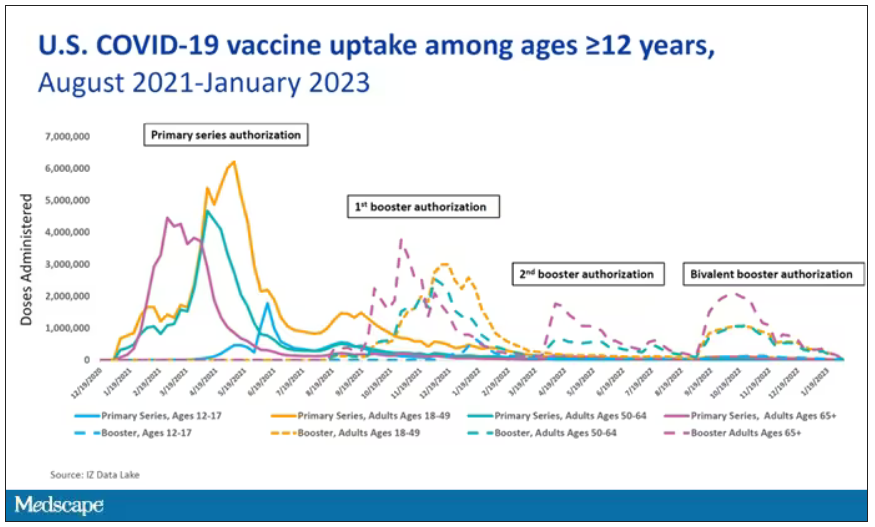
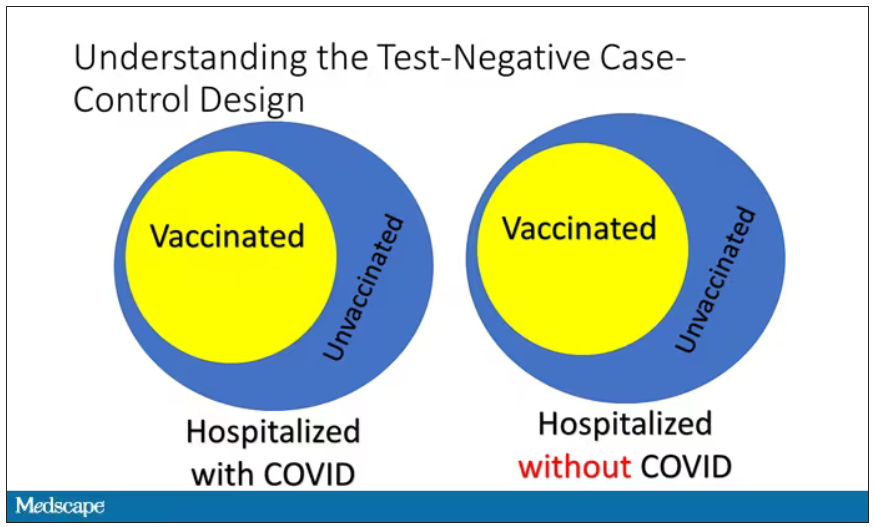
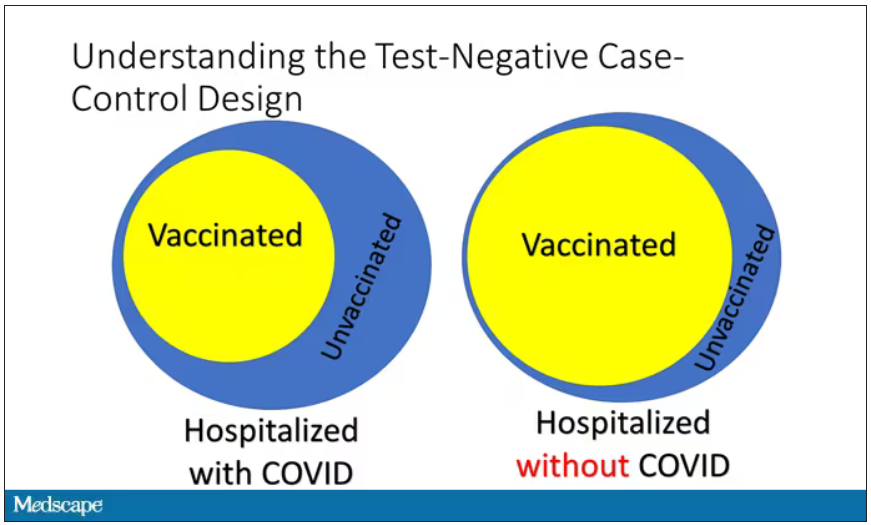
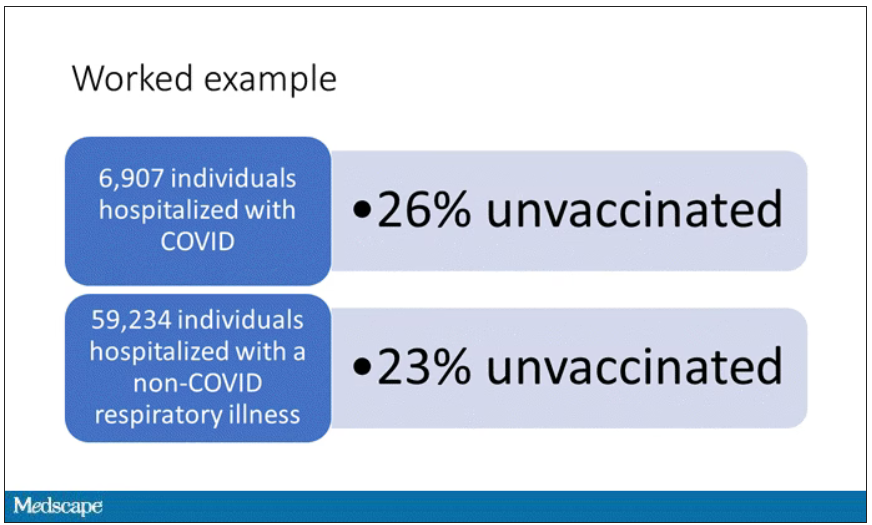
User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
One in 10 people who had Omicron got long COVID: Study
About 10% of people infected with Omicron reported having long COVID, a lower percentage than estimated for people infected with earlier strains of the coronavirus, according to a study published in JAMA.
The research team looked at data from 8,646 adults infected with COVID-19 at different times of the pandemic and 1,118 who did not have COVID.
“Based on a subset of 2,231 patients in this analysis who had a first COVID-19 infection the National Institutes of Health said in a news release.
People who were unvaccinated or got COVID before Omicron were more likely to have long COVID and had more severe cases, the NIH said.
Previous studies have come up with higher figures than 10% for people who have long COVID.
For instance, in June 2022 the CDC said one in five Americans who had COVID reported having long COVID. And a University of Oxford study published in September 2021 found more than a third of patients had long COVID symptoms.
The scientists in the most recent study identified 12 symptoms that distinguished people who did and didn’t have COVID. The scientists developed a scoring system for the symptoms to set a threshold to identify people who had long COVID, the NIH said.
The symptoms were fatigue, brain fog, dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements. Another symptom was postexertional malaise, or worse symptoms after mental or physical exertion.
Scientists still have many questions about long COVID, such as how many people get it and why some people get it and others don’t.
The study was coordinated through the NIH’s RECOVER (Researching COVID to Enhance Recovery) initiative, which aims to find out how to define, detect, and treat long COVID.
“The researchers hope this study is the next step toward potential treatments for long COVID, which affects the health and wellbeing of millions of Americans,” the NIH said.
A version of this article first appeared on WebMD.com.
About 10% of people infected with Omicron reported having long COVID, a lower percentage than estimated for people infected with earlier strains of the coronavirus, according to a study published in JAMA.
The research team looked at data from 8,646 adults infected with COVID-19 at different times of the pandemic and 1,118 who did not have COVID.
“Based on a subset of 2,231 patients in this analysis who had a first COVID-19 infection the National Institutes of Health said in a news release.
People who were unvaccinated or got COVID before Omicron were more likely to have long COVID and had more severe cases, the NIH said.
Previous studies have come up with higher figures than 10% for people who have long COVID.
For instance, in June 2022 the CDC said one in five Americans who had COVID reported having long COVID. And a University of Oxford study published in September 2021 found more than a third of patients had long COVID symptoms.
The scientists in the most recent study identified 12 symptoms that distinguished people who did and didn’t have COVID. The scientists developed a scoring system for the symptoms to set a threshold to identify people who had long COVID, the NIH said.
The symptoms were fatigue, brain fog, dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements. Another symptom was postexertional malaise, or worse symptoms after mental or physical exertion.
Scientists still have many questions about long COVID, such as how many people get it and why some people get it and others don’t.
The study was coordinated through the NIH’s RECOVER (Researching COVID to Enhance Recovery) initiative, which aims to find out how to define, detect, and treat long COVID.
“The researchers hope this study is the next step toward potential treatments for long COVID, which affects the health and wellbeing of millions of Americans,” the NIH said.
A version of this article first appeared on WebMD.com.
About 10% of people infected with Omicron reported having long COVID, a lower percentage than estimated for people infected with earlier strains of the coronavirus, according to a study published in JAMA.
The research team looked at data from 8,646 adults infected with COVID-19 at different times of the pandemic and 1,118 who did not have COVID.
“Based on a subset of 2,231 patients in this analysis who had a first COVID-19 infection the National Institutes of Health said in a news release.
People who were unvaccinated or got COVID before Omicron were more likely to have long COVID and had more severe cases, the NIH said.
Previous studies have come up with higher figures than 10% for people who have long COVID.
For instance, in June 2022 the CDC said one in five Americans who had COVID reported having long COVID. And a University of Oxford study published in September 2021 found more than a third of patients had long COVID symptoms.
The scientists in the most recent study identified 12 symptoms that distinguished people who did and didn’t have COVID. The scientists developed a scoring system for the symptoms to set a threshold to identify people who had long COVID, the NIH said.
The symptoms were fatigue, brain fog, dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements. Another symptom was postexertional malaise, or worse symptoms after mental or physical exertion.
Scientists still have many questions about long COVID, such as how many people get it and why some people get it and others don’t.
The study was coordinated through the NIH’s RECOVER (Researching COVID to Enhance Recovery) initiative, which aims to find out how to define, detect, and treat long COVID.
“The researchers hope this study is the next step toward potential treatments for long COVID, which affects the health and wellbeing of millions of Americans,” the NIH said.
A version of this article first appeared on WebMD.com.
FROM JAMA
Study finds COVID-19 boosters don’t increase miscarriage risk
COVID-19 boosters are not linked to an increased chance of miscarriage, according to a new study in JAMA Network Open.
Researchers were seeking to learn whether a booster in early pregnancy, before 20 weeks, was associated with greater likelihood of spontaneous abortion.
They examined more than 100,000 pregnancies at 6-19 weeks from eight health systems in the Vaccine Safety Datalink (VSD). They found that receiving a COVID-19 booster shot in a 28-day or 42-day exposure window did not increase the chances of miscarriage.
The VSD is a collaboration between the Centers for Disease Control and Prevention’s Immunization Safety Office and large health care systems. The “observational, case-control, surveillance study” was conducted from Nov. 1, 2021, to June 12, 2022.
“COVID infection during pregnancy increases risk of poor outcomes, yet many people who are pregnant or thinking about getting pregnant are hesitant to get a booster dose because of questions about safety,” said Elyse Kharbanda, MD, senior investigator at HealthPartners Institute and lead author of the study in a press release.
The University of Minnesota reported that “previous studies have shown COIVD-19 primary vaccination is safe in pregnancy and not associated with an increased risk for miscarriage. Several studies have also shown COVID-19 can be more severe in pregnancy and lead to worse outcomes for the mother.”
The study was funded by the CDC. Five study authors reported conflicts of interest with Pfizer, Merck, GlaxoSmithKline, AbbVie, and Sanofi Pasteur.
A version of this article first appeared on Medscape.com.
COVID-19 boosters are not linked to an increased chance of miscarriage, according to a new study in JAMA Network Open.
Researchers were seeking to learn whether a booster in early pregnancy, before 20 weeks, was associated with greater likelihood of spontaneous abortion.
They examined more than 100,000 pregnancies at 6-19 weeks from eight health systems in the Vaccine Safety Datalink (VSD). They found that receiving a COVID-19 booster shot in a 28-day or 42-day exposure window did not increase the chances of miscarriage.
The VSD is a collaboration between the Centers for Disease Control and Prevention’s Immunization Safety Office and large health care systems. The “observational, case-control, surveillance study” was conducted from Nov. 1, 2021, to June 12, 2022.
“COVID infection during pregnancy increases risk of poor outcomes, yet many people who are pregnant or thinking about getting pregnant are hesitant to get a booster dose because of questions about safety,” said Elyse Kharbanda, MD, senior investigator at HealthPartners Institute and lead author of the study in a press release.
The University of Minnesota reported that “previous studies have shown COIVD-19 primary vaccination is safe in pregnancy and not associated with an increased risk for miscarriage. Several studies have also shown COVID-19 can be more severe in pregnancy and lead to worse outcomes for the mother.”
The study was funded by the CDC. Five study authors reported conflicts of interest with Pfizer, Merck, GlaxoSmithKline, AbbVie, and Sanofi Pasteur.
A version of this article first appeared on Medscape.com.
COVID-19 boosters are not linked to an increased chance of miscarriage, according to a new study in JAMA Network Open.
Researchers were seeking to learn whether a booster in early pregnancy, before 20 weeks, was associated with greater likelihood of spontaneous abortion.
They examined more than 100,000 pregnancies at 6-19 weeks from eight health systems in the Vaccine Safety Datalink (VSD). They found that receiving a COVID-19 booster shot in a 28-day or 42-day exposure window did not increase the chances of miscarriage.
The VSD is a collaboration between the Centers for Disease Control and Prevention’s Immunization Safety Office and large health care systems. The “observational, case-control, surveillance study” was conducted from Nov. 1, 2021, to June 12, 2022.
“COVID infection during pregnancy increases risk of poor outcomes, yet many people who are pregnant or thinking about getting pregnant are hesitant to get a booster dose because of questions about safety,” said Elyse Kharbanda, MD, senior investigator at HealthPartners Institute and lead author of the study in a press release.
The University of Minnesota reported that “previous studies have shown COIVD-19 primary vaccination is safe in pregnancy and not associated with an increased risk for miscarriage. Several studies have also shown COVID-19 can be more severe in pregnancy and lead to worse outcomes for the mother.”
The study was funded by the CDC. Five study authors reported conflicts of interest with Pfizer, Merck, GlaxoSmithKline, AbbVie, and Sanofi Pasteur.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
SPF is only the start when recommending sunscreens
CHICAGO – at the inaugural Pigmentary Disorders Exchange Symposium.
Among the first factors physicians should consider before recommending sunscreen are a patient’s Fitzpatrick skin type, risks for burning or tanning, underlying skin disorders, and medications the patient is taking, Dr. Taylor, professor of dermatology at the University of Pennsylvania, Philadelphia, said at the meeting, provided by MedscapeLIVE! If patients are on hypertensives, for example, medications can make them more photosensitive.
Consider skin type
Dr. Taylor said she was dismayed by the results of a recent study, which found that 43% of dermatologists who responded to a survey reported that they never, rarely, or only sometimes took a patient’s skin type into account when making sunscreen recommendations. The article is referenced in a 2022 expert panel consensus paper she coauthored on photoprotection “for skin of all color.” But she pointed out that considering skin type alone is inadequate.
Questions for patients in joint decision-making should include lifestyle and work choices such as whether they work inside or outside, and how much sun exposure they get in a typical day. Heat and humidity levels should also be considered as should a patient’s susceptibility to dyspigmentation. “That could be overall darkening of the skin, mottled hyperpigmentation, actinic dyspigmentation, and, of course, propensity for skin cancer,” she said.
Use differs by race
Dr. Taylor, who is also vice chair for diversity, equity and inclusion in the department of dermatology at the University of Pennsylvania, pointed out that sunscreen use differs considerably by race.
In study of 8,952 adults in the United States who reported that they were sun sensitive found that a subset of adults with skin of color were significantly less likely to use sunscreen when compared with non-Hispanic White adults: Non-Hispanic Black (adjusted odds ratio, 0.43); non-Hispanic Asian (aOR. 0.54); and Hispanic (aOR, 0.70) adults.
In the study, non-Hispanic Black and Hispanic adults were significantly less likely to use sunscreens with an SPF greater than 15. In addition, non-Hispanic Black, non-Hispanic Asian, and Hispanic adults were significantly more likely than non-Hispanic Whites to wear long sleeves when outside. Such differences are important to keep in mind when advising patients about sunscreens, she said.
Protection for lighter-colored skin
Dr. Taylor said that, for patients with lighter skin tones, “we really want to protect against ultraviolet B as well as ultraviolet A, particularly ultraviolet A2. Ultraviolet radiation is going to cause DNA damage.” Patients with Fitzpatrick skin types I, II, or III are most susceptible to the effects of UVB with sunburn inflammation, which will cause erythema and tanning, and immunosuppression.
“For those who are I, II, and III, we do want to recommend a broad-spectrum, photostable sunscreen with a critical wavelength of 370 nanometers, which is going to protect from both UVB and UVA2,” she said.
Sunscreen recommendations are meant to be paired with advice to avoid midday sun from 10 a.m. to 2 p.m., wearing protective clothing and accessories, and seeking shade, she noted.
Dr. Taylor said, for those patients with lighter skin who are more susceptible to photodamage and premature aging, physicians should recommend sunscreens that contain DNA repair enzymes such as photolyases and sunscreens that contain antioxidants that can prevent or reverse DNA damage. “The exogenous form of these lyases have been manufactured and added to sunscreens,” Dr. Taylor said. “They’re readily available in the United States. That is something to consider for patients with significant photodamage.”
Retinoids can also help alleviate or reverse photodamage, she added.
Protection for darker-colored skin
“Many people of color do not believe they need sunscreen,” Dr. Taylor said. But studies show that, although there may be more intrinsic protection, sunscreen is still needed.
Over 30 years ago, Halder and colleagues reported that melanin in skin of color can filter two to five times more UV radiation, and in a paper on the photoprotective role of melanin, Kaidbey and colleagues found that skin types V and VI had an intrinsic SPF of 13 when compared with those who have lighter complexions, which had an SPF of 3.
Sunburns seem to occur less frequently in people with skin of color, but that may be because erythema is less apparent in people with darker skin tones or because of differences in personal definitions of sunburn, Dr. Taylor said.
“Skin of color can and does sustain sunburns and sunscreen will help prevent that,” she said, adding that a recommendation of an SPF 30 is likely sufficient for these patients. Dr. Taylor noted that sunscreens for patients with darker skin often cost substantially more than those for lighter skin, and that should be considered in recommendations.
Tinted sunscreens
Dr. Taylor said that, while broad-spectrum photostable sunscreens protect against UVB and UVA 2, they don’t protect from visible light and UVA1. Two methods to add that protection are using inorganic tinted sunscreens that contain iron oxide or pigmentary titanium dioxide. Dr. Taylor was a coauthor of a practical guide to tinted sunscreens published in 2022.
“For iron oxide, we want a concentration of 3% or greater,” she said, adding that the percentage often is not known because if it is contained in a sunscreen, it is listed as an inactive ingredient.
Another method to address visible light and UVA1 is the use of antioxidant-containing sunscreens with vitamin E, vitamin C, or licochalcone A, Dr. Taylor said.
During the question-and-answer period following her presentation, Amit Pandya, MD, adjunct professor of dermatology at University of Texas Southwestern Medical Center, Dallas, asked why “every makeup, every sunscreen, just says iron oxide,” since it is known that visible light will cause pigmentation, especially in those with darker skin tones.
He urged pushing for a law that would require listing the percentage of iron oxide on products to assure it is sufficient, according to what the literature recommends.
Conference Chair Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute of Southern California, Los Angeles, said that she recommends tinted sunscreens almost exclusively for her patients, but those with darker skin colors struggle to match color.
Dr. Taylor referred to an analysis published in 2022 of 58 over-the counter sunscreens, which found that only 38% of tinted sunscreens was available in more than one shade, “which is a problem for many of our patients.” She said that providing samples with different hues and tactile sensations may help patients find the right product.
Dr. Taylor disclosed being on the advisory boards for AbbVie, Avita Medical, Beiersdorf, Biorez, Eli Lily, EPI Health, Evolus, Galderma, Hugel America, Johnson and Johnson, L’Oreal USA, MedScape, Pfizer, Scientis US, UCB, Vichy Laboratories. She is a consultant for Arcutis Biothermapeutics, Beiersdorf, Bristol-Myers Squibb, Cara Therapeutics, Dior, and Sanofi. She has done contracted research for Allergan Aesthetics, Concert Pharmaceuticals, Croma-Pharma, Eli Lilly, and Pfizer, and has an ownership interest in Armis Scientific, GloGetter, and Piction Health.
Medscape and this news organization are owned by the same parent company.
CHICAGO – at the inaugural Pigmentary Disorders Exchange Symposium.
Among the first factors physicians should consider before recommending sunscreen are a patient’s Fitzpatrick skin type, risks for burning or tanning, underlying skin disorders, and medications the patient is taking, Dr. Taylor, professor of dermatology at the University of Pennsylvania, Philadelphia, said at the meeting, provided by MedscapeLIVE! If patients are on hypertensives, for example, medications can make them more photosensitive.
Consider skin type
Dr. Taylor said she was dismayed by the results of a recent study, which found that 43% of dermatologists who responded to a survey reported that they never, rarely, or only sometimes took a patient’s skin type into account when making sunscreen recommendations. The article is referenced in a 2022 expert panel consensus paper she coauthored on photoprotection “for skin of all color.” But she pointed out that considering skin type alone is inadequate.
Questions for patients in joint decision-making should include lifestyle and work choices such as whether they work inside or outside, and how much sun exposure they get in a typical day. Heat and humidity levels should also be considered as should a patient’s susceptibility to dyspigmentation. “That could be overall darkening of the skin, mottled hyperpigmentation, actinic dyspigmentation, and, of course, propensity for skin cancer,” she said.
Use differs by race
Dr. Taylor, who is also vice chair for diversity, equity and inclusion in the department of dermatology at the University of Pennsylvania, pointed out that sunscreen use differs considerably by race.
In study of 8,952 adults in the United States who reported that they were sun sensitive found that a subset of adults with skin of color were significantly less likely to use sunscreen when compared with non-Hispanic White adults: Non-Hispanic Black (adjusted odds ratio, 0.43); non-Hispanic Asian (aOR. 0.54); and Hispanic (aOR, 0.70) adults.
In the study, non-Hispanic Black and Hispanic adults were significantly less likely to use sunscreens with an SPF greater than 15. In addition, non-Hispanic Black, non-Hispanic Asian, and Hispanic adults were significantly more likely than non-Hispanic Whites to wear long sleeves when outside. Such differences are important to keep in mind when advising patients about sunscreens, she said.
Protection for lighter-colored skin
Dr. Taylor said that, for patients with lighter skin tones, “we really want to protect against ultraviolet B as well as ultraviolet A, particularly ultraviolet A2. Ultraviolet radiation is going to cause DNA damage.” Patients with Fitzpatrick skin types I, II, or III are most susceptible to the effects of UVB with sunburn inflammation, which will cause erythema and tanning, and immunosuppression.
“For those who are I, II, and III, we do want to recommend a broad-spectrum, photostable sunscreen with a critical wavelength of 370 nanometers, which is going to protect from both UVB and UVA2,” she said.
Sunscreen recommendations are meant to be paired with advice to avoid midday sun from 10 a.m. to 2 p.m., wearing protective clothing and accessories, and seeking shade, she noted.
Dr. Taylor said, for those patients with lighter skin who are more susceptible to photodamage and premature aging, physicians should recommend sunscreens that contain DNA repair enzymes such as photolyases and sunscreens that contain antioxidants that can prevent or reverse DNA damage. “The exogenous form of these lyases have been manufactured and added to sunscreens,” Dr. Taylor said. “They’re readily available in the United States. That is something to consider for patients with significant photodamage.”
Retinoids can also help alleviate or reverse photodamage, she added.
Protection for darker-colored skin
“Many people of color do not believe they need sunscreen,” Dr. Taylor said. But studies show that, although there may be more intrinsic protection, sunscreen is still needed.
Over 30 years ago, Halder and colleagues reported that melanin in skin of color can filter two to five times more UV radiation, and in a paper on the photoprotective role of melanin, Kaidbey and colleagues found that skin types V and VI had an intrinsic SPF of 13 when compared with those who have lighter complexions, which had an SPF of 3.
Sunburns seem to occur less frequently in people with skin of color, but that may be because erythema is less apparent in people with darker skin tones or because of differences in personal definitions of sunburn, Dr. Taylor said.
“Skin of color can and does sustain sunburns and sunscreen will help prevent that,” she said, adding that a recommendation of an SPF 30 is likely sufficient for these patients. Dr. Taylor noted that sunscreens for patients with darker skin often cost substantially more than those for lighter skin, and that should be considered in recommendations.
Tinted sunscreens
Dr. Taylor said that, while broad-spectrum photostable sunscreens protect against UVB and UVA 2, they don’t protect from visible light and UVA1. Two methods to add that protection are using inorganic tinted sunscreens that contain iron oxide or pigmentary titanium dioxide. Dr. Taylor was a coauthor of a practical guide to tinted sunscreens published in 2022.
“For iron oxide, we want a concentration of 3% or greater,” she said, adding that the percentage often is not known because if it is contained in a sunscreen, it is listed as an inactive ingredient.
Another method to address visible light and UVA1 is the use of antioxidant-containing sunscreens with vitamin E, vitamin C, or licochalcone A, Dr. Taylor said.
During the question-and-answer period following her presentation, Amit Pandya, MD, adjunct professor of dermatology at University of Texas Southwestern Medical Center, Dallas, asked why “every makeup, every sunscreen, just says iron oxide,” since it is known that visible light will cause pigmentation, especially in those with darker skin tones.
He urged pushing for a law that would require listing the percentage of iron oxide on products to assure it is sufficient, according to what the literature recommends.
Conference Chair Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute of Southern California, Los Angeles, said that she recommends tinted sunscreens almost exclusively for her patients, but those with darker skin colors struggle to match color.
Dr. Taylor referred to an analysis published in 2022 of 58 over-the counter sunscreens, which found that only 38% of tinted sunscreens was available in more than one shade, “which is a problem for many of our patients.” She said that providing samples with different hues and tactile sensations may help patients find the right product.
Dr. Taylor disclosed being on the advisory boards for AbbVie, Avita Medical, Beiersdorf, Biorez, Eli Lily, EPI Health, Evolus, Galderma, Hugel America, Johnson and Johnson, L’Oreal USA, MedScape, Pfizer, Scientis US, UCB, Vichy Laboratories. She is a consultant for Arcutis Biothermapeutics, Beiersdorf, Bristol-Myers Squibb, Cara Therapeutics, Dior, and Sanofi. She has done contracted research for Allergan Aesthetics, Concert Pharmaceuticals, Croma-Pharma, Eli Lilly, and Pfizer, and has an ownership interest in Armis Scientific, GloGetter, and Piction Health.
Medscape and this news organization are owned by the same parent company.
CHICAGO – at the inaugural Pigmentary Disorders Exchange Symposium.
Among the first factors physicians should consider before recommending sunscreen are a patient’s Fitzpatrick skin type, risks for burning or tanning, underlying skin disorders, and medications the patient is taking, Dr. Taylor, professor of dermatology at the University of Pennsylvania, Philadelphia, said at the meeting, provided by MedscapeLIVE! If patients are on hypertensives, for example, medications can make them more photosensitive.
Consider skin type
Dr. Taylor said she was dismayed by the results of a recent study, which found that 43% of dermatologists who responded to a survey reported that they never, rarely, or only sometimes took a patient’s skin type into account when making sunscreen recommendations. The article is referenced in a 2022 expert panel consensus paper she coauthored on photoprotection “for skin of all color.” But she pointed out that considering skin type alone is inadequate.
Questions for patients in joint decision-making should include lifestyle and work choices such as whether they work inside or outside, and how much sun exposure they get in a typical day. Heat and humidity levels should also be considered as should a patient’s susceptibility to dyspigmentation. “That could be overall darkening of the skin, mottled hyperpigmentation, actinic dyspigmentation, and, of course, propensity for skin cancer,” she said.
Use differs by race
Dr. Taylor, who is also vice chair for diversity, equity and inclusion in the department of dermatology at the University of Pennsylvania, pointed out that sunscreen use differs considerably by race.
In study of 8,952 adults in the United States who reported that they were sun sensitive found that a subset of adults with skin of color were significantly less likely to use sunscreen when compared with non-Hispanic White adults: Non-Hispanic Black (adjusted odds ratio, 0.43); non-Hispanic Asian (aOR. 0.54); and Hispanic (aOR, 0.70) adults.
In the study, non-Hispanic Black and Hispanic adults were significantly less likely to use sunscreens with an SPF greater than 15. In addition, non-Hispanic Black, non-Hispanic Asian, and Hispanic adults were significantly more likely than non-Hispanic Whites to wear long sleeves when outside. Such differences are important to keep in mind when advising patients about sunscreens, she said.
Protection for lighter-colored skin
Dr. Taylor said that, for patients with lighter skin tones, “we really want to protect against ultraviolet B as well as ultraviolet A, particularly ultraviolet A2. Ultraviolet radiation is going to cause DNA damage.” Patients with Fitzpatrick skin types I, II, or III are most susceptible to the effects of UVB with sunburn inflammation, which will cause erythema and tanning, and immunosuppression.
“For those who are I, II, and III, we do want to recommend a broad-spectrum, photostable sunscreen with a critical wavelength of 370 nanometers, which is going to protect from both UVB and UVA2,” she said.
Sunscreen recommendations are meant to be paired with advice to avoid midday sun from 10 a.m. to 2 p.m., wearing protective clothing and accessories, and seeking shade, she noted.
Dr. Taylor said, for those patients with lighter skin who are more susceptible to photodamage and premature aging, physicians should recommend sunscreens that contain DNA repair enzymes such as photolyases and sunscreens that contain antioxidants that can prevent or reverse DNA damage. “The exogenous form of these lyases have been manufactured and added to sunscreens,” Dr. Taylor said. “They’re readily available in the United States. That is something to consider for patients with significant photodamage.”
Retinoids can also help alleviate or reverse photodamage, she added.
Protection for darker-colored skin
“Many people of color do not believe they need sunscreen,” Dr. Taylor said. But studies show that, although there may be more intrinsic protection, sunscreen is still needed.
Over 30 years ago, Halder and colleagues reported that melanin in skin of color can filter two to five times more UV radiation, and in a paper on the photoprotective role of melanin, Kaidbey and colleagues found that skin types V and VI had an intrinsic SPF of 13 when compared with those who have lighter complexions, which had an SPF of 3.
Sunburns seem to occur less frequently in people with skin of color, but that may be because erythema is less apparent in people with darker skin tones or because of differences in personal definitions of sunburn, Dr. Taylor said.
“Skin of color can and does sustain sunburns and sunscreen will help prevent that,” she said, adding that a recommendation of an SPF 30 is likely sufficient for these patients. Dr. Taylor noted that sunscreens for patients with darker skin often cost substantially more than those for lighter skin, and that should be considered in recommendations.
Tinted sunscreens
Dr. Taylor said that, while broad-spectrum photostable sunscreens protect against UVB and UVA 2, they don’t protect from visible light and UVA1. Two methods to add that protection are using inorganic tinted sunscreens that contain iron oxide or pigmentary titanium dioxide. Dr. Taylor was a coauthor of a practical guide to tinted sunscreens published in 2022.
“For iron oxide, we want a concentration of 3% or greater,” she said, adding that the percentage often is not known because if it is contained in a sunscreen, it is listed as an inactive ingredient.
Another method to address visible light and UVA1 is the use of antioxidant-containing sunscreens with vitamin E, vitamin C, or licochalcone A, Dr. Taylor said.
During the question-and-answer period following her presentation, Amit Pandya, MD, adjunct professor of dermatology at University of Texas Southwestern Medical Center, Dallas, asked why “every makeup, every sunscreen, just says iron oxide,” since it is known that visible light will cause pigmentation, especially in those with darker skin tones.
He urged pushing for a law that would require listing the percentage of iron oxide on products to assure it is sufficient, according to what the literature recommends.
Conference Chair Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute of Southern California, Los Angeles, said that she recommends tinted sunscreens almost exclusively for her patients, but those with darker skin colors struggle to match color.
Dr. Taylor referred to an analysis published in 2022 of 58 over-the counter sunscreens, which found that only 38% of tinted sunscreens was available in more than one shade, “which is a problem for many of our patients.” She said that providing samples with different hues and tactile sensations may help patients find the right product.
Dr. Taylor disclosed being on the advisory boards for AbbVie, Avita Medical, Beiersdorf, Biorez, Eli Lily, EPI Health, Evolus, Galderma, Hugel America, Johnson and Johnson, L’Oreal USA, MedScape, Pfizer, Scientis US, UCB, Vichy Laboratories. She is a consultant for Arcutis Biothermapeutics, Beiersdorf, Bristol-Myers Squibb, Cara Therapeutics, Dior, and Sanofi. She has done contracted research for Allergan Aesthetics, Concert Pharmaceuticals, Croma-Pharma, Eli Lilly, and Pfizer, and has an ownership interest in Armis Scientific, GloGetter, and Piction Health.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! PIGMENTARY DISORDERS SYMPOSIUM
FDA warns people to avoid compounded semaglutide medicines
Compounded medicines are not FDA approved but are allowed to be made during an official drug shortage. Ozempic and Wegovy are currently on the FDA’s shortage list, but the federal agency warned that it has received reports of people experiencing “adverse events” after using compounded versions of the drugs. (The FDA did not provide details of those events or where the drugs involved were compounded.)
Agency officials are concerned that the compounded versions may contain ingredients that sound like the brand name drugs’ active ingredient, semaglutide, but are different because the ingredients are in salt form.
“Patients should be aware that some products sold as ‘semaglutide’ may not contain the same active ingredient as FDA-approved semaglutide products and may be the salt formulations,” the FDA warning stated. “Products containing these salts, such as semaglutide sodium and semaglutide acetate, have not been shown to be safe and effective.”
The agency said salt forms don’t meet the criteria for compounding during a shortage and sent a letter to the National Association of Boards of Pharmacy expressing “concerns with use of the salt forms in compounded products.”
Patients and health care providers should be aware that “compounded drugs are not FDA approved, and the agency does not verify the safety or effectiveness of compounded drugs,” the FDA explained in its statement.
The Alliance for Pharmacy Compounding’s board of directors said in a statement that some compounders’ arguments for the suitability of semaglutide sodium are “worthy of discussion,” but the board did not endorse those arguments.
For people who use an online pharmacy, the FDA recommends checking the FDA’s website BeSafeRx to check its credentials.
A version of this article first appeared on WebMD.com.
Compounded medicines are not FDA approved but are allowed to be made during an official drug shortage. Ozempic and Wegovy are currently on the FDA’s shortage list, but the federal agency warned that it has received reports of people experiencing “adverse events” after using compounded versions of the drugs. (The FDA did not provide details of those events or where the drugs involved were compounded.)
Agency officials are concerned that the compounded versions may contain ingredients that sound like the brand name drugs’ active ingredient, semaglutide, but are different because the ingredients are in salt form.
“Patients should be aware that some products sold as ‘semaglutide’ may not contain the same active ingredient as FDA-approved semaglutide products and may be the salt formulations,” the FDA warning stated. “Products containing these salts, such as semaglutide sodium and semaglutide acetate, have not been shown to be safe and effective.”
The agency said salt forms don’t meet the criteria for compounding during a shortage and sent a letter to the National Association of Boards of Pharmacy expressing “concerns with use of the salt forms in compounded products.”
Patients and health care providers should be aware that “compounded drugs are not FDA approved, and the agency does not verify the safety or effectiveness of compounded drugs,” the FDA explained in its statement.
The Alliance for Pharmacy Compounding’s board of directors said in a statement that some compounders’ arguments for the suitability of semaglutide sodium are “worthy of discussion,” but the board did not endorse those arguments.
For people who use an online pharmacy, the FDA recommends checking the FDA’s website BeSafeRx to check its credentials.
A version of this article first appeared on WebMD.com.
Compounded medicines are not FDA approved but are allowed to be made during an official drug shortage. Ozempic and Wegovy are currently on the FDA’s shortage list, but the federal agency warned that it has received reports of people experiencing “adverse events” after using compounded versions of the drugs. (The FDA did not provide details of those events or where the drugs involved were compounded.)
Agency officials are concerned that the compounded versions may contain ingredients that sound like the brand name drugs’ active ingredient, semaglutide, but are different because the ingredients are in salt form.
“Patients should be aware that some products sold as ‘semaglutide’ may not contain the same active ingredient as FDA-approved semaglutide products and may be the salt formulations,” the FDA warning stated. “Products containing these salts, such as semaglutide sodium and semaglutide acetate, have not been shown to be safe and effective.”
The agency said salt forms don’t meet the criteria for compounding during a shortage and sent a letter to the National Association of Boards of Pharmacy expressing “concerns with use of the salt forms in compounded products.”
Patients and health care providers should be aware that “compounded drugs are not FDA approved, and the agency does not verify the safety or effectiveness of compounded drugs,” the FDA explained in its statement.
The Alliance for Pharmacy Compounding’s board of directors said in a statement that some compounders’ arguments for the suitability of semaglutide sodium are “worthy of discussion,” but the board did not endorse those arguments.
For people who use an online pharmacy, the FDA recommends checking the FDA’s website BeSafeRx to check its credentials.
A version of this article first appeared on WebMD.com.
Sucralose damages DNA, linked to leaky gut: Study
Sucralose is sold under the brand name Splenda and is also used as an ingredient in packaged foods and beverages.
The findings were published in the Journal of Toxicology and Environmental Health, Part B. The researchers conducted a series of laboratory experiments exposing human blood cells and gut tissue to sucralose-6-acetate. The findings build on previous research that linked sucralose to gut health problems.
The researchers found that sucralose causes DNA to break apart, putting people at risk for disease. They also linked sucralose to leaky gut syndrome, which means the lining of the intestines are worn down and become permeable. Symptoms are a burning sensation, painful digestion, diarrhea, gas, and bloating.
When a substance damages DNA, it is called genotoxic. Researchers have found that eating sucralose results in the body producing a substance called sucralose-6-acetate, which the new study now shows is genotoxic. The researchers also found sucralose-6-acetate in trace amounts in off-the-shelf products that are so high, they would exceed the safety levels currently allowed in Europe.
“It’s time to revisit the safety and regulatory status of sucralose because the evidence is mounting that it carries significant risks. If nothing else, I encourage people to avoid products containing sucralose,” researcher Susan Schiffman, PhD, adjunct professor of biomedical engineering at North Carolina State University, Raleigh, said in a statement. “It’s something you should not be eating.”
The FDA says sucralose is safe, describing it as 600 times sweeter than table sugar and used in “baked goods, beverages, chewing gum, gelatins, and frozen dairy desserts.”
“To determine the safety of sucralose, the FDA reviewed more than 110 studies designed to identify possible toxic effects, including studies on the reproductive and nervous systems, carcinogenicity, and metabolism,” the agency explained on its website. “The FDA also reviewed human clinical trials to address metabolism and effects on patients with diabetes.”
The study authors reported that they had no conflicts of interest.A version of this article first appeared on WebMD.com.
Sucralose is sold under the brand name Splenda and is also used as an ingredient in packaged foods and beverages.
The findings were published in the Journal of Toxicology and Environmental Health, Part B. The researchers conducted a series of laboratory experiments exposing human blood cells and gut tissue to sucralose-6-acetate. The findings build on previous research that linked sucralose to gut health problems.
The researchers found that sucralose causes DNA to break apart, putting people at risk for disease. They also linked sucralose to leaky gut syndrome, which means the lining of the intestines are worn down and become permeable. Symptoms are a burning sensation, painful digestion, diarrhea, gas, and bloating.
When a substance damages DNA, it is called genotoxic. Researchers have found that eating sucralose results in the body producing a substance called sucralose-6-acetate, which the new study now shows is genotoxic. The researchers also found sucralose-6-acetate in trace amounts in off-the-shelf products that are so high, they would exceed the safety levels currently allowed in Europe.
“It’s time to revisit the safety and regulatory status of sucralose because the evidence is mounting that it carries significant risks. If nothing else, I encourage people to avoid products containing sucralose,” researcher Susan Schiffman, PhD, adjunct professor of biomedical engineering at North Carolina State University, Raleigh, said in a statement. “It’s something you should not be eating.”
The FDA says sucralose is safe, describing it as 600 times sweeter than table sugar and used in “baked goods, beverages, chewing gum, gelatins, and frozen dairy desserts.”
“To determine the safety of sucralose, the FDA reviewed more than 110 studies designed to identify possible toxic effects, including studies on the reproductive and nervous systems, carcinogenicity, and metabolism,” the agency explained on its website. “The FDA also reviewed human clinical trials to address metabolism and effects on patients with diabetes.”
The study authors reported that they had no conflicts of interest.A version of this article first appeared on WebMD.com.
Sucralose is sold under the brand name Splenda and is also used as an ingredient in packaged foods and beverages.
The findings were published in the Journal of Toxicology and Environmental Health, Part B. The researchers conducted a series of laboratory experiments exposing human blood cells and gut tissue to sucralose-6-acetate. The findings build on previous research that linked sucralose to gut health problems.
The researchers found that sucralose causes DNA to break apart, putting people at risk for disease. They also linked sucralose to leaky gut syndrome, which means the lining of the intestines are worn down and become permeable. Symptoms are a burning sensation, painful digestion, diarrhea, gas, and bloating.
When a substance damages DNA, it is called genotoxic. Researchers have found that eating sucralose results in the body producing a substance called sucralose-6-acetate, which the new study now shows is genotoxic. The researchers also found sucralose-6-acetate in trace amounts in off-the-shelf products that are so high, they would exceed the safety levels currently allowed in Europe.
“It’s time to revisit the safety and regulatory status of sucralose because the evidence is mounting that it carries significant risks. If nothing else, I encourage people to avoid products containing sucralose,” researcher Susan Schiffman, PhD, adjunct professor of biomedical engineering at North Carolina State University, Raleigh, said in a statement. “It’s something you should not be eating.”
The FDA says sucralose is safe, describing it as 600 times sweeter than table sugar and used in “baked goods, beverages, chewing gum, gelatins, and frozen dairy desserts.”
“To determine the safety of sucralose, the FDA reviewed more than 110 studies designed to identify possible toxic effects, including studies on the reproductive and nervous systems, carcinogenicity, and metabolism,” the agency explained on its website. “The FDA also reviewed human clinical trials to address metabolism and effects on patients with diabetes.”
The study authors reported that they had no conflicts of interest.A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF TOXICOLOGY AND ENVIRONMENTAL HEALTH, PART B
Positive top-line results for cannabinoid-based med for nerve pain
, new top-line results released by Zelira Therapeutics suggest.
“The implications of these results for patients are incredibly promising,” principal investigator Bryan Doner, DO, medical director of HealthyWays Integrated Wellness Solutions, Gibsonia, Pa., said in a news release.
“Through this rigorously designed study, we have demonstrated that ZLT-L-007 is a safe, effective, and well-tolerated alternative for patients who would typically seek a Lyrica-level of pain relief,” he added.
The observational, nonblinded trial tested the efficacy, safety, and tolerability of ZLT-L-007 against pregabalin in 60 adults with diabetic nerve pain.
The study had three groups with 20 patients each (pregabalin alone, pregabalin plus ZLT-L-007, and ZLT-L-007 alone).
Top-line results show the study met its primary endpoint for change in daily pain severity as measured by the percent change from baseline at 30, 60, and 90 days on the Numerical Rating Scale.
For the pregabalin-only group, there was a reduction in symptom severity at all follow-up points, ranging from 20% to 35% (median percent change from baseline), the company said.
For the ZLT-L-007 only group, there was about a 33% reduction in symptom severity at 30 days, and 71% and 78% reduction, respectively, at 60 and 90 days, suggesting a larger improvement in symptom severity than with pregabalin alone, the company said.
For the pregabalin plus ZLT-L-007 group, there was a moderate 20% reduction in symptom severity at 30 days, but a larger reduction at 60 and 90 days (50% and 72%, respectively), which indicates substantially greater improvement in symptom severity than with pregabalin alone, the company said.
The study also met secondary endpoints, including significant decreases in daily pain severity as measured by the Visual Analog Scale and measurable changes in the short-form McGill Pain Questionnaire and Neuropathic Pain Symptom Inventory.
Dr. Doner noted that the top-line data showed “no serious adverse events, and participants’ blood pressure and other safety vitals remained unaffected throughout. This confirms that ZLT-L-007 is a well-tolerated product that delivers statistically significant pain relief, surpassing the levels achieved by Lyrica.”
The company plans to report additional insights from the full study, as they become available, during fiscal year 2023-2024.
A version of this article first appeared on Medscape.com.
, new top-line results released by Zelira Therapeutics suggest.
“The implications of these results for patients are incredibly promising,” principal investigator Bryan Doner, DO, medical director of HealthyWays Integrated Wellness Solutions, Gibsonia, Pa., said in a news release.
“Through this rigorously designed study, we have demonstrated that ZLT-L-007 is a safe, effective, and well-tolerated alternative for patients who would typically seek a Lyrica-level of pain relief,” he added.
The observational, nonblinded trial tested the efficacy, safety, and tolerability of ZLT-L-007 against pregabalin in 60 adults with diabetic nerve pain.
The study had three groups with 20 patients each (pregabalin alone, pregabalin plus ZLT-L-007, and ZLT-L-007 alone).
Top-line results show the study met its primary endpoint for change in daily pain severity as measured by the percent change from baseline at 30, 60, and 90 days on the Numerical Rating Scale.
For the pregabalin-only group, there was a reduction in symptom severity at all follow-up points, ranging from 20% to 35% (median percent change from baseline), the company said.
For the ZLT-L-007 only group, there was about a 33% reduction in symptom severity at 30 days, and 71% and 78% reduction, respectively, at 60 and 90 days, suggesting a larger improvement in symptom severity than with pregabalin alone, the company said.
For the pregabalin plus ZLT-L-007 group, there was a moderate 20% reduction in symptom severity at 30 days, but a larger reduction at 60 and 90 days (50% and 72%, respectively), which indicates substantially greater improvement in symptom severity than with pregabalin alone, the company said.
The study also met secondary endpoints, including significant decreases in daily pain severity as measured by the Visual Analog Scale and measurable changes in the short-form McGill Pain Questionnaire and Neuropathic Pain Symptom Inventory.
Dr. Doner noted that the top-line data showed “no serious adverse events, and participants’ blood pressure and other safety vitals remained unaffected throughout. This confirms that ZLT-L-007 is a well-tolerated product that delivers statistically significant pain relief, surpassing the levels achieved by Lyrica.”
The company plans to report additional insights from the full study, as they become available, during fiscal year 2023-2024.
A version of this article first appeared on Medscape.com.
, new top-line results released by Zelira Therapeutics suggest.
“The implications of these results for patients are incredibly promising,” principal investigator Bryan Doner, DO, medical director of HealthyWays Integrated Wellness Solutions, Gibsonia, Pa., said in a news release.
“Through this rigorously designed study, we have demonstrated that ZLT-L-007 is a safe, effective, and well-tolerated alternative for patients who would typically seek a Lyrica-level of pain relief,” he added.
The observational, nonblinded trial tested the efficacy, safety, and tolerability of ZLT-L-007 against pregabalin in 60 adults with diabetic nerve pain.
The study had three groups with 20 patients each (pregabalin alone, pregabalin plus ZLT-L-007, and ZLT-L-007 alone).
Top-line results show the study met its primary endpoint for change in daily pain severity as measured by the percent change from baseline at 30, 60, and 90 days on the Numerical Rating Scale.
For the pregabalin-only group, there was a reduction in symptom severity at all follow-up points, ranging from 20% to 35% (median percent change from baseline), the company said.
For the ZLT-L-007 only group, there was about a 33% reduction in symptom severity at 30 days, and 71% and 78% reduction, respectively, at 60 and 90 days, suggesting a larger improvement in symptom severity than with pregabalin alone, the company said.
For the pregabalin plus ZLT-L-007 group, there was a moderate 20% reduction in symptom severity at 30 days, but a larger reduction at 60 and 90 days (50% and 72%, respectively), which indicates substantially greater improvement in symptom severity than with pregabalin alone, the company said.
The study also met secondary endpoints, including significant decreases in daily pain severity as measured by the Visual Analog Scale and measurable changes in the short-form McGill Pain Questionnaire and Neuropathic Pain Symptom Inventory.
Dr. Doner noted that the top-line data showed “no serious adverse events, and participants’ blood pressure and other safety vitals remained unaffected throughout. This confirms that ZLT-L-007 is a well-tolerated product that delivers statistically significant pain relief, surpassing the levels achieved by Lyrica.”
The company plans to report additional insights from the full study, as they become available, during fiscal year 2023-2024.
A version of this article first appeared on Medscape.com.
Troponin to ID diabetes patients with silent heart disease?
– based on data from a representative sample of more than 10,000 U.S. adults.
The finding suggests hs-cTnT maybe a useful marker for adults with diabetes who could benefit from more aggressive CVD risk reduction despite having no clinical indications of CVD.
The results “highlight the substantial burden of subclinical CVD in persons with diabetes and emphasize the importance of early detection and treatment of CVD for this high-risk population,” say the authors of the research, published in the Journal of the American Heart Association.
“This is the first study to examine subclinical CVD, defined by elevated cardiac biomarkers, in a nationally representative population of adults with or without diabetes. It provides novel information on the high burden of subclinical CVD [in American adults with diabetes] and the potential utility of hs-cTnT for monitoring this risk in people with diabetes,” said Elizabeth Selvin, PhD, senior author and a professor of epidemiology at Johns Hopkins University, Baltimore.
“What we are seeing is that many people with type 2 diabetes who have not had a heart attack or a history of cardiovascular disease are at high risk for cardiovascular complications,” added Dr. Selvin in an AHA press release. “When we look at the whole population of people diagnosed with type 2 diabetes, about 27 million adults in the U.S., according to the [Centers for Disease Control and Prevention], some are at low risk and some are at high risk for cardiovascular disease, so the open question is: ‘Who is most at risk?’ These cardiac biomarkers give us a window into cardiovascular risk in people who otherwise might not be recognized as highest risk.”
“Our results provide evidence to support use of cardiac biomarkers for routine risk monitoring in high-risk populations such as people with diabetes,” Dr. Selvin noted in an interview.
Need for aggressive CVD risk reduction
The findings also indicate that people with diabetes and an elevated hs-cTnT “should be targeted for aggressive cardiovascular risk reduction, including lifestyle interventions, weight loss, and treatment with statins, blood pressure medications, and cardioprotective therapies such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists,” Dr. Selvin added.
“Cholesterol is often the factor that we target to reduce the risk of cardiovascular disease in people with type 2 diabetes,” she observed. “However, type 2 diabetes may have a direct effect on the heart not related to cholesterol levels. If type 2 diabetes is directly causing damage to the small vessels in the heart unrelated to cholesterol plaque buildup, then cholesterol-lowering medications are not going to prevent cardiac damage,” Dr. Selvin explained. “Our research suggests that additional non–statin-related therapies are needed to lower the cardiovascular disease risk in people with type 2 diabetes.”
However, she noted that a necessary step prior to formally recommending such a strategy is to run clinical trials to assess the efficacy of specific treatments, such as SGLT-2 inhibitors and GLP-1 agonists, in people with diabetes and elevated hs-cTnT.
“Randomized controlled trials would be best to test the relevance of measuring these biomarkers to assess risk in asymptomatic people with diabetes,” as well as prospective study of the value of hs-cTnT to guide treatment, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado at Denver, Aurora.
“I doubt measurements [of hs-cTnT] would be reimbursed [by third-party payers] if carried out without such outcome data,” he added.
Dr. Eckel also highlights the need to further validate in additional cohorts the link between elevations in hs-cTnT and CVD events in adults with diabetes, and to confirm that elevated levels of another cardiac biomarker – N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) – do not work as well as troponin as a risk marker for people with diabetes, another finding of the study.
ADA report already recommends testing these biomarkers for HF
However, a consensus report published in 2022 by the American Diabetes Association laid out the case for routinely and regularly measuring levels of both high sensitivity cardiac troponin and natriuretic peptides in people with diabetes for early identification of incident heart failure.
“Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure,” noted the ADA consensus report on heart failure.
The new study run by Dr. Selvin and coauthors used data collected by the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004 from U.S. adults who were at least 20 years old and had no history of CVD: myocardial infarction, stroke, coronary heart disease, or heart failure. This included 9,273 people without diabetes and 1,031 with diabetes, defined as a prior diagnosis or hemoglobin A1c of at least 6.5%.
“Cardiovascular risk varies substantially in adults with type 2 diabetes, highlighting the need for accurate risk stratification,” the authors observed.
All study participants had recorded measures of hs-cTnT and NT-proBNP.
The researchers considered an hs-cTnT level of greater than 14 ng/L and an NT-proBNP level of greater than 125 pg/mL as indicators of subclinical CVD.
The crude prevalence of elevated NT-proBNP was 33.4% among those with diabetes and 16.1% in those without diabetes. Elevated hs-cTnT occurred in 19% of those with diabetes and in 5% of those without diabetes. Elevated levels of both markers existed in 9% of those with diabetes and in 3% of those without diabetes.
“Approximately one in three adults with diabetes had subclinical CVD, with 19% having elevated levels of hs-cTnT, 23% having elevated NT-proBNP, and 9% having elevations in both cardiac biomarkers,” the researchers noted.
Diabetes linked with a doubled prevalence of elevated hs-cTnT
After adjustment for several demographic variables as well as traditional CVD risk factors, people with diabetes had a significant 98% higher rate of elevated hs-cTnT, compared with those without diabetes. But after similar adjustments, the rate of elevated NT-proBNP was significantly lower among people with diabetes, compared with controls, by a relative reduction of 24%.
“Our findings suggest that, in people with diabetes, hs-cTnT may be more useful [than NT-proBNP] for general risk monitoring, as its interpretation is less complicated,” said Dr. Selvin, who explained that “NT-proBNP is affected by overweight and obesity.”
In people with diabetes, the age-adjusted prevalence of elevated hs-cTnT ran higher in those with longer duration diabetes, and in those with less well-controlled diabetes based on a higher level of A1c. Neither of these factors showed any significant relationship with measured levels of NT-proBNP.
Further analysis linked the NHANES findings during 1999-2004 with U.S. national death records through the end of 2019. This showed that elevated levels of both hs-cTnT and NT-proBNP significantly linked with subsequently higher rates of all-cause mortality among people with diabetes. Elevated hs-cTnT linked with a 77% increased mortality and NT-proBNP linked with a 78% increased rate, compared with people with diabetes and no elevations in these markers, after adjustment for demographic variables and CVD risk factors.
However, for the outcome of cardiovascular death, elevated hs-cTnT linked with a nonsignificant 54% relative increase, while elevated NT-proBNP linked with a significant 2.46-fold relative increase.
The study “adds new data on biomarkers that are not routinely measured in asymptomatic people with or without diabetes” and the relationships of these markers to CVD mortality and all-cause mortality, Dr. Eckel concluded.
The study received no commercial funding, but used reagents donated by Abbott Laboratories, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin and Dr. Eckel had no disclosures.
A version of this article first appeared on Medscape.com.
– based on data from a representative sample of more than 10,000 U.S. adults.
The finding suggests hs-cTnT maybe a useful marker for adults with diabetes who could benefit from more aggressive CVD risk reduction despite having no clinical indications of CVD.
The results “highlight the substantial burden of subclinical CVD in persons with diabetes and emphasize the importance of early detection and treatment of CVD for this high-risk population,” say the authors of the research, published in the Journal of the American Heart Association.
“This is the first study to examine subclinical CVD, defined by elevated cardiac biomarkers, in a nationally representative population of adults with or without diabetes. It provides novel information on the high burden of subclinical CVD [in American adults with diabetes] and the potential utility of hs-cTnT for monitoring this risk in people with diabetes,” said Elizabeth Selvin, PhD, senior author and a professor of epidemiology at Johns Hopkins University, Baltimore.
“What we are seeing is that many people with type 2 diabetes who have not had a heart attack or a history of cardiovascular disease are at high risk for cardiovascular complications,” added Dr. Selvin in an AHA press release. “When we look at the whole population of people diagnosed with type 2 diabetes, about 27 million adults in the U.S., according to the [Centers for Disease Control and Prevention], some are at low risk and some are at high risk for cardiovascular disease, so the open question is: ‘Who is most at risk?’ These cardiac biomarkers give us a window into cardiovascular risk in people who otherwise might not be recognized as highest risk.”
“Our results provide evidence to support use of cardiac biomarkers for routine risk monitoring in high-risk populations such as people with diabetes,” Dr. Selvin noted in an interview.
Need for aggressive CVD risk reduction
The findings also indicate that people with diabetes and an elevated hs-cTnT “should be targeted for aggressive cardiovascular risk reduction, including lifestyle interventions, weight loss, and treatment with statins, blood pressure medications, and cardioprotective therapies such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists,” Dr. Selvin added.
“Cholesterol is often the factor that we target to reduce the risk of cardiovascular disease in people with type 2 diabetes,” she observed. “However, type 2 diabetes may have a direct effect on the heart not related to cholesterol levels. If type 2 diabetes is directly causing damage to the small vessels in the heart unrelated to cholesterol plaque buildup, then cholesterol-lowering medications are not going to prevent cardiac damage,” Dr. Selvin explained. “Our research suggests that additional non–statin-related therapies are needed to lower the cardiovascular disease risk in people with type 2 diabetes.”
However, she noted that a necessary step prior to formally recommending such a strategy is to run clinical trials to assess the efficacy of specific treatments, such as SGLT-2 inhibitors and GLP-1 agonists, in people with diabetes and elevated hs-cTnT.
“Randomized controlled trials would be best to test the relevance of measuring these biomarkers to assess risk in asymptomatic people with diabetes,” as well as prospective study of the value of hs-cTnT to guide treatment, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado at Denver, Aurora.
“I doubt measurements [of hs-cTnT] would be reimbursed [by third-party payers] if carried out without such outcome data,” he added.
Dr. Eckel also highlights the need to further validate in additional cohorts the link between elevations in hs-cTnT and CVD events in adults with diabetes, and to confirm that elevated levels of another cardiac biomarker – N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) – do not work as well as troponin as a risk marker for people with diabetes, another finding of the study.
ADA report already recommends testing these biomarkers for HF
However, a consensus report published in 2022 by the American Diabetes Association laid out the case for routinely and regularly measuring levels of both high sensitivity cardiac troponin and natriuretic peptides in people with diabetes for early identification of incident heart failure.
“Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure,” noted the ADA consensus report on heart failure.
The new study run by Dr. Selvin and coauthors used data collected by the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004 from U.S. adults who were at least 20 years old and had no history of CVD: myocardial infarction, stroke, coronary heart disease, or heart failure. This included 9,273 people without diabetes and 1,031 with diabetes, defined as a prior diagnosis or hemoglobin A1c of at least 6.5%.
“Cardiovascular risk varies substantially in adults with type 2 diabetes, highlighting the need for accurate risk stratification,” the authors observed.
All study participants had recorded measures of hs-cTnT and NT-proBNP.
The researchers considered an hs-cTnT level of greater than 14 ng/L and an NT-proBNP level of greater than 125 pg/mL as indicators of subclinical CVD.
The crude prevalence of elevated NT-proBNP was 33.4% among those with diabetes and 16.1% in those without diabetes. Elevated hs-cTnT occurred in 19% of those with diabetes and in 5% of those without diabetes. Elevated levels of both markers existed in 9% of those with diabetes and in 3% of those without diabetes.
“Approximately one in three adults with diabetes had subclinical CVD, with 19% having elevated levels of hs-cTnT, 23% having elevated NT-proBNP, and 9% having elevations in both cardiac biomarkers,” the researchers noted.
Diabetes linked with a doubled prevalence of elevated hs-cTnT
After adjustment for several demographic variables as well as traditional CVD risk factors, people with diabetes had a significant 98% higher rate of elevated hs-cTnT, compared with those without diabetes. But after similar adjustments, the rate of elevated NT-proBNP was significantly lower among people with diabetes, compared with controls, by a relative reduction of 24%.
“Our findings suggest that, in people with diabetes, hs-cTnT may be more useful [than NT-proBNP] for general risk monitoring, as its interpretation is less complicated,” said Dr. Selvin, who explained that “NT-proBNP is affected by overweight and obesity.”
In people with diabetes, the age-adjusted prevalence of elevated hs-cTnT ran higher in those with longer duration diabetes, and in those with less well-controlled diabetes based on a higher level of A1c. Neither of these factors showed any significant relationship with measured levels of NT-proBNP.
Further analysis linked the NHANES findings during 1999-2004 with U.S. national death records through the end of 2019. This showed that elevated levels of both hs-cTnT and NT-proBNP significantly linked with subsequently higher rates of all-cause mortality among people with diabetes. Elevated hs-cTnT linked with a 77% increased mortality and NT-proBNP linked with a 78% increased rate, compared with people with diabetes and no elevations in these markers, after adjustment for demographic variables and CVD risk factors.
However, for the outcome of cardiovascular death, elevated hs-cTnT linked with a nonsignificant 54% relative increase, while elevated NT-proBNP linked with a significant 2.46-fold relative increase.
The study “adds new data on biomarkers that are not routinely measured in asymptomatic people with or without diabetes” and the relationships of these markers to CVD mortality and all-cause mortality, Dr. Eckel concluded.
The study received no commercial funding, but used reagents donated by Abbott Laboratories, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin and Dr. Eckel had no disclosures.
A version of this article first appeared on Medscape.com.
– based on data from a representative sample of more than 10,000 U.S. adults.
The finding suggests hs-cTnT maybe a useful marker for adults with diabetes who could benefit from more aggressive CVD risk reduction despite having no clinical indications of CVD.
The results “highlight the substantial burden of subclinical CVD in persons with diabetes and emphasize the importance of early detection and treatment of CVD for this high-risk population,” say the authors of the research, published in the Journal of the American Heart Association.
“This is the first study to examine subclinical CVD, defined by elevated cardiac biomarkers, in a nationally representative population of adults with or without diabetes. It provides novel information on the high burden of subclinical CVD [in American adults with diabetes] and the potential utility of hs-cTnT for monitoring this risk in people with diabetes,” said Elizabeth Selvin, PhD, senior author and a professor of epidemiology at Johns Hopkins University, Baltimore.
“What we are seeing is that many people with type 2 diabetes who have not had a heart attack or a history of cardiovascular disease are at high risk for cardiovascular complications,” added Dr. Selvin in an AHA press release. “When we look at the whole population of people diagnosed with type 2 diabetes, about 27 million adults in the U.S., according to the [Centers for Disease Control and Prevention], some are at low risk and some are at high risk for cardiovascular disease, so the open question is: ‘Who is most at risk?’ These cardiac biomarkers give us a window into cardiovascular risk in people who otherwise might not be recognized as highest risk.”
“Our results provide evidence to support use of cardiac biomarkers for routine risk monitoring in high-risk populations such as people with diabetes,” Dr. Selvin noted in an interview.
Need for aggressive CVD risk reduction
The findings also indicate that people with diabetes and an elevated hs-cTnT “should be targeted for aggressive cardiovascular risk reduction, including lifestyle interventions, weight loss, and treatment with statins, blood pressure medications, and cardioprotective therapies such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists,” Dr. Selvin added.
“Cholesterol is often the factor that we target to reduce the risk of cardiovascular disease in people with type 2 diabetes,” she observed. “However, type 2 diabetes may have a direct effect on the heart not related to cholesterol levels. If type 2 diabetes is directly causing damage to the small vessels in the heart unrelated to cholesterol plaque buildup, then cholesterol-lowering medications are not going to prevent cardiac damage,” Dr. Selvin explained. “Our research suggests that additional non–statin-related therapies are needed to lower the cardiovascular disease risk in people with type 2 diabetes.”
However, she noted that a necessary step prior to formally recommending such a strategy is to run clinical trials to assess the efficacy of specific treatments, such as SGLT-2 inhibitors and GLP-1 agonists, in people with diabetes and elevated hs-cTnT.
“Randomized controlled trials would be best to test the relevance of measuring these biomarkers to assess risk in asymptomatic people with diabetes,” as well as prospective study of the value of hs-cTnT to guide treatment, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado at Denver, Aurora.
“I doubt measurements [of hs-cTnT] would be reimbursed [by third-party payers] if carried out without such outcome data,” he added.
Dr. Eckel also highlights the need to further validate in additional cohorts the link between elevations in hs-cTnT and CVD events in adults with diabetes, and to confirm that elevated levels of another cardiac biomarker – N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) – do not work as well as troponin as a risk marker for people with diabetes, another finding of the study.
ADA report already recommends testing these biomarkers for HF
However, a consensus report published in 2022 by the American Diabetes Association laid out the case for routinely and regularly measuring levels of both high sensitivity cardiac troponin and natriuretic peptides in people with diabetes for early identification of incident heart failure.
“Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure,” noted the ADA consensus report on heart failure.
The new study run by Dr. Selvin and coauthors used data collected by the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004 from U.S. adults who were at least 20 years old and had no history of CVD: myocardial infarction, stroke, coronary heart disease, or heart failure. This included 9,273 people without diabetes and 1,031 with diabetes, defined as a prior diagnosis or hemoglobin A1c of at least 6.5%.
“Cardiovascular risk varies substantially in adults with type 2 diabetes, highlighting the need for accurate risk stratification,” the authors observed.
All study participants had recorded measures of hs-cTnT and NT-proBNP.
The researchers considered an hs-cTnT level of greater than 14 ng/L and an NT-proBNP level of greater than 125 pg/mL as indicators of subclinical CVD.
The crude prevalence of elevated NT-proBNP was 33.4% among those with diabetes and 16.1% in those without diabetes. Elevated hs-cTnT occurred in 19% of those with diabetes and in 5% of those without diabetes. Elevated levels of both markers existed in 9% of those with diabetes and in 3% of those without diabetes.
“Approximately one in three adults with diabetes had subclinical CVD, with 19% having elevated levels of hs-cTnT, 23% having elevated NT-proBNP, and 9% having elevations in both cardiac biomarkers,” the researchers noted.
Diabetes linked with a doubled prevalence of elevated hs-cTnT
After adjustment for several demographic variables as well as traditional CVD risk factors, people with diabetes had a significant 98% higher rate of elevated hs-cTnT, compared with those without diabetes. But after similar adjustments, the rate of elevated NT-proBNP was significantly lower among people with diabetes, compared with controls, by a relative reduction of 24%.
“Our findings suggest that, in people with diabetes, hs-cTnT may be more useful [than NT-proBNP] for general risk monitoring, as its interpretation is less complicated,” said Dr. Selvin, who explained that “NT-proBNP is affected by overweight and obesity.”
In people with diabetes, the age-adjusted prevalence of elevated hs-cTnT ran higher in those with longer duration diabetes, and in those with less well-controlled diabetes based on a higher level of A1c. Neither of these factors showed any significant relationship with measured levels of NT-proBNP.
Further analysis linked the NHANES findings during 1999-2004 with U.S. national death records through the end of 2019. This showed that elevated levels of both hs-cTnT and NT-proBNP significantly linked with subsequently higher rates of all-cause mortality among people with diabetes. Elevated hs-cTnT linked with a 77% increased mortality and NT-proBNP linked with a 78% increased rate, compared with people with diabetes and no elevations in these markers, after adjustment for demographic variables and CVD risk factors.
However, for the outcome of cardiovascular death, elevated hs-cTnT linked with a nonsignificant 54% relative increase, while elevated NT-proBNP linked with a significant 2.46-fold relative increase.
The study “adds new data on biomarkers that are not routinely measured in asymptomatic people with or without diabetes” and the relationships of these markers to CVD mortality and all-cause mortality, Dr. Eckel concluded.
The study received no commercial funding, but used reagents donated by Abbott Laboratories, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin and Dr. Eckel had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
COVID boosters effective, but not for long
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Overweight in heterozygous FH tied to even higher CAD risk
MANNHEIM, GERMANY – – rates that appear to have a substantial impact on these patients’ already increased risk of coronary artery disease, a registry analysis suggests.
Data on almost 36,000 individuals with FH were collated from an international registry, revealing that 55% of adults and 25% of children and adolescents with the homozygous form of FH had overweight or obesity. The figures for heterozygous FH were 52% and 27%, respectively.
Crucially, overweight or obesity was associated with substantially increased rates of coronary artery disease, particularly in persons with heterozygous FH, among whom adults with obesity faced a twofold increased risk, rising to more than sixfold in children and adolescents.
Moreover, “obesity is associated with a worse lipid profile, even from childhood, regardless of whether a patient is on medication,” said study presenter Amany Elshorbagy, DPhil, Cardiovascular Epidemiologist, department of primary care and public health, Imperial College London.
She added that, with the increased risk of coronary artery disease associated with heterozygous FH, the results showed that “together with lipid-lowering medication, weight management is needed.”
The research was presented at the annual meeting of the European Atherosclerosis Society.
Tended to be thin
Alberico L. Catapano, MD, PhD, director of cardiovascular research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, and past president of the EAS, said in an interview that, historically, few FH patients were overweight or obese; rather, they tended to be thin.
However, there is now “a trend for people with FH to show more diabetes and obesity,” with the “bottom line” being that, as they are already at increased risk of coronary artery disease, it pushes their risk up even further.
In other words, if a risk factor such as obesity is added “on top of the strongest risk factor, that is LDL cholesterol, it is not one plus one makes two, it is one plus one makes three,” he said.
As such, Dr. Catapano believes that the study is “very interesting,” because it further underlines the importance of weight management for individuals with increased LDL cholesterol, “especially when you have genetic forms, like FH.”
Dr. Catapano’s comments were echoed by session co-chair Ulrike Schatz, MD, leader of the lipidology specialty department at the University Hospital Carl Gustav Carus, Technical University of Dresden (Germany).
Indeed, she told Dr. Elshorbagy before her presentation that she finds “a lot of my FH patients have a tendency towards anorexia.”
In an interview, Dr. Elshorbagy said that that reaction was typical of “most of the clinicians” she had spoken to. Upon seeing her data, especially for homozygous FH patients, they say, “They are on the lean side.”
Consequently, the research team went into the study “with the expectation that they might have a lower prevalence of obesity and overweight than the general population,” but “that’s not what we’re seeing.”
Dr. Elshorbagy noted that it would be helpful to have longitudinal data to determine whether, 50 years ago, patients with HF “were leaner, along with the rest of the population.”
The registry data are cross-sectional, and the team is now reaching out to the respective national lead investigators to submit follow-up data on their patients, with the aim of looking at changes in body weight and the impact on outcomes over time.
Another key question for the researchers is in regard to fat distribution, as body mass index “is not the best predictor of heart disease,” Dr. Elshorbagy said, but is rather central obesity.
Although they have also asked investigators to share waist circumference data, she conceded that it is a measurement that “is a lot harder to standardize across centers and countries; it’s not like putting patients on a scale.”
Overall, Dr. Elshorbagy believes that her findings indicate that clinicians should take a broader, more holistic approach toward their patients – in other words, an approach in which lipid lowering medication is “key but is just one of several things we need to do to make sure the coronary event rate goes down.”
More with than without
Dr. Elshorbagy began her presentation by highlighting that the prevalence of overweight and obesity ranges from 50% to 70% and that it is “the only health condition where you’ve got more people worldwide with the condition than without.”
Crucially, overweight increases the risk of coronary artery disease by approximately 20%. Among patients with obesity, the risk rises to 50%.
Given that FH patients “already have a very high risk of cardiovascular disease from their high cholesterol levels,” the team set out to determine rates of obesity and overweight in this population and their impact on coronary artery disease risk.
They used cross-sectional data from the EAS FH Studies Collaboration Global Registry, which involves 29,262 adults aged greater than or equal to 18 years and 6,275 children and adolescents aged 5 to 17 years with heterozygous FH, and 325 adults and 57 children with homozygous FH.
Dividing the adults into standard BMI categories, they found that 16% of heterozygous and 23% of homozygous FH patients had obesity, while 52% and 55%, respectively, had overweight or obesity.
For children, the team used World Health Organization z score cutoffs, which indicated that 9% of patients with heterozygous FH and 7% of patients with homozygous FH had obesity. Rates of overweight or obesity were 27% and 25%, respectively.
Among patients with heterozygous FH, rates of overweight or obesity among adults were 50% in high-income countries and 63% in other countries; among children, the rates were and 27% and 29%, respectively.
Stratified by region, the team found that the lowest rate of overweight or obesity among adult patients with heterozygous FH was in Eastern Asia, at 27%, while the highest was in Northern Africa/Western Asia (the Middle East), at 82%.
In North America, 56% of adult patients had overweight or obesity. The prevalence of coronary artery disease rose with increasing BMI.
Among adult patients with heterozygous FH, 11.3% of those with normal weight had coronary artery disease; the percentage rose to 22.9% among those with overweight, and 30.9% among those with obesity. Among children, the corresponding figures were 0.1%, 0.2%, and 0.7%.
Putting adults and children with homozygous FH together, the researchers found that 29.0% of patients with normal weight had coronary artery disease, compared with 31.3% of those with overweight and 49.3% of those with obesity.
Moreover, the results showed that levels of LDL and remnant cholesterol were significantly associated with BMI in adults and children with heterozygous FH, even after adjusting for age, sex, and lipid-lowering medication (P < .001 for all).
Multivariate analysis that took into account age, sex, lipid-lowering medication, and LDL cholesterol revealed that having obesity, compared with not having obesity, was associated with a substantial increase in the risk of coronary artery disease among patients with heterozygous FH.
Among adults with the condition, the odds ratio was 2.16 (95% confidence interval, 1.97-2.36), while among children and adolescents, it was 6.87 (95% CI, 1.55-30.46).
The results remained similar after further adjustment for the presence of diabetes and when considering peripheral artery disease and stroke.
No funding for the study was declared. Dr. Elshorbagy has relationships with Amgen, Daiichi Sankyo, and Regeneron.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY – – rates that appear to have a substantial impact on these patients’ already increased risk of coronary artery disease, a registry analysis suggests.
Data on almost 36,000 individuals with FH were collated from an international registry, revealing that 55% of adults and 25% of children and adolescents with the homozygous form of FH had overweight or obesity. The figures for heterozygous FH were 52% and 27%, respectively.
Crucially, overweight or obesity was associated with substantially increased rates of coronary artery disease, particularly in persons with heterozygous FH, among whom adults with obesity faced a twofold increased risk, rising to more than sixfold in children and adolescents.
Moreover, “obesity is associated with a worse lipid profile, even from childhood, regardless of whether a patient is on medication,” said study presenter Amany Elshorbagy, DPhil, Cardiovascular Epidemiologist, department of primary care and public health, Imperial College London.
She added that, with the increased risk of coronary artery disease associated with heterozygous FH, the results showed that “together with lipid-lowering medication, weight management is needed.”
The research was presented at the annual meeting of the European Atherosclerosis Society.
Tended to be thin
Alberico L. Catapano, MD, PhD, director of cardiovascular research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, and past president of the EAS, said in an interview that, historically, few FH patients were overweight or obese; rather, they tended to be thin.
However, there is now “a trend for people with FH to show more diabetes and obesity,” with the “bottom line” being that, as they are already at increased risk of coronary artery disease, it pushes their risk up even further.
In other words, if a risk factor such as obesity is added “on top of the strongest risk factor, that is LDL cholesterol, it is not one plus one makes two, it is one plus one makes three,” he said.
As such, Dr. Catapano believes that the study is “very interesting,” because it further underlines the importance of weight management for individuals with increased LDL cholesterol, “especially when you have genetic forms, like FH.”
Dr. Catapano’s comments were echoed by session co-chair Ulrike Schatz, MD, leader of the lipidology specialty department at the University Hospital Carl Gustav Carus, Technical University of Dresden (Germany).
Indeed, she told Dr. Elshorbagy before her presentation that she finds “a lot of my FH patients have a tendency towards anorexia.”
In an interview, Dr. Elshorbagy said that that reaction was typical of “most of the clinicians” she had spoken to. Upon seeing her data, especially for homozygous FH patients, they say, “They are on the lean side.”
Consequently, the research team went into the study “with the expectation that they might have a lower prevalence of obesity and overweight than the general population,” but “that’s not what we’re seeing.”
Dr. Elshorbagy noted that it would be helpful to have longitudinal data to determine whether, 50 years ago, patients with HF “were leaner, along with the rest of the population.”
The registry data are cross-sectional, and the team is now reaching out to the respective national lead investigators to submit follow-up data on their patients, with the aim of looking at changes in body weight and the impact on outcomes over time.
Another key question for the researchers is in regard to fat distribution, as body mass index “is not the best predictor of heart disease,” Dr. Elshorbagy said, but is rather central obesity.
Although they have also asked investigators to share waist circumference data, she conceded that it is a measurement that “is a lot harder to standardize across centers and countries; it’s not like putting patients on a scale.”
Overall, Dr. Elshorbagy believes that her findings indicate that clinicians should take a broader, more holistic approach toward their patients – in other words, an approach in which lipid lowering medication is “key but is just one of several things we need to do to make sure the coronary event rate goes down.”
More with than without
Dr. Elshorbagy began her presentation by highlighting that the prevalence of overweight and obesity ranges from 50% to 70% and that it is “the only health condition where you’ve got more people worldwide with the condition than without.”
Crucially, overweight increases the risk of coronary artery disease by approximately 20%. Among patients with obesity, the risk rises to 50%.
Given that FH patients “already have a very high risk of cardiovascular disease from their high cholesterol levels,” the team set out to determine rates of obesity and overweight in this population and their impact on coronary artery disease risk.
They used cross-sectional data from the EAS FH Studies Collaboration Global Registry, which involves 29,262 adults aged greater than or equal to 18 years and 6,275 children and adolescents aged 5 to 17 years with heterozygous FH, and 325 adults and 57 children with homozygous FH.
Dividing the adults into standard BMI categories, they found that 16% of heterozygous and 23% of homozygous FH patients had obesity, while 52% and 55%, respectively, had overweight or obesity.
For children, the team used World Health Organization z score cutoffs, which indicated that 9% of patients with heterozygous FH and 7% of patients with homozygous FH had obesity. Rates of overweight or obesity were 27% and 25%, respectively.
Among patients with heterozygous FH, rates of overweight or obesity among adults were 50% in high-income countries and 63% in other countries; among children, the rates were and 27% and 29%, respectively.
Stratified by region, the team found that the lowest rate of overweight or obesity among adult patients with heterozygous FH was in Eastern Asia, at 27%, while the highest was in Northern Africa/Western Asia (the Middle East), at 82%.
In North America, 56% of adult patients had overweight or obesity. The prevalence of coronary artery disease rose with increasing BMI.
Among adult patients with heterozygous FH, 11.3% of those with normal weight had coronary artery disease; the percentage rose to 22.9% among those with overweight, and 30.9% among those with obesity. Among children, the corresponding figures were 0.1%, 0.2%, and 0.7%.
Putting adults and children with homozygous FH together, the researchers found that 29.0% of patients with normal weight had coronary artery disease, compared with 31.3% of those with overweight and 49.3% of those with obesity.
Moreover, the results showed that levels of LDL and remnant cholesterol were significantly associated with BMI in adults and children with heterozygous FH, even after adjusting for age, sex, and lipid-lowering medication (P < .001 for all).
Multivariate analysis that took into account age, sex, lipid-lowering medication, and LDL cholesterol revealed that having obesity, compared with not having obesity, was associated with a substantial increase in the risk of coronary artery disease among patients with heterozygous FH.
Among adults with the condition, the odds ratio was 2.16 (95% confidence interval, 1.97-2.36), while among children and adolescents, it was 6.87 (95% CI, 1.55-30.46).
The results remained similar after further adjustment for the presence of diabetes and when considering peripheral artery disease and stroke.
No funding for the study was declared. Dr. Elshorbagy has relationships with Amgen, Daiichi Sankyo, and Regeneron.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY – – rates that appear to have a substantial impact on these patients’ already increased risk of coronary artery disease, a registry analysis suggests.
Data on almost 36,000 individuals with FH were collated from an international registry, revealing that 55% of adults and 25% of children and adolescents with the homozygous form of FH had overweight or obesity. The figures for heterozygous FH were 52% and 27%, respectively.
Crucially, overweight or obesity was associated with substantially increased rates of coronary artery disease, particularly in persons with heterozygous FH, among whom adults with obesity faced a twofold increased risk, rising to more than sixfold in children and adolescents.
Moreover, “obesity is associated with a worse lipid profile, even from childhood, regardless of whether a patient is on medication,” said study presenter Amany Elshorbagy, DPhil, Cardiovascular Epidemiologist, department of primary care and public health, Imperial College London.
She added that, with the increased risk of coronary artery disease associated with heterozygous FH, the results showed that “together with lipid-lowering medication, weight management is needed.”
The research was presented at the annual meeting of the European Atherosclerosis Society.
Tended to be thin
Alberico L. Catapano, MD, PhD, director of cardiovascular research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, and past president of the EAS, said in an interview that, historically, few FH patients were overweight or obese; rather, they tended to be thin.
However, there is now “a trend for people with FH to show more diabetes and obesity,” with the “bottom line” being that, as they are already at increased risk of coronary artery disease, it pushes their risk up even further.
In other words, if a risk factor such as obesity is added “on top of the strongest risk factor, that is LDL cholesterol, it is not one plus one makes two, it is one plus one makes three,” he said.
As such, Dr. Catapano believes that the study is “very interesting,” because it further underlines the importance of weight management for individuals with increased LDL cholesterol, “especially when you have genetic forms, like FH.”
Dr. Catapano’s comments were echoed by session co-chair Ulrike Schatz, MD, leader of the lipidology specialty department at the University Hospital Carl Gustav Carus, Technical University of Dresden (Germany).
Indeed, she told Dr. Elshorbagy before her presentation that she finds “a lot of my FH patients have a tendency towards anorexia.”
In an interview, Dr. Elshorbagy said that that reaction was typical of “most of the clinicians” she had spoken to. Upon seeing her data, especially for homozygous FH patients, they say, “They are on the lean side.”
Consequently, the research team went into the study “with the expectation that they might have a lower prevalence of obesity and overweight than the general population,” but “that’s not what we’re seeing.”
Dr. Elshorbagy noted that it would be helpful to have longitudinal data to determine whether, 50 years ago, patients with HF “were leaner, along with the rest of the population.”
The registry data are cross-sectional, and the team is now reaching out to the respective national lead investigators to submit follow-up data on their patients, with the aim of looking at changes in body weight and the impact on outcomes over time.
Another key question for the researchers is in regard to fat distribution, as body mass index “is not the best predictor of heart disease,” Dr. Elshorbagy said, but is rather central obesity.
Although they have also asked investigators to share waist circumference data, she conceded that it is a measurement that “is a lot harder to standardize across centers and countries; it’s not like putting patients on a scale.”
Overall, Dr. Elshorbagy believes that her findings indicate that clinicians should take a broader, more holistic approach toward their patients – in other words, an approach in which lipid lowering medication is “key but is just one of several things we need to do to make sure the coronary event rate goes down.”
More with than without
Dr. Elshorbagy began her presentation by highlighting that the prevalence of overweight and obesity ranges from 50% to 70% and that it is “the only health condition where you’ve got more people worldwide with the condition than without.”
Crucially, overweight increases the risk of coronary artery disease by approximately 20%. Among patients with obesity, the risk rises to 50%.
Given that FH patients “already have a very high risk of cardiovascular disease from their high cholesterol levels,” the team set out to determine rates of obesity and overweight in this population and their impact on coronary artery disease risk.
They used cross-sectional data from the EAS FH Studies Collaboration Global Registry, which involves 29,262 adults aged greater than or equal to 18 years and 6,275 children and adolescents aged 5 to 17 years with heterozygous FH, and 325 adults and 57 children with homozygous FH.
Dividing the adults into standard BMI categories, they found that 16% of heterozygous and 23% of homozygous FH patients had obesity, while 52% and 55%, respectively, had overweight or obesity.
For children, the team used World Health Organization z score cutoffs, which indicated that 9% of patients with heterozygous FH and 7% of patients with homozygous FH had obesity. Rates of overweight or obesity were 27% and 25%, respectively.
Among patients with heterozygous FH, rates of overweight or obesity among adults were 50% in high-income countries and 63% in other countries; among children, the rates were and 27% and 29%, respectively.
Stratified by region, the team found that the lowest rate of overweight or obesity among adult patients with heterozygous FH was in Eastern Asia, at 27%, while the highest was in Northern Africa/Western Asia (the Middle East), at 82%.
In North America, 56% of adult patients had overweight or obesity. The prevalence of coronary artery disease rose with increasing BMI.
Among adult patients with heterozygous FH, 11.3% of those with normal weight had coronary artery disease; the percentage rose to 22.9% among those with overweight, and 30.9% among those with obesity. Among children, the corresponding figures were 0.1%, 0.2%, and 0.7%.
Putting adults and children with homozygous FH together, the researchers found that 29.0% of patients with normal weight had coronary artery disease, compared with 31.3% of those with overweight and 49.3% of those with obesity.
Moreover, the results showed that levels of LDL and remnant cholesterol were significantly associated with BMI in adults and children with heterozygous FH, even after adjusting for age, sex, and lipid-lowering medication (P < .001 for all).
Multivariate analysis that took into account age, sex, lipid-lowering medication, and LDL cholesterol revealed that having obesity, compared with not having obesity, was associated with a substantial increase in the risk of coronary artery disease among patients with heterozygous FH.
Among adults with the condition, the odds ratio was 2.16 (95% confidence interval, 1.97-2.36), while among children and adolescents, it was 6.87 (95% CI, 1.55-30.46).
The results remained similar after further adjustment for the presence of diabetes and when considering peripheral artery disease and stroke.
No funding for the study was declared. Dr. Elshorbagy has relationships with Amgen, Daiichi Sankyo, and Regeneron.
A version of this article first appeared on Medscape.com.
AT EAS 2023
Itchy scaling rash
A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013

A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013