User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
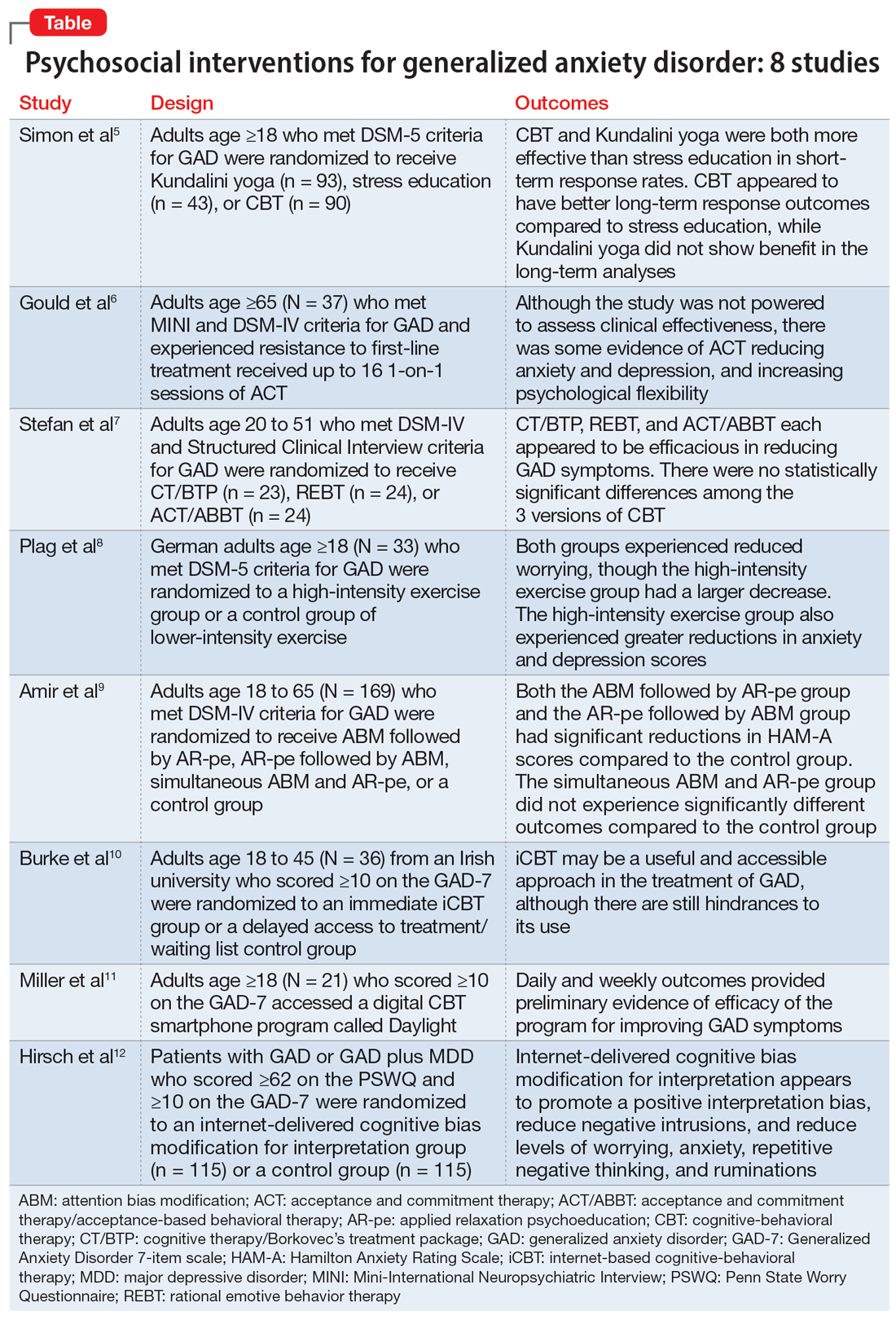
Generalized anxiety disorder: 8 studies of psychosocial interventions
SECOND OF 2 PARTS
For patients with generalized anxiety disorder (GAD), the intensity, duration, and frequency of an individual’s anxiety and worry are out of proportion to the actual likelihood or impact of an anticipated event, and they often find it difficult to prevent worrisome thoughts from interfering with daily life.1 Successful treatment for GAD is patient-specific and requires clinicians to consider all available psychotherapeutic and pharmacologic options.
In a 2020 meta-analysis of 79 randomized controlled trials (RCTs) with 11,002 participants diagnosed with GAD, Carl et al2 focused on pooled effect sizes of evidence-based psychotherapies and medications for GAD. Their analysis showed a medium to large effect size (Hedges g = 0.76) for psychotherapy, compared to a small effect size (Hedges g = 0.38) for medication on GAD outcomes. Other meta-analyses have shown that evidence-based psychotherapies have large effect sizes on GAD outcomes.3
However, in most of the studies included in these meta-analyses, the 2 treatment modalities—psychotherapy and pharmacotherapy—use different control types. The pharmacotherapy trials used a placebo, while psychotherapy studies often had a waitlist control. Thus, the findings of these meta-analyses should not lead to the conclusion that psychotherapy is necessarily more effective for GAD symptoms than pharmacotherapy. However, there is clear evidence that psychosocial interventions are at least as effective as medications for treating GAD. Also, patients often prefer psychosocial treatment over medication.
Part 1 (
1. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
Cognitive-behavioral therapy (CBT) is a first-line therapy for GAD.13 However, patients may not pursue CBT due to fiscal and logistical constraints, as well as the stigma associated with it. Yoga is a common complementary health practice used by adults in the United States,14 although evidence has been inconclusive for its use in treating anxiety. Simon et al5 examined the efficacy of Kundalini yoga (KY) vs stress education (SE) and CBT for treating GAD.
Study design
- A prospective, parallel-group, randomized-controlled, single-blind trial in 2 academic centers evaluated 226 adults age ≥18 who met DSM-5 criteria for GAD.
- Participants were randomized into 3 groups: KY (n = 93), SE (n = 43), or CBT (n = 90), and monitored for 12 weeks to determine the efficacy of each therapy.
- Exclusion criteria included current posttraumatic stress disorder, eating disorders, substance use disorders, significant suicidal ideation, mental disorder due to a medical or neurocognitive condition, lifetime psychosis, bipolar disorder (BD), developmental disorders, and having completed more than 5 yoga or CBT sessions in the past 5 years. Additionally, patients were either not taking medication for ≥2 weeks prior to the trial or had a stable regimen for ≥6 weeks.
- Each therapy was guided by 2 instructors during 12 120-minute sessions with 20 minutes of daily assignments and presented in cohorts of 4 to 6 participants.
- The primary outcome was an improvement in score on the Clinical Global Impression–Improvement scale from baseline at Week 12. Secondary measures included scores on the Meta-Cognitions Questionnaire and the Five Facet Mindfulness Questionnaire.
Outcomes
- A total of 155 participants finished the posttreatment assessment, with similar completion rates between the groups, and 123 participants completed the 6-month follow-up assessment.
- The KY group had a significantly higher response rate (54.2%) than the SE group (33%) at posttreatment, with a number needed to treat (NNT) of 4.59. At 6-month follow-up, the response rate in the KY group was not significantly higher than that of the SE group.
- The CBT group had a significantly higher response rate (70.8%) than the SE group (33%) at posttreatment, with a NNT of 2.62. At 6-month follow-up, the CBT response rate (76.7%) was significantly higher than the SE group (48%), with a NNT of 3.51.
- KY was not found to be as effective as CBT on noninferiority testing.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT and KY were both more effective than SE as assessed by short-term response rates.
- The authors did not find KY to be as effective as CBT at posttreatment or the 6-month follow-up. Additionally, CBT appeared to have better long-term response outcomes compared to SE, while KY did not display a benefit in follow-up analyses. Overall, KY appears to have a less robust efficacy compared to CBT in the treatment of GAD.
- These findings may not generalize to how CBT and yoga are approached in the community. Future studies can assess community-based methods.
2. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
Older adults with GAD may experience treatment resistance to first-line therapies, such as selective serotonin reuptake inhibitors and CBT. Gould et al6 assessed whether acceptance and commitment therapy (ACT) could be a cost-effective option for older adults with treatment-resistant GAD (TR-GAD).
Study design
- In Stage 1 (intervention planning), individual interviews were conducted with 15 participants (11 female) with TR-GAD and 31 health care professionals, as well as 5 academic clinicians. The objective was to assess intervention preferences and priorities.
- Stage 2 included 37 participants, 8 clinicians, and 15 therapists, with the goal of assessing intervention design and feedback on the interventions.
- Participants were age ≥65 and met Mini-International Neuropsychiatric Interview (MINI) and DSM-IV criteria for GAD. They were living in the community and had not responded to the 3 steps of the stepped-care approach for GAD (ie, 6 weeks of an age-appropriate dose of antidepressant or a course of individual psychotherapy). Patients with dementia were excluded.
- Patients received ≤16 1-on-1 sessions of ACT.
- Self-reported outcomes were assessed at baseline and Week 20.
- The primary outcomes for Stage 2 were acceptability (attendance and satisfaction with ACT) and feasibility (recruitment and retention).
Outcomes
- ACT had high feasibility, with a recruitment rate of 93% and a retention rate of 81%.
- It also had high acceptability, with 70% of participants attending ≥10 sessions and 60% of participants showing satisfaction with therapy by scoring ≥21 points on the Satisfaction with Therapy subscale of the Satisfaction with Therapy and Therapist Scale-Revised. However, 80% of participants had not finished their ACT sessions when scores were collected.
- At Week 20, 13 patients showed reliable improvement on the Geriatric Anxiety Inventory, and 15 showed no reliable change. Seven participants showed reliable improvement in Geriatric Depression Scale-15 scores and 22 showed no reliable change. Seven participants showed improvement in the Action and Acceptance Questionnaire-II and 19 showed no reliable change.
Conclusions/limitations
- ACT had high levels of feasibility and acceptability, and large RCTs warrant further assessment of the benefits of this intervention.
- There was some evidence of reductions in anxiety and depression, as well as improvement with psychological flexibility.
- The study was not powered to assess clinical effectiveness, and recruitment for Stage 2 was limited to London.
Continue to: #3
3. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
Previous studies have demonstrated the efficacy of CBT for treating GAD.15,16 However, CBT involves varying approaches, which make it difficult to conclude which model of CBT is more effective. Stefan et al7 aimed to assess the efficacy of 3 versions of CBT for GAD.
Study design
- This RCT investigated 3 versions of CBT: cognitive therapy/Borkovec’s treatment package (CT/BTP), rational emotive behavior therapy (REBT), and acceptance and commitment therapy/acceptance-based behavioral therapy (ACT/ABBT).
- A total of 75 adults (60 women) age 20 to 51 and diagnosed with GAD by the Structured Clinical Interview for DSM-IV were initially randomized to one of the treatment arms for 20 sessions; 4 dropped out before receiving the allocated intervention. Exclusion criteria included panic disorder, severe major depressive disorder (MDD), BD, substance use or dependence, psychotic disorders, suicidal or homicidal ideation, organic brain syndrome, disabling medical conditions, intellectual disability, treatment with a psychotropic drug within the past 3 months, and psychotherapy provided outside the trial.
- The primary outcomes were scores on the Generalized Anxiety Disorder Questionnaire IV (GAD-Q-IV) and the Penn State Worry Questionnaire (PSWQ). A secondary outcome included assessing negative automatic thoughts by the Automatic Thoughts Questionnaire.
Outcomes
- There were no significant differences among the 3 treatment groups with regards to demographic data.
- Approximately 70% of patients (16 of 23) in the CT/BTP group had scores below the cutoff point for response (9) on the GAD-Q-IV, approximately 71% of patients (17 of 24) in the REBT group scored below the cutoff point, and approximately 79% of patients (19 of 24) in the ACT/ABBT group scored below the cutoff point.
- Approximately 83% of patients in the CT/BTP scored below the cutoff point for response (65) on the PSWQ, approximately 83% of patients in the REBT group scored below the cutoff point, and approximately 80% of patients in the ACT/ABBT group scored below the cutoff point.
- There were positive correlations between pre-post changes in GAD symptoms and dysfunctional automatic thoughts in each group.
- There was no statistically significant difference among the 3 versions of CBT.
Conclusions/limitations
- CT/BTP, REBT, and ACT/ABBT each appear to be efficacious in reducing GAD symptoms, allowing the choice of treatment to be determined by patient and clinician preference.
- The study’s small sample size may have prevented differences between the groups from being detected.
- There was no control group, and only 39 of 75 individuals completed the study in its entirety.
4. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.10231
Research has shown the efficacy of aerobic exercise for various anxiety disorders,17-19 but differs regarding the type of exercise and its intensity, frequency, and duration. There is evidence that high-intensity interval training (HIIT) may be beneficial in treating serious mental illness.20 Plag et al8 examined the efficacy and acceptance of HIIT in patients with GAD.
Continue to: Study design
Study design
- A total of 33 German adults (24 women) age ≥18 who met DSM-5 criteria for GAD were enrolled in a parallel-group, assessor-blinded RCT. Participants were blinded to the hypotheses of the trial, but not to the intervention.
- Participants were randomized to a HIIT group (engaged in HIIT on a bicycle ergometer every second day within 12 days, with each session lasting 20 minutes and consisting of alternating sessions of 77% to 95% maximum heart rate and <70% maximum heart rate) or a control group of lower-intensity exercise (LIT; consisted of 6 30-minute sessions within 12 days involving stretching and adapted yoga positions with heart rate <70% maximum heart rate).
- Exclusion criteria included severe depression, schizophrenia, borderline personality disorder (BPD), substance use disorder, suicidality, epilepsy, severe respiratory or cardiovascular diseases, and current psychotherapy. The use of medications was allowed if the patient was stable ≥4 weeks prior to the trial and remained stable during the trial.
- The primary outcome of worrying was assessed by the PSWQ. Other assessment tools included the Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HAM-D), Anxiety Control Questionnaire, and Screening for Somatoform Symptoms-7 (SOMS-7).
Outcomes
- Baseline PSWQ scores in both groups were >60, indicating “high worriers.”
- Both groups experienced reductions in worrying as measured by PSWQ scores. However, the HIIT group had a larger decrease in worrying compared to the LIT group (P < .02). Post-hoc analyses showed significant reductions in symptom severity from baseline to poststudy (P < .01; d = 0.68), and at 30-day follow-up (P < .01; d = 0.62) in the HIIT group. There was no significant difference in the LIT group from baseline to poststudy or at follow-up.
- Secondary outcome measures included a greater reduction in anxiety and depression as determined by change in HAM-A and HAM-D scores in the HIIT group compared to the LIT group.
- All measures showed improvement in the HIIT group, whereas the LIT group showed improvement in HAM-A and HAM-D scores poststudy and at follow-up, as well as SOMS-7 scores at follow-up.
Conclusions/limitations
- HIIT demonstrated a large treatment effect for treating GAD, including somatic symptoms and worrying.
- HIIT displayed a fast onset of action and low cancellation rate, which suggests it is tolerable.
- This study had a small sample size consisting of participants from only 1 institution, which limits generalizability, and did not look at the long-term effects of the interventions.
5. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
Many patients with anxiety disorders do not receive treatment, and logistical factors such as limited time, expertise, and available resources hinder patients from obtaining quality CBT. Attention bias modification (ABM) is a computer-based approach in which patients complete tasks guiding their attention away from threat-relevant cues.21 Applied relaxation psychoeducation (AR-pe) is another empirically supported treatment that can be administered via computer. Amir et al9 examined the feasibility and effectiveness of a home-based computerized regimen of sequenced or simultaneous ABM and AR-pe in patients with GAD.
Study design
- A total of 169 adults age 18 to 65 who met DSM-IV criteria for GAD were randomized into 4 groups: ABM followed by AR-pe, AR-pe followed by ABM, simultaneous ABM and AR-pe, or a clinical monitoring assessment only control group (CM).
- Participants were expected to complete up to 24 30-minute sessions on their home computer over 12 weeks.
- Exclusion criteria included current psychotropic medications/CBT initiated 3 months prior to the study, BD, schizophrenia, or substance use disorder.
- The primary outcome measure was anxiety symptoms as assessed by the HAM-A (remission was defined as a score ≤7 at Week 13). Other measures included the PSWQ, Spielberger State-Trait Anxiety Inventory, Sheehan Disability Scale, and Beck Depression Inventory.
- Participants were assessed at Month 3, Month 6, and Month 12 poststudy.
Continue to: Outcomes
Outcomes
- Baseline characteristics did not significantly differ between groups.
- In the active groups, 41% of participants met remission criteria, compared to 19% in the CM group.
- The ABM followed by AR-pe group and the AR-pe followed by ABM group had significant reductions in HAM-A scores (P = .003 and P = .020) compared to the CM group.
- The simultaneous ABM and AR-pe group did not have a significant difference in outcomes compared to the CM group (P = .081).
- On the PSWQ, the CM group had a larger decrease in worry than all active cohorts combined, with follow-up analysis indicating the CM group surpassed the ABM group (P = .019).
Conclusions/limitations
- Sequential delivery of ABM and AR-pe may be a viable, easy-to-access treatment option for patients with GAD who have limited access to other therapies.
- Individuals assigned to receive simultaneous ABM and AR-pe appeared to complete fewer tasks compared to those in the sequential groups, which suggests that participants were less inclined to complete all tasks despite being allowed more time.
- This study did not examine the effects of ABM only or AR-pe only.
- This study was unable to accurately assess home usage of the program.
6. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
Patients with GAD may not be able to obtain adequate treatment due to financial or logistical constraints. Internet-delivered interventions are easily accessible and provide an opportunity for patients who cannot or do not want to seek traditional therapy options. Burke et al10 aimed to better understand the useful and impeding events of internet-based cognitive-behavioral therapy (iCBT).
Study design
- A total of 36 adults (25 women) age 18 to 45 from an Irish university were randomized to an immediate iCBT treatment group or a delayed access to treatment/waiting list control group. The iCBT program, called Calming Anxiety, involved 6 modules of CBT for GAD.
- Participants initially scored ≥10 on the Generalized Anxiety Disorder 7-item scale (GAD-7).
- The study employed the Helpful and Hindering Aspects of Therapy (HAT) questionnaire to assess the most useful and impeding events in therapy.
- The data were divided into 4 domains: helpful events, helpful impacts, hindering events, and hindering impacts.
Outcomes
- Of the 8 helpful events identified, the top 3 were psychoeducation, supporter interaction, and monitoring.
- Of the 5 helpful impacts identified, the top 3 were support and validation, applying coping strategies/behavioral change, and clarification, awareness, and insight.
- The 2 identified hindering events were treatment content/form and amount of work/technical issues.
- The 3 identified hindering impacts were frustration/irritation, increased anxiety, and isolation.
Continue to: Conclusions/limitations
Conclusions/limitations
- iCBT may be a useful and accessible approach for treating GAD, although there are still hindrances to its use.
- This study was qualitative and did not comment on the efficacy of the applied intervention.
- The benefits of iCBT may differ depending on the patient’s level of computer literacy.
7. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
Access to CBT is limited due to cost, dearth of trained therapists, scheduling availability, stigma, and transportation. Digital CBT may help overcome these obstacles. Miller et al11 studied the feasibility and efficacy of a new automated, digital CBT intervention named Daylight.
Study design
- This randomized, multiple-baseline, single-case, experimental trial included 21 adults (20 women) age ≥18 who scored ≥10 on the GAD-7 and screened positive for GAD on MINI version 7 for DSM-5.
- Participants were not taking psychotropic medications or had been on a stable medication regimen for ≥4 weeks.
- Exclusion criteria included past or present psychosis, schizophrenia, BD, seizure disorder, substance use disorder, trauma to the head or brain damage, severe cognitive impairment, serious physical health concerns necessitating surgery or with prognosis <6 months, and pregnancy.
- Participants were randomized to 1 of 3 baseline durations: 2 weeks, 4 weeks, or 6 weeks. They then could access the smartphone program Daylight. The trial lasted for 12 to 16 weeks.
- Primary anxiety outcomes were assessed daily and weekly, while secondary outcomes (depressive symptoms, sleep) were measured weekly.
- Postintervention was defined as 6 weeks after the start of the intervention and follow-up was 10 weeks after the start of the intervention.
- Participants were deemed not to have clinically significant anxiety if they scored <10 on GAD-7; not to have significant depressive symptoms if they scored <10 on the Patient Health Questionnaire-9 (PHQ-9); and not to have sleep difficulty if they scored >16 on the Sleep Condition Indicator (SCI-8). The change was considered reliable if patients scored below the previously discussed thresholds and showed a difference in score greater than the known unreliability of the questionnaire (GAD-7 reductions ≥5, PHQ-9 reductions ≥6, SCI-8 increases ≥7).
Outcomes
- In terms of feasibility, 76% of participants completed all 4 modules, 81% completed 3 modules, 86% completed 2 modules, and all participants completed at least 1 module.
- No serious adverse events were observed, but 43% of participants reported unwanted symptoms such as agitation, fatigue, low mood, or reduced motivation.
- As evaluated by the Credibility/Expectancy Questionnaire, the program received moderate to high credibility scores. Participants indicated they were mostly satisfied with the program, although some expressed technical difficulties and a lack of specificity to their anxiety symptoms.
- Overall daily anxiety scores significantly decreased from baseline to postintervention (P < .001). Weekly anxiety scores significantly decreased from baseline to postintervention (P = .024), and follow-up (P = .017) as measured by the GAD-7.
- For participants with anxiety, 70% no longer had clinically significant anxiety symptoms postintervention, and 65% had both clinically significant and reliable change at postintervention. Eighty percent had clinically significant and reliable change at follow-up.
- For participants with depressive symptoms, 61% had clinical and reliable change at postintervention and 44% maintained both at follow-up.
- For participants with sleep disturbances, 35% had clinical and reliable improvement at postintervention and 40% had clinical and reliable change at follow-up.
Conclusions/limitations
- Daylight appears to be a feasible program with regards to acceptability, engagement, credibility, satisfaction, and safety.
- The daily and weekly outcomes support preliminary evidence of program efficacy in improving GAD symptoms.
- Most participants identified as female and were recruited online, which limits generalizability, and the study had a small sample size.
Continue to: #8
8. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
The cognitive model of pathological worry posits that worry in GAD occurs due to various factors, including automatic cognitive bias in which ambiguous events are perceived as threatening to the individual.22 Cognitive bias modification for interpretation (CBM) is an approach that assesses an individual’s interpretation bias and resolves ambiguity through the individual’s reading or listening to multiple ambiguous situations.12 Hirsch et al12 examined if an internet-delivered CBM approach would promote positive interpretations and reduce worry and anxiety in patients with GAD.
Study design
- In this dual-arm, parallel group, single-blind RCT, adult participants were randomized to a CBM group (n = 115) or a control group (n = 115); only 186 participants were included in the analyses.
- Patients with GAD only and those with GAD comorbid with MDD who scored ≥62 on the PSWQ and ≥10 on the GAD-7 were recruited. Patients receiving psychotropic medication had to be stable on their regimen for ≥3 months prior to the trial.
- Exclusion criteria included residing outside the United Kingdom, severe depression as measured by a PHQ-9 score ≥23, self-harm in the past 12 months or suicide attempt in past 2 years, a PHQ-9 suicidal ideation score >1, concurrent psychosis, BD, BPD, substance abuse, and current or recent (within the past 6 months) psychological treatment.
- The groups completed up to 10 online training (CBM) or control (listened to ambiguous scenarios but not asked to resolve the ambiguity) sessions in 1 month.
- Primary outcome measures included the scrambled sentences test (SST) and a recognition test (RT) to assess interpretation bias.
- Secondary outcome measures included a breathing focus task (BFT), PSWQ and PSWQ-past week, Ruminative Response Scale (RRS), Repetitive Thinking Questionnaire-trait (RTQ-T), PHQ-9, and GAD-7.
- Scores were assessed preintervention (T0), postintervention (T1), 1 month postintervention (T2), and 3 months postintervention (T3).
Outcomes
- CBM was associated with a more positive interpretation at T1 than the control sessions (P < .001 on both SST and RT).
- CBM was associated with significantly reduced negative intrusions as per BFTs at T1.
- The CBM group had significant less worry as per PSWQ, and significantly less anxiety as per GAD-7 at T1, T2, and T3.
- The CBM group had significantly fewer depressive symptoms as per PHQ-9 at T1, T2, and T3.
- The CBM group had significantly lower levels of ruminations as per RRS at T1, T2, and T3.
- The CBM group had significantly lower levels of general repetitive negative thinking (RNT) as per RTQ-T at T1 and T2, but not T3.
Conclusions/limitations
- Digital CBM appears to promote a positive interpretation bias.
- CBM appears to reduce negative intrusions after the intervention, as well as reduced levels of worrying, anxiety, RNT, and ruminations, with effects lasting ≤3 months except for the RNT.
- CBM appears to be an efficacious, low-intensity, easily accessible intervention that can help individuals with GAD.
- The study recruited participants via advertisements rather than clinical services, and excluded individuals with severe depression.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Carl E, Witcraft SM, Kauffman BY, et al. Psychological and pharmacological treatments for generalized anxiety disorder (GAD): a meta-analysis of randomized controlled trials. Cogn Behav Ther. 2020;49(1):1-21. doi:10.1080/16506073.2018.1560358
3. Cuijpers P, Cristea IA, Karyotaki E, et al. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry. 2016;15(3):245-258. doi:10.1002/wps.20346
4. Saeed SA, Majarwitz DJ. Generalized anxiety disorder: 8 studies of biological interventions. Current Psychiatry. 2022;21(7):10-12,20,22-27. doi:10.12788/cp.02645
5. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
6. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
7. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
8. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.102311
9. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
10. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
11. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
12. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
13. Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632. doi:10.4088/jcp.v69n0415
14. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
15. Carpenter JK, Andrews LA, Witcraft SM, et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
16. Covin R, Ouimet AJ, Seeds PM, et al. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord. 2008;22(1):108-116. doi:10.1016/j.janxdis.2007.01.002
17. Merom D, Phongsavan P, Wagner R, et al. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. J Anxiety Disord. 2008;22(6):959-968. doi:10.1016/j.janxdis.2007.09.010
18. Herring MP, Jacob ML, Suveg C, et al. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21-28. doi:10.1159/000327898
19. Bischoff S, Wieder G, Einsle F, et al. Running for extinction? Aerobic exercise as an augmentation of exposure therapy in panic disorder with agoraphobia. J Psychiatr Res. 2018;101:34-41. doi:10.1016/j.jpsychires.2018.03.001
20. Korman N, Armour M, Chapman J, et al. High Intensity Interval training (HIIT) for people with severe mental illness: a systematic review & meta-analysis of intervention studies- considering diverse approaches for mental and physical recovery. Psychiatry Res. 2020;284:112601. doi:10.1016/j.psychres.2019.112601
21. Amir N, Beard C, Cobb M, et al. Attention modification program in individuals with generalized anxiety disorder. J Abnorm Psychol. 2009;118(1):28-33. doi:10.1037/a0012589
22. Hirsh CR, Mathews A. A cognitive model of pathological worry. Behav Res Ther. 2012;50(10):636-646. doi:10.1016/j.brat.2012.007
SECOND OF 2 PARTS
For patients with generalized anxiety disorder (GAD), the intensity, duration, and frequency of an individual’s anxiety and worry are out of proportion to the actual likelihood or impact of an anticipated event, and they often find it difficult to prevent worrisome thoughts from interfering with daily life.1 Successful treatment for GAD is patient-specific and requires clinicians to consider all available psychotherapeutic and pharmacologic options.
In a 2020 meta-analysis of 79 randomized controlled trials (RCTs) with 11,002 participants diagnosed with GAD, Carl et al2 focused on pooled effect sizes of evidence-based psychotherapies and medications for GAD. Their analysis showed a medium to large effect size (Hedges g = 0.76) for psychotherapy, compared to a small effect size (Hedges g = 0.38) for medication on GAD outcomes. Other meta-analyses have shown that evidence-based psychotherapies have large effect sizes on GAD outcomes.3
However, in most of the studies included in these meta-analyses, the 2 treatment modalities—psychotherapy and pharmacotherapy—use different control types. The pharmacotherapy trials used a placebo, while psychotherapy studies often had a waitlist control. Thus, the findings of these meta-analyses should not lead to the conclusion that psychotherapy is necessarily more effective for GAD symptoms than pharmacotherapy. However, there is clear evidence that psychosocial interventions are at least as effective as medications for treating GAD. Also, patients often prefer psychosocial treatment over medication.
Part 1 (

1. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
Cognitive-behavioral therapy (CBT) is a first-line therapy for GAD.13 However, patients may not pursue CBT due to fiscal and logistical constraints, as well as the stigma associated with it. Yoga is a common complementary health practice used by adults in the United States,14 although evidence has been inconclusive for its use in treating anxiety. Simon et al5 examined the efficacy of Kundalini yoga (KY) vs stress education (SE) and CBT for treating GAD.
Study design
- A prospective, parallel-group, randomized-controlled, single-blind trial in 2 academic centers evaluated 226 adults age ≥18 who met DSM-5 criteria for GAD.
- Participants were randomized into 3 groups: KY (n = 93), SE (n = 43), or CBT (n = 90), and monitored for 12 weeks to determine the efficacy of each therapy.
- Exclusion criteria included current posttraumatic stress disorder, eating disorders, substance use disorders, significant suicidal ideation, mental disorder due to a medical or neurocognitive condition, lifetime psychosis, bipolar disorder (BD), developmental disorders, and having completed more than 5 yoga or CBT sessions in the past 5 years. Additionally, patients were either not taking medication for ≥2 weeks prior to the trial or had a stable regimen for ≥6 weeks.
- Each therapy was guided by 2 instructors during 12 120-minute sessions with 20 minutes of daily assignments and presented in cohorts of 4 to 6 participants.
- The primary outcome was an improvement in score on the Clinical Global Impression–Improvement scale from baseline at Week 12. Secondary measures included scores on the Meta-Cognitions Questionnaire and the Five Facet Mindfulness Questionnaire.
Outcomes
- A total of 155 participants finished the posttreatment assessment, with similar completion rates between the groups, and 123 participants completed the 6-month follow-up assessment.
- The KY group had a significantly higher response rate (54.2%) than the SE group (33%) at posttreatment, with a number needed to treat (NNT) of 4.59. At 6-month follow-up, the response rate in the KY group was not significantly higher than that of the SE group.
- The CBT group had a significantly higher response rate (70.8%) than the SE group (33%) at posttreatment, with a NNT of 2.62. At 6-month follow-up, the CBT response rate (76.7%) was significantly higher than the SE group (48%), with a NNT of 3.51.
- KY was not found to be as effective as CBT on noninferiority testing.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT and KY were both more effective than SE as assessed by short-term response rates.
- The authors did not find KY to be as effective as CBT at posttreatment or the 6-month follow-up. Additionally, CBT appeared to have better long-term response outcomes compared to SE, while KY did not display a benefit in follow-up analyses. Overall, KY appears to have a less robust efficacy compared to CBT in the treatment of GAD.
- These findings may not generalize to how CBT and yoga are approached in the community. Future studies can assess community-based methods.
2. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
Older adults with GAD may experience treatment resistance to first-line therapies, such as selective serotonin reuptake inhibitors and CBT. Gould et al6 assessed whether acceptance and commitment therapy (ACT) could be a cost-effective option for older adults with treatment-resistant GAD (TR-GAD).
Study design
- In Stage 1 (intervention planning), individual interviews were conducted with 15 participants (11 female) with TR-GAD and 31 health care professionals, as well as 5 academic clinicians. The objective was to assess intervention preferences and priorities.
- Stage 2 included 37 participants, 8 clinicians, and 15 therapists, with the goal of assessing intervention design and feedback on the interventions.
- Participants were age ≥65 and met Mini-International Neuropsychiatric Interview (MINI) and DSM-IV criteria for GAD. They were living in the community and had not responded to the 3 steps of the stepped-care approach for GAD (ie, 6 weeks of an age-appropriate dose of antidepressant or a course of individual psychotherapy). Patients with dementia were excluded.
- Patients received ≤16 1-on-1 sessions of ACT.
- Self-reported outcomes were assessed at baseline and Week 20.
- The primary outcomes for Stage 2 were acceptability (attendance and satisfaction with ACT) and feasibility (recruitment and retention).
Outcomes
- ACT had high feasibility, with a recruitment rate of 93% and a retention rate of 81%.
- It also had high acceptability, with 70% of participants attending ≥10 sessions and 60% of participants showing satisfaction with therapy by scoring ≥21 points on the Satisfaction with Therapy subscale of the Satisfaction with Therapy and Therapist Scale-Revised. However, 80% of participants had not finished their ACT sessions when scores were collected.
- At Week 20, 13 patients showed reliable improvement on the Geriatric Anxiety Inventory, and 15 showed no reliable change. Seven participants showed reliable improvement in Geriatric Depression Scale-15 scores and 22 showed no reliable change. Seven participants showed improvement in the Action and Acceptance Questionnaire-II and 19 showed no reliable change.
Conclusions/limitations
- ACT had high levels of feasibility and acceptability, and large RCTs warrant further assessment of the benefits of this intervention.
- There was some evidence of reductions in anxiety and depression, as well as improvement with psychological flexibility.
- The study was not powered to assess clinical effectiveness, and recruitment for Stage 2 was limited to London.
Continue to: #3
3. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
Previous studies have demonstrated the efficacy of CBT for treating GAD.15,16 However, CBT involves varying approaches, which make it difficult to conclude which model of CBT is more effective. Stefan et al7 aimed to assess the efficacy of 3 versions of CBT for GAD.
Study design
- This RCT investigated 3 versions of CBT: cognitive therapy/Borkovec’s treatment package (CT/BTP), rational emotive behavior therapy (REBT), and acceptance and commitment therapy/acceptance-based behavioral therapy (ACT/ABBT).
- A total of 75 adults (60 women) age 20 to 51 and diagnosed with GAD by the Structured Clinical Interview for DSM-IV were initially randomized to one of the treatment arms for 20 sessions; 4 dropped out before receiving the allocated intervention. Exclusion criteria included panic disorder, severe major depressive disorder (MDD), BD, substance use or dependence, psychotic disorders, suicidal or homicidal ideation, organic brain syndrome, disabling medical conditions, intellectual disability, treatment with a psychotropic drug within the past 3 months, and psychotherapy provided outside the trial.
- The primary outcomes were scores on the Generalized Anxiety Disorder Questionnaire IV (GAD-Q-IV) and the Penn State Worry Questionnaire (PSWQ). A secondary outcome included assessing negative automatic thoughts by the Automatic Thoughts Questionnaire.
Outcomes
- There were no significant differences among the 3 treatment groups with regards to demographic data.
- Approximately 70% of patients (16 of 23) in the CT/BTP group had scores below the cutoff point for response (9) on the GAD-Q-IV, approximately 71% of patients (17 of 24) in the REBT group scored below the cutoff point, and approximately 79% of patients (19 of 24) in the ACT/ABBT group scored below the cutoff point.
- Approximately 83% of patients in the CT/BTP scored below the cutoff point for response (65) on the PSWQ, approximately 83% of patients in the REBT group scored below the cutoff point, and approximately 80% of patients in the ACT/ABBT group scored below the cutoff point.
- There were positive correlations between pre-post changes in GAD symptoms and dysfunctional automatic thoughts in each group.
- There was no statistically significant difference among the 3 versions of CBT.
Conclusions/limitations
- CT/BTP, REBT, and ACT/ABBT each appear to be efficacious in reducing GAD symptoms, allowing the choice of treatment to be determined by patient and clinician preference.
- The study’s small sample size may have prevented differences between the groups from being detected.
- There was no control group, and only 39 of 75 individuals completed the study in its entirety.
4. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.10231
Research has shown the efficacy of aerobic exercise for various anxiety disorders,17-19 but differs regarding the type of exercise and its intensity, frequency, and duration. There is evidence that high-intensity interval training (HIIT) may be beneficial in treating serious mental illness.20 Plag et al8 examined the efficacy and acceptance of HIIT in patients with GAD.
Continue to: Study design
Study design
- A total of 33 German adults (24 women) age ≥18 who met DSM-5 criteria for GAD were enrolled in a parallel-group, assessor-blinded RCT. Participants were blinded to the hypotheses of the trial, but not to the intervention.
- Participants were randomized to a HIIT group (engaged in HIIT on a bicycle ergometer every second day within 12 days, with each session lasting 20 minutes and consisting of alternating sessions of 77% to 95% maximum heart rate and <70% maximum heart rate) or a control group of lower-intensity exercise (LIT; consisted of 6 30-minute sessions within 12 days involving stretching and adapted yoga positions with heart rate <70% maximum heart rate).
- Exclusion criteria included severe depression, schizophrenia, borderline personality disorder (BPD), substance use disorder, suicidality, epilepsy, severe respiratory or cardiovascular diseases, and current psychotherapy. The use of medications was allowed if the patient was stable ≥4 weeks prior to the trial and remained stable during the trial.
- The primary outcome of worrying was assessed by the PSWQ. Other assessment tools included the Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HAM-D), Anxiety Control Questionnaire, and Screening for Somatoform Symptoms-7 (SOMS-7).
Outcomes
- Baseline PSWQ scores in both groups were >60, indicating “high worriers.”
- Both groups experienced reductions in worrying as measured by PSWQ scores. However, the HIIT group had a larger decrease in worrying compared to the LIT group (P < .02). Post-hoc analyses showed significant reductions in symptom severity from baseline to poststudy (P < .01; d = 0.68), and at 30-day follow-up (P < .01; d = 0.62) in the HIIT group. There was no significant difference in the LIT group from baseline to poststudy or at follow-up.
- Secondary outcome measures included a greater reduction in anxiety and depression as determined by change in HAM-A and HAM-D scores in the HIIT group compared to the LIT group.
- All measures showed improvement in the HIIT group, whereas the LIT group showed improvement in HAM-A and HAM-D scores poststudy and at follow-up, as well as SOMS-7 scores at follow-up.
Conclusions/limitations
- HIIT demonstrated a large treatment effect for treating GAD, including somatic symptoms and worrying.
- HIIT displayed a fast onset of action and low cancellation rate, which suggests it is tolerable.
- This study had a small sample size consisting of participants from only 1 institution, which limits generalizability, and did not look at the long-term effects of the interventions.
5. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
Many patients with anxiety disorders do not receive treatment, and logistical factors such as limited time, expertise, and available resources hinder patients from obtaining quality CBT. Attention bias modification (ABM) is a computer-based approach in which patients complete tasks guiding their attention away from threat-relevant cues.21 Applied relaxation psychoeducation (AR-pe) is another empirically supported treatment that can be administered via computer. Amir et al9 examined the feasibility and effectiveness of a home-based computerized regimen of sequenced or simultaneous ABM and AR-pe in patients with GAD.
Study design
- A total of 169 adults age 18 to 65 who met DSM-IV criteria for GAD were randomized into 4 groups: ABM followed by AR-pe, AR-pe followed by ABM, simultaneous ABM and AR-pe, or a clinical monitoring assessment only control group (CM).
- Participants were expected to complete up to 24 30-minute sessions on their home computer over 12 weeks.
- Exclusion criteria included current psychotropic medications/CBT initiated 3 months prior to the study, BD, schizophrenia, or substance use disorder.
- The primary outcome measure was anxiety symptoms as assessed by the HAM-A (remission was defined as a score ≤7 at Week 13). Other measures included the PSWQ, Spielberger State-Trait Anxiety Inventory, Sheehan Disability Scale, and Beck Depression Inventory.
- Participants were assessed at Month 3, Month 6, and Month 12 poststudy.
Continue to: Outcomes
Outcomes
- Baseline characteristics did not significantly differ between groups.
- In the active groups, 41% of participants met remission criteria, compared to 19% in the CM group.
- The ABM followed by AR-pe group and the AR-pe followed by ABM group had significant reductions in HAM-A scores (P = .003 and P = .020) compared to the CM group.
- The simultaneous ABM and AR-pe group did not have a significant difference in outcomes compared to the CM group (P = .081).
- On the PSWQ, the CM group had a larger decrease in worry than all active cohorts combined, with follow-up analysis indicating the CM group surpassed the ABM group (P = .019).
Conclusions/limitations
- Sequential delivery of ABM and AR-pe may be a viable, easy-to-access treatment option for patients with GAD who have limited access to other therapies.
- Individuals assigned to receive simultaneous ABM and AR-pe appeared to complete fewer tasks compared to those in the sequential groups, which suggests that participants were less inclined to complete all tasks despite being allowed more time.
- This study did not examine the effects of ABM only or AR-pe only.
- This study was unable to accurately assess home usage of the program.
6. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
Patients with GAD may not be able to obtain adequate treatment due to financial or logistical constraints. Internet-delivered interventions are easily accessible and provide an opportunity for patients who cannot or do not want to seek traditional therapy options. Burke et al10 aimed to better understand the useful and impeding events of internet-based cognitive-behavioral therapy (iCBT).
Study design
- A total of 36 adults (25 women) age 18 to 45 from an Irish university were randomized to an immediate iCBT treatment group or a delayed access to treatment/waiting list control group. The iCBT program, called Calming Anxiety, involved 6 modules of CBT for GAD.
- Participants initially scored ≥10 on the Generalized Anxiety Disorder 7-item scale (GAD-7).
- The study employed the Helpful and Hindering Aspects of Therapy (HAT) questionnaire to assess the most useful and impeding events in therapy.
- The data were divided into 4 domains: helpful events, helpful impacts, hindering events, and hindering impacts.
Outcomes
- Of the 8 helpful events identified, the top 3 were psychoeducation, supporter interaction, and monitoring.
- Of the 5 helpful impacts identified, the top 3 were support and validation, applying coping strategies/behavioral change, and clarification, awareness, and insight.
- The 2 identified hindering events were treatment content/form and amount of work/technical issues.
- The 3 identified hindering impacts were frustration/irritation, increased anxiety, and isolation.
Continue to: Conclusions/limitations
Conclusions/limitations
- iCBT may be a useful and accessible approach for treating GAD, although there are still hindrances to its use.
- This study was qualitative and did not comment on the efficacy of the applied intervention.
- The benefits of iCBT may differ depending on the patient’s level of computer literacy.
7. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
Access to CBT is limited due to cost, dearth of trained therapists, scheduling availability, stigma, and transportation. Digital CBT may help overcome these obstacles. Miller et al11 studied the feasibility and efficacy of a new automated, digital CBT intervention named Daylight.
Study design
- This randomized, multiple-baseline, single-case, experimental trial included 21 adults (20 women) age ≥18 who scored ≥10 on the GAD-7 and screened positive for GAD on MINI version 7 for DSM-5.
- Participants were not taking psychotropic medications or had been on a stable medication regimen for ≥4 weeks.
- Exclusion criteria included past or present psychosis, schizophrenia, BD, seizure disorder, substance use disorder, trauma to the head or brain damage, severe cognitive impairment, serious physical health concerns necessitating surgery or with prognosis <6 months, and pregnancy.
- Participants were randomized to 1 of 3 baseline durations: 2 weeks, 4 weeks, or 6 weeks. They then could access the smartphone program Daylight. The trial lasted for 12 to 16 weeks.
- Primary anxiety outcomes were assessed daily and weekly, while secondary outcomes (depressive symptoms, sleep) were measured weekly.
- Postintervention was defined as 6 weeks after the start of the intervention and follow-up was 10 weeks after the start of the intervention.
- Participants were deemed not to have clinically significant anxiety if they scored <10 on GAD-7; not to have significant depressive symptoms if they scored <10 on the Patient Health Questionnaire-9 (PHQ-9); and not to have sleep difficulty if they scored >16 on the Sleep Condition Indicator (SCI-8). The change was considered reliable if patients scored below the previously discussed thresholds and showed a difference in score greater than the known unreliability of the questionnaire (GAD-7 reductions ≥5, PHQ-9 reductions ≥6, SCI-8 increases ≥7).
Outcomes
- In terms of feasibility, 76% of participants completed all 4 modules, 81% completed 3 modules, 86% completed 2 modules, and all participants completed at least 1 module.
- No serious adverse events were observed, but 43% of participants reported unwanted symptoms such as agitation, fatigue, low mood, or reduced motivation.
- As evaluated by the Credibility/Expectancy Questionnaire, the program received moderate to high credibility scores. Participants indicated they were mostly satisfied with the program, although some expressed technical difficulties and a lack of specificity to their anxiety symptoms.
- Overall daily anxiety scores significantly decreased from baseline to postintervention (P < .001). Weekly anxiety scores significantly decreased from baseline to postintervention (P = .024), and follow-up (P = .017) as measured by the GAD-7.
- For participants with anxiety, 70% no longer had clinically significant anxiety symptoms postintervention, and 65% had both clinically significant and reliable change at postintervention. Eighty percent had clinically significant and reliable change at follow-up.
- For participants with depressive symptoms, 61% had clinical and reliable change at postintervention and 44% maintained both at follow-up.
- For participants with sleep disturbances, 35% had clinical and reliable improvement at postintervention and 40% had clinical and reliable change at follow-up.
Conclusions/limitations
- Daylight appears to be a feasible program with regards to acceptability, engagement, credibility, satisfaction, and safety.
- The daily and weekly outcomes support preliminary evidence of program efficacy in improving GAD symptoms.
- Most participants identified as female and were recruited online, which limits generalizability, and the study had a small sample size.
Continue to: #8
8. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
The cognitive model of pathological worry posits that worry in GAD occurs due to various factors, including automatic cognitive bias in which ambiguous events are perceived as threatening to the individual.22 Cognitive bias modification for interpretation (CBM) is an approach that assesses an individual’s interpretation bias and resolves ambiguity through the individual’s reading or listening to multiple ambiguous situations.12 Hirsch et al12 examined if an internet-delivered CBM approach would promote positive interpretations and reduce worry and anxiety in patients with GAD.
Study design
- In this dual-arm, parallel group, single-blind RCT, adult participants were randomized to a CBM group (n = 115) or a control group (n = 115); only 186 participants were included in the analyses.
- Patients with GAD only and those with GAD comorbid with MDD who scored ≥62 on the PSWQ and ≥10 on the GAD-7 were recruited. Patients receiving psychotropic medication had to be stable on their regimen for ≥3 months prior to the trial.
- Exclusion criteria included residing outside the United Kingdom, severe depression as measured by a PHQ-9 score ≥23, self-harm in the past 12 months or suicide attempt in past 2 years, a PHQ-9 suicidal ideation score >1, concurrent psychosis, BD, BPD, substance abuse, and current or recent (within the past 6 months) psychological treatment.
- The groups completed up to 10 online training (CBM) or control (listened to ambiguous scenarios but not asked to resolve the ambiguity) sessions in 1 month.
- Primary outcome measures included the scrambled sentences test (SST) and a recognition test (RT) to assess interpretation bias.
- Secondary outcome measures included a breathing focus task (BFT), PSWQ and PSWQ-past week, Ruminative Response Scale (RRS), Repetitive Thinking Questionnaire-trait (RTQ-T), PHQ-9, and GAD-7.
- Scores were assessed preintervention (T0), postintervention (T1), 1 month postintervention (T2), and 3 months postintervention (T3).
Outcomes
- CBM was associated with a more positive interpretation at T1 than the control sessions (P < .001 on both SST and RT).
- CBM was associated with significantly reduced negative intrusions as per BFTs at T1.
- The CBM group had significant less worry as per PSWQ, and significantly less anxiety as per GAD-7 at T1, T2, and T3.
- The CBM group had significantly fewer depressive symptoms as per PHQ-9 at T1, T2, and T3.
- The CBM group had significantly lower levels of ruminations as per RRS at T1, T2, and T3.
- The CBM group had significantly lower levels of general repetitive negative thinking (RNT) as per RTQ-T at T1 and T2, but not T3.
Conclusions/limitations
- Digital CBM appears to promote a positive interpretation bias.
- CBM appears to reduce negative intrusions after the intervention, as well as reduced levels of worrying, anxiety, RNT, and ruminations, with effects lasting ≤3 months except for the RNT.
- CBM appears to be an efficacious, low-intensity, easily accessible intervention that can help individuals with GAD.
- The study recruited participants via advertisements rather than clinical services, and excluded individuals with severe depression.
SECOND OF 2 PARTS
For patients with generalized anxiety disorder (GAD), the intensity, duration, and frequency of an individual’s anxiety and worry are out of proportion to the actual likelihood or impact of an anticipated event, and they often find it difficult to prevent worrisome thoughts from interfering with daily life.1 Successful treatment for GAD is patient-specific and requires clinicians to consider all available psychotherapeutic and pharmacologic options.
In a 2020 meta-analysis of 79 randomized controlled trials (RCTs) with 11,002 participants diagnosed with GAD, Carl et al2 focused on pooled effect sizes of evidence-based psychotherapies and medications for GAD. Their analysis showed a medium to large effect size (Hedges g = 0.76) for psychotherapy, compared to a small effect size (Hedges g = 0.38) for medication on GAD outcomes. Other meta-analyses have shown that evidence-based psychotherapies have large effect sizes on GAD outcomes.3
However, in most of the studies included in these meta-analyses, the 2 treatment modalities—psychotherapy and pharmacotherapy—use different control types. The pharmacotherapy trials used a placebo, while psychotherapy studies often had a waitlist control. Thus, the findings of these meta-analyses should not lead to the conclusion that psychotherapy is necessarily more effective for GAD symptoms than pharmacotherapy. However, there is clear evidence that psychosocial interventions are at least as effective as medications for treating GAD. Also, patients often prefer psychosocial treatment over medication.
Part 1 (

1. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
Cognitive-behavioral therapy (CBT) is a first-line therapy for GAD.13 However, patients may not pursue CBT due to fiscal and logistical constraints, as well as the stigma associated with it. Yoga is a common complementary health practice used by adults in the United States,14 although evidence has been inconclusive for its use in treating anxiety. Simon et al5 examined the efficacy of Kundalini yoga (KY) vs stress education (SE) and CBT for treating GAD.
Study design
- A prospective, parallel-group, randomized-controlled, single-blind trial in 2 academic centers evaluated 226 adults age ≥18 who met DSM-5 criteria for GAD.
- Participants were randomized into 3 groups: KY (n = 93), SE (n = 43), or CBT (n = 90), and monitored for 12 weeks to determine the efficacy of each therapy.
- Exclusion criteria included current posttraumatic stress disorder, eating disorders, substance use disorders, significant suicidal ideation, mental disorder due to a medical or neurocognitive condition, lifetime psychosis, bipolar disorder (BD), developmental disorders, and having completed more than 5 yoga or CBT sessions in the past 5 years. Additionally, patients were either not taking medication for ≥2 weeks prior to the trial or had a stable regimen for ≥6 weeks.
- Each therapy was guided by 2 instructors during 12 120-minute sessions with 20 minutes of daily assignments and presented in cohorts of 4 to 6 participants.
- The primary outcome was an improvement in score on the Clinical Global Impression–Improvement scale from baseline at Week 12. Secondary measures included scores on the Meta-Cognitions Questionnaire and the Five Facet Mindfulness Questionnaire.
Outcomes
- A total of 155 participants finished the posttreatment assessment, with similar completion rates between the groups, and 123 participants completed the 6-month follow-up assessment.
- The KY group had a significantly higher response rate (54.2%) than the SE group (33%) at posttreatment, with a number needed to treat (NNT) of 4.59. At 6-month follow-up, the response rate in the KY group was not significantly higher than that of the SE group.
- The CBT group had a significantly higher response rate (70.8%) than the SE group (33%) at posttreatment, with a NNT of 2.62. At 6-month follow-up, the CBT response rate (76.7%) was significantly higher than the SE group (48%), with a NNT of 3.51.
- KY was not found to be as effective as CBT on noninferiority testing.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT and KY were both more effective than SE as assessed by short-term response rates.
- The authors did not find KY to be as effective as CBT at posttreatment or the 6-month follow-up. Additionally, CBT appeared to have better long-term response outcomes compared to SE, while KY did not display a benefit in follow-up analyses. Overall, KY appears to have a less robust efficacy compared to CBT in the treatment of GAD.
- These findings may not generalize to how CBT and yoga are approached in the community. Future studies can assess community-based methods.
2. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
Older adults with GAD may experience treatment resistance to first-line therapies, such as selective serotonin reuptake inhibitors and CBT. Gould et al6 assessed whether acceptance and commitment therapy (ACT) could be a cost-effective option for older adults with treatment-resistant GAD (TR-GAD).
Study design
- In Stage 1 (intervention planning), individual interviews were conducted with 15 participants (11 female) with TR-GAD and 31 health care professionals, as well as 5 academic clinicians. The objective was to assess intervention preferences and priorities.
- Stage 2 included 37 participants, 8 clinicians, and 15 therapists, with the goal of assessing intervention design and feedback on the interventions.
- Participants were age ≥65 and met Mini-International Neuropsychiatric Interview (MINI) and DSM-IV criteria for GAD. They were living in the community and had not responded to the 3 steps of the stepped-care approach for GAD (ie, 6 weeks of an age-appropriate dose of antidepressant or a course of individual psychotherapy). Patients with dementia were excluded.
- Patients received ≤16 1-on-1 sessions of ACT.
- Self-reported outcomes were assessed at baseline and Week 20.
- The primary outcomes for Stage 2 were acceptability (attendance and satisfaction with ACT) and feasibility (recruitment and retention).
Outcomes
- ACT had high feasibility, with a recruitment rate of 93% and a retention rate of 81%.
- It also had high acceptability, with 70% of participants attending ≥10 sessions and 60% of participants showing satisfaction with therapy by scoring ≥21 points on the Satisfaction with Therapy subscale of the Satisfaction with Therapy and Therapist Scale-Revised. However, 80% of participants had not finished their ACT sessions when scores were collected.
- At Week 20, 13 patients showed reliable improvement on the Geriatric Anxiety Inventory, and 15 showed no reliable change. Seven participants showed reliable improvement in Geriatric Depression Scale-15 scores and 22 showed no reliable change. Seven participants showed improvement in the Action and Acceptance Questionnaire-II and 19 showed no reliable change.
Conclusions/limitations
- ACT had high levels of feasibility and acceptability, and large RCTs warrant further assessment of the benefits of this intervention.
- There was some evidence of reductions in anxiety and depression, as well as improvement with psychological flexibility.
- The study was not powered to assess clinical effectiveness, and recruitment for Stage 2 was limited to London.
Continue to: #3
3. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
Previous studies have demonstrated the efficacy of CBT for treating GAD.15,16 However, CBT involves varying approaches, which make it difficult to conclude which model of CBT is more effective. Stefan et al7 aimed to assess the efficacy of 3 versions of CBT for GAD.
Study design
- This RCT investigated 3 versions of CBT: cognitive therapy/Borkovec’s treatment package (CT/BTP), rational emotive behavior therapy (REBT), and acceptance and commitment therapy/acceptance-based behavioral therapy (ACT/ABBT).
- A total of 75 adults (60 women) age 20 to 51 and diagnosed with GAD by the Structured Clinical Interview for DSM-IV were initially randomized to one of the treatment arms for 20 sessions; 4 dropped out before receiving the allocated intervention. Exclusion criteria included panic disorder, severe major depressive disorder (MDD), BD, substance use or dependence, psychotic disorders, suicidal or homicidal ideation, organic brain syndrome, disabling medical conditions, intellectual disability, treatment with a psychotropic drug within the past 3 months, and psychotherapy provided outside the trial.
- The primary outcomes were scores on the Generalized Anxiety Disorder Questionnaire IV (GAD-Q-IV) and the Penn State Worry Questionnaire (PSWQ). A secondary outcome included assessing negative automatic thoughts by the Automatic Thoughts Questionnaire.
Outcomes
- There were no significant differences among the 3 treatment groups with regards to demographic data.
- Approximately 70% of patients (16 of 23) in the CT/BTP group had scores below the cutoff point for response (9) on the GAD-Q-IV, approximately 71% of patients (17 of 24) in the REBT group scored below the cutoff point, and approximately 79% of patients (19 of 24) in the ACT/ABBT group scored below the cutoff point.
- Approximately 83% of patients in the CT/BTP scored below the cutoff point for response (65) on the PSWQ, approximately 83% of patients in the REBT group scored below the cutoff point, and approximately 80% of patients in the ACT/ABBT group scored below the cutoff point.
- There were positive correlations between pre-post changes in GAD symptoms and dysfunctional automatic thoughts in each group.
- There was no statistically significant difference among the 3 versions of CBT.
Conclusions/limitations
- CT/BTP, REBT, and ACT/ABBT each appear to be efficacious in reducing GAD symptoms, allowing the choice of treatment to be determined by patient and clinician preference.
- The study’s small sample size may have prevented differences between the groups from being detected.
- There was no control group, and only 39 of 75 individuals completed the study in its entirety.
4. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.10231
Research has shown the efficacy of aerobic exercise for various anxiety disorders,17-19 but differs regarding the type of exercise and its intensity, frequency, and duration. There is evidence that high-intensity interval training (HIIT) may be beneficial in treating serious mental illness.20 Plag et al8 examined the efficacy and acceptance of HIIT in patients with GAD.
Continue to: Study design
Study design
- A total of 33 German adults (24 women) age ≥18 who met DSM-5 criteria for GAD were enrolled in a parallel-group, assessor-blinded RCT. Participants were blinded to the hypotheses of the trial, but not to the intervention.
- Participants were randomized to a HIIT group (engaged in HIIT on a bicycle ergometer every second day within 12 days, with each session lasting 20 minutes and consisting of alternating sessions of 77% to 95% maximum heart rate and <70% maximum heart rate) or a control group of lower-intensity exercise (LIT; consisted of 6 30-minute sessions within 12 days involving stretching and adapted yoga positions with heart rate <70% maximum heart rate).
- Exclusion criteria included severe depression, schizophrenia, borderline personality disorder (BPD), substance use disorder, suicidality, epilepsy, severe respiratory or cardiovascular diseases, and current psychotherapy. The use of medications was allowed if the patient was stable ≥4 weeks prior to the trial and remained stable during the trial.
- The primary outcome of worrying was assessed by the PSWQ. Other assessment tools included the Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HAM-D), Anxiety Control Questionnaire, and Screening for Somatoform Symptoms-7 (SOMS-7).
Outcomes
- Baseline PSWQ scores in both groups were >60, indicating “high worriers.”
- Both groups experienced reductions in worrying as measured by PSWQ scores. However, the HIIT group had a larger decrease in worrying compared to the LIT group (P < .02). Post-hoc analyses showed significant reductions in symptom severity from baseline to poststudy (P < .01; d = 0.68), and at 30-day follow-up (P < .01; d = 0.62) in the HIIT group. There was no significant difference in the LIT group from baseline to poststudy or at follow-up.
- Secondary outcome measures included a greater reduction in anxiety and depression as determined by change in HAM-A and HAM-D scores in the HIIT group compared to the LIT group.
- All measures showed improvement in the HIIT group, whereas the LIT group showed improvement in HAM-A and HAM-D scores poststudy and at follow-up, as well as SOMS-7 scores at follow-up.
Conclusions/limitations
- HIIT demonstrated a large treatment effect for treating GAD, including somatic symptoms and worrying.
- HIIT displayed a fast onset of action and low cancellation rate, which suggests it is tolerable.
- This study had a small sample size consisting of participants from only 1 institution, which limits generalizability, and did not look at the long-term effects of the interventions.
5. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
Many patients with anxiety disorders do not receive treatment, and logistical factors such as limited time, expertise, and available resources hinder patients from obtaining quality CBT. Attention bias modification (ABM) is a computer-based approach in which patients complete tasks guiding their attention away from threat-relevant cues.21 Applied relaxation psychoeducation (AR-pe) is another empirically supported treatment that can be administered via computer. Amir et al9 examined the feasibility and effectiveness of a home-based computerized regimen of sequenced or simultaneous ABM and AR-pe in patients with GAD.
Study design
- A total of 169 adults age 18 to 65 who met DSM-IV criteria for GAD were randomized into 4 groups: ABM followed by AR-pe, AR-pe followed by ABM, simultaneous ABM and AR-pe, or a clinical monitoring assessment only control group (CM).
- Participants were expected to complete up to 24 30-minute sessions on their home computer over 12 weeks.
- Exclusion criteria included current psychotropic medications/CBT initiated 3 months prior to the study, BD, schizophrenia, or substance use disorder.
- The primary outcome measure was anxiety symptoms as assessed by the HAM-A (remission was defined as a score ≤7 at Week 13). Other measures included the PSWQ, Spielberger State-Trait Anxiety Inventory, Sheehan Disability Scale, and Beck Depression Inventory.
- Participants were assessed at Month 3, Month 6, and Month 12 poststudy.
Continue to: Outcomes
Outcomes
- Baseline characteristics did not significantly differ between groups.
- In the active groups, 41% of participants met remission criteria, compared to 19% in the CM group.
- The ABM followed by AR-pe group and the AR-pe followed by ABM group had significant reductions in HAM-A scores (P = .003 and P = .020) compared to the CM group.
- The simultaneous ABM and AR-pe group did not have a significant difference in outcomes compared to the CM group (P = .081).
- On the PSWQ, the CM group had a larger decrease in worry than all active cohorts combined, with follow-up analysis indicating the CM group surpassed the ABM group (P = .019).
Conclusions/limitations
- Sequential delivery of ABM and AR-pe may be a viable, easy-to-access treatment option for patients with GAD who have limited access to other therapies.
- Individuals assigned to receive simultaneous ABM and AR-pe appeared to complete fewer tasks compared to those in the sequential groups, which suggests that participants were less inclined to complete all tasks despite being allowed more time.
- This study did not examine the effects of ABM only or AR-pe only.
- This study was unable to accurately assess home usage of the program.
6. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
Patients with GAD may not be able to obtain adequate treatment due to financial or logistical constraints. Internet-delivered interventions are easily accessible and provide an opportunity for patients who cannot or do not want to seek traditional therapy options. Burke et al10 aimed to better understand the useful and impeding events of internet-based cognitive-behavioral therapy (iCBT).
Study design
- A total of 36 adults (25 women) age 18 to 45 from an Irish university were randomized to an immediate iCBT treatment group or a delayed access to treatment/waiting list control group. The iCBT program, called Calming Anxiety, involved 6 modules of CBT for GAD.
- Participants initially scored ≥10 on the Generalized Anxiety Disorder 7-item scale (GAD-7).
- The study employed the Helpful and Hindering Aspects of Therapy (HAT) questionnaire to assess the most useful and impeding events in therapy.
- The data were divided into 4 domains: helpful events, helpful impacts, hindering events, and hindering impacts.
Outcomes
- Of the 8 helpful events identified, the top 3 were psychoeducation, supporter interaction, and monitoring.
- Of the 5 helpful impacts identified, the top 3 were support and validation, applying coping strategies/behavioral change, and clarification, awareness, and insight.
- The 2 identified hindering events were treatment content/form and amount of work/technical issues.
- The 3 identified hindering impacts were frustration/irritation, increased anxiety, and isolation.
Continue to: Conclusions/limitations
Conclusions/limitations
- iCBT may be a useful and accessible approach for treating GAD, although there are still hindrances to its use.
- This study was qualitative and did not comment on the efficacy of the applied intervention.
- The benefits of iCBT may differ depending on the patient’s level of computer literacy.
7. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
Access to CBT is limited due to cost, dearth of trained therapists, scheduling availability, stigma, and transportation. Digital CBT may help overcome these obstacles. Miller et al11 studied the feasibility and efficacy of a new automated, digital CBT intervention named Daylight.
Study design
- This randomized, multiple-baseline, single-case, experimental trial included 21 adults (20 women) age ≥18 who scored ≥10 on the GAD-7 and screened positive for GAD on MINI version 7 for DSM-5.
- Participants were not taking psychotropic medications or had been on a stable medication regimen for ≥4 weeks.
- Exclusion criteria included past or present psychosis, schizophrenia, BD, seizure disorder, substance use disorder, trauma to the head or brain damage, severe cognitive impairment, serious physical health concerns necessitating surgery or with prognosis <6 months, and pregnancy.
- Participants were randomized to 1 of 3 baseline durations: 2 weeks, 4 weeks, or 6 weeks. They then could access the smartphone program Daylight. The trial lasted for 12 to 16 weeks.
- Primary anxiety outcomes were assessed daily and weekly, while secondary outcomes (depressive symptoms, sleep) were measured weekly.
- Postintervention was defined as 6 weeks after the start of the intervention and follow-up was 10 weeks after the start of the intervention.
- Participants were deemed not to have clinically significant anxiety if they scored <10 on GAD-7; not to have significant depressive symptoms if they scored <10 on the Patient Health Questionnaire-9 (PHQ-9); and not to have sleep difficulty if they scored >16 on the Sleep Condition Indicator (SCI-8). The change was considered reliable if patients scored below the previously discussed thresholds and showed a difference in score greater than the known unreliability of the questionnaire (GAD-7 reductions ≥5, PHQ-9 reductions ≥6, SCI-8 increases ≥7).
Outcomes
- In terms of feasibility, 76% of participants completed all 4 modules, 81% completed 3 modules, 86% completed 2 modules, and all participants completed at least 1 module.
- No serious adverse events were observed, but 43% of participants reported unwanted symptoms such as agitation, fatigue, low mood, or reduced motivation.
- As evaluated by the Credibility/Expectancy Questionnaire, the program received moderate to high credibility scores. Participants indicated they were mostly satisfied with the program, although some expressed technical difficulties and a lack of specificity to their anxiety symptoms.
- Overall daily anxiety scores significantly decreased from baseline to postintervention (P < .001). Weekly anxiety scores significantly decreased from baseline to postintervention (P = .024), and follow-up (P = .017) as measured by the GAD-7.
- For participants with anxiety, 70% no longer had clinically significant anxiety symptoms postintervention, and 65% had both clinically significant and reliable change at postintervention. Eighty percent had clinically significant and reliable change at follow-up.
- For participants with depressive symptoms, 61% had clinical and reliable change at postintervention and 44% maintained both at follow-up.
- For participants with sleep disturbances, 35% had clinical and reliable improvement at postintervention and 40% had clinical and reliable change at follow-up.
Conclusions/limitations
- Daylight appears to be a feasible program with regards to acceptability, engagement, credibility, satisfaction, and safety.
- The daily and weekly outcomes support preliminary evidence of program efficacy in improving GAD symptoms.
- Most participants identified as female and were recruited online, which limits generalizability, and the study had a small sample size.
Continue to: #8
8. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
The cognitive model of pathological worry posits that worry in GAD occurs due to various factors, including automatic cognitive bias in which ambiguous events are perceived as threatening to the individual.22 Cognitive bias modification for interpretation (CBM) is an approach that assesses an individual’s interpretation bias and resolves ambiguity through the individual’s reading or listening to multiple ambiguous situations.12 Hirsch et al12 examined if an internet-delivered CBM approach would promote positive interpretations and reduce worry and anxiety in patients with GAD.
Study design
- In this dual-arm, parallel group, single-blind RCT, adult participants were randomized to a CBM group (n = 115) or a control group (n = 115); only 186 participants were included in the analyses.
- Patients with GAD only and those with GAD comorbid with MDD who scored ≥62 on the PSWQ and ≥10 on the GAD-7 were recruited. Patients receiving psychotropic medication had to be stable on their regimen for ≥3 months prior to the trial.
- Exclusion criteria included residing outside the United Kingdom, severe depression as measured by a PHQ-9 score ≥23, self-harm in the past 12 months or suicide attempt in past 2 years, a PHQ-9 suicidal ideation score >1, concurrent psychosis, BD, BPD, substance abuse, and current or recent (within the past 6 months) psychological treatment.
- The groups completed up to 10 online training (CBM) or control (listened to ambiguous scenarios but not asked to resolve the ambiguity) sessions in 1 month.
- Primary outcome measures included the scrambled sentences test (SST) and a recognition test (RT) to assess interpretation bias.
- Secondary outcome measures included a breathing focus task (BFT), PSWQ and PSWQ-past week, Ruminative Response Scale (RRS), Repetitive Thinking Questionnaire-trait (RTQ-T), PHQ-9, and GAD-7.
- Scores were assessed preintervention (T0), postintervention (T1), 1 month postintervention (T2), and 3 months postintervention (T3).
Outcomes
- CBM was associated with a more positive interpretation at T1 than the control sessions (P < .001 on both SST and RT).
- CBM was associated with significantly reduced negative intrusions as per BFTs at T1.
- The CBM group had significant less worry as per PSWQ, and significantly less anxiety as per GAD-7 at T1, T2, and T3.
- The CBM group had significantly fewer depressive symptoms as per PHQ-9 at T1, T2, and T3.
- The CBM group had significantly lower levels of ruminations as per RRS at T1, T2, and T3.
- The CBM group had significantly lower levels of general repetitive negative thinking (RNT) as per RTQ-T at T1 and T2, but not T3.
Conclusions/limitations
- Digital CBM appears to promote a positive interpretation bias.
- CBM appears to reduce negative intrusions after the intervention, as well as reduced levels of worrying, anxiety, RNT, and ruminations, with effects lasting ≤3 months except for the RNT.
- CBM appears to be an efficacious, low-intensity, easily accessible intervention that can help individuals with GAD.
- The study recruited participants via advertisements rather than clinical services, and excluded individuals with severe depression.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Carl E, Witcraft SM, Kauffman BY, et al. Psychological and pharmacological treatments for generalized anxiety disorder (GAD): a meta-analysis of randomized controlled trials. Cogn Behav Ther. 2020;49(1):1-21. doi:10.1080/16506073.2018.1560358
3. Cuijpers P, Cristea IA, Karyotaki E, et al. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry. 2016;15(3):245-258. doi:10.1002/wps.20346
4. Saeed SA, Majarwitz DJ. Generalized anxiety disorder: 8 studies of biological interventions. Current Psychiatry. 2022;21(7):10-12,20,22-27. doi:10.12788/cp.02645
5. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
6. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
7. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
8. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.102311
9. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
10. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
11. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
12. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
13. Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632. doi:10.4088/jcp.v69n0415
14. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
15. Carpenter JK, Andrews LA, Witcraft SM, et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
16. Covin R, Ouimet AJ, Seeds PM, et al. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord. 2008;22(1):108-116. doi:10.1016/j.janxdis.2007.01.002
17. Merom D, Phongsavan P, Wagner R, et al. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. J Anxiety Disord. 2008;22(6):959-968. doi:10.1016/j.janxdis.2007.09.010
18. Herring MP, Jacob ML, Suveg C, et al. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21-28. doi:10.1159/000327898
19. Bischoff S, Wieder G, Einsle F, et al. Running for extinction? Aerobic exercise as an augmentation of exposure therapy in panic disorder with agoraphobia. J Psychiatr Res. 2018;101:34-41. doi:10.1016/j.jpsychires.2018.03.001
20. Korman N, Armour M, Chapman J, et al. High Intensity Interval training (HIIT) for people with severe mental illness: a systematic review & meta-analysis of intervention studies- considering diverse approaches for mental and physical recovery. Psychiatry Res. 2020;284:112601. doi:10.1016/j.psychres.2019.112601
21. Amir N, Beard C, Cobb M, et al. Attention modification program in individuals with generalized anxiety disorder. J Abnorm Psychol. 2009;118(1):28-33. doi:10.1037/a0012589
22. Hirsh CR, Mathews A. A cognitive model of pathological worry. Behav Res Ther. 2012;50(10):636-646. doi:10.1016/j.brat.2012.007
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Carl E, Witcraft SM, Kauffman BY, et al. Psychological and pharmacological treatments for generalized anxiety disorder (GAD): a meta-analysis of randomized controlled trials. Cogn Behav Ther. 2020;49(1):1-21. doi:10.1080/16506073.2018.1560358
3. Cuijpers P, Cristea IA, Karyotaki E, et al. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry. 2016;15(3):245-258. doi:10.1002/wps.20346
4. Saeed SA, Majarwitz DJ. Generalized anxiety disorder: 8 studies of biological interventions. Current Psychiatry. 2022;21(7):10-12,20,22-27. doi:10.12788/cp.02645
5. Simon NM, Hofmann SG, Rosenfield D, et al. Efficacy of yoga vs cognitive behavioral therapy vs stress education for the treatment of generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78(1):13-20. doi:10.1001/jamapsychiatry.2020.2496
6. Gould RL, Wetherell JL, Serfaty MA, et al. Acceptance and commitment therapy for older people with treatment-resistant generalised anxiety disorder: the FACTOID feasibility study. Health Technol Assess. 2021;25(54):1-150. doi:10.3310/hta25540
7. Stefan S, Cristea IA, Szentagotai Tatar A, et al. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol. 2019;75(7):1188-1202. doi:10.1002/jclp.22779
8. Plag J, Schmidt-Hellinger P, Klippstein T, et al. Working out the worries: a randomized controlled trial of high intensity interval training in generalized anxiety disorder. J Anxiety Disord. 2020;76:102311. doi:10.1016/j.janxdis.2020.102311
9. Amir N, Taboas W, Montero M. Feasibility and dissemination of a computerized home-based treatment for generalized anxiety disorder: a randomized clinical trial. Behav Res Ther. 2019;120:103446. doi:10.1016/j.brat.2019.103446
10. Burke J, Richards D, Timulak L. Helpful and hindering events in internet-delivered cognitive behavioural treatment for generalized anxiety. Behav Cogn Psychother. 2019;47(3):386-399. doi:10.1017/S1352465818000504
11. Miller CB, Gu J, Henry AL, et al. Feasibility and efficacy of a digital CBT intervention for symptoms of generalized anxiety disorder: a randomized multiple-baseline study. J Behav Ther Exp Psychiatry. 2021;70:101609. doi:10.1016/j.jbtep.2020.101609
12. Hirsch CR, Krahé C, Whyte J, et al. Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: a randomized controlled experiment. J Consult Clin Psychol. 2021;89(7):575-589. doi:10.1037/ccp0000660
13. Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621-632. doi:10.4088/jcp.v69n0415
14. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
15. Carpenter JK, Andrews LA, Witcraft SM, et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
16. Covin R, Ouimet AJ, Seeds PM, et al. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord. 2008;22(1):108-116. doi:10.1016/j.janxdis.2007.01.002
17. Merom D, Phongsavan P, Wagner R, et al. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. J Anxiety Disord. 2008;22(6):959-968. doi:10.1016/j.janxdis.2007.09.010
18. Herring MP, Jacob ML, Suveg C, et al. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21-28. doi:10.1159/000327898
19. Bischoff S, Wieder G, Einsle F, et al. Running for extinction? Aerobic exercise as an augmentation of exposure therapy in panic disorder with agoraphobia. J Psychiatr Res. 2018;101:34-41. doi:10.1016/j.jpsychires.2018.03.001
20. Korman N, Armour M, Chapman J, et al. High Intensity Interval training (HIIT) for people with severe mental illness: a systematic review & meta-analysis of intervention studies- considering diverse approaches for mental and physical recovery. Psychiatry Res. 2020;284:112601. doi:10.1016/j.psychres.2019.112601
21. Amir N, Beard C, Cobb M, et al. Attention modification program in individuals with generalized anxiety disorder. J Abnorm Psychol. 2009;118(1):28-33. doi:10.1037/a0012589
22. Hirsh CR, Mathews A. A cognitive model of pathological worry. Behav Res Ther. 2012;50(10):636-646. doi:10.1016/j.brat.2012.007
Using SNRIs to prevent migraines in patients with depression
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.
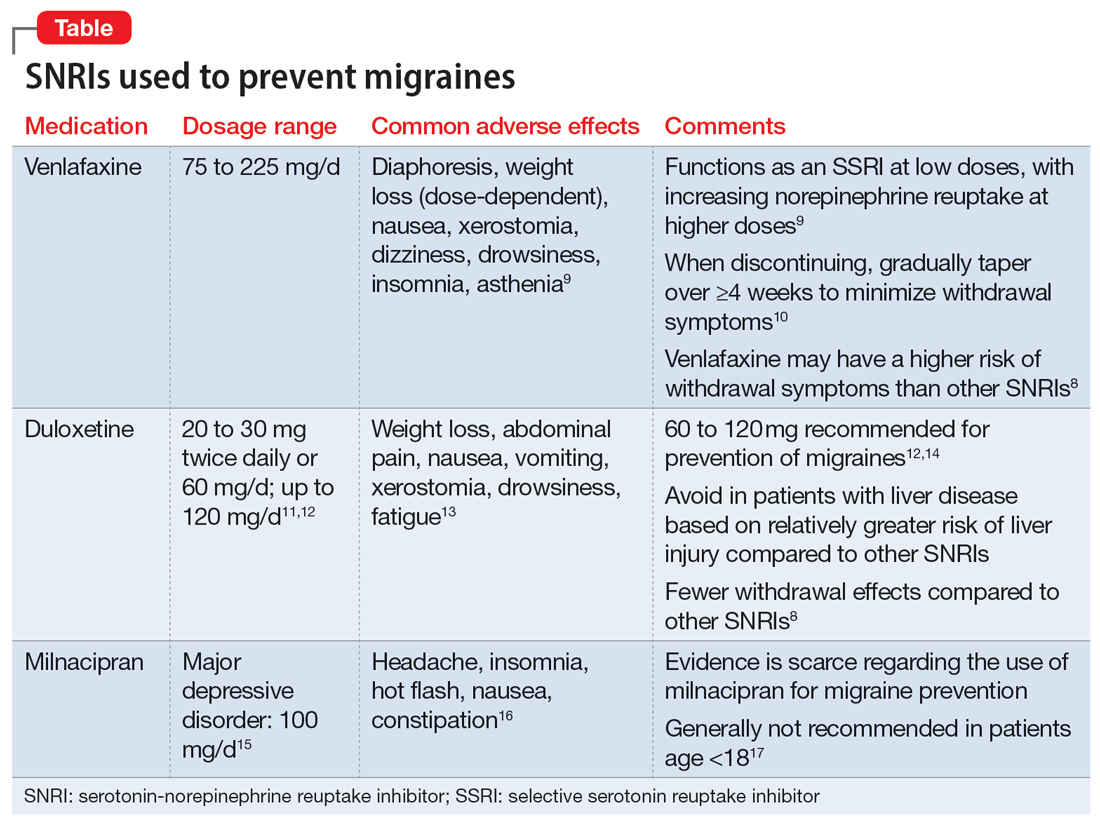
Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.

Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.

Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
Medical record documentation: What to do, and what to avoid
Medical record documentation serves as a reminder of previous discussions with patients and what happened during their visits, a reimbursement justification for services, a communication tool to coordinate care with current and future clinicians, and a basis for defense in legal or regulatory matters.1,2 Documentation should be thorough, accurate, timely, and objective, with the ultimate goal of communicating our thoughts in an easily understood manner to other clinicians or attorneys.2 If we fail to achieve this goal, we may inadvertently give the impression that our care was hurried, incomplete, or thoughtless.2
Although not an exhaustive list, this article outlines strategies to employ and practices to avoid in our documentation efforts so we may enhance our defense in case of litigation and ensure the smooth transition of care for our patients.
Strategies to employ
Proper and accurate documentation details the course of patient care, and we should describe our thoughts in a clear and logical manner. Doing so minimizes the risk of misinterpretation by other clinicians or attorneys. Make sure the documentation of each appointment details the reason(s) for the patient’s visit, the effectiveness of treatment, possible treatment nonadherence, our clinical assessment, treatment consent, changes to the patient’s treatment plan, follow-up plans, reasons for not pursuing certain actions (eg, hospitalization), and a suicide risk assessment (and/or a violence risk assessment, if clinically indicated).2 Document missed or rescheduled appointments, and telephone and electronic contact with patients. Also be sure to use only commonly approved abbreviations.2 Document these items sooner rather than later because doing so improves the credibility of your charting.1 If you are handwriting notes, add the date and time to each encounter and make sure your handwriting is legible. Describe the behaviors of patients in objective and nonjudgmental terms.3 Documenting quotes from patients can convey crucial information about what was considered when making clinical decisions.1
Practices to avoid
If there is a need to make changes to previous entries, ensure these corrections are not mistaken for alterations. Each health care institution has its own policy for making corrections and addenda to medical records. Corrections to a patient’s medical record are acceptable, provided they are done appropriately, as I outlined in a previous Pearls article.4 Minimize or eliminate the copying and pasting of information; doing so can improve the efficiency of our documentation, but the practice can undermine the quality of the medical record, increase the risk of outdated and repetitive information being included, lead to clinical errors, and lead to overbilling of services.5 Finally, be sure to avoid speculation, personal commentary about patients and their family members, and language with negative connotations (unless such language is a direct quote from the patient).2,3
1. Mossman D. Tips to make documentation easier, faster, and more satisfying. Current Psychiatry. 2008;7(2):80,84-86.
2. Staus C. Documentation: your very best defense. Psychiatric News. 2022;57(4):7,19.
3. Nelson KJ. How to use patient-centered language in documentation. Current Psychiatry. 2011;10(10):70.
4. Joshi KG. Metadata, malpractice claims, and making changes to the EHR. Current Psychiatry. 2021;20(3):e1-e3. doi:10.12788/cp.0106
5. Neal D. Do’s and don’ts of electronic documentation. Psychiatric News. 2021;56(8):7.
Medical record documentation serves as a reminder of previous discussions with patients and what happened during their visits, a reimbursement justification for services, a communication tool to coordinate care with current and future clinicians, and a basis for defense in legal or regulatory matters.1,2 Documentation should be thorough, accurate, timely, and objective, with the ultimate goal of communicating our thoughts in an easily understood manner to other clinicians or attorneys.2 If we fail to achieve this goal, we may inadvertently give the impression that our care was hurried, incomplete, or thoughtless.2
Although not an exhaustive list, this article outlines strategies to employ and practices to avoid in our documentation efforts so we may enhance our defense in case of litigation and ensure the smooth transition of care for our patients.
Strategies to employ
Proper and accurate documentation details the course of patient care, and we should describe our thoughts in a clear and logical manner. Doing so minimizes the risk of misinterpretation by other clinicians or attorneys. Make sure the documentation of each appointment details the reason(s) for the patient’s visit, the effectiveness of treatment, possible treatment nonadherence, our clinical assessment, treatment consent, changes to the patient’s treatment plan, follow-up plans, reasons for not pursuing certain actions (eg, hospitalization), and a suicide risk assessment (and/or a violence risk assessment, if clinically indicated).2 Document missed or rescheduled appointments, and telephone and electronic contact with patients. Also be sure to use only commonly approved abbreviations.2 Document these items sooner rather than later because doing so improves the credibility of your charting.1 If you are handwriting notes, add the date and time to each encounter and make sure your handwriting is legible. Describe the behaviors of patients in objective and nonjudgmental terms.3 Documenting quotes from patients can convey crucial information about what was considered when making clinical decisions.1
Practices to avoid
If there is a need to make changes to previous entries, ensure these corrections are not mistaken for alterations. Each health care institution has its own policy for making corrections and addenda to medical records. Corrections to a patient’s medical record are acceptable, provided they are done appropriately, as I outlined in a previous Pearls article.4 Minimize or eliminate the copying and pasting of information; doing so can improve the efficiency of our documentation, but the practice can undermine the quality of the medical record, increase the risk of outdated and repetitive information being included, lead to clinical errors, and lead to overbilling of services.5 Finally, be sure to avoid speculation, personal commentary about patients and their family members, and language with negative connotations (unless such language is a direct quote from the patient).2,3
Medical record documentation serves as a reminder of previous discussions with patients and what happened during their visits, a reimbursement justification for services, a communication tool to coordinate care with current and future clinicians, and a basis for defense in legal or regulatory matters.1,2 Documentation should be thorough, accurate, timely, and objective, with the ultimate goal of communicating our thoughts in an easily understood manner to other clinicians or attorneys.2 If we fail to achieve this goal, we may inadvertently give the impression that our care was hurried, incomplete, or thoughtless.2
Although not an exhaustive list, this article outlines strategies to employ and practices to avoid in our documentation efforts so we may enhance our defense in case of litigation and ensure the smooth transition of care for our patients.
Strategies to employ
Proper and accurate documentation details the course of patient care, and we should describe our thoughts in a clear and logical manner. Doing so minimizes the risk of misinterpretation by other clinicians or attorneys. Make sure the documentation of each appointment details the reason(s) for the patient’s visit, the effectiveness of treatment, possible treatment nonadherence, our clinical assessment, treatment consent, changes to the patient’s treatment plan, follow-up plans, reasons for not pursuing certain actions (eg, hospitalization), and a suicide risk assessment (and/or a violence risk assessment, if clinically indicated).2 Document missed or rescheduled appointments, and telephone and electronic contact with patients. Also be sure to use only commonly approved abbreviations.2 Document these items sooner rather than later because doing so improves the credibility of your charting.1 If you are handwriting notes, add the date and time to each encounter and make sure your handwriting is legible. Describe the behaviors of patients in objective and nonjudgmental terms.3 Documenting quotes from patients can convey crucial information about what was considered when making clinical decisions.1
Practices to avoid
If there is a need to make changes to previous entries, ensure these corrections are not mistaken for alterations. Each health care institution has its own policy for making corrections and addenda to medical records. Corrections to a patient’s medical record are acceptable, provided they are done appropriately, as I outlined in a previous Pearls article.4 Minimize or eliminate the copying and pasting of information; doing so can improve the efficiency of our documentation, but the practice can undermine the quality of the medical record, increase the risk of outdated and repetitive information being included, lead to clinical errors, and lead to overbilling of services.5 Finally, be sure to avoid speculation, personal commentary about patients and their family members, and language with negative connotations (unless such language is a direct quote from the patient).2,3
1. Mossman D. Tips to make documentation easier, faster, and more satisfying. Current Psychiatry. 2008;7(2):80,84-86.
2. Staus C. Documentation: your very best defense. Psychiatric News. 2022;57(4):7,19.
3. Nelson KJ. How to use patient-centered language in documentation. Current Psychiatry. 2011;10(10):70.
4. Joshi KG. Metadata, malpractice claims, and making changes to the EHR. Current Psychiatry. 2021;20(3):e1-e3. doi:10.12788/cp.0106
5. Neal D. Do’s and don’ts of electronic documentation. Psychiatric News. 2021;56(8):7.
1. Mossman D. Tips to make documentation easier, faster, and more satisfying. Current Psychiatry. 2008;7(2):80,84-86.
2. Staus C. Documentation: your very best defense. Psychiatric News. 2022;57(4):7,19.
3. Nelson KJ. How to use patient-centered language in documentation. Current Psychiatry. 2011;10(10):70.
4. Joshi KG. Metadata, malpractice claims, and making changes to the EHR. Current Psychiatry. 2021;20(3):e1-e3. doi:10.12788/cp.0106
5. Neal D. Do’s and don’ts of electronic documentation. Psychiatric News. 2021;56(8):7.
Lithium, valproate, and suicide risk: Analysis of 98,831 cases
The current academic psychiatry paradigm reinforces that lithium reduces suicide risk, more so than other medications, including valproate. However, data from multiple sources contradict this “evidence-based” belief.
Data do not support lithium’s supposed advantage
An 8-year prospective study in Sweden by Song et al1 tracked 51,535 patients with bipolar disorder from 2005 to 2013. In their conclusions, the authors of this study omitted some surprising numbers that contradict the dominant paradigm. There were 230 (1.089%) completed suicides in the lithium group (N = 21,129), 99 (1.177%) in the valproate group (N = 8,411), and 308 (1.195%) in the “other medication” group (N = 25,780). This difference of .088% is too small (95% CI, -.180% to .358%) to substantiate the purported advantage of lithium over valproate. More important is that in terms of suicide-related events, the medication group excluding lithium and valproate had 2,018 (7.8%) events vs lithium 2,142 (10.1%) and valproate 1,105 (13.1%). The difference of 2.3% is statistically significant (95% CI, 1.8% to 2.8%). These numbers reflect fewer suicide-related events with psychiatric medications other than lithium and valproate. Compounding the problem is a design flaw in which 3,785 patients were counted twice in the lithium and valproate groups (21,129 + 8,411 + 25,780 = 55,320, which is more than the 51,535 patients in the study). By falsely inflating the denominator (N) for the lithium and valproate groups, the respective published rates are deceptively lower than the actual rates. Song et al1 did not provide an adequate explanation for these findings and omitted them from their conclusions.
In Schatzberg’s Manual of Clinical Psychopharmacology, the authors cited Song et al1 but omitted these findings as well, and stated “lithium is clearly effective in preventing suicide attempts and completions in bipolar patients.”2 In Stahl’s Essential Psychopharmacology, the author wrote “lithium actually reduces suicide in patients with bipolar disorder.”3 In a review article,
In an overlapping period, National Poison Data System (NPDS) data of single substance exposures painted a different picture in the United States.6 During 2006-2013, the lithium group (N = 26,144) had 32 deaths (all causes) (.122%), and the valproate group (N = 25,630) had 16 deaths (.062%). During 2006-2020, the lithium group (N = 52,262) had 55 deaths (.105%), and the valproate group (N = 46,569) had 31 deaths (.067%). Clearly there is a major disconnect between lithium’s advertised ability to reduce suicide risk and the actual mortality rate, as evidenced by 98,831 cases reported to NPDS during 2006-2020. One would expect a lower rate in the lithium group, but data show it is higher than in the valproate group. This underscores the common fallacy of most lithium studies: each is based on a very small sample (N < 100), and the statistical inference about the entire population is tenuous. If lithium truly reduces suicide risk 5-fold, it would be seen in a sample of 98,831. The law of large numbers and central limit theorem state that as N increases, the variability of the rate progressively decreases. This can be easily demonstrated with computer simulation models and simple Python code, or on the average fuel economy display of most cars.
What about the relative lethality?
The APA Textbook of Suicide Risk Assessment and Management stated that it is important to consider the relative lethality (RL) of prescription medications.7 The RL equation (RL = 310x / LD50) represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person (x is the daily dose and LD50 is the rat oral lethal dose 50).8 Time series analysis shows that the lithium relative morbidity (RM) is consistently double that of valproate (Figure6). Regression models have shown high correlation and causality between RL and RM.9-11 It is surprising that valproate (RL = 1,666%) has a lower RM than lithium (RL = 1,063%). This paradox can be easily explained with clinical insight. The RL equation compares medications at the maximum daily dose, but in routine practice valproate is commonly prescribed at 1,000 mg/d (28% of the maximum 3,600 mg/d). Lithium is commonly prescribed at 1,200 mg/d (67% of the maximum 1,800 mg/d). Within these dosing parameters, the effective RL is valproate 463% and lithium 709%. The 2020 RM is valproate 22% and lithium 43%.12 The COVID-19 pandemic did not affect the predicted RM. Confirming these numbers, Song et al1 acknowledged “greater safety in case of overdose for valproate in clinical practice.” Baldessarini et al4 asserted “the fatality risk of lithium overdose is only moderate, and very similar to modern antidepressants and second-generation antipsychotics.”4 This claim is contradicted by the RL equation and regression models.7-11 Lithium’s RL is 19 times higher than that of fluoxetine, and 30 times higher than that of olanzapine.8 Lithium’s RM is nearly identical to amitriptyline (42%), vs fluoxetine (12%).12
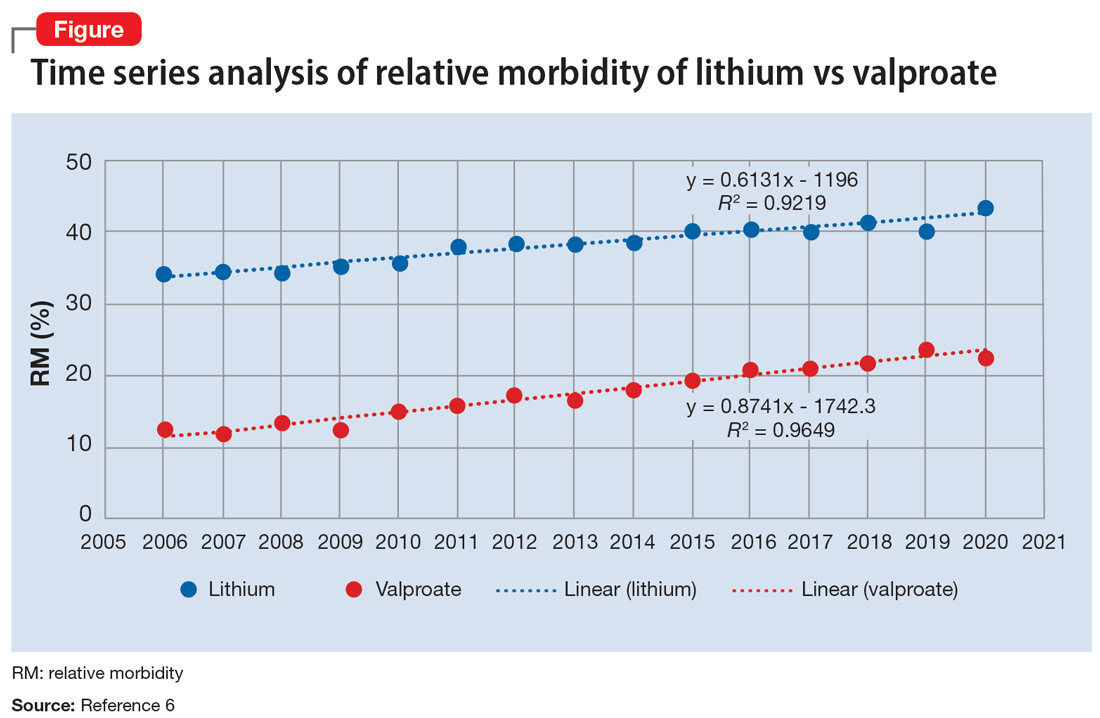
Data-driven analysis shows that lithium has higher rates of morbidity and mortality than valproate, as evidenced by 98,831 NPDS cases during 2006-2020. These hard numbers speak for themselves and contradict the dominant paradigm, which proclaims lithium’s superiority in reducing suicide risk.
1. Song J, Sjölander A, Joas E, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017;174(8):795-802.
2. Schatzberg AF, DeBattista C. Schatzberg’s Manual of Clinical Psychopharmacology. 9th ed. American Psychiatric Association Publishing; 2019:335.
3. Stahl SM. Stahl’s Essential Psychopharmacology. 4th ed. Cambridge University Press; 2013:372.
4. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625-639.
5. Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056.
6. American Association of Poison Control Centers. Annual reports. Accessed August 25, 2022. https://aapcc.org/annual-reports
7. Gold LH, Frierson RL (eds). The American Psychiatric Association Publishing Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020:17-19.
8. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
9. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
10. Giurca D. Time series analysis of poison control data. Current Psychiatry. 2020;19(6):e5-e9.
11. Giurca D, Hodgman MJ. Relative lethality of hypertension drugs. J Med Toxicol. 2022;18(2):81. 2022 American College of Medical Toxicology Annual Scientific Meeting abstract 020.
12. Gummin DD, Mowry JB, Beuhler MD, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282-1501.
The current academic psychiatry paradigm reinforces that lithium reduces suicide risk, more so than other medications, including valproate. However, data from multiple sources contradict this “evidence-based” belief.
Data do not support lithium’s supposed advantage
An 8-year prospective study in Sweden by Song et al1 tracked 51,535 patients with bipolar disorder from 2005 to 2013. In their conclusions, the authors of this study omitted some surprising numbers that contradict the dominant paradigm. There were 230 (1.089%) completed suicides in the lithium group (N = 21,129), 99 (1.177%) in the valproate group (N = 8,411), and 308 (1.195%) in the “other medication” group (N = 25,780). This difference of .088% is too small (95% CI, -.180% to .358%) to substantiate the purported advantage of lithium over valproate. More important is that in terms of suicide-related events, the medication group excluding lithium and valproate had 2,018 (7.8%) events vs lithium 2,142 (10.1%) and valproate 1,105 (13.1%). The difference of 2.3% is statistically significant (95% CI, 1.8% to 2.8%). These numbers reflect fewer suicide-related events with psychiatric medications other than lithium and valproate. Compounding the problem is a design flaw in which 3,785 patients were counted twice in the lithium and valproate groups (21,129 + 8,411 + 25,780 = 55,320, which is more than the 51,535 patients in the study). By falsely inflating the denominator (N) for the lithium and valproate groups, the respective published rates are deceptively lower than the actual rates. Song et al1 did not provide an adequate explanation for these findings and omitted them from their conclusions.
In Schatzberg’s Manual of Clinical Psychopharmacology, the authors cited Song et al1 but omitted these findings as well, and stated “lithium is clearly effective in preventing suicide attempts and completions in bipolar patients.”2 In Stahl’s Essential Psychopharmacology, the author wrote “lithium actually reduces suicide in patients with bipolar disorder.”3 In a review article,
In an overlapping period, National Poison Data System (NPDS) data of single substance exposures painted a different picture in the United States.6 During 2006-2013, the lithium group (N = 26,144) had 32 deaths (all causes) (.122%), and the valproate group (N = 25,630) had 16 deaths (.062%). During 2006-2020, the lithium group (N = 52,262) had 55 deaths (.105%), and the valproate group (N = 46,569) had 31 deaths (.067%). Clearly there is a major disconnect between lithium’s advertised ability to reduce suicide risk and the actual mortality rate, as evidenced by 98,831 cases reported to NPDS during 2006-2020. One would expect a lower rate in the lithium group, but data show it is higher than in the valproate group. This underscores the common fallacy of most lithium studies: each is based on a very small sample (N < 100), and the statistical inference about the entire population is tenuous. If lithium truly reduces suicide risk 5-fold, it would be seen in a sample of 98,831. The law of large numbers and central limit theorem state that as N increases, the variability of the rate progressively decreases. This can be easily demonstrated with computer simulation models and simple Python code, or on the average fuel economy display of most cars.
What about the relative lethality?
The APA Textbook of Suicide Risk Assessment and Management stated that it is important to consider the relative lethality (RL) of prescription medications.7 The RL equation (RL = 310x / LD50) represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person (x is the daily dose and LD50 is the rat oral lethal dose 50).8 Time series analysis shows that the lithium relative morbidity (RM) is consistently double that of valproate (Figure6). Regression models have shown high correlation and causality between RL and RM.9-11 It is surprising that valproate (RL = 1,666%) has a lower RM than lithium (RL = 1,063%). This paradox can be easily explained with clinical insight. The RL equation compares medications at the maximum daily dose, but in routine practice valproate is commonly prescribed at 1,000 mg/d (28% of the maximum 3,600 mg/d). Lithium is commonly prescribed at 1,200 mg/d (67% of the maximum 1,800 mg/d). Within these dosing parameters, the effective RL is valproate 463% and lithium 709%. The 2020 RM is valproate 22% and lithium 43%.12 The COVID-19 pandemic did not affect the predicted RM. Confirming these numbers, Song et al1 acknowledged “greater safety in case of overdose for valproate in clinical practice.” Baldessarini et al4 asserted “the fatality risk of lithium overdose is only moderate, and very similar to modern antidepressants and second-generation antipsychotics.”4 This claim is contradicted by the RL equation and regression models.7-11 Lithium’s RL is 19 times higher than that of fluoxetine, and 30 times higher than that of olanzapine.8 Lithium’s RM is nearly identical to amitriptyline (42%), vs fluoxetine (12%).12

Data-driven analysis shows that lithium has higher rates of morbidity and mortality than valproate, as evidenced by 98,831 NPDS cases during 2006-2020. These hard numbers speak for themselves and contradict the dominant paradigm, which proclaims lithium’s superiority in reducing suicide risk.
The current academic psychiatry paradigm reinforces that lithium reduces suicide risk, more so than other medications, including valproate. However, data from multiple sources contradict this “evidence-based” belief.
Data do not support lithium’s supposed advantage
An 8-year prospective study in Sweden by Song et al1 tracked 51,535 patients with bipolar disorder from 2005 to 2013. In their conclusions, the authors of this study omitted some surprising numbers that contradict the dominant paradigm. There were 230 (1.089%) completed suicides in the lithium group (N = 21,129), 99 (1.177%) in the valproate group (N = 8,411), and 308 (1.195%) in the “other medication” group (N = 25,780). This difference of .088% is too small (95% CI, -.180% to .358%) to substantiate the purported advantage of lithium over valproate. More important is that in terms of suicide-related events, the medication group excluding lithium and valproate had 2,018 (7.8%) events vs lithium 2,142 (10.1%) and valproate 1,105 (13.1%). The difference of 2.3% is statistically significant (95% CI, 1.8% to 2.8%). These numbers reflect fewer suicide-related events with psychiatric medications other than lithium and valproate. Compounding the problem is a design flaw in which 3,785 patients were counted twice in the lithium and valproate groups (21,129 + 8,411 + 25,780 = 55,320, which is more than the 51,535 patients in the study). By falsely inflating the denominator (N) for the lithium and valproate groups, the respective published rates are deceptively lower than the actual rates. Song et al1 did not provide an adequate explanation for these findings and omitted them from their conclusions.
In Schatzberg’s Manual of Clinical Psychopharmacology, the authors cited Song et al1 but omitted these findings as well, and stated “lithium is clearly effective in preventing suicide attempts and completions in bipolar patients.”2 In Stahl’s Essential Psychopharmacology, the author wrote “lithium actually reduces suicide in patients with bipolar disorder.”3 In a review article,
In an overlapping period, National Poison Data System (NPDS) data of single substance exposures painted a different picture in the United States.6 During 2006-2013, the lithium group (N = 26,144) had 32 deaths (all causes) (.122%), and the valproate group (N = 25,630) had 16 deaths (.062%). During 2006-2020, the lithium group (N = 52,262) had 55 deaths (.105%), and the valproate group (N = 46,569) had 31 deaths (.067%). Clearly there is a major disconnect between lithium’s advertised ability to reduce suicide risk and the actual mortality rate, as evidenced by 98,831 cases reported to NPDS during 2006-2020. One would expect a lower rate in the lithium group, but data show it is higher than in the valproate group. This underscores the common fallacy of most lithium studies: each is based on a very small sample (N < 100), and the statistical inference about the entire population is tenuous. If lithium truly reduces suicide risk 5-fold, it would be seen in a sample of 98,831. The law of large numbers and central limit theorem state that as N increases, the variability of the rate progressively decreases. This can be easily demonstrated with computer simulation models and simple Python code, or on the average fuel economy display of most cars.
What about the relative lethality?
The APA Textbook of Suicide Risk Assessment and Management stated that it is important to consider the relative lethality (RL) of prescription medications.7 The RL equation (RL = 310x / LD50) represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person (x is the daily dose and LD50 is the rat oral lethal dose 50).8 Time series analysis shows that the lithium relative morbidity (RM) is consistently double that of valproate (Figure6). Regression models have shown high correlation and causality between RL and RM.9-11 It is surprising that valproate (RL = 1,666%) has a lower RM than lithium (RL = 1,063%). This paradox can be easily explained with clinical insight. The RL equation compares medications at the maximum daily dose, but in routine practice valproate is commonly prescribed at 1,000 mg/d (28% of the maximum 3,600 mg/d). Lithium is commonly prescribed at 1,200 mg/d (67% of the maximum 1,800 mg/d). Within these dosing parameters, the effective RL is valproate 463% and lithium 709%. The 2020 RM is valproate 22% and lithium 43%.12 The COVID-19 pandemic did not affect the predicted RM. Confirming these numbers, Song et al1 acknowledged “greater safety in case of overdose for valproate in clinical practice.” Baldessarini et al4 asserted “the fatality risk of lithium overdose is only moderate, and very similar to modern antidepressants and second-generation antipsychotics.”4 This claim is contradicted by the RL equation and regression models.7-11 Lithium’s RL is 19 times higher than that of fluoxetine, and 30 times higher than that of olanzapine.8 Lithium’s RM is nearly identical to amitriptyline (42%), vs fluoxetine (12%).12

Data-driven analysis shows that lithium has higher rates of morbidity and mortality than valproate, as evidenced by 98,831 NPDS cases during 2006-2020. These hard numbers speak for themselves and contradict the dominant paradigm, which proclaims lithium’s superiority in reducing suicide risk.
1. Song J, Sjölander A, Joas E, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017;174(8):795-802.
2. Schatzberg AF, DeBattista C. Schatzberg’s Manual of Clinical Psychopharmacology. 9th ed. American Psychiatric Association Publishing; 2019:335.
3. Stahl SM. Stahl’s Essential Psychopharmacology. 4th ed. Cambridge University Press; 2013:372.
4. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625-639.
5. Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056.
6. American Association of Poison Control Centers. Annual reports. Accessed August 25, 2022. https://aapcc.org/annual-reports
7. Gold LH, Frierson RL (eds). The American Psychiatric Association Publishing Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020:17-19.
8. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
9. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
10. Giurca D. Time series analysis of poison control data. Current Psychiatry. 2020;19(6):e5-e9.
11. Giurca D, Hodgman MJ. Relative lethality of hypertension drugs. J Med Toxicol. 2022;18(2):81. 2022 American College of Medical Toxicology Annual Scientific Meeting abstract 020.
12. Gummin DD, Mowry JB, Beuhler MD, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282-1501.
1. Song J, Sjölander A, Joas E, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017;174(8):795-802.
2. Schatzberg AF, DeBattista C. Schatzberg’s Manual of Clinical Psychopharmacology. 9th ed. American Psychiatric Association Publishing; 2019:335.
3. Stahl SM. Stahl’s Essential Psychopharmacology. 4th ed. Cambridge University Press; 2013:372.
4. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625-639.
5. Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056.
6. American Association of Poison Control Centers. Annual reports. Accessed August 25, 2022. https://aapcc.org/annual-reports
7. Gold LH, Frierson RL (eds). The American Psychiatric Association Publishing Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020:17-19.
8. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
9. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
10. Giurca D. Time series analysis of poison control data. Current Psychiatry. 2020;19(6):e5-e9.
11. Giurca D, Hodgman MJ. Relative lethality of hypertension drugs. J Med Toxicol. 2022;18(2):81. 2022 American College of Medical Toxicology Annual Scientific Meeting abstract 020.
12. Gummin DD, Mowry JB, Beuhler MD, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282-1501.
Postop analgesia in Saudi Arabia and the United States: A resident’s perspective
I had the opportunity to experience first-hand acute postoperative pain management in both the United States and Saudi Arabia. In this article, I discuss some of the differences in how postop pain is managed in each location, potential reasons for these differences, how they may impact patients over time, and the psychiatrist’s role in raising awareness about the hazards of overprescribing analgesic medications.
Vast differences in postop opioid prescribing
From personal observation and literature review, I was appalled by the amount of oxycodone tablets patients are typically discharged home with after a surgical procedure in the United States. Depending on the extent of the surgical procedure, opioid-naïve patients were routinely discharged with 40 to 120 tablets of oxycodone 5 mg. A ventral hernia repair or laparotomy was on the high end of how much oxycodone was provided, and a laparoscopic cholecystectomy or inguinal hernia repair was on the low end. At least one study has supported this observation, finding a wide variation and excessive doses of opioids prescribed postop.1 Notably, among opioids obtained by postsurgical patients, 42% to 71% of all tablets went unused.2 Nevertheless, prescribing in this manner became the standard for postop pain management—possibly in an effort to maximize patient satisfaction on surveys. Additionally, marketing and promotion by the pharmaceutical industry appears to have considerably amplified the prescription, sales, and availability of opioids.3
Signing those prescriptions always left a bad taste in my mouth out of concern for the potential for initiating chronic opioid use.4 Personally, I would prescribe the lowest reasonable number of narcotic tablets for my patients, along with acetaminophen and ibuprofen, knowing that nonsteroidal anti-inflammatory drugs are sufficient for treating postop pain and will decrease opioid requirements, therefore minimizing opiate-induced adverse events.5 Overtreatment of pain with narcotics as first-line therapy is particularly problematic when treating postop pain in children after minor procedures, such as an umbilical hernia repair.Allowing children to resort to a narcotic analgesic agent as a first-line therapy had the potential to develop into an opioid use disorder (OUD) later in life if environmental factors tipped the scales.6
In the hospital in Saudi Arabia where I initially trained, surgery residents were not permitted to prescribe narcotics. The standard of care was to discharge patients with acetaminophen and ibuprofen. In cases where there was an indication for pain treatment with narcotics, stringent regulations were in place. For example, in my experience, which is corroborated by one study,6 special “narcotic forms” are required in the Middle East. In most of these countries, access to these forms is restricted.7 Moreover, pharmacists would only accept this special form when attested to by the surgery consultant (the equivalent of an attending physician in the United States). These consultants would typically write a prescription for 9 to 15 oxycodone 5 mg tablets. Patients receiving such medications were closely watched and followed up in the surgery clinic 3 to 5 days after discharge. Patients were also required to fill out a form detailing their contact information, including their home address and national ID number, to be able to pick up their prescription. Furthermore, apart from 2 Middle East countries, opioids were only available from hospital pharmacies, which were independent of the general hospital pharmacy in location and staff training.8
The psychiatrist’s role
Adapting similar stringent practices for prescribing narcotics in the United States might reduce 1 risk factor for OUD in postop patients. Surgeons attempt to provide the best care by maximizing analgesia, but psychiatrists see firsthand the consequences of overprescribing, and play a direct role in managing patients’ OUDs. As psychiatrists, we have a duty to continue to raise awareness and alert other clinicians about the hazards of overprescribing narcotic analgesic agents.
1. Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714.
2. Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071.
3. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227.
4. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
5. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi: 10.1007/s11916-016-0591-7
6. Pollini RA, Banta-Green CJ, Cuevas-Mota J, et al. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173-180.
7. Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol. 2013;24 Suppl 11:xi51-xi59. doi: 10.1093/annonc/mdt503
8. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44.
I had the opportunity to experience first-hand acute postoperative pain management in both the United States and Saudi Arabia. In this article, I discuss some of the differences in how postop pain is managed in each location, potential reasons for these differences, how they may impact patients over time, and the psychiatrist’s role in raising awareness about the hazards of overprescribing analgesic medications.
Vast differences in postop opioid prescribing
From personal observation and literature review, I was appalled by the amount of oxycodone tablets patients are typically discharged home with after a surgical procedure in the United States. Depending on the extent of the surgical procedure, opioid-naïve patients were routinely discharged with 40 to 120 tablets of oxycodone 5 mg. A ventral hernia repair or laparotomy was on the high end of how much oxycodone was provided, and a laparoscopic cholecystectomy or inguinal hernia repair was on the low end. At least one study has supported this observation, finding a wide variation and excessive doses of opioids prescribed postop.1 Notably, among opioids obtained by postsurgical patients, 42% to 71% of all tablets went unused.2 Nevertheless, prescribing in this manner became the standard for postop pain management—possibly in an effort to maximize patient satisfaction on surveys. Additionally, marketing and promotion by the pharmaceutical industry appears to have considerably amplified the prescription, sales, and availability of opioids.3
Signing those prescriptions always left a bad taste in my mouth out of concern for the potential for initiating chronic opioid use.4 Personally, I would prescribe the lowest reasonable number of narcotic tablets for my patients, along with acetaminophen and ibuprofen, knowing that nonsteroidal anti-inflammatory drugs are sufficient for treating postop pain and will decrease opioid requirements, therefore minimizing opiate-induced adverse events.5 Overtreatment of pain with narcotics as first-line therapy is particularly problematic when treating postop pain in children after minor procedures, such as an umbilical hernia repair.Allowing children to resort to a narcotic analgesic agent as a first-line therapy had the potential to develop into an opioid use disorder (OUD) later in life if environmental factors tipped the scales.6
In the hospital in Saudi Arabia where I initially trained, surgery residents were not permitted to prescribe narcotics. The standard of care was to discharge patients with acetaminophen and ibuprofen. In cases where there was an indication for pain treatment with narcotics, stringent regulations were in place. For example, in my experience, which is corroborated by one study,6 special “narcotic forms” are required in the Middle East. In most of these countries, access to these forms is restricted.7 Moreover, pharmacists would only accept this special form when attested to by the surgery consultant (the equivalent of an attending physician in the United States). These consultants would typically write a prescription for 9 to 15 oxycodone 5 mg tablets. Patients receiving such medications were closely watched and followed up in the surgery clinic 3 to 5 days after discharge. Patients were also required to fill out a form detailing their contact information, including their home address and national ID number, to be able to pick up their prescription. Furthermore, apart from 2 Middle East countries, opioids were only available from hospital pharmacies, which were independent of the general hospital pharmacy in location and staff training.8
The psychiatrist’s role
Adapting similar stringent practices for prescribing narcotics in the United States might reduce 1 risk factor for OUD in postop patients. Surgeons attempt to provide the best care by maximizing analgesia, but psychiatrists see firsthand the consequences of overprescribing, and play a direct role in managing patients’ OUDs. As psychiatrists, we have a duty to continue to raise awareness and alert other clinicians about the hazards of overprescribing narcotic analgesic agents.
I had the opportunity to experience first-hand acute postoperative pain management in both the United States and Saudi Arabia. In this article, I discuss some of the differences in how postop pain is managed in each location, potential reasons for these differences, how they may impact patients over time, and the psychiatrist’s role in raising awareness about the hazards of overprescribing analgesic medications.
Vast differences in postop opioid prescribing
From personal observation and literature review, I was appalled by the amount of oxycodone tablets patients are typically discharged home with after a surgical procedure in the United States. Depending on the extent of the surgical procedure, opioid-naïve patients were routinely discharged with 40 to 120 tablets of oxycodone 5 mg. A ventral hernia repair or laparotomy was on the high end of how much oxycodone was provided, and a laparoscopic cholecystectomy or inguinal hernia repair was on the low end. At least one study has supported this observation, finding a wide variation and excessive doses of opioids prescribed postop.1 Notably, among opioids obtained by postsurgical patients, 42% to 71% of all tablets went unused.2 Nevertheless, prescribing in this manner became the standard for postop pain management—possibly in an effort to maximize patient satisfaction on surveys. Additionally, marketing and promotion by the pharmaceutical industry appears to have considerably amplified the prescription, sales, and availability of opioids.3
Signing those prescriptions always left a bad taste in my mouth out of concern for the potential for initiating chronic opioid use.4 Personally, I would prescribe the lowest reasonable number of narcotic tablets for my patients, along with acetaminophen and ibuprofen, knowing that nonsteroidal anti-inflammatory drugs are sufficient for treating postop pain and will decrease opioid requirements, therefore minimizing opiate-induced adverse events.5 Overtreatment of pain with narcotics as first-line therapy is particularly problematic when treating postop pain in children after minor procedures, such as an umbilical hernia repair.Allowing children to resort to a narcotic analgesic agent as a first-line therapy had the potential to develop into an opioid use disorder (OUD) later in life if environmental factors tipped the scales.6
In the hospital in Saudi Arabia where I initially trained, surgery residents were not permitted to prescribe narcotics. The standard of care was to discharge patients with acetaminophen and ibuprofen. In cases where there was an indication for pain treatment with narcotics, stringent regulations were in place. For example, in my experience, which is corroborated by one study,6 special “narcotic forms” are required in the Middle East. In most of these countries, access to these forms is restricted.7 Moreover, pharmacists would only accept this special form when attested to by the surgery consultant (the equivalent of an attending physician in the United States). These consultants would typically write a prescription for 9 to 15 oxycodone 5 mg tablets. Patients receiving such medications were closely watched and followed up in the surgery clinic 3 to 5 days after discharge. Patients were also required to fill out a form detailing their contact information, including their home address and national ID number, to be able to pick up their prescription. Furthermore, apart from 2 Middle East countries, opioids were only available from hospital pharmacies, which were independent of the general hospital pharmacy in location and staff training.8
The psychiatrist’s role
Adapting similar stringent practices for prescribing narcotics in the United States might reduce 1 risk factor for OUD in postop patients. Surgeons attempt to provide the best care by maximizing analgesia, but psychiatrists see firsthand the consequences of overprescribing, and play a direct role in managing patients’ OUDs. As psychiatrists, we have a duty to continue to raise awareness and alert other clinicians about the hazards of overprescribing narcotic analgesic agents.
1. Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714.
2. Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071.
3. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227.
4. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
5. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi: 10.1007/s11916-016-0591-7
6. Pollini RA, Banta-Green CJ, Cuevas-Mota J, et al. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173-180.
7. Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol. 2013;24 Suppl 11:xi51-xi59. doi: 10.1093/annonc/mdt503
8. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44.
1. Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714.
2. Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071.
3. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227.
4. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
5. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi: 10.1007/s11916-016-0591-7
6. Pollini RA, Banta-Green CJ, Cuevas-Mota J, et al. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173-180.
7. Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol. 2013;24 Suppl 11:xi51-xi59. doi: 10.1093/annonc/mdt503
8. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44.
Neuropsychiatric symptoms after stroke
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.
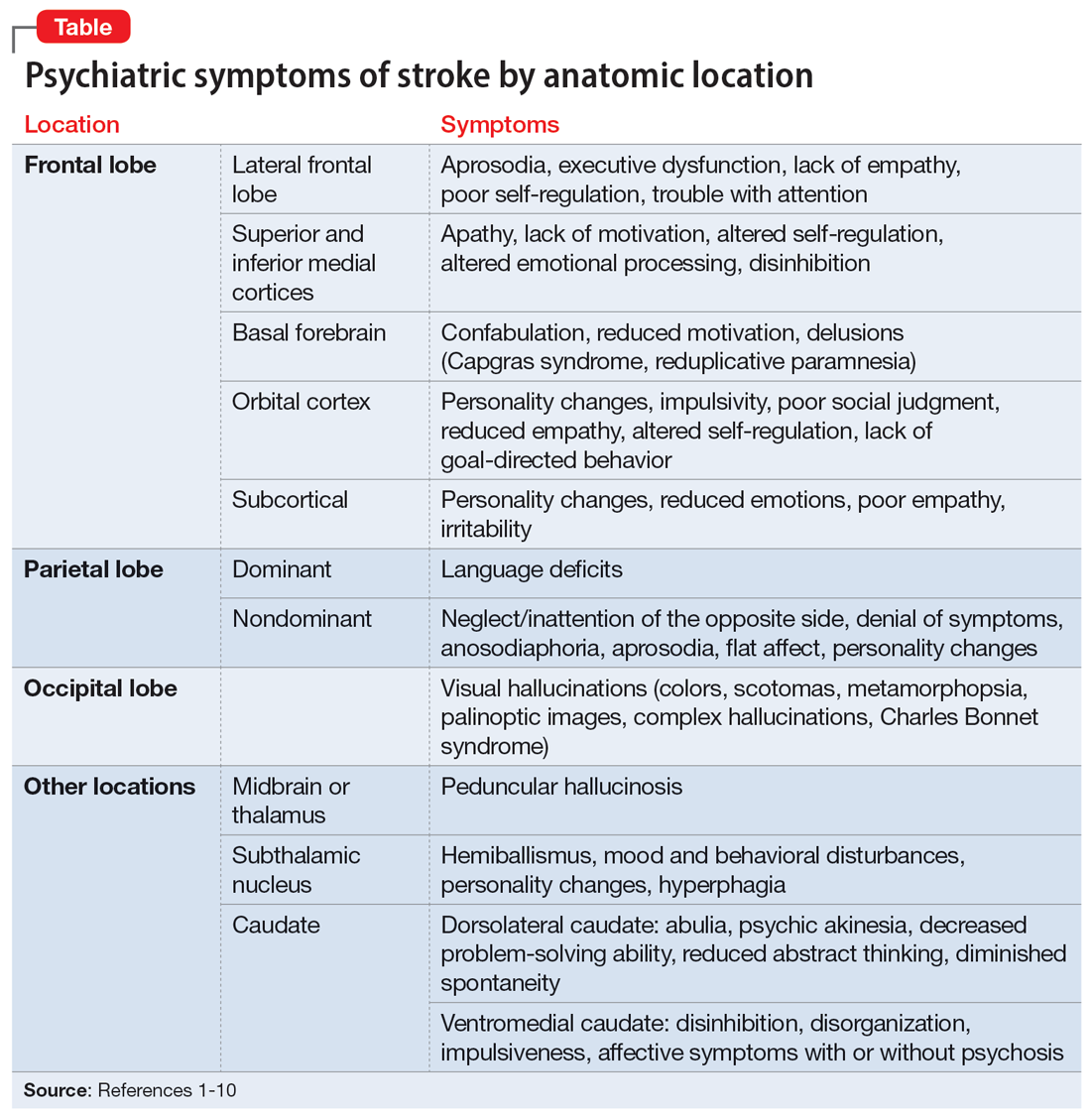
Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.

Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.

Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
Laboratory monitoring for patients on buprenorphine: 10 questions
The opioid use disorder (OUD) epidemic is a major public health crisis in the United States.1 Naltrexone, methadone, and buprenorphine are first-line therapies for OUD and have high success rates.2 While studies have shown that naltrexone is effective, patients must achieve opioid detoxification and maintain 7 to 10 days of total abstinence to avoid a precipitated opioid withdrawal before it can be prescribed.3 Methadone does not require detoxification or a period of complete abstinence, but must be prescribed in special clinics and requires daily observed dosing for the first 90 days,4 though these requirements have been relaxed during the COVID-19 pandemic. In contrast, buprenorphine (with or without naloxone) can be used in office-based settings, which significantly improves the accessibility and availability of treatment for patients with OUD. Clinician knowledge and comfort prescribing buprenorphine are limiting factors to treatment.5 Increasing the number of clinicians proficient with buprenorphine management can improve access to effective treatment and recovery services, which is critical for patients with OUD.
Multiple resources are available for clinicians to learn how to prescribe buprenorphine, but clear guidance on laboratory testing for patients receiving buprenorphine is limited. To safely and effectively prescribe buprenorphine, clinicians need to understand its pharmacology (Box 16-9) and how laboratory testing influences treatment. In an effort to increase clinician knowledge of and proficiency with buprenorphine, this article answers 10 common questions about laboratory monitoring of patients receiving this medication.
Box 1
For patients with opioid use disorder, buprenorphine is indicated for opioid detoxification and maintenance. Oral formulations of buprenorphine (including tablets and buccal films) have long durations of action, and when dosed daily can prevent opioid withdrawal for at least 48 hours.6 The recommended formulation is a combination of buprenorphine and naloxone, because this formulation is associated with a lower risk of misuse and diversion compared to formulations containing only buprenorphine.7 However, buprenorphine alone can be effective in patients who experience adverse effects from or are unable to tolerate the combination buprenorphine/naloxone formulation.7 Despite the addition of naloxone, buprenorphine prescriptions may still be misused and diverted, so close monitoring is necessary.
Buprenorphine is metabolized by the cytochrome P450 system (CYP) (primarily CYP3A4) to its active metabolite, norbuprenorphine, both of which are primarily excreted in feces.8 However, small quantities of buprenorphine and norbuprenorphine are excreted in the urine,9 which makes urine specimen the best choice to monitor buprenorphine use for therapeutic purposes.
1. Why is laboratory monitoring important?
Proper laboratory monitoring discourages illicit substance use, encourages medication adherence, and influences treatment modifications. Patient self-reporting on medication compliance may be inaccurate or unreliable.10 Patients who relapse or use other illicit substances may also be reluctant to disclose their substance use.11
On the other hand, laboratory tests are objective markers of treatment outcome and adherence, and can verify a patient’s self-report.12 When used appropriately, laboratory monitoring can be therapeutic. It holds patients accountable, especially when used in conjunction with contingency management or other behavioral therapies.13 Laboratory monitoring is the most reliable method of determining if patients are abstaining from opioids and other illicit substances, or if the treatment plan requires revision.
2. Which tests should I order?
When initiating or maintaining a patient on buprenorphine, order a general urine drug screen (UDS), urine opioid screen (availability varies by institution), urine creatinine levels, urine buprenorphine/norbuprenorphine/naloxone/creatinine levels, urine alcohol metabolite levels, and a urine general toxicology test. It is also recommended to obtain a comprehensive metabolic panel (CMP) before starting buprenorphine,14,15 and to monitor CMP values at least once annually following treatment. Patients with a history of IV drug use or other high-risk factors should also be screened for hepatitis B, hepatitis C, and HIV.14,15
A general UDS can determine if opiates, amphetamines, cocaine, marijuana, or other common illicit substances are present to identify additional substance use. The proficiency of a general UDS may vary depending on the panels used at the respective institution. Some clinics use point-of-care UDS as part of their clinical management; these tests are inexpensive and provide immediate results.16 A basic UDS typically does not detect synthetic opioids due to the specificity of conventional immunoassays. As a result, specific tests for opioids such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, and methadone should also be considered, depending on their availability. Though buprenorphine treatment may trigger a positive opiate or other opioid screen,17 buprenorphine adherence should be confirmed using several urine tests, including creatinine, buprenorphine, norbuprenorphine, and naloxone urine levels.
In addition to screening for illicit substances and buprenorphine adherence, it is important to also screen for alcohol. Alcohol use disorder (AUD) is highly comorbid with OUD,18 and is associated with worse OUD treatment outcomes.19 Alcohol use may also affect liver function necessary for buprenorphine metabolism,8 so urine alcohol metabolites such as ethyl glucuronide and ethyl sulfate, serum transaminases, and gamma-glutamyl transferase should also be obtained.
Continue to: How frequently should patients be tested?
3. How frequently should patients be tested?
As part of the initial assessment, it is recommended to order CMP, UDS, and urine general toxicology.14 If indicated, specific laboratory tests such as specific opioid and alcohol metabolites screens can be ordered. After starting buprenorphine, the frequency of monitoring urine laboratory tests—including UDS, general drug toxicology, buprenorphine/norbuprenorphine/naloxone/creatinine, and alcohol and its metabolites—depends on a variety of factors, including a patient’s treatment response and stability as well as availability and cost of the tests. Ultimately, the frequency of laboratory monitoring should be determined on a patient-by-patient basis and clinicians should use their judgment.
The American Society of Addiction Medicine suggests testing more frequently earlier in the course of treatment (eg, weekly or biweekly), then spacing it out over time (eg, monthly or quarterly) as the patient’s recovery progresses.14,15 To conserve resources and reduce spending, some clinicians and guidelines recommend random monitoring as opposed to monitoring at every follow-up visit (eg, once out of every 3 to 5 visits, on average), which allows for longer intervals between testing while ensuring consistency with medication and abstinence from illicit substances.15,16 We suggest screening every 2 weeks for the first month, then spacing out to monthly and quarterly as patients demonstrate stability, with random screening as indicated. Monitoring of liver function should be done at least once annually.
4. How should urine buprenorphine and other results be interpreted?
There are several issues to consider when interpreting laboratory results. The clinician needs to know what to expect in the sample, and what approximate levels should be detected. To check treatment adherence, laboratory data should include stable urine buprenorphine and norbuprenorphine levels and negative urine screening for other illicit substances.14,15 While urine buprenorphine and norbuprenorphine levels have great interindividual variability due to genetic differences in hepatic metabolism, unusually high levels of buprenorphine (≥700 ng/mL) without norbuprenorphine suggests “urine spiking,” where patients put buprenorphine directly into their urine sample.20,21 Abnormally low or undetectable levels raise concern for medication nonadherence or diversion.
Though urine buprenorphine levels do not reliably correlate with dose, because there is typically not much intraindividual variability, patients should have relatively stable levels on each screen once a maintenance dose has been established.22 Furthermore, the buprenorphine-to-norbuprenorphine ratio (ie, “the metabolic ratio”) typically ranges from 1:2 to 1:4 across all individuals,20,21,23 regardless of dose or metabolic rate. Urine naloxone levels, which typically are included in commercial urine buprenorphine laboratory panels, also may aid in identifying tampered urine specimens when buprenorphine-to-norbuprenorphine ratios are abnormal or inconsistent with an individual’s prior ratio. Naloxone is typically (but not always) poorly absorbed and minimally detected in urine specimens.20 A high level of naloxone coupled with unusually high buprenorphine levels, particularly in the absence of norbuprenorphine in the urine, may indicate urine spiking.20,21,23
Urine creatinine is used to establish the reliability of the specimen. When urine creatinine concentration is <20 mg/dL, the concentration of most substances typically falls to subthreshold levels of detection.24 If a UDS is negative and the urine has a creatinine concentration <20 mg/dL, the patient should provide a new sample, because the urine was likely too diluted to detect any substances.
Continue to: The presence of alcohol...
The presence of alcohol metabolites can alert the clinician to recent alcohol use and possible AUD, which should be assessed and treated if indicated.
Liver enzymes should be normal or unchanged with short- and long-term buprenorphine use when taken as prescribed.25,26 However, acute liver injury may occur if patients inject buprenorphine intravenously, especially in those with underlying hepatitis C.25
5. What can cause a false negative result on UDS?
Laboratory monitoring may occasionally yield false negative drug screens. For urine buprenorphine levels, false negatives may occur in patients who are “rapid metabolizers,” infrequent or as-needed usage of the medication, patient mix-up, or laboratory error.27 For other substances, a false negative result may occur if the patient used the substance(s) outside the window of detection. The most common causes of false negative results, however, are overly diluted urine samples (eg, due to rapid water ingestion), or the use of an inappropriate test to measure a specific opioid or substance.27
Many laboratories use conventional immunoassays with morphine antibodies that react with various opioid substrates to determine the presence of a specific opioid. Some opioids—particularly synthetics such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, buprenorphine, and methadone—have poor cross-reactivity with the morphine antibody due to their distinct chemical structures, so standard immunoassays used to detect opioids may result in a false negative result.28 In such situations, a discussion with a clinical pathologist familiar with the laboratory detection method can help ensure proper testing. Additional tests for specific opioids should be ordered to more specifically target substances prone to false negative results.27
6. What can cause a false positive result on UDS?
The cross-reactivity of the morphine substrate may also result in a false positive result.28 Other over-the-counter (OTC) or prescription medications that have cross-reactivity with the morphine antibody include dextromethorphan, verapamil, quinine, fluoroquinolones, and rifampin, which can normally be found in urine 2 to 3 days after consumption.17,27 Poppy seeds have long been known to result in positive opiate screens on urine testing, particularly when laboratories use lower cutoff values (eg, 300 ng/mL), so advise patients to avoid consuming poppy seeds.29
Continue to: For other drugs of abuse...
For other drugs of abuse, false positives are typically caused by cross-reactivity with other prescription or OTC medications. Numerous substances cross-react with amphetamines and produce false positive results on amphetamine immunoassays, including amantadine, bupropion, ephedrine, labetalol, phentermine, pseudoephedrine, ranitidine, selegiline, and trazodone.27 Sertraline and efavirenz are known to produce false positive results on benzodiazepine UDS, and ibuprofen, naproxen, and efavirenz can produce false positive results for cannabinoids.27
7. How do I communicate the results to patients?
Effectively communicating test results to patients is just as important as the results themselves. A trusting, therapeutic alliance between patient and clinician is highly predictive of successful treatment,30 and how the clinician communicates affects the strength of this collaboration. A principle of addiction treatment is the use of neutral language when discussing laboratory results.31,32 To avoid unintentional shaming or moral judgment, use words such as “positive” or “negative” rather than stigmatizing terms such as “clean” or “dirty.”33
Additionally, make it clear that laboratory findings are not used to punish patients, but rather to improve treatment.34 Reassuring the patient that a positive screen will not result in withdrawal of care encourages a working relationship.14 All patients who receive buprenorphine treatment should be informed that collecting a UDS is the standard of care used to monitor their progress. You might want to compare using UDS in patients with OUD to monitoring HbA1c levels in patients with diabetes as an example to demonstrate how laboratory values inform treatment.35,36
Before reporting the results, a helpful strategy to maintain the therapeutic alliance in the face of a positive UDS is to ask the patient what they expect their UDS to show. When the patient has been reassured that treatment will not be withdrawn due to a positive result, they may be more likely to fully disclose substance use. This allows them the opportunity to self-disclose rather than be “called out” by the clinician.35
8. What happens when a patient tests positive for drugs of abuse?
If a patient tests positive for opioids or other drugs of abuse, convey this information to them, ideally by asking them what they expect to see on laboratory findings. Patients may have “slip ups” or relapses, or use certain prescription medications for medical reasons with the intention of establishing abstinence. It is essential to convey laboratory findings in a nonjudgmental tone while maintaining a supportive stance with clear boundaries.
Continue to: Though addiction specialists...
Though addiction specialists often advise complete abstinence from all substances, including alcohol, cannabis, and tobacco, the harm-reduction model emphasizes “meeting patients where they are” in terms of continued substance use.37 If a patient can reduce their substance use or abstain from some substances while continuing others, these accomplishments should be acknowledged.
For patients who continue to test positive for illicit substances (>3 instances) without a clear explanation, schedule an appointment to re-educate them about buprenorphine treatment and reassess the patient’s treatment goals. Consider changing the current treatment plan, such as by having more frequent follow-ups, increasing the dose of the buprenorphine for patients whose cravings are not sufficiently suppressed, switching to another medication such as methadone or naltrexone, or referring the patient to a higher level of care, such as intensive outpatient or residential treatment.
9. What should I do if the results indicate abnormal levels of buprenorphine, norbuprenorphine, and naloxone?
When urine buprenorphine, norbuprenorphine, or naloxone levels appear low or the results indicate a likely “spiking,” clarify whether the sample tampering is due to poor adherence or diversion. Similar to dealing with a positive result for substances of abuse, ask the patient what they expect to find in their urine, and discuss the results in a nonjudgmental manner. Patients who admit to difficulty following their medication regimen may require additional psychoeducation and motivational interviewing to identify and address barriers. Strategies to improve adherence include setting an alarm, involving the family, using a pillbox, or simplifying the regimen.38 A long-acting injectable form of buprenorphine is also available.
If you suspect diversion, refer to your clinic’s policy and use other clinical management skills, such as increasing the frequency of visits, random pill counts, and supervised medication administration in the clinic.39 If diversion occurs repetitively and the patient is not appropriate for or benefiting from buprenorphine treatment, it may make sense to terminate treatment and consider other treatment options (such as methadone or residential treatment).39
10. What should I do if a patient disagrees with laboratory findings?
It is common for patients to disagree with laboratory results. Maintaining an attitude of neutrality and allowing the patient to speak and provide explanations is necessary to ensure they feel heard. Explanations patients frequently provide include passive exposure (“I was around someone who was using it”) or accidental ingestion, when a patient reports taking a medication they were not aware was a substance of concern. In a calm and nonjudgmental manner, provide education on what leads to a positive drug screen, including the possibility of false positive findings.
Continue to: Because a screening test...
Because a screening test has high sensitivity and low specificity, false positives may occur.17,27 Therefore, when a result is in dispute, the use of a high-specificity confirmatory test is often needed (many laboratories have reflex confirmatory testing). However, in the case of diluted urine (urine creatinine concentrations <20 mg/dL), patients should be told the findings are physiologically implausible, and a new urine sample should be obtained.24
Goals of laboratory monitoring
Laboratory monitoring, including UDS and urine buprenorphine levels, is a mainstay of treatment for patients with OUD. The increased use of telehealth has affected how laboratory testing is conducted (Box 240,41). The goal of laboratory testing is to influence treatment and improve patient outcomes. Clinical data such as clinician assessment, patient self-reporting, and collateral information provide essential details for patient management. However, laboratory monitoring is often the most reliable and objective source by which to influence treatment.
Box 2
While delivering therapy via telehealth has been shown to decrease the stigma that surrounds treatment, reduce no-show rates, increase retention in care, improve treatment access for patients who have difficulty commuting, and allow for continuity of outpatient treatment during the COVID-19 pandemic, there are also challenges.40,41 Inducing patients on buprenorphine via telehealth, as well as managing complex treatment cases or repeated failed urine drug screen tests, can be especially challenging. However, treatment standards should be followed as much as possible, and laboratory monitoring as clinically indicated should still be used to improve treatment outcomes.
If needed, patients may be directed to community labs for urine screening and should have results sent to their clinicians prior to the telehealth visit. Complex treatment cases (eg, repeat positive opioid screens, or negative urine buprenorphine screens with comorbid psychiatric conditions) should be handled on an individual basis and in-person appointments may be needed. Video assessment is always preferable to telephone. For patients who are unable to use video and have difficulty maintaining negative drug screens, an in-person visit should be requested.
An increased understanding of recommended laboratory monitoring practices may improve your comfort with OUD treatment and motivate more clinicians to offer buprenorphine, a life-saving and disease-modifying treatment for OUD. Doing so would increase access to OUD treatment for patients to reduce the individual and public health risks associated with untreated OUD.
Bottom Line
Laboratory monitoring, particularly urine drug screens and urine buprenorphine levels, is the most reliable source of information in the treatment of patients with opioid use disorder (OUD). An increased understanding of monitoring practices may improve a clinician’s willingness to offer buprenorphine as an option for therapy and their ability to properly treat patients with OUD.
Related Resources
- Li X, Moore S, Olson C. Urine drug tests: how to make the most of them. Current Psychiatry. 2019;18(8):10-18,20.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49. doi:10.12788/cp.0244
Drug Brand Names
Amantadine • Gocovri
Buprenorphine • Subutex, Sublocade
Bupropion • Wellbutrin, Zyban
Efavirenz • Sustiva
Fentanyl • Actiq
Hydrocodone • Hysingla
Hydromorphone • Dilaudid
Methadone • Methadose
Naloxone • Evzio
Naltrexone • Vivitrol
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Ionamin
Quinine • Qualaquin
Ranitidine • Zantac
Rifampin • Rifadin
Selegiline • Eldepryl
Sertraline • Zoloft
Trazodone • Oleptro
Verapamil • Verelan
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication PEP19-5068, NSDUH Series H-54. May 2019. https://www.samhsa.gov/data/
2. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. doi:10.1056/NEJMp1402780
3. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/S0140-6736(17)32812-X
4. Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi:10.1007/s11920-017-0783-9
5. DeFlavio JR, Rolin SA, Nordstrom BR, et al. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:3019. doi:10.22605/rrh3019
6. Kuhlman JJ Jr, Lalani S, Magluiolo J Jr, et al. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958. doi:10.1056/NEJMoa022164
8. Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. doi:10.1097/ALN.0b013e318238fea0
9. Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577-581.
10. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi:10.1007/s13142-015-0315-2
11. Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95 Suppl 3:S347-S360. doi:10.1080/09652140020004278
12. Preston KL, Silverman K, Schuster CR, et al. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130-145.
13. Knezevic NN, Khan OM, Beiranvand A, et al. Repeated quantitative urine toxicology analysis may improve chronic pain patient compliance with opioid therapy. Pain Physician. 2017;20(2S):S135-S145. doi:10.36076/ppj.2017.s145
14. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
15. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. doi:10.1097/ADM.0000000000000633
16. McDonell MG, Graves MC, West II, et al. Utility of point-of-care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196-201. doi:10.1097/ADM.0000000000000220
17. Algren DA, Christian MR. Buyer beware: pitfalls in toxicology laboratory testing. Mo Med. 2015;112(3):206-210.
18. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123. doi:10.1016/j.jsat.2010.05.008
19. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61. doi:10.1016/j.drugalcdep.2009.09.007
20. Warrington JS, Warrington GS, Francis-Fath S, et al. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med. 2020;14(6):e344-e349. doi:10.1097/ADM.0000000000000676
21. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51. doi:10.1016/j.drugalcdep.2017.07.040
22. Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358-369. doi:10.1016/j.clinthera.2015.12.016
23. Suzuki J, Zinser J, Issa M, et al. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abus. 2017;38(4):504-507. doi:10.1080/08897077.2017.1356796
24. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25( Pt 3):259-263. doi:10.1177/000456328802500312
25. Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi:10.1016/j.drugalcdep.2012.08.002
26. Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345-350. doi:10.1097/ADM.0000000000000057
27. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
28. Keary CJ, Wang Y, Moran JR, et al. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4).PCC.12f01371 doi:10.4088/PCC.12f01371
29. Zebelman AM, Troyer BL, Randall GL, et al. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
30. Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304-316. doi:10.1111/j.1360-0443.2004.00935.x
31. Kelly JF, Saitz R, Wakeman S. Language, substance use disorders, and policy: the need to reach consensus on an “addiction-ary.” Alcohol Treat Q. 2016;34(1):116-123. doi:10.1080/07347324.2016.1113103
32. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217-221. doi:10.1080/08897077.2014.930372
33. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128(1):8-9. doi:10.1016/j.amjmed.2014.07.043
34. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
35. Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi:10.7326/M18-1652
36. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 Suppl):S76-S82.
37. Klein A. Harm reduction works: evidence and inclusion in drug policy and advocacy. Health Care Anal. 2020;28(4):404-414. doi:10.1007/s10728-020-00406-w
38. Patel MX, David AS. Medication adherence: predictive factors and enhancement strategies. Psychiatry. 2007;6(9):357-361. doi:10.1016/j.mppsy.2007.06.003
39. Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. doi:10.1097/ADM.0000000000000045
40. Wang L, Weiss J, Ryan EB, et al. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat. 2021;124:108272. doi:10.1016/ j.jsat.2020.108272
41. Harris MTH, Lambert AM, Maschke AD, et al. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135:108655. doi:10.1016/j.jsat.2021.108655
The opioid use disorder (OUD) epidemic is a major public health crisis in the United States.1 Naltrexone, methadone, and buprenorphine are first-line therapies for OUD and have high success rates.2 While studies have shown that naltrexone is effective, patients must achieve opioid detoxification and maintain 7 to 10 days of total abstinence to avoid a precipitated opioid withdrawal before it can be prescribed.3 Methadone does not require detoxification or a period of complete abstinence, but must be prescribed in special clinics and requires daily observed dosing for the first 90 days,4 though these requirements have been relaxed during the COVID-19 pandemic. In contrast, buprenorphine (with or without naloxone) can be used in office-based settings, which significantly improves the accessibility and availability of treatment for patients with OUD. Clinician knowledge and comfort prescribing buprenorphine are limiting factors to treatment.5 Increasing the number of clinicians proficient with buprenorphine management can improve access to effective treatment and recovery services, which is critical for patients with OUD.
Multiple resources are available for clinicians to learn how to prescribe buprenorphine, but clear guidance on laboratory testing for patients receiving buprenorphine is limited. To safely and effectively prescribe buprenorphine, clinicians need to understand its pharmacology (Box 16-9) and how laboratory testing influences treatment. In an effort to increase clinician knowledge of and proficiency with buprenorphine, this article answers 10 common questions about laboratory monitoring of patients receiving this medication.
Box 1
For patients with opioid use disorder, buprenorphine is indicated for opioid detoxification and maintenance. Oral formulations of buprenorphine (including tablets and buccal films) have long durations of action, and when dosed daily can prevent opioid withdrawal for at least 48 hours.6 The recommended formulation is a combination of buprenorphine and naloxone, because this formulation is associated with a lower risk of misuse and diversion compared to formulations containing only buprenorphine.7 However, buprenorphine alone can be effective in patients who experience adverse effects from or are unable to tolerate the combination buprenorphine/naloxone formulation.7 Despite the addition of naloxone, buprenorphine prescriptions may still be misused and diverted, so close monitoring is necessary.
Buprenorphine is metabolized by the cytochrome P450 system (CYP) (primarily CYP3A4) to its active metabolite, norbuprenorphine, both of which are primarily excreted in feces.8 However, small quantities of buprenorphine and norbuprenorphine are excreted in the urine,9 which makes urine specimen the best choice to monitor buprenorphine use for therapeutic purposes.
1. Why is laboratory monitoring important?
Proper laboratory monitoring discourages illicit substance use, encourages medication adherence, and influences treatment modifications. Patient self-reporting on medication compliance may be inaccurate or unreliable.10 Patients who relapse or use other illicit substances may also be reluctant to disclose their substance use.11
On the other hand, laboratory tests are objective markers of treatment outcome and adherence, and can verify a patient’s self-report.12 When used appropriately, laboratory monitoring can be therapeutic. It holds patients accountable, especially when used in conjunction with contingency management or other behavioral therapies.13 Laboratory monitoring is the most reliable method of determining if patients are abstaining from opioids and other illicit substances, or if the treatment plan requires revision.
2. Which tests should I order?
When initiating or maintaining a patient on buprenorphine, order a general urine drug screen (UDS), urine opioid screen (availability varies by institution), urine creatinine levels, urine buprenorphine/norbuprenorphine/naloxone/creatinine levels, urine alcohol metabolite levels, and a urine general toxicology test. It is also recommended to obtain a comprehensive metabolic panel (CMP) before starting buprenorphine,14,15 and to monitor CMP values at least once annually following treatment. Patients with a history of IV drug use or other high-risk factors should also be screened for hepatitis B, hepatitis C, and HIV.14,15
A general UDS can determine if opiates, amphetamines, cocaine, marijuana, or other common illicit substances are present to identify additional substance use. The proficiency of a general UDS may vary depending on the panels used at the respective institution. Some clinics use point-of-care UDS as part of their clinical management; these tests are inexpensive and provide immediate results.16 A basic UDS typically does not detect synthetic opioids due to the specificity of conventional immunoassays. As a result, specific tests for opioids such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, and methadone should also be considered, depending on their availability. Though buprenorphine treatment may trigger a positive opiate or other opioid screen,17 buprenorphine adherence should be confirmed using several urine tests, including creatinine, buprenorphine, norbuprenorphine, and naloxone urine levels.
In addition to screening for illicit substances and buprenorphine adherence, it is important to also screen for alcohol. Alcohol use disorder (AUD) is highly comorbid with OUD,18 and is associated with worse OUD treatment outcomes.19 Alcohol use may also affect liver function necessary for buprenorphine metabolism,8 so urine alcohol metabolites such as ethyl glucuronide and ethyl sulfate, serum transaminases, and gamma-glutamyl transferase should also be obtained.
Continue to: How frequently should patients be tested?
3. How frequently should patients be tested?
As part of the initial assessment, it is recommended to order CMP, UDS, and urine general toxicology.14 If indicated, specific laboratory tests such as specific opioid and alcohol metabolites screens can be ordered. After starting buprenorphine, the frequency of monitoring urine laboratory tests—including UDS, general drug toxicology, buprenorphine/norbuprenorphine/naloxone/creatinine, and alcohol and its metabolites—depends on a variety of factors, including a patient’s treatment response and stability as well as availability and cost of the tests. Ultimately, the frequency of laboratory monitoring should be determined on a patient-by-patient basis and clinicians should use their judgment.
The American Society of Addiction Medicine suggests testing more frequently earlier in the course of treatment (eg, weekly or biweekly), then spacing it out over time (eg, monthly or quarterly) as the patient’s recovery progresses.14,15 To conserve resources and reduce spending, some clinicians and guidelines recommend random monitoring as opposed to monitoring at every follow-up visit (eg, once out of every 3 to 5 visits, on average), which allows for longer intervals between testing while ensuring consistency with medication and abstinence from illicit substances.15,16 We suggest screening every 2 weeks for the first month, then spacing out to monthly and quarterly as patients demonstrate stability, with random screening as indicated. Monitoring of liver function should be done at least once annually.
4. How should urine buprenorphine and other results be interpreted?
There are several issues to consider when interpreting laboratory results. The clinician needs to know what to expect in the sample, and what approximate levels should be detected. To check treatment adherence, laboratory data should include stable urine buprenorphine and norbuprenorphine levels and negative urine screening for other illicit substances.14,15 While urine buprenorphine and norbuprenorphine levels have great interindividual variability due to genetic differences in hepatic metabolism, unusually high levels of buprenorphine (≥700 ng/mL) without norbuprenorphine suggests “urine spiking,” where patients put buprenorphine directly into their urine sample.20,21 Abnormally low or undetectable levels raise concern for medication nonadherence or diversion.
Though urine buprenorphine levels do not reliably correlate with dose, because there is typically not much intraindividual variability, patients should have relatively stable levels on each screen once a maintenance dose has been established.22 Furthermore, the buprenorphine-to-norbuprenorphine ratio (ie, “the metabolic ratio”) typically ranges from 1:2 to 1:4 across all individuals,20,21,23 regardless of dose or metabolic rate. Urine naloxone levels, which typically are included in commercial urine buprenorphine laboratory panels, also may aid in identifying tampered urine specimens when buprenorphine-to-norbuprenorphine ratios are abnormal or inconsistent with an individual’s prior ratio. Naloxone is typically (but not always) poorly absorbed and minimally detected in urine specimens.20 A high level of naloxone coupled with unusually high buprenorphine levels, particularly in the absence of norbuprenorphine in the urine, may indicate urine spiking.20,21,23
Urine creatinine is used to establish the reliability of the specimen. When urine creatinine concentration is <20 mg/dL, the concentration of most substances typically falls to subthreshold levels of detection.24 If a UDS is negative and the urine has a creatinine concentration <20 mg/dL, the patient should provide a new sample, because the urine was likely too diluted to detect any substances.
Continue to: The presence of alcohol...
The presence of alcohol metabolites can alert the clinician to recent alcohol use and possible AUD, which should be assessed and treated if indicated.
Liver enzymes should be normal or unchanged with short- and long-term buprenorphine use when taken as prescribed.25,26 However, acute liver injury may occur if patients inject buprenorphine intravenously, especially in those with underlying hepatitis C.25
5. What can cause a false negative result on UDS?
Laboratory monitoring may occasionally yield false negative drug screens. For urine buprenorphine levels, false negatives may occur in patients who are “rapid metabolizers,” infrequent or as-needed usage of the medication, patient mix-up, or laboratory error.27 For other substances, a false negative result may occur if the patient used the substance(s) outside the window of detection. The most common causes of false negative results, however, are overly diluted urine samples (eg, due to rapid water ingestion), or the use of an inappropriate test to measure a specific opioid or substance.27
Many laboratories use conventional immunoassays with morphine antibodies that react with various opioid substrates to determine the presence of a specific opioid. Some opioids—particularly synthetics such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, buprenorphine, and methadone—have poor cross-reactivity with the morphine antibody due to their distinct chemical structures, so standard immunoassays used to detect opioids may result in a false negative result.28 In such situations, a discussion with a clinical pathologist familiar with the laboratory detection method can help ensure proper testing. Additional tests for specific opioids should be ordered to more specifically target substances prone to false negative results.27
6. What can cause a false positive result on UDS?
The cross-reactivity of the morphine substrate may also result in a false positive result.28 Other over-the-counter (OTC) or prescription medications that have cross-reactivity with the morphine antibody include dextromethorphan, verapamil, quinine, fluoroquinolones, and rifampin, which can normally be found in urine 2 to 3 days after consumption.17,27 Poppy seeds have long been known to result in positive opiate screens on urine testing, particularly when laboratories use lower cutoff values (eg, 300 ng/mL), so advise patients to avoid consuming poppy seeds.29
Continue to: For other drugs of abuse...
For other drugs of abuse, false positives are typically caused by cross-reactivity with other prescription or OTC medications. Numerous substances cross-react with amphetamines and produce false positive results on amphetamine immunoassays, including amantadine, bupropion, ephedrine, labetalol, phentermine, pseudoephedrine, ranitidine, selegiline, and trazodone.27 Sertraline and efavirenz are known to produce false positive results on benzodiazepine UDS, and ibuprofen, naproxen, and efavirenz can produce false positive results for cannabinoids.27
7. How do I communicate the results to patients?
Effectively communicating test results to patients is just as important as the results themselves. A trusting, therapeutic alliance between patient and clinician is highly predictive of successful treatment,30 and how the clinician communicates affects the strength of this collaboration. A principle of addiction treatment is the use of neutral language when discussing laboratory results.31,32 To avoid unintentional shaming or moral judgment, use words such as “positive” or “negative” rather than stigmatizing terms such as “clean” or “dirty.”33
Additionally, make it clear that laboratory findings are not used to punish patients, but rather to improve treatment.34 Reassuring the patient that a positive screen will not result in withdrawal of care encourages a working relationship.14 All patients who receive buprenorphine treatment should be informed that collecting a UDS is the standard of care used to monitor their progress. You might want to compare using UDS in patients with OUD to monitoring HbA1c levels in patients with diabetes as an example to demonstrate how laboratory values inform treatment.35,36
Before reporting the results, a helpful strategy to maintain the therapeutic alliance in the face of a positive UDS is to ask the patient what they expect their UDS to show. When the patient has been reassured that treatment will not be withdrawn due to a positive result, they may be more likely to fully disclose substance use. This allows them the opportunity to self-disclose rather than be “called out” by the clinician.35
8. What happens when a patient tests positive for drugs of abuse?
If a patient tests positive for opioids or other drugs of abuse, convey this information to them, ideally by asking them what they expect to see on laboratory findings. Patients may have “slip ups” or relapses, or use certain prescription medications for medical reasons with the intention of establishing abstinence. It is essential to convey laboratory findings in a nonjudgmental tone while maintaining a supportive stance with clear boundaries.
Continue to: Though addiction specialists...
Though addiction specialists often advise complete abstinence from all substances, including alcohol, cannabis, and tobacco, the harm-reduction model emphasizes “meeting patients where they are” in terms of continued substance use.37 If a patient can reduce their substance use or abstain from some substances while continuing others, these accomplishments should be acknowledged.
For patients who continue to test positive for illicit substances (>3 instances) without a clear explanation, schedule an appointment to re-educate them about buprenorphine treatment and reassess the patient’s treatment goals. Consider changing the current treatment plan, such as by having more frequent follow-ups, increasing the dose of the buprenorphine for patients whose cravings are not sufficiently suppressed, switching to another medication such as methadone or naltrexone, or referring the patient to a higher level of care, such as intensive outpatient or residential treatment.
9. What should I do if the results indicate abnormal levels of buprenorphine, norbuprenorphine, and naloxone?
When urine buprenorphine, norbuprenorphine, or naloxone levels appear low or the results indicate a likely “spiking,” clarify whether the sample tampering is due to poor adherence or diversion. Similar to dealing with a positive result for substances of abuse, ask the patient what they expect to find in their urine, and discuss the results in a nonjudgmental manner. Patients who admit to difficulty following their medication regimen may require additional psychoeducation and motivational interviewing to identify and address barriers. Strategies to improve adherence include setting an alarm, involving the family, using a pillbox, or simplifying the regimen.38 A long-acting injectable form of buprenorphine is also available.
If you suspect diversion, refer to your clinic’s policy and use other clinical management skills, such as increasing the frequency of visits, random pill counts, and supervised medication administration in the clinic.39 If diversion occurs repetitively and the patient is not appropriate for or benefiting from buprenorphine treatment, it may make sense to terminate treatment and consider other treatment options (such as methadone or residential treatment).39
10. What should I do if a patient disagrees with laboratory findings?
It is common for patients to disagree with laboratory results. Maintaining an attitude of neutrality and allowing the patient to speak and provide explanations is necessary to ensure they feel heard. Explanations patients frequently provide include passive exposure (“I was around someone who was using it”) or accidental ingestion, when a patient reports taking a medication they were not aware was a substance of concern. In a calm and nonjudgmental manner, provide education on what leads to a positive drug screen, including the possibility of false positive findings.
Continue to: Because a screening test...
Because a screening test has high sensitivity and low specificity, false positives may occur.17,27 Therefore, when a result is in dispute, the use of a high-specificity confirmatory test is often needed (many laboratories have reflex confirmatory testing). However, in the case of diluted urine (urine creatinine concentrations <20 mg/dL), patients should be told the findings are physiologically implausible, and a new urine sample should be obtained.24
Goals of laboratory monitoring
Laboratory monitoring, including UDS and urine buprenorphine levels, is a mainstay of treatment for patients with OUD. The increased use of telehealth has affected how laboratory testing is conducted (Box 240,41). The goal of laboratory testing is to influence treatment and improve patient outcomes. Clinical data such as clinician assessment, patient self-reporting, and collateral information provide essential details for patient management. However, laboratory monitoring is often the most reliable and objective source by which to influence treatment.
Box 2
While delivering therapy via telehealth has been shown to decrease the stigma that surrounds treatment, reduce no-show rates, increase retention in care, improve treatment access for patients who have difficulty commuting, and allow for continuity of outpatient treatment during the COVID-19 pandemic, there are also challenges.40,41 Inducing patients on buprenorphine via telehealth, as well as managing complex treatment cases or repeated failed urine drug screen tests, can be especially challenging. However, treatment standards should be followed as much as possible, and laboratory monitoring as clinically indicated should still be used to improve treatment outcomes.
If needed, patients may be directed to community labs for urine screening and should have results sent to their clinicians prior to the telehealth visit. Complex treatment cases (eg, repeat positive opioid screens, or negative urine buprenorphine screens with comorbid psychiatric conditions) should be handled on an individual basis and in-person appointments may be needed. Video assessment is always preferable to telephone. For patients who are unable to use video and have difficulty maintaining negative drug screens, an in-person visit should be requested.
An increased understanding of recommended laboratory monitoring practices may improve your comfort with OUD treatment and motivate more clinicians to offer buprenorphine, a life-saving and disease-modifying treatment for OUD. Doing so would increase access to OUD treatment for patients to reduce the individual and public health risks associated with untreated OUD.
Bottom Line
Laboratory monitoring, particularly urine drug screens and urine buprenorphine levels, is the most reliable source of information in the treatment of patients with opioid use disorder (OUD). An increased understanding of monitoring practices may improve a clinician’s willingness to offer buprenorphine as an option for therapy and their ability to properly treat patients with OUD.
Related Resources
- Li X, Moore S, Olson C. Urine drug tests: how to make the most of them. Current Psychiatry. 2019;18(8):10-18,20.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49. doi:10.12788/cp.0244
Drug Brand Names
Amantadine • Gocovri
Buprenorphine • Subutex, Sublocade
Bupropion • Wellbutrin, Zyban
Efavirenz • Sustiva
Fentanyl • Actiq
Hydrocodone • Hysingla
Hydromorphone • Dilaudid
Methadone • Methadose
Naloxone • Evzio
Naltrexone • Vivitrol
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Ionamin
Quinine • Qualaquin
Ranitidine • Zantac
Rifampin • Rifadin
Selegiline • Eldepryl
Sertraline • Zoloft
Trazodone • Oleptro
Verapamil • Verelan
The opioid use disorder (OUD) epidemic is a major public health crisis in the United States.1 Naltrexone, methadone, and buprenorphine are first-line therapies for OUD and have high success rates.2 While studies have shown that naltrexone is effective, patients must achieve opioid detoxification and maintain 7 to 10 days of total abstinence to avoid a precipitated opioid withdrawal before it can be prescribed.3 Methadone does not require detoxification or a period of complete abstinence, but must be prescribed in special clinics and requires daily observed dosing for the first 90 days,4 though these requirements have been relaxed during the COVID-19 pandemic. In contrast, buprenorphine (with or without naloxone) can be used in office-based settings, which significantly improves the accessibility and availability of treatment for patients with OUD. Clinician knowledge and comfort prescribing buprenorphine are limiting factors to treatment.5 Increasing the number of clinicians proficient with buprenorphine management can improve access to effective treatment and recovery services, which is critical for patients with OUD.
Multiple resources are available for clinicians to learn how to prescribe buprenorphine, but clear guidance on laboratory testing for patients receiving buprenorphine is limited. To safely and effectively prescribe buprenorphine, clinicians need to understand its pharmacology (Box 16-9) and how laboratory testing influences treatment. In an effort to increase clinician knowledge of and proficiency with buprenorphine, this article answers 10 common questions about laboratory monitoring of patients receiving this medication.
Box 1
For patients with opioid use disorder, buprenorphine is indicated for opioid detoxification and maintenance. Oral formulations of buprenorphine (including tablets and buccal films) have long durations of action, and when dosed daily can prevent opioid withdrawal for at least 48 hours.6 The recommended formulation is a combination of buprenorphine and naloxone, because this formulation is associated with a lower risk of misuse and diversion compared to formulations containing only buprenorphine.7 However, buprenorphine alone can be effective in patients who experience adverse effects from or are unable to tolerate the combination buprenorphine/naloxone formulation.7 Despite the addition of naloxone, buprenorphine prescriptions may still be misused and diverted, so close monitoring is necessary.
Buprenorphine is metabolized by the cytochrome P450 system (CYP) (primarily CYP3A4) to its active metabolite, norbuprenorphine, both of which are primarily excreted in feces.8 However, small quantities of buprenorphine and norbuprenorphine are excreted in the urine,9 which makes urine specimen the best choice to monitor buprenorphine use for therapeutic purposes.
1. Why is laboratory monitoring important?
Proper laboratory monitoring discourages illicit substance use, encourages medication adherence, and influences treatment modifications. Patient self-reporting on medication compliance may be inaccurate or unreliable.10 Patients who relapse or use other illicit substances may also be reluctant to disclose their substance use.11
On the other hand, laboratory tests are objective markers of treatment outcome and adherence, and can verify a patient’s self-report.12 When used appropriately, laboratory monitoring can be therapeutic. It holds patients accountable, especially when used in conjunction with contingency management or other behavioral therapies.13 Laboratory monitoring is the most reliable method of determining if patients are abstaining from opioids and other illicit substances, or if the treatment plan requires revision.
2. Which tests should I order?
When initiating or maintaining a patient on buprenorphine, order a general urine drug screen (UDS), urine opioid screen (availability varies by institution), urine creatinine levels, urine buprenorphine/norbuprenorphine/naloxone/creatinine levels, urine alcohol metabolite levels, and a urine general toxicology test. It is also recommended to obtain a comprehensive metabolic panel (CMP) before starting buprenorphine,14,15 and to monitor CMP values at least once annually following treatment. Patients with a history of IV drug use or other high-risk factors should also be screened for hepatitis B, hepatitis C, and HIV.14,15
A general UDS can determine if opiates, amphetamines, cocaine, marijuana, or other common illicit substances are present to identify additional substance use. The proficiency of a general UDS may vary depending on the panels used at the respective institution. Some clinics use point-of-care UDS as part of their clinical management; these tests are inexpensive and provide immediate results.16 A basic UDS typically does not detect synthetic opioids due to the specificity of conventional immunoassays. As a result, specific tests for opioids such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, and methadone should also be considered, depending on their availability. Though buprenorphine treatment may trigger a positive opiate or other opioid screen,17 buprenorphine adherence should be confirmed using several urine tests, including creatinine, buprenorphine, norbuprenorphine, and naloxone urine levels.
In addition to screening for illicit substances and buprenorphine adherence, it is important to also screen for alcohol. Alcohol use disorder (AUD) is highly comorbid with OUD,18 and is associated with worse OUD treatment outcomes.19 Alcohol use may also affect liver function necessary for buprenorphine metabolism,8 so urine alcohol metabolites such as ethyl glucuronide and ethyl sulfate, serum transaminases, and gamma-glutamyl transferase should also be obtained.
Continue to: How frequently should patients be tested?
3. How frequently should patients be tested?
As part of the initial assessment, it is recommended to order CMP, UDS, and urine general toxicology.14 If indicated, specific laboratory tests such as specific opioid and alcohol metabolites screens can be ordered. After starting buprenorphine, the frequency of monitoring urine laboratory tests—including UDS, general drug toxicology, buprenorphine/norbuprenorphine/naloxone/creatinine, and alcohol and its metabolites—depends on a variety of factors, including a patient’s treatment response and stability as well as availability and cost of the tests. Ultimately, the frequency of laboratory monitoring should be determined on a patient-by-patient basis and clinicians should use their judgment.
The American Society of Addiction Medicine suggests testing more frequently earlier in the course of treatment (eg, weekly or biweekly), then spacing it out over time (eg, monthly or quarterly) as the patient’s recovery progresses.14,15 To conserve resources and reduce spending, some clinicians and guidelines recommend random monitoring as opposed to monitoring at every follow-up visit (eg, once out of every 3 to 5 visits, on average), which allows for longer intervals between testing while ensuring consistency with medication and abstinence from illicit substances.15,16 We suggest screening every 2 weeks for the first month, then spacing out to monthly and quarterly as patients demonstrate stability, with random screening as indicated. Monitoring of liver function should be done at least once annually.
4. How should urine buprenorphine and other results be interpreted?
There are several issues to consider when interpreting laboratory results. The clinician needs to know what to expect in the sample, and what approximate levels should be detected. To check treatment adherence, laboratory data should include stable urine buprenorphine and norbuprenorphine levels and negative urine screening for other illicit substances.14,15 While urine buprenorphine and norbuprenorphine levels have great interindividual variability due to genetic differences in hepatic metabolism, unusually high levels of buprenorphine (≥700 ng/mL) without norbuprenorphine suggests “urine spiking,” where patients put buprenorphine directly into their urine sample.20,21 Abnormally low or undetectable levels raise concern for medication nonadherence or diversion.
Though urine buprenorphine levels do not reliably correlate with dose, because there is typically not much intraindividual variability, patients should have relatively stable levels on each screen once a maintenance dose has been established.22 Furthermore, the buprenorphine-to-norbuprenorphine ratio (ie, “the metabolic ratio”) typically ranges from 1:2 to 1:4 across all individuals,20,21,23 regardless of dose or metabolic rate. Urine naloxone levels, which typically are included in commercial urine buprenorphine laboratory panels, also may aid in identifying tampered urine specimens when buprenorphine-to-norbuprenorphine ratios are abnormal or inconsistent with an individual’s prior ratio. Naloxone is typically (but not always) poorly absorbed and minimally detected in urine specimens.20 A high level of naloxone coupled with unusually high buprenorphine levels, particularly in the absence of norbuprenorphine in the urine, may indicate urine spiking.20,21,23
Urine creatinine is used to establish the reliability of the specimen. When urine creatinine concentration is <20 mg/dL, the concentration of most substances typically falls to subthreshold levels of detection.24 If a UDS is negative and the urine has a creatinine concentration <20 mg/dL, the patient should provide a new sample, because the urine was likely too diluted to detect any substances.
Continue to: The presence of alcohol...
The presence of alcohol metabolites can alert the clinician to recent alcohol use and possible AUD, which should be assessed and treated if indicated.
Liver enzymes should be normal or unchanged with short- and long-term buprenorphine use when taken as prescribed.25,26 However, acute liver injury may occur if patients inject buprenorphine intravenously, especially in those with underlying hepatitis C.25
5. What can cause a false negative result on UDS?
Laboratory monitoring may occasionally yield false negative drug screens. For urine buprenorphine levels, false negatives may occur in patients who are “rapid metabolizers,” infrequent or as-needed usage of the medication, patient mix-up, or laboratory error.27 For other substances, a false negative result may occur if the patient used the substance(s) outside the window of detection. The most common causes of false negative results, however, are overly diluted urine samples (eg, due to rapid water ingestion), or the use of an inappropriate test to measure a specific opioid or substance.27
Many laboratories use conventional immunoassays with morphine antibodies that react with various opioid substrates to determine the presence of a specific opioid. Some opioids—particularly synthetics such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, buprenorphine, and methadone—have poor cross-reactivity with the morphine antibody due to their distinct chemical structures, so standard immunoassays used to detect opioids may result in a false negative result.28 In such situations, a discussion with a clinical pathologist familiar with the laboratory detection method can help ensure proper testing. Additional tests for specific opioids should be ordered to more specifically target substances prone to false negative results.27
6. What can cause a false positive result on UDS?
The cross-reactivity of the morphine substrate may also result in a false positive result.28 Other over-the-counter (OTC) or prescription medications that have cross-reactivity with the morphine antibody include dextromethorphan, verapamil, quinine, fluoroquinolones, and rifampin, which can normally be found in urine 2 to 3 days after consumption.17,27 Poppy seeds have long been known to result in positive opiate screens on urine testing, particularly when laboratories use lower cutoff values (eg, 300 ng/mL), so advise patients to avoid consuming poppy seeds.29
Continue to: For other drugs of abuse...
For other drugs of abuse, false positives are typically caused by cross-reactivity with other prescription or OTC medications. Numerous substances cross-react with amphetamines and produce false positive results on amphetamine immunoassays, including amantadine, bupropion, ephedrine, labetalol, phentermine, pseudoephedrine, ranitidine, selegiline, and trazodone.27 Sertraline and efavirenz are known to produce false positive results on benzodiazepine UDS, and ibuprofen, naproxen, and efavirenz can produce false positive results for cannabinoids.27
7. How do I communicate the results to patients?
Effectively communicating test results to patients is just as important as the results themselves. A trusting, therapeutic alliance between patient and clinician is highly predictive of successful treatment,30 and how the clinician communicates affects the strength of this collaboration. A principle of addiction treatment is the use of neutral language when discussing laboratory results.31,32 To avoid unintentional shaming or moral judgment, use words such as “positive” or “negative” rather than stigmatizing terms such as “clean” or “dirty.”33
Additionally, make it clear that laboratory findings are not used to punish patients, but rather to improve treatment.34 Reassuring the patient that a positive screen will not result in withdrawal of care encourages a working relationship.14 All patients who receive buprenorphine treatment should be informed that collecting a UDS is the standard of care used to monitor their progress. You might want to compare using UDS in patients with OUD to monitoring HbA1c levels in patients with diabetes as an example to demonstrate how laboratory values inform treatment.35,36
Before reporting the results, a helpful strategy to maintain the therapeutic alliance in the face of a positive UDS is to ask the patient what they expect their UDS to show. When the patient has been reassured that treatment will not be withdrawn due to a positive result, they may be more likely to fully disclose substance use. This allows them the opportunity to self-disclose rather than be “called out” by the clinician.35
8. What happens when a patient tests positive for drugs of abuse?
If a patient tests positive for opioids or other drugs of abuse, convey this information to them, ideally by asking them what they expect to see on laboratory findings. Patients may have “slip ups” or relapses, or use certain prescription medications for medical reasons with the intention of establishing abstinence. It is essential to convey laboratory findings in a nonjudgmental tone while maintaining a supportive stance with clear boundaries.
Continue to: Though addiction specialists...
Though addiction specialists often advise complete abstinence from all substances, including alcohol, cannabis, and tobacco, the harm-reduction model emphasizes “meeting patients where they are” in terms of continued substance use.37 If a patient can reduce their substance use or abstain from some substances while continuing others, these accomplishments should be acknowledged.
For patients who continue to test positive for illicit substances (>3 instances) without a clear explanation, schedule an appointment to re-educate them about buprenorphine treatment and reassess the patient’s treatment goals. Consider changing the current treatment plan, such as by having more frequent follow-ups, increasing the dose of the buprenorphine for patients whose cravings are not sufficiently suppressed, switching to another medication such as methadone or naltrexone, or referring the patient to a higher level of care, such as intensive outpatient or residential treatment.
9. What should I do if the results indicate abnormal levels of buprenorphine, norbuprenorphine, and naloxone?
When urine buprenorphine, norbuprenorphine, or naloxone levels appear low or the results indicate a likely “spiking,” clarify whether the sample tampering is due to poor adherence or diversion. Similar to dealing with a positive result for substances of abuse, ask the patient what they expect to find in their urine, and discuss the results in a nonjudgmental manner. Patients who admit to difficulty following their medication regimen may require additional psychoeducation and motivational interviewing to identify and address barriers. Strategies to improve adherence include setting an alarm, involving the family, using a pillbox, or simplifying the regimen.38 A long-acting injectable form of buprenorphine is also available.
If you suspect diversion, refer to your clinic’s policy and use other clinical management skills, such as increasing the frequency of visits, random pill counts, and supervised medication administration in the clinic.39 If diversion occurs repetitively and the patient is not appropriate for or benefiting from buprenorphine treatment, it may make sense to terminate treatment and consider other treatment options (such as methadone or residential treatment).39
10. What should I do if a patient disagrees with laboratory findings?
It is common for patients to disagree with laboratory results. Maintaining an attitude of neutrality and allowing the patient to speak and provide explanations is necessary to ensure they feel heard. Explanations patients frequently provide include passive exposure (“I was around someone who was using it”) or accidental ingestion, when a patient reports taking a medication they were not aware was a substance of concern. In a calm and nonjudgmental manner, provide education on what leads to a positive drug screen, including the possibility of false positive findings.
Continue to: Because a screening test...
Because a screening test has high sensitivity and low specificity, false positives may occur.17,27 Therefore, when a result is in dispute, the use of a high-specificity confirmatory test is often needed (many laboratories have reflex confirmatory testing). However, in the case of diluted urine (urine creatinine concentrations <20 mg/dL), patients should be told the findings are physiologically implausible, and a new urine sample should be obtained.24
Goals of laboratory monitoring
Laboratory monitoring, including UDS and urine buprenorphine levels, is a mainstay of treatment for patients with OUD. The increased use of telehealth has affected how laboratory testing is conducted (Box 240,41). The goal of laboratory testing is to influence treatment and improve patient outcomes. Clinical data such as clinician assessment, patient self-reporting, and collateral information provide essential details for patient management. However, laboratory monitoring is often the most reliable and objective source by which to influence treatment.
Box 2
While delivering therapy via telehealth has been shown to decrease the stigma that surrounds treatment, reduce no-show rates, increase retention in care, improve treatment access for patients who have difficulty commuting, and allow for continuity of outpatient treatment during the COVID-19 pandemic, there are also challenges.40,41 Inducing patients on buprenorphine via telehealth, as well as managing complex treatment cases or repeated failed urine drug screen tests, can be especially challenging. However, treatment standards should be followed as much as possible, and laboratory monitoring as clinically indicated should still be used to improve treatment outcomes.
If needed, patients may be directed to community labs for urine screening and should have results sent to their clinicians prior to the telehealth visit. Complex treatment cases (eg, repeat positive opioid screens, or negative urine buprenorphine screens with comorbid psychiatric conditions) should be handled on an individual basis and in-person appointments may be needed. Video assessment is always preferable to telephone. For patients who are unable to use video and have difficulty maintaining negative drug screens, an in-person visit should be requested.
An increased understanding of recommended laboratory monitoring practices may improve your comfort with OUD treatment and motivate more clinicians to offer buprenorphine, a life-saving and disease-modifying treatment for OUD. Doing so would increase access to OUD treatment for patients to reduce the individual and public health risks associated with untreated OUD.
Bottom Line
Laboratory monitoring, particularly urine drug screens and urine buprenorphine levels, is the most reliable source of information in the treatment of patients with opioid use disorder (OUD). An increased understanding of monitoring practices may improve a clinician’s willingness to offer buprenorphine as an option for therapy and their ability to properly treat patients with OUD.
Related Resources
- Li X, Moore S, Olson C. Urine drug tests: how to make the most of them. Current Psychiatry. 2019;18(8):10-18,20.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49. doi:10.12788/cp.0244
Drug Brand Names
Amantadine • Gocovri
Buprenorphine • Subutex, Sublocade
Bupropion • Wellbutrin, Zyban
Efavirenz • Sustiva
Fentanyl • Actiq
Hydrocodone • Hysingla
Hydromorphone • Dilaudid
Methadone • Methadose
Naloxone • Evzio
Naltrexone • Vivitrol
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Ionamin
Quinine • Qualaquin
Ranitidine • Zantac
Rifampin • Rifadin
Selegiline • Eldepryl
Sertraline • Zoloft
Trazodone • Oleptro
Verapamil • Verelan
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication PEP19-5068, NSDUH Series H-54. May 2019. https://www.samhsa.gov/data/
2. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. doi:10.1056/NEJMp1402780
3. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/S0140-6736(17)32812-X
4. Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi:10.1007/s11920-017-0783-9
5. DeFlavio JR, Rolin SA, Nordstrom BR, et al. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:3019. doi:10.22605/rrh3019
6. Kuhlman JJ Jr, Lalani S, Magluiolo J Jr, et al. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958. doi:10.1056/NEJMoa022164
8. Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. doi:10.1097/ALN.0b013e318238fea0
9. Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577-581.
10. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi:10.1007/s13142-015-0315-2
11. Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95 Suppl 3:S347-S360. doi:10.1080/09652140020004278
12. Preston KL, Silverman K, Schuster CR, et al. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130-145.
13. Knezevic NN, Khan OM, Beiranvand A, et al. Repeated quantitative urine toxicology analysis may improve chronic pain patient compliance with opioid therapy. Pain Physician. 2017;20(2S):S135-S145. doi:10.36076/ppj.2017.s145
14. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
15. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. doi:10.1097/ADM.0000000000000633
16. McDonell MG, Graves MC, West II, et al. Utility of point-of-care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196-201. doi:10.1097/ADM.0000000000000220
17. Algren DA, Christian MR. Buyer beware: pitfalls in toxicology laboratory testing. Mo Med. 2015;112(3):206-210.
18. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123. doi:10.1016/j.jsat.2010.05.008
19. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61. doi:10.1016/j.drugalcdep.2009.09.007
20. Warrington JS, Warrington GS, Francis-Fath S, et al. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med. 2020;14(6):e344-e349. doi:10.1097/ADM.0000000000000676
21. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51. doi:10.1016/j.drugalcdep.2017.07.040
22. Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358-369. doi:10.1016/j.clinthera.2015.12.016
23. Suzuki J, Zinser J, Issa M, et al. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abus. 2017;38(4):504-507. doi:10.1080/08897077.2017.1356796
24. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25( Pt 3):259-263. doi:10.1177/000456328802500312
25. Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi:10.1016/j.drugalcdep.2012.08.002
26. Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345-350. doi:10.1097/ADM.0000000000000057
27. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
28. Keary CJ, Wang Y, Moran JR, et al. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4).PCC.12f01371 doi:10.4088/PCC.12f01371
29. Zebelman AM, Troyer BL, Randall GL, et al. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
30. Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304-316. doi:10.1111/j.1360-0443.2004.00935.x
31. Kelly JF, Saitz R, Wakeman S. Language, substance use disorders, and policy: the need to reach consensus on an “addiction-ary.” Alcohol Treat Q. 2016;34(1):116-123. doi:10.1080/07347324.2016.1113103
32. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217-221. doi:10.1080/08897077.2014.930372
33. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128(1):8-9. doi:10.1016/j.amjmed.2014.07.043
34. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
35. Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi:10.7326/M18-1652
36. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 Suppl):S76-S82.
37. Klein A. Harm reduction works: evidence and inclusion in drug policy and advocacy. Health Care Anal. 2020;28(4):404-414. doi:10.1007/s10728-020-00406-w
38. Patel MX, David AS. Medication adherence: predictive factors and enhancement strategies. Psychiatry. 2007;6(9):357-361. doi:10.1016/j.mppsy.2007.06.003
39. Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. doi:10.1097/ADM.0000000000000045
40. Wang L, Weiss J, Ryan EB, et al. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat. 2021;124:108272. doi:10.1016/ j.jsat.2020.108272
41. Harris MTH, Lambert AM, Maschke AD, et al. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135:108655. doi:10.1016/j.jsat.2021.108655
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication PEP19-5068, NSDUH Series H-54. May 2019. https://www.samhsa.gov/data/
2. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. doi:10.1056/NEJMp1402780
3. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/S0140-6736(17)32812-X
4. Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi:10.1007/s11920-017-0783-9
5. DeFlavio JR, Rolin SA, Nordstrom BR, et al. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:3019. doi:10.22605/rrh3019
6. Kuhlman JJ Jr, Lalani S, Magluiolo J Jr, et al. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958. doi:10.1056/NEJMoa022164
8. Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. doi:10.1097/ALN.0b013e318238fea0
9. Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577-581.
10. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi:10.1007/s13142-015-0315-2
11. Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95 Suppl 3:S347-S360. doi:10.1080/09652140020004278
12. Preston KL, Silverman K, Schuster CR, et al. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130-145.
13. Knezevic NN, Khan OM, Beiranvand A, et al. Repeated quantitative urine toxicology analysis may improve chronic pain patient compliance with opioid therapy. Pain Physician. 2017;20(2S):S135-S145. doi:10.36076/ppj.2017.s145
14. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
15. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. doi:10.1097/ADM.0000000000000633
16. McDonell MG, Graves MC, West II, et al. Utility of point-of-care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196-201. doi:10.1097/ADM.0000000000000220
17. Algren DA, Christian MR. Buyer beware: pitfalls in toxicology laboratory testing. Mo Med. 2015;112(3):206-210.
18. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123. doi:10.1016/j.jsat.2010.05.008
19. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61. doi:10.1016/j.drugalcdep.2009.09.007
20. Warrington JS, Warrington GS, Francis-Fath S, et al. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med. 2020;14(6):e344-e349. doi:10.1097/ADM.0000000000000676
21. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51. doi:10.1016/j.drugalcdep.2017.07.040
22. Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358-369. doi:10.1016/j.clinthera.2015.12.016
23. Suzuki J, Zinser J, Issa M, et al. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abus. 2017;38(4):504-507. doi:10.1080/08897077.2017.1356796
24. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25( Pt 3):259-263. doi:10.1177/000456328802500312
25. Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi:10.1016/j.drugalcdep.2012.08.002
26. Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345-350. doi:10.1097/ADM.0000000000000057
27. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
28. Keary CJ, Wang Y, Moran JR, et al. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4).PCC.12f01371 doi:10.4088/PCC.12f01371
29. Zebelman AM, Troyer BL, Randall GL, et al. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
30. Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304-316. doi:10.1111/j.1360-0443.2004.00935.x
31. Kelly JF, Saitz R, Wakeman S. Language, substance use disorders, and policy: the need to reach consensus on an “addiction-ary.” Alcohol Treat Q. 2016;34(1):116-123. doi:10.1080/07347324.2016.1113103
32. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217-221. doi:10.1080/08897077.2014.930372
33. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128(1):8-9. doi:10.1016/j.amjmed.2014.07.043
34. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
35. Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi:10.7326/M18-1652
36. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 Suppl):S76-S82.
37. Klein A. Harm reduction works: evidence and inclusion in drug policy and advocacy. Health Care Anal. 2020;28(4):404-414. doi:10.1007/s10728-020-00406-w
38. Patel MX, David AS. Medication adherence: predictive factors and enhancement strategies. Psychiatry. 2007;6(9):357-361. doi:10.1016/j.mppsy.2007.06.003
39. Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. doi:10.1097/ADM.0000000000000045
40. Wang L, Weiss J, Ryan EB, et al. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat. 2021;124:108272. doi:10.1016/ j.jsat.2020.108272
41. Harris MTH, Lambert AM, Maschke AD, et al. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135:108655. doi:10.1016/j.jsat.2021.108655
From neuroplasticity to psychoplasticity: Psilocybin may reverse personality disorders and political fanaticism
One of psychiatry’s long-standing dogmas is that personality disorders are enduring, unchangeable, and not amenable to treatment with potent psychotropics or intensive psychotherapy. I propose that this dogma may soon be shattered.
Several other dogmas in psychiatry have been demolished over the past several decades:
- that “insanity” is completely irreversible and requires lifetime institutionalization. The serendipitous discovery of chlorpromazine1 annihilated this centuries-old dogma
- that chronic, severe, refractory depression (with ongoing suicidal urges) that fails to improve with pharmacotherapy or electroconvulsive therapy (ECT) is hopeless and untreatable, until ketamine not only pulverized this dogma, but did it with lightning speed, dazzling us all2
- that dissociative agents such as ketamine are dangerous and condemnable drugs of abuse, until the therapeutic effect of ketamine slayed that dragon3
- that ECT “fries” the brain (as malevolently propagated by antipsychiatry cults), which was completely disproven by neuroimaging studies that show the hippocampus (which shrinks during depression) actually grows by >10% after a few ECT sessions4
- that psychotherapy is not a “real” treatment because talking cannot reverse a psychiatric brain disorder, until studies showed significant neuroplasticity with psychotherapy and decrease in inflammatory biomarkers with cognitive-behavioral therapy (CBT)5
- that persons with refractory hallucinations and delusions are doomed to a life of disability, until clozapine torpedoed that pessimistic dogma6
- that hallucinogens/psychedelics are dangerous and should be banned, until a jarring paradigm shift occurred with the discovery of psilocybin’s transformative effects, and the remarkable therapeutic effects of its mystical trips.7
Psilocybin’s therapeutic effects
Psilocybin has already proved to have a strong and lasting effect on depression and promises to have therapeutic benefits for patients with substance use disorders, posttraumatic stress disorder (PTSD), and anxiety.8 In addition, when the multiple psychological and neurobiological effects of psilocybin (and of other psychedelics) are examined, I see a very promising path to amelioration of severe personality disorders such as psychopathy, antisocial behavior, and narcissism. The mechanism(s) of action of psilocybin on the human brain are drastically different from any man-made psychotropic agent. As a psychiatric neuroscientist, I envision the neurologic impact of psilocybin to be conducive to a complete transformation of a patient’s view of themself, other people, and the meaning of life. It is reminiscent of religious conversion.
The psychological effects of psilocybin in humans have been described as follows:
- emotional breakthrough9
- increased psychological flexibility,10,11 a very cortical effect
- mystical experience,12 which results in sudden and significant changes in behavior and perception and includes the following dimensions: sacredness, noetic quality, deeply felt positive mood, ineffability, paradoxicality, and transcendence of time and space13
- oceanic boundlessness, feeling “one with the universe”14
- universal interconnectedness, insightfulness, blissful state, spiritual experience14
- ego dissolution,15 with loss of one’s personal identity
- increased neuroplasticity16
- changes in cognition and increase in insight.17
The neurobiological effects of psilocybin are mediated by serotonin 5HT2A agonism and include the following18:
- reduction in the activity of the medial prefrontal cortex, which regulates memory, attention, inhibitory control, and habit
- a decrease in the connectivity between the medial prefrontal cortex and the posterior cingulate cortex, which regulates memory and emotions
- reducing the default mode network, which is active during rest, stimulating internal thoughts and reminiscing about previous feelings and events, sometimes including ruminations. Psilocybin reverses those processes to thinking about others, not just the self, and becoming more open-minded about the world and other people. This can be therapeutic for depression, which is often associated with negative ruminations but also with entrenched habits (addictive behaviors), anxiety, PTSD, and obsessive-compulsive disorders
- increased global functional connectivity among various brain networks, leading to stronger functional integration of behavior
- collapse of major cortical oscillatory rhythms such as alpha and others that perpetuate “prior” beliefs
- extensive neuroplasticity and recalibration of thought processes and decomposition of pathological beliefs, referred to as REBUS (relaxed beliefs under psychedelics).
The bottom line is psilocybin and other psychedelics can dramatically alter, reshape, and relax rigid beliefs and personality traits by decreasing “neuroticism” and increasing “extraversion,” insightfulness, openness, and possibly conscientiousness.19 Although no studies of psychedelics in psychopathic, antisocial, or narcissistic personality disorders have been conducted, it is very reasonable to speculate that psilocybin may reverse traits of these disorders such as callousness, lack of empathy, and pathological self-centeredness.
Going further, a preliminary report suggests psilocybin can modify political views by decreasing authoritarianism and increasing libertarianism.20,21 In the current political zeitgeist, could psychedelics such as psilocybin reduce or even eliminate political extremism and visceral hatred on all sides? It would be remarkable research to carry out to heal a politically divided populace.The dogma of untreatable personality disorders or hopelessly entrenched political extremism is on the chopping block, and psychedelics offer hope to splinter those beliefs by concurrently remodeling brain tissue (neuroplasticity) and rectifying the mindset (psychoplasticity).
1. Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104-112.
2. Walsh Z, Mollaahmetoglu OM, Rootman, J, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2021;8(1):e19. doi:10.1192/bjo.2021.1061
3. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
4. Ayers B, Leaver A, Woods RP, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
5. Cao B, Li R, Ding L, Xu J, et al. Does cognitive behaviour therapy affect peripheral inflammation of depression? A protocol for the systematic review and meta-analysis. BMJ Open. 2021;11(12):e048162. doi:10.1136/bmjopen-2020-048162
6. Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487.
7. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844-851.
8. Pearson C, Siegel J, Gold JA. Psilocybin-assisted psychotherapy for depression: emerging research on a psychedelic compound with a rich history. J Neurol Sci. 2022;434:120096. doi:10.1016/j.jns.2021.120096
9. Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the Emotional Breakthrough Inventory. J Psychopharmacol. 2019;33(9):1076-1087.
10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39-45.
11. Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1-25.
12. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165-1180.
13. Stace WT. Mysticism and Philosophy. Macmillan Pub Ltd; 1960:37.
14. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393-430.
15. Nour MM, Evans L, Nutt D, et al. Ego-dissolution and psychedelics: validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci. 2016;10:269. doi:10.3389/fnhum.2016.00269
16. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563-567.
17. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
18. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
19. Erritzoe D, Roseman L, Nour MM, et al. Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand. 2018;138(5):368-378.
20. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. 2018;32(7):811-819.
21. Nour MM, Evans L, Carhart-Harris RL. Psychedelics, personality and political perspectives. J Psychoactive Drugs. 2017;49(3):182-191.
One of psychiatry’s long-standing dogmas is that personality disorders are enduring, unchangeable, and not amenable to treatment with potent psychotropics or intensive psychotherapy. I propose that this dogma may soon be shattered.
Several other dogmas in psychiatry have been demolished over the past several decades:
- that “insanity” is completely irreversible and requires lifetime institutionalization. The serendipitous discovery of chlorpromazine1 annihilated this centuries-old dogma
- that chronic, severe, refractory depression (with ongoing suicidal urges) that fails to improve with pharmacotherapy or electroconvulsive therapy (ECT) is hopeless and untreatable, until ketamine not only pulverized this dogma, but did it with lightning speed, dazzling us all2
- that dissociative agents such as ketamine are dangerous and condemnable drugs of abuse, until the therapeutic effect of ketamine slayed that dragon3
- that ECT “fries” the brain (as malevolently propagated by antipsychiatry cults), which was completely disproven by neuroimaging studies that show the hippocampus (which shrinks during depression) actually grows by >10% after a few ECT sessions4
- that psychotherapy is not a “real” treatment because talking cannot reverse a psychiatric brain disorder, until studies showed significant neuroplasticity with psychotherapy and decrease in inflammatory biomarkers with cognitive-behavioral therapy (CBT)5
- that persons with refractory hallucinations and delusions are doomed to a life of disability, until clozapine torpedoed that pessimistic dogma6
- that hallucinogens/psychedelics are dangerous and should be banned, until a jarring paradigm shift occurred with the discovery of psilocybin’s transformative effects, and the remarkable therapeutic effects of its mystical trips.7
Psilocybin’s therapeutic effects
Psilocybin has already proved to have a strong and lasting effect on depression and promises to have therapeutic benefits for patients with substance use disorders, posttraumatic stress disorder (PTSD), and anxiety.8 In addition, when the multiple psychological and neurobiological effects of psilocybin (and of other psychedelics) are examined, I see a very promising path to amelioration of severe personality disorders such as psychopathy, antisocial behavior, and narcissism. The mechanism(s) of action of psilocybin on the human brain are drastically different from any man-made psychotropic agent. As a psychiatric neuroscientist, I envision the neurologic impact of psilocybin to be conducive to a complete transformation of a patient’s view of themself, other people, and the meaning of life. It is reminiscent of religious conversion.
The psychological effects of psilocybin in humans have been described as follows:
- emotional breakthrough9
- increased psychological flexibility,10,11 a very cortical effect
- mystical experience,12 which results in sudden and significant changes in behavior and perception and includes the following dimensions: sacredness, noetic quality, deeply felt positive mood, ineffability, paradoxicality, and transcendence of time and space13
- oceanic boundlessness, feeling “one with the universe”14
- universal interconnectedness, insightfulness, blissful state, spiritual experience14
- ego dissolution,15 with loss of one’s personal identity
- increased neuroplasticity16
- changes in cognition and increase in insight.17
The neurobiological effects of psilocybin are mediated by serotonin 5HT2A agonism and include the following18:
- reduction in the activity of the medial prefrontal cortex, which regulates memory, attention, inhibitory control, and habit
- a decrease in the connectivity between the medial prefrontal cortex and the posterior cingulate cortex, which regulates memory and emotions
- reducing the default mode network, which is active during rest, stimulating internal thoughts and reminiscing about previous feelings and events, sometimes including ruminations. Psilocybin reverses those processes to thinking about others, not just the self, and becoming more open-minded about the world and other people. This can be therapeutic for depression, which is often associated with negative ruminations but also with entrenched habits (addictive behaviors), anxiety, PTSD, and obsessive-compulsive disorders
- increased global functional connectivity among various brain networks, leading to stronger functional integration of behavior
- collapse of major cortical oscillatory rhythms such as alpha and others that perpetuate “prior” beliefs
- extensive neuroplasticity and recalibration of thought processes and decomposition of pathological beliefs, referred to as REBUS (relaxed beliefs under psychedelics).
The bottom line is psilocybin and other psychedelics can dramatically alter, reshape, and relax rigid beliefs and personality traits by decreasing “neuroticism” and increasing “extraversion,” insightfulness, openness, and possibly conscientiousness.19 Although no studies of psychedelics in psychopathic, antisocial, or narcissistic personality disorders have been conducted, it is very reasonable to speculate that psilocybin may reverse traits of these disorders such as callousness, lack of empathy, and pathological self-centeredness.
Going further, a preliminary report suggests psilocybin can modify political views by decreasing authoritarianism and increasing libertarianism.20,21 In the current political zeitgeist, could psychedelics such as psilocybin reduce or even eliminate political extremism and visceral hatred on all sides? It would be remarkable research to carry out to heal a politically divided populace.The dogma of untreatable personality disorders or hopelessly entrenched political extremism is on the chopping block, and psychedelics offer hope to splinter those beliefs by concurrently remodeling brain tissue (neuroplasticity) and rectifying the mindset (psychoplasticity).
One of psychiatry’s long-standing dogmas is that personality disorders are enduring, unchangeable, and not amenable to treatment with potent psychotropics or intensive psychotherapy. I propose that this dogma may soon be shattered.
Several other dogmas in psychiatry have been demolished over the past several decades:
- that “insanity” is completely irreversible and requires lifetime institutionalization. The serendipitous discovery of chlorpromazine1 annihilated this centuries-old dogma
- that chronic, severe, refractory depression (with ongoing suicidal urges) that fails to improve with pharmacotherapy or electroconvulsive therapy (ECT) is hopeless and untreatable, until ketamine not only pulverized this dogma, but did it with lightning speed, dazzling us all2
- that dissociative agents such as ketamine are dangerous and condemnable drugs of abuse, until the therapeutic effect of ketamine slayed that dragon3
- that ECT “fries” the brain (as malevolently propagated by antipsychiatry cults), which was completely disproven by neuroimaging studies that show the hippocampus (which shrinks during depression) actually grows by >10% after a few ECT sessions4
- that psychotherapy is not a “real” treatment because talking cannot reverse a psychiatric brain disorder, until studies showed significant neuroplasticity with psychotherapy and decrease in inflammatory biomarkers with cognitive-behavioral therapy (CBT)5
- that persons with refractory hallucinations and delusions are doomed to a life of disability, until clozapine torpedoed that pessimistic dogma6
- that hallucinogens/psychedelics are dangerous and should be banned, until a jarring paradigm shift occurred with the discovery of psilocybin’s transformative effects, and the remarkable therapeutic effects of its mystical trips.7
Psilocybin’s therapeutic effects
Psilocybin has already proved to have a strong and lasting effect on depression and promises to have therapeutic benefits for patients with substance use disorders, posttraumatic stress disorder (PTSD), and anxiety.8 In addition, when the multiple psychological and neurobiological effects of psilocybin (and of other psychedelics) are examined, I see a very promising path to amelioration of severe personality disorders such as psychopathy, antisocial behavior, and narcissism. The mechanism(s) of action of psilocybin on the human brain are drastically different from any man-made psychotropic agent. As a psychiatric neuroscientist, I envision the neurologic impact of psilocybin to be conducive to a complete transformation of a patient’s view of themself, other people, and the meaning of life. It is reminiscent of religious conversion.
The psychological effects of psilocybin in humans have been described as follows:
- emotional breakthrough9
- increased psychological flexibility,10,11 a very cortical effect
- mystical experience,12 which results in sudden and significant changes in behavior and perception and includes the following dimensions: sacredness, noetic quality, deeply felt positive mood, ineffability, paradoxicality, and transcendence of time and space13
- oceanic boundlessness, feeling “one with the universe”14
- universal interconnectedness, insightfulness, blissful state, spiritual experience14
- ego dissolution,15 with loss of one’s personal identity
- increased neuroplasticity16
- changes in cognition and increase in insight.17
The neurobiological effects of psilocybin are mediated by serotonin 5HT2A agonism and include the following18:
- reduction in the activity of the medial prefrontal cortex, which regulates memory, attention, inhibitory control, and habit
- a decrease in the connectivity between the medial prefrontal cortex and the posterior cingulate cortex, which regulates memory and emotions
- reducing the default mode network, which is active during rest, stimulating internal thoughts and reminiscing about previous feelings and events, sometimes including ruminations. Psilocybin reverses those processes to thinking about others, not just the self, and becoming more open-minded about the world and other people. This can be therapeutic for depression, which is often associated with negative ruminations but also with entrenched habits (addictive behaviors), anxiety, PTSD, and obsessive-compulsive disorders
- increased global functional connectivity among various brain networks, leading to stronger functional integration of behavior
- collapse of major cortical oscillatory rhythms such as alpha and others that perpetuate “prior” beliefs
- extensive neuroplasticity and recalibration of thought processes and decomposition of pathological beliefs, referred to as REBUS (relaxed beliefs under psychedelics).
The bottom line is psilocybin and other psychedelics can dramatically alter, reshape, and relax rigid beliefs and personality traits by decreasing “neuroticism” and increasing “extraversion,” insightfulness, openness, and possibly conscientiousness.19 Although no studies of psychedelics in psychopathic, antisocial, or narcissistic personality disorders have been conducted, it is very reasonable to speculate that psilocybin may reverse traits of these disorders such as callousness, lack of empathy, and pathological self-centeredness.
Going further, a preliminary report suggests psilocybin can modify political views by decreasing authoritarianism and increasing libertarianism.20,21 In the current political zeitgeist, could psychedelics such as psilocybin reduce or even eliminate political extremism and visceral hatred on all sides? It would be remarkable research to carry out to heal a politically divided populace.The dogma of untreatable personality disorders or hopelessly entrenched political extremism is on the chopping block, and psychedelics offer hope to splinter those beliefs by concurrently remodeling brain tissue (neuroplasticity) and rectifying the mindset (psychoplasticity).
1. Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104-112.
2. Walsh Z, Mollaahmetoglu OM, Rootman, J, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2021;8(1):e19. doi:10.1192/bjo.2021.1061
3. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
4. Ayers B, Leaver A, Woods RP, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
5. Cao B, Li R, Ding L, Xu J, et al. Does cognitive behaviour therapy affect peripheral inflammation of depression? A protocol for the systematic review and meta-analysis. BMJ Open. 2021;11(12):e048162. doi:10.1136/bmjopen-2020-048162
6. Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487.
7. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844-851.
8. Pearson C, Siegel J, Gold JA. Psilocybin-assisted psychotherapy for depression: emerging research on a psychedelic compound with a rich history. J Neurol Sci. 2022;434:120096. doi:10.1016/j.jns.2021.120096
9. Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the Emotional Breakthrough Inventory. J Psychopharmacol. 2019;33(9):1076-1087.
10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39-45.
11. Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1-25.
12. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165-1180.
13. Stace WT. Mysticism and Philosophy. Macmillan Pub Ltd; 1960:37.
14. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393-430.
15. Nour MM, Evans L, Nutt D, et al. Ego-dissolution and psychedelics: validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci. 2016;10:269. doi:10.3389/fnhum.2016.00269
16. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563-567.
17. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
18. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
19. Erritzoe D, Roseman L, Nour MM, et al. Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand. 2018;138(5):368-378.
20. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. 2018;32(7):811-819.
21. Nour MM, Evans L, Carhart-Harris RL. Psychedelics, personality and political perspectives. J Psychoactive Drugs. 2017;49(3):182-191.
1. Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104-112.
2. Walsh Z, Mollaahmetoglu OM, Rootman, J, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2021;8(1):e19. doi:10.1192/bjo.2021.1061
3. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
4. Ayers B, Leaver A, Woods RP, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
5. Cao B, Li R, Ding L, Xu J, et al. Does cognitive behaviour therapy affect peripheral inflammation of depression? A protocol for the systematic review and meta-analysis. BMJ Open. 2021;11(12):e048162. doi:10.1136/bmjopen-2020-048162
6. Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487.
7. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844-851.
8. Pearson C, Siegel J, Gold JA. Psilocybin-assisted psychotherapy for depression: emerging research on a psychedelic compound with a rich history. J Neurol Sci. 2022;434:120096. doi:10.1016/j.jns.2021.120096
9. Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the Emotional Breakthrough Inventory. J Psychopharmacol. 2019;33(9):1076-1087.
10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39-45.
11. Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1-25.
12. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165-1180.
13. Stace WT. Mysticism and Philosophy. Macmillan Pub Ltd; 1960:37.
14. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393-430.
15. Nour MM, Evans L, Nutt D, et al. Ego-dissolution and psychedelics: validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci. 2016;10:269. doi:10.3389/fnhum.2016.00269
16. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563-567.
17. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
18. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
19. Erritzoe D, Roseman L, Nour MM, et al. Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand. 2018;138(5):368-378.
20. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. 2018;32(7):811-819.
21. Nour MM, Evans L, Carhart-Harris RL. Psychedelics, personality and political perspectives. J Psychoactive Drugs. 2017;49(3):182-191.
More on neurotransmitters
The series “Neurotransmitter-based diagnosis and treatment: A hypothesis” (Part 1:
The presentation of abnormal neurotransmission may occur along a continuum. For example, extreme dopamine deficiency can present as catatonia, moderate deficiency may present with inattention, normal activity permits adaptive functioning, and excitatory delirium and sudden death may be at the extreme end of dopaminergic excess.1
The amplitude, rate of change, and location of neurotransmitter dysfunction may determine which specialty takes the primary treatment role. Fatigue, pain, sleep difficulty, and emotional distress require clinicians to understand the whole patient, which is why health care professionals need cross training in psychiatry, and psychiatry recognizes multisystem pathology.
The recognition and treatment of substance use disorders requires an understanding of neurotransmitter symptoms, in terms of both acute drug effects and withdrawal. Fallows2 provides this information in an accessible chart. Discussions of neurotransmitters also have value in managing psychotropic medication withdrawal.3
Acetylcholine is another neurotransmitter of importance; it is essential to normal motor, cognitive, and emotional function. Extreme cholinergic deficiency or anticholinergic crisis has symptoms of pupillary dilation, psychosis, and delirium.4-6 The progressive decline seen in certain dementias is related in part to cholinergic deficit. Dominance of cholinergic activity is associated with depression and biomarkers such as increased rapid eye movement (REM) density, a measure of the frequency of rapid eye movements during REM sleep.7 Cholinergic excess or cholinergic crisis may present with symptoms of salivation, lacrimation, muscle weakness, delirium, or paralysis.8
The articles alluded to the interaction of neurotransmitter systems (eg, “dopamine blockade helps with endorphin suppression”). Isolating the effects of a single neurotransmitter is useful, but covariance of neurotransmitter activity also has diagnostic and treatment implications.9-11 Abnormalities in these interactions may be part of the causal process in fundamental cognitive functions.12 If endorphin suppression is insensitive to dopamine blockade, a relative endorphin excess may create symptoms. If acetylcholine changes are normally balanced by a relative increase in dopamine and norepinephrine, then a weak catecholamine response would fit the catecholamine-cholinergic balance hypothesis of depression. Neurotransmitter interactions are well worked out in the neurology of the basal ganglia but less clear in the frontal and limbic systems.13
Quantification has been applied in some areas of clinical care. Morphine equivalents are used to express opiate potency, and there are algorithms to summarize multiple medication effects on respiratory depression/overdose risk.14,15 Chlorpromazine equivalents were used to translate a range of antipsychotic potencies in the early days of antipsychotic treatment. Adverse effects and some treatment responses partially corresponded to the level of dopamine blockade, but not without noise. There is a wide range of variance as antipsychotic potency is assessed for clinical efficacy.16 We are still working on the array of medication potency and selectivity across neurotransmitter systems.17,18 For example, paroxetine is a potent serotonin reuptake blocker but less selective than citalopram, particularly antagonizing cholinergic muscarinic receptors.
The authors noted their hypothesis needs further elaboration and quantification as psychiatry moves from impressionistic practice to firmer science. Measurement of neurotransmitter activity is an area of intense research. Biomeasures have yet to add much value to the clinical practice of psychiatry, but we hope for progress. Functional neuroimaging with sophisticated algorithms is beginning to detail neocortical activity.19 CSF measurement of dopamine and serotonin metabolites seem to correlate with severe depression and suicidal behavior. Noninvasive, wearable technologies to measure galvanic skin response, oxygenation, and neurotransmitter metabolic products may add to neuro-transmitter-based assessment and treatment.
Neurotransmitters are one aspect of brain function. Other processes, such as hormonal neuromodulation20 and ion channels, may be over- or underactive. Channelopathies are of particular interest in cardiology and neurology but are also notable in pain and emotional disorders.21-26 Voltage-gated sodium channels are thought to be involved in general anesthesia.27 Adverse effects of some psychotropic medications are best understood as ion channel dysfunction.28 Using the strategy of this hypothesis applied to activation or inactivation of sodium, potassium, and calcium channels can guide useful diagnostic and treatment ideas for further study.
Mark C. Chandler, MD
Triangle Neuropsychiatry
Durham, North Carolina
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in his letter, or with manufacturers of competing products.
References
1. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
2. Fallows Z. MIT MedLinks. Accessed August 8, 2022. http://web.mit.edu/zakf/www/drugchart/drugchart11.html
3. Groot PC, van Os J. How user knowledge of psychotropic drug withdrawal resulted in the development of person-specific tapering medication. Ther Adv Psychopharmacol. 2020;10:2045125320932452. doi:10.1177/2045125320932452
4. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129.
5. Nair VP, Hunter JM. Anticholinesterases and anticholinergic drugs. Continuing Education in Anaesthesia Critical Care & Pain. 2004;4(5):164-168.
6. Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium--theory, evidence and practice. Br J Clin Pharmacol. 2016;81(3):516-524.
7. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709.
8. Adeyinka A, Kondamudi NP. Cholinergic Crisis. StatPearls Publishing; 2022.
9. El Mansari M, Guiard BP, Chernoloz O, et al. Relevance of norepinephrine-dopamine interactions in the treatment of major depressive disorder. CNS Neurosci Ther. 2010;16(3):e1-e17.
10. Esposito E. Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Curr Drug Targets. 2006;7(2):177-185.
11. Kringelbach ML, Cruzat J, Cabral J, et al. Dynamic coupling of whole-brain neuronal and neurotransmitter systems. Proc Natl Acad Sci U S A. 2020;117(17):9566-9576.
12. Thiele A, Bellgrove MA. Neuromodulation of attention. Neuron. 2018;97(4):769-785.
13. Muñoz A, Lopez-Lopez A, Labandeira CM, et al. Interactions between the serotonergic and other neurotransmitter systems in the basal ganglia: role in Parkinson’s disease and adverse effects of L-DOPA. Front Neuroanat. 2020;14:26.
14. Nielsen S, Degenhardt L, Hoban B, et al. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737.
15. Lo-Ciganic WH, Huang JL, Zhang HH, et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among Medicare beneficiaries with opioid prescriptions. JAMA Netw Open. 2019;2(3):e190968. doi:10.1001/jamanetworkopen.2019.0968
16. Dewan MJ, Koss M. The clinical impact of reported variance in potency of antipsychotic agents. Acta Psychiatr Scand. 1995;91(4):229-232.
17. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
18. Hayasaka Y, Purgato M, Magni LR, et al. Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord. 2015;180:179-184.
19. Hansen JY, Shafiei G, Markello RD, et al. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. bioRxiv. 2021. https://doi.org/10.1101/2021.10.28.466336
20. Hwang WJ, Lee TY, Kim NS, et al. The role of estrogen receptors and their signaling across psychiatric disorders. Int J Mol Sci. 2020;22(1):373.
21. Lawrence JH, Tomaselli GF, Marban E. Ion channels: structure and function. Heart Dis Stroke. 1993;2(1):75-80.
22. Fedele F, Severino P, Bruno N, et al. Role of ion channels in coronary microcirculation: a review of the literature. Future Cardiol. 2013;9(6):897-905.
23. Kumar P, Kumar D, Jha SK, et al. Ion channels in neurological disorders. Adv Protein Chem Struct Biol. 2016;103:97-136.
24. Quagliato LA, Nardi AE. The role of convergent ion channel pathways in microglial phenotypes: a systematic review of the implications for neurological and psychiatric disorders. Transl Psychiatry. 2018;8(1):259.
25. Bianchi MT, Botzolakis EJ. Targeting ligand-gated ion channels in neurology and psychiatry: is pharmacological promiscuity an obstacle or an opportunity? BMC Pharmacol. 2010;10:3.
26. Imbrici P, Camerino DC, Tricarico D. Major channels involved in neuropsychiatric disorders and therapeutic perspectives. Front Genet. 2013;4:76.
27. Xiao J, Chen Z, Yu B. A potential mechanism of sodium channel mediating the general anesthesia induced by propofol. Front Cell Neurosci. 2020;14:593050. doi:10.3389/fncel.2020.593050
28. Kamei S, Sato N, Harayama Y, et al. Molecular analysis of potassium ion channel genes in sudden death cases among patients administered psychotropic drug therapy: are polymorphisms in LQT genes a potential risk factor? J Hum Genet. 2014;59(2):95-99.
The authors respond
Thank you for your thoughtful commentary. Our conceptual article was not designed to cover enough ground to be completely thorough. Everything you wrote adds to what we wanted to bring to the reader’s attention. The mechanisms of disease in psychiatry are numerous and still elusive, and the brain’s complexity is staggering. Our main goal was to point out possible correlations between specific symptoms and specific neurotransmitter activity. We had to oversimplify to make the article concise enough for publication. Neurotransmitter effects are based on their synthesis, storage, release, reuptake, and degradation. A receptor’s quantity and quality of function, inhibitors, inducers, and many other factors are involved in neurotransmitter performance. And, of course, there are additional fundamental neurotransmitters beyond the 6 we touched on. Our ability to sort through all of this is still rudimentary. You also reflect on the emerging methods to objectively measure neurotransmitter activity, which will eventually find their way to clinical practice and become invaluable. Still, we treat people, not tests or pictures, so diagnostic thinking based on clinical presentation will forever remain a cornerstone of dealing with individual patients.
We hope scientists and clinicians such as yourself will improve our concept and make it truly practical.
Dmitry M. Arbuck, MD
Clinical Assistant Professor of Psychiatry and Medicine
Indiana University School of Medicine
Indianapolis, Indiana
President and Medical Director
Indiana Polyclinic
Carmel, Indiana
José Miguel Salmerón, MD
Professor
Department of Psychiatry
Universidad del Valle School of Medicine/Hospital
Universitario del Valle
Cali, Colombia
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their response, or with manufacturers of competing products.
The series “Neurotransmitter-based diagnosis and treatment: A hypothesis” (Part 1:
The presentation of abnormal neurotransmission may occur along a continuum. For example, extreme dopamine deficiency can present as catatonia, moderate deficiency may present with inattention, normal activity permits adaptive functioning, and excitatory delirium and sudden death may be at the extreme end of dopaminergic excess.1
The amplitude, rate of change, and location of neurotransmitter dysfunction may determine which specialty takes the primary treatment role. Fatigue, pain, sleep difficulty, and emotional distress require clinicians to understand the whole patient, which is why health care professionals need cross training in psychiatry, and psychiatry recognizes multisystem pathology.
The recognition and treatment of substance use disorders requires an understanding of neurotransmitter symptoms, in terms of both acute drug effects and withdrawal. Fallows2 provides this information in an accessible chart. Discussions of neurotransmitters also have value in managing psychotropic medication withdrawal.3
Acetylcholine is another neurotransmitter of importance; it is essential to normal motor, cognitive, and emotional function. Extreme cholinergic deficiency or anticholinergic crisis has symptoms of pupillary dilation, psychosis, and delirium.4-6 The progressive decline seen in certain dementias is related in part to cholinergic deficit. Dominance of cholinergic activity is associated with depression and biomarkers such as increased rapid eye movement (REM) density, a measure of the frequency of rapid eye movements during REM sleep.7 Cholinergic excess or cholinergic crisis may present with symptoms of salivation, lacrimation, muscle weakness, delirium, or paralysis.8
The articles alluded to the interaction of neurotransmitter systems (eg, “dopamine blockade helps with endorphin suppression”). Isolating the effects of a single neurotransmitter is useful, but covariance of neurotransmitter activity also has diagnostic and treatment implications.9-11 Abnormalities in these interactions may be part of the causal process in fundamental cognitive functions.12 If endorphin suppression is insensitive to dopamine blockade, a relative endorphin excess may create symptoms. If acetylcholine changes are normally balanced by a relative increase in dopamine and norepinephrine, then a weak catecholamine response would fit the catecholamine-cholinergic balance hypothesis of depression. Neurotransmitter interactions are well worked out in the neurology of the basal ganglia but less clear in the frontal and limbic systems.13
Quantification has been applied in some areas of clinical care. Morphine equivalents are used to express opiate potency, and there are algorithms to summarize multiple medication effects on respiratory depression/overdose risk.14,15 Chlorpromazine equivalents were used to translate a range of antipsychotic potencies in the early days of antipsychotic treatment. Adverse effects and some treatment responses partially corresponded to the level of dopamine blockade, but not without noise. There is a wide range of variance as antipsychotic potency is assessed for clinical efficacy.16 We are still working on the array of medication potency and selectivity across neurotransmitter systems.17,18 For example, paroxetine is a potent serotonin reuptake blocker but less selective than citalopram, particularly antagonizing cholinergic muscarinic receptors.
The authors noted their hypothesis needs further elaboration and quantification as psychiatry moves from impressionistic practice to firmer science. Measurement of neurotransmitter activity is an area of intense research. Biomeasures have yet to add much value to the clinical practice of psychiatry, but we hope for progress. Functional neuroimaging with sophisticated algorithms is beginning to detail neocortical activity.19 CSF measurement of dopamine and serotonin metabolites seem to correlate with severe depression and suicidal behavior. Noninvasive, wearable technologies to measure galvanic skin response, oxygenation, and neurotransmitter metabolic products may add to neuro-transmitter-based assessment and treatment.
Neurotransmitters are one aspect of brain function. Other processes, such as hormonal neuromodulation20 and ion channels, may be over- or underactive. Channelopathies are of particular interest in cardiology and neurology but are also notable in pain and emotional disorders.21-26 Voltage-gated sodium channels are thought to be involved in general anesthesia.27 Adverse effects of some psychotropic medications are best understood as ion channel dysfunction.28 Using the strategy of this hypothesis applied to activation or inactivation of sodium, potassium, and calcium channels can guide useful diagnostic and treatment ideas for further study.
Mark C. Chandler, MD
Triangle Neuropsychiatry
Durham, North Carolina
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in his letter, or with manufacturers of competing products.
References
1. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
2. Fallows Z. MIT MedLinks. Accessed August 8, 2022. http://web.mit.edu/zakf/www/drugchart/drugchart11.html
3. Groot PC, van Os J. How user knowledge of psychotropic drug withdrawal resulted in the development of person-specific tapering medication. Ther Adv Psychopharmacol. 2020;10:2045125320932452. doi:10.1177/2045125320932452
4. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129.
5. Nair VP, Hunter JM. Anticholinesterases and anticholinergic drugs. Continuing Education in Anaesthesia Critical Care & Pain. 2004;4(5):164-168.
6. Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium--theory, evidence and practice. Br J Clin Pharmacol. 2016;81(3):516-524.
7. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709.
8. Adeyinka A, Kondamudi NP. Cholinergic Crisis. StatPearls Publishing; 2022.
9. El Mansari M, Guiard BP, Chernoloz O, et al. Relevance of norepinephrine-dopamine interactions in the treatment of major depressive disorder. CNS Neurosci Ther. 2010;16(3):e1-e17.
10. Esposito E. Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Curr Drug Targets. 2006;7(2):177-185.
11. Kringelbach ML, Cruzat J, Cabral J, et al. Dynamic coupling of whole-brain neuronal and neurotransmitter systems. Proc Natl Acad Sci U S A. 2020;117(17):9566-9576.
12. Thiele A, Bellgrove MA. Neuromodulation of attention. Neuron. 2018;97(4):769-785.
13. Muñoz A, Lopez-Lopez A, Labandeira CM, et al. Interactions between the serotonergic and other neurotransmitter systems in the basal ganglia: role in Parkinson’s disease and adverse effects of L-DOPA. Front Neuroanat. 2020;14:26.
14. Nielsen S, Degenhardt L, Hoban B, et al. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737.
15. Lo-Ciganic WH, Huang JL, Zhang HH, et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among Medicare beneficiaries with opioid prescriptions. JAMA Netw Open. 2019;2(3):e190968. doi:10.1001/jamanetworkopen.2019.0968
16. Dewan MJ, Koss M. The clinical impact of reported variance in potency of antipsychotic agents. Acta Psychiatr Scand. 1995;91(4):229-232.
17. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
18. Hayasaka Y, Purgato M, Magni LR, et al. Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord. 2015;180:179-184.
19. Hansen JY, Shafiei G, Markello RD, et al. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. bioRxiv. 2021. https://doi.org/10.1101/2021.10.28.466336
20. Hwang WJ, Lee TY, Kim NS, et al. The role of estrogen receptors and their signaling across psychiatric disorders. Int J Mol Sci. 2020;22(1):373.
21. Lawrence JH, Tomaselli GF, Marban E. Ion channels: structure and function. Heart Dis Stroke. 1993;2(1):75-80.
22. Fedele F, Severino P, Bruno N, et al. Role of ion channels in coronary microcirculation: a review of the literature. Future Cardiol. 2013;9(6):897-905.
23. Kumar P, Kumar D, Jha SK, et al. Ion channels in neurological disorders. Adv Protein Chem Struct Biol. 2016;103:97-136.
24. Quagliato LA, Nardi AE. The role of convergent ion channel pathways in microglial phenotypes: a systematic review of the implications for neurological and psychiatric disorders. Transl Psychiatry. 2018;8(1):259.
25. Bianchi MT, Botzolakis EJ. Targeting ligand-gated ion channels in neurology and psychiatry: is pharmacological promiscuity an obstacle or an opportunity? BMC Pharmacol. 2010;10:3.
26. Imbrici P, Camerino DC, Tricarico D. Major channels involved in neuropsychiatric disorders and therapeutic perspectives. Front Genet. 2013;4:76.
27. Xiao J, Chen Z, Yu B. A potential mechanism of sodium channel mediating the general anesthesia induced by propofol. Front Cell Neurosci. 2020;14:593050. doi:10.3389/fncel.2020.593050
28. Kamei S, Sato N, Harayama Y, et al. Molecular analysis of potassium ion channel genes in sudden death cases among patients administered psychotropic drug therapy: are polymorphisms in LQT genes a potential risk factor? J Hum Genet. 2014;59(2):95-99.
The authors respond
Thank you for your thoughtful commentary. Our conceptual article was not designed to cover enough ground to be completely thorough. Everything you wrote adds to what we wanted to bring to the reader’s attention. The mechanisms of disease in psychiatry are numerous and still elusive, and the brain’s complexity is staggering. Our main goal was to point out possible correlations between specific symptoms and specific neurotransmitter activity. We had to oversimplify to make the article concise enough for publication. Neurotransmitter effects are based on their synthesis, storage, release, reuptake, and degradation. A receptor’s quantity and quality of function, inhibitors, inducers, and many other factors are involved in neurotransmitter performance. And, of course, there are additional fundamental neurotransmitters beyond the 6 we touched on. Our ability to sort through all of this is still rudimentary. You also reflect on the emerging methods to objectively measure neurotransmitter activity, which will eventually find their way to clinical practice and become invaluable. Still, we treat people, not tests or pictures, so diagnostic thinking based on clinical presentation will forever remain a cornerstone of dealing with individual patients.
We hope scientists and clinicians such as yourself will improve our concept and make it truly practical.
Dmitry M. Arbuck, MD
Clinical Assistant Professor of Psychiatry and Medicine
Indiana University School of Medicine
Indianapolis, Indiana
President and Medical Director
Indiana Polyclinic
Carmel, Indiana
José Miguel Salmerón, MD
Professor
Department of Psychiatry
Universidad del Valle School of Medicine/Hospital
Universitario del Valle
Cali, Colombia
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their response, or with manufacturers of competing products.
The series “Neurotransmitter-based diagnosis and treatment: A hypothesis” (Part 1:
The presentation of abnormal neurotransmission may occur along a continuum. For example, extreme dopamine deficiency can present as catatonia, moderate deficiency may present with inattention, normal activity permits adaptive functioning, and excitatory delirium and sudden death may be at the extreme end of dopaminergic excess.1
The amplitude, rate of change, and location of neurotransmitter dysfunction may determine which specialty takes the primary treatment role. Fatigue, pain, sleep difficulty, and emotional distress require clinicians to understand the whole patient, which is why health care professionals need cross training in psychiatry, and psychiatry recognizes multisystem pathology.
The recognition and treatment of substance use disorders requires an understanding of neurotransmitter symptoms, in terms of both acute drug effects and withdrawal. Fallows2 provides this information in an accessible chart. Discussions of neurotransmitters also have value in managing psychotropic medication withdrawal.3
Acetylcholine is another neurotransmitter of importance; it is essential to normal motor, cognitive, and emotional function. Extreme cholinergic deficiency or anticholinergic crisis has symptoms of pupillary dilation, psychosis, and delirium.4-6 The progressive decline seen in certain dementias is related in part to cholinergic deficit. Dominance of cholinergic activity is associated with depression and biomarkers such as increased rapid eye movement (REM) density, a measure of the frequency of rapid eye movements during REM sleep.7 Cholinergic excess or cholinergic crisis may present with symptoms of salivation, lacrimation, muscle weakness, delirium, or paralysis.8
The articles alluded to the interaction of neurotransmitter systems (eg, “dopamine blockade helps with endorphin suppression”). Isolating the effects of a single neurotransmitter is useful, but covariance of neurotransmitter activity also has diagnostic and treatment implications.9-11 Abnormalities in these interactions may be part of the causal process in fundamental cognitive functions.12 If endorphin suppression is insensitive to dopamine blockade, a relative endorphin excess may create symptoms. If acetylcholine changes are normally balanced by a relative increase in dopamine and norepinephrine, then a weak catecholamine response would fit the catecholamine-cholinergic balance hypothesis of depression. Neurotransmitter interactions are well worked out in the neurology of the basal ganglia but less clear in the frontal and limbic systems.13
Quantification has been applied in some areas of clinical care. Morphine equivalents are used to express opiate potency, and there are algorithms to summarize multiple medication effects on respiratory depression/overdose risk.14,15 Chlorpromazine equivalents were used to translate a range of antipsychotic potencies in the early days of antipsychotic treatment. Adverse effects and some treatment responses partially corresponded to the level of dopamine blockade, but not without noise. There is a wide range of variance as antipsychotic potency is assessed for clinical efficacy.16 We are still working on the array of medication potency and selectivity across neurotransmitter systems.17,18 For example, paroxetine is a potent serotonin reuptake blocker but less selective than citalopram, particularly antagonizing cholinergic muscarinic receptors.
The authors noted their hypothesis needs further elaboration and quantification as psychiatry moves from impressionistic practice to firmer science. Measurement of neurotransmitter activity is an area of intense research. Biomeasures have yet to add much value to the clinical practice of psychiatry, but we hope for progress. Functional neuroimaging with sophisticated algorithms is beginning to detail neocortical activity.19 CSF measurement of dopamine and serotonin metabolites seem to correlate with severe depression and suicidal behavior. Noninvasive, wearable technologies to measure galvanic skin response, oxygenation, and neurotransmitter metabolic products may add to neuro-transmitter-based assessment and treatment.
Neurotransmitters are one aspect of brain function. Other processes, such as hormonal neuromodulation20 and ion channels, may be over- or underactive. Channelopathies are of particular interest in cardiology and neurology but are also notable in pain and emotional disorders.21-26 Voltage-gated sodium channels are thought to be involved in general anesthesia.27 Adverse effects of some psychotropic medications are best understood as ion channel dysfunction.28 Using the strategy of this hypothesis applied to activation or inactivation of sodium, potassium, and calcium channels can guide useful diagnostic and treatment ideas for further study.
Mark C. Chandler, MD
Triangle Neuropsychiatry
Durham, North Carolina
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in his letter, or with manufacturers of competing products.
References
1. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
2. Fallows Z. MIT MedLinks. Accessed August 8, 2022. http://web.mit.edu/zakf/www/drugchart/drugchart11.html
3. Groot PC, van Os J. How user knowledge of psychotropic drug withdrawal resulted in the development of person-specific tapering medication. Ther Adv Psychopharmacol. 2020;10:2045125320932452. doi:10.1177/2045125320932452
4. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129.
5. Nair VP, Hunter JM. Anticholinesterases and anticholinergic drugs. Continuing Education in Anaesthesia Critical Care & Pain. 2004;4(5):164-168.
6. Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium--theory, evidence and practice. Br J Clin Pharmacol. 2016;81(3):516-524.
7. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709.
8. Adeyinka A, Kondamudi NP. Cholinergic Crisis. StatPearls Publishing; 2022.
9. El Mansari M, Guiard BP, Chernoloz O, et al. Relevance of norepinephrine-dopamine interactions in the treatment of major depressive disorder. CNS Neurosci Ther. 2010;16(3):e1-e17.
10. Esposito E. Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Curr Drug Targets. 2006;7(2):177-185.
11. Kringelbach ML, Cruzat J, Cabral J, et al. Dynamic coupling of whole-brain neuronal and neurotransmitter systems. Proc Natl Acad Sci U S A. 2020;117(17):9566-9576.
12. Thiele A, Bellgrove MA. Neuromodulation of attention. Neuron. 2018;97(4):769-785.
13. Muñoz A, Lopez-Lopez A, Labandeira CM, et al. Interactions between the serotonergic and other neurotransmitter systems in the basal ganglia: role in Parkinson’s disease and adverse effects of L-DOPA. Front Neuroanat. 2020;14:26.
14. Nielsen S, Degenhardt L, Hoban B, et al. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737.
15. Lo-Ciganic WH, Huang JL, Zhang HH, et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among Medicare beneficiaries with opioid prescriptions. JAMA Netw Open. 2019;2(3):e190968. doi:10.1001/jamanetworkopen.2019.0968
16. Dewan MJ, Koss M. The clinical impact of reported variance in potency of antipsychotic agents. Acta Psychiatr Scand. 1995;91(4):229-232.
17. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
18. Hayasaka Y, Purgato M, Magni LR, et al. Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord. 2015;180:179-184.
19. Hansen JY, Shafiei G, Markello RD, et al. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. bioRxiv. 2021. https://doi.org/10.1101/2021.10.28.466336
20. Hwang WJ, Lee TY, Kim NS, et al. The role of estrogen receptors and their signaling across psychiatric disorders. Int J Mol Sci. 2020;22(1):373.
21. Lawrence JH, Tomaselli GF, Marban E. Ion channels: structure and function. Heart Dis Stroke. 1993;2(1):75-80.
22. Fedele F, Severino P, Bruno N, et al. Role of ion channels in coronary microcirculation: a review of the literature. Future Cardiol. 2013;9(6):897-905.
23. Kumar P, Kumar D, Jha SK, et al. Ion channels in neurological disorders. Adv Protein Chem Struct Biol. 2016;103:97-136.
24. Quagliato LA, Nardi AE. The role of convergent ion channel pathways in microglial phenotypes: a systematic review of the implications for neurological and psychiatric disorders. Transl Psychiatry. 2018;8(1):259.
25. Bianchi MT, Botzolakis EJ. Targeting ligand-gated ion channels in neurology and psychiatry: is pharmacological promiscuity an obstacle or an opportunity? BMC Pharmacol. 2010;10:3.
26. Imbrici P, Camerino DC, Tricarico D. Major channels involved in neuropsychiatric disorders and therapeutic perspectives. Front Genet. 2013;4:76.
27. Xiao J, Chen Z, Yu B. A potential mechanism of sodium channel mediating the general anesthesia induced by propofol. Front Cell Neurosci. 2020;14:593050. doi:10.3389/fncel.2020.593050
28. Kamei S, Sato N, Harayama Y, et al. Molecular analysis of potassium ion channel genes in sudden death cases among patients administered psychotropic drug therapy: are polymorphisms in LQT genes a potential risk factor? J Hum Genet. 2014;59(2):95-99.
The authors respond
Thank you for your thoughtful commentary. Our conceptual article was not designed to cover enough ground to be completely thorough. Everything you wrote adds to what we wanted to bring to the reader’s attention. The mechanisms of disease in psychiatry are numerous and still elusive, and the brain’s complexity is staggering. Our main goal was to point out possible correlations between specific symptoms and specific neurotransmitter activity. We had to oversimplify to make the article concise enough for publication. Neurotransmitter effects are based on their synthesis, storage, release, reuptake, and degradation. A receptor’s quantity and quality of function, inhibitors, inducers, and many other factors are involved in neurotransmitter performance. And, of course, there are additional fundamental neurotransmitters beyond the 6 we touched on. Our ability to sort through all of this is still rudimentary. You also reflect on the emerging methods to objectively measure neurotransmitter activity, which will eventually find their way to clinical practice and become invaluable. Still, we treat people, not tests or pictures, so diagnostic thinking based on clinical presentation will forever remain a cornerstone of dealing with individual patients.
We hope scientists and clinicians such as yourself will improve our concept and make it truly practical.
Dmitry M. Arbuck, MD
Clinical Assistant Professor of Psychiatry and Medicine
Indiana University School of Medicine
Indianapolis, Indiana
President and Medical Director
Indiana Polyclinic
Carmel, Indiana
José Miguel Salmerón, MD
Professor
Department of Psychiatry
Universidad del Valle School of Medicine/Hospital
Universitario del Valle
Cali, Colombia
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their response, or with manufacturers of competing products.