better or best, but a matter of what works best for each patient at that moment in time, depending on the side-effect profile and other issues such as access,” Dr. Kim said. “It will be great to have another option for an incurable disease that requires chronic management.”
Mycosis fungoides (MF)/CTCL is considered an orphan disease, and the treatment has received orphan drug and fast track designations from the FDA, and orphan designation from the European Medicines Agency, according to a press release from its developer, Soligenix. The company is anticipating potential approval in the second half of 2023 and is targeting early 2024 for a U.S. launch, the statement said.
Phase 3 results
The pivotal trial involved 169 patients at 39 academic and community-based U.S. medical centers and consisted of several 6-week cycles of twice-weekly treatment punctuated by 2-week breaks. In cycle 1, patients were randomized 2:1 to receive hypericin or placebo treatment of three index lesions. Cycle 2 involved the crossover of placebo patients to active treatment of index lesions, and cycle 3 (optional) involved open-label treatment of all desired lesions (index and nonindex).
The trial defined the primary endpoint in phase 1 as 50% or greater improvement in the modified Composite Assessment of Index Lesion Severity score – a tool that’s endorsed by U.S. and international MF/CTCL specialty group consensus guidelines. For cycles 2 and 3, open-label response rates were secondary endpoints. Responses were assessed after 2-week rest periods to allow for treatment-induced skin reactions to subside.
After one cycle of treatment, topical hypericin PDT was more effective than placebo (an index lesion response rate of 16% vs. 4%; P =.04). The index lesion response rate with treatment increased to 40% after two cycles and 49% after three cycles. All were statistically significant changes.
Response rates were similar in patch and plaque-type lesions and regardless of age, sex, race, stage IA versus IB, time since diagnosis, and number of prior therapies. Adverse events were primarily mild application-site skin reactions. No serious drug-related adverse events occurred, Dr. Kim said, and “we had a low drop-out rate overall.”
Into the real world
The 24-week phase 3 trial duration is short, considering that “typically, phototherapy takes between 4 to 24 months [to achieve] full responses in CTCL,” Dr. Kim said in the interview.
So with real-world application, she said, “we’ll want to see where the overall response peaks with longer treatment, what the effects are of continuous treatment without any built-in breaks, and whether we will indeed see less skin cancer development in patients who are at higher risk of developing skin cancers from light treatment.”
Such questions will be explored as part of a new 4-year, 50-patient, open-label, multicenter study with the primary aim of investigating home-based hypericin PDT therapy in a supervised setting, said Dr. Kim, principal investigator of this study. Patients who are doing well after 6 weeks of twice-weekly therapy will be given at-home light units to continue therapy and achieve 1 year of treatment with no breaks. They will be monitored with video-based telemedicine.
“Long term, having a home unit should really improve patient access and compliance and hopefully effectiveness,” Dr. Kim said. Based on the phase 3 experience, “we think that continuous treatment will be well tolerated and that we may see greater responses.”
On Dec. 19, Soligenix announced that enrollment had begun in a phase 2a study of synthetic hypericin for treating patients with mild to moderate psoriasis.
Dr. Kim reported to JAMA Dermatology grants from Innate Pharma and Galderma; consulting/advisory fees from Almirall, Galderma, and Helsinn; and honoraria from Ology and UptoDate.
based on phase 3 findings published in JAMA Dermatology.
The treatment employs an ointment formulation of synthetic hypericin (HyBryte), a photosensitizer, that is preferentially absorbed into malignant cells and activated with visible light – rather than ultraviolet light – approximately 24 hours later. Investigators saw significant clinical responses in both patch and plaque type lesions and across races during the 24-week placebo-controlled, double-blinded, phase 3, randomized clinical trial.
“Traditional phototherapy, ultraviolet B phototherapy, has a limited depth of penetration, so patients with thicker plaque lesions don’t respond as well ... and UVB phototherapy typically is less effective in penetrating pigmented skin,” Ellen J. Kim, MD, lead author of the FLASH phase 3 trial, said in an interview.
Visible light in the yellow-red spectrum (500-650 nm) “penetrates deeper into the skin” and is nonmutagenic in vitro, so “theoretically it should have a much more favorable long-term safety profile,” said Dr. Kim, a dermatologist at the University of Pennsylvania, Philadelphia.
Currently, she said, the risk of secondary malignancies inherent with UV PDT, including melanoma, is a deterrent for some patients, especially “patients with really fair skin and a history of skin cancer.”
Hypericin PDT also seems well suited for use with an at-home light unit. “In our field, it’s not about which therapy is [universally] better or best, but a matter of what works best for each patient at that moment in time, depending on the side-effect profile and other issues such as access,” Dr. Kim said. “It will be great to have another option for an incurable disease that requires chronic management.”
Mycosis fungoides (MF)/CTCL is considered an orphan disease, and the treatment has received orphan drug and fast track designations from the FDA, and orphan designation from the European Medicines Agency, according to a press release from its developer, Soligenix. The company is anticipating potential approval in the second half of 2023 and is targeting early 2024 for a U.S. launch, the statement said.
Phase 3 results
The pivotal trial involved 169 patients at 39 academic and community-based U.S. medical centers and consisted of several 6-week cycles of twice-weekly treatment punctuated by 2-week breaks. In cycle 1, patients were randomized 2:1 to receive hypericin or placebo treatment of three index lesions. Cycle 2 involved the crossover of placebo patients to active treatment of index lesions, and cycle 3 (optional) involved open-label treatment of all desired lesions (index and nonindex).
The trial defined the primary endpoint in phase 1 as 50% or greater improvement in the modified Composite Assessment of Index Lesion Severity score – a tool that’s endorsed by U.S. and international MF/CTCL specialty group consensus guidelines. For cycles 2 and 3, open-label response rates were secondary endpoints. Responses were assessed after 2-week rest periods to allow for treatment-induced skin reactions to subside.
After one cycle of treatment, topical hypericin PDT was more effective than placebo (an index lesion response rate of 16% vs. 4%; P =.04). The index lesion response rate with treatment increased to 40% after two cycles and 49% after three cycles. All were statistically significant changes.
Response rates were similar in patch and plaque-type lesions and regardless of age, sex, race, stage IA versus IB, time since diagnosis, and number of prior therapies. Adverse events were primarily mild application-site skin reactions. No serious drug-related adverse events occurred, Dr. Kim said, and “we had a low drop-out rate overall.”
Into the real world
The 24-week phase 3 trial duration is short, considering that “typically, phototherapy takes between 4 to 24 months [to achieve] full responses in CTCL,” Dr. Kim said in the interview.
So with real-world application, she said, “we’ll want to see where the overall response peaks with longer treatment, what the effects are of continuous treatment without any built-in breaks, and whether we will indeed see less skin cancer development in patients who are at higher risk of developing skin cancers from light treatment.”
Such questions will be explored as part of a new 4-year, 50-patient, open-label, multicenter study with the primary aim of investigating home-based hypericin PDT therapy in a supervised setting, said Dr. Kim, principal investigator of this study. Patients who are doing well after 6 weeks of twice-weekly therapy will be given at-home light units to continue therapy and achieve 1 year of treatment with no breaks. They will be monitored with video-based telemedicine.
“Long term, having a home unit should really improve patient access and compliance and hopefully effectiveness,” Dr. Kim said. Based on the phase 3 experience, “we think that continuous treatment will be well tolerated and that we may see greater responses.”
On Dec. 19, Soligenix announced that enrollment had begun in a phase 2a study of synthetic hypericin for treating patients with mild to moderate psoriasis.
Dr. Kim reported to JAMA Dermatology grants from Innate Pharma and Galderma; consulting/advisory fees from Almirall, Galderma, and Helsinn; and honoraria from Ology and UptoDate.
Rise of the fungi: Pandemic tied to increasing fungal infections
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
Rosacea and the gut: Looking into SIBO
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
REPORTING FROM IDS 2022
Advanced practice providers – an evolving role in pulmonary medicine
The integration of advanced practice providers (APPs) into pulmonology practice is in flux and deepening across numerous settings, from outpatient clinics to intensive care and inpatient pulmonary consult services – and as it evolves, so are issues of training.
Some institutions are developing pulmonary fellowship programs for APPs. This is a good indication that team-based pulmonology may be moving toward a time in the future when nurse practitioners (NPs) and physician assistants (PAs) join pulmonologists in practice after having undergone formal education in the subspecialty, rather than learning solely on the job from dedicated mentors.
Neither NPs nor PAs, who comprise almost all of the APP workforce in pulmonology, currently have a pulmonary tract for training. “Weight falls on the employer’s shoulders to train and educate their APPs,” said Corinne R. Young, MSN, FNP-C, FCCP, director of APP and clinical services at Colorado Springs Pulmonary Consultants and founder and president of the Association of Pulmonary Advanced Practice Providers, which launched in 2018.
The role that an APP plays and their scope of practice is determined not only by state policies and regulations – and by their prior experience, knowledge and motivation – but by “how much work a practice puts into [education and training],” she said.
An estimated 3,000-8,000 APPs are working in pulmonology, according to an analysis done by a marketing agency that has worked for the American College of Chest Physicians, Ms. Young said.
A 2021 APAPP survey of its several hundred members at the time showed them working in hospital systems (41%), private practice (28%), university systems (10%), and other health care systems (21%). They indicated practicing in pulmonary medicine, sleep medicine, or critical care – or some combination of these areas – and the vast majority (82%) indicated they were seeing both new and established patients in their roles.
“Nobody knows exactly how many of us are out there,” Ms. Young said. “But CHEST and APAPP are making great efforts to be beacons to APPs working in this realm and to bring them together to have a voice.”
The APAPP also wants to “close the education gap” and to “eventually develop a certification program to vet our knowledge in this area,” she said. “Right now, the closest we can get to vetting our knowledge is to become an FCCP through CHEST.”
Earning trust, seeking training
Omar Hussain, DO, has been practicing with an NP for over a decade in his role as an intensivist and knows what it’s like to train, supervise, and grow together. He and his private practice colleagues have a contract with Advocate Condell Hospital in Libertyville, Ill., to cover its ICU, and they hired their NP primarily to help care for shorter-stay, non–critically ill patients in the ICU (for example, patients receiving postoperative monitoring).
The NP has been invaluable. “We literally sit next to each other and in the mornings we make a game plan of which patients she will tackle first and which ones I’ll see first,” Dr. Hussain said. “When we’re called by the nurse for an ICU evaluation [on the floor], we’ll decide in real time who goes.”
The NP ensures that all guidelines and quality measures are followed in the ICU and, with a Monday-Friday schedule, she provides valuable continuity when there are handoffs from one intensivist to another, said Dr. Hussain, who serves as cochair of the joint CHEST/American Thoracic Society clinical practice committee, which deals with issues of physician-APP collaboration.
After working collaboratively for some time, Dr. Hussain and his partners decided to teach the NP how to intubate. It was a thoughtful and deliberate process, and “we used the same kind of mindset we’d used when we’ve supervised residents at other institutions,” he said.
Dr. Hussain and his partners have been fortunate in having such a long-term relationship with an APP. Their NP had worked as a nurse in the ICU before training as an adult gerontology–acute care NP and joining Dr. Hussain’s practice, so she was also “well known to us,” he added.
Rachel Adney, CPNP-PC, a certified pediatric NP in the division of pediatric pulmonology at Stanford (Calif.) Medicine Children’s Health, is an APP who actively sought advanced training. She joined Stanford in 2011 to provide ambulatory care, primarily, and having years of prior experience in asthma management and education, she fast became known as “the asthma person.”
After a physician colleague one day objected to her caring for a patient without asthma, Ms. Adney, the first APP in the division, approached John D. Mark, MD, program director of the pediatric fellowship program at Stanford, and inquired about training “so I could have more breadth and depth across the whole pulmonary milieu.”
Together they designed a “mini pediatric pulmonary fellowship” for Ms. Adney, incorporating elements of the first year of Stanford’s pediatric fellowship program as well as training materials from the University of Arizona’s Pediatric Pulmonary Center, Tucson, one of six federally funded PCCs that train various health care providers to care for pediatric patients with chronic pulmonary conditions. (Dr. Mark had previously been an educator at the center while serving on the University of Arizona faculty.)
Her curriculum consisted of 1,000 total hours of training, including 125 hours of didactic learning and 400 hours of both inpatient and outpatient clinical training in areas such as cystic fibrosis, sleep medicine, bronchopulmonary dysplasia (BPD), neuromuscular disorders, and general pulmonary medicine. “Rachel rotated through clinics, first as an observer, then as a trainee ... and she attended lectures that my fellows attended,” said Dr. Mark, who has long been a preceptor for APPs. “She became like a 1-year fellow in my division.”
Today, Ms. Adney sees patients independently in four outreach clinics along California’s central coast. “She sees very complicated pediatric pulmonary patients now” overall, and has become integral to Stanford’s interdisciplinary CRIB program (cardiac and respiratory care for infants with BPD), Dr. Mark said. “She follows these patients at Stanford along with the whole CRIB group, then sees them on her own for follow-up.”
As a result of her training, Ms. Adney said, “knowing that I have the knowledge and experience to take on more complex patients, my colleagues now trust me and are confident in my skills. They feel comfortable sending [patients] to me much earlier. ... And they know that if there’s something I need help with I will go to them instantly.”
Pulmonology “really spoke to my heart,” she said, recalling her pre-Stanford journey as an in-hospital medical-surgical nurse, and then, after her NP training, as a outpatient primary care PNP. “For the most part, it’s like putting a puzzle together, and being able to really impact the quality of life these patients have,” said Ms. Adney, who serves on the APAPP’s pediatric subcommittee.
It’s clear, Dr. Mark said, that “things are changing around the country” with increasing institutional interest in developing formal APP specialty training programs. “There’s no way [for an APP] to walk into a specialty and play an active role without additional training,” and institutions are frustrated with turnover and the loss of APPs who decide after 6-9 months of on-the-job training that they’re not interested in the field.
Stanford Medicine Children’s Health, in fact, has launched an internal Pediatric APP Fellowship Program that is training its first cohort of six newly graduated NPs and PAs in two clinical tracks, including a medical/surgical track that incorporates rotations in pulmonary medicine, said Raji Koppolu, CPNP-PC/AC, manager of advanced practice professional development for Stanford Medicine Children’s Health.
APP fellowship programs have been in existence since 2007 in a variety of clinical settings, she said, but more institutions are developing them as a way of recruiting and retaining APPs in areas of high need and of equipping them for successful transitions to their APP roles. Various national bodies accredit APP fellowship programs.
Most pulmonary fellowship programs, Ms. Young said, are also internal programs providing postgraduate education to their own newly hired APPs or recent NP/PA graduates. This limits their reach, but “it’s a step in the right direction toward standardizing education for pulmonary APPs.”
Defining APP competencies
In interventional pulmonology, training may soon be guided by newly defined “core clinical competencies” for APPs. The soon-to-be published and distributed competencies – the first such national APP competencies in pulmonology – were developed by an APP Leadership Council within the American Association of Bronchology and Interventional Pulmonology and cover the most common disease processes and practices in IP, from COPD and bronchoscopic lung volume reduction to pleural effusion and lung cancer screening.
Rebecca Priebe, ACNP-BC, who cochairs the AABIP’s APP chapter, organized the effort several years ago, bringing together a group of APPs and physician experts in advanced bronchoscopy and IP (some but not all of whom have worked with APPs), after fielding questions from pulmonologists at AABIP meetings about what to look for in an AAP and how to train them.
Physicians and institutions who are hiring and training APPs for IP can use any or all of the 11 core competencies to personalize and evaluate the training process for each APP’s needs, she said. “Someone looking to hire an APP for pleural disease, for instance, can pull up the content on plural effusion.”
APP interest in interventional pulmonology is growing rapidly, Ms. Priebe said, noting growth in the AABIP’s APP chapter from about 7-8 APPs 5 years ago to at least 60 currently.
Ms. Priebe was hired by Henry Ford Health in Detroit about 5 years ago to help establish and run an inpatient IP consult service, and more recently, she helped establish their inpatient pleural disease service and a bronchoscopic lung volume reduction program.
For the inpatient IP service, after several months of side-by-side training with an IP fellow and attending physicians, she began independently evaluating new patients, writing notes, and making recommendations.
For patients with pleural disease, she performs ultrasound examinations, chest tube insertions, and bedside thoracentesis independently. And for the bronchoscopic lung volume reduction program, she evaluates patients for candidate status, participates in valve placement, and sees patients independently through a year of follow-up.
“Physician colleagues often aren’t sure what an APP’s education and scope of practice is,” said Ms. Priebe, who was an ICU nurse before training as an acute care NP and then worked first with a private practice inpatient service and then with the University of Michigan, Ann Arbor, where she established and grew an APP-run program managing lung transplant patients and a step-down ICU unit.
“It’s a matter of knowing [your state’s policies], treating them like a fellow you would train, and then using them to the fullest extent of their education and training. If they’re given an opportunity to learn a subspecialty skill set, they can be an asset to any pulmonary program.”
‘We’re here to support,’ not replace
In her own practice, Ms. Young is one of seven APPs who work with nine physicians on a full range of inpatient care, outpatient care, critical care, sleep medicine, and procedures. Many new patients are seen first by the APP, who does the workup and orders tests, and by the physician on a follow-up visit. Most patients needing routine management of asthma and COPD are seen by the physician every third or fourth visit, she said.
Ms. Young also directs a 24-hour in-house APP service recently established by the practice, and she participates in research. In a practice across town, she noted, APPs see mainly established patients and do not practice as autonomously as the state permits. “Part of that difference may [stem from] the lack of a standard of education and variable amounts of work the practice puts into their APPs.”
The American Medical Association’s #StopScopeCreep social media messaging feels divisive and “sheds a negative light on APPs working in any area,” Ms. Young said. “One of the biggest things we want to convey [at APAPP] is that we’re not here for [physicians’] jobs.”
“We’re here to support those who are practicing, to support underserved populations, and to help bridge gaps” created by an aging pulmonologist workforce and real and projected physician shortages, Ms. Young said, referring to a 2016 report from the Health Resources and Services Administration and a 2017 report from Merritt Hawkins indicating that 73% of U.S. pulmonologists (the largest percentage of all subspecialties) were at least 55 years old.
Dr. Hussain said he has “seen scope creep” first-hand in his hospitals, in the form of noncollaborative practices and tasks performed by APPs without adequate training – most likely often stemming from poor decisions and oversight by physicians. But when constructed thoughtfully, APP-physician teams are “serving great needs” in many types of care, he said, from follow-up care and management of chronic conditions to inpatient rounding. “My [colleagues] are having great success,” he said.
He is watching with interest – and some concern – pending reimbursement changes from the Centers for Medicare & Medicaid Services that will make time the only defining feature of the “substantive” portion of a split/shared visit involving physicians and APPs in a facility setting. Medical decision-making will no longer be applicable.
For time-based services like critical care, time alone is currently the metric. (And in the nonfacility setting, physician-APP teams may still apply “incident to” billing practices). But in the facility setting, said Amy M. Ahasic, MD, MPH, a pulmonologist in Norwalk, Conn., who coauthored a 2022 commentary on the issue, the change (now planned for 2024) could be problematic for employed physicians whose contracts are based on productivity, and could create tension and possibly lead to reduced use of APPs rather than supporting collaborative care.
“The team model has been evolving so well over the past 10-15 years,” said Dr. Ahasic, who serves on the CHEST Health Policy and Advocacy Reimbursement Workgroup and cochairs the CHEST/American Thoracic Society clinical practice committee with Dr. Hussain. “It’s good for patient safety to have more [providers] involved ... and because APP salaries are lower health systems could do it and be able to have better care and better coverage.”
The pulmonology culture, said Dr. Hussain, has been increasingly embracing APPs and “it’s collegial.” Pulmonologists are “coming to CHEST meetings with their APPs. They’re learning the same things we’re learning, to manage the same patients we manage.”
The article sources reported that they had no relevant financial conflicts of interest to disclose.
The integration of advanced practice providers (APPs) into pulmonology practice is in flux and deepening across numerous settings, from outpatient clinics to intensive care and inpatient pulmonary consult services – and as it evolves, so are issues of training.
Some institutions are developing pulmonary fellowship programs for APPs. This is a good indication that team-based pulmonology may be moving toward a time in the future when nurse practitioners (NPs) and physician assistants (PAs) join pulmonologists in practice after having undergone formal education in the subspecialty, rather than learning solely on the job from dedicated mentors.
Neither NPs nor PAs, who comprise almost all of the APP workforce in pulmonology, currently have a pulmonary tract for training. “Weight falls on the employer’s shoulders to train and educate their APPs,” said Corinne R. Young, MSN, FNP-C, FCCP, director of APP and clinical services at Colorado Springs Pulmonary Consultants and founder and president of the Association of Pulmonary Advanced Practice Providers, which launched in 2018.
The role that an APP plays and their scope of practice is determined not only by state policies and regulations – and by their prior experience, knowledge and motivation – but by “how much work a practice puts into [education and training],” she said.
An estimated 3,000-8,000 APPs are working in pulmonology, according to an analysis done by a marketing agency that has worked for the American College of Chest Physicians, Ms. Young said.
A 2021 APAPP survey of its several hundred members at the time showed them working in hospital systems (41%), private practice (28%), university systems (10%), and other health care systems (21%). They indicated practicing in pulmonary medicine, sleep medicine, or critical care – or some combination of these areas – and the vast majority (82%) indicated they were seeing both new and established patients in their roles.
“Nobody knows exactly how many of us are out there,” Ms. Young said. “But CHEST and APAPP are making great efforts to be beacons to APPs working in this realm and to bring them together to have a voice.”
The APAPP also wants to “close the education gap” and to “eventually develop a certification program to vet our knowledge in this area,” she said. “Right now, the closest we can get to vetting our knowledge is to become an FCCP through CHEST.”
Earning trust, seeking training
Omar Hussain, DO, has been practicing with an NP for over a decade in his role as an intensivist and knows what it’s like to train, supervise, and grow together. He and his private practice colleagues have a contract with Advocate Condell Hospital in Libertyville, Ill., to cover its ICU, and they hired their NP primarily to help care for shorter-stay, non–critically ill patients in the ICU (for example, patients receiving postoperative monitoring).
The NP has been invaluable. “We literally sit next to each other and in the mornings we make a game plan of which patients she will tackle first and which ones I’ll see first,” Dr. Hussain said. “When we’re called by the nurse for an ICU evaluation [on the floor], we’ll decide in real time who goes.”
The NP ensures that all guidelines and quality measures are followed in the ICU and, with a Monday-Friday schedule, she provides valuable continuity when there are handoffs from one intensivist to another, said Dr. Hussain, who serves as cochair of the joint CHEST/American Thoracic Society clinical practice committee, which deals with issues of physician-APP collaboration.
After working collaboratively for some time, Dr. Hussain and his partners decided to teach the NP how to intubate. It was a thoughtful and deliberate process, and “we used the same kind of mindset we’d used when we’ve supervised residents at other institutions,” he said.
Dr. Hussain and his partners have been fortunate in having such a long-term relationship with an APP. Their NP had worked as a nurse in the ICU before training as an adult gerontology–acute care NP and joining Dr. Hussain’s practice, so she was also “well known to us,” he added.
Rachel Adney, CPNP-PC, a certified pediatric NP in the division of pediatric pulmonology at Stanford (Calif.) Medicine Children’s Health, is an APP who actively sought advanced training. She joined Stanford in 2011 to provide ambulatory care, primarily, and having years of prior experience in asthma management and education, she fast became known as “the asthma person.”
After a physician colleague one day objected to her caring for a patient without asthma, Ms. Adney, the first APP in the division, approached John D. Mark, MD, program director of the pediatric fellowship program at Stanford, and inquired about training “so I could have more breadth and depth across the whole pulmonary milieu.”
Together they designed a “mini pediatric pulmonary fellowship” for Ms. Adney, incorporating elements of the first year of Stanford’s pediatric fellowship program as well as training materials from the University of Arizona’s Pediatric Pulmonary Center, Tucson, one of six federally funded PCCs that train various health care providers to care for pediatric patients with chronic pulmonary conditions. (Dr. Mark had previously been an educator at the center while serving on the University of Arizona faculty.)
Her curriculum consisted of 1,000 total hours of training, including 125 hours of didactic learning and 400 hours of both inpatient and outpatient clinical training in areas such as cystic fibrosis, sleep medicine, bronchopulmonary dysplasia (BPD), neuromuscular disorders, and general pulmonary medicine. “Rachel rotated through clinics, first as an observer, then as a trainee ... and she attended lectures that my fellows attended,” said Dr. Mark, who has long been a preceptor for APPs. “She became like a 1-year fellow in my division.”
Today, Ms. Adney sees patients independently in four outreach clinics along California’s central coast. “She sees very complicated pediatric pulmonary patients now” overall, and has become integral to Stanford’s interdisciplinary CRIB program (cardiac and respiratory care for infants with BPD), Dr. Mark said. “She follows these patients at Stanford along with the whole CRIB group, then sees them on her own for follow-up.”
As a result of her training, Ms. Adney said, “knowing that I have the knowledge and experience to take on more complex patients, my colleagues now trust me and are confident in my skills. They feel comfortable sending [patients] to me much earlier. ... And they know that if there’s something I need help with I will go to them instantly.”
Pulmonology “really spoke to my heart,” she said, recalling her pre-Stanford journey as an in-hospital medical-surgical nurse, and then, after her NP training, as a outpatient primary care PNP. “For the most part, it’s like putting a puzzle together, and being able to really impact the quality of life these patients have,” said Ms. Adney, who serves on the APAPP’s pediatric subcommittee.
It’s clear, Dr. Mark said, that “things are changing around the country” with increasing institutional interest in developing formal APP specialty training programs. “There’s no way [for an APP] to walk into a specialty and play an active role without additional training,” and institutions are frustrated with turnover and the loss of APPs who decide after 6-9 months of on-the-job training that they’re not interested in the field.
Stanford Medicine Children’s Health, in fact, has launched an internal Pediatric APP Fellowship Program that is training its first cohort of six newly graduated NPs and PAs in two clinical tracks, including a medical/surgical track that incorporates rotations in pulmonary medicine, said Raji Koppolu, CPNP-PC/AC, manager of advanced practice professional development for Stanford Medicine Children’s Health.
APP fellowship programs have been in existence since 2007 in a variety of clinical settings, she said, but more institutions are developing them as a way of recruiting and retaining APPs in areas of high need and of equipping them for successful transitions to their APP roles. Various national bodies accredit APP fellowship programs.
Most pulmonary fellowship programs, Ms. Young said, are also internal programs providing postgraduate education to their own newly hired APPs or recent NP/PA graduates. This limits their reach, but “it’s a step in the right direction toward standardizing education for pulmonary APPs.”
Defining APP competencies
In interventional pulmonology, training may soon be guided by newly defined “core clinical competencies” for APPs. The soon-to-be published and distributed competencies – the first such national APP competencies in pulmonology – were developed by an APP Leadership Council within the American Association of Bronchology and Interventional Pulmonology and cover the most common disease processes and practices in IP, from COPD and bronchoscopic lung volume reduction to pleural effusion and lung cancer screening.
Rebecca Priebe, ACNP-BC, who cochairs the AABIP’s APP chapter, organized the effort several years ago, bringing together a group of APPs and physician experts in advanced bronchoscopy and IP (some but not all of whom have worked with APPs), after fielding questions from pulmonologists at AABIP meetings about what to look for in an AAP and how to train them.
Physicians and institutions who are hiring and training APPs for IP can use any or all of the 11 core competencies to personalize and evaluate the training process for each APP’s needs, she said. “Someone looking to hire an APP for pleural disease, for instance, can pull up the content on plural effusion.”
APP interest in interventional pulmonology is growing rapidly, Ms. Priebe said, noting growth in the AABIP’s APP chapter from about 7-8 APPs 5 years ago to at least 60 currently.
Ms. Priebe was hired by Henry Ford Health in Detroit about 5 years ago to help establish and run an inpatient IP consult service, and more recently, she helped establish their inpatient pleural disease service and a bronchoscopic lung volume reduction program.
For the inpatient IP service, after several months of side-by-side training with an IP fellow and attending physicians, she began independently evaluating new patients, writing notes, and making recommendations.
For patients with pleural disease, she performs ultrasound examinations, chest tube insertions, and bedside thoracentesis independently. And for the bronchoscopic lung volume reduction program, she evaluates patients for candidate status, participates in valve placement, and sees patients independently through a year of follow-up.
“Physician colleagues often aren’t sure what an APP’s education and scope of practice is,” said Ms. Priebe, who was an ICU nurse before training as an acute care NP and then worked first with a private practice inpatient service and then with the University of Michigan, Ann Arbor, where she established and grew an APP-run program managing lung transplant patients and a step-down ICU unit.
“It’s a matter of knowing [your state’s policies], treating them like a fellow you would train, and then using them to the fullest extent of their education and training. If they’re given an opportunity to learn a subspecialty skill set, they can be an asset to any pulmonary program.”
‘We’re here to support,’ not replace
In her own practice, Ms. Young is one of seven APPs who work with nine physicians on a full range of inpatient care, outpatient care, critical care, sleep medicine, and procedures. Many new patients are seen first by the APP, who does the workup and orders tests, and by the physician on a follow-up visit. Most patients needing routine management of asthma and COPD are seen by the physician every third or fourth visit, she said.
Ms. Young also directs a 24-hour in-house APP service recently established by the practice, and she participates in research. In a practice across town, she noted, APPs see mainly established patients and do not practice as autonomously as the state permits. “Part of that difference may [stem from] the lack of a standard of education and variable amounts of work the practice puts into their APPs.”
The American Medical Association’s #StopScopeCreep social media messaging feels divisive and “sheds a negative light on APPs working in any area,” Ms. Young said. “One of the biggest things we want to convey [at APAPP] is that we’re not here for [physicians’] jobs.”
“We’re here to support those who are practicing, to support underserved populations, and to help bridge gaps” created by an aging pulmonologist workforce and real and projected physician shortages, Ms. Young said, referring to a 2016 report from the Health Resources and Services Administration and a 2017 report from Merritt Hawkins indicating that 73% of U.S. pulmonologists (the largest percentage of all subspecialties) were at least 55 years old.
Dr. Hussain said he has “seen scope creep” first-hand in his hospitals, in the form of noncollaborative practices and tasks performed by APPs without adequate training – most likely often stemming from poor decisions and oversight by physicians. But when constructed thoughtfully, APP-physician teams are “serving great needs” in many types of care, he said, from follow-up care and management of chronic conditions to inpatient rounding. “My [colleagues] are having great success,” he said.
He is watching with interest – and some concern – pending reimbursement changes from the Centers for Medicare & Medicaid Services that will make time the only defining feature of the “substantive” portion of a split/shared visit involving physicians and APPs in a facility setting. Medical decision-making will no longer be applicable.
For time-based services like critical care, time alone is currently the metric. (And in the nonfacility setting, physician-APP teams may still apply “incident to” billing practices). But in the facility setting, said Amy M. Ahasic, MD, MPH, a pulmonologist in Norwalk, Conn., who coauthored a 2022 commentary on the issue, the change (now planned for 2024) could be problematic for employed physicians whose contracts are based on productivity, and could create tension and possibly lead to reduced use of APPs rather than supporting collaborative care.
“The team model has been evolving so well over the past 10-15 years,” said Dr. Ahasic, who serves on the CHEST Health Policy and Advocacy Reimbursement Workgroup and cochairs the CHEST/American Thoracic Society clinical practice committee with Dr. Hussain. “It’s good for patient safety to have more [providers] involved ... and because APP salaries are lower health systems could do it and be able to have better care and better coverage.”
The pulmonology culture, said Dr. Hussain, has been increasingly embracing APPs and “it’s collegial.” Pulmonologists are “coming to CHEST meetings with their APPs. They’re learning the same things we’re learning, to manage the same patients we manage.”
The article sources reported that they had no relevant financial conflicts of interest to disclose.
The integration of advanced practice providers (APPs) into pulmonology practice is in flux and deepening across numerous settings, from outpatient clinics to intensive care and inpatient pulmonary consult services – and as it evolves, so are issues of training.
Some institutions are developing pulmonary fellowship programs for APPs. This is a good indication that team-based pulmonology may be moving toward a time in the future when nurse practitioners (NPs) and physician assistants (PAs) join pulmonologists in practice after having undergone formal education in the subspecialty, rather than learning solely on the job from dedicated mentors.
Neither NPs nor PAs, who comprise almost all of the APP workforce in pulmonology, currently have a pulmonary tract for training. “Weight falls on the employer’s shoulders to train and educate their APPs,” said Corinne R. Young, MSN, FNP-C, FCCP, director of APP and clinical services at Colorado Springs Pulmonary Consultants and founder and president of the Association of Pulmonary Advanced Practice Providers, which launched in 2018.
The role that an APP plays and their scope of practice is determined not only by state policies and regulations – and by their prior experience, knowledge and motivation – but by “how much work a practice puts into [education and training],” she said.
An estimated 3,000-8,000 APPs are working in pulmonology, according to an analysis done by a marketing agency that has worked for the American College of Chest Physicians, Ms. Young said.
A 2021 APAPP survey of its several hundred members at the time showed them working in hospital systems (41%), private practice (28%), university systems (10%), and other health care systems (21%). They indicated practicing in pulmonary medicine, sleep medicine, or critical care – or some combination of these areas – and the vast majority (82%) indicated they were seeing both new and established patients in their roles.
“Nobody knows exactly how many of us are out there,” Ms. Young said. “But CHEST and APAPP are making great efforts to be beacons to APPs working in this realm and to bring them together to have a voice.”
The APAPP also wants to “close the education gap” and to “eventually develop a certification program to vet our knowledge in this area,” she said. “Right now, the closest we can get to vetting our knowledge is to become an FCCP through CHEST.”
Earning trust, seeking training
Omar Hussain, DO, has been practicing with an NP for over a decade in his role as an intensivist and knows what it’s like to train, supervise, and grow together. He and his private practice colleagues have a contract with Advocate Condell Hospital in Libertyville, Ill., to cover its ICU, and they hired their NP primarily to help care for shorter-stay, non–critically ill patients in the ICU (for example, patients receiving postoperative monitoring).
The NP has been invaluable. “We literally sit next to each other and in the mornings we make a game plan of which patients she will tackle first and which ones I’ll see first,” Dr. Hussain said. “When we’re called by the nurse for an ICU evaluation [on the floor], we’ll decide in real time who goes.”
The NP ensures that all guidelines and quality measures are followed in the ICU and, with a Monday-Friday schedule, she provides valuable continuity when there are handoffs from one intensivist to another, said Dr. Hussain, who serves as cochair of the joint CHEST/American Thoracic Society clinical practice committee, which deals with issues of physician-APP collaboration.
After working collaboratively for some time, Dr. Hussain and his partners decided to teach the NP how to intubate. It was a thoughtful and deliberate process, and “we used the same kind of mindset we’d used when we’ve supervised residents at other institutions,” he said.
Dr. Hussain and his partners have been fortunate in having such a long-term relationship with an APP. Their NP had worked as a nurse in the ICU before training as an adult gerontology–acute care NP and joining Dr. Hussain’s practice, so she was also “well known to us,” he added.
Rachel Adney, CPNP-PC, a certified pediatric NP in the division of pediatric pulmonology at Stanford (Calif.) Medicine Children’s Health, is an APP who actively sought advanced training. She joined Stanford in 2011 to provide ambulatory care, primarily, and having years of prior experience in asthma management and education, she fast became known as “the asthma person.”
After a physician colleague one day objected to her caring for a patient without asthma, Ms. Adney, the first APP in the division, approached John D. Mark, MD, program director of the pediatric fellowship program at Stanford, and inquired about training “so I could have more breadth and depth across the whole pulmonary milieu.”
Together they designed a “mini pediatric pulmonary fellowship” for Ms. Adney, incorporating elements of the first year of Stanford’s pediatric fellowship program as well as training materials from the University of Arizona’s Pediatric Pulmonary Center, Tucson, one of six federally funded PCCs that train various health care providers to care for pediatric patients with chronic pulmonary conditions. (Dr. Mark had previously been an educator at the center while serving on the University of Arizona faculty.)
Her curriculum consisted of 1,000 total hours of training, including 125 hours of didactic learning and 400 hours of both inpatient and outpatient clinical training in areas such as cystic fibrosis, sleep medicine, bronchopulmonary dysplasia (BPD), neuromuscular disorders, and general pulmonary medicine. “Rachel rotated through clinics, first as an observer, then as a trainee ... and she attended lectures that my fellows attended,” said Dr. Mark, who has long been a preceptor for APPs. “She became like a 1-year fellow in my division.”
Today, Ms. Adney sees patients independently in four outreach clinics along California’s central coast. “She sees very complicated pediatric pulmonary patients now” overall, and has become integral to Stanford’s interdisciplinary CRIB program (cardiac and respiratory care for infants with BPD), Dr. Mark said. “She follows these patients at Stanford along with the whole CRIB group, then sees them on her own for follow-up.”
As a result of her training, Ms. Adney said, “knowing that I have the knowledge and experience to take on more complex patients, my colleagues now trust me and are confident in my skills. They feel comfortable sending [patients] to me much earlier. ... And they know that if there’s something I need help with I will go to them instantly.”
Pulmonology “really spoke to my heart,” she said, recalling her pre-Stanford journey as an in-hospital medical-surgical nurse, and then, after her NP training, as a outpatient primary care PNP. “For the most part, it’s like putting a puzzle together, and being able to really impact the quality of life these patients have,” said Ms. Adney, who serves on the APAPP’s pediatric subcommittee.
It’s clear, Dr. Mark said, that “things are changing around the country” with increasing institutional interest in developing formal APP specialty training programs. “There’s no way [for an APP] to walk into a specialty and play an active role without additional training,” and institutions are frustrated with turnover and the loss of APPs who decide after 6-9 months of on-the-job training that they’re not interested in the field.
Stanford Medicine Children’s Health, in fact, has launched an internal Pediatric APP Fellowship Program that is training its first cohort of six newly graduated NPs and PAs in two clinical tracks, including a medical/surgical track that incorporates rotations in pulmonary medicine, said Raji Koppolu, CPNP-PC/AC, manager of advanced practice professional development for Stanford Medicine Children’s Health.
APP fellowship programs have been in existence since 2007 in a variety of clinical settings, she said, but more institutions are developing them as a way of recruiting and retaining APPs in areas of high need and of equipping them for successful transitions to their APP roles. Various national bodies accredit APP fellowship programs.
Most pulmonary fellowship programs, Ms. Young said, are also internal programs providing postgraduate education to their own newly hired APPs or recent NP/PA graduates. This limits their reach, but “it’s a step in the right direction toward standardizing education for pulmonary APPs.”
Defining APP competencies
In interventional pulmonology, training may soon be guided by newly defined “core clinical competencies” for APPs. The soon-to-be published and distributed competencies – the first such national APP competencies in pulmonology – were developed by an APP Leadership Council within the American Association of Bronchology and Interventional Pulmonology and cover the most common disease processes and practices in IP, from COPD and bronchoscopic lung volume reduction to pleural effusion and lung cancer screening.
Rebecca Priebe, ACNP-BC, who cochairs the AABIP’s APP chapter, organized the effort several years ago, bringing together a group of APPs and physician experts in advanced bronchoscopy and IP (some but not all of whom have worked with APPs), after fielding questions from pulmonologists at AABIP meetings about what to look for in an AAP and how to train them.
Physicians and institutions who are hiring and training APPs for IP can use any or all of the 11 core competencies to personalize and evaluate the training process for each APP’s needs, she said. “Someone looking to hire an APP for pleural disease, for instance, can pull up the content on plural effusion.”
APP interest in interventional pulmonology is growing rapidly, Ms. Priebe said, noting growth in the AABIP’s APP chapter from about 7-8 APPs 5 years ago to at least 60 currently.
Ms. Priebe was hired by Henry Ford Health in Detroit about 5 years ago to help establish and run an inpatient IP consult service, and more recently, she helped establish their inpatient pleural disease service and a bronchoscopic lung volume reduction program.
For the inpatient IP service, after several months of side-by-side training with an IP fellow and attending physicians, she began independently evaluating new patients, writing notes, and making recommendations.
For patients with pleural disease, she performs ultrasound examinations, chest tube insertions, and bedside thoracentesis independently. And for the bronchoscopic lung volume reduction program, she evaluates patients for candidate status, participates in valve placement, and sees patients independently through a year of follow-up.
“Physician colleagues often aren’t sure what an APP’s education and scope of practice is,” said Ms. Priebe, who was an ICU nurse before training as an acute care NP and then worked first with a private practice inpatient service and then with the University of Michigan, Ann Arbor, where she established and grew an APP-run program managing lung transplant patients and a step-down ICU unit.
“It’s a matter of knowing [your state’s policies], treating them like a fellow you would train, and then using them to the fullest extent of their education and training. If they’re given an opportunity to learn a subspecialty skill set, they can be an asset to any pulmonary program.”
‘We’re here to support,’ not replace
In her own practice, Ms. Young is one of seven APPs who work with nine physicians on a full range of inpatient care, outpatient care, critical care, sleep medicine, and procedures. Many new patients are seen first by the APP, who does the workup and orders tests, and by the physician on a follow-up visit. Most patients needing routine management of asthma and COPD are seen by the physician every third or fourth visit, she said.
Ms. Young also directs a 24-hour in-house APP service recently established by the practice, and she participates in research. In a practice across town, she noted, APPs see mainly established patients and do not practice as autonomously as the state permits. “Part of that difference may [stem from] the lack of a standard of education and variable amounts of work the practice puts into their APPs.”
The American Medical Association’s #StopScopeCreep social media messaging feels divisive and “sheds a negative light on APPs working in any area,” Ms. Young said. “One of the biggest things we want to convey [at APAPP] is that we’re not here for [physicians’] jobs.”
“We’re here to support those who are practicing, to support underserved populations, and to help bridge gaps” created by an aging pulmonologist workforce and real and projected physician shortages, Ms. Young said, referring to a 2016 report from the Health Resources and Services Administration and a 2017 report from Merritt Hawkins indicating that 73% of U.S. pulmonologists (the largest percentage of all subspecialties) were at least 55 years old.
Dr. Hussain said he has “seen scope creep” first-hand in his hospitals, in the form of noncollaborative practices and tasks performed by APPs without adequate training – most likely often stemming from poor decisions and oversight by physicians. But when constructed thoughtfully, APP-physician teams are “serving great needs” in many types of care, he said, from follow-up care and management of chronic conditions to inpatient rounding. “My [colleagues] are having great success,” he said.
He is watching with interest – and some concern – pending reimbursement changes from the Centers for Medicare & Medicaid Services that will make time the only defining feature of the “substantive” portion of a split/shared visit involving physicians and APPs in a facility setting. Medical decision-making will no longer be applicable.
For time-based services like critical care, time alone is currently the metric. (And in the nonfacility setting, physician-APP teams may still apply “incident to” billing practices). But in the facility setting, said Amy M. Ahasic, MD, MPH, a pulmonologist in Norwalk, Conn., who coauthored a 2022 commentary on the issue, the change (now planned for 2024) could be problematic for employed physicians whose contracts are based on productivity, and could create tension and possibly lead to reduced use of APPs rather than supporting collaborative care.
“The team model has been evolving so well over the past 10-15 years,” said Dr. Ahasic, who serves on the CHEST Health Policy and Advocacy Reimbursement Workgroup and cochairs the CHEST/American Thoracic Society clinical practice committee with Dr. Hussain. “It’s good for patient safety to have more [providers] involved ... and because APP salaries are lower health systems could do it and be able to have better care and better coverage.”
The pulmonology culture, said Dr. Hussain, has been increasingly embracing APPs and “it’s collegial.” Pulmonologists are “coming to CHEST meetings with their APPs. They’re learning the same things we’re learning, to manage the same patients we manage.”
The article sources reported that they had no relevant financial conflicts of interest to disclose.
Breaking the itch-scratch cycle with mindfulness
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
FROM IDS 2022
An integrative approach to atopic dermatitis features a long list of options
to probiotics and acupressure – that he encourages patients to try as they use the big guns, or as they attempt to wean off of them or avoid their use altogether.
During a presentation at the annual Integrative Dermatology Symposium, Dr. Lio said that he uses “5 pillars” to guide his integrative treatment plans: The skin barrier, the psyche, the microbiome, inflammation, and itch. “I try to flag approaches that predominantly address the categories that I think need the most help,” he said. “And I tell patients [which pillar or pillars] each treatment is addressing.”
Most commonly, the greatest challenge with AD – and the “single biggest weakness of conventional Western medicine” – lies not with getting patients clear in the first place, but in keeping them clear safely, he said. “I don’t think that using immunosuppressive [medications] is okay for the long-term unless there is no other choice,” said Dr. Lio, who cofounded the Chicago Integrative Eczema Center about 6 years ago and is clinical assistant professor of dermatology and pediatrics at Northwestern University, Chicago. Oftentimes, he said, complementary approaches, including dietary changes, can also serve as supportive adjunctive therapy to biologics and JAK inhibitors.
He has three main criteria, or “filters,” for evaluating these treatments before recommending them to patients: At least some clinical evidence for efficacy (preferably randomized trials but not necessarily), safety, and practicality. The “only way we’re going to move things forward [for AD and other conditions] is to try out less tested treatments ... to open up to them,” Dr. Lio said in an interview after the meeting. And in doing so, he said, dermatologists “can connect with a lot of patients whom naysayers can’t connect with.”
An integrative menu
Dr. Lio individualizes plans, suggesting treatments after “listening to patients’ stories” and considering their age, history, symptoms and skin presentation, and other factors. He said he “goes little by little,” telling a patient, for instance, “I’d love for us to try adding a little hemp oil to your diet.”
If patients aren’t pleased with or are tired of treatments, he said in the interview, “we move on and try something else.”
At the meeting, he described some of the treatments on his menu and the supporting evidence for those treatments:
Oral hempseed oil. A randomized crossover study of 20 adult patients with AD found that daily consumption of 2 tablespoons of hempseed oil decreased skin dryness, itchiness, and use of topical medications compared with consumption of olive oil. “It was statistically significant and seemed clinically meaningful,” likely resulting from the high concentration of polyunsaturated fatty acids in the oil, Dr. Lio said.
Topical vitamin B12. In a phase 3 randomized controlled trial of topical B12 applied twice a day for 8 weeks, patients experienced significant improvements in the extent and severity of AD compared with placebo. Another study in children with AD aged 6 months to 18 years found significant improvement in as early as 2 weeks of use. “It really does help, and is very gentle in babies,” Dr. Lio said.
Black tea compresses. “It’s absolutely my favorite kind of compress,” he said. “It was studied on the face and eyelids but I use it all over the body for adults and kids.” A German study of 22 patients with AD or contact facial dermatitis showed significant improvements in facial dermatitis within the first 3 days of treatment with application of black tea dressings plus an emollient cream, with significant reductions in four disease activity scores (the Facial Eczema Area and Severity Index, visual analog scale for pruritus, Investigator’s Global Assessment score, and Patient’s Self-Assessment Score) that continued through day 6.
Oolong tea. In a 2001 study, after 1 month of drinking oolong tea after each meal, 64% of patients with recalcitrant AD who continued with their regular treatment showed marked to moderate improvements in AD, with a beneficial effect first noticed after 1-2 weeks. At 6 months, 54% still had a good response to treatment. “It’s super cheap and accessible,” Dr. Lio said.
Coconut oil. One of the greatest benefits of coconut oil is on the microbiome and the dysbiosis that can result from a disrupted, or “leaky,” skin barrier – especially overgrowth of Staphylococcus aureus, which “drives AD,” Dr. Lio said. In a study of adults with AD from the Philippines, topically applied coconut oil decreased S. aureus colonization by 95% when applied twice daily for 4 weeks, compared with a 50% decrease in an olive oil control group. Other research has shown coconut oil to be superior to mineral oil as a moisturizer, he said at the meeting.
Acupressure. After a pilot study conducted by Dr. Lio and colleagues showed greater decreases in itch (per the visual analogue scale) in adults with AD who applied an acupressure bead at the LI11 point (near the elbow) for 3 minutes three times a week for 4 weeks, than among those who did not use the acupressure tool, Dr. Lio began trying it with some of his patients. “Now I use it broadly,” he added in the interview. “Kids over 10 can figure out how to use it and teenagers love it [to relief itch]. Some don’t use the beads anymore, they just use their fingertips.”
Advice on diet, vitamin D, and probiotics
AD severity is “powerfully” correlated with IgE food allergy, but Dr. Lio said at the meeting that he currently takes a cautious approach toward strict elimination diets.
There is a growing school of thought among allergists, he said, that positive IgE tests without evidence of acute reactions may not indicate true allergy, but rather sensitivity – and may not warrant food eliminations. And as has been shown with peanuts, there can be a serious downside to elimination, as food avoidance can lead to serious allergy later on, he said.
“More and more people are thinking that if you can tolerate [a food], continue it,” he added in the interview. In the absence of clear reactions, the only way to really know if a food is making eczema worse is to do a double-blind, placebo-controlled food challenge test, he noted.
Patients often come to see him believing that food is the “root cause” of their eczema and feeling frustrated, even anxious, about strict dietary restrictions they’ve implemented. But for many of these patients, the right question “would be to ask, why is my eczema causing my food allergy?” he said at the meeting, referring to the epithelial barrier hypothesis, which posits that skin barrier dysfunction can lead to asthma, allergic rhinitis, and food allergy.
Dr. Lio often recommends the Autoimmune Protocol (AIP) diet, a “close cousin” of the paleo diet for patients with AD, as general guidance to be followed “holistically” and often without the strict eliminations it prescribes. Minimizing processed foods and dairy and grains, which “can be inflammatory in some people,” and focusing on whole, nutrient-rich foods – all in keeping with the AIP principles – should have positive effects on the microbiome, overall health, and likely AD as well, he said.
Across the board, Dr. Lio recommends vitamin D (at nationally recommended dosages) and probiotics. Vitamin D has been shown to significantly help a small percentage of patients with eczema, he said, so he advises patients that it’s worth a trial. “I tell patients that I don’t know how to pick that small group out, so let’s try for a few months and see,” he said. “Inevitably, a percentage of patients come back and say it makes a huge difference.”
Dr. Lio’s understanding and use of probiotics has been “dynamic” over the years. “The “best, most reliable evidence” that probiotics can improve AD symptoms comes with the use of multiple probiotic strains together, he said. Based on limited but growing literature, he ensures that recommended formulations for babies include Lactobacillus rhamnosus, and that formulations for adults include Lactobacillus salivarius.
Dr. Lio works closely with dietitians, hypnotherapists, and psychologists – and will occasionally refer interested patients with AD to a Chinese medicine practitioner who personalizes the use of herbal formulations.
He reported no relevant disclosures.
to probiotics and acupressure – that he encourages patients to try as they use the big guns, or as they attempt to wean off of them or avoid their use altogether.
During a presentation at the annual Integrative Dermatology Symposium, Dr. Lio said that he uses “5 pillars” to guide his integrative treatment plans: The skin barrier, the psyche, the microbiome, inflammation, and itch. “I try to flag approaches that predominantly address the categories that I think need the most help,” he said. “And I tell patients [which pillar or pillars] each treatment is addressing.”
Most commonly, the greatest challenge with AD – and the “single biggest weakness of conventional Western medicine” – lies not with getting patients clear in the first place, but in keeping them clear safely, he said. “I don’t think that using immunosuppressive [medications] is okay for the long-term unless there is no other choice,” said Dr. Lio, who cofounded the Chicago Integrative Eczema Center about 6 years ago and is clinical assistant professor of dermatology and pediatrics at Northwestern University, Chicago. Oftentimes, he said, complementary approaches, including dietary changes, can also serve as supportive adjunctive therapy to biologics and JAK inhibitors.
He has three main criteria, or “filters,” for evaluating these treatments before recommending them to patients: At least some clinical evidence for efficacy (preferably randomized trials but not necessarily), safety, and practicality. The “only way we’re going to move things forward [for AD and other conditions] is to try out less tested treatments ... to open up to them,” Dr. Lio said in an interview after the meeting. And in doing so, he said, dermatologists “can connect with a lot of patients whom naysayers can’t connect with.”
An integrative menu
Dr. Lio individualizes plans, suggesting treatments after “listening to patients’ stories” and considering their age, history, symptoms and skin presentation, and other factors. He said he “goes little by little,” telling a patient, for instance, “I’d love for us to try adding a little hemp oil to your diet.”
If patients aren’t pleased with or are tired of treatments, he said in the interview, “we move on and try something else.”
At the meeting, he described some of the treatments on his menu and the supporting evidence for those treatments:
Oral hempseed oil. A randomized crossover study of 20 adult patients with AD found that daily consumption of 2 tablespoons of hempseed oil decreased skin dryness, itchiness, and use of topical medications compared with consumption of olive oil. “It was statistically significant and seemed clinically meaningful,” likely resulting from the high concentration of polyunsaturated fatty acids in the oil, Dr. Lio said.
Topical vitamin B12. In a phase 3 randomized controlled trial of topical B12 applied twice a day for 8 weeks, patients experienced significant improvements in the extent and severity of AD compared with placebo. Another study in children with AD aged 6 months to 18 years found significant improvement in as early as 2 weeks of use. “It really does help, and is very gentle in babies,” Dr. Lio said.
Black tea compresses. “It’s absolutely my favorite kind of compress,” he said. “It was studied on the face and eyelids but I use it all over the body for adults and kids.” A German study of 22 patients with AD or contact facial dermatitis showed significant improvements in facial dermatitis within the first 3 days of treatment with application of black tea dressings plus an emollient cream, with significant reductions in four disease activity scores (the Facial Eczema Area and Severity Index, visual analog scale for pruritus, Investigator’s Global Assessment score, and Patient’s Self-Assessment Score) that continued through day 6.
Oolong tea. In a 2001 study, after 1 month of drinking oolong tea after each meal, 64% of patients with recalcitrant AD who continued with their regular treatment showed marked to moderate improvements in AD, with a beneficial effect first noticed after 1-2 weeks. At 6 months, 54% still had a good response to treatment. “It’s super cheap and accessible,” Dr. Lio said.
Coconut oil. One of the greatest benefits of coconut oil is on the microbiome and the dysbiosis that can result from a disrupted, or “leaky,” skin barrier – especially overgrowth of Staphylococcus aureus, which “drives AD,” Dr. Lio said. In a study of adults with AD from the Philippines, topically applied coconut oil decreased S. aureus colonization by 95% when applied twice daily for 4 weeks, compared with a 50% decrease in an olive oil control group. Other research has shown coconut oil to be superior to mineral oil as a moisturizer, he said at the meeting.
Acupressure. After a pilot study conducted by Dr. Lio and colleagues showed greater decreases in itch (per the visual analogue scale) in adults with AD who applied an acupressure bead at the LI11 point (near the elbow) for 3 minutes three times a week for 4 weeks, than among those who did not use the acupressure tool, Dr. Lio began trying it with some of his patients. “Now I use it broadly,” he added in the interview. “Kids over 10 can figure out how to use it and teenagers love it [to relief itch]. Some don’t use the beads anymore, they just use their fingertips.”
Advice on diet, vitamin D, and probiotics
AD severity is “powerfully” correlated with IgE food allergy, but Dr. Lio said at the meeting that he currently takes a cautious approach toward strict elimination diets.
There is a growing school of thought among allergists, he said, that positive IgE tests without evidence of acute reactions may not indicate true allergy, but rather sensitivity – and may not warrant food eliminations. And as has been shown with peanuts, there can be a serious downside to elimination, as food avoidance can lead to serious allergy later on, he said.
“More and more people are thinking that if you can tolerate [a food], continue it,” he added in the interview. In the absence of clear reactions, the only way to really know if a food is making eczema worse is to do a double-blind, placebo-controlled food challenge test, he noted.
Patients often come to see him believing that food is the “root cause” of their eczema and feeling frustrated, even anxious, about strict dietary restrictions they’ve implemented. But for many of these patients, the right question “would be to ask, why is my eczema causing my food allergy?” he said at the meeting, referring to the epithelial barrier hypothesis, which posits that skin barrier dysfunction can lead to asthma, allergic rhinitis, and food allergy.
Dr. Lio often recommends the Autoimmune Protocol (AIP) diet, a “close cousin” of the paleo diet for patients with AD, as general guidance to be followed “holistically” and often without the strict eliminations it prescribes. Minimizing processed foods and dairy and grains, which “can be inflammatory in some people,” and focusing on whole, nutrient-rich foods – all in keeping with the AIP principles – should have positive effects on the microbiome, overall health, and likely AD as well, he said.
Across the board, Dr. Lio recommends vitamin D (at nationally recommended dosages) and probiotics. Vitamin D has been shown to significantly help a small percentage of patients with eczema, he said, so he advises patients that it’s worth a trial. “I tell patients that I don’t know how to pick that small group out, so let’s try for a few months and see,” he said. “Inevitably, a percentage of patients come back and say it makes a huge difference.”
Dr. Lio’s understanding and use of probiotics has been “dynamic” over the years. “The “best, most reliable evidence” that probiotics can improve AD symptoms comes with the use of multiple probiotic strains together, he said. Based on limited but growing literature, he ensures that recommended formulations for babies include Lactobacillus rhamnosus, and that formulations for adults include Lactobacillus salivarius.
Dr. Lio works closely with dietitians, hypnotherapists, and psychologists – and will occasionally refer interested patients with AD to a Chinese medicine practitioner who personalizes the use of herbal formulations.
He reported no relevant disclosures.
to probiotics and acupressure – that he encourages patients to try as they use the big guns, or as they attempt to wean off of them or avoid their use altogether.
During a presentation at the annual Integrative Dermatology Symposium, Dr. Lio said that he uses “5 pillars” to guide his integrative treatment plans: The skin barrier, the psyche, the microbiome, inflammation, and itch. “I try to flag approaches that predominantly address the categories that I think need the most help,” he said. “And I tell patients [which pillar or pillars] each treatment is addressing.”
Most commonly, the greatest challenge with AD – and the “single biggest weakness of conventional Western medicine” – lies not with getting patients clear in the first place, but in keeping them clear safely, he said. “I don’t think that using immunosuppressive [medications] is okay for the long-term unless there is no other choice,” said Dr. Lio, who cofounded the Chicago Integrative Eczema Center about 6 years ago and is clinical assistant professor of dermatology and pediatrics at Northwestern University, Chicago. Oftentimes, he said, complementary approaches, including dietary changes, can also serve as supportive adjunctive therapy to biologics and JAK inhibitors.
He has three main criteria, or “filters,” for evaluating these treatments before recommending them to patients: At least some clinical evidence for efficacy (preferably randomized trials but not necessarily), safety, and practicality. The “only way we’re going to move things forward [for AD and other conditions] is to try out less tested treatments ... to open up to them,” Dr. Lio said in an interview after the meeting. And in doing so, he said, dermatologists “can connect with a lot of patients whom naysayers can’t connect with.”
An integrative menu
Dr. Lio individualizes plans, suggesting treatments after “listening to patients’ stories” and considering their age, history, symptoms and skin presentation, and other factors. He said he “goes little by little,” telling a patient, for instance, “I’d love for us to try adding a little hemp oil to your diet.”
If patients aren’t pleased with or are tired of treatments, he said in the interview, “we move on and try something else.”
At the meeting, he described some of the treatments on his menu and the supporting evidence for those treatments:
Oral hempseed oil. A randomized crossover study of 20 adult patients with AD found that daily consumption of 2 tablespoons of hempseed oil decreased skin dryness, itchiness, and use of topical medications compared with consumption of olive oil. “It was statistically significant and seemed clinically meaningful,” likely resulting from the high concentration of polyunsaturated fatty acids in the oil, Dr. Lio said.
Topical vitamin B12. In a phase 3 randomized controlled trial of topical B12 applied twice a day for 8 weeks, patients experienced significant improvements in the extent and severity of AD compared with placebo. Another study in children with AD aged 6 months to 18 years found significant improvement in as early as 2 weeks of use. “It really does help, and is very gentle in babies,” Dr. Lio said.
Black tea compresses. “It’s absolutely my favorite kind of compress,” he said. “It was studied on the face and eyelids but I use it all over the body for adults and kids.” A German study of 22 patients with AD or contact facial dermatitis showed significant improvements in facial dermatitis within the first 3 days of treatment with application of black tea dressings plus an emollient cream, with significant reductions in four disease activity scores (the Facial Eczema Area and Severity Index, visual analog scale for pruritus, Investigator’s Global Assessment score, and Patient’s Self-Assessment Score) that continued through day 6.
Oolong tea. In a 2001 study, after 1 month of drinking oolong tea after each meal, 64% of patients with recalcitrant AD who continued with their regular treatment showed marked to moderate improvements in AD, with a beneficial effect first noticed after 1-2 weeks. At 6 months, 54% still had a good response to treatment. “It’s super cheap and accessible,” Dr. Lio said.
Coconut oil. One of the greatest benefits of coconut oil is on the microbiome and the dysbiosis that can result from a disrupted, or “leaky,” skin barrier – especially overgrowth of Staphylococcus aureus, which “drives AD,” Dr. Lio said. In a study of adults with AD from the Philippines, topically applied coconut oil decreased S. aureus colonization by 95% when applied twice daily for 4 weeks, compared with a 50% decrease in an olive oil control group. Other research has shown coconut oil to be superior to mineral oil as a moisturizer, he said at the meeting.
Acupressure. After a pilot study conducted by Dr. Lio and colleagues showed greater decreases in itch (per the visual analogue scale) in adults with AD who applied an acupressure bead at the LI11 point (near the elbow) for 3 minutes three times a week for 4 weeks, than among those who did not use the acupressure tool, Dr. Lio began trying it with some of his patients. “Now I use it broadly,” he added in the interview. “Kids over 10 can figure out how to use it and teenagers love it [to relief itch]. Some don’t use the beads anymore, they just use their fingertips.”
Advice on diet, vitamin D, and probiotics
AD severity is “powerfully” correlated with IgE food allergy, but Dr. Lio said at the meeting that he currently takes a cautious approach toward strict elimination diets.
There is a growing school of thought among allergists, he said, that positive IgE tests without evidence of acute reactions may not indicate true allergy, but rather sensitivity – and may not warrant food eliminations. And as has been shown with peanuts, there can be a serious downside to elimination, as food avoidance can lead to serious allergy later on, he said.
“More and more people are thinking that if you can tolerate [a food], continue it,” he added in the interview. In the absence of clear reactions, the only way to really know if a food is making eczema worse is to do a double-blind, placebo-controlled food challenge test, he noted.
Patients often come to see him believing that food is the “root cause” of their eczema and feeling frustrated, even anxious, about strict dietary restrictions they’ve implemented. But for many of these patients, the right question “would be to ask, why is my eczema causing my food allergy?” he said at the meeting, referring to the epithelial barrier hypothesis, which posits that skin barrier dysfunction can lead to asthma, allergic rhinitis, and food allergy.
Dr. Lio often recommends the Autoimmune Protocol (AIP) diet, a “close cousin” of the paleo diet for patients with AD, as general guidance to be followed “holistically” and often without the strict eliminations it prescribes. Minimizing processed foods and dairy and grains, which “can be inflammatory in some people,” and focusing on whole, nutrient-rich foods – all in keeping with the AIP principles – should have positive effects on the microbiome, overall health, and likely AD as well, he said.
Across the board, Dr. Lio recommends vitamin D (at nationally recommended dosages) and probiotics. Vitamin D has been shown to significantly help a small percentage of patients with eczema, he said, so he advises patients that it’s worth a trial. “I tell patients that I don’t know how to pick that small group out, so let’s try for a few months and see,” he said. “Inevitably, a percentage of patients come back and say it makes a huge difference.”
Dr. Lio’s understanding and use of probiotics has been “dynamic” over the years. “The “best, most reliable evidence” that probiotics can improve AD symptoms comes with the use of multiple probiotic strains together, he said. Based on limited but growing literature, he ensures that recommended formulations for babies include Lactobacillus rhamnosus, and that formulations for adults include Lactobacillus salivarius.
Dr. Lio works closely with dietitians, hypnotherapists, and psychologists – and will occasionally refer interested patients with AD to a Chinese medicine practitioner who personalizes the use of herbal formulations.
He reported no relevant disclosures.
FROM IDS 2022
Climate change: Commentary in four dermatology journals calls for emergency action
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.