User login
Herpes zoster infection with MS treatment higher in women?
a new study of adverse event reports on a variety of DMTs suggests.
DMTs are known to be associated with a potentially increased risk of opportunistic infections, including HZV. However, data are lacking on issues such as the relative frequency of HZV and the distribution of cases among age and gender groups, said senior author Ahmed Zayed Obeidat, MD, PhD, assistant professor at the Medical College of Wisconsin, Milwaukee.
“In my practice, we noticed patients being treated with DMTs were developing shingles at much younger ages than would be typical, so we were interested in looking at the distribution of cases among people treated with DMTs,” he said.
For the study, which was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers, Dr. Obeidat, first author Nicola Carlisle, MD, also of the Medical College of Wisconsin, and their colleagues turned to data from the Food and Drug Administration’s Adverse Event Reporting System.
They analyzed reports on adverse events involving HZV and varicella among patients with MS received between January 1999 and June 2019. The reports involved a range of MS DMTs, including interferon-beta, glatiramer acetate, natalizumab, fingolimod, teriflunomide, dimethyl fumarate, alemtuzumab, or ocrelizumab. Recently approved DMTs including cladribine and siponimod were excluded because of an insufficient number of reports.
Among 3,335 reports that were identified, they found highest mean annual report rates of HZV were for natalizumab, at 115.4, and lowest for glatiramer acetate, with just 5.3 reports. The mean annual report rates for HZV among the other DMTs were ocrelizumab, 88.3; dimethyl fumarate, 73.4; fingolimod, 72.9; interferon beta, 32.9; alemtuzumab, 21.7; and teriflunomide, 13.9.
Overall, the reports of HZV were 4.5 times more common among females, ranging from 2.1 times greater with alemtuzumab to 11.4 times greater for females with interferon-beta. The highest percentages of reports involved people in their 50s, with the exceptions of fingolimod, which had the highest rate of reports among patients in their 40s, and alemtuzumab, in which the highest percentage of reports involved patients in their 30s.
Meanwhile, as many as 25.7% of cases occurred in people under the age of 40 years, while 77.6% of total reports of HZV were in age groups between 31 years and 60 years.
“These rates are different than what is expected in the shingles population, which usually involves people over 60,” Dr. Obeidat said. He noted that, while MS is known to affect more women than men, the fivefold increase in HZV well exceeds the female-male ratio in MS, which is about 2.5:1.
Dr. Obeidat speculated that one factor explaining the higher reports of younger patients could be that fewer older patients are taking DMTs. “Many of our patients with MS may not be treated with DMTs when they are older or they may be on older DMTs that don’t have as much of a risk of opportunistic infections or activation, or some older patients may not be on medications anymore, so this may be why we are seeing this,” he said.
In commenting on the study, Joshua Katz, MD, codirector of the Elliot Lewis Center for Multiple Sclerosis Care, Wellesley, Mass., speculated that numerous factors could explain the higher rates of women developing HZV.
“One wonders, for instance, did pregnancy play a role, were some of the women on prior medications?”
The statistical difference is interesting, he said, “but it’s hard to see what the explanation could be.”
While DMTs typically can be effective in suppressing an MS flare even if a patient develops shingles, the risks of the shingles, itself, is a concern, Dr. Katz added. “Just about any infection that stimulates an inflammatory response has some risk of worsening symptoms with MS; however, the bigger risk is probably the shingles itself and getting postherpetic neuralgia,” he explained.
“Sometimes there can be independent neurological problems just from MS, and that’s probably a bigger risk than worsening the MS,” he said. Clinicians should therefore keep shingles on their radar before starting patients on DMTs, Dr. Katz added.
“For many of the medications that are immunosuppressive, you want to check patients’ baseline levels of antibodies for zoster and if they don’t have antibodies, then you do want to vaccinate them.”
He noted that the new HZV vaccine is not a live vaccine and has a high efficacy rate, “so we think we can safely administer it in most cases.
“A concern is whether some DMTs may render the vaccine less effective, and we are looking at studying that with ocrelizumab and maybe some other B-cell depleting treatments.”
Dr. Obeidat disclosed relationships with Alexion, Biogen, Bristol-Myers Squibb, Celgene, EMD Serono, Genentech, Sanofi and Novartis. Dr. Katz has been a speaker for Biogen, Genetech, Sanofi, and EMD Serono.
This article first appeared on Medscape.com.
a new study of adverse event reports on a variety of DMTs suggests.
DMTs are known to be associated with a potentially increased risk of opportunistic infections, including HZV. However, data are lacking on issues such as the relative frequency of HZV and the distribution of cases among age and gender groups, said senior author Ahmed Zayed Obeidat, MD, PhD, assistant professor at the Medical College of Wisconsin, Milwaukee.
“In my practice, we noticed patients being treated with DMTs were developing shingles at much younger ages than would be typical, so we were interested in looking at the distribution of cases among people treated with DMTs,” he said.
For the study, which was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers, Dr. Obeidat, first author Nicola Carlisle, MD, also of the Medical College of Wisconsin, and their colleagues turned to data from the Food and Drug Administration’s Adverse Event Reporting System.
They analyzed reports on adverse events involving HZV and varicella among patients with MS received between January 1999 and June 2019. The reports involved a range of MS DMTs, including interferon-beta, glatiramer acetate, natalizumab, fingolimod, teriflunomide, dimethyl fumarate, alemtuzumab, or ocrelizumab. Recently approved DMTs including cladribine and siponimod were excluded because of an insufficient number of reports.
Among 3,335 reports that were identified, they found highest mean annual report rates of HZV were for natalizumab, at 115.4, and lowest for glatiramer acetate, with just 5.3 reports. The mean annual report rates for HZV among the other DMTs were ocrelizumab, 88.3; dimethyl fumarate, 73.4; fingolimod, 72.9; interferon beta, 32.9; alemtuzumab, 21.7; and teriflunomide, 13.9.
Overall, the reports of HZV were 4.5 times more common among females, ranging from 2.1 times greater with alemtuzumab to 11.4 times greater for females with interferon-beta. The highest percentages of reports involved people in their 50s, with the exceptions of fingolimod, which had the highest rate of reports among patients in their 40s, and alemtuzumab, in which the highest percentage of reports involved patients in their 30s.
Meanwhile, as many as 25.7% of cases occurred in people under the age of 40 years, while 77.6% of total reports of HZV were in age groups between 31 years and 60 years.
“These rates are different than what is expected in the shingles population, which usually involves people over 60,” Dr. Obeidat said. He noted that, while MS is known to affect more women than men, the fivefold increase in HZV well exceeds the female-male ratio in MS, which is about 2.5:1.
Dr. Obeidat speculated that one factor explaining the higher reports of younger patients could be that fewer older patients are taking DMTs. “Many of our patients with MS may not be treated with DMTs when they are older or they may be on older DMTs that don’t have as much of a risk of opportunistic infections or activation, or some older patients may not be on medications anymore, so this may be why we are seeing this,” he said.
In commenting on the study, Joshua Katz, MD, codirector of the Elliot Lewis Center for Multiple Sclerosis Care, Wellesley, Mass., speculated that numerous factors could explain the higher rates of women developing HZV.
“One wonders, for instance, did pregnancy play a role, were some of the women on prior medications?”
The statistical difference is interesting, he said, “but it’s hard to see what the explanation could be.”
While DMTs typically can be effective in suppressing an MS flare even if a patient develops shingles, the risks of the shingles, itself, is a concern, Dr. Katz added. “Just about any infection that stimulates an inflammatory response has some risk of worsening symptoms with MS; however, the bigger risk is probably the shingles itself and getting postherpetic neuralgia,” he explained.
“Sometimes there can be independent neurological problems just from MS, and that’s probably a bigger risk than worsening the MS,” he said. Clinicians should therefore keep shingles on their radar before starting patients on DMTs, Dr. Katz added.
“For many of the medications that are immunosuppressive, you want to check patients’ baseline levels of antibodies for zoster and if they don’t have antibodies, then you do want to vaccinate them.”
He noted that the new HZV vaccine is not a live vaccine and has a high efficacy rate, “so we think we can safely administer it in most cases.
“A concern is whether some DMTs may render the vaccine less effective, and we are looking at studying that with ocrelizumab and maybe some other B-cell depleting treatments.”
Dr. Obeidat disclosed relationships with Alexion, Biogen, Bristol-Myers Squibb, Celgene, EMD Serono, Genentech, Sanofi and Novartis. Dr. Katz has been a speaker for Biogen, Genetech, Sanofi, and EMD Serono.
This article first appeared on Medscape.com.
a new study of adverse event reports on a variety of DMTs suggests.
DMTs are known to be associated with a potentially increased risk of opportunistic infections, including HZV. However, data are lacking on issues such as the relative frequency of HZV and the distribution of cases among age and gender groups, said senior author Ahmed Zayed Obeidat, MD, PhD, assistant professor at the Medical College of Wisconsin, Milwaukee.
“In my practice, we noticed patients being treated with DMTs were developing shingles at much younger ages than would be typical, so we were interested in looking at the distribution of cases among people treated with DMTs,” he said.
For the study, which was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers, Dr. Obeidat, first author Nicola Carlisle, MD, also of the Medical College of Wisconsin, and their colleagues turned to data from the Food and Drug Administration’s Adverse Event Reporting System.
They analyzed reports on adverse events involving HZV and varicella among patients with MS received between January 1999 and June 2019. The reports involved a range of MS DMTs, including interferon-beta, glatiramer acetate, natalizumab, fingolimod, teriflunomide, dimethyl fumarate, alemtuzumab, or ocrelizumab. Recently approved DMTs including cladribine and siponimod were excluded because of an insufficient number of reports.
Among 3,335 reports that were identified, they found highest mean annual report rates of HZV were for natalizumab, at 115.4, and lowest for glatiramer acetate, with just 5.3 reports. The mean annual report rates for HZV among the other DMTs were ocrelizumab, 88.3; dimethyl fumarate, 73.4; fingolimod, 72.9; interferon beta, 32.9; alemtuzumab, 21.7; and teriflunomide, 13.9.
Overall, the reports of HZV were 4.5 times more common among females, ranging from 2.1 times greater with alemtuzumab to 11.4 times greater for females with interferon-beta. The highest percentages of reports involved people in their 50s, with the exceptions of fingolimod, which had the highest rate of reports among patients in their 40s, and alemtuzumab, in which the highest percentage of reports involved patients in their 30s.
Meanwhile, as many as 25.7% of cases occurred in people under the age of 40 years, while 77.6% of total reports of HZV were in age groups between 31 years and 60 years.
“These rates are different than what is expected in the shingles population, which usually involves people over 60,” Dr. Obeidat said. He noted that, while MS is known to affect more women than men, the fivefold increase in HZV well exceeds the female-male ratio in MS, which is about 2.5:1.
Dr. Obeidat speculated that one factor explaining the higher reports of younger patients could be that fewer older patients are taking DMTs. “Many of our patients with MS may not be treated with DMTs when they are older or they may be on older DMTs that don’t have as much of a risk of opportunistic infections or activation, or some older patients may not be on medications anymore, so this may be why we are seeing this,” he said.
In commenting on the study, Joshua Katz, MD, codirector of the Elliot Lewis Center for Multiple Sclerosis Care, Wellesley, Mass., speculated that numerous factors could explain the higher rates of women developing HZV.
“One wonders, for instance, did pregnancy play a role, were some of the women on prior medications?”
The statistical difference is interesting, he said, “but it’s hard to see what the explanation could be.”
While DMTs typically can be effective in suppressing an MS flare even if a patient develops shingles, the risks of the shingles, itself, is a concern, Dr. Katz added. “Just about any infection that stimulates an inflammatory response has some risk of worsening symptoms with MS; however, the bigger risk is probably the shingles itself and getting postherpetic neuralgia,” he explained.
“Sometimes there can be independent neurological problems just from MS, and that’s probably a bigger risk than worsening the MS,” he said. Clinicians should therefore keep shingles on their radar before starting patients on DMTs, Dr. Katz added.
“For many of the medications that are immunosuppressive, you want to check patients’ baseline levels of antibodies for zoster and if they don’t have antibodies, then you do want to vaccinate them.”
He noted that the new HZV vaccine is not a live vaccine and has a high efficacy rate, “so we think we can safely administer it in most cases.
“A concern is whether some DMTs may render the vaccine less effective, and we are looking at studying that with ocrelizumab and maybe some other B-cell depleting treatments.”
Dr. Obeidat disclosed relationships with Alexion, Biogen, Bristol-Myers Squibb, Celgene, EMD Serono, Genentech, Sanofi and Novartis. Dr. Katz has been a speaker for Biogen, Genetech, Sanofi, and EMD Serono.
This article first appeared on Medscape.com.
Constraint-induced movement therapy may boost neuroplasticity in MS
, compared with alternative interventions with medicines. “The findings suggest for the first time that physical behavioral change therapy can significantly stimulate cortical neuroplasticity in a degenerative central nervous system disorder,” said the authors of research presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
CIMT, an intervention involving 3.5 hours/day of therapist-supervised treatment over 10 consecutive weekdays, has been shown to significantly improve paretic limb use for patients with progressive MS, and the effects are long lasting.
For patients with asymmetric upper limb nonuse, the treatment “is highly successful for promoting increased use by the more-affected arm for everyday activities,” said lead author Victor W. Mark, MD, an associate professor and medical director of the Constraint-Induced Movement Therapy Research Programs at the University of Alabama at Birmingham. “The improvements after CIMT can be found to remain as much as 1 year after the completion of the treatment, and even later. That by itself is novel for MS,” he said.
The team’s previous research in Neurorehabilitation and Neural Repair showed that the CIMT intervention is associated with statistically significant changes in white matter integrity in the brain. In this new study, Dr. Mark and colleagues sought to determine whether the effects would also translate to improvements in cortical gray matter.
Promoting neuroplasticity, improving motor function
For their study, they enrolled 20 adults with chronic MS who were matched with respect to unilateral arm disability. The participants were randomly assigned to receive 35 hours of either CIMT or a holistic complementary alternative medicine program, which included yoga, aquatic therapy, massage, and/or relaxation techniques, over the course of 2 weeks.
Both groups expressed the same degree of expectancy of benefits from the intervention. Those who received CIMT showed a significantly larger effect size on the Motor Activity Log, a measure that has been validated against real-world upper-limb accelerometry, compared with the control group (d = 3.2, vs. d = 0.7).
Imaging with tensor-based morphometry showed an increase in the thickness of the primary motor cortex in patients who underwent CIMT but not those who received the alternative medicine treatment. Furthermore, a change in the primary motor cortex was observed in the CIMT group on voxel-based morphometry, suggesting an increase in cortical density or volume, or both. Similar changes were not seen in the alternative medicine group.
“We evaluated the density of the brain cortical gray area before and after treatment, and we found increased gray matter in the area of the brain that is concentrated with voluntary limb movement (the motor cortex),” Dr. Mark said. “As in (previous) studies, we did not find such changes, or any changes, after the other form of treatment,” he said.
The results are important, Dr. Mark noted, “because CIMT seems to specifically promote neuroplasticity changes that appear to be healthy, for what is otherwise a chronically progressive degenerative neurological disorder.”
In addition to the improvements in MS, CIMT has led to improvement in motor function for patients who have experienced other central nervous system injuries, including stroke, traumatic brain injury, cerebral palsy, and, in musicians, focal hand dystonia.
The new findings offer intriguing insights into the effects in progressive MS, commented rehabilitation specialist Patricia Bobryk, MHS, a physical therapist with the UCHealth Yampa Valley Medical Center, in Colorado Springs.
“There is more evidence for CIMT in the area of stroke, which is more acute onset and with more potential recovery, especially early on, so this is exciting initial work in terms of MS,” she said.
“If we’re trying to find new avenues in the brain for better pathways, rather than using something that’s damaged in MS, it makes perfect sense that CIMT really forces and drives those connections, because you’re doing a repetitive, high-intensity patterning throughout the day, so you set up that environment for things to progress, especially in motor functioning,” she said.
Repetition, ‘prevention of compensation’
CIMT was developed at the University of Alabama, Birmingham, 30 years ago and involves four components. The first, described as “massed practice,” involves intensive, repetitive arm movements of the affected arm. The second component involves “shaping,” in which the patient is encouraged to perform his or her best attempts at the movements.
For the third component, described as “prevention of compensation,” the patient’s more-functional arm is inhibited from being used in everyday activities by wearing a padded mitt.
“This permits the patient to brace him- or herself whenever needed, but the better hand nonetheless lacks the dexterity to take over the activities that should be performed by the worse arm,” Dr. Mark explained.
“The patient wears the padded mitt after hours, too, except when using water or when sleeping,” he said.
The fourth component is a set of behavioral enforcement techniques involving goal-setting; daily interviews and discussion of progress and challenges; nightly homework; diary keeping; and telephone follow-up.
Dr. Mark noted that the intervention could have benefits that are secondary to motor and movement function. “We consider that the improvement of limb activity in a motor-challenged person with MS could afford a way to offset the deleterious effects of inactivity that can occur, such as weight gain, diabetes, osteoporosis, cardiac disease, and other conditions associated with prolonged inactivity,” he said.
Although it was developed at the University of Alabama, CIMT is currently more widely practiced in Europe than the United States, likely because of differences in care support, which in Europe is provided through socialized medicine, Dr. Mark pointed out.
Although the detailed methods for conducting CIMT are published in peer-reviewed journals, Dr. Mark recommends hands-on and interactive teaching. Such training is offered to clinicians and affiliated physical therapists and occupational therapists through Mark’s program at the University of Alabama in a semiannual, week-long training course, which includes hands-on treatment practice with actual patients.
Proof of principle
In further commenting on the study, Kathy M. Zackowski, PhD, of the National MS Society, said the findings provide an intriguing proof of concept that should be tested in a larger cohort. “The question of how much a behavioral (therapy) can impact true brain structural change or change in the pathologic mechanism is intriguing and of high importance,” she said.
“It is important to take this information as ‘proof of principle’ of the importance of CIMT for improving upper limb activity,” according to Dr. Zackowski, senior director, patient management, care and rehabilitation research at the society.
“Importantly, this team needs to move forward testing their hypothesis in a larger randomized, clinical trial with a full control group in order to show causal evidence that one intervention caused the structural brain changes seen,” she said in an interview.
Dr. Zackowski added that a caveat of CIMT is that the approach assumes one limb is more impaired than the other, which is always the case in stroke but is true only in some cases of MS. “Therefore, this method may not be effective for everyone with MS, but offers another option for tailoring an intervention to a person’s abilities and interests,” she said.
“Another important detail is that CIMT is also being explored for lower extremity use,” she added. “This is exciting, as lower extremity dysfunction is a very common problem in MS, and may be useful in treating walking disability.”
The authors, Ms. Bobryk, and Dr. Zackowski have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, compared with alternative interventions with medicines. “The findings suggest for the first time that physical behavioral change therapy can significantly stimulate cortical neuroplasticity in a degenerative central nervous system disorder,” said the authors of research presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
CIMT, an intervention involving 3.5 hours/day of therapist-supervised treatment over 10 consecutive weekdays, has been shown to significantly improve paretic limb use for patients with progressive MS, and the effects are long lasting.
For patients with asymmetric upper limb nonuse, the treatment “is highly successful for promoting increased use by the more-affected arm for everyday activities,” said lead author Victor W. Mark, MD, an associate professor and medical director of the Constraint-Induced Movement Therapy Research Programs at the University of Alabama at Birmingham. “The improvements after CIMT can be found to remain as much as 1 year after the completion of the treatment, and even later. That by itself is novel for MS,” he said.
The team’s previous research in Neurorehabilitation and Neural Repair showed that the CIMT intervention is associated with statistically significant changes in white matter integrity in the brain. In this new study, Dr. Mark and colleagues sought to determine whether the effects would also translate to improvements in cortical gray matter.
Promoting neuroplasticity, improving motor function
For their study, they enrolled 20 adults with chronic MS who were matched with respect to unilateral arm disability. The participants were randomly assigned to receive 35 hours of either CIMT or a holistic complementary alternative medicine program, which included yoga, aquatic therapy, massage, and/or relaxation techniques, over the course of 2 weeks.
Both groups expressed the same degree of expectancy of benefits from the intervention. Those who received CIMT showed a significantly larger effect size on the Motor Activity Log, a measure that has been validated against real-world upper-limb accelerometry, compared with the control group (d = 3.2, vs. d = 0.7).
Imaging with tensor-based morphometry showed an increase in the thickness of the primary motor cortex in patients who underwent CIMT but not those who received the alternative medicine treatment. Furthermore, a change in the primary motor cortex was observed in the CIMT group on voxel-based morphometry, suggesting an increase in cortical density or volume, or both. Similar changes were not seen in the alternative medicine group.
“We evaluated the density of the brain cortical gray area before and after treatment, and we found increased gray matter in the area of the brain that is concentrated with voluntary limb movement (the motor cortex),” Dr. Mark said. “As in (previous) studies, we did not find such changes, or any changes, after the other form of treatment,” he said.
The results are important, Dr. Mark noted, “because CIMT seems to specifically promote neuroplasticity changes that appear to be healthy, for what is otherwise a chronically progressive degenerative neurological disorder.”
In addition to the improvements in MS, CIMT has led to improvement in motor function for patients who have experienced other central nervous system injuries, including stroke, traumatic brain injury, cerebral palsy, and, in musicians, focal hand dystonia.
The new findings offer intriguing insights into the effects in progressive MS, commented rehabilitation specialist Patricia Bobryk, MHS, a physical therapist with the UCHealth Yampa Valley Medical Center, in Colorado Springs.
“There is more evidence for CIMT in the area of stroke, which is more acute onset and with more potential recovery, especially early on, so this is exciting initial work in terms of MS,” she said.
“If we’re trying to find new avenues in the brain for better pathways, rather than using something that’s damaged in MS, it makes perfect sense that CIMT really forces and drives those connections, because you’re doing a repetitive, high-intensity patterning throughout the day, so you set up that environment for things to progress, especially in motor functioning,” she said.
Repetition, ‘prevention of compensation’
CIMT was developed at the University of Alabama, Birmingham, 30 years ago and involves four components. The first, described as “massed practice,” involves intensive, repetitive arm movements of the affected arm. The second component involves “shaping,” in which the patient is encouraged to perform his or her best attempts at the movements.
For the third component, described as “prevention of compensation,” the patient’s more-functional arm is inhibited from being used in everyday activities by wearing a padded mitt.
“This permits the patient to brace him- or herself whenever needed, but the better hand nonetheless lacks the dexterity to take over the activities that should be performed by the worse arm,” Dr. Mark explained.
“The patient wears the padded mitt after hours, too, except when using water or when sleeping,” he said.
The fourth component is a set of behavioral enforcement techniques involving goal-setting; daily interviews and discussion of progress and challenges; nightly homework; diary keeping; and telephone follow-up.
Dr. Mark noted that the intervention could have benefits that are secondary to motor and movement function. “We consider that the improvement of limb activity in a motor-challenged person with MS could afford a way to offset the deleterious effects of inactivity that can occur, such as weight gain, diabetes, osteoporosis, cardiac disease, and other conditions associated with prolonged inactivity,” he said.
Although it was developed at the University of Alabama, CIMT is currently more widely practiced in Europe than the United States, likely because of differences in care support, which in Europe is provided through socialized medicine, Dr. Mark pointed out.
Although the detailed methods for conducting CIMT are published in peer-reviewed journals, Dr. Mark recommends hands-on and interactive teaching. Such training is offered to clinicians and affiliated physical therapists and occupational therapists through Mark’s program at the University of Alabama in a semiannual, week-long training course, which includes hands-on treatment practice with actual patients.
Proof of principle
In further commenting on the study, Kathy M. Zackowski, PhD, of the National MS Society, said the findings provide an intriguing proof of concept that should be tested in a larger cohort. “The question of how much a behavioral (therapy) can impact true brain structural change or change in the pathologic mechanism is intriguing and of high importance,” she said.
“It is important to take this information as ‘proof of principle’ of the importance of CIMT for improving upper limb activity,” according to Dr. Zackowski, senior director, patient management, care and rehabilitation research at the society.
“Importantly, this team needs to move forward testing their hypothesis in a larger randomized, clinical trial with a full control group in order to show causal evidence that one intervention caused the structural brain changes seen,” she said in an interview.
Dr. Zackowski added that a caveat of CIMT is that the approach assumes one limb is more impaired than the other, which is always the case in stroke but is true only in some cases of MS. “Therefore, this method may not be effective for everyone with MS, but offers another option for tailoring an intervention to a person’s abilities and interests,” she said.
“Another important detail is that CIMT is also being explored for lower extremity use,” she added. “This is exciting, as lower extremity dysfunction is a very common problem in MS, and may be useful in treating walking disability.”
The authors, Ms. Bobryk, and Dr. Zackowski have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, compared with alternative interventions with medicines. “The findings suggest for the first time that physical behavioral change therapy can significantly stimulate cortical neuroplasticity in a degenerative central nervous system disorder,” said the authors of research presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
CIMT, an intervention involving 3.5 hours/day of therapist-supervised treatment over 10 consecutive weekdays, has been shown to significantly improve paretic limb use for patients with progressive MS, and the effects are long lasting.
For patients with asymmetric upper limb nonuse, the treatment “is highly successful for promoting increased use by the more-affected arm for everyday activities,” said lead author Victor W. Mark, MD, an associate professor and medical director of the Constraint-Induced Movement Therapy Research Programs at the University of Alabama at Birmingham. “The improvements after CIMT can be found to remain as much as 1 year after the completion of the treatment, and even later. That by itself is novel for MS,” he said.
The team’s previous research in Neurorehabilitation and Neural Repair showed that the CIMT intervention is associated with statistically significant changes in white matter integrity in the brain. In this new study, Dr. Mark and colleagues sought to determine whether the effects would also translate to improvements in cortical gray matter.
Promoting neuroplasticity, improving motor function
For their study, they enrolled 20 adults with chronic MS who were matched with respect to unilateral arm disability. The participants were randomly assigned to receive 35 hours of either CIMT or a holistic complementary alternative medicine program, which included yoga, aquatic therapy, massage, and/or relaxation techniques, over the course of 2 weeks.
Both groups expressed the same degree of expectancy of benefits from the intervention. Those who received CIMT showed a significantly larger effect size on the Motor Activity Log, a measure that has been validated against real-world upper-limb accelerometry, compared with the control group (d = 3.2, vs. d = 0.7).
Imaging with tensor-based morphometry showed an increase in the thickness of the primary motor cortex in patients who underwent CIMT but not those who received the alternative medicine treatment. Furthermore, a change in the primary motor cortex was observed in the CIMT group on voxel-based morphometry, suggesting an increase in cortical density or volume, or both. Similar changes were not seen in the alternative medicine group.
“We evaluated the density of the brain cortical gray area before and after treatment, and we found increased gray matter in the area of the brain that is concentrated with voluntary limb movement (the motor cortex),” Dr. Mark said. “As in (previous) studies, we did not find such changes, or any changes, after the other form of treatment,” he said.
The results are important, Dr. Mark noted, “because CIMT seems to specifically promote neuroplasticity changes that appear to be healthy, for what is otherwise a chronically progressive degenerative neurological disorder.”
In addition to the improvements in MS, CIMT has led to improvement in motor function for patients who have experienced other central nervous system injuries, including stroke, traumatic brain injury, cerebral palsy, and, in musicians, focal hand dystonia.
The new findings offer intriguing insights into the effects in progressive MS, commented rehabilitation specialist Patricia Bobryk, MHS, a physical therapist with the UCHealth Yampa Valley Medical Center, in Colorado Springs.
“There is more evidence for CIMT in the area of stroke, which is more acute onset and with more potential recovery, especially early on, so this is exciting initial work in terms of MS,” she said.
“If we’re trying to find new avenues in the brain for better pathways, rather than using something that’s damaged in MS, it makes perfect sense that CIMT really forces and drives those connections, because you’re doing a repetitive, high-intensity patterning throughout the day, so you set up that environment for things to progress, especially in motor functioning,” she said.
Repetition, ‘prevention of compensation’
CIMT was developed at the University of Alabama, Birmingham, 30 years ago and involves four components. The first, described as “massed practice,” involves intensive, repetitive arm movements of the affected arm. The second component involves “shaping,” in which the patient is encouraged to perform his or her best attempts at the movements.
For the third component, described as “prevention of compensation,” the patient’s more-functional arm is inhibited from being used in everyday activities by wearing a padded mitt.
“This permits the patient to brace him- or herself whenever needed, but the better hand nonetheless lacks the dexterity to take over the activities that should be performed by the worse arm,” Dr. Mark explained.
“The patient wears the padded mitt after hours, too, except when using water or when sleeping,” he said.
The fourth component is a set of behavioral enforcement techniques involving goal-setting; daily interviews and discussion of progress and challenges; nightly homework; diary keeping; and telephone follow-up.
Dr. Mark noted that the intervention could have benefits that are secondary to motor and movement function. “We consider that the improvement of limb activity in a motor-challenged person with MS could afford a way to offset the deleterious effects of inactivity that can occur, such as weight gain, diabetes, osteoporosis, cardiac disease, and other conditions associated with prolonged inactivity,” he said.
Although it was developed at the University of Alabama, CIMT is currently more widely practiced in Europe than the United States, likely because of differences in care support, which in Europe is provided through socialized medicine, Dr. Mark pointed out.
Although the detailed methods for conducting CIMT are published in peer-reviewed journals, Dr. Mark recommends hands-on and interactive teaching. Such training is offered to clinicians and affiliated physical therapists and occupational therapists through Mark’s program at the University of Alabama in a semiannual, week-long training course, which includes hands-on treatment practice with actual patients.
Proof of principle
In further commenting on the study, Kathy M. Zackowski, PhD, of the National MS Society, said the findings provide an intriguing proof of concept that should be tested in a larger cohort. “The question of how much a behavioral (therapy) can impact true brain structural change or change in the pathologic mechanism is intriguing and of high importance,” she said.
“It is important to take this information as ‘proof of principle’ of the importance of CIMT for improving upper limb activity,” according to Dr. Zackowski, senior director, patient management, care and rehabilitation research at the society.
“Importantly, this team needs to move forward testing their hypothesis in a larger randomized, clinical trial with a full control group in order to show causal evidence that one intervention caused the structural brain changes seen,” she said in an interview.
Dr. Zackowski added that a caveat of CIMT is that the approach assumes one limb is more impaired than the other, which is always the case in stroke but is true only in some cases of MS. “Therefore, this method may not be effective for everyone with MS, but offers another option for tailoring an intervention to a person’s abilities and interests,” she said.
“Another important detail is that CIMT is also being explored for lower extremity use,” she added. “This is exciting, as lower extremity dysfunction is a very common problem in MS, and may be useful in treating walking disability.”
The authors, Ms. Bobryk, and Dr. Zackowski have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM CMSC 2020
Ofatumumab shows high elimination of disease activity in MS
, a new study shows.
The drug, which is already approved for the treatment of chronic lymphocytic leukemia, is currently under review for relapsing MS as a once-per-month self-injected therapy that could offer a convenient alternative to DMTs that require in-office infusion.
The new findings are from a pooled analysis from the phase 3 ASCLEPIOS I/II trials of the use of ofatumumab for patients with relapsing MS. There were 927 patients in the ASCLEPIOS I trial and 955 in the ASCLEPIOS II trial. The trials were conducted in 37 countries and involved patients aged 18-55 years.
The late-breaking research was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The studies compared patients who were treated with subcutaneous ofatumumab 20 mg with patients treated with oral teriflunomide 14 mg once daily for up to 30 months. The average duration of follow-up was 18 months.
NEDA-3, commonly used to determine treatment outcomes for patients with relapsing MS, was defined as a composite of having no worsening of disability over a 6-month period (6mCDW), no confirmed MS relapse, no new/enlarging T2 lesions, and no gadolinium-enhancing T1 lesions.
The pooled results showed that the odds of achieving NEDA-3 during the first 12 months were three times greater with ofatumumab than with teriflunomide (47.0% vs. 24.5%; odds ratio [OR], 3.36; P < .001) and were more than eight times greater from months 12 to 24 (87.8% vs. 48.2%; OR, 8.09; P < .001).
In addition, compared with patients who received teriflunomide, a higher proportion of patients who received ofatumumab were free from 6mCDW over 2 years (91.9% vs. 88.9%), as well as from relapses (82.3% vs 69.2%) and lesion activity (54.1% vs. 27.5%).
There was a significantly greater reduction in annualized relapse rate with ofatumumab compared with teriflunomide at all cumulative time intervals, including months 0 to 3 (P = .011), and at all subsequent time intervals from month 0 to 27 (P < .001).
The pooled findings further showed that ofatumumab reduced the mean number of gadolinium-enhancing T1 lesions per scan by 95.9% compared with teriflunomide (P < .001).
“Ofatumumab increased the probability of achieving NEDA-3 and demonstrated superior efficacy vs teriflunomide in patients with relapsing MS,” said the authors, led by Stephen L. Hauser, MD, of the department of neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco.
Ofatumumab superior in primary, secondary outcomes
As previously reported, subcutaneous ofatumumab also demonstrated superior efficacy over oral teriflunomide in the primary and secondary endpoints in the ASCLEPIOS I/II trials. The annualized relapse rate was reduced by 0.22 in the teriflunomide group, vs 0.11 in the ofatumumab group (50.5% relative reduction; P < .001) in the ASCLEPIOS I trial, and by 0.25 vs. 0.10 (58.5% relative reduction P < .001) in ASCLEPIOS II.
Ofatumumab also reduced the number of gadolinium-enhancing T1 lesions and new or enlarging T2 lesions compared with teriflunomide (all P < .001). It reduced the risk for disability progression by 34.4% over 3 months and by 32.5% over 6 months.
In the studies, the rate of serious infection with ofatumumab was 2.5%, compared with 1.8% with teriflunomide. Rates of malignancies were 0.5% and 0.3%, respectively.
“Ofatumumab demonstrated superior efficacy versus teriflunomide, with an acceptable safety profile, in patients with relapsing MS,” the authors reported.
Adherence rates with self-injection encouraging
An additional analysis from the two trials presented virtually in a separate abstract at the CMSC showed greater adherence to the self-administered regimen.
The analysis shows that in the ASCLEPIOS I study, 86.0% patients who were randomly assigned to receive ofatumumab and 77.7% who received teriflunomide completed the study on the assigned study drug. The proportion of patients who received ofatumumab and who discontinued treatment was 14.0%, versus 21.2% for those in the teriflunomide group. The most common reasons for discontinuation were patient/guardian decision (ofatumumab, 4.9%; teriflunomide, 8.2%), adverse event (ofatumumab, 5.2%; teriflunomide, 5.0%), and physician decision (ofatumumab, 2.2%; teriflunomide, 6.5%).
In the ASCLEPIOS II study, the rates were similar in all measures.
“In ASCLEPIOS trials, compliance with home-administered subcutaneous ofatumumab was high, and fewer patients discontinued ofatumumab as compared to teriflunomide,” the authors concluded.
Comparator drug a weak choice?
In commenting on the research, Stephen Kamin, MD, professor, vice chair, and chief of service, department of neurology, New Jersey Medical School, in Newark, noted that a limitation of the ASCLEPIOS trials is the comparison with teriflunomide.
“The comparator drug, teriflunomide, is one of the least effective DMTs, and one that some clinicians, including myself, don’t use,” he said.
Previously, when asked in an interview about the choice of teriflunomide as the comparator, Dr. Hauser noted that considerable discussion had gone into the decision. “The rationale was that we wanted to have a comparator that would be present not only against focal disease activity but also potentially against progression, and we were also able to blind the study successfully,” he said at the time.
Dr. Kamin said that ofatumumab will nevertheless likely represent a welcome addition to the tool kit of treatment options for MS. “Any new drug is helpful in adding to our choices as a general rule,” he said. “Subcutaneous injection does have increased convenience.”
It is not likely that the drug will be a game changer, he added, although the treatment’s efficacy compared with other drugs remains to be seen. “It all depends upon the relative efficacy of ofatumumab versus ocrelizumab or siponimod,” Dr. Kamin said.
“There has been another subcutaneous monoclonal for MS, daclizumab, although this was withdrawn from the market due to severe adverse effects not related to route of administration,” he added.
Dr. Hauser has relationships with Alector, Annexon, Bionure, Molecular Stethoscope, Symbiotix, and F. Hoffmann-La Roche. Dr. Kamin has received research support from Biogen, Novartis and CMSC.
A version of this article originally appeared on Medscape.com.
, a new study shows.
The drug, which is already approved for the treatment of chronic lymphocytic leukemia, is currently under review for relapsing MS as a once-per-month self-injected therapy that could offer a convenient alternative to DMTs that require in-office infusion.
The new findings are from a pooled analysis from the phase 3 ASCLEPIOS I/II trials of the use of ofatumumab for patients with relapsing MS. There were 927 patients in the ASCLEPIOS I trial and 955 in the ASCLEPIOS II trial. The trials were conducted in 37 countries and involved patients aged 18-55 years.
The late-breaking research was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The studies compared patients who were treated with subcutaneous ofatumumab 20 mg with patients treated with oral teriflunomide 14 mg once daily for up to 30 months. The average duration of follow-up was 18 months.
NEDA-3, commonly used to determine treatment outcomes for patients with relapsing MS, was defined as a composite of having no worsening of disability over a 6-month period (6mCDW), no confirmed MS relapse, no new/enlarging T2 lesions, and no gadolinium-enhancing T1 lesions.
The pooled results showed that the odds of achieving NEDA-3 during the first 12 months were three times greater with ofatumumab than with teriflunomide (47.0% vs. 24.5%; odds ratio [OR], 3.36; P < .001) and were more than eight times greater from months 12 to 24 (87.8% vs. 48.2%; OR, 8.09; P < .001).
In addition, compared with patients who received teriflunomide, a higher proportion of patients who received ofatumumab were free from 6mCDW over 2 years (91.9% vs. 88.9%), as well as from relapses (82.3% vs 69.2%) and lesion activity (54.1% vs. 27.5%).
There was a significantly greater reduction in annualized relapse rate with ofatumumab compared with teriflunomide at all cumulative time intervals, including months 0 to 3 (P = .011), and at all subsequent time intervals from month 0 to 27 (P < .001).
The pooled findings further showed that ofatumumab reduced the mean number of gadolinium-enhancing T1 lesions per scan by 95.9% compared with teriflunomide (P < .001).
“Ofatumumab increased the probability of achieving NEDA-3 and demonstrated superior efficacy vs teriflunomide in patients with relapsing MS,” said the authors, led by Stephen L. Hauser, MD, of the department of neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco.
Ofatumumab superior in primary, secondary outcomes
As previously reported, subcutaneous ofatumumab also demonstrated superior efficacy over oral teriflunomide in the primary and secondary endpoints in the ASCLEPIOS I/II trials. The annualized relapse rate was reduced by 0.22 in the teriflunomide group, vs 0.11 in the ofatumumab group (50.5% relative reduction; P < .001) in the ASCLEPIOS I trial, and by 0.25 vs. 0.10 (58.5% relative reduction P < .001) in ASCLEPIOS II.
Ofatumumab also reduced the number of gadolinium-enhancing T1 lesions and new or enlarging T2 lesions compared with teriflunomide (all P < .001). It reduced the risk for disability progression by 34.4% over 3 months and by 32.5% over 6 months.
In the studies, the rate of serious infection with ofatumumab was 2.5%, compared with 1.8% with teriflunomide. Rates of malignancies were 0.5% and 0.3%, respectively.
“Ofatumumab demonstrated superior efficacy versus teriflunomide, with an acceptable safety profile, in patients with relapsing MS,” the authors reported.
Adherence rates with self-injection encouraging
An additional analysis from the two trials presented virtually in a separate abstract at the CMSC showed greater adherence to the self-administered regimen.
The analysis shows that in the ASCLEPIOS I study, 86.0% patients who were randomly assigned to receive ofatumumab and 77.7% who received teriflunomide completed the study on the assigned study drug. The proportion of patients who received ofatumumab and who discontinued treatment was 14.0%, versus 21.2% for those in the teriflunomide group. The most common reasons for discontinuation were patient/guardian decision (ofatumumab, 4.9%; teriflunomide, 8.2%), adverse event (ofatumumab, 5.2%; teriflunomide, 5.0%), and physician decision (ofatumumab, 2.2%; teriflunomide, 6.5%).
In the ASCLEPIOS II study, the rates were similar in all measures.
“In ASCLEPIOS trials, compliance with home-administered subcutaneous ofatumumab was high, and fewer patients discontinued ofatumumab as compared to teriflunomide,” the authors concluded.
Comparator drug a weak choice?
In commenting on the research, Stephen Kamin, MD, professor, vice chair, and chief of service, department of neurology, New Jersey Medical School, in Newark, noted that a limitation of the ASCLEPIOS trials is the comparison with teriflunomide.
“The comparator drug, teriflunomide, is one of the least effective DMTs, and one that some clinicians, including myself, don’t use,” he said.
Previously, when asked in an interview about the choice of teriflunomide as the comparator, Dr. Hauser noted that considerable discussion had gone into the decision. “The rationale was that we wanted to have a comparator that would be present not only against focal disease activity but also potentially against progression, and we were also able to blind the study successfully,” he said at the time.
Dr. Kamin said that ofatumumab will nevertheless likely represent a welcome addition to the tool kit of treatment options for MS. “Any new drug is helpful in adding to our choices as a general rule,” he said. “Subcutaneous injection does have increased convenience.”
It is not likely that the drug will be a game changer, he added, although the treatment’s efficacy compared with other drugs remains to be seen. “It all depends upon the relative efficacy of ofatumumab versus ocrelizumab or siponimod,” Dr. Kamin said.
“There has been another subcutaneous monoclonal for MS, daclizumab, although this was withdrawn from the market due to severe adverse effects not related to route of administration,” he added.
Dr. Hauser has relationships with Alector, Annexon, Bionure, Molecular Stethoscope, Symbiotix, and F. Hoffmann-La Roche. Dr. Kamin has received research support from Biogen, Novartis and CMSC.
A version of this article originally appeared on Medscape.com.
, a new study shows.
The drug, which is already approved for the treatment of chronic lymphocytic leukemia, is currently under review for relapsing MS as a once-per-month self-injected therapy that could offer a convenient alternative to DMTs that require in-office infusion.
The new findings are from a pooled analysis from the phase 3 ASCLEPIOS I/II trials of the use of ofatumumab for patients with relapsing MS. There were 927 patients in the ASCLEPIOS I trial and 955 in the ASCLEPIOS II trial. The trials were conducted in 37 countries and involved patients aged 18-55 years.
The late-breaking research was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The studies compared patients who were treated with subcutaneous ofatumumab 20 mg with patients treated with oral teriflunomide 14 mg once daily for up to 30 months. The average duration of follow-up was 18 months.
NEDA-3, commonly used to determine treatment outcomes for patients with relapsing MS, was defined as a composite of having no worsening of disability over a 6-month period (6mCDW), no confirmed MS relapse, no new/enlarging T2 lesions, and no gadolinium-enhancing T1 lesions.
The pooled results showed that the odds of achieving NEDA-3 during the first 12 months were three times greater with ofatumumab than with teriflunomide (47.0% vs. 24.5%; odds ratio [OR], 3.36; P < .001) and were more than eight times greater from months 12 to 24 (87.8% vs. 48.2%; OR, 8.09; P < .001).
In addition, compared with patients who received teriflunomide, a higher proportion of patients who received ofatumumab were free from 6mCDW over 2 years (91.9% vs. 88.9%), as well as from relapses (82.3% vs 69.2%) and lesion activity (54.1% vs. 27.5%).
There was a significantly greater reduction in annualized relapse rate with ofatumumab compared with teriflunomide at all cumulative time intervals, including months 0 to 3 (P = .011), and at all subsequent time intervals from month 0 to 27 (P < .001).
The pooled findings further showed that ofatumumab reduced the mean number of gadolinium-enhancing T1 lesions per scan by 95.9% compared with teriflunomide (P < .001).
“Ofatumumab increased the probability of achieving NEDA-3 and demonstrated superior efficacy vs teriflunomide in patients with relapsing MS,” said the authors, led by Stephen L. Hauser, MD, of the department of neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco.
Ofatumumab superior in primary, secondary outcomes
As previously reported, subcutaneous ofatumumab also demonstrated superior efficacy over oral teriflunomide in the primary and secondary endpoints in the ASCLEPIOS I/II trials. The annualized relapse rate was reduced by 0.22 in the teriflunomide group, vs 0.11 in the ofatumumab group (50.5% relative reduction; P < .001) in the ASCLEPIOS I trial, and by 0.25 vs. 0.10 (58.5% relative reduction P < .001) in ASCLEPIOS II.
Ofatumumab also reduced the number of gadolinium-enhancing T1 lesions and new or enlarging T2 lesions compared with teriflunomide (all P < .001). It reduced the risk for disability progression by 34.4% over 3 months and by 32.5% over 6 months.
In the studies, the rate of serious infection with ofatumumab was 2.5%, compared with 1.8% with teriflunomide. Rates of malignancies were 0.5% and 0.3%, respectively.
“Ofatumumab demonstrated superior efficacy versus teriflunomide, with an acceptable safety profile, in patients with relapsing MS,” the authors reported.
Adherence rates with self-injection encouraging
An additional analysis from the two trials presented virtually in a separate abstract at the CMSC showed greater adherence to the self-administered regimen.
The analysis shows that in the ASCLEPIOS I study, 86.0% patients who were randomly assigned to receive ofatumumab and 77.7% who received teriflunomide completed the study on the assigned study drug. The proportion of patients who received ofatumumab and who discontinued treatment was 14.0%, versus 21.2% for those in the teriflunomide group. The most common reasons for discontinuation were patient/guardian decision (ofatumumab, 4.9%; teriflunomide, 8.2%), adverse event (ofatumumab, 5.2%; teriflunomide, 5.0%), and physician decision (ofatumumab, 2.2%; teriflunomide, 6.5%).
In the ASCLEPIOS II study, the rates were similar in all measures.
“In ASCLEPIOS trials, compliance with home-administered subcutaneous ofatumumab was high, and fewer patients discontinued ofatumumab as compared to teriflunomide,” the authors concluded.
Comparator drug a weak choice?
In commenting on the research, Stephen Kamin, MD, professor, vice chair, and chief of service, department of neurology, New Jersey Medical School, in Newark, noted that a limitation of the ASCLEPIOS trials is the comparison with teriflunomide.
“The comparator drug, teriflunomide, is one of the least effective DMTs, and one that some clinicians, including myself, don’t use,” he said.
Previously, when asked in an interview about the choice of teriflunomide as the comparator, Dr. Hauser noted that considerable discussion had gone into the decision. “The rationale was that we wanted to have a comparator that would be present not only against focal disease activity but also potentially against progression, and we were also able to blind the study successfully,” he said at the time.
Dr. Kamin said that ofatumumab will nevertheless likely represent a welcome addition to the tool kit of treatment options for MS. “Any new drug is helpful in adding to our choices as a general rule,” he said. “Subcutaneous injection does have increased convenience.”
It is not likely that the drug will be a game changer, he added, although the treatment’s efficacy compared with other drugs remains to be seen. “It all depends upon the relative efficacy of ofatumumab versus ocrelizumab or siponimod,” Dr. Kamin said.
“There has been another subcutaneous monoclonal for MS, daclizumab, although this was withdrawn from the market due to severe adverse effects not related to route of administration,” he added.
Dr. Hauser has relationships with Alector, Annexon, Bionure, Molecular Stethoscope, Symbiotix, and F. Hoffmann-La Roche. Dr. Kamin has received research support from Biogen, Novartis and CMSC.
A version of this article originally appeared on Medscape.com.
From CMSC 2020
Natalizumab switch to moderate-efficacy DMT increases disability risk
, new research shows.
“Owing to the vast number of available DMTs, not only understanding DMT performance but answering the question of what can come next if a patient needs to discontinue treatment due to safety or breakthrough disease is important,” said lead author Carrie M. Hersh, DO, of the Lou Ruvo Center for Brain Health, Cleveland Clinic, Las Vegas.
The study shows that, “patients transitioning from natalizumab to another high-efficacy therapy have better inflammatory and disability outcomes compared with those who de-escalate their therapy to a moderate-efficacy DMT,” she said.
Natalizumab (Tysabri) offers significant benefits in the treatment of relapsing forms of MS, however, its long-term use is associated with safety concerns, notably an increased risk of progressive multifocal leukoencephalopathy (PML). Although the risk can be reduced with a switch to a different DMT, the transition can have risks of its own, including a rebound of disease activity that could prove to be worse than the pre-natalizumab treatment period, and there is a lack of consensus on the safest avenues for switching to another DMT following discontinuation of natalizumab.
In research presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers, Dr. Hersh and colleagues explored the issue in a real-world population of 556 patients discontinuing natalizumab at two MS centers. Of these, 270 switched to a moderate DMT (dimethyl fumarate, n = 130; or fingolimod, n = 140) and 130 switched to a highly effective DMT (ocrelizumab, n = 106; rituximab, n = 17; or alemtuzumab, n = 7).
Reasons for switching included a PML risk for 54.9%, breakthrough disease for 15.3%, and adverse effects for 17.3%.
At 24-month follow-up after the switch and after adjustment for propensity score matching, no differences were seen between the moderate and highly effective DMT groups in terms of the annualized relapse rate (ARR; P = 0.33) or the time to first relapse (P = 0.09).
However, significantly higher proportions of patients switching to moderate DMTs showed new T2 lesions (odds ratio, 2.15; P = .01), as well as new gadolinium-enhancing lesions (OR, 1.99; P = .02), and a 20% worsening of the timed 25-foot walk test (T25FW; OR, 1.83; P = .04) and 9-hole peg test (9-HPT; OR, 1.81; P = .04)
Those switching to moderate DMTs also had significantly lower rates of absence of disease activity over the 24 months (OR, 0.41, P = .004), and they had a higher risk of earlier time-to-first gadolinium-enhancing lesion (hazard ratio, 6.67, P = .002), compared with those switching to a high-efficacy DMT.
Other factors that have previously been shown to be associated with rebounds that are worse than pre-natalizumab treatment include washout periods that are longer than 3 months.
The authors noted that there were no significant differences between the groups in terms of mean washout duration, which were relatively short (moderate DMT, 1.4 months; highly effective treatment, 1.8 months; P = .34), In addition, there were no significant differences between the groups in terms of the average duration of natalizumab treatment.
Dr. Hersh speculated that the lack of ARR differences may reflect that the measure is not as objective as the more specific determinants of performance. “One could consider the comparable ARR as a little surprising, but relapse evaluation in a retrospective manner is limited,” she explained.
“Historically, radiographic markers of new inflammation via brain magnetic resonance imaging (MRI) and neuroperformance measures (T25FW and 9-HPT) are more objective compared to assessing clinical relapses, especially in a retrospective cohort where relapses cannot be validated by a central agency or the principal investigator. Therefore, one can surmise that patients transitioning from natalizumab to another high-efficacy DMT fare better than de-escalating treatment to a moderate-efficacy DMT.”
Dr. Hersh and team plan a larger, multicenter study to investigate the short- and long-term effects of post-natalizumab DMT sequencing to help validate the current findings.
Commenting on the research, Stephen Kamin, MD, professor, vice chair and chief of service, department of neurology, New Jersey Medical School, Newark, said the results are consistent with natalizumab’s general profile.
“In general, natalizumab has been used in patients with highly active disease, so I would expect fewer patients with no evidence of disease activity when switched to a moderately active drug rather than a highly active one,” he said in an interview.
Caveats of the findings include the trial’s observational nature, meaning potential confounding factors of baseline characteristics among patients who switched regimens are not known, noted Dr. Kamin, who was not involved with the study.
“Also, the patients were switched to a variety of drugs and even within a class there may be differences in outcome,” he explained.
Dr. Hersh reported consulting or research relationships with Biogen, Genentech, EMD Serono, Genzyme, Novartis, and PCORI. Dr. Kamin has received research support from Biogen, Novartis, and the Consortium of Multiple Sclerosis Centers.
This article first appeared on Medscape.com.
, new research shows.
“Owing to the vast number of available DMTs, not only understanding DMT performance but answering the question of what can come next if a patient needs to discontinue treatment due to safety or breakthrough disease is important,” said lead author Carrie M. Hersh, DO, of the Lou Ruvo Center for Brain Health, Cleveland Clinic, Las Vegas.
The study shows that, “patients transitioning from natalizumab to another high-efficacy therapy have better inflammatory and disability outcomes compared with those who de-escalate their therapy to a moderate-efficacy DMT,” she said.
Natalizumab (Tysabri) offers significant benefits in the treatment of relapsing forms of MS, however, its long-term use is associated with safety concerns, notably an increased risk of progressive multifocal leukoencephalopathy (PML). Although the risk can be reduced with a switch to a different DMT, the transition can have risks of its own, including a rebound of disease activity that could prove to be worse than the pre-natalizumab treatment period, and there is a lack of consensus on the safest avenues for switching to another DMT following discontinuation of natalizumab.
In research presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers, Dr. Hersh and colleagues explored the issue in a real-world population of 556 patients discontinuing natalizumab at two MS centers. Of these, 270 switched to a moderate DMT (dimethyl fumarate, n = 130; or fingolimod, n = 140) and 130 switched to a highly effective DMT (ocrelizumab, n = 106; rituximab, n = 17; or alemtuzumab, n = 7).
Reasons for switching included a PML risk for 54.9%, breakthrough disease for 15.3%, and adverse effects for 17.3%.
At 24-month follow-up after the switch and after adjustment for propensity score matching, no differences were seen between the moderate and highly effective DMT groups in terms of the annualized relapse rate (ARR; P = 0.33) or the time to first relapse (P = 0.09).
However, significantly higher proportions of patients switching to moderate DMTs showed new T2 lesions (odds ratio, 2.15; P = .01), as well as new gadolinium-enhancing lesions (OR, 1.99; P = .02), and a 20% worsening of the timed 25-foot walk test (T25FW; OR, 1.83; P = .04) and 9-hole peg test (9-HPT; OR, 1.81; P = .04)
Those switching to moderate DMTs also had significantly lower rates of absence of disease activity over the 24 months (OR, 0.41, P = .004), and they had a higher risk of earlier time-to-first gadolinium-enhancing lesion (hazard ratio, 6.67, P = .002), compared with those switching to a high-efficacy DMT.
Other factors that have previously been shown to be associated with rebounds that are worse than pre-natalizumab treatment include washout periods that are longer than 3 months.
The authors noted that there were no significant differences between the groups in terms of mean washout duration, which were relatively short (moderate DMT, 1.4 months; highly effective treatment, 1.8 months; P = .34), In addition, there were no significant differences between the groups in terms of the average duration of natalizumab treatment.
Dr. Hersh speculated that the lack of ARR differences may reflect that the measure is not as objective as the more specific determinants of performance. “One could consider the comparable ARR as a little surprising, but relapse evaluation in a retrospective manner is limited,” she explained.
“Historically, radiographic markers of new inflammation via brain magnetic resonance imaging (MRI) and neuroperformance measures (T25FW and 9-HPT) are more objective compared to assessing clinical relapses, especially in a retrospective cohort where relapses cannot be validated by a central agency or the principal investigator. Therefore, one can surmise that patients transitioning from natalizumab to another high-efficacy DMT fare better than de-escalating treatment to a moderate-efficacy DMT.”
Dr. Hersh and team plan a larger, multicenter study to investigate the short- and long-term effects of post-natalizumab DMT sequencing to help validate the current findings.
Commenting on the research, Stephen Kamin, MD, professor, vice chair and chief of service, department of neurology, New Jersey Medical School, Newark, said the results are consistent with natalizumab’s general profile.
“In general, natalizumab has been used in patients with highly active disease, so I would expect fewer patients with no evidence of disease activity when switched to a moderately active drug rather than a highly active one,” he said in an interview.
Caveats of the findings include the trial’s observational nature, meaning potential confounding factors of baseline characteristics among patients who switched regimens are not known, noted Dr. Kamin, who was not involved with the study.
“Also, the patients were switched to a variety of drugs and even within a class there may be differences in outcome,” he explained.
Dr. Hersh reported consulting or research relationships with Biogen, Genentech, EMD Serono, Genzyme, Novartis, and PCORI. Dr. Kamin has received research support from Biogen, Novartis, and the Consortium of Multiple Sclerosis Centers.
This article first appeared on Medscape.com.
, new research shows.
“Owing to the vast number of available DMTs, not only understanding DMT performance but answering the question of what can come next if a patient needs to discontinue treatment due to safety or breakthrough disease is important,” said lead author Carrie M. Hersh, DO, of the Lou Ruvo Center for Brain Health, Cleveland Clinic, Las Vegas.
The study shows that, “patients transitioning from natalizumab to another high-efficacy therapy have better inflammatory and disability outcomes compared with those who de-escalate their therapy to a moderate-efficacy DMT,” she said.
Natalizumab (Tysabri) offers significant benefits in the treatment of relapsing forms of MS, however, its long-term use is associated with safety concerns, notably an increased risk of progressive multifocal leukoencephalopathy (PML). Although the risk can be reduced with a switch to a different DMT, the transition can have risks of its own, including a rebound of disease activity that could prove to be worse than the pre-natalizumab treatment period, and there is a lack of consensus on the safest avenues for switching to another DMT following discontinuation of natalizumab.
In research presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers, Dr. Hersh and colleagues explored the issue in a real-world population of 556 patients discontinuing natalizumab at two MS centers. Of these, 270 switched to a moderate DMT (dimethyl fumarate, n = 130; or fingolimod, n = 140) and 130 switched to a highly effective DMT (ocrelizumab, n = 106; rituximab, n = 17; or alemtuzumab, n = 7).
Reasons for switching included a PML risk for 54.9%, breakthrough disease for 15.3%, and adverse effects for 17.3%.
At 24-month follow-up after the switch and after adjustment for propensity score matching, no differences were seen between the moderate and highly effective DMT groups in terms of the annualized relapse rate (ARR; P = 0.33) or the time to first relapse (P = 0.09).
However, significantly higher proportions of patients switching to moderate DMTs showed new T2 lesions (odds ratio, 2.15; P = .01), as well as new gadolinium-enhancing lesions (OR, 1.99; P = .02), and a 20% worsening of the timed 25-foot walk test (T25FW; OR, 1.83; P = .04) and 9-hole peg test (9-HPT; OR, 1.81; P = .04)
Those switching to moderate DMTs also had significantly lower rates of absence of disease activity over the 24 months (OR, 0.41, P = .004), and they had a higher risk of earlier time-to-first gadolinium-enhancing lesion (hazard ratio, 6.67, P = .002), compared with those switching to a high-efficacy DMT.
Other factors that have previously been shown to be associated with rebounds that are worse than pre-natalizumab treatment include washout periods that are longer than 3 months.
The authors noted that there were no significant differences between the groups in terms of mean washout duration, which were relatively short (moderate DMT, 1.4 months; highly effective treatment, 1.8 months; P = .34), In addition, there were no significant differences between the groups in terms of the average duration of natalizumab treatment.
Dr. Hersh speculated that the lack of ARR differences may reflect that the measure is not as objective as the more specific determinants of performance. “One could consider the comparable ARR as a little surprising, but relapse evaluation in a retrospective manner is limited,” she explained.
“Historically, radiographic markers of new inflammation via brain magnetic resonance imaging (MRI) and neuroperformance measures (T25FW and 9-HPT) are more objective compared to assessing clinical relapses, especially in a retrospective cohort where relapses cannot be validated by a central agency or the principal investigator. Therefore, one can surmise that patients transitioning from natalizumab to another high-efficacy DMT fare better than de-escalating treatment to a moderate-efficacy DMT.”
Dr. Hersh and team plan a larger, multicenter study to investigate the short- and long-term effects of post-natalizumab DMT sequencing to help validate the current findings.
Commenting on the research, Stephen Kamin, MD, professor, vice chair and chief of service, department of neurology, New Jersey Medical School, Newark, said the results are consistent with natalizumab’s general profile.
“In general, natalizumab has been used in patients with highly active disease, so I would expect fewer patients with no evidence of disease activity when switched to a moderately active drug rather than a highly active one,” he said in an interview.
Caveats of the findings include the trial’s observational nature, meaning potential confounding factors of baseline characteristics among patients who switched regimens are not known, noted Dr. Kamin, who was not involved with the study.
“Also, the patients were switched to a variety of drugs and even within a class there may be differences in outcome,” he explained.
Dr. Hersh reported consulting or research relationships with Biogen, Genentech, EMD Serono, Genzyme, Novartis, and PCORI. Dr. Kamin has received research support from Biogen, Novartis, and the Consortium of Multiple Sclerosis Centers.
This article first appeared on Medscape.com.
Endocrinologists’ pay remains at lower end of physician scale
U.S. endocrinologists reported an average income that continues to be among the lowest of all specialist groups, according to results from the latest Medscape Annual Compensation Report.
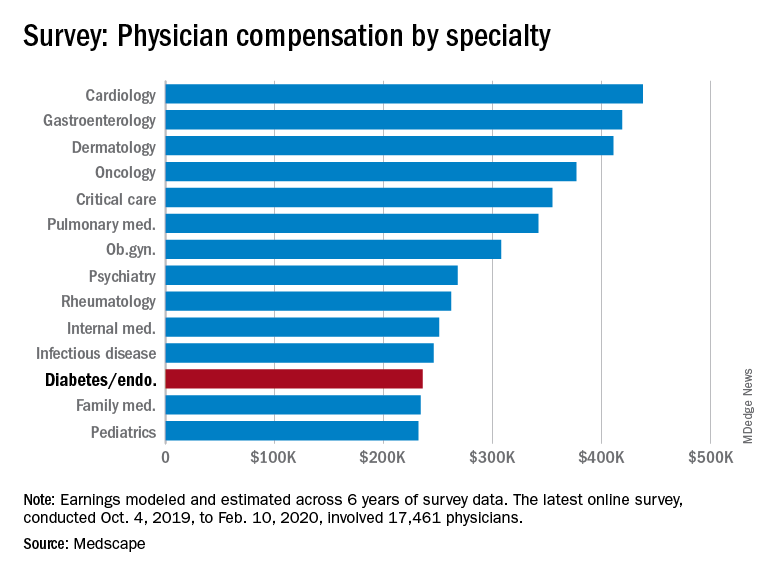
In the survey, which represents the responses of over 17,000 physicians in more than 30 specialties, endocrinologists report an average annual income of $236,000, which is unchanged from that detailed last year.
The report reflects data collected from Oct. 4, 2019 to Feb. 10, 2020, so does not take into account any effects of the COVID-19 pandemic.
It puts the diabetes and endocrinology specialty above family medicine, public health and preventive medicine, and pediatrics but nevertheless among the lowest-earning specialties.
At the opposite end of the earnings scale, orthopedics is at the top, with those doctors earning an average of $511,000 per year, followed by plastic surgery, otolaryngology, cardiology, and radiology.
The reported compensation reflects full-time salaries with patient care, including salary, bonus, and profit-sharing contributions for employed physicians, and earnings after taxes and deductible business expenses for self-employed physicians practicing medicine in the United States.
A gender gap in compensation is still apparent, with male endocrinologists earning about 23% more ($258,000) than their female counterparts ($209,000).
Overall, male specialists earn 31% more than women, which is a slight improvement on the 33% gender pay gap reported in 2019.
Survey respondents were 64% male and 34% female, with 2% declining to respond.
Half happy with pay, most would still choose same path
Around half (49%) of endocrinologists reported feeling fairly compensated for their work, an increase from last year’s rate of 42%.
In all, 82% said – if given another chance – they would choose a career in medicine again, higher than the rate of 77% of physicians overall. And as many as 80% said they would remain in the specialty of endocrinology.
For 35% of endocrinologists, the most rewarding part of their job is gratitude and relationships with patients. The most challenging part is “having so many rules and regulations,” cited by 25% of respondents, followed by working with an EHR system, reported by 20%.
Endocrinologists spent an average of about 34 hours per week seeing patients, lower than the 37.9 hours per week reported among all physicians. And the average of 16.5 hours per week spent on paperwork and administration by endocrinologists is similar to the 15.6 hours reported by physicians overall.
In terms of Medicare and Medicaid patients, 71% of endocrinologists said they had no plans to stop providing services to Medicare and Medicaid patients, which is similar to the overall rate of 73%.
About half of endocrinologists (51%) reported using nurse practitioners and 25% used physician assistants, while 42% used neither.
Among those using nurse practitioners or physician assistants, 50% reported that the assistance increased profitability; 44% said the staffers had no effect on profitability, and 6% reported decreased profitability.
Only about 13% of endocrinologists reported having claims denied or needing to be resubmitted, well below the highest levels of 28% and 22% reported in plastic surgery and emergency medicine, respectively.
COVID-19 suppresses compensation, but boosts telemedicine
Subsequent compensation surveys can be expected to reflect the heavy toll that COVID-19 pandemic has taken on nearly all professions in health care – as well as global economies as a whole.
Specialist practices in general report as much as a 55% decrease in revenue, on average, and a 60% decrease in patient volume since the beginning of the crisis, according to the report.
As many as 43,000 U.S. health care workers were reportedly laid off in March 2020 alone, as hospitals and physician groups announced layoffs, furloughs, and pay cuts in response to the fallout from the pandemic. And a reported 9% of independent medical practices have had to close, at least temporarily.
Meanwhile, the use of remote technologies for patient engagement has increased by 225%.
Specialties that rely heavily on elective procedures that were for the most part delayed during the pandemic have been particularly hard-hit, notably in those practicing orthopedics, plastic surgery, dermatology, cardiology, and ophthalmology.
“The health impact of COVID-19 has been grave, and the financial fallout is widespread,” according to the Medscape report.
A version of this article originally appeared on Medscape.com.
U.S. endocrinologists reported an average income that continues to be among the lowest of all specialist groups, according to results from the latest Medscape Annual Compensation Report.

In the survey, which represents the responses of over 17,000 physicians in more than 30 specialties, endocrinologists report an average annual income of $236,000, which is unchanged from that detailed last year.
The report reflects data collected from Oct. 4, 2019 to Feb. 10, 2020, so does not take into account any effects of the COVID-19 pandemic.
It puts the diabetes and endocrinology specialty above family medicine, public health and preventive medicine, and pediatrics but nevertheless among the lowest-earning specialties.
At the opposite end of the earnings scale, orthopedics is at the top, with those doctors earning an average of $511,000 per year, followed by plastic surgery, otolaryngology, cardiology, and radiology.
The reported compensation reflects full-time salaries with patient care, including salary, bonus, and profit-sharing contributions for employed physicians, and earnings after taxes and deductible business expenses for self-employed physicians practicing medicine in the United States.
A gender gap in compensation is still apparent, with male endocrinologists earning about 23% more ($258,000) than their female counterparts ($209,000).
Overall, male specialists earn 31% more than women, which is a slight improvement on the 33% gender pay gap reported in 2019.
Survey respondents were 64% male and 34% female, with 2% declining to respond.
Half happy with pay, most would still choose same path
Around half (49%) of endocrinologists reported feeling fairly compensated for their work, an increase from last year’s rate of 42%.
In all, 82% said – if given another chance – they would choose a career in medicine again, higher than the rate of 77% of physicians overall. And as many as 80% said they would remain in the specialty of endocrinology.
For 35% of endocrinologists, the most rewarding part of their job is gratitude and relationships with patients. The most challenging part is “having so many rules and regulations,” cited by 25% of respondents, followed by working with an EHR system, reported by 20%.
Endocrinologists spent an average of about 34 hours per week seeing patients, lower than the 37.9 hours per week reported among all physicians. And the average of 16.5 hours per week spent on paperwork and administration by endocrinologists is similar to the 15.6 hours reported by physicians overall.
In terms of Medicare and Medicaid patients, 71% of endocrinologists said they had no plans to stop providing services to Medicare and Medicaid patients, which is similar to the overall rate of 73%.
About half of endocrinologists (51%) reported using nurse practitioners and 25% used physician assistants, while 42% used neither.
Among those using nurse practitioners or physician assistants, 50% reported that the assistance increased profitability; 44% said the staffers had no effect on profitability, and 6% reported decreased profitability.
Only about 13% of endocrinologists reported having claims denied or needing to be resubmitted, well below the highest levels of 28% and 22% reported in plastic surgery and emergency medicine, respectively.
COVID-19 suppresses compensation, but boosts telemedicine
Subsequent compensation surveys can be expected to reflect the heavy toll that COVID-19 pandemic has taken on nearly all professions in health care – as well as global economies as a whole.
Specialist practices in general report as much as a 55% decrease in revenue, on average, and a 60% decrease in patient volume since the beginning of the crisis, according to the report.
As many as 43,000 U.S. health care workers were reportedly laid off in March 2020 alone, as hospitals and physician groups announced layoffs, furloughs, and pay cuts in response to the fallout from the pandemic. And a reported 9% of independent medical practices have had to close, at least temporarily.
Meanwhile, the use of remote technologies for patient engagement has increased by 225%.
Specialties that rely heavily on elective procedures that were for the most part delayed during the pandemic have been particularly hard-hit, notably in those practicing orthopedics, plastic surgery, dermatology, cardiology, and ophthalmology.
“The health impact of COVID-19 has been grave, and the financial fallout is widespread,” according to the Medscape report.
A version of this article originally appeared on Medscape.com.
U.S. endocrinologists reported an average income that continues to be among the lowest of all specialist groups, according to results from the latest Medscape Annual Compensation Report.

In the survey, which represents the responses of over 17,000 physicians in more than 30 specialties, endocrinologists report an average annual income of $236,000, which is unchanged from that detailed last year.
The report reflects data collected from Oct. 4, 2019 to Feb. 10, 2020, so does not take into account any effects of the COVID-19 pandemic.
It puts the diabetes and endocrinology specialty above family medicine, public health and preventive medicine, and pediatrics but nevertheless among the lowest-earning specialties.
At the opposite end of the earnings scale, orthopedics is at the top, with those doctors earning an average of $511,000 per year, followed by plastic surgery, otolaryngology, cardiology, and radiology.
The reported compensation reflects full-time salaries with patient care, including salary, bonus, and profit-sharing contributions for employed physicians, and earnings after taxes and deductible business expenses for self-employed physicians practicing medicine in the United States.
A gender gap in compensation is still apparent, with male endocrinologists earning about 23% more ($258,000) than their female counterparts ($209,000).
Overall, male specialists earn 31% more than women, which is a slight improvement on the 33% gender pay gap reported in 2019.
Survey respondents were 64% male and 34% female, with 2% declining to respond.
Half happy with pay, most would still choose same path
Around half (49%) of endocrinologists reported feeling fairly compensated for their work, an increase from last year’s rate of 42%.
In all, 82% said – if given another chance – they would choose a career in medicine again, higher than the rate of 77% of physicians overall. And as many as 80% said they would remain in the specialty of endocrinology.
For 35% of endocrinologists, the most rewarding part of their job is gratitude and relationships with patients. The most challenging part is “having so many rules and regulations,” cited by 25% of respondents, followed by working with an EHR system, reported by 20%.
Endocrinologists spent an average of about 34 hours per week seeing patients, lower than the 37.9 hours per week reported among all physicians. And the average of 16.5 hours per week spent on paperwork and administration by endocrinologists is similar to the 15.6 hours reported by physicians overall.
In terms of Medicare and Medicaid patients, 71% of endocrinologists said they had no plans to stop providing services to Medicare and Medicaid patients, which is similar to the overall rate of 73%.
About half of endocrinologists (51%) reported using nurse practitioners and 25% used physician assistants, while 42% used neither.
Among those using nurse practitioners or physician assistants, 50% reported that the assistance increased profitability; 44% said the staffers had no effect on profitability, and 6% reported decreased profitability.
Only about 13% of endocrinologists reported having claims denied or needing to be resubmitted, well below the highest levels of 28% and 22% reported in plastic surgery and emergency medicine, respectively.
COVID-19 suppresses compensation, but boosts telemedicine
Subsequent compensation surveys can be expected to reflect the heavy toll that COVID-19 pandemic has taken on nearly all professions in health care – as well as global economies as a whole.
Specialist practices in general report as much as a 55% decrease in revenue, on average, and a 60% decrease in patient volume since the beginning of the crisis, according to the report.
As many as 43,000 U.S. health care workers were reportedly laid off in March 2020 alone, as hospitals and physician groups announced layoffs, furloughs, and pay cuts in response to the fallout from the pandemic. And a reported 9% of independent medical practices have had to close, at least temporarily.
Meanwhile, the use of remote technologies for patient engagement has increased by 225%.
Specialties that rely heavily on elective procedures that were for the most part delayed during the pandemic have been particularly hard-hit, notably in those practicing orthopedics, plastic surgery, dermatology, cardiology, and ophthalmology.
“The health impact of COVID-19 has been grave, and the financial fallout is widespread,” according to the Medscape report.
A version of this article originally appeared on Medscape.com.
Large COVID-19 dataset: Kidney injury in >35% of those in hospital
As a new report shows that over a third of U.S. patients hospitalized with COVID-19 developed acute kidney injury (AKI), and nearly 15% of these patients needed dialysis, experts in the field are calling for more robust research into multiple aspects of this increasingly important issue.
Among 5,449 patients admitted to 13 Northwell Health New York–based hospitals between March and April 2020, 36.6% (1,993) developed AKI.
– the rate of kidney injury was 89.7% among ventilated patients, compared with 21.7% among other patients.
AKI in COVID-19 was also linked to a poor prognosis: 35% of those who developed AKI had died at the time of publication.
The study includes the largest defined cohort of hospitalized COVID-19 patients to date with a focus on AKI, says Jamie S. Hirsch, MD, of Northwell Health in Great Neck, N.Y., and colleagues in their article published online in Kidney International.
The findings track with those of a study of New York hospitals published online in The Lancet. In that dataset, just under a third (31%) of critically ill patients developed severe kidney damage and needed dialysis.
Both of these studies help solidify the experiences of clinicians on the ground, with many U.S. hospitals in the early phases of the pandemic underestimating the problem of AKI and having to scramble to find enough dialysis machines and dialysate solution to treat the most severely affected patients.
“We hope to learn more about the COVID-19–related AKI in the coming weeks, and that by sharing what we have learned from our patients, other doctors and their patients can benefit,” said senior author of the new study, Kenar D. Jhaveri, MD, associated chief of nephrology at Hofstra/Northwell.
The new report also comes as scientists from the National Institute of Diabetes and Digestive and Kidney Diseases highlighted the importance of AKI as a sequela of COVID-19 in an editorial published in Diabetes Care.
They, too, said that it is vitally important to better understand what is happening, as more and more hospitals will face COVID-19 patients with this complication.
“The natural history and heterogeneity of the kidney disease caused by COVID-19 need to be unraveled,” one of the authors, Robert A. Star, MD, director of the division of kidney, urologic, and hematologic diseases at NIDDK, said in an interview.
Such research is key because “low kidney function is an exclusion criterion in current studies” examining antiviral medications in COVID-19, he said. “Clinical trials are needed to test therapeutic interventions to prevent or treat COVID-19–induced AKI.”
Extremely ill patients develop AKI as their condition deteriorates
Identifying risk factors for the development of AKI in COVID-19 will be critical in helping shed more light on diagnostic and predictive biomarkers, Dr. Star said.
Dr. Hirsch and colleagues said that extremely ill patients often develop kidney failure as their condition deteriorates, and this happens quickly. Indeed, the clearest risk factors for the development of AKI were “the need for ventilator support or vasopressor drug treatment.”
Other independent predictors of AKI were older age, black race, diabetes, hypertension, and cardiovascular disease.
Of those on mechanical ventilation overall in the more than 5,000-patient study, almost a quarter (23.2%) developed AKI and needed renal replacement therapy, which consisted of either intermittent or continuous hemodialysis.
Dr. Star and associates wrote that these numbers are important because of the knock-on effects.
“Hemodialysis in critically ill infected patients is associated with significant clotting complications and mortality as well as increased infection risk to staff,” they pointed out.
Dr. Star said that “the incidence rate of AKI reported in this study is higher than what had been previously reported by others in the United States and China and may reflect differences in population demographics, severity of illness, prevalence of comorbidities, socioeconomic factors, patient volume overwhelming hospital capacity, or other factors not yet determined.
“It may be caused by dehydration (volume depletion), heart failure, the inflammatory response to the virus (cytokine storm), respiratory failure, clotting of blood vessels (hypercoagulation), muscle tissue breakdown (rhabdomyolysis), and/or a direct viral infection of the kidney,” he said.
Renal biopsies from patients with AKI may help shed some light
The editorialists went on to say that findings from kidney biopsies of COVID-19 patients with AKI may help shed some light on this condition.
“While difficult to perform, kidney biopsies from patients with early AKI could help us understand the underlying pathophysiologies at the cellular and molecular level and begin to target specific treatments to specific subgroups of patients,” they wrote.
The authors noted that, as part of funding opportunities provided by the National Institutes of Health for COVID-19 research, the NIDDK has published a Notice of Special Interest outlining the most urgent areas in need of research, with one of the focuses being on the kidney.
“As the research community emerges from the crisis situation, there should be renewed efforts for multidisciplinary research to conduct integrated basic, translational, and clinical studies aimed at greatly increasing the knowledge base to understand how both the current COVID-19 threat and future health threats affect both healthy people and people with chronic diseases and conditions,” the editorials noted.
The authors of the Diabetes Care editorial have reported no relevant financial relationships. Dr. Jhaveri has reported being a consultant for Astex Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
As a new report shows that over a third of U.S. patients hospitalized with COVID-19 developed acute kidney injury (AKI), and nearly 15% of these patients needed dialysis, experts in the field are calling for more robust research into multiple aspects of this increasingly important issue.
Among 5,449 patients admitted to 13 Northwell Health New York–based hospitals between March and April 2020, 36.6% (1,993) developed AKI.
– the rate of kidney injury was 89.7% among ventilated patients, compared with 21.7% among other patients.
AKI in COVID-19 was also linked to a poor prognosis: 35% of those who developed AKI had died at the time of publication.
The study includes the largest defined cohort of hospitalized COVID-19 patients to date with a focus on AKI, says Jamie S. Hirsch, MD, of Northwell Health in Great Neck, N.Y., and colleagues in their article published online in Kidney International.
The findings track with those of a study of New York hospitals published online in The Lancet. In that dataset, just under a third (31%) of critically ill patients developed severe kidney damage and needed dialysis.
Both of these studies help solidify the experiences of clinicians on the ground, with many U.S. hospitals in the early phases of the pandemic underestimating the problem of AKI and having to scramble to find enough dialysis machines and dialysate solution to treat the most severely affected patients.
“We hope to learn more about the COVID-19–related AKI in the coming weeks, and that by sharing what we have learned from our patients, other doctors and their patients can benefit,” said senior author of the new study, Kenar D. Jhaveri, MD, associated chief of nephrology at Hofstra/Northwell.
The new report also comes as scientists from the National Institute of Diabetes and Digestive and Kidney Diseases highlighted the importance of AKI as a sequela of COVID-19 in an editorial published in Diabetes Care.
They, too, said that it is vitally important to better understand what is happening, as more and more hospitals will face COVID-19 patients with this complication.
“The natural history and heterogeneity of the kidney disease caused by COVID-19 need to be unraveled,” one of the authors, Robert A. Star, MD, director of the division of kidney, urologic, and hematologic diseases at NIDDK, said in an interview.
Such research is key because “low kidney function is an exclusion criterion in current studies” examining antiviral medications in COVID-19, he said. “Clinical trials are needed to test therapeutic interventions to prevent or treat COVID-19–induced AKI.”
Extremely ill patients develop AKI as their condition deteriorates
Identifying risk factors for the development of AKI in COVID-19 will be critical in helping shed more light on diagnostic and predictive biomarkers, Dr. Star said.
Dr. Hirsch and colleagues said that extremely ill patients often develop kidney failure as their condition deteriorates, and this happens quickly. Indeed, the clearest risk factors for the development of AKI were “the need for ventilator support or vasopressor drug treatment.”
Other independent predictors of AKI were older age, black race, diabetes, hypertension, and cardiovascular disease.
Of those on mechanical ventilation overall in the more than 5,000-patient study, almost a quarter (23.2%) developed AKI and needed renal replacement therapy, which consisted of either intermittent or continuous hemodialysis.
Dr. Star and associates wrote that these numbers are important because of the knock-on effects.
“Hemodialysis in critically ill infected patients is associated with significant clotting complications and mortality as well as increased infection risk to staff,” they pointed out.
Dr. Star said that “the incidence rate of AKI reported in this study is higher than what had been previously reported by others in the United States and China and may reflect differences in population demographics, severity of illness, prevalence of comorbidities, socioeconomic factors, patient volume overwhelming hospital capacity, or other factors not yet determined.
“It may be caused by dehydration (volume depletion), heart failure, the inflammatory response to the virus (cytokine storm), respiratory failure, clotting of blood vessels (hypercoagulation), muscle tissue breakdown (rhabdomyolysis), and/or a direct viral infection of the kidney,” he said.
Renal biopsies from patients with AKI may help shed some light
The editorialists went on to say that findings from kidney biopsies of COVID-19 patients with AKI may help shed some light on this condition.
“While difficult to perform, kidney biopsies from patients with early AKI could help us understand the underlying pathophysiologies at the cellular and molecular level and begin to target specific treatments to specific subgroups of patients,” they wrote.
The authors noted that, as part of funding opportunities provided by the National Institutes of Health for COVID-19 research, the NIDDK has published a Notice of Special Interest outlining the most urgent areas in need of research, with one of the focuses being on the kidney.
“As the research community emerges from the crisis situation, there should be renewed efforts for multidisciplinary research to conduct integrated basic, translational, and clinical studies aimed at greatly increasing the knowledge base to understand how both the current COVID-19 threat and future health threats affect both healthy people and people with chronic diseases and conditions,” the editorials noted.
The authors of the Diabetes Care editorial have reported no relevant financial relationships. Dr. Jhaveri has reported being a consultant for Astex Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
As a new report shows that over a third of U.S. patients hospitalized with COVID-19 developed acute kidney injury (AKI), and nearly 15% of these patients needed dialysis, experts in the field are calling for more robust research into multiple aspects of this increasingly important issue.
Among 5,449 patients admitted to 13 Northwell Health New York–based hospitals between March and April 2020, 36.6% (1,993) developed AKI.
– the rate of kidney injury was 89.7% among ventilated patients, compared with 21.7% among other patients.
AKI in COVID-19 was also linked to a poor prognosis: 35% of those who developed AKI had died at the time of publication.
The study includes the largest defined cohort of hospitalized COVID-19 patients to date with a focus on AKI, says Jamie S. Hirsch, MD, of Northwell Health in Great Neck, N.Y., and colleagues in their article published online in Kidney International.
The findings track with those of a study of New York hospitals published online in The Lancet. In that dataset, just under a third (31%) of critically ill patients developed severe kidney damage and needed dialysis.
Both of these studies help solidify the experiences of clinicians on the ground, with many U.S. hospitals in the early phases of the pandemic underestimating the problem of AKI and having to scramble to find enough dialysis machines and dialysate solution to treat the most severely affected patients.
“We hope to learn more about the COVID-19–related AKI in the coming weeks, and that by sharing what we have learned from our patients, other doctors and their patients can benefit,” said senior author of the new study, Kenar D. Jhaveri, MD, associated chief of nephrology at Hofstra/Northwell.
The new report also comes as scientists from the National Institute of Diabetes and Digestive and Kidney Diseases highlighted the importance of AKI as a sequela of COVID-19 in an editorial published in Diabetes Care.
They, too, said that it is vitally important to better understand what is happening, as more and more hospitals will face COVID-19 patients with this complication.
“The natural history and heterogeneity of the kidney disease caused by COVID-19 need to be unraveled,” one of the authors, Robert A. Star, MD, director of the division of kidney, urologic, and hematologic diseases at NIDDK, said in an interview.
Such research is key because “low kidney function is an exclusion criterion in current studies” examining antiviral medications in COVID-19, he said. “Clinical trials are needed to test therapeutic interventions to prevent or treat COVID-19–induced AKI.”
Extremely ill patients develop AKI as their condition deteriorates
Identifying risk factors for the development of AKI in COVID-19 will be critical in helping shed more light on diagnostic and predictive biomarkers, Dr. Star said.
Dr. Hirsch and colleagues said that extremely ill patients often develop kidney failure as their condition deteriorates, and this happens quickly. Indeed, the clearest risk factors for the development of AKI were “the need for ventilator support or vasopressor drug treatment.”
Other independent predictors of AKI were older age, black race, diabetes, hypertension, and cardiovascular disease.
Of those on mechanical ventilation overall in the more than 5,000-patient study, almost a quarter (23.2%) developed AKI and needed renal replacement therapy, which consisted of either intermittent or continuous hemodialysis.
Dr. Star and associates wrote that these numbers are important because of the knock-on effects.
“Hemodialysis in critically ill infected patients is associated with significant clotting complications and mortality as well as increased infection risk to staff,” they pointed out.
Dr. Star said that “the incidence rate of AKI reported in this study is higher than what had been previously reported by others in the United States and China and may reflect differences in population demographics, severity of illness, prevalence of comorbidities, socioeconomic factors, patient volume overwhelming hospital capacity, or other factors not yet determined.
“It may be caused by dehydration (volume depletion), heart failure, the inflammatory response to the virus (cytokine storm), respiratory failure, clotting of blood vessels (hypercoagulation), muscle tissue breakdown (rhabdomyolysis), and/or a direct viral infection of the kidney,” he said.
Renal biopsies from patients with AKI may help shed some light
The editorialists went on to say that findings from kidney biopsies of COVID-19 patients with AKI may help shed some light on this condition.
“While difficult to perform, kidney biopsies from patients with early AKI could help us understand the underlying pathophysiologies at the cellular and molecular level and begin to target specific treatments to specific subgroups of patients,” they wrote.
The authors noted that, as part of funding opportunities provided by the National Institutes of Health for COVID-19 research, the NIDDK has published a Notice of Special Interest outlining the most urgent areas in need of research, with one of the focuses being on the kidney.
“As the research community emerges from the crisis situation, there should be renewed efforts for multidisciplinary research to conduct integrated basic, translational, and clinical studies aimed at greatly increasing the knowledge base to understand how both the current COVID-19 threat and future health threats affect both healthy people and people with chronic diseases and conditions,” the editorials noted.
The authors of the Diabetes Care editorial have reported no relevant financial relationships. Dr. Jhaveri has reported being a consultant for Astex Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
Endocrinologists adapt to telehealth, deferrals during COVID-19
James V. Hennessey, MD, has been working from home, like so many others, since the lockdowns went into effect. The director of clinical endocrinology at Beth Israel Deaconess Medical Center in Boston has felt surprisingly heartened by his experience.
“So far, these [video-based] discussions have been reassuring,” he said in an interview. “The images generating the referral have been available for review, and we’ve been able to reassure the patients that there are no danger signs in their histories.” Dr. Hennessey noted that for patients who agree to thyroid nodule consultations via video consult, the arrangement has allowed for the assessment of difficulty swallowing and other obvious difficulties.
While Dr. Hennessey has not yet encountered anything serious during his virtual consults, such as a rapidly growing anaplastic thyroid cancer, “it will only take some time before we hear of one, I’m sure,” he observed.
Surprisingly productive
During the COVID-19 pandemic, many physicians have been forced to innovate and turn aspects of their practices virtual. Use of telehealth services has increased by 50% in the United States since the start of the pandemic, according to research by Frost and Sullivan consultants. Three endocrinologists report that telehealth, although not always ideal, may provide more information than expected.
Recent recommendations say physicians should defer biopsies of asymptomatic thyroid nodules until the risk for COVID-19 has passed. As a result, some patients may experience increased anxiety because of such delays. But cases determined to require more urgent care should not be delayed, says the guidance.
Trevor E. Angell, MD, concurred that it’s possible to safely defer thyroid nodule assessment. “I would agree that with appropriate risk stratification by symptom assessment, ultrasound, and lab testing, thyroid nodules can be safely triaged for delayed evaluation,” said Dr. Angell, an assistant professor of clinical medicine and associate medical director of the thyroid center at the University of Southern California, in Los Angeles.
“I have found that patients with thyroid nodules that are not highly suspicious are reasonably reassured that the delay in obtaining FNA [fine needle aspiration] is very unlikely to have an impact on the ultimate outcome,” he said in an interview.
But he does have concerns that many of the investigations needed to make a decision about whether treatment can be deferred are also on hold.
“In many settings, including my own institution, nonurgent radiology studies are not being performed,” he noted. And “patients are reluctant to go to an ultrasound evaluation or a laboratory to perform testing” because of worries about COVID-19. “For possible thyroid nodules that have not yet been evaluated, this presents difficulty in getting the accurate ultrasound risk stratification and/or calcitonin testing that would help determine the need for immediate FNA biopsy or surgical consideration.”
And for patients in whom surgery has already been recommended, because of “indeterminate FNA cytology or suspicious molecular test result,” there is likely to be even more anxiety, Dr. Angell said.
“Most patients happy” with video visits
Victor J. Bernet, MD, American Thyroid Association president-elect, noted that, while his practice already had planned to start doing some video visits in April, the process was by necessity jump-started in March.
“Currently, about 90%-95% of our appointments are video visit or phone-call based,” he said in an interview. “Most patients seem to actually be happy with the video visit experience,” he said. “Some patients were very happy that they were given the opportunity to not have to come into the clinic in person at this point in time.”
Dr. Bernet agreed that video consults can be productive in identifying some important clinical information. “I have had at least a few patients with obvious goiter or nodule,” said Dr. Bernet, an associate professor and chair of the division of endocrinology at the Mayo Clinic in Jacksonville, Fla. “I also had a patient with hyperthyroidism from Graves disease and we were able to assess her for tremor over video.”
In the latter case, “we had the patient place a sheet of paper on the top of her hand while her arm was extended, which is the technique that can be used in person as well, and helps amplify the ability to detect if a fine tremor is present.”
“It was obvious that this patient had a tremor by video,” Dr. Bernet said.
In addition to tremor, video visits can be helpful in “looking for the presence of thyroid eye disease, enlarged thyroid, and possibly even skin changes.” Video may also be useful in evaluating respiratory effort and some cognitive behaviors, Dr. Bernet noted.
Starting to think about elective procedures again
As centers in the United States begin to reopen and permit elective procedures again, patients being considered for FNAs will likely be reintroduced according to the level of need, Dr. Hennessey noted. “Those with clear indications for FNA will be the first (to be scheduled),” while those who had very-low-risk findings, such as small nodules and cysts, “have already been deferred to 6- or 12-month follow-up ultrasounds, and decisions regarding FNAs will be made based on clinical course.”
Dr. Angell agrees that efforts to address the most urgent cases once centers reopen will be of the utmost importance. “To the greatest extent possible, providers should work together to coordinate the rescheduling of patients for FNA or surgery to avoid any further delay for those at most risk,” he said.Dr. Hennessey, Dr. Angell, and Dr. Bernet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
James V. Hennessey, MD, has been working from home, like so many others, since the lockdowns went into effect. The director of clinical endocrinology at Beth Israel Deaconess Medical Center in Boston has felt surprisingly heartened by his experience.
“So far, these [video-based] discussions have been reassuring,” he said in an interview. “The images generating the referral have been available for review, and we’ve been able to reassure the patients that there are no danger signs in their histories.” Dr. Hennessey noted that for patients who agree to thyroid nodule consultations via video consult, the arrangement has allowed for the assessment of difficulty swallowing and other obvious difficulties.
While Dr. Hennessey has not yet encountered anything serious during his virtual consults, such as a rapidly growing anaplastic thyroid cancer, “it will only take some time before we hear of one, I’m sure,” he observed.
Surprisingly productive
During the COVID-19 pandemic, many physicians have been forced to innovate and turn aspects of their practices virtual. Use of telehealth services has increased by 50% in the United States since the start of the pandemic, according to research by Frost and Sullivan consultants. Three endocrinologists report that telehealth, although not always ideal, may provide more information than expected.
Recent recommendations say physicians should defer biopsies of asymptomatic thyroid nodules until the risk for COVID-19 has passed. As a result, some patients may experience increased anxiety because of such delays. But cases determined to require more urgent care should not be delayed, says the guidance.
Trevor E. Angell, MD, concurred that it’s possible to safely defer thyroid nodule assessment. “I would agree that with appropriate risk stratification by symptom assessment, ultrasound, and lab testing, thyroid nodules can be safely triaged for delayed evaluation,” said Dr. Angell, an assistant professor of clinical medicine and associate medical director of the thyroid center at the University of Southern California, in Los Angeles.
“I have found that patients with thyroid nodules that are not highly suspicious are reasonably reassured that the delay in obtaining FNA [fine needle aspiration] is very unlikely to have an impact on the ultimate outcome,” he said in an interview.
But he does have concerns that many of the investigations needed to make a decision about whether treatment can be deferred are also on hold.
“In many settings, including my own institution, nonurgent radiology studies are not being performed,” he noted. And “patients are reluctant to go to an ultrasound evaluation or a laboratory to perform testing” because of worries about COVID-19. “For possible thyroid nodules that have not yet been evaluated, this presents difficulty in getting the accurate ultrasound risk stratification and/or calcitonin testing that would help determine the need for immediate FNA biopsy or surgical consideration.”
And for patients in whom surgery has already been recommended, because of “indeterminate FNA cytology or suspicious molecular test result,” there is likely to be even more anxiety, Dr. Angell said.
“Most patients happy” with video visits
Victor J. Bernet, MD, American Thyroid Association president-elect, noted that, while his practice already had planned to start doing some video visits in April, the process was by necessity jump-started in March.
“Currently, about 90%-95% of our appointments are video visit or phone-call based,” he said in an interview. “Most patients seem to actually be happy with the video visit experience,” he said. “Some patients were very happy that they were given the opportunity to not have to come into the clinic in person at this point in time.”
Dr. Bernet agreed that video consults can be productive in identifying some important clinical information. “I have had at least a few patients with obvious goiter or nodule,” said Dr. Bernet, an associate professor and chair of the division of endocrinology at the Mayo Clinic in Jacksonville, Fla. “I also had a patient with hyperthyroidism from Graves disease and we were able to assess her for tremor over video.”
In the latter case, “we had the patient place a sheet of paper on the top of her hand while her arm was extended, which is the technique that can be used in person as well, and helps amplify the ability to detect if a fine tremor is present.”
“It was obvious that this patient had a tremor by video,” Dr. Bernet said.
In addition to tremor, video visits can be helpful in “looking for the presence of thyroid eye disease, enlarged thyroid, and possibly even skin changes.” Video may also be useful in evaluating respiratory effort and some cognitive behaviors, Dr. Bernet noted.
Starting to think about elective procedures again
As centers in the United States begin to reopen and permit elective procedures again, patients being considered for FNAs will likely be reintroduced according to the level of need, Dr. Hennessey noted. “Those with clear indications for FNA will be the first (to be scheduled),” while those who had very-low-risk findings, such as small nodules and cysts, “have already been deferred to 6- or 12-month follow-up ultrasounds, and decisions regarding FNAs will be made based on clinical course.”
Dr. Angell agrees that efforts to address the most urgent cases once centers reopen will be of the utmost importance. “To the greatest extent possible, providers should work together to coordinate the rescheduling of patients for FNA or surgery to avoid any further delay for those at most risk,” he said.Dr. Hennessey, Dr. Angell, and Dr. Bernet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
James V. Hennessey, MD, has been working from home, like so many others, since the lockdowns went into effect. The director of clinical endocrinology at Beth Israel Deaconess Medical Center in Boston has felt surprisingly heartened by his experience.
“So far, these [video-based] discussions have been reassuring,” he said in an interview. “The images generating the referral have been available for review, and we’ve been able to reassure the patients that there are no danger signs in their histories.” Dr. Hennessey noted that for patients who agree to thyroid nodule consultations via video consult, the arrangement has allowed for the assessment of difficulty swallowing and other obvious difficulties.
While Dr. Hennessey has not yet encountered anything serious during his virtual consults, such as a rapidly growing anaplastic thyroid cancer, “it will only take some time before we hear of one, I’m sure,” he observed.
Surprisingly productive
During the COVID-19 pandemic, many physicians have been forced to innovate and turn aspects of their practices virtual. Use of telehealth services has increased by 50% in the United States since the start of the pandemic, according to research by Frost and Sullivan consultants. Three endocrinologists report that telehealth, although not always ideal, may provide more information than expected.
Recent recommendations say physicians should defer biopsies of asymptomatic thyroid nodules until the risk for COVID-19 has passed. As a result, some patients may experience increased anxiety because of such delays. But cases determined to require more urgent care should not be delayed, says the guidance.
Trevor E. Angell, MD, concurred that it’s possible to safely defer thyroid nodule assessment. “I would agree that with appropriate risk stratification by symptom assessment, ultrasound, and lab testing, thyroid nodules can be safely triaged for delayed evaluation,” said Dr. Angell, an assistant professor of clinical medicine and associate medical director of the thyroid center at the University of Southern California, in Los Angeles.
“I have found that patients with thyroid nodules that are not highly suspicious are reasonably reassured that the delay in obtaining FNA [fine needle aspiration] is very unlikely to have an impact on the ultimate outcome,” he said in an interview.
But he does have concerns that many of the investigations needed to make a decision about whether treatment can be deferred are also on hold.
“In many settings, including my own institution, nonurgent radiology studies are not being performed,” he noted. And “patients are reluctant to go to an ultrasound evaluation or a laboratory to perform testing” because of worries about COVID-19. “For possible thyroid nodules that have not yet been evaluated, this presents difficulty in getting the accurate ultrasound risk stratification and/or calcitonin testing that would help determine the need for immediate FNA biopsy or surgical consideration.”
And for patients in whom surgery has already been recommended, because of “indeterminate FNA cytology or suspicious molecular test result,” there is likely to be even more anxiety, Dr. Angell said.
“Most patients happy” with video visits
Victor J. Bernet, MD, American Thyroid Association president-elect, noted that, while his practice already had planned to start doing some video visits in April, the process was by necessity jump-started in March.
“Currently, about 90%-95% of our appointments are video visit or phone-call based,” he said in an interview. “Most patients seem to actually be happy with the video visit experience,” he said. “Some patients were very happy that they were given the opportunity to not have to come into the clinic in person at this point in time.”
Dr. Bernet agreed that video consults can be productive in identifying some important clinical information. “I have had at least a few patients with obvious goiter or nodule,” said Dr. Bernet, an associate professor and chair of the division of endocrinology at the Mayo Clinic in Jacksonville, Fla. “I also had a patient with hyperthyroidism from Graves disease and we were able to assess her for tremor over video.”
In the latter case, “we had the patient place a sheet of paper on the top of her hand while her arm was extended, which is the technique that can be used in person as well, and helps amplify the ability to detect if a fine tremor is present.”
“It was obvious that this patient had a tremor by video,” Dr. Bernet said.
In addition to tremor, video visits can be helpful in “looking for the presence of thyroid eye disease, enlarged thyroid, and possibly even skin changes.” Video may also be useful in evaluating respiratory effort and some cognitive behaviors, Dr. Bernet noted.
Starting to think about elective procedures again
As centers in the United States begin to reopen and permit elective procedures again, patients being considered for FNAs will likely be reintroduced according to the level of need, Dr. Hennessey noted. “Those with clear indications for FNA will be the first (to be scheduled),” while those who had very-low-risk findings, such as small nodules and cysts, “have already been deferred to 6- or 12-month follow-up ultrasounds, and decisions regarding FNAs will be made based on clinical course.”
Dr. Angell agrees that efforts to address the most urgent cases once centers reopen will be of the utmost importance. “To the greatest extent possible, providers should work together to coordinate the rescheduling of patients for FNA or surgery to avoid any further delay for those at most risk,” he said.Dr. Hennessey, Dr. Angell, and Dr. Bernet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19: Defer ‘bread and butter’ procedure for thyroid nodules
With a few notable exceptions, the majority of fine needle aspiration (FNA) biopsies of thyroid nodules should be delayed until the risk of COVID-19, and the burden on resources, has lessened, according to expert consensus.
“Our group recommends that FNA biopsy of most asymptomatic thyroid nodules – taking into account the sonographic characteristics and patients’ clinical picture – be deferred to a later time, when risk of exposure to COVID-19 is more manageable and resource restriction is no longer a concern,” said the endocrinologists, writing in a guest editorial in Clinical Thyroidology.
All elective procedures have been canceled under guidance of the Centers for Disease Control and Prevention, in conjunction with the U.S. surgeon general, in response to the COVID-19 pandemic. However, thyroid nodule FNAs, though elective, fall into the category of being considered medically necessary and potentially prolonging life expectancy
Yet, with approximately 90% of asymptomatic thyroid nodules turning out to be benign and little evidence that early detection and treatment affects disease outcomes, there is a strong argument for deferral in most cases, stressed Ming Lee, MD, and colleagues, of the endocrinology division at Phoenix (Ariz.) Veterans Affairs Health Care System (PVAHCS), who convened a multidisciplinary meeting to address the urgent issue.
Patients should instead be interviewed by an endocrinologist (preferably via telehealth) to collect their clinical history as well as assess their perception of the disease and risk of malignancy, senior author S. Mitchell Harman, MD, chief of PVAHCS, said in an interview.
“The principal guiding factor should be the objectively assessed likelihood of malignancy of the individual patient’s nodule(s),” he said.
“In my opinion, we should also factor in the patient’s level of anxiety, since some patients are more sanguine about risk than others and our goal is to provide relief of anxiety as well as to determine need for, and course of, subsequent treatment,” Dr. Harman added.
Vast majority of malignant thyroid nodules are DTC, which is ‘indolent’
Dr. Lee and colleagues noted that, even of the 10% of thyroid nodules that do prove to be malignant, the vast majority of these (90%) are differentiated thyroid cancers (DTC). In general, patients with DTC “follow an indolent course and have excellent outcomes.”
“There is little evidence that early detection and treatment of DTC significantly alters disease outcomes as the overall mortality rate for DTC has remained low, at around 0.5%,” they wrote.
They also noted that ultrasound features of thyroid nodules can help guide priority for the future timing of an FNA procedure, but should not be the sole basis for deciding on immediate thyroid FNA or surgery.
Exceptions to the rule
Exceptions for considering FNA include more urgent thyroid disease diagnoses, including those that are symptomatic:
Suspected medullary thyroid cancer
“Regarding medullary thyroid cancer (MTC), early diagnosis and surgery do significantly improve outcomes, therefore, delaying FNA of nodules harboring MTC could be potentially injurious,” the authors said.
They suggested, however, measuring calcitonin levels instead, which they noted “is still controversial” in the United States, but “we feel it would be justified in patients with thyroid nodules that would usually be indicated for FNA.”
Those with a family history of MTC, or nodules located in the posterior upper third of lateral lobes (the usual location of MTC), should have calcitonin levels measured.
If calcitonin levels are above 10 pg/mL, “FNA should be offered as early as possible.”
“Significantly elevated serum calcitonin levels (e.g., > 100 pg/mL) should be considered an indication for surgery without cytologic confirmation by FNA,” they added.
Anaplastic thyroid cancer
Anaplastic thyroid cancer, though rare, “is one of the few occasions when thyroid surgery should be performed on an urgent basis, as this condition can worsen very rapidly.
“Patients typically present with a rapidly enlarging thyroid mass that is associated with compressive symptoms, such as dysphagia and dyspnea,” they observed.
In this instance, although FNA is part of the preoperative work-up, it is often nondiagnostic and could require additional sampling.
“At the time of this pandemic, it is reasonable that after a multidisciplinary discussion, such patients with the appropriate clinical scenario be referred for thyroid surgery, with or without prior FNA, based on the team’s judgment,” the authors recommended.
Long-standing thyroid masses
These are usually large and/or closely associated with vital structures, such as the trachea and esophagus, and when such masses cause compressive symptoms, thyroid surgery typically is warranted.
And although prior FNA is helpful to obtain a cytologic diagnosis, as this may change the extent of surgery, it may not always be essential.
Broadly, symptomatic patients with compressive symptoms threatening vital structures can be directly referred to a surgeon, with the timing for surgery jointly decided based on the severity of symptoms, rapidity of disease progression, local COVID-19 status, and available resources.
“During the pandemic, we believe that the vast majority of thyroid FNAs should be considered optional, and extent of surgery can be determined by pathological analysis of frozen sections intraoperatively,” they wrote.
“The value of FNA in these situations is less compelling in the current COVID-19 setting, as the basis of decision for surgery has been already determined,” the authors explained.
If urgent FNA needed, screen patient for COVID-19 and use PPE
Should the need for an urgent thyroid FNA occur, patients should be screened and tested for COVID-19 by a clinician wearing personal protective equipment (PPE), said Dr. Lee and colleagues.
“It is crucial to carefully weigh the risks of COVID-19 exposure, availability of resources, and urgency of these procedures for each patient in our individual practice settings,” they noted.
As restrictions eventually loosen, precautions will still be necessary to some degree, Dr. Harman said.
“I do not consider FNA a ‘high-risk’ procedure in the era of COVID-19, since it does not routinely result in profuse aerosolization of respiratory fluids,” he said in an interview.
“However, patients do sometimes cough or choke due to pressure on the neck and the operator is, of necessity, very close to the patient’s face. Therefore, when we resume FNA, patients will be screened for symptoms of COVID-19 infection and both the operator and the patient will be masked,” Dr. Harman continued.
“We routinely wear gloves, [and] whether the operator will wear a surgical or an N95 mask, disposable gown, etc, will depend on CDC guidance and guidance received from our VA infectious disease experts as it is applied specifically to each patient evaluation.”
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
With a few notable exceptions, the majority of fine needle aspiration (FNA) biopsies of thyroid nodules should be delayed until the risk of COVID-19, and the burden on resources, has lessened, according to expert consensus.
“Our group recommends that FNA biopsy of most asymptomatic thyroid nodules – taking into account the sonographic characteristics and patients’ clinical picture – be deferred to a later time, when risk of exposure to COVID-19 is more manageable and resource restriction is no longer a concern,” said the endocrinologists, writing in a guest editorial in Clinical Thyroidology.
All elective procedures have been canceled under guidance of the Centers for Disease Control and Prevention, in conjunction with the U.S. surgeon general, in response to the COVID-19 pandemic. However, thyroid nodule FNAs, though elective, fall into the category of being considered medically necessary and potentially prolonging life expectancy
Yet, with approximately 90% of asymptomatic thyroid nodules turning out to be benign and little evidence that early detection and treatment affects disease outcomes, there is a strong argument for deferral in most cases, stressed Ming Lee, MD, and colleagues, of the endocrinology division at Phoenix (Ariz.) Veterans Affairs Health Care System (PVAHCS), who convened a multidisciplinary meeting to address the urgent issue.
Patients should instead be interviewed by an endocrinologist (preferably via telehealth) to collect their clinical history as well as assess their perception of the disease and risk of malignancy, senior author S. Mitchell Harman, MD, chief of PVAHCS, said in an interview.
“The principal guiding factor should be the objectively assessed likelihood of malignancy of the individual patient’s nodule(s),” he said.
“In my opinion, we should also factor in the patient’s level of anxiety, since some patients are more sanguine about risk than others and our goal is to provide relief of anxiety as well as to determine need for, and course of, subsequent treatment,” Dr. Harman added.
Vast majority of malignant thyroid nodules are DTC, which is ‘indolent’
Dr. Lee and colleagues noted that, even of the 10% of thyroid nodules that do prove to be malignant, the vast majority of these (90%) are differentiated thyroid cancers (DTC). In general, patients with DTC “follow an indolent course and have excellent outcomes.”
“There is little evidence that early detection and treatment of DTC significantly alters disease outcomes as the overall mortality rate for DTC has remained low, at around 0.5%,” they wrote.
They also noted that ultrasound features of thyroid nodules can help guide priority for the future timing of an FNA procedure, but should not be the sole basis for deciding on immediate thyroid FNA or surgery.
Exceptions to the rule
Exceptions for considering FNA include more urgent thyroid disease diagnoses, including those that are symptomatic:
Suspected medullary thyroid cancer
“Regarding medullary thyroid cancer (MTC), early diagnosis and surgery do significantly improve outcomes, therefore, delaying FNA of nodules harboring MTC could be potentially injurious,” the authors said.
They suggested, however, measuring calcitonin levels instead, which they noted “is still controversial” in the United States, but “we feel it would be justified in patients with thyroid nodules that would usually be indicated for FNA.”
Those with a family history of MTC, or nodules located in the posterior upper third of lateral lobes (the usual location of MTC), should have calcitonin levels measured.
If calcitonin levels are above 10 pg/mL, “FNA should be offered as early as possible.”
“Significantly elevated serum calcitonin levels (e.g., > 100 pg/mL) should be considered an indication for surgery without cytologic confirmation by FNA,” they added.
Anaplastic thyroid cancer
Anaplastic thyroid cancer, though rare, “is one of the few occasions when thyroid surgery should be performed on an urgent basis, as this condition can worsen very rapidly.
“Patients typically present with a rapidly enlarging thyroid mass that is associated with compressive symptoms, such as dysphagia and dyspnea,” they observed.
In this instance, although FNA is part of the preoperative work-up, it is often nondiagnostic and could require additional sampling.
“At the time of this pandemic, it is reasonable that after a multidisciplinary discussion, such patients with the appropriate clinical scenario be referred for thyroid surgery, with or without prior FNA, based on the team’s judgment,” the authors recommended.
Long-standing thyroid masses
These are usually large and/or closely associated with vital structures, such as the trachea and esophagus, and when such masses cause compressive symptoms, thyroid surgery typically is warranted.
And although prior FNA is helpful to obtain a cytologic diagnosis, as this may change the extent of surgery, it may not always be essential.
Broadly, symptomatic patients with compressive symptoms threatening vital structures can be directly referred to a surgeon, with the timing for surgery jointly decided based on the severity of symptoms, rapidity of disease progression, local COVID-19 status, and available resources.
“During the pandemic, we believe that the vast majority of thyroid FNAs should be considered optional, and extent of surgery can be determined by pathological analysis of frozen sections intraoperatively,” they wrote.
“The value of FNA in these situations is less compelling in the current COVID-19 setting, as the basis of decision for surgery has been already determined,” the authors explained.
If urgent FNA needed, screen patient for COVID-19 and use PPE
Should the need for an urgent thyroid FNA occur, patients should be screened and tested for COVID-19 by a clinician wearing personal protective equipment (PPE), said Dr. Lee and colleagues.
“It is crucial to carefully weigh the risks of COVID-19 exposure, availability of resources, and urgency of these procedures for each patient in our individual practice settings,” they noted.
As restrictions eventually loosen, precautions will still be necessary to some degree, Dr. Harman said.
“I do not consider FNA a ‘high-risk’ procedure in the era of COVID-19, since it does not routinely result in profuse aerosolization of respiratory fluids,” he said in an interview.
“However, patients do sometimes cough or choke due to pressure on the neck and the operator is, of necessity, very close to the patient’s face. Therefore, when we resume FNA, patients will be screened for symptoms of COVID-19 infection and both the operator and the patient will be masked,” Dr. Harman continued.
“We routinely wear gloves, [and] whether the operator will wear a surgical or an N95 mask, disposable gown, etc, will depend on CDC guidance and guidance received from our VA infectious disease experts as it is applied specifically to each patient evaluation.”
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
With a few notable exceptions, the majority of fine needle aspiration (FNA) biopsies of thyroid nodules should be delayed until the risk of COVID-19, and the burden on resources, has lessened, according to expert consensus.
“Our group recommends that FNA biopsy of most asymptomatic thyroid nodules – taking into account the sonographic characteristics and patients’ clinical picture – be deferred to a later time, when risk of exposure to COVID-19 is more manageable and resource restriction is no longer a concern,” said the endocrinologists, writing in a guest editorial in Clinical Thyroidology.
All elective procedures have been canceled under guidance of the Centers for Disease Control and Prevention, in conjunction with the U.S. surgeon general, in response to the COVID-19 pandemic. However, thyroid nodule FNAs, though elective, fall into the category of being considered medically necessary and potentially prolonging life expectancy
Yet, with approximately 90% of asymptomatic thyroid nodules turning out to be benign and little evidence that early detection and treatment affects disease outcomes, there is a strong argument for deferral in most cases, stressed Ming Lee, MD, and colleagues, of the endocrinology division at Phoenix (Ariz.) Veterans Affairs Health Care System (PVAHCS), who convened a multidisciplinary meeting to address the urgent issue.
Patients should instead be interviewed by an endocrinologist (preferably via telehealth) to collect their clinical history as well as assess their perception of the disease and risk of malignancy, senior author S. Mitchell Harman, MD, chief of PVAHCS, said in an interview.
“The principal guiding factor should be the objectively assessed likelihood of malignancy of the individual patient’s nodule(s),” he said.
“In my opinion, we should also factor in the patient’s level of anxiety, since some patients are more sanguine about risk than others and our goal is to provide relief of anxiety as well as to determine need for, and course of, subsequent treatment,” Dr. Harman added.
Vast majority of malignant thyroid nodules are DTC, which is ‘indolent’
Dr. Lee and colleagues noted that, even of the 10% of thyroid nodules that do prove to be malignant, the vast majority of these (90%) are differentiated thyroid cancers (DTC). In general, patients with DTC “follow an indolent course and have excellent outcomes.”
“There is little evidence that early detection and treatment of DTC significantly alters disease outcomes as the overall mortality rate for DTC has remained low, at around 0.5%,” they wrote.
They also noted that ultrasound features of thyroid nodules can help guide priority for the future timing of an FNA procedure, but should not be the sole basis for deciding on immediate thyroid FNA or surgery.
Exceptions to the rule
Exceptions for considering FNA include more urgent thyroid disease diagnoses, including those that are symptomatic:
Suspected medullary thyroid cancer
“Regarding medullary thyroid cancer (MTC), early diagnosis and surgery do significantly improve outcomes, therefore, delaying FNA of nodules harboring MTC could be potentially injurious,” the authors said.
They suggested, however, measuring calcitonin levels instead, which they noted “is still controversial” in the United States, but “we feel it would be justified in patients with thyroid nodules that would usually be indicated for FNA.”
Those with a family history of MTC, or nodules located in the posterior upper third of lateral lobes (the usual location of MTC), should have calcitonin levels measured.
If calcitonin levels are above 10 pg/mL, “FNA should be offered as early as possible.”
“Significantly elevated serum calcitonin levels (e.g., > 100 pg/mL) should be considered an indication for surgery without cytologic confirmation by FNA,” they added.
Anaplastic thyroid cancer
Anaplastic thyroid cancer, though rare, “is one of the few occasions when thyroid surgery should be performed on an urgent basis, as this condition can worsen very rapidly.
“Patients typically present with a rapidly enlarging thyroid mass that is associated with compressive symptoms, such as dysphagia and dyspnea,” they observed.
In this instance, although FNA is part of the preoperative work-up, it is often nondiagnostic and could require additional sampling.
“At the time of this pandemic, it is reasonable that after a multidisciplinary discussion, such patients with the appropriate clinical scenario be referred for thyroid surgery, with or without prior FNA, based on the team’s judgment,” the authors recommended.
Long-standing thyroid masses
These are usually large and/or closely associated with vital structures, such as the trachea and esophagus, and when such masses cause compressive symptoms, thyroid surgery typically is warranted.
And although prior FNA is helpful to obtain a cytologic diagnosis, as this may change the extent of surgery, it may not always be essential.
Broadly, symptomatic patients with compressive symptoms threatening vital structures can be directly referred to a surgeon, with the timing for surgery jointly decided based on the severity of symptoms, rapidity of disease progression, local COVID-19 status, and available resources.
“During the pandemic, we believe that the vast majority of thyroid FNAs should be considered optional, and extent of surgery can be determined by pathological analysis of frozen sections intraoperatively,” they wrote.
“The value of FNA in these situations is less compelling in the current COVID-19 setting, as the basis of decision for surgery has been already determined,” the authors explained.
If urgent FNA needed, screen patient for COVID-19 and use PPE
Should the need for an urgent thyroid FNA occur, patients should be screened and tested for COVID-19 by a clinician wearing personal protective equipment (PPE), said Dr. Lee and colleagues.
“It is crucial to carefully weigh the risks of COVID-19 exposure, availability of resources, and urgency of these procedures for each patient in our individual practice settings,” they noted.
As restrictions eventually loosen, precautions will still be necessary to some degree, Dr. Harman said.
“I do not consider FNA a ‘high-risk’ procedure in the era of COVID-19, since it does not routinely result in profuse aerosolization of respiratory fluids,” he said in an interview.
“However, patients do sometimes cough or choke due to pressure on the neck and the operator is, of necessity, very close to the patient’s face. Therefore, when we resume FNA, patients will be screened for symptoms of COVID-19 infection and both the operator and the patient will be masked,” Dr. Harman continued.
“We routinely wear gloves, [and] whether the operator will wear a surgical or an N95 mask, disposable gown, etc, will depend on CDC guidance and guidance received from our VA infectious disease experts as it is applied specifically to each patient evaluation.”
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Rapid response to PTSD therapy may predict long-term improvement
Patients who experience a rapid response to cognitive processing therapy (CPT) for posttraumatic stress disorder have a greater likelihood of sustained improvement, new research suggests.
A study of 136 veterans with PTSD showed that those who responded quickly to a 3-week CPT program were significantly more likely to report lower symptom scores 3 months post treatment, compared with those participants who responded more slowly.
The results add to previous evidence that intensified, short-term treatment programs can accomplish long-term benefits, noted the investigators, led by Jenna Bagley, Rush University Medical Center, Chicago.
“These findings show promise for the success of condensed evidence-based, trauma-focused interventions,” they added.
Ms. Bagley was scheduled to present the study in March at the Anxiety and Depression Association of America (ADAA) Conference 2020. That conference was canceled because of the coronavirus pandemic.
Reducing high dropout rates
PTSD treatments such as CPT and prolonged exposure have been shown to have high efficacy, but they also have been shown to have a nearly 40% dropout rate, the researchers note.
This problem has prompted a focus on shorter-term interventions that can deliver intensified treatment before participants drop out. However, evidence has been lacking as to the sustainability of symptom improvements that occur in a short period.
The researchers evaluated data on 136 veterans (66% men; mean age, 41 years) with PTSD who completed a non–Veterans Affairs, 3-week CPT-based intensive treatment program. Follow-up assessments were carried out at 3 months.
Symptom reduction rates represented the number of days from intake in the program to the first day a reduction was reported of at least 15 points on the PTSD Checklist for DSM-5 (PCL-5), which was indicative of a clinically meaningful improvement.
Results showed representing greater ongoing symptoms (P = .04).
The amount of time to reach the 15-point reduction also predicted symptom reductions at the 3-month follow-up, even when controlling for the total change in PCL-5 score during the program (P = .03) and when controlling for type of trauma, such as combat or military sexual trauma.
Another puzzle piece?
Commenting on the study, David C. Rozek, PhD, assistant professor at the University of Central Florida, Orlando, said the findings are encouraging, particularly in terms of improvements seen with shorter treatment programs.
“Sudden gains are important to look at in all treatments,” said Dr. Rozek, who was not involved with the research.
“Seeing that these sudden gains occur in intensive treatment and predict long-term outcome provides another piece of the puzzle and provides additional support to intensive treatments,” he added.
Dr. Rozek noted that he has also observed this with patients. However, they, along with mental health practitioners, often question whether short-term improvements will last.
“There is some concern that a rapid drop in a patient’s symptoms could be an increased risk for a rebound,” he said.
“However, that is when the skills learned in therapy can kick in and provide [patients] tools to use in their everyday life and to help continue recovering,” he added.
Dr. Rozek was scheduled to report results from a pilot study on his own experience at the canceled ADAA meeting. The study was about an even shorter, 7-day intensive CPT program (CPT-7) conducted through the National Center for Veterans Studies.
The program involved one daily individual CPT-7 session with a mental health provider in the morning followed by optional group recreational activities in the afternoon. Twelve military personnel in two cohorts with either PTSD or subthreshold PTSD, defined as having all but one symptom cluster present, were included in the study.
Keep patients engaged
Preliminary results showed reductions in PCL-5 scores from pre- to posttreatment of approximately 40% (P < .001).
“Just over 50% of patients left [the program] with symptoms below probable PTSD diagnosis on a self-report measure,” Dr. Rozek said.
Importantly, none of the participants dropped out of the treatment, but Dr. Rozek said that this was not necessarily surprising because of the nature of the program.
“These patients are brought in as a cohort and form some bonds, as they all have experienced traumatic events, although often [they have had] very different traumas,” he said.
“We’ve found that by doing daily treatment, it is more accessible and removes barriers, as it is often easier to take a week or a few weeks off at a time and participate in treatment than the logistics of weekly treatment,” he added.
Dr. Rozek said he suspects two key factors may predict treatment response in such programs – cognitive flexibility and emotion regulation.
“Patients who come into treatment and are extremely rigid in their thinking and are unable to manage their emotions may be slower to respond to treatment,” he noted.
“That being said, there are a variety of treatments that target these factors in different ways. Now we need to do the work to determine which treatments work for whom.”
The findings on longer-term durability of rapid improvement bode well for the program. “Although the work in treatment is hard, they patients really start to see the gains quickly, within a week or weeks instead of months,” Dr. Rozek said.
“This is rewarding in itself, and I would say is a strong factor for keeping patients engaged,” he concluded.
Dr. Rozek has received research funding from the National Institutes of Health, the Department of Defense, the Bob Woodruff Foundation, and the Boeing Corporation.
A version of this article originally appeared on Medscape.com.
Patients who experience a rapid response to cognitive processing therapy (CPT) for posttraumatic stress disorder have a greater likelihood of sustained improvement, new research suggests.
A study of 136 veterans with PTSD showed that those who responded quickly to a 3-week CPT program were significantly more likely to report lower symptom scores 3 months post treatment, compared with those participants who responded more slowly.
The results add to previous evidence that intensified, short-term treatment programs can accomplish long-term benefits, noted the investigators, led by Jenna Bagley, Rush University Medical Center, Chicago.
“These findings show promise for the success of condensed evidence-based, trauma-focused interventions,” they added.
Ms. Bagley was scheduled to present the study in March at the Anxiety and Depression Association of America (ADAA) Conference 2020. That conference was canceled because of the coronavirus pandemic.
Reducing high dropout rates
PTSD treatments such as CPT and prolonged exposure have been shown to have high efficacy, but they also have been shown to have a nearly 40% dropout rate, the researchers note.
This problem has prompted a focus on shorter-term interventions that can deliver intensified treatment before participants drop out. However, evidence has been lacking as to the sustainability of symptom improvements that occur in a short period.
The researchers evaluated data on 136 veterans (66% men; mean age, 41 years) with PTSD who completed a non–Veterans Affairs, 3-week CPT-based intensive treatment program. Follow-up assessments were carried out at 3 months.
Symptom reduction rates represented the number of days from intake in the program to the first day a reduction was reported of at least 15 points on the PTSD Checklist for DSM-5 (PCL-5), which was indicative of a clinically meaningful improvement.
Results showed representing greater ongoing symptoms (P = .04).
The amount of time to reach the 15-point reduction also predicted symptom reductions at the 3-month follow-up, even when controlling for the total change in PCL-5 score during the program (P = .03) and when controlling for type of trauma, such as combat or military sexual trauma.
Another puzzle piece?
Commenting on the study, David C. Rozek, PhD, assistant professor at the University of Central Florida, Orlando, said the findings are encouraging, particularly in terms of improvements seen with shorter treatment programs.
“Sudden gains are important to look at in all treatments,” said Dr. Rozek, who was not involved with the research.
“Seeing that these sudden gains occur in intensive treatment and predict long-term outcome provides another piece of the puzzle and provides additional support to intensive treatments,” he added.
Dr. Rozek noted that he has also observed this with patients. However, they, along with mental health practitioners, often question whether short-term improvements will last.
“There is some concern that a rapid drop in a patient’s symptoms could be an increased risk for a rebound,” he said.
“However, that is when the skills learned in therapy can kick in and provide [patients] tools to use in their everyday life and to help continue recovering,” he added.
Dr. Rozek was scheduled to report results from a pilot study on his own experience at the canceled ADAA meeting. The study was about an even shorter, 7-day intensive CPT program (CPT-7) conducted through the National Center for Veterans Studies.
The program involved one daily individual CPT-7 session with a mental health provider in the morning followed by optional group recreational activities in the afternoon. Twelve military personnel in two cohorts with either PTSD or subthreshold PTSD, defined as having all but one symptom cluster present, were included in the study.
Keep patients engaged
Preliminary results showed reductions in PCL-5 scores from pre- to posttreatment of approximately 40% (P < .001).
“Just over 50% of patients left [the program] with symptoms below probable PTSD diagnosis on a self-report measure,” Dr. Rozek said.
Importantly, none of the participants dropped out of the treatment, but Dr. Rozek said that this was not necessarily surprising because of the nature of the program.
“These patients are brought in as a cohort and form some bonds, as they all have experienced traumatic events, although often [they have had] very different traumas,” he said.
“We’ve found that by doing daily treatment, it is more accessible and removes barriers, as it is often easier to take a week or a few weeks off at a time and participate in treatment than the logistics of weekly treatment,” he added.
Dr. Rozek said he suspects two key factors may predict treatment response in such programs – cognitive flexibility and emotion regulation.
“Patients who come into treatment and are extremely rigid in their thinking and are unable to manage their emotions may be slower to respond to treatment,” he noted.
“That being said, there are a variety of treatments that target these factors in different ways. Now we need to do the work to determine which treatments work for whom.”
The findings on longer-term durability of rapid improvement bode well for the program. “Although the work in treatment is hard, they patients really start to see the gains quickly, within a week or weeks instead of months,” Dr. Rozek said.
“This is rewarding in itself, and I would say is a strong factor for keeping patients engaged,” he concluded.
Dr. Rozek has received research funding from the National Institutes of Health, the Department of Defense, the Bob Woodruff Foundation, and the Boeing Corporation.
A version of this article originally appeared on Medscape.com.
Patients who experience a rapid response to cognitive processing therapy (CPT) for posttraumatic stress disorder have a greater likelihood of sustained improvement, new research suggests.
A study of 136 veterans with PTSD showed that those who responded quickly to a 3-week CPT program were significantly more likely to report lower symptom scores 3 months post treatment, compared with those participants who responded more slowly.
The results add to previous evidence that intensified, short-term treatment programs can accomplish long-term benefits, noted the investigators, led by Jenna Bagley, Rush University Medical Center, Chicago.
“These findings show promise for the success of condensed evidence-based, trauma-focused interventions,” they added.
Ms. Bagley was scheduled to present the study in March at the Anxiety and Depression Association of America (ADAA) Conference 2020. That conference was canceled because of the coronavirus pandemic.
Reducing high dropout rates
PTSD treatments such as CPT and prolonged exposure have been shown to have high efficacy, but they also have been shown to have a nearly 40% dropout rate, the researchers note.
This problem has prompted a focus on shorter-term interventions that can deliver intensified treatment before participants drop out. However, evidence has been lacking as to the sustainability of symptom improvements that occur in a short period.
The researchers evaluated data on 136 veterans (66% men; mean age, 41 years) with PTSD who completed a non–Veterans Affairs, 3-week CPT-based intensive treatment program. Follow-up assessments were carried out at 3 months.
Symptom reduction rates represented the number of days from intake in the program to the first day a reduction was reported of at least 15 points on the PTSD Checklist for DSM-5 (PCL-5), which was indicative of a clinically meaningful improvement.
Results showed representing greater ongoing symptoms (P = .04).
The amount of time to reach the 15-point reduction also predicted symptom reductions at the 3-month follow-up, even when controlling for the total change in PCL-5 score during the program (P = .03) and when controlling for type of trauma, such as combat or military sexual trauma.
Another puzzle piece?
Commenting on the study, David C. Rozek, PhD, assistant professor at the University of Central Florida, Orlando, said the findings are encouraging, particularly in terms of improvements seen with shorter treatment programs.
“Sudden gains are important to look at in all treatments,” said Dr. Rozek, who was not involved with the research.
“Seeing that these sudden gains occur in intensive treatment and predict long-term outcome provides another piece of the puzzle and provides additional support to intensive treatments,” he added.
Dr. Rozek noted that he has also observed this with patients. However, they, along with mental health practitioners, often question whether short-term improvements will last.
“There is some concern that a rapid drop in a patient’s symptoms could be an increased risk for a rebound,” he said.
“However, that is when the skills learned in therapy can kick in and provide [patients] tools to use in their everyday life and to help continue recovering,” he added.
Dr. Rozek was scheduled to report results from a pilot study on his own experience at the canceled ADAA meeting. The study was about an even shorter, 7-day intensive CPT program (CPT-7) conducted through the National Center for Veterans Studies.
The program involved one daily individual CPT-7 session with a mental health provider in the morning followed by optional group recreational activities in the afternoon. Twelve military personnel in two cohorts with either PTSD or subthreshold PTSD, defined as having all but one symptom cluster present, were included in the study.
Keep patients engaged
Preliminary results showed reductions in PCL-5 scores from pre- to posttreatment of approximately 40% (P < .001).
“Just over 50% of patients left [the program] with symptoms below probable PTSD diagnosis on a self-report measure,” Dr. Rozek said.
Importantly, none of the participants dropped out of the treatment, but Dr. Rozek said that this was not necessarily surprising because of the nature of the program.
“These patients are brought in as a cohort and form some bonds, as they all have experienced traumatic events, although often [they have had] very different traumas,” he said.
“We’ve found that by doing daily treatment, it is more accessible and removes barriers, as it is often easier to take a week or a few weeks off at a time and participate in treatment than the logistics of weekly treatment,” he added.
Dr. Rozek said he suspects two key factors may predict treatment response in such programs – cognitive flexibility and emotion regulation.
“Patients who come into treatment and are extremely rigid in their thinking and are unable to manage their emotions may be slower to respond to treatment,” he noted.
“That being said, there are a variety of treatments that target these factors in different ways. Now we need to do the work to determine which treatments work for whom.”
The findings on longer-term durability of rapid improvement bode well for the program. “Although the work in treatment is hard, they patients really start to see the gains quickly, within a week or weeks instead of months,” Dr. Rozek said.
“This is rewarding in itself, and I would say is a strong factor for keeping patients engaged,” he concluded.
Dr. Rozek has received research funding from the National Institutes of Health, the Department of Defense, the Bob Woodruff Foundation, and the Boeing Corporation.
A version of this article originally appeared on Medscape.com.
IV esketamine, ketamine equally effective for resistant depression
Intravenous (IV) esketamine is as safe and effective as IV ketamine for patients with treatment-resistant depression, new research suggests.
“Our study was the first randomized clinical trial directly comparing ketamine and esketamine in treatment-resistant depression,” senior investigator Lucas C. Quarantini, MD, PhD, division of psychiatry, Professor Edgard Santos University Hospital, Federal University of Bahia, Salvador, Brazil, said in an interview.
The findings showed that esketamine was not inferior to ketamine in remission of depressive symptoms 24 hours after a single IV dose, and the two treatments had similar side effect profiles, Dr. Quarantini said.
Furthermore, “our results showed that only the number of treatment failures was an important factor for the remission of symptoms,” he added.
The findings were scheduled to be presented at the Anxiety and Depression Association of America (ADAA) Conference 2020, along with publication in the Journal of Affective Disorders (2020 Mar 1;264:527-34). However, the ADAA conference was canceled in the wake of the coronavirus pandemic.
More treatment options
The randomized, double-blind noninferiority trial compared IV racemic ketamine and esketamine, two formulations of the glutamate NMDA receptor modulator drug. It included 63 participants (61.9% women; mean age, 47 years) with treatment-resistant major depressive disorder, as determined by DSM-5 criteria.
Participants were enrolled between March 2017 and June 2018 and randomized to receive a single subanesthetic dose of racemic ketamine (0.5 mg/kg; n = 29) or esketamine (0.25 mg/kg; n = 34) for 40 minutes.
Results showed esketamine to be noninferior to ketamine as determined by the Montgomery-Åsberg Depression Rating Scale (MADRS).
The difference of just 5.3% confirmed noninferiority.
Although ketamine showed a tendency to have a longer-lasting antidepressant effect compared with esketamine, the difference did not reach statistical significance and should be evaluated in future studies, the investigators noted.
Both treatments were safe and well tolerated. Consistent with previous studies, the most frequent side effects were dissociative symptoms, including derealization, depersonalization, and cardiovascular changes, and increased blood pressure and heart rate, which occurred equally in both groups. There were no serious adverse events in either study group.
The investigators noted that most of the previous research examining antidepressant effects of ketamine has used the IV racemic type. The current findings are particularly important for situations in which ketamine or intranasal esketamine, which was recently approved by the Food and Drug Administration, are unavailable, Dr. Quarantini said.
“What our study adds to what has been previously published is that the only way to really analyze if two drugs are equivalent is to compare them in a head-to-head trial; and that was what we did,” he said.
“Our findings bring a greater basis for practitioners from locations where intravenous esketamine is more easily obtainable than ketamine to use it as an affordable option for treating depressive patients,” Dr. Quarantini added.
“Since this [lack of availability] is the scenario here in Brazil, and probably in many other countries, all patients from these locations will benefit from this finding,” he said.
While further evaluating the study results to determine which clinical characteristics were predictive of remission of depressive symptoms, the researchers assessed several key factors. The median duration of disease progression was 12 months, median number of depressive episodes was five, and median number of therapeutic treatment failures was three.
The investigators also looked at the number of suicide attempts and degree of dissociative behavior.
Of these factors, the number of therapeutic failures was the only significant predictor of symptom remission, with an odds ratio of 1.46 for each prior therapeutic failure (95% CI, 1.08-1.99).
“To date, we have not found [other] studies with similar data,” Dr. Quarantini noted.
“Identifying remission predictors may contribute to selecting more suitable candidates for the intervention and result in more individualized and effective patient management,” the investigators wrote.
Consistent findings
Commenting on the findings, Gerard Sanacora, MD, PhD, professor of psychiatry at Yale University, New Haven, Conn., noted that key study limitations include the small sample size and lack of a placebo group.
Nevertheless, “I think it is fair to say that it is unlikely that the treatments are markedly different in their effects on depression over 24 hours,” he said in an interview.
Dr. Sanacora, director of the Yale Depression Research Program, was not involved with the current research.
The findings are “consistent with what we can extrapolate from other clinical trials examining racemic ketamine and esketamine separately,” he said.
Dr. Sanacora noted that because esketamine has been previously shown to be a more potent anesthetic than arketamine, the other component of racemic ketamine, it is “the primary form of ketamine used as an anesthetic agent in several regions of the world with the idea that it may be more selective for the desired anesthetic effect.”
Even with its limitations, the study does offer some notable yet preliminary insights, he added.
“It is interesting to see varying degrees of numerical differences between the two treatments at different time points,” Dr. Sanacora said. In addition, “there may be some differing effects between the two treatments over time, but we really do not have enough data to say much of anything [about that] with confidence at this point.”
The study was supported by the Programa de Pesquisa para o SUS through Fundação de Amparo à Pesquisa do Estado da Bahia. Dr. Quarantini has reported receiving consulting fees from Allergan, Abbott, Janssen Pharmaceuticals, and Lundbeck, and research fees from Janssen Pharmaceuticals. The other study authors’ disclosures are listed in the published article. Dr. Sanacora has reported consulting and/or conducting research from several pharmaceutical companies. He also holds shares in BioHaven Pharmaceuticals and is coinventor on a patent called “Glutamate Agents in the Treatment of Mental Disorders.”
A version of this article originally appeared on Medscape.com.
Intravenous (IV) esketamine is as safe and effective as IV ketamine for patients with treatment-resistant depression, new research suggests.
“Our study was the first randomized clinical trial directly comparing ketamine and esketamine in treatment-resistant depression,” senior investigator Lucas C. Quarantini, MD, PhD, division of psychiatry, Professor Edgard Santos University Hospital, Federal University of Bahia, Salvador, Brazil, said in an interview.
The findings showed that esketamine was not inferior to ketamine in remission of depressive symptoms 24 hours after a single IV dose, and the two treatments had similar side effect profiles, Dr. Quarantini said.
Furthermore, “our results showed that only the number of treatment failures was an important factor for the remission of symptoms,” he added.
The findings were scheduled to be presented at the Anxiety and Depression Association of America (ADAA) Conference 2020, along with publication in the Journal of Affective Disorders (2020 Mar 1;264:527-34). However, the ADAA conference was canceled in the wake of the coronavirus pandemic.
More treatment options
The randomized, double-blind noninferiority trial compared IV racemic ketamine and esketamine, two formulations of the glutamate NMDA receptor modulator drug. It included 63 participants (61.9% women; mean age, 47 years) with treatment-resistant major depressive disorder, as determined by DSM-5 criteria.
Participants were enrolled between March 2017 and June 2018 and randomized to receive a single subanesthetic dose of racemic ketamine (0.5 mg/kg; n = 29) or esketamine (0.25 mg/kg; n = 34) for 40 minutes.
Results showed esketamine to be noninferior to ketamine as determined by the Montgomery-Åsberg Depression Rating Scale (MADRS).
The difference of just 5.3% confirmed noninferiority.
Although ketamine showed a tendency to have a longer-lasting antidepressant effect compared with esketamine, the difference did not reach statistical significance and should be evaluated in future studies, the investigators noted.
Both treatments were safe and well tolerated. Consistent with previous studies, the most frequent side effects were dissociative symptoms, including derealization, depersonalization, and cardiovascular changes, and increased blood pressure and heart rate, which occurred equally in both groups. There were no serious adverse events in either study group.
The investigators noted that most of the previous research examining antidepressant effects of ketamine has used the IV racemic type. The current findings are particularly important for situations in which ketamine or intranasal esketamine, which was recently approved by the Food and Drug Administration, are unavailable, Dr. Quarantini said.
“What our study adds to what has been previously published is that the only way to really analyze if two drugs are equivalent is to compare them in a head-to-head trial; and that was what we did,” he said.
“Our findings bring a greater basis for practitioners from locations where intravenous esketamine is more easily obtainable than ketamine to use it as an affordable option for treating depressive patients,” Dr. Quarantini added.
“Since this [lack of availability] is the scenario here in Brazil, and probably in many other countries, all patients from these locations will benefit from this finding,” he said.
While further evaluating the study results to determine which clinical characteristics were predictive of remission of depressive symptoms, the researchers assessed several key factors. The median duration of disease progression was 12 months, median number of depressive episodes was five, and median number of therapeutic treatment failures was three.
The investigators also looked at the number of suicide attempts and degree of dissociative behavior.
Of these factors, the number of therapeutic failures was the only significant predictor of symptom remission, with an odds ratio of 1.46 for each prior therapeutic failure (95% CI, 1.08-1.99).
“To date, we have not found [other] studies with similar data,” Dr. Quarantini noted.
“Identifying remission predictors may contribute to selecting more suitable candidates for the intervention and result in more individualized and effective patient management,” the investigators wrote.
Consistent findings
Commenting on the findings, Gerard Sanacora, MD, PhD, professor of psychiatry at Yale University, New Haven, Conn., noted that key study limitations include the small sample size and lack of a placebo group.
Nevertheless, “I think it is fair to say that it is unlikely that the treatments are markedly different in their effects on depression over 24 hours,” he said in an interview.
Dr. Sanacora, director of the Yale Depression Research Program, was not involved with the current research.
The findings are “consistent with what we can extrapolate from other clinical trials examining racemic ketamine and esketamine separately,” he said.
Dr. Sanacora noted that because esketamine has been previously shown to be a more potent anesthetic than arketamine, the other component of racemic ketamine, it is “the primary form of ketamine used as an anesthetic agent in several regions of the world with the idea that it may be more selective for the desired anesthetic effect.”
Even with its limitations, the study does offer some notable yet preliminary insights, he added.
“It is interesting to see varying degrees of numerical differences between the two treatments at different time points,” Dr. Sanacora said. In addition, “there may be some differing effects between the two treatments over time, but we really do not have enough data to say much of anything [about that] with confidence at this point.”
The study was supported by the Programa de Pesquisa para o SUS through Fundação de Amparo à Pesquisa do Estado da Bahia. Dr. Quarantini has reported receiving consulting fees from Allergan, Abbott, Janssen Pharmaceuticals, and Lundbeck, and research fees from Janssen Pharmaceuticals. The other study authors’ disclosures are listed in the published article. Dr. Sanacora has reported consulting and/or conducting research from several pharmaceutical companies. He also holds shares in BioHaven Pharmaceuticals and is coinventor on a patent called “Glutamate Agents in the Treatment of Mental Disorders.”
A version of this article originally appeared on Medscape.com.
Intravenous (IV) esketamine is as safe and effective as IV ketamine for patients with treatment-resistant depression, new research suggests.
“Our study was the first randomized clinical trial directly comparing ketamine and esketamine in treatment-resistant depression,” senior investigator Lucas C. Quarantini, MD, PhD, division of psychiatry, Professor Edgard Santos University Hospital, Federal University of Bahia, Salvador, Brazil, said in an interview.
The findings showed that esketamine was not inferior to ketamine in remission of depressive symptoms 24 hours after a single IV dose, and the two treatments had similar side effect profiles, Dr. Quarantini said.
Furthermore, “our results showed that only the number of treatment failures was an important factor for the remission of symptoms,” he added.
The findings were scheduled to be presented at the Anxiety and Depression Association of America (ADAA) Conference 2020, along with publication in the Journal of Affective Disorders (2020 Mar 1;264:527-34). However, the ADAA conference was canceled in the wake of the coronavirus pandemic.
More treatment options
The randomized, double-blind noninferiority trial compared IV racemic ketamine and esketamine, two formulations of the glutamate NMDA receptor modulator drug. It included 63 participants (61.9% women; mean age, 47 years) with treatment-resistant major depressive disorder, as determined by DSM-5 criteria.
Participants were enrolled between March 2017 and June 2018 and randomized to receive a single subanesthetic dose of racemic ketamine (0.5 mg/kg; n = 29) or esketamine (0.25 mg/kg; n = 34) for 40 minutes.
Results showed esketamine to be noninferior to ketamine as determined by the Montgomery-Åsberg Depression Rating Scale (MADRS).
The difference of just 5.3% confirmed noninferiority.
Although ketamine showed a tendency to have a longer-lasting antidepressant effect compared with esketamine, the difference did not reach statistical significance and should be evaluated in future studies, the investigators noted.
Both treatments were safe and well tolerated. Consistent with previous studies, the most frequent side effects were dissociative symptoms, including derealization, depersonalization, and cardiovascular changes, and increased blood pressure and heart rate, which occurred equally in both groups. There were no serious adverse events in either study group.
The investigators noted that most of the previous research examining antidepressant effects of ketamine has used the IV racemic type. The current findings are particularly important for situations in which ketamine or intranasal esketamine, which was recently approved by the Food and Drug Administration, are unavailable, Dr. Quarantini said.
“What our study adds to what has been previously published is that the only way to really analyze if two drugs are equivalent is to compare them in a head-to-head trial; and that was what we did,” he said.
“Our findings bring a greater basis for practitioners from locations where intravenous esketamine is more easily obtainable than ketamine to use it as an affordable option for treating depressive patients,” Dr. Quarantini added.
“Since this [lack of availability] is the scenario here in Brazil, and probably in many other countries, all patients from these locations will benefit from this finding,” he said.
While further evaluating the study results to determine which clinical characteristics were predictive of remission of depressive symptoms, the researchers assessed several key factors. The median duration of disease progression was 12 months, median number of depressive episodes was five, and median number of therapeutic treatment failures was three.
The investigators also looked at the number of suicide attempts and degree of dissociative behavior.
Of these factors, the number of therapeutic failures was the only significant predictor of symptom remission, with an odds ratio of 1.46 for each prior therapeutic failure (95% CI, 1.08-1.99).
“To date, we have not found [other] studies with similar data,” Dr. Quarantini noted.
“Identifying remission predictors may contribute to selecting more suitable candidates for the intervention and result in more individualized and effective patient management,” the investigators wrote.
Consistent findings
Commenting on the findings, Gerard Sanacora, MD, PhD, professor of psychiatry at Yale University, New Haven, Conn., noted that key study limitations include the small sample size and lack of a placebo group.
Nevertheless, “I think it is fair to say that it is unlikely that the treatments are markedly different in their effects on depression over 24 hours,” he said in an interview.
Dr. Sanacora, director of the Yale Depression Research Program, was not involved with the current research.
The findings are “consistent with what we can extrapolate from other clinical trials examining racemic ketamine and esketamine separately,” he said.
Dr. Sanacora noted that because esketamine has been previously shown to be a more potent anesthetic than arketamine, the other component of racemic ketamine, it is “the primary form of ketamine used as an anesthetic agent in several regions of the world with the idea that it may be more selective for the desired anesthetic effect.”
Even with its limitations, the study does offer some notable yet preliminary insights, he added.
“It is interesting to see varying degrees of numerical differences between the two treatments at different time points,” Dr. Sanacora said. In addition, “there may be some differing effects between the two treatments over time, but we really do not have enough data to say much of anything [about that] with confidence at this point.”
The study was supported by the Programa de Pesquisa para o SUS through Fundação de Amparo à Pesquisa do Estado da Bahia. Dr. Quarantini has reported receiving consulting fees from Allergan, Abbott, Janssen Pharmaceuticals, and Lundbeck, and research fees from Janssen Pharmaceuticals. The other study authors’ disclosures are listed in the published article. Dr. Sanacora has reported consulting and/or conducting research from several pharmaceutical companies. He also holds shares in BioHaven Pharmaceuticals and is coinventor on a patent called “Glutamate Agents in the Treatment of Mental Disorders.”
A version of this article originally appeared on Medscape.com.





