User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
GI symptoms during menopause deserve attention
This transcript has been edited for clarity.
Welcome back to another GI Common Concerns.
Today, I want to highlight some information about menopause.
Approximately 1.5 million women in the United States per year enter into menopause. Hysterectomy is also one of the most common surgeries for women worldwide, with an estimated 20%-40% undergoing this procedure by the age of 60.
Therefore, whether it’s because of biologic onset with age or surgical induction, menopause is a very common condition, and it’s important that we understand its symptoms and the latest information around it.
Impact on GI motility
One of the clearest functional symptoms to be aware of with menopause relates to alterations in hormonal balance. This has an impact on gastrointestinal (GI) motility by increasing abdominal muscle stimulation related to different patterns of secretion and can result in a number of symptomatic changes.
One such change that can occur is food intolerance. It is believed that menopause-associated food intolerance has multiple possible causes and may be related more to alterations to the microbiome, which can be contributed to by diet, activity, sleep cycle, and other factors.
When food intolerances are triggered in the perimenopausal or menopausal patient, it may lead you to recommend the well-established FODMAP diet, which is known to reduce symptoms. But the answer for every patient is not simply placing them on a FODMAP diet and telling them they have irritable bowel syndrome.
Other approaches can be considered for addressing food intolerance in these patients. The data are quite strong that adjunctive use of a dietitian is tremendously helpful in this particular population.
When it comes to menopausal patients, however, we need to consider other changes in their activity or adverse contributors to their mental health, such as stress or anxiety. These all contribute to more of a multifactorial composite in this population, for which irritable bowel syndrome serves as a similar example.
This means that we may need to expand our horizons rather than to focus on solely on antispasmodic or diet-related interventions.
Instead, we can start to consider more of a multidimensional treatment approach consisting of education, relaxation, cognitive-behavioral therapy, and physical activity. Certainly, there are now behavioral interventions using Internet-based digital formats to increase the acceptability and sustainability among patients.
Choosing such a multidisciplinary approach can be quite helpful.
The metabolic consequences of altering hormonal balance
Recent data from a rat model study investigated the metabolic impact of changing hormonal balance.
Investigators looked at ovariectomized rats and found that there was a biologic change in the diversity of the general GI biome. There were also noteworthy associations with weight fluctuations and dramatic changes in the spatial memory and cognitive performance characteristics of these rats, which was subsequently improved by supplemental estrogen.
This indicates that we may be able to remediate these effects with the similar use of supplemental hormone replacement treatments.
Another recent study looked at nonalcoholic fatty liver disease, which is very common in the general population and has a > 20% worldwide prevalence in postmenopausal women. Albeit small in numbers, this was a very interesting study.
Investigators looked at the delivery method for menopausal hormone therapy, which was transdermal for 75 patients and oral for 293 patients. Then, they looked at ultrasound definition of nonalcoholic fatty liver disease after 1 year as the endpoint. They found an approximate 7% reduction in the patients who received the transdermal administration compared with a 4% increase in the patients who received it orally.
Again, we have to remember this is a relatively small study, but the results indicate that the route of estrogen administration may be an important consideration in nonalcoholic fatty liver disease.
Sleep disturbances: fragmentation, duration, and quality
Sleep is something that’s near and dear to my heart and is the focus of a lot of our research.
Sleep disturbances are really part and parcel of menopause and are observed with hormonal imbalances and temperature intolerances. Disturbances such as sleep fragmentation, shorter sleep duration, and poorer sleep quality have a dramatic effect not only on the biome but also on sensory thresholds.
Therefore, as we start to look at mitigating strategies here, we need to focus on sleep and ask the right questions.
In my own practice, I try not to just ask, “How did you sleep last night?” That’s because sleep can be somewhat amnestic. You may have a cognitive awakening or a noncognitive awakening but still have experienced fragmentation.
As a result, my focus is on next-day function. I ask my patients, “When you get up in the morning, are you refreshed? Do you have the ability to perform daytime activities? Do you experience early fatigue or cognitive changes that occur?”
These questions can provide good insights into the sleep efficiency of the previous night.
The effect of the microbiome on osteoporosis
One final topic I found very interesting pertains to the effects of menopause on osteoporosis.
We certainly know that postmenopausal women have a very high prevalence of osteopenia, and that osteoporosis is a progression of that, as well as that increased bone-related disease affects fractures and related morbidity and mortality.
However, there’s accumulating evidence on the osteoporotic effects of biomarker changes in menopause, which shows that the biome regulates the pathophysiologic process of at least a large degree of osteoporosis.
This starts to make sense when you look at the pro-inflammatory factors that increase with changes in biome diversity, in particular tumor necrosis factor alpha (which is something we also see in inflammatory bowel disease), interleukin-1, and increased activated osteoclasts.
Therefore, when it comes to decreasing bone loss among patients who are perimenopausal or postmenopausal, we don’t yet have a clear answer. Hormone therapy, diet, activity, vitamin D supplementation, and other things may positively change the biome. They are worthy topics for patients to bring up with their ob.gyns. or primary care doctors.
Although it may be a little bit outside the scope of gastroenterology, in my opinion there are a number of new findings relating to menopause that we as a field need to be more proactive in addressing.
Ask the right questions when these people come in to you, irrespective of why they’re there. Start to ask about the quality of their sleep. What are their other functional symptoms? What are their other potential osteoporosis-related risks?
We must do a better job about individualizing care. Rather than treating patients as disease states, we must start to do specific patient-focused care.
I hope this gives you some provocative thoughts when you have your next session with a patient in the perimenopausal or menopausal state. There are lots of things that we continue to learn.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Va., and a past president of the American College of Gastroenterology. He serves as an adviser to ISOThrive and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back to another GI Common Concerns.
Today, I want to highlight some information about menopause.
Approximately 1.5 million women in the United States per year enter into menopause. Hysterectomy is also one of the most common surgeries for women worldwide, with an estimated 20%-40% undergoing this procedure by the age of 60.
Therefore, whether it’s because of biologic onset with age or surgical induction, menopause is a very common condition, and it’s important that we understand its symptoms and the latest information around it.
Impact on GI motility
One of the clearest functional symptoms to be aware of with menopause relates to alterations in hormonal balance. This has an impact on gastrointestinal (GI) motility by increasing abdominal muscle stimulation related to different patterns of secretion and can result in a number of symptomatic changes.
One such change that can occur is food intolerance. It is believed that menopause-associated food intolerance has multiple possible causes and may be related more to alterations to the microbiome, which can be contributed to by diet, activity, sleep cycle, and other factors.
When food intolerances are triggered in the perimenopausal or menopausal patient, it may lead you to recommend the well-established FODMAP diet, which is known to reduce symptoms. But the answer for every patient is not simply placing them on a FODMAP diet and telling them they have irritable bowel syndrome.
Other approaches can be considered for addressing food intolerance in these patients. The data are quite strong that adjunctive use of a dietitian is tremendously helpful in this particular population.
When it comes to menopausal patients, however, we need to consider other changes in their activity or adverse contributors to their mental health, such as stress or anxiety. These all contribute to more of a multifactorial composite in this population, for which irritable bowel syndrome serves as a similar example.
This means that we may need to expand our horizons rather than to focus on solely on antispasmodic or diet-related interventions.
Instead, we can start to consider more of a multidimensional treatment approach consisting of education, relaxation, cognitive-behavioral therapy, and physical activity. Certainly, there are now behavioral interventions using Internet-based digital formats to increase the acceptability and sustainability among patients.
Choosing such a multidisciplinary approach can be quite helpful.
The metabolic consequences of altering hormonal balance
Recent data from a rat model study investigated the metabolic impact of changing hormonal balance.
Investigators looked at ovariectomized rats and found that there was a biologic change in the diversity of the general GI biome. There were also noteworthy associations with weight fluctuations and dramatic changes in the spatial memory and cognitive performance characteristics of these rats, which was subsequently improved by supplemental estrogen.
This indicates that we may be able to remediate these effects with the similar use of supplemental hormone replacement treatments.
Another recent study looked at nonalcoholic fatty liver disease, which is very common in the general population and has a > 20% worldwide prevalence in postmenopausal women. Albeit small in numbers, this was a very interesting study.
Investigators looked at the delivery method for menopausal hormone therapy, which was transdermal for 75 patients and oral for 293 patients. Then, they looked at ultrasound definition of nonalcoholic fatty liver disease after 1 year as the endpoint. They found an approximate 7% reduction in the patients who received the transdermal administration compared with a 4% increase in the patients who received it orally.
Again, we have to remember this is a relatively small study, but the results indicate that the route of estrogen administration may be an important consideration in nonalcoholic fatty liver disease.
Sleep disturbances: fragmentation, duration, and quality
Sleep is something that’s near and dear to my heart and is the focus of a lot of our research.
Sleep disturbances are really part and parcel of menopause and are observed with hormonal imbalances and temperature intolerances. Disturbances such as sleep fragmentation, shorter sleep duration, and poorer sleep quality have a dramatic effect not only on the biome but also on sensory thresholds.
Therefore, as we start to look at mitigating strategies here, we need to focus on sleep and ask the right questions.
In my own practice, I try not to just ask, “How did you sleep last night?” That’s because sleep can be somewhat amnestic. You may have a cognitive awakening or a noncognitive awakening but still have experienced fragmentation.
As a result, my focus is on next-day function. I ask my patients, “When you get up in the morning, are you refreshed? Do you have the ability to perform daytime activities? Do you experience early fatigue or cognitive changes that occur?”
These questions can provide good insights into the sleep efficiency of the previous night.
The effect of the microbiome on osteoporosis
One final topic I found very interesting pertains to the effects of menopause on osteoporosis.
We certainly know that postmenopausal women have a very high prevalence of osteopenia, and that osteoporosis is a progression of that, as well as that increased bone-related disease affects fractures and related morbidity and mortality.
However, there’s accumulating evidence on the osteoporotic effects of biomarker changes in menopause, which shows that the biome regulates the pathophysiologic process of at least a large degree of osteoporosis.
This starts to make sense when you look at the pro-inflammatory factors that increase with changes in biome diversity, in particular tumor necrosis factor alpha (which is something we also see in inflammatory bowel disease), interleukin-1, and increased activated osteoclasts.
Therefore, when it comes to decreasing bone loss among patients who are perimenopausal or postmenopausal, we don’t yet have a clear answer. Hormone therapy, diet, activity, vitamin D supplementation, and other things may positively change the biome. They are worthy topics for patients to bring up with their ob.gyns. or primary care doctors.
Although it may be a little bit outside the scope of gastroenterology, in my opinion there are a number of new findings relating to menopause that we as a field need to be more proactive in addressing.
Ask the right questions when these people come in to you, irrespective of why they’re there. Start to ask about the quality of their sleep. What are their other functional symptoms? What are their other potential osteoporosis-related risks?
We must do a better job about individualizing care. Rather than treating patients as disease states, we must start to do specific patient-focused care.
I hope this gives you some provocative thoughts when you have your next session with a patient in the perimenopausal or menopausal state. There are lots of things that we continue to learn.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Va., and a past president of the American College of Gastroenterology. He serves as an adviser to ISOThrive and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back to another GI Common Concerns.
Today, I want to highlight some information about menopause.
Approximately 1.5 million women in the United States per year enter into menopause. Hysterectomy is also one of the most common surgeries for women worldwide, with an estimated 20%-40% undergoing this procedure by the age of 60.
Therefore, whether it’s because of biologic onset with age or surgical induction, menopause is a very common condition, and it’s important that we understand its symptoms and the latest information around it.
Impact on GI motility
One of the clearest functional symptoms to be aware of with menopause relates to alterations in hormonal balance. This has an impact on gastrointestinal (GI) motility by increasing abdominal muscle stimulation related to different patterns of secretion and can result in a number of symptomatic changes.
One such change that can occur is food intolerance. It is believed that menopause-associated food intolerance has multiple possible causes and may be related more to alterations to the microbiome, which can be contributed to by diet, activity, sleep cycle, and other factors.
When food intolerances are triggered in the perimenopausal or menopausal patient, it may lead you to recommend the well-established FODMAP diet, which is known to reduce symptoms. But the answer for every patient is not simply placing them on a FODMAP diet and telling them they have irritable bowel syndrome.
Other approaches can be considered for addressing food intolerance in these patients. The data are quite strong that adjunctive use of a dietitian is tremendously helpful in this particular population.
When it comes to menopausal patients, however, we need to consider other changes in their activity or adverse contributors to their mental health, such as stress or anxiety. These all contribute to more of a multifactorial composite in this population, for which irritable bowel syndrome serves as a similar example.
This means that we may need to expand our horizons rather than to focus on solely on antispasmodic or diet-related interventions.
Instead, we can start to consider more of a multidimensional treatment approach consisting of education, relaxation, cognitive-behavioral therapy, and physical activity. Certainly, there are now behavioral interventions using Internet-based digital formats to increase the acceptability and sustainability among patients.
Choosing such a multidisciplinary approach can be quite helpful.
The metabolic consequences of altering hormonal balance
Recent data from a rat model study investigated the metabolic impact of changing hormonal balance.
Investigators looked at ovariectomized rats and found that there was a biologic change in the diversity of the general GI biome. There were also noteworthy associations with weight fluctuations and dramatic changes in the spatial memory and cognitive performance characteristics of these rats, which was subsequently improved by supplemental estrogen.
This indicates that we may be able to remediate these effects with the similar use of supplemental hormone replacement treatments.
Another recent study looked at nonalcoholic fatty liver disease, which is very common in the general population and has a > 20% worldwide prevalence in postmenopausal women. Albeit small in numbers, this was a very interesting study.
Investigators looked at the delivery method for menopausal hormone therapy, which was transdermal for 75 patients and oral for 293 patients. Then, they looked at ultrasound definition of nonalcoholic fatty liver disease after 1 year as the endpoint. They found an approximate 7% reduction in the patients who received the transdermal administration compared with a 4% increase in the patients who received it orally.
Again, we have to remember this is a relatively small study, but the results indicate that the route of estrogen administration may be an important consideration in nonalcoholic fatty liver disease.
Sleep disturbances: fragmentation, duration, and quality
Sleep is something that’s near and dear to my heart and is the focus of a lot of our research.
Sleep disturbances are really part and parcel of menopause and are observed with hormonal imbalances and temperature intolerances. Disturbances such as sleep fragmentation, shorter sleep duration, and poorer sleep quality have a dramatic effect not only on the biome but also on sensory thresholds.
Therefore, as we start to look at mitigating strategies here, we need to focus on sleep and ask the right questions.
In my own practice, I try not to just ask, “How did you sleep last night?” That’s because sleep can be somewhat amnestic. You may have a cognitive awakening or a noncognitive awakening but still have experienced fragmentation.
As a result, my focus is on next-day function. I ask my patients, “When you get up in the morning, are you refreshed? Do you have the ability to perform daytime activities? Do you experience early fatigue or cognitive changes that occur?”
These questions can provide good insights into the sleep efficiency of the previous night.
The effect of the microbiome on osteoporosis
One final topic I found very interesting pertains to the effects of menopause on osteoporosis.
We certainly know that postmenopausal women have a very high prevalence of osteopenia, and that osteoporosis is a progression of that, as well as that increased bone-related disease affects fractures and related morbidity and mortality.
However, there’s accumulating evidence on the osteoporotic effects of biomarker changes in menopause, which shows that the biome regulates the pathophysiologic process of at least a large degree of osteoporosis.
This starts to make sense when you look at the pro-inflammatory factors that increase with changes in biome diversity, in particular tumor necrosis factor alpha (which is something we also see in inflammatory bowel disease), interleukin-1, and increased activated osteoclasts.
Therefore, when it comes to decreasing bone loss among patients who are perimenopausal or postmenopausal, we don’t yet have a clear answer. Hormone therapy, diet, activity, vitamin D supplementation, and other things may positively change the biome. They are worthy topics for patients to bring up with their ob.gyns. or primary care doctors.
Although it may be a little bit outside the scope of gastroenterology, in my opinion there are a number of new findings relating to menopause that we as a field need to be more proactive in addressing.
Ask the right questions when these people come in to you, irrespective of why they’re there. Start to ask about the quality of their sleep. What are their other functional symptoms? What are their other potential osteoporosis-related risks?
We must do a better job about individualizing care. Rather than treating patients as disease states, we must start to do specific patient-focused care.
I hope this gives you some provocative thoughts when you have your next session with a patient in the perimenopausal or menopausal state. There are lots of things that we continue to learn.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Va., and a past president of the American College of Gastroenterology. He serves as an adviser to ISOThrive and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
More weight loss with time-restricted eating
TOPLINE:
, compared with calorie restriction, while hemoglobin A1c levels dropped with both approaches, compared with no intervention.
METHODOLOGY:
- Six-month clinical trial of 75 adult participants with type 2 diabetes and obesity, randomly assigned to either 8-hour TRE (noon to 8 p.m. only) without calorie counting, a 25% daily calorie restriction, or control.
TAKEAWAY:
- The primary outcome, change in body weight at month 6, was –3.56% (P = .004) with TRE vs. –1.78% with calorie restriction (P = .06), compared with controls.
- The mean calorie deficit over the 6 months was –313 kcal/day with TRE, –197 kcal/day with calorie restriction, and –16 kcal/day for controls.
- Self-reported adherence to the regimens was 87% of days with 8-hour TRE vs. 68% reporting adherence with calorie goals over the 6 months.
- A1c levels were reduced significantly by 0.91% in the TRE group and 0.94% in the calorie-restriction group, relative to controls, with no differences between the two intervention groups.
- No serious adverse events were reported.
- Hypoglycemia and hyperglycemia occurrences didn’t differ between groups.
IN PRACTICE:
“Our findings ... show that TRE is safe in patients who are using either diet alone or medications to control their [type 2 diabetes]. However, for people using sulfonylureas and/or insulin, adopting a TRE regimen will require medication changes and regular monitoring, particularly in the initial stages of the diet.”
SOURCE:
The study was conducted by Vasiliki Pavlou, MS, RD, of the department of kinesiology and nutrition, University of Illinois at Chicago, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
- Relatively short trial duration.
- Lack of blinding.
- A higher proportion in the TRE group were using newer type 2 diabetes medications at baseline.
- Self-reported dietary intake.
DISCLOSURES:
The study was supported by the University of Illinois at Chicago, and by grants from the National Institutes of Health. Ms. Pavlou reports no relevant financial relationships. Several authors reported relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
, compared with calorie restriction, while hemoglobin A1c levels dropped with both approaches, compared with no intervention.
METHODOLOGY:
- Six-month clinical trial of 75 adult participants with type 2 diabetes and obesity, randomly assigned to either 8-hour TRE (noon to 8 p.m. only) without calorie counting, a 25% daily calorie restriction, or control.
TAKEAWAY:
- The primary outcome, change in body weight at month 6, was –3.56% (P = .004) with TRE vs. –1.78% with calorie restriction (P = .06), compared with controls.
- The mean calorie deficit over the 6 months was –313 kcal/day with TRE, –197 kcal/day with calorie restriction, and –16 kcal/day for controls.
- Self-reported adherence to the regimens was 87% of days with 8-hour TRE vs. 68% reporting adherence with calorie goals over the 6 months.
- A1c levels were reduced significantly by 0.91% in the TRE group and 0.94% in the calorie-restriction group, relative to controls, with no differences between the two intervention groups.
- No serious adverse events were reported.
- Hypoglycemia and hyperglycemia occurrences didn’t differ between groups.
IN PRACTICE:
“Our findings ... show that TRE is safe in patients who are using either diet alone or medications to control their [type 2 diabetes]. However, for people using sulfonylureas and/or insulin, adopting a TRE regimen will require medication changes and regular monitoring, particularly in the initial stages of the diet.”
SOURCE:
The study was conducted by Vasiliki Pavlou, MS, RD, of the department of kinesiology and nutrition, University of Illinois at Chicago, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
- Relatively short trial duration.
- Lack of blinding.
- A higher proportion in the TRE group were using newer type 2 diabetes medications at baseline.
- Self-reported dietary intake.
DISCLOSURES:
The study was supported by the University of Illinois at Chicago, and by grants from the National Institutes of Health. Ms. Pavlou reports no relevant financial relationships. Several authors reported relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
, compared with calorie restriction, while hemoglobin A1c levels dropped with both approaches, compared with no intervention.
METHODOLOGY:
- Six-month clinical trial of 75 adult participants with type 2 diabetes and obesity, randomly assigned to either 8-hour TRE (noon to 8 p.m. only) without calorie counting, a 25% daily calorie restriction, or control.
TAKEAWAY:
- The primary outcome, change in body weight at month 6, was –3.56% (P = .004) with TRE vs. –1.78% with calorie restriction (P = .06), compared with controls.
- The mean calorie deficit over the 6 months was –313 kcal/day with TRE, –197 kcal/day with calorie restriction, and –16 kcal/day for controls.
- Self-reported adherence to the regimens was 87% of days with 8-hour TRE vs. 68% reporting adherence with calorie goals over the 6 months.
- A1c levels were reduced significantly by 0.91% in the TRE group and 0.94% in the calorie-restriction group, relative to controls, with no differences between the two intervention groups.
- No serious adverse events were reported.
- Hypoglycemia and hyperglycemia occurrences didn’t differ between groups.
IN PRACTICE:
“Our findings ... show that TRE is safe in patients who are using either diet alone or medications to control their [type 2 diabetes]. However, for people using sulfonylureas and/or insulin, adopting a TRE regimen will require medication changes and regular monitoring, particularly in the initial stages of the diet.”
SOURCE:
The study was conducted by Vasiliki Pavlou, MS, RD, of the department of kinesiology and nutrition, University of Illinois at Chicago, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
- Relatively short trial duration.
- Lack of blinding.
- A higher proportion in the TRE group were using newer type 2 diabetes medications at baseline.
- Self-reported dietary intake.
DISCLOSURES:
The study was supported by the University of Illinois at Chicago, and by grants from the National Institutes of Health. Ms. Pavlou reports no relevant financial relationships. Several authors reported relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Portfolio diet tied to lower risk for CVD, stroke
TOPLINE:
Close adherence to the Portfolio dietary pattern, including foods that have been shown to actively lower cholesterol (for example, plant proteins, nuts, viscous fiber, phytosterols, and plant monounsaturated fats) is associated with a 14% lower risk for total cardiovascular disease (CVD), coronary heart disease (CHD), and stroke, pooled results from three large observational studies suggest.
METHODOLOGY:
- The study included 73,924 women from the Nurses’ Health Study (NHS), 92,346 women from the Nurses’ Health Study II (NHSII), and 43,970 men from the Health Professionals Follow-Up Study (HPFS) without CVD at baseline who were followed biennially on lifestyle, medical history, and other health-related factors.
- From food-frequency questionnaires (FFQs) completed every 4 years, researchers categorized foods into the six components of the Portfolio diet:
- Plant protein such as legumes, beans, tofu, peas, and soymilk
- Nuts and seeds
- Fiber sources such as bran, oats, berries, and eggplant
- Phytosterols
- Monounsaturated fat (MUFA) sources such as olive oil and avocado
- High saturated fat and cholesterol sources such as whole-fat dairy and red and processed meats
- They scored each from 1 (least adherent) to 5 (most adherent), with a higher score indicating higher consumption.
- Researchers examined the association of this Portfolio Diet Score (PDS) with total CVD, CHD, and stroke, in the three cohorts, and associations with plasma levels of lipid and inflammatory biomarkers in a subpopulation of the cohorts.
TAKEAWAY:
- During up to 30 years of follow-up, there were 16,917 incident CVD cases, including 10,666 CHD cases and 6,473 strokes.
- In a pooled analysis of the three cohorts, the fully adjusted hazard ratio for total CVD comparing the highest with the lowest quintile of the PDS was 0.86 (95% confidence interval, 0.81-0.92; P for trend < .001).
- Also comparing extreme quintiles, the pooled HR for CHD was 0.86 (95% CI, 0.80-0.93; P for trend = .0001) and for stroke, it was 0.86 (95% CI, 0.78-0.95; P for trend = .0003).
- A higher PDS was also associated with a more favorable lipid profile and lower levels of inflammation.
IN PRACTICE:
which aligns with American Heart Association guidelines promoting consumption of whole grains, fruits and vegetables, plant-based proteins, minimally processed foods, and healthy unsaturated plant oils, the authors conclude.
SOURCE:
The study was conducted by Andrea J. Glenn, PhD, RD, department of nutrition, Harvard T.H. Chan School of Public Health, Boston, and colleagues. It was published online in the journal Circulation.
LIMITATIONS:
As the study was observational, residual confounding can’t be ruled out. Diet was self-reported, which may have resulted in measurement errors. Consumption of some recommended foods was low, even in the top quintiles, so the association with CVD risk may be underestimated. Information on a few key Portfolio diet foods, including barley and okra, was unavailable, potentially leading to underestimation of intake, which may also attenuate the findings.
DISCLOSURES:
The study was supported by the Diabetes Canada End Diabetes 100 Award. The NH and HPFS studies are supported by the National Institutes of Health. Dr. Glenn is supported by a Canadian Institutes of Health Research fellowship; she has received honoraria or travel support from the Soy Nutrition Institute Global, Vinasoy, and the Academy of Nutrition and Dietetics. See original article for disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
Close adherence to the Portfolio dietary pattern, including foods that have been shown to actively lower cholesterol (for example, plant proteins, nuts, viscous fiber, phytosterols, and plant monounsaturated fats) is associated with a 14% lower risk for total cardiovascular disease (CVD), coronary heart disease (CHD), and stroke, pooled results from three large observational studies suggest.
METHODOLOGY:
- The study included 73,924 women from the Nurses’ Health Study (NHS), 92,346 women from the Nurses’ Health Study II (NHSII), and 43,970 men from the Health Professionals Follow-Up Study (HPFS) without CVD at baseline who were followed biennially on lifestyle, medical history, and other health-related factors.
- From food-frequency questionnaires (FFQs) completed every 4 years, researchers categorized foods into the six components of the Portfolio diet:
- Plant protein such as legumes, beans, tofu, peas, and soymilk
- Nuts and seeds
- Fiber sources such as bran, oats, berries, and eggplant
- Phytosterols
- Monounsaturated fat (MUFA) sources such as olive oil and avocado
- High saturated fat and cholesterol sources such as whole-fat dairy and red and processed meats
- They scored each from 1 (least adherent) to 5 (most adherent), with a higher score indicating higher consumption.
- Researchers examined the association of this Portfolio Diet Score (PDS) with total CVD, CHD, and stroke, in the three cohorts, and associations with plasma levels of lipid and inflammatory biomarkers in a subpopulation of the cohorts.
TAKEAWAY:
- During up to 30 years of follow-up, there were 16,917 incident CVD cases, including 10,666 CHD cases and 6,473 strokes.
- In a pooled analysis of the three cohorts, the fully adjusted hazard ratio for total CVD comparing the highest with the lowest quintile of the PDS was 0.86 (95% confidence interval, 0.81-0.92; P for trend < .001).
- Also comparing extreme quintiles, the pooled HR for CHD was 0.86 (95% CI, 0.80-0.93; P for trend = .0001) and for stroke, it was 0.86 (95% CI, 0.78-0.95; P for trend = .0003).
- A higher PDS was also associated with a more favorable lipid profile and lower levels of inflammation.
IN PRACTICE:
which aligns with American Heart Association guidelines promoting consumption of whole grains, fruits and vegetables, plant-based proteins, minimally processed foods, and healthy unsaturated plant oils, the authors conclude.
SOURCE:
The study was conducted by Andrea J. Glenn, PhD, RD, department of nutrition, Harvard T.H. Chan School of Public Health, Boston, and colleagues. It was published online in the journal Circulation.
LIMITATIONS:
As the study was observational, residual confounding can’t be ruled out. Diet was self-reported, which may have resulted in measurement errors. Consumption of some recommended foods was low, even in the top quintiles, so the association with CVD risk may be underestimated. Information on a few key Portfolio diet foods, including barley and okra, was unavailable, potentially leading to underestimation of intake, which may also attenuate the findings.
DISCLOSURES:
The study was supported by the Diabetes Canada End Diabetes 100 Award. The NH and HPFS studies are supported by the National Institutes of Health. Dr. Glenn is supported by a Canadian Institutes of Health Research fellowship; she has received honoraria or travel support from the Soy Nutrition Institute Global, Vinasoy, and the Academy of Nutrition and Dietetics. See original article for disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
Close adherence to the Portfolio dietary pattern, including foods that have been shown to actively lower cholesterol (for example, plant proteins, nuts, viscous fiber, phytosterols, and plant monounsaturated fats) is associated with a 14% lower risk for total cardiovascular disease (CVD), coronary heart disease (CHD), and stroke, pooled results from three large observational studies suggest.
METHODOLOGY:
- The study included 73,924 women from the Nurses’ Health Study (NHS), 92,346 women from the Nurses’ Health Study II (NHSII), and 43,970 men from the Health Professionals Follow-Up Study (HPFS) without CVD at baseline who were followed biennially on lifestyle, medical history, and other health-related factors.
- From food-frequency questionnaires (FFQs) completed every 4 years, researchers categorized foods into the six components of the Portfolio diet:
- Plant protein such as legumes, beans, tofu, peas, and soymilk
- Nuts and seeds
- Fiber sources such as bran, oats, berries, and eggplant
- Phytosterols
- Monounsaturated fat (MUFA) sources such as olive oil and avocado
- High saturated fat and cholesterol sources such as whole-fat dairy and red and processed meats
- They scored each from 1 (least adherent) to 5 (most adherent), with a higher score indicating higher consumption.
- Researchers examined the association of this Portfolio Diet Score (PDS) with total CVD, CHD, and stroke, in the three cohorts, and associations with plasma levels of lipid and inflammatory biomarkers in a subpopulation of the cohorts.
TAKEAWAY:
- During up to 30 years of follow-up, there were 16,917 incident CVD cases, including 10,666 CHD cases and 6,473 strokes.
- In a pooled analysis of the three cohorts, the fully adjusted hazard ratio for total CVD comparing the highest with the lowest quintile of the PDS was 0.86 (95% confidence interval, 0.81-0.92; P for trend < .001).
- Also comparing extreme quintiles, the pooled HR for CHD was 0.86 (95% CI, 0.80-0.93; P for trend = .0001) and for stroke, it was 0.86 (95% CI, 0.78-0.95; P for trend = .0003).
- A higher PDS was also associated with a more favorable lipid profile and lower levels of inflammation.
IN PRACTICE:
which aligns with American Heart Association guidelines promoting consumption of whole grains, fruits and vegetables, plant-based proteins, minimally processed foods, and healthy unsaturated plant oils, the authors conclude.
SOURCE:
The study was conducted by Andrea J. Glenn, PhD, RD, department of nutrition, Harvard T.H. Chan School of Public Health, Boston, and colleagues. It was published online in the journal Circulation.
LIMITATIONS:
As the study was observational, residual confounding can’t be ruled out. Diet was self-reported, which may have resulted in measurement errors. Consumption of some recommended foods was low, even in the top quintiles, so the association with CVD risk may be underestimated. Information on a few key Portfolio diet foods, including barley and okra, was unavailable, potentially leading to underestimation of intake, which may also attenuate the findings.
DISCLOSURES:
The study was supported by the Diabetes Canada End Diabetes 100 Award. The NH and HPFS studies are supported by the National Institutes of Health. Dr. Glenn is supported by a Canadian Institutes of Health Research fellowship; she has received honoraria or travel support from the Soy Nutrition Institute Global, Vinasoy, and the Academy of Nutrition and Dietetics. See original article for disclosures of other authors.
A version of this article first appeared on Medscape.com.
Experts question finding that 70% cancer deaths are preventable
A new global analysis highlights the substantial burden of premature deaths from cancer around the world – a burden that could potentially be averted through prevention, early detection, and timely treatment.
According to the analysis, in 2020, over half of all cancer deaths – 5.28 million of 9.96 million – occurred prematurely (before age 70), leading to a loss of roughly 183 million life-years from the disease worldwide.
More than two-thirds of premature cancer-related deaths – 3.6 million, or 68% – were potentially preventable through lifestyle changes or early detection efforts, such as cancer screening, dietary changes, or smoking cessation, and about one-third – 1.65 million, or 31% – may have been treatable.
But two biostatisticians not involved in the study who took a deep dive into it urged caution in interpreting the data.
Nilanjan Chatterjee, PhD, Bloomberg Distinguished Professor, Bloomberg School of Public Health at Johns Hopkins University, Baltimore, said the study does a “great job in bringing a lot of diverse data together to show there is very high potential for preventing premature deaths due to cancer worldwide.”
However, for a variety of reasons, Dr. Chatterjee explained, one should not “overinterpret” the high percentage of potentially preventable cancer deaths.
Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong, in Australia, agreed.
“It’s likely many cancer deaths are, in theory, preventable, but the numbers around just how many are necessarily vague,” said Dr. Meyerowitz-Katz. “Also, ‘in theory preventable’ doesn’t necessarily mean that we can actually do it in practice.”
Invest in cancer prevention
The study, led by researchers from the World Health Organization’s International Agency for Research on Cancer and partners, provides estimates of premature deaths from 36 cancers across 185 countries.
The findings, published in The Lancet Global Health along with a new Lancet Commission report – “Women, Power, and Cancer” also highlighted the “underrecognized” cancer burden among women around the world.
Cancer ranks in the top three causes of premature mortality among women in almost all countries worldwide, but it is often “deprioritized,” the Lancet Commission report explained.
Of the nearly 5.3 million premature cancer deaths in 2020, 2.9 million occurred in men, and 2.3 million occurred in women, the investigators found. Of the premature deaths among women, 1.5 million could have potentially been avoided through prevention or detection efforts, while the remaining 800,000 might have been averted “if all women everywhere could access optimal cancer care,” the authors said.
Lung cancer was the leading contributor to preventable premature years of life lost in countries that have medium to very high scores on the Human Development Index (HDI), whereas cervical cancer was the leading contributor in low-HDI countries. HDI rankings are based on life expectancy, education, and gross national income.
Among women, as many as 72% of cancer death were premature in low-HDI countries, vs 36% in very high-HDI countries.
Overall, across all four tiers of HDI, colorectal and breast cancers represented the major treatable cancers.
Reducing exposure to four main risk factors – tobacco smoking, alcohol consumption, high body weight, and infections – would go a long way toward reducing potentially preventable premature cancer-related deaths, the authors said.
“Globally, there are marked inequalities between countries in reaching the target of reducing premature mortality from noncommunicable diseases, including cancer,” author Isabelle Soerjomataram, MD, PhD, deputy head of cancer surveillance at the International Agency for Research on Cancer, said in a press release.
“Greater investments in cancer prevention programs can reduce the prevalence of key risk factors for cancer, and increased coverage of vaccination alongside early diagnosis and screening linked to timely treatment can and must address the current cancer inequalities that are seen worldwide,” she added.
Caveats and cautionary notes
The authors acknowledge that the study has limitations related to its methodology and underlying assumptions. For instance, some premature cancer deaths that were classified as preventable may have been averted through curative therapy as well.
The findings also represent a snapshot of premature mortality in 2020 but do not necessarily predict progress in cancer control over time.
In Dr. Chatterjee’s view, this is “an excellent descriptive study that gives a good overall picture about the potential for saving a very large fraction of premature death due to cancer by implementing what is now known about primary and secondary interventions, and treatments.”
However, estimates for the effects of various risk factors and interventions are often derived from observational nonrandomized studies, which can have various types of biases, he said.
“Additionally, availability of data, observational or randomized, are often limited from many countries in Africa, Latin America, and Asia, where the cancer burdens are increasing,” Dr. Chatterjee told this news organization. “Therefore, extrapolating evidence generated mostly from North America and European countries to other understudied settings could be problematic due to difference in background in genetics, environment, socioeconomic, and cultural differences.”
Dr. Meyerowitz-Katz said the issue with this “very complex” article is that it includes “models built upon models, all of which include layers of assumptions that aren’t always obvious and may be wrong.”
On top of that, he said, “there are questions over whether the modifiable risk factors are really modifiable. Can we really get rid of 100% of ‘lack of physical exercise’? What would that even look like?”
Overall, Dr. Meyerowitz-Katz noted, “Yes, some proportion of these cancers could be prevented, and that percentage may be large, but the exact 70% estimate is very uncertain in my opinion.”
The study had no commercial funding. The authors, Dr. Chatterjee, and Dr. Meyerowitz-Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new global analysis highlights the substantial burden of premature deaths from cancer around the world – a burden that could potentially be averted through prevention, early detection, and timely treatment.
According to the analysis, in 2020, over half of all cancer deaths – 5.28 million of 9.96 million – occurred prematurely (before age 70), leading to a loss of roughly 183 million life-years from the disease worldwide.
More than two-thirds of premature cancer-related deaths – 3.6 million, or 68% – were potentially preventable through lifestyle changes or early detection efforts, such as cancer screening, dietary changes, or smoking cessation, and about one-third – 1.65 million, or 31% – may have been treatable.
But two biostatisticians not involved in the study who took a deep dive into it urged caution in interpreting the data.
Nilanjan Chatterjee, PhD, Bloomberg Distinguished Professor, Bloomberg School of Public Health at Johns Hopkins University, Baltimore, said the study does a “great job in bringing a lot of diverse data together to show there is very high potential for preventing premature deaths due to cancer worldwide.”
However, for a variety of reasons, Dr. Chatterjee explained, one should not “overinterpret” the high percentage of potentially preventable cancer deaths.
Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong, in Australia, agreed.
“It’s likely many cancer deaths are, in theory, preventable, but the numbers around just how many are necessarily vague,” said Dr. Meyerowitz-Katz. “Also, ‘in theory preventable’ doesn’t necessarily mean that we can actually do it in practice.”
Invest in cancer prevention
The study, led by researchers from the World Health Organization’s International Agency for Research on Cancer and partners, provides estimates of premature deaths from 36 cancers across 185 countries.
The findings, published in The Lancet Global Health along with a new Lancet Commission report – “Women, Power, and Cancer” also highlighted the “underrecognized” cancer burden among women around the world.
Cancer ranks in the top three causes of premature mortality among women in almost all countries worldwide, but it is often “deprioritized,” the Lancet Commission report explained.
Of the nearly 5.3 million premature cancer deaths in 2020, 2.9 million occurred in men, and 2.3 million occurred in women, the investigators found. Of the premature deaths among women, 1.5 million could have potentially been avoided through prevention or detection efforts, while the remaining 800,000 might have been averted “if all women everywhere could access optimal cancer care,” the authors said.
Lung cancer was the leading contributor to preventable premature years of life lost in countries that have medium to very high scores on the Human Development Index (HDI), whereas cervical cancer was the leading contributor in low-HDI countries. HDI rankings are based on life expectancy, education, and gross national income.
Among women, as many as 72% of cancer death were premature in low-HDI countries, vs 36% in very high-HDI countries.
Overall, across all four tiers of HDI, colorectal and breast cancers represented the major treatable cancers.
Reducing exposure to four main risk factors – tobacco smoking, alcohol consumption, high body weight, and infections – would go a long way toward reducing potentially preventable premature cancer-related deaths, the authors said.
“Globally, there are marked inequalities between countries in reaching the target of reducing premature mortality from noncommunicable diseases, including cancer,” author Isabelle Soerjomataram, MD, PhD, deputy head of cancer surveillance at the International Agency for Research on Cancer, said in a press release.
“Greater investments in cancer prevention programs can reduce the prevalence of key risk factors for cancer, and increased coverage of vaccination alongside early diagnosis and screening linked to timely treatment can and must address the current cancer inequalities that are seen worldwide,” she added.
Caveats and cautionary notes
The authors acknowledge that the study has limitations related to its methodology and underlying assumptions. For instance, some premature cancer deaths that were classified as preventable may have been averted through curative therapy as well.
The findings also represent a snapshot of premature mortality in 2020 but do not necessarily predict progress in cancer control over time.
In Dr. Chatterjee’s view, this is “an excellent descriptive study that gives a good overall picture about the potential for saving a very large fraction of premature death due to cancer by implementing what is now known about primary and secondary interventions, and treatments.”
However, estimates for the effects of various risk factors and interventions are often derived from observational nonrandomized studies, which can have various types of biases, he said.
“Additionally, availability of data, observational or randomized, are often limited from many countries in Africa, Latin America, and Asia, where the cancer burdens are increasing,” Dr. Chatterjee told this news organization. “Therefore, extrapolating evidence generated mostly from North America and European countries to other understudied settings could be problematic due to difference in background in genetics, environment, socioeconomic, and cultural differences.”
Dr. Meyerowitz-Katz said the issue with this “very complex” article is that it includes “models built upon models, all of which include layers of assumptions that aren’t always obvious and may be wrong.”
On top of that, he said, “there are questions over whether the modifiable risk factors are really modifiable. Can we really get rid of 100% of ‘lack of physical exercise’? What would that even look like?”
Overall, Dr. Meyerowitz-Katz noted, “Yes, some proportion of these cancers could be prevented, and that percentage may be large, but the exact 70% estimate is very uncertain in my opinion.”
The study had no commercial funding. The authors, Dr. Chatterjee, and Dr. Meyerowitz-Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new global analysis highlights the substantial burden of premature deaths from cancer around the world – a burden that could potentially be averted through prevention, early detection, and timely treatment.
According to the analysis, in 2020, over half of all cancer deaths – 5.28 million of 9.96 million – occurred prematurely (before age 70), leading to a loss of roughly 183 million life-years from the disease worldwide.
More than two-thirds of premature cancer-related deaths – 3.6 million, or 68% – were potentially preventable through lifestyle changes or early detection efforts, such as cancer screening, dietary changes, or smoking cessation, and about one-third – 1.65 million, or 31% – may have been treatable.
But two biostatisticians not involved in the study who took a deep dive into it urged caution in interpreting the data.
Nilanjan Chatterjee, PhD, Bloomberg Distinguished Professor, Bloomberg School of Public Health at Johns Hopkins University, Baltimore, said the study does a “great job in bringing a lot of diverse data together to show there is very high potential for preventing premature deaths due to cancer worldwide.”
However, for a variety of reasons, Dr. Chatterjee explained, one should not “overinterpret” the high percentage of potentially preventable cancer deaths.
Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong, in Australia, agreed.
“It’s likely many cancer deaths are, in theory, preventable, but the numbers around just how many are necessarily vague,” said Dr. Meyerowitz-Katz. “Also, ‘in theory preventable’ doesn’t necessarily mean that we can actually do it in practice.”
Invest in cancer prevention
The study, led by researchers from the World Health Organization’s International Agency for Research on Cancer and partners, provides estimates of premature deaths from 36 cancers across 185 countries.
The findings, published in The Lancet Global Health along with a new Lancet Commission report – “Women, Power, and Cancer” also highlighted the “underrecognized” cancer burden among women around the world.
Cancer ranks in the top three causes of premature mortality among women in almost all countries worldwide, but it is often “deprioritized,” the Lancet Commission report explained.
Of the nearly 5.3 million premature cancer deaths in 2020, 2.9 million occurred in men, and 2.3 million occurred in women, the investigators found. Of the premature deaths among women, 1.5 million could have potentially been avoided through prevention or detection efforts, while the remaining 800,000 might have been averted “if all women everywhere could access optimal cancer care,” the authors said.
Lung cancer was the leading contributor to preventable premature years of life lost in countries that have medium to very high scores on the Human Development Index (HDI), whereas cervical cancer was the leading contributor in low-HDI countries. HDI rankings are based on life expectancy, education, and gross national income.
Among women, as many as 72% of cancer death were premature in low-HDI countries, vs 36% in very high-HDI countries.
Overall, across all four tiers of HDI, colorectal and breast cancers represented the major treatable cancers.
Reducing exposure to four main risk factors – tobacco smoking, alcohol consumption, high body weight, and infections – would go a long way toward reducing potentially preventable premature cancer-related deaths, the authors said.
“Globally, there are marked inequalities between countries in reaching the target of reducing premature mortality from noncommunicable diseases, including cancer,” author Isabelle Soerjomataram, MD, PhD, deputy head of cancer surveillance at the International Agency for Research on Cancer, said in a press release.
“Greater investments in cancer prevention programs can reduce the prevalence of key risk factors for cancer, and increased coverage of vaccination alongside early diagnosis and screening linked to timely treatment can and must address the current cancer inequalities that are seen worldwide,” she added.
Caveats and cautionary notes
The authors acknowledge that the study has limitations related to its methodology and underlying assumptions. For instance, some premature cancer deaths that were classified as preventable may have been averted through curative therapy as well.
The findings also represent a snapshot of premature mortality in 2020 but do not necessarily predict progress in cancer control over time.
In Dr. Chatterjee’s view, this is “an excellent descriptive study that gives a good overall picture about the potential for saving a very large fraction of premature death due to cancer by implementing what is now known about primary and secondary interventions, and treatments.”
However, estimates for the effects of various risk factors and interventions are often derived from observational nonrandomized studies, which can have various types of biases, he said.
“Additionally, availability of data, observational or randomized, are often limited from many countries in Africa, Latin America, and Asia, where the cancer burdens are increasing,” Dr. Chatterjee told this news organization. “Therefore, extrapolating evidence generated mostly from North America and European countries to other understudied settings could be problematic due to difference in background in genetics, environment, socioeconomic, and cultural differences.”
Dr. Meyerowitz-Katz said the issue with this “very complex” article is that it includes “models built upon models, all of which include layers of assumptions that aren’t always obvious and may be wrong.”
On top of that, he said, “there are questions over whether the modifiable risk factors are really modifiable. Can we really get rid of 100% of ‘lack of physical exercise’? What would that even look like?”
Overall, Dr. Meyerowitz-Katz noted, “Yes, some proportion of these cancers could be prevented, and that percentage may be large, but the exact 70% estimate is very uncertain in my opinion.”
The study had no commercial funding. The authors, Dr. Chatterjee, and Dr. Meyerowitz-Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET GLOBAL HEALTH
Postmenopausal testosterone for low libido only, doctors say
Your patients may see ads claiming that testosterone replacement therapy (TRT) offers postmenopausal women health benefits beyond restored sex drive: that TRT can improve their mood, energy, and thinking and give them stronger bones and bigger muscles.
How accurate are these claims? According to six experts who talked with this news organization, not very.
“Right now in this country and around the world, testosterone’s only use in postmenopausal women is for libido,” said Adrian Sandra Dobs, MD, MHS, professor of medicine and director of the Johns Hopkins Clinical Research Network at Johns Hopkins Medicine, Baltimore.
“Treating postmenopausal women with testosterone is a rarity. Some physicians and some wellness centers make their money out of prescribing estrogen and testosterone to women in patches, gels, creams, capsules, pellets, and other forms. she added by phone.
“One has to be very careful about using testosterone in women,” Dr. Dobs cautioned. “There’s a lot of hype out there.”
Low testosterone in women has not been well studied, and no testosterone treatments for this condition have been approved by the U.S. Food and Drug Administration. Providers need to adjust male treatment data to their female patients, who require significantly lower doses than males. Contraindications and long-term side effects are poorly understood, said Mary Rosser, MD, PhD, assistant professor of women’s health and director of integrated women’s health at Columbia University Irving Medical Center, New York.
“Despite this preponderance of scientific evidence and recommendations, the myths about testosterone die hard, including that it improves women’s muscle function, endurance, and well-being,” Dr. Rosser said.
“Websites that use compounded products or pellets are not FDA-regulated; therefore, they have no responsibility to prove their claims. They can entice women into using this stuff with all kinds of promises about ‘hormone balancing’ and other meaningless terms. The Endocrine Society statement reviewed the clinical studies on testosterone for various indications surrounding physical endurance, well-being, and mental health – and the studies do not support its use,” Dr. Rosser added.
According to the Australasian Menopause Society, women’s blood testosterone levels tend to peak in their 20s, slowly decline to around 25% of peak levels at menopause, then rise again in later years.
Susan Davis, PhD, and her colleagues at Monash University, Melbourne, found in a study that TRT in postmenopausal women may improve sexual well-being and that side effects include acne and increased hair growth. But they found no benefits for cognition, bone mineral density, body composition, muscle strength, or psychological well-being, and they note that more data are needed on long-term safety.
Postmenopausal testosterone recommended for libido only
“Hypoactive sexual desire disorder (HSDD) is really the only indication for postmenopausal testosterone use,” Nanette F. Santoro, MD, professor and chair of obstetrics and gynecology at the University of Colorado School of Medicine, Aurora, noted by email. “In clinical studies using androgen gel containing testosterone, testosterone treatment has resulted in a mean of one more satisfying sexual encounter per month. Consensus statements issued by the Endocrine Society, The International Menopause Society, and the North American Menopause Society have come to similar conclusions: The only indication for androgen therapy for women is HSDD,” added Santoro, an author of the Endocrine Society statement.
“Sexual health and the sense of well-being are very much related,” Sandra Ann Carson, MD, professor of obstetrics and gynecology at Yale Medicine, New Haven, Conn., said by phone. “So we give testosterone to increase sexual desire. Testosterone is not a treatment for decreased sense of well-being alone. Women who lose their sense of well-being due to depression or other factors need to have a mental health evaluation, not testosterone.”
“Because no female product is presently approved by a national regulatory body, male formulations can be judiciously used in female doses and blood testosterone concentrations must be monitored regularly,” Dr. Rosser said. “The recommendation is for considering use of compounded testosterone for hypoactive sexual desire only; it is against use for overall health and wellness.”
“The real mischief occurs when women are exposed to doses that are supraphysiologic,” Dr. Rosser cautioned. “At high doses that approach and sometimes exceed men’s levels of testosterone, women can have deepening of the voice, adverse changes in cholesterol, and even breast atrophy. This can occur with bioidentical compounded testosterone and with testosterone pellets. The National Academies of Science, Engineering, and Medicine recommend unequivocally that such preparations not be used.”
Not all postmenopausal women should take TRT, said Meredith McClure, MD, assistant professor in the department of obstetrics and gynecology of UT Southwestern Medical School, Dallas, because it has only been shown in trials to help with HSDD.
She advised clinicians to avoid prescribing testosterone to patients who “can’t take estrogen, including if [they] have hormone-sensitive cancer, blood clot risk, liver disease, heart attack, stroke, or undiagnosed genital bleeding.”
TRT for non-libido issues may sometimes be appropriate
“Perhaps women with hip fracture or cancer cachexia could benefit from testosterone to build muscle mass,” said Dr. Dobbs, who is involved in an ongoing study of testosterone treatment in women with hip fracture. “But as yet, we have no proof that testosterone helps.”
In rare cases, Stanley G. Korenman, MD, a reproductive endocrinologist and associate dean for ethics at UCLA Health, treats postmenopausal patients with TRT for reasons other than low libido. “I have a very specialized practice in reproductive endocrinology and internal medicine and am one of very few people in the country who do this kind of management,” he said in an interview. “If my postmenopausal patients have low testosterone and lack energy, I’m willing to give them low doses. If they feel more energetic, we continue, but if they don’t, we stop. I don’t think there’s any risk whatsoever at the low level I prescribe.
“I prescribe standard gel that comes in a squirt bottle, and I suggest they take half a squirt every other day – about one-eighth of a male dose – on the sole of the foot, where hair does not grow.
“I would not prescribe testosterone for bone health. We have bisphosphonates and other much better treatments for that. And I would not prescribe it to someone who is seriously emotionally disturbed or seriously depressed. This is not a treatment for depression.”
“Postmenopausal testosterone is not ‘the latest greatest thing,’ but being very low risk, it’s worth trying once in a while, in the appropriate patient, at the right dose,” Dr. Korenman advised. He cautioned people to “avoid the longevity salespeople who sell all sorts of things in all sorts of doses to try to keep us alive forever.”
All contributors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Your patients may see ads claiming that testosterone replacement therapy (TRT) offers postmenopausal women health benefits beyond restored sex drive: that TRT can improve their mood, energy, and thinking and give them stronger bones and bigger muscles.
How accurate are these claims? According to six experts who talked with this news organization, not very.
“Right now in this country and around the world, testosterone’s only use in postmenopausal women is for libido,” said Adrian Sandra Dobs, MD, MHS, professor of medicine and director of the Johns Hopkins Clinical Research Network at Johns Hopkins Medicine, Baltimore.
“Treating postmenopausal women with testosterone is a rarity. Some physicians and some wellness centers make their money out of prescribing estrogen and testosterone to women in patches, gels, creams, capsules, pellets, and other forms. she added by phone.
“One has to be very careful about using testosterone in women,” Dr. Dobs cautioned. “There’s a lot of hype out there.”
Low testosterone in women has not been well studied, and no testosterone treatments for this condition have been approved by the U.S. Food and Drug Administration. Providers need to adjust male treatment data to their female patients, who require significantly lower doses than males. Contraindications and long-term side effects are poorly understood, said Mary Rosser, MD, PhD, assistant professor of women’s health and director of integrated women’s health at Columbia University Irving Medical Center, New York.
“Despite this preponderance of scientific evidence and recommendations, the myths about testosterone die hard, including that it improves women’s muscle function, endurance, and well-being,” Dr. Rosser said.
“Websites that use compounded products or pellets are not FDA-regulated; therefore, they have no responsibility to prove their claims. They can entice women into using this stuff with all kinds of promises about ‘hormone balancing’ and other meaningless terms. The Endocrine Society statement reviewed the clinical studies on testosterone for various indications surrounding physical endurance, well-being, and mental health – and the studies do not support its use,” Dr. Rosser added.
According to the Australasian Menopause Society, women’s blood testosterone levels tend to peak in their 20s, slowly decline to around 25% of peak levels at menopause, then rise again in later years.
Susan Davis, PhD, and her colleagues at Monash University, Melbourne, found in a study that TRT in postmenopausal women may improve sexual well-being and that side effects include acne and increased hair growth. But they found no benefits for cognition, bone mineral density, body composition, muscle strength, or psychological well-being, and they note that more data are needed on long-term safety.
Postmenopausal testosterone recommended for libido only
“Hypoactive sexual desire disorder (HSDD) is really the only indication for postmenopausal testosterone use,” Nanette F. Santoro, MD, professor and chair of obstetrics and gynecology at the University of Colorado School of Medicine, Aurora, noted by email. “In clinical studies using androgen gel containing testosterone, testosterone treatment has resulted in a mean of one more satisfying sexual encounter per month. Consensus statements issued by the Endocrine Society, The International Menopause Society, and the North American Menopause Society have come to similar conclusions: The only indication for androgen therapy for women is HSDD,” added Santoro, an author of the Endocrine Society statement.
“Sexual health and the sense of well-being are very much related,” Sandra Ann Carson, MD, professor of obstetrics and gynecology at Yale Medicine, New Haven, Conn., said by phone. “So we give testosterone to increase sexual desire. Testosterone is not a treatment for decreased sense of well-being alone. Women who lose their sense of well-being due to depression or other factors need to have a mental health evaluation, not testosterone.”
“Because no female product is presently approved by a national regulatory body, male formulations can be judiciously used in female doses and blood testosterone concentrations must be monitored regularly,” Dr. Rosser said. “The recommendation is for considering use of compounded testosterone for hypoactive sexual desire only; it is against use for overall health and wellness.”
“The real mischief occurs when women are exposed to doses that are supraphysiologic,” Dr. Rosser cautioned. “At high doses that approach and sometimes exceed men’s levels of testosterone, women can have deepening of the voice, adverse changes in cholesterol, and even breast atrophy. This can occur with bioidentical compounded testosterone and with testosterone pellets. The National Academies of Science, Engineering, and Medicine recommend unequivocally that such preparations not be used.”
Not all postmenopausal women should take TRT, said Meredith McClure, MD, assistant professor in the department of obstetrics and gynecology of UT Southwestern Medical School, Dallas, because it has only been shown in trials to help with HSDD.
She advised clinicians to avoid prescribing testosterone to patients who “can’t take estrogen, including if [they] have hormone-sensitive cancer, blood clot risk, liver disease, heart attack, stroke, or undiagnosed genital bleeding.”
TRT for non-libido issues may sometimes be appropriate
“Perhaps women with hip fracture or cancer cachexia could benefit from testosterone to build muscle mass,” said Dr. Dobbs, who is involved in an ongoing study of testosterone treatment in women with hip fracture. “But as yet, we have no proof that testosterone helps.”
In rare cases, Stanley G. Korenman, MD, a reproductive endocrinologist and associate dean for ethics at UCLA Health, treats postmenopausal patients with TRT for reasons other than low libido. “I have a very specialized practice in reproductive endocrinology and internal medicine and am one of very few people in the country who do this kind of management,” he said in an interview. “If my postmenopausal patients have low testosterone and lack energy, I’m willing to give them low doses. If they feel more energetic, we continue, but if they don’t, we stop. I don’t think there’s any risk whatsoever at the low level I prescribe.
“I prescribe standard gel that comes in a squirt bottle, and I suggest they take half a squirt every other day – about one-eighth of a male dose – on the sole of the foot, where hair does not grow.
“I would not prescribe testosterone for bone health. We have bisphosphonates and other much better treatments for that. And I would not prescribe it to someone who is seriously emotionally disturbed or seriously depressed. This is not a treatment for depression.”
“Postmenopausal testosterone is not ‘the latest greatest thing,’ but being very low risk, it’s worth trying once in a while, in the appropriate patient, at the right dose,” Dr. Korenman advised. He cautioned people to “avoid the longevity salespeople who sell all sorts of things in all sorts of doses to try to keep us alive forever.”
All contributors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Your patients may see ads claiming that testosterone replacement therapy (TRT) offers postmenopausal women health benefits beyond restored sex drive: that TRT can improve their mood, energy, and thinking and give them stronger bones and bigger muscles.
How accurate are these claims? According to six experts who talked with this news organization, not very.
“Right now in this country and around the world, testosterone’s only use in postmenopausal women is for libido,” said Adrian Sandra Dobs, MD, MHS, professor of medicine and director of the Johns Hopkins Clinical Research Network at Johns Hopkins Medicine, Baltimore.
“Treating postmenopausal women with testosterone is a rarity. Some physicians and some wellness centers make their money out of prescribing estrogen and testosterone to women in patches, gels, creams, capsules, pellets, and other forms. she added by phone.
“One has to be very careful about using testosterone in women,” Dr. Dobs cautioned. “There’s a lot of hype out there.”
Low testosterone in women has not been well studied, and no testosterone treatments for this condition have been approved by the U.S. Food and Drug Administration. Providers need to adjust male treatment data to their female patients, who require significantly lower doses than males. Contraindications and long-term side effects are poorly understood, said Mary Rosser, MD, PhD, assistant professor of women’s health and director of integrated women’s health at Columbia University Irving Medical Center, New York.
“Despite this preponderance of scientific evidence and recommendations, the myths about testosterone die hard, including that it improves women’s muscle function, endurance, and well-being,” Dr. Rosser said.
“Websites that use compounded products or pellets are not FDA-regulated; therefore, they have no responsibility to prove their claims. They can entice women into using this stuff with all kinds of promises about ‘hormone balancing’ and other meaningless terms. The Endocrine Society statement reviewed the clinical studies on testosterone for various indications surrounding physical endurance, well-being, and mental health – and the studies do not support its use,” Dr. Rosser added.
According to the Australasian Menopause Society, women’s blood testosterone levels tend to peak in their 20s, slowly decline to around 25% of peak levels at menopause, then rise again in later years.
Susan Davis, PhD, and her colleagues at Monash University, Melbourne, found in a study that TRT in postmenopausal women may improve sexual well-being and that side effects include acne and increased hair growth. But they found no benefits for cognition, bone mineral density, body composition, muscle strength, or psychological well-being, and they note that more data are needed on long-term safety.
Postmenopausal testosterone recommended for libido only
“Hypoactive sexual desire disorder (HSDD) is really the only indication for postmenopausal testosterone use,” Nanette F. Santoro, MD, professor and chair of obstetrics and gynecology at the University of Colorado School of Medicine, Aurora, noted by email. “In clinical studies using androgen gel containing testosterone, testosterone treatment has resulted in a mean of one more satisfying sexual encounter per month. Consensus statements issued by the Endocrine Society, The International Menopause Society, and the North American Menopause Society have come to similar conclusions: The only indication for androgen therapy for women is HSDD,” added Santoro, an author of the Endocrine Society statement.
“Sexual health and the sense of well-being are very much related,” Sandra Ann Carson, MD, professor of obstetrics and gynecology at Yale Medicine, New Haven, Conn., said by phone. “So we give testosterone to increase sexual desire. Testosterone is not a treatment for decreased sense of well-being alone. Women who lose their sense of well-being due to depression or other factors need to have a mental health evaluation, not testosterone.”
“Because no female product is presently approved by a national regulatory body, male formulations can be judiciously used in female doses and blood testosterone concentrations must be monitored regularly,” Dr. Rosser said. “The recommendation is for considering use of compounded testosterone for hypoactive sexual desire only; it is against use for overall health and wellness.”
“The real mischief occurs when women are exposed to doses that are supraphysiologic,” Dr. Rosser cautioned. “At high doses that approach and sometimes exceed men’s levels of testosterone, women can have deepening of the voice, adverse changes in cholesterol, and even breast atrophy. This can occur with bioidentical compounded testosterone and with testosterone pellets. The National Academies of Science, Engineering, and Medicine recommend unequivocally that such preparations not be used.”
Not all postmenopausal women should take TRT, said Meredith McClure, MD, assistant professor in the department of obstetrics and gynecology of UT Southwestern Medical School, Dallas, because it has only been shown in trials to help with HSDD.
She advised clinicians to avoid prescribing testosterone to patients who “can’t take estrogen, including if [they] have hormone-sensitive cancer, blood clot risk, liver disease, heart attack, stroke, or undiagnosed genital bleeding.”
TRT for non-libido issues may sometimes be appropriate
“Perhaps women with hip fracture or cancer cachexia could benefit from testosterone to build muscle mass,” said Dr. Dobbs, who is involved in an ongoing study of testosterone treatment in women with hip fracture. “But as yet, we have no proof that testosterone helps.”
In rare cases, Stanley G. Korenman, MD, a reproductive endocrinologist and associate dean for ethics at UCLA Health, treats postmenopausal patients with TRT for reasons other than low libido. “I have a very specialized practice in reproductive endocrinology and internal medicine and am one of very few people in the country who do this kind of management,” he said in an interview. “If my postmenopausal patients have low testosterone and lack energy, I’m willing to give them low doses. If they feel more energetic, we continue, but if they don’t, we stop. I don’t think there’s any risk whatsoever at the low level I prescribe.
“I prescribe standard gel that comes in a squirt bottle, and I suggest they take half a squirt every other day – about one-eighth of a male dose – on the sole of the foot, where hair does not grow.
“I would not prescribe testosterone for bone health. We have bisphosphonates and other much better treatments for that. And I would not prescribe it to someone who is seriously emotionally disturbed or seriously depressed. This is not a treatment for depression.”
“Postmenopausal testosterone is not ‘the latest greatest thing,’ but being very low risk, it’s worth trying once in a while, in the appropriate patient, at the right dose,” Dr. Korenman advised. He cautioned people to “avoid the longevity salespeople who sell all sorts of things in all sorts of doses to try to keep us alive forever.”
All contributors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clustered Vesicles on the Neck
The Diagnosis: Microcystic Lymphatic Malformation
A punch biopsy demonstrated anastomosing fluidfilled spaces within the papillary and reticular dermal layers (Figure), confirming the diagnosis of microcystic lymphatic malformation (LM)(formerly known as lymphangioma circumscriptum), a congenital vascular malformation composed of slow-flow lymphatic channels.1 The patient underwent serial excisions with improvement of the LM, though the treatment course was complicated by hypertrophic scar formation.
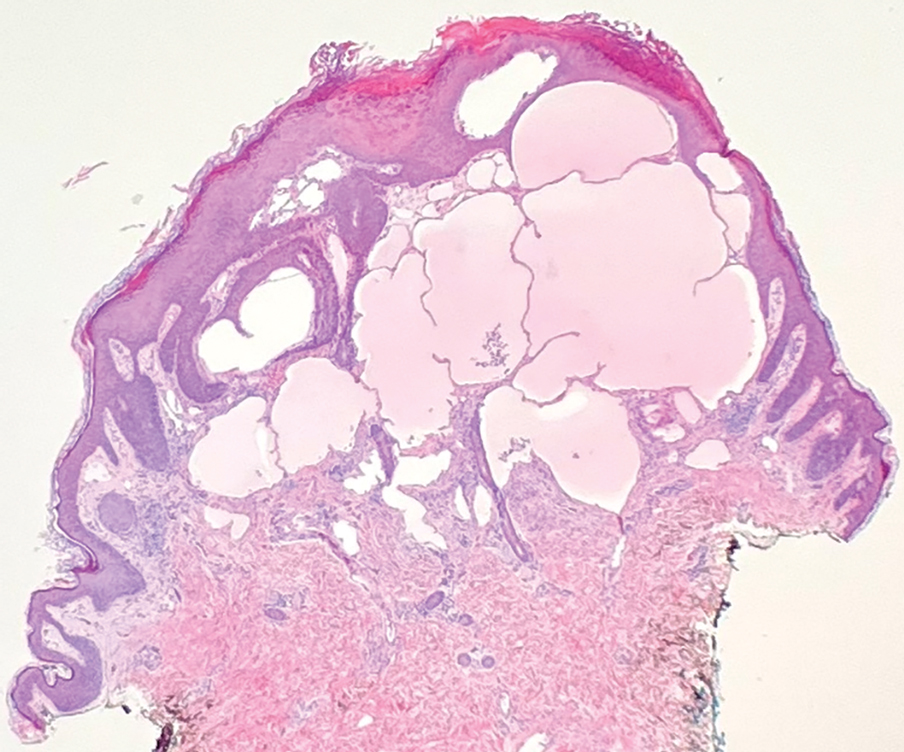
The classic clinical presentation of microcystic LM includes a crop of vesicles containing clear or hemorrhagic fluid with associated oozing or bleeding.2 When cutaneous lesions resembling microcystic LM develop in response to lymphatic damage and resulting stasis, such as from prior radiotherapy or surgery, the term lymphangiectasia is used to distinguish this entity from congenital microcystic LM.3
Microcystic LMs are histologically indistinguishable from macrocystic LMs; however, macrocystic LMs typically are clinically evident at birth as ill-defined subcutaneous masses.2,4-6 Dermatitis herpetiformis, a dermatologic manifestation of gluten sensitivity, causes intensely pruritic vesicles in a symmetric distribution on the elbows, knees, and buttocks. Histopathology shows neutrophilic microabscesses in the dermal papillae with subepidermal blistering. Direct immunofluorescence demonstrates the deposition of IgA along the basement membrane with dermal papillae aggregates.6 The underlying dermis also may contain a lymphohistiocytic infiltrate rich in neutrophils. The vesicles of herpes zoster virus are painful and present in a dermatomal distribution. A viral cytopathic effect often is observed in keratinocytes, specifically with multinucleation, molding, and margination of chromatin material. The lesions are accompanied by variable lymphocytic inflammation and epithelial necrosis resulting in intraepidermal blistering.7 Extragenital lichen sclerosus presents as polygonal white papules merging to form plaques and may include hemorrhagic blisters in some instances. Histopathology shows hyperkeratosis, epidermal atrophy with flattened rete ridges, vacuolar interface changes, loss of elastic fibers, and hyalinization of the lamina propria with lymphocytic infiltrate.8
Endothelial cells in LM exhibit activating mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PIK3CA, which may lead to proliferation and overgrowth of the lymphatic vasculature, as well as increased production of cyclic guanosine monophosphate.9,10 Phosphodiesterase 5 (PDE5) is expressed in the perivascular smooth muscle adjacent to lymphatic spaces in LMs but not in the their vasculature. 10 This pattern of PDE5 expression may cause perilesional vasculature to constrict, preventing lymphatic fluid from draining into the veins.11 It is theorized that the PDE5 inhibitor sildenafil leads to relaxation of the vasculature adjacent to LMs, allowing the outflow of the accumulated lymphatic fluid and thus decompression.11-13
Management of LM should not only take into account the depth and location of involvement but also any associated symptoms or complications, such as pruritus, pain, bleeding, or secondary infections. Magnetic resonance imaging (MRI) typically has been considered the gold standard for determining the size and depth of involvement of the malformation.1,3,4 However, ultrasonography with Doppler flow may be considered an initial diagnostic and screening test, as it can distinguish between macrocystic and microcystic components and provide superior images of microcystic lesions, which are below the resolution capacity of MRI.4 Notably, our patient’s LM was undetectable on ultrasonography and was found to be largely superficial in nature on MRI.
Serial excision of the microcystic LM was conducted in our patient, but there currently is no consensus on optimal treatment of LM, and many treatment options are complicated by high recurrence rates or complications.5 Procedural approaches may include excision, cryotherapy, radiotherapy, sclerotherapy, or laser therapy, while pharmacologic approaches may include sildenafil for its inhibition of PDE5 or sirolimus (oral or topical) for its inhibition of mammalian target of rapamycin.5,12-14 Because recurrence is highly likely, patients may require repeat treatments or a combination approach to therapy.1,5 The development of targeted therapies may lead to a shift in management of LMs in the future, as successful use of the PIK3CA inhibitor alpelisib recently has been reported to lead to clinical improvement of PIK3CA-related LMs, including in patients with PIK3CA-related overgrowth syndromes.15
- Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353-374. doi:10.1016/j.jaad.2006.05.069
- Alrashdan MS, Hammad HM, Alzumaili BAI, et al. Lymphangioma circumscriptum of the tongue: a case with marked hemorrhagic component. J Cutan Pathol. 2018;45:278-281. doi:10.1111/cup.13101
- Osborne GE, Chinn RJ, Francis ND, et al. Magnetic resonance imaging in the investigation of penile lymphangioma circumscriptum. Br J Dermatol. 2000;143:467-468. doi:10.1046/j.1365-2133.2000.03695.x
- Davies D, Rogers M, Lam A, et al. Localized microcystic lymphatic malformations—ultrasound diagnosis. Pediatr Dermatol. 1999;16: 423-429. doi:10.1046/j.1525-1470.1999.00110.x
- García-Montero P, Del Boz J, Baselga-Torres E, et al. Use of topical rapamycin in the treatment of superficial lymphatic malformations. J Am Acad Dermatol. 2019;80:508-515. doi:10.1016/j.jaad.2018.09.050
- Clarindo MV, Possebon AT, Soligo EM, et al. Dermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865-875; quiz 876-877. doi:10.1590/abd1806-4841.20142966
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Shiver M, Papasakelariou C, Brown JA, et al. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31:383-385. doi:10.1111 /pde.12025
- Blesinger H, Kaulfuß S, Aung T, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations [published online July 9, 2018]. PLoS One. 2018;13:E0200343. doi:10.1371/journal.pone.0200343
- Green JS, Prok L, Bruckner AL. Expression of phosphodiesterase-5 in lymphatic malformation tissue. JAMA Dermatol. 2014;150:455-456. doi:10.1001/jamadermatol.2013.7002
- Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366:384-386. doi:10.1056 /NEJMc1112482
- Tu JH, Tafoya E, Jeng M, et al. Long-term follow-up of lymphatic malformations in children treated with sildenafil. Pediatr Dermatol. 2017;34:559-565. doi:10.1111/pde.13237
- Maruani A, Tavernier E, Boccara O, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021;157:1289-1298. doi:10.1001/jamadermatol.2021.3459
- Delestre F, Venot Q, Bayard C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. 2021;13:eabg0809. doi:10.1126/scitranslmed .abg0809
- Garreta Fontelles G, Pardo Pastor J, Grande Moreillo C. Alpelisib to treat CLOVES syndrome, a member of the PIK3CA-related overgrowth syndrome spectrum [published online February 21, 2022]. Br J Clin Pharmacol. 2022;88:3891-3895. doi:10.1111/bcp.15270
The Diagnosis: Microcystic Lymphatic Malformation
A punch biopsy demonstrated anastomosing fluidfilled spaces within the papillary and reticular dermal layers (Figure), confirming the diagnosis of microcystic lymphatic malformation (LM)(formerly known as lymphangioma circumscriptum), a congenital vascular malformation composed of slow-flow lymphatic channels.1 The patient underwent serial excisions with improvement of the LM, though the treatment course was complicated by hypertrophic scar formation.

The classic clinical presentation of microcystic LM includes a crop of vesicles containing clear or hemorrhagic fluid with associated oozing or bleeding.2 When cutaneous lesions resembling microcystic LM develop in response to lymphatic damage and resulting stasis, such as from prior radiotherapy or surgery, the term lymphangiectasia is used to distinguish this entity from congenital microcystic LM.3
Microcystic LMs are histologically indistinguishable from macrocystic LMs; however, macrocystic LMs typically are clinically evident at birth as ill-defined subcutaneous masses.2,4-6 Dermatitis herpetiformis, a dermatologic manifestation of gluten sensitivity, causes intensely pruritic vesicles in a symmetric distribution on the elbows, knees, and buttocks. Histopathology shows neutrophilic microabscesses in the dermal papillae with subepidermal blistering. Direct immunofluorescence demonstrates the deposition of IgA along the basement membrane with dermal papillae aggregates.6 The underlying dermis also may contain a lymphohistiocytic infiltrate rich in neutrophils. The vesicles of herpes zoster virus are painful and present in a dermatomal distribution. A viral cytopathic effect often is observed in keratinocytes, specifically with multinucleation, molding, and margination of chromatin material. The lesions are accompanied by variable lymphocytic inflammation and epithelial necrosis resulting in intraepidermal blistering.7 Extragenital lichen sclerosus presents as polygonal white papules merging to form plaques and may include hemorrhagic blisters in some instances. Histopathology shows hyperkeratosis, epidermal atrophy with flattened rete ridges, vacuolar interface changes, loss of elastic fibers, and hyalinization of the lamina propria with lymphocytic infiltrate.8
Endothelial cells in LM exhibit activating mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PIK3CA, which may lead to proliferation and overgrowth of the lymphatic vasculature, as well as increased production of cyclic guanosine monophosphate.9,10 Phosphodiesterase 5 (PDE5) is expressed in the perivascular smooth muscle adjacent to lymphatic spaces in LMs but not in the their vasculature. 10 This pattern of PDE5 expression may cause perilesional vasculature to constrict, preventing lymphatic fluid from draining into the veins.11 It is theorized that the PDE5 inhibitor sildenafil leads to relaxation of the vasculature adjacent to LMs, allowing the outflow of the accumulated lymphatic fluid and thus decompression.11-13
Management of LM should not only take into account the depth and location of involvement but also any associated symptoms or complications, such as pruritus, pain, bleeding, or secondary infections. Magnetic resonance imaging (MRI) typically has been considered the gold standard for determining the size and depth of involvement of the malformation.1,3,4 However, ultrasonography with Doppler flow may be considered an initial diagnostic and screening test, as it can distinguish between macrocystic and microcystic components and provide superior images of microcystic lesions, which are below the resolution capacity of MRI.4 Notably, our patient’s LM was undetectable on ultrasonography and was found to be largely superficial in nature on MRI.
Serial excision of the microcystic LM was conducted in our patient, but there currently is no consensus on optimal treatment of LM, and many treatment options are complicated by high recurrence rates or complications.5 Procedural approaches may include excision, cryotherapy, radiotherapy, sclerotherapy, or laser therapy, while pharmacologic approaches may include sildenafil for its inhibition of PDE5 or sirolimus (oral or topical) for its inhibition of mammalian target of rapamycin.5,12-14 Because recurrence is highly likely, patients may require repeat treatments or a combination approach to therapy.1,5 The development of targeted therapies may lead to a shift in management of LMs in the future, as successful use of the PIK3CA inhibitor alpelisib recently has been reported to lead to clinical improvement of PIK3CA-related LMs, including in patients with PIK3CA-related overgrowth syndromes.15
The Diagnosis: Microcystic Lymphatic Malformation
A punch biopsy demonstrated anastomosing fluidfilled spaces within the papillary and reticular dermal layers (Figure), confirming the diagnosis of microcystic lymphatic malformation (LM)(formerly known as lymphangioma circumscriptum), a congenital vascular malformation composed of slow-flow lymphatic channels.1 The patient underwent serial excisions with improvement of the LM, though the treatment course was complicated by hypertrophic scar formation.

The classic clinical presentation of microcystic LM includes a crop of vesicles containing clear or hemorrhagic fluid with associated oozing or bleeding.2 When cutaneous lesions resembling microcystic LM develop in response to lymphatic damage and resulting stasis, such as from prior radiotherapy or surgery, the term lymphangiectasia is used to distinguish this entity from congenital microcystic LM.3
Microcystic LMs are histologically indistinguishable from macrocystic LMs; however, macrocystic LMs typically are clinically evident at birth as ill-defined subcutaneous masses.2,4-6 Dermatitis herpetiformis, a dermatologic manifestation of gluten sensitivity, causes intensely pruritic vesicles in a symmetric distribution on the elbows, knees, and buttocks. Histopathology shows neutrophilic microabscesses in the dermal papillae with subepidermal blistering. Direct immunofluorescence demonstrates the deposition of IgA along the basement membrane with dermal papillae aggregates.6 The underlying dermis also may contain a lymphohistiocytic infiltrate rich in neutrophils. The vesicles of herpes zoster virus are painful and present in a dermatomal distribution. A viral cytopathic effect often is observed in keratinocytes, specifically with multinucleation, molding, and margination of chromatin material. The lesions are accompanied by variable lymphocytic inflammation and epithelial necrosis resulting in intraepidermal blistering.7 Extragenital lichen sclerosus presents as polygonal white papules merging to form plaques and may include hemorrhagic blisters in some instances. Histopathology shows hyperkeratosis, epidermal atrophy with flattened rete ridges, vacuolar interface changes, loss of elastic fibers, and hyalinization of the lamina propria with lymphocytic infiltrate.8
Endothelial cells in LM exhibit activating mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PIK3CA, which may lead to proliferation and overgrowth of the lymphatic vasculature, as well as increased production of cyclic guanosine monophosphate.9,10 Phosphodiesterase 5 (PDE5) is expressed in the perivascular smooth muscle adjacent to lymphatic spaces in LMs but not in the their vasculature. 10 This pattern of PDE5 expression may cause perilesional vasculature to constrict, preventing lymphatic fluid from draining into the veins.11 It is theorized that the PDE5 inhibitor sildenafil leads to relaxation of the vasculature adjacent to LMs, allowing the outflow of the accumulated lymphatic fluid and thus decompression.11-13
Management of LM should not only take into account the depth and location of involvement but also any associated symptoms or complications, such as pruritus, pain, bleeding, or secondary infections. Magnetic resonance imaging (MRI) typically has been considered the gold standard for determining the size and depth of involvement of the malformation.1,3,4 However, ultrasonography with Doppler flow may be considered an initial diagnostic and screening test, as it can distinguish between macrocystic and microcystic components and provide superior images of microcystic lesions, which are below the resolution capacity of MRI.4 Notably, our patient’s LM was undetectable on ultrasonography and was found to be largely superficial in nature on MRI.
Serial excision of the microcystic LM was conducted in our patient, but there currently is no consensus on optimal treatment of LM, and many treatment options are complicated by high recurrence rates or complications.5 Procedural approaches may include excision, cryotherapy, radiotherapy, sclerotherapy, or laser therapy, while pharmacologic approaches may include sildenafil for its inhibition of PDE5 or sirolimus (oral or topical) for its inhibition of mammalian target of rapamycin.5,12-14 Because recurrence is highly likely, patients may require repeat treatments or a combination approach to therapy.1,5 The development of targeted therapies may lead to a shift in management of LMs in the future, as successful use of the PIK3CA inhibitor alpelisib recently has been reported to lead to clinical improvement of PIK3CA-related LMs, including in patients with PIK3CA-related overgrowth syndromes.15
- Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353-374. doi:10.1016/j.jaad.2006.05.069
- Alrashdan MS, Hammad HM, Alzumaili BAI, et al. Lymphangioma circumscriptum of the tongue: a case with marked hemorrhagic component. J Cutan Pathol. 2018;45:278-281. doi:10.1111/cup.13101
- Osborne GE, Chinn RJ, Francis ND, et al. Magnetic resonance imaging in the investigation of penile lymphangioma circumscriptum. Br J Dermatol. 2000;143:467-468. doi:10.1046/j.1365-2133.2000.03695.x
- Davies D, Rogers M, Lam A, et al. Localized microcystic lymphatic malformations—ultrasound diagnosis. Pediatr Dermatol. 1999;16: 423-429. doi:10.1046/j.1525-1470.1999.00110.x
- García-Montero P, Del Boz J, Baselga-Torres E, et al. Use of topical rapamycin in the treatment of superficial lymphatic malformations. J Am Acad Dermatol. 2019;80:508-515. doi:10.1016/j.jaad.2018.09.050
- Clarindo MV, Possebon AT, Soligo EM, et al. Dermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865-875; quiz 876-877. doi:10.1590/abd1806-4841.20142966
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Shiver M, Papasakelariou C, Brown JA, et al. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31:383-385. doi:10.1111 /pde.12025
- Blesinger H, Kaulfuß S, Aung T, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations [published online July 9, 2018]. PLoS One. 2018;13:E0200343. doi:10.1371/journal.pone.0200343
- Green JS, Prok L, Bruckner AL. Expression of phosphodiesterase-5 in lymphatic malformation tissue. JAMA Dermatol. 2014;150:455-456. doi:10.1001/jamadermatol.2013.7002
- Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366:384-386. doi:10.1056 /NEJMc1112482
- Tu JH, Tafoya E, Jeng M, et al. Long-term follow-up of lymphatic malformations in children treated with sildenafil. Pediatr Dermatol. 2017;34:559-565. doi:10.1111/pde.13237
- Maruani A, Tavernier E, Boccara O, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021;157:1289-1298. doi:10.1001/jamadermatol.2021.3459
- Delestre F, Venot Q, Bayard C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. 2021;13:eabg0809. doi:10.1126/scitranslmed .abg0809
- Garreta Fontelles G, Pardo Pastor J, Grande Moreillo C. Alpelisib to treat CLOVES syndrome, a member of the PIK3CA-related overgrowth syndrome spectrum [published online February 21, 2022]. Br J Clin Pharmacol. 2022;88:3891-3895. doi:10.1111/bcp.15270
- Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353-374. doi:10.1016/j.jaad.2006.05.069
- Alrashdan MS, Hammad HM, Alzumaili BAI, et al. Lymphangioma circumscriptum of the tongue: a case with marked hemorrhagic component. J Cutan Pathol. 2018;45:278-281. doi:10.1111/cup.13101
- Osborne GE, Chinn RJ, Francis ND, et al. Magnetic resonance imaging in the investigation of penile lymphangioma circumscriptum. Br J Dermatol. 2000;143:467-468. doi:10.1046/j.1365-2133.2000.03695.x
- Davies D, Rogers M, Lam A, et al. Localized microcystic lymphatic malformations—ultrasound diagnosis. Pediatr Dermatol. 1999;16: 423-429. doi:10.1046/j.1525-1470.1999.00110.x
- García-Montero P, Del Boz J, Baselga-Torres E, et al. Use of topical rapamycin in the treatment of superficial lymphatic malformations. J Am Acad Dermatol. 2019;80:508-515. doi:10.1016/j.jaad.2018.09.050
- Clarindo MV, Possebon AT, Soligo EM, et al. Dermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865-875; quiz 876-877. doi:10.1590/abd1806-4841.20142966
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Shiver M, Papasakelariou C, Brown JA, et al. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31:383-385. doi:10.1111 /pde.12025
- Blesinger H, Kaulfuß S, Aung T, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations [published online July 9, 2018]. PLoS One. 2018;13:E0200343. doi:10.1371/journal.pone.0200343
- Green JS, Prok L, Bruckner AL. Expression of phosphodiesterase-5 in lymphatic malformation tissue. JAMA Dermatol. 2014;150:455-456. doi:10.1001/jamadermatol.2013.7002
- Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366:384-386. doi:10.1056 /NEJMc1112482
- Tu JH, Tafoya E, Jeng M, et al. Long-term follow-up of lymphatic malformations in children treated with sildenafil. Pediatr Dermatol. 2017;34:559-565. doi:10.1111/pde.13237
- Maruani A, Tavernier E, Boccara O, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021;157:1289-1298. doi:10.1001/jamadermatol.2021.3459
- Delestre F, Venot Q, Bayard C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. 2021;13:eabg0809. doi:10.1126/scitranslmed .abg0809
- Garreta Fontelles G, Pardo Pastor J, Grande Moreillo C. Alpelisib to treat CLOVES syndrome, a member of the PIK3CA-related overgrowth syndrome spectrum [published online February 21, 2022]. Br J Clin Pharmacol. 2022;88:3891-3895. doi:10.1111/bcp.15270
A 6-year-old girl presented to the dermatology clinic with a rash on the right side of the neck that was noted at birth as a small raised lesion but slowly increased over time in size and number of lesions. She reported pruritus and irritation, particularly when rubbed or scratched. There was no family history of similar skin abnormalities. Her medical history was notable for a left-sided cholesteatoma on tympanomastoidectomy. Physical examination revealed clustered vesicles on the right side of the neck with underlying erythema. The vesicles contained mostly clear fluid with a few focal areas of hemorrhagic fluid. Ultrasonography was unremarkable, and magnetic resonance imaging revealed superficial T2 hyperintense nonenhancing cutaneous and subcutaneous lesions overlying the right lateral neck with minimal extension into the superficial right supraclavicular soft tissues.
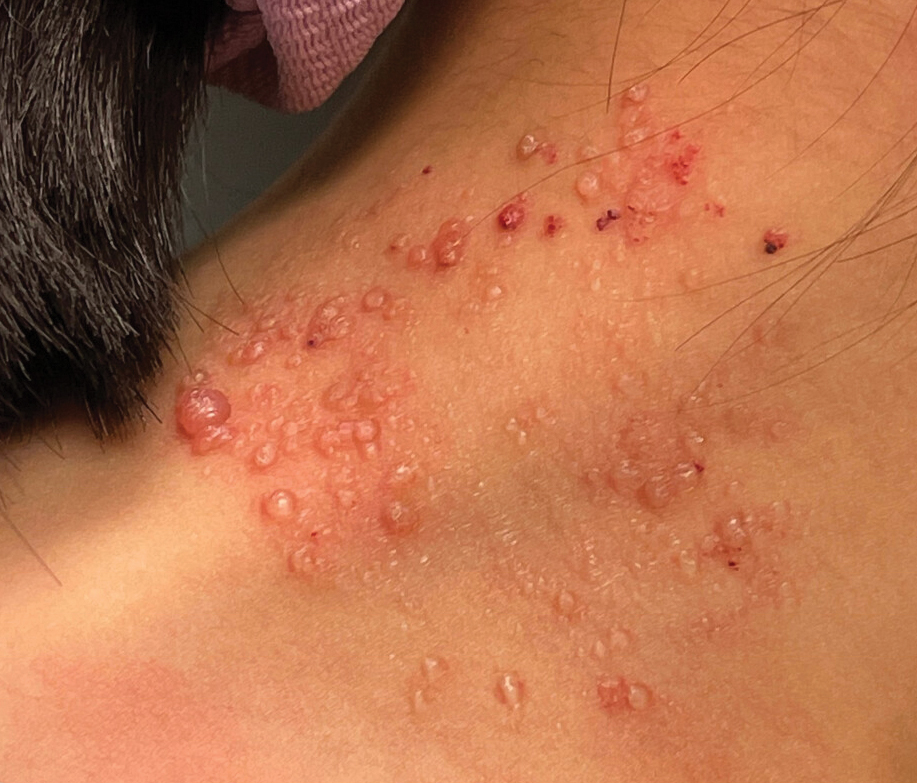
Endoscopic sinus surgery for chronic rhinosinusitis has no impact on comorbid asthma
Endoscopic sinus surgery (ESS) has no significant impact on asthma symptoms for patients with chronic rhinosinusitis up to a year after the procedure, a study of 64 patients shows.
, Anyull Dayanna Bohórquez Caballero said in a presentation at the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) 2023 annual meeting.
The study “offers a unique approach to explore the effects of endoscopic sinus surgery in a real-world context, with valuable insights that differ from previous research,” Dr. Bohórquez Caballero, an international medical graduate and research fellow of the Mayo Clinic, Jacksonville, Fla., said in an interview.
Under the leadership of senior author Angela Donaldson, MD, Dr. Bohórquez Caballero and colleagues at the Mayo Clinic in Jacksonville analyzed data from 185 adults with both asthma and chronic rhinosinusitis who underwent ESS at the clinic between 2013 and 2023. Asthma severity was evaluated up to 3 months before and 1 year after surgery. Patients’ asthma severity was classified as mild, moderate, or severe on the basis of current Global Initiative for Asthma guidelines using medication requirements.
The final study population included 64 patients; 42 of these (66.7%) had chronic rhinosinusitis with nasal polyps. Outcomes included differences in asthma severity, asthma medication doses, and the number of medications.
Overall, there was no significant difference in measures of mild, moderate, or severe asthma before and after ESS in a McNemar paired test (P values: .130, .999, and .288, respectively). Similarly, no difference was found before and after ESS in terms of total inhaled corticosteroid dose (P = .999), number of medications prescribed (P = .157), or control of the disease (P = .078).
The findings were limited by the relatively small number of patients. The study is the first known to assess the real-world impact of ESS on asthma severity, said Bohórquez Caballero.
Expected reduction in asthma severity not seen
Past studies have suggested that ESS improves parameters such as pulmonary function test results or sinonasal outcomes, Dr. Bohórquez Caballero told this news organization. “Our findings indicate that ESS does not significantly impact asthma severity or trends in treatment, including the number and/or dose of medications, in everyday practice.
Our study also identified crucial opportunities to reinforce interdisciplinary follow-up after ESS,” she noted, and it provides a comprehensive depiction of the outcomes experienced by patients with chronic rhinosinusitis and asthma who undergo ESS.
“We were expecting a reduction in severity or a decrease in the dose of inhaled corticosteroid therapies, and we expected to see a translation from previous evidence into clinical practice; however, we did not,” said Dr. Bohórquez Caballero.
“The take-home message is that while there is a strong correlation between CRS and asthma, it does not appear that ESS alone improves real-world treatment based on asthma severity,” she said. “However, our findings have shown that patients may experience a longer period without the need for a reliever medication in the early postoperative period.”
Looking ahead, “We want to explore what happens 5 or 6 months after sinus surgery that would explain the sudden need for a reliever medication,” she added. “Future studies are warranted to investigate the long-term effects of ESS on asthma severity as it relates to modifications of asthma regimens.”
Data important for patient discussions
The current study is important because of the frequency of comorbid asthma among patients with chronic rhinosinusitis, Megan Durr, MD, of the University of California, San Francisco, said in an interview.
“When we are considering functional endoscopy sinus surgery with patients, we are often asked if the surgery will impact the severity of their asthma symptoms,” said Dr. Durr, who served as a moderator for the session in which the study was presented.
“I am surprised the study did not see any difference in asthma severity after sinus surgery, as we often talk to patients about the unified airway that refers to the shared epidemiologic and pathophysiologic relationship between the upper and lower airways,” she told this news organization.
“This study will allow us to have a more informed evidenced-based discussion with patients and their primary care providers and/or pulmonologists” about what to expect for asthma outcomes following surgery, she said.
The study received no outside funding. Dr. Durr has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Endoscopic sinus surgery (ESS) has no significant impact on asthma symptoms for patients with chronic rhinosinusitis up to a year after the procedure, a study of 64 patients shows.
, Anyull Dayanna Bohórquez Caballero said in a presentation at the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) 2023 annual meeting.
The study “offers a unique approach to explore the effects of endoscopic sinus surgery in a real-world context, with valuable insights that differ from previous research,” Dr. Bohórquez Caballero, an international medical graduate and research fellow of the Mayo Clinic, Jacksonville, Fla., said in an interview.
Under the leadership of senior author Angela Donaldson, MD, Dr. Bohórquez Caballero and colleagues at the Mayo Clinic in Jacksonville analyzed data from 185 adults with both asthma and chronic rhinosinusitis who underwent ESS at the clinic between 2013 and 2023. Asthma severity was evaluated up to 3 months before and 1 year after surgery. Patients’ asthma severity was classified as mild, moderate, or severe on the basis of current Global Initiative for Asthma guidelines using medication requirements.
The final study population included 64 patients; 42 of these (66.7%) had chronic rhinosinusitis with nasal polyps. Outcomes included differences in asthma severity, asthma medication doses, and the number of medications.
Overall, there was no significant difference in measures of mild, moderate, or severe asthma before and after ESS in a McNemar paired test (P values: .130, .999, and .288, respectively). Similarly, no difference was found before and after ESS in terms of total inhaled corticosteroid dose (P = .999), number of medications prescribed (P = .157), or control of the disease (P = .078).
The findings were limited by the relatively small number of patients. The study is the first known to assess the real-world impact of ESS on asthma severity, said Bohórquez Caballero.
Expected reduction in asthma severity not seen
Past studies have suggested that ESS improves parameters such as pulmonary function test results or sinonasal outcomes, Dr. Bohórquez Caballero told this news organization. “Our findings indicate that ESS does not significantly impact asthma severity or trends in treatment, including the number and/or dose of medications, in everyday practice.
Our study also identified crucial opportunities to reinforce interdisciplinary follow-up after ESS,” she noted, and it provides a comprehensive depiction of the outcomes experienced by patients with chronic rhinosinusitis and asthma who undergo ESS.
“We were expecting a reduction in severity or a decrease in the dose of inhaled corticosteroid therapies, and we expected to see a translation from previous evidence into clinical practice; however, we did not,” said Dr. Bohórquez Caballero.
“The take-home message is that while there is a strong correlation between CRS and asthma, it does not appear that ESS alone improves real-world treatment based on asthma severity,” she said. “However, our findings have shown that patients may experience a longer period without the need for a reliever medication in the early postoperative period.”
Looking ahead, “We want to explore what happens 5 or 6 months after sinus surgery that would explain the sudden need for a reliever medication,” she added. “Future studies are warranted to investigate the long-term effects of ESS on asthma severity as it relates to modifications of asthma regimens.”
Data important for patient discussions
The current study is important because of the frequency of comorbid asthma among patients with chronic rhinosinusitis, Megan Durr, MD, of the University of California, San Francisco, said in an interview.
“When we are considering functional endoscopy sinus surgery with patients, we are often asked if the surgery will impact the severity of their asthma symptoms,” said Dr. Durr, who served as a moderator for the session in which the study was presented.
“I am surprised the study did not see any difference in asthma severity after sinus surgery, as we often talk to patients about the unified airway that refers to the shared epidemiologic and pathophysiologic relationship between the upper and lower airways,” she told this news organization.
“This study will allow us to have a more informed evidenced-based discussion with patients and their primary care providers and/or pulmonologists” about what to expect for asthma outcomes following surgery, she said.
The study received no outside funding. Dr. Durr has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Endoscopic sinus surgery (ESS) has no significant impact on asthma symptoms for patients with chronic rhinosinusitis up to a year after the procedure, a study of 64 patients shows.
, Anyull Dayanna Bohórquez Caballero said in a presentation at the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) 2023 annual meeting.
The study “offers a unique approach to explore the effects of endoscopic sinus surgery in a real-world context, with valuable insights that differ from previous research,” Dr. Bohórquez Caballero, an international medical graduate and research fellow of the Mayo Clinic, Jacksonville, Fla., said in an interview.
Under the leadership of senior author Angela Donaldson, MD, Dr. Bohórquez Caballero and colleagues at the Mayo Clinic in Jacksonville analyzed data from 185 adults with both asthma and chronic rhinosinusitis who underwent ESS at the clinic between 2013 and 2023. Asthma severity was evaluated up to 3 months before and 1 year after surgery. Patients’ asthma severity was classified as mild, moderate, or severe on the basis of current Global Initiative for Asthma guidelines using medication requirements.
The final study population included 64 patients; 42 of these (66.7%) had chronic rhinosinusitis with nasal polyps. Outcomes included differences in asthma severity, asthma medication doses, and the number of medications.
Overall, there was no significant difference in measures of mild, moderate, or severe asthma before and after ESS in a McNemar paired test (P values: .130, .999, and .288, respectively). Similarly, no difference was found before and after ESS in terms of total inhaled corticosteroid dose (P = .999), number of medications prescribed (P = .157), or control of the disease (P = .078).
The findings were limited by the relatively small number of patients. The study is the first known to assess the real-world impact of ESS on asthma severity, said Bohórquez Caballero.
Expected reduction in asthma severity not seen
Past studies have suggested that ESS improves parameters such as pulmonary function test results or sinonasal outcomes, Dr. Bohórquez Caballero told this news organization. “Our findings indicate that ESS does not significantly impact asthma severity or trends in treatment, including the number and/or dose of medications, in everyday practice.
Our study also identified crucial opportunities to reinforce interdisciplinary follow-up after ESS,” she noted, and it provides a comprehensive depiction of the outcomes experienced by patients with chronic rhinosinusitis and asthma who undergo ESS.
“We were expecting a reduction in severity or a decrease in the dose of inhaled corticosteroid therapies, and we expected to see a translation from previous evidence into clinical practice; however, we did not,” said Dr. Bohórquez Caballero.
“The take-home message is that while there is a strong correlation between CRS and asthma, it does not appear that ESS alone improves real-world treatment based on asthma severity,” she said. “However, our findings have shown that patients may experience a longer period without the need for a reliever medication in the early postoperative period.”
Looking ahead, “We want to explore what happens 5 or 6 months after sinus surgery that would explain the sudden need for a reliever medication,” she added. “Future studies are warranted to investigate the long-term effects of ESS on asthma severity as it relates to modifications of asthma regimens.”
Data important for patient discussions
The current study is important because of the frequency of comorbid asthma among patients with chronic rhinosinusitis, Megan Durr, MD, of the University of California, San Francisco, said in an interview.
“When we are considering functional endoscopy sinus surgery with patients, we are often asked if the surgery will impact the severity of their asthma symptoms,” said Dr. Durr, who served as a moderator for the session in which the study was presented.
“I am surprised the study did not see any difference in asthma severity after sinus surgery, as we often talk to patients about the unified airway that refers to the shared epidemiologic and pathophysiologic relationship between the upper and lower airways,” she told this news organization.
“This study will allow us to have a more informed evidenced-based discussion with patients and their primary care providers and/or pulmonologists” about what to expect for asthma outcomes following surgery, she said.
The study received no outside funding. Dr. Durr has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAO-HNSF 2023
Systematic review spotlights the use of nutraceuticals for acne
.
“While many topical and systemic prescription options are available for the treatment of acne, some patients may be interested in natural and complementary therapies as either an adjunctive or an alternative to prescription medications,” researchers led by John S. Barbieri, MD, MBA, of the department of dermatology at Brigham and Women’s Hospital, Boston, wrote in their study, which was published online in JAMA Dermatology. The researchers defined nutraceuticals as products derived from food sources that provide both nutritional and medicinal benefits, such as vitamins, dietary supplements, and herbal products. “Although patients may be interested in nutraceuticals as a potential treatment option for acne, there is uncertainty regarding the efficacy and safety of these products,” they wrote.
For the systematic review, they searched the PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science databases from inception through January 30, 2023, to identify randomized clinical trials that evaluated oral nutraceutical interventions such as vitamins and minerals, botanical extracts, prebiotics, and probiotics in individuals with acne. They extracted clinician-reported outcomes, patient-reported outcomes, and adverse events from the included studies, and used the Cochrane Risk of Bias checklist tool to assess the quality of evidence in randomized clinical trials. Based on this tool, they used Agency for Healthcare Research and Quality standards to categorize the articles as good, fair, or poor quality.
The search yielded 42 unique studies with 3,346 participants. Of these 42 studies, 27 were considered poor quality, 11 were considered fair quality, and 4 were considered good quality. The good-quality studies separately evaluated four interventions: vitamin D, green tea extract, probiotics, and cheongsangbangpoong-tang, an herbal formula approved for use in acne by the Korea Food and Drug Administration.
The 11 fair-quality studies suggested potential effectiveness for pantothenic acid (vitamin B5), the fatty acids omega-3 (eicosapentaenoic acid [EPA] and/or docosahexaenoic acid [DHA]) and omega-6 (gamma-linoleic acid), and probiotics.
Zinc was the most studied nutraceutical identified in the review, but “there was substantial heterogeneity in the results, with only slightly greater than one-half of studies finding zinc to be efficacious,” the authors noted. “Studies using higher doses more often found zinc to be efficacious,” they said, adding that zinc “had the highest rate of adverse effect reporting of any nutraceuticals assessed in this review.”
Dr. Barbieri and colleagues acknowledged limitations of their analysis, including the fact that few of the nutraceuticals considered to have good or fair evidence for their use were evaluated in more than one study. “In addition, some studies had inconsistent results depending on the outcome measure assessed,” they wrote. “For instance, although green tea extract led to statistically significant improvements in lesion counts, it did not result in statistically significant improvements in quality of life, suggesting the observed lesion count differences may not be clinically meaningful to patients.”
And while probiotics had the most studies supporting their efficacy, they were generally of very small sample size.
Asked to comment on the study, Jonette Keri, MD, PhD, a dermatologist who directs the Acne and Rosacea Treatment Center at the University of Miami, who was not involved with the study, said that while the review was exhaustive, more research is needed to better determine the efficacy and side effects of the products studied. “The real strength of this wonderful review is now we have all of this information in one place, and this will serve as a great patient care resource,” she told this news organization.
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Keri disclosed that she is a consultant for L’Oréal.
.
“While many topical and systemic prescription options are available for the treatment of acne, some patients may be interested in natural and complementary therapies as either an adjunctive or an alternative to prescription medications,” researchers led by John S. Barbieri, MD, MBA, of the department of dermatology at Brigham and Women’s Hospital, Boston, wrote in their study, which was published online in JAMA Dermatology. The researchers defined nutraceuticals as products derived from food sources that provide both nutritional and medicinal benefits, such as vitamins, dietary supplements, and herbal products. “Although patients may be interested in nutraceuticals as a potential treatment option for acne, there is uncertainty regarding the efficacy and safety of these products,” they wrote.
For the systematic review, they searched the PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science databases from inception through January 30, 2023, to identify randomized clinical trials that evaluated oral nutraceutical interventions such as vitamins and minerals, botanical extracts, prebiotics, and probiotics in individuals with acne. They extracted clinician-reported outcomes, patient-reported outcomes, and adverse events from the included studies, and used the Cochrane Risk of Bias checklist tool to assess the quality of evidence in randomized clinical trials. Based on this tool, they used Agency for Healthcare Research and Quality standards to categorize the articles as good, fair, or poor quality.
The search yielded 42 unique studies with 3,346 participants. Of these 42 studies, 27 were considered poor quality, 11 were considered fair quality, and 4 were considered good quality. The good-quality studies separately evaluated four interventions: vitamin D, green tea extract, probiotics, and cheongsangbangpoong-tang, an herbal formula approved for use in acne by the Korea Food and Drug Administration.
The 11 fair-quality studies suggested potential effectiveness for pantothenic acid (vitamin B5), the fatty acids omega-3 (eicosapentaenoic acid [EPA] and/or docosahexaenoic acid [DHA]) and omega-6 (gamma-linoleic acid), and probiotics.
Zinc was the most studied nutraceutical identified in the review, but “there was substantial heterogeneity in the results, with only slightly greater than one-half of studies finding zinc to be efficacious,” the authors noted. “Studies using higher doses more often found zinc to be efficacious,” they said, adding that zinc “had the highest rate of adverse effect reporting of any nutraceuticals assessed in this review.”
Dr. Barbieri and colleagues acknowledged limitations of their analysis, including the fact that few of the nutraceuticals considered to have good or fair evidence for their use were evaluated in more than one study. “In addition, some studies had inconsistent results depending on the outcome measure assessed,” they wrote. “For instance, although green tea extract led to statistically significant improvements in lesion counts, it did not result in statistically significant improvements in quality of life, suggesting the observed lesion count differences may not be clinically meaningful to patients.”
And while probiotics had the most studies supporting their efficacy, they were generally of very small sample size.
Asked to comment on the study, Jonette Keri, MD, PhD, a dermatologist who directs the Acne and Rosacea Treatment Center at the University of Miami, who was not involved with the study, said that while the review was exhaustive, more research is needed to better determine the efficacy and side effects of the products studied. “The real strength of this wonderful review is now we have all of this information in one place, and this will serve as a great patient care resource,” she told this news organization.
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Keri disclosed that she is a consultant for L’Oréal.
.
“While many topical and systemic prescription options are available for the treatment of acne, some patients may be interested in natural and complementary therapies as either an adjunctive or an alternative to prescription medications,” researchers led by John S. Barbieri, MD, MBA, of the department of dermatology at Brigham and Women’s Hospital, Boston, wrote in their study, which was published online in JAMA Dermatology. The researchers defined nutraceuticals as products derived from food sources that provide both nutritional and medicinal benefits, such as vitamins, dietary supplements, and herbal products. “Although patients may be interested in nutraceuticals as a potential treatment option for acne, there is uncertainty regarding the efficacy and safety of these products,” they wrote.
For the systematic review, they searched the PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science databases from inception through January 30, 2023, to identify randomized clinical trials that evaluated oral nutraceutical interventions such as vitamins and minerals, botanical extracts, prebiotics, and probiotics in individuals with acne. They extracted clinician-reported outcomes, patient-reported outcomes, and adverse events from the included studies, and used the Cochrane Risk of Bias checklist tool to assess the quality of evidence in randomized clinical trials. Based on this tool, they used Agency for Healthcare Research and Quality standards to categorize the articles as good, fair, or poor quality.
The search yielded 42 unique studies with 3,346 participants. Of these 42 studies, 27 were considered poor quality, 11 were considered fair quality, and 4 were considered good quality. The good-quality studies separately evaluated four interventions: vitamin D, green tea extract, probiotics, and cheongsangbangpoong-tang, an herbal formula approved for use in acne by the Korea Food and Drug Administration.
The 11 fair-quality studies suggested potential effectiveness for pantothenic acid (vitamin B5), the fatty acids omega-3 (eicosapentaenoic acid [EPA] and/or docosahexaenoic acid [DHA]) and omega-6 (gamma-linoleic acid), and probiotics.
Zinc was the most studied nutraceutical identified in the review, but “there was substantial heterogeneity in the results, with only slightly greater than one-half of studies finding zinc to be efficacious,” the authors noted. “Studies using higher doses more often found zinc to be efficacious,” they said, adding that zinc “had the highest rate of adverse effect reporting of any nutraceuticals assessed in this review.”
Dr. Barbieri and colleagues acknowledged limitations of their analysis, including the fact that few of the nutraceuticals considered to have good or fair evidence for their use were evaluated in more than one study. “In addition, some studies had inconsistent results depending on the outcome measure assessed,” they wrote. “For instance, although green tea extract led to statistically significant improvements in lesion counts, it did not result in statistically significant improvements in quality of life, suggesting the observed lesion count differences may not be clinically meaningful to patients.”
And while probiotics had the most studies supporting their efficacy, they were generally of very small sample size.
Asked to comment on the study, Jonette Keri, MD, PhD, a dermatologist who directs the Acne and Rosacea Treatment Center at the University of Miami, who was not involved with the study, said that while the review was exhaustive, more research is needed to better determine the efficacy and side effects of the products studied. “The real strength of this wonderful review is now we have all of this information in one place, and this will serve as a great patient care resource,” she told this news organization.
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Keri disclosed that she is a consultant for L’Oréal.
FROM JAMA DERMATOLOGY
mRNA vaccine cuts COVID-related Guillain-Barré risk
TOPLINE:
, according to a new study that also showed receipt of the Pfizer-BioNTech mRNA vaccine reduced GSB risk by 59%.
METHODOLOGY:
- The nested-case control study analyzed data from the largest healthcare provider in Israel for 3.2 million patients aged 16 years and older, with no history of GBS.
- GBS cases (n = 76) were identified based on hospital discharge data from January 2021 to June 2022.
- For every GBS case, investigators chose 10 controls at random, matched for age, gender, and follow-up duration (n = 760).
- Investigators examined the association between GBS and SARS-CoV-2 infection, established through documentation of prior positive SARS-CoV-2 test (PCR or antigen), and any COVID-19 vaccine administration.
TAKEAWAY:
- Among those diagnosed with GBS, 8 were exposed to SARS-CoV-2 infection only, 7 were exposed to COVID-19 vaccination only, and 1 patient was exposed to both SARS-CoV-2 infection and COVID-19 vaccination in the prior 6 weeks, leaving 60 GBS patients without exposure to either infection or vaccination.
- All COVID-19 vaccine doses administered in GBS cases within 6 weeks of the index date, and all but two doses administered in controls in the same timeframe, were Pfizer-BioNTech vaccines.
- Compared with people without GBS, those with the condition were more than six times as likely to have had SARS-CoV-2 infection within 6 weeks of GBS diagnosis (adjusted odds ratio, 6.30; 95% confidence interval, 2.55-15.56).
- People who received the COVID-19 vaccine were 59% less likely to develop GBS than those who did not get the vaccine (aOR, 0.41; 95% CI, 0.17-0.96).
IN PRACTICE:
“While Guillain-Barré is extremely rare, people should be aware that having a COVID infection can increase their risk of developing the disorder, and receiving an mRNA vaccine can decrease their risk,” study author Anat Arbel, MD, of Lady Davis Carmel Medical Center and the Technion-Israel Institute of Technology, Haifa, Israel, said in a press release.
SOURCE:
In addition to Dr. Arbel, the other lead author is Haya Bishara, MD, of Lady Davis Carmel Medical Center. The research was published online in the journal Neurology.
LIMITATIONS:
There is a possibility of misclassification of SARS-CoV-2 infection, which could lead to an overestimation of the magnitude of association between infection and GBS. The diagnosis of GBS relied solely on ICD-9 coding, which has been shown in prior studies to contain errors.
DISCLOSURES:
The study was unfunded. Dr. Bishara and Dr. Arbel report no relevant financial relationships. One co-author, Eitan Auriel, MD, has received lecturer fees from Novo Nordisk, Pfizer, Boehringer Ingelheim, and Medison.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a new study that also showed receipt of the Pfizer-BioNTech mRNA vaccine reduced GSB risk by 59%.
METHODOLOGY:
- The nested-case control study analyzed data from the largest healthcare provider in Israel for 3.2 million patients aged 16 years and older, with no history of GBS.
- GBS cases (n = 76) were identified based on hospital discharge data from January 2021 to June 2022.
- For every GBS case, investigators chose 10 controls at random, matched for age, gender, and follow-up duration (n = 760).
- Investigators examined the association between GBS and SARS-CoV-2 infection, established through documentation of prior positive SARS-CoV-2 test (PCR or antigen), and any COVID-19 vaccine administration.
TAKEAWAY:
- Among those diagnosed with GBS, 8 were exposed to SARS-CoV-2 infection only, 7 were exposed to COVID-19 vaccination only, and 1 patient was exposed to both SARS-CoV-2 infection and COVID-19 vaccination in the prior 6 weeks, leaving 60 GBS patients without exposure to either infection or vaccination.
- All COVID-19 vaccine doses administered in GBS cases within 6 weeks of the index date, and all but two doses administered in controls in the same timeframe, were Pfizer-BioNTech vaccines.
- Compared with people without GBS, those with the condition were more than six times as likely to have had SARS-CoV-2 infection within 6 weeks of GBS diagnosis (adjusted odds ratio, 6.30; 95% confidence interval, 2.55-15.56).
- People who received the COVID-19 vaccine were 59% less likely to develop GBS than those who did not get the vaccine (aOR, 0.41; 95% CI, 0.17-0.96).
IN PRACTICE:
“While Guillain-Barré is extremely rare, people should be aware that having a COVID infection can increase their risk of developing the disorder, and receiving an mRNA vaccine can decrease their risk,” study author Anat Arbel, MD, of Lady Davis Carmel Medical Center and the Technion-Israel Institute of Technology, Haifa, Israel, said in a press release.
SOURCE:
In addition to Dr. Arbel, the other lead author is Haya Bishara, MD, of Lady Davis Carmel Medical Center. The research was published online in the journal Neurology.
LIMITATIONS:
There is a possibility of misclassification of SARS-CoV-2 infection, which could lead to an overestimation of the magnitude of association between infection and GBS. The diagnosis of GBS relied solely on ICD-9 coding, which has been shown in prior studies to contain errors.
DISCLOSURES:
The study was unfunded. Dr. Bishara and Dr. Arbel report no relevant financial relationships. One co-author, Eitan Auriel, MD, has received lecturer fees from Novo Nordisk, Pfizer, Boehringer Ingelheim, and Medison.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a new study that also showed receipt of the Pfizer-BioNTech mRNA vaccine reduced GSB risk by 59%.
METHODOLOGY:
- The nested-case control study analyzed data from the largest healthcare provider in Israel for 3.2 million patients aged 16 years and older, with no history of GBS.
- GBS cases (n = 76) were identified based on hospital discharge data from January 2021 to June 2022.
- For every GBS case, investigators chose 10 controls at random, matched for age, gender, and follow-up duration (n = 760).
- Investigators examined the association between GBS and SARS-CoV-2 infection, established through documentation of prior positive SARS-CoV-2 test (PCR or antigen), and any COVID-19 vaccine administration.
TAKEAWAY:
- Among those diagnosed with GBS, 8 were exposed to SARS-CoV-2 infection only, 7 were exposed to COVID-19 vaccination only, and 1 patient was exposed to both SARS-CoV-2 infection and COVID-19 vaccination in the prior 6 weeks, leaving 60 GBS patients without exposure to either infection or vaccination.
- All COVID-19 vaccine doses administered in GBS cases within 6 weeks of the index date, and all but two doses administered in controls in the same timeframe, were Pfizer-BioNTech vaccines.
- Compared with people without GBS, those with the condition were more than six times as likely to have had SARS-CoV-2 infection within 6 weeks of GBS diagnosis (adjusted odds ratio, 6.30; 95% confidence interval, 2.55-15.56).
- People who received the COVID-19 vaccine were 59% less likely to develop GBS than those who did not get the vaccine (aOR, 0.41; 95% CI, 0.17-0.96).
IN PRACTICE:
“While Guillain-Barré is extremely rare, people should be aware that having a COVID infection can increase their risk of developing the disorder, and receiving an mRNA vaccine can decrease their risk,” study author Anat Arbel, MD, of Lady Davis Carmel Medical Center and the Technion-Israel Institute of Technology, Haifa, Israel, said in a press release.
SOURCE:
In addition to Dr. Arbel, the other lead author is Haya Bishara, MD, of Lady Davis Carmel Medical Center. The research was published online in the journal Neurology.
LIMITATIONS:
There is a possibility of misclassification of SARS-CoV-2 infection, which could lead to an overestimation of the magnitude of association between infection and GBS. The diagnosis of GBS relied solely on ICD-9 coding, which has been shown in prior studies to contain errors.
DISCLOSURES:
The study was unfunded. Dr. Bishara and Dr. Arbel report no relevant financial relationships. One co-author, Eitan Auriel, MD, has received lecturer fees from Novo Nordisk, Pfizer, Boehringer Ingelheim, and Medison.
A version of this article first appeared on Medscape.com.
Higher weight loss on tirzepatide links to seven factors
TOPLINE:
Among the 3,188 people with type 2 diabetes who were adherent to their tirzepatide (Mounjaro, Lilly) regimen in four pivotal trials of the agent, a quarter achieved at least a 15% cut from their baseline body weight after 40-42 weeks of treatment, and researchers found seven baseline variables that were significantly linked with a higher incidence of this level of weight loss.
say the authors.
METHODOLOGY:
- Investigators conducted a post hoc analysis of data collected from a total of 3,188 people with type 2 diabetes who had been adherent to their assigned tirzepatide regimen for 40-42 weeks in any one of four pivotal trials of the agent.
- The researchers aimed to identify predictors of a reduction in body weight of at least 15% with tirzepatide treatment at any of the three tested doses – 5 mg, 10 mg, or 15 mg – which were administered by subcutaneous injection once a week.
- All four trials that provided data prohibited concurrent therapy that would promote weight loss, and the people included in the analysis did not receive any rescue medications for controlling glycemia.
- The primary efficacy measure in all four studies was the ability of tirzepatide to improve glycemic control (measured by A1c level), compared with placebo, semaglutide (Ozempic) 1 mg SC once weekly, insulin degludec (Tresiba, Novo Nordisk), or insulin glargine (Basaglar, Lilly).
TAKEAWAY:
- Among the 3,188 people who remained adherent to their tirzepatide regimen for 40-42 weeks, 792 (25%) experienced a weight reduction of at least 15% from baseline.
- Multivariate analysis of baseline covariates showed that these seven factors were significantly linked with greater than or equal to 15% weight loss: higher tirzepatide dose, being female, being of White or Asian race, being of younger age, undergoing treatment with metformin, having better glycemic control (based on lower A1c and lower fasting serum glucose), and having lower non–high-density lipoprotein cholesterol level.
- During follow-up, achievement of at least a 15% cut in baseline body weight was significantly associated with greater reductions in A1c, fasting serum glucose level, waist circumference, blood pressure, serum triglyceride level, and serum level of the liver enzyme alanine transaminase.
IN PRACTICE:
“These findings may provide valuable information to clinicians and people with type 2 diabetes regarding the likelihood of achieving substantial body weight reduction with tirzepatide and also help to signal likely improvements to be seen in a range of cardiometabolic risk parameters with tirzepatide-induced weight loss,” the authors concluded in their report.
SOURCE:
The study was largely run by researchers who are employees of Lilly, the company that markets tirzepatide (Mounjaro). It was published in Diabetes Care.
LIMITATIONS:
- The analysis was post hoc.
- The follow-up was limited.
- The analysis focused entirely on baseline parameters as potential predictors of weight loss magnitude.
DISCLOSURES:
The study was funded by Eli Lilly, the company that markets tirzepatide (Mounjaro) and that sponsored the SURPASS trials. Six authors are employees of Lilly, one is a contractor for Lilly, and the two remaining authors have had financial relationships with Lilly and with several other companies.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among the 3,188 people with type 2 diabetes who were adherent to their tirzepatide (Mounjaro, Lilly) regimen in four pivotal trials of the agent, a quarter achieved at least a 15% cut from their baseline body weight after 40-42 weeks of treatment, and researchers found seven baseline variables that were significantly linked with a higher incidence of this level of weight loss.
say the authors.
METHODOLOGY:
- Investigators conducted a post hoc analysis of data collected from a total of 3,188 people with type 2 diabetes who had been adherent to their assigned tirzepatide regimen for 40-42 weeks in any one of four pivotal trials of the agent.
- The researchers aimed to identify predictors of a reduction in body weight of at least 15% with tirzepatide treatment at any of the three tested doses – 5 mg, 10 mg, or 15 mg – which were administered by subcutaneous injection once a week.
- All four trials that provided data prohibited concurrent therapy that would promote weight loss, and the people included in the analysis did not receive any rescue medications for controlling glycemia.
- The primary efficacy measure in all four studies was the ability of tirzepatide to improve glycemic control (measured by A1c level), compared with placebo, semaglutide (Ozempic) 1 mg SC once weekly, insulin degludec (Tresiba, Novo Nordisk), or insulin glargine (Basaglar, Lilly).
TAKEAWAY:
- Among the 3,188 people who remained adherent to their tirzepatide regimen for 40-42 weeks, 792 (25%) experienced a weight reduction of at least 15% from baseline.
- Multivariate analysis of baseline covariates showed that these seven factors were significantly linked with greater than or equal to 15% weight loss: higher tirzepatide dose, being female, being of White or Asian race, being of younger age, undergoing treatment with metformin, having better glycemic control (based on lower A1c and lower fasting serum glucose), and having lower non–high-density lipoprotein cholesterol level.
- During follow-up, achievement of at least a 15% cut in baseline body weight was significantly associated with greater reductions in A1c, fasting serum glucose level, waist circumference, blood pressure, serum triglyceride level, and serum level of the liver enzyme alanine transaminase.
IN PRACTICE:
“These findings may provide valuable information to clinicians and people with type 2 diabetes regarding the likelihood of achieving substantial body weight reduction with tirzepatide and also help to signal likely improvements to be seen in a range of cardiometabolic risk parameters with tirzepatide-induced weight loss,” the authors concluded in their report.
SOURCE:
The study was largely run by researchers who are employees of Lilly, the company that markets tirzepatide (Mounjaro). It was published in Diabetes Care.
LIMITATIONS:
- The analysis was post hoc.
- The follow-up was limited.
- The analysis focused entirely on baseline parameters as potential predictors of weight loss magnitude.
DISCLOSURES:
The study was funded by Eli Lilly, the company that markets tirzepatide (Mounjaro) and that sponsored the SURPASS trials. Six authors are employees of Lilly, one is a contractor for Lilly, and the two remaining authors have had financial relationships with Lilly and with several other companies.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among the 3,188 people with type 2 diabetes who were adherent to their tirzepatide (Mounjaro, Lilly) regimen in four pivotal trials of the agent, a quarter achieved at least a 15% cut from their baseline body weight after 40-42 weeks of treatment, and researchers found seven baseline variables that were significantly linked with a higher incidence of this level of weight loss.
say the authors.
METHODOLOGY:
- Investigators conducted a post hoc analysis of data collected from a total of 3,188 people with type 2 diabetes who had been adherent to their assigned tirzepatide regimen for 40-42 weeks in any one of four pivotal trials of the agent.
- The researchers aimed to identify predictors of a reduction in body weight of at least 15% with tirzepatide treatment at any of the three tested doses – 5 mg, 10 mg, or 15 mg – which were administered by subcutaneous injection once a week.
- All four trials that provided data prohibited concurrent therapy that would promote weight loss, and the people included in the analysis did not receive any rescue medications for controlling glycemia.
- The primary efficacy measure in all four studies was the ability of tirzepatide to improve glycemic control (measured by A1c level), compared with placebo, semaglutide (Ozempic) 1 mg SC once weekly, insulin degludec (Tresiba, Novo Nordisk), or insulin glargine (Basaglar, Lilly).
TAKEAWAY:
- Among the 3,188 people who remained adherent to their tirzepatide regimen for 40-42 weeks, 792 (25%) experienced a weight reduction of at least 15% from baseline.
- Multivariate analysis of baseline covariates showed that these seven factors were significantly linked with greater than or equal to 15% weight loss: higher tirzepatide dose, being female, being of White or Asian race, being of younger age, undergoing treatment with metformin, having better glycemic control (based on lower A1c and lower fasting serum glucose), and having lower non–high-density lipoprotein cholesterol level.
- During follow-up, achievement of at least a 15% cut in baseline body weight was significantly associated with greater reductions in A1c, fasting serum glucose level, waist circumference, blood pressure, serum triglyceride level, and serum level of the liver enzyme alanine transaminase.
IN PRACTICE:
“These findings may provide valuable information to clinicians and people with type 2 diabetes regarding the likelihood of achieving substantial body weight reduction with tirzepatide and also help to signal likely improvements to be seen in a range of cardiometabolic risk parameters with tirzepatide-induced weight loss,” the authors concluded in their report.
SOURCE:
The study was largely run by researchers who are employees of Lilly, the company that markets tirzepatide (Mounjaro). It was published in Diabetes Care.
LIMITATIONS:
- The analysis was post hoc.
- The follow-up was limited.
- The analysis focused entirely on baseline parameters as potential predictors of weight loss magnitude.
DISCLOSURES:
The study was funded by Eli Lilly, the company that markets tirzepatide (Mounjaro) and that sponsored the SURPASS trials. Six authors are employees of Lilly, one is a contractor for Lilly, and the two remaining authors have had financial relationships with Lilly and with several other companies.
A version of this article first appeared on Medscape.com.



