User login
Things We Do for No Reason – The “48 Hour Rule-out” for Well-Appearing Febrile Infants
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 3-week-old, full-term term male febrile infant was evaluated in the emergency department (ED). On the day of admission, he was noted to feel warm to the touch and was found to have a rectal temperature of 101.3°F (38.3°C) at home.
In the ED, the patient was well appearing and had normal physical exam findings. His workup in the ED included a normal chest radiograph, complete blood count (CBC) with differential count, cerebrospinal fluid (CSF) analysis (cell count, protein, and glucose), and urinalysis. Blood, CSF, and catheterized urine cultures were collected, and he was admitted to the hospital on parenteral antibiotics. His provider informed the parents that the infant would be observed in the hospital for 48 hours while monitoring the bacterial cultures. Is it necessary for the hospitalization of this child to last a full 48 hours?
INTRODUCTION
Evaluation and management of fever (T ≥ 38°C) is a common cause of emergency department visits and accounts for up to 20% of pediatric emergency visits.2
Why You Might Think Hospitalization for at Least 48 Hours is Necessary
The evaluation and management of fever in infants aged less than 90 days is challenging due to concern for occult serious bacterial infections. In particular, providers may be concerned that the physical exam lacks sensitivity.9
There is also a perceived risk of poor outcomes in young infants if a serious bacterial infection is missed. For these reasons, the evaluation and management of febrile infants has been characterized by practice variability in both outpatient10 and ED3 settings.
Commonly used febrile infant management protocols vary in approach and do not provide clear guidelines on the recommended duration of hospitalization and empiric antimicrobial treatment.11-14 Length of hospitalization was widely studied in infants between 1979 and 1999, and results showed that the majority of clinically important bacterial pathogens can be detected within 48 hours.15-17 Many textbooks and online references, based on this literature, continue to support 48 to 72 hours of observation and empiric antimicrobial treatment for febrile infants.18,19 A 2012 AAP Clinical Report advocated for limiting the antimicrobial treatment in low-risk infants suspected of early-onset sepsis to 48 hours.20
Why Shorten the Period of In-Hospital Observation to a Maximum of 36 Hours of Culture Incubation
Discharge of low-risk infants with negative enhanced urinalysis and negative bacterial cultures at 36 hours or earlier can reduce costs21 and potentially preventable harm (eg, intravenous catheter complications, nosocomial infections) without negatively impacting patient outcomes.22 Early discharge is also patient-centered, given the stress and indirect costs associated with hospitalization, including potential separation of a breastfeeding infant and mother, lost wages from time off work, or childcare for well siblings.23
Initial studies that evaluated the time-to-positivity (TTP) of bacterial cultures in febrile infants predate the use of continuous monitoring systems for blood cultures. Traditional bacterial culturing techniques require direct observation of broth turbidity and subsequent subculturing onto chocolate and sheep blood agar, typically occurring only once daily.24 Current commercially available continuous monitoring bacterial culture systems decrease TTP by immediately alerting laboratory technicians to bacterial growth through the detection of 14CO2 released by organisms utilizing radiolabeled glucose in growth media.24 In addition, many studies supporting the evaluation of febrile infants in the hospital for a 48-hour period include those in ICU settings,25 with medically complex histories,24 and aged < 28 days admitted in the NICU,15 where pathogens with longer incubation times are frequently seen.
In a recent single-center retrospective study, infant blood cultures with TTP longer than 36 hours are 7.8 times more likely to be identified as contaminant bacteria compared with cultures that tested positive in <36 hours.26 Even if bacterial cultures were unexpectedly positive after 36 hours, which occurs in less than 1.1% of all infants and 0.3% of low-risk infants,1 these patients do not have adverse outcomes. Infants who were deemed low risk based on established criteria and who had bacterial cultures positive for pathogenic bacteria were treated at that time and recovered uneventfully.7, 31
CSF and urine cultures are often reviewed only once or twice daily in most institutions, and this practice artificially prolongs the TTP for pathogenic bacteria. Small sample-sized studies have demonstrated the low detection rate of pathogens in CSF and urine cultures beyond 36 hours. Evans et al. found that in infants aged 0-28 days, 0.03% of urine cultures and no CSF cultures tested positive after 36 hours.26 In a retrospective study of infants aged 28-90 days in the ED setting, Kaplan et al. found that 0.9% of urine cultures and no CSF cultures were positive at >24 hours.1 For well-appearing infants who have reassuring initial CSF studies, the risk of meningitis is extremely low.7 Management criteria for febrile infants provide guidance for determining those infants with abnormal CSF results who may benefit from longer periods of observation.
Urinary tract infections are common serious bacterial infections in this age group. Enhanced urinalysis, in which cell count and Gram stain analysis are performed on uncentrifuged urine, shows 96% sensitivity of predicting urinary tract infection and can provide additional reassurance for well-appearing infants who are discharged prior to 48 hours.27
When a Longer Observation Period May Be Warranted
What You Should Do Instead: Limit Hospitalization to a Maximum of 36 Hours
For well-appearing febrile infants between 0–90 days of age hospitalized for observation and awaiting bacterial culture results, providers should consider discharge at 36 hours or less, rather than 48 hours, if blood, urine, and CSF cultures do not show bacterial growth. In a large health system, researchers implemented an evidence-based care process model for febrile infants to provide specific guidelines for laboratory testing, criteria for admission, and recommendation for discontinuation of empiric antibiotics and discharge after 36 hours in infants with negative bacterial cultures. These changes led to a 27% reduction in the length of hospital stay and 23% reduction in inpatient costs without any cases of missed bacteremia.21 The reduction in the in-hospital observation duration to 24 hours of culture incubation for well-appearing febrile infants has been advocated 32 and is a common practice for infants with appropriate follow up and parental assurance. This recommendation is supported by the following:
- Recent data showing the overwhelming majority of pathogens will be identified by blood culture <24 hours in infants aged 0-90 days32 with blood culture TTP in infants aged 0-30 days being either no different26 or potentially shorter32
- Studies showing that infants meeting low-risk clinical and laboratory profiles further reduce the likelihood of identifying serious bacterial infection after 24 hours to 0.3%.1
RECOMMENDATIONS
- Determine if febrile infants aged 0-90 days are at low risk for serious bacterial infection and obtain appropriate bacterial cultures.
- If hospitalized for observation, discharge low-risk febrile infants aged 0–90 days after 36 hours or less if bacterial cultures remain negative.
- If hospitalized for observation, consider reducing the length of inpatient observation for low-risk febrile infants aged 0–90 days with reliable follow-up to 24 hours or less when the culture results are negative.
CONCLUSION
Monitoring patients in the hospital for greater than 36 hours of bacterial culture incubation is unnecessary for patients similar to the 3 week-old full-term infant in the case presentation, who are at low risk for serious bacterial infection based on available scoring systems and have negative cultures. If patients are not deemed low risk, have an incomplete laboratory evaluation, or have had prior antibiotic treatment, longer observation in the hospital may be warranted. Close reassessment of the rare patients whose blood cultures return positive after 36 hours is necessary, but their outcomes are excellent, especially in well-appearing infants.7,33
What do you do?
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
There are no conflicts of interest relevant to this work reported by any of the authors.
1. Kaplan RL, Harper MB, Baskin MN, Macone AB, Mandl KD. Time to detection of positive cultures in 28- to 90-day-old febrile infants. Pediatrics 2000;106(6):E74. PubMed
2. Fleisher GR, Ludwig S, Henretig FM. Textbook of Pediatric Emergency Medicine: Lippincott Williams & Wilkins; 2006.
3. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants </=56 days of age. J Hosp Med. 2015;10(6):358-365. PubMed
4. Hui C, Neto G, Tsertsvadze A, et al. Diagnosis and management of febrile infants (0-3 months). Evid Rep Technol Assess. 2012;205:1-297. PubMed
5. Garcia S, Mintegi S, Gomez B, et al. Is 15 days an appropriate cut-off age for considering serious bacterial infection in the management of febrile infants? Pediatr Infect Dis J. 2012;31(5):455-458. PubMed
6. Schwartz S, Raveh D, Toker O, Segal G, Godovitch N, Schlesinger Y. A week-by-week analysis of the low-risk criteria for serious bacterial infection in febrile neonates. Arch Dis Child. 2009;94(4):287-292. PubMed
7. Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics 2010;125(2):228-233. PubMed
8. Baskin MN. The prevalence of serious bacterial infections by age in febrile infants during the first 3 months of life. Pediatr Ann. 1993;22(8):462-466. PubMed
9. Nigrovic LE, Mahajan PV, Blumberg SM, et al. The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics 2017;140(1):e20170695. PubMed
10. Bergman DA, Mayer ML, Pantell RH, Finch SA, Wasserman RC. Does clinical presentation explain practice variability in the treatment of febrile infants? Pediatrics 2006;117(3):787-795. PubMed
11. Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329(20):1437-1441. PubMed
12. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection--an appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics 1994;94(3):390-396. PubMed
13. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr. 1992;120(1):22-27. PubMed
14. Bachur RG, Harper MB. Predictive model for serious bacterial infections among infants younger than 3 months of age. Pediatrics 2001;108(2):311-316. PubMed
15. Pichichero ME, Todd JK. Detection of neonatal bacteremia. J Pediatr. 1979;94(6):958-960. PubMed
16. Hurst MK, Yoder BA. Detection of bacteremia in young infants: is 48 hours adequate? Pediatr Infect Dis J. 1995;14(8):711-713. PubMed
17. Friedman J, Matlow A. Time to identification of positive bacterial cultures in infants under three months of age hospitalized to rule out sepsis. Paediatr Child Health 1999;4(5):331-334. PubMed
18. Kliegman R, Behrman RE, Nelson WE. Nelson textbook of pediatrics. Edition 20 / ed. Philadelphia, PA: Elsevier; 2016.
19. Fever in infants and children. Merck Sharp & Dohme Corp, 2016. (Accessed 27 Nov 2016, 2016, at https://www.merckmanuals.com/professional/pediatrics/symptoms-in-infants-and-children/fever-in-infants-and-children.)
20. Polin RA, Committee on F, Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012;129(5):1006-1015. PubMed
21. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics 2012;130(1):e16-e24. PubMed
22. DeAngelis C, Joffe A, Wilson M, Willis E. Iatrogenic risks and financial costs of hospitalizing febrile infants. Am J Dis Child. 1983;137(12):1146-1149. PubMed
23. Nizam M, Norzila MZ. Stress among parents with acutely ill children. Med J Malaysia. 2001;56(4):428-434. PubMed
24. Rowley AH, Wald ER. The incubation period necessary for detection of bacteremia in immunocompetent children with fever. Implications for the clinician. Clin Pediatr (Phila). 1986;25(10):485-489. PubMed
25. La Scolea LJ, Jr., Dryja D, Sullivan TD, Mosovich L, Ellerstein N, Neter E. Diagnosis of bacteremia in children by quantitative direct plating and a radiometric procedure. J Clin Microbiol. 1981;13(3):478-482. PubMed
26. Evans RC, Fine BR. Time to detection of bacterial cultures in infants aged 0 to 90 days. Hosp Pediatr. 2013;3(2):97-102. PubMed
27. Herr SM, Wald ER, Pitetti RD, Choi SS. Enhanced urinalysis improves identification of febrile infants ages 60 days and younger at low risk for serious bacterial illness. Pediatrics 2001;108(4):866-871. PubMed
28. Nigrovic LE, Kuppermann N, Macias CG, et al. Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis. JAMA. 2007;297(1):52-60. PubMed
29. Doby EH, Stockmann C, Korgenski EK, Blaschke AJ, Byington CL. Cerebrospinal fluid pleocytosis in febrile infants 1-90 days with urinary tract infection. Pediatr Infect Dis J. 2013;32(9):1024-1026. PubMed
30. Bhansali P, Wiedermann BL, Pastor W, McMillan J, Shah N. Management of hospitalized febrile neonates without CSF analysis: A study of US pediatric hospitals. Hosp Pediatr. 2015;5(10):528-533. PubMed
31. Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics 2001;108(5):1169-1174. PubMed
32. Biondi EA, Mischler M, Jerardi KE, et al. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. PubMed
33. Moher D HC, Neto G, Tsertsvadze A. Diagnosis and Management of Febrile Infants (0–3 Months). Evidence Report/Technology Assessment No. 205. In: Center OE-bP, ed. Rockville, MD: Agency for Healthcare Research and Quality; 2012. PubMed
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 3-week-old, full-term term male febrile infant was evaluated in the emergency department (ED). On the day of admission, he was noted to feel warm to the touch and was found to have a rectal temperature of 101.3°F (38.3°C) at home.
In the ED, the patient was well appearing and had normal physical exam findings. His workup in the ED included a normal chest radiograph, complete blood count (CBC) with differential count, cerebrospinal fluid (CSF) analysis (cell count, protein, and glucose), and urinalysis. Blood, CSF, and catheterized urine cultures were collected, and he was admitted to the hospital on parenteral antibiotics. His provider informed the parents that the infant would be observed in the hospital for 48 hours while monitoring the bacterial cultures. Is it necessary for the hospitalization of this child to last a full 48 hours?
INTRODUCTION
Evaluation and management of fever (T ≥ 38°C) is a common cause of emergency department visits and accounts for up to 20% of pediatric emergency visits.2
Why You Might Think Hospitalization for at Least 48 Hours is Necessary
The evaluation and management of fever in infants aged less than 90 days is challenging due to concern for occult serious bacterial infections. In particular, providers may be concerned that the physical exam lacks sensitivity.9
There is also a perceived risk of poor outcomes in young infants if a serious bacterial infection is missed. For these reasons, the evaluation and management of febrile infants has been characterized by practice variability in both outpatient10 and ED3 settings.
Commonly used febrile infant management protocols vary in approach and do not provide clear guidelines on the recommended duration of hospitalization and empiric antimicrobial treatment.11-14 Length of hospitalization was widely studied in infants between 1979 and 1999, and results showed that the majority of clinically important bacterial pathogens can be detected within 48 hours.15-17 Many textbooks and online references, based on this literature, continue to support 48 to 72 hours of observation and empiric antimicrobial treatment for febrile infants.18,19 A 2012 AAP Clinical Report advocated for limiting the antimicrobial treatment in low-risk infants suspected of early-onset sepsis to 48 hours.20
Why Shorten the Period of In-Hospital Observation to a Maximum of 36 Hours of Culture Incubation
Discharge of low-risk infants with negative enhanced urinalysis and negative bacterial cultures at 36 hours or earlier can reduce costs21 and potentially preventable harm (eg, intravenous catheter complications, nosocomial infections) without negatively impacting patient outcomes.22 Early discharge is also patient-centered, given the stress and indirect costs associated with hospitalization, including potential separation of a breastfeeding infant and mother, lost wages from time off work, or childcare for well siblings.23
Initial studies that evaluated the time-to-positivity (TTP) of bacterial cultures in febrile infants predate the use of continuous monitoring systems for blood cultures. Traditional bacterial culturing techniques require direct observation of broth turbidity and subsequent subculturing onto chocolate and sheep blood agar, typically occurring only once daily.24 Current commercially available continuous monitoring bacterial culture systems decrease TTP by immediately alerting laboratory technicians to bacterial growth through the detection of 14CO2 released by organisms utilizing radiolabeled glucose in growth media.24 In addition, many studies supporting the evaluation of febrile infants in the hospital for a 48-hour period include those in ICU settings,25 with medically complex histories,24 and aged < 28 days admitted in the NICU,15 where pathogens with longer incubation times are frequently seen.
In a recent single-center retrospective study, infant blood cultures with TTP longer than 36 hours are 7.8 times more likely to be identified as contaminant bacteria compared with cultures that tested positive in <36 hours.26 Even if bacterial cultures were unexpectedly positive after 36 hours, which occurs in less than 1.1% of all infants and 0.3% of low-risk infants,1 these patients do not have adverse outcomes. Infants who were deemed low risk based on established criteria and who had bacterial cultures positive for pathogenic bacteria were treated at that time and recovered uneventfully.7, 31
CSF and urine cultures are often reviewed only once or twice daily in most institutions, and this practice artificially prolongs the TTP for pathogenic bacteria. Small sample-sized studies have demonstrated the low detection rate of pathogens in CSF and urine cultures beyond 36 hours. Evans et al. found that in infants aged 0-28 days, 0.03% of urine cultures and no CSF cultures tested positive after 36 hours.26 In a retrospective study of infants aged 28-90 days in the ED setting, Kaplan et al. found that 0.9% of urine cultures and no CSF cultures were positive at >24 hours.1 For well-appearing infants who have reassuring initial CSF studies, the risk of meningitis is extremely low.7 Management criteria for febrile infants provide guidance for determining those infants with abnormal CSF results who may benefit from longer periods of observation.
Urinary tract infections are common serious bacterial infections in this age group. Enhanced urinalysis, in which cell count and Gram stain analysis are performed on uncentrifuged urine, shows 96% sensitivity of predicting urinary tract infection and can provide additional reassurance for well-appearing infants who are discharged prior to 48 hours.27
When a Longer Observation Period May Be Warranted
What You Should Do Instead: Limit Hospitalization to a Maximum of 36 Hours
For well-appearing febrile infants between 0–90 days of age hospitalized for observation and awaiting bacterial culture results, providers should consider discharge at 36 hours or less, rather than 48 hours, if blood, urine, and CSF cultures do not show bacterial growth. In a large health system, researchers implemented an evidence-based care process model for febrile infants to provide specific guidelines for laboratory testing, criteria for admission, and recommendation for discontinuation of empiric antibiotics and discharge after 36 hours in infants with negative bacterial cultures. These changes led to a 27% reduction in the length of hospital stay and 23% reduction in inpatient costs without any cases of missed bacteremia.21 The reduction in the in-hospital observation duration to 24 hours of culture incubation for well-appearing febrile infants has been advocated 32 and is a common practice for infants with appropriate follow up and parental assurance. This recommendation is supported by the following:
- Recent data showing the overwhelming majority of pathogens will be identified by blood culture <24 hours in infants aged 0-90 days32 with blood culture TTP in infants aged 0-30 days being either no different26 or potentially shorter32
- Studies showing that infants meeting low-risk clinical and laboratory profiles further reduce the likelihood of identifying serious bacterial infection after 24 hours to 0.3%.1
RECOMMENDATIONS
- Determine if febrile infants aged 0-90 days are at low risk for serious bacterial infection and obtain appropriate bacterial cultures.
- If hospitalized for observation, discharge low-risk febrile infants aged 0–90 days after 36 hours or less if bacterial cultures remain negative.
- If hospitalized for observation, consider reducing the length of inpatient observation for low-risk febrile infants aged 0–90 days with reliable follow-up to 24 hours or less when the culture results are negative.
CONCLUSION
Monitoring patients in the hospital for greater than 36 hours of bacterial culture incubation is unnecessary for patients similar to the 3 week-old full-term infant in the case presentation, who are at low risk for serious bacterial infection based on available scoring systems and have negative cultures. If patients are not deemed low risk, have an incomplete laboratory evaluation, or have had prior antibiotic treatment, longer observation in the hospital may be warranted. Close reassessment of the rare patients whose blood cultures return positive after 36 hours is necessary, but their outcomes are excellent, especially in well-appearing infants.7,33
What do you do?
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
There are no conflicts of interest relevant to this work reported by any of the authors.
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 3-week-old, full-term term male febrile infant was evaluated in the emergency department (ED). On the day of admission, he was noted to feel warm to the touch and was found to have a rectal temperature of 101.3°F (38.3°C) at home.
In the ED, the patient was well appearing and had normal physical exam findings. His workup in the ED included a normal chest radiograph, complete blood count (CBC) with differential count, cerebrospinal fluid (CSF) analysis (cell count, protein, and glucose), and urinalysis. Blood, CSF, and catheterized urine cultures were collected, and he was admitted to the hospital on parenteral antibiotics. His provider informed the parents that the infant would be observed in the hospital for 48 hours while monitoring the bacterial cultures. Is it necessary for the hospitalization of this child to last a full 48 hours?
INTRODUCTION
Evaluation and management of fever (T ≥ 38°C) is a common cause of emergency department visits and accounts for up to 20% of pediatric emergency visits.2
Why You Might Think Hospitalization for at Least 48 Hours is Necessary
The evaluation and management of fever in infants aged less than 90 days is challenging due to concern for occult serious bacterial infections. In particular, providers may be concerned that the physical exam lacks sensitivity.9
There is also a perceived risk of poor outcomes in young infants if a serious bacterial infection is missed. For these reasons, the evaluation and management of febrile infants has been characterized by practice variability in both outpatient10 and ED3 settings.
Commonly used febrile infant management protocols vary in approach and do not provide clear guidelines on the recommended duration of hospitalization and empiric antimicrobial treatment.11-14 Length of hospitalization was widely studied in infants between 1979 and 1999, and results showed that the majority of clinically important bacterial pathogens can be detected within 48 hours.15-17 Many textbooks and online references, based on this literature, continue to support 48 to 72 hours of observation and empiric antimicrobial treatment for febrile infants.18,19 A 2012 AAP Clinical Report advocated for limiting the antimicrobial treatment in low-risk infants suspected of early-onset sepsis to 48 hours.20
Why Shorten the Period of In-Hospital Observation to a Maximum of 36 Hours of Culture Incubation
Discharge of low-risk infants with negative enhanced urinalysis and negative bacterial cultures at 36 hours or earlier can reduce costs21 and potentially preventable harm (eg, intravenous catheter complications, nosocomial infections) without negatively impacting patient outcomes.22 Early discharge is also patient-centered, given the stress and indirect costs associated with hospitalization, including potential separation of a breastfeeding infant and mother, lost wages from time off work, or childcare for well siblings.23
Initial studies that evaluated the time-to-positivity (TTP) of bacterial cultures in febrile infants predate the use of continuous monitoring systems for blood cultures. Traditional bacterial culturing techniques require direct observation of broth turbidity and subsequent subculturing onto chocolate and sheep blood agar, typically occurring only once daily.24 Current commercially available continuous monitoring bacterial culture systems decrease TTP by immediately alerting laboratory technicians to bacterial growth through the detection of 14CO2 released by organisms utilizing radiolabeled glucose in growth media.24 In addition, many studies supporting the evaluation of febrile infants in the hospital for a 48-hour period include those in ICU settings,25 with medically complex histories,24 and aged < 28 days admitted in the NICU,15 where pathogens with longer incubation times are frequently seen.
In a recent single-center retrospective study, infant blood cultures with TTP longer than 36 hours are 7.8 times more likely to be identified as contaminant bacteria compared with cultures that tested positive in <36 hours.26 Even if bacterial cultures were unexpectedly positive after 36 hours, which occurs in less than 1.1% of all infants and 0.3% of low-risk infants,1 these patients do not have adverse outcomes. Infants who were deemed low risk based on established criteria and who had bacterial cultures positive for pathogenic bacteria were treated at that time and recovered uneventfully.7, 31
CSF and urine cultures are often reviewed only once or twice daily in most institutions, and this practice artificially prolongs the TTP for pathogenic bacteria. Small sample-sized studies have demonstrated the low detection rate of pathogens in CSF and urine cultures beyond 36 hours. Evans et al. found that in infants aged 0-28 days, 0.03% of urine cultures and no CSF cultures tested positive after 36 hours.26 In a retrospective study of infants aged 28-90 days in the ED setting, Kaplan et al. found that 0.9% of urine cultures and no CSF cultures were positive at >24 hours.1 For well-appearing infants who have reassuring initial CSF studies, the risk of meningitis is extremely low.7 Management criteria for febrile infants provide guidance for determining those infants with abnormal CSF results who may benefit from longer periods of observation.
Urinary tract infections are common serious bacterial infections in this age group. Enhanced urinalysis, in which cell count and Gram stain analysis are performed on uncentrifuged urine, shows 96% sensitivity of predicting urinary tract infection and can provide additional reassurance for well-appearing infants who are discharged prior to 48 hours.27
When a Longer Observation Period May Be Warranted
What You Should Do Instead: Limit Hospitalization to a Maximum of 36 Hours
For well-appearing febrile infants between 0–90 days of age hospitalized for observation and awaiting bacterial culture results, providers should consider discharge at 36 hours or less, rather than 48 hours, if blood, urine, and CSF cultures do not show bacterial growth. In a large health system, researchers implemented an evidence-based care process model for febrile infants to provide specific guidelines for laboratory testing, criteria for admission, and recommendation for discontinuation of empiric antibiotics and discharge after 36 hours in infants with negative bacterial cultures. These changes led to a 27% reduction in the length of hospital stay and 23% reduction in inpatient costs without any cases of missed bacteremia.21 The reduction in the in-hospital observation duration to 24 hours of culture incubation for well-appearing febrile infants has been advocated 32 and is a common practice for infants with appropriate follow up and parental assurance. This recommendation is supported by the following:
- Recent data showing the overwhelming majority of pathogens will be identified by blood culture <24 hours in infants aged 0-90 days32 with blood culture TTP in infants aged 0-30 days being either no different26 or potentially shorter32
- Studies showing that infants meeting low-risk clinical and laboratory profiles further reduce the likelihood of identifying serious bacterial infection after 24 hours to 0.3%.1
RECOMMENDATIONS
- Determine if febrile infants aged 0-90 days are at low risk for serious bacterial infection and obtain appropriate bacterial cultures.
- If hospitalized for observation, discharge low-risk febrile infants aged 0–90 days after 36 hours or less if bacterial cultures remain negative.
- If hospitalized for observation, consider reducing the length of inpatient observation for low-risk febrile infants aged 0–90 days with reliable follow-up to 24 hours or less when the culture results are negative.
CONCLUSION
Monitoring patients in the hospital for greater than 36 hours of bacterial culture incubation is unnecessary for patients similar to the 3 week-old full-term infant in the case presentation, who are at low risk for serious bacterial infection based on available scoring systems and have negative cultures. If patients are not deemed low risk, have an incomplete laboratory evaluation, or have had prior antibiotic treatment, longer observation in the hospital may be warranted. Close reassessment of the rare patients whose blood cultures return positive after 36 hours is necessary, but their outcomes are excellent, especially in well-appearing infants.7,33
What do you do?
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
There are no conflicts of interest relevant to this work reported by any of the authors.
1. Kaplan RL, Harper MB, Baskin MN, Macone AB, Mandl KD. Time to detection of positive cultures in 28- to 90-day-old febrile infants. Pediatrics 2000;106(6):E74. PubMed
2. Fleisher GR, Ludwig S, Henretig FM. Textbook of Pediatric Emergency Medicine: Lippincott Williams & Wilkins; 2006.
3. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants </=56 days of age. J Hosp Med. 2015;10(6):358-365. PubMed
4. Hui C, Neto G, Tsertsvadze A, et al. Diagnosis and management of febrile infants (0-3 months). Evid Rep Technol Assess. 2012;205:1-297. PubMed
5. Garcia S, Mintegi S, Gomez B, et al. Is 15 days an appropriate cut-off age for considering serious bacterial infection in the management of febrile infants? Pediatr Infect Dis J. 2012;31(5):455-458. PubMed
6. Schwartz S, Raveh D, Toker O, Segal G, Godovitch N, Schlesinger Y. A week-by-week analysis of the low-risk criteria for serious bacterial infection in febrile neonates. Arch Dis Child. 2009;94(4):287-292. PubMed
7. Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics 2010;125(2):228-233. PubMed
8. Baskin MN. The prevalence of serious bacterial infections by age in febrile infants during the first 3 months of life. Pediatr Ann. 1993;22(8):462-466. PubMed
9. Nigrovic LE, Mahajan PV, Blumberg SM, et al. The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics 2017;140(1):e20170695. PubMed
10. Bergman DA, Mayer ML, Pantell RH, Finch SA, Wasserman RC. Does clinical presentation explain practice variability in the treatment of febrile infants? Pediatrics 2006;117(3):787-795. PubMed
11. Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329(20):1437-1441. PubMed
12. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection--an appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics 1994;94(3):390-396. PubMed
13. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr. 1992;120(1):22-27. PubMed
14. Bachur RG, Harper MB. Predictive model for serious bacterial infections among infants younger than 3 months of age. Pediatrics 2001;108(2):311-316. PubMed
15. Pichichero ME, Todd JK. Detection of neonatal bacteremia. J Pediatr. 1979;94(6):958-960. PubMed
16. Hurst MK, Yoder BA. Detection of bacteremia in young infants: is 48 hours adequate? Pediatr Infect Dis J. 1995;14(8):711-713. PubMed
17. Friedman J, Matlow A. Time to identification of positive bacterial cultures in infants under three months of age hospitalized to rule out sepsis. Paediatr Child Health 1999;4(5):331-334. PubMed
18. Kliegman R, Behrman RE, Nelson WE. Nelson textbook of pediatrics. Edition 20 / ed. Philadelphia, PA: Elsevier; 2016.
19. Fever in infants and children. Merck Sharp & Dohme Corp, 2016. (Accessed 27 Nov 2016, 2016, at https://www.merckmanuals.com/professional/pediatrics/symptoms-in-infants-and-children/fever-in-infants-and-children.)
20. Polin RA, Committee on F, Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012;129(5):1006-1015. PubMed
21. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics 2012;130(1):e16-e24. PubMed
22. DeAngelis C, Joffe A, Wilson M, Willis E. Iatrogenic risks and financial costs of hospitalizing febrile infants. Am J Dis Child. 1983;137(12):1146-1149. PubMed
23. Nizam M, Norzila MZ. Stress among parents with acutely ill children. Med J Malaysia. 2001;56(4):428-434. PubMed
24. Rowley AH, Wald ER. The incubation period necessary for detection of bacteremia in immunocompetent children with fever. Implications for the clinician. Clin Pediatr (Phila). 1986;25(10):485-489. PubMed
25. La Scolea LJ, Jr., Dryja D, Sullivan TD, Mosovich L, Ellerstein N, Neter E. Diagnosis of bacteremia in children by quantitative direct plating and a radiometric procedure. J Clin Microbiol. 1981;13(3):478-482. PubMed
26. Evans RC, Fine BR. Time to detection of bacterial cultures in infants aged 0 to 90 days. Hosp Pediatr. 2013;3(2):97-102. PubMed
27. Herr SM, Wald ER, Pitetti RD, Choi SS. Enhanced urinalysis improves identification of febrile infants ages 60 days and younger at low risk for serious bacterial illness. Pediatrics 2001;108(4):866-871. PubMed
28. Nigrovic LE, Kuppermann N, Macias CG, et al. Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis. JAMA. 2007;297(1):52-60. PubMed
29. Doby EH, Stockmann C, Korgenski EK, Blaschke AJ, Byington CL. Cerebrospinal fluid pleocytosis in febrile infants 1-90 days with urinary tract infection. Pediatr Infect Dis J. 2013;32(9):1024-1026. PubMed
30. Bhansali P, Wiedermann BL, Pastor W, McMillan J, Shah N. Management of hospitalized febrile neonates without CSF analysis: A study of US pediatric hospitals. Hosp Pediatr. 2015;5(10):528-533. PubMed
31. Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics 2001;108(5):1169-1174. PubMed
32. Biondi EA, Mischler M, Jerardi KE, et al. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. PubMed
33. Moher D HC, Neto G, Tsertsvadze A. Diagnosis and Management of Febrile Infants (0–3 Months). Evidence Report/Technology Assessment No. 205. In: Center OE-bP, ed. Rockville, MD: Agency for Healthcare Research and Quality; 2012. PubMed
1. Kaplan RL, Harper MB, Baskin MN, Macone AB, Mandl KD. Time to detection of positive cultures in 28- to 90-day-old febrile infants. Pediatrics 2000;106(6):E74. PubMed
2. Fleisher GR, Ludwig S, Henretig FM. Textbook of Pediatric Emergency Medicine: Lippincott Williams & Wilkins; 2006.
3. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants </=56 days of age. J Hosp Med. 2015;10(6):358-365. PubMed
4. Hui C, Neto G, Tsertsvadze A, et al. Diagnosis and management of febrile infants (0-3 months). Evid Rep Technol Assess. 2012;205:1-297. PubMed
5. Garcia S, Mintegi S, Gomez B, et al. Is 15 days an appropriate cut-off age for considering serious bacterial infection in the management of febrile infants? Pediatr Infect Dis J. 2012;31(5):455-458. PubMed
6. Schwartz S, Raveh D, Toker O, Segal G, Godovitch N, Schlesinger Y. A week-by-week analysis of the low-risk criteria for serious bacterial infection in febrile neonates. Arch Dis Child. 2009;94(4):287-292. PubMed
7. Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics 2010;125(2):228-233. PubMed
8. Baskin MN. The prevalence of serious bacterial infections by age in febrile infants during the first 3 months of life. Pediatr Ann. 1993;22(8):462-466. PubMed
9. Nigrovic LE, Mahajan PV, Blumberg SM, et al. The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics 2017;140(1):e20170695. PubMed
10. Bergman DA, Mayer ML, Pantell RH, Finch SA, Wasserman RC. Does clinical presentation explain practice variability in the treatment of febrile infants? Pediatrics 2006;117(3):787-795. PubMed
11. Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329(20):1437-1441. PubMed
12. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection--an appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics 1994;94(3):390-396. PubMed
13. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr. 1992;120(1):22-27. PubMed
14. Bachur RG, Harper MB. Predictive model for serious bacterial infections among infants younger than 3 months of age. Pediatrics 2001;108(2):311-316. PubMed
15. Pichichero ME, Todd JK. Detection of neonatal bacteremia. J Pediatr. 1979;94(6):958-960. PubMed
16. Hurst MK, Yoder BA. Detection of bacteremia in young infants: is 48 hours adequate? Pediatr Infect Dis J. 1995;14(8):711-713. PubMed
17. Friedman J, Matlow A. Time to identification of positive bacterial cultures in infants under three months of age hospitalized to rule out sepsis. Paediatr Child Health 1999;4(5):331-334. PubMed
18. Kliegman R, Behrman RE, Nelson WE. Nelson textbook of pediatrics. Edition 20 / ed. Philadelphia, PA: Elsevier; 2016.
19. Fever in infants and children. Merck Sharp & Dohme Corp, 2016. (Accessed 27 Nov 2016, 2016, at https://www.merckmanuals.com/professional/pediatrics/symptoms-in-infants-and-children/fever-in-infants-and-children.)
20. Polin RA, Committee on F, Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012;129(5):1006-1015. PubMed
21. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics 2012;130(1):e16-e24. PubMed
22. DeAngelis C, Joffe A, Wilson M, Willis E. Iatrogenic risks and financial costs of hospitalizing febrile infants. Am J Dis Child. 1983;137(12):1146-1149. PubMed
23. Nizam M, Norzila MZ. Stress among parents with acutely ill children. Med J Malaysia. 2001;56(4):428-434. PubMed
24. Rowley AH, Wald ER. The incubation period necessary for detection of bacteremia in immunocompetent children with fever. Implications for the clinician. Clin Pediatr (Phila). 1986;25(10):485-489. PubMed
25. La Scolea LJ, Jr., Dryja D, Sullivan TD, Mosovich L, Ellerstein N, Neter E. Diagnosis of bacteremia in children by quantitative direct plating and a radiometric procedure. J Clin Microbiol. 1981;13(3):478-482. PubMed
26. Evans RC, Fine BR. Time to detection of bacterial cultures in infants aged 0 to 90 days. Hosp Pediatr. 2013;3(2):97-102. PubMed
27. Herr SM, Wald ER, Pitetti RD, Choi SS. Enhanced urinalysis improves identification of febrile infants ages 60 days and younger at low risk for serious bacterial illness. Pediatrics 2001;108(4):866-871. PubMed
28. Nigrovic LE, Kuppermann N, Macias CG, et al. Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis. JAMA. 2007;297(1):52-60. PubMed
29. Doby EH, Stockmann C, Korgenski EK, Blaschke AJ, Byington CL. Cerebrospinal fluid pleocytosis in febrile infants 1-90 days with urinary tract infection. Pediatr Infect Dis J. 2013;32(9):1024-1026. PubMed
30. Bhansali P, Wiedermann BL, Pastor W, McMillan J, Shah N. Management of hospitalized febrile neonates without CSF analysis: A study of US pediatric hospitals. Hosp Pediatr. 2015;5(10):528-533. PubMed
31. Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics 2001;108(5):1169-1174. PubMed
32. Biondi EA, Mischler M, Jerardi KE, et al. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. PubMed
33. Moher D HC, Neto G, Tsertsvadze A. Diagnosis and Management of Febrile Infants (0–3 Months). Evidence Report/Technology Assessment No. 205. In: Center OE-bP, ed. Rockville, MD: Agency for Healthcare Research and Quality; 2012. PubMed
© 2018 Society of Hospital Medicine
When Should You Suspect Kawasaki Disease as the Cause of Fever in an Infant?
Case
A seven-week-old Hispanic female with a history of prematurity (born at 35 weeks by C-section) presents to the ED with four days of fever as high as 102°F and new-onset cyanotic spells. Cultures of blood, urine, and cerebrospinal fluid obtained 48 hours prior to admission were negative, but she continued to have intermittent fevers and developed a macular, non-pruritic rash on her hands and feet, with associated non-bilious emesis. One day prior to admission, she began to have episodes of apnea, with color change and cyanosis of her lips and eyelids. In the ED, her vital signs include a rectal temperature of 38.4°C, heart rate of 178/min, respiratory rate of 27/min, and blood pressure of 79/66. Examination reveals a non-toxic-appearing infant, with no conjunctival or oropharyngeal abnormalities, unremarkable heart and lung exam, and a blanching, erythematous macular rash on her hands, lower legs, and feet.
When should you suspect Kawasaki disease (KD) as the cause of fever in an infant?
Background
KD is an acute systemic vasculitis of unknown etiology that occurs in children. Affecting the small- and medium-sized arteries, with a striking predilection for coronary arteries, it is the leading cause of acquired pediatric heart disease in Japan and the U.S.1 Occurring predominantly in children younger than five years, KD has been diagnosed in infants and in young adults.2 The incidence of KD is lowest among white children and highest among Asians and Pacific Islanders, with the highest incidence in children of Japanese descent.
A recent epidemiologic study performed in Taiwan showed an incidence of 69 cases per 100,000 per year among children younger than five years, with a male/female ratio of 1.62:1.3 The peak of mortality occurs 15-45 days after onset of fever, although sudden cardiac death may occur many years later. Recurrence rate is approximately 3%. In the U.S., the estimated incidence ranges from nine to 18 per 100,000 children younger than five years per year.4
Review of Data
Because there is no specific diagnostic test or pathognomonic clinical feature, clinical diagnostic criteria have been established to guide physicians. KD diagnosis traditionally requires fever for at least five days and the presence of at least four of the following five principal features:
- bilateral conjunctival injection;
- changes in the mucous membranes of the upper respiratory tract (injected pharynx, infected, fissured lips, strawberry tongue);
- polymorphous rash;
- changes of the extremities (peripheral edema, erythema, periungual desquamation); and
- cervical lymphadenopathy.5
The fever, which is remittent, typically peaks at 39ºC to 40ºC. The mean duration of untreated fever is 11 days; with prompt treatment, fever typically subsides in two days. Bilateral painless non-exudative conjunctival injection begins shortly after onset of fever, involves typically bulbar conjunctiva, and is not associated with edema.
Erythematous rash usually appears within five days of onset of fever and is often a diffuse, nonspecific maculopapular eruption that is commonly pronounced in the perineal region. The appearance might be urticarial, micropustular, or erythema multiforme-like. Changes in extremities include erythema of palms and soles and tender induration of the hands and feet. Subsequently, desquamation begins in the periungual area within two to three weeks after the onset of fever. Typically, peeling begins around the nail folds of fingers, followed by the toes. The least common of the principal clinical features is tender unilateral anterior cervical lymphadenopathy (1.5 cm or greater in diameter).
When a patient presents with a history, examination, and laboratory findings consistent with KD without meeting the typical diagnostic standard, incomplete KD should be considered. The term “incomplete” is favored over “atypical” for this pre-sentation, because these patients are otherwise similar to other patients with KD. Patients with fever for five or fewer days and fewer than four principal features can be diagnosed as having KD when coronary artery disease is detected by two-dimensional echocardiography or coronary angiography (see Figure 1, p. 10). In the presence of four or more principal criteria, KD can be diagnosed before day four of the illness by an experienced clinician.6 Features less consistent with KD include the presence of exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy.
If clinical features are consistent with KD, further risk stratification with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) will determine whether patients are followed daily (if low) or if supplementary laboratory tests should be done (see Figure 1, p. 10). If three or more of supplementary laboratory criteria are present (albumin ≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase (ALT), platelet count after seven days is 450 000/mm3 or greater, white blood cell count is 15,000/mm3 or greater, and urinary sediment containing 10 white blood cells/high-power field or more), echocardiogram should be performed and treatment initiated if abnormal.6
Young infants are more likely to manifest an incomplete presentation of KD, with a polymorphous rash being the most common symptom other than fever in this age group.7 Acute phase symptoms were also more likely to progress rapidly in this age group, with a higher risk of developing cardiac sequelae.8 As a result, any infant under the age of six months with fever for more than seven days and no other clear etiology should be evaluated for KD even in the absence of other diagnostic criteria.9
Other clinical manifestations of KD may include:
- Irritability: more notable in KD than in other febrile illnesses;
- Arthralgia and arthritis: may occur in the first week;
- Gastrointestinal complaints and findings: hepatomegaly, jaundice; and
- Abnormal chest X-ray findings: may be present in as many as 15% of patients.
Cardiovascular manifestations can be prominent in the acute phase of KD and are the leading cause of long-term morbidity and mortality. Coronary artery aneurysms occur in 20% of affected children with KD. Other cardiovascular complications include myocardial ischemia and ensuing depressed contractility and arrhythmias, as well as vascular obstruction in peripheral arteries.
A subset of KD patients develops hemodynamic instability requiring management in a critical care setting. This phenomenon has been named Kawasaki disease shock syndrome, where hemodynamic instability is not related to administration of intravenous immunoglobulin (IVIG). Patients are more likely to be female, to have laboratory findings consistent with greater inflammation, and to have impaired systolic and diastolic function. They also exhibit resistance to IVIG more often and have higher rates of coronary artery dilation and aneurysm formation.10
Differential diagnoses for KD may include viral infections, scarlet fever, staphylococcal scalded skin syndrome, toxic shock syndrome, Rocky Mountain spotted fever, cervical lymphadenitis, drug hypersensitivity, Stevens-Johnson syndrome, systemic idiopathic arthritis, leptospirosis, and mercury hypersensitivity reaction.11
Work-Up
Laboratory evaluation of a patient with suspected KD should include:
- Complete blood count (CBC) with differential: leukocytosis, anemia, thrombocytosis that peaks in the third week is characteristic. A manual differential may reveal an increase in band forms.
- Acute phase reactants: If C-reactive protein (CRP) is 3 mg/dL or greater and erythrocyte sedimentation rate (ESR) is 40 mm/hr or greater, supplementary laboratory work-up should be done. Make sure not to cloud classic with incomplete KD; the stepwise lab evaluation only pertains to the latter.
- Liver panel: Elevated ALT and gamma-glutamyl transferase (GGT), mild hyperbilirubinemia, or hypoalbuminemia may be present.
- Urinalysis: Sterile pyuria may be present; if present, it may be of urethral origin, and catheterized samples could miss this finding.12
Lack of elevated inflammatory markers (CRP is less than 3 mg/dl and ESR is less than 40 mm/hr) and the presence of two or three principal clinical features warrant ongoing daily monitoring of ESR, CRP, and fever until day seven of illness. If the fever resolves but is followed by peeling of extremities, an echocardiogram should be done. Lumbar puncture might help differentiate from CNS infectious etiologies, but about 50% of KD patients have a cerebrospinal fluid pleocytosis.
Echocardiography is the preferred imaging modality for the initial cardiovascular evaluation and follow-up.1 It has a sensitivity of 100% and specificity of 96% for the detection of proximal coronary aneurysms.13 Coronary aneurysms are clinically silent in most cases and can manifest with delayed complications, such as myocardial infarction or sudden death. Imaging plays an important role in the early diagnosis of these aneurysms and in estimating their number, size, and location, important elements in making a therapeutic decision.14
Although the echocardiography should be done as soon as KD is suspected, definitive treatment must not be delayed. Evaluation of all coronary artery segments, as well as cardiac contractility and presence of effusion, should be noted on echocardiography. In the absence of complications, echocardiography is performed at the time of diagnosis and at two weeks and six to eight weeks after disease onset.11
Treatment
Treatment goals for Kawasaki disease in the acute phase are reduction of systemic and coronary arterial inflammation and prevention of coronary thrombosis. The long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.6 The current standard of care for the treatment of children in the U.S. is anti-inflammatory therapy with:
- immunoglobulin (IVIG) in a single 2 g/kg/dose infused over 10–12 hours, accompanied by;
- high-dose aspirin (80–100 mg/kg/day orally in four divided doses).6,15
IVIG administration within 10 days of the onset of fever results in more favorable outcomes. Live virus vaccines should be delayed to 11 months after administration of IVIG. Both aspirin and IVIG have anti-inflammatory effects. This regimen applies to patients without abnormalities on initial echocardiography. High-dose aspirin typically is continued for 48-72 hours after the child becomes afebrile. Thereafter, low-dose aspirin (3-5 mg/kg/day) is prescribed until patient shows no evidence of coronary changes, typically by six to eight weeks after onset of illness. Children with coronary abnormalities should continue aspirin indefinitely.
Approximately 10% of patients are IVIG-resistant and have persistent or recurrent fever for at least 36 hours after completion of the infusion. The current recommendation is to re-treat with IVIG at the same dose. If the patient has fever 36 hours after the second dose of IVIG, this is considered true treatment failure.
Other possible treatments for KD refractory to IVIG include IV methylprednisolone (30 mg/kg over two to three hours daily for three days) or infliximab.16 Even with prompt treatment, 5% of children who have KD develop coronary artery dilation, and 1% develop giant aneurysms.
Back to the Case
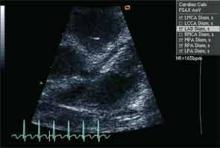
Initial laboratory evaluation revealed white blood cell count of 19.0×103 cells/mm3, hemoglobin of 8.9 gm/dL, CRP of 17.9 mg/dL, and ESR of 73 mm/hr. Because of persistent fevers for 48 hours after admission in the absence of another cause to explain the illness, the KD service was consulted. Echocardiography revealed dilatation of the left main (z-score 4.23) and proximal right (z-score 2.59), confirming the diagnosis of KD. Ejection fraction was read as qualitatively normal.
The infant received infliximab and IVIG, as well as high-dose aspirin, clopidogrel, and propranolol. This treatment regimen was directed by a KD expert and was more aggressive than typical therapy due to the severity of presentation. She received blood transfusions for worsening symptomatic anemia (hemoglobin 7.0 gm/dL) with hypoxia.
Following her IVIG infusion, she remained afebrile with progressive reduction in her CRP. She was discharged on hospital day seven on aspirin until her next follow-up, with propranolol for three days to limit potential tachycardia. At her three-week follow-up visit, her ESR had improved to 8 mm/hr. Her echocardiogram revealed a normal ejection fraction. Echocardiography revealed resolution of all abnormalities except for a borderline prominence of the right coronary artery (z-score 2.11). At this time it was recommended that her aspirin be discontinued.
She continues to be followed by the KD service as an outpatient and has done well without cardiovascular symptoms four months after her diagnosis.
Bottom Line
KD can manifest an incomplete presentation, especially in infants under the age of six months. Clinicians should maintain a high level of suspicion for KD in young infants with unexplained fevers lasting more than seven days.
Dr. Gurevich-Panigrahi is a fellow in pediatric hospital medicine at Cleveland Clinic Children’s Hospital. Dr. Kanegaye is a clinical professor of pediatrics at the University of California San Diego (UCSD) School of Medicine and attending physician in the emergency care center at Rady Children’s Hospital San Diego. Dr. Chang is associate clinical professor of pediatrics and medicine at UCSD School of Medicine, a pediatric hospitalist at Rady Children’s, and pediatric editor of The Hospitalist.
References
- Hendaoui L, Stanson AW, Habib Bouhaouala M, Joffre F, eds. Systemic Vasculitis: Imaging Features. New York: Springer; 2012.
- Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124(3):e410-e415.
- Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
- Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-438.
- Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Shiozawa Y, Inuzuka R, Harita Y, Kagawa J. Age-related differences in the course of the acute phase symptoms of Kawasaki disease. Pediatr Infect Dis J. 2013;32(9):e365-369.
- Genizi J, Miron D, Spiegel R, Fink D, Horowitz Y. Kawasaki disease in very young infants: high prevalence of atypical presentation and coronary arteritis. Clin Pediatr (Phila.). 2003;42(3):263-267.
- Sundel R. Incomplete (atypical) Kawasaki disease. UpToDate. Available at: http://www.uptodate.com/contents/incomplete-atypical-kawasaki-disease. Accessed June 9, 2014.
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783-e789.
- Fimbres AM, Shulman ST. Kawasaki disease. Pediatr Rev. 2008;29(9):308-315.
- Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440-443.
- Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355-360.
- Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27-31.
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544.
- Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2(2):71-76.
Case
A seven-week-old Hispanic female with a history of prematurity (born at 35 weeks by C-section) presents to the ED with four days of fever as high as 102°F and new-onset cyanotic spells. Cultures of blood, urine, and cerebrospinal fluid obtained 48 hours prior to admission were negative, but she continued to have intermittent fevers and developed a macular, non-pruritic rash on her hands and feet, with associated non-bilious emesis. One day prior to admission, she began to have episodes of apnea, with color change and cyanosis of her lips and eyelids. In the ED, her vital signs include a rectal temperature of 38.4°C, heart rate of 178/min, respiratory rate of 27/min, and blood pressure of 79/66. Examination reveals a non-toxic-appearing infant, with no conjunctival or oropharyngeal abnormalities, unremarkable heart and lung exam, and a blanching, erythematous macular rash on her hands, lower legs, and feet.
When should you suspect Kawasaki disease (KD) as the cause of fever in an infant?
Background
KD is an acute systemic vasculitis of unknown etiology that occurs in children. Affecting the small- and medium-sized arteries, with a striking predilection for coronary arteries, it is the leading cause of acquired pediatric heart disease in Japan and the U.S.1 Occurring predominantly in children younger than five years, KD has been diagnosed in infants and in young adults.2 The incidence of KD is lowest among white children and highest among Asians and Pacific Islanders, with the highest incidence in children of Japanese descent.
A recent epidemiologic study performed in Taiwan showed an incidence of 69 cases per 100,000 per year among children younger than five years, with a male/female ratio of 1.62:1.3 The peak of mortality occurs 15-45 days after onset of fever, although sudden cardiac death may occur many years later. Recurrence rate is approximately 3%. In the U.S., the estimated incidence ranges from nine to 18 per 100,000 children younger than five years per year.4
Review of Data
Because there is no specific diagnostic test or pathognomonic clinical feature, clinical diagnostic criteria have been established to guide physicians. KD diagnosis traditionally requires fever for at least five days and the presence of at least four of the following five principal features:
- bilateral conjunctival injection;
- changes in the mucous membranes of the upper respiratory tract (injected pharynx, infected, fissured lips, strawberry tongue);
- polymorphous rash;
- changes of the extremities (peripheral edema, erythema, periungual desquamation); and
- cervical lymphadenopathy.5
The fever, which is remittent, typically peaks at 39ºC to 40ºC. The mean duration of untreated fever is 11 days; with prompt treatment, fever typically subsides in two days. Bilateral painless non-exudative conjunctival injection begins shortly after onset of fever, involves typically bulbar conjunctiva, and is not associated with edema.
Erythematous rash usually appears within five days of onset of fever and is often a diffuse, nonspecific maculopapular eruption that is commonly pronounced in the perineal region. The appearance might be urticarial, micropustular, or erythema multiforme-like. Changes in extremities include erythema of palms and soles and tender induration of the hands and feet. Subsequently, desquamation begins in the periungual area within two to three weeks after the onset of fever. Typically, peeling begins around the nail folds of fingers, followed by the toes. The least common of the principal clinical features is tender unilateral anterior cervical lymphadenopathy (1.5 cm or greater in diameter).
When a patient presents with a history, examination, and laboratory findings consistent with KD without meeting the typical diagnostic standard, incomplete KD should be considered. The term “incomplete” is favored over “atypical” for this pre-sentation, because these patients are otherwise similar to other patients with KD. Patients with fever for five or fewer days and fewer than four principal features can be diagnosed as having KD when coronary artery disease is detected by two-dimensional echocardiography or coronary angiography (see Figure 1, p. 10). In the presence of four or more principal criteria, KD can be diagnosed before day four of the illness by an experienced clinician.6 Features less consistent with KD include the presence of exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy.
If clinical features are consistent with KD, further risk stratification with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) will determine whether patients are followed daily (if low) or if supplementary laboratory tests should be done (see Figure 1, p. 10). If three or more of supplementary laboratory criteria are present (albumin ≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase (ALT), platelet count after seven days is 450 000/mm3 or greater, white blood cell count is 15,000/mm3 or greater, and urinary sediment containing 10 white blood cells/high-power field or more), echocardiogram should be performed and treatment initiated if abnormal.6
Young infants are more likely to manifest an incomplete presentation of KD, with a polymorphous rash being the most common symptom other than fever in this age group.7 Acute phase symptoms were also more likely to progress rapidly in this age group, with a higher risk of developing cardiac sequelae.8 As a result, any infant under the age of six months with fever for more than seven days and no other clear etiology should be evaluated for KD even in the absence of other diagnostic criteria.9
Other clinical manifestations of KD may include:
- Irritability: more notable in KD than in other febrile illnesses;
- Arthralgia and arthritis: may occur in the first week;
- Gastrointestinal complaints and findings: hepatomegaly, jaundice; and
- Abnormal chest X-ray findings: may be present in as many as 15% of patients.
Cardiovascular manifestations can be prominent in the acute phase of KD and are the leading cause of long-term morbidity and mortality. Coronary artery aneurysms occur in 20% of affected children with KD. Other cardiovascular complications include myocardial ischemia and ensuing depressed contractility and arrhythmias, as well as vascular obstruction in peripheral arteries.
A subset of KD patients develops hemodynamic instability requiring management in a critical care setting. This phenomenon has been named Kawasaki disease shock syndrome, where hemodynamic instability is not related to administration of intravenous immunoglobulin (IVIG). Patients are more likely to be female, to have laboratory findings consistent with greater inflammation, and to have impaired systolic and diastolic function. They also exhibit resistance to IVIG more often and have higher rates of coronary artery dilation and aneurysm formation.10
Differential diagnoses for KD may include viral infections, scarlet fever, staphylococcal scalded skin syndrome, toxic shock syndrome, Rocky Mountain spotted fever, cervical lymphadenitis, drug hypersensitivity, Stevens-Johnson syndrome, systemic idiopathic arthritis, leptospirosis, and mercury hypersensitivity reaction.11
Work-Up
Laboratory evaluation of a patient with suspected KD should include:
- Complete blood count (CBC) with differential: leukocytosis, anemia, thrombocytosis that peaks in the third week is characteristic. A manual differential may reveal an increase in band forms.
- Acute phase reactants: If C-reactive protein (CRP) is 3 mg/dL or greater and erythrocyte sedimentation rate (ESR) is 40 mm/hr or greater, supplementary laboratory work-up should be done. Make sure not to cloud classic with incomplete KD; the stepwise lab evaluation only pertains to the latter.
- Liver panel: Elevated ALT and gamma-glutamyl transferase (GGT), mild hyperbilirubinemia, or hypoalbuminemia may be present.
- Urinalysis: Sterile pyuria may be present; if present, it may be of urethral origin, and catheterized samples could miss this finding.12
Lack of elevated inflammatory markers (CRP is less than 3 mg/dl and ESR is less than 40 mm/hr) and the presence of two or three principal clinical features warrant ongoing daily monitoring of ESR, CRP, and fever until day seven of illness. If the fever resolves but is followed by peeling of extremities, an echocardiogram should be done. Lumbar puncture might help differentiate from CNS infectious etiologies, but about 50% of KD patients have a cerebrospinal fluid pleocytosis.
Echocardiography is the preferred imaging modality for the initial cardiovascular evaluation and follow-up.1 It has a sensitivity of 100% and specificity of 96% for the detection of proximal coronary aneurysms.13 Coronary aneurysms are clinically silent in most cases and can manifest with delayed complications, such as myocardial infarction or sudden death. Imaging plays an important role in the early diagnosis of these aneurysms and in estimating their number, size, and location, important elements in making a therapeutic decision.14
Although the echocardiography should be done as soon as KD is suspected, definitive treatment must not be delayed. Evaluation of all coronary artery segments, as well as cardiac contractility and presence of effusion, should be noted on echocardiography. In the absence of complications, echocardiography is performed at the time of diagnosis and at two weeks and six to eight weeks after disease onset.11
Treatment
Treatment goals for Kawasaki disease in the acute phase are reduction of systemic and coronary arterial inflammation and prevention of coronary thrombosis. The long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.6 The current standard of care for the treatment of children in the U.S. is anti-inflammatory therapy with:
- immunoglobulin (IVIG) in a single 2 g/kg/dose infused over 10–12 hours, accompanied by;
- high-dose aspirin (80–100 mg/kg/day orally in four divided doses).6,15
IVIG administration within 10 days of the onset of fever results in more favorable outcomes. Live virus vaccines should be delayed to 11 months after administration of IVIG. Both aspirin and IVIG have anti-inflammatory effects. This regimen applies to patients without abnormalities on initial echocardiography. High-dose aspirin typically is continued for 48-72 hours after the child becomes afebrile. Thereafter, low-dose aspirin (3-5 mg/kg/day) is prescribed until patient shows no evidence of coronary changes, typically by six to eight weeks after onset of illness. Children with coronary abnormalities should continue aspirin indefinitely.
Approximately 10% of patients are IVIG-resistant and have persistent or recurrent fever for at least 36 hours after completion of the infusion. The current recommendation is to re-treat with IVIG at the same dose. If the patient has fever 36 hours after the second dose of IVIG, this is considered true treatment failure.
Other possible treatments for KD refractory to IVIG include IV methylprednisolone (30 mg/kg over two to three hours daily for three days) or infliximab.16 Even with prompt treatment, 5% of children who have KD develop coronary artery dilation, and 1% develop giant aneurysms.
Back to the Case
Initial laboratory evaluation revealed white blood cell count of 19.0×103 cells/mm3, hemoglobin of 8.9 gm/dL, CRP of 17.9 mg/dL, and ESR of 73 mm/hr. Because of persistent fevers for 48 hours after admission in the absence of another cause to explain the illness, the KD service was consulted. Echocardiography revealed dilatation of the left main (z-score 4.23) and proximal right (z-score 2.59), confirming the diagnosis of KD. Ejection fraction was read as qualitatively normal.
The infant received infliximab and IVIG, as well as high-dose aspirin, clopidogrel, and propranolol. This treatment regimen was directed by a KD expert and was more aggressive than typical therapy due to the severity of presentation. She received blood transfusions for worsening symptomatic anemia (hemoglobin 7.0 gm/dL) with hypoxia.
Following her IVIG infusion, she remained afebrile with progressive reduction in her CRP. She was discharged on hospital day seven on aspirin until her next follow-up, with propranolol for three days to limit potential tachycardia. At her three-week follow-up visit, her ESR had improved to 8 mm/hr. Her echocardiogram revealed a normal ejection fraction. Echocardiography revealed resolution of all abnormalities except for a borderline prominence of the right coronary artery (z-score 2.11). At this time it was recommended that her aspirin be discontinued.
She continues to be followed by the KD service as an outpatient and has done well without cardiovascular symptoms four months after her diagnosis.
Bottom Line
KD can manifest an incomplete presentation, especially in infants under the age of six months. Clinicians should maintain a high level of suspicion for KD in young infants with unexplained fevers lasting more than seven days.
Dr. Gurevich-Panigrahi is a fellow in pediatric hospital medicine at Cleveland Clinic Children’s Hospital. Dr. Kanegaye is a clinical professor of pediatrics at the University of California San Diego (UCSD) School of Medicine and attending physician in the emergency care center at Rady Children’s Hospital San Diego. Dr. Chang is associate clinical professor of pediatrics and medicine at UCSD School of Medicine, a pediatric hospitalist at Rady Children’s, and pediatric editor of The Hospitalist.
References
- Hendaoui L, Stanson AW, Habib Bouhaouala M, Joffre F, eds. Systemic Vasculitis: Imaging Features. New York: Springer; 2012.
- Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124(3):e410-e415.
- Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
- Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-438.
- Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Shiozawa Y, Inuzuka R, Harita Y, Kagawa J. Age-related differences in the course of the acute phase symptoms of Kawasaki disease. Pediatr Infect Dis J. 2013;32(9):e365-369.
- Genizi J, Miron D, Spiegel R, Fink D, Horowitz Y. Kawasaki disease in very young infants: high prevalence of atypical presentation and coronary arteritis. Clin Pediatr (Phila.). 2003;42(3):263-267.
- Sundel R. Incomplete (atypical) Kawasaki disease. UpToDate. Available at: http://www.uptodate.com/contents/incomplete-atypical-kawasaki-disease. Accessed June 9, 2014.
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783-e789.
- Fimbres AM, Shulman ST. Kawasaki disease. Pediatr Rev. 2008;29(9):308-315.
- Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440-443.
- Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355-360.
- Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27-31.
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544.
- Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2(2):71-76.
Case
A seven-week-old Hispanic female with a history of prematurity (born at 35 weeks by C-section) presents to the ED with four days of fever as high as 102°F and new-onset cyanotic spells. Cultures of blood, urine, and cerebrospinal fluid obtained 48 hours prior to admission were negative, but she continued to have intermittent fevers and developed a macular, non-pruritic rash on her hands and feet, with associated non-bilious emesis. One day prior to admission, she began to have episodes of apnea, with color change and cyanosis of her lips and eyelids. In the ED, her vital signs include a rectal temperature of 38.4°C, heart rate of 178/min, respiratory rate of 27/min, and blood pressure of 79/66. Examination reveals a non-toxic-appearing infant, with no conjunctival or oropharyngeal abnormalities, unremarkable heart and lung exam, and a blanching, erythematous macular rash on her hands, lower legs, and feet.
When should you suspect Kawasaki disease (KD) as the cause of fever in an infant?
Background
KD is an acute systemic vasculitis of unknown etiology that occurs in children. Affecting the small- and medium-sized arteries, with a striking predilection for coronary arteries, it is the leading cause of acquired pediatric heart disease in Japan and the U.S.1 Occurring predominantly in children younger than five years, KD has been diagnosed in infants and in young adults.2 The incidence of KD is lowest among white children and highest among Asians and Pacific Islanders, with the highest incidence in children of Japanese descent.
A recent epidemiologic study performed in Taiwan showed an incidence of 69 cases per 100,000 per year among children younger than five years, with a male/female ratio of 1.62:1.3 The peak of mortality occurs 15-45 days after onset of fever, although sudden cardiac death may occur many years later. Recurrence rate is approximately 3%. In the U.S., the estimated incidence ranges from nine to 18 per 100,000 children younger than five years per year.4
Review of Data
Because there is no specific diagnostic test or pathognomonic clinical feature, clinical diagnostic criteria have been established to guide physicians. KD diagnosis traditionally requires fever for at least five days and the presence of at least four of the following five principal features:
- bilateral conjunctival injection;
- changes in the mucous membranes of the upper respiratory tract (injected pharynx, infected, fissured lips, strawberry tongue);
- polymorphous rash;
- changes of the extremities (peripheral edema, erythema, periungual desquamation); and
- cervical lymphadenopathy.5
The fever, which is remittent, typically peaks at 39ºC to 40ºC. The mean duration of untreated fever is 11 days; with prompt treatment, fever typically subsides in two days. Bilateral painless non-exudative conjunctival injection begins shortly after onset of fever, involves typically bulbar conjunctiva, and is not associated with edema.
Erythematous rash usually appears within five days of onset of fever and is often a diffuse, nonspecific maculopapular eruption that is commonly pronounced in the perineal region. The appearance might be urticarial, micropustular, or erythema multiforme-like. Changes in extremities include erythema of palms and soles and tender induration of the hands and feet. Subsequently, desquamation begins in the periungual area within two to three weeks after the onset of fever. Typically, peeling begins around the nail folds of fingers, followed by the toes. The least common of the principal clinical features is tender unilateral anterior cervical lymphadenopathy (1.5 cm or greater in diameter).
When a patient presents with a history, examination, and laboratory findings consistent with KD without meeting the typical diagnostic standard, incomplete KD should be considered. The term “incomplete” is favored over “atypical” for this pre-sentation, because these patients are otherwise similar to other patients with KD. Patients with fever for five or fewer days and fewer than four principal features can be diagnosed as having KD when coronary artery disease is detected by two-dimensional echocardiography or coronary angiography (see Figure 1, p. 10). In the presence of four or more principal criteria, KD can be diagnosed before day four of the illness by an experienced clinician.6 Features less consistent with KD include the presence of exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy.
If clinical features are consistent with KD, further risk stratification with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) will determine whether patients are followed daily (if low) or if supplementary laboratory tests should be done (see Figure 1, p. 10). If three or more of supplementary laboratory criteria are present (albumin ≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase (ALT), platelet count after seven days is 450 000/mm3 or greater, white blood cell count is 15,000/mm3 or greater, and urinary sediment containing 10 white blood cells/high-power field or more), echocardiogram should be performed and treatment initiated if abnormal.6
Young infants are more likely to manifest an incomplete presentation of KD, with a polymorphous rash being the most common symptom other than fever in this age group.7 Acute phase symptoms were also more likely to progress rapidly in this age group, with a higher risk of developing cardiac sequelae.8 As a result, any infant under the age of six months with fever for more than seven days and no other clear etiology should be evaluated for KD even in the absence of other diagnostic criteria.9
Other clinical manifestations of KD may include:
- Irritability: more notable in KD than in other febrile illnesses;
- Arthralgia and arthritis: may occur in the first week;
- Gastrointestinal complaints and findings: hepatomegaly, jaundice; and
- Abnormal chest X-ray findings: may be present in as many as 15% of patients.
Cardiovascular manifestations can be prominent in the acute phase of KD and are the leading cause of long-term morbidity and mortality. Coronary artery aneurysms occur in 20% of affected children with KD. Other cardiovascular complications include myocardial ischemia and ensuing depressed contractility and arrhythmias, as well as vascular obstruction in peripheral arteries.
A subset of KD patients develops hemodynamic instability requiring management in a critical care setting. This phenomenon has been named Kawasaki disease shock syndrome, where hemodynamic instability is not related to administration of intravenous immunoglobulin (IVIG). Patients are more likely to be female, to have laboratory findings consistent with greater inflammation, and to have impaired systolic and diastolic function. They also exhibit resistance to IVIG more often and have higher rates of coronary artery dilation and aneurysm formation.10
Differential diagnoses for KD may include viral infections, scarlet fever, staphylococcal scalded skin syndrome, toxic shock syndrome, Rocky Mountain spotted fever, cervical lymphadenitis, drug hypersensitivity, Stevens-Johnson syndrome, systemic idiopathic arthritis, leptospirosis, and mercury hypersensitivity reaction.11
Work-Up
Laboratory evaluation of a patient with suspected KD should include:
- Complete blood count (CBC) with differential: leukocytosis, anemia, thrombocytosis that peaks in the third week is characteristic. A manual differential may reveal an increase in band forms.
- Acute phase reactants: If C-reactive protein (CRP) is 3 mg/dL or greater and erythrocyte sedimentation rate (ESR) is 40 mm/hr or greater, supplementary laboratory work-up should be done. Make sure not to cloud classic with incomplete KD; the stepwise lab evaluation only pertains to the latter.
- Liver panel: Elevated ALT and gamma-glutamyl transferase (GGT), mild hyperbilirubinemia, or hypoalbuminemia may be present.
- Urinalysis: Sterile pyuria may be present; if present, it may be of urethral origin, and catheterized samples could miss this finding.12
Lack of elevated inflammatory markers (CRP is less than 3 mg/dl and ESR is less than 40 mm/hr) and the presence of two or three principal clinical features warrant ongoing daily monitoring of ESR, CRP, and fever until day seven of illness. If the fever resolves but is followed by peeling of extremities, an echocardiogram should be done. Lumbar puncture might help differentiate from CNS infectious etiologies, but about 50% of KD patients have a cerebrospinal fluid pleocytosis.
Echocardiography is the preferred imaging modality for the initial cardiovascular evaluation and follow-up.1 It has a sensitivity of 100% and specificity of 96% for the detection of proximal coronary aneurysms.13 Coronary aneurysms are clinically silent in most cases and can manifest with delayed complications, such as myocardial infarction or sudden death. Imaging plays an important role in the early diagnosis of these aneurysms and in estimating their number, size, and location, important elements in making a therapeutic decision.14
Although the echocardiography should be done as soon as KD is suspected, definitive treatment must not be delayed. Evaluation of all coronary artery segments, as well as cardiac contractility and presence of effusion, should be noted on echocardiography. In the absence of complications, echocardiography is performed at the time of diagnosis and at two weeks and six to eight weeks after disease onset.11
Treatment
Treatment goals for Kawasaki disease in the acute phase are reduction of systemic and coronary arterial inflammation and prevention of coronary thrombosis. The long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.6 The current standard of care for the treatment of children in the U.S. is anti-inflammatory therapy with:
- immunoglobulin (IVIG) in a single 2 g/kg/dose infused over 10–12 hours, accompanied by;
- high-dose aspirin (80–100 mg/kg/day orally in four divided doses).6,15
IVIG administration within 10 days of the onset of fever results in more favorable outcomes. Live virus vaccines should be delayed to 11 months after administration of IVIG. Both aspirin and IVIG have anti-inflammatory effects. This regimen applies to patients without abnormalities on initial echocardiography. High-dose aspirin typically is continued for 48-72 hours after the child becomes afebrile. Thereafter, low-dose aspirin (3-5 mg/kg/day) is prescribed until patient shows no evidence of coronary changes, typically by six to eight weeks after onset of illness. Children with coronary abnormalities should continue aspirin indefinitely.
Approximately 10% of patients are IVIG-resistant and have persistent or recurrent fever for at least 36 hours after completion of the infusion. The current recommendation is to re-treat with IVIG at the same dose. If the patient has fever 36 hours after the second dose of IVIG, this is considered true treatment failure.
Other possible treatments for KD refractory to IVIG include IV methylprednisolone (30 mg/kg over two to three hours daily for three days) or infliximab.16 Even with prompt treatment, 5% of children who have KD develop coronary artery dilation, and 1% develop giant aneurysms.
Back to the Case
Initial laboratory evaluation revealed white blood cell count of 19.0×103 cells/mm3, hemoglobin of 8.9 gm/dL, CRP of 17.9 mg/dL, and ESR of 73 mm/hr. Because of persistent fevers for 48 hours after admission in the absence of another cause to explain the illness, the KD service was consulted. Echocardiography revealed dilatation of the left main (z-score 4.23) and proximal right (z-score 2.59), confirming the diagnosis of KD. Ejection fraction was read as qualitatively normal.
The infant received infliximab and IVIG, as well as high-dose aspirin, clopidogrel, and propranolol. This treatment regimen was directed by a KD expert and was more aggressive than typical therapy due to the severity of presentation. She received blood transfusions for worsening symptomatic anemia (hemoglobin 7.0 gm/dL) with hypoxia.
Following her IVIG infusion, she remained afebrile with progressive reduction in her CRP. She was discharged on hospital day seven on aspirin until her next follow-up, with propranolol for three days to limit potential tachycardia. At her three-week follow-up visit, her ESR had improved to 8 mm/hr. Her echocardiogram revealed a normal ejection fraction. Echocardiography revealed resolution of all abnormalities except for a borderline prominence of the right coronary artery (z-score 2.11). At this time it was recommended that her aspirin be discontinued.
She continues to be followed by the KD service as an outpatient and has done well without cardiovascular symptoms four months after her diagnosis.
Bottom Line
KD can manifest an incomplete presentation, especially in infants under the age of six months. Clinicians should maintain a high level of suspicion for KD in young infants with unexplained fevers lasting more than seven days.
Dr. Gurevich-Panigrahi is a fellow in pediatric hospital medicine at Cleveland Clinic Children’s Hospital. Dr. Kanegaye is a clinical professor of pediatrics at the University of California San Diego (UCSD) School of Medicine and attending physician in the emergency care center at Rady Children’s Hospital San Diego. Dr. Chang is associate clinical professor of pediatrics and medicine at UCSD School of Medicine, a pediatric hospitalist at Rady Children’s, and pediatric editor of The Hospitalist.
References
- Hendaoui L, Stanson AW, Habib Bouhaouala M, Joffre F, eds. Systemic Vasculitis: Imaging Features. New York: Springer; 2012.
- Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124(3):e410-e415.
- Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
- Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-438.
- Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Shiozawa Y, Inuzuka R, Harita Y, Kagawa J. Age-related differences in the course of the acute phase symptoms of Kawasaki disease. Pediatr Infect Dis J. 2013;32(9):e365-369.
- Genizi J, Miron D, Spiegel R, Fink D, Horowitz Y. Kawasaki disease in very young infants: high prevalence of atypical presentation and coronary arteritis. Clin Pediatr (Phila.). 2003;42(3):263-267.
- Sundel R. Incomplete (atypical) Kawasaki disease. UpToDate. Available at: http://www.uptodate.com/contents/incomplete-atypical-kawasaki-disease. Accessed June 9, 2014.
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783-e789.
- Fimbres AM, Shulman ST. Kawasaki disease. Pediatr Rev. 2008;29(9):308-315.
- Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440-443.
- Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355-360.
- Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27-31.
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544.
- Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2(2):71-76.
Hands-On Training Helps Prepare Hospitalists for Procedures
Sally Wang MD, FHM, director of procedure education at Brigham and Women’s Hospital in Boston, and Brad Rosen, MD, MBA, FHM, medical director of the Inpatient Specialty Program (ISP) at Cedars-Sinai Hospital in Los Angeles, led another rapid-fire pre-course in ultrasound-guided procedures for the hospitalist at HM13.
Drs. Wang, Rosen, and a veteran group of faculty and trainers brought hands-on training in core bedside procedures, plus training in relatively new procedures to hospitalists such as intraosseous lines and skin biopsies. All attendees received close interaction with faculty and trainers, and participated in training exercises on tissue models, training models, and live models.
Additional discussion was focused on developing a proceduralist program. Experts explained the required commitment to proficiency and ongoing data collection, quality improvement, and “customer service” to stakeholders. But the basics of ongoing experiential learning are invaluable, they said, and often begin with simulation exercises.
Hospitalists thinking of becoming a proceduralist should start by making sure that they are proficient and experienced, and have invested the time necessary to maintain that experience. Beyond personal interest in procedures, administering an HM program that encourages and fosters procedural experience requires input from multiple stakeholders, as well as ongoing efforts to promote a climate of safety surrounding bedside procedures. TH
Dr. Chang is med-peds hospitalist at Univeristy of California San Diego, and a Team Hospitalist member.
Sally Wang MD, FHM, director of procedure education at Brigham and Women’s Hospital in Boston, and Brad Rosen, MD, MBA, FHM, medical director of the Inpatient Specialty Program (ISP) at Cedars-Sinai Hospital in Los Angeles, led another rapid-fire pre-course in ultrasound-guided procedures for the hospitalist at HM13.
Drs. Wang, Rosen, and a veteran group of faculty and trainers brought hands-on training in core bedside procedures, plus training in relatively new procedures to hospitalists such as intraosseous lines and skin biopsies. All attendees received close interaction with faculty and trainers, and participated in training exercises on tissue models, training models, and live models.
Additional discussion was focused on developing a proceduralist program. Experts explained the required commitment to proficiency and ongoing data collection, quality improvement, and “customer service” to stakeholders. But the basics of ongoing experiential learning are invaluable, they said, and often begin with simulation exercises.
Hospitalists thinking of becoming a proceduralist should start by making sure that they are proficient and experienced, and have invested the time necessary to maintain that experience. Beyond personal interest in procedures, administering an HM program that encourages and fosters procedural experience requires input from multiple stakeholders, as well as ongoing efforts to promote a climate of safety surrounding bedside procedures. TH
Dr. Chang is med-peds hospitalist at Univeristy of California San Diego, and a Team Hospitalist member.
Sally Wang MD, FHM, director of procedure education at Brigham and Women’s Hospital in Boston, and Brad Rosen, MD, MBA, FHM, medical director of the Inpatient Specialty Program (ISP) at Cedars-Sinai Hospital in Los Angeles, led another rapid-fire pre-course in ultrasound-guided procedures for the hospitalist at HM13.
Drs. Wang, Rosen, and a veteran group of faculty and trainers brought hands-on training in core bedside procedures, plus training in relatively new procedures to hospitalists such as intraosseous lines and skin biopsies. All attendees received close interaction with faculty and trainers, and participated in training exercises on tissue models, training models, and live models.
Additional discussion was focused on developing a proceduralist program. Experts explained the required commitment to proficiency and ongoing data collection, quality improvement, and “customer service” to stakeholders. But the basics of ongoing experiential learning are invaluable, they said, and often begin with simulation exercises.
Hospitalists thinking of becoming a proceduralist should start by making sure that they are proficient and experienced, and have invested the time necessary to maintain that experience. Beyond personal interest in procedures, administering an HM program that encourages and fosters procedural experience requires input from multiple stakeholders, as well as ongoing efforts to promote a climate of safety surrounding bedside procedures. TH
Dr. Chang is med-peds hospitalist at Univeristy of California San Diego, and a Team Hospitalist member.
Is Hospitalist Proficiency in Bedside Procedures in Decline?
It’s 3:30 p.m. You’ve seen your old patients, holdovers, and an admission, but you haven’t finished your notes yet. Lunch was an afterthought between emails about schedule changes for the upcoming year. Two pages ring happily from your belt, the first from you-know-who in the ED, and the next from a nurse: “THORA SUPPLIES AT BEDSIDE SINCE THIS AM—WHEN WILL THIS HAPPEN?” The phone number on the wall for the on-call radiologist beckons...
An all-too-familiar situation for hospitalists across the country, this awkward moment raises a series of difficult questions:
Should I set aside time from my day to perform a procedure that could be time-consuming?
- Do I feel confident I can perform this procedure safely?
- Am I really the best physician to provide this service?
- As hospitalists are tasked with an ever-increasing array of responsibilities, answering the call of duty for bedside procedures is becoming more difficult for some.
A Core Competency
“The Core Competencies in Hospital Medicine,” authored by a group of HM thought leaders, was published as a supplement to the January/February 2006 issue of the Journal of Hospital Medicine. The core competencies include such bedside procedures as arthrocentesis, paracentesis, thoracentesis, lumbar puncture, and vascular (arterial and central venous) access (see “Core Competencies in Hospital Medicine: Procedures,” below). Although the authors stressed that the core competencies are to be viewed as a resource rather than as a set of requirements, the inclusion of bedside procedures emphasized the importance of procedural skills for future hospitalists.
“[Hospitalists] are in a perfect spot to continue to perform procedures in a structured manner,” says Joshua Lenchus, DO, RPh, FACP, FHM, associate director of the University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety. “As agents of quality and safety, hospitalists should continue to perform this clinically necessary service.”
Jeffrey Barsuk, MD, FHM, associate professor of medicine at Northwestern Feinberg School of Medicine in Chicago and an academic hospitalist at Northwestern Memorial Hospital (NMH), not only agrees that bedside procedures should be a core competency, but he also says hospitalists are the most appropriate providers of these services.
“I think this is part of hospital medicine. We’re in the hospital, [and] that’s what we do,” Dr. Barsuk says. Other providers, such as interventional radiologists, “really don’t understand why I’m doing [a procedure]. They understand it’s safe to do it, but they might not understand all the indications for it, and they certainly don’t understand the interpretation of the tests they’re sending.”
Despite the goals set forth by the core competencies and authorities in procedural safety, the reality of who actually performs bedside procedures is somewhat murky and varies greatly by institution. Many point to HM program setting (urban vs. rural) or structure (academic vs. community) to explain variance, but often it is other factors that determine whether hospitalists are actually preforming bedside procedures regularly.
Where Does HM Perform Procedures?
Community hospitalists, with strong support from interventional radiologists and subspecialists, often find it more efficient—even necessary, considering their patient volumes—to leave procedures to others. Community hospitalists with ICU admitting privileges, intensivists, and other HM subgroups say that being able to perform procedures should be a prerequisite for employment. Hospitalists in rural communities say they are doing procedures because they are “the only game in town.”
“Sometimes you are the only one available, and you are called upon to stretch your abilities,” says Beatrice Szantyr, MD, FAAP, a community hospitalist and pediatrician in Lincoln, Maine, who has practiced most of her career in rural settings.
Academic hospitalists in large, research-based HM programs can, paradoxically, find themselves performing fewer procedures as residents often take the lead on the majority of such cases. Conversely, academic hospitalists in large, nonteaching programs often find themselves called on to perform more bedside procedures.
No matter the setting, the simplicity of being the physician to recognize the need for a procedure, perform it, and interpret the results is undeniably efficient and “clean,” according to authorities on inpatient bedside procedures. Having to consult other physicians, optimize the patient’s lab values to their standards (a common issue with interventional radiologists), and adhere to their work schedules can often delay procedures unnecessarily.
—Joshua Lenchus, DO, RPh, FACP, FHM, associate director, University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety
“Hospitalists care for floor and ICU patients in many hospitals, and the inability to perform bedside procedures delays patient care,” says Dr. Nilam Soni, an academic hospitalist at the University of Chicago and a recognized expert on procedural safety.
Dr. Soni notes that when it comes to current techniques, many hospitalists suffer from a knowledge deficit. “The introduction of ultrasound for guidance of bedside procedures has been shown to improve the success and safety of certain procedures,” he says, “but the majority of practicing hospitalists did not learn how to use ultrasound for procedure guidance during residency.”
Heterogeneity of Training, Experience, and Skill
While all hospitalists draw upon different bases of training and experience, the heterogeneity of training, confidence, and inherent skill is greatest when it comes to bedside procedures. Mirroring the heterogeneity at the individual level, hospitalist programs vary greatly on the requirements placed on their staffs in regards to procedural skill and privileging.
Such research-driven programs as Brigham and Women’s Hospital (BWH) in Boston often find requiring maintenance of privileges in bedside procedures to be difficult, says Sally Wang, MD, FHM, director of procedural education at BWH. In fact, a new procedure service being created there will be staffed mainly with ED physicians. On the flipside, most community hospitalist programs leave the task of procedural “policing” to the hospital’s medical staff affairs office.
At the University of California at San Diego (UCSD) Medical Center, the HM group is instituting a division standard in which hospitalists maintain privileging and proficiency in a core group of bedside procedures. Other large hospitalist groups have created “proceduralist” subgroups that shoulder the burden of trainee education, as well as provide a resource for less skilled or less experienced inpatient providers.
“If you have a big group, you could have a dedicated procedure service and have a core group of hospitalists who are experts in procedure,” Dr. Barsuk says. “But it needs to be self-sustaining.” Once started, Dr. Barsuk says, proceduralist groups would continue to provide hospitals with ongoing return-on-investment (ROI) benefit.
Variability in procedure volume and payor mix, however, can make it hard for HM groups to demonstrate to hospital leadership a satisfactory ROI for a proceduralist program. Financial backing from grant support or a high-volume procedure—such as paracentesis in hospitals with large hepatology programs—can nurture starting proceduralist programs until all procedural revenues can justify the costs. Lower ROI can also be justified by showing improvement in quality indices—such as CLABSI rates—reduced time to procedures, and reduced costs compared to other subspecialists offering similar services.
“I’m of the firm belief that we can reduce costs by doing the procedures at the bedside rather than referring them to departments such as interventional radiology (IR),” Dr. Barsuk says. “What you would have to do is show the institution that it costs more money to have IR do [bedside procedures].”
National Response

—Jeffrey Barsuk, MD, MS, FHM, associate professor of medicine, Northwestern Feinberg School of Medicine, academic hospitalist, Northwestern Memorial Hospital, Chicago
Filling in the procedural training gaps found on the local level, such national organizations as SHM have stepped in to provide education and support for hospitalists yearning for training. Since its inception, an SHM annual meeting pre-course that focuses on hand-held ultrasound and invasive procedures has consistently been one of the first to sell out. Other national organizations, such as ACP and its annual meeting, have seen similar interest in their courses on ultrasound-guided procedures.
The popularity of this continuing education bears out a worrisome trend: Hospitalists feel they are losing their procedural skills. An online survey conducted by The Hospitalist in May 2011 found that a majority of respondents (62%) had experienced deterioration of their procedural skills in the past five years; only 25% said they experienced improvement over the same period.
Historically, general internists have claimed bedside procedures as their domain. As stated dispassionately in the 1978 book The House of God, “There is no body cavity that cannot be reached with a #14G needle and a good strong arm.”1 Yet much has changed since Samuel Shem’s apocryphal description of medical residency training.
Most notably, the Accreditation Council of Graduate Medical Education (ACGME) has not only progressively restricted inpatient hours and patient loads for residents, but also increased the requirements for outpatient training. Some feel the balance of inpatient and outpatient training has tipped too far toward the latter in medicine residency programs, especially in light of the growing popularity of the hospitalist career path amongst new residency program graduates. This stands in contrast to ED training programs, which have embraced focused procedures training more readily.
“Adult care appears to be diverging into two career tacks as a result of external forces, of which we have limited control over, “ says Michael Beck, MD, a pediatric and adult hospitalist at Milton S. Hershey Medical Center in Hershey, Pa. “With new career choices emerging for graduates, the same square-peg, round-hole residency training should not exist.”
Dr. Beck advocates continuing an ongoing trend of “track” creation in residency programs, which allow trainees to focus training on their planned career path. Hospitalist tracks already exist in many medicine programs, including those at Cleveland Clinic and Northwestern. But many other factors limit the opportunity for trainees to obtain experience with bedside procedures, including competition with nurse practitioners and physician assistants. Even the increasing availability of ancillary phlebotomy and IV-start teams can increase a resident’s anxiety about procedures.
“By the time my residency was over [in 1993] and the work restrictions were beginning, hospital employees were doing all these tasks, making the residents less comfortable with hurting a patient when it was therapeutically necessary,” says Katharine Deiss, MD, assistant clinical professor of medicine at the University of Rochester Medical Center in New York. Interns who came from medical schools without extensive ancillary services in their teaching hospitals, she adds, were more comfortable with invasive procedures.
ACGME has sent a subtle message by decreasing emphasis on procedural skills by eradicating the requirement of showing manual proficiency in most bedside procedures as a requirement for certification. The omission has left individual residency programs and hospitalist groups to determine training and proficiency requirements for more invasive bedside procedures without a national standard.
In an editorial in the March 2007 issue of the Annals of Internal Medicine, F. Daniel Duffy, MD, and Eric Holmboe, MD, wrote that the American Board of Internal Medicine (ABIM) could only give a “qualified ‘yes’” to the question of whether residents should be trained in procedures they may not perform in practice. Although the authors asserted that the relaxed ABIM policy was “an important but small step toward revamping procedure skill training during residency,” others say it portrays an image of the ABIM de-emphasizing the importance of procedural training.
In addition, the recently established Focused Practice in Hospital Medicine (FPHM) pathway to ABIM Maintenance of Certification (MOC) has no requirement to show proficiency in bedside procedures.
“The absence of the procedural requirement in no way constitutes a statement that procedural skills are not important,” says Jeff Wiese, MD, FACP, SFHM, associate professor of medicine and residency program director at Tulane University Health Sciences Center in New Orleans, chair of the ABIM Hospital Medicine MOC Question Writing Committee, and former SHM president. “Rather, it is merely a practical issue with respect to making the MOC process applicable to all physicians engaged in hospital medicine (i.e. many hospitalists do not do procedures) while still making the MOC focused on the skill sets that are common for physicians doing hospital medicine.”
Once released into the world, even if trained well in residency, hospitalists can find it difficult to maintain their skills. In community and nonteaching settings, the pressure to admit and discharge in a timely manner can make procedures seem like the easiest corner to cut. Before long, it has been months since they have laid eyes on a needle of any sort. Many begin to develop performance anxiety.
In teaching hospitals, academic hospitalists often are called upon to participate in quality improvement (QI) and research efforts, which take time away from clinical rotations. Once there, it can be easy for a ward attending to rely upon a well-trained resident to supervise interns doing procedures. The lack of first-hand or even supervisory experience can lead to many academic hospitalists losing facility with procedures, with potentially disastrous results.
“In order to supervise a group of residents, the attending needs to be technically proficient and able to salvage a botched, or failed, procedure,” UM-JMH’s Dr. Lenchus says. “To this end, we strictly limit who can attend on the service.”
So what’s a residency or HM program director to do in the face of wavering support nationally, and sometimes locally, for maintaining procedural skills for hospitalists and trainees? Many hospitalists in teaching hospitals say it’s critical for clinicians to “get their own house in order,” to maintain procedural standards of proficiency with ongoing training, education, and verification.
“The profession now needs to redesign procedural training across the continuum of education and a lifetime of practice,” Drs. Duffy and Holmboe editorialized in the March 2007 Annals paper. “This approach would recognize the varied settings of internal-medicine practice and offer manual skills training to those whose practice settings require such skills.” Hospitalists can partner with medicine residency program leaders to provide procedural education and training to residents, either as a standalone elective or as a more general resource.
Hospitalists in such teaching hospitals as UCSD, Brigham and Women’s, UM-JMH, and Northwestern are leading efforts to provide procedural education to medical students, residents, and attendings. Training takes many forms, including formal procedural electives, required procedure rotations, or even brief one- or two-day courses in procedural skills at a simulation center.
Utilizing simulation training has been shown in many studies to be helpful in establishing procedural skills in learners of all training levels. Dr. Barsuk and his colleagues at Northwestern published studies in the Journal of Hospital Medicine in 2008 and 2009 showing that simulation training of residents was effective in improving skills in thoracentesis and central venous catheterization, respectively.3,4
In the community hospital setting, requirements for procedural skills can vary greatly based on the institution. For those community programs requiring procedural skills of their hospitalists, the clear definition of procedural training and requirements at the time of hiring is critical. Even after vetting a hospitalist’s procedural skills at hire, however, community programs should consider monitoring procedural skills and provide ongoing time and money for CME focused on procedural skills.
Currently, most hospitals depend on the honesty of individual physicians during the privileging process for bedside procedures. Even when the skills of physicians begin to wane, most are reluctant to voluntarily give up their procedure privileges.
“I think it would be pretty unusual for a hospitalist to relinquish their privileges,” Dr. Barsuk admits. But ideally, physicians who relinquish their privileges due to lack of experience could get retrained in simulation centers, then reproctored in order to regain their privileges. Northwestern established the Center for Simulation Technology and Immersive Learning as a resource for simulation training both locally and nationally.
Establishing an environment that supports hospitalists performing bedside procedures is critical. This includes the need to limit hospitalist workload to ensure adequate time to meet the procedural needs of patients. Providing easy access to the tools necessary to perform bedside procedures (e.g. portable ultrasound and pre-packaged procedure trays) helps avoid additional hurdles.
Academic hospitalist programs can serve as a regional resource by developing ongoing procedure mastery programs for hospitalists in their communities, as many smaller institutions do not have the resources to provide ongoing training in bedside procedures. This process can be tedious, but it should not be humiliating.
If the popularity of the SHM pre-course in bedside ultrasound and procedures is any indication, when given the opportunity to receive protected time for procedure training, most hospitalists will likely jump at the chance.
Dr. Chang is an associate clinical professor of medicine in the division of hospital medicine at Diego Medical Center. He is also a member of Team Hospitalist.
References
- Shem S. The House of God. New York: Dell Publishing; 1978.
- Duffy FD, Holmboe ES. What procedures should internists do? Ann Intern Med. 2007;146(5):392-393.
- Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54.
- Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403.
It’s 3:30 p.m. You’ve seen your old patients, holdovers, and an admission, but you haven’t finished your notes yet. Lunch was an afterthought between emails about schedule changes for the upcoming year. Two pages ring happily from your belt, the first from you-know-who in the ED, and the next from a nurse: “THORA SUPPLIES AT BEDSIDE SINCE THIS AM—WHEN WILL THIS HAPPEN?” The phone number on the wall for the on-call radiologist beckons...
An all-too-familiar situation for hospitalists across the country, this awkward moment raises a series of difficult questions:
Should I set aside time from my day to perform a procedure that could be time-consuming?
- Do I feel confident I can perform this procedure safely?
- Am I really the best physician to provide this service?
- As hospitalists are tasked with an ever-increasing array of responsibilities, answering the call of duty for bedside procedures is becoming more difficult for some.
A Core Competency
“The Core Competencies in Hospital Medicine,” authored by a group of HM thought leaders, was published as a supplement to the January/February 2006 issue of the Journal of Hospital Medicine. The core competencies include such bedside procedures as arthrocentesis, paracentesis, thoracentesis, lumbar puncture, and vascular (arterial and central venous) access (see “Core Competencies in Hospital Medicine: Procedures,” below). Although the authors stressed that the core competencies are to be viewed as a resource rather than as a set of requirements, the inclusion of bedside procedures emphasized the importance of procedural skills for future hospitalists.
“[Hospitalists] are in a perfect spot to continue to perform procedures in a structured manner,” says Joshua Lenchus, DO, RPh, FACP, FHM, associate director of the University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety. “As agents of quality and safety, hospitalists should continue to perform this clinically necessary service.”
Jeffrey Barsuk, MD, FHM, associate professor of medicine at Northwestern Feinberg School of Medicine in Chicago and an academic hospitalist at Northwestern Memorial Hospital (NMH), not only agrees that bedside procedures should be a core competency, but he also says hospitalists are the most appropriate providers of these services.
“I think this is part of hospital medicine. We’re in the hospital, [and] that’s what we do,” Dr. Barsuk says. Other providers, such as interventional radiologists, “really don’t understand why I’m doing [a procedure]. They understand it’s safe to do it, but they might not understand all the indications for it, and they certainly don’t understand the interpretation of the tests they’re sending.”
Despite the goals set forth by the core competencies and authorities in procedural safety, the reality of who actually performs bedside procedures is somewhat murky and varies greatly by institution. Many point to HM program setting (urban vs. rural) or structure (academic vs. community) to explain variance, but often it is other factors that determine whether hospitalists are actually preforming bedside procedures regularly.
Where Does HM Perform Procedures?
Community hospitalists, with strong support from interventional radiologists and subspecialists, often find it more efficient—even necessary, considering their patient volumes—to leave procedures to others. Community hospitalists with ICU admitting privileges, intensivists, and other HM subgroups say that being able to perform procedures should be a prerequisite for employment. Hospitalists in rural communities say they are doing procedures because they are “the only game in town.”
“Sometimes you are the only one available, and you are called upon to stretch your abilities,” says Beatrice Szantyr, MD, FAAP, a community hospitalist and pediatrician in Lincoln, Maine, who has practiced most of her career in rural settings.
Academic hospitalists in large, research-based HM programs can, paradoxically, find themselves performing fewer procedures as residents often take the lead on the majority of such cases. Conversely, academic hospitalists in large, nonteaching programs often find themselves called on to perform more bedside procedures.
No matter the setting, the simplicity of being the physician to recognize the need for a procedure, perform it, and interpret the results is undeniably efficient and “clean,” according to authorities on inpatient bedside procedures. Having to consult other physicians, optimize the patient’s lab values to their standards (a common issue with interventional radiologists), and adhere to their work schedules can often delay procedures unnecessarily.
—Joshua Lenchus, DO, RPh, FACP, FHM, associate director, University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety
“Hospitalists care for floor and ICU patients in many hospitals, and the inability to perform bedside procedures delays patient care,” says Dr. Nilam Soni, an academic hospitalist at the University of Chicago and a recognized expert on procedural safety.
Dr. Soni notes that when it comes to current techniques, many hospitalists suffer from a knowledge deficit. “The introduction of ultrasound for guidance of bedside procedures has been shown to improve the success and safety of certain procedures,” he says, “but the majority of practicing hospitalists did not learn how to use ultrasound for procedure guidance during residency.”
Heterogeneity of Training, Experience, and Skill
While all hospitalists draw upon different bases of training and experience, the heterogeneity of training, confidence, and inherent skill is greatest when it comes to bedside procedures. Mirroring the heterogeneity at the individual level, hospitalist programs vary greatly on the requirements placed on their staffs in regards to procedural skill and privileging.
Such research-driven programs as Brigham and Women’s Hospital (BWH) in Boston often find requiring maintenance of privileges in bedside procedures to be difficult, says Sally Wang, MD, FHM, director of procedural education at BWH. In fact, a new procedure service being created there will be staffed mainly with ED physicians. On the flipside, most community hospitalist programs leave the task of procedural “policing” to the hospital’s medical staff affairs office.
At the University of California at San Diego (UCSD) Medical Center, the HM group is instituting a division standard in which hospitalists maintain privileging and proficiency in a core group of bedside procedures. Other large hospitalist groups have created “proceduralist” subgroups that shoulder the burden of trainee education, as well as provide a resource for less skilled or less experienced inpatient providers.
“If you have a big group, you could have a dedicated procedure service and have a core group of hospitalists who are experts in procedure,” Dr. Barsuk says. “But it needs to be self-sustaining.” Once started, Dr. Barsuk says, proceduralist groups would continue to provide hospitals with ongoing return-on-investment (ROI) benefit.
Variability in procedure volume and payor mix, however, can make it hard for HM groups to demonstrate to hospital leadership a satisfactory ROI for a proceduralist program. Financial backing from grant support or a high-volume procedure—such as paracentesis in hospitals with large hepatology programs—can nurture starting proceduralist programs until all procedural revenues can justify the costs. Lower ROI can also be justified by showing improvement in quality indices—such as CLABSI rates—reduced time to procedures, and reduced costs compared to other subspecialists offering similar services.
“I’m of the firm belief that we can reduce costs by doing the procedures at the bedside rather than referring them to departments such as interventional radiology (IR),” Dr. Barsuk says. “What you would have to do is show the institution that it costs more money to have IR do [bedside procedures].”
National Response

—Jeffrey Barsuk, MD, MS, FHM, associate professor of medicine, Northwestern Feinberg School of Medicine, academic hospitalist, Northwestern Memorial Hospital, Chicago
Filling in the procedural training gaps found on the local level, such national organizations as SHM have stepped in to provide education and support for hospitalists yearning for training. Since its inception, an SHM annual meeting pre-course that focuses on hand-held ultrasound and invasive procedures has consistently been one of the first to sell out. Other national organizations, such as ACP and its annual meeting, have seen similar interest in their courses on ultrasound-guided procedures.
The popularity of this continuing education bears out a worrisome trend: Hospitalists feel they are losing their procedural skills. An online survey conducted by The Hospitalist in May 2011 found that a majority of respondents (62%) had experienced deterioration of their procedural skills in the past five years; only 25% said they experienced improvement over the same period.
Historically, general internists have claimed bedside procedures as their domain. As stated dispassionately in the 1978 book The House of God, “There is no body cavity that cannot be reached with a #14G needle and a good strong arm.”1 Yet much has changed since Samuel Shem’s apocryphal description of medical residency training.
Most notably, the Accreditation Council of Graduate Medical Education (ACGME) has not only progressively restricted inpatient hours and patient loads for residents, but also increased the requirements for outpatient training. Some feel the balance of inpatient and outpatient training has tipped too far toward the latter in medicine residency programs, especially in light of the growing popularity of the hospitalist career path amongst new residency program graduates. This stands in contrast to ED training programs, which have embraced focused procedures training more readily.
“Adult care appears to be diverging into two career tacks as a result of external forces, of which we have limited control over, “ says Michael Beck, MD, a pediatric and adult hospitalist at Milton S. Hershey Medical Center in Hershey, Pa. “With new career choices emerging for graduates, the same square-peg, round-hole residency training should not exist.”
Dr. Beck advocates continuing an ongoing trend of “track” creation in residency programs, which allow trainees to focus training on their planned career path. Hospitalist tracks already exist in many medicine programs, including those at Cleveland Clinic and Northwestern. But many other factors limit the opportunity for trainees to obtain experience with bedside procedures, including competition with nurse practitioners and physician assistants. Even the increasing availability of ancillary phlebotomy and IV-start teams can increase a resident’s anxiety about procedures.
“By the time my residency was over [in 1993] and the work restrictions were beginning, hospital employees were doing all these tasks, making the residents less comfortable with hurting a patient when it was therapeutically necessary,” says Katharine Deiss, MD, assistant clinical professor of medicine at the University of Rochester Medical Center in New York. Interns who came from medical schools without extensive ancillary services in their teaching hospitals, she adds, were more comfortable with invasive procedures.
ACGME has sent a subtle message by decreasing emphasis on procedural skills by eradicating the requirement of showing manual proficiency in most bedside procedures as a requirement for certification. The omission has left individual residency programs and hospitalist groups to determine training and proficiency requirements for more invasive bedside procedures without a national standard.
In an editorial in the March 2007 issue of the Annals of Internal Medicine, F. Daniel Duffy, MD, and Eric Holmboe, MD, wrote that the American Board of Internal Medicine (ABIM) could only give a “qualified ‘yes’” to the question of whether residents should be trained in procedures they may not perform in practice. Although the authors asserted that the relaxed ABIM policy was “an important but small step toward revamping procedure skill training during residency,” others say it portrays an image of the ABIM de-emphasizing the importance of procedural training.
In addition, the recently established Focused Practice in Hospital Medicine (FPHM) pathway to ABIM Maintenance of Certification (MOC) has no requirement to show proficiency in bedside procedures.
“The absence of the procedural requirement in no way constitutes a statement that procedural skills are not important,” says Jeff Wiese, MD, FACP, SFHM, associate professor of medicine and residency program director at Tulane University Health Sciences Center in New Orleans, chair of the ABIM Hospital Medicine MOC Question Writing Committee, and former SHM president. “Rather, it is merely a practical issue with respect to making the MOC process applicable to all physicians engaged in hospital medicine (i.e. many hospitalists do not do procedures) while still making the MOC focused on the skill sets that are common for physicians doing hospital medicine.”
Once released into the world, even if trained well in residency, hospitalists can find it difficult to maintain their skills. In community and nonteaching settings, the pressure to admit and discharge in a timely manner can make procedures seem like the easiest corner to cut. Before long, it has been months since they have laid eyes on a needle of any sort. Many begin to develop performance anxiety.
In teaching hospitals, academic hospitalists often are called upon to participate in quality improvement (QI) and research efforts, which take time away from clinical rotations. Once there, it can be easy for a ward attending to rely upon a well-trained resident to supervise interns doing procedures. The lack of first-hand or even supervisory experience can lead to many academic hospitalists losing facility with procedures, with potentially disastrous results.
“In order to supervise a group of residents, the attending needs to be technically proficient and able to salvage a botched, or failed, procedure,” UM-JMH’s Dr. Lenchus says. “To this end, we strictly limit who can attend on the service.”
So what’s a residency or HM program director to do in the face of wavering support nationally, and sometimes locally, for maintaining procedural skills for hospitalists and trainees? Many hospitalists in teaching hospitals say it’s critical for clinicians to “get their own house in order,” to maintain procedural standards of proficiency with ongoing training, education, and verification.
“The profession now needs to redesign procedural training across the continuum of education and a lifetime of practice,” Drs. Duffy and Holmboe editorialized in the March 2007 Annals paper. “This approach would recognize the varied settings of internal-medicine practice and offer manual skills training to those whose practice settings require such skills.” Hospitalists can partner with medicine residency program leaders to provide procedural education and training to residents, either as a standalone elective or as a more general resource.
Hospitalists in such teaching hospitals as UCSD, Brigham and Women’s, UM-JMH, and Northwestern are leading efforts to provide procedural education to medical students, residents, and attendings. Training takes many forms, including formal procedural electives, required procedure rotations, or even brief one- or two-day courses in procedural skills at a simulation center.
Utilizing simulation training has been shown in many studies to be helpful in establishing procedural skills in learners of all training levels. Dr. Barsuk and his colleagues at Northwestern published studies in the Journal of Hospital Medicine in 2008 and 2009 showing that simulation training of residents was effective in improving skills in thoracentesis and central venous catheterization, respectively.3,4
In the community hospital setting, requirements for procedural skills can vary greatly based on the institution. For those community programs requiring procedural skills of their hospitalists, the clear definition of procedural training and requirements at the time of hiring is critical. Even after vetting a hospitalist’s procedural skills at hire, however, community programs should consider monitoring procedural skills and provide ongoing time and money for CME focused on procedural skills.
Currently, most hospitals depend on the honesty of individual physicians during the privileging process for bedside procedures. Even when the skills of physicians begin to wane, most are reluctant to voluntarily give up their procedure privileges.
“I think it would be pretty unusual for a hospitalist to relinquish their privileges,” Dr. Barsuk admits. But ideally, physicians who relinquish their privileges due to lack of experience could get retrained in simulation centers, then reproctored in order to regain their privileges. Northwestern established the Center for Simulation Technology and Immersive Learning as a resource for simulation training both locally and nationally.
Establishing an environment that supports hospitalists performing bedside procedures is critical. This includes the need to limit hospitalist workload to ensure adequate time to meet the procedural needs of patients. Providing easy access to the tools necessary to perform bedside procedures (e.g. portable ultrasound and pre-packaged procedure trays) helps avoid additional hurdles.
Academic hospitalist programs can serve as a regional resource by developing ongoing procedure mastery programs for hospitalists in their communities, as many smaller institutions do not have the resources to provide ongoing training in bedside procedures. This process can be tedious, but it should not be humiliating.
If the popularity of the SHM pre-course in bedside ultrasound and procedures is any indication, when given the opportunity to receive protected time for procedure training, most hospitalists will likely jump at the chance.
Dr. Chang is an associate clinical professor of medicine in the division of hospital medicine at Diego Medical Center. He is also a member of Team Hospitalist.
References
- Shem S. The House of God. New York: Dell Publishing; 1978.
- Duffy FD, Holmboe ES. What procedures should internists do? Ann Intern Med. 2007;146(5):392-393.
- Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54.
- Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403.
It’s 3:30 p.m. You’ve seen your old patients, holdovers, and an admission, but you haven’t finished your notes yet. Lunch was an afterthought between emails about schedule changes for the upcoming year. Two pages ring happily from your belt, the first from you-know-who in the ED, and the next from a nurse: “THORA SUPPLIES AT BEDSIDE SINCE THIS AM—WHEN WILL THIS HAPPEN?” The phone number on the wall for the on-call radiologist beckons...
An all-too-familiar situation for hospitalists across the country, this awkward moment raises a series of difficult questions:
Should I set aside time from my day to perform a procedure that could be time-consuming?
- Do I feel confident I can perform this procedure safely?
- Am I really the best physician to provide this service?
- As hospitalists are tasked with an ever-increasing array of responsibilities, answering the call of duty for bedside procedures is becoming more difficult for some.
A Core Competency
“The Core Competencies in Hospital Medicine,” authored by a group of HM thought leaders, was published as a supplement to the January/February 2006 issue of the Journal of Hospital Medicine. The core competencies include such bedside procedures as arthrocentesis, paracentesis, thoracentesis, lumbar puncture, and vascular (arterial and central venous) access (see “Core Competencies in Hospital Medicine: Procedures,” below). Although the authors stressed that the core competencies are to be viewed as a resource rather than as a set of requirements, the inclusion of bedside procedures emphasized the importance of procedural skills for future hospitalists.
“[Hospitalists] are in a perfect spot to continue to perform procedures in a structured manner,” says Joshua Lenchus, DO, RPh, FACP, FHM, associate director of the University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety. “As agents of quality and safety, hospitalists should continue to perform this clinically necessary service.”
Jeffrey Barsuk, MD, FHM, associate professor of medicine at Northwestern Feinberg School of Medicine in Chicago and an academic hospitalist at Northwestern Memorial Hospital (NMH), not only agrees that bedside procedures should be a core competency, but he also says hospitalists are the most appropriate providers of these services.
“I think this is part of hospital medicine. We’re in the hospital, [and] that’s what we do,” Dr. Barsuk says. Other providers, such as interventional radiologists, “really don’t understand why I’m doing [a procedure]. They understand it’s safe to do it, but they might not understand all the indications for it, and they certainly don’t understand the interpretation of the tests they’re sending.”
Despite the goals set forth by the core competencies and authorities in procedural safety, the reality of who actually performs bedside procedures is somewhat murky and varies greatly by institution. Many point to HM program setting (urban vs. rural) or structure (academic vs. community) to explain variance, but often it is other factors that determine whether hospitalists are actually preforming bedside procedures regularly.
Where Does HM Perform Procedures?
Community hospitalists, with strong support from interventional radiologists and subspecialists, often find it more efficient—even necessary, considering their patient volumes—to leave procedures to others. Community hospitalists with ICU admitting privileges, intensivists, and other HM subgroups say that being able to perform procedures should be a prerequisite for employment. Hospitalists in rural communities say they are doing procedures because they are “the only game in town.”
“Sometimes you are the only one available, and you are called upon to stretch your abilities,” says Beatrice Szantyr, MD, FAAP, a community hospitalist and pediatrician in Lincoln, Maine, who has practiced most of her career in rural settings.
Academic hospitalists in large, research-based HM programs can, paradoxically, find themselves performing fewer procedures as residents often take the lead on the majority of such cases. Conversely, academic hospitalists in large, nonteaching programs often find themselves called on to perform more bedside procedures.
No matter the setting, the simplicity of being the physician to recognize the need for a procedure, perform it, and interpret the results is undeniably efficient and “clean,” according to authorities on inpatient bedside procedures. Having to consult other physicians, optimize the patient’s lab values to their standards (a common issue with interventional radiologists), and adhere to their work schedules can often delay procedures unnecessarily.
—Joshua Lenchus, DO, RPh, FACP, FHM, associate director, University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety
“Hospitalists care for floor and ICU patients in many hospitals, and the inability to perform bedside procedures delays patient care,” says Dr. Nilam Soni, an academic hospitalist at the University of Chicago and a recognized expert on procedural safety.
Dr. Soni notes that when it comes to current techniques, many hospitalists suffer from a knowledge deficit. “The introduction of ultrasound for guidance of bedside procedures has been shown to improve the success and safety of certain procedures,” he says, “but the majority of practicing hospitalists did not learn how to use ultrasound for procedure guidance during residency.”
Heterogeneity of Training, Experience, and Skill
While all hospitalists draw upon different bases of training and experience, the heterogeneity of training, confidence, and inherent skill is greatest when it comes to bedside procedures. Mirroring the heterogeneity at the individual level, hospitalist programs vary greatly on the requirements placed on their staffs in regards to procedural skill and privileging.
Such research-driven programs as Brigham and Women’s Hospital (BWH) in Boston often find requiring maintenance of privileges in bedside procedures to be difficult, says Sally Wang, MD, FHM, director of procedural education at BWH. In fact, a new procedure service being created there will be staffed mainly with ED physicians. On the flipside, most community hospitalist programs leave the task of procedural “policing” to the hospital’s medical staff affairs office.
At the University of California at San Diego (UCSD) Medical Center, the HM group is instituting a division standard in which hospitalists maintain privileging and proficiency in a core group of bedside procedures. Other large hospitalist groups have created “proceduralist” subgroups that shoulder the burden of trainee education, as well as provide a resource for less skilled or less experienced inpatient providers.
“If you have a big group, you could have a dedicated procedure service and have a core group of hospitalists who are experts in procedure,” Dr. Barsuk says. “But it needs to be self-sustaining.” Once started, Dr. Barsuk says, proceduralist groups would continue to provide hospitals with ongoing return-on-investment (ROI) benefit.
Variability in procedure volume and payor mix, however, can make it hard for HM groups to demonstrate to hospital leadership a satisfactory ROI for a proceduralist program. Financial backing from grant support or a high-volume procedure—such as paracentesis in hospitals with large hepatology programs—can nurture starting proceduralist programs until all procedural revenues can justify the costs. Lower ROI can also be justified by showing improvement in quality indices—such as CLABSI rates—reduced time to procedures, and reduced costs compared to other subspecialists offering similar services.
“I’m of the firm belief that we can reduce costs by doing the procedures at the bedside rather than referring them to departments such as interventional radiology (IR),” Dr. Barsuk says. “What you would have to do is show the institution that it costs more money to have IR do [bedside procedures].”
National Response

—Jeffrey Barsuk, MD, MS, FHM, associate professor of medicine, Northwestern Feinberg School of Medicine, academic hospitalist, Northwestern Memorial Hospital, Chicago
Filling in the procedural training gaps found on the local level, such national organizations as SHM have stepped in to provide education and support for hospitalists yearning for training. Since its inception, an SHM annual meeting pre-course that focuses on hand-held ultrasound and invasive procedures has consistently been one of the first to sell out. Other national organizations, such as ACP and its annual meeting, have seen similar interest in their courses on ultrasound-guided procedures.
The popularity of this continuing education bears out a worrisome trend: Hospitalists feel they are losing their procedural skills. An online survey conducted by The Hospitalist in May 2011 found that a majority of respondents (62%) had experienced deterioration of their procedural skills in the past five years; only 25% said they experienced improvement over the same period.
Historically, general internists have claimed bedside procedures as their domain. As stated dispassionately in the 1978 book The House of God, “There is no body cavity that cannot be reached with a #14G needle and a good strong arm.”1 Yet much has changed since Samuel Shem’s apocryphal description of medical residency training.
Most notably, the Accreditation Council of Graduate Medical Education (ACGME) has not only progressively restricted inpatient hours and patient loads for residents, but also increased the requirements for outpatient training. Some feel the balance of inpatient and outpatient training has tipped too far toward the latter in medicine residency programs, especially in light of the growing popularity of the hospitalist career path amongst new residency program graduates. This stands in contrast to ED training programs, which have embraced focused procedures training more readily.
“Adult care appears to be diverging into two career tacks as a result of external forces, of which we have limited control over, “ says Michael Beck, MD, a pediatric and adult hospitalist at Milton S. Hershey Medical Center in Hershey, Pa. “With new career choices emerging for graduates, the same square-peg, round-hole residency training should not exist.”
Dr. Beck advocates continuing an ongoing trend of “track” creation in residency programs, which allow trainees to focus training on their planned career path. Hospitalist tracks already exist in many medicine programs, including those at Cleveland Clinic and Northwestern. But many other factors limit the opportunity for trainees to obtain experience with bedside procedures, including competition with nurse practitioners and physician assistants. Even the increasing availability of ancillary phlebotomy and IV-start teams can increase a resident’s anxiety about procedures.
“By the time my residency was over [in 1993] and the work restrictions were beginning, hospital employees were doing all these tasks, making the residents less comfortable with hurting a patient when it was therapeutically necessary,” says Katharine Deiss, MD, assistant clinical professor of medicine at the University of Rochester Medical Center in New York. Interns who came from medical schools without extensive ancillary services in their teaching hospitals, she adds, were more comfortable with invasive procedures.
ACGME has sent a subtle message by decreasing emphasis on procedural skills by eradicating the requirement of showing manual proficiency in most bedside procedures as a requirement for certification. The omission has left individual residency programs and hospitalist groups to determine training and proficiency requirements for more invasive bedside procedures without a national standard.
In an editorial in the March 2007 issue of the Annals of Internal Medicine, F. Daniel Duffy, MD, and Eric Holmboe, MD, wrote that the American Board of Internal Medicine (ABIM) could only give a “qualified ‘yes’” to the question of whether residents should be trained in procedures they may not perform in practice. Although the authors asserted that the relaxed ABIM policy was “an important but small step toward revamping procedure skill training during residency,” others say it portrays an image of the ABIM de-emphasizing the importance of procedural training.
In addition, the recently established Focused Practice in Hospital Medicine (FPHM) pathway to ABIM Maintenance of Certification (MOC) has no requirement to show proficiency in bedside procedures.
“The absence of the procedural requirement in no way constitutes a statement that procedural skills are not important,” says Jeff Wiese, MD, FACP, SFHM, associate professor of medicine and residency program director at Tulane University Health Sciences Center in New Orleans, chair of the ABIM Hospital Medicine MOC Question Writing Committee, and former SHM president. “Rather, it is merely a practical issue with respect to making the MOC process applicable to all physicians engaged in hospital medicine (i.e. many hospitalists do not do procedures) while still making the MOC focused on the skill sets that are common for physicians doing hospital medicine.”
Once released into the world, even if trained well in residency, hospitalists can find it difficult to maintain their skills. In community and nonteaching settings, the pressure to admit and discharge in a timely manner can make procedures seem like the easiest corner to cut. Before long, it has been months since they have laid eyes on a needle of any sort. Many begin to develop performance anxiety.
In teaching hospitals, academic hospitalists often are called upon to participate in quality improvement (QI) and research efforts, which take time away from clinical rotations. Once there, it can be easy for a ward attending to rely upon a well-trained resident to supervise interns doing procedures. The lack of first-hand or even supervisory experience can lead to many academic hospitalists losing facility with procedures, with potentially disastrous results.
“In order to supervise a group of residents, the attending needs to be technically proficient and able to salvage a botched, or failed, procedure,” UM-JMH’s Dr. Lenchus says. “To this end, we strictly limit who can attend on the service.”
So what’s a residency or HM program director to do in the face of wavering support nationally, and sometimes locally, for maintaining procedural skills for hospitalists and trainees? Many hospitalists in teaching hospitals say it’s critical for clinicians to “get their own house in order,” to maintain procedural standards of proficiency with ongoing training, education, and verification.
“The profession now needs to redesign procedural training across the continuum of education and a lifetime of practice,” Drs. Duffy and Holmboe editorialized in the March 2007 Annals paper. “This approach would recognize the varied settings of internal-medicine practice and offer manual skills training to those whose practice settings require such skills.” Hospitalists can partner with medicine residency program leaders to provide procedural education and training to residents, either as a standalone elective or as a more general resource.
Hospitalists in such teaching hospitals as UCSD, Brigham and Women’s, UM-JMH, and Northwestern are leading efforts to provide procedural education to medical students, residents, and attendings. Training takes many forms, including formal procedural electives, required procedure rotations, or even brief one- or two-day courses in procedural skills at a simulation center.
Utilizing simulation training has been shown in many studies to be helpful in establishing procedural skills in learners of all training levels. Dr. Barsuk and his colleagues at Northwestern published studies in the Journal of Hospital Medicine in 2008 and 2009 showing that simulation training of residents was effective in improving skills in thoracentesis and central venous catheterization, respectively.3,4
In the community hospital setting, requirements for procedural skills can vary greatly based on the institution. For those community programs requiring procedural skills of their hospitalists, the clear definition of procedural training and requirements at the time of hiring is critical. Even after vetting a hospitalist’s procedural skills at hire, however, community programs should consider monitoring procedural skills and provide ongoing time and money for CME focused on procedural skills.
Currently, most hospitals depend on the honesty of individual physicians during the privileging process for bedside procedures. Even when the skills of physicians begin to wane, most are reluctant to voluntarily give up their procedure privileges.
“I think it would be pretty unusual for a hospitalist to relinquish their privileges,” Dr. Barsuk admits. But ideally, physicians who relinquish their privileges due to lack of experience could get retrained in simulation centers, then reproctored in order to regain their privileges. Northwestern established the Center for Simulation Technology and Immersive Learning as a resource for simulation training both locally and nationally.
Establishing an environment that supports hospitalists performing bedside procedures is critical. This includes the need to limit hospitalist workload to ensure adequate time to meet the procedural needs of patients. Providing easy access to the tools necessary to perform bedside procedures (e.g. portable ultrasound and pre-packaged procedure trays) helps avoid additional hurdles.
Academic hospitalist programs can serve as a regional resource by developing ongoing procedure mastery programs for hospitalists in their communities, as many smaller institutions do not have the resources to provide ongoing training in bedside procedures. This process can be tedious, but it should not be humiliating.
If the popularity of the SHM pre-course in bedside ultrasound and procedures is any indication, when given the opportunity to receive protected time for procedure training, most hospitalists will likely jump at the chance.
Dr. Chang is an associate clinical professor of medicine in the division of hospital medicine at Diego Medical Center. He is also a member of Team Hospitalist.
References
- Shem S. The House of God. New York: Dell Publishing; 1978.
- Duffy FD, Holmboe ES. What procedures should internists do? Ann Intern Med. 2007;146(5):392-393.
- Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54.
- Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403.
HM12 SESSION ANALYSIS: Innovative Scheduling as Quality Improvement
Shalini Chandra, MD, MS, Gregory Harlan, MD, FAAP, MPH, Brian Donovan, MD, MMM, FACP, SFHM, and Judy Shumway, DO, MPH, led a standing-room only morning breakout session on Monday at HM12 that focused on the challenges and opportunities of scheduling and rounding.
Dr. Harlan, a hospitalist at IPC, introduced the topic of innovative scheduling by placing the issue in a framework easily understood by hospitalists: quality improvement. He advocated identifying the salient problems faced by each individual group and then applying changes that make sense to each facility and group.
Dr. Chandra, a hospitalist at Johns Hopkins Bayview Medical Center, further elaborated on this by explaining how the PDSA (plan, do, study, act) approach can be used to initiate and assess the changes implemented in scheduling. Metrics such as hospitalist morale, patient satisfaction, length of stay, and time of discharge, can be used to assess the effect of each scheduling change.
Dr. Donovan, medical director of IPC, described a “zone” approach to scheduling. This rounding scheme assigns a hospitalist to a geographic unit, allowing for greater accessibility and higher efficiency. Closer relationships with multidisciplinary personnel can be achieved with this model.
Takeaways
- Test your scheduling changes with PDSA methods of quality improvement.
- Multidisciplinary rounds are critical to success.
- "Zone" rounding allows the development of physician leaders in each zone, and enable more efficiency.
- Engaging stakeholders in the success of physician scheduling is critical; this may enable more support and resources for these changes from administration.
Dr. Chang is a pediatric hospitalist with the University of San Diego Medical Center and Rady Children's Hospital, San Diego.
Shalini Chandra, MD, MS, Gregory Harlan, MD, FAAP, MPH, Brian Donovan, MD, MMM, FACP, SFHM, and Judy Shumway, DO, MPH, led a standing-room only morning breakout session on Monday at HM12 that focused on the challenges and opportunities of scheduling and rounding.
Dr. Harlan, a hospitalist at IPC, introduced the topic of innovative scheduling by placing the issue in a framework easily understood by hospitalists: quality improvement. He advocated identifying the salient problems faced by each individual group and then applying changes that make sense to each facility and group.
Dr. Chandra, a hospitalist at Johns Hopkins Bayview Medical Center, further elaborated on this by explaining how the PDSA (plan, do, study, act) approach can be used to initiate and assess the changes implemented in scheduling. Metrics such as hospitalist morale, patient satisfaction, length of stay, and time of discharge, can be used to assess the effect of each scheduling change.
Dr. Donovan, medical director of IPC, described a “zone” approach to scheduling. This rounding scheme assigns a hospitalist to a geographic unit, allowing for greater accessibility and higher efficiency. Closer relationships with multidisciplinary personnel can be achieved with this model.
Takeaways
- Test your scheduling changes with PDSA methods of quality improvement.
- Multidisciplinary rounds are critical to success.
- "Zone" rounding allows the development of physician leaders in each zone, and enable more efficiency.
- Engaging stakeholders in the success of physician scheduling is critical; this may enable more support and resources for these changes from administration.
Dr. Chang is a pediatric hospitalist with the University of San Diego Medical Center and Rady Children's Hospital, San Diego.
Shalini Chandra, MD, MS, Gregory Harlan, MD, FAAP, MPH, Brian Donovan, MD, MMM, FACP, SFHM, and Judy Shumway, DO, MPH, led a standing-room only morning breakout session on Monday at HM12 that focused on the challenges and opportunities of scheduling and rounding.
Dr. Harlan, a hospitalist at IPC, introduced the topic of innovative scheduling by placing the issue in a framework easily understood by hospitalists: quality improvement. He advocated identifying the salient problems faced by each individual group and then applying changes that make sense to each facility and group.
Dr. Chandra, a hospitalist at Johns Hopkins Bayview Medical Center, further elaborated on this by explaining how the PDSA (plan, do, study, act) approach can be used to initiate and assess the changes implemented in scheduling. Metrics such as hospitalist morale, patient satisfaction, length of stay, and time of discharge, can be used to assess the effect of each scheduling change.
Dr. Donovan, medical director of IPC, described a “zone” approach to scheduling. This rounding scheme assigns a hospitalist to a geographic unit, allowing for greater accessibility and higher efficiency. Closer relationships with multidisciplinary personnel can be achieved with this model.
Takeaways
- Test your scheduling changes with PDSA methods of quality improvement.
- Multidisciplinary rounds are critical to success.
- "Zone" rounding allows the development of physician leaders in each zone, and enable more efficiency.
- Engaging stakeholders in the success of physician scheduling is critical; this may enable more support and resources for these changes from administration.
Dr. Chang is a pediatric hospitalist with the University of San Diego Medical Center and Rady Children's Hospital, San Diego.