User login
Imatinib cuts mast cells in severe asthma
Imatinib decreased airway mast-cell counts and airway hyperresponsiveness in adults with asthma, who were not responding well to maximal therapy, according to a report published online May 17 in the New England Journal of Medicine.
Imatinib is an inhibitor of the stem-cell factor receptor KIT, which is essential for mast-cell development and survival in bodily tissues. This study’s findings suggest that KIT-dependent processes and mast cells contribute to the pathobiology of severe asthma.
The researchers undertook this study because imatinib is known to reduce bone-marrow mast cells and tryptase levels in chronic myeloid leukemia and to reduce serum tryptase in patients with pulmonary hypertension. Tryptase is a marker of mast-cell burden and activation when detected in extracellular fluids, and it is elevated in the bronchoalveolar lavage fluid from patients with uncontrolled asthma.
To examine whether imatinib would decrease mast-cell counts and activation in the airways of adults with severe, refractory asthma, the investigators performed the randomized double-blind proof-of-principle trial at seven academic centers across the United States over the course of 5 years. A total of 62 patients were assigned to 24 weeks of either oral imatinib (32 participants) or a matching placebo (30 participants). Fifty patients, 24 in the imatinib group and 26 in the placebo group, completed the trial.
The primary outcome measure was the change in airway hyperresponsiveness at 6 months, as measured by the increase in the concentration of methacholine that causes significant bronchoconstriction (PC20). Imatinib decreased airway hyperresponsiveness to a greater degree than did placebo. Imatinib increased PC20 by a mean of 1.20 doubling doses at 3 months and by a mean of 1.73 doubling doses at 6 months, compared with 0.03 and 1.07, respectively, for placebo.
The small improvement in the placebo group is consistent with a phenomenon reported in other studies, in which patients show a delayed improvement in airway hyperresponsiveness for several months after they started inhaled glucocorticoids, Dr. Cahill and her associates noted (N Engl J Med. 2017 May 18. doi: 10.1056/NEJMoa1613125).
Imatinib also reduced mast-cell activity as measured by serum and airway levels of tryptase. Serum tryptase decreased by 43% in the imatinib group, compared with a 12% decline in the placebo group. And tryptase levels in bronchoalveolar lavage fluid tended to decrease in the imatinib group but to increase in the placebo group.
Imatinib also increased mean forced expiratory volume in 1 second (FEV1).
“Although the increase in FEV1 may not seem substantial, it suggests that mast-cell–dependent processes contribute to airway obstruction in these patients despite high-dose, anti-inflammatory glucocorticoid therapy. The near–50-mL difference in the change in baseline FEV1 between the imatinib and placebo groups is small, but it is likely to be important in light of the population we studied,” Dr. Cahill and her associates wrote.
In addition, exploratory analyses showed that the reduction in airway hyperresponsiveness with imatinib “negatively correlated with baseline blood eosinophil counts, and baseline numbers of neutrophils in bronchoalveolar lavage fluid were strongly correlated with increases in FEV1. Together, these findings support a role for mast cells in noneosinophilic asthma. Since almost half of the patients with severe asthma have neutrophilic airway inflammation, we speculate that KIT inhibition might represent an important approach to treatment for this group,” they said.
This study was supported by the National Heart, Lung, and Blood Institute, the National Institute of Allergy and Infectious Diseases, the Vinik family, and the Kaye family; Novartis provided imatinib free of charge. The authors’ financial disclosures are available at www.nejm.org.
As Cahill et al. noted, these data suggest that the role of mast cells should be studied further, but cautiously.
It is not yet time to target mast cells in patients with asthma. Evolution has given us these cells for a reason. They appear to assist in host defense against parasites and play a role in other innate and adaptive immune responses. And they likely have other beneficial effects that haven’t been discovered yet.
However, in the unfortunate patients in whom mast cells can be strongly implicated as contributing to disease, either reducing their numbers or suppressing their function may confer more benefit than harm. This is particularly true in the case of asthma, where mast cells can be targeted locally, in the airways.
Stephen J. Galli, MD, is the Mary Hewitt Loveless, MD, Professor in the school of medicine, and is at the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University. His financial disclosures are available at www.nejm.org. Dr. Galli made these remarks in an editorial accompanying Dr. Cahill’s report (N Engl J Med. 2017 May 18. doi: 10.1056/NEJMe1702653).
As Cahill et al. noted, these data suggest that the role of mast cells should be studied further, but cautiously.
It is not yet time to target mast cells in patients with asthma. Evolution has given us these cells for a reason. They appear to assist in host defense against parasites and play a role in other innate and adaptive immune responses. And they likely have other beneficial effects that haven’t been discovered yet.
However, in the unfortunate patients in whom mast cells can be strongly implicated as contributing to disease, either reducing their numbers or suppressing their function may confer more benefit than harm. This is particularly true in the case of asthma, where mast cells can be targeted locally, in the airways.
Stephen J. Galli, MD, is the Mary Hewitt Loveless, MD, Professor in the school of medicine, and is at the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University. His financial disclosures are available at www.nejm.org. Dr. Galli made these remarks in an editorial accompanying Dr. Cahill’s report (N Engl J Med. 2017 May 18. doi: 10.1056/NEJMe1702653).
As Cahill et al. noted, these data suggest that the role of mast cells should be studied further, but cautiously.
It is not yet time to target mast cells in patients with asthma. Evolution has given us these cells for a reason. They appear to assist in host defense against parasites and play a role in other innate and adaptive immune responses. And they likely have other beneficial effects that haven’t been discovered yet.
However, in the unfortunate patients in whom mast cells can be strongly implicated as contributing to disease, either reducing their numbers or suppressing their function may confer more benefit than harm. This is particularly true in the case of asthma, where mast cells can be targeted locally, in the airways.
Stephen J. Galli, MD, is the Mary Hewitt Loveless, MD, Professor in the school of medicine, and is at the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University. His financial disclosures are available at www.nejm.org. Dr. Galli made these remarks in an editorial accompanying Dr. Cahill’s report (N Engl J Med. 2017 May 18. doi: 10.1056/NEJMe1702653).
Imatinib decreased airway mast-cell counts and airway hyperresponsiveness in adults with asthma, who were not responding well to maximal therapy, according to a report published online May 17 in the New England Journal of Medicine.
Imatinib is an inhibitor of the stem-cell factor receptor KIT, which is essential for mast-cell development and survival in bodily tissues. This study’s findings suggest that KIT-dependent processes and mast cells contribute to the pathobiology of severe asthma.
The researchers undertook this study because imatinib is known to reduce bone-marrow mast cells and tryptase levels in chronic myeloid leukemia and to reduce serum tryptase in patients with pulmonary hypertension. Tryptase is a marker of mast-cell burden and activation when detected in extracellular fluids, and it is elevated in the bronchoalveolar lavage fluid from patients with uncontrolled asthma.
To examine whether imatinib would decrease mast-cell counts and activation in the airways of adults with severe, refractory asthma, the investigators performed the randomized double-blind proof-of-principle trial at seven academic centers across the United States over the course of 5 years. A total of 62 patients were assigned to 24 weeks of either oral imatinib (32 participants) or a matching placebo (30 participants). Fifty patients, 24 in the imatinib group and 26 in the placebo group, completed the trial.
The primary outcome measure was the change in airway hyperresponsiveness at 6 months, as measured by the increase in the concentration of methacholine that causes significant bronchoconstriction (PC20). Imatinib decreased airway hyperresponsiveness to a greater degree than did placebo. Imatinib increased PC20 by a mean of 1.20 doubling doses at 3 months and by a mean of 1.73 doubling doses at 6 months, compared with 0.03 and 1.07, respectively, for placebo.
The small improvement in the placebo group is consistent with a phenomenon reported in other studies, in which patients show a delayed improvement in airway hyperresponsiveness for several months after they started inhaled glucocorticoids, Dr. Cahill and her associates noted (N Engl J Med. 2017 May 18. doi: 10.1056/NEJMoa1613125).
Imatinib also reduced mast-cell activity as measured by serum and airway levels of tryptase. Serum tryptase decreased by 43% in the imatinib group, compared with a 12% decline in the placebo group. And tryptase levels in bronchoalveolar lavage fluid tended to decrease in the imatinib group but to increase in the placebo group.
Imatinib also increased mean forced expiratory volume in 1 second (FEV1).
“Although the increase in FEV1 may not seem substantial, it suggests that mast-cell–dependent processes contribute to airway obstruction in these patients despite high-dose, anti-inflammatory glucocorticoid therapy. The near–50-mL difference in the change in baseline FEV1 between the imatinib and placebo groups is small, but it is likely to be important in light of the population we studied,” Dr. Cahill and her associates wrote.
In addition, exploratory analyses showed that the reduction in airway hyperresponsiveness with imatinib “negatively correlated with baseline blood eosinophil counts, and baseline numbers of neutrophils in bronchoalveolar lavage fluid were strongly correlated with increases in FEV1. Together, these findings support a role for mast cells in noneosinophilic asthma. Since almost half of the patients with severe asthma have neutrophilic airway inflammation, we speculate that KIT inhibition might represent an important approach to treatment for this group,” they said.
This study was supported by the National Heart, Lung, and Blood Institute, the National Institute of Allergy and Infectious Diseases, the Vinik family, and the Kaye family; Novartis provided imatinib free of charge. The authors’ financial disclosures are available at www.nejm.org.
Imatinib decreased airway mast-cell counts and airway hyperresponsiveness in adults with asthma, who were not responding well to maximal therapy, according to a report published online May 17 in the New England Journal of Medicine.
Imatinib is an inhibitor of the stem-cell factor receptor KIT, which is essential for mast-cell development and survival in bodily tissues. This study’s findings suggest that KIT-dependent processes and mast cells contribute to the pathobiology of severe asthma.
The researchers undertook this study because imatinib is known to reduce bone-marrow mast cells and tryptase levels in chronic myeloid leukemia and to reduce serum tryptase in patients with pulmonary hypertension. Tryptase is a marker of mast-cell burden and activation when detected in extracellular fluids, and it is elevated in the bronchoalveolar lavage fluid from patients with uncontrolled asthma.
To examine whether imatinib would decrease mast-cell counts and activation in the airways of adults with severe, refractory asthma, the investigators performed the randomized double-blind proof-of-principle trial at seven academic centers across the United States over the course of 5 years. A total of 62 patients were assigned to 24 weeks of either oral imatinib (32 participants) or a matching placebo (30 participants). Fifty patients, 24 in the imatinib group and 26 in the placebo group, completed the trial.
The primary outcome measure was the change in airway hyperresponsiveness at 6 months, as measured by the increase in the concentration of methacholine that causes significant bronchoconstriction (PC20). Imatinib decreased airway hyperresponsiveness to a greater degree than did placebo. Imatinib increased PC20 by a mean of 1.20 doubling doses at 3 months and by a mean of 1.73 doubling doses at 6 months, compared with 0.03 and 1.07, respectively, for placebo.
The small improvement in the placebo group is consistent with a phenomenon reported in other studies, in which patients show a delayed improvement in airway hyperresponsiveness for several months after they started inhaled glucocorticoids, Dr. Cahill and her associates noted (N Engl J Med. 2017 May 18. doi: 10.1056/NEJMoa1613125).
Imatinib also reduced mast-cell activity as measured by serum and airway levels of tryptase. Serum tryptase decreased by 43% in the imatinib group, compared with a 12% decline in the placebo group. And tryptase levels in bronchoalveolar lavage fluid tended to decrease in the imatinib group but to increase in the placebo group.
Imatinib also increased mean forced expiratory volume in 1 second (FEV1).
“Although the increase in FEV1 may not seem substantial, it suggests that mast-cell–dependent processes contribute to airway obstruction in these patients despite high-dose, anti-inflammatory glucocorticoid therapy. The near–50-mL difference in the change in baseline FEV1 between the imatinib and placebo groups is small, but it is likely to be important in light of the population we studied,” Dr. Cahill and her associates wrote.
In addition, exploratory analyses showed that the reduction in airway hyperresponsiveness with imatinib “negatively correlated with baseline blood eosinophil counts, and baseline numbers of neutrophils in bronchoalveolar lavage fluid were strongly correlated with increases in FEV1. Together, these findings support a role for mast cells in noneosinophilic asthma. Since almost half of the patients with severe asthma have neutrophilic airway inflammation, we speculate that KIT inhibition might represent an important approach to treatment for this group,” they said.
This study was supported by the National Heart, Lung, and Blood Institute, the National Institute of Allergy and Infectious Diseases, the Vinik family, and the Kaye family; Novartis provided imatinib free of charge. The authors’ financial disclosures are available at www.nejm.org.
Key clinical point: Imatinib, a KIT inhibitor, reduced mast cell counts and airway hyperresponsiveness in severe asthma.
Major finding: Imatinib increased PC20 by a mean of 1.20 doubling doses at 3 months and by a mean of 1.73 doubling doses at 6 months, compared with 0.03 and 1.07, respectively, for placebo.
Data source: A randomized, double-blind, placebo-controlled proof-of-principle trial involving 62 adults treated for 24 weeks.
Disclosures: This study was supported by the National Heart, Lung, and Blood Institute, the National Institute of Allergy and Infectious Diseases, the Vinik family, and the Kaye family; Novartis provided imatinib free of charge. The researchers’ financial disclosures are available at www.nejm.org.
Oral iron of no benefit in heart failure with iron deficiency
High-dose oral iron therapy doesn’t improve exercise capacity in the estimated 50% of patients with symptomatic heart failure who also have iron deficiency, according to a report published online May 16 in JAMA.
Iron deficiency in patients with HF, regardless of their hemoglobin status, is associated with reduced functional capacity, poorer quality of life, and increased mortality. Iron plays a crucial role in the delivery and utilization of oxygen, and “cells with high-energy demands, including skeletal and cardiac myocytes, are particularly sensitive to depleted iron stores,” said Gregory D. Lewis, MD, of the pulmonary critical care unit of Massachusetts General Hospital, Boston, and his associates.
The IRONOUT study was conducted at 23 U.S. medical centers, where outcomes after 16 weeks of oral iron therapy (150 mg twice daily) were compared against matching placebo in 225 patients. The median patient age was 63 years, and the median duration of HF was 5.7 years. Ischemic heart disease was the primary cause of HF in 78% of the study participants.
These patients had low LVEF and poor exercise capacity, despite having high rates of guideline-directed treatment with medications.
The primary endpoint was a change in peak oxygen uptake (peak VO2) at the conclusion of treatment, a measure that “reflects the multiple mechanisms by which iron repletion is expected to improve systemic oxygen delivery and utilization.” Change in peak VO2 was not significantly different between the 111 participants who took oral iron supplements (+23 mL/min) and the 114 who took placebo (–2 mL/min), the investigators wrote (JAMA Pediatr. 2017 May 16. doi: 10.1001/jama.2017.5427).
In subgroup analyses, oral iron also failed to improve peak VO2 in any subgroup of patients: neither men nor women; neither those with decreased hemoglobin nor those with normal hemoglobin levels; nor patients with or without venous congestion at baseline. Oral iron also failed to improve secondary endpoints including 6-minute walk distance, quality of life scores, NT-proBNP levels, and ventilatory efficiency.
In contrast to previous studies of IV iron repletion, oral iron supplementation “produced minimal improvement in iron stores, implicating the route of administration rather than the strategy of iron repletion in the lack of clinical benefit,” Dr. Lewis and his associates said.
This study was funded by the National Heart, Lung, and Blood Institute, which also conceived, designed, and conducted the trial. Dr. Lewis reported ties to Abbott, Novartis, Shape Systems, Stealth Bio Therapeutics, Ironwood, Cheetah Medical, Luitpold, and SoniVie. His associates reported ties to numerous industry sources.
High-dose oral iron therapy doesn’t improve exercise capacity in the estimated 50% of patients with symptomatic heart failure who also have iron deficiency, according to a report published online May 16 in JAMA.
Iron deficiency in patients with HF, regardless of their hemoglobin status, is associated with reduced functional capacity, poorer quality of life, and increased mortality. Iron plays a crucial role in the delivery and utilization of oxygen, and “cells with high-energy demands, including skeletal and cardiac myocytes, are particularly sensitive to depleted iron stores,” said Gregory D. Lewis, MD, of the pulmonary critical care unit of Massachusetts General Hospital, Boston, and his associates.
The IRONOUT study was conducted at 23 U.S. medical centers, where outcomes after 16 weeks of oral iron therapy (150 mg twice daily) were compared against matching placebo in 225 patients. The median patient age was 63 years, and the median duration of HF was 5.7 years. Ischemic heart disease was the primary cause of HF in 78% of the study participants.
These patients had low LVEF and poor exercise capacity, despite having high rates of guideline-directed treatment with medications.
The primary endpoint was a change in peak oxygen uptake (peak VO2) at the conclusion of treatment, a measure that “reflects the multiple mechanisms by which iron repletion is expected to improve systemic oxygen delivery and utilization.” Change in peak VO2 was not significantly different between the 111 participants who took oral iron supplements (+23 mL/min) and the 114 who took placebo (–2 mL/min), the investigators wrote (JAMA Pediatr. 2017 May 16. doi: 10.1001/jama.2017.5427).
In subgroup analyses, oral iron also failed to improve peak VO2 in any subgroup of patients: neither men nor women; neither those with decreased hemoglobin nor those with normal hemoglobin levels; nor patients with or without venous congestion at baseline. Oral iron also failed to improve secondary endpoints including 6-minute walk distance, quality of life scores, NT-proBNP levels, and ventilatory efficiency.
In contrast to previous studies of IV iron repletion, oral iron supplementation “produced minimal improvement in iron stores, implicating the route of administration rather than the strategy of iron repletion in the lack of clinical benefit,” Dr. Lewis and his associates said.
This study was funded by the National Heart, Lung, and Blood Institute, which also conceived, designed, and conducted the trial. Dr. Lewis reported ties to Abbott, Novartis, Shape Systems, Stealth Bio Therapeutics, Ironwood, Cheetah Medical, Luitpold, and SoniVie. His associates reported ties to numerous industry sources.
High-dose oral iron therapy doesn’t improve exercise capacity in the estimated 50% of patients with symptomatic heart failure who also have iron deficiency, according to a report published online May 16 in JAMA.
Iron deficiency in patients with HF, regardless of their hemoglobin status, is associated with reduced functional capacity, poorer quality of life, and increased mortality. Iron plays a crucial role in the delivery and utilization of oxygen, and “cells with high-energy demands, including skeletal and cardiac myocytes, are particularly sensitive to depleted iron stores,” said Gregory D. Lewis, MD, of the pulmonary critical care unit of Massachusetts General Hospital, Boston, and his associates.
The IRONOUT study was conducted at 23 U.S. medical centers, where outcomes after 16 weeks of oral iron therapy (150 mg twice daily) were compared against matching placebo in 225 patients. The median patient age was 63 years, and the median duration of HF was 5.7 years. Ischemic heart disease was the primary cause of HF in 78% of the study participants.
These patients had low LVEF and poor exercise capacity, despite having high rates of guideline-directed treatment with medications.
The primary endpoint was a change in peak oxygen uptake (peak VO2) at the conclusion of treatment, a measure that “reflects the multiple mechanisms by which iron repletion is expected to improve systemic oxygen delivery and utilization.” Change in peak VO2 was not significantly different between the 111 participants who took oral iron supplements (+23 mL/min) and the 114 who took placebo (–2 mL/min), the investigators wrote (JAMA Pediatr. 2017 May 16. doi: 10.1001/jama.2017.5427).
In subgroup analyses, oral iron also failed to improve peak VO2 in any subgroup of patients: neither men nor women; neither those with decreased hemoglobin nor those with normal hemoglobin levels; nor patients with or without venous congestion at baseline. Oral iron also failed to improve secondary endpoints including 6-minute walk distance, quality of life scores, NT-proBNP levels, and ventilatory efficiency.
In contrast to previous studies of IV iron repletion, oral iron supplementation “produced minimal improvement in iron stores, implicating the route of administration rather than the strategy of iron repletion in the lack of clinical benefit,” Dr. Lewis and his associates said.
This study was funded by the National Heart, Lung, and Blood Institute, which also conceived, designed, and conducted the trial. Dr. Lewis reported ties to Abbott, Novartis, Shape Systems, Stealth Bio Therapeutics, Ironwood, Cheetah Medical, Luitpold, and SoniVie. His associates reported ties to numerous industry sources.
Key clinical point: High-dose oral iron therapy doesn’t improve exercise capacity in the estimated 50% of patients with symptomatic heart failure and iron deficiency.
Major finding: Change in peak VO2 was not significantly different between the 111 participants who took oral iron supplements (+23 mL/min) and the 114 who took placebo (–2 mL/min).
Data source: A multicenter, randomized, double-blind, placebo-controlled phase II trial involving 225 patients treated for 16 weeks.
Disclosures: This study was funded by the National Heart, Lung, and Blood Institute (NCT02188784), which also conceived, designed, and conducted the trial. Dr. Lewis reported ties to Abbott, Novartis, Shape Systems, Stealth Bio Therapeutics, Ironwood, Cheetah Medical, Luitpold, and SoniVie. His associates reported ties to numerous industry sources.
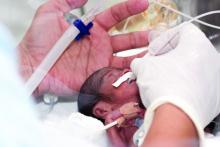
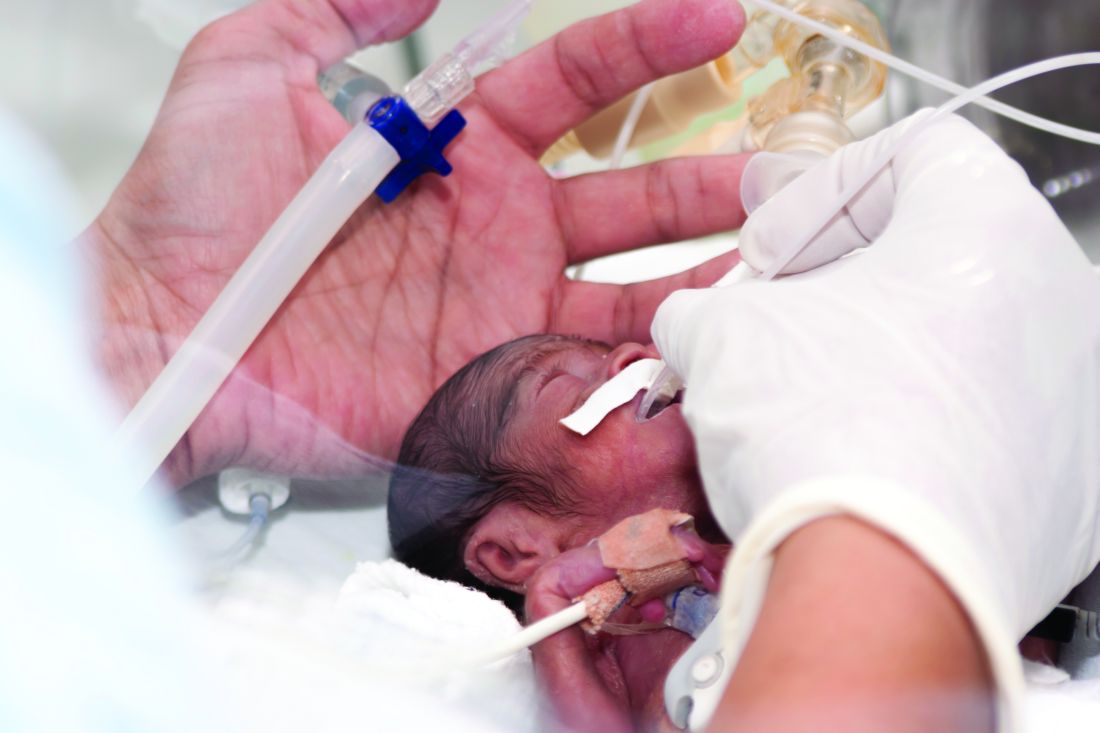
Corticosteroids effective just hours before preterm delivery
Antenatal corticosteroids may significantly decrease neonatal mortality and morbidity even when they are given just hours before preterm delivery, according to a report published online May 15 in JAMA Pediatrics.
In women at risk of preterm delivery, antenatal corticosteroids given between 1 and 7 days before birth reduce infant mortality by an estimated 31%, respiratory distress syndrome by 34%, intraventricular hemorrhage by 46%, and necrotizing enterocolitis by 54%. But until now their effect when given less than 24 hours before preterm birth has been described as “suboptimal,” “partial,” or “incomplete,” said Mikael Norman, MD, PhD, of Karolinska Institutet in Stockholm, Sweden, and his associates.
“Our findings challenge current thinking about the optimal timing” of antenatal corticosteroids and encourage “a more proactive management of women at risk for imminent preterm birth, which may help reduce infant mortality and severe neonatal brain injury,” the investigators said.
To further examine this issue, they assessed the effects of antenatal corticosteroids when given at different intervals before preterm birth, using data from a prospective cohort study of perinatal intensive care. That study involved 10,329 very preterm births throughout 11 countries in Europe during a 1-year period.
For their analysis, Dr. Norman and his associates focused on 4,594 singleton births at 24-31 weeks’ gestation. They classified the timing of antenatal corticosteroids into four categories: no injections (662 infants, or 14.4% of the study population), first injection at less than 24 hours before birth (1,111 infants, or 24.2%), first injection at the recommended 1-7 days before birth (1,871 infants, or 40.7%), and first injection more than 7 days before birth (950 infants, or 20.7%).
Receiving antenatal corticosteroids at any interval before preterm birth was associated with lower infant mortality, a lower rate of severe neonatal morbidity, and a lower rate of severe neonatal brain injury, compared with not receiving any antenatal corticosteroids. The largest reduction in risk (more than 50%) occurred at the recommended interval of 1-7 days before birth. However, receiving the treatment less than 24 hours before birth also significantly reduced these risks (JAMA Pediatr. 2017 May 15. doi: 10.1001/jamapediatrics.2017.0602).
Using their findings on treatment intervals and effectiveness, the investigators created a simulation model for the 661 infants in this cohort who did not receive any antenatal corticosteroids. Their model predicted that if these infants had received treatment at least 3 hours before delivery, overall mortality would have decreased by 26%. If they had received treatment 3-5 hours before delivery, mortality would have decreased by 37%, and if they had received treatment at 6-12 hours before delivery it would have decreased by 51%.
At the other end of the timing spectrum, infant mortality increased 40% when corticosteroids were given more than 7 days before delivery, compared with when they were given within the recommended 1-7 days. This represents a substantial number of infants – approximately 20% of the study cohort.
The study was supported by the European Union, the French Institute of Public Health, the Polish Ministry of Science and Higher Education, the Karolinska Institutet, and other nonindustry sources. Dr. Norman and his associates reported having no relevant financial disclosures.
Antenatal corticosteroids may significantly decrease neonatal mortality and morbidity even when they are given just hours before preterm delivery, according to a report published online May 15 in JAMA Pediatrics.
In women at risk of preterm delivery, antenatal corticosteroids given between 1 and 7 days before birth reduce infant mortality by an estimated 31%, respiratory distress syndrome by 34%, intraventricular hemorrhage by 46%, and necrotizing enterocolitis by 54%. But until now their effect when given less than 24 hours before preterm birth has been described as “suboptimal,” “partial,” or “incomplete,” said Mikael Norman, MD, PhD, of Karolinska Institutet in Stockholm, Sweden, and his associates.
“Our findings challenge current thinking about the optimal timing” of antenatal corticosteroids and encourage “a more proactive management of women at risk for imminent preterm birth, which may help reduce infant mortality and severe neonatal brain injury,” the investigators said.
To further examine this issue, they assessed the effects of antenatal corticosteroids when given at different intervals before preterm birth, using data from a prospective cohort study of perinatal intensive care. That study involved 10,329 very preterm births throughout 11 countries in Europe during a 1-year period.
For their analysis, Dr. Norman and his associates focused on 4,594 singleton births at 24-31 weeks’ gestation. They classified the timing of antenatal corticosteroids into four categories: no injections (662 infants, or 14.4% of the study population), first injection at less than 24 hours before birth (1,111 infants, or 24.2%), first injection at the recommended 1-7 days before birth (1,871 infants, or 40.7%), and first injection more than 7 days before birth (950 infants, or 20.7%).
Receiving antenatal corticosteroids at any interval before preterm birth was associated with lower infant mortality, a lower rate of severe neonatal morbidity, and a lower rate of severe neonatal brain injury, compared with not receiving any antenatal corticosteroids. The largest reduction in risk (more than 50%) occurred at the recommended interval of 1-7 days before birth. However, receiving the treatment less than 24 hours before birth also significantly reduced these risks (JAMA Pediatr. 2017 May 15. doi: 10.1001/jamapediatrics.2017.0602).
Using their findings on treatment intervals and effectiveness, the investigators created a simulation model for the 661 infants in this cohort who did not receive any antenatal corticosteroids. Their model predicted that if these infants had received treatment at least 3 hours before delivery, overall mortality would have decreased by 26%. If they had received treatment 3-5 hours before delivery, mortality would have decreased by 37%, and if they had received treatment at 6-12 hours before delivery it would have decreased by 51%.
At the other end of the timing spectrum, infant mortality increased 40% when corticosteroids were given more than 7 days before delivery, compared with when they were given within the recommended 1-7 days. This represents a substantial number of infants – approximately 20% of the study cohort.
The study was supported by the European Union, the French Institute of Public Health, the Polish Ministry of Science and Higher Education, the Karolinska Institutet, and other nonindustry sources. Dr. Norman and his associates reported having no relevant financial disclosures.
Antenatal corticosteroids may significantly decrease neonatal mortality and morbidity even when they are given just hours before preterm delivery, according to a report published online May 15 in JAMA Pediatrics.
In women at risk of preterm delivery, antenatal corticosteroids given between 1 and 7 days before birth reduce infant mortality by an estimated 31%, respiratory distress syndrome by 34%, intraventricular hemorrhage by 46%, and necrotizing enterocolitis by 54%. But until now their effect when given less than 24 hours before preterm birth has been described as “suboptimal,” “partial,” or “incomplete,” said Mikael Norman, MD, PhD, of Karolinska Institutet in Stockholm, Sweden, and his associates.
“Our findings challenge current thinking about the optimal timing” of antenatal corticosteroids and encourage “a more proactive management of women at risk for imminent preterm birth, which may help reduce infant mortality and severe neonatal brain injury,” the investigators said.
To further examine this issue, they assessed the effects of antenatal corticosteroids when given at different intervals before preterm birth, using data from a prospective cohort study of perinatal intensive care. That study involved 10,329 very preterm births throughout 11 countries in Europe during a 1-year period.
For their analysis, Dr. Norman and his associates focused on 4,594 singleton births at 24-31 weeks’ gestation. They classified the timing of antenatal corticosteroids into four categories: no injections (662 infants, or 14.4% of the study population), first injection at less than 24 hours before birth (1,111 infants, or 24.2%), first injection at the recommended 1-7 days before birth (1,871 infants, or 40.7%), and first injection more than 7 days before birth (950 infants, or 20.7%).
Receiving antenatal corticosteroids at any interval before preterm birth was associated with lower infant mortality, a lower rate of severe neonatal morbidity, and a lower rate of severe neonatal brain injury, compared with not receiving any antenatal corticosteroids. The largest reduction in risk (more than 50%) occurred at the recommended interval of 1-7 days before birth. However, receiving the treatment less than 24 hours before birth also significantly reduced these risks (JAMA Pediatr. 2017 May 15. doi: 10.1001/jamapediatrics.2017.0602).
Using their findings on treatment intervals and effectiveness, the investigators created a simulation model for the 661 infants in this cohort who did not receive any antenatal corticosteroids. Their model predicted that if these infants had received treatment at least 3 hours before delivery, overall mortality would have decreased by 26%. If they had received treatment 3-5 hours before delivery, mortality would have decreased by 37%, and if they had received treatment at 6-12 hours before delivery it would have decreased by 51%.
At the other end of the timing spectrum, infant mortality increased 40% when corticosteroids were given more than 7 days before delivery, compared with when they were given within the recommended 1-7 days. This represents a substantial number of infants – approximately 20% of the study cohort.
The study was supported by the European Union, the French Institute of Public Health, the Polish Ministry of Science and Higher Education, the Karolinska Institutet, and other nonindustry sources. Dr. Norman and his associates reported having no relevant financial disclosures.
Key clinical point:
Major finding: A simulation model predicted that if untreated infants had received antenatal corticosteroids 6-12 hours before delivery, overall mortality would have decreased by 51%.
Data source: A secondary analysis of data from a population-based cohort study of perinatal intensive care across Europe, involving 4,594 preterm singleton births.
Disclosures: The study was supported by the European Union, the French Institute of Public Health, the Polish Ministry of Science and Higher Education, the Karolinska Institutet, and other nonindustry sources. Dr. Norman and his associates reported having no relevant financial disclosures.
Deep infiltrating endometriosis lesions harbor cancer-driver mutations
Deep infiltrating endometriosis lesions were found to harbor several genetic mutations, including well-known cancer-driver mutations in the ARID1A, PIK3CA, KRAS, and PPP2R1A genes, investigators reported in the May 11 issue of the New England Journal of Medicine.
This finding was possible only through the use of a combination of next-generation sequencing tools, together with highly sensitive digital genomic assays. A finding of cancer-related mutations in noncancerous lesions – and, “in particular, in nonovarian deep infiltrating lesions that rarely (if ever) transform into cancer” – was very “surprising” and raises new questions about the pathobiology of endometriosis, said Michael S. Anglesio, PhD, of the University of British Columbia, Vancouver, and his associates.
All three subtypes of endometriosis exhibit “benign clinical behavior and normal-appearing histologic features,” and are considered to be nonmalignant inflammatory lesions, Dr. Anglesio and his associates said. However, they also “recapitulate some features of malignant neoplasms, including local invasion and resistance to apoptosis,” the investigators noted.
To further explore whether deep infiltrating endometriosis lesions harbor somatic mutations, they performed a series of genetic analyses on samples obtained from three independent cohorts of 27 affected patients in New York, Japan, and Vancouver. Whole-exome sequencing of lesions and adjacent normal tissue from 24 women revealed 80 different somatic mutations in 19 (79%) of the lesions.
The number of mutations per lesion varied widely, from 0 to 17, with a mean of 3.3 mutations per lesion. Five of these lesions harbored mutations in the cancer-driver genes ARID1A, PIK3CA, KRAS, or PPP2R1A.
In targeted-sequencing analyses of samples from three more patients, more activating mutations in the KRAS gene were found in two (66%). One woman had two different activating KRAS mutations, and the other had the same KRAS mutation in three separate lesions.
A further analysis of samples from an additional 12 patients showed KRAS mutations in 3 (25%) of them.
The data indicate that these mutations were very unlikely to have arisen by chance alone. In addition, “because cancer-driver mutations were present only in the epithelium but not the stroma of the same endometriosis lesion, we can assume that the observed mutations provide some key selective advantage to endometriotic epithelial cells,” Dr. Anglesio and his associates said (N Engl J Med. 2017 May 11. doi: 10.1056/NEJMoa1614814).
Their findings are consistent with those of recent studies of other organ systems, which reported cancer-driver mutations in benign lesions and in normal tissues from skin, neuronal, and ovarian samples, they added.
“Our findings challenge the current understanding of this invasive subtype of endometriosis and open the discussion on whether deep infiltrating endometriosis can be considered as a benign neoplasm,” the investigators wrote.
This study was sponsored by 27 nonindustry sources, including the Johns Hopkins University, the Virginia and D.K. Ludwig Fund for Cancer Research, the Ephraim and Wilma Shaw Roseman Foundation, the Endometriosis Foundation of America, and the National Institutes of Health. Dr. Anglesio reported having no relevant financial disclosures; some of his associates reported ties to several industry sources.
The variable patterns of somatic mutations discovered by Anglesio et al. are “intriguing” and raise many questions.
Do specific mutations in deep infiltrating endometriosis lesions contribute to the severity and progression of the disorder? Are such mutations to be found in other types of lesions? In endometrial precursor cells? Do specific mutations account for different presentations in different patients?
Exploring these questions will not be straightforward because of the difficulties in obtaining sufficient endometriotic tissue for genetic studies. Not only is it hard to get samples from peritoneal lesions, but the mutations occur in only one cell type – epithelium – which is even harder to isolate from mixed tissue samples.
Grant W. Montgomery, PhD, is at the Institute for Molecular Bioscience at the University of Queensland, Brisbane, Australia, and reported having no relevant financial disclosures. Linda C. Giudice, MD, PhD, is in the department of ob.gyn. and reproductive sciences at the University of California, San Francisco, and reported ties to AbbVie and Bayer. Dr. Montgomery and Dr. Giudice made these remarks in an editorial accompanying Dr. Anglesio’s report (N Engl J Med. 2017 May 11. doi: 10.1056/NEJMe1701700).
The variable patterns of somatic mutations discovered by Anglesio et al. are “intriguing” and raise many questions.
Do specific mutations in deep infiltrating endometriosis lesions contribute to the severity and progression of the disorder? Are such mutations to be found in other types of lesions? In endometrial precursor cells? Do specific mutations account for different presentations in different patients?
Exploring these questions will not be straightforward because of the difficulties in obtaining sufficient endometriotic tissue for genetic studies. Not only is it hard to get samples from peritoneal lesions, but the mutations occur in only one cell type – epithelium – which is even harder to isolate from mixed tissue samples.
Grant W. Montgomery, PhD, is at the Institute for Molecular Bioscience at the University of Queensland, Brisbane, Australia, and reported having no relevant financial disclosures. Linda C. Giudice, MD, PhD, is in the department of ob.gyn. and reproductive sciences at the University of California, San Francisco, and reported ties to AbbVie and Bayer. Dr. Montgomery and Dr. Giudice made these remarks in an editorial accompanying Dr. Anglesio’s report (N Engl J Med. 2017 May 11. doi: 10.1056/NEJMe1701700).
The variable patterns of somatic mutations discovered by Anglesio et al. are “intriguing” and raise many questions.
Do specific mutations in deep infiltrating endometriosis lesions contribute to the severity and progression of the disorder? Are such mutations to be found in other types of lesions? In endometrial precursor cells? Do specific mutations account for different presentations in different patients?
Exploring these questions will not be straightforward because of the difficulties in obtaining sufficient endometriotic tissue for genetic studies. Not only is it hard to get samples from peritoneal lesions, but the mutations occur in only one cell type – epithelium – which is even harder to isolate from mixed tissue samples.
Grant W. Montgomery, PhD, is at the Institute for Molecular Bioscience at the University of Queensland, Brisbane, Australia, and reported having no relevant financial disclosures. Linda C. Giudice, MD, PhD, is in the department of ob.gyn. and reproductive sciences at the University of California, San Francisco, and reported ties to AbbVie and Bayer. Dr. Montgomery and Dr. Giudice made these remarks in an editorial accompanying Dr. Anglesio’s report (N Engl J Med. 2017 May 11. doi: 10.1056/NEJMe1701700).
Deep infiltrating endometriosis lesions were found to harbor several genetic mutations, including well-known cancer-driver mutations in the ARID1A, PIK3CA, KRAS, and PPP2R1A genes, investigators reported in the May 11 issue of the New England Journal of Medicine.
This finding was possible only through the use of a combination of next-generation sequencing tools, together with highly sensitive digital genomic assays. A finding of cancer-related mutations in noncancerous lesions – and, “in particular, in nonovarian deep infiltrating lesions that rarely (if ever) transform into cancer” – was very “surprising” and raises new questions about the pathobiology of endometriosis, said Michael S. Anglesio, PhD, of the University of British Columbia, Vancouver, and his associates.
All three subtypes of endometriosis exhibit “benign clinical behavior and normal-appearing histologic features,” and are considered to be nonmalignant inflammatory lesions, Dr. Anglesio and his associates said. However, they also “recapitulate some features of malignant neoplasms, including local invasion and resistance to apoptosis,” the investigators noted.
To further explore whether deep infiltrating endometriosis lesions harbor somatic mutations, they performed a series of genetic analyses on samples obtained from three independent cohorts of 27 affected patients in New York, Japan, and Vancouver. Whole-exome sequencing of lesions and adjacent normal tissue from 24 women revealed 80 different somatic mutations in 19 (79%) of the lesions.
The number of mutations per lesion varied widely, from 0 to 17, with a mean of 3.3 mutations per lesion. Five of these lesions harbored mutations in the cancer-driver genes ARID1A, PIK3CA, KRAS, or PPP2R1A.
In targeted-sequencing analyses of samples from three more patients, more activating mutations in the KRAS gene were found in two (66%). One woman had two different activating KRAS mutations, and the other had the same KRAS mutation in three separate lesions.
A further analysis of samples from an additional 12 patients showed KRAS mutations in 3 (25%) of them.
The data indicate that these mutations were very unlikely to have arisen by chance alone. In addition, “because cancer-driver mutations were present only in the epithelium but not the stroma of the same endometriosis lesion, we can assume that the observed mutations provide some key selective advantage to endometriotic epithelial cells,” Dr. Anglesio and his associates said (N Engl J Med. 2017 May 11. doi: 10.1056/NEJMoa1614814).
Their findings are consistent with those of recent studies of other organ systems, which reported cancer-driver mutations in benign lesions and in normal tissues from skin, neuronal, and ovarian samples, they added.
“Our findings challenge the current understanding of this invasive subtype of endometriosis and open the discussion on whether deep infiltrating endometriosis can be considered as a benign neoplasm,” the investigators wrote.
This study was sponsored by 27 nonindustry sources, including the Johns Hopkins University, the Virginia and D.K. Ludwig Fund for Cancer Research, the Ephraim and Wilma Shaw Roseman Foundation, the Endometriosis Foundation of America, and the National Institutes of Health. Dr. Anglesio reported having no relevant financial disclosures; some of his associates reported ties to several industry sources.
Deep infiltrating endometriosis lesions were found to harbor several genetic mutations, including well-known cancer-driver mutations in the ARID1A, PIK3CA, KRAS, and PPP2R1A genes, investigators reported in the May 11 issue of the New England Journal of Medicine.
This finding was possible only through the use of a combination of next-generation sequencing tools, together with highly sensitive digital genomic assays. A finding of cancer-related mutations in noncancerous lesions – and, “in particular, in nonovarian deep infiltrating lesions that rarely (if ever) transform into cancer” – was very “surprising” and raises new questions about the pathobiology of endometriosis, said Michael S. Anglesio, PhD, of the University of British Columbia, Vancouver, and his associates.
All three subtypes of endometriosis exhibit “benign clinical behavior and normal-appearing histologic features,” and are considered to be nonmalignant inflammatory lesions, Dr. Anglesio and his associates said. However, they also “recapitulate some features of malignant neoplasms, including local invasion and resistance to apoptosis,” the investigators noted.
To further explore whether deep infiltrating endometriosis lesions harbor somatic mutations, they performed a series of genetic analyses on samples obtained from three independent cohorts of 27 affected patients in New York, Japan, and Vancouver. Whole-exome sequencing of lesions and adjacent normal tissue from 24 women revealed 80 different somatic mutations in 19 (79%) of the lesions.
The number of mutations per lesion varied widely, from 0 to 17, with a mean of 3.3 mutations per lesion. Five of these lesions harbored mutations in the cancer-driver genes ARID1A, PIK3CA, KRAS, or PPP2R1A.
In targeted-sequencing analyses of samples from three more patients, more activating mutations in the KRAS gene were found in two (66%). One woman had two different activating KRAS mutations, and the other had the same KRAS mutation in three separate lesions.
A further analysis of samples from an additional 12 patients showed KRAS mutations in 3 (25%) of them.
The data indicate that these mutations were very unlikely to have arisen by chance alone. In addition, “because cancer-driver mutations were present only in the epithelium but not the stroma of the same endometriosis lesion, we can assume that the observed mutations provide some key selective advantage to endometriotic epithelial cells,” Dr. Anglesio and his associates said (N Engl J Med. 2017 May 11. doi: 10.1056/NEJMoa1614814).
Their findings are consistent with those of recent studies of other organ systems, which reported cancer-driver mutations in benign lesions and in normal tissues from skin, neuronal, and ovarian samples, they added.
“Our findings challenge the current understanding of this invasive subtype of endometriosis and open the discussion on whether deep infiltrating endometriosis can be considered as a benign neoplasm,” the investigators wrote.
This study was sponsored by 27 nonindustry sources, including the Johns Hopkins University, the Virginia and D.K. Ludwig Fund for Cancer Research, the Ephraim and Wilma Shaw Roseman Foundation, the Endometriosis Foundation of America, and the National Institutes of Health. Dr. Anglesio reported having no relevant financial disclosures; some of his associates reported ties to several industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Deep infiltrating endometriosis lesions were found to harbor several genetic mutations, including well-known cancer-driver mutations in the ARID1A, PIK3CA, KRAS, and PPP2R1A genes.
Major finding: Endometriosis samples from 24 women revealed 80 different somatic mutations in 19 (79%) of the lesions; the number of mutations per lesion varied widely, from 0 to 17, with a mean of 3.3 mutations per lesion.
Data source: A series of genetic analyses of archived endometriosis samples from 27 women, and an additional analysis of KRAS mutations in samples from 12 more women.
Disclosures: This study was sponsored by 27 nonindustry sources, including Johns Hopkins University, the Virginia and D.K. Ludwig Fund for Cancer Research, the Ephraim and Wilma Shaw Roseman Foundation, the Endometriosis Foundation of America, and the National Institutes of Health. Dr. Anglesio reported having no relevant financial disclosures; some of his associates reported ties to several industry sources.
Isothiazolinone allergy frequent and underdiagnosed in children
Sensitization to the isothiazolinones MCI (methylchloroisothiazolinone) and MI (methylisothiazolinone), which are used as preservatives in a wide variety of personal and household products, is both frequent and underdiagnosed in U.S. children, according to a report published in Pediatric Dermatology.
These agents are compatible with surfactants and emulsifiers, and because they maintain biocidal activity across a broad range of pH levels they are frequently used as preservatives in products such as wet wipes; shampoos and hair conditioners; soaps, cleansers, and disinfectants; and laundry products. However, they are known to cause contact dermatitis very frequently, and are among the top five contact allergens identified in infants’ patch tests.
A recent survey showed that among 152 pediatric skin care products available at major retail stores, 20% contained MI. These were specifically targeted to infants and children, advertised as being “hypoallergenic,” “natural,” good for “sensitive” skin, and containing “gentle ingredients,” said Alina Goldenberg, MD, of the department of dermatology at the University of California, San Diego, and her associates.
During the past 10 years, only 35 U.S. cases of a positive patch-test reaction to MCI and/or MI have been reported in the literature. To get a more accurate estimate of the true prevalence of pediatric sensitization to MCI and MI, the investigators analyzed information in a database of patch-test results, the Provider Contact Dermatitis Registry. They focused on 1,056 patch tests performed during a 1-year period.
They found 37 positive reactions to combined MCI/MI and another 39 reactions that were negative to combined MCI/MI but positive to MI alone. This shows how important it is to test for sensitization to both formulations separately, Dr. Goldenberg and her associates noted (Pediatr Dermatol. 2017 Mar;34[2]:138-43).
In stark contrast to the reported 35 cases across the entire country during a 10-year period, the investigators found 76 cases (1%) in 1,056 patch tests during a 1-year period.
When test results for MCI/MI and MI alone were compared with those for all other allergens, children sensitized to the isothiazolinones showed marked differences: They were significantly younger, and the location of their dermatitis was more likely to involve the groin and buttocks. This probably is due to the increased use of wet wipes containing MCI and MI being used to clean up urinary and fecal accidents in young children, the researchers said.
The Society for Pediatric Dermatology supported the work. Dr. Goldenberg reported having no relevant financial disclosures; an associate reported serving as a consultant for Johnson & Johnson.
Sensitization to the isothiazolinones MCI (methylchloroisothiazolinone) and MI (methylisothiazolinone), which are used as preservatives in a wide variety of personal and household products, is both frequent and underdiagnosed in U.S. children, according to a report published in Pediatric Dermatology.
These agents are compatible with surfactants and emulsifiers, and because they maintain biocidal activity across a broad range of pH levels they are frequently used as preservatives in products such as wet wipes; shampoos and hair conditioners; soaps, cleansers, and disinfectants; and laundry products. However, they are known to cause contact dermatitis very frequently, and are among the top five contact allergens identified in infants’ patch tests.
A recent survey showed that among 152 pediatric skin care products available at major retail stores, 20% contained MI. These were specifically targeted to infants and children, advertised as being “hypoallergenic,” “natural,” good for “sensitive” skin, and containing “gentle ingredients,” said Alina Goldenberg, MD, of the department of dermatology at the University of California, San Diego, and her associates.
During the past 10 years, only 35 U.S. cases of a positive patch-test reaction to MCI and/or MI have been reported in the literature. To get a more accurate estimate of the true prevalence of pediatric sensitization to MCI and MI, the investigators analyzed information in a database of patch-test results, the Provider Contact Dermatitis Registry. They focused on 1,056 patch tests performed during a 1-year period.
They found 37 positive reactions to combined MCI/MI and another 39 reactions that were negative to combined MCI/MI but positive to MI alone. This shows how important it is to test for sensitization to both formulations separately, Dr. Goldenberg and her associates noted (Pediatr Dermatol. 2017 Mar;34[2]:138-43).
In stark contrast to the reported 35 cases across the entire country during a 10-year period, the investigators found 76 cases (1%) in 1,056 patch tests during a 1-year period.
When test results for MCI/MI and MI alone were compared with those for all other allergens, children sensitized to the isothiazolinones showed marked differences: They were significantly younger, and the location of their dermatitis was more likely to involve the groin and buttocks. This probably is due to the increased use of wet wipes containing MCI and MI being used to clean up urinary and fecal accidents in young children, the researchers said.
The Society for Pediatric Dermatology supported the work. Dr. Goldenberg reported having no relevant financial disclosures; an associate reported serving as a consultant for Johnson & Johnson.
Sensitization to the isothiazolinones MCI (methylchloroisothiazolinone) and MI (methylisothiazolinone), which are used as preservatives in a wide variety of personal and household products, is both frequent and underdiagnosed in U.S. children, according to a report published in Pediatric Dermatology.
These agents are compatible with surfactants and emulsifiers, and because they maintain biocidal activity across a broad range of pH levels they are frequently used as preservatives in products such as wet wipes; shampoos and hair conditioners; soaps, cleansers, and disinfectants; and laundry products. However, they are known to cause contact dermatitis very frequently, and are among the top five contact allergens identified in infants’ patch tests.
A recent survey showed that among 152 pediatric skin care products available at major retail stores, 20% contained MI. These were specifically targeted to infants and children, advertised as being “hypoallergenic,” “natural,” good for “sensitive” skin, and containing “gentle ingredients,” said Alina Goldenberg, MD, of the department of dermatology at the University of California, San Diego, and her associates.
During the past 10 years, only 35 U.S. cases of a positive patch-test reaction to MCI and/or MI have been reported in the literature. To get a more accurate estimate of the true prevalence of pediatric sensitization to MCI and MI, the investigators analyzed information in a database of patch-test results, the Provider Contact Dermatitis Registry. They focused on 1,056 patch tests performed during a 1-year period.
They found 37 positive reactions to combined MCI/MI and another 39 reactions that were negative to combined MCI/MI but positive to MI alone. This shows how important it is to test for sensitization to both formulations separately, Dr. Goldenberg and her associates noted (Pediatr Dermatol. 2017 Mar;34[2]:138-43).
In stark contrast to the reported 35 cases across the entire country during a 10-year period, the investigators found 76 cases (1%) in 1,056 patch tests during a 1-year period.
When test results for MCI/MI and MI alone were compared with those for all other allergens, children sensitized to the isothiazolinones showed marked differences: They were significantly younger, and the location of their dermatitis was more likely to involve the groin and buttocks. This probably is due to the increased use of wet wipes containing MCI and MI being used to clean up urinary and fecal accidents in young children, the researchers said.
The Society for Pediatric Dermatology supported the work. Dr. Goldenberg reported having no relevant financial disclosures; an associate reported serving as a consultant for Johnson & Johnson.
FROM PEDIATRIC DERMATOLOGY
Key clinical point:
Major finding: There were 37 positive patch-test reactions to combined MCI/MI and another 39 reactions that were negative to combined MCI/MI but positive to MI alone.
Data source: An analysis of 1,056 patch-test results recorded in a database by clinicians during a 1-year period.
Disclosures: The Society for Pediatric Dermatology supported the work. Dr. Goldenberg reported having no relevant financial disclosures; an associate reported serving as a consultant for Johnson & Johnson.
USPSTF: No thyroid cancer screening for asymptomatic adults
The U.S. Preventive Services Task Force recommends against screening asymptomatic adults for thyroid cancer, because the harms of such screening outweigh the benefits, according to a recommendation statement published May 9 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the recommendation statement addresses screening of adults who have no signs or symptoms of thyroid cancer by using neck palpation or ultrasonography, said Kirsten Bibbins-Domingo, MD, PhD, chair of the organization and lead author of the recommendation statement, and her associates (JAMA. 2017 May 9;317[18]:1882-7).
This document is an update of the previous USPSTF recommendation statement issued in 1996 and was undertaken because there have been several major advances related to the disease since that time.
However, despite a comprehensive review of the current literature, the group found no direct evidence supporting a change to their original advice against such screening.
They emphasized that this applies only to asymptomatic adults, not to those who have hoarseness, throat pain, difficulty swallowing, lumps in the neck, swelling, or asymmetry of the neck; nor to those who have a history of exposure to ionizing radiation, a family history of thyroid cancer, or a genetic susceptibility to the disease.
The results of the literature review were summarized in an evidence report by Jennifer S. Lin, MD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates. They examined 67 studies involving nearly 584,000 patients, including 10 studies that addressed screening test performance, 3 that addressed the possible harms of screening, 2 that addressed treatment benefits, and 52 that addressed treatment harms.
No good-quality studies assessed the net benefit of thyroid cancer screening, nor whether “early” treatment of screen-detected cancers improved patient outcomes. However, the preponderance of evidence suggested that most thyroid cancers detected by screening are indolent.
So, treatment is likely unnecessary but exposes patients to “nontrivial” harms, including an increased risk of second primary malignancy, permanent adverse effects on the salivary glands, laryngeal nerve injury, hypoparathyroidism, and the need for lifelong thyroid replacement therapy and monitoring for cancer recurrence, Dr. Lin and her associates said in their evidence report (JAMA. 2017 May 9;317[18]:1888-1903).
The task force noted that no professional medical society currently recommends population-based screening for thyroid cancer. The American Cancer Society, American Thyroid Association, American Association of Clinical Endocrinologists, and American College of Endocrinology all have no specific recommendations for screening asymptomatic patients, while the American Academy of Family Physicians recommends against such screening, said Dr. Bibbins-Domingo, who is also a professor of medicine at the University of California, San Francisco, and her associates.
Further information regarding the recommendation statement and the evidence report is available at www.uspreventiveservicestaskforce.org.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
The rationale for recommending against screening asymptomatic people for thyroid cancer is compelling, and the evidence clearly points to the harms outweighing the benefits.
But that doesn’t mean that the conversation about screening should stop. What the field needs is not a better means to detect thyroid nodules, but a noninvasive measure to distinguish nodules whose cells will leave the thyroid capsule and cause morbidity from nodules whose cells will not. That will spare patients with indolent cancers from unnecessary treatment, while steering the minority of patients with more aggressive cancers to early treatment.
Anne R. Cappola, MD, is in the division of endocrinology, diabetes, and metabolism at the University of Pennsylvania, Philadelphia, and is an associate editor of JAMA. She reported having no relevant financial disclosures. Dr. Cappola made these remarks in an editorial accompanying the recommendation statement and the evidence report (JAMA. 2017 May 9;317[18]:1840-1).
The rationale for recommending against screening asymptomatic people for thyroid cancer is compelling, and the evidence clearly points to the harms outweighing the benefits.
But that doesn’t mean that the conversation about screening should stop. What the field needs is not a better means to detect thyroid nodules, but a noninvasive measure to distinguish nodules whose cells will leave the thyroid capsule and cause morbidity from nodules whose cells will not. That will spare patients with indolent cancers from unnecessary treatment, while steering the minority of patients with more aggressive cancers to early treatment.
Anne R. Cappola, MD, is in the division of endocrinology, diabetes, and metabolism at the University of Pennsylvania, Philadelphia, and is an associate editor of JAMA. She reported having no relevant financial disclosures. Dr. Cappola made these remarks in an editorial accompanying the recommendation statement and the evidence report (JAMA. 2017 May 9;317[18]:1840-1).
The rationale for recommending against screening asymptomatic people for thyroid cancer is compelling, and the evidence clearly points to the harms outweighing the benefits.
But that doesn’t mean that the conversation about screening should stop. What the field needs is not a better means to detect thyroid nodules, but a noninvasive measure to distinguish nodules whose cells will leave the thyroid capsule and cause morbidity from nodules whose cells will not. That will spare patients with indolent cancers from unnecessary treatment, while steering the minority of patients with more aggressive cancers to early treatment.
Anne R. Cappola, MD, is in the division of endocrinology, diabetes, and metabolism at the University of Pennsylvania, Philadelphia, and is an associate editor of JAMA. She reported having no relevant financial disclosures. Dr. Cappola made these remarks in an editorial accompanying the recommendation statement and the evidence report (JAMA. 2017 May 9;317[18]:1840-1).
The U.S. Preventive Services Task Force recommends against screening asymptomatic adults for thyroid cancer, because the harms of such screening outweigh the benefits, according to a recommendation statement published May 9 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the recommendation statement addresses screening of adults who have no signs or symptoms of thyroid cancer by using neck palpation or ultrasonography, said Kirsten Bibbins-Domingo, MD, PhD, chair of the organization and lead author of the recommendation statement, and her associates (JAMA. 2017 May 9;317[18]:1882-7).
This document is an update of the previous USPSTF recommendation statement issued in 1996 and was undertaken because there have been several major advances related to the disease since that time.
However, despite a comprehensive review of the current literature, the group found no direct evidence supporting a change to their original advice against such screening.
They emphasized that this applies only to asymptomatic adults, not to those who have hoarseness, throat pain, difficulty swallowing, lumps in the neck, swelling, or asymmetry of the neck; nor to those who have a history of exposure to ionizing radiation, a family history of thyroid cancer, or a genetic susceptibility to the disease.
The results of the literature review were summarized in an evidence report by Jennifer S. Lin, MD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates. They examined 67 studies involving nearly 584,000 patients, including 10 studies that addressed screening test performance, 3 that addressed the possible harms of screening, 2 that addressed treatment benefits, and 52 that addressed treatment harms.
No good-quality studies assessed the net benefit of thyroid cancer screening, nor whether “early” treatment of screen-detected cancers improved patient outcomes. However, the preponderance of evidence suggested that most thyroid cancers detected by screening are indolent.
So, treatment is likely unnecessary but exposes patients to “nontrivial” harms, including an increased risk of second primary malignancy, permanent adverse effects on the salivary glands, laryngeal nerve injury, hypoparathyroidism, and the need for lifelong thyroid replacement therapy and monitoring for cancer recurrence, Dr. Lin and her associates said in their evidence report (JAMA. 2017 May 9;317[18]:1888-1903).
The task force noted that no professional medical society currently recommends population-based screening for thyroid cancer. The American Cancer Society, American Thyroid Association, American Association of Clinical Endocrinologists, and American College of Endocrinology all have no specific recommendations for screening asymptomatic patients, while the American Academy of Family Physicians recommends against such screening, said Dr. Bibbins-Domingo, who is also a professor of medicine at the University of California, San Francisco, and her associates.
Further information regarding the recommendation statement and the evidence report is available at www.uspreventiveservicestaskforce.org.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
The U.S. Preventive Services Task Force recommends against screening asymptomatic adults for thyroid cancer, because the harms of such screening outweigh the benefits, according to a recommendation statement published May 9 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the recommendation statement addresses screening of adults who have no signs or symptoms of thyroid cancer by using neck palpation or ultrasonography, said Kirsten Bibbins-Domingo, MD, PhD, chair of the organization and lead author of the recommendation statement, and her associates (JAMA. 2017 May 9;317[18]:1882-7).
This document is an update of the previous USPSTF recommendation statement issued in 1996 and was undertaken because there have been several major advances related to the disease since that time.
However, despite a comprehensive review of the current literature, the group found no direct evidence supporting a change to their original advice against such screening.
They emphasized that this applies only to asymptomatic adults, not to those who have hoarseness, throat pain, difficulty swallowing, lumps in the neck, swelling, or asymmetry of the neck; nor to those who have a history of exposure to ionizing radiation, a family history of thyroid cancer, or a genetic susceptibility to the disease.
The results of the literature review were summarized in an evidence report by Jennifer S. Lin, MD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates. They examined 67 studies involving nearly 584,000 patients, including 10 studies that addressed screening test performance, 3 that addressed the possible harms of screening, 2 that addressed treatment benefits, and 52 that addressed treatment harms.
No good-quality studies assessed the net benefit of thyroid cancer screening, nor whether “early” treatment of screen-detected cancers improved patient outcomes. However, the preponderance of evidence suggested that most thyroid cancers detected by screening are indolent.
So, treatment is likely unnecessary but exposes patients to “nontrivial” harms, including an increased risk of second primary malignancy, permanent adverse effects on the salivary glands, laryngeal nerve injury, hypoparathyroidism, and the need for lifelong thyroid replacement therapy and monitoring for cancer recurrence, Dr. Lin and her associates said in their evidence report (JAMA. 2017 May 9;317[18]:1888-1903).
The task force noted that no professional medical society currently recommends population-based screening for thyroid cancer. The American Cancer Society, American Thyroid Association, American Association of Clinical Endocrinologists, and American College of Endocrinology all have no specific recommendations for screening asymptomatic patients, while the American Academy of Family Physicians recommends against such screening, said Dr. Bibbins-Domingo, who is also a professor of medicine at the University of California, San Francisco, and her associates.
Further information regarding the recommendation statement and the evidence report is available at www.uspreventiveservicestaskforce.org.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
FROM JAMA
Key clinical point: The USPSTF recommends against screening asymptomatic adults for thyroid cancer because the harms outweigh the benefits.
Major finding: None of the 67 studies reviewed in the evidence report directly assessed the net benefit of thyroid cancer screening, nor whether “early” treatment of screen-detected cancers improved patient outcomes.
Data source: A recommendation statement based on a review of 67 studies published during 1996-2016 involving 583,914 patients.
Disclosures: The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
Subcutaneous rituximab safe, effective for follicular lymphoma
Efficacy and safety profiles were similar for subcutaneous and standard IV rituximab when given as first-line therapy to adults with follicular lymphoma, based on results of a phase III clinical trial published online in Lancet Haematology.
Administering rituximab by IV infusion can take up to 6 hours to complete and requires continuous monitoring. Subcutaneous delivery takes approximately 6 minutes using a new rituximab formulation that is 12 times more concentrated to reduce the administered volume. The new formulation is expected to reduce the burden of treatment for patients, as well as for the health care system, said Andrew Davies, PhD, of the Cancer Research UK Centre, Southampton, and his associates.
They compared the two agents in an international open-label trial funded by Hoffmann-La Roche, maker of the subcutaneous formulation. Adult patients at 113 medical centers in 30 countries were randomly assigned to receive either IV (205 patients) or subcutaneous (205 patients) rituximab during induction therapy with six to eight cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CVP (cyclophosphamide, vincristine, and prednisone). They continued with rituximab as maintenance therapy every 2 months for 2 years. The median duration of treatment was 27 months, and median follow-up was 37 months.
The primary efficacy end point – overall (complete or partial) response rate at the end of induction, based on investigator assessment confirmed by an independent review panel of radiologists – was 84.9% with IV and 84.4% with subcutaneous rituximab, a nonsignificant difference. Similarly, the overall response rate at the end of maintenance therapy was not significantly different between the two groups, at 78.1% and 77.9%, respectively.
Progression-free survival (hazard ratio, 0.84) and event-free survival (HR, 0.91) also did not differ significantly between the two study groups, the investigators said (Lancet Haematol. 2017 doi: 10.1016/S2352.3026(17)30078-9).
The rates of adverse events, grade 3 or higher adverse events, and serious adverse events also were similar for IV and subcutaneous formulations of rituximab. “Administration-related reactions were more common in the subcutaneous group but were predominantly mild-to-moderate local injection-site reactions, such as mild pain, swelling and erythema, reflecting the expected change in safety profile when switching to the subcutaneous route of administration,” Dr. Davies and his associates said.
These results indicate that subcutaneous administration of rituximab along with chemotherapy doesn’t compromise the agent’s antilymphoma activity, they added.
Efficacy and safety profiles were similar for subcutaneous and standard IV rituximab when given as first-line therapy to adults with follicular lymphoma, based on results of a phase III clinical trial published online in Lancet Haematology.
Administering rituximab by IV infusion can take up to 6 hours to complete and requires continuous monitoring. Subcutaneous delivery takes approximately 6 minutes using a new rituximab formulation that is 12 times more concentrated to reduce the administered volume. The new formulation is expected to reduce the burden of treatment for patients, as well as for the health care system, said Andrew Davies, PhD, of the Cancer Research UK Centre, Southampton, and his associates.
They compared the two agents in an international open-label trial funded by Hoffmann-La Roche, maker of the subcutaneous formulation. Adult patients at 113 medical centers in 30 countries were randomly assigned to receive either IV (205 patients) or subcutaneous (205 patients) rituximab during induction therapy with six to eight cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CVP (cyclophosphamide, vincristine, and prednisone). They continued with rituximab as maintenance therapy every 2 months for 2 years. The median duration of treatment was 27 months, and median follow-up was 37 months.
The primary efficacy end point – overall (complete or partial) response rate at the end of induction, based on investigator assessment confirmed by an independent review panel of radiologists – was 84.9% with IV and 84.4% with subcutaneous rituximab, a nonsignificant difference. Similarly, the overall response rate at the end of maintenance therapy was not significantly different between the two groups, at 78.1% and 77.9%, respectively.
Progression-free survival (hazard ratio, 0.84) and event-free survival (HR, 0.91) also did not differ significantly between the two study groups, the investigators said (Lancet Haematol. 2017 doi: 10.1016/S2352.3026(17)30078-9).
The rates of adverse events, grade 3 or higher adverse events, and serious adverse events also were similar for IV and subcutaneous formulations of rituximab. “Administration-related reactions were more common in the subcutaneous group but were predominantly mild-to-moderate local injection-site reactions, such as mild pain, swelling and erythema, reflecting the expected change in safety profile when switching to the subcutaneous route of administration,” Dr. Davies and his associates said.
These results indicate that subcutaneous administration of rituximab along with chemotherapy doesn’t compromise the agent’s antilymphoma activity, they added.
Efficacy and safety profiles were similar for subcutaneous and standard IV rituximab when given as first-line therapy to adults with follicular lymphoma, based on results of a phase III clinical trial published online in Lancet Haematology.
Administering rituximab by IV infusion can take up to 6 hours to complete and requires continuous monitoring. Subcutaneous delivery takes approximately 6 minutes using a new rituximab formulation that is 12 times more concentrated to reduce the administered volume. The new formulation is expected to reduce the burden of treatment for patients, as well as for the health care system, said Andrew Davies, PhD, of the Cancer Research UK Centre, Southampton, and his associates.
They compared the two agents in an international open-label trial funded by Hoffmann-La Roche, maker of the subcutaneous formulation. Adult patients at 113 medical centers in 30 countries were randomly assigned to receive either IV (205 patients) or subcutaneous (205 patients) rituximab during induction therapy with six to eight cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CVP (cyclophosphamide, vincristine, and prednisone). They continued with rituximab as maintenance therapy every 2 months for 2 years. The median duration of treatment was 27 months, and median follow-up was 37 months.
The primary efficacy end point – overall (complete or partial) response rate at the end of induction, based on investigator assessment confirmed by an independent review panel of radiologists – was 84.9% with IV and 84.4% with subcutaneous rituximab, a nonsignificant difference. Similarly, the overall response rate at the end of maintenance therapy was not significantly different between the two groups, at 78.1% and 77.9%, respectively.
Progression-free survival (hazard ratio, 0.84) and event-free survival (HR, 0.91) also did not differ significantly between the two study groups, the investigators said (Lancet Haematol. 2017 doi: 10.1016/S2352.3026(17)30078-9).
The rates of adverse events, grade 3 or higher adverse events, and serious adverse events also were similar for IV and subcutaneous formulations of rituximab. “Administration-related reactions were more common in the subcutaneous group but were predominantly mild-to-moderate local injection-site reactions, such as mild pain, swelling and erythema, reflecting the expected change in safety profile when switching to the subcutaneous route of administration,” Dr. Davies and his associates said.
These results indicate that subcutaneous administration of rituximab along with chemotherapy doesn’t compromise the agent’s antilymphoma activity, they added.
FROM LANCET HAEMATOLOGY
Key clinical point: Subcutaneous rituximab had efficacy and safety profiles similar to those of standard IV rituximab when given as first-line therapy to adults with follicular lymphoma.
Major finding: The primary efficacy end point – overall response rate at the end of induction – was 84.9% with IV and 84.4% with subcutaneous rituximab.
Data source: An international randomized controlled phase III trial involving 410 adults followed for 3 years.
Disclosures: This trial was funded by Hoffmann-La Roche, maker of the subcutaneous formulation of rituximab. The pharmaceutical company also was involved in the design and conduct of the trial, collection and interpretation of the data, and writing of the results. Dr. Davies reported ties to Hoffmann-La Roche and numerous other drug companies.
Updated osteoporosis guideline advocates bisphosphonates, nixes estrogens
The American College of Physicians’ updated clinical practice guideline for treating osteoporosis strongly advocates bisphosphonates and strongly advises against estrogens or raloxifene. It was presented online May 8 in the Annals of Internal Medicine.
The new guideline is based on a review of the evidence published since the previous (2008) ACP guideline for preventing fractures in women and men with osteoporosis or low bone density and is intended to replace that document. It offers six main recommendations and several additional pointers for both clinicians and the approximately 50% of Americans older than age 50 years who are at risk for osteoporotic fracture, said Amir Qaseem, MD, PhD, lead author and vice president of clinical policy at the ACP, Philadelphia, and his associates.
The strongest recommendation, based on extensive high-quality evidence, is that clinicians offer women known to have osteoporosis pharmacologic therapy with the bisphosphonates alendronate, risedronate, or zoledronic acid, or the biologic agent denosumab, to reduce their risk of hip and vertebral fracture. Counseling on the importance of adherence despite the relative inconvenience of taking the medications and their possible adverse effects is urged, as is prescribing generic formulations whenever possible.
Raloxifene, ibandronate, and teriparatide have not been shown to reduce the risks of all types of fractures, so they are not recommended as first-line therapy. The updated guideline no longer addresses the use of calcitonin, because it is no longer used widely for osteoporosis. Similarly, it no longer addresses the use of etidronate or pamidronate, which are not approved by the Food and Drug Administration for this indication. The updated guideline added denosumab, a new biologic agent recently approved by the FDA.
Estrogens or raloxifene are not advised for established osteoporosis because good evidence shows they do not reduce fracture risk, according to the guideline. Moreover, these agents can be associated with serious harms that outweigh any potential benefits, including stroke and venous thromboembolism. This recommendation directly refutes one in the previous guideline that favored estrogen therapy, based on newer evidence, Dr. Qaseem and his associates said (Ann. Intern. Med. 2017 May 8.doi: 10.7326/M15-1361).
Four additional recommendations in the updated guideline are weaker because they are based on low-quality evidence.
One advises that the duration of pharmacologic therapy should be 5 years, although there is considerable variation in response to treatment and many patients may benefit from longer treatment. Another recommendation advises against bone mineral density monitoring during this 5-year period because current evidence shows no benefit for it.
Another recommendation advises that men who have clinically recognized osteoporosis should be offered bisphosphonate therapy to reduce their risk of vertebral fracture. The final recommendation advises clinicians to decide whether to treat “osteopenic women 65 years of age or older who are at a high risk for fracture,” based on patient preferences and the “benefits, harms, and costs of medications.”
The guideline notes that, for women who have normal bone mineral density, frequent monitoring for osteoporosis is unnecessary, because current evidence shows those with normal dual-energy x-ray absorptiometry scores do not progress to osteoporosis within 15 years. And it cautions that even though the World Health Organization’s Fracture Risk Assessment Tool scores are widely used, there is no evidence that they accurately reflect treatment efficacy.
The most important recommendation in this updated ACP guideline is for clinicians to offer bisphosphonates or denosumab to women with osteoporosis.
Even though large, well-designed, randomized controlled trials amply demonstrate the effectiveness of these drugs in preventing fractures, undertreatment is rampant and is actually increasing. It appears that patients and perhaps some clinicians have the mistaken impression that bisphosphonates frequently cause serious adverse effects.
In truth, the rate of adverse effects and of serious adverse events with bisphosphonates is very low. Clinicians should educate patients about the safety of these agents.
Eric S. Orwoll, MD, is at Oregon Health and Science University, Portland. Dr. Orwoll made these remarks in an editorial accompanying the updated guideline (Ann. Intern. Med. 2017 May 8. doi: 10.7326/M17-0957). His financial disclosures are available at www.acponline.org
The most important recommendation in this updated ACP guideline is for clinicians to offer bisphosphonates or denosumab to women with osteoporosis.
Even though large, well-designed, randomized controlled trials amply demonstrate the effectiveness of these drugs in preventing fractures, undertreatment is rampant and is actually increasing. It appears that patients and perhaps some clinicians have the mistaken impression that bisphosphonates frequently cause serious adverse effects.
In truth, the rate of adverse effects and of serious adverse events with bisphosphonates is very low. Clinicians should educate patients about the safety of these agents.
Eric S. Orwoll, MD, is at Oregon Health and Science University, Portland. Dr. Orwoll made these remarks in an editorial accompanying the updated guideline (Ann. Intern. Med. 2017 May 8. doi: 10.7326/M17-0957). His financial disclosures are available at www.acponline.org
The most important recommendation in this updated ACP guideline is for clinicians to offer bisphosphonates or denosumab to women with osteoporosis.
Even though large, well-designed, randomized controlled trials amply demonstrate the effectiveness of these drugs in preventing fractures, undertreatment is rampant and is actually increasing. It appears that patients and perhaps some clinicians have the mistaken impression that bisphosphonates frequently cause serious adverse effects.
In truth, the rate of adverse effects and of serious adverse events with bisphosphonates is very low. Clinicians should educate patients about the safety of these agents.
Eric S. Orwoll, MD, is at Oregon Health and Science University, Portland. Dr. Orwoll made these remarks in an editorial accompanying the updated guideline (Ann. Intern. Med. 2017 May 8. doi: 10.7326/M17-0957). His financial disclosures are available at www.acponline.org
The American College of Physicians’ updated clinical practice guideline for treating osteoporosis strongly advocates bisphosphonates and strongly advises against estrogens or raloxifene. It was presented online May 8 in the Annals of Internal Medicine.
The new guideline is based on a review of the evidence published since the previous (2008) ACP guideline for preventing fractures in women and men with osteoporosis or low bone density and is intended to replace that document. It offers six main recommendations and several additional pointers for both clinicians and the approximately 50% of Americans older than age 50 years who are at risk for osteoporotic fracture, said Amir Qaseem, MD, PhD, lead author and vice president of clinical policy at the ACP, Philadelphia, and his associates.
The strongest recommendation, based on extensive high-quality evidence, is that clinicians offer women known to have osteoporosis pharmacologic therapy with the bisphosphonates alendronate, risedronate, or zoledronic acid, or the biologic agent denosumab, to reduce their risk of hip and vertebral fracture. Counseling on the importance of adherence despite the relative inconvenience of taking the medications and their possible adverse effects is urged, as is prescribing generic formulations whenever possible.
Raloxifene, ibandronate, and teriparatide have not been shown to reduce the risks of all types of fractures, so they are not recommended as first-line therapy. The updated guideline no longer addresses the use of calcitonin, because it is no longer used widely for osteoporosis. Similarly, it no longer addresses the use of etidronate or pamidronate, which are not approved by the Food and Drug Administration for this indication. The updated guideline added denosumab, a new biologic agent recently approved by the FDA.
Estrogens or raloxifene are not advised for established osteoporosis because good evidence shows they do not reduce fracture risk, according to the guideline. Moreover, these agents can be associated with serious harms that outweigh any potential benefits, including stroke and venous thromboembolism. This recommendation directly refutes one in the previous guideline that favored estrogen therapy, based on newer evidence, Dr. Qaseem and his associates said (Ann. Intern. Med. 2017 May 8.doi: 10.7326/M15-1361).
Four additional recommendations in the updated guideline are weaker because they are based on low-quality evidence.
One advises that the duration of pharmacologic therapy should be 5 years, although there is considerable variation in response to treatment and many patients may benefit from longer treatment. Another recommendation advises against bone mineral density monitoring during this 5-year period because current evidence shows no benefit for it.
Another recommendation advises that men who have clinically recognized osteoporosis should be offered bisphosphonate therapy to reduce their risk of vertebral fracture. The final recommendation advises clinicians to decide whether to treat “osteopenic women 65 years of age or older who are at a high risk for fracture,” based on patient preferences and the “benefits, harms, and costs of medications.”
The guideline notes that, for women who have normal bone mineral density, frequent monitoring for osteoporosis is unnecessary, because current evidence shows those with normal dual-energy x-ray absorptiometry scores do not progress to osteoporosis within 15 years. And it cautions that even though the World Health Organization’s Fracture Risk Assessment Tool scores are widely used, there is no evidence that they accurately reflect treatment efficacy.
The American College of Physicians’ updated clinical practice guideline for treating osteoporosis strongly advocates bisphosphonates and strongly advises against estrogens or raloxifene. It was presented online May 8 in the Annals of Internal Medicine.
The new guideline is based on a review of the evidence published since the previous (2008) ACP guideline for preventing fractures in women and men with osteoporosis or low bone density and is intended to replace that document. It offers six main recommendations and several additional pointers for both clinicians and the approximately 50% of Americans older than age 50 years who are at risk for osteoporotic fracture, said Amir Qaseem, MD, PhD, lead author and vice president of clinical policy at the ACP, Philadelphia, and his associates.
The strongest recommendation, based on extensive high-quality evidence, is that clinicians offer women known to have osteoporosis pharmacologic therapy with the bisphosphonates alendronate, risedronate, or zoledronic acid, or the biologic agent denosumab, to reduce their risk of hip and vertebral fracture. Counseling on the importance of adherence despite the relative inconvenience of taking the medications and their possible adverse effects is urged, as is prescribing generic formulations whenever possible.
Raloxifene, ibandronate, and teriparatide have not been shown to reduce the risks of all types of fractures, so they are not recommended as first-line therapy. The updated guideline no longer addresses the use of calcitonin, because it is no longer used widely for osteoporosis. Similarly, it no longer addresses the use of etidronate or pamidronate, which are not approved by the Food and Drug Administration for this indication. The updated guideline added denosumab, a new biologic agent recently approved by the FDA.
Estrogens or raloxifene are not advised for established osteoporosis because good evidence shows they do not reduce fracture risk, according to the guideline. Moreover, these agents can be associated with serious harms that outweigh any potential benefits, including stroke and venous thromboembolism. This recommendation directly refutes one in the previous guideline that favored estrogen therapy, based on newer evidence, Dr. Qaseem and his associates said (Ann. Intern. Med. 2017 May 8.doi: 10.7326/M15-1361).
Four additional recommendations in the updated guideline are weaker because they are based on low-quality evidence.
One advises that the duration of pharmacologic therapy should be 5 years, although there is considerable variation in response to treatment and many patients may benefit from longer treatment. Another recommendation advises against bone mineral density monitoring during this 5-year period because current evidence shows no benefit for it.
Another recommendation advises that men who have clinically recognized osteoporosis should be offered bisphosphonate therapy to reduce their risk of vertebral fracture. The final recommendation advises clinicians to decide whether to treat “osteopenic women 65 years of age or older who are at a high risk for fracture,” based on patient preferences and the “benefits, harms, and costs of medications.”
The guideline notes that, for women who have normal bone mineral density, frequent monitoring for osteoporosis is unnecessary, because current evidence shows those with normal dual-energy x-ray absorptiometry scores do not progress to osteoporosis within 15 years. And it cautions that even though the World Health Organization’s Fracture Risk Assessment Tool scores are widely used, there is no evidence that they accurately reflect treatment efficacy.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point:
Major finding: The guideline offers six main recommendations and several additional pointers for clinicians and for those over age 50 years who are at risk for osteoporotic fracture.
Data source: A review of the evidence since the previous (2008) ACP guideline for treating osteoporosis.
Disclosures: This work was supported exclusively by the American College of Physicians. The authors’ financial disclosures are available at www.acponline.org
Breast milk bacteria seed infant gut microbiome
Beneficial bacteria in the mother’s breast milk and on the breast skin seed the infant gut microbiome and maintain it even after solid foods are introduced, according to a report published in JAMA Pediatrics.
Noting that little is known about the vertical transfer of breast milk microbes from mother to infant, researchers examined bacteria in samples from breast milk, areolar skin swabs, and infant stools in 107 healthy mother-infant pairs. The samples were obtained during a 5-year period from mothers and infants living in the community in Los Angeles and St. Petersburg, Fla., said Pia S. Pannaraj, MD, of the division of infectious diseases, Children’s Hospital Los Angeles, and her associates.
The transferred bacteria are known to “have prominent carbohydrate, amino acid, and energy metabolism functions.” In addition, “The amount of daily breastfeeding as a proportion of total milk intake continued to influence the infant stool microbiome diversity and membership even after solid foods were introduced,” Dr. Pannaraj and her associates said.
These findings highlight “the importance of breastfeeding in the assembly of the infant gut microbiome.” They also support current World Health Organization and American Academy of Pediatrics recommendations “for exclusive breastfeeding during the first 6 months, with continued breastfeeding until at least 12 months,” the investigators added.
Beneficial bacteria in the mother’s breast milk and on the breast skin seed the infant gut microbiome and maintain it even after solid foods are introduced, according to a report published in JAMA Pediatrics.
Noting that little is known about the vertical transfer of breast milk microbes from mother to infant, researchers examined bacteria in samples from breast milk, areolar skin swabs, and infant stools in 107 healthy mother-infant pairs. The samples were obtained during a 5-year period from mothers and infants living in the community in Los Angeles and St. Petersburg, Fla., said Pia S. Pannaraj, MD, of the division of infectious diseases, Children’s Hospital Los Angeles, and her associates.
The transferred bacteria are known to “have prominent carbohydrate, amino acid, and energy metabolism functions.” In addition, “The amount of daily breastfeeding as a proportion of total milk intake continued to influence the infant stool microbiome diversity and membership even after solid foods were introduced,” Dr. Pannaraj and her associates said.
These findings highlight “the importance of breastfeeding in the assembly of the infant gut microbiome.” They also support current World Health Organization and American Academy of Pediatrics recommendations “for exclusive breastfeeding during the first 6 months, with continued breastfeeding until at least 12 months,” the investigators added.
Beneficial bacteria in the mother’s breast milk and on the breast skin seed the infant gut microbiome and maintain it even after solid foods are introduced, according to a report published in JAMA Pediatrics.
Noting that little is known about the vertical transfer of breast milk microbes from mother to infant, researchers examined bacteria in samples from breast milk, areolar skin swabs, and infant stools in 107 healthy mother-infant pairs. The samples were obtained during a 5-year period from mothers and infants living in the community in Los Angeles and St. Petersburg, Fla., said Pia S. Pannaraj, MD, of the division of infectious diseases, Children’s Hospital Los Angeles, and her associates.
The transferred bacteria are known to “have prominent carbohydrate, amino acid, and energy metabolism functions.” In addition, “The amount of daily breastfeeding as a proportion of total milk intake continued to influence the infant stool microbiome diversity and membership even after solid foods were introduced,” Dr. Pannaraj and her associates said.
These findings highlight “the importance of breastfeeding in the assembly of the infant gut microbiome.” They also support current World Health Organization and American Academy of Pediatrics recommendations “for exclusive breastfeeding during the first 6 months, with continued breastfeeding until at least 12 months,” the investigators added.
FROM JAMA PEDIATRICS
Key clinical point: Beneficial bacteria in the mother’s breast milk and on the areola seed and maintain the infant gut microbiome.
Major finding: During their first 30 days of life, breastfed infants obtained a mean of 28% of their gut bacteria from breast milk and 10% from the mothers’ areolar skin.
Data source: A prospective longitudinal study involving 107 healthy mother-infant pairs.
Disclosures: This study was supported by the National Institutes of Health and the University of Pennsylvania Center for AIDS Research. Dr. Pannaraj reported ties to AstraZeneca and Pfizer.
AGA Clinical Practice Update: Expert review recommendations on post-SVR hepatitis C care
The AGA Institute issued a clinical practice update for managing hepatitis C virus–infected patients who achieve a sustained virologic response after antiviral therapy, who still require ongoing care for their liver disease. The expert review appears in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.018).
Even though direct-acting antiviral regimens have produced remarkably high sustained virologic response (SVR) rates and it appears that fewer than 1% of patients relapse, and even though liver fibrosis and cirrhosis may regress with this therapy, continued surveillance and even intervention may be needed “to reduce complications arising from liver damage that has already accrued by the time SVR was attained,” said Ira M. Jacobson, MD, AGAF, chair of the department of medicine, Mount Sinai Beth Israel Medical Center, New York, and his associates.
Dr. Jacobson and his associates at the AGA Institute reviewed the current literature and expert opinion to formulate 11 best-practice recommendations for managing this patient population. Among their recommendations:
SVR should be confirmed by hepatitis C virus RNA testing at 12 weeks after completion of an all-oral direct-acting antiviral regimen, and routine confirmation after 48 weeks is also “prudent.” Further testing for later virologic relapse is not supported by the available evidence. However, further periodic testing is advised for patients at risk for reinfection, such as those who continue to use IV drugs.
All patients with stage 3 or higher liver fibrosis or cirrhosis before achieving SVR should continue to be monitored by liver imaging (with or without serum alpha fetoprotein testing) twice a year “for an indefinite duration.” At present, there is no evidence of a finite point beyond which the risk of hepatocellular carcinoma is reduced to the level of people who don’t have a history of liver disease. And there have been documented cases of hepatocellular carcinoma developing more than 5 years after attaining SVR.
Regardless of SVR status, all patients with liver cirrhosis should undergo endoscopic screening for esophagogastric varices. If no varices or only small varices are detected, repeat endoscopy should be done 2-3 years after achieving SVR. If no varices are identified then, “cessation of further endoscopic screening may be considered on an individual patient basis if there are no risk factors for progressive cirrhosis.”
Noninvasive assessment of fibrosis, such as liver elastography, may be considered on an individual basis after SVR is attained, to assess whether fibrosis has progressed or regressed or to guide clinical management.
All patients who achieve SVR must be counseled regarding factors that could further injure the liver and contribute to the progression of fibrosis, hepatic decompensation, or the development of hepatocellular carcinoma. These include alcohol consumption, fatty liver, diabetes, and potential toxins. If serum liver enzyme levels rise, all patients should be evaluated for possible liver injury.
The AGA Institute issued a clinical practice update for managing hepatitis C virus–infected patients who achieve a sustained virologic response after antiviral therapy, who still require ongoing care for their liver disease. The expert review appears in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.018).
Even though direct-acting antiviral regimens have produced remarkably high sustained virologic response (SVR) rates and it appears that fewer than 1% of patients relapse, and even though liver fibrosis and cirrhosis may regress with this therapy, continued surveillance and even intervention may be needed “to reduce complications arising from liver damage that has already accrued by the time SVR was attained,” said Ira M. Jacobson, MD, AGAF, chair of the department of medicine, Mount Sinai Beth Israel Medical Center, New York, and his associates.
Dr. Jacobson and his associates at the AGA Institute reviewed the current literature and expert opinion to formulate 11 best-practice recommendations for managing this patient population. Among their recommendations:
SVR should be confirmed by hepatitis C virus RNA testing at 12 weeks after completion of an all-oral direct-acting antiviral regimen, and routine confirmation after 48 weeks is also “prudent.” Further testing for later virologic relapse is not supported by the available evidence. However, further periodic testing is advised for patients at risk for reinfection, such as those who continue to use IV drugs.
All patients with stage 3 or higher liver fibrosis or cirrhosis before achieving SVR should continue to be monitored by liver imaging (with or without serum alpha fetoprotein testing) twice a year “for an indefinite duration.” At present, there is no evidence of a finite point beyond which the risk of hepatocellular carcinoma is reduced to the level of people who don’t have a history of liver disease. And there have been documented cases of hepatocellular carcinoma developing more than 5 years after attaining SVR.
Regardless of SVR status, all patients with liver cirrhosis should undergo endoscopic screening for esophagogastric varices. If no varices or only small varices are detected, repeat endoscopy should be done 2-3 years after achieving SVR. If no varices are identified then, “cessation of further endoscopic screening may be considered on an individual patient basis if there are no risk factors for progressive cirrhosis.”
Noninvasive assessment of fibrosis, such as liver elastography, may be considered on an individual basis after SVR is attained, to assess whether fibrosis has progressed or regressed or to guide clinical management.
All patients who achieve SVR must be counseled regarding factors that could further injure the liver and contribute to the progression of fibrosis, hepatic decompensation, or the development of hepatocellular carcinoma. These include alcohol consumption, fatty liver, diabetes, and potential toxins. If serum liver enzyme levels rise, all patients should be evaluated for possible liver injury.
The AGA Institute issued a clinical practice update for managing hepatitis C virus–infected patients who achieve a sustained virologic response after antiviral therapy, who still require ongoing care for their liver disease. The expert review appears in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.018).
Even though direct-acting antiviral regimens have produced remarkably high sustained virologic response (SVR) rates and it appears that fewer than 1% of patients relapse, and even though liver fibrosis and cirrhosis may regress with this therapy, continued surveillance and even intervention may be needed “to reduce complications arising from liver damage that has already accrued by the time SVR was attained,” said Ira M. Jacobson, MD, AGAF, chair of the department of medicine, Mount Sinai Beth Israel Medical Center, New York, and his associates.
Dr. Jacobson and his associates at the AGA Institute reviewed the current literature and expert opinion to formulate 11 best-practice recommendations for managing this patient population. Among their recommendations:
SVR should be confirmed by hepatitis C virus RNA testing at 12 weeks after completion of an all-oral direct-acting antiviral regimen, and routine confirmation after 48 weeks is also “prudent.” Further testing for later virologic relapse is not supported by the available evidence. However, further periodic testing is advised for patients at risk for reinfection, such as those who continue to use IV drugs.
All patients with stage 3 or higher liver fibrosis or cirrhosis before achieving SVR should continue to be monitored by liver imaging (with or without serum alpha fetoprotein testing) twice a year “for an indefinite duration.” At present, there is no evidence of a finite point beyond which the risk of hepatocellular carcinoma is reduced to the level of people who don’t have a history of liver disease. And there have been documented cases of hepatocellular carcinoma developing more than 5 years after attaining SVR.
Regardless of SVR status, all patients with liver cirrhosis should undergo endoscopic screening for esophagogastric varices. If no varices or only small varices are detected, repeat endoscopy should be done 2-3 years after achieving SVR. If no varices are identified then, “cessation of further endoscopic screening may be considered on an individual patient basis if there are no risk factors for progressive cirrhosis.”
Noninvasive assessment of fibrosis, such as liver elastography, may be considered on an individual basis after SVR is attained, to assess whether fibrosis has progressed or regressed or to guide clinical management.
All patients who achieve SVR must be counseled regarding factors that could further injure the liver and contribute to the progression of fibrosis, hepatic decompensation, or the development of hepatocellular carcinoma. These include alcohol consumption, fatty liver, diabetes, and potential toxins. If serum liver enzyme levels rise, all patients should be evaluated for possible liver injury.
Key clinical point: The AGA Institute issued a clinical practice update for managing HCV patients who achieve a sustained virologic response after antiviral therapy, who still require ongoing care for their liver disease.
Major finding: SVR should be confirmed by HCV RNA testing at 12 weeks after completion of an all-oral direct-acting antiviral regimen, and routine confirmation after 48 weeks is also “prudent.”
Data source: A review of the literature and of expert opinion to compile 11 best-practice recommendations for managing post-SVR HCV care.
Disclosures: This work was supported by the AGA Institute. Dr. Jacobson reported ties to AbbVie, Bristol-Myers Squibb, Gilead, Intercept, Janssen, Merck, and Trek; one of his associates reported ties to those groups and to Target PharmaSolutions.