User login
Verbal Communication at Discharge
Hospital discharge can be hazardous because discontinuity and fragmentation of care increase risks to the patient. Inadequate communication has been identified as a major etiology for errors and adverse events occurring shortly after discharge.1, 2 Another potential result of a failed hospital discharge is patient dissatisfaction. Increased patient involvement in care improves health outcomes, and may improve patient satisfaction.3 To engage patients in their care, healthcare providers must collaborate with patients to coordinate care across settings.
In this study, we sought to determine what patients and their caregivers view as essential elements of a safe and high‐quality discharge process. We developed a survey with a broad range of questions related to the hospital discharge process (see Supporting Information, Appendix A, in the online version of this article). The survey included several questions derived from Project BOOST (Better Outcomes for Older adults through Safe Transitions) discharge care plans.4
METHODS
Study Design
We surveyed patients on the second day of admission to the internal medicine wards at the University of Washington Medical Center (a 450‐bed tertiary care teaching hospital) and Harborview Medical Center (a 412‐bed county teaching hospital) from June 1, 2010 to August 1, 2010. All patients 18 years old who were admitted during weekdays were considered for participation. Any potential participant unable to manually fill out the survey was offered the opportunity to use a proxy to help complete the survey. A proxy was any adult support person who was present in the room at the time the patient was approached with the opportunity to participate. Patients were excluded only if they (or their proxies) could not read English. The second day of hospitalization was chosen for several reasons: 1) to attempt to assess patients at a similar point in their hospital stay; 2) to avoid the day of discharge, as this may have introduced confounders such as patients who were actively engaged in the discharge process; and 3) to avoid the day of admission to increase the likelihood that patients would be medically stable at the time of the survey.
The Survey
The study protocol was reviewed and approved by the University of Washington Committee for the Protection of Human Subjects. All subjects gave verbal informed consent. The survey consisted of 3 sections: demographics, questions gauging the importance of various key points in the discharge process to patients, and open‐ended questions. Responses to questions used a Likert scale. Responses to open‐ended questions were handwritten on the paper survey.
Statistical Analysis
The quantitative data were classified categorically and analyzed using Fisher's exact test. Three investigators (M.S., S.E.M., M.B.J.) individually reviewed and coded all written patient or proxy comments using grounded theory methodology.5 Discrepant coding was identified and reconciled. The reconciled coded comments were aggregated into themes.
RESULTS
Demographics
We screened 240 patients or proxies and 200 completed the survey; 10.4% were ineligible due to language barrier, and 6.3% refused. Ninety‐two percent of patients completed the surveys. A majority were male (62.5%), 1859 years old (80%); spoke English as their first language (66%); were community‐dwelling prior to hospitalization (59%); were followed by a primary care provider (PCP) (53%), and many had at least a 4‐year‐college education (45%). One hundred eighty‐five surveys (92.5%) were completed by patients, and 15 (7.5%) were completed by proxies. Ninety surveys were completed at the county teaching hospital, and 110 surveys were completed at the tertiary teaching hospital. See Table 1 for detailed demographic information.
| Patient age, n (%) | |
| 1859 yr | 160 (80) |
| 6069 yr | 30 (15) |
| 7079 yr | 5 (2.5) |
| 80 and older | 5 (2.5) |
| Patient gender, n (%) | |
| Male | 125 (62.5) |
| Female | 75 (37.5) |
| Patient schooling, n (%) | |
| Less than high school | 20 (10) |
| High school | 50 (25) |
| Two‐year college | 40 (20) |
| Four‐year college | 70 (35) |
| Graduate education | 20 (10) |
| English is patient's first language, n (%) | |
| Yes | 132 (66) |
| No | 68 (34) |
| Patient has a primary care doctor, n (%) | |
| Yes | 106 (53) |
| No | 94 (47) |
| Patient's residence before hospitalization, n (%) | |
| Home without home health | 64 (32) |
| Home with home health | 54 (27) |
| Skilled nursing facility | 52 (26) |
| Shelter | 30 (15) |
Survey Results
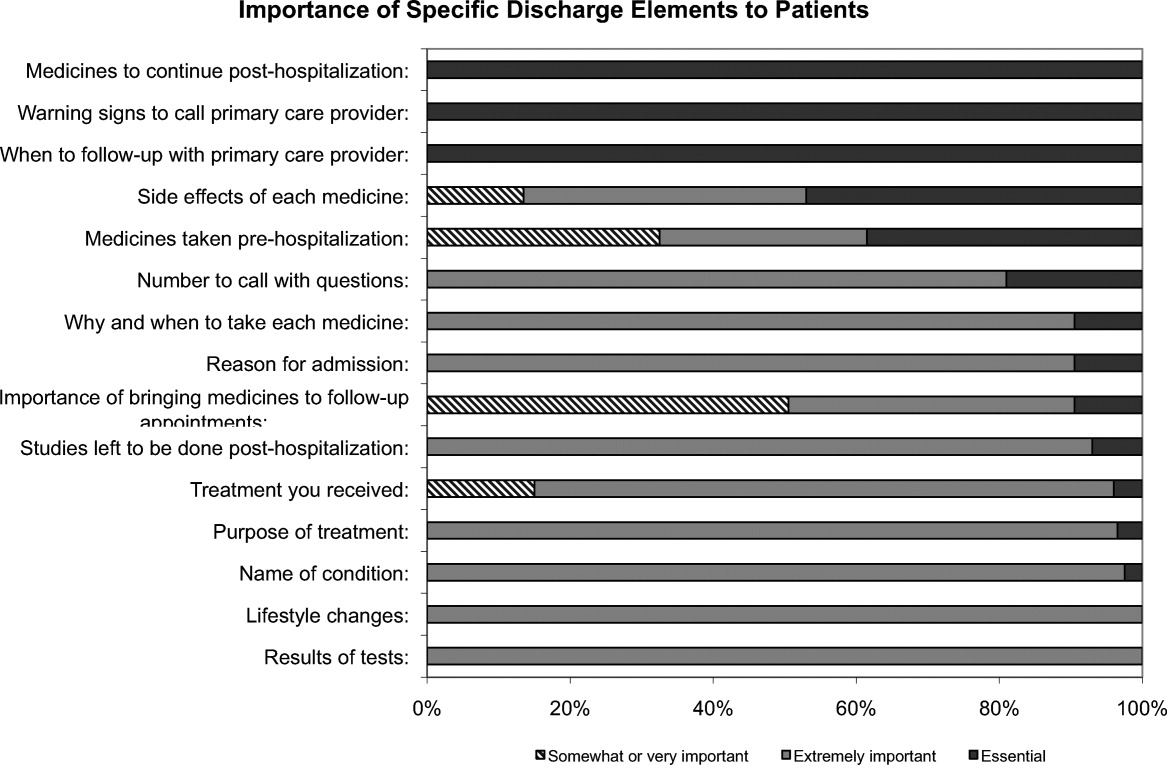
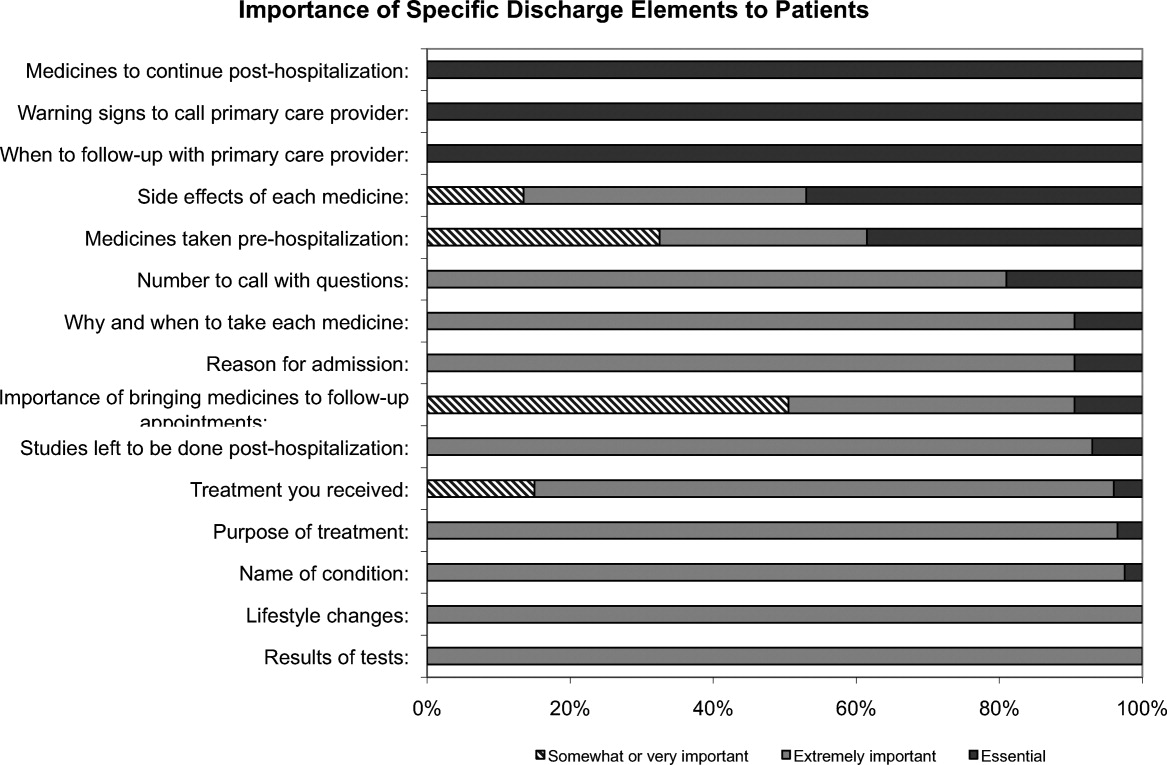
One hundred percent of patients rated the following items as essential (highest category on Likert Scale): when you need to follow‐up with primary care doctor, warning signs to call primary care doctor, and medicines to continue post‐hospitalization (Figure 1). Patients rated the following items as less important (these items were not unanimously rated as extremely important or essential): treatment you received, medicines you took pre‐hospitalization, importance of bringing all your medicines to follow‐up appointments, and given the side effect of each medication. One hundred percent of patients wanted a lot of explanation (highest category on Likert Scale) about my condition and my test results. Only 39% of patients wanted a lot of explanation about discharge medications. Sixty‐one percent wanted somewhat of an explanation about discharge medications. When asked to choose the most important piece of information, 67.5% of patients chose lifestyle changes. See Figure 1 for the relative importance of the items.
The majority of patients surveyed, 173 (86.5%), wanted verbal discharge instructions with or without written discharge instructions, with only 10.5% requesting only written discharge instructions (P < 0.0001). The majority of patients, 168 (84%), wanted resources to read about their medical condition, with 97 (57%) requesting brochures and 62 (36.9%) requesting Web sites. One hundred percent of patients thought that personal communication between the inpatient provider and the primary care doctor was extremely important or essential.
We identified 4 major themes in our qualitative review of the patients' and proxies' comments: verbal communication, frustration, opacity of system, and too many physicians. Participant quotes related to the 4 major themes are presented in Table 2. Many participants expressed a desire for verbal, rather than written, communication at the time of discharge with their healthcare team; patients particularly requested time for verbal communication with their physician. In the frustration theme, many patients and caregivers expressed frustration that the healthcare team was not carefully listening to them. In the theme of too many physicians, many patients expressed feeling overwhelmed by the number of different doctors involved in their care; particularly at discharge, patients did not know to whom to direct questions. Finally, as part of the opacity of system theme, patient comments included concerns regarding how information will be passed to outside doctors, and that the system of communication is not clear.
| Verbal communication |
| Can we just stop and talk? Everybody is rushing in and out. |
| I just want my doctor to stop by before I go home and tell me what the plan is. |
| Sometimes I feel like no one is talking to me. All they do is give me paperwork. |
| I want my doctors to sit down with me before I leave the hospital and tell me exactly what I need to do so that I don't come back. |
| I don't want papers, I want people. I want to talk to someone and not read my problems from a sheet of paper. |
| Frustration |
| I wonder sometimes if anyone is listening to me I seem to be part of a very elaborate organization that has its own rules and regulations and will not alter its ways. |
| Why do I have to keep retelling my story? It gets tiring. I wish my story could just be told once. |
| Too many physicians |
| I saw lots of doctors during my time here, but I didn't see them again when I was leaving. |
| I see so many doctors I have no idea who is in charge and who I should direct my questions to. |
| I feel overwhelmed by the number of doctors I see every time I come into the hospital. |
| I want my main doctor to talk to me. I get so confused when I hear from more than one doctor. |
| I miss the days when my primary doctor came in to check on me. He knew exactly what I needed. Now, I meet new people every time I go into the hospital. |
| Opacity of system |
| I wonder if all my doctors talk to each other. Sometimes, it seems like they don't. |
| Who keeps track of all this information? Is there someone who will pass on what happened to me here to the outside world? |
DISCUSSION
Discharge is a period of transition from hospital to home that involves a transfer in responsibility from the inpatient care team to the patient and/or caregivers and primary care physician. Ineffective communication, planning, and coordination of care can undermine patient satisfaction, increase adverse events, and contribute to more frequent hospital readmissions.
The patients we surveyed uniformly placed high value on verbal (more than written) communication about discharge care plans. Protected time during the discharge process for hospital staff to provide verbal recommendations to patients, especially about when they should return for follow‐up, warning signs to contact PCP sooner, and medications to continue after discharge, may improve patient satisfaction.
In open‐ended comments, several subjects suggested that physicians should sit down in the patient's room and provide verbal discharge instructions. Although it is well recognized that verbal communication alone has limitations and that providing patients with written instructions remains crucial, verbal reinforcement may highlight the most important instructions.
Interestingly, subjects valued information about lifestyle changes over detailed information about their medications. This may suggest that hospitalized patients are particularly receptive to information about lifestyle changes such as smoking cessation or importance of compliance with medical appointments.
Lastly, patients we surveyed value personal communication between inpatient and outpatient providers. It is plausible that this would improve transitions of care, and previous studies have suggested that direct communication between inpatient and outpatient providers occurs infrequently, with only 20% of primary care providers in 1 study reporting that they are always notified when their patient is being discharged from a hospitalist service.6
The themes that emerged from our open‐ended questions also highlight the importance of direct verbal communication with patients and careful coordination of care with outside physicians. Because patients may be unlikely to fully remember verbal instructions at discharge, providers may consider providing patients and family members with patient‐centered written materials to take home in order to reinforce important self‐care instructions. The patient comments further suggest that patients may be more satisfied, and that discharges may be smoother, if 1 or 2 physicians were always identified to the patients and their caregivers as the leaders of the care team throughout the hospital course and discharge process.
Our study had several limitations. We only surveyed patients on general medicine services, so our findings might not apply to other populations. We did not enroll participants on weekends and holidays; it is possible that this led to some bias in the enrollment of subjects. We also only surveyed patients and/or proxies who could speak and read English, and this was a fairly highly educated population, with almost half having completed 4 years of college. Finally, we relied on participant self‐report for demographic information because we did not have access to the electronic medical record. This study was conducted at 2 large academic medical centers that include resident physicians in the daily care of patients; thus, these results may not be generalizable to other settings.
Effective verbal communication between physicians, outpatient providers, patients, and their caregivers about discharge care plans might improve patients' understanding of their hospitalizations, increase their satisfaction with care, and reduce readmissions. In addition, physicians should recognize that patients value advice about lifestyle interventions that might improve their health, as part of the discharge care plan. Intervention studies are necessary to test these hypotheses in large, diverse populations.
Acknowledgements
Disclosure: Nothing to report.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,.Expanding patient involvement in care. Effects on patient outcomes.Ann Intern Med.1985;102(4):520–528.
- Society of Hospital Medicine. Project BOOST, Better Outcomes for Older adults through Safe Transitions. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Home1998.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111(9B):15S–20S.
Hospital discharge can be hazardous because discontinuity and fragmentation of care increase risks to the patient. Inadequate communication has been identified as a major etiology for errors and adverse events occurring shortly after discharge.1, 2 Another potential result of a failed hospital discharge is patient dissatisfaction. Increased patient involvement in care improves health outcomes, and may improve patient satisfaction.3 To engage patients in their care, healthcare providers must collaborate with patients to coordinate care across settings.
In this study, we sought to determine what patients and their caregivers view as essential elements of a safe and high‐quality discharge process. We developed a survey with a broad range of questions related to the hospital discharge process (see Supporting Information, Appendix A, in the online version of this article). The survey included several questions derived from Project BOOST (Better Outcomes for Older adults through Safe Transitions) discharge care plans.4
METHODS
Study Design
We surveyed patients on the second day of admission to the internal medicine wards at the University of Washington Medical Center (a 450‐bed tertiary care teaching hospital) and Harborview Medical Center (a 412‐bed county teaching hospital) from June 1, 2010 to August 1, 2010. All patients 18 years old who were admitted during weekdays were considered for participation. Any potential participant unable to manually fill out the survey was offered the opportunity to use a proxy to help complete the survey. A proxy was any adult support person who was present in the room at the time the patient was approached with the opportunity to participate. Patients were excluded only if they (or their proxies) could not read English. The second day of hospitalization was chosen for several reasons: 1) to attempt to assess patients at a similar point in their hospital stay; 2) to avoid the day of discharge, as this may have introduced confounders such as patients who were actively engaged in the discharge process; and 3) to avoid the day of admission to increase the likelihood that patients would be medically stable at the time of the survey.
The Survey
The study protocol was reviewed and approved by the University of Washington Committee for the Protection of Human Subjects. All subjects gave verbal informed consent. The survey consisted of 3 sections: demographics, questions gauging the importance of various key points in the discharge process to patients, and open‐ended questions. Responses to questions used a Likert scale. Responses to open‐ended questions were handwritten on the paper survey.
Statistical Analysis
The quantitative data were classified categorically and analyzed using Fisher's exact test. Three investigators (M.S., S.E.M., M.B.J.) individually reviewed and coded all written patient or proxy comments using grounded theory methodology.5 Discrepant coding was identified and reconciled. The reconciled coded comments were aggregated into themes.
RESULTS
Demographics
We screened 240 patients or proxies and 200 completed the survey; 10.4% were ineligible due to language barrier, and 6.3% refused. Ninety‐two percent of patients completed the surveys. A majority were male (62.5%), 1859 years old (80%); spoke English as their first language (66%); were community‐dwelling prior to hospitalization (59%); were followed by a primary care provider (PCP) (53%), and many had at least a 4‐year‐college education (45%). One hundred eighty‐five surveys (92.5%) were completed by patients, and 15 (7.5%) were completed by proxies. Ninety surveys were completed at the county teaching hospital, and 110 surveys were completed at the tertiary teaching hospital. See Table 1 for detailed demographic information.
| Patient age, n (%) | |
| 1859 yr | 160 (80) |
| 6069 yr | 30 (15) |
| 7079 yr | 5 (2.5) |
| 80 and older | 5 (2.5) |
| Patient gender, n (%) | |
| Male | 125 (62.5) |
| Female | 75 (37.5) |
| Patient schooling, n (%) | |
| Less than high school | 20 (10) |
| High school | 50 (25) |
| Two‐year college | 40 (20) |
| Four‐year college | 70 (35) |
| Graduate education | 20 (10) |
| English is patient's first language, n (%) | |
| Yes | 132 (66) |
| No | 68 (34) |
| Patient has a primary care doctor, n (%) | |
| Yes | 106 (53) |
| No | 94 (47) |
| Patient's residence before hospitalization, n (%) | |
| Home without home health | 64 (32) |
| Home with home health | 54 (27) |
| Skilled nursing facility | 52 (26) |
| Shelter | 30 (15) |
Survey Results
One hundred percent of patients rated the following items as essential (highest category on Likert Scale): when you need to follow‐up with primary care doctor, warning signs to call primary care doctor, and medicines to continue post‐hospitalization (Figure 1). Patients rated the following items as less important (these items were not unanimously rated as extremely important or essential): treatment you received, medicines you took pre‐hospitalization, importance of bringing all your medicines to follow‐up appointments, and given the side effect of each medication. One hundred percent of patients wanted a lot of explanation (highest category on Likert Scale) about my condition and my test results. Only 39% of patients wanted a lot of explanation about discharge medications. Sixty‐one percent wanted somewhat of an explanation about discharge medications. When asked to choose the most important piece of information, 67.5% of patients chose lifestyle changes. See Figure 1 for the relative importance of the items.

The majority of patients surveyed, 173 (86.5%), wanted verbal discharge instructions with or without written discharge instructions, with only 10.5% requesting only written discharge instructions (P < 0.0001). The majority of patients, 168 (84%), wanted resources to read about their medical condition, with 97 (57%) requesting brochures and 62 (36.9%) requesting Web sites. One hundred percent of patients thought that personal communication between the inpatient provider and the primary care doctor was extremely important or essential.
We identified 4 major themes in our qualitative review of the patients' and proxies' comments: verbal communication, frustration, opacity of system, and too many physicians. Participant quotes related to the 4 major themes are presented in Table 2. Many participants expressed a desire for verbal, rather than written, communication at the time of discharge with their healthcare team; patients particularly requested time for verbal communication with their physician. In the frustration theme, many patients and caregivers expressed frustration that the healthcare team was not carefully listening to them. In the theme of too many physicians, many patients expressed feeling overwhelmed by the number of different doctors involved in their care; particularly at discharge, patients did not know to whom to direct questions. Finally, as part of the opacity of system theme, patient comments included concerns regarding how information will be passed to outside doctors, and that the system of communication is not clear.
| Verbal communication |
| Can we just stop and talk? Everybody is rushing in and out. |
| I just want my doctor to stop by before I go home and tell me what the plan is. |
| Sometimes I feel like no one is talking to me. All they do is give me paperwork. |
| I want my doctors to sit down with me before I leave the hospital and tell me exactly what I need to do so that I don't come back. |
| I don't want papers, I want people. I want to talk to someone and not read my problems from a sheet of paper. |
| Frustration |
| I wonder sometimes if anyone is listening to me I seem to be part of a very elaborate organization that has its own rules and regulations and will not alter its ways. |
| Why do I have to keep retelling my story? It gets tiring. I wish my story could just be told once. |
| Too many physicians |
| I saw lots of doctors during my time here, but I didn't see them again when I was leaving. |
| I see so many doctors I have no idea who is in charge and who I should direct my questions to. |
| I feel overwhelmed by the number of doctors I see every time I come into the hospital. |
| I want my main doctor to talk to me. I get so confused when I hear from more than one doctor. |
| I miss the days when my primary doctor came in to check on me. He knew exactly what I needed. Now, I meet new people every time I go into the hospital. |
| Opacity of system |
| I wonder if all my doctors talk to each other. Sometimes, it seems like they don't. |
| Who keeps track of all this information? Is there someone who will pass on what happened to me here to the outside world? |
DISCUSSION
Discharge is a period of transition from hospital to home that involves a transfer in responsibility from the inpatient care team to the patient and/or caregivers and primary care physician. Ineffective communication, planning, and coordination of care can undermine patient satisfaction, increase adverse events, and contribute to more frequent hospital readmissions.
The patients we surveyed uniformly placed high value on verbal (more than written) communication about discharge care plans. Protected time during the discharge process for hospital staff to provide verbal recommendations to patients, especially about when they should return for follow‐up, warning signs to contact PCP sooner, and medications to continue after discharge, may improve patient satisfaction.
In open‐ended comments, several subjects suggested that physicians should sit down in the patient's room and provide verbal discharge instructions. Although it is well recognized that verbal communication alone has limitations and that providing patients with written instructions remains crucial, verbal reinforcement may highlight the most important instructions.
Interestingly, subjects valued information about lifestyle changes over detailed information about their medications. This may suggest that hospitalized patients are particularly receptive to information about lifestyle changes such as smoking cessation or importance of compliance with medical appointments.
Lastly, patients we surveyed value personal communication between inpatient and outpatient providers. It is plausible that this would improve transitions of care, and previous studies have suggested that direct communication between inpatient and outpatient providers occurs infrequently, with only 20% of primary care providers in 1 study reporting that they are always notified when their patient is being discharged from a hospitalist service.6
The themes that emerged from our open‐ended questions also highlight the importance of direct verbal communication with patients and careful coordination of care with outside physicians. Because patients may be unlikely to fully remember verbal instructions at discharge, providers may consider providing patients and family members with patient‐centered written materials to take home in order to reinforce important self‐care instructions. The patient comments further suggest that patients may be more satisfied, and that discharges may be smoother, if 1 or 2 physicians were always identified to the patients and their caregivers as the leaders of the care team throughout the hospital course and discharge process.
Our study had several limitations. We only surveyed patients on general medicine services, so our findings might not apply to other populations. We did not enroll participants on weekends and holidays; it is possible that this led to some bias in the enrollment of subjects. We also only surveyed patients and/or proxies who could speak and read English, and this was a fairly highly educated population, with almost half having completed 4 years of college. Finally, we relied on participant self‐report for demographic information because we did not have access to the electronic medical record. This study was conducted at 2 large academic medical centers that include resident physicians in the daily care of patients; thus, these results may not be generalizable to other settings.
Effective verbal communication between physicians, outpatient providers, patients, and their caregivers about discharge care plans might improve patients' understanding of their hospitalizations, increase their satisfaction with care, and reduce readmissions. In addition, physicians should recognize that patients value advice about lifestyle interventions that might improve their health, as part of the discharge care plan. Intervention studies are necessary to test these hypotheses in large, diverse populations.
Acknowledgements
Disclosure: Nothing to report.
Hospital discharge can be hazardous because discontinuity and fragmentation of care increase risks to the patient. Inadequate communication has been identified as a major etiology for errors and adverse events occurring shortly after discharge.1, 2 Another potential result of a failed hospital discharge is patient dissatisfaction. Increased patient involvement in care improves health outcomes, and may improve patient satisfaction.3 To engage patients in their care, healthcare providers must collaborate with patients to coordinate care across settings.
In this study, we sought to determine what patients and their caregivers view as essential elements of a safe and high‐quality discharge process. We developed a survey with a broad range of questions related to the hospital discharge process (see Supporting Information, Appendix A, in the online version of this article). The survey included several questions derived from Project BOOST (Better Outcomes for Older adults through Safe Transitions) discharge care plans.4
METHODS
Study Design
We surveyed patients on the second day of admission to the internal medicine wards at the University of Washington Medical Center (a 450‐bed tertiary care teaching hospital) and Harborview Medical Center (a 412‐bed county teaching hospital) from June 1, 2010 to August 1, 2010. All patients 18 years old who were admitted during weekdays were considered for participation. Any potential participant unable to manually fill out the survey was offered the opportunity to use a proxy to help complete the survey. A proxy was any adult support person who was present in the room at the time the patient was approached with the opportunity to participate. Patients were excluded only if they (or their proxies) could not read English. The second day of hospitalization was chosen for several reasons: 1) to attempt to assess patients at a similar point in their hospital stay; 2) to avoid the day of discharge, as this may have introduced confounders such as patients who were actively engaged in the discharge process; and 3) to avoid the day of admission to increase the likelihood that patients would be medically stable at the time of the survey.
The Survey
The study protocol was reviewed and approved by the University of Washington Committee for the Protection of Human Subjects. All subjects gave verbal informed consent. The survey consisted of 3 sections: demographics, questions gauging the importance of various key points in the discharge process to patients, and open‐ended questions. Responses to questions used a Likert scale. Responses to open‐ended questions were handwritten on the paper survey.
Statistical Analysis
The quantitative data were classified categorically and analyzed using Fisher's exact test. Three investigators (M.S., S.E.M., M.B.J.) individually reviewed and coded all written patient or proxy comments using grounded theory methodology.5 Discrepant coding was identified and reconciled. The reconciled coded comments were aggregated into themes.
RESULTS
Demographics
We screened 240 patients or proxies and 200 completed the survey; 10.4% were ineligible due to language barrier, and 6.3% refused. Ninety‐two percent of patients completed the surveys. A majority were male (62.5%), 1859 years old (80%); spoke English as their first language (66%); were community‐dwelling prior to hospitalization (59%); were followed by a primary care provider (PCP) (53%), and many had at least a 4‐year‐college education (45%). One hundred eighty‐five surveys (92.5%) were completed by patients, and 15 (7.5%) were completed by proxies. Ninety surveys were completed at the county teaching hospital, and 110 surveys were completed at the tertiary teaching hospital. See Table 1 for detailed demographic information.
| Patient age, n (%) | |
| 1859 yr | 160 (80) |
| 6069 yr | 30 (15) |
| 7079 yr | 5 (2.5) |
| 80 and older | 5 (2.5) |
| Patient gender, n (%) | |
| Male | 125 (62.5) |
| Female | 75 (37.5) |
| Patient schooling, n (%) | |
| Less than high school | 20 (10) |
| High school | 50 (25) |
| Two‐year college | 40 (20) |
| Four‐year college | 70 (35) |
| Graduate education | 20 (10) |
| English is patient's first language, n (%) | |
| Yes | 132 (66) |
| No | 68 (34) |
| Patient has a primary care doctor, n (%) | |
| Yes | 106 (53) |
| No | 94 (47) |
| Patient's residence before hospitalization, n (%) | |
| Home without home health | 64 (32) |
| Home with home health | 54 (27) |
| Skilled nursing facility | 52 (26) |
| Shelter | 30 (15) |
Survey Results
One hundred percent of patients rated the following items as essential (highest category on Likert Scale): when you need to follow‐up with primary care doctor, warning signs to call primary care doctor, and medicines to continue post‐hospitalization (Figure 1). Patients rated the following items as less important (these items were not unanimously rated as extremely important or essential): treatment you received, medicines you took pre‐hospitalization, importance of bringing all your medicines to follow‐up appointments, and given the side effect of each medication. One hundred percent of patients wanted a lot of explanation (highest category on Likert Scale) about my condition and my test results. Only 39% of patients wanted a lot of explanation about discharge medications. Sixty‐one percent wanted somewhat of an explanation about discharge medications. When asked to choose the most important piece of information, 67.5% of patients chose lifestyle changes. See Figure 1 for the relative importance of the items.

The majority of patients surveyed, 173 (86.5%), wanted verbal discharge instructions with or without written discharge instructions, with only 10.5% requesting only written discharge instructions (P < 0.0001). The majority of patients, 168 (84%), wanted resources to read about their medical condition, with 97 (57%) requesting brochures and 62 (36.9%) requesting Web sites. One hundred percent of patients thought that personal communication between the inpatient provider and the primary care doctor was extremely important or essential.
We identified 4 major themes in our qualitative review of the patients' and proxies' comments: verbal communication, frustration, opacity of system, and too many physicians. Participant quotes related to the 4 major themes are presented in Table 2. Many participants expressed a desire for verbal, rather than written, communication at the time of discharge with their healthcare team; patients particularly requested time for verbal communication with their physician. In the frustration theme, many patients and caregivers expressed frustration that the healthcare team was not carefully listening to them. In the theme of too many physicians, many patients expressed feeling overwhelmed by the number of different doctors involved in their care; particularly at discharge, patients did not know to whom to direct questions. Finally, as part of the opacity of system theme, patient comments included concerns regarding how information will be passed to outside doctors, and that the system of communication is not clear.
| Verbal communication |
| Can we just stop and talk? Everybody is rushing in and out. |
| I just want my doctor to stop by before I go home and tell me what the plan is. |
| Sometimes I feel like no one is talking to me. All they do is give me paperwork. |
| I want my doctors to sit down with me before I leave the hospital and tell me exactly what I need to do so that I don't come back. |
| I don't want papers, I want people. I want to talk to someone and not read my problems from a sheet of paper. |
| Frustration |
| I wonder sometimes if anyone is listening to me I seem to be part of a very elaborate organization that has its own rules and regulations and will not alter its ways. |
| Why do I have to keep retelling my story? It gets tiring. I wish my story could just be told once. |
| Too many physicians |
| I saw lots of doctors during my time here, but I didn't see them again when I was leaving. |
| I see so many doctors I have no idea who is in charge and who I should direct my questions to. |
| I feel overwhelmed by the number of doctors I see every time I come into the hospital. |
| I want my main doctor to talk to me. I get so confused when I hear from more than one doctor. |
| I miss the days when my primary doctor came in to check on me. He knew exactly what I needed. Now, I meet new people every time I go into the hospital. |
| Opacity of system |
| I wonder if all my doctors talk to each other. Sometimes, it seems like they don't. |
| Who keeps track of all this information? Is there someone who will pass on what happened to me here to the outside world? |
DISCUSSION
Discharge is a period of transition from hospital to home that involves a transfer in responsibility from the inpatient care team to the patient and/or caregivers and primary care physician. Ineffective communication, planning, and coordination of care can undermine patient satisfaction, increase adverse events, and contribute to more frequent hospital readmissions.
The patients we surveyed uniformly placed high value on verbal (more than written) communication about discharge care plans. Protected time during the discharge process for hospital staff to provide verbal recommendations to patients, especially about when they should return for follow‐up, warning signs to contact PCP sooner, and medications to continue after discharge, may improve patient satisfaction.
In open‐ended comments, several subjects suggested that physicians should sit down in the patient's room and provide verbal discharge instructions. Although it is well recognized that verbal communication alone has limitations and that providing patients with written instructions remains crucial, verbal reinforcement may highlight the most important instructions.
Interestingly, subjects valued information about lifestyle changes over detailed information about their medications. This may suggest that hospitalized patients are particularly receptive to information about lifestyle changes such as smoking cessation or importance of compliance with medical appointments.
Lastly, patients we surveyed value personal communication between inpatient and outpatient providers. It is plausible that this would improve transitions of care, and previous studies have suggested that direct communication between inpatient and outpatient providers occurs infrequently, with only 20% of primary care providers in 1 study reporting that they are always notified when their patient is being discharged from a hospitalist service.6
The themes that emerged from our open‐ended questions also highlight the importance of direct verbal communication with patients and careful coordination of care with outside physicians. Because patients may be unlikely to fully remember verbal instructions at discharge, providers may consider providing patients and family members with patient‐centered written materials to take home in order to reinforce important self‐care instructions. The patient comments further suggest that patients may be more satisfied, and that discharges may be smoother, if 1 or 2 physicians were always identified to the patients and their caregivers as the leaders of the care team throughout the hospital course and discharge process.
Our study had several limitations. We only surveyed patients on general medicine services, so our findings might not apply to other populations. We did not enroll participants on weekends and holidays; it is possible that this led to some bias in the enrollment of subjects. We also only surveyed patients and/or proxies who could speak and read English, and this was a fairly highly educated population, with almost half having completed 4 years of college. Finally, we relied on participant self‐report for demographic information because we did not have access to the electronic medical record. This study was conducted at 2 large academic medical centers that include resident physicians in the daily care of patients; thus, these results may not be generalizable to other settings.
Effective verbal communication between physicians, outpatient providers, patients, and their caregivers about discharge care plans might improve patients' understanding of their hospitalizations, increase their satisfaction with care, and reduce readmissions. In addition, physicians should recognize that patients value advice about lifestyle interventions that might improve their health, as part of the discharge care plan. Intervention studies are necessary to test these hypotheses in large, diverse populations.
Acknowledgements
Disclosure: Nothing to report.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,.Expanding patient involvement in care. Effects on patient outcomes.Ann Intern Med.1985;102(4):520–528.
- Society of Hospital Medicine. Project BOOST, Better Outcomes for Older adults through Safe Transitions. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Home1998.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111(9B):15S–20S.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,.Expanding patient involvement in care. Effects on patient outcomes.Ann Intern Med.1985;102(4):520–528.
- Society of Hospital Medicine. Project BOOST, Better Outcomes for Older adults through Safe Transitions. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Home1998.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111(9B):15S–20S.
What Is the Best Therapy for Acute Hepatic Encephalopathy?
Case
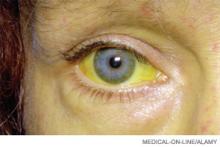
A 56-year-old man with a history of cirrhosis, complicated by esophageal varices and ongoing alcohol abuse, is admitted after his wife found him lethargic and disoriented in bed. His wife said he’d been increasingly irritable and agitated, with slurred speech, the past two days. On exam, he is somnolent but arousable; spider telangiectasias and asterixis are noted. Laboratory studies are consistent with chronic liver disease.
What is the best therapy for his acute hepatic encephalopathy?
Overview
Hepatic encephalopathy (HE) describes the spectrum of potentially reversible neuropsychiatric abnormalities seen in patients with liver dysfunction. The wide range of neuropsychiatric presentations led to the development of consensus HE classification terminology by the World Congress of Gastroenterology in 2002.
The primary tenet of all HE pathogenesis theories is firmly established: Nitrogenous substances derived from the gut adversely affect brain function. These compounds access the systemic circulation via decreased hepatic function or portal-systemic shunts. In the brain, they alter neurotransmission, which affects consciousness and behavior.
HE patients usually have advanced cirrhosis and, hence, many of the physical findings associated with severe hepatic dysfunction: muscle-wasting, jaundice, ascites, palmar erythema, edema, spider telangiectasias, and fetor hepaticus. Encephalopathy progresses from reversal of the sleep-wake cycle and mild mental status changes to irritability, confusion, and slurred speech.
Advanced neurologic features include asterixis or tongue fasciculations, bradykinesia, hyperreflexia, and ultimately coma. History and laboratory data can reveal a precipitating cause (see Table 2, p. 19). Measurement of ammonia concentration remains controversial. The value may be useful for monitoring the efficacy of ammonia-lowering therapy, but elevated levels are not required to make the diagnosis.
Multiple treatments have been used to manage HE, yet few well-designed randomized trials have assessed efficacy due to challenges inherent in measuring the wide range of neuropsychiatric presentations. Nonetheless, a critical appraisal of available data delineates a rational approach to therapy.
Review of the Data
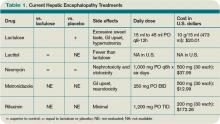
In addition to supportive care and the reversal of any precipitating factors, the treatment of acute HE is aimed at reducing or inhibiting intestinal ammonia production or increasing its removal (see Table 1, left).
Nonabsorbable disaccharides (NAD): Lactulose (beta-galactosidofructose) and lactitol (beta-galactosidosorbitol) are used as first-line agents for the treatment of HE and lead to symptomatic improvement in 67% to 87% of patients.1 They reduce the concentration of ammoniogenic substrates in the colonic lumen in two ways—first, by facilitating bacterial fermentation and secondary organic acid production (lowering colonic pH) and, second, by direct osmotic catharsis.
NAD are administered orally or via nasogastric tube at an initial dose of 45 ml, followed by repeated hourly doses until the patient has a bowel movement. For patients at risk of aspiration, NAD can be administered via enema (300 ml in 700 ml of water) every two hours as needed until mental function improves. Once the risk of aspiration is minimized, NAD can be administered orally and titrated to achieve two to three soft bowel movements daily (the usual oral dosage is 15 ml to 45 ml every eight to 12 hours).
Common side effects of NAD include an excessively sweet taste, flatulence, abdominal cramping, and electrolyte imbalance, particularly hypernatremia, which may further deteriorate mental status.
Als-Nielsen et al demonstrated in a systematic review that NAD were more effective than placebo in improving HE, but NAD had no significant benefit on mortality.1 However, the effect on HE no longer reached statistical significance when the analysis was confined to studies with the highest methodological quality. In a randomized, double-blind comparison, Morgan et al showed that lactitol was more tolerable than lactulose and produced fewer side effects.2 Lactitol is not currently available for use in the U.S.
Antibiotics: Certain oral antibiotics (e.g., neomycin, rifaximin, and metronidazole) reduce urease-producing intestinal bacteria, which results in decreased ammonia production and absorption through the gastrointestinal tract. Antibiotics generally are used in patients who do not tolerate NAD or who remain symptomatic despite NAD. The combined use of NAD and antibiotics is a subject of significant clinical relevance, though data are limited.
Neomycin is approved by the FDA for treatment of acute HE. It can be administered orally at a dose of 1,000 mg every six hours for up to six days. A randomized, controlled trial of neomycin versus placebo in 39 patients with acute HE demonstrated no significant difference in time to symptom improvement.3 Another study of 80 patients receiving neomycin and lactulose demonstrated no benefit against placebo, though some data suggest that the combination of lactulose and neomycin therapy might be more effective than either agent alone against placebo.4
Rifaximin was granted an orphan drug designation by the FDA for use in HE cases and has been compared with NAD. The recommended dose is 1,200 mg three times per day. It has minimal side effects and no reported drug interactions. A study of rifaximin versus lactitol administered for five to 10 days showed approximately 80% symptomatic improvement in both groups.5 Another trial demonstrated significantly greater improvement in blood ammonia concentrations, electroencephalographic (EEG) abnormalities, and mental status with rifaximin compared with lactulose.6 Studies comparing rifaximin and lactulose, either alone or in combination, have demonstrated that rifaximin is at least similar to lactulose, and in some cases superior in reversing encephalopathy, with better tolerability reported in the antibiotic group.7
Metronidazole is not approved by the FDA for the treatment of HE but has been evaluated. The recommended oral dose of metronidazole for chronic use is 250 mg twice per day. Prolonged administration of metronidazole can be associated with gastrointestinal disturbance and neurotoxicity. In a report of 11 HE patients with mild to moderate symptoms and seven chronically affected HE cirrhotic patients treated with metronidazole for one week, Morgan and colleagues showed metronidazole to be as effective as neomycin.8
Diet: Historically, patients with HE were placed on protein-restricted diets to reduce the production of intestinal ammonia. Recent evidence suggests that excessive restriction can raise serum ammonia levels as a result of reduced muscular ammonia metabolism. Furthermore, restricting protein intake worsens nutritional status and does not improve the outcome.9
In patients with established cirrhosis, the minimal daily dietary protein intake required to maintain nitrogen balance is 0.8 g/kg to 1.0 g/kg. At this time, a normoprotein diet for HE patients is considered the standard of care.
Other agents: L-ornithine L-aspartate (LOLA), a stable salt of ornithine and aspartic acid, provides crucial substrates for glutamine and urea synthesis—key pathways in deammonation. In patients with cirrhosis and HE, oral LOLA reduces serum ammonia and improves clinical manifestations of HE, including EEG abnormalities.10 LOLA, however, is not available in the U.S.
Sodium benzoate might be beneficial in the treatment of acute HE; it increases urinary excretion of ammonia. A prospective, randomized, double-blind study of 74 patients with acute HE found that treatment with sodium benzoate 5 g twice daily, compared with lactulose, resulted in equivalent improvements in encephalopathy. There was no placebo group.11 Routine use has been limited due to concerns regarding sodium load and increased frequency of adverse gastrointestinal symptoms, particularly nausea.
Flumazenil, a short-acting benzodiazepine receptor antagonist, has been utilized on the basis of observed increases in benzodiazepine receptor activation among cirrhotic HE patients. In a systematic review of 12 controlled trials (765 patients), Als-Nielsen and colleagues found flumazenil to be associated with significant improvement.12 Flumazenil is not used routinely as an HE therapy because of significant side effects, namely seizures, nausea, vomiting, dizziness, and agitation.
Such therapies as L-carnitine, branched amino acids (BCAA), probiotics, bromocriptine, acarbose, and zinc are among the many experimental agents currently under evaluation. Few have been tested in clinical trials.
Back to the Case
Our patient has severe HE manifested by worsening somnolence. It is postulated that ongoing alcohol abuse led to medication nonadherence, precipitating his HE, but as HE has many causes, a complete workup for infection and metabolic derangement is performed. However, it is unrevealing.
The best initial action is the prescription of lactulose, the mainstay of HE therapy. Given concern for aspiration in patients with somnolence, a feeding tube is placed for administration. The lactulose dosage will be titrated to achieve two to three soft stools per day. If the patient remains symptomatic or develops significant side effects on lactulose, the addition of an antibiotic is recommended. Neomycin, a low-cost medicine approved by the FDA for HE treatment, is a good choice. The patient will be maintained on a normal protein diet.
Bottom Line
The first-line agents used to treat episodes of acute HE are the nonabsorbable disaccharides, lactulose or lactitol. TH
Dr. Shoeb is a resident in the Department of Medicine at the University of Washington in Seattle. Dr. Best is assistant professor of medicine in the Division of General Internal Medicine at the University of Washington.
References
- Als-Nielsen B, Gluud L, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;2:CD003044.
- Morgan MY, Hawley KE. Lactitol v. lactulose in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double-blind, randomized trial. Hepatology. 1987; 7(6):1278-1284.
- Blanc P, Daurès JP, Liautard J, et al. Lactulose-neomycin combination versus placebo in the treatment of acute hepatic encephalopathy. Results of a randomized controlled trial. Gastroenterol Clin Biol. 1994;18(12):1063-1068.
- Mas A, Rodés J, Sunyer L, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38(1):51-58.
- Paik YH, Lee KS, Han KH, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46(3):399-407.
- Massa P, Vallerino E, Dodero M. Treatment of hepatic encephalopathy with rifaximin: double blind, double dummy study versus lactulose. Eur J Clin Res. 1993;4:7-18.
- Williams R, James OF, Warnes TW, Morgan MY. Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol. 2000;12(2):203-208.
- Morgan MH, Read AE, Speller DC. Treatment of hepatic encephalopathy with metronidazole. Gut. 1982;23(1):1-7.
- Córdoba J, López-Hellín J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38-43.
- Poo JL, Gongora J, Sánchez-Avila F, et al. Efficacy of oral L-ornithine-L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Results of a randomized, lactulose-controlled study. Ann Hepatol. 2006;5(4):281-288.
- Sushma S, Dasarathy S, Tandon RK, Jain S, Gupta S, Bhist MS. Sodium benzoate in the treatment of acute hepatic encephalopathy: a double-blind randomized trial. Hepatology. 1992;16(16):138-144.
- Als-Nielsen B, Kjaergard LL, Gluud C. Benzodiazepine receptor antagonists for acute and chronic hepatic encephalopathy. Cochrane Database Syst Rev. 2001;4:CD002798.
Case
A 56-year-old man with a history of cirrhosis, complicated by esophageal varices and ongoing alcohol abuse, is admitted after his wife found him lethargic and disoriented in bed. His wife said he’d been increasingly irritable and agitated, with slurred speech, the past two days. On exam, he is somnolent but arousable; spider telangiectasias and asterixis are noted. Laboratory studies are consistent with chronic liver disease.
What is the best therapy for his acute hepatic encephalopathy?
Overview
Hepatic encephalopathy (HE) describes the spectrum of potentially reversible neuropsychiatric abnormalities seen in patients with liver dysfunction. The wide range of neuropsychiatric presentations led to the development of consensus HE classification terminology by the World Congress of Gastroenterology in 2002.
The primary tenet of all HE pathogenesis theories is firmly established: Nitrogenous substances derived from the gut adversely affect brain function. These compounds access the systemic circulation via decreased hepatic function or portal-systemic shunts. In the brain, they alter neurotransmission, which affects consciousness and behavior.
HE patients usually have advanced cirrhosis and, hence, many of the physical findings associated with severe hepatic dysfunction: muscle-wasting, jaundice, ascites, palmar erythema, edema, spider telangiectasias, and fetor hepaticus. Encephalopathy progresses from reversal of the sleep-wake cycle and mild mental status changes to irritability, confusion, and slurred speech.
Advanced neurologic features include asterixis or tongue fasciculations, bradykinesia, hyperreflexia, and ultimately coma. History and laboratory data can reveal a precipitating cause (see Table 2, p. 19). Measurement of ammonia concentration remains controversial. The value may be useful for monitoring the efficacy of ammonia-lowering therapy, but elevated levels are not required to make the diagnosis.
Multiple treatments have been used to manage HE, yet few well-designed randomized trials have assessed efficacy due to challenges inherent in measuring the wide range of neuropsychiatric presentations. Nonetheless, a critical appraisal of available data delineates a rational approach to therapy.
Review of the Data
In addition to supportive care and the reversal of any precipitating factors, the treatment of acute HE is aimed at reducing or inhibiting intestinal ammonia production or increasing its removal (see Table 1, left).
Nonabsorbable disaccharides (NAD): Lactulose (beta-galactosidofructose) and lactitol (beta-galactosidosorbitol) are used as first-line agents for the treatment of HE and lead to symptomatic improvement in 67% to 87% of patients.1 They reduce the concentration of ammoniogenic substrates in the colonic lumen in two ways—first, by facilitating bacterial fermentation and secondary organic acid production (lowering colonic pH) and, second, by direct osmotic catharsis.
NAD are administered orally or via nasogastric tube at an initial dose of 45 ml, followed by repeated hourly doses until the patient has a bowel movement. For patients at risk of aspiration, NAD can be administered via enema (300 ml in 700 ml of water) every two hours as needed until mental function improves. Once the risk of aspiration is minimized, NAD can be administered orally and titrated to achieve two to three soft bowel movements daily (the usual oral dosage is 15 ml to 45 ml every eight to 12 hours).
Common side effects of NAD include an excessively sweet taste, flatulence, abdominal cramping, and electrolyte imbalance, particularly hypernatremia, which may further deteriorate mental status.
Als-Nielsen et al demonstrated in a systematic review that NAD were more effective than placebo in improving HE, but NAD had no significant benefit on mortality.1 However, the effect on HE no longer reached statistical significance when the analysis was confined to studies with the highest methodological quality. In a randomized, double-blind comparison, Morgan et al showed that lactitol was more tolerable than lactulose and produced fewer side effects.2 Lactitol is not currently available for use in the U.S.
Antibiotics: Certain oral antibiotics (e.g., neomycin, rifaximin, and metronidazole) reduce urease-producing intestinal bacteria, which results in decreased ammonia production and absorption through the gastrointestinal tract. Antibiotics generally are used in patients who do not tolerate NAD or who remain symptomatic despite NAD. The combined use of NAD and antibiotics is a subject of significant clinical relevance, though data are limited.
Neomycin is approved by the FDA for treatment of acute HE. It can be administered orally at a dose of 1,000 mg every six hours for up to six days. A randomized, controlled trial of neomycin versus placebo in 39 patients with acute HE demonstrated no significant difference in time to symptom improvement.3 Another study of 80 patients receiving neomycin and lactulose demonstrated no benefit against placebo, though some data suggest that the combination of lactulose and neomycin therapy might be more effective than either agent alone against placebo.4
Rifaximin was granted an orphan drug designation by the FDA for use in HE cases and has been compared with NAD. The recommended dose is 1,200 mg three times per day. It has minimal side effects and no reported drug interactions. A study of rifaximin versus lactitol administered for five to 10 days showed approximately 80% symptomatic improvement in both groups.5 Another trial demonstrated significantly greater improvement in blood ammonia concentrations, electroencephalographic (EEG) abnormalities, and mental status with rifaximin compared with lactulose.6 Studies comparing rifaximin and lactulose, either alone or in combination, have demonstrated that rifaximin is at least similar to lactulose, and in some cases superior in reversing encephalopathy, with better tolerability reported in the antibiotic group.7
Metronidazole is not approved by the FDA for the treatment of HE but has been evaluated. The recommended oral dose of metronidazole for chronic use is 250 mg twice per day. Prolonged administration of metronidazole can be associated with gastrointestinal disturbance and neurotoxicity. In a report of 11 HE patients with mild to moderate symptoms and seven chronically affected HE cirrhotic patients treated with metronidazole for one week, Morgan and colleagues showed metronidazole to be as effective as neomycin.8
Diet: Historically, patients with HE were placed on protein-restricted diets to reduce the production of intestinal ammonia. Recent evidence suggests that excessive restriction can raise serum ammonia levels as a result of reduced muscular ammonia metabolism. Furthermore, restricting protein intake worsens nutritional status and does not improve the outcome.9
In patients with established cirrhosis, the minimal daily dietary protein intake required to maintain nitrogen balance is 0.8 g/kg to 1.0 g/kg. At this time, a normoprotein diet for HE patients is considered the standard of care.
Other agents: L-ornithine L-aspartate (LOLA), a stable salt of ornithine and aspartic acid, provides crucial substrates for glutamine and urea synthesis—key pathways in deammonation. In patients with cirrhosis and HE, oral LOLA reduces serum ammonia and improves clinical manifestations of HE, including EEG abnormalities.10 LOLA, however, is not available in the U.S.
Sodium benzoate might be beneficial in the treatment of acute HE; it increases urinary excretion of ammonia. A prospective, randomized, double-blind study of 74 patients with acute HE found that treatment with sodium benzoate 5 g twice daily, compared with lactulose, resulted in equivalent improvements in encephalopathy. There was no placebo group.11 Routine use has been limited due to concerns regarding sodium load and increased frequency of adverse gastrointestinal symptoms, particularly nausea.
Flumazenil, a short-acting benzodiazepine receptor antagonist, has been utilized on the basis of observed increases in benzodiazepine receptor activation among cirrhotic HE patients. In a systematic review of 12 controlled trials (765 patients), Als-Nielsen and colleagues found flumazenil to be associated with significant improvement.12 Flumazenil is not used routinely as an HE therapy because of significant side effects, namely seizures, nausea, vomiting, dizziness, and agitation.
Such therapies as L-carnitine, branched amino acids (BCAA), probiotics, bromocriptine, acarbose, and zinc are among the many experimental agents currently under evaluation. Few have been tested in clinical trials.
Back to the Case
Our patient has severe HE manifested by worsening somnolence. It is postulated that ongoing alcohol abuse led to medication nonadherence, precipitating his HE, but as HE has many causes, a complete workup for infection and metabolic derangement is performed. However, it is unrevealing.
The best initial action is the prescription of lactulose, the mainstay of HE therapy. Given concern for aspiration in patients with somnolence, a feeding tube is placed for administration. The lactulose dosage will be titrated to achieve two to three soft stools per day. If the patient remains symptomatic or develops significant side effects on lactulose, the addition of an antibiotic is recommended. Neomycin, a low-cost medicine approved by the FDA for HE treatment, is a good choice. The patient will be maintained on a normal protein diet.
Bottom Line
The first-line agents used to treat episodes of acute HE are the nonabsorbable disaccharides, lactulose or lactitol. TH
Dr. Shoeb is a resident in the Department of Medicine at the University of Washington in Seattle. Dr. Best is assistant professor of medicine in the Division of General Internal Medicine at the University of Washington.
References
- Als-Nielsen B, Gluud L, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;2:CD003044.
- Morgan MY, Hawley KE. Lactitol v. lactulose in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double-blind, randomized trial. Hepatology. 1987; 7(6):1278-1284.
- Blanc P, Daurès JP, Liautard J, et al. Lactulose-neomycin combination versus placebo in the treatment of acute hepatic encephalopathy. Results of a randomized controlled trial. Gastroenterol Clin Biol. 1994;18(12):1063-1068.
- Mas A, Rodés J, Sunyer L, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38(1):51-58.
- Paik YH, Lee KS, Han KH, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46(3):399-407.
- Massa P, Vallerino E, Dodero M. Treatment of hepatic encephalopathy with rifaximin: double blind, double dummy study versus lactulose. Eur J Clin Res. 1993;4:7-18.
- Williams R, James OF, Warnes TW, Morgan MY. Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol. 2000;12(2):203-208.
- Morgan MH, Read AE, Speller DC. Treatment of hepatic encephalopathy with metronidazole. Gut. 1982;23(1):1-7.
- Córdoba J, López-Hellín J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38-43.
- Poo JL, Gongora J, Sánchez-Avila F, et al. Efficacy of oral L-ornithine-L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Results of a randomized, lactulose-controlled study. Ann Hepatol. 2006;5(4):281-288.
- Sushma S, Dasarathy S, Tandon RK, Jain S, Gupta S, Bhist MS. Sodium benzoate in the treatment of acute hepatic encephalopathy: a double-blind randomized trial. Hepatology. 1992;16(16):138-144.
- Als-Nielsen B, Kjaergard LL, Gluud C. Benzodiazepine receptor antagonists for acute and chronic hepatic encephalopathy. Cochrane Database Syst Rev. 2001;4:CD002798.
Case
A 56-year-old man with a history of cirrhosis, complicated by esophageal varices and ongoing alcohol abuse, is admitted after his wife found him lethargic and disoriented in bed. His wife said he’d been increasingly irritable and agitated, with slurred speech, the past two days. On exam, he is somnolent but arousable; spider telangiectasias and asterixis are noted. Laboratory studies are consistent with chronic liver disease.
What is the best therapy for his acute hepatic encephalopathy?
Overview
Hepatic encephalopathy (HE) describes the spectrum of potentially reversible neuropsychiatric abnormalities seen in patients with liver dysfunction. The wide range of neuropsychiatric presentations led to the development of consensus HE classification terminology by the World Congress of Gastroenterology in 2002.
The primary tenet of all HE pathogenesis theories is firmly established: Nitrogenous substances derived from the gut adversely affect brain function. These compounds access the systemic circulation via decreased hepatic function or portal-systemic shunts. In the brain, they alter neurotransmission, which affects consciousness and behavior.
HE patients usually have advanced cirrhosis and, hence, many of the physical findings associated with severe hepatic dysfunction: muscle-wasting, jaundice, ascites, palmar erythema, edema, spider telangiectasias, and fetor hepaticus. Encephalopathy progresses from reversal of the sleep-wake cycle and mild mental status changes to irritability, confusion, and slurred speech.
Advanced neurologic features include asterixis or tongue fasciculations, bradykinesia, hyperreflexia, and ultimately coma. History and laboratory data can reveal a precipitating cause (see Table 2, p. 19). Measurement of ammonia concentration remains controversial. The value may be useful for monitoring the efficacy of ammonia-lowering therapy, but elevated levels are not required to make the diagnosis.
Multiple treatments have been used to manage HE, yet few well-designed randomized trials have assessed efficacy due to challenges inherent in measuring the wide range of neuropsychiatric presentations. Nonetheless, a critical appraisal of available data delineates a rational approach to therapy.
Review of the Data
In addition to supportive care and the reversal of any precipitating factors, the treatment of acute HE is aimed at reducing or inhibiting intestinal ammonia production or increasing its removal (see Table 1, left).
Nonabsorbable disaccharides (NAD): Lactulose (beta-galactosidofructose) and lactitol (beta-galactosidosorbitol) are used as first-line agents for the treatment of HE and lead to symptomatic improvement in 67% to 87% of patients.1 They reduce the concentration of ammoniogenic substrates in the colonic lumen in two ways—first, by facilitating bacterial fermentation and secondary organic acid production (lowering colonic pH) and, second, by direct osmotic catharsis.
NAD are administered orally or via nasogastric tube at an initial dose of 45 ml, followed by repeated hourly doses until the patient has a bowel movement. For patients at risk of aspiration, NAD can be administered via enema (300 ml in 700 ml of water) every two hours as needed until mental function improves. Once the risk of aspiration is minimized, NAD can be administered orally and titrated to achieve two to three soft bowel movements daily (the usual oral dosage is 15 ml to 45 ml every eight to 12 hours).
Common side effects of NAD include an excessively sweet taste, flatulence, abdominal cramping, and electrolyte imbalance, particularly hypernatremia, which may further deteriorate mental status.
Als-Nielsen et al demonstrated in a systematic review that NAD were more effective than placebo in improving HE, but NAD had no significant benefit on mortality.1 However, the effect on HE no longer reached statistical significance when the analysis was confined to studies with the highest methodological quality. In a randomized, double-blind comparison, Morgan et al showed that lactitol was more tolerable than lactulose and produced fewer side effects.2 Lactitol is not currently available for use in the U.S.
Antibiotics: Certain oral antibiotics (e.g., neomycin, rifaximin, and metronidazole) reduce urease-producing intestinal bacteria, which results in decreased ammonia production and absorption through the gastrointestinal tract. Antibiotics generally are used in patients who do not tolerate NAD or who remain symptomatic despite NAD. The combined use of NAD and antibiotics is a subject of significant clinical relevance, though data are limited.
Neomycin is approved by the FDA for treatment of acute HE. It can be administered orally at a dose of 1,000 mg every six hours for up to six days. A randomized, controlled trial of neomycin versus placebo in 39 patients with acute HE demonstrated no significant difference in time to symptom improvement.3 Another study of 80 patients receiving neomycin and lactulose demonstrated no benefit against placebo, though some data suggest that the combination of lactulose and neomycin therapy might be more effective than either agent alone against placebo.4
Rifaximin was granted an orphan drug designation by the FDA for use in HE cases and has been compared with NAD. The recommended dose is 1,200 mg three times per day. It has minimal side effects and no reported drug interactions. A study of rifaximin versus lactitol administered for five to 10 days showed approximately 80% symptomatic improvement in both groups.5 Another trial demonstrated significantly greater improvement in blood ammonia concentrations, electroencephalographic (EEG) abnormalities, and mental status with rifaximin compared with lactulose.6 Studies comparing rifaximin and lactulose, either alone or in combination, have demonstrated that rifaximin is at least similar to lactulose, and in some cases superior in reversing encephalopathy, with better tolerability reported in the antibiotic group.7
Metronidazole is not approved by the FDA for the treatment of HE but has been evaluated. The recommended oral dose of metronidazole for chronic use is 250 mg twice per day. Prolonged administration of metronidazole can be associated with gastrointestinal disturbance and neurotoxicity. In a report of 11 HE patients with mild to moderate symptoms and seven chronically affected HE cirrhotic patients treated with metronidazole for one week, Morgan and colleagues showed metronidazole to be as effective as neomycin.8
Diet: Historically, patients with HE were placed on protein-restricted diets to reduce the production of intestinal ammonia. Recent evidence suggests that excessive restriction can raise serum ammonia levels as a result of reduced muscular ammonia metabolism. Furthermore, restricting protein intake worsens nutritional status and does not improve the outcome.9
In patients with established cirrhosis, the minimal daily dietary protein intake required to maintain nitrogen balance is 0.8 g/kg to 1.0 g/kg. At this time, a normoprotein diet for HE patients is considered the standard of care.
Other agents: L-ornithine L-aspartate (LOLA), a stable salt of ornithine and aspartic acid, provides crucial substrates for glutamine and urea synthesis—key pathways in deammonation. In patients with cirrhosis and HE, oral LOLA reduces serum ammonia and improves clinical manifestations of HE, including EEG abnormalities.10 LOLA, however, is not available in the U.S.
Sodium benzoate might be beneficial in the treatment of acute HE; it increases urinary excretion of ammonia. A prospective, randomized, double-blind study of 74 patients with acute HE found that treatment with sodium benzoate 5 g twice daily, compared with lactulose, resulted in equivalent improvements in encephalopathy. There was no placebo group.11 Routine use has been limited due to concerns regarding sodium load and increased frequency of adverse gastrointestinal symptoms, particularly nausea.
Flumazenil, a short-acting benzodiazepine receptor antagonist, has been utilized on the basis of observed increases in benzodiazepine receptor activation among cirrhotic HE patients. In a systematic review of 12 controlled trials (765 patients), Als-Nielsen and colleagues found flumazenil to be associated with significant improvement.12 Flumazenil is not used routinely as an HE therapy because of significant side effects, namely seizures, nausea, vomiting, dizziness, and agitation.
Such therapies as L-carnitine, branched amino acids (BCAA), probiotics, bromocriptine, acarbose, and zinc are among the many experimental agents currently under evaluation. Few have been tested in clinical trials.
Back to the Case
Our patient has severe HE manifested by worsening somnolence. It is postulated that ongoing alcohol abuse led to medication nonadherence, precipitating his HE, but as HE has many causes, a complete workup for infection and metabolic derangement is performed. However, it is unrevealing.
The best initial action is the prescription of lactulose, the mainstay of HE therapy. Given concern for aspiration in patients with somnolence, a feeding tube is placed for administration. The lactulose dosage will be titrated to achieve two to three soft stools per day. If the patient remains symptomatic or develops significant side effects on lactulose, the addition of an antibiotic is recommended. Neomycin, a low-cost medicine approved by the FDA for HE treatment, is a good choice. The patient will be maintained on a normal protein diet.
Bottom Line
The first-line agents used to treat episodes of acute HE are the nonabsorbable disaccharides, lactulose or lactitol. TH
Dr. Shoeb is a resident in the Department of Medicine at the University of Washington in Seattle. Dr. Best is assistant professor of medicine in the Division of General Internal Medicine at the University of Washington.
References
- Als-Nielsen B, Gluud L, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;2:CD003044.
- Morgan MY, Hawley KE. Lactitol v. lactulose in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double-blind, randomized trial. Hepatology. 1987; 7(6):1278-1284.
- Blanc P, Daurès JP, Liautard J, et al. Lactulose-neomycin combination versus placebo in the treatment of acute hepatic encephalopathy. Results of a randomized controlled trial. Gastroenterol Clin Biol. 1994;18(12):1063-1068.
- Mas A, Rodés J, Sunyer L, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38(1):51-58.
- Paik YH, Lee KS, Han KH, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46(3):399-407.
- Massa P, Vallerino E, Dodero M. Treatment of hepatic encephalopathy with rifaximin: double blind, double dummy study versus lactulose. Eur J Clin Res. 1993;4:7-18.
- Williams R, James OF, Warnes TW, Morgan MY. Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol. 2000;12(2):203-208.
- Morgan MH, Read AE, Speller DC. Treatment of hepatic encephalopathy with metronidazole. Gut. 1982;23(1):1-7.
- Córdoba J, López-Hellín J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38-43.
- Poo JL, Gongora J, Sánchez-Avila F, et al. Efficacy of oral L-ornithine-L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Results of a randomized, lactulose-controlled study. Ann Hepatol. 2006;5(4):281-288.
- Sushma S, Dasarathy S, Tandon RK, Jain S, Gupta S, Bhist MS. Sodium benzoate in the treatment of acute hepatic encephalopathy: a double-blind randomized trial. Hepatology. 1992;16(16):138-144.
- Als-Nielsen B, Kjaergard LL, Gluud C. Benzodiazepine receptor antagonists for acute and chronic hepatic encephalopathy. Cochrane Database Syst Rev. 2001;4:CD002798.