User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Understanding the Evaluation and Management Add-on Complexity Code
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
PRACTICE POINTS
- The add-on code G2211 went into effect on January 1, 2024, and can be applied to outpatient evaluation and management visits that fulfill certain criteria.
- This code should be utilized when one is serving as the continuing focal point for all of the patient's health care needs or providing ongoing medical care related to a patient’s single, serious or complex condition.
A Structured Approach for the Management of Orodynia (Burning Mouth Syndrome)
Practice Gap
Orodynia (OD)—together with glossodynia colloquially termed “burning mouth syndrome”—is a chronic disorder characterized by a burning sensation within the oral cavity without objective clinical signs. It is most common in perimenopausal and postmenopausal women.1,2
Orodynia is a diagnosis of exclusion and is considered after 4 to 6 months of normal imaging and laboratory test results.1,2 Its pathophysiology is poorly understood, as it can be intermittent or continuous, manifest with a variety of symptoms, and affect various entities of the oral cavity.3,4 The most common structure affected is the tongue, and symptoms may include xerostomia, dysgeusia, and discomfort.1,2 Orodynia is a frustrating condition, as many patients do not respond to treatment and experience symptoms for years.1-4
The current approach to management of OD typically involves a combination of psychosocial strategies and pharmacologic agents. The psychosocial component consists of coping mechanisms (eg, stress management techniques and behavioral therapies) aimed at alleviating the psychological impact of the condition. Pharmacologic agents such as antidepressants, anticonvulsants, and topical medications often are prescribed to address neuropathic pain and dry mouth symptoms.1,2 Additionally, oral rinses, saliva substitutes, and dietary supplements may be recommended to counteract the discomfort associated with xerostomia.1,2 However, there is no stepwise protocol, leaving these treatments to be trialed in a disorganized manner.2
The Tools
In our unique approach to managing OD, physicians may employ a variety of tools, including autoantibody profiles, noninvasive salivary gland analysis, saliva analysis, patch testing for allergens, and—if deemed necessary—a minor salivary gland biopsy. The use of specific prescription medications is included in the later stages of our approach.
The Technique
First, exclude inflammatory conditions such as geographic tongue, oral lichen planus, autoimmune bullous disorders, and other treatable conditions such as dyspepsia and Sjögren syndrome using the tools described above. Noninvasive modalities should be exhausted first, and dermatologists/clinicians should exercise clinical judgement to determine whether all options should be trialed, including more invasive/costly ones.
If symptoms persist, clinicians may want to obtain a culture for oral candida. If results are positive, candida may be treated quickly with oral fluconazole. If that treatment fails and fissuring is present, advise the patient on treating the tongue; we recommend lightly brushing the tongue once daily with a hydrogen peroxide 3% solution, followed by rinsing. Next, the patient can allow an active probiotic yogurt to sit on the tongue for at least 1 minute to repopulate it with healthy oral bacteria.
If symptoms persist, prescribe gabapentin 100 to 300 mg to be taken at bedtime. Cevimeline 30 mg 3 times daily can be added to treat symptoms of xerostomia. As a last resort, a low daily dose of trifluoperazine 1 to 2 mg may alleviate the dysesthesia of OD. Because this medication is an antipsychotic, there is an increased risk for adverse effects such as tardive dyskinesia; however, given that we recommend using at most one-twentieth of the dose recommended for psychiatric illnesses such as schizophrenia, the risk appears to be minimal.5
We have found this protocol to be more structured, and in our practice, it has led to better outcomes than previously described therapeutic interventions.
Practice Implications
As a chronic condition, OD can be frustrating for patients, as many of them have attempted multiple treatments without success. It also may be challenging for dermatologists who are unfamiliar with its management. This approach to OD provides simple step-by-step diagnostic and therapeutic plans for a condition with an often-uncertain etiology and stubborn response to initial treatments. By following this protocol, dermatologists can be confident in their ability to help patients find relief from OD.
- Klein B, Thoppay JR, De Rossi SS, et al. Burning mouth syndrome. Dermatol Clin. 2020;38:477-483. doi:10.1016/j.det.2020.05.008
- Bender SD. Burning mouth syndrome. Dent Clin North Am. 2018;62:585-596. doi:10.1016/j.cden.2018.05.006
- Javali MA. Burning mouth syndrome: an enigmatic disorder. Kathmandu Univ Med J. 2013;11:175-178. doi:10.3126/kumj.v11i2.12498
- Sardella A, Lodi G, Demarosi F, et al. Burning mouth syndrome: a retrospective study investigating spontaneous remission and response to treatments. Oral Dis. 2006;12:152-155. doi:10.1111/j.1601-0825.2005.01174
- Macdonald R, Watts TP. Trifluoperazine dihydrochloride (stelazine) in paranoid schizophrenia. Br Med J. 1959;1:549-550. doi:10.1136/bmj.1.5121.549
Practice Gap
Orodynia (OD)—together with glossodynia colloquially termed “burning mouth syndrome”—is a chronic disorder characterized by a burning sensation within the oral cavity without objective clinical signs. It is most common in perimenopausal and postmenopausal women.1,2
Orodynia is a diagnosis of exclusion and is considered after 4 to 6 months of normal imaging and laboratory test results.1,2 Its pathophysiology is poorly understood, as it can be intermittent or continuous, manifest with a variety of symptoms, and affect various entities of the oral cavity.3,4 The most common structure affected is the tongue, and symptoms may include xerostomia, dysgeusia, and discomfort.1,2 Orodynia is a frustrating condition, as many patients do not respond to treatment and experience symptoms for years.1-4
The current approach to management of OD typically involves a combination of psychosocial strategies and pharmacologic agents. The psychosocial component consists of coping mechanisms (eg, stress management techniques and behavioral therapies) aimed at alleviating the psychological impact of the condition. Pharmacologic agents such as antidepressants, anticonvulsants, and topical medications often are prescribed to address neuropathic pain and dry mouth symptoms.1,2 Additionally, oral rinses, saliva substitutes, and dietary supplements may be recommended to counteract the discomfort associated with xerostomia.1,2 However, there is no stepwise protocol, leaving these treatments to be trialed in a disorganized manner.2
The Tools
In our unique approach to managing OD, physicians may employ a variety of tools, including autoantibody profiles, noninvasive salivary gland analysis, saliva analysis, patch testing for allergens, and—if deemed necessary—a minor salivary gland biopsy. The use of specific prescription medications is included in the later stages of our approach.
The Technique
First, exclude inflammatory conditions such as geographic tongue, oral lichen planus, autoimmune bullous disorders, and other treatable conditions such as dyspepsia and Sjögren syndrome using the tools described above. Noninvasive modalities should be exhausted first, and dermatologists/clinicians should exercise clinical judgement to determine whether all options should be trialed, including more invasive/costly ones.
If symptoms persist, clinicians may want to obtain a culture for oral candida. If results are positive, candida may be treated quickly with oral fluconazole. If that treatment fails and fissuring is present, advise the patient on treating the tongue; we recommend lightly brushing the tongue once daily with a hydrogen peroxide 3% solution, followed by rinsing. Next, the patient can allow an active probiotic yogurt to sit on the tongue for at least 1 minute to repopulate it with healthy oral bacteria.
If symptoms persist, prescribe gabapentin 100 to 300 mg to be taken at bedtime. Cevimeline 30 mg 3 times daily can be added to treat symptoms of xerostomia. As a last resort, a low daily dose of trifluoperazine 1 to 2 mg may alleviate the dysesthesia of OD. Because this medication is an antipsychotic, there is an increased risk for adverse effects such as tardive dyskinesia; however, given that we recommend using at most one-twentieth of the dose recommended for psychiatric illnesses such as schizophrenia, the risk appears to be minimal.5
We have found this protocol to be more structured, and in our practice, it has led to better outcomes than previously described therapeutic interventions.
Practice Implications
As a chronic condition, OD can be frustrating for patients, as many of them have attempted multiple treatments without success. It also may be challenging for dermatologists who are unfamiliar with its management. This approach to OD provides simple step-by-step diagnostic and therapeutic plans for a condition with an often-uncertain etiology and stubborn response to initial treatments. By following this protocol, dermatologists can be confident in their ability to help patients find relief from OD.
Practice Gap
Orodynia (OD)—together with glossodynia colloquially termed “burning mouth syndrome”—is a chronic disorder characterized by a burning sensation within the oral cavity without objective clinical signs. It is most common in perimenopausal and postmenopausal women.1,2
Orodynia is a diagnosis of exclusion and is considered after 4 to 6 months of normal imaging and laboratory test results.1,2 Its pathophysiology is poorly understood, as it can be intermittent or continuous, manifest with a variety of symptoms, and affect various entities of the oral cavity.3,4 The most common structure affected is the tongue, and symptoms may include xerostomia, dysgeusia, and discomfort.1,2 Orodynia is a frustrating condition, as many patients do not respond to treatment and experience symptoms for years.1-4
The current approach to management of OD typically involves a combination of psychosocial strategies and pharmacologic agents. The psychosocial component consists of coping mechanisms (eg, stress management techniques and behavioral therapies) aimed at alleviating the psychological impact of the condition. Pharmacologic agents such as antidepressants, anticonvulsants, and topical medications often are prescribed to address neuropathic pain and dry mouth symptoms.1,2 Additionally, oral rinses, saliva substitutes, and dietary supplements may be recommended to counteract the discomfort associated with xerostomia.1,2 However, there is no stepwise protocol, leaving these treatments to be trialed in a disorganized manner.2
The Tools
In our unique approach to managing OD, physicians may employ a variety of tools, including autoantibody profiles, noninvasive salivary gland analysis, saliva analysis, patch testing for allergens, and—if deemed necessary—a minor salivary gland biopsy. The use of specific prescription medications is included in the later stages of our approach.
The Technique
First, exclude inflammatory conditions such as geographic tongue, oral lichen planus, autoimmune bullous disorders, and other treatable conditions such as dyspepsia and Sjögren syndrome using the tools described above. Noninvasive modalities should be exhausted first, and dermatologists/clinicians should exercise clinical judgement to determine whether all options should be trialed, including more invasive/costly ones.
If symptoms persist, clinicians may want to obtain a culture for oral candida. If results are positive, candida may be treated quickly with oral fluconazole. If that treatment fails and fissuring is present, advise the patient on treating the tongue; we recommend lightly brushing the tongue once daily with a hydrogen peroxide 3% solution, followed by rinsing. Next, the patient can allow an active probiotic yogurt to sit on the tongue for at least 1 minute to repopulate it with healthy oral bacteria.
If symptoms persist, prescribe gabapentin 100 to 300 mg to be taken at bedtime. Cevimeline 30 mg 3 times daily can be added to treat symptoms of xerostomia. As a last resort, a low daily dose of trifluoperazine 1 to 2 mg may alleviate the dysesthesia of OD. Because this medication is an antipsychotic, there is an increased risk for adverse effects such as tardive dyskinesia; however, given that we recommend using at most one-twentieth of the dose recommended for psychiatric illnesses such as schizophrenia, the risk appears to be minimal.5
We have found this protocol to be more structured, and in our practice, it has led to better outcomes than previously described therapeutic interventions.
Practice Implications
As a chronic condition, OD can be frustrating for patients, as many of them have attempted multiple treatments without success. It also may be challenging for dermatologists who are unfamiliar with its management. This approach to OD provides simple step-by-step diagnostic and therapeutic plans for a condition with an often-uncertain etiology and stubborn response to initial treatments. By following this protocol, dermatologists can be confident in their ability to help patients find relief from OD.
- Klein B, Thoppay JR, De Rossi SS, et al. Burning mouth syndrome. Dermatol Clin. 2020;38:477-483. doi:10.1016/j.det.2020.05.008
- Bender SD. Burning mouth syndrome. Dent Clin North Am. 2018;62:585-596. doi:10.1016/j.cden.2018.05.006
- Javali MA. Burning mouth syndrome: an enigmatic disorder. Kathmandu Univ Med J. 2013;11:175-178. doi:10.3126/kumj.v11i2.12498
- Sardella A, Lodi G, Demarosi F, et al. Burning mouth syndrome: a retrospective study investigating spontaneous remission and response to treatments. Oral Dis. 2006;12:152-155. doi:10.1111/j.1601-0825.2005.01174
- Macdonald R, Watts TP. Trifluoperazine dihydrochloride (stelazine) in paranoid schizophrenia. Br Med J. 1959;1:549-550. doi:10.1136/bmj.1.5121.549
- Klein B, Thoppay JR, De Rossi SS, et al. Burning mouth syndrome. Dermatol Clin. 2020;38:477-483. doi:10.1016/j.det.2020.05.008
- Bender SD. Burning mouth syndrome. Dent Clin North Am. 2018;62:585-596. doi:10.1016/j.cden.2018.05.006
- Javali MA. Burning mouth syndrome: an enigmatic disorder. Kathmandu Univ Med J. 2013;11:175-178. doi:10.3126/kumj.v11i2.12498
- Sardella A, Lodi G, Demarosi F, et al. Burning mouth syndrome: a retrospective study investigating spontaneous remission and response to treatments. Oral Dis. 2006;12:152-155. doi:10.1111/j.1601-0825.2005.01174
- Macdonald R, Watts TP. Trifluoperazine dihydrochloride (stelazine) in paranoid schizophrenia. Br Med J. 1959;1:549-550. doi:10.1136/bmj.1.5121.549
The Burden of Skin Cancer in the Military Health System, 2017-2022
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.
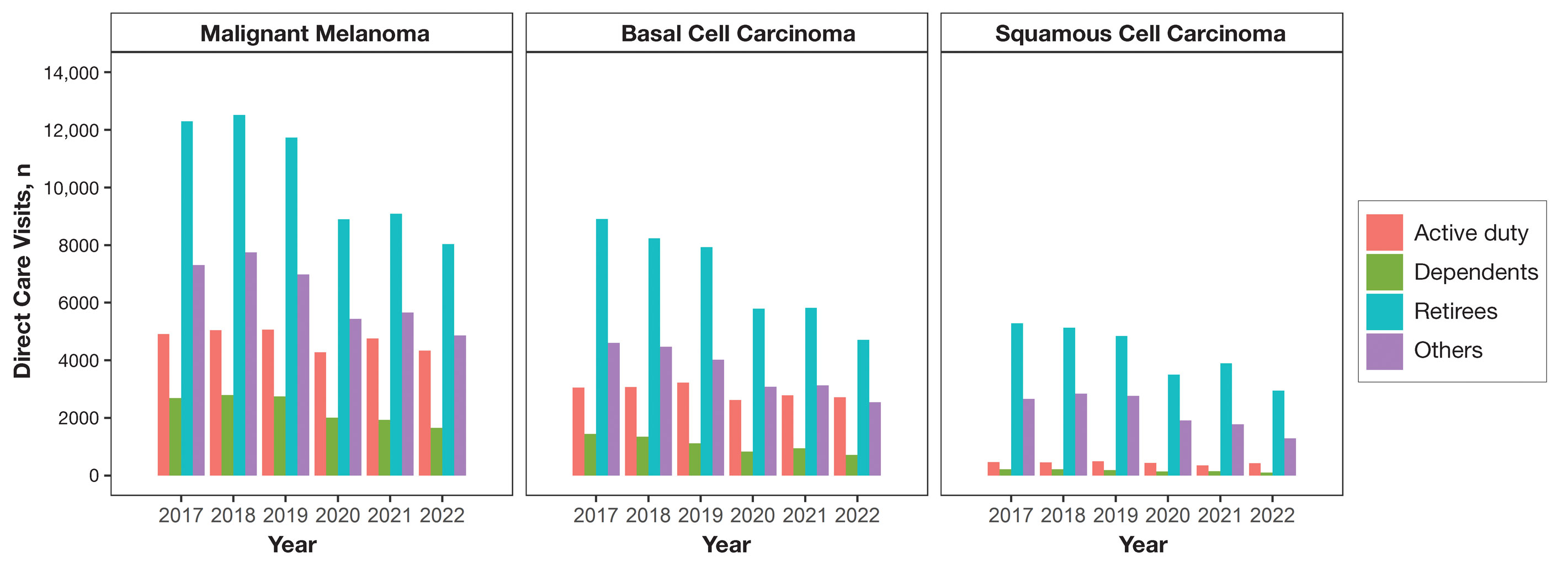
Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.
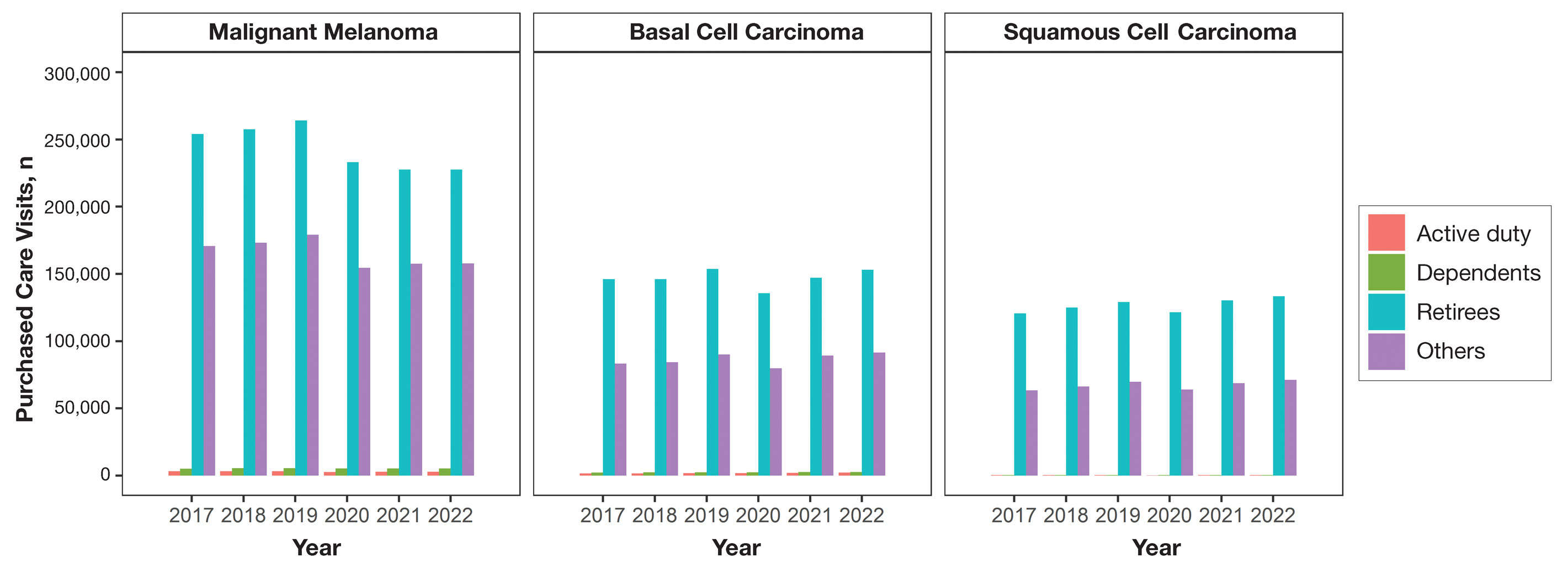
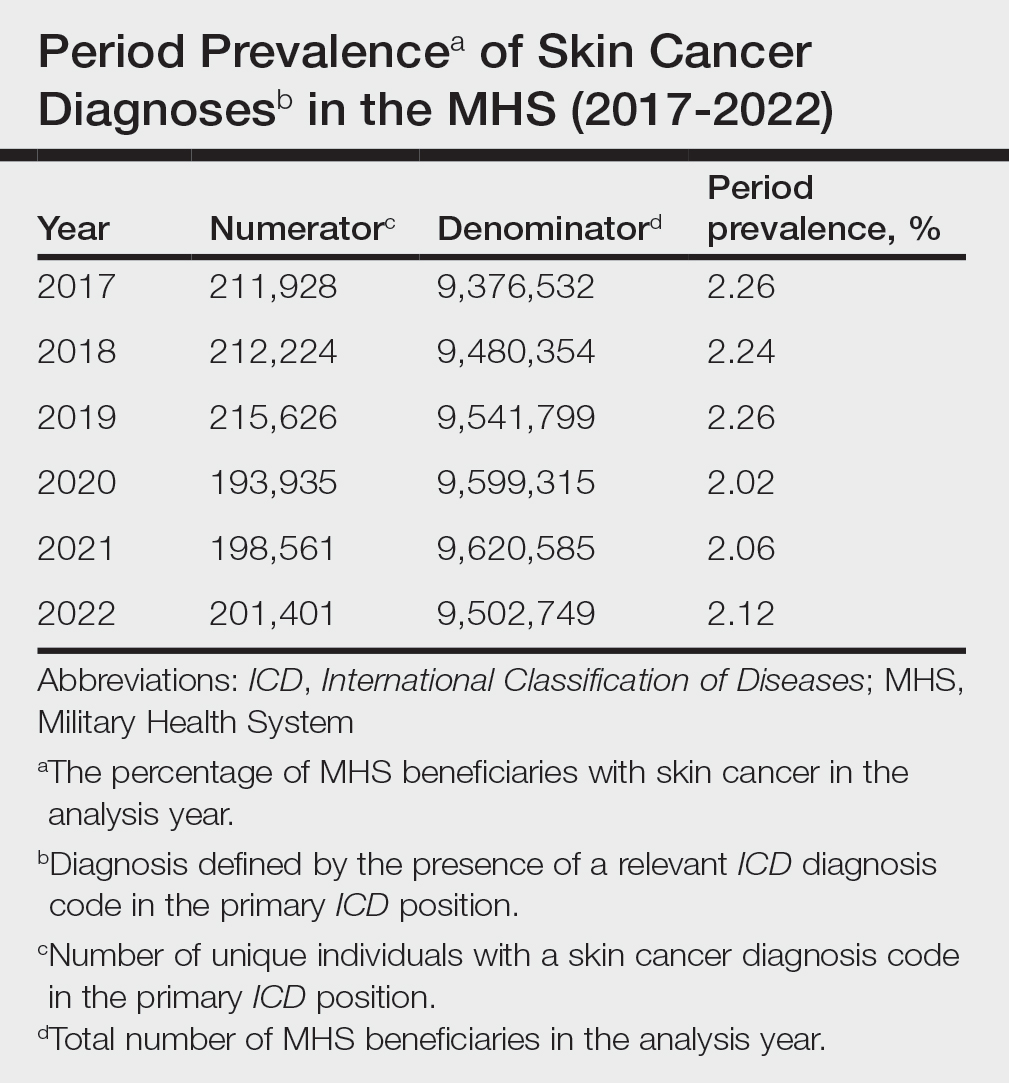
When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.
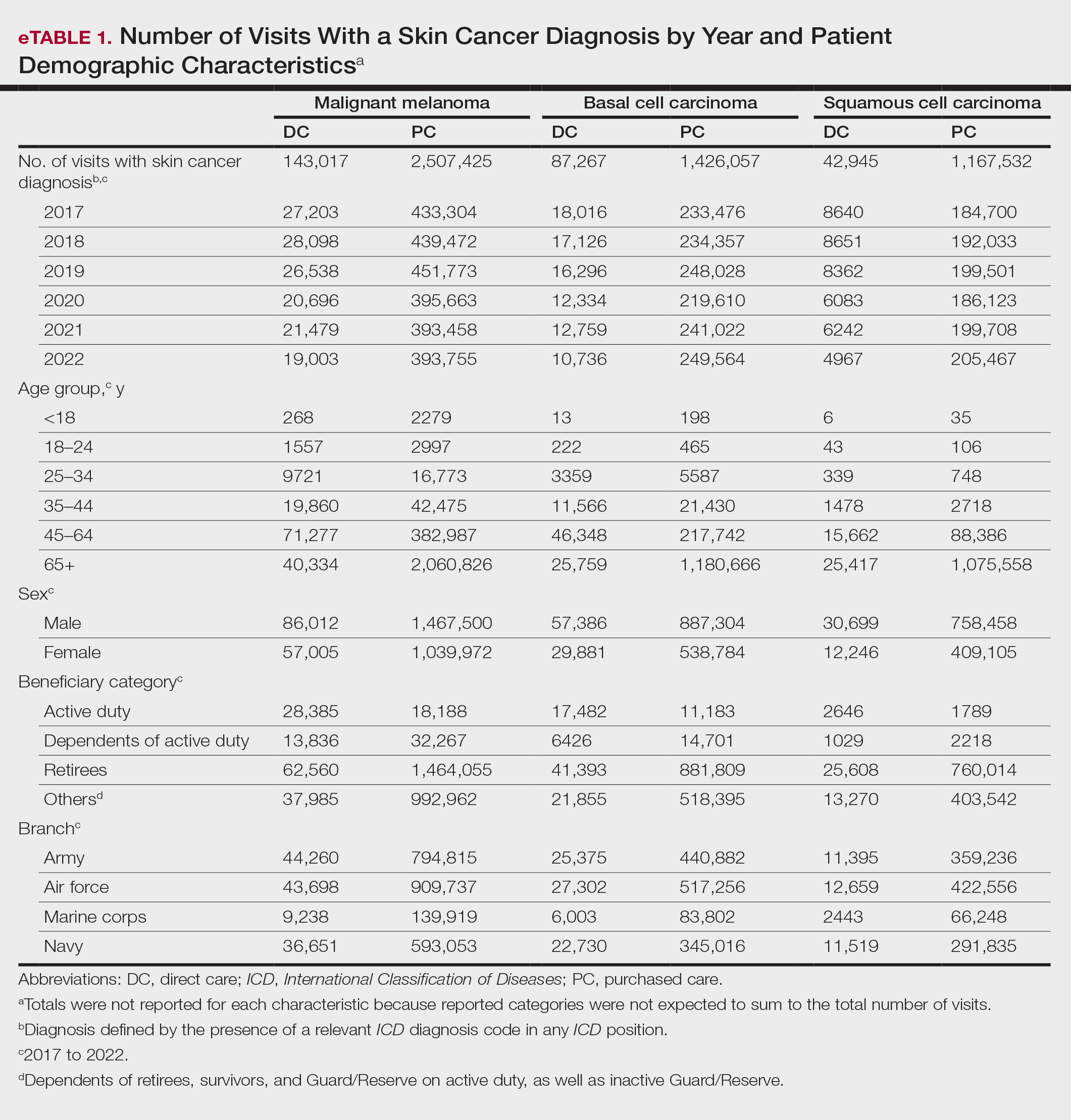
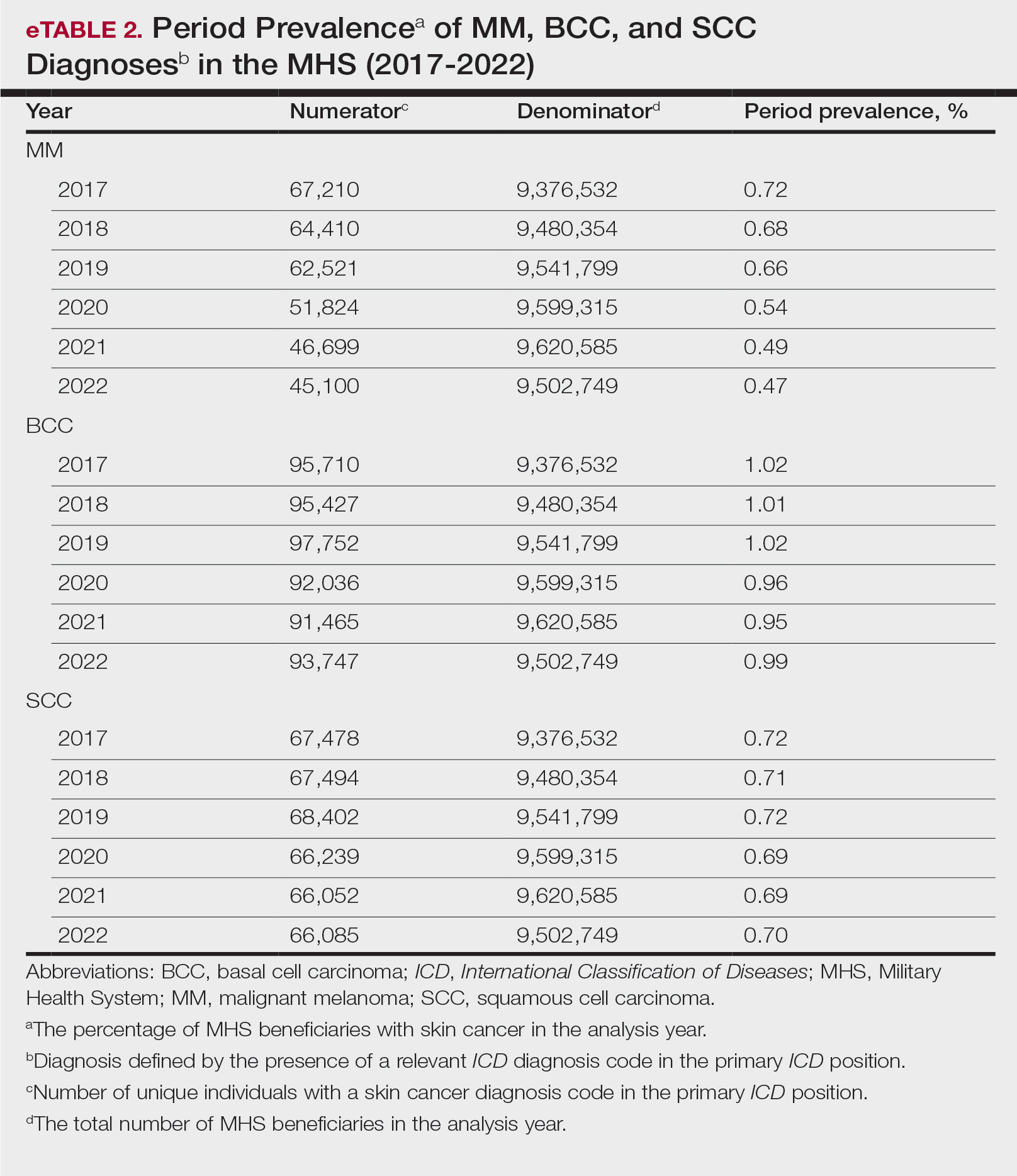
The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.
Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.

Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.

When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.

The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.


Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
This retrospective observational study investigates skin cancer prevalence and care patterns within the Military Health System (MHS) from 2017 to 2022. Utilizing the MHS Management Analysis and Reporting Tool (most commonly called M2), we analyzed more than 5 million patient encounters and documented skin cancer prevalence in the MHS beneficiary population utilizing available demographic data. Notable findings included an increased prevalence of skin cancer in the military population compared with the civilian population, a substantial decline in direct care (DC) visits at military treatment facilities compared with civilian purchased care (PC) visits, and a decreased total number of visits during COVID-19 restrictions.
The Military Health System (MHS) is a worldwide health care delivery system that serves 9.6 million beneficiaries, including military service members, retirees, and their families.1 Its mission is 2-fold: provide a medically ready force, and provide a medical benefit in keeping with the service and sacrifice of active-duty personnel, military retirees, and their families. For fiscal year (FY) 2022, active-duty service members and their families comprised 16.7% and 19.9% of beneficiaries, respectively, while retired service members and their families comprised 27% and 32% of beneficiaries, respectively.
The MHS operates under the authority of the Department of Defense (DoD) and is supported by an annual budget of approximately $50 billion.1 Health care provision within the MHS is managed by TRICARE regional networks.2 Within these networks, MHS beneficiaries may receive health care in 2 categories: direct care (DC) and purchased care (PC). Direct care is rendered in military treatment facilities by military or civilian providers contracted by the DoD, and PC is administered by civilian providers at civilian health care facilities within the TRICARE network, which is comprised of individual providers, clinics, and hospitals that have agreed to accept TRICARE beneficiaries.1 Purchased care is fee-for-service and paid for by the MHS. Of note, the MHS differs from the Veterans Affairs health care system in that the MHS through DC and PC sees only active-duty service members, active-duty dependents, retirees, and retirees’ dependents (primarily spouses), whereas Veterans Affairs sees only veterans (not necessarily retirees) discharged from military service with compensable medical conditions or disabilities.
Skin cancer presents a notable concern for the US Military, as the risk for skin cancer is thought to be higher than in the general population.3,4 This elevated risk is attributed to numerous factors inherent to active-duty service, including time spent in tropical environments, increased exposure to UV radiation, time spent at high altitudes, and decreased rates of sun-protective behaviors.3 Although numerous studies have explored the mechanisms that contribute to service members’ increased skin cancer risk, there are few (if any) that discuss the burden of skin cancer on the MHS and where its beneficiaries receive their skin cancer care. This study evaluated the burden of skin cancer within the MHS, as demonstrated by the period prevalence of skin cancer among its beneficiaries and the number and distribution of patient visits for skin cancer across both DC and PC from 2017 to 2022.
Methods
Data Collection—This retrospective observational study was designed to describe trends in outpatient visits with a skin cancer diagnosis and annual prevalence of skin cancer types in the MHS. Data are from all MHS beneficiaries who were eligible or enrolled in the analysis year. Our data source was the MHS Management Analysis and Reporting Tool (most commonly called M2), a query tool that contains the current and most recent 5 full FYs of Defense Health Agency corporate health care data including aggregated FY and calendar-year counts of MHS beneficiaries from 2017 to 2022 using encounter and claims data tables from both DC and PC. Data in M2 are coded using a pseudo-person identification number, and queries performed for this study were limited to de-identified visit and patient counts.
Skin cancer diagnoses were defined by relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes recorded from outpatient visits in DC and PC. The M2 database was queried to find aggregate counts of visits and unique MHS beneficiaries with one or more diagnoses of a skin cancer type of interest (defined by relevant ICD-10-CM code) over the study period stratified by year and by patient demographic characteristics. Skin cancer types by ICD-10-CM code group included basal cell carcinoma (BCC), squamous cell carcinoma (SCC), malignant melanoma (MM), and other (including Merkel cell carcinoma and sebaceous carcinoma). Demographic strata included age, sex, military status (active duty, dependents of active duty, retired, or all others), sponsor military rank, and sponsor branch (army, air force, marine corps, or navy). Visit counts included diagnoses from any ICD position (for encounters that contained multiple ICD codes) to describe the total volume of care that addressed a diagnosed skin cancer. Counts of unique patients in prevalence analyses included relevant diagnoses in the primary ICD position only to increase the specificity of prevalence estimates.
Data Analysis—Descriptive analyses included the total number of outpatient visits with a skin cancer diagnosis in DC and PC over the study period, with percentages of total visits by year and by demographic strata. Separate analyses estimated annual prevalences of skin cancer types in the MHS by study year and within 2022 by demographic strata. Numerators in prevalence analyses were defined as the number of unique individuals with one or more relevant ICD codes in the analysis year. Denominators were defined as the total number of MHS beneficiaries in the analysis year and resulting period prevalences reported. Observed prevalences were qualitatively described, and trends were compared with prevalences in nonmilitary populations reported in the literature.
Ethics—This study was conducted as part of a study using secondary analyses of de-identified data from the M2 database. The study was reviewed and approved by the Walter Reed National Military Medical Center institutional review board.

Results
Encounter data were analyzed from a total of 5,374,348 visits between DC and PC over the study period for each cancer type of interest. Figures 1 and 2 show temporal trends in DC visits compared with PC visits in each beneficiary category. The percentage of total DC visits subsequently declined each year throughout the study period, with percentage decreases from 2017 to 2022 of 1.45% or 8200 fewer visits for MM, 3.41% or 7280 fewer visits for BCC, and 2.26% or 3673 fewer visits for SCC.

When stratified by beneficiary category, this trend remained consistent among dependents and retirees, with the most notable annual percentage decrease from 2019 to 2020. A higher proportion of younger adults and active-duty beneficiaries was seen in DC relative to PC, in which most visits were among retirees and others (primarily dependents of retirees, survivors, and Guard/Reserve on active duty, as well as inactive Guard/Reserve). No linear trends over time were apparent for active duty in DC and for dependents and retirees in PC. eTable 1 summarizes the demographic characteristics of MHS beneficiaries being seen in DC and PC over the study period for each cancer type of interest.

The Table shows the period prevalence of skin cancer diagnoses within the MHS beneficiary population from 2017 to 2022. These data were further analyzed by MM, BCC, and SCC (eTable 2) and demographics of interest for the year 2022. By beneficiary category, the period prevalence of MM was 0.08% in active duty, 0.06% in dependents, 0.48% in others, and 1.10% in retirees; the period prevalence of BCC was 0.12% in active duty, 0.07% in dependents, 0.91% in others, and 2.50% in retirees; and the period prevalence of SCC was 0.02% in active duty, 0.01% in dependents, 0.63% in others, and 1.87% in retirees. By sponsor branch, the period prevalence of MM was 0.35% in the army, 0.62% in the air force, 0.35% in the marine corps, and 0.65% in the navy; the period prevalence of BCC was 0.74% in the army, 1.30% in the air force, 0.74% in the marine corps, and 1.36% in the navy; and the period prevalence of SCC was 0.52% in the army, 0.92% in the air force, 0.51% in the marine corps, and 0.97% in the navy.


Comment
This study aimed to provide insight into the burden of skin cancer within the MHS beneficiary population and to identify temporal trends in where these beneficiaries receive their care. We examined patient encounter data from more than 9.6 million MHS beneficiaries.
The utilization of ICD codes from patient encounters to estimate the prevalence of nonmelanoma skin cancer (NMSC) has demonstrated a high positive predictive value. In one study, NMSC cases were confirmed in 96.5% of ICD code–identified patients.5 We presented an extensive collection of epidemiologic data on BCC and SCC, which posed unique challenges for tracking, as they are not reported to or monitored by cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) Program.6
MHS Compared to the US Population—A study using the Global Burden of Disease 2019 database revealed an increasing trend in the incidence and prevalence of NMSC and melanoma since 1990. The same study found the period prevalence in 2019 of MM, SCC, and BCC in the general US population to be 0.13%, 0.31%, and 0.05%, respectively.7 In contrast, among MHS beneficiaries, we observed a higher prevalence in the same year, with figures of 0.66% for MM, 0.72% for SCC, and 1.02% for BCC. According to the SEER database, the period prevalence of MM within the general US population in 2020 was 0.4%.8 That same year, we identified a higher period prevalence of MM—0.54%—within the MHS beneficiary population. Specifically, within the MHS retiree population, the prevalence in 2022 was double that of the general MHS population, with a rate of 1.10%, underscoring the importance of skin cancer screening in older, at-risk adult populations. Prior studies similarly found increased rates of skin cancer within the military beneficiary population. Further studies are needed to compare age-adjusted rates in the MHS vs US population.9-11
COVID-19 Trends—Our data showed an overall decreasing prevalence of skin cancer in the MHS from 2019 to 2021. We suspect that the apparent decrease in skin cancer prevalence may be attributed to underdiagnosis from COVID-19 pandemic restrictions. During that time, many dermatology clinics at military treatment facilities underwent temporary closures, and some dermatologists were sent on nondermatologic utilization tours. Likewise, a US multi-institutional study described declining rates of new melanomas from 2020 to 2021, with an increased proportion of patient presentations with advanced melanoma, suggesting an underdiagnosis of melanoma cases during pandemic restrictions. That study also noted an increased rate of patient-identified melanomas and a decreased rate of provider-identified melanomas during that time.12 Contributing factors may include excess hospital demand, increased patient complexity and acute care needs, and long outpatient clinic backlogs during this time.13Financial Burden—Over our 5-year study period, there were 5,374,348 patient encounters addressing skin cancer, both in DC and PC (Figures 1 and 2; eTable 1). In 2016 to 2018, the average annual cost of treating skin cancer in the US civilian, noninstitutionalized population was $1243 for NMSC (BCC and SCC) and $2430 for melanoma.6 Using this metric, the estimated total cost of care rendered in the MHS in 2018 for NMSC and melanoma was $202,510,803 and $156,516,300, respectively.
Trends in DC vs PC—In the years examined, we found a notable decrease in the number of beneficiaries receiving treatment for MM, BCC, and SCC in DC. Simultaneously, there has been an increase in the number of beneficiaries receiving PC for BCC and SCC, though this trend was not apparent for MM.
Our data provided interesting insights into the percentage of PC compared with DC offered within the MHS. Importantly, our findings suggested that the majority of skin cancer in active-duty service members is managed with DC within the military treatment facility setting (61% DC management over the period analyzed). This finding was true across all years of data analyzed, suggesting that the COVID-19 pandemic did not result in a quantifiable shift in care of skin cancer within the active-duty component to outside providers. One of the critical roles of dermatologists in the MHS is to diagnose and treat skin cancer, and our study suggested that the current global manning and staffing for MHS dermatologists may not be sufficient to meet the burden of skin cancers encountered within our active-duty troops, as only 61% are managed with DC. In particular, service members in more austere and/or overseas locations may not have ready access to a dermatologist.
The burden of skin cancer shifts dramatically when analyzing care of all other populations included in these data, including dependents of active-duty service members, retirees, and the category of “other” (ie, principally dependents of retirees). Within these populations, the rate of DC falls to 30%, with 70% of active-duty dependent care being deferred to network. The findings are even more noticeable for retirees and others within these 2 cohorts in all types of skin cancer analyzed, where DC only accounted for 5.2% of those skin cancers encountered and managed across TRICARE-eligible beneficiaries. For MM, BCC, and SCC, percentages of DC were 5.4%, 5.8%, and 3.5%, respectively. Although it is interesting to note the lower percentage of SCC managed via DC, our data did not allow for extrapolation as to why more SCC cases may be deferred to network. The shift to PC may align with DoD initiatives to increase the private sector’s involvement in military medicine and transition to civilianizing the MHS.14 In the end, the findings are remarkable, with approximately 95% of skin cancer care and management provided overall via PC.
These findings differ from previously published data regarding DC and PC from other specialty areas. Results from an analysis of DC vs PC for plastic surgery for the entire MHS from 2016 to 2019 found 83.2% of cases were deferred to network.15 A similar publication in the orthopedics literature examined TRICARE claims for patients who underwent total hip or knee arthroplasties between 2006 and 2019 and found 84.6% of cases were referred for PC. Notably, the authors utilized generalized linear models for cost analysis and found that DC was more expensive than PC, though this likely was a result of higher rates of hospital readmission within DC cases.16 Lastly, an article on the DC vs PC disposition of MHS patients with breast cancer from 2003 to 2008 found 46% of cases managed with DC vs 26.% with PC and 27.8% receiving a combination. In this case, the authors found a reduced cost associated with DC vs PC.17
Little additional literature exists regarding the costs of DC vs PC. An article published in 2016 designed to assess costs of DC vs PC showed that almost all military treatment facilities have higher costs than their private sector counterparts, with a few exceptions.18 This does not assess the costs of specific procedures, however, and only the overall cost to maintain a treatment facility. Importantly, this study was based on data from FY 2014 and has not been updated to reflect complex changes within the MHS system and the private health care system. Indeed, a US Government Accountability Office FY 2023 study highlighted staffing and efficiency issues within this transition to civilian medicine; subsequently, the 2024 President’s Budget suspended all planned clinical medical military end strength divestitures, underscoring the potential ineffectiveness of a civilianized MHS at meeting the health care needs of its beneficiaries.19,20 Future research on a national scale will be necessary to see if there is a reversal of this trend to PC and if doing so has any impact on access to DC for active-duty troops or active-duty dependents.
In addition to PC vs DC trends, we also can get a sense of the impact of the COVID pandemic restrictions on access to DC vs PC by assessing the change in rates seen in the data from the pre-COVID years (2017-2019) to the “post-COVID” years (2020-2022) included. Overall, rates of DC decreased uniformly from their already low percentages. In our study, rates of DC decreased from 5.8% in 2019 to 4.8% in 2022 for MM, from 6.6% to 4.3% for BCC, and from 4.2% to 2.9% for SCC. Although these changes seem small at first, they represent a 30.6% overall decrease in DC for BCC and an overall decrease of 55.4% in DC for SCC. Although our data do not allow us to extrapolate the real cost of this reduction across a nationwide health care system and more than 5 million care encounters, the financial and personal (ie, lost man-hours) costs of this decrease in DC likely are substantial.
In addition to costs, qualitative aspects that contribute to the burden of skin cancer include treatment-related morbidity, such as scarring, pain, and time spent away from family, work, and hobbies, as well as overall patient satisfaction with the quality of care they receive.21 Future work is critical to assess the real cost of this immense burden of PC for the treatment and management of skin cancers within the DoD beneficiary population.
Limitations—This study is limited by its observational nature. Given the mechanism of our data collection, we may have underestimated disease prevalence, as not all patients are seen for their diagnosis annually. Furthermore, reported demographic strata (eg, age, sex) were limited to those available and valid in the M2 reporting system. Finally, our study only collected data from those service members or former service members seen within the MHS and does not reflect any care rendered to those who are no longer active duty but did not officially retire from the military (ie, nonretired service members receiving care in the Veterans Affairs system for skin cancer).
Conclusion
We describe the annual burden of care for skin cancer in the MHS beneficiary population. Noteworthy findings observed were an overall decrease in beneficiaries being treated for skin cancer through DC; a decreasing annual prevalence of skin cancer diagnosis between 2019 and 2021, which may represent underdiagnosis or decreased follow-up in the setting of the COVID-19 pandemic; and a higher rate of skin cancer in the military beneficiary population compared to the civilian population.
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
- US Department of Defense. Military health. Accessed October 5, 2023. https://www.defense.gov/
- Wooten NR, Brittingham JA, Pitner RO, et al. Purchased behavioral health care received by Military Health System beneficiaries in civilian medical facilities, 2000-2014. Mil Med. 2018;183:E278-E290. doi:10.1093/milmed/usx101
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- American Academy of Dermatology. Skin cancer. Updated April 22, 2022. Accessed April 17, 2024. https://www.aad.org/media/stats-skin-cancer
- Eide MJ, Krajenta R, Johnson D, et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171:123-128. doi:10.1093/aje/kwp352
- Kao SYZ, Ekwueme DU, Holman DM, et al. Economic burden of skin cancer treatment in the USA: an analysis of the Medical Expenditure Panel Survey Data, 2012-2018. Cancer Causes Control. 2023;34:205-212. doi:10.1007/s10552-022-01644-0
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- SEER*Explorer. SEER Incidence Data, November 2023 Submission (1975-2021). National Cancer Institute; 2024. Accessed April 17, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=1
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663. doi:10.1111/j.1365-4362.1984.tb01228.x
- Page WF, Whiteman D, Murphy M. A comparison of melanoma mortality among WWII veterans of the Pacific and European theaters. Ann Epidemiol. 2000;10:192-195. doi:10.1016/s1047-2797(99)00050-2
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737. doi:10.1016/0190-9622(93)70102-Y
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Gibbs A. COVID-19 shutdowns caused delays in melanoma diagnoses, study finds. OHSU News. August 4, 2022. Accessed April 17, 2024. https://news.ohsu.edu/2022/08/04/covid-19-shutdowns-caused-delays-in-melanoma-diagnoses-study-finds
- Kime P. Pentagon budget calls for ‘civilianizing’ military hospitals. Military Times. Published February 10, 2020. Accessed April 17, 2024. https://www.militarytimes.com/news/your-military/2020/02/10/pentagon-budget-calls-for-civilianizing-military-hospitals/
- O’Reilly EB, Norris E, Ortiz-Pomales YT, et al. A comparison of direct care at military medical treatment facilities with purchased care in plastic surgery operative volume. Plast Reconstr Surg Glob Open. 2022;10(10 suppl):124-125. doi:10.1097/01.GOX.0000898976.03344.62
- Haag A, Hosein S, Lyon S, et al. Outcomes for arthroplasties in military health: a retrospective analysis of direct versus purchased care. Mil Med. 2023;188(suppl 6):45-51. doi:10.1093/milmed/usac441
- Eaglehouse YL, Georg MW, Richard P, et al. Cost-efficiency of breast cancer care in the US Military Health System: an economic evaluation in direct and purchased care. Mil Med. 2019;184:e494-e501. doi:10.1093/milmed/usz025
- Lurie PM. Comparing the cost of military treatment facilities with private sector care. Institute for Defense Analyses; February 2016. Accessed April 17, 2024. https://www.ida.org/research-and-publications/publications/all/c/co/comparing-the-costs-of-military-treatment-facilities-with-private-sector-care
- Defense Health Program. Fiscal Year (FY) 2024 President’s Budget: Operation and Maintenance Procurement Research, Development, Test and Evaluation. Department of Defense; March 2023. Accessed April 17, 2024. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2024/budget_justification/pdfs/09_Defense_Health_Program/00-DHP_Vols_I_II_and_III_PB24.pdf
- US Government Accountability Office. Defense Health Care. DOD should reevaluate market structure for military medical treatment facility management. Published August 21, 2023. Accessed April 17, 2024. https://www.gao.gov/products/gao-23-105441
- Rosenberg A, Cho S. We can do better at protecting our service members from skin cancer. Mil Med. 2022;187:311-313. doi:10.1093/milmed/usac198
PRACTICE POINTS
- Study data showed an overall decreasing prevalence of skin cancer in the Military Health System (MHS) from 2019 to 2021, possibly attributable to underdiagnosis resulting from the COVID-19 pandemic. Providers should be mindful of this trend when screening patients who have experienced interruptions in care.
- An overall increased prevalence of skin cancer was noted in the military beneficiary population compared with publicly available civilian data—and thus this diagnosis should be given special consideration within this population.
An Update on Cutaneous Angiosarcoma Diagnosis and Treatment
Angiosarcomas are aggressive endothelial cell tumors of vascular origin that account for 1% to 2% of all soft tissue sarcomas in the United States.1,2 They can affect any organ in the body but most commonly affect the skin and soft tissue. Cutaneous angiosarcoma (CAS) is a rare type of skin cancer that can present in 2 forms: primary and secondary.
Dermatologists may be responsible for the initial diagnosis and management of CAS. They must be familiar with its presentation, as this condition can be difficult to diagnose and mimics other diseases. Additionally, dermatologists must understand the role of varying treatment modalities including Mohs micrographic surgery (MMS) in the management of CAS. This review will provide an overview of the epidemiology, presentation, and pathologic features of CAS and will discuss both emerging and existing treatments.
Epidemiology
Cutaneous angiosarcoma may present in various locations in the body, predominantly on the head and neck.4,5 Approximately 85% of cases arise in patients older than 60 years, and most of these patients are White men.1,4,5 The risk factors for the development of CAS include prior radiation exposure; chronic lymphedema (ie, Stewart-Treves syndrome); and familial syndromes including neurofibromatosis 1, BRCA1 or BRCA2 mutations, Maffucci syndrome, and Klippel-Trenaunay syndrome. Exogenous exposure to toxins such as vinyl chloride, thorium dioxide, or anabolic steroids also is associated with angiosarcoma, primarily in the form of visceral disease such as hepatic angiosarcoma.6
The average tumor size is approximately 4 to 5 cm; however, some tumors may grow larger than 10 cm.7,8 Metastasis through hematogenous or lymphatic spread is fairly common, occurring in approximately 16% to 35% of patients. The lungs and liver are the most common sites of metastasis.9,10 The age-adjusted incidence rate of CAS is decreasing for patients younger than 50 years, from 1.30 in 1995 to 2004 to 1.10 in 2005 to 2014, but increasing for individuals older than 70 years, from 2.53 in 1995 to 2004 to 2.87 in 2005 to 2014.4 The incidence of angiosarcoma also has grown in the female population, likely due to the increasing use of radiotherapy for the treatment of breast cancer.11
The high rates of CAS on the head and neck may be explained by the increased vascularity and UV exposure in these locations.12 In a Surveillance, Epidemiology, and End Results population-based study (N=811), 43% of patients with CAS had a history of other malignancies such as breast, prostate, genitourinary, gastrointestinal tract, and respiratory tract cancers.4 Cutaneous angiosarcoma can develop secondary to the primary cancer treatment, as seen in patients who develop CAS following radiation therapy.11
The underlying mechanism of CAS is believed to involve dysregulation of angiogenesis due to the vascular origin of these tumors. Studies have identified overexpression of vascular endothelial growth factor (VEGF), TP53 mutations, and RAS pathway mutations as potential contributing factors to the pathogenesis of angiosarcoma.6 Molecular differences between primary and secondary angiosarcomas are not well documented; however, radiation-associated CAS has been found to have higher expression of LYN and PRKCΘ, while non–radiation-induced lesions express FTL1 and AKT3.2 Chromosomal abnormalities have been identified in a small set of primary CAS patients, but the specific role of these abnormalities in the pathogenesis of CAS remains unclear.7
Prognosis
Cutaneous angiosarcoma has a poor prognosis, with 3-year disease-specific survival rates as low as 40% and 5-year rates as low as 17%.4,5,13,14 Survival rates increased from 1985 to 2014, likely due to earlier diagnoses and more effective treatments.4 Several factors are associated with worse prognosis, including metastatic disease, increasing age, scalp and neck tumor location, tumor size greater than 5 cm, necrosis, multiple skin lesions, and nodular and epithelioid morphology.4,5,10,13-16 Factors including sex, race, and presence of another malignancy do not affect survival.4,5 Prognosis in CAS may be evaluated by TNM tumor staging. The American Joint Committee on Cancer Staging Manual (8th edition) for soft tissue sarcoma (STS) commonly is used; however, CAS is not included in this staging system because it does not share the same behavior and natural history as other types of STS. This staging system provides separate guidelines for STS of the head and neck and STS of the extremities and trunk because of the smaller size but paradoxically higher risk for head and neck tumors.17 Given that there is no agreed-upon staging system for CAS, prognosis and communication among providers may be complicated.
Clinical Presentation
Early CAS typically presents as single or multifocal ill-defined, enlarging, violaceous or dusky red macules or patches (Figure 1). Lesions often rapidly develop into raised nodules and plaques that may bleed and ulcerate. Other common symptoms include pain, edema, neuropathy, anemia, and weight loss; however, it is not uncommon for lesions to be asymptomatic.8,18-20 Nodular lesions are more common on the scalp, and patches are more common on the face and neck.16 Tumors typically extend into the dermis, and aggressive cancers may invade the subcutaneous tissue and fascia.2
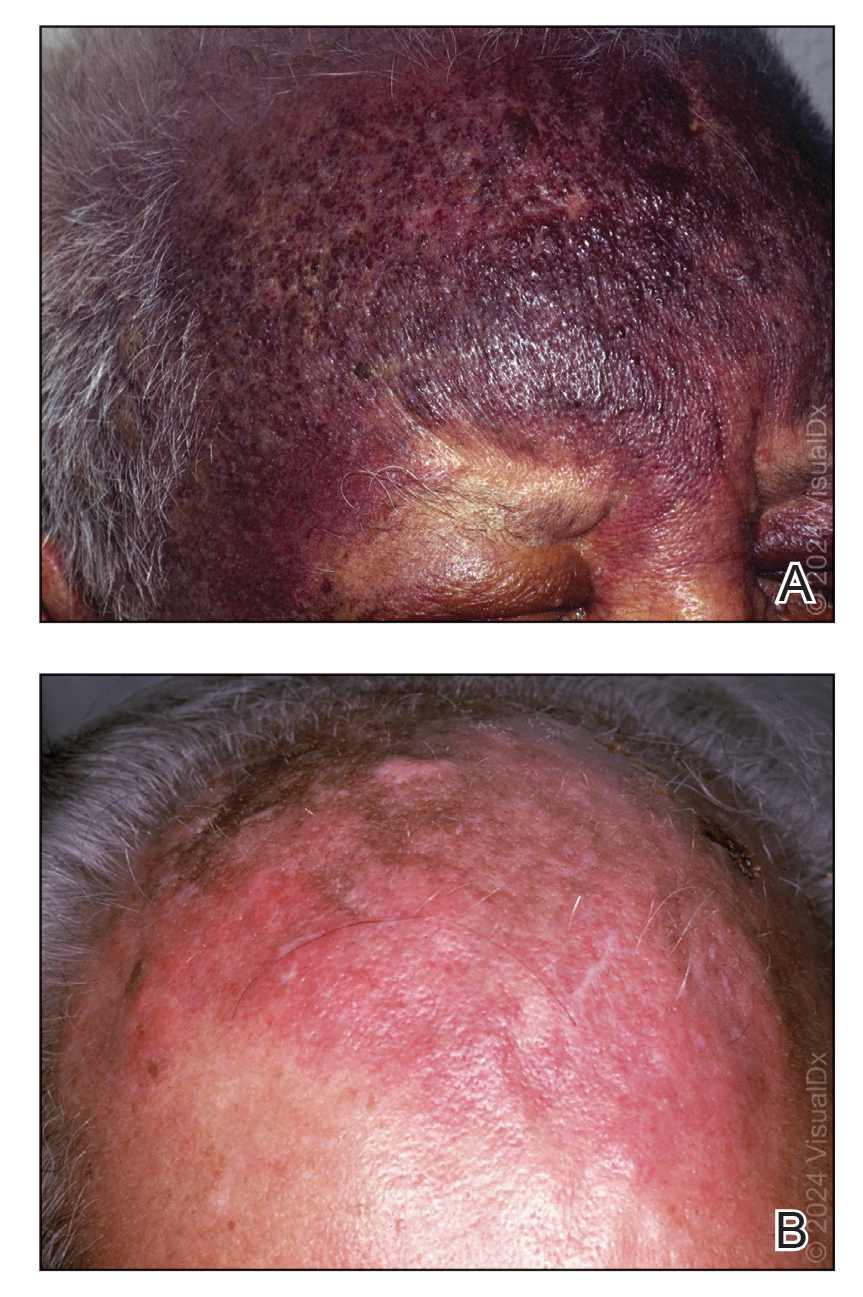
Cutaneous angiosarcoma may mimic ecchymosis, hemangioma, lymphangioma, edema, cellulitis, or scarring alopecia. Its nonspecific features make it difficult to recognize without dermoscopy or ultrasonography, which often results in delayed diagnosis and treatment. The median delay typically is 5 to 7 months and up to 1 year for some patients.7,16 Cutaneous angiosarcoma of the scalp tends to have a longer diagnostic delay than other areas of the body, which may be attributable to challenges in tumor identification and visualization by patients.16
Dermoscopy and ultrasonography can aid in the diagnosis of CAS. Dermoscopy may demonstrate a range of colors with yellow, brown, or red areas in a violaceous background. Other reported features include white veils and lines, purple ovals, pink-purple “steamlike” areas, and atypical vessels (Figure 2).21-23 Dermoscopic findings may appear similar to other vascular tumors, such as hemangioma and Kaposi sarcoma, or nonvascular tumors, including amelanotic melanoma, Merkel cell carcinoma, and primary cutaneous B-cell lymphoma. Ultrasonography may show ill-defined, hypoechoic areas with anechoic reticular channels and a hypoechoic subepidermal layer.21 Other radiologic modalities, such as computed tomography, magnetic resonance imaging, or positron emission tomography, are nonspecific and are more useful in evaluating the extent of tumor spread in visceral angiosarcoma. Magnetic resonance imaging in CAS may indicate malignancy with the presence of high T2 and T1 signal intensity and high-flow serpentine vessels.24
Histopathology
Histologically, angiosarcoma is characterized by anastomosing irregular vascular channels lined by a single layer of endothelial cells displaying slight to moderate atypia.25 These vascular channels dissect between collagen bundles and adipocytes. Monocyte infiltration may be observed.6 The neoplastic endothelial cells may present as spindle-shaped, round, polygonal, or epithelioid with eosinophilic cytoplasm. Histologic features differ based on the type of clinical lesion (Figure 3). In a study of CAS in Asian populations, nodular tumors showed solid sheets of pleomorphic spindle cells, many mitotic figures, and widely hemorrhagic spaces, whereas nonnodular tumors showed irregular vascular spaces dissecting collagen.16 Poorly differentiated tumors may present with hyperchromatic nuclei and prominent nucleoli, papillary endothelial formations, mitoses, and possible hemorrhage or necrosis.2,6,8 Histologic specimens also may reveal calcified bodies and hemosiderin particles.19 Angiosarcomas typically are invasive without a clear capsule or border.6
Secondary CAS in the setting of lymphedema and radiation therapy has MYC amplification and is positive for MYC via immunohistochemistry, which is uncommon in primary angiosarcoma.26 Immunohistochemical staining of tumor specimens is helpful to confirm the diagnosis of CAS. These markers include CD31, CD34, CD117, cytokeratin, vimentin, epithelial membrane antigen, factor VIII–related antigen, Ulex europaeus agglutinin-1, von Willebrand factor, and VEGF.6,19,27,28 Notably, advanced angiosarcomas with progressive dedifferentiation often lose these markers.
Treatment
Surgery—The majority of patients treated for CAS undergo surgical resection, as surgery has been shown to have the best prognosis for patients.5,9,10,13,15 Achieving R0 resection (microscopically negative margins) is the most important factor in determining the success of treatment, with incomplete surgical resection resulting in higher rates of systemic and local spread.29 Abraham et al8 found that the median disease-specific survival of patients with microscopically negative margins was 83.7 months; patients with microscopically positive and grossly positive margins had median disease-specific survival of 63.4 and 18.1 months, respectively. In a case series of patients undergoing resection with negative surgical margins, 4 patients demonstrated no evidence of local recurrence or systemic disease at an average of 4.3 years after therapy, and the other 4 patients each had 1 local recurrence but were disease free an average of 4.8 years after removal of the recurrent lesion. In a series of 27 patients with positive surgical margins, there was local recurrence within 2 years for most patients.12
Large tumors invading nearby structures may not be amenable to surgical resection because of extensive local growth, propensity for skip lesions, and localization near vital organs of the head and neck.5,7 The extended delay in diagnosis often seen in CAS allows for advanced local progression, resulting in large areas of resection. In a case series (N=8), the average surgical defect measured 14.3×11.8 cm, necessitating reconstruction with either a tissue flap or split-thickness skin graft in every case because primary closure was not possible. More than 80% of patients in this study still had positive margins after surgery, necessitating the use of additional chemotherapy or radiation to eradicate remaining disease.7 In several studies, multimodality therapy was associated with improved overall survival.7,14,30
Mohs Micrographic Surgery—Mohs micrographic surgery is the standard of care for many aggressive cutaneous malignancies on the head, but its utility for the treatment of CAS is uncertain. Only a few studies have compared the efficacy of MMS vs wide local excision (WLE). There have been reports of recurrence-free follow-up at 12, 16, 18, 20, and 72 months after MMS.31-36 The latter case showed a patient who underwent MMS with a 72-month relapse-free survival, whereas other patients who underwent WLE only survived 5 to 7 months without recurrence.36 In another study, there was a local recurrence rate of 42.9% after a median follow-up of 4 years in 7 patients with CAS treated with complete circumferential peripheral and deep margin assessment, which is less than the reported recurrence rates of 72% to 84% after standard excisional procedures.28,37
Houpe et al38 conducted a systematic review of the use of WLE vs MMS; the median overall survival was longest for WLE in conjunction with chemotherapy, radiotherapy, and immunotherapy at 39.3 months, followed by MMS alone at 37 months. Mohs micrographic surgery in conjunction with chemotherapy and radiotherapy was used in 1 patient, with a median overall survival of 82 months. Wide local excision alone resulted in a median overall survival of 19.8 months. Although these data are promising and suggest that the combination of surgery with adjuvant therapy may be more beneficial than surgery alone, it is important to note that there were only 9 cases treated with MMS compared with 825 cases treated with WLE.38
Several studies have documented that paraffin-embedded sections may be more useful than frozen sections in the determination of margin positivity from a surgical specimen, as frozen sections showed a poor negative predictive value of 33.3%.7,35 Mohs micrographic surgery has been proposed for tumors measuring less than 5 cm; however, the most recent appropriate use criteria for MMS of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery deemed the use of MMS for angiosarcoma uncertain.32,33,37 Further research is necessary to elucidate the role of MMS in the management of CAS.
Radiotherapy—Radiotherapy is a common adjuvant to surgical resection but has been used palliatively in patients with tumors that are unresectable. Improved local control and disease-free survival have been observed with the combination of radiation and surgery. A dose response to radiotherapy has been demonstrated,18,30 with 1 study showing that patients who received more than 5000 cGy of radiotherapy achieved better local control than patients who received 4500 cGy or less.18 Pawlik et al7 showed a decreased chance of death with the addition of adjunctive radiotherapy, and patients who underwent postoperative radiotherapy demonstrated a median survival almost 4-times longer than patients who did not receive radiation. Morrison et al39 reported that radiation therapy administered to patients with no clinically evident disease after surgical resection resulted in improved local control and overall survival vs patients who were irradiated with clinically evident disease.
Complications of radiotherapy for angiosarcoma have been reported, including xerostomia, nonfunctionally significant fibrosis, chronic ulceration/cellulitis of the scalp, necrosis requiring debridement, severe ocular complications, and fibrosis of the eyelids requiring surgical intervention.14 Radiation therapy also poses unique risks to patients with radiation-induced angiosarcoma of the breast, as many of these patients have already received the maximum recommended dose of radiation in the affected areas and additional radiation could exacerbate their CAS.
Chemotherapy—Chemotherapy occasionally is used as an adjunct to surgical resection with positive margins or as palliative care when surgical resection is not possible. Unfortunately, STSs have a response rate of less than 40% to standard chemotherapy.40 Studies in which the use of chemotherapy is evaluated for CAS have mixed results. Mark et al18 reported no significant overall survival benefit when comparing CAS treated with surgery plus radiotherapy with or without chemotherapy. Torres et al41 evaluated radiation-induced angiosarcoma of the breast and found a reduced risk for local recurrence in patients receiving chemotherapy in addition to surgery, indicating that chemotherapy may be useful in this subset of patients when radiation is not recommended.
Cytotoxic chemotherapy agents such as paclitaxel, doxorubicin, or doxorubicin in combination with mesna and ifosfamide (MAI) are common.39 Median progression-free survival is 5.4 months, 4 to 5.6 months, and 3.9 months for MAI, paclitaxel, and doxorubicin, respectively.8,9,42-46 Improved prognosis with MAI may indicate that combination chemotherapy regimens are more effective than single-agent regimens. Cutaneous angiosarcomas may respond better to paclitaxel than doxorubicin, and angiosarcomas of the scalp and face have shown a better response to paclitaxel.47,48
Other Therapies—Although there have not been large-scale studies performed on alternative treatments, there are several case reports on the use of immune modulators, biologics, β-blockers, and various other therapies in the treatment of CAS. The following studies include small sample sizes of patients with metastatic or locally aggressive disease not amenable to surgical resection, which may affect reported outcomes and survival times.49-57 In addition, several studies include patients with visceral angiosarcoma, which may not be generalizable to the CAS population. Even so, these treatment alternatives should not be overlooked because there are few agents that are truly efficacious in the treatment of CAS.
Results on the use of VEGF and tyrosine kinase inhibitors have been disappointing. There have been reports of median progression-free survival of only 3.8 months with sorafenib treatment, 3 months with pazopanib, and 6 months with bevacizumab.49-51 However, one study of patients who were treated with bevacizumab combined with radiation and surgery resulted in a complete response in 2 patients, with no evidence of residual disease at the last follow-up of 8.5 months and 2.1 years.52
Studies on the utility of β-blockers in the treatment of CAS have shown mixed results. Pasquier et al53 evaluated the use of adjunctive therapy with propranolol and vinblastine-based chemotherapy, with a promising median progression-free survival of 11 months compared with an average of 3 to 6 months with conventional chemotherapy regimens. However, in vitro studies reported by Pasquier et al53 indicated that the addition of propranolol to doxorubicin or paclitaxel did not result in increased efficacy. Chow et al54 demonstrated that propranolol monotherapy resulted in a reduction of the proliferative index of scalp angiosarcoma by 34% after only 1 week of treatment. This was followed by combination therapy of propranolol, paclitaxel, and radiation, which resulted in substantial tumor regression and no evidence of metastasis after 8 months of therapy.54
Immune checkpoint inhibitors have been a recent subject of interest in the treatment of angiosarcoma. Two case reports showed improvement in CAS of the face and primary pleural angiosarcoma with a course of pembrolizumab.55,56 In another case series, investigators used immune checkpoint inhibitors in 7 patients with cutaneous, breast, or radiation-associated angiosarcoma and found partial response in several patients treated with pembrolizumab and ipilimumab-nivolumab and complete response in 1 patient treated with anti–cytotoxic T-lymphocyte–associated protein 4 antibodies. The authors of this study hypothesized that treatment response was associated with the mutational profile of tumors, including mutational signatures of UV radiation with a large number of C-to-T substitutions similar to melanomas.57
Conclusion
Cutaneous angiosarcoma is a rare and aggressive tumor with a poor prognosis due to delayed detection. A thorough skin examination and heightened awareness of CAS by dermatologists may result in early biopsy and shortened time to a definitive diagnosis. Until quality evidence allows for the creation of consensus guidelines, care at a cancer center that specializes in rare and difficult-to-treat tumors and employs a multidisciplinary approach is essential to optimizing patient outcomes. Current knowledge supports surgery with negative margins as the mainstay of treatment, with adjuvant radiation, chemotherapy, and targeted therapies as possible additions for extensive disease. The role of MMS is uncertain, and because of the lack of contiguity in CAS, it may not be an optimal treatment.
- Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627.
- Goldblum JR, Folpe AL, Weiss SW. Enzinger & Weiss’s Soft Tissue Tumors. 7th ed. Elsevier Inc; 2020.
- Arora TK, Terracina KP, Soong J, et al. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014;3:28-34.
- Conic RRZ, Damiani G, Frigerio A, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population-based study. J Am Acad Dermatol. 2020;83:809-816.
- Chang C, Wu SP, Hu K, et al. Patterns of care and survival of cutaneous angiosarcoma of the head and neck. Otolaryngol Head Neck Surg. 2020;162:881-887.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241-247.
- Lindet C, Neuville A, Penel N, et al. Localised angiosarcomas: the identification of prognostic factors and analysis of treatment impact. a retrospective analysis from the French Sarcoma Group (GSF/GETO). Eur J Cancer. 2013;49:369-376.
- Mery CM, George S, Bertagnolli MM, et al. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer. 2009;115:4055-4063.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Dettenborn T, Wermker K, Schulze HJ, et al. Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg. 2014;42:1623-1628.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Moon IJ, Kim YJ, Won CH, et al. Clinicopathological and survival analyses of primary cutaneous angiosarcoma in an Asian population: prognostic value of the clinical features of skin lesions. Int J Dermatol. 2020;59:582-589.
- Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. a report of 67 patients and a review of the literature. Cancer. 1996;77:2400-2406.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. a review of 99 cases. Cancer. 1995;75:989-996.
- Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030-2036.
- Oranges T, Janowska A, Vitali S, et al. Dermatoscopic and ultra-high frequency ultrasound evaluation in cutaneous postradiation angiosarcoma. J Eur Acad Dermatol Venereol. 2020;34:e741.
- Figueroa-Silva O, Argenziano G, Lallas A, et al. Dermoscopic pattern of radiation-induced angiosarcoma (RIA). J Am Acad Dermatol. 2015;73:E51-E55.
- Cole DW, Huerta T, Andea A, et al. Purpuric plaques-dermoscopic and histopathological correlation of cutaneous angiosarcoma. Dermatol Pract Concept. 2020;10:E2020084. doi:10.5826/dpc.1004a84
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. Vol 2. 4th ed. Elsevier; 2018.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39. doi:10.2353/ajpath.2010.090637
- Ohsawa M, Naka N, Tomita Y, et al. Use of immunohistochemical procedures in diagnosing angiosarcoma. Evaluation of 98 cases. Cancer. 1995;75:2867-2874.
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850.
- Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16:2502-2509.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Kofler L, Breuninger H, Schulz C, et al. Local recurrence rates of skin tumors after resection with complete circumferential peripheral and deep margin assessment-identification of high-risk entities. Dermatol Surg. 2021;47:E31-E36.
- Houpe JE, Seger EW, Neill BC, et al. Treatment of angiosarcoma of the head and neck: a systematic review. Cutis. 2023;111:247-251. doi:10.12788/cutis.0767
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Gonzalez MJ, Koehler MM, Satter EK. Angiosarcoma of the scalp: a case report and review of current and novel therapeutic regimens. Dermatol Surg. 2009;35:679-684.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269-5274.
- Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012;23:517-523.
- Young RJ, Natukunda A, Litière S, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50:3178-3186.
- Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104:361-366.
- Fata F, O’Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034-2037.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012;118:3330-3336.
- Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44:2433-2436.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017;56:88-92.
- Koontz BF, Miles EF, Rubio MA, et al. Preoperative radiotherapy and bevacizumab for angiosarcoma of the head and neck: two case studies. Head Neck. 2008;30:262-266.
- Pasquier E, André N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Wang X, Wei J, Zeng Z, et al. Primary pleural epithelioid angiosarcoma treated successfully with anti-PD-1 therapy: a rare case report. Medicine (Baltimore). 2021;100:E27132.
- Sindhu S, Gimber LH, Cranmer L, et al. Angiosarcoma treated successfully with anti-PD-1 therapy—a case report. J Immunother Cancer. 2017;5:58.
- Florou V, Rosenberg AE, Wieder E, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7:213.
Angiosarcomas are aggressive endothelial cell tumors of vascular origin that account for 1% to 2% of all soft tissue sarcomas in the United States.1,2 They can affect any organ in the body but most commonly affect the skin and soft tissue. Cutaneous angiosarcoma (CAS) is a rare type of skin cancer that can present in 2 forms: primary and secondary.
Dermatologists may be responsible for the initial diagnosis and management of CAS. They must be familiar with its presentation, as this condition can be difficult to diagnose and mimics other diseases. Additionally, dermatologists must understand the role of varying treatment modalities including Mohs micrographic surgery (MMS) in the management of CAS. This review will provide an overview of the epidemiology, presentation, and pathologic features of CAS and will discuss both emerging and existing treatments.
Epidemiology
Cutaneous angiosarcoma may present in various locations in the body, predominantly on the head and neck.4,5 Approximately 85% of cases arise in patients older than 60 years, and most of these patients are White men.1,4,5 The risk factors for the development of CAS include prior radiation exposure; chronic lymphedema (ie, Stewart-Treves syndrome); and familial syndromes including neurofibromatosis 1, BRCA1 or BRCA2 mutations, Maffucci syndrome, and Klippel-Trenaunay syndrome. Exogenous exposure to toxins such as vinyl chloride, thorium dioxide, or anabolic steroids also is associated with angiosarcoma, primarily in the form of visceral disease such as hepatic angiosarcoma.6
The average tumor size is approximately 4 to 5 cm; however, some tumors may grow larger than 10 cm.7,8 Metastasis through hematogenous or lymphatic spread is fairly common, occurring in approximately 16% to 35% of patients. The lungs and liver are the most common sites of metastasis.9,10 The age-adjusted incidence rate of CAS is decreasing for patients younger than 50 years, from 1.30 in 1995 to 2004 to 1.10 in 2005 to 2014, but increasing for individuals older than 70 years, from 2.53 in 1995 to 2004 to 2.87 in 2005 to 2014.4 The incidence of angiosarcoma also has grown in the female population, likely due to the increasing use of radiotherapy for the treatment of breast cancer.11
The high rates of CAS on the head and neck may be explained by the increased vascularity and UV exposure in these locations.12 In a Surveillance, Epidemiology, and End Results population-based study (N=811), 43% of patients with CAS had a history of other malignancies such as breast, prostate, genitourinary, gastrointestinal tract, and respiratory tract cancers.4 Cutaneous angiosarcoma can develop secondary to the primary cancer treatment, as seen in patients who develop CAS following radiation therapy.11
The underlying mechanism of CAS is believed to involve dysregulation of angiogenesis due to the vascular origin of these tumors. Studies have identified overexpression of vascular endothelial growth factor (VEGF), TP53 mutations, and RAS pathway mutations as potential contributing factors to the pathogenesis of angiosarcoma.6 Molecular differences between primary and secondary angiosarcomas are not well documented; however, radiation-associated CAS has been found to have higher expression of LYN and PRKCΘ, while non–radiation-induced lesions express FTL1 and AKT3.2 Chromosomal abnormalities have been identified in a small set of primary CAS patients, but the specific role of these abnormalities in the pathogenesis of CAS remains unclear.7
Prognosis
Cutaneous angiosarcoma has a poor prognosis, with 3-year disease-specific survival rates as low as 40% and 5-year rates as low as 17%.4,5,13,14 Survival rates increased from 1985 to 2014, likely due to earlier diagnoses and more effective treatments.4 Several factors are associated with worse prognosis, including metastatic disease, increasing age, scalp and neck tumor location, tumor size greater than 5 cm, necrosis, multiple skin lesions, and nodular and epithelioid morphology.4,5,10,13-16 Factors including sex, race, and presence of another malignancy do not affect survival.4,5 Prognosis in CAS may be evaluated by TNM tumor staging. The American Joint Committee on Cancer Staging Manual (8th edition) for soft tissue sarcoma (STS) commonly is used; however, CAS is not included in this staging system because it does not share the same behavior and natural history as other types of STS. This staging system provides separate guidelines for STS of the head and neck and STS of the extremities and trunk because of the smaller size but paradoxically higher risk for head and neck tumors.17 Given that there is no agreed-upon staging system for CAS, prognosis and communication among providers may be complicated.
Clinical Presentation
Early CAS typically presents as single or multifocal ill-defined, enlarging, violaceous or dusky red macules or patches (Figure 1). Lesions often rapidly develop into raised nodules and plaques that may bleed and ulcerate. Other common symptoms include pain, edema, neuropathy, anemia, and weight loss; however, it is not uncommon for lesions to be asymptomatic.8,18-20 Nodular lesions are more common on the scalp, and patches are more common on the face and neck.16 Tumors typically extend into the dermis, and aggressive cancers may invade the subcutaneous tissue and fascia.2

Cutaneous angiosarcoma may mimic ecchymosis, hemangioma, lymphangioma, edema, cellulitis, or scarring alopecia. Its nonspecific features make it difficult to recognize without dermoscopy or ultrasonography, which often results in delayed diagnosis and treatment. The median delay typically is 5 to 7 months and up to 1 year for some patients.7,16 Cutaneous angiosarcoma of the scalp tends to have a longer diagnostic delay than other areas of the body, which may be attributable to challenges in tumor identification and visualization by patients.16
Dermoscopy and ultrasonography can aid in the diagnosis of CAS. Dermoscopy may demonstrate a range of colors with yellow, brown, or red areas in a violaceous background. Other reported features include white veils and lines, purple ovals, pink-purple “steamlike” areas, and atypical vessels (Figure 2).21-23 Dermoscopic findings may appear similar to other vascular tumors, such as hemangioma and Kaposi sarcoma, or nonvascular tumors, including amelanotic melanoma, Merkel cell carcinoma, and primary cutaneous B-cell lymphoma. Ultrasonography may show ill-defined, hypoechoic areas with anechoic reticular channels and a hypoechoic subepidermal layer.21 Other radiologic modalities, such as computed tomography, magnetic resonance imaging, or positron emission tomography, are nonspecific and are more useful in evaluating the extent of tumor spread in visceral angiosarcoma. Magnetic resonance imaging in CAS may indicate malignancy with the presence of high T2 and T1 signal intensity and high-flow serpentine vessels.24

Histopathology
Histologically, angiosarcoma is characterized by anastomosing irregular vascular channels lined by a single layer of endothelial cells displaying slight to moderate atypia.25 These vascular channels dissect between collagen bundles and adipocytes. Monocyte infiltration may be observed.6 The neoplastic endothelial cells may present as spindle-shaped, round, polygonal, or epithelioid with eosinophilic cytoplasm. Histologic features differ based on the type of clinical lesion (Figure 3). In a study of CAS in Asian populations, nodular tumors showed solid sheets of pleomorphic spindle cells, many mitotic figures, and widely hemorrhagic spaces, whereas nonnodular tumors showed irregular vascular spaces dissecting collagen.16 Poorly differentiated tumors may present with hyperchromatic nuclei and prominent nucleoli, papillary endothelial formations, mitoses, and possible hemorrhage or necrosis.2,6,8 Histologic specimens also may reveal calcified bodies and hemosiderin particles.19 Angiosarcomas typically are invasive without a clear capsule or border.6

Secondary CAS in the setting of lymphedema and radiation therapy has MYC amplification and is positive for MYC via immunohistochemistry, which is uncommon in primary angiosarcoma.26 Immunohistochemical staining of tumor specimens is helpful to confirm the diagnosis of CAS. These markers include CD31, CD34, CD117, cytokeratin, vimentin, epithelial membrane antigen, factor VIII–related antigen, Ulex europaeus agglutinin-1, von Willebrand factor, and VEGF.6,19,27,28 Notably, advanced angiosarcomas with progressive dedifferentiation often lose these markers.
Treatment
Surgery—The majority of patients treated for CAS undergo surgical resection, as surgery has been shown to have the best prognosis for patients.5,9,10,13,15 Achieving R0 resection (microscopically negative margins) is the most important factor in determining the success of treatment, with incomplete surgical resection resulting in higher rates of systemic and local spread.29 Abraham et al8 found that the median disease-specific survival of patients with microscopically negative margins was 83.7 months; patients with microscopically positive and grossly positive margins had median disease-specific survival of 63.4 and 18.1 months, respectively. In a case series of patients undergoing resection with negative surgical margins, 4 patients demonstrated no evidence of local recurrence or systemic disease at an average of 4.3 years after therapy, and the other 4 patients each had 1 local recurrence but were disease free an average of 4.8 years after removal of the recurrent lesion. In a series of 27 patients with positive surgical margins, there was local recurrence within 2 years for most patients.12
Large tumors invading nearby structures may not be amenable to surgical resection because of extensive local growth, propensity for skip lesions, and localization near vital organs of the head and neck.5,7 The extended delay in diagnosis often seen in CAS allows for advanced local progression, resulting in large areas of resection. In a case series (N=8), the average surgical defect measured 14.3×11.8 cm, necessitating reconstruction with either a tissue flap or split-thickness skin graft in every case because primary closure was not possible. More than 80% of patients in this study still had positive margins after surgery, necessitating the use of additional chemotherapy or radiation to eradicate remaining disease.7 In several studies, multimodality therapy was associated with improved overall survival.7,14,30
Mohs Micrographic Surgery—Mohs micrographic surgery is the standard of care for many aggressive cutaneous malignancies on the head, but its utility for the treatment of CAS is uncertain. Only a few studies have compared the efficacy of MMS vs wide local excision (WLE). There have been reports of recurrence-free follow-up at 12, 16, 18, 20, and 72 months after MMS.31-36 The latter case showed a patient who underwent MMS with a 72-month relapse-free survival, whereas other patients who underwent WLE only survived 5 to 7 months without recurrence.36 In another study, there was a local recurrence rate of 42.9% after a median follow-up of 4 years in 7 patients with CAS treated with complete circumferential peripheral and deep margin assessment, which is less than the reported recurrence rates of 72% to 84% after standard excisional procedures.28,37
Houpe et al38 conducted a systematic review of the use of WLE vs MMS; the median overall survival was longest for WLE in conjunction with chemotherapy, radiotherapy, and immunotherapy at 39.3 months, followed by MMS alone at 37 months. Mohs micrographic surgery in conjunction with chemotherapy and radiotherapy was used in 1 patient, with a median overall survival of 82 months. Wide local excision alone resulted in a median overall survival of 19.8 months. Although these data are promising and suggest that the combination of surgery with adjuvant therapy may be more beneficial than surgery alone, it is important to note that there were only 9 cases treated with MMS compared with 825 cases treated with WLE.38
Several studies have documented that paraffin-embedded sections may be more useful than frozen sections in the determination of margin positivity from a surgical specimen, as frozen sections showed a poor negative predictive value of 33.3%.7,35 Mohs micrographic surgery has been proposed for tumors measuring less than 5 cm; however, the most recent appropriate use criteria for MMS of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery deemed the use of MMS for angiosarcoma uncertain.32,33,37 Further research is necessary to elucidate the role of MMS in the management of CAS.
Radiotherapy—Radiotherapy is a common adjuvant to surgical resection but has been used palliatively in patients with tumors that are unresectable. Improved local control and disease-free survival have been observed with the combination of radiation and surgery. A dose response to radiotherapy has been demonstrated,18,30 with 1 study showing that patients who received more than 5000 cGy of radiotherapy achieved better local control than patients who received 4500 cGy or less.18 Pawlik et al7 showed a decreased chance of death with the addition of adjunctive radiotherapy, and patients who underwent postoperative radiotherapy demonstrated a median survival almost 4-times longer than patients who did not receive radiation. Morrison et al39 reported that radiation therapy administered to patients with no clinically evident disease after surgical resection resulted in improved local control and overall survival vs patients who were irradiated with clinically evident disease.
Complications of radiotherapy for angiosarcoma have been reported, including xerostomia, nonfunctionally significant fibrosis, chronic ulceration/cellulitis of the scalp, necrosis requiring debridement, severe ocular complications, and fibrosis of the eyelids requiring surgical intervention.14 Radiation therapy also poses unique risks to patients with radiation-induced angiosarcoma of the breast, as many of these patients have already received the maximum recommended dose of radiation in the affected areas and additional radiation could exacerbate their CAS.
Chemotherapy—Chemotherapy occasionally is used as an adjunct to surgical resection with positive margins or as palliative care when surgical resection is not possible. Unfortunately, STSs have a response rate of less than 40% to standard chemotherapy.40 Studies in which the use of chemotherapy is evaluated for CAS have mixed results. Mark et al18 reported no significant overall survival benefit when comparing CAS treated with surgery plus radiotherapy with or without chemotherapy. Torres et al41 evaluated radiation-induced angiosarcoma of the breast and found a reduced risk for local recurrence in patients receiving chemotherapy in addition to surgery, indicating that chemotherapy may be useful in this subset of patients when radiation is not recommended.
Cytotoxic chemotherapy agents such as paclitaxel, doxorubicin, or doxorubicin in combination with mesna and ifosfamide (MAI) are common.39 Median progression-free survival is 5.4 months, 4 to 5.6 months, and 3.9 months for MAI, paclitaxel, and doxorubicin, respectively.8,9,42-46 Improved prognosis with MAI may indicate that combination chemotherapy regimens are more effective than single-agent regimens. Cutaneous angiosarcomas may respond better to paclitaxel than doxorubicin, and angiosarcomas of the scalp and face have shown a better response to paclitaxel.47,48
Other Therapies—Although there have not been large-scale studies performed on alternative treatments, there are several case reports on the use of immune modulators, biologics, β-blockers, and various other therapies in the treatment of CAS. The following studies include small sample sizes of patients with metastatic or locally aggressive disease not amenable to surgical resection, which may affect reported outcomes and survival times.49-57 In addition, several studies include patients with visceral angiosarcoma, which may not be generalizable to the CAS population. Even so, these treatment alternatives should not be overlooked because there are few agents that are truly efficacious in the treatment of CAS.
Results on the use of VEGF and tyrosine kinase inhibitors have been disappointing. There have been reports of median progression-free survival of only 3.8 months with sorafenib treatment, 3 months with pazopanib, and 6 months with bevacizumab.49-51 However, one study of patients who were treated with bevacizumab combined with radiation and surgery resulted in a complete response in 2 patients, with no evidence of residual disease at the last follow-up of 8.5 months and 2.1 years.52
Studies on the utility of β-blockers in the treatment of CAS have shown mixed results. Pasquier et al53 evaluated the use of adjunctive therapy with propranolol and vinblastine-based chemotherapy, with a promising median progression-free survival of 11 months compared with an average of 3 to 6 months with conventional chemotherapy regimens. However, in vitro studies reported by Pasquier et al53 indicated that the addition of propranolol to doxorubicin or paclitaxel did not result in increased efficacy. Chow et al54 demonstrated that propranolol monotherapy resulted in a reduction of the proliferative index of scalp angiosarcoma by 34% after only 1 week of treatment. This was followed by combination therapy of propranolol, paclitaxel, and radiation, which resulted in substantial tumor regression and no evidence of metastasis after 8 months of therapy.54
Immune checkpoint inhibitors have been a recent subject of interest in the treatment of angiosarcoma. Two case reports showed improvement in CAS of the face and primary pleural angiosarcoma with a course of pembrolizumab.55,56 In another case series, investigators used immune checkpoint inhibitors in 7 patients with cutaneous, breast, or radiation-associated angiosarcoma and found partial response in several patients treated with pembrolizumab and ipilimumab-nivolumab and complete response in 1 patient treated with anti–cytotoxic T-lymphocyte–associated protein 4 antibodies. The authors of this study hypothesized that treatment response was associated with the mutational profile of tumors, including mutational signatures of UV radiation with a large number of C-to-T substitutions similar to melanomas.57
Conclusion
Cutaneous angiosarcoma is a rare and aggressive tumor with a poor prognosis due to delayed detection. A thorough skin examination and heightened awareness of CAS by dermatologists may result in early biopsy and shortened time to a definitive diagnosis. Until quality evidence allows for the creation of consensus guidelines, care at a cancer center that specializes in rare and difficult-to-treat tumors and employs a multidisciplinary approach is essential to optimizing patient outcomes. Current knowledge supports surgery with negative margins as the mainstay of treatment, with adjuvant radiation, chemotherapy, and targeted therapies as possible additions for extensive disease. The role of MMS is uncertain, and because of the lack of contiguity in CAS, it may not be an optimal treatment.
Angiosarcomas are aggressive endothelial cell tumors of vascular origin that account for 1% to 2% of all soft tissue sarcomas in the United States.1,2 They can affect any organ in the body but most commonly affect the skin and soft tissue. Cutaneous angiosarcoma (CAS) is a rare type of skin cancer that can present in 2 forms: primary and secondary.
Dermatologists may be responsible for the initial diagnosis and management of CAS. They must be familiar with its presentation, as this condition can be difficult to diagnose and mimics other diseases. Additionally, dermatologists must understand the role of varying treatment modalities including Mohs micrographic surgery (MMS) in the management of CAS. This review will provide an overview of the epidemiology, presentation, and pathologic features of CAS and will discuss both emerging and existing treatments.
Epidemiology
Cutaneous angiosarcoma may present in various locations in the body, predominantly on the head and neck.4,5 Approximately 85% of cases arise in patients older than 60 years, and most of these patients are White men.1,4,5 The risk factors for the development of CAS include prior radiation exposure; chronic lymphedema (ie, Stewart-Treves syndrome); and familial syndromes including neurofibromatosis 1, BRCA1 or BRCA2 mutations, Maffucci syndrome, and Klippel-Trenaunay syndrome. Exogenous exposure to toxins such as vinyl chloride, thorium dioxide, or anabolic steroids also is associated with angiosarcoma, primarily in the form of visceral disease such as hepatic angiosarcoma.6
The average tumor size is approximately 4 to 5 cm; however, some tumors may grow larger than 10 cm.7,8 Metastasis through hematogenous or lymphatic spread is fairly common, occurring in approximately 16% to 35% of patients. The lungs and liver are the most common sites of metastasis.9,10 The age-adjusted incidence rate of CAS is decreasing for patients younger than 50 years, from 1.30 in 1995 to 2004 to 1.10 in 2005 to 2014, but increasing for individuals older than 70 years, from 2.53 in 1995 to 2004 to 2.87 in 2005 to 2014.4 The incidence of angiosarcoma also has grown in the female population, likely due to the increasing use of radiotherapy for the treatment of breast cancer.11
The high rates of CAS on the head and neck may be explained by the increased vascularity and UV exposure in these locations.12 In a Surveillance, Epidemiology, and End Results population-based study (N=811), 43% of patients with CAS had a history of other malignancies such as breast, prostate, genitourinary, gastrointestinal tract, and respiratory tract cancers.4 Cutaneous angiosarcoma can develop secondary to the primary cancer treatment, as seen in patients who develop CAS following radiation therapy.11
The underlying mechanism of CAS is believed to involve dysregulation of angiogenesis due to the vascular origin of these tumors. Studies have identified overexpression of vascular endothelial growth factor (VEGF), TP53 mutations, and RAS pathway mutations as potential contributing factors to the pathogenesis of angiosarcoma.6 Molecular differences between primary and secondary angiosarcomas are not well documented; however, radiation-associated CAS has been found to have higher expression of LYN and PRKCΘ, while non–radiation-induced lesions express FTL1 and AKT3.2 Chromosomal abnormalities have been identified in a small set of primary CAS patients, but the specific role of these abnormalities in the pathogenesis of CAS remains unclear.7
Prognosis
Cutaneous angiosarcoma has a poor prognosis, with 3-year disease-specific survival rates as low as 40% and 5-year rates as low as 17%.4,5,13,14 Survival rates increased from 1985 to 2014, likely due to earlier diagnoses and more effective treatments.4 Several factors are associated with worse prognosis, including metastatic disease, increasing age, scalp and neck tumor location, tumor size greater than 5 cm, necrosis, multiple skin lesions, and nodular and epithelioid morphology.4,5,10,13-16 Factors including sex, race, and presence of another malignancy do not affect survival.4,5 Prognosis in CAS may be evaluated by TNM tumor staging. The American Joint Committee on Cancer Staging Manual (8th edition) for soft tissue sarcoma (STS) commonly is used; however, CAS is not included in this staging system because it does not share the same behavior and natural history as other types of STS. This staging system provides separate guidelines for STS of the head and neck and STS of the extremities and trunk because of the smaller size but paradoxically higher risk for head and neck tumors.17 Given that there is no agreed-upon staging system for CAS, prognosis and communication among providers may be complicated.
Clinical Presentation
Early CAS typically presents as single or multifocal ill-defined, enlarging, violaceous or dusky red macules or patches (Figure 1). Lesions often rapidly develop into raised nodules and plaques that may bleed and ulcerate. Other common symptoms include pain, edema, neuropathy, anemia, and weight loss; however, it is not uncommon for lesions to be asymptomatic.8,18-20 Nodular lesions are more common on the scalp, and patches are more common on the face and neck.16 Tumors typically extend into the dermis, and aggressive cancers may invade the subcutaneous tissue and fascia.2

Cutaneous angiosarcoma may mimic ecchymosis, hemangioma, lymphangioma, edema, cellulitis, or scarring alopecia. Its nonspecific features make it difficult to recognize without dermoscopy or ultrasonography, which often results in delayed diagnosis and treatment. The median delay typically is 5 to 7 months and up to 1 year for some patients.7,16 Cutaneous angiosarcoma of the scalp tends to have a longer diagnostic delay than other areas of the body, which may be attributable to challenges in tumor identification and visualization by patients.16
Dermoscopy and ultrasonography can aid in the diagnosis of CAS. Dermoscopy may demonstrate a range of colors with yellow, brown, or red areas in a violaceous background. Other reported features include white veils and lines, purple ovals, pink-purple “steamlike” areas, and atypical vessels (Figure 2).21-23 Dermoscopic findings may appear similar to other vascular tumors, such as hemangioma and Kaposi sarcoma, or nonvascular tumors, including amelanotic melanoma, Merkel cell carcinoma, and primary cutaneous B-cell lymphoma. Ultrasonography may show ill-defined, hypoechoic areas with anechoic reticular channels and a hypoechoic subepidermal layer.21 Other radiologic modalities, such as computed tomography, magnetic resonance imaging, or positron emission tomography, are nonspecific and are more useful in evaluating the extent of tumor spread in visceral angiosarcoma. Magnetic resonance imaging in CAS may indicate malignancy with the presence of high T2 and T1 signal intensity and high-flow serpentine vessels.24

Histopathology
Histologically, angiosarcoma is characterized by anastomosing irregular vascular channels lined by a single layer of endothelial cells displaying slight to moderate atypia.25 These vascular channels dissect between collagen bundles and adipocytes. Monocyte infiltration may be observed.6 The neoplastic endothelial cells may present as spindle-shaped, round, polygonal, or epithelioid with eosinophilic cytoplasm. Histologic features differ based on the type of clinical lesion (Figure 3). In a study of CAS in Asian populations, nodular tumors showed solid sheets of pleomorphic spindle cells, many mitotic figures, and widely hemorrhagic spaces, whereas nonnodular tumors showed irregular vascular spaces dissecting collagen.16 Poorly differentiated tumors may present with hyperchromatic nuclei and prominent nucleoli, papillary endothelial formations, mitoses, and possible hemorrhage or necrosis.2,6,8 Histologic specimens also may reveal calcified bodies and hemosiderin particles.19 Angiosarcomas typically are invasive without a clear capsule or border.6

Secondary CAS in the setting of lymphedema and radiation therapy has MYC amplification and is positive for MYC via immunohistochemistry, which is uncommon in primary angiosarcoma.26 Immunohistochemical staining of tumor specimens is helpful to confirm the diagnosis of CAS. These markers include CD31, CD34, CD117, cytokeratin, vimentin, epithelial membrane antigen, factor VIII–related antigen, Ulex europaeus agglutinin-1, von Willebrand factor, and VEGF.6,19,27,28 Notably, advanced angiosarcomas with progressive dedifferentiation often lose these markers.
Treatment
Surgery—The majority of patients treated for CAS undergo surgical resection, as surgery has been shown to have the best prognosis for patients.5,9,10,13,15 Achieving R0 resection (microscopically negative margins) is the most important factor in determining the success of treatment, with incomplete surgical resection resulting in higher rates of systemic and local spread.29 Abraham et al8 found that the median disease-specific survival of patients with microscopically negative margins was 83.7 months; patients with microscopically positive and grossly positive margins had median disease-specific survival of 63.4 and 18.1 months, respectively. In a case series of patients undergoing resection with negative surgical margins, 4 patients demonstrated no evidence of local recurrence or systemic disease at an average of 4.3 years after therapy, and the other 4 patients each had 1 local recurrence but were disease free an average of 4.8 years after removal of the recurrent lesion. In a series of 27 patients with positive surgical margins, there was local recurrence within 2 years for most patients.12
Large tumors invading nearby structures may not be amenable to surgical resection because of extensive local growth, propensity for skip lesions, and localization near vital organs of the head and neck.5,7 The extended delay in diagnosis often seen in CAS allows for advanced local progression, resulting in large areas of resection. In a case series (N=8), the average surgical defect measured 14.3×11.8 cm, necessitating reconstruction with either a tissue flap or split-thickness skin graft in every case because primary closure was not possible. More than 80% of patients in this study still had positive margins after surgery, necessitating the use of additional chemotherapy or radiation to eradicate remaining disease.7 In several studies, multimodality therapy was associated with improved overall survival.7,14,30
Mohs Micrographic Surgery—Mohs micrographic surgery is the standard of care for many aggressive cutaneous malignancies on the head, but its utility for the treatment of CAS is uncertain. Only a few studies have compared the efficacy of MMS vs wide local excision (WLE). There have been reports of recurrence-free follow-up at 12, 16, 18, 20, and 72 months after MMS.31-36 The latter case showed a patient who underwent MMS with a 72-month relapse-free survival, whereas other patients who underwent WLE only survived 5 to 7 months without recurrence.36 In another study, there was a local recurrence rate of 42.9% after a median follow-up of 4 years in 7 patients with CAS treated with complete circumferential peripheral and deep margin assessment, which is less than the reported recurrence rates of 72% to 84% after standard excisional procedures.28,37
Houpe et al38 conducted a systematic review of the use of WLE vs MMS; the median overall survival was longest for WLE in conjunction with chemotherapy, radiotherapy, and immunotherapy at 39.3 months, followed by MMS alone at 37 months. Mohs micrographic surgery in conjunction with chemotherapy and radiotherapy was used in 1 patient, with a median overall survival of 82 months. Wide local excision alone resulted in a median overall survival of 19.8 months. Although these data are promising and suggest that the combination of surgery with adjuvant therapy may be more beneficial than surgery alone, it is important to note that there were only 9 cases treated with MMS compared with 825 cases treated with WLE.38
Several studies have documented that paraffin-embedded sections may be more useful than frozen sections in the determination of margin positivity from a surgical specimen, as frozen sections showed a poor negative predictive value of 33.3%.7,35 Mohs micrographic surgery has been proposed for tumors measuring less than 5 cm; however, the most recent appropriate use criteria for MMS of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery deemed the use of MMS for angiosarcoma uncertain.32,33,37 Further research is necessary to elucidate the role of MMS in the management of CAS.
Radiotherapy—Radiotherapy is a common adjuvant to surgical resection but has been used palliatively in patients with tumors that are unresectable. Improved local control and disease-free survival have been observed with the combination of radiation and surgery. A dose response to radiotherapy has been demonstrated,18,30 with 1 study showing that patients who received more than 5000 cGy of radiotherapy achieved better local control than patients who received 4500 cGy or less.18 Pawlik et al7 showed a decreased chance of death with the addition of adjunctive radiotherapy, and patients who underwent postoperative radiotherapy demonstrated a median survival almost 4-times longer than patients who did not receive radiation. Morrison et al39 reported that radiation therapy administered to patients with no clinically evident disease after surgical resection resulted in improved local control and overall survival vs patients who were irradiated with clinically evident disease.
Complications of radiotherapy for angiosarcoma have been reported, including xerostomia, nonfunctionally significant fibrosis, chronic ulceration/cellulitis of the scalp, necrosis requiring debridement, severe ocular complications, and fibrosis of the eyelids requiring surgical intervention.14 Radiation therapy also poses unique risks to patients with radiation-induced angiosarcoma of the breast, as many of these patients have already received the maximum recommended dose of radiation in the affected areas and additional radiation could exacerbate their CAS.
Chemotherapy—Chemotherapy occasionally is used as an adjunct to surgical resection with positive margins or as palliative care when surgical resection is not possible. Unfortunately, STSs have a response rate of less than 40% to standard chemotherapy.40 Studies in which the use of chemotherapy is evaluated for CAS have mixed results. Mark et al18 reported no significant overall survival benefit when comparing CAS treated with surgery plus radiotherapy with or without chemotherapy. Torres et al41 evaluated radiation-induced angiosarcoma of the breast and found a reduced risk for local recurrence in patients receiving chemotherapy in addition to surgery, indicating that chemotherapy may be useful in this subset of patients when radiation is not recommended.
Cytotoxic chemotherapy agents such as paclitaxel, doxorubicin, or doxorubicin in combination with mesna and ifosfamide (MAI) are common.39 Median progression-free survival is 5.4 months, 4 to 5.6 months, and 3.9 months for MAI, paclitaxel, and doxorubicin, respectively.8,9,42-46 Improved prognosis with MAI may indicate that combination chemotherapy regimens are more effective than single-agent regimens. Cutaneous angiosarcomas may respond better to paclitaxel than doxorubicin, and angiosarcomas of the scalp and face have shown a better response to paclitaxel.47,48
Other Therapies—Although there have not been large-scale studies performed on alternative treatments, there are several case reports on the use of immune modulators, biologics, β-blockers, and various other therapies in the treatment of CAS. The following studies include small sample sizes of patients with metastatic or locally aggressive disease not amenable to surgical resection, which may affect reported outcomes and survival times.49-57 In addition, several studies include patients with visceral angiosarcoma, which may not be generalizable to the CAS population. Even so, these treatment alternatives should not be overlooked because there are few agents that are truly efficacious in the treatment of CAS.
Results on the use of VEGF and tyrosine kinase inhibitors have been disappointing. There have been reports of median progression-free survival of only 3.8 months with sorafenib treatment, 3 months with pazopanib, and 6 months with bevacizumab.49-51 However, one study of patients who were treated with bevacizumab combined with radiation and surgery resulted in a complete response in 2 patients, with no evidence of residual disease at the last follow-up of 8.5 months and 2.1 years.52
Studies on the utility of β-blockers in the treatment of CAS have shown mixed results. Pasquier et al53 evaluated the use of adjunctive therapy with propranolol and vinblastine-based chemotherapy, with a promising median progression-free survival of 11 months compared with an average of 3 to 6 months with conventional chemotherapy regimens. However, in vitro studies reported by Pasquier et al53 indicated that the addition of propranolol to doxorubicin or paclitaxel did not result in increased efficacy. Chow et al54 demonstrated that propranolol monotherapy resulted in a reduction of the proliferative index of scalp angiosarcoma by 34% after only 1 week of treatment. This was followed by combination therapy of propranolol, paclitaxel, and radiation, which resulted in substantial tumor regression and no evidence of metastasis after 8 months of therapy.54
Immune checkpoint inhibitors have been a recent subject of interest in the treatment of angiosarcoma. Two case reports showed improvement in CAS of the face and primary pleural angiosarcoma with a course of pembrolizumab.55,56 In another case series, investigators used immune checkpoint inhibitors in 7 patients with cutaneous, breast, or radiation-associated angiosarcoma and found partial response in several patients treated with pembrolizumab and ipilimumab-nivolumab and complete response in 1 patient treated with anti–cytotoxic T-lymphocyte–associated protein 4 antibodies. The authors of this study hypothesized that treatment response was associated with the mutational profile of tumors, including mutational signatures of UV radiation with a large number of C-to-T substitutions similar to melanomas.57
Conclusion
Cutaneous angiosarcoma is a rare and aggressive tumor with a poor prognosis due to delayed detection. A thorough skin examination and heightened awareness of CAS by dermatologists may result in early biopsy and shortened time to a definitive diagnosis. Until quality evidence allows for the creation of consensus guidelines, care at a cancer center that specializes in rare and difficult-to-treat tumors and employs a multidisciplinary approach is essential to optimizing patient outcomes. Current knowledge supports surgery with negative margins as the mainstay of treatment, with adjuvant radiation, chemotherapy, and targeted therapies as possible additions for extensive disease. The role of MMS is uncertain, and because of the lack of contiguity in CAS, it may not be an optimal treatment.
- Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627.
- Goldblum JR, Folpe AL, Weiss SW. Enzinger & Weiss’s Soft Tissue Tumors. 7th ed. Elsevier Inc; 2020.
- Arora TK, Terracina KP, Soong J, et al. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014;3:28-34.
- Conic RRZ, Damiani G, Frigerio A, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population-based study. J Am Acad Dermatol. 2020;83:809-816.
- Chang C, Wu SP, Hu K, et al. Patterns of care and survival of cutaneous angiosarcoma of the head and neck. Otolaryngol Head Neck Surg. 2020;162:881-887.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241-247.
- Lindet C, Neuville A, Penel N, et al. Localised angiosarcomas: the identification of prognostic factors and analysis of treatment impact. a retrospective analysis from the French Sarcoma Group (GSF/GETO). Eur J Cancer. 2013;49:369-376.
- Mery CM, George S, Bertagnolli MM, et al. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer. 2009;115:4055-4063.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Dettenborn T, Wermker K, Schulze HJ, et al. Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg. 2014;42:1623-1628.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Moon IJ, Kim YJ, Won CH, et al. Clinicopathological and survival analyses of primary cutaneous angiosarcoma in an Asian population: prognostic value of the clinical features of skin lesions. Int J Dermatol. 2020;59:582-589.
- Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. a report of 67 patients and a review of the literature. Cancer. 1996;77:2400-2406.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. a review of 99 cases. Cancer. 1995;75:989-996.
- Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030-2036.
- Oranges T, Janowska A, Vitali S, et al. Dermatoscopic and ultra-high frequency ultrasound evaluation in cutaneous postradiation angiosarcoma. J Eur Acad Dermatol Venereol. 2020;34:e741.
- Figueroa-Silva O, Argenziano G, Lallas A, et al. Dermoscopic pattern of radiation-induced angiosarcoma (RIA). J Am Acad Dermatol. 2015;73:E51-E55.
- Cole DW, Huerta T, Andea A, et al. Purpuric plaques-dermoscopic and histopathological correlation of cutaneous angiosarcoma. Dermatol Pract Concept. 2020;10:E2020084. doi:10.5826/dpc.1004a84
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. Vol 2. 4th ed. Elsevier; 2018.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39. doi:10.2353/ajpath.2010.090637
- Ohsawa M, Naka N, Tomita Y, et al. Use of immunohistochemical procedures in diagnosing angiosarcoma. Evaluation of 98 cases. Cancer. 1995;75:2867-2874.
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850.
- Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16:2502-2509.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Kofler L, Breuninger H, Schulz C, et al. Local recurrence rates of skin tumors after resection with complete circumferential peripheral and deep margin assessment-identification of high-risk entities. Dermatol Surg. 2021;47:E31-E36.
- Houpe JE, Seger EW, Neill BC, et al. Treatment of angiosarcoma of the head and neck: a systematic review. Cutis. 2023;111:247-251. doi:10.12788/cutis.0767
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Gonzalez MJ, Koehler MM, Satter EK. Angiosarcoma of the scalp: a case report and review of current and novel therapeutic regimens. Dermatol Surg. 2009;35:679-684.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269-5274.
- Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012;23:517-523.
- Young RJ, Natukunda A, Litière S, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50:3178-3186.
- Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104:361-366.
- Fata F, O’Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034-2037.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012;118:3330-3336.
- Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44:2433-2436.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017;56:88-92.
- Koontz BF, Miles EF, Rubio MA, et al. Preoperative radiotherapy and bevacizumab for angiosarcoma of the head and neck: two case studies. Head Neck. 2008;30:262-266.
- Pasquier E, André N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Wang X, Wei J, Zeng Z, et al. Primary pleural epithelioid angiosarcoma treated successfully with anti-PD-1 therapy: a rare case report. Medicine (Baltimore). 2021;100:E27132.
- Sindhu S, Gimber LH, Cranmer L, et al. Angiosarcoma treated successfully with anti-PD-1 therapy—a case report. J Immunother Cancer. 2017;5:58.
- Florou V, Rosenberg AE, Wieder E, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7:213.
- Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627.
- Goldblum JR, Folpe AL, Weiss SW. Enzinger & Weiss’s Soft Tissue Tumors. 7th ed. Elsevier Inc; 2020.
- Arora TK, Terracina KP, Soong J, et al. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014;3:28-34.
- Conic RRZ, Damiani G, Frigerio A, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population-based study. J Am Acad Dermatol. 2020;83:809-816.
- Chang C, Wu SP, Hu K, et al. Patterns of care and survival of cutaneous angiosarcoma of the head and neck. Otolaryngol Head Neck Surg. 2020;162:881-887.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241-247.
- Lindet C, Neuville A, Penel N, et al. Localised angiosarcomas: the identification of prognostic factors and analysis of treatment impact. a retrospective analysis from the French Sarcoma Group (GSF/GETO). Eur J Cancer. 2013;49:369-376.
- Mery CM, George S, Bertagnolli MM, et al. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer. 2009;115:4055-4063.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Dettenborn T, Wermker K, Schulze HJ, et al. Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg. 2014;42:1623-1628.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Moon IJ, Kim YJ, Won CH, et al. Clinicopathological and survival analyses of primary cutaneous angiosarcoma in an Asian population: prognostic value of the clinical features of skin lesions. Int J Dermatol. 2020;59:582-589.
- Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. a report of 67 patients and a review of the literature. Cancer. 1996;77:2400-2406.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. a review of 99 cases. Cancer. 1995;75:989-996.
- Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030-2036.
- Oranges T, Janowska A, Vitali S, et al. Dermatoscopic and ultra-high frequency ultrasound evaluation in cutaneous postradiation angiosarcoma. J Eur Acad Dermatol Venereol. 2020;34:e741.
- Figueroa-Silva O, Argenziano G, Lallas A, et al. Dermoscopic pattern of radiation-induced angiosarcoma (RIA). J Am Acad Dermatol. 2015;73:E51-E55.
- Cole DW, Huerta T, Andea A, et al. Purpuric plaques-dermoscopic and histopathological correlation of cutaneous angiosarcoma. Dermatol Pract Concept. 2020;10:E2020084. doi:10.5826/dpc.1004a84
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. Vol 2. 4th ed. Elsevier; 2018.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39. doi:10.2353/ajpath.2010.090637
- Ohsawa M, Naka N, Tomita Y, et al. Use of immunohistochemical procedures in diagnosing angiosarcoma. Evaluation of 98 cases. Cancer. 1995;75:2867-2874.
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850.
- Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16:2502-2509.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Kofler L, Breuninger H, Schulz C, et al. Local recurrence rates of skin tumors after resection with complete circumferential peripheral and deep margin assessment-identification of high-risk entities. Dermatol Surg. 2021;47:E31-E36.
- Houpe JE, Seger EW, Neill BC, et al. Treatment of angiosarcoma of the head and neck: a systematic review. Cutis. 2023;111:247-251. doi:10.12788/cutis.0767
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Gonzalez MJ, Koehler MM, Satter EK. Angiosarcoma of the scalp: a case report and review of current and novel therapeutic regimens. Dermatol Surg. 2009;35:679-684.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269-5274.
- Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012;23:517-523.
- Young RJ, Natukunda A, Litière S, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50:3178-3186.
- Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104:361-366.
- Fata F, O’Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034-2037.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012;118:3330-3336.
- Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44:2433-2436.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017;56:88-92.
- Koontz BF, Miles EF, Rubio MA, et al. Preoperative radiotherapy and bevacizumab for angiosarcoma of the head and neck: two case studies. Head Neck. 2008;30:262-266.
- Pasquier E, André N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Wang X, Wei J, Zeng Z, et al. Primary pleural epithelioid angiosarcoma treated successfully with anti-PD-1 therapy: a rare case report. Medicine (Baltimore). 2021;100:E27132.
- Sindhu S, Gimber LH, Cranmer L, et al. Angiosarcoma treated successfully with anti-PD-1 therapy—a case report. J Immunother Cancer. 2017;5:58.
- Florou V, Rosenberg AE, Wieder E, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7:213.
PRACTICE POINTS
- Dermatologists should be aware of challenges in diagnosing cutaneous angiosarcoma (CAS) due to its clinical similarity to benign entities such as ecchymosis and hemangioma.
- Surgery with negative margins is the first-line treatment of CAS with the best prognosis.
- Mohs micrographic surgery is useful for well-defined lesions measuring less than 5 cm on the head and neck; however, further studies are needed to determine its use in other areas.
- Paraffin-embedded sections may be more reliable than frozen sections in determining margin clearance.
Recurrence Rates of Mohs Micrographic Surgery vs Radiation Therapy for Basal Cell Carcinoma of the Ear
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. In this retrospective analysis, we evaluated recurrence rates of BCC of the ear in 102 patients who underwent treatment with Mohs micrographic surgery (MMS) or radiation therapy (RT) at a single institution between January 2017 and December 2019. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. Recurrence rates were assessed over a mean follow-up time of 2.8 years. Although MMS is the gold standard for treatment of BCC of the ear, RT may be a suitable alternative for nonsurgical candidates.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. Given that these aggressive histologic subtypes—defined as morpheaform, basosquamous, sclerosing, infiltrative, or micronodular in any portion of the tumor—have been reported as independent predictors of recurrence,1,2 BCC of the ear may be more likely to recur.
Mohs micrographic surgery (MMS) is the gold standard for the treatment of BCC of the ear. For nonsurgical candidates—those with high bleeding risk, low life expectancy, or other medical or social factors—definitive radiation therapy (RT) may be an option. Our study sought to examine recurrence rates in patients with BCC of the ear treated with MMS vs RT.
Methods
A retrospective review of patients undergoing treatment of BCC of the ear at Bighorn Mohs Surgery and Dermatology Center (San Diego, California) between January 2017 and December 2019 was conducted. A total of 507 medical records were reviewed, and 102 patients were included in the study. Inclusion criteria consisted of biopsy-confirmed BCC of the ear that was treated with MMS, RT, or both. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. This retrospective review of medical records was exempt from institutional review board approval, as it did not involve direct human research subjects, solely entailing a retrospective examination of existing data.
Results
Of the 102 patients included, 82 were male and 20 were female, with an average age of 71 years. All patients were White with the exception of 1 patient whose race was unknown. Two patients were immunocompromised. The helix was identified as the most frequently involved site on the ear (Table). Most of the tumors (56/102) exhibited aggressive histologic subtypes; 36 tumors had nonaggressive histology, and 10 had no subtype listed. Two of the BCCs demonstrated perineural invasion on biopsy. Mohs micrographic surgery was used to treat 96 BCCs, definitive RT was used to treat 5 BCCs (all of which occurred in nonsurgical candidates), and MMS and adjuvant RT were used in 1 patient given multifocal perineural involvement. All 5 patients treated with definitive RT received electron beam radiation therapy; the total dose ranged from 5100 to 6000 cGy divided into 17 to 24 fractions. The final MMS defects ranged from 6 to 55 mm in size. The average follow-up time was 2.8 years. One of the BCCs on the helix that was treated with MMS recurred after 1.3 years. The overall recurrence rate was 0.98%. None of the patients treated with definitive RT experienced recurrence after the mean follow-up time of 2.8 years.
Comment
Basal cell carcinoma is the most commonly diagnosed cancer in the United States, with approximately 2 million new cases each year.1 Treatment modalities for localized BCC include MMS, surgical excision, electrodesiccation and curettage, topical and intralesional medications, laser therapy, and RT. For high-risk BCCs, MMS is associated with the lowest recurrence rates4 and remains the gold standard for treatment. For patients with contraindications to surgery, definitive RT is an alternative treatment for high-risk BCC.1
Definitive RT can be employed for patients who are poor surgical candidates or when surgery would result in substantial morbidity, impaired function, and/or poor cosmesis.3 Radiation therapy for skin cancers of the ear commonly is administered using high-energy electrons that produce double-strand breaks in the DNA of malignant cells, leading to cell death.4 Disadvantages of RT compared to MMS include a longer treatment course (3 to 6 weeks), possible minimal long-term cosmetic sequelae (eg, color or texture mismatch), lack of pathologic confirmation of margin control, and small risk for secondary malignancy in the treatment field over 2 to 3 decades. For patients with incurable or metastatic disease, palliative RT can provide local control and/or symptomatic relief to improve quality of life.4 Adjuvant RT may be indicated if there is substantial perineural involvement or positive margins after MMS when margins are unable to be achieved or in patients who may not tolerate prolonged or extensive surgical procedures.3
Basal cell carcinoma of the ear is considered a high-risk anatomic location independent of other prognostic factors. Basal cell carcinomas of the ear have a higher propensity for more aggressive histologic subtypes and subclinical spread.5 Our study demonstrated a higher proportion of aggressive histologic subtypes (56/102 [54.9%]) compared with nonaggressive subtypes (36/102 [35.3%]). There was 1 recurrence of a nodular, sclerosing, and infiltrative BCC on the helix treated with MMS after 1.3 years.
Limitations of our study include that it was conducted at a single institution with a homogenous study population and with relatively short follow-up.
Conclusion
Our study further validates the well-known utility of MMS for the treatment of BCC of the ears. Definitive RT is a suitable alternative for patients who are not surgical candidates. Adjuvant RT may be considered for substantial perineural involvement or positive margins after MMS.3
- Lee CT, Lehrer EJ, Aphale A, et al. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: an international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582-3594.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part II: when is radiation therapy indicated? J Am Acad Dermatol. 2021;85:551-562.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part I: diagnostic modalities and applications. J Am Acad Dermatol. 2021;85:539-548.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. In this retrospective analysis, we evaluated recurrence rates of BCC of the ear in 102 patients who underwent treatment with Mohs micrographic surgery (MMS) or radiation therapy (RT) at a single institution between January 2017 and December 2019. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. Recurrence rates were assessed over a mean follow-up time of 2.8 years. Although MMS is the gold standard for treatment of BCC of the ear, RT may be a suitable alternative for nonsurgical candidates.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. Given that these aggressive histologic subtypes—defined as morpheaform, basosquamous, sclerosing, infiltrative, or micronodular in any portion of the tumor—have been reported as independent predictors of recurrence,1,2 BCC of the ear may be more likely to recur.
Mohs micrographic surgery (MMS) is the gold standard for the treatment of BCC of the ear. For nonsurgical candidates—those with high bleeding risk, low life expectancy, or other medical or social factors—definitive radiation therapy (RT) may be an option. Our study sought to examine recurrence rates in patients with BCC of the ear treated with MMS vs RT.
Methods
A retrospective review of patients undergoing treatment of BCC of the ear at Bighorn Mohs Surgery and Dermatology Center (San Diego, California) between January 2017 and December 2019 was conducted. A total of 507 medical records were reviewed, and 102 patients were included in the study. Inclusion criteria consisted of biopsy-confirmed BCC of the ear that was treated with MMS, RT, or both. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. This retrospective review of medical records was exempt from institutional review board approval, as it did not involve direct human research subjects, solely entailing a retrospective examination of existing data.
Results
Of the 102 patients included, 82 were male and 20 were female, with an average age of 71 years. All patients were White with the exception of 1 patient whose race was unknown. Two patients were immunocompromised. The helix was identified as the most frequently involved site on the ear (Table). Most of the tumors (56/102) exhibited aggressive histologic subtypes; 36 tumors had nonaggressive histology, and 10 had no subtype listed. Two of the BCCs demonstrated perineural invasion on biopsy. Mohs micrographic surgery was used to treat 96 BCCs, definitive RT was used to treat 5 BCCs (all of which occurred in nonsurgical candidates), and MMS and adjuvant RT were used in 1 patient given multifocal perineural involvement. All 5 patients treated with definitive RT received electron beam radiation therapy; the total dose ranged from 5100 to 6000 cGy divided into 17 to 24 fractions. The final MMS defects ranged from 6 to 55 mm in size. The average follow-up time was 2.8 years. One of the BCCs on the helix that was treated with MMS recurred after 1.3 years. The overall recurrence rate was 0.98%. None of the patients treated with definitive RT experienced recurrence after the mean follow-up time of 2.8 years.

Comment
Basal cell carcinoma is the most commonly diagnosed cancer in the United States, with approximately 2 million new cases each year.1 Treatment modalities for localized BCC include MMS, surgical excision, electrodesiccation and curettage, topical and intralesional medications, laser therapy, and RT. For high-risk BCCs, MMS is associated with the lowest recurrence rates4 and remains the gold standard for treatment. For patients with contraindications to surgery, definitive RT is an alternative treatment for high-risk BCC.1
Definitive RT can be employed for patients who are poor surgical candidates or when surgery would result in substantial morbidity, impaired function, and/or poor cosmesis.3 Radiation therapy for skin cancers of the ear commonly is administered using high-energy electrons that produce double-strand breaks in the DNA of malignant cells, leading to cell death.4 Disadvantages of RT compared to MMS include a longer treatment course (3 to 6 weeks), possible minimal long-term cosmetic sequelae (eg, color or texture mismatch), lack of pathologic confirmation of margin control, and small risk for secondary malignancy in the treatment field over 2 to 3 decades. For patients with incurable or metastatic disease, palliative RT can provide local control and/or symptomatic relief to improve quality of life.4 Adjuvant RT may be indicated if there is substantial perineural involvement or positive margins after MMS when margins are unable to be achieved or in patients who may not tolerate prolonged or extensive surgical procedures.3
Basal cell carcinoma of the ear is considered a high-risk anatomic location independent of other prognostic factors. Basal cell carcinomas of the ear have a higher propensity for more aggressive histologic subtypes and subclinical spread.5 Our study demonstrated a higher proportion of aggressive histologic subtypes (56/102 [54.9%]) compared with nonaggressive subtypes (36/102 [35.3%]). There was 1 recurrence of a nodular, sclerosing, and infiltrative BCC on the helix treated with MMS after 1.3 years.
Limitations of our study include that it was conducted at a single institution with a homogenous study population and with relatively short follow-up.
Conclusion
Our study further validates the well-known utility of MMS for the treatment of BCC of the ears. Definitive RT is a suitable alternative for patients who are not surgical candidates. Adjuvant RT may be considered for substantial perineural involvement or positive margins after MMS.3
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. In this retrospective analysis, we evaluated recurrence rates of BCC of the ear in 102 patients who underwent treatment with Mohs micrographic surgery (MMS) or radiation therapy (RT) at a single institution between January 2017 and December 2019. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. Recurrence rates were assessed over a mean follow-up time of 2.8 years. Although MMS is the gold standard for treatment of BCC of the ear, RT may be a suitable alternative for nonsurgical candidates.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. Given that these aggressive histologic subtypes—defined as morpheaform, basosquamous, sclerosing, infiltrative, or micronodular in any portion of the tumor—have been reported as independent predictors of recurrence,1,2 BCC of the ear may be more likely to recur.
Mohs micrographic surgery (MMS) is the gold standard for the treatment of BCC of the ear. For nonsurgical candidates—those with high bleeding risk, low life expectancy, or other medical or social factors—definitive radiation therapy (RT) may be an option. Our study sought to examine recurrence rates in patients with BCC of the ear treated with MMS vs RT.
Methods
A retrospective review of patients undergoing treatment of BCC of the ear at Bighorn Mohs Surgery and Dermatology Center (San Diego, California) between January 2017 and December 2019 was conducted. A total of 507 medical records were reviewed, and 102 patients were included in the study. Inclusion criteria consisted of biopsy-confirmed BCC of the ear that was treated with MMS, RT, or both. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. This retrospective review of medical records was exempt from institutional review board approval, as it did not involve direct human research subjects, solely entailing a retrospective examination of existing data.
Results
Of the 102 patients included, 82 were male and 20 were female, with an average age of 71 years. All patients were White with the exception of 1 patient whose race was unknown. Two patients were immunocompromised. The helix was identified as the most frequently involved site on the ear (Table). Most of the tumors (56/102) exhibited aggressive histologic subtypes; 36 tumors had nonaggressive histology, and 10 had no subtype listed. Two of the BCCs demonstrated perineural invasion on biopsy. Mohs micrographic surgery was used to treat 96 BCCs, definitive RT was used to treat 5 BCCs (all of which occurred in nonsurgical candidates), and MMS and adjuvant RT were used in 1 patient given multifocal perineural involvement. All 5 patients treated with definitive RT received electron beam radiation therapy; the total dose ranged from 5100 to 6000 cGy divided into 17 to 24 fractions. The final MMS defects ranged from 6 to 55 mm in size. The average follow-up time was 2.8 years. One of the BCCs on the helix that was treated with MMS recurred after 1.3 years. The overall recurrence rate was 0.98%. None of the patients treated with definitive RT experienced recurrence after the mean follow-up time of 2.8 years.

Comment
Basal cell carcinoma is the most commonly diagnosed cancer in the United States, with approximately 2 million new cases each year.1 Treatment modalities for localized BCC include MMS, surgical excision, electrodesiccation and curettage, topical and intralesional medications, laser therapy, and RT. For high-risk BCCs, MMS is associated with the lowest recurrence rates4 and remains the gold standard for treatment. For patients with contraindications to surgery, definitive RT is an alternative treatment for high-risk BCC.1
Definitive RT can be employed for patients who are poor surgical candidates or when surgery would result in substantial morbidity, impaired function, and/or poor cosmesis.3 Radiation therapy for skin cancers of the ear commonly is administered using high-energy electrons that produce double-strand breaks in the DNA of malignant cells, leading to cell death.4 Disadvantages of RT compared to MMS include a longer treatment course (3 to 6 weeks), possible minimal long-term cosmetic sequelae (eg, color or texture mismatch), lack of pathologic confirmation of margin control, and small risk for secondary malignancy in the treatment field over 2 to 3 decades. For patients with incurable or metastatic disease, palliative RT can provide local control and/or symptomatic relief to improve quality of life.4 Adjuvant RT may be indicated if there is substantial perineural involvement or positive margins after MMS when margins are unable to be achieved or in patients who may not tolerate prolonged or extensive surgical procedures.3
Basal cell carcinoma of the ear is considered a high-risk anatomic location independent of other prognostic factors. Basal cell carcinomas of the ear have a higher propensity for more aggressive histologic subtypes and subclinical spread.5 Our study demonstrated a higher proportion of aggressive histologic subtypes (56/102 [54.9%]) compared with nonaggressive subtypes (36/102 [35.3%]). There was 1 recurrence of a nodular, sclerosing, and infiltrative BCC on the helix treated with MMS after 1.3 years.
Limitations of our study include that it was conducted at a single institution with a homogenous study population and with relatively short follow-up.
Conclusion
Our study further validates the well-known utility of MMS for the treatment of BCC of the ears. Definitive RT is a suitable alternative for patients who are not surgical candidates. Adjuvant RT may be considered for substantial perineural involvement or positive margins after MMS.3
- Lee CT, Lehrer EJ, Aphale A, et al. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: an international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582-3594.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part II: when is radiation therapy indicated? J Am Acad Dermatol. 2021;85:551-562.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part I: diagnostic modalities and applications. J Am Acad Dermatol. 2021;85:539-548.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597.
- Lee CT, Lehrer EJ, Aphale A, et al. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: an international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582-3594.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part II: when is radiation therapy indicated? J Am Acad Dermatol. 2021;85:551-562.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part I: diagnostic modalities and applications. J Am Acad Dermatol. 2021;85:539-548.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597.
PRACTICE POINTS
- Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations, highlighting the importance of careful management and follow-up.
- Although Mohs micrographic surgery remains the gold standard for treating BCC of the ear, radiation therapy can be considered as a suitable alternative for nonsurgical candidates.
The Clinical Utility of Teledermatology in Triaging and Diagnosing Skin Malignancies: Case Series
With the increasing utilization of telemedicine since the COVID-19 pandemic, it is critical that clinicians have an appropriate understanding of the application of virtual care resources, including teledermatology. We present a case series of 3 patients to demonstrate the clinical utility of teledermatology in reducing the time to diagnosis of various rare and/or aggressive cutaneous malignancies, including Merkel cell carcinoma, malignant melanoma, and atypical fibroxanthoma. Cases were obtained from one large Midwestern medical center during the month of July 2021. Each case presented includes a description of the initial teledermatology presentation and reviews the clinical timeline from initial consultation submission to in-person clinic visit with lesion biopsy. This case series demonstrates real-world examples of how teledermatology can be utilized to expedite the care of specific vulnerable patient populations.
Teledermatology is a rapidly growing digital resource with specific utility in triaging patients to determine those requiring in-person evaluation for early and accurate detection of skin malignancies. Approximately one-third of teledermatology consultations result in face-to-face clinical encounters, with malignant neoplasms being the leading cause for biopsy.1,2 For specific populations, such as geriatric and immunocompromised patients, teledermatology may serve as a valuable tool, particularly in the wake of the COVID-19 pandemic. Furthermore, telemedicine may aid in addressing health disparities within the field of medicine and ultimately may improve access to care for vulnerable populations.3 Along with increasing access to specific subspecialty expertise, the use of teledermatology may reduce health care costs and improve the overall quality of care delivered to patients.4,5
We describe the clinical utility of teledermatology in triaging and diagnosing skin malignancies through a series of 3 cases obtained from digital image review at one large Midwestern medical center during the month of July 2021. Three unique cases with a final diagnosis of a rare or aggressive skin cancer were selected as examples, including a 75-year-old man with Merkle cell carcinoma, a 55-year-old man with aggressive pT3b malignant melanoma, and a 72-year-old man with an atypical fibroxanthoma. A clinical timeline of each case is presented, including the time intervals from initial image submission to image review, image submission to face-to-face clinical encounter, and image submission to final diagnosis. In all cases, the primary care provider submitted an order for teledermatology, and the teledermatology team obtained the images.
Case Series
Patient 1—Images of the right hand of a 75-year-old man with a medical history of basal cell carcinoma were submitted for teledermatology consultation utilizing store-and-forward image-capturing technology (day 1). The patient history provided with image submission indicated that the lesion had been present for 6 months and there were no associated symptoms. Clinical imaging demonstrated a pink-red pearly papule located on the proximal fourth digit of the dorsal aspect of the right hand (Figure 1). One day following the teledermatology request (day 2), the patient’s case was reviewed and triaged for an in-person visit. The patient was brought to clinic on day 34, and a biopsy was performed. On day 36, dermatopathology results indicated a diagnosis of Merkel cell carcinoma. On day 37, the patient was referred to surgical oncology, and on day 44, the patient underwent an initial surgical oncology visit with a plan for wide local excision of the right fourth digit with right axillary sentinel lymph node biopsy.
Patient 2—Images of the left flank of a 55-year-old man were submitted for teledermatology consultation via store-and-forward technology (day 1). A patient history provided with the image indicated that the lesion had been present for months to years and there were no associated symptoms, but the lesion recently had changed in color and size. Teledermatology images were reviewed on day 3 and demonstrated a 2- to 3-cm brown plaque on the left flank with color variegation and a prominent red papule protruding centrally (Figure 2). The patient was scheduled for an urgent in-person visit with biopsy. On day 6, the patient presented to clinic and an excision biopsy was performed. Dermatopathology was ordered with a RUSH indication, with results on day 7 revealing a pT3b malignant melanoma. An urgent consultation to surgical oncology was placed on the same day, and the patient underwent an initial surgical oncology visit on day 24 with a plan for wide local excision with left axillary and inguinal sentinel lymph node biopsy.
Patient 3—Images of the left ear of a 72-year-old man were submitted for teledermatology consultation utilizing review via store-and-forward technology (day 1). A patient history indicated that the lesion had been present for 3 months with associated bleeding. Image review demonstrated a solitary pearly pink papule located on the crura of the antihelix (Figure 3). Initial teledermatology consultation was reviewed on day 2 with notification of the need for in-person evaluation. The patient presented to clinic on day 33 for a biopsy, with dermatopathology results on day 36 consistent with an atypical fibroxanthoma. The patient was scheduled for Mohs micrographic surgery on day 37 and underwent surgical treatment on day 64.
Comment
Teledermatology consultations from all patients demonstrated adequate image quality to be able to evaluate the lesion of concern and yielded a request for in-person evaluation with possible biopsy (Table). In this case series, the average time interval from teledermatology consultation placement to teledermatology image report was 2 days (range, 1–3 days). The average time from teledermatology consultation placement to face-to-face encounter with biopsy was 24.3 days for the 3 cases presented in this series (range, 6–34 days). The initial surgical oncology visits took place an average of 34 days after the initial teledermatology consultation was placed for the 2 patients requiring referral (44 days for patient 1; 24 days for patient 2). For patient 3, Mohs micrographic surgery was required for treatment, which was scheduled by day 37 and subsequently performed on day 64.
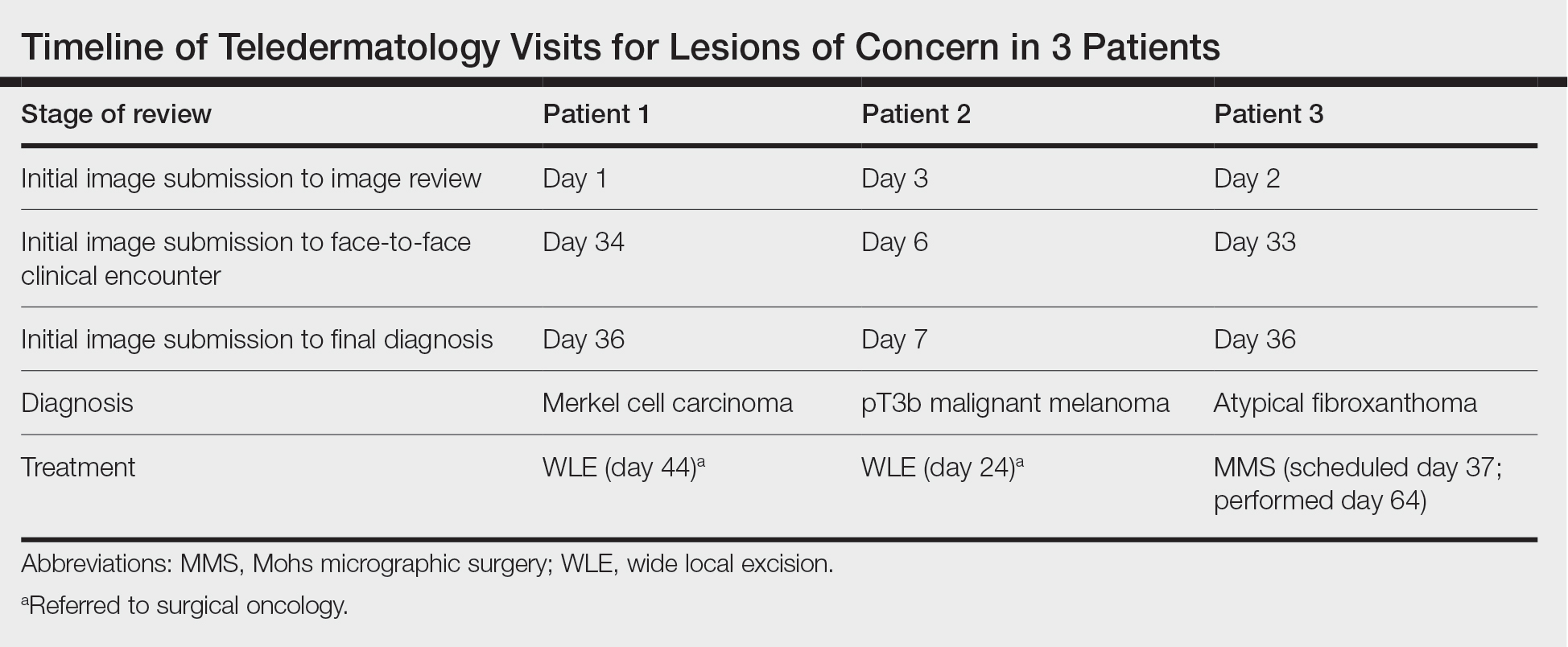
When specifically looking at the diagnosis of cutaneous malignancies, studies have found that the incidence of skin cancer detection is similar for teledermatology compared to in-person clinic visits.6,7 Creighton-Smith et al6 performed a retrospective cohort study comparing prebiopsy and postbiopsy diagnostic accuracy and detection rates of skin cancer between store-and-forward technology and face-to-face consultation. When adjusting for possible compounding factors including personal and family history of skin cancer, there was no notable difference in detection rates of any skin cancer, including melanoma and nonmelanoma skin cancers. Furthermore, the 2 cohorts of patients were found to have similar prebiopsy and postbiopsy diagnostic concordance, with similar times from consultation being placed to requested biopsy and time from biopsy to final treatment.6
Clarke et al7 similarly analyzed the accuracy of store-and-forward teledermatology and found that there was overall concordance in diagnosis when comparing clinical dermatologists to teledermatologists. Moreover, when melanocytic lesions were excluded from the study, the decision to biopsy did not differ substantially.7
Areas of further study include determining what percentage of teledermatology lesions of concern for malignancy were proven to be skin cancer after in-person evaluation and biopsy, as well as investigating the effectiveness of teledermatology for melanocytic lesions, which frequently are removed from analysis in large-scale teledermatology studies.
Although teledermatology has substantial clinical utility and may serve as a great resource for specific populations, including geriatric patients and those who are immunocompromised, it is important to recognize notable limitations. Specifically, brief history and image review should not serve as replacements for a face-to-face visit with physical examination in cases where the diagnosis remains uncertain or when high-risk skin malignancies are suspected or included in the differential. Certain aggressive cutaneous malignancies such as Merkel cell carcinoma may appear as less aggressive via teledermatology due to restrictions of technology.
Conclusion
Teledermatology has had a major impact on the way health care is delivered to patients and may increase access to care, reducing unnecessary in-person visits and decreasing the number of in-person visit no-shows. With the appropriate use of a brief clinical history and image review, teledermatology can be effective to evaluate specific lesions of concern. We report 3 unique cases identified during a 1-month period at a large Midwestern medical center. These cases serve as important examples of the application of teledermatology in reducing the time to diagnosis of aggressive skin malignancies. Further research on the clinical utility of teledermatology is warranted.
Acknowledgments—The authors thank the additional providers from the University of Wisconsin and William S. Middleton Memorial Veterans Hospital (both in Madison, Wisconsin) involved in the medical care of the patients included in this case series.
- Bianchi MG, Santos A, Cordioli E. Benefits of teledermatology for geriatric patients: population-based cross-sectional study. J Med Internet Res. 2020;22:E16700.
- Mortimer S, Rosin A. A retrospective review of incidental malignancies in veterans seen for face-to-face follow-up after teledermatology consultation. J Am Acad Dermatol. 2021;84:1130-1132.
- Costello CM, Cumsky HJL, Maly CJ, et al. Improving access to care through the establishment of a local, teledermatology network. Telemed J E Health. 2020;26:935-940. doi:10.1089/tmj.2019.0051
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260. doi:10.1007/s40257-017-0317-6
- Hadeler E, Beer J, Nouri K. The influence of teledermatology on health care access and equity. J Am Acad Dermatol. 2021;84:E219-E220. doi:10.1016/j.jaad.2020.12.036
- Creighton-Smith M, Murgia RD 3rd, Konnikov N, et al. Incidence of melanoma and keratinocytic carcinomas in patients evaluated by store-and-forward teledermatology vs dermatology clinic. Int J Dermatol. 2017;56:1026-1031. doi:10.1111/ijd.13672
- Clarke EL, Reichenberg JS, Ahmed AM, et al. The utility of teledermatology in the evaluation of skin lesions. J Telemed Telecare. 2023;29:382-389. doi:10.1177/1357633X20987423
With the increasing utilization of telemedicine since the COVID-19 pandemic, it is critical that clinicians have an appropriate understanding of the application of virtual care resources, including teledermatology. We present a case series of 3 patients to demonstrate the clinical utility of teledermatology in reducing the time to diagnosis of various rare and/or aggressive cutaneous malignancies, including Merkel cell carcinoma, malignant melanoma, and atypical fibroxanthoma. Cases were obtained from one large Midwestern medical center during the month of July 2021. Each case presented includes a description of the initial teledermatology presentation and reviews the clinical timeline from initial consultation submission to in-person clinic visit with lesion biopsy. This case series demonstrates real-world examples of how teledermatology can be utilized to expedite the care of specific vulnerable patient populations.
Teledermatology is a rapidly growing digital resource with specific utility in triaging patients to determine those requiring in-person evaluation for early and accurate detection of skin malignancies. Approximately one-third of teledermatology consultations result in face-to-face clinical encounters, with malignant neoplasms being the leading cause for biopsy.1,2 For specific populations, such as geriatric and immunocompromised patients, teledermatology may serve as a valuable tool, particularly in the wake of the COVID-19 pandemic. Furthermore, telemedicine may aid in addressing health disparities within the field of medicine and ultimately may improve access to care for vulnerable populations.3 Along with increasing access to specific subspecialty expertise, the use of teledermatology may reduce health care costs and improve the overall quality of care delivered to patients.4,5
We describe the clinical utility of teledermatology in triaging and diagnosing skin malignancies through a series of 3 cases obtained from digital image review at one large Midwestern medical center during the month of July 2021. Three unique cases with a final diagnosis of a rare or aggressive skin cancer were selected as examples, including a 75-year-old man with Merkle cell carcinoma, a 55-year-old man with aggressive pT3b malignant melanoma, and a 72-year-old man with an atypical fibroxanthoma. A clinical timeline of each case is presented, including the time intervals from initial image submission to image review, image submission to face-to-face clinical encounter, and image submission to final diagnosis. In all cases, the primary care provider submitted an order for teledermatology, and the teledermatology team obtained the images.
Case Series
Patient 1—Images of the right hand of a 75-year-old man with a medical history of basal cell carcinoma were submitted for teledermatology consultation utilizing store-and-forward image-capturing technology (day 1). The patient history provided with image submission indicated that the lesion had been present for 6 months and there were no associated symptoms. Clinical imaging demonstrated a pink-red pearly papule located on the proximal fourth digit of the dorsal aspect of the right hand (Figure 1). One day following the teledermatology request (day 2), the patient’s case was reviewed and triaged for an in-person visit. The patient was brought to clinic on day 34, and a biopsy was performed. On day 36, dermatopathology results indicated a diagnosis of Merkel cell carcinoma. On day 37, the patient was referred to surgical oncology, and on day 44, the patient underwent an initial surgical oncology visit with a plan for wide local excision of the right fourth digit with right axillary sentinel lymph node biopsy.

Patient 2—Images of the left flank of a 55-year-old man were submitted for teledermatology consultation via store-and-forward technology (day 1). A patient history provided with the image indicated that the lesion had been present for months to years and there were no associated symptoms, but the lesion recently had changed in color and size. Teledermatology images were reviewed on day 3 and demonstrated a 2- to 3-cm brown plaque on the left flank with color variegation and a prominent red papule protruding centrally (Figure 2). The patient was scheduled for an urgent in-person visit with biopsy. On day 6, the patient presented to clinic and an excision biopsy was performed. Dermatopathology was ordered with a RUSH indication, with results on day 7 revealing a pT3b malignant melanoma. An urgent consultation to surgical oncology was placed on the same day, and the patient underwent an initial surgical oncology visit on day 24 with a plan for wide local excision with left axillary and inguinal sentinel lymph node biopsy.

Patient 3—Images of the left ear of a 72-year-old man were submitted for teledermatology consultation utilizing review via store-and-forward technology (day 1). A patient history indicated that the lesion had been present for 3 months with associated bleeding. Image review demonstrated a solitary pearly pink papule located on the crura of the antihelix (Figure 3). Initial teledermatology consultation was reviewed on day 2 with notification of the need for in-person evaluation. The patient presented to clinic on day 33 for a biopsy, with dermatopathology results on day 36 consistent with an atypical fibroxanthoma. The patient was scheduled for Mohs micrographic surgery on day 37 and underwent surgical treatment on day 64.

Comment
Teledermatology consultations from all patients demonstrated adequate image quality to be able to evaluate the lesion of concern and yielded a request for in-person evaluation with possible biopsy (Table). In this case series, the average time interval from teledermatology consultation placement to teledermatology image report was 2 days (range, 1–3 days). The average time from teledermatology consultation placement to face-to-face encounter with biopsy was 24.3 days for the 3 cases presented in this series (range, 6–34 days). The initial surgical oncology visits took place an average of 34 days after the initial teledermatology consultation was placed for the 2 patients requiring referral (44 days for patient 1; 24 days for patient 2). For patient 3, Mohs micrographic surgery was required for treatment, which was scheduled by day 37 and subsequently performed on day 64.

When specifically looking at the diagnosis of cutaneous malignancies, studies have found that the incidence of skin cancer detection is similar for teledermatology compared to in-person clinic visits.6,7 Creighton-Smith et al6 performed a retrospective cohort study comparing prebiopsy and postbiopsy diagnostic accuracy and detection rates of skin cancer between store-and-forward technology and face-to-face consultation. When adjusting for possible compounding factors including personal and family history of skin cancer, there was no notable difference in detection rates of any skin cancer, including melanoma and nonmelanoma skin cancers. Furthermore, the 2 cohorts of patients were found to have similar prebiopsy and postbiopsy diagnostic concordance, with similar times from consultation being placed to requested biopsy and time from biopsy to final treatment.6
Clarke et al7 similarly analyzed the accuracy of store-and-forward teledermatology and found that there was overall concordance in diagnosis when comparing clinical dermatologists to teledermatologists. Moreover, when melanocytic lesions were excluded from the study, the decision to biopsy did not differ substantially.7
Areas of further study include determining what percentage of teledermatology lesions of concern for malignancy were proven to be skin cancer after in-person evaluation and biopsy, as well as investigating the effectiveness of teledermatology for melanocytic lesions, which frequently are removed from analysis in large-scale teledermatology studies.
Although teledermatology has substantial clinical utility and may serve as a great resource for specific populations, including geriatric patients and those who are immunocompromised, it is important to recognize notable limitations. Specifically, brief history and image review should not serve as replacements for a face-to-face visit with physical examination in cases where the diagnosis remains uncertain or when high-risk skin malignancies are suspected or included in the differential. Certain aggressive cutaneous malignancies such as Merkel cell carcinoma may appear as less aggressive via teledermatology due to restrictions of technology.
Conclusion
Teledermatology has had a major impact on the way health care is delivered to patients and may increase access to care, reducing unnecessary in-person visits and decreasing the number of in-person visit no-shows. With the appropriate use of a brief clinical history and image review, teledermatology can be effective to evaluate specific lesions of concern. We report 3 unique cases identified during a 1-month period at a large Midwestern medical center. These cases serve as important examples of the application of teledermatology in reducing the time to diagnosis of aggressive skin malignancies. Further research on the clinical utility of teledermatology is warranted.
Acknowledgments—The authors thank the additional providers from the University of Wisconsin and William S. Middleton Memorial Veterans Hospital (both in Madison, Wisconsin) involved in the medical care of the patients included in this case series.
With the increasing utilization of telemedicine since the COVID-19 pandemic, it is critical that clinicians have an appropriate understanding of the application of virtual care resources, including teledermatology. We present a case series of 3 patients to demonstrate the clinical utility of teledermatology in reducing the time to diagnosis of various rare and/or aggressive cutaneous malignancies, including Merkel cell carcinoma, malignant melanoma, and atypical fibroxanthoma. Cases were obtained from one large Midwestern medical center during the month of July 2021. Each case presented includes a description of the initial teledermatology presentation and reviews the clinical timeline from initial consultation submission to in-person clinic visit with lesion biopsy. This case series demonstrates real-world examples of how teledermatology can be utilized to expedite the care of specific vulnerable patient populations.
Teledermatology is a rapidly growing digital resource with specific utility in triaging patients to determine those requiring in-person evaluation for early and accurate detection of skin malignancies. Approximately one-third of teledermatology consultations result in face-to-face clinical encounters, with malignant neoplasms being the leading cause for biopsy.1,2 For specific populations, such as geriatric and immunocompromised patients, teledermatology may serve as a valuable tool, particularly in the wake of the COVID-19 pandemic. Furthermore, telemedicine may aid in addressing health disparities within the field of medicine and ultimately may improve access to care for vulnerable populations.3 Along with increasing access to specific subspecialty expertise, the use of teledermatology may reduce health care costs and improve the overall quality of care delivered to patients.4,5
We describe the clinical utility of teledermatology in triaging and diagnosing skin malignancies through a series of 3 cases obtained from digital image review at one large Midwestern medical center during the month of July 2021. Three unique cases with a final diagnosis of a rare or aggressive skin cancer were selected as examples, including a 75-year-old man with Merkle cell carcinoma, a 55-year-old man with aggressive pT3b malignant melanoma, and a 72-year-old man with an atypical fibroxanthoma. A clinical timeline of each case is presented, including the time intervals from initial image submission to image review, image submission to face-to-face clinical encounter, and image submission to final diagnosis. In all cases, the primary care provider submitted an order for teledermatology, and the teledermatology team obtained the images.
Case Series
Patient 1—Images of the right hand of a 75-year-old man with a medical history of basal cell carcinoma were submitted for teledermatology consultation utilizing store-and-forward image-capturing technology (day 1). The patient history provided with image submission indicated that the lesion had been present for 6 months and there were no associated symptoms. Clinical imaging demonstrated a pink-red pearly papule located on the proximal fourth digit of the dorsal aspect of the right hand (Figure 1). One day following the teledermatology request (day 2), the patient’s case was reviewed and triaged for an in-person visit. The patient was brought to clinic on day 34, and a biopsy was performed. On day 36, dermatopathology results indicated a diagnosis of Merkel cell carcinoma. On day 37, the patient was referred to surgical oncology, and on day 44, the patient underwent an initial surgical oncology visit with a plan for wide local excision of the right fourth digit with right axillary sentinel lymph node biopsy.

Patient 2—Images of the left flank of a 55-year-old man were submitted for teledermatology consultation via store-and-forward technology (day 1). A patient history provided with the image indicated that the lesion had been present for months to years and there were no associated symptoms, but the lesion recently had changed in color and size. Teledermatology images were reviewed on day 3 and demonstrated a 2- to 3-cm brown plaque on the left flank with color variegation and a prominent red papule protruding centrally (Figure 2). The patient was scheduled for an urgent in-person visit with biopsy. On day 6, the patient presented to clinic and an excision biopsy was performed. Dermatopathology was ordered with a RUSH indication, with results on day 7 revealing a pT3b malignant melanoma. An urgent consultation to surgical oncology was placed on the same day, and the patient underwent an initial surgical oncology visit on day 24 with a plan for wide local excision with left axillary and inguinal sentinel lymph node biopsy.

Patient 3—Images of the left ear of a 72-year-old man were submitted for teledermatology consultation utilizing review via store-and-forward technology (day 1). A patient history indicated that the lesion had been present for 3 months with associated bleeding. Image review demonstrated a solitary pearly pink papule located on the crura of the antihelix (Figure 3). Initial teledermatology consultation was reviewed on day 2 with notification of the need for in-person evaluation. The patient presented to clinic on day 33 for a biopsy, with dermatopathology results on day 36 consistent with an atypical fibroxanthoma. The patient was scheduled for Mohs micrographic surgery on day 37 and underwent surgical treatment on day 64.

Comment
Teledermatology consultations from all patients demonstrated adequate image quality to be able to evaluate the lesion of concern and yielded a request for in-person evaluation with possible biopsy (Table). In this case series, the average time interval from teledermatology consultation placement to teledermatology image report was 2 days (range, 1–3 days). The average time from teledermatology consultation placement to face-to-face encounter with biopsy was 24.3 days for the 3 cases presented in this series (range, 6–34 days). The initial surgical oncology visits took place an average of 34 days after the initial teledermatology consultation was placed for the 2 patients requiring referral (44 days for patient 1; 24 days for patient 2). For patient 3, Mohs micrographic surgery was required for treatment, which was scheduled by day 37 and subsequently performed on day 64.

When specifically looking at the diagnosis of cutaneous malignancies, studies have found that the incidence of skin cancer detection is similar for teledermatology compared to in-person clinic visits.6,7 Creighton-Smith et al6 performed a retrospective cohort study comparing prebiopsy and postbiopsy diagnostic accuracy and detection rates of skin cancer between store-and-forward technology and face-to-face consultation. When adjusting for possible compounding factors including personal and family history of skin cancer, there was no notable difference in detection rates of any skin cancer, including melanoma and nonmelanoma skin cancers. Furthermore, the 2 cohorts of patients were found to have similar prebiopsy and postbiopsy diagnostic concordance, with similar times from consultation being placed to requested biopsy and time from biopsy to final treatment.6
Clarke et al7 similarly analyzed the accuracy of store-and-forward teledermatology and found that there was overall concordance in diagnosis when comparing clinical dermatologists to teledermatologists. Moreover, when melanocytic lesions were excluded from the study, the decision to biopsy did not differ substantially.7
Areas of further study include determining what percentage of teledermatology lesions of concern for malignancy were proven to be skin cancer after in-person evaluation and biopsy, as well as investigating the effectiveness of teledermatology for melanocytic lesions, which frequently are removed from analysis in large-scale teledermatology studies.
Although teledermatology has substantial clinical utility and may serve as a great resource for specific populations, including geriatric patients and those who are immunocompromised, it is important to recognize notable limitations. Specifically, brief history and image review should not serve as replacements for a face-to-face visit with physical examination in cases where the diagnosis remains uncertain or when high-risk skin malignancies are suspected or included in the differential. Certain aggressive cutaneous malignancies such as Merkel cell carcinoma may appear as less aggressive via teledermatology due to restrictions of technology.
Conclusion
Teledermatology has had a major impact on the way health care is delivered to patients and may increase access to care, reducing unnecessary in-person visits and decreasing the number of in-person visit no-shows. With the appropriate use of a brief clinical history and image review, teledermatology can be effective to evaluate specific lesions of concern. We report 3 unique cases identified during a 1-month period at a large Midwestern medical center. These cases serve as important examples of the application of teledermatology in reducing the time to diagnosis of aggressive skin malignancies. Further research on the clinical utility of teledermatology is warranted.
Acknowledgments—The authors thank the additional providers from the University of Wisconsin and William S. Middleton Memorial Veterans Hospital (both in Madison, Wisconsin) involved in the medical care of the patients included in this case series.
- Bianchi MG, Santos A, Cordioli E. Benefits of teledermatology for geriatric patients: population-based cross-sectional study. J Med Internet Res. 2020;22:E16700.
- Mortimer S, Rosin A. A retrospective review of incidental malignancies in veterans seen for face-to-face follow-up after teledermatology consultation. J Am Acad Dermatol. 2021;84:1130-1132.
- Costello CM, Cumsky HJL, Maly CJ, et al. Improving access to care through the establishment of a local, teledermatology network. Telemed J E Health. 2020;26:935-940. doi:10.1089/tmj.2019.0051
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260. doi:10.1007/s40257-017-0317-6
- Hadeler E, Beer J, Nouri K. The influence of teledermatology on health care access and equity. J Am Acad Dermatol. 2021;84:E219-E220. doi:10.1016/j.jaad.2020.12.036
- Creighton-Smith M, Murgia RD 3rd, Konnikov N, et al. Incidence of melanoma and keratinocytic carcinomas in patients evaluated by store-and-forward teledermatology vs dermatology clinic. Int J Dermatol. 2017;56:1026-1031. doi:10.1111/ijd.13672
- Clarke EL, Reichenberg JS, Ahmed AM, et al. The utility of teledermatology in the evaluation of skin lesions. J Telemed Telecare. 2023;29:382-389. doi:10.1177/1357633X20987423
- Bianchi MG, Santos A, Cordioli E. Benefits of teledermatology for geriatric patients: population-based cross-sectional study. J Med Internet Res. 2020;22:E16700.
- Mortimer S, Rosin A. A retrospective review of incidental malignancies in veterans seen for face-to-face follow-up after teledermatology consultation. J Am Acad Dermatol. 2021;84:1130-1132.
- Costello CM, Cumsky HJL, Maly CJ, et al. Improving access to care through the establishment of a local, teledermatology network. Telemed J E Health. 2020;26:935-940. doi:10.1089/tmj.2019.0051
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260. doi:10.1007/s40257-017-0317-6
- Hadeler E, Beer J, Nouri K. The influence of teledermatology on health care access and equity. J Am Acad Dermatol. 2021;84:E219-E220. doi:10.1016/j.jaad.2020.12.036
- Creighton-Smith M, Murgia RD 3rd, Konnikov N, et al. Incidence of melanoma and keratinocytic carcinomas in patients evaluated by store-and-forward teledermatology vs dermatology clinic. Int J Dermatol. 2017;56:1026-1031. doi:10.1111/ijd.13672
- Clarke EL, Reichenberg JS, Ahmed AM, et al. The utility of teledermatology in the evaluation of skin lesions. J Telemed Telecare. 2023;29:382-389. doi:10.1177/1357633X20987423
Practice Points
- Teledermatology via store-and-forward technology has been demonstrated to be effective in assessing and triaging various cutaneous malignancies.
- The use of teledermatology has increased because of the COVID-19 pandemic and may be useful for specific vulnerable populations.
- When used appropriately, teledermatology may function as a useful resource to triage patients requiring in-person evaluation for the diagnosis of aggressive skin malignancies and may aid in reducing the time to diagnosis of various skin cancers.
Sunscreen Safety: 2024 Updates
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
Clinical Manifestation of Degos Disease: Painful Penile Ulcers
To the Editor:
A 56-year-old man was referred to our Grand Rounds by another dermatologist in our health system for evaluation of a red scaly rash on the trunk that had been present for more than a year. More recently, over the course of approximately 9 months he experienced recurrent painful penile ulcers that lasted for approximately 4 weeks and then self-resolved. He had a medical history of central retinal vein occlusion, primary hyperparathyroidism, and nonspecific colitis. A family history was notable for lung cancer in the patient’s father and myelodysplastic syndrome and breast cancer in his mother; however, there was no family history of a similar rash. A bacterial culture of the penile ulcer was negative. Testing for antibodies against HIV and herpes simplex virus (HSV) types 1 and 2 was negative. Results of a serum VDRL test were nonreactive, which ruled out syphilis. The patient was treated by the referring dermatologist with azithromycin for possible chancroid without relief.
The patient was being followed by the referring dermatologist who initially was concerned for Degos disease based on clinical examination findings, prompting biopsy of a lesion on the back, which revealed vacuolar interface dermatitis, a sparse superficial perivascular lymphocytic infiltrate, and increased mucin—all highly suspicious for connective tissue disease (Figure 1). An antinuclear antibody test was positive, with a titer of 1:640. The patient was started on prednisone and referred to rheumatology; however, further evaluation by rheumatology for an autoimmune process—including anticardiolipin antibodies—was unremarkable. A few months prior to the current presentation, he also had mildly elevated liver function test results. A colonoscopy was performed, and a biopsy revealed nonspecific colitis. A biopsy of the penile ulcer also was nonspecific, showing only ulceration and acute and chronic inflammation. No epidermal interface change was seen. Results from a Grocott-Gomori methenamine-silver stain, Treponema pallidum immunostain, and HSV polymerase chain reaction were negative for fungal organisms, spirochetes, and HSV, respectively. The differential diagnosis included trauma, aphthous ulceration, and Behçet disease. Behçet disease was suspected by the referring dermatologist, and the patient was treated with colchicine, prednisone, pimecrolimus cream, and topical lidocaine; however, the lesions persisted, and he was subsequently referred to our Grand Rounds for further evaluation.
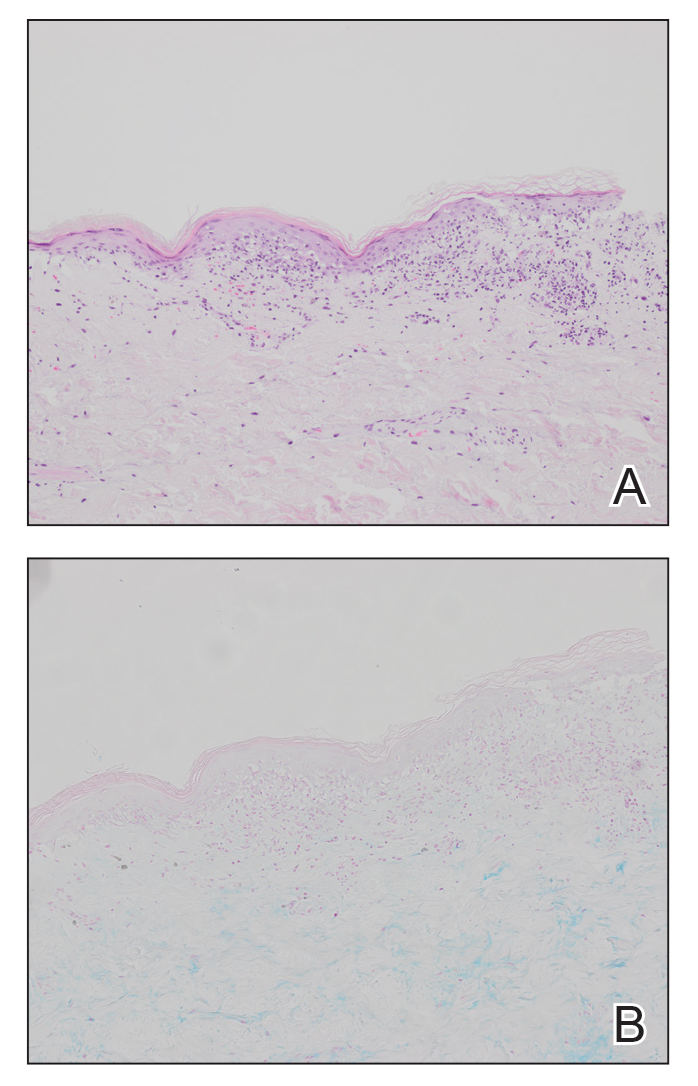
At the current presentation, physical examination revealed several small papules with white atrophic centers and erythematous rims on the trunk and extremities (Figure 2A). An ulceration was noted on the penile shaft (Figure 2B). Further evaluation for Behçet disease, including testing for pathergy and HLA-B51, was negative. Degos disease was strongly suspected clinically, and a repeat biopsy was performed of a lesion on the abdomen, which revealed central epidermal necrosis, atrophy, and parakeratosis with an underlying wedge-shaped dermal infarct surrounded by multiple small occluded dermal vessels, perivascular inflammation, and dermal edema (Figure 3). Direct immunofluorescence was performed using antibodies against IgG, IgA, IgM, fibrinogen, albumin, and C3, which was negative. These findings from direct immunofluorescence and histopathology as well as the clinical presentation were considered compatible with Degos disease. The patient was started on aspirin and pentoxifylline. Pentoxifylline 400 mg twice daily appeared to lessen some of the pain. Pain management specialists started the patient on gabapentin.

Approximately 4 months after the Grand Rounds evaluation, during which time he continued treatment with pentoxifylline, he was admitted to the hospital for intractable nausea and vomiting. His condition acutely declined due to bowel perforation, and he was started on eculizumab 1200 mg every 14 days. Because of an increased risk for meningococcal meningitis while on this medication, he also was given erythromycin 500 mg twice daily prophylactically. He was being followed by hematology for the vasculopathy, and they were planning to monitor for any disease changes with computed tomography of the chest, abdomen, and pelvis every 3 months, as well as echocardiogram every 6 months for any development of pericardial or pleural fibrosis. Approximately 1 month later, the patient was admitted to the hospital again but died after 1 week from gastrointestinal complications (approximately 22 months after the onset of the rash).
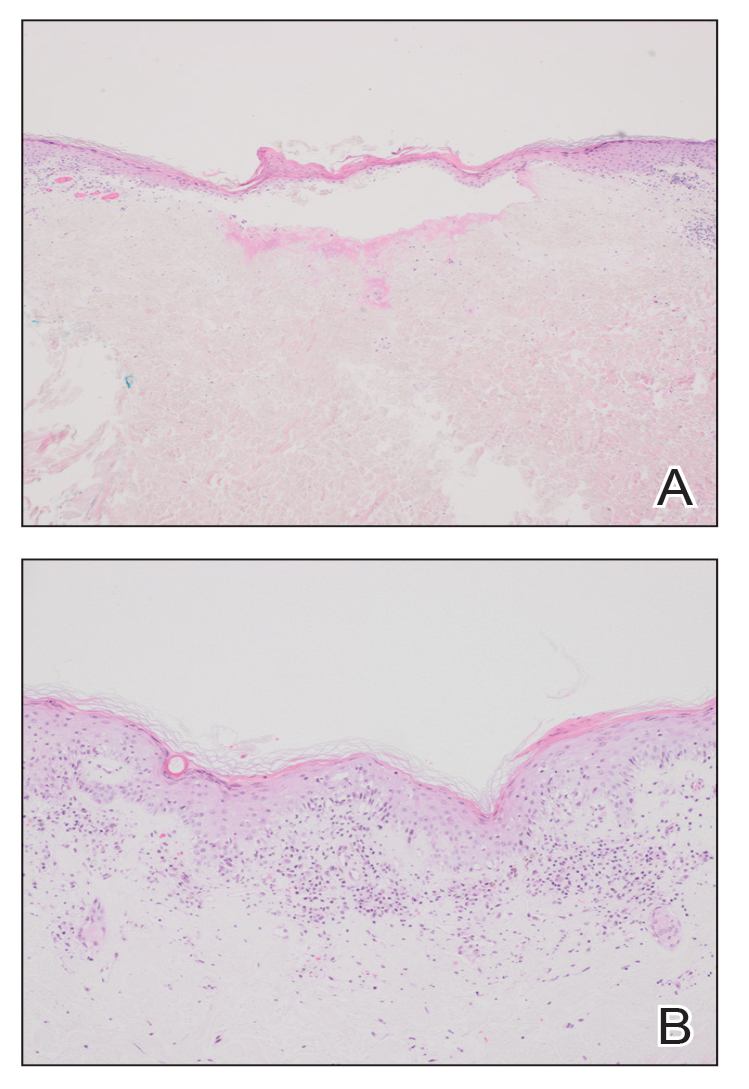
Degos disease (atrophic papulosis) is a rare small vessel vasculopathy of unknown etiology, but complement-mediated endothelial injury plays a role.1,2 It typically occurs in the fourth decade of life, with a slight female predominance.3,4 The skin lesions are characteristic and described as 5- to 10-mm papules with atrophic white centers and erythematous telangiectatic rims, most commonly on the upper body and typically sparing the head, palms, and soles.1 Penile ulceration is an uncommon cutaneous feature, with only a few cases reported in the literature.5,6 Approximately one-third of patients will have only skin lesions, but two-thirds will develop systemic involvement 1 to 2 years after onset, with the gastrointestinal tract and central nervous system most commonly involved. For those with systemic involvement, the 5-year survival rate is approximately 55%, and the most common causes of death are bowel perforation, peritonitis, and stroke.3,4 Because some patients appear to never develop systemic complications, Theodoridis et al4 proposed that the disease be classified as either malignant atrophic papulosis or benign atrophic papulosis to indicate the malignant systemic form and the benign cutaneous form, respectively.
The histopathology of Degos disease changes as the lesions evolve.7 Early lesions show a superficial and deep perivascular and periadnexal lymphocytic infiltrate, possible interface dermatitis, and dermal mucin resembling lupus. The more fully developed lesions show a greater degree of inflammation and interface change as well as lymphocytic vasculitis. This stage also may have epidermal atrophy and early papillary dermal sclerosis resembling lichen sclerosus. The late-stage lesions, clinically observed as papules with atrophic white centers and surrounding erythema, show the classic pathology of wedge-shaped dermal sclerosis and central epidermal atrophy with surrounding hyperkeratosis. Interface dermatitis and dermal mucin can be seen in all stages, though mucin is diminished in the later stage.
Effective treatment options are limited; however, antithrombotics or compounds that facilitate blood perfusion, such as aspirin or pentoxifylline, initially can be used.1 Eculizumab, a humanized monoclonal antibody that prevents the cleavage of C5, has been used for salvage therapy,8 as in our case. Treprostinil, a prostacyclin analog that causes arterial vasodilation and inhibition of platelet aggregation, has been reported to improve bowel and cutaneous lesions, functional status, and neurologic symptoms.9
Our case highlights important features of Degos disease. First, it is important for both the clinician and the pathologist to recognize that the histopathology of Degos disease changes as the lesions evolve. In our case, although the lesions were characteristic of Degos disease clinically, the initial biopsy was suspicious for connective tissue disease, which led to an autoimmune evaluation that ultimately was unremarkable. Recognizing that early lesions of Degos disease can resemble connective tissue disease histologically could have prevented this delay in diagnosis. However, Degos disease has been reported in association with autoimmune diseases.10 Second, although penile ulceration is uncommon, it can be a prominent cutaneous manifestation of the disease. Finally, eculizumab and treprostinil are therapeutic options that have shown some efficacy in improving symptoms and cutaneous lesions.8,9
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)—a review. Orphanet J Rare Dis. 2013;8:10. doi:10.1186/1750-1172-8-10
- Magro CM, Poe JC, Kim C, et al. Degos disease: a C5b-9/interferon-α-mediated endotheliopathy syndrome. Am J Clin Pathol. 2011;135:599-610. doi:10.1309/AJCP66QIMFARLZKI
- Hu P, Mao Z, Liu C, et al. Malignant atrophic papulosis with motor aphasia and intestinal perforation: a case report and review of published works. J Dermatol. 2018;45:723-726. doi:10.1111/1346-8138.14280
- Theodoridis A, Konstantinidou A, Makrantonaki E, et al. Malignant and benign forms of atrophic papulosis (Köhlmeier-Degos disease): systemic involvement determines the prognosis. Br J Dermatol. 2014;170:110-115. doi:10.1111/bjd.12642
- Thomson KF, Highet AS. Penile ulceration in fatal malignant atrophic papulosis (Degos’ disease). Br J Dermatol. 2000;143:1320-1322. doi:10.1046/j.1365-2133.2000.03911.x
- Aydogan K, Alkan G, Karadogan Koran S, et al. Painful penile ulceration in a patient with malignant atrophic papulosis. J Eur Acad Dermatol Venereol. 2005;19:612-616. doi:10.1111/j.1468-3083.2005.01227.x
- Harvell JD, Williford PL, White WL. Benign cutaneous Degos’ disease: a case report with emphasis on histopathology as papules chronologically evolve. Am J Dermatopathol. 2001;23:116-123. doi:10.1097/00000372-200104000-00006
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277. doi:10.1016/j.jaad.2016.09.015
- Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil—early experience. Orphanet J Rare Dis. 2013;8:52. doi:10.1186/1750-1172-8-52
- Burgin S, Stone JH, Shenoy-Bhangle AS, et al. Case records of the Massachusetts General Hospital. Case 18-2014. A 32-year-old man with a rash, myalgia, and weakness. N Engl J Med. 2014;370:2327-2337. doi:10.1056/NEJMcpc1304161
To the Editor:
A 56-year-old man was referred to our Grand Rounds by another dermatologist in our health system for evaluation of a red scaly rash on the trunk that had been present for more than a year. More recently, over the course of approximately 9 months he experienced recurrent painful penile ulcers that lasted for approximately 4 weeks and then self-resolved. He had a medical history of central retinal vein occlusion, primary hyperparathyroidism, and nonspecific colitis. A family history was notable for lung cancer in the patient’s father and myelodysplastic syndrome and breast cancer in his mother; however, there was no family history of a similar rash. A bacterial culture of the penile ulcer was negative. Testing for antibodies against HIV and herpes simplex virus (HSV) types 1 and 2 was negative. Results of a serum VDRL test were nonreactive, which ruled out syphilis. The patient was treated by the referring dermatologist with azithromycin for possible chancroid without relief.
The patient was being followed by the referring dermatologist who initially was concerned for Degos disease based on clinical examination findings, prompting biopsy of a lesion on the back, which revealed vacuolar interface dermatitis, a sparse superficial perivascular lymphocytic infiltrate, and increased mucin—all highly suspicious for connective tissue disease (Figure 1). An antinuclear antibody test was positive, with a titer of 1:640. The patient was started on prednisone and referred to rheumatology; however, further evaluation by rheumatology for an autoimmune process—including anticardiolipin antibodies—was unremarkable. A few months prior to the current presentation, he also had mildly elevated liver function test results. A colonoscopy was performed, and a biopsy revealed nonspecific colitis. A biopsy of the penile ulcer also was nonspecific, showing only ulceration and acute and chronic inflammation. No epidermal interface change was seen. Results from a Grocott-Gomori methenamine-silver stain, Treponema pallidum immunostain, and HSV polymerase chain reaction were negative for fungal organisms, spirochetes, and HSV, respectively. The differential diagnosis included trauma, aphthous ulceration, and Behçet disease. Behçet disease was suspected by the referring dermatologist, and the patient was treated with colchicine, prednisone, pimecrolimus cream, and topical lidocaine; however, the lesions persisted, and he was subsequently referred to our Grand Rounds for further evaluation.

At the current presentation, physical examination revealed several small papules with white atrophic centers and erythematous rims on the trunk and extremities (Figure 2A). An ulceration was noted on the penile shaft (Figure 2B). Further evaluation for Behçet disease, including testing for pathergy and HLA-B51, was negative. Degos disease was strongly suspected clinically, and a repeat biopsy was performed of a lesion on the abdomen, which revealed central epidermal necrosis, atrophy, and parakeratosis with an underlying wedge-shaped dermal infarct surrounded by multiple small occluded dermal vessels, perivascular inflammation, and dermal edema (Figure 3). Direct immunofluorescence was performed using antibodies against IgG, IgA, IgM, fibrinogen, albumin, and C3, which was negative. These findings from direct immunofluorescence and histopathology as well as the clinical presentation were considered compatible with Degos disease. The patient was started on aspirin and pentoxifylline. Pentoxifylline 400 mg twice daily appeared to lessen some of the pain. Pain management specialists started the patient on gabapentin.

Approximately 4 months after the Grand Rounds evaluation, during which time he continued treatment with pentoxifylline, he was admitted to the hospital for intractable nausea and vomiting. His condition acutely declined due to bowel perforation, and he was started on eculizumab 1200 mg every 14 days. Because of an increased risk for meningococcal meningitis while on this medication, he also was given erythromycin 500 mg twice daily prophylactically. He was being followed by hematology for the vasculopathy, and they were planning to monitor for any disease changes with computed tomography of the chest, abdomen, and pelvis every 3 months, as well as echocardiogram every 6 months for any development of pericardial or pleural fibrosis. Approximately 1 month later, the patient was admitted to the hospital again but died after 1 week from gastrointestinal complications (approximately 22 months after the onset of the rash).

Degos disease (atrophic papulosis) is a rare small vessel vasculopathy of unknown etiology, but complement-mediated endothelial injury plays a role.1,2 It typically occurs in the fourth decade of life, with a slight female predominance.3,4 The skin lesions are characteristic and described as 5- to 10-mm papules with atrophic white centers and erythematous telangiectatic rims, most commonly on the upper body and typically sparing the head, palms, and soles.1 Penile ulceration is an uncommon cutaneous feature, with only a few cases reported in the literature.5,6 Approximately one-third of patients will have only skin lesions, but two-thirds will develop systemic involvement 1 to 2 years after onset, with the gastrointestinal tract and central nervous system most commonly involved. For those with systemic involvement, the 5-year survival rate is approximately 55%, and the most common causes of death are bowel perforation, peritonitis, and stroke.3,4 Because some patients appear to never develop systemic complications, Theodoridis et al4 proposed that the disease be classified as either malignant atrophic papulosis or benign atrophic papulosis to indicate the malignant systemic form and the benign cutaneous form, respectively.
The histopathology of Degos disease changes as the lesions evolve.7 Early lesions show a superficial and deep perivascular and periadnexal lymphocytic infiltrate, possible interface dermatitis, and dermal mucin resembling lupus. The more fully developed lesions show a greater degree of inflammation and interface change as well as lymphocytic vasculitis. This stage also may have epidermal atrophy and early papillary dermal sclerosis resembling lichen sclerosus. The late-stage lesions, clinically observed as papules with atrophic white centers and surrounding erythema, show the classic pathology of wedge-shaped dermal sclerosis and central epidermal atrophy with surrounding hyperkeratosis. Interface dermatitis and dermal mucin can be seen in all stages, though mucin is diminished in the later stage.
Effective treatment options are limited; however, antithrombotics or compounds that facilitate blood perfusion, such as aspirin or pentoxifylline, initially can be used.1 Eculizumab, a humanized monoclonal antibody that prevents the cleavage of C5, has been used for salvage therapy,8 as in our case. Treprostinil, a prostacyclin analog that causes arterial vasodilation and inhibition of platelet aggregation, has been reported to improve bowel and cutaneous lesions, functional status, and neurologic symptoms.9
Our case highlights important features of Degos disease. First, it is important for both the clinician and the pathologist to recognize that the histopathology of Degos disease changes as the lesions evolve. In our case, although the lesions were characteristic of Degos disease clinically, the initial biopsy was suspicious for connective tissue disease, which led to an autoimmune evaluation that ultimately was unremarkable. Recognizing that early lesions of Degos disease can resemble connective tissue disease histologically could have prevented this delay in diagnosis. However, Degos disease has been reported in association with autoimmune diseases.10 Second, although penile ulceration is uncommon, it can be a prominent cutaneous manifestation of the disease. Finally, eculizumab and treprostinil are therapeutic options that have shown some efficacy in improving symptoms and cutaneous lesions.8,9
To the Editor:
A 56-year-old man was referred to our Grand Rounds by another dermatologist in our health system for evaluation of a red scaly rash on the trunk that had been present for more than a year. More recently, over the course of approximately 9 months he experienced recurrent painful penile ulcers that lasted for approximately 4 weeks and then self-resolved. He had a medical history of central retinal vein occlusion, primary hyperparathyroidism, and nonspecific colitis. A family history was notable for lung cancer in the patient’s father and myelodysplastic syndrome and breast cancer in his mother; however, there was no family history of a similar rash. A bacterial culture of the penile ulcer was negative. Testing for antibodies against HIV and herpes simplex virus (HSV) types 1 and 2 was negative. Results of a serum VDRL test were nonreactive, which ruled out syphilis. The patient was treated by the referring dermatologist with azithromycin for possible chancroid without relief.
The patient was being followed by the referring dermatologist who initially was concerned for Degos disease based on clinical examination findings, prompting biopsy of a lesion on the back, which revealed vacuolar interface dermatitis, a sparse superficial perivascular lymphocytic infiltrate, and increased mucin—all highly suspicious for connective tissue disease (Figure 1). An antinuclear antibody test was positive, with a titer of 1:640. The patient was started on prednisone and referred to rheumatology; however, further evaluation by rheumatology for an autoimmune process—including anticardiolipin antibodies—was unremarkable. A few months prior to the current presentation, he also had mildly elevated liver function test results. A colonoscopy was performed, and a biopsy revealed nonspecific colitis. A biopsy of the penile ulcer also was nonspecific, showing only ulceration and acute and chronic inflammation. No epidermal interface change was seen. Results from a Grocott-Gomori methenamine-silver stain, Treponema pallidum immunostain, and HSV polymerase chain reaction were negative for fungal organisms, spirochetes, and HSV, respectively. The differential diagnosis included trauma, aphthous ulceration, and Behçet disease. Behçet disease was suspected by the referring dermatologist, and the patient was treated with colchicine, prednisone, pimecrolimus cream, and topical lidocaine; however, the lesions persisted, and he was subsequently referred to our Grand Rounds for further evaluation.

At the current presentation, physical examination revealed several small papules with white atrophic centers and erythematous rims on the trunk and extremities (Figure 2A). An ulceration was noted on the penile shaft (Figure 2B). Further evaluation for Behçet disease, including testing for pathergy and HLA-B51, was negative. Degos disease was strongly suspected clinically, and a repeat biopsy was performed of a lesion on the abdomen, which revealed central epidermal necrosis, atrophy, and parakeratosis with an underlying wedge-shaped dermal infarct surrounded by multiple small occluded dermal vessels, perivascular inflammation, and dermal edema (Figure 3). Direct immunofluorescence was performed using antibodies against IgG, IgA, IgM, fibrinogen, albumin, and C3, which was negative. These findings from direct immunofluorescence and histopathology as well as the clinical presentation were considered compatible with Degos disease. The patient was started on aspirin and pentoxifylline. Pentoxifylline 400 mg twice daily appeared to lessen some of the pain. Pain management specialists started the patient on gabapentin.

Approximately 4 months after the Grand Rounds evaluation, during which time he continued treatment with pentoxifylline, he was admitted to the hospital for intractable nausea and vomiting. His condition acutely declined due to bowel perforation, and he was started on eculizumab 1200 mg every 14 days. Because of an increased risk for meningococcal meningitis while on this medication, he also was given erythromycin 500 mg twice daily prophylactically. He was being followed by hematology for the vasculopathy, and they were planning to monitor for any disease changes with computed tomography of the chest, abdomen, and pelvis every 3 months, as well as echocardiogram every 6 months for any development of pericardial or pleural fibrosis. Approximately 1 month later, the patient was admitted to the hospital again but died after 1 week from gastrointestinal complications (approximately 22 months after the onset of the rash).

Degos disease (atrophic papulosis) is a rare small vessel vasculopathy of unknown etiology, but complement-mediated endothelial injury plays a role.1,2 It typically occurs in the fourth decade of life, with a slight female predominance.3,4 The skin lesions are characteristic and described as 5- to 10-mm papules with atrophic white centers and erythematous telangiectatic rims, most commonly on the upper body and typically sparing the head, palms, and soles.1 Penile ulceration is an uncommon cutaneous feature, with only a few cases reported in the literature.5,6 Approximately one-third of patients will have only skin lesions, but two-thirds will develop systemic involvement 1 to 2 years after onset, with the gastrointestinal tract and central nervous system most commonly involved. For those with systemic involvement, the 5-year survival rate is approximately 55%, and the most common causes of death are bowel perforation, peritonitis, and stroke.3,4 Because some patients appear to never develop systemic complications, Theodoridis et al4 proposed that the disease be classified as either malignant atrophic papulosis or benign atrophic papulosis to indicate the malignant systemic form and the benign cutaneous form, respectively.
The histopathology of Degos disease changes as the lesions evolve.7 Early lesions show a superficial and deep perivascular and periadnexal lymphocytic infiltrate, possible interface dermatitis, and dermal mucin resembling lupus. The more fully developed lesions show a greater degree of inflammation and interface change as well as lymphocytic vasculitis. This stage also may have epidermal atrophy and early papillary dermal sclerosis resembling lichen sclerosus. The late-stage lesions, clinically observed as papules with atrophic white centers and surrounding erythema, show the classic pathology of wedge-shaped dermal sclerosis and central epidermal atrophy with surrounding hyperkeratosis. Interface dermatitis and dermal mucin can be seen in all stages, though mucin is diminished in the later stage.
Effective treatment options are limited; however, antithrombotics or compounds that facilitate blood perfusion, such as aspirin or pentoxifylline, initially can be used.1 Eculizumab, a humanized monoclonal antibody that prevents the cleavage of C5, has been used for salvage therapy,8 as in our case. Treprostinil, a prostacyclin analog that causes arterial vasodilation and inhibition of platelet aggregation, has been reported to improve bowel and cutaneous lesions, functional status, and neurologic symptoms.9
Our case highlights important features of Degos disease. First, it is important for both the clinician and the pathologist to recognize that the histopathology of Degos disease changes as the lesions evolve. In our case, although the lesions were characteristic of Degos disease clinically, the initial biopsy was suspicious for connective tissue disease, which led to an autoimmune evaluation that ultimately was unremarkable. Recognizing that early lesions of Degos disease can resemble connective tissue disease histologically could have prevented this delay in diagnosis. However, Degos disease has been reported in association with autoimmune diseases.10 Second, although penile ulceration is uncommon, it can be a prominent cutaneous manifestation of the disease. Finally, eculizumab and treprostinil are therapeutic options that have shown some efficacy in improving symptoms and cutaneous lesions.8,9
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)—a review. Orphanet J Rare Dis. 2013;8:10. doi:10.1186/1750-1172-8-10
- Magro CM, Poe JC, Kim C, et al. Degos disease: a C5b-9/interferon-α-mediated endotheliopathy syndrome. Am J Clin Pathol. 2011;135:599-610. doi:10.1309/AJCP66QIMFARLZKI
- Hu P, Mao Z, Liu C, et al. Malignant atrophic papulosis with motor aphasia and intestinal perforation: a case report and review of published works. J Dermatol. 2018;45:723-726. doi:10.1111/1346-8138.14280
- Theodoridis A, Konstantinidou A, Makrantonaki E, et al. Malignant and benign forms of atrophic papulosis (Köhlmeier-Degos disease): systemic involvement determines the prognosis. Br J Dermatol. 2014;170:110-115. doi:10.1111/bjd.12642
- Thomson KF, Highet AS. Penile ulceration in fatal malignant atrophic papulosis (Degos’ disease). Br J Dermatol. 2000;143:1320-1322. doi:10.1046/j.1365-2133.2000.03911.x
- Aydogan K, Alkan G, Karadogan Koran S, et al. Painful penile ulceration in a patient with malignant atrophic papulosis. J Eur Acad Dermatol Venereol. 2005;19:612-616. doi:10.1111/j.1468-3083.2005.01227.x
- Harvell JD, Williford PL, White WL. Benign cutaneous Degos’ disease: a case report with emphasis on histopathology as papules chronologically evolve. Am J Dermatopathol. 2001;23:116-123. doi:10.1097/00000372-200104000-00006
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277. doi:10.1016/j.jaad.2016.09.015
- Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil—early experience. Orphanet J Rare Dis. 2013;8:52. doi:10.1186/1750-1172-8-52
- Burgin S, Stone JH, Shenoy-Bhangle AS, et al. Case records of the Massachusetts General Hospital. Case 18-2014. A 32-year-old man with a rash, myalgia, and weakness. N Engl J Med. 2014;370:2327-2337. doi:10.1056/NEJMcpc1304161
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)—a review. Orphanet J Rare Dis. 2013;8:10. doi:10.1186/1750-1172-8-10
- Magro CM, Poe JC, Kim C, et al. Degos disease: a C5b-9/interferon-α-mediated endotheliopathy syndrome. Am J Clin Pathol. 2011;135:599-610. doi:10.1309/AJCP66QIMFARLZKI
- Hu P, Mao Z, Liu C, et al. Malignant atrophic papulosis with motor aphasia and intestinal perforation: a case report and review of published works. J Dermatol. 2018;45:723-726. doi:10.1111/1346-8138.14280
- Theodoridis A, Konstantinidou A, Makrantonaki E, et al. Malignant and benign forms of atrophic papulosis (Köhlmeier-Degos disease): systemic involvement determines the prognosis. Br J Dermatol. 2014;170:110-115. doi:10.1111/bjd.12642
- Thomson KF, Highet AS. Penile ulceration in fatal malignant atrophic papulosis (Degos’ disease). Br J Dermatol. 2000;143:1320-1322. doi:10.1046/j.1365-2133.2000.03911.x
- Aydogan K, Alkan G, Karadogan Koran S, et al. Painful penile ulceration in a patient with malignant atrophic papulosis. J Eur Acad Dermatol Venereol. 2005;19:612-616. doi:10.1111/j.1468-3083.2005.01227.x
- Harvell JD, Williford PL, White WL. Benign cutaneous Degos’ disease: a case report with emphasis on histopathology as papules chronologically evolve. Am J Dermatopathol. 2001;23:116-123. doi:10.1097/00000372-200104000-00006
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277. doi:10.1016/j.jaad.2016.09.015
- Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil—early experience. Orphanet J Rare Dis. 2013;8:52. doi:10.1186/1750-1172-8-52
- Burgin S, Stone JH, Shenoy-Bhangle AS, et al. Case records of the Massachusetts General Hospital. Case 18-2014. A 32-year-old man with a rash, myalgia, and weakness. N Engl J Med. 2014;370:2327-2337. doi:10.1056/NEJMcpc1304161
PRACTICE POINTS
- Papules with atrophic white centers and erythematous telangiectatic rims are the characteristic skin lesions found in Degos disease.
- A painful penile ulceration also may occur in Degos disease, though it is uncommon.
- The histopathology of skin lesions changes as the lesions evolve. Early lesions may resemble connective tissue disease. Late lesions show the classic pathology of wedge-shaped dermal sclerosis.
Chronic Cribriform Ulcerated Plaque on the Left Calf
The Diagnosis: Nodular Basal Cell Carcinoma
Histopathology of the lesion showed a large basaloid lobule with focal epidermal attachment, peripheral nuclear palisading with cleft formation between the tumor and surrounding stroma, fibromyxoid stroma and mild pleomorphism, and variable mitotic activity and apoptosis (Figure). Based on the clinical presentation and histopathology, the patient was diagnosed with nodular basal cell carcinoma (BCC). He underwent a wide local excision of the affected area that was repaired with a splitthickness skin graft.
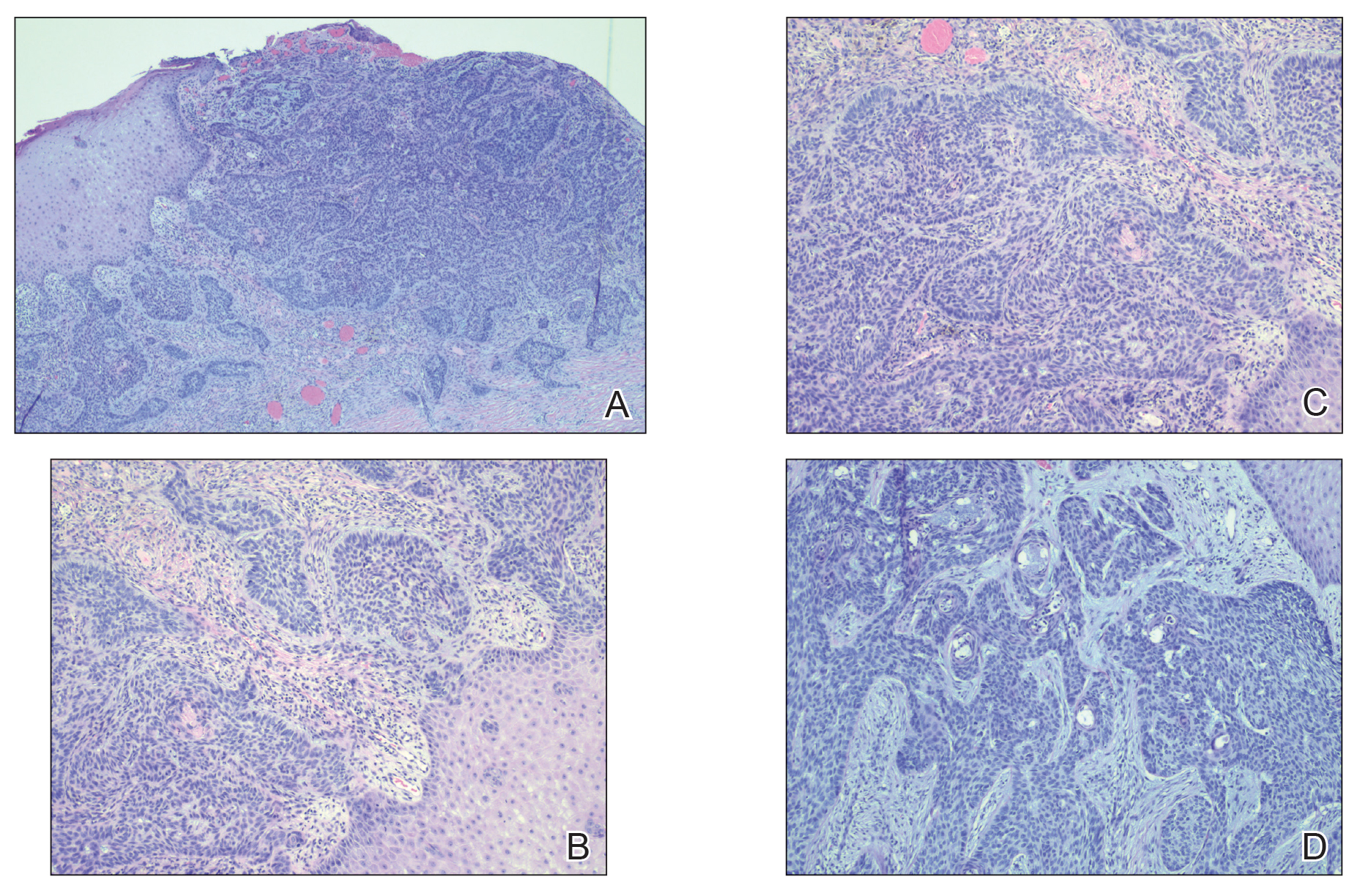
Basal cell carcinoma is the most common skin cancer worldwide and typically occurs due to years of UV radiation damage on sun-exposed skin, which accounts for a higher frequency of BCC occurring in patients residing in geographic locations with greater UV exposure (eg, higher and lower latitudes). In addition to cumulative UV dose, the duration of the exposure as well as its intensity also play a role in the development of BCC, particularly in early childhood and adolescence. Nevertheless, UV exposure is not the only risk factor, as 20% of BCCs arise in skin that is not exposed to the sun. Other risk factors include exposure to ionizing radiation and arsenic, immunosuppression, and genetic predisposition.1 Although these malignancies typically do not metastasize, growth can lead to local tissue destruction and major disfigurement if not treated in a timely fashion.2
In our patient, the differential diagnosis included pyoderma gangrenosum (PG) given the clinical appearance of the cribriform base and violaceous undermined rim of the ulcer. Pyoderma gangrenosum is a rare neutrophilic disorder that often results in ulcers that have been associated with various systemic autoimmune and inflammatory conditions, such as inflammatory bowel disease. There are 4 main subtypes of PG: the classic ulcerative type (our patient); the pustular type, which most often is seen in patients with inflammatory bowel disease; the bullous type, which can be seen in patients with an associated lymphoproliferative disorder; and the vegetative type. It frequently is thought of as both a clinical and histologic diagnosis of exclusion due to the nonspecific histopathologic features; most lesions demonstrate an infiltrate of neutrophils in the dermis. A biopsy was crucial in our patient, considering that diagnosis and treatment would have been further delayed had the patient been empirically treated with oral and topical steroids for presumed PG, which is precisely why PG is a diagnosis of exclusion. It is imperative for clinicians to rule out other pathologies, such as infection or malignancy, as demonstrated in our patient. The progressive onset and slow evolution of the lesion over years along with a lack of pain were more suggestive of BCC rather than PG. However, there is a report in the literature of PG mimicking BCC with both clinical and dermoscopic findings.3
Venous or stasis ulcers are painless, and although they rarely occur on the calf, they typically are seen lower on the leg such as on the medial ankles. Our patient endorsed occasional swelling of the affected leg and presented with edema, but overlying stasis change and other signs of venous insufficiency were absent.
Buruli ulcer is a painless chronic debilitating cutaneous disease resulting in indolent necrotizing skin as well as subcutaneous and bone lesions. It is caused by the environmental organism Mycobacterium ulcerans and typically is reported in Africa, Central/South America, the Western Pacific Region, and Australia.4 Histopathology usually demonstrates necrosis of subcutaneous tissue and dermal collagen accompanied by inflammation and acidfast bacilli highlighted by Ziehl-Neelsen stain.5 Smears of the lesions as well as culture and polymerase chain reaction for acid-fast bacilli also can be performed. Our patient reported no recent travel to any endemic areas and had no other risk factors or exposures to the pathogen responsible for this condition.
Traumatic ulcer also was included in the differential diagnosis, but the patient denied preceding trauma to the area, and the contralateral foot prosthesis did not rub on or impact the affected leg.
Basal cell carcinoma typically is treated surgically, but choice of treatment can depend on the subtype, size, tumor site, and/or patient preference.1 Other treatment modalities include electrodesiccation and curettage, cryosurgical destruction, photodynamic therapy, radiation, topical therapies, and systemic medications. Radiotherapy can be considered as a primary treatment option for BCC if surgery is contraindicated or declined by the patient, but it also is useful as an adjuvant therapy when there is perineural invasion of the tumor or positive margins. Hedgehog pathway inhibitors such as vismodegib currently are indicated for patients who are not candidates for surgery or radiation as well as for those with metastatic or locally advanced, recurrent BCC. There is no single treatment method ideal for every lesion or patient. Specific populations such as the elderly, the immunosuppressed, or those with poor baseline functional status may warrant a nonsurgical approach. The clinician must take into consideration all factors while at the same time thinking about how to best accomplish the goals of recurrencefree tumor removal, correction of any underlying functional impairment from the tumor, and maintenance of cosmesis.1
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. In: StatPearls. StatPearls; 2022.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Rosina P, Papagrigoraki A, Colato C. A case of superficial granulomatous pyoderma mimicking a basal cell carcinoma. Acta Dermatovenerol Croat. 2014;22:48-51.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256.
- Guarner J, Bartlett J, Whitney EA, et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg Infect Dis. 2003;9:651-656.
The Diagnosis: Nodular Basal Cell Carcinoma
Histopathology of the lesion showed a large basaloid lobule with focal epidermal attachment, peripheral nuclear palisading with cleft formation between the tumor and surrounding stroma, fibromyxoid stroma and mild pleomorphism, and variable mitotic activity and apoptosis (Figure). Based on the clinical presentation and histopathology, the patient was diagnosed with nodular basal cell carcinoma (BCC). He underwent a wide local excision of the affected area that was repaired with a splitthickness skin graft.

Basal cell carcinoma is the most common skin cancer worldwide and typically occurs due to years of UV radiation damage on sun-exposed skin, which accounts for a higher frequency of BCC occurring in patients residing in geographic locations with greater UV exposure (eg, higher and lower latitudes). In addition to cumulative UV dose, the duration of the exposure as well as its intensity also play a role in the development of BCC, particularly in early childhood and adolescence. Nevertheless, UV exposure is not the only risk factor, as 20% of BCCs arise in skin that is not exposed to the sun. Other risk factors include exposure to ionizing radiation and arsenic, immunosuppression, and genetic predisposition.1 Although these malignancies typically do not metastasize, growth can lead to local tissue destruction and major disfigurement if not treated in a timely fashion.2
In our patient, the differential diagnosis included pyoderma gangrenosum (PG) given the clinical appearance of the cribriform base and violaceous undermined rim of the ulcer. Pyoderma gangrenosum is a rare neutrophilic disorder that often results in ulcers that have been associated with various systemic autoimmune and inflammatory conditions, such as inflammatory bowel disease. There are 4 main subtypes of PG: the classic ulcerative type (our patient); the pustular type, which most often is seen in patients with inflammatory bowel disease; the bullous type, which can be seen in patients with an associated lymphoproliferative disorder; and the vegetative type. It frequently is thought of as both a clinical and histologic diagnosis of exclusion due to the nonspecific histopathologic features; most lesions demonstrate an infiltrate of neutrophils in the dermis. A biopsy was crucial in our patient, considering that diagnosis and treatment would have been further delayed had the patient been empirically treated with oral and topical steroids for presumed PG, which is precisely why PG is a diagnosis of exclusion. It is imperative for clinicians to rule out other pathologies, such as infection or malignancy, as demonstrated in our patient. The progressive onset and slow evolution of the lesion over years along with a lack of pain were more suggestive of BCC rather than PG. However, there is a report in the literature of PG mimicking BCC with both clinical and dermoscopic findings.3
Venous or stasis ulcers are painless, and although they rarely occur on the calf, they typically are seen lower on the leg such as on the medial ankles. Our patient endorsed occasional swelling of the affected leg and presented with edema, but overlying stasis change and other signs of venous insufficiency were absent.
Buruli ulcer is a painless chronic debilitating cutaneous disease resulting in indolent necrotizing skin as well as subcutaneous and bone lesions. It is caused by the environmental organism Mycobacterium ulcerans and typically is reported in Africa, Central/South America, the Western Pacific Region, and Australia.4 Histopathology usually demonstrates necrosis of subcutaneous tissue and dermal collagen accompanied by inflammation and acidfast bacilli highlighted by Ziehl-Neelsen stain.5 Smears of the lesions as well as culture and polymerase chain reaction for acid-fast bacilli also can be performed. Our patient reported no recent travel to any endemic areas and had no other risk factors or exposures to the pathogen responsible for this condition.
Traumatic ulcer also was included in the differential diagnosis, but the patient denied preceding trauma to the area, and the contralateral foot prosthesis did not rub on or impact the affected leg.
Basal cell carcinoma typically is treated surgically, but choice of treatment can depend on the subtype, size, tumor site, and/or patient preference.1 Other treatment modalities include electrodesiccation and curettage, cryosurgical destruction, photodynamic therapy, radiation, topical therapies, and systemic medications. Radiotherapy can be considered as a primary treatment option for BCC if surgery is contraindicated or declined by the patient, but it also is useful as an adjuvant therapy when there is perineural invasion of the tumor or positive margins. Hedgehog pathway inhibitors such as vismodegib currently are indicated for patients who are not candidates for surgery or radiation as well as for those with metastatic or locally advanced, recurrent BCC. There is no single treatment method ideal for every lesion or patient. Specific populations such as the elderly, the immunosuppressed, or those with poor baseline functional status may warrant a nonsurgical approach. The clinician must take into consideration all factors while at the same time thinking about how to best accomplish the goals of recurrencefree tumor removal, correction of any underlying functional impairment from the tumor, and maintenance of cosmesis.1
The Diagnosis: Nodular Basal Cell Carcinoma
Histopathology of the lesion showed a large basaloid lobule with focal epidermal attachment, peripheral nuclear palisading with cleft formation between the tumor and surrounding stroma, fibromyxoid stroma and mild pleomorphism, and variable mitotic activity and apoptosis (Figure). Based on the clinical presentation and histopathology, the patient was diagnosed with nodular basal cell carcinoma (BCC). He underwent a wide local excision of the affected area that was repaired with a splitthickness skin graft.

Basal cell carcinoma is the most common skin cancer worldwide and typically occurs due to years of UV radiation damage on sun-exposed skin, which accounts for a higher frequency of BCC occurring in patients residing in geographic locations with greater UV exposure (eg, higher and lower latitudes). In addition to cumulative UV dose, the duration of the exposure as well as its intensity also play a role in the development of BCC, particularly in early childhood and adolescence. Nevertheless, UV exposure is not the only risk factor, as 20% of BCCs arise in skin that is not exposed to the sun. Other risk factors include exposure to ionizing radiation and arsenic, immunosuppression, and genetic predisposition.1 Although these malignancies typically do not metastasize, growth can lead to local tissue destruction and major disfigurement if not treated in a timely fashion.2
In our patient, the differential diagnosis included pyoderma gangrenosum (PG) given the clinical appearance of the cribriform base and violaceous undermined rim of the ulcer. Pyoderma gangrenosum is a rare neutrophilic disorder that often results in ulcers that have been associated with various systemic autoimmune and inflammatory conditions, such as inflammatory bowel disease. There are 4 main subtypes of PG: the classic ulcerative type (our patient); the pustular type, which most often is seen in patients with inflammatory bowel disease; the bullous type, which can be seen in patients with an associated lymphoproliferative disorder; and the vegetative type. It frequently is thought of as both a clinical and histologic diagnosis of exclusion due to the nonspecific histopathologic features; most lesions demonstrate an infiltrate of neutrophils in the dermis. A biopsy was crucial in our patient, considering that diagnosis and treatment would have been further delayed had the patient been empirically treated with oral and topical steroids for presumed PG, which is precisely why PG is a diagnosis of exclusion. It is imperative for clinicians to rule out other pathologies, such as infection or malignancy, as demonstrated in our patient. The progressive onset and slow evolution of the lesion over years along with a lack of pain were more suggestive of BCC rather than PG. However, there is a report in the literature of PG mimicking BCC with both clinical and dermoscopic findings.3
Venous or stasis ulcers are painless, and although they rarely occur on the calf, they typically are seen lower on the leg such as on the medial ankles. Our patient endorsed occasional swelling of the affected leg and presented with edema, but overlying stasis change and other signs of venous insufficiency were absent.
Buruli ulcer is a painless chronic debilitating cutaneous disease resulting in indolent necrotizing skin as well as subcutaneous and bone lesions. It is caused by the environmental organism Mycobacterium ulcerans and typically is reported in Africa, Central/South America, the Western Pacific Region, and Australia.4 Histopathology usually demonstrates necrosis of subcutaneous tissue and dermal collagen accompanied by inflammation and acidfast bacilli highlighted by Ziehl-Neelsen stain.5 Smears of the lesions as well as culture and polymerase chain reaction for acid-fast bacilli also can be performed. Our patient reported no recent travel to any endemic areas and had no other risk factors or exposures to the pathogen responsible for this condition.
Traumatic ulcer also was included in the differential diagnosis, but the patient denied preceding trauma to the area, and the contralateral foot prosthesis did not rub on or impact the affected leg.
Basal cell carcinoma typically is treated surgically, but choice of treatment can depend on the subtype, size, tumor site, and/or patient preference.1 Other treatment modalities include electrodesiccation and curettage, cryosurgical destruction, photodynamic therapy, radiation, topical therapies, and systemic medications. Radiotherapy can be considered as a primary treatment option for BCC if surgery is contraindicated or declined by the patient, but it also is useful as an adjuvant therapy when there is perineural invasion of the tumor or positive margins. Hedgehog pathway inhibitors such as vismodegib currently are indicated for patients who are not candidates for surgery or radiation as well as for those with metastatic or locally advanced, recurrent BCC. There is no single treatment method ideal for every lesion or patient. Specific populations such as the elderly, the immunosuppressed, or those with poor baseline functional status may warrant a nonsurgical approach. The clinician must take into consideration all factors while at the same time thinking about how to best accomplish the goals of recurrencefree tumor removal, correction of any underlying functional impairment from the tumor, and maintenance of cosmesis.1
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. In: StatPearls. StatPearls; 2022.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Rosina P, Papagrigoraki A, Colato C. A case of superficial granulomatous pyoderma mimicking a basal cell carcinoma. Acta Dermatovenerol Croat. 2014;22:48-51.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256.
- Guarner J, Bartlett J, Whitney EA, et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg Infect Dis. 2003;9:651-656.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. In: StatPearls. StatPearls; 2022.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Rosina P, Papagrigoraki A, Colato C. A case of superficial granulomatous pyoderma mimicking a basal cell carcinoma. Acta Dermatovenerol Croat. 2014;22:48-51.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256.
- Guarner J, Bartlett J, Whitney EA, et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg Infect Dis. 2003;9:651-656.
A 61-year-old man presented to the dermatology clinic for evaluation of a painless nonhealing wound on the left calf of 4 years’ duration. The patient had a history of amputation of the right foot as an infant, for which he wore an orthopedic prosthesis. He also had chronic lymphedema of the left leg, hyperlipidemia, and osteoarthritis of the right hip. There was no history of gastrointestinal tract issues. The lesion initially was small, then grew and began to ulcerate and bleed. His presentation to dermatology was delayed due to office closures during the COVID-19 pandemic. Physical examination revealed a 5-cm, erythematous, cribriform ulcer with a violaceous undermined rim. A punch biopsy was performed on the edge of the ulcer.
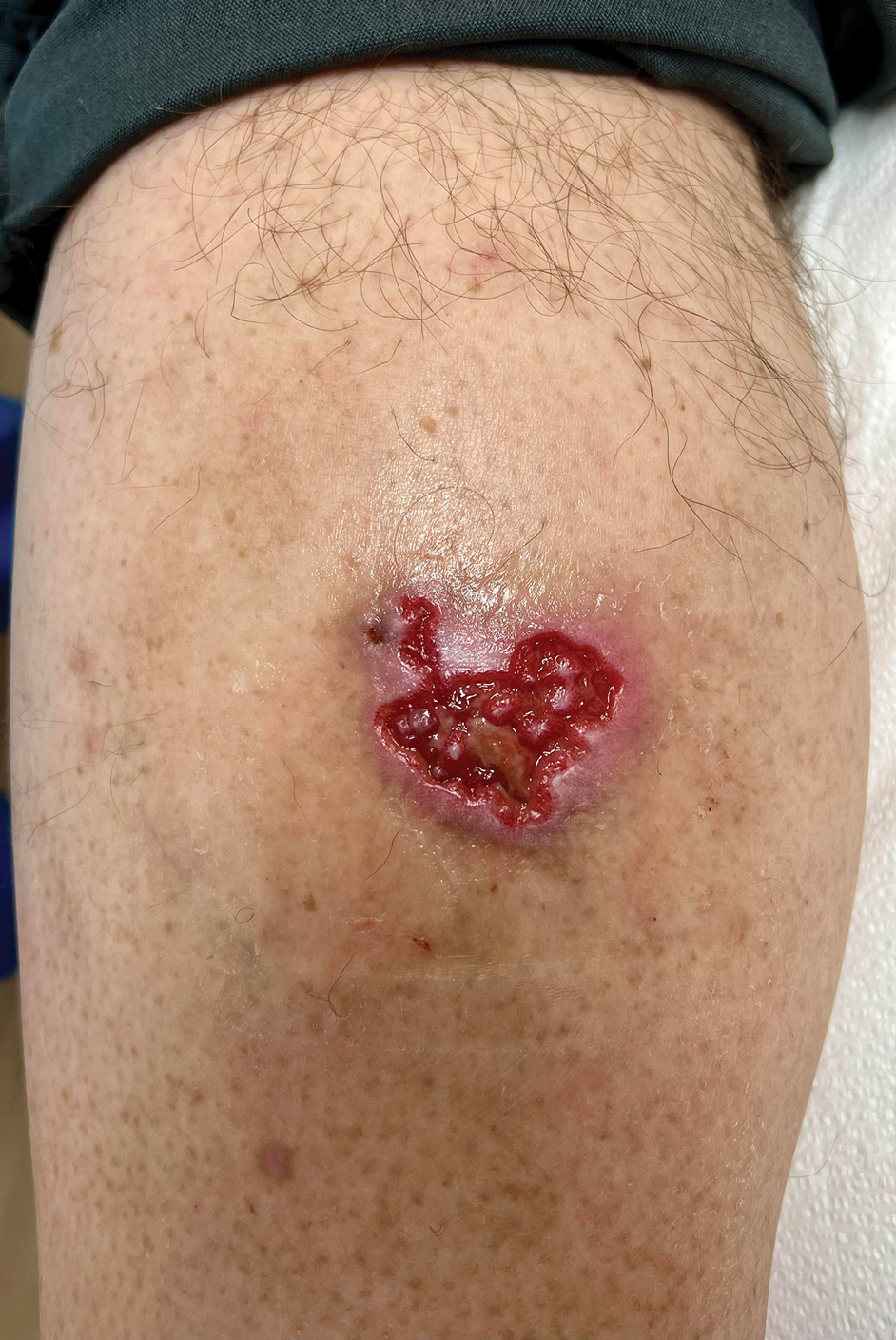
Periorbital Changes Induced by Prostaglandin Eye Drops
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.
The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.

The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.

The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
PRACTICE POINTS
- Ask patients to provide photographs taken prior to noticed changes to assess progression if they are new to your practice.
- Take photographs of patients in good light against a solid-colored background to have a baseline. It may be helpful to update patient images annually.
- Discuss with patients the aesthetic changes that may occur with the use of prescription medications. Provide pamphlets with images to educate them on what to expect.