User login
In Case You Missed It: COVID
Study finds COVID-19 boosters don’t increase miscarriage risk
COVID-19 boosters are not linked to an increased chance of miscarriage, according to a new study in JAMA Network Open.
Researchers were seeking to learn whether a booster in early pregnancy, before 20 weeks, was associated with greater likelihood of spontaneous abortion.
They examined more than 100,000 pregnancies at 6-19 weeks from eight health systems in the Vaccine Safety Datalink (VSD). They found that receiving a COVID-19 booster shot in a 28-day or 42-day exposure window did not increase the chances of miscarriage.
The VSD is a collaboration between the Centers for Disease Control and Prevention’s Immunization Safety Office and large health care systems. The “observational, case-control, surveillance study” was conducted from Nov. 1, 2021, to June 12, 2022.
“COVID infection during pregnancy increases risk of poor outcomes, yet many people who are pregnant or thinking about getting pregnant are hesitant to get a booster dose because of questions about safety,” said Elyse Kharbanda, MD, senior investigator at HealthPartners Institute and lead author of the study in a press release.
The University of Minnesota reported that “previous studies have shown COIVD-19 primary vaccination is safe in pregnancy and not associated with an increased risk for miscarriage. Several studies have also shown COVID-19 can be more severe in pregnancy and lead to worse outcomes for the mother.”
The study was funded by the CDC. Five study authors reported conflicts of interest with Pfizer, Merck, GlaxoSmithKline, AbbVie, and Sanofi Pasteur.
A version of this article first appeared on Medscape.com.
COVID-19 boosters are not linked to an increased chance of miscarriage, according to a new study in JAMA Network Open.
Researchers were seeking to learn whether a booster in early pregnancy, before 20 weeks, was associated with greater likelihood of spontaneous abortion.
They examined more than 100,000 pregnancies at 6-19 weeks from eight health systems in the Vaccine Safety Datalink (VSD). They found that receiving a COVID-19 booster shot in a 28-day or 42-day exposure window did not increase the chances of miscarriage.
The VSD is a collaboration between the Centers for Disease Control and Prevention’s Immunization Safety Office and large health care systems. The “observational, case-control, surveillance study” was conducted from Nov. 1, 2021, to June 12, 2022.
“COVID infection during pregnancy increases risk of poor outcomes, yet many people who are pregnant or thinking about getting pregnant are hesitant to get a booster dose because of questions about safety,” said Elyse Kharbanda, MD, senior investigator at HealthPartners Institute and lead author of the study in a press release.
The University of Minnesota reported that “previous studies have shown COIVD-19 primary vaccination is safe in pregnancy and not associated with an increased risk for miscarriage. Several studies have also shown COVID-19 can be more severe in pregnancy and lead to worse outcomes for the mother.”
The study was funded by the CDC. Five study authors reported conflicts of interest with Pfizer, Merck, GlaxoSmithKline, AbbVie, and Sanofi Pasteur.
A version of this article first appeared on Medscape.com.
COVID-19 boosters are not linked to an increased chance of miscarriage, according to a new study in JAMA Network Open.
Researchers were seeking to learn whether a booster in early pregnancy, before 20 weeks, was associated with greater likelihood of spontaneous abortion.
They examined more than 100,000 pregnancies at 6-19 weeks from eight health systems in the Vaccine Safety Datalink (VSD). They found that receiving a COVID-19 booster shot in a 28-day or 42-day exposure window did not increase the chances of miscarriage.
The VSD is a collaboration between the Centers for Disease Control and Prevention’s Immunization Safety Office and large health care systems. The “observational, case-control, surveillance study” was conducted from Nov. 1, 2021, to June 12, 2022.
“COVID infection during pregnancy increases risk of poor outcomes, yet many people who are pregnant or thinking about getting pregnant are hesitant to get a booster dose because of questions about safety,” said Elyse Kharbanda, MD, senior investigator at HealthPartners Institute and lead author of the study in a press release.
The University of Minnesota reported that “previous studies have shown COIVD-19 primary vaccination is safe in pregnancy and not associated with an increased risk for miscarriage. Several studies have also shown COVID-19 can be more severe in pregnancy and lead to worse outcomes for the mother.”
The study was funded by the CDC. Five study authors reported conflicts of interest with Pfizer, Merck, GlaxoSmithKline, AbbVie, and Sanofi Pasteur.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
COVID boosters effective, but not for long
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
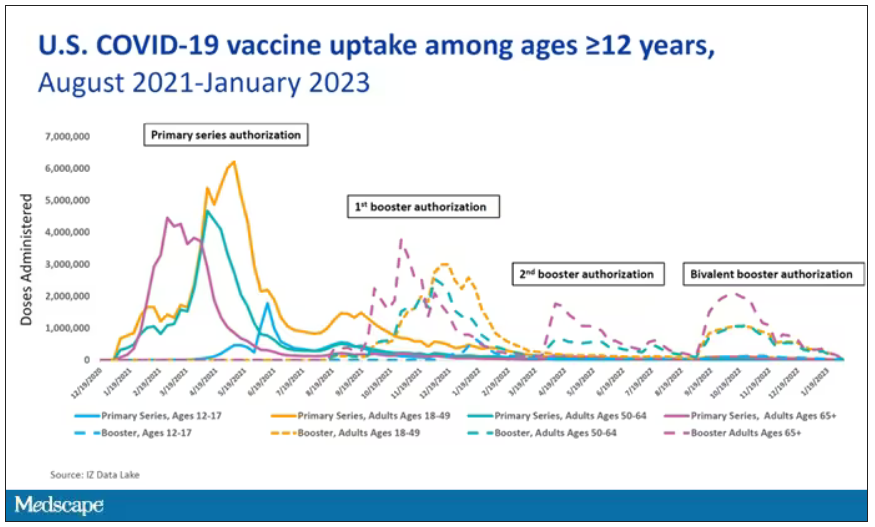
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
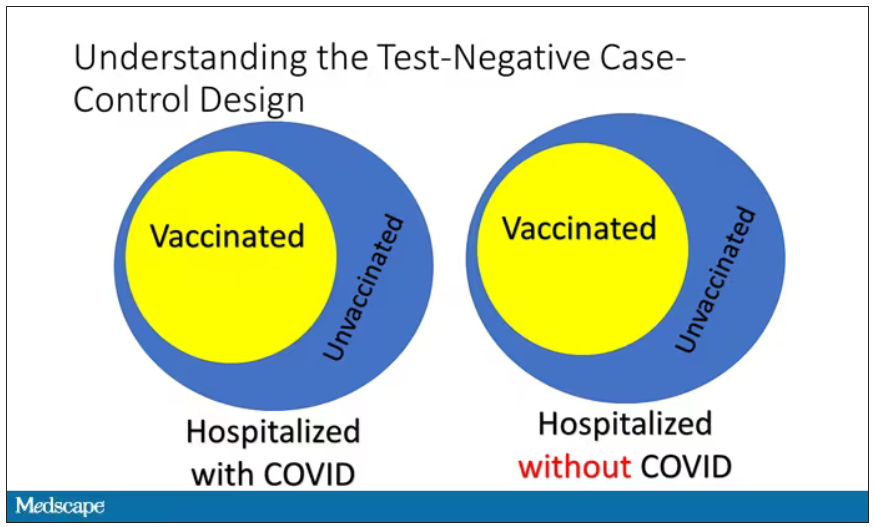
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
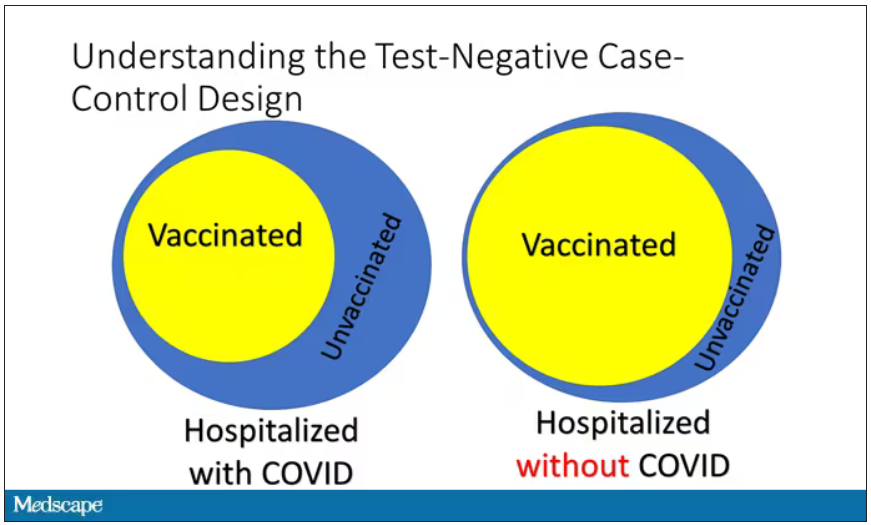
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
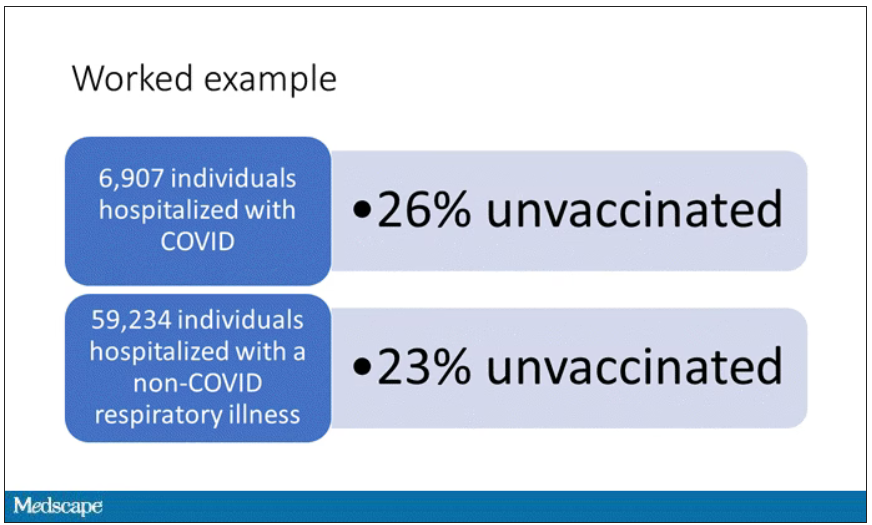
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
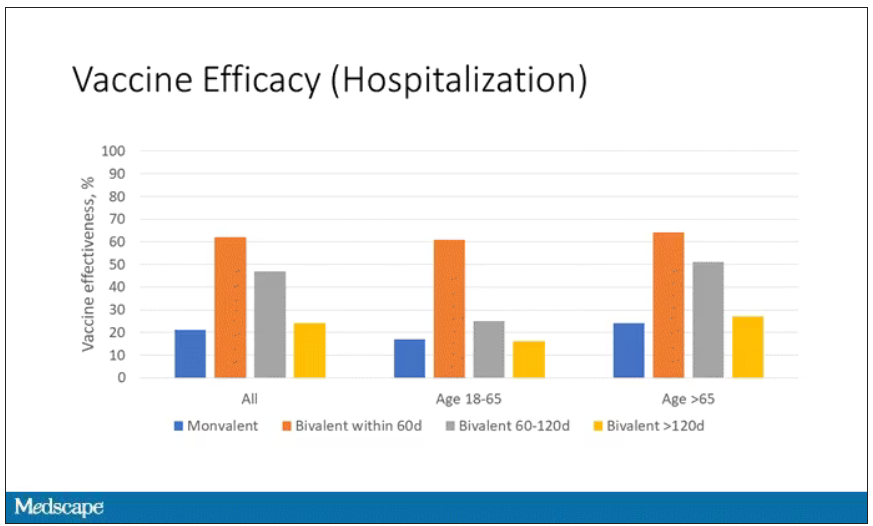
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
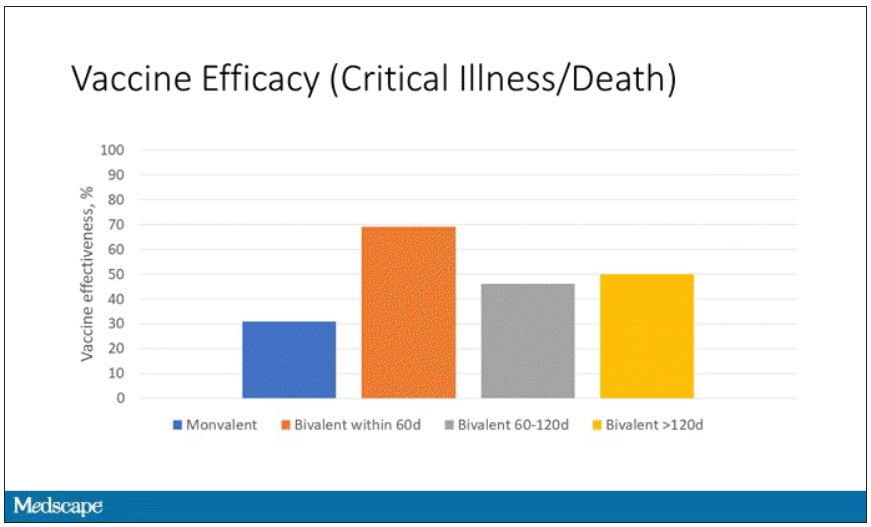
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
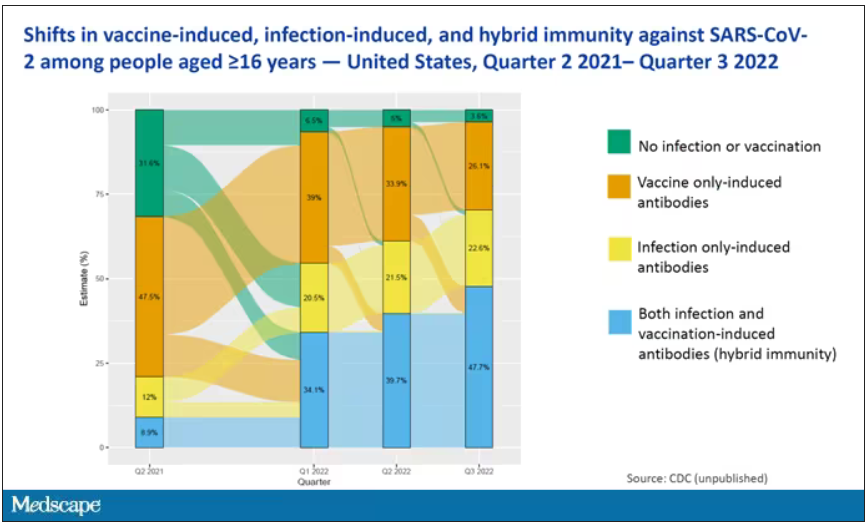
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Review supports continued mask-wearing in health care visits
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
The study said one reason people should wear masks to medical settings is because “health care personnel are notorious for coming to work while ill.” Transmission from patient to staff and staff to patient is still possible, but rare, when both are masked.
The review authors reported no conflicts of interest. Dr. Palmore has received grants from the NIH, Rigel, Gilead, and AbbVie, and Dr. Henderson is a past president of the Society for Healthcare Epidemiology of America.
A version of this article first appeared on WebMD.com.
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
The study said one reason people should wear masks to medical settings is because “health care personnel are notorious for coming to work while ill.” Transmission from patient to staff and staff to patient is still possible, but rare, when both are masked.
The review authors reported no conflicts of interest. Dr. Palmore has received grants from the NIH, Rigel, Gilead, and AbbVie, and Dr. Henderson is a past president of the Society for Healthcare Epidemiology of America.
A version of this article first appeared on WebMD.com.
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
The study said one reason people should wear masks to medical settings is because “health care personnel are notorious for coming to work while ill.” Transmission from patient to staff and staff to patient is still possible, but rare, when both are masked.
The review authors reported no conflicts of interest. Dr. Palmore has received grants from the NIH, Rigel, Gilead, and AbbVie, and Dr. Henderson is a past president of the Society for Healthcare Epidemiology of America.
A version of this article first appeared on WebMD.com.
FROM ANNALS OF INTERNAL MEDICINE
Could vitamin D supplementation help in long COVID?
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECE 2023
Clinical trials: Top priority for long COVID
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
Study of hospitalizations in Canada quantifies benefit of COVID-19 vaccine to reduce death, ICU admissions
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
New drugs in primary care: Lessons learned from COVID-19
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
AT INTERNAL MEDICINE 2023
White House to end COVID vaccine mandate for federal workers
The move means vaccines will no longer be required for workers who are federal employees, federal contractors, Head Start early education employees, workers at Medicare-certified health care facilities, and those who work at U.S. borders. International air travelers will no longer be required to prove their vaccination status. The requirement will be lifted at the end of the day on May 11, which is also when the federal public health emergency declaration ends.
“While vaccination remains one of the most important tools in advancing the health and safety of employees and promoting the efficiency of workplaces, we are now in a different phase of our response when these measures are no longer necessary,” an announcement from the White House stated.
White House officials credited vaccine requirements with saving millions of lives, noting that the rules ensured “the safety of workers in critical workforces including those in the healthcare and education sectors, protecting themselves and the populations they serve, and strengthening their ability to provide services without disruptions to operations.”
More than 100 million people were subject to the vaccine requirement, The Associated Press reported. All but 2% of those covered by the mandate had received at least one dose or had a pending or approved exception on file by January 2022, the Biden administration said, noting that COVID deaths have dropped 95% since January 2021 and hospitalizations are down nearly 91%.
In January, vaccine requirements were lifted for U.S. military members.
On the government-run website Safer Federal Workforce, which helped affected organizations put federal COVID rules into place, agencies were told to “take no action to implement or enforce the COVID-19 vaccination requirement” at this time.
A version of this article first appeared on WebMD.com.
The move means vaccines will no longer be required for workers who are federal employees, federal contractors, Head Start early education employees, workers at Medicare-certified health care facilities, and those who work at U.S. borders. International air travelers will no longer be required to prove their vaccination status. The requirement will be lifted at the end of the day on May 11, which is also when the federal public health emergency declaration ends.
“While vaccination remains one of the most important tools in advancing the health and safety of employees and promoting the efficiency of workplaces, we are now in a different phase of our response when these measures are no longer necessary,” an announcement from the White House stated.
White House officials credited vaccine requirements with saving millions of lives, noting that the rules ensured “the safety of workers in critical workforces including those in the healthcare and education sectors, protecting themselves and the populations they serve, and strengthening their ability to provide services without disruptions to operations.”
More than 100 million people were subject to the vaccine requirement, The Associated Press reported. All but 2% of those covered by the mandate had received at least one dose or had a pending or approved exception on file by January 2022, the Biden administration said, noting that COVID deaths have dropped 95% since January 2021 and hospitalizations are down nearly 91%.
In January, vaccine requirements were lifted for U.S. military members.
On the government-run website Safer Federal Workforce, which helped affected organizations put federal COVID rules into place, agencies were told to “take no action to implement or enforce the COVID-19 vaccination requirement” at this time.
A version of this article first appeared on WebMD.com.
The move means vaccines will no longer be required for workers who are federal employees, federal contractors, Head Start early education employees, workers at Medicare-certified health care facilities, and those who work at U.S. borders. International air travelers will no longer be required to prove their vaccination status. The requirement will be lifted at the end of the day on May 11, which is also when the federal public health emergency declaration ends.
“While vaccination remains one of the most important tools in advancing the health and safety of employees and promoting the efficiency of workplaces, we are now in a different phase of our response when these measures are no longer necessary,” an announcement from the White House stated.
White House officials credited vaccine requirements with saving millions of lives, noting that the rules ensured “the safety of workers in critical workforces including those in the healthcare and education sectors, protecting themselves and the populations they serve, and strengthening their ability to provide services without disruptions to operations.”
More than 100 million people were subject to the vaccine requirement, The Associated Press reported. All but 2% of those covered by the mandate had received at least one dose or had a pending or approved exception on file by January 2022, the Biden administration said, noting that COVID deaths have dropped 95% since January 2021 and hospitalizations are down nearly 91%.
In January, vaccine requirements were lifted for U.S. military members.
On the government-run website Safer Federal Workforce, which helped affected organizations put federal COVID rules into place, agencies were told to “take no action to implement or enforce the COVID-19 vaccination requirement” at this time.
A version of this article first appeared on WebMD.com.
Long-COVID rate may be higher with rheumatic diseases
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
AT BSR 2023
Researchers seek to understand post-COVID autoimmune disease risk
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.