User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Are they ‘antiobesity medications’ or ‘weight-loss drugs’?
A simple Google search for the terms “weight-loss pens,” “weight-loss drugs,” and “weight-loss medications” displays seven times more results than a search for terms like “antiobesity medications,” “antiobesity drugs,” or “drugs (or medications) to treat obesity.” The same search applied to academic databases yields the opposite results: fewer than 500 results for “weight-loss drugs/agents/medications” and 19,000 results for “antiobesity agents,” for example.
To highlight the importance of the language used to talk about obesity treatment, researchers affiliated with the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Association for the Study of Obesity and Metabolic Syndrome (ABESO) released a statement on the subject at the Brazilian Congress of Update in Endocrinology and Metabolism 2023. On the basis of the study by the ABESO and the SBEM, the statement proposes abandoning the use of the term “weight-loss medications” in scientific publications and, most importantly, in the media.
“Put together, we believe that the common use of the term ‘weight-loss medications’ by media and the public, as well as by doctors and the scientific community, contributes to stigma, and certainly that language matters,” study author Paulo Augusto Carvalho Miranda, MD, PhD, chair of SBEM, said in an interview.
“When we refer to these medications as ‘weight-loss drugs,’ we are using derogatory terms to refer to medications that were extensively studied before their launch onto the market and approved by a regulatory authority to treat a disease called obesity,” said study author Márcio Mancini, MD, PhD, deputy chair of the SBEM’s Obesity Department.
Beyond semantics
Another article published by this news organization presents the initiative of a global task force comprising 60 leaders in the clinical management of obesity, who proposed a new name for the disease. According to the leader of the project, Francesco Rubino, MD, “The word is so stigmatized, with so much misunderstanding and misperception, that some might say the only solution is to change the name.” Following this same logic, the authors of the Brazilian study believe that changing how we refer to medications may improve perceptions of health care professionals and patients toward prevention and treatment strategies for obesity.
According to Dr. Miranda, the first step is “remembering that how we refer to people, diseases, and treatments makes all the difference, especially in situations like obesity, a stigmatized disease loaded with misconceptions. It is not merely an issue of semantics, but also an issue of reducing the stigma surrounding the subject.”
According to Dr. Miranda, the primary purpose of the statement is to highlight the uniqueness of the situation and the importance of encouraging the use of the expressions “antiobesity medications” and “medications to treat obesity” to help reduce the stigma and improve adherence and persistence in obesity treatment.
Impact in practice
The statement also emphasizes that obesity pharmacotherapy is widely underused in patients with obesity and that, in the United States, it is prescribed only for approximately 3% of adults with the disease. Weight management programs for this patient population stress implementing lifestyle changes, and only 1.1% of participants are prescribed medications.
According to the statement, the term “weight-loss medications” contributes to the concept that their use has an aesthetic goal and can be consumed by anyone who desires to lose weight.
In addition to ensuring the correct use of language, Dr. Mancini adds that it is essential for doctors to seek and present pharmacologic treatment for obesity as something that will improve patient health. This means stressing that obesity can be controlled with a 10% loss in body weight, just as other chronic diseases, such as diabetes, can be controlled. Moreover, it is important to point out that medications also have a crucial role in optimizing weight maintenance in the long term.
Another issue Dr. Mancini raised is the prejudice that many doctors have against people with obesity. Health professionals should recognize they are also subject to weight bias and that the way they communicate with patients could have a profound effect on health-related outcomes.
“The stigma surrounding obesity can lead to bullying, even in the patient’s home by their relatives; this is very common. Weight stigma is so strong that it hinders patient health and decreases the likelihood of the patient seeking specialized care,” Dr. Mancini warned.
According to the authors, it is of utmost importance to understand that an individual should not be defined by his or disease (as by the use of the terms “obese” or “diabetic”) but rather understood to live with this disease (“individual with obesity” or “with diabetes”). Dr. Mancini suggests the following strategies that health care professionals can adopt while caring for patients with obesity:
- Speak to patients with empathy and respect, avoiding the use of judgmental words.
- Ask if they would like to discuss the “weight issue,” “BMI issue,” terms that are better received by the public, instead of saying “excess fat” or “excess weight.”
- If the patient agrees to talk about the subject, reinforce that this is a chronic health problem that requires longterm treatment and give him or her short, medium, and longterm options.
Lastly, the authors highlighted the importance of differentiating between regulatory agency–approved medications and over-the-counter drugs and supplements that are often sold as “weight-loss agents” and are responsible for an unacceptably high rate of emergency visits.
This article was translated from the Medscape Portuguese Edition. A version appeared on Medscape.com.
A simple Google search for the terms “weight-loss pens,” “weight-loss drugs,” and “weight-loss medications” displays seven times more results than a search for terms like “antiobesity medications,” “antiobesity drugs,” or “drugs (or medications) to treat obesity.” The same search applied to academic databases yields the opposite results: fewer than 500 results for “weight-loss drugs/agents/medications” and 19,000 results for “antiobesity agents,” for example.
To highlight the importance of the language used to talk about obesity treatment, researchers affiliated with the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Association for the Study of Obesity and Metabolic Syndrome (ABESO) released a statement on the subject at the Brazilian Congress of Update in Endocrinology and Metabolism 2023. On the basis of the study by the ABESO and the SBEM, the statement proposes abandoning the use of the term “weight-loss medications” in scientific publications and, most importantly, in the media.
“Put together, we believe that the common use of the term ‘weight-loss medications’ by media and the public, as well as by doctors and the scientific community, contributes to stigma, and certainly that language matters,” study author Paulo Augusto Carvalho Miranda, MD, PhD, chair of SBEM, said in an interview.
“When we refer to these medications as ‘weight-loss drugs,’ we are using derogatory terms to refer to medications that were extensively studied before their launch onto the market and approved by a regulatory authority to treat a disease called obesity,” said study author Márcio Mancini, MD, PhD, deputy chair of the SBEM’s Obesity Department.
Beyond semantics
Another article published by this news organization presents the initiative of a global task force comprising 60 leaders in the clinical management of obesity, who proposed a new name for the disease. According to the leader of the project, Francesco Rubino, MD, “The word is so stigmatized, with so much misunderstanding and misperception, that some might say the only solution is to change the name.” Following this same logic, the authors of the Brazilian study believe that changing how we refer to medications may improve perceptions of health care professionals and patients toward prevention and treatment strategies for obesity.
According to Dr. Miranda, the first step is “remembering that how we refer to people, diseases, and treatments makes all the difference, especially in situations like obesity, a stigmatized disease loaded with misconceptions. It is not merely an issue of semantics, but also an issue of reducing the stigma surrounding the subject.”
According to Dr. Miranda, the primary purpose of the statement is to highlight the uniqueness of the situation and the importance of encouraging the use of the expressions “antiobesity medications” and “medications to treat obesity” to help reduce the stigma and improve adherence and persistence in obesity treatment.
Impact in practice
The statement also emphasizes that obesity pharmacotherapy is widely underused in patients with obesity and that, in the United States, it is prescribed only for approximately 3% of adults with the disease. Weight management programs for this patient population stress implementing lifestyle changes, and only 1.1% of participants are prescribed medications.
According to the statement, the term “weight-loss medications” contributes to the concept that their use has an aesthetic goal and can be consumed by anyone who desires to lose weight.
In addition to ensuring the correct use of language, Dr. Mancini adds that it is essential for doctors to seek and present pharmacologic treatment for obesity as something that will improve patient health. This means stressing that obesity can be controlled with a 10% loss in body weight, just as other chronic diseases, such as diabetes, can be controlled. Moreover, it is important to point out that medications also have a crucial role in optimizing weight maintenance in the long term.
Another issue Dr. Mancini raised is the prejudice that many doctors have against people with obesity. Health professionals should recognize they are also subject to weight bias and that the way they communicate with patients could have a profound effect on health-related outcomes.
“The stigma surrounding obesity can lead to bullying, even in the patient’s home by their relatives; this is very common. Weight stigma is so strong that it hinders patient health and decreases the likelihood of the patient seeking specialized care,” Dr. Mancini warned.
According to the authors, it is of utmost importance to understand that an individual should not be defined by his or disease (as by the use of the terms “obese” or “diabetic”) but rather understood to live with this disease (“individual with obesity” or “with diabetes”). Dr. Mancini suggests the following strategies that health care professionals can adopt while caring for patients with obesity:
- Speak to patients with empathy and respect, avoiding the use of judgmental words.
- Ask if they would like to discuss the “weight issue,” “BMI issue,” terms that are better received by the public, instead of saying “excess fat” or “excess weight.”
- If the patient agrees to talk about the subject, reinforce that this is a chronic health problem that requires longterm treatment and give him or her short, medium, and longterm options.
Lastly, the authors highlighted the importance of differentiating between regulatory agency–approved medications and over-the-counter drugs and supplements that are often sold as “weight-loss agents” and are responsible for an unacceptably high rate of emergency visits.
This article was translated from the Medscape Portuguese Edition. A version appeared on Medscape.com.
A simple Google search for the terms “weight-loss pens,” “weight-loss drugs,” and “weight-loss medications” displays seven times more results than a search for terms like “antiobesity medications,” “antiobesity drugs,” or “drugs (or medications) to treat obesity.” The same search applied to academic databases yields the opposite results: fewer than 500 results for “weight-loss drugs/agents/medications” and 19,000 results for “antiobesity agents,” for example.
To highlight the importance of the language used to talk about obesity treatment, researchers affiliated with the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Association for the Study of Obesity and Metabolic Syndrome (ABESO) released a statement on the subject at the Brazilian Congress of Update in Endocrinology and Metabolism 2023. On the basis of the study by the ABESO and the SBEM, the statement proposes abandoning the use of the term “weight-loss medications” in scientific publications and, most importantly, in the media.
“Put together, we believe that the common use of the term ‘weight-loss medications’ by media and the public, as well as by doctors and the scientific community, contributes to stigma, and certainly that language matters,” study author Paulo Augusto Carvalho Miranda, MD, PhD, chair of SBEM, said in an interview.
“When we refer to these medications as ‘weight-loss drugs,’ we are using derogatory terms to refer to medications that were extensively studied before their launch onto the market and approved by a regulatory authority to treat a disease called obesity,” said study author Márcio Mancini, MD, PhD, deputy chair of the SBEM’s Obesity Department.
Beyond semantics
Another article published by this news organization presents the initiative of a global task force comprising 60 leaders in the clinical management of obesity, who proposed a new name for the disease. According to the leader of the project, Francesco Rubino, MD, “The word is so stigmatized, with so much misunderstanding and misperception, that some might say the only solution is to change the name.” Following this same logic, the authors of the Brazilian study believe that changing how we refer to medications may improve perceptions of health care professionals and patients toward prevention and treatment strategies for obesity.
According to Dr. Miranda, the first step is “remembering that how we refer to people, diseases, and treatments makes all the difference, especially in situations like obesity, a stigmatized disease loaded with misconceptions. It is not merely an issue of semantics, but also an issue of reducing the stigma surrounding the subject.”
According to Dr. Miranda, the primary purpose of the statement is to highlight the uniqueness of the situation and the importance of encouraging the use of the expressions “antiobesity medications” and “medications to treat obesity” to help reduce the stigma and improve adherence and persistence in obesity treatment.
Impact in practice
The statement also emphasizes that obesity pharmacotherapy is widely underused in patients with obesity and that, in the United States, it is prescribed only for approximately 3% of adults with the disease. Weight management programs for this patient population stress implementing lifestyle changes, and only 1.1% of participants are prescribed medications.
According to the statement, the term “weight-loss medications” contributes to the concept that their use has an aesthetic goal and can be consumed by anyone who desires to lose weight.
In addition to ensuring the correct use of language, Dr. Mancini adds that it is essential for doctors to seek and present pharmacologic treatment for obesity as something that will improve patient health. This means stressing that obesity can be controlled with a 10% loss in body weight, just as other chronic diseases, such as diabetes, can be controlled. Moreover, it is important to point out that medications also have a crucial role in optimizing weight maintenance in the long term.
Another issue Dr. Mancini raised is the prejudice that many doctors have against people with obesity. Health professionals should recognize they are also subject to weight bias and that the way they communicate with patients could have a profound effect on health-related outcomes.
“The stigma surrounding obesity can lead to bullying, even in the patient’s home by their relatives; this is very common. Weight stigma is so strong that it hinders patient health and decreases the likelihood of the patient seeking specialized care,” Dr. Mancini warned.
According to the authors, it is of utmost importance to understand that an individual should not be defined by his or disease (as by the use of the terms “obese” or “diabetic”) but rather understood to live with this disease (“individual with obesity” or “with diabetes”). Dr. Mancini suggests the following strategies that health care professionals can adopt while caring for patients with obesity:
- Speak to patients with empathy and respect, avoiding the use of judgmental words.
- Ask if they would like to discuss the “weight issue,” “BMI issue,” terms that are better received by the public, instead of saying “excess fat” or “excess weight.”
- If the patient agrees to talk about the subject, reinforce that this is a chronic health problem that requires longterm treatment and give him or her short, medium, and longterm options.
Lastly, the authors highlighted the importance of differentiating between regulatory agency–approved medications and over-the-counter drugs and supplements that are often sold as “weight-loss agents” and are responsible for an unacceptably high rate of emergency visits.
This article was translated from the Medscape Portuguese Edition. A version appeared on Medscape.com.
‘Vaginal dryness’ can be fatal. No, really.
This transcript has been edited for clarity.
What do you mean, Dr. Rubin? How is vaginal dryness killing women? We minimize the term vaginal dryness. When women come to our offices and complain of a little vaginal dryness – or they don’t even come to our office to complain of it because the doctor can’t be bothered with a little vaginal dryness — what they don’t understand is that this “little vaginal dryness” is really something called genitourinary syndrome of menopause (GSM). They don’t know that because they’ve never heard of it, and you may have never heard of it either. In 2014, we changed the terms vaginal dryness and vulvovaginal atrophy or atrophic vaginitis to GSM to make it short and simple.
GSM – what does it mean? It’s not just a little vaginal dryness. It turns out that all of the genital and urinary symptoms from menopause just get worse over time. The bladder, the urethra, and the vagina have lots of hormone receptors, including estrogen and testosterone. When the body no longer makes those hormones, the system doesn’t work very well, and genital and urinary symptoms occur that just get worse over time without treatment. Unlike hot flashes, which tend to go away, GSM does not.
What are the symptoms of GSM? Some are sexual: a little vaginal dryness, pain with sex, and worsening orgasm. But there are also genital and urinary symptoms that get worse: itching, burning irritation, rawness, an awareness of their genitals that the patient has never had before. And as a urologist, we see frequency, urgency, and leakage.
The thing that kills women is recurrent urinary tract infections (UTIs). Did you know that UTIs account for 7 million visits and hospitalizations annually and 25% of all infections in older people? In fact, apparently one-third of the total Medicare expenditure is around UTIs. Not preventing UTIs is costing our health care system an enormous amount of money and resources.
Did you know we’ve had safe and effective treatment options for GSM since the 1970s? Vaginal hormones have existed since the 1970s, but we’re using them only for pain with sex and not for GSM. In fact, data show that by using vaginal hormones, we can prevent UTIs by more than 50%. We can save lives using safe, effective, local, low-dose vaginal hormone strategies. And they are safe and effective for all of our patients in pre- and post menopause.
There are five different treatment options: vaginal estrogen inserts, vaginal estrogen creams, vaginal dehydroepiandrosterone (DHEA), low-dose vaginal estrogen rings, and an oral pill option called ospemifene (Osphena). All are used to treat GSM and will only work if your patient actually uses them and continues to use them.
These treatments are safe. They are effective. They do not increase the level of systemic hormones in the bloodstream. I have many patients with breast cancer who use these products as well. The only patients you may want to talk to your oncology colleagues about is women on active aromatase inhibitors.
We have to understand that UTIs kill people and having GSM is debilitating, often requiring pain medication because it can hurt to sit or to wear pads and our patients’ quality of life is severely affected. So please consider learning how to treat GSM. It turns out you don’t have to do exams. You don’t have to do follow-up. You can give these therapies, and women can use them for life.
Now, if your patient has vaginal bleeding, of course they need to see their gynecologist. But this is something every primary care doctor can and should do. As a urologist, we prescribe a lot of tamsulosin (Flomax) for our male patients to help with urination. Vaginal estrogen or DHEA is basically like Flomax for women, but it prevents UTIs and actually works like sildenafil (Viagra) because it can help orgasm and reduce pain with sex.
You have access to affordable, safe, effective treatment options to treat GSM. So check them out and hopefully change the world.
Dr. Rubin is an assistant clinical professor in the department of urology at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
What do you mean, Dr. Rubin? How is vaginal dryness killing women? We minimize the term vaginal dryness. When women come to our offices and complain of a little vaginal dryness – or they don’t even come to our office to complain of it because the doctor can’t be bothered with a little vaginal dryness — what they don’t understand is that this “little vaginal dryness” is really something called genitourinary syndrome of menopause (GSM). They don’t know that because they’ve never heard of it, and you may have never heard of it either. In 2014, we changed the terms vaginal dryness and vulvovaginal atrophy or atrophic vaginitis to GSM to make it short and simple.
GSM – what does it mean? It’s not just a little vaginal dryness. It turns out that all of the genital and urinary symptoms from menopause just get worse over time. The bladder, the urethra, and the vagina have lots of hormone receptors, including estrogen and testosterone. When the body no longer makes those hormones, the system doesn’t work very well, and genital and urinary symptoms occur that just get worse over time without treatment. Unlike hot flashes, which tend to go away, GSM does not.
What are the symptoms of GSM? Some are sexual: a little vaginal dryness, pain with sex, and worsening orgasm. But there are also genital and urinary symptoms that get worse: itching, burning irritation, rawness, an awareness of their genitals that the patient has never had before. And as a urologist, we see frequency, urgency, and leakage.
The thing that kills women is recurrent urinary tract infections (UTIs). Did you know that UTIs account for 7 million visits and hospitalizations annually and 25% of all infections in older people? In fact, apparently one-third of the total Medicare expenditure is around UTIs. Not preventing UTIs is costing our health care system an enormous amount of money and resources.
Did you know we’ve had safe and effective treatment options for GSM since the 1970s? Vaginal hormones have existed since the 1970s, but we’re using them only for pain with sex and not for GSM. In fact, data show that by using vaginal hormones, we can prevent UTIs by more than 50%. We can save lives using safe, effective, local, low-dose vaginal hormone strategies. And they are safe and effective for all of our patients in pre- and post menopause.
There are five different treatment options: vaginal estrogen inserts, vaginal estrogen creams, vaginal dehydroepiandrosterone (DHEA), low-dose vaginal estrogen rings, and an oral pill option called ospemifene (Osphena). All are used to treat GSM and will only work if your patient actually uses them and continues to use them.
These treatments are safe. They are effective. They do not increase the level of systemic hormones in the bloodstream. I have many patients with breast cancer who use these products as well. The only patients you may want to talk to your oncology colleagues about is women on active aromatase inhibitors.
We have to understand that UTIs kill people and having GSM is debilitating, often requiring pain medication because it can hurt to sit or to wear pads and our patients’ quality of life is severely affected. So please consider learning how to treat GSM. It turns out you don’t have to do exams. You don’t have to do follow-up. You can give these therapies, and women can use them for life.
Now, if your patient has vaginal bleeding, of course they need to see their gynecologist. But this is something every primary care doctor can and should do. As a urologist, we prescribe a lot of tamsulosin (Flomax) for our male patients to help with urination. Vaginal estrogen or DHEA is basically like Flomax for women, but it prevents UTIs and actually works like sildenafil (Viagra) because it can help orgasm and reduce pain with sex.
You have access to affordable, safe, effective treatment options to treat GSM. So check them out and hopefully change the world.
Dr. Rubin is an assistant clinical professor in the department of urology at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
What do you mean, Dr. Rubin? How is vaginal dryness killing women? We minimize the term vaginal dryness. When women come to our offices and complain of a little vaginal dryness – or they don’t even come to our office to complain of it because the doctor can’t be bothered with a little vaginal dryness — what they don’t understand is that this “little vaginal dryness” is really something called genitourinary syndrome of menopause (GSM). They don’t know that because they’ve never heard of it, and you may have never heard of it either. In 2014, we changed the terms vaginal dryness and vulvovaginal atrophy or atrophic vaginitis to GSM to make it short and simple.
GSM – what does it mean? It’s not just a little vaginal dryness. It turns out that all of the genital and urinary symptoms from menopause just get worse over time. The bladder, the urethra, and the vagina have lots of hormone receptors, including estrogen and testosterone. When the body no longer makes those hormones, the system doesn’t work very well, and genital and urinary symptoms occur that just get worse over time without treatment. Unlike hot flashes, which tend to go away, GSM does not.
What are the symptoms of GSM? Some are sexual: a little vaginal dryness, pain with sex, and worsening orgasm. But there are also genital and urinary symptoms that get worse: itching, burning irritation, rawness, an awareness of their genitals that the patient has never had before. And as a urologist, we see frequency, urgency, and leakage.
The thing that kills women is recurrent urinary tract infections (UTIs). Did you know that UTIs account for 7 million visits and hospitalizations annually and 25% of all infections in older people? In fact, apparently one-third of the total Medicare expenditure is around UTIs. Not preventing UTIs is costing our health care system an enormous amount of money and resources.
Did you know we’ve had safe and effective treatment options for GSM since the 1970s? Vaginal hormones have existed since the 1970s, but we’re using them only for pain with sex and not for GSM. In fact, data show that by using vaginal hormones, we can prevent UTIs by more than 50%. We can save lives using safe, effective, local, low-dose vaginal hormone strategies. And they are safe and effective for all of our patients in pre- and post menopause.
There are five different treatment options: vaginal estrogen inserts, vaginal estrogen creams, vaginal dehydroepiandrosterone (DHEA), low-dose vaginal estrogen rings, and an oral pill option called ospemifene (Osphena). All are used to treat GSM and will only work if your patient actually uses them and continues to use them.
These treatments are safe. They are effective. They do not increase the level of systemic hormones in the bloodstream. I have many patients with breast cancer who use these products as well. The only patients you may want to talk to your oncology colleagues about is women on active aromatase inhibitors.
We have to understand that UTIs kill people and having GSM is debilitating, often requiring pain medication because it can hurt to sit or to wear pads and our patients’ quality of life is severely affected. So please consider learning how to treat GSM. It turns out you don’t have to do exams. You don’t have to do follow-up. You can give these therapies, and women can use them for life.
Now, if your patient has vaginal bleeding, of course they need to see their gynecologist. But this is something every primary care doctor can and should do. As a urologist, we prescribe a lot of tamsulosin (Flomax) for our male patients to help with urination. Vaginal estrogen or DHEA is basically like Flomax for women, but it prevents UTIs and actually works like sildenafil (Viagra) because it can help orgasm and reduce pain with sex.
You have access to affordable, safe, effective treatment options to treat GSM. So check them out and hopefully change the world.
Dr. Rubin is an assistant clinical professor in the department of urology at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
Many young adults with type 2 diabetes skip medications
Young adults who developed type 2 diabetes as children often do not take medications to control blood pressure or cholesterol, according to a new study in JAMA Network Open. Researchers expressed alarm that young people who forgo these medications increase their chances of developing kidney disease or having a stroke.
“We’re learning more and more that those with youth onset [type 2 diabetes] really differ from those with adult onset: It looks like a more virulent form of the disease because kids are getting complications and comorbidities at much earlier ages and more severe levels,” said study author Paula Trief, PhD, a professor of psychiatry and behavioral science at State University of New York, Syracuse.
Participants in the new study were on average aged 26 years. They also had previously been part of the Treating Options for Type 2 Diabetes in Adolescents and Youth study, known as TODAY, which took place from 2004 to 2011. TODAY enrolled children between ages 10 and 17 years with type 2 diabetes who received either metformin, metformin plus rosiglitazone, or metformin plus a lifestyle intervention.
The study included extensive education and contact from medical professionals to the participants about managing diabetes.
“This cohort was followed a long time and they had a lot of support. It may be better than the real world where people haven’t had the history of this much attention,” said Lorraine Katz, MD, who specializes in endocrinology and diabetes at the Children’s Hospital of Philadelphia. Dr. Katz has enrolled participants in TODAY and published about medication adherence rates but was not part of the recent analysis.
Unannounced pill counts, addressing concerns about medication
The analysis, known as iCount, included 243 participants from the original TODAY study (159 girls) who had hypertension, neuropathy, or dyslipidemia that required ongoing medication. As the TODAY study was concluding between 2017 and 2019, researchers made unannounced phone calls to participants to request the numbers of pills they had prescribed, number of refills, and the refill date. Participants also counted aloud every pill in their possession twice.
Those phone calls continued for 3 consecutive months after iCount began and again at the same intervals 1 year later.
If the number of pills counted at a later time was at least 80% of the starting total, researchers considered this rate as low adherence. Anything less than 80% was considered high adherence.
“That’s kind of an arbitrary cutoff, but it’s one that’s used consistently in the literature” to measure medication adherence for many conditions including cancer and heart disease, Dr. Trief said. Unannounced calls to initiate pill counts were first used to understand how often people took medications for HIV, and this method was found to be a more reliable method than are self-reports.
Of 196 participants with hypertension or neuropathy, 157 (80.1%) had low adherence. And of the 146 people with high cholesterol, 137 (93.8%) had low adherence. Ninety-nine people with high cholesterol also had neuropathy or diabetes.
“This is new to the literature: We don’t really know as much about this age group,” because medication adherence studies of people who have had diabetes for more than a decade and are still in their 20s are rare, Dr. Katz said.
During the core TODAY study period, all medications were provided for free. In contrast, in the current study, participants had to obtain their prescriptions on their own. The researchers found that many participants who showed low adherence to blood pressure medications reported sometimes having trouble obtaining food (n = 62), struggling with securing stable housing (n = 47), or lacking reliable health care insurance (n = 28), all factors linked to medication adherence success, according to the analysis authors.
Researchers also assessed the impact of concerns that taking blood pressure medications may be harmful and found that people with these concerns were 37% less likely to maintain high adherence than others were by the 1-year follow-up point (odds ratio, 0.63; 95% confidence interval, 0.40-0.96; P = .01).
To some extent, the reasons people avoid medications are understandable, according to pediatric endocrinologist Tamara Hannon, MD, of Indiana University, Indianapolis.
“Rather than taking a medicine to feel better, you’re taking one not to have a problem in the future: You might not feel blood pressure, you certainly don’t feel cholesterol,” Dr. Hannon, who was not involved in the analysis, said. “Scolding them or telling them you’re going to be sorry one day doesn’t generally work.”
Dr. Hannon added that education alone about the benefits of medications does not generally drive people to adherence but that adding reminders to their phone calendar when refills are due could help. Or, the clinician could reach out to a trusted person in the patient’s life and enlist their support in taking medications consistently.
Dr. Trief advised that clinicians should carve out time for people to express their concerns about medications rather than simply writing a prescription and sending them on their way and to ask patients open-ended questions.
“If you just say to people do you have any questions, they usually say, ‘no.’ ”
No disclosures were reported.
A version of this article first appeared on Medscape.com.
Young adults who developed type 2 diabetes as children often do not take medications to control blood pressure or cholesterol, according to a new study in JAMA Network Open. Researchers expressed alarm that young people who forgo these medications increase their chances of developing kidney disease or having a stroke.
“We’re learning more and more that those with youth onset [type 2 diabetes] really differ from those with adult onset: It looks like a more virulent form of the disease because kids are getting complications and comorbidities at much earlier ages and more severe levels,” said study author Paula Trief, PhD, a professor of psychiatry and behavioral science at State University of New York, Syracuse.
Participants in the new study were on average aged 26 years. They also had previously been part of the Treating Options for Type 2 Diabetes in Adolescents and Youth study, known as TODAY, which took place from 2004 to 2011. TODAY enrolled children between ages 10 and 17 years with type 2 diabetes who received either metformin, metformin plus rosiglitazone, or metformin plus a lifestyle intervention.
The study included extensive education and contact from medical professionals to the participants about managing diabetes.
“This cohort was followed a long time and they had a lot of support. It may be better than the real world where people haven’t had the history of this much attention,” said Lorraine Katz, MD, who specializes in endocrinology and diabetes at the Children’s Hospital of Philadelphia. Dr. Katz has enrolled participants in TODAY and published about medication adherence rates but was not part of the recent analysis.
Unannounced pill counts, addressing concerns about medication
The analysis, known as iCount, included 243 participants from the original TODAY study (159 girls) who had hypertension, neuropathy, or dyslipidemia that required ongoing medication. As the TODAY study was concluding between 2017 and 2019, researchers made unannounced phone calls to participants to request the numbers of pills they had prescribed, number of refills, and the refill date. Participants also counted aloud every pill in their possession twice.
Those phone calls continued for 3 consecutive months after iCount began and again at the same intervals 1 year later.
If the number of pills counted at a later time was at least 80% of the starting total, researchers considered this rate as low adherence. Anything less than 80% was considered high adherence.
“That’s kind of an arbitrary cutoff, but it’s one that’s used consistently in the literature” to measure medication adherence for many conditions including cancer and heart disease, Dr. Trief said. Unannounced calls to initiate pill counts were first used to understand how often people took medications for HIV, and this method was found to be a more reliable method than are self-reports.
Of 196 participants with hypertension or neuropathy, 157 (80.1%) had low adherence. And of the 146 people with high cholesterol, 137 (93.8%) had low adherence. Ninety-nine people with high cholesterol also had neuropathy or diabetes.
“This is new to the literature: We don’t really know as much about this age group,” because medication adherence studies of people who have had diabetes for more than a decade and are still in their 20s are rare, Dr. Katz said.
During the core TODAY study period, all medications were provided for free. In contrast, in the current study, participants had to obtain their prescriptions on their own. The researchers found that many participants who showed low adherence to blood pressure medications reported sometimes having trouble obtaining food (n = 62), struggling with securing stable housing (n = 47), or lacking reliable health care insurance (n = 28), all factors linked to medication adherence success, according to the analysis authors.
Researchers also assessed the impact of concerns that taking blood pressure medications may be harmful and found that people with these concerns were 37% less likely to maintain high adherence than others were by the 1-year follow-up point (odds ratio, 0.63; 95% confidence interval, 0.40-0.96; P = .01).
To some extent, the reasons people avoid medications are understandable, according to pediatric endocrinologist Tamara Hannon, MD, of Indiana University, Indianapolis.
“Rather than taking a medicine to feel better, you’re taking one not to have a problem in the future: You might not feel blood pressure, you certainly don’t feel cholesterol,” Dr. Hannon, who was not involved in the analysis, said. “Scolding them or telling them you’re going to be sorry one day doesn’t generally work.”
Dr. Hannon added that education alone about the benefits of medications does not generally drive people to adherence but that adding reminders to their phone calendar when refills are due could help. Or, the clinician could reach out to a trusted person in the patient’s life and enlist their support in taking medications consistently.
Dr. Trief advised that clinicians should carve out time for people to express their concerns about medications rather than simply writing a prescription and sending them on their way and to ask patients open-ended questions.
“If you just say to people do you have any questions, they usually say, ‘no.’ ”
No disclosures were reported.
A version of this article first appeared on Medscape.com.
Young adults who developed type 2 diabetes as children often do not take medications to control blood pressure or cholesterol, according to a new study in JAMA Network Open. Researchers expressed alarm that young people who forgo these medications increase their chances of developing kidney disease or having a stroke.
“We’re learning more and more that those with youth onset [type 2 diabetes] really differ from those with adult onset: It looks like a more virulent form of the disease because kids are getting complications and comorbidities at much earlier ages and more severe levels,” said study author Paula Trief, PhD, a professor of psychiatry and behavioral science at State University of New York, Syracuse.
Participants in the new study were on average aged 26 years. They also had previously been part of the Treating Options for Type 2 Diabetes in Adolescents and Youth study, known as TODAY, which took place from 2004 to 2011. TODAY enrolled children between ages 10 and 17 years with type 2 diabetes who received either metformin, metformin plus rosiglitazone, or metformin plus a lifestyle intervention.
The study included extensive education and contact from medical professionals to the participants about managing diabetes.
“This cohort was followed a long time and they had a lot of support. It may be better than the real world where people haven’t had the history of this much attention,” said Lorraine Katz, MD, who specializes in endocrinology and diabetes at the Children’s Hospital of Philadelphia. Dr. Katz has enrolled participants in TODAY and published about medication adherence rates but was not part of the recent analysis.
Unannounced pill counts, addressing concerns about medication
The analysis, known as iCount, included 243 participants from the original TODAY study (159 girls) who had hypertension, neuropathy, or dyslipidemia that required ongoing medication. As the TODAY study was concluding between 2017 and 2019, researchers made unannounced phone calls to participants to request the numbers of pills they had prescribed, number of refills, and the refill date. Participants also counted aloud every pill in their possession twice.
Those phone calls continued for 3 consecutive months after iCount began and again at the same intervals 1 year later.
If the number of pills counted at a later time was at least 80% of the starting total, researchers considered this rate as low adherence. Anything less than 80% was considered high adherence.
“That’s kind of an arbitrary cutoff, but it’s one that’s used consistently in the literature” to measure medication adherence for many conditions including cancer and heart disease, Dr. Trief said. Unannounced calls to initiate pill counts were first used to understand how often people took medications for HIV, and this method was found to be a more reliable method than are self-reports.
Of 196 participants with hypertension or neuropathy, 157 (80.1%) had low adherence. And of the 146 people with high cholesterol, 137 (93.8%) had low adherence. Ninety-nine people with high cholesterol also had neuropathy or diabetes.
“This is new to the literature: We don’t really know as much about this age group,” because medication adherence studies of people who have had diabetes for more than a decade and are still in their 20s are rare, Dr. Katz said.
During the core TODAY study period, all medications were provided for free. In contrast, in the current study, participants had to obtain their prescriptions on their own. The researchers found that many participants who showed low adherence to blood pressure medications reported sometimes having trouble obtaining food (n = 62), struggling with securing stable housing (n = 47), or lacking reliable health care insurance (n = 28), all factors linked to medication adherence success, according to the analysis authors.
Researchers also assessed the impact of concerns that taking blood pressure medications may be harmful and found that people with these concerns were 37% less likely to maintain high adherence than others were by the 1-year follow-up point (odds ratio, 0.63; 95% confidence interval, 0.40-0.96; P = .01).
To some extent, the reasons people avoid medications are understandable, according to pediatric endocrinologist Tamara Hannon, MD, of Indiana University, Indianapolis.
“Rather than taking a medicine to feel better, you’re taking one not to have a problem in the future: You might not feel blood pressure, you certainly don’t feel cholesterol,” Dr. Hannon, who was not involved in the analysis, said. “Scolding them or telling them you’re going to be sorry one day doesn’t generally work.”
Dr. Hannon added that education alone about the benefits of medications does not generally drive people to adherence but that adding reminders to their phone calendar when refills are due could help. Or, the clinician could reach out to a trusted person in the patient’s life and enlist their support in taking medications consistently.
Dr. Trief advised that clinicians should carve out time for people to express their concerns about medications rather than simply writing a prescription and sending them on their way and to ask patients open-ended questions.
“If you just say to people do you have any questions, they usually say, ‘no.’ ”
No disclosures were reported.
A version of this article first appeared on Medscape.com.
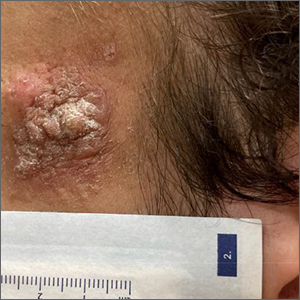
Patch testing finds higher prevalence of ACD among children with AD
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Redefining CVD risk: Cardiovascular-kidney-metabolic (CKM) syndrome
“This work was prompted by the fact that CKM syndrome leads to premature morbidity and mortality, primarily because of a higher burden of CVD,” writing committee chair Chiadi Ndumele, MD, PhD, said in an interview.
“While CKM syndrome is a public health emergency, there is also great potential for improving CKM health in the population, with an increasing number of therapies that favorably impact metabolic risk factors, risk for adverse kidney events, or both, which also protect against CVD,” added Dr. Ndumele, director of obesity and cardiometabolic research in the division of cardiology at Johns Hopkins University, Baltimore.
The AHA presidential advisory and accompanying scientific statement, which provides a synopsis of evidence for the science and clinical management of CKM, were published online in the journal Circulation.
CKM syndrome staging
According to the AHA, one in three U.S. adults have three or more risk factors that contribute to CVD, metabolic disorders, and/or kidney disease.
In addition to defining CKM syndrome, the advisory provides a “staging construct, to be used in both adults and youth, that reflects the progressive pathophysiology and risk within CKM syndrome, with therapeutic guidance tied to CKM stages,” Dr. Ndumele told this news organization.
The AHA outlines four stages of CKM syndrome:
Stage 0: At this stage, no CKM risk factors are present, and the goal is to prevent CKM syndrome (particularly unhealthy weight gain) by achieving and maintaining ideal health based on the AHA’s Life’s Essential 8 recommendations. Adults in this stage should be screened every 3-5 years to assess lipids, blood pressure, and blood sugar.
Stage 1: At this stage, excess weight, abdominal obesity, or dysfunctional adipose tissue (clinically manifest as impaired glucose tolerance or prediabetes) is present without other metabolic risk factors or CVD. Management includes providing support for healthy lifestyle changes (healthy eating and regular physical activity), with a goal of at least 5% weight loss and addressing glucose intolerance if needed. Screening adults with stage 1 CKM every 2-3 years is advised to assess blood pressure, triglycerides, cholesterol, and blood sugar.
Stage 2: At this stage, metabolic risk factors (hypertriglyceridemia, hypertension, metabolic syndrome, diabetes) and kidney disease are present. The goal is to address risk factors to prevent progression to CVD and kidney failure. Screening for stage 2 CKM syndrome aligns with AHA/ACC guidelines, which include yearly assessment of blood pressure, triglycerides, cholesterol, blood sugar, and kidney function. More frequent kidney screening is recommended for individuals with increased risk of kidney failure based on kidney function assessments.
Stage 3: This stage describes individuals with subclinical CVD with metabolic risk factors or kidney disease or those at high predicted risk for CVD. The goal is to intensify efforts to prevent progression to symptomatic CVD and kidney failure. This may involve increasing or changing medications, and additional focus on lifestyle changes. Coronary artery calcium (CAC) measurement in some adults is recommended to assess narrowing of the arteries when treatment decisions are unclear.
Stage 4: Individuals with stage 4 CKM syndrome have symptomatic CVD, excess body fat, metabolic risk factors, or kidney disease. Stage 4 CKM syndrome is divided into two subcategories: (4a) no kidney failure and (4b) kidney failure. In this stage, patients may have already had a myocardial infarction (MI) or stroke or may already have heart failure. They also may have additional CV conditions such as peripheral artery disease or atrial fibrillation. The goal of care is individualized treatment for CVD with consideration for CKM syndrome conditions.
The advisory also describes CKM syndrome regression, “an important concept and public health message in which people making healthy lifestyle changes and achieving weight loss may regress to lower CKM syndrome stages and a better state of health,” the AHA says in a news release.
They note that a “critical” next step is to update the pooled cohort equation (PCE) risk prediction algorithm to include measures of kidney function, type 2 diabetes control, and social determinants of health for a more comprehensive risk estimate.
The advisory also recommends risk calculator updates be expanded to assess risk in people as young as age 30 and to calculate both 10- and 30-year CVD risk.
“Clearly defining the patient with CKM syndrome, and providing new approaches for CKM syndrome staging and risk prediction, will help health care professionals to identify these individuals earlier and to provide timely, holistic, and patient-centered care,” Dr. Ndumele said.
This presidential advisory was prepared by the volunteer writing group on behalf of the AHA . The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“This work was prompted by the fact that CKM syndrome leads to premature morbidity and mortality, primarily because of a higher burden of CVD,” writing committee chair Chiadi Ndumele, MD, PhD, said in an interview.
“While CKM syndrome is a public health emergency, there is also great potential for improving CKM health in the population, with an increasing number of therapies that favorably impact metabolic risk factors, risk for adverse kidney events, or both, which also protect against CVD,” added Dr. Ndumele, director of obesity and cardiometabolic research in the division of cardiology at Johns Hopkins University, Baltimore.
The AHA presidential advisory and accompanying scientific statement, which provides a synopsis of evidence for the science and clinical management of CKM, were published online in the journal Circulation.
CKM syndrome staging
According to the AHA, one in three U.S. adults have three or more risk factors that contribute to CVD, metabolic disorders, and/or kidney disease.
In addition to defining CKM syndrome, the advisory provides a “staging construct, to be used in both adults and youth, that reflects the progressive pathophysiology and risk within CKM syndrome, with therapeutic guidance tied to CKM stages,” Dr. Ndumele told this news organization.
The AHA outlines four stages of CKM syndrome:
Stage 0: At this stage, no CKM risk factors are present, and the goal is to prevent CKM syndrome (particularly unhealthy weight gain) by achieving and maintaining ideal health based on the AHA’s Life’s Essential 8 recommendations. Adults in this stage should be screened every 3-5 years to assess lipids, blood pressure, and blood sugar.
Stage 1: At this stage, excess weight, abdominal obesity, or dysfunctional adipose tissue (clinically manifest as impaired glucose tolerance or prediabetes) is present without other metabolic risk factors or CVD. Management includes providing support for healthy lifestyle changes (healthy eating and regular physical activity), with a goal of at least 5% weight loss and addressing glucose intolerance if needed. Screening adults with stage 1 CKM every 2-3 years is advised to assess blood pressure, triglycerides, cholesterol, and blood sugar.
Stage 2: At this stage, metabolic risk factors (hypertriglyceridemia, hypertension, metabolic syndrome, diabetes) and kidney disease are present. The goal is to address risk factors to prevent progression to CVD and kidney failure. Screening for stage 2 CKM syndrome aligns with AHA/ACC guidelines, which include yearly assessment of blood pressure, triglycerides, cholesterol, blood sugar, and kidney function. More frequent kidney screening is recommended for individuals with increased risk of kidney failure based on kidney function assessments.
Stage 3: This stage describes individuals with subclinical CVD with metabolic risk factors or kidney disease or those at high predicted risk for CVD. The goal is to intensify efforts to prevent progression to symptomatic CVD and kidney failure. This may involve increasing or changing medications, and additional focus on lifestyle changes. Coronary artery calcium (CAC) measurement in some adults is recommended to assess narrowing of the arteries when treatment decisions are unclear.
Stage 4: Individuals with stage 4 CKM syndrome have symptomatic CVD, excess body fat, metabolic risk factors, or kidney disease. Stage 4 CKM syndrome is divided into two subcategories: (4a) no kidney failure and (4b) kidney failure. In this stage, patients may have already had a myocardial infarction (MI) or stroke or may already have heart failure. They also may have additional CV conditions such as peripheral artery disease or atrial fibrillation. The goal of care is individualized treatment for CVD with consideration for CKM syndrome conditions.
The advisory also describes CKM syndrome regression, “an important concept and public health message in which people making healthy lifestyle changes and achieving weight loss may regress to lower CKM syndrome stages and a better state of health,” the AHA says in a news release.
They note that a “critical” next step is to update the pooled cohort equation (PCE) risk prediction algorithm to include measures of kidney function, type 2 diabetes control, and social determinants of health for a more comprehensive risk estimate.
The advisory also recommends risk calculator updates be expanded to assess risk in people as young as age 30 and to calculate both 10- and 30-year CVD risk.
“Clearly defining the patient with CKM syndrome, and providing new approaches for CKM syndrome staging and risk prediction, will help health care professionals to identify these individuals earlier and to provide timely, holistic, and patient-centered care,” Dr. Ndumele said.
This presidential advisory was prepared by the volunteer writing group on behalf of the AHA . The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“This work was prompted by the fact that CKM syndrome leads to premature morbidity and mortality, primarily because of a higher burden of CVD,” writing committee chair Chiadi Ndumele, MD, PhD, said in an interview.
“While CKM syndrome is a public health emergency, there is also great potential for improving CKM health in the population, with an increasing number of therapies that favorably impact metabolic risk factors, risk for adverse kidney events, or both, which also protect against CVD,” added Dr. Ndumele, director of obesity and cardiometabolic research in the division of cardiology at Johns Hopkins University, Baltimore.
The AHA presidential advisory and accompanying scientific statement, which provides a synopsis of evidence for the science and clinical management of CKM, were published online in the journal Circulation.
CKM syndrome staging
According to the AHA, one in three U.S. adults have three or more risk factors that contribute to CVD, metabolic disorders, and/or kidney disease.
In addition to defining CKM syndrome, the advisory provides a “staging construct, to be used in both adults and youth, that reflects the progressive pathophysiology and risk within CKM syndrome, with therapeutic guidance tied to CKM stages,” Dr. Ndumele told this news organization.
The AHA outlines four stages of CKM syndrome:
Stage 0: At this stage, no CKM risk factors are present, and the goal is to prevent CKM syndrome (particularly unhealthy weight gain) by achieving and maintaining ideal health based on the AHA’s Life’s Essential 8 recommendations. Adults in this stage should be screened every 3-5 years to assess lipids, blood pressure, and blood sugar.
Stage 1: At this stage, excess weight, abdominal obesity, or dysfunctional adipose tissue (clinically manifest as impaired glucose tolerance or prediabetes) is present without other metabolic risk factors or CVD. Management includes providing support for healthy lifestyle changes (healthy eating and regular physical activity), with a goal of at least 5% weight loss and addressing glucose intolerance if needed. Screening adults with stage 1 CKM every 2-3 years is advised to assess blood pressure, triglycerides, cholesterol, and blood sugar.
Stage 2: At this stage, metabolic risk factors (hypertriglyceridemia, hypertension, metabolic syndrome, diabetes) and kidney disease are present. The goal is to address risk factors to prevent progression to CVD and kidney failure. Screening for stage 2 CKM syndrome aligns with AHA/ACC guidelines, which include yearly assessment of blood pressure, triglycerides, cholesterol, blood sugar, and kidney function. More frequent kidney screening is recommended for individuals with increased risk of kidney failure based on kidney function assessments.
Stage 3: This stage describes individuals with subclinical CVD with metabolic risk factors or kidney disease or those at high predicted risk for CVD. The goal is to intensify efforts to prevent progression to symptomatic CVD and kidney failure. This may involve increasing or changing medications, and additional focus on lifestyle changes. Coronary artery calcium (CAC) measurement in some adults is recommended to assess narrowing of the arteries when treatment decisions are unclear.
Stage 4: Individuals with stage 4 CKM syndrome have symptomatic CVD, excess body fat, metabolic risk factors, or kidney disease. Stage 4 CKM syndrome is divided into two subcategories: (4a) no kidney failure and (4b) kidney failure. In this stage, patients may have already had a myocardial infarction (MI) or stroke or may already have heart failure. They also may have additional CV conditions such as peripheral artery disease or atrial fibrillation. The goal of care is individualized treatment for CVD with consideration for CKM syndrome conditions.
The advisory also describes CKM syndrome regression, “an important concept and public health message in which people making healthy lifestyle changes and achieving weight loss may regress to lower CKM syndrome stages and a better state of health,” the AHA says in a news release.
They note that a “critical” next step is to update the pooled cohort equation (PCE) risk prediction algorithm to include measures of kidney function, type 2 diabetes control, and social determinants of health for a more comprehensive risk estimate.
The advisory also recommends risk calculator updates be expanded to assess risk in people as young as age 30 and to calculate both 10- and 30-year CVD risk.
“Clearly defining the patient with CKM syndrome, and providing new approaches for CKM syndrome staging and risk prediction, will help health care professionals to identify these individuals earlier and to provide timely, holistic, and patient-centered care,” Dr. Ndumele said.
This presidential advisory was prepared by the volunteer writing group on behalf of the AHA . The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
New hyperglycemia emergency guidance updates DKA definition
HAMBURG, GERMANY – along with many other updates to the last statement on the topic, published 14 years ago.
Based on extensive literature reviews and observations of current trends, the new document – due to be published soon – will cover diagnosis and management of the two most serious acute hyperglycemic emergencies seen in adults, DKA and hyperosmolar hyperglycemic state (HHS).
New to the 2023 version will be a strong emphasis on the excess morbidity and mortality risks associated with the increasingly common “hybrid” presentation of the two conditions together, now seen in about a third of cases.
The new report will also more strongly urge clinicians to investigate why the person experienced the emergency.
While new-onset diabetes and infection are recognized precipitating causes for DKA, insulin omission related to finances, mental health, and social determinants should be identified, and patients directed to appropriate resources, said experts previewing the upcoming new report at the annual meeting of the European Association for the Study of Diabetes.
“The challenge is, although we were making progress for a long time in terms of those hyperglycemic crises, we’ve really plateaued and there are still people being admitted in large numbers, and when you look more globally even more so,” said American Diabetes Association Chief Science and Medical Officer Robert A. Gabbay, MD, PhD.
The new consensus report will be jointly endorsed by the ADA, the EASD, the American Association of Clinical Endocrinology, the Diabetes Technology Society, and the Joint British Diabetes Societies for Inpatient Care. The previous consensus statement on the subject was published in 2009 by the ADA alone.
New DKA and HHS definitions reflect emerging trends
The statement will revise the definition of DKA, partly spurred by the increasing occurrence and recognition of euglycemic ketoacidosis arising from the use of sodium-glucose cotransporter 2 (SGLT2) inhibitors. For all patients with hyperglycemic crisis, the hyperglycemia cutoff is now lowered to 200 mg/dL (11.1 mmol/L) from the previous 250 mg/dL.
However, the glucose cutoff has been removed entirely for people with a history of diabetes.
“Both of these changes are recognizing the wide range of glucose levels at the presence of DKA. Approximately 10% of DKA occurs with euglycemia or near-normoglycemia,” noted coauthor Shivani Misra, MD, PhD, senior clinical lecturer and honorary consultant in Metabolic Medicine at Imperial College, London.
For assessing ketosis in DKA, the new statement strongly recommends use of beta-hydroxybutyrate – either via point-of-care test or serum level measured in a laboratory – with a low cutoff of ≥ 3.0 mmol/L. Alternatively, a urine ketone strip value of 2+ or greater can be used.
However, beta-hydroxybutyrate testing is more widely available now than it was in 2009 and is strongly preferred over urine ketone measurement because it’s the predominant ketone during acidosis. Moreover, urine acetoacetate – measured by the strips – paradoxically increases during resolution of DKA, and drug interferences can occur with urine ketone measurement, Dr. Misra noted.
Metabolic acidosis is now defined as a pH < 7.3 and/or a bicarbonate concentration < 18 mmol/L, up from 15 in some prior guidelines including the United Kingdom’s. Also, anion gap has been removed from the main definition but, the document will say, can still be used in settings where ketone testing is unavailable.
As previously, the new statement will classify DKA by mild, moderate, and severe but now for the first time there are recommendations of care for each of those levels, as well as for HHS.
For HHS, the glucose cutoff of ≥ 600 mg/dL will stay the same. But now, the effective serum osmolality has been lowered from > 320 to > 300 mOsml/L to account for the effect of dehydration, along with an alternative criteria of total serum osmolality > 320 mOsm/L. The same two changes as with DKA for both ketones and acidosis have also been included for HHS.
Asked to comment, session audience member and independent diabetes industry consultant Charles Alexander, MD, told this news organization, “I liked the proposal to eliminate the anion gap in decision-making and to focus on measurement of blood ketones, principally beta-hydroxybutyrate, in the diagnosis of DKA and monitoring the effect of treatment.
“If someone is on an SGLT2 inhibitor, there is no need to look at blood glucose levels, which may be normal or near normal in the setting of DKA.”
But Dr. Alexander thinks that they should have eliminated glucose levels entirely as part of the DKA/HHS definition even for people without diabetes.
“The problem is that medical education for many years has taught us that DKA is a condition of high blood glucose, but it may not be. It is good that they said blood glucose levels were not important if the patient had a history of diabetes. However, a glucose of 200mg/dL may not be low enough if someone is on an SGLT2 inhibitor. There needs to be a much lower threshold for measuring blood ketones in anyone with nausea, vomiting, and abdominal pain, regardless of the blood glucose level.”
Acute management: IV fluids, insulin, and potassium
Like the 2009 statement, the new one will include detailed management flowcharts for DKA and HHS, but this time in color. This new statement includes individual algorithms for management with intravenous fluids, insulin, and potassium. Bicarbonate has been removed and relegated to a note at the bottom saying that it should only be considered if pH is < 7.0.
Under fluid treatment, the new statement offers more information about using crystalloids to treat dehydration and a recommendation to add dextrose to IV fluid therapy as a substrate when the glucose drops below 250 mg/dL, in order to prevent hypoglycemia. For euglycemic DKA, the recommendation is to include dextrose and normal saline simultaneously.
And for the first time, subcutaneous rather than IV insulin is considered acceptable for mild, but not moderate or severe, DKA.
Two options are suggested for IV insulin in HHS: The fluid can be given first and low-dose fixed-rate insulin infusion added, or fluids and insulin can be given at the same time.
Criteria for resolution of DKA are a venous pH of ≥ 7.3 or bicarbonate > 18 mmol/L, ketones < 0.6 mmol/L, and glucose ideally < 200 mg/dL (11.0 mmol/L). For HHS, resolution is suggested when the measured or calculated serum osmolality falls to < 300 mosm/kg, blood glucose is < 250mg/dL (13.9 mmol/L), urine output > 0.5 mL/kg/hour, and cognitive status is improved.
The statement also will provide detailed recommended options for transitioning from IV to subcutaneous insulin, but defers to clinical judgment for deciding when the patient can be discharged. The initiation or continuation of SGLT2 inhibitors is not recommended at any time during hospitalization for hyperglycemic crises.
Mitigating complications, preventing recurrence
In addition to listing potential complications of treating hyperglycemic crises, just as the 2009 statement did, the new one will offer mitigation strategies for some of the more common ones. For preventing hypoglycemia, frequent blood glucose monitoring is advised along with adding dextrose to the IV fluids when glucose drops below 250 mg/dL.
For prevention of hypokalemia, which occurs in about half of patients treated for DKA and HHS, the statement recommends potassium monitoring every 4 hours and replacement added to fluids.
Acute kidney injury, also occurring in about half of people treated for DKA and/or HHS, usually resolves with hydration. Daily renal function monitoring is advised.
Preventing recurrence: Many factors beyond clinical
Prevention of recurrence with readmission for DKA and/or HHS, occurring in up to 22% of U.S. patients within 30 days, entails close follow-up within 2-4 weeks after discharge (including via telemedicine), and assessment of possible causes, including mental health disorders and social determinants of health.
Appropriate education should be provided, including “structured education” involving problem-solving, sick day rules, injection techniques, a review of insulin doses, consideration of continuous glucose monitoring (CGM), and home ketone testing.
Patients should be provided with an adequate supply of insulin and durable diabetes equipment, along with contact information for health care professionals who can assist them. Social service professionals can be helpful for patients who lack reliable access.
Dr. Gabbay told this news organization, “The eye-opening thing is we tend to typically think of DKA as how people tend to get diagnosed with diabetes and, yes, that’s true, but that’s only a minority of people. Those might be preventable by early screening, but all these other people and the number of recurrent episodes, that’s an area where it’s really a failure of the system where we can do better in ensuring that doesn’t happen.”
Education is only part of it, he stressed. “It’s not just an intelligence thing. It’s social factors, and there can be complex psychological issues and mental health issues. We need to screen for those things when we see someone coming back the second, third, fifth, or sixth time. We’ve all seen that. Just educating them to take their insulin is not the answer. …You’ve got to ask the questions and engage them to go a little deeper.”
Dr. Gabbay is an employee of the ADA. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum. Dr. Misra has received speaker fees from Sanofi and ABCD and an investigator-initiated research grant from Dexcom, and is a trustee for the Diabetes Research and Wellness Foundation in the United Kingdom.
A version of this article appeared on Medscape.com.
HAMBURG, GERMANY – along with many other updates to the last statement on the topic, published 14 years ago.
Based on extensive literature reviews and observations of current trends, the new document – due to be published soon – will cover diagnosis and management of the two most serious acute hyperglycemic emergencies seen in adults, DKA and hyperosmolar hyperglycemic state (HHS).
New to the 2023 version will be a strong emphasis on the excess morbidity and mortality risks associated with the increasingly common “hybrid” presentation of the two conditions together, now seen in about a third of cases.
The new report will also more strongly urge clinicians to investigate why the person experienced the emergency.
While new-onset diabetes and infection are recognized precipitating causes for DKA, insulin omission related to finances, mental health, and social determinants should be identified, and patients directed to appropriate resources, said experts previewing the upcoming new report at the annual meeting of the European Association for the Study of Diabetes.
“The challenge is, although we were making progress for a long time in terms of those hyperglycemic crises, we’ve really plateaued and there are still people being admitted in large numbers, and when you look more globally even more so,” said American Diabetes Association Chief Science and Medical Officer Robert A. Gabbay, MD, PhD.
The new consensus report will be jointly endorsed by the ADA, the EASD, the American Association of Clinical Endocrinology, the Diabetes Technology Society, and the Joint British Diabetes Societies for Inpatient Care. The previous consensus statement on the subject was published in 2009 by the ADA alone.
New DKA and HHS definitions reflect emerging trends
The statement will revise the definition of DKA, partly spurred by the increasing occurrence and recognition of euglycemic ketoacidosis arising from the use of sodium-glucose cotransporter 2 (SGLT2) inhibitors. For all patients with hyperglycemic crisis, the hyperglycemia cutoff is now lowered to 200 mg/dL (11.1 mmol/L) from the previous 250 mg/dL.
However, the glucose cutoff has been removed entirely for people with a history of diabetes.
“Both of these changes are recognizing the wide range of glucose levels at the presence of DKA. Approximately 10% of DKA occurs with euglycemia or near-normoglycemia,” noted coauthor Shivani Misra, MD, PhD, senior clinical lecturer and honorary consultant in Metabolic Medicine at Imperial College, London.
For assessing ketosis in DKA, the new statement strongly recommends use of beta-hydroxybutyrate – either via point-of-care test or serum level measured in a laboratory – with a low cutoff of ≥ 3.0 mmol/L. Alternatively, a urine ketone strip value of 2+ or greater can be used.
However, beta-hydroxybutyrate testing is more widely available now than it was in 2009 and is strongly preferred over urine ketone measurement because it’s the predominant ketone during acidosis. Moreover, urine acetoacetate – measured by the strips – paradoxically increases during resolution of DKA, and drug interferences can occur with urine ketone measurement, Dr. Misra noted.
Metabolic acidosis is now defined as a pH < 7.3 and/or a bicarbonate concentration < 18 mmol/L, up from 15 in some prior guidelines including the United Kingdom’s. Also, anion gap has been removed from the main definition but, the document will say, can still be used in settings where ketone testing is unavailable.
As previously, the new statement will classify DKA by mild, moderate, and severe but now for the first time there are recommendations of care for each of those levels, as well as for HHS.
For HHS, the glucose cutoff of ≥ 600 mg/dL will stay the same. But now, the effective serum osmolality has been lowered from > 320 to > 300 mOsml/L to account for the effect of dehydration, along with an alternative criteria of total serum osmolality > 320 mOsm/L. The same two changes as with DKA for both ketones and acidosis have also been included for HHS.
Asked to comment, session audience member and independent diabetes industry consultant Charles Alexander, MD, told this news organization, “I liked the proposal to eliminate the anion gap in decision-making and to focus on measurement of blood ketones, principally beta-hydroxybutyrate, in the diagnosis of DKA and monitoring the effect of treatment.
“If someone is on an SGLT2 inhibitor, there is no need to look at blood glucose levels, which may be normal or near normal in the setting of DKA.”
But Dr. Alexander thinks that they should have eliminated glucose levels entirely as part of the DKA/HHS definition even for people without diabetes.
“The problem is that medical education for many years has taught us that DKA is a condition of high blood glucose, but it may not be. It is good that they said blood glucose levels were not important if the patient had a history of diabetes. However, a glucose of 200mg/dL may not be low enough if someone is on an SGLT2 inhibitor. There needs to be a much lower threshold for measuring blood ketones in anyone with nausea, vomiting, and abdominal pain, regardless of the blood glucose level.”
Acute management: IV fluids, insulin, and potassium
Like the 2009 statement, the new one will include detailed management flowcharts for DKA and HHS, but this time in color. This new statement includes individual algorithms for management with intravenous fluids, insulin, and potassium. Bicarbonate has been removed and relegated to a note at the bottom saying that it should only be considered if pH is < 7.0.
Under fluid treatment, the new statement offers more information about using crystalloids to treat dehydration and a recommendation to add dextrose to IV fluid therapy as a substrate when the glucose drops below 250 mg/dL, in order to prevent hypoglycemia. For euglycemic DKA, the recommendation is to include dextrose and normal saline simultaneously.
And for the first time, subcutaneous rather than IV insulin is considered acceptable for mild, but not moderate or severe, DKA.
Two options are suggested for IV insulin in HHS: The fluid can be given first and low-dose fixed-rate insulin infusion added, or fluids and insulin can be given at the same time.
Criteria for resolution of DKA are a venous pH of ≥ 7.3 or bicarbonate > 18 mmol/L, ketones < 0.6 mmol/L, and glucose ideally < 200 mg/dL (11.0 mmol/L). For HHS, resolution is suggested when the measured or calculated serum osmolality falls to < 300 mosm/kg, blood glucose is < 250mg/dL (13.9 mmol/L), urine output > 0.5 mL/kg/hour, and cognitive status is improved.
The statement also will provide detailed recommended options for transitioning from IV to subcutaneous insulin, but defers to clinical judgment for deciding when the patient can be discharged. The initiation or continuation of SGLT2 inhibitors is not recommended at any time during hospitalization for hyperglycemic crises.
Mitigating complications, preventing recurrence
In addition to listing potential complications of treating hyperglycemic crises, just as the 2009 statement did, the new one will offer mitigation strategies for some of the more common ones. For preventing hypoglycemia, frequent blood glucose monitoring is advised along with adding dextrose to the IV fluids when glucose drops below 250 mg/dL.
For prevention of hypokalemia, which occurs in about half of patients treated for DKA and HHS, the statement recommends potassium monitoring every 4 hours and replacement added to fluids.
Acute kidney injury, also occurring in about half of people treated for DKA and/or HHS, usually resolves with hydration. Daily renal function monitoring is advised.
Preventing recurrence: Many factors beyond clinical
Prevention of recurrence with readmission for DKA and/or HHS, occurring in up to 22% of U.S. patients within 30 days, entails close follow-up within 2-4 weeks after discharge (including via telemedicine), and assessment of possible causes, including mental health disorders and social determinants of health.
Appropriate education should be provided, including “structured education” involving problem-solving, sick day rules, injection techniques, a review of insulin doses, consideration of continuous glucose monitoring (CGM), and home ketone testing.
Patients should be provided with an adequate supply of insulin and durable diabetes equipment, along with contact information for health care professionals who can assist them. Social service professionals can be helpful for patients who lack reliable access.
Dr. Gabbay told this news organization, “The eye-opening thing is we tend to typically think of DKA as how people tend to get diagnosed with diabetes and, yes, that’s true, but that’s only a minority of people. Those might be preventable by early screening, but all these other people and the number of recurrent episodes, that’s an area where it’s really a failure of the system where we can do better in ensuring that doesn’t happen.”
Education is only part of it, he stressed. “It’s not just an intelligence thing. It’s social factors, and there can be complex psychological issues and mental health issues. We need to screen for those things when we see someone coming back the second, third, fifth, or sixth time. We’ve all seen that. Just educating them to take their insulin is not the answer. …You’ve got to ask the questions and engage them to go a little deeper.”
Dr. Gabbay is an employee of the ADA. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum. Dr. Misra has received speaker fees from Sanofi and ABCD and an investigator-initiated research grant from Dexcom, and is a trustee for the Diabetes Research and Wellness Foundation in the United Kingdom.
A version of this article appeared on Medscape.com.
HAMBURG, GERMANY – along with many other updates to the last statement on the topic, published 14 years ago.
Based on extensive literature reviews and observations of current trends, the new document – due to be published soon – will cover diagnosis and management of the two most serious acute hyperglycemic emergencies seen in adults, DKA and hyperosmolar hyperglycemic state (HHS).
New to the 2023 version will be a strong emphasis on the excess morbidity and mortality risks associated with the increasingly common “hybrid” presentation of the two conditions together, now seen in about a third of cases.
The new report will also more strongly urge clinicians to investigate why the person experienced the emergency.
While new-onset diabetes and infection are recognized precipitating causes for DKA, insulin omission related to finances, mental health, and social determinants should be identified, and patients directed to appropriate resources, said experts previewing the upcoming new report at the annual meeting of the European Association for the Study of Diabetes.
“The challenge is, although we were making progress for a long time in terms of those hyperglycemic crises, we’ve really plateaued and there are still people being admitted in large numbers, and when you look more globally even more so,” said American Diabetes Association Chief Science and Medical Officer Robert A. Gabbay, MD, PhD.
The new consensus report will be jointly endorsed by the ADA, the EASD, the American Association of Clinical Endocrinology, the Diabetes Technology Society, and the Joint British Diabetes Societies for Inpatient Care. The previous consensus statement on the subject was published in 2009 by the ADA alone.
New DKA and HHS definitions reflect emerging trends
The statement will revise the definition of DKA, partly spurred by the increasing occurrence and recognition of euglycemic ketoacidosis arising from the use of sodium-glucose cotransporter 2 (SGLT2) inhibitors. For all patients with hyperglycemic crisis, the hyperglycemia cutoff is now lowered to 200 mg/dL (11.1 mmol/L) from the previous 250 mg/dL.
However, the glucose cutoff has been removed entirely for people with a history of diabetes.
“Both of these changes are recognizing the wide range of glucose levels at the presence of DKA. Approximately 10% of DKA occurs with euglycemia or near-normoglycemia,” noted coauthor Shivani Misra, MD, PhD, senior clinical lecturer and honorary consultant in Metabolic Medicine at Imperial College, London.
For assessing ketosis in DKA, the new statement strongly recommends use of beta-hydroxybutyrate – either via point-of-care test or serum level measured in a laboratory – with a low cutoff of ≥ 3.0 mmol/L. Alternatively, a urine ketone strip value of 2+ or greater can be used.
However, beta-hydroxybutyrate testing is more widely available now than it was in 2009 and is strongly preferred over urine ketone measurement because it’s the predominant ketone during acidosis. Moreover, urine acetoacetate – measured by the strips – paradoxically increases during resolution of DKA, and drug interferences can occur with urine ketone measurement, Dr. Misra noted.
Metabolic acidosis is now defined as a pH < 7.3 and/or a bicarbonate concentration < 18 mmol/L, up from 15 in some prior guidelines including the United Kingdom’s. Also, anion gap has been removed from the main definition but, the document will say, can still be used in settings where ketone testing is unavailable.
As previously, the new statement will classify DKA by mild, moderate, and severe but now for the first time there are recommendations of care for each of those levels, as well as for HHS.
For HHS, the glucose cutoff of ≥ 600 mg/dL will stay the same. But now, the effective serum osmolality has been lowered from > 320 to > 300 mOsml/L to account for the effect of dehydration, along with an alternative criteria of total serum osmolality > 320 mOsm/L. The same two changes as with DKA for both ketones and acidosis have also been included for HHS.
Asked to comment, session audience member and independent diabetes industry consultant Charles Alexander, MD, told this news organization, “I liked the proposal to eliminate the anion gap in decision-making and to focus on measurement of blood ketones, principally beta-hydroxybutyrate, in the diagnosis of DKA and monitoring the effect of treatment.
“If someone is on an SGLT2 inhibitor, there is no need to look at blood glucose levels, which may be normal or near normal in the setting of DKA.”
But Dr. Alexander thinks that they should have eliminated glucose levels entirely as part of the DKA/HHS definition even for people without diabetes.
“The problem is that medical education for many years has taught us that DKA is a condition of high blood glucose, but it may not be. It is good that they said blood glucose levels were not important if the patient had a history of diabetes. However, a glucose of 200mg/dL may not be low enough if someone is on an SGLT2 inhibitor. There needs to be a much lower threshold for measuring blood ketones in anyone with nausea, vomiting, and abdominal pain, regardless of the blood glucose level.”
Acute management: IV fluids, insulin, and potassium
Like the 2009 statement, the new one will include detailed management flowcharts for DKA and HHS, but this time in color. This new statement includes individual algorithms for management with intravenous fluids, insulin, and potassium. Bicarbonate has been removed and relegated to a note at the bottom saying that it should only be considered if pH is < 7.0.
Under fluid treatment, the new statement offers more information about using crystalloids to treat dehydration and a recommendation to add dextrose to IV fluid therapy as a substrate when the glucose drops below 250 mg/dL, in order to prevent hypoglycemia. For euglycemic DKA, the recommendation is to include dextrose and normal saline simultaneously.
And for the first time, subcutaneous rather than IV insulin is considered acceptable for mild, but not moderate or severe, DKA.
Two options are suggested for IV insulin in HHS: The fluid can be given first and low-dose fixed-rate insulin infusion added, or fluids and insulin can be given at the same time.
Criteria for resolution of DKA are a venous pH of ≥ 7.3 or bicarbonate > 18 mmol/L, ketones < 0.6 mmol/L, and glucose ideally < 200 mg/dL (11.0 mmol/L). For HHS, resolution is suggested when the measured or calculated serum osmolality falls to < 300 mosm/kg, blood glucose is < 250mg/dL (13.9 mmol/L), urine output > 0.5 mL/kg/hour, and cognitive status is improved.
The statement also will provide detailed recommended options for transitioning from IV to subcutaneous insulin, but defers to clinical judgment for deciding when the patient can be discharged. The initiation or continuation of SGLT2 inhibitors is not recommended at any time during hospitalization for hyperglycemic crises.
Mitigating complications, preventing recurrence
In addition to listing potential complications of treating hyperglycemic crises, just as the 2009 statement did, the new one will offer mitigation strategies for some of the more common ones. For preventing hypoglycemia, frequent blood glucose monitoring is advised along with adding dextrose to the IV fluids when glucose drops below 250 mg/dL.
For prevention of hypokalemia, which occurs in about half of patients treated for DKA and HHS, the statement recommends potassium monitoring every 4 hours and replacement added to fluids.
Acute kidney injury, also occurring in about half of people treated for DKA and/or HHS, usually resolves with hydration. Daily renal function monitoring is advised.
Preventing recurrence: Many factors beyond clinical
Prevention of recurrence with readmission for DKA and/or HHS, occurring in up to 22% of U.S. patients within 30 days, entails close follow-up within 2-4 weeks after discharge (including via telemedicine), and assessment of possible causes, including mental health disorders and social determinants of health.
Appropriate education should be provided, including “structured education” involving problem-solving, sick day rules, injection techniques, a review of insulin doses, consideration of continuous glucose monitoring (CGM), and home ketone testing.
Patients should be provided with an adequate supply of insulin and durable diabetes equipment, along with contact information for health care professionals who can assist them. Social service professionals can be helpful for patients who lack reliable access.
Dr. Gabbay told this news organization, “The eye-opening thing is we tend to typically think of DKA as how people tend to get diagnosed with diabetes and, yes, that’s true, but that’s only a minority of people. Those might be preventable by early screening, but all these other people and the number of recurrent episodes, that’s an area where it’s really a failure of the system where we can do better in ensuring that doesn’t happen.”
Education is only part of it, he stressed. “It’s not just an intelligence thing. It’s social factors, and there can be complex psychological issues and mental health issues. We need to screen for those things when we see someone coming back the second, third, fifth, or sixth time. We’ve all seen that. Just educating them to take their insulin is not the answer. …You’ve got to ask the questions and engage them to go a little deeper.”
Dr. Gabbay is an employee of the ADA. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum. Dr. Misra has received speaker fees from Sanofi and ABCD and an investigator-initiated research grant from Dexcom, and is a trustee for the Diabetes Research and Wellness Foundation in the United Kingdom.
A version of this article appeared on Medscape.com.
AT EASD 2023
Take a closer look at sleep’s role in GERD
This transcript has been edited for clarity.
The ongoing longitudinal Nurses’ Health Study has served as an incredible database for evaluating disease states prospectively over decades, thanks to the robust input of its participants. Most recently, this allowed for an important analysis of the association between gastroesophageal reflux (GER) symptoms and sleep quality, the results of which were published in JAMA Network Open.
Approximately 49,000 women with a median age of 59 years (range, 48-69 years) provided data for this analysis. Starting in 2005, they were asked about their experience of GER symptoms. In 2017, they were also asked to respond to a questionnaire, a modified Pittsburgh Sleep Quality Index (PSQI). This is a tool we’ve used a lot in prospective studies looking at gastrointestinal diseases and sleep-related abnormalities. It’s unique in that it looks not only at sleep but also at next-day function and daytime sleepiness, which is important here for its implications related to reflux disease and sleep fragmentation.
For those with GER symptoms occurring once a week and more than once a week, the approximate relative risk increased by 30% and 53%, respectively. Clearly, the association of GER symptoms and relative sleep quality was really important.
It should be noted that the PSQI is a disease-independent, validated instrument. It’s not specific to GER disease or any diseases. It’s cross validated across 17 different languages. I think what’s most important about its use in the assessment here is the incorporation of next-day function and asking participants about daytime sleepiness, which we’ll discuss in more detail shortly.
The many causes of interrupted sleep
We’ve all experienced sleep fragmentation, whether in the form of having been on call during our medical training or common experiences like hearing a child cry in the night, a noisy truck pass by, or a dog barking. You may or may not remember that these happened the next day, but they’ve nonetheless interrupted your sleep efficiency.
When you transition laterally across the stages of sleep, that’s what establishes the circadian rhythm and ensures sleep hygiene. Typically, we require approximately 7 hours of restful sleep to do that. But if you fragment or interrupt this process, you more or less move your way erratically through the night, disrupting sleep hygiene and efficiency.
If you have a cognitive awakening during those disruptions, you may recall those events the next day. Or, you may not remember it at all, and such amnestic events are normal for some people with sleep disruptions.
You may also have a sensory arousal, whether it’s due to GER symptoms, auditory stimuli, bumping your toe, or whatever disruptive event. Any of these can cause you to lose that laterality of smooth transition through sleep.
Approximately 20% of the U.S. population have reported GER symptoms at least once a week. Incident data indicate that number may be increasing by as much as 5% a year. Much of that increase is tied to obesity. But nonetheless, it’s a problem on the rise.
It’s important to know this as we start to look at sleep. If GER is acting as a trigger to sleep disruption, you need to ask your patients with this condition about next-day function.
In particular, the next-day function questions to ask are, “How do you feel when you get up? Are you awake and refreshed? Do you have early fatigue? Do you drag yourself out of bed, have daytime somnolence, loss of concentration, or irritability?”
Those are key parameters we can use for looking back to the night before and gauging sleep efficiency. If you’re not asking those questions, you may miss out on identifying a patient having sleep fragmentation.
Sleep’s role in inflammatory disease processes
I now perform an interval assessment of this type not just in my patients with GER disease but across all my patients. I do so because sleep is physiologically important in so many ways.
In patients who have nonalcoholic fatty liver disease and a variety of other liver diseases, we’re finding an increased association with sleep fragmentation outside of sleep apnea.
The same is true with irritable bowel and other functional diseases.
When you have sleep fragmentation in inflammatory bowel disease, you turn on a variety of inflammatory proteins (e.g., C-reactive protein) and cytokines, such as interleukins and tumor necrosis factor alpha. These processes may actually tip somebody over to a pro-inflammatory state.
When it comes to what might be considered a relatively simpler condition like GER disease, Ronnie Fass and colleagues showed a number of years ago via Bernstein testing performed in patients with both fragmented and normal sleep that the sensory thresholds all get lowered in the former group. This is irrespective of whether you have a functional symptom or you’re awakened by bumping your toe, a headache, or having heartburn; your sensory thresholds are lower. As a result, the same stimulus provides a higher sense of awareness. By ramping up that awareness, you increase the interference with the next-day function.
We’ve shown that sleep fragmentation affects a variety of things, including immune function. This may be why many people get sick when they travel in between time zones.
There are also implications relating to things like obesity. When you have sleep dysfunction, you have effects on leptin and ghrelin, contrary to what you would normally want to have. This, in turn, causes adverse effects on stimulation or suppression of satiety or appetite. These are things that I counsel my patients about when I talk about reflux as well as those trying to lose weight.
Sleep disruption affects cortisol stimulation and has a significant correlation with type 2 diabetes, cardiovascular diseases, and even mortality statistics.
Advice for counseling patients
This latest analysis from the Nurses’ Health Study reminds us that a lot of people have reflux and a lot of people have sleep fragmentation. We need to do better in asking our patients if they have symptoms specific not only to reflux but also to potentially sleep-related complications.
The more we do that, the more we individualize patient treatment rather than treating them as a disease state. This, in turn, will allow us to practice personalized medicine. The more we can engage our patients with reflux disease by asking the right questions about next-day function, the better we can do in improving their outcomes.
It’s time for us all to open our eyes to the value of closing them. Let’s talk to our patients with reflux disease in a little bit of a different light, providing a new perspective on strategies we can use to mitigate and deal with those symptoms, thereby preventing the consequences of sleep fragmentation.
Hopefully, this overview gives you some guidance the next time you have a conversation with your patients. It will apply across many, many disease states, and in almost everything we do in gastroenterology.
David A. Johnson, MD, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, Va., and a past president of the American College of Gastroenterology. He reported advising with ISOTHRIVE and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
The ongoing longitudinal Nurses’ Health Study has served as an incredible database for evaluating disease states prospectively over decades, thanks to the robust input of its participants. Most recently, this allowed for an important analysis of the association between gastroesophageal reflux (GER) symptoms and sleep quality, the results of which were published in JAMA Network Open.
Approximately 49,000 women with a median age of 59 years (range, 48-69 years) provided data for this analysis. Starting in 2005, they were asked about their experience of GER symptoms. In 2017, they were also asked to respond to a questionnaire, a modified Pittsburgh Sleep Quality Index (PSQI). This is a tool we’ve used a lot in prospective studies looking at gastrointestinal diseases and sleep-related abnormalities. It’s unique in that it looks not only at sleep but also at next-day function and daytime sleepiness, which is important here for its implications related to reflux disease and sleep fragmentation.
For those with GER symptoms occurring once a week and more than once a week, the approximate relative risk increased by 30% and 53%, respectively. Clearly, the association of GER symptoms and relative sleep quality was really important.
It should be noted that the PSQI is a disease-independent, validated instrument. It’s not specific to GER disease or any diseases. It’s cross validated across 17 different languages. I think what’s most important about its use in the assessment here is the incorporation of next-day function and asking participants about daytime sleepiness, which we’ll discuss in more detail shortly.
The many causes of interrupted sleep
We’ve all experienced sleep fragmentation, whether in the form of having been on call during our medical training or common experiences like hearing a child cry in the night, a noisy truck pass by, or a dog barking. You may or may not remember that these happened the next day, but they’ve nonetheless interrupted your sleep efficiency.
When you transition laterally across the stages of sleep, that’s what establishes the circadian rhythm and ensures sleep hygiene. Typically, we require approximately 7 hours of restful sleep to do that. But if you fragment or interrupt this process, you more or less move your way erratically through the night, disrupting sleep hygiene and efficiency.
If you have a cognitive awakening during those disruptions, you may recall those events the next day. Or, you may not remember it at all, and such amnestic events are normal for some people with sleep disruptions.
You may also have a sensory arousal, whether it’s due to GER symptoms, auditory stimuli, bumping your toe, or whatever disruptive event. Any of these can cause you to lose that laterality of smooth transition through sleep.
Approximately 20% of the U.S. population have reported GER symptoms at least once a week. Incident data indicate that number may be increasing by as much as 5% a year. Much of that increase is tied to obesity. But nonetheless, it’s a problem on the rise.
It’s important to know this as we start to look at sleep. If GER is acting as a trigger to sleep disruption, you need to ask your patients with this condition about next-day function.
In particular, the next-day function questions to ask are, “How do you feel when you get up? Are you awake and refreshed? Do you have early fatigue? Do you drag yourself out of bed, have daytime somnolence, loss of concentration, or irritability?”
Those are key parameters we can use for looking back to the night before and gauging sleep efficiency. If you’re not asking those questions, you may miss out on identifying a patient having sleep fragmentation.
Sleep’s role in inflammatory disease processes
I now perform an interval assessment of this type not just in my patients with GER disease but across all my patients. I do so because sleep is physiologically important in so many ways.
In patients who have nonalcoholic fatty liver disease and a variety of other liver diseases, we’re finding an increased association with sleep fragmentation outside of sleep apnea.
The same is true with irritable bowel and other functional diseases.
When you have sleep fragmentation in inflammatory bowel disease, you turn on a variety of inflammatory proteins (e.g., C-reactive protein) and cytokines, such as interleukins and tumor necrosis factor alpha. These processes may actually tip somebody over to a pro-inflammatory state.
When it comes to what might be considered a relatively simpler condition like GER disease, Ronnie Fass and colleagues showed a number of years ago via Bernstein testing performed in patients with both fragmented and normal sleep that the sensory thresholds all get lowered in the former group. This is irrespective of whether you have a functional symptom or you’re awakened by bumping your toe, a headache, or having heartburn; your sensory thresholds are lower. As a result, the same stimulus provides a higher sense of awareness. By ramping up that awareness, you increase the interference with the next-day function.
We’ve shown that sleep fragmentation affects a variety of things, including immune function. This may be why many people get sick when they travel in between time zones.
There are also implications relating to things like obesity. When you have sleep dysfunction, you have effects on leptin and ghrelin, contrary to what you would normally want to have. This, in turn, causes adverse effects on stimulation or suppression of satiety or appetite. These are things that I counsel my patients about when I talk about reflux as well as those trying to lose weight.
Sleep disruption affects cortisol stimulation and has a significant correlation with type 2 diabetes, cardiovascular diseases, and even mortality statistics.
Advice for counseling patients
This latest analysis from the Nurses’ Health Study reminds us that a lot of people have reflux and a lot of people have sleep fragmentation. We need to do better in asking our patients if they have symptoms specific not only to reflux but also to potentially sleep-related complications.
The more we do that, the more we individualize patient treatment rather than treating them as a disease state. This, in turn, will allow us to practice personalized medicine. The more we can engage our patients with reflux disease by asking the right questions about next-day function, the better we can do in improving their outcomes.
It’s time for us all to open our eyes to the value of closing them. Let’s talk to our patients with reflux disease in a little bit of a different light, providing a new perspective on strategies we can use to mitigate and deal with those symptoms, thereby preventing the consequences of sleep fragmentation.
Hopefully, this overview gives you some guidance the next time you have a conversation with your patients. It will apply across many, many disease states, and in almost everything we do in gastroenterology.
David A. Johnson, MD, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, Va., and a past president of the American College of Gastroenterology. He reported advising with ISOTHRIVE and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
The ongoing longitudinal Nurses’ Health Study has served as an incredible database for evaluating disease states prospectively over decades, thanks to the robust input of its participants. Most recently, this allowed for an important analysis of the association between gastroesophageal reflux (GER) symptoms and sleep quality, the results of which were published in JAMA Network Open.
Approximately 49,000 women with a median age of 59 years (range, 48-69 years) provided data for this analysis. Starting in 2005, they were asked about their experience of GER symptoms. In 2017, they were also asked to respond to a questionnaire, a modified Pittsburgh Sleep Quality Index (PSQI). This is a tool we’ve used a lot in prospective studies looking at gastrointestinal diseases and sleep-related abnormalities. It’s unique in that it looks not only at sleep but also at next-day function and daytime sleepiness, which is important here for its implications related to reflux disease and sleep fragmentation.
For those with GER symptoms occurring once a week and more than once a week, the approximate relative risk increased by 30% and 53%, respectively. Clearly, the association of GER symptoms and relative sleep quality was really important.
It should be noted that the PSQI is a disease-independent, validated instrument. It’s not specific to GER disease or any diseases. It’s cross validated across 17 different languages. I think what’s most important about its use in the assessment here is the incorporation of next-day function and asking participants about daytime sleepiness, which we’ll discuss in more detail shortly.
The many causes of interrupted sleep
We’ve all experienced sleep fragmentation, whether in the form of having been on call during our medical training or common experiences like hearing a child cry in the night, a noisy truck pass by, or a dog barking. You may or may not remember that these happened the next day, but they’ve nonetheless interrupted your sleep efficiency.
When you transition laterally across the stages of sleep, that’s what establishes the circadian rhythm and ensures sleep hygiene. Typically, we require approximately 7 hours of restful sleep to do that. But if you fragment or interrupt this process, you more or less move your way erratically through the night, disrupting sleep hygiene and efficiency.
If you have a cognitive awakening during those disruptions, you may recall those events the next day. Or, you may not remember it at all, and such amnestic events are normal for some people with sleep disruptions.
You may also have a sensory arousal, whether it’s due to GER symptoms, auditory stimuli, bumping your toe, or whatever disruptive event. Any of these can cause you to lose that laterality of smooth transition through sleep.
Approximately 20% of the U.S. population have reported GER symptoms at least once a week. Incident data indicate that number may be increasing by as much as 5% a year. Much of that increase is tied to obesity. But nonetheless, it’s a problem on the rise.
It’s important to know this as we start to look at sleep. If GER is acting as a trigger to sleep disruption, you need to ask your patients with this condition about next-day function.
In particular, the next-day function questions to ask are, “How do you feel when you get up? Are you awake and refreshed? Do you have early fatigue? Do you drag yourself out of bed, have daytime somnolence, loss of concentration, or irritability?”
Those are key parameters we can use for looking back to the night before and gauging sleep efficiency. If you’re not asking those questions, you may miss out on identifying a patient having sleep fragmentation.
Sleep’s role in inflammatory disease processes
I now perform an interval assessment of this type not just in my patients with GER disease but across all my patients. I do so because sleep is physiologically important in so many ways.
In patients who have nonalcoholic fatty liver disease and a variety of other liver diseases, we’re finding an increased association with sleep fragmentation outside of sleep apnea.
The same is true with irritable bowel and other functional diseases.
When you have sleep fragmentation in inflammatory bowel disease, you turn on a variety of inflammatory proteins (e.g., C-reactive protein) and cytokines, such as interleukins and tumor necrosis factor alpha. These processes may actually tip somebody over to a pro-inflammatory state.
When it comes to what might be considered a relatively simpler condition like GER disease, Ronnie Fass and colleagues showed a number of years ago via Bernstein testing performed in patients with both fragmented and normal sleep that the sensory thresholds all get lowered in the former group. This is irrespective of whether you have a functional symptom or you’re awakened by bumping your toe, a headache, or having heartburn; your sensory thresholds are lower. As a result, the same stimulus provides a higher sense of awareness. By ramping up that awareness, you increase the interference with the next-day function.
We’ve shown that sleep fragmentation affects a variety of things, including immune function. This may be why many people get sick when they travel in between time zones.
There are also implications relating to things like obesity. When you have sleep dysfunction, you have effects on leptin and ghrelin, contrary to what you would normally want to have. This, in turn, causes adverse effects on stimulation or suppression of satiety or appetite. These are things that I counsel my patients about when I talk about reflux as well as those trying to lose weight.
Sleep disruption affects cortisol stimulation and has a significant correlation with type 2 diabetes, cardiovascular diseases, and even mortality statistics.
Advice for counseling patients
This latest analysis from the Nurses’ Health Study reminds us that a lot of people have reflux and a lot of people have sleep fragmentation. We need to do better in asking our patients if they have symptoms specific not only to reflux but also to potentially sleep-related complications.
The more we do that, the more we individualize patient treatment rather than treating them as a disease state. This, in turn, will allow us to practice personalized medicine. The more we can engage our patients with reflux disease by asking the right questions about next-day function, the better we can do in improving their outcomes.
It’s time for us all to open our eyes to the value of closing them. Let’s talk to our patients with reflux disease in a little bit of a different light, providing a new perspective on strategies we can use to mitigate and deal with those symptoms, thereby preventing the consequences of sleep fragmentation.
Hopefully, this overview gives you some guidance the next time you have a conversation with your patients. It will apply across many, many disease states, and in almost everything we do in gastroenterology.
David A. Johnson, MD, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, Va., and a past president of the American College of Gastroenterology. He reported advising with ISOTHRIVE and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
Paxlovid tied to benefits in high-risk patients with COVID
In a cohort study from British Columbia that included nearly 7,000 patients with COVID-19, nirmatrelvir-ritonavir was associated with a 2.5% reduction in risk for death or emergency hospitalization in clinically extremely vulnerable (CEV) patients who were severely immunocompromised. No significant benefit was observed in patients who were not immunocompromised.
“This finding could help substantially limit unnecessary use of nirmatrelvir and ritonavir in older, otherwise healthy individuals,” lead author Colin R. Dormuth, ScD, associate professor of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, told this news organization. “Another finding that was surprising and might help place the role of nirmatrelvir and ritonavir in context is that even in severely immunocompromised individuals who did not take [the drug], the risk of death or hospitalization with COVID-19 was less than 4% in our study population.”
The study was published online in JAMA Network Open.
Who benefits?
The investigators analyzed medical records for 6,866 patients in British Columbia (median age, 70 years; 57% women) who presented between Feb. 1, 2022, and Feb. 3, 2023. Eligible patients belonged to one of four higher-risk groups who received priority for COVID-19 vaccination.
Two groups included CEV patients who were severely (CEV1) or moderately (CEV2) immunocompromised. The CEV3 group was not immunocompromised but had medical conditions associated with a high risk for complications from COVID-19. A fourth expanded eligibility (EXEL) group included higher-risk patients who were not in one of the other groups, such as unvaccinated patients older than age 70 years.
The investigators matched treated patients to untreated patients in the same vulnerability group according to age, sex, and month of infection. The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Treatment with nirmatrelvir-ritonavir was associated with statistically significant relative reductions in the primary outcome, compared with no treatment, for patients in the CEV1 (risk difference, −2.5%) and CEV2 (RD, −1.7%) groups. In the CEV3 group, the RD of −1.3% was not statistically significant. In the EXEL group, treatment was associated with a higher risk for the primary outcome (RD, 1.0%), but the result was not statistically significant.
The results were “robust across sex and older vs. younger age,” the authors note. “No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.”
The combination of nirmatrelvir-ritonavir was approved for use in Canada based on interim efficacy and safety data from the Evaluation of Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, said Dr. Dormuth.
British Columbia’s eligibility criteria for nirmatrelvir-ritonavir coverage differ substantially from the criteria for participants in the EPIC-HR trial, he noted. Those patients were unvaccinated, had no natural immunity from a previous COVID-19 infection, and were infected with COVID-19 variants that were different from those now circulating. The current study was prompted by the need to look at a broader population of individuals in British Columbia with varying risks of complications from COVID-19 infection.
Before the study, a common view was that patients aged 70 and older would benefit from the drug, said Dr. Dormuth. “Our study, which accounted for medical conditions related to an individual’s vulnerability to complications, showed that older age on its own was not a reason to use nirmatrelvir and ritonavir once relevant medical conditions were taken into consideration.”
The researchers are working on a study to identify with greater specificity which comorbid conditions are most associated with nirmatrelvir-ritonavir effectiveness, he added. “It could be that a relatively small number of conditions can be used to identify most individuals who would benefit from the drug.”
‘Signal toward benefit’
Commenting on the findings for this news organization, Abhijit Duggal, MD, vice chair of critical care at the Cleveland Clinic, who was not involved in this study, said, “I’m always very wary when we look at observational data and we start saying the effectiveness is not really as high as was seen in other studies. We are seeing an effect with all these studies that seems to be in the right direction.
“Having said that,” he added, “is the effect going to be potentially more in patients at higher risk? Absolutely. I think these postmarket studies are really showing that after vaccination, if someone does get infected, this is a secondary option available to us that can prevent progression of the disease, which would likely be more severe in immunocompromised patients.”
Dr. Duggal was a coinvestigator on a recent study of more than 68,000 patients that showed that nirmatrelvir-ritonavir or molnupiravir was associated with reductions in mortality and hospitalization in nonhospitalized patients infected with the Omicron variant, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions.
“In all groups, there was a signal toward benefit,” said Dr. Duggal. “These studies tell us that these drugs do remain valid options. But their use needs to be discussed on a case-by-case basis with patients we feel are deteriorating or at a higher risk because of underlying disease processes.”
The study was supported by funding from the British Columbia Ministry of Health. Dr. Dormuth and Dr. Duggal report no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a cohort study from British Columbia that included nearly 7,000 patients with COVID-19, nirmatrelvir-ritonavir was associated with a 2.5% reduction in risk for death or emergency hospitalization in clinically extremely vulnerable (CEV) patients who were severely immunocompromised. No significant benefit was observed in patients who were not immunocompromised.
“This finding could help substantially limit unnecessary use of nirmatrelvir and ritonavir in older, otherwise healthy individuals,” lead author Colin R. Dormuth, ScD, associate professor of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, told this news organization. “Another finding that was surprising and might help place the role of nirmatrelvir and ritonavir in context is that even in severely immunocompromised individuals who did not take [the drug], the risk of death or hospitalization with COVID-19 was less than 4% in our study population.”
The study was published online in JAMA Network Open.
Who benefits?
The investigators analyzed medical records for 6,866 patients in British Columbia (median age, 70 years; 57% women) who presented between Feb. 1, 2022, and Feb. 3, 2023. Eligible patients belonged to one of four higher-risk groups who received priority for COVID-19 vaccination.
Two groups included CEV patients who were severely (CEV1) or moderately (CEV2) immunocompromised. The CEV3 group was not immunocompromised but had medical conditions associated with a high risk for complications from COVID-19. A fourth expanded eligibility (EXEL) group included higher-risk patients who were not in one of the other groups, such as unvaccinated patients older than age 70 years.
The investigators matched treated patients to untreated patients in the same vulnerability group according to age, sex, and month of infection. The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Treatment with nirmatrelvir-ritonavir was associated with statistically significant relative reductions in the primary outcome, compared with no treatment, for patients in the CEV1 (risk difference, −2.5%) and CEV2 (RD, −1.7%) groups. In the CEV3 group, the RD of −1.3% was not statistically significant. In the EXEL group, treatment was associated with a higher risk for the primary outcome (RD, 1.0%), but the result was not statistically significant.
The results were “robust across sex and older vs. younger age,” the authors note. “No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.”
The combination of nirmatrelvir-ritonavir was approved for use in Canada based on interim efficacy and safety data from the Evaluation of Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, said Dr. Dormuth.
British Columbia’s eligibility criteria for nirmatrelvir-ritonavir coverage differ substantially from the criteria for participants in the EPIC-HR trial, he noted. Those patients were unvaccinated, had no natural immunity from a previous COVID-19 infection, and were infected with COVID-19 variants that were different from those now circulating. The current study was prompted by the need to look at a broader population of individuals in British Columbia with varying risks of complications from COVID-19 infection.
Before the study, a common view was that patients aged 70 and older would benefit from the drug, said Dr. Dormuth. “Our study, which accounted for medical conditions related to an individual’s vulnerability to complications, showed that older age on its own was not a reason to use nirmatrelvir and ritonavir once relevant medical conditions were taken into consideration.”
The researchers are working on a study to identify with greater specificity which comorbid conditions are most associated with nirmatrelvir-ritonavir effectiveness, he added. “It could be that a relatively small number of conditions can be used to identify most individuals who would benefit from the drug.”
‘Signal toward benefit’
Commenting on the findings for this news organization, Abhijit Duggal, MD, vice chair of critical care at the Cleveland Clinic, who was not involved in this study, said, “I’m always very wary when we look at observational data and we start saying the effectiveness is not really as high as was seen in other studies. We are seeing an effect with all these studies that seems to be in the right direction.
“Having said that,” he added, “is the effect going to be potentially more in patients at higher risk? Absolutely. I think these postmarket studies are really showing that after vaccination, if someone does get infected, this is a secondary option available to us that can prevent progression of the disease, which would likely be more severe in immunocompromised patients.”
Dr. Duggal was a coinvestigator on a recent study of more than 68,000 patients that showed that nirmatrelvir-ritonavir or molnupiravir was associated with reductions in mortality and hospitalization in nonhospitalized patients infected with the Omicron variant, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions.
“In all groups, there was a signal toward benefit,” said Dr. Duggal. “These studies tell us that these drugs do remain valid options. But their use needs to be discussed on a case-by-case basis with patients we feel are deteriorating or at a higher risk because of underlying disease processes.”
The study was supported by funding from the British Columbia Ministry of Health. Dr. Dormuth and Dr. Duggal report no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a cohort study from British Columbia that included nearly 7,000 patients with COVID-19, nirmatrelvir-ritonavir was associated with a 2.5% reduction in risk for death or emergency hospitalization in clinically extremely vulnerable (CEV) patients who were severely immunocompromised. No significant benefit was observed in patients who were not immunocompromised.
“This finding could help substantially limit unnecessary use of nirmatrelvir and ritonavir in older, otherwise healthy individuals,” lead author Colin R. Dormuth, ScD, associate professor of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, told this news organization. “Another finding that was surprising and might help place the role of nirmatrelvir and ritonavir in context is that even in severely immunocompromised individuals who did not take [the drug], the risk of death or hospitalization with COVID-19 was less than 4% in our study population.”
The study was published online in JAMA Network Open.
Who benefits?
The investigators analyzed medical records for 6,866 patients in British Columbia (median age, 70 years; 57% women) who presented between Feb. 1, 2022, and Feb. 3, 2023. Eligible patients belonged to one of four higher-risk groups who received priority for COVID-19 vaccination.
Two groups included CEV patients who were severely (CEV1) or moderately (CEV2) immunocompromised. The CEV3 group was not immunocompromised but had medical conditions associated with a high risk for complications from COVID-19. A fourth expanded eligibility (EXEL) group included higher-risk patients who were not in one of the other groups, such as unvaccinated patients older than age 70 years.
The investigators matched treated patients to untreated patients in the same vulnerability group according to age, sex, and month of infection. The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Treatment with nirmatrelvir-ritonavir was associated with statistically significant relative reductions in the primary outcome, compared with no treatment, for patients in the CEV1 (risk difference, −2.5%) and CEV2 (RD, −1.7%) groups. In the CEV3 group, the RD of −1.3% was not statistically significant. In the EXEL group, treatment was associated with a higher risk for the primary outcome (RD, 1.0%), but the result was not statistically significant.
The results were “robust across sex and older vs. younger age,” the authors note. “No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.”
The combination of nirmatrelvir-ritonavir was approved for use in Canada based on interim efficacy and safety data from the Evaluation of Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, said Dr. Dormuth.
British Columbia’s eligibility criteria for nirmatrelvir-ritonavir coverage differ substantially from the criteria for participants in the EPIC-HR trial, he noted. Those patients were unvaccinated, had no natural immunity from a previous COVID-19 infection, and were infected with COVID-19 variants that were different from those now circulating. The current study was prompted by the need to look at a broader population of individuals in British Columbia with varying risks of complications from COVID-19 infection.
Before the study, a common view was that patients aged 70 and older would benefit from the drug, said Dr. Dormuth. “Our study, which accounted for medical conditions related to an individual’s vulnerability to complications, showed that older age on its own was not a reason to use nirmatrelvir and ritonavir once relevant medical conditions were taken into consideration.”
The researchers are working on a study to identify with greater specificity which comorbid conditions are most associated with nirmatrelvir-ritonavir effectiveness, he added. “It could be that a relatively small number of conditions can be used to identify most individuals who would benefit from the drug.”
‘Signal toward benefit’
Commenting on the findings for this news organization, Abhijit Duggal, MD, vice chair of critical care at the Cleveland Clinic, who was not involved in this study, said, “I’m always very wary when we look at observational data and we start saying the effectiveness is not really as high as was seen in other studies. We are seeing an effect with all these studies that seems to be in the right direction.
“Having said that,” he added, “is the effect going to be potentially more in patients at higher risk? Absolutely. I think these postmarket studies are really showing that after vaccination, if someone does get infected, this is a secondary option available to us that can prevent progression of the disease, which would likely be more severe in immunocompromised patients.”
Dr. Duggal was a coinvestigator on a recent study of more than 68,000 patients that showed that nirmatrelvir-ritonavir or molnupiravir was associated with reductions in mortality and hospitalization in nonhospitalized patients infected with the Omicron variant, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions.
“In all groups, there was a signal toward benefit,” said Dr. Duggal. “These studies tell us that these drugs do remain valid options. But their use needs to be discussed on a case-by-case basis with patients we feel are deteriorating or at a higher risk because of underlying disease processes.”
The study was supported by funding from the British Columbia Ministry of Health. Dr. Dormuth and Dr. Duggal report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Enlarging lesion on temple
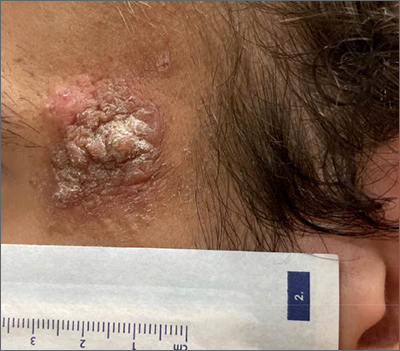
A shave biopsy revealed acanthosis, papillomatosis, hyperkeratosis, hypergranulosis, parakeratosis, and cytoplasmic viral-like inclusions without atypia, consistent with a diagnosis of a common wart. The biopsy ruled out other possible diagnoses, which included keratoacanthoma, seborrheic keratosis, and squamous cell carcinoma.
Cutaneous warts can manifest as common warts (verruca vulgaris), plantar warts (verruca plantaris), or plane warts (verruca plana). These benign skin lesions are caused by human papillomavirus and can manifest in areas of skin trauma; this is known as the Koebner phenomenon. Most warts can be diagnosed through clinical history and examination. Dermoscopy, if performed, may reveal thrombosed capillaries as dotted structures, but there is an increased risk of cross-contamination.1 That said, some dermatoscopes have disposable covers or can be cleaned with antiviral, antibacterial wipes. If the diagnosis is unclear or the exam is clinically suspicious, a biopsy may be required.
Cases with progressive enlargement and extensive involvement of the skin (as was seen here) are generally associated with certain predisposing conditions, such as atopic dermatitis and immunosuppression.2 Our patient screened negative for HIV infection, and further evaluation did not reveal any concerns for immunosuppression.
Treatment for a common wart depends on patient characteristics, preferences, cost, and possible adverse effects. Standard treatment options are topical salicylic acid and cryotherapy with liquid nitrogen. Depending on the location and type of the wart, multiple treatments may be required, and recurrences are common. Intralesional injection with bleomycin, 5‐fluorouracil, or cidofovir is often used for recurrent and refractory warts.
Patients unable to tolerate cryotherapy or local injections may benefit from thermotherapy by heating the wart with a pulsed dye laser.3 Observation is also a reasonable course of action for new warts, as they may spontaneously resolve within a year.
In this case, the patient opted for over-the-counter salicylic acid 17% to be applied nightly until resolution. Cryosurgery would be a next step for him if the lesion does not resolve after 3 months of treatment.
Image courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Mun JH, Park SM, Ko HC, et al. Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion. Dermatol Pract Concept. 2013;3:33-34. doi: 10.5826/dpc.0304a07
2. Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030-1048. doi: 10.1016/j.jaci.2012.07.049
3. Zhu P, Qi RQ, Yang Y, et al. Clinical guideline for the diagnosis and treatment of cutaneous warts (2022). J Evid Based Med. 2022;15:284-301. doi: 10.1111/jebm.12494

A shave biopsy revealed acanthosis, papillomatosis, hyperkeratosis, hypergranulosis, parakeratosis, and cytoplasmic viral-like inclusions without atypia, consistent with a diagnosis of a common wart. The biopsy ruled out other possible diagnoses, which included keratoacanthoma, seborrheic keratosis, and squamous cell carcinoma.
Cutaneous warts can manifest as common warts (verruca vulgaris), plantar warts (verruca plantaris), or plane warts (verruca plana). These benign skin lesions are caused by human papillomavirus and can manifest in areas of skin trauma; this is known as the Koebner phenomenon. Most warts can be diagnosed through clinical history and examination. Dermoscopy, if performed, may reveal thrombosed capillaries as dotted structures, but there is an increased risk of cross-contamination.1 That said, some dermatoscopes have disposable covers or can be cleaned with antiviral, antibacterial wipes. If the diagnosis is unclear or the exam is clinically suspicious, a biopsy may be required.
Cases with progressive enlargement and extensive involvement of the skin (as was seen here) are generally associated with certain predisposing conditions, such as atopic dermatitis and immunosuppression.2 Our patient screened negative for HIV infection, and further evaluation did not reveal any concerns for immunosuppression.
Treatment for a common wart depends on patient characteristics, preferences, cost, and possible adverse effects. Standard treatment options are topical salicylic acid and cryotherapy with liquid nitrogen. Depending on the location and type of the wart, multiple treatments may be required, and recurrences are common. Intralesional injection with bleomycin, 5‐fluorouracil, or cidofovir is often used for recurrent and refractory warts.
Patients unable to tolerate cryotherapy or local injections may benefit from thermotherapy by heating the wart with a pulsed dye laser.3 Observation is also a reasonable course of action for new warts, as they may spontaneously resolve within a year.
In this case, the patient opted for over-the-counter salicylic acid 17% to be applied nightly until resolution. Cryosurgery would be a next step for him if the lesion does not resolve after 3 months of treatment.
Image courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

A shave biopsy revealed acanthosis, papillomatosis, hyperkeratosis, hypergranulosis, parakeratosis, and cytoplasmic viral-like inclusions without atypia, consistent with a diagnosis of a common wart. The biopsy ruled out other possible diagnoses, which included keratoacanthoma, seborrheic keratosis, and squamous cell carcinoma.
Cutaneous warts can manifest as common warts (verruca vulgaris), plantar warts (verruca plantaris), or plane warts (verruca plana). These benign skin lesions are caused by human papillomavirus and can manifest in areas of skin trauma; this is known as the Koebner phenomenon. Most warts can be diagnosed through clinical history and examination. Dermoscopy, if performed, may reveal thrombosed capillaries as dotted structures, but there is an increased risk of cross-contamination.1 That said, some dermatoscopes have disposable covers or can be cleaned with antiviral, antibacterial wipes. If the diagnosis is unclear or the exam is clinically suspicious, a biopsy may be required.
Cases with progressive enlargement and extensive involvement of the skin (as was seen here) are generally associated with certain predisposing conditions, such as atopic dermatitis and immunosuppression.2 Our patient screened negative for HIV infection, and further evaluation did not reveal any concerns for immunosuppression.
Treatment for a common wart depends on patient characteristics, preferences, cost, and possible adverse effects. Standard treatment options are topical salicylic acid and cryotherapy with liquid nitrogen. Depending on the location and type of the wart, multiple treatments may be required, and recurrences are common. Intralesional injection with bleomycin, 5‐fluorouracil, or cidofovir is often used for recurrent and refractory warts.
Patients unable to tolerate cryotherapy or local injections may benefit from thermotherapy by heating the wart with a pulsed dye laser.3 Observation is also a reasonable course of action for new warts, as they may spontaneously resolve within a year.
In this case, the patient opted for over-the-counter salicylic acid 17% to be applied nightly until resolution. Cryosurgery would be a next step for him if the lesion does not resolve after 3 months of treatment.
Image courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Mun JH, Park SM, Ko HC, et al. Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion. Dermatol Pract Concept. 2013;3:33-34. doi: 10.5826/dpc.0304a07
2. Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030-1048. doi: 10.1016/j.jaci.2012.07.049
3. Zhu P, Qi RQ, Yang Y, et al. Clinical guideline for the diagnosis and treatment of cutaneous warts (2022). J Evid Based Med. 2022;15:284-301. doi: 10.1111/jebm.12494
1. Mun JH, Park SM, Ko HC, et al. Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion. Dermatol Pract Concept. 2013;3:33-34. doi: 10.5826/dpc.0304a07
2. Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030-1048. doi: 10.1016/j.jaci.2012.07.049
3. Zhu P, Qi RQ, Yang Y, et al. Clinical guideline for the diagnosis and treatment of cutaneous warts (2022). J Evid Based Med. 2022;15:284-301. doi: 10.1111/jebm.12494
Don’t fear POTS: Tips for diagnosis and treatment
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here in Amsterdam at the European Society of Cardiology (ESC) Congress 2023. Joining me for a great discussion is my friend Dr. Pam Taub, who is a cardiologist and a professor of medicine at UC San Diego. She has a particular interest in postural orthostatic tachycardia syndrome (POTS), so that’s what we’ll be talking about today.
Thanks for joining me, Pam. When we think about POTS, for those who are not familiar with the term, what does it actually mean and how do you diagnose it?
No tilt table required
Pam R. Taub, MD: That’s typically associated with symptoms such as lightheadedness, dizziness, and cognitive difficulties such as brain fog. The diagnosis can be made by tilt-table testing, but it can also be made in the office with simple orthostats.
In my clinic, I have people lie down for 3-5 minutes. At the end of that period, you get a heart rate and blood pressure. Then you have them stand up for 3-5 minutes and then get heart rate and blood pressure, and you look at the differences. If the heart rate goes up by 30 points – so maybe they’re 80 beats/min when they’re lying down and when they stand up, it goes to 110 beats/min – that’s POTS, so very objective criteria. Typically, these people don’t have what we call orthostatic hypotension, where there is a significant decrease in the blood pressure. It’s more a heart rate issue.
Dr. O’Donoghue: How symptomatically do they usually present?
Dr. Taub: It’s a spectrum. Some people have mild symptoms. After they’re in the upright position for maybe 10 minutes, they get symptoms. There are some people who, when they go from a lying to standing position, they’re extremely symptomatic and can’t really do any activities. There are some people that are even wheelchair-bound because the symptoms are so debilitating. There’s a wide spectrum.
Dr. O’Donoghue: There has been more discussion, I feel like, about the rising prevalence of POTS as a diagnosis, and in particular since the COVID pandemic. What’s our understanding of the relationship between COVID and POTS and what the mechanism might be?
Dr. Taub: We’ve known that POTS can be triggered by a viral infection. Before COVID, we knew that in certain individuals that we think have an underlying genetic predisposition, usually some autoimmune substrate, when they get certain types of infections, whether it’s influenza or mononucleosis, they get POTS.
Typically, when they get an infection, they start getting deconditioned. They don’t feel well, so they’re on bed rest. When they get long periods of bed rest, when they start to become active, they start to have overactivation of their sympathetic nervous system, and they have a large amount of cardiovascular deconditioning. It’s a cycle that is often triggered after an infection.
A huge increase of POTS has been seen after COVID-19 because we had so many people exposed to this virus. With COVID-19, there is a period where people don’t feel great and they are getting bed rest, so they’re getting deconditioned. We’ve seen so many patients referred for post-COVID POTS and also long COVID or the post-acute sequelae of COVID-19, where POTS is a part of that presentation.
Female sex and autoimmune conditions
Dr. O’Donoghue: We know that POTS seems to disproportionately affect women. Is that understood? Is it thought that that’s related to the perhaps the autoimmune component of that illness?
Dr. Taub: Yes. The theory is because women tend to have more autoimmune conditions, that’s why they’re more predisposed. There’s a large amount of genetic susceptibility. For instance, we know that there’s an association between POTS and conditions like Ehlers-Danlos syndrome and between POTS and mast cell activation. Some of those conditions are more prevalent in women as well.
Dr. O’Donoghue: I feel like many physicians don’t know how to manage POTS, and they’re actually a little fearful perhaps to take it on. Fortunately, there have been a growing number of POTS clinics with specialists that focus on that area. For the average practitioner who maybe can’t refer to a POTS clinic, how should they approach that?
Dr. Taub: The first thing is its diagnosis. When someone tells you that they have symptoms of orthostatic intolerance – so, activities that involve standing – you need to first have that on your differential diagnosis. You can make the diagnosis in the office with orthostats. You don’t need a tilt table. It’s sometimes helpful if you’re unsure about the diagnosis, but you can make the diagnosis.
Many times, you’re finding people that have very mild symptoms. You can treat that with some good lifestyle recommendations, such as increased hydration, increasing salt in their diet, and compression. And the exercise component is really important.
Many people with POTS are told to go exercise, go for a run, or go for a walk. That’s incorrect, because these people have symptoms when they’re in the upright position. The type of exercise they need to do initially is exercise in the lying or seated position – so exercises like rowing or a seated bike, and strength training. As they start to feel better, then they can do upright exercise.
You should never tell a person that has POTS to just initially start with upright exercise, because they’re going to feel so much worse and then they’re never going to want to exercise. It’s really important to give them the right exercise recommendations. I find that for many of these mild cases, if they do the right exercise and engage in the right lifestyle strategies, they get better.
Compression wear and drug therapy
Dr. O’Donoghue: When it comes to compression stockings, do you usually start with a particular length?
Dr. Taub: It’s interesting. There are many different compression stockings, medical grade. Through patients with POTS, I’ve gotten feedback on certain types of athletic wear that have built-in compression, and that’s a little bit easier for people to wear every day because they can do their errands and it doesn’t look like they’re wearing medical-grade compression stockings.
Basically, I’ve collected all the different recommendations that patients say help, and I give them a list. The medical-grade compression stockings sometimes are very challenging to put on, and sometimes people just need light compression or even just socks. Any kind of compression is going to help.
Dr. O’Donoghue: That’s a great tip, because I know there are many patients who refuse to wear the compression stockings. If there’s a fashionable alternative, that’s always good to reach for.
Dr. Taub: Another thing that patients have told me is that abdominal compression is also very helpful. There are many commercially available abdominal compression options, like shapewear. Many patients with POTS use that and that helps, too.
Dr. O’Donoghue: Good. For those patients with POTS that is refractory to the measures you’ve already discussed, what are the next steps after that?
Dr. Taub: Pharmacotherapy is very synergistic with lifestyle, and there are many different pharmacotherapy options. One of the first things that you want to think about is lowering that heart rate. The reason people feel horrible is because their heart rate is usually very high when they’re upright. If they’re upright for long periods of time and they’re having very high heart rates, they’re going to get really tired because it’s like they’re exercising for hours when they’re upright.
Heart rate lowering is the cornerstone of therapy. Traditionally, we’ve used beta-blockers for heart rate lowering. The problem is they also lower blood pressure. They can also cause fatigue, so not the ideal agent for patients with POTS.
One of the clinical trials that I led was with a drug called ivabradine, which selectively works on the SA node and decreases heart rate without affecting blood pressure. What’s really elegant about ivabradine is it has a more potent effect when the heart rate is higher. When the patient is standing, it’s going to have a more potent effect on heart rate lowering. It’s really well tolerated in patients with POTS. In our study, we showed an improvement in quality of life metrics. That’s one of the first-line drugs that I use for patients with POTS.
The other thing is some of them will also have a concomitant lowering of blood pressure. You can think about medications that increase blood pressure, like midodrine, fludrocortisone, and droxidopa. Sometimes that combination of a heart rate-lowering medication and a medication that increases blood pressure really works well.
Dr. O’Donoghue: That’s very helpful. I think that those kinds of practical tips are the ones that practitioners really want to reach for, because they need to have that algorithm in their mind to take on this condition. Thanks again for walking us through that.
I think it’s a very interesting space, and there’s more that we’re going to be learning over the next few years as we further flesh out these post-COVID cases and what we learn from that as well.
Dr. Taub: There are many clinical trials now starting in POTS, so it’s exciting.
Dr. O’Donoghue: Absolutely. Thank you again for joining me today. Signing off, this is Dr Michelle O’Donoghue.
Dr. O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Dr. O’Donoghue loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. She disclosed ties with Amgen, AstraZeneca Pharmaceuticals LP, CVS Minute Clinic, Eisai, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novartis, and The Medicines Company. Dr. Taub is professor of Medicine, University of California San Diego Health, La Jolla. She disclosed ties with Amgen, Bayer, Boehringer Ingelheim, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here in Amsterdam at the European Society of Cardiology (ESC) Congress 2023. Joining me for a great discussion is my friend Dr. Pam Taub, who is a cardiologist and a professor of medicine at UC San Diego. She has a particular interest in postural orthostatic tachycardia syndrome (POTS), so that’s what we’ll be talking about today.
Thanks for joining me, Pam. When we think about POTS, for those who are not familiar with the term, what does it actually mean and how do you diagnose it?
No tilt table required
Pam R. Taub, MD: That’s typically associated with symptoms such as lightheadedness, dizziness, and cognitive difficulties such as brain fog. The diagnosis can be made by tilt-table testing, but it can also be made in the office with simple orthostats.
In my clinic, I have people lie down for 3-5 minutes. At the end of that period, you get a heart rate and blood pressure. Then you have them stand up for 3-5 minutes and then get heart rate and blood pressure, and you look at the differences. If the heart rate goes up by 30 points – so maybe they’re 80 beats/min when they’re lying down and when they stand up, it goes to 110 beats/min – that’s POTS, so very objective criteria. Typically, these people don’t have what we call orthostatic hypotension, where there is a significant decrease in the blood pressure. It’s more a heart rate issue.
Dr. O’Donoghue: How symptomatically do they usually present?
Dr. Taub: It’s a spectrum. Some people have mild symptoms. After they’re in the upright position for maybe 10 minutes, they get symptoms. There are some people who, when they go from a lying to standing position, they’re extremely symptomatic and can’t really do any activities. There are some people that are even wheelchair-bound because the symptoms are so debilitating. There’s a wide spectrum.
Dr. O’Donoghue: There has been more discussion, I feel like, about the rising prevalence of POTS as a diagnosis, and in particular since the COVID pandemic. What’s our understanding of the relationship between COVID and POTS and what the mechanism might be?
Dr. Taub: We’ve known that POTS can be triggered by a viral infection. Before COVID, we knew that in certain individuals that we think have an underlying genetic predisposition, usually some autoimmune substrate, when they get certain types of infections, whether it’s influenza or mononucleosis, they get POTS.
Typically, when they get an infection, they start getting deconditioned. They don’t feel well, so they’re on bed rest. When they get long periods of bed rest, when they start to become active, they start to have overactivation of their sympathetic nervous system, and they have a large amount of cardiovascular deconditioning. It’s a cycle that is often triggered after an infection.
A huge increase of POTS has been seen after COVID-19 because we had so many people exposed to this virus. With COVID-19, there is a period where people don’t feel great and they are getting bed rest, so they’re getting deconditioned. We’ve seen so many patients referred for post-COVID POTS and also long COVID or the post-acute sequelae of COVID-19, where POTS is a part of that presentation.
Female sex and autoimmune conditions
Dr. O’Donoghue: We know that POTS seems to disproportionately affect women. Is that understood? Is it thought that that’s related to the perhaps the autoimmune component of that illness?
Dr. Taub: Yes. The theory is because women tend to have more autoimmune conditions, that’s why they’re more predisposed. There’s a large amount of genetic susceptibility. For instance, we know that there’s an association between POTS and conditions like Ehlers-Danlos syndrome and between POTS and mast cell activation. Some of those conditions are more prevalent in women as well.
Dr. O’Donoghue: I feel like many physicians don’t know how to manage POTS, and they’re actually a little fearful perhaps to take it on. Fortunately, there have been a growing number of POTS clinics with specialists that focus on that area. For the average practitioner who maybe can’t refer to a POTS clinic, how should they approach that?
Dr. Taub: The first thing is its diagnosis. When someone tells you that they have symptoms of orthostatic intolerance – so, activities that involve standing – you need to first have that on your differential diagnosis. You can make the diagnosis in the office with orthostats. You don’t need a tilt table. It’s sometimes helpful if you’re unsure about the diagnosis, but you can make the diagnosis.
Many times, you’re finding people that have very mild symptoms. You can treat that with some good lifestyle recommendations, such as increased hydration, increasing salt in their diet, and compression. And the exercise component is really important.
Many people with POTS are told to go exercise, go for a run, or go for a walk. That’s incorrect, because these people have symptoms when they’re in the upright position. The type of exercise they need to do initially is exercise in the lying or seated position – so exercises like rowing or a seated bike, and strength training. As they start to feel better, then they can do upright exercise.
You should never tell a person that has POTS to just initially start with upright exercise, because they’re going to feel so much worse and then they’re never going to want to exercise. It’s really important to give them the right exercise recommendations. I find that for many of these mild cases, if they do the right exercise and engage in the right lifestyle strategies, they get better.
Compression wear and drug therapy
Dr. O’Donoghue: When it comes to compression stockings, do you usually start with a particular length?
Dr. Taub: It’s interesting. There are many different compression stockings, medical grade. Through patients with POTS, I’ve gotten feedback on certain types of athletic wear that have built-in compression, and that’s a little bit easier for people to wear every day because they can do their errands and it doesn’t look like they’re wearing medical-grade compression stockings.
Basically, I’ve collected all the different recommendations that patients say help, and I give them a list. The medical-grade compression stockings sometimes are very challenging to put on, and sometimes people just need light compression or even just socks. Any kind of compression is going to help.
Dr. O’Donoghue: That’s a great tip, because I know there are many patients who refuse to wear the compression stockings. If there’s a fashionable alternative, that’s always good to reach for.
Dr. Taub: Another thing that patients have told me is that abdominal compression is also very helpful. There are many commercially available abdominal compression options, like shapewear. Many patients with POTS use that and that helps, too.
Dr. O’Donoghue: Good. For those patients with POTS that is refractory to the measures you’ve already discussed, what are the next steps after that?
Dr. Taub: Pharmacotherapy is very synergistic with lifestyle, and there are many different pharmacotherapy options. One of the first things that you want to think about is lowering that heart rate. The reason people feel horrible is because their heart rate is usually very high when they’re upright. If they’re upright for long periods of time and they’re having very high heart rates, they’re going to get really tired because it’s like they’re exercising for hours when they’re upright.
Heart rate lowering is the cornerstone of therapy. Traditionally, we’ve used beta-blockers for heart rate lowering. The problem is they also lower blood pressure. They can also cause fatigue, so not the ideal agent for patients with POTS.
One of the clinical trials that I led was with a drug called ivabradine, which selectively works on the SA node and decreases heart rate without affecting blood pressure. What’s really elegant about ivabradine is it has a more potent effect when the heart rate is higher. When the patient is standing, it’s going to have a more potent effect on heart rate lowering. It’s really well tolerated in patients with POTS. In our study, we showed an improvement in quality of life metrics. That’s one of the first-line drugs that I use for patients with POTS.
The other thing is some of them will also have a concomitant lowering of blood pressure. You can think about medications that increase blood pressure, like midodrine, fludrocortisone, and droxidopa. Sometimes that combination of a heart rate-lowering medication and a medication that increases blood pressure really works well.
Dr. O’Donoghue: That’s very helpful. I think that those kinds of practical tips are the ones that practitioners really want to reach for, because they need to have that algorithm in their mind to take on this condition. Thanks again for walking us through that.
I think it’s a very interesting space, and there’s more that we’re going to be learning over the next few years as we further flesh out these post-COVID cases and what we learn from that as well.
Dr. Taub: There are many clinical trials now starting in POTS, so it’s exciting.
Dr. O’Donoghue: Absolutely. Thank you again for joining me today. Signing off, this is Dr Michelle O’Donoghue.
Dr. O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Dr. O’Donoghue loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. She disclosed ties with Amgen, AstraZeneca Pharmaceuticals LP, CVS Minute Clinic, Eisai, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novartis, and The Medicines Company. Dr. Taub is professor of Medicine, University of California San Diego Health, La Jolla. She disclosed ties with Amgen, Bayer, Boehringer Ingelheim, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here in Amsterdam at the European Society of Cardiology (ESC) Congress 2023. Joining me for a great discussion is my friend Dr. Pam Taub, who is a cardiologist and a professor of medicine at UC San Diego. She has a particular interest in postural orthostatic tachycardia syndrome (POTS), so that’s what we’ll be talking about today.
Thanks for joining me, Pam. When we think about POTS, for those who are not familiar with the term, what does it actually mean and how do you diagnose it?
No tilt table required
Pam R. Taub, MD: That’s typically associated with symptoms such as lightheadedness, dizziness, and cognitive difficulties such as brain fog. The diagnosis can be made by tilt-table testing, but it can also be made in the office with simple orthostats.
In my clinic, I have people lie down for 3-5 minutes. At the end of that period, you get a heart rate and blood pressure. Then you have them stand up for 3-5 minutes and then get heart rate and blood pressure, and you look at the differences. If the heart rate goes up by 30 points – so maybe they’re 80 beats/min when they’re lying down and when they stand up, it goes to 110 beats/min – that’s POTS, so very objective criteria. Typically, these people don’t have what we call orthostatic hypotension, where there is a significant decrease in the blood pressure. It’s more a heart rate issue.
Dr. O’Donoghue: How symptomatically do they usually present?
Dr. Taub: It’s a spectrum. Some people have mild symptoms. After they’re in the upright position for maybe 10 minutes, they get symptoms. There are some people who, when they go from a lying to standing position, they’re extremely symptomatic and can’t really do any activities. There are some people that are even wheelchair-bound because the symptoms are so debilitating. There’s a wide spectrum.
Dr. O’Donoghue: There has been more discussion, I feel like, about the rising prevalence of POTS as a diagnosis, and in particular since the COVID pandemic. What’s our understanding of the relationship between COVID and POTS and what the mechanism might be?
Dr. Taub: We’ve known that POTS can be triggered by a viral infection. Before COVID, we knew that in certain individuals that we think have an underlying genetic predisposition, usually some autoimmune substrate, when they get certain types of infections, whether it’s influenza or mononucleosis, they get POTS.
Typically, when they get an infection, they start getting deconditioned. They don’t feel well, so they’re on bed rest. When they get long periods of bed rest, when they start to become active, they start to have overactivation of their sympathetic nervous system, and they have a large amount of cardiovascular deconditioning. It’s a cycle that is often triggered after an infection.
A huge increase of POTS has been seen after COVID-19 because we had so many people exposed to this virus. With COVID-19, there is a period where people don’t feel great and they are getting bed rest, so they’re getting deconditioned. We’ve seen so many patients referred for post-COVID POTS and also long COVID or the post-acute sequelae of COVID-19, where POTS is a part of that presentation.
Female sex and autoimmune conditions
Dr. O’Donoghue: We know that POTS seems to disproportionately affect women. Is that understood? Is it thought that that’s related to the perhaps the autoimmune component of that illness?
Dr. Taub: Yes. The theory is because women tend to have more autoimmune conditions, that’s why they’re more predisposed. There’s a large amount of genetic susceptibility. For instance, we know that there’s an association between POTS and conditions like Ehlers-Danlos syndrome and between POTS and mast cell activation. Some of those conditions are more prevalent in women as well.
Dr. O’Donoghue: I feel like many physicians don’t know how to manage POTS, and they’re actually a little fearful perhaps to take it on. Fortunately, there have been a growing number of POTS clinics with specialists that focus on that area. For the average practitioner who maybe can’t refer to a POTS clinic, how should they approach that?
Dr. Taub: The first thing is its diagnosis. When someone tells you that they have symptoms of orthostatic intolerance – so, activities that involve standing – you need to first have that on your differential diagnosis. You can make the diagnosis in the office with orthostats. You don’t need a tilt table. It’s sometimes helpful if you’re unsure about the diagnosis, but you can make the diagnosis.
Many times, you’re finding people that have very mild symptoms. You can treat that with some good lifestyle recommendations, such as increased hydration, increasing salt in their diet, and compression. And the exercise component is really important.
Many people with POTS are told to go exercise, go for a run, or go for a walk. That’s incorrect, because these people have symptoms when they’re in the upright position. The type of exercise they need to do initially is exercise in the lying or seated position – so exercises like rowing or a seated bike, and strength training. As they start to feel better, then they can do upright exercise.
You should never tell a person that has POTS to just initially start with upright exercise, because they’re going to feel so much worse and then they’re never going to want to exercise. It’s really important to give them the right exercise recommendations. I find that for many of these mild cases, if they do the right exercise and engage in the right lifestyle strategies, they get better.
Compression wear and drug therapy
Dr. O’Donoghue: When it comes to compression stockings, do you usually start with a particular length?
Dr. Taub: It’s interesting. There are many different compression stockings, medical grade. Through patients with POTS, I’ve gotten feedback on certain types of athletic wear that have built-in compression, and that’s a little bit easier for people to wear every day because they can do their errands and it doesn’t look like they’re wearing medical-grade compression stockings.
Basically, I’ve collected all the different recommendations that patients say help, and I give them a list. The medical-grade compression stockings sometimes are very challenging to put on, and sometimes people just need light compression or even just socks. Any kind of compression is going to help.
Dr. O’Donoghue: That’s a great tip, because I know there are many patients who refuse to wear the compression stockings. If there’s a fashionable alternative, that’s always good to reach for.
Dr. Taub: Another thing that patients have told me is that abdominal compression is also very helpful. There are many commercially available abdominal compression options, like shapewear. Many patients with POTS use that and that helps, too.
Dr. O’Donoghue: Good. For those patients with POTS that is refractory to the measures you’ve already discussed, what are the next steps after that?
Dr. Taub: Pharmacotherapy is very synergistic with lifestyle, and there are many different pharmacotherapy options. One of the first things that you want to think about is lowering that heart rate. The reason people feel horrible is because their heart rate is usually very high when they’re upright. If they’re upright for long periods of time and they’re having very high heart rates, they’re going to get really tired because it’s like they’re exercising for hours when they’re upright.
Heart rate lowering is the cornerstone of therapy. Traditionally, we’ve used beta-blockers for heart rate lowering. The problem is they also lower blood pressure. They can also cause fatigue, so not the ideal agent for patients with POTS.
One of the clinical trials that I led was with a drug called ivabradine, which selectively works on the SA node and decreases heart rate without affecting blood pressure. What’s really elegant about ivabradine is it has a more potent effect when the heart rate is higher. When the patient is standing, it’s going to have a more potent effect on heart rate lowering. It’s really well tolerated in patients with POTS. In our study, we showed an improvement in quality of life metrics. That’s one of the first-line drugs that I use for patients with POTS.
The other thing is some of them will also have a concomitant lowering of blood pressure. You can think about medications that increase blood pressure, like midodrine, fludrocortisone, and droxidopa. Sometimes that combination of a heart rate-lowering medication and a medication that increases blood pressure really works well.
Dr. O’Donoghue: That’s very helpful. I think that those kinds of practical tips are the ones that practitioners really want to reach for, because they need to have that algorithm in their mind to take on this condition. Thanks again for walking us through that.
I think it’s a very interesting space, and there’s more that we’re going to be learning over the next few years as we further flesh out these post-COVID cases and what we learn from that as well.
Dr. Taub: There are many clinical trials now starting in POTS, so it’s exciting.
Dr. O’Donoghue: Absolutely. Thank you again for joining me today. Signing off, this is Dr Michelle O’Donoghue.
Dr. O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Dr. O’Donoghue loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. She disclosed ties with Amgen, AstraZeneca Pharmaceuticals LP, CVS Minute Clinic, Eisai, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novartis, and The Medicines Company. Dr. Taub is professor of Medicine, University of California San Diego Health, La Jolla. She disclosed ties with Amgen, Bayer, Boehringer Ingelheim, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi.
A version of this article appeared on Medscape.com.