User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
GVHD raises vitiligo risk in transplant recipients
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
FROM JAMA DERMATOLOGY
AI-Aided Stethoscope Beats PCP in Detecting Valvular HD
, a new study shows.
The results suggest collecting relevant sounds through a stethoscope (auscultation) using AI-powered technology is an important primary care tool to detect VHD, study author Moshe A. Rancier, MD, medical director, Massachusetts General Brigham Community Physicians, Lawrence, Massachusetts, said in an interview.
“Incorporating this AI-assisted device into the primary care exam will help identify patients at risk for VHD earlier and eventually decrease costs in our healthcare system,” he said, because timely detection could avoid emergency room visits and surgeries.
The findings were presented at the annual scientific sessions of the American Heart Association.
VHD Common
Clinically significant VHD, indicating structural damage to heart valves, affects 1 in 10 adults older than 65 years. Patients may be asymptomatic or present to their PCP with an unspecific symptom like fatigue or malaise.
If VHD is undiagnosed and left untreated, patients could develop more severe symptoms, even be at risk for death, and their quality of life is significantly affected, said Dr. Rancier.
Cardiac auscultation, the current point-of-care clinical standard, has relatively low sensitivity for detecting VHD, leaving most patients undiagnosed.
The deep learning–based AI tool uses sound data to detect cardiac murmurs associated with clinically significant VHD. The device used in the study (Eko; Eko Health) is approved by the US Food and Drug Administration and is on the market.
The tool identifies background sounds that might affect the evaluation. “If there’s any noise or breath sounds, it tells me this is not a good heart sound, and asks me to record again,” said Dr. Rancier.
A doctor using the AI-assisted stethoscope carries out the auscultation exam with the sound data captured by a smartphone or tablet and sent to the AI server. “I get an answer in a second as to if there’s a murmur or not,” said Dr. Rancier.
Not only that, but the tool can determine if it’s a systolic or diastolic murmur, he added.
Real-World Population
The study enrolled a “real-world” population of 368 patients, median age 70 years, 61% female, 70% White, and 18% Hispanic without a prior VHD diagnosis or history of murmur, from three primary care clinics in Queens, New York, and Lawrence and Haverhill, Massachusetts.
About 79% of the cohort had hypertension, 68% had dyslipidemia, and 38% had diabetes, “which aligns with the population in the US,” said Dr. Rancier.
Each study participant had a regular exam carried out by Dr. Rancier using a traditional stethoscope to detect murmurs and an exam by a technician with a digital stethoscope that collected phonocardiogram (PCG) data for analysis by AI.
In addition, each patient received an echocardiogram 1-2 weeks later to confirm whether clinically significant VHD was present. An expert panel of cardiologists also reviewed the patient’s PCG recordings to confirm the presence of audible murmurs.
Dr. Rancier and the expert panel were blinded to AI and echocardiogram results.
Researchers calculated performance metrics for both PCP auscultation and the AI in detecting audible VHD.
The study showed that AI improved sensitivity to detect audible VHD by over twofold compared with PCP auscultation (94.1% vs 41.2%), with limited impact on specificity (84.5% vs 95.5%).
Dr. Rancier stressed the importance of sensitivity because clinicians tend to under-detect murmurs. “You don’t want to miss those patients because the consequences of undiagnosed VHD are dire.”
The AI tool identified 22 patients with moderate or greater VHD who were previously undiagnosed, whereas PCPs identified eight previously undiagnosed patients with VHD.
Dr. Rancier sees this tool being used beyond primary care, perhaps by emergency room personnel.
The authors plan to follow study participants and assess outcomes at for 6-12 months. They also aim to include more patients to increase the study’s power.
Expanding the Technology
They are also interested to see whether the technology can determine which valve is affected; for example, whether the issue is aortic stenosis or mitral regurgitation.
A limitation of the study was its small sample size.
Commenting on the findings, Dan Roden, MD, professor of medicine, pharmacology, and biomedical informatics, senior vice president for personalized medicine at Vanderbilt University Medical Center, Nashville, Tennessee, and chair of the American Heart Association Council on Genomic and Precision Medicine, noted that it demonstrated the AI-based stethoscope “did extraordinarily well” in predicting VHD.
“I see this as an emerging technology — using an AI-enabled stethoscope and perhaps combining it with other imaging modalities, like an AI-enabled echocardiogram built into your stethoscope,” said Dr. Roden.
“Use of these new tools to detect the presence of valvular disease, as well as the extent of valvular disease and the extent of other kinds of heart disease, will likely help to transform CVD care.”
The study was funded by Eko Health Inc. Dr. Rancier and Dr. Roden have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
, a new study shows.
The results suggest collecting relevant sounds through a stethoscope (auscultation) using AI-powered technology is an important primary care tool to detect VHD, study author Moshe A. Rancier, MD, medical director, Massachusetts General Brigham Community Physicians, Lawrence, Massachusetts, said in an interview.
“Incorporating this AI-assisted device into the primary care exam will help identify patients at risk for VHD earlier and eventually decrease costs in our healthcare system,” he said, because timely detection could avoid emergency room visits and surgeries.
The findings were presented at the annual scientific sessions of the American Heart Association.
VHD Common
Clinically significant VHD, indicating structural damage to heart valves, affects 1 in 10 adults older than 65 years. Patients may be asymptomatic or present to their PCP with an unspecific symptom like fatigue or malaise.
If VHD is undiagnosed and left untreated, patients could develop more severe symptoms, even be at risk for death, and their quality of life is significantly affected, said Dr. Rancier.
Cardiac auscultation, the current point-of-care clinical standard, has relatively low sensitivity for detecting VHD, leaving most patients undiagnosed.
The deep learning–based AI tool uses sound data to detect cardiac murmurs associated with clinically significant VHD. The device used in the study (Eko; Eko Health) is approved by the US Food and Drug Administration and is on the market.
The tool identifies background sounds that might affect the evaluation. “If there’s any noise or breath sounds, it tells me this is not a good heart sound, and asks me to record again,” said Dr. Rancier.
A doctor using the AI-assisted stethoscope carries out the auscultation exam with the sound data captured by a smartphone or tablet and sent to the AI server. “I get an answer in a second as to if there’s a murmur or not,” said Dr. Rancier.
Not only that, but the tool can determine if it’s a systolic or diastolic murmur, he added.
Real-World Population
The study enrolled a “real-world” population of 368 patients, median age 70 years, 61% female, 70% White, and 18% Hispanic without a prior VHD diagnosis or history of murmur, from three primary care clinics in Queens, New York, and Lawrence and Haverhill, Massachusetts.
About 79% of the cohort had hypertension, 68% had dyslipidemia, and 38% had diabetes, “which aligns with the population in the US,” said Dr. Rancier.
Each study participant had a regular exam carried out by Dr. Rancier using a traditional stethoscope to detect murmurs and an exam by a technician with a digital stethoscope that collected phonocardiogram (PCG) data for analysis by AI.
In addition, each patient received an echocardiogram 1-2 weeks later to confirm whether clinically significant VHD was present. An expert panel of cardiologists also reviewed the patient’s PCG recordings to confirm the presence of audible murmurs.
Dr. Rancier and the expert panel were blinded to AI and echocardiogram results.
Researchers calculated performance metrics for both PCP auscultation and the AI in detecting audible VHD.
The study showed that AI improved sensitivity to detect audible VHD by over twofold compared with PCP auscultation (94.1% vs 41.2%), with limited impact on specificity (84.5% vs 95.5%).
Dr. Rancier stressed the importance of sensitivity because clinicians tend to under-detect murmurs. “You don’t want to miss those patients because the consequences of undiagnosed VHD are dire.”
The AI tool identified 22 patients with moderate or greater VHD who were previously undiagnosed, whereas PCPs identified eight previously undiagnosed patients with VHD.
Dr. Rancier sees this tool being used beyond primary care, perhaps by emergency room personnel.
The authors plan to follow study participants and assess outcomes at for 6-12 months. They also aim to include more patients to increase the study’s power.
Expanding the Technology
They are also interested to see whether the technology can determine which valve is affected; for example, whether the issue is aortic stenosis or mitral regurgitation.
A limitation of the study was its small sample size.
Commenting on the findings, Dan Roden, MD, professor of medicine, pharmacology, and biomedical informatics, senior vice president for personalized medicine at Vanderbilt University Medical Center, Nashville, Tennessee, and chair of the American Heart Association Council on Genomic and Precision Medicine, noted that it demonstrated the AI-based stethoscope “did extraordinarily well” in predicting VHD.
“I see this as an emerging technology — using an AI-enabled stethoscope and perhaps combining it with other imaging modalities, like an AI-enabled echocardiogram built into your stethoscope,” said Dr. Roden.
“Use of these new tools to detect the presence of valvular disease, as well as the extent of valvular disease and the extent of other kinds of heart disease, will likely help to transform CVD care.”
The study was funded by Eko Health Inc. Dr. Rancier and Dr. Roden have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
, a new study shows.
The results suggest collecting relevant sounds through a stethoscope (auscultation) using AI-powered technology is an important primary care tool to detect VHD, study author Moshe A. Rancier, MD, medical director, Massachusetts General Brigham Community Physicians, Lawrence, Massachusetts, said in an interview.
“Incorporating this AI-assisted device into the primary care exam will help identify patients at risk for VHD earlier and eventually decrease costs in our healthcare system,” he said, because timely detection could avoid emergency room visits and surgeries.
The findings were presented at the annual scientific sessions of the American Heart Association.
VHD Common
Clinically significant VHD, indicating structural damage to heart valves, affects 1 in 10 adults older than 65 years. Patients may be asymptomatic or present to their PCP with an unspecific symptom like fatigue or malaise.
If VHD is undiagnosed and left untreated, patients could develop more severe symptoms, even be at risk for death, and their quality of life is significantly affected, said Dr. Rancier.
Cardiac auscultation, the current point-of-care clinical standard, has relatively low sensitivity for detecting VHD, leaving most patients undiagnosed.
The deep learning–based AI tool uses sound data to detect cardiac murmurs associated with clinically significant VHD. The device used in the study (Eko; Eko Health) is approved by the US Food and Drug Administration and is on the market.
The tool identifies background sounds that might affect the evaluation. “If there’s any noise or breath sounds, it tells me this is not a good heart sound, and asks me to record again,” said Dr. Rancier.
A doctor using the AI-assisted stethoscope carries out the auscultation exam with the sound data captured by a smartphone or tablet and sent to the AI server. “I get an answer in a second as to if there’s a murmur or not,” said Dr. Rancier.
Not only that, but the tool can determine if it’s a systolic or diastolic murmur, he added.
Real-World Population
The study enrolled a “real-world” population of 368 patients, median age 70 years, 61% female, 70% White, and 18% Hispanic without a prior VHD diagnosis or history of murmur, from three primary care clinics in Queens, New York, and Lawrence and Haverhill, Massachusetts.
About 79% of the cohort had hypertension, 68% had dyslipidemia, and 38% had diabetes, “which aligns with the population in the US,” said Dr. Rancier.
Each study participant had a regular exam carried out by Dr. Rancier using a traditional stethoscope to detect murmurs and an exam by a technician with a digital stethoscope that collected phonocardiogram (PCG) data for analysis by AI.
In addition, each patient received an echocardiogram 1-2 weeks later to confirm whether clinically significant VHD was present. An expert panel of cardiologists also reviewed the patient’s PCG recordings to confirm the presence of audible murmurs.
Dr. Rancier and the expert panel were blinded to AI and echocardiogram results.
Researchers calculated performance metrics for both PCP auscultation and the AI in detecting audible VHD.
The study showed that AI improved sensitivity to detect audible VHD by over twofold compared with PCP auscultation (94.1% vs 41.2%), with limited impact on specificity (84.5% vs 95.5%).
Dr. Rancier stressed the importance of sensitivity because clinicians tend to under-detect murmurs. “You don’t want to miss those patients because the consequences of undiagnosed VHD are dire.”
The AI tool identified 22 patients with moderate or greater VHD who were previously undiagnosed, whereas PCPs identified eight previously undiagnosed patients with VHD.
Dr. Rancier sees this tool being used beyond primary care, perhaps by emergency room personnel.
The authors plan to follow study participants and assess outcomes at for 6-12 months. They also aim to include more patients to increase the study’s power.
Expanding the Technology
They are also interested to see whether the technology can determine which valve is affected; for example, whether the issue is aortic stenosis or mitral regurgitation.
A limitation of the study was its small sample size.
Commenting on the findings, Dan Roden, MD, professor of medicine, pharmacology, and biomedical informatics, senior vice president for personalized medicine at Vanderbilt University Medical Center, Nashville, Tennessee, and chair of the American Heart Association Council on Genomic and Precision Medicine, noted that it demonstrated the AI-based stethoscope “did extraordinarily well” in predicting VHD.
“I see this as an emerging technology — using an AI-enabled stethoscope and perhaps combining it with other imaging modalities, like an AI-enabled echocardiogram built into your stethoscope,” said Dr. Roden.
“Use of these new tools to detect the presence of valvular disease, as well as the extent of valvular disease and the extent of other kinds of heart disease, will likely help to transform CVD care.”
The study was funded by Eko Health Inc. Dr. Rancier and Dr. Roden have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM AHA 2023
Report: CKD Severity Linked to Thinning of Retina, Choroid Layers
Changes in tissue thickness in the back of the eye can correlate with worsening or improvement of renal problems and could help predict who will have worsening of kidney function, a new analysis report finds.
The research, published in the journal Nature Communications, is the first to show an association between chronic kidney disease (CKD) and the thickness of the retinal and choroidal layers in the back of the eye as measured by optical coherence tomography (OCT), a noninvasive imaging technology commonly used to evaluate eye diseases such as age-related macular degeneration (AMD), diabetic eye disease, and retinal detachments.
“These are common scans that people get at the opticians and now in many hospitals,” said Neeraj Dhaun, MD, PhD, a professor of nephrology at the University of Edinburgh, Scotland. (Opticians in the United Kingdom are the equivalent of optometrists in North America.)
CKD Severity Equals Thinner Retinas
“We scanned the back of eye of healthy people as well as patients with various types and degrees of kidney disease, and we found that two layers in the back of eye, the retina and the choroid, were thinner in patients with kidney disease compared to people who are healthy, and that the extent of this thinning predicts whether kidney function would decline going forward over a period of 2 or 3 years,” Dr. Dhaun, the corresponding author of the new paper, said.
The publication is a report of four different studies. The first study measured OCT metrics in 112 patients with CKD, 92 patients with a functional kidney transplant, and 86 control volunteers. The researchers found the retina was 5% thinner in patients with CKD than in healthy controls. They also found that patients with CKD had reduced macular volume: 8.44 ± .44 mm3 vs 8.73 ± .36 mm3 (P < .001). The choroid was also found to be thinner at each of three macular locations measured in patients with CKD vs control volunteers. At baseline, CKD and transplant patients had significantly lower estimated glomerular filtration rate (eGFR) at 55 ± 27 and 55 ± 24 mL/min/1.73 m2 compared with control volunteers at 97 ± 14 mL/min/1.73 m2.
The second study reported on OCT measurements and kidney histologic injury in 50 patients who had a kidney biopsy within 30 days of their OCT. It found that choroidal thinning at all three macular locations was independently associated with more extensive kidney scarring.
The third study focused on 25 patients with kidney failure who had a kidney transplant. Their eGFR improved from 8 ± 3 to 58 ± 21 mL/min/1.73 m2 in the first week after the transplant. The choroid in these patients thickened about 5% at 1 week and by about 10% at 1 month posttransplant. OCT of 22 kidney donors showed thickening of the choroid a week after nephrectomy before a tendency to thinning over the next year.
The fourth study found that for patients with stable CKD, every 1 mm3 decrease in macular volume correlated to an increased odds of a decline in eGFR by more than 10% at 1 year (2.48; 95% CI, 1.26-5.08; P = .01) and by more than 20% at 2 years (3.75; 95% CI, 1.26-5.08; P = .004).
Exploring the Kidney-Eye Connection
The potential explanation for the correlation between retinal and choroidal thickness and kidney function is unclear, Dr. Dhaun said.
“We don’t know the exact mechanisms, and these are difficult to define from studies in patients, which is why we are doing more work in animal models of kidney disease to see if we can establish the pathways that lead to the changes in the eye,” he said.
“However,” Dr. Dhaun added, “what we do know is that kidney disease affects the whole body. For example, kidney disease can lead to high blood pressure and heart disease, as well as diseases in the brain, and it is these effects of kidney disease on the body as whole that we are probably picking up in the back of the eye.”
OCT has the potential to make the monitoring of patients with CKD and kidney transplant more convenient than it is now, Dr. Dhaun said. “These scanners are available in the community, and what would be ideal at some point in the future is to be able to do a patient’s kidney health check in the community potentially incorporating OCT scanning alongside blood-pressure monitoring and other healthcare measures,” he said.
“The findings provide an exciting example of how noninvasive retinal imaging using OCT can provide quantitative biomarkers of systemic disease,” Amir Kashani, MD, PhD, the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University in Baltimore, told this news organization. “It is striking that their findings demonstrate some potential of reversible changes in choroidal perfusion after kidney transplantation.”
The finding that choroidal thickness changes in CKD are at least partly reversible with kidney transplantation is a revelation, Dr. Kashani said, and may point to a greater role for ophthalmologists in managing systemic disease.
“Ophthalmologists can and should use their unique experience and understanding of the eye to help monitor and manage systemic conditions in collaboration with our medicine colleagues,” he said. “There are many systemic diseases that can impact the eye and ophthalmologist are uniquely positioned to help interpret those findings.”
Dr. Kashani noted that a particular strength of the report was the comparison of choroidal measurements in patients who had kidney transplantation and those that had a nephrectomy. “The consistent direction of changes in these two groups suggests the study findings are real and meaningful,” he said.
The study was independently supported. Dr. Dhaun and co-authors report no relevant financial relationships. Dr. Kashani disclosed a financial relationship with Carl Zeiss Meditec.
A version of this article first appeared on Medscape.com.
Changes in tissue thickness in the back of the eye can correlate with worsening or improvement of renal problems and could help predict who will have worsening of kidney function, a new analysis report finds.
The research, published in the journal Nature Communications, is the first to show an association between chronic kidney disease (CKD) and the thickness of the retinal and choroidal layers in the back of the eye as measured by optical coherence tomography (OCT), a noninvasive imaging technology commonly used to evaluate eye diseases such as age-related macular degeneration (AMD), diabetic eye disease, and retinal detachments.
“These are common scans that people get at the opticians and now in many hospitals,” said Neeraj Dhaun, MD, PhD, a professor of nephrology at the University of Edinburgh, Scotland. (Opticians in the United Kingdom are the equivalent of optometrists in North America.)
CKD Severity Equals Thinner Retinas
“We scanned the back of eye of healthy people as well as patients with various types and degrees of kidney disease, and we found that two layers in the back of eye, the retina and the choroid, were thinner in patients with kidney disease compared to people who are healthy, and that the extent of this thinning predicts whether kidney function would decline going forward over a period of 2 or 3 years,” Dr. Dhaun, the corresponding author of the new paper, said.
The publication is a report of four different studies. The first study measured OCT metrics in 112 patients with CKD, 92 patients with a functional kidney transplant, and 86 control volunteers. The researchers found the retina was 5% thinner in patients with CKD than in healthy controls. They also found that patients with CKD had reduced macular volume: 8.44 ± .44 mm3 vs 8.73 ± .36 mm3 (P < .001). The choroid was also found to be thinner at each of three macular locations measured in patients with CKD vs control volunteers. At baseline, CKD and transplant patients had significantly lower estimated glomerular filtration rate (eGFR) at 55 ± 27 and 55 ± 24 mL/min/1.73 m2 compared with control volunteers at 97 ± 14 mL/min/1.73 m2.
The second study reported on OCT measurements and kidney histologic injury in 50 patients who had a kidney biopsy within 30 days of their OCT. It found that choroidal thinning at all three macular locations was independently associated with more extensive kidney scarring.
The third study focused on 25 patients with kidney failure who had a kidney transplant. Their eGFR improved from 8 ± 3 to 58 ± 21 mL/min/1.73 m2 in the first week after the transplant. The choroid in these patients thickened about 5% at 1 week and by about 10% at 1 month posttransplant. OCT of 22 kidney donors showed thickening of the choroid a week after nephrectomy before a tendency to thinning over the next year.
The fourth study found that for patients with stable CKD, every 1 mm3 decrease in macular volume correlated to an increased odds of a decline in eGFR by more than 10% at 1 year (2.48; 95% CI, 1.26-5.08; P = .01) and by more than 20% at 2 years (3.75; 95% CI, 1.26-5.08; P = .004).
Exploring the Kidney-Eye Connection
The potential explanation for the correlation between retinal and choroidal thickness and kidney function is unclear, Dr. Dhaun said.
“We don’t know the exact mechanisms, and these are difficult to define from studies in patients, which is why we are doing more work in animal models of kidney disease to see if we can establish the pathways that lead to the changes in the eye,” he said.
“However,” Dr. Dhaun added, “what we do know is that kidney disease affects the whole body. For example, kidney disease can lead to high blood pressure and heart disease, as well as diseases in the brain, and it is these effects of kidney disease on the body as whole that we are probably picking up in the back of the eye.”
OCT has the potential to make the monitoring of patients with CKD and kidney transplant more convenient than it is now, Dr. Dhaun said. “These scanners are available in the community, and what would be ideal at some point in the future is to be able to do a patient’s kidney health check in the community potentially incorporating OCT scanning alongside blood-pressure monitoring and other healthcare measures,” he said.
“The findings provide an exciting example of how noninvasive retinal imaging using OCT can provide quantitative biomarkers of systemic disease,” Amir Kashani, MD, PhD, the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University in Baltimore, told this news organization. “It is striking that their findings demonstrate some potential of reversible changes in choroidal perfusion after kidney transplantation.”
The finding that choroidal thickness changes in CKD are at least partly reversible with kidney transplantation is a revelation, Dr. Kashani said, and may point to a greater role for ophthalmologists in managing systemic disease.
“Ophthalmologists can and should use their unique experience and understanding of the eye to help monitor and manage systemic conditions in collaboration with our medicine colleagues,” he said. “There are many systemic diseases that can impact the eye and ophthalmologist are uniquely positioned to help interpret those findings.”
Dr. Kashani noted that a particular strength of the report was the comparison of choroidal measurements in patients who had kidney transplantation and those that had a nephrectomy. “The consistent direction of changes in these two groups suggests the study findings are real and meaningful,” he said.
The study was independently supported. Dr. Dhaun and co-authors report no relevant financial relationships. Dr. Kashani disclosed a financial relationship with Carl Zeiss Meditec.
A version of this article first appeared on Medscape.com.
Changes in tissue thickness in the back of the eye can correlate with worsening or improvement of renal problems and could help predict who will have worsening of kidney function, a new analysis report finds.
The research, published in the journal Nature Communications, is the first to show an association between chronic kidney disease (CKD) and the thickness of the retinal and choroidal layers in the back of the eye as measured by optical coherence tomography (OCT), a noninvasive imaging technology commonly used to evaluate eye diseases such as age-related macular degeneration (AMD), diabetic eye disease, and retinal detachments.
“These are common scans that people get at the opticians and now in many hospitals,” said Neeraj Dhaun, MD, PhD, a professor of nephrology at the University of Edinburgh, Scotland. (Opticians in the United Kingdom are the equivalent of optometrists in North America.)
CKD Severity Equals Thinner Retinas
“We scanned the back of eye of healthy people as well as patients with various types and degrees of kidney disease, and we found that two layers in the back of eye, the retina and the choroid, were thinner in patients with kidney disease compared to people who are healthy, and that the extent of this thinning predicts whether kidney function would decline going forward over a period of 2 or 3 years,” Dr. Dhaun, the corresponding author of the new paper, said.
The publication is a report of four different studies. The first study measured OCT metrics in 112 patients with CKD, 92 patients with a functional kidney transplant, and 86 control volunteers. The researchers found the retina was 5% thinner in patients with CKD than in healthy controls. They also found that patients with CKD had reduced macular volume: 8.44 ± .44 mm3 vs 8.73 ± .36 mm3 (P < .001). The choroid was also found to be thinner at each of three macular locations measured in patients with CKD vs control volunteers. At baseline, CKD and transplant patients had significantly lower estimated glomerular filtration rate (eGFR) at 55 ± 27 and 55 ± 24 mL/min/1.73 m2 compared with control volunteers at 97 ± 14 mL/min/1.73 m2.
The second study reported on OCT measurements and kidney histologic injury in 50 patients who had a kidney biopsy within 30 days of their OCT. It found that choroidal thinning at all three macular locations was independently associated with more extensive kidney scarring.
The third study focused on 25 patients with kidney failure who had a kidney transplant. Their eGFR improved from 8 ± 3 to 58 ± 21 mL/min/1.73 m2 in the first week after the transplant. The choroid in these patients thickened about 5% at 1 week and by about 10% at 1 month posttransplant. OCT of 22 kidney donors showed thickening of the choroid a week after nephrectomy before a tendency to thinning over the next year.
The fourth study found that for patients with stable CKD, every 1 mm3 decrease in macular volume correlated to an increased odds of a decline in eGFR by more than 10% at 1 year (2.48; 95% CI, 1.26-5.08; P = .01) and by more than 20% at 2 years (3.75; 95% CI, 1.26-5.08; P = .004).
Exploring the Kidney-Eye Connection
The potential explanation for the correlation between retinal and choroidal thickness and kidney function is unclear, Dr. Dhaun said.
“We don’t know the exact mechanisms, and these are difficult to define from studies in patients, which is why we are doing more work in animal models of kidney disease to see if we can establish the pathways that lead to the changes in the eye,” he said.
“However,” Dr. Dhaun added, “what we do know is that kidney disease affects the whole body. For example, kidney disease can lead to high blood pressure and heart disease, as well as diseases in the brain, and it is these effects of kidney disease on the body as whole that we are probably picking up in the back of the eye.”
OCT has the potential to make the monitoring of patients with CKD and kidney transplant more convenient than it is now, Dr. Dhaun said. “These scanners are available in the community, and what would be ideal at some point in the future is to be able to do a patient’s kidney health check in the community potentially incorporating OCT scanning alongside blood-pressure monitoring and other healthcare measures,” he said.
“The findings provide an exciting example of how noninvasive retinal imaging using OCT can provide quantitative biomarkers of systemic disease,” Amir Kashani, MD, PhD, the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University in Baltimore, told this news organization. “It is striking that their findings demonstrate some potential of reversible changes in choroidal perfusion after kidney transplantation.”
The finding that choroidal thickness changes in CKD are at least partly reversible with kidney transplantation is a revelation, Dr. Kashani said, and may point to a greater role for ophthalmologists in managing systemic disease.
“Ophthalmologists can and should use their unique experience and understanding of the eye to help monitor and manage systemic conditions in collaboration with our medicine colleagues,” he said. “There are many systemic diseases that can impact the eye and ophthalmologist are uniquely positioned to help interpret those findings.”
Dr. Kashani noted that a particular strength of the report was the comparison of choroidal measurements in patients who had kidney transplantation and those that had a nephrectomy. “The consistent direction of changes in these two groups suggests the study findings are real and meaningful,” he said.
The study was independently supported. Dr. Dhaun and co-authors report no relevant financial relationships. Dr. Kashani disclosed a financial relationship with Carl Zeiss Meditec.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Slow-to-moderate weight loss better than rapid with antiobesity drugs in OA
TOPLINE:
Individuals with overweight or obesity and knee or hip osteoarthritis (OA) who used antiobesity medications and achieved slow-to-moderate weight loss had a lower risk for all-cause mortality than did those with weight gain or stable weight in a population-based cohort study emulating a randomized controlled trial. Patients who rapidly lost weight had mortality similar to those with weight gain or stable weight.
METHODOLOGY:
- The researchers used the IQVIA Medical Research Database to identify overweight or obese individuals with knee or hip OA; they conducted a hypothetical trial comparing the effects of slow-to-moderate weight loss (defined as 2%-10% of body weight) and rapid weight loss (defined as 5% or more of body weight) within 1 year of starting antiobesity medications.
- The final analysis included patients with a mean age of 60.9 years who met the criteria for treatment adherence to orlistat (n = 3028), sibutramine (n = 2919), or rimonabant (n = 797).
- The primary outcome was all-cause mortality over a 5-year follow-up period; secondary outcomes included hypertension, type 2 diabetes, and venous thromboembolism.
TAKEAWAY:
- All-cause mortality at 5 years was 5.3% with weight gain or stable weight, 4.0% with slow to moderate weight loss, and 5.4% with rapid weight loss.
- Hazard ratios for all-cause mortality were 0.72 (95% CI, 0.56-0.92) for slow to moderate weight loss and 0.99 (95% CI, 0.67-1.44) for the rapid weight loss group.
- Weight loss was associated with the secondary outcomes of reduced hypertension, type 2 diabetes, and venous thromboembolism in a dose-dependent manner.
- A slightly increased risk for cardiovascular disease occurred in the rapid weight loss group, compared with the weight gain or stable group, but this difference was not significant.
IN PRACTICE:
“Our finding that gradual weight loss by antiobesity medications lowers all-cause mortality, if confirmed by future studies, could guide policy-making and improve the well-being of patients with overweight or obesity and knee or hip OA,” the researchers wrote.
SOURCE:
The lead author on the study was Jie Wei, MD, of Central South University, Changsha, China. The study was published online in Arthritis & Rheumatology.
LIMITATIONS:
Study limitations included the inability to control for factors such as exercise, diet, and disease severity; the inability to assess the risk for cause-specific mortality; and the inability to account for the impact of pain reduction and improved function as a result of weight loss.
DISCLOSURES:
The study was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, the Natural Science Foundation of Hunan Province, the Central South University Innovation-Driven Research Programme, and the Science and Technology Innovation Program of Hunan Province. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Individuals with overweight or obesity and knee or hip osteoarthritis (OA) who used antiobesity medications and achieved slow-to-moderate weight loss had a lower risk for all-cause mortality than did those with weight gain or stable weight in a population-based cohort study emulating a randomized controlled trial. Patients who rapidly lost weight had mortality similar to those with weight gain or stable weight.
METHODOLOGY:
- The researchers used the IQVIA Medical Research Database to identify overweight or obese individuals with knee or hip OA; they conducted a hypothetical trial comparing the effects of slow-to-moderate weight loss (defined as 2%-10% of body weight) and rapid weight loss (defined as 5% or more of body weight) within 1 year of starting antiobesity medications.
- The final analysis included patients with a mean age of 60.9 years who met the criteria for treatment adherence to orlistat (n = 3028), sibutramine (n = 2919), or rimonabant (n = 797).
- The primary outcome was all-cause mortality over a 5-year follow-up period; secondary outcomes included hypertension, type 2 diabetes, and venous thromboembolism.
TAKEAWAY:
- All-cause mortality at 5 years was 5.3% with weight gain or stable weight, 4.0% with slow to moderate weight loss, and 5.4% with rapid weight loss.
- Hazard ratios for all-cause mortality were 0.72 (95% CI, 0.56-0.92) for slow to moderate weight loss and 0.99 (95% CI, 0.67-1.44) for the rapid weight loss group.
- Weight loss was associated with the secondary outcomes of reduced hypertension, type 2 diabetes, and venous thromboembolism in a dose-dependent manner.
- A slightly increased risk for cardiovascular disease occurred in the rapid weight loss group, compared with the weight gain or stable group, but this difference was not significant.
IN PRACTICE:
“Our finding that gradual weight loss by antiobesity medications lowers all-cause mortality, if confirmed by future studies, could guide policy-making and improve the well-being of patients with overweight or obesity and knee or hip OA,” the researchers wrote.
SOURCE:
The lead author on the study was Jie Wei, MD, of Central South University, Changsha, China. The study was published online in Arthritis & Rheumatology.
LIMITATIONS:
Study limitations included the inability to control for factors such as exercise, diet, and disease severity; the inability to assess the risk for cause-specific mortality; and the inability to account for the impact of pain reduction and improved function as a result of weight loss.
DISCLOSURES:
The study was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, the Natural Science Foundation of Hunan Province, the Central South University Innovation-Driven Research Programme, and the Science and Technology Innovation Program of Hunan Province. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Individuals with overweight or obesity and knee or hip osteoarthritis (OA) who used antiobesity medications and achieved slow-to-moderate weight loss had a lower risk for all-cause mortality than did those with weight gain or stable weight in a population-based cohort study emulating a randomized controlled trial. Patients who rapidly lost weight had mortality similar to those with weight gain or stable weight.
METHODOLOGY:
- The researchers used the IQVIA Medical Research Database to identify overweight or obese individuals with knee or hip OA; they conducted a hypothetical trial comparing the effects of slow-to-moderate weight loss (defined as 2%-10% of body weight) and rapid weight loss (defined as 5% or more of body weight) within 1 year of starting antiobesity medications.
- The final analysis included patients with a mean age of 60.9 years who met the criteria for treatment adherence to orlistat (n = 3028), sibutramine (n = 2919), or rimonabant (n = 797).
- The primary outcome was all-cause mortality over a 5-year follow-up period; secondary outcomes included hypertension, type 2 diabetes, and venous thromboembolism.
TAKEAWAY:
- All-cause mortality at 5 years was 5.3% with weight gain or stable weight, 4.0% with slow to moderate weight loss, and 5.4% with rapid weight loss.
- Hazard ratios for all-cause mortality were 0.72 (95% CI, 0.56-0.92) for slow to moderate weight loss and 0.99 (95% CI, 0.67-1.44) for the rapid weight loss group.
- Weight loss was associated with the secondary outcomes of reduced hypertension, type 2 diabetes, and venous thromboembolism in a dose-dependent manner.
- A slightly increased risk for cardiovascular disease occurred in the rapid weight loss group, compared with the weight gain or stable group, but this difference was not significant.
IN PRACTICE:
“Our finding that gradual weight loss by antiobesity medications lowers all-cause mortality, if confirmed by future studies, could guide policy-making and improve the well-being of patients with overweight or obesity and knee or hip OA,” the researchers wrote.
SOURCE:
The lead author on the study was Jie Wei, MD, of Central South University, Changsha, China. The study was published online in Arthritis & Rheumatology.
LIMITATIONS:
Study limitations included the inability to control for factors such as exercise, diet, and disease severity; the inability to assess the risk for cause-specific mortality; and the inability to account for the impact of pain reduction and improved function as a result of weight loss.
DISCLOSURES:
The study was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, the Natural Science Foundation of Hunan Province, the Central South University Innovation-Driven Research Programme, and the Science and Technology Innovation Program of Hunan Province. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
What if a single GLP-1 shot could last for months?
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
FROM CELL REPORTS MEDICINE
How to prescribe Zepbound
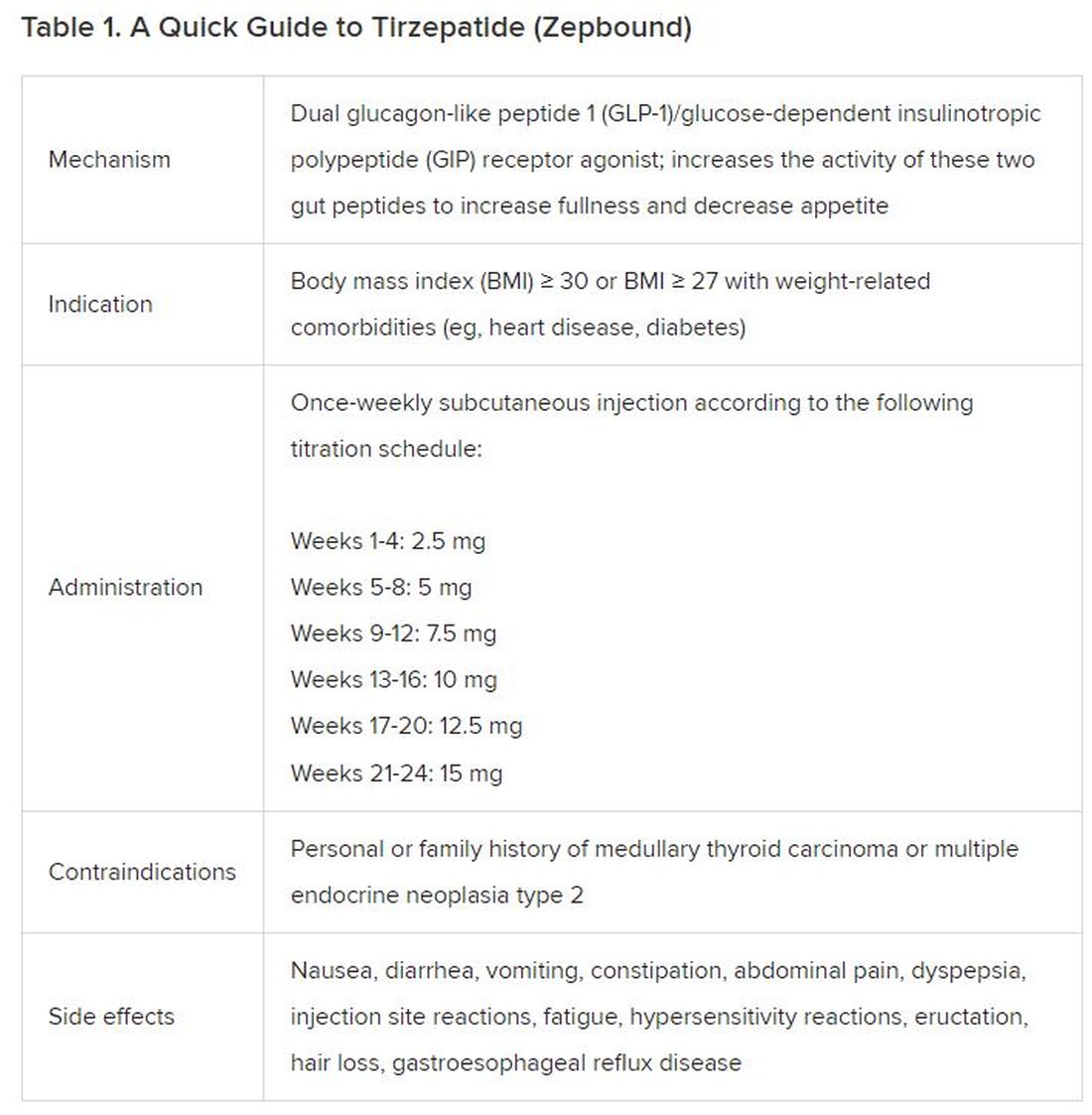
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes,
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).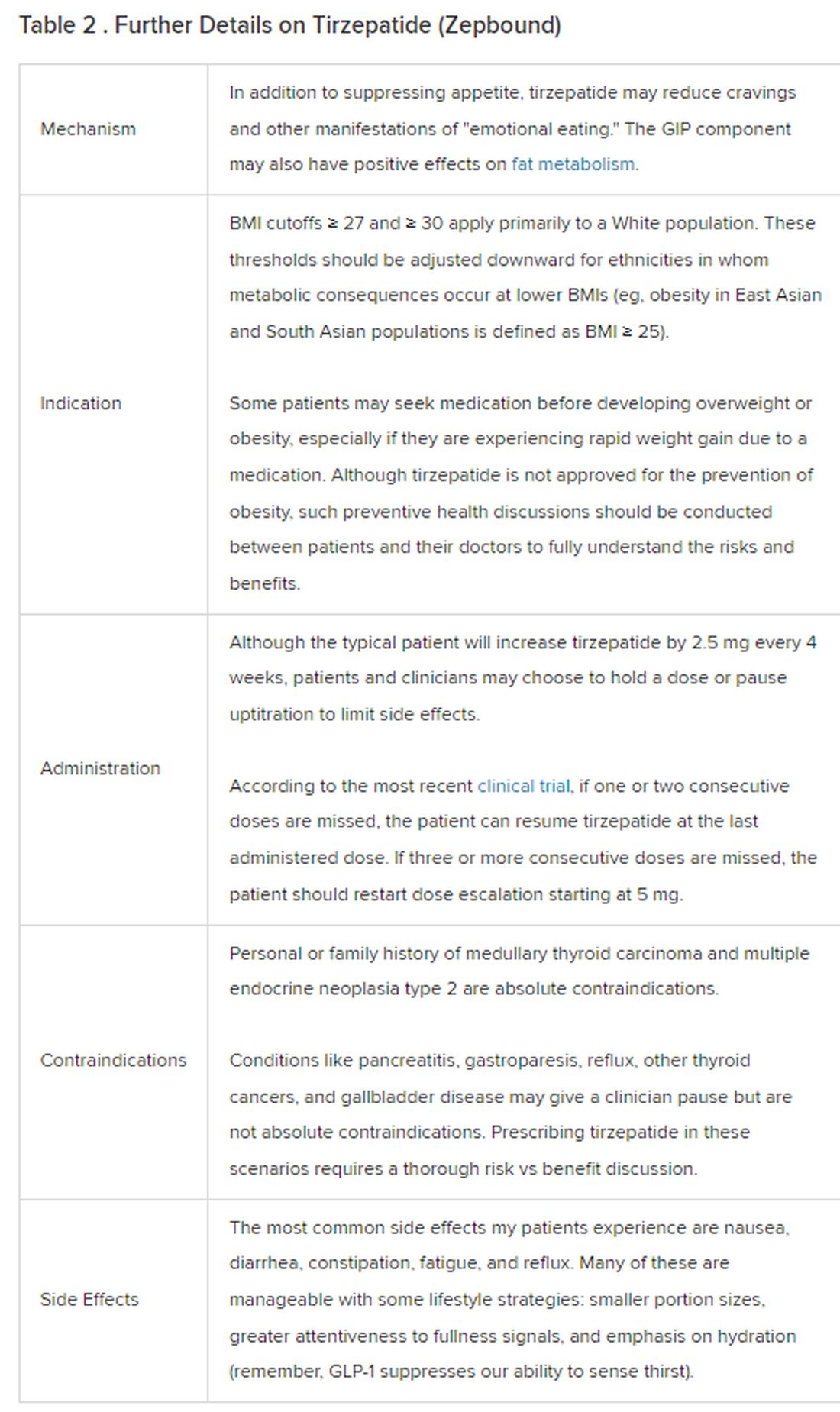
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes,
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes,
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
What is the link between cellphones and male fertility?
Infertility affects approximately one in six couples worldwide. More than half the time, it is the man’s low sperm quality that is to blame. Over the last three decades, sperm quality seems to have declined for no clearly identifiable reason. Theories are running rampant without anyone having the proof to back them up.
Potential Causes
The environment, lifestyle, excess weight or obesity, smoking, alcohol consumption, and psychological stress have all been alternately offered up as potential causes, following low-quality epidemiological studies. Cellphones are not exempt from this list, due to their emission of high-frequency (800-2200 MHz) electromagnetic waves that can be absorbed by the body.
Clinical trials conducted in rats or mice suggest that these waves can affect sperm quality and lead to histological changes to the testicles, bearing in mind that the conditions met in these trials are very far from our day-to-day exposure to electromagnetic waves, mostly via our cellphones.
The same observation can be made about experiments conducted on human sperm in vitro, but changes to the latter caused by electromagnetic waves leave doubts. Observational studies are rare, carried out in small cohorts, and marred by largely conflicting results. Publication bias plays a major role, just as much as the abundance of potential confounding factors does.
Swiss Observational Study
An observational study carried out in Switzerland had the benefit of involving a large cohort of 2886 young men who were representative of the general population. The participants completed an online questionnaire describing their relationship with their cellphone in detail and in qualitative and quantitative terms.
The study was launched in 2005, before cellphone use became so widespread, and this timeline was considered when looking for a link between cellphone exposure and sperm quality. In addition, multiple adjustments were made in the multivariate analyses to account for as many potential confounding factors as possible.
The participants, aged between 18 and 22 years, were recruited during a 3-day period to assess their suitability for military service. Each year, this cohort makes up 97% of the male population in Switzerland in this age range, with the remaining 3% being excluded from the selection process due to disability or chronic illness.
Regardless of the review board’s decision, subjects wishing to take part in the study were given a detailed description of what it involved, a consent form, and two questionnaires. The first focused on the individual directly, asking questions about his health and lifestyle. The second, intended for his parents, dealt with the period before conception.
This recruitment, which took place between September 2005 and November 2018, involved the researchers contacting 106,924 men. Ultimately, only 5.3% of subjects contacted returned the completed documentation. In the end, the study involved 2886 participants (3.1%) who provided all the necessary information, especially the laboratory testing (including a sperm analysis) needed to meet the study objectives. The number of hours spent on a smartphone and how it was used were routinely considered, as was sperm quality (volume, concentration, and total sperm count, as well as sperm mobility and morphology).
Significant Associations
A data analysis using an adjusted linear model revealed a significant association between frequent phone use (> 20 times per day) and lower sperm concentration (in mL) (adjusted β: -0.152, 95% CI -0.316 to 0.011). The same was found for their total concentration in ejaculate (adjusted β: -0.271, 95% CI -0.515 to -0.027).
An adjusted logistic regression analysis estimated that the risk for subnormal male fertility levels, as determined by the World Health Organization (WHO), was increased by at most 30%, when referring to the concentration of sperm per mL (21% in terms of total concentration). This inverse link was shown to be more pronounced during the first phase of the study (2005-2007), compared with the other two phases (2008-2011 and 2012-2018). Yet no links involving sperm mobility or morphology were found, and carrying a cellphone in a trouser pocket had no impact on the results.
This study certainly involves a large cohort of nearly 3000 young men. It is, nonetheless, retrospective, and its methodology, despite being better than that of previous studies, is still open to criticism. Its results can only fuel hypotheses, nothing more. Only prospective cohort studies will allow conclusions to be drawn and, in the meantime,
This article was translated from JIM, which is part of the Medscape professional network. A version of this article appeared on Medscape.com.
Infertility affects approximately one in six couples worldwide. More than half the time, it is the man’s low sperm quality that is to blame. Over the last three decades, sperm quality seems to have declined for no clearly identifiable reason. Theories are running rampant without anyone having the proof to back them up.
Potential Causes
The environment, lifestyle, excess weight or obesity, smoking, alcohol consumption, and psychological stress have all been alternately offered up as potential causes, following low-quality epidemiological studies. Cellphones are not exempt from this list, due to their emission of high-frequency (800-2200 MHz) electromagnetic waves that can be absorbed by the body.
Clinical trials conducted in rats or mice suggest that these waves can affect sperm quality and lead to histological changes to the testicles, bearing in mind that the conditions met in these trials are very far from our day-to-day exposure to electromagnetic waves, mostly via our cellphones.
The same observation can be made about experiments conducted on human sperm in vitro, but changes to the latter caused by electromagnetic waves leave doubts. Observational studies are rare, carried out in small cohorts, and marred by largely conflicting results. Publication bias plays a major role, just as much as the abundance of potential confounding factors does.
Swiss Observational Study
An observational study carried out in Switzerland had the benefit of involving a large cohort of 2886 young men who were representative of the general population. The participants completed an online questionnaire describing their relationship with their cellphone in detail and in qualitative and quantitative terms.
The study was launched in 2005, before cellphone use became so widespread, and this timeline was considered when looking for a link between cellphone exposure and sperm quality. In addition, multiple adjustments were made in the multivariate analyses to account for as many potential confounding factors as possible.
The participants, aged between 18 and 22 years, were recruited during a 3-day period to assess their suitability for military service. Each year, this cohort makes up 97% of the male population in Switzerland in this age range, with the remaining 3% being excluded from the selection process due to disability or chronic illness.
Regardless of the review board’s decision, subjects wishing to take part in the study were given a detailed description of what it involved, a consent form, and two questionnaires. The first focused on the individual directly, asking questions about his health and lifestyle. The second, intended for his parents, dealt with the period before conception.
This recruitment, which took place between September 2005 and November 2018, involved the researchers contacting 106,924 men. Ultimately, only 5.3% of subjects contacted returned the completed documentation. In the end, the study involved 2886 participants (3.1%) who provided all the necessary information, especially the laboratory testing (including a sperm analysis) needed to meet the study objectives. The number of hours spent on a smartphone and how it was used were routinely considered, as was sperm quality (volume, concentration, and total sperm count, as well as sperm mobility and morphology).
Significant Associations
A data analysis using an adjusted linear model revealed a significant association between frequent phone use (> 20 times per day) and lower sperm concentration (in mL) (adjusted β: -0.152, 95% CI -0.316 to 0.011). The same was found for their total concentration in ejaculate (adjusted β: -0.271, 95% CI -0.515 to -0.027).
An adjusted logistic regression analysis estimated that the risk for subnormal male fertility levels, as determined by the World Health Organization (WHO), was increased by at most 30%, when referring to the concentration of sperm per mL (21% in terms of total concentration). This inverse link was shown to be more pronounced during the first phase of the study (2005-2007), compared with the other two phases (2008-2011 and 2012-2018). Yet no links involving sperm mobility or morphology were found, and carrying a cellphone in a trouser pocket had no impact on the results.
This study certainly involves a large cohort of nearly 3000 young men. It is, nonetheless, retrospective, and its methodology, despite being better than that of previous studies, is still open to criticism. Its results can only fuel hypotheses, nothing more. Only prospective cohort studies will allow conclusions to be drawn and, in the meantime,
This article was translated from JIM, which is part of the Medscape professional network. A version of this article appeared on Medscape.com.
Infertility affects approximately one in six couples worldwide. More than half the time, it is the man’s low sperm quality that is to blame. Over the last three decades, sperm quality seems to have declined for no clearly identifiable reason. Theories are running rampant without anyone having the proof to back them up.
Potential Causes
The environment, lifestyle, excess weight or obesity, smoking, alcohol consumption, and psychological stress have all been alternately offered up as potential causes, following low-quality epidemiological studies. Cellphones are not exempt from this list, due to their emission of high-frequency (800-2200 MHz) electromagnetic waves that can be absorbed by the body.
Clinical trials conducted in rats or mice suggest that these waves can affect sperm quality and lead to histological changes to the testicles, bearing in mind that the conditions met in these trials are very far from our day-to-day exposure to electromagnetic waves, mostly via our cellphones.
The same observation can be made about experiments conducted on human sperm in vitro, but changes to the latter caused by electromagnetic waves leave doubts. Observational studies are rare, carried out in small cohorts, and marred by largely conflicting results. Publication bias plays a major role, just as much as the abundance of potential confounding factors does.
Swiss Observational Study
An observational study carried out in Switzerland had the benefit of involving a large cohort of 2886 young men who were representative of the general population. The participants completed an online questionnaire describing their relationship with their cellphone in detail and in qualitative and quantitative terms.
The study was launched in 2005, before cellphone use became so widespread, and this timeline was considered when looking for a link between cellphone exposure and sperm quality. In addition, multiple adjustments were made in the multivariate analyses to account for as many potential confounding factors as possible.
The participants, aged between 18 and 22 years, were recruited during a 3-day period to assess their suitability for military service. Each year, this cohort makes up 97% of the male population in Switzerland in this age range, with the remaining 3% being excluded from the selection process due to disability or chronic illness.
Regardless of the review board’s decision, subjects wishing to take part in the study were given a detailed description of what it involved, a consent form, and two questionnaires. The first focused on the individual directly, asking questions about his health and lifestyle. The second, intended for his parents, dealt with the period before conception.
This recruitment, which took place between September 2005 and November 2018, involved the researchers contacting 106,924 men. Ultimately, only 5.3% of subjects contacted returned the completed documentation. In the end, the study involved 2886 participants (3.1%) who provided all the necessary information, especially the laboratory testing (including a sperm analysis) needed to meet the study objectives. The number of hours spent on a smartphone and how it was used were routinely considered, as was sperm quality (volume, concentration, and total sperm count, as well as sperm mobility and morphology).
Significant Associations
A data analysis using an adjusted linear model revealed a significant association between frequent phone use (> 20 times per day) and lower sperm concentration (in mL) (adjusted β: -0.152, 95% CI -0.316 to 0.011). The same was found for their total concentration in ejaculate (adjusted β: -0.271, 95% CI -0.515 to -0.027).
An adjusted logistic regression analysis estimated that the risk for subnormal male fertility levels, as determined by the World Health Organization (WHO), was increased by at most 30%, when referring to the concentration of sperm per mL (21% in terms of total concentration). This inverse link was shown to be more pronounced during the first phase of the study (2005-2007), compared with the other two phases (2008-2011 and 2012-2018). Yet no links involving sperm mobility or morphology were found, and carrying a cellphone in a trouser pocket had no impact on the results.
This study certainly involves a large cohort of nearly 3000 young men. It is, nonetheless, retrospective, and its methodology, despite being better than that of previous studies, is still open to criticism. Its results can only fuel hypotheses, nothing more. Only prospective cohort studies will allow conclusions to be drawn and, in the meantime,
This article was translated from JIM, which is part of the Medscape professional network. A version of this article appeared on Medscape.com.
Federal program offers free COVID, flu at-home tests, treatments
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
Children who are overweight at risk for chronic kidney disease
TOPLINE
, with the association, though weaker, still significant among those who do not develop type 2 diabetes or hypertension, in a large cohort study.
METHODOLOGY
- The study included data on 593,660 adolescents aged 16-20, born after January 1, 1975, who had medical assessments as part of mandatory military service in Israel.
- The mean age at study entry was 17.2 and 54.5% were male.
- Early CKD was defined as stage 1 to 2 CKD with moderately or severely increased albuminuria, with an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher.
- The study excluded those with kidney pathology, albuminuria, hypertension, dysglycemia, or missing blood pressure or BMI data.
- Participants were followed up until early CKD onset, death, the last day insured, or August 23, 2020.
TAKEAWAY
- With a mean follow-up of 13.4 years, 1963 adolescents (0.3%) overall developed early chronic kidney disease. Among males, an increased risk of developing CKD was observed with a high-normal BMI in adolescence (hazard ratio [HR], 1.8); with overweight BMI (HR, 4.0); with mild obesity (HR, 6.7); and severe obesity (HR, 9.4).
- Among females, the increased risk was also observed with high-normal BMI (HR 1.4); overweight (HR, 2.3); mild obesity (HR, 2.7); and severe obesity (HR, 4.3).
- In excluding those who developed diabetes or hypertension, the overall rate of early CKD in the cohort was 0.2%.
- For males without diabetes or hypertension, the adjusted HR for early CKD with high-normal weight was 1.2; for overweight, HR 1.6; for mild obesity, HR 2.2; and for severe obesity, HR 2.7.
- For females without diabetes or hypertension, the corresponding increased risk for early CKD was HR 1.2 for high-normal BMI; HR 1.8 for overweight; 1.5 for mild obesity and 2.3 for severe obesity.
IN PRACTICE
“These findings suggest that adolescent obesity is a major risk factor for early CKD in young adulthood; this underscores the importance of mitigating adolescent obesity rates and managing risk factors for kidney disease in adolescents with high BMI,” the authors report.
“The association was evident even in persons with high-normal BMI in adolescence, was more pronounced in men, and appeared before the age of 30 years,” they say.
“Given the increasing obesity rates among adolescents, our findings are a harbinger of the potentially preventable increasing burden of CKD and subsequent cardiovascular disease.”
SOURCE
The study was conducted by first author Avishai M. Tsur, MD, of the Israel Defense Forces, Medical Corps, Tel Hashomer, Ramat Gan, Israel and Department of Military Medicine, Hebrew University of Jerusalem Faculty of Medicine, Jerusalem, Israel, and colleagues. The study was published online in JAMA Pediatrics.
LIMITATIONS
The study lacked longitudinal data on clinical and lifestyle factors, including stress, diet and physical activity. While adolescents were screened using urine dipstick, a lack of serum creatinine measurements could have missed some adolescents with reduced eGFR at the study entry. The generalizability of the results is limited by the lack of people from West Africa and East Asia in the study population.
DISCLOSURES
Coauthor Josef Coresh, MD, reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE
, with the association, though weaker, still significant among those who do not develop type 2 diabetes or hypertension, in a large cohort study.
METHODOLOGY
- The study included data on 593,660 adolescents aged 16-20, born after January 1, 1975, who had medical assessments as part of mandatory military service in Israel.
- The mean age at study entry was 17.2 and 54.5% were male.
- Early CKD was defined as stage 1 to 2 CKD with moderately or severely increased albuminuria, with an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher.
- The study excluded those with kidney pathology, albuminuria, hypertension, dysglycemia, or missing blood pressure or BMI data.
- Participants were followed up until early CKD onset, death, the last day insured, or August 23, 2020.
TAKEAWAY
- With a mean follow-up of 13.4 years, 1963 adolescents (0.3%) overall developed early chronic kidney disease. Among males, an increased risk of developing CKD was observed with a high-normal BMI in adolescence (hazard ratio [HR], 1.8); with overweight BMI (HR, 4.0); with mild obesity (HR, 6.7); and severe obesity (HR, 9.4).
- Among females, the increased risk was also observed with high-normal BMI (HR 1.4); overweight (HR, 2.3); mild obesity (HR, 2.7); and severe obesity (HR, 4.3).
- In excluding those who developed diabetes or hypertension, the overall rate of early CKD in the cohort was 0.2%.
- For males without diabetes or hypertension, the adjusted HR for early CKD with high-normal weight was 1.2; for overweight, HR 1.6; for mild obesity, HR 2.2; and for severe obesity, HR 2.7.
- For females without diabetes or hypertension, the corresponding increased risk for early CKD was HR 1.2 for high-normal BMI; HR 1.8 for overweight; 1.5 for mild obesity and 2.3 for severe obesity.
IN PRACTICE
“These findings suggest that adolescent obesity is a major risk factor for early CKD in young adulthood; this underscores the importance of mitigating adolescent obesity rates and managing risk factors for kidney disease in adolescents with high BMI,” the authors report.
“The association was evident even in persons with high-normal BMI in adolescence, was more pronounced in men, and appeared before the age of 30 years,” they say.
“Given the increasing obesity rates among adolescents, our findings are a harbinger of the potentially preventable increasing burden of CKD and subsequent cardiovascular disease.”
SOURCE
The study was conducted by first author Avishai M. Tsur, MD, of the Israel Defense Forces, Medical Corps, Tel Hashomer, Ramat Gan, Israel and Department of Military Medicine, Hebrew University of Jerusalem Faculty of Medicine, Jerusalem, Israel, and colleagues. The study was published online in JAMA Pediatrics.
LIMITATIONS
The study lacked longitudinal data on clinical and lifestyle factors, including stress, diet and physical activity. While adolescents were screened using urine dipstick, a lack of serum creatinine measurements could have missed some adolescents with reduced eGFR at the study entry. The generalizability of the results is limited by the lack of people from West Africa and East Asia in the study population.
DISCLOSURES
Coauthor Josef Coresh, MD, reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE
, with the association, though weaker, still significant among those who do not develop type 2 diabetes or hypertension, in a large cohort study.
METHODOLOGY
- The study included data on 593,660 adolescents aged 16-20, born after January 1, 1975, who had medical assessments as part of mandatory military service in Israel.
- The mean age at study entry was 17.2 and 54.5% were male.
- Early CKD was defined as stage 1 to 2 CKD with moderately or severely increased albuminuria, with an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher.
- The study excluded those with kidney pathology, albuminuria, hypertension, dysglycemia, or missing blood pressure or BMI data.
- Participants were followed up until early CKD onset, death, the last day insured, or August 23, 2020.
TAKEAWAY
- With a mean follow-up of 13.4 years, 1963 adolescents (0.3%) overall developed early chronic kidney disease. Among males, an increased risk of developing CKD was observed with a high-normal BMI in adolescence (hazard ratio [HR], 1.8); with overweight BMI (HR, 4.0); with mild obesity (HR, 6.7); and severe obesity (HR, 9.4).
- Among females, the increased risk was also observed with high-normal BMI (HR 1.4); overweight (HR, 2.3); mild obesity (HR, 2.7); and severe obesity (HR, 4.3).
- In excluding those who developed diabetes or hypertension, the overall rate of early CKD in the cohort was 0.2%.
- For males without diabetes or hypertension, the adjusted HR for early CKD with high-normal weight was 1.2; for overweight, HR 1.6; for mild obesity, HR 2.2; and for severe obesity, HR 2.7.
- For females without diabetes or hypertension, the corresponding increased risk for early CKD was HR 1.2 for high-normal BMI; HR 1.8 for overweight; 1.5 for mild obesity and 2.3 for severe obesity.
IN PRACTICE
“These findings suggest that adolescent obesity is a major risk factor for early CKD in young adulthood; this underscores the importance of mitigating adolescent obesity rates and managing risk factors for kidney disease in adolescents with high BMI,” the authors report.
“The association was evident even in persons with high-normal BMI in adolescence, was more pronounced in men, and appeared before the age of 30 years,” they say.
“Given the increasing obesity rates among adolescents, our findings are a harbinger of the potentially preventable increasing burden of CKD and subsequent cardiovascular disease.”
SOURCE
The study was conducted by first author Avishai M. Tsur, MD, of the Israel Defense Forces, Medical Corps, Tel Hashomer, Ramat Gan, Israel and Department of Military Medicine, Hebrew University of Jerusalem Faculty of Medicine, Jerusalem, Israel, and colleagues. The study was published online in JAMA Pediatrics.
LIMITATIONS
The study lacked longitudinal data on clinical and lifestyle factors, including stress, diet and physical activity. While adolescents were screened using urine dipstick, a lack of serum creatinine measurements could have missed some adolescents with reduced eGFR at the study entry. The generalizability of the results is limited by the lack of people from West Africa and East Asia in the study population.
DISCLOSURES
Coauthor Josef Coresh, MD, reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
Is migraine really a female disorder?
BARCELONA, SPAIN — Migraine is widely considered a predominantly female disorder. Its frequency, duration, and severity tend to be higher in women, and women are also more likely than men to receive a migraine diagnosis. However, gender expectations, differences in the likelihood of self-reporting, and problems with how migraine is classified make it difficult to estimate its true prevalence in men and women.
Different Symptoms
Headache disorders are estimated to affect 50% of the general population ; tension-type headache and migraine are the two most common. According to epidemiologic studies, migraine is more prevalent in women, with a female-to-male ratio of 3:1. There are numerous studies of why this might be, most of which focus largely on female-related factors, such as hormones and the menstrual cycle.
“Despite many years of research, there isn’t one clear factor explaining this substantial difference between women and men,” said Tobias Kurth of Charité – Universitätsmedizin Berlin, Germany. “So the question is: Are we missing something else?”
One factor in these perceived sex differences in migraine is that women seem to report their migraines differently from men, and they also have different symptoms. For example, women are more likely than men to report severe pain, and their migraine attacks are more often accompanied by photophobia, phonophobia, and nausea, whereas men’s migraines are more often accompanied by aura.
“By favoring female symptoms, the classification system may not be picking up male symptoms because they’re not being classified in the right way,” Dr. Kurth said, with one consequence being that migraine is underdiagnosed in men. “Before trying to understand the biological and behavioral reasons for these sex differences, we first need to consider these methodological challenges that we all apply knowingly or unknowingly.”
Christian Lampl, professor of neurology at Konventhospital der Barmherzigen Brüder Linz, Austria, and president of the European Headache Federation, said in an interview, “I’m convinced that this 3:1 ratio which has been stated for decades is wrong, but we still don’t have the data. The criteria we have [for classifying migraine] are useful for clinical trials, but they are useless for determining the male-to-female ratio.
“We need a new definition of migraine,” he added. “Migraine is an episode, not an attack. Attacks have a sudden onset, and migraine onset is not sudden — it is an episode with a headache attack.”
Inadequate Menopause Services
Professor Anne MacGregor of St. Bartholomew’s Hospital in London, United Kingdom, specializes in migraine and women’s health. She presented data showing that migraine is underdiagnosed in women; one reason being that the disorder receives inadequate attention from healthcare professionals at specialist menopause services.
Menopause is associated with an increased prevalence of migraine, but women do not discuss headache symptoms at specialist menopause services, Dr. MacGregor said.
She then described unpublished results from a survey of 117 women attending the specialist menopause service at St. Bartholomew’s Hospital. Among the respondents, 34% reported experiencing episodic migraine and an additional 8% reported having chronic migraine.
“Within this population of women who were not reporting headache as a symptom [to the menopause service until asked in the survey], 42% of them were positive for a diagnosis of migraine,” said Dr. MacGregor. “They were mostly relying on prescribed paracetamol and codeine, or buying it over the counter, and only 22% of them were receiving triptans.
“They are clearly being undertreated,” she added. “Part of this issue is that they didn’t spontaneously report headache as a menopause symptom, so they weren’t consulting for headache to their primary care physicians.”
Correct diagnosis by a consultant is a prerequisite for receiving appropriate migraine treatment. Yet, according to a US study published in 2012, only 45.5% of women with episodic migraine consulted a prescribing healthcare professional. Of those who consulted, 89% were diagnosed correctly, and only 68% of those received the appropriate treatment.
A larger, more recent study confirmed that there is a massive unmet need for improving care in this patient population. The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, which analyzed data from nearly 90,000 participants, showed that just 4.8% of people with chronic migraine received consultation, correct diagnosis, and treatment, with 89% of women with chronic migraine left undiagnosed.
The OVERCOME Study further revealed that although many people with migraine were repeat consulters, they were consulting their physicians for other health problems.
“This makes it very clear that people in other specialties need to be more aware about picking up and diagnosing headache,” said MacGregor. “That’s where the real need is in managing headache. We have the treatments, but if the patients can’t access them, they’re not much good to them.”
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — Migraine is widely considered a predominantly female disorder. Its frequency, duration, and severity tend to be higher in women, and women are also more likely than men to receive a migraine diagnosis. However, gender expectations, differences in the likelihood of self-reporting, and problems with how migraine is classified make it difficult to estimate its true prevalence in men and women.
Different Symptoms
Headache disorders are estimated to affect 50% of the general population ; tension-type headache and migraine are the two most common. According to epidemiologic studies, migraine is more prevalent in women, with a female-to-male ratio of 3:1. There are numerous studies of why this might be, most of which focus largely on female-related factors, such as hormones and the menstrual cycle.
“Despite many years of research, there isn’t one clear factor explaining this substantial difference between women and men,” said Tobias Kurth of Charité – Universitätsmedizin Berlin, Germany. “So the question is: Are we missing something else?”
One factor in these perceived sex differences in migraine is that women seem to report their migraines differently from men, and they also have different symptoms. For example, women are more likely than men to report severe pain, and their migraine attacks are more often accompanied by photophobia, phonophobia, and nausea, whereas men’s migraines are more often accompanied by aura.
“By favoring female symptoms, the classification system may not be picking up male symptoms because they’re not being classified in the right way,” Dr. Kurth said, with one consequence being that migraine is underdiagnosed in men. “Before trying to understand the biological and behavioral reasons for these sex differences, we first need to consider these methodological challenges that we all apply knowingly or unknowingly.”
Christian Lampl, professor of neurology at Konventhospital der Barmherzigen Brüder Linz, Austria, and president of the European Headache Federation, said in an interview, “I’m convinced that this 3:1 ratio which has been stated for decades is wrong, but we still don’t have the data. The criteria we have [for classifying migraine] are useful for clinical trials, but they are useless for determining the male-to-female ratio.
“We need a new definition of migraine,” he added. “Migraine is an episode, not an attack. Attacks have a sudden onset, and migraine onset is not sudden — it is an episode with a headache attack.”
Inadequate Menopause Services
Professor Anne MacGregor of St. Bartholomew’s Hospital in London, United Kingdom, specializes in migraine and women’s health. She presented data showing that migraine is underdiagnosed in women; one reason being that the disorder receives inadequate attention from healthcare professionals at specialist menopause services.
Menopause is associated with an increased prevalence of migraine, but women do not discuss headache symptoms at specialist menopause services, Dr. MacGregor said.
She then described unpublished results from a survey of 117 women attending the specialist menopause service at St. Bartholomew’s Hospital. Among the respondents, 34% reported experiencing episodic migraine and an additional 8% reported having chronic migraine.
“Within this population of women who were not reporting headache as a symptom [to the menopause service until asked in the survey], 42% of them were positive for a diagnosis of migraine,” said Dr. MacGregor. “They were mostly relying on prescribed paracetamol and codeine, or buying it over the counter, and only 22% of them were receiving triptans.
“They are clearly being undertreated,” she added. “Part of this issue is that they didn’t spontaneously report headache as a menopause symptom, so they weren’t consulting for headache to their primary care physicians.”
Correct diagnosis by a consultant is a prerequisite for receiving appropriate migraine treatment. Yet, according to a US study published in 2012, only 45.5% of women with episodic migraine consulted a prescribing healthcare professional. Of those who consulted, 89% were diagnosed correctly, and only 68% of those received the appropriate treatment.
A larger, more recent study confirmed that there is a massive unmet need for improving care in this patient population. The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, which analyzed data from nearly 90,000 participants, showed that just 4.8% of people with chronic migraine received consultation, correct diagnosis, and treatment, with 89% of women with chronic migraine left undiagnosed.
The OVERCOME Study further revealed that although many people with migraine were repeat consulters, they were consulting their physicians for other health problems.
“This makes it very clear that people in other specialties need to be more aware about picking up and diagnosing headache,” said MacGregor. “That’s where the real need is in managing headache. We have the treatments, but if the patients can’t access them, they’re not much good to them.”
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — Migraine is widely considered a predominantly female disorder. Its frequency, duration, and severity tend to be higher in women, and women are also more likely than men to receive a migraine diagnosis. However, gender expectations, differences in the likelihood of self-reporting, and problems with how migraine is classified make it difficult to estimate its true prevalence in men and women.
Different Symptoms
Headache disorders are estimated to affect 50% of the general population ; tension-type headache and migraine are the two most common. According to epidemiologic studies, migraine is more prevalent in women, with a female-to-male ratio of 3:1. There are numerous studies of why this might be, most of which focus largely on female-related factors, such as hormones and the menstrual cycle.
“Despite many years of research, there isn’t one clear factor explaining this substantial difference between women and men,” said Tobias Kurth of Charité – Universitätsmedizin Berlin, Germany. “So the question is: Are we missing something else?”
One factor in these perceived sex differences in migraine is that women seem to report their migraines differently from men, and they also have different symptoms. For example, women are more likely than men to report severe pain, and their migraine attacks are more often accompanied by photophobia, phonophobia, and nausea, whereas men’s migraines are more often accompanied by aura.
“By favoring female symptoms, the classification system may not be picking up male symptoms because they’re not being classified in the right way,” Dr. Kurth said, with one consequence being that migraine is underdiagnosed in men. “Before trying to understand the biological and behavioral reasons for these sex differences, we first need to consider these methodological challenges that we all apply knowingly or unknowingly.”
Christian Lampl, professor of neurology at Konventhospital der Barmherzigen Brüder Linz, Austria, and president of the European Headache Federation, said in an interview, “I’m convinced that this 3:1 ratio which has been stated for decades is wrong, but we still don’t have the data. The criteria we have [for classifying migraine] are useful for clinical trials, but they are useless for determining the male-to-female ratio.
“We need a new definition of migraine,” he added. “Migraine is an episode, not an attack. Attacks have a sudden onset, and migraine onset is not sudden — it is an episode with a headache attack.”
Inadequate Menopause Services
Professor Anne MacGregor of St. Bartholomew’s Hospital in London, United Kingdom, specializes in migraine and women’s health. She presented data showing that migraine is underdiagnosed in women; one reason being that the disorder receives inadequate attention from healthcare professionals at specialist menopause services.
Menopause is associated with an increased prevalence of migraine, but women do not discuss headache symptoms at specialist menopause services, Dr. MacGregor said.
She then described unpublished results from a survey of 117 women attending the specialist menopause service at St. Bartholomew’s Hospital. Among the respondents, 34% reported experiencing episodic migraine and an additional 8% reported having chronic migraine.
“Within this population of women who were not reporting headache as a symptom [to the menopause service until asked in the survey], 42% of them were positive for a diagnosis of migraine,” said Dr. MacGregor. “They were mostly relying on prescribed paracetamol and codeine, or buying it over the counter, and only 22% of them were receiving triptans.
“They are clearly being undertreated,” she added. “Part of this issue is that they didn’t spontaneously report headache as a menopause symptom, so they weren’t consulting for headache to their primary care physicians.”
Correct diagnosis by a consultant is a prerequisite for receiving appropriate migraine treatment. Yet, according to a US study published in 2012, only 45.5% of women with episodic migraine consulted a prescribing healthcare professional. Of those who consulted, 89% were diagnosed correctly, and only 68% of those received the appropriate treatment.
A larger, more recent study confirmed that there is a massive unmet need for improving care in this patient population. The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, which analyzed data from nearly 90,000 participants, showed that just 4.8% of people with chronic migraine received consultation, correct diagnosis, and treatment, with 89% of women with chronic migraine left undiagnosed.
The OVERCOME Study further revealed that although many people with migraine were repeat consulters, they were consulting their physicians for other health problems.
“This makes it very clear that people in other specialties need to be more aware about picking up and diagnosing headache,” said MacGregor. “That’s where the real need is in managing headache. We have the treatments, but if the patients can’t access them, they’re not much good to them.”
A version of this article appeared on Medscape.com.
FROM EHC 2023