User login
Youngest children more likely to spread SARS-CoV-2 to family: Study
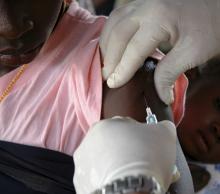
Young children are more likely than are their older siblings to transmit SARS-CoV-2 in their households, according to an analysis of public health records in Ontario, Canada – a finding that upends the common belief that children play a minimal role in COVID-19 spread.
The study by researchers from Public Health Ontario, published online in JAMA Pediatrics, found that teenagers (14- to 17-year-olds) were more likely than were their younger siblings to bring the virus into the household, while infants and toddlers (up to age 3) were about 43% more likely than were the older teens to spread it to others in the home.
Children or teens were the source of SARS-CoV-2 in about 1 in 13 Ontario households between June and December 2020, the study shows. The researchers analyzed health records from 6,280 households with a pediatric COVID-19 case and a subset of 1,717 households in which a child up to age 17 was the source of transmission in a household.
When analyzing the data, the researchers controlled for gender differences, month of disease onset, testing delay, and mean family size.
The role of young children in transmission seemed logical to some experts who have been tracking the evolution of the pandemic. “I think what was more surprising was how long the narrative persisted that children weren’t transmitting SARS-CoV-2,” said Samuel Scarpino, PhD, managing director of pathogen surveillance at the Rockefeller Foundation.
Meanwhile, less mask-wearing, the return to school and activities, and the onslaught of the Delta variant have changed the dynamics of spread, said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah.
“Adolescents and high-school-aged kids have had much, much higher rates of infection in the past,” he said. “Now when we look at the rates of school-aged kids, they are the same as high-school-aged kids, and we’re seeing more and more in the preschool age groups.”
Cases may be underestimated
If anything, the study may underestimate the role young children play in spreading COVID-19 in families, since it included only symptomatic cases as the initial source and young children are more likely to be asymptomatic, Dr. Pavia said.
The Delta variant heightens the concern; it is more than twice as infectious as previous strains and has spurred a rise in pediatric cases, including some coinfection with other circulating respiratory diseases, such as respiratory syncytial virus (RSV).
The Ontario study covers a period before vaccination and the spread of the Delta variant. “As the number of pediatric cases increases worldwide, the role of children in household transmission will continue to grow,” the authors concluded.
Following recommended respiratory hygiene is clearly more difficult with very young children. For example, parents, caregivers, and older siblings aren’t going to stay 6 feet away from a sick baby or toddler, Susan Coffin, MD, MPH, a pediatric infectious disease physician, and David Rubin, MD, a pediatrician and director of PolicyLab at Children’s Hospital of Philadelphia, noted in an accompanying commentary.
“Cuddling and touching are part and parcel of taking care of a sick young child, and that will obviously come with an increased risk of transmission to parents as well as to older siblings who may be helping to care for their sick brother or sister,” they wrote.
While parents may wash their hands more frequently when caring for a sick child, they aren’t likely to wear a mask, said William Schaffner, MD, an infectious disease specialist at Vanderbilt University, Nashville, Tenn.
“I imagine some moms even take a sick child into bed with them,” he said. “It’s probably just the extensive contact one has with a sick, very small child that augments their capacity to transmit this infection.”
What can be done
What can be done, then, to reduce the household spread of COVID-19? “The obvious solution to protect a household with a sick young infant or toddler is to make sure that all eligible members of the household are vaccinated,” Dr. Coffin and Dr. Rubin stated in their commentary.
The American Academy of Pediatrics recently wrote to Janet Woodcock, MD, acting commissioner of the Food and Drug Administration, asking for the agency to authorize use of SARS-CoV-2 vaccines for children under age 12 “as soon as possible,” noting that “the Delta variant has created a new and pressing risk to children and adolescents across this country, as it has also done for unvaccinated adults.”
The FDA reportedly asked vaccine makers Pfizer and Moderna to expand the clinical trials of children, which may delay authorization for younger age groups. Pfizer has said it plans to submit a request for emergency use authorization of its vaccine for 5- to 11-year-olds in September or October.
As with adult vaccination, hesitancy is likely to be a barrier. Less than half of parents said they are very or somewhat likely to have their children get a COVID-19 vaccine, according to a national survey conducted by researchers at the University of California, Los Angeles.
The Ontario study provides valuable evidence to support taking steps to protect children from transmission in schools, including mask requirements, frequent testing, and improved ventilation, said Dr. Scarpino.
“We’re not going to be able to control COVID without vaccinating younger individuals,” he said.
Dr. Pavia has consulted for GlaxoSmithKline on non–COVID-19–related issues. Sarah Buchan, PhD, study author and scientist at Public Health Ontario, reported grants from the Canadian Institutes of Health Research for research on influenza, RSV, and COVID-19, and grants from the Canadian Immunity Task Force for COVID-19 outside the submitted work. Dr. Coffin reported grants as a Centers for Disease Control and Prevention coinvestigator at a Vaccine and Treatment Evaluation Unit site conducting COVID-19 vaccine trials in children. Dr. Scarpino holds unexercised options in ILiAD Biotechnologies, which is focused on the prevention and treatment of pertussis. Dr. Schaffner is a consultant for VBI Vaccines.
A version of this article first appeared on Medscape.com.
Young children are more likely than are their older siblings to transmit SARS-CoV-2 in their households, according to an analysis of public health records in Ontario, Canada – a finding that upends the common belief that children play a minimal role in COVID-19 spread.
The study by researchers from Public Health Ontario, published online in JAMA Pediatrics, found that teenagers (14- to 17-year-olds) were more likely than were their younger siblings to bring the virus into the household, while infants and toddlers (up to age 3) were about 43% more likely than were the older teens to spread it to others in the home.
Children or teens were the source of SARS-CoV-2 in about 1 in 13 Ontario households between June and December 2020, the study shows. The researchers analyzed health records from 6,280 households with a pediatric COVID-19 case and a subset of 1,717 households in which a child up to age 17 was the source of transmission in a household.
When analyzing the data, the researchers controlled for gender differences, month of disease onset, testing delay, and mean family size.
The role of young children in transmission seemed logical to some experts who have been tracking the evolution of the pandemic. “I think what was more surprising was how long the narrative persisted that children weren’t transmitting SARS-CoV-2,” said Samuel Scarpino, PhD, managing director of pathogen surveillance at the Rockefeller Foundation.
Meanwhile, less mask-wearing, the return to school and activities, and the onslaught of the Delta variant have changed the dynamics of spread, said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah.
“Adolescents and high-school-aged kids have had much, much higher rates of infection in the past,” he said. “Now when we look at the rates of school-aged kids, they are the same as high-school-aged kids, and we’re seeing more and more in the preschool age groups.”
Cases may be underestimated
If anything, the study may underestimate the role young children play in spreading COVID-19 in families, since it included only symptomatic cases as the initial source and young children are more likely to be asymptomatic, Dr. Pavia said.
The Delta variant heightens the concern; it is more than twice as infectious as previous strains and has spurred a rise in pediatric cases, including some coinfection with other circulating respiratory diseases, such as respiratory syncytial virus (RSV).
The Ontario study covers a period before vaccination and the spread of the Delta variant. “As the number of pediatric cases increases worldwide, the role of children in household transmission will continue to grow,” the authors concluded.
Following recommended respiratory hygiene is clearly more difficult with very young children. For example, parents, caregivers, and older siblings aren’t going to stay 6 feet away from a sick baby or toddler, Susan Coffin, MD, MPH, a pediatric infectious disease physician, and David Rubin, MD, a pediatrician and director of PolicyLab at Children’s Hospital of Philadelphia, noted in an accompanying commentary.
“Cuddling and touching are part and parcel of taking care of a sick young child, and that will obviously come with an increased risk of transmission to parents as well as to older siblings who may be helping to care for their sick brother or sister,” they wrote.
While parents may wash their hands more frequently when caring for a sick child, they aren’t likely to wear a mask, said William Schaffner, MD, an infectious disease specialist at Vanderbilt University, Nashville, Tenn.
“I imagine some moms even take a sick child into bed with them,” he said. “It’s probably just the extensive contact one has with a sick, very small child that augments their capacity to transmit this infection.”
What can be done
What can be done, then, to reduce the household spread of COVID-19? “The obvious solution to protect a household with a sick young infant or toddler is to make sure that all eligible members of the household are vaccinated,” Dr. Coffin and Dr. Rubin stated in their commentary.
The American Academy of Pediatrics recently wrote to Janet Woodcock, MD, acting commissioner of the Food and Drug Administration, asking for the agency to authorize use of SARS-CoV-2 vaccines for children under age 12 “as soon as possible,” noting that “the Delta variant has created a new and pressing risk to children and adolescents across this country, as it has also done for unvaccinated adults.”
The FDA reportedly asked vaccine makers Pfizer and Moderna to expand the clinical trials of children, which may delay authorization for younger age groups. Pfizer has said it plans to submit a request for emergency use authorization of its vaccine for 5- to 11-year-olds in September or October.
As with adult vaccination, hesitancy is likely to be a barrier. Less than half of parents said they are very or somewhat likely to have their children get a COVID-19 vaccine, according to a national survey conducted by researchers at the University of California, Los Angeles.
The Ontario study provides valuable evidence to support taking steps to protect children from transmission in schools, including mask requirements, frequent testing, and improved ventilation, said Dr. Scarpino.
“We’re not going to be able to control COVID without vaccinating younger individuals,” he said.
Dr. Pavia has consulted for GlaxoSmithKline on non–COVID-19–related issues. Sarah Buchan, PhD, study author and scientist at Public Health Ontario, reported grants from the Canadian Institutes of Health Research for research on influenza, RSV, and COVID-19, and grants from the Canadian Immunity Task Force for COVID-19 outside the submitted work. Dr. Coffin reported grants as a Centers for Disease Control and Prevention coinvestigator at a Vaccine and Treatment Evaluation Unit site conducting COVID-19 vaccine trials in children. Dr. Scarpino holds unexercised options in ILiAD Biotechnologies, which is focused on the prevention and treatment of pertussis. Dr. Schaffner is a consultant for VBI Vaccines.
A version of this article first appeared on Medscape.com.
Young children are more likely than are their older siblings to transmit SARS-CoV-2 in their households, according to an analysis of public health records in Ontario, Canada – a finding that upends the common belief that children play a minimal role in COVID-19 spread.
The study by researchers from Public Health Ontario, published online in JAMA Pediatrics, found that teenagers (14- to 17-year-olds) were more likely than were their younger siblings to bring the virus into the household, while infants and toddlers (up to age 3) were about 43% more likely than were the older teens to spread it to others in the home.
Children or teens were the source of SARS-CoV-2 in about 1 in 13 Ontario households between June and December 2020, the study shows. The researchers analyzed health records from 6,280 households with a pediatric COVID-19 case and a subset of 1,717 households in which a child up to age 17 was the source of transmission in a household.
When analyzing the data, the researchers controlled for gender differences, month of disease onset, testing delay, and mean family size.
The role of young children in transmission seemed logical to some experts who have been tracking the evolution of the pandemic. “I think what was more surprising was how long the narrative persisted that children weren’t transmitting SARS-CoV-2,” said Samuel Scarpino, PhD, managing director of pathogen surveillance at the Rockefeller Foundation.
Meanwhile, less mask-wearing, the return to school and activities, and the onslaught of the Delta variant have changed the dynamics of spread, said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah.
“Adolescents and high-school-aged kids have had much, much higher rates of infection in the past,” he said. “Now when we look at the rates of school-aged kids, they are the same as high-school-aged kids, and we’re seeing more and more in the preschool age groups.”
Cases may be underestimated
If anything, the study may underestimate the role young children play in spreading COVID-19 in families, since it included only symptomatic cases as the initial source and young children are more likely to be asymptomatic, Dr. Pavia said.
The Delta variant heightens the concern; it is more than twice as infectious as previous strains and has spurred a rise in pediatric cases, including some coinfection with other circulating respiratory diseases, such as respiratory syncytial virus (RSV).
The Ontario study covers a period before vaccination and the spread of the Delta variant. “As the number of pediatric cases increases worldwide, the role of children in household transmission will continue to grow,” the authors concluded.
Following recommended respiratory hygiene is clearly more difficult with very young children. For example, parents, caregivers, and older siblings aren’t going to stay 6 feet away from a sick baby or toddler, Susan Coffin, MD, MPH, a pediatric infectious disease physician, and David Rubin, MD, a pediatrician and director of PolicyLab at Children’s Hospital of Philadelphia, noted in an accompanying commentary.
“Cuddling and touching are part and parcel of taking care of a sick young child, and that will obviously come with an increased risk of transmission to parents as well as to older siblings who may be helping to care for their sick brother or sister,” they wrote.
While parents may wash their hands more frequently when caring for a sick child, they aren’t likely to wear a mask, said William Schaffner, MD, an infectious disease specialist at Vanderbilt University, Nashville, Tenn.
“I imagine some moms even take a sick child into bed with them,” he said. “It’s probably just the extensive contact one has with a sick, very small child that augments their capacity to transmit this infection.”
What can be done
What can be done, then, to reduce the household spread of COVID-19? “The obvious solution to protect a household with a sick young infant or toddler is to make sure that all eligible members of the household are vaccinated,” Dr. Coffin and Dr. Rubin stated in their commentary.
The American Academy of Pediatrics recently wrote to Janet Woodcock, MD, acting commissioner of the Food and Drug Administration, asking for the agency to authorize use of SARS-CoV-2 vaccines for children under age 12 “as soon as possible,” noting that “the Delta variant has created a new and pressing risk to children and adolescents across this country, as it has also done for unvaccinated adults.”
The FDA reportedly asked vaccine makers Pfizer and Moderna to expand the clinical trials of children, which may delay authorization for younger age groups. Pfizer has said it plans to submit a request for emergency use authorization of its vaccine for 5- to 11-year-olds in September or October.
As with adult vaccination, hesitancy is likely to be a barrier. Less than half of parents said they are very or somewhat likely to have their children get a COVID-19 vaccine, according to a national survey conducted by researchers at the University of California, Los Angeles.
The Ontario study provides valuable evidence to support taking steps to protect children from transmission in schools, including mask requirements, frequent testing, and improved ventilation, said Dr. Scarpino.
“We’re not going to be able to control COVID without vaccinating younger individuals,” he said.
Dr. Pavia has consulted for GlaxoSmithKline on non–COVID-19–related issues. Sarah Buchan, PhD, study author and scientist at Public Health Ontario, reported grants from the Canadian Institutes of Health Research for research on influenza, RSV, and COVID-19, and grants from the Canadian Immunity Task Force for COVID-19 outside the submitted work. Dr. Coffin reported grants as a Centers for Disease Control and Prevention coinvestigator at a Vaccine and Treatment Evaluation Unit site conducting COVID-19 vaccine trials in children. Dr. Scarpino holds unexercised options in ILiAD Biotechnologies, which is focused on the prevention and treatment of pertussis. Dr. Schaffner is a consultant for VBI Vaccines.
A version of this article first appeared on Medscape.com.
FDA to add myocarditis warning to mRNA COVID-19 vaccines
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
Milk is overtaking nuts as top food allergy threat
When Lesley Solomon’s son was 10 years old, he was standing in an unlucky spot on the playground when a schoolmate kicked over a cup of hot chocolate, sending droplets flying into the air. For the young boy with a severe milk allergy, the hot liquid splattering was less of a hazard for him than the dairy stirred into the drink.
Ms. Solomon’s son quickly washed the fluids off his clothes and skin, took some Benadryl, and called his parents. But on the car ride home, his throat began to close and his pulse raced. It was one of about a dozen times he has needed an epinephrine injection, which increases blood flow, reduces swelling, and reverses anaphylaxis.
“Until you see a child going through that anaphylaxis and not being able to breathe, or throwing up so much that they can’t breathe, you don’t understand” how serious food allergies can be, said Ms. Solomon, who is senior vice president and chief innovation officer of the Dana-Farber Cancer Institute in Boston and cofounder of the Food Allergy Science Initiative, an independent nonprofit that funds food allergy research.
The rate of children hospitalized for food-induced anaphylaxis rose by 25% from 2006 to 2012 – from 1.2 to 1.5 per 100,000 – according to a 2019 analysis of data from pediatric hospitals in the United States. And severe symptoms were more often linked to milk than to peanuts or tree nuts, the study showed.
Cow’s milk is the most common food allergy in children aged younger than 5 years, and accounts for about half of all food allergies in children younger than 1. Most children grow out of it, but when milk allergy persists into the teenage years and adulthood, it is more likely to cause severe reactions.
A dangerous allergy
“Cow’s milk allergy is the most distressing of the food allergies. Many people are unaware that it can cause anaphylaxis that is so severe,” said Carla Davis, MD, director of the food allergy program at the Texas Children’s Hospital in Houston. “People do not think about how much of this is in our food.”
And cow’s milk was shown to be the food allergy most likely to lead to death in school-aged children in the United Kingdom, according to an analysis of national data reported by this news organization.
Lack of awareness is what makes milk allergy so dangerous, said Paul Turner, BMBCh, PhD, a pediatric allergist and immunologist from Imperial College London, who was involved in the British analysis. “We need to get that information out to the public and businesses so they take the same level of care that they have with nuts, and when someone says they have milk allergy, they take it seriously.
In food allergy, the body treats certain proteins, such as the casein and whey in milk, as invaders, mounting an immune response. Antibodies known as IgE – which normally protect against bacteria, viruses, and parasites – trigger inflammation, the release of histamine, and can lead to symptoms, typically within minutes, ranging from rash and swelling to vomiting, difficulty swallowing, and difficulty breathing.
So, the very thing that makes milk a healthy choice for kids – its high protein content – can cause serious reactions in a small portion of children and adults. “You don’t need much milk to get a decent dose” of the allergen, Dr. Turner pointed out.
The mechanisms of milk allergy are complex, even compared with other food allergies. The IgE antibody can be detected with a skin-prick test or IgE blood test, but some people have positive results even though they are not allergic. To complicate things further, people can also have non–IgE-mediated milk allergy, which cannot be detected with testing and can lead to symptoms that emerge hours or even days after exposure.
More serious than lactose intolerance
Unfortunately, milk allergy is often confused with a milk-related digestive problem. Globally, about 70% of people lack the enzyme to break down the sugar in milk; the condition, known as lactose intolerance, can cause bloating, abdominal cramps, and diarrhea but is not life-threatening.
“Because lactose intolerance is so common, people don’t think of milk allergy as something that can be significant or severe,” said Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at the Northwestern University, Chicago.
In babies, colic, the regurgitation of milk-based formula, and rash are sometimes misinterpreted as a milk allergy, leading parents to buy expensive, specialized formula unnecessarily.
Frustrated by a lack of data about food allergies, Dr. Gupta and colleagues launched a nationally representative survey of 38,408 American parents in 2009, which was updated in 2015 and 2016.
On average, children with milk allergy had their first reaction before the age of 2, most commonly vomiting, diarrhea, hives, and eczema; this is a younger age of onset than for other food allergies. And children with milk allergy were twice as likely as children with other allergies to grow out of it.
Yet about one-third of milk-allergic children in the updated study were 11 years and older. And in a similar survey of adults who self-reported symptoms, milk allergy was as common as peanut allergy (1.9% vs 1.8%). “We don’t know why milk allergy is becoming more persistent,” Dr. Gupta said. And, she warned, only one in four children with a milk allergy had a current prescription for an epinephrine autoinjector, compared with about 70% of children with peanut allergy.
Food allergy can’t be caused by genetics alone, said Christine Olsen, MD, cofounder and CEO of the Food Allergy Science Initiative at the Broad Institute in Cambridge, Mass. “There may be a genetic predisposition, but there must be something environmental” that has influenced the development of food allergies.
One theory is that the body’s natural defense against noxious substances has been disrupted in the modern world by processed foods, chemical additives, and hygienic surroundings.
Dr. Olson’s own son vomited when he had his first small taste of hummus as a baby; he is severely allergic to sesame. The immediacy of his bodily reaction made Dr. Olsen think that the response involved neurons, not just a misguided immune system.
Researchers are currently looking for drug targets that could shut off the immune response as quickly as it starts. If you think of the fact that some kids outgrow their allergies and some adults get allergies, that suggests there’s some lever that you can turn on and off,” said Dr. Olsen, who is also a radiation oncologist.
Preventing allergy
The approach to food allergy prevention has already been transformed by the Learning Early About Peanut Allergy (LEAP) study conducted in the United Kingdom. LEAP investigators randomly assigned 640 infants to ingest regular amounts of peanut snacks or peanut butter or to avoid peanut products until they reached 5 years of age. The babies who had regular exposure to peanut from an early age were much less likely to develop a peanut allergy than those who avoided peanuts.
The National Institute of Allergy and Infectious Diseases revised its guidelines and now recommends that all babies be exposed to peanut-containing food at around 6 months of age; for high-risk babies, that can start as early as 4 months.
Allergy experts are planning to study that concept again with other foods, including cow’s milk. The 5-year iREACH study, launched by the Center for Food Allergy & Asthma Research at Northwestern and Lurie Children’s Hospital in Chicago, is currently enrolling 10,500 infants to test early exposure to peanuts, milk, egg, and cashew. A portion of the infants will have severe eczema, putting them at high risk for food allergies, and others will be low risk, said Dr. Gupta, who is the principal iREACH investigator.
“Hopefully in the next 5 years we will have data showing whether this prevention technique will work for other common food allergens, in addition to peanuts,” she said.
Introducing foods early “promotes tolerance rather than early sensitization,” explained Stephanie Leeds, MD, an allergist and immunologist at Yale University, New Haven, Conn. In the future, rather than just diagnosing and treating food allergies, allergists might work with pediatricians to help prevent them from ever happening.
A version of this article first appeared on Medscape.com.
When Lesley Solomon’s son was 10 years old, he was standing in an unlucky spot on the playground when a schoolmate kicked over a cup of hot chocolate, sending droplets flying into the air. For the young boy with a severe milk allergy, the hot liquid splattering was less of a hazard for him than the dairy stirred into the drink.
Ms. Solomon’s son quickly washed the fluids off his clothes and skin, took some Benadryl, and called his parents. But on the car ride home, his throat began to close and his pulse raced. It was one of about a dozen times he has needed an epinephrine injection, which increases blood flow, reduces swelling, and reverses anaphylaxis.
“Until you see a child going through that anaphylaxis and not being able to breathe, or throwing up so much that they can’t breathe, you don’t understand” how serious food allergies can be, said Ms. Solomon, who is senior vice president and chief innovation officer of the Dana-Farber Cancer Institute in Boston and cofounder of the Food Allergy Science Initiative, an independent nonprofit that funds food allergy research.
The rate of children hospitalized for food-induced anaphylaxis rose by 25% from 2006 to 2012 – from 1.2 to 1.5 per 100,000 – according to a 2019 analysis of data from pediatric hospitals in the United States. And severe symptoms were more often linked to milk than to peanuts or tree nuts, the study showed.
Cow’s milk is the most common food allergy in children aged younger than 5 years, and accounts for about half of all food allergies in children younger than 1. Most children grow out of it, but when milk allergy persists into the teenage years and adulthood, it is more likely to cause severe reactions.
A dangerous allergy
“Cow’s milk allergy is the most distressing of the food allergies. Many people are unaware that it can cause anaphylaxis that is so severe,” said Carla Davis, MD, director of the food allergy program at the Texas Children’s Hospital in Houston. “People do not think about how much of this is in our food.”
And cow’s milk was shown to be the food allergy most likely to lead to death in school-aged children in the United Kingdom, according to an analysis of national data reported by this news organization.
Lack of awareness is what makes milk allergy so dangerous, said Paul Turner, BMBCh, PhD, a pediatric allergist and immunologist from Imperial College London, who was involved in the British analysis. “We need to get that information out to the public and businesses so they take the same level of care that they have with nuts, and when someone says they have milk allergy, they take it seriously.
In food allergy, the body treats certain proteins, such as the casein and whey in milk, as invaders, mounting an immune response. Antibodies known as IgE – which normally protect against bacteria, viruses, and parasites – trigger inflammation, the release of histamine, and can lead to symptoms, typically within minutes, ranging from rash and swelling to vomiting, difficulty swallowing, and difficulty breathing.
So, the very thing that makes milk a healthy choice for kids – its high protein content – can cause serious reactions in a small portion of children and adults. “You don’t need much milk to get a decent dose” of the allergen, Dr. Turner pointed out.
The mechanisms of milk allergy are complex, even compared with other food allergies. The IgE antibody can be detected with a skin-prick test or IgE blood test, but some people have positive results even though they are not allergic. To complicate things further, people can also have non–IgE-mediated milk allergy, which cannot be detected with testing and can lead to symptoms that emerge hours or even days after exposure.
More serious than lactose intolerance
Unfortunately, milk allergy is often confused with a milk-related digestive problem. Globally, about 70% of people lack the enzyme to break down the sugar in milk; the condition, known as lactose intolerance, can cause bloating, abdominal cramps, and diarrhea but is not life-threatening.
“Because lactose intolerance is so common, people don’t think of milk allergy as something that can be significant or severe,” said Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at the Northwestern University, Chicago.
In babies, colic, the regurgitation of milk-based formula, and rash are sometimes misinterpreted as a milk allergy, leading parents to buy expensive, specialized formula unnecessarily.
Frustrated by a lack of data about food allergies, Dr. Gupta and colleagues launched a nationally representative survey of 38,408 American parents in 2009, which was updated in 2015 and 2016.
On average, children with milk allergy had their first reaction before the age of 2, most commonly vomiting, diarrhea, hives, and eczema; this is a younger age of onset than for other food allergies. And children with milk allergy were twice as likely as children with other allergies to grow out of it.
Yet about one-third of milk-allergic children in the updated study were 11 years and older. And in a similar survey of adults who self-reported symptoms, milk allergy was as common as peanut allergy (1.9% vs 1.8%). “We don’t know why milk allergy is becoming more persistent,” Dr. Gupta said. And, she warned, only one in four children with a milk allergy had a current prescription for an epinephrine autoinjector, compared with about 70% of children with peanut allergy.
Food allergy can’t be caused by genetics alone, said Christine Olsen, MD, cofounder and CEO of the Food Allergy Science Initiative at the Broad Institute in Cambridge, Mass. “There may be a genetic predisposition, but there must be something environmental” that has influenced the development of food allergies.
One theory is that the body’s natural defense against noxious substances has been disrupted in the modern world by processed foods, chemical additives, and hygienic surroundings.
Dr. Olson’s own son vomited when he had his first small taste of hummus as a baby; he is severely allergic to sesame. The immediacy of his bodily reaction made Dr. Olsen think that the response involved neurons, not just a misguided immune system.
Researchers are currently looking for drug targets that could shut off the immune response as quickly as it starts. If you think of the fact that some kids outgrow their allergies and some adults get allergies, that suggests there’s some lever that you can turn on and off,” said Dr. Olsen, who is also a radiation oncologist.
Preventing allergy
The approach to food allergy prevention has already been transformed by the Learning Early About Peanut Allergy (LEAP) study conducted in the United Kingdom. LEAP investigators randomly assigned 640 infants to ingest regular amounts of peanut snacks or peanut butter or to avoid peanut products until they reached 5 years of age. The babies who had regular exposure to peanut from an early age were much less likely to develop a peanut allergy than those who avoided peanuts.
The National Institute of Allergy and Infectious Diseases revised its guidelines and now recommends that all babies be exposed to peanut-containing food at around 6 months of age; for high-risk babies, that can start as early as 4 months.
Allergy experts are planning to study that concept again with other foods, including cow’s milk. The 5-year iREACH study, launched by the Center for Food Allergy & Asthma Research at Northwestern and Lurie Children’s Hospital in Chicago, is currently enrolling 10,500 infants to test early exposure to peanuts, milk, egg, and cashew. A portion of the infants will have severe eczema, putting them at high risk for food allergies, and others will be low risk, said Dr. Gupta, who is the principal iREACH investigator.
“Hopefully in the next 5 years we will have data showing whether this prevention technique will work for other common food allergens, in addition to peanuts,” she said.
Introducing foods early “promotes tolerance rather than early sensitization,” explained Stephanie Leeds, MD, an allergist and immunologist at Yale University, New Haven, Conn. In the future, rather than just diagnosing and treating food allergies, allergists might work with pediatricians to help prevent them from ever happening.
A version of this article first appeared on Medscape.com.
When Lesley Solomon’s son was 10 years old, he was standing in an unlucky spot on the playground when a schoolmate kicked over a cup of hot chocolate, sending droplets flying into the air. For the young boy with a severe milk allergy, the hot liquid splattering was less of a hazard for him than the dairy stirred into the drink.
Ms. Solomon’s son quickly washed the fluids off his clothes and skin, took some Benadryl, and called his parents. But on the car ride home, his throat began to close and his pulse raced. It was one of about a dozen times he has needed an epinephrine injection, which increases blood flow, reduces swelling, and reverses anaphylaxis.
“Until you see a child going through that anaphylaxis and not being able to breathe, or throwing up so much that they can’t breathe, you don’t understand” how serious food allergies can be, said Ms. Solomon, who is senior vice president and chief innovation officer of the Dana-Farber Cancer Institute in Boston and cofounder of the Food Allergy Science Initiative, an independent nonprofit that funds food allergy research.
The rate of children hospitalized for food-induced anaphylaxis rose by 25% from 2006 to 2012 – from 1.2 to 1.5 per 100,000 – according to a 2019 analysis of data from pediatric hospitals in the United States. And severe symptoms were more often linked to milk than to peanuts or tree nuts, the study showed.
Cow’s milk is the most common food allergy in children aged younger than 5 years, and accounts for about half of all food allergies in children younger than 1. Most children grow out of it, but when milk allergy persists into the teenage years and adulthood, it is more likely to cause severe reactions.
A dangerous allergy
“Cow’s milk allergy is the most distressing of the food allergies. Many people are unaware that it can cause anaphylaxis that is so severe,” said Carla Davis, MD, director of the food allergy program at the Texas Children’s Hospital in Houston. “People do not think about how much of this is in our food.”
And cow’s milk was shown to be the food allergy most likely to lead to death in school-aged children in the United Kingdom, according to an analysis of national data reported by this news organization.
Lack of awareness is what makes milk allergy so dangerous, said Paul Turner, BMBCh, PhD, a pediatric allergist and immunologist from Imperial College London, who was involved in the British analysis. “We need to get that information out to the public and businesses so they take the same level of care that they have with nuts, and when someone says they have milk allergy, they take it seriously.
In food allergy, the body treats certain proteins, such as the casein and whey in milk, as invaders, mounting an immune response. Antibodies known as IgE – which normally protect against bacteria, viruses, and parasites – trigger inflammation, the release of histamine, and can lead to symptoms, typically within minutes, ranging from rash and swelling to vomiting, difficulty swallowing, and difficulty breathing.
So, the very thing that makes milk a healthy choice for kids – its high protein content – can cause serious reactions in a small portion of children and adults. “You don’t need much milk to get a decent dose” of the allergen, Dr. Turner pointed out.
The mechanisms of milk allergy are complex, even compared with other food allergies. The IgE antibody can be detected with a skin-prick test or IgE blood test, but some people have positive results even though they are not allergic. To complicate things further, people can also have non–IgE-mediated milk allergy, which cannot be detected with testing and can lead to symptoms that emerge hours or even days after exposure.
More serious than lactose intolerance
Unfortunately, milk allergy is often confused with a milk-related digestive problem. Globally, about 70% of people lack the enzyme to break down the sugar in milk; the condition, known as lactose intolerance, can cause bloating, abdominal cramps, and diarrhea but is not life-threatening.
“Because lactose intolerance is so common, people don’t think of milk allergy as something that can be significant or severe,” said Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at the Northwestern University, Chicago.
In babies, colic, the regurgitation of milk-based formula, and rash are sometimes misinterpreted as a milk allergy, leading parents to buy expensive, specialized formula unnecessarily.
Frustrated by a lack of data about food allergies, Dr. Gupta and colleagues launched a nationally representative survey of 38,408 American parents in 2009, which was updated in 2015 and 2016.
On average, children with milk allergy had their first reaction before the age of 2, most commonly vomiting, diarrhea, hives, and eczema; this is a younger age of onset than for other food allergies. And children with milk allergy were twice as likely as children with other allergies to grow out of it.
Yet about one-third of milk-allergic children in the updated study were 11 years and older. And in a similar survey of adults who self-reported symptoms, milk allergy was as common as peanut allergy (1.9% vs 1.8%). “We don’t know why milk allergy is becoming more persistent,” Dr. Gupta said. And, she warned, only one in four children with a milk allergy had a current prescription for an epinephrine autoinjector, compared with about 70% of children with peanut allergy.
Food allergy can’t be caused by genetics alone, said Christine Olsen, MD, cofounder and CEO of the Food Allergy Science Initiative at the Broad Institute in Cambridge, Mass. “There may be a genetic predisposition, but there must be something environmental” that has influenced the development of food allergies.
One theory is that the body’s natural defense against noxious substances has been disrupted in the modern world by processed foods, chemical additives, and hygienic surroundings.
Dr. Olson’s own son vomited when he had his first small taste of hummus as a baby; he is severely allergic to sesame. The immediacy of his bodily reaction made Dr. Olsen think that the response involved neurons, not just a misguided immune system.
Researchers are currently looking for drug targets that could shut off the immune response as quickly as it starts. If you think of the fact that some kids outgrow their allergies and some adults get allergies, that suggests there’s some lever that you can turn on and off,” said Dr. Olsen, who is also a radiation oncologist.
Preventing allergy
The approach to food allergy prevention has already been transformed by the Learning Early About Peanut Allergy (LEAP) study conducted in the United Kingdom. LEAP investigators randomly assigned 640 infants to ingest regular amounts of peanut snacks or peanut butter or to avoid peanut products until they reached 5 years of age. The babies who had regular exposure to peanut from an early age were much less likely to develop a peanut allergy than those who avoided peanuts.
The National Institute of Allergy and Infectious Diseases revised its guidelines and now recommends that all babies be exposed to peanut-containing food at around 6 months of age; for high-risk babies, that can start as early as 4 months.
Allergy experts are planning to study that concept again with other foods, including cow’s milk. The 5-year iREACH study, launched by the Center for Food Allergy & Asthma Research at Northwestern and Lurie Children’s Hospital in Chicago, is currently enrolling 10,500 infants to test early exposure to peanuts, milk, egg, and cashew. A portion of the infants will have severe eczema, putting them at high risk for food allergies, and others will be low risk, said Dr. Gupta, who is the principal iREACH investigator.
“Hopefully in the next 5 years we will have data showing whether this prevention technique will work for other common food allergens, in addition to peanuts,” she said.
Introducing foods early “promotes tolerance rather than early sensitization,” explained Stephanie Leeds, MD, an allergist and immunologist at Yale University, New Haven, Conn. In the future, rather than just diagnosing and treating food allergies, allergists might work with pediatricians to help prevent them from ever happening.
A version of this article first appeared on Medscape.com.
Everyday chemicals are linked to declines in human fertility
Chemicals that pervade our modern world – plastics, pesticides, stain repellents, components of personal hygiene products – are contributing to a decades-long decline in fertility and could pose health risks even into future generations, according to an explosive new book by Shanna Swan, PhD, an environmental and reproductive epidemiologist at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Swan laid out the case that endocrine-disrupting chemicals (EDCs) such as phthalates and bisphenol A (BPA) threaten human existence, a conclusion that stems in part from her 2017 meta-analysis that showed a 52% drop in sperm counts from 1973 to 2011 in men in North America, Europe, and Australia.
“This alarming rate of decline could mean the human race will be unable to reproduce itself if the trend continues,” Dr. Swan said in her book, “Count Down: How Our Modern World Is Threatening Sperm Counts, Altering Male and Female Reproductive Development, and Imperiling the Future of the Human Race,” (New York: Scribner, 2021) coauthored with health journalist Stacey Colino.
Her premise that EDCs pose a risk to both male and female fertility is underscored by new research. A March 2021 article in Human Reproduction links prenatal chemical exposures to lowered fertility in a study of 1,045 Swiss military conscripts.
The Swiss men, aged 18-22 years, were significantly more likely to have low semen volume and low total sperm count if their mothers reported that they had occupational exposures to four or more endocrine-disrupting chemicals while they were pregnant. These EDCs, which mimic natural hormones, included pesticides, heavy metals, phthalates, alkylphenolic compounds, and solvents that can be found in agricultural work or hair and beauty salons.
These chemicals are not so-called “forever chemicals” that persist in the human body. But the Swiss study still showed an association between exposure during pregnancy and the future fertility of the male children. “Those apparently small exposures that pass quickly can affect development,” said Dr. Swan, who was not affiliated with the research. “It takes very little in terms of time and amount of chemicals to alter fetal development.”
Health risks beyond reproduction
While Count Down is placing a new spotlight on chemical hazards, some major medical organizations have already taken positions on the risks. “Reducing exposure to toxic environmental agents is a critical area of intervention for ob.gyns.,” the American College of Obstetricians and Gynecologists said in an environmental policy priority. “The Endocrine Society is concerned that human health is at risk because the current extensive scientific knowledge on EDCs and their health effects is not effectively translated to regulatory policies that fully protect populations from EDC exposures.”
But for the medical community, addressing the impact of EDCs goes beyond advocacy for regulatory and legislative changes, Dr. Swan said in an interview. Physicians should talk to patients about the importance of reducing their chemical exposure to safeguard their overall health.
“Reproductive health and particularly sperm count, subfertility, and infertility are predictors of lifelong health,” she said. That includes associations between reproductive disorders and “the risk of heart disease, obesity, reproductive cancers and, perhaps most dramatically, with a shortened lifespan.”
Some medical schools are including information on environmental health and exposure risks in the curriculum, said Tracey Woodruff, PhD, MPH, director of the program on reproductive health and the environment at the University of California, San Francisco. She urged physicians to ask patients about potential occupational exposures to hazardous chemicals and provide information about ways to reduce everyday exposures.
For example, safer options include buying organic produce, microwaving food in glass rather than plastic containers and avoiding products that contain phthalate or BPA. “If you’re going to talk to people about what they eat, that’s a perfect venue for talking about the environment,” said Dr. Woodruff, who coedited the textbook, Environmental Impacts on Reproductive Health and Fertility (Cambridge University Press: Cambridge, England, 2010).
The UCSF program provides patient guides in English and Spanish with suggestions of ways to reduce chemical exposures at work and at home.
Limits in the data
Michael Eisenberg, MD, a urologist and director of male reproductive medicine and surgery at Stanford (Calif.) University Medical Center, often gets questions from patients about how lifestyle and environmental exposures affect male fertility. (In her book, Dr. Swan also discusses how factors such as diet, exercise, smoking, and stress can affect male and female fertility.)
He found the evidence convincing that certain chemicals impact fertility – although, of course, it isn’t ethically possible to do randomized, controlled trials in which some people are intentionally exposed to chemicals to measure the effects. Along with adopting other healthy habits, he advised patients to avoid chemical exposures.
“It’s reasonable to try to eat organic and be mindful of where some of these exposures come from and try to minimize them to the extent possible,” he said.
Rebecca Sokol, MD, MPH, an endocrinologist and expert in male reproductive health, demonstrated the toxic effects of lead on sperm production in studies conducted on rats. But she views low-dose chemical exposure from everyday products as just one aspect of modern reproductive risks, some of which have stronger associations. For example, testosterone therapy impairs sperm production, and finasteride (a medication for male pattern baldness) has been linked to a reversible decline in sperm count.
“When it comes to these ubiquitous chemicals like phthalate and BPA, we explain to the patient that maybe they’re harmful, but we can’t say for sure,” because of the lack of causal data, said Dr. Sokol, professor emerita at the University of Southern California, who was on the panel that drafted the American Urological Association and American Society of Reproductive Medicine guideline on the diagnosis and treatment of male infertility.
Nonetheless, she advised patients to try to reduce exposures. “I don’t see us eradicating all the chemicals that might be bad for us unless we go back to another era. But we can do the best we can to avoid what we can.”
A call to action
Dr. Swan likened awareness of the health threat of chemical exposures to the gradual recognition of the climate crisis as a global imperative. Yet in some ways, the scientific work on chemical effects is even more daunting. The Environmental Protection Agency lists more than 86,000 chemicals on its inventory of chemical substances manufactured or imported into the United States.
Little is known about the potential effects of many chemicals that we inhale, ingest or absorb through our skin, Dr. Swan said. In her book, she noted the impact on wildlife – for example, reproductive abnormalities in frogs, alligators, and birds that were exposed to EDCs.
Yet Dr. Swan also takes solace in the lessons from the animal kingdom. Decades after the pesticide DDT, a neurotoxin and endocrine-disruptor, was banned in the United States in 1972, the bald eagle has made a comeback from near-extinction. She also pointed to a 2018 study which found that, while mice exposed to bisphenols passed on reproductive effects to offspring, when the exposures stopped, the effects disappeared after several generations.
“If we stop poisoning ourselves, we can turn this around,” said Dr. Swan. “That’s what I want people to know.”
Count Down frames the issues in language that is much starker than typically found in academic publications. But that is what’s necessary to draw attention to the effects of chemical exposures on human health and reproduction, Dr. Swan said. “I’m saying this in fairly extreme terms to alarm people, to make them realize it is a crisis and they have to act.”
No disclosures were reported.
Chemicals that pervade our modern world – plastics, pesticides, stain repellents, components of personal hygiene products – are contributing to a decades-long decline in fertility and could pose health risks even into future generations, according to an explosive new book by Shanna Swan, PhD, an environmental and reproductive epidemiologist at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Swan laid out the case that endocrine-disrupting chemicals (EDCs) such as phthalates and bisphenol A (BPA) threaten human existence, a conclusion that stems in part from her 2017 meta-analysis that showed a 52% drop in sperm counts from 1973 to 2011 in men in North America, Europe, and Australia.
“This alarming rate of decline could mean the human race will be unable to reproduce itself if the trend continues,” Dr. Swan said in her book, “Count Down: How Our Modern World Is Threatening Sperm Counts, Altering Male and Female Reproductive Development, and Imperiling the Future of the Human Race,” (New York: Scribner, 2021) coauthored with health journalist Stacey Colino.
Her premise that EDCs pose a risk to both male and female fertility is underscored by new research. A March 2021 article in Human Reproduction links prenatal chemical exposures to lowered fertility in a study of 1,045 Swiss military conscripts.
The Swiss men, aged 18-22 years, were significantly more likely to have low semen volume and low total sperm count if their mothers reported that they had occupational exposures to four or more endocrine-disrupting chemicals while they were pregnant. These EDCs, which mimic natural hormones, included pesticides, heavy metals, phthalates, alkylphenolic compounds, and solvents that can be found in agricultural work or hair and beauty salons.
These chemicals are not so-called “forever chemicals” that persist in the human body. But the Swiss study still showed an association between exposure during pregnancy and the future fertility of the male children. “Those apparently small exposures that pass quickly can affect development,” said Dr. Swan, who was not affiliated with the research. “It takes very little in terms of time and amount of chemicals to alter fetal development.”
Health risks beyond reproduction
While Count Down is placing a new spotlight on chemical hazards, some major medical organizations have already taken positions on the risks. “Reducing exposure to toxic environmental agents is a critical area of intervention for ob.gyns.,” the American College of Obstetricians and Gynecologists said in an environmental policy priority. “The Endocrine Society is concerned that human health is at risk because the current extensive scientific knowledge on EDCs and their health effects is not effectively translated to regulatory policies that fully protect populations from EDC exposures.”
But for the medical community, addressing the impact of EDCs goes beyond advocacy for regulatory and legislative changes, Dr. Swan said in an interview. Physicians should talk to patients about the importance of reducing their chemical exposure to safeguard their overall health.
“Reproductive health and particularly sperm count, subfertility, and infertility are predictors of lifelong health,” she said. That includes associations between reproductive disorders and “the risk of heart disease, obesity, reproductive cancers and, perhaps most dramatically, with a shortened lifespan.”
Some medical schools are including information on environmental health and exposure risks in the curriculum, said Tracey Woodruff, PhD, MPH, director of the program on reproductive health and the environment at the University of California, San Francisco. She urged physicians to ask patients about potential occupational exposures to hazardous chemicals and provide information about ways to reduce everyday exposures.
For example, safer options include buying organic produce, microwaving food in glass rather than plastic containers and avoiding products that contain phthalate or BPA. “If you’re going to talk to people about what they eat, that’s a perfect venue for talking about the environment,” said Dr. Woodruff, who coedited the textbook, Environmental Impacts on Reproductive Health and Fertility (Cambridge University Press: Cambridge, England, 2010).
The UCSF program provides patient guides in English and Spanish with suggestions of ways to reduce chemical exposures at work and at home.
Limits in the data
Michael Eisenberg, MD, a urologist and director of male reproductive medicine and surgery at Stanford (Calif.) University Medical Center, often gets questions from patients about how lifestyle and environmental exposures affect male fertility. (In her book, Dr. Swan also discusses how factors such as diet, exercise, smoking, and stress can affect male and female fertility.)
He found the evidence convincing that certain chemicals impact fertility – although, of course, it isn’t ethically possible to do randomized, controlled trials in which some people are intentionally exposed to chemicals to measure the effects. Along with adopting other healthy habits, he advised patients to avoid chemical exposures.
“It’s reasonable to try to eat organic and be mindful of where some of these exposures come from and try to minimize them to the extent possible,” he said.
Rebecca Sokol, MD, MPH, an endocrinologist and expert in male reproductive health, demonstrated the toxic effects of lead on sperm production in studies conducted on rats. But she views low-dose chemical exposure from everyday products as just one aspect of modern reproductive risks, some of which have stronger associations. For example, testosterone therapy impairs sperm production, and finasteride (a medication for male pattern baldness) has been linked to a reversible decline in sperm count.
“When it comes to these ubiquitous chemicals like phthalate and BPA, we explain to the patient that maybe they’re harmful, but we can’t say for sure,” because of the lack of causal data, said Dr. Sokol, professor emerita at the University of Southern California, who was on the panel that drafted the American Urological Association and American Society of Reproductive Medicine guideline on the diagnosis and treatment of male infertility.
Nonetheless, she advised patients to try to reduce exposures. “I don’t see us eradicating all the chemicals that might be bad for us unless we go back to another era. But we can do the best we can to avoid what we can.”
A call to action
Dr. Swan likened awareness of the health threat of chemical exposures to the gradual recognition of the climate crisis as a global imperative. Yet in some ways, the scientific work on chemical effects is even more daunting. The Environmental Protection Agency lists more than 86,000 chemicals on its inventory of chemical substances manufactured or imported into the United States.
Little is known about the potential effects of many chemicals that we inhale, ingest or absorb through our skin, Dr. Swan said. In her book, she noted the impact on wildlife – for example, reproductive abnormalities in frogs, alligators, and birds that were exposed to EDCs.
Yet Dr. Swan also takes solace in the lessons from the animal kingdom. Decades after the pesticide DDT, a neurotoxin and endocrine-disruptor, was banned in the United States in 1972, the bald eagle has made a comeback from near-extinction. She also pointed to a 2018 study which found that, while mice exposed to bisphenols passed on reproductive effects to offspring, when the exposures stopped, the effects disappeared after several generations.
“If we stop poisoning ourselves, we can turn this around,” said Dr. Swan. “That’s what I want people to know.”
Count Down frames the issues in language that is much starker than typically found in academic publications. But that is what’s necessary to draw attention to the effects of chemical exposures on human health and reproduction, Dr. Swan said. “I’m saying this in fairly extreme terms to alarm people, to make them realize it is a crisis and they have to act.”
No disclosures were reported.
Chemicals that pervade our modern world – plastics, pesticides, stain repellents, components of personal hygiene products – are contributing to a decades-long decline in fertility and could pose health risks even into future generations, according to an explosive new book by Shanna Swan, PhD, an environmental and reproductive epidemiologist at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Swan laid out the case that endocrine-disrupting chemicals (EDCs) such as phthalates and bisphenol A (BPA) threaten human existence, a conclusion that stems in part from her 2017 meta-analysis that showed a 52% drop in sperm counts from 1973 to 2011 in men in North America, Europe, and Australia.
“This alarming rate of decline could mean the human race will be unable to reproduce itself if the trend continues,” Dr. Swan said in her book, “Count Down: How Our Modern World Is Threatening Sperm Counts, Altering Male and Female Reproductive Development, and Imperiling the Future of the Human Race,” (New York: Scribner, 2021) coauthored with health journalist Stacey Colino.
Her premise that EDCs pose a risk to both male and female fertility is underscored by new research. A March 2021 article in Human Reproduction links prenatal chemical exposures to lowered fertility in a study of 1,045 Swiss military conscripts.
The Swiss men, aged 18-22 years, were significantly more likely to have low semen volume and low total sperm count if their mothers reported that they had occupational exposures to four or more endocrine-disrupting chemicals while they were pregnant. These EDCs, which mimic natural hormones, included pesticides, heavy metals, phthalates, alkylphenolic compounds, and solvents that can be found in agricultural work or hair and beauty salons.
These chemicals are not so-called “forever chemicals” that persist in the human body. But the Swiss study still showed an association between exposure during pregnancy and the future fertility of the male children. “Those apparently small exposures that pass quickly can affect development,” said Dr. Swan, who was not affiliated with the research. “It takes very little in terms of time and amount of chemicals to alter fetal development.”
Health risks beyond reproduction
While Count Down is placing a new spotlight on chemical hazards, some major medical organizations have already taken positions on the risks. “Reducing exposure to toxic environmental agents is a critical area of intervention for ob.gyns.,” the American College of Obstetricians and Gynecologists said in an environmental policy priority. “The Endocrine Society is concerned that human health is at risk because the current extensive scientific knowledge on EDCs and their health effects is not effectively translated to regulatory policies that fully protect populations from EDC exposures.”
But for the medical community, addressing the impact of EDCs goes beyond advocacy for regulatory and legislative changes, Dr. Swan said in an interview. Physicians should talk to patients about the importance of reducing their chemical exposure to safeguard their overall health.
“Reproductive health and particularly sperm count, subfertility, and infertility are predictors of lifelong health,” she said. That includes associations between reproductive disorders and “the risk of heart disease, obesity, reproductive cancers and, perhaps most dramatically, with a shortened lifespan.”
Some medical schools are including information on environmental health and exposure risks in the curriculum, said Tracey Woodruff, PhD, MPH, director of the program on reproductive health and the environment at the University of California, San Francisco. She urged physicians to ask patients about potential occupational exposures to hazardous chemicals and provide information about ways to reduce everyday exposures.
For example, safer options include buying organic produce, microwaving food in glass rather than plastic containers and avoiding products that contain phthalate or BPA. “If you’re going to talk to people about what they eat, that’s a perfect venue for talking about the environment,” said Dr. Woodruff, who coedited the textbook, Environmental Impacts on Reproductive Health and Fertility (Cambridge University Press: Cambridge, England, 2010).
The UCSF program provides patient guides in English and Spanish with suggestions of ways to reduce chemical exposures at work and at home.
Limits in the data
Michael Eisenberg, MD, a urologist and director of male reproductive medicine and surgery at Stanford (Calif.) University Medical Center, often gets questions from patients about how lifestyle and environmental exposures affect male fertility. (In her book, Dr. Swan also discusses how factors such as diet, exercise, smoking, and stress can affect male and female fertility.)
He found the evidence convincing that certain chemicals impact fertility – although, of course, it isn’t ethically possible to do randomized, controlled trials in which some people are intentionally exposed to chemicals to measure the effects. Along with adopting other healthy habits, he advised patients to avoid chemical exposures.
“It’s reasonable to try to eat organic and be mindful of where some of these exposures come from and try to minimize them to the extent possible,” he said.
Rebecca Sokol, MD, MPH, an endocrinologist and expert in male reproductive health, demonstrated the toxic effects of lead on sperm production in studies conducted on rats. But she views low-dose chemical exposure from everyday products as just one aspect of modern reproductive risks, some of which have stronger associations. For example, testosterone therapy impairs sperm production, and finasteride (a medication for male pattern baldness) has been linked to a reversible decline in sperm count.
“When it comes to these ubiquitous chemicals like phthalate and BPA, we explain to the patient that maybe they’re harmful, but we can’t say for sure,” because of the lack of causal data, said Dr. Sokol, professor emerita at the University of Southern California, who was on the panel that drafted the American Urological Association and American Society of Reproductive Medicine guideline on the diagnosis and treatment of male infertility.
Nonetheless, she advised patients to try to reduce exposures. “I don’t see us eradicating all the chemicals that might be bad for us unless we go back to another era. But we can do the best we can to avoid what we can.”
A call to action
Dr. Swan likened awareness of the health threat of chemical exposures to the gradual recognition of the climate crisis as a global imperative. Yet in some ways, the scientific work on chemical effects is even more daunting. The Environmental Protection Agency lists more than 86,000 chemicals on its inventory of chemical substances manufactured or imported into the United States.
Little is known about the potential effects of many chemicals that we inhale, ingest or absorb through our skin, Dr. Swan said. In her book, she noted the impact on wildlife – for example, reproductive abnormalities in frogs, alligators, and birds that were exposed to EDCs.
Yet Dr. Swan also takes solace in the lessons from the animal kingdom. Decades after the pesticide DDT, a neurotoxin and endocrine-disruptor, was banned in the United States in 1972, the bald eagle has made a comeback from near-extinction. She also pointed to a 2018 study which found that, while mice exposed to bisphenols passed on reproductive effects to offspring, when the exposures stopped, the effects disappeared after several generations.
“If we stop poisoning ourselves, we can turn this around,” said Dr. Swan. “That’s what I want people to know.”
Count Down frames the issues in language that is much starker than typically found in academic publications. But that is what’s necessary to draw attention to the effects of chemical exposures on human health and reproduction, Dr. Swan said. “I’m saying this in fairly extreme terms to alarm people, to make them realize it is a crisis and they have to act.”
No disclosures were reported.
How to talk to patients reluctant to get a COVID-19 vaccine
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Vagisil offered teens a vaginal ‘glow up.’ Docs cry foul
Late one night in early February, Jen Gunter, MD, was scrolling online when she discovered a new “feminine hygiene” product being marketed for teen girls. The new vanilla clementine scented wipes and cleansers with confetti-colored packaging and a cute name (OMV!) irked Dr. Gunter because they are designed for girls to use to “freshen” their vaginal area.
Dr. Gunter, a San Francisco-based gynecologist and author of “The Vagina Bible,” has built a reputation as a fierce advocate for women’s health and debunker of pseudoscience. She has called out jade eggs and “detox pearls” and various other items that promise to improve the vagina but that she and other doctors warn could actually be harmful. And, in her view, this product is no different.
She fired off a tweet that became the first volley in a vociferous social media countercampaign: “Hey @vagisil going to call you out here for this predatory line of products aimed at teen girls. Why do you think teen vulvas need special cleaning? To be prepped for men? Because they are dirty. Anxiously awaiting your answer as are all my followers.”
Vagisil responded on Instagram that “we want to clarify any confusion or the underlying belief that OMV! was developed because there is something wrong with teens or that vulvas/vaginas are inherently dirty. That is not the case. All-Day Fresh Wash is an all-over body wash, that is safe, gentle, and pH-balanced for sensitive vulvar area skin.”
Dr. Gunter’s Feb. 4 tweet attracted more than 8,300 likes, 1,300 retweets and hundreds of comments, but that was just the beginning. Dr. Gunter has continued to tweet about the OMV! product line – and has inspired dozens of other gynecologists to join in.
‘Your vagina is fine’
Dr. Gunter and other gynecologists have long delivered the message that water alone is sufficient to cleanse the vulvar area and that the vagina itself is self-cleaning. Research into the vaginal microbiome reveals the role of lactobacilli in preventing urogenital diseases. “Disturbances in your vagina microbiome are hard to undo,” says Jocelyn Fitzgerald, MD, a urogynecologist and pelvic reconstructive surgeon at Magee-Womens Hospital at the University of Pittsburgh Medical Center.
To underscore that message, Dr. Fitzgerald recently tweeted in support of Dr. Gunter’s Twitter thread: “Honestly, the @vagisil marketing campaign is a brilliant one because using their products while your vagina is perfectly fine will destroy your microbiome, give you real Bacterial Vaginosis, and prompt you to buy more Vagisil. DON’T FALL FOR IT GIRLS YOUR VAGINA IS FINE.”
In an emailed response to this news organization, a Vagisil spokesperson said, “We follow industry best practices for testing and OMV! products are rigorously assessed for safety and quality. In addition, we work with respected, independent clinical labs that follow strict testing protocols, using board-certified gynecologists and dermatologists to test our products before launch.”
However, beyond the potential for irritation or misuse, the gynecologists zeroed in on the underlying message that girls would feel more confident if they used the wipes and cleanser. For example, the company suggested that teens could use the wipes to get rid of “period funk.”
“There is no such thing as period funk!” gynecologist Danielle Jones, MD, exclaimed in a video on YouTube, where she has a channel called Mama Doctor Jones – with 700,000 subscribers. “All you need is ordinary hygiene. Period funk is not a thing! And if you feel like something is going on because there’s an odor that is abnormal, you need to talk to your doctor.”
Adult women often use wipes and special cleansers in the vaginal area. An online survey of 1,435 Canadian women, published in BMC Women’s Health in 2018, found 42% had used vaginal wipes, 12% had used vaginal washes or cleansers – and 4% had used them internally.
When it launched OMV! in July, Vagisil said it had engaged 2,500 teens and their mothers in creating the product, which it said was “designed to meet the cleansing and care needs of a new generation of young women.”
That extension of a product most commonly used by adult women to teenagers – who often feel self-conscious about their bodies – is exactly what bothers Dr. Gunter. “BTW I am sorry I am subjecting you all to my @vagisil outrage, but preying on teens and amplifying patriarchal shame of normal bodily functions to sell an irritating product is not acceptable. I’m not stopping until they take that OMV! product line down everywhere,” she said in a Feb. 8 tweet that attracted more than 7,900 likes.
No ‘glow up’ needed
Dr. Gunter’s tweets tapped into collective anger over the shaming of women’s bodies. The OMV! marketing suggested that teens could get a “glow up” with the products.
“Your vulva doesn’t need a ‘glow up.’ It’s fine like it is. And if it’s not, talk to your doctor,” Dr. Jones said in her Feb. 8 video, which has had almost 350,000 views, with 28,000 likes and only 149 dislikes.
“They’re very clearly pathologizing normal physiology,” Dr. Jones says. “They’re creating language that makes people feel as though their normal bodily functions have to be somehow fixed or changed.”
Dr. Gunter says she specifically wanted to prevent Vagisil from leveraging social media to influence teen girls. With her stream of tweets and support from colleagues around the country, she has sparked a prolonged online conversation.
“I am encouraged by the strong response on social media from both other enraged ob.gyns. and health care professionals as well the response from a lot of women and men,” Dr. Gunter said in an interview. “We have effectively blocked [Vagisil] from using social media.”
In its response to this news organization, Vagisil noted, “We are a brand run by women with daughters of our own.” While defending the products, Vagisil acknowledged the criticisms: “We are always listening to our consumers and our expert partners so that we continuously evolve. We appreciate the perspective that our language choice surrounding periods may perpetuate an old idea and have already begun to make changes to address this.”
Dr. Gunter says she plans to stay on topic. “Given the number of people outraged, I suspect if they venture out on social media again the reaction will be swift,” she said. “Hopefully we have made OMV! toxic for influencers as well.”
In fact, she’s ready to take on “the entire predatory feminine hygiene market. I’m sick of their false claims about balancing pH and not-so-subtle suggestions that vaginas and vulvas and menstruation stink. These products cause psychological harm as well as physical harm from their irritants,” she said.
A version of this article first appeared on Medscape.com.
Late one night in early February, Jen Gunter, MD, was scrolling online when she discovered a new “feminine hygiene” product being marketed for teen girls. The new vanilla clementine scented wipes and cleansers with confetti-colored packaging and a cute name (OMV!) irked Dr. Gunter because they are designed for girls to use to “freshen” their vaginal area.
Dr. Gunter, a San Francisco-based gynecologist and author of “The Vagina Bible,” has built a reputation as a fierce advocate for women’s health and debunker of pseudoscience. She has called out jade eggs and “detox pearls” and various other items that promise to improve the vagina but that she and other doctors warn could actually be harmful. And, in her view, this product is no different.
She fired off a tweet that became the first volley in a vociferous social media countercampaign: “Hey @vagisil going to call you out here for this predatory line of products aimed at teen girls. Why do you think teen vulvas need special cleaning? To be prepped for men? Because they are dirty. Anxiously awaiting your answer as are all my followers.”
Vagisil responded on Instagram that “we want to clarify any confusion or the underlying belief that OMV! was developed because there is something wrong with teens or that vulvas/vaginas are inherently dirty. That is not the case. All-Day Fresh Wash is an all-over body wash, that is safe, gentle, and pH-balanced for sensitive vulvar area skin.”
Dr. Gunter’s Feb. 4 tweet attracted more than 8,300 likes, 1,300 retweets and hundreds of comments, but that was just the beginning. Dr. Gunter has continued to tweet about the OMV! product line – and has inspired dozens of other gynecologists to join in.
‘Your vagina is fine’
Dr. Gunter and other gynecologists have long delivered the message that water alone is sufficient to cleanse the vulvar area and that the vagina itself is self-cleaning. Research into the vaginal microbiome reveals the role of lactobacilli in preventing urogenital diseases. “Disturbances in your vagina microbiome are hard to undo,” says Jocelyn Fitzgerald, MD, a urogynecologist and pelvic reconstructive surgeon at Magee-Womens Hospital at the University of Pittsburgh Medical Center.
To underscore that message, Dr. Fitzgerald recently tweeted in support of Dr. Gunter’s Twitter thread: “Honestly, the @vagisil marketing campaign is a brilliant one because using their products while your vagina is perfectly fine will destroy your microbiome, give you real Bacterial Vaginosis, and prompt you to buy more Vagisil. DON’T FALL FOR IT GIRLS YOUR VAGINA IS FINE.”
In an emailed response to this news organization, a Vagisil spokesperson said, “We follow industry best practices for testing and OMV! products are rigorously assessed for safety and quality. In addition, we work with respected, independent clinical labs that follow strict testing protocols, using board-certified gynecologists and dermatologists to test our products before launch.”
However, beyond the potential for irritation or misuse, the gynecologists zeroed in on the underlying message that girls would feel more confident if they used the wipes and cleanser. For example, the company suggested that teens could use the wipes to get rid of “period funk.”
“There is no such thing as period funk!” gynecologist Danielle Jones, MD, exclaimed in a video on YouTube, where she has a channel called Mama Doctor Jones – with 700,000 subscribers. “All you need is ordinary hygiene. Period funk is not a thing! And if you feel like something is going on because there’s an odor that is abnormal, you need to talk to your doctor.”
Adult women often use wipes and special cleansers in the vaginal area. An online survey of 1,435 Canadian women, published in BMC Women’s Health in 2018, found 42% had used vaginal wipes, 12% had used vaginal washes or cleansers – and 4% had used them internally.
When it launched OMV! in July, Vagisil said it had engaged 2,500 teens and their mothers in creating the product, which it said was “designed to meet the cleansing and care needs of a new generation of young women.”
That extension of a product most commonly used by adult women to teenagers – who often feel self-conscious about their bodies – is exactly what bothers Dr. Gunter. “BTW I am sorry I am subjecting you all to my @vagisil outrage, but preying on teens and amplifying patriarchal shame of normal bodily functions to sell an irritating product is not acceptable. I’m not stopping until they take that OMV! product line down everywhere,” she said in a Feb. 8 tweet that attracted more than 7,900 likes.
No ‘glow up’ needed
Dr. Gunter’s tweets tapped into collective anger over the shaming of women’s bodies. The OMV! marketing suggested that teens could get a “glow up” with the products.
“Your vulva doesn’t need a ‘glow up.’ It’s fine like it is. And if it’s not, talk to your doctor,” Dr. Jones said in her Feb. 8 video, which has had almost 350,000 views, with 28,000 likes and only 149 dislikes.
“They’re very clearly pathologizing normal physiology,” Dr. Jones says. “They’re creating language that makes people feel as though their normal bodily functions have to be somehow fixed or changed.”
Dr. Gunter says she specifically wanted to prevent Vagisil from leveraging social media to influence teen girls. With her stream of tweets and support from colleagues around the country, she has sparked a prolonged online conversation.
“I am encouraged by the strong response on social media from both other enraged ob.gyns. and health care professionals as well the response from a lot of women and men,” Dr. Gunter said in an interview. “We have effectively blocked [Vagisil] from using social media.”
In its response to this news organization, Vagisil noted, “We are a brand run by women with daughters of our own.” While defending the products, Vagisil acknowledged the criticisms: “We are always listening to our consumers and our expert partners so that we continuously evolve. We appreciate the perspective that our language choice surrounding periods may perpetuate an old idea and have already begun to make changes to address this.”
Dr. Gunter says she plans to stay on topic. “Given the number of people outraged, I suspect if they venture out on social media again the reaction will be swift,” she said. “Hopefully we have made OMV! toxic for influencers as well.”
In fact, she’s ready to take on “the entire predatory feminine hygiene market. I’m sick of their false claims about balancing pH and not-so-subtle suggestions that vaginas and vulvas and menstruation stink. These products cause psychological harm as well as physical harm from their irritants,” she said.
A version of this article first appeared on Medscape.com.
Late one night in early February, Jen Gunter, MD, was scrolling online when she discovered a new “feminine hygiene” product being marketed for teen girls. The new vanilla clementine scented wipes and cleansers with confetti-colored packaging and a cute name (OMV!) irked Dr. Gunter because they are designed for girls to use to “freshen” their vaginal area.
Dr. Gunter, a San Francisco-based gynecologist and author of “The Vagina Bible,” has built a reputation as a fierce advocate for women’s health and debunker of pseudoscience. She has called out jade eggs and “detox pearls” and various other items that promise to improve the vagina but that she and other doctors warn could actually be harmful. And, in her view, this product is no different.
She fired off a tweet that became the first volley in a vociferous social media countercampaign: “Hey @vagisil going to call you out here for this predatory line of products aimed at teen girls. Why do you think teen vulvas need special cleaning? To be prepped for men? Because they are dirty. Anxiously awaiting your answer as are all my followers.”
Vagisil responded on Instagram that “we want to clarify any confusion or the underlying belief that OMV! was developed because there is something wrong with teens or that vulvas/vaginas are inherently dirty. That is not the case. All-Day Fresh Wash is an all-over body wash, that is safe, gentle, and pH-balanced for sensitive vulvar area skin.”
Dr. Gunter’s Feb. 4 tweet attracted more than 8,300 likes, 1,300 retweets and hundreds of comments, but that was just the beginning. Dr. Gunter has continued to tweet about the OMV! product line – and has inspired dozens of other gynecologists to join in.
‘Your vagina is fine’
Dr. Gunter and other gynecologists have long delivered the message that water alone is sufficient to cleanse the vulvar area and that the vagina itself is self-cleaning. Research into the vaginal microbiome reveals the role of lactobacilli in preventing urogenital diseases. “Disturbances in your vagina microbiome are hard to undo,” says Jocelyn Fitzgerald, MD, a urogynecologist and pelvic reconstructive surgeon at Magee-Womens Hospital at the University of Pittsburgh Medical Center.
To underscore that message, Dr. Fitzgerald recently tweeted in support of Dr. Gunter’s Twitter thread: “Honestly, the @vagisil marketing campaign is a brilliant one because using their products while your vagina is perfectly fine will destroy your microbiome, give you real Bacterial Vaginosis, and prompt you to buy more Vagisil. DON’T FALL FOR IT GIRLS YOUR VAGINA IS FINE.”
In an emailed response to this news organization, a Vagisil spokesperson said, “We follow industry best practices for testing and OMV! products are rigorously assessed for safety and quality. In addition, we work with respected, independent clinical labs that follow strict testing protocols, using board-certified gynecologists and dermatologists to test our products before launch.”
However, beyond the potential for irritation or misuse, the gynecologists zeroed in on the underlying message that girls would feel more confident if they used the wipes and cleanser. For example, the company suggested that teens could use the wipes to get rid of “period funk.”
“There is no such thing as period funk!” gynecologist Danielle Jones, MD, exclaimed in a video on YouTube, where she has a channel called Mama Doctor Jones – with 700,000 subscribers. “All you need is ordinary hygiene. Period funk is not a thing! And if you feel like something is going on because there’s an odor that is abnormal, you need to talk to your doctor.”
Adult women often use wipes and special cleansers in the vaginal area. An online survey of 1,435 Canadian women, published in BMC Women’s Health in 2018, found 42% had used vaginal wipes, 12% had used vaginal washes or cleansers – and 4% had used them internally.
When it launched OMV! in July, Vagisil said it had engaged 2,500 teens and their mothers in creating the product, which it said was “designed to meet the cleansing and care needs of a new generation of young women.”
That extension of a product most commonly used by adult women to teenagers – who often feel self-conscious about their bodies – is exactly what bothers Dr. Gunter. “BTW I am sorry I am subjecting you all to my @vagisil outrage, but preying on teens and amplifying patriarchal shame of normal bodily functions to sell an irritating product is not acceptable. I’m not stopping until they take that OMV! product line down everywhere,” she said in a Feb. 8 tweet that attracted more than 7,900 likes.
No ‘glow up’ needed
Dr. Gunter’s tweets tapped into collective anger over the shaming of women’s bodies. The OMV! marketing suggested that teens could get a “glow up” with the products.
“Your vulva doesn’t need a ‘glow up.’ It’s fine like it is. And if it’s not, talk to your doctor,” Dr. Jones said in her Feb. 8 video, which has had almost 350,000 views, with 28,000 likes and only 149 dislikes.
“They’re very clearly pathologizing normal physiology,” Dr. Jones says. “They’re creating language that makes people feel as though their normal bodily functions have to be somehow fixed or changed.”
Dr. Gunter says she specifically wanted to prevent Vagisil from leveraging social media to influence teen girls. With her stream of tweets and support from colleagues around the country, she has sparked a prolonged online conversation.
“I am encouraged by the strong response on social media from both other enraged ob.gyns. and health care professionals as well the response from a lot of women and men,” Dr. Gunter said in an interview. “We have effectively blocked [Vagisil] from using social media.”
In its response to this news organization, Vagisil noted, “We are a brand run by women with daughters of our own.” While defending the products, Vagisil acknowledged the criticisms: “We are always listening to our consumers and our expert partners so that we continuously evolve. We appreciate the perspective that our language choice surrounding periods may perpetuate an old idea and have already begun to make changes to address this.”
Dr. Gunter says she plans to stay on topic. “Given the number of people outraged, I suspect if they venture out on social media again the reaction will be swift,” she said. “Hopefully we have made OMV! toxic for influencers as well.”
In fact, she’s ready to take on “the entire predatory feminine hygiene market. I’m sick of their false claims about balancing pH and not-so-subtle suggestions that vaginas and vulvas and menstruation stink. These products cause psychological harm as well as physical harm from their irritants,” she said.
A version of this article first appeared on Medscape.com.
Teenagers get in the queue for COVID-19 vaccines
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Some COVID-19 vaccine reactions could be pseudoallergic, experts say
On Jan. 13, 2 days after a drive-through vaccination “superstation” opened in San Diego, six people were treated for anaphylaxis after they received the Moderna vaccine, leading the California state epidemiologist to recommend pausing the administration of that particular lot.
A group of allergy and immunology experts and public health officials reviewed the cases, as well as an incident that occurred the day before, and concluded that at least some of the responses were angioedema, or swelling — a serious allergic reaction — but none were actually anaphylaxis. No similar clusters had occurred with the same vaccine lot in other states, and California resumed using the doses.
Yet questions remain about the reactions and the mechanisms for them. Some might have been triggered by an allergy to a vaccine component, most likely the polyethylene glycol (PEG) that stabilizes the lipid surrounding the mRNA, the key vaccine component in both the Moderna and Pfizer vaccines. Another possible explanation is that some could be pseudoallergic reactions to a blood protein known as complement, a little-understood process that resembles an antigen-based reaction but doesn’t leave an immune memory and might not recur.
Cases of complement-activation-related pseudoallergy look like a severe allergic reaction but occur through a different mechanism and don’t require previous exposure to an allergen.
“It has the same signs and symptoms and is treated the same way, but it occurs through a different pathway,” explained Neal Halsey, MD, director emeritus of the Institute for Vaccine Safety and emeritus professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Pseudoallergies are not well understood, but they have been associated with reactions to the contrast media used in imaging, such as with MRI. “If people have had an anaphylaxis-type reaction following the injection of contrast-dye material, that is a strong signal that it might be a complement-activation-related pseudoallergy,” said Dr. Halsey, a member of the Clinical Immunization Safety Assessment Network. “Those are the people who definitely need to consider seeing an allergist before getting the COVID vaccines.”
When Aleena Banerji, MD, clinical director of the allergy and clinical immunology unit at Massachusetts General Hospital in Boston, talks to patients about vaccine reactions, she addresses the risk for COVID-19 infection. All of the people who developed allergies after the Pfizer and Moderna vaccines recovered, but more than 445,000 Americans have died from COVID-19.
Most people with common allergies, such as to food or oral medications, don’t need to worry about reactions, said Dr. Banerji, lead author of a review that assessed the risk for allergic reactions to the Pfizer and Moderna vaccines.
Investigating reactions
As investigators search for the answers to what causes reactions, transparency is crucial to trust, said Kathryn Edwards, MD, principal investigator of the Clinical Immunization Safety Assessment Project, a vaccine safety network funded by the Centers for Disease Control and Prevention.
“Unless the public knows that we’re really investigating and we’re taking this seriously, then I think the vaccine hesitancy is going to increase,” said Dr. Edwards, professor of pediatrics at Vanderbilt University Medical Center and scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn.
First reports of anaphylaxis came quickly after COVID-19 vaccinations began. In the 2 weeks before the holidays, almost 2 million health care workers received the Pfizer vaccine, and 21 of them developed anaphylaxis, according to CDC researchers who reviewed case reports from the Vaccine Adverse Event Reporting System (VAERS). That rate of about 1 in 100,000 is 10 times higher than the occurrence with other vaccines. No deaths from anaphylaxis were reported.
As the vaccinations ramped up, the rate declined. As of Jan. 18, 50 cases of anaphylaxis were reported to VAERS after the administration of 9,943,247 Pfizer doses, for a rate of 5.0 per million, according to data presented at the Jan. 27 meeting of the CDC Advisory Committee on Immunization Practices. And 21 cases of anaphylaxis were reported to VAERS after the administration of 7,581,429 Moderna doses, for a rate of 2.8 per million.
The anaphylaxis occurred almost exclusively in women; only three of the VAERS anaphylaxis reports were from men. Only 24% had a history of anaphylaxis.
The earlier CDC report explored the potential link to allergies. One person with anaphylaxis had a history of allergy to iodinated contrast media, and others had allergies to various medications, vaccines, foods, and animals. The researchers reported 86 nonanaphylaxis allergic reactions and 61 nonallergic adverse events among the 175 case reports they reviewed as possible cases of severe allergic reaction.
Of 1,266 reports that VAERS received from Dec. 21 to Jan. 10, the CDC identified 108 possible cases of severe allergic reaction after the Moderna vaccine. Only 10 met the case definition of anaphylaxis put forward by the Brighton Collaboration, a vaccine safety organization. All but one case involved a history of allergies or allergic reactions; only five had a previously experienced anaphylaxis.
There were 47 nonanaphylaxis allergic reactions.
The San Diego cluster also met the Brighton case definition for anaphylaxis, Dr. Edwards reported. This discrepancy highlights the difficulties in characterizing vaccine reactions.
Measuring a pseudoallergic reaction is a challenge. It requires that a blood sample be drawn soon after the incident and then frozen to protect heat-sensitive blood markers, Dr. Edwards explained.
And as vaccinations rise, so do adverse-event reports. But unlike in clinical trials, there is no control group for comparison. That is why vaccine safety experts urge caution when evaluating events and, where possible, advise looking at background rates.
“A major way to determine whether the adverse event is causally related is to assess the incidence of the adverse event in vaccines versus nonvaccines,” said Walter Orenstein, MD, who directed the U.S. Immunization Program from 1988 to 2004 and is now associate director of the Emory Vaccine Center and professor of infectious diseases at Emory University in Atlanta. Public health officials could then identify vaccine risk factors, he said.
When a reaction occurs almost immediately after vaccination, vaccine safety investigators look for probable triggers. If allergy to PEG is the culprit in anaphylactic reactions, then the individuals would have had a previous exposure, perhaps from injectable medications, Dr. Edwards said.
It might be feasible to perform a skin test for allergy to PEG. “If the skin testing is negative, that doesn’t completely rule out allergy, but it can be used in the decision-making about giving the first or second vaccine dose,” Dr. Banerji said.
Other vaccines, such as childhood vaccines, contain polysorbate as a stabilizer, which has a similar chemical structure, and it’s not clear why someone would react to PEG but not to polysorbate, Dr. Edwards said.
Meanwhile, other illnesses and even deaths sometimes occur in the days after vaccination, but that doesn’t mean the vaccine caused them, cautioned Steve Black, MD, emeritus professor of pediatrics at Cincinnati Children’s Hospital and cofounder of the Global Vaccine Data Network, an international vaccine safety collaboration.
“Different events and clusters of events will occur by chance alone, as these events can occur without vaccines. We need to not immediately assume that they’re due to the vaccine,” he said. “You don’t want to undermine the whole vaccine program every time something comes up and assume that it’s associated with the vaccine.”
The CDC only has three contraindications for the vaccines:
- Severe allergic reaction (such as anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components.
- Immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including PEG).
- Immediate allergic reaction of any severity to polysorbate (due to potential cross-reactive hypersensitivity with PEG).
People who have had an immediate allergic reaction to other vaccines or injectable therapies should consider consulting with an allergist or immunologist before getting the Pfizer or Moderna vaccines, the CDC advises.
The CDC also says that people with a history of anaphylaxis from any cause should be observed for 30 minutes after vaccination. Vaccination protocol calls for everyone else to wait on site for 15 minutes after vaccination.
A version of this article first appeared on Medscape.com.
On Jan. 13, 2 days after a drive-through vaccination “superstation” opened in San Diego, six people were treated for anaphylaxis after they received the Moderna vaccine, leading the California state epidemiologist to recommend pausing the administration of that particular lot.
A group of allergy and immunology experts and public health officials reviewed the cases, as well as an incident that occurred the day before, and concluded that at least some of the responses were angioedema, or swelling — a serious allergic reaction — but none were actually anaphylaxis. No similar clusters had occurred with the same vaccine lot in other states, and California resumed using the doses.
Yet questions remain about the reactions and the mechanisms for them. Some might have been triggered by an allergy to a vaccine component, most likely the polyethylene glycol (PEG) that stabilizes the lipid surrounding the mRNA, the key vaccine component in both the Moderna and Pfizer vaccines. Another possible explanation is that some could be pseudoallergic reactions to a blood protein known as complement, a little-understood process that resembles an antigen-based reaction but doesn’t leave an immune memory and might not recur.
Cases of complement-activation-related pseudoallergy look like a severe allergic reaction but occur through a different mechanism and don’t require previous exposure to an allergen.
“It has the same signs and symptoms and is treated the same way, but it occurs through a different pathway,” explained Neal Halsey, MD, director emeritus of the Institute for Vaccine Safety and emeritus professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Pseudoallergies are not well understood, but they have been associated with reactions to the contrast media used in imaging, such as with MRI. “If people have had an anaphylaxis-type reaction following the injection of contrast-dye material, that is a strong signal that it might be a complement-activation-related pseudoallergy,” said Dr. Halsey, a member of the Clinical Immunization Safety Assessment Network. “Those are the people who definitely need to consider seeing an allergist before getting the COVID vaccines.”
When Aleena Banerji, MD, clinical director of the allergy and clinical immunology unit at Massachusetts General Hospital in Boston, talks to patients about vaccine reactions, she addresses the risk for COVID-19 infection. All of the people who developed allergies after the Pfizer and Moderna vaccines recovered, but more than 445,000 Americans have died from COVID-19.
Most people with common allergies, such as to food or oral medications, don’t need to worry about reactions, said Dr. Banerji, lead author of a review that assessed the risk for allergic reactions to the Pfizer and Moderna vaccines.
Investigating reactions
As investigators search for the answers to what causes reactions, transparency is crucial to trust, said Kathryn Edwards, MD, principal investigator of the Clinical Immunization Safety Assessment Project, a vaccine safety network funded by the Centers for Disease Control and Prevention.
“Unless the public knows that we’re really investigating and we’re taking this seriously, then I think the vaccine hesitancy is going to increase,” said Dr. Edwards, professor of pediatrics at Vanderbilt University Medical Center and scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn.
First reports of anaphylaxis came quickly after COVID-19 vaccinations began. In the 2 weeks before the holidays, almost 2 million health care workers received the Pfizer vaccine, and 21 of them developed anaphylaxis, according to CDC researchers who reviewed case reports from the Vaccine Adverse Event Reporting System (VAERS). That rate of about 1 in 100,000 is 10 times higher than the occurrence with other vaccines. No deaths from anaphylaxis were reported.
As the vaccinations ramped up, the rate declined. As of Jan. 18, 50 cases of anaphylaxis were reported to VAERS after the administration of 9,943,247 Pfizer doses, for a rate of 5.0 per million, according to data presented at the Jan. 27 meeting of the CDC Advisory Committee on Immunization Practices. And 21 cases of anaphylaxis were reported to VAERS after the administration of 7,581,429 Moderna doses, for a rate of 2.8 per million.
The anaphylaxis occurred almost exclusively in women; only three of the VAERS anaphylaxis reports were from men. Only 24% had a history of anaphylaxis.
The earlier CDC report explored the potential link to allergies. One person with anaphylaxis had a history of allergy to iodinated contrast media, and others had allergies to various medications, vaccines, foods, and animals. The researchers reported 86 nonanaphylaxis allergic reactions and 61 nonallergic adverse events among the 175 case reports they reviewed as possible cases of severe allergic reaction.
Of 1,266 reports that VAERS received from Dec. 21 to Jan. 10, the CDC identified 108 possible cases of severe allergic reaction after the Moderna vaccine. Only 10 met the case definition of anaphylaxis put forward by the Brighton Collaboration, a vaccine safety organization. All but one case involved a history of allergies or allergic reactions; only five had a previously experienced anaphylaxis.
There were 47 nonanaphylaxis allergic reactions.
The San Diego cluster also met the Brighton case definition for anaphylaxis, Dr. Edwards reported. This discrepancy highlights the difficulties in characterizing vaccine reactions.
Measuring a pseudoallergic reaction is a challenge. It requires that a blood sample be drawn soon after the incident and then frozen to protect heat-sensitive blood markers, Dr. Edwards explained.
And as vaccinations rise, so do adverse-event reports. But unlike in clinical trials, there is no control group for comparison. That is why vaccine safety experts urge caution when evaluating events and, where possible, advise looking at background rates.
“A major way to determine whether the adverse event is causally related is to assess the incidence of the adverse event in vaccines versus nonvaccines,” said Walter Orenstein, MD, who directed the U.S. Immunization Program from 1988 to 2004 and is now associate director of the Emory Vaccine Center and professor of infectious diseases at Emory University in Atlanta. Public health officials could then identify vaccine risk factors, he said.
When a reaction occurs almost immediately after vaccination, vaccine safety investigators look for probable triggers. If allergy to PEG is the culprit in anaphylactic reactions, then the individuals would have had a previous exposure, perhaps from injectable medications, Dr. Edwards said.
It might be feasible to perform a skin test for allergy to PEG. “If the skin testing is negative, that doesn’t completely rule out allergy, but it can be used in the decision-making about giving the first or second vaccine dose,” Dr. Banerji said.
Other vaccines, such as childhood vaccines, contain polysorbate as a stabilizer, which has a similar chemical structure, and it’s not clear why someone would react to PEG but not to polysorbate, Dr. Edwards said.
Meanwhile, other illnesses and even deaths sometimes occur in the days after vaccination, but that doesn’t mean the vaccine caused them, cautioned Steve Black, MD, emeritus professor of pediatrics at Cincinnati Children’s Hospital and cofounder of the Global Vaccine Data Network, an international vaccine safety collaboration.
“Different events and clusters of events will occur by chance alone, as these events can occur without vaccines. We need to not immediately assume that they’re due to the vaccine,” he said. “You don’t want to undermine the whole vaccine program every time something comes up and assume that it’s associated with the vaccine.”
The CDC only has three contraindications for the vaccines:
- Severe allergic reaction (such as anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components.
- Immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including PEG).
- Immediate allergic reaction of any severity to polysorbate (due to potential cross-reactive hypersensitivity with PEG).
People who have had an immediate allergic reaction to other vaccines or injectable therapies should consider consulting with an allergist or immunologist before getting the Pfizer or Moderna vaccines, the CDC advises.
The CDC also says that people with a history of anaphylaxis from any cause should be observed for 30 minutes after vaccination. Vaccination protocol calls for everyone else to wait on site for 15 minutes after vaccination.
A version of this article first appeared on Medscape.com.
On Jan. 13, 2 days after a drive-through vaccination “superstation” opened in San Diego, six people were treated for anaphylaxis after they received the Moderna vaccine, leading the California state epidemiologist to recommend pausing the administration of that particular lot.
A group of allergy and immunology experts and public health officials reviewed the cases, as well as an incident that occurred the day before, and concluded that at least some of the responses were angioedema, or swelling — a serious allergic reaction — but none were actually anaphylaxis. No similar clusters had occurred with the same vaccine lot in other states, and California resumed using the doses.
Yet questions remain about the reactions and the mechanisms for them. Some might have been triggered by an allergy to a vaccine component, most likely the polyethylene glycol (PEG) that stabilizes the lipid surrounding the mRNA, the key vaccine component in both the Moderna and Pfizer vaccines. Another possible explanation is that some could be pseudoallergic reactions to a blood protein known as complement, a little-understood process that resembles an antigen-based reaction but doesn’t leave an immune memory and might not recur.
Cases of complement-activation-related pseudoallergy look like a severe allergic reaction but occur through a different mechanism and don’t require previous exposure to an allergen.
“It has the same signs and symptoms and is treated the same way, but it occurs through a different pathway,” explained Neal Halsey, MD, director emeritus of the Institute for Vaccine Safety and emeritus professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Pseudoallergies are not well understood, but they have been associated with reactions to the contrast media used in imaging, such as with MRI. “If people have had an anaphylaxis-type reaction following the injection of contrast-dye material, that is a strong signal that it might be a complement-activation-related pseudoallergy,” said Dr. Halsey, a member of the Clinical Immunization Safety Assessment Network. “Those are the people who definitely need to consider seeing an allergist before getting the COVID vaccines.”
When Aleena Banerji, MD, clinical director of the allergy and clinical immunology unit at Massachusetts General Hospital in Boston, talks to patients about vaccine reactions, she addresses the risk for COVID-19 infection. All of the people who developed allergies after the Pfizer and Moderna vaccines recovered, but more than 445,000 Americans have died from COVID-19.
Most people with common allergies, such as to food or oral medications, don’t need to worry about reactions, said Dr. Banerji, lead author of a review that assessed the risk for allergic reactions to the Pfizer and Moderna vaccines.
Investigating reactions
As investigators search for the answers to what causes reactions, transparency is crucial to trust, said Kathryn Edwards, MD, principal investigator of the Clinical Immunization Safety Assessment Project, a vaccine safety network funded by the Centers for Disease Control and Prevention.
“Unless the public knows that we’re really investigating and we’re taking this seriously, then I think the vaccine hesitancy is going to increase,” said Dr. Edwards, professor of pediatrics at Vanderbilt University Medical Center and scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn.
First reports of anaphylaxis came quickly after COVID-19 vaccinations began. In the 2 weeks before the holidays, almost 2 million health care workers received the Pfizer vaccine, and 21 of them developed anaphylaxis, according to CDC researchers who reviewed case reports from the Vaccine Adverse Event Reporting System (VAERS). That rate of about 1 in 100,000 is 10 times higher than the occurrence with other vaccines. No deaths from anaphylaxis were reported.
As the vaccinations ramped up, the rate declined. As of Jan. 18, 50 cases of anaphylaxis were reported to VAERS after the administration of 9,943,247 Pfizer doses, for a rate of 5.0 per million, according to data presented at the Jan. 27 meeting of the CDC Advisory Committee on Immunization Practices. And 21 cases of anaphylaxis were reported to VAERS after the administration of 7,581,429 Moderna doses, for a rate of 2.8 per million.
The anaphylaxis occurred almost exclusively in women; only three of the VAERS anaphylaxis reports were from men. Only 24% had a history of anaphylaxis.
The earlier CDC report explored the potential link to allergies. One person with anaphylaxis had a history of allergy to iodinated contrast media, and others had allergies to various medications, vaccines, foods, and animals. The researchers reported 86 nonanaphylaxis allergic reactions and 61 nonallergic adverse events among the 175 case reports they reviewed as possible cases of severe allergic reaction.
Of 1,266 reports that VAERS received from Dec. 21 to Jan. 10, the CDC identified 108 possible cases of severe allergic reaction after the Moderna vaccine. Only 10 met the case definition of anaphylaxis put forward by the Brighton Collaboration, a vaccine safety organization. All but one case involved a history of allergies or allergic reactions; only five had a previously experienced anaphylaxis.
There were 47 nonanaphylaxis allergic reactions.
The San Diego cluster also met the Brighton case definition for anaphylaxis, Dr. Edwards reported. This discrepancy highlights the difficulties in characterizing vaccine reactions.
Measuring a pseudoallergic reaction is a challenge. It requires that a blood sample be drawn soon after the incident and then frozen to protect heat-sensitive blood markers, Dr. Edwards explained.
And as vaccinations rise, so do adverse-event reports. But unlike in clinical trials, there is no control group for comparison. That is why vaccine safety experts urge caution when evaluating events and, where possible, advise looking at background rates.
“A major way to determine whether the adverse event is causally related is to assess the incidence of the adverse event in vaccines versus nonvaccines,” said Walter Orenstein, MD, who directed the U.S. Immunization Program from 1988 to 2004 and is now associate director of the Emory Vaccine Center and professor of infectious diseases at Emory University in Atlanta. Public health officials could then identify vaccine risk factors, he said.
When a reaction occurs almost immediately after vaccination, vaccine safety investigators look for probable triggers. If allergy to PEG is the culprit in anaphylactic reactions, then the individuals would have had a previous exposure, perhaps from injectable medications, Dr. Edwards said.
It might be feasible to perform a skin test for allergy to PEG. “If the skin testing is negative, that doesn’t completely rule out allergy, but it can be used in the decision-making about giving the first or second vaccine dose,” Dr. Banerji said.
Other vaccines, such as childhood vaccines, contain polysorbate as a stabilizer, which has a similar chemical structure, and it’s not clear why someone would react to PEG but not to polysorbate, Dr. Edwards said.
Meanwhile, other illnesses and even deaths sometimes occur in the days after vaccination, but that doesn’t mean the vaccine caused them, cautioned Steve Black, MD, emeritus professor of pediatrics at Cincinnati Children’s Hospital and cofounder of the Global Vaccine Data Network, an international vaccine safety collaboration.
“Different events and clusters of events will occur by chance alone, as these events can occur without vaccines. We need to not immediately assume that they’re due to the vaccine,” he said. “You don’t want to undermine the whole vaccine program every time something comes up and assume that it’s associated with the vaccine.”
The CDC only has three contraindications for the vaccines:
- Severe allergic reaction (such as anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components.
- Immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including PEG).
- Immediate allergic reaction of any severity to polysorbate (due to potential cross-reactive hypersensitivity with PEG).
People who have had an immediate allergic reaction to other vaccines or injectable therapies should consider consulting with an allergist or immunologist before getting the Pfizer or Moderna vaccines, the CDC advises.
The CDC also says that people with a history of anaphylaxis from any cause should be observed for 30 minutes after vaccination. Vaccination protocol calls for everyone else to wait on site for 15 minutes after vaccination.
A version of this article first appeared on Medscape.com.
Call to arms: vaccinating the health workforce of 21 million strong
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
Hospitals poised to launch first COVID-19 vaccines in clinicians
At first, when news spread of a 28-year-old doctor on the COVID-19 front lines in Brazil who died after receiving an experimental vaccine, doubts arose about the safety of one of the most promising coronavirus vaccine candidates. But then the story flipped. Although the vaccine maker wouldn’t confirm it, the doctor appeared to have been in the control group and had received a dose of an established meningitis vaccine. The danger came from exposure to the coronavirus itself.
That tragedy underscores the ongoing risk of COVID-19 to healthcare workers, who have been designated by US advisory panels as part of phase 1A – the first to receive doses of any approved vaccine. The Centers for Disease Control and Prevention (CDC) recently reported that 6% of adults hospitalized with COVID from March to May were healthcare workers. The report was based on surveillance data from 13 states. The average age of the patients was 49 years. The agency set a November 15 vaccination “readiness date” for jurisdictions, such as state health departments, even though a vaccine isn’t likely to be authorized by then.
As hospitals scramble to prepare, their watchword is flexibility. They don’t yet know how many initial doses they will get, of which vaccine, or in what time frame. They have a sophisticated infrastructure to deliver flu vaccines each fall, but that framework doesn’t align with the likely scenarios of limited supply, additional reporting requirements, two-dose regimens, and differing storage needs.
“Healthcare organizations have consistently risen to the challenge. I wholeheartedly believe in their potential to do this,” Anna Legreid Dopp, PharmD, senior director of quality improvement and guidelines for the American Society of Health-System Pharmacists, told Medscape Medical News.
Healthcare workers won’t face a vaccine mandate
Even after months of caring for COVID patients, most clinicians remain vulnerable to infection – at work and in their communities. That was what occupational medicine physician Kevin Smith, MD, realized when his health system, Toledo, Ohio–based ProMedica, offered antibody testing to all its 50,000 employees. About 2% of the 6933 tests given came back positive, he says.
Yet many physicians, nurses, and other healthcare workers share the public’s skepticism about the safety and effectiveness of a vaccine that receives swift US Food and Drug Administration (FDA) approval for emergency use. About half of nurses (47%) and almost 1 in 3 physicians (30%) say that they don’t want to get the vaccine when it first becomes available or that they’re unsure about vaccination, according to a Medscape survey.
Because vaccination of healthcare workers will set the stage for public acceptance of the vaccine, hospital epidemiologists are concerned. “We know that there will be some hesitancy in the healthcare workforce, just as there will be in the broader public,” said Marci Drees, MD, chief infection prevention officer and hospital epidemiologist for ChristianaCare in Newark, Delaware, and liaison from the Society for Healthcare Epidemiology of America to the CDC’s Advisory Committee on Immunization Practices.* “I do not think we can expect anyone to be vaccinated if we’re not willing to vaccinate ourselves.”
Healthcare workers are typically required to receive a range of vaccines, including measles, mumps, and rubella (MMR) and pertussis shots. Each year, close to half of US healthcare workers receive a flu vaccine under a workplace mandate. But COVID-19 will be different. The FDA requires anyone given products under an emergency use authorization (EUA) to receive information about risks and benefits and to have the option to decline. Hospitals instead will rely on education as they offer a novel vaccine (or more than one) that will have a minimum effectiveness of 50%.
ProMedica doesn’t require employees to be vaccinated against flu, but employees who decline must get a note from a doctor indicating that they have talked about the risks and benefits of the vaccine. A similar approach may be used with a COVID-19 vaccine, in which employees may be required to learn about the vaccine before they decline, Smith says. “I do believe some people will say they don’t want to get it,” he added.
Like colleagues across the country, Smith is identifying healthcare workers who are involved in direct care of COVID-19 patients and are at highest risk for exposure. Even within the top tier, those performing the riskiest tasks, such as respiratory therapists who provide breathing treatments that spread aerosols and droplets, will be tagged as a priority group, he says. Healthcare workers who spend the most time in proximity to COVID patients, such as nurses in a COVID unit, also are likely to get the first doses, he says.
Swirl, don’t shake, the vaccine
Hospitals are adept at ramping up vaccination campaigns. For example, last year, Vanderbilt University Medical Center, in Nashville, Tennessee, vaccinated nearly 16,000 employees against influenza in their 1-day “Flulapalooza” event. The medical center even earned a Guinness world record in 2011 at the first Flulapalooza for giving the most vaccinations ever within 8 hours.
The 10th anniversary of the event was canceled this year because of COVID restrictions. Instead, nurses, pharmacists, and other clinicians pitched in to vaccinate their coworkers against influenza. Now, plans for COVID-19 vaccination move forward amid uncertainty.
Instead of holding a mass event, “the delivery mechanisms will need to be more targeted and focused,” said Lori Rolando, MD, MPH, director of the Vanderbilt Occupational Health Clinic. In the CDC’s most recent version of its vaccination program “playbook,” the agency recommends giving the vaccines in an area that allows people to remain 6 feet apart and for them to wait for 15 minutes after receiving the shot to make sure they don’t faint, a potential risk common to almost all vaccines.
That’s the easy part. Planning becomes more complex, given the uncertainty as to which vaccines will receive approval and which one a hospital will receive.
If the Pfizer/BioNTech vaccine receives EUA in 2020, about 10 to 20 million doses could be available in November and 20 to 30 million doses in December. The ultracold containers used to ship the vaccines have to be replenished with dry ice within 24 hours of receipt and every 5 days thereafter. Hospitals will need temperature probes to monitor storage in the containers. The five-dose vials can be refrigerated before administering, but only for 5 days. The product must be diluted, and it then must be used within 6 hours.
The Moderna vaccine will be somewhat less plentiful at first. About 10 million doses are expected in November and 15 million doses by the end of December. The 10-dose vials are stored in a freezer. Once they are placed in a refrigerator to thaw, they have to be used within 7 days, and once they’re removed from the refrigerator, they have to be used within 12 hours. The pharmacist or other vaccinator must swirl – but not shake! – the vial before delivering a dose, according to the CDC playbook.
As more information emerges about the vaccines, instructions may change, and Smith is steeled for shifting scenarios. “These are all draft plans. We’re going to modify as we go along,” he says.
The Pfizer vaccine requires a second dose at 21 days, and the Moderna vaccine targets the second dose at 28 days. In addition to using information systems to track vaccinations and any adverse effects, hospitals will give employees a card indicating what vaccine they received, the date it was administered, and the date on which they need to return. (At this point, the time frame for the second dose doesn’t appear to be flexible.)
Regardless of the vaccine, one message stays the same: COVID precautions must continue. That means mask wearing, social distancing, and hand washing – practices that also must be followed by healthcare workers who test positive for naturally acquired antibodies.
“I don’t think anyone expects the COVID vaccine to be 100% effective at preventing COVID,” says Rolando. “So all of the other tools in our toolbox are going to need to be continued to be used as well.”
*Correction, 11/12/20: An earlier version of this article misstated the name of Dr. Drees' institution.
This article first appeared on Medscape.com.
At first, when news spread of a 28-year-old doctor on the COVID-19 front lines in Brazil who died after receiving an experimental vaccine, doubts arose about the safety of one of the most promising coronavirus vaccine candidates. But then the story flipped. Although the vaccine maker wouldn’t confirm it, the doctor appeared to have been in the control group and had received a dose of an established meningitis vaccine. The danger came from exposure to the coronavirus itself.
That tragedy underscores the ongoing risk of COVID-19 to healthcare workers, who have been designated by US advisory panels as part of phase 1A – the first to receive doses of any approved vaccine. The Centers for Disease Control and Prevention (CDC) recently reported that 6% of adults hospitalized with COVID from March to May were healthcare workers. The report was based on surveillance data from 13 states. The average age of the patients was 49 years. The agency set a November 15 vaccination “readiness date” for jurisdictions, such as state health departments, even though a vaccine isn’t likely to be authorized by then.
As hospitals scramble to prepare, their watchword is flexibility. They don’t yet know how many initial doses they will get, of which vaccine, or in what time frame. They have a sophisticated infrastructure to deliver flu vaccines each fall, but that framework doesn’t align with the likely scenarios of limited supply, additional reporting requirements, two-dose regimens, and differing storage needs.
“Healthcare organizations have consistently risen to the challenge. I wholeheartedly believe in their potential to do this,” Anna Legreid Dopp, PharmD, senior director of quality improvement and guidelines for the American Society of Health-System Pharmacists, told Medscape Medical News.
Healthcare workers won’t face a vaccine mandate
Even after months of caring for COVID patients, most clinicians remain vulnerable to infection – at work and in their communities. That was what occupational medicine physician Kevin Smith, MD, realized when his health system, Toledo, Ohio–based ProMedica, offered antibody testing to all its 50,000 employees. About 2% of the 6933 tests given came back positive, he says.
Yet many physicians, nurses, and other healthcare workers share the public’s skepticism about the safety and effectiveness of a vaccine that receives swift US Food and Drug Administration (FDA) approval for emergency use. About half of nurses (47%) and almost 1 in 3 physicians (30%) say that they don’t want to get the vaccine when it first becomes available or that they’re unsure about vaccination, according to a Medscape survey.
Because vaccination of healthcare workers will set the stage for public acceptance of the vaccine, hospital epidemiologists are concerned. “We know that there will be some hesitancy in the healthcare workforce, just as there will be in the broader public,” said Marci Drees, MD, chief infection prevention officer and hospital epidemiologist for ChristianaCare in Newark, Delaware, and liaison from the Society for Healthcare Epidemiology of America to the CDC’s Advisory Committee on Immunization Practices.* “I do not think we can expect anyone to be vaccinated if we’re not willing to vaccinate ourselves.”
Healthcare workers are typically required to receive a range of vaccines, including measles, mumps, and rubella (MMR) and pertussis shots. Each year, close to half of US healthcare workers receive a flu vaccine under a workplace mandate. But COVID-19 will be different. The FDA requires anyone given products under an emergency use authorization (EUA) to receive information about risks and benefits and to have the option to decline. Hospitals instead will rely on education as they offer a novel vaccine (or more than one) that will have a minimum effectiveness of 50%.
ProMedica doesn’t require employees to be vaccinated against flu, but employees who decline must get a note from a doctor indicating that they have talked about the risks and benefits of the vaccine. A similar approach may be used with a COVID-19 vaccine, in which employees may be required to learn about the vaccine before they decline, Smith says. “I do believe some people will say they don’t want to get it,” he added.
Like colleagues across the country, Smith is identifying healthcare workers who are involved in direct care of COVID-19 patients and are at highest risk for exposure. Even within the top tier, those performing the riskiest tasks, such as respiratory therapists who provide breathing treatments that spread aerosols and droplets, will be tagged as a priority group, he says. Healthcare workers who spend the most time in proximity to COVID patients, such as nurses in a COVID unit, also are likely to get the first doses, he says.
Swirl, don’t shake, the vaccine
Hospitals are adept at ramping up vaccination campaigns. For example, last year, Vanderbilt University Medical Center, in Nashville, Tennessee, vaccinated nearly 16,000 employees against influenza in their 1-day “Flulapalooza” event. The medical center even earned a Guinness world record in 2011 at the first Flulapalooza for giving the most vaccinations ever within 8 hours.
The 10th anniversary of the event was canceled this year because of COVID restrictions. Instead, nurses, pharmacists, and other clinicians pitched in to vaccinate their coworkers against influenza. Now, plans for COVID-19 vaccination move forward amid uncertainty.
Instead of holding a mass event, “the delivery mechanisms will need to be more targeted and focused,” said Lori Rolando, MD, MPH, director of the Vanderbilt Occupational Health Clinic. In the CDC’s most recent version of its vaccination program “playbook,” the agency recommends giving the vaccines in an area that allows people to remain 6 feet apart and for them to wait for 15 minutes after receiving the shot to make sure they don’t faint, a potential risk common to almost all vaccines.
That’s the easy part. Planning becomes more complex, given the uncertainty as to which vaccines will receive approval and which one a hospital will receive.
If the Pfizer/BioNTech vaccine receives EUA in 2020, about 10 to 20 million doses could be available in November and 20 to 30 million doses in December. The ultracold containers used to ship the vaccines have to be replenished with dry ice within 24 hours of receipt and every 5 days thereafter. Hospitals will need temperature probes to monitor storage in the containers. The five-dose vials can be refrigerated before administering, but only for 5 days. The product must be diluted, and it then must be used within 6 hours.
The Moderna vaccine will be somewhat less plentiful at first. About 10 million doses are expected in November and 15 million doses by the end of December. The 10-dose vials are stored in a freezer. Once they are placed in a refrigerator to thaw, they have to be used within 7 days, and once they’re removed from the refrigerator, they have to be used within 12 hours. The pharmacist or other vaccinator must swirl – but not shake! – the vial before delivering a dose, according to the CDC playbook.
As more information emerges about the vaccines, instructions may change, and Smith is steeled for shifting scenarios. “These are all draft plans. We’re going to modify as we go along,” he says.
The Pfizer vaccine requires a second dose at 21 days, and the Moderna vaccine targets the second dose at 28 days. In addition to using information systems to track vaccinations and any adverse effects, hospitals will give employees a card indicating what vaccine they received, the date it was administered, and the date on which they need to return. (At this point, the time frame for the second dose doesn’t appear to be flexible.)
Regardless of the vaccine, one message stays the same: COVID precautions must continue. That means mask wearing, social distancing, and hand washing – practices that also must be followed by healthcare workers who test positive for naturally acquired antibodies.
“I don’t think anyone expects the COVID vaccine to be 100% effective at preventing COVID,” says Rolando. “So all of the other tools in our toolbox are going to need to be continued to be used as well.”
*Correction, 11/12/20: An earlier version of this article misstated the name of Dr. Drees' institution.
This article first appeared on Medscape.com.
At first, when news spread of a 28-year-old doctor on the COVID-19 front lines in Brazil who died after receiving an experimental vaccine, doubts arose about the safety of one of the most promising coronavirus vaccine candidates. But then the story flipped. Although the vaccine maker wouldn’t confirm it, the doctor appeared to have been in the control group and had received a dose of an established meningitis vaccine. The danger came from exposure to the coronavirus itself.
That tragedy underscores the ongoing risk of COVID-19 to healthcare workers, who have been designated by US advisory panels as part of phase 1A – the first to receive doses of any approved vaccine. The Centers for Disease Control and Prevention (CDC) recently reported that 6% of adults hospitalized with COVID from March to May were healthcare workers. The report was based on surveillance data from 13 states. The average age of the patients was 49 years. The agency set a November 15 vaccination “readiness date” for jurisdictions, such as state health departments, even though a vaccine isn’t likely to be authorized by then.
As hospitals scramble to prepare, their watchword is flexibility. They don’t yet know how many initial doses they will get, of which vaccine, or in what time frame. They have a sophisticated infrastructure to deliver flu vaccines each fall, but that framework doesn’t align with the likely scenarios of limited supply, additional reporting requirements, two-dose regimens, and differing storage needs.
“Healthcare organizations have consistently risen to the challenge. I wholeheartedly believe in their potential to do this,” Anna Legreid Dopp, PharmD, senior director of quality improvement and guidelines for the American Society of Health-System Pharmacists, told Medscape Medical News.
Healthcare workers won’t face a vaccine mandate
Even after months of caring for COVID patients, most clinicians remain vulnerable to infection – at work and in their communities. That was what occupational medicine physician Kevin Smith, MD, realized when his health system, Toledo, Ohio–based ProMedica, offered antibody testing to all its 50,000 employees. About 2% of the 6933 tests given came back positive, he says.
Yet many physicians, nurses, and other healthcare workers share the public’s skepticism about the safety and effectiveness of a vaccine that receives swift US Food and Drug Administration (FDA) approval for emergency use. About half of nurses (47%) and almost 1 in 3 physicians (30%) say that they don’t want to get the vaccine when it first becomes available or that they’re unsure about vaccination, according to a Medscape survey.
Because vaccination of healthcare workers will set the stage for public acceptance of the vaccine, hospital epidemiologists are concerned. “We know that there will be some hesitancy in the healthcare workforce, just as there will be in the broader public,” said Marci Drees, MD, chief infection prevention officer and hospital epidemiologist for ChristianaCare in Newark, Delaware, and liaison from the Society for Healthcare Epidemiology of America to the CDC’s Advisory Committee on Immunization Practices.* “I do not think we can expect anyone to be vaccinated if we’re not willing to vaccinate ourselves.”
Healthcare workers are typically required to receive a range of vaccines, including measles, mumps, and rubella (MMR) and pertussis shots. Each year, close to half of US healthcare workers receive a flu vaccine under a workplace mandate. But COVID-19 will be different. The FDA requires anyone given products under an emergency use authorization (EUA) to receive information about risks and benefits and to have the option to decline. Hospitals instead will rely on education as they offer a novel vaccine (or more than one) that will have a minimum effectiveness of 50%.
ProMedica doesn’t require employees to be vaccinated against flu, but employees who decline must get a note from a doctor indicating that they have talked about the risks and benefits of the vaccine. A similar approach may be used with a COVID-19 vaccine, in which employees may be required to learn about the vaccine before they decline, Smith says. “I do believe some people will say they don’t want to get it,” he added.
Like colleagues across the country, Smith is identifying healthcare workers who are involved in direct care of COVID-19 patients and are at highest risk for exposure. Even within the top tier, those performing the riskiest tasks, such as respiratory therapists who provide breathing treatments that spread aerosols and droplets, will be tagged as a priority group, he says. Healthcare workers who spend the most time in proximity to COVID patients, such as nurses in a COVID unit, also are likely to get the first doses, he says.
Swirl, don’t shake, the vaccine
Hospitals are adept at ramping up vaccination campaigns. For example, last year, Vanderbilt University Medical Center, in Nashville, Tennessee, vaccinated nearly 16,000 employees against influenza in their 1-day “Flulapalooza” event. The medical center even earned a Guinness world record in 2011 at the first Flulapalooza for giving the most vaccinations ever within 8 hours.
The 10th anniversary of the event was canceled this year because of COVID restrictions. Instead, nurses, pharmacists, and other clinicians pitched in to vaccinate their coworkers against influenza. Now, plans for COVID-19 vaccination move forward amid uncertainty.
Instead of holding a mass event, “the delivery mechanisms will need to be more targeted and focused,” said Lori Rolando, MD, MPH, director of the Vanderbilt Occupational Health Clinic. In the CDC’s most recent version of its vaccination program “playbook,” the agency recommends giving the vaccines in an area that allows people to remain 6 feet apart and for them to wait for 15 minutes after receiving the shot to make sure they don’t faint, a potential risk common to almost all vaccines.
That’s the easy part. Planning becomes more complex, given the uncertainty as to which vaccines will receive approval and which one a hospital will receive.
If the Pfizer/BioNTech vaccine receives EUA in 2020, about 10 to 20 million doses could be available in November and 20 to 30 million doses in December. The ultracold containers used to ship the vaccines have to be replenished with dry ice within 24 hours of receipt and every 5 days thereafter. Hospitals will need temperature probes to monitor storage in the containers. The five-dose vials can be refrigerated before administering, but only for 5 days. The product must be diluted, and it then must be used within 6 hours.
The Moderna vaccine will be somewhat less plentiful at first. About 10 million doses are expected in November and 15 million doses by the end of December. The 10-dose vials are stored in a freezer. Once they are placed in a refrigerator to thaw, they have to be used within 7 days, and once they’re removed from the refrigerator, they have to be used within 12 hours. The pharmacist or other vaccinator must swirl – but not shake! – the vial before delivering a dose, according to the CDC playbook.
As more information emerges about the vaccines, instructions may change, and Smith is steeled for shifting scenarios. “These are all draft plans. We’re going to modify as we go along,” he says.
The Pfizer vaccine requires a second dose at 21 days, and the Moderna vaccine targets the second dose at 28 days. In addition to using information systems to track vaccinations and any adverse effects, hospitals will give employees a card indicating what vaccine they received, the date it was administered, and the date on which they need to return. (At this point, the time frame for the second dose doesn’t appear to be flexible.)
Regardless of the vaccine, one message stays the same: COVID precautions must continue. That means mask wearing, social distancing, and hand washing – practices that also must be followed by healthcare workers who test positive for naturally acquired antibodies.
“I don’t think anyone expects the COVID vaccine to be 100% effective at preventing COVID,” says Rolando. “So all of the other tools in our toolbox are going to need to be continued to be used as well.”
*Correction, 11/12/20: An earlier version of this article misstated the name of Dr. Drees' institution.
This article first appeared on Medscape.com.