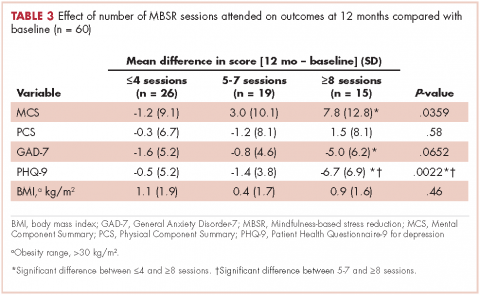
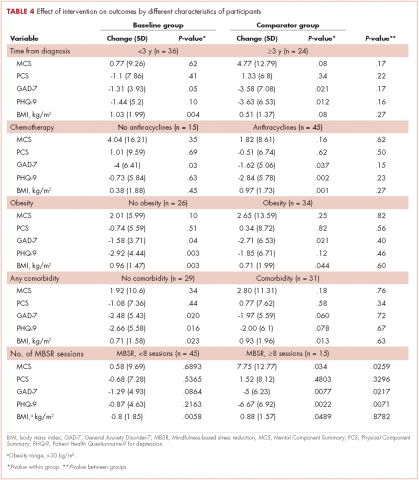
User login
Research and Reviews for the Practicing Oncologist
Two cases of possible remission in metastatic triple-negative breast cancer
Triple-negative breast cancer (TNBC) has been shown to generally have a poor prognosis. Within the first 3-5 years of diagnosis, the mortality rate is the highest of all the subtypes of breast cancer, although late relapses are less common.1,2 TNBC is markedly heterogeneous tumor, and the individual prognosis can vary widely.1,3 Metastatic TNBC is generally considered a noncurable disease. The median time from recurrence to death for metastatic disease is about 9 months, compared with 20 months for patients with other subtypes of breast cancers.4,5 The median survival time for patients with metastatic TNBC is about 13 months.3
New targeted therapies are emerging for breast cancer, but there are currently no effective targeted therapies for patients with TNBC. In addition, few reports in the literature that discuss long-term complete remissions in patients who have metastatic TNBC. Here, we describe two cases in which patients with metastatic TNBC achieved sustained complete response on conventional chemotherapy regimens.
Case presentations and summaries
Case 1
A 59-year-old woman (age in 2015) had been diagnosed on biopsy in February 2005 with locally advanced right breast cancer (stage T2N2bM0). She underwent lumpectomy, and the results of her pathology tests revealed a triple-negative invasive ductal carcinoma. She was started on 4 cycles of neoadjuvant doxorubicin (60 mg/m2 IV) and cyclophosphamide (600 mg/m2 IV)
In November 2007, the patient was found to have right chest wall metastasis confirmed by ultrasound-guided needle biopsy, and underwent right-side chest wall and partial sternum resection. In May 2008, she had recurrence in the left axilla, and biopsy results showed that she had TNBC disease. She was started on weekly paclitaxel (90 mg/m2) and bevacizumab (10 mg/kg every 2 weeks) continued until July 2008. Chemotherapy was stopped in July 2008 because of a methicillin-resistant Staphylococcus aureus (MRSA) infection of the chest wall and was not resumed after the infection had resolved.
A follow-up positron-emission tomography– computed tomography (PET-CT) scan in June 2009, showed no evidence of disease and the scan was negative for disease in her left axilla. Another PET scan about a year later, in September 2010, was also negative for any disease recurrence.
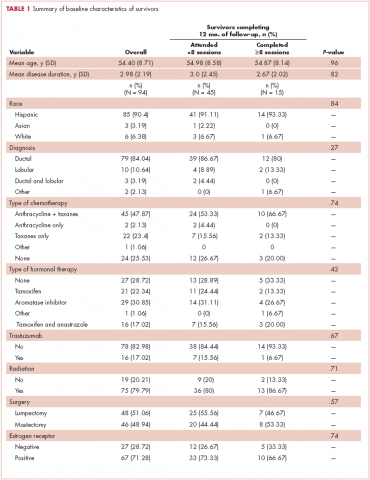
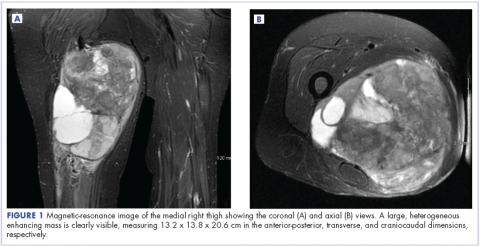
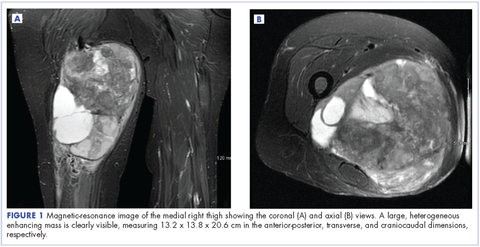
The patient has continued her follow-up with physical examinations and imaging scans. A CT scan of the abdomen and pelvis (December 2010), an MRI of the breasts (February 2011, August 2015), and a PET-CT scan (April 2015, Figure 1) were all negative for any evidence of disease. In September 2011, she had a CT-guided biopsy of a medial right clavicle and costal junction lesion; and in November 2011 and January 2013, surgical biopsies of the right chest wall and first rib lesions, all negative for any evidence for malignancy. At her last follow-up in January 2017, the patient remained in remission.
Case 2
A 68-year old woman (age in 2015) had been diagnosed in Russia in 2004 with infiltrating ductal carcinoma of the right breast (T4N1M0; receptor status unknown at that time). She underwent a right modified radical mastectomy and received adjuvant chemotherapy with 4 cycles of cyclophosphamide (100 mg/m2 day 1 to day 14), methotrexate (40 mg/m2 IV day 1 and day 8), and fluorouracil (600 mg/m2 IV, day 1 and day 8) followed by 2 cycles of docetaxel (75 mg/m2 IV) and anthracycline adriyamycin (50 mg/m2 IV). The patient later received radiation therapy (radiation dose not known, treatment was received in Russia), and completed her treatment in November 2004.
The patient moved to the United States and was started on 25 mg daily exemestane in February 2005. In March 2009, she was diagnosed by biopsy to have recurrence in her internal mammary and hilar lymph nodes and sternum. The cancer was found to be ER- and PR-negative and HER2-neu–negative. The patient was treated with radiation therapy (37.5 Gy in 15 fractions) to sternum and hilar and internal mammary lymph nodes with improvement in pain and shrinkage of lymph nodes size. In May 2009, she was started on 1,500 mg oral twice a day capecitabine (3 cycles). The therapy was started after completion of radiation treatment due to progression of disease. She developed hand-and-foot syndrome as side effect of the capecitabine, so the dose was reduced. She was switched to gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle) as a single-agent therapy and completed 3 cycles. A follow-up PET-CT scan in February 2010 showed no evidence of disease.
In May 2010, the patient had a recurrence in the same metastatic foci as before, and she was again started on gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle). She continued gemcitabine until there was evidence of disease progression on a PET-CT scan in October 2010, which showed new areas of disease in the left parasternal region, left sternum, prevascular mediastinal nodes, and left supraclavicular, hilar and axillary adenopathy, and fourth thoracic vertebra. Gemcitabine was discontinued and patient was started on weekly paclitaxel (90 mg/m2) for 6 cycles. Paclitaxel was discontinued after 6 weeks because she developed a drug-related rash. A follow-up PET-CT scan in December 2010 again showed complete resolution of disease in terms of response.
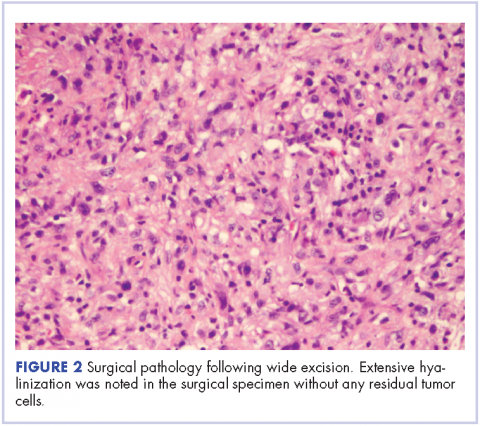
In March 2011, PET imaging showed progression of disease in the left chest wall and axillary lymph nodes, so the patient was started on eribulin therapy (1.4 mg/m2 on days 1 and 8 every 21-day cycle) and completed 3 cycles. In May 2011, PET imaging showed complete response to treatment with no evidence of recurrent or metastatic disease. The patient has not had chemotherapy since November 2011, and surveillance PET imaging has not demonstrated any recurrence of disease (Figure 2). Following her last follow-up in November 2016, the patient remains in remission.
Discussion
Triple-negative breast cancers (TNBCs) are defined as tumors that lack expression of estrogen receptor (ER), progesterone receptor (PR), and HER2, and represent about 12%-17% of breast cancer cases.1,6 TNBCs tend to be larger in size at diagnosis than are other subtypes, are usually high-grade (poorly differentiated), and are more likely to be invasive ductal carcinomas.1,7 TNBC and the basal-like breast cancers as a group are associated with an adverse prognosis.1,7 There is no standard preferred chemotherapy and no biologic therapy available for TNBC.1,6-7 A sharp decline in survival outcome during the first 3-5 years after diagnosis initial is observed in TNBC, although the distant relapses after this time are less common.1 Beyond 10 years from diagnosis, the relapses are seen more common among patients with ER-positive cancers than among those with ER-negative subtype cancers. Therefore, although TNBCs are biologically aggressive, many are possibly curable, and this reflects their interesting characteristic heterogeneity.1,6
Chemotherapy is currently the mainstay of systemic medical treatment. Although patients with TNBC have a worse outcome after chemotherapy than patients with breast cancers of other subtypes, it still improves their outcome to a greater extent than in patients with ER-positive subtypes.1,6,7 Considering the heterogeneity of TNBC, it is difficult to predict which patients will benefit more from chemotherapy. The same has been observed in previous studies when subgroups of women with TNBC were extremely sensitive to chemotherapy, whereas in others it was of uncertain benefit.1
Currently, there is no preferred standard form of chemotherapy for TNBC. There are few case reports that demonstrate long-term survival and complete remission in metastatic TNBC. Shakir has reported on a significant clinical response to nab-paclitaxel monotherapy in a patient with triple-negative BRCA1-positive breast cancer, although patient survived a little more than 5 years and died with central nervous system recurrence.8 Montero and Gluck have described a patient with metastatic TNBC who was treated with nab-paclitaxel, gemcitabine, and bevacizumab and who also survived for 5 years after diagnosis.9 Different retrospective analyses have suggested that the addition of docetaxel or paclitaxel to anthracycline-containing adjuvant regimens may be of greater benefit for the treatment of TNBC than for ER-positive tumors.10 A meta-analysis of trials comparing the effects of cyclophosphamide, methotrexate, and fluorouracil (CMF, which was used in Case 2) with anthracycline-containing regimens has suggested that the latter therapy regimen is more effective against TNBC,11 although another retrospective analysis of a separate trial suggested the opposite for basal-like breast cancers. 12 The authors of the latter analysis concluded that anthracycline-containing adjuvant chemotherapy regimens are inferior to adjuvant CMF in women with basal breast cancer.12
Miller and colleagues have shown that the addition of bevacizumab (angiogenesis inhibitor) to paclitaxel (used in Case 1) improved progression-free survival (median PFS, 11.8 vs 5.9 months; hazard ratio [HR] for progression, 0.60; P < .001) in women with TNBC as it did in the overall study group (HR, 0.53 and 0.60, respectively), although the overall survival rate was similar in the two groups (median OS, 26.7 vs 25.2 months; HR, 0.88; P = .16).13
An interesting clinical target in TNBC is the enzyme poly (adenosine diphosphate– ribose) polymerase (PARP), which is involved in base-excision repair after DNA damage. PARP inhibitors have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and in sporadic TNBC cancers.14 Similarly, the use of an oral PARP inhibitor, olaparib, resulted in tumor regression in up to 41% of patients carrying BRCA mutations, most of whom had TNBC.15
Conclusion
TNBC and basal-like breast cancers show aggressive clinical behavior, but a subgroup of these cancers may be markedly sensitive to chemotherapy and associated with a good prognosis when treated with conventional chemotherapy regimens. The two cases presented here show that some patients can get a prolonged disease control from chemotherapy, even after progressing on multiple previous chemotherapy regimens and that after, 5 years or so, these rare patients could be in true long-term remission. Novel approaches, for example PARP inhibitors, have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and as well as sporadic TNBC.
1. Foulkes WD, Smith IE, Reis-Filho JS, Triple-negative breast cancer. N Engl J Med. 2010;363:1938-1948.
2. Pogoda K, Niwińska A, Murawska M, Pieńkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol. 2013;30(1):388.
3. Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9(1):29-33.
4. Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2010;15(suppl 5):39-48.
5. Rakha EA, Chan S. Metastatic triple-negative breast cancer. Clin Oncol (R Coll Radiol). 2011;23(9):587-600.
6. Williams N, Harris L. Triple-negative breast cancer in the post-genomic era. Oncology (Williston Park). 2013;27(9):859-860, 864.
7. Randhawa SK, Venur VA, Kawsar H, et al. A retrospective comparison of the characteristics and recurrence outcome of triple-negative and triple-positive breast cancer. J Clin Oncol. 2013;31(suppl; abstr 1038).
8. Shakir AR. Strong and sustained response to treatment with carboplatin plus nab-paclitaxel in a patient with metastatic, triple-negative, BRCA1-positive breast cancer. Case Rep Oncol. 2014;7(1)252-259.
9. Montero A, Glück S. Long-term complete remission with nab-paclitaxel, bevacizumab, and gemcitabine combination therapy in a patient with triple-negative metastatic breast cancer. Case Rep Oncol. 2012;5(3):687-692.
10. Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496-1506.
11. Di Leo A, Isola J, Piette F, et al. A meta- analysis of phase III trials evaluating the predictive value of HER2 and topoisomerase alpha in early breast cancer patients treated with CMF or anthracycline-based adjuvant therapy [SABCS, abstract 705]. http://cancerres.aacrjournals.org/content/69/2_Supplement/705. Published 2008. Accessed May 4, 2017.
12. Cheang M, Chia SK, Tu D, et al. Anthracycline in basal breast cancer: the NCIC-CTG trial MA5 comparing adjuvant CMF to CEF [ASCO; abstract 519]. http://meetinglibrary.asco.org/content/35150-65. Published 2009. Accessed May 4, 2017.
13. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676.
14. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123-134.
15. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235-244.
Triple-negative breast cancer (TNBC) has been shown to generally have a poor prognosis. Within the first 3-5 years of diagnosis, the mortality rate is the highest of all the subtypes of breast cancer, although late relapses are less common.1,2 TNBC is markedly heterogeneous tumor, and the individual prognosis can vary widely.1,3 Metastatic TNBC is generally considered a noncurable disease. The median time from recurrence to death for metastatic disease is about 9 months, compared with 20 months for patients with other subtypes of breast cancers.4,5 The median survival time for patients with metastatic TNBC is about 13 months.3
New targeted therapies are emerging for breast cancer, but there are currently no effective targeted therapies for patients with TNBC. In addition, few reports in the literature that discuss long-term complete remissions in patients who have metastatic TNBC. Here, we describe two cases in which patients with metastatic TNBC achieved sustained complete response on conventional chemotherapy regimens.
Case presentations and summaries
Case 1
A 59-year-old woman (age in 2015) had been diagnosed on biopsy in February 2005 with locally advanced right breast cancer (stage T2N2bM0). She underwent lumpectomy, and the results of her pathology tests revealed a triple-negative invasive ductal carcinoma. She was started on 4 cycles of neoadjuvant doxorubicin (60 mg/m2 IV) and cyclophosphamide (600 mg/m2 IV)
In November 2007, the patient was found to have right chest wall metastasis confirmed by ultrasound-guided needle biopsy, and underwent right-side chest wall and partial sternum resection. In May 2008, she had recurrence in the left axilla, and biopsy results showed that she had TNBC disease. She was started on weekly paclitaxel (90 mg/m2) and bevacizumab (10 mg/kg every 2 weeks) continued until July 2008. Chemotherapy was stopped in July 2008 because of a methicillin-resistant Staphylococcus aureus (MRSA) infection of the chest wall and was not resumed after the infection had resolved.
A follow-up positron-emission tomography– computed tomography (PET-CT) scan in June 2009, showed no evidence of disease and the scan was negative for disease in her left axilla. Another PET scan about a year later, in September 2010, was also negative for any disease recurrence.
The patient has continued her follow-up with physical examinations and imaging scans. A CT scan of the abdomen and pelvis (December 2010), an MRI of the breasts (February 2011, August 2015), and a PET-CT scan (April 2015, Figure 1) were all negative for any evidence of disease. In September 2011, she had a CT-guided biopsy of a medial right clavicle and costal junction lesion; and in November 2011 and January 2013, surgical biopsies of the right chest wall and first rib lesions, all negative for any evidence for malignancy. At her last follow-up in January 2017, the patient remained in remission.
Case 2
A 68-year old woman (age in 2015) had been diagnosed in Russia in 2004 with infiltrating ductal carcinoma of the right breast (T4N1M0; receptor status unknown at that time). She underwent a right modified radical mastectomy and received adjuvant chemotherapy with 4 cycles of cyclophosphamide (100 mg/m2 day 1 to day 14), methotrexate (40 mg/m2 IV day 1 and day 8), and fluorouracil (600 mg/m2 IV, day 1 and day 8) followed by 2 cycles of docetaxel (75 mg/m2 IV) and anthracycline adriyamycin (50 mg/m2 IV). The patient later received radiation therapy (radiation dose not known, treatment was received in Russia), and completed her treatment in November 2004.
The patient moved to the United States and was started on 25 mg daily exemestane in February 2005. In March 2009, she was diagnosed by biopsy to have recurrence in her internal mammary and hilar lymph nodes and sternum. The cancer was found to be ER- and PR-negative and HER2-neu–negative. The patient was treated with radiation therapy (37.5 Gy in 15 fractions) to sternum and hilar and internal mammary lymph nodes with improvement in pain and shrinkage of lymph nodes size. In May 2009, she was started on 1,500 mg oral twice a day capecitabine (3 cycles). The therapy was started after completion of radiation treatment due to progression of disease. She developed hand-and-foot syndrome as side effect of the capecitabine, so the dose was reduced. She was switched to gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle) as a single-agent therapy and completed 3 cycles. A follow-up PET-CT scan in February 2010 showed no evidence of disease.
In May 2010, the patient had a recurrence in the same metastatic foci as before, and she was again started on gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle). She continued gemcitabine until there was evidence of disease progression on a PET-CT scan in October 2010, which showed new areas of disease in the left parasternal region, left sternum, prevascular mediastinal nodes, and left supraclavicular, hilar and axillary adenopathy, and fourth thoracic vertebra. Gemcitabine was discontinued and patient was started on weekly paclitaxel (90 mg/m2) for 6 cycles. Paclitaxel was discontinued after 6 weeks because she developed a drug-related rash. A follow-up PET-CT scan in December 2010 again showed complete resolution of disease in terms of response.
In March 2011, PET imaging showed progression of disease in the left chest wall and axillary lymph nodes, so the patient was started on eribulin therapy (1.4 mg/m2 on days 1 and 8 every 21-day cycle) and completed 3 cycles. In May 2011, PET imaging showed complete response to treatment with no evidence of recurrent or metastatic disease. The patient has not had chemotherapy since November 2011, and surveillance PET imaging has not demonstrated any recurrence of disease (Figure 2). Following her last follow-up in November 2016, the patient remains in remission.
Discussion
Triple-negative breast cancers (TNBCs) are defined as tumors that lack expression of estrogen receptor (ER), progesterone receptor (PR), and HER2, and represent about 12%-17% of breast cancer cases.1,6 TNBCs tend to be larger in size at diagnosis than are other subtypes, are usually high-grade (poorly differentiated), and are more likely to be invasive ductal carcinomas.1,7 TNBC and the basal-like breast cancers as a group are associated with an adverse prognosis.1,7 There is no standard preferred chemotherapy and no biologic therapy available for TNBC.1,6-7 A sharp decline in survival outcome during the first 3-5 years after diagnosis initial is observed in TNBC, although the distant relapses after this time are less common.1 Beyond 10 years from diagnosis, the relapses are seen more common among patients with ER-positive cancers than among those with ER-negative subtype cancers. Therefore, although TNBCs are biologically aggressive, many are possibly curable, and this reflects their interesting characteristic heterogeneity.1,6
Chemotherapy is currently the mainstay of systemic medical treatment. Although patients with TNBC have a worse outcome after chemotherapy than patients with breast cancers of other subtypes, it still improves their outcome to a greater extent than in patients with ER-positive subtypes.1,6,7 Considering the heterogeneity of TNBC, it is difficult to predict which patients will benefit more from chemotherapy. The same has been observed in previous studies when subgroups of women with TNBC were extremely sensitive to chemotherapy, whereas in others it was of uncertain benefit.1
Currently, there is no preferred standard form of chemotherapy for TNBC. There are few case reports that demonstrate long-term survival and complete remission in metastatic TNBC. Shakir has reported on a significant clinical response to nab-paclitaxel monotherapy in a patient with triple-negative BRCA1-positive breast cancer, although patient survived a little more than 5 years and died with central nervous system recurrence.8 Montero and Gluck have described a patient with metastatic TNBC who was treated with nab-paclitaxel, gemcitabine, and bevacizumab and who also survived for 5 years after diagnosis.9 Different retrospective analyses have suggested that the addition of docetaxel or paclitaxel to anthracycline-containing adjuvant regimens may be of greater benefit for the treatment of TNBC than for ER-positive tumors.10 A meta-analysis of trials comparing the effects of cyclophosphamide, methotrexate, and fluorouracil (CMF, which was used in Case 2) with anthracycline-containing regimens has suggested that the latter therapy regimen is more effective against TNBC,11 although another retrospective analysis of a separate trial suggested the opposite for basal-like breast cancers. 12 The authors of the latter analysis concluded that anthracycline-containing adjuvant chemotherapy regimens are inferior to adjuvant CMF in women with basal breast cancer.12
Miller and colleagues have shown that the addition of bevacizumab (angiogenesis inhibitor) to paclitaxel (used in Case 1) improved progression-free survival (median PFS, 11.8 vs 5.9 months; hazard ratio [HR] for progression, 0.60; P < .001) in women with TNBC as it did in the overall study group (HR, 0.53 and 0.60, respectively), although the overall survival rate was similar in the two groups (median OS, 26.7 vs 25.2 months; HR, 0.88; P = .16).13
An interesting clinical target in TNBC is the enzyme poly (adenosine diphosphate– ribose) polymerase (PARP), which is involved in base-excision repair after DNA damage. PARP inhibitors have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and in sporadic TNBC cancers.14 Similarly, the use of an oral PARP inhibitor, olaparib, resulted in tumor regression in up to 41% of patients carrying BRCA mutations, most of whom had TNBC.15
Conclusion
TNBC and basal-like breast cancers show aggressive clinical behavior, but a subgroup of these cancers may be markedly sensitive to chemotherapy and associated with a good prognosis when treated with conventional chemotherapy regimens. The two cases presented here show that some patients can get a prolonged disease control from chemotherapy, even after progressing on multiple previous chemotherapy regimens and that after, 5 years or so, these rare patients could be in true long-term remission. Novel approaches, for example PARP inhibitors, have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and as well as sporadic TNBC.
Triple-negative breast cancer (TNBC) has been shown to generally have a poor prognosis. Within the first 3-5 years of diagnosis, the mortality rate is the highest of all the subtypes of breast cancer, although late relapses are less common.1,2 TNBC is markedly heterogeneous tumor, and the individual prognosis can vary widely.1,3 Metastatic TNBC is generally considered a noncurable disease. The median time from recurrence to death for metastatic disease is about 9 months, compared with 20 months for patients with other subtypes of breast cancers.4,5 The median survival time for patients with metastatic TNBC is about 13 months.3
New targeted therapies are emerging for breast cancer, but there are currently no effective targeted therapies for patients with TNBC. In addition, few reports in the literature that discuss long-term complete remissions in patients who have metastatic TNBC. Here, we describe two cases in which patients with metastatic TNBC achieved sustained complete response on conventional chemotherapy regimens.
Case presentations and summaries
Case 1
A 59-year-old woman (age in 2015) had been diagnosed on biopsy in February 2005 with locally advanced right breast cancer (stage T2N2bM0). She underwent lumpectomy, and the results of her pathology tests revealed a triple-negative invasive ductal carcinoma. She was started on 4 cycles of neoadjuvant doxorubicin (60 mg/m2 IV) and cyclophosphamide (600 mg/m2 IV)
In November 2007, the patient was found to have right chest wall metastasis confirmed by ultrasound-guided needle biopsy, and underwent right-side chest wall and partial sternum resection. In May 2008, she had recurrence in the left axilla, and biopsy results showed that she had TNBC disease. She was started on weekly paclitaxel (90 mg/m2) and bevacizumab (10 mg/kg every 2 weeks) continued until July 2008. Chemotherapy was stopped in July 2008 because of a methicillin-resistant Staphylococcus aureus (MRSA) infection of the chest wall and was not resumed after the infection had resolved.
A follow-up positron-emission tomography– computed tomography (PET-CT) scan in June 2009, showed no evidence of disease and the scan was negative for disease in her left axilla. Another PET scan about a year later, in September 2010, was also negative for any disease recurrence.
The patient has continued her follow-up with physical examinations and imaging scans. A CT scan of the abdomen and pelvis (December 2010), an MRI of the breasts (February 2011, August 2015), and a PET-CT scan (April 2015, Figure 1) were all negative for any evidence of disease. In September 2011, she had a CT-guided biopsy of a medial right clavicle and costal junction lesion; and in November 2011 and January 2013, surgical biopsies of the right chest wall and first rib lesions, all negative for any evidence for malignancy. At her last follow-up in January 2017, the patient remained in remission.
Case 2
A 68-year old woman (age in 2015) had been diagnosed in Russia in 2004 with infiltrating ductal carcinoma of the right breast (T4N1M0; receptor status unknown at that time). She underwent a right modified radical mastectomy and received adjuvant chemotherapy with 4 cycles of cyclophosphamide (100 mg/m2 day 1 to day 14), methotrexate (40 mg/m2 IV day 1 and day 8), and fluorouracil (600 mg/m2 IV, day 1 and day 8) followed by 2 cycles of docetaxel (75 mg/m2 IV) and anthracycline adriyamycin (50 mg/m2 IV). The patient later received radiation therapy (radiation dose not known, treatment was received in Russia), and completed her treatment in November 2004.
The patient moved to the United States and was started on 25 mg daily exemestane in February 2005. In March 2009, she was diagnosed by biopsy to have recurrence in her internal mammary and hilar lymph nodes and sternum. The cancer was found to be ER- and PR-negative and HER2-neu–negative. The patient was treated with radiation therapy (37.5 Gy in 15 fractions) to sternum and hilar and internal mammary lymph nodes with improvement in pain and shrinkage of lymph nodes size. In May 2009, she was started on 1,500 mg oral twice a day capecitabine (3 cycles). The therapy was started after completion of radiation treatment due to progression of disease. She developed hand-and-foot syndrome as side effect of the capecitabine, so the dose was reduced. She was switched to gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle) as a single-agent therapy and completed 3 cycles. A follow-up PET-CT scan in February 2010 showed no evidence of disease.
In May 2010, the patient had a recurrence in the same metastatic foci as before, and she was again started on gemcitabine (1,000 mg/m2 on days 1, 8, and 15, every 28-day cycle). She continued gemcitabine until there was evidence of disease progression on a PET-CT scan in October 2010, which showed new areas of disease in the left parasternal region, left sternum, prevascular mediastinal nodes, and left supraclavicular, hilar and axillary adenopathy, and fourth thoracic vertebra. Gemcitabine was discontinued and patient was started on weekly paclitaxel (90 mg/m2) for 6 cycles. Paclitaxel was discontinued after 6 weeks because she developed a drug-related rash. A follow-up PET-CT scan in December 2010 again showed complete resolution of disease in terms of response.
In March 2011, PET imaging showed progression of disease in the left chest wall and axillary lymph nodes, so the patient was started on eribulin therapy (1.4 mg/m2 on days 1 and 8 every 21-day cycle) and completed 3 cycles. In May 2011, PET imaging showed complete response to treatment with no evidence of recurrent or metastatic disease. The patient has not had chemotherapy since November 2011, and surveillance PET imaging has not demonstrated any recurrence of disease (Figure 2). Following her last follow-up in November 2016, the patient remains in remission.
Discussion
Triple-negative breast cancers (TNBCs) are defined as tumors that lack expression of estrogen receptor (ER), progesterone receptor (PR), and HER2, and represent about 12%-17% of breast cancer cases.1,6 TNBCs tend to be larger in size at diagnosis than are other subtypes, are usually high-grade (poorly differentiated), and are more likely to be invasive ductal carcinomas.1,7 TNBC and the basal-like breast cancers as a group are associated with an adverse prognosis.1,7 There is no standard preferred chemotherapy and no biologic therapy available for TNBC.1,6-7 A sharp decline in survival outcome during the first 3-5 years after diagnosis initial is observed in TNBC, although the distant relapses after this time are less common.1 Beyond 10 years from diagnosis, the relapses are seen more common among patients with ER-positive cancers than among those with ER-negative subtype cancers. Therefore, although TNBCs are biologically aggressive, many are possibly curable, and this reflects their interesting characteristic heterogeneity.1,6
Chemotherapy is currently the mainstay of systemic medical treatment. Although patients with TNBC have a worse outcome after chemotherapy than patients with breast cancers of other subtypes, it still improves their outcome to a greater extent than in patients with ER-positive subtypes.1,6,7 Considering the heterogeneity of TNBC, it is difficult to predict which patients will benefit more from chemotherapy. The same has been observed in previous studies when subgroups of women with TNBC were extremely sensitive to chemotherapy, whereas in others it was of uncertain benefit.1
Currently, there is no preferred standard form of chemotherapy for TNBC. There are few case reports that demonstrate long-term survival and complete remission in metastatic TNBC. Shakir has reported on a significant clinical response to nab-paclitaxel monotherapy in a patient with triple-negative BRCA1-positive breast cancer, although patient survived a little more than 5 years and died with central nervous system recurrence.8 Montero and Gluck have described a patient with metastatic TNBC who was treated with nab-paclitaxel, gemcitabine, and bevacizumab and who also survived for 5 years after diagnosis.9 Different retrospective analyses have suggested that the addition of docetaxel or paclitaxel to anthracycline-containing adjuvant regimens may be of greater benefit for the treatment of TNBC than for ER-positive tumors.10 A meta-analysis of trials comparing the effects of cyclophosphamide, methotrexate, and fluorouracil (CMF, which was used in Case 2) with anthracycline-containing regimens has suggested that the latter therapy regimen is more effective against TNBC,11 although another retrospective analysis of a separate trial suggested the opposite for basal-like breast cancers. 12 The authors of the latter analysis concluded that anthracycline-containing adjuvant chemotherapy regimens are inferior to adjuvant CMF in women with basal breast cancer.12
Miller and colleagues have shown that the addition of bevacizumab (angiogenesis inhibitor) to paclitaxel (used in Case 1) improved progression-free survival (median PFS, 11.8 vs 5.9 months; hazard ratio [HR] for progression, 0.60; P < .001) in women with TNBC as it did in the overall study group (HR, 0.53 and 0.60, respectively), although the overall survival rate was similar in the two groups (median OS, 26.7 vs 25.2 months; HR, 0.88; P = .16).13
An interesting clinical target in TNBC is the enzyme poly (adenosine diphosphate– ribose) polymerase (PARP), which is involved in base-excision repair after DNA damage. PARP inhibitors have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and in sporadic TNBC cancers.14 Similarly, the use of an oral PARP inhibitor, olaparib, resulted in tumor regression in up to 41% of patients carrying BRCA mutations, most of whom had TNBC.15
Conclusion
TNBC and basal-like breast cancers show aggressive clinical behavior, but a subgroup of these cancers may be markedly sensitive to chemotherapy and associated with a good prognosis when treated with conventional chemotherapy regimens. The two cases presented here show that some patients can get a prolonged disease control from chemotherapy, even after progressing on multiple previous chemotherapy regimens and that after, 5 years or so, these rare patients could be in true long-term remission. Novel approaches, for example PARP inhibitors, have shown encouraging clinical activity in trials of tumors arising in BRCA mutation carriers and as well as sporadic TNBC.
1. Foulkes WD, Smith IE, Reis-Filho JS, Triple-negative breast cancer. N Engl J Med. 2010;363:1938-1948.
2. Pogoda K, Niwińska A, Murawska M, Pieńkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol. 2013;30(1):388.
3. Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9(1):29-33.
4. Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2010;15(suppl 5):39-48.
5. Rakha EA, Chan S. Metastatic triple-negative breast cancer. Clin Oncol (R Coll Radiol). 2011;23(9):587-600.
6. Williams N, Harris L. Triple-negative breast cancer in the post-genomic era. Oncology (Williston Park). 2013;27(9):859-860, 864.
7. Randhawa SK, Venur VA, Kawsar H, et al. A retrospective comparison of the characteristics and recurrence outcome of triple-negative and triple-positive breast cancer. J Clin Oncol. 2013;31(suppl; abstr 1038).
8. Shakir AR. Strong and sustained response to treatment with carboplatin plus nab-paclitaxel in a patient with metastatic, triple-negative, BRCA1-positive breast cancer. Case Rep Oncol. 2014;7(1)252-259.
9. Montero A, Glück S. Long-term complete remission with nab-paclitaxel, bevacizumab, and gemcitabine combination therapy in a patient with triple-negative metastatic breast cancer. Case Rep Oncol. 2012;5(3):687-692.
10. Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496-1506.
11. Di Leo A, Isola J, Piette F, et al. A meta- analysis of phase III trials evaluating the predictive value of HER2 and topoisomerase alpha in early breast cancer patients treated with CMF or anthracycline-based adjuvant therapy [SABCS, abstract 705]. http://cancerres.aacrjournals.org/content/69/2_Supplement/705. Published 2008. Accessed May 4, 2017.
12. Cheang M, Chia SK, Tu D, et al. Anthracycline in basal breast cancer: the NCIC-CTG trial MA5 comparing adjuvant CMF to CEF [ASCO; abstract 519]. http://meetinglibrary.asco.org/content/35150-65. Published 2009. Accessed May 4, 2017.
13. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676.
14. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123-134.
15. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235-244.
1. Foulkes WD, Smith IE, Reis-Filho JS, Triple-negative breast cancer. N Engl J Med. 2010;363:1938-1948.
2. Pogoda K, Niwińska A, Murawska M, Pieńkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol. 2013;30(1):388.
3. Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9(1):29-33.
4. Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2010;15(suppl 5):39-48.
5. Rakha EA, Chan S. Metastatic triple-negative breast cancer. Clin Oncol (R Coll Radiol). 2011;23(9):587-600.
6. Williams N, Harris L. Triple-negative breast cancer in the post-genomic era. Oncology (Williston Park). 2013;27(9):859-860, 864.
7. Randhawa SK, Venur VA, Kawsar H, et al. A retrospective comparison of the characteristics and recurrence outcome of triple-negative and triple-positive breast cancer. J Clin Oncol. 2013;31(suppl; abstr 1038).
8. Shakir AR. Strong and sustained response to treatment with carboplatin plus nab-paclitaxel in a patient with metastatic, triple-negative, BRCA1-positive breast cancer. Case Rep Oncol. 2014;7(1)252-259.
9. Montero A, Glück S. Long-term complete remission with nab-paclitaxel, bevacizumab, and gemcitabine combination therapy in a patient with triple-negative metastatic breast cancer. Case Rep Oncol. 2012;5(3):687-692.
10. Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496-1506.
11. Di Leo A, Isola J, Piette F, et al. A meta- analysis of phase III trials evaluating the predictive value of HER2 and topoisomerase alpha in early breast cancer patients treated with CMF or anthracycline-based adjuvant therapy [SABCS, abstract 705]. http://cancerres.aacrjournals.org/content/69/2_Supplement/705. Published 2008. Accessed May 4, 2017.
12. Cheang M, Chia SK, Tu D, et al. Anthracycline in basal breast cancer: the NCIC-CTG trial MA5 comparing adjuvant CMF to CEF [ASCO; abstract 519]. http://meetinglibrary.asco.org/content/35150-65. Published 2009. Accessed May 4, 2017.
13. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676.
14. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123-134.
15. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235-244.
Making Practice Perfect Download
Please click either of the links below to download your free eBook
Onecount Call To Arms
Bone remodeling associated with CTLA-4 inhibition: an unreported side effect
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is an important component of the immune checkpoint pathway. CTLA-4 inhibition causes T-cell activation and proliferation, increases T-cell responsiveness, and enhances the anti-tumor immune response. CTLA-4 inhibition also results in immune-related adverse reactions such as colitis, hepatitis, and endocrinopathies. Preclinical investigations have recently shown that CTLA-4 inhibition can cause cytokine-mediated increase in bone remodeling.1,2(p4) Ipilimumab, a recombinant IgG1 kappa antibody against human CTLA-4, has been approved for use in unresectable or metastatic melanoma. We hypothesize that ipilumumab results in increase in bone remodeling manifesting as an autoimmune reaction.
Methods
We conducted a retrospective case-control study of patients with stage III/IV melanoma treated at the University of New Mexico Comprehensive Cancer Center during April 2009-July 2014. The university’s Institutional Review Board approved the study.
Two cohorts were compared: an ipilumimab cohort receiving ipilumimab at 3 mg/kg every 3 weeks, and a chemotherapy cohort receiving an investigational chemotherapy regimen: carboplatin IV at an area under curve of 5 on day 1, paclitaxel IV at 175 mg/m2 on day 1, and temozolomide orally at 125 mg/m2 daily on days 2 to 6 every 21 days. Patients receiving at least 1 cycle of treatment were included. Those with known hepatic disease or concurrent malignancy were excluded from the study.
Serum ALP level (normal range, 38-150 international units per liter [IU/L]) and patient-reported bone pain measured by the 11-point numeric rating scale (NRS) for pain assessment were recorded before treatment initiation, on each cycle, and upon treatment completion.3 Clinical response was assessed per RECIST guidelines.4 Bone pain was dichotomized into Absent (pain intensity of 0 on the NRS, meaning no pain) or Present (pain intensity of 1-10 on the NRS, with 1 = mild pain and 10 = worst imaginable pain). Patients with a complete or partial response to the therapy were categorized as responders, and those with progressive or stable disease were categorized as nonresponders.
Descriptive statistics were generated for demographic and clinical characteristics. The primary outcome variables of interest were bone pain and mean ALP levels. Generalized linear mixed-effect models for proportion of patients with bone pain (with logit link function) and mean ALP levels (with identify link function) were used to evaluate for a difference in trends between the two cohorts over time. We used the Kenward-Roger approach to adjust for the small size of the degrees of freedom. To assess the significance of difference of the proportions of patients with bone pain and the mean ALP levels between responders and nonresponders in the ipilumimab cohort, the Fisher exact test and Wilcoxon rank-sum test were used, respectively. Statistical analyses were performed with statistical packages R (v3.1.3) and SAS (v9.4).
Results
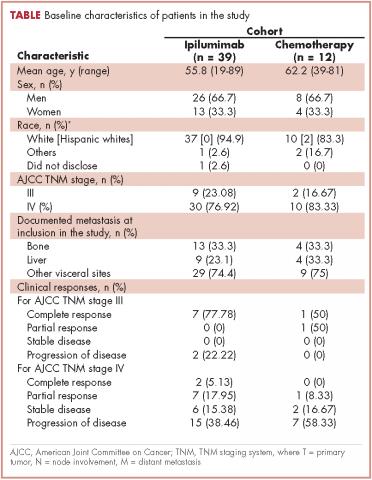
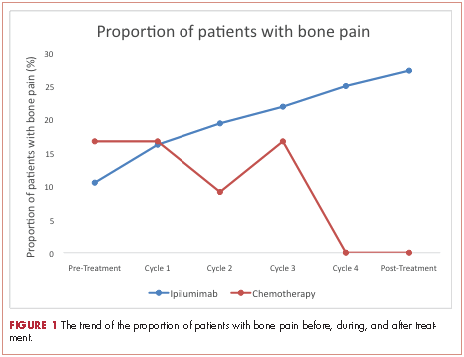
A total of 281 patients were screened, and 51 met the inclusion criteria (39 in the ipilumimab and 12 in chemotherapy cohorts). Baseline parameters were well matched between the cohorts (Table). Of the 39 patients in the ipilimumab cohort, 14 (35.9%) had bone pain during at least one of the treatment cycles, compared with 3 of the 12 patients (25%) in the chemotherapy cohort. At baseline, 4 of 38 ipilimumab patients (10.5%; 95% confidence interval [CI], 2.9-24.8) and 2 of 12 chemotherapy patients (16.7%; 95% CI, 2.1-48.4) had bone pain. Upon treatment completion, 9 of 33 ipilimumab patients (27.3%; 95% CI, 13.3-45.5) and 0 of 12 chemotherapy patients (0%; 95% CI, 0-26.5) had bone pain. The trend of proportion of patients with bone pain over time was statistically significant between the two cohorts (P = .023, Figure 1). The trends of proportion of patients with bone pain were not statistically significant when stratified by the presence of bone metastasis at inclusion in the study (P = .418) or disease progression at treatment completion (P = .500).
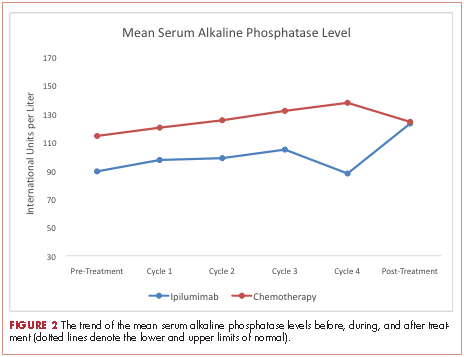
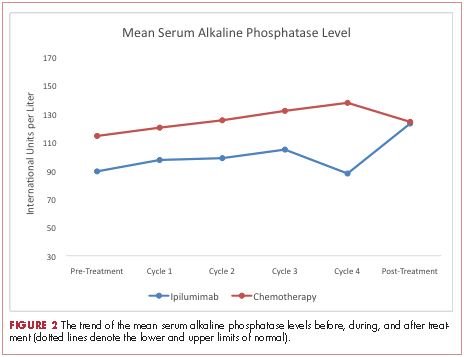
At baseline, the mean ALP level was 89.39 IU/L (95% CI, 81.03-97.75) in the ipilumimab cohort and 114.33 IU/L (95% CI, 69.48-159.19) in the chemotherapy cohort. Upon treatment completion, the mean ALP level was 123.09 IU/L (95% C.I. 80.78-165.41) in the ipilumimab cohort and 124.24 IU/L (95% C.I. 90.88-157.62) in the chemotherapy cohort. The trend of mean ALP level over time was not statistically significant between the 2 cohorts (P = .653, Figure 2).
Discussion
Immune checkpoints are inhibitory pathways that are critical for maintenance of self-tolerance and regulation of appropriate immune response. CTLA-4 is present exclusively on T cells and interacts with its ligands B7.1 and B7.2. CTLA-4 competes with CD28 in binding with B7, leading to dampening of T-cell activation and function.5,6 Development of checkpoint inhibitors such as ipilumimab have heralded a new era of immune targeted therapies for various malignancies including malignant melanoma.
Bone remodeling involves 4 distinct but overlapping phases. The first phase involves detection of loss of bone continuity by osteocytes and activation of osteoclast precursors derived from progenitors of the monocyte-macrophage lineage. The second phase involves osteoclast-medicated bone resorption and concurrent recruitment of mesenchymal stem cells and osteoprogenitors. The third phase involves osteoblast differentiation and osteoid synthesis, and the fourth phase results in mineralization of osteoid and termination of bone remodeling.7,8
The role of T-lymphocytes and cytokines, such as IL-1 and TNF-α, and receptor activator of NF-κB ligand (RANK-L) in osteoclastogenesis is well studied. RANK-L is considered to be the final downstream effector of this process.9 T-lymphocytes have also been shown to promote osteoblast maturation and function.9,10 These findings suggest a significant interaction between immune system activation and bone remodeling.
The search for a reliable biomarker for immune therapy is ongoing. Although ipilumimab-associated immune-related adverse events have been suggested to predict response to therapy,11 there is considerable debate on the subject. Ipilumimab’s impact on bone remodeling could offer a solution.
In the current study, there was a statistically significant difference in proportion of patients with bone pain in the 2 cohorts. This was preserved with stratification based on bone metastasis at inclusion and disease progression on treatment completion making new or worsening skeletal metastasis. Furthermore, the proportion of patients with bone pain increased with each cycle for ipilumimab cohort. However, we were unable to detect an association between bone pain and response to ipilimumab.
We were not able to detect a difference in trend of mean ALP level with treatment in the two cohorts. Although it is possible that no such association exists, we believe our study was not powered to detect it. Finally, we were not able to study markers for osteoblast (bone-specific ALP) and osteoclasts (N- and C-telopeptides of type 1 collagen, deoxypyridinoline, etc) to better assess this interaction because they are not commonly clinically used.
Regarding the limitations of our study, we chose to dichotomize the patient-reported bone pain because it is a subjective measure and there is a significant variability of the perceived pain intensity among patients. We also excluded patients with hepatitis from receiving the ipilumimab therapy and those with known hepatic disease from the study to reduce the impact of hepatic ALP on total serum ALP levels.
In conclusion, as far as we know, this is the first clinical report suggesting a possible relationship between CTLA-4 inhibition and bone remodeling. Supported by a strong preclinical rationale, this side effect remains under-studied and under-recognized by clinicians. A prospective assessment of this interaction using bone specific markers is planned.
1. Bozec A, Zaiss MM, Kagwiria R, et al. T-cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
2. Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F. EphB4 promotes osteogenesis of CTLA 4-modified bone marrow-derived mesenchymal stem cells through cross talk with wnt pathway in xenotransplantation. Tissue Eng Part A. 2015;21(17-18):2404-2416.
3. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158.
4. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
6. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
7. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139.
8. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-145.
9. Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9(2):103.
10. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109-117.
11. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681-6688.
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is an important component of the immune checkpoint pathway. CTLA-4 inhibition causes T-cell activation and proliferation, increases T-cell responsiveness, and enhances the anti-tumor immune response. CTLA-4 inhibition also results in immune-related adverse reactions such as colitis, hepatitis, and endocrinopathies. Preclinical investigations have recently shown that CTLA-4 inhibition can cause cytokine-mediated increase in bone remodeling.1,2(p4) Ipilimumab, a recombinant IgG1 kappa antibody against human CTLA-4, has been approved for use in unresectable or metastatic melanoma. We hypothesize that ipilumumab results in increase in bone remodeling manifesting as an autoimmune reaction.
Methods
We conducted a retrospective case-control study of patients with stage III/IV melanoma treated at the University of New Mexico Comprehensive Cancer Center during April 2009-July 2014. The university’s Institutional Review Board approved the study.
Two cohorts were compared: an ipilumimab cohort receiving ipilumimab at 3 mg/kg every 3 weeks, and a chemotherapy cohort receiving an investigational chemotherapy regimen: carboplatin IV at an area under curve of 5 on day 1, paclitaxel IV at 175 mg/m2 on day 1, and temozolomide orally at 125 mg/m2 daily on days 2 to 6 every 21 days. Patients receiving at least 1 cycle of treatment were included. Those with known hepatic disease or concurrent malignancy were excluded from the study.
Serum ALP level (normal range, 38-150 international units per liter [IU/L]) and patient-reported bone pain measured by the 11-point numeric rating scale (NRS) for pain assessment were recorded before treatment initiation, on each cycle, and upon treatment completion.3 Clinical response was assessed per RECIST guidelines.4 Bone pain was dichotomized into Absent (pain intensity of 0 on the NRS, meaning no pain) or Present (pain intensity of 1-10 on the NRS, with 1 = mild pain and 10 = worst imaginable pain). Patients with a complete or partial response to the therapy were categorized as responders, and those with progressive or stable disease were categorized as nonresponders.
Descriptive statistics were generated for demographic and clinical characteristics. The primary outcome variables of interest were bone pain and mean ALP levels. Generalized linear mixed-effect models for proportion of patients with bone pain (with logit link function) and mean ALP levels (with identify link function) were used to evaluate for a difference in trends between the two cohorts over time. We used the Kenward-Roger approach to adjust for the small size of the degrees of freedom. To assess the significance of difference of the proportions of patients with bone pain and the mean ALP levels between responders and nonresponders in the ipilumimab cohort, the Fisher exact test and Wilcoxon rank-sum test were used, respectively. Statistical analyses were performed with statistical packages R (v3.1.3) and SAS (v9.4).
Results
A total of 281 patients were screened, and 51 met the inclusion criteria (39 in the ipilumimab and 12 in chemotherapy cohorts). Baseline parameters were well matched between the cohorts (Table). Of the 39 patients in the ipilimumab cohort, 14 (35.9%) had bone pain during at least one of the treatment cycles, compared with 3 of the 12 patients (25%) in the chemotherapy cohort. At baseline, 4 of 38 ipilimumab patients (10.5%; 95% confidence interval [CI], 2.9-24.8) and 2 of 12 chemotherapy patients (16.7%; 95% CI, 2.1-48.4) had bone pain. Upon treatment completion, 9 of 33 ipilimumab patients (27.3%; 95% CI, 13.3-45.5) and 0 of 12 chemotherapy patients (0%; 95% CI, 0-26.5) had bone pain. The trend of proportion of patients with bone pain over time was statistically significant between the two cohorts (P = .023, Figure 1). The trends of proportion of patients with bone pain were not statistically significant when stratified by the presence of bone metastasis at inclusion in the study (P = .418) or disease progression at treatment completion (P = .500).
At baseline, the mean ALP level was 89.39 IU/L (95% CI, 81.03-97.75) in the ipilumimab cohort and 114.33 IU/L (95% CI, 69.48-159.19) in the chemotherapy cohort. Upon treatment completion, the mean ALP level was 123.09 IU/L (95% C.I. 80.78-165.41) in the ipilumimab cohort and 124.24 IU/L (95% C.I. 90.88-157.62) in the chemotherapy cohort. The trend of mean ALP level over time was not statistically significant between the 2 cohorts (P = .653, Figure 2).
Discussion
Immune checkpoints are inhibitory pathways that are critical for maintenance of self-tolerance and regulation of appropriate immune response. CTLA-4 is present exclusively on T cells and interacts with its ligands B7.1 and B7.2. CTLA-4 competes with CD28 in binding with B7, leading to dampening of T-cell activation and function.5,6 Development of checkpoint inhibitors such as ipilumimab have heralded a new era of immune targeted therapies for various malignancies including malignant melanoma.
Bone remodeling involves 4 distinct but overlapping phases. The first phase involves detection of loss of bone continuity by osteocytes and activation of osteoclast precursors derived from progenitors of the monocyte-macrophage lineage. The second phase involves osteoclast-medicated bone resorption and concurrent recruitment of mesenchymal stem cells and osteoprogenitors. The third phase involves osteoblast differentiation and osteoid synthesis, and the fourth phase results in mineralization of osteoid and termination of bone remodeling.7,8
The role of T-lymphocytes and cytokines, such as IL-1 and TNF-α, and receptor activator of NF-κB ligand (RANK-L) in osteoclastogenesis is well studied. RANK-L is considered to be the final downstream effector of this process.9 T-lymphocytes have also been shown to promote osteoblast maturation and function.9,10 These findings suggest a significant interaction between immune system activation and bone remodeling.
The search for a reliable biomarker for immune therapy is ongoing. Although ipilumimab-associated immune-related adverse events have been suggested to predict response to therapy,11 there is considerable debate on the subject. Ipilumimab’s impact on bone remodeling could offer a solution.
In the current study, there was a statistically significant difference in proportion of patients with bone pain in the 2 cohorts. This was preserved with stratification based on bone metastasis at inclusion and disease progression on treatment completion making new or worsening skeletal metastasis. Furthermore, the proportion of patients with bone pain increased with each cycle for ipilumimab cohort. However, we were unable to detect an association between bone pain and response to ipilimumab.
We were not able to detect a difference in trend of mean ALP level with treatment in the two cohorts. Although it is possible that no such association exists, we believe our study was not powered to detect it. Finally, we were not able to study markers for osteoblast (bone-specific ALP) and osteoclasts (N- and C-telopeptides of type 1 collagen, deoxypyridinoline, etc) to better assess this interaction because they are not commonly clinically used.
Regarding the limitations of our study, we chose to dichotomize the patient-reported bone pain because it is a subjective measure and there is a significant variability of the perceived pain intensity among patients. We also excluded patients with hepatitis from receiving the ipilumimab therapy and those with known hepatic disease from the study to reduce the impact of hepatic ALP on total serum ALP levels.
In conclusion, as far as we know, this is the first clinical report suggesting a possible relationship between CTLA-4 inhibition and bone remodeling. Supported by a strong preclinical rationale, this side effect remains under-studied and under-recognized by clinicians. A prospective assessment of this interaction using bone specific markers is planned.
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is an important component of the immune checkpoint pathway. CTLA-4 inhibition causes T-cell activation and proliferation, increases T-cell responsiveness, and enhances the anti-tumor immune response. CTLA-4 inhibition also results in immune-related adverse reactions such as colitis, hepatitis, and endocrinopathies. Preclinical investigations have recently shown that CTLA-4 inhibition can cause cytokine-mediated increase in bone remodeling.1,2(p4) Ipilimumab, a recombinant IgG1 kappa antibody against human CTLA-4, has been approved for use in unresectable or metastatic melanoma. We hypothesize that ipilumumab results in increase in bone remodeling manifesting as an autoimmune reaction.
Methods
We conducted a retrospective case-control study of patients with stage III/IV melanoma treated at the University of New Mexico Comprehensive Cancer Center during April 2009-July 2014. The university’s Institutional Review Board approved the study.
Two cohorts were compared: an ipilumimab cohort receiving ipilumimab at 3 mg/kg every 3 weeks, and a chemotherapy cohort receiving an investigational chemotherapy regimen: carboplatin IV at an area under curve of 5 on day 1, paclitaxel IV at 175 mg/m2 on day 1, and temozolomide orally at 125 mg/m2 daily on days 2 to 6 every 21 days. Patients receiving at least 1 cycle of treatment were included. Those with known hepatic disease or concurrent malignancy were excluded from the study.
Serum ALP level (normal range, 38-150 international units per liter [IU/L]) and patient-reported bone pain measured by the 11-point numeric rating scale (NRS) for pain assessment were recorded before treatment initiation, on each cycle, and upon treatment completion.3 Clinical response was assessed per RECIST guidelines.4 Bone pain was dichotomized into Absent (pain intensity of 0 on the NRS, meaning no pain) or Present (pain intensity of 1-10 on the NRS, with 1 = mild pain and 10 = worst imaginable pain). Patients with a complete or partial response to the therapy were categorized as responders, and those with progressive or stable disease were categorized as nonresponders.
Descriptive statistics were generated for demographic and clinical characteristics. The primary outcome variables of interest were bone pain and mean ALP levels. Generalized linear mixed-effect models for proportion of patients with bone pain (with logit link function) and mean ALP levels (with identify link function) were used to evaluate for a difference in trends between the two cohorts over time. We used the Kenward-Roger approach to adjust for the small size of the degrees of freedom. To assess the significance of difference of the proportions of patients with bone pain and the mean ALP levels between responders and nonresponders in the ipilumimab cohort, the Fisher exact test and Wilcoxon rank-sum test were used, respectively. Statistical analyses were performed with statistical packages R (v3.1.3) and SAS (v9.4).
Results
A total of 281 patients were screened, and 51 met the inclusion criteria (39 in the ipilumimab and 12 in chemotherapy cohorts). Baseline parameters were well matched between the cohorts (Table). Of the 39 patients in the ipilimumab cohort, 14 (35.9%) had bone pain during at least one of the treatment cycles, compared with 3 of the 12 patients (25%) in the chemotherapy cohort. At baseline, 4 of 38 ipilimumab patients (10.5%; 95% confidence interval [CI], 2.9-24.8) and 2 of 12 chemotherapy patients (16.7%; 95% CI, 2.1-48.4) had bone pain. Upon treatment completion, 9 of 33 ipilimumab patients (27.3%; 95% CI, 13.3-45.5) and 0 of 12 chemotherapy patients (0%; 95% CI, 0-26.5) had bone pain. The trend of proportion of patients with bone pain over time was statistically significant between the two cohorts (P = .023, Figure 1). The trends of proportion of patients with bone pain were not statistically significant when stratified by the presence of bone metastasis at inclusion in the study (P = .418) or disease progression at treatment completion (P = .500).
At baseline, the mean ALP level was 89.39 IU/L (95% CI, 81.03-97.75) in the ipilumimab cohort and 114.33 IU/L (95% CI, 69.48-159.19) in the chemotherapy cohort. Upon treatment completion, the mean ALP level was 123.09 IU/L (95% C.I. 80.78-165.41) in the ipilumimab cohort and 124.24 IU/L (95% C.I. 90.88-157.62) in the chemotherapy cohort. The trend of mean ALP level over time was not statistically significant between the 2 cohorts (P = .653, Figure 2).
Discussion
Immune checkpoints are inhibitory pathways that are critical for maintenance of self-tolerance and regulation of appropriate immune response. CTLA-4 is present exclusively on T cells and interacts with its ligands B7.1 and B7.2. CTLA-4 competes with CD28 in binding with B7, leading to dampening of T-cell activation and function.5,6 Development of checkpoint inhibitors such as ipilumimab have heralded a new era of immune targeted therapies for various malignancies including malignant melanoma.
Bone remodeling involves 4 distinct but overlapping phases. The first phase involves detection of loss of bone continuity by osteocytes and activation of osteoclast precursors derived from progenitors of the monocyte-macrophage lineage. The second phase involves osteoclast-medicated bone resorption and concurrent recruitment of mesenchymal stem cells and osteoprogenitors. The third phase involves osteoblast differentiation and osteoid synthesis, and the fourth phase results in mineralization of osteoid and termination of bone remodeling.7,8
The role of T-lymphocytes and cytokines, such as IL-1 and TNF-α, and receptor activator of NF-κB ligand (RANK-L) in osteoclastogenesis is well studied. RANK-L is considered to be the final downstream effector of this process.9 T-lymphocytes have also been shown to promote osteoblast maturation and function.9,10 These findings suggest a significant interaction between immune system activation and bone remodeling.
The search for a reliable biomarker for immune therapy is ongoing. Although ipilumimab-associated immune-related adverse events have been suggested to predict response to therapy,11 there is considerable debate on the subject. Ipilumimab’s impact on bone remodeling could offer a solution.
In the current study, there was a statistically significant difference in proportion of patients with bone pain in the 2 cohorts. This was preserved with stratification based on bone metastasis at inclusion and disease progression on treatment completion making new or worsening skeletal metastasis. Furthermore, the proportion of patients with bone pain increased with each cycle for ipilumimab cohort. However, we were unable to detect an association between bone pain and response to ipilimumab.
We were not able to detect a difference in trend of mean ALP level with treatment in the two cohorts. Although it is possible that no such association exists, we believe our study was not powered to detect it. Finally, we were not able to study markers for osteoblast (bone-specific ALP) and osteoclasts (N- and C-telopeptides of type 1 collagen, deoxypyridinoline, etc) to better assess this interaction because they are not commonly clinically used.
Regarding the limitations of our study, we chose to dichotomize the patient-reported bone pain because it is a subjective measure and there is a significant variability of the perceived pain intensity among patients. We also excluded patients with hepatitis from receiving the ipilumimab therapy and those with known hepatic disease from the study to reduce the impact of hepatic ALP on total serum ALP levels.
In conclusion, as far as we know, this is the first clinical report suggesting a possible relationship between CTLA-4 inhibition and bone remodeling. Supported by a strong preclinical rationale, this side effect remains under-studied and under-recognized by clinicians. A prospective assessment of this interaction using bone specific markers is planned.
1. Bozec A, Zaiss MM, Kagwiria R, et al. T-cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
2. Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F. EphB4 promotes osteogenesis of CTLA 4-modified bone marrow-derived mesenchymal stem cells through cross talk with wnt pathway in xenotransplantation. Tissue Eng Part A. 2015;21(17-18):2404-2416.
3. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158.
4. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
6. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
7. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139.
8. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-145.
9. Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9(2):103.
10. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109-117.
11. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681-6688.
1. Bozec A, Zaiss MM, Kagwiria R, et al. T-cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
2. Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F. EphB4 promotes osteogenesis of CTLA 4-modified bone marrow-derived mesenchymal stem cells through cross talk with wnt pathway in xenotransplantation. Tissue Eng Part A. 2015;21(17-18):2404-2416.
3. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158.
4. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
6. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
7. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139.
8. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-145.
9. Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9(2):103.
10. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109-117.
11. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681-6688.
Management of polycythemia vera in the community oncology setting
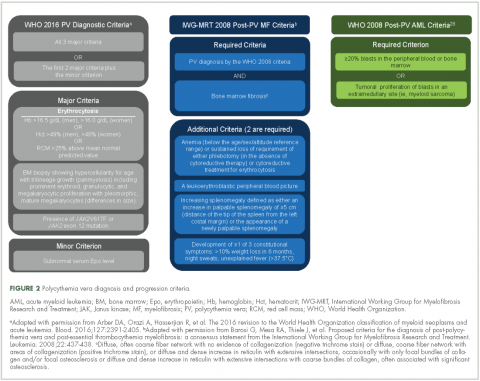
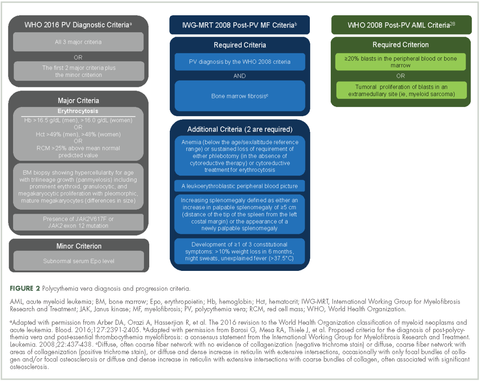
Polycythemia vera, classified as a myeloproliferative neoplasm (MPN) and characterized by uncontrolled, clonal, myeloid expansion with predominant erythrocytosis,1 affects about 100,000 individuals in the United States.2 It is a chronic and burdensome disease associated with shortened survival.3 Patients are at an increased risk of cardiovascular events, solid tumors, and transformation to myelofibrosis (MF) and/or acute myeloid leukemia (AML).4,5 Furthermore, patients generally have a reduced quality of life (QoL) stemming from prevalent and occasionally severe polycythemia vera–related signs and symptoms, including fatigue, pruritus, and splenomegaly.6 In general, the classical Philadelphia chromosome-negative MPNs are associated with driver mutations in the following three genes: Janus kinase 2 (JAK2), calreticulin (CALR), and myeloproliferative leukemia virus oncogene (MPL).7 Almost all patients with polycythemia vera have an activating mutation in the cytoplasmic signal transduction protein JAK2.4 Patients with essential thrombocythemia (ET) or MF can have mutations in JAK2, CALR, or MPL. However, CALR and MPL mutations are absent or exceedingly rare in patients with polycythemia vera.7 Diagnosis can be challenging and is currently based on 2016 World Health Organization (WHO) diagnostic criteria.1
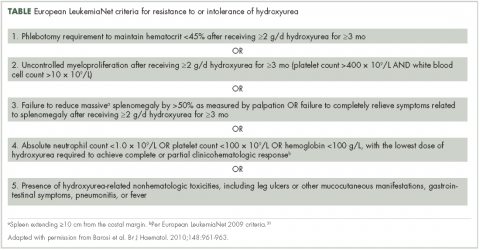
Management strategies include the use of aspirin, phlebotomy, and cytoreductive therapy. Ruxolitinib is a newer treatment option available for patients with polycythemia vera who are either resistant to or intolerant of hydroxyurea8,9— a population that previously had few treatment options. It is important for community oncologists and other treating clinicians to understand current diagnostic strategy and management options based on established guidelines, recent clinical evidence, and regulatory updates.
Search and selection process for research sources
In September 2016, we searched PubMed for articles published since 2006 with polycythemia vera included in the abstract or title. The initial 1,730 publications were screened by eye to select 46 key articles that guide current management of polycythemia vera. Four studies published before 2006 were also included based on their continued relevance.
Epidemiology and pathophysiology
Based on a meta-analysis of patients from Europe and the United States, the annual incidence of polycythemia vera estimated to be between 0.7 and 2.6 per 100,000 people.10 The age-adjusted prevalence of polycythemia vera in the United States is about 45-57 per 100,000 people,2 however, the true prevalence might be considerably greater.
Patients with polycythemia vera are at increased risk of cardiovascular events, thrombosis, and death.3-5 Risk is highest among patients older than 60 years or with a history of thrombosis.11 Uncontrolled myeloproliferation has also been identified as a risk factor for cardiovascular mortality and thrombosis. This was demonstrated in the prospective Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial, which reported more cardiovascular events in patients with hematocrit levels of 45%-50%, compared with those whose hematocrit levels were <45%.12 In addition, retrospective data suggest leukocytosis is a potential risk factor for thromboembolic events and poor outcomes.13
Dysregulated JAK2 signaling is the principal driver of polycythemia vera pathophysiology. About 95% of patients with polycythemia vera will have an identifiable JAK2 V617F exon 14 mutation, with an additional 3%-5% demonstrating a JAK2 exon 12 mutation.4,14 Under physiologic conditions, JAK2 interacts with the STAT family of signal transduction proteins and serves as an important regulator of normal hematopoiesis.15 Mutated, constitutively activated JAK2 signaling promotes the various polycythemia vera disease manifestations, including excessive myeloproliferation, splenomegaly,15 and constitutional symptoms.14,16,17
Burden of disease for the individual
Mortality
Patients with polycythemia vera have an increased risk of mortality compared with an unaffected, age- and gender-matched cohort of the general population.3 A retrospective study of Medicare patients with polycythemia vera (mean age at diagnosis, 76.1 years) reported a median survival of 5.4 years, compared with 8.7 years for a matched cohort.3 A second retrospective study reported a median survival of 13.5 years (median age at diagnosis, 64 years; median follow-up time, 11.8 years).18
Leading causes of death for patients with polycythemia vera include cardiovascular and thrombotic events, the development of secondary solid tumors, and disease transformation to MF and/or AML. In the prospective European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP) study of 1,638 patients, 45% of deaths (74/164) resulted from cardiovascular causes (1.7 per 100 patient-years).5 Thirteen percent of deaths were related to either leukemic or myelofibrotic transformation, and 20% of deaths were attributed to secondary solid tumors.5 In a retrospective analysis of 1,545 patients with polycythemia vera followed for a median of 6.9 years after diagnosis, 347 had died, primarily from acute leukemia (10%), secondary malignancies (10%), and thrombotic events (9%).4 Arterial and venous thrombotic events occurred in 12% and 9% of patients, respectively, with disease transformation to MF and AML occurring in 9% and 3% of patients. Further support of an increased risk of secondary malignancies comes from a retrospective analysis of a large Swedish cancer registry (1958–2006) that found an increased risk of secondary endocrine, renal, and skin malignancies; MF; and leukemia among patients with polycythemia vera.19
Symptoms and quality of life
Symptoms of polycythemia vera vary in severity, and patients often fail to attribute symptoms to the disease.20 Moreover, clinicians may underestimate a patient’s true disease burden or the effect it has on QoL.20 Point-of-care metrics, such as the MPN Symptom Assessment Form (MPN-SAF), were developed to aid in identifying and grading symptom burden. Studies using this metric have reported fatigue as the most common and most severe symptom (incidence, 73%-92%), with a variety of other symptoms also affecting a majority of patients (Figure 1).6,21-24 Although fatigue, pruritus, and a higher MPN-SAF total symptom score are significantly correlated with reduced QoL,22,25 the recent MPN Landmark survey suggests that even patients with low symptom severity scores have a reduction in their QoL.6 This study also highlighted that polycythemia vera can adversely affect multiple aspects of daily living: 48% of patients reported disease interfering with daily activities; 63% with family or social life; and 37% with employment, feeling compelled to work reduced hours.6
Splenomegaly is a common feature of polycythemia vera, affecting an estimated one in three patients, which may result in discomfort and early satiety.4
Identification and diagnosis
Most patients diagnosed with polycythemia vera are between the ages of 60 and 76 years,3-5 although about 25% are diagnosed before age 50.4 Tefferi and colleagues reported in a retrospective study that common features at presentation include JAK2 mutations (98%), elevated hemoglobin (73%), endogenous erythroid colony growth (73%), white blood cell count of >10.5 × 109/L (49%), and platelet count of ≥450 × 109/L (53%).4 In that same study, about a third of patients presented with a palpable spleen or polycythemia vera–related symptoms, including pruritus and vasomotor symptoms. However, many patients were asymptomatic at presentation, diagnosed incidentally by abnormal laboratory values.4 Patients can present with vascular thrombosis, occasionally involving atypical sites (eg, Budd-Chiari syndrome, other abdominal blood clots),26 thus, a heightened awareness and testing for JAK2 mutations may be appropriate in the evaluation of such individuals.
Evidence suggests many clinicians may not rigidly apply the WHO diagnostic criteria to establish a diagnosis.1,27,28 A recent retrospective claims analysis showed that only 40% of 121 patients diagnosed with polycythemia vera met the 2008 WHO diagnostic criteria, and for some patients, the diagnosis was based solely on the presence of the JAK2 V617F mutation.29 One should be aware of individuals with “masked” polycythemia vera, who may present with characteristic polycythemia vera features but have hemoglobin levels below those established by the WHO in 2008, typically owing to iron deficiency and/or a disproportionate expansion of plasma volume.30 To improve polycythemia vera diagnosis, the WHO diagnostic criteria were updated in 2016 with reduced hemoglobin diagnostic thresholds (Figure 2).1
Management strategy
Treatment goals
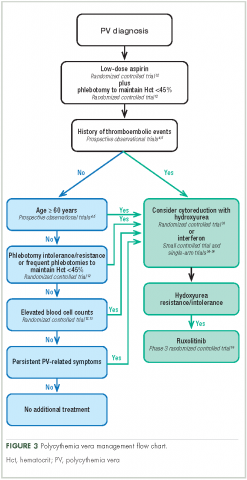
The primary polycythemia vera–treatment goals are to reduce the risk of cardiovascular, thrombotic, and hemorrhagic events; reduce the risk of fibrotic and/or leukemic transformation; and alleviate polycythemia vera–related symptoms.11,31
Traditional treatment options
Aspirin. To reduce the risk of death from cardiovascular events, patients with polycythemia vera should receive low-dose aspirin32 and undergo phlebotomy to maintain a target hematocrit <45%, as established by the ECLAP and CYTO-PV trials (Figure 3).4,5,12,13,16,32-36 Higher doses of aspirin (ie, 325 mg 2 or more times a week) are associated with a dose-dependent increased risk of gastrointestinal bleeding.37 Low-dose aspirin is generally well tolerated; however, patients with extreme thrombocytosis may develop bleeding as a consequence of a well-described, thrombocytosis-associated acquired von Willebrand disease.11,38
Phlebotomy. This procedure is generally tolerated by most patients, although it can occasionally engender extreme anxiety in some patients39 and may promote clinical manifestations of iron deficiency, including restless leg syndrome,40 impaired cognition, and worsening of fatigue.41 In the CYTO-PV study, 28% of patients with a target hematocrit 45%-50% discontinued phlebotomy treatment, although the percentage that discontinued because of poor tolerance was not reported.12 To avoid potential complications in patients with underlying cardiovascular disease, smaller-volume phlebotomies are often pursued.42
Cytoreductive therapy. Cytoreductive therapy with hydroxyurea or interferon (IFN) is recommended for high-risk patients (ie, those with a history of thrombosis or older than 60 years) as well as those with intolerable symptoms, progressive splenomegaly, or a burdensome phlebotomy requirement.11,31 Hydroxyurea is the typical first-line cytoreductive therapy11 based on clinical benefit,33,43 low cost, and feasibility of long-term treatment.33
Most patients benefit from long-term treatment with hydroxyurea; however, 25% develop resistance to or intolerance of hydroxyurea therapy.44 Intolerance typically manifests as leg ulcers or other mucocutaneous toxicity, gastrointestinal side effects, or fever.44 Resistance to hydroxyurea is defined as failure to achieve phlebotomy independence, persistent leukothrombocytosis or splenomegaly despite adequate doses of hydroxyurea, or inability to deliver the drug owing to dose-limiting cytopenias. The European LeukemiaNet (ELN) formally codified and published a definition of hydroxyurea resistance/intolerance45 (Table), which can be used to identify patients at high risk of poor outcomes.44 In a retrospective chart review of 261 patients with polycythemia vera, those meeting the ELN definition of hydroxyurea resistance had a 5.6-fold greater risk of mortality and a 6.8-fold increased risk of fibrotic and/or leukemic disease transformation.44
The use of IFN-α and pegylated variants are associated with clinical benefit, including normalization of blood counts, reduction of splenomegaly, symptom mitigation, and reduction in JAK2 V617F allele burden.46 However, poor tolerance46 and an inconvenient route of administration often preclude the long-term use of these agents. Adverse events associated with IFN-α include chills, depression, diarrhea, fatigue, fever, headache, musculoskeletal pain, myalgia, nausea, and weight loss.46 In clinical trials, recombinant IFN-α discontinuation rates within the first year of administration were as high as 29% and may have been dose dependent.46
Traditional treatment options may not effectively alleviate polycythemia vera–related symptoms.23,47 Two prospective studies failed to show an improvement in patient-reported MPN-SAF scores after treatment with hydroxyurea, aspirin, phlebotomy, IFN-α, busulfan, or radiophosphorus,23,47 and symptoms may worsen with the use of IFN-α.47
Allogenic transplantation. Although allogeneic transplantation is a potentially curative treatment option, it has been reserved primarily for younger patients with MPNs (age <60 years31). Furthermore, a recent systematic review concluded that overall survival was worse following allogeneic transplantation compared with a nontransplant approach (ie, phlebotomy and aspirin).48
Ruxolitinib. The oral JAK1/JAK2 inhibitor ruxolitinib has been approved by the US Food and Drug Administration (FDA) for the treatment of patients with polycythemia vera who have had an inadequate response to or are intolerant of hydroxyurea,8 and by the European Medicines Agency (EMA) for adult patients with polycythemia vera who are resistant to or intolerant of hydroxyurea.9 Ruxolitinib is also approved by the FDA for patients with intermediate- or high-risk MF, including primary MF, post-polycythemia vera MF, and post-essential thrombocythemia MF,8 and for similar patient populations by the EMA.9
Approval of ruxolitinib for the treatment of patients with polycythemia vera was based on the phase 3 randomized, open-label, multicenter RESPONSE trial in which 222 patients with polycythemia vera who met the modified ELN criteria for hydroxyurea resistance or intolerance (Table)16,31 were randomized to ruxolitinib or best available therapy (BAT). Compared with BAT, a greater proportion of patients treated with ruxolitinib achieved the primary composite endpoint of hematocrit control without the need for phlebotomy and ≥35% reduction in spleen volume by week 32 (22.7% vs 0.9%; P < .001).16,49 When looked at individually, hematocrit control and reduction in spleen size favored ruxolitinib over BAT (hematocrit control, 60.0% vs 18.8%, ruxolitinib and BAT, respectively; ≥35% reduction in spleen volume, 40.0% vs 0.9%). Furthermore, more patients receiving ruxolitinib achieved the key secondary endpoint of complete hematologic remission than did those receiving BAT (ie, normalization of blood counts; 23.6% vs 8.0%; P = .0016).16,49 Of note is that most patients who achieved primary treatment responses maintained disease control for ≥80 weeks.49
Results from RESPONSE indicate that ruxolitinib may substantially improve polycythemia vera–related symptoms. Treatment with ruxolitinib was associated with a greater improvement in nearly all symptoms evaluated by the MPN-SAF as well as greater improvements in QoL and functional measures with the EORTC QLQ-C30 trial metric compared with BAT (Figure 1).16 In addition, a post hoc exploratory analysis of RESPONSE indicated that patients receiving ruxolitinib showed a rapid normalization of abnormal iron indices at baseline, compared with those receiving BAT.50
Treatment safety and tolerability are particularly important considerations for patients with polycythemia vera, given the long natural history of the disease. In a preplanned analysis of RESPONSE at 80 weeks, 83% of patients randomized to receive treatment with ruxolitinib remained on treatment (median exposure, 111 weeks).49 Most adverse events reported in both treatment arms were grade 1/2.16,49 The most frequent nonhematologic adverse events (per 100 patient-years of exposure) in the ruxolitinib arm were headache (10.5%), diarrhea (9.7%), pruritus (9.7%), and fatigue (8.3%). The most common grade 3/4 nonhematologic adverse events (occurring at a rate of ≥0.9 per 100 patient-years of exposure) were limited to dyspnea (1.3%), abdominal pain (0.9%), headache (0.9%), and herpes zoster (0.9%).49 Hematologic adverse event rates in the ruxolitinib and BAT arms included anemia (any grade, 27.2% vs 47.6%, respectively; grade 3/4, 0.9% vs 0%), lymphopenia (27.2% vs 78.8%; 9.7% vs 27.2%), and thrombocytopenia (14.9% vs 29.9%; 2.6% vs 5.4%).49 Herpes zoster infections occurred more frequently in the ruxolitinib arm (any grade, 5.3%; grade 3/4, 0.9%) compared with the BAT arm (no herpes zoster events).49 There was a higher rate of nonmelanoma skin cancer (NMSC) in the ruxolitinib arm (4.4%), compared with the BAT arm (2.7%),49 most of which occurred in patients with a history of NMSC or precancerous skin lesions.16 Grade 1 or 2 elevations in serum lipids and cholesterol were observed with ruxolitinib but not BAT; however, subsequent effects on patient outcomes have not been determined.8,16 The rates of MF and AML transformations were 1.3% and 0.4%, respectively, in patients randomized to receive ruxolitinib,49 similar to previously published reports for patients with polycythemia vera.44
Additional insight regarding the effect of ruxolitinib on polycythemia vera–related symptoms is available from the RELIEF trial, a randomized, multicenter, double-blind, double-dummy, phase 3b clinical trial. In RELIEF, 110 patients were randomized to receive ruxolitinib or a stable dose of hydroxyurea and were then asked to record disease-related symptoms.51 Although the study failed to meet its primary endpoint (a ≥50% improvement by week 16 in MPN-SAF total symptom score for the cytokine symptom cluster [sum of individual scores for tiredness, itching, muscle aches, night sweats, and sweats while awake]), a numerically greater proportion of patients receiving ruxolitinib achieved the primary endpoint compared with those receiving hydroxyurea (43.4% and 29.6%, respectively; P = .139; odds ratio, 1.82; 95% confidence interval, 0.82-4.04). Similarly, the proportion of patients reporting a ≥50% improvement in pruritus and fatigue favored ruxolitinib over hydroxyurea (itching, 40.0% vs 26.4%; tiredness, 54.2% vs 32.0%). The safety profile for ruxolitinib was similar to that reported in the RESPONSE trial.
Possible future treatment options
Other possible treatment options for patients with polycythemia vera that are currently in clinical development include three pegylated IFN-α (PEG-IFN-α) variants and the telomerase inhibitor, imetelstat.
Pegylated interferon-α. PEG–IFN-α has the advantage of a longer plasma half-life compared with conventional IFN-α, permitting administration once per week or less often.46 Currently, three variants are under active investigation in phase 3 clinical trials: PEG–IFN-α2a (NCT01259856 and NCT01387763), PEG–IFN-α2b (NCT01387763), and AOP2014/P1101 (NCT02218047, NCT02523638, and NCT01949805).
Imetelstat. The telomerase inhibitor imetelstat is in clinical development for patients with MPNs. Clinical benefit was previously observed in patients with primary MF as well as post-polycythemia vera and post-ET MF.52 Imetelstat was evaluated in a phase 2 trial in patients with polycythemia vera or ET who required cytoreductive therapy and were resistant to or intolerant of ≥1 previous line of therapy or who refused standard therapy (NCT01243073). Results in patients with ET were published,53 however, findings from the polycythemia vera cohort have not been reported.
Community oncologist role in managing disease burden
Most patients with polycythemia vera are managed in the community setting. Consequently, the community oncologist plays a critical role in the initial diagnosis, risk stratification, patient education, and disease management.
Early disease recognition allows prompt therapeutic intervention with low-dose aspirin and phlebotomy, interventions shown to reduce the risk of cardiovascular events based on the ECLAP32 and CYTO-PV trials,12 respectively. The diagnosis of polycythemia vera is facilitated by applying the WHO diagnostic criteria (Figure 2);1 however, one should be aware of atypical presentations, including “masked” polycythemia vera,30 as well as the development of thrombosis at atypical sites.26
Optimal management strategies must include the frequent assessment of symptom burden and its effect on a patient’s QoL, with a keen awareness of the nonspecific nature of polycythemia vera–related symptoms, and the potential for patients and clinicians to minimize that effect.20 Patient-reported symptom severity and QoL should be assessed at each office visit with validated instruments, such as the MPN-SAF 10-item questionnaire.22It is important for the community oncologist to define treatment goals and implement a plan that reduces disease-associated morbidity and mortality. A critical treatment goal is to maintain hematocrit <45% by the appropriate use of phlebotomy12 and/or cytoreductive agents.12,16,46 Continued reassessment is important to identify patients with progressive disease and those who fail to achieve stated treatment goals or require an adjustment in cytoreductive therapy. Oncologists should be familiar with the concept of hydroxyurea resistance/intolerance as defined by the ELN (Table)31,45 to allow early identification of those patients who are most likely to benefit from a treatment change for continued optimal outcome.
Conclusions
Polycythemia vera is a clonal myeloproliferative neoplasm associated with significant disease-related morbidity and mortality. Appropriate management includes early diagnosis and implementation of appropriate therapy according to patient risk and therapeutic tolerance. Patients should initially receive aspirin32 and phlebotomy,12 with the goal of maintaining hematocrit <45%. Higher-risk patients and those who had inadequate disease control with phlebotomy alone require cytoreduction, typically with hydroxyurea. Although most patients will achieve adequate disease control with hydroxyurea,33,43 one in four patients will develop drug resistance or intolerance.44 Ruxolitinib is approved by the FDA and the EMA for the treatment of patients with polycythemia vera who are resistant to or intolerant of hydroxyurea. Compared with BAT, ruxolitinib is associated with improved hematocrit control, reductions in spleen size, a greater probability of blood count normalization, and improvement in polycythemia vera–related symptoms.16
Acknowledgments
Writing assistance was provided by Cory Pfeiffenberger, PhD, of Complete Healthcare Communications LLC, and was funded by Incyte Corporation, the maker of ruxolitinib.
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
2. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55:595-600.
3. Price GL, Davis KL, Karve S, Pohl G, Walgren RA. Survival patterns in United States (US) Medicare enrollees with non-CML myeloproliferative neoplasms (MPN). PLoS ONE. 2014;9:e90299.
4. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27:1874-1881.
5. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224-2232.
6. Mesa R, Miller CB, Thyne M, et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the MPN Landmark survey. BMC Cancer. 2016;16:167.
7. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379-2390.
8. JAKAFI (ruxolitinib). Full Prescribing Information, Incyte Corporation, Wilmington, DE, USA, 2016.
9. JAKAVI (ruxolitinib). Summary of Product Characteristics, Novartis Pharma GmbH, Nuremberg, Germany, 2015.
10. Johansson P. Epidemiology of the myeloproliferative disorders polycythemia vera and essential thrombocythemia. Semin Thromb Hemost. 2006;32:171-173.
11. Vannucchi AM. How I treat polycythemia vera. Blood. 2014;124:3212-3220.
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22-33.
13. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126:560-561.
14. Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24:1574-1579.
15. Quintás-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10:127-140.
16. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372:426-435.
17. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952-1959.
18. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly-annotated essential thrombocythemia, polycythemia vera and myelofibrosis. Blood. 2014;124:2507-2513.
19. Fallah M, Kharazmi E, Sundquist J, Hemminki K. Higher risk of primary cancers after polycythaemia vera and vice versa. Br J Haematol. 2011;153:283-285.
20. Mesa R, Miller C, Thyne M, et al. Differences in treatment goals and perception of symptom burden between patients with MPNs and hematologists/oncologists in the United States: findings from the MPN landmark survey. Cancer. 2017;123:449-458.
21. Abelsson J, Andreasson B, Samuelsson J, et al. Patients with polycythemia vera have the worst impairment of quality of life among patients with newly diagnosed myeloproliferative neoplasms. Leuk Lymphoma. 2013;54:2226-2230.
22. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative Neoplasm (MPN) Symptom Assessment Form Total Symptom Score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098-4103.
23. Johansson P, Mesa R, Scherber R, et al. Association between quality of life and clinical parameters in patients with myeloproliferative neoplasms. Leuk Lymphoma. 2012;53:441-444.
24. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401-408.
25. Siegel FP, Tauscher J, Petrides PE. Aquagenic pruritus in polycythemia vera: characteristics and influence on quality of life in 441 patients. Am J Hematol. 2013;88:665-669.
26. Smalberg JH, Arends LR, Valla DC, Kiladjian JJ, Janssen HL, Leebeek FW. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood. 2012;120:4921-4928.
27. Barosi G, Mesa RA, Thiele J, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22:437-438.
28. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937-951.
29. Roda P, Ferrari A, Tang X, et al. Determination of accuracy of polycythemia vera diagnoses and use of the JAK2V617F test in the diagnostic scheme. Ann Hematol. 2014;93:1467-1472.
30. Barbui T, Thiele J, Gisslinger H, et al. Masked polycythemia vera (mPV): results of an international study. Am J Hematol. 2014;89:52-54.
31. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29:761-770.
32. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:114-124.
33. Kiladjian JJ, Chevret S, Dosquet C, Chomienne C, Rain JD. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol. 2011;29:3907-3913.
34. Quintás-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27:5418-5424.
35. Sacchi S, Leoni P, Liberati M, et al. A prospective comparison between treatment with phlebotomy alone and with interferon-alpha in patients with polycythemia vera. Ann Hematol. 1994;68:247-250.
36. Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer. 2006;107:451-458.
37. Huang ES, Strate LL, Ho WW, Lee SS, Chan AT. Long-term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011;124:426-433.
38. Shetty S, Kasatkar P, Ghosh K. Pathophysiology of acquired von Willebrand disease: a concise review. Eur J Haematol. 2011;87:99-106.
39. Deacon B, Abramowitz J. Fear of needles and vasovagal reactions among phlebotomy patients. J Anxiety Disord. 2006;20:946-960.
40. Tobiasson M, Alyass B, Soderlund S, Birgegard G. High prevalence of restless legs syndrome among patients with polycytemia vera treated with venesectio. Med Oncol. 2010;27:105-107.
41. Greig AJ, Patterson AJ, Collins CE, Chalmers KA. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.
42. Passamonti F. How I treat polycythemia vera. Blood. 2012;120:275-284.
43. Fruchtman SM, Mack K, Kaplan ME, Peterson P, Berk PD, Wasserman LR. From efficacy to safety: a Polycythemia Vera Study Group report on hydroxyurea in patients with polycythemia vera. Semin Hematol. 1997;34:17-23.
44. Alvarez-Lárran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood. 2012;119:1363-1369.
45. Barosi G, Birgegard G, Finazzi G, et al. A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: results of a European LeukemiaNet (ELN) consensus process. Br J Haematol. 2010;148:961-963.
46. Hasselbalch HC. A new era for IFN-α in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. Expert Rev Hematol. 2011;4:637-655.
47. Emanuel R, Dueck AC, Kiladjian JJ, et al. Conventional therapeutic options have limited impact on MPN symptoms: insights from a prospective analysis of the MPN-SAF [abstract 366]. Presented at: European Hematology Association, June 14-17, 2012; Amsterdam, Netherlands.
48. Lacevic J, Reljic T, El Jurdi N, et al. Conservative management vs. allogeneic hematopoietic cell transplantation for polycythemia vera: a systematic review and decision-analysis. Blood. 2013;122:abstract 5372.
49. Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80 week follow-up from the RESPONSE trial. Haematologica. 2016;101:821-829.
50. Verstovsek S, Harrison CN, Kiladjian J-J, et al. Effect of ruxolitinib on markers of iron deficiency: an analysis of the RESPONSE trial. Haematologica (EHA Annual Meeting Abstracts). 2015;100:abstract P672.
51. Mesa R, Vannucchi AM, Yacoub A, et al. The efficacy and safety of continued hydroxyurea therapy versus switching to ruxolitinib in patients with polycythemia vera: a randomized, double-blind, double-dummy, symptom study (RELIEF). Blood (ASH Annual Meeting Abstracts). 2014;124:abstract 3168.
52. Tefferi A, Lasho TL, Begna KH, et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N Engl J Med. 2015;373:908-919.
53. Baerlocher GM, Oppliger Leibundgut E, Ottmann OG, et al. Telomerase inhibitor imetelstat in patients with essential thrombocythemia. N Engl J Med. 2015;373:920-928.
Polycythemia vera, classified as a myeloproliferative neoplasm (MPN) and characterized by uncontrolled, clonal, myeloid expansion with predominant erythrocytosis,1 affects about 100,000 individuals in the United States.2 It is a chronic and burdensome disease associated with shortened survival.3 Patients are at an increased risk of cardiovascular events, solid tumors, and transformation to myelofibrosis (MF) and/or acute myeloid leukemia (AML).4,5 Furthermore, patients generally have a reduced quality of life (QoL) stemming from prevalent and occasionally severe polycythemia vera–related signs and symptoms, including fatigue, pruritus, and splenomegaly.6 In general, the classical Philadelphia chromosome-negative MPNs are associated with driver mutations in the following three genes: Janus kinase 2 (JAK2), calreticulin (CALR), and myeloproliferative leukemia virus oncogene (MPL).7 Almost all patients with polycythemia vera have an activating mutation in the cytoplasmic signal transduction protein JAK2.4 Patients with essential thrombocythemia (ET) or MF can have mutations in JAK2, CALR, or MPL. However, CALR and MPL mutations are absent or exceedingly rare in patients with polycythemia vera.7 Diagnosis can be challenging and is currently based on 2016 World Health Organization (WHO) diagnostic criteria.1
Management strategies include the use of aspirin, phlebotomy, and cytoreductive therapy. Ruxolitinib is a newer treatment option available for patients with polycythemia vera who are either resistant to or intolerant of hydroxyurea8,9— a population that previously had few treatment options. It is important for community oncologists and other treating clinicians to understand current diagnostic strategy and management options based on established guidelines, recent clinical evidence, and regulatory updates.
Search and selection process for research sources
In September 2016, we searched PubMed for articles published since 2006 with polycythemia vera included in the abstract or title. The initial 1,730 publications were screened by eye to select 46 key articles that guide current management of polycythemia vera. Four studies published before 2006 were also included based on their continued relevance.
Epidemiology and pathophysiology
Based on a meta-analysis of patients from Europe and the United States, the annual incidence of polycythemia vera estimated to be between 0.7 and 2.6 per 100,000 people.10 The age-adjusted prevalence of polycythemia vera in the United States is about 45-57 per 100,000 people,2 however, the true prevalence might be considerably greater.
Patients with polycythemia vera are at increased risk of cardiovascular events, thrombosis, and death.3-5 Risk is highest among patients older than 60 years or with a history of thrombosis.11 Uncontrolled myeloproliferation has also been identified as a risk factor for cardiovascular mortality and thrombosis. This was demonstrated in the prospective Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial, which reported more cardiovascular events in patients with hematocrit levels of 45%-50%, compared with those whose hematocrit levels were <45%.12 In addition, retrospective data suggest leukocytosis is a potential risk factor for thromboembolic events and poor outcomes.13
Dysregulated JAK2 signaling is the principal driver of polycythemia vera pathophysiology. About 95% of patients with polycythemia vera will have an identifiable JAK2 V617F exon 14 mutation, with an additional 3%-5% demonstrating a JAK2 exon 12 mutation.4,14 Under physiologic conditions, JAK2 interacts with the STAT family of signal transduction proteins and serves as an important regulator of normal hematopoiesis.15 Mutated, constitutively activated JAK2 signaling promotes the various polycythemia vera disease manifestations, including excessive myeloproliferation, splenomegaly,15 and constitutional symptoms.14,16,17
Burden of disease for the individual
Mortality
Patients with polycythemia vera have an increased risk of mortality compared with an unaffected, age- and gender-matched cohort of the general population.3 A retrospective study of Medicare patients with polycythemia vera (mean age at diagnosis, 76.1 years) reported a median survival of 5.4 years, compared with 8.7 years for a matched cohort.3 A second retrospective study reported a median survival of 13.5 years (median age at diagnosis, 64 years; median follow-up time, 11.8 years).18
Leading causes of death for patients with polycythemia vera include cardiovascular and thrombotic events, the development of secondary solid tumors, and disease transformation to MF and/or AML. In the prospective European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP) study of 1,638 patients, 45% of deaths (74/164) resulted from cardiovascular causes (1.7 per 100 patient-years).5 Thirteen percent of deaths were related to either leukemic or myelofibrotic transformation, and 20% of deaths were attributed to secondary solid tumors.5 In a retrospective analysis of 1,545 patients with polycythemia vera followed for a median of 6.9 years after diagnosis, 347 had died, primarily from acute leukemia (10%), secondary malignancies (10%), and thrombotic events (9%).4 Arterial and venous thrombotic events occurred in 12% and 9% of patients, respectively, with disease transformation to MF and AML occurring in 9% and 3% of patients. Further support of an increased risk of secondary malignancies comes from a retrospective analysis of a large Swedish cancer registry (1958–2006) that found an increased risk of secondary endocrine, renal, and skin malignancies; MF; and leukemia among patients with polycythemia vera.19
Symptoms and quality of life
Symptoms of polycythemia vera vary in severity, and patients often fail to attribute symptoms to the disease.20 Moreover, clinicians may underestimate a patient’s true disease burden or the effect it has on QoL.20 Point-of-care metrics, such as the MPN Symptom Assessment Form (MPN-SAF), were developed to aid in identifying and grading symptom burden. Studies using this metric have reported fatigue as the most common and most severe symptom (incidence, 73%-92%), with a variety of other symptoms also affecting a majority of patients (Figure 1).6,21-24 Although fatigue, pruritus, and a higher MPN-SAF total symptom score are significantly correlated with reduced QoL,22,25 the recent MPN Landmark survey suggests that even patients with low symptom severity scores have a reduction in their QoL.6 This study also highlighted that polycythemia vera can adversely affect multiple aspects of daily living: 48% of patients reported disease interfering with daily activities; 63% with family or social life; and 37% with employment, feeling compelled to work reduced hours.6
Splenomegaly is a common feature of polycythemia vera, affecting an estimated one in three patients, which may result in discomfort and early satiety.4
Identification and diagnosis
Most patients diagnosed with polycythemia vera are between the ages of 60 and 76 years,3-5 although about 25% are diagnosed before age 50.4 Tefferi and colleagues reported in a retrospective study that common features at presentation include JAK2 mutations (98%), elevated hemoglobin (73%), endogenous erythroid colony growth (73%), white blood cell count of >10.5 × 109/L (49%), and platelet count of ≥450 × 109/L (53%).4 In that same study, about a third of patients presented with a palpable spleen or polycythemia vera–related symptoms, including pruritus and vasomotor symptoms. However, many patients were asymptomatic at presentation, diagnosed incidentally by abnormal laboratory values.4 Patients can present with vascular thrombosis, occasionally involving atypical sites (eg, Budd-Chiari syndrome, other abdominal blood clots),26 thus, a heightened awareness and testing for JAK2 mutations may be appropriate in the evaluation of such individuals.
Evidence suggests many clinicians may not rigidly apply the WHO diagnostic criteria to establish a diagnosis.1,27,28 A recent retrospective claims analysis showed that only 40% of 121 patients diagnosed with polycythemia vera met the 2008 WHO diagnostic criteria, and for some patients, the diagnosis was based solely on the presence of the JAK2 V617F mutation.29 One should be aware of individuals with “masked” polycythemia vera, who may present with characteristic polycythemia vera features but have hemoglobin levels below those established by the WHO in 2008, typically owing to iron deficiency and/or a disproportionate expansion of plasma volume.30 To improve polycythemia vera diagnosis, the WHO diagnostic criteria were updated in 2016 with reduced hemoglobin diagnostic thresholds (Figure 2).1
Management strategy
Treatment goals
The primary polycythemia vera–treatment goals are to reduce the risk of cardiovascular, thrombotic, and hemorrhagic events; reduce the risk of fibrotic and/or leukemic transformation; and alleviate polycythemia vera–related symptoms.11,31
Traditional treatment options
Aspirin. To reduce the risk of death from cardiovascular events, patients with polycythemia vera should receive low-dose aspirin32 and undergo phlebotomy to maintain a target hematocrit <45%, as established by the ECLAP and CYTO-PV trials (Figure 3).4,5,12,13,16,32-36 Higher doses of aspirin (ie, 325 mg 2 or more times a week) are associated with a dose-dependent increased risk of gastrointestinal bleeding.37 Low-dose aspirin is generally well tolerated; however, patients with extreme thrombocytosis may develop bleeding as a consequence of a well-described, thrombocytosis-associated acquired von Willebrand disease.11,38
Phlebotomy. This procedure is generally tolerated by most patients, although it can occasionally engender extreme anxiety in some patients39 and may promote clinical manifestations of iron deficiency, including restless leg syndrome,40 impaired cognition, and worsening of fatigue.41 In the CYTO-PV study, 28% of patients with a target hematocrit 45%-50% discontinued phlebotomy treatment, although the percentage that discontinued because of poor tolerance was not reported.12 To avoid potential complications in patients with underlying cardiovascular disease, smaller-volume phlebotomies are often pursued.42
Cytoreductive therapy. Cytoreductive therapy with hydroxyurea or interferon (IFN) is recommended for high-risk patients (ie, those with a history of thrombosis or older than 60 years) as well as those with intolerable symptoms, progressive splenomegaly, or a burdensome phlebotomy requirement.11,31 Hydroxyurea is the typical first-line cytoreductive therapy11 based on clinical benefit,33,43 low cost, and feasibility of long-term treatment.33
Most patients benefit from long-term treatment with hydroxyurea; however, 25% develop resistance to or intolerance of hydroxyurea therapy.44 Intolerance typically manifests as leg ulcers or other mucocutaneous toxicity, gastrointestinal side effects, or fever.44 Resistance to hydroxyurea is defined as failure to achieve phlebotomy independence, persistent leukothrombocytosis or splenomegaly despite adequate doses of hydroxyurea, or inability to deliver the drug owing to dose-limiting cytopenias. The European LeukemiaNet (ELN) formally codified and published a definition of hydroxyurea resistance/intolerance45 (Table), which can be used to identify patients at high risk of poor outcomes.44 In a retrospective chart review of 261 patients with polycythemia vera, those meeting the ELN definition of hydroxyurea resistance had a 5.6-fold greater risk of mortality and a 6.8-fold increased risk of fibrotic and/or leukemic disease transformation.44
The use of IFN-α and pegylated variants are associated with clinical benefit, including normalization of blood counts, reduction of splenomegaly, symptom mitigation, and reduction in JAK2 V617F allele burden.46 However, poor tolerance46 and an inconvenient route of administration often preclude the long-term use of these agents. Adverse events associated with IFN-α include chills, depression, diarrhea, fatigue, fever, headache, musculoskeletal pain, myalgia, nausea, and weight loss.46 In clinical trials, recombinant IFN-α discontinuation rates within the first year of administration were as high as 29% and may have been dose dependent.46
Traditional treatment options may not effectively alleviate polycythemia vera–related symptoms.23,47 Two prospective studies failed to show an improvement in patient-reported MPN-SAF scores after treatment with hydroxyurea, aspirin, phlebotomy, IFN-α, busulfan, or radiophosphorus,23,47 and symptoms may worsen with the use of IFN-α.47
Allogenic transplantation. Although allogeneic transplantation is a potentially curative treatment option, it has been reserved primarily for younger patients with MPNs (age <60 years31). Furthermore, a recent systematic review concluded that overall survival was worse following allogeneic transplantation compared with a nontransplant approach (ie, phlebotomy and aspirin).48
Ruxolitinib. The oral JAK1/JAK2 inhibitor ruxolitinib has been approved by the US Food and Drug Administration (FDA) for the treatment of patients with polycythemia vera who have had an inadequate response to or are intolerant of hydroxyurea,8 and by the European Medicines Agency (EMA) for adult patients with polycythemia vera who are resistant to or intolerant of hydroxyurea.9 Ruxolitinib is also approved by the FDA for patients with intermediate- or high-risk MF, including primary MF, post-polycythemia vera MF, and post-essential thrombocythemia MF,8 and for similar patient populations by the EMA.9
Approval of ruxolitinib for the treatment of patients with polycythemia vera was based on the phase 3 randomized, open-label, multicenter RESPONSE trial in which 222 patients with polycythemia vera who met the modified ELN criteria for hydroxyurea resistance or intolerance (Table)16,31 were randomized to ruxolitinib or best available therapy (BAT). Compared with BAT, a greater proportion of patients treated with ruxolitinib achieved the primary composite endpoint of hematocrit control without the need for phlebotomy and ≥35% reduction in spleen volume by week 32 (22.7% vs 0.9%; P < .001).16,49 When looked at individually, hematocrit control and reduction in spleen size favored ruxolitinib over BAT (hematocrit control, 60.0% vs 18.8%, ruxolitinib and BAT, respectively; ≥35% reduction in spleen volume, 40.0% vs 0.9%). Furthermore, more patients receiving ruxolitinib achieved the key secondary endpoint of complete hematologic remission than did those receiving BAT (ie, normalization of blood counts; 23.6% vs 8.0%; P = .0016).16,49 Of note is that most patients who achieved primary treatment responses maintained disease control for ≥80 weeks.49
Results from RESPONSE indicate that ruxolitinib may substantially improve polycythemia vera–related symptoms. Treatment with ruxolitinib was associated with a greater improvement in nearly all symptoms evaluated by the MPN-SAF as well as greater improvements in QoL and functional measures with the EORTC QLQ-C30 trial metric compared with BAT (Figure 1).16 In addition, a post hoc exploratory analysis of RESPONSE indicated that patients receiving ruxolitinib showed a rapid normalization of abnormal iron indices at baseline, compared with those receiving BAT.50
Treatment safety and tolerability are particularly important considerations for patients with polycythemia vera, given the long natural history of the disease. In a preplanned analysis of RESPONSE at 80 weeks, 83% of patients randomized to receive treatment with ruxolitinib remained on treatment (median exposure, 111 weeks).49 Most adverse events reported in both treatment arms were grade 1/2.16,49 The most frequent nonhematologic adverse events (per 100 patient-years of exposure) in the ruxolitinib arm were headache (10.5%), diarrhea (9.7%), pruritus (9.7%), and fatigue (8.3%). The most common grade 3/4 nonhematologic adverse events (occurring at a rate of ≥0.9 per 100 patient-years of exposure) were limited to dyspnea (1.3%), abdominal pain (0.9%), headache (0.9%), and herpes zoster (0.9%).49 Hematologic adverse event rates in the ruxolitinib and BAT arms included anemia (any grade, 27.2% vs 47.6%, respectively; grade 3/4, 0.9% vs 0%), lymphopenia (27.2% vs 78.8%; 9.7% vs 27.2%), and thrombocytopenia (14.9% vs 29.9%; 2.6% vs 5.4%).49 Herpes zoster infections occurred more frequently in the ruxolitinib arm (any grade, 5.3%; grade 3/4, 0.9%) compared with the BAT arm (no herpes zoster events).49 There was a higher rate of nonmelanoma skin cancer (NMSC) in the ruxolitinib arm (4.4%), compared with the BAT arm (2.7%),49 most of which occurred in patients with a history of NMSC or precancerous skin lesions.16 Grade 1 or 2 elevations in serum lipids and cholesterol were observed with ruxolitinib but not BAT; however, subsequent effects on patient outcomes have not been determined.8,16 The rates of MF and AML transformations were 1.3% and 0.4%, respectively, in patients randomized to receive ruxolitinib,49 similar to previously published reports for patients with polycythemia vera.44
Additional insight regarding the effect of ruxolitinib on polycythemia vera–related symptoms is available from the RELIEF trial, a randomized, multicenter, double-blind, double-dummy, phase 3b clinical trial. In RELIEF, 110 patients were randomized to receive ruxolitinib or a stable dose of hydroxyurea and were then asked to record disease-related symptoms.51 Although the study failed to meet its primary endpoint (a ≥50% improvement by week 16 in MPN-SAF total symptom score for the cytokine symptom cluster [sum of individual scores for tiredness, itching, muscle aches, night sweats, and sweats while awake]), a numerically greater proportion of patients receiving ruxolitinib achieved the primary endpoint compared with those receiving hydroxyurea (43.4% and 29.6%, respectively; P = .139; odds ratio, 1.82; 95% confidence interval, 0.82-4.04). Similarly, the proportion of patients reporting a ≥50% improvement in pruritus and fatigue favored ruxolitinib over hydroxyurea (itching, 40.0% vs 26.4%; tiredness, 54.2% vs 32.0%). The safety profile for ruxolitinib was similar to that reported in the RESPONSE trial.
Possible future treatment options
Other possible treatment options for patients with polycythemia vera that are currently in clinical development include three pegylated IFN-α (PEG-IFN-α) variants and the telomerase inhibitor, imetelstat.
Pegylated interferon-α. PEG–IFN-α has the advantage of a longer plasma half-life compared with conventional IFN-α, permitting administration once per week or less often.46 Currently, three variants are under active investigation in phase 3 clinical trials: PEG–IFN-α2a (NCT01259856 and NCT01387763), PEG–IFN-α2b (NCT01387763), and AOP2014/P1101 (NCT02218047, NCT02523638, and NCT01949805).
Imetelstat. The telomerase inhibitor imetelstat is in clinical development for patients with MPNs. Clinical benefit was previously observed in patients with primary MF as well as post-polycythemia vera and post-ET MF.52 Imetelstat was evaluated in a phase 2 trial in patients with polycythemia vera or ET who required cytoreductive therapy and were resistant to or intolerant of ≥1 previous line of therapy or who refused standard therapy (NCT01243073). Results in patients with ET were published,53 however, findings from the polycythemia vera cohort have not been reported.
Community oncologist role in managing disease burden
Most patients with polycythemia vera are managed in the community setting. Consequently, the community oncologist plays a critical role in the initial diagnosis, risk stratification, patient education, and disease management.
Early disease recognition allows prompt therapeutic intervention with low-dose aspirin and phlebotomy, interventions shown to reduce the risk of cardiovascular events based on the ECLAP32 and CYTO-PV trials,12 respectively. The diagnosis of polycythemia vera is facilitated by applying the WHO diagnostic criteria (Figure 2);1 however, one should be aware of atypical presentations, including “masked” polycythemia vera,30 as well as the development of thrombosis at atypical sites.26
Optimal management strategies must include the frequent assessment of symptom burden and its effect on a patient’s QoL, with a keen awareness of the nonspecific nature of polycythemia vera–related symptoms, and the potential for patients and clinicians to minimize that effect.20 Patient-reported symptom severity and QoL should be assessed at each office visit with validated instruments, such as the MPN-SAF 10-item questionnaire.22It is important for the community oncologist to define treatment goals and implement a plan that reduces disease-associated morbidity and mortality. A critical treatment goal is to maintain hematocrit <45% by the appropriate use of phlebotomy12 and/or cytoreductive agents.12,16,46 Continued reassessment is important to identify patients with progressive disease and those who fail to achieve stated treatment goals or require an adjustment in cytoreductive therapy. Oncologists should be familiar with the concept of hydroxyurea resistance/intolerance as defined by the ELN (Table)31,45 to allow early identification of those patients who are most likely to benefit from a treatment change for continued optimal outcome.
Conclusions
Polycythemia vera is a clonal myeloproliferative neoplasm associated with significant disease-related morbidity and mortality. Appropriate management includes early diagnosis and implementation of appropriate therapy according to patient risk and therapeutic tolerance. Patients should initially receive aspirin32 and phlebotomy,12 with the goal of maintaining hematocrit <45%. Higher-risk patients and those who had inadequate disease control with phlebotomy alone require cytoreduction, typically with hydroxyurea. Although most patients will achieve adequate disease control with hydroxyurea,33,43 one in four patients will develop drug resistance or intolerance.44 Ruxolitinib is approved by the FDA and the EMA for the treatment of patients with polycythemia vera who are resistant to or intolerant of hydroxyurea. Compared with BAT, ruxolitinib is associated with improved hematocrit control, reductions in spleen size, a greater probability of blood count normalization, and improvement in polycythemia vera–related symptoms.16
Acknowledgments
Writing assistance was provided by Cory Pfeiffenberger, PhD, of Complete Healthcare Communications LLC, and was funded by Incyte Corporation, the maker of ruxolitinib.
Polycythemia vera, classified as a myeloproliferative neoplasm (MPN) and characterized by uncontrolled, clonal, myeloid expansion with predominant erythrocytosis,1 affects about 100,000 individuals in the United States.2 It is a chronic and burdensome disease associated with shortened survival.3 Patients are at an increased risk of cardiovascular events, solid tumors, and transformation to myelofibrosis (MF) and/or acute myeloid leukemia (AML).4,5 Furthermore, patients generally have a reduced quality of life (QoL) stemming from prevalent and occasionally severe polycythemia vera–related signs and symptoms, including fatigue, pruritus, and splenomegaly.6 In general, the classical Philadelphia chromosome-negative MPNs are associated with driver mutations in the following three genes: Janus kinase 2 (JAK2), calreticulin (CALR), and myeloproliferative leukemia virus oncogene (MPL).7 Almost all patients with polycythemia vera have an activating mutation in the cytoplasmic signal transduction protein JAK2.4 Patients with essential thrombocythemia (ET) or MF can have mutations in JAK2, CALR, or MPL. However, CALR and MPL mutations are absent or exceedingly rare in patients with polycythemia vera.7 Diagnosis can be challenging and is currently based on 2016 World Health Organization (WHO) diagnostic criteria.1
Management strategies include the use of aspirin, phlebotomy, and cytoreductive therapy. Ruxolitinib is a newer treatment option available for patients with polycythemia vera who are either resistant to or intolerant of hydroxyurea8,9— a population that previously had few treatment options. It is important for community oncologists and other treating clinicians to understand current diagnostic strategy and management options based on established guidelines, recent clinical evidence, and regulatory updates.
Search and selection process for research sources
In September 2016, we searched PubMed for articles published since 2006 with polycythemia vera included in the abstract or title. The initial 1,730 publications were screened by eye to select 46 key articles that guide current management of polycythemia vera. Four studies published before 2006 were also included based on their continued relevance.
Epidemiology and pathophysiology
Based on a meta-analysis of patients from Europe and the United States, the annual incidence of polycythemia vera estimated to be between 0.7 and 2.6 per 100,000 people.10 The age-adjusted prevalence of polycythemia vera in the United States is about 45-57 per 100,000 people,2 however, the true prevalence might be considerably greater.
Patients with polycythemia vera are at increased risk of cardiovascular events, thrombosis, and death.3-5 Risk is highest among patients older than 60 years or with a history of thrombosis.11 Uncontrolled myeloproliferation has also been identified as a risk factor for cardiovascular mortality and thrombosis. This was demonstrated in the prospective Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial, which reported more cardiovascular events in patients with hematocrit levels of 45%-50%, compared with those whose hematocrit levels were <45%.12 In addition, retrospective data suggest leukocytosis is a potential risk factor for thromboembolic events and poor outcomes.13
Dysregulated JAK2 signaling is the principal driver of polycythemia vera pathophysiology. About 95% of patients with polycythemia vera will have an identifiable JAK2 V617F exon 14 mutation, with an additional 3%-5% demonstrating a JAK2 exon 12 mutation.4,14 Under physiologic conditions, JAK2 interacts with the STAT family of signal transduction proteins and serves as an important regulator of normal hematopoiesis.15 Mutated, constitutively activated JAK2 signaling promotes the various polycythemia vera disease manifestations, including excessive myeloproliferation, splenomegaly,15 and constitutional symptoms.14,16,17
Burden of disease for the individual
Mortality
Patients with polycythemia vera have an increased risk of mortality compared with an unaffected, age- and gender-matched cohort of the general population.3 A retrospective study of Medicare patients with polycythemia vera (mean age at diagnosis, 76.1 years) reported a median survival of 5.4 years, compared with 8.7 years for a matched cohort.3 A second retrospective study reported a median survival of 13.5 years (median age at diagnosis, 64 years; median follow-up time, 11.8 years).18
Leading causes of death for patients with polycythemia vera include cardiovascular and thrombotic events, the development of secondary solid tumors, and disease transformation to MF and/or AML. In the prospective European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP) study of 1,638 patients, 45% of deaths (74/164) resulted from cardiovascular causes (1.7 per 100 patient-years).5 Thirteen percent of deaths were related to either leukemic or myelofibrotic transformation, and 20% of deaths were attributed to secondary solid tumors.5 In a retrospective analysis of 1,545 patients with polycythemia vera followed for a median of 6.9 years after diagnosis, 347 had died, primarily from acute leukemia (10%), secondary malignancies (10%), and thrombotic events (9%).4 Arterial and venous thrombotic events occurred in 12% and 9% of patients, respectively, with disease transformation to MF and AML occurring in 9% and 3% of patients. Further support of an increased risk of secondary malignancies comes from a retrospective analysis of a large Swedish cancer registry (1958–2006) that found an increased risk of secondary endocrine, renal, and skin malignancies; MF; and leukemia among patients with polycythemia vera.19
Symptoms and quality of life
Symptoms of polycythemia vera vary in severity, and patients often fail to attribute symptoms to the disease.20 Moreover, clinicians may underestimate a patient’s true disease burden or the effect it has on QoL.20 Point-of-care metrics, such as the MPN Symptom Assessment Form (MPN-SAF), were developed to aid in identifying and grading symptom burden. Studies using this metric have reported fatigue as the most common and most severe symptom (incidence, 73%-92%), with a variety of other symptoms also affecting a majority of patients (Figure 1).6,21-24 Although fatigue, pruritus, and a higher MPN-SAF total symptom score are significantly correlated with reduced QoL,22,25 the recent MPN Landmark survey suggests that even patients with low symptom severity scores have a reduction in their QoL.6 This study also highlighted that polycythemia vera can adversely affect multiple aspects of daily living: 48% of patients reported disease interfering with daily activities; 63% with family or social life; and 37% with employment, feeling compelled to work reduced hours.6
Splenomegaly is a common feature of polycythemia vera, affecting an estimated one in three patients, which may result in discomfort and early satiety.4
Identification and diagnosis
Most patients diagnosed with polycythemia vera are between the ages of 60 and 76 years,3-5 although about 25% are diagnosed before age 50.4 Tefferi and colleagues reported in a retrospective study that common features at presentation include JAK2 mutations (98%), elevated hemoglobin (73%), endogenous erythroid colony growth (73%), white blood cell count of >10.5 × 109/L (49%), and platelet count of ≥450 × 109/L (53%).4 In that same study, about a third of patients presented with a palpable spleen or polycythemia vera–related symptoms, including pruritus and vasomotor symptoms. However, many patients were asymptomatic at presentation, diagnosed incidentally by abnormal laboratory values.4 Patients can present with vascular thrombosis, occasionally involving atypical sites (eg, Budd-Chiari syndrome, other abdominal blood clots),26 thus, a heightened awareness and testing for JAK2 mutations may be appropriate in the evaluation of such individuals.
Evidence suggests many clinicians may not rigidly apply the WHO diagnostic criteria to establish a diagnosis.1,27,28 A recent retrospective claims analysis showed that only 40% of 121 patients diagnosed with polycythemia vera met the 2008 WHO diagnostic criteria, and for some patients, the diagnosis was based solely on the presence of the JAK2 V617F mutation.29 One should be aware of individuals with “masked” polycythemia vera, who may present with characteristic polycythemia vera features but have hemoglobin levels below those established by the WHO in 2008, typically owing to iron deficiency and/or a disproportionate expansion of plasma volume.30 To improve polycythemia vera diagnosis, the WHO diagnostic criteria were updated in 2016 with reduced hemoglobin diagnostic thresholds (Figure 2).1
Management strategy
Treatment goals
The primary polycythemia vera–treatment goals are to reduce the risk of cardiovascular, thrombotic, and hemorrhagic events; reduce the risk of fibrotic and/or leukemic transformation; and alleviate polycythemia vera–related symptoms.11,31
Traditional treatment options
Aspirin. To reduce the risk of death from cardiovascular events, patients with polycythemia vera should receive low-dose aspirin32 and undergo phlebotomy to maintain a target hematocrit <45%, as established by the ECLAP and CYTO-PV trials (Figure 3).4,5,12,13,16,32-36 Higher doses of aspirin (ie, 325 mg 2 or more times a week) are associated with a dose-dependent increased risk of gastrointestinal bleeding.37 Low-dose aspirin is generally well tolerated; however, patients with extreme thrombocytosis may develop bleeding as a consequence of a well-described, thrombocytosis-associated acquired von Willebrand disease.11,38
Phlebotomy. This procedure is generally tolerated by most patients, although it can occasionally engender extreme anxiety in some patients39 and may promote clinical manifestations of iron deficiency, including restless leg syndrome,40 impaired cognition, and worsening of fatigue.41 In the CYTO-PV study, 28% of patients with a target hematocrit 45%-50% discontinued phlebotomy treatment, although the percentage that discontinued because of poor tolerance was not reported.12 To avoid potential complications in patients with underlying cardiovascular disease, smaller-volume phlebotomies are often pursued.42
Cytoreductive therapy. Cytoreductive therapy with hydroxyurea or interferon (IFN) is recommended for high-risk patients (ie, those with a history of thrombosis or older than 60 years) as well as those with intolerable symptoms, progressive splenomegaly, or a burdensome phlebotomy requirement.11,31 Hydroxyurea is the typical first-line cytoreductive therapy11 based on clinical benefit,33,43 low cost, and feasibility of long-term treatment.33
Most patients benefit from long-term treatment with hydroxyurea; however, 25% develop resistance to or intolerance of hydroxyurea therapy.44 Intolerance typically manifests as leg ulcers or other mucocutaneous toxicity, gastrointestinal side effects, or fever.44 Resistance to hydroxyurea is defined as failure to achieve phlebotomy independence, persistent leukothrombocytosis or splenomegaly despite adequate doses of hydroxyurea, or inability to deliver the drug owing to dose-limiting cytopenias. The European LeukemiaNet (ELN) formally codified and published a definition of hydroxyurea resistance/intolerance45 (Table), which can be used to identify patients at high risk of poor outcomes.44 In a retrospective chart review of 261 patients with polycythemia vera, those meeting the ELN definition of hydroxyurea resistance had a 5.6-fold greater risk of mortality and a 6.8-fold increased risk of fibrotic and/or leukemic disease transformation.44
The use of IFN-α and pegylated variants are associated with clinical benefit, including normalization of blood counts, reduction of splenomegaly, symptom mitigation, and reduction in JAK2 V617F allele burden.46 However, poor tolerance46 and an inconvenient route of administration often preclude the long-term use of these agents. Adverse events associated with IFN-α include chills, depression, diarrhea, fatigue, fever, headache, musculoskeletal pain, myalgia, nausea, and weight loss.46 In clinical trials, recombinant IFN-α discontinuation rates within the first year of administration were as high as 29% and may have been dose dependent.46
Traditional treatment options may not effectively alleviate polycythemia vera–related symptoms.23,47 Two prospective studies failed to show an improvement in patient-reported MPN-SAF scores after treatment with hydroxyurea, aspirin, phlebotomy, IFN-α, busulfan, or radiophosphorus,23,47 and symptoms may worsen with the use of IFN-α.47
Allogenic transplantation. Although allogeneic transplantation is a potentially curative treatment option, it has been reserved primarily for younger patients with MPNs (age <60 years31). Furthermore, a recent systematic review concluded that overall survival was worse following allogeneic transplantation compared with a nontransplant approach (ie, phlebotomy and aspirin).48
Ruxolitinib. The oral JAK1/JAK2 inhibitor ruxolitinib has been approved by the US Food and Drug Administration (FDA) for the treatment of patients with polycythemia vera who have had an inadequate response to or are intolerant of hydroxyurea,8 and by the European Medicines Agency (EMA) for adult patients with polycythemia vera who are resistant to or intolerant of hydroxyurea.9 Ruxolitinib is also approved by the FDA for patients with intermediate- or high-risk MF, including primary MF, post-polycythemia vera MF, and post-essential thrombocythemia MF,8 and for similar patient populations by the EMA.9
Approval of ruxolitinib for the treatment of patients with polycythemia vera was based on the phase 3 randomized, open-label, multicenter RESPONSE trial in which 222 patients with polycythemia vera who met the modified ELN criteria for hydroxyurea resistance or intolerance (Table)16,31 were randomized to ruxolitinib or best available therapy (BAT). Compared with BAT, a greater proportion of patients treated with ruxolitinib achieved the primary composite endpoint of hematocrit control without the need for phlebotomy and ≥35% reduction in spleen volume by week 32 (22.7% vs 0.9%; P < .001).16,49 When looked at individually, hematocrit control and reduction in spleen size favored ruxolitinib over BAT (hematocrit control, 60.0% vs 18.8%, ruxolitinib and BAT, respectively; ≥35% reduction in spleen volume, 40.0% vs 0.9%). Furthermore, more patients receiving ruxolitinib achieved the key secondary endpoint of complete hematologic remission than did those receiving BAT (ie, normalization of blood counts; 23.6% vs 8.0%; P = .0016).16,49 Of note is that most patients who achieved primary treatment responses maintained disease control for ≥80 weeks.49
Results from RESPONSE indicate that ruxolitinib may substantially improve polycythemia vera–related symptoms. Treatment with ruxolitinib was associated with a greater improvement in nearly all symptoms evaluated by the MPN-SAF as well as greater improvements in QoL and functional measures with the EORTC QLQ-C30 trial metric compared with BAT (Figure 1).16 In addition, a post hoc exploratory analysis of RESPONSE indicated that patients receiving ruxolitinib showed a rapid normalization of abnormal iron indices at baseline, compared with those receiving BAT.50
Treatment safety and tolerability are particularly important considerations for patients with polycythemia vera, given the long natural history of the disease. In a preplanned analysis of RESPONSE at 80 weeks, 83% of patients randomized to receive treatment with ruxolitinib remained on treatment (median exposure, 111 weeks).49 Most adverse events reported in both treatment arms were grade 1/2.16,49 The most frequent nonhematologic adverse events (per 100 patient-years of exposure) in the ruxolitinib arm were headache (10.5%), diarrhea (9.7%), pruritus (9.7%), and fatigue (8.3%). The most common grade 3/4 nonhematologic adverse events (occurring at a rate of ≥0.9 per 100 patient-years of exposure) were limited to dyspnea (1.3%), abdominal pain (0.9%), headache (0.9%), and herpes zoster (0.9%).49 Hematologic adverse event rates in the ruxolitinib and BAT arms included anemia (any grade, 27.2% vs 47.6%, respectively; grade 3/4, 0.9% vs 0%), lymphopenia (27.2% vs 78.8%; 9.7% vs 27.2%), and thrombocytopenia (14.9% vs 29.9%; 2.6% vs 5.4%).49 Herpes zoster infections occurred more frequently in the ruxolitinib arm (any grade, 5.3%; grade 3/4, 0.9%) compared with the BAT arm (no herpes zoster events).49 There was a higher rate of nonmelanoma skin cancer (NMSC) in the ruxolitinib arm (4.4%), compared with the BAT arm (2.7%),49 most of which occurred in patients with a history of NMSC or precancerous skin lesions.16 Grade 1 or 2 elevations in serum lipids and cholesterol were observed with ruxolitinib but not BAT; however, subsequent effects on patient outcomes have not been determined.8,16 The rates of MF and AML transformations were 1.3% and 0.4%, respectively, in patients randomized to receive ruxolitinib,49 similar to previously published reports for patients with polycythemia vera.44
Additional insight regarding the effect of ruxolitinib on polycythemia vera–related symptoms is available from the RELIEF trial, a randomized, multicenter, double-blind, double-dummy, phase 3b clinical trial. In RELIEF, 110 patients were randomized to receive ruxolitinib or a stable dose of hydroxyurea and were then asked to record disease-related symptoms.51 Although the study failed to meet its primary endpoint (a ≥50% improvement by week 16 in MPN-SAF total symptom score for the cytokine symptom cluster [sum of individual scores for tiredness, itching, muscle aches, night sweats, and sweats while awake]), a numerically greater proportion of patients receiving ruxolitinib achieved the primary endpoint compared with those receiving hydroxyurea (43.4% and 29.6%, respectively; P = .139; odds ratio, 1.82; 95% confidence interval, 0.82-4.04). Similarly, the proportion of patients reporting a ≥50% improvement in pruritus and fatigue favored ruxolitinib over hydroxyurea (itching, 40.0% vs 26.4%; tiredness, 54.2% vs 32.0%). The safety profile for ruxolitinib was similar to that reported in the RESPONSE trial.
Possible future treatment options
Other possible treatment options for patients with polycythemia vera that are currently in clinical development include three pegylated IFN-α (PEG-IFN-α) variants and the telomerase inhibitor, imetelstat.
Pegylated interferon-α. PEG–IFN-α has the advantage of a longer plasma half-life compared with conventional IFN-α, permitting administration once per week or less often.46 Currently, three variants are under active investigation in phase 3 clinical trials: PEG–IFN-α2a (NCT01259856 and NCT01387763), PEG–IFN-α2b (NCT01387763), and AOP2014/P1101 (NCT02218047, NCT02523638, and NCT01949805).
Imetelstat. The telomerase inhibitor imetelstat is in clinical development for patients with MPNs. Clinical benefit was previously observed in patients with primary MF as well as post-polycythemia vera and post-ET MF.52 Imetelstat was evaluated in a phase 2 trial in patients with polycythemia vera or ET who required cytoreductive therapy and were resistant to or intolerant of ≥1 previous line of therapy or who refused standard therapy (NCT01243073). Results in patients with ET were published,53 however, findings from the polycythemia vera cohort have not been reported.
Community oncologist role in managing disease burden
Most patients with polycythemia vera are managed in the community setting. Consequently, the community oncologist plays a critical role in the initial diagnosis, risk stratification, patient education, and disease management.
Early disease recognition allows prompt therapeutic intervention with low-dose aspirin and phlebotomy, interventions shown to reduce the risk of cardiovascular events based on the ECLAP32 and CYTO-PV trials,12 respectively. The diagnosis of polycythemia vera is facilitated by applying the WHO diagnostic criteria (Figure 2);1 however, one should be aware of atypical presentations, including “masked” polycythemia vera,30 as well as the development of thrombosis at atypical sites.26
Optimal management strategies must include the frequent assessment of symptom burden and its effect on a patient’s QoL, with a keen awareness of the nonspecific nature of polycythemia vera–related symptoms, and the potential for patients and clinicians to minimize that effect.20 Patient-reported symptom severity and QoL should be assessed at each office visit with validated instruments, such as the MPN-SAF 10-item questionnaire.22It is important for the community oncologist to define treatment goals and implement a plan that reduces disease-associated morbidity and mortality. A critical treatment goal is to maintain hematocrit <45% by the appropriate use of phlebotomy12 and/or cytoreductive agents.12,16,46 Continued reassessment is important to identify patients with progressive disease and those who fail to achieve stated treatment goals or require an adjustment in cytoreductive therapy. Oncologists should be familiar with the concept of hydroxyurea resistance/intolerance as defined by the ELN (Table)31,45 to allow early identification of those patients who are most likely to benefit from a treatment change for continued optimal outcome.
Conclusions
Polycythemia vera is a clonal myeloproliferative neoplasm associated with significant disease-related morbidity and mortality. Appropriate management includes early diagnosis and implementation of appropriate therapy according to patient risk and therapeutic tolerance. Patients should initially receive aspirin32 and phlebotomy,12 with the goal of maintaining hematocrit <45%. Higher-risk patients and those who had inadequate disease control with phlebotomy alone require cytoreduction, typically with hydroxyurea. Although most patients will achieve adequate disease control with hydroxyurea,33,43 one in four patients will develop drug resistance or intolerance.44 Ruxolitinib is approved by the FDA and the EMA for the treatment of patients with polycythemia vera who are resistant to or intolerant of hydroxyurea. Compared with BAT, ruxolitinib is associated with improved hematocrit control, reductions in spleen size, a greater probability of blood count normalization, and improvement in polycythemia vera–related symptoms.16
Acknowledgments
Writing assistance was provided by Cory Pfeiffenberger, PhD, of Complete Healthcare Communications LLC, and was funded by Incyte Corporation, the maker of ruxolitinib.
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
2. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55:595-600.
3. Price GL, Davis KL, Karve S, Pohl G, Walgren RA. Survival patterns in United States (US) Medicare enrollees with non-CML myeloproliferative neoplasms (MPN). PLoS ONE. 2014;9:e90299.
4. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27:1874-1881.
5. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224-2232.
6. Mesa R, Miller CB, Thyne M, et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the MPN Landmark survey. BMC Cancer. 2016;16:167.
7. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379-2390.
8. JAKAFI (ruxolitinib). Full Prescribing Information, Incyte Corporation, Wilmington, DE, USA, 2016.
9. JAKAVI (ruxolitinib). Summary of Product Characteristics, Novartis Pharma GmbH, Nuremberg, Germany, 2015.
10. Johansson P. Epidemiology of the myeloproliferative disorders polycythemia vera and essential thrombocythemia. Semin Thromb Hemost. 2006;32:171-173.
11. Vannucchi AM. How I treat polycythemia vera. Blood. 2014;124:3212-3220.
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22-33.
13. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126:560-561.
14. Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24:1574-1579.
15. Quintás-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10:127-140.
16. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372:426-435.
17. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952-1959.
18. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly-annotated essential thrombocythemia, polycythemia vera and myelofibrosis. Blood. 2014;124:2507-2513.
19. Fallah M, Kharazmi E, Sundquist J, Hemminki K. Higher risk of primary cancers after polycythaemia vera and vice versa. Br J Haematol. 2011;153:283-285.
20. Mesa R, Miller C, Thyne M, et al. Differences in treatment goals and perception of symptom burden between patients with MPNs and hematologists/oncologists in the United States: findings from the MPN landmark survey. Cancer. 2017;123:449-458.
21. Abelsson J, Andreasson B, Samuelsson J, et al. Patients with polycythemia vera have the worst impairment of quality of life among patients with newly diagnosed myeloproliferative neoplasms. Leuk Lymphoma. 2013;54:2226-2230.
22. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative Neoplasm (MPN) Symptom Assessment Form Total Symptom Score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098-4103.
23. Johansson P, Mesa R, Scherber R, et al. Association between quality of life and clinical parameters in patients with myeloproliferative neoplasms. Leuk Lymphoma. 2012;53:441-444.
24. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401-408.
25. Siegel FP, Tauscher J, Petrides PE. Aquagenic pruritus in polycythemia vera: characteristics and influence on quality of life in 441 patients. Am J Hematol. 2013;88:665-669.
26. Smalberg JH, Arends LR, Valla DC, Kiladjian JJ, Janssen HL, Leebeek FW. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood. 2012;120:4921-4928.
27. Barosi G, Mesa RA, Thiele J, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22:437-438.
28. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937-951.
29. Roda P, Ferrari A, Tang X, et al. Determination of accuracy of polycythemia vera diagnoses and use of the JAK2V617F test in the diagnostic scheme. Ann Hematol. 2014;93:1467-1472.
30. Barbui T, Thiele J, Gisslinger H, et al. Masked polycythemia vera (mPV): results of an international study. Am J Hematol. 2014;89:52-54.
31. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29:761-770.
32. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:114-124.
33. Kiladjian JJ, Chevret S, Dosquet C, Chomienne C, Rain JD. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol. 2011;29:3907-3913.
34. Quintás-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27:5418-5424.
35. Sacchi S, Leoni P, Liberati M, et al. A prospective comparison between treatment with phlebotomy alone and with interferon-alpha in patients with polycythemia vera. Ann Hematol. 1994;68:247-250.
36. Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer. 2006;107:451-458.
37. Huang ES, Strate LL, Ho WW, Lee SS, Chan AT. Long-term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011;124:426-433.
38. Shetty S, Kasatkar P, Ghosh K. Pathophysiology of acquired von Willebrand disease: a concise review. Eur J Haematol. 2011;87:99-106.
39. Deacon B, Abramowitz J. Fear of needles and vasovagal reactions among phlebotomy patients. J Anxiety Disord. 2006;20:946-960.
40. Tobiasson M, Alyass B, Soderlund S, Birgegard G. High prevalence of restless legs syndrome among patients with polycytemia vera treated with venesectio. Med Oncol. 2010;27:105-107.
41. Greig AJ, Patterson AJ, Collins CE, Chalmers KA. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.
42. Passamonti F. How I treat polycythemia vera. Blood. 2012;120:275-284.
43. Fruchtman SM, Mack K, Kaplan ME, Peterson P, Berk PD, Wasserman LR. From efficacy to safety: a Polycythemia Vera Study Group report on hydroxyurea in patients with polycythemia vera. Semin Hematol. 1997;34:17-23.
44. Alvarez-Lárran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood. 2012;119:1363-1369.
45. Barosi G, Birgegard G, Finazzi G, et al. A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: results of a European LeukemiaNet (ELN) consensus process. Br J Haematol. 2010;148:961-963.
46. Hasselbalch HC. A new era for IFN-α in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. Expert Rev Hematol. 2011;4:637-655.
47. Emanuel R, Dueck AC, Kiladjian JJ, et al. Conventional therapeutic options have limited impact on MPN symptoms: insights from a prospective analysis of the MPN-SAF [abstract 366]. Presented at: European Hematology Association, June 14-17, 2012; Amsterdam, Netherlands.
48. Lacevic J, Reljic T, El Jurdi N, et al. Conservative management vs. allogeneic hematopoietic cell transplantation for polycythemia vera: a systematic review and decision-analysis. Blood. 2013;122:abstract 5372.
49. Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80 week follow-up from the RESPONSE trial. Haematologica. 2016;101:821-829.
50. Verstovsek S, Harrison CN, Kiladjian J-J, et al. Effect of ruxolitinib on markers of iron deficiency: an analysis of the RESPONSE trial. Haematologica (EHA Annual Meeting Abstracts). 2015;100:abstract P672.
51. Mesa R, Vannucchi AM, Yacoub A, et al. The efficacy and safety of continued hydroxyurea therapy versus switching to ruxolitinib in patients with polycythemia vera: a randomized, double-blind, double-dummy, symptom study (RELIEF). Blood (ASH Annual Meeting Abstracts). 2014;124:abstract 3168.
52. Tefferi A, Lasho TL, Begna KH, et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N Engl J Med. 2015;373:908-919.
53. Baerlocher GM, Oppliger Leibundgut E, Ottmann OG, et al. Telomerase inhibitor imetelstat in patients with essential thrombocythemia. N Engl J Med. 2015;373:920-928.
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
2. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55:595-600.
3. Price GL, Davis KL, Karve S, Pohl G, Walgren RA. Survival patterns in United States (US) Medicare enrollees with non-CML myeloproliferative neoplasms (MPN). PLoS ONE. 2014;9:e90299.
4. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27:1874-1881.
5. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224-2232.
6. Mesa R, Miller CB, Thyne M, et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the MPN Landmark survey. BMC Cancer. 2016;16:167.
7. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379-2390.
8. JAKAFI (ruxolitinib). Full Prescribing Information, Incyte Corporation, Wilmington, DE, USA, 2016.
9. JAKAVI (ruxolitinib). Summary of Product Characteristics, Novartis Pharma GmbH, Nuremberg, Germany, 2015.
10. Johansson P. Epidemiology of the myeloproliferative disorders polycythemia vera and essential thrombocythemia. Semin Thromb Hemost. 2006;32:171-173.
11. Vannucchi AM. How I treat polycythemia vera. Blood. 2014;124:3212-3220.
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22-33.
13. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126:560-561.
14. Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24:1574-1579.
15. Quintás-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10:127-140.
16. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372:426-435.
17. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952-1959.
18. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly-annotated essential thrombocythemia, polycythemia vera and myelofibrosis. Blood. 2014;124:2507-2513.
19. Fallah M, Kharazmi E, Sundquist J, Hemminki K. Higher risk of primary cancers after polycythaemia vera and vice versa. Br J Haematol. 2011;153:283-285.
20. Mesa R, Miller C, Thyne M, et al. Differences in treatment goals and perception of symptom burden between patients with MPNs and hematologists/oncologists in the United States: findings from the MPN landmark survey. Cancer. 2017;123:449-458.
21. Abelsson J, Andreasson B, Samuelsson J, et al. Patients with polycythemia vera have the worst impairment of quality of life among patients with newly diagnosed myeloproliferative neoplasms. Leuk Lymphoma. 2013;54:2226-2230.
22. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative Neoplasm (MPN) Symptom Assessment Form Total Symptom Score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098-4103.
23. Johansson P, Mesa R, Scherber R, et al. Association between quality of life and clinical parameters in patients with myeloproliferative neoplasms. Leuk Lymphoma. 2012;53:441-444.
24. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401-408.
25. Siegel FP, Tauscher J, Petrides PE. Aquagenic pruritus in polycythemia vera: characteristics and influence on quality of life in 441 patients. Am J Hematol. 2013;88:665-669.
26. Smalberg JH, Arends LR, Valla DC, Kiladjian JJ, Janssen HL, Leebeek FW. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood. 2012;120:4921-4928.
27. Barosi G, Mesa RA, Thiele J, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22:437-438.
28. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937-951.
29. Roda P, Ferrari A, Tang X, et al. Determination of accuracy of polycythemia vera diagnoses and use of the JAK2V617F test in the diagnostic scheme. Ann Hematol. 2014;93:1467-1472.
30. Barbui T, Thiele J, Gisslinger H, et al. Masked polycythemia vera (mPV): results of an international study. Am J Hematol. 2014;89:52-54.
31. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29:761-770.
32. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:114-124.
33. Kiladjian JJ, Chevret S, Dosquet C, Chomienne C, Rain JD. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol. 2011;29:3907-3913.
34. Quintás-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27:5418-5424.
35. Sacchi S, Leoni P, Liberati M, et al. A prospective comparison between treatment with phlebotomy alone and with interferon-alpha in patients with polycythemia vera. Ann Hematol. 1994;68:247-250.
36. Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer. 2006;107:451-458.
37. Huang ES, Strate LL, Ho WW, Lee SS, Chan AT. Long-term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011;124:426-433.
38. Shetty S, Kasatkar P, Ghosh K. Pathophysiology of acquired von Willebrand disease: a concise review. Eur J Haematol. 2011;87:99-106.
39. Deacon B, Abramowitz J. Fear of needles and vasovagal reactions among phlebotomy patients. J Anxiety Disord. 2006;20:946-960.
40. Tobiasson M, Alyass B, Soderlund S, Birgegard G. High prevalence of restless legs syndrome among patients with polycytemia vera treated with venesectio. Med Oncol. 2010;27:105-107.
41. Greig AJ, Patterson AJ, Collins CE, Chalmers KA. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.
42. Passamonti F. How I treat polycythemia vera. Blood. 2012;120:275-284.
43. Fruchtman SM, Mack K, Kaplan ME, Peterson P, Berk PD, Wasserman LR. From efficacy to safety: a Polycythemia Vera Study Group report on hydroxyurea in patients with polycythemia vera. Semin Hematol. 1997;34:17-23.
44. Alvarez-Lárran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood. 2012;119:1363-1369.
45. Barosi G, Birgegard G, Finazzi G, et al. A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: results of a European LeukemiaNet (ELN) consensus process. Br J Haematol. 2010;148:961-963.
46. Hasselbalch HC. A new era for IFN-α in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. Expert Rev Hematol. 2011;4:637-655.
47. Emanuel R, Dueck AC, Kiladjian JJ, et al. Conventional therapeutic options have limited impact on MPN symptoms: insights from a prospective analysis of the MPN-SAF [abstract 366]. Presented at: European Hematology Association, June 14-17, 2012; Amsterdam, Netherlands.
48. Lacevic J, Reljic T, El Jurdi N, et al. Conservative management vs. allogeneic hematopoietic cell transplantation for polycythemia vera: a systematic review and decision-analysis. Blood. 2013;122:abstract 5372.
49. Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80 week follow-up from the RESPONSE trial. Haematologica. 2016;101:821-829.
50. Verstovsek S, Harrison CN, Kiladjian J-J, et al. Effect of ruxolitinib on markers of iron deficiency: an analysis of the RESPONSE trial. Haematologica (EHA Annual Meeting Abstracts). 2015;100:abstract P672.
51. Mesa R, Vannucchi AM, Yacoub A, et al. The efficacy and safety of continued hydroxyurea therapy versus switching to ruxolitinib in patients with polycythemia vera: a randomized, double-blind, double-dummy, symptom study (RELIEF). Blood (ASH Annual Meeting Abstracts). 2014;124:abstract 3168.
52. Tefferi A, Lasho TL, Begna KH, et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N Engl J Med. 2015;373:908-919.
53. Baerlocher GM, Oppliger Leibundgut E, Ottmann OG, et al. Telomerase inhibitor imetelstat in patients with essential thrombocythemia. N Engl J Med. 2015;373:920-928.
Assessing a multidisciplinary survivorship program in a group of predominantly Hispanic women with breast cancer
Breast cancer survivors comprise the most prevalent cancer survivor population in the United States.1 The number of breast cancer survivors is increasing because of early detection and diagnosis, and advances in treatment have resulted in increased life expectancy. Therefore, greater attention is needed to improve the long-term quality of life of these survivors and to help them re-adjust to normal life. For many women, although the medical treatment may have been completed, the recovery process may have not.2 The prevalence of long-term mental and physical illness is significant among many breast cancer survivors. Long-term mental consequences may include memory problems, anxiety, depression, and fear of recurrence3, and long-term physical consequences may include pain, fatigue, and lymphedema, among others.4
El Paso, Texas, is the fourth most populous city in Texas and has a Hispanic majority. This provides an opportunity to conduct clinical research targeting participants of Hispanic descent. Several studies have noted the influence of race/ethnicity on the psychosocial function of breast cancer survivors.5,6 We have previously reported that Hispanic breast cancer survivors might experience decreased mental and physical health-related quality of life (QoL) which limit their normal social functioning.6Other studies have similarly reported poor outcomes of breast cancer survivors and higher rates of fatigue and depression among Hispanic patients.7 However, there is a paucity of research addressing specific interventions needed to improve these outcomes and provide better QoL for breast cancer survivors.8,9 In addition, a few survivorship care interventions have focused on minorities. We sought to assess whether a multidisciplinary cancer survivorship program in a primarily Hispanic populated area would lead to improved QoL and reduce anxiety and depressive symptoms among breast cancer survivors.
Methods
After obtaining Institutional Review Board approval, we recruited consecutive patients who were treated at our institution during October 2013-October 2014 and obtained informed consent from them. The participants were within the first 5 years after diagnosis with stages I-III breast cancer and had completed surgery, chemotherapy, and/or radiation therapy. We sought to determine whether breast cancer survivors would benefit from this intervention as determined by improvement of performance at 12 months compared with baseline based on the following self-reported validated questionnaires: Patient Health Questionnaire-9 (PHQ-9) for depression; General Anxiety Disorder-7 (GAD-7); and Short-Form Health Survey-36 (SF-36, version 2) for patient quality of life. The participants were enrolled in a comprehensive survivorship program staffed by an oncologist, an oncology nurse practitioner, a nutritionist, and a certified clinical psychologist who had trained in mindfulness-based stress reduction (MBSR).
Interventions
The participants received a one-on-one individual psychological consultation visit every 3 months for 20-45 minutes during which the psychologist addressed each patient’s emotional and psychological issues in depth, discussed relaxation techniques, and provided psychosocial counselling. In addition, all participants were asked to attend an 8-week-course (in Spanish or English) using MBSR, an interventional program in which participants receive training to promote reduction of stress by self-regulating mindfulness practice.3,10 Our institution’s MBSR program consists of a weekly 2-hour class for 8 sessions or more. The program is provided 3 times a year, in English and Spanish. It includes the following components:
- Learning various mindfulness meditation techniques (eg, body scans, awareness of breathing, sitting/walking meditations);
- Practicing the mindfulness techniques in class; and
- Practicing techniques at home through audiorecordings of mindfulness meditation exercises and daily diary writing.
Participants were provided with a workbook on MBSR in their preferred language.11 In addition to the psychological component, they were also provided with oncologic evaluations by an oncology nurse practitioner. The nurse practitioner met with participants every 3 months and provided each one with a personalized summary of all the treatments received and routine oncology follow-up care in consultation with the patients’ regular oncologists. This care also addresses the long-term sequelae of treatment, including arthritis and osteoporosis, referrals to receive screening for other cancers (eg, cervical and colon cancer), and genetic counselling as appropriate. In addition, a nutritionist provided general dietary advice in individual and group sessions every 3 months.
The self-administered questionnaires, PHQ-9, GAD-7, and SF-36, were completed at baseline, and every 3 months for 12 months. The scores were reviewed by the psychologist and the oncologist. The PHQ-9 was used to initially screen survivors for depression and monitor their improvement after the intervention. The PHQ-9 is a reliable and validated self-administered depression module.12 The PQH-9 exclusively focuses on the 9 diagnostic criteria for DSM-IV depression disorder and it can be used as a useful measure for monitoring outcomes of depression therapy. A score of 5-14 suggests mild-moderate depression, and a score of >15 suggests severe depression
The survivors were screened for anxiety using the GAD-7, a brief 7-item self-report scale to identify probable cases of anxiety disorder that has been shown to be an efficient tool for screening and assessing the severity of anxiety.13 For GAD-7, a score of 5 or higher is suggestive of anxiety. Scores of 5, 10, and 15 represent cut-off points for mild, moderate, and severe anxiety, respectively.
Survivor QoL was evaluated using the SF-36 questionnaire, a multipurpose survey containing 36 questions. It ranges from 0-100 and a score that is <50.0 is considered low. The lower the score, the worse the mental or physical function.14 The SF-36 yields a patient profile of 8 health domains – vitality, physical functioning, bodily pain, general health perceptions, physical, emotional, and social role functioning; and mental health.15,16 A score of 50.0 on either the Physical Component Summary (PCS – vitality, physical functioning, bodily pain, general health perceptions, physical role functioning) or Mental Component Summary (MCS – emotional and social role functioning, and mental health) is consistent with the US norm.
Statistical analysis
In this study, the primary objective was to use the MBSR survivorship program to improve the survivors’ outcomes at 12 months compared with baseline using the following measures: PHQ-9 for depression, GAD-7 for anxiety, and SF-36 for QoL using the PCS and MCS. Quantitative data were described using the mean and standard deviation, and categorical data were described using frequency and percentage. The outcome measures were compared between patients who completed 12-month follow-up and those who did not, using unpaired t test. The change in outcome measures at 12 months from baseline was evaluated using paired t test. The effect of intervention was summarized using relative percentage change. The “dose” of the intervention was categorized the number of MBSR sessions – ≤4 sessions, 5-7 sessions, or ≤8 sessions. The change in outcome measures were compared among three groups using 1-way analysis of variance (ANOVA) followed by post hoc multiple comparison using the Bonferroni adjustment. In addition, the effect of intervention on each outcome was evaluated by important baseline characteristics of patients. In each subgroup, the changes were compared with baseline measures using the paired t test, whereas changes in outcome between groups were compared using the unpaired t test. Statistical analyses were conducted using SAS 9.3. P-values less than 5% were considered to be significant.
Results
A total of 94 survivors of breast cancer were included in this study and 60 (63.8%) completed the 12 months of follow-up. The average age of the participants was 54.4 years (SD, 8.7), and 90.4% were Hispanic (Table 1). Tumor characteristics were as follows: invasive ductal carcinoma (84.04%), estrogen receptor–positive (ER-, 71.28%), progesterone receptor–positive (PR-, 58.51%), and HER2-neu–positive (20%). In regard to therapy received, 48% of the participants had received anthracycline- and taxane-based adjuvant chemotherapy and 23%, nonanthracycline-based chemotherapy; 71% had received anti-estrogen (hormonal) therapy and 80%, radiation therapy. In regard to surgery, half of the participants had a lumpectomy, and half, a mastectomy. The trends in the outcome measures over the follow-up period are show in the Figure 1.
The effect of survivorship program intervention on SF-36 (PCS and MCS), anxiety (GAD-7), and (PHQ-9) at 12 months are shown in Table 2, which also includes the 12-month effects on the body-mass index (BMI). The P-values correspond to the comparison of mean change in scores between baseline and 12-month follow-up. Significant improvement from baseline was observed for PHQ-9 (P = .0031) and GAD-7 (P = .0027). There was a significant trend toward improvement (14%) relative to baseline in the SF-36 MCS at 12 months (P = .097). Although the SF-36 PCS improved numerically, it did not reach to a statistical significance level (P = .896). The BMI at 12 months was found to be statistically significantly increased compared with baseline (P = .0007).
The effect of the number of MBSR sessions attended on the outcome measures is summarized in Table 3. There were significant improvements in the 12-month MCS scores for patients who completed 5-7 sessions of MBSR or ≥8 sessions, compared with patients who completed ≤4 sessions of MBSR. There was an improvement observed in PCS scores only among patients who received at least 8 sessions of MBSR. There was a marked improvement observed in GAD-7 and PHQ-9 among patients who received ≥8 sessions. There was no statistically significant change in the GAD-7 or PHQ-9 scores between patients who received ≤4 sessions and 5-7 sessions. No significant association was obtained between number of MBSR sessions attended and BMI.
The effect of survivorship program intervention on all outcomes according to important baseline cofactors is shown in Table 4. As such, there were no significant differences in changes in the outcome measures after intervention according to any considered baseline characteristics. However, the effect of survivorship program intervention was more pronounced in patients who were ≥3 years away from their initial diagnosis and who had attended a minimum of 80% of the 3-monthly visits and received a minimum of 8 MBSR sessions.
The mean baseline PCS and MCS scores of the SF-36 were 43.7 and 45.8, respectively, indicating that the participants’ scores were significantly less than half the standard deviation below the US norm (50.0; SD, 10). The SF-36 health-related QoL categories showed that, on an average, scores improved by more than 4 units for emotional and physical role functions, vitality, and mental health compared with baseline. In addition, scores improved by about 2 units for general health and social functioning compared with baseline data. In all, 65% of survivors had difficulty preforming work at baseline, but that dropped to 55% after enrollment in the program; and 60% had originally reduced the amount of time spent on work, but that increased to 50% after the intervention. Also of note is that 70% of survivors reported accomplishing less than they would like to have (role physical) before the intervention, but that was reduced to 57% after the intervention. Similarly, 77% of survivors felt worn out at baseline, compared with 65% at the 12-month follow-up; and 88% felt tired at baseline, but that percentage was reduced to 68% after the intervention. Before the intervention, 60% of the participants reported that they had been very nervous, and 45% said they had been so down in the dumps that nothing could cheer them up, but those percentages were reduced to 43% and 32%, respectively, after intervention. Before intervention, 63% of the women said they felt depressed and that was reduced to 50% after the intervention.
Discussion
In this study, we showed that a group of predominately Hispanic breast cancer survivors benefited from participating in a multidisciplinary cancer survivorship program that emphasized in-depth psychological care and MBSR. They also benefited from an education effort that included providing survivors with personalized summaries of their treatment and oncology survivorship care, addressing potential long-term side effects of treatment, referral for genetic counselling and screening for other cancers as appropriate, as well dietary advice. We found significant improvement compared with baseline in both mental and physical determinants of the patient-reported outcomes, including anxiety (GAD-7), depression (PHQ-9), and HR-QoL (PCS) and (MCS). Survivors demonstrated significant improvement on the MCS and PHQ-9 if they attended 5 or more sessions of the 8-week MBSR course, and attending 8 sessions was associated with significant improvement in GAD-7 and PCS. This might suggest that survivors who are more motivated do benefit the most from such program.
To our knowledge, this study is the first to address the benefit of the MBSR intervention in Hispanic breast cancer survivors. In a randomized controlled trial that included breast cancer survivors with stages 0-III breast cancer who completed surgery, adjunctive radiation, and/or chemotherapy, MBSR was shown to reduce the symptoms of depression and anxiety and increase energy and physical functioning compared with participants who received “usual care”.3 Furthermore, Bower and colleagues have reported improvements in sleep, fatigue, and pro-inflammatory signaling in younger survivors of breast cancer.17 A similar standardized MBSR program was tested on Danish women who had been treated for stage I-III breast cancer18 and the results showed reduced levels of anxiety and depression at the 12-month follow-up. A similar study by Hoffman and colleagues19 reported improved mood, breast- and endocrine-related quality of life, and well-being with MBSR compared with standard care in women with stage 0-III breast cancer.
Several theories have been suggested to explain how MBSR reduces symptoms of depression, anxiety, and fear of recurrence in breast cancer survivors, one of which is that it provides supportive interaction between group members to practice meditation and apply mindfulness in daily situations.3 In addition, evidence is beginning to emerge that stress-reducing interventions such as MBSR may improve telomere length (TL) and telomerase activity (TA), the markers for cellular aging, psychological stress, and disease risk.20-24 Lengacher and colleagues conducted a randomized controlled study to investigate the effects of MBSR on TL and TA in women with breast cancer, and suggested that MBSR increases telomere length and telomerase activity.25 The 142 patients with stages 0-III breast cancer had completed adjuvant treatment with radiation and/or chemotherapy at least 2 weeks before enrollment and within 2 years of completion of treatment with lumpectomy and/or mastectomy. They were randomly assigned to either a 6-week MBSR for breast cancer program or usual care.25 Assessments of TA and TL were obtained along with psychological measurements at baseline, 6 weeks, and 12 weeks after the patients had completed the MBSR program. The mean age of the participants was 55.3 years; 72% were non-Hispanic white; 78% had stage I or II cancer; and 36% received both chemotherapy and radiation. In analyses adjusted for baseline TA and psychological status, TA increased steadily by about 17% over 12 weeks in the MBSR group, compared with about 3% (P < .01) in the control group. No difference was observed for TL (P = .92). The authors concluded that the data provide preliminary evidence that MBSR increases TA in peripheral blood mononuclear cells from breast cancer patients and have implications for understanding how MBSR may extend cell longevity at the cellular level.
In another study among healthy volunteers who were randomly assigned to a 3-month meditation retreat or a control group, the 30 participants in the meditation group had higher TA compared with controls.20 In a nonrandomized study among prostate cancer patients, TA increased and psychological stress decreased following a stress-reducing, lifestyle-modification program.21 The results of another intervention study among overweight women showed improvement in distress, eating behavior, and metabolic health in women participating in a MBSR program, all of which correlated with increases in TA.22 Most recently, researchers explored the impact on TA of a Kirtan Kriya yogic meditation intervention compared with exposure to relaxing music in 39 dementia family caregivers. The yogic-meditation intervention group had a 43% increase in TA after the 8-week intervention period compared with 3.7% the music group (P < .05).23 Finally, among 22 patients with cervical cancer who were randomized to a psychosocial telephone counseling intervention,24 investigators found a significant association between increased TL and changes in psychological distress.20 Findings from other studies have assessed interventions to improve outcome of breast cancer survivors, such as the Taking CHARGE self-management intervention that is designed to facilitate the transition to survivorship after breast cancer treatment.8 Another intervention using home-based physical activity was shown in a randomized controlled trial to improve self-reported physical activity, body-mass index, and health-related QoL.9 Findings from another study suggested that a combined exercise and psychological counselling program might improve QoL more than a single entity intervention.26 As noted previously, these studies did not focus on minority breast cancer survivors’ population, and it is not clear if they are generalizable to Hispanics.
In addition to the MBSR component, our program has also included one-on-one psychological assessment for long-term treatment complications and provided participants with appropriate care and follow-up plans, adding the benefits of self-awareness and self-attention for the survivors, which can effectively reduce the fear of recurrence.3 Furthermore, we included dietary consults based on general cancer survivor guidelines recommending a high fruit and vegetable diet that is low in fat and sugar.27 Healthier dietary lifestyle has been reported to improve breast cancer prognosis, metabolic disease, and cardiovascular outcomes among Hispanic breast cancer survivors.28
Our study has some limitations, including a relatively small sample size. It did not include an exercise program, which would have been helpful in addressing the issue of overweight and obesity we encountered in the most of the Hispanic breast cancer survivors (baseline average BMI, 31.32 kg/m2; obesity range, >30 kg/m2). Because of the small sample size and nonrandomized design of the study, it is hard to evaluate the confounding effect of time on intervention effect. However, a subgroup analysis by MBSR number of sessions showed that the survivors who completed the full course of MBSR sessions (8 sessions) achieved superior benefit, compared with those who did not complete the full course, which indicates that the intervention did weigh in regardless of time. Despite these limitations, the participants in this interventional program showed improved outcomes, including less anxiety and depression and improved MCS score of the SF-36. A larger and longer follow-up prospective, randomized study is needed to validate the findings of this study. Implementing cancer survivorship as an integral component of cancer care during and after treatment is essential to improve the quality of life of cancer survivors and empower them in their transition from cancer treatment to survivorship.
1. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012 [published correction in CA Cancer J Clin. 2012;62(5):348].CA Cancer J Clin. 2012;62(4):220-241.
2. Williams F, Jeanetta SC. Lived experiences of breast cancer survivors after diagnosis, treatment and beyond: qualitative study. Health Expect. 2016;19(3):631-642.
3. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261-1272.
4. Feiten S, Dünnebacke J, Friesenhahn V, et al. Follow-up reality for breast cancer patients - standardised survey of patients and physicians and analysis of treatment data. Geburtshilfe Frauenheilkd. 2016;76(5):557-563.
5. Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85-95.
6. Nahleh ZA, Dwivedi A, Khang T, et al. Decreased health related quality of life among hispanic breast cancer survivors. http://medcraveonline.com/MOJWH/MOJWH-01-00016.php. Published January 28, 2016. Accessed July 25, 2017.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-254.
8. Cimprich B, Janz NK, Northouse L, Wren PA, Given B, Given CW. Taking CHARGE: a self-management program for women following breast cancer treatment. Psychooncology. 2005;14(9):704-717.
9. Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2258-5. Published 2016. Accessed July 25, 2017.
10. Huang J, Shi L. The effectiveness of mindfulness-based stress reduction (MBSR) for survivors of breast cancer: study protocol for a randomized controlled trial. Trials. 2016;17(1):209.
11. Stahl B and Goldstein E, A mindfulness-based stress reduction workbook. 2010: New Harbinger Publications.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
13. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
14. Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264-279.
15. Gandek B, Sinclair SJ, Kosinski M, Ware JE Jr. Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev. 2004;25(4):5-25.
16. Ruta D, Garratt A, Abdalla M, Buckingham K, Russell I. The SF-36 health survey questionnaire. A valid measure of health status. BMJ. 1993;307(6901):448-449.
17. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231-1240.
18. Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49(6):1365-1373.
19. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335-1342.
20. Jacobs TL, Epel ES, Lin J, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664-681.
21. Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048-1057.
22. Daubenmier J, Lin J, Blackburn E, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37(7):917-928.
23. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
24. Biegler KA, Anderson AK, Wenzel LB, Osann K, Nelson EL. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: implications for cancer prevention. Cancer Prev Res (Phila). 2012;5(10):1173-1182.
25. Lengacher CA, Reich RR, Kip KE. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC). Biol Res Nurs. 2014;16(4):438-447.
26. Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10(5):188-194.
27. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30-67.
28. Greenlee H, Gaffney AO, Aycinena AC, et al. Cocinar para su salud!: randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. J Acad Nutr Diet. 2015;115(5):709-723.e3.
Breast cancer survivors comprise the most prevalent cancer survivor population in the United States.1 The number of breast cancer survivors is increasing because of early detection and diagnosis, and advances in treatment have resulted in increased life expectancy. Therefore, greater attention is needed to improve the long-term quality of life of these survivors and to help them re-adjust to normal life. For many women, although the medical treatment may have been completed, the recovery process may have not.2 The prevalence of long-term mental and physical illness is significant among many breast cancer survivors. Long-term mental consequences may include memory problems, anxiety, depression, and fear of recurrence3, and long-term physical consequences may include pain, fatigue, and lymphedema, among others.4
El Paso, Texas, is the fourth most populous city in Texas and has a Hispanic majority. This provides an opportunity to conduct clinical research targeting participants of Hispanic descent. Several studies have noted the influence of race/ethnicity on the psychosocial function of breast cancer survivors.5,6 We have previously reported that Hispanic breast cancer survivors might experience decreased mental and physical health-related quality of life (QoL) which limit their normal social functioning.6Other studies have similarly reported poor outcomes of breast cancer survivors and higher rates of fatigue and depression among Hispanic patients.7 However, there is a paucity of research addressing specific interventions needed to improve these outcomes and provide better QoL for breast cancer survivors.8,9 In addition, a few survivorship care interventions have focused on minorities. We sought to assess whether a multidisciplinary cancer survivorship program in a primarily Hispanic populated area would lead to improved QoL and reduce anxiety and depressive symptoms among breast cancer survivors.
Methods
After obtaining Institutional Review Board approval, we recruited consecutive patients who were treated at our institution during October 2013-October 2014 and obtained informed consent from them. The participants were within the first 5 years after diagnosis with stages I-III breast cancer and had completed surgery, chemotherapy, and/or radiation therapy. We sought to determine whether breast cancer survivors would benefit from this intervention as determined by improvement of performance at 12 months compared with baseline based on the following self-reported validated questionnaires: Patient Health Questionnaire-9 (PHQ-9) for depression; General Anxiety Disorder-7 (GAD-7); and Short-Form Health Survey-36 (SF-36, version 2) for patient quality of life. The participants were enrolled in a comprehensive survivorship program staffed by an oncologist, an oncology nurse practitioner, a nutritionist, and a certified clinical psychologist who had trained in mindfulness-based stress reduction (MBSR).
Interventions
The participants received a one-on-one individual psychological consultation visit every 3 months for 20-45 minutes during which the psychologist addressed each patient’s emotional and psychological issues in depth, discussed relaxation techniques, and provided psychosocial counselling. In addition, all participants were asked to attend an 8-week-course (in Spanish or English) using MBSR, an interventional program in which participants receive training to promote reduction of stress by self-regulating mindfulness practice.3,10 Our institution’s MBSR program consists of a weekly 2-hour class for 8 sessions or more. The program is provided 3 times a year, in English and Spanish. It includes the following components:
- Learning various mindfulness meditation techniques (eg, body scans, awareness of breathing, sitting/walking meditations);
- Practicing the mindfulness techniques in class; and
- Practicing techniques at home through audiorecordings of mindfulness meditation exercises and daily diary writing.
Participants were provided with a workbook on MBSR in their preferred language.11 In addition to the psychological component, they were also provided with oncologic evaluations by an oncology nurse practitioner. The nurse practitioner met with participants every 3 months and provided each one with a personalized summary of all the treatments received and routine oncology follow-up care in consultation with the patients’ regular oncologists. This care also addresses the long-term sequelae of treatment, including arthritis and osteoporosis, referrals to receive screening for other cancers (eg, cervical and colon cancer), and genetic counselling as appropriate. In addition, a nutritionist provided general dietary advice in individual and group sessions every 3 months.
The self-administered questionnaires, PHQ-9, GAD-7, and SF-36, were completed at baseline, and every 3 months for 12 months. The scores were reviewed by the psychologist and the oncologist. The PHQ-9 was used to initially screen survivors for depression and monitor their improvement after the intervention. The PHQ-9 is a reliable and validated self-administered depression module.12 The PQH-9 exclusively focuses on the 9 diagnostic criteria for DSM-IV depression disorder and it can be used as a useful measure for monitoring outcomes of depression therapy. A score of 5-14 suggests mild-moderate depression, and a score of >15 suggests severe depression
The survivors were screened for anxiety using the GAD-7, a brief 7-item self-report scale to identify probable cases of anxiety disorder that has been shown to be an efficient tool for screening and assessing the severity of anxiety.13 For GAD-7, a score of 5 or higher is suggestive of anxiety. Scores of 5, 10, and 15 represent cut-off points for mild, moderate, and severe anxiety, respectively.
Survivor QoL was evaluated using the SF-36 questionnaire, a multipurpose survey containing 36 questions. It ranges from 0-100 and a score that is <50.0 is considered low. The lower the score, the worse the mental or physical function.14 The SF-36 yields a patient profile of 8 health domains – vitality, physical functioning, bodily pain, general health perceptions, physical, emotional, and social role functioning; and mental health.15,16 A score of 50.0 on either the Physical Component Summary (PCS – vitality, physical functioning, bodily pain, general health perceptions, physical role functioning) or Mental Component Summary (MCS – emotional and social role functioning, and mental health) is consistent with the US norm.
Statistical analysis
In this study, the primary objective was to use the MBSR survivorship program to improve the survivors’ outcomes at 12 months compared with baseline using the following measures: PHQ-9 for depression, GAD-7 for anxiety, and SF-36 for QoL using the PCS and MCS. Quantitative data were described using the mean and standard deviation, and categorical data were described using frequency and percentage. The outcome measures were compared between patients who completed 12-month follow-up and those who did not, using unpaired t test. The change in outcome measures at 12 months from baseline was evaluated using paired t test. The effect of intervention was summarized using relative percentage change. The “dose” of the intervention was categorized the number of MBSR sessions – ≤4 sessions, 5-7 sessions, or ≤8 sessions. The change in outcome measures were compared among three groups using 1-way analysis of variance (ANOVA) followed by post hoc multiple comparison using the Bonferroni adjustment. In addition, the effect of intervention on each outcome was evaluated by important baseline characteristics of patients. In each subgroup, the changes were compared with baseline measures using the paired t test, whereas changes in outcome between groups were compared using the unpaired t test. Statistical analyses were conducted using SAS 9.3. P-values less than 5% were considered to be significant.
Results
A total of 94 survivors of breast cancer were included in this study and 60 (63.8%) completed the 12 months of follow-up. The average age of the participants was 54.4 years (SD, 8.7), and 90.4% were Hispanic (Table 1). Tumor characteristics were as follows: invasive ductal carcinoma (84.04%), estrogen receptor–positive (ER-, 71.28%), progesterone receptor–positive (PR-, 58.51%), and HER2-neu–positive (20%). In regard to therapy received, 48% of the participants had received anthracycline- and taxane-based adjuvant chemotherapy and 23%, nonanthracycline-based chemotherapy; 71% had received anti-estrogen (hormonal) therapy and 80%, radiation therapy. In regard to surgery, half of the participants had a lumpectomy, and half, a mastectomy. The trends in the outcome measures over the follow-up period are show in the Figure 1.
The effect of survivorship program intervention on SF-36 (PCS and MCS), anxiety (GAD-7), and (PHQ-9) at 12 months are shown in Table 2, which also includes the 12-month effects on the body-mass index (BMI). The P-values correspond to the comparison of mean change in scores between baseline and 12-month follow-up. Significant improvement from baseline was observed for PHQ-9 (P = .0031) and GAD-7 (P = .0027). There was a significant trend toward improvement (14%) relative to baseline in the SF-36 MCS at 12 months (P = .097). Although the SF-36 PCS improved numerically, it did not reach to a statistical significance level (P = .896). The BMI at 12 months was found to be statistically significantly increased compared with baseline (P = .0007).
The effect of the number of MBSR sessions attended on the outcome measures is summarized in Table 3. There were significant improvements in the 12-month MCS scores for patients who completed 5-7 sessions of MBSR or ≥8 sessions, compared with patients who completed ≤4 sessions of MBSR. There was an improvement observed in PCS scores only among patients who received at least 8 sessions of MBSR. There was a marked improvement observed in GAD-7 and PHQ-9 among patients who received ≥8 sessions. There was no statistically significant change in the GAD-7 or PHQ-9 scores between patients who received ≤4 sessions and 5-7 sessions. No significant association was obtained between number of MBSR sessions attended and BMI.
The effect of survivorship program intervention on all outcomes according to important baseline cofactors is shown in Table 4. As such, there were no significant differences in changes in the outcome measures after intervention according to any considered baseline characteristics. However, the effect of survivorship program intervention was more pronounced in patients who were ≥3 years away from their initial diagnosis and who had attended a minimum of 80% of the 3-monthly visits and received a minimum of 8 MBSR sessions.
The mean baseline PCS and MCS scores of the SF-36 were 43.7 and 45.8, respectively, indicating that the participants’ scores were significantly less than half the standard deviation below the US norm (50.0; SD, 10). The SF-36 health-related QoL categories showed that, on an average, scores improved by more than 4 units for emotional and physical role functions, vitality, and mental health compared with baseline. In addition, scores improved by about 2 units for general health and social functioning compared with baseline data. In all, 65% of survivors had difficulty preforming work at baseline, but that dropped to 55% after enrollment in the program; and 60% had originally reduced the amount of time spent on work, but that increased to 50% after the intervention. Also of note is that 70% of survivors reported accomplishing less than they would like to have (role physical) before the intervention, but that was reduced to 57% after the intervention. Similarly, 77% of survivors felt worn out at baseline, compared with 65% at the 12-month follow-up; and 88% felt tired at baseline, but that percentage was reduced to 68% after the intervention. Before the intervention, 60% of the participants reported that they had been very nervous, and 45% said they had been so down in the dumps that nothing could cheer them up, but those percentages were reduced to 43% and 32%, respectively, after intervention. Before intervention, 63% of the women said they felt depressed and that was reduced to 50% after the intervention.
Discussion
In this study, we showed that a group of predominately Hispanic breast cancer survivors benefited from participating in a multidisciplinary cancer survivorship program that emphasized in-depth psychological care and MBSR. They also benefited from an education effort that included providing survivors with personalized summaries of their treatment and oncology survivorship care, addressing potential long-term side effects of treatment, referral for genetic counselling and screening for other cancers as appropriate, as well dietary advice. We found significant improvement compared with baseline in both mental and physical determinants of the patient-reported outcomes, including anxiety (GAD-7), depression (PHQ-9), and HR-QoL (PCS) and (MCS). Survivors demonstrated significant improvement on the MCS and PHQ-9 if they attended 5 or more sessions of the 8-week MBSR course, and attending 8 sessions was associated with significant improvement in GAD-7 and PCS. This might suggest that survivors who are more motivated do benefit the most from such program.
To our knowledge, this study is the first to address the benefit of the MBSR intervention in Hispanic breast cancer survivors. In a randomized controlled trial that included breast cancer survivors with stages 0-III breast cancer who completed surgery, adjunctive radiation, and/or chemotherapy, MBSR was shown to reduce the symptoms of depression and anxiety and increase energy and physical functioning compared with participants who received “usual care”.3 Furthermore, Bower and colleagues have reported improvements in sleep, fatigue, and pro-inflammatory signaling in younger survivors of breast cancer.17 A similar standardized MBSR program was tested on Danish women who had been treated for stage I-III breast cancer18 and the results showed reduced levels of anxiety and depression at the 12-month follow-up. A similar study by Hoffman and colleagues19 reported improved mood, breast- and endocrine-related quality of life, and well-being with MBSR compared with standard care in women with stage 0-III breast cancer.
Several theories have been suggested to explain how MBSR reduces symptoms of depression, anxiety, and fear of recurrence in breast cancer survivors, one of which is that it provides supportive interaction between group members to practice meditation and apply mindfulness in daily situations.3 In addition, evidence is beginning to emerge that stress-reducing interventions such as MBSR may improve telomere length (TL) and telomerase activity (TA), the markers for cellular aging, psychological stress, and disease risk.20-24 Lengacher and colleagues conducted a randomized controlled study to investigate the effects of MBSR on TL and TA in women with breast cancer, and suggested that MBSR increases telomere length and telomerase activity.25 The 142 patients with stages 0-III breast cancer had completed adjuvant treatment with radiation and/or chemotherapy at least 2 weeks before enrollment and within 2 years of completion of treatment with lumpectomy and/or mastectomy. They were randomly assigned to either a 6-week MBSR for breast cancer program or usual care.25 Assessments of TA and TL were obtained along with psychological measurements at baseline, 6 weeks, and 12 weeks after the patients had completed the MBSR program. The mean age of the participants was 55.3 years; 72% were non-Hispanic white; 78% had stage I or II cancer; and 36% received both chemotherapy and radiation. In analyses adjusted for baseline TA and psychological status, TA increased steadily by about 17% over 12 weeks in the MBSR group, compared with about 3% (P < .01) in the control group. No difference was observed for TL (P = .92). The authors concluded that the data provide preliminary evidence that MBSR increases TA in peripheral blood mononuclear cells from breast cancer patients and have implications for understanding how MBSR may extend cell longevity at the cellular level.
In another study among healthy volunteers who were randomly assigned to a 3-month meditation retreat or a control group, the 30 participants in the meditation group had higher TA compared with controls.20 In a nonrandomized study among prostate cancer patients, TA increased and psychological stress decreased following a stress-reducing, lifestyle-modification program.21 The results of another intervention study among overweight women showed improvement in distress, eating behavior, and metabolic health in women participating in a MBSR program, all of which correlated with increases in TA.22 Most recently, researchers explored the impact on TA of a Kirtan Kriya yogic meditation intervention compared with exposure to relaxing music in 39 dementia family caregivers. The yogic-meditation intervention group had a 43% increase in TA after the 8-week intervention period compared with 3.7% the music group (P < .05).23 Finally, among 22 patients with cervical cancer who were randomized to a psychosocial telephone counseling intervention,24 investigators found a significant association between increased TL and changes in psychological distress.20 Findings from other studies have assessed interventions to improve outcome of breast cancer survivors, such as the Taking CHARGE self-management intervention that is designed to facilitate the transition to survivorship after breast cancer treatment.8 Another intervention using home-based physical activity was shown in a randomized controlled trial to improve self-reported physical activity, body-mass index, and health-related QoL.9 Findings from another study suggested that a combined exercise and psychological counselling program might improve QoL more than a single entity intervention.26 As noted previously, these studies did not focus on minority breast cancer survivors’ population, and it is not clear if they are generalizable to Hispanics.
In addition to the MBSR component, our program has also included one-on-one psychological assessment for long-term treatment complications and provided participants with appropriate care and follow-up plans, adding the benefits of self-awareness and self-attention for the survivors, which can effectively reduce the fear of recurrence.3 Furthermore, we included dietary consults based on general cancer survivor guidelines recommending a high fruit and vegetable diet that is low in fat and sugar.27 Healthier dietary lifestyle has been reported to improve breast cancer prognosis, metabolic disease, and cardiovascular outcomes among Hispanic breast cancer survivors.28
Our study has some limitations, including a relatively small sample size. It did not include an exercise program, which would have been helpful in addressing the issue of overweight and obesity we encountered in the most of the Hispanic breast cancer survivors (baseline average BMI, 31.32 kg/m2; obesity range, >30 kg/m2). Because of the small sample size and nonrandomized design of the study, it is hard to evaluate the confounding effect of time on intervention effect. However, a subgroup analysis by MBSR number of sessions showed that the survivors who completed the full course of MBSR sessions (8 sessions) achieved superior benefit, compared with those who did not complete the full course, which indicates that the intervention did weigh in regardless of time. Despite these limitations, the participants in this interventional program showed improved outcomes, including less anxiety and depression and improved MCS score of the SF-36. A larger and longer follow-up prospective, randomized study is needed to validate the findings of this study. Implementing cancer survivorship as an integral component of cancer care during and after treatment is essential to improve the quality of life of cancer survivors and empower them in their transition from cancer treatment to survivorship.
Breast cancer survivors comprise the most prevalent cancer survivor population in the United States.1 The number of breast cancer survivors is increasing because of early detection and diagnosis, and advances in treatment have resulted in increased life expectancy. Therefore, greater attention is needed to improve the long-term quality of life of these survivors and to help them re-adjust to normal life. For many women, although the medical treatment may have been completed, the recovery process may have not.2 The prevalence of long-term mental and physical illness is significant among many breast cancer survivors. Long-term mental consequences may include memory problems, anxiety, depression, and fear of recurrence3, and long-term physical consequences may include pain, fatigue, and lymphedema, among others.4
El Paso, Texas, is the fourth most populous city in Texas and has a Hispanic majority. This provides an opportunity to conduct clinical research targeting participants of Hispanic descent. Several studies have noted the influence of race/ethnicity on the psychosocial function of breast cancer survivors.5,6 We have previously reported that Hispanic breast cancer survivors might experience decreased mental and physical health-related quality of life (QoL) which limit their normal social functioning.6Other studies have similarly reported poor outcomes of breast cancer survivors and higher rates of fatigue and depression among Hispanic patients.7 However, there is a paucity of research addressing specific interventions needed to improve these outcomes and provide better QoL for breast cancer survivors.8,9 In addition, a few survivorship care interventions have focused on minorities. We sought to assess whether a multidisciplinary cancer survivorship program in a primarily Hispanic populated area would lead to improved QoL and reduce anxiety and depressive symptoms among breast cancer survivors.
Methods
After obtaining Institutional Review Board approval, we recruited consecutive patients who were treated at our institution during October 2013-October 2014 and obtained informed consent from them. The participants were within the first 5 years after diagnosis with stages I-III breast cancer and had completed surgery, chemotherapy, and/or radiation therapy. We sought to determine whether breast cancer survivors would benefit from this intervention as determined by improvement of performance at 12 months compared with baseline based on the following self-reported validated questionnaires: Patient Health Questionnaire-9 (PHQ-9) for depression; General Anxiety Disorder-7 (GAD-7); and Short-Form Health Survey-36 (SF-36, version 2) for patient quality of life. The participants were enrolled in a comprehensive survivorship program staffed by an oncologist, an oncology nurse practitioner, a nutritionist, and a certified clinical psychologist who had trained in mindfulness-based stress reduction (MBSR).
Interventions
The participants received a one-on-one individual psychological consultation visit every 3 months for 20-45 minutes during which the psychologist addressed each patient’s emotional and psychological issues in depth, discussed relaxation techniques, and provided psychosocial counselling. In addition, all participants were asked to attend an 8-week-course (in Spanish or English) using MBSR, an interventional program in which participants receive training to promote reduction of stress by self-regulating mindfulness practice.3,10 Our institution’s MBSR program consists of a weekly 2-hour class for 8 sessions or more. The program is provided 3 times a year, in English and Spanish. It includes the following components:
- Learning various mindfulness meditation techniques (eg, body scans, awareness of breathing, sitting/walking meditations);
- Practicing the mindfulness techniques in class; and
- Practicing techniques at home through audiorecordings of mindfulness meditation exercises and daily diary writing.
Participants were provided with a workbook on MBSR in their preferred language.11 In addition to the psychological component, they were also provided with oncologic evaluations by an oncology nurse practitioner. The nurse practitioner met with participants every 3 months and provided each one with a personalized summary of all the treatments received and routine oncology follow-up care in consultation with the patients’ regular oncologists. This care also addresses the long-term sequelae of treatment, including arthritis and osteoporosis, referrals to receive screening for other cancers (eg, cervical and colon cancer), and genetic counselling as appropriate. In addition, a nutritionist provided general dietary advice in individual and group sessions every 3 months.
The self-administered questionnaires, PHQ-9, GAD-7, and SF-36, were completed at baseline, and every 3 months for 12 months. The scores were reviewed by the psychologist and the oncologist. The PHQ-9 was used to initially screen survivors for depression and monitor their improvement after the intervention. The PHQ-9 is a reliable and validated self-administered depression module.12 The PQH-9 exclusively focuses on the 9 diagnostic criteria for DSM-IV depression disorder and it can be used as a useful measure for monitoring outcomes of depression therapy. A score of 5-14 suggests mild-moderate depression, and a score of >15 suggests severe depression
The survivors were screened for anxiety using the GAD-7, a brief 7-item self-report scale to identify probable cases of anxiety disorder that has been shown to be an efficient tool for screening and assessing the severity of anxiety.13 For GAD-7, a score of 5 or higher is suggestive of anxiety. Scores of 5, 10, and 15 represent cut-off points for mild, moderate, and severe anxiety, respectively.
Survivor QoL was evaluated using the SF-36 questionnaire, a multipurpose survey containing 36 questions. It ranges from 0-100 and a score that is <50.0 is considered low. The lower the score, the worse the mental or physical function.14 The SF-36 yields a patient profile of 8 health domains – vitality, physical functioning, bodily pain, general health perceptions, physical, emotional, and social role functioning; and mental health.15,16 A score of 50.0 on either the Physical Component Summary (PCS – vitality, physical functioning, bodily pain, general health perceptions, physical role functioning) or Mental Component Summary (MCS – emotional and social role functioning, and mental health) is consistent with the US norm.
Statistical analysis
In this study, the primary objective was to use the MBSR survivorship program to improve the survivors’ outcomes at 12 months compared with baseline using the following measures: PHQ-9 for depression, GAD-7 for anxiety, and SF-36 for QoL using the PCS and MCS. Quantitative data were described using the mean and standard deviation, and categorical data were described using frequency and percentage. The outcome measures were compared between patients who completed 12-month follow-up and those who did not, using unpaired t test. The change in outcome measures at 12 months from baseline was evaluated using paired t test. The effect of intervention was summarized using relative percentage change. The “dose” of the intervention was categorized the number of MBSR sessions – ≤4 sessions, 5-7 sessions, or ≤8 sessions. The change in outcome measures were compared among three groups using 1-way analysis of variance (ANOVA) followed by post hoc multiple comparison using the Bonferroni adjustment. In addition, the effect of intervention on each outcome was evaluated by important baseline characteristics of patients. In each subgroup, the changes were compared with baseline measures using the paired t test, whereas changes in outcome between groups were compared using the unpaired t test. Statistical analyses were conducted using SAS 9.3. P-values less than 5% were considered to be significant.
Results
A total of 94 survivors of breast cancer were included in this study and 60 (63.8%) completed the 12 months of follow-up. The average age of the participants was 54.4 years (SD, 8.7), and 90.4% were Hispanic (Table 1). Tumor characteristics were as follows: invasive ductal carcinoma (84.04%), estrogen receptor–positive (ER-, 71.28%), progesterone receptor–positive (PR-, 58.51%), and HER2-neu–positive (20%). In regard to therapy received, 48% of the participants had received anthracycline- and taxane-based adjuvant chemotherapy and 23%, nonanthracycline-based chemotherapy; 71% had received anti-estrogen (hormonal) therapy and 80%, radiation therapy. In regard to surgery, half of the participants had a lumpectomy, and half, a mastectomy. The trends in the outcome measures over the follow-up period are show in the Figure 1.
The effect of survivorship program intervention on SF-36 (PCS and MCS), anxiety (GAD-7), and (PHQ-9) at 12 months are shown in Table 2, which also includes the 12-month effects on the body-mass index (BMI). The P-values correspond to the comparison of mean change in scores between baseline and 12-month follow-up. Significant improvement from baseline was observed for PHQ-9 (P = .0031) and GAD-7 (P = .0027). There was a significant trend toward improvement (14%) relative to baseline in the SF-36 MCS at 12 months (P = .097). Although the SF-36 PCS improved numerically, it did not reach to a statistical significance level (P = .896). The BMI at 12 months was found to be statistically significantly increased compared with baseline (P = .0007).
The effect of the number of MBSR sessions attended on the outcome measures is summarized in Table 3. There were significant improvements in the 12-month MCS scores for patients who completed 5-7 sessions of MBSR or ≥8 sessions, compared with patients who completed ≤4 sessions of MBSR. There was an improvement observed in PCS scores only among patients who received at least 8 sessions of MBSR. There was a marked improvement observed in GAD-7 and PHQ-9 among patients who received ≥8 sessions. There was no statistically significant change in the GAD-7 or PHQ-9 scores between patients who received ≤4 sessions and 5-7 sessions. No significant association was obtained between number of MBSR sessions attended and BMI.
The effect of survivorship program intervention on all outcomes according to important baseline cofactors is shown in Table 4. As such, there were no significant differences in changes in the outcome measures after intervention according to any considered baseline characteristics. However, the effect of survivorship program intervention was more pronounced in patients who were ≥3 years away from their initial diagnosis and who had attended a minimum of 80% of the 3-monthly visits and received a minimum of 8 MBSR sessions.
The mean baseline PCS and MCS scores of the SF-36 were 43.7 and 45.8, respectively, indicating that the participants’ scores were significantly less than half the standard deviation below the US norm (50.0; SD, 10). The SF-36 health-related QoL categories showed that, on an average, scores improved by more than 4 units for emotional and physical role functions, vitality, and mental health compared with baseline. In addition, scores improved by about 2 units for general health and social functioning compared with baseline data. In all, 65% of survivors had difficulty preforming work at baseline, but that dropped to 55% after enrollment in the program; and 60% had originally reduced the amount of time spent on work, but that increased to 50% after the intervention. Also of note is that 70% of survivors reported accomplishing less than they would like to have (role physical) before the intervention, but that was reduced to 57% after the intervention. Similarly, 77% of survivors felt worn out at baseline, compared with 65% at the 12-month follow-up; and 88% felt tired at baseline, but that percentage was reduced to 68% after the intervention. Before the intervention, 60% of the participants reported that they had been very nervous, and 45% said they had been so down in the dumps that nothing could cheer them up, but those percentages were reduced to 43% and 32%, respectively, after intervention. Before intervention, 63% of the women said they felt depressed and that was reduced to 50% after the intervention.
Discussion
In this study, we showed that a group of predominately Hispanic breast cancer survivors benefited from participating in a multidisciplinary cancer survivorship program that emphasized in-depth psychological care and MBSR. They also benefited from an education effort that included providing survivors with personalized summaries of their treatment and oncology survivorship care, addressing potential long-term side effects of treatment, referral for genetic counselling and screening for other cancers as appropriate, as well dietary advice. We found significant improvement compared with baseline in both mental and physical determinants of the patient-reported outcomes, including anxiety (GAD-7), depression (PHQ-9), and HR-QoL (PCS) and (MCS). Survivors demonstrated significant improvement on the MCS and PHQ-9 if they attended 5 or more sessions of the 8-week MBSR course, and attending 8 sessions was associated with significant improvement in GAD-7 and PCS. This might suggest that survivors who are more motivated do benefit the most from such program.
To our knowledge, this study is the first to address the benefit of the MBSR intervention in Hispanic breast cancer survivors. In a randomized controlled trial that included breast cancer survivors with stages 0-III breast cancer who completed surgery, adjunctive radiation, and/or chemotherapy, MBSR was shown to reduce the symptoms of depression and anxiety and increase energy and physical functioning compared with participants who received “usual care”.3 Furthermore, Bower and colleagues have reported improvements in sleep, fatigue, and pro-inflammatory signaling in younger survivors of breast cancer.17 A similar standardized MBSR program was tested on Danish women who had been treated for stage I-III breast cancer18 and the results showed reduced levels of anxiety and depression at the 12-month follow-up. A similar study by Hoffman and colleagues19 reported improved mood, breast- and endocrine-related quality of life, and well-being with MBSR compared with standard care in women with stage 0-III breast cancer.
Several theories have been suggested to explain how MBSR reduces symptoms of depression, anxiety, and fear of recurrence in breast cancer survivors, one of which is that it provides supportive interaction between group members to practice meditation and apply mindfulness in daily situations.3 In addition, evidence is beginning to emerge that stress-reducing interventions such as MBSR may improve telomere length (TL) and telomerase activity (TA), the markers for cellular aging, psychological stress, and disease risk.20-24 Lengacher and colleagues conducted a randomized controlled study to investigate the effects of MBSR on TL and TA in women with breast cancer, and suggested that MBSR increases telomere length and telomerase activity.25 The 142 patients with stages 0-III breast cancer had completed adjuvant treatment with radiation and/or chemotherapy at least 2 weeks before enrollment and within 2 years of completion of treatment with lumpectomy and/or mastectomy. They were randomly assigned to either a 6-week MBSR for breast cancer program or usual care.25 Assessments of TA and TL were obtained along with psychological measurements at baseline, 6 weeks, and 12 weeks after the patients had completed the MBSR program. The mean age of the participants was 55.3 years; 72% were non-Hispanic white; 78% had stage I or II cancer; and 36% received both chemotherapy and radiation. In analyses adjusted for baseline TA and psychological status, TA increased steadily by about 17% over 12 weeks in the MBSR group, compared with about 3% (P < .01) in the control group. No difference was observed for TL (P = .92). The authors concluded that the data provide preliminary evidence that MBSR increases TA in peripheral blood mononuclear cells from breast cancer patients and have implications for understanding how MBSR may extend cell longevity at the cellular level.
In another study among healthy volunteers who were randomly assigned to a 3-month meditation retreat or a control group, the 30 participants in the meditation group had higher TA compared with controls.20 In a nonrandomized study among prostate cancer patients, TA increased and psychological stress decreased following a stress-reducing, lifestyle-modification program.21 The results of another intervention study among overweight women showed improvement in distress, eating behavior, and metabolic health in women participating in a MBSR program, all of which correlated with increases in TA.22 Most recently, researchers explored the impact on TA of a Kirtan Kriya yogic meditation intervention compared with exposure to relaxing music in 39 dementia family caregivers. The yogic-meditation intervention group had a 43% increase in TA after the 8-week intervention period compared with 3.7% the music group (P < .05).23 Finally, among 22 patients with cervical cancer who were randomized to a psychosocial telephone counseling intervention,24 investigators found a significant association between increased TL and changes in psychological distress.20 Findings from other studies have assessed interventions to improve outcome of breast cancer survivors, such as the Taking CHARGE self-management intervention that is designed to facilitate the transition to survivorship after breast cancer treatment.8 Another intervention using home-based physical activity was shown in a randomized controlled trial to improve self-reported physical activity, body-mass index, and health-related QoL.9 Findings from another study suggested that a combined exercise and psychological counselling program might improve QoL more than a single entity intervention.26 As noted previously, these studies did not focus on minority breast cancer survivors’ population, and it is not clear if they are generalizable to Hispanics.
In addition to the MBSR component, our program has also included one-on-one psychological assessment for long-term treatment complications and provided participants with appropriate care and follow-up plans, adding the benefits of self-awareness and self-attention for the survivors, which can effectively reduce the fear of recurrence.3 Furthermore, we included dietary consults based on general cancer survivor guidelines recommending a high fruit and vegetable diet that is low in fat and sugar.27 Healthier dietary lifestyle has been reported to improve breast cancer prognosis, metabolic disease, and cardiovascular outcomes among Hispanic breast cancer survivors.28
Our study has some limitations, including a relatively small sample size. It did not include an exercise program, which would have been helpful in addressing the issue of overweight and obesity we encountered in the most of the Hispanic breast cancer survivors (baseline average BMI, 31.32 kg/m2; obesity range, >30 kg/m2). Because of the small sample size and nonrandomized design of the study, it is hard to evaluate the confounding effect of time on intervention effect. However, a subgroup analysis by MBSR number of sessions showed that the survivors who completed the full course of MBSR sessions (8 sessions) achieved superior benefit, compared with those who did not complete the full course, which indicates that the intervention did weigh in regardless of time. Despite these limitations, the participants in this interventional program showed improved outcomes, including less anxiety and depression and improved MCS score of the SF-36. A larger and longer follow-up prospective, randomized study is needed to validate the findings of this study. Implementing cancer survivorship as an integral component of cancer care during and after treatment is essential to improve the quality of life of cancer survivors and empower them in their transition from cancer treatment to survivorship.
1. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012 [published correction in CA Cancer J Clin. 2012;62(5):348].CA Cancer J Clin. 2012;62(4):220-241.
2. Williams F, Jeanetta SC. Lived experiences of breast cancer survivors after diagnosis, treatment and beyond: qualitative study. Health Expect. 2016;19(3):631-642.
3. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261-1272.
4. Feiten S, Dünnebacke J, Friesenhahn V, et al. Follow-up reality for breast cancer patients - standardised survey of patients and physicians and analysis of treatment data. Geburtshilfe Frauenheilkd. 2016;76(5):557-563.
5. Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85-95.
6. Nahleh ZA, Dwivedi A, Khang T, et al. Decreased health related quality of life among hispanic breast cancer survivors. http://medcraveonline.com/MOJWH/MOJWH-01-00016.php. Published January 28, 2016. Accessed July 25, 2017.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-254.
8. Cimprich B, Janz NK, Northouse L, Wren PA, Given B, Given CW. Taking CHARGE: a self-management program for women following breast cancer treatment. Psychooncology. 2005;14(9):704-717.
9. Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2258-5. Published 2016. Accessed July 25, 2017.
10. Huang J, Shi L. The effectiveness of mindfulness-based stress reduction (MBSR) for survivors of breast cancer: study protocol for a randomized controlled trial. Trials. 2016;17(1):209.
11. Stahl B and Goldstein E, A mindfulness-based stress reduction workbook. 2010: New Harbinger Publications.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
13. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
14. Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264-279.
15. Gandek B, Sinclair SJ, Kosinski M, Ware JE Jr. Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev. 2004;25(4):5-25.
16. Ruta D, Garratt A, Abdalla M, Buckingham K, Russell I. The SF-36 health survey questionnaire. A valid measure of health status. BMJ. 1993;307(6901):448-449.
17. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231-1240.
18. Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49(6):1365-1373.
19. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335-1342.
20. Jacobs TL, Epel ES, Lin J, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664-681.
21. Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048-1057.
22. Daubenmier J, Lin J, Blackburn E, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37(7):917-928.
23. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
24. Biegler KA, Anderson AK, Wenzel LB, Osann K, Nelson EL. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: implications for cancer prevention. Cancer Prev Res (Phila). 2012;5(10):1173-1182.
25. Lengacher CA, Reich RR, Kip KE. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC). Biol Res Nurs. 2014;16(4):438-447.
26. Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10(5):188-194.
27. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30-67.
28. Greenlee H, Gaffney AO, Aycinena AC, et al. Cocinar para su salud!: randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. J Acad Nutr Diet. 2015;115(5):709-723.e3.
1. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012 [published correction in CA Cancer J Clin. 2012;62(5):348].CA Cancer J Clin. 2012;62(4):220-241.
2. Williams F, Jeanetta SC. Lived experiences of breast cancer survivors after diagnosis, treatment and beyond: qualitative study. Health Expect. 2016;19(3):631-642.
3. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261-1272.
4. Feiten S, Dünnebacke J, Friesenhahn V, et al. Follow-up reality for breast cancer patients - standardised survey of patients and physicians and analysis of treatment data. Geburtshilfe Frauenheilkd. 2016;76(5):557-563.
5. Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85-95.
6. Nahleh ZA, Dwivedi A, Khang T, et al. Decreased health related quality of life among hispanic breast cancer survivors. http://medcraveonline.com/MOJWH/MOJWH-01-00016.php. Published January 28, 2016. Accessed July 25, 2017.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-254.
8. Cimprich B, Janz NK, Northouse L, Wren PA, Given B, Given CW. Taking CHARGE: a self-management program for women following breast cancer treatment. Psychooncology. 2005;14(9):704-717.
9. Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2258-5. Published 2016. Accessed July 25, 2017.
10. Huang J, Shi L. The effectiveness of mindfulness-based stress reduction (MBSR) for survivors of breast cancer: study protocol for a randomized controlled trial. Trials. 2016;17(1):209.
11. Stahl B and Goldstein E, A mindfulness-based stress reduction workbook. 2010: New Harbinger Publications.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
13. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
14. Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264-279.
15. Gandek B, Sinclair SJ, Kosinski M, Ware JE Jr. Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev. 2004;25(4):5-25.
16. Ruta D, Garratt A, Abdalla M, Buckingham K, Russell I. The SF-36 health survey questionnaire. A valid measure of health status. BMJ. 1993;307(6901):448-449.
17. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231-1240.
18. Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49(6):1365-1373.
19. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335-1342.
20. Jacobs TL, Epel ES, Lin J, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664-681.
21. Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048-1057.
22. Daubenmier J, Lin J, Blackburn E, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37(7):917-928.
23. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
24. Biegler KA, Anderson AK, Wenzel LB, Osann K, Nelson EL. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: implications for cancer prevention. Cancer Prev Res (Phila). 2012;5(10):1173-1182.
25. Lengacher CA, Reich RR, Kip KE. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC). Biol Res Nurs. 2014;16(4):438-447.
26. Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10(5):188-194.
27. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30-67.
28. Greenlee H, Gaffney AO, Aycinena AC, et al. Cocinar para su salud!: randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. J Acad Nutr Diet. 2015;115(5):709-723.e3.
Goals-of-care discussions
Goals-of-care conversations led by the oncologist are a key opportunity to improve advance care planning and end-of-life care,1 but our patients are not understanding the essentials. Findings from a study of patients’ expectations about chemotherapy showed that more than two-thirds of patients with lung or colorectal cancers thought their palliative chemotherapy,2 radiation, 3 and/or surgery4 could cure them. Failure to effectively educate patients can lead to end-of-life care associated with poor quality of care, including over-aggressive care, poor quality of life with suboptimal symptom management, caregiver distress, and other potentially preventable problems.5
Palliative care routinely includes goals-of-care (GoC) discussions as part of the standard note. At one large urban hospital, the 30-day readmission rate was 10% if palliative care consultation was done, compared with 15% if no consultation was obtained.6 Patients who had consultations that included GoC discussions in addition to a symptom management consultation had a lower hospital readmission rate of 5%, compared with 15% in patients who received symptom management consultations alone (adjusted odds ratio [AOR], 0.36; confidence interval [CI], 0.27-0.48; P < .001). 6 Findings from another study showed that the use of aggressive end-of-life care was reduced by one-third when the patient and provider had a GoC planning session close to the time of diagnosis, instead of at the end of life.7
We prepared a template to be incorporated into the electronic medical record (EMR) to facilitate GoC discussions between the oncologist and patient as part of a randomized phase 3 trial of early versus delayed palliative care for phase 1 clinical trial patients. Documentation is one of the most important elements of efforts to improve end-of-life care, along with advance care planning, GoC discussions, and understanding the system of care.8 Because we have found this approach useful in our everyday oncology practice, we are sharing this simple, doable template in the hope that others will find it effective.
The GoC template
What does it look like?
We developed a document for oncology GoC discussions that was incorporated into the EMR (Table). We use the GoC document in much the same way we’d review a computed-tomography scan with our patients and their families: we bring it up on the computer screen, show them the categories, and type in responses on the allotted spaces on the right-hand side of the document. The input can be done in real time or after the conversation, or even after the patient has left. In our practice, we access the Patient Instructions part of the EMR, use a SmartPhrase to copy the Word template into the chart, and start typing.
The completed GoC sheet can be used in several ways. Most commonly, we print it out for the patient and family, so that everyone has access to the same information. Printing it out as part of the after-visit patient summary also satisfies the meaningful use requirement for EMRs. It is also possible to cut and paste the document into a letter to the care team, or directly into the Progress Note, so that it will be available for members of the care team to see.
The GoC form does take some time to complete. Palliative care teams that have reported on what was done during the palliative care visit have noted that the initial and subsequent visits took about an hour, with about 20 minutes devoted to symptom management, 15 minutes to patient and family coping, and 10 minutes to illness understanding and education, including prognosis.9,10 In our own practice, we do questions 1-9 first (Table, shaded area) because it takes less time than anticipated, just like code status discussions,11 and it is much easier with a script.
We use this template for in- and out-patient consultations and for routine visits when a GoC discussion is warranted. It is important to remember that doing this counts as advance care planning and can be billed using the ACP codes.
How do you use template?
It is critically important to make sure that the patient and family are ready for this discussion. To further facilitate the discussion, we have devised a temporary palliative care tattoo with a script, or prompts, for what questions to ask and in what order they should be asked (Figure). The easy-to-read tattoo is worn on the inner forearm so that it is readily visible to the oncologist or advanced practice nurse.12
We always start by asking, “How do you like to get medical information?” (Table, question 1) and follow up with something like, “Are you the sort of person who wants all the details, or not?” (Table). If the response is yes, they want all the details, then we follow up with another question, “Does that include talking about prognosis and what might happen?” (Table). Most patients will want full disclosure of their circumstances and prognosis, but some will not, and will feel overwhelmed and disempowered if you proceed.
After reviewing the answers to question 1 and any follow-up questions you may have asked, you will be able to gauge whether you should continue with questions 2-4 in the template. Once you know more about the patient and the family’s understanding of the situation (Table, question 2), what is important to them (question 3), what they are hoping for (question 4), and have spoken to them about disease progression, recapped their treatment to date, and checked to see if they might be eligible for any clinical trials (question 5), it will be easier to move on to the next questions, about progressive disease and advance care planning: “You are doing OK now, but have you thought about a time when you could be sicker [and need] a living will or advance directive [question 6, see next section of this article]?” For people unwilling to have this discussion, or have it at that moment, there is an excellent article that outlines the process to help practitioners increase prognostic awareness.14 Patient readiness will change over time as they adjust to the life changes forced by serious illness, and one can put off the discussion until they are more accepting. Just remember that patients are not likely to broach the subject themselves, and part of our job is to offer guidance.
As Singh and colleagues have noted, many patients with incurable disease have poor “prognosis awareness,” 15 so it is important for the oncologist to have a GoC conversation with the patient to be able to guage the patient’s understanding of the prognosis after a scan that shows progressive disease. Singh and his colleagues reported that of 64 taped oncologist-patient conversations about scan results, only 4 included frank discussions about prognosis. The authors suggested asking the question, “Would you like to talk about what this means?” after showing the patient the scan to allow the patient some control and to get permission to disclose crucial information based on the reading of the scan.15
Getting started on the GoC discussions may be the hardest part. Some useful introductory lines might include: “In my experience, it is easier to talk about our goals of care while people are still doing well. I know the future can be more uncertain. That’s why I want us to discuss these things now,” or “I am worried about you, after looking at these scans. I think it is time to have another discussion about what the future holds.”
Starting the hospice information visit
For patients with progressive disease, how do you know when to initiate a discussion about hospice and specifically, the hospice information visit, in a timely manner? It turns out oncologists are fairly adept at sensing when the prognosis has changed, but one useful tip is to routinely ask yourself the “surprise” question: “Would you be surprised if this patient were to die within the next 6 months?”.16 We have developed this hospice information visit practice to ensure that hospice is brought up as part of a natural transition to end-of-life care. We have not formally tested the tool, but every oncologist we know who has adopted the practice has continued it.
If the answer to the “surprise” question is, “No, I would not be surprised if the patient were to die within 6 months,” then we will make a referral to hospice for a hospice information visit. This allows for a timely, carefully planned transition to a known team, working with the oncologist, at some reasonably predictable point on the illness trajectory, usually while the patient is still on treatment. This transition can be difficult for patients and their families, and often they will voice concerns about feeling abandoned by the treating oncologist: “Dr Smith took care of us for seven years, and now – when Mom is the sickest and likely dying – he is sending us to someone whom we have never met before.” The timing of this transition is important, because the tendency is to delay for as long as possible, as demonstrated in a study by O’Connor and colleagues of admissions to hospice, which found that 16% of cancer patients were on hospice for 3 days or less.17
After we have discussed hospice care with the patient, we ask the hospice team to call the patient to set up the hospice information visit. We have been surprised to see that most patients, after the initial shock of talking about hospice care, have indicated that they found this planned transition visit with the hospice team informative and helpful.
One way in which you could broach the topic of hospice care with a patient to avoid the feelings of abandonment, is to say, “I want you to meet the people who will be helping me take care of you if and when we need them.” This emphasizes the continuity of care and the desire for a smooth, planned transition. We try to do this when we think the patient has about 6 months to live. Then, when the patient’s performance status changes or the disease progresses, we will activate the referral and will say, “Remember nurse Bob and the social worker Clare who came out to your house 3 months ago? I think it’s time we touch base with them again. It’s time to change goals from fighting the cancer to maintaining your quality of life. In my experience, hospice support will help us do just that.”
Adelson and colleagues developed standardized criteria (triggers or prompts) for use in palliative care consultation to help improve overall quality of care.18 The criteria included any solid tumor patient with: stage IV solid malignancy or stage III lung or pancreatic cancer; a hospitalization of >7 days; hospitalized in the last 30 days (not including routine chemotherapy); and uncontrolled symptoms (pain, nausea/vomiting, dyspnea, delirium, psychological distress). The investigators showed that use of the standardized criteria for palliative care consultation was associated with a decline in 30-day readmission rates (35% for the intervention group, 13% for the control group), the use of chemotherapy after discharge (18% and 44%, respectively), and use of support services after discharge.
Discussion
We see this approach with GoC discussions as part of our TEAM approach (Time, Education, Assessment, and Management) to care19 that is patient and family centered, education centered, and symptom centered. Other institutions where similar prompt systems have been used have also shown improvements in advance care planning. Temel and colleagues found in a 2010 retrospective review of the EMRs and longitudinal medical records of 2,498 patients with metastatic cancer that only 20% patients had a documented code status, despite their advanced disease state.20 A second study at the same institution, also by Temel and colleagues, showed that during 2009-2011, e-mail prompts encouraging physicians to document their patients’ code status resulted in a doubling of the rate of code status documentation, from 14.5% for historical controls to 33.7% after introducing the prompt system.21
In a study of a disease-management pilot program in Medicare patients with cancer, US Oncology adopted the best practice model of appointing someone in the practice, usually a nurse, to review advance care planning within the first visits of diagnosis of a life-limiting illness, and increased advance care planning to more than 80%.22 In another US Oncology study, Neubauer and colleagues reported that the implementation of an ACP process at 38 member sites resulted in a 15.6% increase in the incidence of code status documentation, and although the incidence of documentation varied considerably, it was as high as 89% at some sites.23
For how long should a terminally ill cancer patient be enrolled in hospice? Von Gunten has suggested increasing length of stay (LoS) in hospice as a quality improvement task. He reported on a study in which oncologists in Ohio were given the LoS recommendations from the state’s Oncology Clinical Guidance Council (LoS, 45-90 days), the national LoS average (43 days), and their peers (19.7 days) at baseline, including a chart showing the median LoS by oncologist. Follow-up with the medical oncologist after a year, showed that there was a doubling of hospice LoS, from the baseline 19.7 days to 39.6 days.24
Patients who have timely end-of-life discussions addressing GoC and understanding of their illness, are more likely to be satisfied with their quality of care, receive care that is closer to their stated preferences, and die at the place of their choosing, and their family members will be less distressed.25 In addition, Enzinger and colleagues have shown that patients who had prognostic discussions with their oncologists revised their self-reported estimates of their survival downward by 17.2 months, which brought them closer to a more realistic expectation of life expectancy, without having a negative impact on their emotional well-being (sadness, anxiety) or relationship with the physician.26 However, it is important to remember, that we, as the oncologist, have to start the GoC, hospice, and EoL conversations, because patients understandably rarely bring it up of their own free will.
1. Bernacki RE, Block SD; American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994-2003.
2. Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616-25.
3. Chen AB, Cronin A, Weeks JC, et al. Expectations about the effectiveness of radiation therapy among patients with incurable lung cancer. J Clin Oncol. 2013;31(21):2730-2735.
4. Kim Y, Winner M, Page A, et al. Patient perceptions regarding the likelihood of cure after surgical resection of lung and colorectal cancer. Cancer. 2015;121(20):3564-3573.
5. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
6. O’Connor NR, Moyer ME, Behta M, Casarett DJ. The impact of inpatient palliative care consultations on 30-day hospital readmissions. J Palliat Med. 2015;18(11):956-961.
7. Ahluwalia SC, Tisnado DM, Walling AM, et al. Association of early patient-physician care planning discussions and end-of-life care intensity in advanced cancer. J Palliat Med. 2015;18(10):834-841.
8. Sinuff T, Dodek P, You JJ, et al. Improving end-of-life communication and decision making: the development of a conceptual framework and quality indicators. J Pain Symptom Manag. 2015;49:1070-1080.
9. [Behind paywall] Jacobsen J, Jackson V, Dahlin C, et al. Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med. 2011;14(4):459-464.
10. [Behind paywall] Jorgenson A, Sidebottom AC, Richards H, Kirven J. A description of inpatient palliative care actions for patients with acute heart failure. Am J Hosp Palliat Care. 2016;33(9):863-870.
11. Smith TJ, Desch CE, Hackney MH, Shaw JE. How long does it take to get a ‘do not resuscitate’ order? J Palliat Care. 1997;13(1):5-8. [Available only as PDF on interlibrary loan.]
12. Leong M, Shah M, Smith TJ. How to avoid late chemotherapy. J Oncol Pract. 2016;12(12):1208-1210.
13. [Behind paywall] Spencer JC, Wheeler SB. A systematic review of motivational interviewing interventions in cancer patients and survivors. Patient Educ Couns. 2016;99(7):1099-1105.
14. [Behind paywall] Jackson VA, Jacobsen J, Greer JA, Pirl WF, Temel JS, Back AL. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: a communication guide. J Palliat Med. 2013;16(8):894-900.
15. [Behind paywall] Singh S, Cortez D, Maynard D, Cleary JF, DuBenske L, Campbell TC. Characterizing the nature of scan results discussions: insights into why patients misunderstand their prognosis. J Oncol Pract. 2017;13(3):e231-e239.
16. [Behind paywall] Gómez-Batiste X, Martínez-Muñoz M, Blay C, et al. Utility of the NECPAL CCOMS-ICO© tool and the Surprise Question as screening tools for early palliative care and to predict mortality in patients with advanced chronic conditions: A cohort study. http://journals.sagepub.com/doi/abs/10.1177/0269216316676647. Published online November 4, 2016. Accessed August 4, 2017.
17. O’Connor NR, Hu R, Harris PS, Ache K, Casarett DJ. Hospice admissions for cancer in the final days of life: independent predictors and implications for quality measures. J Clin Oncol. 2014;32(28):3184-3179.
18. [Behind paywall] Adelson K, Paris J, Horton JR, et al. Standardized criteria for palliative care consultation on a solid tumor oncology service reduces downstream health care use. J Oncol Pract. 2017;13(5):e431-e440.
19. Bakitas MA, El-Jawahri A, Farquhar M, et al. The TEAM approach to improving oncology outcomes by incorporating palliative care in practice. J Onc Pract. In press.
20. Temel JS, Greer JA, Admane S, et al. Code status documentation in the outpatient electronic medical records of patients with metastatic cancer. J Gen Intern Med. 2010;25(2):150-153.
21. Temel JS, Greer JA, Gallagher ER, et al. Electronic prompt to improve outpatient code status documentation for patients with advanced lung cancer. J Clin Oncol. 2013;31(6):710-715.
22. Neubauer MA, Hoverman RA, Jameson M, et al. A disease management pilot program in a Medicare-age population with cancer [abstract 6505]. http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.6505. Published May 2016. Accessed August 4, 2016.
23. Neubauer MA, Taniguchi CB, Hoverman JR. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-e266.
24. Von Gunten CF. A quality improvement approach to oncologist referrals for hospice care. http://ascopubs.org/doi/abs/10.1200/jco.2016.34.26_suppl.4. Published October 2016. Accessed August 4, 2017.
25. Kumar P, Temel JS. End-of-life care discussions in patients with advanced cancer. J Clin Oncol. 2013;31(27):3315-3319.
26. Enzinger AC, Zhang B, Schrag D, Prigerson HG. Outcomes of prognostic disclosure: associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol. 2015;33(32):3809-3816.
27. Varon J, Walsh GL, Marik PE, Fromm RE. Should a cancer patient be resuscitated following an in-hospital cardiac arrest? Resuscitation. 1998;36(3):165-168.
28. Wallace S, Ewer MS, Price KJ, Feeley TW. Outcome and cost implications of cardiopulmonary resuscitation in the medical intensive care unit of a comprehensive cancer center. Support Care Cancer. 2002;10(5):425-429.
Goals-of-care conversations led by the oncologist are a key opportunity to improve advance care planning and end-of-life care,1 but our patients are not understanding the essentials. Findings from a study of patients’ expectations about chemotherapy showed that more than two-thirds of patients with lung or colorectal cancers thought their palliative chemotherapy,2 radiation, 3 and/or surgery4 could cure them. Failure to effectively educate patients can lead to end-of-life care associated with poor quality of care, including over-aggressive care, poor quality of life with suboptimal symptom management, caregiver distress, and other potentially preventable problems.5
Palliative care routinely includes goals-of-care (GoC) discussions as part of the standard note. At one large urban hospital, the 30-day readmission rate was 10% if palliative care consultation was done, compared with 15% if no consultation was obtained.6 Patients who had consultations that included GoC discussions in addition to a symptom management consultation had a lower hospital readmission rate of 5%, compared with 15% in patients who received symptom management consultations alone (adjusted odds ratio [AOR], 0.36; confidence interval [CI], 0.27-0.48; P < .001). 6 Findings from another study showed that the use of aggressive end-of-life care was reduced by one-third when the patient and provider had a GoC planning session close to the time of diagnosis, instead of at the end of life.7
We prepared a template to be incorporated into the electronic medical record (EMR) to facilitate GoC discussions between the oncologist and patient as part of a randomized phase 3 trial of early versus delayed palliative care for phase 1 clinical trial patients. Documentation is one of the most important elements of efforts to improve end-of-life care, along with advance care planning, GoC discussions, and understanding the system of care.8 Because we have found this approach useful in our everyday oncology practice, we are sharing this simple, doable template in the hope that others will find it effective.
The GoC template
What does it look like?
We developed a document for oncology GoC discussions that was incorporated into the EMR (Table). We use the GoC document in much the same way we’d review a computed-tomography scan with our patients and their families: we bring it up on the computer screen, show them the categories, and type in responses on the allotted spaces on the right-hand side of the document. The input can be done in real time or after the conversation, or even after the patient has left. In our practice, we access the Patient Instructions part of the EMR, use a SmartPhrase to copy the Word template into the chart, and start typing.
The completed GoC sheet can be used in several ways. Most commonly, we print it out for the patient and family, so that everyone has access to the same information. Printing it out as part of the after-visit patient summary also satisfies the meaningful use requirement for EMRs. It is also possible to cut and paste the document into a letter to the care team, or directly into the Progress Note, so that it will be available for members of the care team to see.
The GoC form does take some time to complete. Palliative care teams that have reported on what was done during the palliative care visit have noted that the initial and subsequent visits took about an hour, with about 20 minutes devoted to symptom management, 15 minutes to patient and family coping, and 10 minutes to illness understanding and education, including prognosis.9,10 In our own practice, we do questions 1-9 first (Table, shaded area) because it takes less time than anticipated, just like code status discussions,11 and it is much easier with a script.
We use this template for in- and out-patient consultations and for routine visits when a GoC discussion is warranted. It is important to remember that doing this counts as advance care planning and can be billed using the ACP codes.
How do you use template?
It is critically important to make sure that the patient and family are ready for this discussion. To further facilitate the discussion, we have devised a temporary palliative care tattoo with a script, or prompts, for what questions to ask and in what order they should be asked (Figure). The easy-to-read tattoo is worn on the inner forearm so that it is readily visible to the oncologist or advanced practice nurse.12
We always start by asking, “How do you like to get medical information?” (Table, question 1) and follow up with something like, “Are you the sort of person who wants all the details, or not?” (Table). If the response is yes, they want all the details, then we follow up with another question, “Does that include talking about prognosis and what might happen?” (Table). Most patients will want full disclosure of their circumstances and prognosis, but some will not, and will feel overwhelmed and disempowered if you proceed.
After reviewing the answers to question 1 and any follow-up questions you may have asked, you will be able to gauge whether you should continue with questions 2-4 in the template. Once you know more about the patient and the family’s understanding of the situation (Table, question 2), what is important to them (question 3), what they are hoping for (question 4), and have spoken to them about disease progression, recapped their treatment to date, and checked to see if they might be eligible for any clinical trials (question 5), it will be easier to move on to the next questions, about progressive disease and advance care planning: “You are doing OK now, but have you thought about a time when you could be sicker [and need] a living will or advance directive [question 6, see next section of this article]?” For people unwilling to have this discussion, or have it at that moment, there is an excellent article that outlines the process to help practitioners increase prognostic awareness.14 Patient readiness will change over time as they adjust to the life changes forced by serious illness, and one can put off the discussion until they are more accepting. Just remember that patients are not likely to broach the subject themselves, and part of our job is to offer guidance.
As Singh and colleagues have noted, many patients with incurable disease have poor “prognosis awareness,” 15 so it is important for the oncologist to have a GoC conversation with the patient to be able to guage the patient’s understanding of the prognosis after a scan that shows progressive disease. Singh and his colleagues reported that of 64 taped oncologist-patient conversations about scan results, only 4 included frank discussions about prognosis. The authors suggested asking the question, “Would you like to talk about what this means?” after showing the patient the scan to allow the patient some control and to get permission to disclose crucial information based on the reading of the scan.15
Getting started on the GoC discussions may be the hardest part. Some useful introductory lines might include: “In my experience, it is easier to talk about our goals of care while people are still doing well. I know the future can be more uncertain. That’s why I want us to discuss these things now,” or “I am worried about you, after looking at these scans. I think it is time to have another discussion about what the future holds.”
Starting the hospice information visit
For patients with progressive disease, how do you know when to initiate a discussion about hospice and specifically, the hospice information visit, in a timely manner? It turns out oncologists are fairly adept at sensing when the prognosis has changed, but one useful tip is to routinely ask yourself the “surprise” question: “Would you be surprised if this patient were to die within the next 6 months?”.16 We have developed this hospice information visit practice to ensure that hospice is brought up as part of a natural transition to end-of-life care. We have not formally tested the tool, but every oncologist we know who has adopted the practice has continued it.
If the answer to the “surprise” question is, “No, I would not be surprised if the patient were to die within 6 months,” then we will make a referral to hospice for a hospice information visit. This allows for a timely, carefully planned transition to a known team, working with the oncologist, at some reasonably predictable point on the illness trajectory, usually while the patient is still on treatment. This transition can be difficult for patients and their families, and often they will voice concerns about feeling abandoned by the treating oncologist: “Dr Smith took care of us for seven years, and now – when Mom is the sickest and likely dying – he is sending us to someone whom we have never met before.” The timing of this transition is important, because the tendency is to delay for as long as possible, as demonstrated in a study by O’Connor and colleagues of admissions to hospice, which found that 16% of cancer patients were on hospice for 3 days or less.17
After we have discussed hospice care with the patient, we ask the hospice team to call the patient to set up the hospice information visit. We have been surprised to see that most patients, after the initial shock of talking about hospice care, have indicated that they found this planned transition visit with the hospice team informative and helpful.
One way in which you could broach the topic of hospice care with a patient to avoid the feelings of abandonment, is to say, “I want you to meet the people who will be helping me take care of you if and when we need them.” This emphasizes the continuity of care and the desire for a smooth, planned transition. We try to do this when we think the patient has about 6 months to live. Then, when the patient’s performance status changes or the disease progresses, we will activate the referral and will say, “Remember nurse Bob and the social worker Clare who came out to your house 3 months ago? I think it’s time we touch base with them again. It’s time to change goals from fighting the cancer to maintaining your quality of life. In my experience, hospice support will help us do just that.”
Adelson and colleagues developed standardized criteria (triggers or prompts) for use in palliative care consultation to help improve overall quality of care.18 The criteria included any solid tumor patient with: stage IV solid malignancy or stage III lung or pancreatic cancer; a hospitalization of >7 days; hospitalized in the last 30 days (not including routine chemotherapy); and uncontrolled symptoms (pain, nausea/vomiting, dyspnea, delirium, psychological distress). The investigators showed that use of the standardized criteria for palliative care consultation was associated with a decline in 30-day readmission rates (35% for the intervention group, 13% for the control group), the use of chemotherapy after discharge (18% and 44%, respectively), and use of support services after discharge.
Discussion
We see this approach with GoC discussions as part of our TEAM approach (Time, Education, Assessment, and Management) to care19 that is patient and family centered, education centered, and symptom centered. Other institutions where similar prompt systems have been used have also shown improvements in advance care planning. Temel and colleagues found in a 2010 retrospective review of the EMRs and longitudinal medical records of 2,498 patients with metastatic cancer that only 20% patients had a documented code status, despite their advanced disease state.20 A second study at the same institution, also by Temel and colleagues, showed that during 2009-2011, e-mail prompts encouraging physicians to document their patients’ code status resulted in a doubling of the rate of code status documentation, from 14.5% for historical controls to 33.7% after introducing the prompt system.21
In a study of a disease-management pilot program in Medicare patients with cancer, US Oncology adopted the best practice model of appointing someone in the practice, usually a nurse, to review advance care planning within the first visits of diagnosis of a life-limiting illness, and increased advance care planning to more than 80%.22 In another US Oncology study, Neubauer and colleagues reported that the implementation of an ACP process at 38 member sites resulted in a 15.6% increase in the incidence of code status documentation, and although the incidence of documentation varied considerably, it was as high as 89% at some sites.23
For how long should a terminally ill cancer patient be enrolled in hospice? Von Gunten has suggested increasing length of stay (LoS) in hospice as a quality improvement task. He reported on a study in which oncologists in Ohio were given the LoS recommendations from the state’s Oncology Clinical Guidance Council (LoS, 45-90 days), the national LoS average (43 days), and their peers (19.7 days) at baseline, including a chart showing the median LoS by oncologist. Follow-up with the medical oncologist after a year, showed that there was a doubling of hospice LoS, from the baseline 19.7 days to 39.6 days.24
Patients who have timely end-of-life discussions addressing GoC and understanding of their illness, are more likely to be satisfied with their quality of care, receive care that is closer to their stated preferences, and die at the place of their choosing, and their family members will be less distressed.25 In addition, Enzinger and colleagues have shown that patients who had prognostic discussions with their oncologists revised their self-reported estimates of their survival downward by 17.2 months, which brought them closer to a more realistic expectation of life expectancy, without having a negative impact on their emotional well-being (sadness, anxiety) or relationship with the physician.26 However, it is important to remember, that we, as the oncologist, have to start the GoC, hospice, and EoL conversations, because patients understandably rarely bring it up of their own free will.
Goals-of-care conversations led by the oncologist are a key opportunity to improve advance care planning and end-of-life care,1 but our patients are not understanding the essentials. Findings from a study of patients’ expectations about chemotherapy showed that more than two-thirds of patients with lung or colorectal cancers thought their palliative chemotherapy,2 radiation, 3 and/or surgery4 could cure them. Failure to effectively educate patients can lead to end-of-life care associated with poor quality of care, including over-aggressive care, poor quality of life with suboptimal symptom management, caregiver distress, and other potentially preventable problems.5
Palliative care routinely includes goals-of-care (GoC) discussions as part of the standard note. At one large urban hospital, the 30-day readmission rate was 10% if palliative care consultation was done, compared with 15% if no consultation was obtained.6 Patients who had consultations that included GoC discussions in addition to a symptom management consultation had a lower hospital readmission rate of 5%, compared with 15% in patients who received symptom management consultations alone (adjusted odds ratio [AOR], 0.36; confidence interval [CI], 0.27-0.48; P < .001). 6 Findings from another study showed that the use of aggressive end-of-life care was reduced by one-third when the patient and provider had a GoC planning session close to the time of diagnosis, instead of at the end of life.7
We prepared a template to be incorporated into the electronic medical record (EMR) to facilitate GoC discussions between the oncologist and patient as part of a randomized phase 3 trial of early versus delayed palliative care for phase 1 clinical trial patients. Documentation is one of the most important elements of efforts to improve end-of-life care, along with advance care planning, GoC discussions, and understanding the system of care.8 Because we have found this approach useful in our everyday oncology practice, we are sharing this simple, doable template in the hope that others will find it effective.
The GoC template
What does it look like?
We developed a document for oncology GoC discussions that was incorporated into the EMR (Table). We use the GoC document in much the same way we’d review a computed-tomography scan with our patients and their families: we bring it up on the computer screen, show them the categories, and type in responses on the allotted spaces on the right-hand side of the document. The input can be done in real time or after the conversation, or even after the patient has left. In our practice, we access the Patient Instructions part of the EMR, use a SmartPhrase to copy the Word template into the chart, and start typing.
The completed GoC sheet can be used in several ways. Most commonly, we print it out for the patient and family, so that everyone has access to the same information. Printing it out as part of the after-visit patient summary also satisfies the meaningful use requirement for EMRs. It is also possible to cut and paste the document into a letter to the care team, or directly into the Progress Note, so that it will be available for members of the care team to see.
The GoC form does take some time to complete. Palliative care teams that have reported on what was done during the palliative care visit have noted that the initial and subsequent visits took about an hour, with about 20 minutes devoted to symptom management, 15 minutes to patient and family coping, and 10 minutes to illness understanding and education, including prognosis.9,10 In our own practice, we do questions 1-9 first (Table, shaded area) because it takes less time than anticipated, just like code status discussions,11 and it is much easier with a script.
We use this template for in- and out-patient consultations and for routine visits when a GoC discussion is warranted. It is important to remember that doing this counts as advance care planning and can be billed using the ACP codes.
How do you use template?
It is critically important to make sure that the patient and family are ready for this discussion. To further facilitate the discussion, we have devised a temporary palliative care tattoo with a script, or prompts, for what questions to ask and in what order they should be asked (Figure). The easy-to-read tattoo is worn on the inner forearm so that it is readily visible to the oncologist or advanced practice nurse.12
We always start by asking, “How do you like to get medical information?” (Table, question 1) and follow up with something like, “Are you the sort of person who wants all the details, or not?” (Table). If the response is yes, they want all the details, then we follow up with another question, “Does that include talking about prognosis and what might happen?” (Table). Most patients will want full disclosure of their circumstances and prognosis, but some will not, and will feel overwhelmed and disempowered if you proceed.
After reviewing the answers to question 1 and any follow-up questions you may have asked, you will be able to gauge whether you should continue with questions 2-4 in the template. Once you know more about the patient and the family’s understanding of the situation (Table, question 2), what is important to them (question 3), what they are hoping for (question 4), and have spoken to them about disease progression, recapped their treatment to date, and checked to see if they might be eligible for any clinical trials (question 5), it will be easier to move on to the next questions, about progressive disease and advance care planning: “You are doing OK now, but have you thought about a time when you could be sicker [and need] a living will or advance directive [question 6, see next section of this article]?” For people unwilling to have this discussion, or have it at that moment, there is an excellent article that outlines the process to help practitioners increase prognostic awareness.14 Patient readiness will change over time as they adjust to the life changes forced by serious illness, and one can put off the discussion until they are more accepting. Just remember that patients are not likely to broach the subject themselves, and part of our job is to offer guidance.
As Singh and colleagues have noted, many patients with incurable disease have poor “prognosis awareness,” 15 so it is important for the oncologist to have a GoC conversation with the patient to be able to guage the patient’s understanding of the prognosis after a scan that shows progressive disease. Singh and his colleagues reported that of 64 taped oncologist-patient conversations about scan results, only 4 included frank discussions about prognosis. The authors suggested asking the question, “Would you like to talk about what this means?” after showing the patient the scan to allow the patient some control and to get permission to disclose crucial information based on the reading of the scan.15
Getting started on the GoC discussions may be the hardest part. Some useful introductory lines might include: “In my experience, it is easier to talk about our goals of care while people are still doing well. I know the future can be more uncertain. That’s why I want us to discuss these things now,” or “I am worried about you, after looking at these scans. I think it is time to have another discussion about what the future holds.”
Starting the hospice information visit
For patients with progressive disease, how do you know when to initiate a discussion about hospice and specifically, the hospice information visit, in a timely manner? It turns out oncologists are fairly adept at sensing when the prognosis has changed, but one useful tip is to routinely ask yourself the “surprise” question: “Would you be surprised if this patient were to die within the next 6 months?”.16 We have developed this hospice information visit practice to ensure that hospice is brought up as part of a natural transition to end-of-life care. We have not formally tested the tool, but every oncologist we know who has adopted the practice has continued it.
If the answer to the “surprise” question is, “No, I would not be surprised if the patient were to die within 6 months,” then we will make a referral to hospice for a hospice information visit. This allows for a timely, carefully planned transition to a known team, working with the oncologist, at some reasonably predictable point on the illness trajectory, usually while the patient is still on treatment. This transition can be difficult for patients and their families, and often they will voice concerns about feeling abandoned by the treating oncologist: “Dr Smith took care of us for seven years, and now – when Mom is the sickest and likely dying – he is sending us to someone whom we have never met before.” The timing of this transition is important, because the tendency is to delay for as long as possible, as demonstrated in a study by O’Connor and colleagues of admissions to hospice, which found that 16% of cancer patients were on hospice for 3 days or less.17
After we have discussed hospice care with the patient, we ask the hospice team to call the patient to set up the hospice information visit. We have been surprised to see that most patients, after the initial shock of talking about hospice care, have indicated that they found this planned transition visit with the hospice team informative and helpful.
One way in which you could broach the topic of hospice care with a patient to avoid the feelings of abandonment, is to say, “I want you to meet the people who will be helping me take care of you if and when we need them.” This emphasizes the continuity of care and the desire for a smooth, planned transition. We try to do this when we think the patient has about 6 months to live. Then, when the patient’s performance status changes or the disease progresses, we will activate the referral and will say, “Remember nurse Bob and the social worker Clare who came out to your house 3 months ago? I think it’s time we touch base with them again. It’s time to change goals from fighting the cancer to maintaining your quality of life. In my experience, hospice support will help us do just that.”
Adelson and colleagues developed standardized criteria (triggers or prompts) for use in palliative care consultation to help improve overall quality of care.18 The criteria included any solid tumor patient with: stage IV solid malignancy or stage III lung or pancreatic cancer; a hospitalization of >7 days; hospitalized in the last 30 days (not including routine chemotherapy); and uncontrolled symptoms (pain, nausea/vomiting, dyspnea, delirium, psychological distress). The investigators showed that use of the standardized criteria for palliative care consultation was associated with a decline in 30-day readmission rates (35% for the intervention group, 13% for the control group), the use of chemotherapy after discharge (18% and 44%, respectively), and use of support services after discharge.
Discussion
We see this approach with GoC discussions as part of our TEAM approach (Time, Education, Assessment, and Management) to care19 that is patient and family centered, education centered, and symptom centered. Other institutions where similar prompt systems have been used have also shown improvements in advance care planning. Temel and colleagues found in a 2010 retrospective review of the EMRs and longitudinal medical records of 2,498 patients with metastatic cancer that only 20% patients had a documented code status, despite their advanced disease state.20 A second study at the same institution, also by Temel and colleagues, showed that during 2009-2011, e-mail prompts encouraging physicians to document their patients’ code status resulted in a doubling of the rate of code status documentation, from 14.5% for historical controls to 33.7% after introducing the prompt system.21
In a study of a disease-management pilot program in Medicare patients with cancer, US Oncology adopted the best practice model of appointing someone in the practice, usually a nurse, to review advance care planning within the first visits of diagnosis of a life-limiting illness, and increased advance care planning to more than 80%.22 In another US Oncology study, Neubauer and colleagues reported that the implementation of an ACP process at 38 member sites resulted in a 15.6% increase in the incidence of code status documentation, and although the incidence of documentation varied considerably, it was as high as 89% at some sites.23
For how long should a terminally ill cancer patient be enrolled in hospice? Von Gunten has suggested increasing length of stay (LoS) in hospice as a quality improvement task. He reported on a study in which oncologists in Ohio were given the LoS recommendations from the state’s Oncology Clinical Guidance Council (LoS, 45-90 days), the national LoS average (43 days), and their peers (19.7 days) at baseline, including a chart showing the median LoS by oncologist. Follow-up with the medical oncologist after a year, showed that there was a doubling of hospice LoS, from the baseline 19.7 days to 39.6 days.24
Patients who have timely end-of-life discussions addressing GoC and understanding of their illness, are more likely to be satisfied with their quality of care, receive care that is closer to their stated preferences, and die at the place of their choosing, and their family members will be less distressed.25 In addition, Enzinger and colleagues have shown that patients who had prognostic discussions with their oncologists revised their self-reported estimates of their survival downward by 17.2 months, which brought them closer to a more realistic expectation of life expectancy, without having a negative impact on their emotional well-being (sadness, anxiety) or relationship with the physician.26 However, it is important to remember, that we, as the oncologist, have to start the GoC, hospice, and EoL conversations, because patients understandably rarely bring it up of their own free will.
1. Bernacki RE, Block SD; American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994-2003.
2. Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616-25.
3. Chen AB, Cronin A, Weeks JC, et al. Expectations about the effectiveness of radiation therapy among patients with incurable lung cancer. J Clin Oncol. 2013;31(21):2730-2735.
4. Kim Y, Winner M, Page A, et al. Patient perceptions regarding the likelihood of cure after surgical resection of lung and colorectal cancer. Cancer. 2015;121(20):3564-3573.
5. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
6. O’Connor NR, Moyer ME, Behta M, Casarett DJ. The impact of inpatient palliative care consultations on 30-day hospital readmissions. J Palliat Med. 2015;18(11):956-961.
7. Ahluwalia SC, Tisnado DM, Walling AM, et al. Association of early patient-physician care planning discussions and end-of-life care intensity in advanced cancer. J Palliat Med. 2015;18(10):834-841.
8. Sinuff T, Dodek P, You JJ, et al. Improving end-of-life communication and decision making: the development of a conceptual framework and quality indicators. J Pain Symptom Manag. 2015;49:1070-1080.
9. [Behind paywall] Jacobsen J, Jackson V, Dahlin C, et al. Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med. 2011;14(4):459-464.
10. [Behind paywall] Jorgenson A, Sidebottom AC, Richards H, Kirven J. A description of inpatient palliative care actions for patients with acute heart failure. Am J Hosp Palliat Care. 2016;33(9):863-870.
11. Smith TJ, Desch CE, Hackney MH, Shaw JE. How long does it take to get a ‘do not resuscitate’ order? J Palliat Care. 1997;13(1):5-8. [Available only as PDF on interlibrary loan.]
12. Leong M, Shah M, Smith TJ. How to avoid late chemotherapy. J Oncol Pract. 2016;12(12):1208-1210.
13. [Behind paywall] Spencer JC, Wheeler SB. A systematic review of motivational interviewing interventions in cancer patients and survivors. Patient Educ Couns. 2016;99(7):1099-1105.
14. [Behind paywall] Jackson VA, Jacobsen J, Greer JA, Pirl WF, Temel JS, Back AL. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: a communication guide. J Palliat Med. 2013;16(8):894-900.
15. [Behind paywall] Singh S, Cortez D, Maynard D, Cleary JF, DuBenske L, Campbell TC. Characterizing the nature of scan results discussions: insights into why patients misunderstand their prognosis. J Oncol Pract. 2017;13(3):e231-e239.
16. [Behind paywall] Gómez-Batiste X, Martínez-Muñoz M, Blay C, et al. Utility of the NECPAL CCOMS-ICO© tool and the Surprise Question as screening tools for early palliative care and to predict mortality in patients with advanced chronic conditions: A cohort study. http://journals.sagepub.com/doi/abs/10.1177/0269216316676647. Published online November 4, 2016. Accessed August 4, 2017.
17. O’Connor NR, Hu R, Harris PS, Ache K, Casarett DJ. Hospice admissions for cancer in the final days of life: independent predictors and implications for quality measures. J Clin Oncol. 2014;32(28):3184-3179.
18. [Behind paywall] Adelson K, Paris J, Horton JR, et al. Standardized criteria for palliative care consultation on a solid tumor oncology service reduces downstream health care use. J Oncol Pract. 2017;13(5):e431-e440.
19. Bakitas MA, El-Jawahri A, Farquhar M, et al. The TEAM approach to improving oncology outcomes by incorporating palliative care in practice. J Onc Pract. In press.
20. Temel JS, Greer JA, Admane S, et al. Code status documentation in the outpatient electronic medical records of patients with metastatic cancer. J Gen Intern Med. 2010;25(2):150-153.
21. Temel JS, Greer JA, Gallagher ER, et al. Electronic prompt to improve outpatient code status documentation for patients with advanced lung cancer. J Clin Oncol. 2013;31(6):710-715.
22. Neubauer MA, Hoverman RA, Jameson M, et al. A disease management pilot program in a Medicare-age population with cancer [abstract 6505]. http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.6505. Published May 2016. Accessed August 4, 2016.
23. Neubauer MA, Taniguchi CB, Hoverman JR. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-e266.
24. Von Gunten CF. A quality improvement approach to oncologist referrals for hospice care. http://ascopubs.org/doi/abs/10.1200/jco.2016.34.26_suppl.4. Published October 2016. Accessed August 4, 2017.
25. Kumar P, Temel JS. End-of-life care discussions in patients with advanced cancer. J Clin Oncol. 2013;31(27):3315-3319.
26. Enzinger AC, Zhang B, Schrag D, Prigerson HG. Outcomes of prognostic disclosure: associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol. 2015;33(32):3809-3816.
27. Varon J, Walsh GL, Marik PE, Fromm RE. Should a cancer patient be resuscitated following an in-hospital cardiac arrest? Resuscitation. 1998;36(3):165-168.
28. Wallace S, Ewer MS, Price KJ, Feeley TW. Outcome and cost implications of cardiopulmonary resuscitation in the medical intensive care unit of a comprehensive cancer center. Support Care Cancer. 2002;10(5):425-429.
1. Bernacki RE, Block SD; American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994-2003.
2. Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616-25.
3. Chen AB, Cronin A, Weeks JC, et al. Expectations about the effectiveness of radiation therapy among patients with incurable lung cancer. J Clin Oncol. 2013;31(21):2730-2735.
4. Kim Y, Winner M, Page A, et al. Patient perceptions regarding the likelihood of cure after surgical resection of lung and colorectal cancer. Cancer. 2015;121(20):3564-3573.
5. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
6. O’Connor NR, Moyer ME, Behta M, Casarett DJ. The impact of inpatient palliative care consultations on 30-day hospital readmissions. J Palliat Med. 2015;18(11):956-961.
7. Ahluwalia SC, Tisnado DM, Walling AM, et al. Association of early patient-physician care planning discussions and end-of-life care intensity in advanced cancer. J Palliat Med. 2015;18(10):834-841.
8. Sinuff T, Dodek P, You JJ, et al. Improving end-of-life communication and decision making: the development of a conceptual framework and quality indicators. J Pain Symptom Manag. 2015;49:1070-1080.
9. [Behind paywall] Jacobsen J, Jackson V, Dahlin C, et al. Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med. 2011;14(4):459-464.
10. [Behind paywall] Jorgenson A, Sidebottom AC, Richards H, Kirven J. A description of inpatient palliative care actions for patients with acute heart failure. Am J Hosp Palliat Care. 2016;33(9):863-870.
11. Smith TJ, Desch CE, Hackney MH, Shaw JE. How long does it take to get a ‘do not resuscitate’ order? J Palliat Care. 1997;13(1):5-8. [Available only as PDF on interlibrary loan.]
12. Leong M, Shah M, Smith TJ. How to avoid late chemotherapy. J Oncol Pract. 2016;12(12):1208-1210.
13. [Behind paywall] Spencer JC, Wheeler SB. A systematic review of motivational interviewing interventions in cancer patients and survivors. Patient Educ Couns. 2016;99(7):1099-1105.
14. [Behind paywall] Jackson VA, Jacobsen J, Greer JA, Pirl WF, Temel JS, Back AL. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: a communication guide. J Palliat Med. 2013;16(8):894-900.
15. [Behind paywall] Singh S, Cortez D, Maynard D, Cleary JF, DuBenske L, Campbell TC. Characterizing the nature of scan results discussions: insights into why patients misunderstand their prognosis. J Oncol Pract. 2017;13(3):e231-e239.
16. [Behind paywall] Gómez-Batiste X, Martínez-Muñoz M, Blay C, et al. Utility of the NECPAL CCOMS-ICO© tool and the Surprise Question as screening tools for early palliative care and to predict mortality in patients with advanced chronic conditions: A cohort study. http://journals.sagepub.com/doi/abs/10.1177/0269216316676647. Published online November 4, 2016. Accessed August 4, 2017.
17. O’Connor NR, Hu R, Harris PS, Ache K, Casarett DJ. Hospice admissions for cancer in the final days of life: independent predictors and implications for quality measures. J Clin Oncol. 2014;32(28):3184-3179.
18. [Behind paywall] Adelson K, Paris J, Horton JR, et al. Standardized criteria for palliative care consultation on a solid tumor oncology service reduces downstream health care use. J Oncol Pract. 2017;13(5):e431-e440.
19. Bakitas MA, El-Jawahri A, Farquhar M, et al. The TEAM approach to improving oncology outcomes by incorporating palliative care in practice. J Onc Pract. In press.
20. Temel JS, Greer JA, Admane S, et al. Code status documentation in the outpatient electronic medical records of patients with metastatic cancer. J Gen Intern Med. 2010;25(2):150-153.
21. Temel JS, Greer JA, Gallagher ER, et al. Electronic prompt to improve outpatient code status documentation for patients with advanced lung cancer. J Clin Oncol. 2013;31(6):710-715.
22. Neubauer MA, Hoverman RA, Jameson M, et al. A disease management pilot program in a Medicare-age population with cancer [abstract 6505]. http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.6505. Published May 2016. Accessed August 4, 2016.
23. Neubauer MA, Taniguchi CB, Hoverman JR. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-e266.
24. Von Gunten CF. A quality improvement approach to oncologist referrals for hospice care. http://ascopubs.org/doi/abs/10.1200/jco.2016.34.26_suppl.4. Published October 2016. Accessed August 4, 2017.
25. Kumar P, Temel JS. End-of-life care discussions in patients with advanced cancer. J Clin Oncol. 2013;31(27):3315-3319.
26. Enzinger AC, Zhang B, Schrag D, Prigerson HG. Outcomes of prognostic disclosure: associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol. 2015;33(32):3809-3816.
27. Varon J, Walsh GL, Marik PE, Fromm RE. Should a cancer patient be resuscitated following an in-hospital cardiac arrest? Resuscitation. 1998;36(3):165-168.
28. Wallace S, Ewer MS, Price KJ, Feeley TW. Outcome and cost implications of cardiopulmonary resuscitation in the medical intensive care unit of a comprehensive cancer center. Support Care Cancer. 2002;10(5):425-429.
An ASCO 2017 recap: significant advances continue
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
‘Making a difference in cancer care’
Survival improves when patients with cancer self-report symptoms
Key clinical point Patients with metastatic cancer who self-reported symptoms experienced significant improvement in overall survival. Major finding Median overall survival with self-reporting of symptoms compared with usual care was 31.2 and 26 months, respectively. Data source A randomized controlled clinical trial of 766 patients. Funding and disclosures This study was supported by the National Institutes of Health and the Conquer Cancer Foundation of the American Society of Clinical Oncology. Dr Basch and Dr Burstein each reported having no disclosures.
Patients with metastatic cancer who self-reported symptoms during routine cancer treatment experienced a number of benefits, including a statistically significant improvement in overall survival, according to findings from a randomized, controlled clinical trial (see p. e184). The median overall survival in 441 patients receiving treatment for metastatic breast, lung, genitourinary, or gynecologic cancer who were randomized to the self-reporting intervention arm was more than 5 months longer (a nearly 20% increase) than in 325 patients receiving standard care (31.2 vs. 26 months), Ethan Basch, MD, of the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill, said at the meeting. “Another way to think of this is [in terms of] 5-year survival. At 5 years, 8% more patients were alive in the self-reporting group,” he said.
In addition, 31% of patients in the intervention arm had better quality of life/physical functioning, compared with those in the control arm, and 7% fewer patients in the intervention arm visited an emergency room during the study. The duration of potentially life-prolonging chemotherapy was increased by an average of 2 months in the intervention arm, he said (JAMA. 2017 Jun 4. doi: 10.1001/jama.2017.7156).
Symptoms such as nausea, pain, and fatigue are common among patients with metastatic cancer and can often go undetected by doctors and nurses until they become severe and physically debilitating, Dr Basch added, noting that patients are often hesitant to call the office between visits to report symptoms.
Dr Basch and his colleagues hypothesized that self-reporting of patient symptoms between visits or before a visit while the patient was in the clinic waiting area would prompt earlier intervention and improve symptom control and outcomes.
Study participants were patients at Memorial Sloan Kettering Cancer Center who had advanced solid genitourinary, gynecologic, breast, or lung tumors and who were receiving outpatient chemotherapy. Those assigned to the intervention group used tablet computers and an online web survey system to report on 12 symptoms commonly experienced during chemotherapy. The system triggers an alert to a nurse when a severe or worsening symptom is reported. Patients in the usual care group discussed symptoms during office visits and were encouraged to call the office between visits if they were concerned about symptoms.
Patients remained on the study until discontinuation of all cancer treatment, hospice, or death.
One possible explanation for the findings is that this self-reporting approach prompts clinicians to manage symptoms before they cause serious downstream complications, Dr Basch said.
The approach may also keep patients more physically functional, which is known from previous studies to have a strong association with better survival, and it may also improve management of chemotherapy side effects, enabling longer duration of beneficial cancer treatment. “In oncology, we often are limited in our ability to give life-prolonging treatment because people don’t tolerate it well,” Dr Basch explained.
“This approach should be considered for inclusion in standard symptoms management as a component of high-quality cancer care,” he concluded, noting that efforts are underway to test the next generation of systems to improve communication between patients and care teams and to figure out how best to integrate these tools into oncology practice.
The system used in the this study was designed for research, but a number of companies have tools currently available for patient-reported outcomes, and others are being developed, Dr Basch said. A National Cancer Institute questionnaire, the PRO-CTCAE, is publicly available and can be loaded into patients’ electronic health records for this purpose as well.
Harold J Burstein, MD, of Dana-Farber Cancer Institute, Boston, said the study findings validate the feeling among many clinicians that patient-focused, team-based care can improve outcomes in a meaningful way for patients. If this were a drug … it would be worth tens, if not hundreds of thousands, of dollars per year … We don’t have those same kinds of dollars to help implement these into our electronic health records or our systems. We need to find ways to support that and make it happen,” he said.
— Sharon Worcester
TRK inhibitor shows ‘striking’ activity and durability across diverse cancers
Key clinical point Larotrectinib has good, durable efficacy when used to treat advanced cancers harboring TRK fusions. Major finding The overall response rate was 76%, and 79% of responses were still ongoing at 12 months. Data source An integrated analysis of phase 1 and 2 trials among 55 children and adults having 17 discrete types of advanced cancer with TRK fusions. Funding and disclosures Loxo Oncology funded the study. Dr Hyman disclosed that he has a consulting or advisory role with Atara Biotherapeutics, Chugai Pharma, and CytomX Therapeutics, and that he receives research funding from AstraZeneca and Puma Biotechnology.
Larotrectinib, an oral inhibitor of tropomyosin receptor kinase (TRK), has durable efficacy across diverse adult and pediatric cancers that harbor a genetic aberration known as TRK fusion, according to findings from an analysis of 3 trials reported at the meeting (see p. e184). Fusion of a TRK gene with an unrelated gene leads to uncontrolled signaling in the TRK pathway, potentially causing tumor growth and addiction to this input, David Hyman, MD, chief of early drug development at Memorial Sloan Kettering Cancer Center in New York, explained in a press briefing.
“One of the defining features of TRK fusions is that they are not just found in one cancer type, but in dozens of different cancer types, and not just in adults, but children as well, spanning the entire lifetime of the person,” he noted. They are rare in common cancers and nearly universal in certain uncommon cancers; collectively, they are present in possibly 5,000 cancers diagnosed each year in the United States.
Dr Hyman and his colleagues analyzed data from 55 patients having 17 discrete types of advanced cancer harboring TRK fusions who were treated with larotrectinib in phase 1 and 2 trials. Results showed an overall response rate of 76%, and the majority of responses were still ongoing at 12 months. “I believe these data support larotrectinib as a potential new standard of care for these patients,” he said. “However, I want to emphasize that really recognizing this benefit in the community will require that we test patients more universally for the presence of TRK fusions or other tumor-agnostic biomarkers, such as microsatellite instability.”
Study details
The investigators analyzed data from 3 trials in which patients with advanced TRK fusion-positive solid cancers received larotrectinib (LOXO-101): a phase 1 trial among 8 adult patients, a phase 1/2 trial among 12 pediatric patients (SCOUT), and a phase 2 “basket” trial among 35 adult and adolescent patients (NAVIGATE).
“These patients were identified by local testing,” Dr Hyman noted. “We did not perform central screening to find the TRK fusions, and in fact, 50 different laboratories identified the 55 patients. So in a sense, this really represents the real-world identification of these patients.”
In an integrated analysis, the overall rate of confirmed response as assessed by investigators was 76%, with complete response in 12% of patients and partial response in 64%. Two patients had such deep tumor regression that they experienced downstaging enabling them to undergo potentially curative surgery. Efficacy was consistent regardless of tumor type, which TRK gene was affected, and the fusion partner gene.
Median time to response was 1.8 months. “This is just a reflection of when the first scan was obtained. But in the clinic, patients reported dramatic improvement of their symptoms within days of starting therapy,” Dr Hyman said.
With a median follow-up of 5.8 months, the median duration of response was not yet reached. In all, 79% of responses were still ongoing at 12 months. Median progression-free survival (PFS) was likewise not reached; the 12-month rate was 63%.
The leading treatment-emergent adverse events were fatigue (38%), dizziness (27%), nausea (26%), and anemia (26%). “This is an extremely well tolerated therapy with only 13% of patients requiring any form of dose modification and not a single patient discontinuing due to adverse events,” he said.
It is not clear why some patients had apparent primary resistance to larotrectinib, but their TRK fusion test results may have been incorrect, Dr Hyman speculated. In all, 6 patients developed acquired resistance to larotrectinib; 5 of them were found to have an identical resistance mutation, and 2 went on to receive and have a response to LOXO-195, a next-generation TRK inhibitor that seems to retain activity in the presence of this mutation (Cancer Discov. 2017 June 3. doi: 10.1158/2159-8290.CD-17-0507).
TRK testing
Several next-generation sequencing-based tests already available clinically can pick up TRK fusions, Dr Hyman pointed out. “But it is important for the ordering physician to understand whether the tests they are ordering include fusion detection and, if it’s an option, to select it. Otherwise, they will not find TRK fusions. “The list price for these tests is in the low thousands of dollars,” he noted. In cancers in which sequential single-gene testing is already being done as standard of care, there is “minimal” incremental cost of instead using comprehensive testing that would detect TRK fusions.
Oncologists should be aware that obtaining test results can take weeks, Dr Hyman stressed. “This [testing] should be more broadly adopted and should be adopted at a point in the patient’s treatment [so that they] don’t become too sick, then don’t have an opportunity to be treated even when the test results come back positive.”
— Susan London
QoL preserved with ribociclib-letrozole for advanced breast cancer
Key clinical point Patients who took ribociclib plus letrozole had less pain and no drop in QoL compared with letrozole alone. Major finding QoL was sustained and pain scores decreased when ribociclib was added to letrozole for patients with advanced breast cancer. Data source Double-blind, placebo-controlled phase 3 trial of letrozole plus ribociclib compared with letrozole plus placebo in 668 patients with advanced hormone receptor–positive, HER2-negative breast cancer. Disclosures Dr Verma reported financial relationships with Novartis, which markets ribociclib, and other firms.
Patients with advanced breast cancer whose aromatase inhibitor therapy was supplemented with a cycline-dependent kinase inhibitor had better PFS with no drop in quality of life (QoL). Health-related QoL for patients on the combination therapy was equivalent to that of patients on monotherapy in most aspects, but patients receiving both therapies had a sustained and clinically meaningful decrease in pain.
In addition, the time to definitive deterioration by 10% or more of the global health status/QoL scale score was similar between treatment arms (hazard ratio [HR], 0.944; 95% confidence interval, 0.720-1.237).
The MONALEESA-2 trial had previously shown that the CDK4/6 inhibitor ribociclib, when added to the aromatase inhibitor letrozole, significantly improved PFS for postmenopausal patients with hormone receptor–positive, HER2-negative advanced breast cancer, when compared with letrozole in combination with placebo.
Sunil Verma, MD, reported on health-related QoL and symptoms in the 2 arms of MONALEESA-2, showing change from baseline, time to a definitive 10% deterioration, and the mean scores for on-treatment time compared with end of treatment on the global health-status QoL subscale of the European Organization for Research and Treatment of Cancer's (EORTC's) 30-item core QoL questionnaire.
“During treatment, overall health-related quality of life was maintained from baseline and was similar in both arms,” said Dr Verma, the study’s first author. Changes during treatment were not statistically significant, and did not reach the predetermined threshold for a clinically meaningful difference. The effect of key symptoms such as fatigue, nausea, and vomiting on QoL was similar for patients receiving ribociclib or placebo, he said. Although symptom scores were slightly higher for patients in the active arm of the study, the results were not clinically significant.
Reporting validated, cancer-specific patient-reported outcomes from the trial, Dr Verma, professor and head of the department of oncology at the University of Calgary in Alberta, Canada, sought to “highlight the patient experience with a focus on health-related quality of life and symptoms,” he said during his presentation at the meeting. “A clinically meaningful – more than 5 points – improvement from baseline in pain score was maintained up to and including cycle 15 in the ribociclib plus letrozole arm.” The placebo arm had a mild, clinically insignificant improvement at most assessment points. For both treatment arms, pain scores increased a bit above baseline levels at the time of disease progression or end of therapy, he said.
Patients completed the EORTC 30-item core QoL questionnaire at their screening visit, and then every 8 weeks for the first 18 months. Then, they completed the questionnaires every 12 weeks until they experienced disease progression, died, were lost to follow-up or withdrew from the study, or stopped treatment.
Statistical analysis of the questionnaire results took into account the patients’ baseline scores, treatments received, and how they were stratified. Investigators assessed both statistical significance and the clinical meaningfulness of changes, defined as a change of 5-10 points.
In MONALEESA-2, 334 patients each were allocated to the ribociclib-letrozole arm and the placebo-letrozole arm. Patients in both arms, said Dr Verma, were very compliant with questionnaire completion. More than 90% of patients who were eligible completed questionnaire through cycle 19 of ribociclib or placebo. He explained that the overall numbers completing the questionnaire declined with time, as more patients had disease progression. Dr Verma said it is important to include those measures, because patients and their families care about “the quality of the time gained,” so patient-reported outcomes should be a part of risk-benefit assessments for new cancer therapies. “While delaying disease progression may help maintain patient quality of life, the addition of novel treatments to existing therapies can add toxicities, which may diminish quality of life,” he said.
— Kari Oakes
Ibrutinib and beyond: optimizing therapy in relapsed CLL
Key clinical point Ibrutinib had durable efficacy in relapsed CLL, and combinations with other targeted agents or with CAR-T cells are promising. Major finding Long-term PFS was better with ibrutinib than with ofatumumab (HR, 0.133). The overall response rate with the triplet of ibrutinib, ublituximab, and umbralisib was 100%. In all, 89% of patients achieved no evidence of disease in marrow when anti-CD19 CAR-T cells were added to ibrutinib. Data source An update of a phase 3 randomized trial among 391 patients with previously treated CLL or SLL (RESONATE). A phase 1/1b trial including 19 patients with mainly relapsed or refractory CLL or SLL. A pilot trial among 10 patients with previously treated, mainly higher-risk, CLL or SLL. Disclosures See article text.
Ibrutinib monotherapy
In the phase 3 randomized RESONATE trial, investigators compared ibrutinib with ofatumumab, an anti-CD20 antibody, among 391 patients with CLL or small lymphocytic lymphoma (SLL), with crossover allowed. Initial results favored ibrutinib.
Investigators led by John C Byrd, MD, director of the division of hematology at the Ohio State University Comprehensive Cancer Center in Columbus, reported updated data in a poster session at the meeting, now with a median 44 months of follow-up in the ibrutinib arm.
Median PFS was not reached with ibrutinib, compared with 8.1 months with ofatumumab (HR, 0.133). The 3-year rate of PFS for ibrutinib and ofatumumab was 59% and 3%, respectively.
The pattern was generally similar across patients stratified by cytogenetics (deletion of 17p, deletion of 11q, or neither), IGHV and TP53 mutation status, and previous lines of therapy, reported Dr Byrd. The 3-year overall survival rate for ibrutinib was 74%. In analyses adjusted for crossover, patients given the inhibitor had a markedly lower risk of death than did peers given the antibody (HR, 0.37). The overall response rate with ibrutinib was 91%. Although the rate of complete response increased with follow-up, it was still just 9%.
The most common grade 3 or worse adverse events were neutropenia (23%), pneumonia (17%), and anemia, thrombocytopenia, and hypertension (8% each). Major hemorrhage was reported in 2 patients (1%) in the ibrutinib group, and 3 (2%) in the ofatumumab group.
The investigators emphasized that these long-term results “show that extended treatment with ibrutinib is tolerable and continues to show sustained PFS in previously treated patients with CLL regardless of high-risk cytogenetics, adding that traditional poor prognostic factors for survival with chemoimmunotherapy, including del(17p) and del(11q), were not significant factors predictive of PFS outcomes with ibrutinib.
Funding and disclosures: Pharmacyclics funded the trial. Dr Byrd disclosed that he receives research funding from Pharmacyclics and other companies.
Ibrutinib plus ublituximab and umbralisib
Investigators led by Loretta J Nastoupil, MD, tested a triplet consisting of ibrutinib with ublituximab (another anti-CD20 antibody) and umbralisib (TGR-1202), an oral PI3 kinase-delta inhibitor, in a phase 1/1b trial of 38 patients with generally heavily pretreated leukemias and lymphomas, including 20 with CLL or SLL. Notably, 8 patients with CLL (50%) had a 17p or 11q deletion.
The median time on study was 11.1 months, reported Dr Nastoupil, of the department of lymphoma/myeloma at the University of Texas MD Anderson Cancer Center, Houston. The overall response rate for the 19 evaluable patients with CLL or SLL was 100% (complete response in 32%, partial response in 68%). The main grade 3 or 4 adverse events in the entire trial population were neutropenia (18%) and pyrexia (8%). Only two patients discontinued treatment because of adverse events.
“An expansion cohort is ongoing at the highest dose, and we will clearly need more patients treated and longer follow-up to really know the efficacy,” commented invited discussant Jennifer R Brown, MD, PhD, director of the Chronic Lymphocytic Leukemia Center at the Dana-Farber Cancer Institute in Boston.
Funding and disclosures: TG Therapeutics funded the trial. Dr Nastoupil has received honoraria and research funding from TG Therapeutics, and honoraria from Pharmacyclics.
Ibrutinib plus CAR-T cells
Investigators in a pilot trial led by Saar Gill, MD, PhD, of the hospital of the University of Pennsylvania in Philadelphia, tested the combination of ibrutinib with chimeric antigen receptor-T cells (CAR-T cells) against CD19, on the basis of preclinical evidence of synergy. The trial participants were 10 patients with CLL or SLL who had not achieved complete response with ibrutinib. All had a 17p deletion or a p53 mutation, or a complex karyotype, and some had a known ibrutinib resistance mutation.
At 3 months, 8 of 9 evaluable patients (89%) had no evidence of disease in bone marrow, reported Dr Gill. Seven patients (78%) achieved a complete or partial radiographic response in the spleen and lymph nodes. Overall, the treatment was well tolerated. One patient developed grade 4 tumor lysis syndrome, and two developed grade 3 cytokine release syndrome. But, none required anticytokine therapy.
Most patients remain on ibrutinib and are being monitored. In addition, the researchers plan to treat 25 more patients with the same combination.
Funding and disclosures: Novartis funded the trial. Dr Gill disclosed that he receives research funding from Novartis (institutional) and has patents for CAR-T cells for AML.
— Susan London
Pazopanib falls short as adjuvant therapy for high-risk RCC
Key clinical point Pazopanib is not efficacious for treating resected high-risk locally advanced RCC. Major finding Compared with placebo, pazopanib started at 600 mg daily did not significantly reduce the risk of DFS events (HR, 0.86; P = .16). Data source A phase 3 randomized controlled trial among 1,538 patients who had undergone nephrectomy for high-risk locally advanced RCC (PROTECT trial). Disclosures Novartis Oncology funded the trial. Dr Motzer disclosed that he is a consultant to Novartis and receives research funding from Novartis (institutional).
The antiangiogenic agent pazopanib is not efficacious when used as adjuvant therapy for resected renal cell carcinoma (RCC) with features that confer a high risk of recurrence, investigators in the PROTECT investigators reported at the meeting. “The trial did not meet its primary endpoint,” concluded lead investigator Robert J Motzer, MD, of Memorial Sloan-Kettering Cancer Center in New York. “Pazopanib is not recommended for adjuvant therapy following resection of locally advanced RCC.”
Adjuvant use of other agents in this class has yielded mixed results, Dr Motzer noted, citing the earlier ASSURE trial found that neither sunitinib nor sorafenib improved disease-free survival (DFS) or overall survival (Lancet 2016;387:2008-2016), and the S-TRAC trial that found that sunitinib improved DFS (N Engl J Med. 2016;375:2246-2254).
Pazopanib is an oral multitargeted tyrosine kinase inhibitor active against the vascular endothelial growth factor receptor (VEGFR). The PROTECT trial included 1,538 patients with resected pT2 (high grade), pT3, or greater nonmetastatic clear cell RCC, a highly vascular tumor typically reliant on aberrant signaling in the VEGF pathway. The patients were assigned evenly to receive pazopanib or placebo for 1 year. The starting dose was lowered from 800 mg daily to 600 mg daily (with escalation permitted) after 403 patients had been treated.
In intention-to-treat analysis among patients started on the 600-mg dose and having a median follow-up of about 31 months, DFS was better with pazopanib but not significantly so (HR, 0.86; P = .16). In secondary analyses, pazopanib had a significant DFS benefit in patients started on the 800-mg dose (HR, 0.69; P = .02) and among the entire trial population started on either dose (HR, 0.80; P = .01).
One possible explanation for the differing results seen with the 2 doses was the difference in follow-up, because the 800-mg group was treated earlier in the trial, said Dr Motzer. But with an additional year of blinded follow-up, the benefit in the 600-mg group diminished, but not in the 800-mg group.
Another possibility was the somewhat better performance of the placebo group used for comparison with the lower dose: the 3-year DFS rate with placebo was 64% for the 600-mg comparison but 56% for the 800-mg comparison. “One factor that could explain differences in the outcomes of the placebo groups includes unidentified patient demographic characteristics,” Dr Motzer added.
Overall survival was statistically indistinguishable between the pazopanib and placebo groups, regardless of dose. However, “the results are inconclusive as the data are not yet mature,” he said, with a definitive analysis planned for 2019.
Patients started on the 600-mg dose of pazopanib had a higher rate of grade 3 or 4 adverse events overall compared with those given placebo (60% vs 21%, respectively), driven in part by higher rates of hypertension and increased alanine aminotransferase levels. “Although the intent of modifying the protocol dose of pazopanib from 800 mg to 600 mg was to reduce the rate of discontinuation and improve the safety profile ... both cohorts had similar discontinuation rates and safety profiles,” Dr Motzer noted.
A QoL analysis for the 600-mg group using the 19-item Functional Assessment of Cancer Therapy Kidney Symptom Index showed values were consistently lower with the drug than with placebo during treatment, with a crossing of the threshold for a minimally important difference at week 8. Pharmacokinetic analyses from the trial, reported in a poster at the meeting, showed that in the group starting pazopanib at 600 mg, DFS was longer in patients who achieved higher drug trough concentrations at week 3 or 5.
— Susan London
Survival improves when patients with cancer self-report symptoms
Key clinical point Patients with metastatic cancer who self-reported symptoms experienced significant improvement in overall survival. Major finding Median overall survival with self-reporting of symptoms compared with usual care was 31.2 and 26 months, respectively. Data source A randomized controlled clinical trial of 766 patients. Funding and disclosures This study was supported by the National Institutes of Health and the Conquer Cancer Foundation of the American Society of Clinical Oncology. Dr Basch and Dr Burstein each reported having no disclosures.
Patients with metastatic cancer who self-reported symptoms during routine cancer treatment experienced a number of benefits, including a statistically significant improvement in overall survival, according to findings from a randomized, controlled clinical trial (see p. e184). The median overall survival in 441 patients receiving treatment for metastatic breast, lung, genitourinary, or gynecologic cancer who were randomized to the self-reporting intervention arm was more than 5 months longer (a nearly 20% increase) than in 325 patients receiving standard care (31.2 vs. 26 months), Ethan Basch, MD, of the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill, said at the meeting. “Another way to think of this is [in terms of] 5-year survival. At 5 years, 8% more patients were alive in the self-reporting group,” he said.
In addition, 31% of patients in the intervention arm had better quality of life/physical functioning, compared with those in the control arm, and 7% fewer patients in the intervention arm visited an emergency room during the study. The duration of potentially life-prolonging chemotherapy was increased by an average of 2 months in the intervention arm, he said (JAMA. 2017 Jun 4. doi: 10.1001/jama.2017.7156).
Symptoms such as nausea, pain, and fatigue are common among patients with metastatic cancer and can often go undetected by doctors and nurses until they become severe and physically debilitating, Dr Basch added, noting that patients are often hesitant to call the office between visits to report symptoms.
Dr Basch and his colleagues hypothesized that self-reporting of patient symptoms between visits or before a visit while the patient was in the clinic waiting area would prompt earlier intervention and improve symptom control and outcomes.
Study participants were patients at Memorial Sloan Kettering Cancer Center who had advanced solid genitourinary, gynecologic, breast, or lung tumors and who were receiving outpatient chemotherapy. Those assigned to the intervention group used tablet computers and an online web survey system to report on 12 symptoms commonly experienced during chemotherapy. The system triggers an alert to a nurse when a severe or worsening symptom is reported. Patients in the usual care group discussed symptoms during office visits and were encouraged to call the office between visits if they were concerned about symptoms.
Patients remained on the study until discontinuation of all cancer treatment, hospice, or death.
One possible explanation for the findings is that this self-reporting approach prompts clinicians to manage symptoms before they cause serious downstream complications, Dr Basch said.
The approach may also keep patients more physically functional, which is known from previous studies to have a strong association with better survival, and it may also improve management of chemotherapy side effects, enabling longer duration of beneficial cancer treatment. “In oncology, we often are limited in our ability to give life-prolonging treatment because people don’t tolerate it well,” Dr Basch explained.
“This approach should be considered for inclusion in standard symptoms management as a component of high-quality cancer care,” he concluded, noting that efforts are underway to test the next generation of systems to improve communication between patients and care teams and to figure out how best to integrate these tools into oncology practice.
The system used in the this study was designed for research, but a number of companies have tools currently available for patient-reported outcomes, and others are being developed, Dr Basch said. A National Cancer Institute questionnaire, the PRO-CTCAE, is publicly available and can be loaded into patients’ electronic health records for this purpose as well.
Harold J Burstein, MD, of Dana-Farber Cancer Institute, Boston, said the study findings validate the feeling among many clinicians that patient-focused, team-based care can improve outcomes in a meaningful way for patients. If this were a drug … it would be worth tens, if not hundreds of thousands, of dollars per year … We don’t have those same kinds of dollars to help implement these into our electronic health records or our systems. We need to find ways to support that and make it happen,” he said.
— Sharon Worcester
TRK inhibitor shows ‘striking’ activity and durability across diverse cancers
Key clinical point Larotrectinib has good, durable efficacy when used to treat advanced cancers harboring TRK fusions. Major finding The overall response rate was 76%, and 79% of responses were still ongoing at 12 months. Data source An integrated analysis of phase 1 and 2 trials among 55 children and adults having 17 discrete types of advanced cancer with TRK fusions. Funding and disclosures Loxo Oncology funded the study. Dr Hyman disclosed that he has a consulting or advisory role with Atara Biotherapeutics, Chugai Pharma, and CytomX Therapeutics, and that he receives research funding from AstraZeneca and Puma Biotechnology.
Larotrectinib, an oral inhibitor of tropomyosin receptor kinase (TRK), has durable efficacy across diverse adult and pediatric cancers that harbor a genetic aberration known as TRK fusion, according to findings from an analysis of 3 trials reported at the meeting (see p. e184). Fusion of a TRK gene with an unrelated gene leads to uncontrolled signaling in the TRK pathway, potentially causing tumor growth and addiction to this input, David Hyman, MD, chief of early drug development at Memorial Sloan Kettering Cancer Center in New York, explained in a press briefing.
“One of the defining features of TRK fusions is that they are not just found in one cancer type, but in dozens of different cancer types, and not just in adults, but children as well, spanning the entire lifetime of the person,” he noted. They are rare in common cancers and nearly universal in certain uncommon cancers; collectively, they are present in possibly 5,000 cancers diagnosed each year in the United States.
Dr Hyman and his colleagues analyzed data from 55 patients having 17 discrete types of advanced cancer harboring TRK fusions who were treated with larotrectinib in phase 1 and 2 trials. Results showed an overall response rate of 76%, and the majority of responses were still ongoing at 12 months. “I believe these data support larotrectinib as a potential new standard of care for these patients,” he said. “However, I want to emphasize that really recognizing this benefit in the community will require that we test patients more universally for the presence of TRK fusions or other tumor-agnostic biomarkers, such as microsatellite instability.”
Study details
The investigators analyzed data from 3 trials in which patients with advanced TRK fusion-positive solid cancers received larotrectinib (LOXO-101): a phase 1 trial among 8 adult patients, a phase 1/2 trial among 12 pediatric patients (SCOUT), and a phase 2 “basket” trial among 35 adult and adolescent patients (NAVIGATE).
“These patients were identified by local testing,” Dr Hyman noted. “We did not perform central screening to find the TRK fusions, and in fact, 50 different laboratories identified the 55 patients. So in a sense, this really represents the real-world identification of these patients.”
In an integrated analysis, the overall rate of confirmed response as assessed by investigators was 76%, with complete response in 12% of patients and partial response in 64%. Two patients had such deep tumor regression that they experienced downstaging enabling them to undergo potentially curative surgery. Efficacy was consistent regardless of tumor type, which TRK gene was affected, and the fusion partner gene.
Median time to response was 1.8 months. “This is just a reflection of when the first scan was obtained. But in the clinic, patients reported dramatic improvement of their symptoms within days of starting therapy,” Dr Hyman said.
With a median follow-up of 5.8 months, the median duration of response was not yet reached. In all, 79% of responses were still ongoing at 12 months. Median progression-free survival (PFS) was likewise not reached; the 12-month rate was 63%.
The leading treatment-emergent adverse events were fatigue (38%), dizziness (27%), nausea (26%), and anemia (26%). “This is an extremely well tolerated therapy with only 13% of patients requiring any form of dose modification and not a single patient discontinuing due to adverse events,” he said.
It is not clear why some patients had apparent primary resistance to larotrectinib, but their TRK fusion test results may have been incorrect, Dr Hyman speculated. In all, 6 patients developed acquired resistance to larotrectinib; 5 of them were found to have an identical resistance mutation, and 2 went on to receive and have a response to LOXO-195, a next-generation TRK inhibitor that seems to retain activity in the presence of this mutation (Cancer Discov. 2017 June 3. doi: 10.1158/2159-8290.CD-17-0507).
TRK testing
Several next-generation sequencing-based tests already available clinically can pick up TRK fusions, Dr Hyman pointed out. “But it is important for the ordering physician to understand whether the tests they are ordering include fusion detection and, if it’s an option, to select it. Otherwise, they will not find TRK fusions. “The list price for these tests is in the low thousands of dollars,” he noted. In cancers in which sequential single-gene testing is already being done as standard of care, there is “minimal” incremental cost of instead using comprehensive testing that would detect TRK fusions.
Oncologists should be aware that obtaining test results can take weeks, Dr Hyman stressed. “This [testing] should be more broadly adopted and should be adopted at a point in the patient’s treatment [so that they] don’t become too sick, then don’t have an opportunity to be treated even when the test results come back positive.”
— Susan London
QoL preserved with ribociclib-letrozole for advanced breast cancer
Key clinical point Patients who took ribociclib plus letrozole had less pain and no drop in QoL compared with letrozole alone. Major finding QoL was sustained and pain scores decreased when ribociclib was added to letrozole for patients with advanced breast cancer. Data source Double-blind, placebo-controlled phase 3 trial of letrozole plus ribociclib compared with letrozole plus placebo in 668 patients with advanced hormone receptor–positive, HER2-negative breast cancer. Disclosures Dr Verma reported financial relationships with Novartis, which markets ribociclib, and other firms.
Patients with advanced breast cancer whose aromatase inhibitor therapy was supplemented with a cycline-dependent kinase inhibitor had better PFS with no drop in quality of life (QoL). Health-related QoL for patients on the combination therapy was equivalent to that of patients on monotherapy in most aspects, but patients receiving both therapies had a sustained and clinically meaningful decrease in pain.
In addition, the time to definitive deterioration by 10% or more of the global health status/QoL scale score was similar between treatment arms (hazard ratio [HR], 0.944; 95% confidence interval, 0.720-1.237).
The MONALEESA-2 trial had previously shown that the CDK4/6 inhibitor ribociclib, when added to the aromatase inhibitor letrozole, significantly improved PFS for postmenopausal patients with hormone receptor–positive, HER2-negative advanced breast cancer, when compared with letrozole in combination with placebo.
Sunil Verma, MD, reported on health-related QoL and symptoms in the 2 arms of MONALEESA-2, showing change from baseline, time to a definitive 10% deterioration, and the mean scores for on-treatment time compared with end of treatment on the global health-status QoL subscale of the European Organization for Research and Treatment of Cancer's (EORTC's) 30-item core QoL questionnaire.
“During treatment, overall health-related quality of life was maintained from baseline and was similar in both arms,” said Dr Verma, the study’s first author. Changes during treatment were not statistically significant, and did not reach the predetermined threshold for a clinically meaningful difference. The effect of key symptoms such as fatigue, nausea, and vomiting on QoL was similar for patients receiving ribociclib or placebo, he said. Although symptom scores were slightly higher for patients in the active arm of the study, the results were not clinically significant.
Reporting validated, cancer-specific patient-reported outcomes from the trial, Dr Verma, professor and head of the department of oncology at the University of Calgary in Alberta, Canada, sought to “highlight the patient experience with a focus on health-related quality of life and symptoms,” he said during his presentation at the meeting. “A clinically meaningful – more than 5 points – improvement from baseline in pain score was maintained up to and including cycle 15 in the ribociclib plus letrozole arm.” The placebo arm had a mild, clinically insignificant improvement at most assessment points. For both treatment arms, pain scores increased a bit above baseline levels at the time of disease progression or end of therapy, he said.
Patients completed the EORTC 30-item core QoL questionnaire at their screening visit, and then every 8 weeks for the first 18 months. Then, they completed the questionnaires every 12 weeks until they experienced disease progression, died, were lost to follow-up or withdrew from the study, or stopped treatment.
Statistical analysis of the questionnaire results took into account the patients’ baseline scores, treatments received, and how they were stratified. Investigators assessed both statistical significance and the clinical meaningfulness of changes, defined as a change of 5-10 points.
In MONALEESA-2, 334 patients each were allocated to the ribociclib-letrozole arm and the placebo-letrozole arm. Patients in both arms, said Dr Verma, were very compliant with questionnaire completion. More than 90% of patients who were eligible completed questionnaire through cycle 19 of ribociclib or placebo. He explained that the overall numbers completing the questionnaire declined with time, as more patients had disease progression. Dr Verma said it is important to include those measures, because patients and their families care about “the quality of the time gained,” so patient-reported outcomes should be a part of risk-benefit assessments for new cancer therapies. “While delaying disease progression may help maintain patient quality of life, the addition of novel treatments to existing therapies can add toxicities, which may diminish quality of life,” he said.
— Kari Oakes
Ibrutinib and beyond: optimizing therapy in relapsed CLL
Key clinical point Ibrutinib had durable efficacy in relapsed CLL, and combinations with other targeted agents or with CAR-T cells are promising. Major finding Long-term PFS was better with ibrutinib than with ofatumumab (HR, 0.133). The overall response rate with the triplet of ibrutinib, ublituximab, and umbralisib was 100%. In all, 89% of patients achieved no evidence of disease in marrow when anti-CD19 CAR-T cells were added to ibrutinib. Data source An update of a phase 3 randomized trial among 391 patients with previously treated CLL or SLL (RESONATE). A phase 1/1b trial including 19 patients with mainly relapsed or refractory CLL or SLL. A pilot trial among 10 patients with previously treated, mainly higher-risk, CLL or SLL. Disclosures See article text.
Ibrutinib monotherapy
In the phase 3 randomized RESONATE trial, investigators compared ibrutinib with ofatumumab, an anti-CD20 antibody, among 391 patients with CLL or small lymphocytic lymphoma (SLL), with crossover allowed. Initial results favored ibrutinib.
Investigators led by John C Byrd, MD, director of the division of hematology at the Ohio State University Comprehensive Cancer Center in Columbus, reported updated data in a poster session at the meeting, now with a median 44 months of follow-up in the ibrutinib arm.
Median PFS was not reached with ibrutinib, compared with 8.1 months with ofatumumab (HR, 0.133). The 3-year rate of PFS for ibrutinib and ofatumumab was 59% and 3%, respectively.
The pattern was generally similar across patients stratified by cytogenetics (deletion of 17p, deletion of 11q, or neither), IGHV and TP53 mutation status, and previous lines of therapy, reported Dr Byrd. The 3-year overall survival rate for ibrutinib was 74%. In analyses adjusted for crossover, patients given the inhibitor had a markedly lower risk of death than did peers given the antibody (HR, 0.37). The overall response rate with ibrutinib was 91%. Although the rate of complete response increased with follow-up, it was still just 9%.
The most common grade 3 or worse adverse events were neutropenia (23%), pneumonia (17%), and anemia, thrombocytopenia, and hypertension (8% each). Major hemorrhage was reported in 2 patients (1%) in the ibrutinib group, and 3 (2%) in the ofatumumab group.
The investigators emphasized that these long-term results “show that extended treatment with ibrutinib is tolerable and continues to show sustained PFS in previously treated patients with CLL regardless of high-risk cytogenetics, adding that traditional poor prognostic factors for survival with chemoimmunotherapy, including del(17p) and del(11q), were not significant factors predictive of PFS outcomes with ibrutinib.
Funding and disclosures: Pharmacyclics funded the trial. Dr Byrd disclosed that he receives research funding from Pharmacyclics and other companies.
Ibrutinib plus ublituximab and umbralisib
Investigators led by Loretta J Nastoupil, MD, tested a triplet consisting of ibrutinib with ublituximab (another anti-CD20 antibody) and umbralisib (TGR-1202), an oral PI3 kinase-delta inhibitor, in a phase 1/1b trial of 38 patients with generally heavily pretreated leukemias and lymphomas, including 20 with CLL or SLL. Notably, 8 patients with CLL (50%) had a 17p or 11q deletion.
The median time on study was 11.1 months, reported Dr Nastoupil, of the department of lymphoma/myeloma at the University of Texas MD Anderson Cancer Center, Houston. The overall response rate for the 19 evaluable patients with CLL or SLL was 100% (complete response in 32%, partial response in 68%). The main grade 3 or 4 adverse events in the entire trial population were neutropenia (18%) and pyrexia (8%). Only two patients discontinued treatment because of adverse events.
“An expansion cohort is ongoing at the highest dose, and we will clearly need more patients treated and longer follow-up to really know the efficacy,” commented invited discussant Jennifer R Brown, MD, PhD, director of the Chronic Lymphocytic Leukemia Center at the Dana-Farber Cancer Institute in Boston.
Funding and disclosures: TG Therapeutics funded the trial. Dr Nastoupil has received honoraria and research funding from TG Therapeutics, and honoraria from Pharmacyclics.
Ibrutinib plus CAR-T cells
Investigators in a pilot trial led by Saar Gill, MD, PhD, of the hospital of the University of Pennsylvania in Philadelphia, tested the combination of ibrutinib with chimeric antigen receptor-T cells (CAR-T cells) against CD19, on the basis of preclinical evidence of synergy. The trial participants were 10 patients with CLL or SLL who had not achieved complete response with ibrutinib. All had a 17p deletion or a p53 mutation, or a complex karyotype, and some had a known ibrutinib resistance mutation.
At 3 months, 8 of 9 evaluable patients (89%) had no evidence of disease in bone marrow, reported Dr Gill. Seven patients (78%) achieved a complete or partial radiographic response in the spleen and lymph nodes. Overall, the treatment was well tolerated. One patient developed grade 4 tumor lysis syndrome, and two developed grade 3 cytokine release syndrome. But, none required anticytokine therapy.
Most patients remain on ibrutinib and are being monitored. In addition, the researchers plan to treat 25 more patients with the same combination.
Funding and disclosures: Novartis funded the trial. Dr Gill disclosed that he receives research funding from Novartis (institutional) and has patents for CAR-T cells for AML.
— Susan London
Pazopanib falls short as adjuvant therapy for high-risk RCC
Key clinical point Pazopanib is not efficacious for treating resected high-risk locally advanced RCC. Major finding Compared with placebo, pazopanib started at 600 mg daily did not significantly reduce the risk of DFS events (HR, 0.86; P = .16). Data source A phase 3 randomized controlled trial among 1,538 patients who had undergone nephrectomy for high-risk locally advanced RCC (PROTECT trial). Disclosures Novartis Oncology funded the trial. Dr Motzer disclosed that he is a consultant to Novartis and receives research funding from Novartis (institutional).
The antiangiogenic agent pazopanib is not efficacious when used as adjuvant therapy for resected renal cell carcinoma (RCC) with features that confer a high risk of recurrence, investigators in the PROTECT investigators reported at the meeting. “The trial did not meet its primary endpoint,” concluded lead investigator Robert J Motzer, MD, of Memorial Sloan-Kettering Cancer Center in New York. “Pazopanib is not recommended for adjuvant therapy following resection of locally advanced RCC.”
Adjuvant use of other agents in this class has yielded mixed results, Dr Motzer noted, citing the earlier ASSURE trial found that neither sunitinib nor sorafenib improved disease-free survival (DFS) or overall survival (Lancet 2016;387:2008-2016), and the S-TRAC trial that found that sunitinib improved DFS (N Engl J Med. 2016;375:2246-2254).
Pazopanib is an oral multitargeted tyrosine kinase inhibitor active against the vascular endothelial growth factor receptor (VEGFR). The PROTECT trial included 1,538 patients with resected pT2 (high grade), pT3, or greater nonmetastatic clear cell RCC, a highly vascular tumor typically reliant on aberrant signaling in the VEGF pathway. The patients were assigned evenly to receive pazopanib or placebo for 1 year. The starting dose was lowered from 800 mg daily to 600 mg daily (with escalation permitted) after 403 patients had been treated.
In intention-to-treat analysis among patients started on the 600-mg dose and having a median follow-up of about 31 months, DFS was better with pazopanib but not significantly so (HR, 0.86; P = .16). In secondary analyses, pazopanib had a significant DFS benefit in patients started on the 800-mg dose (HR, 0.69; P = .02) and among the entire trial population started on either dose (HR, 0.80; P = .01).
One possible explanation for the differing results seen with the 2 doses was the difference in follow-up, because the 800-mg group was treated earlier in the trial, said Dr Motzer. But with an additional year of blinded follow-up, the benefit in the 600-mg group diminished, but not in the 800-mg group.
Another possibility was the somewhat better performance of the placebo group used for comparison with the lower dose: the 3-year DFS rate with placebo was 64% for the 600-mg comparison but 56% for the 800-mg comparison. “One factor that could explain differences in the outcomes of the placebo groups includes unidentified patient demographic characteristics,” Dr Motzer added.
Overall survival was statistically indistinguishable between the pazopanib and placebo groups, regardless of dose. However, “the results are inconclusive as the data are not yet mature,” he said, with a definitive analysis planned for 2019.
Patients started on the 600-mg dose of pazopanib had a higher rate of grade 3 or 4 adverse events overall compared with those given placebo (60% vs 21%, respectively), driven in part by higher rates of hypertension and increased alanine aminotransferase levels. “Although the intent of modifying the protocol dose of pazopanib from 800 mg to 600 mg was to reduce the rate of discontinuation and improve the safety profile ... both cohorts had similar discontinuation rates and safety profiles,” Dr Motzer noted.
A QoL analysis for the 600-mg group using the 19-item Functional Assessment of Cancer Therapy Kidney Symptom Index showed values were consistently lower with the drug than with placebo during treatment, with a crossing of the threshold for a minimally important difference at week 8. Pharmacokinetic analyses from the trial, reported in a poster at the meeting, showed that in the group starting pazopanib at 600 mg, DFS was longer in patients who achieved higher drug trough concentrations at week 3 or 5.
— Susan London
Survival improves when patients with cancer self-report symptoms
Key clinical point Patients with metastatic cancer who self-reported symptoms experienced significant improvement in overall survival. Major finding Median overall survival with self-reporting of symptoms compared with usual care was 31.2 and 26 months, respectively. Data source A randomized controlled clinical trial of 766 patients. Funding and disclosures This study was supported by the National Institutes of Health and the Conquer Cancer Foundation of the American Society of Clinical Oncology. Dr Basch and Dr Burstein each reported having no disclosures.
Patients with metastatic cancer who self-reported symptoms during routine cancer treatment experienced a number of benefits, including a statistically significant improvement in overall survival, according to findings from a randomized, controlled clinical trial (see p. e184). The median overall survival in 441 patients receiving treatment for metastatic breast, lung, genitourinary, or gynecologic cancer who were randomized to the self-reporting intervention arm was more than 5 months longer (a nearly 20% increase) than in 325 patients receiving standard care (31.2 vs. 26 months), Ethan Basch, MD, of the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill, said at the meeting. “Another way to think of this is [in terms of] 5-year survival. At 5 years, 8% more patients were alive in the self-reporting group,” he said.
In addition, 31% of patients in the intervention arm had better quality of life/physical functioning, compared with those in the control arm, and 7% fewer patients in the intervention arm visited an emergency room during the study. The duration of potentially life-prolonging chemotherapy was increased by an average of 2 months in the intervention arm, he said (JAMA. 2017 Jun 4. doi: 10.1001/jama.2017.7156).
Symptoms such as nausea, pain, and fatigue are common among patients with metastatic cancer and can often go undetected by doctors and nurses until they become severe and physically debilitating, Dr Basch added, noting that patients are often hesitant to call the office between visits to report symptoms.
Dr Basch and his colleagues hypothesized that self-reporting of patient symptoms between visits or before a visit while the patient was in the clinic waiting area would prompt earlier intervention and improve symptom control and outcomes.
Study participants were patients at Memorial Sloan Kettering Cancer Center who had advanced solid genitourinary, gynecologic, breast, or lung tumors and who were receiving outpatient chemotherapy. Those assigned to the intervention group used tablet computers and an online web survey system to report on 12 symptoms commonly experienced during chemotherapy. The system triggers an alert to a nurse when a severe or worsening symptom is reported. Patients in the usual care group discussed symptoms during office visits and were encouraged to call the office between visits if they were concerned about symptoms.
Patients remained on the study until discontinuation of all cancer treatment, hospice, or death.
One possible explanation for the findings is that this self-reporting approach prompts clinicians to manage symptoms before they cause serious downstream complications, Dr Basch said.
The approach may also keep patients more physically functional, which is known from previous studies to have a strong association with better survival, and it may also improve management of chemotherapy side effects, enabling longer duration of beneficial cancer treatment. “In oncology, we often are limited in our ability to give life-prolonging treatment because people don’t tolerate it well,” Dr Basch explained.
“This approach should be considered for inclusion in standard symptoms management as a component of high-quality cancer care,” he concluded, noting that efforts are underway to test the next generation of systems to improve communication between patients and care teams and to figure out how best to integrate these tools into oncology practice.
The system used in the this study was designed for research, but a number of companies have tools currently available for patient-reported outcomes, and others are being developed, Dr Basch said. A National Cancer Institute questionnaire, the PRO-CTCAE, is publicly available and can be loaded into patients’ electronic health records for this purpose as well.
Harold J Burstein, MD, of Dana-Farber Cancer Institute, Boston, said the study findings validate the feeling among many clinicians that patient-focused, team-based care can improve outcomes in a meaningful way for patients. If this were a drug … it would be worth tens, if not hundreds of thousands, of dollars per year … We don’t have those same kinds of dollars to help implement these into our electronic health records or our systems. We need to find ways to support that and make it happen,” he said.
— Sharon Worcester
TRK inhibitor shows ‘striking’ activity and durability across diverse cancers
Key clinical point Larotrectinib has good, durable efficacy when used to treat advanced cancers harboring TRK fusions. Major finding The overall response rate was 76%, and 79% of responses were still ongoing at 12 months. Data source An integrated analysis of phase 1 and 2 trials among 55 children and adults having 17 discrete types of advanced cancer with TRK fusions. Funding and disclosures Loxo Oncology funded the study. Dr Hyman disclosed that he has a consulting or advisory role with Atara Biotherapeutics, Chugai Pharma, and CytomX Therapeutics, and that he receives research funding from AstraZeneca and Puma Biotechnology.
Larotrectinib, an oral inhibitor of tropomyosin receptor kinase (TRK), has durable efficacy across diverse adult and pediatric cancers that harbor a genetic aberration known as TRK fusion, according to findings from an analysis of 3 trials reported at the meeting (see p. e184). Fusion of a TRK gene with an unrelated gene leads to uncontrolled signaling in the TRK pathway, potentially causing tumor growth and addiction to this input, David Hyman, MD, chief of early drug development at Memorial Sloan Kettering Cancer Center in New York, explained in a press briefing.
“One of the defining features of TRK fusions is that they are not just found in one cancer type, but in dozens of different cancer types, and not just in adults, but children as well, spanning the entire lifetime of the person,” he noted. They are rare in common cancers and nearly universal in certain uncommon cancers; collectively, they are present in possibly 5,000 cancers diagnosed each year in the United States.
Dr Hyman and his colleagues analyzed data from 55 patients having 17 discrete types of advanced cancer harboring TRK fusions who were treated with larotrectinib in phase 1 and 2 trials. Results showed an overall response rate of 76%, and the majority of responses were still ongoing at 12 months. “I believe these data support larotrectinib as a potential new standard of care for these patients,” he said. “However, I want to emphasize that really recognizing this benefit in the community will require that we test patients more universally for the presence of TRK fusions or other tumor-agnostic biomarkers, such as microsatellite instability.”
Study details
The investigators analyzed data from 3 trials in which patients with advanced TRK fusion-positive solid cancers received larotrectinib (LOXO-101): a phase 1 trial among 8 adult patients, a phase 1/2 trial among 12 pediatric patients (SCOUT), and a phase 2 “basket” trial among 35 adult and adolescent patients (NAVIGATE).
“These patients were identified by local testing,” Dr Hyman noted. “We did not perform central screening to find the TRK fusions, and in fact, 50 different laboratories identified the 55 patients. So in a sense, this really represents the real-world identification of these patients.”
In an integrated analysis, the overall rate of confirmed response as assessed by investigators was 76%, with complete response in 12% of patients and partial response in 64%. Two patients had such deep tumor regression that they experienced downstaging enabling them to undergo potentially curative surgery. Efficacy was consistent regardless of tumor type, which TRK gene was affected, and the fusion partner gene.
Median time to response was 1.8 months. “This is just a reflection of when the first scan was obtained. But in the clinic, patients reported dramatic improvement of their symptoms within days of starting therapy,” Dr Hyman said.
With a median follow-up of 5.8 months, the median duration of response was not yet reached. In all, 79% of responses were still ongoing at 12 months. Median progression-free survival (PFS) was likewise not reached; the 12-month rate was 63%.
The leading treatment-emergent adverse events were fatigue (38%), dizziness (27%), nausea (26%), and anemia (26%). “This is an extremely well tolerated therapy with only 13% of patients requiring any form of dose modification and not a single patient discontinuing due to adverse events,” he said.
It is not clear why some patients had apparent primary resistance to larotrectinib, but their TRK fusion test results may have been incorrect, Dr Hyman speculated. In all, 6 patients developed acquired resistance to larotrectinib; 5 of them were found to have an identical resistance mutation, and 2 went on to receive and have a response to LOXO-195, a next-generation TRK inhibitor that seems to retain activity in the presence of this mutation (Cancer Discov. 2017 June 3. doi: 10.1158/2159-8290.CD-17-0507).
TRK testing
Several next-generation sequencing-based tests already available clinically can pick up TRK fusions, Dr Hyman pointed out. “But it is important for the ordering physician to understand whether the tests they are ordering include fusion detection and, if it’s an option, to select it. Otherwise, they will not find TRK fusions. “The list price for these tests is in the low thousands of dollars,” he noted. In cancers in which sequential single-gene testing is already being done as standard of care, there is “minimal” incremental cost of instead using comprehensive testing that would detect TRK fusions.
Oncologists should be aware that obtaining test results can take weeks, Dr Hyman stressed. “This [testing] should be more broadly adopted and should be adopted at a point in the patient’s treatment [so that they] don’t become too sick, then don’t have an opportunity to be treated even when the test results come back positive.”
— Susan London
QoL preserved with ribociclib-letrozole for advanced breast cancer
Key clinical point Patients who took ribociclib plus letrozole had less pain and no drop in QoL compared with letrozole alone. Major finding QoL was sustained and pain scores decreased when ribociclib was added to letrozole for patients with advanced breast cancer. Data source Double-blind, placebo-controlled phase 3 trial of letrozole plus ribociclib compared with letrozole plus placebo in 668 patients with advanced hormone receptor–positive, HER2-negative breast cancer. Disclosures Dr Verma reported financial relationships with Novartis, which markets ribociclib, and other firms.
Patients with advanced breast cancer whose aromatase inhibitor therapy was supplemented with a cycline-dependent kinase inhibitor had better PFS with no drop in quality of life (QoL). Health-related QoL for patients on the combination therapy was equivalent to that of patients on monotherapy in most aspects, but patients receiving both therapies had a sustained and clinically meaningful decrease in pain.
In addition, the time to definitive deterioration by 10% or more of the global health status/QoL scale score was similar between treatment arms (hazard ratio [HR], 0.944; 95% confidence interval, 0.720-1.237).
The MONALEESA-2 trial had previously shown that the CDK4/6 inhibitor ribociclib, when added to the aromatase inhibitor letrozole, significantly improved PFS for postmenopausal patients with hormone receptor–positive, HER2-negative advanced breast cancer, when compared with letrozole in combination with placebo.
Sunil Verma, MD, reported on health-related QoL and symptoms in the 2 arms of MONALEESA-2, showing change from baseline, time to a definitive 10% deterioration, and the mean scores for on-treatment time compared with end of treatment on the global health-status QoL subscale of the European Organization for Research and Treatment of Cancer's (EORTC's) 30-item core QoL questionnaire.
“During treatment, overall health-related quality of life was maintained from baseline and was similar in both arms,” said Dr Verma, the study’s first author. Changes during treatment were not statistically significant, and did not reach the predetermined threshold for a clinically meaningful difference. The effect of key symptoms such as fatigue, nausea, and vomiting on QoL was similar for patients receiving ribociclib or placebo, he said. Although symptom scores were slightly higher for patients in the active arm of the study, the results were not clinically significant.
Reporting validated, cancer-specific patient-reported outcomes from the trial, Dr Verma, professor and head of the department of oncology at the University of Calgary in Alberta, Canada, sought to “highlight the patient experience with a focus on health-related quality of life and symptoms,” he said during his presentation at the meeting. “A clinically meaningful – more than 5 points – improvement from baseline in pain score was maintained up to and including cycle 15 in the ribociclib plus letrozole arm.” The placebo arm had a mild, clinically insignificant improvement at most assessment points. For both treatment arms, pain scores increased a bit above baseline levels at the time of disease progression or end of therapy, he said.
Patients completed the EORTC 30-item core QoL questionnaire at their screening visit, and then every 8 weeks for the first 18 months. Then, they completed the questionnaires every 12 weeks until they experienced disease progression, died, were lost to follow-up or withdrew from the study, or stopped treatment.
Statistical analysis of the questionnaire results took into account the patients’ baseline scores, treatments received, and how they were stratified. Investigators assessed both statistical significance and the clinical meaningfulness of changes, defined as a change of 5-10 points.
In MONALEESA-2, 334 patients each were allocated to the ribociclib-letrozole arm and the placebo-letrozole arm. Patients in both arms, said Dr Verma, were very compliant with questionnaire completion. More than 90% of patients who were eligible completed questionnaire through cycle 19 of ribociclib or placebo. He explained that the overall numbers completing the questionnaire declined with time, as more patients had disease progression. Dr Verma said it is important to include those measures, because patients and their families care about “the quality of the time gained,” so patient-reported outcomes should be a part of risk-benefit assessments for new cancer therapies. “While delaying disease progression may help maintain patient quality of life, the addition of novel treatments to existing therapies can add toxicities, which may diminish quality of life,” he said.
— Kari Oakes
Ibrutinib and beyond: optimizing therapy in relapsed CLL
Key clinical point Ibrutinib had durable efficacy in relapsed CLL, and combinations with other targeted agents or with CAR-T cells are promising. Major finding Long-term PFS was better with ibrutinib than with ofatumumab (HR, 0.133). The overall response rate with the triplet of ibrutinib, ublituximab, and umbralisib was 100%. In all, 89% of patients achieved no evidence of disease in marrow when anti-CD19 CAR-T cells were added to ibrutinib. Data source An update of a phase 3 randomized trial among 391 patients with previously treated CLL or SLL (RESONATE). A phase 1/1b trial including 19 patients with mainly relapsed or refractory CLL or SLL. A pilot trial among 10 patients with previously treated, mainly higher-risk, CLL or SLL. Disclosures See article text.
Ibrutinib monotherapy
In the phase 3 randomized RESONATE trial, investigators compared ibrutinib with ofatumumab, an anti-CD20 antibody, among 391 patients with CLL or small lymphocytic lymphoma (SLL), with crossover allowed. Initial results favored ibrutinib.
Investigators led by John C Byrd, MD, director of the division of hematology at the Ohio State University Comprehensive Cancer Center in Columbus, reported updated data in a poster session at the meeting, now with a median 44 months of follow-up in the ibrutinib arm.
Median PFS was not reached with ibrutinib, compared with 8.1 months with ofatumumab (HR, 0.133). The 3-year rate of PFS for ibrutinib and ofatumumab was 59% and 3%, respectively.
The pattern was generally similar across patients stratified by cytogenetics (deletion of 17p, deletion of 11q, or neither), IGHV and TP53 mutation status, and previous lines of therapy, reported Dr Byrd. The 3-year overall survival rate for ibrutinib was 74%. In analyses adjusted for crossover, patients given the inhibitor had a markedly lower risk of death than did peers given the antibody (HR, 0.37). The overall response rate with ibrutinib was 91%. Although the rate of complete response increased with follow-up, it was still just 9%.
The most common grade 3 or worse adverse events were neutropenia (23%), pneumonia (17%), and anemia, thrombocytopenia, and hypertension (8% each). Major hemorrhage was reported in 2 patients (1%) in the ibrutinib group, and 3 (2%) in the ofatumumab group.
The investigators emphasized that these long-term results “show that extended treatment with ibrutinib is tolerable and continues to show sustained PFS in previously treated patients with CLL regardless of high-risk cytogenetics, adding that traditional poor prognostic factors for survival with chemoimmunotherapy, including del(17p) and del(11q), were not significant factors predictive of PFS outcomes with ibrutinib.
Funding and disclosures: Pharmacyclics funded the trial. Dr Byrd disclosed that he receives research funding from Pharmacyclics and other companies.
Ibrutinib plus ublituximab and umbralisib
Investigators led by Loretta J Nastoupil, MD, tested a triplet consisting of ibrutinib with ublituximab (another anti-CD20 antibody) and umbralisib (TGR-1202), an oral PI3 kinase-delta inhibitor, in a phase 1/1b trial of 38 patients with generally heavily pretreated leukemias and lymphomas, including 20 with CLL or SLL. Notably, 8 patients with CLL (50%) had a 17p or 11q deletion.
The median time on study was 11.1 months, reported Dr Nastoupil, of the department of lymphoma/myeloma at the University of Texas MD Anderson Cancer Center, Houston. The overall response rate for the 19 evaluable patients with CLL or SLL was 100% (complete response in 32%, partial response in 68%). The main grade 3 or 4 adverse events in the entire trial population were neutropenia (18%) and pyrexia (8%). Only two patients discontinued treatment because of adverse events.
“An expansion cohort is ongoing at the highest dose, and we will clearly need more patients treated and longer follow-up to really know the efficacy,” commented invited discussant Jennifer R Brown, MD, PhD, director of the Chronic Lymphocytic Leukemia Center at the Dana-Farber Cancer Institute in Boston.
Funding and disclosures: TG Therapeutics funded the trial. Dr Nastoupil has received honoraria and research funding from TG Therapeutics, and honoraria from Pharmacyclics.
Ibrutinib plus CAR-T cells
Investigators in a pilot trial led by Saar Gill, MD, PhD, of the hospital of the University of Pennsylvania in Philadelphia, tested the combination of ibrutinib with chimeric antigen receptor-T cells (CAR-T cells) against CD19, on the basis of preclinical evidence of synergy. The trial participants were 10 patients with CLL or SLL who had not achieved complete response with ibrutinib. All had a 17p deletion or a p53 mutation, or a complex karyotype, and some had a known ibrutinib resistance mutation.
At 3 months, 8 of 9 evaluable patients (89%) had no evidence of disease in bone marrow, reported Dr Gill. Seven patients (78%) achieved a complete or partial radiographic response in the spleen and lymph nodes. Overall, the treatment was well tolerated. One patient developed grade 4 tumor lysis syndrome, and two developed grade 3 cytokine release syndrome. But, none required anticytokine therapy.
Most patients remain on ibrutinib and are being monitored. In addition, the researchers plan to treat 25 more patients with the same combination.
Funding and disclosures: Novartis funded the trial. Dr Gill disclosed that he receives research funding from Novartis (institutional) and has patents for CAR-T cells for AML.
— Susan London
Pazopanib falls short as adjuvant therapy for high-risk RCC
Key clinical point Pazopanib is not efficacious for treating resected high-risk locally advanced RCC. Major finding Compared with placebo, pazopanib started at 600 mg daily did not significantly reduce the risk of DFS events (HR, 0.86; P = .16). Data source A phase 3 randomized controlled trial among 1,538 patients who had undergone nephrectomy for high-risk locally advanced RCC (PROTECT trial). Disclosures Novartis Oncology funded the trial. Dr Motzer disclosed that he is a consultant to Novartis and receives research funding from Novartis (institutional).
The antiangiogenic agent pazopanib is not efficacious when used as adjuvant therapy for resected renal cell carcinoma (RCC) with features that confer a high risk of recurrence, investigators in the PROTECT investigators reported at the meeting. “The trial did not meet its primary endpoint,” concluded lead investigator Robert J Motzer, MD, of Memorial Sloan-Kettering Cancer Center in New York. “Pazopanib is not recommended for adjuvant therapy following resection of locally advanced RCC.”
Adjuvant use of other agents in this class has yielded mixed results, Dr Motzer noted, citing the earlier ASSURE trial found that neither sunitinib nor sorafenib improved disease-free survival (DFS) or overall survival (Lancet 2016;387:2008-2016), and the S-TRAC trial that found that sunitinib improved DFS (N Engl J Med. 2016;375:2246-2254).
Pazopanib is an oral multitargeted tyrosine kinase inhibitor active against the vascular endothelial growth factor receptor (VEGFR). The PROTECT trial included 1,538 patients with resected pT2 (high grade), pT3, or greater nonmetastatic clear cell RCC, a highly vascular tumor typically reliant on aberrant signaling in the VEGF pathway. The patients were assigned evenly to receive pazopanib or placebo for 1 year. The starting dose was lowered from 800 mg daily to 600 mg daily (with escalation permitted) after 403 patients had been treated.
In intention-to-treat analysis among patients started on the 600-mg dose and having a median follow-up of about 31 months, DFS was better with pazopanib but not significantly so (HR, 0.86; P = .16). In secondary analyses, pazopanib had a significant DFS benefit in patients started on the 800-mg dose (HR, 0.69; P = .02) and among the entire trial population started on either dose (HR, 0.80; P = .01).
One possible explanation for the differing results seen with the 2 doses was the difference in follow-up, because the 800-mg group was treated earlier in the trial, said Dr Motzer. But with an additional year of blinded follow-up, the benefit in the 600-mg group diminished, but not in the 800-mg group.
Another possibility was the somewhat better performance of the placebo group used for comparison with the lower dose: the 3-year DFS rate with placebo was 64% for the 600-mg comparison but 56% for the 800-mg comparison. “One factor that could explain differences in the outcomes of the placebo groups includes unidentified patient demographic characteristics,” Dr Motzer added.
Overall survival was statistically indistinguishable between the pazopanib and placebo groups, regardless of dose. However, “the results are inconclusive as the data are not yet mature,” he said, with a definitive analysis planned for 2019.
Patients started on the 600-mg dose of pazopanib had a higher rate of grade 3 or 4 adverse events overall compared with those given placebo (60% vs 21%, respectively), driven in part by higher rates of hypertension and increased alanine aminotransferase levels. “Although the intent of modifying the protocol dose of pazopanib from 800 mg to 600 mg was to reduce the rate of discontinuation and improve the safety profile ... both cohorts had similar discontinuation rates and safety profiles,” Dr Motzer noted.
A QoL analysis for the 600-mg group using the 19-item Functional Assessment of Cancer Therapy Kidney Symptom Index showed values were consistently lower with the drug than with placebo during treatment, with a crossing of the threshold for a minimally important difference at week 8. Pharmacokinetic analyses from the trial, reported in a poster at the meeting, showed that in the group starting pazopanib at 600 mg, DFS was longer in patients who achieved higher drug trough concentrations at week 3 or 5.
— Susan London
Management of high-grade pleomorphic sarcoma with colon metastasis
Soft tissue sarcomas (STS) are a heterogeneous group of tumors of mesenchymal origin that represent a rare form of adult malignancy. According to the World Health Organization classification system, there are more than 100 histologic subtypes of sarcoma based on tissue of origin. Staging criteria most commonly use the American Joint Committee on Cancer’s TNM Classification of Malignant Tumours. About 50% of soft tissue sarcomas originate in the lower extremity.1 Advancements in the use of multimodal therapy have reduced the need for amputation and allowed for equally effective treatment strategies that use limb-sparing surgical resections.
Although most sarcoma metastases spread in a hematogenous fashion, nodal spread is underestimated. Certain histologic subtypes carry a higher predilection for nodal involvement: these include rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, vascular sarcoma, and clear-cell sarcoma.2-4 Fong and colleagues have reported that 2.6% of sarcomas have lymphatic spread.3 In the current report, we describe a rare observation of locoregional pelvic nodal metastases from a large undifferentiated pleomorphic sarcoma of the right thigh.
Case presentation and summary
A 63-year-old white woman had a 1-year history of a right thigh mass and an unintentional weight loss of 40 lb. After a year of chiropractic care, she was referred to a physician because of palpable inguinal adenopathy and a 20-cm mass in the medial compartment of the right thigh, with heterogeneous appearance on a magnetic-resonance imaging scan (Figure 1). The patient was referred to the sarcoma transdisciplinary team for evaluation. She was diagnosed by core needle biopsy with a high-grade malignant epithelioid and spindle-cell neoplasm, favoring pleomorphic sarcoma. The metastatic work-up confirmed locoregional right inguinal and retroperitoneal lymph node disease, with 2 lung nodules that were too small to characterize. She also had a paraneoplastic leukocytosis with a white blood cell count of 45,500 cells/ml (normal 10,500 cells/ml).
About 8 months after surgery, she presented to the emergency room with a 3-day history of blood per rectum, anemia, and fatigue. She also reported a weight loss of 10 lb in the previous month. She was admitted for hydration and monitoring. Although computed-tomography (CT) scans of the abdomen and chest done during the previous month and had been interpreted as having no evidence of recurrence or any lymph node disease, the results of an inpatient colonoscopy revealed 2 colonic masses, 1 in the ascending colon and another in the transverse colon. The biopsy findings were consistent with undifferentiated pleomorphic sarcoma, favoring epithelioid histology. The CT scans were re-evaluated in light of these colonoscopic findings. These masses were visible retrospectively on imaging but had been interpreted as stool given the lack of abnormality on imaging 3 months before.
Adequate re-staging was complete, and without other evidence of disease, an extended right hemicolectomy was performed. The postoperative pathology report described geographically 2 distinct masses: a 7-cm mass in the ascending colon, about 3 cm from the ileocecal valve; and a 4-cm mass in the transverse colon, about 7.5 cm from the distal margin of resection. Both masses were identified as high-grade pleomorphic sarcoma. Again, all nodes recovered were negative for malignancy (0/5). Of note is that the background colonic mucosa showed active multifocal colitis with deep inflammatory activity likely consistent with a paraneoplastic syndrome.
Discussion
Surgery remains the primary treatment modality for localized soft tissue sarcoma. Obtaining a margin-free resection, while maintaining optimal function, is the objective with extremity sarcoma. In addition to surgical resection, doxorubicin-based adjuvant chemotherapy remains the standard of care with modest improvement in overall survival and disease-free survival, especially in sarcomas of the extremities.5,6 Gemcitabine also has activity in soft tissue sarcomas7 and might synergize with docetaxel. High response rates of 53% with fixed dose infusion rates of these agents in uterine leiomyosarcoma led the Sarcoma Alliance for Research through Collaboration investigators to consider this regimen for other STS.8 An overall response rate of 16% was noted across all sarcoma subtypes, and undifferentiated pleomorphic sarcoma had a response rate of 32%.
In our experience, a modified schedule of gemcitabine and docetaxel is better tolerated than the standard every 3 weeks regimen or doxorubicin-based chemotherapies. As previously described, gemcitabine and docetaxel were administered to this patient every 2 weeks.9 This regimen, in our experience, is less toxic and preserves the dose intensity of the regimen. A complete pathologic necrosis of the primary tumor and regional nodal basin was observed, as well as pulmonary nodule regression, enabling an R0-wide excision and regional lymphadenectomy. The colonic recurrence was surprising, but easily managed with surgical resection.
Pathologic complete response after neoadjuvant chemotherapy is a rare event, observed in about 10% of patients. However, when observed, complete pathologic necrosis (>95%) provided a distant recurrence-free survival of 100% at 3 years.10-12
Our patient, despite achieving a complete pathologic response and excellent initial local control, ultimately experienced an isolated metastatic recurrence in the colon within 1 year of therapy. Data supports performing metastatectomy for stage IV extremity sarcoma for isolated pulmonary or hepatic burden in selected patients with improved survival rates.13,14 As far as we know, there is no published literature describing isolated colon metastases in the absence of liver burden from lower extremity soft tissue sarcomas, or the outcome of surgical resection and adjuvant therapy in these cases.
In conclusion, high-grade pleomorphic sarcoma often follows an aggressive clinical course with ultimately local and distant recurrence. Use of multimodal therapy may have a role in improving local control. Complete pathologic necrosis is a rare event that is predictive of improved outcome. Our case represents an unusual pattern of recurrence among patients with a complete pathologic response to neoadjuvant therapy with isolated colon metastases. Timely, comprehensive management together with vigilant surveillance remain key priorities in the long-term management of high-risk sarcoma.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349-359.
2. Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004:129-134.
3. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77.
4. Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800-1808.
5. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647-1654.
6. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft tissue sarcoma. Cancer. 2008;113:573-581.
7. Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483-3489.
8. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755-2763.
9. Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785-790.
10. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147-1153.
11. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203-3209.
12. Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911-3915.
13. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111-117.
14. Pfannschmidt J, Hoffmann H, Schneider T, Dienemann H. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res. 2009;179:321-336.
Soft tissue sarcomas (STS) are a heterogeneous group of tumors of mesenchymal origin that represent a rare form of adult malignancy. According to the World Health Organization classification system, there are more than 100 histologic subtypes of sarcoma based on tissue of origin. Staging criteria most commonly use the American Joint Committee on Cancer’s TNM Classification of Malignant Tumours. About 50% of soft tissue sarcomas originate in the lower extremity.1 Advancements in the use of multimodal therapy have reduced the need for amputation and allowed for equally effective treatment strategies that use limb-sparing surgical resections.
Although most sarcoma metastases spread in a hematogenous fashion, nodal spread is underestimated. Certain histologic subtypes carry a higher predilection for nodal involvement: these include rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, vascular sarcoma, and clear-cell sarcoma.2-4 Fong and colleagues have reported that 2.6% of sarcomas have lymphatic spread.3 In the current report, we describe a rare observation of locoregional pelvic nodal metastases from a large undifferentiated pleomorphic sarcoma of the right thigh.
Case presentation and summary
A 63-year-old white woman had a 1-year history of a right thigh mass and an unintentional weight loss of 40 lb. After a year of chiropractic care, she was referred to a physician because of palpable inguinal adenopathy and a 20-cm mass in the medial compartment of the right thigh, with heterogeneous appearance on a magnetic-resonance imaging scan (Figure 1). The patient was referred to the sarcoma transdisciplinary team for evaluation. She was diagnosed by core needle biopsy with a high-grade malignant epithelioid and spindle-cell neoplasm, favoring pleomorphic sarcoma. The metastatic work-up confirmed locoregional right inguinal and retroperitoneal lymph node disease, with 2 lung nodules that were too small to characterize. She also had a paraneoplastic leukocytosis with a white blood cell count of 45,500 cells/ml (normal 10,500 cells/ml).
About 8 months after surgery, she presented to the emergency room with a 3-day history of blood per rectum, anemia, and fatigue. She also reported a weight loss of 10 lb in the previous month. She was admitted for hydration and monitoring. Although computed-tomography (CT) scans of the abdomen and chest done during the previous month and had been interpreted as having no evidence of recurrence or any lymph node disease, the results of an inpatient colonoscopy revealed 2 colonic masses, 1 in the ascending colon and another in the transverse colon. The biopsy findings were consistent with undifferentiated pleomorphic sarcoma, favoring epithelioid histology. The CT scans were re-evaluated in light of these colonoscopic findings. These masses were visible retrospectively on imaging but had been interpreted as stool given the lack of abnormality on imaging 3 months before.
Adequate re-staging was complete, and without other evidence of disease, an extended right hemicolectomy was performed. The postoperative pathology report described geographically 2 distinct masses: a 7-cm mass in the ascending colon, about 3 cm from the ileocecal valve; and a 4-cm mass in the transverse colon, about 7.5 cm from the distal margin of resection. Both masses were identified as high-grade pleomorphic sarcoma. Again, all nodes recovered were negative for malignancy (0/5). Of note is that the background colonic mucosa showed active multifocal colitis with deep inflammatory activity likely consistent with a paraneoplastic syndrome.
Discussion
Surgery remains the primary treatment modality for localized soft tissue sarcoma. Obtaining a margin-free resection, while maintaining optimal function, is the objective with extremity sarcoma. In addition to surgical resection, doxorubicin-based adjuvant chemotherapy remains the standard of care with modest improvement in overall survival and disease-free survival, especially in sarcomas of the extremities.5,6 Gemcitabine also has activity in soft tissue sarcomas7 and might synergize with docetaxel. High response rates of 53% with fixed dose infusion rates of these agents in uterine leiomyosarcoma led the Sarcoma Alliance for Research through Collaboration investigators to consider this regimen for other STS.8 An overall response rate of 16% was noted across all sarcoma subtypes, and undifferentiated pleomorphic sarcoma had a response rate of 32%.
In our experience, a modified schedule of gemcitabine and docetaxel is better tolerated than the standard every 3 weeks regimen or doxorubicin-based chemotherapies. As previously described, gemcitabine and docetaxel were administered to this patient every 2 weeks.9 This regimen, in our experience, is less toxic and preserves the dose intensity of the regimen. A complete pathologic necrosis of the primary tumor and regional nodal basin was observed, as well as pulmonary nodule regression, enabling an R0-wide excision and regional lymphadenectomy. The colonic recurrence was surprising, but easily managed with surgical resection.
Pathologic complete response after neoadjuvant chemotherapy is a rare event, observed in about 10% of patients. However, when observed, complete pathologic necrosis (>95%) provided a distant recurrence-free survival of 100% at 3 years.10-12
Our patient, despite achieving a complete pathologic response and excellent initial local control, ultimately experienced an isolated metastatic recurrence in the colon within 1 year of therapy. Data supports performing metastatectomy for stage IV extremity sarcoma for isolated pulmonary or hepatic burden in selected patients with improved survival rates.13,14 As far as we know, there is no published literature describing isolated colon metastases in the absence of liver burden from lower extremity soft tissue sarcomas, or the outcome of surgical resection and adjuvant therapy in these cases.
In conclusion, high-grade pleomorphic sarcoma often follows an aggressive clinical course with ultimately local and distant recurrence. Use of multimodal therapy may have a role in improving local control. Complete pathologic necrosis is a rare event that is predictive of improved outcome. Our case represents an unusual pattern of recurrence among patients with a complete pathologic response to neoadjuvant therapy with isolated colon metastases. Timely, comprehensive management together with vigilant surveillance remain key priorities in the long-term management of high-risk sarcoma.
Soft tissue sarcomas (STS) are a heterogeneous group of tumors of mesenchymal origin that represent a rare form of adult malignancy. According to the World Health Organization classification system, there are more than 100 histologic subtypes of sarcoma based on tissue of origin. Staging criteria most commonly use the American Joint Committee on Cancer’s TNM Classification of Malignant Tumours. About 50% of soft tissue sarcomas originate in the lower extremity.1 Advancements in the use of multimodal therapy have reduced the need for amputation and allowed for equally effective treatment strategies that use limb-sparing surgical resections.
Although most sarcoma metastases spread in a hematogenous fashion, nodal spread is underestimated. Certain histologic subtypes carry a higher predilection for nodal involvement: these include rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, vascular sarcoma, and clear-cell sarcoma.2-4 Fong and colleagues have reported that 2.6% of sarcomas have lymphatic spread.3 In the current report, we describe a rare observation of locoregional pelvic nodal metastases from a large undifferentiated pleomorphic sarcoma of the right thigh.
Case presentation and summary
A 63-year-old white woman had a 1-year history of a right thigh mass and an unintentional weight loss of 40 lb. After a year of chiropractic care, she was referred to a physician because of palpable inguinal adenopathy and a 20-cm mass in the medial compartment of the right thigh, with heterogeneous appearance on a magnetic-resonance imaging scan (Figure 1). The patient was referred to the sarcoma transdisciplinary team for evaluation. She was diagnosed by core needle biopsy with a high-grade malignant epithelioid and spindle-cell neoplasm, favoring pleomorphic sarcoma. The metastatic work-up confirmed locoregional right inguinal and retroperitoneal lymph node disease, with 2 lung nodules that were too small to characterize. She also had a paraneoplastic leukocytosis with a white blood cell count of 45,500 cells/ml (normal 10,500 cells/ml).
About 8 months after surgery, she presented to the emergency room with a 3-day history of blood per rectum, anemia, and fatigue. She also reported a weight loss of 10 lb in the previous month. She was admitted for hydration and monitoring. Although computed-tomography (CT) scans of the abdomen and chest done during the previous month and had been interpreted as having no evidence of recurrence or any lymph node disease, the results of an inpatient colonoscopy revealed 2 colonic masses, 1 in the ascending colon and another in the transverse colon. The biopsy findings were consistent with undifferentiated pleomorphic sarcoma, favoring epithelioid histology. The CT scans were re-evaluated in light of these colonoscopic findings. These masses were visible retrospectively on imaging but had been interpreted as stool given the lack of abnormality on imaging 3 months before.
Adequate re-staging was complete, and without other evidence of disease, an extended right hemicolectomy was performed. The postoperative pathology report described geographically 2 distinct masses: a 7-cm mass in the ascending colon, about 3 cm from the ileocecal valve; and a 4-cm mass in the transverse colon, about 7.5 cm from the distal margin of resection. Both masses were identified as high-grade pleomorphic sarcoma. Again, all nodes recovered were negative for malignancy (0/5). Of note is that the background colonic mucosa showed active multifocal colitis with deep inflammatory activity likely consistent with a paraneoplastic syndrome.
Discussion
Surgery remains the primary treatment modality for localized soft tissue sarcoma. Obtaining a margin-free resection, while maintaining optimal function, is the objective with extremity sarcoma. In addition to surgical resection, doxorubicin-based adjuvant chemotherapy remains the standard of care with modest improvement in overall survival and disease-free survival, especially in sarcomas of the extremities.5,6 Gemcitabine also has activity in soft tissue sarcomas7 and might synergize with docetaxel. High response rates of 53% with fixed dose infusion rates of these agents in uterine leiomyosarcoma led the Sarcoma Alliance for Research through Collaboration investigators to consider this regimen for other STS.8 An overall response rate of 16% was noted across all sarcoma subtypes, and undifferentiated pleomorphic sarcoma had a response rate of 32%.
In our experience, a modified schedule of gemcitabine and docetaxel is better tolerated than the standard every 3 weeks regimen or doxorubicin-based chemotherapies. As previously described, gemcitabine and docetaxel were administered to this patient every 2 weeks.9 This regimen, in our experience, is less toxic and preserves the dose intensity of the regimen. A complete pathologic necrosis of the primary tumor and regional nodal basin was observed, as well as pulmonary nodule regression, enabling an R0-wide excision and regional lymphadenectomy. The colonic recurrence was surprising, but easily managed with surgical resection.
Pathologic complete response after neoadjuvant chemotherapy is a rare event, observed in about 10% of patients. However, when observed, complete pathologic necrosis (>95%) provided a distant recurrence-free survival of 100% at 3 years.10-12
Our patient, despite achieving a complete pathologic response and excellent initial local control, ultimately experienced an isolated metastatic recurrence in the colon within 1 year of therapy. Data supports performing metastatectomy for stage IV extremity sarcoma for isolated pulmonary or hepatic burden in selected patients with improved survival rates.13,14 As far as we know, there is no published literature describing isolated colon metastases in the absence of liver burden from lower extremity soft tissue sarcomas, or the outcome of surgical resection and adjuvant therapy in these cases.
In conclusion, high-grade pleomorphic sarcoma often follows an aggressive clinical course with ultimately local and distant recurrence. Use of multimodal therapy may have a role in improving local control. Complete pathologic necrosis is a rare event that is predictive of improved outcome. Our case represents an unusual pattern of recurrence among patients with a complete pathologic response to neoadjuvant therapy with isolated colon metastases. Timely, comprehensive management together with vigilant surveillance remain key priorities in the long-term management of high-risk sarcoma.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349-359.
2. Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004:129-134.
3. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77.
4. Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800-1808.
5. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647-1654.
6. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft tissue sarcoma. Cancer. 2008;113:573-581.
7. Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483-3489.
8. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755-2763.
9. Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785-790.
10. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147-1153.
11. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203-3209.
12. Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911-3915.
13. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111-117.
14. Pfannschmidt J, Hoffmann H, Schneider T, Dienemann H. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res. 2009;179:321-336.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349-359.
2. Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004:129-134.
3. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77.
4. Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800-1808.
5. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647-1654.
6. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft tissue sarcoma. Cancer. 2008;113:573-581.
7. Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483-3489.
8. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755-2763.
9. Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785-790.
10. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147-1153.
11. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203-3209.
12. Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911-3915.
13. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111-117.
14. Pfannschmidt J, Hoffmann H, Schneider T, Dienemann H. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res. 2009;179:321-336.