User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
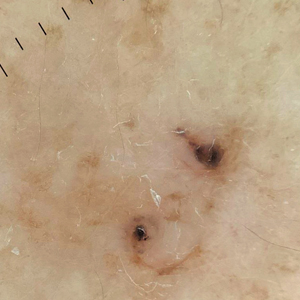
Pigmented Lesion on the Left Shoulder in an Older Woman
The Diagnosis: Pigmented Nodular Basal Cell Carcinoma
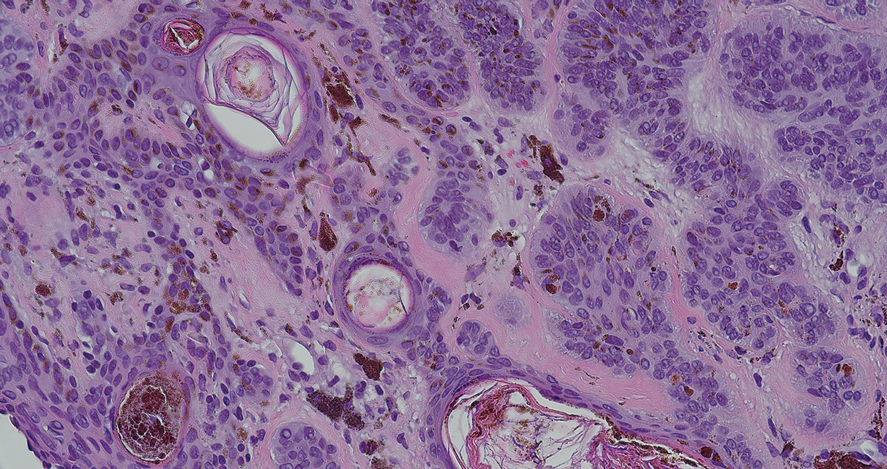
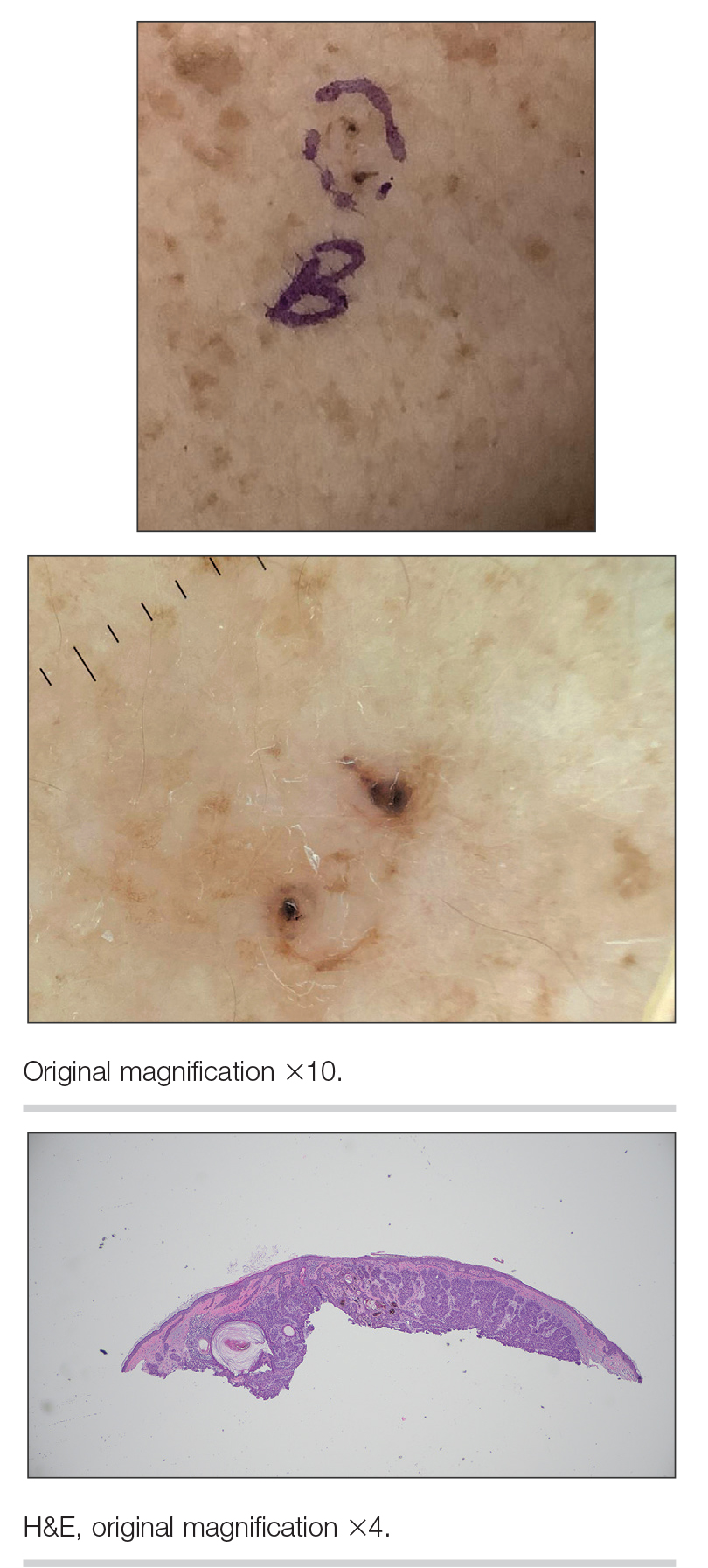
Dermoscopy of our patient’s irregular dark brown papule revealed large blue clustered clods and radial lines converging to a central dot (middle quiz image). Histopathology revealed nests of basaloid cells with peripheral palisading, small horn pseudocysts, and deposits of melanin extending into the dermis (Figure). These findings were consistent with a diagnosis of pigmented nodular basal cell carcinoma (BCC).
Nodular BCC represents 60% to 80% of all BCC cases; pigmented BCC represents 6% of BCC cases.1 Basal cell carcinomas frequently manifest as pearly papules with areas of pigment, surface telangiectases, and foci of ulceration. Dermoscopic features include fine arborizing vessels, blue-gray ovoid nests, spoke wheel–like structures, leaflike structures, and focal ulceration.1 Histopathology shows well-defined dermal nodules comprising basaloid epithelial cells with peripheral palisading, mucinous stroma, focal melanin deposits, and surrounding clefting.2 Arborizing vessels correspond to dilated vessels in the dermis.3 Blue-gray ovoid nests are wellcircumscribed ovoid or elongated structures that correspond histologically to well-defined large tumor nests with melanin aggregates invading the dermis. Spoke wheel–like structures are well-circumscribed radial projections connected to a pigmented central axis that correspond histologically to tumor nests near the epidermis and that appear as fingerlike projections with centrally located melanin deposits.3
The differential diagnosis of our patient’s lesion included nodular melanoma, lentigo maligna melanoma, deep penetrating nevus, and cellular blue nevus. Nodular melanoma is an invasive melanoma that lacks a radial growth phase. Dermoscopically, the more common features are a bluewhite veil, atypical vascular pattern, asymmetric pigmentation, atypical pigment network, and peripheral black globules.4 Histopathology reveals atypical melanocytes and architectural disorder.2 Pigmented nodular BCC also can display dark globules on dermoscopy but typically has smaller and more arborizing blood vessels and does not have a pigmented network. Furthermore, BCC would not have atypical melanocytes on histopathology.4,5
Dermoscopy of lentigo maligna melanoma displays hyperpigmented follicular openings, an annular-granular pattern, pigmented rhomboidal structures, and obliterated hair follicles.6 Histopathology demonstrates epidermal atrophy, increased pigmentation in basal keratinocytes, prominent solar elastosis, and an increased number of melanocytes that extend beyond the epidermis. 7 Pigmented nodular BCC can be distinguished from lentigo maligna melanoma dermoscopically by the presence of arborizing vessels, blue-gray ovoid nests, and lack of a pigment network.
Deep penetrating nevus is a darkly pigmented melanocytic lesion that infiltrates deeply into the reticular dermis.8 Specific dermoscopic features have not been well established; however, a uniformly dark blue or black pattern is common. Histologically, this type of nevus is symmetric and wedge shaped with a broad base extending to the deep dermis and subcutaneous fat.8 Melanocytes do not exhibit atypia or bizarre mitoses. Although pigmented nodular BCC can appear similar to deep penetrating nevus, histologically there will be atypical basaloid epithelial cells in BCC.
Blue nevi clinically appear as a smooth blue-gray lesion with a steel blue ground-glass pattern on dermoscopy. Histopathology shows spindle-shaped melanocytes in the dermis, which distinguishes this lesion from BCC.9
Consider pigmented BCC when a patient presents with a pigmented lesion. Dermoscopy can help appreciate a pigmented BCC by looking for features such as a spoke wheel– like pattern, blue ovoid nests, arborizing blood vessels, and lack of a pigment network. Because pigmented BCC constitutes a small fraction of all BCCs, it is important to be familiar with its presentation and dermoscopic features.
- Heath MS, Bar A. Basal cell carcinoma. Dermatol Clin. 2023;41:13-21. doi:10.1016/j.det.2022.07.005
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28:1005-1012.
- Wozniak-Rito A, Zalaudek I, Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-247. doi:10.1111/ced.13387
- Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic valuation of nodular melanoma. JAMA Dermatol. 2013;149:699-709. doi:10.1001 /jamadermatol.2013.2466
- Pizzichetta MA, Kittler H, Stanganelli I, et al; Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-114. doi:10.1111/bjd.13861
- Pralong P, Bathelier E, Dalle S, et al. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280-287. doi:10.1111/j.1365-2133.2012.10932.x
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838-841. doi:10.5858/2011-0051-RAIR.1
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240. doi:10.1016/j .jaad.2014.07.026
- Ferrera G, Argenziano G. Blue nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al, eds. Color Atlas of Melanocytic Lesions of the Skin. Springer; 2007:78-86.
The Diagnosis: Pigmented Nodular Basal Cell Carcinoma
Dermoscopy of our patient’s irregular dark brown papule revealed large blue clustered clods and radial lines converging to a central dot (middle quiz image). Histopathology revealed nests of basaloid cells with peripheral palisading, small horn pseudocysts, and deposits of melanin extending into the dermis (Figure). These findings were consistent with a diagnosis of pigmented nodular basal cell carcinoma (BCC).
Nodular BCC represents 60% to 80% of all BCC cases; pigmented BCC represents 6% of BCC cases.1 Basal cell carcinomas frequently manifest as pearly papules with areas of pigment, surface telangiectases, and foci of ulceration. Dermoscopic features include fine arborizing vessels, blue-gray ovoid nests, spoke wheel–like structures, leaflike structures, and focal ulceration.1 Histopathology shows well-defined dermal nodules comprising basaloid epithelial cells with peripheral palisading, mucinous stroma, focal melanin deposits, and surrounding clefting.2 Arborizing vessels correspond to dilated vessels in the dermis.3 Blue-gray ovoid nests are wellcircumscribed ovoid or elongated structures that correspond histologically to well-defined large tumor nests with melanin aggregates invading the dermis. Spoke wheel–like structures are well-circumscribed radial projections connected to a pigmented central axis that correspond histologically to tumor nests near the epidermis and that appear as fingerlike projections with centrally located melanin deposits.3
The differential diagnosis of our patient’s lesion included nodular melanoma, lentigo maligna melanoma, deep penetrating nevus, and cellular blue nevus. Nodular melanoma is an invasive melanoma that lacks a radial growth phase. Dermoscopically, the more common features are a bluewhite veil, atypical vascular pattern, asymmetric pigmentation, atypical pigment network, and peripheral black globules.4 Histopathology reveals atypical melanocytes and architectural disorder.2 Pigmented nodular BCC also can display dark globules on dermoscopy but typically has smaller and more arborizing blood vessels and does not have a pigmented network. Furthermore, BCC would not have atypical melanocytes on histopathology.4,5
Dermoscopy of lentigo maligna melanoma displays hyperpigmented follicular openings, an annular-granular pattern, pigmented rhomboidal structures, and obliterated hair follicles.6 Histopathology demonstrates epidermal atrophy, increased pigmentation in basal keratinocytes, prominent solar elastosis, and an increased number of melanocytes that extend beyond the epidermis. 7 Pigmented nodular BCC can be distinguished from lentigo maligna melanoma dermoscopically by the presence of arborizing vessels, blue-gray ovoid nests, and lack of a pigment network.

Deep penetrating nevus is a darkly pigmented melanocytic lesion that infiltrates deeply into the reticular dermis.8 Specific dermoscopic features have not been well established; however, a uniformly dark blue or black pattern is common. Histologically, this type of nevus is symmetric and wedge shaped with a broad base extending to the deep dermis and subcutaneous fat.8 Melanocytes do not exhibit atypia or bizarre mitoses. Although pigmented nodular BCC can appear similar to deep penetrating nevus, histologically there will be atypical basaloid epithelial cells in BCC.
Blue nevi clinically appear as a smooth blue-gray lesion with a steel blue ground-glass pattern on dermoscopy. Histopathology shows spindle-shaped melanocytes in the dermis, which distinguishes this lesion from BCC.9
Consider pigmented BCC when a patient presents with a pigmented lesion. Dermoscopy can help appreciate a pigmented BCC by looking for features such as a spoke wheel– like pattern, blue ovoid nests, arborizing blood vessels, and lack of a pigment network. Because pigmented BCC constitutes a small fraction of all BCCs, it is important to be familiar with its presentation and dermoscopic features.
The Diagnosis: Pigmented Nodular Basal Cell Carcinoma
Dermoscopy of our patient’s irregular dark brown papule revealed large blue clustered clods and radial lines converging to a central dot (middle quiz image). Histopathology revealed nests of basaloid cells with peripheral palisading, small horn pseudocysts, and deposits of melanin extending into the dermis (Figure). These findings were consistent with a diagnosis of pigmented nodular basal cell carcinoma (BCC).
Nodular BCC represents 60% to 80% of all BCC cases; pigmented BCC represents 6% of BCC cases.1 Basal cell carcinomas frequently manifest as pearly papules with areas of pigment, surface telangiectases, and foci of ulceration. Dermoscopic features include fine arborizing vessels, blue-gray ovoid nests, spoke wheel–like structures, leaflike structures, and focal ulceration.1 Histopathology shows well-defined dermal nodules comprising basaloid epithelial cells with peripheral palisading, mucinous stroma, focal melanin deposits, and surrounding clefting.2 Arborizing vessels correspond to dilated vessels in the dermis.3 Blue-gray ovoid nests are wellcircumscribed ovoid or elongated structures that correspond histologically to well-defined large tumor nests with melanin aggregates invading the dermis. Spoke wheel–like structures are well-circumscribed radial projections connected to a pigmented central axis that correspond histologically to tumor nests near the epidermis and that appear as fingerlike projections with centrally located melanin deposits.3
The differential diagnosis of our patient’s lesion included nodular melanoma, lentigo maligna melanoma, deep penetrating nevus, and cellular blue nevus. Nodular melanoma is an invasive melanoma that lacks a radial growth phase. Dermoscopically, the more common features are a bluewhite veil, atypical vascular pattern, asymmetric pigmentation, atypical pigment network, and peripheral black globules.4 Histopathology reveals atypical melanocytes and architectural disorder.2 Pigmented nodular BCC also can display dark globules on dermoscopy but typically has smaller and more arborizing blood vessels and does not have a pigmented network. Furthermore, BCC would not have atypical melanocytes on histopathology.4,5
Dermoscopy of lentigo maligna melanoma displays hyperpigmented follicular openings, an annular-granular pattern, pigmented rhomboidal structures, and obliterated hair follicles.6 Histopathology demonstrates epidermal atrophy, increased pigmentation in basal keratinocytes, prominent solar elastosis, and an increased number of melanocytes that extend beyond the epidermis. 7 Pigmented nodular BCC can be distinguished from lentigo maligna melanoma dermoscopically by the presence of arborizing vessels, blue-gray ovoid nests, and lack of a pigment network.

Deep penetrating nevus is a darkly pigmented melanocytic lesion that infiltrates deeply into the reticular dermis.8 Specific dermoscopic features have not been well established; however, a uniformly dark blue or black pattern is common. Histologically, this type of nevus is symmetric and wedge shaped with a broad base extending to the deep dermis and subcutaneous fat.8 Melanocytes do not exhibit atypia or bizarre mitoses. Although pigmented nodular BCC can appear similar to deep penetrating nevus, histologically there will be atypical basaloid epithelial cells in BCC.
Blue nevi clinically appear as a smooth blue-gray lesion with a steel blue ground-glass pattern on dermoscopy. Histopathology shows spindle-shaped melanocytes in the dermis, which distinguishes this lesion from BCC.9
Consider pigmented BCC when a patient presents with a pigmented lesion. Dermoscopy can help appreciate a pigmented BCC by looking for features such as a spoke wheel– like pattern, blue ovoid nests, arborizing blood vessels, and lack of a pigment network. Because pigmented BCC constitutes a small fraction of all BCCs, it is important to be familiar with its presentation and dermoscopic features.
- Heath MS, Bar A. Basal cell carcinoma. Dermatol Clin. 2023;41:13-21. doi:10.1016/j.det.2022.07.005
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28:1005-1012.
- Wozniak-Rito A, Zalaudek I, Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-247. doi:10.1111/ced.13387
- Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic valuation of nodular melanoma. JAMA Dermatol. 2013;149:699-709. doi:10.1001 /jamadermatol.2013.2466
- Pizzichetta MA, Kittler H, Stanganelli I, et al; Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-114. doi:10.1111/bjd.13861
- Pralong P, Bathelier E, Dalle S, et al. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280-287. doi:10.1111/j.1365-2133.2012.10932.x
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838-841. doi:10.5858/2011-0051-RAIR.1
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240. doi:10.1016/j .jaad.2014.07.026
- Ferrera G, Argenziano G. Blue nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al, eds. Color Atlas of Melanocytic Lesions of the Skin. Springer; 2007:78-86.
- Heath MS, Bar A. Basal cell carcinoma. Dermatol Clin. 2023;41:13-21. doi:10.1016/j.det.2022.07.005
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28:1005-1012.
- Wozniak-Rito A, Zalaudek I, Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-247. doi:10.1111/ced.13387
- Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic valuation of nodular melanoma. JAMA Dermatol. 2013;149:699-709. doi:10.1001 /jamadermatol.2013.2466
- Pizzichetta MA, Kittler H, Stanganelli I, et al; Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-114. doi:10.1111/bjd.13861
- Pralong P, Bathelier E, Dalle S, et al. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280-287. doi:10.1111/j.1365-2133.2012.10932.x
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838-841. doi:10.5858/2011-0051-RAIR.1
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240. doi:10.1016/j .jaad.2014.07.026
- Ferrera G, Argenziano G. Blue nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al, eds. Color Atlas of Melanocytic Lesions of the Skin. Springer; 2007:78-86.
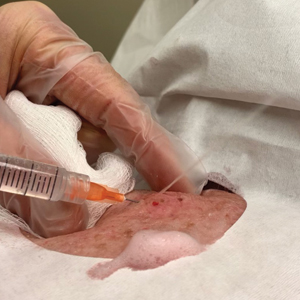
A 92-year-old woman presented to dermatology as a new patient for a full-body skin examination. She had a history of sarcoidosis and a liposarcoma that had been excised more than 20 years prior. She had no history of skin cancer; however, her granddaughter recently was diagnosed with melanoma. Physical examination revealed a 5-mm, irregular, dark brown papule on the left shoulder (top) that was evaluated by dermoscopy (middle). A tangential biopsy was performed for histopathologic analysis (bottom).
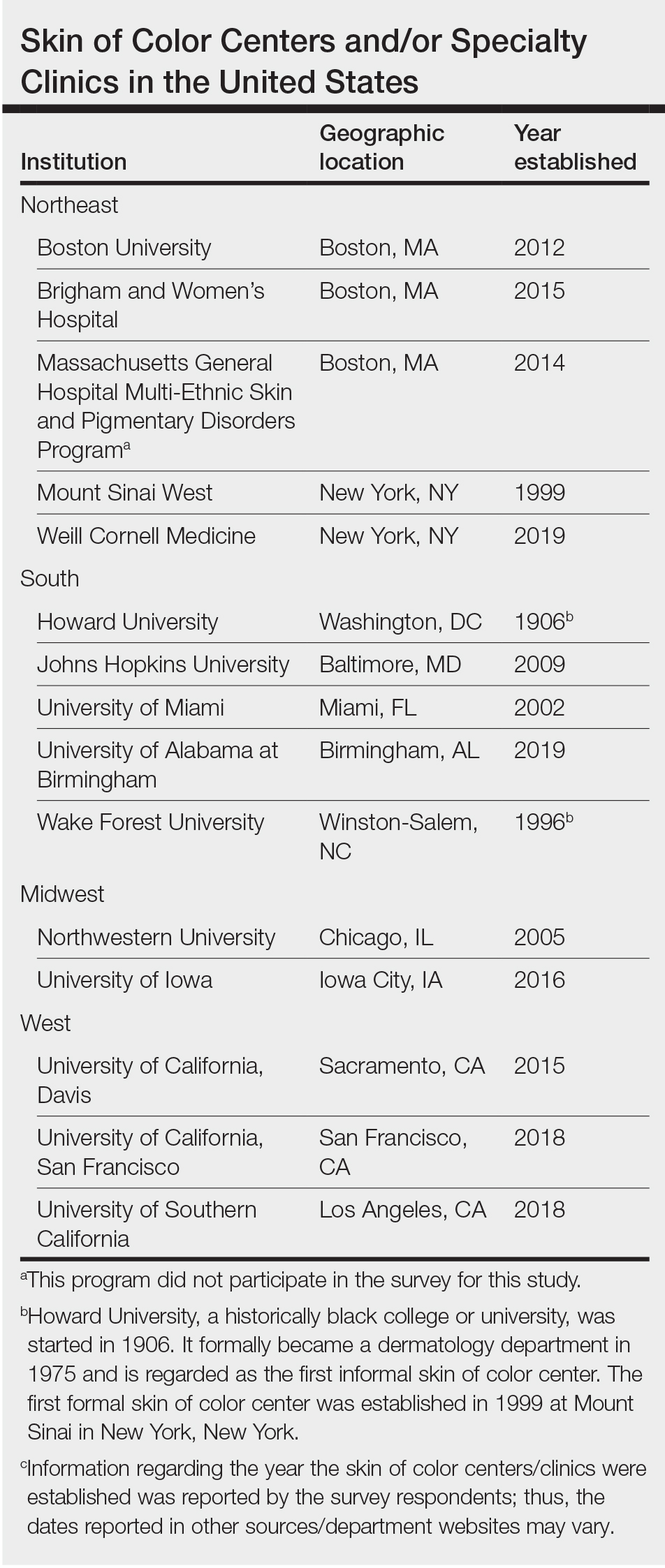
The State of Skin of Color Centers in the United States: A Cross-Sectional Survey Study
Although individuals with skin of color (SoC) are expected to become at least half of the US population by the year 2044, there remains a paucity of education and exposure to treatment of patients with SoC at many dermatology residency programs across the country.1 One way to improve SoC education has been the formation of specialized clinics, centers, and programs. The first SoC center (SoCC) was established in 1999 at Mount Sinai–St. Luke’s Roosevelt in New York, New York2; since then, at least 13 additional formal SoCCs or SoC specialty clinics (SoCSCs) at US academic dermatology programs have been established.
Skin of color centers serve several important purposes: they improve dermatologic care in patients with SoC, increase research efforts focused on SoC dermatologic conditions, and educate dermatology resident and fellow trainees about SoC. Improving dermatologic care of patients with SoC in the United States is important in providing equitable health care and improving health disparities. Studies have shown that patient-physician racial and cultural concordance can positively impact patient care, increase patient trust and rapport, and improve patient-physician communication, and it can even influence patient decision-making to seek care.3,4 Unfortunately, even though the US population continues to diversify, the racial/ethnic backgrounds of dermatologists do not parallel this trend; Hispanic and Black physicians comprise 18.9% and 13.6% of the general population, respectively, but represent only 4.2% and 3.0% of dermatologists, respectively.5-7 This deficit is mirrored by resident and faculty representation, with Black and Latino representation ranging from 3% to 7%.8-10
Many SoCC’s engage in research focused on dermatologic conditions affecting patients with SoC, which is vital to improving the dermatologic care in this underserved population. Despite increasing recognition of the importance of SoC research, there remains a paucity of clinical trials and research specifically focused on or demonstrating equitable representation of SoC.11,12
The education and training of future dermatologists is another important area that can be improved by SoCCs. A 2008 study involving 63 chief residents showed that approximately half (52.4% [33/63]) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and 30.2% (19/63) reported having a dedicated rotation where they gained specific experience treating patients with SoC.13 A later study in 2022 (N=125) found that 63.2% of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and only 11.2% reported having a dedicated rotation where they gained experience treating patients with SoC.14 These findings suggest that in the last 14 years, formal SoC education—specifically SoC clinical training—has not increased sufficiently.
We conducted a cross-sectional survey study to provide an in-depth analysis of SoCCs and SoCSCs in the United States, including their patient care focus, research, and program diversity.
Methods
We conducted an investigator-initiated, multicenter, cross-sectional survey study of all SoCCs in the United States and their respective academic residency programs. Fifteen formal SoCCs and/or SoCSCs were identified by dermatology program websites and an article by Tull et al2 on the state of ethnic skin centers. All programs and centers identified were associated with a dermatology residency program accredited by the Accreditation Council for Graduate Medical Education.
A 42-item questionnaire was sent via email to the directors of these centers and clinics with the intent to collect descriptive information about each of the SoCCs, the diversity of the faculty and residents of the associated dermatology department, current research and funding, diversity and inclusion initiatives, and trainee education from March through April 2020. Data were analyzed using Excel and SPSS statistical software to obtain descriptive statistics including the mean value numeric trends across programs.
This study underwent expedited review and was approved by the University of Southern California (Los Angeles, California) institutional review board (IRB #HS-20-00113). Patient consent was not applicable, as no information was collected about patients.
Results
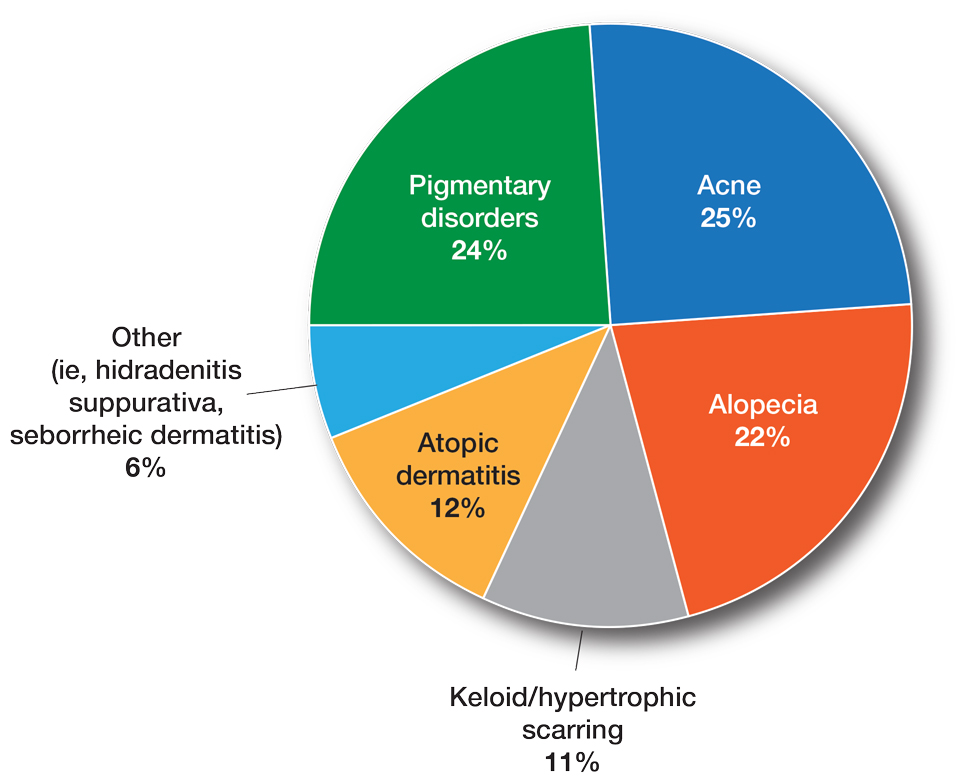
Fourteen directors from SoCCs/SoCSCs completed the questionnaire (93.3% response rate). Most centers were located in urban areas (12/14 [85.71%]), except for 2 in rural or suburban settings (Table). Most of the SoCCs/SoCSCs were located in the South (5/14 [35.71%]), followed by the Northeast (4/14 [28.57%]), West (3/14 [21.43%]), and Midwest (2/14 [14.29%])(Table). Six (42.86%) of the programs had a SoCSC, 3 (21.43%) had a formal SoCC, and 5 (35.71%) had both. Across all centers, the most common population seen and treated was Black/African American followed by Hispanic/Latino and Asian, respectively. The most commonly seen dermatologic conditions were acne, pigmentary disorders, alopecia, and atopic dermatitis (Figure). The most common cosmetic practice performed for patients with SoC was dermatosis papulosa nigra/seborrheic keratosis removal, followed by laser treatments, skin tag removal, chemical peels, and neuromodulator injections, respectively.
Faculty and Resident Demographics and Areas of Focus—The demographics and diversity of the dermatology faculty and residents at each individual institution also were assessed. The average number of full-time faculty at each institution was 19.4 (range, 2–48), while the average number of full-time faculty who identified as underrepresented in medicine (URiM) was 2.1 (range, 0–5). The average number of residents at each institution was 17.1 (range, 10–31), while the average number of URiM residents was 1.7 (range, 1–3).
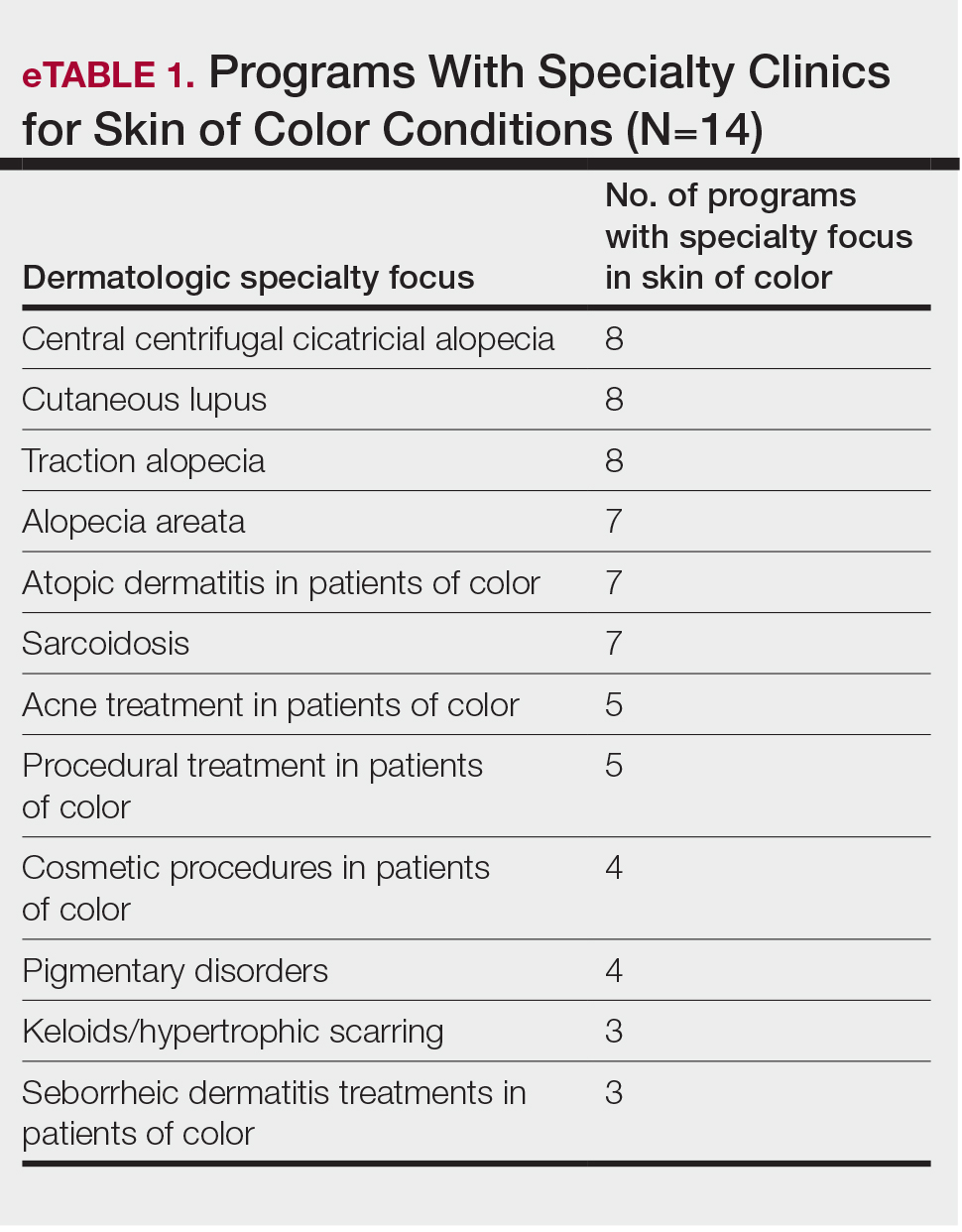
The average number of full-time faculty members at each SoCC was 1.6 (range, 1–4). The majority of program directors reported having other specialists in their department that also treated dermatologic conditions predominantly affecting patients with SoC (10/14 [71.43%]). The 3 most common areas of expertise were alopecia, including central centrifugal cicatricial alopecia (CCCA); cutaneous lupus; and traction alopecia (eTable 1).
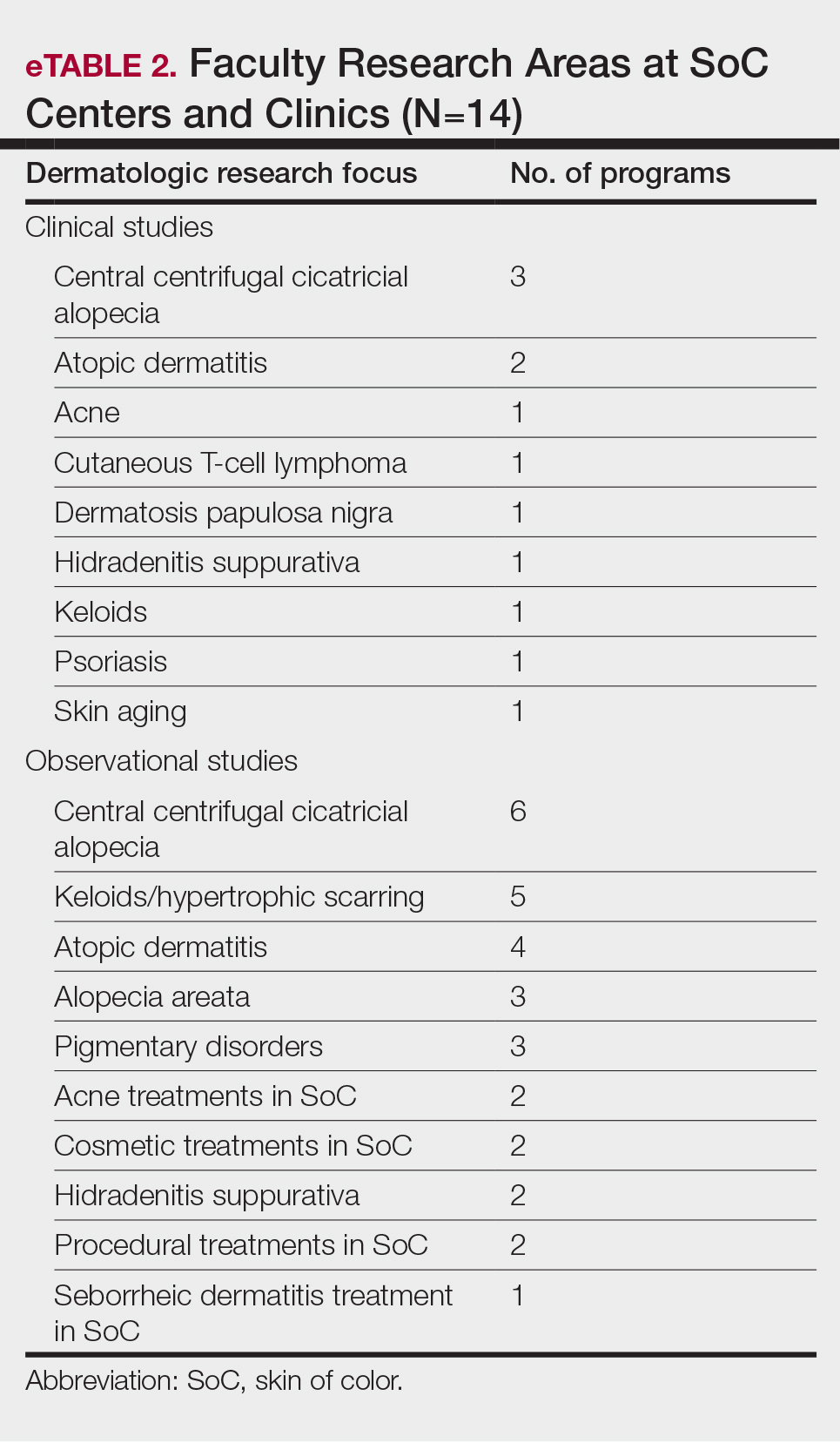
Faculty SoC Research—Only a minority of programs had active clinical trials related to SoC (5/14 [35.71%]). Clinical research was the most common type of research being conducted (11/14 [78.57%]), followed by basic science/translational (4/14 [28.57%]) and epidemiologic research (2/14 [14.29%]). The most commonly investigated conditions for observational studies included CCCA, keloids/hypertrophic scarring, and atopic dermatitis (eTable 2). Only 8 of 14 programs had formal SoC research opportunities for residents (57.14%), while 9 had opportunities for medical students (64.29%).
Few institutions had internal funding (3/14 [21.43%]) or external funding (4/14 [28.57%]) for SoC research. Extramural fun ding sources included the Skin of Color Society, the Dermatology Foundation, and the Radiation Oncology Institute, as well as industry funding. No federal funding was received by any of the sites.
Skin of Color Education and Diversity Initiatives—All 14 programs had residents rotating through their SoCC and/or SoCSCs. The vast majority (12/14 [85.71%]) indicated resident exposure to clinical training at the SoCC and/or SoCSC during all 3 years of training. Residents at most of the programs spent 1 to 3 months rotating at the SoCC/SoCSC (6/14 [42.86%]). The other programs indicated residents spent 3 to 6 months (3/14 [21.43%]) or longer than 6 months (3/14 [21.4%]), and only 2 programs (14.29%) indicated that residents spent less than 1 month in the SoCC/SoCSC.
The majority of programs offered a SoC didactic curriculum for residents (10/14 [71.43%]), with an average of 3.3 SoC-related lectures per year (range, 0–5). Almost all programs (13/14 [92.86%]) invited SoC specialists from outside institutions as guest lecturers. Half of the programs (7/14 [50.0%]) used a SoC textbook for resident education. Only 3 programs (21.43%) offered at least 1 introductory SoC dermatology lecture as part of the preclinical medical student dermatology curriculum.
Home institution medical students were able to rotate at their respective SoCC/SoCSC at 11 of 14 institutions (78.57%), while visiting students were able to rotate at half of the programs (7/14 [50.0%]). At some programs, rotating at the SoCC/SoCSC was optional and was not formally integrated into the medical student rotation schedule for both home and visiting students (1/14 [7.14%] and 4/14 [28.57%], respectively). A majority of the programs (8/14 [57.14%]) offered scholarships and/or grants for home and/or visiting URiM students to help fund away rotations.
Despite their SoC focus, only half of the programs with SoCCs/SoCSCs had a formal committee focused on diversity and inclusion (7/14 [50.0%]) Additionally, only 5 of 14 (35.71%) programs had any URiM outreach programs with the medical school and/or the local community.
Comment
As the number of SoCCs/SoCSCs in the United States continues to grow, it is important to highlight their programmatic, research, and educational accomplishments to show the benefits of such programs, including their ability to increase access to culturally competent and inclusive care for diverse patient populations. One study found that nearly 92% of patients in the United States seen by dermatologists are White.15 Although studies have shown that Hispanic/Latino and Black patients are less likely to seek care from a dermatologist,16,17 there is no indication that these patients have a lesser need for such specialty care. Additionally, outcomes of common dermatologic conditions often are poorer in SoC populations.15 The dermatologists leading SoCCs/SoCSCs are actively working to reverse these trends, with Black and Hispanic/Latino patients representing the majority of their patients.
Faculty and Resident Demographics and Areas of Focus—Although there are increased diversity efforts in dermatology and the medical profession more broadly, there still is much work to be done. While individuals with SoC now comprise more than 35% of the US population, only 12% of dermatology residents and 6% of academic dermatology faculty identify as either Black or Hispanic/Latino.5,8,10 These numbers are even more discouraging when considering other URiM racial groups such as Pacific Islander/Native Hawaiians or Native American/American Indians who represent 0% and 0.1% of dermatology faculty, respectively.8,10 Academic programs with SoCCs/SoCSCs are working to create a space in which these discrepancies in representation can begin to be addressed. Compared to the national 6.8% rate of URiM faculty at academic institutions, those with SoCCs/SoCSCs report closer to 10% of faculty identifying as URiM.18 Moreover, almost all programs had faculty specialized in at least 1 condition that predominantly affects patients with SoC. This is of critical importance, as the conditions that most commonly affect SoC populations—such as CCCA, hidradenitis suppurativa, and cutaneous lupus—often are understudied, underfunded, underdiagnosed, and undertreated.19-22
Faculty SoC Research—An important step in narrowing the knowledge gap and improving health care disparities in patients with SoC is to increase SoC research and/or to increase the representation of patients with SoC in research studies. In a 2021 study, a PubMed search of articles indexed for MEDLINE using the terms race/ethnicity, dyschromia, atopic dermatitis, and acne was conducted to investigate publications pertaining to the top 3 most common chief concerns in patients with SoC. Only 1.6% of studies analyzed (N=74,941) had a specific focus on SoC.12 A similar study found that among the top 5 dermatology-focused research journals, only 3.4% of all research (N=11,003) on the top 3 most common chief concerns in patients with SOC was conducted in patients with SoC.23 Research efforts focused on dermatologic issues that affect patients with SoC are a priority at SoCCs/SoCSCs. In our study, all respondents indicated that they had at least 1 ongoing observational study; the most commonly studied conditions were CCCA, keloids/hypertrophic scarring, and atopic dermatitis, all of which are conditions that either occur in high frequency or primarily occur in SoC. Only 35.71% (5/14) of respondents had active clinical trials related to SoC, and only 21.43% (3/14) and 28.57% (4/14) had internal and external funding, respectively. Although research efforts are a priority at SoCCs/SoCSCs, our survey study highlights the continued paucity of formal clinical trials as well as funding for SoC-focused research. Improved research efforts for SoC must address these deficits in funding, academic support, and other resources.
It also is of great importance for institutions to provide support for trainees wanting to pursue SoC research. Encouragingly, more than half (57.14%) of SoCCs/SoCSCs have developed formal research opportunities for residents, and nearly 64.29% have formal opportunities for medical students. These efforts to provide early experiences in SoC research are especially impactful by cultivating interest in working with populations with SoC and hopefully inspiring future dermatologists to engage in further SoC research.
SoC Education and Diversity Initiatives—Although it is important to increase representation of URiM physicians in dermatology and to train more SoC specialists, it is imperative that all dermatologists feel comfortable recognizing and treating dermatologic conditions in patients of all skin tones and all racial/ethnic backgrounds; however, many studies suggest that residents not only lack formal didactics and education in SoC, but even more unsettling, they also lack confidence in treating SoC.13,24 However, one study showed that this can be changed; Mhlaba et al25 assessed a SoC curriculum for dermatology residents, and indeed all of the residents indicated that the curriculum improved their ability to treat SoC patients. This deficit in dermatology residency training is specifically addressed by SoCCs/SoCSCs. In our study, all respondents indicated that residents rotate through their centers. Moreover, our study found that most of the academic institutions with SoCCs/SoCSCs provide a SoC didactic curriculum for residents, and almost all of the programs invited SoC specialists to give guest lectures. This is in contrast to a 2022 study showing that 63.2% (N=125) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures.14 These findings highlight the critical role that SoCCs/SoCSCs can provide in dermatology residency training.
Although SoCCs/SoCSCs have made considerable progress, there is still much room for improvement. Namely, only half of the respondents in our study indicated that their program has formally incorporated a SoC textbook into resident education (eTable 3). Representation of SoC in the textbooks that dermatology residents use is critically important because these images form the foundation of the morphologic aids of diagnosis. Numerous studies have analyzed popular dermatologic textbooks used by residency programs nationwide, finding the number of SoC images across dermatology textbooks ranging from 4% to 18%.26,27 The use of standard dermatology textbooks is not enough to train residents to be competent in diagnosing and treating patients with SoC. There should be a concerted effort across the field of dermatology to encourage the development of a SoC educational curriculum at every academic dermatology program, including SoC textbooks, Kodachromes, and online/electronic resources.
Efforts to increase diversity in dermatology and dermatologic training should start in medical school preclinical curriculums and medical student rotations. Although our survey did not assess current medical student curricula, the benefits of academic institutions with SoCCs/SoCSCs are highlighted by the ability for both home and visiting medical students to rotate through the centers and gain early exposure to SoC dermatology. Most of the programs even provide scholarships and/or grants for URiM students to help fund their rotations, which is of critical importance considering the mounting data that the financial burden of visiting rotations disproportionately affects URiM students.28
Study Limitations—Although we did an extensive search and believe to have correctly identified all 15 formal SoCCs/SoCSCs with a high response rate (93.3%), there are institutions that do not have formalized SoCCs/SoCSCs but are known to serve SoC populations. Likewise, there are private dermatology practices not associated with academic centers that have SoC specialists and positively contribute to SoC patient care, research, and education that were not included in this study. Additionally, the data for this study were collected in 2020 and analyzed in 2021, so it is possible that not all SoCCs, divisions, or clinics were included in this study, particularly if established after 2021.
Conclusion
As the United States continues to diversify, the proportion of patients with SoC will continue to grow, and it is imperative that this racial, ethnic, and cultural diversity is reflected in the dermatology workforce as well as research and training. The current deficits in medical training related to SoC populations and the importance for patients with SoC to find dermatologists who can appropriately treat them is well known.29 Skin of color centers/SoCSCs strive to increase access to care for patients with SoC, improve cultural competency, promote diversity among faculty and trainees, and encourage SoC research and education at all levels. We urge academic dermatology training programs to make SoC education, research, and patient care a departmental priority. Important first steps include departmental diversification at all levels, incorporating SoC into curricula for residents, providing and securing funding for SoC research, and supporting the establishment of more formal SoCCs and/or SoCSCs to help reduce dermatologic health care disparities among patients with SoC and improve health equity.
Appendix

- Colby SL, Jennifer JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. March 3, 2015. Accessed June 18, 2024. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020? J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117-140. doi:10.1007/s40615-017-0350-4
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Quick Facts: United States. US Census Bureau website. Accessed June 18, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045221
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49. doi:10.1016/j.jisp.2017.07.001
- Association of American Medical Colleges. Table B5. number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b5-md-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table B6. number of active DO residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b6-do-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table 16. U.S. medical school faculty by gender, race/ethnicity, and department, 2022. Accessed June 24, 2024. https://www.aamc.org/media/8456/download
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/jamadermatol.2021.5596
- Montgomery SNB, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in patients withskinof color. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618. doi:10.1016/j.jaad.2008.06.024
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291. doi:10.1001/jamadermatol.2018.3114
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 202;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Okeke CAV, Perry JD, Simmonds FC, et al. Clinical trials and skin of color: the example of hidradenitis suppurativa. dermatology. 2022;238:180-184. doi:10.1159/000516467
- Robles J, Anim T, Wusu MH, et al. An Approach to Faculty Development for Underrepresented Minorities in Medicine. South Med J. 2021;114(9):579-582. doi:10.14423/SMJ.0000000000001290
- Serrano L, Ulschmid C, Szabo A, et al. Racial disparities of delay in diagnosis and dermatologic care for hidradenitis suppurativa. J Natl Med Assoc. 2022;114:613-616. doi:10.1016/j.jnma.2022.08.002
- Drenkard C, Lim SS. Update on lupus epidemiology: advancinghealth disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689-696. doi:10.1097/BOR.0000000000000646
- Militello M, Szeto MD, Presley CL, et al. A quantitative analysis of research publications focused on skin of color: representation in academic dermatology journals. J Am Acad Dermatol. 2021;85:E189-E192. doi:10.1016/j.jaad.2021.04.053
- Cline A, Winter RP, Kourosh S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Mhlaba JM, Pontes DS, Patterson SS, et al. Evaluation of a skin of color curriculum for dermatology residents. J Drugs Dermatol. 2021;20:786-789. doi:10.36849/JDD.6193
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Harp T, Militello M, McCarver V, et al. Further analysis of skin of color representation in dermatology textbooks used by residents. J Am Acad Dermatol. 2022;87:E39-E41. doi:10.1016/j.jaad.2022.02.069
- Muzumdar S, Grant-Kels JM, Feng H. Strategies to improve medical student visiting rotations. Clin Dermatol. 2021;39:727-728. doi:10.1016/j.clindermatol.2020.11.001
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
Although individuals with skin of color (SoC) are expected to become at least half of the US population by the year 2044, there remains a paucity of education and exposure to treatment of patients with SoC at many dermatology residency programs across the country.1 One way to improve SoC education has been the formation of specialized clinics, centers, and programs. The first SoC center (SoCC) was established in 1999 at Mount Sinai–St. Luke’s Roosevelt in New York, New York2; since then, at least 13 additional formal SoCCs or SoC specialty clinics (SoCSCs) at US academic dermatology programs have been established.
Skin of color centers serve several important purposes: they improve dermatologic care in patients with SoC, increase research efforts focused on SoC dermatologic conditions, and educate dermatology resident and fellow trainees about SoC. Improving dermatologic care of patients with SoC in the United States is important in providing equitable health care and improving health disparities. Studies have shown that patient-physician racial and cultural concordance can positively impact patient care, increase patient trust and rapport, and improve patient-physician communication, and it can even influence patient decision-making to seek care.3,4 Unfortunately, even though the US population continues to diversify, the racial/ethnic backgrounds of dermatologists do not parallel this trend; Hispanic and Black physicians comprise 18.9% and 13.6% of the general population, respectively, but represent only 4.2% and 3.0% of dermatologists, respectively.5-7 This deficit is mirrored by resident and faculty representation, with Black and Latino representation ranging from 3% to 7%.8-10
Many SoCC’s engage in research focused on dermatologic conditions affecting patients with SoC, which is vital to improving the dermatologic care in this underserved population. Despite increasing recognition of the importance of SoC research, there remains a paucity of clinical trials and research specifically focused on or demonstrating equitable representation of SoC.11,12
The education and training of future dermatologists is another important area that can be improved by SoCCs. A 2008 study involving 63 chief residents showed that approximately half (52.4% [33/63]) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and 30.2% (19/63) reported having a dedicated rotation where they gained specific experience treating patients with SoC.13 A later study in 2022 (N=125) found that 63.2% of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and only 11.2% reported having a dedicated rotation where they gained experience treating patients with SoC.14 These findings suggest that in the last 14 years, formal SoC education—specifically SoC clinical training—has not increased sufficiently.
We conducted a cross-sectional survey study to provide an in-depth analysis of SoCCs and SoCSCs in the United States, including their patient care focus, research, and program diversity.
Methods
We conducted an investigator-initiated, multicenter, cross-sectional survey study of all SoCCs in the United States and their respective academic residency programs. Fifteen formal SoCCs and/or SoCSCs were identified by dermatology program websites and an article by Tull et al2 on the state of ethnic skin centers. All programs and centers identified were associated with a dermatology residency program accredited by the Accreditation Council for Graduate Medical Education.
A 42-item questionnaire was sent via email to the directors of these centers and clinics with the intent to collect descriptive information about each of the SoCCs, the diversity of the faculty and residents of the associated dermatology department, current research and funding, diversity and inclusion initiatives, and trainee education from March through April 2020. Data were analyzed using Excel and SPSS statistical software to obtain descriptive statistics including the mean value numeric trends across programs.
This study underwent expedited review and was approved by the University of Southern California (Los Angeles, California) institutional review board (IRB #HS-20-00113). Patient consent was not applicable, as no information was collected about patients.
Results
Fourteen directors from SoCCs/SoCSCs completed the questionnaire (93.3% response rate). Most centers were located in urban areas (12/14 [85.71%]), except for 2 in rural or suburban settings (Table). Most of the SoCCs/SoCSCs were located in the South (5/14 [35.71%]), followed by the Northeast (4/14 [28.57%]), West (3/14 [21.43%]), and Midwest (2/14 [14.29%])(Table). Six (42.86%) of the programs had a SoCSC, 3 (21.43%) had a formal SoCC, and 5 (35.71%) had both. Across all centers, the most common population seen and treated was Black/African American followed by Hispanic/Latino and Asian, respectively. The most commonly seen dermatologic conditions were acne, pigmentary disorders, alopecia, and atopic dermatitis (Figure). The most common cosmetic practice performed for patients with SoC was dermatosis papulosa nigra/seborrheic keratosis removal, followed by laser treatments, skin tag removal, chemical peels, and neuromodulator injections, respectively.

Faculty and Resident Demographics and Areas of Focus—The demographics and diversity of the dermatology faculty and residents at each individual institution also were assessed. The average number of full-time faculty at each institution was 19.4 (range, 2–48), while the average number of full-time faculty who identified as underrepresented in medicine (URiM) was 2.1 (range, 0–5). The average number of residents at each institution was 17.1 (range, 10–31), while the average number of URiM residents was 1.7 (range, 1–3).

The average number of full-time faculty members at each SoCC was 1.6 (range, 1–4). The majority of program directors reported having other specialists in their department that also treated dermatologic conditions predominantly affecting patients with SoC (10/14 [71.43%]). The 3 most common areas of expertise were alopecia, including central centrifugal cicatricial alopecia (CCCA); cutaneous lupus; and traction alopecia (eTable 1).
Faculty SoC Research—Only a minority of programs had active clinical trials related to SoC (5/14 [35.71%]). Clinical research was the most common type of research being conducted (11/14 [78.57%]), followed by basic science/translational (4/14 [28.57%]) and epidemiologic research (2/14 [14.29%]). The most commonly investigated conditions for observational studies included CCCA, keloids/hypertrophic scarring, and atopic dermatitis (eTable 2). Only 8 of 14 programs had formal SoC research opportunities for residents (57.14%), while 9 had opportunities for medical students (64.29%).
Few institutions had internal funding (3/14 [21.43%]) or external funding (4/14 [28.57%]) for SoC research. Extramural fun ding sources included the Skin of Color Society, the Dermatology Foundation, and the Radiation Oncology Institute, as well as industry funding. No federal funding was received by any of the sites.
Skin of Color Education and Diversity Initiatives—All 14 programs had residents rotating through their SoCC and/or SoCSCs. The vast majority (12/14 [85.71%]) indicated resident exposure to clinical training at the SoCC and/or SoCSC during all 3 years of training. Residents at most of the programs spent 1 to 3 months rotating at the SoCC/SoCSC (6/14 [42.86%]). The other programs indicated residents spent 3 to 6 months (3/14 [21.43%]) or longer than 6 months (3/14 [21.4%]), and only 2 programs (14.29%) indicated that residents spent less than 1 month in the SoCC/SoCSC.
The majority of programs offered a SoC didactic curriculum for residents (10/14 [71.43%]), with an average of 3.3 SoC-related lectures per year (range, 0–5). Almost all programs (13/14 [92.86%]) invited SoC specialists from outside institutions as guest lecturers. Half of the programs (7/14 [50.0%]) used a SoC textbook for resident education. Only 3 programs (21.43%) offered at least 1 introductory SoC dermatology lecture as part of the preclinical medical student dermatology curriculum.
Home institution medical students were able to rotate at their respective SoCC/SoCSC at 11 of 14 institutions (78.57%), while visiting students were able to rotate at half of the programs (7/14 [50.0%]). At some programs, rotating at the SoCC/SoCSC was optional and was not formally integrated into the medical student rotation schedule for both home and visiting students (1/14 [7.14%] and 4/14 [28.57%], respectively). A majority of the programs (8/14 [57.14%]) offered scholarships and/or grants for home and/or visiting URiM students to help fund away rotations.
Despite their SoC focus, only half of the programs with SoCCs/SoCSCs had a formal committee focused on diversity and inclusion (7/14 [50.0%]) Additionally, only 5 of 14 (35.71%) programs had any URiM outreach programs with the medical school and/or the local community.
Comment
As the number of SoCCs/SoCSCs in the United States continues to grow, it is important to highlight their programmatic, research, and educational accomplishments to show the benefits of such programs, including their ability to increase access to culturally competent and inclusive care for diverse patient populations. One study found that nearly 92% of patients in the United States seen by dermatologists are White.15 Although studies have shown that Hispanic/Latino and Black patients are less likely to seek care from a dermatologist,16,17 there is no indication that these patients have a lesser need for such specialty care. Additionally, outcomes of common dermatologic conditions often are poorer in SoC populations.15 The dermatologists leading SoCCs/SoCSCs are actively working to reverse these trends, with Black and Hispanic/Latino patients representing the majority of their patients.
Faculty and Resident Demographics and Areas of Focus—Although there are increased diversity efforts in dermatology and the medical profession more broadly, there still is much work to be done. While individuals with SoC now comprise more than 35% of the US population, only 12% of dermatology residents and 6% of academic dermatology faculty identify as either Black or Hispanic/Latino.5,8,10 These numbers are even more discouraging when considering other URiM racial groups such as Pacific Islander/Native Hawaiians or Native American/American Indians who represent 0% and 0.1% of dermatology faculty, respectively.8,10 Academic programs with SoCCs/SoCSCs are working to create a space in which these discrepancies in representation can begin to be addressed. Compared to the national 6.8% rate of URiM faculty at academic institutions, those with SoCCs/SoCSCs report closer to 10% of faculty identifying as URiM.18 Moreover, almost all programs had faculty specialized in at least 1 condition that predominantly affects patients with SoC. This is of critical importance, as the conditions that most commonly affect SoC populations—such as CCCA, hidradenitis suppurativa, and cutaneous lupus—often are understudied, underfunded, underdiagnosed, and undertreated.19-22
Faculty SoC Research—An important step in narrowing the knowledge gap and improving health care disparities in patients with SoC is to increase SoC research and/or to increase the representation of patients with SoC in research studies. In a 2021 study, a PubMed search of articles indexed for MEDLINE using the terms race/ethnicity, dyschromia, atopic dermatitis, and acne was conducted to investigate publications pertaining to the top 3 most common chief concerns in patients with SoC. Only 1.6% of studies analyzed (N=74,941) had a specific focus on SoC.12 A similar study found that among the top 5 dermatology-focused research journals, only 3.4% of all research (N=11,003) on the top 3 most common chief concerns in patients with SOC was conducted in patients with SoC.23 Research efforts focused on dermatologic issues that affect patients with SoC are a priority at SoCCs/SoCSCs. In our study, all respondents indicated that they had at least 1 ongoing observational study; the most commonly studied conditions were CCCA, keloids/hypertrophic scarring, and atopic dermatitis, all of which are conditions that either occur in high frequency or primarily occur in SoC. Only 35.71% (5/14) of respondents had active clinical trials related to SoC, and only 21.43% (3/14) and 28.57% (4/14) had internal and external funding, respectively. Although research efforts are a priority at SoCCs/SoCSCs, our survey study highlights the continued paucity of formal clinical trials as well as funding for SoC-focused research. Improved research efforts for SoC must address these deficits in funding, academic support, and other resources.
It also is of great importance for institutions to provide support for trainees wanting to pursue SoC research. Encouragingly, more than half (57.14%) of SoCCs/SoCSCs have developed formal research opportunities for residents, and nearly 64.29% have formal opportunities for medical students. These efforts to provide early experiences in SoC research are especially impactful by cultivating interest in working with populations with SoC and hopefully inspiring future dermatologists to engage in further SoC research.
SoC Education and Diversity Initiatives—Although it is important to increase representation of URiM physicians in dermatology and to train more SoC specialists, it is imperative that all dermatologists feel comfortable recognizing and treating dermatologic conditions in patients of all skin tones and all racial/ethnic backgrounds; however, many studies suggest that residents not only lack formal didactics and education in SoC, but even more unsettling, they also lack confidence in treating SoC.13,24 However, one study showed that this can be changed; Mhlaba et al25 assessed a SoC curriculum for dermatology residents, and indeed all of the residents indicated that the curriculum improved their ability to treat SoC patients. This deficit in dermatology residency training is specifically addressed by SoCCs/SoCSCs. In our study, all respondents indicated that residents rotate through their centers. Moreover, our study found that most of the academic institutions with SoCCs/SoCSCs provide a SoC didactic curriculum for residents, and almost all of the programs invited SoC specialists to give guest lectures. This is in contrast to a 2022 study showing that 63.2% (N=125) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures.14 These findings highlight the critical role that SoCCs/SoCSCs can provide in dermatology residency training.
Although SoCCs/SoCSCs have made considerable progress, there is still much room for improvement. Namely, only half of the respondents in our study indicated that their program has formally incorporated a SoC textbook into resident education (eTable 3). Representation of SoC in the textbooks that dermatology residents use is critically important because these images form the foundation of the morphologic aids of diagnosis. Numerous studies have analyzed popular dermatologic textbooks used by residency programs nationwide, finding the number of SoC images across dermatology textbooks ranging from 4% to 18%.26,27 The use of standard dermatology textbooks is not enough to train residents to be competent in diagnosing and treating patients with SoC. There should be a concerted effort across the field of dermatology to encourage the development of a SoC educational curriculum at every academic dermatology program, including SoC textbooks, Kodachromes, and online/electronic resources.
Efforts to increase diversity in dermatology and dermatologic training should start in medical school preclinical curriculums and medical student rotations. Although our survey did not assess current medical student curricula, the benefits of academic institutions with SoCCs/SoCSCs are highlighted by the ability for both home and visiting medical students to rotate through the centers and gain early exposure to SoC dermatology. Most of the programs even provide scholarships and/or grants for URiM students to help fund their rotations, which is of critical importance considering the mounting data that the financial burden of visiting rotations disproportionately affects URiM students.28
Study Limitations—Although we did an extensive search and believe to have correctly identified all 15 formal SoCCs/SoCSCs with a high response rate (93.3%), there are institutions that do not have formalized SoCCs/SoCSCs but are known to serve SoC populations. Likewise, there are private dermatology practices not associated with academic centers that have SoC specialists and positively contribute to SoC patient care, research, and education that were not included in this study. Additionally, the data for this study were collected in 2020 and analyzed in 2021, so it is possible that not all SoCCs, divisions, or clinics were included in this study, particularly if established after 2021.
Conclusion
As the United States continues to diversify, the proportion of patients with SoC will continue to grow, and it is imperative that this racial, ethnic, and cultural diversity is reflected in the dermatology workforce as well as research and training. The current deficits in medical training related to SoC populations and the importance for patients with SoC to find dermatologists who can appropriately treat them is well known.29 Skin of color centers/SoCSCs strive to increase access to care for patients with SoC, improve cultural competency, promote diversity among faculty and trainees, and encourage SoC research and education at all levels. We urge academic dermatology training programs to make SoC education, research, and patient care a departmental priority. Important first steps include departmental diversification at all levels, incorporating SoC into curricula for residents, providing and securing funding for SoC research, and supporting the establishment of more formal SoCCs and/or SoCSCs to help reduce dermatologic health care disparities among patients with SoC and improve health equity.
Appendix



Although individuals with skin of color (SoC) are expected to become at least half of the US population by the year 2044, there remains a paucity of education and exposure to treatment of patients with SoC at many dermatology residency programs across the country.1 One way to improve SoC education has been the formation of specialized clinics, centers, and programs. The first SoC center (SoCC) was established in 1999 at Mount Sinai–St. Luke’s Roosevelt in New York, New York2; since then, at least 13 additional formal SoCCs or SoC specialty clinics (SoCSCs) at US academic dermatology programs have been established.
Skin of color centers serve several important purposes: they improve dermatologic care in patients with SoC, increase research efforts focused on SoC dermatologic conditions, and educate dermatology resident and fellow trainees about SoC. Improving dermatologic care of patients with SoC in the United States is important in providing equitable health care and improving health disparities. Studies have shown that patient-physician racial and cultural concordance can positively impact patient care, increase patient trust and rapport, and improve patient-physician communication, and it can even influence patient decision-making to seek care.3,4 Unfortunately, even though the US population continues to diversify, the racial/ethnic backgrounds of dermatologists do not parallel this trend; Hispanic and Black physicians comprise 18.9% and 13.6% of the general population, respectively, but represent only 4.2% and 3.0% of dermatologists, respectively.5-7 This deficit is mirrored by resident and faculty representation, with Black and Latino representation ranging from 3% to 7%.8-10
Many SoCC’s engage in research focused on dermatologic conditions affecting patients with SoC, which is vital to improving the dermatologic care in this underserved population. Despite increasing recognition of the importance of SoC research, there remains a paucity of clinical trials and research specifically focused on or demonstrating equitable representation of SoC.11,12
The education and training of future dermatologists is another important area that can be improved by SoCCs. A 2008 study involving 63 chief residents showed that approximately half (52.4% [33/63]) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and 30.2% (19/63) reported having a dedicated rotation where they gained specific experience treating patients with SoC.13 A later study in 2022 (N=125) found that 63.2% of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and only 11.2% reported having a dedicated rotation where they gained experience treating patients with SoC.14 These findings suggest that in the last 14 years, formal SoC education—specifically SoC clinical training—has not increased sufficiently.
We conducted a cross-sectional survey study to provide an in-depth analysis of SoCCs and SoCSCs in the United States, including their patient care focus, research, and program diversity.
Methods
We conducted an investigator-initiated, multicenter, cross-sectional survey study of all SoCCs in the United States and their respective academic residency programs. Fifteen formal SoCCs and/or SoCSCs were identified by dermatology program websites and an article by Tull et al2 on the state of ethnic skin centers. All programs and centers identified were associated with a dermatology residency program accredited by the Accreditation Council for Graduate Medical Education.
A 42-item questionnaire was sent via email to the directors of these centers and clinics with the intent to collect descriptive information about each of the SoCCs, the diversity of the faculty and residents of the associated dermatology department, current research and funding, diversity and inclusion initiatives, and trainee education from March through April 2020. Data were analyzed using Excel and SPSS statistical software to obtain descriptive statistics including the mean value numeric trends across programs.
This study underwent expedited review and was approved by the University of Southern California (Los Angeles, California) institutional review board (IRB #HS-20-00113). Patient consent was not applicable, as no information was collected about patients.
Results
Fourteen directors from SoCCs/SoCSCs completed the questionnaire (93.3% response rate). Most centers were located in urban areas (12/14 [85.71%]), except for 2 in rural or suburban settings (Table). Most of the SoCCs/SoCSCs were located in the South (5/14 [35.71%]), followed by the Northeast (4/14 [28.57%]), West (3/14 [21.43%]), and Midwest (2/14 [14.29%])(Table). Six (42.86%) of the programs had a SoCSC, 3 (21.43%) had a formal SoCC, and 5 (35.71%) had both. Across all centers, the most common population seen and treated was Black/African American followed by Hispanic/Latino and Asian, respectively. The most commonly seen dermatologic conditions were acne, pigmentary disorders, alopecia, and atopic dermatitis (Figure). The most common cosmetic practice performed for patients with SoC was dermatosis papulosa nigra/seborrheic keratosis removal, followed by laser treatments, skin tag removal, chemical peels, and neuromodulator injections, respectively.

Faculty and Resident Demographics and Areas of Focus—The demographics and diversity of the dermatology faculty and residents at each individual institution also were assessed. The average number of full-time faculty at each institution was 19.4 (range, 2–48), while the average number of full-time faculty who identified as underrepresented in medicine (URiM) was 2.1 (range, 0–5). The average number of residents at each institution was 17.1 (range, 10–31), while the average number of URiM residents was 1.7 (range, 1–3).

The average number of full-time faculty members at each SoCC was 1.6 (range, 1–4). The majority of program directors reported having other specialists in their department that also treated dermatologic conditions predominantly affecting patients with SoC (10/14 [71.43%]). The 3 most common areas of expertise were alopecia, including central centrifugal cicatricial alopecia (CCCA); cutaneous lupus; and traction alopecia (eTable 1).
Faculty SoC Research—Only a minority of programs had active clinical trials related to SoC (5/14 [35.71%]). Clinical research was the most common type of research being conducted (11/14 [78.57%]), followed by basic science/translational (4/14 [28.57%]) and epidemiologic research (2/14 [14.29%]). The most commonly investigated conditions for observational studies included CCCA, keloids/hypertrophic scarring, and atopic dermatitis (eTable 2). Only 8 of 14 programs had formal SoC research opportunities for residents (57.14%), while 9 had opportunities for medical students (64.29%).
Few institutions had internal funding (3/14 [21.43%]) or external funding (4/14 [28.57%]) for SoC research. Extramural fun ding sources included the Skin of Color Society, the Dermatology Foundation, and the Radiation Oncology Institute, as well as industry funding. No federal funding was received by any of the sites.
Skin of Color Education and Diversity Initiatives—All 14 programs had residents rotating through their SoCC and/or SoCSCs. The vast majority (12/14 [85.71%]) indicated resident exposure to clinical training at the SoCC and/or SoCSC during all 3 years of training. Residents at most of the programs spent 1 to 3 months rotating at the SoCC/SoCSC (6/14 [42.86%]). The other programs indicated residents spent 3 to 6 months (3/14 [21.43%]) or longer than 6 months (3/14 [21.4%]), and only 2 programs (14.29%) indicated that residents spent less than 1 month in the SoCC/SoCSC.
The majority of programs offered a SoC didactic curriculum for residents (10/14 [71.43%]), with an average of 3.3 SoC-related lectures per year (range, 0–5). Almost all programs (13/14 [92.86%]) invited SoC specialists from outside institutions as guest lecturers. Half of the programs (7/14 [50.0%]) used a SoC textbook for resident education. Only 3 programs (21.43%) offered at least 1 introductory SoC dermatology lecture as part of the preclinical medical student dermatology curriculum.
Home institution medical students were able to rotate at their respective SoCC/SoCSC at 11 of 14 institutions (78.57%), while visiting students were able to rotate at half of the programs (7/14 [50.0%]). At some programs, rotating at the SoCC/SoCSC was optional and was not formally integrated into the medical student rotation schedule for both home and visiting students (1/14 [7.14%] and 4/14 [28.57%], respectively). A majority of the programs (8/14 [57.14%]) offered scholarships and/or grants for home and/or visiting URiM students to help fund away rotations.
Despite their SoC focus, only half of the programs with SoCCs/SoCSCs had a formal committee focused on diversity and inclusion (7/14 [50.0%]) Additionally, only 5 of 14 (35.71%) programs had any URiM outreach programs with the medical school and/or the local community.
Comment
As the number of SoCCs/SoCSCs in the United States continues to grow, it is important to highlight their programmatic, research, and educational accomplishments to show the benefits of such programs, including their ability to increase access to culturally competent and inclusive care for diverse patient populations. One study found that nearly 92% of patients in the United States seen by dermatologists are White.15 Although studies have shown that Hispanic/Latino and Black patients are less likely to seek care from a dermatologist,16,17 there is no indication that these patients have a lesser need for such specialty care. Additionally, outcomes of common dermatologic conditions often are poorer in SoC populations.15 The dermatologists leading SoCCs/SoCSCs are actively working to reverse these trends, with Black and Hispanic/Latino patients representing the majority of their patients.
Faculty and Resident Demographics and Areas of Focus—Although there are increased diversity efforts in dermatology and the medical profession more broadly, there still is much work to be done. While individuals with SoC now comprise more than 35% of the US population, only 12% of dermatology residents and 6% of academic dermatology faculty identify as either Black or Hispanic/Latino.5,8,10 These numbers are even more discouraging when considering other URiM racial groups such as Pacific Islander/Native Hawaiians or Native American/American Indians who represent 0% and 0.1% of dermatology faculty, respectively.8,10 Academic programs with SoCCs/SoCSCs are working to create a space in which these discrepancies in representation can begin to be addressed. Compared to the national 6.8% rate of URiM faculty at academic institutions, those with SoCCs/SoCSCs report closer to 10% of faculty identifying as URiM.18 Moreover, almost all programs had faculty specialized in at least 1 condition that predominantly affects patients with SoC. This is of critical importance, as the conditions that most commonly affect SoC populations—such as CCCA, hidradenitis suppurativa, and cutaneous lupus—often are understudied, underfunded, underdiagnosed, and undertreated.19-22
Faculty SoC Research—An important step in narrowing the knowledge gap and improving health care disparities in patients with SoC is to increase SoC research and/or to increase the representation of patients with SoC in research studies. In a 2021 study, a PubMed search of articles indexed for MEDLINE using the terms race/ethnicity, dyschromia, atopic dermatitis, and acne was conducted to investigate publications pertaining to the top 3 most common chief concerns in patients with SoC. Only 1.6% of studies analyzed (N=74,941) had a specific focus on SoC.12 A similar study found that among the top 5 dermatology-focused research journals, only 3.4% of all research (N=11,003) on the top 3 most common chief concerns in patients with SOC was conducted in patients with SoC.23 Research efforts focused on dermatologic issues that affect patients with SoC are a priority at SoCCs/SoCSCs. In our study, all respondents indicated that they had at least 1 ongoing observational study; the most commonly studied conditions were CCCA, keloids/hypertrophic scarring, and atopic dermatitis, all of which are conditions that either occur in high frequency or primarily occur in SoC. Only 35.71% (5/14) of respondents had active clinical trials related to SoC, and only 21.43% (3/14) and 28.57% (4/14) had internal and external funding, respectively. Although research efforts are a priority at SoCCs/SoCSCs, our survey study highlights the continued paucity of formal clinical trials as well as funding for SoC-focused research. Improved research efforts for SoC must address these deficits in funding, academic support, and other resources.
It also is of great importance for institutions to provide support for trainees wanting to pursue SoC research. Encouragingly, more than half (57.14%) of SoCCs/SoCSCs have developed formal research opportunities for residents, and nearly 64.29% have formal opportunities for medical students. These efforts to provide early experiences in SoC research are especially impactful by cultivating interest in working with populations with SoC and hopefully inspiring future dermatologists to engage in further SoC research.
SoC Education and Diversity Initiatives—Although it is important to increase representation of URiM physicians in dermatology and to train more SoC specialists, it is imperative that all dermatologists feel comfortable recognizing and treating dermatologic conditions in patients of all skin tones and all racial/ethnic backgrounds; however, many studies suggest that residents not only lack formal didactics and education in SoC, but even more unsettling, they also lack confidence in treating SoC.13,24 However, one study showed that this can be changed; Mhlaba et al25 assessed a SoC curriculum for dermatology residents, and indeed all of the residents indicated that the curriculum improved their ability to treat SoC patients. This deficit in dermatology residency training is specifically addressed by SoCCs/SoCSCs. In our study, all respondents indicated that residents rotate through their centers. Moreover, our study found that most of the academic institutions with SoCCs/SoCSCs provide a SoC didactic curriculum for residents, and almost all of the programs invited SoC specialists to give guest lectures. This is in contrast to a 2022 study showing that 63.2% (N=125) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures.14 These findings highlight the critical role that SoCCs/SoCSCs can provide in dermatology residency training.
Although SoCCs/SoCSCs have made considerable progress, there is still much room for improvement. Namely, only half of the respondents in our study indicated that their program has formally incorporated a SoC textbook into resident education (eTable 3). Representation of SoC in the textbooks that dermatology residents use is critically important because these images form the foundation of the morphologic aids of diagnosis. Numerous studies have analyzed popular dermatologic textbooks used by residency programs nationwide, finding the number of SoC images across dermatology textbooks ranging from 4% to 18%.26,27 The use of standard dermatology textbooks is not enough to train residents to be competent in diagnosing and treating patients with SoC. There should be a concerted effort across the field of dermatology to encourage the development of a SoC educational curriculum at every academic dermatology program, including SoC textbooks, Kodachromes, and online/electronic resources.
Efforts to increase diversity in dermatology and dermatologic training should start in medical school preclinical curriculums and medical student rotations. Although our survey did not assess current medical student curricula, the benefits of academic institutions with SoCCs/SoCSCs are highlighted by the ability for both home and visiting medical students to rotate through the centers and gain early exposure to SoC dermatology. Most of the programs even provide scholarships and/or grants for URiM students to help fund their rotations, which is of critical importance considering the mounting data that the financial burden of visiting rotations disproportionately affects URiM students.28
Study Limitations—Although we did an extensive search and believe to have correctly identified all 15 formal SoCCs/SoCSCs with a high response rate (93.3%), there are institutions that do not have formalized SoCCs/SoCSCs but are known to serve SoC populations. Likewise, there are private dermatology practices not associated with academic centers that have SoC specialists and positively contribute to SoC patient care, research, and education that were not included in this study. Additionally, the data for this study were collected in 2020 and analyzed in 2021, so it is possible that not all SoCCs, divisions, or clinics were included in this study, particularly if established after 2021.
Conclusion
As the United States continues to diversify, the proportion of patients with SoC will continue to grow, and it is imperative that this racial, ethnic, and cultural diversity is reflected in the dermatology workforce as well as research and training. The current deficits in medical training related to SoC populations and the importance for patients with SoC to find dermatologists who can appropriately treat them is well known.29 Skin of color centers/SoCSCs strive to increase access to care for patients with SoC, improve cultural competency, promote diversity among faculty and trainees, and encourage SoC research and education at all levels. We urge academic dermatology training programs to make SoC education, research, and patient care a departmental priority. Important first steps include departmental diversification at all levels, incorporating SoC into curricula for residents, providing and securing funding for SoC research, and supporting the establishment of more formal SoCCs and/or SoCSCs to help reduce dermatologic health care disparities among patients with SoC and improve health equity.
Appendix



- Colby SL, Jennifer JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. March 3, 2015. Accessed June 18, 2024. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020? J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117-140. doi:10.1007/s40615-017-0350-4
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Quick Facts: United States. US Census Bureau website. Accessed June 18, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045221
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49. doi:10.1016/j.jisp.2017.07.001
- Association of American Medical Colleges. Table B5. number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b5-md-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table B6. number of active DO residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b6-do-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table 16. U.S. medical school faculty by gender, race/ethnicity, and department, 2022. Accessed June 24, 2024. https://www.aamc.org/media/8456/download
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/jamadermatol.2021.5596
- Montgomery SNB, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in patients withskinof color. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618. doi:10.1016/j.jaad.2008.06.024
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291. doi:10.1001/jamadermatol.2018.3114
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 202;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Okeke CAV, Perry JD, Simmonds FC, et al. Clinical trials and skin of color: the example of hidradenitis suppurativa. dermatology. 2022;238:180-184. doi:10.1159/000516467
- Robles J, Anim T, Wusu MH, et al. An Approach to Faculty Development for Underrepresented Minorities in Medicine. South Med J. 2021;114(9):579-582. doi:10.14423/SMJ.0000000000001290
- Serrano L, Ulschmid C, Szabo A, et al. Racial disparities of delay in diagnosis and dermatologic care for hidradenitis suppurativa. J Natl Med Assoc. 2022;114:613-616. doi:10.1016/j.jnma.2022.08.002
- Drenkard C, Lim SS. Update on lupus epidemiology: advancinghealth disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689-696. doi:10.1097/BOR.0000000000000646
- Militello M, Szeto MD, Presley CL, et al. A quantitative analysis of research publications focused on skin of color: representation in academic dermatology journals. J Am Acad Dermatol. 2021;85:E189-E192. doi:10.1016/j.jaad.2021.04.053
- Cline A, Winter RP, Kourosh S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Mhlaba JM, Pontes DS, Patterson SS, et al. Evaluation of a skin of color curriculum for dermatology residents. J Drugs Dermatol. 2021;20:786-789. doi:10.36849/JDD.6193
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Harp T, Militello M, McCarver V, et al. Further analysis of skin of color representation in dermatology textbooks used by residents. J Am Acad Dermatol. 2022;87:E39-E41. doi:10.1016/j.jaad.2022.02.069
- Muzumdar S, Grant-Kels JM, Feng H. Strategies to improve medical student visiting rotations. Clin Dermatol. 2021;39:727-728. doi:10.1016/j.clindermatol.2020.11.001
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Colby SL, Jennifer JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. March 3, 2015. Accessed June 18, 2024. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020? J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117-140. doi:10.1007/s40615-017-0350-4
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Quick Facts: United States. US Census Bureau website. Accessed June 18, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045221
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49. doi:10.1016/j.jisp.2017.07.001
- Association of American Medical Colleges. Table B5. number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b5-md-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table B6. number of active DO residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b6-do-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table 16. U.S. medical school faculty by gender, race/ethnicity, and department, 2022. Accessed June 24, 2024. https://www.aamc.org/media/8456/download
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/jamadermatol.2021.5596
- Montgomery SNB, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in patients withskinof color. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618. doi:10.1016/j.jaad.2008.06.024
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291. doi:10.1001/jamadermatol.2018.3114
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 202;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Okeke CAV, Perry JD, Simmonds FC, et al. Clinical trials and skin of color: the example of hidradenitis suppurativa. dermatology. 2022;238:180-184. doi:10.1159/000516467
- Robles J, Anim T, Wusu MH, et al. An Approach to Faculty Development for Underrepresented Minorities in Medicine. South Med J. 2021;114(9):579-582. doi:10.14423/SMJ.0000000000001290
- Serrano L, Ulschmid C, Szabo A, et al. Racial disparities of delay in diagnosis and dermatologic care for hidradenitis suppurativa. J Natl Med Assoc. 2022;114:613-616. doi:10.1016/j.jnma.2022.08.002
- Drenkard C, Lim SS. Update on lupus epidemiology: advancinghealth disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689-696. doi:10.1097/BOR.0000000000000646
- Militello M, Szeto MD, Presley CL, et al. A quantitative analysis of research publications focused on skin of color: representation in academic dermatology journals. J Am Acad Dermatol. 2021;85:E189-E192. doi:10.1016/j.jaad.2021.04.053
- Cline A, Winter RP, Kourosh S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Mhlaba JM, Pontes DS, Patterson SS, et al. Evaluation of a skin of color curriculum for dermatology residents. J Drugs Dermatol. 2021;20:786-789. doi:10.36849/JDD.6193
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Harp T, Militello M, McCarver V, et al. Further analysis of skin of color representation in dermatology textbooks used by residents. J Am Acad Dermatol. 2022;87:E39-E41. doi:10.1016/j.jaad.2022.02.069
- Muzumdar S, Grant-Kels JM, Feng H. Strategies to improve medical student visiting rotations. Clin Dermatol. 2021;39:727-728. doi:10.1016/j.clindermatol.2020.11.001
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
Practice Points
- Skin of color centers in the United States work to reverse the paucity of research, education, and training in skin of color dermatology and promote the diversification of residents and faculty.
- Skin of color centers expand access to culturally competent and inclusive care for diverse patient populations.
Generational Differences in Isotretinoin Prescribing Habits: A Cross-Sectional Analysis
To the Editor:
Prescriptions for isotretinoin may be influenced by patient demographics, medical comorbidities, and drug safety programs.1,2 In 1982, isotretinoin was approved by the US Food and Drug Administration for treatment of severe recalcitrant nodulocystic acne that is nonresponsive to conventional therapies such as antibiotics; however, prescriber beliefs regarding the necessity of oral antibiotic failure before isotretinoin is prescribed may be influenced by the provider’s generational age.3 Currently, there is a knowledge gap regarding the impact of provider characteristics, including the year providers completed training, on isotretinoin utilization. The aim of our cross-sectional study was to characterize generational isotretinoin prescribing habits in a large-scale midwestern private practice dermatology group.
Modernizing Medicine (https://www.modmed.com), an electronic medical record software, was queried for all encounters that included both an International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis code L70.0 (acne vulgaris) and a medication prescription from May 2021 to May 2022. Data were collected from a large private practice group with locations across the state of Ohio. Exclusion criteria included provider-patient prescription pairs that included non–acne medication prescriptions, patients seen by multiple providers, and providers who treated fewer than 5 patients with acne during the study period. A mixed-effect multiple logistic regression was performed to analyze whether a patient was ever prescribed isotretinoin, adjusting for individual prescriber, prescriber generation (millennial [1981–1996], Generation X [1965–1980], and baby boomer [1946–1964]),4 and patient sex; spironolactone and oral antibiotic prescriptions during the study period were included as additional covariates in a subsequent post hoc analysis. This study utilized data that was fully deidentified in accordance with the US Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Approval from an institutional review board was not required.
A total of 18,089 provider-patient prescription pairs were included in our analysis (Table). In our most robust model, female patients were significantly less likely to receive isotretinoin compared with male patients (adjusted OR [aOR], 0.394; P<.01). Millennial providers were significantly more likely to utilize isotretinoin in patients who did not receive antibiotics compared with patients who did receive antibiotics (aOR, 1.693; P<.01). When compared with both Generation X and baby boomers, millennial providers were more likely to prescribe isotretinoin in patients who received antibiotics (aOR, 2.227 [P=.02] and 3.638 [P<.01], respectively).
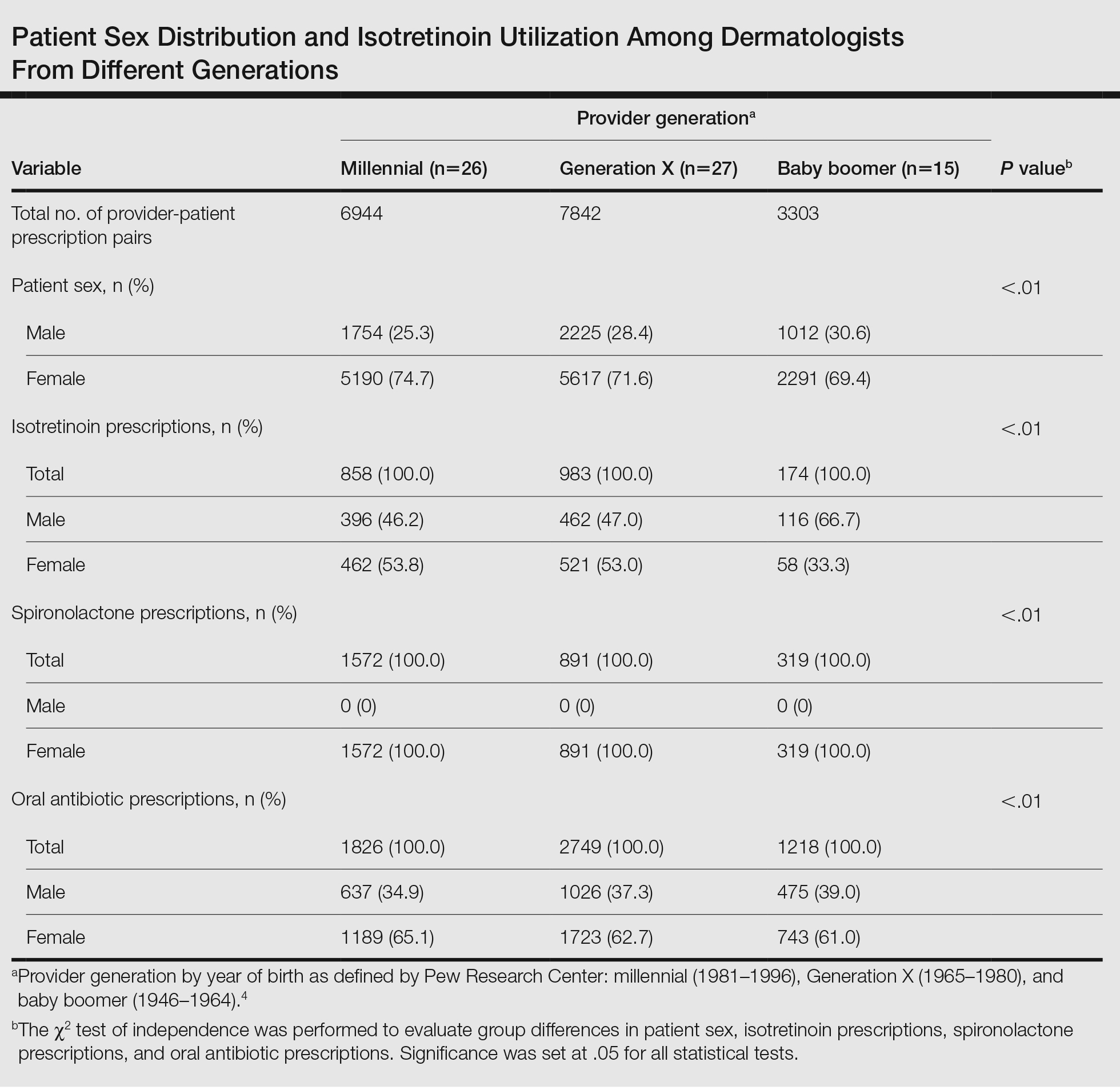
In 2018, the American Academy of Dermatology and the Global Alliance to Improve Outcomes in Acne updated thir guidelines to recommend isotretinoin as a first-line therapy for severe nodular acne, treatment-resistant moderate acne, or acne that produces scarring or psychosocial distress.5 Our study results suggest that millennial providers are adhering to these guidelines and readily prescribing isotretinoin in patients who did not receive antibiotics, which corroborates survey findings by Nagler and Orlow.3 Our results also revealed that prescriber generation may influence isotretinoin usage, with millennials utilizing isotretinoin more in patients who received oral antibiotic therapy than their older counterparts. In part, this may be due to beliefs among older generations that failure of oral antibiotics is necessary before pursuing isotretinoin.3 Additionally, this finding suggests that millennials, if utilizing antibiotics for acne, may have a lower threshold for starting isotretinoin in patients who received oral antibiotic therapy.
Generational prescribing variation appears not to be unique to isotretinoin and also may be present in the use of spironolactone. Over the past decade, utilization of spironolactone for acne treatment has increased, likely in response to new data demonstrating that routine use is safe and effective.6 Several large cohort and retrospective studies have debunked the historical concerns for tumorigenicity in those with breast cancer history as well as the need for routine laboratory monitoring for hyperkalemia.7,8 Although spironolactone use for the treatment of acne has increased, it still remains relatively underutilized,6 suggesting there may be a knowledge gap similar to that of isotretinoin, with younger generations utilizing spironolactone more readily than older generations.
Our study analyzed generational differences in isotretinoin utilization for acne over 1 calendar year. Limitations include sampling from a midwestern patient cohort and private practice–based providers. Due to limitations of our data set, we were unable to capture acne medication usage prior to May 2021, temporal sequencing of acne medication usage, and stratification of patients by acne severity. Furthermore, we were unable to capture female patients who were pregnant or planning pregnancy at the time of their encounter, which would exclude isotretinoin usage.
Overall, millennial providers may be utilizing isotretinoin more in line with the updated acne guidelines5 compared with providers from older generations. Further research is necessary to elucidate how these prescribing habits may change based on acne severity.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22. doi:10.1001/jamadermatol.2019.3270
- Nagler AR, Orlow SJ. Dermatologists’ attitudes, prescription, and counseling patterns for isotretinoin: a questionnaire-based study. J Drugs Dermatol. 2015;14:184-189.
- Dimock M. Where Millennials end and Generation Z begins. Pew Research Center website. January 17, 2019. Accessed June 17, 2024. https://www.pewresearch.org/fact-tank/2019/01/17/where-millennials-end-and-generation-z-begins/
- Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 suppl 1):S1-S23.e1. doi:10.1016/j.jaad.2017.09.078
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
To the Editor:
Prescriptions for isotretinoin may be influenced by patient demographics, medical comorbidities, and drug safety programs.1,2 In 1982, isotretinoin was approved by the US Food and Drug Administration for treatment of severe recalcitrant nodulocystic acne that is nonresponsive to conventional therapies such as antibiotics; however, prescriber beliefs regarding the necessity of oral antibiotic failure before isotretinoin is prescribed may be influenced by the provider’s generational age.3 Currently, there is a knowledge gap regarding the impact of provider characteristics, including the year providers completed training, on isotretinoin utilization. The aim of our cross-sectional study was to characterize generational isotretinoin prescribing habits in a large-scale midwestern private practice dermatology group.
Modernizing Medicine (https://www.modmed.com), an electronic medical record software, was queried for all encounters that included both an International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis code L70.0 (acne vulgaris) and a medication prescription from May 2021 to May 2022. Data were collected from a large private practice group with locations across the state of Ohio. Exclusion criteria included provider-patient prescription pairs that included non–acne medication prescriptions, patients seen by multiple providers, and providers who treated fewer than 5 patients with acne during the study period. A mixed-effect multiple logistic regression was performed to analyze whether a patient was ever prescribed isotretinoin, adjusting for individual prescriber, prescriber generation (millennial [1981–1996], Generation X [1965–1980], and baby boomer [1946–1964]),4 and patient sex; spironolactone and oral antibiotic prescriptions during the study period were included as additional covariates in a subsequent post hoc analysis. This study utilized data that was fully deidentified in accordance with the US Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Approval from an institutional review board was not required.
A total of 18,089 provider-patient prescription pairs were included in our analysis (Table). In our most robust model, female patients were significantly less likely to receive isotretinoin compared with male patients (adjusted OR [aOR], 0.394; P<.01). Millennial providers were significantly more likely to utilize isotretinoin in patients who did not receive antibiotics compared with patients who did receive antibiotics (aOR, 1.693; P<.01). When compared with both Generation X and baby boomers, millennial providers were more likely to prescribe isotretinoin in patients who received antibiotics (aOR, 2.227 [P=.02] and 3.638 [P<.01], respectively).

In 2018, the American Academy of Dermatology and the Global Alliance to Improve Outcomes in Acne updated thir guidelines to recommend isotretinoin as a first-line therapy for severe nodular acne, treatment-resistant moderate acne, or acne that produces scarring or psychosocial distress.5 Our study results suggest that millennial providers are adhering to these guidelines and readily prescribing isotretinoin in patients who did not receive antibiotics, which corroborates survey findings by Nagler and Orlow.3 Our results also revealed that prescriber generation may influence isotretinoin usage, with millennials utilizing isotretinoin more in patients who received oral antibiotic therapy than their older counterparts. In part, this may be due to beliefs among older generations that failure of oral antibiotics is necessary before pursuing isotretinoin.3 Additionally, this finding suggests that millennials, if utilizing antibiotics for acne, may have a lower threshold for starting isotretinoin in patients who received oral antibiotic therapy.
Generational prescribing variation appears not to be unique to isotretinoin and also may be present in the use of spironolactone. Over the past decade, utilization of spironolactone for acne treatment has increased, likely in response to new data demonstrating that routine use is safe and effective.6 Several large cohort and retrospective studies have debunked the historical concerns for tumorigenicity in those with breast cancer history as well as the need for routine laboratory monitoring for hyperkalemia.7,8 Although spironolactone use for the treatment of acne has increased, it still remains relatively underutilized,6 suggesting there may be a knowledge gap similar to that of isotretinoin, with younger generations utilizing spironolactone more readily than older generations.
Our study analyzed generational differences in isotretinoin utilization for acne over 1 calendar year. Limitations include sampling from a midwestern patient cohort and private practice–based providers. Due to limitations of our data set, we were unable to capture acne medication usage prior to May 2021, temporal sequencing of acne medication usage, and stratification of patients by acne severity. Furthermore, we were unable to capture female patients who were pregnant or planning pregnancy at the time of their encounter, which would exclude isotretinoin usage.
Overall, millennial providers may be utilizing isotretinoin more in line with the updated acne guidelines5 compared with providers from older generations. Further research is necessary to elucidate how these prescribing habits may change based on acne severity.
To the Editor:
Prescriptions for isotretinoin may be influenced by patient demographics, medical comorbidities, and drug safety programs.1,2 In 1982, isotretinoin was approved by the US Food and Drug Administration for treatment of severe recalcitrant nodulocystic acne that is nonresponsive to conventional therapies such as antibiotics; however, prescriber beliefs regarding the necessity of oral antibiotic failure before isotretinoin is prescribed may be influenced by the provider’s generational age.3 Currently, there is a knowledge gap regarding the impact of provider characteristics, including the year providers completed training, on isotretinoin utilization. The aim of our cross-sectional study was to characterize generational isotretinoin prescribing habits in a large-scale midwestern private practice dermatology group.
Modernizing Medicine (https://www.modmed.com), an electronic medical record software, was queried for all encounters that included both an International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis code L70.0 (acne vulgaris) and a medication prescription from May 2021 to May 2022. Data were collected from a large private practice group with locations across the state of Ohio. Exclusion criteria included provider-patient prescription pairs that included non–acne medication prescriptions, patients seen by multiple providers, and providers who treated fewer than 5 patients with acne during the study period. A mixed-effect multiple logistic regression was performed to analyze whether a patient was ever prescribed isotretinoin, adjusting for individual prescriber, prescriber generation (millennial [1981–1996], Generation X [1965–1980], and baby boomer [1946–1964]),4 and patient sex; spironolactone and oral antibiotic prescriptions during the study period were included as additional covariates in a subsequent post hoc analysis. This study utilized data that was fully deidentified in accordance with the US Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Approval from an institutional review board was not required.
A total of 18,089 provider-patient prescription pairs were included in our analysis (Table). In our most robust model, female patients were significantly less likely to receive isotretinoin compared with male patients (adjusted OR [aOR], 0.394; P<.01). Millennial providers were significantly more likely to utilize isotretinoin in patients who did not receive antibiotics compared with patients who did receive antibiotics (aOR, 1.693; P<.01). When compared with both Generation X and baby boomers, millennial providers were more likely to prescribe isotretinoin in patients who received antibiotics (aOR, 2.227 [P=.02] and 3.638 [P<.01], respectively).

In 2018, the American Academy of Dermatology and the Global Alliance to Improve Outcomes in Acne updated thir guidelines to recommend isotretinoin as a first-line therapy for severe nodular acne, treatment-resistant moderate acne, or acne that produces scarring or psychosocial distress.5 Our study results suggest that millennial providers are adhering to these guidelines and readily prescribing isotretinoin in patients who did not receive antibiotics, which corroborates survey findings by Nagler and Orlow.3 Our results also revealed that prescriber generation may influence isotretinoin usage, with millennials utilizing isotretinoin more in patients who received oral antibiotic therapy than their older counterparts. In part, this may be due to beliefs among older generations that failure of oral antibiotics is necessary before pursuing isotretinoin.3 Additionally, this finding suggests that millennials, if utilizing antibiotics for acne, may have a lower threshold for starting isotretinoin in patients who received oral antibiotic therapy.
Generational prescribing variation appears not to be unique to isotretinoin and also may be present in the use of spironolactone. Over the past decade, utilization of spironolactone for acne treatment has increased, likely in response to new data demonstrating that routine use is safe and effective.6 Several large cohort and retrospective studies have debunked the historical concerns for tumorigenicity in those with breast cancer history as well as the need for routine laboratory monitoring for hyperkalemia.7,8 Although spironolactone use for the treatment of acne has increased, it still remains relatively underutilized,6 suggesting there may be a knowledge gap similar to that of isotretinoin, with younger generations utilizing spironolactone more readily than older generations.
Our study analyzed generational differences in isotretinoin utilization for acne over 1 calendar year. Limitations include sampling from a midwestern patient cohort and private practice–based providers. Due to limitations of our data set, we were unable to capture acne medication usage prior to May 2021, temporal sequencing of acne medication usage, and stratification of patients by acne severity. Furthermore, we were unable to capture female patients who were pregnant or planning pregnancy at the time of their encounter, which would exclude isotretinoin usage.
Overall, millennial providers may be utilizing isotretinoin more in line with the updated acne guidelines5 compared with providers from older generations. Further research is necessary to elucidate how these prescribing habits may change based on acne severity.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22. doi:10.1001/jamadermatol.2019.3270
- Nagler AR, Orlow SJ. Dermatologists’ attitudes, prescription, and counseling patterns for isotretinoin: a questionnaire-based study. J Drugs Dermatol. 2015;14:184-189.
- Dimock M. Where Millennials end and Generation Z begins. Pew Research Center website. January 17, 2019. Accessed June 17, 2024. https://www.pewresearch.org/fact-tank/2019/01/17/where-millennials-end-and-generation-z-begins/
- Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 suppl 1):S1-S23.e1. doi:10.1016/j.jaad.2017.09.078
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22. doi:10.1001/jamadermatol.2019.3270
- Nagler AR, Orlow SJ. Dermatologists’ attitudes, prescription, and counseling patterns for isotretinoin: a questionnaire-based study. J Drugs Dermatol. 2015;14:184-189.
- Dimock M. Where Millennials end and Generation Z begins. Pew Research Center website. January 17, 2019. Accessed June 17, 2024. https://www.pewresearch.org/fact-tank/2019/01/17/where-millennials-end-and-generation-z-begins/
- Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 suppl 1):S1-S23.e1. doi:10.1016/j.jaad.2017.09.078
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
Practice Points
- Provider generational age appears to impact utilization of isotretinoin for the treatment of acne.
- Millennial providers seem to adhere more readily to guidelines for precribing isotretinoin vs older generations and also may have a lower threshold for starting isotretinoin in patients who received oral antibiotic therapy for acne treatment.
Transgender and Gender Diverse Health Care in the US Military: What Dermatologists Need to Know
People whose gender identity differs from the sex assigned at birth are referred to as transgender. For some, gender identity may not fit into the binary constructs of male and female but rather falls between, within, or outside this construct. These people often consider themselves nonbinary or gender diverse. As the terminology continues to evolve, current recommendations include referring to this patient population as transgender and gender diverse (TGD) to ensure the broadest inclusivity.1 In this article, the following terms are used as defined below:
- The terms transgender woman and trans feminine describe persons who were assigned male gender at birth but their affirmed gender is female or nonmasculine.
- The terms transgender man and trans masculine describe persons who were assigned female gender at birth but their affirmed gender is male or nonfeminine.
The US Military’s policies on the service of TGD persons have evolved considerably over the past decade. Initial military policies barred TGD service members (TSMs) from service all together, leading to challenges in accessing necessary health care. The first official memorandum explicitly allowing military service by TGD persons was released on June 30, 2016.2 The intention of this memorandum was 2-fold: (1) to allow TGD persons to serve in the military so long as they meet “the rigorous standards for military service and readiness” by fulfilling the same standards and procedures as other military service members, including medical fitness for duty, physical fitness, uniform and grooming, deployability, and retention, and (2) to direct the establishment of new or updated policies to specific departments and prescribe procedures for retention standards, separation from service, in-service transition, and medical coverage.2 Several other official policies were released following this initial memorandum that provided more specific guidance on how to implement these policies at the level of the force, unit, and individual service member.
Modifications to the original 2016 policies had varying impacts on transgender health care provision and access.3 At the time of publication of this article, the current policy—the Department of Defense Instruction 1300.284—among others, establishes standards and procedures for the process by which active and reserve TSMs may medically, socially, and legally transition genders within the military. The current policy applies to all military branches and serves as the framework by which each branch currently organizes their gender-affirmation processes (GAP).4
There currently are several different GAP models among the military branches.5 Each branch has a different model or approach to implementing the current policy, with varying service-specific processes in place for TSMs to access gender-affirming care; however, this may be changing. The Defense Health Agency is in the process of consolidating and streamlining the GAP across the Department of Defense branches in an effort to optimize costs and ensure uniformity of care. Per the Defense Health Agency Procedural Instruction Number 6025.21 published in May 2023, the proposed consolidated model likely will entail a single central transgender health center that provides oversight and guidance for several regional joint-service gender-affirming medical hubs. Patients would either be managed at the level of the hub or be referred to the central site.5
Herein, we discuss the importance of gender-affirming care and how military and civilian dermatologists can contribute. We also review disparities in health care and identify areas of improvement.
Benefits of Gender-Affirming Care
Gender-affirming procedures are critical for aligning physical appearance with gender identity. Physical appearance is essential for psychological well-being, operational readiness, and the safety of TSMs.6 It is well documented that TGD persons experience suicidal ideation, depression, stigma, discrimination and violence at higher rates than their cisgender peers.7,8 It is important to recognize that transgender identity is not a mental illness, and these elevated rates have been linked to complex trauma, societal stigma, violence, and discrimination.1 Other studies have suggested that increased access to gender-affirming interventions may ameliorate these mental health concerns.1,7-9
The major components of gender-affirming care include hormone therapy, gender confirmation surgery, and mental health care, if needed. These are covered by TRICARE, the health care program for military service members; however, at the time of publication, many of the dermatologic gender-affirming procedures are not covered by TRICARE because they are considered “cosmetic procedures,” which is a term used by insurance companies but does not accurately indicate whether a procedure is medically necessary or not. Newer literature has demonstrated that gender-affirming care positively affects the lives of TGD patients, strengthening the argument that gender-affirming care is a medical necessity and not just cosmetic.1
Aesthetic Procedures in Gender-Affirming Care
Surgeons, including those within the specialties of oto-laryngology, oral and maxillofacial surgery, urology, gynecology, and plastic surgery, provide major gender-affirming interventions; however, dermatologists may offer less invasive solutions that can serve as a temporary experience prior to undergoing more permanent procedures.Hormonally driven disorders including acne, hair loss, and melasma also are managed by dermatologists, along with scar treatment following surgeries.
Because human variation is expansive and subjective, what is considered feminine or masculine may vary by person, group, culture, and country; therefore, it is imperative to ask patients about their individual aesthetic goals and tailor their treatment accordingly. Feminine and masculine are terms that will be used to describe prototypical appearances and are not meant to define a patient’s current state or ultimate goals. The following procedures and medical interventions are where dermatologists can play an important role in TGD persons’ GAPs.
Botulinum Toxin Injections—Botulinum toxin injection is the most common nonsurgical aesthetic procedure performed around the world.10 The selective paralysis afforded by botulinum toxin has several uses for people undergoing transition. Aesthetically, the feminine eyebrow tends to be positioned above the orbital rim and is arched with its apex between the lateral limbus and lateral canthus,11 while the masculine eyebrow tends to be flatter and fuller and runs over the orbital rim without a peak. For people seeking a more feminine appearance, an eyebrow lift with botulinum toxin can help reshape the typical flatter masculine eyebrow to give it lateral lift that often is considered more feminine. The targeted muscle is the superolateral orbicularis oculi, which serves as a depressor on the eyebrow. This can be combined with purposefully avoiding total lateral frontalis paralysis, which leads to a “Spock” brow for extra lift. Conversely, a naturally arched and higher eyebrow can be flattened and lowered by selectively targeting areas of the frontalis muscle.
Broad square jawlines typically are considered a masculine feature and are another area where botulinum toxin can be used to feminize a patient’s facial features. Targeting the masseter muscle induces muscle weakness, which ultimately may result in atrophy after one or more treatment sessions. This atrophy may lead to narrowing of the lower face and thus may lead to a fuller-appearing midface or overall more heart-shaped face. Every individual’s aesthetic goals are unique and therefore should be discussed prior to any treatment.
Dermal Fillers—Dermal fillers are gel-like substances injected under the skin for subtle contouring of the face. Fillers also can be used to help promote a more masculine or feminine appearance. Filler can be placed in the lips to create a fuller, more projected, feminine-appearing lip. Malar cheek and central lower chin filler can be used to help define a heart-shaped face by accentuating the upper portion of the face and creating a more pointed chin, respectively. Alternatively, filler can be used to masculinize the chin by placing it where it can increase jawline squareness and increase anterior jaw projection. Additionally, filler at the angle of the jaw can help accentuate a square facial shape and a more defined jawline. Although not as widely practiced, lateral brow filler can create a heavier-appearing and broader forehead for a more masculine appearance. These procedures can be combined with the previously mentioned botulinum toxin procedures for a synergistic effect.
Deoxycholic Acid—Deoxycholic acid is an injectable product used to selectively remove unwanted fat. It currently is approved by the US Food and Drug Administration for submental fat, but some providers are experimenting with off-label uses. Buccal fat pad removal—or in this case reduction by dissolution—tends to give a thinner, more feminine facial appearance.12 Reducing fat around the axillae also can help promote a more masculine upper torso.13 The safety of deoxycholic acid in these areas has not been adequately tested; thus, caution should be used when discussing these off-label uses with patients.
Hair and Tattoo Removal—Hair removal may be desired by TGD persons for a variety of reasons. Because cisgender females tend to have less body hair overall, transgender people in pursuit of a more feminine appearance often desire removal of facial, neck, and body hair. Although shaving and other modalities such as waxing and chemical depilatories are readily available at-home options, they are not permanent and may lead to folliculitis or pseudofolliculitis barbae. Laser hair removal (LHR) and electrolysis are modalities provided by dermatologists that tend to be more permanent and lead to better outcomes, including less irritation and better aesthetic appearance. It is important to keep in mind that not every person and not every body site can be safely treated with LHR. Patients with lighter skin types and darker hair tend to have the most effective response with a higher margin of safety, as these features allow the laser energy to be selectively absorbed by the melanin in the hair bulb and not by the background skin pigmentation.14,15 Inappropriate patient selection or improper settings for wavelength, pulse width, or fluences can lead to burns and permanent scarring.14,15 Electrolysis is an alternative to hair removal within tattoos and is more effective for those individuals with blonde, red, or white hair.16
Another novel treatment for unwanted hair is eflornithine hydrochloride cream, which works by blocking ornithine decarboxylase, the enzyme that stimulates hair growth. It currently is approved to reduce unwanted hair on the face and adjacent areas under the chin; however the effects of this medication are modest and the medication can be expensive.17
Cosmetic hair and tattoo removal are not currently covered by TRICARE, except in cases of surgical and donor-site preparation for some GAPs. Individuals may desire removal of tattoos at surgery sites to obtain more natural-appearing skin. Currently, GAPs such as vaginoplasty, phalloplasty, and metoidioplasty—often referred to by patients as “bottom surgeries”—include insurance coverage for tattoo removal, LHR, and/or electrolysis.
Management of Hormonal Adverse Effects
Acne—Individuals on testosterone supplementation tend to develop acne for the first several years of treatment, but it may improve with time.18 Acne is treated in individuals receiving testosterone the same way as it is treated in cisgender men, with numerous options for topical and oral medications. In trans masculine persons, spironolactone therapy typically is avoided because it may interfere with the actions of exogenous testosterone administered as part of gender-affirming medical treatment and may lead to other undesired adverse effects such as impotence and gynecomastia.1
Although acne typically improves after starting estrogen therapy, patients receiving estrogens may still develop acne. Most trans feminine patients will already be on an estrogen and an antiandrogen, often spironolactone.1 Spironolactone often is used as monotherapy for acne control in cisgender women. Additionally, an important factor to consider with spironolactone is the possible adverse effect of increased micturition. Currently, the military rarely has gender-inclusive restroom options, which can create a challenge for TSMs who find themselves needing to use the restroom more frequently in the workplace.
If planning therapy with isotretinoin, dermatologists should discuss several important factors with all patients, including TGD patients. One consideration is the patient’s planned future surgeries. Although new literature shows that isotretinoin does not adversely affect wound healing,19 some surgeons still adhere to an isotretinoin washout period of 6 to 12 months prior to performing any elective procedures due to concerns about wound healing.20,21 Second, be sure to properly assess and document pregnancy potential in TGD persons. Providers should not assume that a patient is not pregnant or is not trying to become pregnant just because they are trans masculine. It also is important to note that testosterone is not a reliable birth control method.1 If a patient still has ovaries, fallopian tubes, and a uterus, they are considered medically capable of pregnancy, and providers should keep this in mind regarding all procedures in the TGD population.
Another newer acne treatment modality is the 1762-nm laser, which targets sebaceous glands.22 This device allows for targeted treatment of acne-prone areas without systemic therapy such as retinoids or antiandrogens. The 1762-nm laser is not widely available but may become a regular treatment option once its benefits are proven over time.
Alopecia and Hyperpigmentation—Androgens, whether endogenously or exogenously derived, can lead to androgenetic alopecia (AGA) in genetically susceptible individuals. Trans masculine persons and others receiving androgen therapy are at higher risk for AGA, which often is undesirable and may be considered gender affirming by some TGD persons. Standard AGA treatments for cisgender men also can be used in trans masculine persons. Some of the most common anti-AGA medications are topical minoxidil, oral finasteride, and oral minoxidil. Although Coleman et al1 recently reported that finasteride may be an appropriate treatment option in trans masculine persons experiencing alopecia, treatment with 5α-reductase inhibitors may impair clitoral growth and the development of facial and body hair. Further studies are needed to assess the efficacy and safety of 5α-reductase inhibitors in transgender populations.1 Dutasteride may be used off-label and comes with a similar potential adverse-event profile as finasteride, which includes depression, decreased libido, erectile dysfunction, ejaculation disorders, and gynecomastia.
Conversely, AGA tends to improve in trans feminine persons and others receiving estrogen and antiandrogen therapy. Natural testosterone production is suppressed by estrogens and spironolactone as well as in patients who undergo orchiectomy.1 Although spironolactone is not approved for acne, AGA, or hirsutism, it is a standard treatment of AGA in cisgender women because it functions to block the effects of androgens, including at the hair follicle. Finasteride may be used for AGA in cisgender women but it is not recommended for trans feminine persons.1
There are many other modalities available for the treatment of AGA that are less commonly used—some may be cost prohibitive or do not have robust supporting evidence, or both. One example is hair
Melasma is a hyperpigmentation disorder related to estrogens, UV light exposure, and sometimes medication use (eg, hormonal birth control, spironolactone).24 The mainstay of treatment is prevention, including sun avoidance as well as use of sun-protective clothing and broad-spectrum sunscreens. Dermatologists tend to recommend physical sunscreens containing zinc oxide, titanium dioxide, and/or iron oxide, as they cover a wider UV spectrum and also provide some protection from visible light. Once melasma is present, dermatologists still have several treatment options. Topical hydroquinone is a proven treatment; however, it must be used with caution to avoid ochronosis. With careful patient selection, chemical peels also are effective treatment options for dyspigmentation and hyperpigmentation. Energy devices such as intense pulsed light and tattoo removal lasers—Q-switched lasers and picosecond pulse widths—also can be used to treat hyperpigmentation. Oral, intralesional, and topical tranexamic acid are newer treatment options for melasma that still are being studied and have shown promising results. Further studies are needed to determine long-term safety and optimal treatment regimens.24,25
Many insurance carriers, including TRICARE, do not routinely cover medical management of AGA or melasma. Patients should be advised that they likely will have to pay for any medications prescribed and procedures undertaken for these purposes; however, some medication costs can be offset by ordering larger prescription quantities, such as a 90-day supply vs a 30-day supply, as well as utilizing pharmacy discount programs.
Scar Management Following Surgery
In TSMs who undergo gender-affirming surgeries, dermatologists play an important role when scar symptoms develop, including pruritus, tenderness, and/or paresthesia. In the military, some common treatment modalities for symptomatic scars include intralesional steroids with or without 5-fluouroruacil and the fractionated CO2 laser. There also are numerous experimental treatment options for scars, including intralesional or perilesional botulinum toxin, the pulsed dye laser, or nonablative fractionated lasers. These modalities also may be used on hypertrophic scars or keloids. Another option for keloids is scar excision followed by superficial radiation therapy.26
Mental Health Considerations
Providers must take psychological adverse effects into consideration when considering medical therapies for dermatologic conditions in TGD patients. In particular, it is important to consider the risks for increased rates of depression and suicidal ideation formerly associated with the use of isotretinoin and finasteride, though much of the evidence regarding these risks has been called into question in recent years.27,28 Nonetheless, it remains prominent in lay media and may be a more important consideration in patients at higher baseline risk.27 Although there are no known studies that have expressly assessed rates of depression or suicidal ideation in TGD patients taking isotretinoin or finasteride, it is well established that TGD persons are at higher baseline risk for depression and suicidality.1,7,8 All patients should be carefully assessed for depression and suicidal ideation as well as counseled regarding these risks prior to initiating these therapies. If concerns for untreated mental health issues arise during screening and counseling, patients should be referred for assessment by a behavioral health specialist prior to starting therapy.
Future Directions
The future of TGD health care in the military could see an expansion of covered benefits and the development of new dermatologic procedures or medications. Research and policy evolution are necessary to bridge the current gaps in care; however, it is unlikely that all procedures currently considered to be cosmetic will become covered benefits.
Facial LHR is a promising candidate for future coverage for trans feminine persons. When cisgender men develop adverse effects from mandatory daily shaving, LHR is already a covered benefit. Two arguments in support of adding LHR for TGD patients revolve around achieving and maintaining an appearance congruent with their gender along with avoiding unwanted adverse effects related to daily shaving. Visual conformity with one’s affirmed gender has been associated with improvements in well-being, quality of life, and some mental health conditions.29
Scar prevention, treatment, and reduction are additional areas under active research in which dermatologists likely will play a crucial role.30,31 As more dermatologic procedures are performed on TGD persons, the published data and collective knowledge regarding best practices in this population will continue to grow, which will lead to improved cosmetic and safety outcomes.
Final Thoughts
Although dermatologists do not directly perform gender-affirming surgeries or hormone management, they do play an important role in enhancing a TGD person’s desired appearance and managing possible adverse effects resulting from gender-affirming interventions. There have been considerable advancements in TGD health care over the past decade, but there likely are more changes on the way. As policies and understanding of TGD health care needs evolve, it is crucial that the military health care system adapts to provide comprehensive, accessible, and equitable care, which includes expanding the range of covered dermatologic treatments to fully support the health and readiness of TSMs.
Acknowledgment—We would like to extend our sincere appreciation to the invaluable contributions and editorial support provided by Allison Higgins, JD (San Antonio, Texas), throughout the writing of this article.
- Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S260. doi:10.1080/26895269.2022.2100644
- Secretary of Defense. DTM 16-005—military service of transgender service members. June 30, 2016. Accessed June 17, 2024. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DTM-16-005.pdf
- Office of the Deputy Secretary of Defense. DTM 19-004—military service by transgender persons and persons with gender dysphoria. March 17, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction (DODI) 1300.28. in-service transition for transgender service members. September 4, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/09/04/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Defense Health Agency Procedural Instruction Number 6025.21, Guidance for Gender-Affirming Health Care of Transgender and Gender-Diverse Active and Reserve Component Service Members, May 12, 2023. https://www.health.mil/Reference-Center/DHA-Publications/2023/05/12/DHA-PI-6015-21
- Elders MJ, Brown GR, Coleman E, et al. Medical aspects of transgender military service. Armed Forces Soc. 2015;41:199-220. doi:10.1177/0095327X14545625.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156:611-618.
- Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:E220978. doi:10.1001/jamanetworkopen.2022.0978
- Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431-436. doi:10.1001/jamapediatrics.2017.5440
- Top non-invasive cosmetic procedures worldwide 2022. Statista website. February 8, 2024. Accessed June 13, 2024. https://www.statista.com/statistics/293449/leading-nonsurgical-cosmetic-procedures/
- Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, et al. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29:154-168. doi:10.1016/j.joco.2017.04.001
- Thomas MK, D’Silva JA, Borole AJ. Injection lipolysis: a systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222-228. doi:10.4103/JCAS.JCAS_117_18
- Jegasothy SM. Deoxycholic acid injections for bra-line lipolysis. Dermatol Surg. 2018;44:757-760. doi:10.1097/DSS.0000000000001311
- Dierickx CC. Hair removal by lasers and intense pulsed light sources. Dermatol Clin. 2002;20:135-146. doi:10.1016/s0733-8635(03)00052-4
- Lepselter J, Elman M. Biological and clinical aspects in laser hair removal. J Dermatolog Treat. 2004;15:72-83. doi:10.1080/09546630310023152
- Yuan N, Feldman AT, Chin P, et al. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545. doi:10.1016/j.esxm.2022.100545
- Kumar A, Naguib YW, Shi YC, et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23:1495-1501. doi:10.3109/10717544.2014.951746
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102:3869-3903.
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- Rubenstein R, Roenigk HH Jr, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2 pt 1):280-285.
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913-914. doi:10.1111/ced.14292
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology: 2-Volume Set. Elsevier; 2024:1130.
- Konisky H, Balazic E, Jaller JA, et al. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22:1197-1206. doi:10.1111/jocd.15589
- Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi:10.1186/s13643-023-02192-7
- Kridin K, Ludwig RJ. Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J Am Acad Dermatol. 2023;88:388-394. doi:10.1016/j.jaad.2022.10.031
- Dyson TE, Cantrell MA, Lund BC. Lack of association between 5α-reductase inhibitors and depression. J Urol. 2020;204:793-798. doi:10.1097/JU.0000000000001079
- To M, Zhang Q, Bradlyn A, et al. Visual conformity with affirmed gender or “passing”: its distribution and association with depression and anxiety in a cohort of transgender people. J Sex Med. 2020;17:2084-2092. doi:10.1016/j.jsxm.2020.07.019
- Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22-33. doi:10.1016/j.actbio.2022.07.042
- Kolli H, Moy RL. Prevention of scarring with intraoperative erbium:YAG laser treatment. J Drugs Dermatol. 2020;19:1040-1043. doi:10.36849/JDD.2020.5244
People whose gender identity differs from the sex assigned at birth are referred to as transgender. For some, gender identity may not fit into the binary constructs of male and female but rather falls between, within, or outside this construct. These people often consider themselves nonbinary or gender diverse. As the terminology continues to evolve, current recommendations include referring to this patient population as transgender and gender diverse (TGD) to ensure the broadest inclusivity.1 In this article, the following terms are used as defined below:
- The terms transgender woman and trans feminine describe persons who were assigned male gender at birth but their affirmed gender is female or nonmasculine.
- The terms transgender man and trans masculine describe persons who were assigned female gender at birth but their affirmed gender is male or nonfeminine.
The US Military’s policies on the service of TGD persons have evolved considerably over the past decade. Initial military policies barred TGD service members (TSMs) from service all together, leading to challenges in accessing necessary health care. The first official memorandum explicitly allowing military service by TGD persons was released on June 30, 2016.2 The intention of this memorandum was 2-fold: (1) to allow TGD persons to serve in the military so long as they meet “the rigorous standards for military service and readiness” by fulfilling the same standards and procedures as other military service members, including medical fitness for duty, physical fitness, uniform and grooming, deployability, and retention, and (2) to direct the establishment of new or updated policies to specific departments and prescribe procedures for retention standards, separation from service, in-service transition, and medical coverage.2 Several other official policies were released following this initial memorandum that provided more specific guidance on how to implement these policies at the level of the force, unit, and individual service member.
Modifications to the original 2016 policies had varying impacts on transgender health care provision and access.3 At the time of publication of this article, the current policy—the Department of Defense Instruction 1300.284—among others, establishes standards and procedures for the process by which active and reserve TSMs may medically, socially, and legally transition genders within the military. The current policy applies to all military branches and serves as the framework by which each branch currently organizes their gender-affirmation processes (GAP).4
There currently are several different GAP models among the military branches.5 Each branch has a different model or approach to implementing the current policy, with varying service-specific processes in place for TSMs to access gender-affirming care; however, this may be changing. The Defense Health Agency is in the process of consolidating and streamlining the GAP across the Department of Defense branches in an effort to optimize costs and ensure uniformity of care. Per the Defense Health Agency Procedural Instruction Number 6025.21 published in May 2023, the proposed consolidated model likely will entail a single central transgender health center that provides oversight and guidance for several regional joint-service gender-affirming medical hubs. Patients would either be managed at the level of the hub or be referred to the central site.5
Herein, we discuss the importance of gender-affirming care and how military and civilian dermatologists can contribute. We also review disparities in health care and identify areas of improvement.
Benefits of Gender-Affirming Care
Gender-affirming procedures are critical for aligning physical appearance with gender identity. Physical appearance is essential for psychological well-being, operational readiness, and the safety of TSMs.6 It is well documented that TGD persons experience suicidal ideation, depression, stigma, discrimination and violence at higher rates than their cisgender peers.7,8 It is important to recognize that transgender identity is not a mental illness, and these elevated rates have been linked to complex trauma, societal stigma, violence, and discrimination.1 Other studies have suggested that increased access to gender-affirming interventions may ameliorate these mental health concerns.1,7-9
The major components of gender-affirming care include hormone therapy, gender confirmation surgery, and mental health care, if needed. These are covered by TRICARE, the health care program for military service members; however, at the time of publication, many of the dermatologic gender-affirming procedures are not covered by TRICARE because they are considered “cosmetic procedures,” which is a term used by insurance companies but does not accurately indicate whether a procedure is medically necessary or not. Newer literature has demonstrated that gender-affirming care positively affects the lives of TGD patients, strengthening the argument that gender-affirming care is a medical necessity and not just cosmetic.1
Aesthetic Procedures in Gender-Affirming Care
Surgeons, including those within the specialties of oto-laryngology, oral and maxillofacial surgery, urology, gynecology, and plastic surgery, provide major gender-affirming interventions; however, dermatologists may offer less invasive solutions that can serve as a temporary experience prior to undergoing more permanent procedures.Hormonally driven disorders including acne, hair loss, and melasma also are managed by dermatologists, along with scar treatment following surgeries.
Because human variation is expansive and subjective, what is considered feminine or masculine may vary by person, group, culture, and country; therefore, it is imperative to ask patients about their individual aesthetic goals and tailor their treatment accordingly. Feminine and masculine are terms that will be used to describe prototypical appearances and are not meant to define a patient’s current state or ultimate goals. The following procedures and medical interventions are where dermatologists can play an important role in TGD persons’ GAPs.
Botulinum Toxin Injections—Botulinum toxin injection is the most common nonsurgical aesthetic procedure performed around the world.10 The selective paralysis afforded by botulinum toxin has several uses for people undergoing transition. Aesthetically, the feminine eyebrow tends to be positioned above the orbital rim and is arched with its apex between the lateral limbus and lateral canthus,11 while the masculine eyebrow tends to be flatter and fuller and runs over the orbital rim without a peak. For people seeking a more feminine appearance, an eyebrow lift with botulinum toxin can help reshape the typical flatter masculine eyebrow to give it lateral lift that often is considered more feminine. The targeted muscle is the superolateral orbicularis oculi, which serves as a depressor on the eyebrow. This can be combined with purposefully avoiding total lateral frontalis paralysis, which leads to a “Spock” brow for extra lift. Conversely, a naturally arched and higher eyebrow can be flattened and lowered by selectively targeting areas of the frontalis muscle.
Broad square jawlines typically are considered a masculine feature and are another area where botulinum toxin can be used to feminize a patient’s facial features. Targeting the masseter muscle induces muscle weakness, which ultimately may result in atrophy after one or more treatment sessions. This atrophy may lead to narrowing of the lower face and thus may lead to a fuller-appearing midface or overall more heart-shaped face. Every individual’s aesthetic goals are unique and therefore should be discussed prior to any treatment.
Dermal Fillers—Dermal fillers are gel-like substances injected under the skin for subtle contouring of the face. Fillers also can be used to help promote a more masculine or feminine appearance. Filler can be placed in the lips to create a fuller, more projected, feminine-appearing lip. Malar cheek and central lower chin filler can be used to help define a heart-shaped face by accentuating the upper portion of the face and creating a more pointed chin, respectively. Alternatively, filler can be used to masculinize the chin by placing it where it can increase jawline squareness and increase anterior jaw projection. Additionally, filler at the angle of the jaw can help accentuate a square facial shape and a more defined jawline. Although not as widely practiced, lateral brow filler can create a heavier-appearing and broader forehead for a more masculine appearance. These procedures can be combined with the previously mentioned botulinum toxin procedures for a synergistic effect.
Deoxycholic Acid—Deoxycholic acid is an injectable product used to selectively remove unwanted fat. It currently is approved by the US Food and Drug Administration for submental fat, but some providers are experimenting with off-label uses. Buccal fat pad removal—or in this case reduction by dissolution—tends to give a thinner, more feminine facial appearance.12 Reducing fat around the axillae also can help promote a more masculine upper torso.13 The safety of deoxycholic acid in these areas has not been adequately tested; thus, caution should be used when discussing these off-label uses with patients.
Hair and Tattoo Removal—Hair removal may be desired by TGD persons for a variety of reasons. Because cisgender females tend to have less body hair overall, transgender people in pursuit of a more feminine appearance often desire removal of facial, neck, and body hair. Although shaving and other modalities such as waxing and chemical depilatories are readily available at-home options, they are not permanent and may lead to folliculitis or pseudofolliculitis barbae. Laser hair removal (LHR) and electrolysis are modalities provided by dermatologists that tend to be more permanent and lead to better outcomes, including less irritation and better aesthetic appearance. It is important to keep in mind that not every person and not every body site can be safely treated with LHR. Patients with lighter skin types and darker hair tend to have the most effective response with a higher margin of safety, as these features allow the laser energy to be selectively absorbed by the melanin in the hair bulb and not by the background skin pigmentation.14,15 Inappropriate patient selection or improper settings for wavelength, pulse width, or fluences can lead to burns and permanent scarring.14,15 Electrolysis is an alternative to hair removal within tattoos and is more effective for those individuals with blonde, red, or white hair.16
Another novel treatment for unwanted hair is eflornithine hydrochloride cream, which works by blocking ornithine decarboxylase, the enzyme that stimulates hair growth. It currently is approved to reduce unwanted hair on the face and adjacent areas under the chin; however the effects of this medication are modest and the medication can be expensive.17
Cosmetic hair and tattoo removal are not currently covered by TRICARE, except in cases of surgical and donor-site preparation for some GAPs. Individuals may desire removal of tattoos at surgery sites to obtain more natural-appearing skin. Currently, GAPs such as vaginoplasty, phalloplasty, and metoidioplasty—often referred to by patients as “bottom surgeries”—include insurance coverage for tattoo removal, LHR, and/or electrolysis.
Management of Hormonal Adverse Effects
Acne—Individuals on testosterone supplementation tend to develop acne for the first several years of treatment, but it may improve with time.18 Acne is treated in individuals receiving testosterone the same way as it is treated in cisgender men, with numerous options for topical and oral medications. In trans masculine persons, spironolactone therapy typically is avoided because it may interfere with the actions of exogenous testosterone administered as part of gender-affirming medical treatment and may lead to other undesired adverse effects such as impotence and gynecomastia.1
Although acne typically improves after starting estrogen therapy, patients receiving estrogens may still develop acne. Most trans feminine patients will already be on an estrogen and an antiandrogen, often spironolactone.1 Spironolactone often is used as monotherapy for acne control in cisgender women. Additionally, an important factor to consider with spironolactone is the possible adverse effect of increased micturition. Currently, the military rarely has gender-inclusive restroom options, which can create a challenge for TSMs who find themselves needing to use the restroom more frequently in the workplace.
If planning therapy with isotretinoin, dermatologists should discuss several important factors with all patients, including TGD patients. One consideration is the patient’s planned future surgeries. Although new literature shows that isotretinoin does not adversely affect wound healing,19 some surgeons still adhere to an isotretinoin washout period of 6 to 12 months prior to performing any elective procedures due to concerns about wound healing.20,21 Second, be sure to properly assess and document pregnancy potential in TGD persons. Providers should not assume that a patient is not pregnant or is not trying to become pregnant just because they are trans masculine. It also is important to note that testosterone is not a reliable birth control method.1 If a patient still has ovaries, fallopian tubes, and a uterus, they are considered medically capable of pregnancy, and providers should keep this in mind regarding all procedures in the TGD population.
Another newer acne treatment modality is the 1762-nm laser, which targets sebaceous glands.22 This device allows for targeted treatment of acne-prone areas without systemic therapy such as retinoids or antiandrogens. The 1762-nm laser is not widely available but may become a regular treatment option once its benefits are proven over time.
Alopecia and Hyperpigmentation—Androgens, whether endogenously or exogenously derived, can lead to androgenetic alopecia (AGA) in genetically susceptible individuals. Trans masculine persons and others receiving androgen therapy are at higher risk for AGA, which often is undesirable and may be considered gender affirming by some TGD persons. Standard AGA treatments for cisgender men also can be used in trans masculine persons. Some of the most common anti-AGA medications are topical minoxidil, oral finasteride, and oral minoxidil. Although Coleman et al1 recently reported that finasteride may be an appropriate treatment option in trans masculine persons experiencing alopecia, treatment with 5α-reductase inhibitors may impair clitoral growth and the development of facial and body hair. Further studies are needed to assess the efficacy and safety of 5α-reductase inhibitors in transgender populations.1 Dutasteride may be used off-label and comes with a similar potential adverse-event profile as finasteride, which includes depression, decreased libido, erectile dysfunction, ejaculation disorders, and gynecomastia.
Conversely, AGA tends to improve in trans feminine persons and others receiving estrogen and antiandrogen therapy. Natural testosterone production is suppressed by estrogens and spironolactone as well as in patients who undergo orchiectomy.1 Although spironolactone is not approved for acne, AGA, or hirsutism, it is a standard treatment of AGA in cisgender women because it functions to block the effects of androgens, including at the hair follicle. Finasteride may be used for AGA in cisgender women but it is not recommended for trans feminine persons.1
There are many other modalities available for the treatment of AGA that are less commonly used—some may be cost prohibitive or do not have robust supporting evidence, or both. One example is hair
Melasma is a hyperpigmentation disorder related to estrogens, UV light exposure, and sometimes medication use (eg, hormonal birth control, spironolactone).24 The mainstay of treatment is prevention, including sun avoidance as well as use of sun-protective clothing and broad-spectrum sunscreens. Dermatologists tend to recommend physical sunscreens containing zinc oxide, titanium dioxide, and/or iron oxide, as they cover a wider UV spectrum and also provide some protection from visible light. Once melasma is present, dermatologists still have several treatment options. Topical hydroquinone is a proven treatment; however, it must be used with caution to avoid ochronosis. With careful patient selection, chemical peels also are effective treatment options for dyspigmentation and hyperpigmentation. Energy devices such as intense pulsed light and tattoo removal lasers—Q-switched lasers and picosecond pulse widths—also can be used to treat hyperpigmentation. Oral, intralesional, and topical tranexamic acid are newer treatment options for melasma that still are being studied and have shown promising results. Further studies are needed to determine long-term safety and optimal treatment regimens.24,25
Many insurance carriers, including TRICARE, do not routinely cover medical management of AGA or melasma. Patients should be advised that they likely will have to pay for any medications prescribed and procedures undertaken for these purposes; however, some medication costs can be offset by ordering larger prescription quantities, such as a 90-day supply vs a 30-day supply, as well as utilizing pharmacy discount programs.
Scar Management Following Surgery
In TSMs who undergo gender-affirming surgeries, dermatologists play an important role when scar symptoms develop, including pruritus, tenderness, and/or paresthesia. In the military, some common treatment modalities for symptomatic scars include intralesional steroids with or without 5-fluouroruacil and the fractionated CO2 laser. There also are numerous experimental treatment options for scars, including intralesional or perilesional botulinum toxin, the pulsed dye laser, or nonablative fractionated lasers. These modalities also may be used on hypertrophic scars or keloids. Another option for keloids is scar excision followed by superficial radiation therapy.26
Mental Health Considerations
Providers must take psychological adverse effects into consideration when considering medical therapies for dermatologic conditions in TGD patients. In particular, it is important to consider the risks for increased rates of depression and suicidal ideation formerly associated with the use of isotretinoin and finasteride, though much of the evidence regarding these risks has been called into question in recent years.27,28 Nonetheless, it remains prominent in lay media and may be a more important consideration in patients at higher baseline risk.27 Although there are no known studies that have expressly assessed rates of depression or suicidal ideation in TGD patients taking isotretinoin or finasteride, it is well established that TGD persons are at higher baseline risk for depression and suicidality.1,7,8 All patients should be carefully assessed for depression and suicidal ideation as well as counseled regarding these risks prior to initiating these therapies. If concerns for untreated mental health issues arise during screening and counseling, patients should be referred for assessment by a behavioral health specialist prior to starting therapy.
Future Directions
The future of TGD health care in the military could see an expansion of covered benefits and the development of new dermatologic procedures or medications. Research and policy evolution are necessary to bridge the current gaps in care; however, it is unlikely that all procedures currently considered to be cosmetic will become covered benefits.
Facial LHR is a promising candidate for future coverage for trans feminine persons. When cisgender men develop adverse effects from mandatory daily shaving, LHR is already a covered benefit. Two arguments in support of adding LHR for TGD patients revolve around achieving and maintaining an appearance congruent with their gender along with avoiding unwanted adverse effects related to daily shaving. Visual conformity with one’s affirmed gender has been associated with improvements in well-being, quality of life, and some mental health conditions.29
Scar prevention, treatment, and reduction are additional areas under active research in which dermatologists likely will play a crucial role.30,31 As more dermatologic procedures are performed on TGD persons, the published data and collective knowledge regarding best practices in this population will continue to grow, which will lead to improved cosmetic and safety outcomes.
Final Thoughts
Although dermatologists do not directly perform gender-affirming surgeries or hormone management, they do play an important role in enhancing a TGD person’s desired appearance and managing possible adverse effects resulting from gender-affirming interventions. There have been considerable advancements in TGD health care over the past decade, but there likely are more changes on the way. As policies and understanding of TGD health care needs evolve, it is crucial that the military health care system adapts to provide comprehensive, accessible, and equitable care, which includes expanding the range of covered dermatologic treatments to fully support the health and readiness of TSMs.
Acknowledgment—We would like to extend our sincere appreciation to the invaluable contributions and editorial support provided by Allison Higgins, JD (San Antonio, Texas), throughout the writing of this article.
People whose gender identity differs from the sex assigned at birth are referred to as transgender. For some, gender identity may not fit into the binary constructs of male and female but rather falls between, within, or outside this construct. These people often consider themselves nonbinary or gender diverse. As the terminology continues to evolve, current recommendations include referring to this patient population as transgender and gender diverse (TGD) to ensure the broadest inclusivity.1 In this article, the following terms are used as defined below:
- The terms transgender woman and trans feminine describe persons who were assigned male gender at birth but their affirmed gender is female or nonmasculine.
- The terms transgender man and trans masculine describe persons who were assigned female gender at birth but their affirmed gender is male or nonfeminine.
The US Military’s policies on the service of TGD persons have evolved considerably over the past decade. Initial military policies barred TGD service members (TSMs) from service all together, leading to challenges in accessing necessary health care. The first official memorandum explicitly allowing military service by TGD persons was released on June 30, 2016.2 The intention of this memorandum was 2-fold: (1) to allow TGD persons to serve in the military so long as they meet “the rigorous standards for military service and readiness” by fulfilling the same standards and procedures as other military service members, including medical fitness for duty, physical fitness, uniform and grooming, deployability, and retention, and (2) to direct the establishment of new or updated policies to specific departments and prescribe procedures for retention standards, separation from service, in-service transition, and medical coverage.2 Several other official policies were released following this initial memorandum that provided more specific guidance on how to implement these policies at the level of the force, unit, and individual service member.
Modifications to the original 2016 policies had varying impacts on transgender health care provision and access.3 At the time of publication of this article, the current policy—the Department of Defense Instruction 1300.284—among others, establishes standards and procedures for the process by which active and reserve TSMs may medically, socially, and legally transition genders within the military. The current policy applies to all military branches and serves as the framework by which each branch currently organizes their gender-affirmation processes (GAP).4
There currently are several different GAP models among the military branches.5 Each branch has a different model or approach to implementing the current policy, with varying service-specific processes in place for TSMs to access gender-affirming care; however, this may be changing. The Defense Health Agency is in the process of consolidating and streamlining the GAP across the Department of Defense branches in an effort to optimize costs and ensure uniformity of care. Per the Defense Health Agency Procedural Instruction Number 6025.21 published in May 2023, the proposed consolidated model likely will entail a single central transgender health center that provides oversight and guidance for several regional joint-service gender-affirming medical hubs. Patients would either be managed at the level of the hub or be referred to the central site.5
Herein, we discuss the importance of gender-affirming care and how military and civilian dermatologists can contribute. We also review disparities in health care and identify areas of improvement.
Benefits of Gender-Affirming Care
Gender-affirming procedures are critical for aligning physical appearance with gender identity. Physical appearance is essential for psychological well-being, operational readiness, and the safety of TSMs.6 It is well documented that TGD persons experience suicidal ideation, depression, stigma, discrimination and violence at higher rates than their cisgender peers.7,8 It is important to recognize that transgender identity is not a mental illness, and these elevated rates have been linked to complex trauma, societal stigma, violence, and discrimination.1 Other studies have suggested that increased access to gender-affirming interventions may ameliorate these mental health concerns.1,7-9
The major components of gender-affirming care include hormone therapy, gender confirmation surgery, and mental health care, if needed. These are covered by TRICARE, the health care program for military service members; however, at the time of publication, many of the dermatologic gender-affirming procedures are not covered by TRICARE because they are considered “cosmetic procedures,” which is a term used by insurance companies but does not accurately indicate whether a procedure is medically necessary or not. Newer literature has demonstrated that gender-affirming care positively affects the lives of TGD patients, strengthening the argument that gender-affirming care is a medical necessity and not just cosmetic.1
Aesthetic Procedures in Gender-Affirming Care
Surgeons, including those within the specialties of oto-laryngology, oral and maxillofacial surgery, urology, gynecology, and plastic surgery, provide major gender-affirming interventions; however, dermatologists may offer less invasive solutions that can serve as a temporary experience prior to undergoing more permanent procedures.Hormonally driven disorders including acne, hair loss, and melasma also are managed by dermatologists, along with scar treatment following surgeries.
Because human variation is expansive and subjective, what is considered feminine or masculine may vary by person, group, culture, and country; therefore, it is imperative to ask patients about their individual aesthetic goals and tailor their treatment accordingly. Feminine and masculine are terms that will be used to describe prototypical appearances and are not meant to define a patient’s current state or ultimate goals. The following procedures and medical interventions are where dermatologists can play an important role in TGD persons’ GAPs.
Botulinum Toxin Injections—Botulinum toxin injection is the most common nonsurgical aesthetic procedure performed around the world.10 The selective paralysis afforded by botulinum toxin has several uses for people undergoing transition. Aesthetically, the feminine eyebrow tends to be positioned above the orbital rim and is arched with its apex between the lateral limbus and lateral canthus,11 while the masculine eyebrow tends to be flatter and fuller and runs over the orbital rim without a peak. For people seeking a more feminine appearance, an eyebrow lift with botulinum toxin can help reshape the typical flatter masculine eyebrow to give it lateral lift that often is considered more feminine. The targeted muscle is the superolateral orbicularis oculi, which serves as a depressor on the eyebrow. This can be combined with purposefully avoiding total lateral frontalis paralysis, which leads to a “Spock” brow for extra lift. Conversely, a naturally arched and higher eyebrow can be flattened and lowered by selectively targeting areas of the frontalis muscle.
Broad square jawlines typically are considered a masculine feature and are another area where botulinum toxin can be used to feminize a patient’s facial features. Targeting the masseter muscle induces muscle weakness, which ultimately may result in atrophy after one or more treatment sessions. This atrophy may lead to narrowing of the lower face and thus may lead to a fuller-appearing midface or overall more heart-shaped face. Every individual’s aesthetic goals are unique and therefore should be discussed prior to any treatment.
Dermal Fillers—Dermal fillers are gel-like substances injected under the skin for subtle contouring of the face. Fillers also can be used to help promote a more masculine or feminine appearance. Filler can be placed in the lips to create a fuller, more projected, feminine-appearing lip. Malar cheek and central lower chin filler can be used to help define a heart-shaped face by accentuating the upper portion of the face and creating a more pointed chin, respectively. Alternatively, filler can be used to masculinize the chin by placing it where it can increase jawline squareness and increase anterior jaw projection. Additionally, filler at the angle of the jaw can help accentuate a square facial shape and a more defined jawline. Although not as widely practiced, lateral brow filler can create a heavier-appearing and broader forehead for a more masculine appearance. These procedures can be combined with the previously mentioned botulinum toxin procedures for a synergistic effect.
Deoxycholic Acid—Deoxycholic acid is an injectable product used to selectively remove unwanted fat. It currently is approved by the US Food and Drug Administration for submental fat, but some providers are experimenting with off-label uses. Buccal fat pad removal—or in this case reduction by dissolution—tends to give a thinner, more feminine facial appearance.12 Reducing fat around the axillae also can help promote a more masculine upper torso.13 The safety of deoxycholic acid in these areas has not been adequately tested; thus, caution should be used when discussing these off-label uses with patients.
Hair and Tattoo Removal—Hair removal may be desired by TGD persons for a variety of reasons. Because cisgender females tend to have less body hair overall, transgender people in pursuit of a more feminine appearance often desire removal of facial, neck, and body hair. Although shaving and other modalities such as waxing and chemical depilatories are readily available at-home options, they are not permanent and may lead to folliculitis or pseudofolliculitis barbae. Laser hair removal (LHR) and electrolysis are modalities provided by dermatologists that tend to be more permanent and lead to better outcomes, including less irritation and better aesthetic appearance. It is important to keep in mind that not every person and not every body site can be safely treated with LHR. Patients with lighter skin types and darker hair tend to have the most effective response with a higher margin of safety, as these features allow the laser energy to be selectively absorbed by the melanin in the hair bulb and not by the background skin pigmentation.14,15 Inappropriate patient selection or improper settings for wavelength, pulse width, or fluences can lead to burns and permanent scarring.14,15 Electrolysis is an alternative to hair removal within tattoos and is more effective for those individuals with blonde, red, or white hair.16
Another novel treatment for unwanted hair is eflornithine hydrochloride cream, which works by blocking ornithine decarboxylase, the enzyme that stimulates hair growth. It currently is approved to reduce unwanted hair on the face and adjacent areas under the chin; however the effects of this medication are modest and the medication can be expensive.17
Cosmetic hair and tattoo removal are not currently covered by TRICARE, except in cases of surgical and donor-site preparation for some GAPs. Individuals may desire removal of tattoos at surgery sites to obtain more natural-appearing skin. Currently, GAPs such as vaginoplasty, phalloplasty, and metoidioplasty—often referred to by patients as “bottom surgeries”—include insurance coverage for tattoo removal, LHR, and/or electrolysis.
Management of Hormonal Adverse Effects
Acne—Individuals on testosterone supplementation tend to develop acne for the first several years of treatment, but it may improve with time.18 Acne is treated in individuals receiving testosterone the same way as it is treated in cisgender men, with numerous options for topical and oral medications. In trans masculine persons, spironolactone therapy typically is avoided because it may interfere with the actions of exogenous testosterone administered as part of gender-affirming medical treatment and may lead to other undesired adverse effects such as impotence and gynecomastia.1
Although acne typically improves after starting estrogen therapy, patients receiving estrogens may still develop acne. Most trans feminine patients will already be on an estrogen and an antiandrogen, often spironolactone.1 Spironolactone often is used as monotherapy for acne control in cisgender women. Additionally, an important factor to consider with spironolactone is the possible adverse effect of increased micturition. Currently, the military rarely has gender-inclusive restroom options, which can create a challenge for TSMs who find themselves needing to use the restroom more frequently in the workplace.
If planning therapy with isotretinoin, dermatologists should discuss several important factors with all patients, including TGD patients. One consideration is the patient’s planned future surgeries. Although new literature shows that isotretinoin does not adversely affect wound healing,19 some surgeons still adhere to an isotretinoin washout period of 6 to 12 months prior to performing any elective procedures due to concerns about wound healing.20,21 Second, be sure to properly assess and document pregnancy potential in TGD persons. Providers should not assume that a patient is not pregnant or is not trying to become pregnant just because they are trans masculine. It also is important to note that testosterone is not a reliable birth control method.1 If a patient still has ovaries, fallopian tubes, and a uterus, they are considered medically capable of pregnancy, and providers should keep this in mind regarding all procedures in the TGD population.
Another newer acne treatment modality is the 1762-nm laser, which targets sebaceous glands.22 This device allows for targeted treatment of acne-prone areas without systemic therapy such as retinoids or antiandrogens. The 1762-nm laser is not widely available but may become a regular treatment option once its benefits are proven over time.
Alopecia and Hyperpigmentation—Androgens, whether endogenously or exogenously derived, can lead to androgenetic alopecia (AGA) in genetically susceptible individuals. Trans masculine persons and others receiving androgen therapy are at higher risk for AGA, which often is undesirable and may be considered gender affirming by some TGD persons. Standard AGA treatments for cisgender men also can be used in trans masculine persons. Some of the most common anti-AGA medications are topical minoxidil, oral finasteride, and oral minoxidil. Although Coleman et al1 recently reported that finasteride may be an appropriate treatment option in trans masculine persons experiencing alopecia, treatment with 5α-reductase inhibitors may impair clitoral growth and the development of facial and body hair. Further studies are needed to assess the efficacy and safety of 5α-reductase inhibitors in transgender populations.1 Dutasteride may be used off-label and comes with a similar potential adverse-event profile as finasteride, which includes depression, decreased libido, erectile dysfunction, ejaculation disorders, and gynecomastia.
Conversely, AGA tends to improve in trans feminine persons and others receiving estrogen and antiandrogen therapy. Natural testosterone production is suppressed by estrogens and spironolactone as well as in patients who undergo orchiectomy.1 Although spironolactone is not approved for acne, AGA, or hirsutism, it is a standard treatment of AGA in cisgender women because it functions to block the effects of androgens, including at the hair follicle. Finasteride may be used for AGA in cisgender women but it is not recommended for trans feminine persons.1
There are many other modalities available for the treatment of AGA that are less commonly used—some may be cost prohibitive or do not have robust supporting evidence, or both. One example is hair
Melasma is a hyperpigmentation disorder related to estrogens, UV light exposure, and sometimes medication use (eg, hormonal birth control, spironolactone).24 The mainstay of treatment is prevention, including sun avoidance as well as use of sun-protective clothing and broad-spectrum sunscreens. Dermatologists tend to recommend physical sunscreens containing zinc oxide, titanium dioxide, and/or iron oxide, as they cover a wider UV spectrum and also provide some protection from visible light. Once melasma is present, dermatologists still have several treatment options. Topical hydroquinone is a proven treatment; however, it must be used with caution to avoid ochronosis. With careful patient selection, chemical peels also are effective treatment options for dyspigmentation and hyperpigmentation. Energy devices such as intense pulsed light and tattoo removal lasers—Q-switched lasers and picosecond pulse widths—also can be used to treat hyperpigmentation. Oral, intralesional, and topical tranexamic acid are newer treatment options for melasma that still are being studied and have shown promising results. Further studies are needed to determine long-term safety and optimal treatment regimens.24,25
Many insurance carriers, including TRICARE, do not routinely cover medical management of AGA or melasma. Patients should be advised that they likely will have to pay for any medications prescribed and procedures undertaken for these purposes; however, some medication costs can be offset by ordering larger prescription quantities, such as a 90-day supply vs a 30-day supply, as well as utilizing pharmacy discount programs.
Scar Management Following Surgery
In TSMs who undergo gender-affirming surgeries, dermatologists play an important role when scar symptoms develop, including pruritus, tenderness, and/or paresthesia. In the military, some common treatment modalities for symptomatic scars include intralesional steroids with or without 5-fluouroruacil and the fractionated CO2 laser. There also are numerous experimental treatment options for scars, including intralesional or perilesional botulinum toxin, the pulsed dye laser, or nonablative fractionated lasers. These modalities also may be used on hypertrophic scars or keloids. Another option for keloids is scar excision followed by superficial radiation therapy.26
Mental Health Considerations
Providers must take psychological adverse effects into consideration when considering medical therapies for dermatologic conditions in TGD patients. In particular, it is important to consider the risks for increased rates of depression and suicidal ideation formerly associated with the use of isotretinoin and finasteride, though much of the evidence regarding these risks has been called into question in recent years.27,28 Nonetheless, it remains prominent in lay media and may be a more important consideration in patients at higher baseline risk.27 Although there are no known studies that have expressly assessed rates of depression or suicidal ideation in TGD patients taking isotretinoin or finasteride, it is well established that TGD persons are at higher baseline risk for depression and suicidality.1,7,8 All patients should be carefully assessed for depression and suicidal ideation as well as counseled regarding these risks prior to initiating these therapies. If concerns for untreated mental health issues arise during screening and counseling, patients should be referred for assessment by a behavioral health specialist prior to starting therapy.
Future Directions
The future of TGD health care in the military could see an expansion of covered benefits and the development of new dermatologic procedures or medications. Research and policy evolution are necessary to bridge the current gaps in care; however, it is unlikely that all procedures currently considered to be cosmetic will become covered benefits.
Facial LHR is a promising candidate for future coverage for trans feminine persons. When cisgender men develop adverse effects from mandatory daily shaving, LHR is already a covered benefit. Two arguments in support of adding LHR for TGD patients revolve around achieving and maintaining an appearance congruent with their gender along with avoiding unwanted adverse effects related to daily shaving. Visual conformity with one’s affirmed gender has been associated with improvements in well-being, quality of life, and some mental health conditions.29
Scar prevention, treatment, and reduction are additional areas under active research in which dermatologists likely will play a crucial role.30,31 As more dermatologic procedures are performed on TGD persons, the published data and collective knowledge regarding best practices in this population will continue to grow, which will lead to improved cosmetic and safety outcomes.
Final Thoughts
Although dermatologists do not directly perform gender-affirming surgeries or hormone management, they do play an important role in enhancing a TGD person’s desired appearance and managing possible adverse effects resulting from gender-affirming interventions. There have been considerable advancements in TGD health care over the past decade, but there likely are more changes on the way. As policies and understanding of TGD health care needs evolve, it is crucial that the military health care system adapts to provide comprehensive, accessible, and equitable care, which includes expanding the range of covered dermatologic treatments to fully support the health and readiness of TSMs.
Acknowledgment—We would like to extend our sincere appreciation to the invaluable contributions and editorial support provided by Allison Higgins, JD (San Antonio, Texas), throughout the writing of this article.
- Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S260. doi:10.1080/26895269.2022.2100644
- Secretary of Defense. DTM 16-005—military service of transgender service members. June 30, 2016. Accessed June 17, 2024. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DTM-16-005.pdf
- Office of the Deputy Secretary of Defense. DTM 19-004—military service by transgender persons and persons with gender dysphoria. March 17, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction (DODI) 1300.28. in-service transition for transgender service members. September 4, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/09/04/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Defense Health Agency Procedural Instruction Number 6025.21, Guidance for Gender-Affirming Health Care of Transgender and Gender-Diverse Active and Reserve Component Service Members, May 12, 2023. https://www.health.mil/Reference-Center/DHA-Publications/2023/05/12/DHA-PI-6015-21
- Elders MJ, Brown GR, Coleman E, et al. Medical aspects of transgender military service. Armed Forces Soc. 2015;41:199-220. doi:10.1177/0095327X14545625.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156:611-618.
- Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:E220978. doi:10.1001/jamanetworkopen.2022.0978
- Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431-436. doi:10.1001/jamapediatrics.2017.5440
- Top non-invasive cosmetic procedures worldwide 2022. Statista website. February 8, 2024. Accessed June 13, 2024. https://www.statista.com/statistics/293449/leading-nonsurgical-cosmetic-procedures/
- Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, et al. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29:154-168. doi:10.1016/j.joco.2017.04.001
- Thomas MK, D’Silva JA, Borole AJ. Injection lipolysis: a systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222-228. doi:10.4103/JCAS.JCAS_117_18
- Jegasothy SM. Deoxycholic acid injections for bra-line lipolysis. Dermatol Surg. 2018;44:757-760. doi:10.1097/DSS.0000000000001311
- Dierickx CC. Hair removal by lasers and intense pulsed light sources. Dermatol Clin. 2002;20:135-146. doi:10.1016/s0733-8635(03)00052-4
- Lepselter J, Elman M. Biological and clinical aspects in laser hair removal. J Dermatolog Treat. 2004;15:72-83. doi:10.1080/09546630310023152
- Yuan N, Feldman AT, Chin P, et al. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545. doi:10.1016/j.esxm.2022.100545
- Kumar A, Naguib YW, Shi YC, et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23:1495-1501. doi:10.3109/10717544.2014.951746
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102:3869-3903.
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- Rubenstein R, Roenigk HH Jr, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2 pt 1):280-285.
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913-914. doi:10.1111/ced.14292
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology: 2-Volume Set. Elsevier; 2024:1130.
- Konisky H, Balazic E, Jaller JA, et al. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22:1197-1206. doi:10.1111/jocd.15589
- Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi:10.1186/s13643-023-02192-7
- Kridin K, Ludwig RJ. Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J Am Acad Dermatol. 2023;88:388-394. doi:10.1016/j.jaad.2022.10.031
- Dyson TE, Cantrell MA, Lund BC. Lack of association between 5α-reductase inhibitors and depression. J Urol. 2020;204:793-798. doi:10.1097/JU.0000000000001079
- To M, Zhang Q, Bradlyn A, et al. Visual conformity with affirmed gender or “passing”: its distribution and association with depression and anxiety in a cohort of transgender people. J Sex Med. 2020;17:2084-2092. doi:10.1016/j.jsxm.2020.07.019
- Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22-33. doi:10.1016/j.actbio.2022.07.042
- Kolli H, Moy RL. Prevention of scarring with intraoperative erbium:YAG laser treatment. J Drugs Dermatol. 2020;19:1040-1043. doi:10.36849/JDD.2020.5244
- Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S260. doi:10.1080/26895269.2022.2100644
- Secretary of Defense. DTM 16-005—military service of transgender service members. June 30, 2016. Accessed June 17, 2024. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DTM-16-005.pdf
- Office of the Deputy Secretary of Defense. DTM 19-004—military service by transgender persons and persons with gender dysphoria. March 17, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction (DODI) 1300.28. in-service transition for transgender service members. September 4, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/09/04/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Defense Health Agency Procedural Instruction Number 6025.21, Guidance for Gender-Affirming Health Care of Transgender and Gender-Diverse Active and Reserve Component Service Members, May 12, 2023. https://www.health.mil/Reference-Center/DHA-Publications/2023/05/12/DHA-PI-6015-21
- Elders MJ, Brown GR, Coleman E, et al. Medical aspects of transgender military service. Armed Forces Soc. 2015;41:199-220. doi:10.1177/0095327X14545625.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156:611-618.
- Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:E220978. doi:10.1001/jamanetworkopen.2022.0978
- Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431-436. doi:10.1001/jamapediatrics.2017.5440
- Top non-invasive cosmetic procedures worldwide 2022. Statista website. February 8, 2024. Accessed June 13, 2024. https://www.statista.com/statistics/293449/leading-nonsurgical-cosmetic-procedures/
- Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, et al. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29:154-168. doi:10.1016/j.joco.2017.04.001
- Thomas MK, D’Silva JA, Borole AJ. Injection lipolysis: a systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222-228. doi:10.4103/JCAS.JCAS_117_18
- Jegasothy SM. Deoxycholic acid injections for bra-line lipolysis. Dermatol Surg. 2018;44:757-760. doi:10.1097/DSS.0000000000001311
- Dierickx CC. Hair removal by lasers and intense pulsed light sources. Dermatol Clin. 2002;20:135-146. doi:10.1016/s0733-8635(03)00052-4
- Lepselter J, Elman M. Biological and clinical aspects in laser hair removal. J Dermatolog Treat. 2004;15:72-83. doi:10.1080/09546630310023152
- Yuan N, Feldman AT, Chin P, et al. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545. doi:10.1016/j.esxm.2022.100545
- Kumar A, Naguib YW, Shi YC, et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23:1495-1501. doi:10.3109/10717544.2014.951746
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102:3869-3903.
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- Rubenstein R, Roenigk HH Jr, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2 pt 1):280-285.
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913-914. doi:10.1111/ced.14292
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology: 2-Volume Set. Elsevier; 2024:1130.
- Konisky H, Balazic E, Jaller JA, et al. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22:1197-1206. doi:10.1111/jocd.15589
- Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi:10.1186/s13643-023-02192-7
- Kridin K, Ludwig RJ. Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J Am Acad Dermatol. 2023;88:388-394. doi:10.1016/j.jaad.2022.10.031
- Dyson TE, Cantrell MA, Lund BC. Lack of association between 5α-reductase inhibitors and depression. J Urol. 2020;204:793-798. doi:10.1097/JU.0000000000001079
- To M, Zhang Q, Bradlyn A, et al. Visual conformity with affirmed gender or “passing”: its distribution and association with depression and anxiety in a cohort of transgender people. J Sex Med. 2020;17:2084-2092. doi:10.1016/j.jsxm.2020.07.019
- Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22-33. doi:10.1016/j.actbio.2022.07.042
- Kolli H, Moy RL. Prevention of scarring with intraoperative erbium:YAG laser treatment. J Drugs Dermatol. 2020;19:1040-1043. doi:10.36849/JDD.2020.5244
Practice Points
- Transgender and gender diverse (TGD) health care is multidisciplinary, and both military and civilian dermatologists can serve an important role.
- Although dermatologists do not directly perform gender-affirming surgeries or hormone management, there are a number of dermatologic procedures and medical interventions that can enhance a TGD person’s desired appearance.
- Dermatologists also can help manage possible adverse effects from gender-affirming interventions.
Benzoyl Peroxide, Benzene, and Lots of Unanswered Questions: Where Are We Now?
March 2024 proved to be a busy month for benzoyl peroxide in the media! We are now at almost 4 months since Valisure, an independent analytical laboratory located in Connecticut, filed a Citizen Petition on benzene in benzoyl peroxide drug products with the US Food and Drug Administration (FDA) on March 5, 2024.1 This petition was filed shortly before the annual meeting of the American Academy of Dermatology was held in San Diego, California, creating quite a stir of concern in the dermatology world. Further information on the degradation of benzoyl peroxide with production of benzene was published in the medical literature in March 2024.2 As benzene is recognized as a human carcinogen, manufacturing regulations exist to assure that it does not appear in topical products either through contamination or degradation over the course of a product’s shelf-life.3
As anticipated, several opinions and commentaries appeared quickly, both on video and in various articles. The American Acne & Rosacea Society (AARS) released a statement on this issue on March 20, 2024.4 The safety of the public is the overarching primary concern. This AARS statement does include some general suggestions related to benzoyl peroxide use based on the best assessment to date while awaiting further guidance from the FDA on this issue. Benzoyl peroxide is approved for use by the FDA as an over-the-counter (OTC) topical product for acne and also is in several FDA-approved prescription topical products.5,6
The following reflects my personal viewpoint as both a dermatologist and a grandfather who has grandchildren who use acne products. My views are not necessarily those of AARS. Since early March 2024, I have read several documents and spoken to several dermatologists, scientists, and formulators with knowledge in this area, including contacts at Valisure. I was hoping to get to some reasonable definitive answer but have not been able to achieve this to my full satisfaction. There are many opinions and concerns, and each one makes sense based on the vantage point of the presenter. However, several unanswered questions remain related to what testing and data are currently required of companies to gain FDA approval of a benzoyl peroxide product, including:
- assessment of stability and degradation products (including benzene),
- validation of testing methods,
- the issue of benzoyl peroxide stability in commercial products, and
- the relevant magnitude of resultant benzene exposures, especially as we are all exposed to benzene from several sources each day.
I am certain that companies with benzoyl peroxide products will evaluate their already-approved products and also do further testing. However, in this situation, which impacts millions of people on so many levels, I feel there needs to be an organized approach to evaluate and resolve the issue, otherwise the likelihood of continued confusion and uncertainty is high. As the FDA is the approval body, I am hoping it will provide definitive guidance within a reasonable timeline so that clinicians, patients, and manufacturers of benzoyl peroxide can proceed with full confidence. Right now, we all remain in a state of limbo. It is time for less talk and more definitive action to sort out this issue.
- Valisure Citizen Petition on Benzene in Benzoyl Peroxide Products. March 5, 2024. Accessed June 5, 2024. https://assets-global.website-files.com/6215052733f8bb8fea016220/65e8560962ed23f744902a7b_Valisure%20Citizen%20Petition%20on%20Benzene%20in%20Benzoyl%20Peroxide%20Drug%20Products.pdf
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:37702. doi:10.1289/EHP13984
- US Food and Drug Administration. Reformulating drug products that contain carbomers manufactured with benzene. December 2023. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reformulating-drug-products-contain-carbomers-manufactured-benzene
- American Acne & Rosacea Society. Response Statement from the AARS to the Valisure Citizen Petition on Benzene in Benzoyl Peroxide Drug Products. March 20, 2024. Accessed June 12, 2024. https://www.einpresswire.com/article/697481595/response-statement-from-the-aars-to-the-valisure-citizen-petition-on-benzene-in-benzoyl-peroxide-drug-products
- Department of Health and Human Services. Classification of benzoyl peroxide as safe and effective and revision of labeling to drug facts format; topical acne drug products for over-the-counter human use; Final Rule. Fed Registr. 2010;75:9767-9777.
- US Food and Drug Administration. Topical acne drug products for over-the-counter human use—revision of labeling and classification of benzoyl peroxide as safe and effective. June 2011. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/topical-acne-drug-products-over-counter-human-use-revision-labeling-and-classification-benzoyl
March 2024 proved to be a busy month for benzoyl peroxide in the media! We are now at almost 4 months since Valisure, an independent analytical laboratory located in Connecticut, filed a Citizen Petition on benzene in benzoyl peroxide drug products with the US Food and Drug Administration (FDA) on March 5, 2024.1 This petition was filed shortly before the annual meeting of the American Academy of Dermatology was held in San Diego, California, creating quite a stir of concern in the dermatology world. Further information on the degradation of benzoyl peroxide with production of benzene was published in the medical literature in March 2024.2 As benzene is recognized as a human carcinogen, manufacturing regulations exist to assure that it does not appear in topical products either through contamination or degradation over the course of a product’s shelf-life.3
As anticipated, several opinions and commentaries appeared quickly, both on video and in various articles. The American Acne & Rosacea Society (AARS) released a statement on this issue on March 20, 2024.4 The safety of the public is the overarching primary concern. This AARS statement does include some general suggestions related to benzoyl peroxide use based on the best assessment to date while awaiting further guidance from the FDA on this issue. Benzoyl peroxide is approved for use by the FDA as an over-the-counter (OTC) topical product for acne and also is in several FDA-approved prescription topical products.5,6
The following reflects my personal viewpoint as both a dermatologist and a grandfather who has grandchildren who use acne products. My views are not necessarily those of AARS. Since early March 2024, I have read several documents and spoken to several dermatologists, scientists, and formulators with knowledge in this area, including contacts at Valisure. I was hoping to get to some reasonable definitive answer but have not been able to achieve this to my full satisfaction. There are many opinions and concerns, and each one makes sense based on the vantage point of the presenter. However, several unanswered questions remain related to what testing and data are currently required of companies to gain FDA approval of a benzoyl peroxide product, including:
- assessment of stability and degradation products (including benzene),
- validation of testing methods,
- the issue of benzoyl peroxide stability in commercial products, and
- the relevant magnitude of resultant benzene exposures, especially as we are all exposed to benzene from several sources each day.
I am certain that companies with benzoyl peroxide products will evaluate their already-approved products and also do further testing. However, in this situation, which impacts millions of people on so many levels, I feel there needs to be an organized approach to evaluate and resolve the issue, otherwise the likelihood of continued confusion and uncertainty is high. As the FDA is the approval body, I am hoping it will provide definitive guidance within a reasonable timeline so that clinicians, patients, and manufacturers of benzoyl peroxide can proceed with full confidence. Right now, we all remain in a state of limbo. It is time for less talk and more definitive action to sort out this issue.
March 2024 proved to be a busy month for benzoyl peroxide in the media! We are now at almost 4 months since Valisure, an independent analytical laboratory located in Connecticut, filed a Citizen Petition on benzene in benzoyl peroxide drug products with the US Food and Drug Administration (FDA) on March 5, 2024.1 This petition was filed shortly before the annual meeting of the American Academy of Dermatology was held in San Diego, California, creating quite a stir of concern in the dermatology world. Further information on the degradation of benzoyl peroxide with production of benzene was published in the medical literature in March 2024.2 As benzene is recognized as a human carcinogen, manufacturing regulations exist to assure that it does not appear in topical products either through contamination or degradation over the course of a product’s shelf-life.3
As anticipated, several opinions and commentaries appeared quickly, both on video and in various articles. The American Acne & Rosacea Society (AARS) released a statement on this issue on March 20, 2024.4 The safety of the public is the overarching primary concern. This AARS statement does include some general suggestions related to benzoyl peroxide use based on the best assessment to date while awaiting further guidance from the FDA on this issue. Benzoyl peroxide is approved for use by the FDA as an over-the-counter (OTC) topical product for acne and also is in several FDA-approved prescription topical products.5,6
The following reflects my personal viewpoint as both a dermatologist and a grandfather who has grandchildren who use acne products. My views are not necessarily those of AARS. Since early March 2024, I have read several documents and spoken to several dermatologists, scientists, and formulators with knowledge in this area, including contacts at Valisure. I was hoping to get to some reasonable definitive answer but have not been able to achieve this to my full satisfaction. There are many opinions and concerns, and each one makes sense based on the vantage point of the presenter. However, several unanswered questions remain related to what testing and data are currently required of companies to gain FDA approval of a benzoyl peroxide product, including:
- assessment of stability and degradation products (including benzene),
- validation of testing methods,
- the issue of benzoyl peroxide stability in commercial products, and
- the relevant magnitude of resultant benzene exposures, especially as we are all exposed to benzene from several sources each day.
I am certain that companies with benzoyl peroxide products will evaluate their already-approved products and also do further testing. However, in this situation, which impacts millions of people on so many levels, I feel there needs to be an organized approach to evaluate and resolve the issue, otherwise the likelihood of continued confusion and uncertainty is high. As the FDA is the approval body, I am hoping it will provide definitive guidance within a reasonable timeline so that clinicians, patients, and manufacturers of benzoyl peroxide can proceed with full confidence. Right now, we all remain in a state of limbo. It is time for less talk and more definitive action to sort out this issue.
- Valisure Citizen Petition on Benzene in Benzoyl Peroxide Products. March 5, 2024. Accessed June 5, 2024. https://assets-global.website-files.com/6215052733f8bb8fea016220/65e8560962ed23f744902a7b_Valisure%20Citizen%20Petition%20on%20Benzene%20in%20Benzoyl%20Peroxide%20Drug%20Products.pdf
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:37702. doi:10.1289/EHP13984
- US Food and Drug Administration. Reformulating drug products that contain carbomers manufactured with benzene. December 2023. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reformulating-drug-products-contain-carbomers-manufactured-benzene
- American Acne & Rosacea Society. Response Statement from the AARS to the Valisure Citizen Petition on Benzene in Benzoyl Peroxide Drug Products. March 20, 2024. Accessed June 12, 2024. https://www.einpresswire.com/article/697481595/response-statement-from-the-aars-to-the-valisure-citizen-petition-on-benzene-in-benzoyl-peroxide-drug-products
- Department of Health and Human Services. Classification of benzoyl peroxide as safe and effective and revision of labeling to drug facts format; topical acne drug products for over-the-counter human use; Final Rule. Fed Registr. 2010;75:9767-9777.
- US Food and Drug Administration. Topical acne drug products for over-the-counter human use—revision of labeling and classification of benzoyl peroxide as safe and effective. June 2011. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/topical-acne-drug-products-over-counter-human-use-revision-labeling-and-classification-benzoyl
- Valisure Citizen Petition on Benzene in Benzoyl Peroxide Products. March 5, 2024. Accessed June 5, 2024. https://assets-global.website-files.com/6215052733f8bb8fea016220/65e8560962ed23f744902a7b_Valisure%20Citizen%20Petition%20on%20Benzene%20in%20Benzoyl%20Peroxide%20Drug%20Products.pdf
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:37702. doi:10.1289/EHP13984
- US Food and Drug Administration. Reformulating drug products that contain carbomers manufactured with benzene. December 2023. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reformulating-drug-products-contain-carbomers-manufactured-benzene
- American Acne & Rosacea Society. Response Statement from the AARS to the Valisure Citizen Petition on Benzene in Benzoyl Peroxide Drug Products. March 20, 2024. Accessed June 12, 2024. https://www.einpresswire.com/article/697481595/response-statement-from-the-aars-to-the-valisure-citizen-petition-on-benzene-in-benzoyl-peroxide-drug-products
- Department of Health and Human Services. Classification of benzoyl peroxide as safe and effective and revision of labeling to drug facts format; topical acne drug products for over-the-counter human use; Final Rule. Fed Registr. 2010;75:9767-9777.
- US Food and Drug Administration. Topical acne drug products for over-the-counter human use—revision of labeling and classification of benzoyl peroxide as safe and effective. June 2011. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/topical-acne-drug-products-over-counter-human-use-revision-labeling-and-classification-benzoyl
Isotretinoin-Induced Skin Fragility in an Aerialist
Isotretinoin was introduced more than 3 decades ago and marked a major advancement in the treatment of severe refractory cystic acne. The most common adverse effects linked to isotretinoin usage are mucocutaneous in nature, manifesting as xerosis and cheilitis.1 Skin fragility and poor wound healing also have been reported.2-6 Current recommendations for avoiding these adverse effects include refraining from waxing, laser procedures, and other elective cutaneous procedures for at least 6 months.7 We present a case of isotretinoin-induced cutaneous fragility resulting in blistering and erosions on the palms of a competitive aerial trapeze artist.
Case Report
A 25-year-old woman presented for follow-up during week 12 of isotretinoin therapy (40 mg twice daily) prescribed for acne. She reported peeling of the skin on the palms following intense aerial acrobatic workouts. She had been a performing aerialist for many years and had never sustained a similar injury. The wounds were painful and led to decreased activity. She had no notable medical history. Physical examination of the palms revealed erosions in a distribution that corresponded to horizontal bar contact and friction (Figure). The patient was advised on proper wound care, application of emollients, and minimizing friction. She completed the course of isotretinoin and has continued aerialist activity without recurrence of skin fragility.
Comment
Skin fragility is a well-known adverse effect of isotretinoin therapy.8 Pavlis and Lieblich9 reported skin fragility in a young wrestler who experienced similar skin erosions due to isotretinoin therapy. The proposed mechanism of isotretinoin-induced skin fragility is multifactorial. It involves an apoptotic effect on sebocytes,5 which results in reduced stratum corneum hydration and an associated increase in transepidermal water loss.6,10,11 Retinoids also are known to cause thinning of the skin, likely due to the disadhesion of both the epidermis and the stratum corneum, which was demonstrated by the easy removal of cornified cells through tape stripping in hairless mice treated with isotretinoin.12 In further investigations, human patients and hairless mice treated with isotretinoin readily developed friction blisters through pencil eraser abrasion.13 Examination of the friction blisters using light and electron microscopy revealed fraying or loss of the stratum corneum and viable epidermis as well as loss of desmosomes and tonofilaments. Additionally, intracellular and intercellular deposits of an unidentified amorphous material were noted.13
Overall, the origin of skin fragility induced by isotretinoin is supported by its effect on sebocytes, increased transepidermal water loss, and profound disruption of the integrity of the epidermis, resulting in an elevated risk for inadvertent skin damage. Patients were encouraged to avoid cosmetic procedures in prior case reports,14-16 and because our case demonstrates the risk for cutaneous injury in athletes due to isotretinoin-induced skin fragility, we propose an extension of these warnings to encompass athletes receiving isotretinoin treatment. Offering early guidance on wound prevention is of paramount importance in maintaining athletic performance and minimizing painful injuries.
- Rajput I, Anjankar VP. Side effects of treating acne vulgaris with isotretinoin: a systematic review. Cureus. 2024;16:E55946. doi:10.7759/cureus.55946
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- McDonald KA, Shelley AJ, Alavi A. A systematic review on oral isotretinoin therapy and clinically observable wound healing in acne patients. J Cutan Med Surg. 2017;21:325-333. doi:10.1177/1203475417701419
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169. doi:10.4161/derm.1.3.9364
- Zouboulis CC. Isotretinoin revisited: pluripotent effects on human sebaceous gland cells. J Invest Dermatol. 2006;126:2154-2156. doi:10.1038/sj.jid.5700418
- Kmiec´ ML, Pajor A, Broniarczyk-Dyła G. Evaluation of biophysical skin parameters and assessment of hair growth in patients with acne treated with isotretinoin. Postepy Dermatol Alergol. 2013;30:343-349. doi:10.5114/pdia.2013.39432
- Waldman A, Bolotin D, Arndt KA, et al. ASDS Guidelines Task Force: Consensus recommendations regarding the safety of lasers, dermabrasion, chemical peels, energy devices, and skin surgery during and after isotretinoin use. Dermatolog Surg. 2017;43:1249-1262. doi:10.1097/DSS.0000000000001166
- Aksoy H, Aksoy B, Calikoglu E. Systemic retinoids and scar dehiscence. Indian J Dermatol. 2019;64:68. doi:10.4103/ijd.IJD_148_18
- Pavlis MB, Lieblich L. Isotretinoin-induced skin fragility in a teenaged athlete: a case report. Cutis. 2013;92:33-34.
- Herane MI, Fuenzalida H, Zegpi E, et al. Specific gel-cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase—a double-blind, randomized, placebo-controlled study. J Cosmet Dermatol. 2009;8:181-185. doi:10.1111/j.1473-2165.2009.00455.x
- Park KY, Ko EJ, Kim IS, et al. The effect of evening primrose oil for the prevention of xerotic cheilitis in acne patients being treated with isotretinoin: a pilot study. Ann Dermatol. 2014;26:706-712. doi:10.5021/ad.2014.26.6.706
- Elias PM, Fritsch PO, Lampe M, et al. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981;44:531-540.
- Williams ML, Elias PM. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981;117:611-619.
- Rubenstein R, Roenigk HH, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15:280-285. doi:10.1016/S0190-9622(86)70167-9
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706. doi:10.1111/j.1365-2133.1988.tb02574.x
- Katz BE, Mac Farlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853. doi:10.1016/S0190-9622(94)70096-6
Isotretinoin was introduced more than 3 decades ago and marked a major advancement in the treatment of severe refractory cystic acne. The most common adverse effects linked to isotretinoin usage are mucocutaneous in nature, manifesting as xerosis and cheilitis.1 Skin fragility and poor wound healing also have been reported.2-6 Current recommendations for avoiding these adverse effects include refraining from waxing, laser procedures, and other elective cutaneous procedures for at least 6 months.7 We present a case of isotretinoin-induced cutaneous fragility resulting in blistering and erosions on the palms of a competitive aerial trapeze artist.
Case Report
A 25-year-old woman presented for follow-up during week 12 of isotretinoin therapy (40 mg twice daily) prescribed for acne. She reported peeling of the skin on the palms following intense aerial acrobatic workouts. She had been a performing aerialist for many years and had never sustained a similar injury. The wounds were painful and led to decreased activity. She had no notable medical history. Physical examination of the palms revealed erosions in a distribution that corresponded to horizontal bar contact and friction (Figure). The patient was advised on proper wound care, application of emollients, and minimizing friction. She completed the course of isotretinoin and has continued aerialist activity without recurrence of skin fragility.
Comment
Skin fragility is a well-known adverse effect of isotretinoin therapy.8 Pavlis and Lieblich9 reported skin fragility in a young wrestler who experienced similar skin erosions due to isotretinoin therapy. The proposed mechanism of isotretinoin-induced skin fragility is multifactorial. It involves an apoptotic effect on sebocytes,5 which results in reduced stratum corneum hydration and an associated increase in transepidermal water loss.6,10,11 Retinoids also are known to cause thinning of the skin, likely due to the disadhesion of both the epidermis and the stratum corneum, which was demonstrated by the easy removal of cornified cells through tape stripping in hairless mice treated with isotretinoin.12 In further investigations, human patients and hairless mice treated with isotretinoin readily developed friction blisters through pencil eraser abrasion.13 Examination of the friction blisters using light and electron microscopy revealed fraying or loss of the stratum corneum and viable epidermis as well as loss of desmosomes and tonofilaments. Additionally, intracellular and intercellular deposits of an unidentified amorphous material were noted.13

Overall, the origin of skin fragility induced by isotretinoin is supported by its effect on sebocytes, increased transepidermal water loss, and profound disruption of the integrity of the epidermis, resulting in an elevated risk for inadvertent skin damage. Patients were encouraged to avoid cosmetic procedures in prior case reports,14-16 and because our case demonstrates the risk for cutaneous injury in athletes due to isotretinoin-induced skin fragility, we propose an extension of these warnings to encompass athletes receiving isotretinoin treatment. Offering early guidance on wound prevention is of paramount importance in maintaining athletic performance and minimizing painful injuries.
Isotretinoin was introduced more than 3 decades ago and marked a major advancement in the treatment of severe refractory cystic acne. The most common adverse effects linked to isotretinoin usage are mucocutaneous in nature, manifesting as xerosis and cheilitis.1 Skin fragility and poor wound healing also have been reported.2-6 Current recommendations for avoiding these adverse effects include refraining from waxing, laser procedures, and other elective cutaneous procedures for at least 6 months.7 We present a case of isotretinoin-induced cutaneous fragility resulting in blistering and erosions on the palms of a competitive aerial trapeze artist.
Case Report
A 25-year-old woman presented for follow-up during week 12 of isotretinoin therapy (40 mg twice daily) prescribed for acne. She reported peeling of the skin on the palms following intense aerial acrobatic workouts. She had been a performing aerialist for many years and had never sustained a similar injury. The wounds were painful and led to decreased activity. She had no notable medical history. Physical examination of the palms revealed erosions in a distribution that corresponded to horizontal bar contact and friction (Figure). The patient was advised on proper wound care, application of emollients, and minimizing friction. She completed the course of isotretinoin and has continued aerialist activity without recurrence of skin fragility.
Comment
Skin fragility is a well-known adverse effect of isotretinoin therapy.8 Pavlis and Lieblich9 reported skin fragility in a young wrestler who experienced similar skin erosions due to isotretinoin therapy. The proposed mechanism of isotretinoin-induced skin fragility is multifactorial. It involves an apoptotic effect on sebocytes,5 which results in reduced stratum corneum hydration and an associated increase in transepidermal water loss.6,10,11 Retinoids also are known to cause thinning of the skin, likely due to the disadhesion of both the epidermis and the stratum corneum, which was demonstrated by the easy removal of cornified cells through tape stripping in hairless mice treated with isotretinoin.12 In further investigations, human patients and hairless mice treated with isotretinoin readily developed friction blisters through pencil eraser abrasion.13 Examination of the friction blisters using light and electron microscopy revealed fraying or loss of the stratum corneum and viable epidermis as well as loss of desmosomes and tonofilaments. Additionally, intracellular and intercellular deposits of an unidentified amorphous material were noted.13

Overall, the origin of skin fragility induced by isotretinoin is supported by its effect on sebocytes, increased transepidermal water loss, and profound disruption of the integrity of the epidermis, resulting in an elevated risk for inadvertent skin damage. Patients were encouraged to avoid cosmetic procedures in prior case reports,14-16 and because our case demonstrates the risk for cutaneous injury in athletes due to isotretinoin-induced skin fragility, we propose an extension of these warnings to encompass athletes receiving isotretinoin treatment. Offering early guidance on wound prevention is of paramount importance in maintaining athletic performance and minimizing painful injuries.
- Rajput I, Anjankar VP. Side effects of treating acne vulgaris with isotretinoin: a systematic review. Cureus. 2024;16:E55946. doi:10.7759/cureus.55946
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- McDonald KA, Shelley AJ, Alavi A. A systematic review on oral isotretinoin therapy and clinically observable wound healing in acne patients. J Cutan Med Surg. 2017;21:325-333. doi:10.1177/1203475417701419
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169. doi:10.4161/derm.1.3.9364
- Zouboulis CC. Isotretinoin revisited: pluripotent effects on human sebaceous gland cells. J Invest Dermatol. 2006;126:2154-2156. doi:10.1038/sj.jid.5700418
- Kmiec´ ML, Pajor A, Broniarczyk-Dyła G. Evaluation of biophysical skin parameters and assessment of hair growth in patients with acne treated with isotretinoin. Postepy Dermatol Alergol. 2013;30:343-349. doi:10.5114/pdia.2013.39432
- Waldman A, Bolotin D, Arndt KA, et al. ASDS Guidelines Task Force: Consensus recommendations regarding the safety of lasers, dermabrasion, chemical peels, energy devices, and skin surgery during and after isotretinoin use. Dermatolog Surg. 2017;43:1249-1262. doi:10.1097/DSS.0000000000001166
- Aksoy H, Aksoy B, Calikoglu E. Systemic retinoids and scar dehiscence. Indian J Dermatol. 2019;64:68. doi:10.4103/ijd.IJD_148_18
- Pavlis MB, Lieblich L. Isotretinoin-induced skin fragility in a teenaged athlete: a case report. Cutis. 2013;92:33-34.
- Herane MI, Fuenzalida H, Zegpi E, et al. Specific gel-cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase—a double-blind, randomized, placebo-controlled study. J Cosmet Dermatol. 2009;8:181-185. doi:10.1111/j.1473-2165.2009.00455.x
- Park KY, Ko EJ, Kim IS, et al. The effect of evening primrose oil for the prevention of xerotic cheilitis in acne patients being treated with isotretinoin: a pilot study. Ann Dermatol. 2014;26:706-712. doi:10.5021/ad.2014.26.6.706
- Elias PM, Fritsch PO, Lampe M, et al. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981;44:531-540.
- Williams ML, Elias PM. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981;117:611-619.
- Rubenstein R, Roenigk HH, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15:280-285. doi:10.1016/S0190-9622(86)70167-9
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706. doi:10.1111/j.1365-2133.1988.tb02574.x
- Katz BE, Mac Farlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853. doi:10.1016/S0190-9622(94)70096-6
- Rajput I, Anjankar VP. Side effects of treating acne vulgaris with isotretinoin: a systematic review. Cureus. 2024;16:E55946. doi:10.7759/cureus.55946
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- McDonald KA, Shelley AJ, Alavi A. A systematic review on oral isotretinoin therapy and clinically observable wound healing in acne patients. J Cutan Med Surg. 2017;21:325-333. doi:10.1177/1203475417701419
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169. doi:10.4161/derm.1.3.9364
- Zouboulis CC. Isotretinoin revisited: pluripotent effects on human sebaceous gland cells. J Invest Dermatol. 2006;126:2154-2156. doi:10.1038/sj.jid.5700418
- Kmiec´ ML, Pajor A, Broniarczyk-Dyła G. Evaluation of biophysical skin parameters and assessment of hair growth in patients with acne treated with isotretinoin. Postepy Dermatol Alergol. 2013;30:343-349. doi:10.5114/pdia.2013.39432
- Waldman A, Bolotin D, Arndt KA, et al. ASDS Guidelines Task Force: Consensus recommendations regarding the safety of lasers, dermabrasion, chemical peels, energy devices, and skin surgery during and after isotretinoin use. Dermatolog Surg. 2017;43:1249-1262. doi:10.1097/DSS.0000000000001166
- Aksoy H, Aksoy B, Calikoglu E. Systemic retinoids and scar dehiscence. Indian J Dermatol. 2019;64:68. doi:10.4103/ijd.IJD_148_18
- Pavlis MB, Lieblich L. Isotretinoin-induced skin fragility in a teenaged athlete: a case report. Cutis. 2013;92:33-34.
- Herane MI, Fuenzalida H, Zegpi E, et al. Specific gel-cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase—a double-blind, randomized, placebo-controlled study. J Cosmet Dermatol. 2009;8:181-185. doi:10.1111/j.1473-2165.2009.00455.x
- Park KY, Ko EJ, Kim IS, et al. The effect of evening primrose oil for the prevention of xerotic cheilitis in acne patients being treated with isotretinoin: a pilot study. Ann Dermatol. 2014;26:706-712. doi:10.5021/ad.2014.26.6.706
- Elias PM, Fritsch PO, Lampe M, et al. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981;44:531-540.
- Williams ML, Elias PM. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981;117:611-619.
- Rubenstein R, Roenigk HH, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15:280-285. doi:10.1016/S0190-9622(86)70167-9
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706. doi:10.1111/j.1365-2133.1988.tb02574.x
- Katz BE, Mac Farlane DF. Atypical facial scarring after isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852-853. doi:10.1016/S0190-9622(94)70096-6
Practice Points
- Isotretinoin is used to treat severe nodulocystic acne but can cause adverse effects such as skin fragility, xerosis, and poor wound healing.
- Dermatologists should inform athletes of heightened skin vulnerability while undergoing isotretinoin treatment.
- Isotretinoin-induced skin fragility involves the effects of isotretinoin on sebocytes, transepidermal water loss, and disruption of the integrity of the epidermis.
Two Techniques to Avoid Cyst Spray During Excision
Practice Gap
Epidermoid cysts are asymptomatic, well-circumscribed, mobile, subcutaneous masses that elevate the skin. Also known as epidermal, keratin, or infundibular cysts, epidermoid cysts are caused by proliferation of surface epidermoid cells within the dermis and can arise anywhere on the body, most commonly on the face, neck, and trunk.1 Cutaneous cysts often contain fluid or semifluid contents and can be aesthetically displeasing or cause mild pain, prompting patients to seek removal. Definitive treatment of epidermoid cysts is complete surgical removal,2 which can be performed in office in a sterile or clean manner by either dermatologists or primary care providers.
Prior to incision, a local anesthetic—commonly lidocaine with epinephrine—is injected in the region surrounding the cyst sac so as not to rupture the cyst wall. Maintaining the cyst wall throughout the procedure ensures total cyst removal and minimizes the risk for recurrence. However, it often is difficult to approximate the cyst border because it cannot be visualized prior to incision.
Throughout the duration of the procedure, cyst contents may suddenly spray out of the area and pose a risk to providers and their staff (Figure, A). Even with careful application around the periphery, either puncture or pericystic anesthesia between the cyst wall and the dermis can lead to splatter. Larger and wider peripheral anesthesia may not be possible given a shortage of lidocaine and a desire to minimize injection. Even with meticulous use of personal protective equipment in cutaneous surgery, infectious organisms found in ruptured cysts and abscesses may spray the surgical field.3 Therefore, it is in our best interest to minimize the trajectory of cyst spray contents.
The Tools
We have employed 2 simple techniques using equipment normally found on a standard surgical tray for easy safe injection of cysts. Supplies needed include 4×4-inch gauze pads, alcohol and chlorhexidine, a marker, all instruments necessary for cyst excision, and a small clear biohazard bag.
The Technique
Prior to covering the cyst, care is taken to locate the cyst opening. At times, a comedo or punctum can be seen overlying the cyst bulge. We mark the lumen and cyst opening with a surgical marker. If the pore is not easily identified, we draw an 8-mm circle around the mound of the cyst.
One option is to apply a gauze pad over the cyst to allow for stabilization of the surgical field and blanket the area from splatter (Figure, B). Then we cover the cyst using antiseptic-soaked gauze as a protective barrier to avoid potentially contaminated spray. This tool can be constructed from a 4×4-inch gauze pad with the addition of alcohol and chlorhexidine. When the cyst is covered, the surgeon can inject the lesion and surrounding tissue without biohazard splatter.
Another method is to cover the cyst with a small clear biohazard bag (Figure, C). When injecting anesthetic through the bag, the spray is captured by the bag and does not reach the surgeon or staff. This method is potentially more effective given that the cyst can still be visualized fully for more accurate injection.
Practice Implications
Outpatient surgical excision is a common effective procedure for epidermoid cysts. However, it is not uncommon for cyst contents to spray during the injection of anesthetic, posing a nuisance to the surgeon, health care staff, and patient. The technique of covering the lesion with antiseptic-soaked gauze or a small clear biohazard bag prevents cyst contents from spraying and reduces risk for contamination. In addition to these protective benefits, the use of readily available items replaces the need to order a splatter control shield.
Limitations—Although we seldom see spray using our technique, covering the cyst with gauze may disguise the region of interest and interfere with accurate incision. Marking the lesion prior to anesthesia administration or using a clear biohazard bag minimizes difficulty visualizing the cyst opening.
- Zito PM, Scharf R. Epidermoid cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK499974
- Weir CB, St. Hilaire NJ. Epidermal inclusion cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532310/
- Kuniyuki S, Yoshida Y, Maekawa N, et al. Bacteriological study of epidermal cysts. Acta Derm Venereol. 2018;88:23-25. doi:10.2340/00015555-0348
Practice Gap
Epidermoid cysts are asymptomatic, well-circumscribed, mobile, subcutaneous masses that elevate the skin. Also known as epidermal, keratin, or infundibular cysts, epidermoid cysts are caused by proliferation of surface epidermoid cells within the dermis and can arise anywhere on the body, most commonly on the face, neck, and trunk.1 Cutaneous cysts often contain fluid or semifluid contents and can be aesthetically displeasing or cause mild pain, prompting patients to seek removal. Definitive treatment of epidermoid cysts is complete surgical removal,2 which can be performed in office in a sterile or clean manner by either dermatologists or primary care providers.
Prior to incision, a local anesthetic—commonly lidocaine with epinephrine—is injected in the region surrounding the cyst sac so as not to rupture the cyst wall. Maintaining the cyst wall throughout the procedure ensures total cyst removal and minimizes the risk for recurrence. However, it often is difficult to approximate the cyst border because it cannot be visualized prior to incision.
Throughout the duration of the procedure, cyst contents may suddenly spray out of the area and pose a risk to providers and their staff (Figure, A). Even with careful application around the periphery, either puncture or pericystic anesthesia between the cyst wall and the dermis can lead to splatter. Larger and wider peripheral anesthesia may not be possible given a shortage of lidocaine and a desire to minimize injection. Even with meticulous use of personal protective equipment in cutaneous surgery, infectious organisms found in ruptured cysts and abscesses may spray the surgical field.3 Therefore, it is in our best interest to minimize the trajectory of cyst spray contents.
The Tools
We have employed 2 simple techniques using equipment normally found on a standard surgical tray for easy safe injection of cysts. Supplies needed include 4×4-inch gauze pads, alcohol and chlorhexidine, a marker, all instruments necessary for cyst excision, and a small clear biohazard bag.
The Technique
Prior to covering the cyst, care is taken to locate the cyst opening. At times, a comedo or punctum can be seen overlying the cyst bulge. We mark the lumen and cyst opening with a surgical marker. If the pore is not easily identified, we draw an 8-mm circle around the mound of the cyst.
One option is to apply a gauze pad over the cyst to allow for stabilization of the surgical field and blanket the area from splatter (Figure, B). Then we cover the cyst using antiseptic-soaked gauze as a protective barrier to avoid potentially contaminated spray. This tool can be constructed from a 4×4-inch gauze pad with the addition of alcohol and chlorhexidine. When the cyst is covered, the surgeon can inject the lesion and surrounding tissue without biohazard splatter.

Another method is to cover the cyst with a small clear biohazard bag (Figure, C). When injecting anesthetic through the bag, the spray is captured by the bag and does not reach the surgeon or staff. This method is potentially more effective given that the cyst can still be visualized fully for more accurate injection.
Practice Implications
Outpatient surgical excision is a common effective procedure for epidermoid cysts. However, it is not uncommon for cyst contents to spray during the injection of anesthetic, posing a nuisance to the surgeon, health care staff, and patient. The technique of covering the lesion with antiseptic-soaked gauze or a small clear biohazard bag prevents cyst contents from spraying and reduces risk for contamination. In addition to these protective benefits, the use of readily available items replaces the need to order a splatter control shield.
Limitations—Although we seldom see spray using our technique, covering the cyst with gauze may disguise the region of interest and interfere with accurate incision. Marking the lesion prior to anesthesia administration or using a clear biohazard bag minimizes difficulty visualizing the cyst opening.
Practice Gap
Epidermoid cysts are asymptomatic, well-circumscribed, mobile, subcutaneous masses that elevate the skin. Also known as epidermal, keratin, or infundibular cysts, epidermoid cysts are caused by proliferation of surface epidermoid cells within the dermis and can arise anywhere on the body, most commonly on the face, neck, and trunk.1 Cutaneous cysts often contain fluid or semifluid contents and can be aesthetically displeasing or cause mild pain, prompting patients to seek removal. Definitive treatment of epidermoid cysts is complete surgical removal,2 which can be performed in office in a sterile or clean manner by either dermatologists or primary care providers.
Prior to incision, a local anesthetic—commonly lidocaine with epinephrine—is injected in the region surrounding the cyst sac so as not to rupture the cyst wall. Maintaining the cyst wall throughout the procedure ensures total cyst removal and minimizes the risk for recurrence. However, it often is difficult to approximate the cyst border because it cannot be visualized prior to incision.
Throughout the duration of the procedure, cyst contents may suddenly spray out of the area and pose a risk to providers and their staff (Figure, A). Even with careful application around the periphery, either puncture or pericystic anesthesia between the cyst wall and the dermis can lead to splatter. Larger and wider peripheral anesthesia may not be possible given a shortage of lidocaine and a desire to minimize injection. Even with meticulous use of personal protective equipment in cutaneous surgery, infectious organisms found in ruptured cysts and abscesses may spray the surgical field.3 Therefore, it is in our best interest to minimize the trajectory of cyst spray contents.
The Tools
We have employed 2 simple techniques using equipment normally found on a standard surgical tray for easy safe injection of cysts. Supplies needed include 4×4-inch gauze pads, alcohol and chlorhexidine, a marker, all instruments necessary for cyst excision, and a small clear biohazard bag.
The Technique
Prior to covering the cyst, care is taken to locate the cyst opening. At times, a comedo or punctum can be seen overlying the cyst bulge. We mark the lumen and cyst opening with a surgical marker. If the pore is not easily identified, we draw an 8-mm circle around the mound of the cyst.
One option is to apply a gauze pad over the cyst to allow for stabilization of the surgical field and blanket the area from splatter (Figure, B). Then we cover the cyst using antiseptic-soaked gauze as a protective barrier to avoid potentially contaminated spray. This tool can be constructed from a 4×4-inch gauze pad with the addition of alcohol and chlorhexidine. When the cyst is covered, the surgeon can inject the lesion and surrounding tissue without biohazard splatter.

Another method is to cover the cyst with a small clear biohazard bag (Figure, C). When injecting anesthetic through the bag, the spray is captured by the bag and does not reach the surgeon or staff. This method is potentially more effective given that the cyst can still be visualized fully for more accurate injection.
Practice Implications
Outpatient surgical excision is a common effective procedure for epidermoid cysts. However, it is not uncommon for cyst contents to spray during the injection of anesthetic, posing a nuisance to the surgeon, health care staff, and patient. The technique of covering the lesion with antiseptic-soaked gauze or a small clear biohazard bag prevents cyst contents from spraying and reduces risk for contamination. In addition to these protective benefits, the use of readily available items replaces the need to order a splatter control shield.
Limitations—Although we seldom see spray using our technique, covering the cyst with gauze may disguise the region of interest and interfere with accurate incision. Marking the lesion prior to anesthesia administration or using a clear biohazard bag minimizes difficulty visualizing the cyst opening.
- Zito PM, Scharf R. Epidermoid cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK499974
- Weir CB, St. Hilaire NJ. Epidermal inclusion cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532310/
- Kuniyuki S, Yoshida Y, Maekawa N, et al. Bacteriological study of epidermal cysts. Acta Derm Venereol. 2018;88:23-25. doi:10.2340/00015555-0348
- Zito PM, Scharf R. Epidermoid cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK499974
- Weir CB, St. Hilaire NJ. Epidermal inclusion cyst. StatPearls [Internet]. Updated August 8, 2023. Accessed June3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532310/
- Kuniyuki S, Yoshida Y, Maekawa N, et al. Bacteriological study of epidermal cysts. Acta Derm Venereol. 2018;88:23-25. doi:10.2340/00015555-0348
Vascular Mass on the Posterior Neck in a Newborn
The Diagnosis: Congenital Hemangioma
Surgical resection of the mass was performed at 4 months of age without complication (Figure 1). Histopathology revealed a lobular endothelial cell proliferation within a densely fibrotic stroma, multiple thin-walled vessels, and negative immunoreactivity to glucose transporter type 1 (GLUT-1)(Figures 2 and 3). Combined with the patient’s clinical history and findings on imaging (Figure 4), the most accurate diagnosis was a congenital hemangioma (CH). The mass was determined to be a noninvoluting congenital hemangioma (NICH).
A variety of vascular anomalies manifest in newborns and can be differentiated by the patient’s clinical history—particularly whether the lesion is present at birth or develops after birth. Imaging and histopathology of the lesion(s) may be utilized when clinical examination alone is not sufficient to make a diagnosis. Histopathology and immunohistochemistry further aid in differentiating the type of vascular lesion.

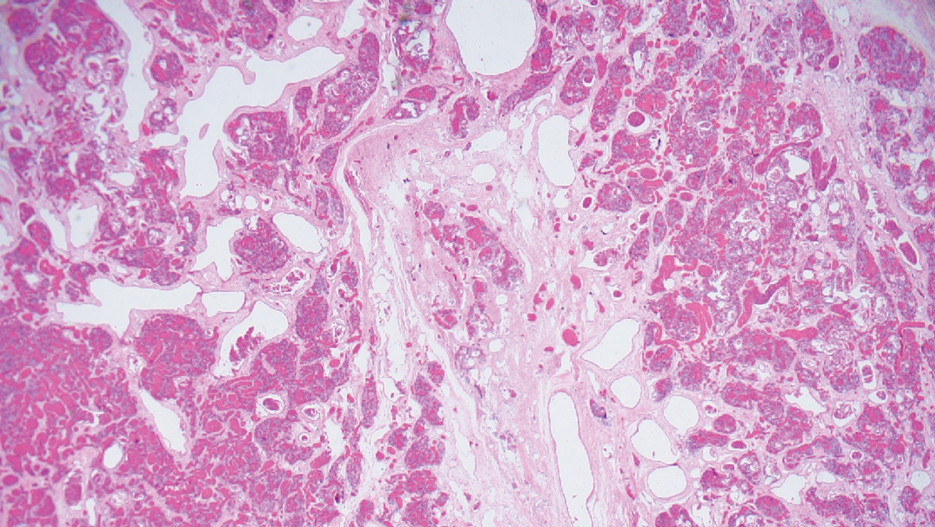
Overall, vascular anomalies are classified broadly into 2 categories based on their pathogenesis: tumors and malformations. Vascular tumors are composed of proliferating endothelial cells that have the potential to resolve spontaneously over time. Examples include CH, infantile hemangioma (IH), kaposiform hemangioendothelioma (KHE), and tufted angioma (TA). In contrast, vascular malformations (ie, arteriovenous malformations) are composed of dysplastic vessels with normal endothelial cell turnover and do not resolve without intervention.1
Congenital hemangiomas are rare vascular tumors that are fully developed at birth. These tumors proliferate in utero, enabling prenatal detection via ultrasonography as early as 12 weeks’ gestation for large heterogeneous vascular masses.2-4 Congenital hemangiomas are described as solitary, well-circumscribed, raised, violaceous lesions most commonly located in the head and neck region.4-6 Histopathologically, they are characterized by lobules of proliferating capillaries surrounded by fibrous stroma and dysplastic vascular channels.6,7
Congenital hemangiomas are categorized based on their postnatal involution patterns.2 Fetally involuting CH both develops and begins regression in utero and often is completely regressed at birth.8 Rapidly involuting CH begins regression in the first few weeks of life and usually is completely involuted by 14 months of age.6,9-11 Conversely, NICH does not regress, often requiring surgical excision due to functional and cosmetic issues.12,13 Partially involuting CH is intermediary, beginning as rapidly involuting but not involuting completely and persisting as lesions that resemble NICH.14-16 Although generally benign and asymptomatic, these tumors can cause transient thrombocytopenia and coagulopathy at birth, as seen in our patient.17,18
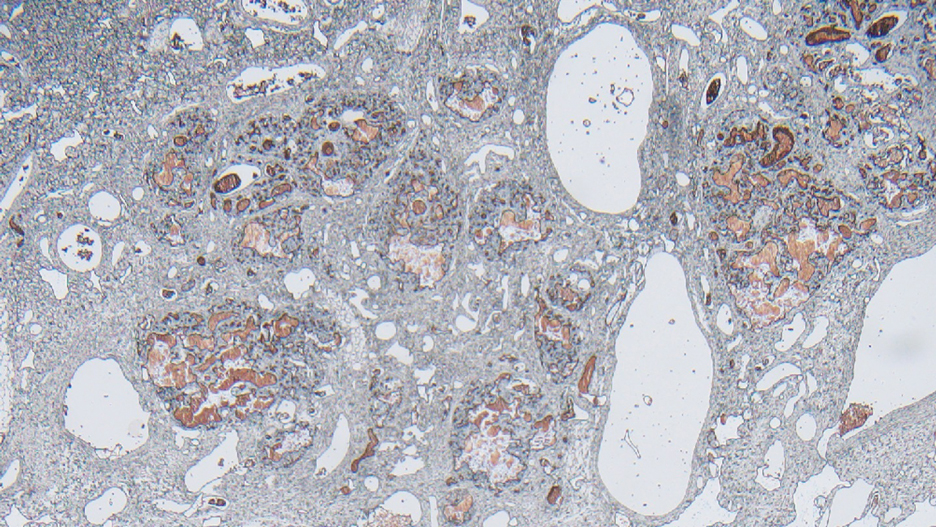
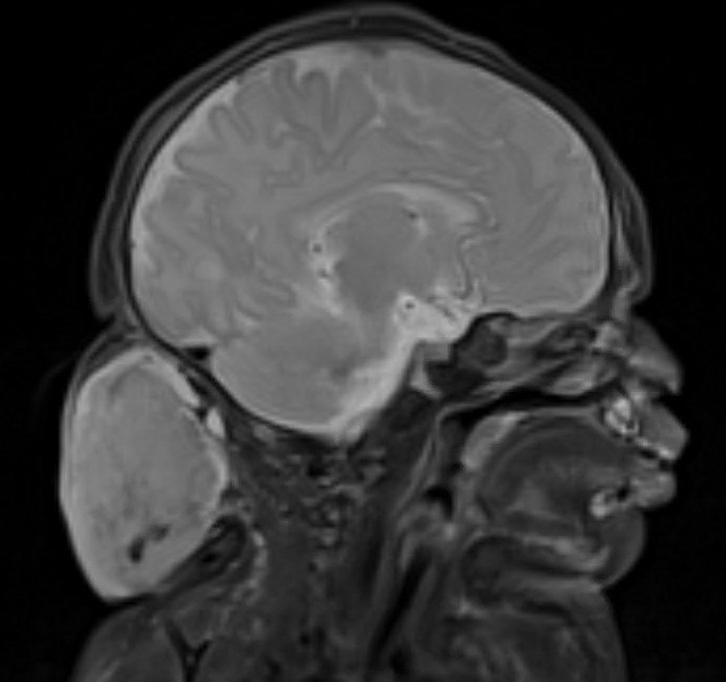
Infantile hemangioma is the most common vascular tumor of infancy.19-21 Although a precursor lesion may be present at birth, generally this tumor becomes apparent after the first few weeks of life as a solitary vascular plaque or nodule with a predilection for the head and neck.22-25 Once it arises, IH quickly enters a period of rapid growth, followed by a period of slower continued growth, with most reaching maximum size by 3 months.22 Thereafter, IH enters a slow period of involution (range, 3–9 years)26; more recent data suggest near resolution by 5 years of age.27 Infantile hemangioma is categorized based on its depth in the skin and subcutaneous tissues and can be classified as superficial, mixed, or deep.22,24,28,29 Superficial IH appears as a red plaque and may exhibit lobulation, while deep IH can be identified as flesh-colored or blue subcutaneous masses. Mixed IH may manifest with both superficial and deep features depending on the extent of its involvement in the dermal and subcutaneous layers. The pattern of involvement may be focal, segmental, or indeterminate.24 In contrast, CH typically is a solitary vascular mass with prominent telangiectases, nodules, and radiating veins.6 Histologically, IH is composed of proliferative plump endothelial cells that form capillaries, and the lesion stains positively for GLUT-1, whereas CH does not.30
Kaposiform hemangioendothelioma is classified as a locally aggressive vascular tumor that manifests either prenatally or in early infancy.31 It is described as a solitary, ill-defined, firm, purple plaque most commonly located on the extremities and retroperitoneum.32-34 Histopathologically, these lesions are characterized by dilated lymphatic channels and irregular sheets or lobules of spindle-shaped endothelial cells infiltrating the dermis and subcutaneous fat.33,35 In contrast to CH, KHE lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein.36,37 Notably, 70% of these tumors are complicated by the presence of Kasabach-Merritt phenomenon, a potentially life-threatening emergency that occurs when platelets are trapped within a vascular tumor, leading to the consumption of clotting factors, intralesional bleeding, and rapid enlargement of the tumor.32 The Kasabach-Merritt phenomenon manifests clinically as microangiopathic hemolytic anemia, severe thrombocytopenia, and disseminated intravascular coagulation. 38 Although CH lesions also can be associated with thrombocytopenia and coagulopathy, they generally are mild and self-limited.18
Tufted angioma is a vascular tumor that arises within the first 5 years of life as firm violaceous papules or plaques, often with associated hyperhidrosis or hypertrichosis.39,40 Although TA grows slowly for a period of time, it eventually stabilizes and persists, rarely regressing completely.41 These tumors share many similarities with KHE, and it has been suggested that they may be part of the same spectrum. 42 As with KHE, TA lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein, which are negative in CH.36,37 Although TA also can be complicated by Kasabach-Merritt phenomenon, the incidence is much lower (up to 38%).43,44 As such, TAs tend to be recognized as more superficial benign lesions. However, they still can cause notable cosmetic and functional impairment and should be monitored closely, especially in the presence of associated symptoms or complications.
Arteriovenous malformation is a vascular lesion that results from errors during the embryonic development of vascular channels.45 Although present at birth, it may not become clinically apparent until later in life. Arteriovenous malformations enlarge postnatally, and their growth is proportional to the developmental growth of the affected individual rather than the result of endothelial proliferation.46 In infants, AVM may manifest as a faint vascular stain that can evolve over time into a pink patch associated with a palpable thrill during adolescence. 4 On Doppler flow imaging, AVMs are identified as fast-flow anomalies arising from an abnormal communication between high-pressure arterial systems and low-pressure venous systems without the presence of a capillary bed.47 One of the differentiating factors between AVM and CH is that AVMs do not regress spontaneously and tend to have high recurrence rates, even with intervention. 48 In contrast, CH can be categorized based on its postnatal involution pattern. Another distinguishing factor is that AVMs tend to be larger and more invasive than CHs.46 Therefore, early diagnosis and intervention are crucial to prevent complications such as bleeding, seizures, or neurologic deficits associated with AVMs.1
- Enjolras O, Wassef M, Chapot R. Introduction: ISSVA Classification. In: Enjolras O, Wassef M, Chapot R, eds. Color Atlas of Vascular Tumors and Vascular Malformations. Cambridge University Press; 2007:3-11.
- Fadell MF, Jones BV, Adams DM. Prenatal diagnosis and postnatal follow-up of rapidly involuting congenital hemangioma (RICH). Pediatr Radiol. 2011;41:1057-1060.
- Feygin T, Khalek N, Moldenhauer JS. Fetal brain, head, and neck tumors: prenatal imaging and management. Prenat Diagn. 2020;40:1203-1219.
- Foley LS, Kulungowski AM. Vascular anomalies in pediatrics. Adv Pediatr. 2015;62:227-255.
- Bruder E, Alaggio R, Kozakewich HPW, et al. Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Dev Pathol. 2012;15:26-61.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Maguiness S, Uihlein LC, Liang MG, et al. Rapidly involuting congenital hemangioma with fetal involution. Pediatr Dermatol. 2015;32:321-326.
- Keating LJ, Soares GM, Muratore CS. Rapidly involuting congenital hemangioma. Med Health R I. 2012;95:149-152.
- Schafer F, Tapia M, Pinto C. Rapidly involuting congenital haemangioma. Arch Dis Child Fetal Neonatal Ed. 2014;99:F422.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Liang MG, Frieden IJ. Infantile and congenital hemangiomas. Semin Pediatr Surg. 2014;23:162-167.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:E203-E214.
- Boull C, Maguiness SM. Congenital hemangiomas. Semin Cutan Med Surg. 2016;35:124-127.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Baselga E, Cordisco MR, Garzon M, et al. Rapidly involuting congenital haemangioma associated with transient thrombocytopenia and coagulopathy: a case series. Br J Dermatol. 2008;158:1363-1370.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Hidano A, Nakajima S. Earliest features of the strawberry mark in the newborn. Br J Dermatol. 1972;87:138-144.
- Martinez-Perez D, Fein NA, Boon LM, et al. Not all hemangiomas look like strawberries: uncommon presentations of the most common tumor of infancy. Pediatr Dermatol. 1995;12:1-6.
- Payne MM, Moyer F, Marcks KM, et al. The precursor to the hemangioma. Plast Reconstr Surg. 1966;38:64-67.
- Bowers RE, Graham EA, Tomlinson KM. The natural history of the strawberry nevus. Arch Dermatol. 1960;82:667-680.
- Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624.
- Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173-181.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567-1576.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
- Gruman A, Liang MG, Mulliken JB, et al. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52:616-622.
- Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach- Merritt phenomenon in 107 referrals. J Pediatr. 2013;162:142-147.
- Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. an aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321-328.
- Mac-Moune Lai F, To KF, Choi PC, et al. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol. 2001;14:1087-1092.
- O’Rafferty C, O’Regan GM, Irvine AD, et al. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol. 2015;171:38-51.
- Le Huu AR, Jokinen CH, Rubin BP, et al. Expression of prox1, lymphatic endothelial nuclear transcription factor, in kaposiform hemangioendothelioma and tufted angioma. Am J Surg Pathol. 2010;34:1563-1573.
- Debelenko LV, Perez-Atayde AR, Mulliken JB, et al. D2-40 immuno-histochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. 2005;18:1454-1460.
- Haisley-Royster C, Enjolras O, Frieden IJ, et al. Kasabach-Merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459-462.
- Wilmer A, Kaatz M, Bocker T, et al. Tufted angioma. Eur J Dermatol. 1999;9:51-53.
- Herron MD, Coffin CM, Vanderhooft SL. Tufted angiomas: variability of the clinical morphology. Pediatr Dermatol. 2002;19:394-401.
- North PE. Pediatric vascular tumors and malformations. Surg Pathol Clin. 2010,3:455-494.
- Chu CY, Hsiao CH, Chiu HC. Transformation between kaposiform hemangioendothelioma and tufted angioma. Dermatology. 2003;206:334-337.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Johnson EF, Davis DM, Tollefson MM, et al. Vascular tumors in infants: case report and review of clinical, histopathologic, and immunohistochemical characteristics of infantile hemangioma, pyogenic granuloma, noninvoluting congenital hemangioma, tufted angioma, and kaposiform hemangioendothelioma. Am J Dermatopathol. 2018;40:231-239.
- Christison-Lagay ER, Fishman SJ. Vascular anomalies. Surg Clin North Am. 2006;86:393-425.
- Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010;125:1185-1194.
- Young AE, Mulliken JB. Arteriovenous malformations. In: Mulliken JB, Young AE, eds. Vascular Birthmarks: Haemangiomas and Malformations. WB Saunders; 1988:228-245.
- Duggan EM, Fishman SJ. Vascular anomalies. In: Holcomb GW III, Murphy JP, St Peter SD, eds. Holcomb and Ashcraft’s Pediatric Surgery. 7th edition. Elsevier; 2019:1147-1170.
The Diagnosis: Congenital Hemangioma
Surgical resection of the mass was performed at 4 months of age without complication (Figure 1). Histopathology revealed a lobular endothelial cell proliferation within a densely fibrotic stroma, multiple thin-walled vessels, and negative immunoreactivity to glucose transporter type 1 (GLUT-1)(Figures 2 and 3). Combined with the patient’s clinical history and findings on imaging (Figure 4), the most accurate diagnosis was a congenital hemangioma (CH). The mass was determined to be a noninvoluting congenital hemangioma (NICH).
A variety of vascular anomalies manifest in newborns and can be differentiated by the patient’s clinical history—particularly whether the lesion is present at birth or develops after birth. Imaging and histopathology of the lesion(s) may be utilized when clinical examination alone is not sufficient to make a diagnosis. Histopathology and immunohistochemistry further aid in differentiating the type of vascular lesion.


Overall, vascular anomalies are classified broadly into 2 categories based on their pathogenesis: tumors and malformations. Vascular tumors are composed of proliferating endothelial cells that have the potential to resolve spontaneously over time. Examples include CH, infantile hemangioma (IH), kaposiform hemangioendothelioma (KHE), and tufted angioma (TA). In contrast, vascular malformations (ie, arteriovenous malformations) are composed of dysplastic vessels with normal endothelial cell turnover and do not resolve without intervention.1
Congenital hemangiomas are rare vascular tumors that are fully developed at birth. These tumors proliferate in utero, enabling prenatal detection via ultrasonography as early as 12 weeks’ gestation for large heterogeneous vascular masses.2-4 Congenital hemangiomas are described as solitary, well-circumscribed, raised, violaceous lesions most commonly located in the head and neck region.4-6 Histopathologically, they are characterized by lobules of proliferating capillaries surrounded by fibrous stroma and dysplastic vascular channels.6,7
Congenital hemangiomas are categorized based on their postnatal involution patterns.2 Fetally involuting CH both develops and begins regression in utero and often is completely regressed at birth.8 Rapidly involuting CH begins regression in the first few weeks of life and usually is completely involuted by 14 months of age.6,9-11 Conversely, NICH does not regress, often requiring surgical excision due to functional and cosmetic issues.12,13 Partially involuting CH is intermediary, beginning as rapidly involuting but not involuting completely and persisting as lesions that resemble NICH.14-16 Although generally benign and asymptomatic, these tumors can cause transient thrombocytopenia and coagulopathy at birth, as seen in our patient.17,18


Infantile hemangioma is the most common vascular tumor of infancy.19-21 Although a precursor lesion may be present at birth, generally this tumor becomes apparent after the first few weeks of life as a solitary vascular plaque or nodule with a predilection for the head and neck.22-25 Once it arises, IH quickly enters a period of rapid growth, followed by a period of slower continued growth, with most reaching maximum size by 3 months.22 Thereafter, IH enters a slow period of involution (range, 3–9 years)26; more recent data suggest near resolution by 5 years of age.27 Infantile hemangioma is categorized based on its depth in the skin and subcutaneous tissues and can be classified as superficial, mixed, or deep.22,24,28,29 Superficial IH appears as a red plaque and may exhibit lobulation, while deep IH can be identified as flesh-colored or blue subcutaneous masses. Mixed IH may manifest with both superficial and deep features depending on the extent of its involvement in the dermal and subcutaneous layers. The pattern of involvement may be focal, segmental, or indeterminate.24 In contrast, CH typically is a solitary vascular mass with prominent telangiectases, nodules, and radiating veins.6 Histologically, IH is composed of proliferative plump endothelial cells that form capillaries, and the lesion stains positively for GLUT-1, whereas CH does not.30
Kaposiform hemangioendothelioma is classified as a locally aggressive vascular tumor that manifests either prenatally or in early infancy.31 It is described as a solitary, ill-defined, firm, purple plaque most commonly located on the extremities and retroperitoneum.32-34 Histopathologically, these lesions are characterized by dilated lymphatic channels and irregular sheets or lobules of spindle-shaped endothelial cells infiltrating the dermis and subcutaneous fat.33,35 In contrast to CH, KHE lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein.36,37 Notably, 70% of these tumors are complicated by the presence of Kasabach-Merritt phenomenon, a potentially life-threatening emergency that occurs when platelets are trapped within a vascular tumor, leading to the consumption of clotting factors, intralesional bleeding, and rapid enlargement of the tumor.32 The Kasabach-Merritt phenomenon manifests clinically as microangiopathic hemolytic anemia, severe thrombocytopenia, and disseminated intravascular coagulation. 38 Although CH lesions also can be associated with thrombocytopenia and coagulopathy, they generally are mild and self-limited.18
Tufted angioma is a vascular tumor that arises within the first 5 years of life as firm violaceous papules or plaques, often with associated hyperhidrosis or hypertrichosis.39,40 Although TA grows slowly for a period of time, it eventually stabilizes and persists, rarely regressing completely.41 These tumors share many similarities with KHE, and it has been suggested that they may be part of the same spectrum. 42 As with KHE, TA lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein, which are negative in CH.36,37 Although TA also can be complicated by Kasabach-Merritt phenomenon, the incidence is much lower (up to 38%).43,44 As such, TAs tend to be recognized as more superficial benign lesions. However, they still can cause notable cosmetic and functional impairment and should be monitored closely, especially in the presence of associated symptoms or complications.
Arteriovenous malformation is a vascular lesion that results from errors during the embryonic development of vascular channels.45 Although present at birth, it may not become clinically apparent until later in life. Arteriovenous malformations enlarge postnatally, and their growth is proportional to the developmental growth of the affected individual rather than the result of endothelial proliferation.46 In infants, AVM may manifest as a faint vascular stain that can evolve over time into a pink patch associated with a palpable thrill during adolescence. 4 On Doppler flow imaging, AVMs are identified as fast-flow anomalies arising from an abnormal communication between high-pressure arterial systems and low-pressure venous systems without the presence of a capillary bed.47 One of the differentiating factors between AVM and CH is that AVMs do not regress spontaneously and tend to have high recurrence rates, even with intervention. 48 In contrast, CH can be categorized based on its postnatal involution pattern. Another distinguishing factor is that AVMs tend to be larger and more invasive than CHs.46 Therefore, early diagnosis and intervention are crucial to prevent complications such as bleeding, seizures, or neurologic deficits associated with AVMs.1
The Diagnosis: Congenital Hemangioma
Surgical resection of the mass was performed at 4 months of age without complication (Figure 1). Histopathology revealed a lobular endothelial cell proliferation within a densely fibrotic stroma, multiple thin-walled vessels, and negative immunoreactivity to glucose transporter type 1 (GLUT-1)(Figures 2 and 3). Combined with the patient’s clinical history and findings on imaging (Figure 4), the most accurate diagnosis was a congenital hemangioma (CH). The mass was determined to be a noninvoluting congenital hemangioma (NICH).
A variety of vascular anomalies manifest in newborns and can be differentiated by the patient’s clinical history—particularly whether the lesion is present at birth or develops after birth. Imaging and histopathology of the lesion(s) may be utilized when clinical examination alone is not sufficient to make a diagnosis. Histopathology and immunohistochemistry further aid in differentiating the type of vascular lesion.


Overall, vascular anomalies are classified broadly into 2 categories based on their pathogenesis: tumors and malformations. Vascular tumors are composed of proliferating endothelial cells that have the potential to resolve spontaneously over time. Examples include CH, infantile hemangioma (IH), kaposiform hemangioendothelioma (KHE), and tufted angioma (TA). In contrast, vascular malformations (ie, arteriovenous malformations) are composed of dysplastic vessels with normal endothelial cell turnover and do not resolve without intervention.1
Congenital hemangiomas are rare vascular tumors that are fully developed at birth. These tumors proliferate in utero, enabling prenatal detection via ultrasonography as early as 12 weeks’ gestation for large heterogeneous vascular masses.2-4 Congenital hemangiomas are described as solitary, well-circumscribed, raised, violaceous lesions most commonly located in the head and neck region.4-6 Histopathologically, they are characterized by lobules of proliferating capillaries surrounded by fibrous stroma and dysplastic vascular channels.6,7
Congenital hemangiomas are categorized based on their postnatal involution patterns.2 Fetally involuting CH both develops and begins regression in utero and often is completely regressed at birth.8 Rapidly involuting CH begins regression in the first few weeks of life and usually is completely involuted by 14 months of age.6,9-11 Conversely, NICH does not regress, often requiring surgical excision due to functional and cosmetic issues.12,13 Partially involuting CH is intermediary, beginning as rapidly involuting but not involuting completely and persisting as lesions that resemble NICH.14-16 Although generally benign and asymptomatic, these tumors can cause transient thrombocytopenia and coagulopathy at birth, as seen in our patient.17,18


Infantile hemangioma is the most common vascular tumor of infancy.19-21 Although a precursor lesion may be present at birth, generally this tumor becomes apparent after the first few weeks of life as a solitary vascular plaque or nodule with a predilection for the head and neck.22-25 Once it arises, IH quickly enters a period of rapid growth, followed by a period of slower continued growth, with most reaching maximum size by 3 months.22 Thereafter, IH enters a slow period of involution (range, 3–9 years)26; more recent data suggest near resolution by 5 years of age.27 Infantile hemangioma is categorized based on its depth in the skin and subcutaneous tissues and can be classified as superficial, mixed, or deep.22,24,28,29 Superficial IH appears as a red plaque and may exhibit lobulation, while deep IH can be identified as flesh-colored or blue subcutaneous masses. Mixed IH may manifest with both superficial and deep features depending on the extent of its involvement in the dermal and subcutaneous layers. The pattern of involvement may be focal, segmental, or indeterminate.24 In contrast, CH typically is a solitary vascular mass with prominent telangiectases, nodules, and radiating veins.6 Histologically, IH is composed of proliferative plump endothelial cells that form capillaries, and the lesion stains positively for GLUT-1, whereas CH does not.30
Kaposiform hemangioendothelioma is classified as a locally aggressive vascular tumor that manifests either prenatally or in early infancy.31 It is described as a solitary, ill-defined, firm, purple plaque most commonly located on the extremities and retroperitoneum.32-34 Histopathologically, these lesions are characterized by dilated lymphatic channels and irregular sheets or lobules of spindle-shaped endothelial cells infiltrating the dermis and subcutaneous fat.33,35 In contrast to CH, KHE lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein.36,37 Notably, 70% of these tumors are complicated by the presence of Kasabach-Merritt phenomenon, a potentially life-threatening emergency that occurs when platelets are trapped within a vascular tumor, leading to the consumption of clotting factors, intralesional bleeding, and rapid enlargement of the tumor.32 The Kasabach-Merritt phenomenon manifests clinically as microangiopathic hemolytic anemia, severe thrombocytopenia, and disseminated intravascular coagulation. 38 Although CH lesions also can be associated with thrombocytopenia and coagulopathy, they generally are mild and self-limited.18
Tufted angioma is a vascular tumor that arises within the first 5 years of life as firm violaceous papules or plaques, often with associated hyperhidrosis or hypertrichosis.39,40 Although TA grows slowly for a period of time, it eventually stabilizes and persists, rarely regressing completely.41 These tumors share many similarities with KHE, and it has been suggested that they may be part of the same spectrum. 42 As with KHE, TA lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein, which are negative in CH.36,37 Although TA also can be complicated by Kasabach-Merritt phenomenon, the incidence is much lower (up to 38%).43,44 As such, TAs tend to be recognized as more superficial benign lesions. However, they still can cause notable cosmetic and functional impairment and should be monitored closely, especially in the presence of associated symptoms or complications.
Arteriovenous malformation is a vascular lesion that results from errors during the embryonic development of vascular channels.45 Although present at birth, it may not become clinically apparent until later in life. Arteriovenous malformations enlarge postnatally, and their growth is proportional to the developmental growth of the affected individual rather than the result of endothelial proliferation.46 In infants, AVM may manifest as a faint vascular stain that can evolve over time into a pink patch associated with a palpable thrill during adolescence. 4 On Doppler flow imaging, AVMs are identified as fast-flow anomalies arising from an abnormal communication between high-pressure arterial systems and low-pressure venous systems without the presence of a capillary bed.47 One of the differentiating factors between AVM and CH is that AVMs do not regress spontaneously and tend to have high recurrence rates, even with intervention. 48 In contrast, CH can be categorized based on its postnatal involution pattern. Another distinguishing factor is that AVMs tend to be larger and more invasive than CHs.46 Therefore, early diagnosis and intervention are crucial to prevent complications such as bleeding, seizures, or neurologic deficits associated with AVMs.1
- Enjolras O, Wassef M, Chapot R. Introduction: ISSVA Classification. In: Enjolras O, Wassef M, Chapot R, eds. Color Atlas of Vascular Tumors and Vascular Malformations. Cambridge University Press; 2007:3-11.
- Fadell MF, Jones BV, Adams DM. Prenatal diagnosis and postnatal follow-up of rapidly involuting congenital hemangioma (RICH). Pediatr Radiol. 2011;41:1057-1060.
- Feygin T, Khalek N, Moldenhauer JS. Fetal brain, head, and neck tumors: prenatal imaging and management. Prenat Diagn. 2020;40:1203-1219.
- Foley LS, Kulungowski AM. Vascular anomalies in pediatrics. Adv Pediatr. 2015;62:227-255.
- Bruder E, Alaggio R, Kozakewich HPW, et al. Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Dev Pathol. 2012;15:26-61.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Maguiness S, Uihlein LC, Liang MG, et al. Rapidly involuting congenital hemangioma with fetal involution. Pediatr Dermatol. 2015;32:321-326.
- Keating LJ, Soares GM, Muratore CS. Rapidly involuting congenital hemangioma. Med Health R I. 2012;95:149-152.
- Schafer F, Tapia M, Pinto C. Rapidly involuting congenital haemangioma. Arch Dis Child Fetal Neonatal Ed. 2014;99:F422.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Liang MG, Frieden IJ. Infantile and congenital hemangiomas. Semin Pediatr Surg. 2014;23:162-167.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:E203-E214.
- Boull C, Maguiness SM. Congenital hemangiomas. Semin Cutan Med Surg. 2016;35:124-127.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Baselga E, Cordisco MR, Garzon M, et al. Rapidly involuting congenital haemangioma associated with transient thrombocytopenia and coagulopathy: a case series. Br J Dermatol. 2008;158:1363-1370.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Hidano A, Nakajima S. Earliest features of the strawberry mark in the newborn. Br J Dermatol. 1972;87:138-144.
- Martinez-Perez D, Fein NA, Boon LM, et al. Not all hemangiomas look like strawberries: uncommon presentations of the most common tumor of infancy. Pediatr Dermatol. 1995;12:1-6.
- Payne MM, Moyer F, Marcks KM, et al. The precursor to the hemangioma. Plast Reconstr Surg. 1966;38:64-67.
- Bowers RE, Graham EA, Tomlinson KM. The natural history of the strawberry nevus. Arch Dermatol. 1960;82:667-680.
- Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624.
- Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173-181.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567-1576.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
- Gruman A, Liang MG, Mulliken JB, et al. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52:616-622.
- Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach- Merritt phenomenon in 107 referrals. J Pediatr. 2013;162:142-147.
- Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. an aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321-328.
- Mac-Moune Lai F, To KF, Choi PC, et al. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol. 2001;14:1087-1092.
- O’Rafferty C, O’Regan GM, Irvine AD, et al. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol. 2015;171:38-51.
- Le Huu AR, Jokinen CH, Rubin BP, et al. Expression of prox1, lymphatic endothelial nuclear transcription factor, in kaposiform hemangioendothelioma and tufted angioma. Am J Surg Pathol. 2010;34:1563-1573.
- Debelenko LV, Perez-Atayde AR, Mulliken JB, et al. D2-40 immuno-histochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. 2005;18:1454-1460.
- Haisley-Royster C, Enjolras O, Frieden IJ, et al. Kasabach-Merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459-462.
- Wilmer A, Kaatz M, Bocker T, et al. Tufted angioma. Eur J Dermatol. 1999;9:51-53.
- Herron MD, Coffin CM, Vanderhooft SL. Tufted angiomas: variability of the clinical morphology. Pediatr Dermatol. 2002;19:394-401.
- North PE. Pediatric vascular tumors and malformations. Surg Pathol Clin. 2010,3:455-494.
- Chu CY, Hsiao CH, Chiu HC. Transformation between kaposiform hemangioendothelioma and tufted angioma. Dermatology. 2003;206:334-337.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Johnson EF, Davis DM, Tollefson MM, et al. Vascular tumors in infants: case report and review of clinical, histopathologic, and immunohistochemical characteristics of infantile hemangioma, pyogenic granuloma, noninvoluting congenital hemangioma, tufted angioma, and kaposiform hemangioendothelioma. Am J Dermatopathol. 2018;40:231-239.
- Christison-Lagay ER, Fishman SJ. Vascular anomalies. Surg Clin North Am. 2006;86:393-425.
- Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010;125:1185-1194.
- Young AE, Mulliken JB. Arteriovenous malformations. In: Mulliken JB, Young AE, eds. Vascular Birthmarks: Haemangiomas and Malformations. WB Saunders; 1988:228-245.
- Duggan EM, Fishman SJ. Vascular anomalies. In: Holcomb GW III, Murphy JP, St Peter SD, eds. Holcomb and Ashcraft’s Pediatric Surgery. 7th edition. Elsevier; 2019:1147-1170.
- Enjolras O, Wassef M, Chapot R. Introduction: ISSVA Classification. In: Enjolras O, Wassef M, Chapot R, eds. Color Atlas of Vascular Tumors and Vascular Malformations. Cambridge University Press; 2007:3-11.
- Fadell MF, Jones BV, Adams DM. Prenatal diagnosis and postnatal follow-up of rapidly involuting congenital hemangioma (RICH). Pediatr Radiol. 2011;41:1057-1060.
- Feygin T, Khalek N, Moldenhauer JS. Fetal brain, head, and neck tumors: prenatal imaging and management. Prenat Diagn. 2020;40:1203-1219.
- Foley LS, Kulungowski AM. Vascular anomalies in pediatrics. Adv Pediatr. 2015;62:227-255.
- Bruder E, Alaggio R, Kozakewich HPW, et al. Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Dev Pathol. 2012;15:26-61.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Maguiness S, Uihlein LC, Liang MG, et al. Rapidly involuting congenital hemangioma with fetal involution. Pediatr Dermatol. 2015;32:321-326.
- Keating LJ, Soares GM, Muratore CS. Rapidly involuting congenital hemangioma. Med Health R I. 2012;95:149-152.
- Schafer F, Tapia M, Pinto C. Rapidly involuting congenital haemangioma. Arch Dis Child Fetal Neonatal Ed. 2014;99:F422.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Liang MG, Frieden IJ. Infantile and congenital hemangiomas. Semin Pediatr Surg. 2014;23:162-167.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:E203-E214.
- Boull C, Maguiness SM. Congenital hemangiomas. Semin Cutan Med Surg. 2016;35:124-127.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Baselga E, Cordisco MR, Garzon M, et al. Rapidly involuting congenital haemangioma associated with transient thrombocytopenia and coagulopathy: a case series. Br J Dermatol. 2008;158:1363-1370.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Hidano A, Nakajima S. Earliest features of the strawberry mark in the newborn. Br J Dermatol. 1972;87:138-144.
- Martinez-Perez D, Fein NA, Boon LM, et al. Not all hemangiomas look like strawberries: uncommon presentations of the most common tumor of infancy. Pediatr Dermatol. 1995;12:1-6.
- Payne MM, Moyer F, Marcks KM, et al. The precursor to the hemangioma. Plast Reconstr Surg. 1966;38:64-67.
- Bowers RE, Graham EA, Tomlinson KM. The natural history of the strawberry nevus. Arch Dermatol. 1960;82:667-680.
- Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624.
- Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173-181.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567-1576.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
- Gruman A, Liang MG, Mulliken JB, et al. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52:616-622.
- Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach- Merritt phenomenon in 107 referrals. J Pediatr. 2013;162:142-147.
- Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. an aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321-328.
- Mac-Moune Lai F, To KF, Choi PC, et al. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol. 2001;14:1087-1092.
- O’Rafferty C, O’Regan GM, Irvine AD, et al. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol. 2015;171:38-51.
- Le Huu AR, Jokinen CH, Rubin BP, et al. Expression of prox1, lymphatic endothelial nuclear transcription factor, in kaposiform hemangioendothelioma and tufted angioma. Am J Surg Pathol. 2010;34:1563-1573.
- Debelenko LV, Perez-Atayde AR, Mulliken JB, et al. D2-40 immuno-histochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. 2005;18:1454-1460.
- Haisley-Royster C, Enjolras O, Frieden IJ, et al. Kasabach-Merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459-462.
- Wilmer A, Kaatz M, Bocker T, et al. Tufted angioma. Eur J Dermatol. 1999;9:51-53.
- Herron MD, Coffin CM, Vanderhooft SL. Tufted angiomas: variability of the clinical morphology. Pediatr Dermatol. 2002;19:394-401.
- North PE. Pediatric vascular tumors and malformations. Surg Pathol Clin. 2010,3:455-494.
- Chu CY, Hsiao CH, Chiu HC. Transformation between kaposiform hemangioendothelioma and tufted angioma. Dermatology. 2003;206:334-337.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Johnson EF, Davis DM, Tollefson MM, et al. Vascular tumors in infants: case report and review of clinical, histopathologic, and immunohistochemical characteristics of infantile hemangioma, pyogenic granuloma, noninvoluting congenital hemangioma, tufted angioma, and kaposiform hemangioendothelioma. Am J Dermatopathol. 2018;40:231-239.
- Christison-Lagay ER, Fishman SJ. Vascular anomalies. Surg Clin North Am. 2006;86:393-425.
- Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010;125:1185-1194.
- Young AE, Mulliken JB. Arteriovenous malformations. In: Mulliken JB, Young AE, eds. Vascular Birthmarks: Haemangiomas and Malformations. WB Saunders; 1988:228-245.
- Duggan EM, Fishman SJ. Vascular anomalies. In: Holcomb GW III, Murphy JP, St Peter SD, eds. Holcomb and Ashcraft’s Pediatric Surgery. 7th edition. Elsevier; 2019:1147-1170.
A newborn male was delivered via cesarean section at 38 weeks 5 days’ gestation with a large vascular mass on the posterior neck. The mass previously had been identified on a 23-week prenatal ultrasound. Physical examination by dermatology at birth revealed a well-defined violaceous mass measuring 6×5 cm with prominent radiating veins, coarse telangiectases, and a pale rim. Magnetic resonance imaging demonstrated a well-circumscribed mass with avid arterial phase enhancement. The patient experienced transient thrombocytopenia that resolved following administration of methylprednisolone. No evidence of rapid involution was noted after 3 months of observation.
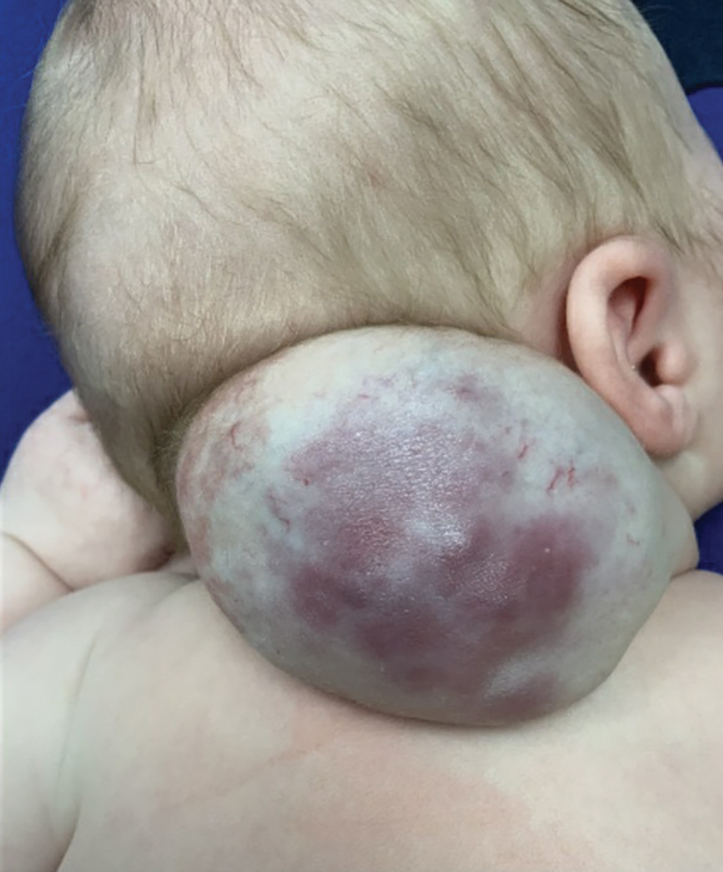
Extensive Multidrug-Resistant Dermatophytosis From Trichophyton indotineae
To the Editor:
Historically, commonly available antifungal medications have been effective for treating dermatophytosis (tinea). However, recent tinea outbreaks caused by Trichophyton indotineae—a dermatophyte often resistant to terbinafine and sometimes to other antifungals—have been reported in South Asia, Europe, the Middle East, Southeast Asia, and Australia.1-5
Three confirmed cases of T indotineae dermatophytosis in the United States were reported in 2023 in New York3,6; a fourth confirmed case was reported in 2024 in Pennsylvania.7 Post hoc laboratory testing of fungal isolates in New York in 2022 and 2023 identified an additional 11 cases.8 We present a case of extensive multidrug-resistant tinea caused by T indotineae in a man in California.
An otherwise healthy 65-year-old man who had traveled to Europe in the past 3 months presented to his primary care physician with a widespread pruritic rash (Figure 1). He was treated with 2 weeks of oral terbinafine 250 mg/d and topical medicines, including clotrimazole cream 1%, fluocinonide ointment 0.05%, and clobetasol ointment 0.05% without improvement. Subsequently, 2 weeks of oral griseofulvin microsize 500 mg/d also proved ineffective. An antibody test was negative for HIV. His hemoglobin A1c was 6.2% (reference range, ≤5.6%). The patient was referred to dermatology.
Erythematous plaques—many scaly throughout and some annular with central clearing—were present on the arms, legs, and torso as well as in the groin. Honey crust was present on some plaques on the leg. A potassium hydroxide preparation showed abundant fungal hyphae. Material for fungal and bacterial cultures was collected. The patient was treated again with oral terbinafine 250 mg/d, an oral prednisone taper starting at 60 mg/d for a presumed id reaction, and various oral antihistamines for pruritus; all were ineffective. A bacterial culture showed only mixed skin flora. Oral fluconazole 200 mg/d was prescribed. A skin biopsy specimen showed compact orthokeratosis and parakeratosis of the stratum corneum with few neutrophils and focal pustule formation (Figure 2). Superficial perivascular inflammation, including lymphocytes, histiocytes, and few neutrophils, was present. A periodic acid–Schiff stain showed fungal hyphae in the stratum corneum and a hair follicle (Figure 3). After approximately 2 weeks, mold was identified in the fungal culture. Approximately 2 weeks thereafter, the organism was reported as Trichophyton species.
The rash did not improve; resistance to terbinafine, griseofulvin, and fluconazole was suspected clinically. The fungal isolate was sent to a reference laboratory (University of Texas Health Science Center, San Antonio). Meanwhile, oral itraconazole 200 mg twice daily and ketoconazole cream 2% were prescribed; the rash began to improve. A serum itraconazole trough level obtained 4 days after treatment initiation was 0.5 μg/mL (reference range, ≥0.6 μg/mL). The evening itraconazole dose was increased to 300 mg; a subsequent trough level was 0.8 μg/mL.
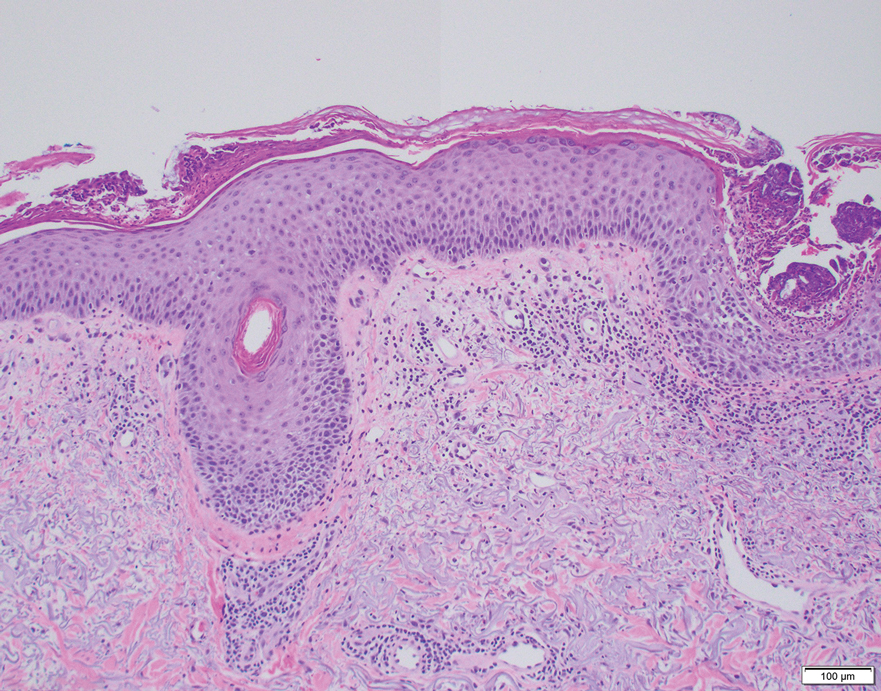
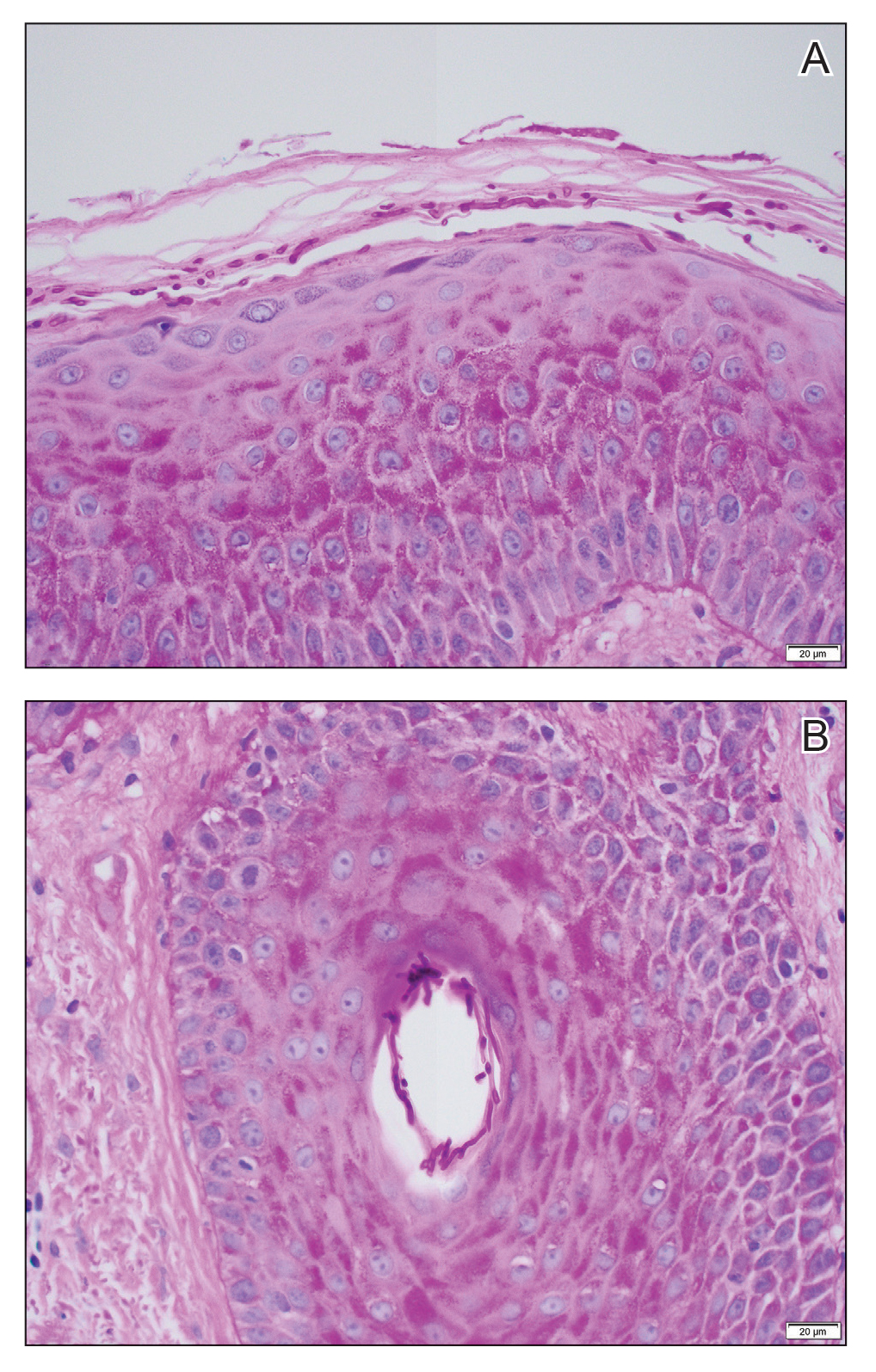
Approximately 1 month after the fungal isolate was sent to the reference laboratory, T indotineae was confirmed based on polymerase chain reaction (PCR) testing of internal transcribed spacer region sequences. Minimum inhibitory concentrations (MICs) obtained through antifungal susceptibility testing (AFST) were reported for fluconazole (8 μg/mL), griseofulvin (2 μg/mL), itraconazole (≤0.03 μg/mL), posaconazole (≤0.03 μg/mL), terbinafine (≥2 μg/mL), and voriconazole (0.125 μg/mL).
Approximately 7 weeks after itraconazole and ketoconazole were started, the rash had completely resolved. Nearly 8 months later (at the time this article was written), the rash had not recurred.
We report a unique case of T indotineae in a patient residing in California. Post hoc laboratory testing of dermatophyte isolates sent to the University of Texas reference laboratory identified terbinafine-resistant T indotineae specimens from the United States and Canada dating to 2017; clinical characteristics of patients from whom those isolates were obtained were unavailable.9
Trichophyton indotineae dermatophytosis typically is more extensive, inflamed, and pruritic, as well as likely more contagious, than tinea caused by other dermatophytes.5 Previously called Trichophyton mentagrophytes genotype VIII when first isolated in 2017, the pathogen was renamed T indotineae in 2020 after important genetic differences were discovered between it and other T mentagrophytes species.5 The emergence of T indotineae has been attributed to concomitant use of topical steroids and antifungals,5,10 inappropriate prescribing of antifungals,5 and nonadherence to antifungal treatment.5
Likely risk factors for T indotineae infection include suboptimal hygiene, overcrowded conditions, hot and humid environments, and tight-fitting synthetic clothing.4 Transmission from family members appears common,5 especially when fomites are shared.4 A case reported in Pennsylvania likely was acquired through sexual contact.7 Travel to South Asia has been associated with acquisition of T indotineae infection,3,5-7 though our patient and some others had not traveled there.3,8 It is not clear whether immunosuppression and diabetes mellitus are associated with T indotineae infection.4,5,8Trichophyton indotineae also can affect animals,11 though zoonotic transmission has not been reported.4
Not all T indotineae isolates are resistant to one or more antifungals; furthermore, antifungal resistance in other dermatophyte species has been reported.5 Terbinafine resistance in T indotineae is conferred by mutations in the gene encoding squalene epoxidase, which helps synthesize ergosterol—a component of the cell membrane in fungi.2,4,5,12 Although clinical cut-points for MIC obtained by AFST are not well established, T indotineae MICs for terbinafine of 0.5 μg/mL or more correlate with resistance.9 Resistance to azoles has been linked to overexpression of transporter genes, which increase azole efflux from cells, as well as to mutations in the gene encoding lanosterol 14α demethylase.4,12,13
Potassium hydroxide preparations and fungal cultures cannot differentiate T indotineae from other dermatophytes that typically cause tinea.5,14 Histopathologic findings in our case were no different than those of non–T indotineae dermatophytes. Only molecular testing using PCR assays to sequence internal transcribed spacer genes can confirm T indotineae infection. However, PCR assays and AFST are not available in many US laboratories.5 Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry has shown promise in distinguishing T indotineae from other dermatophytes, though its clinical use is limited and it cannot assess terbinafine sensitivity.15,16 Clinicians in the United States who want to test specimens from cases suspicious for T indotineae infection should contact their local or state health department or the Centers for Disease Control and Prevention for assistance.3,5
Systemic treatment typically is necessary for T indotineae infection.5 Combinations of oral and topical azoles have been used, as well as topical ciclopirox, amorolfine (not available in the United States), and luliconazole.1,5,17-21
Itraconazole has emerged as the treatment of choice for T indotineae tinea, typically at 200 mg/d and often for courses of more than 3 months.5 Testing for serum itraconazole trough levels, as done for our patient, typically is not recommended. Clinicians should counsel patients to take itraconazole with high-fat foods and an acidic beverage to increase bioavailability.5 Potential adverse effects of itraconazole include heart failure and numerous drug-drug interactions.5,22 Patients with T indotineae dermatophytosis should avoid sharing personal belongings and having skin-to-skin contact of affected areas with others.4
Dermatologists who suspect T indotineae infection should work with public health agencies that can assist with testing and undertake infection surveillance, prevention, and control.5,23 Challenges to diagnosing and managing T indotineae infection include lack of awareness among dermatology providers, the need for specialized laboratory testing to confirm infection, lack of established clinical cut-points for MICs from AFST, the need for longer duration of treatment vs what is needed for typical tinea, and potential challenges with insurance coverage for testing and treatment. Empiric treatment with itraconazole should be considered when terbinafine-resistant dermatophytosis is suspected or when terbinafine-resistant T indotineae infection is confirmed.
Acknowledgments—Jeremy Gold, MD; Dallas J. Smith, PharmD; and Shawn Lockhart, PhD, all of the Centers for Disease Control and Prevention, Mycotic Diseases Branch (Atlanta, Georgia), provided helpful comments to the authors in preparing the manuscript of this article.
- Uhrlaß S, Verma SB, Gräser Y, al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Jabet A, Brun S, Normand A-C, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233. doi:10.3201/eid2801.210883
- Caplan AS, Chaturvedi S, Zhu Y, et al. Notes from the field. First reported U.S. cases of tinea caused by Trichophyton indotineae—New York City, December 2021-March 2023. MMWR Morb Mortal Wkly Rep. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Jabet A, Normand A-C, Brun S, et al. Trichophyton indotineae, from epidemiology to therapeutic. J Mycol Med. 2023;33:101383. doi:10.1016/j.mycmed.2023.101383
- Hill RC, Caplan AS, Elewski B, et al. Expert panel review of skin and hair dermatophytoses in an era of antifungal resistance. Am J Clin Dermatol. 2024;25:359-389. doi:10.1007/s40257-024-00848-1
- Caplan AS, Zakhem GA, Pomeranz MK. Trichophyton mentagrophytes internal transcribed spacer genotype VIII. JAMA Dermatol. 2023;159:1130. doi:10.1001/jamadermatol.2023.2645
- Spivack S, Gold JAW, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807-809. doi:10.3201/eid3004.240115
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1126
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:e0056223. doi:10.1128/jcm.00562-23
- Gupta AK, Venkataraman M, Hall DC, et al. The emergence of Trichophyton indotineae: implications for clinical practice. Int J Dermatol. 2023;62:857-861.
- Oladzad V, Nasrollahi Omran A, Haghani I, et al. Multi-drug resistance Trichophyton indotineae in a stray dog. Res Vet Sci. 2024;166:105105. doi:10.1016/j.rvsc.2023.105105
- Martinez-Rossi NM, Bitencourt TA, Peres NTA, et al. Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front Microbiol. 2018;9:1108. doi:10.3389/fmicb.2018.01108
- Sacheli R, Hayette MP. Antifungal resistance in dermatophytes: genetic considerations, clinical presentations and alternative therapies. J Fungi (Basel). 2021;711:983. doi:10.3390/jof7110983
- Gupta AK, Cooper EA. Dermatophytosis (tinea) and other superficial fungal infections. In: Hospenthal DR, Rinaldi MG, eds. Diagnosis and Treatment of Human Mycoses. Humana Press; 2008:355-381.
- Normand A-C, Moreno-Sabater A, Jabet A, et al. MALDI-TOF mass spectrometry online identification of Trichophyton indotineae using the MSI-2 application. J Fungi (Basel). 2022;8:1103. doi:10.3390/jof8101103
- De Paepe R, Normand A-C, Uhrlaß S, et al. Resistance profile, terbinafine resistance screening and MALDI-TOF MS identification of the emerging pathogen Trichophyton indotineae. Mycopathologia. 2024;189:29. doi:10.1007/s11046-024-00835-4
- Rajagopalan M, Inamadar A, Mittal A, et al. Expert consensus on the management of dermatophytosis in India (ECTODERM India). BMC Dermatol. 2018;18:6. doi:10.1186/s12895-018-0073-1
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: III. Antifungal resistance and treatment options. Indian J Dermatol Venereol Leprol. 2021;87:468-482. doi:10.25259/IJDVL_303_20
- Shaw D, Singh S, Dogra S, et al. MIC and upper limit of wild-type distribution for 13 antifungal agents against a Trichophyton mentagrophytes–Trichophyton interdigitale complex of Indian origin. Antimicrob Agents Chemother. 2020;64:E01964-19. doi:10.1128/AAC.01964-19
- Burmester A, Hipler U-C, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180. doi:10.1111/myc.13150
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278. doi:10.1001/jamadermatol.2022.3745
- Itraconazole capsule. DailyMed [Internet]. Updated June 3, 2024. Accessed June 19, 2024. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2ab38a8a-3708-4b97-9f7f-8e554a15348d
- Bui TS, Katz KA. Resistant Trichophyton indotineae dermatophytosis—an emerging pandemic, now in the US. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1125
To the Editor:
Historically, commonly available antifungal medications have been effective for treating dermatophytosis (tinea). However, recent tinea outbreaks caused by Trichophyton indotineae—a dermatophyte often resistant to terbinafine and sometimes to other antifungals—have been reported in South Asia, Europe, the Middle East, Southeast Asia, and Australia.1-5
Three confirmed cases of T indotineae dermatophytosis in the United States were reported in 2023 in New York3,6; a fourth confirmed case was reported in 2024 in Pennsylvania.7 Post hoc laboratory testing of fungal isolates in New York in 2022 and 2023 identified an additional 11 cases.8 We present a case of extensive multidrug-resistant tinea caused by T indotineae in a man in California.
An otherwise healthy 65-year-old man who had traveled to Europe in the past 3 months presented to his primary care physician with a widespread pruritic rash (Figure 1). He was treated with 2 weeks of oral terbinafine 250 mg/d and topical medicines, including clotrimazole cream 1%, fluocinonide ointment 0.05%, and clobetasol ointment 0.05% without improvement. Subsequently, 2 weeks of oral griseofulvin microsize 500 mg/d also proved ineffective. An antibody test was negative for HIV. His hemoglobin A1c was 6.2% (reference range, ≤5.6%). The patient was referred to dermatology.
Erythematous plaques—many scaly throughout and some annular with central clearing—were present on the arms, legs, and torso as well as in the groin. Honey crust was present on some plaques on the leg. A potassium hydroxide preparation showed abundant fungal hyphae. Material for fungal and bacterial cultures was collected. The patient was treated again with oral terbinafine 250 mg/d, an oral prednisone taper starting at 60 mg/d for a presumed id reaction, and various oral antihistamines for pruritus; all were ineffective. A bacterial culture showed only mixed skin flora. Oral fluconazole 200 mg/d was prescribed. A skin biopsy specimen showed compact orthokeratosis and parakeratosis of the stratum corneum with few neutrophils and focal pustule formation (Figure 2). Superficial perivascular inflammation, including lymphocytes, histiocytes, and few neutrophils, was present. A periodic acid–Schiff stain showed fungal hyphae in the stratum corneum and a hair follicle (Figure 3). After approximately 2 weeks, mold was identified in the fungal culture. Approximately 2 weeks thereafter, the organism was reported as Trichophyton species.

The rash did not improve; resistance to terbinafine, griseofulvin, and fluconazole was suspected clinically. The fungal isolate was sent to a reference laboratory (University of Texas Health Science Center, San Antonio). Meanwhile, oral itraconazole 200 mg twice daily and ketoconazole cream 2% were prescribed; the rash began to improve. A serum itraconazole trough level obtained 4 days after treatment initiation was 0.5 μg/mL (reference range, ≥0.6 μg/mL). The evening itraconazole dose was increased to 300 mg; a subsequent trough level was 0.8 μg/mL.


Approximately 1 month after the fungal isolate was sent to the reference laboratory, T indotineae was confirmed based on polymerase chain reaction (PCR) testing of internal transcribed spacer region sequences. Minimum inhibitory concentrations (MICs) obtained through antifungal susceptibility testing (AFST) were reported for fluconazole (8 μg/mL), griseofulvin (2 μg/mL), itraconazole (≤0.03 μg/mL), posaconazole (≤0.03 μg/mL), terbinafine (≥2 μg/mL), and voriconazole (0.125 μg/mL).
Approximately 7 weeks after itraconazole and ketoconazole were started, the rash had completely resolved. Nearly 8 months later (at the time this article was written), the rash had not recurred.
We report a unique case of T indotineae in a patient residing in California. Post hoc laboratory testing of dermatophyte isolates sent to the University of Texas reference laboratory identified terbinafine-resistant T indotineae specimens from the United States and Canada dating to 2017; clinical characteristics of patients from whom those isolates were obtained were unavailable.9
Trichophyton indotineae dermatophytosis typically is more extensive, inflamed, and pruritic, as well as likely more contagious, than tinea caused by other dermatophytes.5 Previously called Trichophyton mentagrophytes genotype VIII when first isolated in 2017, the pathogen was renamed T indotineae in 2020 after important genetic differences were discovered between it and other T mentagrophytes species.5 The emergence of T indotineae has been attributed to concomitant use of topical steroids and antifungals,5,10 inappropriate prescribing of antifungals,5 and nonadherence to antifungal treatment.5
Likely risk factors for T indotineae infection include suboptimal hygiene, overcrowded conditions, hot and humid environments, and tight-fitting synthetic clothing.4 Transmission from family members appears common,5 especially when fomites are shared.4 A case reported in Pennsylvania likely was acquired through sexual contact.7 Travel to South Asia has been associated with acquisition of T indotineae infection,3,5-7 though our patient and some others had not traveled there.3,8 It is not clear whether immunosuppression and diabetes mellitus are associated with T indotineae infection.4,5,8Trichophyton indotineae also can affect animals,11 though zoonotic transmission has not been reported.4
Not all T indotineae isolates are resistant to one or more antifungals; furthermore, antifungal resistance in other dermatophyte species has been reported.5 Terbinafine resistance in T indotineae is conferred by mutations in the gene encoding squalene epoxidase, which helps synthesize ergosterol—a component of the cell membrane in fungi.2,4,5,12 Although clinical cut-points for MIC obtained by AFST are not well established, T indotineae MICs for terbinafine of 0.5 μg/mL or more correlate with resistance.9 Resistance to azoles has been linked to overexpression of transporter genes, which increase azole efflux from cells, as well as to mutations in the gene encoding lanosterol 14α demethylase.4,12,13
Potassium hydroxide preparations and fungal cultures cannot differentiate T indotineae from other dermatophytes that typically cause tinea.5,14 Histopathologic findings in our case were no different than those of non–T indotineae dermatophytes. Only molecular testing using PCR assays to sequence internal transcribed spacer genes can confirm T indotineae infection. However, PCR assays and AFST are not available in many US laboratories.5 Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry has shown promise in distinguishing T indotineae from other dermatophytes, though its clinical use is limited and it cannot assess terbinafine sensitivity.15,16 Clinicians in the United States who want to test specimens from cases suspicious for T indotineae infection should contact their local or state health department or the Centers for Disease Control and Prevention for assistance.3,5
Systemic treatment typically is necessary for T indotineae infection.5 Combinations of oral and topical azoles have been used, as well as topical ciclopirox, amorolfine (not available in the United States), and luliconazole.1,5,17-21
Itraconazole has emerged as the treatment of choice for T indotineae tinea, typically at 200 mg/d and often for courses of more than 3 months.5 Testing for serum itraconazole trough levels, as done for our patient, typically is not recommended. Clinicians should counsel patients to take itraconazole with high-fat foods and an acidic beverage to increase bioavailability.5 Potential adverse effects of itraconazole include heart failure and numerous drug-drug interactions.5,22 Patients with T indotineae dermatophytosis should avoid sharing personal belongings and having skin-to-skin contact of affected areas with others.4
Dermatologists who suspect T indotineae infection should work with public health agencies that can assist with testing and undertake infection surveillance, prevention, and control.5,23 Challenges to diagnosing and managing T indotineae infection include lack of awareness among dermatology providers, the need for specialized laboratory testing to confirm infection, lack of established clinical cut-points for MICs from AFST, the need for longer duration of treatment vs what is needed for typical tinea, and potential challenges with insurance coverage for testing and treatment. Empiric treatment with itraconazole should be considered when terbinafine-resistant dermatophytosis is suspected or when terbinafine-resistant T indotineae infection is confirmed.
Acknowledgments—Jeremy Gold, MD; Dallas J. Smith, PharmD; and Shawn Lockhart, PhD, all of the Centers for Disease Control and Prevention, Mycotic Diseases Branch (Atlanta, Georgia), provided helpful comments to the authors in preparing the manuscript of this article.
To the Editor:
Historically, commonly available antifungal medications have been effective for treating dermatophytosis (tinea). However, recent tinea outbreaks caused by Trichophyton indotineae—a dermatophyte often resistant to terbinafine and sometimes to other antifungals—have been reported in South Asia, Europe, the Middle East, Southeast Asia, and Australia.1-5
Three confirmed cases of T indotineae dermatophytosis in the United States were reported in 2023 in New York3,6; a fourth confirmed case was reported in 2024 in Pennsylvania.7 Post hoc laboratory testing of fungal isolates in New York in 2022 and 2023 identified an additional 11 cases.8 We present a case of extensive multidrug-resistant tinea caused by T indotineae in a man in California.
An otherwise healthy 65-year-old man who had traveled to Europe in the past 3 months presented to his primary care physician with a widespread pruritic rash (Figure 1). He was treated with 2 weeks of oral terbinafine 250 mg/d and topical medicines, including clotrimazole cream 1%, fluocinonide ointment 0.05%, and clobetasol ointment 0.05% without improvement. Subsequently, 2 weeks of oral griseofulvin microsize 500 mg/d also proved ineffective. An antibody test was negative for HIV. His hemoglobin A1c was 6.2% (reference range, ≤5.6%). The patient was referred to dermatology.
Erythematous plaques—many scaly throughout and some annular with central clearing—were present on the arms, legs, and torso as well as in the groin. Honey crust was present on some plaques on the leg. A potassium hydroxide preparation showed abundant fungal hyphae. Material for fungal and bacterial cultures was collected. The patient was treated again with oral terbinafine 250 mg/d, an oral prednisone taper starting at 60 mg/d for a presumed id reaction, and various oral antihistamines for pruritus; all were ineffective. A bacterial culture showed only mixed skin flora. Oral fluconazole 200 mg/d was prescribed. A skin biopsy specimen showed compact orthokeratosis and parakeratosis of the stratum corneum with few neutrophils and focal pustule formation (Figure 2). Superficial perivascular inflammation, including lymphocytes, histiocytes, and few neutrophils, was present. A periodic acid–Schiff stain showed fungal hyphae in the stratum corneum and a hair follicle (Figure 3). After approximately 2 weeks, mold was identified in the fungal culture. Approximately 2 weeks thereafter, the organism was reported as Trichophyton species.

The rash did not improve; resistance to terbinafine, griseofulvin, and fluconazole was suspected clinically. The fungal isolate was sent to a reference laboratory (University of Texas Health Science Center, San Antonio). Meanwhile, oral itraconazole 200 mg twice daily and ketoconazole cream 2% were prescribed; the rash began to improve. A serum itraconazole trough level obtained 4 days after treatment initiation was 0.5 μg/mL (reference range, ≥0.6 μg/mL). The evening itraconazole dose was increased to 300 mg; a subsequent trough level was 0.8 μg/mL.


Approximately 1 month after the fungal isolate was sent to the reference laboratory, T indotineae was confirmed based on polymerase chain reaction (PCR) testing of internal transcribed spacer region sequences. Minimum inhibitory concentrations (MICs) obtained through antifungal susceptibility testing (AFST) were reported for fluconazole (8 μg/mL), griseofulvin (2 μg/mL), itraconazole (≤0.03 μg/mL), posaconazole (≤0.03 μg/mL), terbinafine (≥2 μg/mL), and voriconazole (0.125 μg/mL).
Approximately 7 weeks after itraconazole and ketoconazole were started, the rash had completely resolved. Nearly 8 months later (at the time this article was written), the rash had not recurred.
We report a unique case of T indotineae in a patient residing in California. Post hoc laboratory testing of dermatophyte isolates sent to the University of Texas reference laboratory identified terbinafine-resistant T indotineae specimens from the United States and Canada dating to 2017; clinical characteristics of patients from whom those isolates were obtained were unavailable.9
Trichophyton indotineae dermatophytosis typically is more extensive, inflamed, and pruritic, as well as likely more contagious, than tinea caused by other dermatophytes.5 Previously called Trichophyton mentagrophytes genotype VIII when first isolated in 2017, the pathogen was renamed T indotineae in 2020 after important genetic differences were discovered between it and other T mentagrophytes species.5 The emergence of T indotineae has been attributed to concomitant use of topical steroids and antifungals,5,10 inappropriate prescribing of antifungals,5 and nonadherence to antifungal treatment.5
Likely risk factors for T indotineae infection include suboptimal hygiene, overcrowded conditions, hot and humid environments, and tight-fitting synthetic clothing.4 Transmission from family members appears common,5 especially when fomites are shared.4 A case reported in Pennsylvania likely was acquired through sexual contact.7 Travel to South Asia has been associated with acquisition of T indotineae infection,3,5-7 though our patient and some others had not traveled there.3,8 It is not clear whether immunosuppression and diabetes mellitus are associated with T indotineae infection.4,5,8Trichophyton indotineae also can affect animals,11 though zoonotic transmission has not been reported.4
Not all T indotineae isolates are resistant to one or more antifungals; furthermore, antifungal resistance in other dermatophyte species has been reported.5 Terbinafine resistance in T indotineae is conferred by mutations in the gene encoding squalene epoxidase, which helps synthesize ergosterol—a component of the cell membrane in fungi.2,4,5,12 Although clinical cut-points for MIC obtained by AFST are not well established, T indotineae MICs for terbinafine of 0.5 μg/mL or more correlate with resistance.9 Resistance to azoles has been linked to overexpression of transporter genes, which increase azole efflux from cells, as well as to mutations in the gene encoding lanosterol 14α demethylase.4,12,13
Potassium hydroxide preparations and fungal cultures cannot differentiate T indotineae from other dermatophytes that typically cause tinea.5,14 Histopathologic findings in our case were no different than those of non–T indotineae dermatophytes. Only molecular testing using PCR assays to sequence internal transcribed spacer genes can confirm T indotineae infection. However, PCR assays and AFST are not available in many US laboratories.5 Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry has shown promise in distinguishing T indotineae from other dermatophytes, though its clinical use is limited and it cannot assess terbinafine sensitivity.15,16 Clinicians in the United States who want to test specimens from cases suspicious for T indotineae infection should contact their local or state health department or the Centers for Disease Control and Prevention for assistance.3,5
Systemic treatment typically is necessary for T indotineae infection.5 Combinations of oral and topical azoles have been used, as well as topical ciclopirox, amorolfine (not available in the United States), and luliconazole.1,5,17-21
Itraconazole has emerged as the treatment of choice for T indotineae tinea, typically at 200 mg/d and often for courses of more than 3 months.5 Testing for serum itraconazole trough levels, as done for our patient, typically is not recommended. Clinicians should counsel patients to take itraconazole with high-fat foods and an acidic beverage to increase bioavailability.5 Potential adverse effects of itraconazole include heart failure and numerous drug-drug interactions.5,22 Patients with T indotineae dermatophytosis should avoid sharing personal belongings and having skin-to-skin contact of affected areas with others.4
Dermatologists who suspect T indotineae infection should work with public health agencies that can assist with testing and undertake infection surveillance, prevention, and control.5,23 Challenges to diagnosing and managing T indotineae infection include lack of awareness among dermatology providers, the need for specialized laboratory testing to confirm infection, lack of established clinical cut-points for MICs from AFST, the need for longer duration of treatment vs what is needed for typical tinea, and potential challenges with insurance coverage for testing and treatment. Empiric treatment with itraconazole should be considered when terbinafine-resistant dermatophytosis is suspected or when terbinafine-resistant T indotineae infection is confirmed.
Acknowledgments—Jeremy Gold, MD; Dallas J. Smith, PharmD; and Shawn Lockhart, PhD, all of the Centers for Disease Control and Prevention, Mycotic Diseases Branch (Atlanta, Georgia), provided helpful comments to the authors in preparing the manuscript of this article.
- Uhrlaß S, Verma SB, Gräser Y, al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Jabet A, Brun S, Normand A-C, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233. doi:10.3201/eid2801.210883
- Caplan AS, Chaturvedi S, Zhu Y, et al. Notes from the field. First reported U.S. cases of tinea caused by Trichophyton indotineae—New York City, December 2021-March 2023. MMWR Morb Mortal Wkly Rep. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Jabet A, Normand A-C, Brun S, et al. Trichophyton indotineae, from epidemiology to therapeutic. J Mycol Med. 2023;33:101383. doi:10.1016/j.mycmed.2023.101383
- Hill RC, Caplan AS, Elewski B, et al. Expert panel review of skin and hair dermatophytoses in an era of antifungal resistance. Am J Clin Dermatol. 2024;25:359-389. doi:10.1007/s40257-024-00848-1
- Caplan AS, Zakhem GA, Pomeranz MK. Trichophyton mentagrophytes internal transcribed spacer genotype VIII. JAMA Dermatol. 2023;159:1130. doi:10.1001/jamadermatol.2023.2645
- Spivack S, Gold JAW, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807-809. doi:10.3201/eid3004.240115
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1126
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:e0056223. doi:10.1128/jcm.00562-23
- Gupta AK, Venkataraman M, Hall DC, et al. The emergence of Trichophyton indotineae: implications for clinical practice. Int J Dermatol. 2023;62:857-861.
- Oladzad V, Nasrollahi Omran A, Haghani I, et al. Multi-drug resistance Trichophyton indotineae in a stray dog. Res Vet Sci. 2024;166:105105. doi:10.1016/j.rvsc.2023.105105
- Martinez-Rossi NM, Bitencourt TA, Peres NTA, et al. Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front Microbiol. 2018;9:1108. doi:10.3389/fmicb.2018.01108
- Sacheli R, Hayette MP. Antifungal resistance in dermatophytes: genetic considerations, clinical presentations and alternative therapies. J Fungi (Basel). 2021;711:983. doi:10.3390/jof7110983
- Gupta AK, Cooper EA. Dermatophytosis (tinea) and other superficial fungal infections. In: Hospenthal DR, Rinaldi MG, eds. Diagnosis and Treatment of Human Mycoses. Humana Press; 2008:355-381.
- Normand A-C, Moreno-Sabater A, Jabet A, et al. MALDI-TOF mass spectrometry online identification of Trichophyton indotineae using the MSI-2 application. J Fungi (Basel). 2022;8:1103. doi:10.3390/jof8101103
- De Paepe R, Normand A-C, Uhrlaß S, et al. Resistance profile, terbinafine resistance screening and MALDI-TOF MS identification of the emerging pathogen Trichophyton indotineae. Mycopathologia. 2024;189:29. doi:10.1007/s11046-024-00835-4
- Rajagopalan M, Inamadar A, Mittal A, et al. Expert consensus on the management of dermatophytosis in India (ECTODERM India). BMC Dermatol. 2018;18:6. doi:10.1186/s12895-018-0073-1
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: III. Antifungal resistance and treatment options. Indian J Dermatol Venereol Leprol. 2021;87:468-482. doi:10.25259/IJDVL_303_20
- Shaw D, Singh S, Dogra S, et al. MIC and upper limit of wild-type distribution for 13 antifungal agents against a Trichophyton mentagrophytes–Trichophyton interdigitale complex of Indian origin. Antimicrob Agents Chemother. 2020;64:E01964-19. doi:10.1128/AAC.01964-19
- Burmester A, Hipler U-C, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180. doi:10.1111/myc.13150
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278. doi:10.1001/jamadermatol.2022.3745
- Itraconazole capsule. DailyMed [Internet]. Updated June 3, 2024. Accessed June 19, 2024. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2ab38a8a-3708-4b97-9f7f-8e554a15348d
- Bui TS, Katz KA. Resistant Trichophyton indotineae dermatophytosis—an emerging pandemic, now in the US. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1125
- Uhrlaß S, Verma SB, Gräser Y, al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Jabet A, Brun S, Normand A-C, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233. doi:10.3201/eid2801.210883
- Caplan AS, Chaturvedi S, Zhu Y, et al. Notes from the field. First reported U.S. cases of tinea caused by Trichophyton indotineae—New York City, December 2021-March 2023. MMWR Morb Mortal Wkly Rep. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Jabet A, Normand A-C, Brun S, et al. Trichophyton indotineae, from epidemiology to therapeutic. J Mycol Med. 2023;33:101383. doi:10.1016/j.mycmed.2023.101383
- Hill RC, Caplan AS, Elewski B, et al. Expert panel review of skin and hair dermatophytoses in an era of antifungal resistance. Am J Clin Dermatol. 2024;25:359-389. doi:10.1007/s40257-024-00848-1
- Caplan AS, Zakhem GA, Pomeranz MK. Trichophyton mentagrophytes internal transcribed spacer genotype VIII. JAMA Dermatol. 2023;159:1130. doi:10.1001/jamadermatol.2023.2645
- Spivack S, Gold JAW, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807-809. doi:10.3201/eid3004.240115
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1126
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:e0056223. doi:10.1128/jcm.00562-23
- Gupta AK, Venkataraman M, Hall DC, et al. The emergence of Trichophyton indotineae: implications for clinical practice. Int J Dermatol. 2023;62:857-861.
- Oladzad V, Nasrollahi Omran A, Haghani I, et al. Multi-drug resistance Trichophyton indotineae in a stray dog. Res Vet Sci. 2024;166:105105. doi:10.1016/j.rvsc.2023.105105
- Martinez-Rossi NM, Bitencourt TA, Peres NTA, et al. Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front Microbiol. 2018;9:1108. doi:10.3389/fmicb.2018.01108
- Sacheli R, Hayette MP. Antifungal resistance in dermatophytes: genetic considerations, clinical presentations and alternative therapies. J Fungi (Basel). 2021;711:983. doi:10.3390/jof7110983
- Gupta AK, Cooper EA. Dermatophytosis (tinea) and other superficial fungal infections. In: Hospenthal DR, Rinaldi MG, eds. Diagnosis and Treatment of Human Mycoses. Humana Press; 2008:355-381.
- Normand A-C, Moreno-Sabater A, Jabet A, et al. MALDI-TOF mass spectrometry online identification of Trichophyton indotineae using the MSI-2 application. J Fungi (Basel). 2022;8:1103. doi:10.3390/jof8101103
- De Paepe R, Normand A-C, Uhrlaß S, et al. Resistance profile, terbinafine resistance screening and MALDI-TOF MS identification of the emerging pathogen Trichophyton indotineae. Mycopathologia. 2024;189:29. doi:10.1007/s11046-024-00835-4
- Rajagopalan M, Inamadar A, Mittal A, et al. Expert consensus on the management of dermatophytosis in India (ECTODERM India). BMC Dermatol. 2018;18:6. doi:10.1186/s12895-018-0073-1
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: III. Antifungal resistance and treatment options. Indian J Dermatol Venereol Leprol. 2021;87:468-482. doi:10.25259/IJDVL_303_20
- Shaw D, Singh S, Dogra S, et al. MIC and upper limit of wild-type distribution for 13 antifungal agents against a Trichophyton mentagrophytes–Trichophyton interdigitale complex of Indian origin. Antimicrob Agents Chemother. 2020;64:E01964-19. doi:10.1128/AAC.01964-19
- Burmester A, Hipler U-C, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180. doi:10.1111/myc.13150
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278. doi:10.1001/jamadermatol.2022.3745
- Itraconazole capsule. DailyMed [Internet]. Updated June 3, 2024. Accessed June 19, 2024. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2ab38a8a-3708-4b97-9f7f-8e554a15348d
- Bui TS, Katz KA. Resistant Trichophyton indotineae dermatophytosis—an emerging pandemic, now in the US. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1125
Practice Points
- Trichophyton indotineae can cause extensive dermatophytosis that often is resistant to terbinafine and in some cases to other antifungals.
- Only molecular testing, which is not widely available, can distinguish T indotineae from other dermatophytes.
- Suspected or confirmed cases of T indotineae dermatophytosis should be reported to public health agencies to provide assistance with testing, as well as surveillance, prevention, and control of infection.