User login
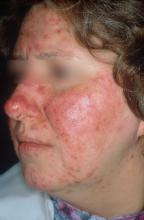
National Rosacea Society designates April as “Rosacea Awareness Month”
April has been designated “Rosacea Awareness Month” by the National Rosacea Society (NRS), with the aim of educating the public about the disease.
“During April and throughout the year, people who suspect they may have rosacea can contact the NRS for more information,” according to a press release issued April 2 by the NRS.
The press release refers to the new standard diagnostic guidelines for rosacea, developed by the National Rosacea Society Expert Committee, which were released online in 2017 (J Am Acad Dermatol. 2018 Jan;78[1]:148-55).
More information for patients is available on the NRS website, at www.rosacea.org, via email at rosaceas@aol.com, or by calling 888-NO-BLUSH (662-5874).
April has been designated “Rosacea Awareness Month” by the National Rosacea Society (NRS), with the aim of educating the public about the disease.
“During April and throughout the year, people who suspect they may have rosacea can contact the NRS for more information,” according to a press release issued April 2 by the NRS.
The press release refers to the new standard diagnostic guidelines for rosacea, developed by the National Rosacea Society Expert Committee, which were released online in 2017 (J Am Acad Dermatol. 2018 Jan;78[1]:148-55).
More information for patients is available on the NRS website, at www.rosacea.org, via email at rosaceas@aol.com, or by calling 888-NO-BLUSH (662-5874).
April has been designated “Rosacea Awareness Month” by the National Rosacea Society (NRS), with the aim of educating the public about the disease.
“During April and throughout the year, people who suspect they may have rosacea can contact the NRS for more information,” according to a press release issued April 2 by the NRS.
The press release refers to the new standard diagnostic guidelines for rosacea, developed by the National Rosacea Society Expert Committee, which were released online in 2017 (J Am Acad Dermatol. 2018 Jan;78[1]:148-55).
More information for patients is available on the NRS website, at www.rosacea.org, via email at rosaceas@aol.com, or by calling 888-NO-BLUSH (662-5874).
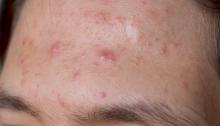
Review finds some evidence of efficacy for nonpharmacological acne therapies
A systematic review of 33 studies evaluating nonpharmacological treatments for acne provided what was described as “circumstantial” evidence of efficacy by the authors who conducted the analysis.
The 33 studies evaluated three types of treatments: laser-based and light-based treatments (20), chemical peels (11), and fractional microneedling radiofrequency (2); most were associated with significant reductions in acne lesions in the studies. The evidence for efficacy was “strong” for glycolic acid at concentrations from 10% to 40%, and “moderate” for amino fruit acids at concentrations of 20%-60%, for intense pulsed light (IPL: 400-700 and 870-1,200 nm), and for the diode laser 1450 nm, according to F.M.C. de Vries, MD, of the department of dermatology, Radboud University, Nijmegen, the Netherlands, and coauthors.
However, they added, “although a high rate of statistically significant results was found in most of the studies, indicating efficacy of nonpharmacological therapies, the low methodological quality of the included studies made it difficult to draw clear conclusions.” Most of the studies were limited by factors that included a small number of enrolled participants, short follow-up, and lack of or possibly inadequate blinding in participants and/or clinicians.
Their review of three electronic databases (MEDLINE, Cochrane library, CINAHL) identified the 33 studies evaluating these treatments in 1,404 participants with acne, published between January 2000 and May 2017, which met their inclusion criteria.
Most of the studies on laser- and light-based treatments found a “significant reduction in acne lesions,” including eight that had a control group. However, with two exceptions, “suboptimal methodologic quality of the majority of these studies resulted in limited evidence of efficacy,” they added. The exceptions were two studies that were the basis of their “moderate” rating for IPL and the diode laser: a randomized study of IPL (40-700 and 870-1,200 nm, 100 ms, 20 J/cm2, 20 ms, 18 J/cm2), which found that treatment with IPL resulted in a significant reduction in papules, pustules, and comedones, compared with controls; and a randomized controlled study that found that treatment with a diode laser (1,450 nm, 9.5-11.0 J/cm2, 29-30 ms) resulted in reductions in inflammatory acne that were statistically significant.
The agents studied in the 11 chemical peel trials included salicylic acid, glycolic acid, Jessner solution, trichloroacetic acid, mandelic acid, amino fruit acid, and lipohydroxy acids. “Strong evidence of efficacy” was evident in two studies of glycolic acid (10% and 40% concentrations), which were double-blind, randomized, placebo-controlled studies that were of “high methodological quality,” the authors wrote.
Both studies of fractional microneedling radiofrequency, which were split-face randomized controlled studies, found statistically significant effects of treatment on inflammatory and noninflammatory acne in one study, and a “substantial” reduction in papules and pustules, compared with baseline in the other study. But this evidence was considered limited, because of the “suboptimal methodological quality” of both studies, they noted.
Erythema, pain (described as “tolerable”), purpura, edema, and hyperpigmentation were among the most common adverse effects associated with the nonpharmacological treatments and were described as mild and transient in most cases.
The review provided “circumstantial evidence for nonpharmacological therapies in the treatment of acne vulgaris” and “has created order and structure in resulting outcomes in which a first step towards future research is generated,” the authors concluded. “The large amount of studies performed in the area of acne treatment and the frequent application of these therapies in daily practice indicates a great interest in this topic and the urgent demand for effective nonpharmacological treatment options for acne in addition to the use of conventional therapies,” they added.
The authors had no disclosures. Of the six authors, Dr. de Vries and three other authors are affiliated with HU University of Applied Sciences, Utrecht, the Netherlands, which funded the study.
emechcatie@frontlinemedcom.com
SOURCE: de Vries FMC et al. J Eur Acad Dermatol Venereol. 2018 Feb 14. doi: 10.1111/jdv.14881.
A systematic review of 33 studies evaluating nonpharmacological treatments for acne provided what was described as “circumstantial” evidence of efficacy by the authors who conducted the analysis.
The 33 studies evaluated three types of treatments: laser-based and light-based treatments (20), chemical peels (11), and fractional microneedling radiofrequency (2); most were associated with significant reductions in acne lesions in the studies. The evidence for efficacy was “strong” for glycolic acid at concentrations from 10% to 40%, and “moderate” for amino fruit acids at concentrations of 20%-60%, for intense pulsed light (IPL: 400-700 and 870-1,200 nm), and for the diode laser 1450 nm, according to F.M.C. de Vries, MD, of the department of dermatology, Radboud University, Nijmegen, the Netherlands, and coauthors.
However, they added, “although a high rate of statistically significant results was found in most of the studies, indicating efficacy of nonpharmacological therapies, the low methodological quality of the included studies made it difficult to draw clear conclusions.” Most of the studies were limited by factors that included a small number of enrolled participants, short follow-up, and lack of or possibly inadequate blinding in participants and/or clinicians.
Their review of three electronic databases (MEDLINE, Cochrane library, CINAHL) identified the 33 studies evaluating these treatments in 1,404 participants with acne, published between January 2000 and May 2017, which met their inclusion criteria.
Most of the studies on laser- and light-based treatments found a “significant reduction in acne lesions,” including eight that had a control group. However, with two exceptions, “suboptimal methodologic quality of the majority of these studies resulted in limited evidence of efficacy,” they added. The exceptions were two studies that were the basis of their “moderate” rating for IPL and the diode laser: a randomized study of IPL (40-700 and 870-1,200 nm, 100 ms, 20 J/cm2, 20 ms, 18 J/cm2), which found that treatment with IPL resulted in a significant reduction in papules, pustules, and comedones, compared with controls; and a randomized controlled study that found that treatment with a diode laser (1,450 nm, 9.5-11.0 J/cm2, 29-30 ms) resulted in reductions in inflammatory acne that were statistically significant.
The agents studied in the 11 chemical peel trials included salicylic acid, glycolic acid, Jessner solution, trichloroacetic acid, mandelic acid, amino fruit acid, and lipohydroxy acids. “Strong evidence of efficacy” was evident in two studies of glycolic acid (10% and 40% concentrations), which were double-blind, randomized, placebo-controlled studies that were of “high methodological quality,” the authors wrote.
Both studies of fractional microneedling radiofrequency, which were split-face randomized controlled studies, found statistically significant effects of treatment on inflammatory and noninflammatory acne in one study, and a “substantial” reduction in papules and pustules, compared with baseline in the other study. But this evidence was considered limited, because of the “suboptimal methodological quality” of both studies, they noted.
Erythema, pain (described as “tolerable”), purpura, edema, and hyperpigmentation were among the most common adverse effects associated with the nonpharmacological treatments and were described as mild and transient in most cases.
The review provided “circumstantial evidence for nonpharmacological therapies in the treatment of acne vulgaris” and “has created order and structure in resulting outcomes in which a first step towards future research is generated,” the authors concluded. “The large amount of studies performed in the area of acne treatment and the frequent application of these therapies in daily practice indicates a great interest in this topic and the urgent demand for effective nonpharmacological treatment options for acne in addition to the use of conventional therapies,” they added.
The authors had no disclosures. Of the six authors, Dr. de Vries and three other authors are affiliated with HU University of Applied Sciences, Utrecht, the Netherlands, which funded the study.
emechcatie@frontlinemedcom.com
SOURCE: de Vries FMC et al. J Eur Acad Dermatol Venereol. 2018 Feb 14. doi: 10.1111/jdv.14881.
A systematic review of 33 studies evaluating nonpharmacological treatments for acne provided what was described as “circumstantial” evidence of efficacy by the authors who conducted the analysis.
The 33 studies evaluated three types of treatments: laser-based and light-based treatments (20), chemical peels (11), and fractional microneedling radiofrequency (2); most were associated with significant reductions in acne lesions in the studies. The evidence for efficacy was “strong” for glycolic acid at concentrations from 10% to 40%, and “moderate” for amino fruit acids at concentrations of 20%-60%, for intense pulsed light (IPL: 400-700 and 870-1,200 nm), and for the diode laser 1450 nm, according to F.M.C. de Vries, MD, of the department of dermatology, Radboud University, Nijmegen, the Netherlands, and coauthors.
However, they added, “although a high rate of statistically significant results was found in most of the studies, indicating efficacy of nonpharmacological therapies, the low methodological quality of the included studies made it difficult to draw clear conclusions.” Most of the studies were limited by factors that included a small number of enrolled participants, short follow-up, and lack of or possibly inadequate blinding in participants and/or clinicians.
Their review of three electronic databases (MEDLINE, Cochrane library, CINAHL) identified the 33 studies evaluating these treatments in 1,404 participants with acne, published between January 2000 and May 2017, which met their inclusion criteria.
Most of the studies on laser- and light-based treatments found a “significant reduction in acne lesions,” including eight that had a control group. However, with two exceptions, “suboptimal methodologic quality of the majority of these studies resulted in limited evidence of efficacy,” they added. The exceptions were two studies that were the basis of their “moderate” rating for IPL and the diode laser: a randomized study of IPL (40-700 and 870-1,200 nm, 100 ms, 20 J/cm2, 20 ms, 18 J/cm2), which found that treatment with IPL resulted in a significant reduction in papules, pustules, and comedones, compared with controls; and a randomized controlled study that found that treatment with a diode laser (1,450 nm, 9.5-11.0 J/cm2, 29-30 ms) resulted in reductions in inflammatory acne that were statistically significant.
The agents studied in the 11 chemical peel trials included salicylic acid, glycolic acid, Jessner solution, trichloroacetic acid, mandelic acid, amino fruit acid, and lipohydroxy acids. “Strong evidence of efficacy” was evident in two studies of glycolic acid (10% and 40% concentrations), which were double-blind, randomized, placebo-controlled studies that were of “high methodological quality,” the authors wrote.
Both studies of fractional microneedling radiofrequency, which were split-face randomized controlled studies, found statistically significant effects of treatment on inflammatory and noninflammatory acne in one study, and a “substantial” reduction in papules and pustules, compared with baseline in the other study. But this evidence was considered limited, because of the “suboptimal methodological quality” of both studies, they noted.
Erythema, pain (described as “tolerable”), purpura, edema, and hyperpigmentation were among the most common adverse effects associated with the nonpharmacological treatments and were described as mild and transient in most cases.
The review provided “circumstantial evidence for nonpharmacological therapies in the treatment of acne vulgaris” and “has created order and structure in resulting outcomes in which a first step towards future research is generated,” the authors concluded. “The large amount of studies performed in the area of acne treatment and the frequent application of these therapies in daily practice indicates a great interest in this topic and the urgent demand for effective nonpharmacological treatment options for acne in addition to the use of conventional therapies,” they added.
The authors had no disclosures. Of the six authors, Dr. de Vries and three other authors are affiliated with HU University of Applied Sciences, Utrecht, the Netherlands, which funded the study.
emechcatie@frontlinemedcom.com
SOURCE: de Vries FMC et al. J Eur Acad Dermatol Venereol. 2018 Feb 14. doi: 10.1111/jdv.14881.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: Certain nonpharmacological acne treatments are effective but better data are needed.
Major finding: There is moderate to strong evidence of efficacy for glycolic acid, IPL, and the diode laser in treating acne.
Study details: A systematic review of 33 studies evaluating nonpharmacological treatments in 1,404 participants with acne.
Disclosures: The authors had no disclosures; three of the six authors are affiliated with HU University of Applied Sciences, Utrecht, which funded the study.
Source: de Vries FMC et al. J Eur Acad Dermatol Venereol. 2018 Feb 14. doi: 10.1111/jdv.14881.
VIDEO: Device-based therapy for onychomycosis
REPORTING FROM AAD 18
SAN DIEGO – which has been studied in two clinical trials and case series, Shari Lipner, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she presented on this topic.
“Something that we’re looking at is plasma treatment of onychomycosis basically using ionized gas,” which has been shown to inhibit the growth of Trichophyton rubrum in vitro, added Dr. Lipner of the department of dermatology, Cornell University, New York.
In a pilot study of 19 patients with onychomycosis, she and her associates found that the clinical cure with nonthermal plasma was about 50% and the mycological cure rate was 15%, “and we’re now trying to improve efficacy using this device,” she said (Clin Exp Dermatol. 2017 Apr;42[3]:295-8). With a dielectric insulator, “nonthermal plasma is created by short pulses (about 10 ns) of strong (about 20 kV/mm peak) electric field that ionizes air molecules, creating ions and electrons, as well as ozone, hydroxyl radicals and nitric oxide,” according to the description in the study.
Other device-based therapies include iontophoresis, using electrical currents to increase drug delivery, and creating small punch biopsies or using a device to create “microholes” in the nails to increase delivery of topical medication across the nail, Dr. Lipner said.
Patients often ask about another device-based treatment, laser therapy, which she pointed out is not approved by the Food and Drug Administration for cure, but for a temporary increase in clear nail in patients with onychomycosis, “very different” than the criteria used for topical and systemic medications, making it difficult to compare efficacy data between lasers and medications, she noted.
Dr. Lipner reported receiving grants/research funding from MOE Medical Devices.
REPORTING FROM AAD 18
SAN DIEGO – which has been studied in two clinical trials and case series, Shari Lipner, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she presented on this topic.
“Something that we’re looking at is plasma treatment of onychomycosis basically using ionized gas,” which has been shown to inhibit the growth of Trichophyton rubrum in vitro, added Dr. Lipner of the department of dermatology, Cornell University, New York.
In a pilot study of 19 patients with onychomycosis, she and her associates found that the clinical cure with nonthermal plasma was about 50% and the mycological cure rate was 15%, “and we’re now trying to improve efficacy using this device,” she said (Clin Exp Dermatol. 2017 Apr;42[3]:295-8). With a dielectric insulator, “nonthermal plasma is created by short pulses (about 10 ns) of strong (about 20 kV/mm peak) electric field that ionizes air molecules, creating ions and electrons, as well as ozone, hydroxyl radicals and nitric oxide,” according to the description in the study.
Other device-based therapies include iontophoresis, using electrical currents to increase drug delivery, and creating small punch biopsies or using a device to create “microholes” in the nails to increase delivery of topical medication across the nail, Dr. Lipner said.
Patients often ask about another device-based treatment, laser therapy, which she pointed out is not approved by the Food and Drug Administration for cure, but for a temporary increase in clear nail in patients with onychomycosis, “very different” than the criteria used for topical and systemic medications, making it difficult to compare efficacy data between lasers and medications, she noted.
Dr. Lipner reported receiving grants/research funding from MOE Medical Devices.
REPORTING FROM AAD 18
SAN DIEGO – which has been studied in two clinical trials and case series, Shari Lipner, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she presented on this topic.
“Something that we’re looking at is plasma treatment of onychomycosis basically using ionized gas,” which has been shown to inhibit the growth of Trichophyton rubrum in vitro, added Dr. Lipner of the department of dermatology, Cornell University, New York.
In a pilot study of 19 patients with onychomycosis, she and her associates found that the clinical cure with nonthermal plasma was about 50% and the mycological cure rate was 15%, “and we’re now trying to improve efficacy using this device,” she said (Clin Exp Dermatol. 2017 Apr;42[3]:295-8). With a dielectric insulator, “nonthermal plasma is created by short pulses (about 10 ns) of strong (about 20 kV/mm peak) electric field that ionizes air molecules, creating ions and electrons, as well as ozone, hydroxyl radicals and nitric oxide,” according to the description in the study.
Other device-based therapies include iontophoresis, using electrical currents to increase drug delivery, and creating small punch biopsies or using a device to create “microholes” in the nails to increase delivery of topical medication across the nail, Dr. Lipner said.
Patients often ask about another device-based treatment, laser therapy, which she pointed out is not approved by the Food and Drug Administration for cure, but for a temporary increase in clear nail in patients with onychomycosis, “very different” than the criteria used for topical and systemic medications, making it difficult to compare efficacy data between lasers and medications, she noted.
Dr. Lipner reported receiving grants/research funding from MOE Medical Devices.
VIDEO: Cannabinoids in dermatology
SAN DIEGO – To date, most of the research on cannabinoids has been outside of dermatology, but these agents may eventually play an important role in the treatment of dermatologic diseases, according to Adam Friedman, MD, director of translational research, department of dermatology, at George Washington University, Washington.
for diseases like dermatomyositis, scleroderma, and lupus, Dr. Friedman said in a video interview at the annual meeting of the American Academy of Dermatology.
In this area, most progress has been made with a synthetic cannabinoid, ajulemic acid (also known as anabasum), which is designed to go after CB2 cannabinoid receptors, which have the anti-inflammatory effects, and not the CB1 receptors, which have the psychoactive effects, he explained. Results of phase 2 studies of ajulemic acid in dermatomyositis and systemic sclerosis have been “very promising,” he noted.
In collaboration with Albert Einstein College of Medicine, New York, he and his associates have studied the topical application of an endocannabinoid, anandamide (AEA), in nanoparticles in an animal model of cutaneous lupus. “We found that we can actually reverse the very classic, almost chronic cutaneous-like symptoms that we see in these animals if they go untreated,” he said.
In the interview, Dr. Friedman, who spoke about the potential of cannabinoids for the treatment of inflammatory and neoplastic diseases of the skin at the meeting, said that it is actually surprising that most research with cannabinoids to date has been outside of dermatology, “because our skin is chock full of cannabinoids; chock full of expression of cannabinoid receptors.”
Dr. Friedman disclosed that he has invented the nanotechnology licensed to Zylo Therapeutics. He is a member of the Dermatology News advisory board.
SAN DIEGO – To date, most of the research on cannabinoids has been outside of dermatology, but these agents may eventually play an important role in the treatment of dermatologic diseases, according to Adam Friedman, MD, director of translational research, department of dermatology, at George Washington University, Washington.
for diseases like dermatomyositis, scleroderma, and lupus, Dr. Friedman said in a video interview at the annual meeting of the American Academy of Dermatology.
In this area, most progress has been made with a synthetic cannabinoid, ajulemic acid (also known as anabasum), which is designed to go after CB2 cannabinoid receptors, which have the anti-inflammatory effects, and not the CB1 receptors, which have the psychoactive effects, he explained. Results of phase 2 studies of ajulemic acid in dermatomyositis and systemic sclerosis have been “very promising,” he noted.
In collaboration with Albert Einstein College of Medicine, New York, he and his associates have studied the topical application of an endocannabinoid, anandamide (AEA), in nanoparticles in an animal model of cutaneous lupus. “We found that we can actually reverse the very classic, almost chronic cutaneous-like symptoms that we see in these animals if they go untreated,” he said.
In the interview, Dr. Friedman, who spoke about the potential of cannabinoids for the treatment of inflammatory and neoplastic diseases of the skin at the meeting, said that it is actually surprising that most research with cannabinoids to date has been outside of dermatology, “because our skin is chock full of cannabinoids; chock full of expression of cannabinoid receptors.”
Dr. Friedman disclosed that he has invented the nanotechnology licensed to Zylo Therapeutics. He is a member of the Dermatology News advisory board.
SAN DIEGO – To date, most of the research on cannabinoids has been outside of dermatology, but these agents may eventually play an important role in the treatment of dermatologic diseases, according to Adam Friedman, MD, director of translational research, department of dermatology, at George Washington University, Washington.
for diseases like dermatomyositis, scleroderma, and lupus, Dr. Friedman said in a video interview at the annual meeting of the American Academy of Dermatology.
In this area, most progress has been made with a synthetic cannabinoid, ajulemic acid (also known as anabasum), which is designed to go after CB2 cannabinoid receptors, which have the anti-inflammatory effects, and not the CB1 receptors, which have the psychoactive effects, he explained. Results of phase 2 studies of ajulemic acid in dermatomyositis and systemic sclerosis have been “very promising,” he noted.
In collaboration with Albert Einstein College of Medicine, New York, he and his associates have studied the topical application of an endocannabinoid, anandamide (AEA), in nanoparticles in an animal model of cutaneous lupus. “We found that we can actually reverse the very classic, almost chronic cutaneous-like symptoms that we see in these animals if they go untreated,” he said.
In the interview, Dr. Friedman, who spoke about the potential of cannabinoids for the treatment of inflammatory and neoplastic diseases of the skin at the meeting, said that it is actually surprising that most research with cannabinoids to date has been outside of dermatology, “because our skin is chock full of cannabinoids; chock full of expression of cannabinoid receptors.”
Dr. Friedman disclosed that he has invented the nanotechnology licensed to Zylo Therapeutics. He is a member of the Dermatology News advisory board.
REPORTING FROM AAD 18
VIDEO: Considering systemic disease in dermatology patients
SAN DIEGO – Be mindful of what lies below the skin.
That was the message of Joseph Merola, MD, during a session on “rheumatology for the dermatologist” at the annual meeting of the American Academy of Dermatology.
“The idea is really to start to try to get our dermatology colleagues thinking more systemically and outside of just the skin,” said Dr. Merola, a rheumatologist and dermatologist who is codirector of the center for skin and related musculoskeletal diseases at Brigham and Women’s Hospital, Boston.
“ in up to 30% of patients,” he noted.
He urged his colleagues to ask patients functional questions; for example, those pertaining to sicca symptoms; and how to parse out whether a patient’s joint pain is inflammatory or non-inflammatory.
In a video interview, Dr. Merola also discussed lab tests used to evaluate patients with lupus, the value of a simple urine test, and recent work on the development of the first international classification criteria set for discoid type skin lupus.
SAN DIEGO – Be mindful of what lies below the skin.
That was the message of Joseph Merola, MD, during a session on “rheumatology for the dermatologist” at the annual meeting of the American Academy of Dermatology.
“The idea is really to start to try to get our dermatology colleagues thinking more systemically and outside of just the skin,” said Dr. Merola, a rheumatologist and dermatologist who is codirector of the center for skin and related musculoskeletal diseases at Brigham and Women’s Hospital, Boston.
“ in up to 30% of patients,” he noted.
He urged his colleagues to ask patients functional questions; for example, those pertaining to sicca symptoms; and how to parse out whether a patient’s joint pain is inflammatory or non-inflammatory.
In a video interview, Dr. Merola also discussed lab tests used to evaluate patients with lupus, the value of a simple urine test, and recent work on the development of the first international classification criteria set for discoid type skin lupus.
SAN DIEGO – Be mindful of what lies below the skin.
That was the message of Joseph Merola, MD, during a session on “rheumatology for the dermatologist” at the annual meeting of the American Academy of Dermatology.
“The idea is really to start to try to get our dermatology colleagues thinking more systemically and outside of just the skin,” said Dr. Merola, a rheumatologist and dermatologist who is codirector of the center for skin and related musculoskeletal diseases at Brigham and Women’s Hospital, Boston.
“ in up to 30% of patients,” he noted.
He urged his colleagues to ask patients functional questions; for example, those pertaining to sicca symptoms; and how to parse out whether a patient’s joint pain is inflammatory or non-inflammatory.
In a video interview, Dr. Merola also discussed lab tests used to evaluate patients with lupus, the value of a simple urine test, and recent work on the development of the first international classification criteria set for discoid type skin lupus.
REPORTING FROM AAD 18
VIDEO: PPACMAN aims to advance the combined rheum-derm clinic approach in the community
SAN DIEGO – A new endeavor that aims to promote the concept of the combined clinic approach to caring for psoriatic patients is now underway.
PPACMAN (Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network) is made up of dermatologists and rheumatologists who play a key role in the management of psoriatic disease and are interested in combined clinics, with the mission “to nucleate psoriatic disease combined clinics and centers to advance a multilevel approach to psoriatic patients, increase disease awareness, and accelerate management,” according to Joseph Merola, MD, codirector of the center for skin and related musculoskeletal diseases at Brigham and Women’s Hospital, Boston.
There are now about 12 centers in North America with formal rheumatology-dermatology clinics for patients with psoriasis and psoriatic arthritis, including the one at Brigham and Women’s, where Dr. Merola and his colleagues have seen the “myriad benefits that come with having a combined clinic,” he said in a video interview at the annual meeting of the American Academy of Dermatology. The idea behind starting PPACMAN was to help form new clinics at academic centers but, also, “to start to catalyze local-regional partnerships in the community so we could get dermatologists and rheumatologists in the community to start interacting, communicating, [and] sharing patients,” he explained.
“The group is really very much focused on this mission of getting combined ... treatment models out there,” added Dr. Merola, president and chair of the board of PPACMAN, which is a 501c3 nonprofit organization.
In the interview, he discusses other benefits of the combined clinic model and other elements of the PPACMAN mission, including education and the potential for shared EMR templates.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – A new endeavor that aims to promote the concept of the combined clinic approach to caring for psoriatic patients is now underway.
PPACMAN (Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network) is made up of dermatologists and rheumatologists who play a key role in the management of psoriatic disease and are interested in combined clinics, with the mission “to nucleate psoriatic disease combined clinics and centers to advance a multilevel approach to psoriatic patients, increase disease awareness, and accelerate management,” according to Joseph Merola, MD, codirector of the center for skin and related musculoskeletal diseases at Brigham and Women’s Hospital, Boston.
There are now about 12 centers in North America with formal rheumatology-dermatology clinics for patients with psoriasis and psoriatic arthritis, including the one at Brigham and Women’s, where Dr. Merola and his colleagues have seen the “myriad benefits that come with having a combined clinic,” he said in a video interview at the annual meeting of the American Academy of Dermatology. The idea behind starting PPACMAN was to help form new clinics at academic centers but, also, “to start to catalyze local-regional partnerships in the community so we could get dermatologists and rheumatologists in the community to start interacting, communicating, [and] sharing patients,” he explained.
“The group is really very much focused on this mission of getting combined ... treatment models out there,” added Dr. Merola, president and chair of the board of PPACMAN, which is a 501c3 nonprofit organization.
In the interview, he discusses other benefits of the combined clinic model and other elements of the PPACMAN mission, including education and the potential for shared EMR templates.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – A new endeavor that aims to promote the concept of the combined clinic approach to caring for psoriatic patients is now underway.
PPACMAN (Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network) is made up of dermatologists and rheumatologists who play a key role in the management of psoriatic disease and are interested in combined clinics, with the mission “to nucleate psoriatic disease combined clinics and centers to advance a multilevel approach to psoriatic patients, increase disease awareness, and accelerate management,” according to Joseph Merola, MD, codirector of the center for skin and related musculoskeletal diseases at Brigham and Women’s Hospital, Boston.
There are now about 12 centers in North America with formal rheumatology-dermatology clinics for patients with psoriasis and psoriatic arthritis, including the one at Brigham and Women’s, where Dr. Merola and his colleagues have seen the “myriad benefits that come with having a combined clinic,” he said in a video interview at the annual meeting of the American Academy of Dermatology. The idea behind starting PPACMAN was to help form new clinics at academic centers but, also, “to start to catalyze local-regional partnerships in the community so we could get dermatologists and rheumatologists in the community to start interacting, communicating, [and] sharing patients,” he explained.
“The group is really very much focused on this mission of getting combined ... treatment models out there,” added Dr. Merola, president and chair of the board of PPACMAN, which is a 501c3 nonprofit organization.
In the interview, he discusses other benefits of the combined clinic model and other elements of the PPACMAN mission, including education and the potential for shared EMR templates.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
REPORTING FROM AAD 18
VIDEO: Vulvar disorders in preadolescent patients
SAN DIEGO – Over the past few years, pediatric dermatologist Kalyani Marathe, MD, has been seeing young patients with vulvar diseases in a multidisciplinary vulvar dermatology clinic at Children’s National Health System, in Washington, DC.
When Dr. Marathe started, it was her first experience treating such patients and there still are not much data in this population. She and Veronica Gomez-Lobo, MD, a pediatric and adolescent gynecologist at Children’s, “have now been doing the clinic every month for the last three and a half years,” and counsel and treat patients together. With longitudinal follow-up, “we’re learning so much about these conditions in children,” most of whom are about ages 3-11 years.
In a video interview at the annual meeting of the American Academy of Dermatology, Dr. Marathe discussed some of what she and Dr. Gomez-Lobo have learned over the past 3 years, with algorithms for treatment for the most common conditions they encounter in the clinic: non-specific vulvovaginitis, lichen sclerosus, and vitiligo.
Dr. Marathe had no relevant disclosures. She is a Dermatology News editorial board advisor.
emechcatie@frontlinemedcom.com
SOURCE: Marathe, K. et al, Session U018
SAN DIEGO – Over the past few years, pediatric dermatologist Kalyani Marathe, MD, has been seeing young patients with vulvar diseases in a multidisciplinary vulvar dermatology clinic at Children’s National Health System, in Washington, DC.
When Dr. Marathe started, it was her first experience treating such patients and there still are not much data in this population. She and Veronica Gomez-Lobo, MD, a pediatric and adolescent gynecologist at Children’s, “have now been doing the clinic every month for the last three and a half years,” and counsel and treat patients together. With longitudinal follow-up, “we’re learning so much about these conditions in children,” most of whom are about ages 3-11 years.
In a video interview at the annual meeting of the American Academy of Dermatology, Dr. Marathe discussed some of what she and Dr. Gomez-Lobo have learned over the past 3 years, with algorithms for treatment for the most common conditions they encounter in the clinic: non-specific vulvovaginitis, lichen sclerosus, and vitiligo.
Dr. Marathe had no relevant disclosures. She is a Dermatology News editorial board advisor.
emechcatie@frontlinemedcom.com
SOURCE: Marathe, K. et al, Session U018
SAN DIEGO – Over the past few years, pediatric dermatologist Kalyani Marathe, MD, has been seeing young patients with vulvar diseases in a multidisciplinary vulvar dermatology clinic at Children’s National Health System, in Washington, DC.
When Dr. Marathe started, it was her first experience treating such patients and there still are not much data in this population. She and Veronica Gomez-Lobo, MD, a pediatric and adolescent gynecologist at Children’s, “have now been doing the clinic every month for the last three and a half years,” and counsel and treat patients together. With longitudinal follow-up, “we’re learning so much about these conditions in children,” most of whom are about ages 3-11 years.
In a video interview at the annual meeting of the American Academy of Dermatology, Dr. Marathe discussed some of what she and Dr. Gomez-Lobo have learned over the past 3 years, with algorithms for treatment for the most common conditions they encounter in the clinic: non-specific vulvovaginitis, lichen sclerosus, and vitiligo.
Dr. Marathe had no relevant disclosures. She is a Dermatology News editorial board advisor.
emechcatie@frontlinemedcom.com
SOURCE: Marathe, K. et al, Session U018
REPORTING FROM AAD 18
Oral treatment for menorrhagia shows promise for melasma
SAN DIEGO – The use of , according to Amit Pandya, MD.
Tranexamic acid is also used to treat intraoperative hemorrhage, is available over the counter in some countries, and is used widely for treating melasma in East Asia, Dr. Pandya said at a meeting of the Skin of Color Society, held the day before the annual meeting of the American Academy of Dermatology.
Dr. Pandya, professor of dermatology at the University of Texas Southwestern, Dallas, described a woman with a 20-year history of melasma who had been treated with triple combination creams, chemical peels, and Fraxel lasers “to no avail,” similar to patients he sees in his practice every week. But after 3 months of treatment with 325 mg of tranexamic acid twice daily, triple combination cream, and visible light sunscreen, she was clearer than she had been for some time, he said.
Tranexamic acid “ blocks keratinocytes from causing plasminogen to go into plasmin,” and plasmin stimulates fibroblast growth factor production, which is “one of the most potent stimulants of melanin,” he explained.
In a retrospective study published in 2016, conducted by investigators at the National Skin Center in Singapore, 561 patients with melasma were treated with oral tranexamic acid for a median of 4 months, almost 90% of the patients improved. There was one serious adverse event, a deep vein thrombosis (J Am Acad Dermatol. 2016 Aug;75[2]:385-92). The other adverse effects were mild. When Dr. Pandya spoke with the investigators about this patient, he was told that the patient had not disclosed her true medical history, which included protein S deficiency and a strong family history of thrombotic events, which would have excluded her from treatment.
Of 2,000 published cases of melasma treated with tranexamic acid to date, “this is the only severe event ever seen with tranexamic acid,” he noted.
Dr. Pandya and his associates recently published the results of a study evaluating tranexamic acid in 44 Latino women with moderate to severe melasma, which he said was the first study of tranexamic acid in the Western hemisphere. For 3 months, the women were treated with 250 mg of tranexamic acid or placebo in combination with sunscreen in both groups, then sunscreen only for 3 months in both groups. The primary outcome was the change in the modified Melasma Area and Severity Index (mMASI) score.
“Results were spectacular,” he said. At 3 months, the mMASI score had improved by 49% among those in the tranexamic acid group, compared with 18% among those on placebo and sunscreen. After 3 months on sunscreen only, there was a 26% reduction in the mMASI score from baseline among those treated with tranexamic acid, compared with 19% in the placebo group. None of the patients in either group had severe adverse events (J Am Acad Dermatol. 2018 Feb;78[2]:363-9). Side effects include GI upset, reduced menstrual flow, myalgias, and headache.
Rebound after cessation of therapy is an issue, however, and was worse in the treated group “because more melanocytes are actually created when you reduce melanin. So once you stop the tranexamic acid, it rebounds,” Dr. Pandya said. Patients should use triple combination cream when they stop taking tranexamic acid, he advised.
However, he said that patients have called him within 1 month of stopping tranexamic acid, asking to restart treatment. He has had patients on tranexamic acid for 1 year or longer, without any side effects.
Women who are pregnant or nursing, have had two or more spontaneous abortions, are on oral contraceptives or other hormone-based birth control, have a history of thrombosis, are on blood thinners, are smokers, or have significant cardiovascular or respiratory disease, subarachnoid hemorrhage, any DVT, or a strong family history of thromboembolic events should not be treated with tranexamic acid.
Dr. Pandya pointed out that the 250-mg dose used in the study is not available in the United States, where only the 650-mg dose is available. So he writes a prescription for 650 mg a day, and tells patients to cut the pill in half and take a 325-mg dose twice a day (half in the morning and half at night).
At the Skin of Color Society meeting, Nahla Shihab, MD, of Universitas Indonesia, Jakarta, presented the results of a randomized, placebo-controlled study evaluating oral tranexamic acid plus hydroquinone cream in patients with moderate to severe melasma, in collaboration with UT Southwestern and Dr. Pandya. Patients were randomized to treatment with topical hydroquinone 4% cream, sunscreen, and tranexamic acid (250 mg twice a day), or hydroquinone 4% cream, sunscreen, and placebo for 3 months, followed by 3 months of sunscreen only.
At 12 weeks, those in the tranexamic acid group had a 55% decrease in the mMASI score, compared with 10.9% in the control group. After stopping treatment, some patients experienced relapses, similar to what has been observed in other studies, but “the severity was still lower than baseline,” Dr. Shihab reported.
In addition, the improvement in the mMASI score was higher than that seen in other studies, which could be due to a synergistic effect of the fibrin inhibitor with hydroquinone, she added. Another important finding was that improvements were noticeable after 2 weeks of treatment, “which suggests that the combination of oral tranexamic acid and hydroquinone has a rapid onset of action,” she said.
In both groups, 6% of the patients experienced erythema and pruritus, which resolved with continued use of hydroquinone, and one woman on tranexamic acid had menstrual cycle changes. Further studies should evaluate a longer duration of treatment and follow-up, with tranexamic acid and hydroquinone, and in combination with other treatments, Dr. Shihab said.
Dr. Pandya reported that he is a consultant to Aclaris Therapeutics and Pfizer, and has received grants/research funding from Incyte Corp. Dr. Shihab had no disclosures.
SAN DIEGO – The use of , according to Amit Pandya, MD.
Tranexamic acid is also used to treat intraoperative hemorrhage, is available over the counter in some countries, and is used widely for treating melasma in East Asia, Dr. Pandya said at a meeting of the Skin of Color Society, held the day before the annual meeting of the American Academy of Dermatology.
Dr. Pandya, professor of dermatology at the University of Texas Southwestern, Dallas, described a woman with a 20-year history of melasma who had been treated with triple combination creams, chemical peels, and Fraxel lasers “to no avail,” similar to patients he sees in his practice every week. But after 3 months of treatment with 325 mg of tranexamic acid twice daily, triple combination cream, and visible light sunscreen, she was clearer than she had been for some time, he said.
Tranexamic acid “ blocks keratinocytes from causing plasminogen to go into plasmin,” and plasmin stimulates fibroblast growth factor production, which is “one of the most potent stimulants of melanin,” he explained.
In a retrospective study published in 2016, conducted by investigators at the National Skin Center in Singapore, 561 patients with melasma were treated with oral tranexamic acid for a median of 4 months, almost 90% of the patients improved. There was one serious adverse event, a deep vein thrombosis (J Am Acad Dermatol. 2016 Aug;75[2]:385-92). The other adverse effects were mild. When Dr. Pandya spoke with the investigators about this patient, he was told that the patient had not disclosed her true medical history, which included protein S deficiency and a strong family history of thrombotic events, which would have excluded her from treatment.
Of 2,000 published cases of melasma treated with tranexamic acid to date, “this is the only severe event ever seen with tranexamic acid,” he noted.
Dr. Pandya and his associates recently published the results of a study evaluating tranexamic acid in 44 Latino women with moderate to severe melasma, which he said was the first study of tranexamic acid in the Western hemisphere. For 3 months, the women were treated with 250 mg of tranexamic acid or placebo in combination with sunscreen in both groups, then sunscreen only for 3 months in both groups. The primary outcome was the change in the modified Melasma Area and Severity Index (mMASI) score.
“Results were spectacular,” he said. At 3 months, the mMASI score had improved by 49% among those in the tranexamic acid group, compared with 18% among those on placebo and sunscreen. After 3 months on sunscreen only, there was a 26% reduction in the mMASI score from baseline among those treated with tranexamic acid, compared with 19% in the placebo group. None of the patients in either group had severe adverse events (J Am Acad Dermatol. 2018 Feb;78[2]:363-9). Side effects include GI upset, reduced menstrual flow, myalgias, and headache.
Rebound after cessation of therapy is an issue, however, and was worse in the treated group “because more melanocytes are actually created when you reduce melanin. So once you stop the tranexamic acid, it rebounds,” Dr. Pandya said. Patients should use triple combination cream when they stop taking tranexamic acid, he advised.
However, he said that patients have called him within 1 month of stopping tranexamic acid, asking to restart treatment. He has had patients on tranexamic acid for 1 year or longer, without any side effects.
Women who are pregnant or nursing, have had two or more spontaneous abortions, are on oral contraceptives or other hormone-based birth control, have a history of thrombosis, are on blood thinners, are smokers, or have significant cardiovascular or respiratory disease, subarachnoid hemorrhage, any DVT, or a strong family history of thromboembolic events should not be treated with tranexamic acid.
Dr. Pandya pointed out that the 250-mg dose used in the study is not available in the United States, where only the 650-mg dose is available. So he writes a prescription for 650 mg a day, and tells patients to cut the pill in half and take a 325-mg dose twice a day (half in the morning and half at night).
At the Skin of Color Society meeting, Nahla Shihab, MD, of Universitas Indonesia, Jakarta, presented the results of a randomized, placebo-controlled study evaluating oral tranexamic acid plus hydroquinone cream in patients with moderate to severe melasma, in collaboration with UT Southwestern and Dr. Pandya. Patients were randomized to treatment with topical hydroquinone 4% cream, sunscreen, and tranexamic acid (250 mg twice a day), or hydroquinone 4% cream, sunscreen, and placebo for 3 months, followed by 3 months of sunscreen only.
At 12 weeks, those in the tranexamic acid group had a 55% decrease in the mMASI score, compared with 10.9% in the control group. After stopping treatment, some patients experienced relapses, similar to what has been observed in other studies, but “the severity was still lower than baseline,” Dr. Shihab reported.
In addition, the improvement in the mMASI score was higher than that seen in other studies, which could be due to a synergistic effect of the fibrin inhibitor with hydroquinone, she added. Another important finding was that improvements were noticeable after 2 weeks of treatment, “which suggests that the combination of oral tranexamic acid and hydroquinone has a rapid onset of action,” she said.
In both groups, 6% of the patients experienced erythema and pruritus, which resolved with continued use of hydroquinone, and one woman on tranexamic acid had menstrual cycle changes. Further studies should evaluate a longer duration of treatment and follow-up, with tranexamic acid and hydroquinone, and in combination with other treatments, Dr. Shihab said.
Dr. Pandya reported that he is a consultant to Aclaris Therapeutics and Pfizer, and has received grants/research funding from Incyte Corp. Dr. Shihab had no disclosures.
SAN DIEGO – The use of , according to Amit Pandya, MD.
Tranexamic acid is also used to treat intraoperative hemorrhage, is available over the counter in some countries, and is used widely for treating melasma in East Asia, Dr. Pandya said at a meeting of the Skin of Color Society, held the day before the annual meeting of the American Academy of Dermatology.
Dr. Pandya, professor of dermatology at the University of Texas Southwestern, Dallas, described a woman with a 20-year history of melasma who had been treated with triple combination creams, chemical peels, and Fraxel lasers “to no avail,” similar to patients he sees in his practice every week. But after 3 months of treatment with 325 mg of tranexamic acid twice daily, triple combination cream, and visible light sunscreen, she was clearer than she had been for some time, he said.
Tranexamic acid “ blocks keratinocytes from causing plasminogen to go into plasmin,” and plasmin stimulates fibroblast growth factor production, which is “one of the most potent stimulants of melanin,” he explained.
In a retrospective study published in 2016, conducted by investigators at the National Skin Center in Singapore, 561 patients with melasma were treated with oral tranexamic acid for a median of 4 months, almost 90% of the patients improved. There was one serious adverse event, a deep vein thrombosis (J Am Acad Dermatol. 2016 Aug;75[2]:385-92). The other adverse effects were mild. When Dr. Pandya spoke with the investigators about this patient, he was told that the patient had not disclosed her true medical history, which included protein S deficiency and a strong family history of thrombotic events, which would have excluded her from treatment.
Of 2,000 published cases of melasma treated with tranexamic acid to date, “this is the only severe event ever seen with tranexamic acid,” he noted.
Dr. Pandya and his associates recently published the results of a study evaluating tranexamic acid in 44 Latino women with moderate to severe melasma, which he said was the first study of tranexamic acid in the Western hemisphere. For 3 months, the women were treated with 250 mg of tranexamic acid or placebo in combination with sunscreen in both groups, then sunscreen only for 3 months in both groups. The primary outcome was the change in the modified Melasma Area and Severity Index (mMASI) score.
“Results were spectacular,” he said. At 3 months, the mMASI score had improved by 49% among those in the tranexamic acid group, compared with 18% among those on placebo and sunscreen. After 3 months on sunscreen only, there was a 26% reduction in the mMASI score from baseline among those treated with tranexamic acid, compared with 19% in the placebo group. None of the patients in either group had severe adverse events (J Am Acad Dermatol. 2018 Feb;78[2]:363-9). Side effects include GI upset, reduced menstrual flow, myalgias, and headache.
Rebound after cessation of therapy is an issue, however, and was worse in the treated group “because more melanocytes are actually created when you reduce melanin. So once you stop the tranexamic acid, it rebounds,” Dr. Pandya said. Patients should use triple combination cream when they stop taking tranexamic acid, he advised.
However, he said that patients have called him within 1 month of stopping tranexamic acid, asking to restart treatment. He has had patients on tranexamic acid for 1 year or longer, without any side effects.
Women who are pregnant or nursing, have had two or more spontaneous abortions, are on oral contraceptives or other hormone-based birth control, have a history of thrombosis, are on blood thinners, are smokers, or have significant cardiovascular or respiratory disease, subarachnoid hemorrhage, any DVT, or a strong family history of thromboembolic events should not be treated with tranexamic acid.
Dr. Pandya pointed out that the 250-mg dose used in the study is not available in the United States, where only the 650-mg dose is available. So he writes a prescription for 650 mg a day, and tells patients to cut the pill in half and take a 325-mg dose twice a day (half in the morning and half at night).
At the Skin of Color Society meeting, Nahla Shihab, MD, of Universitas Indonesia, Jakarta, presented the results of a randomized, placebo-controlled study evaluating oral tranexamic acid plus hydroquinone cream in patients with moderate to severe melasma, in collaboration with UT Southwestern and Dr. Pandya. Patients were randomized to treatment with topical hydroquinone 4% cream, sunscreen, and tranexamic acid (250 mg twice a day), or hydroquinone 4% cream, sunscreen, and placebo for 3 months, followed by 3 months of sunscreen only.
At 12 weeks, those in the tranexamic acid group had a 55% decrease in the mMASI score, compared with 10.9% in the control group. After stopping treatment, some patients experienced relapses, similar to what has been observed in other studies, but “the severity was still lower than baseline,” Dr. Shihab reported.
In addition, the improvement in the mMASI score was higher than that seen in other studies, which could be due to a synergistic effect of the fibrin inhibitor with hydroquinone, she added. Another important finding was that improvements were noticeable after 2 weeks of treatment, “which suggests that the combination of oral tranexamic acid and hydroquinone has a rapid onset of action,” she said.
In both groups, 6% of the patients experienced erythema and pruritus, which resolved with continued use of hydroquinone, and one woman on tranexamic acid had menstrual cycle changes. Further studies should evaluate a longer duration of treatment and follow-up, with tranexamic acid and hydroquinone, and in combination with other treatments, Dr. Shihab said.
Dr. Pandya reported that he is a consultant to Aclaris Therapeutics and Pfizer, and has received grants/research funding from Incyte Corp. Dr. Shihab had no disclosures.
REPORTING FROM THE SKIN OF COLOR SOCIETY SCIENTIFIC SYMPOSIUM
FDA approves IL-17A antagonist for treating psoriatic arthritis
The interleukin-17A antagonist ixekizumab has been approved by the Food and Drug Administration for treating adults with active psoriatic arthritis (PsA), based on two phase 3 studies, the manufacturer announced in a written statement Dec. 1.
The Eli Lilly statement noted that the approval is based on two randomized, double-blind, placebo-controlled studies; one compared ixekizumab to placebo in patients with active PsA never treated with a biologic (SPIRIT-P1) and another tested the drug in those who had been treated with a tumor necrosis factor inhibitor (TNFi) previously (SPIRIT-P2).
Ixekizumab, marketed as Taltz by Eli Lilly, was first approved by the FDA in 2016 for treating adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
The statement did not provide information on dermatologic endpoints, but treatment with ixekizumab “resulted in an improvement in psoriatic skin lesions in patients with PsA,” as well as “in dactylitis and enthesitis in patients with pre-existing dactylitis or enthesitis,” according to the prescribing information.
The recommended dose for patients with psoriatic arthritis is 160 mg by subcutaneous injection (two 80 mg injections) at baseline, followed by 80 mg every 4 weeks. When patients with psoriatic arthritis also have moderate-to-severe plaque psoriasis, then the prescribing information recommends following the dosing for psoriasis, which is 160 mg (two 80 mg injections) at baseline, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.
The most common adverse reactions associated with ixekizumab are injection site reactions, upper respiratory tract infections, nausea, and tinea infections, according to the warnings and precautions section of the drug’s prescribing information, which lists the potential for serious infections, tuberculosis, and serious allergic reactions. Prescriptions come with a Medication Guide for patients.
The interleukin-17A antagonist ixekizumab has been approved by the Food and Drug Administration for treating adults with active psoriatic arthritis (PsA), based on two phase 3 studies, the manufacturer announced in a written statement Dec. 1.
The Eli Lilly statement noted that the approval is based on two randomized, double-blind, placebo-controlled studies; one compared ixekizumab to placebo in patients with active PsA never treated with a biologic (SPIRIT-P1) and another tested the drug in those who had been treated with a tumor necrosis factor inhibitor (TNFi) previously (SPIRIT-P2).
Ixekizumab, marketed as Taltz by Eli Lilly, was first approved by the FDA in 2016 for treating adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
The statement did not provide information on dermatologic endpoints, but treatment with ixekizumab “resulted in an improvement in psoriatic skin lesions in patients with PsA,” as well as “in dactylitis and enthesitis in patients with pre-existing dactylitis or enthesitis,” according to the prescribing information.
The recommended dose for patients with psoriatic arthritis is 160 mg by subcutaneous injection (two 80 mg injections) at baseline, followed by 80 mg every 4 weeks. When patients with psoriatic arthritis also have moderate-to-severe plaque psoriasis, then the prescribing information recommends following the dosing for psoriasis, which is 160 mg (two 80 mg injections) at baseline, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.
The most common adverse reactions associated with ixekizumab are injection site reactions, upper respiratory tract infections, nausea, and tinea infections, according to the warnings and precautions section of the drug’s prescribing information, which lists the potential for serious infections, tuberculosis, and serious allergic reactions. Prescriptions come with a Medication Guide for patients.
The interleukin-17A antagonist ixekizumab has been approved by the Food and Drug Administration for treating adults with active psoriatic arthritis (PsA), based on two phase 3 studies, the manufacturer announced in a written statement Dec. 1.
The Eli Lilly statement noted that the approval is based on two randomized, double-blind, placebo-controlled studies; one compared ixekizumab to placebo in patients with active PsA never treated with a biologic (SPIRIT-P1) and another tested the drug in those who had been treated with a tumor necrosis factor inhibitor (TNFi) previously (SPIRIT-P2).
Ixekizumab, marketed as Taltz by Eli Lilly, was first approved by the FDA in 2016 for treating adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
The statement did not provide information on dermatologic endpoints, but treatment with ixekizumab “resulted in an improvement in psoriatic skin lesions in patients with PsA,” as well as “in dactylitis and enthesitis in patients with pre-existing dactylitis or enthesitis,” according to the prescribing information.
The recommended dose for patients with psoriatic arthritis is 160 mg by subcutaneous injection (two 80 mg injections) at baseline, followed by 80 mg every 4 weeks. When patients with psoriatic arthritis also have moderate-to-severe plaque psoriasis, then the prescribing information recommends following the dosing for psoriasis, which is 160 mg (two 80 mg injections) at baseline, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.
The most common adverse reactions associated with ixekizumab are injection site reactions, upper respiratory tract infections, nausea, and tinea infections, according to the warnings and precautions section of the drug’s prescribing information, which lists the potential for serious infections, tuberculosis, and serious allergic reactions. Prescriptions come with a Medication Guide for patients.
Biologic approved for moderate to severe psoriasis in adolescents
The Food and Drug Administration approval of ustekinumab has been expanded to include adolescents aged 12 and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, based on the results of a phase 3 study.
The manufacturer, Janssen Biotech, announced the expanded indication in a press release on Oct. 13.
Ustekinumab, an interleukin-12 and -23 antagonist administered subcutaneously, was first approved by the FDA in 2009 for the same indication in adults; it is also approved for adults with active psoriatic arthritis, and for adults with moderately to severely active Crohn’s disease.
Ustekinumab is marketed as Stelara.
The Food and Drug Administration approval of ustekinumab has been expanded to include adolescents aged 12 and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, based on the results of a phase 3 study.
The manufacturer, Janssen Biotech, announced the expanded indication in a press release on Oct. 13.
Ustekinumab, an interleukin-12 and -23 antagonist administered subcutaneously, was first approved by the FDA in 2009 for the same indication in adults; it is also approved for adults with active psoriatic arthritis, and for adults with moderately to severely active Crohn’s disease.
Ustekinumab is marketed as Stelara.
The Food and Drug Administration approval of ustekinumab has been expanded to include adolescents aged 12 and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, based on the results of a phase 3 study.
The manufacturer, Janssen Biotech, announced the expanded indication in a press release on Oct. 13.
Ustekinumab, an interleukin-12 and -23 antagonist administered subcutaneously, was first approved by the FDA in 2009 for the same indication in adults; it is also approved for adults with active psoriatic arthritis, and for adults with moderately to severely active Crohn’s disease.
Ustekinumab is marketed as Stelara.