User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Greater fracture risk reduction seen with denosumab vs. zoledronic acid in postmenopausal women
VANCOUVER –
A previous head-to-head comparison showed that denosumab increased bone mineral density at key skeletal sites compared with zoledronic acid, but only a single, small observational study has examined fracture risk, and it found no difference.
The new study, presented at the annual meeting of the American Society for Bone and Mineral Research, used a relatively new method of real-world comparative effectiveness analysis called negative control outcome (NCO) to analyze Medicare fee-for-service data.
NCO analysis takes extra pains to remove bias through data that might be linked to potential confounders but could not reasonably be attributed to a drug. For example, people who have greater contact with the health care system may be more likely to get one drug or another. The researchers used the frequency of receiving a flu or pneumonia vaccine as a proxy for this. If the two comparison groups had a significant difference in a proxy, it suggested a hidden bias and forced the researchers to abandon those groupings. Another example used car accidents as a proxy for cognitive impairment.
“If you find meaningful differences between the two groups, and you can say there’s no way a bone drug could account for these differences, then we shouldn’t do this analysis because these groups just aren’t comparable. They probably differ by that confounding factor we couldn’t measure,” said Jeffrey Curtis, MD, who presented the study. He is a professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham.
The study strongly suggests superiority for denosumab. “There was a significant difference in multiple different groupings of fractures – beginning at year 2, extending to year 3 and even out to year 5 – that showed that there is a significant reduction in fracture risk if you get treated with denosumab [that was greater] than if you get treated with zoledronic acid,” Dr. Curtis said.
The researchers weighed 118 covariates and ultimately identified a population of 90,805 women taking denosumab and 37,328 taking zoledronic acid that was equally balanced in all patient characteristics. The mean age was about 75 years in the denosumab group and 74 in the zoledronic acid group.
The researchers found a 34% lower risk for hip fracture in the denosumab group by 5 years (relative risk, 0.66; 95% confidence interval, 0.43-0.90).
Similar patterns in fracture risk reduction were observed at 5 years for nonvertebral fracture (RR, 0.67; 95% CI, 0.52-0.82), nonhip nonvertebral fracture (RR, 0.69; 95% CI, 0.50-0.88), and major osteoporotic fracture (RR, 0.74; 95% CI, 0.59-0.89).
During the Q&A session after the talk, one audience member commented that the study was limited because the researchers only followed patients who received zoledronic acid for 60 days, which could have missed potential long-term benefits of the drug, especially since bisphosphonates have a lengthy skeletal retention time. Dr. Curtis acknowledged the point but said, “Usually, that’s not something we do, but these are different enough mechanisms of action that it may be warranted at least as a sensitivity analysis,” he said.
The study and its methodology were impressive, according to Yumie Rhee, MD, who comoderated the session where the study was presented. “I think they did a really good job by doing the negative control analysis. We’re not going to have a head-to-head clinical trial, so we don’t know the real fracture reduction differences [between denosumab and zoledronic acid]. [The NCO analysis] is more than the propensity matching score that we do usually,” said Dr. Rhee, who is a professor of endocrinology at Yonsei University College of Medicine in Seoul, South Korea.
In particular, the study showed a significantly greater reduction in hip fractures with denosumab. “Even in the RCTs, it was really hard to see the reduction in hip fracture, so I think this is showing much stronger data for denosumab. Especially in patients who have more [general fracture] risk and patients with higher hip fracture risk, I would go with denosumab,” Dr. Rhee said.
Her comoderator, Maria Zanchetta, MD, agreed. “It can have clinical implication, because we think denosumab is better than [zoledronic acid] for higher-risk patients, but we didn’t have the evidence. So at least we have a new [study] to look at, and I think it’s very important for our practice,” said Dr. Zanchetta, who is a professor of osteology at the Institute of Diagnostics and Metabolic Research, Universidad del Salvador, Buenos Aires.
The study was funded by Amgen, which markets denosumab. Dr. Curtis has consulted for Amgen. Dr. Rhee and Dr. Zanchetta report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VANCOUVER –
A previous head-to-head comparison showed that denosumab increased bone mineral density at key skeletal sites compared with zoledronic acid, but only a single, small observational study has examined fracture risk, and it found no difference.
The new study, presented at the annual meeting of the American Society for Bone and Mineral Research, used a relatively new method of real-world comparative effectiveness analysis called negative control outcome (NCO) to analyze Medicare fee-for-service data.
NCO analysis takes extra pains to remove bias through data that might be linked to potential confounders but could not reasonably be attributed to a drug. For example, people who have greater contact with the health care system may be more likely to get one drug or another. The researchers used the frequency of receiving a flu or pneumonia vaccine as a proxy for this. If the two comparison groups had a significant difference in a proxy, it suggested a hidden bias and forced the researchers to abandon those groupings. Another example used car accidents as a proxy for cognitive impairment.
“If you find meaningful differences between the two groups, and you can say there’s no way a bone drug could account for these differences, then we shouldn’t do this analysis because these groups just aren’t comparable. They probably differ by that confounding factor we couldn’t measure,” said Jeffrey Curtis, MD, who presented the study. He is a professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham.
The study strongly suggests superiority for denosumab. “There was a significant difference in multiple different groupings of fractures – beginning at year 2, extending to year 3 and even out to year 5 – that showed that there is a significant reduction in fracture risk if you get treated with denosumab [that was greater] than if you get treated with zoledronic acid,” Dr. Curtis said.
The researchers weighed 118 covariates and ultimately identified a population of 90,805 women taking denosumab and 37,328 taking zoledronic acid that was equally balanced in all patient characteristics. The mean age was about 75 years in the denosumab group and 74 in the zoledronic acid group.
The researchers found a 34% lower risk for hip fracture in the denosumab group by 5 years (relative risk, 0.66; 95% confidence interval, 0.43-0.90).
Similar patterns in fracture risk reduction were observed at 5 years for nonvertebral fracture (RR, 0.67; 95% CI, 0.52-0.82), nonhip nonvertebral fracture (RR, 0.69; 95% CI, 0.50-0.88), and major osteoporotic fracture (RR, 0.74; 95% CI, 0.59-0.89).
During the Q&A session after the talk, one audience member commented that the study was limited because the researchers only followed patients who received zoledronic acid for 60 days, which could have missed potential long-term benefits of the drug, especially since bisphosphonates have a lengthy skeletal retention time. Dr. Curtis acknowledged the point but said, “Usually, that’s not something we do, but these are different enough mechanisms of action that it may be warranted at least as a sensitivity analysis,” he said.
The study and its methodology were impressive, according to Yumie Rhee, MD, who comoderated the session where the study was presented. “I think they did a really good job by doing the negative control analysis. We’re not going to have a head-to-head clinical trial, so we don’t know the real fracture reduction differences [between denosumab and zoledronic acid]. [The NCO analysis] is more than the propensity matching score that we do usually,” said Dr. Rhee, who is a professor of endocrinology at Yonsei University College of Medicine in Seoul, South Korea.
In particular, the study showed a significantly greater reduction in hip fractures with denosumab. “Even in the RCTs, it was really hard to see the reduction in hip fracture, so I think this is showing much stronger data for denosumab. Especially in patients who have more [general fracture] risk and patients with higher hip fracture risk, I would go with denosumab,” Dr. Rhee said.
Her comoderator, Maria Zanchetta, MD, agreed. “It can have clinical implication, because we think denosumab is better than [zoledronic acid] for higher-risk patients, but we didn’t have the evidence. So at least we have a new [study] to look at, and I think it’s very important for our practice,” said Dr. Zanchetta, who is a professor of osteology at the Institute of Diagnostics and Metabolic Research, Universidad del Salvador, Buenos Aires.
The study was funded by Amgen, which markets denosumab. Dr. Curtis has consulted for Amgen. Dr. Rhee and Dr. Zanchetta report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VANCOUVER –
A previous head-to-head comparison showed that denosumab increased bone mineral density at key skeletal sites compared with zoledronic acid, but only a single, small observational study has examined fracture risk, and it found no difference.
The new study, presented at the annual meeting of the American Society for Bone and Mineral Research, used a relatively new method of real-world comparative effectiveness analysis called negative control outcome (NCO) to analyze Medicare fee-for-service data.
NCO analysis takes extra pains to remove bias through data that might be linked to potential confounders but could not reasonably be attributed to a drug. For example, people who have greater contact with the health care system may be more likely to get one drug or another. The researchers used the frequency of receiving a flu or pneumonia vaccine as a proxy for this. If the two comparison groups had a significant difference in a proxy, it suggested a hidden bias and forced the researchers to abandon those groupings. Another example used car accidents as a proxy for cognitive impairment.
“If you find meaningful differences between the two groups, and you can say there’s no way a bone drug could account for these differences, then we shouldn’t do this analysis because these groups just aren’t comparable. They probably differ by that confounding factor we couldn’t measure,” said Jeffrey Curtis, MD, who presented the study. He is a professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham.
The study strongly suggests superiority for denosumab. “There was a significant difference in multiple different groupings of fractures – beginning at year 2, extending to year 3 and even out to year 5 – that showed that there is a significant reduction in fracture risk if you get treated with denosumab [that was greater] than if you get treated with zoledronic acid,” Dr. Curtis said.
The researchers weighed 118 covariates and ultimately identified a population of 90,805 women taking denosumab and 37,328 taking zoledronic acid that was equally balanced in all patient characteristics. The mean age was about 75 years in the denosumab group and 74 in the zoledronic acid group.
The researchers found a 34% lower risk for hip fracture in the denosumab group by 5 years (relative risk, 0.66; 95% confidence interval, 0.43-0.90).
Similar patterns in fracture risk reduction were observed at 5 years for nonvertebral fracture (RR, 0.67; 95% CI, 0.52-0.82), nonhip nonvertebral fracture (RR, 0.69; 95% CI, 0.50-0.88), and major osteoporotic fracture (RR, 0.74; 95% CI, 0.59-0.89).
During the Q&A session after the talk, one audience member commented that the study was limited because the researchers only followed patients who received zoledronic acid for 60 days, which could have missed potential long-term benefits of the drug, especially since bisphosphonates have a lengthy skeletal retention time. Dr. Curtis acknowledged the point but said, “Usually, that’s not something we do, but these are different enough mechanisms of action that it may be warranted at least as a sensitivity analysis,” he said.
The study and its methodology were impressive, according to Yumie Rhee, MD, who comoderated the session where the study was presented. “I think they did a really good job by doing the negative control analysis. We’re not going to have a head-to-head clinical trial, so we don’t know the real fracture reduction differences [between denosumab and zoledronic acid]. [The NCO analysis] is more than the propensity matching score that we do usually,” said Dr. Rhee, who is a professor of endocrinology at Yonsei University College of Medicine in Seoul, South Korea.
In particular, the study showed a significantly greater reduction in hip fractures with denosumab. “Even in the RCTs, it was really hard to see the reduction in hip fracture, so I think this is showing much stronger data for denosumab. Especially in patients who have more [general fracture] risk and patients with higher hip fracture risk, I would go with denosumab,” Dr. Rhee said.
Her comoderator, Maria Zanchetta, MD, agreed. “It can have clinical implication, because we think denosumab is better than [zoledronic acid] for higher-risk patients, but we didn’t have the evidence. So at least we have a new [study] to look at, and I think it’s very important for our practice,” said Dr. Zanchetta, who is a professor of osteology at the Institute of Diagnostics and Metabolic Research, Universidad del Salvador, Buenos Aires.
The study was funded by Amgen, which markets denosumab. Dr. Curtis has consulted for Amgen. Dr. Rhee and Dr. Zanchetta report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ASBMR 2023
CKD linked to cardiac arrest in Hispanic, Latinx patients
TOPLINE:
new data show, suggesting early identification of CKD may provide an opportunity to reduce the risk in these groups. Other predictors included heavy drinking, atrial fibrillation, coronary artery disease, heart failure and diabetes.
METHODOLOGY:
- The study included 295 Hispanic or Latinx patients with out-of-hospital SCA from the PRESTO study in Ventura County, California, and 590 frequency-matched controls from the San Diego site of the population-based HCHS/SOL (Hispanic Community Health Survey/Study of Latinos); in both cohorts, men made up 70% of participants, and the median age was about 63 years.
- Researchers collected data on demographics, medical history, and current health conditions. Of note, 51.2% of SCA cases and 8.8% of control participants had CKD, and 20.0% of cases and 0.7% of the control group were on dialysis.
- Pre-SCA echocardiograms were available for 48% of SCA cases and baseline echocardiograms for more than 99% of control participants.
TAKEAWAY:
- In analyses adjusted for age, sex, and clinical variables, predictors significantly associated with higher odds of SCA included: CKD (odds ratio, 7.3; 95% confidence interval, 3.8-14.3; P < .001), heavy drinking (OR, 4.5), stroke (OR, 3.1), atrial fibrillation (OR, 3.7), coronary artery disease (OR, 2.9), heart failure (OR, 2.5), and diabetes (OR, 1.5).
- Hypertension, hyperlipemia, body mass index, and current smoking status were not significantly associated with SCA.
- In adjusted analyses, heart rate (OR, 1.8 per one standard deviation [1-SD] increase), QTc interval (OR, 2.5 per 1-SD increase) and left ventricular ejection fraction (OR, 4.4 per 1-SD decrease) were significantly associated with SCA, suggesting echocardiogram evaluations could help identify Hispanic or Latinx individuals at increased risk for SCA, wrote the authors.
IN PRACTICE:
“Our study, the first to include feasible numbers of Hispanic or Latino individuals, highlights the importance of renal dysfunction as a risk factor for SCA in the community,” the authors wrote, adding that early identification and management of chronic kidney disease could reduce risk for SCA in this population.
SOURCE:
The study was conducted by Kyndaron Reinier, PhD, MPH, Cedars-Sinai Health System, Los Angeles, and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
Most participants from the HCHS/SOL study were born outside the United States, compared with about half the SCA cases, which could have influenced cardiovascular disease risk, although results did not change considerably when models were adjusted for place of birth. Study participants were predominantly of Mexican heritage, so results may not be generalizable to Hispanic or Latinx individuals from other regions. As medical history was assessed differently in the two studies, there could be some error in estimating the strength of associations. Results from echocardiographic data should be viewed as hypothesis generating because of the potential for residual bias.
DISCLOSURES:
The Ventura PRESTO study was funded, in part, by the National Institutes of Health, and National Heart, Lung, and Blood Institute. The HCHS/SOL was carried out as a collaborative study supported by contracts from the NHLBI.
A version of this article first appeared on Medscape.com.
TOPLINE:
new data show, suggesting early identification of CKD may provide an opportunity to reduce the risk in these groups. Other predictors included heavy drinking, atrial fibrillation, coronary artery disease, heart failure and diabetes.
METHODOLOGY:
- The study included 295 Hispanic or Latinx patients with out-of-hospital SCA from the PRESTO study in Ventura County, California, and 590 frequency-matched controls from the San Diego site of the population-based HCHS/SOL (Hispanic Community Health Survey/Study of Latinos); in both cohorts, men made up 70% of participants, and the median age was about 63 years.
- Researchers collected data on demographics, medical history, and current health conditions. Of note, 51.2% of SCA cases and 8.8% of control participants had CKD, and 20.0% of cases and 0.7% of the control group were on dialysis.
- Pre-SCA echocardiograms were available for 48% of SCA cases and baseline echocardiograms for more than 99% of control participants.
TAKEAWAY:
- In analyses adjusted for age, sex, and clinical variables, predictors significantly associated with higher odds of SCA included: CKD (odds ratio, 7.3; 95% confidence interval, 3.8-14.3; P < .001), heavy drinking (OR, 4.5), stroke (OR, 3.1), atrial fibrillation (OR, 3.7), coronary artery disease (OR, 2.9), heart failure (OR, 2.5), and diabetes (OR, 1.5).
- Hypertension, hyperlipemia, body mass index, and current smoking status were not significantly associated with SCA.
- In adjusted analyses, heart rate (OR, 1.8 per one standard deviation [1-SD] increase), QTc interval (OR, 2.5 per 1-SD increase) and left ventricular ejection fraction (OR, 4.4 per 1-SD decrease) were significantly associated with SCA, suggesting echocardiogram evaluations could help identify Hispanic or Latinx individuals at increased risk for SCA, wrote the authors.
IN PRACTICE:
“Our study, the first to include feasible numbers of Hispanic or Latino individuals, highlights the importance of renal dysfunction as a risk factor for SCA in the community,” the authors wrote, adding that early identification and management of chronic kidney disease could reduce risk for SCA in this population.
SOURCE:
The study was conducted by Kyndaron Reinier, PhD, MPH, Cedars-Sinai Health System, Los Angeles, and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
Most participants from the HCHS/SOL study were born outside the United States, compared with about half the SCA cases, which could have influenced cardiovascular disease risk, although results did not change considerably when models were adjusted for place of birth. Study participants were predominantly of Mexican heritage, so results may not be generalizable to Hispanic or Latinx individuals from other regions. As medical history was assessed differently in the two studies, there could be some error in estimating the strength of associations. Results from echocardiographic data should be viewed as hypothesis generating because of the potential for residual bias.
DISCLOSURES:
The Ventura PRESTO study was funded, in part, by the National Institutes of Health, and National Heart, Lung, and Blood Institute. The HCHS/SOL was carried out as a collaborative study supported by contracts from the NHLBI.
A version of this article first appeared on Medscape.com.
TOPLINE:
new data show, suggesting early identification of CKD may provide an opportunity to reduce the risk in these groups. Other predictors included heavy drinking, atrial fibrillation, coronary artery disease, heart failure and diabetes.
METHODOLOGY:
- The study included 295 Hispanic or Latinx patients with out-of-hospital SCA from the PRESTO study in Ventura County, California, and 590 frequency-matched controls from the San Diego site of the population-based HCHS/SOL (Hispanic Community Health Survey/Study of Latinos); in both cohorts, men made up 70% of participants, and the median age was about 63 years.
- Researchers collected data on demographics, medical history, and current health conditions. Of note, 51.2% of SCA cases and 8.8% of control participants had CKD, and 20.0% of cases and 0.7% of the control group were on dialysis.
- Pre-SCA echocardiograms were available for 48% of SCA cases and baseline echocardiograms for more than 99% of control participants.
TAKEAWAY:
- In analyses adjusted for age, sex, and clinical variables, predictors significantly associated with higher odds of SCA included: CKD (odds ratio, 7.3; 95% confidence interval, 3.8-14.3; P < .001), heavy drinking (OR, 4.5), stroke (OR, 3.1), atrial fibrillation (OR, 3.7), coronary artery disease (OR, 2.9), heart failure (OR, 2.5), and diabetes (OR, 1.5).
- Hypertension, hyperlipemia, body mass index, and current smoking status were not significantly associated with SCA.
- In adjusted analyses, heart rate (OR, 1.8 per one standard deviation [1-SD] increase), QTc interval (OR, 2.5 per 1-SD increase) and left ventricular ejection fraction (OR, 4.4 per 1-SD decrease) were significantly associated with SCA, suggesting echocardiogram evaluations could help identify Hispanic or Latinx individuals at increased risk for SCA, wrote the authors.
IN PRACTICE:
“Our study, the first to include feasible numbers of Hispanic or Latino individuals, highlights the importance of renal dysfunction as a risk factor for SCA in the community,” the authors wrote, adding that early identification and management of chronic kidney disease could reduce risk for SCA in this population.
SOURCE:
The study was conducted by Kyndaron Reinier, PhD, MPH, Cedars-Sinai Health System, Los Angeles, and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
Most participants from the HCHS/SOL study were born outside the United States, compared with about half the SCA cases, which could have influenced cardiovascular disease risk, although results did not change considerably when models were adjusted for place of birth. Study participants were predominantly of Mexican heritage, so results may not be generalizable to Hispanic or Latinx individuals from other regions. As medical history was assessed differently in the two studies, there could be some error in estimating the strength of associations. Results from echocardiographic data should be viewed as hypothesis generating because of the potential for residual bias.
DISCLOSURES:
The Ventura PRESTO study was funded, in part, by the National Institutes of Health, and National Heart, Lung, and Blood Institute. The HCHS/SOL was carried out as a collaborative study supported by contracts from the NHLBI.
A version of this article first appeared on Medscape.com.
Metformin, weight management to stop type 2 diabetes in kids
TOPLINE:
Nearly one in five adolescents are living with prediabetes, a condition where blood glucose levels are elevated, but are not high enough for a type 2 diabetes (T2D) diagnosis. According to a new study, higher levels of nonfasting glucose and hemoglobin A1c, and worsening obesity are important predictors of progression to T2D. In addition, metformin and weight stabilization may prove to be important interventions for preventing T2D in kids.
METHODOLOGY:
- Researchers did a retrospective chart review of patient data from Vanderbilt University Medical Center Pediatric Prediabetes Clinic, Nashville, Tenn., from May 2015 to August 2022.
- The study included 552 children with prediabetes, defined as abnormal blood glucose (fasting plasma glucose [FPG] ≥ 100 mg/dL, random glucose ≥ 150 mg/dL), or hemoglobin A1c equal to or greater than 5.9%.
- Based on follow-up visits, patients were classified as having progressed to T2D, or nonprogression.
- Researchers analyzed the patients’ initial visit A1c, fasting C-peptide, 2-hour glucose, fasting glucose, and body mass index (BMI), among other baseline characteristics.
TAKEAWAY:
- Thirty-six children (6.5%) progressed to T2D during the duration of the study period.
- The average time to T2D diagnosis was much longer in patients taking metformin (43 months), compared with those not taking the prescribed medication (28 months).
- Worsening obesity was strongly associated with T2D progression – patients who progressed to T2D had a higher BMI at baseline and had continued weight gain.
- A higher baseline A1c, fasting C-peptide, and 2-hour glucose were also associated with progression to T2D.
- In the multivariable analysis, both A1c and 2-hour glucose were strong independent predictors of progression.
- Fasting plasma glucose was not associated with progression to T2D.
IN PRACTICE:
“Weight stabilization and metformin therapy could be important interventions for diabetes prevention in children,” study author Ashley H. Shoemaker, MD, MSci, a pediatric endocrinologist at Vanderbilt University Medical Center in Nashville, Tenn., said in a press release.
In addition, A1c plus a nonfasting glucose may be a feasible way to identify high-risk pediatric patients in a clinical setting.
SOURCE:
This study was performed by Natasha Belsky, Jaclyn Tamaroff, and Ashley H. Shoemaker of the Vanderbilt University Medical Center and the Vanderbilt University School of Medicine in Nashville, Tenn. It was published October 12, 2023, in the Journal of the Endocrine Society
LIMITATIONS:
Additional patients who developed T2D may have been lost to follow-up, since the authors did not contact patients to confirm their disease status. The authors were also unable to establish racial differences in the progression to T2D because of missing data.
DISCLOSURES:
Funding for this study was provided by the National Center for Advancing Translational Sciences. One author has research contracts with Novo Nordisk and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
TOPLINE:
Nearly one in five adolescents are living with prediabetes, a condition where blood glucose levels are elevated, but are not high enough for a type 2 diabetes (T2D) diagnosis. According to a new study, higher levels of nonfasting glucose and hemoglobin A1c, and worsening obesity are important predictors of progression to T2D. In addition, metformin and weight stabilization may prove to be important interventions for preventing T2D in kids.
METHODOLOGY:
- Researchers did a retrospective chart review of patient data from Vanderbilt University Medical Center Pediatric Prediabetes Clinic, Nashville, Tenn., from May 2015 to August 2022.
- The study included 552 children with prediabetes, defined as abnormal blood glucose (fasting plasma glucose [FPG] ≥ 100 mg/dL, random glucose ≥ 150 mg/dL), or hemoglobin A1c equal to or greater than 5.9%.
- Based on follow-up visits, patients were classified as having progressed to T2D, or nonprogression.
- Researchers analyzed the patients’ initial visit A1c, fasting C-peptide, 2-hour glucose, fasting glucose, and body mass index (BMI), among other baseline characteristics.
TAKEAWAY:
- Thirty-six children (6.5%) progressed to T2D during the duration of the study period.
- The average time to T2D diagnosis was much longer in patients taking metformin (43 months), compared with those not taking the prescribed medication (28 months).
- Worsening obesity was strongly associated with T2D progression – patients who progressed to T2D had a higher BMI at baseline and had continued weight gain.
- A higher baseline A1c, fasting C-peptide, and 2-hour glucose were also associated with progression to T2D.
- In the multivariable analysis, both A1c and 2-hour glucose were strong independent predictors of progression.
- Fasting plasma glucose was not associated with progression to T2D.
IN PRACTICE:
“Weight stabilization and metformin therapy could be important interventions for diabetes prevention in children,” study author Ashley H. Shoemaker, MD, MSci, a pediatric endocrinologist at Vanderbilt University Medical Center in Nashville, Tenn., said in a press release.
In addition, A1c plus a nonfasting glucose may be a feasible way to identify high-risk pediatric patients in a clinical setting.
SOURCE:
This study was performed by Natasha Belsky, Jaclyn Tamaroff, and Ashley H. Shoemaker of the Vanderbilt University Medical Center and the Vanderbilt University School of Medicine in Nashville, Tenn. It was published October 12, 2023, in the Journal of the Endocrine Society
LIMITATIONS:
Additional patients who developed T2D may have been lost to follow-up, since the authors did not contact patients to confirm their disease status. The authors were also unable to establish racial differences in the progression to T2D because of missing data.
DISCLOSURES:
Funding for this study was provided by the National Center for Advancing Translational Sciences. One author has research contracts with Novo Nordisk and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
TOPLINE:
Nearly one in five adolescents are living with prediabetes, a condition where blood glucose levels are elevated, but are not high enough for a type 2 diabetes (T2D) diagnosis. According to a new study, higher levels of nonfasting glucose and hemoglobin A1c, and worsening obesity are important predictors of progression to T2D. In addition, metformin and weight stabilization may prove to be important interventions for preventing T2D in kids.
METHODOLOGY:
- Researchers did a retrospective chart review of patient data from Vanderbilt University Medical Center Pediatric Prediabetes Clinic, Nashville, Tenn., from May 2015 to August 2022.
- The study included 552 children with prediabetes, defined as abnormal blood glucose (fasting plasma glucose [FPG] ≥ 100 mg/dL, random glucose ≥ 150 mg/dL), or hemoglobin A1c equal to or greater than 5.9%.
- Based on follow-up visits, patients were classified as having progressed to T2D, or nonprogression.
- Researchers analyzed the patients’ initial visit A1c, fasting C-peptide, 2-hour glucose, fasting glucose, and body mass index (BMI), among other baseline characteristics.
TAKEAWAY:
- Thirty-six children (6.5%) progressed to T2D during the duration of the study period.
- The average time to T2D diagnosis was much longer in patients taking metformin (43 months), compared with those not taking the prescribed medication (28 months).
- Worsening obesity was strongly associated with T2D progression – patients who progressed to T2D had a higher BMI at baseline and had continued weight gain.
- A higher baseline A1c, fasting C-peptide, and 2-hour glucose were also associated with progression to T2D.
- In the multivariable analysis, both A1c and 2-hour glucose were strong independent predictors of progression.
- Fasting plasma glucose was not associated with progression to T2D.
IN PRACTICE:
“Weight stabilization and metformin therapy could be important interventions for diabetes prevention in children,” study author Ashley H. Shoemaker, MD, MSci, a pediatric endocrinologist at Vanderbilt University Medical Center in Nashville, Tenn., said in a press release.
In addition, A1c plus a nonfasting glucose may be a feasible way to identify high-risk pediatric patients in a clinical setting.
SOURCE:
This study was performed by Natasha Belsky, Jaclyn Tamaroff, and Ashley H. Shoemaker of the Vanderbilt University Medical Center and the Vanderbilt University School of Medicine in Nashville, Tenn. It was published October 12, 2023, in the Journal of the Endocrine Society
LIMITATIONS:
Additional patients who developed T2D may have been lost to follow-up, since the authors did not contact patients to confirm their disease status. The authors were also unable to establish racial differences in the progression to T2D because of missing data.
DISCLOSURES:
Funding for this study was provided by the National Center for Advancing Translational Sciences. One author has research contracts with Novo Nordisk and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
New ‘twincretin’ pemvidutide: Another option for obesity
HAMBURG, GERMANY – , adding a different type of “twincretin” to a growing mix of incretin-based weight-loss drugs in development that also offer additional benefits.
Pemvidutide (Altimmune Inc) is a long-acting “balanced” dual agonist of both glucagon-like peptide 1 (GLP-1) and glucagon that is in development for the treatment of obesity and nonalcoholic steatohepatitis (NASH) but not type 2 diabetes, as its effect on glucose is neutral. Phase 1 data for pemvidutide’s liver effect were presented in 2022.
In contrast, the dual GLP-1-glucose-dependent insulinotropic polypeptide (GIP) agonist tirzepatide (Mounjaro, Lilly) has been approved for the treatment of type 2 diabetes. It awaits an indication for obesity.
“When you look [at] the results for any given agent, think about obesity as a series of problems. Some overlap, and some don’t. While about 20%-25% of people with obesity also have type 2 diabetes, not everybody does. So the compounds that don’t lower glucose ... those will be great for others who have [fatty liver disease] or hyperlipidemia. ... It’s not going to be one compound for everybody,” said Louis J. Aronne, MD, director of the center for weight management and metabolic clinical research, Weill Cornell Medicine, New York.
Results of a new 24-week interim analysis of data from the phase 2 pemvidutide trial, called MOMENTUM, were presented at the annual meeting of the European Association for the Study of Diabetes by Dr. Aronne.
Included in that session were encore presentations of data for another GLP-1-glucagon dual agonist, survodutide, as well as data for Eli Lilly’s GLP-1-GIP-glucagon “triagonist,” retatrutide. Retatrutide is in development to induce weight loss, while survodutide (Boehringer Ingelheim and Zealand Pharma), like pemvidutide, is in development to induce weight loss and treat fatty liver disease.
Added Dr. Aronne, “As good as [the triple agonist] retatrutide looks, I doubt that every single person with obesity in the world will be treated with it. ... Think about this as a field, the way you treat diabetes and every other chronic illness.”
Asked to comment, session moderator Rajna Golubic, PhD, of the Oxford (England) Centre for Diabetes, Endocrinology and Metabolism, told this news organization, “We need to think in terms of treating beyond weight loss. ... We need to look at the person holistically and at other aspects of cardiometabolic health and treat in a personalized way and choose treatments according to the comorbidities people have.”
Regarding the dual GLP-1-glucagon agonists, including pemvidutide, Dr. Golubic pointed out that the glucagon agonism does the opposite of glucose-lowering agents but that the compound is “balanced for greater affinity for the GLP-1 receptor vs. glucagon, so that the beneficial effects outweigh the effect for glucose but it still harnesses the benefits of glucagon on liver with a decrease in liver fat, with positive effects on heart, positive effects on kidneys, and other beneficial metabolic effects.”
Pemvidutide lowers weight, LDL cholesterol, triglycerides, and blood pressure
Dr. Aronne began his presentation by noting that dyslipidemia, fatty liver disease, and hypertension are the most significant comorbidities of obesity, occurring in 66%-70%, 58%-75%, and 45%-55% of patients, respectively, while type 2 diabetes is less common, at 19%-23%.
Pemvidutide’s GLP-1 receptor agonism reduces appetite, inflammation, and gastric emptying, while glucagon agonism increases lipolysis, mobilizes fat, and increases energy expenditure, Dr. Aronne explained.
The 48-week phase 2 MOMENTUM trial randomly assigned 320 participants with overweight or obesity and at least one obesity-related comorbidity but not diabetes to receive weekly doses of 1.2 mg, 1.8 mg, or 2.4 mg of pemvidutide or placebo. The two lower pemvidutide doses were initiated immediately without titration, while the 2.4-mg dose was titrated rapidly over 4 weeks.
In a prespecified interim analysis of 160 participants, the percent body weight loss at 24 weeks was 10.7%, 9.4%, and 7.3% with the 2.4-mg, 1.8-mg, and 1.2-mg doses, respectively (P < .001). All weight loss values were significant; weight loss with placebo was a nonsignificant 1%.
The proportions of patients who lost at least 5% of their body weight were 84.6%, 66.7%, and 66.7%, respectively, vs. 25% with placebo. Half of the patients who received the 2.4-mg and 1.8-mg doses lost at least 10% of their body weight. Reductions in waist circumference followed suit; the patients who received the 2.4-mg dose lost an average of 10.2 cm, or “in the U.S., about 4 inches or 4 belt loops. That’s pretty good, you need a new belt,” Dr. Aronne commented.
Significant reductions in total cholesterol and triglyceride levels were also seen at week 24 by 16.5% and 25.0%, respectively, with the 2.4-mg dose. Low-density lipoprotein cholesterol levels also dropped, although not significantly; high-density lipoprotein levels dropped significantly.
Systolic blood pressure dropped by 5.5 mm Hg, and diastolic blood pressure dropped by 1.8 mm Hg in the 2.4-mg group and by lesser degrees among the patients who received lower doses. There were no significant changes in heart rate, Dr. Aronne noted.
Glucose homeostasis was preserved in all groups throughout the 24 weeks.
As with all drugs in the incretin class, gastrointestinal adverse events were common. Severe vomiting occurred in one person in the 1.8-mg group and in four with 2.4 mg. Efforts will be made to reduce that in subsequent trials, Dr. Aronne said.
“We have learned over time that going more gradually in titrating up these agents is a better strategy, allowing dose reduction may be a better strategy, and allowing antiemetics temporarily as we increase the dose is a lesson that many have learned doing these trials and of course in our clinical practices,” he commented.
Dr. Golubic told this news organization that the recent emergence of potent incretin-based weight loss drugs is “a huge paradigm shift. The prevalence of obesity will be 35% or higher by 2035. Bariatric surgery isn’t feasible for everyone, and it’s very expensive, so we need drugs to provide benefits in terms of lowering weight, glucose, and other cardiometabolic risk factors.”
The full 48-week data for MOMENTUM will be announced in the fourth quarter of 2023.
Dr. Aronne has received consulting fees from and serves on advisory boards for Allurion, Altimmune, Atria, Gelesis, Jamieson Wellness, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Novo Nordisk, Pfizer, Optum, Eli Lilly, Senda Biosciences, and Versanis; has received research funding from Allurion, AstraZeneca, Gelesis, Janssen Pharmaceuticals, Novo Nordisk, and Eli Lilly; has equity interests in Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, Jamieson Wellness, and Myos Corp; and serves on a board of directors for ERX Pharmaceuticals, Intellihealth, and Jamieson Wellness. Dr. Golubic has received research support from AstraZeneca.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – , adding a different type of “twincretin” to a growing mix of incretin-based weight-loss drugs in development that also offer additional benefits.
Pemvidutide (Altimmune Inc) is a long-acting “balanced” dual agonist of both glucagon-like peptide 1 (GLP-1) and glucagon that is in development for the treatment of obesity and nonalcoholic steatohepatitis (NASH) but not type 2 diabetes, as its effect on glucose is neutral. Phase 1 data for pemvidutide’s liver effect were presented in 2022.
In contrast, the dual GLP-1-glucose-dependent insulinotropic polypeptide (GIP) agonist tirzepatide (Mounjaro, Lilly) has been approved for the treatment of type 2 diabetes. It awaits an indication for obesity.
“When you look [at] the results for any given agent, think about obesity as a series of problems. Some overlap, and some don’t. While about 20%-25% of people with obesity also have type 2 diabetes, not everybody does. So the compounds that don’t lower glucose ... those will be great for others who have [fatty liver disease] or hyperlipidemia. ... It’s not going to be one compound for everybody,” said Louis J. Aronne, MD, director of the center for weight management and metabolic clinical research, Weill Cornell Medicine, New York.
Results of a new 24-week interim analysis of data from the phase 2 pemvidutide trial, called MOMENTUM, were presented at the annual meeting of the European Association for the Study of Diabetes by Dr. Aronne.
Included in that session were encore presentations of data for another GLP-1-glucagon dual agonist, survodutide, as well as data for Eli Lilly’s GLP-1-GIP-glucagon “triagonist,” retatrutide. Retatrutide is in development to induce weight loss, while survodutide (Boehringer Ingelheim and Zealand Pharma), like pemvidutide, is in development to induce weight loss and treat fatty liver disease.
Added Dr. Aronne, “As good as [the triple agonist] retatrutide looks, I doubt that every single person with obesity in the world will be treated with it. ... Think about this as a field, the way you treat diabetes and every other chronic illness.”
Asked to comment, session moderator Rajna Golubic, PhD, of the Oxford (England) Centre for Diabetes, Endocrinology and Metabolism, told this news organization, “We need to think in terms of treating beyond weight loss. ... We need to look at the person holistically and at other aspects of cardiometabolic health and treat in a personalized way and choose treatments according to the comorbidities people have.”
Regarding the dual GLP-1-glucagon agonists, including pemvidutide, Dr. Golubic pointed out that the glucagon agonism does the opposite of glucose-lowering agents but that the compound is “balanced for greater affinity for the GLP-1 receptor vs. glucagon, so that the beneficial effects outweigh the effect for glucose but it still harnesses the benefits of glucagon on liver with a decrease in liver fat, with positive effects on heart, positive effects on kidneys, and other beneficial metabolic effects.”
Pemvidutide lowers weight, LDL cholesterol, triglycerides, and blood pressure
Dr. Aronne began his presentation by noting that dyslipidemia, fatty liver disease, and hypertension are the most significant comorbidities of obesity, occurring in 66%-70%, 58%-75%, and 45%-55% of patients, respectively, while type 2 diabetes is less common, at 19%-23%.
Pemvidutide’s GLP-1 receptor agonism reduces appetite, inflammation, and gastric emptying, while glucagon agonism increases lipolysis, mobilizes fat, and increases energy expenditure, Dr. Aronne explained.
The 48-week phase 2 MOMENTUM trial randomly assigned 320 participants with overweight or obesity and at least one obesity-related comorbidity but not diabetes to receive weekly doses of 1.2 mg, 1.8 mg, or 2.4 mg of pemvidutide or placebo. The two lower pemvidutide doses were initiated immediately without titration, while the 2.4-mg dose was titrated rapidly over 4 weeks.
In a prespecified interim analysis of 160 participants, the percent body weight loss at 24 weeks was 10.7%, 9.4%, and 7.3% with the 2.4-mg, 1.8-mg, and 1.2-mg doses, respectively (P < .001). All weight loss values were significant; weight loss with placebo was a nonsignificant 1%.
The proportions of patients who lost at least 5% of their body weight were 84.6%, 66.7%, and 66.7%, respectively, vs. 25% with placebo. Half of the patients who received the 2.4-mg and 1.8-mg doses lost at least 10% of their body weight. Reductions in waist circumference followed suit; the patients who received the 2.4-mg dose lost an average of 10.2 cm, or “in the U.S., about 4 inches or 4 belt loops. That’s pretty good, you need a new belt,” Dr. Aronne commented.
Significant reductions in total cholesterol and triglyceride levels were also seen at week 24 by 16.5% and 25.0%, respectively, with the 2.4-mg dose. Low-density lipoprotein cholesterol levels also dropped, although not significantly; high-density lipoprotein levels dropped significantly.
Systolic blood pressure dropped by 5.5 mm Hg, and diastolic blood pressure dropped by 1.8 mm Hg in the 2.4-mg group and by lesser degrees among the patients who received lower doses. There were no significant changes in heart rate, Dr. Aronne noted.
Glucose homeostasis was preserved in all groups throughout the 24 weeks.
As with all drugs in the incretin class, gastrointestinal adverse events were common. Severe vomiting occurred in one person in the 1.8-mg group and in four with 2.4 mg. Efforts will be made to reduce that in subsequent trials, Dr. Aronne said.
“We have learned over time that going more gradually in titrating up these agents is a better strategy, allowing dose reduction may be a better strategy, and allowing antiemetics temporarily as we increase the dose is a lesson that many have learned doing these trials and of course in our clinical practices,” he commented.
Dr. Golubic told this news organization that the recent emergence of potent incretin-based weight loss drugs is “a huge paradigm shift. The prevalence of obesity will be 35% or higher by 2035. Bariatric surgery isn’t feasible for everyone, and it’s very expensive, so we need drugs to provide benefits in terms of lowering weight, glucose, and other cardiometabolic risk factors.”
The full 48-week data for MOMENTUM will be announced in the fourth quarter of 2023.
Dr. Aronne has received consulting fees from and serves on advisory boards for Allurion, Altimmune, Atria, Gelesis, Jamieson Wellness, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Novo Nordisk, Pfizer, Optum, Eli Lilly, Senda Biosciences, and Versanis; has received research funding from Allurion, AstraZeneca, Gelesis, Janssen Pharmaceuticals, Novo Nordisk, and Eli Lilly; has equity interests in Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, Jamieson Wellness, and Myos Corp; and serves on a board of directors for ERX Pharmaceuticals, Intellihealth, and Jamieson Wellness. Dr. Golubic has received research support from AstraZeneca.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – , adding a different type of “twincretin” to a growing mix of incretin-based weight-loss drugs in development that also offer additional benefits.
Pemvidutide (Altimmune Inc) is a long-acting “balanced” dual agonist of both glucagon-like peptide 1 (GLP-1) and glucagon that is in development for the treatment of obesity and nonalcoholic steatohepatitis (NASH) but not type 2 diabetes, as its effect on glucose is neutral. Phase 1 data for pemvidutide’s liver effect were presented in 2022.
In contrast, the dual GLP-1-glucose-dependent insulinotropic polypeptide (GIP) agonist tirzepatide (Mounjaro, Lilly) has been approved for the treatment of type 2 diabetes. It awaits an indication for obesity.
“When you look [at] the results for any given agent, think about obesity as a series of problems. Some overlap, and some don’t. While about 20%-25% of people with obesity also have type 2 diabetes, not everybody does. So the compounds that don’t lower glucose ... those will be great for others who have [fatty liver disease] or hyperlipidemia. ... It’s not going to be one compound for everybody,” said Louis J. Aronne, MD, director of the center for weight management and metabolic clinical research, Weill Cornell Medicine, New York.
Results of a new 24-week interim analysis of data from the phase 2 pemvidutide trial, called MOMENTUM, were presented at the annual meeting of the European Association for the Study of Diabetes by Dr. Aronne.
Included in that session were encore presentations of data for another GLP-1-glucagon dual agonist, survodutide, as well as data for Eli Lilly’s GLP-1-GIP-glucagon “triagonist,” retatrutide. Retatrutide is in development to induce weight loss, while survodutide (Boehringer Ingelheim and Zealand Pharma), like pemvidutide, is in development to induce weight loss and treat fatty liver disease.
Added Dr. Aronne, “As good as [the triple agonist] retatrutide looks, I doubt that every single person with obesity in the world will be treated with it. ... Think about this as a field, the way you treat diabetes and every other chronic illness.”
Asked to comment, session moderator Rajna Golubic, PhD, of the Oxford (England) Centre for Diabetes, Endocrinology and Metabolism, told this news organization, “We need to think in terms of treating beyond weight loss. ... We need to look at the person holistically and at other aspects of cardiometabolic health and treat in a personalized way and choose treatments according to the comorbidities people have.”
Regarding the dual GLP-1-glucagon agonists, including pemvidutide, Dr. Golubic pointed out that the glucagon agonism does the opposite of glucose-lowering agents but that the compound is “balanced for greater affinity for the GLP-1 receptor vs. glucagon, so that the beneficial effects outweigh the effect for glucose but it still harnesses the benefits of glucagon on liver with a decrease in liver fat, with positive effects on heart, positive effects on kidneys, and other beneficial metabolic effects.”
Pemvidutide lowers weight, LDL cholesterol, triglycerides, and blood pressure
Dr. Aronne began his presentation by noting that dyslipidemia, fatty liver disease, and hypertension are the most significant comorbidities of obesity, occurring in 66%-70%, 58%-75%, and 45%-55% of patients, respectively, while type 2 diabetes is less common, at 19%-23%.
Pemvidutide’s GLP-1 receptor agonism reduces appetite, inflammation, and gastric emptying, while glucagon agonism increases lipolysis, mobilizes fat, and increases energy expenditure, Dr. Aronne explained.
The 48-week phase 2 MOMENTUM trial randomly assigned 320 participants with overweight or obesity and at least one obesity-related comorbidity but not diabetes to receive weekly doses of 1.2 mg, 1.8 mg, or 2.4 mg of pemvidutide or placebo. The two lower pemvidutide doses were initiated immediately without titration, while the 2.4-mg dose was titrated rapidly over 4 weeks.
In a prespecified interim analysis of 160 participants, the percent body weight loss at 24 weeks was 10.7%, 9.4%, and 7.3% with the 2.4-mg, 1.8-mg, and 1.2-mg doses, respectively (P < .001). All weight loss values were significant; weight loss with placebo was a nonsignificant 1%.
The proportions of patients who lost at least 5% of their body weight were 84.6%, 66.7%, and 66.7%, respectively, vs. 25% with placebo. Half of the patients who received the 2.4-mg and 1.8-mg doses lost at least 10% of their body weight. Reductions in waist circumference followed suit; the patients who received the 2.4-mg dose lost an average of 10.2 cm, or “in the U.S., about 4 inches or 4 belt loops. That’s pretty good, you need a new belt,” Dr. Aronne commented.
Significant reductions in total cholesterol and triglyceride levels were also seen at week 24 by 16.5% and 25.0%, respectively, with the 2.4-mg dose. Low-density lipoprotein cholesterol levels also dropped, although not significantly; high-density lipoprotein levels dropped significantly.
Systolic blood pressure dropped by 5.5 mm Hg, and diastolic blood pressure dropped by 1.8 mm Hg in the 2.4-mg group and by lesser degrees among the patients who received lower doses. There were no significant changes in heart rate, Dr. Aronne noted.
Glucose homeostasis was preserved in all groups throughout the 24 weeks.
As with all drugs in the incretin class, gastrointestinal adverse events were common. Severe vomiting occurred in one person in the 1.8-mg group and in four with 2.4 mg. Efforts will be made to reduce that in subsequent trials, Dr. Aronne said.
“We have learned over time that going more gradually in titrating up these agents is a better strategy, allowing dose reduction may be a better strategy, and allowing antiemetics temporarily as we increase the dose is a lesson that many have learned doing these trials and of course in our clinical practices,” he commented.
Dr. Golubic told this news organization that the recent emergence of potent incretin-based weight loss drugs is “a huge paradigm shift. The prevalence of obesity will be 35% or higher by 2035. Bariatric surgery isn’t feasible for everyone, and it’s very expensive, so we need drugs to provide benefits in terms of lowering weight, glucose, and other cardiometabolic risk factors.”
The full 48-week data for MOMENTUM will be announced in the fourth quarter of 2023.
Dr. Aronne has received consulting fees from and serves on advisory boards for Allurion, Altimmune, Atria, Gelesis, Jamieson Wellness, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Novo Nordisk, Pfizer, Optum, Eli Lilly, Senda Biosciences, and Versanis; has received research funding from Allurion, AstraZeneca, Gelesis, Janssen Pharmaceuticals, Novo Nordisk, and Eli Lilly; has equity interests in Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, Jamieson Wellness, and Myos Corp; and serves on a board of directors for ERX Pharmaceuticals, Intellihealth, and Jamieson Wellness. Dr. Golubic has received research support from AstraZeneca.
A version of this article first appeared on Medscape.com.
AT EASD 2023
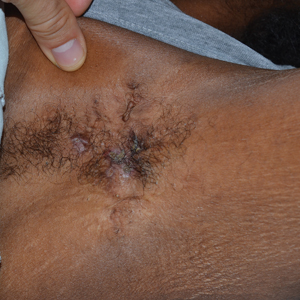
Tender Nodular Lesions in the Axilla and Vulva
The Diagnosis: Cutaneous Langerhans Cell Histiocytosis
Histopathologic findings of the left axillary lesion included a diffuse infiltrate of irregular hematolymphoid cells with reniform nuclei that strongly and diffusely stained positively with CD1a and S-100 but were negative for CD138 and CD163 (Figure). Numerous eosinophils also were present. The surrounding lymphocytic infiltrate stained positively with CD45. Polymerase chain reaction of the vaginal lesion was negative for herpes simplex virus types 1 and 2. Biopsy of the vaginal lesion revealed a mildly acanthotic epidermis and an aggregation of epithelioid cells with reniform nuclei in the papillary dermis. Positron emission tomography revealed widely disseminated disease. Sequencing of the mitogen-activated protein kinase/extracellular signalregulated kinase pathway showed amplified expression of these genes but found no mutations. These results led to a diagnosis of cutaneous Langerhans cell histiocytosis (LCH) with a background of hidradenitis suppurativa (HS). Our patient has since initiated therapy with trametinib leading to disease improvement without known recurrence.

Langerhans cell histiocytosis is a rare disease of clonal dendritic cells (Langerhans cells) that can present in any organ.1 Most LCH diagnoses are made in pediatric patients, most often presenting in the bones, with other presentations in the skin, hypophysis, liver, lymph nodes, lungs, and spleen occurring less commonly.2 Proto-oncogene BRAF V600E mutations are a common determinant of LCH, with half of cases linked with this mutation that leads to enhanced activation of the mitogen-activated protein kinase pathway, though other mutations have been reported.3,4 These genetic alterations suggest LCH is neoplastic in nature; however, this is controversial, as spontaneous regression among pulmonary LCH has been observed, pointing to a reactive inflammatory process.5 Cutaneous LCH can present as a distinct papular or nodular lesion or multiple lesions with possible ulceration, but it is rare that LCH first presents on the skin.2,6 There is a substantial association of cutaneous LCH with the development of systemically disseminated LCH as well as other blood tumors, such as myelomonocytic leukemia, histiocytic sarcoma, and multiple lymphomas; this association is thought to be due to the common origin of LCH and other blood diseases in the bone marrow.6
Histopathology of LCH shows a diffuse papillary dermal infiltrate of clonal proliferation of reniform or cleaved histiocytes.5 Epidermal ulceration and epidermotropism also are common. Neoplastic cells are found admixed with variable levels of eosinophils, lymphocytes, plasma cells, and neutrophils, though eosinophils typically are elevated. Immunohistochemistry characteristically shows the expression of CD1a, S-100, and/or CD207, and the absence of CD163 expression.
Treatment of LCH is primarily dependent on disease dissemination status, with splenic and hepatic involvement, genetic panel results, and central nervous system risk considered in the treatment plan.5 Langerhans cell histiocytosis localized to the skin may require follow-up and monitoring, as spontaneous regression of cutaneous LCH is common. However, topical steroids or psoralen and long-wave UV radiation are potential treatments. Physicians who diagnose unifocal cutaneous LCH should have high clinical suspicion of disseminated LCH, and laboratory and radiographic evaluation may be necessary to rule out systemic disease, as more than 40% of patients with cutaneous LCH have systemic disease upon full evaluation.7 With systemic involvement, systemic chemotherapy may reduce morbidity and mortality, but clinical response should be monitored after 6 weeks of treatment, as results are variably effective. Vinblastine is the most common chemotherapy regimen, with an 84% survival rate and 51.5% event-free survival rate after 8 years.8 Targeted therapy for common genetic mutations also is possible, as vemurafenib has been used to treat patients with the BRAF V600E mutation.
Due to the variable clinical presentation of cutaneous LCH, the lesions can mimic other common skin diseases such as eczema or seborrheic dermatitis.7 However, there are limited data on LCH presenting in infiltrative skin disease. Langerhans cell histiocytosis that was misdiagnosed as HS has been reported,9-11 but LCH presenting alongside long-standing HS is rare. Although LCH often mimics infiltrative skin diseases, its simultaneous presentation with a previously confirmed diagnosis of HS was notable in our patient.
In our patient, the differential diagnosis included HS, Actinomyces infection, lymphomatoid papulosis, and dermatofibrosarcoma protuberans. Cutaneous findings in HS include chronic acneform nodules with follicular plugging, ruptured ducts leading to epithelized sinuses, inflammation, and abscesses in the axillae or inguinal and perineal areas.11 Histopathology reveals follicular occlusion and hyperkeratinization, which cause destruction of the pilosebaceous glands. Hidradenitis suppurativa features on immunohistochemistry often are conflicting, but there consistently is co-localization of keratinocyte hyperplasia with CD3-, CD4-, CD8-, and CD68-positive staining of cells that produce tumor necrosis factor α, IL-12, IL-23, and IL-32, with CD1a staining variable.12 An infection with Actinomyces, a slow-progressing anaerobic or microaerophilic bacteria, may present in the skin with chronic suppurative inflammation on the neck, trunk, and abdomen. The classic presentation is subcutaneous nodules with localized infiltration of abscesses, fistulas, and draining sinuses.13 Morphologically, Actinomyces causes chronic granulomatous infection with 0.1- to 1-mm sulfur granules, which are seen as basophilic masses with eosinophilic terminal clubs on hematoxylin and eosin staining.14 Histopathology reveals grampositive filamentous Actinomyces bacteria that branch at the edge of the granules. Lymphomatoid papulosis, a nonaggressive T-cell lymphoma, presents as papulonodular and sometimes necrotic disseminated lesions that spontaneously can regress or can cause a higher risk for the development of more aggressive lymphomas.15 Histopathology shows consistently dense, dermal, lymphocytic infiltration. Immunohistochemistry is characterized by lymphocytes expressing CD30 of varying degrees: type A with many CD30 staining cells, type B presenting similar to mycosis fungoides with little CD30 staining, and type C with lymphocytic CD30-staining plaques. Dermatofibrosarcoma protuberans is a low-grade soft-tissue malignant tumor with extensive local infiltration characterized by asymptomatic plaques on the trunk and proximal extremities that are indurated and adhered to the skin.16 Histopathology shows extensive invasion into the adjacent tissue far from the original focus of the tumor.
- Girschikofsky M, Arico M, Castillo D, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis. 2013;8:72. doi:10.1186/1750-1172-8-72
- Flores-Terry MA, Sanz-Trenado JL, García-Arpa M, et al. Cutaneous Langerhans cell histiocytosis presenting in adulthood. Actas Dermosifiliogr (Engl Ed). 2019;110:167-169. doi:10.1016/j .adengl.2018.12.005
- Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
- Bohn OL, Teruya-Feldstein J, Sanchez-Sosa S. Skin biopsy diagnosis of Langerhans cell neoplasms. In: Fernando S, ed. Skin Biopsy: Diagnosis and Treatment [Internet]. InTechOpen; 2013. http://dx.doi .org/10.5772/55893
- Edelbroek JR, Vermeer MH, Jansen PM, et al. Langerhans cell histiocytosis first presenting in the skin in adults: frequent association with a second haematological malignancy. Br J Dermatol. 2012;167:1287-1294. doi:10.1111/j.1365-2133.2012.11169.x
- Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165: 990-996. doi:10.1016/j.jpeds.2014.07.063
- Yag˘ ci B, Varan A, Cag˘ lar M, et al. Langerhans cell histiocytosis: retrospective analysis of 217 cases in a single center. Pediatr Hematol Oncol. 2008;25:399-408. doi:10.1080/08880010802107356
- Kalen JE, Shokeen D, Mislankar M, et al. Langerhans cell histiocytosis with clinical and histologic features of hidradenitis suppurativa: brief report and review. Am J Dermatopathol. 2018;40:502-505. doi:10.1097/dad.0000000000001005
- Chertoff J, Chung J, Ataya A. Adult Langerhans cell histiocytosis masquerading as hidradenitis suppurativa. Am J Respir Crit Care Med. 2017;195:E34-E36. doi:10.1164/rccm.201610-2082IM
- St. Claire K, Bunney R, Ashack KA, et al. Langerhans cell histiocytosis: a great imitator. Clin Dermatol. 2020;38:223-234. doi:10.1016/j.clindermatol.2019.10.007
- Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F1000Research. 2019;7:1923. doi:10.12688/f1000research.17268.2
- Robati RM, Niknezhad N, Bidari-Zerehpoush F, et al. Primary cutaneous actinomycosis along with the surgical scar on the hand [published online November 9, 2016]. Case Rep Infect Dis. doi:10.1155/2016/5943932
- Ferry T, Valour F, Karsenty J, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Res. 2014;2014:183-197. doi:10.2147/idr.s39601
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182 /blood-2004-09-3502
- Tsai Y, Lin P, Chew K, et al. Dermatofibrosarcoma protuberans in children and adolescents: clinical presentation, histology, treatment, and review of the literature. J Plast Reconstr Aesthet Surg. 2014;67:1222-1229. doi:10.1016/j.bjps.2014.05.03
The Diagnosis: Cutaneous Langerhans Cell Histiocytosis
Histopathologic findings of the left axillary lesion included a diffuse infiltrate of irregular hematolymphoid cells with reniform nuclei that strongly and diffusely stained positively with CD1a and S-100 but were negative for CD138 and CD163 (Figure). Numerous eosinophils also were present. The surrounding lymphocytic infiltrate stained positively with CD45. Polymerase chain reaction of the vaginal lesion was negative for herpes simplex virus types 1 and 2. Biopsy of the vaginal lesion revealed a mildly acanthotic epidermis and an aggregation of epithelioid cells with reniform nuclei in the papillary dermis. Positron emission tomography revealed widely disseminated disease. Sequencing of the mitogen-activated protein kinase/extracellular signalregulated kinase pathway showed amplified expression of these genes but found no mutations. These results led to a diagnosis of cutaneous Langerhans cell histiocytosis (LCH) with a background of hidradenitis suppurativa (HS). Our patient has since initiated therapy with trametinib leading to disease improvement without known recurrence.

Langerhans cell histiocytosis is a rare disease of clonal dendritic cells (Langerhans cells) that can present in any organ.1 Most LCH diagnoses are made in pediatric patients, most often presenting in the bones, with other presentations in the skin, hypophysis, liver, lymph nodes, lungs, and spleen occurring less commonly.2 Proto-oncogene BRAF V600E mutations are a common determinant of LCH, with half of cases linked with this mutation that leads to enhanced activation of the mitogen-activated protein kinase pathway, though other mutations have been reported.3,4 These genetic alterations suggest LCH is neoplastic in nature; however, this is controversial, as spontaneous regression among pulmonary LCH has been observed, pointing to a reactive inflammatory process.5 Cutaneous LCH can present as a distinct papular or nodular lesion or multiple lesions with possible ulceration, but it is rare that LCH first presents on the skin.2,6 There is a substantial association of cutaneous LCH with the development of systemically disseminated LCH as well as other blood tumors, such as myelomonocytic leukemia, histiocytic sarcoma, and multiple lymphomas; this association is thought to be due to the common origin of LCH and other blood diseases in the bone marrow.6
Histopathology of LCH shows a diffuse papillary dermal infiltrate of clonal proliferation of reniform or cleaved histiocytes.5 Epidermal ulceration and epidermotropism also are common. Neoplastic cells are found admixed with variable levels of eosinophils, lymphocytes, plasma cells, and neutrophils, though eosinophils typically are elevated. Immunohistochemistry characteristically shows the expression of CD1a, S-100, and/or CD207, and the absence of CD163 expression.
Treatment of LCH is primarily dependent on disease dissemination status, with splenic and hepatic involvement, genetic panel results, and central nervous system risk considered in the treatment plan.5 Langerhans cell histiocytosis localized to the skin may require follow-up and monitoring, as spontaneous regression of cutaneous LCH is common. However, topical steroids or psoralen and long-wave UV radiation are potential treatments. Physicians who diagnose unifocal cutaneous LCH should have high clinical suspicion of disseminated LCH, and laboratory and radiographic evaluation may be necessary to rule out systemic disease, as more than 40% of patients with cutaneous LCH have systemic disease upon full evaluation.7 With systemic involvement, systemic chemotherapy may reduce morbidity and mortality, but clinical response should be monitored after 6 weeks of treatment, as results are variably effective. Vinblastine is the most common chemotherapy regimen, with an 84% survival rate and 51.5% event-free survival rate after 8 years.8 Targeted therapy for common genetic mutations also is possible, as vemurafenib has been used to treat patients with the BRAF V600E mutation.
Due to the variable clinical presentation of cutaneous LCH, the lesions can mimic other common skin diseases such as eczema or seborrheic dermatitis.7 However, there are limited data on LCH presenting in infiltrative skin disease. Langerhans cell histiocytosis that was misdiagnosed as HS has been reported,9-11 but LCH presenting alongside long-standing HS is rare. Although LCH often mimics infiltrative skin diseases, its simultaneous presentation with a previously confirmed diagnosis of HS was notable in our patient.
In our patient, the differential diagnosis included HS, Actinomyces infection, lymphomatoid papulosis, and dermatofibrosarcoma protuberans. Cutaneous findings in HS include chronic acneform nodules with follicular plugging, ruptured ducts leading to epithelized sinuses, inflammation, and abscesses in the axillae or inguinal and perineal areas.11 Histopathology reveals follicular occlusion and hyperkeratinization, which cause destruction of the pilosebaceous glands. Hidradenitis suppurativa features on immunohistochemistry often are conflicting, but there consistently is co-localization of keratinocyte hyperplasia with CD3-, CD4-, CD8-, and CD68-positive staining of cells that produce tumor necrosis factor α, IL-12, IL-23, and IL-32, with CD1a staining variable.12 An infection with Actinomyces, a slow-progressing anaerobic or microaerophilic bacteria, may present in the skin with chronic suppurative inflammation on the neck, trunk, and abdomen. The classic presentation is subcutaneous nodules with localized infiltration of abscesses, fistulas, and draining sinuses.13 Morphologically, Actinomyces causes chronic granulomatous infection with 0.1- to 1-mm sulfur granules, which are seen as basophilic masses with eosinophilic terminal clubs on hematoxylin and eosin staining.14 Histopathology reveals grampositive filamentous Actinomyces bacteria that branch at the edge of the granules. Lymphomatoid papulosis, a nonaggressive T-cell lymphoma, presents as papulonodular and sometimes necrotic disseminated lesions that spontaneously can regress or can cause a higher risk for the development of more aggressive lymphomas.15 Histopathology shows consistently dense, dermal, lymphocytic infiltration. Immunohistochemistry is characterized by lymphocytes expressing CD30 of varying degrees: type A with many CD30 staining cells, type B presenting similar to mycosis fungoides with little CD30 staining, and type C with lymphocytic CD30-staining plaques. Dermatofibrosarcoma protuberans is a low-grade soft-tissue malignant tumor with extensive local infiltration characterized by asymptomatic plaques on the trunk and proximal extremities that are indurated and adhered to the skin.16 Histopathology shows extensive invasion into the adjacent tissue far from the original focus of the tumor.
The Diagnosis: Cutaneous Langerhans Cell Histiocytosis
Histopathologic findings of the left axillary lesion included a diffuse infiltrate of irregular hematolymphoid cells with reniform nuclei that strongly and diffusely stained positively with CD1a and S-100 but were negative for CD138 and CD163 (Figure). Numerous eosinophils also were present. The surrounding lymphocytic infiltrate stained positively with CD45. Polymerase chain reaction of the vaginal lesion was negative for herpes simplex virus types 1 and 2. Biopsy of the vaginal lesion revealed a mildly acanthotic epidermis and an aggregation of epithelioid cells with reniform nuclei in the papillary dermis. Positron emission tomography revealed widely disseminated disease. Sequencing of the mitogen-activated protein kinase/extracellular signalregulated kinase pathway showed amplified expression of these genes but found no mutations. These results led to a diagnosis of cutaneous Langerhans cell histiocytosis (LCH) with a background of hidradenitis suppurativa (HS). Our patient has since initiated therapy with trametinib leading to disease improvement without known recurrence.

Langerhans cell histiocytosis is a rare disease of clonal dendritic cells (Langerhans cells) that can present in any organ.1 Most LCH diagnoses are made in pediatric patients, most often presenting in the bones, with other presentations in the skin, hypophysis, liver, lymph nodes, lungs, and spleen occurring less commonly.2 Proto-oncogene BRAF V600E mutations are a common determinant of LCH, with half of cases linked with this mutation that leads to enhanced activation of the mitogen-activated protein kinase pathway, though other mutations have been reported.3,4 These genetic alterations suggest LCH is neoplastic in nature; however, this is controversial, as spontaneous regression among pulmonary LCH has been observed, pointing to a reactive inflammatory process.5 Cutaneous LCH can present as a distinct papular or nodular lesion or multiple lesions with possible ulceration, but it is rare that LCH first presents on the skin.2,6 There is a substantial association of cutaneous LCH with the development of systemically disseminated LCH as well as other blood tumors, such as myelomonocytic leukemia, histiocytic sarcoma, and multiple lymphomas; this association is thought to be due to the common origin of LCH and other blood diseases in the bone marrow.6
Histopathology of LCH shows a diffuse papillary dermal infiltrate of clonal proliferation of reniform or cleaved histiocytes.5 Epidermal ulceration and epidermotropism also are common. Neoplastic cells are found admixed with variable levels of eosinophils, lymphocytes, plasma cells, and neutrophils, though eosinophils typically are elevated. Immunohistochemistry characteristically shows the expression of CD1a, S-100, and/or CD207, and the absence of CD163 expression.
Treatment of LCH is primarily dependent on disease dissemination status, with splenic and hepatic involvement, genetic panel results, and central nervous system risk considered in the treatment plan.5 Langerhans cell histiocytosis localized to the skin may require follow-up and monitoring, as spontaneous regression of cutaneous LCH is common. However, topical steroids or psoralen and long-wave UV radiation are potential treatments. Physicians who diagnose unifocal cutaneous LCH should have high clinical suspicion of disseminated LCH, and laboratory and radiographic evaluation may be necessary to rule out systemic disease, as more than 40% of patients with cutaneous LCH have systemic disease upon full evaluation.7 With systemic involvement, systemic chemotherapy may reduce morbidity and mortality, but clinical response should be monitored after 6 weeks of treatment, as results are variably effective. Vinblastine is the most common chemotherapy regimen, with an 84% survival rate and 51.5% event-free survival rate after 8 years.8 Targeted therapy for common genetic mutations also is possible, as vemurafenib has been used to treat patients with the BRAF V600E mutation.
Due to the variable clinical presentation of cutaneous LCH, the lesions can mimic other common skin diseases such as eczema or seborrheic dermatitis.7 However, there are limited data on LCH presenting in infiltrative skin disease. Langerhans cell histiocytosis that was misdiagnosed as HS has been reported,9-11 but LCH presenting alongside long-standing HS is rare. Although LCH often mimics infiltrative skin diseases, its simultaneous presentation with a previously confirmed diagnosis of HS was notable in our patient.
In our patient, the differential diagnosis included HS, Actinomyces infection, lymphomatoid papulosis, and dermatofibrosarcoma protuberans. Cutaneous findings in HS include chronic acneform nodules with follicular plugging, ruptured ducts leading to epithelized sinuses, inflammation, and abscesses in the axillae or inguinal and perineal areas.11 Histopathology reveals follicular occlusion and hyperkeratinization, which cause destruction of the pilosebaceous glands. Hidradenitis suppurativa features on immunohistochemistry often are conflicting, but there consistently is co-localization of keratinocyte hyperplasia with CD3-, CD4-, CD8-, and CD68-positive staining of cells that produce tumor necrosis factor α, IL-12, IL-23, and IL-32, with CD1a staining variable.12 An infection with Actinomyces, a slow-progressing anaerobic or microaerophilic bacteria, may present in the skin with chronic suppurative inflammation on the neck, trunk, and abdomen. The classic presentation is subcutaneous nodules with localized infiltration of abscesses, fistulas, and draining sinuses.13 Morphologically, Actinomyces causes chronic granulomatous infection with 0.1- to 1-mm sulfur granules, which are seen as basophilic masses with eosinophilic terminal clubs on hematoxylin and eosin staining.14 Histopathology reveals grampositive filamentous Actinomyces bacteria that branch at the edge of the granules. Lymphomatoid papulosis, a nonaggressive T-cell lymphoma, presents as papulonodular and sometimes necrotic disseminated lesions that spontaneously can regress or can cause a higher risk for the development of more aggressive lymphomas.15 Histopathology shows consistently dense, dermal, lymphocytic infiltration. Immunohistochemistry is characterized by lymphocytes expressing CD30 of varying degrees: type A with many CD30 staining cells, type B presenting similar to mycosis fungoides with little CD30 staining, and type C with lymphocytic CD30-staining plaques. Dermatofibrosarcoma protuberans is a low-grade soft-tissue malignant tumor with extensive local infiltration characterized by asymptomatic plaques on the trunk and proximal extremities that are indurated and adhered to the skin.16 Histopathology shows extensive invasion into the adjacent tissue far from the original focus of the tumor.
- Girschikofsky M, Arico M, Castillo D, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis. 2013;8:72. doi:10.1186/1750-1172-8-72
- Flores-Terry MA, Sanz-Trenado JL, García-Arpa M, et al. Cutaneous Langerhans cell histiocytosis presenting in adulthood. Actas Dermosifiliogr (Engl Ed). 2019;110:167-169. doi:10.1016/j .adengl.2018.12.005
- Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
- Bohn OL, Teruya-Feldstein J, Sanchez-Sosa S. Skin biopsy diagnosis of Langerhans cell neoplasms. In: Fernando S, ed. Skin Biopsy: Diagnosis and Treatment [Internet]. InTechOpen; 2013. http://dx.doi .org/10.5772/55893
- Edelbroek JR, Vermeer MH, Jansen PM, et al. Langerhans cell histiocytosis first presenting in the skin in adults: frequent association with a second haematological malignancy. Br J Dermatol. 2012;167:1287-1294. doi:10.1111/j.1365-2133.2012.11169.x
- Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165: 990-996. doi:10.1016/j.jpeds.2014.07.063
- Yag˘ ci B, Varan A, Cag˘ lar M, et al. Langerhans cell histiocytosis: retrospective analysis of 217 cases in a single center. Pediatr Hematol Oncol. 2008;25:399-408. doi:10.1080/08880010802107356
- Kalen JE, Shokeen D, Mislankar M, et al. Langerhans cell histiocytosis with clinical and histologic features of hidradenitis suppurativa: brief report and review. Am J Dermatopathol. 2018;40:502-505. doi:10.1097/dad.0000000000001005
- Chertoff J, Chung J, Ataya A. Adult Langerhans cell histiocytosis masquerading as hidradenitis suppurativa. Am J Respir Crit Care Med. 2017;195:E34-E36. doi:10.1164/rccm.201610-2082IM
- St. Claire K, Bunney R, Ashack KA, et al. Langerhans cell histiocytosis: a great imitator. Clin Dermatol. 2020;38:223-234. doi:10.1016/j.clindermatol.2019.10.007
- Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F1000Research. 2019;7:1923. doi:10.12688/f1000research.17268.2
- Robati RM, Niknezhad N, Bidari-Zerehpoush F, et al. Primary cutaneous actinomycosis along with the surgical scar on the hand [published online November 9, 2016]. Case Rep Infect Dis. doi:10.1155/2016/5943932
- Ferry T, Valour F, Karsenty J, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Res. 2014;2014:183-197. doi:10.2147/idr.s39601
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182 /blood-2004-09-3502
- Tsai Y, Lin P, Chew K, et al. Dermatofibrosarcoma protuberans in children and adolescents: clinical presentation, histology, treatment, and review of the literature. J Plast Reconstr Aesthet Surg. 2014;67:1222-1229. doi:10.1016/j.bjps.2014.05.03
- Girschikofsky M, Arico M, Castillo D, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis. 2013;8:72. doi:10.1186/1750-1172-8-72
- Flores-Terry MA, Sanz-Trenado JL, García-Arpa M, et al. Cutaneous Langerhans cell histiocytosis presenting in adulthood. Actas Dermosifiliogr (Engl Ed). 2019;110:167-169. doi:10.1016/j .adengl.2018.12.005
- Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
- Bohn OL, Teruya-Feldstein J, Sanchez-Sosa S. Skin biopsy diagnosis of Langerhans cell neoplasms. In: Fernando S, ed. Skin Biopsy: Diagnosis and Treatment [Internet]. InTechOpen; 2013. http://dx.doi .org/10.5772/55893
- Edelbroek JR, Vermeer MH, Jansen PM, et al. Langerhans cell histiocytosis first presenting in the skin in adults: frequent association with a second haematological malignancy. Br J Dermatol. 2012;167:1287-1294. doi:10.1111/j.1365-2133.2012.11169.x
- Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165: 990-996. doi:10.1016/j.jpeds.2014.07.063
- Yag˘ ci B, Varan A, Cag˘ lar M, et al. Langerhans cell histiocytosis: retrospective analysis of 217 cases in a single center. Pediatr Hematol Oncol. 2008;25:399-408. doi:10.1080/08880010802107356
- Kalen JE, Shokeen D, Mislankar M, et al. Langerhans cell histiocytosis with clinical and histologic features of hidradenitis suppurativa: brief report and review. Am J Dermatopathol. 2018;40:502-505. doi:10.1097/dad.0000000000001005
- Chertoff J, Chung J, Ataya A. Adult Langerhans cell histiocytosis masquerading as hidradenitis suppurativa. Am J Respir Crit Care Med. 2017;195:E34-E36. doi:10.1164/rccm.201610-2082IM
- St. Claire K, Bunney R, Ashack KA, et al. Langerhans cell histiocytosis: a great imitator. Clin Dermatol. 2020;38:223-234. doi:10.1016/j.clindermatol.2019.10.007
- Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F1000Research. 2019;7:1923. doi:10.12688/f1000research.17268.2
- Robati RM, Niknezhad N, Bidari-Zerehpoush F, et al. Primary cutaneous actinomycosis along with the surgical scar on the hand [published online November 9, 2016]. Case Rep Infect Dis. doi:10.1155/2016/5943932
- Ferry T, Valour F, Karsenty J, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Res. 2014;2014:183-197. doi:10.2147/idr.s39601
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182 /blood-2004-09-3502
- Tsai Y, Lin P, Chew K, et al. Dermatofibrosarcoma protuberans in children and adolescents: clinical presentation, histology, treatment, and review of the literature. J Plast Reconstr Aesthet Surg. 2014;67:1222-1229. doi:10.1016/j.bjps.2014.05.03
A 28-year-old woman presented with tender burning lesions of the left axillary and vaginal skin that had worsened over the last year. Her medical history was notable for hidradenitis suppurativa, which had been present since adolescence, as well as pulmonary Langerhans cell histiocytosis diagnosed 7 years prior to the current presentation after a spontaneous pneumothorax that eventually led to a pulmonary transplantation 3 years prior. The patient’s Langerhans cell histiocytosis was believed to have resolved without treatment after smoking cessation. Physical examination revealed nodular inflammation and scarring with deep undermining along the left axilla as well as swelling of the mons pubis with erosive skin lesions in the surrounding vaginal area. Bilateral cervical, axillary, inguinal, supraclavicular, and femoral lymph node chains were negative for adenopathy. A shave biopsy was performed on the axillary nodule.
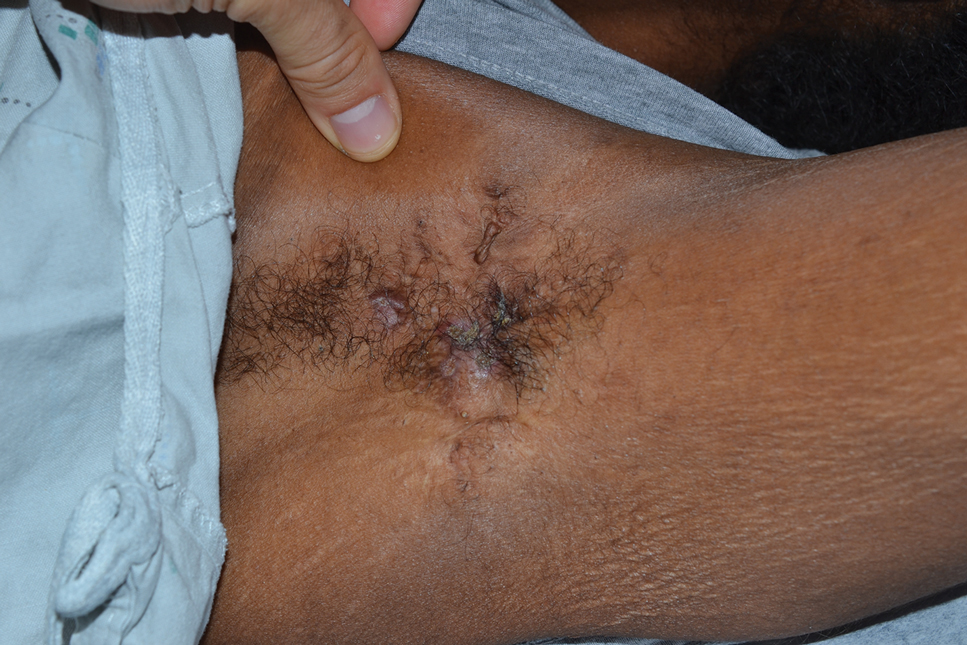
Roflumilast side effect benefits patients with psoriasis and overweight/obesity
BERLIN – .
Reporting secondary outcomes from the investigator-led trial at the annual congress of the European Academy of Dermatology and Venereology, Alexander Egeberg, MD, PhD, DMSc, noted that “clinically significant weight loss” was seen among patients who were treated with oral roflumilast, 500 mcg once daily, versus those receiving placebo.
Indeed, after 12 weeks of therapy, one in three patients treated with oral roflumilast experienced at least a 5% drop in their baseline body weight vs no patients who received placebo (35% vs. 0%; P < .05).
Additionally, a respective 17% versus 0% of patients lost 10% or more of their body weight, and 4% versus 0% lost 15% or more of their baseline body weight at 12 weeks.
After 24 weeks’ treatment, a substantial percentage of patients still had greater than or equal to 5%, greater than or equal to 10%, or greater than or equal to 15% weight loss, at 30%, 17%, and 13% for oral roflumilast, compared with 9%, 0%, and 0% for placebo, respectively.
“We saw that the higher baseline weight correlated with the proportion of weight loss, so that the more heavy patients at baseline also were the ones who experienced the greatest weight loss,” said Dr. Egeberg, who is professor of dermatology at the University of Copenhagen and a senior consultant at the department of dermatology at Bispebjerg Hospital, Copenhagen.
A beneficial side effect in psoriasis?
“You may have heard in psoriasis about topical roflumilast, but oral roflumilast is actually also shown to be effective in treating psoriasis,” said Egeberg.
Topical roflumilast is approved in the United States and Canada for treating plaque psoriasis.
Efficacy results from the PSORRO study were published earlier this year and showed a significantly greater improvement in Psoriasis Area and Severity Index (PASI) 75 with oral roflumilast vs. placebo at 12 weeks (35% vs. 0%), with a sustained effect seen at 24 weeks (44% vs. 40%).
Weight loss was among the most common side effects seen, leading Dr. Egeberg and fellow PSORRO investigators to wonder whether this may actually be a beneficial effect in patients with psoriasis.
“Oral roflumilast is actually a drug that has been on the market for quite a number of years,” Dr. Egeberg said.
Although only currently licensed for chronic obstructive pulmonary disease (COPD) in the United States, oral roflumilast, a phosphodiesterase (PDE) 4 inhibitor, is available as a generic, “which also means that it is extremely affordable,” suggested Dr. Edeberg.
Weight loss may be a problem in patients with COPD, he acknowledged; these patients tend to be underweight as a result of their poor state of health caused by the lung condition. Weight loss could be an advantage in patients with psoriasis who are overweight or living with obesity and have poor cardiometabolic parameters.
The psoriasis treatment with oral roflumilast study
The PSORRO study was a phase 2, multicenter, placebo-controlled, randomized trial performed between 2021 and 2022. A total of 46 adults with plaque psoriasis participated; half were initially treated with oral roflumilast and half with placebo.
Treatment was double-blind for the first 12 weeks, with all patients then receiving open-label treatment with roflumilast for 12 weeks.
The primary endpoint was the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75). A host of secondary endpoints were studied, including weight and cardiometabolic parameters, which Dr. Egeberg reported at the EADV meeting.
Looking at the baseline characteristics of the oral roflumilast and placebo groups, the mean age was a respective 38 and 39 years, 65% and 83% were men, and the mean starting body weight was 102 kg and 105.1 kg.
After 12 weeks of treatment, body weight fell by a mean of 5.4 kg in the oral roflumilast group, with a further decrease of 1.4 kg by 24 weeks, bringing the total average weight loss to 6.8 kg. By comparison, weight loss among those in the placebo group was 0 kg at 12 weeks and around 2 kg at 24 weeks.
The majority of participants in both groups had high baseline BMIs; 70% of those who received oral roflumilast and 61% of those who received placebo had a BMI of 30 or higher.
“We wanted to investigate the impact of body weight, [so] we didn’t allow patients to be underweight when they were included,” Dr. Egeberg explained. Thus, for inclusion, patients had to have a BMI of 20 or higher.
An “extraordinary” finding was how some patients’ weight status based on their BMI changed throughout the study.
“We could see people that went from obese class 3, all the way to obese class 1. And we could see people going from being overweight to normal weight, which is really extraordinary for patients with psoriasis,” Dr. Egeberg said.
“But most importantly,” he added, “we didn’t have any patients who became underweight, suggesting that it actually is safe to use also in normal-weight patients.”
Reduced appetite behind benefit?
Trying to see why the weight loss occurred, Dr. Egeberg noted that it looked like it could be a result of a reduced appetite.
In common with other PDE-4 inhibitors, oral roflumilast treatment was associated with gastrointestinal symptoms – nausea, diarrhea, and abdominal pain – but all of these “decrease to placebo levels again, quite quickly,” he said.
“This really suggests that it’s not because of diarrhea, it’s not because of nausea and abdominal pain; it is because of a reduced appetite that patients actually lose weight when treated with roflumilast,” Dr. Egeberg said. It’s a potential bonus for the drug’s effects on the skin and could afford clinicians an opportunity to help motivate patients to eat well when they do eat, he observed.
Other cardiometabolic parameters assessed included blood pressure, glycated hemoglobin, total cholesterol and other key lipids, creatinine, alanine aminotransferase, and high-sensitivity C-reactive protein, but there were no noteworthy differences between the groups.
Roflumilast is an inexpensive drug because it is generic, Dr. Egeberg observed, but that also means that its use is likely to be off-label.
“It will be up to the treating physician to decide if this is an optimal therapy for their patients,” he suggested.
Cardiometabolic comorbidities important to target
Obesity is a cardiometabolic comorbidity that is important to consider when treating your patients with psoriasis, Paolo Gisondi, MD, of the University of Verona (Italy), said at a separate presentation at the EADV meeting.
While not directly commenting on the roflumilast study, he noted that moderate to severe psoriasis was “frequently associated” with metabolic disorders that put people at additional risk for cardiovascular and fatty liver diseases.
The PSORRO study was an investigator-initiated and investigator-led study and received no commercial funding. Research funding came from the Danish Psoriasis Foundation, Herlev and Gentofte Hospital, and several charitable and humanitarian organizations. Dr. Egeberg acknowledged acting as the principal investigator, speaker, and/or consultant to multiple pharma companies, all of which were unrelated to the study he presented. Dr. Gisondi’s comments were from a separate presentation, and he was not involved in the study.
A version of this article first appeared on Medscape.com.
BERLIN – .
Reporting secondary outcomes from the investigator-led trial at the annual congress of the European Academy of Dermatology and Venereology, Alexander Egeberg, MD, PhD, DMSc, noted that “clinically significant weight loss” was seen among patients who were treated with oral roflumilast, 500 mcg once daily, versus those receiving placebo.
Indeed, after 12 weeks of therapy, one in three patients treated with oral roflumilast experienced at least a 5% drop in their baseline body weight vs no patients who received placebo (35% vs. 0%; P < .05).
Additionally, a respective 17% versus 0% of patients lost 10% or more of their body weight, and 4% versus 0% lost 15% or more of their baseline body weight at 12 weeks.
After 24 weeks’ treatment, a substantial percentage of patients still had greater than or equal to 5%, greater than or equal to 10%, or greater than or equal to 15% weight loss, at 30%, 17%, and 13% for oral roflumilast, compared with 9%, 0%, and 0% for placebo, respectively.
“We saw that the higher baseline weight correlated with the proportion of weight loss, so that the more heavy patients at baseline also were the ones who experienced the greatest weight loss,” said Dr. Egeberg, who is professor of dermatology at the University of Copenhagen and a senior consultant at the department of dermatology at Bispebjerg Hospital, Copenhagen.
A beneficial side effect in psoriasis?
“You may have heard in psoriasis about topical roflumilast, but oral roflumilast is actually also shown to be effective in treating psoriasis,” said Egeberg.
Topical roflumilast is approved in the United States and Canada for treating plaque psoriasis.
Efficacy results from the PSORRO study were published earlier this year and showed a significantly greater improvement in Psoriasis Area and Severity Index (PASI) 75 with oral roflumilast vs. placebo at 12 weeks (35% vs. 0%), with a sustained effect seen at 24 weeks (44% vs. 40%).
Weight loss was among the most common side effects seen, leading Dr. Egeberg and fellow PSORRO investigators to wonder whether this may actually be a beneficial effect in patients with psoriasis.
“Oral roflumilast is actually a drug that has been on the market for quite a number of years,” Dr. Egeberg said.
Although only currently licensed for chronic obstructive pulmonary disease (COPD) in the United States, oral roflumilast, a phosphodiesterase (PDE) 4 inhibitor, is available as a generic, “which also means that it is extremely affordable,” suggested Dr. Edeberg.
Weight loss may be a problem in patients with COPD, he acknowledged; these patients tend to be underweight as a result of their poor state of health caused by the lung condition. Weight loss could be an advantage in patients with psoriasis who are overweight or living with obesity and have poor cardiometabolic parameters.
The psoriasis treatment with oral roflumilast study
The PSORRO study was a phase 2, multicenter, placebo-controlled, randomized trial performed between 2021 and 2022. A total of 46 adults with plaque psoriasis participated; half were initially treated with oral roflumilast and half with placebo.
Treatment was double-blind for the first 12 weeks, with all patients then receiving open-label treatment with roflumilast for 12 weeks.
The primary endpoint was the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75). A host of secondary endpoints were studied, including weight and cardiometabolic parameters, which Dr. Egeberg reported at the EADV meeting.
Looking at the baseline characteristics of the oral roflumilast and placebo groups, the mean age was a respective 38 and 39 years, 65% and 83% were men, and the mean starting body weight was 102 kg and 105.1 kg.
After 12 weeks of treatment, body weight fell by a mean of 5.4 kg in the oral roflumilast group, with a further decrease of 1.4 kg by 24 weeks, bringing the total average weight loss to 6.8 kg. By comparison, weight loss among those in the placebo group was 0 kg at 12 weeks and around 2 kg at 24 weeks.
The majority of participants in both groups had high baseline BMIs; 70% of those who received oral roflumilast and 61% of those who received placebo had a BMI of 30 or higher.
“We wanted to investigate the impact of body weight, [so] we didn’t allow patients to be underweight when they were included,” Dr. Egeberg explained. Thus, for inclusion, patients had to have a BMI of 20 or higher.
An “extraordinary” finding was how some patients’ weight status based on their BMI changed throughout the study.
“We could see people that went from obese class 3, all the way to obese class 1. And we could see people going from being overweight to normal weight, which is really extraordinary for patients with psoriasis,” Dr. Egeberg said.
“But most importantly,” he added, “we didn’t have any patients who became underweight, suggesting that it actually is safe to use also in normal-weight patients.”
Reduced appetite behind benefit?
Trying to see why the weight loss occurred, Dr. Egeberg noted that it looked like it could be a result of a reduced appetite.
In common with other PDE-4 inhibitors, oral roflumilast treatment was associated with gastrointestinal symptoms – nausea, diarrhea, and abdominal pain – but all of these “decrease to placebo levels again, quite quickly,” he said.
“This really suggests that it’s not because of diarrhea, it’s not because of nausea and abdominal pain; it is because of a reduced appetite that patients actually lose weight when treated with roflumilast,” Dr. Egeberg said. It’s a potential bonus for the drug’s effects on the skin and could afford clinicians an opportunity to help motivate patients to eat well when they do eat, he observed.
Other cardiometabolic parameters assessed included blood pressure, glycated hemoglobin, total cholesterol and other key lipids, creatinine, alanine aminotransferase, and high-sensitivity C-reactive protein, but there were no noteworthy differences between the groups.
Roflumilast is an inexpensive drug because it is generic, Dr. Egeberg observed, but that also means that its use is likely to be off-label.
“It will be up to the treating physician to decide if this is an optimal therapy for their patients,” he suggested.
Cardiometabolic comorbidities important to target
Obesity is a cardiometabolic comorbidity that is important to consider when treating your patients with psoriasis, Paolo Gisondi, MD, of the University of Verona (Italy), said at a separate presentation at the EADV meeting.
While not directly commenting on the roflumilast study, he noted that moderate to severe psoriasis was “frequently associated” with metabolic disorders that put people at additional risk for cardiovascular and fatty liver diseases.
The PSORRO study was an investigator-initiated and investigator-led study and received no commercial funding. Research funding came from the Danish Psoriasis Foundation, Herlev and Gentofte Hospital, and several charitable and humanitarian organizations. Dr. Egeberg acknowledged acting as the principal investigator, speaker, and/or consultant to multiple pharma companies, all of which were unrelated to the study he presented. Dr. Gisondi’s comments were from a separate presentation, and he was not involved in the study.
A version of this article first appeared on Medscape.com.
BERLIN – .
Reporting secondary outcomes from the investigator-led trial at the annual congress of the European Academy of Dermatology and Venereology, Alexander Egeberg, MD, PhD, DMSc, noted that “clinically significant weight loss” was seen among patients who were treated with oral roflumilast, 500 mcg once daily, versus those receiving placebo.
Indeed, after 12 weeks of therapy, one in three patients treated with oral roflumilast experienced at least a 5% drop in their baseline body weight vs no patients who received placebo (35% vs. 0%; P < .05).
Additionally, a respective 17% versus 0% of patients lost 10% or more of their body weight, and 4% versus 0% lost 15% or more of their baseline body weight at 12 weeks.
After 24 weeks’ treatment, a substantial percentage of patients still had greater than or equal to 5%, greater than or equal to 10%, or greater than or equal to 15% weight loss, at 30%, 17%, and 13% for oral roflumilast, compared with 9%, 0%, and 0% for placebo, respectively.
“We saw that the higher baseline weight correlated with the proportion of weight loss, so that the more heavy patients at baseline also were the ones who experienced the greatest weight loss,” said Dr. Egeberg, who is professor of dermatology at the University of Copenhagen and a senior consultant at the department of dermatology at Bispebjerg Hospital, Copenhagen.
A beneficial side effect in psoriasis?
“You may have heard in psoriasis about topical roflumilast, but oral roflumilast is actually also shown to be effective in treating psoriasis,” said Egeberg.
Topical roflumilast is approved in the United States and Canada for treating plaque psoriasis.
Efficacy results from the PSORRO study were published earlier this year and showed a significantly greater improvement in Psoriasis Area and Severity Index (PASI) 75 with oral roflumilast vs. placebo at 12 weeks (35% vs. 0%), with a sustained effect seen at 24 weeks (44% vs. 40%).
Weight loss was among the most common side effects seen, leading Dr. Egeberg and fellow PSORRO investigators to wonder whether this may actually be a beneficial effect in patients with psoriasis.
“Oral roflumilast is actually a drug that has been on the market for quite a number of years,” Dr. Egeberg said.
Although only currently licensed for chronic obstructive pulmonary disease (COPD) in the United States, oral roflumilast, a phosphodiesterase (PDE) 4 inhibitor, is available as a generic, “which also means that it is extremely affordable,” suggested Dr. Edeberg.
Weight loss may be a problem in patients with COPD, he acknowledged; these patients tend to be underweight as a result of their poor state of health caused by the lung condition. Weight loss could be an advantage in patients with psoriasis who are overweight or living with obesity and have poor cardiometabolic parameters.
The psoriasis treatment with oral roflumilast study
The PSORRO study was a phase 2, multicenter, placebo-controlled, randomized trial performed between 2021 and 2022. A total of 46 adults with plaque psoriasis participated; half were initially treated with oral roflumilast and half with placebo.
Treatment was double-blind for the first 12 weeks, with all patients then receiving open-label treatment with roflumilast for 12 weeks.
The primary endpoint was the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75). A host of secondary endpoints were studied, including weight and cardiometabolic parameters, which Dr. Egeberg reported at the EADV meeting.
Looking at the baseline characteristics of the oral roflumilast and placebo groups, the mean age was a respective 38 and 39 years, 65% and 83% were men, and the mean starting body weight was 102 kg and 105.1 kg.
After 12 weeks of treatment, body weight fell by a mean of 5.4 kg in the oral roflumilast group, with a further decrease of 1.4 kg by 24 weeks, bringing the total average weight loss to 6.8 kg. By comparison, weight loss among those in the placebo group was 0 kg at 12 weeks and around 2 kg at 24 weeks.
The majority of participants in both groups had high baseline BMIs; 70% of those who received oral roflumilast and 61% of those who received placebo had a BMI of 30 or higher.
“We wanted to investigate the impact of body weight, [so] we didn’t allow patients to be underweight when they were included,” Dr. Egeberg explained. Thus, for inclusion, patients had to have a BMI of 20 or higher.
An “extraordinary” finding was how some patients’ weight status based on their BMI changed throughout the study.
“We could see people that went from obese class 3, all the way to obese class 1. And we could see people going from being overweight to normal weight, which is really extraordinary for patients with psoriasis,” Dr. Egeberg said.
“But most importantly,” he added, “we didn’t have any patients who became underweight, suggesting that it actually is safe to use also in normal-weight patients.”
Reduced appetite behind benefit?
Trying to see why the weight loss occurred, Dr. Egeberg noted that it looked like it could be a result of a reduced appetite.
In common with other PDE-4 inhibitors, oral roflumilast treatment was associated with gastrointestinal symptoms – nausea, diarrhea, and abdominal pain – but all of these “decrease to placebo levels again, quite quickly,” he said.
“This really suggests that it’s not because of diarrhea, it’s not because of nausea and abdominal pain; it is because of a reduced appetite that patients actually lose weight when treated with roflumilast,” Dr. Egeberg said. It’s a potential bonus for the drug’s effects on the skin and could afford clinicians an opportunity to help motivate patients to eat well when they do eat, he observed.
Other cardiometabolic parameters assessed included blood pressure, glycated hemoglobin, total cholesterol and other key lipids, creatinine, alanine aminotransferase, and high-sensitivity C-reactive protein, but there were no noteworthy differences between the groups.
Roflumilast is an inexpensive drug because it is generic, Dr. Egeberg observed, but that also means that its use is likely to be off-label.
“It will be up to the treating physician to decide if this is an optimal therapy for their patients,” he suggested.
Cardiometabolic comorbidities important to target
Obesity is a cardiometabolic comorbidity that is important to consider when treating your patients with psoriasis, Paolo Gisondi, MD, of the University of Verona (Italy), said at a separate presentation at the EADV meeting.
While not directly commenting on the roflumilast study, he noted that moderate to severe psoriasis was “frequently associated” with metabolic disorders that put people at additional risk for cardiovascular and fatty liver diseases.
The PSORRO study was an investigator-initiated and investigator-led study and received no commercial funding. Research funding came from the Danish Psoriasis Foundation, Herlev and Gentofte Hospital, and several charitable and humanitarian organizations. Dr. Egeberg acknowledged acting as the principal investigator, speaker, and/or consultant to multiple pharma companies, all of which were unrelated to the study he presented. Dr. Gisondi’s comments were from a separate presentation, and he was not involved in the study.
A version of this article first appeared on Medscape.com.
AT THE EADV CONGRESS
Novel triple-threat approach to acne beats placebo
TOPLINE:
A topical fixed-dose combination of three approved acne treatments significantly improves moderate to severe acne with a strong safety profile.
METHODOLOGY:
- The two multicenter studies included 363 individuals aged 9 years and older with moderate to severe acne from 30 centers, including 15 in North America.
- Moderate to severe acne was defined as having 30-100 inflammatory lesions (papules, pustules, or nodules), 35-150 noninflammatory lesions (open or closed comedones), and at least two nodules.
- Participants were randomly assigned to receive treatment with a combination gel containing phosphate 1.2%, 0.15%, and 3.1% (known as IDP-126) or a vehicle gel for once-daily application for 12 weeks.
- Treatment success was defined as a reduction of at least two grades from baseline on the Evaluator’s Global Severity Score (EGSS) and lesion counts of clear (0) or almost clear (1) at weeks 2, 4, 8, and 12.
TAKEAWAY:
- Treatment success occurred in 49.6% of the IDP-126 group, vs 24.9% of the vehicle group in study 1, and in 50.5% of the IDP-126 group, vs 20.5% of the vehicle group in study 2. Overall treatment compliance was 93.7% and 91.3% for studies 1 and 2, respectively (P < .01 for both).
- Patients in the IDP-126 groups for both studies 1 and 2 had significantly greater absolute mean reductions in both inflammatory and noninflammatory lesions from baseline to week 12 compared to the vehicle patients (P ≤ .001 for all).
- Significantly more patients in the IDP-126 group achieved a grade reduction of 2 or more in EGSS compared with those who received the vehicle, with treatment differences of approximately 32% in both studies. Changes in lesion reductions between the treatment and the vehicle groups were significantly greater as early as week 4.
- The most common treatment-related adverse events among patients treated with IDP-126 were erythema, application-site pain, dryness, irritation, and exfoliation. Discontinuation of the study drug as a result of adverse events occurred in 2.5% and 3.3% of these patients in studies 1 and 2, respectively.
IN PRACTICE:
“With its simple treatment regimen containing 3 recommended acne treatments (benzoyl peroxide, a topical retinoid, and a topical antibiotic), IDP-126 is a potential new treatment option for acne,” the researchers concluded.
SOURCE:
The study was led by Linda Stein Gold, MD, of Henry Ford Hospital, Detroit. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
In both studies, treatment duration was short, and the studies may not reflect patients’ real-world experiences. The results may be affected by interobserver bias or variation in assessment of acne severity.
DISCLOSURES:
Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Other study coauthors have relationships with multiple companies, including Ortho Dermatologics, which provided medical writing support for the study.
A version of this article first appeared on Medscape.com.
TOPLINE:
A topical fixed-dose combination of three approved acne treatments significantly improves moderate to severe acne with a strong safety profile.
METHODOLOGY:
- The two multicenter studies included 363 individuals aged 9 years and older with moderate to severe acne from 30 centers, including 15 in North America.
- Moderate to severe acne was defined as having 30-100 inflammatory lesions (papules, pustules, or nodules), 35-150 noninflammatory lesions (open or closed comedones), and at least two nodules.
- Participants were randomly assigned to receive treatment with a combination gel containing phosphate 1.2%, 0.15%, and 3.1% (known as IDP-126) or a vehicle gel for once-daily application for 12 weeks.
- Treatment success was defined as a reduction of at least two grades from baseline on the Evaluator’s Global Severity Score (EGSS) and lesion counts of clear (0) or almost clear (1) at weeks 2, 4, 8, and 12.
TAKEAWAY:
- Treatment success occurred in 49.6% of the IDP-126 group, vs 24.9% of the vehicle group in study 1, and in 50.5% of the IDP-126 group, vs 20.5% of the vehicle group in study 2. Overall treatment compliance was 93.7% and 91.3% for studies 1 and 2, respectively (P < .01 for both).
- Patients in the IDP-126 groups for both studies 1 and 2 had significantly greater absolute mean reductions in both inflammatory and noninflammatory lesions from baseline to week 12 compared to the vehicle patients (P ≤ .001 for all).
- Significantly more patients in the IDP-126 group achieved a grade reduction of 2 or more in EGSS compared with those who received the vehicle, with treatment differences of approximately 32% in both studies. Changes in lesion reductions between the treatment and the vehicle groups were significantly greater as early as week 4.
- The most common treatment-related adverse events among patients treated with IDP-126 were erythema, application-site pain, dryness, irritation, and exfoliation. Discontinuation of the study drug as a result of adverse events occurred in 2.5% and 3.3% of these patients in studies 1 and 2, respectively.
IN PRACTICE:
“With its simple treatment regimen containing 3 recommended acne treatments (benzoyl peroxide, a topical retinoid, and a topical antibiotic), IDP-126 is a potential new treatment option for acne,” the researchers concluded.
SOURCE:
The study was led by Linda Stein Gold, MD, of Henry Ford Hospital, Detroit. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
In both studies, treatment duration was short, and the studies may not reflect patients’ real-world experiences. The results may be affected by interobserver bias or variation in assessment of acne severity.
DISCLOSURES:
Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Other study coauthors have relationships with multiple companies, including Ortho Dermatologics, which provided medical writing support for the study.
A version of this article first appeared on Medscape.com.
TOPLINE:
A topical fixed-dose combination of three approved acne treatments significantly improves moderate to severe acne with a strong safety profile.
METHODOLOGY:
- The two multicenter studies included 363 individuals aged 9 years and older with moderate to severe acne from 30 centers, including 15 in North America.
- Moderate to severe acne was defined as having 30-100 inflammatory lesions (papules, pustules, or nodules), 35-150 noninflammatory lesions (open or closed comedones), and at least two nodules.
- Participants were randomly assigned to receive treatment with a combination gel containing phosphate 1.2%, 0.15%, and 3.1% (known as IDP-126) or a vehicle gel for once-daily application for 12 weeks.
- Treatment success was defined as a reduction of at least two grades from baseline on the Evaluator’s Global Severity Score (EGSS) and lesion counts of clear (0) or almost clear (1) at weeks 2, 4, 8, and 12.
TAKEAWAY:
- Treatment success occurred in 49.6% of the IDP-126 group, vs 24.9% of the vehicle group in study 1, and in 50.5% of the IDP-126 group, vs 20.5% of the vehicle group in study 2. Overall treatment compliance was 93.7% and 91.3% for studies 1 and 2, respectively (P < .01 for both).
- Patients in the IDP-126 groups for both studies 1 and 2 had significantly greater absolute mean reductions in both inflammatory and noninflammatory lesions from baseline to week 12 compared to the vehicle patients (P ≤ .001 for all).
- Significantly more patients in the IDP-126 group achieved a grade reduction of 2 or more in EGSS compared with those who received the vehicle, with treatment differences of approximately 32% in both studies. Changes in lesion reductions between the treatment and the vehicle groups were significantly greater as early as week 4.
- The most common treatment-related adverse events among patients treated with IDP-126 were erythema, application-site pain, dryness, irritation, and exfoliation. Discontinuation of the study drug as a result of adverse events occurred in 2.5% and 3.3% of these patients in studies 1 and 2, respectively.
IN PRACTICE:
“With its simple treatment regimen containing 3 recommended acne treatments (benzoyl peroxide, a topical retinoid, and a topical antibiotic), IDP-126 is a potential new treatment option for acne,” the researchers concluded.
SOURCE:
The study was led by Linda Stein Gold, MD, of Henry Ford Hospital, Detroit. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
In both studies, treatment duration was short, and the studies may not reflect patients’ real-world experiences. The results may be affected by interobserver bias or variation in assessment of acne severity.
DISCLOSURES:
Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Other study coauthors have relationships with multiple companies, including Ortho Dermatologics, which provided medical writing support for the study.
A version of this article first appeared on Medscape.com.
Antibiotics ‘like gold’ for some, driving inappropriate use
Personal beliefs and health care system barriers contribute to inappropriate antibiotic use by patients, report researchers presenting results at an annual scientific meeting on infectious diseases.
Nonprescription antibiotic use includes accessing medication left over from a prior prescribed course, obtained from social networks, and purchased over-the-counter in other countries or illegally in stores and markets in the United States.
Overuse and misuse of antibiotics contributes to a growing threat of antimicrobial resistance, and it is tough to say how common it is, Lindsey A. Laytner, PhD, MPH, with Baylor College of Medicine, Houston, pointed out in her presentation.
“This is an understudied area. We don’t routinely collect these data, so we don’t actually know what the true prevalence is. The factors that contribute to this unsafe practice in the U.S. are also underexplored,” Dr. Laytner said.
To investigate, the researchers conducted in-depth interviews with 86 adults (median age, 49 years; 62% women) to identify patients’ motivations to use antibiotics without a prescription. All of them answered “yes” when asked in a previous survey whether they would use antibiotics without contacting a doctor, nurse, dentist, or clinic.
Dr. Laytner said several prominent themes emerged.
Nearly all interviewees reported nonprescription antibiotic use for symptoms that mostly do not warrant antibiotics. These included symptoms of COVID-19, influenza, and the common cold, as well as for pain management, allergies, and even wounds.
Ineffectively treating symptoms
Many felt they “knew their body, knew what they had, and knew how to treat themselves” without a health care provider, Dr. Laytner said.
They also felt the over-the-counter medicines “don’t always work and that antibiotics are like gold or this cure-all and because they are difficult to get a prescription for, they should be kept on hand,” she explained.
A variety of health care system barriers also contribute to inappropriate antibiotic use, including long wait times to schedule appointments and to see the doctor while at their appointments; high costs for clinic visits and prescriptions; and transportation issues.
Many patients opted to use nonprescription antibiotics out of “convenience,” Laytner added.
She explains that the findings could help inform community-level education efforts on inappropriate use of antibiotics and help shape policies to promote antibiotic stewardship.
Access to care, education
Commenting on the study, Emily Sydnor Spivak, MD, associate professor of medicine at University of Utah, Salt Lake City, said she “wasn’t totally surprised by the results, but found it very interesting how there was a theme of autonomy, or ‘I know my body,’ that seemed to drive patients to get antibiotics for relief of symptoms.”
said Dr. Spivak, who is also medical director of antimicrobial stewardship programs at University of Utah Health and VA Salt Lake City Health Care System.
“Given the lack of access to health care as a reason some patients use nonprescription antibiotics, we need to think about access to the health care system and process changes and policy changes to allow better access. Without better access or interaction with the health care system, we can’t educate patients,” Dr. Spivak said.
The study had no commercial funding. Dr. Laytner and Dr. Spivak report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Personal beliefs and health care system barriers contribute to inappropriate antibiotic use by patients, report researchers presenting results at an annual scientific meeting on infectious diseases.
Nonprescription antibiotic use includes accessing medication left over from a prior prescribed course, obtained from social networks, and purchased over-the-counter in other countries or illegally in stores and markets in the United States.
Overuse and misuse of antibiotics contributes to a growing threat of antimicrobial resistance, and it is tough to say how common it is, Lindsey A. Laytner, PhD, MPH, with Baylor College of Medicine, Houston, pointed out in her presentation.
“This is an understudied area. We don’t routinely collect these data, so we don’t actually know what the true prevalence is. The factors that contribute to this unsafe practice in the U.S. are also underexplored,” Dr. Laytner said.
To investigate, the researchers conducted in-depth interviews with 86 adults (median age, 49 years; 62% women) to identify patients’ motivations to use antibiotics without a prescription. All of them answered “yes” when asked in a previous survey whether they would use antibiotics without contacting a doctor, nurse, dentist, or clinic.
Dr. Laytner said several prominent themes emerged.
Nearly all interviewees reported nonprescription antibiotic use for symptoms that mostly do not warrant antibiotics. These included symptoms of COVID-19, influenza, and the common cold, as well as for pain management, allergies, and even wounds.
Ineffectively treating symptoms
Many felt they “knew their body, knew what they had, and knew how to treat themselves” without a health care provider, Dr. Laytner said.
They also felt the over-the-counter medicines “don’t always work and that antibiotics are like gold or this cure-all and because they are difficult to get a prescription for, they should be kept on hand,” she explained.
A variety of health care system barriers also contribute to inappropriate antibiotic use, including long wait times to schedule appointments and to see the doctor while at their appointments; high costs for clinic visits and prescriptions; and transportation issues.
Many patients opted to use nonprescription antibiotics out of “convenience,” Laytner added.
She explains that the findings could help inform community-level education efforts on inappropriate use of antibiotics and help shape policies to promote antibiotic stewardship.
Access to care, education
Commenting on the study, Emily Sydnor Spivak, MD, associate professor of medicine at University of Utah, Salt Lake City, said she “wasn’t totally surprised by the results, but found it very interesting how there was a theme of autonomy, or ‘I know my body,’ that seemed to drive patients to get antibiotics for relief of symptoms.”
said Dr. Spivak, who is also medical director of antimicrobial stewardship programs at University of Utah Health and VA Salt Lake City Health Care System.
“Given the lack of access to health care as a reason some patients use nonprescription antibiotics, we need to think about access to the health care system and process changes and policy changes to allow better access. Without better access or interaction with the health care system, we can’t educate patients,” Dr. Spivak said.
The study had no commercial funding. Dr. Laytner and Dr. Spivak report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Personal beliefs and health care system barriers contribute to inappropriate antibiotic use by patients, report researchers presenting results at an annual scientific meeting on infectious diseases.
Nonprescription antibiotic use includes accessing medication left over from a prior prescribed course, obtained from social networks, and purchased over-the-counter in other countries or illegally in stores and markets in the United States.
Overuse and misuse of antibiotics contributes to a growing threat of antimicrobial resistance, and it is tough to say how common it is, Lindsey A. Laytner, PhD, MPH, with Baylor College of Medicine, Houston, pointed out in her presentation.
“This is an understudied area. We don’t routinely collect these data, so we don’t actually know what the true prevalence is. The factors that contribute to this unsafe practice in the U.S. are also underexplored,” Dr. Laytner said.
To investigate, the researchers conducted in-depth interviews with 86 adults (median age, 49 years; 62% women) to identify patients’ motivations to use antibiotics without a prescription. All of them answered “yes” when asked in a previous survey whether they would use antibiotics without contacting a doctor, nurse, dentist, or clinic.
Dr. Laytner said several prominent themes emerged.
Nearly all interviewees reported nonprescription antibiotic use for symptoms that mostly do not warrant antibiotics. These included symptoms of COVID-19, influenza, and the common cold, as well as for pain management, allergies, and even wounds.
Ineffectively treating symptoms
Many felt they “knew their body, knew what they had, and knew how to treat themselves” without a health care provider, Dr. Laytner said.
They also felt the over-the-counter medicines “don’t always work and that antibiotics are like gold or this cure-all and because they are difficult to get a prescription for, they should be kept on hand,” she explained.
A variety of health care system barriers also contribute to inappropriate antibiotic use, including long wait times to schedule appointments and to see the doctor while at their appointments; high costs for clinic visits and prescriptions; and transportation issues.
Many patients opted to use nonprescription antibiotics out of “convenience,” Laytner added.
She explains that the findings could help inform community-level education efforts on inappropriate use of antibiotics and help shape policies to promote antibiotic stewardship.
Access to care, education
Commenting on the study, Emily Sydnor Spivak, MD, associate professor of medicine at University of Utah, Salt Lake City, said she “wasn’t totally surprised by the results, but found it very interesting how there was a theme of autonomy, or ‘I know my body,’ that seemed to drive patients to get antibiotics for relief of symptoms.”
said Dr. Spivak, who is also medical director of antimicrobial stewardship programs at University of Utah Health and VA Salt Lake City Health Care System.
“Given the lack of access to health care as a reason some patients use nonprescription antibiotics, we need to think about access to the health care system and process changes and policy changes to allow better access. Without better access or interaction with the health care system, we can’t educate patients,” Dr. Spivak said.
The study had no commercial funding. Dr. Laytner and Dr. Spivak report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
IDWEEK 2023
Bone degradation measure can sway osteoporosis diagnosis
Assessing a key aspect of bone architecture, for which clinicians can now be reimbursed under Medicare, can significantly improve the ability to predict a patient’s risk for bone fracture.
Although bone mineral density (BMD) is traditionally used to identify patients with osteoporosis or low bone mass, some physicians have begun incorporating the trabecular bone score (TBS) into their exams.
At the Cleveland Clinic Center for Specialized Women’s Health, factoring in the TBS changed the diagnosis for 16% of 432 patients, according to Holly Thacker, MD, the center’s director.
“Importantly, 11% got worse diagnoses, and I use that in terms of prioritizing treatment,” Dr. Thacker said in an interview. The ability to determine how degraded the bone microarchitecture is through a software system “is a huge advance.”
Dr. Thacker described her center’s experience with the technology at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
While BMD captures the amount of minerals like calcium in the skeleton, TBS assesses the underlying microarchitecture by looking at the distribution of shades of gray on dual-energy x-ray absorptiometry (DXA) scans.
Based on the TBS, patients’ bones are classified as normal, partially degraded, or degraded. Among the 432 patients who received a TBS analysis in 2022, 3% shifted from a normal diagnosis to osteopenia, 8% worsened from osteopenia to osteoporosis, 4% went from osteopenia to normal, and 1.6% downgraded from osteoporosis to osteopenia, Dr. Thacker reported.
The new test may also provide some reassurance for female patients who have thinner bones, which may raise alarms based on BMD. TBS, however, may show that the structure of the bone looks normal.
“When you know that the microarchitecture is normal, you’re a lot less concerned that they actually have a bone disease of osteoporosis,” Dr. Thacker said.
Conversely, unexpectedly degraded bone raises questions.
“That makes you go back and say [to the patient]: ‘Have you been on steroids? Were you malnourished? Is there some other metabolic problem? Have you had some calcium disorder?’ ” Dr. Thacker said.
Dr. Thacker leverages the TBS to help patients obtain effective therapy, typically an anabolic agent followed by antiresorptive medication.
“When I see a patient who not only has osteoporosis on bone density but has completely degraded bone architecture, it’s a lot easier for me to make the argument to the insurance company that this patient is at grave risk for a low trauma fracture and bad outcome without the best treatment,” Dr. Thacker said.
10-year-old tech, recently covered
The Food and Drug Administration approved TBS software in 2012, but Medicare only recently started paying for it.
Medimaps Group, a company that markets imaging software to calculate TBS, announced in 2022 that reimbursement from the Centers for Medicare & Medicaid Services was available, at $41.53 on the Physician Fee Schedule and $82.61 on the Hospital Outpatient Prospective Payment Schedule.
“Reimbursement through CMS is an important endorsement of the clinical value of TBS for clinicians and their patients,” Didier Hans, PhD, MBA, the CEO of Medimaps, said in a statement at the time. He noted that more than 600,000 TBS procedures were being performed in the United States each year.
Nevertheless, the initial investment in purchasing the software may be a barrier for health systems.
“We are the first and only site in our health system to offer TBS, as this is an extra expense and not uniformly reimbursed by insurers,” Dr. Thacker reported at the meeting.
Potential drawbacks
The TBS software used in Dr. Thacker’s study has been validated only in Asian and White patients between certain ages and weights, meaning the system is not designed to be used for other populations. Other researchers have highlighted a need for trabecular bone scoring to be validated more broadly. The authors of a recent analysis, however, suggest that TBS can be used the same way no matter a patient’s race.
TBS “is going to be most helpful in those with osteopenia who are right near the threshold for treatment,” said Marcella Donovan Walker, MD, MS, in a presentation on bone quality at the meeting.
Many studies have shown that TBS “provides additive information to bone density,” said Dr. Walker, a professor of medicine in the division of endocrinology at Columbia University, New York. For example, a large study of women in Manitoba found that, regardless of whether their bone density was normal, osteopenic, or osteoporotic, those with a low TBS had a much higher risk for fracture.
‘Opportunistic screening’ with CT?
TBS relies on the same DXA scans that are used to calculate bone mineral density, so obtaining the score does not add time or radiation to the scanning process, Dr. Thacker said.
But many patients who should receive DXA scans do not, which adds to the promise of “opportunistic screening” for osteoporosis, Dr. Walker said. With this approach, physicians would analyze a CT scan that a patient received for another purpose, such as to investigate abdominal pain or chest pain.
“In these images is information about the bone,” Dr. Walker said.
Researchers have used high-resolution peripheral quantitative CT to perform finite element analysis, where a computer program simulates compression of the bone to create a measure of bone stiffness and determine the load required for a break.
One study found that including those elements predicted fractures better than bone mineral density or the Fracture Risk Assessment Tool alone, Dr. Walker noted.
Other aspects of bone quality include how many cracks are in the bone, the amount of adipose in the marrow space, and the rate at which bone is broken down and rebuilt. But Dr. Walker suggested that the longstanding focus on bone mineral density in clinical practice makes sense.
“By far, bone mass is the most important bone quality,” Dr. Walker said.
Dr. Thacker is the executive director of the nonprofit Speaking of Women’s Health. Dr. Walker reported receiving funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and Amgen.
A version of this article first appeared on Medscape.com.
Assessing a key aspect of bone architecture, for which clinicians can now be reimbursed under Medicare, can significantly improve the ability to predict a patient’s risk for bone fracture.
Although bone mineral density (BMD) is traditionally used to identify patients with osteoporosis or low bone mass, some physicians have begun incorporating the trabecular bone score (TBS) into their exams.
At the Cleveland Clinic Center for Specialized Women’s Health, factoring in the TBS changed the diagnosis for 16% of 432 patients, according to Holly Thacker, MD, the center’s director.
“Importantly, 11% got worse diagnoses, and I use that in terms of prioritizing treatment,” Dr. Thacker said in an interview. The ability to determine how degraded the bone microarchitecture is through a software system “is a huge advance.”
Dr. Thacker described her center’s experience with the technology at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
While BMD captures the amount of minerals like calcium in the skeleton, TBS assesses the underlying microarchitecture by looking at the distribution of shades of gray on dual-energy x-ray absorptiometry (DXA) scans.
Based on the TBS, patients’ bones are classified as normal, partially degraded, or degraded. Among the 432 patients who received a TBS analysis in 2022, 3% shifted from a normal diagnosis to osteopenia, 8% worsened from osteopenia to osteoporosis, 4% went from osteopenia to normal, and 1.6% downgraded from osteoporosis to osteopenia, Dr. Thacker reported.
The new test may also provide some reassurance for female patients who have thinner bones, which may raise alarms based on BMD. TBS, however, may show that the structure of the bone looks normal.
“When you know that the microarchitecture is normal, you’re a lot less concerned that they actually have a bone disease of osteoporosis,” Dr. Thacker said.
Conversely, unexpectedly degraded bone raises questions.
“That makes you go back and say [to the patient]: ‘Have you been on steroids? Were you malnourished? Is there some other metabolic problem? Have you had some calcium disorder?’ ” Dr. Thacker said.
Dr. Thacker leverages the TBS to help patients obtain effective therapy, typically an anabolic agent followed by antiresorptive medication.
“When I see a patient who not only has osteoporosis on bone density but has completely degraded bone architecture, it’s a lot easier for me to make the argument to the insurance company that this patient is at grave risk for a low trauma fracture and bad outcome without the best treatment,” Dr. Thacker said.
10-year-old tech, recently covered
The Food and Drug Administration approved TBS software in 2012, but Medicare only recently started paying for it.
Medimaps Group, a company that markets imaging software to calculate TBS, announced in 2022 that reimbursement from the Centers for Medicare & Medicaid Services was available, at $41.53 on the Physician Fee Schedule and $82.61 on the Hospital Outpatient Prospective Payment Schedule.
“Reimbursement through CMS is an important endorsement of the clinical value of TBS for clinicians and their patients,” Didier Hans, PhD, MBA, the CEO of Medimaps, said in a statement at the time. He noted that more than 600,000 TBS procedures were being performed in the United States each year.
Nevertheless, the initial investment in purchasing the software may be a barrier for health systems.
“We are the first and only site in our health system to offer TBS, as this is an extra expense and not uniformly reimbursed by insurers,” Dr. Thacker reported at the meeting.
Potential drawbacks
The TBS software used in Dr. Thacker’s study has been validated only in Asian and White patients between certain ages and weights, meaning the system is not designed to be used for other populations. Other researchers have highlighted a need for trabecular bone scoring to be validated more broadly. The authors of a recent analysis, however, suggest that TBS can be used the same way no matter a patient’s race.
TBS “is going to be most helpful in those with osteopenia who are right near the threshold for treatment,” said Marcella Donovan Walker, MD, MS, in a presentation on bone quality at the meeting.
Many studies have shown that TBS “provides additive information to bone density,” said Dr. Walker, a professor of medicine in the division of endocrinology at Columbia University, New York. For example, a large study of women in Manitoba found that, regardless of whether their bone density was normal, osteopenic, or osteoporotic, those with a low TBS had a much higher risk for fracture.
‘Opportunistic screening’ with CT?
TBS relies on the same DXA scans that are used to calculate bone mineral density, so obtaining the score does not add time or radiation to the scanning process, Dr. Thacker said.
But many patients who should receive DXA scans do not, which adds to the promise of “opportunistic screening” for osteoporosis, Dr. Walker said. With this approach, physicians would analyze a CT scan that a patient received for another purpose, such as to investigate abdominal pain or chest pain.
“In these images is information about the bone,” Dr. Walker said.
Researchers have used high-resolution peripheral quantitative CT to perform finite element analysis, where a computer program simulates compression of the bone to create a measure of bone stiffness and determine the load required for a break.
One study found that including those elements predicted fractures better than bone mineral density or the Fracture Risk Assessment Tool alone, Dr. Walker noted.
Other aspects of bone quality include how many cracks are in the bone, the amount of adipose in the marrow space, and the rate at which bone is broken down and rebuilt. But Dr. Walker suggested that the longstanding focus on bone mineral density in clinical practice makes sense.
“By far, bone mass is the most important bone quality,” Dr. Walker said.
Dr. Thacker is the executive director of the nonprofit Speaking of Women’s Health. Dr. Walker reported receiving funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and Amgen.
A version of this article first appeared on Medscape.com.
Assessing a key aspect of bone architecture, for which clinicians can now be reimbursed under Medicare, can significantly improve the ability to predict a patient’s risk for bone fracture.
Although bone mineral density (BMD) is traditionally used to identify patients with osteoporosis or low bone mass, some physicians have begun incorporating the trabecular bone score (TBS) into their exams.
At the Cleveland Clinic Center for Specialized Women’s Health, factoring in the TBS changed the diagnosis for 16% of 432 patients, according to Holly Thacker, MD, the center’s director.
“Importantly, 11% got worse diagnoses, and I use that in terms of prioritizing treatment,” Dr. Thacker said in an interview. The ability to determine how degraded the bone microarchitecture is through a software system “is a huge advance.”
Dr. Thacker described her center’s experience with the technology at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
While BMD captures the amount of minerals like calcium in the skeleton, TBS assesses the underlying microarchitecture by looking at the distribution of shades of gray on dual-energy x-ray absorptiometry (DXA) scans.
Based on the TBS, patients’ bones are classified as normal, partially degraded, or degraded. Among the 432 patients who received a TBS analysis in 2022, 3% shifted from a normal diagnosis to osteopenia, 8% worsened from osteopenia to osteoporosis, 4% went from osteopenia to normal, and 1.6% downgraded from osteoporosis to osteopenia, Dr. Thacker reported.
The new test may also provide some reassurance for female patients who have thinner bones, which may raise alarms based on BMD. TBS, however, may show that the structure of the bone looks normal.
“When you know that the microarchitecture is normal, you’re a lot less concerned that they actually have a bone disease of osteoporosis,” Dr. Thacker said.
Conversely, unexpectedly degraded bone raises questions.
“That makes you go back and say [to the patient]: ‘Have you been on steroids? Were you malnourished? Is there some other metabolic problem? Have you had some calcium disorder?’ ” Dr. Thacker said.
Dr. Thacker leverages the TBS to help patients obtain effective therapy, typically an anabolic agent followed by antiresorptive medication.
“When I see a patient who not only has osteoporosis on bone density but has completely degraded bone architecture, it’s a lot easier for me to make the argument to the insurance company that this patient is at grave risk for a low trauma fracture and bad outcome without the best treatment,” Dr. Thacker said.
10-year-old tech, recently covered
The Food and Drug Administration approved TBS software in 2012, but Medicare only recently started paying for it.
Medimaps Group, a company that markets imaging software to calculate TBS, announced in 2022 that reimbursement from the Centers for Medicare & Medicaid Services was available, at $41.53 on the Physician Fee Schedule and $82.61 on the Hospital Outpatient Prospective Payment Schedule.
“Reimbursement through CMS is an important endorsement of the clinical value of TBS for clinicians and their patients,” Didier Hans, PhD, MBA, the CEO of Medimaps, said in a statement at the time. He noted that more than 600,000 TBS procedures were being performed in the United States each year.
Nevertheless, the initial investment in purchasing the software may be a barrier for health systems.
“We are the first and only site in our health system to offer TBS, as this is an extra expense and not uniformly reimbursed by insurers,” Dr. Thacker reported at the meeting.
Potential drawbacks
The TBS software used in Dr. Thacker’s study has been validated only in Asian and White patients between certain ages and weights, meaning the system is not designed to be used for other populations. Other researchers have highlighted a need for trabecular bone scoring to be validated more broadly. The authors of a recent analysis, however, suggest that TBS can be used the same way no matter a patient’s race.
TBS “is going to be most helpful in those with osteopenia who are right near the threshold for treatment,” said Marcella Donovan Walker, MD, MS, in a presentation on bone quality at the meeting.
Many studies have shown that TBS “provides additive information to bone density,” said Dr. Walker, a professor of medicine in the division of endocrinology at Columbia University, New York. For example, a large study of women in Manitoba found that, regardless of whether their bone density was normal, osteopenic, or osteoporotic, those with a low TBS had a much higher risk for fracture.
‘Opportunistic screening’ with CT?
TBS relies on the same DXA scans that are used to calculate bone mineral density, so obtaining the score does not add time or radiation to the scanning process, Dr. Thacker said.
But many patients who should receive DXA scans do not, which adds to the promise of “opportunistic screening” for osteoporosis, Dr. Walker said. With this approach, physicians would analyze a CT scan that a patient received for another purpose, such as to investigate abdominal pain or chest pain.
“In these images is information about the bone,” Dr. Walker said.
Researchers have used high-resolution peripheral quantitative CT to perform finite element analysis, where a computer program simulates compression of the bone to create a measure of bone stiffness and determine the load required for a break.
One study found that including those elements predicted fractures better than bone mineral density or the Fracture Risk Assessment Tool alone, Dr. Walker noted.
Other aspects of bone quality include how many cracks are in the bone, the amount of adipose in the marrow space, and the rate at which bone is broken down and rebuilt. But Dr. Walker suggested that the longstanding focus on bone mineral density in clinical practice makes sense.
“By far, bone mass is the most important bone quality,” Dr. Walker said.
Dr. Thacker is the executive director of the nonprofit Speaking of Women’s Health. Dr. Walker reported receiving funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and Amgen.
A version of this article first appeared on Medscape.com.
FROM NAMS 2023
Mixed CRC screening messaging. Confusing? Some docs think so
Recently updated colorectal cancer (CRC) screening guidance from the American College of Physicians is raising concerns among some specialists.
The ACP’s clinical guidance, published in Annals of Internal Medicine, called for CRC screenings to start at age 50 in average-risk individuals who are asymptomatic. This recommendation, however, conflicts with guidelines from the American Cancer Society and the U.S. Preventive Services Task Force, which, in 2021, officially lowered the recommended initial age of screening to 45.
Following the ACP’s announcement, several professional organizations, such as the American College of Radiology, criticized the new guidelines, calling them “a step backward” and warning they may hinder recent gains against CRC.
Some physicians believe the discordance will confuse patients and lead to varying referral practices among primary care physicians. And while insurers will likely continue to pay for screening procedures based on the USPSTF guidelines, which dictate insurance coverage, some physicians worry that insurers could create additional roadblocks for CRC screening coverage, such as requiring prior authorization.
“We’re in a conflicted space on this issue as a country,” said John L. Marshall, MD, a GI oncologist and director of The Ruesch Center for the Cure of GI Cancers at Georgetown University, Washington.
Ultimately, the physician community wants an inexpensive screening test that’s effective at preventing cancer and deaths, but the evidence thus far doesn’t necessarily support colonoscopy as that test, said Dr. Marshall, also chief medical officer for Lombardi Comprehensive Cancer Center.
Although colonoscopy can prevent CRC by removing precancerous polyps and can reduce deaths from cancer, it has not been shown to lower all-cause mortality, Dr. Marshall explained. A recent meta-analysis, for example, found that, aside from sigmoidoscopy for colon cancer screening, no other cancer screening modalities meaningfully changed life expectancy.
“That’s why we’re struggling,” Dr. Marshall said. “We’re emotionally invested in having screening available to younger people because we’re seeing colon cancer in younger people. So, we want it to move earlier, but it’s expensive and it’s invasive.”
Docs debate differing guidance
The new ACP guidance, based on a critical review of existing guidelines, evidence, and modeling studies, argues that the potential harms of screening average-risk individuals under age 50 may outweigh the potential benefits.
The benefits of screening, of course, include identifying and removing precancerous lesions or localized cancer, while the potential harms include false positives that may lead to unnecessary additional tests, treatments, and costs. More invasive screening procedures, such as colonoscopy, can also come with their own risks, including serious bleeding and perforation.
For colonoscopy, for instance, the ACP team determined that starting screening at age 45 vs. 50 could prevent three additional CRC cases per 1,000 individuals screened (58 vs. 61) and one CRC death (27 vs. 28) over the recommended screening time frame. On the flip side, screening starting at age 45 could increase the incidence of gastrointestinal or cardiovascular events (14 vs. 16).
“Even if we assumed the modeling study had no limitations and accepted the results at face value, we would conclude that the small estimated benefits and harms roughly balance each other out, resulting in an inadequate net benefit to warrant CRC screening in average-risk adults aged 45 to 49 years,” Amir Qaseem, MD, PhD, and ACP coauthors write.
Family physician Kenny Lin, MD, MPH, believes the updated ACP guidelines are reasonable, and points out the ACP is not the first group to disagree with the USPSTF’s recommendations.
“I think the [ACP] guidelines make a lot of sense,” said Dr. Lin, who practices in Lancaster, Pa. The American Academy of Family Physicians “also did not endorse the recommendations to start screenings at 45.” In its 2021 updated guidance, the AAFP recommended screening for CRC starting at age 50, concluding there was “insufficient evidence to assess the benefits and harms of screening” in the 45 to 49 population.
However, Jason R. Woloski, MD, a family physician based in Wilkes-Barre, Pa., expressed concern that the differing guidelines will confuse patients as well as present challenges for primary care physicians.
“I feel like we took the last couple of years convincing people that earlier is better,” said Dr. Woloski, an associate professor of family medicine at Geisinger Commonwealth School of Medicine, Scranton, Pa. “It can send a mixed message to a patient after we’ve been stressing the importance of earlier [screening], and then saying, ‘Maybe we got it wrong; maybe we were okay the first time.’ ”
Mark A. Lewis, MD, a GI oncologist, had a similar initial reaction upon hearing about the updated guidelines: “The lack of synchronization across groups is going to create confusion among patients.”
Although he could not say definitively whether the recommendations will affect GI oncologists, because he only sees patients with advanced CRC, he does see the demands in primary care and gastroenterology shifting.
“I think the much bigger impact will be on primary care physicians and gastroenterologists,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “My best guess is that the procedural burden on the latter will be mitigated by more stool testing ordered by primary care physicians. Patients may understandably prefer the convenience and lack of invasiveness of home-based fecal testing, but a positive FIT [fecal immunochemical test] without a follow-up scope is an incomplete screening.”
Dr. Marshall, however, had a different take. He does not envision the updated guidelines having much of a practical impact on physician practice. Most of the country is already not receiving proper colon cancer screenings, he said. Research shows more than 40% of Americans skip standard CRC screenings. Even anecdotally, he noted, friends in their 60s come to him and admit they haven’t had a colonoscopy yet.
Potential impact on patient outcomes, costs
Beyond mixed messaging, some experts worry that pushing CRC screening later could mean cancers are caught later, when they’re more advanced.
Finding cancers earlier, when they are easier and less expensive to treat, make earlier CRC screenings worthwhile, Dr. Woloski explained.
Dr. Lewis sees earlier screening as a way to stop a tumor from progressing before it can really pick up steam.
“To me the biggest advantage of colonoscopy is the interruption of the adenoma-to-carcinoma sequence, whereby a polyp that is completely removed cannot become an invasive adenocarcinoma,” Dr. Lewis said. “We’ve also had evidence for well over a decade that flexible sigmoidoscopy, which doesn’t come close to visualizing the entire colon, can confer a survival benefit.”
Another concern is the potential effect on insurance coverage.
Medicare and other insurers use USPSTF guidelines to make coverage decisions. However, because of this mixed message, Dr. Woloski questioned whether there would be more challenges regarding insurance coverage. “Does it mean primary care doctors are going to have to preauthorize a lot of these screenings even if you have shared decision-making with the patient?” he asked.
When it comes to screening referrals, Douglas A. Corley, MD, PhD, a gastroenterologist at Kaiser Permanente in northern California, said it’s critical for primary care physicians to educate patients about the differing views on screening benefits and harms as well as the different screening options.
“Given the different opinions, it is important to let people in this age group know that screening is an option recommended by some groups,” Dr. Corley said. “Colorectal cancer screening is very effective for decreasing the risk for death from colorectal cancer, which is the second leading cause of cancer death in the United States. Making sure all eligible people know this is an option provides the best way for patients to have an informed choice.”
Dr. Lin has already begun talking with patients about the differing recommendations. He said it’s helpful to simplify the issue and focus the conversation on what patients value most. For more assertive patients whose priority is finding every possible cancer early, starting screenings at age 45 may be reasonable, he said, whereas other patients may not find the process or possible side effects worth it.
“And then you have the middle group that decides, ‘Yes, I want to start at 45, but I want the fecal test. I don’t want to just jump into colonoscopy.’ ” Dr. Lin said. “That would be kind of a compromise where you’d be starting screening earlier, but not subjecting yourself to something that has more potential for harms.”
Dr. Woloski said he plans to continue making referrals based on the USPSTF recommendations.
“With every screening, it is about informed decision-making with the patient, but I think for now, since USPSTF still supports the earlier screening, I will probably stick with offering it earlier,” he said.
But when deciding on the appropriate timing for evaluating CRC, the most important distinction is between screening and diagnosis, Dr. Lewis added.
“The former is only appropriate in patients who are truly asymptomatic and who are truly average-risk,” he said. “The latter is critical in any patient with symptoms. I cannot count the number of times I have seen blood in the stool discounted as hemorrhoids without even an exam, digital rectal, or scope, to demonstrate that hemorrhoids are present and the culprit for blood loss.”
A version of this article first appeared on Medscape.com.
Recently updated colorectal cancer (CRC) screening guidance from the American College of Physicians is raising concerns among some specialists.
The ACP’s clinical guidance, published in Annals of Internal Medicine, called for CRC screenings to start at age 50 in average-risk individuals who are asymptomatic. This recommendation, however, conflicts with guidelines from the American Cancer Society and the U.S. Preventive Services Task Force, which, in 2021, officially lowered the recommended initial age of screening to 45.
Following the ACP’s announcement, several professional organizations, such as the American College of Radiology, criticized the new guidelines, calling them “a step backward” and warning they may hinder recent gains against CRC.
Some physicians believe the discordance will confuse patients and lead to varying referral practices among primary care physicians. And while insurers will likely continue to pay for screening procedures based on the USPSTF guidelines, which dictate insurance coverage, some physicians worry that insurers could create additional roadblocks for CRC screening coverage, such as requiring prior authorization.
“We’re in a conflicted space on this issue as a country,” said John L. Marshall, MD, a GI oncologist and director of The Ruesch Center for the Cure of GI Cancers at Georgetown University, Washington.
Ultimately, the physician community wants an inexpensive screening test that’s effective at preventing cancer and deaths, but the evidence thus far doesn’t necessarily support colonoscopy as that test, said Dr. Marshall, also chief medical officer for Lombardi Comprehensive Cancer Center.
Although colonoscopy can prevent CRC by removing precancerous polyps and can reduce deaths from cancer, it has not been shown to lower all-cause mortality, Dr. Marshall explained. A recent meta-analysis, for example, found that, aside from sigmoidoscopy for colon cancer screening, no other cancer screening modalities meaningfully changed life expectancy.
“That’s why we’re struggling,” Dr. Marshall said. “We’re emotionally invested in having screening available to younger people because we’re seeing colon cancer in younger people. So, we want it to move earlier, but it’s expensive and it’s invasive.”
Docs debate differing guidance
The new ACP guidance, based on a critical review of existing guidelines, evidence, and modeling studies, argues that the potential harms of screening average-risk individuals under age 50 may outweigh the potential benefits.
The benefits of screening, of course, include identifying and removing precancerous lesions or localized cancer, while the potential harms include false positives that may lead to unnecessary additional tests, treatments, and costs. More invasive screening procedures, such as colonoscopy, can also come with their own risks, including serious bleeding and perforation.
For colonoscopy, for instance, the ACP team determined that starting screening at age 45 vs. 50 could prevent three additional CRC cases per 1,000 individuals screened (58 vs. 61) and one CRC death (27 vs. 28) over the recommended screening time frame. On the flip side, screening starting at age 45 could increase the incidence of gastrointestinal or cardiovascular events (14 vs. 16).
“Even if we assumed the modeling study had no limitations and accepted the results at face value, we would conclude that the small estimated benefits and harms roughly balance each other out, resulting in an inadequate net benefit to warrant CRC screening in average-risk adults aged 45 to 49 years,” Amir Qaseem, MD, PhD, and ACP coauthors write.
Family physician Kenny Lin, MD, MPH, believes the updated ACP guidelines are reasonable, and points out the ACP is not the first group to disagree with the USPSTF’s recommendations.
“I think the [ACP] guidelines make a lot of sense,” said Dr. Lin, who practices in Lancaster, Pa. The American Academy of Family Physicians “also did not endorse the recommendations to start screenings at 45.” In its 2021 updated guidance, the AAFP recommended screening for CRC starting at age 50, concluding there was “insufficient evidence to assess the benefits and harms of screening” in the 45 to 49 population.
However, Jason R. Woloski, MD, a family physician based in Wilkes-Barre, Pa., expressed concern that the differing guidelines will confuse patients as well as present challenges for primary care physicians.
“I feel like we took the last couple of years convincing people that earlier is better,” said Dr. Woloski, an associate professor of family medicine at Geisinger Commonwealth School of Medicine, Scranton, Pa. “It can send a mixed message to a patient after we’ve been stressing the importance of earlier [screening], and then saying, ‘Maybe we got it wrong; maybe we were okay the first time.’ ”
Mark A. Lewis, MD, a GI oncologist, had a similar initial reaction upon hearing about the updated guidelines: “The lack of synchronization across groups is going to create confusion among patients.”
Although he could not say definitively whether the recommendations will affect GI oncologists, because he only sees patients with advanced CRC, he does see the demands in primary care and gastroenterology shifting.
“I think the much bigger impact will be on primary care physicians and gastroenterologists,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “My best guess is that the procedural burden on the latter will be mitigated by more stool testing ordered by primary care physicians. Patients may understandably prefer the convenience and lack of invasiveness of home-based fecal testing, but a positive FIT [fecal immunochemical test] without a follow-up scope is an incomplete screening.”
Dr. Marshall, however, had a different take. He does not envision the updated guidelines having much of a practical impact on physician practice. Most of the country is already not receiving proper colon cancer screenings, he said. Research shows more than 40% of Americans skip standard CRC screenings. Even anecdotally, he noted, friends in their 60s come to him and admit they haven’t had a colonoscopy yet.
Potential impact on patient outcomes, costs
Beyond mixed messaging, some experts worry that pushing CRC screening later could mean cancers are caught later, when they’re more advanced.
Finding cancers earlier, when they are easier and less expensive to treat, make earlier CRC screenings worthwhile, Dr. Woloski explained.
Dr. Lewis sees earlier screening as a way to stop a tumor from progressing before it can really pick up steam.
“To me the biggest advantage of colonoscopy is the interruption of the adenoma-to-carcinoma sequence, whereby a polyp that is completely removed cannot become an invasive adenocarcinoma,” Dr. Lewis said. “We’ve also had evidence for well over a decade that flexible sigmoidoscopy, which doesn’t come close to visualizing the entire colon, can confer a survival benefit.”
Another concern is the potential effect on insurance coverage.
Medicare and other insurers use USPSTF guidelines to make coverage decisions. However, because of this mixed message, Dr. Woloski questioned whether there would be more challenges regarding insurance coverage. “Does it mean primary care doctors are going to have to preauthorize a lot of these screenings even if you have shared decision-making with the patient?” he asked.
When it comes to screening referrals, Douglas A. Corley, MD, PhD, a gastroenterologist at Kaiser Permanente in northern California, said it’s critical for primary care physicians to educate patients about the differing views on screening benefits and harms as well as the different screening options.
“Given the different opinions, it is important to let people in this age group know that screening is an option recommended by some groups,” Dr. Corley said. “Colorectal cancer screening is very effective for decreasing the risk for death from colorectal cancer, which is the second leading cause of cancer death in the United States. Making sure all eligible people know this is an option provides the best way for patients to have an informed choice.”
Dr. Lin has already begun talking with patients about the differing recommendations. He said it’s helpful to simplify the issue and focus the conversation on what patients value most. For more assertive patients whose priority is finding every possible cancer early, starting screenings at age 45 may be reasonable, he said, whereas other patients may not find the process or possible side effects worth it.
“And then you have the middle group that decides, ‘Yes, I want to start at 45, but I want the fecal test. I don’t want to just jump into colonoscopy.’ ” Dr. Lin said. “That would be kind of a compromise where you’d be starting screening earlier, but not subjecting yourself to something that has more potential for harms.”
Dr. Woloski said he plans to continue making referrals based on the USPSTF recommendations.
“With every screening, it is about informed decision-making with the patient, but I think for now, since USPSTF still supports the earlier screening, I will probably stick with offering it earlier,” he said.
But when deciding on the appropriate timing for evaluating CRC, the most important distinction is between screening and diagnosis, Dr. Lewis added.
“The former is only appropriate in patients who are truly asymptomatic and who are truly average-risk,” he said. “The latter is critical in any patient with symptoms. I cannot count the number of times I have seen blood in the stool discounted as hemorrhoids without even an exam, digital rectal, or scope, to demonstrate that hemorrhoids are present and the culprit for blood loss.”
A version of this article first appeared on Medscape.com.
Recently updated colorectal cancer (CRC) screening guidance from the American College of Physicians is raising concerns among some specialists.
The ACP’s clinical guidance, published in Annals of Internal Medicine, called for CRC screenings to start at age 50 in average-risk individuals who are asymptomatic. This recommendation, however, conflicts with guidelines from the American Cancer Society and the U.S. Preventive Services Task Force, which, in 2021, officially lowered the recommended initial age of screening to 45.
Following the ACP’s announcement, several professional organizations, such as the American College of Radiology, criticized the new guidelines, calling them “a step backward” and warning they may hinder recent gains against CRC.
Some physicians believe the discordance will confuse patients and lead to varying referral practices among primary care physicians. And while insurers will likely continue to pay for screening procedures based on the USPSTF guidelines, which dictate insurance coverage, some physicians worry that insurers could create additional roadblocks for CRC screening coverage, such as requiring prior authorization.
“We’re in a conflicted space on this issue as a country,” said John L. Marshall, MD, a GI oncologist and director of The Ruesch Center for the Cure of GI Cancers at Georgetown University, Washington.
Ultimately, the physician community wants an inexpensive screening test that’s effective at preventing cancer and deaths, but the evidence thus far doesn’t necessarily support colonoscopy as that test, said Dr. Marshall, also chief medical officer for Lombardi Comprehensive Cancer Center.
Although colonoscopy can prevent CRC by removing precancerous polyps and can reduce deaths from cancer, it has not been shown to lower all-cause mortality, Dr. Marshall explained. A recent meta-analysis, for example, found that, aside from sigmoidoscopy for colon cancer screening, no other cancer screening modalities meaningfully changed life expectancy.
“That’s why we’re struggling,” Dr. Marshall said. “We’re emotionally invested in having screening available to younger people because we’re seeing colon cancer in younger people. So, we want it to move earlier, but it’s expensive and it’s invasive.”
Docs debate differing guidance
The new ACP guidance, based on a critical review of existing guidelines, evidence, and modeling studies, argues that the potential harms of screening average-risk individuals under age 50 may outweigh the potential benefits.
The benefits of screening, of course, include identifying and removing precancerous lesions or localized cancer, while the potential harms include false positives that may lead to unnecessary additional tests, treatments, and costs. More invasive screening procedures, such as colonoscopy, can also come with their own risks, including serious bleeding and perforation.
For colonoscopy, for instance, the ACP team determined that starting screening at age 45 vs. 50 could prevent three additional CRC cases per 1,000 individuals screened (58 vs. 61) and one CRC death (27 vs. 28) over the recommended screening time frame. On the flip side, screening starting at age 45 could increase the incidence of gastrointestinal or cardiovascular events (14 vs. 16).
“Even if we assumed the modeling study had no limitations and accepted the results at face value, we would conclude that the small estimated benefits and harms roughly balance each other out, resulting in an inadequate net benefit to warrant CRC screening in average-risk adults aged 45 to 49 years,” Amir Qaseem, MD, PhD, and ACP coauthors write.
Family physician Kenny Lin, MD, MPH, believes the updated ACP guidelines are reasonable, and points out the ACP is not the first group to disagree with the USPSTF’s recommendations.
“I think the [ACP] guidelines make a lot of sense,” said Dr. Lin, who practices in Lancaster, Pa. The American Academy of Family Physicians “also did not endorse the recommendations to start screenings at 45.” In its 2021 updated guidance, the AAFP recommended screening for CRC starting at age 50, concluding there was “insufficient evidence to assess the benefits and harms of screening” in the 45 to 49 population.
However, Jason R. Woloski, MD, a family physician based in Wilkes-Barre, Pa., expressed concern that the differing guidelines will confuse patients as well as present challenges for primary care physicians.
“I feel like we took the last couple of years convincing people that earlier is better,” said Dr. Woloski, an associate professor of family medicine at Geisinger Commonwealth School of Medicine, Scranton, Pa. “It can send a mixed message to a patient after we’ve been stressing the importance of earlier [screening], and then saying, ‘Maybe we got it wrong; maybe we were okay the first time.’ ”
Mark A. Lewis, MD, a GI oncologist, had a similar initial reaction upon hearing about the updated guidelines: “The lack of synchronization across groups is going to create confusion among patients.”
Although he could not say definitively whether the recommendations will affect GI oncologists, because he only sees patients with advanced CRC, he does see the demands in primary care and gastroenterology shifting.
“I think the much bigger impact will be on primary care physicians and gastroenterologists,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “My best guess is that the procedural burden on the latter will be mitigated by more stool testing ordered by primary care physicians. Patients may understandably prefer the convenience and lack of invasiveness of home-based fecal testing, but a positive FIT [fecal immunochemical test] without a follow-up scope is an incomplete screening.”
Dr. Marshall, however, had a different take. He does not envision the updated guidelines having much of a practical impact on physician practice. Most of the country is already not receiving proper colon cancer screenings, he said. Research shows more than 40% of Americans skip standard CRC screenings. Even anecdotally, he noted, friends in their 60s come to him and admit they haven’t had a colonoscopy yet.
Potential impact on patient outcomes, costs
Beyond mixed messaging, some experts worry that pushing CRC screening later could mean cancers are caught later, when they’re more advanced.
Finding cancers earlier, when they are easier and less expensive to treat, make earlier CRC screenings worthwhile, Dr. Woloski explained.
Dr. Lewis sees earlier screening as a way to stop a tumor from progressing before it can really pick up steam.
“To me the biggest advantage of colonoscopy is the interruption of the adenoma-to-carcinoma sequence, whereby a polyp that is completely removed cannot become an invasive adenocarcinoma,” Dr. Lewis said. “We’ve also had evidence for well over a decade that flexible sigmoidoscopy, which doesn’t come close to visualizing the entire colon, can confer a survival benefit.”
Another concern is the potential effect on insurance coverage.
Medicare and other insurers use USPSTF guidelines to make coverage decisions. However, because of this mixed message, Dr. Woloski questioned whether there would be more challenges regarding insurance coverage. “Does it mean primary care doctors are going to have to preauthorize a lot of these screenings even if you have shared decision-making with the patient?” he asked.
When it comes to screening referrals, Douglas A. Corley, MD, PhD, a gastroenterologist at Kaiser Permanente in northern California, said it’s critical for primary care physicians to educate patients about the differing views on screening benefits and harms as well as the different screening options.
“Given the different opinions, it is important to let people in this age group know that screening is an option recommended by some groups,” Dr. Corley said. “Colorectal cancer screening is very effective for decreasing the risk for death from colorectal cancer, which is the second leading cause of cancer death in the United States. Making sure all eligible people know this is an option provides the best way for patients to have an informed choice.”
Dr. Lin has already begun talking with patients about the differing recommendations. He said it’s helpful to simplify the issue and focus the conversation on what patients value most. For more assertive patients whose priority is finding every possible cancer early, starting screenings at age 45 may be reasonable, he said, whereas other patients may not find the process or possible side effects worth it.
“And then you have the middle group that decides, ‘Yes, I want to start at 45, but I want the fecal test. I don’t want to just jump into colonoscopy.’ ” Dr. Lin said. “That would be kind of a compromise where you’d be starting screening earlier, but not subjecting yourself to something that has more potential for harms.”
Dr. Woloski said he plans to continue making referrals based on the USPSTF recommendations.
“With every screening, it is about informed decision-making with the patient, but I think for now, since USPSTF still supports the earlier screening, I will probably stick with offering it earlier,” he said.
But when deciding on the appropriate timing for evaluating CRC, the most important distinction is between screening and diagnosis, Dr. Lewis added.
“The former is only appropriate in patients who are truly asymptomatic and who are truly average-risk,” he said. “The latter is critical in any patient with symptoms. I cannot count the number of times I have seen blood in the stool discounted as hemorrhoids without even an exam, digital rectal, or scope, to demonstrate that hemorrhoids are present and the culprit for blood loss.”
A version of this article first appeared on Medscape.com.