User login
VIDEO: Don’t be afraid to treat acne in pregnant patients
DENVER – When it comes to treating pregnant women with acne, Dr. Jonette Keri’s goal is to prevent scarring. Her main message: there are several safe treatment options for women before, during, and after pregnancy, so don’t be afraid to use them.
Dr. Keri, associate professor of dermatology at the University of Miami Miller School of Medicine and chief of the dermatology service at Miami VA Hospital, shares her practice pearls in this video interview.
On Twitter @naseemmiller
DENVER – When it comes to treating pregnant women with acne, Dr. Jonette Keri’s goal is to prevent scarring. Her main message: there are several safe treatment options for women before, during, and after pregnancy, so don’t be afraid to use them.
Dr. Keri, associate professor of dermatology at the University of Miami Miller School of Medicine and chief of the dermatology service at Miami VA Hospital, shares her practice pearls in this video interview.
On Twitter @naseemmiller
DENVER – When it comes to treating pregnant women with acne, Dr. Jonette Keri’s goal is to prevent scarring. Her main message: there are several safe treatment options for women before, during, and after pregnancy, so don’t be afraid to use them.
Dr. Keri, associate professor of dermatology at the University of Miami Miller School of Medicine and chief of the dermatology service at Miami VA Hospital, shares her practice pearls in this video interview.
On Twitter @naseemmiller
AT THE AAD ANNUAL MEETING
Dr. Henry W. Lim previews AAD 2014
DENVER – As the annual meeting of the American Academy of Dermatology kicks off, we asked the meeting’s Scientific Assembly chair, Dr. Henry W. Lim, to give us an overview.
Dr. Lim, professor and chairman of dermatology at Henry Ford Hospital in Detroit, speaks about hot clinical topics, types of sessions, and important policy issues addressed during the meeting.
On Twitter @NaseemMiller
DENVER – As the annual meeting of the American Academy of Dermatology kicks off, we asked the meeting’s Scientific Assembly chair, Dr. Henry W. Lim, to give us an overview.
Dr. Lim, professor and chairman of dermatology at Henry Ford Hospital in Detroit, speaks about hot clinical topics, types of sessions, and important policy issues addressed during the meeting.
On Twitter @NaseemMiller
DENVER – As the annual meeting of the American Academy of Dermatology kicks off, we asked the meeting’s Scientific Assembly chair, Dr. Henry W. Lim, to give us an overview.
Dr. Lim, professor and chairman of dermatology at Henry Ford Hospital in Detroit, speaks about hot clinical topics, types of sessions, and important policy issues addressed during the meeting.
On Twitter @NaseemMiller
AT THE AAD ANNUAL MEETING
VIDEO: Vitiligo gene hunt could open door to eventual drug treatment
DENVER – Vitiligo has been known for thousands of years, but only in the past six decades have researchers begun to better understand it and uncover genes associated with the disorder.
Dr. Richard A. Spritz, one of the leaders in vitiligo research, says that scientists are very close to understanding the condition’s pathogenesis. He is embarking on another analysis of data from a large genetic study, which could double the number of known vitiligo genes, putting the disorder on par with other autoimmune diseases such as type 1 diabetes and rheumatoid arthritis.
At the Society of Pediatric Dermatology’s annual meeting, held immediately before the annual meeting of the American Academy of Dermatology, Dr. Spritz spoke about what is known so far about vitiligo and what the future holds. Dr. Spritz is professor and director of the Human Medical Genetics and Genomics Program at the University of Colorado, Aurora.
On Twitter @naseemsmiller
DENVER – Vitiligo has been known for thousands of years, but only in the past six decades have researchers begun to better understand it and uncover genes associated with the disorder.
Dr. Richard A. Spritz, one of the leaders in vitiligo research, says that scientists are very close to understanding the condition’s pathogenesis. He is embarking on another analysis of data from a large genetic study, which could double the number of known vitiligo genes, putting the disorder on par with other autoimmune diseases such as type 1 diabetes and rheumatoid arthritis.
At the Society of Pediatric Dermatology’s annual meeting, held immediately before the annual meeting of the American Academy of Dermatology, Dr. Spritz spoke about what is known so far about vitiligo and what the future holds. Dr. Spritz is professor and director of the Human Medical Genetics and Genomics Program at the University of Colorado, Aurora.
On Twitter @naseemsmiller
DENVER – Vitiligo has been known for thousands of years, but only in the past six decades have researchers begun to better understand it and uncover genes associated with the disorder.
Dr. Richard A. Spritz, one of the leaders in vitiligo research, says that scientists are very close to understanding the condition’s pathogenesis. He is embarking on another analysis of data from a large genetic study, which could double the number of known vitiligo genes, putting the disorder on par with other autoimmune diseases such as type 1 diabetes and rheumatoid arthritis.
At the Society of Pediatric Dermatology’s annual meeting, held immediately before the annual meeting of the American Academy of Dermatology, Dr. Spritz spoke about what is known so far about vitiligo and what the future holds. Dr. Spritz is professor and director of the Human Medical Genetics and Genomics Program at the University of Colorado, Aurora.
On Twitter @naseemsmiller
AT A MEETING OF THE SOCIETY OF PEDIATRIC DERMATOLOGY
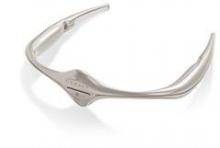
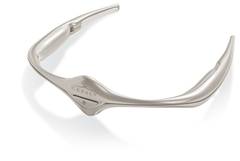
FDA okays first migraine prevention device
The Food and Drug Administration has authorized the marketing of Cefaly, a transcutaneous electrical nerve stimulation device that is designed for use before the onset of migraine headaches.
Cefaly would be first device of its kind in the United States, and it "provides an alternative to medication for migraine prevention," Christy Foreman, director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health, said in a statement. "This may help patients who cannot tolerate current migraine medications for preventing migraines or treating attacks."
The device, which resembles a futuristic tiara for the forehead, is battery powered and will be available via prescription for patients aged 18 years or older. It generates an electric current that stimulates branches of the trigeminal nerve, which has been associated with migraine headaches, and it should only be used once per day for 20 minutes, according to the FDA.
The device was approved based on a randomized, controlled trial of 67 patients, and a satisfaction study of 2,300 users, a little more than half of whom said they were satisfied. There were no serious adverse events in either study.
The 67-patient study showed that the mean number of migraine days, monthly migraine attacks, monthly headache days, and monthly acute antimigraine drug intake were significantly reduced among the patients who received the device, but not among those who were using the placebo device.
Meanwhile, in the 2,300-patient satisfaction study, the most common patient complaint was dislike of the feeling that the device generated. Users also reported sleepiness during the treatment session, and headache afterward.
The Cefaly kit sells for about $350 on the company’s website.
On Twitter @naseemsmiller
The Food and Drug Administration has authorized the marketing of Cefaly, a transcutaneous electrical nerve stimulation device that is designed for use before the onset of migraine headaches.
Cefaly would be first device of its kind in the United States, and it "provides an alternative to medication for migraine prevention," Christy Foreman, director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health, said in a statement. "This may help patients who cannot tolerate current migraine medications for preventing migraines or treating attacks."
The device, which resembles a futuristic tiara for the forehead, is battery powered and will be available via prescription for patients aged 18 years or older. It generates an electric current that stimulates branches of the trigeminal nerve, which has been associated with migraine headaches, and it should only be used once per day for 20 minutes, according to the FDA.
The device was approved based on a randomized, controlled trial of 67 patients, and a satisfaction study of 2,300 users, a little more than half of whom said they were satisfied. There were no serious adverse events in either study.
The 67-patient study showed that the mean number of migraine days, monthly migraine attacks, monthly headache days, and monthly acute antimigraine drug intake were significantly reduced among the patients who received the device, but not among those who were using the placebo device.
Meanwhile, in the 2,300-patient satisfaction study, the most common patient complaint was dislike of the feeling that the device generated. Users also reported sleepiness during the treatment session, and headache afterward.
The Cefaly kit sells for about $350 on the company’s website.
On Twitter @naseemsmiller
The Food and Drug Administration has authorized the marketing of Cefaly, a transcutaneous electrical nerve stimulation device that is designed for use before the onset of migraine headaches.
Cefaly would be first device of its kind in the United States, and it "provides an alternative to medication for migraine prevention," Christy Foreman, director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health, said in a statement. "This may help patients who cannot tolerate current migraine medications for preventing migraines or treating attacks."
The device, which resembles a futuristic tiara for the forehead, is battery powered and will be available via prescription for patients aged 18 years or older. It generates an electric current that stimulates branches of the trigeminal nerve, which has been associated with migraine headaches, and it should only be used once per day for 20 minutes, according to the FDA.
The device was approved based on a randomized, controlled trial of 67 patients, and a satisfaction study of 2,300 users, a little more than half of whom said they were satisfied. There were no serious adverse events in either study.
The 67-patient study showed that the mean number of migraine days, monthly migraine attacks, monthly headache days, and monthly acute antimigraine drug intake were significantly reduced among the patients who received the device, but not among those who were using the placebo device.
Meanwhile, in the 2,300-patient satisfaction study, the most common patient complaint was dislike of the feeling that the device generated. Users also reported sleepiness during the treatment session, and headache afterward.
The Cefaly kit sells for about $350 on the company’s website.
On Twitter @naseemsmiller
VIDEO: Despite privacy concerns, some physicians optimistic about Google Glass
ORLANDO – Google Glass will soon be available to the public, but its future role in medicine is still hazy.
Glass, which is among the growing number of wearable technologies, has several applications to health care settings, experts say. Just last year, a surgeon at Ohio State University’s Wexner Medical Center in Columbus live-streamed an ACL procedure via his Google Glass. And most recently, Rhode Island Hospital’s emergency department began using the device’s video capabilities to consult with off-site specialists.
But Glass is not HIPAA compliant, raising patient privacy concerns and dissuading hospitals from incorporating it into their growing list of mobile technologies. Still, technology enthusiasts are charging ahead, envisioning a future in which Glass could be yet another piece in physicians’ toolkits.
At the annual meeting of the Healthcare Information and Management Systems Society, Dr. Jonathan A. Handler, chief medical information officer for M*Modal, spoke about Glass's potential in medicine.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemsmiller
ORLANDO – Google Glass will soon be available to the public, but its future role in medicine is still hazy.
Glass, which is among the growing number of wearable technologies, has several applications to health care settings, experts say. Just last year, a surgeon at Ohio State University’s Wexner Medical Center in Columbus live-streamed an ACL procedure via his Google Glass. And most recently, Rhode Island Hospital’s emergency department began using the device’s video capabilities to consult with off-site specialists.
But Glass is not HIPAA compliant, raising patient privacy concerns and dissuading hospitals from incorporating it into their growing list of mobile technologies. Still, technology enthusiasts are charging ahead, envisioning a future in which Glass could be yet another piece in physicians’ toolkits.
At the annual meeting of the Healthcare Information and Management Systems Society, Dr. Jonathan A. Handler, chief medical information officer for M*Modal, spoke about Glass's potential in medicine.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemsmiller
ORLANDO – Google Glass will soon be available to the public, but its future role in medicine is still hazy.
Glass, which is among the growing number of wearable technologies, has several applications to health care settings, experts say. Just last year, a surgeon at Ohio State University’s Wexner Medical Center in Columbus live-streamed an ACL procedure via his Google Glass. And most recently, Rhode Island Hospital’s emergency department began using the device’s video capabilities to consult with off-site specialists.
But Glass is not HIPAA compliant, raising patient privacy concerns and dissuading hospitals from incorporating it into their growing list of mobile technologies. Still, technology enthusiasts are charging ahead, envisioning a future in which Glass could be yet another piece in physicians’ toolkits.
At the annual meeting of the Healthcare Information and Management Systems Society, Dr. Jonathan A. Handler, chief medical information officer for M*Modal, spoke about Glass's potential in medicine.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemsmiller
AT HIMSS14
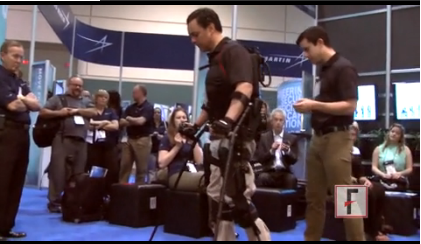
VIDEO: Bionic exoskeleton helps paralyzed patients walk
ORLANDO – It’s a bionic suit, a battery-powered exoskeleton, or as Chris Tagatac calls it, a wearable robot.
Mr. Tagatac, who is paralyzed from the lower ribs down, demonstrated the computer- and battery-operated Ekso Bionics suit at the annual meeting of the Healthcare Information and Management Systems Society, and he spoke with us about his experience. Watch the video to learn more about him and the technology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
On Twitter @naseemsmiller
ORLANDO – It’s a bionic suit, a battery-powered exoskeleton, or as Chris Tagatac calls it, a wearable robot.
Mr. Tagatac, who is paralyzed from the lower ribs down, demonstrated the computer- and battery-operated Ekso Bionics suit at the annual meeting of the Healthcare Information and Management Systems Society, and he spoke with us about his experience. Watch the video to learn more about him and the technology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
On Twitter @naseemsmiller
ORLANDO – It’s a bionic suit, a battery-powered exoskeleton, or as Chris Tagatac calls it, a wearable robot.
Mr. Tagatac, who is paralyzed from the lower ribs down, demonstrated the computer- and battery-operated Ekso Bionics suit at the annual meeting of the Healthcare Information and Management Systems Society, and he spoke with us about his experience. Watch the video to learn more about him and the technology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
On Twitter @naseemsmiller
AT HIMSS14
Transcranial ultrasound method outdoes echocardiography for finding PFO
SAN DIEGO – Transcranial Doppler saline studies were superior to transesophageal echocardiography in diagnosing a hole in the heart, according to a prospective study of patients who had a cryptogenic stroke.
Researchers analyzed data from 340 patients with cryptogenic stroke suspected of paradoxical embolism who had had their right-left shunt confirmed with transcranial Doppler saline study (TCDSS). Transesophageal echocardiography (TEE) failed to show a shunt such as patent foramen ovale (PFO) in 15% of the cases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. J. David Spence, Robarts Research Institute
"I was surprised by the number," said Dr. J. David Spence, senior researcher of the study, who presented the findings at the International Stroke Conference, sponsored by the American Heart Association. "I had realized that some of the patients with shunts were missed by TEE, ... but I was surprised by how often that happened."
Nearly a quarter of the population has a PFO, and 4.0%-5.5% of strokes are due to paradoxical embolism through a right-left shunt. But patching the shunts isn’t the simple answer, because the procedure is not without complications, and so far, the studies haven’t been able to make a strong case for the procedure.
TCDSS is an ultrasound method in which 1 mL of tiny bubbles is injected into the vein and if detected in the brain, could give clues to a right-left shunt. The method is safer, the equipment is cheaper, and the training is faster than for TEE, Dr. Spence said.
He added that one reason that TCDSS may be more sensitive than TEE is that sedation for TEE may prevent an adequate Valsalva maneuver. He also showed in a video that detecting the bubbles in TCDSS is rather straightforward.
Given the findings of his study, Dr. Spence said that there’s a need to identify which PFO patients are more likely to have paradoxical embolism and are more likely to respond to patching.
One solution is paying attention to the clinical clues of paradoxical embolism (J. Neurol. Sci. 2008;275:121-7). And Dr. Spence’s recent findings may add another tool to help with this prediction.
Aside from the superiority of TCDSS to TEE in identifying PFO, Dr. Spence and his colleagues found that TCDSS was also better for risk stratification of PFOs. The analysis found that 25% of the shunts that were missed on TEE were high-grade shunts (Spencer grade 3 or higher). Patients with a shunt grade of 3 or higher were significantly more likely to have a stroke or transient ischemic attack in the 4-year follow-up period (P = .028), said Dr. Spence, director of the Stroke Prevention & Atherosclerosis Research Centre at the University of Western Ontario, London. This was not predicted by the presence of shunt on TEE, nor was it predicted by mobile atrial septum or septal aneurysm. TEE missed nearly 46% of grade 1 shunts, 32% of grade 2, 13% of grade 3, 7% of grade 4, and almost 5% of grade 5.
"This doesn’t mean that everyone should go pack up their TEE machines, because we still need it for diagnosis of other cardioembolism," said Dr. Spence. "But these techniques can be complementary."
He added that it’s too soon to use these findings as grounds for change in clinical practice, and there’s a need for further studies such as randomized trials.
The majority (62%) of the study’s 340 patients were female. The patients had a mean age of 53 years and underwent follow-up for a median of 420 days. All the patients, who visited the center between 2000 and 2013, had cryptogenic stroke and were suspected of having paradoxical embolism. A total of 280 cases had TEE data available.
Dr. Spence said he had no disclosures relevant to the study.
On Twitter @naseemsmiller
SAN DIEGO – Transcranial Doppler saline studies were superior to transesophageal echocardiography in diagnosing a hole in the heart, according to a prospective study of patients who had a cryptogenic stroke.
Researchers analyzed data from 340 patients with cryptogenic stroke suspected of paradoxical embolism who had had their right-left shunt confirmed with transcranial Doppler saline study (TCDSS). Transesophageal echocardiography (TEE) failed to show a shunt such as patent foramen ovale (PFO) in 15% of the cases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. J. David Spence, Robarts Research Institute
"I was surprised by the number," said Dr. J. David Spence, senior researcher of the study, who presented the findings at the International Stroke Conference, sponsored by the American Heart Association. "I had realized that some of the patients with shunts were missed by TEE, ... but I was surprised by how often that happened."
Nearly a quarter of the population has a PFO, and 4.0%-5.5% of strokes are due to paradoxical embolism through a right-left shunt. But patching the shunts isn’t the simple answer, because the procedure is not without complications, and so far, the studies haven’t been able to make a strong case for the procedure.
TCDSS is an ultrasound method in which 1 mL of tiny bubbles is injected into the vein and if detected in the brain, could give clues to a right-left shunt. The method is safer, the equipment is cheaper, and the training is faster than for TEE, Dr. Spence said.
He added that one reason that TCDSS may be more sensitive than TEE is that sedation for TEE may prevent an adequate Valsalva maneuver. He also showed in a video that detecting the bubbles in TCDSS is rather straightforward.
Given the findings of his study, Dr. Spence said that there’s a need to identify which PFO patients are more likely to have paradoxical embolism and are more likely to respond to patching.
One solution is paying attention to the clinical clues of paradoxical embolism (J. Neurol. Sci. 2008;275:121-7). And Dr. Spence’s recent findings may add another tool to help with this prediction.
Aside from the superiority of TCDSS to TEE in identifying PFO, Dr. Spence and his colleagues found that TCDSS was also better for risk stratification of PFOs. The analysis found that 25% of the shunts that were missed on TEE were high-grade shunts (Spencer grade 3 or higher). Patients with a shunt grade of 3 or higher were significantly more likely to have a stroke or transient ischemic attack in the 4-year follow-up period (P = .028), said Dr. Spence, director of the Stroke Prevention & Atherosclerosis Research Centre at the University of Western Ontario, London. This was not predicted by the presence of shunt on TEE, nor was it predicted by mobile atrial septum or septal aneurysm. TEE missed nearly 46% of grade 1 shunts, 32% of grade 2, 13% of grade 3, 7% of grade 4, and almost 5% of grade 5.
"This doesn’t mean that everyone should go pack up their TEE machines, because we still need it for diagnosis of other cardioembolism," said Dr. Spence. "But these techniques can be complementary."
He added that it’s too soon to use these findings as grounds for change in clinical practice, and there’s a need for further studies such as randomized trials.
The majority (62%) of the study’s 340 patients were female. The patients had a mean age of 53 years and underwent follow-up for a median of 420 days. All the patients, who visited the center between 2000 and 2013, had cryptogenic stroke and were suspected of having paradoxical embolism. A total of 280 cases had TEE data available.
Dr. Spence said he had no disclosures relevant to the study.
On Twitter @naseemsmiller
SAN DIEGO – Transcranial Doppler saline studies were superior to transesophageal echocardiography in diagnosing a hole in the heart, according to a prospective study of patients who had a cryptogenic stroke.
Researchers analyzed data from 340 patients with cryptogenic stroke suspected of paradoxical embolism who had had their right-left shunt confirmed with transcranial Doppler saline study (TCDSS). Transesophageal echocardiography (TEE) failed to show a shunt such as patent foramen ovale (PFO) in 15% of the cases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. J. David Spence, Robarts Research Institute
"I was surprised by the number," said Dr. J. David Spence, senior researcher of the study, who presented the findings at the International Stroke Conference, sponsored by the American Heart Association. "I had realized that some of the patients with shunts were missed by TEE, ... but I was surprised by how often that happened."
Nearly a quarter of the population has a PFO, and 4.0%-5.5% of strokes are due to paradoxical embolism through a right-left shunt. But patching the shunts isn’t the simple answer, because the procedure is not without complications, and so far, the studies haven’t been able to make a strong case for the procedure.
TCDSS is an ultrasound method in which 1 mL of tiny bubbles is injected into the vein and if detected in the brain, could give clues to a right-left shunt. The method is safer, the equipment is cheaper, and the training is faster than for TEE, Dr. Spence said.
He added that one reason that TCDSS may be more sensitive than TEE is that sedation for TEE may prevent an adequate Valsalva maneuver. He also showed in a video that detecting the bubbles in TCDSS is rather straightforward.
Given the findings of his study, Dr. Spence said that there’s a need to identify which PFO patients are more likely to have paradoxical embolism and are more likely to respond to patching.
One solution is paying attention to the clinical clues of paradoxical embolism (J. Neurol. Sci. 2008;275:121-7). And Dr. Spence’s recent findings may add another tool to help with this prediction.
Aside from the superiority of TCDSS to TEE in identifying PFO, Dr. Spence and his colleagues found that TCDSS was also better for risk stratification of PFOs. The analysis found that 25% of the shunts that were missed on TEE were high-grade shunts (Spencer grade 3 or higher). Patients with a shunt grade of 3 or higher were significantly more likely to have a stroke or transient ischemic attack in the 4-year follow-up period (P = .028), said Dr. Spence, director of the Stroke Prevention & Atherosclerosis Research Centre at the University of Western Ontario, London. This was not predicted by the presence of shunt on TEE, nor was it predicted by mobile atrial septum or septal aneurysm. TEE missed nearly 46% of grade 1 shunts, 32% of grade 2, 13% of grade 3, 7% of grade 4, and almost 5% of grade 5.
"This doesn’t mean that everyone should go pack up their TEE machines, because we still need it for diagnosis of other cardioembolism," said Dr. Spence. "But these techniques can be complementary."
He added that it’s too soon to use these findings as grounds for change in clinical practice, and there’s a need for further studies such as randomized trials.
The majority (62%) of the study’s 340 patients were female. The patients had a mean age of 53 years and underwent follow-up for a median of 420 days. All the patients, who visited the center between 2000 and 2013, had cryptogenic stroke and were suspected of having paradoxical embolism. A total of 280 cases had TEE data available.
Dr. Spence said he had no disclosures relevant to the study.
On Twitter @naseemsmiller
AT THE INTERNATIONAL STROKE CONFERENCE
Major finding: Echocardiography (TEE) failed to show right-to-left shunts, such as patent foramen ovale, which were identified by TCDSS, in 15% of the cases.
Data source: Prospective study of 340 patients who had a cryptogenic stroke.
Disclosures: Dr. Spence had no disclosures relevant to the study.
IT survey underlines move to mobile health
ORLANDO – Most health IT executives provided a mobile device to point-of-care clinicians in their organizations, according to the results of the third annual HIMSS Analytics Mobile Survey.
Ranging from computers on wheels to smartphones and tablets, most clinicians used the provided devices primarily to access patient information, clinical practice guidelines, and reference materials, based on the survey results released at the annual meeting of the Healthcare Information Management Systems Society.
"The mobile health market is one of the fastest-growing areas in the health IT space," said David Collins, senior director of mHIMSS, in a statement. "We recognize the growing importance of mobile technologies and its impact to transform the delivery of patient care."
The survey of health IT executives, conducted between December 2013 and January 2014, showed a projected increase in the use of tablet computers while hinting at the demise of the pager.
While 67% of the respondents said they provided pagers to their staff, only 8% said that they’ll add or expand the use of the devices. Laptop computers remained the most common (87%) mobile device provided to clinicians.
Among other findings:
• 36% of respondents said physicians in their organizations used the technology to collect data at bedside.
• Organizations were most likely to leverage mobile technology for pharmacy management, such as medication reminders, preventive support care, continuing of care, and telehealth interventions.
• 35% said that their organization provided apps for their patients for monitoring chronic conditions, physical activity, or nutrition. More than half said they were planning to update or launch new apps next year.
• 22% said that most of their data was integrated into the organization’s electronic medical records.
• 95% said they’ve used at least one security tool to secure data on mobile devices. Passwords were the most widespread form of security.
The complete survey can be viewed here.
On Twitter @naseemsmiller
ORLANDO – Most health IT executives provided a mobile device to point-of-care clinicians in their organizations, according to the results of the third annual HIMSS Analytics Mobile Survey.
Ranging from computers on wheels to smartphones and tablets, most clinicians used the provided devices primarily to access patient information, clinical practice guidelines, and reference materials, based on the survey results released at the annual meeting of the Healthcare Information Management Systems Society.
"The mobile health market is one of the fastest-growing areas in the health IT space," said David Collins, senior director of mHIMSS, in a statement. "We recognize the growing importance of mobile technologies and its impact to transform the delivery of patient care."
The survey of health IT executives, conducted between December 2013 and January 2014, showed a projected increase in the use of tablet computers while hinting at the demise of the pager.
While 67% of the respondents said they provided pagers to their staff, only 8% said that they’ll add or expand the use of the devices. Laptop computers remained the most common (87%) mobile device provided to clinicians.
Among other findings:
• 36% of respondents said physicians in their organizations used the technology to collect data at bedside.
• Organizations were most likely to leverage mobile technology for pharmacy management, such as medication reminders, preventive support care, continuing of care, and telehealth interventions.
• 35% said that their organization provided apps for their patients for monitoring chronic conditions, physical activity, or nutrition. More than half said they were planning to update or launch new apps next year.
• 22% said that most of their data was integrated into the organization’s electronic medical records.
• 95% said they’ve used at least one security tool to secure data on mobile devices. Passwords were the most widespread form of security.
The complete survey can be viewed here.
On Twitter @naseemsmiller
ORLANDO – Most health IT executives provided a mobile device to point-of-care clinicians in their organizations, according to the results of the third annual HIMSS Analytics Mobile Survey.
Ranging from computers on wheels to smartphones and tablets, most clinicians used the provided devices primarily to access patient information, clinical practice guidelines, and reference materials, based on the survey results released at the annual meeting of the Healthcare Information Management Systems Society.
"The mobile health market is one of the fastest-growing areas in the health IT space," said David Collins, senior director of mHIMSS, in a statement. "We recognize the growing importance of mobile technologies and its impact to transform the delivery of patient care."
The survey of health IT executives, conducted between December 2013 and January 2014, showed a projected increase in the use of tablet computers while hinting at the demise of the pager.
While 67% of the respondents said they provided pagers to their staff, only 8% said that they’ll add or expand the use of the devices. Laptop computers remained the most common (87%) mobile device provided to clinicians.
Among other findings:
• 36% of respondents said physicians in their organizations used the technology to collect data at bedside.
• Organizations were most likely to leverage mobile technology for pharmacy management, such as medication reminders, preventive support care, continuing of care, and telehealth interventions.
• 35% said that their organization provided apps for their patients for monitoring chronic conditions, physical activity, or nutrition. More than half said they were planning to update or launch new apps next year.
• 22% said that most of their data was integrated into the organization’s electronic medical records.
• 95% said they’ve used at least one security tool to secure data on mobile devices. Passwords were the most widespread form of security.
The complete survey can be viewed here.
On Twitter @naseemsmiller
AT HIMSS14
Midpoint safety results for intraventricular hemorrhage thrombolysis trial promising
SAN DIEGO – The midpoint results of the CLEAR III trial that is investigating the safety and benefit of using recombinant tissue plasminogen activator to lyse intraventricular clots formed after intracerebral hemorrhage didn’t set off any safety alarms.
"The mortality, bleeding, and infection rates are lower than expected, indicating that the study protocol is safe for patients with severe intraventricular hemorrhage," said Dr. Wendy Ziai, chair of the trial’s safety event committee and the principal investigator of the trial at Johns Hopkins University, Baltimore, which is one of 70 sites involved in the trial.
There are currently very few treatments available for the treatment of intraventricular hemorrhage (IVH) and intracerebral hemorrhage (ICH). Clot lysis with recombinant tissue plasminogen activator (TPA) is one treatment option that has reached phase III, and it could have practice-changing implications, depending on the final results.
But for now, "we still don’t have enough information to change patient care," said Dr. Darin Zahuranec of the department of neurology at the University of Michigan, Ann Arbor. "These findings mean that we need to eagerly await the final results," said Dr. Zahuranec, who was not involved in the study.
The double-blind, randomized placebo-controlled CLEAR III trial (Clot Lysis: Evaluation of Accelerated Resolution of Interventricular Hemorrhage Phase III) is evaluating the benefit, safety, and best protocol for extraventricular drainage use plus 1-mg recombinant TPA (Alteplase) every 8 hours, in patients with IVH. The control group had extraventricular drainage in place, but received placebo.
The midpoint results, which included 250 patients in 61 centers, also showed that prophylactic heparin during and immediately after dosing may be associated with a higher risk of intracranial bleeding. Thrombotic events, however, may be more frequent in patients who were not receiving prophylaxis, said Dr. Ziai, who presented her findings at the International Stroke Conference, sponsored by the American Heart Association.
The CLEAR III trial monitored three prespecified safety endpoint thresholds: symptomatic hemorrhage within 72 hours of the agent, brain infections within 30 days of randomization, and 30-day mortality.
The results for all patients showed that there were 36 hemorrhages at 72 hours after the last dose of the study agent, 3 (1.2%) of which were symptomatic. Also, 22 (61%) of the hemorrhages occurred in the setting of prophylactic heparin.
Within 30 days of randomization, there were 60 brain hemorrhages in 44 patients at a rate of 17.5% (10% were symptomatic and 90% were asymptomatic). Mortality was 12.4%, bacterial ventriculitis was 2%, and nonbacterial ventriculitis was 5.2%.
"These are well below the [Data Safety Monitoring Board’s] safety thresholds," Dr. Ziai said.
Of 215 patients not on antiplatelet therapy prior to diagnosis, 15% had a bleeding event 72 hours after randomization, compared with 11% of the 35 patients who were on antiplatelet therapy. The association was not significant.
Also, of the 116 patients not on prophylactic heparin, 12% had a hemorrhagic event, compared with 16% of the 134 patients who were receiving the therapy, showing no significant association.
Meanwhile, 23% of the patients who were not on prophylactic heparin had a thrombotic event by day 30, while 9% of those on heparin had such an event (P = .005).
There were 259 severe adverse events within 1-year follow-up, 19 (7%) of which were possibly related to the study agent. Ventriculoperitoneal shunts were placed in 53 patients.
"It should be remembered that intraventricular rTPA is not currently the standard of care, and it’s hoped that CLEAR III trial will demonstrate the benefit and safety of this therapy," Dr. Ziai said. "The safety, however, cannot be achieved without strict protocols for stabilizing hemorrhages, concurrent drug use, and careful tracking of adverse events through an adjudication process."
Dr. Ziai and Dr. Zahuranec said they had no relevant financial disclosures. The study is funded by the National Institute of Neurological Disorders and Stroke.
On Twitter @naseemsmiller
SAN DIEGO – The midpoint results of the CLEAR III trial that is investigating the safety and benefit of using recombinant tissue plasminogen activator to lyse intraventricular clots formed after intracerebral hemorrhage didn’t set off any safety alarms.
"The mortality, bleeding, and infection rates are lower than expected, indicating that the study protocol is safe for patients with severe intraventricular hemorrhage," said Dr. Wendy Ziai, chair of the trial’s safety event committee and the principal investigator of the trial at Johns Hopkins University, Baltimore, which is one of 70 sites involved in the trial.
There are currently very few treatments available for the treatment of intraventricular hemorrhage (IVH) and intracerebral hemorrhage (ICH). Clot lysis with recombinant tissue plasminogen activator (TPA) is one treatment option that has reached phase III, and it could have practice-changing implications, depending on the final results.
But for now, "we still don’t have enough information to change patient care," said Dr. Darin Zahuranec of the department of neurology at the University of Michigan, Ann Arbor. "These findings mean that we need to eagerly await the final results," said Dr. Zahuranec, who was not involved in the study.
The double-blind, randomized placebo-controlled CLEAR III trial (Clot Lysis: Evaluation of Accelerated Resolution of Interventricular Hemorrhage Phase III) is evaluating the benefit, safety, and best protocol for extraventricular drainage use plus 1-mg recombinant TPA (Alteplase) every 8 hours, in patients with IVH. The control group had extraventricular drainage in place, but received placebo.
The midpoint results, which included 250 patients in 61 centers, also showed that prophylactic heparin during and immediately after dosing may be associated with a higher risk of intracranial bleeding. Thrombotic events, however, may be more frequent in patients who were not receiving prophylaxis, said Dr. Ziai, who presented her findings at the International Stroke Conference, sponsored by the American Heart Association.
The CLEAR III trial monitored three prespecified safety endpoint thresholds: symptomatic hemorrhage within 72 hours of the agent, brain infections within 30 days of randomization, and 30-day mortality.
The results for all patients showed that there were 36 hemorrhages at 72 hours after the last dose of the study agent, 3 (1.2%) of which were symptomatic. Also, 22 (61%) of the hemorrhages occurred in the setting of prophylactic heparin.
Within 30 days of randomization, there were 60 brain hemorrhages in 44 patients at a rate of 17.5% (10% were symptomatic and 90% were asymptomatic). Mortality was 12.4%, bacterial ventriculitis was 2%, and nonbacterial ventriculitis was 5.2%.
"These are well below the [Data Safety Monitoring Board’s] safety thresholds," Dr. Ziai said.
Of 215 patients not on antiplatelet therapy prior to diagnosis, 15% had a bleeding event 72 hours after randomization, compared with 11% of the 35 patients who were on antiplatelet therapy. The association was not significant.
Also, of the 116 patients not on prophylactic heparin, 12% had a hemorrhagic event, compared with 16% of the 134 patients who were receiving the therapy, showing no significant association.
Meanwhile, 23% of the patients who were not on prophylactic heparin had a thrombotic event by day 30, while 9% of those on heparin had such an event (P = .005).
There were 259 severe adverse events within 1-year follow-up, 19 (7%) of which were possibly related to the study agent. Ventriculoperitoneal shunts were placed in 53 patients.
"It should be remembered that intraventricular rTPA is not currently the standard of care, and it’s hoped that CLEAR III trial will demonstrate the benefit and safety of this therapy," Dr. Ziai said. "The safety, however, cannot be achieved without strict protocols for stabilizing hemorrhages, concurrent drug use, and careful tracking of adverse events through an adjudication process."
Dr. Ziai and Dr. Zahuranec said they had no relevant financial disclosures. The study is funded by the National Institute of Neurological Disorders and Stroke.
On Twitter @naseemsmiller
SAN DIEGO – The midpoint results of the CLEAR III trial that is investigating the safety and benefit of using recombinant tissue plasminogen activator to lyse intraventricular clots formed after intracerebral hemorrhage didn’t set off any safety alarms.
"The mortality, bleeding, and infection rates are lower than expected, indicating that the study protocol is safe for patients with severe intraventricular hemorrhage," said Dr. Wendy Ziai, chair of the trial’s safety event committee and the principal investigator of the trial at Johns Hopkins University, Baltimore, which is one of 70 sites involved in the trial.
There are currently very few treatments available for the treatment of intraventricular hemorrhage (IVH) and intracerebral hemorrhage (ICH). Clot lysis with recombinant tissue plasminogen activator (TPA) is one treatment option that has reached phase III, and it could have practice-changing implications, depending on the final results.
But for now, "we still don’t have enough information to change patient care," said Dr. Darin Zahuranec of the department of neurology at the University of Michigan, Ann Arbor. "These findings mean that we need to eagerly await the final results," said Dr. Zahuranec, who was not involved in the study.
The double-blind, randomized placebo-controlled CLEAR III trial (Clot Lysis: Evaluation of Accelerated Resolution of Interventricular Hemorrhage Phase III) is evaluating the benefit, safety, and best protocol for extraventricular drainage use plus 1-mg recombinant TPA (Alteplase) every 8 hours, in patients with IVH. The control group had extraventricular drainage in place, but received placebo.
The midpoint results, which included 250 patients in 61 centers, also showed that prophylactic heparin during and immediately after dosing may be associated with a higher risk of intracranial bleeding. Thrombotic events, however, may be more frequent in patients who were not receiving prophylaxis, said Dr. Ziai, who presented her findings at the International Stroke Conference, sponsored by the American Heart Association.
The CLEAR III trial monitored three prespecified safety endpoint thresholds: symptomatic hemorrhage within 72 hours of the agent, brain infections within 30 days of randomization, and 30-day mortality.
The results for all patients showed that there were 36 hemorrhages at 72 hours after the last dose of the study agent, 3 (1.2%) of which were symptomatic. Also, 22 (61%) of the hemorrhages occurred in the setting of prophylactic heparin.
Within 30 days of randomization, there were 60 brain hemorrhages in 44 patients at a rate of 17.5% (10% were symptomatic and 90% were asymptomatic). Mortality was 12.4%, bacterial ventriculitis was 2%, and nonbacterial ventriculitis was 5.2%.
"These are well below the [Data Safety Monitoring Board’s] safety thresholds," Dr. Ziai said.
Of 215 patients not on antiplatelet therapy prior to diagnosis, 15% had a bleeding event 72 hours after randomization, compared with 11% of the 35 patients who were on antiplatelet therapy. The association was not significant.
Also, of the 116 patients not on prophylactic heparin, 12% had a hemorrhagic event, compared with 16% of the 134 patients who were receiving the therapy, showing no significant association.
Meanwhile, 23% of the patients who were not on prophylactic heparin had a thrombotic event by day 30, while 9% of those on heparin had such an event (P = .005).
There were 259 severe adverse events within 1-year follow-up, 19 (7%) of which were possibly related to the study agent. Ventriculoperitoneal shunts were placed in 53 patients.
"It should be remembered that intraventricular rTPA is not currently the standard of care, and it’s hoped that CLEAR III trial will demonstrate the benefit and safety of this therapy," Dr. Ziai said. "The safety, however, cannot be achieved without strict protocols for stabilizing hemorrhages, concurrent drug use, and careful tracking of adverse events through an adjudication process."
Dr. Ziai and Dr. Zahuranec said they had no relevant financial disclosures. The study is funded by the National Institute of Neurological Disorders and Stroke.
On Twitter @naseemsmiller
AT THE INTERNATIONAL STROKE CONFERENCE
Major finding: Results showed that within 30 days, mortality was 12.4%, bacterial ventriculitis was 2%, and nonbacterial ventriculitis was 5.2%, all below the safety threshold.
Data source: Midpoint results of a phase III, double-blind, randomized placebo-controlled trial of 250 patients with IVH.
Disclosures: Dr. Ziai and Dr. Zahuranec said they had no relevant financial disclosures. The study is funded by the National Institute of Neurological Disorders and Stroke.
Meet Dr. Victor Dzau, new IOM president
Beginning in July, Dr. Victor J. Dzau will take the helm at the Institute of Medicine, succeeding Dr. Harvey V. Fineberg, who has been the institute’s president for the past 12 years.
Dr. Dzau will be on leave of absence from his post as chancellor for health affairs at Duke University, Durham, N.C., and president and CEO for Duke University Health System.
He’s also in the process of resigning from several corporation boards, including PepsiCo, he said in an interview.
"I think at IOM we need to be independent, and there shouldn’t be any potential concerns over these issues," said Dr. Dzau.
Dr. Dzau spoke about the most pressing issues in health care, and his vision for his presidency, in an interview.
Dr. Dzau, who was born in China and grew up in Hong Kong, brings with him experience in academic health systems, national health policy, health care innovation, and global health. He is recognized at Duke for his "transformational leadership," which "renewed academic, research, and clinical care enterprises."
While at Duke, he has also been conducting research focused on the molecular and genetic mechanisms of cardiovascular disease and the development of new gene and stem cell–based therapies to regenerate and repair tissue damage from heart attack and heart disease, according to the university.
"He has guided Duke Medicine through a rapidly changing health care landscape with strength, imagination and unflagging energy. He has been an outstanding citizen of the university, the city, and the region, and a major voice for health care innovation globally through the World Economic Forum," Duke University President Richard H. Brodhead said in a statement.
Dr. Dzau isn’t new to the institute. He was elected to IOM Council in 1998.
"As a physician-scientist and leader in academic medicine, Victor has consistently demonstrated inspirational leadership, innovative thinking, and multifaceted achievement. Now, all of us at the IOM, both members and staff, will benefit more fully from his leadership," Mr. Fineberg said in a statement.
"Many people have been telling me, ‘You have to make a lot of sacrifices,’ but I’m prepared to make those sacrifices," said Dr. Dzau. "I think it’s an enormous opportunity to make a difference, and it’s not about me. It’s what we can together to make the society better."
You can read more about his background and accolades here.
On Twitter @naseemsmiller
Beginning in July, Dr. Victor J. Dzau will take the helm at the Institute of Medicine, succeeding Dr. Harvey V. Fineberg, who has been the institute’s president for the past 12 years.
Dr. Dzau will be on leave of absence from his post as chancellor for health affairs at Duke University, Durham, N.C., and president and CEO for Duke University Health System.
He’s also in the process of resigning from several corporation boards, including PepsiCo, he said in an interview.
"I think at IOM we need to be independent, and there shouldn’t be any potential concerns over these issues," said Dr. Dzau.
Dr. Dzau spoke about the most pressing issues in health care, and his vision for his presidency, in an interview.
Dr. Dzau, who was born in China and grew up in Hong Kong, brings with him experience in academic health systems, national health policy, health care innovation, and global health. He is recognized at Duke for his "transformational leadership," which "renewed academic, research, and clinical care enterprises."
While at Duke, he has also been conducting research focused on the molecular and genetic mechanisms of cardiovascular disease and the development of new gene and stem cell–based therapies to regenerate and repair tissue damage from heart attack and heart disease, according to the university.
"He has guided Duke Medicine through a rapidly changing health care landscape with strength, imagination and unflagging energy. He has been an outstanding citizen of the university, the city, and the region, and a major voice for health care innovation globally through the World Economic Forum," Duke University President Richard H. Brodhead said in a statement.
Dr. Dzau isn’t new to the institute. He was elected to IOM Council in 1998.
"As a physician-scientist and leader in academic medicine, Victor has consistently demonstrated inspirational leadership, innovative thinking, and multifaceted achievement. Now, all of us at the IOM, both members and staff, will benefit more fully from his leadership," Mr. Fineberg said in a statement.
"Many people have been telling me, ‘You have to make a lot of sacrifices,’ but I’m prepared to make those sacrifices," said Dr. Dzau. "I think it’s an enormous opportunity to make a difference, and it’s not about me. It’s what we can together to make the society better."
You can read more about his background and accolades here.
On Twitter @naseemsmiller
Beginning in July, Dr. Victor J. Dzau will take the helm at the Institute of Medicine, succeeding Dr. Harvey V. Fineberg, who has been the institute’s president for the past 12 years.
Dr. Dzau will be on leave of absence from his post as chancellor for health affairs at Duke University, Durham, N.C., and president and CEO for Duke University Health System.
He’s also in the process of resigning from several corporation boards, including PepsiCo, he said in an interview.
"I think at IOM we need to be independent, and there shouldn’t be any potential concerns over these issues," said Dr. Dzau.
Dr. Dzau spoke about the most pressing issues in health care, and his vision for his presidency, in an interview.
Dr. Dzau, who was born in China and grew up in Hong Kong, brings with him experience in academic health systems, national health policy, health care innovation, and global health. He is recognized at Duke for his "transformational leadership," which "renewed academic, research, and clinical care enterprises."
While at Duke, he has also been conducting research focused on the molecular and genetic mechanisms of cardiovascular disease and the development of new gene and stem cell–based therapies to regenerate and repair tissue damage from heart attack and heart disease, according to the university.
"He has guided Duke Medicine through a rapidly changing health care landscape with strength, imagination and unflagging energy. He has been an outstanding citizen of the university, the city, and the region, and a major voice for health care innovation globally through the World Economic Forum," Duke University President Richard H. Brodhead said in a statement.
Dr. Dzau isn’t new to the institute. He was elected to IOM Council in 1998.
"As a physician-scientist and leader in academic medicine, Victor has consistently demonstrated inspirational leadership, innovative thinking, and multifaceted achievement. Now, all of us at the IOM, both members and staff, will benefit more fully from his leadership," Mr. Fineberg said in a statement.
"Many people have been telling me, ‘You have to make a lot of sacrifices,’ but I’m prepared to make those sacrifices," said Dr. Dzau. "I think it’s an enormous opportunity to make a difference, and it’s not about me. It’s what we can together to make the society better."
You can read more about his background and accolades here.
On Twitter @naseemsmiller