User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
‘Water fasting’ benefits don’t last
Health benefits of prolonged “water fasting” (zero calories) or Buchinger fasting (200-300 calories/day) don’t last, according to authors of a review of eight studies.
Five days of fasting lowered weight by about 6%, but this weight was regained after 3 months of regular eating, the investigators found. The article was published in Nutrition Reviews.
“Water fasting led to improvements in blood pressure, cholesterol, and blood sugar levels, but these were short-lived,” senior author Krista A. Varady, PhD, told this news organization.
“Levels returned to baseline ... quickly after participants started eating. Most benefits disappeared in 3-4 months,” said Dr. Varady, professor of nutrition at the University of Illinois, Chicago.
“My overall conclusion,” she said, “is that I guess you could try it, but it just seems like a lot of work, and all those metabolic benefits disappear. I would encourage someone hoping to lose weight to try intermittent fasting instead of water fasting, because there’s a lot more data to show it can help with weight management.
“People should consult their doctor if they have diabetes or any other major obesity-related conditions before doing water fasting,” Dr. Varady cautioned.
“Healthy people with obesity can probably fast safely for 5 days on their own (if they don’t have any other conditions). However, no one should undertake one of these fasts for more than 5 days without medical supervision,” she stressed.
Eight studies of water and Buchinger fasting
Although several favorable effects of prolonged fasting have been observed, benefits must be weighed against risks, Dr. Varady and her coauthors wrote.
Most medically supervised fasting programs have reported only minor adverse events, which included hunger, headaches, nausea, vomiting, dry mouth, and fatigue. However, more severe events have been documented, including edema, abnormal results on liver function tests, decreased bone density, and metabolic acidosis.
The researchers aimed to determine the effect of prolonged fasting on weight, blood pressure, lipid levels, and glycemic control, as well as safety and the effects of refeeding.
They examined two types of prolonged fasting: water fasting and Buchinger fasting, which involves consuming 250 mL of fruit or vegetable juice for lunch and 250 mL of soup for dinner every day of the 5- to 20-day fast.
Buchinger fasting is popular in Central Europe. Water fasting “institutes” exist in the United States, such as one in California, Dr. Varady noted.
The researchers excluded fasting during Ramadan or fasting practiced by Seventh Day Adventists.
They identified four studies of water fasting and four studies of Buchinger fasting (of which one study of 1,422 participants assessed fasting for 5, 10, 15, and 20 days).
The review showed that prolonged fasting for 5-20 days produced large increases in circulating ketones, weight loss of 2%-10%, and decreases in systolic and diastolic blood pressure.
People who fasted 5 days typically lost 4%-6% of their weight; those who fasted 7-10 days lost 2%-10% of their weight; and those who fasted 15-20 days lost 7%-10% of their weight.
LDL cholesterol and triglyceride levels decreased in some trials.
Fasting glucose levels, fasting insulin levels, insulin resistance, and A1c decreased in adults without diabetes but remained unchanged in patients with type 1 or type 2 diabetes.
Some participants experienced metabolic acidosis, headaches, insomnia, or hunger.
About two-thirds of the weight lost was of lean mass, and one-third was of fat mass. The loss of lean mass loss suggests that prolonged fasting may increase the breakdown of muscle proteins, which is a concern, the researchers noted.
Few of the trials examined the effects of refeeding. In one study, normal-weight adults lost 6% of their weight after 5 days of water-only fasting but then gained it all back after 3 months of eating regularly.
In three trials, participants regained 1%-2% of their weight 2-4 months after fasting; however, those trials instructed participants to follow a calorie-restricted diet during the refeeding period.
Three to 4 months after the fast was completed, none of the metabolic benefits were maintained, even when weight loss was maintained.
The study did not receive external funding. Dr. Varady has received author fees from Hachette Book Group for “The Every Other Day Diet” and from Pan Macmillan Press for “The Fastest Diet.” The other authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Health benefits of prolonged “water fasting” (zero calories) or Buchinger fasting (200-300 calories/day) don’t last, according to authors of a review of eight studies.
Five days of fasting lowered weight by about 6%, but this weight was regained after 3 months of regular eating, the investigators found. The article was published in Nutrition Reviews.
“Water fasting led to improvements in blood pressure, cholesterol, and blood sugar levels, but these were short-lived,” senior author Krista A. Varady, PhD, told this news organization.
“Levels returned to baseline ... quickly after participants started eating. Most benefits disappeared in 3-4 months,” said Dr. Varady, professor of nutrition at the University of Illinois, Chicago.
“My overall conclusion,” she said, “is that I guess you could try it, but it just seems like a lot of work, and all those metabolic benefits disappear. I would encourage someone hoping to lose weight to try intermittent fasting instead of water fasting, because there’s a lot more data to show it can help with weight management.
“People should consult their doctor if they have diabetes or any other major obesity-related conditions before doing water fasting,” Dr. Varady cautioned.
“Healthy people with obesity can probably fast safely for 5 days on their own (if they don’t have any other conditions). However, no one should undertake one of these fasts for more than 5 days without medical supervision,” she stressed.
Eight studies of water and Buchinger fasting
Although several favorable effects of prolonged fasting have been observed, benefits must be weighed against risks, Dr. Varady and her coauthors wrote.
Most medically supervised fasting programs have reported only minor adverse events, which included hunger, headaches, nausea, vomiting, dry mouth, and fatigue. However, more severe events have been documented, including edema, abnormal results on liver function tests, decreased bone density, and metabolic acidosis.
The researchers aimed to determine the effect of prolonged fasting on weight, blood pressure, lipid levels, and glycemic control, as well as safety and the effects of refeeding.
They examined two types of prolonged fasting: water fasting and Buchinger fasting, which involves consuming 250 mL of fruit or vegetable juice for lunch and 250 mL of soup for dinner every day of the 5- to 20-day fast.
Buchinger fasting is popular in Central Europe. Water fasting “institutes” exist in the United States, such as one in California, Dr. Varady noted.
The researchers excluded fasting during Ramadan or fasting practiced by Seventh Day Adventists.
They identified four studies of water fasting and four studies of Buchinger fasting (of which one study of 1,422 participants assessed fasting for 5, 10, 15, and 20 days).
The review showed that prolonged fasting for 5-20 days produced large increases in circulating ketones, weight loss of 2%-10%, and decreases in systolic and diastolic blood pressure.
People who fasted 5 days typically lost 4%-6% of their weight; those who fasted 7-10 days lost 2%-10% of their weight; and those who fasted 15-20 days lost 7%-10% of their weight.
LDL cholesterol and triglyceride levels decreased in some trials.
Fasting glucose levels, fasting insulin levels, insulin resistance, and A1c decreased in adults without diabetes but remained unchanged in patients with type 1 or type 2 diabetes.
Some participants experienced metabolic acidosis, headaches, insomnia, or hunger.
About two-thirds of the weight lost was of lean mass, and one-third was of fat mass. The loss of lean mass loss suggests that prolonged fasting may increase the breakdown of muscle proteins, which is a concern, the researchers noted.
Few of the trials examined the effects of refeeding. In one study, normal-weight adults lost 6% of their weight after 5 days of water-only fasting but then gained it all back after 3 months of eating regularly.
In three trials, participants regained 1%-2% of their weight 2-4 months after fasting; however, those trials instructed participants to follow a calorie-restricted diet during the refeeding period.
Three to 4 months after the fast was completed, none of the metabolic benefits were maintained, even when weight loss was maintained.
The study did not receive external funding. Dr. Varady has received author fees from Hachette Book Group for “The Every Other Day Diet” and from Pan Macmillan Press for “The Fastest Diet.” The other authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Health benefits of prolonged “water fasting” (zero calories) or Buchinger fasting (200-300 calories/day) don’t last, according to authors of a review of eight studies.
Five days of fasting lowered weight by about 6%, but this weight was regained after 3 months of regular eating, the investigators found. The article was published in Nutrition Reviews.
“Water fasting led to improvements in blood pressure, cholesterol, and blood sugar levels, but these were short-lived,” senior author Krista A. Varady, PhD, told this news organization.
“Levels returned to baseline ... quickly after participants started eating. Most benefits disappeared in 3-4 months,” said Dr. Varady, professor of nutrition at the University of Illinois, Chicago.
“My overall conclusion,” she said, “is that I guess you could try it, but it just seems like a lot of work, and all those metabolic benefits disappear. I would encourage someone hoping to lose weight to try intermittent fasting instead of water fasting, because there’s a lot more data to show it can help with weight management.
“People should consult their doctor if they have diabetes or any other major obesity-related conditions before doing water fasting,” Dr. Varady cautioned.
“Healthy people with obesity can probably fast safely for 5 days on their own (if they don’t have any other conditions). However, no one should undertake one of these fasts for more than 5 days without medical supervision,” she stressed.
Eight studies of water and Buchinger fasting
Although several favorable effects of prolonged fasting have been observed, benefits must be weighed against risks, Dr. Varady and her coauthors wrote.
Most medically supervised fasting programs have reported only minor adverse events, which included hunger, headaches, nausea, vomiting, dry mouth, and fatigue. However, more severe events have been documented, including edema, abnormal results on liver function tests, decreased bone density, and metabolic acidosis.
The researchers aimed to determine the effect of prolonged fasting on weight, blood pressure, lipid levels, and glycemic control, as well as safety and the effects of refeeding.
They examined two types of prolonged fasting: water fasting and Buchinger fasting, which involves consuming 250 mL of fruit or vegetable juice for lunch and 250 mL of soup for dinner every day of the 5- to 20-day fast.
Buchinger fasting is popular in Central Europe. Water fasting “institutes” exist in the United States, such as one in California, Dr. Varady noted.
The researchers excluded fasting during Ramadan or fasting practiced by Seventh Day Adventists.
They identified four studies of water fasting and four studies of Buchinger fasting (of which one study of 1,422 participants assessed fasting for 5, 10, 15, and 20 days).
The review showed that prolonged fasting for 5-20 days produced large increases in circulating ketones, weight loss of 2%-10%, and decreases in systolic and diastolic blood pressure.
People who fasted 5 days typically lost 4%-6% of their weight; those who fasted 7-10 days lost 2%-10% of their weight; and those who fasted 15-20 days lost 7%-10% of their weight.
LDL cholesterol and triglyceride levels decreased in some trials.
Fasting glucose levels, fasting insulin levels, insulin resistance, and A1c decreased in adults without diabetes but remained unchanged in patients with type 1 or type 2 diabetes.
Some participants experienced metabolic acidosis, headaches, insomnia, or hunger.
About two-thirds of the weight lost was of lean mass, and one-third was of fat mass. The loss of lean mass loss suggests that prolonged fasting may increase the breakdown of muscle proteins, which is a concern, the researchers noted.
Few of the trials examined the effects of refeeding. In one study, normal-weight adults lost 6% of their weight after 5 days of water-only fasting but then gained it all back after 3 months of eating regularly.
In three trials, participants regained 1%-2% of their weight 2-4 months after fasting; however, those trials instructed participants to follow a calorie-restricted diet during the refeeding period.
Three to 4 months after the fast was completed, none of the metabolic benefits were maintained, even when weight loss was maintained.
The study did not receive external funding. Dr. Varady has received author fees from Hachette Book Group for “The Every Other Day Diet” and from Pan Macmillan Press for “The Fastest Diet.” The other authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
New weight loss drugs appeal to half of U.S. adults
A recent survey of more than 1,000 U.S. adults showed that 18% were “somewhat interested” in taking a “safe, effective” weight loss drug, 27% were “very interested,” and 4% said they were already using such an agent, together constituting 49% of the surveyed adults.
The newer, more potent and generally safe agents that work by stimulating receptors to nutrient-stimulated hormones, such as incretins like glucagonlike peptide–1, seem to drive this interest.
When asked: “How much have you heard, if anything, about a new class of drugs being used for weight loss, such as Ozempic [semaglutide formulated and approved for people with type 2 diabetes], Wegovy [semaglutide for weight loss], and Mounjaro [tirzepatide, currently approved for treating only people with type 2 diabetes]?” 43% said they had heard some, or a lot, about these agents.
This was particularly true among people at least 65 years old, who had a 55% prevalence of knowing some, or a lot, about these new weight-loss agents, while an additional 26% had heard at least “a little” about them, reported staff members of KFF (formerly the Kaiser Family Foundation) in a report posted online in early August.
Weight loss drugs garner ‘increasing’ attention
“A new class of prescription drugs, initially developed to treat type 2 diabetes, have been garnering an increasing amount of attention due to their ability to act as highly effective weight loss drugs for overweight or obese adults,” wrote the report’s authors.
However, surveyed interest fell markedly when respondents answered further questions that hinged on certain limitations of the newer weight loss formulations.
For example, the percent interested held nearly steady, at 44%, when told the weight loss agent in question was an oral pill, but when asked about formulations requiring weekly injections the prevalence of people who had some interest, or were very interested, dropped to 23%. And when presented with the premise that they would need to take the drug chronically to keep their weight off and that stopping the agent would mean weight regain, those with “higher levels of interest” in the agent fell to 14% of the study sample.
Other deal breakers for most survey respondents were lack of a weight-loss indication approved by the Food and Drug Administration, a hypothetical that left 16% still somewhat or very interested, and lack of insurance coverage, which also dropped the higher interest levels to 16% of respondents. On the flip side of that sentiment, 80% of survey respondents believe that health insurance should cover the cost for a prescription weight loss drug for people with overweight or obesity.
The survey was designed and analyzed by public-opinion researchers at KFF and run both online and by telephone in both English and Spanish during July 11-19, 2023. The margin of sampling error was plus or minus 3 percentage points for the full sample but may have been even higher for results based on subgroup analyses.
The survey report includes no funding or disclosure information. However, KFF describes itself as “independent” and “nonpartisan” and that it “does everything based on facts and data, and we do so objectively without taking policy positions and without affiliation to any political party or external interest.”
A version of this article first appeared on Medscape.com.
A recent survey of more than 1,000 U.S. adults showed that 18% were “somewhat interested” in taking a “safe, effective” weight loss drug, 27% were “very interested,” and 4% said they were already using such an agent, together constituting 49% of the surveyed adults.
The newer, more potent and generally safe agents that work by stimulating receptors to nutrient-stimulated hormones, such as incretins like glucagonlike peptide–1, seem to drive this interest.
When asked: “How much have you heard, if anything, about a new class of drugs being used for weight loss, such as Ozempic [semaglutide formulated and approved for people with type 2 diabetes], Wegovy [semaglutide for weight loss], and Mounjaro [tirzepatide, currently approved for treating only people with type 2 diabetes]?” 43% said they had heard some, or a lot, about these agents.
This was particularly true among people at least 65 years old, who had a 55% prevalence of knowing some, or a lot, about these new weight-loss agents, while an additional 26% had heard at least “a little” about them, reported staff members of KFF (formerly the Kaiser Family Foundation) in a report posted online in early August.
Weight loss drugs garner ‘increasing’ attention
“A new class of prescription drugs, initially developed to treat type 2 diabetes, have been garnering an increasing amount of attention due to their ability to act as highly effective weight loss drugs for overweight or obese adults,” wrote the report’s authors.
However, surveyed interest fell markedly when respondents answered further questions that hinged on certain limitations of the newer weight loss formulations.
For example, the percent interested held nearly steady, at 44%, when told the weight loss agent in question was an oral pill, but when asked about formulations requiring weekly injections the prevalence of people who had some interest, or were very interested, dropped to 23%. And when presented with the premise that they would need to take the drug chronically to keep their weight off and that stopping the agent would mean weight regain, those with “higher levels of interest” in the agent fell to 14% of the study sample.
Other deal breakers for most survey respondents were lack of a weight-loss indication approved by the Food and Drug Administration, a hypothetical that left 16% still somewhat or very interested, and lack of insurance coverage, which also dropped the higher interest levels to 16% of respondents. On the flip side of that sentiment, 80% of survey respondents believe that health insurance should cover the cost for a prescription weight loss drug for people with overweight or obesity.
The survey was designed and analyzed by public-opinion researchers at KFF and run both online and by telephone in both English and Spanish during July 11-19, 2023. The margin of sampling error was plus or minus 3 percentage points for the full sample but may have been even higher for results based on subgroup analyses.
The survey report includes no funding or disclosure information. However, KFF describes itself as “independent” and “nonpartisan” and that it “does everything based on facts and data, and we do so objectively without taking policy positions and without affiliation to any political party or external interest.”
A version of this article first appeared on Medscape.com.
A recent survey of more than 1,000 U.S. adults showed that 18% were “somewhat interested” in taking a “safe, effective” weight loss drug, 27% were “very interested,” and 4% said they were already using such an agent, together constituting 49% of the surveyed adults.
The newer, more potent and generally safe agents that work by stimulating receptors to nutrient-stimulated hormones, such as incretins like glucagonlike peptide–1, seem to drive this interest.
When asked: “How much have you heard, if anything, about a new class of drugs being used for weight loss, such as Ozempic [semaglutide formulated and approved for people with type 2 diabetes], Wegovy [semaglutide for weight loss], and Mounjaro [tirzepatide, currently approved for treating only people with type 2 diabetes]?” 43% said they had heard some, or a lot, about these agents.
This was particularly true among people at least 65 years old, who had a 55% prevalence of knowing some, or a lot, about these new weight-loss agents, while an additional 26% had heard at least “a little” about them, reported staff members of KFF (formerly the Kaiser Family Foundation) in a report posted online in early August.
Weight loss drugs garner ‘increasing’ attention
“A new class of prescription drugs, initially developed to treat type 2 diabetes, have been garnering an increasing amount of attention due to their ability to act as highly effective weight loss drugs for overweight or obese adults,” wrote the report’s authors.
However, surveyed interest fell markedly when respondents answered further questions that hinged on certain limitations of the newer weight loss formulations.
For example, the percent interested held nearly steady, at 44%, when told the weight loss agent in question was an oral pill, but when asked about formulations requiring weekly injections the prevalence of people who had some interest, or were very interested, dropped to 23%. And when presented with the premise that they would need to take the drug chronically to keep their weight off and that stopping the agent would mean weight regain, those with “higher levels of interest” in the agent fell to 14% of the study sample.
Other deal breakers for most survey respondents were lack of a weight-loss indication approved by the Food and Drug Administration, a hypothetical that left 16% still somewhat or very interested, and lack of insurance coverage, which also dropped the higher interest levels to 16% of respondents. On the flip side of that sentiment, 80% of survey respondents believe that health insurance should cover the cost for a prescription weight loss drug for people with overweight or obesity.
The survey was designed and analyzed by public-opinion researchers at KFF and run both online and by telephone in both English and Spanish during July 11-19, 2023. The margin of sampling error was plus or minus 3 percentage points for the full sample but may have been even higher for results based on subgroup analyses.
The survey report includes no funding or disclosure information. However, KFF describes itself as “independent” and “nonpartisan” and that it “does everything based on facts and data, and we do so objectively without taking policy positions and without affiliation to any political party or external interest.”
A version of this article first appeared on Medscape.com.
New COVID shots will be available in September
The updated vaccine still needs final sign-offs from the Food and Drug Administration and the CDC.
“We anticipate that they are going to be available for most folks by the third or fourth week of September,” Director Mandy Cohen, MD, MPH, said on a podcast hosted by former White House COVID adviser Andy Slavitt. “We are likely to see this as a recommendation as an annual COVID shot, just as we have an annual flu shot. I think that will give folks more clarity on whether they should get one or not.”
For people who are considering now whether they should get the currently available COVID vaccine or wait until the new one comes out, Dr. Cohen said that depends on a person’s individual risk. People who are 65 or older or who have multiple health conditions should go ahead and get the currently available shot if it’s been more than 6-8 months since their last dose. For all other people, it’s OK to wait for the new version.
Analysts expect low demand for the updated vaccine. About 240 million people in the United States got at least one dose when vaccines first became available in 2021, Reuters reported, but that number dropped to less than 50 million getting the most updated shot in the fall of 2022.
“Take a look at what happened last winter. It was 50 million in the U.S., and it seems likely to be lower than that, given that there’s less concern about COVID this year than last year,” Michael Yee, a health care industry analyst for the firm Jefferies, told Reuters.
Dr. Cohen noted during the podcast that the recent uptick in virus activity should be taken in context.
“What we’re seeing right now in August of 2023 are small increases of folks getting COVID. We are still at some of the lowest hospitalizations that we’ve been at in the past 3 years,” she said. “Even a 10% increase on a very, very small number is still very small. My level of concern continues to be low.”
A version of this article was first published on WebMD.com .
The updated vaccine still needs final sign-offs from the Food and Drug Administration and the CDC.
“We anticipate that they are going to be available for most folks by the third or fourth week of September,” Director Mandy Cohen, MD, MPH, said on a podcast hosted by former White House COVID adviser Andy Slavitt. “We are likely to see this as a recommendation as an annual COVID shot, just as we have an annual flu shot. I think that will give folks more clarity on whether they should get one or not.”
For people who are considering now whether they should get the currently available COVID vaccine or wait until the new one comes out, Dr. Cohen said that depends on a person’s individual risk. People who are 65 or older or who have multiple health conditions should go ahead and get the currently available shot if it’s been more than 6-8 months since their last dose. For all other people, it’s OK to wait for the new version.
Analysts expect low demand for the updated vaccine. About 240 million people in the United States got at least one dose when vaccines first became available in 2021, Reuters reported, but that number dropped to less than 50 million getting the most updated shot in the fall of 2022.
“Take a look at what happened last winter. It was 50 million in the U.S., and it seems likely to be lower than that, given that there’s less concern about COVID this year than last year,” Michael Yee, a health care industry analyst for the firm Jefferies, told Reuters.
Dr. Cohen noted during the podcast that the recent uptick in virus activity should be taken in context.
“What we’re seeing right now in August of 2023 are small increases of folks getting COVID. We are still at some of the lowest hospitalizations that we’ve been at in the past 3 years,” she said. “Even a 10% increase on a very, very small number is still very small. My level of concern continues to be low.”
A version of this article was first published on WebMD.com .
The updated vaccine still needs final sign-offs from the Food and Drug Administration and the CDC.
“We anticipate that they are going to be available for most folks by the third or fourth week of September,” Director Mandy Cohen, MD, MPH, said on a podcast hosted by former White House COVID adviser Andy Slavitt. “We are likely to see this as a recommendation as an annual COVID shot, just as we have an annual flu shot. I think that will give folks more clarity on whether they should get one or not.”
For people who are considering now whether they should get the currently available COVID vaccine or wait until the new one comes out, Dr. Cohen said that depends on a person’s individual risk. People who are 65 or older or who have multiple health conditions should go ahead and get the currently available shot if it’s been more than 6-8 months since their last dose. For all other people, it’s OK to wait for the new version.
Analysts expect low demand for the updated vaccine. About 240 million people in the United States got at least one dose when vaccines first became available in 2021, Reuters reported, but that number dropped to less than 50 million getting the most updated shot in the fall of 2022.
“Take a look at what happened last winter. It was 50 million in the U.S., and it seems likely to be lower than that, given that there’s less concern about COVID this year than last year,” Michael Yee, a health care industry analyst for the firm Jefferies, told Reuters.
Dr. Cohen noted during the podcast that the recent uptick in virus activity should be taken in context.
“What we’re seeing right now in August of 2023 are small increases of folks getting COVID. We are still at some of the lowest hospitalizations that we’ve been at in the past 3 years,” she said. “Even a 10% increase on a very, very small number is still very small. My level of concern continues to be low.”
A version of this article was first published on WebMD.com .
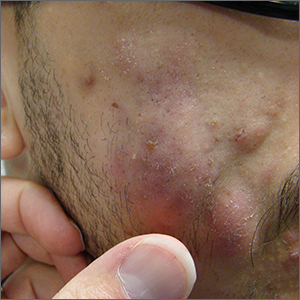
Fluctuant facial lesions
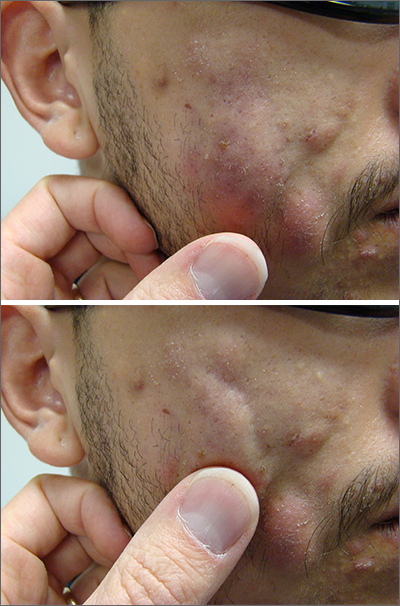
This patient had more than cystic acne; he had acne conglobata. AC is a severe form of inflammatory acne leading to coalescing lesions with purulent sinus tracts under the skin. It can be seen as part of the follicular tetrad syndrome of cystic acne, hidradenitis suppurativa, dissecting cellulitis, and pilonidal disease. AC is thought to be an elevated tumor necrosis factor (TNF)-alpha response to Propionibacterium acnes (now known as Cutibacterium acnes) that leads to excessive inflammation and sterile abscesses.1 Acne fulminans (AF) can also manifest as a purulent form of acne, but AF has associated systemic signs and symptoms that include fevers, chills, and malaise.
Due to the depth of the inflammation, AC is treated with systemic medications, most commonly isotretinoin. Isotretinoin can be started at 0.5 mg/kg (divided twice daily to enhance tolerability) and then increased to 1 mg/kg (divided twice daily) for 5 months. There is some variation in dosing regimens in practice; the target goal is 120 to 150 mg/kg over the course of treatment. In AF, the patient is pretreated with systemic steroids, and in AC, some clinicians will even prescribe systemic steroids (prednisone 0.5 mg/kg daily for the first month) along with isotretinoin.
Second-line medications include dapsone (50-150 mg/d).2 Case reports describe the successful use of the TNF-alpha antagonist adalimumab, although this is not a usual practice in AC treatment.1 Note that all of these medications have the potential for severe adverse effects and require laboratory evaluation prior to initiation.
This patient was counseled, prescribed isotretinoin (dose as above), and enrolled in the IPledge prescribing and monitoring system for isotretinoin. At 20 weeks of use, the purulent drainage ceased. The pus-filled sinus tracts and redness had resolved, although he still had thickened tissue and scarring where the tracts had been. In time, the scars will usually get flatter and softer.
If the patient’s AC were to flare, another 20-week course of isotretinoin could be prescribed after a 2-month hiatus or he could be switched to a second-line medication. Referral for any cosmetic therapy is typically delayed for another 6 months in case there is a need to treat a recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Yiu ZZ, Madan V, Griffiths CE. Acne conglobata and adalimumab: use of tumour necrosis factor-α antagonists in treatment-resistant acne conglobata, and review of the literature. Clin Exp Dermatol. 2015;40:383-386. doi: 10.1111/ced.12540
2. Hafsi W, Arnold DL, Kassardjian M. Acne Conglobata. StatPearls Publishing; 2023.

This patient had more than cystic acne; he had acne conglobata. AC is a severe form of inflammatory acne leading to coalescing lesions with purulent sinus tracts under the skin. It can be seen as part of the follicular tetrad syndrome of cystic acne, hidradenitis suppurativa, dissecting cellulitis, and pilonidal disease. AC is thought to be an elevated tumor necrosis factor (TNF)-alpha response to Propionibacterium acnes (now known as Cutibacterium acnes) that leads to excessive inflammation and sterile abscesses.1 Acne fulminans (AF) can also manifest as a purulent form of acne, but AF has associated systemic signs and symptoms that include fevers, chills, and malaise.
Due to the depth of the inflammation, AC is treated with systemic medications, most commonly isotretinoin. Isotretinoin can be started at 0.5 mg/kg (divided twice daily to enhance tolerability) and then increased to 1 mg/kg (divided twice daily) for 5 months. There is some variation in dosing regimens in practice; the target goal is 120 to 150 mg/kg over the course of treatment. In AF, the patient is pretreated with systemic steroids, and in AC, some clinicians will even prescribe systemic steroids (prednisone 0.5 mg/kg daily for the first month) along with isotretinoin.
Second-line medications include dapsone (50-150 mg/d).2 Case reports describe the successful use of the TNF-alpha antagonist adalimumab, although this is not a usual practice in AC treatment.1 Note that all of these medications have the potential for severe adverse effects and require laboratory evaluation prior to initiation.
This patient was counseled, prescribed isotretinoin (dose as above), and enrolled in the IPledge prescribing and monitoring system for isotretinoin. At 20 weeks of use, the purulent drainage ceased. The pus-filled sinus tracts and redness had resolved, although he still had thickened tissue and scarring where the tracts had been. In time, the scars will usually get flatter and softer.
If the patient’s AC were to flare, another 20-week course of isotretinoin could be prescribed after a 2-month hiatus or he could be switched to a second-line medication. Referral for any cosmetic therapy is typically delayed for another 6 months in case there is a need to treat a recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This patient had more than cystic acne; he had acne conglobata. AC is a severe form of inflammatory acne leading to coalescing lesions with purulent sinus tracts under the skin. It can be seen as part of the follicular tetrad syndrome of cystic acne, hidradenitis suppurativa, dissecting cellulitis, and pilonidal disease. AC is thought to be an elevated tumor necrosis factor (TNF)-alpha response to Propionibacterium acnes (now known as Cutibacterium acnes) that leads to excessive inflammation and sterile abscesses.1 Acne fulminans (AF) can also manifest as a purulent form of acne, but AF has associated systemic signs and symptoms that include fevers, chills, and malaise.
Due to the depth of the inflammation, AC is treated with systemic medications, most commonly isotretinoin. Isotretinoin can be started at 0.5 mg/kg (divided twice daily to enhance tolerability) and then increased to 1 mg/kg (divided twice daily) for 5 months. There is some variation in dosing regimens in practice; the target goal is 120 to 150 mg/kg over the course of treatment. In AF, the patient is pretreated with systemic steroids, and in AC, some clinicians will even prescribe systemic steroids (prednisone 0.5 mg/kg daily for the first month) along with isotretinoin.
Second-line medications include dapsone (50-150 mg/d).2 Case reports describe the successful use of the TNF-alpha antagonist adalimumab, although this is not a usual practice in AC treatment.1 Note that all of these medications have the potential for severe adverse effects and require laboratory evaluation prior to initiation.
This patient was counseled, prescribed isotretinoin (dose as above), and enrolled in the IPledge prescribing and monitoring system for isotretinoin. At 20 weeks of use, the purulent drainage ceased. The pus-filled sinus tracts and redness had resolved, although he still had thickened tissue and scarring where the tracts had been. In time, the scars will usually get flatter and softer.
If the patient’s AC were to flare, another 20-week course of isotretinoin could be prescribed after a 2-month hiatus or he could be switched to a second-line medication. Referral for any cosmetic therapy is typically delayed for another 6 months in case there is a need to treat a recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Yiu ZZ, Madan V, Griffiths CE. Acne conglobata and adalimumab: use of tumour necrosis factor-α antagonists in treatment-resistant acne conglobata, and review of the literature. Clin Exp Dermatol. 2015;40:383-386. doi: 10.1111/ced.12540
2. Hafsi W, Arnold DL, Kassardjian M. Acne Conglobata. StatPearls Publishing; 2023.
1. Yiu ZZ, Madan V, Griffiths CE. Acne conglobata and adalimumab: use of tumour necrosis factor-α antagonists in treatment-resistant acne conglobata, and review of the literature. Clin Exp Dermatol. 2015;40:383-386. doi: 10.1111/ced.12540
2. Hafsi W, Arnold DL, Kassardjian M. Acne Conglobata. StatPearls Publishing; 2023.
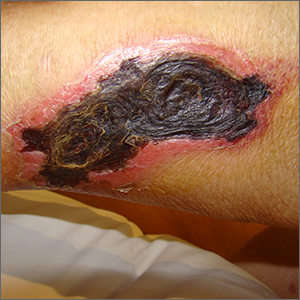
Leathery plaque on thigh
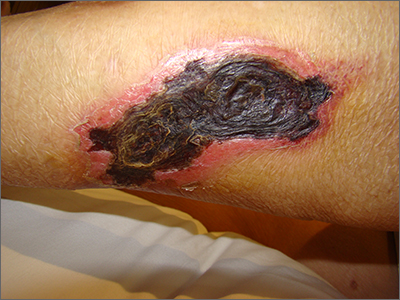
The necrotic eschar on this patient’s thigh is calciphylaxis, also known as calcific uremic arteriolopathy (CUA). Most cases are seen in ESRD and start as painful erythematous, firm lesions that progress to necrotic eschars. Up to 4% of patients with ESRD who are on dialysis develop CUA.1
The exact pathology of CUA is unknown. Calcification of the arterioles leads to ischemia and necrosis of tissue, which is not limited to the skin and can affect tissue elsewhere (eg, muscles, central nervous system, internal organs).2
Morbidity and mortality of CUA is often due to bacterial infections and sepsis related to the necrotic tissue. CUA can be treated with sodium thiosulfate (25 g in 100 mL of normal saline) infused intravenously during the last 30 minutes of dialysis treatment 3 times per week.3 Sodium thiosulfate (which acts as a calcium binder) and cinacalcet (a calcimimetic that leads to lower parathyroid hormone levels) have been used, but evidence of efficacy is limited. In a multicenter observational study involving 89 patients with chronic kidney disease and CUA, 17% of patients experienced complete wound healing, while 56% died over a median follow-up period of 5.8 months.1 (No cause of death data were available; sodium thiosulfate and a calcimimetic were the most widely used treatment strategies.) This extrapolated to a mortality rate of 72 patients per 100 individuals over the course of 1 year (the 100 patient-years rate).1
This patient continued her dialysis regimen and general care. She was seen by the wound care team and treated with topical wound care, including moist dressings for her open lesions. The eschars were not debrided because they showed no sign of active infection. Unfortunately, she was in extremely frail condition and died 1 month after evaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Chinnadurai R, Huckle A, Hegarty J, et al. Calciphylaxis in end-stage kidney disease: outcome data from the United Kingdom Calciphylaxis Study. J Nephrol. 2021;34:1537-1545. doi: 10.1007/s40620-020-00908-9
2. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
3. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034

The necrotic eschar on this patient’s thigh is calciphylaxis, also known as calcific uremic arteriolopathy (CUA). Most cases are seen in ESRD and start as painful erythematous, firm lesions that progress to necrotic eschars. Up to 4% of patients with ESRD who are on dialysis develop CUA.1
The exact pathology of CUA is unknown. Calcification of the arterioles leads to ischemia and necrosis of tissue, which is not limited to the skin and can affect tissue elsewhere (eg, muscles, central nervous system, internal organs).2
Morbidity and mortality of CUA is often due to bacterial infections and sepsis related to the necrotic tissue. CUA can be treated with sodium thiosulfate (25 g in 100 mL of normal saline) infused intravenously during the last 30 minutes of dialysis treatment 3 times per week.3 Sodium thiosulfate (which acts as a calcium binder) and cinacalcet (a calcimimetic that leads to lower parathyroid hormone levels) have been used, but evidence of efficacy is limited. In a multicenter observational study involving 89 patients with chronic kidney disease and CUA, 17% of patients experienced complete wound healing, while 56% died over a median follow-up period of 5.8 months.1 (No cause of death data were available; sodium thiosulfate and a calcimimetic were the most widely used treatment strategies.) This extrapolated to a mortality rate of 72 patients per 100 individuals over the course of 1 year (the 100 patient-years rate).1
This patient continued her dialysis regimen and general care. She was seen by the wound care team and treated with topical wound care, including moist dressings for her open lesions. The eschars were not debrided because they showed no sign of active infection. Unfortunately, she was in extremely frail condition and died 1 month after evaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The necrotic eschar on this patient’s thigh is calciphylaxis, also known as calcific uremic arteriolopathy (CUA). Most cases are seen in ESRD and start as painful erythematous, firm lesions that progress to necrotic eschars. Up to 4% of patients with ESRD who are on dialysis develop CUA.1
The exact pathology of CUA is unknown. Calcification of the arterioles leads to ischemia and necrosis of tissue, which is not limited to the skin and can affect tissue elsewhere (eg, muscles, central nervous system, internal organs).2
Morbidity and mortality of CUA is often due to bacterial infections and sepsis related to the necrotic tissue. CUA can be treated with sodium thiosulfate (25 g in 100 mL of normal saline) infused intravenously during the last 30 minutes of dialysis treatment 3 times per week.3 Sodium thiosulfate (which acts as a calcium binder) and cinacalcet (a calcimimetic that leads to lower parathyroid hormone levels) have been used, but evidence of efficacy is limited. In a multicenter observational study involving 89 patients with chronic kidney disease and CUA, 17% of patients experienced complete wound healing, while 56% died over a median follow-up period of 5.8 months.1 (No cause of death data were available; sodium thiosulfate and a calcimimetic were the most widely used treatment strategies.) This extrapolated to a mortality rate of 72 patients per 100 individuals over the course of 1 year (the 100 patient-years rate).1
This patient continued her dialysis regimen and general care. She was seen by the wound care team and treated with topical wound care, including moist dressings for her open lesions. The eschars were not debrided because they showed no sign of active infection. Unfortunately, she was in extremely frail condition and died 1 month after evaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Chinnadurai R, Huckle A, Hegarty J, et al. Calciphylaxis in end-stage kidney disease: outcome data from the United Kingdom Calciphylaxis Study. J Nephrol. 2021;34:1537-1545. doi: 10.1007/s40620-020-00908-9
2. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
3. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
1. Chinnadurai R, Huckle A, Hegarty J, et al. Calciphylaxis in end-stage kidney disease: outcome data from the United Kingdom Calciphylaxis Study. J Nephrol. 2021;34:1537-1545. doi: 10.1007/s40620-020-00908-9
2. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
3. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
Children and long COVID: How many are affected?
Long COVID most often strikes seniors and adults, but children are also affected, even though they get less attention, new research shows.
Experts noted that the disease poses particular challenges for children and the doctors who treat them.
Parents and doctors need to be on the lookout for symptoms of long COVID in children and teens that might be easily missed or misdiagnosed, according to physicians and family groups.
Children are at lower risk for contracting COVID and often experience milder symptoms. But the virus is now widespread, and a recent study found that around 16% of pediatric patients with COVID go on to develop symptoms that last more than 3 months – the working definition of long COVID.
Parents and doctors are calling for more studies and more awareness.
Diane Sheehan, who lives outside Charlotte, N.C., says she was an active person and is now permanently disabled from long COVID. Her teenage son has it too and is still recovering.
He contracted COVID after a school event, she said. He had a mild case, but then he started experiencing dizziness and would even experience loss of consciousness when he stood up suddenly. After he contracted the virus a second time, he was bedridden for 8 months.
The staff at Hackensack Meridian Health, a pediatric long COVID clinic in New Jersey, has been working with area schools to help teachers and school nurses recognize possible long COVID in children and young people. The clinic is one of about a dozen in the United States that specializes in pediatric cases.
Katherine Clouser, MD, a pediatric hospital medicine specialist, has been with the clinic since it opened in 2021, and she’s seen a steady flow of patients. Some get better, but she sees a few new cases each week.
“We are seeing children who are having a difficult time returning to school and sports,” she said.
The clinic is having success with a mix of approaches, including intensive rehabilitation, talk therapy, and some off-label use of nirmatrelvir (Paxlovid), an antiviral now being studied as a treatment for long COVID through a National Institutes of Health clinical trials initiative that was announced last month.
Treatment depends on symptoms and is determined on a case-by-case basis, Dr. Clouser said.
Families of her patients are grateful, she added.
“We hear a lot of parents who were desperate for someone to believe them – or someone who knows about it,” she said.
A recent review of more than 30 studies with about 15,000 participants concluded that 16.2% (95% confidence interval, 8.5%-28.6%) of the pediatric participants experienced one or more persistent symptoms of long COVID at least 3 months after acute infection.
Estimates of the number of children and youth with long COVID have varied widely. A 2022 study put the number at more than 25% of cases, but the American Academy of Pediatrics notes that estimates of the percentage of children infected with SARS-CoV-2 who go on to have long COVID range from 2% to 66%.
The federal Recover Initiative has enrolled more than 10,000 children and youth – a number it plans to double – and studies of electronic health records are underway. The Recover pediatric team is also setting up a cohort that they plan to follow into 2025.
Some clinics are having luck treating young people with approaches ranging from special diets to off-label medication.
David W. Miller, MD, who runs the long COVID clinic at the UH Rainbow Babies and Children’s Hospital, Cleveland, said he’s seen about 250 patients.
A warning sign of long COVID in children is profound fatigue, he said.
“It’s the most common symptom,” Dr. Miller said. “They feel like they have the flu all the time.”
Many also experience orthostatic hypotension on standing, triggering dizziness.
He said his team targets symptom groups. Initial management consists of a diet without sugar or refined carbohydrates. Skipping pasta and sweets can be hard for young people, but Dr. Miller said sometimes the diet alone helps.
Many have vitamin D and iron deficiencies. Others need help getting a good night’s sleep. He’s treated 50 with off-label low-dose naltrexone.
Some people with long COVID – both young and old – complain about being misdiagnosed as having depression. Dr. Miller says he see a lot of anxiety – some situational and some biochemical – in pediatric patients. But he cautions doctors not to treat their illness solely as a mental health problem.
His advice: If a young person or child experiences a major change in his or her regular level of functioning or has multiple COVID symptoms that don’t go away after several months, parents and doctors should consider long COVID as a possible cause.
Dr. Miller said most of his patients get better over time with some treatments: “We see improvement in the majority of kids who can stick to the regimen,” such as a sugar-free diet, supplements, and adequate sleep. Recovery has been slow and incomplete for Diane Sheehan and her son. She was training as a permanent make-up artist, she said, but now has hand tremors that make work impossible.
She has found doctors who treat some of her symptoms with antihistamines, and her son has benefited from physical therapy.
But for now, her son is passing on a scholarship he was awarded to attend North Carolina State University this year. Instead, he’s living at home and going to a local college.
Ms. Sheehan urges parents to be on the alert for signs that their children might have long COVID, which can be confused with many other conditions.
A version of this article first appeared on Medscape.com.
Long COVID most often strikes seniors and adults, but children are also affected, even though they get less attention, new research shows.
Experts noted that the disease poses particular challenges for children and the doctors who treat them.
Parents and doctors need to be on the lookout for symptoms of long COVID in children and teens that might be easily missed or misdiagnosed, according to physicians and family groups.
Children are at lower risk for contracting COVID and often experience milder symptoms. But the virus is now widespread, and a recent study found that around 16% of pediatric patients with COVID go on to develop symptoms that last more than 3 months – the working definition of long COVID.
Parents and doctors are calling for more studies and more awareness.
Diane Sheehan, who lives outside Charlotte, N.C., says she was an active person and is now permanently disabled from long COVID. Her teenage son has it too and is still recovering.
He contracted COVID after a school event, she said. He had a mild case, but then he started experiencing dizziness and would even experience loss of consciousness when he stood up suddenly. After he contracted the virus a second time, he was bedridden for 8 months.
The staff at Hackensack Meridian Health, a pediatric long COVID clinic in New Jersey, has been working with area schools to help teachers and school nurses recognize possible long COVID in children and young people. The clinic is one of about a dozen in the United States that specializes in pediatric cases.
Katherine Clouser, MD, a pediatric hospital medicine specialist, has been with the clinic since it opened in 2021, and she’s seen a steady flow of patients. Some get better, but she sees a few new cases each week.
“We are seeing children who are having a difficult time returning to school and sports,” she said.
The clinic is having success with a mix of approaches, including intensive rehabilitation, talk therapy, and some off-label use of nirmatrelvir (Paxlovid), an antiviral now being studied as a treatment for long COVID through a National Institutes of Health clinical trials initiative that was announced last month.
Treatment depends on symptoms and is determined on a case-by-case basis, Dr. Clouser said.
Families of her patients are grateful, she added.
“We hear a lot of parents who were desperate for someone to believe them – or someone who knows about it,” she said.
A recent review of more than 30 studies with about 15,000 participants concluded that 16.2% (95% confidence interval, 8.5%-28.6%) of the pediatric participants experienced one or more persistent symptoms of long COVID at least 3 months after acute infection.
Estimates of the number of children and youth with long COVID have varied widely. A 2022 study put the number at more than 25% of cases, but the American Academy of Pediatrics notes that estimates of the percentage of children infected with SARS-CoV-2 who go on to have long COVID range from 2% to 66%.
The federal Recover Initiative has enrolled more than 10,000 children and youth – a number it plans to double – and studies of electronic health records are underway. The Recover pediatric team is also setting up a cohort that they plan to follow into 2025.
Some clinics are having luck treating young people with approaches ranging from special diets to off-label medication.
David W. Miller, MD, who runs the long COVID clinic at the UH Rainbow Babies and Children’s Hospital, Cleveland, said he’s seen about 250 patients.
A warning sign of long COVID in children is profound fatigue, he said.
“It’s the most common symptom,” Dr. Miller said. “They feel like they have the flu all the time.”
Many also experience orthostatic hypotension on standing, triggering dizziness.
He said his team targets symptom groups. Initial management consists of a diet without sugar or refined carbohydrates. Skipping pasta and sweets can be hard for young people, but Dr. Miller said sometimes the diet alone helps.
Many have vitamin D and iron deficiencies. Others need help getting a good night’s sleep. He’s treated 50 with off-label low-dose naltrexone.
Some people with long COVID – both young and old – complain about being misdiagnosed as having depression. Dr. Miller says he see a lot of anxiety – some situational and some biochemical – in pediatric patients. But he cautions doctors not to treat their illness solely as a mental health problem.
His advice: If a young person or child experiences a major change in his or her regular level of functioning or has multiple COVID symptoms that don’t go away after several months, parents and doctors should consider long COVID as a possible cause.
Dr. Miller said most of his patients get better over time with some treatments: “We see improvement in the majority of kids who can stick to the regimen,” such as a sugar-free diet, supplements, and adequate sleep. Recovery has been slow and incomplete for Diane Sheehan and her son. She was training as a permanent make-up artist, she said, but now has hand tremors that make work impossible.
She has found doctors who treat some of her symptoms with antihistamines, and her son has benefited from physical therapy.
But for now, her son is passing on a scholarship he was awarded to attend North Carolina State University this year. Instead, he’s living at home and going to a local college.
Ms. Sheehan urges parents to be on the alert for signs that their children might have long COVID, which can be confused with many other conditions.
A version of this article first appeared on Medscape.com.
Long COVID most often strikes seniors and adults, but children are also affected, even though they get less attention, new research shows.
Experts noted that the disease poses particular challenges for children and the doctors who treat them.
Parents and doctors need to be on the lookout for symptoms of long COVID in children and teens that might be easily missed or misdiagnosed, according to physicians and family groups.
Children are at lower risk for contracting COVID and often experience milder symptoms. But the virus is now widespread, and a recent study found that around 16% of pediatric patients with COVID go on to develop symptoms that last more than 3 months – the working definition of long COVID.
Parents and doctors are calling for more studies and more awareness.
Diane Sheehan, who lives outside Charlotte, N.C., says she was an active person and is now permanently disabled from long COVID. Her teenage son has it too and is still recovering.
He contracted COVID after a school event, she said. He had a mild case, but then he started experiencing dizziness and would even experience loss of consciousness when he stood up suddenly. After he contracted the virus a second time, he was bedridden for 8 months.
The staff at Hackensack Meridian Health, a pediatric long COVID clinic in New Jersey, has been working with area schools to help teachers and school nurses recognize possible long COVID in children and young people. The clinic is one of about a dozen in the United States that specializes in pediatric cases.
Katherine Clouser, MD, a pediatric hospital medicine specialist, has been with the clinic since it opened in 2021, and she’s seen a steady flow of patients. Some get better, but she sees a few new cases each week.
“We are seeing children who are having a difficult time returning to school and sports,” she said.
The clinic is having success with a mix of approaches, including intensive rehabilitation, talk therapy, and some off-label use of nirmatrelvir (Paxlovid), an antiviral now being studied as a treatment for long COVID through a National Institutes of Health clinical trials initiative that was announced last month.
Treatment depends on symptoms and is determined on a case-by-case basis, Dr. Clouser said.
Families of her patients are grateful, she added.
“We hear a lot of parents who were desperate for someone to believe them – or someone who knows about it,” she said.
A recent review of more than 30 studies with about 15,000 participants concluded that 16.2% (95% confidence interval, 8.5%-28.6%) of the pediatric participants experienced one or more persistent symptoms of long COVID at least 3 months after acute infection.
Estimates of the number of children and youth with long COVID have varied widely. A 2022 study put the number at more than 25% of cases, but the American Academy of Pediatrics notes that estimates of the percentage of children infected with SARS-CoV-2 who go on to have long COVID range from 2% to 66%.
The federal Recover Initiative has enrolled more than 10,000 children and youth – a number it plans to double – and studies of electronic health records are underway. The Recover pediatric team is also setting up a cohort that they plan to follow into 2025.
Some clinics are having luck treating young people with approaches ranging from special diets to off-label medication.
David W. Miller, MD, who runs the long COVID clinic at the UH Rainbow Babies and Children’s Hospital, Cleveland, said he’s seen about 250 patients.
A warning sign of long COVID in children is profound fatigue, he said.
“It’s the most common symptom,” Dr. Miller said. “They feel like they have the flu all the time.”
Many also experience orthostatic hypotension on standing, triggering dizziness.
He said his team targets symptom groups. Initial management consists of a diet without sugar or refined carbohydrates. Skipping pasta and sweets can be hard for young people, but Dr. Miller said sometimes the diet alone helps.
Many have vitamin D and iron deficiencies. Others need help getting a good night’s sleep. He’s treated 50 with off-label low-dose naltrexone.
Some people with long COVID – both young and old – complain about being misdiagnosed as having depression. Dr. Miller says he see a lot of anxiety – some situational and some biochemical – in pediatric patients. But he cautions doctors not to treat their illness solely as a mental health problem.
His advice: If a young person or child experiences a major change in his or her regular level of functioning or has multiple COVID symptoms that don’t go away after several months, parents and doctors should consider long COVID as a possible cause.
Dr. Miller said most of his patients get better over time with some treatments: “We see improvement in the majority of kids who can stick to the regimen,” such as a sugar-free diet, supplements, and adequate sleep. Recovery has been slow and incomplete for Diane Sheehan and her son. She was training as a permanent make-up artist, she said, but now has hand tremors that make work impossible.
She has found doctors who treat some of her symptoms with antihistamines, and her son has benefited from physical therapy.
But for now, her son is passing on a scholarship he was awarded to attend North Carolina State University this year. Instead, he’s living at home and going to a local college.
Ms. Sheehan urges parents to be on the alert for signs that their children might have long COVID, which can be confused with many other conditions.
A version of this article first appeared on Medscape.com.
Long COVID–induced activity limitations persist
Approximately one-quarter of adults who experience long COVID report activity limitations that do not change over time, based on data from national sample of nonhospitalized individuals.
Symptoms of long COVID, an ongoing medical condition that occurs in the wake of COVID-19 infection, include respiratory, neurologic, cardiovascular, or other complications that may last for weeks, months, or years after infection.
Current estimates of the incidence of long COVID in the United States range from 7.5% to 41%, according to Nicole D. Ford, PhD, of the Centers for Disease Control and Prevention, Atlanta, and colleagues. Long COVID has shown a significant effect on patients’ quality of life, functional status, and ability to work, but the impact on activity limitation in particular has not been examined, the researchers said.
In a study published in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (MMWR), the researchers reviewed data from surveys conducted between June 1 and 13, 2022, and June 7 and 19, 2023. The data came from the Census Bureau’s Household Pulse Survey (HPS), a cross-sectional national survey designed to measure the social and economic effects of COVID-19 on U.S. households. Surveys were conducted in 2-week cycles (2 weeks on, 2 weeks off). Questions about long COVID were added to the survey beginning on June 1, 2022, and questions about activity limitations from long COVID were added on Sept. 14, 2022, including questions about participants’ abilities to perform daily activities before and after COVID-19 infection.
Overall, the prevalence of long COVID decreased from 7.5% to 6.0% in U.S. adults aged 18 years and older during the study period. However, when stratified by age group, the decline was significant only in adults older than 60 years, and 1 in 10 adults with a history of COVID-19 reported long COVID at the end of the study period.
Among respondents with long COVID, 26.4% of respondents for time period of June 7-19, 2023, reported significant activity limitations, which remained unchanged over time, with no clear pattern in activity limitations across age groups, the researchers said.
Prevalence of long COVID was highest for individuals in middle adulthood (aged 30-39 years, 40-49 years, and 50-59 years) and lowest for younger adults (18-29 years) and older adults (aged 60 years and older). The prevalence of long COVID decreased by 1.16% per survey cycle between the June 1-13 and Jan. 4-16 cycles, but then remained stable, with a decrease of 0.01% per cycle between June 1-13, 2022, and Jan. 4-16, 2023.
Previous studies have shown that activity limitations resulting from long COVID can significantly affect quality of life and functional status, as well as the ability to work or care for others. A recent study in the United Kingdom showed that quality of life scores among long COVID patients were similar to those of individuals with advanced cancer, and more than half of the long COVID patients reported moderately severe functional impairment. “The larger economic and societal impact of long COVID could be far-reaching if working-age adults are unable to maintain employment or care for children or aging parents,” the researchers said.
The current study findings were limited by several factors including potential coverage bias in the survey sample, the relatively low survey response rate, and the inability to collect data on duration of symptoms, COVID-19 vaccination status, treatment during acute infection, and time since COVID-19 illness; any of these factors could affect the reported prevalence of long COVID, the researchers noted.
However, the results suggest the need for continued attention to COVID-19 prevention efforts, including not only staying current with recommended COVID-19 vaccination, but also planning for symptom management and health care service needs of long COVID patients, they concluded.
More data are needed to tease out patterns
“Physicians and patients are still trying to understand long COVID and its implications for the health of affected individuals,” said Noel Deep, MD, in an interview.
The current study shows a prevalence of long COVID in approximately 11% of COVID patients, which is a significant number, said Dr. Deep, a general internist in private practice in Antigo, Wisc., who was not involved in the study. Dr. Deep also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The study also was useful to illustrate a decline in the incidence of people affected by long COVID symptoms in the United States and in other countries, he said.
Dr. Deep noted that despite the persistent prevalence of long COVID symptoms overall, he was encouraged by the findings that older adults “who tend to have other underlying health conditions that could put them at a higher risk for adverse health outcomes” reported fewer long COVID symptoms than younger adults.
However, he noted that the high incidence of long COVID symptoms in able-bodied individuals in their 30s and 40s may affect their the economic situations as well as their ability to care for elderly relatives and children who might be dependent on them.
“Physicians and other clinicians should be aware of the symptoms and impacts caused by long COVID,” Dr. Deep said in an interview. “These individuals usually present with a myriad of vague and varying symptoms. Physicians should be cognizant of this situation, ask about previous infection with COVID-19, and utilize the resources of long COVID clinics where available,” he said.
Several factors can affect the assessment and management of patients with long COVID symptoms in primary care practices, said Dr. Deep. First and foremost are the time constraints of detailed evaluation and testing, he said.
Second, primary care clinicians need to be aware of the different symptoms that may be indicative of long COVID including fatigue, neurocognitive symptoms such as brain fog or memory disturbance, respiratory symptoms, and cardiovascular symptoms, as well as olfactory and gustatory symptoms. “These symptoms can be confounded by underlying health conditions, especially in elderly individuals,” he noted.
“Recommendations and guidelines are evolving regarding the evaluation and management of patients with long COVID that should help physicians and other clinicians in the future,” said Dr. Deep.
In the meantime, having a high index of suspicion, paying attention to the symptoms described by the patient, and taking a proper history with regard to previous COVID-19 infection should help overcome some of these challenges, he said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose and serves on the Editorial Advisory Board of Internal Medicine News.
Approximately one-quarter of adults who experience long COVID report activity limitations that do not change over time, based on data from national sample of nonhospitalized individuals.
Symptoms of long COVID, an ongoing medical condition that occurs in the wake of COVID-19 infection, include respiratory, neurologic, cardiovascular, or other complications that may last for weeks, months, or years after infection.
Current estimates of the incidence of long COVID in the United States range from 7.5% to 41%, according to Nicole D. Ford, PhD, of the Centers for Disease Control and Prevention, Atlanta, and colleagues. Long COVID has shown a significant effect on patients’ quality of life, functional status, and ability to work, but the impact on activity limitation in particular has not been examined, the researchers said.
In a study published in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (MMWR), the researchers reviewed data from surveys conducted between June 1 and 13, 2022, and June 7 and 19, 2023. The data came from the Census Bureau’s Household Pulse Survey (HPS), a cross-sectional national survey designed to measure the social and economic effects of COVID-19 on U.S. households. Surveys were conducted in 2-week cycles (2 weeks on, 2 weeks off). Questions about long COVID were added to the survey beginning on June 1, 2022, and questions about activity limitations from long COVID were added on Sept. 14, 2022, including questions about participants’ abilities to perform daily activities before and after COVID-19 infection.
Overall, the prevalence of long COVID decreased from 7.5% to 6.0% in U.S. adults aged 18 years and older during the study period. However, when stratified by age group, the decline was significant only in adults older than 60 years, and 1 in 10 adults with a history of COVID-19 reported long COVID at the end of the study period.
Among respondents with long COVID, 26.4% of respondents for time period of June 7-19, 2023, reported significant activity limitations, which remained unchanged over time, with no clear pattern in activity limitations across age groups, the researchers said.
Prevalence of long COVID was highest for individuals in middle adulthood (aged 30-39 years, 40-49 years, and 50-59 years) and lowest for younger adults (18-29 years) and older adults (aged 60 years and older). The prevalence of long COVID decreased by 1.16% per survey cycle between the June 1-13 and Jan. 4-16 cycles, but then remained stable, with a decrease of 0.01% per cycle between June 1-13, 2022, and Jan. 4-16, 2023.
Previous studies have shown that activity limitations resulting from long COVID can significantly affect quality of life and functional status, as well as the ability to work or care for others. A recent study in the United Kingdom showed that quality of life scores among long COVID patients were similar to those of individuals with advanced cancer, and more than half of the long COVID patients reported moderately severe functional impairment. “The larger economic and societal impact of long COVID could be far-reaching if working-age adults are unable to maintain employment or care for children or aging parents,” the researchers said.
The current study findings were limited by several factors including potential coverage bias in the survey sample, the relatively low survey response rate, and the inability to collect data on duration of symptoms, COVID-19 vaccination status, treatment during acute infection, and time since COVID-19 illness; any of these factors could affect the reported prevalence of long COVID, the researchers noted.
However, the results suggest the need for continued attention to COVID-19 prevention efforts, including not only staying current with recommended COVID-19 vaccination, but also planning for symptom management and health care service needs of long COVID patients, they concluded.
More data are needed to tease out patterns
“Physicians and patients are still trying to understand long COVID and its implications for the health of affected individuals,” said Noel Deep, MD, in an interview.
The current study shows a prevalence of long COVID in approximately 11% of COVID patients, which is a significant number, said Dr. Deep, a general internist in private practice in Antigo, Wisc., who was not involved in the study. Dr. Deep also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The study also was useful to illustrate a decline in the incidence of people affected by long COVID symptoms in the United States and in other countries, he said.
Dr. Deep noted that despite the persistent prevalence of long COVID symptoms overall, he was encouraged by the findings that older adults “who tend to have other underlying health conditions that could put them at a higher risk for adverse health outcomes” reported fewer long COVID symptoms than younger adults.
However, he noted that the high incidence of long COVID symptoms in able-bodied individuals in their 30s and 40s may affect their the economic situations as well as their ability to care for elderly relatives and children who might be dependent on them.
“Physicians and other clinicians should be aware of the symptoms and impacts caused by long COVID,” Dr. Deep said in an interview. “These individuals usually present with a myriad of vague and varying symptoms. Physicians should be cognizant of this situation, ask about previous infection with COVID-19, and utilize the resources of long COVID clinics where available,” he said.
Several factors can affect the assessment and management of patients with long COVID symptoms in primary care practices, said Dr. Deep. First and foremost are the time constraints of detailed evaluation and testing, he said.
Second, primary care clinicians need to be aware of the different symptoms that may be indicative of long COVID including fatigue, neurocognitive symptoms such as brain fog or memory disturbance, respiratory symptoms, and cardiovascular symptoms, as well as olfactory and gustatory symptoms. “These symptoms can be confounded by underlying health conditions, especially in elderly individuals,” he noted.
“Recommendations and guidelines are evolving regarding the evaluation and management of patients with long COVID that should help physicians and other clinicians in the future,” said Dr. Deep.
In the meantime, having a high index of suspicion, paying attention to the symptoms described by the patient, and taking a proper history with regard to previous COVID-19 infection should help overcome some of these challenges, he said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose and serves on the Editorial Advisory Board of Internal Medicine News.
Approximately one-quarter of adults who experience long COVID report activity limitations that do not change over time, based on data from national sample of nonhospitalized individuals.
Symptoms of long COVID, an ongoing medical condition that occurs in the wake of COVID-19 infection, include respiratory, neurologic, cardiovascular, or other complications that may last for weeks, months, or years after infection.
Current estimates of the incidence of long COVID in the United States range from 7.5% to 41%, according to Nicole D. Ford, PhD, of the Centers for Disease Control and Prevention, Atlanta, and colleagues. Long COVID has shown a significant effect on patients’ quality of life, functional status, and ability to work, but the impact on activity limitation in particular has not been examined, the researchers said.
In a study published in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (MMWR), the researchers reviewed data from surveys conducted between June 1 and 13, 2022, and June 7 and 19, 2023. The data came from the Census Bureau’s Household Pulse Survey (HPS), a cross-sectional national survey designed to measure the social and economic effects of COVID-19 on U.S. households. Surveys were conducted in 2-week cycles (2 weeks on, 2 weeks off). Questions about long COVID were added to the survey beginning on June 1, 2022, and questions about activity limitations from long COVID were added on Sept. 14, 2022, including questions about participants’ abilities to perform daily activities before and after COVID-19 infection.
Overall, the prevalence of long COVID decreased from 7.5% to 6.0% in U.S. adults aged 18 years and older during the study period. However, when stratified by age group, the decline was significant only in adults older than 60 years, and 1 in 10 adults with a history of COVID-19 reported long COVID at the end of the study period.
Among respondents with long COVID, 26.4% of respondents for time period of June 7-19, 2023, reported significant activity limitations, which remained unchanged over time, with no clear pattern in activity limitations across age groups, the researchers said.
Prevalence of long COVID was highest for individuals in middle adulthood (aged 30-39 years, 40-49 years, and 50-59 years) and lowest for younger adults (18-29 years) and older adults (aged 60 years and older). The prevalence of long COVID decreased by 1.16% per survey cycle between the June 1-13 and Jan. 4-16 cycles, but then remained stable, with a decrease of 0.01% per cycle between June 1-13, 2022, and Jan. 4-16, 2023.
Previous studies have shown that activity limitations resulting from long COVID can significantly affect quality of life and functional status, as well as the ability to work or care for others. A recent study in the United Kingdom showed that quality of life scores among long COVID patients were similar to those of individuals with advanced cancer, and more than half of the long COVID patients reported moderately severe functional impairment. “The larger economic and societal impact of long COVID could be far-reaching if working-age adults are unable to maintain employment or care for children or aging parents,” the researchers said.
The current study findings were limited by several factors including potential coverage bias in the survey sample, the relatively low survey response rate, and the inability to collect data on duration of symptoms, COVID-19 vaccination status, treatment during acute infection, and time since COVID-19 illness; any of these factors could affect the reported prevalence of long COVID, the researchers noted.
However, the results suggest the need for continued attention to COVID-19 prevention efforts, including not only staying current with recommended COVID-19 vaccination, but also planning for symptom management and health care service needs of long COVID patients, they concluded.
More data are needed to tease out patterns
“Physicians and patients are still trying to understand long COVID and its implications for the health of affected individuals,” said Noel Deep, MD, in an interview.
The current study shows a prevalence of long COVID in approximately 11% of COVID patients, which is a significant number, said Dr. Deep, a general internist in private practice in Antigo, Wisc., who was not involved in the study. Dr. Deep also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The study also was useful to illustrate a decline in the incidence of people affected by long COVID symptoms in the United States and in other countries, he said.
Dr. Deep noted that despite the persistent prevalence of long COVID symptoms overall, he was encouraged by the findings that older adults “who tend to have other underlying health conditions that could put them at a higher risk for adverse health outcomes” reported fewer long COVID symptoms than younger adults.
However, he noted that the high incidence of long COVID symptoms in able-bodied individuals in their 30s and 40s may affect their the economic situations as well as their ability to care for elderly relatives and children who might be dependent on them.
“Physicians and other clinicians should be aware of the symptoms and impacts caused by long COVID,” Dr. Deep said in an interview. “These individuals usually present with a myriad of vague and varying symptoms. Physicians should be cognizant of this situation, ask about previous infection with COVID-19, and utilize the resources of long COVID clinics where available,” he said.
Several factors can affect the assessment and management of patients with long COVID symptoms in primary care practices, said Dr. Deep. First and foremost are the time constraints of detailed evaluation and testing, he said.
Second, primary care clinicians need to be aware of the different symptoms that may be indicative of long COVID including fatigue, neurocognitive symptoms such as brain fog or memory disturbance, respiratory symptoms, and cardiovascular symptoms, as well as olfactory and gustatory symptoms. “These symptoms can be confounded by underlying health conditions, especially in elderly individuals,” he noted.
“Recommendations and guidelines are evolving regarding the evaluation and management of patients with long COVID that should help physicians and other clinicians in the future,” said Dr. Deep.
In the meantime, having a high index of suspicion, paying attention to the symptoms described by the patient, and taking a proper history with regard to previous COVID-19 infection should help overcome some of these challenges, he said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose and serves on the Editorial Advisory Board of Internal Medicine News.
FROM MMWR
Do you P.U.I.?
In case you are looking for a place to park your discretionary funds, I have recently learned that nonalcoholic beer is the fastest-growing segment of the beer industry. It is just barely outperforming the strong beer market while the standard beer market is flat. The reasons behind this surge in popularity are unclear. While the general population doesn’t seem to grasp the importance of diet and exercise, there seem to be enough folks who are health conscious to support a demand.
Possibly more important has been the emergence of a couple of small breweries that have been able to produce a nonalcoholic product that actually tastes as good as regular beer, and in some cases even better than the real stuff. In Europe, nonalcoholic beer has become popular as a rehydration drink among athletes. We recently found it everywhere we looked while bicycling in France. The large breweries have taken notice and it is hard to find a restaurant here in Maine that doesn’t offer nonalcoholic beer on its menu.
My history with beer goes back to preadolescence, when my father offered me a sip of his beer. I was never sure of his motive but that taste did not immediately whet my appetite for more. However, when I was in high school, New York State’s drinking age was 18 and beer just became part of growing up.
When I went into practice, my routine of having a can or bottle of beer with dinner presented a problem. When I was on call the odds of having to leave the house and see a patient or two was substantial. Back at the beginning I was never much concerned about having alcohol circulating through my brain but I didn’t want to be exhaling its vapors as I interacted with the parents and nurses. As I got older I became more aware that when I was tired, which was always the case at the end of a long office day, even just a glass of beer might impair my decision making. As a result, I drank only nonalcoholic beer when I was on call. Were I still practicing today this wouldn’t have represented a sacrifice on my part. However, until 5 years ago the nonalcoholic beer was not even a close approximation of the alcohol-containing product.
So this brings me to my question. Do you share any of my concerns about practicing under the influence of alcohol (P.U.I.)? And, if you have any concerns, how do you deal with them?
Do you make a distinction between physical and mental impairment? Would you have a drink if you were only fielding phone calls? Would your decision change if you knew you might be called in to perform surgery or start an intravenous on a premie?
Does the prospect of meeting face to face with your patient/parents change your decision? Is practicing telemedicine under the influence any less concerning to you than seeing patients in your office or the emergency room?
Can you imagine any extenuating circumstances? For example, let’s say you are the only pediatric ENT in your county. While you have office hours 4½ days per week, in effect you are on call 24/7 for emergencies. If you made a decision to never practice under the influence, does that mean you will never drink alcohol?
Am I making too big of a thing out of a can of beer or a glass of wine? We have certainly read concerns about patient safety when cared for by house officers working on schedules that leave them practicing while sleep deprived (P.W.S.D.) You don’t hear anything about physicians’ P.U.I. Is it a real problem? Certainly, with marijuana becoming legal in more states alcohol may not be the only influencer to consider.
In the bigger picture I suspect that P.W.S.D. is the bigger problem both for house officers and practicing physicians but it is time we swept away the cloud of silence around P.U.I and had a frank discussion about both among ourselves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
In case you are looking for a place to park your discretionary funds, I have recently learned that nonalcoholic beer is the fastest-growing segment of the beer industry. It is just barely outperforming the strong beer market while the standard beer market is flat. The reasons behind this surge in popularity are unclear. While the general population doesn’t seem to grasp the importance of diet and exercise, there seem to be enough folks who are health conscious to support a demand.
Possibly more important has been the emergence of a couple of small breweries that have been able to produce a nonalcoholic product that actually tastes as good as regular beer, and in some cases even better than the real stuff. In Europe, nonalcoholic beer has become popular as a rehydration drink among athletes. We recently found it everywhere we looked while bicycling in France. The large breweries have taken notice and it is hard to find a restaurant here in Maine that doesn’t offer nonalcoholic beer on its menu.
My history with beer goes back to preadolescence, when my father offered me a sip of his beer. I was never sure of his motive but that taste did not immediately whet my appetite for more. However, when I was in high school, New York State’s drinking age was 18 and beer just became part of growing up.
When I went into practice, my routine of having a can or bottle of beer with dinner presented a problem. When I was on call the odds of having to leave the house and see a patient or two was substantial. Back at the beginning I was never much concerned about having alcohol circulating through my brain but I didn’t want to be exhaling its vapors as I interacted with the parents and nurses. As I got older I became more aware that when I was tired, which was always the case at the end of a long office day, even just a glass of beer might impair my decision making. As a result, I drank only nonalcoholic beer when I was on call. Were I still practicing today this wouldn’t have represented a sacrifice on my part. However, until 5 years ago the nonalcoholic beer was not even a close approximation of the alcohol-containing product.
So this brings me to my question. Do you share any of my concerns about practicing under the influence of alcohol (P.U.I.)? And, if you have any concerns, how do you deal with them?
Do you make a distinction between physical and mental impairment? Would you have a drink if you were only fielding phone calls? Would your decision change if you knew you might be called in to perform surgery or start an intravenous on a premie?
Does the prospect of meeting face to face with your patient/parents change your decision? Is practicing telemedicine under the influence any less concerning to you than seeing patients in your office or the emergency room?
Can you imagine any extenuating circumstances? For example, let’s say you are the only pediatric ENT in your county. While you have office hours 4½ days per week, in effect you are on call 24/7 for emergencies. If you made a decision to never practice under the influence, does that mean you will never drink alcohol?
Am I making too big of a thing out of a can of beer or a glass of wine? We have certainly read concerns about patient safety when cared for by house officers working on schedules that leave them practicing while sleep deprived (P.W.S.D.) You don’t hear anything about physicians’ P.U.I. Is it a real problem? Certainly, with marijuana becoming legal in more states alcohol may not be the only influencer to consider.
In the bigger picture I suspect that P.W.S.D. is the bigger problem both for house officers and practicing physicians but it is time we swept away the cloud of silence around P.U.I and had a frank discussion about both among ourselves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
In case you are looking for a place to park your discretionary funds, I have recently learned that nonalcoholic beer is the fastest-growing segment of the beer industry. It is just barely outperforming the strong beer market while the standard beer market is flat. The reasons behind this surge in popularity are unclear. While the general population doesn’t seem to grasp the importance of diet and exercise, there seem to be enough folks who are health conscious to support a demand.
Possibly more important has been the emergence of a couple of small breweries that have been able to produce a nonalcoholic product that actually tastes as good as regular beer, and in some cases even better than the real stuff. In Europe, nonalcoholic beer has become popular as a rehydration drink among athletes. We recently found it everywhere we looked while bicycling in France. The large breweries have taken notice and it is hard to find a restaurant here in Maine that doesn’t offer nonalcoholic beer on its menu.
My history with beer goes back to preadolescence, when my father offered me a sip of his beer. I was never sure of his motive but that taste did not immediately whet my appetite for more. However, when I was in high school, New York State’s drinking age was 18 and beer just became part of growing up.
When I went into practice, my routine of having a can or bottle of beer with dinner presented a problem. When I was on call the odds of having to leave the house and see a patient or two was substantial. Back at the beginning I was never much concerned about having alcohol circulating through my brain but I didn’t want to be exhaling its vapors as I interacted with the parents and nurses. As I got older I became more aware that when I was tired, which was always the case at the end of a long office day, even just a glass of beer might impair my decision making. As a result, I drank only nonalcoholic beer when I was on call. Were I still practicing today this wouldn’t have represented a sacrifice on my part. However, until 5 years ago the nonalcoholic beer was not even a close approximation of the alcohol-containing product.
So this brings me to my question. Do you share any of my concerns about practicing under the influence of alcohol (P.U.I.)? And, if you have any concerns, how do you deal with them?
Do you make a distinction between physical and mental impairment? Would you have a drink if you were only fielding phone calls? Would your decision change if you knew you might be called in to perform surgery or start an intravenous on a premie?
Does the prospect of meeting face to face with your patient/parents change your decision? Is practicing telemedicine under the influence any less concerning to you than seeing patients in your office or the emergency room?
Can you imagine any extenuating circumstances? For example, let’s say you are the only pediatric ENT in your county. While you have office hours 4½ days per week, in effect you are on call 24/7 for emergencies. If you made a decision to never practice under the influence, does that mean you will never drink alcohol?
Am I making too big of a thing out of a can of beer or a glass of wine? We have certainly read concerns about patient safety when cared for by house officers working on schedules that leave them practicing while sleep deprived (P.W.S.D.) You don’t hear anything about physicians’ P.U.I. Is it a real problem? Certainly, with marijuana becoming legal in more states alcohol may not be the only influencer to consider.
In the bigger picture I suspect that P.W.S.D. is the bigger problem both for house officers and practicing physicians but it is time we swept away the cloud of silence around P.U.I and had a frank discussion about both among ourselves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Despite recent uptick in cases, leprosy is very rare, expert says
, according to Jose A. Lucar, MD.
“Contrary to historical beliefs, leprosy is not highly contagious,” Dr. Lucar, an infectious disease physician and associate professor of medicine at George Washington University, Washington, said in an interview. “For reasons that have to do with the makeup of genes that affect their immune system, most people are not susceptible to acquire leprosy. It’s really a small percentage of the population. It does require prolonged contact with someone with untreated leprosy – over several months – to acquire an infection. So, the risk from any type of casual contact is low.”
According to a research letter published in the CDC’s Emerging Infectious Diseases, the number of reported leprosy cases has more than doubled in the past decade. Of the 159 new cases reported nationwide in 2020, Florida accounted for about one-fifth of cases, with most limited to the central part of the state. “In the U.S., there have been 150-250 cases reported each year over the past several years,” said Dr. Lucar, who was not affiliated with the research letter. “What seems to have changed is that since 2015, there has been a rise in cases in people who are U.S.-born “In the U.S., there have been 150-250 cases reported each year over the past several years,” said Dr. Lucar, who was not affiliated with the research letter. “What seems to have changed is that since 2015, there has been a rise in cases in people who are U.S.-born," and currently, about one-third of leprosy cases are in individuals born in the United States, he noted.
The research letter described a case of leprosy in a 54-year-old man who worked in landscaping, who sought treatment at a dermatology clinic in Central Florida in 2022 for a painful and progressive erythematous rash. The lesions began on his distal extensor extremities and progressed to involve his trunk and face. According to the report, the man denied any domestic or foreign travel, exposure to armadillos (a known source of transmission), prolonged contact with immigrants from leprosy-endemic countries, or connections with someone known to have leprosy. The authors concluded that the case “adds to the growing body of literature suggesting that central Florida represents an endemic location for leprosy. Travel to this area, even in the absence of other risk factors, should prompt consideration of leprosy in the appropriate clinical context.”
Dr. Lucar said that the mechanism of leprosy transmission is not fully understood, but armadillos, which typically traverse the southern United States, are naturally infected with the bacteria that causes leprosy. “It’s possible that they can spread it to people,” he said. “People who have occupations or hobbies that put them in potential contact with wildlife should avoid any close contact with armadillos. There’s also a discussion of whether [the spike in leprosy cases] may have to do with climate change. That is not yet confirmed. It’s not entirely clear why there’s been a recent rise. It remains an area of investigation.”
Meanwhile, clinicians should keep a high level of suspicion in patients who present with skin lesions compatible with leprosy. “These are typically discolored or numb patches on the skin,” Dr. Lucar said. “This can range from a single or a few lesions to very extensive involvement of the skin. The diminished sensation or loss of sensation within those skin patches is an important sign. There’s a loss of skin color but sometimes they can be reddish.” He emphasized that leprosy “does not spread easily from person to person; casual contact will not spread leprosy. It’s important for the public to understand that.”
Dr. Lucar reported no disclosures.
, according to Jose A. Lucar, MD.
“Contrary to historical beliefs, leprosy is not highly contagious,” Dr. Lucar, an infectious disease physician and associate professor of medicine at George Washington University, Washington, said in an interview. “For reasons that have to do with the makeup of genes that affect their immune system, most people are not susceptible to acquire leprosy. It’s really a small percentage of the population. It does require prolonged contact with someone with untreated leprosy – over several months – to acquire an infection. So, the risk from any type of casual contact is low.”
According to a research letter published in the CDC’s Emerging Infectious Diseases, the number of reported leprosy cases has more than doubled in the past decade. Of the 159 new cases reported nationwide in 2020, Florida accounted for about one-fifth of cases, with most limited to the central part of the state. “In the U.S., there have been 150-250 cases reported each year over the past several years,” said Dr. Lucar, who was not affiliated with the research letter. “What seems to have changed is that since 2015, there has been a rise in cases in people who are U.S.-born “In the U.S., there have been 150-250 cases reported each year over the past several years,” said Dr. Lucar, who was not affiliated with the research letter. “What seems to have changed is that since 2015, there has been a rise in cases in people who are U.S.-born," and currently, about one-third of leprosy cases are in individuals born in the United States, he noted.
The research letter described a case of leprosy in a 54-year-old man who worked in landscaping, who sought treatment at a dermatology clinic in Central Florida in 2022 for a painful and progressive erythematous rash. The lesions began on his distal extensor extremities and progressed to involve his trunk and face. According to the report, the man denied any domestic or foreign travel, exposure to armadillos (a known source of transmission), prolonged contact with immigrants from leprosy-endemic countries, or connections with someone known to have leprosy. The authors concluded that the case “adds to the growing body of literature suggesting that central Florida represents an endemic location for leprosy. Travel to this area, even in the absence of other risk factors, should prompt consideration of leprosy in the appropriate clinical context.”
Dr. Lucar said that the mechanism of leprosy transmission is not fully understood, but armadillos, which typically traverse the southern United States, are naturally infected with the bacteria that causes leprosy. “It’s possible that they can spread it to people,” he said. “People who have occupations or hobbies that put them in potential contact with wildlife should avoid any close contact with armadillos. There’s also a discussion of whether [the spike in leprosy cases] may have to do with climate change. That is not yet confirmed. It’s not entirely clear why there’s been a recent rise. It remains an area of investigation.”
Meanwhile, clinicians should keep a high level of suspicion in patients who present with skin lesions compatible with leprosy. “These are typically discolored or numb patches on the skin,” Dr. Lucar said. “This can range from a single or a few lesions to very extensive involvement of the skin. The diminished sensation or loss of sensation within those skin patches is an important sign. There’s a loss of skin color but sometimes they can be reddish.” He emphasized that leprosy “does not spread easily from person to person; casual contact will not spread leprosy. It’s important for the public to understand that.”
Dr. Lucar reported no disclosures.
, according to Jose A. Lucar, MD.
“Contrary to historical beliefs, leprosy is not highly contagious,” Dr. Lucar, an infectious disease physician and associate professor of medicine at George Washington University, Washington, said in an interview. “For reasons that have to do with the makeup of genes that affect their immune system, most people are not susceptible to acquire leprosy. It’s really a small percentage of the population. It does require prolonged contact with someone with untreated leprosy – over several months – to acquire an infection. So, the risk from any type of casual contact is low.”
According to a research letter published in the CDC’s Emerging Infectious Diseases, the number of reported leprosy cases has more than doubled in the past decade. Of the 159 new cases reported nationwide in 2020, Florida accounted for about one-fifth of cases, with most limited to the central part of the state. “In the U.S., there have been 150-250 cases reported each year over the past several years,” said Dr. Lucar, who was not affiliated with the research letter. “What seems to have changed is that since 2015, there has been a rise in cases in people who are U.S.-born “In the U.S., there have been 150-250 cases reported each year over the past several years,” said Dr. Lucar, who was not affiliated with the research letter. “What seems to have changed is that since 2015, there has been a rise in cases in people who are U.S.-born," and currently, about one-third of leprosy cases are in individuals born in the United States, he noted.
The research letter described a case of leprosy in a 54-year-old man who worked in landscaping, who sought treatment at a dermatology clinic in Central Florida in 2022 for a painful and progressive erythematous rash. The lesions began on his distal extensor extremities and progressed to involve his trunk and face. According to the report, the man denied any domestic or foreign travel, exposure to armadillos (a known source of transmission), prolonged contact with immigrants from leprosy-endemic countries, or connections with someone known to have leprosy. The authors concluded that the case “adds to the growing body of literature suggesting that central Florida represents an endemic location for leprosy. Travel to this area, even in the absence of other risk factors, should prompt consideration of leprosy in the appropriate clinical context.”
Dr. Lucar said that the mechanism of leprosy transmission is not fully understood, but armadillos, which typically traverse the southern United States, are naturally infected with the bacteria that causes leprosy. “It’s possible that they can spread it to people,” he said. “People who have occupations or hobbies that put them in potential contact with wildlife should avoid any close contact with armadillos. There’s also a discussion of whether [the spike in leprosy cases] may have to do with climate change. That is not yet confirmed. It’s not entirely clear why there’s been a recent rise. It remains an area of investigation.”
Meanwhile, clinicians should keep a high level of suspicion in patients who present with skin lesions compatible with leprosy. “These are typically discolored or numb patches on the skin,” Dr. Lucar said. “This can range from a single or a few lesions to very extensive involvement of the skin. The diminished sensation or loss of sensation within those skin patches is an important sign. There’s a loss of skin color but sometimes they can be reddish.” He emphasized that leprosy “does not spread easily from person to person; casual contact will not spread leprosy. It’s important for the public to understand that.”
Dr. Lucar reported no disclosures.
Low-dose colchicine for ASCVD: Your questions answered
This transcript has been edited for clarity.
Dr. O’Donoghue: We’re going to discuss a very important and emerging topic, which is the use of low-dose colchicine. I think there’s much interest in the use of this drug, which now has a Food and Drug Administration indication, which we’ll talk about further, and it’s also been written into both European and American guidelines that have been recently released.
who’s been at the forefront of research into anti-inflammatory therapeutics.
Lifestyle lipid-lowering paramount
Dr. O’Donoghue: As we think about the concept behind the use of colchicine, we’ve obviously done a large amount of research into lipid-lowering drugs, but where does colchicine now fit in?
Dr. Ridker: Let’s make sure we get the basics down. Anti-inflammatory therapy is going to be added on top of quality other care. This is not a replacement for lipids; it’s not a change in diet, exercise, and smoking cessation. The new data are really telling us that a patient who’s aggressively treated to guideline-recommended levels can still do much better in terms of preventing heart attack, stroke, cardiovascular death, and revascularization by adding low-dose colchicine as the first proven anti-inflammatory therapy for atherosclerotic disease.
I have to say, Michelle, for me, it’s been a wonderful end of a journey in many ways. This story starts almost 30 years ago for quite a few of us, thinking about inflammation and atherosclerosis. The whole C-reactive protein (CRP) story is still an ongoing one. We recently showed, for example, that residual inflammatory risk in some 30,000 patients, all taking a statin, was a far better predictor of the likelihood of more cardiovascular events, in particular cardiovascular death, than was residual cholesterol risk.
Think about that. We’re all aggressively giving second lipid-lowering drugs in our very sick patients, but that means inflammation is really the untapped piece of this.
The two clinical trials we have in front of us, the COLCOT trial and the LoDoCo2 trial – both New England Journal of Medicine papers, both with roughly 5,000 patients – provide very clear evidence that following a relatively recent myocardial infarction (that’s COLCOT) in chronic stable atherosclerosis (that’s LoDoCo2), we’re getting 25%-30% relative risk reductions in major adverse cardiovascular events (MACEs) on top of aggressive statin therapy. That’s a big deal. It’s safe, it works, and it’s fully consistent with all the information we have about inflammation being part and parcel of atherosclerosis. It’s a pretty exciting time.
Inflammatory pathway
Dr. O’Donoghue: It beautifully proves the inflammatory hypothesis in many ways. You led CANTOS, and that was a much more specific target. Here, in terms of the effects of colchicine, what do we know about how it may work on the inflammatory cascade?
Dr. Ridker: Our CANTOS trial was proof of principle that you could directly target, with a very specific monoclonal antibody, a specific piece of this innate immune cascade and lower cardiovascular event rates.
Colchicine is a more broad-spectrum drug. It does have a number of antineutrophil effects – that’s important, by the way. Neutrophils are really becoming very important in atherosclerotic disease progression. It’s an indirect inhibitor of the so-called NLRP3 inflammasome, which is where both interleukin-1 (that’s the target for canakinumab) and IL-6 are up-regulated. As you know, it’s been used to treat gout and pericarditis in high doses in short, little bursts.
The change here is this use of low-dose colchicine, that’s 0.5 mg once a day for years to treat chronic, stable atherosclerosis. It is very much like using a statin. The idea here is to prevent the progression of the disease by slowing down and maybe stabilizing the plaque so we have fewer heart attacks and strokes down the road.
It’s entering the armamentarium – at least my armamentarium – as chronic, stable secondary prevention. That’s where the new American College of Cardiology/American Heart Association guidelines also put it. It’s really in as a treatment for chronic, stable atherosclerosis. I think that’s where it belongs.
When to start colchicine, and in whom?
Dr. O’Donoghue: To that point, as we think about the efficacy, I think it’s nice, as you outlined, that we have two complementary trials that are both showing a consistent reduction in MACEs, one in the post–acute coronary syndrome (ACS) state and one for more chronic patients.
At what point do you think would be the appropriate time to start therapy, and who would you be starting it for?
Dr. Ridker: Michelle, that’s a great question. There’s a very interesting analysis that just came out from the LoDoCo2 investigators. It’s kind of a landmark analysis. What they show is that 1 year, 2 years, 3 years, and 4 years since the initiating myocardial infarction, the drug is very effective.
In fact, you could think about starting this drug at your clinic in patients with chronic, stable atherosclerotic disease. That’s just like we would start a statin in people who had a heart attack some time ago, and that’s absolutely fine.
I’m using it for what I call my frequent fliers, those patients who just keep coming back. They’re already on aggressive lipid-lowering therapy. I have them on beta-blockers, aspirin, and all the usual things. I say, look, I can get a large risk reduction by starting them on this drug.
There are a few caveats, Michelle. Like all drugs, colchicine comes with some adverse effects. Most of them are pretty rare, but there are some patients I would not give this drug to, just to be very clear. Colchicine is cleared by the kidney and by the liver. Patients who have severe chronic kidney disease and severe liver disease – this is a no-go for those patients. We should talk about where patients in that realm might want to go.
Then there are some unusual drugs. Colchicine is metabolized by the CYP3A4 and the P-glycoprotein pathway. There are a few drugs, such as ketoconazole, fluconazole, and cyclosporine, that if your primary care doctor or internist is going to start for a short term, you probably want to stop your colchicine for a week or two.
In people with familial Mediterranean fever, for whom colchicine is lifesaving and life-changing and who take it for 20, 30, or 40 years, there’s been no increase in risk for cancer. There have been very few adverse effects. I think it’s interesting that we, who practice in North America, basically never see familial Mediterranean fever. If we were practicing in Lebanon, Israel, or North Africa, this would be a very common therapy that we’d all be extremely familiar with.
Dr. O’Donoghue: To that point, it’s interesting to hear that colchicine was even used by the ancient Greeks and ancient Egyptians. It’s a drug that’s been around for a long time.
In terms of its safety, some people have been talking about the fact that an increase in noncardiovascular death was seen in LoDoCo2. What are your thoughts on that? Is that anything that we should be concerned about?
Colchicine safety and contraindications
Dr. Ridker: First, to set the record straight, a meta-analysis has been done of all-cause mortality in the various colchicine trials, and the hazard ratio is 1.04. I’ll remind you, and all of us know, that the hazard ratios for all-cause mortality in the PCSK9 trials, the bempedoic acid trials, and the ezetimibe trials are also essentially neutral. We’re in a state where we don’t let these trials roll long enough to see benefits necessarily on all-cause mortality. Some of us think we probably should, but that’s just the reality of trials.
One of most interesting things that was part of the FDA review, I suspect, was that there was no specific cause of any of this. It was not like there was a set of particular issues. I suspect that most people think this is probably the play of chance and with time, things will get better.
Again, I do want to emphasize this is not a drug for severe chronic kidney disease and severe liver disease, because those patients will get in trouble with this. The other thing that’s worth knowing is when you start a patient on low-dose colchicine – that’s 0.5 mg/d – there will be some patients who get some short-term gastrointestinal upset. That’s very common when you start colchicine at the much higher doses you might use to treat acute gout or pericarditis. In these trials, the vast majority of patients treated through that, and there were very few episodes long-term. I think it’s generally safe. That’s where we’re at.
Dr. O’Donoghue: Paul, you’ve been a leader, certainly, at looking at CRP as a marker of inflammation. Do you, in your practice, consider CRP levels when making a decision about who is appropriate for this therapy?
Dr. Ridker: That’s another terrific question. I do, because I’m trying to distinguish in my own mind patients who have residual inflammatory risk, in whom the high-sensitivity CRP (hsCRP) level remains high despite being on statins versus those with residual cholesterol risk, in whom I’m really predominantly worried about LDL cholesterol, that I haven’t brought it down far enough.
I do measure it, and if the CRP remains high and the LDL cholesterol is low, to me, that’s residual inflammatory risk and that’s the patient I would target this to. Conversely, if the LDL cholesterol was still, say, above some threshold of 75-100 and I’m worried about that, even if the CRP is low, I’ll probably add a second lipid-lowering drug.
The complexity of this, however, is that CRP was not measured in either LoDoCo2 or COLCOT. That’s mostly because they didn’t have much funding. These trials were done really on a shoestring. They were not sponsored by major pharma at all. We know that the median hsCRP in these trials was probably around 3.5-4 mg/L so I’m pretty comfortable doing that. Others have just advocated giving it to many patients. I must say I like to use biomarkers to think through the biology and who might have the best benefit-to-risk ratio. In my practice, I am doing it that way.
Inpatient vs. outpatient initiation
Dr. O’Donoghue: This is perhaps my last question for you before we wrap up. I know you talked about use of low-dose colchicine for patients with more chronic, stable coronary disease. Now obviously, COLCOT studied patients who were early post ACS, and there we certainly think about the anti-inflammatory effects as potentially having more benefit. What are your thoughts about early initiation of colchicine in that setting, the acute hospitalized setting? Do you think it’s more appropriate for an outpatient start?
Dr. Ridker: Today, I think this is all about chronic, stable atherosclerosis. Yes, COLCOT enrolled their patients within 30 days of a recent myocardial infarction, but as we all know, that’s a pretty stable phase. The vast majority were enrolled after 15 days. There were a small number enrolled within 3 days or something like that, but the benefit is about the same in all these patients.
Conversely, there’s been a small number of trials looking at colchicine in acute coronary ischemia and they’ve not been terribly promising. That makes some sense, though, right? We want to get an artery open. In acute ischemia, that’s about revascularization. It’s about oxygenation. It’s about reperfusion injury. My guess is that 3, 4, 5, or 6 days later, when it becomes a stable situation, is when the drug is probably effective.
Again, there will be some ongoing true intervention trials with large sample sizes for acute coronary ischemia. We don’t have those yet. Right now, I think it’s a therapy for chronic, stable angina. That’s many of our patients.
I would say that if you compare the relative benefit in these trials of adding ezetimibe to a statin, that’s a 5% or 6% benefit. For PCSK9 inhibitors – we all use them – it’s about a 15% benefit. These are 25%-30% risk reductions. If we’re going to think about what’s the next drug to give on top of the statin, serious consideration should be given to low-dose colchicine.
Let me also emphasize that this is not an either/or situation. This is about the fact that we now understand atherosclerosis to be a disorder both of lipid accumulation and a proinflammatory systemic response. We can give these drugs together. I suspect that the best patient care is going to be very aggressive lipid-lowering combined with pretty aggressive inflammation inhibition. I suspect that, down the road, that’s where all of us are going to be.
Dr. O’Donoghue: Thank you so much, Paul, for walking us through that today. I think it was a very nice, succinct review of the evidence, and then also just getting our minds more accustomed to the concept that we can now start to target more orthogonal axes that really get at the pathobiology of what’s going on in the atherosclerotic plaque. I think it’s an important topic.
Dr. O’Donoghue is an associate professor of medicine at Harvard Medical School and an associate physician at Brigham and Women’s Hospital, both in Boston. Dr. Ridker is director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital. Both Dr. O’Donoghue and Dr. Ridker reported numerous conflicts of interest.
This transcript has been edited for clarity.
Dr. O’Donoghue: We’re going to discuss a very important and emerging topic, which is the use of low-dose colchicine. I think there’s much interest in the use of this drug, which now has a Food and Drug Administration indication, which we’ll talk about further, and it’s also been written into both European and American guidelines that have been recently released.
who’s been at the forefront of research into anti-inflammatory therapeutics.
Lifestyle lipid-lowering paramount
Dr. O’Donoghue: As we think about the concept behind the use of colchicine, we’ve obviously done a large amount of research into lipid-lowering drugs, but where does colchicine now fit in?
Dr. Ridker: Let’s make sure we get the basics down. Anti-inflammatory therapy is going to be added on top of quality other care. This is not a replacement for lipids; it’s not a change in diet, exercise, and smoking cessation. The new data are really telling us that a patient who’s aggressively treated to guideline-recommended levels can still do much better in terms of preventing heart attack, stroke, cardiovascular death, and revascularization by adding low-dose colchicine as the first proven anti-inflammatory therapy for atherosclerotic disease.
I have to say, Michelle, for me, it’s been a wonderful end of a journey in many ways. This story starts almost 30 years ago for quite a few of us, thinking about inflammation and atherosclerosis. The whole C-reactive protein (CRP) story is still an ongoing one. We recently showed, for example, that residual inflammatory risk in some 30,000 patients, all taking a statin, was a far better predictor of the likelihood of more cardiovascular events, in particular cardiovascular death, than was residual cholesterol risk.
Think about that. We’re all aggressively giving second lipid-lowering drugs in our very sick patients, but that means inflammation is really the untapped piece of this.
The two clinical trials we have in front of us, the COLCOT trial and the LoDoCo2 trial – both New England Journal of Medicine papers, both with roughly 5,000 patients – provide very clear evidence that following a relatively recent myocardial infarction (that’s COLCOT) in chronic stable atherosclerosis (that’s LoDoCo2), we’re getting 25%-30% relative risk reductions in major adverse cardiovascular events (MACEs) on top of aggressive statin therapy. That’s a big deal. It’s safe, it works, and it’s fully consistent with all the information we have about inflammation being part and parcel of atherosclerosis. It’s a pretty exciting time.
Inflammatory pathway
Dr. O’Donoghue: It beautifully proves the inflammatory hypothesis in many ways. You led CANTOS, and that was a much more specific target. Here, in terms of the effects of colchicine, what do we know about how it may work on the inflammatory cascade?
Dr. Ridker: Our CANTOS trial was proof of principle that you could directly target, with a very specific monoclonal antibody, a specific piece of this innate immune cascade and lower cardiovascular event rates.
Colchicine is a more broad-spectrum drug. It does have a number of antineutrophil effects – that’s important, by the way. Neutrophils are really becoming very important in atherosclerotic disease progression. It’s an indirect inhibitor of the so-called NLRP3 inflammasome, which is where both interleukin-1 (that’s the target for canakinumab) and IL-6 are up-regulated. As you know, it’s been used to treat gout and pericarditis in high doses in short, little bursts.
The change here is this use of low-dose colchicine, that’s 0.5 mg once a day for years to treat chronic, stable atherosclerosis. It is very much like using a statin. The idea here is to prevent the progression of the disease by slowing down and maybe stabilizing the plaque so we have fewer heart attacks and strokes down the road.
It’s entering the armamentarium – at least my armamentarium – as chronic, stable secondary prevention. That’s where the new American College of Cardiology/American Heart Association guidelines also put it. It’s really in as a treatment for chronic, stable atherosclerosis. I think that’s where it belongs.
When to start colchicine, and in whom?
Dr. O’Donoghue: To that point, as we think about the efficacy, I think it’s nice, as you outlined, that we have two complementary trials that are both showing a consistent reduction in MACEs, one in the post–acute coronary syndrome (ACS) state and one for more chronic patients.
At what point do you think would be the appropriate time to start therapy, and who would you be starting it for?
Dr. Ridker: Michelle, that’s a great question. There’s a very interesting analysis that just came out from the LoDoCo2 investigators. It’s kind of a landmark analysis. What they show is that 1 year, 2 years, 3 years, and 4 years since the initiating myocardial infarction, the drug is very effective.
In fact, you could think about starting this drug at your clinic in patients with chronic, stable atherosclerotic disease. That’s just like we would start a statin in people who had a heart attack some time ago, and that’s absolutely fine.
I’m using it for what I call my frequent fliers, those patients who just keep coming back. They’re already on aggressive lipid-lowering therapy. I have them on beta-blockers, aspirin, and all the usual things. I say, look, I can get a large risk reduction by starting them on this drug.
There are a few caveats, Michelle. Like all drugs, colchicine comes with some adverse effects. Most of them are pretty rare, but there are some patients I would not give this drug to, just to be very clear. Colchicine is cleared by the kidney and by the liver. Patients who have severe chronic kidney disease and severe liver disease – this is a no-go for those patients. We should talk about where patients in that realm might want to go.
Then there are some unusual drugs. Colchicine is metabolized by the CYP3A4 and the P-glycoprotein pathway. There are a few drugs, such as ketoconazole, fluconazole, and cyclosporine, that if your primary care doctor or internist is going to start for a short term, you probably want to stop your colchicine for a week or two.
In people with familial Mediterranean fever, for whom colchicine is lifesaving and life-changing and who take it for 20, 30, or 40 years, there’s been no increase in risk for cancer. There have been very few adverse effects. I think it’s interesting that we, who practice in North America, basically never see familial Mediterranean fever. If we were practicing in Lebanon, Israel, or North Africa, this would be a very common therapy that we’d all be extremely familiar with.
Dr. O’Donoghue: To that point, it’s interesting to hear that colchicine was even used by the ancient Greeks and ancient Egyptians. It’s a drug that’s been around for a long time.
In terms of its safety, some people have been talking about the fact that an increase in noncardiovascular death was seen in LoDoCo2. What are your thoughts on that? Is that anything that we should be concerned about?
Colchicine safety and contraindications
Dr. Ridker: First, to set the record straight, a meta-analysis has been done of all-cause mortality in the various colchicine trials, and the hazard ratio is 1.04. I’ll remind you, and all of us know, that the hazard ratios for all-cause mortality in the PCSK9 trials, the bempedoic acid trials, and the ezetimibe trials are also essentially neutral. We’re in a state where we don’t let these trials roll long enough to see benefits necessarily on all-cause mortality. Some of us think we probably should, but that’s just the reality of trials.
One of most interesting things that was part of the FDA review, I suspect, was that there was no specific cause of any of this. It was not like there was a set of particular issues. I suspect that most people think this is probably the play of chance and with time, things will get better.
Again, I do want to emphasize this is not a drug for severe chronic kidney disease and severe liver disease, because those patients will get in trouble with this. The other thing that’s worth knowing is when you start a patient on low-dose colchicine – that’s 0.5 mg/d – there will be some patients who get some short-term gastrointestinal upset. That’s very common when you start colchicine at the much higher doses you might use to treat acute gout or pericarditis. In these trials, the vast majority of patients treated through that, and there were very few episodes long-term. I think it’s generally safe. That’s where we’re at.
Dr. O’Donoghue: Paul, you’ve been a leader, certainly, at looking at CRP as a marker of inflammation. Do you, in your practice, consider CRP levels when making a decision about who is appropriate for this therapy?
Dr. Ridker: That’s another terrific question. I do, because I’m trying to distinguish in my own mind patients who have residual inflammatory risk, in whom the high-sensitivity CRP (hsCRP) level remains high despite being on statins versus those with residual cholesterol risk, in whom I’m really predominantly worried about LDL cholesterol, that I haven’t brought it down far enough.
I do measure it, and if the CRP remains high and the LDL cholesterol is low, to me, that’s residual inflammatory risk and that’s the patient I would target this to. Conversely, if the LDL cholesterol was still, say, above some threshold of 75-100 and I’m worried about that, even if the CRP is low, I’ll probably add a second lipid-lowering drug.
The complexity of this, however, is that CRP was not measured in either LoDoCo2 or COLCOT. That’s mostly because they didn’t have much funding. These trials were done really on a shoestring. They were not sponsored by major pharma at all. We know that the median hsCRP in these trials was probably around 3.5-4 mg/L so I’m pretty comfortable doing that. Others have just advocated giving it to many patients. I must say I like to use biomarkers to think through the biology and who might have the best benefit-to-risk ratio. In my practice, I am doing it that way.
Inpatient vs. outpatient initiation
Dr. O’Donoghue: This is perhaps my last question for you before we wrap up. I know you talked about use of low-dose colchicine for patients with more chronic, stable coronary disease. Now obviously, COLCOT studied patients who were early post ACS, and there we certainly think about the anti-inflammatory effects as potentially having more benefit. What are your thoughts about early initiation of colchicine in that setting, the acute hospitalized setting? Do you think it’s more appropriate for an outpatient start?
Dr. Ridker: Today, I think this is all about chronic, stable atherosclerosis. Yes, COLCOT enrolled their patients within 30 days of a recent myocardial infarction, but as we all know, that’s a pretty stable phase. The vast majority were enrolled after 15 days. There were a small number enrolled within 3 days or something like that, but the benefit is about the same in all these patients.
Conversely, there’s been a small number of trials looking at colchicine in acute coronary ischemia and they’ve not been terribly promising. That makes some sense, though, right? We want to get an artery open. In acute ischemia, that’s about revascularization. It’s about oxygenation. It’s about reperfusion injury. My guess is that 3, 4, 5, or 6 days later, when it becomes a stable situation, is when the drug is probably effective.
Again, there will be some ongoing true intervention trials with large sample sizes for acute coronary ischemia. We don’t have those yet. Right now, I think it’s a therapy for chronic, stable angina. That’s many of our patients.
I would say that if you compare the relative benefit in these trials of adding ezetimibe to a statin, that’s a 5% or 6% benefit. For PCSK9 inhibitors – we all use them – it’s about a 15% benefit. These are 25%-30% risk reductions. If we’re going to think about what’s the next drug to give on top of the statin, serious consideration should be given to low-dose colchicine.
Let me also emphasize that this is not an either/or situation. This is about the fact that we now understand atherosclerosis to be a disorder both of lipid accumulation and a proinflammatory systemic response. We can give these drugs together. I suspect that the best patient care is going to be very aggressive lipid-lowering combined with pretty aggressive inflammation inhibition. I suspect that, down the road, that’s where all of us are going to be.
Dr. O’Donoghue: Thank you so much, Paul, for walking us through that today. I think it was a very nice, succinct review of the evidence, and then also just getting our minds more accustomed to the concept that we can now start to target more orthogonal axes that really get at the pathobiology of what’s going on in the atherosclerotic plaque. I think it’s an important topic.
Dr. O’Donoghue is an associate professor of medicine at Harvard Medical School and an associate physician at Brigham and Women’s Hospital, both in Boston. Dr. Ridker is director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital. Both Dr. O’Donoghue and Dr. Ridker reported numerous conflicts of interest.
This transcript has been edited for clarity.
Dr. O’Donoghue: We’re going to discuss a very important and emerging topic, which is the use of low-dose colchicine. I think there’s much interest in the use of this drug, which now has a Food and Drug Administration indication, which we’ll talk about further, and it’s also been written into both European and American guidelines that have been recently released.
who’s been at the forefront of research into anti-inflammatory therapeutics.
Lifestyle lipid-lowering paramount
Dr. O’Donoghue: As we think about the concept behind the use of colchicine, we’ve obviously done a large amount of research into lipid-lowering drugs, but where does colchicine now fit in?
Dr. Ridker: Let’s make sure we get the basics down. Anti-inflammatory therapy is going to be added on top of quality other care. This is not a replacement for lipids; it’s not a change in diet, exercise, and smoking cessation. The new data are really telling us that a patient who’s aggressively treated to guideline-recommended levels can still do much better in terms of preventing heart attack, stroke, cardiovascular death, and revascularization by adding low-dose colchicine as the first proven anti-inflammatory therapy for atherosclerotic disease.
I have to say, Michelle, for me, it’s been a wonderful end of a journey in many ways. This story starts almost 30 years ago for quite a few of us, thinking about inflammation and atherosclerosis. The whole C-reactive protein (CRP) story is still an ongoing one. We recently showed, for example, that residual inflammatory risk in some 30,000 patients, all taking a statin, was a far better predictor of the likelihood of more cardiovascular events, in particular cardiovascular death, than was residual cholesterol risk.
Think about that. We’re all aggressively giving second lipid-lowering drugs in our very sick patients, but that means inflammation is really the untapped piece of this.
The two clinical trials we have in front of us, the COLCOT trial and the LoDoCo2 trial – both New England Journal of Medicine papers, both with roughly 5,000 patients – provide very clear evidence that following a relatively recent myocardial infarction (that’s COLCOT) in chronic stable atherosclerosis (that’s LoDoCo2), we’re getting 25%-30% relative risk reductions in major adverse cardiovascular events (MACEs) on top of aggressive statin therapy. That’s a big deal. It’s safe, it works, and it’s fully consistent with all the information we have about inflammation being part and parcel of atherosclerosis. It’s a pretty exciting time.
Inflammatory pathway
Dr. O’Donoghue: It beautifully proves the inflammatory hypothesis in many ways. You led CANTOS, and that was a much more specific target. Here, in terms of the effects of colchicine, what do we know about how it may work on the inflammatory cascade?
Dr. Ridker: Our CANTOS trial was proof of principle that you could directly target, with a very specific monoclonal antibody, a specific piece of this innate immune cascade and lower cardiovascular event rates.
Colchicine is a more broad-spectrum drug. It does have a number of antineutrophil effects – that’s important, by the way. Neutrophils are really becoming very important in atherosclerotic disease progression. It’s an indirect inhibitor of the so-called NLRP3 inflammasome, which is where both interleukin-1 (that’s the target for canakinumab) and IL-6 are up-regulated. As you know, it’s been used to treat gout and pericarditis in high doses in short, little bursts.
The change here is this use of low-dose colchicine, that’s 0.5 mg once a day for years to treat chronic, stable atherosclerosis. It is very much like using a statin. The idea here is to prevent the progression of the disease by slowing down and maybe stabilizing the plaque so we have fewer heart attacks and strokes down the road.
It’s entering the armamentarium – at least my armamentarium – as chronic, stable secondary prevention. That’s where the new American College of Cardiology/American Heart Association guidelines also put it. It’s really in as a treatment for chronic, stable atherosclerosis. I think that’s where it belongs.
When to start colchicine, and in whom?
Dr. O’Donoghue: To that point, as we think about the efficacy, I think it’s nice, as you outlined, that we have two complementary trials that are both showing a consistent reduction in MACEs, one in the post–acute coronary syndrome (ACS) state and one for more chronic patients.
At what point do you think would be the appropriate time to start therapy, and who would you be starting it for?
Dr. Ridker: Michelle, that’s a great question. There’s a very interesting analysis that just came out from the LoDoCo2 investigators. It’s kind of a landmark analysis. What they show is that 1 year, 2 years, 3 years, and 4 years since the initiating myocardial infarction, the drug is very effective.
In fact, you could think about starting this drug at your clinic in patients with chronic, stable atherosclerotic disease. That’s just like we would start a statin in people who had a heart attack some time ago, and that’s absolutely fine.
I’m using it for what I call my frequent fliers, those patients who just keep coming back. They’re already on aggressive lipid-lowering therapy. I have them on beta-blockers, aspirin, and all the usual things. I say, look, I can get a large risk reduction by starting them on this drug.
There are a few caveats, Michelle. Like all drugs, colchicine comes with some adverse effects. Most of them are pretty rare, but there are some patients I would not give this drug to, just to be very clear. Colchicine is cleared by the kidney and by the liver. Patients who have severe chronic kidney disease and severe liver disease – this is a no-go for those patients. We should talk about where patients in that realm might want to go.
Then there are some unusual drugs. Colchicine is metabolized by the CYP3A4 and the P-glycoprotein pathway. There are a few drugs, such as ketoconazole, fluconazole, and cyclosporine, that if your primary care doctor or internist is going to start for a short term, you probably want to stop your colchicine for a week or two.
In people with familial Mediterranean fever, for whom colchicine is lifesaving and life-changing and who take it for 20, 30, or 40 years, there’s been no increase in risk for cancer. There have been very few adverse effects. I think it’s interesting that we, who practice in North America, basically never see familial Mediterranean fever. If we were practicing in Lebanon, Israel, or North Africa, this would be a very common therapy that we’d all be extremely familiar with.
Dr. O’Donoghue: To that point, it’s interesting to hear that colchicine was even used by the ancient Greeks and ancient Egyptians. It’s a drug that’s been around for a long time.
In terms of its safety, some people have been talking about the fact that an increase in noncardiovascular death was seen in LoDoCo2. What are your thoughts on that? Is that anything that we should be concerned about?
Colchicine safety and contraindications
Dr. Ridker: First, to set the record straight, a meta-analysis has been done of all-cause mortality in the various colchicine trials, and the hazard ratio is 1.04. I’ll remind you, and all of us know, that the hazard ratios for all-cause mortality in the PCSK9 trials, the bempedoic acid trials, and the ezetimibe trials are also essentially neutral. We’re in a state where we don’t let these trials roll long enough to see benefits necessarily on all-cause mortality. Some of us think we probably should, but that’s just the reality of trials.
One of most interesting things that was part of the FDA review, I suspect, was that there was no specific cause of any of this. It was not like there was a set of particular issues. I suspect that most people think this is probably the play of chance and with time, things will get better.
Again, I do want to emphasize this is not a drug for severe chronic kidney disease and severe liver disease, because those patients will get in trouble with this. The other thing that’s worth knowing is when you start a patient on low-dose colchicine – that’s 0.5 mg/d – there will be some patients who get some short-term gastrointestinal upset. That’s very common when you start colchicine at the much higher doses you might use to treat acute gout or pericarditis. In these trials, the vast majority of patients treated through that, and there were very few episodes long-term. I think it’s generally safe. That’s where we’re at.
Dr. O’Donoghue: Paul, you’ve been a leader, certainly, at looking at CRP as a marker of inflammation. Do you, in your practice, consider CRP levels when making a decision about who is appropriate for this therapy?
Dr. Ridker: That’s another terrific question. I do, because I’m trying to distinguish in my own mind patients who have residual inflammatory risk, in whom the high-sensitivity CRP (hsCRP) level remains high despite being on statins versus those with residual cholesterol risk, in whom I’m really predominantly worried about LDL cholesterol, that I haven’t brought it down far enough.
I do measure it, and if the CRP remains high and the LDL cholesterol is low, to me, that’s residual inflammatory risk and that’s the patient I would target this to. Conversely, if the LDL cholesterol was still, say, above some threshold of 75-100 and I’m worried about that, even if the CRP is low, I’ll probably add a second lipid-lowering drug.
The complexity of this, however, is that CRP was not measured in either LoDoCo2 or COLCOT. That’s mostly because they didn’t have much funding. These trials were done really on a shoestring. They were not sponsored by major pharma at all. We know that the median hsCRP in these trials was probably around 3.5-4 mg/L so I’m pretty comfortable doing that. Others have just advocated giving it to many patients. I must say I like to use biomarkers to think through the biology and who might have the best benefit-to-risk ratio. In my practice, I am doing it that way.
Inpatient vs. outpatient initiation
Dr. O’Donoghue: This is perhaps my last question for you before we wrap up. I know you talked about use of low-dose colchicine for patients with more chronic, stable coronary disease. Now obviously, COLCOT studied patients who were early post ACS, and there we certainly think about the anti-inflammatory effects as potentially having more benefit. What are your thoughts about early initiation of colchicine in that setting, the acute hospitalized setting? Do you think it’s more appropriate for an outpatient start?
Dr. Ridker: Today, I think this is all about chronic, stable atherosclerosis. Yes, COLCOT enrolled their patients within 30 days of a recent myocardial infarction, but as we all know, that’s a pretty stable phase. The vast majority were enrolled after 15 days. There were a small number enrolled within 3 days or something like that, but the benefit is about the same in all these patients.
Conversely, there’s been a small number of trials looking at colchicine in acute coronary ischemia and they’ve not been terribly promising. That makes some sense, though, right? We want to get an artery open. In acute ischemia, that’s about revascularization. It’s about oxygenation. It’s about reperfusion injury. My guess is that 3, 4, 5, or 6 days later, when it becomes a stable situation, is when the drug is probably effective.
Again, there will be some ongoing true intervention trials with large sample sizes for acute coronary ischemia. We don’t have those yet. Right now, I think it’s a therapy for chronic, stable angina. That’s many of our patients.
I would say that if you compare the relative benefit in these trials of adding ezetimibe to a statin, that’s a 5% or 6% benefit. For PCSK9 inhibitors – we all use them – it’s about a 15% benefit. These are 25%-30% risk reductions. If we’re going to think about what’s the next drug to give on top of the statin, serious consideration should be given to low-dose colchicine.
Let me also emphasize that this is not an either/or situation. This is about the fact that we now understand atherosclerosis to be a disorder both of lipid accumulation and a proinflammatory systemic response. We can give these drugs together. I suspect that the best patient care is going to be very aggressive lipid-lowering combined with pretty aggressive inflammation inhibition. I suspect that, down the road, that’s where all of us are going to be.
Dr. O’Donoghue: Thank you so much, Paul, for walking us through that today. I think it was a very nice, succinct review of the evidence, and then also just getting our minds more accustomed to the concept that we can now start to target more orthogonal axes that really get at the pathobiology of what’s going on in the atherosclerotic plaque. I think it’s an important topic.
Dr. O’Donoghue is an associate professor of medicine at Harvard Medical School and an associate physician at Brigham and Women’s Hospital, both in Boston. Dr. Ridker is director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital. Both Dr. O’Donoghue and Dr. Ridker reported numerous conflicts of interest.