User login
Lebrikizumab gets European nod for treating moderate-to-severe atopic dermatitis
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
Bipolar disorder may raise risk of polycystic ovarian syndrome
Previous studies suggest that the prevalence of polycystic ovarian syndrome (PCOS) is higher in bipolar disorder (BD) patients compared with individuals not diagnosed with BD, wrote Jieyu Liu, PhD, of the Second Xiangya Hospital of Central South University, Hunan, China, and colleagues.
However, studies have been limited to drug-treated BD patients, and data on the effects of BD on the development of PCOS are limited, they said. Data from previous studies also indicate that serum testosterone levels, serum androstenedione levels, and polycystic ovarian morphology (PCOM) are increased in BD patients compared with women without BD.
In a study published in the Journal of Affective Disorders, the researchers recruited 72 BD patients on long-term medication, 72 drug-naive patients, and 98 healthy controls between March 2022 and November 2022.
PCOM was assessed using ≥ 8 MHz transvaginal transducers to determine the number of follicles and ovarian volume. PCOS was then defined using the Rotterdam criteria, in which patients met two of three qualifications: oligoovulation or anovulation; hyperandrogenemia; or PCOM (excluding other endocrine diseases).
In a multivariate analysis, drug-naive women with BD had significantly higher rates of PCOS compared with healthy controls (odds ratio 3.02). The drug-naive BD patients also had a greater prevalence of oligoamenorrhea compared with healthy controls (36.36% vs. 12.12%) and higher levels of anti-mullerian hormone, luteinizing hormone, and follicle stimulating hormone compared to the controls.
A further regression analysis showed that those on long-term valproate treatment had the highest risk (OR 3.89) and the prevalence of PCOS was significantly higher among patients treated with valproate compared with drug-naive patients (53.3% vs. 30.6%). Younger age and the presence of insulin resistance also were associated with increased risk of PCOS (OR 0.37 and OR 1.73, respectively).
“Unexpectedly, no significant differences in serum androgen levels, including TT, FAI, androstenedione, and [dehydroepiandrosterone sulfate] levels, were observed between drug-naive BD patients and the HCs,” the researchers wrote in their discussion. This difference may stem from multiple causes including demographic variables, inclusion of PCOM as a diagnostic criterion, and the impact of genetic and environmental factors, they said.
The findings were limited by several factors including the small study population, which prevented conclusions of causality and comparison of the effects of different mood stabilizers on PCOS, the researchers noted. Other limitations included the relatively homogeneous population from a single region in China, and the inability to account for the effects of diet and lifestyle.
More research is needed to explore the impact of mediations, but the results suggest that BD patients are susceptible to PCOS; therefore, they should evaluate their reproductive health before starting any medication, and review reproductive health regularly, the researchers concluded.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
Previous studies suggest that the prevalence of polycystic ovarian syndrome (PCOS) is higher in bipolar disorder (BD) patients compared with individuals not diagnosed with BD, wrote Jieyu Liu, PhD, of the Second Xiangya Hospital of Central South University, Hunan, China, and colleagues.
However, studies have been limited to drug-treated BD patients, and data on the effects of BD on the development of PCOS are limited, they said. Data from previous studies also indicate that serum testosterone levels, serum androstenedione levels, and polycystic ovarian morphology (PCOM) are increased in BD patients compared with women without BD.
In a study published in the Journal of Affective Disorders, the researchers recruited 72 BD patients on long-term medication, 72 drug-naive patients, and 98 healthy controls between March 2022 and November 2022.
PCOM was assessed using ≥ 8 MHz transvaginal transducers to determine the number of follicles and ovarian volume. PCOS was then defined using the Rotterdam criteria, in which patients met two of three qualifications: oligoovulation or anovulation; hyperandrogenemia; or PCOM (excluding other endocrine diseases).
In a multivariate analysis, drug-naive women with BD had significantly higher rates of PCOS compared with healthy controls (odds ratio 3.02). The drug-naive BD patients also had a greater prevalence of oligoamenorrhea compared with healthy controls (36.36% vs. 12.12%) and higher levels of anti-mullerian hormone, luteinizing hormone, and follicle stimulating hormone compared to the controls.
A further regression analysis showed that those on long-term valproate treatment had the highest risk (OR 3.89) and the prevalence of PCOS was significantly higher among patients treated with valproate compared with drug-naive patients (53.3% vs. 30.6%). Younger age and the presence of insulin resistance also were associated with increased risk of PCOS (OR 0.37 and OR 1.73, respectively).
“Unexpectedly, no significant differences in serum androgen levels, including TT, FAI, androstenedione, and [dehydroepiandrosterone sulfate] levels, were observed between drug-naive BD patients and the HCs,” the researchers wrote in their discussion. This difference may stem from multiple causes including demographic variables, inclusion of PCOM as a diagnostic criterion, and the impact of genetic and environmental factors, they said.
The findings were limited by several factors including the small study population, which prevented conclusions of causality and comparison of the effects of different mood stabilizers on PCOS, the researchers noted. Other limitations included the relatively homogeneous population from a single region in China, and the inability to account for the effects of diet and lifestyle.
More research is needed to explore the impact of mediations, but the results suggest that BD patients are susceptible to PCOS; therefore, they should evaluate their reproductive health before starting any medication, and review reproductive health regularly, the researchers concluded.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
Previous studies suggest that the prevalence of polycystic ovarian syndrome (PCOS) is higher in bipolar disorder (BD) patients compared with individuals not diagnosed with BD, wrote Jieyu Liu, PhD, of the Second Xiangya Hospital of Central South University, Hunan, China, and colleagues.
However, studies have been limited to drug-treated BD patients, and data on the effects of BD on the development of PCOS are limited, they said. Data from previous studies also indicate that serum testosterone levels, serum androstenedione levels, and polycystic ovarian morphology (PCOM) are increased in BD patients compared with women without BD.
In a study published in the Journal of Affective Disorders, the researchers recruited 72 BD patients on long-term medication, 72 drug-naive patients, and 98 healthy controls between March 2022 and November 2022.
PCOM was assessed using ≥ 8 MHz transvaginal transducers to determine the number of follicles and ovarian volume. PCOS was then defined using the Rotterdam criteria, in which patients met two of three qualifications: oligoovulation or anovulation; hyperandrogenemia; or PCOM (excluding other endocrine diseases).
In a multivariate analysis, drug-naive women with BD had significantly higher rates of PCOS compared with healthy controls (odds ratio 3.02). The drug-naive BD patients also had a greater prevalence of oligoamenorrhea compared with healthy controls (36.36% vs. 12.12%) and higher levels of anti-mullerian hormone, luteinizing hormone, and follicle stimulating hormone compared to the controls.
A further regression analysis showed that those on long-term valproate treatment had the highest risk (OR 3.89) and the prevalence of PCOS was significantly higher among patients treated with valproate compared with drug-naive patients (53.3% vs. 30.6%). Younger age and the presence of insulin resistance also were associated with increased risk of PCOS (OR 0.37 and OR 1.73, respectively).
“Unexpectedly, no significant differences in serum androgen levels, including TT, FAI, androstenedione, and [dehydroepiandrosterone sulfate] levels, were observed between drug-naive BD patients and the HCs,” the researchers wrote in their discussion. This difference may stem from multiple causes including demographic variables, inclusion of PCOM as a diagnostic criterion, and the impact of genetic and environmental factors, they said.
The findings were limited by several factors including the small study population, which prevented conclusions of causality and comparison of the effects of different mood stabilizers on PCOS, the researchers noted. Other limitations included the relatively homogeneous population from a single region in China, and the inability to account for the effects of diet and lifestyle.
More research is needed to explore the impact of mediations, but the results suggest that BD patients are susceptible to PCOS; therefore, they should evaluate their reproductive health before starting any medication, and review reproductive health regularly, the researchers concluded.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Algorithm cuts time to incision in urgent cesarean deliveries
No specific recommended decision-to-incision time exists for cases of unscheduled, nonemergent cesarean deliveries, although a target of 30 minutes is recommended for emergent deliveries, Lina T. Bernal, MD, of Boston University and colleagues wrote.
The researchers developed a quality improvement project in which a multidisciplinary team defined which unscheduled cesarean deliveries should qualify as urgent, and identified a goal of 40 minutes or less for decision-to-incision time in these cases.
“We defined urgent, unscheduled cesarean delivery as cesarean delivery in patients with the following diagnoses: active phase arrest at 6 cm or greater, category II fetal heart rate tracing during labor requiring delivery per the Shields algorithm, but not meeting emergent category III criteria, any unscheduled cesarean delivery complicated by chorioamnionitis, and failed trial of labor after cesarean,” they wrote.
In a study published in Obstetrics & Gynecology, the researchers compared times from decision to incision before and after the implementation of a multidisciplinary algorithm. The study included 199 urgent, unscheduled deliveries in a single center between May 2019 and November 2019, and implementation period with 283 deliveries from December 2019 to September 2020, and a postimplementation period with 160 deliveries between October 2020 and May 2021.
The primary outcome was the mean time from decision to incision; secondary outcomes were neonatal status based on 5-minute Apgar score and quantitative blood loss during delivery.
Overall, the mean decision-to-incision time improved from 88 minutes during the preimplementation period to 50 minutes in the postimplementation period.
For Black non-Hispanic patients, the mean decision-to-incision time improved from 98 minutes during the preimplementation period to 50 minutes in the postimplementation period. Similarly, mean times among Hispanic patients decreased from 84 minutes to 49 minutes during the pre- and postimplementation periods, respectively.
No significant improvement in decision-to-incision time was noted among patients in other racial and ethnic groups.
In cases of cesarean delivery for fetal indications, 5-minute Apgar scores were significantly higher in the postimplementation period compared with the preimplementation period (8.5 vs. 8.8, P < .01).
No significant associations appeared between maternal quantitative blood loss and the implementation of the algorithm across treatment periods.
Over the course of the study, adjustments to the algorithm included clarification of the criteria, streamlined communication, and expanded use of resources. “There are no prior studies regarding the effects of creation of an urgent category on decision-to-incision time or maternal or neonatal outcomes,” the researchers wrote. “As a result of improved outcomes and appreciation of a standardized approach, the urgent cesarean delivery designation has been incorporated into the labor unit work flow.”
The findings were limited by several factors including the retrospective design, use of data from a single medical center, and the inability to address confounding variables such as age, parity, body mass index, time of delivery, and staffing, the researchers noted. Other limitations include a lack of data on measures of maternal morbidity beyond quantitative blood loss and other neonatal morbidities, and lack of data on patient satisfaction.
However, the results support the use of a standard algorithm to successfully reduce decision-to-incision time in urgent and unscheduled cesarean deliveries, and next steps for further improvement of care should identify which patients are most likely to benefit from a more rapid delivery, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
No specific recommended decision-to-incision time exists for cases of unscheduled, nonemergent cesarean deliveries, although a target of 30 minutes is recommended for emergent deliveries, Lina T. Bernal, MD, of Boston University and colleagues wrote.
The researchers developed a quality improvement project in which a multidisciplinary team defined which unscheduled cesarean deliveries should qualify as urgent, and identified a goal of 40 minutes or less for decision-to-incision time in these cases.
“We defined urgent, unscheduled cesarean delivery as cesarean delivery in patients with the following diagnoses: active phase arrest at 6 cm or greater, category II fetal heart rate tracing during labor requiring delivery per the Shields algorithm, but not meeting emergent category III criteria, any unscheduled cesarean delivery complicated by chorioamnionitis, and failed trial of labor after cesarean,” they wrote.
In a study published in Obstetrics & Gynecology, the researchers compared times from decision to incision before and after the implementation of a multidisciplinary algorithm. The study included 199 urgent, unscheduled deliveries in a single center between May 2019 and November 2019, and implementation period with 283 deliveries from December 2019 to September 2020, and a postimplementation period with 160 deliveries between October 2020 and May 2021.
The primary outcome was the mean time from decision to incision; secondary outcomes were neonatal status based on 5-minute Apgar score and quantitative blood loss during delivery.
Overall, the mean decision-to-incision time improved from 88 minutes during the preimplementation period to 50 minutes in the postimplementation period.
For Black non-Hispanic patients, the mean decision-to-incision time improved from 98 minutes during the preimplementation period to 50 minutes in the postimplementation period. Similarly, mean times among Hispanic patients decreased from 84 minutes to 49 minutes during the pre- and postimplementation periods, respectively.
No significant improvement in decision-to-incision time was noted among patients in other racial and ethnic groups.
In cases of cesarean delivery for fetal indications, 5-minute Apgar scores were significantly higher in the postimplementation period compared with the preimplementation period (8.5 vs. 8.8, P < .01).
No significant associations appeared between maternal quantitative blood loss and the implementation of the algorithm across treatment periods.
Over the course of the study, adjustments to the algorithm included clarification of the criteria, streamlined communication, and expanded use of resources. “There are no prior studies regarding the effects of creation of an urgent category on decision-to-incision time or maternal or neonatal outcomes,” the researchers wrote. “As a result of improved outcomes and appreciation of a standardized approach, the urgent cesarean delivery designation has been incorporated into the labor unit work flow.”
The findings were limited by several factors including the retrospective design, use of data from a single medical center, and the inability to address confounding variables such as age, parity, body mass index, time of delivery, and staffing, the researchers noted. Other limitations include a lack of data on measures of maternal morbidity beyond quantitative blood loss and other neonatal morbidities, and lack of data on patient satisfaction.
However, the results support the use of a standard algorithm to successfully reduce decision-to-incision time in urgent and unscheduled cesarean deliveries, and next steps for further improvement of care should identify which patients are most likely to benefit from a more rapid delivery, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
No specific recommended decision-to-incision time exists for cases of unscheduled, nonemergent cesarean deliveries, although a target of 30 minutes is recommended for emergent deliveries, Lina T. Bernal, MD, of Boston University and colleagues wrote.
The researchers developed a quality improvement project in which a multidisciplinary team defined which unscheduled cesarean deliveries should qualify as urgent, and identified a goal of 40 minutes or less for decision-to-incision time in these cases.
“We defined urgent, unscheduled cesarean delivery as cesarean delivery in patients with the following diagnoses: active phase arrest at 6 cm or greater, category II fetal heart rate tracing during labor requiring delivery per the Shields algorithm, but not meeting emergent category III criteria, any unscheduled cesarean delivery complicated by chorioamnionitis, and failed trial of labor after cesarean,” they wrote.
In a study published in Obstetrics & Gynecology, the researchers compared times from decision to incision before and after the implementation of a multidisciplinary algorithm. The study included 199 urgent, unscheduled deliveries in a single center between May 2019 and November 2019, and implementation period with 283 deliveries from December 2019 to September 2020, and a postimplementation period with 160 deliveries between October 2020 and May 2021.
The primary outcome was the mean time from decision to incision; secondary outcomes were neonatal status based on 5-minute Apgar score and quantitative blood loss during delivery.
Overall, the mean decision-to-incision time improved from 88 minutes during the preimplementation period to 50 minutes in the postimplementation period.
For Black non-Hispanic patients, the mean decision-to-incision time improved from 98 minutes during the preimplementation period to 50 minutes in the postimplementation period. Similarly, mean times among Hispanic patients decreased from 84 minutes to 49 minutes during the pre- and postimplementation periods, respectively.
No significant improvement in decision-to-incision time was noted among patients in other racial and ethnic groups.
In cases of cesarean delivery for fetal indications, 5-minute Apgar scores were significantly higher in the postimplementation period compared with the preimplementation period (8.5 vs. 8.8, P < .01).
No significant associations appeared between maternal quantitative blood loss and the implementation of the algorithm across treatment periods.
Over the course of the study, adjustments to the algorithm included clarification of the criteria, streamlined communication, and expanded use of resources. “There are no prior studies regarding the effects of creation of an urgent category on decision-to-incision time or maternal or neonatal outcomes,” the researchers wrote. “As a result of improved outcomes and appreciation of a standardized approach, the urgent cesarean delivery designation has been incorporated into the labor unit work flow.”
The findings were limited by several factors including the retrospective design, use of data from a single medical center, and the inability to address confounding variables such as age, parity, body mass index, time of delivery, and staffing, the researchers noted. Other limitations include a lack of data on measures of maternal morbidity beyond quantitative blood loss and other neonatal morbidities, and lack of data on patient satisfaction.
However, the results support the use of a standard algorithm to successfully reduce decision-to-incision time in urgent and unscheduled cesarean deliveries, and next steps for further improvement of care should identify which patients are most likely to benefit from a more rapid delivery, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY
Breast implants used in double lung transplant post infection
An innovative surgical procedure combining breast implants and an artificial lung may help more patients with severe lung disease survive to receive transplants. The case was described in a press conference sponsored by Northwestern University, Evanston, Ill.
In May 2023, a surgical team at Northwestern removed both infected lungs from David “Davey” Bauer, aged 34 years, and temporarily used breast implants to hold his heart in place until new lungs were available.
In April 2023, Mr. Bauer, a longtime smoker and vaper, experienced shortness of breath. His girlfriend, Susan Gore, took him to an urgent care center, and he returned home, but “the next morning he couldn’t walk,” Ms. Gore said in the press conference. A trip to the ED yielded a diagnosis of influenza A, followed rapidly by a bacterial lung infection that proved resistant to antibiotics. Mr. Bauer had no prior medical history of serious illness, but he was soon in an intensive care unit. His condition continued to decline, and a double lung transplant was his only option.
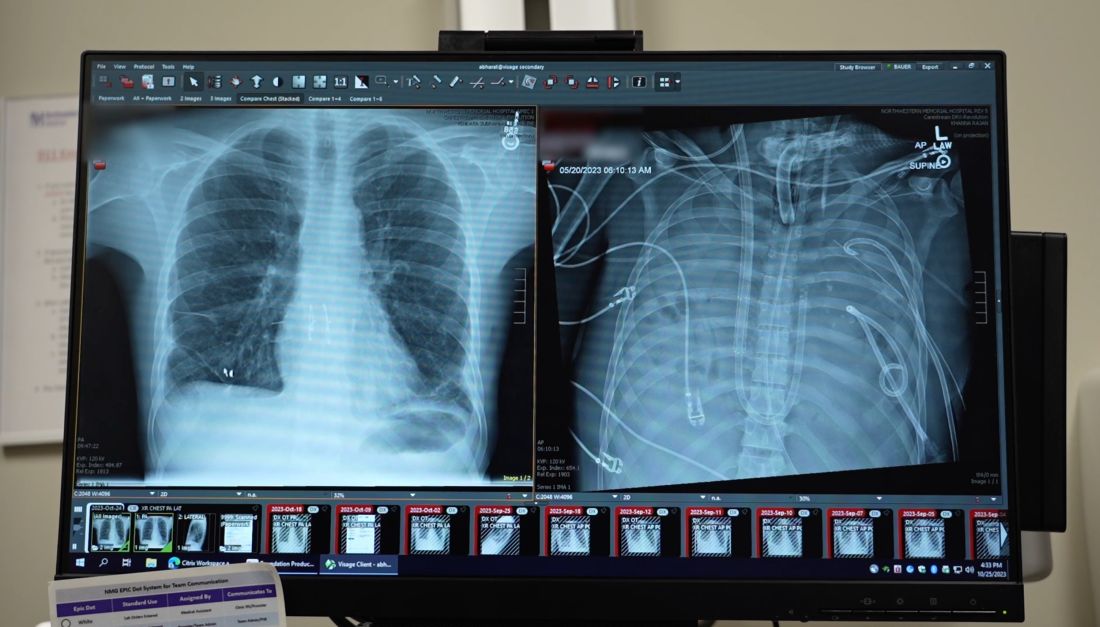
The Northwestern Medicine Canning Thoracic Institute specializes in challenging cases, and Mr. Bauer was transferred there.
Back from the brink
Mr. Bauer made the transfer to Chicago despite being critically ill. He was in dire need of a lung transplant, and the only way to resolve his infection was to remove the lungs, said Ankit Bharat, MD, chief of thoracic surgery and director of Northwestern Medicine Canning Thoracic Institute, in the press conference.
“Something needed to be done right away,” Dr. Bharat said. Mr. Bauer’s lungs were removed and the chest cavity was extensively debrided to remove the infection.
Then it was time for outside-the-box thinking. “With the lungs taken out, we needed something to support the heart,” he said. Breast implants came to mind, and double Ds were the largest available.
In addition, the surgeons created an artificial lung system of conduits to keep Mr. Bauer’s blood pumping. “We wanted to maintain the natural blood flow in the body that would be present if the lungs were there,” Dr. Bharat explained.
Plastic surgeons at Northwestern gave Mr. Bauer’s surgical team “a crash course” in managing the breast implants, Dr. Bharat said. The team anticipated that their novel surgical solution would need to last for weeks, but Mr. Bauer’s condition improved immediately once the infected lungs were removed. He was placed on a double-lung transplant list, and the team received an offer of new lungs within 24 hours.
The breast implants were removed, the new lungs were implanted, and Bauer spent several months in the ICU before his discharge to rehabilitation therapy at the end of September, according to a Northwestern press release.
This type of procedure could help patients with infections who need transplants but are too sick to undergo them, Dr. Bharat said in the press conference. In Mr. Bauer’s case, “a lot of stars aligned,” including Bauer’s rapid improvement and the quick availability of a perfect lung match, Dr. Bharat said. Many patients don’t survive to the point of transplant.
“We were surprised how quickly he recovered once we removed the infected lungs,” Dr. Bharat noted. The quick recovery may be in part because of Bauer’s youth and relative good health, but “this was uncharted territory.”
Mr. Bauer’s case is the first use of this particular surgical technique, although the team drew on lessons learned in other surgical settings, such as removal of both lungs to prevent cross-contamination in patients with cancer, he added.
Causes and effects
As for the factors that contributed to Mr. Bauer’s initial infection, “there is a lot we don’t know, but we can try to put things together,” said Dr. Bharat. Just as many factors lined up to promote Mr. Bauer’s recovery, many factors lined up to cause the problem, including long-standing smoking and vaping. Although some still view vaping as a safer alternative to smoking, patient data and experiences do not support this claim. “We know for a fact that both of them cause harm,” he added.
Mr. Bauer started smoking cigarettes at age 21 and typically smoked a pack of cigarettes each day before switching to vaping in 2014. In addition, Mr. Bauer had not been vaccinated against the flu, and his flu infection was followed by a bacterial infection.
Bacterial infections followed by hospitalizations are not new as an effect of vaping; a series of articles described the ongoing epidemic of e-cigarette or vaping product use–associated lung injury (EVALI). Patients with EVALI often present at urgent care centers, as Bauer did, with symptoms of flu or pneumonia, and they are often given medication and sent home.
Looking ahead: “We expect that Davey will fully recover and live a normal life,” although he will remain in Chicago for another year for monitoring, said Rade Tomic, MD, pulmonologist and medical director of the Northwestern Medicine Canning Thoracic Institute lung transplant program, in the press conference.
Mr. Bauer expressed his thanks to the surgical team, who also presented him with another gift: a T-shirt with his newly chosen nickname, “DD Davey.” “I feel so blessed, I got a second chance at life,” Mr. Bauer said in the press conference. “You should not inhale anything into your lungs except oxygen.”
A version of this article first appeared on Medscape.com.
An innovative surgical procedure combining breast implants and an artificial lung may help more patients with severe lung disease survive to receive transplants. The case was described in a press conference sponsored by Northwestern University, Evanston, Ill.
In May 2023, a surgical team at Northwestern removed both infected lungs from David “Davey” Bauer, aged 34 years, and temporarily used breast implants to hold his heart in place until new lungs were available.
In April 2023, Mr. Bauer, a longtime smoker and vaper, experienced shortness of breath. His girlfriend, Susan Gore, took him to an urgent care center, and he returned home, but “the next morning he couldn’t walk,” Ms. Gore said in the press conference. A trip to the ED yielded a diagnosis of influenza A, followed rapidly by a bacterial lung infection that proved resistant to antibiotics. Mr. Bauer had no prior medical history of serious illness, but he was soon in an intensive care unit. His condition continued to decline, and a double lung transplant was his only option.

The Northwestern Medicine Canning Thoracic Institute specializes in challenging cases, and Mr. Bauer was transferred there.
Back from the brink
Mr. Bauer made the transfer to Chicago despite being critically ill. He was in dire need of a lung transplant, and the only way to resolve his infection was to remove the lungs, said Ankit Bharat, MD, chief of thoracic surgery and director of Northwestern Medicine Canning Thoracic Institute, in the press conference.
“Something needed to be done right away,” Dr. Bharat said. Mr. Bauer’s lungs were removed and the chest cavity was extensively debrided to remove the infection.
Then it was time for outside-the-box thinking. “With the lungs taken out, we needed something to support the heart,” he said. Breast implants came to mind, and double Ds were the largest available.
In addition, the surgeons created an artificial lung system of conduits to keep Mr. Bauer’s blood pumping. “We wanted to maintain the natural blood flow in the body that would be present if the lungs were there,” Dr. Bharat explained.
Plastic surgeons at Northwestern gave Mr. Bauer’s surgical team “a crash course” in managing the breast implants, Dr. Bharat said. The team anticipated that their novel surgical solution would need to last for weeks, but Mr. Bauer’s condition improved immediately once the infected lungs were removed. He was placed on a double-lung transplant list, and the team received an offer of new lungs within 24 hours.
The breast implants were removed, the new lungs were implanted, and Bauer spent several months in the ICU before his discharge to rehabilitation therapy at the end of September, according to a Northwestern press release.
This type of procedure could help patients with infections who need transplants but are too sick to undergo them, Dr. Bharat said in the press conference. In Mr. Bauer’s case, “a lot of stars aligned,” including Bauer’s rapid improvement and the quick availability of a perfect lung match, Dr. Bharat said. Many patients don’t survive to the point of transplant.
“We were surprised how quickly he recovered once we removed the infected lungs,” Dr. Bharat noted. The quick recovery may be in part because of Bauer’s youth and relative good health, but “this was uncharted territory.”
Mr. Bauer’s case is the first use of this particular surgical technique, although the team drew on lessons learned in other surgical settings, such as removal of both lungs to prevent cross-contamination in patients with cancer, he added.
Causes and effects
As for the factors that contributed to Mr. Bauer’s initial infection, “there is a lot we don’t know, but we can try to put things together,” said Dr. Bharat. Just as many factors lined up to promote Mr. Bauer’s recovery, many factors lined up to cause the problem, including long-standing smoking and vaping. Although some still view vaping as a safer alternative to smoking, patient data and experiences do not support this claim. “We know for a fact that both of them cause harm,” he added.
Mr. Bauer started smoking cigarettes at age 21 and typically smoked a pack of cigarettes each day before switching to vaping in 2014. In addition, Mr. Bauer had not been vaccinated against the flu, and his flu infection was followed by a bacterial infection.
Bacterial infections followed by hospitalizations are not new as an effect of vaping; a series of articles described the ongoing epidemic of e-cigarette or vaping product use–associated lung injury (EVALI). Patients with EVALI often present at urgent care centers, as Bauer did, with symptoms of flu or pneumonia, and they are often given medication and sent home.
Looking ahead: “We expect that Davey will fully recover and live a normal life,” although he will remain in Chicago for another year for monitoring, said Rade Tomic, MD, pulmonologist and medical director of the Northwestern Medicine Canning Thoracic Institute lung transplant program, in the press conference.
Mr. Bauer expressed his thanks to the surgical team, who also presented him with another gift: a T-shirt with his newly chosen nickname, “DD Davey.” “I feel so blessed, I got a second chance at life,” Mr. Bauer said in the press conference. “You should not inhale anything into your lungs except oxygen.”
A version of this article first appeared on Medscape.com.
An innovative surgical procedure combining breast implants and an artificial lung may help more patients with severe lung disease survive to receive transplants. The case was described in a press conference sponsored by Northwestern University, Evanston, Ill.
In May 2023, a surgical team at Northwestern removed both infected lungs from David “Davey” Bauer, aged 34 years, and temporarily used breast implants to hold his heart in place until new lungs were available.
In April 2023, Mr. Bauer, a longtime smoker and vaper, experienced shortness of breath. His girlfriend, Susan Gore, took him to an urgent care center, and he returned home, but “the next morning he couldn’t walk,” Ms. Gore said in the press conference. A trip to the ED yielded a diagnosis of influenza A, followed rapidly by a bacterial lung infection that proved resistant to antibiotics. Mr. Bauer had no prior medical history of serious illness, but he was soon in an intensive care unit. His condition continued to decline, and a double lung transplant was his only option.

The Northwestern Medicine Canning Thoracic Institute specializes in challenging cases, and Mr. Bauer was transferred there.
Back from the brink
Mr. Bauer made the transfer to Chicago despite being critically ill. He was in dire need of a lung transplant, and the only way to resolve his infection was to remove the lungs, said Ankit Bharat, MD, chief of thoracic surgery and director of Northwestern Medicine Canning Thoracic Institute, in the press conference.
“Something needed to be done right away,” Dr. Bharat said. Mr. Bauer’s lungs were removed and the chest cavity was extensively debrided to remove the infection.
Then it was time for outside-the-box thinking. “With the lungs taken out, we needed something to support the heart,” he said. Breast implants came to mind, and double Ds were the largest available.
In addition, the surgeons created an artificial lung system of conduits to keep Mr. Bauer’s blood pumping. “We wanted to maintain the natural blood flow in the body that would be present if the lungs were there,” Dr. Bharat explained.
Plastic surgeons at Northwestern gave Mr. Bauer’s surgical team “a crash course” in managing the breast implants, Dr. Bharat said. The team anticipated that their novel surgical solution would need to last for weeks, but Mr. Bauer’s condition improved immediately once the infected lungs were removed. He was placed on a double-lung transplant list, and the team received an offer of new lungs within 24 hours.
The breast implants were removed, the new lungs were implanted, and Bauer spent several months in the ICU before his discharge to rehabilitation therapy at the end of September, according to a Northwestern press release.
This type of procedure could help patients with infections who need transplants but are too sick to undergo them, Dr. Bharat said in the press conference. In Mr. Bauer’s case, “a lot of stars aligned,” including Bauer’s rapid improvement and the quick availability of a perfect lung match, Dr. Bharat said. Many patients don’t survive to the point of transplant.
“We were surprised how quickly he recovered once we removed the infected lungs,” Dr. Bharat noted. The quick recovery may be in part because of Bauer’s youth and relative good health, but “this was uncharted territory.”
Mr. Bauer’s case is the first use of this particular surgical technique, although the team drew on lessons learned in other surgical settings, such as removal of both lungs to prevent cross-contamination in patients with cancer, he added.
Causes and effects
As for the factors that contributed to Mr. Bauer’s initial infection, “there is a lot we don’t know, but we can try to put things together,” said Dr. Bharat. Just as many factors lined up to promote Mr. Bauer’s recovery, many factors lined up to cause the problem, including long-standing smoking and vaping. Although some still view vaping as a safer alternative to smoking, patient data and experiences do not support this claim. “We know for a fact that both of them cause harm,” he added.
Mr. Bauer started smoking cigarettes at age 21 and typically smoked a pack of cigarettes each day before switching to vaping in 2014. In addition, Mr. Bauer had not been vaccinated against the flu, and his flu infection was followed by a bacterial infection.
Bacterial infections followed by hospitalizations are not new as an effect of vaping; a series of articles described the ongoing epidemic of e-cigarette or vaping product use–associated lung injury (EVALI). Patients with EVALI often present at urgent care centers, as Bauer did, with symptoms of flu or pneumonia, and they are often given medication and sent home.
Looking ahead: “We expect that Davey will fully recover and live a normal life,” although he will remain in Chicago for another year for monitoring, said Rade Tomic, MD, pulmonologist and medical director of the Northwestern Medicine Canning Thoracic Institute lung transplant program, in the press conference.
Mr. Bauer expressed his thanks to the surgical team, who also presented him with another gift: a T-shirt with his newly chosen nickname, “DD Davey.” “I feel so blessed, I got a second chance at life,” Mr. Bauer said in the press conference. “You should not inhale anything into your lungs except oxygen.”
A version of this article first appeared on Medscape.com.
FTC considers proposals on mergers and noncompete clauses
Changes may be in store for how physicians do business based on pending proposals from the Federal Trade Commission to ban noncompete clauses and monitor potential merger monopolies.
In January 2023, the FTC announced a rule that would ban noncompete clauses, stating that such clauses reduce workers’ wages and stifle new businesses. Simply put, the rule would ban employers from entering into noncompete clauses with workers, including independent contractors.
Aspects of the rule include whether it should pertain to franchisees, whether senior executives should be exempted, and whether low-wage and high-wage workers should be treated differently.
According to the FTC, banning noncompete clauses would increase workers’ earnings by approximately $300 billion per year, save consumers as much as $148 billion in health care costs, and double the number of companies founded by former workers in the same field.
In June 2023, the FTC and the Department of Justice proposed changes to rules governing mergers, including changes to prenotification forms that would promote more efficient screening of potential mergers. According to a press release from the FTC, the proposed changes include provision of details about investments or corporate relationships, product and services, projected revenue streams, and previous acquisitions.
The proposal also includes a waiting period during which agencies would assess the risk that a merger would lessen competition or tend to create a monopoly.
What the FTC proposals mean for physicians
FTC Chair Lina M. Khan addressed attendees at the American College of Physicians at their annual meeting in October.
In March 2023, ACEP wrote to Ms. Khan in support of the banning of noncompete clauses. The ACEP also stated that the FTC should monitor the effect of a ban on the ability to recruit and maintain a stable physician workforce in rural and underserved areas “and should examine the potential impacts should nonprofit health systems be exempt from a ban.”
However, the American Medical Group Association, a nonprofit trade organization that supports multispecialty medical groups, opposes the ban. In a press release issued in March 2023, AMGA noted that, “As employers, AMGA members rely in part on noncompete agreements to build strong, sustainable care teams that work together to coordinate care for their patients. These care teams emphasize the importance of the doctor-patient relationship, which reasonable noncompete agreements help support.”
The American Medical Association supports the ban on noncompete clauses, detailed in an official AMA policy statement as, “support[ing] policies, regulations, and legislation that prohibits covenants not-to-compete for all physicians in clinical practice who hold employment contracts with for-profit or nonprofit hospital, hospital system, or staffing company employers.”
In regard to the merger guidelines, ACEP wrote a separate letter to Ms. Khan identifying some of the unique aspects of emergency medicine practice. The ACEP stressed the need for caution as the consolidation of medical practices continues, many under the umbrella of private equity investment companies.
“Unchecked mergers that substantially lessen competition in the labor market for emergency physicians, in which the employer is the buyer and the physician is the seller, can impact physicians directly by lowering wages or slowing wage growth, worsening benefits or working conditions, or contributing to other degradations in workplace quality,” according to ACEP.
The AMA also supports the FTC’s draft merger guidelines as protective of physicians and their working environments.
In September 2023, the AMA sent a letter to the FTC commending the agency on the proposed guidelines: “It is our strong contention that the agencies must have merger guidelines that protect physicians against health insurer mergers that may substantially lessen competition for the purchase of physician services and that degrade physician working conditions,” according to the AMA letter.
According the FTC, the proposed changes represent an expansion and reorganization of information along with the addition of new document requirements and represents the first comprehensive review of the Hart-Scott-Rodino Antitrust Improvements Act since 1978.
After soliciting public comments, the FTC is reviewing the proposals, and no specific date for a final vote has been announced.
More specifics on the potential changes to premerger notification, reporting, and waiting period requirements are available on the FTC website.
A version of this article appeared on Medscape.com.
Changes may be in store for how physicians do business based on pending proposals from the Federal Trade Commission to ban noncompete clauses and monitor potential merger monopolies.
In January 2023, the FTC announced a rule that would ban noncompete clauses, stating that such clauses reduce workers’ wages and stifle new businesses. Simply put, the rule would ban employers from entering into noncompete clauses with workers, including independent contractors.
Aspects of the rule include whether it should pertain to franchisees, whether senior executives should be exempted, and whether low-wage and high-wage workers should be treated differently.
According to the FTC, banning noncompete clauses would increase workers’ earnings by approximately $300 billion per year, save consumers as much as $148 billion in health care costs, and double the number of companies founded by former workers in the same field.
In June 2023, the FTC and the Department of Justice proposed changes to rules governing mergers, including changes to prenotification forms that would promote more efficient screening of potential mergers. According to a press release from the FTC, the proposed changes include provision of details about investments or corporate relationships, product and services, projected revenue streams, and previous acquisitions.
The proposal also includes a waiting period during which agencies would assess the risk that a merger would lessen competition or tend to create a monopoly.
What the FTC proposals mean for physicians
FTC Chair Lina M. Khan addressed attendees at the American College of Physicians at their annual meeting in October.
In March 2023, ACEP wrote to Ms. Khan in support of the banning of noncompete clauses. The ACEP also stated that the FTC should monitor the effect of a ban on the ability to recruit and maintain a stable physician workforce in rural and underserved areas “and should examine the potential impacts should nonprofit health systems be exempt from a ban.”
However, the American Medical Group Association, a nonprofit trade organization that supports multispecialty medical groups, opposes the ban. In a press release issued in March 2023, AMGA noted that, “As employers, AMGA members rely in part on noncompete agreements to build strong, sustainable care teams that work together to coordinate care for their patients. These care teams emphasize the importance of the doctor-patient relationship, which reasonable noncompete agreements help support.”
The American Medical Association supports the ban on noncompete clauses, detailed in an official AMA policy statement as, “support[ing] policies, regulations, and legislation that prohibits covenants not-to-compete for all physicians in clinical practice who hold employment contracts with for-profit or nonprofit hospital, hospital system, or staffing company employers.”
In regard to the merger guidelines, ACEP wrote a separate letter to Ms. Khan identifying some of the unique aspects of emergency medicine practice. The ACEP stressed the need for caution as the consolidation of medical practices continues, many under the umbrella of private equity investment companies.
“Unchecked mergers that substantially lessen competition in the labor market for emergency physicians, in which the employer is the buyer and the physician is the seller, can impact physicians directly by lowering wages or slowing wage growth, worsening benefits or working conditions, or contributing to other degradations in workplace quality,” according to ACEP.
The AMA also supports the FTC’s draft merger guidelines as protective of physicians and their working environments.
In September 2023, the AMA sent a letter to the FTC commending the agency on the proposed guidelines: “It is our strong contention that the agencies must have merger guidelines that protect physicians against health insurer mergers that may substantially lessen competition for the purchase of physician services and that degrade physician working conditions,” according to the AMA letter.
According the FTC, the proposed changes represent an expansion and reorganization of information along with the addition of new document requirements and represents the first comprehensive review of the Hart-Scott-Rodino Antitrust Improvements Act since 1978.
After soliciting public comments, the FTC is reviewing the proposals, and no specific date for a final vote has been announced.
More specifics on the potential changes to premerger notification, reporting, and waiting period requirements are available on the FTC website.
A version of this article appeared on Medscape.com.
Changes may be in store for how physicians do business based on pending proposals from the Federal Trade Commission to ban noncompete clauses and monitor potential merger monopolies.
In January 2023, the FTC announced a rule that would ban noncompete clauses, stating that such clauses reduce workers’ wages and stifle new businesses. Simply put, the rule would ban employers from entering into noncompete clauses with workers, including independent contractors.
Aspects of the rule include whether it should pertain to franchisees, whether senior executives should be exempted, and whether low-wage and high-wage workers should be treated differently.
According to the FTC, banning noncompete clauses would increase workers’ earnings by approximately $300 billion per year, save consumers as much as $148 billion in health care costs, and double the number of companies founded by former workers in the same field.
In June 2023, the FTC and the Department of Justice proposed changes to rules governing mergers, including changes to prenotification forms that would promote more efficient screening of potential mergers. According to a press release from the FTC, the proposed changes include provision of details about investments or corporate relationships, product and services, projected revenue streams, and previous acquisitions.
The proposal also includes a waiting period during which agencies would assess the risk that a merger would lessen competition or tend to create a monopoly.
What the FTC proposals mean for physicians
FTC Chair Lina M. Khan addressed attendees at the American College of Physicians at their annual meeting in October.
In March 2023, ACEP wrote to Ms. Khan in support of the banning of noncompete clauses. The ACEP also stated that the FTC should monitor the effect of a ban on the ability to recruit and maintain a stable physician workforce in rural and underserved areas “and should examine the potential impacts should nonprofit health systems be exempt from a ban.”
However, the American Medical Group Association, a nonprofit trade organization that supports multispecialty medical groups, opposes the ban. In a press release issued in March 2023, AMGA noted that, “As employers, AMGA members rely in part on noncompete agreements to build strong, sustainable care teams that work together to coordinate care for their patients. These care teams emphasize the importance of the doctor-patient relationship, which reasonable noncompete agreements help support.”
The American Medical Association supports the ban on noncompete clauses, detailed in an official AMA policy statement as, “support[ing] policies, regulations, and legislation that prohibits covenants not-to-compete for all physicians in clinical practice who hold employment contracts with for-profit or nonprofit hospital, hospital system, or staffing company employers.”
In regard to the merger guidelines, ACEP wrote a separate letter to Ms. Khan identifying some of the unique aspects of emergency medicine practice. The ACEP stressed the need for caution as the consolidation of medical practices continues, many under the umbrella of private equity investment companies.
“Unchecked mergers that substantially lessen competition in the labor market for emergency physicians, in which the employer is the buyer and the physician is the seller, can impact physicians directly by lowering wages or slowing wage growth, worsening benefits or working conditions, or contributing to other degradations in workplace quality,” according to ACEP.
The AMA also supports the FTC’s draft merger guidelines as protective of physicians and their working environments.
In September 2023, the AMA sent a letter to the FTC commending the agency on the proposed guidelines: “It is our strong contention that the agencies must have merger guidelines that protect physicians against health insurer mergers that may substantially lessen competition for the purchase of physician services and that degrade physician working conditions,” according to the AMA letter.
According the FTC, the proposed changes represent an expansion and reorganization of information along with the addition of new document requirements and represents the first comprehensive review of the Hart-Scott-Rodino Antitrust Improvements Act since 1978.
After soliciting public comments, the FTC is reviewing the proposals, and no specific date for a final vote has been announced.
More specifics on the potential changes to premerger notification, reporting, and waiting period requirements are available on the FTC website.
A version of this article appeared on Medscape.com.
Actinic keratoses may predict skin cancers in older adults
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Obinutuzumab promotes renal preservation in lupus nephritis
TOPLINE:
Adults with lupus nephritis (LN) who received obinutuzumab (Gazyva) plus standard of care therapy experienced significantly improved kidney function and fewer flares compared with those given a placebo plus standard of care.
METHODOLOGY:
- Researchers conducted a post hoc analysis of the phase 2 NOBILITY study, a randomized trial in which 63 adults received 1,000 mg of obinutuzumab or placebo by infusion on day 1 and at weeks 2, 24, and 26.
- Outcomes were time to an unfavorable kidney outcome, defined by the first of any of the following events: treatment failure, doubling of serum creatinine, or death; researchers also measured LN flare outcomes including the first 30% and 40% declines in estimated glomerular filtration rate (eGFR) from baseline, chronic eGFR slope, and how many patients achieved complete renal response (CRR) on no more than 7.5 mg of prednisone.
TAKEAWAY:
- Adding obinutuzumab to the treatment of patients with LN reduced the risk of the composite outcome by 60% and reduced the risk for LN flare by 57%.
- The risk of first eGFR 30% and 40% decline was reduced by 80% and 91%, respectively, with obinutuzumab, and patients who took obinutuzumab had a significantly slower eGFR decline than with placebo (annualized eGFR slope advantage, 4.1 mL/min/1.73 m2 /year).
- At 76 weeks (1.5 years), 38% of patients receiving obinutuzumab achieved CRR on 7.5 mg or less of daily prednisone, compared with 16% of placebo patients, but this difference was not statistically significant at 104 weeks (2 years).
- The total numbers of unfavorable kidney outcomes for obinutuzumab vs. placebo were 12 vs. 24 for treatment failure, 1 vs. 6 for creatinine doubling, and 1 vs. 4 for death, respectively.
IN PRACTICE:
“By reducing flare risk, obinutuzumab should decrease the accumulation of chronic parenchymal kidney damage,” the authors wrote.
SOURCE:
The study was presented at the American College of Rheumatology (ACR) 2023 annual meeting and was published online in Arthritis & Rheumatology. The lead author was Brad H. Rovin, MD, of The Ohio State University in Columbus.
LIMITATIONS:
The analyses were post hoc and not prespecified, and the number of events was small, which prevented statistical confirmation, but the analyses are being repeated in an ongoing phase 3 study.
DISCLOSURES:
The study was supported by F. Hoffman–La Roche. Dr. Rovin reported receiving personal fees from F. Hoffman–La Roche during the conduct of the original trial. Several coauthors are F. Hoffman–La Roche employees.
A version of this article first appeared on Medscape.com.
TOPLINE:
Adults with lupus nephritis (LN) who received obinutuzumab (Gazyva) plus standard of care therapy experienced significantly improved kidney function and fewer flares compared with those given a placebo plus standard of care.
METHODOLOGY:
- Researchers conducted a post hoc analysis of the phase 2 NOBILITY study, a randomized trial in which 63 adults received 1,000 mg of obinutuzumab or placebo by infusion on day 1 and at weeks 2, 24, and 26.
- Outcomes were time to an unfavorable kidney outcome, defined by the first of any of the following events: treatment failure, doubling of serum creatinine, or death; researchers also measured LN flare outcomes including the first 30% and 40% declines in estimated glomerular filtration rate (eGFR) from baseline, chronic eGFR slope, and how many patients achieved complete renal response (CRR) on no more than 7.5 mg of prednisone.
TAKEAWAY:
- Adding obinutuzumab to the treatment of patients with LN reduced the risk of the composite outcome by 60% and reduced the risk for LN flare by 57%.
- The risk of first eGFR 30% and 40% decline was reduced by 80% and 91%, respectively, with obinutuzumab, and patients who took obinutuzumab had a significantly slower eGFR decline than with placebo (annualized eGFR slope advantage, 4.1 mL/min/1.73 m2 /year).
- At 76 weeks (1.5 years), 38% of patients receiving obinutuzumab achieved CRR on 7.5 mg or less of daily prednisone, compared with 16% of placebo patients, but this difference was not statistically significant at 104 weeks (2 years).
- The total numbers of unfavorable kidney outcomes for obinutuzumab vs. placebo were 12 vs. 24 for treatment failure, 1 vs. 6 for creatinine doubling, and 1 vs. 4 for death, respectively.
IN PRACTICE:
“By reducing flare risk, obinutuzumab should decrease the accumulation of chronic parenchymal kidney damage,” the authors wrote.
SOURCE:
The study was presented at the American College of Rheumatology (ACR) 2023 annual meeting and was published online in Arthritis & Rheumatology. The lead author was Brad H. Rovin, MD, of The Ohio State University in Columbus.
LIMITATIONS:
The analyses were post hoc and not prespecified, and the number of events was small, which prevented statistical confirmation, but the analyses are being repeated in an ongoing phase 3 study.
DISCLOSURES:
The study was supported by F. Hoffman–La Roche. Dr. Rovin reported receiving personal fees from F. Hoffman–La Roche during the conduct of the original trial. Several coauthors are F. Hoffman–La Roche employees.
A version of this article first appeared on Medscape.com.
TOPLINE:
Adults with lupus nephritis (LN) who received obinutuzumab (Gazyva) plus standard of care therapy experienced significantly improved kidney function and fewer flares compared with those given a placebo plus standard of care.
METHODOLOGY:
- Researchers conducted a post hoc analysis of the phase 2 NOBILITY study, a randomized trial in which 63 adults received 1,000 mg of obinutuzumab or placebo by infusion on day 1 and at weeks 2, 24, and 26.
- Outcomes were time to an unfavorable kidney outcome, defined by the first of any of the following events: treatment failure, doubling of serum creatinine, or death; researchers also measured LN flare outcomes including the first 30% and 40% declines in estimated glomerular filtration rate (eGFR) from baseline, chronic eGFR slope, and how many patients achieved complete renal response (CRR) on no more than 7.5 mg of prednisone.
TAKEAWAY:
- Adding obinutuzumab to the treatment of patients with LN reduced the risk of the composite outcome by 60% and reduced the risk for LN flare by 57%.
- The risk of first eGFR 30% and 40% decline was reduced by 80% and 91%, respectively, with obinutuzumab, and patients who took obinutuzumab had a significantly slower eGFR decline than with placebo (annualized eGFR slope advantage, 4.1 mL/min/1.73 m2 /year).
- At 76 weeks (1.5 years), 38% of patients receiving obinutuzumab achieved CRR on 7.5 mg or less of daily prednisone, compared with 16% of placebo patients, but this difference was not statistically significant at 104 weeks (2 years).
- The total numbers of unfavorable kidney outcomes for obinutuzumab vs. placebo were 12 vs. 24 for treatment failure, 1 vs. 6 for creatinine doubling, and 1 vs. 4 for death, respectively.
IN PRACTICE:
“By reducing flare risk, obinutuzumab should decrease the accumulation of chronic parenchymal kidney damage,” the authors wrote.
SOURCE:
The study was presented at the American College of Rheumatology (ACR) 2023 annual meeting and was published online in Arthritis & Rheumatology. The lead author was Brad H. Rovin, MD, of The Ohio State University in Columbus.
LIMITATIONS:
The analyses were post hoc and not prespecified, and the number of events was small, which prevented statistical confirmation, but the analyses are being repeated in an ongoing phase 3 study.
DISCLOSURES:
The study was supported by F. Hoffman–La Roche. Dr. Rovin reported receiving personal fees from F. Hoffman–La Roche during the conduct of the original trial. Several coauthors are F. Hoffman–La Roche employees.
A version of this article first appeared on Medscape.com.
Short steroid taper tested with tocilizumab for giant cell arteritis
TOPLINE:
A combination of tocilizumab (Actemra) and 8 weeks of tapering prednisone was effective for inducing and maintaining disease remission in adults with giant cell arteritis (GCA).
METHODOLOGY:
- In a single-center, single-arm, open-label pilot study, 30 adults (mean age, 73.7 years) with GCA received 162 mg of tocilizumab as a subcutaneous injection once a week for 52 weeks, plus prednisone starting between 20 mg and 60 mg with a prespecified 8-week taper off the glucocorticoid.
- Patients had to be at least 50 years of age and could have either new-onset (diagnosis within 6 weeks of baseline) or relapsing disease (diagnosis > 6 weeks from baseline).
- The primary endpoint was sustained, prednisone-free remission at 52 weeks, defined by an erythrocyte sedimentation rate of less than 40 mm/h, C-reactive protein level less than 10 mg/L, and adherence to the prednisone taper; secondary endpoints included the proportions of patients in remission and relapse, cumulative prednisone dose, and glucocorticoid toxicity.
TAKEAWAY:
- At 52 weeks, 23 patients (77%) met the criteria for sustained remission after weaning off prednisone within 8 weeks of starting tocilizumab; 7 relapsed after a mean of 15.8 weeks.
- Of the patients who relapsed, six underwent a second prednisone taper for 8 weeks with a mean initial daily dose of 32.1 mg, four regained and maintained remission, and two experienced a second relapse and withdrew from the study.
- The mean cumulative prednisone dose at week 52 was 1,051.5 mg for responders and 1,673.1 mg for nonresponders.
- All 30 patients had at least one adverse event; four patients had a serious adverse event likely related to tocilizumab, prednisone, or both.
IN PRACTICE:
Studies such as this “are highly valuable as proof of concept, but of course cannot be definitive guides to treatment decisions without a comparator group,” according to authors of an editorial accompanying the study.
SOURCE:
The study, by Sebastian Unizony, MD, Harvard Medical School, Boston, and colleagues, was published in The Lancet Rheumatology .
LIMITATIONS:
The small size and open-label design with no control group were limiting factors; more research is needed to confirm the findings before this treatment strategy can be recommended for clinical practice.
DISCLOSURES:
The study was funded by Genentech. Two authors reported financial relationships with pharmaceutical companies outside of this report.
A version of this article first appeared on Medscape.com.
TOPLINE:
A combination of tocilizumab (Actemra) and 8 weeks of tapering prednisone was effective for inducing and maintaining disease remission in adults with giant cell arteritis (GCA).
METHODOLOGY:
- In a single-center, single-arm, open-label pilot study, 30 adults (mean age, 73.7 years) with GCA received 162 mg of tocilizumab as a subcutaneous injection once a week for 52 weeks, plus prednisone starting between 20 mg and 60 mg with a prespecified 8-week taper off the glucocorticoid.
- Patients had to be at least 50 years of age and could have either new-onset (diagnosis within 6 weeks of baseline) or relapsing disease (diagnosis > 6 weeks from baseline).
- The primary endpoint was sustained, prednisone-free remission at 52 weeks, defined by an erythrocyte sedimentation rate of less than 40 mm/h, C-reactive protein level less than 10 mg/L, and adherence to the prednisone taper; secondary endpoints included the proportions of patients in remission and relapse, cumulative prednisone dose, and glucocorticoid toxicity.
TAKEAWAY:
- At 52 weeks, 23 patients (77%) met the criteria for sustained remission after weaning off prednisone within 8 weeks of starting tocilizumab; 7 relapsed after a mean of 15.8 weeks.
- Of the patients who relapsed, six underwent a second prednisone taper for 8 weeks with a mean initial daily dose of 32.1 mg, four regained and maintained remission, and two experienced a second relapse and withdrew from the study.
- The mean cumulative prednisone dose at week 52 was 1,051.5 mg for responders and 1,673.1 mg for nonresponders.
- All 30 patients had at least one adverse event; four patients had a serious adverse event likely related to tocilizumab, prednisone, or both.
IN PRACTICE:
Studies such as this “are highly valuable as proof of concept, but of course cannot be definitive guides to treatment decisions without a comparator group,” according to authors of an editorial accompanying the study.
SOURCE:
The study, by Sebastian Unizony, MD, Harvard Medical School, Boston, and colleagues, was published in The Lancet Rheumatology .
LIMITATIONS:
The small size and open-label design with no control group were limiting factors; more research is needed to confirm the findings before this treatment strategy can be recommended for clinical practice.
DISCLOSURES:
The study was funded by Genentech. Two authors reported financial relationships with pharmaceutical companies outside of this report.
A version of this article first appeared on Medscape.com.
TOPLINE:
A combination of tocilizumab (Actemra) and 8 weeks of tapering prednisone was effective for inducing and maintaining disease remission in adults with giant cell arteritis (GCA).
METHODOLOGY:
- In a single-center, single-arm, open-label pilot study, 30 adults (mean age, 73.7 years) with GCA received 162 mg of tocilizumab as a subcutaneous injection once a week for 52 weeks, plus prednisone starting between 20 mg and 60 mg with a prespecified 8-week taper off the glucocorticoid.
- Patients had to be at least 50 years of age and could have either new-onset (diagnosis within 6 weeks of baseline) or relapsing disease (diagnosis > 6 weeks from baseline).
- The primary endpoint was sustained, prednisone-free remission at 52 weeks, defined by an erythrocyte sedimentation rate of less than 40 mm/h, C-reactive protein level less than 10 mg/L, and adherence to the prednisone taper; secondary endpoints included the proportions of patients in remission and relapse, cumulative prednisone dose, and glucocorticoid toxicity.
TAKEAWAY:
- At 52 weeks, 23 patients (77%) met the criteria for sustained remission after weaning off prednisone within 8 weeks of starting tocilizumab; 7 relapsed after a mean of 15.8 weeks.
- Of the patients who relapsed, six underwent a second prednisone taper for 8 weeks with a mean initial daily dose of 32.1 mg, four regained and maintained remission, and two experienced a second relapse and withdrew from the study.
- The mean cumulative prednisone dose at week 52 was 1,051.5 mg for responders and 1,673.1 mg for nonresponders.
- All 30 patients had at least one adverse event; four patients had a serious adverse event likely related to tocilizumab, prednisone, or both.
IN PRACTICE:
Studies such as this “are highly valuable as proof of concept, but of course cannot be definitive guides to treatment decisions without a comparator group,” according to authors of an editorial accompanying the study.
SOURCE:
The study, by Sebastian Unizony, MD, Harvard Medical School, Boston, and colleagues, was published in The Lancet Rheumatology .
LIMITATIONS:
The small size and open-label design with no control group were limiting factors; more research is needed to confirm the findings before this treatment strategy can be recommended for clinical practice.
DISCLOSURES:
The study was funded by Genentech. Two authors reported financial relationships with pharmaceutical companies outside of this report.
A version of this article first appeared on Medscape.com.
Review estimates acne risk with JAK inhibitor therapy
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
Experts offer guidance on GLP-1 receptor agonists prior to endoscopy
to support the success of endoscopic procedures, according to a new Clinical Practice Update from the American Gastroenterological Association.
Use of glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1 RAs) has been associated with delayed gastric emptying, which raises a clinical concern about performing endoscopic procedures, especially upper endoscopies in patients using these medications, wrote Jana G. Al Hashash, MD, MSc, of the Mayo Clinic, Jacksonville, Fla., and colleagues.
The Clinical Practice Update (CPU), published in Clinical Gastroenterology and Hepatology, reviews the evidence and provides expert advice for clinicians on the evolving landscape of patients taking GLP-1 receptor agonists prior to endoscopic procedures. The CPU reflects on the most recent literature and the experience of the authors, all experts in bariatric medicine and/or endoscopy.
The American Society of Anesthesiologists (ASA) issued guidance that reflects concerns for the risk of aspiration in sedated patients because of delayed gastric motility from the use of GLP-1 RAs. The ASA advises patients on daily doses of GLP-1 RAs to refrain from taking the medications on the day of a procedure; those on weekly dosing should hold the drugs for a week prior to surgery.
However, the ASA suggestions do not differentiate based on the indication for the drug or for the type of procedure, and questions remain as to whether these changes are necessary and/or effective, the CPU authors said. The ASA’s guidance is based mainly on expert opinion, as not enough published evidence on this topic exists for a robust review and formal guideline, they added.
Recently, a multisociety statement from the AGA, AASLD, ACG, ASGE, and NASPGHAN noted that widespread implementation of the ASA guidance could be associated with unintended harms to patients.
Therefore, the AGA CPU suggests an individualized approach to managing patients on GLP-1 RAs in a pre-endoscopic setting.
For patients on GLP-1 RAs for diabetes management, discontinuing prior to endoscopic may not be worth the potential risk. Also, consider not only the dose and frequency of the GLP-1 RAs but also other comorbidities, medications, and potential gastrointestinal side effects.
“If patients taking GLP-1 RAs solely for weight loss can be identified beforehand, a dose of the medication could be withheld prior to endoscopy with likely little harm, though this should not be considered mandatory or evidence-based,” the CPU authors wrote.
However, withholding a single dose of medication may not be enough for an individual’s gastric motility to return to normal, the authors emphasized.
Additionally, the ASA’s suggestions for holding GLP-1 RAs add complexity to periprocedural medication management, which may strain resources and delay care.
The AGA CPU offers the following guidance for patients on GLP-1 RAs prior to endoscopy:
In general, patients using GLP-1 RAs who have followed the standard perioperative procedures, usually an 8-hour solid-food fast and 2-hour liquid fast, and who do not have symptoms such as ongoing nausea, vomiting, or abdominal distension should proceed with upper and/or lower endoscopy.
For symptomatic patients who may experience negative clinical consequences of endoscopy if delayed, consider rapid-sequence intubation, but the authors acknowledge that this option may not be possible in most ambulatory or office-based endoscopy settings.
Finally, consider placing patients on a liquid diet the day before a sedated procedure instead of stopping GLP-1 RAs; this strategy is “more consistent with the holistic approach to preprocedural management of other similar condi-tions,” the authors said.
The current CPU endorses the multi-society statement that puts patient safety first and encourages AGA members to follow best practices when performing endoscopies on patients who are using GLP-1 RAs, in the absence of actionable data, the authors concluded.
The Clinical Practice Update received no outside funding. Lead author Dr. Al Hashash had no financial conflicts to disclose.
to support the success of endoscopic procedures, according to a new Clinical Practice Update from the American Gastroenterological Association.
Use of glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1 RAs) has been associated with delayed gastric emptying, which raises a clinical concern about performing endoscopic procedures, especially upper endoscopies in patients using these medications, wrote Jana G. Al Hashash, MD, MSc, of the Mayo Clinic, Jacksonville, Fla., and colleagues.
The Clinical Practice Update (CPU), published in Clinical Gastroenterology and Hepatology, reviews the evidence and provides expert advice for clinicians on the evolving landscape of patients taking GLP-1 receptor agonists prior to endoscopic procedures. The CPU reflects on the most recent literature and the experience of the authors, all experts in bariatric medicine and/or endoscopy.
The American Society of Anesthesiologists (ASA) issued guidance that reflects concerns for the risk of aspiration in sedated patients because of delayed gastric motility from the use of GLP-1 RAs. The ASA advises patients on daily doses of GLP-1 RAs to refrain from taking the medications on the day of a procedure; those on weekly dosing should hold the drugs for a week prior to surgery.
However, the ASA suggestions do not differentiate based on the indication for the drug or for the type of procedure, and questions remain as to whether these changes are necessary and/or effective, the CPU authors said. The ASA’s guidance is based mainly on expert opinion, as not enough published evidence on this topic exists for a robust review and formal guideline, they added.
Recently, a multisociety statement from the AGA, AASLD, ACG, ASGE, and NASPGHAN noted that widespread implementation of the ASA guidance could be associated with unintended harms to patients.
Therefore, the AGA CPU suggests an individualized approach to managing patients on GLP-1 RAs in a pre-endoscopic setting.
For patients on GLP-1 RAs for diabetes management, discontinuing prior to endoscopic may not be worth the potential risk. Also, consider not only the dose and frequency of the GLP-1 RAs but also other comorbidities, medications, and potential gastrointestinal side effects.
“If patients taking GLP-1 RAs solely for weight loss can be identified beforehand, a dose of the medication could be withheld prior to endoscopy with likely little harm, though this should not be considered mandatory or evidence-based,” the CPU authors wrote.
However, withholding a single dose of medication may not be enough for an individual’s gastric motility to return to normal, the authors emphasized.
Additionally, the ASA’s suggestions for holding GLP-1 RAs add complexity to periprocedural medication management, which may strain resources and delay care.
The AGA CPU offers the following guidance for patients on GLP-1 RAs prior to endoscopy:
In general, patients using GLP-1 RAs who have followed the standard perioperative procedures, usually an 8-hour solid-food fast and 2-hour liquid fast, and who do not have symptoms such as ongoing nausea, vomiting, or abdominal distension should proceed with upper and/or lower endoscopy.
For symptomatic patients who may experience negative clinical consequences of endoscopy if delayed, consider rapid-sequence intubation, but the authors acknowledge that this option may not be possible in most ambulatory or office-based endoscopy settings.
Finally, consider placing patients on a liquid diet the day before a sedated procedure instead of stopping GLP-1 RAs; this strategy is “more consistent with the holistic approach to preprocedural management of other similar condi-tions,” the authors said.
The current CPU endorses the multi-society statement that puts patient safety first and encourages AGA members to follow best practices when performing endoscopies on patients who are using GLP-1 RAs, in the absence of actionable data, the authors concluded.
The Clinical Practice Update received no outside funding. Lead author Dr. Al Hashash had no financial conflicts to disclose.
to support the success of endoscopic procedures, according to a new Clinical Practice Update from the American Gastroenterological Association.
Use of glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1 RAs) has been associated with delayed gastric emptying, which raises a clinical concern about performing endoscopic procedures, especially upper endoscopies in patients using these medications, wrote Jana G. Al Hashash, MD, MSc, of the Mayo Clinic, Jacksonville, Fla., and colleagues.
The Clinical Practice Update (CPU), published in Clinical Gastroenterology and Hepatology, reviews the evidence and provides expert advice for clinicians on the evolving landscape of patients taking GLP-1 receptor agonists prior to endoscopic procedures. The CPU reflects on the most recent literature and the experience of the authors, all experts in bariatric medicine and/or endoscopy.
The American Society of Anesthesiologists (ASA) issued guidance that reflects concerns for the risk of aspiration in sedated patients because of delayed gastric motility from the use of GLP-1 RAs. The ASA advises patients on daily doses of GLP-1 RAs to refrain from taking the medications on the day of a procedure; those on weekly dosing should hold the drugs for a week prior to surgery.
However, the ASA suggestions do not differentiate based on the indication for the drug or for the type of procedure, and questions remain as to whether these changes are necessary and/or effective, the CPU authors said. The ASA’s guidance is based mainly on expert opinion, as not enough published evidence on this topic exists for a robust review and formal guideline, they added.
Recently, a multisociety statement from the AGA, AASLD, ACG, ASGE, and NASPGHAN noted that widespread implementation of the ASA guidance could be associated with unintended harms to patients.
Therefore, the AGA CPU suggests an individualized approach to managing patients on GLP-1 RAs in a pre-endoscopic setting.
For patients on GLP-1 RAs for diabetes management, discontinuing prior to endoscopic may not be worth the potential risk. Also, consider not only the dose and frequency of the GLP-1 RAs but also other comorbidities, medications, and potential gastrointestinal side effects.
“If patients taking GLP-1 RAs solely for weight loss can be identified beforehand, a dose of the medication could be withheld prior to endoscopy with likely little harm, though this should not be considered mandatory or evidence-based,” the CPU authors wrote.
However, withholding a single dose of medication may not be enough for an individual’s gastric motility to return to normal, the authors emphasized.
Additionally, the ASA’s suggestions for holding GLP-1 RAs add complexity to periprocedural medication management, which may strain resources and delay care.
The AGA CPU offers the following guidance for patients on GLP-1 RAs prior to endoscopy:
In general, patients using GLP-1 RAs who have followed the standard perioperative procedures, usually an 8-hour solid-food fast and 2-hour liquid fast, and who do not have symptoms such as ongoing nausea, vomiting, or abdominal distension should proceed with upper and/or lower endoscopy.
For symptomatic patients who may experience negative clinical consequences of endoscopy if delayed, consider rapid-sequence intubation, but the authors acknowledge that this option may not be possible in most ambulatory or office-based endoscopy settings.
Finally, consider placing patients on a liquid diet the day before a sedated procedure instead of stopping GLP-1 RAs; this strategy is “more consistent with the holistic approach to preprocedural management of other similar condi-tions,” the authors said.
The current CPU endorses the multi-society statement that puts patient safety first and encourages AGA members to follow best practices when performing endoscopies on patients who are using GLP-1 RAs, in the absence of actionable data, the authors concluded.
The Clinical Practice Update received no outside funding. Lead author Dr. Al Hashash had no financial conflicts to disclose.
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
