User login
Treatment of Femoroacetabular Impingement: Labrum, Cartilage, Osseous Deformity, and Capsule
Take-Home Points
- Repair the labrum when tissue quality is good.
- Avoid overcorrection of acetabulum by measuring center edge angle.
- Cam resection should be comprehensive and restore a smooth head-neck offset to restore the suction seal.
- Chondral débridement for Outerbridge grade 0-3 and microfracture for grade 4.
- Routine capsular closure to prevent postoperative instability.
The surgical approach of femoroacetabular impingement (FAI) pathology should cover the entire hip joint. Both bony and cartilaginous tissue pathology should be adequately addressed. However, treating soft-tissue abnormalities (acetabular labrum and joint capsule) is also crucial. Overall, any surgical intervention should focus on restoring the hip labrum seal mechanism to ensure successful clinical outcomes. This restoration, combined with the use of biological therapies and rehabilitation, will produce the maximum benefit for the patient.
Management of Acetabular Labrum
The final decision regarding how to surgically approach the acetabular labrum is made during the operation. We focus restoring the labrum seal mechanism, which is crucial for proper function and health of the hip joint.1 The intra-articular hydrostatic pressure loss caused by labral deficiency results in abnormal load distribution and joint microinstability, which have detrimental effects on cartilage and periarticular tissues. A biomechanical study highlighted the role of the hip labrum in maintaining intra-articular fluid pressurization and showed that labral reconstruction restores intra-articular fluid pressure to levels similar to those of the intact state.1
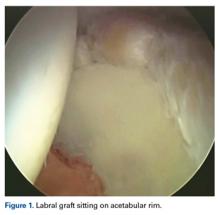
In cases in which the remaining labral tissue is adequate and of good quality (reparable), the labral repair technique is preferred.2 After diagnostic arthroscopy, the labral tear is identified, and a 4.5-mm burr is used to correct (rim-trim) any osseous deformity of the acetabulum to create a “new rim” for labrum reattachment. Suture anchors are placed on the rim about 2 mm to 3 mm below the cartilage surface. Considering the rim angle3 is helpful in avoiding acetabular cartilage damage. Labral sutures can be looped around or pierced through the labrum to secure it to the acetabulum. No difference in clinical outcomes was found between the 2 suture types,4 though biomechanically piercing sutures help restore the labrum seal better.1 When the labrum is deficient and longitudinal fibers remain but are insufficient for seal restoration, the repair can be augmented with adjacent iliotibial band (ITB) tissue. This technique is similar to labral reconstruction but involves placing a graft on top of the remaining labral tissue, and suture around both the native tissue and the graft. The additional tissue gives the labrum the volume it needs to recreate the seal.
The labral reconstruction technique is indicated when the remaining labrum is irreparable, absent, or severely hypotrophic or deficient, or when an irreparable complex tear or poor-quality tissue is present. Different types of grafts can be used to reconstruct the labrum. ITB, semitendinosus, gracilis, and anterior tibialis grafts and the human acetabular labrum exhibit similar cyclic elongation behavior in response to simulated physiologic forces, though there is variability in both elongation and geometry for all graft types.5 We prefer the ITB autograft technique.6 The graft should be about 30% to 40% longer than the labral defect as measured with arthroscopic probe. With the leg in traction, the graft is inserted through the mid-anterior portal, and a suture anchor is used to secure it against the acetabulum medially.
With proper patient selection, these techniques have excellent clinical outcomes.4,7 Severe osteoarthritis (joint space <2 mm) is a contraindication for these procedures.8
Osseous Deformity
On approaching the bony structures of the hip joint, the surgeon should examine the acetabular rim (pincer lesion), the femoral head and neck shape (cam lesion), and the anterior inferior iliac spine (AIIS). Preoperative imaging and physical examination are important for identifying severe bone deformities that can complicate the procedure.9
The acetabular rim can be directly viewed after labrum detachment, but usually complete detachment is not necessary. Pincer deformity causes focal or global overcoverage of the femoral head. Rim trimming is performed with a 4.5-mm round curved burr. Resection is usually performed to the end of rim chondrosis (about 3-5 mm). Using a simple formula, you can calculate how the lateral center edge will be reduced by the amount of rim resected, maintaining a safe margin.2 A new acetabular “bed” is created where the to-be-attached labral tissue will contribute to the suction seal mechanism of the joint.2Cam lesion correction is challenging, and the amount of bone that should be resected is a matter of disagreement. We perform cam osteochondroplasty2 with a 5.5-mm round burr inserted through the anterolateral portal while the hip is positioned in 45° of flexion, neutral rotation, and adduction/abduction. This position allows an osteoplasty from 6 to 10 o’clock on the head–neck junction. Osteoplasty performed between 10 and 12 o’clock requires hip extension and slight traction. The proximal limit of osteochondroplasty is about 15 mm from the labral edge, while distally the resection stops beneath the zona orbicularis. The lateral epiphyseal vessels and the Weitbrecht ligament constitute the lateral and medial borders, respectively.
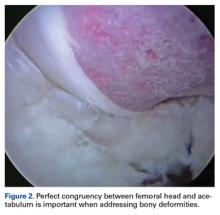
The surgeon should create a smooth head–neck offset that prevents elevation of the labrum during flexion and achieves a nearly perfect anatomical relationship between the femoral head and the acetabular labrum, restoring the hip joint seal (Figure 2).
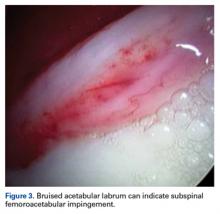
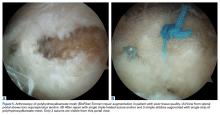
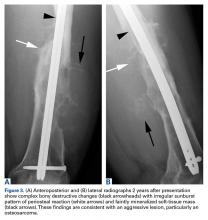
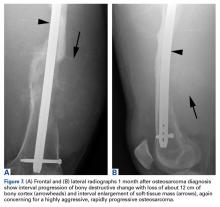
A hypertrophic AIIS can impinge the femur (extra-articular subspinal impingement). Patients present with limited range of motion (especially hip flexion), pain in the AIIS area, and, in some cases, a history of avulsion injury.11 Seeing a bruised labrum (Figure 3) during surgery is common with this pathology.
Treatment of Cartilage Lesions
The indications and contraindications for hip arthroscopy in patients with cartilage lesions are important. Our study’s 5-year outcomes of treating FAI with hip arthroscopy in patients with preserved joint space (>2 mm) were promising, though 86% of patients with limited joint space (≤2 mm) converted to total hip arthroplasty.8 We regard patients with severe osteoarthritis as not being candidates for hip arthroscopy.
As 3 Tesla magnetic resonance imaging has low positive predictive value in identifying severe cartilage damage,13 the cartilage should be examined during surgery to further define the diagnosis. Nearly half of the hip arthroscopy patients in our study had at least 1 Outerbridge grade 3 or 4 cartilage lesion.14 Compared with the femoral head, acetabular cartilage was damaged 3 times more often. More than 90% of acetabular cartilage lesions were in the anterosuperior region.
Grades 0 and 1 cartilage lesions are usually left untreated; no intervention is necessary. Grades 2 and 3 cartilage lesions are reduced by partial débridement and/or thermal shrinkage. With the improved joint microenvironment arising from simple correction of the underlying hip bony abnormalities, these lesions should not produce further symptoms.
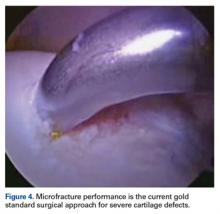
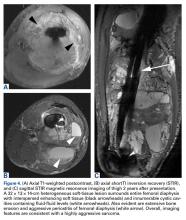
Grade 4 hip cartilage defects are challenging. We prefer microfracture for grade 4 lesions (Figure 4).
A ring curette is used to prepare the defect, and perpendicular borders are created to hold the clot in place. Deep débridement removes the calcified layer while maintaining the integrity of the subchondral plate.15 As a recent study found microfracture performed with small-diameter awls improved cartilage repair more effectively than microfracture with large-diameter awls,16 we prefer making small-diameter holes when establishing the maximum number of holes possible. As it is important to make a perpendicular hole, not a scratch, we use an XL Microfracture Pick (Smith & Nephew) 90° curve, which is suitable for creating a vertical entry point. The 60° curved awl is then used to further deepen the hole. Creation and stability of the marrow clot are ensured by shutting down the infusion pump device and verifying that blood and marrow elements are released from the microfractures.
Capsule Management
The increase in hip arthroscopies performed worldwide has generated interest in proper capsular management and development of iatrogenic microinstability.17 Hip capsulotomy is routinely performed for adequate visualization of the intra-articular compartment. Standard anterosuperior interportal capsulotomy for hip arthroscopic surgery (12 to 3 o’clock) sacrifices the integrity of the iliofemoral ligament (ligament of Bigelow),18 which provides rotational stability. Failure to restore the anatomical and biomechanical properties of the iliofemoral ligament after arthroscopic surgery increases the likelihood of postoperative microinstability or gross instability,19 which can lead to persistent pain and/or sense of an unstable joint, in addition to accelerated cartilage wear.
Capsulotomies are useful in obtaining adequate intraoperative exposure of the central and peripheral compartments. In the past, little attention was given to capsular closure on completion of the procedure. However, concern about postoperative instability from capsular laxity or deficiency made the introduction of capsular repair techniques necessary. Although deciding between capsular closure and plication remains debatable, we routinely perform capsular closure with a Quebec City slider knot.20 Mindful management of the capsule throughout the procedure is important in avoiding irreversible capsular damage, which would complicate capsular closure. Mindful management involves leaving a proximal leaflet of at least 1 cm during the capsulotomy, avoiding capsular thinning during shaver use, and using a cannula to prevent soft-tissue bridging.
Recent evidence suggests that capsule repair restores near native hip joint stability.17 In addition to capsular shift or capsulorrhaphy, 2 to 6 sutures have been used for capsular closure or plication after an interportal or T capsulotomy. Chahla and colleagues21 reported that 2- and 3-suture constructs produced comparable biomechanical failure torques when external rotation forces were applied to conventional hip capsulotomy on cadavers. Three-suture constructs were significantly stronger than 1-suture constructs, but there was no significant difference between 2- and 3-suture constructs. All constructs failed at about 36° of external rotation. Therefore, restricted external rotation is recommended for 3 weeks after surgery.
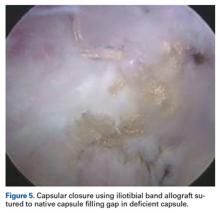
In one study, 35% of revision hip arthroscopy patients had undiagnosed hip instability from iatrogenic injury,22 which can lead to labral and chondral injury.17 Capsular reconstruction is recommended in cases of symptomatic capsular deficiency; capsular deficiency caused by adhesion removal; and pain and range-of-motion limitation caused by capsular adhesions. However, indications need to be further established. We have performed capsular reconstruction with ITB allograft23 (Figure 5).
Biologics
At the end of the procedure, we use platelet-rich plasma and/or bone marrow aspirate injections (individualized to the patient) to potentiate the biological healing of the tissues. Further research is planned to determine how to prepare these biological products to provide the best mix of biological factors for improved healing. Antifibrotic factors are useful in preventing adhesions, and angiotensin II receptor blockers are effective, but clinical studies are needed to establish their use.
Rehabilitation
Immediately after surgery, a postoperative hip brace and antirotational boots are applied to the patient to protect the operative site and reduce pain. The actual postoperative protocol is based on the procedure and individualized to the patient. During microfractures, the patient is kept 20 pounds touch-toe weight-bearing for 4 to 8 weeks. The capsular closure is brace-protected by limiting abduction to 0° to 45° and hip flexion to 0° to 90° while external rotation and extension are prohibited (first 3 weeks). Immediate mobilization with passive rotational movement is crucial in preventing adhesions. Stationary bike exercise and use of a continuous passive motion machine are helpful. Progressive functional and sport-specific rehabilitation help the patient return to full activity, though the decision to return to full activity is based on several factors, both objective (functional tests) and subjective (physician–patient co-decisions).
Conclusion
Although hip arthroscopic techniques have expanded significantly in recent years, our treatment approach is based on restoring the normal anatomy of the hip joint—combining the procedures with biological therapies and a postoperative rehabilitation program that is individualized to the patient’s special needs.
Am J Orthop. 2017;46(1):23-27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
3. Lertwanich P, Ejnisman L, Torry MR, Giphart JE, Philippon MJ. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(suppl):111S-116S.
4. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
5. Ferro FP, Philippon MJ, Rasmussen MT, Smith SD, LaPrade RF, Wijdicks CA. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am J Sports Med. 2015;43(5):1222-1227.
6. Philippon MJ, Briggs KK, Boykin RE. Results of arthroscopic labral reconstruction of the hip in elite athletes: response. Am J Sports Med. 2014;42(10):NP48.
7. Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41(8):1750-1756.
8. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42(5):1127-1133.
9. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
10. Locks R, Chahla J, Mitchell JJ, Soares E, Philippon MJ. Dynamic hip examination for assesment of impingement during hip arthroscopy. Arthroscopy Tech. 2016 Nov 28. http://dx.doi.org/10.1016/j.eats.2016.08.011
11. Nabhan DC, Moreau WJ, McNamara SC, Briggs KK, Philippon MJ. Subspine hip impingement: an unusual cause of hip pain in an elite weightlifter. Curr Sports Med Rep. 2016;15(5):315-319.
12. Philippon MJ, Michalski MP, Campbell KJ, et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am. 2014;96(20):1673-1682.
13. Ho CP, Ommen ND, Bhatia S, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808-1813.
14. Bhatia S, Nowak DD, Briggs KK, Patterson DC, Philippon MJ. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Arthroscopy. 2016;32(5):814-819.
15. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-1831.
16. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209-219.
17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
18. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
19. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
20. Menge TJ, Chahla J, Soares E, Mitchell JJ, Philippon MJ. The Quebec City slider: a technique for capsular closure and plication in hip arthroscopy. Arthrosc Tech. 2016;5(5):e971-e974.
21. Chahla J, Mikula JD, Schon JM, et al. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. doi:10.1177/0363546516666353.
22. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
23. Trindade CA, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71-e74.
Take-Home Points
- Repair the labrum when tissue quality is good.
- Avoid overcorrection of acetabulum by measuring center edge angle.
- Cam resection should be comprehensive and restore a smooth head-neck offset to restore the suction seal.
- Chondral débridement for Outerbridge grade 0-3 and microfracture for grade 4.
- Routine capsular closure to prevent postoperative instability.
The surgical approach of femoroacetabular impingement (FAI) pathology should cover the entire hip joint. Both bony and cartilaginous tissue pathology should be adequately addressed. However, treating soft-tissue abnormalities (acetabular labrum and joint capsule) is also crucial. Overall, any surgical intervention should focus on restoring the hip labrum seal mechanism to ensure successful clinical outcomes. This restoration, combined with the use of biological therapies and rehabilitation, will produce the maximum benefit for the patient.
Management of Acetabular Labrum
The final decision regarding how to surgically approach the acetabular labrum is made during the operation. We focus restoring the labrum seal mechanism, which is crucial for proper function and health of the hip joint.1 The intra-articular hydrostatic pressure loss caused by labral deficiency results in abnormal load distribution and joint microinstability, which have detrimental effects on cartilage and periarticular tissues. A biomechanical study highlighted the role of the hip labrum in maintaining intra-articular fluid pressurization and showed that labral reconstruction restores intra-articular fluid pressure to levels similar to those of the intact state.1
In cases in which the remaining labral tissue is adequate and of good quality (reparable), the labral repair technique is preferred.2 After diagnostic arthroscopy, the labral tear is identified, and a 4.5-mm burr is used to correct (rim-trim) any osseous deformity of the acetabulum to create a “new rim” for labrum reattachment. Suture anchors are placed on the rim about 2 mm to 3 mm below the cartilage surface. Considering the rim angle3 is helpful in avoiding acetabular cartilage damage. Labral sutures can be looped around or pierced through the labrum to secure it to the acetabulum. No difference in clinical outcomes was found between the 2 suture types,4 though biomechanically piercing sutures help restore the labrum seal better.1 When the labrum is deficient and longitudinal fibers remain but are insufficient for seal restoration, the repair can be augmented with adjacent iliotibial band (ITB) tissue. This technique is similar to labral reconstruction but involves placing a graft on top of the remaining labral tissue, and suture around both the native tissue and the graft. The additional tissue gives the labrum the volume it needs to recreate the seal.
The labral reconstruction technique is indicated when the remaining labrum is irreparable, absent, or severely hypotrophic or deficient, or when an irreparable complex tear or poor-quality tissue is present. Different types of grafts can be used to reconstruct the labrum. ITB, semitendinosus, gracilis, and anterior tibialis grafts and the human acetabular labrum exhibit similar cyclic elongation behavior in response to simulated physiologic forces, though there is variability in both elongation and geometry for all graft types.5 We prefer the ITB autograft technique.6 The graft should be about 30% to 40% longer than the labral defect as measured with arthroscopic probe. With the leg in traction, the graft is inserted through the mid-anterior portal, and a suture anchor is used to secure it against the acetabulum medially.
With proper patient selection, these techniques have excellent clinical outcomes.4,7 Severe osteoarthritis (joint space <2 mm) is a contraindication for these procedures.8
Osseous Deformity
On approaching the bony structures of the hip joint, the surgeon should examine the acetabular rim (pincer lesion), the femoral head and neck shape (cam lesion), and the anterior inferior iliac spine (AIIS). Preoperative imaging and physical examination are important for identifying severe bone deformities that can complicate the procedure.9
The acetabular rim can be directly viewed after labrum detachment, but usually complete detachment is not necessary. Pincer deformity causes focal or global overcoverage of the femoral head. Rim trimming is performed with a 4.5-mm round curved burr. Resection is usually performed to the end of rim chondrosis (about 3-5 mm). Using a simple formula, you can calculate how the lateral center edge will be reduced by the amount of rim resected, maintaining a safe margin.2 A new acetabular “bed” is created where the to-be-attached labral tissue will contribute to the suction seal mechanism of the joint.2Cam lesion correction is challenging, and the amount of bone that should be resected is a matter of disagreement. We perform cam osteochondroplasty2 with a 5.5-mm round burr inserted through the anterolateral portal while the hip is positioned in 45° of flexion, neutral rotation, and adduction/abduction. This position allows an osteoplasty from 6 to 10 o’clock on the head–neck junction. Osteoplasty performed between 10 and 12 o’clock requires hip extension and slight traction. The proximal limit of osteochondroplasty is about 15 mm from the labral edge, while distally the resection stops beneath the zona orbicularis. The lateral epiphyseal vessels and the Weitbrecht ligament constitute the lateral and medial borders, respectively.
The surgeon should create a smooth head–neck offset that prevents elevation of the labrum during flexion and achieves a nearly perfect anatomical relationship between the femoral head and the acetabular labrum, restoring the hip joint seal (Figure 2).
A hypertrophic AIIS can impinge the femur (extra-articular subspinal impingement). Patients present with limited range of motion (especially hip flexion), pain in the AIIS area, and, in some cases, a history of avulsion injury.11 Seeing a bruised labrum (Figure 3) during surgery is common with this pathology.
Treatment of Cartilage Lesions
The indications and contraindications for hip arthroscopy in patients with cartilage lesions are important. Our study’s 5-year outcomes of treating FAI with hip arthroscopy in patients with preserved joint space (>2 mm) were promising, though 86% of patients with limited joint space (≤2 mm) converted to total hip arthroplasty.8 We regard patients with severe osteoarthritis as not being candidates for hip arthroscopy.
As 3 Tesla magnetic resonance imaging has low positive predictive value in identifying severe cartilage damage,13 the cartilage should be examined during surgery to further define the diagnosis. Nearly half of the hip arthroscopy patients in our study had at least 1 Outerbridge grade 3 or 4 cartilage lesion.14 Compared with the femoral head, acetabular cartilage was damaged 3 times more often. More than 90% of acetabular cartilage lesions were in the anterosuperior region.
Grades 0 and 1 cartilage lesions are usually left untreated; no intervention is necessary. Grades 2 and 3 cartilage lesions are reduced by partial débridement and/or thermal shrinkage. With the improved joint microenvironment arising from simple correction of the underlying hip bony abnormalities, these lesions should not produce further symptoms.
Grade 4 hip cartilage defects are challenging. We prefer microfracture for grade 4 lesions (Figure 4).
A ring curette is used to prepare the defect, and perpendicular borders are created to hold the clot in place. Deep débridement removes the calcified layer while maintaining the integrity of the subchondral plate.15 As a recent study found microfracture performed with small-diameter awls improved cartilage repair more effectively than microfracture with large-diameter awls,16 we prefer making small-diameter holes when establishing the maximum number of holes possible. As it is important to make a perpendicular hole, not a scratch, we use an XL Microfracture Pick (Smith & Nephew) 90° curve, which is suitable for creating a vertical entry point. The 60° curved awl is then used to further deepen the hole. Creation and stability of the marrow clot are ensured by shutting down the infusion pump device and verifying that blood and marrow elements are released from the microfractures.
Capsule Management
The increase in hip arthroscopies performed worldwide has generated interest in proper capsular management and development of iatrogenic microinstability.17 Hip capsulotomy is routinely performed for adequate visualization of the intra-articular compartment. Standard anterosuperior interportal capsulotomy for hip arthroscopic surgery (12 to 3 o’clock) sacrifices the integrity of the iliofemoral ligament (ligament of Bigelow),18 which provides rotational stability. Failure to restore the anatomical and biomechanical properties of the iliofemoral ligament after arthroscopic surgery increases the likelihood of postoperative microinstability or gross instability,19 which can lead to persistent pain and/or sense of an unstable joint, in addition to accelerated cartilage wear.
Capsulotomies are useful in obtaining adequate intraoperative exposure of the central and peripheral compartments. In the past, little attention was given to capsular closure on completion of the procedure. However, concern about postoperative instability from capsular laxity or deficiency made the introduction of capsular repair techniques necessary. Although deciding between capsular closure and plication remains debatable, we routinely perform capsular closure with a Quebec City slider knot.20 Mindful management of the capsule throughout the procedure is important in avoiding irreversible capsular damage, which would complicate capsular closure. Mindful management involves leaving a proximal leaflet of at least 1 cm during the capsulotomy, avoiding capsular thinning during shaver use, and using a cannula to prevent soft-tissue bridging.
Recent evidence suggests that capsule repair restores near native hip joint stability.17 In addition to capsular shift or capsulorrhaphy, 2 to 6 sutures have been used for capsular closure or plication after an interportal or T capsulotomy. Chahla and colleagues21 reported that 2- and 3-suture constructs produced comparable biomechanical failure torques when external rotation forces were applied to conventional hip capsulotomy on cadavers. Three-suture constructs were significantly stronger than 1-suture constructs, but there was no significant difference between 2- and 3-suture constructs. All constructs failed at about 36° of external rotation. Therefore, restricted external rotation is recommended for 3 weeks after surgery.
In one study, 35% of revision hip arthroscopy patients had undiagnosed hip instability from iatrogenic injury,22 which can lead to labral and chondral injury.17 Capsular reconstruction is recommended in cases of symptomatic capsular deficiency; capsular deficiency caused by adhesion removal; and pain and range-of-motion limitation caused by capsular adhesions. However, indications need to be further established. We have performed capsular reconstruction with ITB allograft23 (Figure 5).
Biologics
At the end of the procedure, we use platelet-rich plasma and/or bone marrow aspirate injections (individualized to the patient) to potentiate the biological healing of the tissues. Further research is planned to determine how to prepare these biological products to provide the best mix of biological factors for improved healing. Antifibrotic factors are useful in preventing adhesions, and angiotensin II receptor blockers are effective, but clinical studies are needed to establish their use.
Rehabilitation
Immediately after surgery, a postoperative hip brace and antirotational boots are applied to the patient to protect the operative site and reduce pain. The actual postoperative protocol is based on the procedure and individualized to the patient. During microfractures, the patient is kept 20 pounds touch-toe weight-bearing for 4 to 8 weeks. The capsular closure is brace-protected by limiting abduction to 0° to 45° and hip flexion to 0° to 90° while external rotation and extension are prohibited (first 3 weeks). Immediate mobilization with passive rotational movement is crucial in preventing adhesions. Stationary bike exercise and use of a continuous passive motion machine are helpful. Progressive functional and sport-specific rehabilitation help the patient return to full activity, though the decision to return to full activity is based on several factors, both objective (functional tests) and subjective (physician–patient co-decisions).
Conclusion
Although hip arthroscopic techniques have expanded significantly in recent years, our treatment approach is based on restoring the normal anatomy of the hip joint—combining the procedures with biological therapies and a postoperative rehabilitation program that is individualized to the patient’s special needs.
Am J Orthop. 2017;46(1):23-27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Repair the labrum when tissue quality is good.
- Avoid overcorrection of acetabulum by measuring center edge angle.
- Cam resection should be comprehensive and restore a smooth head-neck offset to restore the suction seal.
- Chondral débridement for Outerbridge grade 0-3 and microfracture for grade 4.
- Routine capsular closure to prevent postoperative instability.
The surgical approach of femoroacetabular impingement (FAI) pathology should cover the entire hip joint. Both bony and cartilaginous tissue pathology should be adequately addressed. However, treating soft-tissue abnormalities (acetabular labrum and joint capsule) is also crucial. Overall, any surgical intervention should focus on restoring the hip labrum seal mechanism to ensure successful clinical outcomes. This restoration, combined with the use of biological therapies and rehabilitation, will produce the maximum benefit for the patient.
Management of Acetabular Labrum
The final decision regarding how to surgically approach the acetabular labrum is made during the operation. We focus restoring the labrum seal mechanism, which is crucial for proper function and health of the hip joint.1 The intra-articular hydrostatic pressure loss caused by labral deficiency results in abnormal load distribution and joint microinstability, which have detrimental effects on cartilage and periarticular tissues. A biomechanical study highlighted the role of the hip labrum in maintaining intra-articular fluid pressurization and showed that labral reconstruction restores intra-articular fluid pressure to levels similar to those of the intact state.1
In cases in which the remaining labral tissue is adequate and of good quality (reparable), the labral repair technique is preferred.2 After diagnostic arthroscopy, the labral tear is identified, and a 4.5-mm burr is used to correct (rim-trim) any osseous deformity of the acetabulum to create a “new rim” for labrum reattachment. Suture anchors are placed on the rim about 2 mm to 3 mm below the cartilage surface. Considering the rim angle3 is helpful in avoiding acetabular cartilage damage. Labral sutures can be looped around or pierced through the labrum to secure it to the acetabulum. No difference in clinical outcomes was found between the 2 suture types,4 though biomechanically piercing sutures help restore the labrum seal better.1 When the labrum is deficient and longitudinal fibers remain but are insufficient for seal restoration, the repair can be augmented with adjacent iliotibial band (ITB) tissue. This technique is similar to labral reconstruction but involves placing a graft on top of the remaining labral tissue, and suture around both the native tissue and the graft. The additional tissue gives the labrum the volume it needs to recreate the seal.
The labral reconstruction technique is indicated when the remaining labrum is irreparable, absent, or severely hypotrophic or deficient, or when an irreparable complex tear or poor-quality tissue is present. Different types of grafts can be used to reconstruct the labrum. ITB, semitendinosus, gracilis, and anterior tibialis grafts and the human acetabular labrum exhibit similar cyclic elongation behavior in response to simulated physiologic forces, though there is variability in both elongation and geometry for all graft types.5 We prefer the ITB autograft technique.6 The graft should be about 30% to 40% longer than the labral defect as measured with arthroscopic probe. With the leg in traction, the graft is inserted through the mid-anterior portal, and a suture anchor is used to secure it against the acetabulum medially.
With proper patient selection, these techniques have excellent clinical outcomes.4,7 Severe osteoarthritis (joint space <2 mm) is a contraindication for these procedures.8
Osseous Deformity
On approaching the bony structures of the hip joint, the surgeon should examine the acetabular rim (pincer lesion), the femoral head and neck shape (cam lesion), and the anterior inferior iliac spine (AIIS). Preoperative imaging and physical examination are important for identifying severe bone deformities that can complicate the procedure.9
The acetabular rim can be directly viewed after labrum detachment, but usually complete detachment is not necessary. Pincer deformity causes focal or global overcoverage of the femoral head. Rim trimming is performed with a 4.5-mm round curved burr. Resection is usually performed to the end of rim chondrosis (about 3-5 mm). Using a simple formula, you can calculate how the lateral center edge will be reduced by the amount of rim resected, maintaining a safe margin.2 A new acetabular “bed” is created where the to-be-attached labral tissue will contribute to the suction seal mechanism of the joint.2Cam lesion correction is challenging, and the amount of bone that should be resected is a matter of disagreement. We perform cam osteochondroplasty2 with a 5.5-mm round burr inserted through the anterolateral portal while the hip is positioned in 45° of flexion, neutral rotation, and adduction/abduction. This position allows an osteoplasty from 6 to 10 o’clock on the head–neck junction. Osteoplasty performed between 10 and 12 o’clock requires hip extension and slight traction. The proximal limit of osteochondroplasty is about 15 mm from the labral edge, while distally the resection stops beneath the zona orbicularis. The lateral epiphyseal vessels and the Weitbrecht ligament constitute the lateral and medial borders, respectively.
The surgeon should create a smooth head–neck offset that prevents elevation of the labrum during flexion and achieves a nearly perfect anatomical relationship between the femoral head and the acetabular labrum, restoring the hip joint seal (Figure 2).
A hypertrophic AIIS can impinge the femur (extra-articular subspinal impingement). Patients present with limited range of motion (especially hip flexion), pain in the AIIS area, and, in some cases, a history of avulsion injury.11 Seeing a bruised labrum (Figure 3) during surgery is common with this pathology.
Treatment of Cartilage Lesions
The indications and contraindications for hip arthroscopy in patients with cartilage lesions are important. Our study’s 5-year outcomes of treating FAI with hip arthroscopy in patients with preserved joint space (>2 mm) were promising, though 86% of patients with limited joint space (≤2 mm) converted to total hip arthroplasty.8 We regard patients with severe osteoarthritis as not being candidates for hip arthroscopy.
As 3 Tesla magnetic resonance imaging has low positive predictive value in identifying severe cartilage damage,13 the cartilage should be examined during surgery to further define the diagnosis. Nearly half of the hip arthroscopy patients in our study had at least 1 Outerbridge grade 3 or 4 cartilage lesion.14 Compared with the femoral head, acetabular cartilage was damaged 3 times more often. More than 90% of acetabular cartilage lesions were in the anterosuperior region.
Grades 0 and 1 cartilage lesions are usually left untreated; no intervention is necessary. Grades 2 and 3 cartilage lesions are reduced by partial débridement and/or thermal shrinkage. With the improved joint microenvironment arising from simple correction of the underlying hip bony abnormalities, these lesions should not produce further symptoms.
Grade 4 hip cartilage defects are challenging. We prefer microfracture for grade 4 lesions (Figure 4).
A ring curette is used to prepare the defect, and perpendicular borders are created to hold the clot in place. Deep débridement removes the calcified layer while maintaining the integrity of the subchondral plate.15 As a recent study found microfracture performed with small-diameter awls improved cartilage repair more effectively than microfracture with large-diameter awls,16 we prefer making small-diameter holes when establishing the maximum number of holes possible. As it is important to make a perpendicular hole, not a scratch, we use an XL Microfracture Pick (Smith & Nephew) 90° curve, which is suitable for creating a vertical entry point. The 60° curved awl is then used to further deepen the hole. Creation and stability of the marrow clot are ensured by shutting down the infusion pump device and verifying that blood and marrow elements are released from the microfractures.
Capsule Management
The increase in hip arthroscopies performed worldwide has generated interest in proper capsular management and development of iatrogenic microinstability.17 Hip capsulotomy is routinely performed for adequate visualization of the intra-articular compartment. Standard anterosuperior interportal capsulotomy for hip arthroscopic surgery (12 to 3 o’clock) sacrifices the integrity of the iliofemoral ligament (ligament of Bigelow),18 which provides rotational stability. Failure to restore the anatomical and biomechanical properties of the iliofemoral ligament after arthroscopic surgery increases the likelihood of postoperative microinstability or gross instability,19 which can lead to persistent pain and/or sense of an unstable joint, in addition to accelerated cartilage wear.
Capsulotomies are useful in obtaining adequate intraoperative exposure of the central and peripheral compartments. In the past, little attention was given to capsular closure on completion of the procedure. However, concern about postoperative instability from capsular laxity or deficiency made the introduction of capsular repair techniques necessary. Although deciding between capsular closure and plication remains debatable, we routinely perform capsular closure with a Quebec City slider knot.20 Mindful management of the capsule throughout the procedure is important in avoiding irreversible capsular damage, which would complicate capsular closure. Mindful management involves leaving a proximal leaflet of at least 1 cm during the capsulotomy, avoiding capsular thinning during shaver use, and using a cannula to prevent soft-tissue bridging.
Recent evidence suggests that capsule repair restores near native hip joint stability.17 In addition to capsular shift or capsulorrhaphy, 2 to 6 sutures have been used for capsular closure or plication after an interportal or T capsulotomy. Chahla and colleagues21 reported that 2- and 3-suture constructs produced comparable biomechanical failure torques when external rotation forces were applied to conventional hip capsulotomy on cadavers. Three-suture constructs were significantly stronger than 1-suture constructs, but there was no significant difference between 2- and 3-suture constructs. All constructs failed at about 36° of external rotation. Therefore, restricted external rotation is recommended for 3 weeks after surgery.
In one study, 35% of revision hip arthroscopy patients had undiagnosed hip instability from iatrogenic injury,22 which can lead to labral and chondral injury.17 Capsular reconstruction is recommended in cases of symptomatic capsular deficiency; capsular deficiency caused by adhesion removal; and pain and range-of-motion limitation caused by capsular adhesions. However, indications need to be further established. We have performed capsular reconstruction with ITB allograft23 (Figure 5).
Biologics
At the end of the procedure, we use platelet-rich plasma and/or bone marrow aspirate injections (individualized to the patient) to potentiate the biological healing of the tissues. Further research is planned to determine how to prepare these biological products to provide the best mix of biological factors for improved healing. Antifibrotic factors are useful in preventing adhesions, and angiotensin II receptor blockers are effective, but clinical studies are needed to establish their use.
Rehabilitation
Immediately after surgery, a postoperative hip brace and antirotational boots are applied to the patient to protect the operative site and reduce pain. The actual postoperative protocol is based on the procedure and individualized to the patient. During microfractures, the patient is kept 20 pounds touch-toe weight-bearing for 4 to 8 weeks. The capsular closure is brace-protected by limiting abduction to 0° to 45° and hip flexion to 0° to 90° while external rotation and extension are prohibited (first 3 weeks). Immediate mobilization with passive rotational movement is crucial in preventing adhesions. Stationary bike exercise and use of a continuous passive motion machine are helpful. Progressive functional and sport-specific rehabilitation help the patient return to full activity, though the decision to return to full activity is based on several factors, both objective (functional tests) and subjective (physician–patient co-decisions).
Conclusion
Although hip arthroscopic techniques have expanded significantly in recent years, our treatment approach is based on restoring the normal anatomy of the hip joint—combining the procedures with biological therapies and a postoperative rehabilitation program that is individualized to the patient’s special needs.
Am J Orthop. 2017;46(1):23-27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
3. Lertwanich P, Ejnisman L, Torry MR, Giphart JE, Philippon MJ. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(suppl):111S-116S.
4. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
5. Ferro FP, Philippon MJ, Rasmussen MT, Smith SD, LaPrade RF, Wijdicks CA. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am J Sports Med. 2015;43(5):1222-1227.
6. Philippon MJ, Briggs KK, Boykin RE. Results of arthroscopic labral reconstruction of the hip in elite athletes: response. Am J Sports Med. 2014;42(10):NP48.
7. Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41(8):1750-1756.
8. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42(5):1127-1133.
9. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
10. Locks R, Chahla J, Mitchell JJ, Soares E, Philippon MJ. Dynamic hip examination for assesment of impingement during hip arthroscopy. Arthroscopy Tech. 2016 Nov 28. http://dx.doi.org/10.1016/j.eats.2016.08.011
11. Nabhan DC, Moreau WJ, McNamara SC, Briggs KK, Philippon MJ. Subspine hip impingement: an unusual cause of hip pain in an elite weightlifter. Curr Sports Med Rep. 2016;15(5):315-319.
12. Philippon MJ, Michalski MP, Campbell KJ, et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am. 2014;96(20):1673-1682.
13. Ho CP, Ommen ND, Bhatia S, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808-1813.
14. Bhatia S, Nowak DD, Briggs KK, Patterson DC, Philippon MJ. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Arthroscopy. 2016;32(5):814-819.
15. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-1831.
16. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209-219.
17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
18. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
19. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
20. Menge TJ, Chahla J, Soares E, Mitchell JJ, Philippon MJ. The Quebec City slider: a technique for capsular closure and plication in hip arthroscopy. Arthrosc Tech. 2016;5(5):e971-e974.
21. Chahla J, Mikula JD, Schon JM, et al. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. doi:10.1177/0363546516666353.
22. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
23. Trindade CA, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71-e74.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
3. Lertwanich P, Ejnisman L, Torry MR, Giphart JE, Philippon MJ. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(suppl):111S-116S.
4. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
5. Ferro FP, Philippon MJ, Rasmussen MT, Smith SD, LaPrade RF, Wijdicks CA. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am J Sports Med. 2015;43(5):1222-1227.
6. Philippon MJ, Briggs KK, Boykin RE. Results of arthroscopic labral reconstruction of the hip in elite athletes: response. Am J Sports Med. 2014;42(10):NP48.
7. Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41(8):1750-1756.
8. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42(5):1127-1133.
9. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
10. Locks R, Chahla J, Mitchell JJ, Soares E, Philippon MJ. Dynamic hip examination for assesment of impingement during hip arthroscopy. Arthroscopy Tech. 2016 Nov 28. http://dx.doi.org/10.1016/j.eats.2016.08.011
11. Nabhan DC, Moreau WJ, McNamara SC, Briggs KK, Philippon MJ. Subspine hip impingement: an unusual cause of hip pain in an elite weightlifter. Curr Sports Med Rep. 2016;15(5):315-319.
12. Philippon MJ, Michalski MP, Campbell KJ, et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am. 2014;96(20):1673-1682.
13. Ho CP, Ommen ND, Bhatia S, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808-1813.
14. Bhatia S, Nowak DD, Briggs KK, Patterson DC, Philippon MJ. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Arthroscopy. 2016;32(5):814-819.
15. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-1831.
16. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209-219.
17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
18. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
19. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
20. Menge TJ, Chahla J, Soares E, Mitchell JJ, Philippon MJ. The Quebec City slider: a technique for capsular closure and plication in hip arthroscopy. Arthrosc Tech. 2016;5(5):e971-e974.
21. Chahla J, Mikula JD, Schon JM, et al. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. doi:10.1177/0363546516666353.
22. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
23. Trindade CA, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71-e74.
Acute pancreatitis
Dear Colleagues,
Acute pancreatitis has long been one of the “bread and butter” conditions in gastroenterology and having up-to-date knowledge on its management will serve our community well. In this issue of The New Gastroenterologist, Abhishek Gulati and Georgios Papachristou (University of Pittsburgh) provide a comprehensive review of the latest advances in the treatment of acute pancreatitis and its complications, which has direct application to GI clinical practice.
Also included in this issue of The New Gastroenterologist is an article highlighting the importance of diversity in gastroenterology training by Sandra Quezada (University of Maryland) and an article on financial tips to ensure a secure retirement by an experienced contract and tax attorney. Additionally, Peter Liang (New York University), Tatyana Kushner (University of California at San Francisco), and Folasade May (University of California at Los Angeles), who are all members of the AGA Institute Trainee and Early Career Committee, provide an overview of the work that they have done to benefit the early career gastroenterology community and the opportunities that exist for getting involved in related AGA activities.
In prior issues of The New Gastroenterologist, we have typically featured a case from the “Clinical Challenges and Images in GI” section of Gastroenterology. However, in this issue we will instead feature a “Practical Teaching Case,” which is one of Gastroenterology’s newest features with a specific focus on the trainee and early-career gastroenterologist. These new cases are great didactic resources and I hope that they become a part of the regular reading of the early career GI community.
If you enjoy the articles in The New Gastroenterologist, have suggestions for future issues, or are interested in contributing to future issues, please let us know! You can contact me (bryson.katona@uphs.upenn.edu) or the Managing Editor of The New Gastroenterologist, Ryan Farrell (rfarrell@gastro.org).
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Bryson W. Katona is a instructor of medicine in the division of gasteroenterology at the University of Pennsylvania.
Dear Colleagues,
Acute pancreatitis has long been one of the “bread and butter” conditions in gastroenterology and having up-to-date knowledge on its management will serve our community well. In this issue of The New Gastroenterologist, Abhishek Gulati and Georgios Papachristou (University of Pittsburgh) provide a comprehensive review of the latest advances in the treatment of acute pancreatitis and its complications, which has direct application to GI clinical practice.
Also included in this issue of The New Gastroenterologist is an article highlighting the importance of diversity in gastroenterology training by Sandra Quezada (University of Maryland) and an article on financial tips to ensure a secure retirement by an experienced contract and tax attorney. Additionally, Peter Liang (New York University), Tatyana Kushner (University of California at San Francisco), and Folasade May (University of California at Los Angeles), who are all members of the AGA Institute Trainee and Early Career Committee, provide an overview of the work that they have done to benefit the early career gastroenterology community and the opportunities that exist for getting involved in related AGA activities.
In prior issues of The New Gastroenterologist, we have typically featured a case from the “Clinical Challenges and Images in GI” section of Gastroenterology. However, in this issue we will instead feature a “Practical Teaching Case,” which is one of Gastroenterology’s newest features with a specific focus on the trainee and early-career gastroenterologist. These new cases are great didactic resources and I hope that they become a part of the regular reading of the early career GI community.
If you enjoy the articles in The New Gastroenterologist, have suggestions for future issues, or are interested in contributing to future issues, please let us know! You can contact me (bryson.katona@uphs.upenn.edu) or the Managing Editor of The New Gastroenterologist, Ryan Farrell (rfarrell@gastro.org).
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Bryson W. Katona is a instructor of medicine in the division of gasteroenterology at the University of Pennsylvania.
Dear Colleagues,
Acute pancreatitis has long been one of the “bread and butter” conditions in gastroenterology and having up-to-date knowledge on its management will serve our community well. In this issue of The New Gastroenterologist, Abhishek Gulati and Georgios Papachristou (University of Pittsburgh) provide a comprehensive review of the latest advances in the treatment of acute pancreatitis and its complications, which has direct application to GI clinical practice.
Also included in this issue of The New Gastroenterologist is an article highlighting the importance of diversity in gastroenterology training by Sandra Quezada (University of Maryland) and an article on financial tips to ensure a secure retirement by an experienced contract and tax attorney. Additionally, Peter Liang (New York University), Tatyana Kushner (University of California at San Francisco), and Folasade May (University of California at Los Angeles), who are all members of the AGA Institute Trainee and Early Career Committee, provide an overview of the work that they have done to benefit the early career gastroenterology community and the opportunities that exist for getting involved in related AGA activities.
In prior issues of The New Gastroenterologist, we have typically featured a case from the “Clinical Challenges and Images in GI” section of Gastroenterology. However, in this issue we will instead feature a “Practical Teaching Case,” which is one of Gastroenterology’s newest features with a specific focus on the trainee and early-career gastroenterologist. These new cases are great didactic resources and I hope that they become a part of the regular reading of the early career GI community.
If you enjoy the articles in The New Gastroenterologist, have suggestions for future issues, or are interested in contributing to future issues, please let us know! You can contact me (bryson.katona@uphs.upenn.edu) or the Managing Editor of The New Gastroenterologist, Ryan Farrell (rfarrell@gastro.org).
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Bryson W. Katona is a instructor of medicine in the division of gasteroenterology at the University of Pennsylvania.
Pembrolizumab is the first immune checkpoint inhibitor to receive approval for head and neck cancer
The first immune checkpoint inhibitor was approved for the treatment of head and neck cancer approved in August 2016. Pembrolizumab, which targets the programmed cell death 1 (PD-1) protein, is designed to reinstate the anti-tumor immune response to kill cancer cells and was approved for the treatment of recurrent or metastatic disease that progressed during or after platinum-containing chemotherapy.
Click on the PDF icon at the top of this introduction to read the full article.
The first immune checkpoint inhibitor was approved for the treatment of head and neck cancer approved in August 2016. Pembrolizumab, which targets the programmed cell death 1 (PD-1) protein, is designed to reinstate the anti-tumor immune response to kill cancer cells and was approved for the treatment of recurrent or metastatic disease that progressed during or after platinum-containing chemotherapy.
Click on the PDF icon at the top of this introduction to read the full article.
The first immune checkpoint inhibitor was approved for the treatment of head and neck cancer approved in August 2016. Pembrolizumab, which targets the programmed cell death 1 (PD-1) protein, is designed to reinstate the anti-tumor immune response to kill cancer cells and was approved for the treatment of recurrent or metastatic disease that progressed during or after platinum-containing chemotherapy.
Click on the PDF icon at the top of this introduction to read the full article.
Biomechanics of Polyhydroxyalkanoate Mesh–Augmented Single-Row Rotator Cuff Repairs
Healing after rotator cuff repair (RCR) can be challenging, especially in cases of large and massive tears, revision repairs, and tendons with poor tissue quality.1-3 Poor tissue quality is associated with increased risk for recurrent tears, independent of age and tear size.3 Various techniques have been used to improve tendon fixation strength in these difficult situations, including augmented suture configurations (eg, massive cuff stitches, rip-stop stitches) and tissue grafts (eg, acellular dermal matrix).4-9 Clinical studies have found improved healing rates for larger tears and revision repairs using acellular dermal matrix grafts.6,10 Synthetic patches are another option for RCR augmentation, but limited clinical data and biomechanical evidence support use of synthetic grafts as an augment for RCRs.11-13
Polyhydroxyalkanoates (PHAs) are a class of biodegradable polymers that have been used as orthopedic devices, tissue scaffolds, patches, and other applications with increasing frequency over the past decade.14 In the laboratory, these implanted materials have been shown to support cell migration and growth.15 The PHA family of polymers typically degrades by hydrolytic and bacterial depolymerase mechanisms over 52-plus weeks in vivo.14PHA grafts have been studied in the setting of RCR. An expanded polytetrafluoroethylene scaffold was shown to improve repair mechanics when used as a bursal side graft in an in vitro ovine model.11 The graft increased tendon footprint contact pressure and failure loads by almost 180 N. In clinical studies, poly-L-lactic acid augmentations have been used to reinforce massive RCRs. Lenart and colleagues16 found that 38% of 16 patients with such tears had an intact rotator cuff at 1.2-year follow-up, and improvement in clinical scores. Proctor13 reported on use of a poly-L-lactic acid retrograde patch for reinforcement of massive tears with both single- and double-row repairs in 18 patients. The cohort had more favorable rates of intact cuffs at 12 months (83%) and 42 months (78%), and ASES (American Shoulder and Elbow Surgeons) scores improved from 25 before surgery to 82 at latest follow-up after surgery.
RCR augmentation traditionally has been performed with an open or mini-open technique.6 Recently, several authors have reported on arthroscopic techniques for augmentation with either acellular dermal matrix or synthetic grafts.13,17,18 Most techniques have involved “bridging” with a graft or patch used to stress-shield a single-row repair.8,9,13 This bridging typically involves placing several sutures medial to where the anchor repair stitches pass through the tendon. An alternative is to pass the repair stitches through both the tendon and the graft.17-19 The overall volume of tissue incorporated into the repair stitches (rotator cuff plus graft) is increased with the augmented technique relative to the bridging technique. Both can be technically challenging, but the augmented technique may be easier to perform arthroscopically.9,19 Regardless, these techniques are complicated and require a higher level of arthroscopic skills compared with those required in arthroscopic RCR without a graft. Simplifying arthroscopic graft augmentation likely will increase its utility because, even for skilled surgeons, adding a graft can increase operative time by 20 to 30 minutes. Simplification will also extend use of the technique to surgeons with less experience and proficiency with arthroscopic repair.
We developed a simple method for augmenting single-row RCR with a strip of bioresorbable soft-tissue scaffold. We also conducted a study to evaluate the initial biomechanical properties of single-row RCR in cadaveric shoulder specimens augmented with PHA mesh (BioFiber; Tornier) graft as compared with single-row RCR without augmentation. Both cyclic gap formation and ultimate failure loads and displacement were quantified. We hypothesized that the augmented RCRs would have decreased gap formation and increased ultimate failure loads compared with nonaugmented RCRs. This study was exempt from having to obtain Institutional Review B
Methods
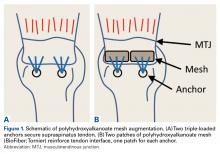
Eight pairs of fresh-frozen cadaver humeri (6 male, 2 female; mean [SD] age, 61 [9] years) were dissected of all soft tissue (except rotator cuff) by Dr. Tashjian, a board-certified, fellowship-trained orthopedic surgeon. There were no qualitative differences in tendon condition between tendons within a pair. The supraspinatus muscle and tendon were separated from the other rotator cuff muscles. The infraspinatus, subscapularis, and teres minor were removed from the humerus. Last, the supraspinatus was resected at its insertion. Humeral pairs were then randomized into augmented and nonaugmented RCRs within each pair.
In the nonaugmented group, the supraspinatus was reattached to its insertion in a single-row RCR with 2 triple-loaded suture anchors (5.5-mm Insite FT Ti, No. 2 Force Fiber suture; Tornier) and 6 simple stitches (Figure 1A). Anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity at about 45° to the bone surface.
In the contralateral shoulders, augmented RCRs were performed. Specimens were prepared exactly as they were for the nonaugmented RCRs, including anchor placement and suture passage. Before knot tying, RCRs were augmented with 2 strips of 13-mm × 23-mm PHA mesh (BioFiber) (Figure 1B). One strip was used to augment the 3 sutures of each anchor, overlying the residual tendon, to reinforce the tendon–knot interface. After each suture was passed through the supraspinatus tendon from the intra-articular surface, the stitch was passed through the strip of PHA mesh. Stitches were separated by 5 mm in each mesh strip. All 6 sutures were then tied with a Revo knot between the free end of each suture leg and the leg that passed through the tendon and mesh.
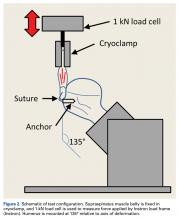
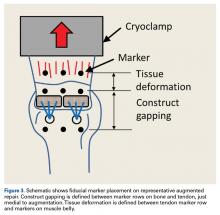
Each humerus was transected at the midshaft and potted and mounted in an Instron 1331 load frame with Model 8800 controller (Instron). A cryoclamp was used to grasp the supraspinatus muscle belly above the musculotendinous junction (Figure 2).
Three rows of 2-mm fiducial markers were affixed to the bone, tendon, and muscle belly with cyanoacrylate for tracking with a digital video system (DMAS Version 6.5; Spicatek) (Figure 3).21
A 0.1-MPa pre-stress (applied force/tendon cross-sectional area) was applied to each construct to determine the starting position for the deformation profile. Each repair underwent 1000 cycles of uniaxial load-controlled displacement between 0.1 and 1.0 MPa of effective stress at 1 Hz. Effective stress was determined as the ratio of applied force to cross-sectional area of the tendon at harvest to normalize the applied loads between tendons of varying size. During cyclic testing, gapping of more than 5 mm was defined as construct failure.22 After cyclic loading, each construct was loaded to failure at 1.0 mm/s. Ultimate failure load was defined as the highest load achieved at the maximum displacement before rapid decline in load supported by the construct.
Statistical Analysis
Paired t tests were used to compare the matched pairs of constructs. For all tests, significance was set at P ≤ .05. Post hoc power was calculated for significant results using G*Power Version 3.1.6.23 All data are presented as means (SDs).
Results
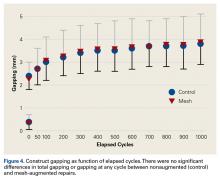
After 1000 cycles of displacement, mean (SD) gapping was 3.8 (0.9) mm for the nonaugmented repairs and 3.9 (1.1) mm for the PHA mesh–augmented repairs (P = .879) (Figure 4).
For the nonaugmented repairs, mean (SD) failure displacement was 6.3 (1.7) mm, and mean (SD) ultimate failure load was 472.1 (120.3) N. For the PHA-augmented repairs, failure displacement was 5.5 (1.9) mm, and ultimate failure load was 571.2 (173.0) N. There was no difference in failure displacement (P = .393), but there was a difference in ultimate failure load (P = .042; power = 0.57). During failure testing, mean (SD) tissue deformation was higher (P = .012; power = 0.83) for the PHA-augmented repairs, 1.2 (0.7) mm, than for the nonaugmented repairs, 0.8 (0.5) mm. Failures, which were consistent within pairs, were caused by tissue failure, with sutures pulling through the tissue (4 pairs) or single anchor pullout before ultimate tissue failure (4 pairs). Of the 4 failures with anchor pullout, 3 had anterior anchor pullout, and 1 had posterior anchor pullout. In all specimens with anchor pullout, the second anchor remained stable, and ultimate failure occurred with tissue tearing at the suture interface. There were no significant differences in any metrics between specimens that failed with intact anchors and specimens with single anchor pullout (P ≥ .122). Therefore, both groups were pooled for the failure analysis.
Discussion
RCR augmentation with a synthetic graft is a viable option for improving fixation strength of supraspinatus repairs, as shown in otherwise healthy tendon in the present study. Our hypothesis that there would be decreased gap formation with graft augmentation was not supported, whereas the hypothesis of increased failure loads with graft augmentation was supported. These findings may also be applicable in cases of large tears, revisions, and tendons with poor tissue quality. Simplification of graft application techniques will allow quick and easy arthroscopic augmentation.
Studies of RCRs for large or massive tears have reported retear rates of 25% to 79%.24-26 Latissimus dorsi tendon transfers also show promise in posterosuperior RCRs, with failure rates near 10%.27,28 Although use of PHA patches in RCR augmentation is relatively new, short-term and midterm failure rates are in the range of 20% to 60% in the few small cohorts currently being studied.13,16 It is possible that these rates may improve as indications, surgical experience, and techniques for use of PHA patches are further refined. Regardless, with PHA currently being used in practice, it is important to quantify the biomechanics of the augmentation as a baseline for its performance in reinforcing the tendon–suture interface.
We determined that the initial fixation strength of single-row repairs was higher with the addition of PHA synthetic grafts using a very simple technique. Single-row triple-loaded anchor repairs already provide high initial mechanical strength, and our results are similar to those of another study of this technique.29 Despite the already high mechanical strength of a triple-loaded anchor repair, PHA mesh increased ultimate strength by about 100 N (~25%). Of note, tissue elongation during failure was higher (P = .012; power = 0.83) in the PHA-augmented group (1.2 mm) than in the nonaugmented group (0.8 mm). This was not surprising—failure loads were almost 100 N higher in the PHA-augmented group than in the nonaugmented group. Consequently, much higher forces were placed on the muscle belly, likely resulting in additional elongation of the intact tissue medial to the repair construct.
The ultimate failure loads in our study compare favorably with the biomechanical strength of augmented repairs reported by others.8,9,18 Barber and colleagues18 evaluated an augmented single-row repair with 2 double-loaded suture anchors and an acellular dermal matrix graft. The ultimate failure load of the augmented repairs was 325 N. In contrast, Omae and colleagues8 tested a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft. Ultimate failure load of the augmented repairs was 560 N, similar to our finding. Last, Shea and colleagues9 evaluated a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft, with ultimate failure load of 429 N. The techniques in all 3 studies can be performed arthroscopically but are challenging and require multiple extra sutures and anchors that need management and tying. Our technique provides similar initial fixation strength, has no requirement for extra sutures or anchors, and is very simple to perform.
The supraspinatus tendon is estimated to fail between 800 N and 1000 N.30,31 Biomechanical shoulder simulators use supraspinatus forces in the range of 20 N to 200 N for scapular plane abduction.32-36 Therefore, the single-row repair failures in our study fell between functional and full-thickness failure loads. Studies on the mechanics of degenerated human supraspinatus tendon are limited, but there is evidence the mechanical properties of these tissues are inferior to those of healthy tendon.37 A 100-N increase in failure loads with PHA augmentation may prove highly significant in reinforcing the suture–tendon interface in degenerated tendons.
Adding the mesh did not have any effect on gapping at the repair site after cyclic loading. This finding suggests that construct gapping under cyclic loading is not a function of a reinforced knot–tendon interface but is instead caused by microtearing and cinching of the suture constructs in relation to the underlying bone. Tissue elongation likely was not a strong contributor to overall cyclic gapping, as elongation did not differ between the nonaugmented and augmented repairs (0.5 mm vs 0.7 mm; P = .276) and was small relative to the nearly 4 mm of construct gapping. Gapping may be affected by healing and integration of the mesh into the repaired tendon over time, but this effect could not be captured in the present study. Patients are initially immobilized and passive shoulder motion gradually introduced, in stark contrast to the immediate loading protocol in the present study. Regardless, the 25% increase in overall strength may be clinically important, especially in cases of difficult repair or poor tissue quality.
Our technique simplifies arthroscopic augmentation—stitches are passed through the rotator cuff in simple fashion. Before being tied, the limbs that were passed through the rotator cuff are removed through a cannula and then passed through the synthetic graft.
Study Limitations
This study had several limitations. First, it was a cadaveric biomechanical study that evaluated only time-zero biomechanical properties. Loads were normalized to tendon size, specimens were randomized between sides, and paired specimens were used to minimize the effects of tendon and bone quality on outcome metrics. In addition, donor tendons were representative of otherwise healthy tissue. Chronic tears and associated resorption/atrophy could have affected the magnitude of forces and gapping detected in this study. Theoretically, over time the tendon tissue will adhere to and grow into the mesh, which could minimize potential differences. Studies are needed to determine the effects of healing on long-term repair strength in affected patients. Last, all constructs were performed in open fashion to improve repeatability of construct placement and provide accessibility for Instron testing. Our technique did not directly replicate the arthroscopic approach, but, unlike other augmentation techniques, it is so simple that transition to all-arthroscopic augmentation is realistic.
Patch augmentation increases the cost of materials and operative time and should be considered a limitation of its utility. We do not recommend augmentation in all RCRs, as it likely is cost-ineffective. Instead, we recommend augmentation in cases of poor tissue quality, which could lead to healing failure, revision surgery, and higher overall patient costs beyond the cost of adding augmentation. Similarly, we recommend augmentation for revision cases in which tendon healing has failed and tissue quality is poor. The goal is to prevent another failure.
Conclusion
PHA graft augmentation of single-row triple-loaded anchor repairs of the supraspinatus tendon improves the overall ultimate load to failure by 25%. There was no difference in gap formation after cyclic loading between augmented and nonaugmented repairs. This technique for arthroscopic augmentation can be used to improve initial biomechanical repair strength in tears at risk for failure.
Am J Orthop. 2016;45(7):E527-E533. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
2. Keener JD, Wei AS, Kim HM, et al. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am. 2010;92(3):590-598.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Barber FA, Herbert MA, Schroeder FA, Aziz-Jacobo J, Mays MM, Rapley JH. Biomechanical advantages of triple-loaded suture anchors compared with double-row rotator cuff repairs. Arthroscopy. 2010;26(3):316-323.
5. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
6. Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41(4):872-879.
7. Ma CB, MacGillivray JD, Clabeaux J, Lee S, Otis JC. Biomechanical evaluation of arthroscopic rotator cuff stitches. J Bone Joint Surg Am. 2004;86(6):1211-1216.
8. Omae H, Steinmann SP, Zhao C, et al. Biomechanical effect of rotator cuff augmentation with an acellular dermal matrix graft: a cadaver study. Clin Biomech. 2012;27(8):789-792.
9. Shea KP, Obopilwe E, Sperling JW, Iannotti JP. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg. 2012;21(8):1072-1079.
10. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
11. Beimers L, Lam PH, Murrell GA. The biomechanical effects of polytetrafluoroethylene suture augmentations in lateral-row rotator cuff repairs in an ovine model. J Shoulder Elbow Surg. 2014;23(10):1545-1552.
12. McCarron JA, Milks RA, Chen X, Iannotti JP, Derwin KA. Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J Shoulder Elbow Surg. 2010;19(5):688-696.
13. Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg. 2014;23(10):1508-1513.
14. Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules. 2006;7(8):2249-2258.
15. Ellis G, Cano P, Jadraque M, et al. Laser microperforated biodegradable microbial polyhydroxyalkanoate substrates for tissue repair strategies: an infrared microspectroscopy study. Anal Bioanal Chem. 2011;399(7):2379-2388.
16. Lenart BA, Martens KA, Kearns KA, Gillespie RJ, Zoga AC, Williams GR. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg. 2015;24(6):915-921.
17. Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
18. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
19. Gilot GJ, Attia AK, Alvarez AM. Arthroscopic repair of rotator cuff tears using extracellular matrix graft. Arthrosc Tech. 2014;3(4):e487-e489.
20. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
21. Kullar RS, Reagan JM, Kolz CW, Burks RT, Henninger HB. Suture placement near the musculotendinous junction in the supraspinatus: implications for rotator cuff repair. Am J Sports Med. 2015;43(1):57-62.
22. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athanasiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
23. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
24. Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24(9):1493-1505.
25. Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear rates after arthroscopic single-row, double-row, and suture bridge rotator cuff repair at a minimum of 1 year of imaging follow-up: a systematic review. Arthroscopy. 2015;31(11):2274-2281.
26. Henry P, Wasserstein D, Park S, et al. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31(12):2472-2480.
27. El-Azab HM, Rott O, Irlenbusch U. Long-term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2015;97(6):462-469.
28. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
29. Coons DA, Barber FA, Herbert MA. Triple-loaded single-anchor stitch configurations: an analysis of cyclically loaded suture–tendon interface security. Arthroscopy. 2006;22(11):1154-1158.
30. Itoi E, Berglund LJ, Grabowski JJ, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13(4):578-584.
31. Matsuhashi T, Hooke AW, Zhao KD, et al. Tensile properties of a morphologically split supraspinatus tendon. Clin Anat. 2014;27(5):702-706.
32. Apreleva M, Parsons IM 4th, Warner JJ, Fu FH, Woo SL. Experimental investigation of reaction forces at the glenohumeral joint during active abduction. J Shoulder Elbow Surg. 2000;9(5):409-417.
33. Giles JW, Ferreira LM, Athwal GS, Johnson JA. Development and performance evaluation of a multi-PID muscle loading driven in vitro active-motion shoulder simulator and application to assessing reverse total shoulder arthroplasty. J Biomech Eng. 2014;136(12):121007.
34. Hansen ML, Otis JC, Johnson JS, Cordasco FA, Craig EV, Warren RF. Biomechanics of massive rotator cuff tears: implications for treatment. J Bone Joint Surg Am. 2008;90(2):316-325.
35. Henninger HB, Barg A, Anderson AE, Bachus KN, Tashjian RZ, Burks RT. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(4):483-490.
36. Mihata T, Gates J, McGarry MH, Lee J, Kinoshita M, Lee TQ. Effect of rotator cuff muscle imbalance on forceful internal impingement and peel-back of the superior labrum: a cadaveric study. Am J Sports Med. 2009;37(11):2222-2227.
37. Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, Uhthoff HK. Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone–tendon complex. J Orthop Res. 1997;15(5):719-726.
Healing after rotator cuff repair (RCR) can be challenging, especially in cases of large and massive tears, revision repairs, and tendons with poor tissue quality.1-3 Poor tissue quality is associated with increased risk for recurrent tears, independent of age and tear size.3 Various techniques have been used to improve tendon fixation strength in these difficult situations, including augmented suture configurations (eg, massive cuff stitches, rip-stop stitches) and tissue grafts (eg, acellular dermal matrix).4-9 Clinical studies have found improved healing rates for larger tears and revision repairs using acellular dermal matrix grafts.6,10 Synthetic patches are another option for RCR augmentation, but limited clinical data and biomechanical evidence support use of synthetic grafts as an augment for RCRs.11-13
Polyhydroxyalkanoates (PHAs) are a class of biodegradable polymers that have been used as orthopedic devices, tissue scaffolds, patches, and other applications with increasing frequency over the past decade.14 In the laboratory, these implanted materials have been shown to support cell migration and growth.15 The PHA family of polymers typically degrades by hydrolytic and bacterial depolymerase mechanisms over 52-plus weeks in vivo.14PHA grafts have been studied in the setting of RCR. An expanded polytetrafluoroethylene scaffold was shown to improve repair mechanics when used as a bursal side graft in an in vitro ovine model.11 The graft increased tendon footprint contact pressure and failure loads by almost 180 N. In clinical studies, poly-L-lactic acid augmentations have been used to reinforce massive RCRs. Lenart and colleagues16 found that 38% of 16 patients with such tears had an intact rotator cuff at 1.2-year follow-up, and improvement in clinical scores. Proctor13 reported on use of a poly-L-lactic acid retrograde patch for reinforcement of massive tears with both single- and double-row repairs in 18 patients. The cohort had more favorable rates of intact cuffs at 12 months (83%) and 42 months (78%), and ASES (American Shoulder and Elbow Surgeons) scores improved from 25 before surgery to 82 at latest follow-up after surgery.
RCR augmentation traditionally has been performed with an open or mini-open technique.6 Recently, several authors have reported on arthroscopic techniques for augmentation with either acellular dermal matrix or synthetic grafts.13,17,18 Most techniques have involved “bridging” with a graft or patch used to stress-shield a single-row repair.8,9,13 This bridging typically involves placing several sutures medial to where the anchor repair stitches pass through the tendon. An alternative is to pass the repair stitches through both the tendon and the graft.17-19 The overall volume of tissue incorporated into the repair stitches (rotator cuff plus graft) is increased with the augmented technique relative to the bridging technique. Both can be technically challenging, but the augmented technique may be easier to perform arthroscopically.9,19 Regardless, these techniques are complicated and require a higher level of arthroscopic skills compared with those required in arthroscopic RCR without a graft. Simplifying arthroscopic graft augmentation likely will increase its utility because, even for skilled surgeons, adding a graft can increase operative time by 20 to 30 minutes. Simplification will also extend use of the technique to surgeons with less experience and proficiency with arthroscopic repair.
We developed a simple method for augmenting single-row RCR with a strip of bioresorbable soft-tissue scaffold. We also conducted a study to evaluate the initial biomechanical properties of single-row RCR in cadaveric shoulder specimens augmented with PHA mesh (BioFiber; Tornier) graft as compared with single-row RCR without augmentation. Both cyclic gap formation and ultimate failure loads and displacement were quantified. We hypothesized that the augmented RCRs would have decreased gap formation and increased ultimate failure loads compared with nonaugmented RCRs. This study was exempt from having to obtain Institutional Review B
Methods
Eight pairs of fresh-frozen cadaver humeri (6 male, 2 female; mean [SD] age, 61 [9] years) were dissected of all soft tissue (except rotator cuff) by Dr. Tashjian, a board-certified, fellowship-trained orthopedic surgeon. There were no qualitative differences in tendon condition between tendons within a pair. The supraspinatus muscle and tendon were separated from the other rotator cuff muscles. The infraspinatus, subscapularis, and teres minor were removed from the humerus. Last, the supraspinatus was resected at its insertion. Humeral pairs were then randomized into augmented and nonaugmented RCRs within each pair.
In the nonaugmented group, the supraspinatus was reattached to its insertion in a single-row RCR with 2 triple-loaded suture anchors (5.5-mm Insite FT Ti, No. 2 Force Fiber suture; Tornier) and 6 simple stitches (Figure 1A). Anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity at about 45° to the bone surface.
In the contralateral shoulders, augmented RCRs were performed. Specimens were prepared exactly as they were for the nonaugmented RCRs, including anchor placement and suture passage. Before knot tying, RCRs were augmented with 2 strips of 13-mm × 23-mm PHA mesh (BioFiber) (Figure 1B). One strip was used to augment the 3 sutures of each anchor, overlying the residual tendon, to reinforce the tendon–knot interface. After each suture was passed through the supraspinatus tendon from the intra-articular surface, the stitch was passed through the strip of PHA mesh. Stitches were separated by 5 mm in each mesh strip. All 6 sutures were then tied with a Revo knot between the free end of each suture leg and the leg that passed through the tendon and mesh.
Each humerus was transected at the midshaft and potted and mounted in an Instron 1331 load frame with Model 8800 controller (Instron). A cryoclamp was used to grasp the supraspinatus muscle belly above the musculotendinous junction (Figure 2).
Three rows of 2-mm fiducial markers were affixed to the bone, tendon, and muscle belly with cyanoacrylate for tracking with a digital video system (DMAS Version 6.5; Spicatek) (Figure 3).21
A 0.1-MPa pre-stress (applied force/tendon cross-sectional area) was applied to each construct to determine the starting position for the deformation profile. Each repair underwent 1000 cycles of uniaxial load-controlled displacement between 0.1 and 1.0 MPa of effective stress at 1 Hz. Effective stress was determined as the ratio of applied force to cross-sectional area of the tendon at harvest to normalize the applied loads between tendons of varying size. During cyclic testing, gapping of more than 5 mm was defined as construct failure.22 After cyclic loading, each construct was loaded to failure at 1.0 mm/s. Ultimate failure load was defined as the highest load achieved at the maximum displacement before rapid decline in load supported by the construct.
Statistical Analysis
Paired t tests were used to compare the matched pairs of constructs. For all tests, significance was set at P ≤ .05. Post hoc power was calculated for significant results using G*Power Version 3.1.6.23 All data are presented as means (SDs).
Results
After 1000 cycles of displacement, mean (SD) gapping was 3.8 (0.9) mm for the nonaugmented repairs and 3.9 (1.1) mm for the PHA mesh–augmented repairs (P = .879) (Figure 4).
For the nonaugmented repairs, mean (SD) failure displacement was 6.3 (1.7) mm, and mean (SD) ultimate failure load was 472.1 (120.3) N. For the PHA-augmented repairs, failure displacement was 5.5 (1.9) mm, and ultimate failure load was 571.2 (173.0) N. There was no difference in failure displacement (P = .393), but there was a difference in ultimate failure load (P = .042; power = 0.57). During failure testing, mean (SD) tissue deformation was higher (P = .012; power = 0.83) for the PHA-augmented repairs, 1.2 (0.7) mm, than for the nonaugmented repairs, 0.8 (0.5) mm. Failures, which were consistent within pairs, were caused by tissue failure, with sutures pulling through the tissue (4 pairs) or single anchor pullout before ultimate tissue failure (4 pairs). Of the 4 failures with anchor pullout, 3 had anterior anchor pullout, and 1 had posterior anchor pullout. In all specimens with anchor pullout, the second anchor remained stable, and ultimate failure occurred with tissue tearing at the suture interface. There were no significant differences in any metrics between specimens that failed with intact anchors and specimens with single anchor pullout (P ≥ .122). Therefore, both groups were pooled for the failure analysis.
Discussion
RCR augmentation with a synthetic graft is a viable option for improving fixation strength of supraspinatus repairs, as shown in otherwise healthy tendon in the present study. Our hypothesis that there would be decreased gap formation with graft augmentation was not supported, whereas the hypothesis of increased failure loads with graft augmentation was supported. These findings may also be applicable in cases of large tears, revisions, and tendons with poor tissue quality. Simplification of graft application techniques will allow quick and easy arthroscopic augmentation.
Studies of RCRs for large or massive tears have reported retear rates of 25% to 79%.24-26 Latissimus dorsi tendon transfers also show promise in posterosuperior RCRs, with failure rates near 10%.27,28 Although use of PHA patches in RCR augmentation is relatively new, short-term and midterm failure rates are in the range of 20% to 60% in the few small cohorts currently being studied.13,16 It is possible that these rates may improve as indications, surgical experience, and techniques for use of PHA patches are further refined. Regardless, with PHA currently being used in practice, it is important to quantify the biomechanics of the augmentation as a baseline for its performance in reinforcing the tendon–suture interface.
We determined that the initial fixation strength of single-row repairs was higher with the addition of PHA synthetic grafts using a very simple technique. Single-row triple-loaded anchor repairs already provide high initial mechanical strength, and our results are similar to those of another study of this technique.29 Despite the already high mechanical strength of a triple-loaded anchor repair, PHA mesh increased ultimate strength by about 100 N (~25%). Of note, tissue elongation during failure was higher (P = .012; power = 0.83) in the PHA-augmented group (1.2 mm) than in the nonaugmented group (0.8 mm). This was not surprising—failure loads were almost 100 N higher in the PHA-augmented group than in the nonaugmented group. Consequently, much higher forces were placed on the muscle belly, likely resulting in additional elongation of the intact tissue medial to the repair construct.
The ultimate failure loads in our study compare favorably with the biomechanical strength of augmented repairs reported by others.8,9,18 Barber and colleagues18 evaluated an augmented single-row repair with 2 double-loaded suture anchors and an acellular dermal matrix graft. The ultimate failure load of the augmented repairs was 325 N. In contrast, Omae and colleagues8 tested a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft. Ultimate failure load of the augmented repairs was 560 N, similar to our finding. Last, Shea and colleagues9 evaluated a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft, with ultimate failure load of 429 N. The techniques in all 3 studies can be performed arthroscopically but are challenging and require multiple extra sutures and anchors that need management and tying. Our technique provides similar initial fixation strength, has no requirement for extra sutures or anchors, and is very simple to perform.
The supraspinatus tendon is estimated to fail between 800 N and 1000 N.30,31 Biomechanical shoulder simulators use supraspinatus forces in the range of 20 N to 200 N for scapular plane abduction.32-36 Therefore, the single-row repair failures in our study fell between functional and full-thickness failure loads. Studies on the mechanics of degenerated human supraspinatus tendon are limited, but there is evidence the mechanical properties of these tissues are inferior to those of healthy tendon.37 A 100-N increase in failure loads with PHA augmentation may prove highly significant in reinforcing the suture–tendon interface in degenerated tendons.
Adding the mesh did not have any effect on gapping at the repair site after cyclic loading. This finding suggests that construct gapping under cyclic loading is not a function of a reinforced knot–tendon interface but is instead caused by microtearing and cinching of the suture constructs in relation to the underlying bone. Tissue elongation likely was not a strong contributor to overall cyclic gapping, as elongation did not differ between the nonaugmented and augmented repairs (0.5 mm vs 0.7 mm; P = .276) and was small relative to the nearly 4 mm of construct gapping. Gapping may be affected by healing and integration of the mesh into the repaired tendon over time, but this effect could not be captured in the present study. Patients are initially immobilized and passive shoulder motion gradually introduced, in stark contrast to the immediate loading protocol in the present study. Regardless, the 25% increase in overall strength may be clinically important, especially in cases of difficult repair or poor tissue quality.
Our technique simplifies arthroscopic augmentation—stitches are passed through the rotator cuff in simple fashion. Before being tied, the limbs that were passed through the rotator cuff are removed through a cannula and then passed through the synthetic graft.
Study Limitations
This study had several limitations. First, it was a cadaveric biomechanical study that evaluated only time-zero biomechanical properties. Loads were normalized to tendon size, specimens were randomized between sides, and paired specimens were used to minimize the effects of tendon and bone quality on outcome metrics. In addition, donor tendons were representative of otherwise healthy tissue. Chronic tears and associated resorption/atrophy could have affected the magnitude of forces and gapping detected in this study. Theoretically, over time the tendon tissue will adhere to and grow into the mesh, which could minimize potential differences. Studies are needed to determine the effects of healing on long-term repair strength in affected patients. Last, all constructs were performed in open fashion to improve repeatability of construct placement and provide accessibility for Instron testing. Our technique did not directly replicate the arthroscopic approach, but, unlike other augmentation techniques, it is so simple that transition to all-arthroscopic augmentation is realistic.
Patch augmentation increases the cost of materials and operative time and should be considered a limitation of its utility. We do not recommend augmentation in all RCRs, as it likely is cost-ineffective. Instead, we recommend augmentation in cases of poor tissue quality, which could lead to healing failure, revision surgery, and higher overall patient costs beyond the cost of adding augmentation. Similarly, we recommend augmentation for revision cases in which tendon healing has failed and tissue quality is poor. The goal is to prevent another failure.
Conclusion
PHA graft augmentation of single-row triple-loaded anchor repairs of the supraspinatus tendon improves the overall ultimate load to failure by 25%. There was no difference in gap formation after cyclic loading between augmented and nonaugmented repairs. This technique for arthroscopic augmentation can be used to improve initial biomechanical repair strength in tears at risk for failure.
Am J Orthop. 2016;45(7):E527-E533. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Healing after rotator cuff repair (RCR) can be challenging, especially in cases of large and massive tears, revision repairs, and tendons with poor tissue quality.1-3 Poor tissue quality is associated with increased risk for recurrent tears, independent of age and tear size.3 Various techniques have been used to improve tendon fixation strength in these difficult situations, including augmented suture configurations (eg, massive cuff stitches, rip-stop stitches) and tissue grafts (eg, acellular dermal matrix).4-9 Clinical studies have found improved healing rates for larger tears and revision repairs using acellular dermal matrix grafts.6,10 Synthetic patches are another option for RCR augmentation, but limited clinical data and biomechanical evidence support use of synthetic grafts as an augment for RCRs.11-13
Polyhydroxyalkanoates (PHAs) are a class of biodegradable polymers that have been used as orthopedic devices, tissue scaffolds, patches, and other applications with increasing frequency over the past decade.14 In the laboratory, these implanted materials have been shown to support cell migration and growth.15 The PHA family of polymers typically degrades by hydrolytic and bacterial depolymerase mechanisms over 52-plus weeks in vivo.14PHA grafts have been studied in the setting of RCR. An expanded polytetrafluoroethylene scaffold was shown to improve repair mechanics when used as a bursal side graft in an in vitro ovine model.11 The graft increased tendon footprint contact pressure and failure loads by almost 180 N. In clinical studies, poly-L-lactic acid augmentations have been used to reinforce massive RCRs. Lenart and colleagues16 found that 38% of 16 patients with such tears had an intact rotator cuff at 1.2-year follow-up, and improvement in clinical scores. Proctor13 reported on use of a poly-L-lactic acid retrograde patch for reinforcement of massive tears with both single- and double-row repairs in 18 patients. The cohort had more favorable rates of intact cuffs at 12 months (83%) and 42 months (78%), and ASES (American Shoulder and Elbow Surgeons) scores improved from 25 before surgery to 82 at latest follow-up after surgery.
RCR augmentation traditionally has been performed with an open or mini-open technique.6 Recently, several authors have reported on arthroscopic techniques for augmentation with either acellular dermal matrix or synthetic grafts.13,17,18 Most techniques have involved “bridging” with a graft or patch used to stress-shield a single-row repair.8,9,13 This bridging typically involves placing several sutures medial to where the anchor repair stitches pass through the tendon. An alternative is to pass the repair stitches through both the tendon and the graft.17-19 The overall volume of tissue incorporated into the repair stitches (rotator cuff plus graft) is increased with the augmented technique relative to the bridging technique. Both can be technically challenging, but the augmented technique may be easier to perform arthroscopically.9,19 Regardless, these techniques are complicated and require a higher level of arthroscopic skills compared with those required in arthroscopic RCR without a graft. Simplifying arthroscopic graft augmentation likely will increase its utility because, even for skilled surgeons, adding a graft can increase operative time by 20 to 30 minutes. Simplification will also extend use of the technique to surgeons with less experience and proficiency with arthroscopic repair.
We developed a simple method for augmenting single-row RCR with a strip of bioresorbable soft-tissue scaffold. We also conducted a study to evaluate the initial biomechanical properties of single-row RCR in cadaveric shoulder specimens augmented with PHA mesh (BioFiber; Tornier) graft as compared with single-row RCR without augmentation. Both cyclic gap formation and ultimate failure loads and displacement were quantified. We hypothesized that the augmented RCRs would have decreased gap formation and increased ultimate failure loads compared with nonaugmented RCRs. This study was exempt from having to obtain Institutional Review B
Methods
Eight pairs of fresh-frozen cadaver humeri (6 male, 2 female; mean [SD] age, 61 [9] years) were dissected of all soft tissue (except rotator cuff) by Dr. Tashjian, a board-certified, fellowship-trained orthopedic surgeon. There were no qualitative differences in tendon condition between tendons within a pair. The supraspinatus muscle and tendon were separated from the other rotator cuff muscles. The infraspinatus, subscapularis, and teres minor were removed from the humerus. Last, the supraspinatus was resected at its insertion. Humeral pairs were then randomized into augmented and nonaugmented RCRs within each pair.
In the nonaugmented group, the supraspinatus was reattached to its insertion in a single-row RCR with 2 triple-loaded suture anchors (5.5-mm Insite FT Ti, No. 2 Force Fiber suture; Tornier) and 6 simple stitches (Figure 1A). Anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity at about 45° to the bone surface.
In the contralateral shoulders, augmented RCRs were performed. Specimens were prepared exactly as they were for the nonaugmented RCRs, including anchor placement and suture passage. Before knot tying, RCRs were augmented with 2 strips of 13-mm × 23-mm PHA mesh (BioFiber) (Figure 1B). One strip was used to augment the 3 sutures of each anchor, overlying the residual tendon, to reinforce the tendon–knot interface. After each suture was passed through the supraspinatus tendon from the intra-articular surface, the stitch was passed through the strip of PHA mesh. Stitches were separated by 5 mm in each mesh strip. All 6 sutures were then tied with a Revo knot between the free end of each suture leg and the leg that passed through the tendon and mesh.
Each humerus was transected at the midshaft and potted and mounted in an Instron 1331 load frame with Model 8800 controller (Instron). A cryoclamp was used to grasp the supraspinatus muscle belly above the musculotendinous junction (Figure 2).
Three rows of 2-mm fiducial markers were affixed to the bone, tendon, and muscle belly with cyanoacrylate for tracking with a digital video system (DMAS Version 6.5; Spicatek) (Figure 3).21
A 0.1-MPa pre-stress (applied force/tendon cross-sectional area) was applied to each construct to determine the starting position for the deformation profile. Each repair underwent 1000 cycles of uniaxial load-controlled displacement between 0.1 and 1.0 MPa of effective stress at 1 Hz. Effective stress was determined as the ratio of applied force to cross-sectional area of the tendon at harvest to normalize the applied loads between tendons of varying size. During cyclic testing, gapping of more than 5 mm was defined as construct failure.22 After cyclic loading, each construct was loaded to failure at 1.0 mm/s. Ultimate failure load was defined as the highest load achieved at the maximum displacement before rapid decline in load supported by the construct.
Statistical Analysis
Paired t tests were used to compare the matched pairs of constructs. For all tests, significance was set at P ≤ .05. Post hoc power was calculated for significant results using G*Power Version 3.1.6.23 All data are presented as means (SDs).
Results
After 1000 cycles of displacement, mean (SD) gapping was 3.8 (0.9) mm for the nonaugmented repairs and 3.9 (1.1) mm for the PHA mesh–augmented repairs (P = .879) (Figure 4).
For the nonaugmented repairs, mean (SD) failure displacement was 6.3 (1.7) mm, and mean (SD) ultimate failure load was 472.1 (120.3) N. For the PHA-augmented repairs, failure displacement was 5.5 (1.9) mm, and ultimate failure load was 571.2 (173.0) N. There was no difference in failure displacement (P = .393), but there was a difference in ultimate failure load (P = .042; power = 0.57). During failure testing, mean (SD) tissue deformation was higher (P = .012; power = 0.83) for the PHA-augmented repairs, 1.2 (0.7) mm, than for the nonaugmented repairs, 0.8 (0.5) mm. Failures, which were consistent within pairs, were caused by tissue failure, with sutures pulling through the tissue (4 pairs) or single anchor pullout before ultimate tissue failure (4 pairs). Of the 4 failures with anchor pullout, 3 had anterior anchor pullout, and 1 had posterior anchor pullout. In all specimens with anchor pullout, the second anchor remained stable, and ultimate failure occurred with tissue tearing at the suture interface. There were no significant differences in any metrics between specimens that failed with intact anchors and specimens with single anchor pullout (P ≥ .122). Therefore, both groups were pooled for the failure analysis.
Discussion
RCR augmentation with a synthetic graft is a viable option for improving fixation strength of supraspinatus repairs, as shown in otherwise healthy tendon in the present study. Our hypothesis that there would be decreased gap formation with graft augmentation was not supported, whereas the hypothesis of increased failure loads with graft augmentation was supported. These findings may also be applicable in cases of large tears, revisions, and tendons with poor tissue quality. Simplification of graft application techniques will allow quick and easy arthroscopic augmentation.
Studies of RCRs for large or massive tears have reported retear rates of 25% to 79%.24-26 Latissimus dorsi tendon transfers also show promise in posterosuperior RCRs, with failure rates near 10%.27,28 Although use of PHA patches in RCR augmentation is relatively new, short-term and midterm failure rates are in the range of 20% to 60% in the few small cohorts currently being studied.13,16 It is possible that these rates may improve as indications, surgical experience, and techniques for use of PHA patches are further refined. Regardless, with PHA currently being used in practice, it is important to quantify the biomechanics of the augmentation as a baseline for its performance in reinforcing the tendon–suture interface.
We determined that the initial fixation strength of single-row repairs was higher with the addition of PHA synthetic grafts using a very simple technique. Single-row triple-loaded anchor repairs already provide high initial mechanical strength, and our results are similar to those of another study of this technique.29 Despite the already high mechanical strength of a triple-loaded anchor repair, PHA mesh increased ultimate strength by about 100 N (~25%). Of note, tissue elongation during failure was higher (P = .012; power = 0.83) in the PHA-augmented group (1.2 mm) than in the nonaugmented group (0.8 mm). This was not surprising—failure loads were almost 100 N higher in the PHA-augmented group than in the nonaugmented group. Consequently, much higher forces were placed on the muscle belly, likely resulting in additional elongation of the intact tissue medial to the repair construct.
The ultimate failure loads in our study compare favorably with the biomechanical strength of augmented repairs reported by others.8,9,18 Barber and colleagues18 evaluated an augmented single-row repair with 2 double-loaded suture anchors and an acellular dermal matrix graft. The ultimate failure load of the augmented repairs was 325 N. In contrast, Omae and colleagues8 tested a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft. Ultimate failure load of the augmented repairs was 560 N, similar to our finding. Last, Shea and colleagues9 evaluated a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft, with ultimate failure load of 429 N. The techniques in all 3 studies can be performed arthroscopically but are challenging and require multiple extra sutures and anchors that need management and tying. Our technique provides similar initial fixation strength, has no requirement for extra sutures or anchors, and is very simple to perform.
The supraspinatus tendon is estimated to fail between 800 N and 1000 N.30,31 Biomechanical shoulder simulators use supraspinatus forces in the range of 20 N to 200 N for scapular plane abduction.32-36 Therefore, the single-row repair failures in our study fell between functional and full-thickness failure loads. Studies on the mechanics of degenerated human supraspinatus tendon are limited, but there is evidence the mechanical properties of these tissues are inferior to those of healthy tendon.37 A 100-N increase in failure loads with PHA augmentation may prove highly significant in reinforcing the suture–tendon interface in degenerated tendons.
Adding the mesh did not have any effect on gapping at the repair site after cyclic loading. This finding suggests that construct gapping under cyclic loading is not a function of a reinforced knot–tendon interface but is instead caused by microtearing and cinching of the suture constructs in relation to the underlying bone. Tissue elongation likely was not a strong contributor to overall cyclic gapping, as elongation did not differ between the nonaugmented and augmented repairs (0.5 mm vs 0.7 mm; P = .276) and was small relative to the nearly 4 mm of construct gapping. Gapping may be affected by healing and integration of the mesh into the repaired tendon over time, but this effect could not be captured in the present study. Patients are initially immobilized and passive shoulder motion gradually introduced, in stark contrast to the immediate loading protocol in the present study. Regardless, the 25% increase in overall strength may be clinically important, especially in cases of difficult repair or poor tissue quality.
Our technique simplifies arthroscopic augmentation—stitches are passed through the rotator cuff in simple fashion. Before being tied, the limbs that were passed through the rotator cuff are removed through a cannula and then passed through the synthetic graft.
Study Limitations
This study had several limitations. First, it was a cadaveric biomechanical study that evaluated only time-zero biomechanical properties. Loads were normalized to tendon size, specimens were randomized between sides, and paired specimens were used to minimize the effects of tendon and bone quality on outcome metrics. In addition, donor tendons were representative of otherwise healthy tissue. Chronic tears and associated resorption/atrophy could have affected the magnitude of forces and gapping detected in this study. Theoretically, over time the tendon tissue will adhere to and grow into the mesh, which could minimize potential differences. Studies are needed to determine the effects of healing on long-term repair strength in affected patients. Last, all constructs were performed in open fashion to improve repeatability of construct placement and provide accessibility for Instron testing. Our technique did not directly replicate the arthroscopic approach, but, unlike other augmentation techniques, it is so simple that transition to all-arthroscopic augmentation is realistic.
Patch augmentation increases the cost of materials and operative time and should be considered a limitation of its utility. We do not recommend augmentation in all RCRs, as it likely is cost-ineffective. Instead, we recommend augmentation in cases of poor tissue quality, which could lead to healing failure, revision surgery, and higher overall patient costs beyond the cost of adding augmentation. Similarly, we recommend augmentation for revision cases in which tendon healing has failed and tissue quality is poor. The goal is to prevent another failure.
Conclusion
PHA graft augmentation of single-row triple-loaded anchor repairs of the supraspinatus tendon improves the overall ultimate load to failure by 25%. There was no difference in gap formation after cyclic loading between augmented and nonaugmented repairs. This technique for arthroscopic augmentation can be used to improve initial biomechanical repair strength in tears at risk for failure.
Am J Orthop. 2016;45(7):E527-E533. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
2. Keener JD, Wei AS, Kim HM, et al. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am. 2010;92(3):590-598.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Barber FA, Herbert MA, Schroeder FA, Aziz-Jacobo J, Mays MM, Rapley JH. Biomechanical advantages of triple-loaded suture anchors compared with double-row rotator cuff repairs. Arthroscopy. 2010;26(3):316-323.
5. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
6. Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41(4):872-879.
7. Ma CB, MacGillivray JD, Clabeaux J, Lee S, Otis JC. Biomechanical evaluation of arthroscopic rotator cuff stitches. J Bone Joint Surg Am. 2004;86(6):1211-1216.
8. Omae H, Steinmann SP, Zhao C, et al. Biomechanical effect of rotator cuff augmentation with an acellular dermal matrix graft: a cadaver study. Clin Biomech. 2012;27(8):789-792.
9. Shea KP, Obopilwe E, Sperling JW, Iannotti JP. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg. 2012;21(8):1072-1079.
10. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
11. Beimers L, Lam PH, Murrell GA. The biomechanical effects of polytetrafluoroethylene suture augmentations in lateral-row rotator cuff repairs in an ovine model. J Shoulder Elbow Surg. 2014;23(10):1545-1552.
12. McCarron JA, Milks RA, Chen X, Iannotti JP, Derwin KA. Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J Shoulder Elbow Surg. 2010;19(5):688-696.
13. Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg. 2014;23(10):1508-1513.
14. Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules. 2006;7(8):2249-2258.
15. Ellis G, Cano P, Jadraque M, et al. Laser microperforated biodegradable microbial polyhydroxyalkanoate substrates for tissue repair strategies: an infrared microspectroscopy study. Anal Bioanal Chem. 2011;399(7):2379-2388.
16. Lenart BA, Martens KA, Kearns KA, Gillespie RJ, Zoga AC, Williams GR. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg. 2015;24(6):915-921.
17. Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
18. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
19. Gilot GJ, Attia AK, Alvarez AM. Arthroscopic repair of rotator cuff tears using extracellular matrix graft. Arthrosc Tech. 2014;3(4):e487-e489.
20. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
21. Kullar RS, Reagan JM, Kolz CW, Burks RT, Henninger HB. Suture placement near the musculotendinous junction in the supraspinatus: implications for rotator cuff repair. Am J Sports Med. 2015;43(1):57-62.
22. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athanasiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
23. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
24. Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24(9):1493-1505.
25. Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear rates after arthroscopic single-row, double-row, and suture bridge rotator cuff repair at a minimum of 1 year of imaging follow-up: a systematic review. Arthroscopy. 2015;31(11):2274-2281.
26. Henry P, Wasserstein D, Park S, et al. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31(12):2472-2480.
27. El-Azab HM, Rott O, Irlenbusch U. Long-term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2015;97(6):462-469.
28. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
29. Coons DA, Barber FA, Herbert MA. Triple-loaded single-anchor stitch configurations: an analysis of cyclically loaded suture–tendon interface security. Arthroscopy. 2006;22(11):1154-1158.
30. Itoi E, Berglund LJ, Grabowski JJ, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13(4):578-584.
31. Matsuhashi T, Hooke AW, Zhao KD, et al. Tensile properties of a morphologically split supraspinatus tendon. Clin Anat. 2014;27(5):702-706.
32. Apreleva M, Parsons IM 4th, Warner JJ, Fu FH, Woo SL. Experimental investigation of reaction forces at the glenohumeral joint during active abduction. J Shoulder Elbow Surg. 2000;9(5):409-417.
33. Giles JW, Ferreira LM, Athwal GS, Johnson JA. Development and performance evaluation of a multi-PID muscle loading driven in vitro active-motion shoulder simulator and application to assessing reverse total shoulder arthroplasty. J Biomech Eng. 2014;136(12):121007.
34. Hansen ML, Otis JC, Johnson JS, Cordasco FA, Craig EV, Warren RF. Biomechanics of massive rotator cuff tears: implications for treatment. J Bone Joint Surg Am. 2008;90(2):316-325.
35. Henninger HB, Barg A, Anderson AE, Bachus KN, Tashjian RZ, Burks RT. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(4):483-490.
36. Mihata T, Gates J, McGarry MH, Lee J, Kinoshita M, Lee TQ. Effect of rotator cuff muscle imbalance on forceful internal impingement and peel-back of the superior labrum: a cadaveric study. Am J Sports Med. 2009;37(11):2222-2227.
37. Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, Uhthoff HK. Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone–tendon complex. J Orthop Res. 1997;15(5):719-726.
1. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
2. Keener JD, Wei AS, Kim HM, et al. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am. 2010;92(3):590-598.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Barber FA, Herbert MA, Schroeder FA, Aziz-Jacobo J, Mays MM, Rapley JH. Biomechanical advantages of triple-loaded suture anchors compared with double-row rotator cuff repairs. Arthroscopy. 2010;26(3):316-323.
5. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
6. Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41(4):872-879.
7. Ma CB, MacGillivray JD, Clabeaux J, Lee S, Otis JC. Biomechanical evaluation of arthroscopic rotator cuff stitches. J Bone Joint Surg Am. 2004;86(6):1211-1216.
8. Omae H, Steinmann SP, Zhao C, et al. Biomechanical effect of rotator cuff augmentation with an acellular dermal matrix graft: a cadaver study. Clin Biomech. 2012;27(8):789-792.
9. Shea KP, Obopilwe E, Sperling JW, Iannotti JP. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg. 2012;21(8):1072-1079.
10. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
11. Beimers L, Lam PH, Murrell GA. The biomechanical effects of polytetrafluoroethylene suture augmentations in lateral-row rotator cuff repairs in an ovine model. J Shoulder Elbow Surg. 2014;23(10):1545-1552.
12. McCarron JA, Milks RA, Chen X, Iannotti JP, Derwin KA. Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J Shoulder Elbow Surg. 2010;19(5):688-696.
13. Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg. 2014;23(10):1508-1513.
14. Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules. 2006;7(8):2249-2258.
15. Ellis G, Cano P, Jadraque M, et al. Laser microperforated biodegradable microbial polyhydroxyalkanoate substrates for tissue repair strategies: an infrared microspectroscopy study. Anal Bioanal Chem. 2011;399(7):2379-2388.
16. Lenart BA, Martens KA, Kearns KA, Gillespie RJ, Zoga AC, Williams GR. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg. 2015;24(6):915-921.
17. Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
18. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
19. Gilot GJ, Attia AK, Alvarez AM. Arthroscopic repair of rotator cuff tears using extracellular matrix graft. Arthrosc Tech. 2014;3(4):e487-e489.
20. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
21. Kullar RS, Reagan JM, Kolz CW, Burks RT, Henninger HB. Suture placement near the musculotendinous junction in the supraspinatus: implications for rotator cuff repair. Am J Sports Med. 2015;43(1):57-62.
22. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athanasiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
23. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
24. Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24(9):1493-1505.
25. Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear rates after arthroscopic single-row, double-row, and suture bridge rotator cuff repair at a minimum of 1 year of imaging follow-up: a systematic review. Arthroscopy. 2015;31(11):2274-2281.
26. Henry P, Wasserstein D, Park S, et al. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31(12):2472-2480.
27. El-Azab HM, Rott O, Irlenbusch U. Long-term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2015;97(6):462-469.
28. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
29. Coons DA, Barber FA, Herbert MA. Triple-loaded single-anchor stitch configurations: an analysis of cyclically loaded suture–tendon interface security. Arthroscopy. 2006;22(11):1154-1158.
30. Itoi E, Berglund LJ, Grabowski JJ, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13(4):578-584.
31. Matsuhashi T, Hooke AW, Zhao KD, et al. Tensile properties of a morphologically split supraspinatus tendon. Clin Anat. 2014;27(5):702-706.
32. Apreleva M, Parsons IM 4th, Warner JJ, Fu FH, Woo SL. Experimental investigation of reaction forces at the glenohumeral joint during active abduction. J Shoulder Elbow Surg. 2000;9(5):409-417.
33. Giles JW, Ferreira LM, Athwal GS, Johnson JA. Development and performance evaluation of a multi-PID muscle loading driven in vitro active-motion shoulder simulator and application to assessing reverse total shoulder arthroplasty. J Biomech Eng. 2014;136(12):121007.
34. Hansen ML, Otis JC, Johnson JS, Cordasco FA, Craig EV, Warren RF. Biomechanics of massive rotator cuff tears: implications for treatment. J Bone Joint Surg Am. 2008;90(2):316-325.
35. Henninger HB, Barg A, Anderson AE, Bachus KN, Tashjian RZ, Burks RT. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(4):483-490.
36. Mihata T, Gates J, McGarry MH, Lee J, Kinoshita M, Lee TQ. Effect of rotator cuff muscle imbalance on forceful internal impingement and peel-back of the superior labrum: a cadaveric study. Am J Sports Med. 2009;37(11):2222-2227.
37. Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, Uhthoff HK. Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone–tendon complex. J Orthop Res. 1997;15(5):719-726.
Patient-Reported Outcome Measures: How Do Digital Tablets Stack Up to Paper Forms? A Randomized, Controlled Study
Over the past several decades, patient-reported outcomes (PROs) have become increasingly important in assessing the quality and effectiveness of medical and surgical care.1,2 The benefit lies in the ability of PROs to characterize the impact of medical interventions on symptoms, function, and other outcomes from the patient’s perspective. Consequently, clinical practices can improve patients’ objective findings (from radiographic and clinical examinations) as well as their preferences in a social-psychological context.2,3 As a patient’s satisfaction with a surgical intervention may not correlate with the surgeon’s objective assessment of outcome, PROs offer unique insight into the patient’s perceptions of well-being.4
Health-related quality-of-life assessments can be made with either general-health or disease-specific instruments. These instruments traditionally are administered with pen and paper—a data collection method with several limitations, chief being the need to manually transfer the data into an electronic medical record, a research database, or both. In addition, administering surveys on paper risks potential disqualification of partially or incorrectly completed surveys. With pen and paper, it is difficult to mandate that every question be answered accurately.
Currently, there is a potential role for electronic medical records and digital tablet devices in survey administration and data collection and storage. Theoretical advantages include direct input of survey data into databases (eliminating manual data entry and associated entry errors), improved accuracy and completion rates, and long-term storage not dependent on paper charts.5To our knowledge, there have been no prospective studies of different orthopedic outcomes collection methods. Some studies have evaluated use of touch-based tablets in data collection. Dy and colleagues6 considered administration of the DASH (Disabilities of the Arm, Shoulder, and Hand) survey on an iPad tablet (Apple Computers) and retrospectively compared the tablet and paper completion rates. The tablet group’s rate (98%) was significantly higher than the paper group’s rate (76%). Aktas and colleagues7 reported a high completion rate for a tablet survey of palliative care outcomes (they did not compare modalities). A handful of other studies have found higher intraclass correlation and validation for digital data collection than for paper collection.7-14 The comparability of the data collected digitally vs on paper was the nidus for our decision to prospectively evaluate the ease and reliability of digital data collection.
We conducted a prospective, randomized study to compare the performance of tablet and paper versions of several general-health and musculoskeletal disease–specific questionnaires. We hypothesized the tablet and paper surveys would have similar completion rates and times.
Methods
This study was approved by our Institutional Review Board. Participants were recruited during their clinic visit to 3 subspecialty orthopedic services (upper extremity, spine, arthroplasty). The questionnaires included basic demographics questions and questions about tablet use (comfort level with computers, measured on a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree), and ownership of a tablet or smartphone). Also included were European Quality of Life–5 Dimensions (EQ-5D, General Health), a disease questionnaire specific to 1 of the 3 subspecialty services, and a satisfaction survey. Patients were asked to complete the Oswestry Disability Index (ODI) for low-back pain, the Neck Disability Index (NDI) for neck pain, the Hip Disability and Osteoarthritis Outcomes Score (HOOS) for hip pain, the Knee Injury and Osteoarthritis Outcomes Score (KOOS) for knee pain, or the QuickDASH survey for upper extremity complaints (subspecialty-specific). After recruitment, a computer-generated randomization technique was used to randomly assign patients to either a paper or an electronic (iPad) data collection group.15 We included all surveys for which patients had sufficient completion time (no clinic staff interruptions) and excluded surveys marked incomplete (because of interruptions for clinic workflow efficiency). For direct input from tablets and for data storage, we used the Research Electronic Data Capture (REDCap) system hosted at our institution.16 Our staff registered patients as REDCap participants, assigned them to their disease-specific study arms, and gave them tablets to use to complete the surveys.
Patients who were randomly assigned to take the surveys on paper were given a packet that included the demographics survey, the EQ-5D, a disease-specific survey, and a satisfaction survey. Their responses were then manually entered by the investigators into the REDCap system.
Patients who were randomly assigned to take the surveys on tablets used the REDCap survey feature, which allowed them to directly input their responses into the database (Figure).
Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction with survey length (Likert scale, 1-5), ease of completion (Likert scale, 1-5), ability to comprehend questions (Likert scale, 1-5), and preference for the other survey modality (Appendix).
We used SPSS statistical software (IBM) to analyze our data, t test to compare continuous variables, χ2 test to compare categorical variables, and linear regression to test the relationship between number of questions and completion rate. Statistical significance was set at P < .05.
Results
Of the 510 patients enrolled in the study, 483 completed the initial demographics questionnaire and were included in the analysis. Patients were excluded if they were unable to complete the initial demographics questionnaire because of clinic workflow (eg, immediate need to be seen by physician, need to transfer to radiology for imaging and not being able to revisit the survey). Mean age was 56 years (range, 14-93 years), and 51% of the respondents were female. Fifty percent owned tablets, 70% owned smartphones, and mean (SD) self-rating of computer skills was 3.13 (1.16) (Likert scale, 1-5). There were no significant demographic differences between the tablet and paper groups (Table 1).
For each disease-specific questionnaire, the instrument’s published instructions for calculating scores were followed; these scores were then compared in order to further characterize the groups. There were significant differences in scores on the EQ-5D descriptive questions, a pain visual analog scale (VAS), and the NDI. Mean EQ-5D score was 0.664 for the tablet group and 0.699 for the paper group (P = .041), mean pain VAS score was 62.5 for the tablet group and 71.6 for the paper group (P < .001), and mean NDI score was 42.8 for the tablet group and 32.4 for the paper group (P = .033).
The overall completion rate for all questionnaires was 84.4%. The KOOS completion rate was 83.3% for the tablet group and 54.5% for the paper group (P = .023). Although it was not statistically significant, there was a trend toward higher rates of completing all disease-specific questionnaires in the tablet group relative to the paper group.
Satisfaction regarding the surveys and their modalities was similar between the groups.
Discussion
Electronic data entry has many advantages over traditional paper-based data collection and can be used with PRO surveys to measure response to treatment. Our study evaluated whether completion rates differed between surveys administered on digital tablets and those administered on traditional paper forms in a clinic setting. We selected general-health and disease-specific instruments commonly used to collect PROs from orthopedic patients. Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction, and survey preferences.
In this study, our tablet and paper groups had similar overall survey completion rates, which suggests digital tablet-based data collection is noninferior to traditional pen-and-paper data collection with respect to patient response rate in the clinical setting. It is worth emphasizing that the tablet surveys were made to resemble and function as much as possible like the paper surveys. For example, patients were allowed to select multiple answers as well as advance without answering a question. Paper surveys were mimicked so we could study inherent differences in patient responsiveness without adding digital features to prevent patients from selecting multiple answers or skipping questions. We postulate that adding these digital features could have introduced a significant difference in patient responsiveness.
Time for survey completion was not significantly different between the tablet and paper groups, demonstrating that data can be digitally collected and the aforementioned advantages realized without significant delay or clinic workflow disruption. In the future, patients may be able to complete their forms digitally, on their own devices, before arriving for their clinic visits—resulting in improved clinic workflow and data collection efficiency.
Scores computed for the health-related quality-of-life questionnaires were not significantly different between the tablet and paper groups, except for EQ-5D and NDI. Although statistically significant, the 0.035 difference between the groups’ EQ-5D scores (0.664, 0.699) is not clinically significant. (Pickard and colleagues17 established that 0.06 is the clinically significant difference between EQ-5D scores in the United States.) If there were any clinical difference in the present study, our paper group patients appeared to be in better health than our tablet group patients.
Patients’ motivation to complete surveys often plays a large role in meaningful rates of completion. On our subjective satisfaction survey, a larger percentage of patients reported they would prefer to use a tablet for future surveys (Table 4). This finding may be driven by the novelty or ease of using a popular device. Nevertheless, we think it is worthwhile to heed patient preferences, as they may point to more successful data collection and compliance.
Several other studies have compared electronic and paper data capture.6,7,9-14,18-22 Dy and colleagues6 reported on administering the DASH survey on an iPad tablet using REDCap in an outpatient setting. They found that the percentage of surveys that could be scored (<3 questions left unanswered) was significantly higher for their tablet group (98%) than their paper group (76%). The larger difference in survey completion rates in their study (vs ours) may be attributable to their use of DASH, which has more survey items (compared with QuickDASH, the instrument we used) and thus may be more sensitive to detecting differences, at the risk of increasing the burden on survey takers.23 Aktas and colleagues7 conducted a similar but smaller study of completion rates, completion times, and overall practicality of using digital tablets to collect PROs in a palliative care clinic (they did not compare tablet and paper modalities). Marsh and colleagues,12 who studied the agreement between data collected on electronic and paper versions of the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index and the SF-12 (12-item Short Form Health Survey, Version 2) after total hip and total knee arthroplasty, found a high intraclass correlation coefficient between the 2 methods. Griffiths-Jones and colleagues11 also found a high degree of agreement between patient data collected on digital and paper surveys. In a similar study, Fanning and McAuley10 compared digital tablet and paper survey administration in an older population and found a higher percentage of preference for tablets, with ease of use and anxiety during survey completion correlating with preference. These findings mirror ours, even with our inclusion of patients in a broader age range.
Strengths of our study included its overall cohort size and the variety of measurement instruments used. In addition, we measured time for survey completion to assess the practicality of tablet-based data collection and refrained from using digital features that could have artificially improved the completion rate for this survey modality.
Our study had a few limitations. First, we recruited unequal numbers of patients from the different subspecialties—a result of each subspecialty having a different number of attending physicians and a different patient volume. Given randomization and use of similar patients across the study arms, however, this likely did not present any significant bias. Second, each patient completed a tablet survey or a paper survey but not both, and therefore we could not compare a patient’s performance on the 2 modalities. However, the burden of completing the same survey more than once likely would have lowered our participation rate and introduced additional biases we wanted to avoid. Third, despite our attempt to mimic the look of a paper survey, the tablet’s user interface presented several potential difficulties. For example, its small text and small answer buttons may have been limiting for patients with poor vision. These design features emphasize the importance of having a user interface that can be adapted to the individual, regardless of handicap. Indeed, adaptability is a potential strength of digital interfaces. For adaptability, an interface designer can use large, scalable text and add audio prompts and other features.
Our findings can be useful in evaluating patient responsiveness to surveys administered on digital tablets in an outpatient clinic setting. In this prospective, randomized study, we found that, for survey completion, use of a tablet device did not require more time than use of a paper form. In addition, the administration modalities had similar completion and error rates for a variety of orthopedic outcomes surveys. We did not activate digital features that would have given unfair advantage to the digital data collection modality. We also found a strong preference for use of technology in PRO data collection, and this may help improve collection rates. Last, though optimizing the flow of patients in our clinic was not a strict research metric, we prioritized making sure patients were not spending any more time completing these surveys than in the past. Given the potential benefits of digital surveys—immediate and accurate transfer of collected data into multiple databases, including the patient’s electronic medical record—our experience supports continuing validation of these instruments for potential wider use.
Am J Orthop. 2016;45(7):E451-E457. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Higginson IJ, Carr AJ. Measuring quality of life: using quality of life measures in the clinical setting. BMJ. 2001;322(7297):1297-1300.
3. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102-109.
4. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622-629.
5. Paudel D, Ahmed M, Pradhan A, Lal Dangol R. Successful use of tablet personal computers and wireless technologies for the 2011 Nepal Demographic and Health Survey. Glob Heal Sci Pract. 2013;1(2):277-284.
6. Dy CJ, Schmicker T, Tran Q, Chadwick B, Daluiski A. The use of a tablet computer to complete the DASH questionnaire. J Hand Surg Am. 2012;37(12):2589-2594.
7. Aktas A, Hullihen B, Shrotriya S, Thomas S, Walsh D, Estfan B. Connected health: cancer symptom and quality-of-life assessment using a tablet computer: a pilot study. Am J Hosp Palliat Care. 2015;32(2):189-197.
8. Basnov M, Kongsved SM, Bech P, Hjollund NH. Reliability of Short Form-36 in an internet- and a pen-and-paper version. Inform Health Soc Care. 2009;34(1):53-58.
9. Bellamy N, Wilson C, Hendrikz J, et al; EDC Study Group. Osteoarthritis Index delivered by mobile phone (m-WOMAC) is valid, reliable, and responsive. J Clin Epidemiol. 2011;64(2):182-190.
10. Fanning J, McAuley E. A comparison of tablet computer and paper-based questionnaires in healthy aging research. JMIR Res Protoc. 2014;3(3):e38.
11. Griffiths-Jones W, Norton MR, Fern ED, Williams DH. The equivalence of remote electronic and paper patient reported outcome (PRO) collection. J Arthroplasty. 2014;29(11):2136-2139.
12. Marsh JD, Bryant DM, Macdonald SJ, Naudie DD. Patients respond similarly to paper and electronic versions of the WOMAC and SF-12 following total joint arthroplasty. J Arthroplasty. 2014;29(4):670-673.
13. Olajos-Clow J, Minard J, Szpiro K, et al. Validation of an electronic version of the Mini Asthma Quality of Life Questionnaire. Respir Med. 2010;104(5):658-667.
14. Shervin N, Dorrwachter J, Bragdon CR, Shervin D, Zurakowski D, Malchau H. Comparison of paper and computer-based questionnaire modes for measuring health outcomes in patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2011;93(3):285-293.
15. Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8-11.
16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
17. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
18. Abdel Messih M, Naylor JM, Descallar J, Manickam A, Mittal R, Harris IA. Mail versus telephone administration of the Oxford Knee and Hip Scores. J Arthroplasty. 2014;29(3):491-494.
19. Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH. Response rate and completeness of questionnaires: a randomized study of internet versus paper-and-pencil versions. J Med Internet Res. 2007;9(3):e25.
20. Theiler R, Bischoff-Ferrari HA, Good M, Bellamy N. Responsiveness of the electronic touch screen WOMAC 3.1 OA Index in a short term clinical trial with rofecoxib. Osteoarthritis Cartilage. 2004;12(11):912-916.
21. Ryan JM, Corry JR, Attewell R, Smithson MJ. A comparison of an electronic version of the SF-36 General Health Questionnaire to the standard paper version. Qual Life Res. 2002;11(1):19-26.
22. Wilson AS, Kitas GD, Carruthers DM, et al. Computerized information-gathering in specialist rheumatology clinics: an initial evaluation of an electronic version of the Short Form 36. Rheumatology. 2002;41(3):268-273.
23. Angst F, Goldhahn J, Drerup S, Flury M, Schwyzer HK, Simmen BR. How sharp is the short QuickDASH? A refined content and validity analysis of the Short Form of the Disabilities of the Shoulder, Arm and Hand questionnaire in the strata of symptoms and function and specific joint conditions. Qual Life Res. 2009;18(8):1043-1051.
Over the past several decades, patient-reported outcomes (PROs) have become increasingly important in assessing the quality and effectiveness of medical and surgical care.1,2 The benefit lies in the ability of PROs to characterize the impact of medical interventions on symptoms, function, and other outcomes from the patient’s perspective. Consequently, clinical practices can improve patients’ objective findings (from radiographic and clinical examinations) as well as their preferences in a social-psychological context.2,3 As a patient’s satisfaction with a surgical intervention may not correlate with the surgeon’s objective assessment of outcome, PROs offer unique insight into the patient’s perceptions of well-being.4
Health-related quality-of-life assessments can be made with either general-health or disease-specific instruments. These instruments traditionally are administered with pen and paper—a data collection method with several limitations, chief being the need to manually transfer the data into an electronic medical record, a research database, or both. In addition, administering surveys on paper risks potential disqualification of partially or incorrectly completed surveys. With pen and paper, it is difficult to mandate that every question be answered accurately.
Currently, there is a potential role for electronic medical records and digital tablet devices in survey administration and data collection and storage. Theoretical advantages include direct input of survey data into databases (eliminating manual data entry and associated entry errors), improved accuracy and completion rates, and long-term storage not dependent on paper charts.5To our knowledge, there have been no prospective studies of different orthopedic outcomes collection methods. Some studies have evaluated use of touch-based tablets in data collection. Dy and colleagues6 considered administration of the DASH (Disabilities of the Arm, Shoulder, and Hand) survey on an iPad tablet (Apple Computers) and retrospectively compared the tablet and paper completion rates. The tablet group’s rate (98%) was significantly higher than the paper group’s rate (76%). Aktas and colleagues7 reported a high completion rate for a tablet survey of palliative care outcomes (they did not compare modalities). A handful of other studies have found higher intraclass correlation and validation for digital data collection than for paper collection.7-14 The comparability of the data collected digitally vs on paper was the nidus for our decision to prospectively evaluate the ease and reliability of digital data collection.
We conducted a prospective, randomized study to compare the performance of tablet and paper versions of several general-health and musculoskeletal disease–specific questionnaires. We hypothesized the tablet and paper surveys would have similar completion rates and times.
Methods
This study was approved by our Institutional Review Board. Participants were recruited during their clinic visit to 3 subspecialty orthopedic services (upper extremity, spine, arthroplasty). The questionnaires included basic demographics questions and questions about tablet use (comfort level with computers, measured on a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree), and ownership of a tablet or smartphone). Also included were European Quality of Life–5 Dimensions (EQ-5D, General Health), a disease questionnaire specific to 1 of the 3 subspecialty services, and a satisfaction survey. Patients were asked to complete the Oswestry Disability Index (ODI) for low-back pain, the Neck Disability Index (NDI) for neck pain, the Hip Disability and Osteoarthritis Outcomes Score (HOOS) for hip pain, the Knee Injury and Osteoarthritis Outcomes Score (KOOS) for knee pain, or the QuickDASH survey for upper extremity complaints (subspecialty-specific). After recruitment, a computer-generated randomization technique was used to randomly assign patients to either a paper or an electronic (iPad) data collection group.15 We included all surveys for which patients had sufficient completion time (no clinic staff interruptions) and excluded surveys marked incomplete (because of interruptions for clinic workflow efficiency). For direct input from tablets and for data storage, we used the Research Electronic Data Capture (REDCap) system hosted at our institution.16 Our staff registered patients as REDCap participants, assigned them to their disease-specific study arms, and gave them tablets to use to complete the surveys.
Patients who were randomly assigned to take the surveys on paper were given a packet that included the demographics survey, the EQ-5D, a disease-specific survey, and a satisfaction survey. Their responses were then manually entered by the investigators into the REDCap system.
Patients who were randomly assigned to take the surveys on tablets used the REDCap survey feature, which allowed them to directly input their responses into the database (Figure).
Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction with survey length (Likert scale, 1-5), ease of completion (Likert scale, 1-5), ability to comprehend questions (Likert scale, 1-5), and preference for the other survey modality (Appendix).
We used SPSS statistical software (IBM) to analyze our data, t test to compare continuous variables, χ2 test to compare categorical variables, and linear regression to test the relationship between number of questions and completion rate. Statistical significance was set at P < .05.
Results
Of the 510 patients enrolled in the study, 483 completed the initial demographics questionnaire and were included in the analysis. Patients were excluded if they were unable to complete the initial demographics questionnaire because of clinic workflow (eg, immediate need to be seen by physician, need to transfer to radiology for imaging and not being able to revisit the survey). Mean age was 56 years (range, 14-93 years), and 51% of the respondents were female. Fifty percent owned tablets, 70% owned smartphones, and mean (SD) self-rating of computer skills was 3.13 (1.16) (Likert scale, 1-5). There were no significant demographic differences between the tablet and paper groups (Table 1).
For each disease-specific questionnaire, the instrument’s published instructions for calculating scores were followed; these scores were then compared in order to further characterize the groups. There were significant differences in scores on the EQ-5D descriptive questions, a pain visual analog scale (VAS), and the NDI. Mean EQ-5D score was 0.664 for the tablet group and 0.699 for the paper group (P = .041), mean pain VAS score was 62.5 for the tablet group and 71.6 for the paper group (P < .001), and mean NDI score was 42.8 for the tablet group and 32.4 for the paper group (P = .033).
The overall completion rate for all questionnaires was 84.4%. The KOOS completion rate was 83.3% for the tablet group and 54.5% for the paper group (P = .023). Although it was not statistically significant, there was a trend toward higher rates of completing all disease-specific questionnaires in the tablet group relative to the paper group.
Satisfaction regarding the surveys and their modalities was similar between the groups.
Discussion
Electronic data entry has many advantages over traditional paper-based data collection and can be used with PRO surveys to measure response to treatment. Our study evaluated whether completion rates differed between surveys administered on digital tablets and those administered on traditional paper forms in a clinic setting. We selected general-health and disease-specific instruments commonly used to collect PROs from orthopedic patients. Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction, and survey preferences.
In this study, our tablet and paper groups had similar overall survey completion rates, which suggests digital tablet-based data collection is noninferior to traditional pen-and-paper data collection with respect to patient response rate in the clinical setting. It is worth emphasizing that the tablet surveys were made to resemble and function as much as possible like the paper surveys. For example, patients were allowed to select multiple answers as well as advance without answering a question. Paper surveys were mimicked so we could study inherent differences in patient responsiveness without adding digital features to prevent patients from selecting multiple answers or skipping questions. We postulate that adding these digital features could have introduced a significant difference in patient responsiveness.
Time for survey completion was not significantly different between the tablet and paper groups, demonstrating that data can be digitally collected and the aforementioned advantages realized without significant delay or clinic workflow disruption. In the future, patients may be able to complete their forms digitally, on their own devices, before arriving for their clinic visits—resulting in improved clinic workflow and data collection efficiency.
Scores computed for the health-related quality-of-life questionnaires were not significantly different between the tablet and paper groups, except for EQ-5D and NDI. Although statistically significant, the 0.035 difference between the groups’ EQ-5D scores (0.664, 0.699) is not clinically significant. (Pickard and colleagues17 established that 0.06 is the clinically significant difference between EQ-5D scores in the United States.) If there were any clinical difference in the present study, our paper group patients appeared to be in better health than our tablet group patients.
Patients’ motivation to complete surveys often plays a large role in meaningful rates of completion. On our subjective satisfaction survey, a larger percentage of patients reported they would prefer to use a tablet for future surveys (Table 4). This finding may be driven by the novelty or ease of using a popular device. Nevertheless, we think it is worthwhile to heed patient preferences, as they may point to more successful data collection and compliance.
Several other studies have compared electronic and paper data capture.6,7,9-14,18-22 Dy and colleagues6 reported on administering the DASH survey on an iPad tablet using REDCap in an outpatient setting. They found that the percentage of surveys that could be scored (<3 questions left unanswered) was significantly higher for their tablet group (98%) than their paper group (76%). The larger difference in survey completion rates in their study (vs ours) may be attributable to their use of DASH, which has more survey items (compared with QuickDASH, the instrument we used) and thus may be more sensitive to detecting differences, at the risk of increasing the burden on survey takers.23 Aktas and colleagues7 conducted a similar but smaller study of completion rates, completion times, and overall practicality of using digital tablets to collect PROs in a palliative care clinic (they did not compare tablet and paper modalities). Marsh and colleagues,12 who studied the agreement between data collected on electronic and paper versions of the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index and the SF-12 (12-item Short Form Health Survey, Version 2) after total hip and total knee arthroplasty, found a high intraclass correlation coefficient between the 2 methods. Griffiths-Jones and colleagues11 also found a high degree of agreement between patient data collected on digital and paper surveys. In a similar study, Fanning and McAuley10 compared digital tablet and paper survey administration in an older population and found a higher percentage of preference for tablets, with ease of use and anxiety during survey completion correlating with preference. These findings mirror ours, even with our inclusion of patients in a broader age range.
Strengths of our study included its overall cohort size and the variety of measurement instruments used. In addition, we measured time for survey completion to assess the practicality of tablet-based data collection and refrained from using digital features that could have artificially improved the completion rate for this survey modality.
Our study had a few limitations. First, we recruited unequal numbers of patients from the different subspecialties—a result of each subspecialty having a different number of attending physicians and a different patient volume. Given randomization and use of similar patients across the study arms, however, this likely did not present any significant bias. Second, each patient completed a tablet survey or a paper survey but not both, and therefore we could not compare a patient’s performance on the 2 modalities. However, the burden of completing the same survey more than once likely would have lowered our participation rate and introduced additional biases we wanted to avoid. Third, despite our attempt to mimic the look of a paper survey, the tablet’s user interface presented several potential difficulties. For example, its small text and small answer buttons may have been limiting for patients with poor vision. These design features emphasize the importance of having a user interface that can be adapted to the individual, regardless of handicap. Indeed, adaptability is a potential strength of digital interfaces. For adaptability, an interface designer can use large, scalable text and add audio prompts and other features.
Our findings can be useful in evaluating patient responsiveness to surveys administered on digital tablets in an outpatient clinic setting. In this prospective, randomized study, we found that, for survey completion, use of a tablet device did not require more time than use of a paper form. In addition, the administration modalities had similar completion and error rates for a variety of orthopedic outcomes surveys. We did not activate digital features that would have given unfair advantage to the digital data collection modality. We also found a strong preference for use of technology in PRO data collection, and this may help improve collection rates. Last, though optimizing the flow of patients in our clinic was not a strict research metric, we prioritized making sure patients were not spending any more time completing these surveys than in the past. Given the potential benefits of digital surveys—immediate and accurate transfer of collected data into multiple databases, including the patient’s electronic medical record—our experience supports continuing validation of these instruments for potential wider use.
Am J Orthop. 2016;45(7):E451-E457. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Over the past several decades, patient-reported outcomes (PROs) have become increasingly important in assessing the quality and effectiveness of medical and surgical care.1,2 The benefit lies in the ability of PROs to characterize the impact of medical interventions on symptoms, function, and other outcomes from the patient’s perspective. Consequently, clinical practices can improve patients’ objective findings (from radiographic and clinical examinations) as well as their preferences in a social-psychological context.2,3 As a patient’s satisfaction with a surgical intervention may not correlate with the surgeon’s objective assessment of outcome, PROs offer unique insight into the patient’s perceptions of well-being.4
Health-related quality-of-life assessments can be made with either general-health or disease-specific instruments. These instruments traditionally are administered with pen and paper—a data collection method with several limitations, chief being the need to manually transfer the data into an electronic medical record, a research database, or both. In addition, administering surveys on paper risks potential disqualification of partially or incorrectly completed surveys. With pen and paper, it is difficult to mandate that every question be answered accurately.
Currently, there is a potential role for electronic medical records and digital tablet devices in survey administration and data collection and storage. Theoretical advantages include direct input of survey data into databases (eliminating manual data entry and associated entry errors), improved accuracy and completion rates, and long-term storage not dependent on paper charts.5To our knowledge, there have been no prospective studies of different orthopedic outcomes collection methods. Some studies have evaluated use of touch-based tablets in data collection. Dy and colleagues6 considered administration of the DASH (Disabilities of the Arm, Shoulder, and Hand) survey on an iPad tablet (Apple Computers) and retrospectively compared the tablet and paper completion rates. The tablet group’s rate (98%) was significantly higher than the paper group’s rate (76%). Aktas and colleagues7 reported a high completion rate for a tablet survey of palliative care outcomes (they did not compare modalities). A handful of other studies have found higher intraclass correlation and validation for digital data collection than for paper collection.7-14 The comparability of the data collected digitally vs on paper was the nidus for our decision to prospectively evaluate the ease and reliability of digital data collection.
We conducted a prospective, randomized study to compare the performance of tablet and paper versions of several general-health and musculoskeletal disease–specific questionnaires. We hypothesized the tablet and paper surveys would have similar completion rates and times.
Methods
This study was approved by our Institutional Review Board. Participants were recruited during their clinic visit to 3 subspecialty orthopedic services (upper extremity, spine, arthroplasty). The questionnaires included basic demographics questions and questions about tablet use (comfort level with computers, measured on a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree), and ownership of a tablet or smartphone). Also included were European Quality of Life–5 Dimensions (EQ-5D, General Health), a disease questionnaire specific to 1 of the 3 subspecialty services, and a satisfaction survey. Patients were asked to complete the Oswestry Disability Index (ODI) for low-back pain, the Neck Disability Index (NDI) for neck pain, the Hip Disability and Osteoarthritis Outcomes Score (HOOS) for hip pain, the Knee Injury and Osteoarthritis Outcomes Score (KOOS) for knee pain, or the QuickDASH survey for upper extremity complaints (subspecialty-specific). After recruitment, a computer-generated randomization technique was used to randomly assign patients to either a paper or an electronic (iPad) data collection group.15 We included all surveys for which patients had sufficient completion time (no clinic staff interruptions) and excluded surveys marked incomplete (because of interruptions for clinic workflow efficiency). For direct input from tablets and for data storage, we used the Research Electronic Data Capture (REDCap) system hosted at our institution.16 Our staff registered patients as REDCap participants, assigned them to their disease-specific study arms, and gave them tablets to use to complete the surveys.
Patients who were randomly assigned to take the surveys on paper were given a packet that included the demographics survey, the EQ-5D, a disease-specific survey, and a satisfaction survey. Their responses were then manually entered by the investigators into the REDCap system.
Patients who were randomly assigned to take the surveys on tablets used the REDCap survey feature, which allowed them to directly input their responses into the database (Figure).
Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction with survey length (Likert scale, 1-5), ease of completion (Likert scale, 1-5), ability to comprehend questions (Likert scale, 1-5), and preference for the other survey modality (Appendix).
We used SPSS statistical software (IBM) to analyze our data, t test to compare continuous variables, χ2 test to compare categorical variables, and linear regression to test the relationship between number of questions and completion rate. Statistical significance was set at P < .05.
Results
Of the 510 patients enrolled in the study, 483 completed the initial demographics questionnaire and were included in the analysis. Patients were excluded if they were unable to complete the initial demographics questionnaire because of clinic workflow (eg, immediate need to be seen by physician, need to transfer to radiology for imaging and not being able to revisit the survey). Mean age was 56 years (range, 14-93 years), and 51% of the respondents were female. Fifty percent owned tablets, 70% owned smartphones, and mean (SD) self-rating of computer skills was 3.13 (1.16) (Likert scale, 1-5). There were no significant demographic differences between the tablet and paper groups (Table 1).
For each disease-specific questionnaire, the instrument’s published instructions for calculating scores were followed; these scores were then compared in order to further characterize the groups. There were significant differences in scores on the EQ-5D descriptive questions, a pain visual analog scale (VAS), and the NDI. Mean EQ-5D score was 0.664 for the tablet group and 0.699 for the paper group (P = .041), mean pain VAS score was 62.5 for the tablet group and 71.6 for the paper group (P < .001), and mean NDI score was 42.8 for the tablet group and 32.4 for the paper group (P = .033).
The overall completion rate for all questionnaires was 84.4%. The KOOS completion rate was 83.3% for the tablet group and 54.5% for the paper group (P = .023). Although it was not statistically significant, there was a trend toward higher rates of completing all disease-specific questionnaires in the tablet group relative to the paper group.
Satisfaction regarding the surveys and their modalities was similar between the groups.
Discussion
Electronic data entry has many advantages over traditional paper-based data collection and can be used with PRO surveys to measure response to treatment. Our study evaluated whether completion rates differed between surveys administered on digital tablets and those administered on traditional paper forms in a clinic setting. We selected general-health and disease-specific instruments commonly used to collect PROs from orthopedic patients. Our primary outcome measure was survey completion rate. Secondary outcome measures were total time for completion, number of questions left unanswered on incomplete surveys, patient satisfaction, and survey preferences.
In this study, our tablet and paper groups had similar overall survey completion rates, which suggests digital tablet-based data collection is noninferior to traditional pen-and-paper data collection with respect to patient response rate in the clinical setting. It is worth emphasizing that the tablet surveys were made to resemble and function as much as possible like the paper surveys. For example, patients were allowed to select multiple answers as well as advance without answering a question. Paper surveys were mimicked so we could study inherent differences in patient responsiveness without adding digital features to prevent patients from selecting multiple answers or skipping questions. We postulate that adding these digital features could have introduced a significant difference in patient responsiveness.
Time for survey completion was not significantly different between the tablet and paper groups, demonstrating that data can be digitally collected and the aforementioned advantages realized without significant delay or clinic workflow disruption. In the future, patients may be able to complete their forms digitally, on their own devices, before arriving for their clinic visits—resulting in improved clinic workflow and data collection efficiency.
Scores computed for the health-related quality-of-life questionnaires were not significantly different between the tablet and paper groups, except for EQ-5D and NDI. Although statistically significant, the 0.035 difference between the groups’ EQ-5D scores (0.664, 0.699) is not clinically significant. (Pickard and colleagues17 established that 0.06 is the clinically significant difference between EQ-5D scores in the United States.) If there were any clinical difference in the present study, our paper group patients appeared to be in better health than our tablet group patients.
Patients’ motivation to complete surveys often plays a large role in meaningful rates of completion. On our subjective satisfaction survey, a larger percentage of patients reported they would prefer to use a tablet for future surveys (Table 4). This finding may be driven by the novelty or ease of using a popular device. Nevertheless, we think it is worthwhile to heed patient preferences, as they may point to more successful data collection and compliance.
Several other studies have compared electronic and paper data capture.6,7,9-14,18-22 Dy and colleagues6 reported on administering the DASH survey on an iPad tablet using REDCap in an outpatient setting. They found that the percentage of surveys that could be scored (<3 questions left unanswered) was significantly higher for their tablet group (98%) than their paper group (76%). The larger difference in survey completion rates in their study (vs ours) may be attributable to their use of DASH, which has more survey items (compared with QuickDASH, the instrument we used) and thus may be more sensitive to detecting differences, at the risk of increasing the burden on survey takers.23 Aktas and colleagues7 conducted a similar but smaller study of completion rates, completion times, and overall practicality of using digital tablets to collect PROs in a palliative care clinic (they did not compare tablet and paper modalities). Marsh and colleagues,12 who studied the agreement between data collected on electronic and paper versions of the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index and the SF-12 (12-item Short Form Health Survey, Version 2) after total hip and total knee arthroplasty, found a high intraclass correlation coefficient between the 2 methods. Griffiths-Jones and colleagues11 also found a high degree of agreement between patient data collected on digital and paper surveys. In a similar study, Fanning and McAuley10 compared digital tablet and paper survey administration in an older population and found a higher percentage of preference for tablets, with ease of use and anxiety during survey completion correlating with preference. These findings mirror ours, even with our inclusion of patients in a broader age range.
Strengths of our study included its overall cohort size and the variety of measurement instruments used. In addition, we measured time for survey completion to assess the practicality of tablet-based data collection and refrained from using digital features that could have artificially improved the completion rate for this survey modality.
Our study had a few limitations. First, we recruited unequal numbers of patients from the different subspecialties—a result of each subspecialty having a different number of attending physicians and a different patient volume. Given randomization and use of similar patients across the study arms, however, this likely did not present any significant bias. Second, each patient completed a tablet survey or a paper survey but not both, and therefore we could not compare a patient’s performance on the 2 modalities. However, the burden of completing the same survey more than once likely would have lowered our participation rate and introduced additional biases we wanted to avoid. Third, despite our attempt to mimic the look of a paper survey, the tablet’s user interface presented several potential difficulties. For example, its small text and small answer buttons may have been limiting for patients with poor vision. These design features emphasize the importance of having a user interface that can be adapted to the individual, regardless of handicap. Indeed, adaptability is a potential strength of digital interfaces. For adaptability, an interface designer can use large, scalable text and add audio prompts and other features.
Our findings can be useful in evaluating patient responsiveness to surveys administered on digital tablets in an outpatient clinic setting. In this prospective, randomized study, we found that, for survey completion, use of a tablet device did not require more time than use of a paper form. In addition, the administration modalities had similar completion and error rates for a variety of orthopedic outcomes surveys. We did not activate digital features that would have given unfair advantage to the digital data collection modality. We also found a strong preference for use of technology in PRO data collection, and this may help improve collection rates. Last, though optimizing the flow of patients in our clinic was not a strict research metric, we prioritized making sure patients were not spending any more time completing these surveys than in the past. Given the potential benefits of digital surveys—immediate and accurate transfer of collected data into multiple databases, including the patient’s electronic medical record—our experience supports continuing validation of these instruments for potential wider use.
Am J Orthop. 2016;45(7):E451-E457. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Higginson IJ, Carr AJ. Measuring quality of life: using quality of life measures in the clinical setting. BMJ. 2001;322(7297):1297-1300.
3. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102-109.
4. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622-629.
5. Paudel D, Ahmed M, Pradhan A, Lal Dangol R. Successful use of tablet personal computers and wireless technologies for the 2011 Nepal Demographic and Health Survey. Glob Heal Sci Pract. 2013;1(2):277-284.
6. Dy CJ, Schmicker T, Tran Q, Chadwick B, Daluiski A. The use of a tablet computer to complete the DASH questionnaire. J Hand Surg Am. 2012;37(12):2589-2594.
7. Aktas A, Hullihen B, Shrotriya S, Thomas S, Walsh D, Estfan B. Connected health: cancer symptom and quality-of-life assessment using a tablet computer: a pilot study. Am J Hosp Palliat Care. 2015;32(2):189-197.
8. Basnov M, Kongsved SM, Bech P, Hjollund NH. Reliability of Short Form-36 in an internet- and a pen-and-paper version. Inform Health Soc Care. 2009;34(1):53-58.
9. Bellamy N, Wilson C, Hendrikz J, et al; EDC Study Group. Osteoarthritis Index delivered by mobile phone (m-WOMAC) is valid, reliable, and responsive. J Clin Epidemiol. 2011;64(2):182-190.
10. Fanning J, McAuley E. A comparison of tablet computer and paper-based questionnaires in healthy aging research. JMIR Res Protoc. 2014;3(3):e38.
11. Griffiths-Jones W, Norton MR, Fern ED, Williams DH. The equivalence of remote electronic and paper patient reported outcome (PRO) collection. J Arthroplasty. 2014;29(11):2136-2139.
12. Marsh JD, Bryant DM, Macdonald SJ, Naudie DD. Patients respond similarly to paper and electronic versions of the WOMAC and SF-12 following total joint arthroplasty. J Arthroplasty. 2014;29(4):670-673.
13. Olajos-Clow J, Minard J, Szpiro K, et al. Validation of an electronic version of the Mini Asthma Quality of Life Questionnaire. Respir Med. 2010;104(5):658-667.
14. Shervin N, Dorrwachter J, Bragdon CR, Shervin D, Zurakowski D, Malchau H. Comparison of paper and computer-based questionnaire modes for measuring health outcomes in patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2011;93(3):285-293.
15. Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8-11.
16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
17. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
18. Abdel Messih M, Naylor JM, Descallar J, Manickam A, Mittal R, Harris IA. Mail versus telephone administration of the Oxford Knee and Hip Scores. J Arthroplasty. 2014;29(3):491-494.
19. Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH. Response rate and completeness of questionnaires: a randomized study of internet versus paper-and-pencil versions. J Med Internet Res. 2007;9(3):e25.
20. Theiler R, Bischoff-Ferrari HA, Good M, Bellamy N. Responsiveness of the electronic touch screen WOMAC 3.1 OA Index in a short term clinical trial with rofecoxib. Osteoarthritis Cartilage. 2004;12(11):912-916.
21. Ryan JM, Corry JR, Attewell R, Smithson MJ. A comparison of an electronic version of the SF-36 General Health Questionnaire to the standard paper version. Qual Life Res. 2002;11(1):19-26.
22. Wilson AS, Kitas GD, Carruthers DM, et al. Computerized information-gathering in specialist rheumatology clinics: an initial evaluation of an electronic version of the Short Form 36. Rheumatology. 2002;41(3):268-273.
23. Angst F, Goldhahn J, Drerup S, Flury M, Schwyzer HK, Simmen BR. How sharp is the short QuickDASH? A refined content and validity analysis of the Short Form of the Disabilities of the Shoulder, Arm and Hand questionnaire in the strata of symptoms and function and specific joint conditions. Qual Life Res. 2009;18(8):1043-1051.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Higginson IJ, Carr AJ. Measuring quality of life: using quality of life measures in the clinical setting. BMJ. 2001;322(7297):1297-1300.
3. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102-109.
4. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622-629.
5. Paudel D, Ahmed M, Pradhan A, Lal Dangol R. Successful use of tablet personal computers and wireless technologies for the 2011 Nepal Demographic and Health Survey. Glob Heal Sci Pract. 2013;1(2):277-284.
6. Dy CJ, Schmicker T, Tran Q, Chadwick B, Daluiski A. The use of a tablet computer to complete the DASH questionnaire. J Hand Surg Am. 2012;37(12):2589-2594.
7. Aktas A, Hullihen B, Shrotriya S, Thomas S, Walsh D, Estfan B. Connected health: cancer symptom and quality-of-life assessment using a tablet computer: a pilot study. Am J Hosp Palliat Care. 2015;32(2):189-197.
8. Basnov M, Kongsved SM, Bech P, Hjollund NH. Reliability of Short Form-36 in an internet- and a pen-and-paper version. Inform Health Soc Care. 2009;34(1):53-58.
9. Bellamy N, Wilson C, Hendrikz J, et al; EDC Study Group. Osteoarthritis Index delivered by mobile phone (m-WOMAC) is valid, reliable, and responsive. J Clin Epidemiol. 2011;64(2):182-190.
10. Fanning J, McAuley E. A comparison of tablet computer and paper-based questionnaires in healthy aging research. JMIR Res Protoc. 2014;3(3):e38.
11. Griffiths-Jones W, Norton MR, Fern ED, Williams DH. The equivalence of remote electronic and paper patient reported outcome (PRO) collection. J Arthroplasty. 2014;29(11):2136-2139.
12. Marsh JD, Bryant DM, Macdonald SJ, Naudie DD. Patients respond similarly to paper and electronic versions of the WOMAC and SF-12 following total joint arthroplasty. J Arthroplasty. 2014;29(4):670-673.
13. Olajos-Clow J, Minard J, Szpiro K, et al. Validation of an electronic version of the Mini Asthma Quality of Life Questionnaire. Respir Med. 2010;104(5):658-667.
14. Shervin N, Dorrwachter J, Bragdon CR, Shervin D, Zurakowski D, Malchau H. Comparison of paper and computer-based questionnaire modes for measuring health outcomes in patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2011;93(3):285-293.
15. Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8-11.
16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
17. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
18. Abdel Messih M, Naylor JM, Descallar J, Manickam A, Mittal R, Harris IA. Mail versus telephone administration of the Oxford Knee and Hip Scores. J Arthroplasty. 2014;29(3):491-494.
19. Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH. Response rate and completeness of questionnaires: a randomized study of internet versus paper-and-pencil versions. J Med Internet Res. 2007;9(3):e25.
20. Theiler R, Bischoff-Ferrari HA, Good M, Bellamy N. Responsiveness of the electronic touch screen WOMAC 3.1 OA Index in a short term clinical trial with rofecoxib. Osteoarthritis Cartilage. 2004;12(11):912-916.
21. Ryan JM, Corry JR, Attewell R, Smithson MJ. A comparison of an electronic version of the SF-36 General Health Questionnaire to the standard paper version. Qual Life Res. 2002;11(1):19-26.
22. Wilson AS, Kitas GD, Carruthers DM, et al. Computerized information-gathering in specialist rheumatology clinics: an initial evaluation of an electronic version of the Short Form 36. Rheumatology. 2002;41(3):268-273.
23. Angst F, Goldhahn J, Drerup S, Flury M, Schwyzer HK, Simmen BR. How sharp is the short QuickDASH? A refined content and validity analysis of the Short Form of the Disabilities of the Shoulder, Arm and Hand questionnaire in the strata of symptoms and function and specific joint conditions. Qual Life Res. 2009;18(8):1043-1051.
Fourth approved indication for ofatumumab in chronic lymphocytic leukemia
The recent decision by the US Food and Drug Administration to approve ofatumumab in combination with fludarabine and cyclophosphamide in relapsed disease marks a fourth approved indication for this drug in patients with chronic lymphocytic leukemia (CLL). Ofatumumab is a fully human monoclonal antibody that targets the CD20 protein on the surface of B cells, first approved for the treatment of CLL back in 2009.
Click on the PDF icon at the top of this introduction to read the full article.
The recent decision by the US Food and Drug Administration to approve ofatumumab in combination with fludarabine and cyclophosphamide in relapsed disease marks a fourth approved indication for this drug in patients with chronic lymphocytic leukemia (CLL). Ofatumumab is a fully human monoclonal antibody that targets the CD20 protein on the surface of B cells, first approved for the treatment of CLL back in 2009.
Click on the PDF icon at the top of this introduction to read the full article.
The recent decision by the US Food and Drug Administration to approve ofatumumab in combination with fludarabine and cyclophosphamide in relapsed disease marks a fourth approved indication for this drug in patients with chronic lymphocytic leukemia (CLL). Ofatumumab is a fully human monoclonal antibody that targets the CD20 protein on the surface of B cells, first approved for the treatment of CLL back in 2009.
Click on the PDF icon at the top of this introduction to read the full article.
Expanding treatment options and ongoing challenges for urologic cancers
Urologic cancers are those that form in organs of the urinary and male reproductive systems, the most significant among them being cancers of the bladder, kidney, prostate, and testicles. Collectively, they are diagnosed in close to 400,000 Americans each year and are responsible for almost 60,000 deaths annually.1 Here, we describe the most recent developments in treating these malignancies.
Update/related article
Atezolizumab approval marks first new treatment option for bladder cancer in more than 3 decades
Click on the PDF icon at the top of this introduction to read the full article.
Urologic cancers are those that form in organs of the urinary and male reproductive systems, the most significant among them being cancers of the bladder, kidney, prostate, and testicles. Collectively, they are diagnosed in close to 400,000 Americans each year and are responsible for almost 60,000 deaths annually.1 Here, we describe the most recent developments in treating these malignancies.
Update/related article
Atezolizumab approval marks first new treatment option for bladder cancer in more than 3 decades
Click on the PDF icon at the top of this introduction to read the full article.
Urologic cancers are those that form in organs of the urinary and male reproductive systems, the most significant among them being cancers of the bladder, kidney, prostate, and testicles. Collectively, they are diagnosed in close to 400,000 Americans each year and are responsible for almost 60,000 deaths annually.1 Here, we describe the most recent developments in treating these malignancies.
Update/related article
Atezolizumab approval marks first new treatment option for bladder cancer in more than 3 decades
Click on the PDF icon at the top of this introduction to read the full article.
Renal cell carcinoma approval adds another notch to cabozantinib’s belt
In April this year, the US Food and Drug Administration awarded regulatory approval to cabozantinib for the treatment of advanced renal cell carcinoma patients previously treated with anti-angiogenic therapy.1 The small-molecule inhibitor, which targets multiple kinases, including the vascular endothelial growth factor receptors (VEGFRs) and the hepatocyte growth factor receptor (MET), had previously been approved for the treatment of medullary thyroid carcinoma in 2012.
Click on the PDF icon below for the full article.
In April this year, the US Food and Drug Administration awarded regulatory approval to cabozantinib for the treatment of advanced renal cell carcinoma patients previously treated with anti-angiogenic therapy.1 The small-molecule inhibitor, which targets multiple kinases, including the vascular endothelial growth factor receptors (VEGFRs) and the hepatocyte growth factor receptor (MET), had previously been approved for the treatment of medullary thyroid carcinoma in 2012.
Click on the PDF icon below for the full article.
In April this year, the US Food and Drug Administration awarded regulatory approval to cabozantinib for the treatment of advanced renal cell carcinoma patients previously treated with anti-angiogenic therapy.1 The small-molecule inhibitor, which targets multiple kinases, including the vascular endothelial growth factor receptors (VEGFRs) and the hepatocyte growth factor receptor (MET), had previously been approved for the treatment of medullary thyroid carcinoma in 2012.
Click on the PDF icon below for the full article.
Incidence of and Risk Factors for Symptomatic Venous Thromboembolism After Shoulder Arthroplasty
Venous thromboembolism (VTE) after shoulder arthroplasty (SA) is relatively uncommon. Reported rates of VTE development are highly variable, ranging from 0.2% to 13% (pulmonary embolism [PE], 0.2%-10.8%; deep venous thrombosis [DVT], 0.1%-13%).1-4 Sources of this variability include different methods of capturing cases (small clinical series vs large database studies, which capture mainly hospital readmissions), differences in defining or detecting VTE, and different patient populations (fracture vs osteoarthritis).1-3 Most studies have also tried to identify factors associated with increased risk for VTE. Risk factors associated with development of VTE after SA include history of VTE, advanced age, prolonged operating room time, higher body mass index (BMI), trauma, history of cancer, female sex, and raised Charlson Comorbidity Index (CCI).1-7 Limitations of clinical series include the smaller number of reporting institutions—a potential source of bias given regional variability.1,3,4,7 Limitations of large state or national databases include capturing only events coded during inpatient admission and capturing readmissions for complications at the same institution. This underreporting may lead to very conservative estimates of VTE incidence.2,5,6,8
In this study, we retrospectively identified all the SAs performed at a single institution over a 13-year period and evaluated the cases for development of VTE (DVT, PE). We hypothesized that the VTE rate would be lower than the very high rates reported by Hoxie and colleagues1 and Willis and colleagues4 but higher than those reported for large state or national databases.2,3 We also evaluated clotting risk factors, including many never analyzed before.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we searched our database for all SAs performed at our institution between January 1999 and May 2012 and identified cases in which symptomatic VTE developed within the first 90 days after surgery. Charts were reviewed for information on medical history, surgical procedure, and in-hospital and out-of-hospital care within the 90-day postoperative period. We recorded data on symptomatic VTE (DVT, PE) as documented by lower or upper extremity duplex ultrasonography (US) or chest computed tomography (CT) angiography. There had been no routine screening of patients; duplex US or CT angiography was performed only if a patient was clinically symptomatic (leg swelling, leg pain, shortness of breath, tachycardia, chest pain) for a potential DVT or PE. For a patient who had repeat SAs on the same shoulder or bilateral SAs at different times, only the first procedure was included in the analysis. Arthroplasties performed for fracture were excluded.
Study data were collected and managed with REDCap (Research Electronic Data Capture) tools hosted at the University of Utah School of Medicine.9 Continuous and discrete data collected on medical history and postoperative course included BMI, age at surgery, preoperative hemoglobin (Hb) and hematocrit (Hct) levels, days in hospital, days until out of bed and days until ambulation (both documented in nursing and physical therapy notes), postoperative Hb and Hct levels, and CCI. Categorical data included sex, diagnosis (primary osteoarthritis, rotator cuff arthropathy, rheumatoid arthritis, failed hemiarthroplasty [HA], failed total SA [TSA], others), attending surgeon, procedure (TSA, HA, reverse TSA, revision SA), anesthesia (general endotracheal anesthesia [GETA] alone, interscalene nerve block alone, GETA plus block), prophylactic use of aspirin after surgery, presence of various medical comorbidities (diabetes, hypertension, cardiac disease, clotting disorders, cancer), hormone replacement therapy, family history of a clotting disorder, and VTE consequences (cardiac events, death).
Statistical Analysis
Descriptive statistics were calculated to summarize aspects of the surgical procedures, the study cohort’s demographics and medical histories, and the incidence of VTE. Logistic regression analysis was performed to explore the association between development of VTE (DVT, PE) and potential risk factors. Unadjusted odds ratios (ORs) were estimated for the risk factors of age, BMI, revision SA, CCI, prophylactic use of aspirin after surgery, preoperative history of VTE, preoperative and postoperative Hb and Hct levels, diabetes, anesthesia (GETA with and without interscalene nerve block), family history of a clotting disorder, days until out of bed, hormone replacement therapy, race, discharge home or to rehabilitation, distance traveled for surgery, hypertension, cardiac disease, cement use, and history of cancer. In addition, ORs were adjusted for age, BMI, and revision SA. For all statistical tests, significance was set at P < .05. All analyses were performed with SAS Version 9.3 (SAS Institute).
Results
We identified 533 SAs: 245 anatomical TSAs, 112 reverse TSAs, 92 HAs, and 84 revision SAs. Three different surgeons performed the procedures, and no patients were lost to follow-up within the first 90 days after surgery. Although SAs were performed for various diagnoses, more than 50% (274) of the SAs were for primary osteoarthritis; 97 were performed for rotator cuff arthropathy, 16 for rheumatoid arthritis, 43 for failed HA, 23 for failed TSA, and 79 for other diagnoses.
Of the 533 patients, 288 were female and 245 were male. Mean age at surgery was 65.2 years (range, 16-93 years). Mean (SD) BMI was 29.2 (6.4) kg/m2. Mean (SD) preoperative Hb level was 13.7 (1.8) g/dL, and mean preoperative Hct level was 40.1% (4.8%). Mean (SD) length of hospital stay was 2.6 (1.5) days. Mean (SD) time before patients were out of bed was 1.1 (0.7) days. On postoperative day 1, mean Hb level was 11.1 (1.7) g/dL, and mean (SD) Hct level was 33.2% (4.8%). Mean (SD) CCI was 1.1 (0.9).
Anesthesia for the 533 patients consisted of GETA (209 patients, 39.0%), interscalene nerve block (2, 0.4%), or GETA with nerve block (314, 59.0%). After surgery, 125 patients (24.3%) received aspirin as prophylaxis. Diabetes was reported by 83 patients, hypertension by 286, cardiac disease by 74, a history of a clotting disorder by 2, a family history of a clotting disorder by 8, ongoing cancer by 4, a history of cancer by 67, and hormone replacement therapy by 104.
For the entire cohort of 533 patients, the symptomatic VTE rate was 2.6% (14 patients), the DVT rate was 0.9% (5), and the PE rate was 2.3% (12). Although VTE did not cause any deaths, there were 3 cardiac events.
Discussion
VTE after SA is rare. We report an overall VTE incidence of 2.6%, with DVT at 0.9% and PE at 2.3%. These rates are similar to those reported in clinical series and significantly higher than those reported for large institutional or national databases.2-7 Our results also support a previously reported trend: The ratio of PE to DVT for SA is significantly higher than historically reported ratios for lower extremity arthroplasty.2,6-8 We have identified many VTE risk factors: raised CCI, preoperative thrombotic event, lower preoperative Hb and Hct levels, lower postoperative Hb level, diabetes, use of GETA without interscalene nerve block, higher BMI, and revision SA. Results of other studies support 3 findings (higher BMI, raised CCI, preoperative thrombotic event); new findings include correlation with Hb and Hct levels, diabetes, type of anesthesia, and revision SA.6,7 Identification of these other factors may be useful in making treatment decisions in patients symptomatic after SA and in lowering the threshold for performing diagnostic tests in these patients at risk for VTE.
Reported rates of VTE after SA are highly variable, ranging from 0.2% to 13%.10 Our rationale for investigating VTE rates at a single institution was to estimate the rates that can be expected in a university-based practice and to determine whether these rates are high enough to warrant routine thromboprophylaxis. The rate variability seems to result in part from variability in the data sources. Most studies that have reported very low VTE rates typically used large state or national databases, which likely were subject to underreporting.
Lyman and colleagues6 found 0.5% DVT and 0.2% PE rates in a New York state hospital database, but only in-hospital immediate postoperative symptomatic complications were included; slightly delayed complications may have been missed. Farng and colleagues5 reported a 0.6% VTE rate, but only inpatient (immediate postoperative or readmission) events were included; all outpatient events were missed. Jameson and colleagues,2 using a national database that included only cases involving inpatient treatment, reported 0% DVT and 0.2% PE rates, again missing outpatient events, and relying on appropriate coding to capture events. Using electronic health records from a large healthcare system, Navarro and colleagues8 queried for VTE cases and reported 0.5% DVT and 0.5% PE rates. The inclusiveness of their data source for the outcome of interest was potentially improved relative to national or statewide databases—and the resulting data reported in their study should reflect that improvement. However, the authors relied on ICD–9 (International Classification of Diseases, Ninth Revision) coding to screen for VTE events and excluded patients with prior VTE, preoperative prophylaxis (enoxaparin or warfarin), or follow-up of <90 days. As patients with prior VTE are those most at risk (present study OR, 6-7), excluding them significantly reduces the overall incidence of clotting reported.
Only 4 studies specifically used information drawn directly from physicians’ clinic notes, vs data retrieved (using code-based queries) from databases.1,3,4,7 These studies may provide a better representation of the rate of VTE after SA, as they were not reliant on codes, included both inpatient and outpatient events, and were inclusive of outpatient follow-up of at least 3 months.
Three of the 4 studies used the Mayo Clinic Total Joint Registry.1,3,4 Hoxie and colleagues1 reported an 11% rate of PE after HA performed for fracture (we excluded SA for fracture). As several other investigators have reported an association between trauma and increased risk for VTE, postoperative anticoagulation should be considered in this patient population (though it was not the focus of the present study).6-8 Sperling and Cofield3 and Singh and colleagues7 reported on the risk for PE among SA patients at the Mayo Clinic. Sperling and Cofield3 included only those events that occurred within the first 7 days after surgery; Singh and colleagues7 included events out to 90 days after surgery. Sperling and Cofield3 reported a 0.17% PE rate; Singh and colleagues7 reported 0.6% PE and 0.1% DVT rates. Sperling and Cofield3 reported on 2885 SAs; Singh and colleagues7 reported on 4019 SAs from the same database. As it is unclear whether these 2 studies had complete information on all patients, underreporting may be an issue. Information was obtained through “clinic visits, medical records and/or standardized mailed and telephone-administered questionnaires.”7The fourth study, a prospective study of 100 patients by Willis and colleagues,4 had the best data on development of symptomatic PE after SA. The authors reported a 2% PE rate and a high (13%) DVT rate. Because US was not performed before the surgical procedures, the number of patients with new and existing DVT cases could not be determined. However, all PEs were new, and the 2% rate found there is similar to the 2.3% in our study. Therefore, we think these rates capture the data most accurately and avoid the underreporting that marks large databases.4Studies have identified various factors that increase the risk for VTE after SA. Singh and colleagues7 identified the risk factors of age over 70 years, female sex, higher BMI (25-29.9 kg/m2), CCI above 1, traumatic etiology, prior history of VTE, and HA. However, their use of univariate regression analysis may have confounded the effects—one factor may have become a surrogate for another (ie, trauma and HA, as most fractures treated with SA during the study period were treated with HA). Lyman and colleagues6 also found advanced age and trauma were associated with higher VTE risk, and reported prior history of cancer as a risk factor as well. Navarro and colleagues8 identified trauma as a risk factor, as in the other 2 studies.6,7 Our data support prior history of VTE, higher BMI, and raised CCI as increasing the risk for VTE.
Other factors identified in the present study are use of GETA without interscalene nerve block, lower preoperative and postoperative Hb levels, diabetes, and revision SA. Because of the limited number of events, only ORs with and without limited control of confounders were performed. Just as in the study by Singh and colleagues,7 uncontrolled confounding could have occurred. A nerve block may be protective, as less postoperative pain may allow patients quicker mobilization and therapy. Diabetes may be a surrogate for other medical comorbidities, as reflected by the higher overall risk with raised CCI. Lower preoperative and postoperative Hb levels were associated with clotting and may be representative of patients with poorer overall health and more complicated surgical procedures (eg, revision SA). In an earlier study, we found increased risk for transfusions in revision SA relative to primary SA.11 Lower preoperative Hb level correlated with development of VTE after lower extremity arthroplasty.12 Postoperative use of aspirin was not found to significantly reduce the incidence of clotting, though this finding may have resulted from lack of power. Therefore, from the present data, there is nothing to conclude about the efficacy of aspirin in preventing thrombosis.
Our findings can be placed in the context of the Virchow triad. Specifically, 3 categories of factors are thought to contribute to thrombosis: hypercoagulability, hemodynamic stasis, and endothelial injury. In grouping factors, we identified prior thrombotic event and obesity as increasing hypercoagulability; revision SA, more comorbidities, lower Hb and Hct levels, diabetes, and GETA as increasing hemodynamic stasis; and revision SA (longer operating room times) as leading to stasis. More comorbidities can be associated with delayed postoperative ambulation, and diabetes and lower Hb and Hct levels can be surrogates for more comorbidities. Surgery performed with the patient under GETA without interscalene nerve block can lead to higher levels of pain and less early mobility.
The present findings have made us more aware of patients at risk for VTE, and we have lowered our threshold for evaluating them for potential clots. Before this study, we used warfarin or enoxaparin for anticoagulation in patients with a history of VTE or active cancer. We are continuing this protocol, but not with other patients. Patients with many comorbidities, lower preoperative Hb level, revision SA, high BMI, or diabetes are carefully monitored for clots early in the postoperative course. Our new threshold for these high-risk patients is to order diagnostic testing, including duplex US or CT angiography. Now, even mild oxygen requirements or mild tachycardia within postoperative week 1 typically prompt a study in these patients. We hope this increased awareness will limit the potential negative consequences associated with development of VTE. Given the present data, we do not think the simple presence of increased comorbidities, lower preoperative Hb, revision SA, high BMI or diabetes should rule out performing SA; rather, it should increase surgeons’ postoperative vigilance in evaluating for potential clots.
Limitations of our study include its retrospective nature and reliance on clinic chart review. Patients were not directly questioned about venous thrombus at follow-up, so all events may not have been captured. Although retrospective review has its drawbacks, it allows for accurate identification of events, even uncoded events. Therefore, more events are likely to be captured with this technique than with large database analyses using only coding information. We tried to identify as many cases as possible by reviewing all outpatient records (orthopedic, nonorthopedic), inpatient records, radiologic studies, and scanned outside records. Another limitation is that having a small number of VTE events limited our ability to perform a multivariate analysis, and uncontrolled confounding likely resulted. Only a very large multi-institutional study can capture enough events to allow a multivariate analysis. A third limitation is that the small number of events may have underpowered the study. Having more patients would have allowed other potential factors to be identified as being significantly associated with VTE. Last, as the study captured only symptomatic VTE events, it may have underreported VTE events. Given our complete review of the medical records, however, most clinically significant events likely were captured.
Conclusion
VTE after SA is rare. In our single-institution study, the symptomatic DVT rate was 0.9%, and the symptomatic PE rate was 2.3%. Risk factors associated with clotting included prior VTE, higher BMI, lower preoperative and postoperative Hb levels, raised CCI, diabetes, use of GETA without interscalene nerve block, and revision SA. Risk factors can be used to identify patients who may benefit from a more scrutinized postoperative evaluation and from increased surgeon awareness of the potential for VTE development. Rates of VTE can be used to counsel SA patients regarding overall surgical risks.
Am J Orthop. 2016;45(6):E379-E385. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism after operative treatment of proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(6):782-783.
2. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):764-770.
3. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
4. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
5. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
6. Lyman S, Sherman S, Carter TI, Bach PB, Mandl LA, Marx RG. Prevalence and risk factors for symptomatic thromboembolic events after shoulder arthroplasty. Clin Orthop Relat Res. 2006;(448):152-156.
7. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
8. Navarro RA, Inacio MC, Burke MF, Costouros JG, Yian EH. Risk of thromboembolism in shoulder arthroplasty: effect of implant type and traumatic indication. Clin Orthop Relat Res. 2013;471(5):1576-1581.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. Saleh HE, Pennings AL, ElMaraghy AW. Venous thromboembolism after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2013;22(10):1440-1448.
11. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
12. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
Venous thromboembolism (VTE) after shoulder arthroplasty (SA) is relatively uncommon. Reported rates of VTE development are highly variable, ranging from 0.2% to 13% (pulmonary embolism [PE], 0.2%-10.8%; deep venous thrombosis [DVT], 0.1%-13%).1-4 Sources of this variability include different methods of capturing cases (small clinical series vs large database studies, which capture mainly hospital readmissions), differences in defining or detecting VTE, and different patient populations (fracture vs osteoarthritis).1-3 Most studies have also tried to identify factors associated with increased risk for VTE. Risk factors associated with development of VTE after SA include history of VTE, advanced age, prolonged operating room time, higher body mass index (BMI), trauma, history of cancer, female sex, and raised Charlson Comorbidity Index (CCI).1-7 Limitations of clinical series include the smaller number of reporting institutions—a potential source of bias given regional variability.1,3,4,7 Limitations of large state or national databases include capturing only events coded during inpatient admission and capturing readmissions for complications at the same institution. This underreporting may lead to very conservative estimates of VTE incidence.2,5,6,8
In this study, we retrospectively identified all the SAs performed at a single institution over a 13-year period and evaluated the cases for development of VTE (DVT, PE). We hypothesized that the VTE rate would be lower than the very high rates reported by Hoxie and colleagues1 and Willis and colleagues4 but higher than those reported for large state or national databases.2,3 We also evaluated clotting risk factors, including many never analyzed before.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we searched our database for all SAs performed at our institution between January 1999 and May 2012 and identified cases in which symptomatic VTE developed within the first 90 days after surgery. Charts were reviewed for information on medical history, surgical procedure, and in-hospital and out-of-hospital care within the 90-day postoperative period. We recorded data on symptomatic VTE (DVT, PE) as documented by lower or upper extremity duplex ultrasonography (US) or chest computed tomography (CT) angiography. There had been no routine screening of patients; duplex US or CT angiography was performed only if a patient was clinically symptomatic (leg swelling, leg pain, shortness of breath, tachycardia, chest pain) for a potential DVT or PE. For a patient who had repeat SAs on the same shoulder or bilateral SAs at different times, only the first procedure was included in the analysis. Arthroplasties performed for fracture were excluded.
Study data were collected and managed with REDCap (Research Electronic Data Capture) tools hosted at the University of Utah School of Medicine.9 Continuous and discrete data collected on medical history and postoperative course included BMI, age at surgery, preoperative hemoglobin (Hb) and hematocrit (Hct) levels, days in hospital, days until out of bed and days until ambulation (both documented in nursing and physical therapy notes), postoperative Hb and Hct levels, and CCI. Categorical data included sex, diagnosis (primary osteoarthritis, rotator cuff arthropathy, rheumatoid arthritis, failed hemiarthroplasty [HA], failed total SA [TSA], others), attending surgeon, procedure (TSA, HA, reverse TSA, revision SA), anesthesia (general endotracheal anesthesia [GETA] alone, interscalene nerve block alone, GETA plus block), prophylactic use of aspirin after surgery, presence of various medical comorbidities (diabetes, hypertension, cardiac disease, clotting disorders, cancer), hormone replacement therapy, family history of a clotting disorder, and VTE consequences (cardiac events, death).
Statistical Analysis
Descriptive statistics were calculated to summarize aspects of the surgical procedures, the study cohort’s demographics and medical histories, and the incidence of VTE. Logistic regression analysis was performed to explore the association between development of VTE (DVT, PE) and potential risk factors. Unadjusted odds ratios (ORs) were estimated for the risk factors of age, BMI, revision SA, CCI, prophylactic use of aspirin after surgery, preoperative history of VTE, preoperative and postoperative Hb and Hct levels, diabetes, anesthesia (GETA with and without interscalene nerve block), family history of a clotting disorder, days until out of bed, hormone replacement therapy, race, discharge home or to rehabilitation, distance traveled for surgery, hypertension, cardiac disease, cement use, and history of cancer. In addition, ORs were adjusted for age, BMI, and revision SA. For all statistical tests, significance was set at P < .05. All analyses were performed with SAS Version 9.3 (SAS Institute).
Results
We identified 533 SAs: 245 anatomical TSAs, 112 reverse TSAs, 92 HAs, and 84 revision SAs. Three different surgeons performed the procedures, and no patients were lost to follow-up within the first 90 days after surgery. Although SAs were performed for various diagnoses, more than 50% (274) of the SAs were for primary osteoarthritis; 97 were performed for rotator cuff arthropathy, 16 for rheumatoid arthritis, 43 for failed HA, 23 for failed TSA, and 79 for other diagnoses.
Of the 533 patients, 288 were female and 245 were male. Mean age at surgery was 65.2 years (range, 16-93 years). Mean (SD) BMI was 29.2 (6.4) kg/m2. Mean (SD) preoperative Hb level was 13.7 (1.8) g/dL, and mean preoperative Hct level was 40.1% (4.8%). Mean (SD) length of hospital stay was 2.6 (1.5) days. Mean (SD) time before patients were out of bed was 1.1 (0.7) days. On postoperative day 1, mean Hb level was 11.1 (1.7) g/dL, and mean (SD) Hct level was 33.2% (4.8%). Mean (SD) CCI was 1.1 (0.9).
Anesthesia for the 533 patients consisted of GETA (209 patients, 39.0%), interscalene nerve block (2, 0.4%), or GETA with nerve block (314, 59.0%). After surgery, 125 patients (24.3%) received aspirin as prophylaxis. Diabetes was reported by 83 patients, hypertension by 286, cardiac disease by 74, a history of a clotting disorder by 2, a family history of a clotting disorder by 8, ongoing cancer by 4, a history of cancer by 67, and hormone replacement therapy by 104.
For the entire cohort of 533 patients, the symptomatic VTE rate was 2.6% (14 patients), the DVT rate was 0.9% (5), and the PE rate was 2.3% (12). Although VTE did not cause any deaths, there were 3 cardiac events.
Discussion
VTE after SA is rare. We report an overall VTE incidence of 2.6%, with DVT at 0.9% and PE at 2.3%. These rates are similar to those reported in clinical series and significantly higher than those reported for large institutional or national databases.2-7 Our results also support a previously reported trend: The ratio of PE to DVT for SA is significantly higher than historically reported ratios for lower extremity arthroplasty.2,6-8 We have identified many VTE risk factors: raised CCI, preoperative thrombotic event, lower preoperative Hb and Hct levels, lower postoperative Hb level, diabetes, use of GETA without interscalene nerve block, higher BMI, and revision SA. Results of other studies support 3 findings (higher BMI, raised CCI, preoperative thrombotic event); new findings include correlation with Hb and Hct levels, diabetes, type of anesthesia, and revision SA.6,7 Identification of these other factors may be useful in making treatment decisions in patients symptomatic after SA and in lowering the threshold for performing diagnostic tests in these patients at risk for VTE.
Reported rates of VTE after SA are highly variable, ranging from 0.2% to 13%.10 Our rationale for investigating VTE rates at a single institution was to estimate the rates that can be expected in a university-based practice and to determine whether these rates are high enough to warrant routine thromboprophylaxis. The rate variability seems to result in part from variability in the data sources. Most studies that have reported very low VTE rates typically used large state or national databases, which likely were subject to underreporting.
Lyman and colleagues6 found 0.5% DVT and 0.2% PE rates in a New York state hospital database, but only in-hospital immediate postoperative symptomatic complications were included; slightly delayed complications may have been missed. Farng and colleagues5 reported a 0.6% VTE rate, but only inpatient (immediate postoperative or readmission) events were included; all outpatient events were missed. Jameson and colleagues,2 using a national database that included only cases involving inpatient treatment, reported 0% DVT and 0.2% PE rates, again missing outpatient events, and relying on appropriate coding to capture events. Using electronic health records from a large healthcare system, Navarro and colleagues8 queried for VTE cases and reported 0.5% DVT and 0.5% PE rates. The inclusiveness of their data source for the outcome of interest was potentially improved relative to national or statewide databases—and the resulting data reported in their study should reflect that improvement. However, the authors relied on ICD–9 (International Classification of Diseases, Ninth Revision) coding to screen for VTE events and excluded patients with prior VTE, preoperative prophylaxis (enoxaparin or warfarin), or follow-up of <90 days. As patients with prior VTE are those most at risk (present study OR, 6-7), excluding them significantly reduces the overall incidence of clotting reported.
Only 4 studies specifically used information drawn directly from physicians’ clinic notes, vs data retrieved (using code-based queries) from databases.1,3,4,7 These studies may provide a better representation of the rate of VTE after SA, as they were not reliant on codes, included both inpatient and outpatient events, and were inclusive of outpatient follow-up of at least 3 months.
Three of the 4 studies used the Mayo Clinic Total Joint Registry.1,3,4 Hoxie and colleagues1 reported an 11% rate of PE after HA performed for fracture (we excluded SA for fracture). As several other investigators have reported an association between trauma and increased risk for VTE, postoperative anticoagulation should be considered in this patient population (though it was not the focus of the present study).6-8 Sperling and Cofield3 and Singh and colleagues7 reported on the risk for PE among SA patients at the Mayo Clinic. Sperling and Cofield3 included only those events that occurred within the first 7 days after surgery; Singh and colleagues7 included events out to 90 days after surgery. Sperling and Cofield3 reported a 0.17% PE rate; Singh and colleagues7 reported 0.6% PE and 0.1% DVT rates. Sperling and Cofield3 reported on 2885 SAs; Singh and colleagues7 reported on 4019 SAs from the same database. As it is unclear whether these 2 studies had complete information on all patients, underreporting may be an issue. Information was obtained through “clinic visits, medical records and/or standardized mailed and telephone-administered questionnaires.”7The fourth study, a prospective study of 100 patients by Willis and colleagues,4 had the best data on development of symptomatic PE after SA. The authors reported a 2% PE rate and a high (13%) DVT rate. Because US was not performed before the surgical procedures, the number of patients with new and existing DVT cases could not be determined. However, all PEs were new, and the 2% rate found there is similar to the 2.3% in our study. Therefore, we think these rates capture the data most accurately and avoid the underreporting that marks large databases.4Studies have identified various factors that increase the risk for VTE after SA. Singh and colleagues7 identified the risk factors of age over 70 years, female sex, higher BMI (25-29.9 kg/m2), CCI above 1, traumatic etiology, prior history of VTE, and HA. However, their use of univariate regression analysis may have confounded the effects—one factor may have become a surrogate for another (ie, trauma and HA, as most fractures treated with SA during the study period were treated with HA). Lyman and colleagues6 also found advanced age and trauma were associated with higher VTE risk, and reported prior history of cancer as a risk factor as well. Navarro and colleagues8 identified trauma as a risk factor, as in the other 2 studies.6,7 Our data support prior history of VTE, higher BMI, and raised CCI as increasing the risk for VTE.
Other factors identified in the present study are use of GETA without interscalene nerve block, lower preoperative and postoperative Hb levels, diabetes, and revision SA. Because of the limited number of events, only ORs with and without limited control of confounders were performed. Just as in the study by Singh and colleagues,7 uncontrolled confounding could have occurred. A nerve block may be protective, as less postoperative pain may allow patients quicker mobilization and therapy. Diabetes may be a surrogate for other medical comorbidities, as reflected by the higher overall risk with raised CCI. Lower preoperative and postoperative Hb levels were associated with clotting and may be representative of patients with poorer overall health and more complicated surgical procedures (eg, revision SA). In an earlier study, we found increased risk for transfusions in revision SA relative to primary SA.11 Lower preoperative Hb level correlated with development of VTE after lower extremity arthroplasty.12 Postoperative use of aspirin was not found to significantly reduce the incidence of clotting, though this finding may have resulted from lack of power. Therefore, from the present data, there is nothing to conclude about the efficacy of aspirin in preventing thrombosis.
Our findings can be placed in the context of the Virchow triad. Specifically, 3 categories of factors are thought to contribute to thrombosis: hypercoagulability, hemodynamic stasis, and endothelial injury. In grouping factors, we identified prior thrombotic event and obesity as increasing hypercoagulability; revision SA, more comorbidities, lower Hb and Hct levels, diabetes, and GETA as increasing hemodynamic stasis; and revision SA (longer operating room times) as leading to stasis. More comorbidities can be associated with delayed postoperative ambulation, and diabetes and lower Hb and Hct levels can be surrogates for more comorbidities. Surgery performed with the patient under GETA without interscalene nerve block can lead to higher levels of pain and less early mobility.
The present findings have made us more aware of patients at risk for VTE, and we have lowered our threshold for evaluating them for potential clots. Before this study, we used warfarin or enoxaparin for anticoagulation in patients with a history of VTE or active cancer. We are continuing this protocol, but not with other patients. Patients with many comorbidities, lower preoperative Hb level, revision SA, high BMI, or diabetes are carefully monitored for clots early in the postoperative course. Our new threshold for these high-risk patients is to order diagnostic testing, including duplex US or CT angiography. Now, even mild oxygen requirements or mild tachycardia within postoperative week 1 typically prompt a study in these patients. We hope this increased awareness will limit the potential negative consequences associated with development of VTE. Given the present data, we do not think the simple presence of increased comorbidities, lower preoperative Hb, revision SA, high BMI or diabetes should rule out performing SA; rather, it should increase surgeons’ postoperative vigilance in evaluating for potential clots.
Limitations of our study include its retrospective nature and reliance on clinic chart review. Patients were not directly questioned about venous thrombus at follow-up, so all events may not have been captured. Although retrospective review has its drawbacks, it allows for accurate identification of events, even uncoded events. Therefore, more events are likely to be captured with this technique than with large database analyses using only coding information. We tried to identify as many cases as possible by reviewing all outpatient records (orthopedic, nonorthopedic), inpatient records, radiologic studies, and scanned outside records. Another limitation is that having a small number of VTE events limited our ability to perform a multivariate analysis, and uncontrolled confounding likely resulted. Only a very large multi-institutional study can capture enough events to allow a multivariate analysis. A third limitation is that the small number of events may have underpowered the study. Having more patients would have allowed other potential factors to be identified as being significantly associated with VTE. Last, as the study captured only symptomatic VTE events, it may have underreported VTE events. Given our complete review of the medical records, however, most clinically significant events likely were captured.
Conclusion
VTE after SA is rare. In our single-institution study, the symptomatic DVT rate was 0.9%, and the symptomatic PE rate was 2.3%. Risk factors associated with clotting included prior VTE, higher BMI, lower preoperative and postoperative Hb levels, raised CCI, diabetes, use of GETA without interscalene nerve block, and revision SA. Risk factors can be used to identify patients who may benefit from a more scrutinized postoperative evaluation and from increased surgeon awareness of the potential for VTE development. Rates of VTE can be used to counsel SA patients regarding overall surgical risks.
Am J Orthop. 2016;45(6):E379-E385. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Venous thromboembolism (VTE) after shoulder arthroplasty (SA) is relatively uncommon. Reported rates of VTE development are highly variable, ranging from 0.2% to 13% (pulmonary embolism [PE], 0.2%-10.8%; deep venous thrombosis [DVT], 0.1%-13%).1-4 Sources of this variability include different methods of capturing cases (small clinical series vs large database studies, which capture mainly hospital readmissions), differences in defining or detecting VTE, and different patient populations (fracture vs osteoarthritis).1-3 Most studies have also tried to identify factors associated with increased risk for VTE. Risk factors associated with development of VTE after SA include history of VTE, advanced age, prolonged operating room time, higher body mass index (BMI), trauma, history of cancer, female sex, and raised Charlson Comorbidity Index (CCI).1-7 Limitations of clinical series include the smaller number of reporting institutions—a potential source of bias given regional variability.1,3,4,7 Limitations of large state or national databases include capturing only events coded during inpatient admission and capturing readmissions for complications at the same institution. This underreporting may lead to very conservative estimates of VTE incidence.2,5,6,8
In this study, we retrospectively identified all the SAs performed at a single institution over a 13-year period and evaluated the cases for development of VTE (DVT, PE). We hypothesized that the VTE rate would be lower than the very high rates reported by Hoxie and colleagues1 and Willis and colleagues4 but higher than those reported for large state or national databases.2,3 We also evaluated clotting risk factors, including many never analyzed before.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we searched our database for all SAs performed at our institution between January 1999 and May 2012 and identified cases in which symptomatic VTE developed within the first 90 days after surgery. Charts were reviewed for information on medical history, surgical procedure, and in-hospital and out-of-hospital care within the 90-day postoperative period. We recorded data on symptomatic VTE (DVT, PE) as documented by lower or upper extremity duplex ultrasonography (US) or chest computed tomography (CT) angiography. There had been no routine screening of patients; duplex US or CT angiography was performed only if a patient was clinically symptomatic (leg swelling, leg pain, shortness of breath, tachycardia, chest pain) for a potential DVT or PE. For a patient who had repeat SAs on the same shoulder or bilateral SAs at different times, only the first procedure was included in the analysis. Arthroplasties performed for fracture were excluded.
Study data were collected and managed with REDCap (Research Electronic Data Capture) tools hosted at the University of Utah School of Medicine.9 Continuous and discrete data collected on medical history and postoperative course included BMI, age at surgery, preoperative hemoglobin (Hb) and hematocrit (Hct) levels, days in hospital, days until out of bed and days until ambulation (both documented in nursing and physical therapy notes), postoperative Hb and Hct levels, and CCI. Categorical data included sex, diagnosis (primary osteoarthritis, rotator cuff arthropathy, rheumatoid arthritis, failed hemiarthroplasty [HA], failed total SA [TSA], others), attending surgeon, procedure (TSA, HA, reverse TSA, revision SA), anesthesia (general endotracheal anesthesia [GETA] alone, interscalene nerve block alone, GETA plus block), prophylactic use of aspirin after surgery, presence of various medical comorbidities (diabetes, hypertension, cardiac disease, clotting disorders, cancer), hormone replacement therapy, family history of a clotting disorder, and VTE consequences (cardiac events, death).
Statistical Analysis
Descriptive statistics were calculated to summarize aspects of the surgical procedures, the study cohort’s demographics and medical histories, and the incidence of VTE. Logistic regression analysis was performed to explore the association between development of VTE (DVT, PE) and potential risk factors. Unadjusted odds ratios (ORs) were estimated for the risk factors of age, BMI, revision SA, CCI, prophylactic use of aspirin after surgery, preoperative history of VTE, preoperative and postoperative Hb and Hct levels, diabetes, anesthesia (GETA with and without interscalene nerve block), family history of a clotting disorder, days until out of bed, hormone replacement therapy, race, discharge home or to rehabilitation, distance traveled for surgery, hypertension, cardiac disease, cement use, and history of cancer. In addition, ORs were adjusted for age, BMI, and revision SA. For all statistical tests, significance was set at P < .05. All analyses were performed with SAS Version 9.3 (SAS Institute).
Results
We identified 533 SAs: 245 anatomical TSAs, 112 reverse TSAs, 92 HAs, and 84 revision SAs. Three different surgeons performed the procedures, and no patients were lost to follow-up within the first 90 days after surgery. Although SAs were performed for various diagnoses, more than 50% (274) of the SAs were for primary osteoarthritis; 97 were performed for rotator cuff arthropathy, 16 for rheumatoid arthritis, 43 for failed HA, 23 for failed TSA, and 79 for other diagnoses.
Of the 533 patients, 288 were female and 245 were male. Mean age at surgery was 65.2 years (range, 16-93 years). Mean (SD) BMI was 29.2 (6.4) kg/m2. Mean (SD) preoperative Hb level was 13.7 (1.8) g/dL, and mean preoperative Hct level was 40.1% (4.8%). Mean (SD) length of hospital stay was 2.6 (1.5) days. Mean (SD) time before patients were out of bed was 1.1 (0.7) days. On postoperative day 1, mean Hb level was 11.1 (1.7) g/dL, and mean (SD) Hct level was 33.2% (4.8%). Mean (SD) CCI was 1.1 (0.9).
Anesthesia for the 533 patients consisted of GETA (209 patients, 39.0%), interscalene nerve block (2, 0.4%), or GETA with nerve block (314, 59.0%). After surgery, 125 patients (24.3%) received aspirin as prophylaxis. Diabetes was reported by 83 patients, hypertension by 286, cardiac disease by 74, a history of a clotting disorder by 2, a family history of a clotting disorder by 8, ongoing cancer by 4, a history of cancer by 67, and hormone replacement therapy by 104.
For the entire cohort of 533 patients, the symptomatic VTE rate was 2.6% (14 patients), the DVT rate was 0.9% (5), and the PE rate was 2.3% (12). Although VTE did not cause any deaths, there were 3 cardiac events.
Discussion
VTE after SA is rare. We report an overall VTE incidence of 2.6%, with DVT at 0.9% and PE at 2.3%. These rates are similar to those reported in clinical series and significantly higher than those reported for large institutional or national databases.2-7 Our results also support a previously reported trend: The ratio of PE to DVT for SA is significantly higher than historically reported ratios for lower extremity arthroplasty.2,6-8 We have identified many VTE risk factors: raised CCI, preoperative thrombotic event, lower preoperative Hb and Hct levels, lower postoperative Hb level, diabetes, use of GETA without interscalene nerve block, higher BMI, and revision SA. Results of other studies support 3 findings (higher BMI, raised CCI, preoperative thrombotic event); new findings include correlation with Hb and Hct levels, diabetes, type of anesthesia, and revision SA.6,7 Identification of these other factors may be useful in making treatment decisions in patients symptomatic after SA and in lowering the threshold for performing diagnostic tests in these patients at risk for VTE.
Reported rates of VTE after SA are highly variable, ranging from 0.2% to 13%.10 Our rationale for investigating VTE rates at a single institution was to estimate the rates that can be expected in a university-based practice and to determine whether these rates are high enough to warrant routine thromboprophylaxis. The rate variability seems to result in part from variability in the data sources. Most studies that have reported very low VTE rates typically used large state or national databases, which likely were subject to underreporting.
Lyman and colleagues6 found 0.5% DVT and 0.2% PE rates in a New York state hospital database, but only in-hospital immediate postoperative symptomatic complications were included; slightly delayed complications may have been missed. Farng and colleagues5 reported a 0.6% VTE rate, but only inpatient (immediate postoperative or readmission) events were included; all outpatient events were missed. Jameson and colleagues,2 using a national database that included only cases involving inpatient treatment, reported 0% DVT and 0.2% PE rates, again missing outpatient events, and relying on appropriate coding to capture events. Using electronic health records from a large healthcare system, Navarro and colleagues8 queried for VTE cases and reported 0.5% DVT and 0.5% PE rates. The inclusiveness of their data source for the outcome of interest was potentially improved relative to national or statewide databases—and the resulting data reported in their study should reflect that improvement. However, the authors relied on ICD–9 (International Classification of Diseases, Ninth Revision) coding to screen for VTE events and excluded patients with prior VTE, preoperative prophylaxis (enoxaparin or warfarin), or follow-up of <90 days. As patients with prior VTE are those most at risk (present study OR, 6-7), excluding them significantly reduces the overall incidence of clotting reported.
Only 4 studies specifically used information drawn directly from physicians’ clinic notes, vs data retrieved (using code-based queries) from databases.1,3,4,7 These studies may provide a better representation of the rate of VTE after SA, as they were not reliant on codes, included both inpatient and outpatient events, and were inclusive of outpatient follow-up of at least 3 months.
Three of the 4 studies used the Mayo Clinic Total Joint Registry.1,3,4 Hoxie and colleagues1 reported an 11% rate of PE after HA performed for fracture (we excluded SA for fracture). As several other investigators have reported an association between trauma and increased risk for VTE, postoperative anticoagulation should be considered in this patient population (though it was not the focus of the present study).6-8 Sperling and Cofield3 and Singh and colleagues7 reported on the risk for PE among SA patients at the Mayo Clinic. Sperling and Cofield3 included only those events that occurred within the first 7 days after surgery; Singh and colleagues7 included events out to 90 days after surgery. Sperling and Cofield3 reported a 0.17% PE rate; Singh and colleagues7 reported 0.6% PE and 0.1% DVT rates. Sperling and Cofield3 reported on 2885 SAs; Singh and colleagues7 reported on 4019 SAs from the same database. As it is unclear whether these 2 studies had complete information on all patients, underreporting may be an issue. Information was obtained through “clinic visits, medical records and/or standardized mailed and telephone-administered questionnaires.”7The fourth study, a prospective study of 100 patients by Willis and colleagues,4 had the best data on development of symptomatic PE after SA. The authors reported a 2% PE rate and a high (13%) DVT rate. Because US was not performed before the surgical procedures, the number of patients with new and existing DVT cases could not be determined. However, all PEs were new, and the 2% rate found there is similar to the 2.3% in our study. Therefore, we think these rates capture the data most accurately and avoid the underreporting that marks large databases.4Studies have identified various factors that increase the risk for VTE after SA. Singh and colleagues7 identified the risk factors of age over 70 years, female sex, higher BMI (25-29.9 kg/m2), CCI above 1, traumatic etiology, prior history of VTE, and HA. However, their use of univariate regression analysis may have confounded the effects—one factor may have become a surrogate for another (ie, trauma and HA, as most fractures treated with SA during the study period were treated with HA). Lyman and colleagues6 also found advanced age and trauma were associated with higher VTE risk, and reported prior history of cancer as a risk factor as well. Navarro and colleagues8 identified trauma as a risk factor, as in the other 2 studies.6,7 Our data support prior history of VTE, higher BMI, and raised CCI as increasing the risk for VTE.
Other factors identified in the present study are use of GETA without interscalene nerve block, lower preoperative and postoperative Hb levels, diabetes, and revision SA. Because of the limited number of events, only ORs with and without limited control of confounders were performed. Just as in the study by Singh and colleagues,7 uncontrolled confounding could have occurred. A nerve block may be protective, as less postoperative pain may allow patients quicker mobilization and therapy. Diabetes may be a surrogate for other medical comorbidities, as reflected by the higher overall risk with raised CCI. Lower preoperative and postoperative Hb levels were associated with clotting and may be representative of patients with poorer overall health and more complicated surgical procedures (eg, revision SA). In an earlier study, we found increased risk for transfusions in revision SA relative to primary SA.11 Lower preoperative Hb level correlated with development of VTE after lower extremity arthroplasty.12 Postoperative use of aspirin was not found to significantly reduce the incidence of clotting, though this finding may have resulted from lack of power. Therefore, from the present data, there is nothing to conclude about the efficacy of aspirin in preventing thrombosis.
Our findings can be placed in the context of the Virchow triad. Specifically, 3 categories of factors are thought to contribute to thrombosis: hypercoagulability, hemodynamic stasis, and endothelial injury. In grouping factors, we identified prior thrombotic event and obesity as increasing hypercoagulability; revision SA, more comorbidities, lower Hb and Hct levels, diabetes, and GETA as increasing hemodynamic stasis; and revision SA (longer operating room times) as leading to stasis. More comorbidities can be associated with delayed postoperative ambulation, and diabetes and lower Hb and Hct levels can be surrogates for more comorbidities. Surgery performed with the patient under GETA without interscalene nerve block can lead to higher levels of pain and less early mobility.
The present findings have made us more aware of patients at risk for VTE, and we have lowered our threshold for evaluating them for potential clots. Before this study, we used warfarin or enoxaparin for anticoagulation in patients with a history of VTE or active cancer. We are continuing this protocol, but not with other patients. Patients with many comorbidities, lower preoperative Hb level, revision SA, high BMI, or diabetes are carefully monitored for clots early in the postoperative course. Our new threshold for these high-risk patients is to order diagnostic testing, including duplex US or CT angiography. Now, even mild oxygen requirements or mild tachycardia within postoperative week 1 typically prompt a study in these patients. We hope this increased awareness will limit the potential negative consequences associated with development of VTE. Given the present data, we do not think the simple presence of increased comorbidities, lower preoperative Hb, revision SA, high BMI or diabetes should rule out performing SA; rather, it should increase surgeons’ postoperative vigilance in evaluating for potential clots.
Limitations of our study include its retrospective nature and reliance on clinic chart review. Patients were not directly questioned about venous thrombus at follow-up, so all events may not have been captured. Although retrospective review has its drawbacks, it allows for accurate identification of events, even uncoded events. Therefore, more events are likely to be captured with this technique than with large database analyses using only coding information. We tried to identify as many cases as possible by reviewing all outpatient records (orthopedic, nonorthopedic), inpatient records, radiologic studies, and scanned outside records. Another limitation is that having a small number of VTE events limited our ability to perform a multivariate analysis, and uncontrolled confounding likely resulted. Only a very large multi-institutional study can capture enough events to allow a multivariate analysis. A third limitation is that the small number of events may have underpowered the study. Having more patients would have allowed other potential factors to be identified as being significantly associated with VTE. Last, as the study captured only symptomatic VTE events, it may have underreported VTE events. Given our complete review of the medical records, however, most clinically significant events likely were captured.
Conclusion
VTE after SA is rare. In our single-institution study, the symptomatic DVT rate was 0.9%, and the symptomatic PE rate was 2.3%. Risk factors associated with clotting included prior VTE, higher BMI, lower preoperative and postoperative Hb levels, raised CCI, diabetes, use of GETA without interscalene nerve block, and revision SA. Risk factors can be used to identify patients who may benefit from a more scrutinized postoperative evaluation and from increased surgeon awareness of the potential for VTE development. Rates of VTE can be used to counsel SA patients regarding overall surgical risks.
Am J Orthop. 2016;45(6):E379-E385. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism after operative treatment of proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(6):782-783.
2. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):764-770.
3. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
4. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
5. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
6. Lyman S, Sherman S, Carter TI, Bach PB, Mandl LA, Marx RG. Prevalence and risk factors for symptomatic thromboembolic events after shoulder arthroplasty. Clin Orthop Relat Res. 2006;(448):152-156.
7. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
8. Navarro RA, Inacio MC, Burke MF, Costouros JG, Yian EH. Risk of thromboembolism in shoulder arthroplasty: effect of implant type and traumatic indication. Clin Orthop Relat Res. 2013;471(5):1576-1581.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. Saleh HE, Pennings AL, ElMaraghy AW. Venous thromboembolism after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2013;22(10):1440-1448.
11. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
12. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
1. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism after operative treatment of proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(6):782-783.
2. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):764-770.
3. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
4. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
5. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
6. Lyman S, Sherman S, Carter TI, Bach PB, Mandl LA, Marx RG. Prevalence and risk factors for symptomatic thromboembolic events after shoulder arthroplasty. Clin Orthop Relat Res. 2006;(448):152-156.
7. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
8. Navarro RA, Inacio MC, Burke MF, Costouros JG, Yian EH. Risk of thromboembolism in shoulder arthroplasty: effect of implant type and traumatic indication. Clin Orthop Relat Res. 2013;471(5):1576-1581.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. Saleh HE, Pennings AL, ElMaraghy AW. Venous thromboembolism after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2013;22(10):1440-1448.
11. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
12. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
Malignant Transformation of an Aneurysmal Bone Cyst to Fibroblastic Osteosarcoma
Aneurysmal bone cysts (ABC) are expansile, hemorrhagic, non-neoplastic lesions that can be locally destructive1 and that can arise either de novo or secondary to another benign or malignant lesion.2 Although primary and secondary ABCs typically are benign, there are cases of malignant degeneration of primary ABCs, though the transformation arises almost exclusively in the context of prior radiation exposure.3-5 Malignant change without history of irradiation is rare; only 6 such cases have been reported.5-10 In 4 of these cases, the transformation was to osteosarcoma.5,8-10
Here we report on an ABC that degenerated into a fibroblastic osteosarcoma—the fifth such case in the medical literature. In addition to reviewing the earlier cases, we describe the radiologic and histologic underpinnings of this diagnosis and the insight that they provide into the pathogenesis of this rare process. Although the prevailing view is that ABCs are benign, it is important to know these lesions have the potential to undergo malignant transformation, even in the absence of prior radiation exposure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy and previously asymptomatic 37-year-old man presented with thigh pain after a minor fall onto a couch. Radiographs showed a diaphyseal femoral pathologic fracture adjacent to a small but benign-appearing cystic lesion (Figures 1A, 1B).
Two years later, the patient had a bicycle accident and, after 2 weeks of significantly increased thigh swelling, presented to the emergency department at the referring institution. Radiographs showed a lytic lesion in the femoral diaphysis that was highly suspicious for malignancy (Figures 3A, 3B).
The initial biopsy specimens were evaluated at our institution and interpreted as being consistent with an ABC, with negative immunohistochemical staining for MDM2 (Figures 5A, 5B).
The patient underwent a 3-month course of neoadjuvant chemotherapy with cisplatin and doxorubicin. Interval-staging contrast-enhanced chest, abdomen, and pelvis computed tomography (CT) showed no evidence of metastatic disease. Preoperative MRI showed a significantly larger heterogeneous mass, now with neurovascular involvement, which precluded limb salvage.
Discussion
Aneurysmal bone cysts are expansile, hemorrhagic, locally destructive lesions that generally develop within the first 3 decades of life. Ever since they were first described by Jaffe and Lichtenstein11 in 1942, the most widely accepted theory of their pathogenesis has been that they begin as a benign reactive vascular process.12 However, more recent molecular studies by Oliveira and colleagues13 and Panoutsakopoulos and colleagues14 have demonstrated a clonal neoplastic basis for primary ABCs related to cytogenetic upregulation of oncogenes USP6 and CDH11 after translocation of 17p13 and 16q22.
Given the clonal nature of these lesions, it is surprising that malignant transformation is so rare. Until now, there have been only 4 reports of an ABC undergoing malignant degeneration to osteosarcoma without prior radiation exposure.
In this article, we have presented a fifth case of a primary ABC degenerating into an osteosarcoma, which in this instance was the fibroblastic subtype. This diagnosis was strongly supported by radiologic and pathologic evidence. From a radiologic perspective, imaging at initial presentation showed absolutely no suspicious features, and the same was true when follow-up radiographs were obtained, 1 month later. Although 1 month is short for a follow-up, the complete lack of radiographic changes would be highly unusual if in fact there had been a coexisting, undetected lesion as aggressive as the one that ultimately developed. Furthermore, imaging at second presentation, almost 2 years later, showed an extremely rapid evolution of findings over 1 month. Extrapolating back in time, we think this time course indicates the malignancy developed not long before its aggressive features were detected.
Genetic evidence suggests that most conventional high-grade osteosarcomas arise de novo from a mesenchymal precursor driven by multiple genetic aberrations. Less often, low-grade osteosarcomas progress to high-grade osteosarcomas. Amplification of 12q13-15 with resulting overexpression of MDM2 and CDK4 proteins is found in low-grade osteosarcomas and persists in examples that progress to higher-grade forms.15 Not only did review of our patient’s initial biopsy sample reveal no evidence of malignant features or abnormal mitotic activity, but the complete absence of MDM2 suggests not even a low-grade osteosarcoma was present at the time. By contrast, the second incisional biopsy specimen, 2 years later, showed markedly different histology and pronounced expression of MDM2 throughout the specimen. This finding suggests the histologically high-grade osteosarcoma did not arise de novo but rather secondarily from a low-grade osteosarcoma that had arisen from an ABC. Results of the final heterogeneous histology of the very large mass, which contained benign ABC areas indistinguishable from the initial biopsy sample, as well as areas of high-grade osteosarcoma, further support a multistep process of de-differentiation. Together, these findings are compelling evidence of malignant transformation of a primary ABC.
We acknowledge that the initial surgery performed at the outside hospital might have properly included frozen-section analysis of the biopsy material and that sampling error may have occurred during the index procedure—possibilities in the absence of complete lesional resection. In this case, however, the radiographic findings and the dominant histologic immunophenotype from medullary canal bone were both consistent with ABC and not osteosarcoma, lending support to malignant degeneration.
We have presented a fifth case of primary ABC degenerating into an osteosarcoma, now with immunohistochemical evidence supporting traditional radiologic and histologic evidence. Despite the rarity of the diagnosis, this case yields considerable insight into the pathogenetic mechanisms underlying malignant degeneration. Despite the widely held view that ABCs are benign, physicians caring for these patients must be aware that malignant transformation can occur.
Am J Orthop. 2016;45(6):E367-E372. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Donaldson WF. Aneurysmal bone cyst. J Bone Joint Surg Am. 1962;44:25-40.
2. Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26(3):615-625.
3. Aho HJ, Aho AJ, Einola S. Aneurysmal bone cyst, a study of ultrastructure and malignant transformation. Virchows Arch A Pathol Anat Histol. 1982;395(2):169-179.
4. Tillman BP, Dahlin DC, Lipscomb PR, Stewart JR. Aneurysmal bone cyst: an analysis of ninety-five cases. Mayo Clin Proc. 1968;43(7):478-495.
5. Kyriakos M, Hardy D. Malignant transformation of aneurysmal bone cyst, with an analysis of the literature. Cancer. 1991;68(8):1770-1780.
6. Mei J, Gao YS, Wang SQ, Cai XS. Malignant transformation of aneurysmal bone cysts: a case report. Chin Med J (Engl). 2009;122(1):110-112.
7. Anract P, de Pinieux G, Jeanrot C, Babinet A, Forest M, Tomeno B. Malignant fibrous histiocytoma at the site of a previously treated aneurysmal bone cyst: a case report. J Bone Joint Surg Am. 2002;84(1):106-111.
8. Hsu CC, Wang JW, Huang CH, Chen WJ. Osteosarcoma at the site of a previously treated aneurysmal bone cyst. A case report. J Bone Joint Surg Am. 2005;87(2):395-398.
9. Wuisman P, Roessner A, Blasius S, Grünert J, Vestering T, Winkelmann W. High malignant surface osteosarcoma arising at the site of a previously treated aneurysmal bone cyst. J Cancer Res Clin Oncol. 1993;119(7):375-378.
10. Brindley GW, Greene JF Jr, Frankel LS. Case reports: malignant transformation of aneurysmal bone cysts. Clin Orthop Relat Res. 2005;(438):282-287.
11. Jaffe HL, Lichtenstein L. Solitary unicameral bone cyst: with emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch Surg. 1942;44:1004-1025.
12. Mirra JM. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
13. Oliveira AM, Chou MM, Perez-Atayde AR, Rosenberg AE. Aneurysmal bone cyst: a neoplasm driven by upregulation of the USP6 oncogene. J Clin Oncol. 2006;24(1):e1.
14. Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999;26(3):265-266.
15. Dujardin F, Binh MB, Bouvier C, et al. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. 2001;24(5):624-637.
Aneurysmal bone cysts (ABC) are expansile, hemorrhagic, non-neoplastic lesions that can be locally destructive1 and that can arise either de novo or secondary to another benign or malignant lesion.2 Although primary and secondary ABCs typically are benign, there are cases of malignant degeneration of primary ABCs, though the transformation arises almost exclusively in the context of prior radiation exposure.3-5 Malignant change without history of irradiation is rare; only 6 such cases have been reported.5-10 In 4 of these cases, the transformation was to osteosarcoma.5,8-10
Here we report on an ABC that degenerated into a fibroblastic osteosarcoma—the fifth such case in the medical literature. In addition to reviewing the earlier cases, we describe the radiologic and histologic underpinnings of this diagnosis and the insight that they provide into the pathogenesis of this rare process. Although the prevailing view is that ABCs are benign, it is important to know these lesions have the potential to undergo malignant transformation, even in the absence of prior radiation exposure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy and previously asymptomatic 37-year-old man presented with thigh pain after a minor fall onto a couch. Radiographs showed a diaphyseal femoral pathologic fracture adjacent to a small but benign-appearing cystic lesion (Figures 1A, 1B).
Two years later, the patient had a bicycle accident and, after 2 weeks of significantly increased thigh swelling, presented to the emergency department at the referring institution. Radiographs showed a lytic lesion in the femoral diaphysis that was highly suspicious for malignancy (Figures 3A, 3B).
The initial biopsy specimens were evaluated at our institution and interpreted as being consistent with an ABC, with negative immunohistochemical staining for MDM2 (Figures 5A, 5B).
The patient underwent a 3-month course of neoadjuvant chemotherapy with cisplatin and doxorubicin. Interval-staging contrast-enhanced chest, abdomen, and pelvis computed tomography (CT) showed no evidence of metastatic disease. Preoperative MRI showed a significantly larger heterogeneous mass, now with neurovascular involvement, which precluded limb salvage.
Discussion
Aneurysmal bone cysts are expansile, hemorrhagic, locally destructive lesions that generally develop within the first 3 decades of life. Ever since they were first described by Jaffe and Lichtenstein11 in 1942, the most widely accepted theory of their pathogenesis has been that they begin as a benign reactive vascular process.12 However, more recent molecular studies by Oliveira and colleagues13 and Panoutsakopoulos and colleagues14 have demonstrated a clonal neoplastic basis for primary ABCs related to cytogenetic upregulation of oncogenes USP6 and CDH11 after translocation of 17p13 and 16q22.
Given the clonal nature of these lesions, it is surprising that malignant transformation is so rare. Until now, there have been only 4 reports of an ABC undergoing malignant degeneration to osteosarcoma without prior radiation exposure.
In this article, we have presented a fifth case of a primary ABC degenerating into an osteosarcoma, which in this instance was the fibroblastic subtype. This diagnosis was strongly supported by radiologic and pathologic evidence. From a radiologic perspective, imaging at initial presentation showed absolutely no suspicious features, and the same was true when follow-up radiographs were obtained, 1 month later. Although 1 month is short for a follow-up, the complete lack of radiographic changes would be highly unusual if in fact there had been a coexisting, undetected lesion as aggressive as the one that ultimately developed. Furthermore, imaging at second presentation, almost 2 years later, showed an extremely rapid evolution of findings over 1 month. Extrapolating back in time, we think this time course indicates the malignancy developed not long before its aggressive features were detected.
Genetic evidence suggests that most conventional high-grade osteosarcomas arise de novo from a mesenchymal precursor driven by multiple genetic aberrations. Less often, low-grade osteosarcomas progress to high-grade osteosarcomas. Amplification of 12q13-15 with resulting overexpression of MDM2 and CDK4 proteins is found in low-grade osteosarcomas and persists in examples that progress to higher-grade forms.15 Not only did review of our patient’s initial biopsy sample reveal no evidence of malignant features or abnormal mitotic activity, but the complete absence of MDM2 suggests not even a low-grade osteosarcoma was present at the time. By contrast, the second incisional biopsy specimen, 2 years later, showed markedly different histology and pronounced expression of MDM2 throughout the specimen. This finding suggests the histologically high-grade osteosarcoma did not arise de novo but rather secondarily from a low-grade osteosarcoma that had arisen from an ABC. Results of the final heterogeneous histology of the very large mass, which contained benign ABC areas indistinguishable from the initial biopsy sample, as well as areas of high-grade osteosarcoma, further support a multistep process of de-differentiation. Together, these findings are compelling evidence of malignant transformation of a primary ABC.
We acknowledge that the initial surgery performed at the outside hospital might have properly included frozen-section analysis of the biopsy material and that sampling error may have occurred during the index procedure—possibilities in the absence of complete lesional resection. In this case, however, the radiographic findings and the dominant histologic immunophenotype from medullary canal bone were both consistent with ABC and not osteosarcoma, lending support to malignant degeneration.
We have presented a fifth case of primary ABC degenerating into an osteosarcoma, now with immunohistochemical evidence supporting traditional radiologic and histologic evidence. Despite the rarity of the diagnosis, this case yields considerable insight into the pathogenetic mechanisms underlying malignant degeneration. Despite the widely held view that ABCs are benign, physicians caring for these patients must be aware that malignant transformation can occur.
Am J Orthop. 2016;45(6):E367-E372. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Aneurysmal bone cysts (ABC) are expansile, hemorrhagic, non-neoplastic lesions that can be locally destructive1 and that can arise either de novo or secondary to another benign or malignant lesion.2 Although primary and secondary ABCs typically are benign, there are cases of malignant degeneration of primary ABCs, though the transformation arises almost exclusively in the context of prior radiation exposure.3-5 Malignant change without history of irradiation is rare; only 6 such cases have been reported.5-10 In 4 of these cases, the transformation was to osteosarcoma.5,8-10
Here we report on an ABC that degenerated into a fibroblastic osteosarcoma—the fifth such case in the medical literature. In addition to reviewing the earlier cases, we describe the radiologic and histologic underpinnings of this diagnosis and the insight that they provide into the pathogenesis of this rare process. Although the prevailing view is that ABCs are benign, it is important to know these lesions have the potential to undergo malignant transformation, even in the absence of prior radiation exposure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy and previously asymptomatic 37-year-old man presented with thigh pain after a minor fall onto a couch. Radiographs showed a diaphyseal femoral pathologic fracture adjacent to a small but benign-appearing cystic lesion (Figures 1A, 1B).
Two years later, the patient had a bicycle accident and, after 2 weeks of significantly increased thigh swelling, presented to the emergency department at the referring institution. Radiographs showed a lytic lesion in the femoral diaphysis that was highly suspicious for malignancy (Figures 3A, 3B).
The initial biopsy specimens were evaluated at our institution and interpreted as being consistent with an ABC, with negative immunohistochemical staining for MDM2 (Figures 5A, 5B).
The patient underwent a 3-month course of neoadjuvant chemotherapy with cisplatin and doxorubicin. Interval-staging contrast-enhanced chest, abdomen, and pelvis computed tomography (CT) showed no evidence of metastatic disease. Preoperative MRI showed a significantly larger heterogeneous mass, now with neurovascular involvement, which precluded limb salvage.
Discussion
Aneurysmal bone cysts are expansile, hemorrhagic, locally destructive lesions that generally develop within the first 3 decades of life. Ever since they were first described by Jaffe and Lichtenstein11 in 1942, the most widely accepted theory of their pathogenesis has been that they begin as a benign reactive vascular process.12 However, more recent molecular studies by Oliveira and colleagues13 and Panoutsakopoulos and colleagues14 have demonstrated a clonal neoplastic basis for primary ABCs related to cytogenetic upregulation of oncogenes USP6 and CDH11 after translocation of 17p13 and 16q22.
Given the clonal nature of these lesions, it is surprising that malignant transformation is so rare. Until now, there have been only 4 reports of an ABC undergoing malignant degeneration to osteosarcoma without prior radiation exposure.
In this article, we have presented a fifth case of a primary ABC degenerating into an osteosarcoma, which in this instance was the fibroblastic subtype. This diagnosis was strongly supported by radiologic and pathologic evidence. From a radiologic perspective, imaging at initial presentation showed absolutely no suspicious features, and the same was true when follow-up radiographs were obtained, 1 month later. Although 1 month is short for a follow-up, the complete lack of radiographic changes would be highly unusual if in fact there had been a coexisting, undetected lesion as aggressive as the one that ultimately developed. Furthermore, imaging at second presentation, almost 2 years later, showed an extremely rapid evolution of findings over 1 month. Extrapolating back in time, we think this time course indicates the malignancy developed not long before its aggressive features were detected.
Genetic evidence suggests that most conventional high-grade osteosarcomas arise de novo from a mesenchymal precursor driven by multiple genetic aberrations. Less often, low-grade osteosarcomas progress to high-grade osteosarcomas. Amplification of 12q13-15 with resulting overexpression of MDM2 and CDK4 proteins is found in low-grade osteosarcomas and persists in examples that progress to higher-grade forms.15 Not only did review of our patient’s initial biopsy sample reveal no evidence of malignant features or abnormal mitotic activity, but the complete absence of MDM2 suggests not even a low-grade osteosarcoma was present at the time. By contrast, the second incisional biopsy specimen, 2 years later, showed markedly different histology and pronounced expression of MDM2 throughout the specimen. This finding suggests the histologically high-grade osteosarcoma did not arise de novo but rather secondarily from a low-grade osteosarcoma that had arisen from an ABC. Results of the final heterogeneous histology of the very large mass, which contained benign ABC areas indistinguishable from the initial biopsy sample, as well as areas of high-grade osteosarcoma, further support a multistep process of de-differentiation. Together, these findings are compelling evidence of malignant transformation of a primary ABC.
We acknowledge that the initial surgery performed at the outside hospital might have properly included frozen-section analysis of the biopsy material and that sampling error may have occurred during the index procedure—possibilities in the absence of complete lesional resection. In this case, however, the radiographic findings and the dominant histologic immunophenotype from medullary canal bone were both consistent with ABC and not osteosarcoma, lending support to malignant degeneration.
We have presented a fifth case of primary ABC degenerating into an osteosarcoma, now with immunohistochemical evidence supporting traditional radiologic and histologic evidence. Despite the rarity of the diagnosis, this case yields considerable insight into the pathogenetic mechanisms underlying malignant degeneration. Despite the widely held view that ABCs are benign, physicians caring for these patients must be aware that malignant transformation can occur.
Am J Orthop. 2016;45(6):E367-E372. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Donaldson WF. Aneurysmal bone cyst. J Bone Joint Surg Am. 1962;44:25-40.
2. Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26(3):615-625.
3. Aho HJ, Aho AJ, Einola S. Aneurysmal bone cyst, a study of ultrastructure and malignant transformation. Virchows Arch A Pathol Anat Histol. 1982;395(2):169-179.
4. Tillman BP, Dahlin DC, Lipscomb PR, Stewart JR. Aneurysmal bone cyst: an analysis of ninety-five cases. Mayo Clin Proc. 1968;43(7):478-495.
5. Kyriakos M, Hardy D. Malignant transformation of aneurysmal bone cyst, with an analysis of the literature. Cancer. 1991;68(8):1770-1780.
6. Mei J, Gao YS, Wang SQ, Cai XS. Malignant transformation of aneurysmal bone cysts: a case report. Chin Med J (Engl). 2009;122(1):110-112.
7. Anract P, de Pinieux G, Jeanrot C, Babinet A, Forest M, Tomeno B. Malignant fibrous histiocytoma at the site of a previously treated aneurysmal bone cyst: a case report. J Bone Joint Surg Am. 2002;84(1):106-111.
8. Hsu CC, Wang JW, Huang CH, Chen WJ. Osteosarcoma at the site of a previously treated aneurysmal bone cyst. A case report. J Bone Joint Surg Am. 2005;87(2):395-398.
9. Wuisman P, Roessner A, Blasius S, Grünert J, Vestering T, Winkelmann W. High malignant surface osteosarcoma arising at the site of a previously treated aneurysmal bone cyst. J Cancer Res Clin Oncol. 1993;119(7):375-378.
10. Brindley GW, Greene JF Jr, Frankel LS. Case reports: malignant transformation of aneurysmal bone cysts. Clin Orthop Relat Res. 2005;(438):282-287.
11. Jaffe HL, Lichtenstein L. Solitary unicameral bone cyst: with emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch Surg. 1942;44:1004-1025.
12. Mirra JM. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
13. Oliveira AM, Chou MM, Perez-Atayde AR, Rosenberg AE. Aneurysmal bone cyst: a neoplasm driven by upregulation of the USP6 oncogene. J Clin Oncol. 2006;24(1):e1.
14. Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999;26(3):265-266.
15. Dujardin F, Binh MB, Bouvier C, et al. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. 2001;24(5):624-637.
1. Donaldson WF. Aneurysmal bone cyst. J Bone Joint Surg Am. 1962;44:25-40.
2. Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26(3):615-625.
3. Aho HJ, Aho AJ, Einola S. Aneurysmal bone cyst, a study of ultrastructure and malignant transformation. Virchows Arch A Pathol Anat Histol. 1982;395(2):169-179.
4. Tillman BP, Dahlin DC, Lipscomb PR, Stewart JR. Aneurysmal bone cyst: an analysis of ninety-five cases. Mayo Clin Proc. 1968;43(7):478-495.
5. Kyriakos M, Hardy D. Malignant transformation of aneurysmal bone cyst, with an analysis of the literature. Cancer. 1991;68(8):1770-1780.
6. Mei J, Gao YS, Wang SQ, Cai XS. Malignant transformation of aneurysmal bone cysts: a case report. Chin Med J (Engl). 2009;122(1):110-112.
7. Anract P, de Pinieux G, Jeanrot C, Babinet A, Forest M, Tomeno B. Malignant fibrous histiocytoma at the site of a previously treated aneurysmal bone cyst: a case report. J Bone Joint Surg Am. 2002;84(1):106-111.
8. Hsu CC, Wang JW, Huang CH, Chen WJ. Osteosarcoma at the site of a previously treated aneurysmal bone cyst. A case report. J Bone Joint Surg Am. 2005;87(2):395-398.
9. Wuisman P, Roessner A, Blasius S, Grünert J, Vestering T, Winkelmann W. High malignant surface osteosarcoma arising at the site of a previously treated aneurysmal bone cyst. J Cancer Res Clin Oncol. 1993;119(7):375-378.
10. Brindley GW, Greene JF Jr, Frankel LS. Case reports: malignant transformation of aneurysmal bone cysts. Clin Orthop Relat Res. 2005;(438):282-287.
11. Jaffe HL, Lichtenstein L. Solitary unicameral bone cyst: with emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch Surg. 1942;44:1004-1025.
12. Mirra JM. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
13. Oliveira AM, Chou MM, Perez-Atayde AR, Rosenberg AE. Aneurysmal bone cyst: a neoplasm driven by upregulation of the USP6 oncogene. J Clin Oncol. 2006;24(1):e1.
14. Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999;26(3):265-266.
15. Dujardin F, Binh MB, Bouvier C, et al. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. 2001;24(5):624-637.