User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
CDC offers guidance on RSV vaccines for adults
Two newly approved respiratory syncytial virus (RSV) vaccines for adults aged 60 years and older may be able to prevent illness in those at risk for severe RSV disease.
Most adult RSV illness occurs among the older age group and results in an estimated 60,000-160,000 hospitalizations and 6,000-10,000 deaths per year among people aged at least 65 years.
Older adults deciding whether to get the vaccines should weigh risks and their own preferences and make the decision in consultation with their clinicians, said authors of a Centers for Disease Control and Prevention report.
Michael Melgar, MD, with the Coronavirus and Other Respiratory Viruses Division at the CDC, was lead author on the report, published in the Morbidity and Mortality Weekly Report.
Two new vaccines
In May, the Food and Drug Administration approved the first of two vaccines for preventing RSV lower respiratory tract disease for adults aged at least 60 years.
On June 21, the Advisory Committee on Immunization Practices (ACIP) recommended that people in that age group receive a single dose of RSV vaccine using shared decision-making.
The recommendation for shared decision-making makes the ACIP decision different from routine and risk-based vaccine recommendations. Rather than targeting all in a particular age group or risk group, the decision calls for consideration of a patients’ risk for disease and their characteristics, preferences, and values; the health care professional’s clinical discretion; and performance of the vaccine.
Dr. Melgar and colleagues reported that vaccination with one dose of the GSK or Pfizer RSV vaccines has proved moderately to highly effective in preventing symptomatic RSV-associated lower respiratory tract disease over two consecutive RSV seasons among people aged 60 and older.
The trials that led to approval weren’t powered to gauge efficacy against RSV-associated hospitalization and death. However, the authors wrote, the prevention of lower respiratory tract disease, including medically attended illness, suggests that the shots might prevent considerable morbidity from RSV disease among those aged 60 and older.
Both vaccines were generally well tolerated with a good safety profile. However, six cases of inflammatory neurologic events (including Guillain-Barré Syndrome, acute disseminated encephalomyelitis, and others) were reported in clinical trials after RSV vaccination.
“Whether these events occurred due to chance, or whether RSV vaccination increases the risk for inflammatory neurologic events, is currently unknown,” the authors wrote.
Postmarketing surveillance may help clarify the existence of any potential risk, but until those results are clearer, the CDC researchers said, RSV vaccinations should be targeted to older adults at highest risk for severe RSV and those most likely to benefit from the shots.
At higher risk
Some adults with certain medical conditions have a higher risk for RSV-associated hospitalization, according to the report.
Those conditions include chronic obstructive pulmonary disease, asthma, heart failure, coronary artery disease, cerebrovascular disease, diabetes mellitus, and chronic kidney disease.
People who are frail and of advanced age also are at higher risk for RSV hospitalization. That risk increases with age and the highest risk is for people aged at least 75 years.
The researchers added that RSV can cause severe disease in those with compromised immunity, including people who have received hematopoietic stem cell transplants and patients taking immunosuppressive drugs such as those used with solid organ transplants, cancer treatment, or other conditions.
As for when physicians should offer the vaccinations, shots are optimally given before the start of the RSV season.
However, the COVID-19 pandemic interrupted the seasonality and the timing has not yet returned to prepandemic patterns.
For the 2023-24 season, this report states, clinicians should offer RSV vaccination to adults aged at least 60 years using shared clinical decision-making as early as vaccine supply is available and should continue to offer vaccination to eligible adults who remain unvaccinated.
RSV vaccines can be administered with other adult vaccines during the same visit, the authors confirmed.
A version of this article first appeared on Medscape.com.
Two newly approved respiratory syncytial virus (RSV) vaccines for adults aged 60 years and older may be able to prevent illness in those at risk for severe RSV disease.
Most adult RSV illness occurs among the older age group and results in an estimated 60,000-160,000 hospitalizations and 6,000-10,000 deaths per year among people aged at least 65 years.
Older adults deciding whether to get the vaccines should weigh risks and their own preferences and make the decision in consultation with their clinicians, said authors of a Centers for Disease Control and Prevention report.
Michael Melgar, MD, with the Coronavirus and Other Respiratory Viruses Division at the CDC, was lead author on the report, published in the Morbidity and Mortality Weekly Report.
Two new vaccines
In May, the Food and Drug Administration approved the first of two vaccines for preventing RSV lower respiratory tract disease for adults aged at least 60 years.
On June 21, the Advisory Committee on Immunization Practices (ACIP) recommended that people in that age group receive a single dose of RSV vaccine using shared decision-making.
The recommendation for shared decision-making makes the ACIP decision different from routine and risk-based vaccine recommendations. Rather than targeting all in a particular age group or risk group, the decision calls for consideration of a patients’ risk for disease and their characteristics, preferences, and values; the health care professional’s clinical discretion; and performance of the vaccine.
Dr. Melgar and colleagues reported that vaccination with one dose of the GSK or Pfizer RSV vaccines has proved moderately to highly effective in preventing symptomatic RSV-associated lower respiratory tract disease over two consecutive RSV seasons among people aged 60 and older.
The trials that led to approval weren’t powered to gauge efficacy against RSV-associated hospitalization and death. However, the authors wrote, the prevention of lower respiratory tract disease, including medically attended illness, suggests that the shots might prevent considerable morbidity from RSV disease among those aged 60 and older.
Both vaccines were generally well tolerated with a good safety profile. However, six cases of inflammatory neurologic events (including Guillain-Barré Syndrome, acute disseminated encephalomyelitis, and others) were reported in clinical trials after RSV vaccination.
“Whether these events occurred due to chance, or whether RSV vaccination increases the risk for inflammatory neurologic events, is currently unknown,” the authors wrote.
Postmarketing surveillance may help clarify the existence of any potential risk, but until those results are clearer, the CDC researchers said, RSV vaccinations should be targeted to older adults at highest risk for severe RSV and those most likely to benefit from the shots.
At higher risk
Some adults with certain medical conditions have a higher risk for RSV-associated hospitalization, according to the report.
Those conditions include chronic obstructive pulmonary disease, asthma, heart failure, coronary artery disease, cerebrovascular disease, diabetes mellitus, and chronic kidney disease.
People who are frail and of advanced age also are at higher risk for RSV hospitalization. That risk increases with age and the highest risk is for people aged at least 75 years.
The researchers added that RSV can cause severe disease in those with compromised immunity, including people who have received hematopoietic stem cell transplants and patients taking immunosuppressive drugs such as those used with solid organ transplants, cancer treatment, or other conditions.
As for when physicians should offer the vaccinations, shots are optimally given before the start of the RSV season.
However, the COVID-19 pandemic interrupted the seasonality and the timing has not yet returned to prepandemic patterns.
For the 2023-24 season, this report states, clinicians should offer RSV vaccination to adults aged at least 60 years using shared clinical decision-making as early as vaccine supply is available and should continue to offer vaccination to eligible adults who remain unvaccinated.
RSV vaccines can be administered with other adult vaccines during the same visit, the authors confirmed.
A version of this article first appeared on Medscape.com.
Two newly approved respiratory syncytial virus (RSV) vaccines for adults aged 60 years and older may be able to prevent illness in those at risk for severe RSV disease.
Most adult RSV illness occurs among the older age group and results in an estimated 60,000-160,000 hospitalizations and 6,000-10,000 deaths per year among people aged at least 65 years.
Older adults deciding whether to get the vaccines should weigh risks and their own preferences and make the decision in consultation with their clinicians, said authors of a Centers for Disease Control and Prevention report.
Michael Melgar, MD, with the Coronavirus and Other Respiratory Viruses Division at the CDC, was lead author on the report, published in the Morbidity and Mortality Weekly Report.
Two new vaccines
In May, the Food and Drug Administration approved the first of two vaccines for preventing RSV lower respiratory tract disease for adults aged at least 60 years.
On June 21, the Advisory Committee on Immunization Practices (ACIP) recommended that people in that age group receive a single dose of RSV vaccine using shared decision-making.
The recommendation for shared decision-making makes the ACIP decision different from routine and risk-based vaccine recommendations. Rather than targeting all in a particular age group or risk group, the decision calls for consideration of a patients’ risk for disease and their characteristics, preferences, and values; the health care professional’s clinical discretion; and performance of the vaccine.
Dr. Melgar and colleagues reported that vaccination with one dose of the GSK or Pfizer RSV vaccines has proved moderately to highly effective in preventing symptomatic RSV-associated lower respiratory tract disease over two consecutive RSV seasons among people aged 60 and older.
The trials that led to approval weren’t powered to gauge efficacy against RSV-associated hospitalization and death. However, the authors wrote, the prevention of lower respiratory tract disease, including medically attended illness, suggests that the shots might prevent considerable morbidity from RSV disease among those aged 60 and older.
Both vaccines were generally well tolerated with a good safety profile. However, six cases of inflammatory neurologic events (including Guillain-Barré Syndrome, acute disseminated encephalomyelitis, and others) were reported in clinical trials after RSV vaccination.
“Whether these events occurred due to chance, or whether RSV vaccination increases the risk for inflammatory neurologic events, is currently unknown,” the authors wrote.
Postmarketing surveillance may help clarify the existence of any potential risk, but until those results are clearer, the CDC researchers said, RSV vaccinations should be targeted to older adults at highest risk for severe RSV and those most likely to benefit from the shots.
At higher risk
Some adults with certain medical conditions have a higher risk for RSV-associated hospitalization, according to the report.
Those conditions include chronic obstructive pulmonary disease, asthma, heart failure, coronary artery disease, cerebrovascular disease, diabetes mellitus, and chronic kidney disease.
People who are frail and of advanced age also are at higher risk for RSV hospitalization. That risk increases with age and the highest risk is for people aged at least 75 years.
The researchers added that RSV can cause severe disease in those with compromised immunity, including people who have received hematopoietic stem cell transplants and patients taking immunosuppressive drugs such as those used with solid organ transplants, cancer treatment, or other conditions.
As for when physicians should offer the vaccinations, shots are optimally given before the start of the RSV season.
However, the COVID-19 pandemic interrupted the seasonality and the timing has not yet returned to prepandemic patterns.
For the 2023-24 season, this report states, clinicians should offer RSV vaccination to adults aged at least 60 years using shared clinical decision-making as early as vaccine supply is available and should continue to offer vaccination to eligible adults who remain unvaccinated.
RSV vaccines can be administered with other adult vaccines during the same visit, the authors confirmed.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
Daily statin cuts cardiovascular risk in HIV
BRISBANE, AUSTRALIA – that show pitavastatin therapy is associated with a significantly lower risk of cardiovascular events than placebo.
“There was a significant 35% lower risk of major adverse cardiovascular events after a median follow-up of 5.1 years “ said Steven Grinspoon, MD, from Massachusetts General Hospital and Harvard Medical School in Boston, who presented the final analysis of data from the REPRIEVE trial at the International AIDS Society Conference on HIV Science.
The results were simultaneously published in the New England Journal of Medicine. The primary endpoint of major adverse cardiovascular events included a composite of outcomes that included cardiovascular death, stroke, myocardial infarction, hospitalization for unstable angina, and transient ischemic attack among those treated with pitavastatin, compared with placebo (95% confidence interval, 0.48-0.90; P = .002).
The REPRIEVE trial was halted earlier this year for efficacy after an interim analysis pointed to a significantly lower rate of cardiovascular events in the treatment group.
The international double-blind, placebo-controlled trial randomly assigned 7,769 people with HIV infection, who were at low to moderate risk of cardiovascular disease, to either 4 mg daily of pitavastatin calcium or placebo.
The secondary outcome – a composite of major cardiovascular events and all-cause mortality – also showed a significant 21% reduction in risk with pitavastatin treatment, compared with placebo (95% CI, 0.65-0.96).
Cardiovascular events in HIV
HIV infection is an independent risk factor for cardiovascular disease, Dr. Grinspoon pointed out, and those living with HIV have about double the risk of myocardial infarction and stroke, compared with the general population.
“There’s an unmet need for people living with HIV who have low to moderate traditional risk, for whom HIV is even considered a risk equivalent but for whom no primary prevention strategy has been tested in a large trial,” Dr. Grinspoon said during an interview.
Those enrolled in the study had a 10-year Atherosclerotic Cardiovascular Disease risk score ranging from 2.1% to 7%, with a median of 4.5%. While LDL cholesterol levels at baseline ranged from 87 to 128 mg/dL, the study showed a similar reduction in cardiovascular risk regardless of LDL.
“These are types of people who, if they came to the doctor’s office right now before REPRIEVE, they would largely be told your risk score is not really making you eligible for a statin,” Dr. Grinspoon said.
He explained that what is most interesting about the reduction in risk is that it was nearly twice what would be expected with LDL lowering, based on what has previously been seen in statin trials in non–HIV-positive populations.
“I think the data are suggesting that it’s certainly in part due to the reduction in LDL – that is very important – but it’s also due to other factors beyond changes in LDL,” Dr. Grinspoon said. He speculated that the statin could be affecting anti-inflammatory and immune pathways, and that this could account for some of the reduction in cardiovascular risk, but “those data are cooking, and they’re being analyzed as we speak.”
In a substudy analysis of REPRIEVE, Markella Zanni, MD, associate professor of medicine at Harvard Medical School and Massachusetts General Hospital, focused on the women in the clinical trial.
Women’s risk
In REPRIEVE, 31.1% of the study population were women. Dr. Zanni and her team investigated whether there are differences in the way HIV affects the risk of developing atherosclerotic cardiovascular disease in women, compared with men.
They found that women have both higher levels of inflammatory markers, such as interleukin-6, C-reactive protein, and D-dimer, but a lower prevalence of coronary artery plaques than men.
“This finding represents an interesting paradox given that high levels of select inflammatory markers have been associated with coronary artery plaque, both among women living with HIV and among men living with HIV,” Dr. Zanni explained.
She says the researchers were hoping to further explore whether inflammation is fueling the increased risk for atherosclerotic disease, and particularly the higher risk evident in women living with HIV, compared with men.
“Women living with HIV should discuss with their treating clinicians heart risks and possible prevention strategies, including statin therapy coupled with healthy lifestyle changes addressing modifiable, traditional metabolic risk factors” she said.
Time for primary prevention?
All patients in the study were on antiretroviral therapy and investigators report that pitavastatin does not interact with these medications. The median CD4 cell count was 621 cells/mm3, and 87.5% of participants had an HIV viral load below the lower limit of quantification.
Participants were enrolled from 12 countries including the United States, Spain, Brazil, South Africa, and Thailand, and around two-thirds were non-White. Individuals of South Asian ethnicity showed the biggest reduction in cardiovascular risk with pitavastatin treatment.
There was a 74% higher rate of muscle pain and weakness in the pitavastatin group – affecting 91 people in the treatment arm and 53 in the placebo arm – but the majority were low grade. The rate of rhabdomyolysis of grade 3 or above was lower in the statin group, with three cases, compared with four cases in the placebo group.
Commenting on the findings, Laura Waters, MD, a genitourinary and HIV medicine consultant at Central and North West London NHS Foundation Trust’s Mortimer Market Centre, said that, while HIV infection was considered a risk factor for cardiovascular disease, risk calculators don’t specifically adjust for HIV infection.
“Now that we’ve got effective HIV drugs and people can enjoy normal life expectancy, cardiovascular disease is a particular issue for people with HIV,” she said.
Dr. Waters, who was not involved with the study, suggested that people living with HIV should discuss the use of statins with their doctor, but she acknowledged there are some barriers to treatment in people living with HIV. “It’s another pill, and when it’s a borderline [decision] it is easy to say, ‘I have to think about it,’ ” she said, with the result that statin treatment is often deferred.
The REPRIEVE study was supported by grants from the National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare. Dr. Grinspoon declared institutional grants from National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare and consultancies unrelated to the study. Dr. Zanni reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BRISBANE, AUSTRALIA – that show pitavastatin therapy is associated with a significantly lower risk of cardiovascular events than placebo.
“There was a significant 35% lower risk of major adverse cardiovascular events after a median follow-up of 5.1 years “ said Steven Grinspoon, MD, from Massachusetts General Hospital and Harvard Medical School in Boston, who presented the final analysis of data from the REPRIEVE trial at the International AIDS Society Conference on HIV Science.
The results were simultaneously published in the New England Journal of Medicine. The primary endpoint of major adverse cardiovascular events included a composite of outcomes that included cardiovascular death, stroke, myocardial infarction, hospitalization for unstable angina, and transient ischemic attack among those treated with pitavastatin, compared with placebo (95% confidence interval, 0.48-0.90; P = .002).
The REPRIEVE trial was halted earlier this year for efficacy after an interim analysis pointed to a significantly lower rate of cardiovascular events in the treatment group.
The international double-blind, placebo-controlled trial randomly assigned 7,769 people with HIV infection, who were at low to moderate risk of cardiovascular disease, to either 4 mg daily of pitavastatin calcium or placebo.
The secondary outcome – a composite of major cardiovascular events and all-cause mortality – also showed a significant 21% reduction in risk with pitavastatin treatment, compared with placebo (95% CI, 0.65-0.96).
Cardiovascular events in HIV
HIV infection is an independent risk factor for cardiovascular disease, Dr. Grinspoon pointed out, and those living with HIV have about double the risk of myocardial infarction and stroke, compared with the general population.
“There’s an unmet need for people living with HIV who have low to moderate traditional risk, for whom HIV is even considered a risk equivalent but for whom no primary prevention strategy has been tested in a large trial,” Dr. Grinspoon said during an interview.
Those enrolled in the study had a 10-year Atherosclerotic Cardiovascular Disease risk score ranging from 2.1% to 7%, with a median of 4.5%. While LDL cholesterol levels at baseline ranged from 87 to 128 mg/dL, the study showed a similar reduction in cardiovascular risk regardless of LDL.
“These are types of people who, if they came to the doctor’s office right now before REPRIEVE, they would largely be told your risk score is not really making you eligible for a statin,” Dr. Grinspoon said.
He explained that what is most interesting about the reduction in risk is that it was nearly twice what would be expected with LDL lowering, based on what has previously been seen in statin trials in non–HIV-positive populations.
“I think the data are suggesting that it’s certainly in part due to the reduction in LDL – that is very important – but it’s also due to other factors beyond changes in LDL,” Dr. Grinspoon said. He speculated that the statin could be affecting anti-inflammatory and immune pathways, and that this could account for some of the reduction in cardiovascular risk, but “those data are cooking, and they’re being analyzed as we speak.”
In a substudy analysis of REPRIEVE, Markella Zanni, MD, associate professor of medicine at Harvard Medical School and Massachusetts General Hospital, focused on the women in the clinical trial.
Women’s risk
In REPRIEVE, 31.1% of the study population were women. Dr. Zanni and her team investigated whether there are differences in the way HIV affects the risk of developing atherosclerotic cardiovascular disease in women, compared with men.
They found that women have both higher levels of inflammatory markers, such as interleukin-6, C-reactive protein, and D-dimer, but a lower prevalence of coronary artery plaques than men.
“This finding represents an interesting paradox given that high levels of select inflammatory markers have been associated with coronary artery plaque, both among women living with HIV and among men living with HIV,” Dr. Zanni explained.
She says the researchers were hoping to further explore whether inflammation is fueling the increased risk for atherosclerotic disease, and particularly the higher risk evident in women living with HIV, compared with men.
“Women living with HIV should discuss with their treating clinicians heart risks and possible prevention strategies, including statin therapy coupled with healthy lifestyle changes addressing modifiable, traditional metabolic risk factors” she said.
Time for primary prevention?
All patients in the study were on antiretroviral therapy and investigators report that pitavastatin does not interact with these medications. The median CD4 cell count was 621 cells/mm3, and 87.5% of participants had an HIV viral load below the lower limit of quantification.
Participants were enrolled from 12 countries including the United States, Spain, Brazil, South Africa, and Thailand, and around two-thirds were non-White. Individuals of South Asian ethnicity showed the biggest reduction in cardiovascular risk with pitavastatin treatment.
There was a 74% higher rate of muscle pain and weakness in the pitavastatin group – affecting 91 people in the treatment arm and 53 in the placebo arm – but the majority were low grade. The rate of rhabdomyolysis of grade 3 or above was lower in the statin group, with three cases, compared with four cases in the placebo group.
Commenting on the findings, Laura Waters, MD, a genitourinary and HIV medicine consultant at Central and North West London NHS Foundation Trust’s Mortimer Market Centre, said that, while HIV infection was considered a risk factor for cardiovascular disease, risk calculators don’t specifically adjust for HIV infection.
“Now that we’ve got effective HIV drugs and people can enjoy normal life expectancy, cardiovascular disease is a particular issue for people with HIV,” she said.
Dr. Waters, who was not involved with the study, suggested that people living with HIV should discuss the use of statins with their doctor, but she acknowledged there are some barriers to treatment in people living with HIV. “It’s another pill, and when it’s a borderline [decision] it is easy to say, ‘I have to think about it,’ ” she said, with the result that statin treatment is often deferred.
The REPRIEVE study was supported by grants from the National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare. Dr. Grinspoon declared institutional grants from National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare and consultancies unrelated to the study. Dr. Zanni reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BRISBANE, AUSTRALIA – that show pitavastatin therapy is associated with a significantly lower risk of cardiovascular events than placebo.
“There was a significant 35% lower risk of major adverse cardiovascular events after a median follow-up of 5.1 years “ said Steven Grinspoon, MD, from Massachusetts General Hospital and Harvard Medical School in Boston, who presented the final analysis of data from the REPRIEVE trial at the International AIDS Society Conference on HIV Science.
The results were simultaneously published in the New England Journal of Medicine. The primary endpoint of major adverse cardiovascular events included a composite of outcomes that included cardiovascular death, stroke, myocardial infarction, hospitalization for unstable angina, and transient ischemic attack among those treated with pitavastatin, compared with placebo (95% confidence interval, 0.48-0.90; P = .002).
The REPRIEVE trial was halted earlier this year for efficacy after an interim analysis pointed to a significantly lower rate of cardiovascular events in the treatment group.
The international double-blind, placebo-controlled trial randomly assigned 7,769 people with HIV infection, who were at low to moderate risk of cardiovascular disease, to either 4 mg daily of pitavastatin calcium or placebo.
The secondary outcome – a composite of major cardiovascular events and all-cause mortality – also showed a significant 21% reduction in risk with pitavastatin treatment, compared with placebo (95% CI, 0.65-0.96).
Cardiovascular events in HIV
HIV infection is an independent risk factor for cardiovascular disease, Dr. Grinspoon pointed out, and those living with HIV have about double the risk of myocardial infarction and stroke, compared with the general population.
“There’s an unmet need for people living with HIV who have low to moderate traditional risk, for whom HIV is even considered a risk equivalent but for whom no primary prevention strategy has been tested in a large trial,” Dr. Grinspoon said during an interview.
Those enrolled in the study had a 10-year Atherosclerotic Cardiovascular Disease risk score ranging from 2.1% to 7%, with a median of 4.5%. While LDL cholesterol levels at baseline ranged from 87 to 128 mg/dL, the study showed a similar reduction in cardiovascular risk regardless of LDL.
“These are types of people who, if they came to the doctor’s office right now before REPRIEVE, they would largely be told your risk score is not really making you eligible for a statin,” Dr. Grinspoon said.
He explained that what is most interesting about the reduction in risk is that it was nearly twice what would be expected with LDL lowering, based on what has previously been seen in statin trials in non–HIV-positive populations.
“I think the data are suggesting that it’s certainly in part due to the reduction in LDL – that is very important – but it’s also due to other factors beyond changes in LDL,” Dr. Grinspoon said. He speculated that the statin could be affecting anti-inflammatory and immune pathways, and that this could account for some of the reduction in cardiovascular risk, but “those data are cooking, and they’re being analyzed as we speak.”
In a substudy analysis of REPRIEVE, Markella Zanni, MD, associate professor of medicine at Harvard Medical School and Massachusetts General Hospital, focused on the women in the clinical trial.
Women’s risk
In REPRIEVE, 31.1% of the study population were women. Dr. Zanni and her team investigated whether there are differences in the way HIV affects the risk of developing atherosclerotic cardiovascular disease in women, compared with men.
They found that women have both higher levels of inflammatory markers, such as interleukin-6, C-reactive protein, and D-dimer, but a lower prevalence of coronary artery plaques than men.
“This finding represents an interesting paradox given that high levels of select inflammatory markers have been associated with coronary artery plaque, both among women living with HIV and among men living with HIV,” Dr. Zanni explained.
She says the researchers were hoping to further explore whether inflammation is fueling the increased risk for atherosclerotic disease, and particularly the higher risk evident in women living with HIV, compared with men.
“Women living with HIV should discuss with their treating clinicians heart risks and possible prevention strategies, including statin therapy coupled with healthy lifestyle changes addressing modifiable, traditional metabolic risk factors” she said.
Time for primary prevention?
All patients in the study were on antiretroviral therapy and investigators report that pitavastatin does not interact with these medications. The median CD4 cell count was 621 cells/mm3, and 87.5% of participants had an HIV viral load below the lower limit of quantification.
Participants were enrolled from 12 countries including the United States, Spain, Brazil, South Africa, and Thailand, and around two-thirds were non-White. Individuals of South Asian ethnicity showed the biggest reduction in cardiovascular risk with pitavastatin treatment.
There was a 74% higher rate of muscle pain and weakness in the pitavastatin group – affecting 91 people in the treatment arm and 53 in the placebo arm – but the majority were low grade. The rate of rhabdomyolysis of grade 3 or above was lower in the statin group, with three cases, compared with four cases in the placebo group.
Commenting on the findings, Laura Waters, MD, a genitourinary and HIV medicine consultant at Central and North West London NHS Foundation Trust’s Mortimer Market Centre, said that, while HIV infection was considered a risk factor for cardiovascular disease, risk calculators don’t specifically adjust for HIV infection.
“Now that we’ve got effective HIV drugs and people can enjoy normal life expectancy, cardiovascular disease is a particular issue for people with HIV,” she said.
Dr. Waters, who was not involved with the study, suggested that people living with HIV should discuss the use of statins with their doctor, but she acknowledged there are some barriers to treatment in people living with HIV. “It’s another pill, and when it’s a borderline [decision] it is easy to say, ‘I have to think about it,’ ” she said, with the result that statin treatment is often deferred.
The REPRIEVE study was supported by grants from the National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare. Dr. Grinspoon declared institutional grants from National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare and consultancies unrelated to the study. Dr. Zanni reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT IAS 2023
Even exercise by ‘weekend warriors’ can cut CV risk
Moderate to vigorous physical activity (MVPA) is a familiar and established approach to reducing cardiovascular (CV) risk, but it’s often believed that the exercise should be spread out across the week rather than concentrated within a couple of days.
A challenge to that view comes from an observational study of accelerometer-confirmed exercise in almost 90,000 people in their 60s. It suggests,
Researchers compared three patterns of MVPA in their subjects who wore accelerometers on their wrists for 1 week. Active WW subjects obtained at least 2.5 hours of exercise weekly, with at least half the amount completed over 1-2 days; “active regular” subjects achieved that exercise level but not mostly during 1 or 2 days; and those who were “inactive” fell short of 2.5 hours of exercise during the week. The group used a median exercise threshold of 3 hours, 50 minutes in a separate analysis.
The “active” groups, compared with inactive subjects, achieved similar and significant reductions in risk for incident atrial fibrillation (AF), myocardial infarction (MI), stroke, and heart failure (HF) over a median follow-up of 6.3 years at both weekly exercise thresholds, the group reported.
“The take-home [message] is that efforts to optimize activity, even if concentrated within just a day or 2 each week, should be expected to result in improved cardiovascular risk profiles,” lead author Shaan Khurshid, MD, MPH, Massachusetts General Hospital, Boston, said in an interview.
The study was published online in JAMA.
The research “provides novel data on patterns of physical activity accumulation and the risk of developing cardiovascular diseases,” observed Peter Katzmarzyk, PhD, Pennington Biomedical Research Center, Baton Rouge, La., in an interview. He was not involved with the research. Its “marked strengths,” he noted, include a large sample population and “use of accelerometers to measure physical activity levels and patterns.”
Moreover, Dr. Katzmarzyk said, its findings are “important” for showing that physical activity “can be accumulated throughout the week in different ways, which opens up more options for busy people to get their physical activity in.”
Current guidelines from the World Health Organization and the American Heart Association recommend at least 150 minutes of MVPA weekly to lower risk for cardiovascular disease and death, but do not specify an optimal exercise time frame. The U.K. National Health Service recommends MVPA daily or spread evenly over perhaps 4-5 days.
“The weekend warrior pattern has been studied previously, but typically relying on self-reported data, which may be biased, or [in studies] too small to look at specific cardiovascular outcomes,” Dr. Khurshid explained.
In the UK Biobank database, he said, “We saw the opportunity to leverage the largest sample of measured activity to date” to address the question of whether exercise time pattern “affects specific major cardiovascular diseases differently,” Dr. Khurshid said
The primary analysis assessed exercise amount in a week based on the guideline-recommended threshold of at least 2.5 hours; a 3-hour, 50-minutes threshold was used in a secondary analysis. The group assessed multiple thresholds because optimal MVPS levels derived from wrist-based accelerometers are “unclear,” he said.
The sample consisted of 89,573 participants with a mean age 62; slightly more than half (56%) were women. Based on the weekly MVPA threshold of 2.5 hours , the WW, active regular, and inactive groups made up 42.2%, 24%, and 33.7% of the population, respectively.
Compared with the inactive group, the two active groups both showed significant risk reductions for the four clinical outcomes, to similar degrees, in multivariate analysis. The results were similar at the 230-minute weekly exercise threshold for incident AF, MI, and HF but not for stroke.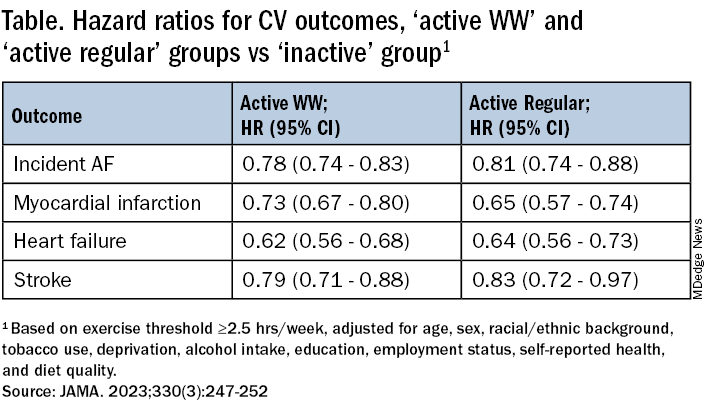
The findings were similarly consistent at the 3-hour, 50-minutes median threshold, although stroke differences were no longer significant.
Patients should be encouraged to exercise at recommended levels, “and should not be discouraged if, for whatever reasons, they are able to focus exercise within only 1 or a few days of the week,” said Dr. Khurshid. “Our findings suggest that it is the volume of activity, rather than the pattern, that matters most.”
The report notes several limitations of the study, including the exercise observation period limited to 1 week and that participants could have modified their behavior during the observation period. Also, the participants were almost all White, so the results may not be generalizable to other populations.
Clinicians should familiarize themselves with the “full range of recommendations” presented in the “Physical Activity Guidelines for Americans, 2nd Edition” “and personalize prescriptions by setting achievable physical activity goals” based on age, physical abilities, and activity levels, states an accompanying editorial from Dr. Katzmarzyk and John M. Jakicic, PhD, University of Kansas Medical Center, Kansas City.
Although MVPA at the recommended level of at least 2.5 hours per week will certainly be beneficial, they write, “the public health message should also clearly convey that every minute counts, especially among the three-quarters of U.S. adults who do not achieve that goal.”
Dr. Khurshid reported no relevant financial relationships; disclosures for the other authors are in the original article. Dr. Katzmarzyk reports no relevant financial relationships. Dr. Jakicic discloses receiving personal fees from Wondr Health, WW International (formerly Weight Watchers), and Educational Initiatives and grants from Epitomee Medical.
A version of this article appeared on Medscape.com.
Moderate to vigorous physical activity (MVPA) is a familiar and established approach to reducing cardiovascular (CV) risk, but it’s often believed that the exercise should be spread out across the week rather than concentrated within a couple of days.
A challenge to that view comes from an observational study of accelerometer-confirmed exercise in almost 90,000 people in their 60s. It suggests,
Researchers compared three patterns of MVPA in their subjects who wore accelerometers on their wrists for 1 week. Active WW subjects obtained at least 2.5 hours of exercise weekly, with at least half the amount completed over 1-2 days; “active regular” subjects achieved that exercise level but not mostly during 1 or 2 days; and those who were “inactive” fell short of 2.5 hours of exercise during the week. The group used a median exercise threshold of 3 hours, 50 minutes in a separate analysis.
The “active” groups, compared with inactive subjects, achieved similar and significant reductions in risk for incident atrial fibrillation (AF), myocardial infarction (MI), stroke, and heart failure (HF) over a median follow-up of 6.3 years at both weekly exercise thresholds, the group reported.
“The take-home [message] is that efforts to optimize activity, even if concentrated within just a day or 2 each week, should be expected to result in improved cardiovascular risk profiles,” lead author Shaan Khurshid, MD, MPH, Massachusetts General Hospital, Boston, said in an interview.
The study was published online in JAMA.
The research “provides novel data on patterns of physical activity accumulation and the risk of developing cardiovascular diseases,” observed Peter Katzmarzyk, PhD, Pennington Biomedical Research Center, Baton Rouge, La., in an interview. He was not involved with the research. Its “marked strengths,” he noted, include a large sample population and “use of accelerometers to measure physical activity levels and patterns.”
Moreover, Dr. Katzmarzyk said, its findings are “important” for showing that physical activity “can be accumulated throughout the week in different ways, which opens up more options for busy people to get their physical activity in.”
Current guidelines from the World Health Organization and the American Heart Association recommend at least 150 minutes of MVPA weekly to lower risk for cardiovascular disease and death, but do not specify an optimal exercise time frame. The U.K. National Health Service recommends MVPA daily or spread evenly over perhaps 4-5 days.
“The weekend warrior pattern has been studied previously, but typically relying on self-reported data, which may be biased, or [in studies] too small to look at specific cardiovascular outcomes,” Dr. Khurshid explained.
In the UK Biobank database, he said, “We saw the opportunity to leverage the largest sample of measured activity to date” to address the question of whether exercise time pattern “affects specific major cardiovascular diseases differently,” Dr. Khurshid said
The primary analysis assessed exercise amount in a week based on the guideline-recommended threshold of at least 2.5 hours; a 3-hour, 50-minutes threshold was used in a secondary analysis. The group assessed multiple thresholds because optimal MVPS levels derived from wrist-based accelerometers are “unclear,” he said.
The sample consisted of 89,573 participants with a mean age 62; slightly more than half (56%) were women. Based on the weekly MVPA threshold of 2.5 hours , the WW, active regular, and inactive groups made up 42.2%, 24%, and 33.7% of the population, respectively.
Compared with the inactive group, the two active groups both showed significant risk reductions for the four clinical outcomes, to similar degrees, in multivariate analysis. The results were similar at the 230-minute weekly exercise threshold for incident AF, MI, and HF but not for stroke.
The findings were similarly consistent at the 3-hour, 50-minutes median threshold, although stroke differences were no longer significant.
Patients should be encouraged to exercise at recommended levels, “and should not be discouraged if, for whatever reasons, they are able to focus exercise within only 1 or a few days of the week,” said Dr. Khurshid. “Our findings suggest that it is the volume of activity, rather than the pattern, that matters most.”
The report notes several limitations of the study, including the exercise observation period limited to 1 week and that participants could have modified their behavior during the observation period. Also, the participants were almost all White, so the results may not be generalizable to other populations.
Clinicians should familiarize themselves with the “full range of recommendations” presented in the “Physical Activity Guidelines for Americans, 2nd Edition” “and personalize prescriptions by setting achievable physical activity goals” based on age, physical abilities, and activity levels, states an accompanying editorial from Dr. Katzmarzyk and John M. Jakicic, PhD, University of Kansas Medical Center, Kansas City.
Although MVPA at the recommended level of at least 2.5 hours per week will certainly be beneficial, they write, “the public health message should also clearly convey that every minute counts, especially among the three-quarters of U.S. adults who do not achieve that goal.”
Dr. Khurshid reported no relevant financial relationships; disclosures for the other authors are in the original article. Dr. Katzmarzyk reports no relevant financial relationships. Dr. Jakicic discloses receiving personal fees from Wondr Health, WW International (formerly Weight Watchers), and Educational Initiatives and grants from Epitomee Medical.
A version of this article appeared on Medscape.com.
Moderate to vigorous physical activity (MVPA) is a familiar and established approach to reducing cardiovascular (CV) risk, but it’s often believed that the exercise should be spread out across the week rather than concentrated within a couple of days.
A challenge to that view comes from an observational study of accelerometer-confirmed exercise in almost 90,000 people in their 60s. It suggests,
Researchers compared three patterns of MVPA in their subjects who wore accelerometers on their wrists for 1 week. Active WW subjects obtained at least 2.5 hours of exercise weekly, with at least half the amount completed over 1-2 days; “active regular” subjects achieved that exercise level but not mostly during 1 or 2 days; and those who were “inactive” fell short of 2.5 hours of exercise during the week. The group used a median exercise threshold of 3 hours, 50 minutes in a separate analysis.
The “active” groups, compared with inactive subjects, achieved similar and significant reductions in risk for incident atrial fibrillation (AF), myocardial infarction (MI), stroke, and heart failure (HF) over a median follow-up of 6.3 years at both weekly exercise thresholds, the group reported.
“The take-home [message] is that efforts to optimize activity, even if concentrated within just a day or 2 each week, should be expected to result in improved cardiovascular risk profiles,” lead author Shaan Khurshid, MD, MPH, Massachusetts General Hospital, Boston, said in an interview.
The study was published online in JAMA.
The research “provides novel data on patterns of physical activity accumulation and the risk of developing cardiovascular diseases,” observed Peter Katzmarzyk, PhD, Pennington Biomedical Research Center, Baton Rouge, La., in an interview. He was not involved with the research. Its “marked strengths,” he noted, include a large sample population and “use of accelerometers to measure physical activity levels and patterns.”
Moreover, Dr. Katzmarzyk said, its findings are “important” for showing that physical activity “can be accumulated throughout the week in different ways, which opens up more options for busy people to get their physical activity in.”
Current guidelines from the World Health Organization and the American Heart Association recommend at least 150 minutes of MVPA weekly to lower risk for cardiovascular disease and death, but do not specify an optimal exercise time frame. The U.K. National Health Service recommends MVPA daily or spread evenly over perhaps 4-5 days.
“The weekend warrior pattern has been studied previously, but typically relying on self-reported data, which may be biased, or [in studies] too small to look at specific cardiovascular outcomes,” Dr. Khurshid explained.
In the UK Biobank database, he said, “We saw the opportunity to leverage the largest sample of measured activity to date” to address the question of whether exercise time pattern “affects specific major cardiovascular diseases differently,” Dr. Khurshid said
The primary analysis assessed exercise amount in a week based on the guideline-recommended threshold of at least 2.5 hours; a 3-hour, 50-minutes threshold was used in a secondary analysis. The group assessed multiple thresholds because optimal MVPS levels derived from wrist-based accelerometers are “unclear,” he said.
The sample consisted of 89,573 participants with a mean age 62; slightly more than half (56%) were women. Based on the weekly MVPA threshold of 2.5 hours , the WW, active regular, and inactive groups made up 42.2%, 24%, and 33.7% of the population, respectively.
Compared with the inactive group, the two active groups both showed significant risk reductions for the four clinical outcomes, to similar degrees, in multivariate analysis. The results were similar at the 230-minute weekly exercise threshold for incident AF, MI, and HF but not for stroke.
The findings were similarly consistent at the 3-hour, 50-minutes median threshold, although stroke differences were no longer significant.
Patients should be encouraged to exercise at recommended levels, “and should not be discouraged if, for whatever reasons, they are able to focus exercise within only 1 or a few days of the week,” said Dr. Khurshid. “Our findings suggest that it is the volume of activity, rather than the pattern, that matters most.”
The report notes several limitations of the study, including the exercise observation period limited to 1 week and that participants could have modified their behavior during the observation period. Also, the participants were almost all White, so the results may not be generalizable to other populations.
Clinicians should familiarize themselves with the “full range of recommendations” presented in the “Physical Activity Guidelines for Americans, 2nd Edition” “and personalize prescriptions by setting achievable physical activity goals” based on age, physical abilities, and activity levels, states an accompanying editorial from Dr. Katzmarzyk and John M. Jakicic, PhD, University of Kansas Medical Center, Kansas City.
Although MVPA at the recommended level of at least 2.5 hours per week will certainly be beneficial, they write, “the public health message should also clearly convey that every minute counts, especially among the three-quarters of U.S. adults who do not achieve that goal.”
Dr. Khurshid reported no relevant financial relationships; disclosures for the other authors are in the original article. Dr. Katzmarzyk reports no relevant financial relationships. Dr. Jakicic discloses receiving personal fees from Wondr Health, WW International (formerly Weight Watchers), and Educational Initiatives and grants from Epitomee Medical.
A version of this article appeared on Medscape.com.
FROM JAMA
EU agency issues positive opinion on ritlecitinib
, paving the way for possible marketing authorization of the drug in the European Union for individuals 12 years of age and older. A final decision is expected in the coming months.
The development, which was announced by the manufacturer, Pfizer, on July 21, 2023, follows approval of ritlecitinib (Litfulo) for the treatment of severe alopecia areata in adults and adolescents 12 years and older by the Food and Drug Administration and the Japanese Ministry of Health, Labour, and Welfare in June 2023. According to a press release from Pfizer, submissions to other regulatory agencies for the use of ritlecitinib in alopecia areata are ongoing.
The Marketing Authorization Application for ritlecitinib was based on results from a randomized, placebo-controlled, double-blind ALLEGRO Phase 2b/3 study.
, paving the way for possible marketing authorization of the drug in the European Union for individuals 12 years of age and older. A final decision is expected in the coming months.
The development, which was announced by the manufacturer, Pfizer, on July 21, 2023, follows approval of ritlecitinib (Litfulo) for the treatment of severe alopecia areata in adults and adolescents 12 years and older by the Food and Drug Administration and the Japanese Ministry of Health, Labour, and Welfare in June 2023. According to a press release from Pfizer, submissions to other regulatory agencies for the use of ritlecitinib in alopecia areata are ongoing.
The Marketing Authorization Application for ritlecitinib was based on results from a randomized, placebo-controlled, double-blind ALLEGRO Phase 2b/3 study.
, paving the way for possible marketing authorization of the drug in the European Union for individuals 12 years of age and older. A final decision is expected in the coming months.
The development, which was announced by the manufacturer, Pfizer, on July 21, 2023, follows approval of ritlecitinib (Litfulo) for the treatment of severe alopecia areata in adults and adolescents 12 years and older by the Food and Drug Administration and the Japanese Ministry of Health, Labour, and Welfare in June 2023. According to a press release from Pfizer, submissions to other regulatory agencies for the use of ritlecitinib in alopecia areata are ongoing.
The Marketing Authorization Application for ritlecitinib was based on results from a randomized, placebo-controlled, double-blind ALLEGRO Phase 2b/3 study.
Affording the cost of new obesity drugs? We can’t afford not to
SAN DIEGO – Although the glucagonlike peptide–1 (GLP-1) receptor agonists, such as liraglutide and semaglutide, have been revolutionary advances for the treatment of obesity, the cost-effectiveness of these agents for treating both obesity and type 2 diabetes remains uncertain based on published analyses.
But potential future changes in the cost-effectiveness dynamics of GLP-1 agonists could tip the balance in their favor. These include
Costs to people with obesity that are generally not part of cost-effectiveness calculations include pain, disability, depression, and bias that affect employment, Carol H. Wysham, MD, said at the recent scientific sessions of the American Diabetes Association.
Other costs to society left out of conventional calculations are items such as the incremental cost for fuel to transport a heavier population and the carbon-footprint costs for the production and transportation of the excess food produced to feed an over-fed population, added Dr. Wysham, an endocrinologist with MultiCare and the Rockwood Clinic in Spokane, Wash.
Analyses should include ‘things we don’t often think about’
“The impact of living with obesity is much greater than what we traditionally calculate in health economics,” commented Naveed Sattar, PhD, speaking from the floor during the session.
“Patient happiness and self-esteem are hard to measure and capture as cost impacts. We need to also add carbon dioxide effects and transportation costs, and governments are starting to get wise to this. How to run proper health economics analyses is the key question; we need to do better than what we currently do,” said Dr. Sattar, a professor of metabolic medicine at the University of Glasgow.
Dr. Sattar is lead author of a recent analysis that highlights the overwhelming importance of improved weight management in adults as they age to reduce their risk of developing a broad range of chronic disorders.
“Most chronic conditions are, to differing extents, caused or exacerbated by excess adiposity,” was a conclusion of his report.
“It’s important to include the costs to society, including things we don’t often think about. No one has ever done a cost analysis that includes all the factors” cited by Dr. Wysham, said Irl B. Hirsch, MD, another speaker at the session. “No one includes obstructive sleep apnea, degenerative arthritis, and the downstream effects of a high body mass index.”
The GLP-1 agonists “are great” for both weight loss and glycemic control, said Dr. Hirsch, an endocrinologist and professor at the University of Washington, Seattle. “We can’t afford not to use them. These agents have been transformational.”
U.S. has the highest drug costs
Another key factor driving cost-effectiveness is, of course, the relatively high cost of the agents in the class, especially in the United States. Dr. Hirsch cited a recently published report in Obesity that quoted monthly U.S. costs of $804 for weekly 2.4-mg injections of semaglutide (Wegovy) and $1418 for daily 3.0-mg injections of liraglutide (Saxenda). Highlighting the relatively high cost of medications in the United States, the report cited a monthly price tag of $95 for the same semaglutide regimen in Turkey and a monthly cost of $252 for the same liraglutide regimen in Norway.
U.S. prices for agents in this class may start to deflate as soon as 2024, when one or more generic versions of liraglutide are expected, following expiration of the U.S. patent later in 2023, Dr. Wysham said.
Another pending trigger for lower costs may be the possible decision by the World Health Organization to designate liraglutide an “essential medicine” later in 2023, she noted. The WHO received an application for this designation from four U.S. clinicians and is considering it as part of its planned 2023 update to the WHO’s Essential Medicines List. Dr. Wysham predicted this designation would “press international pharmaceutical companies to produce [liraglutide] at a much lower cost.”
“I’m not saying that drug companies should not profit, but they should not do it on the backs of patients,” Dr. Wysham declared. “What do we measure by ‘cost-effectiveness?’ There are so many complications of obesity. For patients with diabetes and obesity we need to look for a little different economic policy.”
Dr. Wysham has reported being an adviser to Abbott and CeQur and receiving research funding from Eli Lilly and Novo Nordisk. Dr. Hirsch has reported being a consultant for Abbott, Embecta, and Hagar, and receiving research funding from Dexcom and Insulet. Dr. Sattar has reported receiving consulting fees or speaker honoraria from Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Sanofi.
A version of this article appeared on Medscape.com.
SAN DIEGO – Although the glucagonlike peptide–1 (GLP-1) receptor agonists, such as liraglutide and semaglutide, have been revolutionary advances for the treatment of obesity, the cost-effectiveness of these agents for treating both obesity and type 2 diabetes remains uncertain based on published analyses.
But potential future changes in the cost-effectiveness dynamics of GLP-1 agonists could tip the balance in their favor. These include
Costs to people with obesity that are generally not part of cost-effectiveness calculations include pain, disability, depression, and bias that affect employment, Carol H. Wysham, MD, said at the recent scientific sessions of the American Diabetes Association.
Other costs to society left out of conventional calculations are items such as the incremental cost for fuel to transport a heavier population and the carbon-footprint costs for the production and transportation of the excess food produced to feed an over-fed population, added Dr. Wysham, an endocrinologist with MultiCare and the Rockwood Clinic in Spokane, Wash.
Analyses should include ‘things we don’t often think about’
“The impact of living with obesity is much greater than what we traditionally calculate in health economics,” commented Naveed Sattar, PhD, speaking from the floor during the session.
“Patient happiness and self-esteem are hard to measure and capture as cost impacts. We need to also add carbon dioxide effects and transportation costs, and governments are starting to get wise to this. How to run proper health economics analyses is the key question; we need to do better than what we currently do,” said Dr. Sattar, a professor of metabolic medicine at the University of Glasgow.
Dr. Sattar is lead author of a recent analysis that highlights the overwhelming importance of improved weight management in adults as they age to reduce their risk of developing a broad range of chronic disorders.
“Most chronic conditions are, to differing extents, caused or exacerbated by excess adiposity,” was a conclusion of his report.
“It’s important to include the costs to society, including things we don’t often think about. No one has ever done a cost analysis that includes all the factors” cited by Dr. Wysham, said Irl B. Hirsch, MD, another speaker at the session. “No one includes obstructive sleep apnea, degenerative arthritis, and the downstream effects of a high body mass index.”
The GLP-1 agonists “are great” for both weight loss and glycemic control, said Dr. Hirsch, an endocrinologist and professor at the University of Washington, Seattle. “We can’t afford not to use them. These agents have been transformational.”
U.S. has the highest drug costs
Another key factor driving cost-effectiveness is, of course, the relatively high cost of the agents in the class, especially in the United States. Dr. Hirsch cited a recently published report in Obesity that quoted monthly U.S. costs of $804 for weekly 2.4-mg injections of semaglutide (Wegovy) and $1418 for daily 3.0-mg injections of liraglutide (Saxenda). Highlighting the relatively high cost of medications in the United States, the report cited a monthly price tag of $95 for the same semaglutide regimen in Turkey and a monthly cost of $252 for the same liraglutide regimen in Norway.
U.S. prices for agents in this class may start to deflate as soon as 2024, when one or more generic versions of liraglutide are expected, following expiration of the U.S. patent later in 2023, Dr. Wysham said.
Another pending trigger for lower costs may be the possible decision by the World Health Organization to designate liraglutide an “essential medicine” later in 2023, she noted. The WHO received an application for this designation from four U.S. clinicians and is considering it as part of its planned 2023 update to the WHO’s Essential Medicines List. Dr. Wysham predicted this designation would “press international pharmaceutical companies to produce [liraglutide] at a much lower cost.”
“I’m not saying that drug companies should not profit, but they should not do it on the backs of patients,” Dr. Wysham declared. “What do we measure by ‘cost-effectiveness?’ There are so many complications of obesity. For patients with diabetes and obesity we need to look for a little different economic policy.”
Dr. Wysham has reported being an adviser to Abbott and CeQur and receiving research funding from Eli Lilly and Novo Nordisk. Dr. Hirsch has reported being a consultant for Abbott, Embecta, and Hagar, and receiving research funding from Dexcom and Insulet. Dr. Sattar has reported receiving consulting fees or speaker honoraria from Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Sanofi.
A version of this article appeared on Medscape.com.
SAN DIEGO – Although the glucagonlike peptide–1 (GLP-1) receptor agonists, such as liraglutide and semaglutide, have been revolutionary advances for the treatment of obesity, the cost-effectiveness of these agents for treating both obesity and type 2 diabetes remains uncertain based on published analyses.
But potential future changes in the cost-effectiveness dynamics of GLP-1 agonists could tip the balance in their favor. These include
Costs to people with obesity that are generally not part of cost-effectiveness calculations include pain, disability, depression, and bias that affect employment, Carol H. Wysham, MD, said at the recent scientific sessions of the American Diabetes Association.
Other costs to society left out of conventional calculations are items such as the incremental cost for fuel to transport a heavier population and the carbon-footprint costs for the production and transportation of the excess food produced to feed an over-fed population, added Dr. Wysham, an endocrinologist with MultiCare and the Rockwood Clinic in Spokane, Wash.
Analyses should include ‘things we don’t often think about’
“The impact of living with obesity is much greater than what we traditionally calculate in health economics,” commented Naveed Sattar, PhD, speaking from the floor during the session.
“Patient happiness and self-esteem are hard to measure and capture as cost impacts. We need to also add carbon dioxide effects and transportation costs, and governments are starting to get wise to this. How to run proper health economics analyses is the key question; we need to do better than what we currently do,” said Dr. Sattar, a professor of metabolic medicine at the University of Glasgow.
Dr. Sattar is lead author of a recent analysis that highlights the overwhelming importance of improved weight management in adults as they age to reduce their risk of developing a broad range of chronic disorders.
“Most chronic conditions are, to differing extents, caused or exacerbated by excess adiposity,” was a conclusion of his report.
“It’s important to include the costs to society, including things we don’t often think about. No one has ever done a cost analysis that includes all the factors” cited by Dr. Wysham, said Irl B. Hirsch, MD, another speaker at the session. “No one includes obstructive sleep apnea, degenerative arthritis, and the downstream effects of a high body mass index.”
The GLP-1 agonists “are great” for both weight loss and glycemic control, said Dr. Hirsch, an endocrinologist and professor at the University of Washington, Seattle. “We can’t afford not to use them. These agents have been transformational.”
U.S. has the highest drug costs
Another key factor driving cost-effectiveness is, of course, the relatively high cost of the agents in the class, especially in the United States. Dr. Hirsch cited a recently published report in Obesity that quoted monthly U.S. costs of $804 for weekly 2.4-mg injections of semaglutide (Wegovy) and $1418 for daily 3.0-mg injections of liraglutide (Saxenda). Highlighting the relatively high cost of medications in the United States, the report cited a monthly price tag of $95 for the same semaglutide regimen in Turkey and a monthly cost of $252 for the same liraglutide regimen in Norway.
U.S. prices for agents in this class may start to deflate as soon as 2024, when one or more generic versions of liraglutide are expected, following expiration of the U.S. patent later in 2023, Dr. Wysham said.
Another pending trigger for lower costs may be the possible decision by the World Health Organization to designate liraglutide an “essential medicine” later in 2023, she noted. The WHO received an application for this designation from four U.S. clinicians and is considering it as part of its planned 2023 update to the WHO’s Essential Medicines List. Dr. Wysham predicted this designation would “press international pharmaceutical companies to produce [liraglutide] at a much lower cost.”
“I’m not saying that drug companies should not profit, but they should not do it on the backs of patients,” Dr. Wysham declared. “What do we measure by ‘cost-effectiveness?’ There are so many complications of obesity. For patients with diabetes and obesity we need to look for a little different economic policy.”
Dr. Wysham has reported being an adviser to Abbott and CeQur and receiving research funding from Eli Lilly and Novo Nordisk. Dr. Hirsch has reported being a consultant for Abbott, Embecta, and Hagar, and receiving research funding from Dexcom and Insulet. Dr. Sattar has reported receiving consulting fees or speaker honoraria from Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Sanofi.
A version of this article appeared on Medscape.com.
AT ADA 2023
High-intensity interval training before major surgery may boost postoperative outcomes
TOPLINE:
It cuts the risk of postoperative complications and may shorten hospital length of stay and improve postoperative quality of life.
METHODOLOGY:
Evidence suggests CRF – which improves physical and cognitive function and is associated with a reduction in cardiovascular risk – can be enhanced before major surgeries, but reported postoperative outcomes in previous reviews have been inconsistent.
In the study, HIIT involved repeated aerobic high-intensity exercise intervals at about 80% of maximum heart rate, followed by active recovery.
The meta-analysis included 12 studies with 832 patients (mean age, 67) that compared preoperative HIIT – supervised at hospitals, gyms, or community or physical therapy centers, or unsupervised at home – with standard care for patients slated for major surgery, including liver, lung, colorectal, urologic, and mixed major abdominal operations.
The primary outcome was change in CRF by peak VO2 or 6-minute walk test; other endpoints included change in endurance time and postoperative outcomes.
TAKEAWAY:
Preoperative HIIT (median total, 160 minutes; range, 80-240 minutes; intense exercise during 6-40 sessions) was associated with an increase in peak oxygen consumption (VO2 peak) by 2.59 mL/kg/min (95% confidence interval, 1.52-3.65 mL/kg/min; P < .001), compared with standard care, which represents about a 10% increase in CRF.
In eight studies that involved 770 patients, there was moderate evidence that preoperative HIIT cut the odds ratio for postoperative complications by more than half (OR, 0.44; 95% CI, 0.32-0.60; P < .001); there was a similar apparent benefit in an analysis that was limited to patients who were slated for abdominal surgery (OR, 0.45; 95% CI, 0.29-0.68; P < .001).
An analysis that was limited to studies that reported hospital length of stay showed a clinically relevant but nonsignificant 3-day reduction among patients in the HIIT groups.
Most quality of life assessments did not show post-HIIT improvements; some showed a significant benefit 6 weeks after surgery.
IN PRACTICE:
The results suggest preoperative HIIT may improve postoperative outcomes. By extension, it could be cost-effective and “should be included in prehabilitation programs,” the report states.
SOURCE:
The study was carried out by Kari Clifford, PhD, Otago Medical School, University of Otago, Dunedin, New Zealand, and colleagues. It was published online June 30, 2023, in JAMA Network Open.
LIMITATIONS:
Included studies were heterogeneous in methodology; for example, HIIT definitions and protocols varied across almost every study. Data reporting was incomplete, the samples sizes in the studies were limited, and patients could not be blinded to their intervention. The patients could not be stratified on the basis of frailty. There were limited HIIT data from patients who underwent orthopedic surgeries.
DISCLOSURES:
The study received funding from the University of Otago. The authors reported no conflicts.
A version of this article first appeared on Medscape.com.
TOPLINE:
It cuts the risk of postoperative complications and may shorten hospital length of stay and improve postoperative quality of life.
METHODOLOGY:
Evidence suggests CRF – which improves physical and cognitive function and is associated with a reduction in cardiovascular risk – can be enhanced before major surgeries, but reported postoperative outcomes in previous reviews have been inconsistent.
In the study, HIIT involved repeated aerobic high-intensity exercise intervals at about 80% of maximum heart rate, followed by active recovery.
The meta-analysis included 12 studies with 832 patients (mean age, 67) that compared preoperative HIIT – supervised at hospitals, gyms, or community or physical therapy centers, or unsupervised at home – with standard care for patients slated for major surgery, including liver, lung, colorectal, urologic, and mixed major abdominal operations.
The primary outcome was change in CRF by peak VO2 or 6-minute walk test; other endpoints included change in endurance time and postoperative outcomes.
TAKEAWAY:
Preoperative HIIT (median total, 160 minutes; range, 80-240 minutes; intense exercise during 6-40 sessions) was associated with an increase in peak oxygen consumption (VO2 peak) by 2.59 mL/kg/min (95% confidence interval, 1.52-3.65 mL/kg/min; P < .001), compared with standard care, which represents about a 10% increase in CRF.
In eight studies that involved 770 patients, there was moderate evidence that preoperative HIIT cut the odds ratio for postoperative complications by more than half (OR, 0.44; 95% CI, 0.32-0.60; P < .001); there was a similar apparent benefit in an analysis that was limited to patients who were slated for abdominal surgery (OR, 0.45; 95% CI, 0.29-0.68; P < .001).
An analysis that was limited to studies that reported hospital length of stay showed a clinically relevant but nonsignificant 3-day reduction among patients in the HIIT groups.
Most quality of life assessments did not show post-HIIT improvements; some showed a significant benefit 6 weeks after surgery.
IN PRACTICE:
The results suggest preoperative HIIT may improve postoperative outcomes. By extension, it could be cost-effective and “should be included in prehabilitation programs,” the report states.
SOURCE:
The study was carried out by Kari Clifford, PhD, Otago Medical School, University of Otago, Dunedin, New Zealand, and colleagues. It was published online June 30, 2023, in JAMA Network Open.
LIMITATIONS:
Included studies were heterogeneous in methodology; for example, HIIT definitions and protocols varied across almost every study. Data reporting was incomplete, the samples sizes in the studies were limited, and patients could not be blinded to their intervention. The patients could not be stratified on the basis of frailty. There were limited HIIT data from patients who underwent orthopedic surgeries.
DISCLOSURES:
The study received funding from the University of Otago. The authors reported no conflicts.
A version of this article first appeared on Medscape.com.
TOPLINE:
It cuts the risk of postoperative complications and may shorten hospital length of stay and improve postoperative quality of life.
METHODOLOGY:
Evidence suggests CRF – which improves physical and cognitive function and is associated with a reduction in cardiovascular risk – can be enhanced before major surgeries, but reported postoperative outcomes in previous reviews have been inconsistent.
In the study, HIIT involved repeated aerobic high-intensity exercise intervals at about 80% of maximum heart rate, followed by active recovery.
The meta-analysis included 12 studies with 832 patients (mean age, 67) that compared preoperative HIIT – supervised at hospitals, gyms, or community or physical therapy centers, or unsupervised at home – with standard care for patients slated for major surgery, including liver, lung, colorectal, urologic, and mixed major abdominal operations.
The primary outcome was change in CRF by peak VO2 or 6-minute walk test; other endpoints included change in endurance time and postoperative outcomes.
TAKEAWAY:
Preoperative HIIT (median total, 160 minutes; range, 80-240 minutes; intense exercise during 6-40 sessions) was associated with an increase in peak oxygen consumption (VO2 peak) by 2.59 mL/kg/min (95% confidence interval, 1.52-3.65 mL/kg/min; P < .001), compared with standard care, which represents about a 10% increase in CRF.
In eight studies that involved 770 patients, there was moderate evidence that preoperative HIIT cut the odds ratio for postoperative complications by more than half (OR, 0.44; 95% CI, 0.32-0.60; P < .001); there was a similar apparent benefit in an analysis that was limited to patients who were slated for abdominal surgery (OR, 0.45; 95% CI, 0.29-0.68; P < .001).
An analysis that was limited to studies that reported hospital length of stay showed a clinically relevant but nonsignificant 3-day reduction among patients in the HIIT groups.
Most quality of life assessments did not show post-HIIT improvements; some showed a significant benefit 6 weeks after surgery.
IN PRACTICE:
The results suggest preoperative HIIT may improve postoperative outcomes. By extension, it could be cost-effective and “should be included in prehabilitation programs,” the report states.
SOURCE:
The study was carried out by Kari Clifford, PhD, Otago Medical School, University of Otago, Dunedin, New Zealand, and colleagues. It was published online June 30, 2023, in JAMA Network Open.
LIMITATIONS:
Included studies were heterogeneous in methodology; for example, HIIT definitions and protocols varied across almost every study. Data reporting was incomplete, the samples sizes in the studies were limited, and patients could not be blinded to their intervention. The patients could not be stratified on the basis of frailty. There were limited HIIT data from patients who underwent orthopedic surgeries.
DISCLOSURES:
The study received funding from the University of Otago. The authors reported no conflicts.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Infection-related chronic illness: A new paradigm for research and treatment
Experience with long COVID has shone a spotlight on persistent Lyme disease and other often debilitating chronic illnesses that follow known or suspected infections – and on the urgent need for a common and well-funded research agenda, education of physicians, growth of multidisciplinary clinics, and financially supported clinical care.
“We critically need to understand the epidemiology and pathogenesis of chronic symptoms, and identify more effective ways to manage, treat, and potentially cure these illnesses,” Lyle Petersen, MD, MPH, director of the division of vector-borne diseases at the Centers for Disease Control and Prevention, said at the start of a 2-day National Academies of Science, Engineering, and Medicine (NASEM) workshop, “Toward a Common Research Agenda in Infection-Associated Chronic Illnesses.”
Thinking about infection-associated chronic illnesses as an entity – one predicated on commonalities in chronic symptoms and in leading hypotheses for causes – represents a paradigm shift that researchers and patient advocates said can avoid research redundancies and is essential to address what the NASEM calls an overlooked, growing public health problem.
An estimated 2 million people in the United States are living with what’s called posttreatment Lyme disease (PTLD) – a subset of patients with persistent or chronic Lyme disease – and an estimated 1.7-3.3 million people in the United States have diagnoses of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). More than 700,000 people are living with multiple sclerosis. And as of January 2023, 11% of people in the United States reported having long COVID symptoms; the incidence of long COVID is currently estimated at 10%-30% of nonhospitalized cases of COVID-19.
These illnesses “have come under one umbrella,” said Avindra Nath, MD, clinical director of the National Institute of Neurologic Disorders and Stroke (NINDS), Bethesda, Md.
To date, common ground in the literature has grown largely around long COVID and ME/CFS, the latter of which is often associated with a prior, often unidentified infection.
Symptoms of both have been “rigorously” studied and shown to have overlaps, and the illnesses appear to share underlying biologic abnormalities in metabolism and the gut microbiome, as well as viral reactivation and abnormalities in the immune system, central and autonomic nervous systems, and the cardiovascular and pulmonary systems, said Anthony L. Komaroff, MD, professor of medicine at Harvard Medical School and a senior physician at Brigham & Women’s Hospital, both in Boston. (An estimated half of patients with long COVID meet the diagnostic criteria for ME/CFS.)
Although less thoroughly researched, similar symptoms are experienced by a subset of people following a variety of viral, bacterial, and protozoal infections, Dr. Komaroff said. To be determined, he said, is whether the pathophysiology believed to be shared by long COVID and ME/CFS is also shared with other postinfectious syndromes following acute illness with Ebola, West Nile, dengue, mycoplasma pneumonia, enteroviruses, and other pathogens, he said.
Persistent infection, viral reactivation
RNA viral infections can lead to persistent inflammation and dysregulated immunity, with or without viral persistence over time, Timothy J. Henrich, MD, MMSc, associate professor of medicine at the University of California, San Francisco, said in a keynote address.
Research on Ebola survivors has documented long-lasting inflammation and severe immune dysfunction 2 years after infection, for instance. And it’s well known that HIV-1 leads to aberrant immune responses, inflammation, and organ damage despite antiretroviral therapy, said Dr. Henrich, who leads a laboratory/research group that studies approaches to HIV-1 cure and PET-based imaging approaches to characterize viral reservoirs and immune sequelae.
Viral persistence, which can be difficult to measure, has also been documented in Ebola survivors. And in patients living with HIV-1, HIV-1 RNA and protein expression have been shown to persist, again despite antiretroviral therapy. The UCSF Long-Term Immunological Impact of Novel Coronavirus (LIINC) study, for which Dr. Henrich is the principal investigator, found spike RNA in colorectal tissue more than 22 months post COVID, and other research documented viral protein in gut tissue for up to 6 months, he said.
“I think we’re appreciating now, in at least the scientific and treatment community, that there’s a potential for ‘acute’ infections to exhibit some degree of persistence leading to clinical morbidity,” said Dr. Henrich, one of several speakers to describe reports of pathogen persistence. Regarding long COVID, its “etiology is likely heterogeneous,” he said, but persistence of SARS-CoV-2 “may lay behind” other described mechanisms, from clotting/microvascular dysfunction to inflammation and tissue damage to immune dysregulation.
Reactivation of existing latent viral infections in the setting of new acute microbial illness may also play an etiologic role in chronic illnesses, Dr. Henrich said. Epstein-Barr virus (EBV) reactivation has been shown in some studies, including their UCSF COVID-19 cohort, to be associated with long COVID.
“Physicians have been trained to be skeptical about the role [of latent viral infections],” Michael Peluso, MD, an infectious disease physician and assistant professor at UCSF, said during a talk on viral reactivation. This skepticism needs to be “reexamined and overcome,” he said.
Herpesviruses have frequently been associated with ME/CFS, he noted. And evidence of a strong association between EBV and multiple sclerosis came recently from a prospective study of 10 million military recruits that found a 32-fold increased risk of MS after EBV infection but no increase after infection with other viruses, Dr. Peluso and Dr. Henrich both noted.
Research needs, treatment trials
Research needs are vast: The need to learn more about the mechanisms of pathogen persistence and immune evasion, for instance, and the need for more biomarker studies, more imaging studies and tissue analyses, more study of microbiome composition and activity, and continued development and application of metagenomic next-generation sequencing.
Workshop participants also spoke of the need to better understand the molecular mimicry that can occur between pathogen-produced proteins and self-antigens, for instance, and the effects of inflammation and infection-related immune changes on neuronal and microglial function in the brain.
“We should perform similar forms of analysis [across] patients with different infection-associated chronic conditions,” said Amy Proal, PhD, president of the PolyBio Research Foundation, which funds research on infection-associated chronic infections. And within individual conditions and well-characterized study groups “we should perform many different forms of analysis … so we can define endotypes and get more solid biomarkers so that industry [will have more confidence] to run clinical trials.”
In the meantime, patients need fast-moving treatment trials for long COVID, long Lyme, and other infection-associated chronic illnesses, speakers emphasized. “We all agree that treatment trials are overdue,” said the NINDS’ Dr. Nath. “We can’t afford to wait for another decade until we understand all the mechanisms, but rather we can do clinical trials based on what we understand now and study the pathophysiology in the context of the clinical trials.”
Just as was done with HIV, said Steven G. Deeks, MD, professor of medicine at UCSF, researchers must “practice experimental medicine” and select pathways and mechanisms of interest, interrupt those pathways in a controlled manner, and assess impact. “Much of this can be done by repurposing existing drugs,” he said, like antivirals for persistent viral infection, EBV-directed therapies for EBV reactivation, anti-inflammatory drugs for inflammation, B–cell-directed therapies for autoantibodies, and antiplatelet drugs for microvascular disease.
When done correctly, he said, such “probe” studies can deepen mechanistic understandings, lead to biomarkers, and provide proof-of-concept that “will encourage massive investment in developing new therapies” for long COVID and other infection-associated chronic illnesses.
Trials of treatments for long COVID “are starting, so I’m optimistic,” said Dr. Deeks, an expert on HIV pathogenesis and treatment and a principal investigator of the Researching COVID to Enhance Recovery (RECOVER) study. Among the trials: A study of intravenous immunoglobulin (IVIG) for neurologic long COVID; a study of an anti-SARS-CoV-2 monoclonal antibody that can deplete tissue/cellular reservoirs of viral particles (replicating or not); and a study evaluating baricitinib (Olumiant), a Janus kinase inhibitor, for neurocognitive impairment and cardiopulmonary symptoms of long COVID.
Alessio Fasano, MD, professor of pediatrics at Harvard Medical School and professor of nutrition at the Harvard T.H. Chan School of Public Health, Boston, described at the workshop how he began investigating the use of larazotide acetate – an inhibitor of the protein zonulin, which increases intestinal permeability – in children with COVID-19 Multisystem Inflammatory Syndrome (MIS-C) after learning that SARS-CoV-2 viral particles persist in the gastrointestinal tract, causing dysbiosis and zonulin upregulation.
In an ongoing phase 2, double-blind, placebo-controlled trial, the agent thus far has expedited the resolution of gastrointestinal symptoms and clearance of spike protein from the circulation, he said. A phase 2 trial of the agent for pediatric patients with long COVID and SARS-CoV-2 antigenemia is underway. “What if we were to stop [chains of events] by stopping the passage of elements from the virus into circulation?’ he said.
In the realm of Lyme disease, a recently launched Clinical Trials Network for Lyme and Other Tick-Borne Diseases has awarded pilot study grants to evaluate treatments aimed at a variety of possible disease mechanisms that, notably, are similar to those of other chronic illnesses: persistence of infection or remnants of infection, immune dysregulation and autoimmune reactions, neural dysfunction, and gut microbiome changes. (Microclots and mitochondrial dysfunction have not been as well studied in Lyme.)
Current and upcoming studies include evaluations of transcutaneous auricular vagus nerve stimulation for those with persistent Lyme fatigue, transcranial direct current stimulation with cognitive retraining for Lyme brain fog, and tetracycline for PTLD, said Brian Fallon, MD, MPH, professor of clinical psychiatry at Columbia University, New York, who directs the Lyme & Tick-Borne Diseases Research Center and the coordinating center of the new network.
Moving forward, he said, it is important to loosen exclusion criteria and include patients with “probable or possible” Lyme and those with suspected infections with other tick-borne pathogens. All told, these patients comprise a large portion of those with chronic symptoms and have been neglected in an already thin research space, Dr. Fallon said, noting that “there haven’t been any clinical trials of posttreatment Lyme disease in ages – in 10-15 years.”
(PTLD refers to symptoms lasting for more than 6 months after the completion of standard Infectious Diseases Society of America–recommended antibiotic protocols. It occurs in about 15% of patients, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Research Center, Baltimore, a member of the new clinical trials network.)
Calls for a new NIH center and patient involvement
Patients and patient advocacy organizations have played a vital role in research thus far: They’ve documented post-COVID symptoms that academic researchers said they would not otherwise have known of. Leaders of the Patient-Led Research Collaborative have coauthored published reviews with leading long COVID experts. And patients with tick-borne illnesses have enrolled in the MyLymeData patient registry run by LymeDisease.org, which has documented patient-experienced efficacy of alternative treatments and described antibiotic responders and nonresponders.
At the workshop, they shared findings alongside academic experts, and researchers called for their continued involvement. “Patient engagement at every step of the research process is critical,” Dr. Nath said.
“We need to ensure that research is reflective of lived experiences … and [that we’re] accelerating clinical trials of therapeutics that are of priority to the patient community,” said Lisa McCorkell, cofounder of the long COVID-focused Patient-Led Research Collaborative.
Ms. McCorkell also called for the creation of an office for infection-associated chronic illnesses in the NIH director’s office. Others voiced their support. “I think it’s a great idea to have an NIH center for infection-associated chronic illnesses,” said Dr. Fallon. “I think it would have a profound impact.”
The other great need, of course, is funding. “We have ideas, we have drugs that can be repurposed, we have a highly informed and engaged community that will enroll in and be retained in studies, and we have outcomes we can measure,” Dr. Deeks said. “What we’re missing is industry engagement and funding. We need massive engagement from the NIH.”
Real-world treatment needs
In the meantime, patients are seeking treatment, and “clinicians need to have uncertainty tolerance” and try multiple treatments simultaneously, said David Putrino, PT, PhD, director of rehabilitation innovation for the Mount Sinai Health System and professor of rehabilitation and human performance at the Icahn School of Medicine at Mount Sinai, New York. He oversees a multidisciplinary hybrid clinical care research center that has seen over 1,500 patients with long COVID and is beginning to see patients with other infection-associated chronic illnesses.
It’s a model that should be replicated to help fill the “enormous unmet clinical need” of patients with infection-associated chronic illness, said Peter Rowe, MD, professor of pediatrics at the Johns Hopkins School of Medicine and an expert on ME/CFS. And “as we request [more research funding], we will also need [financial] support for clinical care,” he emphasized, to provide equitable access for patients and to attract treating physicians.
Moreover, said Linda Geng, MD, PhD, the culture of stigma needs to change. Right now, patients with long COVID often feel dismissed not only by friends, families, and coworkers, but by clinicians who find it find it hard “to grasp that this is real and a biological condition.”
And it’s not just conditions such as long COVID that are stigmatized, but treatments as well, she said. For instance, some clinicians view low-dose naltrexone, a treatment increasingly being used for inflammation, with suspicion because it is used for opioid use disorder and alcohol use disorder – or because the “low-dose” label summons mistrust of homeopathy. “Even with therapies, there are preconceived notions and biases,” said Dr. Geng, cofounder and codirector of the Stanford (Calif.) Long COVID program.
“What almost killed me,” said Meghan O’Rourke, who has ongoing effects from long-undiagnosed tick-borne illness, “was the invisibility of the illness.” Ms. O’Rourke teaches at Yale University and is the author of “The Invisible Kingdom: Reimagining Chronic Illness.”
Teaching young physicians about these illnesses would help, she and others said. During a question and answer session, Dr. Putrino shared that the Icahn School of Medicine has recently committed to “create a complex chronic illness medical curriculum” that will impact medical education from the first year of medical school through residencies. Dr. Putrino said his team is also working on materials to help other clinics develop care models similar to those at his Mount Sinai clinic.
The NASEM workshop did not collect or require disclosures of its participants.
Experience with long COVID has shone a spotlight on persistent Lyme disease and other often debilitating chronic illnesses that follow known or suspected infections – and on the urgent need for a common and well-funded research agenda, education of physicians, growth of multidisciplinary clinics, and financially supported clinical care.
“We critically need to understand the epidemiology and pathogenesis of chronic symptoms, and identify more effective ways to manage, treat, and potentially cure these illnesses,” Lyle Petersen, MD, MPH, director of the division of vector-borne diseases at the Centers for Disease Control and Prevention, said at the start of a 2-day National Academies of Science, Engineering, and Medicine (NASEM) workshop, “Toward a Common Research Agenda in Infection-Associated Chronic Illnesses.”
Thinking about infection-associated chronic illnesses as an entity – one predicated on commonalities in chronic symptoms and in leading hypotheses for causes – represents a paradigm shift that researchers and patient advocates said can avoid research redundancies and is essential to address what the NASEM calls an overlooked, growing public health problem.
An estimated 2 million people in the United States are living with what’s called posttreatment Lyme disease (PTLD) – a subset of patients with persistent or chronic Lyme disease – and an estimated 1.7-3.3 million people in the United States have diagnoses of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). More than 700,000 people are living with multiple sclerosis. And as of January 2023, 11% of people in the United States reported having long COVID symptoms; the incidence of long COVID is currently estimated at 10%-30% of nonhospitalized cases of COVID-19.
These illnesses “have come under one umbrella,” said Avindra Nath, MD, clinical director of the National Institute of Neurologic Disorders and Stroke (NINDS), Bethesda, Md.
To date, common ground in the literature has grown largely around long COVID and ME/CFS, the latter of which is often associated with a prior, often unidentified infection.
Symptoms of both have been “rigorously” studied and shown to have overlaps, and the illnesses appear to share underlying biologic abnormalities in metabolism and the gut microbiome, as well as viral reactivation and abnormalities in the immune system, central and autonomic nervous systems, and the cardiovascular and pulmonary systems, said Anthony L. Komaroff, MD, professor of medicine at Harvard Medical School and a senior physician at Brigham & Women’s Hospital, both in Boston. (An estimated half of patients with long COVID meet the diagnostic criteria for ME/CFS.)
Although less thoroughly researched, similar symptoms are experienced by a subset of people following a variety of viral, bacterial, and protozoal infections, Dr. Komaroff said. To be determined, he said, is whether the pathophysiology believed to be shared by long COVID and ME/CFS is also shared with other postinfectious syndromes following acute illness with Ebola, West Nile, dengue, mycoplasma pneumonia, enteroviruses, and other pathogens, he said.
Persistent infection, viral reactivation
RNA viral infections can lead to persistent inflammation and dysregulated immunity, with or without viral persistence over time, Timothy J. Henrich, MD, MMSc, associate professor of medicine at the University of California, San Francisco, said in a keynote address.
Research on Ebola survivors has documented long-lasting inflammation and severe immune dysfunction 2 years after infection, for instance. And it’s well known that HIV-1 leads to aberrant immune responses, inflammation, and organ damage despite antiretroviral therapy, said Dr. Henrich, who leads a laboratory/research group that studies approaches to HIV-1 cure and PET-based imaging approaches to characterize viral reservoirs and immune sequelae.
Viral persistence, which can be difficult to measure, has also been documented in Ebola survivors. And in patients living with HIV-1, HIV-1 RNA and protein expression have been shown to persist, again despite antiretroviral therapy. The UCSF Long-Term Immunological Impact of Novel Coronavirus (LIINC) study, for which Dr. Henrich is the principal investigator, found spike RNA in colorectal tissue more than 22 months post COVID, and other research documented viral protein in gut tissue for up to 6 months, he said.
“I think we’re appreciating now, in at least the scientific and treatment community, that there’s a potential for ‘acute’ infections to exhibit some degree of persistence leading to clinical morbidity,” said Dr. Henrich, one of several speakers to describe reports of pathogen persistence. Regarding long COVID, its “etiology is likely heterogeneous,” he said, but persistence of SARS-CoV-2 “may lay behind” other described mechanisms, from clotting/microvascular dysfunction to inflammation and tissue damage to immune dysregulation.
Reactivation of existing latent viral infections in the setting of new acute microbial illness may also play an etiologic role in chronic illnesses, Dr. Henrich said. Epstein-Barr virus (EBV) reactivation has been shown in some studies, including their UCSF COVID-19 cohort, to be associated with long COVID.
“Physicians have been trained to be skeptical about the role [of latent viral infections],” Michael Peluso, MD, an infectious disease physician and assistant professor at UCSF, said during a talk on viral reactivation. This skepticism needs to be “reexamined and overcome,” he said.
Herpesviruses have frequently been associated with ME/CFS, he noted. And evidence of a strong association between EBV and multiple sclerosis came recently from a prospective study of 10 million military recruits that found a 32-fold increased risk of MS after EBV infection but no increase after infection with other viruses, Dr. Peluso and Dr. Henrich both noted.
Research needs, treatment trials
Research needs are vast: The need to learn more about the mechanisms of pathogen persistence and immune evasion, for instance, and the need for more biomarker studies, more imaging studies and tissue analyses, more study of microbiome composition and activity, and continued development and application of metagenomic next-generation sequencing.
Workshop participants also spoke of the need to better understand the molecular mimicry that can occur between pathogen-produced proteins and self-antigens, for instance, and the effects of inflammation and infection-related immune changes on neuronal and microglial function in the brain.
“We should perform similar forms of analysis [across] patients with different infection-associated chronic conditions,” said Amy Proal, PhD, president of the PolyBio Research Foundation, which funds research on infection-associated chronic infections. And within individual conditions and well-characterized study groups “we should perform many different forms of analysis … so we can define endotypes and get more solid biomarkers so that industry [will have more confidence] to run clinical trials.”
In the meantime, patients need fast-moving treatment trials for long COVID, long Lyme, and other infection-associated chronic illnesses, speakers emphasized. “We all agree that treatment trials are overdue,” said the NINDS’ Dr. Nath. “We can’t afford to wait for another decade until we understand all the mechanisms, but rather we can do clinical trials based on what we understand now and study the pathophysiology in the context of the clinical trials.”
Just as was done with HIV, said Steven G. Deeks, MD, professor of medicine at UCSF, researchers must “practice experimental medicine” and select pathways and mechanisms of interest, interrupt those pathways in a controlled manner, and assess impact. “Much of this can be done by repurposing existing drugs,” he said, like antivirals for persistent viral infection, EBV-directed therapies for EBV reactivation, anti-inflammatory drugs for inflammation, B–cell-directed therapies for autoantibodies, and antiplatelet drugs for microvascular disease.
When done correctly, he said, such “probe” studies can deepen mechanistic understandings, lead to biomarkers, and provide proof-of-concept that “will encourage massive investment in developing new therapies” for long COVID and other infection-associated chronic illnesses.
Trials of treatments for long COVID “are starting, so I’m optimistic,” said Dr. Deeks, an expert on HIV pathogenesis and treatment and a principal investigator of the Researching COVID to Enhance Recovery (RECOVER) study. Among the trials: A study of intravenous immunoglobulin (IVIG) for neurologic long COVID; a study of an anti-SARS-CoV-2 monoclonal antibody that can deplete tissue/cellular reservoirs of viral particles (replicating or not); and a study evaluating baricitinib (Olumiant), a Janus kinase inhibitor, for neurocognitive impairment and cardiopulmonary symptoms of long COVID.
Alessio Fasano, MD, professor of pediatrics at Harvard Medical School and professor of nutrition at the Harvard T.H. Chan School of Public Health, Boston, described at the workshop how he began investigating the use of larazotide acetate – an inhibitor of the protein zonulin, which increases intestinal permeability – in children with COVID-19 Multisystem Inflammatory Syndrome (MIS-C) after learning that SARS-CoV-2 viral particles persist in the gastrointestinal tract, causing dysbiosis and zonulin upregulation.
In an ongoing phase 2, double-blind, placebo-controlled trial, the agent thus far has expedited the resolution of gastrointestinal symptoms and clearance of spike protein from the circulation, he said. A phase 2 trial of the agent for pediatric patients with long COVID and SARS-CoV-2 antigenemia is underway. “What if we were to stop [chains of events] by stopping the passage of elements from the virus into circulation?’ he said.
In the realm of Lyme disease, a recently launched Clinical Trials Network for Lyme and Other Tick-Borne Diseases has awarded pilot study grants to evaluate treatments aimed at a variety of possible disease mechanisms that, notably, are similar to those of other chronic illnesses: persistence of infection or remnants of infection, immune dysregulation and autoimmune reactions, neural dysfunction, and gut microbiome changes. (Microclots and mitochondrial dysfunction have not been as well studied in Lyme.)
Current and upcoming studies include evaluations of transcutaneous auricular vagus nerve stimulation for those with persistent Lyme fatigue, transcranial direct current stimulation with cognitive retraining for Lyme brain fog, and tetracycline for PTLD, said Brian Fallon, MD, MPH, professor of clinical psychiatry at Columbia University, New York, who directs the Lyme & Tick-Borne Diseases Research Center and the coordinating center of the new network.
Moving forward, he said, it is important to loosen exclusion criteria and include patients with “probable or possible” Lyme and those with suspected infections with other tick-borne pathogens. All told, these patients comprise a large portion of those with chronic symptoms and have been neglected in an already thin research space, Dr. Fallon said, noting that “there haven’t been any clinical trials of posttreatment Lyme disease in ages – in 10-15 years.”
(PTLD refers to symptoms lasting for more than 6 months after the completion of standard Infectious Diseases Society of America–recommended antibiotic protocols. It occurs in about 15% of patients, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Research Center, Baltimore, a member of the new clinical trials network.)
Calls for a new NIH center and patient involvement
Patients and patient advocacy organizations have played a vital role in research thus far: They’ve documented post-COVID symptoms that academic researchers said they would not otherwise have known of. Leaders of the Patient-Led Research Collaborative have coauthored published reviews with leading long COVID experts. And patients with tick-borne illnesses have enrolled in the MyLymeData patient registry run by LymeDisease.org, which has documented patient-experienced efficacy of alternative treatments and described antibiotic responders and nonresponders.
At the workshop, they shared findings alongside academic experts, and researchers called for their continued involvement. “Patient engagement at every step of the research process is critical,” Dr. Nath said.
“We need to ensure that research is reflective of lived experiences … and [that we’re] accelerating clinical trials of therapeutics that are of priority to the patient community,” said Lisa McCorkell, cofounder of the long COVID-focused Patient-Led Research Collaborative.
Ms. McCorkell also called for the creation of an office for infection-associated chronic illnesses in the NIH director’s office. Others voiced their support. “I think it’s a great idea to have an NIH center for infection-associated chronic illnesses,” said Dr. Fallon. “I think it would have a profound impact.”
The other great need, of course, is funding. “We have ideas, we have drugs that can be repurposed, we have a highly informed and engaged community that will enroll in and be retained in studies, and we have outcomes we can measure,” Dr. Deeks said. “What we’re missing is industry engagement and funding. We need massive engagement from the NIH.”
Real-world treatment needs
In the meantime, patients are seeking treatment, and “clinicians need to have uncertainty tolerance” and try multiple treatments simultaneously, said David Putrino, PT, PhD, director of rehabilitation innovation for the Mount Sinai Health System and professor of rehabilitation and human performance at the Icahn School of Medicine at Mount Sinai, New York. He oversees a multidisciplinary hybrid clinical care research center that has seen over 1,500 patients with long COVID and is beginning to see patients with other infection-associated chronic illnesses.
It’s a model that should be replicated to help fill the “enormous unmet clinical need” of patients with infection-associated chronic illness, said Peter Rowe, MD, professor of pediatrics at the Johns Hopkins School of Medicine and an expert on ME/CFS. And “as we request [more research funding], we will also need [financial] support for clinical care,” he emphasized, to provide equitable access for patients and to attract treating physicians.
Moreover, said Linda Geng, MD, PhD, the culture of stigma needs to change. Right now, patients with long COVID often feel dismissed not only by friends, families, and coworkers, but by clinicians who find it find it hard “to grasp that this is real and a biological condition.”
And it’s not just conditions such as long COVID that are stigmatized, but treatments as well, she said. For instance, some clinicians view low-dose naltrexone, a treatment increasingly being used for inflammation, with suspicion because it is used for opioid use disorder and alcohol use disorder – or because the “low-dose” label summons mistrust of homeopathy. “Even with therapies, there are preconceived notions and biases,” said Dr. Geng, cofounder and codirector of the Stanford (Calif.) Long COVID program.
“What almost killed me,” said Meghan O’Rourke, who has ongoing effects from long-undiagnosed tick-borne illness, “was the invisibility of the illness.” Ms. O’Rourke teaches at Yale University and is the author of “The Invisible Kingdom: Reimagining Chronic Illness.”
Teaching young physicians about these illnesses would help, she and others said. During a question and answer session, Dr. Putrino shared that the Icahn School of Medicine has recently committed to “create a complex chronic illness medical curriculum” that will impact medical education from the first year of medical school through residencies. Dr. Putrino said his team is also working on materials to help other clinics develop care models similar to those at his Mount Sinai clinic.
The NASEM workshop did not collect or require disclosures of its participants.
Experience with long COVID has shone a spotlight on persistent Lyme disease and other often debilitating chronic illnesses that follow known or suspected infections – and on the urgent need for a common and well-funded research agenda, education of physicians, growth of multidisciplinary clinics, and financially supported clinical care.
“We critically need to understand the epidemiology and pathogenesis of chronic symptoms, and identify more effective ways to manage, treat, and potentially cure these illnesses,” Lyle Petersen, MD, MPH, director of the division of vector-borne diseases at the Centers for Disease Control and Prevention, said at the start of a 2-day National Academies of Science, Engineering, and Medicine (NASEM) workshop, “Toward a Common Research Agenda in Infection-Associated Chronic Illnesses.”
Thinking about infection-associated chronic illnesses as an entity – one predicated on commonalities in chronic symptoms and in leading hypotheses for causes – represents a paradigm shift that researchers and patient advocates said can avoid research redundancies and is essential to address what the NASEM calls an overlooked, growing public health problem.
An estimated 2 million people in the United States are living with what’s called posttreatment Lyme disease (PTLD) – a subset of patients with persistent or chronic Lyme disease – and an estimated 1.7-3.3 million people in the United States have diagnoses of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). More than 700,000 people are living with multiple sclerosis. And as of January 2023, 11% of people in the United States reported having long COVID symptoms; the incidence of long COVID is currently estimated at 10%-30% of nonhospitalized cases of COVID-19.
These illnesses “have come under one umbrella,” said Avindra Nath, MD, clinical director of the National Institute of Neurologic Disorders and Stroke (NINDS), Bethesda, Md.
To date, common ground in the literature has grown largely around long COVID and ME/CFS, the latter of which is often associated with a prior, often unidentified infection.
Symptoms of both have been “rigorously” studied and shown to have overlaps, and the illnesses appear to share underlying biologic abnormalities in metabolism and the gut microbiome, as well as viral reactivation and abnormalities in the immune system, central and autonomic nervous systems, and the cardiovascular and pulmonary systems, said Anthony L. Komaroff, MD, professor of medicine at Harvard Medical School and a senior physician at Brigham & Women’s Hospital, both in Boston. (An estimated half of patients with long COVID meet the diagnostic criteria for ME/CFS.)
Although less thoroughly researched, similar symptoms are experienced by a subset of people following a variety of viral, bacterial, and protozoal infections, Dr. Komaroff said. To be determined, he said, is whether the pathophysiology believed to be shared by long COVID and ME/CFS is also shared with other postinfectious syndromes following acute illness with Ebola, West Nile, dengue, mycoplasma pneumonia, enteroviruses, and other pathogens, he said.
Persistent infection, viral reactivation
RNA viral infections can lead to persistent inflammation and dysregulated immunity, with or without viral persistence over time, Timothy J. Henrich, MD, MMSc, associate professor of medicine at the University of California, San Francisco, said in a keynote address.
Research on Ebola survivors has documented long-lasting inflammation and severe immune dysfunction 2 years after infection, for instance. And it’s well known that HIV-1 leads to aberrant immune responses, inflammation, and organ damage despite antiretroviral therapy, said Dr. Henrich, who leads a laboratory/research group that studies approaches to HIV-1 cure and PET-based imaging approaches to characterize viral reservoirs and immune sequelae.
Viral persistence, which can be difficult to measure, has also been documented in Ebola survivors. And in patients living with HIV-1, HIV-1 RNA and protein expression have been shown to persist, again despite antiretroviral therapy. The UCSF Long-Term Immunological Impact of Novel Coronavirus (LIINC) study, for which Dr. Henrich is the principal investigator, found spike RNA in colorectal tissue more than 22 months post COVID, and other research documented viral protein in gut tissue for up to 6 months, he said.
“I think we’re appreciating now, in at least the scientific and treatment community, that there’s a potential for ‘acute’ infections to exhibit some degree of persistence leading to clinical morbidity,” said Dr. Henrich, one of several speakers to describe reports of pathogen persistence. Regarding long COVID, its “etiology is likely heterogeneous,” he said, but persistence of SARS-CoV-2 “may lay behind” other described mechanisms, from clotting/microvascular dysfunction to inflammation and tissue damage to immune dysregulation.
Reactivation of existing latent viral infections in the setting of new acute microbial illness may also play an etiologic role in chronic illnesses, Dr. Henrich said. Epstein-Barr virus (EBV) reactivation has been shown in some studies, including their UCSF COVID-19 cohort, to be associated with long COVID.
“Physicians have been trained to be skeptical about the role [of latent viral infections],” Michael Peluso, MD, an infectious disease physician and assistant professor at UCSF, said during a talk on viral reactivation. This skepticism needs to be “reexamined and overcome,” he said.
Herpesviruses have frequently been associated with ME/CFS, he noted. And evidence of a strong association between EBV and multiple sclerosis came recently from a prospective study of 10 million military recruits that found a 32-fold increased risk of MS after EBV infection but no increase after infection with other viruses, Dr. Peluso and Dr. Henrich both noted.
Research needs, treatment trials
Research needs are vast: The need to learn more about the mechanisms of pathogen persistence and immune evasion, for instance, and the need for more biomarker studies, more imaging studies and tissue analyses, more study of microbiome composition and activity, and continued development and application of metagenomic next-generation sequencing.
Workshop participants also spoke of the need to better understand the molecular mimicry that can occur between pathogen-produced proteins and self-antigens, for instance, and the effects of inflammation and infection-related immune changes on neuronal and microglial function in the brain.
“We should perform similar forms of analysis [across] patients with different infection-associated chronic conditions,” said Amy Proal, PhD, president of the PolyBio Research Foundation, which funds research on infection-associated chronic infections. And within individual conditions and well-characterized study groups “we should perform many different forms of analysis … so we can define endotypes and get more solid biomarkers so that industry [will have more confidence] to run clinical trials.”
In the meantime, patients need fast-moving treatment trials for long COVID, long Lyme, and other infection-associated chronic illnesses, speakers emphasized. “We all agree that treatment trials are overdue,” said the NINDS’ Dr. Nath. “We can’t afford to wait for another decade until we understand all the mechanisms, but rather we can do clinical trials based on what we understand now and study the pathophysiology in the context of the clinical trials.”
Just as was done with HIV, said Steven G. Deeks, MD, professor of medicine at UCSF, researchers must “practice experimental medicine” and select pathways and mechanisms of interest, interrupt those pathways in a controlled manner, and assess impact. “Much of this can be done by repurposing existing drugs,” he said, like antivirals for persistent viral infection, EBV-directed therapies for EBV reactivation, anti-inflammatory drugs for inflammation, B–cell-directed therapies for autoantibodies, and antiplatelet drugs for microvascular disease.
When done correctly, he said, such “probe” studies can deepen mechanistic understandings, lead to biomarkers, and provide proof-of-concept that “will encourage massive investment in developing new therapies” for long COVID and other infection-associated chronic illnesses.
Trials of treatments for long COVID “are starting, so I’m optimistic,” said Dr. Deeks, an expert on HIV pathogenesis and treatment and a principal investigator of the Researching COVID to Enhance Recovery (RECOVER) study. Among the trials: A study of intravenous immunoglobulin (IVIG) for neurologic long COVID; a study of an anti-SARS-CoV-2 monoclonal antibody that can deplete tissue/cellular reservoirs of viral particles (replicating or not); and a study evaluating baricitinib (Olumiant), a Janus kinase inhibitor, for neurocognitive impairment and cardiopulmonary symptoms of long COVID.
Alessio Fasano, MD, professor of pediatrics at Harvard Medical School and professor of nutrition at the Harvard T.H. Chan School of Public Health, Boston, described at the workshop how he began investigating the use of larazotide acetate – an inhibitor of the protein zonulin, which increases intestinal permeability – in children with COVID-19 Multisystem Inflammatory Syndrome (MIS-C) after learning that SARS-CoV-2 viral particles persist in the gastrointestinal tract, causing dysbiosis and zonulin upregulation.
In an ongoing phase 2, double-blind, placebo-controlled trial, the agent thus far has expedited the resolution of gastrointestinal symptoms and clearance of spike protein from the circulation, he said. A phase 2 trial of the agent for pediatric patients with long COVID and SARS-CoV-2 antigenemia is underway. “What if we were to stop [chains of events] by stopping the passage of elements from the virus into circulation?’ he said.
In the realm of Lyme disease, a recently launched Clinical Trials Network for Lyme and Other Tick-Borne Diseases has awarded pilot study grants to evaluate treatments aimed at a variety of possible disease mechanisms that, notably, are similar to those of other chronic illnesses: persistence of infection or remnants of infection, immune dysregulation and autoimmune reactions, neural dysfunction, and gut microbiome changes. (Microclots and mitochondrial dysfunction have not been as well studied in Lyme.)
Current and upcoming studies include evaluations of transcutaneous auricular vagus nerve stimulation for those with persistent Lyme fatigue, transcranial direct current stimulation with cognitive retraining for Lyme brain fog, and tetracycline for PTLD, said Brian Fallon, MD, MPH, professor of clinical psychiatry at Columbia University, New York, who directs the Lyme & Tick-Borne Diseases Research Center and the coordinating center of the new network.
Moving forward, he said, it is important to loosen exclusion criteria and include patients with “probable or possible” Lyme and those with suspected infections with other tick-borne pathogens. All told, these patients comprise a large portion of those with chronic symptoms and have been neglected in an already thin research space, Dr. Fallon said, noting that “there haven’t been any clinical trials of posttreatment Lyme disease in ages – in 10-15 years.”
(PTLD refers to symptoms lasting for more than 6 months after the completion of standard Infectious Diseases Society of America–recommended antibiotic protocols. It occurs in about 15% of patients, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Research Center, Baltimore, a member of the new clinical trials network.)
Calls for a new NIH center and patient involvement
Patients and patient advocacy organizations have played a vital role in research thus far: They’ve documented post-COVID symptoms that academic researchers said they would not otherwise have known of. Leaders of the Patient-Led Research Collaborative have coauthored published reviews with leading long COVID experts. And patients with tick-borne illnesses have enrolled in the MyLymeData patient registry run by LymeDisease.org, which has documented patient-experienced efficacy of alternative treatments and described antibiotic responders and nonresponders.
At the workshop, they shared findings alongside academic experts, and researchers called for their continued involvement. “Patient engagement at every step of the research process is critical,” Dr. Nath said.
“We need to ensure that research is reflective of lived experiences … and [that we’re] accelerating clinical trials of therapeutics that are of priority to the patient community,” said Lisa McCorkell, cofounder of the long COVID-focused Patient-Led Research Collaborative.
Ms. McCorkell also called for the creation of an office for infection-associated chronic illnesses in the NIH director’s office. Others voiced their support. “I think it’s a great idea to have an NIH center for infection-associated chronic illnesses,” said Dr. Fallon. “I think it would have a profound impact.”
The other great need, of course, is funding. “We have ideas, we have drugs that can be repurposed, we have a highly informed and engaged community that will enroll in and be retained in studies, and we have outcomes we can measure,” Dr. Deeks said. “What we’re missing is industry engagement and funding. We need massive engagement from the NIH.”
Real-world treatment needs
In the meantime, patients are seeking treatment, and “clinicians need to have uncertainty tolerance” and try multiple treatments simultaneously, said David Putrino, PT, PhD, director of rehabilitation innovation for the Mount Sinai Health System and professor of rehabilitation and human performance at the Icahn School of Medicine at Mount Sinai, New York. He oversees a multidisciplinary hybrid clinical care research center that has seen over 1,500 patients with long COVID and is beginning to see patients with other infection-associated chronic illnesses.
It’s a model that should be replicated to help fill the “enormous unmet clinical need” of patients with infection-associated chronic illness, said Peter Rowe, MD, professor of pediatrics at the Johns Hopkins School of Medicine and an expert on ME/CFS. And “as we request [more research funding], we will also need [financial] support for clinical care,” he emphasized, to provide equitable access for patients and to attract treating physicians.
Moreover, said Linda Geng, MD, PhD, the culture of stigma needs to change. Right now, patients with long COVID often feel dismissed not only by friends, families, and coworkers, but by clinicians who find it find it hard “to grasp that this is real and a biological condition.”
And it’s not just conditions such as long COVID that are stigmatized, but treatments as well, she said. For instance, some clinicians view low-dose naltrexone, a treatment increasingly being used for inflammation, with suspicion because it is used for opioid use disorder and alcohol use disorder – or because the “low-dose” label summons mistrust of homeopathy. “Even with therapies, there are preconceived notions and biases,” said Dr. Geng, cofounder and codirector of the Stanford (Calif.) Long COVID program.
“What almost killed me,” said Meghan O’Rourke, who has ongoing effects from long-undiagnosed tick-borne illness, “was the invisibility of the illness.” Ms. O’Rourke teaches at Yale University and is the author of “The Invisible Kingdom: Reimagining Chronic Illness.”
Teaching young physicians about these illnesses would help, she and others said. During a question and answer session, Dr. Putrino shared that the Icahn School of Medicine has recently committed to “create a complex chronic illness medical curriculum” that will impact medical education from the first year of medical school through residencies. Dr. Putrino said his team is also working on materials to help other clinics develop care models similar to those at his Mount Sinai clinic.
The NASEM workshop did not collect or require disclosures of its participants.
FROM A NATIONAL ACADEMIES OF SCIENCE, ENGINEERING, AND MEDICINE WORKSHOP
How a heat wave affects glycemic control
TOPLINE:
, according to research published online May 17 in Science of The Total Environment.
METHODOLOGY:
Researchers in Spain analyzed data from 2,701 adults with type 1 diabetes who had been using intermittently scanned continuous glucose monitoring (CGM) devices during a 2022 heat wave (July 9-26) and 14 days after. Extreme heat claimed nearly 62,000 lives across Europe in the summer of 2022.
TAKEAWAY:
Time in range (between 70 mg/dL and 180 mg/dL of interstitial glucose) decreased by 4%, from 60.8% during the heat wave to 54.8% after (P < .001).
Patients who scanned their CGM results the most during the heat wave (more than 13 scans per day) scanned less often after the weather broke (1.8 fewer scans per day) and experienced the biggest drop in time in range (−5.4%).
More patients met all time-in-range recommendations during the heat wave (10.6% vs. 8.4%, P < .001).
IN PRACTICE:
“We hypothesized that people with diabetes, who are highly vulnerable, have more time for self-management as they spend more time indoors,” study author Jesús Moreno Fernández, MD, PhD, said in an interview. “During the COVID-19 pandemic, something similar was observed among people with diabetes.”
SOURCE:
Moreno Fernández, with the department of endocrinology and nutrition at Ciudad Real General University Hospital in Spain, is the study’s lead author.
LIMITATIONS:
The CGM data were anonymized, so researchers could not examine how individual patient factors like sex, education, or treatment type may have influenced outcomes. Temperatures remained higher than usual even after the heat wave. Worsening glycemic control could be interpreted as a lag effect of prolonged heat exposure, the researchers note.
DISCLOSURES:
The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to research published online May 17 in Science of The Total Environment.
METHODOLOGY:
Researchers in Spain analyzed data from 2,701 adults with type 1 diabetes who had been using intermittently scanned continuous glucose monitoring (CGM) devices during a 2022 heat wave (July 9-26) and 14 days after. Extreme heat claimed nearly 62,000 lives across Europe in the summer of 2022.
TAKEAWAY:
Time in range (between 70 mg/dL and 180 mg/dL of interstitial glucose) decreased by 4%, from 60.8% during the heat wave to 54.8% after (P < .001).
Patients who scanned their CGM results the most during the heat wave (more than 13 scans per day) scanned less often after the weather broke (1.8 fewer scans per day) and experienced the biggest drop in time in range (−5.4%).
More patients met all time-in-range recommendations during the heat wave (10.6% vs. 8.4%, P < .001).
IN PRACTICE:
“We hypothesized that people with diabetes, who are highly vulnerable, have more time for self-management as they spend more time indoors,” study author Jesús Moreno Fernández, MD, PhD, said in an interview. “During the COVID-19 pandemic, something similar was observed among people with diabetes.”
SOURCE:
Moreno Fernández, with the department of endocrinology and nutrition at Ciudad Real General University Hospital in Spain, is the study’s lead author.
LIMITATIONS:
The CGM data were anonymized, so researchers could not examine how individual patient factors like sex, education, or treatment type may have influenced outcomes. Temperatures remained higher than usual even after the heat wave. Worsening glycemic control could be interpreted as a lag effect of prolonged heat exposure, the researchers note.
DISCLOSURES:
The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to research published online May 17 in Science of The Total Environment.
METHODOLOGY:
Researchers in Spain analyzed data from 2,701 adults with type 1 diabetes who had been using intermittently scanned continuous glucose monitoring (CGM) devices during a 2022 heat wave (July 9-26) and 14 days after. Extreme heat claimed nearly 62,000 lives across Europe in the summer of 2022.
TAKEAWAY:
Time in range (between 70 mg/dL and 180 mg/dL of interstitial glucose) decreased by 4%, from 60.8% during the heat wave to 54.8% after (P < .001).
Patients who scanned their CGM results the most during the heat wave (more than 13 scans per day) scanned less often after the weather broke (1.8 fewer scans per day) and experienced the biggest drop in time in range (−5.4%).
More patients met all time-in-range recommendations during the heat wave (10.6% vs. 8.4%, P < .001).
IN PRACTICE:
“We hypothesized that people with diabetes, who are highly vulnerable, have more time for self-management as they spend more time indoors,” study author Jesús Moreno Fernández, MD, PhD, said in an interview. “During the COVID-19 pandemic, something similar was observed among people with diabetes.”
SOURCE:
Moreno Fernández, with the department of endocrinology and nutrition at Ciudad Real General University Hospital in Spain, is the study’s lead author.
LIMITATIONS:
The CGM data were anonymized, so researchers could not examine how individual patient factors like sex, education, or treatment type may have influenced outcomes. Temperatures remained higher than usual even after the heat wave. Worsening glycemic control could be interpreted as a lag effect of prolonged heat exposure, the researchers note.
DISCLOSURES:
The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM SCIENCE OF THE TOTAL ENVIRONMENT
Omega-3s and AFib: No added risk from eating fish but high-dose supplement questions persist
Regular consumption of fish and other foods rich in omega-3 fatty acids (FA) won’t raise an individual’s risk for developing atrial fibrillation (AFib), suggests a meta-analysis of population-based studies.
The finding may alleviate recent concerns about higher-dose omega-3 FA supplement intake in clinical-trial patients at elevated cardiovascular (CV) risk, researchers say.
Indeed, across the 17 cohort studies in the meta-analysis, risk for incident AFib was unaffected by elevated circulating and adipose tissue levels of eicosapentaenoic acid (EPA) from dietary intake. Moreover, the risk appeared to drop significantly with such levels of docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and EPA plus DHA.
The signals of AFib risk associated with high-dose omega-3 FA in supplements or prescription form in some clinical trials “may not necessarily be generalizable to lower-dose habitual dietary omega-3 intakes,” concludes the study’s report published in the Journal of the American College of Cardiology.
Other recent research suggests that any elevated AFib risk from omega-3 FA intake is dose-related and may be associated with omega-3 FA supplement or medication intake in high doses, such as 4 g/day.
“Coupled with the more consistent benefits of these fatty acids in the prevention of adverse coronary events, our study suggests that current dietary guidelines recommending fish/omega-3 fatty acid consumption should be maintained,” conclude the authors of the report, led by Frank Qian, MD, MPH, Harvard T.H. Chan School of Public Health and Beth Israel Deaconess Medical Center, Boston.
The current study is “important in showing that physiologic levels of omega-3s that would accumulate through diet don’t seem to increase the risk of arrhythmia,” preventive cardiologist Sean Heffron, MD, NYU Langone Health and Grossman School of Medicine, told this news organization.
“It also lends credence to the fact that the increased risk is specific to the high dose supplementation, because that’s the only instance in which we’ve seen increased atrial fibrillation in association with omega-3s,” said Dr. Heffron, who wasn’t involved in the meta-analysis.
An accompanying editorial agrees. “Based on present evidence, moderate dietary intake of fish and seafood is unlikely to achieve sufficiently high levels of omega-3-FAs in blood or tissue that would result in increased AFib risk as observed in clinical trials of fish oil supplements and high-dose prescriptions,” write Christie Ballantyne, MD, and Xiaoming Jia, MD, Baylor College of Medicine, Houston.
Therefore, they conclude, “fish should continue to be an important part of the menu of a heart-healthy diet.”
The meta-analysis comprised 54,799 participants from 21 countries worldwide in 17 prospective cohort studies that yielded data on incident AFib, in which there were 7,720 cases of the arrhythmia over a median follow-up of 13 years. It looked at associations between such cases and levels of omega-3 FA in blood and adipose tissue samples.
In multivariable analysis, EPA levels were not associated with incident AFib, with a hazard ratio of 1.00 (95% confidence interval, 0.95-1.05) per interquintile range, which the report describes as the difference between the 90th and 10th percentiles.
In contrast, levels of DPA, DHA, and EPA plus DHA were all associated with reduced AFib incidence at interquintile-range HRs of 0.89 (95% CI, 0.83-0.95) for DPA, 0.90 (95% CI, 0.85-0.96) for DHA and 0.93 (95% CI, 0.87-0.99) for EPA and DHA combined.
“We found little evidence that the associations significantly varied by age, sex, or global region, or across the various lipid compartments,” the report states. “Moreover, the relationship between omega-3 fatty acids and AFib did not significantly differ among individuals at higher CV risk.”
The authors observe that the prevalence of omega-3 FA supplement use in the cohorts was very low, suggesting that the omega-3 FA biomarker levels largely reflected habitual dietary intake.
Most of the meta-analysis population were free of CV disease or at relatively low CV risk, they write, and “it is conceivable that the effects of omega-3 fatty acids on atrial arrhythmias may differ in those with existing CV disease versus without.”
However, they note, in a prespecified subgroup analysis of participants mirroring the REDUCE-IT cohort of people with established CV disease or at elevated CV risk, no association with incident AFib was observed for EPA and inverse associations emerged for DPA, DHA, and EPA plus DHA.
In their editorial, Dr. Ballantyne and Dr. Jia say the meta-analysis “represents the largest epidemiological study assessing laboratory-measured omega-3 fatty acid concentrations and AFib risk in the general population.”
But significant heterogeneity across the studies and their populations is a major limitation of the analysis, they write, and made for differences in protocols, sample preparation, outcomes ascertainment, follow-up time, and other variables.
“Despite a rigorous approach to harmonize the data across cohorts and adjusting for multiple confounders,” note Dr. Ballantyne and Dr. Jia, “observational studies always have potential for residual confounders.”
The findings support fish consumption as heart-healthy, they write, but “clinicians should be aware of and discuss with patients the risks versus benefits when prescribing high-dose omega-3 FA therapies.”
The Fatty Acid Research Institute retrospectively provided a small honorarium to a subset of the analysts who participated in this study, but it had no role in its design, analysis, manuscript writing, or decision to submit for publication, the report states. Dr. Ballantyne received grant/research support through his institution from Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, and Regeneron, as well as consulting fees from Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Esperion, Genentech, Gilead, Matinas BioPharma, Merck, New Amsterdam, Novartis, Pfizer, and Regeneron. Dr. Heffron and Dr. Jia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Regular consumption of fish and other foods rich in omega-3 fatty acids (FA) won’t raise an individual’s risk for developing atrial fibrillation (AFib), suggests a meta-analysis of population-based studies.
The finding may alleviate recent concerns about higher-dose omega-3 FA supplement intake in clinical-trial patients at elevated cardiovascular (CV) risk, researchers say.
Indeed, across the 17 cohort studies in the meta-analysis, risk for incident AFib was unaffected by elevated circulating and adipose tissue levels of eicosapentaenoic acid (EPA) from dietary intake. Moreover, the risk appeared to drop significantly with such levels of docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and EPA plus DHA.
The signals of AFib risk associated with high-dose omega-3 FA in supplements or prescription form in some clinical trials “may not necessarily be generalizable to lower-dose habitual dietary omega-3 intakes,” concludes the study’s report published in the Journal of the American College of Cardiology.
Other recent research suggests that any elevated AFib risk from omega-3 FA intake is dose-related and may be associated with omega-3 FA supplement or medication intake in high doses, such as 4 g/day.
“Coupled with the more consistent benefits of these fatty acids in the prevention of adverse coronary events, our study suggests that current dietary guidelines recommending fish/omega-3 fatty acid consumption should be maintained,” conclude the authors of the report, led by Frank Qian, MD, MPH, Harvard T.H. Chan School of Public Health and Beth Israel Deaconess Medical Center, Boston.
The current study is “important in showing that physiologic levels of omega-3s that would accumulate through diet don’t seem to increase the risk of arrhythmia,” preventive cardiologist Sean Heffron, MD, NYU Langone Health and Grossman School of Medicine, told this news organization.
“It also lends credence to the fact that the increased risk is specific to the high dose supplementation, because that’s the only instance in which we’ve seen increased atrial fibrillation in association with omega-3s,” said Dr. Heffron, who wasn’t involved in the meta-analysis.
An accompanying editorial agrees. “Based on present evidence, moderate dietary intake of fish and seafood is unlikely to achieve sufficiently high levels of omega-3-FAs in blood or tissue that would result in increased AFib risk as observed in clinical trials of fish oil supplements and high-dose prescriptions,” write Christie Ballantyne, MD, and Xiaoming Jia, MD, Baylor College of Medicine, Houston.
Therefore, they conclude, “fish should continue to be an important part of the menu of a heart-healthy diet.”
The meta-analysis comprised 54,799 participants from 21 countries worldwide in 17 prospective cohort studies that yielded data on incident AFib, in which there were 7,720 cases of the arrhythmia over a median follow-up of 13 years. It looked at associations between such cases and levels of omega-3 FA in blood and adipose tissue samples.
In multivariable analysis, EPA levels were not associated with incident AFib, with a hazard ratio of 1.00 (95% confidence interval, 0.95-1.05) per interquintile range, which the report describes as the difference between the 90th and 10th percentiles.
In contrast, levels of DPA, DHA, and EPA plus DHA were all associated with reduced AFib incidence at interquintile-range HRs of 0.89 (95% CI, 0.83-0.95) for DPA, 0.90 (95% CI, 0.85-0.96) for DHA and 0.93 (95% CI, 0.87-0.99) for EPA and DHA combined.
“We found little evidence that the associations significantly varied by age, sex, or global region, or across the various lipid compartments,” the report states. “Moreover, the relationship between omega-3 fatty acids and AFib did not significantly differ among individuals at higher CV risk.”
The authors observe that the prevalence of omega-3 FA supplement use in the cohorts was very low, suggesting that the omega-3 FA biomarker levels largely reflected habitual dietary intake.
Most of the meta-analysis population were free of CV disease or at relatively low CV risk, they write, and “it is conceivable that the effects of omega-3 fatty acids on atrial arrhythmias may differ in those with existing CV disease versus without.”
However, they note, in a prespecified subgroup analysis of participants mirroring the REDUCE-IT cohort of people with established CV disease or at elevated CV risk, no association with incident AFib was observed for EPA and inverse associations emerged for DPA, DHA, and EPA plus DHA.
In their editorial, Dr. Ballantyne and Dr. Jia say the meta-analysis “represents the largest epidemiological study assessing laboratory-measured omega-3 fatty acid concentrations and AFib risk in the general population.”
But significant heterogeneity across the studies and their populations is a major limitation of the analysis, they write, and made for differences in protocols, sample preparation, outcomes ascertainment, follow-up time, and other variables.
“Despite a rigorous approach to harmonize the data across cohorts and adjusting for multiple confounders,” note Dr. Ballantyne and Dr. Jia, “observational studies always have potential for residual confounders.”
The findings support fish consumption as heart-healthy, they write, but “clinicians should be aware of and discuss with patients the risks versus benefits when prescribing high-dose omega-3 FA therapies.”
The Fatty Acid Research Institute retrospectively provided a small honorarium to a subset of the analysts who participated in this study, but it had no role in its design, analysis, manuscript writing, or decision to submit for publication, the report states. Dr. Ballantyne received grant/research support through his institution from Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, and Regeneron, as well as consulting fees from Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Esperion, Genentech, Gilead, Matinas BioPharma, Merck, New Amsterdam, Novartis, Pfizer, and Regeneron. Dr. Heffron and Dr. Jia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Regular consumption of fish and other foods rich in omega-3 fatty acids (FA) won’t raise an individual’s risk for developing atrial fibrillation (AFib), suggests a meta-analysis of population-based studies.
The finding may alleviate recent concerns about higher-dose omega-3 FA supplement intake in clinical-trial patients at elevated cardiovascular (CV) risk, researchers say.
Indeed, across the 17 cohort studies in the meta-analysis, risk for incident AFib was unaffected by elevated circulating and adipose tissue levels of eicosapentaenoic acid (EPA) from dietary intake. Moreover, the risk appeared to drop significantly with such levels of docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and EPA plus DHA.
The signals of AFib risk associated with high-dose omega-3 FA in supplements or prescription form in some clinical trials “may not necessarily be generalizable to lower-dose habitual dietary omega-3 intakes,” concludes the study’s report published in the Journal of the American College of Cardiology.
Other recent research suggests that any elevated AFib risk from omega-3 FA intake is dose-related and may be associated with omega-3 FA supplement or medication intake in high doses, such as 4 g/day.
“Coupled with the more consistent benefits of these fatty acids in the prevention of adverse coronary events, our study suggests that current dietary guidelines recommending fish/omega-3 fatty acid consumption should be maintained,” conclude the authors of the report, led by Frank Qian, MD, MPH, Harvard T.H. Chan School of Public Health and Beth Israel Deaconess Medical Center, Boston.
The current study is “important in showing that physiologic levels of omega-3s that would accumulate through diet don’t seem to increase the risk of arrhythmia,” preventive cardiologist Sean Heffron, MD, NYU Langone Health and Grossman School of Medicine, told this news organization.
“It also lends credence to the fact that the increased risk is specific to the high dose supplementation, because that’s the only instance in which we’ve seen increased atrial fibrillation in association with omega-3s,” said Dr. Heffron, who wasn’t involved in the meta-analysis.
An accompanying editorial agrees. “Based on present evidence, moderate dietary intake of fish and seafood is unlikely to achieve sufficiently high levels of omega-3-FAs in blood or tissue that would result in increased AFib risk as observed in clinical trials of fish oil supplements and high-dose prescriptions,” write Christie Ballantyne, MD, and Xiaoming Jia, MD, Baylor College of Medicine, Houston.
Therefore, they conclude, “fish should continue to be an important part of the menu of a heart-healthy diet.”
The meta-analysis comprised 54,799 participants from 21 countries worldwide in 17 prospective cohort studies that yielded data on incident AFib, in which there were 7,720 cases of the arrhythmia over a median follow-up of 13 years. It looked at associations between such cases and levels of omega-3 FA in blood and adipose tissue samples.
In multivariable analysis, EPA levels were not associated with incident AFib, with a hazard ratio of 1.00 (95% confidence interval, 0.95-1.05) per interquintile range, which the report describes as the difference between the 90th and 10th percentiles.
In contrast, levels of DPA, DHA, and EPA plus DHA were all associated with reduced AFib incidence at interquintile-range HRs of 0.89 (95% CI, 0.83-0.95) for DPA, 0.90 (95% CI, 0.85-0.96) for DHA and 0.93 (95% CI, 0.87-0.99) for EPA and DHA combined.
“We found little evidence that the associations significantly varied by age, sex, or global region, or across the various lipid compartments,” the report states. “Moreover, the relationship between omega-3 fatty acids and AFib did not significantly differ among individuals at higher CV risk.”
The authors observe that the prevalence of omega-3 FA supplement use in the cohorts was very low, suggesting that the omega-3 FA biomarker levels largely reflected habitual dietary intake.
Most of the meta-analysis population were free of CV disease or at relatively low CV risk, they write, and “it is conceivable that the effects of omega-3 fatty acids on atrial arrhythmias may differ in those with existing CV disease versus without.”
However, they note, in a prespecified subgroup analysis of participants mirroring the REDUCE-IT cohort of people with established CV disease or at elevated CV risk, no association with incident AFib was observed for EPA and inverse associations emerged for DPA, DHA, and EPA plus DHA.
In their editorial, Dr. Ballantyne and Dr. Jia say the meta-analysis “represents the largest epidemiological study assessing laboratory-measured omega-3 fatty acid concentrations and AFib risk in the general population.”
But significant heterogeneity across the studies and their populations is a major limitation of the analysis, they write, and made for differences in protocols, sample preparation, outcomes ascertainment, follow-up time, and other variables.
“Despite a rigorous approach to harmonize the data across cohorts and adjusting for multiple confounders,” note Dr. Ballantyne and Dr. Jia, “observational studies always have potential for residual confounders.”
The findings support fish consumption as heart-healthy, they write, but “clinicians should be aware of and discuss with patients the risks versus benefits when prescribing high-dose omega-3 FA therapies.”
The Fatty Acid Research Institute retrospectively provided a small honorarium to a subset of the analysts who participated in this study, but it had no role in its design, analysis, manuscript writing, or decision to submit for publication, the report states. Dr. Ballantyne received grant/research support through his institution from Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, and Regeneron, as well as consulting fees from Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Esperion, Genentech, Gilead, Matinas BioPharma, Merck, New Amsterdam, Novartis, Pfizer, and Regeneron. Dr. Heffron and Dr. Jia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Nurse practitioners sue state over right to use ‘doctor’ title
, saying it violates their first amendment right to use the honorific title without fear of regulatory repercussions.
The case highlights ongoing scope-creep battles as the American Medical Association tries to preserve the physician-led team model and nursing organizations and some lawmakers push for greater autonomy for allied professionals.
In the complaint filed in district court in June, plaintiffs Jacqueline Palmer, DNP, Heather Lewis, DNP, and Rodolfo Jaravata-Hanson, DNP, say they fear the state will sanction them. They note that “Doctor Sarah,” another DNP, was fined nearly $20,000 by the state last November for false advertising and fraud after using the moniker in her online advertising and social media accounts.
The fine was part of a settlement that the DNP, Sarah Erny, reached with the state to resolve allegations that she failed to identify her supervising physician and inform the public that she was not a medical doctor.
Under California’s Medical Practice Act, individuals cannot refer to themselves as “doctor, physician, or any other terms or letters indicating or implying that he or she is a physician and surgeon ... without having ... a certificate as a physician and surgeon.”
Instead, nurse practitioners certified by the California Board of Registered Nursing may use titles like “Certified Nurse Practitioner” and “Advanced Practice Registered Nurse,” corresponding letters such as APRN-CNP, RN, and NP, and phrases like pediatric nurse practitioner to identify specialization.
Individuals who misrepresent themselves are subject to misdemeanor charges and civil penalties.
The nonprofit Pacific Legal Foundation represents the plaintiffs. In court records, its attorneys argue that after “years earning their advanced degrees and qualifications ... they should be able to speak truthfully about them in their workplaces, on their business cards, the Internet, and social media, so long as they clarify that they are nurse practitioners.”
State lawmakers’ attempts to clarify the roles of physicians and nurse practitioners have seen mixed results. Florida legislators recently passed a bill to prevent advanced practice nurses from using the honorific title, reserving it only for MDs and DOs. Gov. Ron DeSantis vetoed it last month.
In May, Georgia lawmakers passed the Health Care Practitioners Truth and Transparency Act. It requires advanced practice nurses and physician assistants with doctoral degrees who refer to themselves as doctors in a clinical setting to state they are not medical doctors or physicians.
Still, some health professionals say that the designation should only be used in academic settings or among peers, and that all doctoral degree holders should ditch the moniker at the bedside to ease patient communications.
Named as defendants in the suit are three state officials: California Attorney General Rob Bonta, state Medical Board President Kristina Lawson, and California Board of Registered Nursing Executive Officer Loretta Melby.
A version of this article first appeared on Medscape.com.
, saying it violates their first amendment right to use the honorific title without fear of regulatory repercussions.
The case highlights ongoing scope-creep battles as the American Medical Association tries to preserve the physician-led team model and nursing organizations and some lawmakers push for greater autonomy for allied professionals.
In the complaint filed in district court in June, plaintiffs Jacqueline Palmer, DNP, Heather Lewis, DNP, and Rodolfo Jaravata-Hanson, DNP, say they fear the state will sanction them. They note that “Doctor Sarah,” another DNP, was fined nearly $20,000 by the state last November for false advertising and fraud after using the moniker in her online advertising and social media accounts.
The fine was part of a settlement that the DNP, Sarah Erny, reached with the state to resolve allegations that she failed to identify her supervising physician and inform the public that she was not a medical doctor.
Under California’s Medical Practice Act, individuals cannot refer to themselves as “doctor, physician, or any other terms or letters indicating or implying that he or she is a physician and surgeon ... without having ... a certificate as a physician and surgeon.”
Instead, nurse practitioners certified by the California Board of Registered Nursing may use titles like “Certified Nurse Practitioner” and “Advanced Practice Registered Nurse,” corresponding letters such as APRN-CNP, RN, and NP, and phrases like pediatric nurse practitioner to identify specialization.
Individuals who misrepresent themselves are subject to misdemeanor charges and civil penalties.
The nonprofit Pacific Legal Foundation represents the plaintiffs. In court records, its attorneys argue that after “years earning their advanced degrees and qualifications ... they should be able to speak truthfully about them in their workplaces, on their business cards, the Internet, and social media, so long as they clarify that they are nurse practitioners.”
State lawmakers’ attempts to clarify the roles of physicians and nurse practitioners have seen mixed results. Florida legislators recently passed a bill to prevent advanced practice nurses from using the honorific title, reserving it only for MDs and DOs. Gov. Ron DeSantis vetoed it last month.
In May, Georgia lawmakers passed the Health Care Practitioners Truth and Transparency Act. It requires advanced practice nurses and physician assistants with doctoral degrees who refer to themselves as doctors in a clinical setting to state they are not medical doctors or physicians.
Still, some health professionals say that the designation should only be used in academic settings or among peers, and that all doctoral degree holders should ditch the moniker at the bedside to ease patient communications.
Named as defendants in the suit are three state officials: California Attorney General Rob Bonta, state Medical Board President Kristina Lawson, and California Board of Registered Nursing Executive Officer Loretta Melby.
A version of this article first appeared on Medscape.com.
, saying it violates their first amendment right to use the honorific title without fear of regulatory repercussions.
The case highlights ongoing scope-creep battles as the American Medical Association tries to preserve the physician-led team model and nursing organizations and some lawmakers push for greater autonomy for allied professionals.
In the complaint filed in district court in June, plaintiffs Jacqueline Palmer, DNP, Heather Lewis, DNP, and Rodolfo Jaravata-Hanson, DNP, say they fear the state will sanction them. They note that “Doctor Sarah,” another DNP, was fined nearly $20,000 by the state last November for false advertising and fraud after using the moniker in her online advertising and social media accounts.
The fine was part of a settlement that the DNP, Sarah Erny, reached with the state to resolve allegations that she failed to identify her supervising physician and inform the public that she was not a medical doctor.
Under California’s Medical Practice Act, individuals cannot refer to themselves as “doctor, physician, or any other terms or letters indicating or implying that he or she is a physician and surgeon ... without having ... a certificate as a physician and surgeon.”
Instead, nurse practitioners certified by the California Board of Registered Nursing may use titles like “Certified Nurse Practitioner” and “Advanced Practice Registered Nurse,” corresponding letters such as APRN-CNP, RN, and NP, and phrases like pediatric nurse practitioner to identify specialization.
Individuals who misrepresent themselves are subject to misdemeanor charges and civil penalties.
The nonprofit Pacific Legal Foundation represents the plaintiffs. In court records, its attorneys argue that after “years earning their advanced degrees and qualifications ... they should be able to speak truthfully about them in their workplaces, on their business cards, the Internet, and social media, so long as they clarify that they are nurse practitioners.”
State lawmakers’ attempts to clarify the roles of physicians and nurse practitioners have seen mixed results. Florida legislators recently passed a bill to prevent advanced practice nurses from using the honorific title, reserving it only for MDs and DOs. Gov. Ron DeSantis vetoed it last month.
In May, Georgia lawmakers passed the Health Care Practitioners Truth and Transparency Act. It requires advanced practice nurses and physician assistants with doctoral degrees who refer to themselves as doctors in a clinical setting to state they are not medical doctors or physicians.
Still, some health professionals say that the designation should only be used in academic settings or among peers, and that all doctoral degree holders should ditch the moniker at the bedside to ease patient communications.
Named as defendants in the suit are three state officials: California Attorney General Rob Bonta, state Medical Board President Kristina Lawson, and California Board of Registered Nursing Executive Officer Loretta Melby.
A version of this article first appeared on Medscape.com.






