User login
Research and Reviews for the Practicing Oncologist
Palliative and supportive interventions to improve patient-reported outcomes in rural residents with cancer
People in rural areas have increased rates of advanced cancer and mortality compared with those who live in more affluent and urban areas.1,2 Indeed, a recent report from the Center for Disease Control found that rural residents have higher mortality rates from 5 leading causes of death, including cancer, compared with their urban counterparts.1 Significant challenges facing rural residents are due largely to not having easy access to cancer care and supportive care services.3 In addition, living in a rural area is associated with: a lower socioeconomic status, inadequate health insurance coverage, and less flexible employment that in turn decreases the ability to obtain the full range of supportive oncology services.4 The closest available specialists may be several hours away. Individuals may be unwilling or unable to travel hundreds of miles or more to see a specialist.3 Traveling places financial burdens on patients because of the cost of traveling and loss of work, which can compound the stress and fatigue associated with cancer treatment. People living in rural areas also may have less social support in commuting between their place of living and hospitals.5
Background
Typically, the primary goals of treatment for individuals with advanced cancer are to control the spread of the disease; maintain important patient-reported outcomes (PROs) such as physical, mental, and psychosocial function; and optimize quality of life (QoL). Health-related QoL (ie, the physical and mental health perceptions) are increasingly being used to assess effectiveness of cancer treatment.6 Palliative care and supportive oncology focus on managing physical, social, psychological, and spiritual needs of patients and have been recommended by the American Society of Clinical Oncology to be integrated into standard oncology care.7
People living in rural areas are less likely to get their care within a single health system. Often, their care is divided across multiple facilities and providers, which increases the chances of miscommunication between providers and can lead to inferior clinical outcomes and decreased patient QoL.8 There is a growing body of research describing the impact of palliative care on people with advanced cancer. Specifically, palliative care has been shown to reduce symptoms, improve QoL, and increase survival.9-11 Differences have been observed in the palliative care needs between people with cancer living in urban and suburban areas.12 It is likely that palliative care needs as well as the impact of palliative care services for people with advanced cancer in rural areas differs from those of their urban and suburban counterparts. Despite the known differences in access to care and impact of cancer between rural and nonrural residents, the impact of palliative care on people with advanced cancer living in rural areas has not been well described in the literature.
The purpose of this systematic review is to examine effect of palliative care and supportive oncology interventions on QoL in people with advanced cancer living in rural areas.
Methods
This systematic review was developed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13
Eligibility criteria
To achieve the objective of a systemic review of studies describing supportive oncology and palliative care interventions in rural communities articles had to meet 4 inclusion criteria:
All research methods were eligible, including mixed-methods and program evaluations, as long as the article met the 4 inclusion criteria. Review articles were ineligible for inclusion as only original research was considered.
Search process
Search terms were developed by the research team with consultation from a medical librarian. Four main search terms were developed and included: palliative care, supportive oncology, rural, and cancer. Synonyms and terms closely related to the main terms were included in the search using the OR command. Examples of closely related search terms include: Palliative care: palliative; Rural: remote; Cancer: neoplasms (Table).
We systematically searched PyschINFO, PubMed, CINHAL, and Scopus for articles that had been published during 1991-2016 and written in English. Databases were chosen to reflect the different subfields that encompass palliative care and supportive oncology: PyschINFO to capture the psychological perspective, CINHAL to capture the nursing perspective, and PubMed to capture the medical perspective. Finally, Scopus was searched to ensure that articles not indexed by the other databases would be included. The search was limited to the past 25 years to capture the most up-to-date literature.
Selection process
In accordance with PRISMA guidelines, articles underwent an initial screening and an eligibility screening for inclusion in the final review.13 After duplicates were removed, 2 research team members reviewed all abstracts to screen for initial eligibility. Articles that successfully passed the screening process were reviewed in full by 4 research team members. Each member made an independent inclusion decision based on the stated inclusion criteria. Disagreements across team members were resolved through discussion and consensus.
Analysis
The articles that met the inclusion criteria were heterogeneous in design and analytic approach. The set of manuscripts identified, therefore, did not meet the statistical assumptions for meta-analytic data analysis. The analytic plan for this review consisted of sorting the results described in the identified articles into meaningful categories, identifying cross cutting themes, and presenting the results of these themes in narrative forms.
Results
Study selection
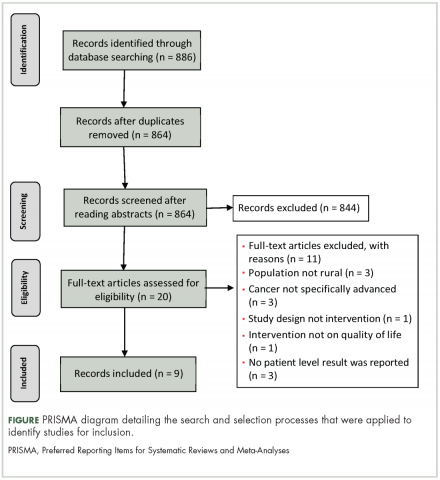
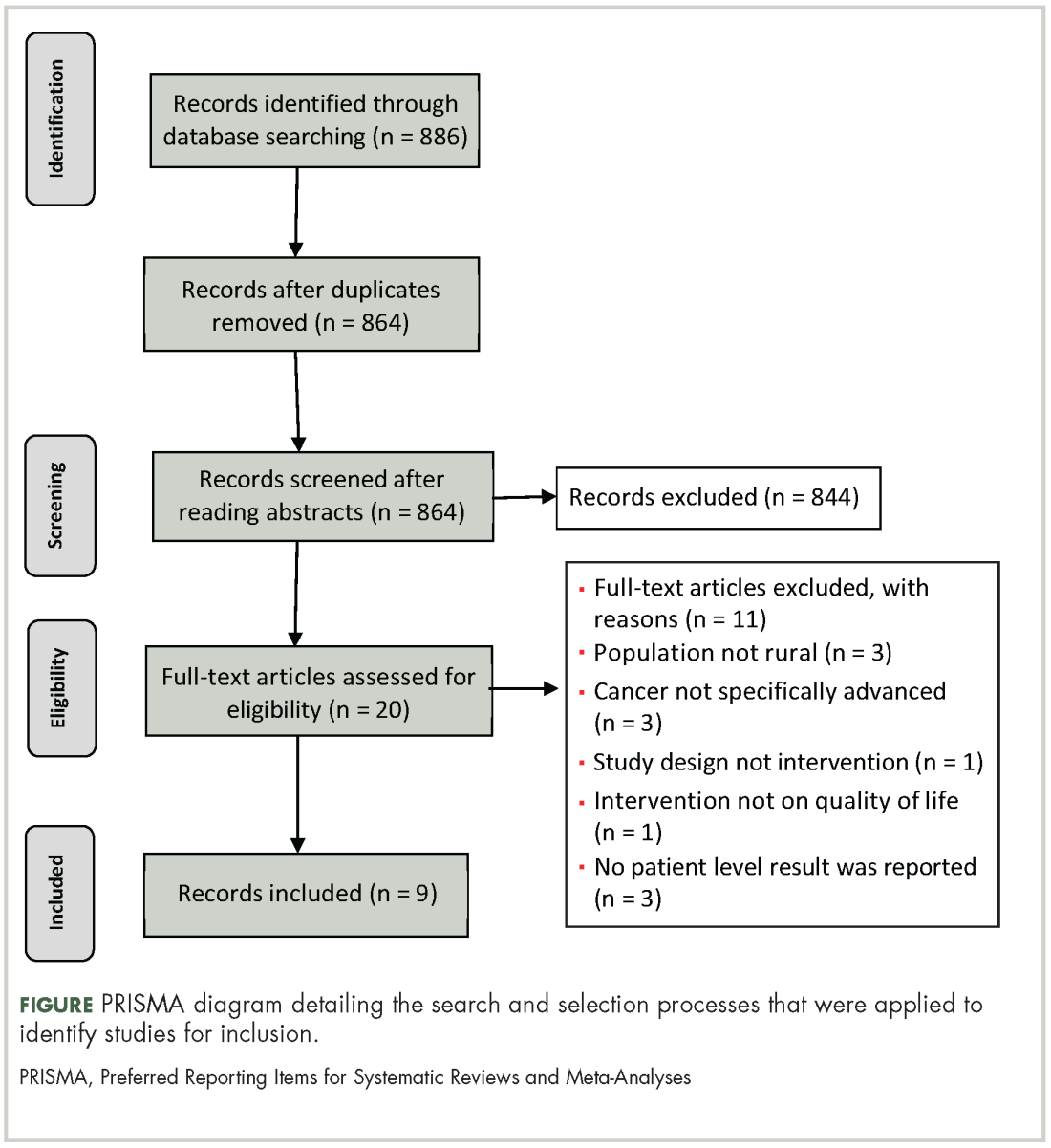
The search strategy resulted in 886 articles across the 4 databases. The breakdown for each database is as follows: PsychINFO (n = 286), PubMed (n = 194), CINHAL (n = 334), Scopus (n = 72). After duplicates were removed, 864 articles were left and were initially screened resulting in 844 articles being excluded. The remaining 20 articles were reviewed and 12 articles failed to meet the inclusion criteria. Reasons for exclusion included: the population was not rural; no advanced cancer in sample; intervention was not specifically palliative care or supportive oncology. Nine articles representing 8 projects (one project published 2 manuscripts included in this review) were included in the final review (Figure).
After reviewing the articles, 2 clear themes arose: PROs, and overall impact of rural palliative care for people and society. The PRO theme included articles that provided information on how an intervention or program improved the personal lives experience of rural cancer patients. PROs, such as decreased symptomology, were often reported. The “overall impact of rural palliative care for people and society” theme included articles that provided information on how an intervention or program improved the lives of rural people and society as a larger group. An example would include results indicating how a program increased access to supportive oncology care in a rural area.
Study characteristics
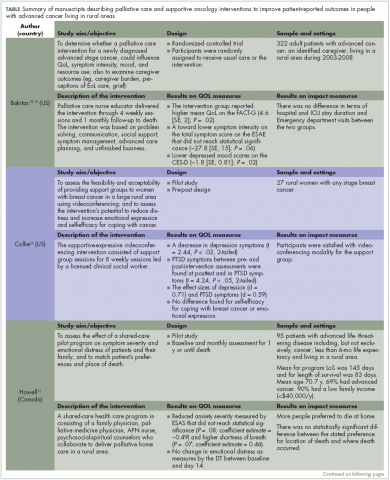
Nine publications, describing 8 projects were included in this review (Table). These projects were conducted in Canada (n = 3)14-16 Australia (n = 1)17 and the United States (n = 5).18-22 All of the the projects used a quantitative approach for the analysis, except 1 that used mixed-methods.16 The studies designs were: 4 feasibilities/pilot studies, 1 randomized control trials (RCT), and 3 program evaluations.
A total of 807 patients participated across the 9 articles. Participants’ age ranged from 20 to 88 years. The average ages for participants ranged from 50.4 to 70.7 years. Overall, there were slightly more men (55%) than women (45%) when all the demographic data were combined across the 9 articles; however, 2 articles exclusively had women as part of the sample.17,20 The cancer types that participants had included: gastrointestinal, genitourinary, breast, lung, brain, kidney, and hematological. Finally, the articles had inconsistent reporting of race/ethnicity data with only 4 studies reporting this information; of the 4 studies, 91% of participants self-identified as white.
The projects targeted multiple PROs, including physical symptoms and psychosocial issues (ie, stress management, grief, mood, emotional distress, coping, self-efficacy, dignity, joy, affection) domains. Publications dates ranged from 1996 to 2013. The sample sizes ranged from 8 to 322; 11.7%-100% of the study population had advanced cancer, and 20%-100% were living in rural area. The duration of the clinical intervention described was 30-120 minutes. The modes of delivery for the palliative intervention were videoconference/videophone (n = 3), telephone/teleconference (n = 3), and in person (n = 2). The interventions were delivered by nurses, psychiatrists, and social workers. In 5 of these studies, participants received palliative care on an individual basis and 2 studies delivered their intervention through groups. The individual basis studies focused on physical aspects of care and the group studies focused on emotional aspects of care.
Patient-reported outcomes
Cancer and its treatments are often associated with physical and emotional sequelae that can have a significant impact on patients and therefore PROs. The interventions reviewed in this article often reported data on the reduction of the physical and/or emotional symptom burden of cancer as well as overall QoL.
Reduction in physical symptoms. Three articles included physical symptoms as an outcome measure. Of those, 2 were pilot or feasibility studies, and 1 was a randomized control trial. Common physical symptoms included: shortness of breath, pain, fatigue, nausea, and appetite change. Across the articles, the Edmonton Symptom Assessment Scale (ESAS), a 10-item inventory of common cancer symptoms, was frequently used to measure of symptom scores in these interventions.14,15,19 The ESAS is an empirically validated measure that is used in palliative care research and clinical practice. Individuals are asked to rank 10 common symptoms on an ascending scale from 1 to 10 (0, the symptom is absent; 10, worst possible severity).23
The findings from these 3 research studies were encouraging. In a large randomized control trial of a supportive education program, researchers reported decreased physical symptom intensity after the intervention, however the change did not reach statistical significance.18 Similar findings were reported in a videoconferencing and a home health program to improve access of palliative and supportive oncology health care.14,15 Physical symptoms that had decreasing trends were pain, tiredness, and appetite, however, trends for shortness of breath found increasing severity.14,15 Although these trends were observed, it is important to note that scores on the ESAS did not reach statistical significance for physical symptoms in any of these studies.
Reduction in emotional symptom reduction. In addition to reducing physical symptoms, researchers also sought to understand the impact of programs on the emotional symptoms of cancer including: anxiety, depression, negative affect, and posttraumatic stress disorder (PTSD). Five articles included emotional symptoms as an outcome measure. Four were pilot or feasibility studies, and 1 was a randomized control trial.
Results across studies indicated an observable decrease in the severity of anxiety and depression for those exposed to an intervention program.14,15,18,19 Again, although trends were found, the results were not statistically significant. Only Watanabe and colleagues14 reported a statistically significant a decrease in anxiety in participants after the implementation of a rural palliative care videoconference consultation program. One report indicated that data on depression severity was collected but was not analyzed because of a small sample size.21
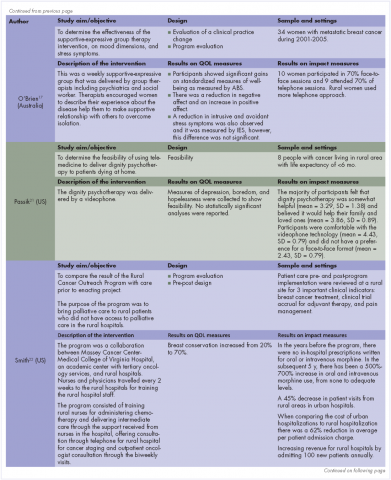
O’Brien and colleagues17 also collected data on negative affect and found that participants who participated in a supportive-expressive therapy group had a reduction in the negative affect as measured by the Derogatis Affects Balance Scale (ABS). Other researchers found no change in emotional distress.15
Finally, Collie and colleagues20 also measured the impact of a videoconference support group of PTSD symptomology for people with breast cancer in rural areas. Their results indicated a statistically significant decrease on the PTSD Checklist-Specific after intervention. Analysis of the data also found a medium effect size. Participants in the intervention group spoke about how participation in the support group allowed them to be generative and share information about breast cancer as well as build an emotional bond with other women with cancer.
Overall quality of life and well-being. Researchers have also looked into impact of intervention on overall QoL. Two articles included QoL or Well-being as an outcome measure. One was a pilot study and 1 was a randomized control trial.
Bakitas and colleagues18,19 found that those enrolled in the intervention arm of their study had higher QoL scores on the Functional Assessment of Cancer Therapy-General (FACT-G) compared with those in the control arm. These results were also found in an analysis of data from participants who subsequently died during the intervention. Improvements in overall well-being were also found by O’Brien and colleagues17 using ABS. They reported that a post hoc comparison of participants’ total positive affect score was significantly higher at the 12-month follow-up. In addition, the authors also noted qualitative improvements in well-being, including increased effort to be at the support group and the low attrition rates.
Overall impact of rural palliative care on individuals and society. In addition to reducing physical and emotional symptoms in patients, several of the articles also addressed other measures of the overall impact of the intervention or program on society as a whole. The authors evaluated patient satisfaction and quality of life, access to health care services, and financial impact on individuals and society at large.
Satisfaction with intervention. In 2 of the articles, individuals or their family members reported to be satisfied with the intervention14,20 and said they would recommend it to others as well.20 Both of those studies used teleconferencing to provide access to the intervention to people in rural communities.
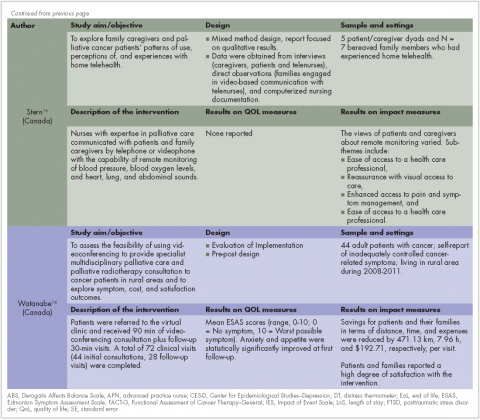
Increasing access to the health care services and quality of care. Four of the articles evaluated the impact of intervention on patient’s access to the health care services.14,16,20,22 Specifically, after the interventions individuals had increased access to palliative care information in rural areas where it had previously been unavailable20 as well as actual delivery of clinical care in their home community, thus eliminating the need to travel to urban areas.14,20,22 This increase of access to health care services in rural area had significant effect on time and distance spent traveling. In 1 study, the amount of saving in terms of distance was 471.13 km and time in, 7.96 hours, for each visit.14
In addition, the quality of overall cancer care in rural area was increased. In an early clinical program, to increase access of palliative care in rural communities, the authors reported an increase in the breast conservation from 20% at the start of the program to 70% 2 years after the program was implemented.22 Breast conservation is not a typical outcome for palliative care studies, but the authors highlighted this practice change because of the improved QoL that is associated with the use of breast conservation therapies. In the same study, the authors reported an increased use of curative therapies for other cancers such as lymphoma as well as an increase use of pain management medication.
Financial impact. Two articles described the financial impact of cancer care costs on the patient and society.14,22 In a study by Watanabe and colleagues in Canada,14 the amount of savings after the intervention in terms of travel expenses was C$192.71 for each visit because patients had previously had to travel from their rural communities to urban tertiary hospitals to receive palliative care. For some patients in that study, the amount of saving for expenses was as high as C$500 a visit. In addition, some individuals were not able to travel and would not have received anything if the intervention had not been available remotely.14 In a study by Smith and colleagues in the United States, there was a 62% decrease in the cost to society for each patient, from US$10,233 to US$3,862.22 The factors contributing to that reduction included increasing outpatient services, engaging nurses and primary care providers instead of specialists, and the lower costs of living in rural areas. In addition, the rural hospitals saw an increase in revenue and profits because of higher admission rates ($500,00 for each hospital annually).22
Discussion
The articles identified in this review provide some evidence of the potential impact that palliative and supportive oncology interventions could have on PROs for rural residents with advanced cancer. Noteworthy results were seen for impact on reducing physical and emotional symptoms, increasing overall QoL and well-being, increasing satisfaction and access to palliative care, and reducing the overall cost of palliative care for individuals and society.14-18,20-22
Although statistical significance was not observed for most of the symptom assessment, trends toward improved symptom reports were observed. A likely explanation for this finding, is the small sample size or inadequate design to evaluate symptoms as an outcome measure. Three studies were pilot or feasibility projects15,20,21 that were not powered to detect the impact of the intervention on symptoms. In contrast, QoL stands out as an outcome that was positively affected by palliative care interventions. Further research is needed to determine if there are important mediating and moderating factors that contribute to improve QoL that are specific to rural residents. Significant outcomes were also reported for participant satisfaction with the interventions, the increase in access to services, and the decrease in costs.
Although there were not enough studies to determine the efficacy of these interventions, these results suggest that palliative and supportive interventions can have an impact on important patient-reported outcomes, such as symptoms and quality of life, and on health care system outcomes, such as cost. Evidence supporting the extent of the effectiveness of palliative care on various PROs in rural people is limited. None of the studies in this review evaluated the different aspects of palliative care specifically in rural residents.
It is interesting to note that all but one of the interventions used a telehealth approach to deliver the intervention. Telehealth interventions seem to be feasible, acceptable to people in rural areas, and show preliminary evidence that they can have an impact on PROs.
Limitations of this review include only inclusion of publications in English. In addition, some studies in this review include populations that were not exclusively rural residents, which makes it difficult for generalization.
Conclusion
Palliative and supportive interventions may improve various PROs in people with advanced cancer living in rural areas. Technologies that support remote access to people in rural areas, such as teleconferencing and videoconferencing, seem particularly promising delivery modalities with their potential to increase access to palliative and supportive interventions in underserved communities. Large-scale studies that are powered to test the impact of palliative care and support oncology interventions on PROs and other aspects of quality care among rural residents with advanced cancer are needed.
The authors thank Jennifer DeBerg, Health Science Librarian at the University of Iowa for her assistance in developing the literature search strategies.
1. Moy E, Garcia MC, Bastian B, et al. Leading causes of death in nonmetropolitan and metropolitan areas – United States, 1999-2014 [published correction at https://www.cdc.gov/mmwr/volumes/66/wr/mm6603a11.htm]. MMWR Surveillance Summaries [serial online]. https://www.cdc.gov/mmwr/volumes/66/ss/ss6601a1.htm?s_cid=ss6601a1_w. Published January 13, 2017. Accessed January 20, 2017.
2. Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US Cancer Mortality: Part I – All cancers and lung cancer and Part II – Colorectal, prostate, breast, and cervical cancers. https://www.hindawi.com/journals/jce/2011/107497/. Published 2011. Accessed April 28, 2017.
3. Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of rural cancer care in the United States. Oncology (Williston Park). 2015;29(9):633-640.
4. Weaver KE, Geiger AM, Lu L, Case LD. Rural‐urban disparities in health status among US cancer survivors. Cancer. 2013;119(5):1050-1057.
5. Fuchsia Howard A, Smillie K, Turnbull K, et al. Access to medical and supportive care for rural and remote cancer survivors in northern British Columbia. J Rural Health. 2014;30(3):311-321.
6. Bottomley A, Aaronson NK. International perspective on health-related quality-of-life research in cancer clinical trials: the European Organisation for Research and Treatment of Cancer experience. J Clin Oncol. 2007;25(32):5082-5086.
7. Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880-887.
8. Baldwin LM, Cai Y, Larson EH, et al. Access to cancer services for rural colorectal cancer patients. J Rural Health. 2008;24(4):390-399.
9. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
10. McCorkle R, Jeon S, Ercolano E, et al. An advanced practice nurse coordinated multidisciplinary intervention for patients with late-stage cancer: a cluster randomized trial. J Palliat Med. 2015;18(11):962-969.
11. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
12. Regn R, Robinson W, Robinson WR. Differences in palliative care needs among cancer survivors in an inner city academic facility versus a suburban community facility. J Clin Oncol. 2015;33(29_suppl):61.
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;339:b2535.
14. Watanabe SM, Fairchild A, Pituskin E, Borgersen P, Hanson J, Fassbender K. Improving access to specialist multidisciplinary palliative care consultation for rural cancer patients by videoconferencing: report of a pilot project. Support Care Cancer. 2013;21(4):1201-1207.
15. Howell D, Marshall D, Brazil K, et al. A shared care model pilot for palliative home care in a rural area: impact on symptoms, distress, and place of death. J Pain Symptom Manage. 2011;42(1):60-75.
16. Stern A, Valaitis R, Weir R, Jadad AR. Use of home telehealth in palliative cancer care: a case study. J Telemed Telecare. 2012;18(5):297-300.
17. O’Brien M, Harris J, King R, O’Brien T. Supportive-expressive group therapy for women with metastatic breast cancer: Improving access for Australian women through use of teleconference. Counselling Psychother Res. 2008;8(1):28-35.
18. Bakitas M, Lyons KD, Hegel MT, et al. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliat Supportive Care. 2009;7(1):75-86.
19. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
20. Collie K, Kreshka MA, Ferrier S, et al. Videoconferencing for delivery of breast cancer support groups to women living in rural communities: a pilot study. Psychooncology. 200
21. Passik SD, Kirsh KL, Leibee S, et al. A feasibility study of dignity psychotherapy delivered via telemedicine. Palliat Support Care. 2004;2(2):149-155.
22. Smith TJ, Desch CE, Grasso MA, et al. The Rural Cancer Outreach Program: clinical and financial analysis of palliative and curative care for an underserved population. Cancer Treat Rev. 1996;22(Suppl A):97-101.
23. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6-9.
People in rural areas have increased rates of advanced cancer and mortality compared with those who live in more affluent and urban areas.1,2 Indeed, a recent report from the Center for Disease Control found that rural residents have higher mortality rates from 5 leading causes of death, including cancer, compared with their urban counterparts.1 Significant challenges facing rural residents are due largely to not having easy access to cancer care and supportive care services.3 In addition, living in a rural area is associated with: a lower socioeconomic status, inadequate health insurance coverage, and less flexible employment that in turn decreases the ability to obtain the full range of supportive oncology services.4 The closest available specialists may be several hours away. Individuals may be unwilling or unable to travel hundreds of miles or more to see a specialist.3 Traveling places financial burdens on patients because of the cost of traveling and loss of work, which can compound the stress and fatigue associated with cancer treatment. People living in rural areas also may have less social support in commuting between their place of living and hospitals.5
Background
Typically, the primary goals of treatment for individuals with advanced cancer are to control the spread of the disease; maintain important patient-reported outcomes (PROs) such as physical, mental, and psychosocial function; and optimize quality of life (QoL). Health-related QoL (ie, the physical and mental health perceptions) are increasingly being used to assess effectiveness of cancer treatment.6 Palliative care and supportive oncology focus on managing physical, social, psychological, and spiritual needs of patients and have been recommended by the American Society of Clinical Oncology to be integrated into standard oncology care.7
People living in rural areas are less likely to get their care within a single health system. Often, their care is divided across multiple facilities and providers, which increases the chances of miscommunication between providers and can lead to inferior clinical outcomes and decreased patient QoL.8 There is a growing body of research describing the impact of palliative care on people with advanced cancer. Specifically, palliative care has been shown to reduce symptoms, improve QoL, and increase survival.9-11 Differences have been observed in the palliative care needs between people with cancer living in urban and suburban areas.12 It is likely that palliative care needs as well as the impact of palliative care services for people with advanced cancer in rural areas differs from those of their urban and suburban counterparts. Despite the known differences in access to care and impact of cancer between rural and nonrural residents, the impact of palliative care on people with advanced cancer living in rural areas has not been well described in the literature.
The purpose of this systematic review is to examine effect of palliative care and supportive oncology interventions on QoL in people with advanced cancer living in rural areas.
Methods
This systematic review was developed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13
Eligibility criteria
To achieve the objective of a systemic review of studies describing supportive oncology and palliative care interventions in rural communities articles had to meet 4 inclusion criteria:
All research methods were eligible, including mixed-methods and program evaluations, as long as the article met the 4 inclusion criteria. Review articles were ineligible for inclusion as only original research was considered.
Search process
Search terms were developed by the research team with consultation from a medical librarian. Four main search terms were developed and included: palliative care, supportive oncology, rural, and cancer. Synonyms and terms closely related to the main terms were included in the search using the OR command. Examples of closely related search terms include: Palliative care: palliative; Rural: remote; Cancer: neoplasms (Table).
We systematically searched PyschINFO, PubMed, CINHAL, and Scopus for articles that had been published during 1991-2016 and written in English. Databases were chosen to reflect the different subfields that encompass palliative care and supportive oncology: PyschINFO to capture the psychological perspective, CINHAL to capture the nursing perspective, and PubMed to capture the medical perspective. Finally, Scopus was searched to ensure that articles not indexed by the other databases would be included. The search was limited to the past 25 years to capture the most up-to-date literature.
Selection process
In accordance with PRISMA guidelines, articles underwent an initial screening and an eligibility screening for inclusion in the final review.13 After duplicates were removed, 2 research team members reviewed all abstracts to screen for initial eligibility. Articles that successfully passed the screening process were reviewed in full by 4 research team members. Each member made an independent inclusion decision based on the stated inclusion criteria. Disagreements across team members were resolved through discussion and consensus.
Analysis
The articles that met the inclusion criteria were heterogeneous in design and analytic approach. The set of manuscripts identified, therefore, did not meet the statistical assumptions for meta-analytic data analysis. The analytic plan for this review consisted of sorting the results described in the identified articles into meaningful categories, identifying cross cutting themes, and presenting the results of these themes in narrative forms.
Results
Study selection
The search strategy resulted in 886 articles across the 4 databases. The breakdown for each database is as follows: PsychINFO (n = 286), PubMed (n = 194), CINHAL (n = 334), Scopus (n = 72). After duplicates were removed, 864 articles were left and were initially screened resulting in 844 articles being excluded. The remaining 20 articles were reviewed and 12 articles failed to meet the inclusion criteria. Reasons for exclusion included: the population was not rural; no advanced cancer in sample; intervention was not specifically palliative care or supportive oncology. Nine articles representing 8 projects (one project published 2 manuscripts included in this review) were included in the final review (Figure).
After reviewing the articles, 2 clear themes arose: PROs, and overall impact of rural palliative care for people and society. The PRO theme included articles that provided information on how an intervention or program improved the personal lives experience of rural cancer patients. PROs, such as decreased symptomology, were often reported. The “overall impact of rural palliative care for people and society” theme included articles that provided information on how an intervention or program improved the lives of rural people and society as a larger group. An example would include results indicating how a program increased access to supportive oncology care in a rural area.
Study characteristics
Nine publications, describing 8 projects were included in this review (Table). These projects were conducted in Canada (n = 3)14-16 Australia (n = 1)17 and the United States (n = 5).18-22 All of the the projects used a quantitative approach for the analysis, except 1 that used mixed-methods.16 The studies designs were: 4 feasibilities/pilot studies, 1 randomized control trials (RCT), and 3 program evaluations.
A total of 807 patients participated across the 9 articles. Participants’ age ranged from 20 to 88 years. The average ages for participants ranged from 50.4 to 70.7 years. Overall, there were slightly more men (55%) than women (45%) when all the demographic data were combined across the 9 articles; however, 2 articles exclusively had women as part of the sample.17,20 The cancer types that participants had included: gastrointestinal, genitourinary, breast, lung, brain, kidney, and hematological. Finally, the articles had inconsistent reporting of race/ethnicity data with only 4 studies reporting this information; of the 4 studies, 91% of participants self-identified as white.
The projects targeted multiple PROs, including physical symptoms and psychosocial issues (ie, stress management, grief, mood, emotional distress, coping, self-efficacy, dignity, joy, affection) domains. Publications dates ranged from 1996 to 2013. The sample sizes ranged from 8 to 322; 11.7%-100% of the study population had advanced cancer, and 20%-100% were living in rural area. The duration of the clinical intervention described was 30-120 minutes. The modes of delivery for the palliative intervention were videoconference/videophone (n = 3), telephone/teleconference (n = 3), and in person (n = 2). The interventions were delivered by nurses, psychiatrists, and social workers. In 5 of these studies, participants received palliative care on an individual basis and 2 studies delivered their intervention through groups. The individual basis studies focused on physical aspects of care and the group studies focused on emotional aspects of care.
Patient-reported outcomes
Cancer and its treatments are often associated with physical and emotional sequelae that can have a significant impact on patients and therefore PROs. The interventions reviewed in this article often reported data on the reduction of the physical and/or emotional symptom burden of cancer as well as overall QoL.
Reduction in physical symptoms. Three articles included physical symptoms as an outcome measure. Of those, 2 were pilot or feasibility studies, and 1 was a randomized control trial. Common physical symptoms included: shortness of breath, pain, fatigue, nausea, and appetite change. Across the articles, the Edmonton Symptom Assessment Scale (ESAS), a 10-item inventory of common cancer symptoms, was frequently used to measure of symptom scores in these interventions.14,15,19 The ESAS is an empirically validated measure that is used in palliative care research and clinical practice. Individuals are asked to rank 10 common symptoms on an ascending scale from 1 to 10 (0, the symptom is absent; 10, worst possible severity).23
The findings from these 3 research studies were encouraging. In a large randomized control trial of a supportive education program, researchers reported decreased physical symptom intensity after the intervention, however the change did not reach statistical significance.18 Similar findings were reported in a videoconferencing and a home health program to improve access of palliative and supportive oncology health care.14,15 Physical symptoms that had decreasing trends were pain, tiredness, and appetite, however, trends for shortness of breath found increasing severity.14,15 Although these trends were observed, it is important to note that scores on the ESAS did not reach statistical significance for physical symptoms in any of these studies.
Reduction in emotional symptom reduction. In addition to reducing physical symptoms, researchers also sought to understand the impact of programs on the emotional symptoms of cancer including: anxiety, depression, negative affect, and posttraumatic stress disorder (PTSD). Five articles included emotional symptoms as an outcome measure. Four were pilot or feasibility studies, and 1 was a randomized control trial.
Results across studies indicated an observable decrease in the severity of anxiety and depression for those exposed to an intervention program.14,15,18,19 Again, although trends were found, the results were not statistically significant. Only Watanabe and colleagues14 reported a statistically significant a decrease in anxiety in participants after the implementation of a rural palliative care videoconference consultation program. One report indicated that data on depression severity was collected but was not analyzed because of a small sample size.21
O’Brien and colleagues17 also collected data on negative affect and found that participants who participated in a supportive-expressive therapy group had a reduction in the negative affect as measured by the Derogatis Affects Balance Scale (ABS). Other researchers found no change in emotional distress.15
Finally, Collie and colleagues20 also measured the impact of a videoconference support group of PTSD symptomology for people with breast cancer in rural areas. Their results indicated a statistically significant decrease on the PTSD Checklist-Specific after intervention. Analysis of the data also found a medium effect size. Participants in the intervention group spoke about how participation in the support group allowed them to be generative and share information about breast cancer as well as build an emotional bond with other women with cancer.
Overall quality of life and well-being. Researchers have also looked into impact of intervention on overall QoL. Two articles included QoL or Well-being as an outcome measure. One was a pilot study and 1 was a randomized control trial.
Bakitas and colleagues18,19 found that those enrolled in the intervention arm of their study had higher QoL scores on the Functional Assessment of Cancer Therapy-General (FACT-G) compared with those in the control arm. These results were also found in an analysis of data from participants who subsequently died during the intervention. Improvements in overall well-being were also found by O’Brien and colleagues17 using ABS. They reported that a post hoc comparison of participants’ total positive affect score was significantly higher at the 12-month follow-up. In addition, the authors also noted qualitative improvements in well-being, including increased effort to be at the support group and the low attrition rates.
Overall impact of rural palliative care on individuals and society. In addition to reducing physical and emotional symptoms in patients, several of the articles also addressed other measures of the overall impact of the intervention or program on society as a whole. The authors evaluated patient satisfaction and quality of life, access to health care services, and financial impact on individuals and society at large.
Satisfaction with intervention. In 2 of the articles, individuals or their family members reported to be satisfied with the intervention14,20 and said they would recommend it to others as well.20 Both of those studies used teleconferencing to provide access to the intervention to people in rural communities.
Increasing access to the health care services and quality of care. Four of the articles evaluated the impact of intervention on patient’s access to the health care services.14,16,20,22 Specifically, after the interventions individuals had increased access to palliative care information in rural areas where it had previously been unavailable20 as well as actual delivery of clinical care in their home community, thus eliminating the need to travel to urban areas.14,20,22 This increase of access to health care services in rural area had significant effect on time and distance spent traveling. In 1 study, the amount of saving in terms of distance was 471.13 km and time in, 7.96 hours, for each visit.14
In addition, the quality of overall cancer care in rural area was increased. In an early clinical program, to increase access of palliative care in rural communities, the authors reported an increase in the breast conservation from 20% at the start of the program to 70% 2 years after the program was implemented.22 Breast conservation is not a typical outcome for palliative care studies, but the authors highlighted this practice change because of the improved QoL that is associated with the use of breast conservation therapies. In the same study, the authors reported an increased use of curative therapies for other cancers such as lymphoma as well as an increase use of pain management medication.
Financial impact. Two articles described the financial impact of cancer care costs on the patient and society.14,22 In a study by Watanabe and colleagues in Canada,14 the amount of savings after the intervention in terms of travel expenses was C$192.71 for each visit because patients had previously had to travel from their rural communities to urban tertiary hospitals to receive palliative care. For some patients in that study, the amount of saving for expenses was as high as C$500 a visit. In addition, some individuals were not able to travel and would not have received anything if the intervention had not been available remotely.14 In a study by Smith and colleagues in the United States, there was a 62% decrease in the cost to society for each patient, from US$10,233 to US$3,862.22 The factors contributing to that reduction included increasing outpatient services, engaging nurses and primary care providers instead of specialists, and the lower costs of living in rural areas. In addition, the rural hospitals saw an increase in revenue and profits because of higher admission rates ($500,00 for each hospital annually).22
Discussion
The articles identified in this review provide some evidence of the potential impact that palliative and supportive oncology interventions could have on PROs for rural residents with advanced cancer. Noteworthy results were seen for impact on reducing physical and emotional symptoms, increasing overall QoL and well-being, increasing satisfaction and access to palliative care, and reducing the overall cost of palliative care for individuals and society.14-18,20-22
Although statistical significance was not observed for most of the symptom assessment, trends toward improved symptom reports were observed. A likely explanation for this finding, is the small sample size or inadequate design to evaluate symptoms as an outcome measure. Three studies were pilot or feasibility projects15,20,21 that were not powered to detect the impact of the intervention on symptoms. In contrast, QoL stands out as an outcome that was positively affected by palliative care interventions. Further research is needed to determine if there are important mediating and moderating factors that contribute to improve QoL that are specific to rural residents. Significant outcomes were also reported for participant satisfaction with the interventions, the increase in access to services, and the decrease in costs.
Although there were not enough studies to determine the efficacy of these interventions, these results suggest that palliative and supportive interventions can have an impact on important patient-reported outcomes, such as symptoms and quality of life, and on health care system outcomes, such as cost. Evidence supporting the extent of the effectiveness of palliative care on various PROs in rural people is limited. None of the studies in this review evaluated the different aspects of palliative care specifically in rural residents.
It is interesting to note that all but one of the interventions used a telehealth approach to deliver the intervention. Telehealth interventions seem to be feasible, acceptable to people in rural areas, and show preliminary evidence that they can have an impact on PROs.
Limitations of this review include only inclusion of publications in English. In addition, some studies in this review include populations that were not exclusively rural residents, which makes it difficult for generalization.
Conclusion
Palliative and supportive interventions may improve various PROs in people with advanced cancer living in rural areas. Technologies that support remote access to people in rural areas, such as teleconferencing and videoconferencing, seem particularly promising delivery modalities with their potential to increase access to palliative and supportive interventions in underserved communities. Large-scale studies that are powered to test the impact of palliative care and support oncology interventions on PROs and other aspects of quality care among rural residents with advanced cancer are needed.
The authors thank Jennifer DeBerg, Health Science Librarian at the University of Iowa for her assistance in developing the literature search strategies.
People in rural areas have increased rates of advanced cancer and mortality compared with those who live in more affluent and urban areas.1,2 Indeed, a recent report from the Center for Disease Control found that rural residents have higher mortality rates from 5 leading causes of death, including cancer, compared with their urban counterparts.1 Significant challenges facing rural residents are due largely to not having easy access to cancer care and supportive care services.3 In addition, living in a rural area is associated with: a lower socioeconomic status, inadequate health insurance coverage, and less flexible employment that in turn decreases the ability to obtain the full range of supportive oncology services.4 The closest available specialists may be several hours away. Individuals may be unwilling or unable to travel hundreds of miles or more to see a specialist.3 Traveling places financial burdens on patients because of the cost of traveling and loss of work, which can compound the stress and fatigue associated with cancer treatment. People living in rural areas also may have less social support in commuting between their place of living and hospitals.5
Background
Typically, the primary goals of treatment for individuals with advanced cancer are to control the spread of the disease; maintain important patient-reported outcomes (PROs) such as physical, mental, and psychosocial function; and optimize quality of life (QoL). Health-related QoL (ie, the physical and mental health perceptions) are increasingly being used to assess effectiveness of cancer treatment.6 Palliative care and supportive oncology focus on managing physical, social, psychological, and spiritual needs of patients and have been recommended by the American Society of Clinical Oncology to be integrated into standard oncology care.7
People living in rural areas are less likely to get their care within a single health system. Often, their care is divided across multiple facilities and providers, which increases the chances of miscommunication between providers and can lead to inferior clinical outcomes and decreased patient QoL.8 There is a growing body of research describing the impact of palliative care on people with advanced cancer. Specifically, palliative care has been shown to reduce symptoms, improve QoL, and increase survival.9-11 Differences have been observed in the palliative care needs between people with cancer living in urban and suburban areas.12 It is likely that palliative care needs as well as the impact of palliative care services for people with advanced cancer in rural areas differs from those of their urban and suburban counterparts. Despite the known differences in access to care and impact of cancer between rural and nonrural residents, the impact of palliative care on people with advanced cancer living in rural areas has not been well described in the literature.
The purpose of this systematic review is to examine effect of palliative care and supportive oncology interventions on QoL in people with advanced cancer living in rural areas.
Methods
This systematic review was developed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13
Eligibility criteria
To achieve the objective of a systemic review of studies describing supportive oncology and palliative care interventions in rural communities articles had to meet 4 inclusion criteria:
All research methods were eligible, including mixed-methods and program evaluations, as long as the article met the 4 inclusion criteria. Review articles were ineligible for inclusion as only original research was considered.
Search process
Search terms were developed by the research team with consultation from a medical librarian. Four main search terms were developed and included: palliative care, supportive oncology, rural, and cancer. Synonyms and terms closely related to the main terms were included in the search using the OR command. Examples of closely related search terms include: Palliative care: palliative; Rural: remote; Cancer: neoplasms (Table).
We systematically searched PyschINFO, PubMed, CINHAL, and Scopus for articles that had been published during 1991-2016 and written in English. Databases were chosen to reflect the different subfields that encompass palliative care and supportive oncology: PyschINFO to capture the psychological perspective, CINHAL to capture the nursing perspective, and PubMed to capture the medical perspective. Finally, Scopus was searched to ensure that articles not indexed by the other databases would be included. The search was limited to the past 25 years to capture the most up-to-date literature.
Selection process
In accordance with PRISMA guidelines, articles underwent an initial screening and an eligibility screening for inclusion in the final review.13 After duplicates were removed, 2 research team members reviewed all abstracts to screen for initial eligibility. Articles that successfully passed the screening process were reviewed in full by 4 research team members. Each member made an independent inclusion decision based on the stated inclusion criteria. Disagreements across team members were resolved through discussion and consensus.
Analysis
The articles that met the inclusion criteria were heterogeneous in design and analytic approach. The set of manuscripts identified, therefore, did not meet the statistical assumptions for meta-analytic data analysis. The analytic plan for this review consisted of sorting the results described in the identified articles into meaningful categories, identifying cross cutting themes, and presenting the results of these themes in narrative forms.
Results
Study selection
The search strategy resulted in 886 articles across the 4 databases. The breakdown for each database is as follows: PsychINFO (n = 286), PubMed (n = 194), CINHAL (n = 334), Scopus (n = 72). After duplicates were removed, 864 articles were left and were initially screened resulting in 844 articles being excluded. The remaining 20 articles were reviewed and 12 articles failed to meet the inclusion criteria. Reasons for exclusion included: the population was not rural; no advanced cancer in sample; intervention was not specifically palliative care or supportive oncology. Nine articles representing 8 projects (one project published 2 manuscripts included in this review) were included in the final review (Figure).
After reviewing the articles, 2 clear themes arose: PROs, and overall impact of rural palliative care for people and society. The PRO theme included articles that provided information on how an intervention or program improved the personal lives experience of rural cancer patients. PROs, such as decreased symptomology, were often reported. The “overall impact of rural palliative care for people and society” theme included articles that provided information on how an intervention or program improved the lives of rural people and society as a larger group. An example would include results indicating how a program increased access to supportive oncology care in a rural area.
Study characteristics
Nine publications, describing 8 projects were included in this review (Table). These projects were conducted in Canada (n = 3)14-16 Australia (n = 1)17 and the United States (n = 5).18-22 All of the the projects used a quantitative approach for the analysis, except 1 that used mixed-methods.16 The studies designs were: 4 feasibilities/pilot studies, 1 randomized control trials (RCT), and 3 program evaluations.
A total of 807 patients participated across the 9 articles. Participants’ age ranged from 20 to 88 years. The average ages for participants ranged from 50.4 to 70.7 years. Overall, there were slightly more men (55%) than women (45%) when all the demographic data were combined across the 9 articles; however, 2 articles exclusively had women as part of the sample.17,20 The cancer types that participants had included: gastrointestinal, genitourinary, breast, lung, brain, kidney, and hematological. Finally, the articles had inconsistent reporting of race/ethnicity data with only 4 studies reporting this information; of the 4 studies, 91% of participants self-identified as white.
The projects targeted multiple PROs, including physical symptoms and psychosocial issues (ie, stress management, grief, mood, emotional distress, coping, self-efficacy, dignity, joy, affection) domains. Publications dates ranged from 1996 to 2013. The sample sizes ranged from 8 to 322; 11.7%-100% of the study population had advanced cancer, and 20%-100% were living in rural area. The duration of the clinical intervention described was 30-120 minutes. The modes of delivery for the palliative intervention were videoconference/videophone (n = 3), telephone/teleconference (n = 3), and in person (n = 2). The interventions were delivered by nurses, psychiatrists, and social workers. In 5 of these studies, participants received palliative care on an individual basis and 2 studies delivered their intervention through groups. The individual basis studies focused on physical aspects of care and the group studies focused on emotional aspects of care.
Patient-reported outcomes
Cancer and its treatments are often associated with physical and emotional sequelae that can have a significant impact on patients and therefore PROs. The interventions reviewed in this article often reported data on the reduction of the physical and/or emotional symptom burden of cancer as well as overall QoL.
Reduction in physical symptoms. Three articles included physical symptoms as an outcome measure. Of those, 2 were pilot or feasibility studies, and 1 was a randomized control trial. Common physical symptoms included: shortness of breath, pain, fatigue, nausea, and appetite change. Across the articles, the Edmonton Symptom Assessment Scale (ESAS), a 10-item inventory of common cancer symptoms, was frequently used to measure of symptom scores in these interventions.14,15,19 The ESAS is an empirically validated measure that is used in palliative care research and clinical practice. Individuals are asked to rank 10 common symptoms on an ascending scale from 1 to 10 (0, the symptom is absent; 10, worst possible severity).23
The findings from these 3 research studies were encouraging. In a large randomized control trial of a supportive education program, researchers reported decreased physical symptom intensity after the intervention, however the change did not reach statistical significance.18 Similar findings were reported in a videoconferencing and a home health program to improve access of palliative and supportive oncology health care.14,15 Physical symptoms that had decreasing trends were pain, tiredness, and appetite, however, trends for shortness of breath found increasing severity.14,15 Although these trends were observed, it is important to note that scores on the ESAS did not reach statistical significance for physical symptoms in any of these studies.
Reduction in emotional symptom reduction. In addition to reducing physical symptoms, researchers also sought to understand the impact of programs on the emotional symptoms of cancer including: anxiety, depression, negative affect, and posttraumatic stress disorder (PTSD). Five articles included emotional symptoms as an outcome measure. Four were pilot or feasibility studies, and 1 was a randomized control trial.
Results across studies indicated an observable decrease in the severity of anxiety and depression for those exposed to an intervention program.14,15,18,19 Again, although trends were found, the results were not statistically significant. Only Watanabe and colleagues14 reported a statistically significant a decrease in anxiety in participants after the implementation of a rural palliative care videoconference consultation program. One report indicated that data on depression severity was collected but was not analyzed because of a small sample size.21
O’Brien and colleagues17 also collected data on negative affect and found that participants who participated in a supportive-expressive therapy group had a reduction in the negative affect as measured by the Derogatis Affects Balance Scale (ABS). Other researchers found no change in emotional distress.15
Finally, Collie and colleagues20 also measured the impact of a videoconference support group of PTSD symptomology for people with breast cancer in rural areas. Their results indicated a statistically significant decrease on the PTSD Checklist-Specific after intervention. Analysis of the data also found a medium effect size. Participants in the intervention group spoke about how participation in the support group allowed them to be generative and share information about breast cancer as well as build an emotional bond with other women with cancer.
Overall quality of life and well-being. Researchers have also looked into impact of intervention on overall QoL. Two articles included QoL or Well-being as an outcome measure. One was a pilot study and 1 was a randomized control trial.
Bakitas and colleagues18,19 found that those enrolled in the intervention arm of their study had higher QoL scores on the Functional Assessment of Cancer Therapy-General (FACT-G) compared with those in the control arm. These results were also found in an analysis of data from participants who subsequently died during the intervention. Improvements in overall well-being were also found by O’Brien and colleagues17 using ABS. They reported that a post hoc comparison of participants’ total positive affect score was significantly higher at the 12-month follow-up. In addition, the authors also noted qualitative improvements in well-being, including increased effort to be at the support group and the low attrition rates.
Overall impact of rural palliative care on individuals and society. In addition to reducing physical and emotional symptoms in patients, several of the articles also addressed other measures of the overall impact of the intervention or program on society as a whole. The authors evaluated patient satisfaction and quality of life, access to health care services, and financial impact on individuals and society at large.
Satisfaction with intervention. In 2 of the articles, individuals or their family members reported to be satisfied with the intervention14,20 and said they would recommend it to others as well.20 Both of those studies used teleconferencing to provide access to the intervention to people in rural communities.
Increasing access to the health care services and quality of care. Four of the articles evaluated the impact of intervention on patient’s access to the health care services.14,16,20,22 Specifically, after the interventions individuals had increased access to palliative care information in rural areas where it had previously been unavailable20 as well as actual delivery of clinical care in their home community, thus eliminating the need to travel to urban areas.14,20,22 This increase of access to health care services in rural area had significant effect on time and distance spent traveling. In 1 study, the amount of saving in terms of distance was 471.13 km and time in, 7.96 hours, for each visit.14
In addition, the quality of overall cancer care in rural area was increased. In an early clinical program, to increase access of palliative care in rural communities, the authors reported an increase in the breast conservation from 20% at the start of the program to 70% 2 years after the program was implemented.22 Breast conservation is not a typical outcome for palliative care studies, but the authors highlighted this practice change because of the improved QoL that is associated with the use of breast conservation therapies. In the same study, the authors reported an increased use of curative therapies for other cancers such as lymphoma as well as an increase use of pain management medication.
Financial impact. Two articles described the financial impact of cancer care costs on the patient and society.14,22 In a study by Watanabe and colleagues in Canada,14 the amount of savings after the intervention in terms of travel expenses was C$192.71 for each visit because patients had previously had to travel from their rural communities to urban tertiary hospitals to receive palliative care. For some patients in that study, the amount of saving for expenses was as high as C$500 a visit. In addition, some individuals were not able to travel and would not have received anything if the intervention had not been available remotely.14 In a study by Smith and colleagues in the United States, there was a 62% decrease in the cost to society for each patient, from US$10,233 to US$3,862.22 The factors contributing to that reduction included increasing outpatient services, engaging nurses and primary care providers instead of specialists, and the lower costs of living in rural areas. In addition, the rural hospitals saw an increase in revenue and profits because of higher admission rates ($500,00 for each hospital annually).22
Discussion
The articles identified in this review provide some evidence of the potential impact that palliative and supportive oncology interventions could have on PROs for rural residents with advanced cancer. Noteworthy results were seen for impact on reducing physical and emotional symptoms, increasing overall QoL and well-being, increasing satisfaction and access to palliative care, and reducing the overall cost of palliative care for individuals and society.14-18,20-22
Although statistical significance was not observed for most of the symptom assessment, trends toward improved symptom reports were observed. A likely explanation for this finding, is the small sample size or inadequate design to evaluate symptoms as an outcome measure. Three studies were pilot or feasibility projects15,20,21 that were not powered to detect the impact of the intervention on symptoms. In contrast, QoL stands out as an outcome that was positively affected by palliative care interventions. Further research is needed to determine if there are important mediating and moderating factors that contribute to improve QoL that are specific to rural residents. Significant outcomes were also reported for participant satisfaction with the interventions, the increase in access to services, and the decrease in costs.
Although there were not enough studies to determine the efficacy of these interventions, these results suggest that palliative and supportive interventions can have an impact on important patient-reported outcomes, such as symptoms and quality of life, and on health care system outcomes, such as cost. Evidence supporting the extent of the effectiveness of palliative care on various PROs in rural people is limited. None of the studies in this review evaluated the different aspects of palliative care specifically in rural residents.
It is interesting to note that all but one of the interventions used a telehealth approach to deliver the intervention. Telehealth interventions seem to be feasible, acceptable to people in rural areas, and show preliminary evidence that they can have an impact on PROs.
Limitations of this review include only inclusion of publications in English. In addition, some studies in this review include populations that were not exclusively rural residents, which makes it difficult for generalization.
Conclusion
Palliative and supportive interventions may improve various PROs in people with advanced cancer living in rural areas. Technologies that support remote access to people in rural areas, such as teleconferencing and videoconferencing, seem particularly promising delivery modalities with their potential to increase access to palliative and supportive interventions in underserved communities. Large-scale studies that are powered to test the impact of palliative care and support oncology interventions on PROs and other aspects of quality care among rural residents with advanced cancer are needed.
The authors thank Jennifer DeBerg, Health Science Librarian at the University of Iowa for her assistance in developing the literature search strategies.
1. Moy E, Garcia MC, Bastian B, et al. Leading causes of death in nonmetropolitan and metropolitan areas – United States, 1999-2014 [published correction at https://www.cdc.gov/mmwr/volumes/66/wr/mm6603a11.htm]. MMWR Surveillance Summaries [serial online]. https://www.cdc.gov/mmwr/volumes/66/ss/ss6601a1.htm?s_cid=ss6601a1_w. Published January 13, 2017. Accessed January 20, 2017.
2. Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US Cancer Mortality: Part I – All cancers and lung cancer and Part II – Colorectal, prostate, breast, and cervical cancers. https://www.hindawi.com/journals/jce/2011/107497/. Published 2011. Accessed April 28, 2017.
3. Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of rural cancer care in the United States. Oncology (Williston Park). 2015;29(9):633-640.
4. Weaver KE, Geiger AM, Lu L, Case LD. Rural‐urban disparities in health status among US cancer survivors. Cancer. 2013;119(5):1050-1057.
5. Fuchsia Howard A, Smillie K, Turnbull K, et al. Access to medical and supportive care for rural and remote cancer survivors in northern British Columbia. J Rural Health. 2014;30(3):311-321.
6. Bottomley A, Aaronson NK. International perspective on health-related quality-of-life research in cancer clinical trials: the European Organisation for Research and Treatment of Cancer experience. J Clin Oncol. 2007;25(32):5082-5086.
7. Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880-887.
8. Baldwin LM, Cai Y, Larson EH, et al. Access to cancer services for rural colorectal cancer patients. J Rural Health. 2008;24(4):390-399.
9. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
10. McCorkle R, Jeon S, Ercolano E, et al. An advanced practice nurse coordinated multidisciplinary intervention for patients with late-stage cancer: a cluster randomized trial. J Palliat Med. 2015;18(11):962-969.
11. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
12. Regn R, Robinson W, Robinson WR. Differences in palliative care needs among cancer survivors in an inner city academic facility versus a suburban community facility. J Clin Oncol. 2015;33(29_suppl):61.
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;339:b2535.
14. Watanabe SM, Fairchild A, Pituskin E, Borgersen P, Hanson J, Fassbender K. Improving access to specialist multidisciplinary palliative care consultation for rural cancer patients by videoconferencing: report of a pilot project. Support Care Cancer. 2013;21(4):1201-1207.
15. Howell D, Marshall D, Brazil K, et al. A shared care model pilot for palliative home care in a rural area: impact on symptoms, distress, and place of death. J Pain Symptom Manage. 2011;42(1):60-75.
16. Stern A, Valaitis R, Weir R, Jadad AR. Use of home telehealth in palliative cancer care: a case study. J Telemed Telecare. 2012;18(5):297-300.
17. O’Brien M, Harris J, King R, O’Brien T. Supportive-expressive group therapy for women with metastatic breast cancer: Improving access for Australian women through use of teleconference. Counselling Psychother Res. 2008;8(1):28-35.
18. Bakitas M, Lyons KD, Hegel MT, et al. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliat Supportive Care. 2009;7(1):75-86.
19. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
20. Collie K, Kreshka MA, Ferrier S, et al. Videoconferencing for delivery of breast cancer support groups to women living in rural communities: a pilot study. Psychooncology. 200
21. Passik SD, Kirsh KL, Leibee S, et al. A feasibility study of dignity psychotherapy delivered via telemedicine. Palliat Support Care. 2004;2(2):149-155.
22. Smith TJ, Desch CE, Grasso MA, et al. The Rural Cancer Outreach Program: clinical and financial analysis of palliative and curative care for an underserved population. Cancer Treat Rev. 1996;22(Suppl A):97-101.
23. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6-9.
1. Moy E, Garcia MC, Bastian B, et al. Leading causes of death in nonmetropolitan and metropolitan areas – United States, 1999-2014 [published correction at https://www.cdc.gov/mmwr/volumes/66/wr/mm6603a11.htm]. MMWR Surveillance Summaries [serial online]. https://www.cdc.gov/mmwr/volumes/66/ss/ss6601a1.htm?s_cid=ss6601a1_w. Published January 13, 2017. Accessed January 20, 2017.
2. Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US Cancer Mortality: Part I – All cancers and lung cancer and Part II – Colorectal, prostate, breast, and cervical cancers. https://www.hindawi.com/journals/jce/2011/107497/. Published 2011. Accessed April 28, 2017.
3. Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of rural cancer care in the United States. Oncology (Williston Park). 2015;29(9):633-640.
4. Weaver KE, Geiger AM, Lu L, Case LD. Rural‐urban disparities in health status among US cancer survivors. Cancer. 2013;119(5):1050-1057.
5. Fuchsia Howard A, Smillie K, Turnbull K, et al. Access to medical and supportive care for rural and remote cancer survivors in northern British Columbia. J Rural Health. 2014;30(3):311-321.
6. Bottomley A, Aaronson NK. International perspective on health-related quality-of-life research in cancer clinical trials: the European Organisation for Research and Treatment of Cancer experience. J Clin Oncol. 2007;25(32):5082-5086.
7. Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880-887.
8. Baldwin LM, Cai Y, Larson EH, et al. Access to cancer services for rural colorectal cancer patients. J Rural Health. 2008;24(4):390-399.
9. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
10. McCorkle R, Jeon S, Ercolano E, et al. An advanced practice nurse coordinated multidisciplinary intervention for patients with late-stage cancer: a cluster randomized trial. J Palliat Med. 2015;18(11):962-969.
11. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
12. Regn R, Robinson W, Robinson WR. Differences in palliative care needs among cancer survivors in an inner city academic facility versus a suburban community facility. J Clin Oncol. 2015;33(29_suppl):61.
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;339:b2535.
14. Watanabe SM, Fairchild A, Pituskin E, Borgersen P, Hanson J, Fassbender K. Improving access to specialist multidisciplinary palliative care consultation for rural cancer patients by videoconferencing: report of a pilot project. Support Care Cancer. 2013;21(4):1201-1207.
15. Howell D, Marshall D, Brazil K, et al. A shared care model pilot for palliative home care in a rural area: impact on symptoms, distress, and place of death. J Pain Symptom Manage. 2011;42(1):60-75.
16. Stern A, Valaitis R, Weir R, Jadad AR. Use of home telehealth in palliative cancer care: a case study. J Telemed Telecare. 2012;18(5):297-300.
17. O’Brien M, Harris J, King R, O’Brien T. Supportive-expressive group therapy for women with metastatic breast cancer: Improving access for Australian women through use of teleconference. Counselling Psychother Res. 2008;8(1):28-35.
18. Bakitas M, Lyons KD, Hegel MT, et al. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliat Supportive Care. 2009;7(1):75-86.
19. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
20. Collie K, Kreshka MA, Ferrier S, et al. Videoconferencing for delivery of breast cancer support groups to women living in rural communities: a pilot study. Psychooncology. 200
21. Passik SD, Kirsh KL, Leibee S, et al. A feasibility study of dignity psychotherapy delivered via telemedicine. Palliat Support Care. 2004;2(2):149-155.
22. Smith TJ, Desch CE, Grasso MA, et al. The Rural Cancer Outreach Program: clinical and financial analysis of palliative and curative care for an underserved population. Cancer Treat Rev. 1996;22(Suppl A):97-101.
23. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6-9.
Adverse events from systemic treatment of cancer and patient-reported quality of life
Adverse events (AEs) from systemic treatment of cancer have a negative impact on patient quality of life (QoL). The extent of this impact is difficult to ascertain, particularly in patients undergoing palliative treatment because of variations in QoL resulting from antitumor effect.1 Patient-reported outcomes (PROs) are the best tool for elicitation of patient preferences, therefore helping cancer patients, oncologists, and health care managers to make better choices. Indeed, analysis of self-reported QoL during cancer chemotherapy provides new insights that are missed by other efficacy outcomes,2 although patient-reported AEs correlate well with AEs reported by clinicians.3 Self-reported symptoms provide better control during cancer treatment.4 However, there are other instruments to measure the impact of treatments on QoL that are based on preferences of members of the general public. Use of that strategy has been strongly debated. The most obvious problem is the difficulty that persons from the general public may have in putting themselves in the patient position.5 In addition, there is evidence that compared with the general public, patients adapt to their illness5,6 and then tend to downplay severity when rating values of health states.7 Therefore, a systematic discrepancy is observed between actual patients and the general public. It is not clear if it reflects the inability of members of the general public to fully grasp the relative severity of health problems or to the adaptation process of patients. This fact may obscure a negative impact on QoL which, in turn, could be detected using the general public as a surrogate. A combination of both approaches has been recommended for rating QoL when the ultimate purpose is making decisions on resource allocation.5 This debate is prolonging in time and it is far from over.8,9
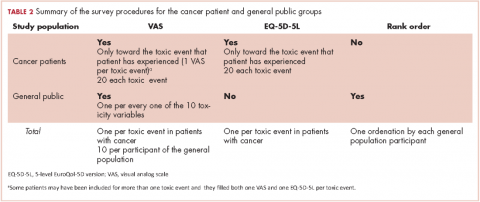
Based on this background, this study investigates the impact of AEs on QoL of cancer patients from the perspective of cancer patients who had experienced the AEs of interest (ex post population) and the perspective of members of the general public. The second group comprised participants imagining themselves as hypothetical cancer patients experiencing the AEs (ex ante population). Previous studies with this dual approach allowing for comparisons between these two populations are small or centered on a few AEs.10 Therefore, a large and comprehensive study on the impact of AEs on QoL is lacking. Supported by previous literature, the investigational hypothesis was that ex post impact would be significantly lower than that imagined in an ex ante setting. The secondary objective is to study the potential use of the EuroQol (EQ-5D) instrument for health-related QoL in the measurement of the impact of AEs in cancer patients. This generic instrument is based on interviews with members of the general public. We tried to investigate to what extent those values relate to the cancer patients’ evaluation of their own health during treatment. The ultimate goal of the study is to assist in increasing the utility that patients derive from the benefits associated with cancer treatment.
Methods
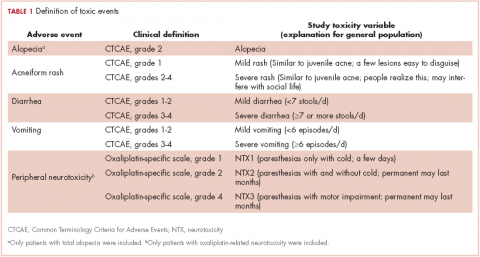
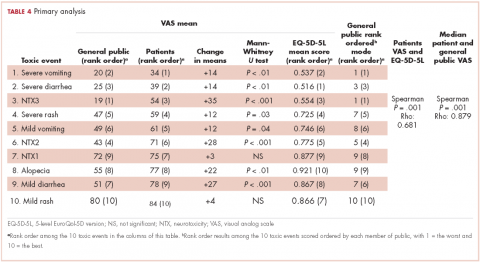
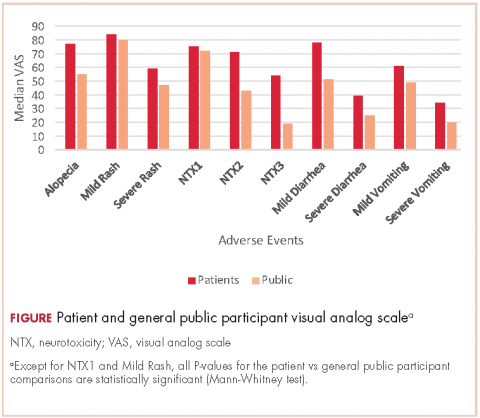
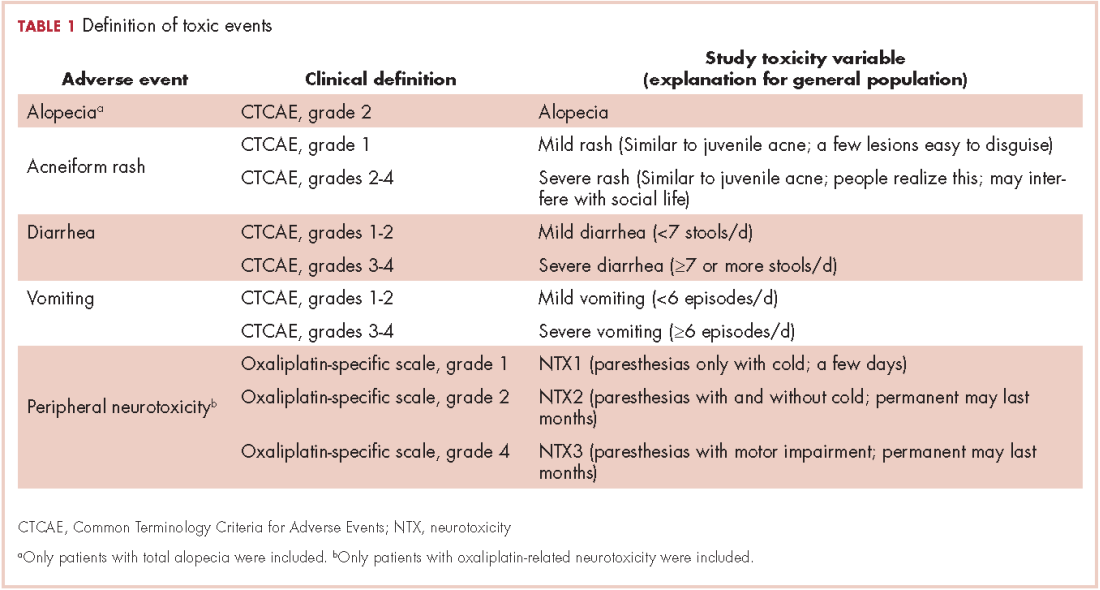
Selection of AEs
Five AEs related to systemic treatment of cancer – alopecia, acneiform rash, oxaliplatin-associated peripheral neuropathy, diarrhea, and vomiting – were selected for the study. Investigators set up different relevant cut-off points for severity, resulting in 10 toxic events that were ad hoc defined as the variables for the study (Table 1). We used the Common Terminology Criteria for Adverse Events (CTCAE, version 4) to classify alopecia, acneiform rash, diarrhea, and vomiting. For oxaliplatin-associated peripheral neuropathy, we adapted Misset’s oxaliplatin-specific scale11 (range, grade 1-4; Table 1) in which grade 1 (neurotoxicity [NTX] 1) = paresthesias only with cold lasting a few days; grade 2 [NTX2] = paresthesias with and without cold that may last months; and grade 4 [NTX3] = paresthesias with functional consequence).
Participants
Two populations were included in the study: cancer patients who had experienced a particular AE and received treatment at the medical oncology departments of Hospital Santa Tecla and Hospital del Vendrell in Tarragona, Spain; and participants from the general public who received care at the Primary Health Care Center-Llevant in the same city.
Cancer patients. These participants had to be 18 years or older and had to have experienced 1 of the 10 toxic events in the 5 years before inclusion in the study; the treatment setting could be either curative attempt (adjuvant, neoadjuvant) or palliative, and patients with ongoing treatment should have received almost 3 months of treatment. Patients were excluded if they had an ECOG PS grade of 3 or more (Eastern Cooperative Oncology Group Performance Status; range, 0-5, where 0 = fully active, 3 = capable of limited self-care; confined to bed or chair more than 50% of waking hours, and 5 = dead). A particular patient with cancer could be included because of more than 1 study toxic event (eg, alopecia and severe vomiting or NTX1 and NTX2) but had to complete separate questionnaires for the different toxic events.
General public group. Participants in this group were selected from the records of general practitioner consultations at the aforementioned primary health care center. They had to be 18 years or older and could not have a history of cancer or symptomatic/severe chronic diseases (eg, they could have hypertension or diabetes without chronic target organ involvement, or they could be patients with either acute nonserious illness or nonserious injuries).
Survey procedures
Cancer patients. Participants in this group filled in 2 questionnaires provided by a medical oncologist in a face-to-face interview: the 5-dimension, 5-level EuroQol (EQ-5D-5L) questionnaire in reference to the days when patients were suffering the toxic event; and a visual analog scale (VAS) answering the question: How do you feel that this AE has impacted on your QoL the days you have experienced it?
VAS scores ranged from 0 (the poorest QoL, the highest impact) to 100 (the better QoL, the lower impact). The EQ-5D-5L has 5 dimensions (Mobility, Self-care, Daily life activities/social performance, Discomfort/pain, and Anxiety/depression) with 5 level response options each (No problem at all, Light problem, Moderate problem, Severe problem, Extreme problem/unable).12 The combination of 5 answers is converted to a single score, which is different for different countries; in the validated version for Spanish population, the score ranges from -0.654 (the worst health state) to 1.000 (the best health state). Patients were asked to make an effort to separate and encapsulate the impact of every adverse event and separate it from others they may have experienced during the same period. Table 2 summarizes survey procedures.
General public group. Two internal medicine residents who administered the questionnaire to the participants of the public group were well trained to carefully explain what each of the 10 the toxic events meant. Some details on these explanations are shown in Table 1, and the full set of explanations is shown in Appendix 1 (online only). Participants in the general public group were asked in a face-to-face interview to imagine they were cancer patients and envision how these toxic events would impact on their QoL if they were undergoing systemic treatment of cancer. They were asked to rate the imagined impact with the VAS (1 VAS/every toxic event = 10 VAS/participant). Then, they were presented with 10 cards, each with the name of 1 of the 10 toxic events (Appendix 2 [online only]), to show them the order of the impact on QoL based on their scores (respecting ties). The participants were asked if they agreed with the order, and if they did not, they were invited to change the scores. Therefore, results in the general public group also show the rank-order of the study toxic events.
Statistical analysis
We calculated the sample size as follows:
Primary outcomes were VAS score in cancer patients and VAS score in the general public. Primary analyses were comparison between VAS in both populations. Secondary outcomes were EQ-5D-5L score in cancer patients, and intra-participant rank-order in the general public group. Secondary analyses were correlation between VAS score and EQ-5D-5L in cancer patients and descriptive analysis of rank-ordered data in the general public group.
It was planned to compare means of quantitative variables with the Mann-Whitney U test and to assess correlation between quantitative variables with the Spearman rho test. All tests for contrast were nonparametric because a normal distribution was not expected from quoted scores with some ceiling or floor effect. A hierarchical generalized cluster analysis was planned to study clusters of variables grouped by VAS score in the public group.
Ethics
The study was conducted in accordance with the Declaration of Helsinki version Fortaleza 2013 and was approved by the institutional review board of the participant institutions. All of the patients provided written informed consent before study entry. Data of the participants of the general public were anonymous, so those participants were asked to provide only oral assent, with the permission of the review board.
Results
Between December 1, 2013 and January 31, 2015, a total of 250 participants of the general public and 139 cancer patients were included in the study. Four participants of the general public had incompletely filled the questionnaire and were excluded from the study, resulting in 246 participants with complete data available. There were no losses in the patient group, of whom 79 (57%) were currently on treatment and 118 (85%) had received the treatment in the previous 2 years. The total number of study toxic events in the 139 cancer patients was 200 (20 by each of the 10 study toxicity variables). Of those, 42 patients (30%) experienced (and were included in the study for) more than 1 toxic event.
Of the 139 patients, 91 (65%) received the treatment with curative intent. The most frequent diagnosis was colorectal cancer in 77 patients (55%), followed by breast cancer (13 patients, 9.4%), and lung cancer (11 patients, 7.9%). Systemic treatment of cancer was one of these options: chemotherapy alone, anti-EGFR [epidermal growth factor receptor] alone, chemotherapy plus anti-EGFR, or chemotherapy plus other biologics. The chemotherapy regimen most frequently administered was mFOLFOX6 [modified leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin] which, alone or in combination, was administered to 51 patients (37%). An anti-EGFR agent was administered to 22 patients (16%): cetuximab (15 patients), panitumumab (4 patients), erlotinib (2 patients) and afatinib (1 patient). The baseline characteristics of the patients and participants in the study are shown in Table 3.
For all 10 toxicity outcomes, the mean VAS score from the general public was numerically lower (lower QoL, more impact) than that resulted from the cancer patients who had actually experienced the toxic event of interest (Table 4 and Figure).
Taking off 2 mild effects (NTX1 and mild rash), for the 8 remaining toxic events, this difference was statistically significant (Mann-Whitney U test; P < .01 for severe vomiting, severe diarrhea, and alopecia; P < .001 for NTX2, NTX3 and mild diarrhea; P = .03 for severe rash; P = .04 for mild vomiting). Severe vomiting resulted in the worst VAS score for cancer patients (median VAS, 34) and NTX3 had the worst VAS score for the general public participants (median, VAS 19). Table 4 summarizes the 4 sets of results (patient and public VAS, and patient EQ-5D-5L and public rank-order). Regarding the results of the esthetic toxicities compared with each other, impact from severe rash was considered higher than that from alopecia for both populations, patients (mean VAS, 59 [rash] vs 77 [alopecia]; mean EQ-5D-5L score, 0.725/rank order 4 vs 0.921/10) and the general public (mean VAS, 47 vs 55; EQ-5D-5L, rank order 5 vs 9). In the group of patients, linear correlation between VAS and EQ-5D-5L score was assessed resulting in a significant positive correlation (Spearman P = .001) with a correlation coefficient Rho 0.681 (Appendix 3 [online only]). Also, a positive linear correlation was observed between the 10 means of the cancer patients’ VAS and the 10 means of the general public participants’ VAS (Spearman P = .001; coefficient Rho 0.879). Both ceiling and floor effect were observed for VAS in the 2 populations, but only ceiling effect for EQ-5D-5L in the patient population. The most important floor effect was for NTX3, with 66 participants (27%) of the general public group scoring VAS 0 (see Appendix 4 [online only] for the frequencies of answers for every level of the 5 dimensions of the EQ-5D-5L). An analysis of the results, considered as an intraparticipant rank-ordered evaluation, was performed in the general public group. Fourteen participants of that group (5.7%) changed their scores after they were presented with the order shown in cards. Mode of the ranks show that NTX3 and severe vomiting were the worst-scored toxic events. The most frequent rank-order for alopecia and severe rash were (from best to worst) the second and the fourth, respectively.
Discussion
The findings in this study show that impact on QoL imagined by members of the general public is higher than that declared by cancer patients who have experienced the AEs. It is worth noting that that result was observed for all 10 toxic events, thus confirming the investigational hypothesis of the study. However, the graph shows a strong parallel between the 2 groups, which means that both populations similarly perceive upward and downward variations in the impact resulting from the different toxic events (Figure).
Three previous studies have addressed the comparison of the impact of different AEs in these 2 populations and findings from all 3 showed the same systematic difference between patients and the general public participants. The first, Calhoun and colleagues used time to trade-off (TTO, a measure of the QoL a person or groups is experiencing) to compare therapies for ovarian cancer in patients and the general population (n = 39 for each group). The results showed that cancer patients valued more the health status associated with toxicity than did the general public participants.13 The design of the second study, by Havrilesky and colleagues, was similar to that of the present study, and they compared toxic events one by one, using VAS and TTO in 13 ovarian cancer patients and 37 women of the general public.14 The investigators found the same results as we did on the parallel of the 2 groups and also a very similar order of the toxic events. Indeed, alopecia was the less bothersome, whereas both motor neuropathy and severe vomiting were among the worst toxic events. Therefore, our results correlate perfectly with theirs. Best and colleagues found that health states values associated with oxaliplatin-related peripheral neuropathy were lower in the general public population compared with those of cancer patients.15 Besides adaptive behavior of the patients, all these results may be explained by an established awareness cancer patients have of the dual outcome of cancer treatments (AEs and benefits from the treatment).9 This awareness is absent in the general public participants, who can only envision the negative outcomes and who do not realize the importance of the benefits.9 Findings from previous studies conducted in several tumor types such as breast cancer16-18 non–small-cell lung cancer,19 thyroid cancer,20 and renal cancer21 have shown that patients are willing to trade-off AEs for treatment benefits.
Alopecia has been considered as one of the most distressing and troublesome AEs of cancer therapy.22 However, in the present study, alopecia was rated inside the range of mild toxic events as it is in the study by Havrilesky and colleagues.14 Our results show that alopecia was placed as the first less damaging toxic event when assessed with the EQ-5D-5L, the second less damaging when assessed with a rank-order system, and the third less damaging when assessed the VAS. This could be related to current fashion trends that promote shaving one’s hair, which minimizes the social stigma of alopecia and its association with cancer treatment.
The other esthetic event we analyzed was acneiform rash associated with anti-EGFR agents. Our results show that severe rash was rated as clearly worse than alopecia by the 2 populations, irrespective of the measuring instrument (VAS, EQ-5D-5L, or ordinal assessment). To our knowledge, the present study is the first to demonstrate the relative impact of total alopecia and severe rash on patient QoL. This result is even more significant considering that we included grade 2 acneiform rash inside the Severe Rash toxic event. Our results show that the worst AEs for both populations were severe vomiting and neurotoxicity with functional impairment. The high impact of severe vomiting, the quintessentially chemotherapy-induced AE, was to be expected because it is strongly supported by a number of previous reports,14,23 as is also true for peripheral motor neuropathy.14,15,24
EQ-5D is a powerful instrument for measuring health status25 and is widely used to describe and evaluate patient health.26 Our results from the 5 dimensions represented by a single score were well correlated with the results of the VAS. However, whereas median VAS scores were evenly distributed in the 0-100 range of the VAS, median EQ-5D-5L scores were distributed mainly in the 0.5-1.0 range (full range, -0.654-1.000, for Spanish population).
The final single score of the original EQ-5D is based on responses from the general public, and we have shown that its use is a valid option when the objective is the evaluation of AEs in patients with cancer. Management of AEs is of the utmost importance in this era of personalized cancer medicine. Basch and colleagues recently reported that an intensive web-based follow-up of AEs during chemotherapy improved overall survival compared with standard follow-up.27 The results of our study show that patients have strongly defined preferences regarding AEs. Therefore, therapeutic strategies with a personalized approach in managing AEs would be associated with increased effectiveness.
There are some limitations in the present study. First, we modified slightly the EQ-5D-5L questionnaire by asking patients to recall and rate the days they experienced the adverse event instead of asking for “today’s feelings.” It is not known how this modification affects internal validity of this study. Second, we asked patients to isolate the toxic event to rate it independently from the other toxic events. We believe that this request may have been difficult for some patients to do because they might have experienced more than 1 toxic event concurrently. Third, using VAS to assess health status may be a weakness because it has been considered to be too straightforward an instrument. Likewise, there are some strengths of the study: it was performed in a face-to-face manner; it displayed cardinal and ordinal results for participants in the public group; and the results are the same as those in a previous study.14
In conclusion, patients with cancer who have experienced AEs perceive a lower impact on their QoL compared with that envisioned by participants from the general public. The EQ-5D-5L is a useful tool for evaluating cancer-therapy–related AEs. The impact of alopecia on QoL was notably low and even lower than that of severe rash. Further investigation on this issue should focus on patients’ and oncologists’ shared choices, which increasingly will be driven by patient preferences.
The Oncologic Association Dr Amadeu Pelegrí (AODAP), a charitable organization led by cancer patients and based in Salou, Spain, provided the financial support needed to conduct this study (www.aodapelegri.com).
1. Mazzotti E, Antonini Cappellini GC, Buconovo S, et al. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer. 2012;20(10):2553-2557.
2. Gunnars B, Nygren P, Glimelius B, SBU-group. Swedish Council of Technology Assessment in Health Care. Assessment of quality of life during chemotherapy. Acta Oncol. 2001;40(2-3):175-184.
3. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051-1059.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Menzel P, Dolan P, Richardson J, Olsen JA. The role of adaptation to disability and disease in health state valuation: a preliminary normative analysis. Soc Sci Med. 2002;55(12):2149-2158.
6. McTaggart-Cowan H, Tsuchiya A, O’Cathain A, Brazier J. Understanding the effect of disease adaptation information on general population values for hypothetical health states. Soc Sci Med. 2011;72(11): 1904-1912.
7. Ubel PA, Loewenstein G, Schwarz N, Smith D. (2005). Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005;24(4 Suppl):S57-62.
8. Brazier J, Akehurst R, Brennan A, et al. Should patients have a greater role in valuing health states? Appl Health Econ Health Policy. 2005;4(4):201-208.
9. Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Qual Life Res. 2003;12(6), 599-607.
10. Shabaruddin FH, Chen LC, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics. 2013;31(4):277-288.
11. Misset JL. Oxaliplatin in practice. Br J Cancer. 1998;77 Suppl 4:4-7.
12. EQ-5D website. About the EQ-5D-5L. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Last updated April 18, 2017. Accessed October 18, 2017.
13. Calhoun EA, Fishman DA, Lurain JR, Welshman EE, Bennett CL. A comparison of ovarian cancer treatments: analysis of utility assessments of ovarian cancer patients, at-risk population, general population, and physicians. Gynecol Oncol. 2004;93(1):164-169.
14. Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216-220.
15. Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preferences values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391-400.
16. Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C. Patient preferences for chemotherapies used in breast cancer. Int J Womens Health. 2012;4:279-287.
17. Beusterien K, Grinspan J, Kuchuk I, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. The Oncologist 2014;19(2):127-134.
18. Kuchuk I, Bouganim N, Beusterien K, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. 2013;142(1):101-107.
19. Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77(1):224-231.
20. Mohamed AF, González JM, Fairchild A. Patient benefit-risk tradeoffs for radioactive Iodine-refractory differentiated thyroid cancer treatments. J Thyroid Res. 2015:438235.
21. Wong MK, Mohamed AF, Hauber AB, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ. 2012;15(6):1139-1148.
22. Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17(4):317-328.
23. Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother. 2013;14(6):757-766.
24. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
25. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343.
26. Greiner W, Weijnen T, Nieuwenhuizen M, et al. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ.2003; 4(3):222-231.
27. Basch E, Deal AM, Dueck AC, et al. Survival results of a trial assessing patient-reported outcomes for symprom monitoring during routine cancer treatment. JAMA 2017. doi:10.1001/jama.2017.7156
Adverse events (AEs) from systemic treatment of cancer have a negative impact on patient quality of life (QoL). The extent of this impact is difficult to ascertain, particularly in patients undergoing palliative treatment because of variations in QoL resulting from antitumor effect.1 Patient-reported outcomes (PROs) are the best tool for elicitation of patient preferences, therefore helping cancer patients, oncologists, and health care managers to make better choices. Indeed, analysis of self-reported QoL during cancer chemotherapy provides new insights that are missed by other efficacy outcomes,2 although patient-reported AEs correlate well with AEs reported by clinicians.3 Self-reported symptoms provide better control during cancer treatment.4 However, there are other instruments to measure the impact of treatments on QoL that are based on preferences of members of the general public. Use of that strategy has been strongly debated. The most obvious problem is the difficulty that persons from the general public may have in putting themselves in the patient position.5 In addition, there is evidence that compared with the general public, patients adapt to their illness5,6 and then tend to downplay severity when rating values of health states.7 Therefore, a systematic discrepancy is observed between actual patients and the general public. It is not clear if it reflects the inability of members of the general public to fully grasp the relative severity of health problems or to the adaptation process of patients. This fact may obscure a negative impact on QoL which, in turn, could be detected using the general public as a surrogate. A combination of both approaches has been recommended for rating QoL when the ultimate purpose is making decisions on resource allocation.5 This debate is prolonging in time and it is far from over.8,9
Based on this background, this study investigates the impact of AEs on QoL of cancer patients from the perspective of cancer patients who had experienced the AEs of interest (ex post population) and the perspective of members of the general public. The second group comprised participants imagining themselves as hypothetical cancer patients experiencing the AEs (ex ante population). Previous studies with this dual approach allowing for comparisons between these two populations are small or centered on a few AEs.10 Therefore, a large and comprehensive study on the impact of AEs on QoL is lacking. Supported by previous literature, the investigational hypothesis was that ex post impact would be significantly lower than that imagined in an ex ante setting. The secondary objective is to study the potential use of the EuroQol (EQ-5D) instrument for health-related QoL in the measurement of the impact of AEs in cancer patients. This generic instrument is based on interviews with members of the general public. We tried to investigate to what extent those values relate to the cancer patients’ evaluation of their own health during treatment. The ultimate goal of the study is to assist in increasing the utility that patients derive from the benefits associated with cancer treatment.
Methods
Selection of AEs
Five AEs related to systemic treatment of cancer – alopecia, acneiform rash, oxaliplatin-associated peripheral neuropathy, diarrhea, and vomiting – were selected for the study. Investigators set up different relevant cut-off points for severity, resulting in 10 toxic events that were ad hoc defined as the variables for the study (Table 1). We used the Common Terminology Criteria for Adverse Events (CTCAE, version 4) to classify alopecia, acneiform rash, diarrhea, and vomiting. For oxaliplatin-associated peripheral neuropathy, we adapted Misset’s oxaliplatin-specific scale11 (range, grade 1-4; Table 1) in which grade 1 (neurotoxicity [NTX] 1) = paresthesias only with cold lasting a few days; grade 2 [NTX2] = paresthesias with and without cold that may last months; and grade 4 [NTX3] = paresthesias with functional consequence).
Participants
Two populations were included in the study: cancer patients who had experienced a particular AE and received treatment at the medical oncology departments of Hospital Santa Tecla and Hospital del Vendrell in Tarragona, Spain; and participants from the general public who received care at the Primary Health Care Center-Llevant in the same city.
Cancer patients. These participants had to be 18 years or older and had to have experienced 1 of the 10 toxic events in the 5 years before inclusion in the study; the treatment setting could be either curative attempt (adjuvant, neoadjuvant) or palliative, and patients with ongoing treatment should have received almost 3 months of treatment. Patients were excluded if they had an ECOG PS grade of 3 or more (Eastern Cooperative Oncology Group Performance Status; range, 0-5, where 0 = fully active, 3 = capable of limited self-care; confined to bed or chair more than 50% of waking hours, and 5 = dead). A particular patient with cancer could be included because of more than 1 study toxic event (eg, alopecia and severe vomiting or NTX1 and NTX2) but had to complete separate questionnaires for the different toxic events.
General public group. Participants in this group were selected from the records of general practitioner consultations at the aforementioned primary health care center. They had to be 18 years or older and could not have a history of cancer or symptomatic/severe chronic diseases (eg, they could have hypertension or diabetes without chronic target organ involvement, or they could be patients with either acute nonserious illness or nonserious injuries).
Survey procedures
Cancer patients. Participants in this group filled in 2 questionnaires provided by a medical oncologist in a face-to-face interview: the 5-dimension, 5-level EuroQol (EQ-5D-5L) questionnaire in reference to the days when patients were suffering the toxic event; and a visual analog scale (VAS) answering the question: How do you feel that this AE has impacted on your QoL the days you have experienced it?
VAS scores ranged from 0 (the poorest QoL, the highest impact) to 100 (the better QoL, the lower impact). The EQ-5D-5L has 5 dimensions (Mobility, Self-care, Daily life activities/social performance, Discomfort/pain, and Anxiety/depression) with 5 level response options each (No problem at all, Light problem, Moderate problem, Severe problem, Extreme problem/unable).12 The combination of 5 answers is converted to a single score, which is different for different countries; in the validated version for Spanish population, the score ranges from -0.654 (the worst health state) to 1.000 (the best health state). Patients were asked to make an effort to separate and encapsulate the impact of every adverse event and separate it from others they may have experienced during the same period. Table 2 summarizes survey procedures.
General public group. Two internal medicine residents who administered the questionnaire to the participants of the public group were well trained to carefully explain what each of the 10 the toxic events meant. Some details on these explanations are shown in Table 1, and the full set of explanations is shown in Appendix 1 (online only). Participants in the general public group were asked in a face-to-face interview to imagine they were cancer patients and envision how these toxic events would impact on their QoL if they were undergoing systemic treatment of cancer. They were asked to rate the imagined impact with the VAS (1 VAS/every toxic event = 10 VAS/participant). Then, they were presented with 10 cards, each with the name of 1 of the 10 toxic events (Appendix 2 [online only]), to show them the order of the impact on QoL based on their scores (respecting ties). The participants were asked if they agreed with the order, and if they did not, they were invited to change the scores. Therefore, results in the general public group also show the rank-order of the study toxic events.
Statistical analysis
We calculated the sample size as follows:
Primary outcomes were VAS score in cancer patients and VAS score in the general public. Primary analyses were comparison between VAS in both populations. Secondary outcomes were EQ-5D-5L score in cancer patients, and intra-participant rank-order in the general public group. Secondary analyses were correlation between VAS score and EQ-5D-5L in cancer patients and descriptive analysis of rank-ordered data in the general public group.
It was planned to compare means of quantitative variables with the Mann-Whitney U test and to assess correlation between quantitative variables with the Spearman rho test. All tests for contrast were nonparametric because a normal distribution was not expected from quoted scores with some ceiling or floor effect. A hierarchical generalized cluster analysis was planned to study clusters of variables grouped by VAS score in the public group.
Ethics
The study was conducted in accordance with the Declaration of Helsinki version Fortaleza 2013 and was approved by the institutional review board of the participant institutions. All of the patients provided written informed consent before study entry. Data of the participants of the general public were anonymous, so those participants were asked to provide only oral assent, with the permission of the review board.
Results
Between December 1, 2013 and January 31, 2015, a total of 250 participants of the general public and 139 cancer patients were included in the study. Four participants of the general public had incompletely filled the questionnaire and were excluded from the study, resulting in 246 participants with complete data available. There were no losses in the patient group, of whom 79 (57%) were currently on treatment and 118 (85%) had received the treatment in the previous 2 years. The total number of study toxic events in the 139 cancer patients was 200 (20 by each of the 10 study toxicity variables). Of those, 42 patients (30%) experienced (and were included in the study for) more than 1 toxic event.
Of the 139 patients, 91 (65%) received the treatment with curative intent. The most frequent diagnosis was colorectal cancer in 77 patients (55%), followed by breast cancer (13 patients, 9.4%), and lung cancer (11 patients, 7.9%). Systemic treatment of cancer was one of these options: chemotherapy alone, anti-EGFR [epidermal growth factor receptor] alone, chemotherapy plus anti-EGFR, or chemotherapy plus other biologics. The chemotherapy regimen most frequently administered was mFOLFOX6 [modified leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin] which, alone or in combination, was administered to 51 patients (37%). An anti-EGFR agent was administered to 22 patients (16%): cetuximab (15 patients), panitumumab (4 patients), erlotinib (2 patients) and afatinib (1 patient). The baseline characteristics of the patients and participants in the study are shown in Table 3.
For all 10 toxicity outcomes, the mean VAS score from the general public was numerically lower (lower QoL, more impact) than that resulted from the cancer patients who had actually experienced the toxic event of interest (Table 4 and Figure).
Taking off 2 mild effects (NTX1 and mild rash), for the 8 remaining toxic events, this difference was statistically significant (Mann-Whitney U test; P < .01 for severe vomiting, severe diarrhea, and alopecia; P < .001 for NTX2, NTX3 and mild diarrhea; P = .03 for severe rash; P = .04 for mild vomiting). Severe vomiting resulted in the worst VAS score for cancer patients (median VAS, 34) and NTX3 had the worst VAS score for the general public participants (median, VAS 19). Table 4 summarizes the 4 sets of results (patient and public VAS, and patient EQ-5D-5L and public rank-order). Regarding the results of the esthetic toxicities compared with each other, impact from severe rash was considered higher than that from alopecia for both populations, patients (mean VAS, 59 [rash] vs 77 [alopecia]; mean EQ-5D-5L score, 0.725/rank order 4 vs 0.921/10) and the general public (mean VAS, 47 vs 55; EQ-5D-5L, rank order 5 vs 9). In the group of patients, linear correlation between VAS and EQ-5D-5L score was assessed resulting in a significant positive correlation (Spearman P = .001) with a correlation coefficient Rho 0.681 (Appendix 3 [online only]). Also, a positive linear correlation was observed between the 10 means of the cancer patients’ VAS and the 10 means of the general public participants’ VAS (Spearman P = .001; coefficient Rho 0.879). Both ceiling and floor effect were observed for VAS in the 2 populations, but only ceiling effect for EQ-5D-5L in the patient population. The most important floor effect was for NTX3, with 66 participants (27%) of the general public group scoring VAS 0 (see Appendix 4 [online only] for the frequencies of answers for every level of the 5 dimensions of the EQ-5D-5L). An analysis of the results, considered as an intraparticipant rank-ordered evaluation, was performed in the general public group. Fourteen participants of that group (5.7%) changed their scores after they were presented with the order shown in cards. Mode of the ranks show that NTX3 and severe vomiting were the worst-scored toxic events. The most frequent rank-order for alopecia and severe rash were (from best to worst) the second and the fourth, respectively.
Discussion
The findings in this study show that impact on QoL imagined by members of the general public is higher than that declared by cancer patients who have experienced the AEs. It is worth noting that that result was observed for all 10 toxic events, thus confirming the investigational hypothesis of the study. However, the graph shows a strong parallel between the 2 groups, which means that both populations similarly perceive upward and downward variations in the impact resulting from the different toxic events (Figure).
Three previous studies have addressed the comparison of the impact of different AEs in these 2 populations and findings from all 3 showed the same systematic difference between patients and the general public participants. The first, Calhoun and colleagues used time to trade-off (TTO, a measure of the QoL a person or groups is experiencing) to compare therapies for ovarian cancer in patients and the general population (n = 39 for each group). The results showed that cancer patients valued more the health status associated with toxicity than did the general public participants.13 The design of the second study, by Havrilesky and colleagues, was similar to that of the present study, and they compared toxic events one by one, using VAS and TTO in 13 ovarian cancer patients and 37 women of the general public.14 The investigators found the same results as we did on the parallel of the 2 groups and also a very similar order of the toxic events. Indeed, alopecia was the less bothersome, whereas both motor neuropathy and severe vomiting were among the worst toxic events. Therefore, our results correlate perfectly with theirs. Best and colleagues found that health states values associated with oxaliplatin-related peripheral neuropathy were lower in the general public population compared with those of cancer patients.15 Besides adaptive behavior of the patients, all these results may be explained by an established awareness cancer patients have of the dual outcome of cancer treatments (AEs and benefits from the treatment).9 This awareness is absent in the general public participants, who can only envision the negative outcomes and who do not realize the importance of the benefits.9 Findings from previous studies conducted in several tumor types such as breast cancer16-18 non–small-cell lung cancer,19 thyroid cancer,20 and renal cancer21 have shown that patients are willing to trade-off AEs for treatment benefits.
Alopecia has been considered as one of the most distressing and troublesome AEs of cancer therapy.22 However, in the present study, alopecia was rated inside the range of mild toxic events as it is in the study by Havrilesky and colleagues.14 Our results show that alopecia was placed as the first less damaging toxic event when assessed with the EQ-5D-5L, the second less damaging when assessed with a rank-order system, and the third less damaging when assessed the VAS. This could be related to current fashion trends that promote shaving one’s hair, which minimizes the social stigma of alopecia and its association with cancer treatment.
The other esthetic event we analyzed was acneiform rash associated with anti-EGFR agents. Our results show that severe rash was rated as clearly worse than alopecia by the 2 populations, irrespective of the measuring instrument (VAS, EQ-5D-5L, or ordinal assessment). To our knowledge, the present study is the first to demonstrate the relative impact of total alopecia and severe rash on patient QoL. This result is even more significant considering that we included grade 2 acneiform rash inside the Severe Rash toxic event. Our results show that the worst AEs for both populations were severe vomiting and neurotoxicity with functional impairment. The high impact of severe vomiting, the quintessentially chemotherapy-induced AE, was to be expected because it is strongly supported by a number of previous reports,14,23 as is also true for peripheral motor neuropathy.14,15,24
EQ-5D is a powerful instrument for measuring health status25 and is widely used to describe and evaluate patient health.26 Our results from the 5 dimensions represented by a single score were well correlated with the results of the VAS. However, whereas median VAS scores were evenly distributed in the 0-100 range of the VAS, median EQ-5D-5L scores were distributed mainly in the 0.5-1.0 range (full range, -0.654-1.000, for Spanish population).
The final single score of the original EQ-5D is based on responses from the general public, and we have shown that its use is a valid option when the objective is the evaluation of AEs in patients with cancer. Management of AEs is of the utmost importance in this era of personalized cancer medicine. Basch and colleagues recently reported that an intensive web-based follow-up of AEs during chemotherapy improved overall survival compared with standard follow-up.27 The results of our study show that patients have strongly defined preferences regarding AEs. Therefore, therapeutic strategies with a personalized approach in managing AEs would be associated with increased effectiveness.
There are some limitations in the present study. First, we modified slightly the EQ-5D-5L questionnaire by asking patients to recall and rate the days they experienced the adverse event instead of asking for “today’s feelings.” It is not known how this modification affects internal validity of this study. Second, we asked patients to isolate the toxic event to rate it independently from the other toxic events. We believe that this request may have been difficult for some patients to do because they might have experienced more than 1 toxic event concurrently. Third, using VAS to assess health status may be a weakness because it has been considered to be too straightforward an instrument. Likewise, there are some strengths of the study: it was performed in a face-to-face manner; it displayed cardinal and ordinal results for participants in the public group; and the results are the same as those in a previous study.14
In conclusion, patients with cancer who have experienced AEs perceive a lower impact on their QoL compared with that envisioned by participants from the general public. The EQ-5D-5L is a useful tool for evaluating cancer-therapy–related AEs. The impact of alopecia on QoL was notably low and even lower than that of severe rash. Further investigation on this issue should focus on patients’ and oncologists’ shared choices, which increasingly will be driven by patient preferences.
The Oncologic Association Dr Amadeu Pelegrí (AODAP), a charitable organization led by cancer patients and based in Salou, Spain, provided the financial support needed to conduct this study (www.aodapelegri.com).
Adverse events (AEs) from systemic treatment of cancer have a negative impact on patient quality of life (QoL). The extent of this impact is difficult to ascertain, particularly in patients undergoing palliative treatment because of variations in QoL resulting from antitumor effect.1 Patient-reported outcomes (PROs) are the best tool for elicitation of patient preferences, therefore helping cancer patients, oncologists, and health care managers to make better choices. Indeed, analysis of self-reported QoL during cancer chemotherapy provides new insights that are missed by other efficacy outcomes,2 although patient-reported AEs correlate well with AEs reported by clinicians.3 Self-reported symptoms provide better control during cancer treatment.4 However, there are other instruments to measure the impact of treatments on QoL that are based on preferences of members of the general public. Use of that strategy has been strongly debated. The most obvious problem is the difficulty that persons from the general public may have in putting themselves in the patient position.5 In addition, there is evidence that compared with the general public, patients adapt to their illness5,6 and then tend to downplay severity when rating values of health states.7 Therefore, a systematic discrepancy is observed between actual patients and the general public. It is not clear if it reflects the inability of members of the general public to fully grasp the relative severity of health problems or to the adaptation process of patients. This fact may obscure a negative impact on QoL which, in turn, could be detected using the general public as a surrogate. A combination of both approaches has been recommended for rating QoL when the ultimate purpose is making decisions on resource allocation.5 This debate is prolonging in time and it is far from over.8,9
Based on this background, this study investigates the impact of AEs on QoL of cancer patients from the perspective of cancer patients who had experienced the AEs of interest (ex post population) and the perspective of members of the general public. The second group comprised participants imagining themselves as hypothetical cancer patients experiencing the AEs (ex ante population). Previous studies with this dual approach allowing for comparisons between these two populations are small or centered on a few AEs.10 Therefore, a large and comprehensive study on the impact of AEs on QoL is lacking. Supported by previous literature, the investigational hypothesis was that ex post impact would be significantly lower than that imagined in an ex ante setting. The secondary objective is to study the potential use of the EuroQol (EQ-5D) instrument for health-related QoL in the measurement of the impact of AEs in cancer patients. This generic instrument is based on interviews with members of the general public. We tried to investigate to what extent those values relate to the cancer patients’ evaluation of their own health during treatment. The ultimate goal of the study is to assist in increasing the utility that patients derive from the benefits associated with cancer treatment.
Methods
Selection of AEs
Five AEs related to systemic treatment of cancer – alopecia, acneiform rash, oxaliplatin-associated peripheral neuropathy, diarrhea, and vomiting – were selected for the study. Investigators set up different relevant cut-off points for severity, resulting in 10 toxic events that were ad hoc defined as the variables for the study (Table 1). We used the Common Terminology Criteria for Adverse Events (CTCAE, version 4) to classify alopecia, acneiform rash, diarrhea, and vomiting. For oxaliplatin-associated peripheral neuropathy, we adapted Misset’s oxaliplatin-specific scale11 (range, grade 1-4; Table 1) in which grade 1 (neurotoxicity [NTX] 1) = paresthesias only with cold lasting a few days; grade 2 [NTX2] = paresthesias with and without cold that may last months; and grade 4 [NTX3] = paresthesias with functional consequence).
Participants
Two populations were included in the study: cancer patients who had experienced a particular AE and received treatment at the medical oncology departments of Hospital Santa Tecla and Hospital del Vendrell in Tarragona, Spain; and participants from the general public who received care at the Primary Health Care Center-Llevant in the same city.
Cancer patients. These participants had to be 18 years or older and had to have experienced 1 of the 10 toxic events in the 5 years before inclusion in the study; the treatment setting could be either curative attempt (adjuvant, neoadjuvant) or palliative, and patients with ongoing treatment should have received almost 3 months of treatment. Patients were excluded if they had an ECOG PS grade of 3 or more (Eastern Cooperative Oncology Group Performance Status; range, 0-5, where 0 = fully active, 3 = capable of limited self-care; confined to bed or chair more than 50% of waking hours, and 5 = dead). A particular patient with cancer could be included because of more than 1 study toxic event (eg, alopecia and severe vomiting or NTX1 and NTX2) but had to complete separate questionnaires for the different toxic events.
General public group. Participants in this group were selected from the records of general practitioner consultations at the aforementioned primary health care center. They had to be 18 years or older and could not have a history of cancer or symptomatic/severe chronic diseases (eg, they could have hypertension or diabetes without chronic target organ involvement, or they could be patients with either acute nonserious illness or nonserious injuries).
Survey procedures
Cancer patients. Participants in this group filled in 2 questionnaires provided by a medical oncologist in a face-to-face interview: the 5-dimension, 5-level EuroQol (EQ-5D-5L) questionnaire in reference to the days when patients were suffering the toxic event; and a visual analog scale (VAS) answering the question: How do you feel that this AE has impacted on your QoL the days you have experienced it?
VAS scores ranged from 0 (the poorest QoL, the highest impact) to 100 (the better QoL, the lower impact). The EQ-5D-5L has 5 dimensions (Mobility, Self-care, Daily life activities/social performance, Discomfort/pain, and Anxiety/depression) with 5 level response options each (No problem at all, Light problem, Moderate problem, Severe problem, Extreme problem/unable).12 The combination of 5 answers is converted to a single score, which is different for different countries; in the validated version for Spanish population, the score ranges from -0.654 (the worst health state) to 1.000 (the best health state). Patients were asked to make an effort to separate and encapsulate the impact of every adverse event and separate it from others they may have experienced during the same period. Table 2 summarizes survey procedures.
General public group. Two internal medicine residents who administered the questionnaire to the participants of the public group were well trained to carefully explain what each of the 10 the toxic events meant. Some details on these explanations are shown in Table 1, and the full set of explanations is shown in Appendix 1 (online only). Participants in the general public group were asked in a face-to-face interview to imagine they were cancer patients and envision how these toxic events would impact on their QoL if they were undergoing systemic treatment of cancer. They were asked to rate the imagined impact with the VAS (1 VAS/every toxic event = 10 VAS/participant). Then, they were presented with 10 cards, each with the name of 1 of the 10 toxic events (Appendix 2 [online only]), to show them the order of the impact on QoL based on their scores (respecting ties). The participants were asked if they agreed with the order, and if they did not, they were invited to change the scores. Therefore, results in the general public group also show the rank-order of the study toxic events.
Statistical analysis
We calculated the sample size as follows:
Primary outcomes were VAS score in cancer patients and VAS score in the general public. Primary analyses were comparison between VAS in both populations. Secondary outcomes were EQ-5D-5L score in cancer patients, and intra-participant rank-order in the general public group. Secondary analyses were correlation between VAS score and EQ-5D-5L in cancer patients and descriptive analysis of rank-ordered data in the general public group.
It was planned to compare means of quantitative variables with the Mann-Whitney U test and to assess correlation between quantitative variables with the Spearman rho test. All tests for contrast were nonparametric because a normal distribution was not expected from quoted scores with some ceiling or floor effect. A hierarchical generalized cluster analysis was planned to study clusters of variables grouped by VAS score in the public group.
Ethics
The study was conducted in accordance with the Declaration of Helsinki version Fortaleza 2013 and was approved by the institutional review board of the participant institutions. All of the patients provided written informed consent before study entry. Data of the participants of the general public were anonymous, so those participants were asked to provide only oral assent, with the permission of the review board.
Results
Between December 1, 2013 and January 31, 2015, a total of 250 participants of the general public and 139 cancer patients were included in the study. Four participants of the general public had incompletely filled the questionnaire and were excluded from the study, resulting in 246 participants with complete data available. There were no losses in the patient group, of whom 79 (57%) were currently on treatment and 118 (85%) had received the treatment in the previous 2 years. The total number of study toxic events in the 139 cancer patients was 200 (20 by each of the 10 study toxicity variables). Of those, 42 patients (30%) experienced (and were included in the study for) more than 1 toxic event.
Of the 139 patients, 91 (65%) received the treatment with curative intent. The most frequent diagnosis was colorectal cancer in 77 patients (55%), followed by breast cancer (13 patients, 9.4%), and lung cancer (11 patients, 7.9%). Systemic treatment of cancer was one of these options: chemotherapy alone, anti-EGFR [epidermal growth factor receptor] alone, chemotherapy plus anti-EGFR, or chemotherapy plus other biologics. The chemotherapy regimen most frequently administered was mFOLFOX6 [modified leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin] which, alone or in combination, was administered to 51 patients (37%). An anti-EGFR agent was administered to 22 patients (16%): cetuximab (15 patients), panitumumab (4 patients), erlotinib (2 patients) and afatinib (1 patient). The baseline characteristics of the patients and participants in the study are shown in Table 3.
For all 10 toxicity outcomes, the mean VAS score from the general public was numerically lower (lower QoL, more impact) than that resulted from the cancer patients who had actually experienced the toxic event of interest (Table 4 and Figure).
Taking off 2 mild effects (NTX1 and mild rash), for the 8 remaining toxic events, this difference was statistically significant (Mann-Whitney U test; P < .01 for severe vomiting, severe diarrhea, and alopecia; P < .001 for NTX2, NTX3 and mild diarrhea; P = .03 for severe rash; P = .04 for mild vomiting). Severe vomiting resulted in the worst VAS score for cancer patients (median VAS, 34) and NTX3 had the worst VAS score for the general public participants (median, VAS 19). Table 4 summarizes the 4 sets of results (patient and public VAS, and patient EQ-5D-5L and public rank-order). Regarding the results of the esthetic toxicities compared with each other, impact from severe rash was considered higher than that from alopecia for both populations, patients (mean VAS, 59 [rash] vs 77 [alopecia]; mean EQ-5D-5L score, 0.725/rank order 4 vs 0.921/10) and the general public (mean VAS, 47 vs 55; EQ-5D-5L, rank order 5 vs 9). In the group of patients, linear correlation between VAS and EQ-5D-5L score was assessed resulting in a significant positive correlation (Spearman P = .001) with a correlation coefficient Rho 0.681 (Appendix 3 [online only]). Also, a positive linear correlation was observed between the 10 means of the cancer patients’ VAS and the 10 means of the general public participants’ VAS (Spearman P = .001; coefficient Rho 0.879). Both ceiling and floor effect were observed for VAS in the 2 populations, but only ceiling effect for EQ-5D-5L in the patient population. The most important floor effect was for NTX3, with 66 participants (27%) of the general public group scoring VAS 0 (see Appendix 4 [online only] for the frequencies of answers for every level of the 5 dimensions of the EQ-5D-5L). An analysis of the results, considered as an intraparticipant rank-ordered evaluation, was performed in the general public group. Fourteen participants of that group (5.7%) changed their scores after they were presented with the order shown in cards. Mode of the ranks show that NTX3 and severe vomiting were the worst-scored toxic events. The most frequent rank-order for alopecia and severe rash were (from best to worst) the second and the fourth, respectively.
Discussion
The findings in this study show that impact on QoL imagined by members of the general public is higher than that declared by cancer patients who have experienced the AEs. It is worth noting that that result was observed for all 10 toxic events, thus confirming the investigational hypothesis of the study. However, the graph shows a strong parallel between the 2 groups, which means that both populations similarly perceive upward and downward variations in the impact resulting from the different toxic events (Figure).
Three previous studies have addressed the comparison of the impact of different AEs in these 2 populations and findings from all 3 showed the same systematic difference between patients and the general public participants. The first, Calhoun and colleagues used time to trade-off (TTO, a measure of the QoL a person or groups is experiencing) to compare therapies for ovarian cancer in patients and the general population (n = 39 for each group). The results showed that cancer patients valued more the health status associated with toxicity than did the general public participants.13 The design of the second study, by Havrilesky and colleagues, was similar to that of the present study, and they compared toxic events one by one, using VAS and TTO in 13 ovarian cancer patients and 37 women of the general public.14 The investigators found the same results as we did on the parallel of the 2 groups and also a very similar order of the toxic events. Indeed, alopecia was the less bothersome, whereas both motor neuropathy and severe vomiting were among the worst toxic events. Therefore, our results correlate perfectly with theirs. Best and colleagues found that health states values associated with oxaliplatin-related peripheral neuropathy were lower in the general public population compared with those of cancer patients.15 Besides adaptive behavior of the patients, all these results may be explained by an established awareness cancer patients have of the dual outcome of cancer treatments (AEs and benefits from the treatment).9 This awareness is absent in the general public participants, who can only envision the negative outcomes and who do not realize the importance of the benefits.9 Findings from previous studies conducted in several tumor types such as breast cancer16-18 non–small-cell lung cancer,19 thyroid cancer,20 and renal cancer21 have shown that patients are willing to trade-off AEs for treatment benefits.
Alopecia has been considered as one of the most distressing and troublesome AEs of cancer therapy.22 However, in the present study, alopecia was rated inside the range of mild toxic events as it is in the study by Havrilesky and colleagues.14 Our results show that alopecia was placed as the first less damaging toxic event when assessed with the EQ-5D-5L, the second less damaging when assessed with a rank-order system, and the third less damaging when assessed the VAS. This could be related to current fashion trends that promote shaving one’s hair, which minimizes the social stigma of alopecia and its association with cancer treatment.
The other esthetic event we analyzed was acneiform rash associated with anti-EGFR agents. Our results show that severe rash was rated as clearly worse than alopecia by the 2 populations, irrespective of the measuring instrument (VAS, EQ-5D-5L, or ordinal assessment). To our knowledge, the present study is the first to demonstrate the relative impact of total alopecia and severe rash on patient QoL. This result is even more significant considering that we included grade 2 acneiform rash inside the Severe Rash toxic event. Our results show that the worst AEs for both populations were severe vomiting and neurotoxicity with functional impairment. The high impact of severe vomiting, the quintessentially chemotherapy-induced AE, was to be expected because it is strongly supported by a number of previous reports,14,23 as is also true for peripheral motor neuropathy.14,15,24
EQ-5D is a powerful instrument for measuring health status25 and is widely used to describe and evaluate patient health.26 Our results from the 5 dimensions represented by a single score were well correlated with the results of the VAS. However, whereas median VAS scores were evenly distributed in the 0-100 range of the VAS, median EQ-5D-5L scores were distributed mainly in the 0.5-1.0 range (full range, -0.654-1.000, for Spanish population).
The final single score of the original EQ-5D is based on responses from the general public, and we have shown that its use is a valid option when the objective is the evaluation of AEs in patients with cancer. Management of AEs is of the utmost importance in this era of personalized cancer medicine. Basch and colleagues recently reported that an intensive web-based follow-up of AEs during chemotherapy improved overall survival compared with standard follow-up.27 The results of our study show that patients have strongly defined preferences regarding AEs. Therefore, therapeutic strategies with a personalized approach in managing AEs would be associated with increased effectiveness.
There are some limitations in the present study. First, we modified slightly the EQ-5D-5L questionnaire by asking patients to recall and rate the days they experienced the adverse event instead of asking for “today’s feelings.” It is not known how this modification affects internal validity of this study. Second, we asked patients to isolate the toxic event to rate it independently from the other toxic events. We believe that this request may have been difficult for some patients to do because they might have experienced more than 1 toxic event concurrently. Third, using VAS to assess health status may be a weakness because it has been considered to be too straightforward an instrument. Likewise, there are some strengths of the study: it was performed in a face-to-face manner; it displayed cardinal and ordinal results for participants in the public group; and the results are the same as those in a previous study.14
In conclusion, patients with cancer who have experienced AEs perceive a lower impact on their QoL compared with that envisioned by participants from the general public. The EQ-5D-5L is a useful tool for evaluating cancer-therapy–related AEs. The impact of alopecia on QoL was notably low and even lower than that of severe rash. Further investigation on this issue should focus on patients’ and oncologists’ shared choices, which increasingly will be driven by patient preferences.
The Oncologic Association Dr Amadeu Pelegrí (AODAP), a charitable organization led by cancer patients and based in Salou, Spain, provided the financial support needed to conduct this study (www.aodapelegri.com).
1. Mazzotti E, Antonini Cappellini GC, Buconovo S, et al. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer. 2012;20(10):2553-2557.
2. Gunnars B, Nygren P, Glimelius B, SBU-group. Swedish Council of Technology Assessment in Health Care. Assessment of quality of life during chemotherapy. Acta Oncol. 2001;40(2-3):175-184.
3. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051-1059.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Menzel P, Dolan P, Richardson J, Olsen JA. The role of adaptation to disability and disease in health state valuation: a preliminary normative analysis. Soc Sci Med. 2002;55(12):2149-2158.
6. McTaggart-Cowan H, Tsuchiya A, O’Cathain A, Brazier J. Understanding the effect of disease adaptation information on general population values for hypothetical health states. Soc Sci Med. 2011;72(11): 1904-1912.
7. Ubel PA, Loewenstein G, Schwarz N, Smith D. (2005). Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005;24(4 Suppl):S57-62.
8. Brazier J, Akehurst R, Brennan A, et al. Should patients have a greater role in valuing health states? Appl Health Econ Health Policy. 2005;4(4):201-208.
9. Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Qual Life Res. 2003;12(6), 599-607.
10. Shabaruddin FH, Chen LC, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics. 2013;31(4):277-288.
11. Misset JL. Oxaliplatin in practice. Br J Cancer. 1998;77 Suppl 4:4-7.
12. EQ-5D website. About the EQ-5D-5L. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Last updated April 18, 2017. Accessed October 18, 2017.
13. Calhoun EA, Fishman DA, Lurain JR, Welshman EE, Bennett CL. A comparison of ovarian cancer treatments: analysis of utility assessments of ovarian cancer patients, at-risk population, general population, and physicians. Gynecol Oncol. 2004;93(1):164-169.
14. Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216-220.
15. Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preferences values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391-400.
16. Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C. Patient preferences for chemotherapies used in breast cancer. Int J Womens Health. 2012;4:279-287.
17. Beusterien K, Grinspan J, Kuchuk I, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. The Oncologist 2014;19(2):127-134.
18. Kuchuk I, Bouganim N, Beusterien K, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. 2013;142(1):101-107.
19. Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77(1):224-231.
20. Mohamed AF, González JM, Fairchild A. Patient benefit-risk tradeoffs for radioactive Iodine-refractory differentiated thyroid cancer treatments. J Thyroid Res. 2015:438235.
21. Wong MK, Mohamed AF, Hauber AB, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ. 2012;15(6):1139-1148.
22. Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17(4):317-328.
23. Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother. 2013;14(6):757-766.
24. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
25. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343.
26. Greiner W, Weijnen T, Nieuwenhuizen M, et al. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ.2003; 4(3):222-231.
27. Basch E, Deal AM, Dueck AC, et al. Survival results of a trial assessing patient-reported outcomes for symprom monitoring during routine cancer treatment. JAMA 2017. doi:10.1001/jama.2017.7156
1. Mazzotti E, Antonini Cappellini GC, Buconovo S, et al. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer. 2012;20(10):2553-2557.
2. Gunnars B, Nygren P, Glimelius B, SBU-group. Swedish Council of Technology Assessment in Health Care. Assessment of quality of life during chemotherapy. Acta Oncol. 2001;40(2-3):175-184.
3. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051-1059.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Menzel P, Dolan P, Richardson J, Olsen JA. The role of adaptation to disability and disease in health state valuation: a preliminary normative analysis. Soc Sci Med. 2002;55(12):2149-2158.
6. McTaggart-Cowan H, Tsuchiya A, O’Cathain A, Brazier J. Understanding the effect of disease adaptation information on general population values for hypothetical health states. Soc Sci Med. 2011;72(11): 1904-1912.
7. Ubel PA, Loewenstein G, Schwarz N, Smith D. (2005). Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005;24(4 Suppl):S57-62.
8. Brazier J, Akehurst R, Brennan A, et al. Should patients have a greater role in valuing health states? Appl Health Econ Health Policy. 2005;4(4):201-208.
9. Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Qual Life Res. 2003;12(6), 599-607.
10. Shabaruddin FH, Chen LC, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics. 2013;31(4):277-288.
11. Misset JL. Oxaliplatin in practice. Br J Cancer. 1998;77 Suppl 4:4-7.
12. EQ-5D website. About the EQ-5D-5L. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Last updated April 18, 2017. Accessed October 18, 2017.
13. Calhoun EA, Fishman DA, Lurain JR, Welshman EE, Bennett CL. A comparison of ovarian cancer treatments: analysis of utility assessments of ovarian cancer patients, at-risk population, general population, and physicians. Gynecol Oncol. 2004;93(1):164-169.
14. Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216-220.
15. Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preferences values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391-400.
16. Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C. Patient preferences for chemotherapies used in breast cancer. Int J Womens Health. 2012;4:279-287.
17. Beusterien K, Grinspan J, Kuchuk I, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. The Oncologist 2014;19(2):127-134.
18. Kuchuk I, Bouganim N, Beusterien K, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. 2013;142(1):101-107.
19. Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77(1):224-231.
20. Mohamed AF, González JM, Fairchild A. Patient benefit-risk tradeoffs for radioactive Iodine-refractory differentiated thyroid cancer treatments. J Thyroid Res. 2015:438235.
21. Wong MK, Mohamed AF, Hauber AB, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ. 2012;15(6):1139-1148.
22. Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17(4):317-328.
23. Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother. 2013;14(6):757-766.
24. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
25. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343.
26. Greiner W, Weijnen T, Nieuwenhuizen M, et al. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ.2003; 4(3):222-231.
27. Basch E, Deal AM, Dueck AC, et al. Survival results of a trial assessing patient-reported outcomes for symprom monitoring during routine cancer treatment. JAMA 2017. doi:10.1001/jama.2017.7156
Familial essential thrombocythemia associated with JAK2 V617F mutation in siblings
Three myeloproliferative neoplasms (MPN), polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), are associated with an abnormal somatic mutation of the JAK2 gene. Essential thrombocythemia is considered when there is a persistent increase in the peripheral blood platelet count, associated with a proliferation of atypical megakaryocytes in the bone marrow. The manifestations of PV, ET, and PMF all typically occur within the sixth or seventh decade of life. A patient may present with an abnormal blood count but be asymptomatic at the time. Over the course and progression of the disease, increases in hematocrit or platelet counts along with symptoms such as headaches, blurred vision, and plethora may occur.1 The JAK2 V617F mutation is responsible for the production of the JAK2 protein, which is continuously activated, promoting the growth and division of cells such as erythrocytes, granulocytes, and platelets. It has been reported that there is a nearly 100% incidence of the JAK2 mutation in patients with polycythemia vera, and a 50% incidence in patients with essential thrombocythemia and primary myelofibrosis.2
The discovery of the JAK2 mutation in PV, ET, and PMF was an important advancement in helping distinguish these disorders from other MPNs, including chronic myelogenous leukemia, but its presence does not explain why some individuals develop ET and others, PV or PMF.3 Although there have been familial cases proven of ET, the somatic JAK2 mutation is acquired and not inherited. In this report, we describe the unusual circumstance of JAK2 V617F mutation in a brother and a sister who were both diagnosed with essential thrombocythemia.
Case presentations and summaries
RS, a 69-year-old white man, was referred to our service in 2006 for continued care of previously diagnosed essential thrombocythemia. At the time of his initial visit to our clinic, his complete blood count was normal, the platelet count being adequately controlled by anagrelide at a daily dose of 4.0 mg. He complained of palpitations and peripheral neuropathy. A bone marrow biopsy was performed, revealing moderate hypercellularity, atypical megakaryocytosis, and a negative BCR-ABL mutation but a positive JAK2 V617F mutation. The patient is now treated with hydroxyurea 1,000 mg daily in divided doses, which better controls his counts and does not have the side effects of anagrelide.
SW, a 73-year-old woman, and brother of RS (they share the same biological mother and father), was noted to have a mild thrombocytosis in 2008. In 2013, her platelet count rose to 865,000 cells/uL (normal, 150,000-450,000 cells/uL, age and sex adjusted) and she was referred to our clinic. A bone marrow biopsy was performed, revealing borderline hypercellularity with atypical megakaryocytosis and the presence of a JAK2 V617F mutation. As with her brother, the BCR-ABL mutation was not present. She has also responded to treatment with hydroxyurea, but at a reduced dosage of 500 mg daily.
A third sibling, AS, again of the same biological mother and father, had died of multiple veno-occlusive cerebral vascular events long before the diagnoses on his younger siblings had been made. The suggestion of any underling hematologic pathology would be interesting, but speculative. Nothing is known abou
Discussion
Much research has been done to understand the pathogenesis of and find a cure for myeloproliferative disorders, but despite some progress, a cure remains elusive. However, there have been some advances that have contributed to partial cures for MPNs. One of the major breakthroughs in MPN research, about 50 years ago, was related to the “sporadic vs familial debate” around the Philadelphia chromosome.4 It led to the discovery of the reciprocal translocation between chromosomes 9 and 22, known as the BCR-ABL mutation, which is found in many CML patients. This discovery allowed researchers to focus their attention on other tyrosine kinase domains, such as the JAK2 V617F mutation, which is presented in the three other MPNs; PV, ET, and PMF. Both the JAK2 V617F and BCR-ABL mutations are active in signaling transcription, more commonly growth of cells.4
Since the discovery of the JAK2 V617F mutation in early 2005, it has become a leading diagnostic criteria for myeloproliferative diseases. The presence of the JAK2 V617F mutation and the measurement of its allele burden can be assessed by examination of either peripheral blood or bone marrow samples.5
The JAK2 V617F mutation is a result of a single change in the DNA nucleotide base pair that causes a substitution of a valine amino acid for a phenylalanine amino acid at the 617 position on exon 14 within the JAK2 kinase regulatory domain. This point mutation disrupts the regular control of the JAK2 by removing its ability to turn off, leading to uncontrolled blood cell growth.6 When the JAK2 V617F mutation cannot be demonstrated in a patient with the hallmarks of an MPN, the detection of other JAK2 and MPL proto-oncogene, thrombopoietin receptor mutations may be used as a diagnostic procedure for other MPNs.7
Other mutations incorporated in JAK2 domain can be detected in the coding portions of the DNA known as exons. One such mutation is the JAK2 exon 12, which is involved in JAK2 V617F-negative PV patients. This mutation is not detected in patients with ET or PMF and is 2%-5% present in patients with PV. There are other somatic mutations in the thrombopoietin receptors that work in accordance with thrombopoietin: MPL W515L and MPL W515K, which are found at chromosome 1p34, are identified in about 5% of PMF and 1% of ET patients, but are not present in PV patients.8.9
Pikman and colleagues reported in 2009 that the JAK2 V617F mutation is not acquired randomly.9 Their findings showed that, only in white populations, does the JAK2 V617F mutation arise preferentially on a specific constitutional JAK2 46/1 haplotype. According to the authors, the preconceived notion a of randomly acquired JAK2 V617F mutation does not account for familial MPN’s. Familial MPNs are thought to be produced by sporadic and extremely penetrant substitutions in genes that still are not identified and the 46/1 haplotype does not explain for the phenotypic diversity correlated with the JAK2 V617F gene. The 46/1 haplotype, however, correlates more frequently with different MPN subtypes. There are two hypotheses that try to explain how an acquired mutation as prevailing as the JAK2 V617F mutation can be associated with certain inherited backgrounds. The first hypothesis asserts the V617F accumulates at a faster rate than other genes because of the fundamentally unstable genetics of the 46/1 haplotype. The second theory is that all the mutated genes, including the V617F, arise at equal rates, but 46/1 may grant a selective advantage to the V617F-positive clone or interacts in some way to increase the likelihood of abnormal blood counts. A study that examined both these hypotheses concluded that the 46/1 haplotype was present more frequently in patients with myeloproliferative disorders than in their control groups and even more so in cases that were proven to be V617F-positive.10
There are very few cases that have reported familial MPN’s, especially as the pedigrees of the familial MPN’s illustrate that inheritance patterns are notably heterogeneous, indicating that there may be a range of different germline mutations driving the susceptibility. With recent data, the JAK2 V617F mutation in tandem with MPL W515L/K and inactivating TET2 mutations still continue to be the most frequently acquired mutations involved in both familial and sporadic MPN. As far as we know, there have been no cases to prove that JAK2 V617F and MPL W515L/K mutations are inherited through the germline, but there are other alleles that may pass through the germline that can be associated with hereditary thrombocytosis.11 Further cytogenetic studies will clarify the pathogenesis of these disorders and possibly lead to effective targeted therapies.
1. Murphy S, Peterson P, Iland H, Laszio J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol. 1997;34:29-39.
2. Zhan H, Spivak JL. The diagnosis and management of polycythemia vera, essential thrombocythemia, and primary myelofibrosis in the JAK2 V617F era. Clin Adv Hematol Oncol. 2009;7:334-342.
3. Higgs JR, Sadek I, Neumann PE, et al. Familial essential thrombocythemia with spontaneous megakaryocyte colony formation and acquired JAK2 mutations. Leukemia. 2008;22:1551-1556.
4. Senyak Z. Eileen Wiggins – out of the blue. http://www.mpnresearchfoundation.org/White-Paper-3A-Nature-2C-Nurture-2C-or-Both-3F. Published October 2010. Accessed May 23, 2017.
5. Cankovic M, Whiteley L, Hawley RC, Zarbo RJ, Chitale D. Clinical performance of JAK2 V617F mutation detection assays in a molecular diagnostics laboratory: evaluation of screening and quantitation methods. Am J Clin Pathol. 2009;132:713-721.
6. Kralovics R, Teo SS, Li S, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108:1377-1380.
7. James C. The JAK2V617F mutation in polycythemia vera and other myeloproliferative disorders: one mutation for three diseases? Hematology Am Soc Hematol Educ Program. 2008:69-75.
8. Pancrazzi A, Guglielmelli P, Ponziani V, et al. A sensitive detection method for MPLW515L or MPLW515K mutation in chronic myeloproliferative disorders with locked nucleic acid-modified probes and real-time polymerase chain reaction. J Mol Diagn. 2008;10:435-441.
9. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:1140-1151.
10. Jones AV, Campbell PJ, Beer PA, et al. The JAK2 46/1 haplotype predisposes to MPL-mutated myeloproliferative neoplasms. Blood. 2010;115:4517-4523.
11. Jones AV, Cross NCP. Inherited predisposition to myeloproliferative neoplasms. Ther Adv Hematol. 2013;4:237-253.
Three myeloproliferative neoplasms (MPN), polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), are associated with an abnormal somatic mutation of the JAK2 gene. Essential thrombocythemia is considered when there is a persistent increase in the peripheral blood platelet count, associated with a proliferation of atypical megakaryocytes in the bone marrow. The manifestations of PV, ET, and PMF all typically occur within the sixth or seventh decade of life. A patient may present with an abnormal blood count but be asymptomatic at the time. Over the course and progression of the disease, increases in hematocrit or platelet counts along with symptoms such as headaches, blurred vision, and plethora may occur.1 The JAK2 V617F mutation is responsible for the production of the JAK2 protein, which is continuously activated, promoting the growth and division of cells such as erythrocytes, granulocytes, and platelets. It has been reported that there is a nearly 100% incidence of the JAK2 mutation in patients with polycythemia vera, and a 50% incidence in patients with essential thrombocythemia and primary myelofibrosis.2
The discovery of the JAK2 mutation in PV, ET, and PMF was an important advancement in helping distinguish these disorders from other MPNs, including chronic myelogenous leukemia, but its presence does not explain why some individuals develop ET and others, PV or PMF.3 Although there have been familial cases proven of ET, the somatic JAK2 mutation is acquired and not inherited. In this report, we describe the unusual circumstance of JAK2 V617F mutation in a brother and a sister who were both diagnosed with essential thrombocythemia.
Case presentations and summaries
RS, a 69-year-old white man, was referred to our service in 2006 for continued care of previously diagnosed essential thrombocythemia. At the time of his initial visit to our clinic, his complete blood count was normal, the platelet count being adequately controlled by anagrelide at a daily dose of 4.0 mg. He complained of palpitations and peripheral neuropathy. A bone marrow biopsy was performed, revealing moderate hypercellularity, atypical megakaryocytosis, and a negative BCR-ABL mutation but a positive JAK2 V617F mutation. The patient is now treated with hydroxyurea 1,000 mg daily in divided doses, which better controls his counts and does not have the side effects of anagrelide.
SW, a 73-year-old woman, and brother of RS (they share the same biological mother and father), was noted to have a mild thrombocytosis in 2008. In 2013, her platelet count rose to 865,000 cells/uL (normal, 150,000-450,000 cells/uL, age and sex adjusted) and she was referred to our clinic. A bone marrow biopsy was performed, revealing borderline hypercellularity with atypical megakaryocytosis and the presence of a JAK2 V617F mutation. As with her brother, the BCR-ABL mutation was not present. She has also responded to treatment with hydroxyurea, but at a reduced dosage of 500 mg daily.
A third sibling, AS, again of the same biological mother and father, had died of multiple veno-occlusive cerebral vascular events long before the diagnoses on his younger siblings had been made. The suggestion of any underling hematologic pathology would be interesting, but speculative. Nothing is known abou
Discussion
Much research has been done to understand the pathogenesis of and find a cure for myeloproliferative disorders, but despite some progress, a cure remains elusive. However, there have been some advances that have contributed to partial cures for MPNs. One of the major breakthroughs in MPN research, about 50 years ago, was related to the “sporadic vs familial debate” around the Philadelphia chromosome.4 It led to the discovery of the reciprocal translocation between chromosomes 9 and 22, known as the BCR-ABL mutation, which is found in many CML patients. This discovery allowed researchers to focus their attention on other tyrosine kinase domains, such as the JAK2 V617F mutation, which is presented in the three other MPNs; PV, ET, and PMF. Both the JAK2 V617F and BCR-ABL mutations are active in signaling transcription, more commonly growth of cells.4
Since the discovery of the JAK2 V617F mutation in early 2005, it has become a leading diagnostic criteria for myeloproliferative diseases. The presence of the JAK2 V617F mutation and the measurement of its allele burden can be assessed by examination of either peripheral blood or bone marrow samples.5
The JAK2 V617F mutation is a result of a single change in the DNA nucleotide base pair that causes a substitution of a valine amino acid for a phenylalanine amino acid at the 617 position on exon 14 within the JAK2 kinase regulatory domain. This point mutation disrupts the regular control of the JAK2 by removing its ability to turn off, leading to uncontrolled blood cell growth.6 When the JAK2 V617F mutation cannot be demonstrated in a patient with the hallmarks of an MPN, the detection of other JAK2 and MPL proto-oncogene, thrombopoietin receptor mutations may be used as a diagnostic procedure for other MPNs.7
Other mutations incorporated in JAK2 domain can be detected in the coding portions of the DNA known as exons. One such mutation is the JAK2 exon 12, which is involved in JAK2 V617F-negative PV patients. This mutation is not detected in patients with ET or PMF and is 2%-5% present in patients with PV. There are other somatic mutations in the thrombopoietin receptors that work in accordance with thrombopoietin: MPL W515L and MPL W515K, which are found at chromosome 1p34, are identified in about 5% of PMF and 1% of ET patients, but are not present in PV patients.8.9
Pikman and colleagues reported in 2009 that the JAK2 V617F mutation is not acquired randomly.9 Their findings showed that, only in white populations, does the JAK2 V617F mutation arise preferentially on a specific constitutional JAK2 46/1 haplotype. According to the authors, the preconceived notion a of randomly acquired JAK2 V617F mutation does not account for familial MPN’s. Familial MPNs are thought to be produced by sporadic and extremely penetrant substitutions in genes that still are not identified and the 46/1 haplotype does not explain for the phenotypic diversity correlated with the JAK2 V617F gene. The 46/1 haplotype, however, correlates more frequently with different MPN subtypes. There are two hypotheses that try to explain how an acquired mutation as prevailing as the JAK2 V617F mutation can be associated with certain inherited backgrounds. The first hypothesis asserts the V617F accumulates at a faster rate than other genes because of the fundamentally unstable genetics of the 46/1 haplotype. The second theory is that all the mutated genes, including the V617F, arise at equal rates, but 46/1 may grant a selective advantage to the V617F-positive clone or interacts in some way to increase the likelihood of abnormal blood counts. A study that examined both these hypotheses concluded that the 46/1 haplotype was present more frequently in patients with myeloproliferative disorders than in their control groups and even more so in cases that were proven to be V617F-positive.10
There are very few cases that have reported familial MPN’s, especially as the pedigrees of the familial MPN’s illustrate that inheritance patterns are notably heterogeneous, indicating that there may be a range of different germline mutations driving the susceptibility. With recent data, the JAK2 V617F mutation in tandem with MPL W515L/K and inactivating TET2 mutations still continue to be the most frequently acquired mutations involved in both familial and sporadic MPN. As far as we know, there have been no cases to prove that JAK2 V617F and MPL W515L/K mutations are inherited through the germline, but there are other alleles that may pass through the germline that can be associated with hereditary thrombocytosis.11 Further cytogenetic studies will clarify the pathogenesis of these disorders and possibly lead to effective targeted therapies.
Three myeloproliferative neoplasms (MPN), polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), are associated with an abnormal somatic mutation of the JAK2 gene. Essential thrombocythemia is considered when there is a persistent increase in the peripheral blood platelet count, associated with a proliferation of atypical megakaryocytes in the bone marrow. The manifestations of PV, ET, and PMF all typically occur within the sixth or seventh decade of life. A patient may present with an abnormal blood count but be asymptomatic at the time. Over the course and progression of the disease, increases in hematocrit or platelet counts along with symptoms such as headaches, blurred vision, and plethora may occur.1 The JAK2 V617F mutation is responsible for the production of the JAK2 protein, which is continuously activated, promoting the growth and division of cells such as erythrocytes, granulocytes, and platelets. It has been reported that there is a nearly 100% incidence of the JAK2 mutation in patients with polycythemia vera, and a 50% incidence in patients with essential thrombocythemia and primary myelofibrosis.2
The discovery of the JAK2 mutation in PV, ET, and PMF was an important advancement in helping distinguish these disorders from other MPNs, including chronic myelogenous leukemia, but its presence does not explain why some individuals develop ET and others, PV or PMF.3 Although there have been familial cases proven of ET, the somatic JAK2 mutation is acquired and not inherited. In this report, we describe the unusual circumstance of JAK2 V617F mutation in a brother and a sister who were both diagnosed with essential thrombocythemia.
Case presentations and summaries
RS, a 69-year-old white man, was referred to our service in 2006 for continued care of previously diagnosed essential thrombocythemia. At the time of his initial visit to our clinic, his complete blood count was normal, the platelet count being adequately controlled by anagrelide at a daily dose of 4.0 mg. He complained of palpitations and peripheral neuropathy. A bone marrow biopsy was performed, revealing moderate hypercellularity, atypical megakaryocytosis, and a negative BCR-ABL mutation but a positive JAK2 V617F mutation. The patient is now treated with hydroxyurea 1,000 mg daily in divided doses, which better controls his counts and does not have the side effects of anagrelide.
SW, a 73-year-old woman, and brother of RS (they share the same biological mother and father), was noted to have a mild thrombocytosis in 2008. In 2013, her platelet count rose to 865,000 cells/uL (normal, 150,000-450,000 cells/uL, age and sex adjusted) and she was referred to our clinic. A bone marrow biopsy was performed, revealing borderline hypercellularity with atypical megakaryocytosis and the presence of a JAK2 V617F mutation. As with her brother, the BCR-ABL mutation was not present. She has also responded to treatment with hydroxyurea, but at a reduced dosage of 500 mg daily.
A third sibling, AS, again of the same biological mother and father, had died of multiple veno-occlusive cerebral vascular events long before the diagnoses on his younger siblings had been made. The suggestion of any underling hematologic pathology would be interesting, but speculative. Nothing is known abou
Discussion
Much research has been done to understand the pathogenesis of and find a cure for myeloproliferative disorders, but despite some progress, a cure remains elusive. However, there have been some advances that have contributed to partial cures for MPNs. One of the major breakthroughs in MPN research, about 50 years ago, was related to the “sporadic vs familial debate” around the Philadelphia chromosome.4 It led to the discovery of the reciprocal translocation between chromosomes 9 and 22, known as the BCR-ABL mutation, which is found in many CML patients. This discovery allowed researchers to focus their attention on other tyrosine kinase domains, such as the JAK2 V617F mutation, which is presented in the three other MPNs; PV, ET, and PMF. Both the JAK2 V617F and BCR-ABL mutations are active in signaling transcription, more commonly growth of cells.4
Since the discovery of the JAK2 V617F mutation in early 2005, it has become a leading diagnostic criteria for myeloproliferative diseases. The presence of the JAK2 V617F mutation and the measurement of its allele burden can be assessed by examination of either peripheral blood or bone marrow samples.5
The JAK2 V617F mutation is a result of a single change in the DNA nucleotide base pair that causes a substitution of a valine amino acid for a phenylalanine amino acid at the 617 position on exon 14 within the JAK2 kinase regulatory domain. This point mutation disrupts the regular control of the JAK2 by removing its ability to turn off, leading to uncontrolled blood cell growth.6 When the JAK2 V617F mutation cannot be demonstrated in a patient with the hallmarks of an MPN, the detection of other JAK2 and MPL proto-oncogene, thrombopoietin receptor mutations may be used as a diagnostic procedure for other MPNs.7
Other mutations incorporated in JAK2 domain can be detected in the coding portions of the DNA known as exons. One such mutation is the JAK2 exon 12, which is involved in JAK2 V617F-negative PV patients. This mutation is not detected in patients with ET or PMF and is 2%-5% present in patients with PV. There are other somatic mutations in the thrombopoietin receptors that work in accordance with thrombopoietin: MPL W515L and MPL W515K, which are found at chromosome 1p34, are identified in about 5% of PMF and 1% of ET patients, but are not present in PV patients.8.9
Pikman and colleagues reported in 2009 that the JAK2 V617F mutation is not acquired randomly.9 Their findings showed that, only in white populations, does the JAK2 V617F mutation arise preferentially on a specific constitutional JAK2 46/1 haplotype. According to the authors, the preconceived notion a of randomly acquired JAK2 V617F mutation does not account for familial MPN’s. Familial MPNs are thought to be produced by sporadic and extremely penetrant substitutions in genes that still are not identified and the 46/1 haplotype does not explain for the phenotypic diversity correlated with the JAK2 V617F gene. The 46/1 haplotype, however, correlates more frequently with different MPN subtypes. There are two hypotheses that try to explain how an acquired mutation as prevailing as the JAK2 V617F mutation can be associated with certain inherited backgrounds. The first hypothesis asserts the V617F accumulates at a faster rate than other genes because of the fundamentally unstable genetics of the 46/1 haplotype. The second theory is that all the mutated genes, including the V617F, arise at equal rates, but 46/1 may grant a selective advantage to the V617F-positive clone or interacts in some way to increase the likelihood of abnormal blood counts. A study that examined both these hypotheses concluded that the 46/1 haplotype was present more frequently in patients with myeloproliferative disorders than in their control groups and even more so in cases that were proven to be V617F-positive.10
There are very few cases that have reported familial MPN’s, especially as the pedigrees of the familial MPN’s illustrate that inheritance patterns are notably heterogeneous, indicating that there may be a range of different germline mutations driving the susceptibility. With recent data, the JAK2 V617F mutation in tandem with MPL W515L/K and inactivating TET2 mutations still continue to be the most frequently acquired mutations involved in both familial and sporadic MPN. As far as we know, there have been no cases to prove that JAK2 V617F and MPL W515L/K mutations are inherited through the germline, but there are other alleles that may pass through the germline that can be associated with hereditary thrombocytosis.11 Further cytogenetic studies will clarify the pathogenesis of these disorders and possibly lead to effective targeted therapies.
1. Murphy S, Peterson P, Iland H, Laszio J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol. 1997;34:29-39.
2. Zhan H, Spivak JL. The diagnosis and management of polycythemia vera, essential thrombocythemia, and primary myelofibrosis in the JAK2 V617F era. Clin Adv Hematol Oncol. 2009;7:334-342.
3. Higgs JR, Sadek I, Neumann PE, et al. Familial essential thrombocythemia with spontaneous megakaryocyte colony formation and acquired JAK2 mutations. Leukemia. 2008;22:1551-1556.
4. Senyak Z. Eileen Wiggins – out of the blue. http://www.mpnresearchfoundation.org/White-Paper-3A-Nature-2C-Nurture-2C-or-Both-3F. Published October 2010. Accessed May 23, 2017.
5. Cankovic M, Whiteley L, Hawley RC, Zarbo RJ, Chitale D. Clinical performance of JAK2 V617F mutation detection assays in a molecular diagnostics laboratory: evaluation of screening and quantitation methods. Am J Clin Pathol. 2009;132:713-721.
6. Kralovics R, Teo SS, Li S, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108:1377-1380.
7. James C. The JAK2V617F mutation in polycythemia vera and other myeloproliferative disorders: one mutation for three diseases? Hematology Am Soc Hematol Educ Program. 2008:69-75.
8. Pancrazzi A, Guglielmelli P, Ponziani V, et al. A sensitive detection method for MPLW515L or MPLW515K mutation in chronic myeloproliferative disorders with locked nucleic acid-modified probes and real-time polymerase chain reaction. J Mol Diagn. 2008;10:435-441.
9. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:1140-1151.
10. Jones AV, Campbell PJ, Beer PA, et al. The JAK2 46/1 haplotype predisposes to MPL-mutated myeloproliferative neoplasms. Blood. 2010;115:4517-4523.
11. Jones AV, Cross NCP. Inherited predisposition to myeloproliferative neoplasms. Ther Adv Hematol. 2013;4:237-253.
1. Murphy S, Peterson P, Iland H, Laszio J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol. 1997;34:29-39.
2. Zhan H, Spivak JL. The diagnosis and management of polycythemia vera, essential thrombocythemia, and primary myelofibrosis in the JAK2 V617F era. Clin Adv Hematol Oncol. 2009;7:334-342.
3. Higgs JR, Sadek I, Neumann PE, et al. Familial essential thrombocythemia with spontaneous megakaryocyte colony formation and acquired JAK2 mutations. Leukemia. 2008;22:1551-1556.
4. Senyak Z. Eileen Wiggins – out of the blue. http://www.mpnresearchfoundation.org/White-Paper-3A-Nature-2C-Nurture-2C-or-Both-3F. Published October 2010. Accessed May 23, 2017.
5. Cankovic M, Whiteley L, Hawley RC, Zarbo RJ, Chitale D. Clinical performance of JAK2 V617F mutation detection assays in a molecular diagnostics laboratory: evaluation of screening and quantitation methods. Am J Clin Pathol. 2009;132:713-721.
6. Kralovics R, Teo SS, Li S, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108:1377-1380.
7. James C. The JAK2V617F mutation in polycythemia vera and other myeloproliferative disorders: one mutation for three diseases? Hematology Am Soc Hematol Educ Program. 2008:69-75.
8. Pancrazzi A, Guglielmelli P, Ponziani V, et al. A sensitive detection method for MPLW515L or MPLW515K mutation in chronic myeloproliferative disorders with locked nucleic acid-modified probes and real-time polymerase chain reaction. J Mol Diagn. 2008;10:435-441.
9. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:1140-1151.
10. Jones AV, Campbell PJ, Beer PA, et al. The JAK2 46/1 haplotype predisposes to MPL-mutated myeloproliferative neoplasms. Blood. 2010;115:4517-4523.
11. Jones AV, Cross NCP. Inherited predisposition to myeloproliferative neoplasms. Ther Adv Hematol. 2013;4:237-253.
Management of tonsillar carcinoma with advanced radiation therapy and chemotherapy techniques
Tonsillar carcinoma is the most common of the oropharyngeal malignancies of the head and neck region after thyroid and laryngeal carcinoma. Squamous cell carcinoma is the most frequent histologic type of these tumors.1 Tonsillar tumors may originate in the oral cavity, oropharynx, hypopharynx, or larynx. In the United States, more than 5,000 new cases of oropharynx cancer are diagnosed annually.2 Men are affected three to four times more often than are women, and the rate of incidence increases after the 4th decade of life.3 Surveillance, Epidemiology, and End Results data from 1975-2004 show that tonsillar squamous cell carcinoma has had one of the largest increases in the male-to-female incidence rate ratios.4 The overall incidence of tonsillar carcinoma is increasing, especially in the younger population, and this may be attributed to increasing rates of human papilloma virus.5,6
Squamous cell carcinoma in the head and neck originate from subsites within the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx.7 Traditionally, alcohol consumption and tobacco use were considered the most significant risk factors for the development of tonsillar cancer.8 More recently, however, the high-risk oncogenic human papilloma virus has emerged as a clinical entity in the pathogenesis of squamous cell carcinoma in the head and neck. Other risk factors include poor oral hygiene, mechanical irritation, chewing of betel quid preparations, and a lack of vegetables and fruits in the diet.9-11 Squamous cell carcinoma of the oropharynx often presents late with lymph node involvement at the time of diagnosis. Nonspecific symptoms such as a sore throat and dysphagia can allow head and neck cancer to evade early detection. Many patients with tonsillar carcinoma present with advanced disease because early lesions are generally asymptomatic when small. This absence of symptoms is responsible for 67%-77% of patients presenting with tumors larger than 2.0 cm and often with regional nodal metastasis. At presentation, 45% of anterior tonsillar pillar lesions and 76% of tonsillar fossa lesions have clinically positive necks.12
Despite significant treatment advances, the management of advanced squamous cell carcinoma of the tonsil remains challenging. Historically, surgery was considered the standard of care for patients with tonsillar carcinoma with or without postoperative adjuvant radiotherapy. In locally advanced tonsillar carcinoma, extensive surgery with major tissue reconstruction was necessary, leading to speech dysfunction, cosmetic deformities, and difficulties in swallowing, all of which are detrimental to patient quality of life.13 Given the critical role of the oropharynx in speech and swallowing, nonsurgical therapy with organ-preserving chemoradiation has gained a greater role in the treatment of tonsil carcinoma.13 Over the past decade, innovations in radiation therapy techniques have led to the introduction of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) for the treatment of various cancers including tonsillar carcinoma.14,15 IMRT is an advanced mode of conformal high-precision radiotherapy that uses computer-controlled multiple small radiation beams of varying intensities to deliver precise radiation doses to the target tissues while sparing adjacent healthy tissues.14 By incorporating three-dimensional computed-tomography (CT) or positron-emission–tomography (PET) imaging technology, IMRT allows the radiation dose to conform more precisely to the three-dimensional shape of the tumor while modulating the intensity of the radiation beam and minimizing its dose to those adjacent sensitive and unaffected organs. IGRT uses a range of two-, three-, and four-dimensional imaging techniques that improve the precision and accuracy of the delivery of the radiation dose to the targeted tumor tissue while minimizing the dose to the surrounding normal tissue during the course of radiation therapy (Figure 1). In this report, we present challenging cases of advanced tonsillar carcinoma and describe our experience in managing the disease using a hyperfractionated IMRT-IGRT based three-dimensional conformal radiation therapy protocol with concurrent chemotherapy.
Case presentations and summaries
Case 1
A 52-year-old white, nonsmoking man who worked in a research chemical laboratory, presented with complaints of throat pain and difficulty in swallowing. The patient had a history of asthma and allergies and had been seen by an ear, nose, and throat (ENT) specialist prior to his visit to our oncology center. A biopsy was performed on a right tonsillar mass measuring 2.7 x 3.6 cm. A computed-tomography (CT) scan showed 2 enlarged inhomogeneous lymph nodes measuring 2.9 cm and 1.7 cm. The nodes were well defined with no soft tissue edema. Neoplasm was favored as a diagnosis and biopsy of the mass was carried out. A biopsy specimen measuring 1.0 x 0.4 x 0.3 cm revealed a moderately differentiated infiltrating squamous cell carcinoma, which extended to the edge of the biopsy specimen. The patient’s Karnofsky performance status was 90% (ie, able to carry on normal activity; minor signs or symptoms of disease).
A CT scan of the chest was clear with no evidence of malignant involvement. A subsequent CT scan of the neck revealed a primary neoplasm of the right faucial tonsil measuring 3.3 x 3.0 cm and associated with right level II, level III, and level IV pathological lymphadenopathy. Positron-emission tomography (PET) imaging of the neck revealed a right tonsillar lesion of 2.7 x 3.0 cm involving the right parapharyngeal space (Figure 2, Case 1). The standardized uptake value (SUV) of the PET scan of the primary lesion was measured at 7.3. A cluster of right level II cervical nodes measuring 3.2 x 2.5 cm had an SUV of 3.5. A 1.0-cm right level III jugular node was also seen with an SUV of 1.6, and a right level IV lymph node measuring 1.5 x 1.0 cm was seen with an SUV of 1.8. No other lesions were noted. The tumor stage was T2N2bM0, a stage IVa disease.
The patient had a percutaneous endoscopic gastrostomy (PEG) tube placement before starting radiation. He underwent a course of hyperfractionated intensity-modulated radiation therapy with image guidance (IMRT-IGRT) in 67 fractions of 120 cGy twice a day to a final tumor dose of 8,040 cGy.16 Concurrently, the patient received systemic chemotherapy with carboplatin at a dose of 240 mg weekly. To optimize the treatment, molecular profiling was performed to identify the sensitive genetic targets to systemic chemotherapy drugs.17, 18 Targets sensitive to paclitaxel and docetaxel were identified by molecular profiling of the tumor tissue, then chemotherapy with paclitaxel or docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off) was also administered to the patient.
The follow-up after 41 months indicated that the patient had no evidence of recurrent disease (Figure 2, Case 1). Posttreatment magnetic-resonance imaging (MRI) of the neck also indicated no evidence of residual tonsillar cancer. The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 2
A 49-year-old black male presented with throat pain and a mass seen initially by his family physician. The patient had a history of tobacco use (at least 1 cigar a day) periodically for about 10 years and had quit cigar smoking 15 years prior to developing his disease. An initial evaluation indicated that the patient had a hypopharyngeal mass in the left inferior pole of his tonsil with near occlusion of the hypopharyngeal airway. His larynx could not be visualized because of the obstructive mass. A neck lymph node measuring 3.0 cm in the left jugulodigastric region was also noted. The patient’s Karnofsky performance status was 90%. Subsequently, the patient underwent excision of the right tonsil and left tonsillar region.
The pathology of the right tonsil was found to be benign. Histology of the left tonsil revealed invasive squamous cell carcinoma. The resected tumor size measured 3.7 x 2.7 x 2.5 cm. The tumor was moderately differentiated involving the deep surgical margins. No lymphovascular invasion was seen. A PET scan revealed a mass arising from the left tonsillar pillar measuring 3.6 x 2.6 x 3.3 cm with deviation of the epiglottis posteriorly nearing the left vallecula. In addition, multiple large cervical nodal lesions in the left level II nodal chain were seen, with the largest measuring 3.1 x 3.0 x 4.5 cm with an SUV of 3.4. Displacement of the left submandibular gland with several further enlarged level II lymph nodes was observed. In the region of left vallecula, there was soft tissue thickening with increased activity measuring 2.7 x 1.5 cm, likely crossing the midline with an SUV of 5.5. The rest of the neck was negative for metastatic involvement (Figure 2, Case 2). The tumor stage was T3N2Mx, a stage IVa disease.
The patient had a Port-A-Cath placed, which caused a hemothorax after placement of the port and delayed initiating his treatment. A pretreatment MRI scan of the neck revealed multiple conglomerate hypodense peripherally enhancing nodular areas in the left neck posterior to the left submandibular gland deep to the parotid tail worrisome for necrotic lymphadenopathy. The patient underwent a course of hyperfractionated IMRT-IGRT in 67 fractions of 120 cGy twice daily for a total dose of 8,040 cGy to the primary tumor site.16 The patient had a port and PEG tube prior to initiating his radiation therapy. He received IMRT-IGRT with concurrent chemotherapy that was selected based on the recommendation of his genomic testing.17,18 The chemotherapy regimen used included carboplatin (300 mg weekly) and docetaxel (400 mg weekly). The patient had a treatment break because he was hospitalized for anemia and pancytopenia from his chemotherapy and he received supportive cancer care with epoetin alfa.A post therapy PET scan was negative for evidence of hypermetabolic malignancy; however, a 3.3 x 2.7 cm calcified lesion representing likely level III jugular lymph node exhibited no measurable activity at that time. The follow-up after 40 months indicated that the patient had no reported recurrence of the disease (Figure 2, Case 2). The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 3
A 53-year-old white man, who had no smoking or tobacco history but who was exposed to chemicals including sulfuric acid, hydrogen chloride gas, and glycols at work, presented initially with a sore throat that became more painful over time. His ENT specialist referred him for a CT scan of the neck, which revealed a left-sided neck mass measuring 2.5 cm in diameter posterior to the submandibular gland and lateral to carotid sheath and anterior to the triangle (Figure 2, Case 3). The mass appeared to be encapsulated. There was a lobulated spherical mass in the left supraglottic area with formation of the airway of the pyriform sinus and additional anterior vascular involvement was noted. The mass measured 3.6 cm in transverse diameter.
A left tonsillar biopsy specimen measuring 1.4 x 0.6 x 0.2 cm was obtained, and its pathology revealed that the patient had a metastatic squamous cell carcinoma. The left neck lymph node mass aspiration also revealed the presence of squamous cell carcinoma. A PET-CT scan staging showed a dominant tonsillar fossa mass extending from the soft palate down to the pyriform sinus measuring 4.2 x 3.8 cm, with an SUV uptake of 7.3. There was a dominant left level II necrotic lymph node presence measuring 5.0 x 3.7 cm, with an SUV of 3.0. The patient’s Karnofsky performance status was 90%. The tumor stage was T4N2M0, a stage IVa disease. The patient received a course of conformal hyperfractionated IMRT-IGRT delivered to the primary tumor in 67 fractions at 120 cGy twice daily for a total dose of 8,040 cGy16 and concurrent carboplatin chemotherapy at a weekly dose of 200 mg.
After completion of his radiation therapy, chemotherapy was changed based on genomic testing from single agent to doublet with carboplatin (area under the curve (AUC) dose of 2 or 200 mg, weekly) plus docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off ).17,18 A PET scan after chemoradiation therapy revealed a marked anatomical improvement in the primary neoplastic disease seen in the faucial tonsil. The tonsillar mass noted previously had almost completely resolved over the interval, with only a mild persistent asymmetrical thickening of around 1.5 cm, with a peak SUV of 2.0. A lymph node of 2.8 x 2.0 cm was present anterior to the left sternocleidomastoid muscle exhibiting SUV of only 1.8. No other abnormal lesions were noted (Figure 2, Case 3). The patient continues to do extremely well without local recurrence of the disease 46 months after radiation therapy (see Table for patient demographics, tumor characteristics, and therapy details.)
Discussion
The management of patients with primary squamous cell carcinoma of the oropharyngeal remains controversial. Traditionally, early-stage tonsillar squamous cell carcinoma was managed by a single modality treatment, either by surgery or radiation therapy, each showing similar efficacy and outcomes.19 For late-stage disease, a combined approach using surgery and radiation therapy was found to be superior to single modality treatment. However, surgery in conjugation with radiation therapy has been associated with significant toxicities compared with the radiation therapy alone.13Therefore, the use of radiation therapy without surgery is becoming more common with increasingly sophisticated radiation therapy techniques and organ preservation approach in patients with squamous cell carcinoma of the tonsil.
Findings from several studies have shown that in stage I or II oropharyngeal cancer, single modality treatment with radiation therapy achieves 80%-90% of local control of the disease, but poorer outcomes are reported for locally advanced stages III/IV with a local control rate of 63%-74%.20 These findings and others have led to a shift to evaluate the clinical benefits of radiation therapy given with concurrent chemotherapy for the primary treatment of advanced stage oropharyngeal squamous cell carcinoma.20,21 Findings from a number of studies have since reported comparable efficacy and toxicity outcomes using this regimen with concurrent chemotherapy in patients with locally advanced head and neck squamous cell cancer.22-24 Synchronous carboplatin chemotherapy was used effectively as an alternative to cisplatin with fewer potential adverse effects in the good prognosis group of patients with oropharyngeal squamous cell carcinoma.25,26 For our 3 patients, we used carboplatin-based chemotherapy with concurrent advanced hyperfractionated radiation therapy techniques to successfully manage tonsillar squamous cell carcinoma and reduce renal toxicity and neuropathy.
Advanced radiation therapy techniques such as IMRT-IGRT are used routinely at the University Cancer and Diagnostic Centers in Houston, Texas, to manage a range of malignant cancers.27 These innovative techniques have the potential to deliver highly conformal dose-intense radiation to targeted regions of disease, while sparing adjacent critical nonmalignant tissue. The improved shaping of high-dose distributions with IMRT-IGRT could mitigate treatment-related toxicities. For example, the use of advanced radiation therapy techniques has been associated with increased preservation of parotid salivary flow.28-30 The use of advanced radiation therapy techniques in head and neck squamous cell carcinoma is growing, and early evidence confirms its ability to secure excellent local and regional disease control.31,32 In this study, we have demonstrated that by using hyperfractionated conformal three-dimensional IMRT-IGRT we were able not only to manage advanced tonsillar squamous cell carcinoma and treat the malignant metastasis, but also spare adjacent critical organs that were not involved in the disease, thus reducing many of the detrimental side effects associated with hyperfractionated chemoradiation.
All 3 patients were followed for between 40 and 46 months. They continue to do extremely well without local recurrence of their disease, indicating a 100% disease control and overall survival rate. The disease control and survival outcomes for our patients with stage IVA disease compare favorably to other published reports in the literature.33,34 Findings from a study by Prestwich and colleagues33 of 41 patients with stage IV tonsillar carcinoma showed that the radiation therapy with concurrent chemotherapy achieved local and regional disease control in 91% of complete responders and an overall survival rate of 66% at 3 years. Similarly, Setton and colleagues34 reported on 442 patients – 50% with tonsillar cancer, 46% with base-of-tongue cancer – who underwent IMRT and concurrent chemotherapy and who achieved a 3-year overall survival of 84.9%. Our study findings demonstrate that hyperfractionated conformal three-dimensional IMRT-IGRT with concurrent chemotherapy can be delivered safely and effectively to patients with advanced tonsillar squamous cell carcinoma.
Acknowledgment
The authors thank Ms June Lyliston, LVN, for editing and proofreading the manuscript.
1. Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45(1):1-20.
2. Lin DT, Cohen SM, Coppit GL, Burkey BB. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clin North Am. 2005;38(1):59-74, viii.
3. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35(2):98-108.
4. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174-1182.
5. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the Incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. 2016.
6. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
7. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489-501.
8. Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748-2754.
9. Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34(4):284-291.
10. Farrow DC, Vaughan TL, Berwick M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
11. Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330-2336.
12. Guay ME, Lavertu P. Tonsillar carcinoma. Eur Arch Otorhinolaryngol. 1995;252(5):259-264.
13. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980.
14. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63(2):410-421.
15. Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29-42.
16. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):13-20.
17. Tomkiewicz C, Hans S, Mucchielli MH, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7(10):e47170.
18. Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1625-1638.
19. Moose BD, Kelly MD, Levine PA, et al. Definitive radiotherapy for T1 and T2 squamous cell carcinoma of the tonsil. Head Neck. 1995;17(4):334-338.
20. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16-21.
21. Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20(19):3964-3971.
22. Jegannathen A, Swindell R, Yap B, et al. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol (R Coll Radiol). 2010;22(3):185-191.
23. Budach V, Becker ET, Boehmer D, et al. Concurrent hyperfractionated accelerated radiotherapy with 5-FU and once weekly cisplatin in locally advanced head and neck cancer. The 10-year results of a prospective phase II trial. Strahlenther Onkol. 2014;190(3):250-255.
24. Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66-74.
25. Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis. Oral Oncol. 2013;49(6):615-619.
26. Benghiat H, Sanghera P, Cashmore1 J, et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer and Clinical Oncology. 2014;3:1-9.
27. D’Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol. 2015;13:77.
28. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007-1014.
29. Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863-4864.
30. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991.
31. Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966-974.
32. Daly ME, Lieskovsky Y, Pawlicki T, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220.
33. Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
34. Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298.
Tonsillar carcinoma is the most common of the oropharyngeal malignancies of the head and neck region after thyroid and laryngeal carcinoma. Squamous cell carcinoma is the most frequent histologic type of these tumors.1 Tonsillar tumors may originate in the oral cavity, oropharynx, hypopharynx, or larynx. In the United States, more than 5,000 new cases of oropharynx cancer are diagnosed annually.2 Men are affected three to four times more often than are women, and the rate of incidence increases after the 4th decade of life.3 Surveillance, Epidemiology, and End Results data from 1975-2004 show that tonsillar squamous cell carcinoma has had one of the largest increases in the male-to-female incidence rate ratios.4 The overall incidence of tonsillar carcinoma is increasing, especially in the younger population, and this may be attributed to increasing rates of human papilloma virus.5,6
Squamous cell carcinoma in the head and neck originate from subsites within the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx.7 Traditionally, alcohol consumption and tobacco use were considered the most significant risk factors for the development of tonsillar cancer.8 More recently, however, the high-risk oncogenic human papilloma virus has emerged as a clinical entity in the pathogenesis of squamous cell carcinoma in the head and neck. Other risk factors include poor oral hygiene, mechanical irritation, chewing of betel quid preparations, and a lack of vegetables and fruits in the diet.9-11 Squamous cell carcinoma of the oropharynx often presents late with lymph node involvement at the time of diagnosis. Nonspecific symptoms such as a sore throat and dysphagia can allow head and neck cancer to evade early detection. Many patients with tonsillar carcinoma present with advanced disease because early lesions are generally asymptomatic when small. This absence of symptoms is responsible for 67%-77% of patients presenting with tumors larger than 2.0 cm and often with regional nodal metastasis. At presentation, 45% of anterior tonsillar pillar lesions and 76% of tonsillar fossa lesions have clinically positive necks.12
Despite significant treatment advances, the management of advanced squamous cell carcinoma of the tonsil remains challenging. Historically, surgery was considered the standard of care for patients with tonsillar carcinoma with or without postoperative adjuvant radiotherapy. In locally advanced tonsillar carcinoma, extensive surgery with major tissue reconstruction was necessary, leading to speech dysfunction, cosmetic deformities, and difficulties in swallowing, all of which are detrimental to patient quality of life.13 Given the critical role of the oropharynx in speech and swallowing, nonsurgical therapy with organ-preserving chemoradiation has gained a greater role in the treatment of tonsil carcinoma.13 Over the past decade, innovations in radiation therapy techniques have led to the introduction of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) for the treatment of various cancers including tonsillar carcinoma.14,15 IMRT is an advanced mode of conformal high-precision radiotherapy that uses computer-controlled multiple small radiation beams of varying intensities to deliver precise radiation doses to the target tissues while sparing adjacent healthy tissues.14 By incorporating three-dimensional computed-tomography (CT) or positron-emission–tomography (PET) imaging technology, IMRT allows the radiation dose to conform more precisely to the three-dimensional shape of the tumor while modulating the intensity of the radiation beam and minimizing its dose to those adjacent sensitive and unaffected organs. IGRT uses a range of two-, three-, and four-dimensional imaging techniques that improve the precision and accuracy of the delivery of the radiation dose to the targeted tumor tissue while minimizing the dose to the surrounding normal tissue during the course of radiation therapy (Figure 1). In this report, we present challenging cases of advanced tonsillar carcinoma and describe our experience in managing the disease using a hyperfractionated IMRT-IGRT based three-dimensional conformal radiation therapy protocol with concurrent chemotherapy.
Case presentations and summaries
Case 1
A 52-year-old white, nonsmoking man who worked in a research chemical laboratory, presented with complaints of throat pain and difficulty in swallowing. The patient had a history of asthma and allergies and had been seen by an ear, nose, and throat (ENT) specialist prior to his visit to our oncology center. A biopsy was performed on a right tonsillar mass measuring 2.7 x 3.6 cm. A computed-tomography (CT) scan showed 2 enlarged inhomogeneous lymph nodes measuring 2.9 cm and 1.7 cm. The nodes were well defined with no soft tissue edema. Neoplasm was favored as a diagnosis and biopsy of the mass was carried out. A biopsy specimen measuring 1.0 x 0.4 x 0.3 cm revealed a moderately differentiated infiltrating squamous cell carcinoma, which extended to the edge of the biopsy specimen. The patient’s Karnofsky performance status was 90% (ie, able to carry on normal activity; minor signs or symptoms of disease).
A CT scan of the chest was clear with no evidence of malignant involvement. A subsequent CT scan of the neck revealed a primary neoplasm of the right faucial tonsil measuring 3.3 x 3.0 cm and associated with right level II, level III, and level IV pathological lymphadenopathy. Positron-emission tomography (PET) imaging of the neck revealed a right tonsillar lesion of 2.7 x 3.0 cm involving the right parapharyngeal space (Figure 2, Case 1). The standardized uptake value (SUV) of the PET scan of the primary lesion was measured at 7.3. A cluster of right level II cervical nodes measuring 3.2 x 2.5 cm had an SUV of 3.5. A 1.0-cm right level III jugular node was also seen with an SUV of 1.6, and a right level IV lymph node measuring 1.5 x 1.0 cm was seen with an SUV of 1.8. No other lesions were noted. The tumor stage was T2N2bM0, a stage IVa disease.
The patient had a percutaneous endoscopic gastrostomy (PEG) tube placement before starting radiation. He underwent a course of hyperfractionated intensity-modulated radiation therapy with image guidance (IMRT-IGRT) in 67 fractions of 120 cGy twice a day to a final tumor dose of 8,040 cGy.16 Concurrently, the patient received systemic chemotherapy with carboplatin at a dose of 240 mg weekly. To optimize the treatment, molecular profiling was performed to identify the sensitive genetic targets to systemic chemotherapy drugs.17, 18 Targets sensitive to paclitaxel and docetaxel were identified by molecular profiling of the tumor tissue, then chemotherapy with paclitaxel or docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off) was also administered to the patient.
The follow-up after 41 months indicated that the patient had no evidence of recurrent disease (Figure 2, Case 1). Posttreatment magnetic-resonance imaging (MRI) of the neck also indicated no evidence of residual tonsillar cancer. The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 2
A 49-year-old black male presented with throat pain and a mass seen initially by his family physician. The patient had a history of tobacco use (at least 1 cigar a day) periodically for about 10 years and had quit cigar smoking 15 years prior to developing his disease. An initial evaluation indicated that the patient had a hypopharyngeal mass in the left inferior pole of his tonsil with near occlusion of the hypopharyngeal airway. His larynx could not be visualized because of the obstructive mass. A neck lymph node measuring 3.0 cm in the left jugulodigastric region was also noted. The patient’s Karnofsky performance status was 90%. Subsequently, the patient underwent excision of the right tonsil and left tonsillar region.
The pathology of the right tonsil was found to be benign. Histology of the left tonsil revealed invasive squamous cell carcinoma. The resected tumor size measured 3.7 x 2.7 x 2.5 cm. The tumor was moderately differentiated involving the deep surgical margins. No lymphovascular invasion was seen. A PET scan revealed a mass arising from the left tonsillar pillar measuring 3.6 x 2.6 x 3.3 cm with deviation of the epiglottis posteriorly nearing the left vallecula. In addition, multiple large cervical nodal lesions in the left level II nodal chain were seen, with the largest measuring 3.1 x 3.0 x 4.5 cm with an SUV of 3.4. Displacement of the left submandibular gland with several further enlarged level II lymph nodes was observed. In the region of left vallecula, there was soft tissue thickening with increased activity measuring 2.7 x 1.5 cm, likely crossing the midline with an SUV of 5.5. The rest of the neck was negative for metastatic involvement (Figure 2, Case 2). The tumor stage was T3N2Mx, a stage IVa disease.
The patient had a Port-A-Cath placed, which caused a hemothorax after placement of the port and delayed initiating his treatment. A pretreatment MRI scan of the neck revealed multiple conglomerate hypodense peripherally enhancing nodular areas in the left neck posterior to the left submandibular gland deep to the parotid tail worrisome for necrotic lymphadenopathy. The patient underwent a course of hyperfractionated IMRT-IGRT in 67 fractions of 120 cGy twice daily for a total dose of 8,040 cGy to the primary tumor site.16 The patient had a port and PEG tube prior to initiating his radiation therapy. He received IMRT-IGRT with concurrent chemotherapy that was selected based on the recommendation of his genomic testing.17,18 The chemotherapy regimen used included carboplatin (300 mg weekly) and docetaxel (400 mg weekly). The patient had a treatment break because he was hospitalized for anemia and pancytopenia from his chemotherapy and he received supportive cancer care with epoetin alfa.A post therapy PET scan was negative for evidence of hypermetabolic malignancy; however, a 3.3 x 2.7 cm calcified lesion representing likely level III jugular lymph node exhibited no measurable activity at that time. The follow-up after 40 months indicated that the patient had no reported recurrence of the disease (Figure 2, Case 2). The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 3
A 53-year-old white man, who had no smoking or tobacco history but who was exposed to chemicals including sulfuric acid, hydrogen chloride gas, and glycols at work, presented initially with a sore throat that became more painful over time. His ENT specialist referred him for a CT scan of the neck, which revealed a left-sided neck mass measuring 2.5 cm in diameter posterior to the submandibular gland and lateral to carotid sheath and anterior to the triangle (Figure 2, Case 3). The mass appeared to be encapsulated. There was a lobulated spherical mass in the left supraglottic area with formation of the airway of the pyriform sinus and additional anterior vascular involvement was noted. The mass measured 3.6 cm in transverse diameter.
A left tonsillar biopsy specimen measuring 1.4 x 0.6 x 0.2 cm was obtained, and its pathology revealed that the patient had a metastatic squamous cell carcinoma. The left neck lymph node mass aspiration also revealed the presence of squamous cell carcinoma. A PET-CT scan staging showed a dominant tonsillar fossa mass extending from the soft palate down to the pyriform sinus measuring 4.2 x 3.8 cm, with an SUV uptake of 7.3. There was a dominant left level II necrotic lymph node presence measuring 5.0 x 3.7 cm, with an SUV of 3.0. The patient’s Karnofsky performance status was 90%. The tumor stage was T4N2M0, a stage IVa disease. The patient received a course of conformal hyperfractionated IMRT-IGRT delivered to the primary tumor in 67 fractions at 120 cGy twice daily for a total dose of 8,040 cGy16 and concurrent carboplatin chemotherapy at a weekly dose of 200 mg.
After completion of his radiation therapy, chemotherapy was changed based on genomic testing from single agent to doublet with carboplatin (area under the curve (AUC) dose of 2 or 200 mg, weekly) plus docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off ).17,18 A PET scan after chemoradiation therapy revealed a marked anatomical improvement in the primary neoplastic disease seen in the faucial tonsil. The tonsillar mass noted previously had almost completely resolved over the interval, with only a mild persistent asymmetrical thickening of around 1.5 cm, with a peak SUV of 2.0. A lymph node of 2.8 x 2.0 cm was present anterior to the left sternocleidomastoid muscle exhibiting SUV of only 1.8. No other abnormal lesions were noted (Figure 2, Case 3). The patient continues to do extremely well without local recurrence of the disease 46 months after radiation therapy (see Table for patient demographics, tumor characteristics, and therapy details.)
Discussion
The management of patients with primary squamous cell carcinoma of the oropharyngeal remains controversial. Traditionally, early-stage tonsillar squamous cell carcinoma was managed by a single modality treatment, either by surgery or radiation therapy, each showing similar efficacy and outcomes.19 For late-stage disease, a combined approach using surgery and radiation therapy was found to be superior to single modality treatment. However, surgery in conjugation with radiation therapy has been associated with significant toxicities compared with the radiation therapy alone.13Therefore, the use of radiation therapy without surgery is becoming more common with increasingly sophisticated radiation therapy techniques and organ preservation approach in patients with squamous cell carcinoma of the tonsil.
Findings from several studies have shown that in stage I or II oropharyngeal cancer, single modality treatment with radiation therapy achieves 80%-90% of local control of the disease, but poorer outcomes are reported for locally advanced stages III/IV with a local control rate of 63%-74%.20 These findings and others have led to a shift to evaluate the clinical benefits of radiation therapy given with concurrent chemotherapy for the primary treatment of advanced stage oropharyngeal squamous cell carcinoma.20,21 Findings from a number of studies have since reported comparable efficacy and toxicity outcomes using this regimen with concurrent chemotherapy in patients with locally advanced head and neck squamous cell cancer.22-24 Synchronous carboplatin chemotherapy was used effectively as an alternative to cisplatin with fewer potential adverse effects in the good prognosis group of patients with oropharyngeal squamous cell carcinoma.25,26 For our 3 patients, we used carboplatin-based chemotherapy with concurrent advanced hyperfractionated radiation therapy techniques to successfully manage tonsillar squamous cell carcinoma and reduce renal toxicity and neuropathy.
Advanced radiation therapy techniques such as IMRT-IGRT are used routinely at the University Cancer and Diagnostic Centers in Houston, Texas, to manage a range of malignant cancers.27 These innovative techniques have the potential to deliver highly conformal dose-intense radiation to targeted regions of disease, while sparing adjacent critical nonmalignant tissue. The improved shaping of high-dose distributions with IMRT-IGRT could mitigate treatment-related toxicities. For example, the use of advanced radiation therapy techniques has been associated with increased preservation of parotid salivary flow.28-30 The use of advanced radiation therapy techniques in head and neck squamous cell carcinoma is growing, and early evidence confirms its ability to secure excellent local and regional disease control.31,32 In this study, we have demonstrated that by using hyperfractionated conformal three-dimensional IMRT-IGRT we were able not only to manage advanced tonsillar squamous cell carcinoma and treat the malignant metastasis, but also spare adjacent critical organs that were not involved in the disease, thus reducing many of the detrimental side effects associated with hyperfractionated chemoradiation.
All 3 patients were followed for between 40 and 46 months. They continue to do extremely well without local recurrence of their disease, indicating a 100% disease control and overall survival rate. The disease control and survival outcomes for our patients with stage IVA disease compare favorably to other published reports in the literature.33,34 Findings from a study by Prestwich and colleagues33 of 41 patients with stage IV tonsillar carcinoma showed that the radiation therapy with concurrent chemotherapy achieved local and regional disease control in 91% of complete responders and an overall survival rate of 66% at 3 years. Similarly, Setton and colleagues34 reported on 442 patients – 50% with tonsillar cancer, 46% with base-of-tongue cancer – who underwent IMRT and concurrent chemotherapy and who achieved a 3-year overall survival of 84.9%. Our study findings demonstrate that hyperfractionated conformal three-dimensional IMRT-IGRT with concurrent chemotherapy can be delivered safely and effectively to patients with advanced tonsillar squamous cell carcinoma.
Acknowledgment
The authors thank Ms June Lyliston, LVN, for editing and proofreading the manuscript.
Tonsillar carcinoma is the most common of the oropharyngeal malignancies of the head and neck region after thyroid and laryngeal carcinoma. Squamous cell carcinoma is the most frequent histologic type of these tumors.1 Tonsillar tumors may originate in the oral cavity, oropharynx, hypopharynx, or larynx. In the United States, more than 5,000 new cases of oropharynx cancer are diagnosed annually.2 Men are affected three to four times more often than are women, and the rate of incidence increases after the 4th decade of life.3 Surveillance, Epidemiology, and End Results data from 1975-2004 show that tonsillar squamous cell carcinoma has had one of the largest increases in the male-to-female incidence rate ratios.4 The overall incidence of tonsillar carcinoma is increasing, especially in the younger population, and this may be attributed to increasing rates of human papilloma virus.5,6
Squamous cell carcinoma in the head and neck originate from subsites within the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx.7 Traditionally, alcohol consumption and tobacco use were considered the most significant risk factors for the development of tonsillar cancer.8 More recently, however, the high-risk oncogenic human papilloma virus has emerged as a clinical entity in the pathogenesis of squamous cell carcinoma in the head and neck. Other risk factors include poor oral hygiene, mechanical irritation, chewing of betel quid preparations, and a lack of vegetables and fruits in the diet.9-11 Squamous cell carcinoma of the oropharynx often presents late with lymph node involvement at the time of diagnosis. Nonspecific symptoms such as a sore throat and dysphagia can allow head and neck cancer to evade early detection. Many patients with tonsillar carcinoma present with advanced disease because early lesions are generally asymptomatic when small. This absence of symptoms is responsible for 67%-77% of patients presenting with tumors larger than 2.0 cm and often with regional nodal metastasis. At presentation, 45% of anterior tonsillar pillar lesions and 76% of tonsillar fossa lesions have clinically positive necks.12
Despite significant treatment advances, the management of advanced squamous cell carcinoma of the tonsil remains challenging. Historically, surgery was considered the standard of care for patients with tonsillar carcinoma with or without postoperative adjuvant radiotherapy. In locally advanced tonsillar carcinoma, extensive surgery with major tissue reconstruction was necessary, leading to speech dysfunction, cosmetic deformities, and difficulties in swallowing, all of which are detrimental to patient quality of life.13 Given the critical role of the oropharynx in speech and swallowing, nonsurgical therapy with organ-preserving chemoradiation has gained a greater role in the treatment of tonsil carcinoma.13 Over the past decade, innovations in radiation therapy techniques have led to the introduction of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) for the treatment of various cancers including tonsillar carcinoma.14,15 IMRT is an advanced mode of conformal high-precision radiotherapy that uses computer-controlled multiple small radiation beams of varying intensities to deliver precise radiation doses to the target tissues while sparing adjacent healthy tissues.14 By incorporating three-dimensional computed-tomography (CT) or positron-emission–tomography (PET) imaging technology, IMRT allows the radiation dose to conform more precisely to the three-dimensional shape of the tumor while modulating the intensity of the radiation beam and minimizing its dose to those adjacent sensitive and unaffected organs. IGRT uses a range of two-, three-, and four-dimensional imaging techniques that improve the precision and accuracy of the delivery of the radiation dose to the targeted tumor tissue while minimizing the dose to the surrounding normal tissue during the course of radiation therapy (Figure 1). In this report, we present challenging cases of advanced tonsillar carcinoma and describe our experience in managing the disease using a hyperfractionated IMRT-IGRT based three-dimensional conformal radiation therapy protocol with concurrent chemotherapy.
Case presentations and summaries
Case 1
A 52-year-old white, nonsmoking man who worked in a research chemical laboratory, presented with complaints of throat pain and difficulty in swallowing. The patient had a history of asthma and allergies and had been seen by an ear, nose, and throat (ENT) specialist prior to his visit to our oncology center. A biopsy was performed on a right tonsillar mass measuring 2.7 x 3.6 cm. A computed-tomography (CT) scan showed 2 enlarged inhomogeneous lymph nodes measuring 2.9 cm and 1.7 cm. The nodes were well defined with no soft tissue edema. Neoplasm was favored as a diagnosis and biopsy of the mass was carried out. A biopsy specimen measuring 1.0 x 0.4 x 0.3 cm revealed a moderately differentiated infiltrating squamous cell carcinoma, which extended to the edge of the biopsy specimen. The patient’s Karnofsky performance status was 90% (ie, able to carry on normal activity; minor signs or symptoms of disease).
A CT scan of the chest was clear with no evidence of malignant involvement. A subsequent CT scan of the neck revealed a primary neoplasm of the right faucial tonsil measuring 3.3 x 3.0 cm and associated with right level II, level III, and level IV pathological lymphadenopathy. Positron-emission tomography (PET) imaging of the neck revealed a right tonsillar lesion of 2.7 x 3.0 cm involving the right parapharyngeal space (Figure 2, Case 1). The standardized uptake value (SUV) of the PET scan of the primary lesion was measured at 7.3. A cluster of right level II cervical nodes measuring 3.2 x 2.5 cm had an SUV of 3.5. A 1.0-cm right level III jugular node was also seen with an SUV of 1.6, and a right level IV lymph node measuring 1.5 x 1.0 cm was seen with an SUV of 1.8. No other lesions were noted. The tumor stage was T2N2bM0, a stage IVa disease.
The patient had a percutaneous endoscopic gastrostomy (PEG) tube placement before starting radiation. He underwent a course of hyperfractionated intensity-modulated radiation therapy with image guidance (IMRT-IGRT) in 67 fractions of 120 cGy twice a day to a final tumor dose of 8,040 cGy.16 Concurrently, the patient received systemic chemotherapy with carboplatin at a dose of 240 mg weekly. To optimize the treatment, molecular profiling was performed to identify the sensitive genetic targets to systemic chemotherapy drugs.17, 18 Targets sensitive to paclitaxel and docetaxel were identified by molecular profiling of the tumor tissue, then chemotherapy with paclitaxel or docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off) was also administered to the patient.
The follow-up after 41 months indicated that the patient had no evidence of recurrent disease (Figure 2, Case 1). Posttreatment magnetic-resonance imaging (MRI) of the neck also indicated no evidence of residual tonsillar cancer. The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 2
A 49-year-old black male presented with throat pain and a mass seen initially by his family physician. The patient had a history of tobacco use (at least 1 cigar a day) periodically for about 10 years and had quit cigar smoking 15 years prior to developing his disease. An initial evaluation indicated that the patient had a hypopharyngeal mass in the left inferior pole of his tonsil with near occlusion of the hypopharyngeal airway. His larynx could not be visualized because of the obstructive mass. A neck lymph node measuring 3.0 cm in the left jugulodigastric region was also noted. The patient’s Karnofsky performance status was 90%. Subsequently, the patient underwent excision of the right tonsil and left tonsillar region.
The pathology of the right tonsil was found to be benign. Histology of the left tonsil revealed invasive squamous cell carcinoma. The resected tumor size measured 3.7 x 2.7 x 2.5 cm. The tumor was moderately differentiated involving the deep surgical margins. No lymphovascular invasion was seen. A PET scan revealed a mass arising from the left tonsillar pillar measuring 3.6 x 2.6 x 3.3 cm with deviation of the epiglottis posteriorly nearing the left vallecula. In addition, multiple large cervical nodal lesions in the left level II nodal chain were seen, with the largest measuring 3.1 x 3.0 x 4.5 cm with an SUV of 3.4. Displacement of the left submandibular gland with several further enlarged level II lymph nodes was observed. In the region of left vallecula, there was soft tissue thickening with increased activity measuring 2.7 x 1.5 cm, likely crossing the midline with an SUV of 5.5. The rest of the neck was negative for metastatic involvement (Figure 2, Case 2). The tumor stage was T3N2Mx, a stage IVa disease.
The patient had a Port-A-Cath placed, which caused a hemothorax after placement of the port and delayed initiating his treatment. A pretreatment MRI scan of the neck revealed multiple conglomerate hypodense peripherally enhancing nodular areas in the left neck posterior to the left submandibular gland deep to the parotid tail worrisome for necrotic lymphadenopathy. The patient underwent a course of hyperfractionated IMRT-IGRT in 67 fractions of 120 cGy twice daily for a total dose of 8,040 cGy to the primary tumor site.16 The patient had a port and PEG tube prior to initiating his radiation therapy. He received IMRT-IGRT with concurrent chemotherapy that was selected based on the recommendation of his genomic testing.17,18 The chemotherapy regimen used included carboplatin (300 mg weekly) and docetaxel (400 mg weekly). The patient had a treatment break because he was hospitalized for anemia and pancytopenia from his chemotherapy and he received supportive cancer care with epoetin alfa.A post therapy PET scan was negative for evidence of hypermetabolic malignancy; however, a 3.3 x 2.7 cm calcified lesion representing likely level III jugular lymph node exhibited no measurable activity at that time. The follow-up after 40 months indicated that the patient had no reported recurrence of the disease (Figure 2, Case 2). The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 3
A 53-year-old white man, who had no smoking or tobacco history but who was exposed to chemicals including sulfuric acid, hydrogen chloride gas, and glycols at work, presented initially with a sore throat that became more painful over time. His ENT specialist referred him for a CT scan of the neck, which revealed a left-sided neck mass measuring 2.5 cm in diameter posterior to the submandibular gland and lateral to carotid sheath and anterior to the triangle (Figure 2, Case 3). The mass appeared to be encapsulated. There was a lobulated spherical mass in the left supraglottic area with formation of the airway of the pyriform sinus and additional anterior vascular involvement was noted. The mass measured 3.6 cm in transverse diameter.
A left tonsillar biopsy specimen measuring 1.4 x 0.6 x 0.2 cm was obtained, and its pathology revealed that the patient had a metastatic squamous cell carcinoma. The left neck lymph node mass aspiration also revealed the presence of squamous cell carcinoma. A PET-CT scan staging showed a dominant tonsillar fossa mass extending from the soft palate down to the pyriform sinus measuring 4.2 x 3.8 cm, with an SUV uptake of 7.3. There was a dominant left level II necrotic lymph node presence measuring 5.0 x 3.7 cm, with an SUV of 3.0. The patient’s Karnofsky performance status was 90%. The tumor stage was T4N2M0, a stage IVa disease. The patient received a course of conformal hyperfractionated IMRT-IGRT delivered to the primary tumor in 67 fractions at 120 cGy twice daily for a total dose of 8,040 cGy16 and concurrent carboplatin chemotherapy at a weekly dose of 200 mg.
After completion of his radiation therapy, chemotherapy was changed based on genomic testing from single agent to doublet with carboplatin (area under the curve (AUC) dose of 2 or 200 mg, weekly) plus docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off ).17,18 A PET scan after chemoradiation therapy revealed a marked anatomical improvement in the primary neoplastic disease seen in the faucial tonsil. The tonsillar mass noted previously had almost completely resolved over the interval, with only a mild persistent asymmetrical thickening of around 1.5 cm, with a peak SUV of 2.0. A lymph node of 2.8 x 2.0 cm was present anterior to the left sternocleidomastoid muscle exhibiting SUV of only 1.8. No other abnormal lesions were noted (Figure 2, Case 3). The patient continues to do extremely well without local recurrence of the disease 46 months after radiation therapy (see Table for patient demographics, tumor characteristics, and therapy details.)
Discussion
The management of patients with primary squamous cell carcinoma of the oropharyngeal remains controversial. Traditionally, early-stage tonsillar squamous cell carcinoma was managed by a single modality treatment, either by surgery or radiation therapy, each showing similar efficacy and outcomes.19 For late-stage disease, a combined approach using surgery and radiation therapy was found to be superior to single modality treatment. However, surgery in conjugation with radiation therapy has been associated with significant toxicities compared with the radiation therapy alone.13Therefore, the use of radiation therapy without surgery is becoming more common with increasingly sophisticated radiation therapy techniques and organ preservation approach in patients with squamous cell carcinoma of the tonsil.
Findings from several studies have shown that in stage I or II oropharyngeal cancer, single modality treatment with radiation therapy achieves 80%-90% of local control of the disease, but poorer outcomes are reported for locally advanced stages III/IV with a local control rate of 63%-74%.20 These findings and others have led to a shift to evaluate the clinical benefits of radiation therapy given with concurrent chemotherapy for the primary treatment of advanced stage oropharyngeal squamous cell carcinoma.20,21 Findings from a number of studies have since reported comparable efficacy and toxicity outcomes using this regimen with concurrent chemotherapy in patients with locally advanced head and neck squamous cell cancer.22-24 Synchronous carboplatin chemotherapy was used effectively as an alternative to cisplatin with fewer potential adverse effects in the good prognosis group of patients with oropharyngeal squamous cell carcinoma.25,26 For our 3 patients, we used carboplatin-based chemotherapy with concurrent advanced hyperfractionated radiation therapy techniques to successfully manage tonsillar squamous cell carcinoma and reduce renal toxicity and neuropathy.
Advanced radiation therapy techniques such as IMRT-IGRT are used routinely at the University Cancer and Diagnostic Centers in Houston, Texas, to manage a range of malignant cancers.27 These innovative techniques have the potential to deliver highly conformal dose-intense radiation to targeted regions of disease, while sparing adjacent critical nonmalignant tissue. The improved shaping of high-dose distributions with IMRT-IGRT could mitigate treatment-related toxicities. For example, the use of advanced radiation therapy techniques has been associated with increased preservation of parotid salivary flow.28-30 The use of advanced radiation therapy techniques in head and neck squamous cell carcinoma is growing, and early evidence confirms its ability to secure excellent local and regional disease control.31,32 In this study, we have demonstrated that by using hyperfractionated conformal three-dimensional IMRT-IGRT we were able not only to manage advanced tonsillar squamous cell carcinoma and treat the malignant metastasis, but also spare adjacent critical organs that were not involved in the disease, thus reducing many of the detrimental side effects associated with hyperfractionated chemoradiation.
All 3 patients were followed for between 40 and 46 months. They continue to do extremely well without local recurrence of their disease, indicating a 100% disease control and overall survival rate. The disease control and survival outcomes for our patients with stage IVA disease compare favorably to other published reports in the literature.33,34 Findings from a study by Prestwich and colleagues33 of 41 patients with stage IV tonsillar carcinoma showed that the radiation therapy with concurrent chemotherapy achieved local and regional disease control in 91% of complete responders and an overall survival rate of 66% at 3 years. Similarly, Setton and colleagues34 reported on 442 patients – 50% with tonsillar cancer, 46% with base-of-tongue cancer – who underwent IMRT and concurrent chemotherapy and who achieved a 3-year overall survival of 84.9%. Our study findings demonstrate that hyperfractionated conformal three-dimensional IMRT-IGRT with concurrent chemotherapy can be delivered safely and effectively to patients with advanced tonsillar squamous cell carcinoma.
Acknowledgment
The authors thank Ms June Lyliston, LVN, for editing and proofreading the manuscript.
1. Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45(1):1-20.
2. Lin DT, Cohen SM, Coppit GL, Burkey BB. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clin North Am. 2005;38(1):59-74, viii.
3. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35(2):98-108.
4. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174-1182.
5. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the Incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. 2016.
6. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
7. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489-501.
8. Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748-2754.
9. Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34(4):284-291.
10. Farrow DC, Vaughan TL, Berwick M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
11. Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330-2336.
12. Guay ME, Lavertu P. Tonsillar carcinoma. Eur Arch Otorhinolaryngol. 1995;252(5):259-264.
13. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980.
14. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63(2):410-421.
15. Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29-42.
16. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):13-20.
17. Tomkiewicz C, Hans S, Mucchielli MH, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7(10):e47170.
18. Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1625-1638.
19. Moose BD, Kelly MD, Levine PA, et al. Definitive radiotherapy for T1 and T2 squamous cell carcinoma of the tonsil. Head Neck. 1995;17(4):334-338.
20. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16-21.
21. Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20(19):3964-3971.
22. Jegannathen A, Swindell R, Yap B, et al. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol (R Coll Radiol). 2010;22(3):185-191.
23. Budach V, Becker ET, Boehmer D, et al. Concurrent hyperfractionated accelerated radiotherapy with 5-FU and once weekly cisplatin in locally advanced head and neck cancer. The 10-year results of a prospective phase II trial. Strahlenther Onkol. 2014;190(3):250-255.
24. Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66-74.
25. Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis. Oral Oncol. 2013;49(6):615-619.
26. Benghiat H, Sanghera P, Cashmore1 J, et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer and Clinical Oncology. 2014;3:1-9.
27. D’Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol. 2015;13:77.
28. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007-1014.
29. Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863-4864.
30. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991.
31. Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966-974.
32. Daly ME, Lieskovsky Y, Pawlicki T, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220.
33. Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
34. Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298.
1. Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45(1):1-20.
2. Lin DT, Cohen SM, Coppit GL, Burkey BB. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clin North Am. 2005;38(1):59-74, viii.
3. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35(2):98-108.
4. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174-1182.
5. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the Incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. 2016.
6. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
7. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489-501.
8. Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748-2754.
9. Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34(4):284-291.
10. Farrow DC, Vaughan TL, Berwick M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
11. Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330-2336.
12. Guay ME, Lavertu P. Tonsillar carcinoma. Eur Arch Otorhinolaryngol. 1995;252(5):259-264.
13. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980.
14. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63(2):410-421.
15. Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29-42.
16. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):13-20.
17. Tomkiewicz C, Hans S, Mucchielli MH, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7(10):e47170.
18. Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1625-1638.
19. Moose BD, Kelly MD, Levine PA, et al. Definitive radiotherapy for T1 and T2 squamous cell carcinoma of the tonsil. Head Neck. 1995;17(4):334-338.
20. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16-21.
21. Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20(19):3964-3971.
22. Jegannathen A, Swindell R, Yap B, et al. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol (R Coll Radiol). 2010;22(3):185-191.
23. Budach V, Becker ET, Boehmer D, et al. Concurrent hyperfractionated accelerated radiotherapy with 5-FU and once weekly cisplatin in locally advanced head and neck cancer. The 10-year results of a prospective phase II trial. Strahlenther Onkol. 2014;190(3):250-255.
24. Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66-74.
25. Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis. Oral Oncol. 2013;49(6):615-619.
26. Benghiat H, Sanghera P, Cashmore1 J, et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer and Clinical Oncology. 2014;3:1-9.
27. D’Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol. 2015;13:77.
28. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007-1014.
29. Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863-4864.
30. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991.
31. Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966-974.
32. Daly ME, Lieskovsky Y, Pawlicki T, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220.
33. Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
34. Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298.
PD-L1-targeting drug atezolizumab nabs approval for non-small cell lung cancer
The approval last fall by the US Food and Drug Administration (FDA) of the immune checkpoint inhibitor atezolizumab for the treatment of metastatic non–small cell lung cancer (NSCLC) marked the second approved indication for the drug in a single year. Atezolizumab, which targets the programmed cell death protein-ligand 1 (PD-L1), has a unique mechanism of action compared with other approved immune checkpoint inhibitors and had been previously approved for the treatment of patients with advanced urothelial carcinoma. The current approval was based on 2 international, randomized, open-label trials, involving more than 1,000 patients, in which atezolizumab outperformed the chemotherapeutic drug docetaxel.
The POPLAR trial1 was a phase 2 study performed at 61 academic centers and community oncology practices across 13 countries in Europe and North America and enrolled 287 patients, while the phase 3 OAK trial2 was carried out at 193 centers in 31 countries and enrolled 850 patients.
Eligibility criteria for the 2 studies were similar; patients were 18 years or older, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, had measurable disease as per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), and adequate hematologic and end-organ function. Patients with asymptomatic or treated central nervous system (CNS) metastases were also eligible.
Patients were excluded from both studies if they had a history of autoimmune disease, pneumonitis or chronic viral diseases, or had received previous treatment with docetaxel, CD137 agonists, anti-CTLA4, anti-PD-L1 or anti-PD-1 therapeutics.
Patients were randomized 1:1 to receive atezolizumab, administered intravenously at a fixed dose of 1,200 mg, or a 75 mg/m2 dose of docetaxel, every 3 weeks on day 1 of each 3-week cycle. Randomization was stratified according to PD-L1 expression, number of previous chemotherapy regimens, and histology (squamous vs nonsquamous). Tumors were assessed at baseline and every 6 weeks for 36 weeks, and every 9 weeks thereafter until progression or, in patients who continued beyond progression, until discontinuation. PD-L1 expression was assessed prospectively on tumor cells and tumor-infiltrating immune cells, using the FDA-approved companion diagnostic, Ventana SP142 PD-L1 immunohistochemistry assay.
The primary endpoint in both studies was overall survival (OS), which was significantly improved in the atezolizumab treatment arm. In the POPLAR trial, over a minimum follow-up of 13 months, the median OS was 12.6 months in atezolizumab-treated patients, compared with 9.7 months in docetaxel-treated patients (hazard ratio [HR], 0.73; P = .04) and in the OAK trial, over a median follow-up of 21 months, the median OS was 15.7 months and 10.3 months, respectively.
The main difference between the 2 trials was that the OS benefit in the POPLAR trial reached statistical significance only in patients with the highest levels of PD-L1 expression, whereas in the OAK study, the OS was significantly improved regardless of PD-L1 expression status, although the greatest benefit was derived in patients with higher levels of PD-L1 expression (20.5 months and 8.9 months, respectively). The OS benefit was observed across all other prespecified subgroups, except in patients with EGFR mutation-positivity in the OAK trial. Progression-free survival and overall response rates were similar in the 2 treatment arms in both studies.
An additional 375 patients were enrolled in the OAK trial and included in the safety analyses. In both studies, the safety profile of atezolizumab was similar to that observed in previous studies of this drug. The most commonly observed adverse events (AEs) with atezolizumab treatment were fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, and constipation. Despite longer treatment duration, there were fewer incidences of grade 3/4 AEs with atezolizumab compared with docetaxel (37% vs 54%, respectively, in the OAK trial), a lower rate of discontinuation with atezolizumab treatment, and no deaths related to the study drug. The most common grade 3/4 AEs included dyspnea, pneumonia, hypoxia, hyponatremia, fatigue, and anemia. Clinically significant immune-related AEs included pneumonitis, hepatitis, colitis, and thyroid disease.
Atezolizumab is marketed as Tecentriq by Genentech and the prescribing information details warnings and precautions about immune-related AEs; pneumonitis, colitis, endocrinopathies (eg, thyroid disorders, adrenal insufficiency, and diabetes mellitus), and others, as well as infections, infusion-related reactions, and embryofetal toxicity.3
Atezolizumab treatment should be withheld for grade 2 pneumonitis; aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels of >3-5 times the upper limit of normal (ULN) or total bilirubin levels of >1.5 and up to 3 times the ULN; grade 2/3 diarrhea or colitis, adrenal insufficiency, hypothyroidism, or grade 3/4 hyperglycemia; grade 2 ocular inflammatory toxicity; grade 2/3 pancreatitis or grade 3/4 increases in amylase or lipase levels; grade 3/4 infection; grade 2 infusion-related reactions; and grade 3 rash. Treatment can then be resumed in patients whose AEs recover to grade 0 or 1.
Treatment should be permanently discontinued in the event of grade 3/4 pneumonitis; AST/ALT levels of >5 times the ULN or bilirubin levels of >3 times the ULN; grade 4 diarrhea or colitis; myasthenic syndrome/myasthenia gravis, Guillain-Barre syndrome or meningoencephalitis (all grades); grade 3/4 ocular inflammatory toxicity; grade 4 or recurrent (any grade) pancreatitis; grade 3/4 infusion-related reactions; or grade 4 rash. Patients should also be advised of the risk of fetal harm.
The approval last fall by the US Food and Drug Administration (FDA) of the immune checkpoint inhibitor atezolizumab for the treatment of metastatic non–small cell lung cancer (NSCLC) marked the second approved indication for the drug in a single year. Atezolizumab, which targets the programmed cell death protein-ligand 1 (PD-L1), has a unique mechanism of action compared with other approved immune checkpoint inhibitors and had been previously approved for the treatment of patients with advanced urothelial carcinoma. The current approval was based on 2 international, randomized, open-label trials, involving more than 1,000 patients, in which atezolizumab outperformed the chemotherapeutic drug docetaxel.
The POPLAR trial1 was a phase 2 study performed at 61 academic centers and community oncology practices across 13 countries in Europe and North America and enrolled 287 patients, while the phase 3 OAK trial2 was carried out at 193 centers in 31 countries and enrolled 850 patients.
Eligibility criteria for the 2 studies were similar; patients were 18 years or older, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, had measurable disease as per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), and adequate hematologic and end-organ function. Patients with asymptomatic or treated central nervous system (CNS) metastases were also eligible.
Patients were excluded from both studies if they had a history of autoimmune disease, pneumonitis or chronic viral diseases, or had received previous treatment with docetaxel, CD137 agonists, anti-CTLA4, anti-PD-L1 or anti-PD-1 therapeutics.
Patients were randomized 1:1 to receive atezolizumab, administered intravenously at a fixed dose of 1,200 mg, or a 75 mg/m2 dose of docetaxel, every 3 weeks on day 1 of each 3-week cycle. Randomization was stratified according to PD-L1 expression, number of previous chemotherapy regimens, and histology (squamous vs nonsquamous). Tumors were assessed at baseline and every 6 weeks for 36 weeks, and every 9 weeks thereafter until progression or, in patients who continued beyond progression, until discontinuation. PD-L1 expression was assessed prospectively on tumor cells and tumor-infiltrating immune cells, using the FDA-approved companion diagnostic, Ventana SP142 PD-L1 immunohistochemistry assay.
The primary endpoint in both studies was overall survival (OS), which was significantly improved in the atezolizumab treatment arm. In the POPLAR trial, over a minimum follow-up of 13 months, the median OS was 12.6 months in atezolizumab-treated patients, compared with 9.7 months in docetaxel-treated patients (hazard ratio [HR], 0.73; P = .04) and in the OAK trial, over a median follow-up of 21 months, the median OS was 15.7 months and 10.3 months, respectively.
The main difference between the 2 trials was that the OS benefit in the POPLAR trial reached statistical significance only in patients with the highest levels of PD-L1 expression, whereas in the OAK study, the OS was significantly improved regardless of PD-L1 expression status, although the greatest benefit was derived in patients with higher levels of PD-L1 expression (20.5 months and 8.9 months, respectively). The OS benefit was observed across all other prespecified subgroups, except in patients with EGFR mutation-positivity in the OAK trial. Progression-free survival and overall response rates were similar in the 2 treatment arms in both studies.
An additional 375 patients were enrolled in the OAK trial and included in the safety analyses. In both studies, the safety profile of atezolizumab was similar to that observed in previous studies of this drug. The most commonly observed adverse events (AEs) with atezolizumab treatment were fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, and constipation. Despite longer treatment duration, there were fewer incidences of grade 3/4 AEs with atezolizumab compared with docetaxel (37% vs 54%, respectively, in the OAK trial), a lower rate of discontinuation with atezolizumab treatment, and no deaths related to the study drug. The most common grade 3/4 AEs included dyspnea, pneumonia, hypoxia, hyponatremia, fatigue, and anemia. Clinically significant immune-related AEs included pneumonitis, hepatitis, colitis, and thyroid disease.
Atezolizumab is marketed as Tecentriq by Genentech and the prescribing information details warnings and precautions about immune-related AEs; pneumonitis, colitis, endocrinopathies (eg, thyroid disorders, adrenal insufficiency, and diabetes mellitus), and others, as well as infections, infusion-related reactions, and embryofetal toxicity.3
Atezolizumab treatment should be withheld for grade 2 pneumonitis; aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels of >3-5 times the upper limit of normal (ULN) or total bilirubin levels of >1.5 and up to 3 times the ULN; grade 2/3 diarrhea or colitis, adrenal insufficiency, hypothyroidism, or grade 3/4 hyperglycemia; grade 2 ocular inflammatory toxicity; grade 2/3 pancreatitis or grade 3/4 increases in amylase or lipase levels; grade 3/4 infection; grade 2 infusion-related reactions; and grade 3 rash. Treatment can then be resumed in patients whose AEs recover to grade 0 or 1.
Treatment should be permanently discontinued in the event of grade 3/4 pneumonitis; AST/ALT levels of >5 times the ULN or bilirubin levels of >3 times the ULN; grade 4 diarrhea or colitis; myasthenic syndrome/myasthenia gravis, Guillain-Barre syndrome or meningoencephalitis (all grades); grade 3/4 ocular inflammatory toxicity; grade 4 or recurrent (any grade) pancreatitis; grade 3/4 infusion-related reactions; or grade 4 rash. Patients should also be advised of the risk of fetal harm.
The approval last fall by the US Food and Drug Administration (FDA) of the immune checkpoint inhibitor atezolizumab for the treatment of metastatic non–small cell lung cancer (NSCLC) marked the second approved indication for the drug in a single year. Atezolizumab, which targets the programmed cell death protein-ligand 1 (PD-L1), has a unique mechanism of action compared with other approved immune checkpoint inhibitors and had been previously approved for the treatment of patients with advanced urothelial carcinoma. The current approval was based on 2 international, randomized, open-label trials, involving more than 1,000 patients, in which atezolizumab outperformed the chemotherapeutic drug docetaxel.
The POPLAR trial1 was a phase 2 study performed at 61 academic centers and community oncology practices across 13 countries in Europe and North America and enrolled 287 patients, while the phase 3 OAK trial2 was carried out at 193 centers in 31 countries and enrolled 850 patients.
Eligibility criteria for the 2 studies were similar; patients were 18 years or older, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, had measurable disease as per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), and adequate hematologic and end-organ function. Patients with asymptomatic or treated central nervous system (CNS) metastases were also eligible.
Patients were excluded from both studies if they had a history of autoimmune disease, pneumonitis or chronic viral diseases, or had received previous treatment with docetaxel, CD137 agonists, anti-CTLA4, anti-PD-L1 or anti-PD-1 therapeutics.
Patients were randomized 1:1 to receive atezolizumab, administered intravenously at a fixed dose of 1,200 mg, or a 75 mg/m2 dose of docetaxel, every 3 weeks on day 1 of each 3-week cycle. Randomization was stratified according to PD-L1 expression, number of previous chemotherapy regimens, and histology (squamous vs nonsquamous). Tumors were assessed at baseline and every 6 weeks for 36 weeks, and every 9 weeks thereafter until progression or, in patients who continued beyond progression, until discontinuation. PD-L1 expression was assessed prospectively on tumor cells and tumor-infiltrating immune cells, using the FDA-approved companion diagnostic, Ventana SP142 PD-L1 immunohistochemistry assay.
The primary endpoint in both studies was overall survival (OS), which was significantly improved in the atezolizumab treatment arm. In the POPLAR trial, over a minimum follow-up of 13 months, the median OS was 12.6 months in atezolizumab-treated patients, compared with 9.7 months in docetaxel-treated patients (hazard ratio [HR], 0.73; P = .04) and in the OAK trial, over a median follow-up of 21 months, the median OS was 15.7 months and 10.3 months, respectively.
The main difference between the 2 trials was that the OS benefit in the POPLAR trial reached statistical significance only in patients with the highest levels of PD-L1 expression, whereas in the OAK study, the OS was significantly improved regardless of PD-L1 expression status, although the greatest benefit was derived in patients with higher levels of PD-L1 expression (20.5 months and 8.9 months, respectively). The OS benefit was observed across all other prespecified subgroups, except in patients with EGFR mutation-positivity in the OAK trial. Progression-free survival and overall response rates were similar in the 2 treatment arms in both studies.
An additional 375 patients were enrolled in the OAK trial and included in the safety analyses. In both studies, the safety profile of atezolizumab was similar to that observed in previous studies of this drug. The most commonly observed adverse events (AEs) with atezolizumab treatment were fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, and constipation. Despite longer treatment duration, there were fewer incidences of grade 3/4 AEs with atezolizumab compared with docetaxel (37% vs 54%, respectively, in the OAK trial), a lower rate of discontinuation with atezolizumab treatment, and no deaths related to the study drug. The most common grade 3/4 AEs included dyspnea, pneumonia, hypoxia, hyponatremia, fatigue, and anemia. Clinically significant immune-related AEs included pneumonitis, hepatitis, colitis, and thyroid disease.
Atezolizumab is marketed as Tecentriq by Genentech and the prescribing information details warnings and precautions about immune-related AEs; pneumonitis, colitis, endocrinopathies (eg, thyroid disorders, adrenal insufficiency, and diabetes mellitus), and others, as well as infections, infusion-related reactions, and embryofetal toxicity.3
Atezolizumab treatment should be withheld for grade 2 pneumonitis; aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels of >3-5 times the upper limit of normal (ULN) or total bilirubin levels of >1.5 and up to 3 times the ULN; grade 2/3 diarrhea or colitis, adrenal insufficiency, hypothyroidism, or grade 3/4 hyperglycemia; grade 2 ocular inflammatory toxicity; grade 2/3 pancreatitis or grade 3/4 increases in amylase or lipase levels; grade 3/4 infection; grade 2 infusion-related reactions; and grade 3 rash. Treatment can then be resumed in patients whose AEs recover to grade 0 or 1.
Treatment should be permanently discontinued in the event of grade 3/4 pneumonitis; AST/ALT levels of >5 times the ULN or bilirubin levels of >3 times the ULN; grade 4 diarrhea or colitis; myasthenic syndrome/myasthenia gravis, Guillain-Barre syndrome or meningoencephalitis (all grades); grade 3/4 ocular inflammatory toxicity; grade 4 or recurrent (any grade) pancreatitis; grade 3/4 infusion-related reactions; or grade 4 rash. Patients should also be advised of the risk of fetal harm.
It's all about the care: new takes on access, quality, consolidation, and outcomes
Timely access to cancer care can translate into improved outcomes and quality of care, better patient quality of life, and cost savings for patients, practices, and society alike. There are many ways to facilitate such access, starting with educating patients and caregivers about its importance and that ensuring routine screening recommendations are followed to increase the likelihood of earlier-stage diagnosis and therefore earlier therapy initiation. But literal access – physically getting to the point of care – can also be an issue. For patients who live in rural or remote areas or those of low socioeconomic status, even in an urban area, the cost and logistics of getting to the point of care for treatment can be prohibitive, especially if the various components of care are not consolidated. Two articles in this issue focus on access to care and the impact on outcomes.
On page e263, Margaret Kemeny reports on the impact on outcomes and quality of care in patients of low socioeconomic status who were treated for breast cancer at a centralized cancer care center. The center was established at a public hospital in Queens, New York, to provide single-site, comprehensive care encompassing the clinical, supportive, and clerical-financial aspects of care during all phases of the disease trajectory. Kemeny compared the data of breast cancer patients treated at the hospital before the center was established with the five-year data of those treated at the cancer care center. She found that several factors changed, among them, that there was an increase in the number of patients diagnosed with earlier-stage breast cancer, an increase in the use of lumpectomies, and an increase in survival for patients with stage 3 disease. Not only are the findings of this study compelling, but the introduction and discussion sections provide a useful review of related literature as well as some practical “how-to” pointers for anyone thinking about setting up a similar center.
Gilbertson-White and colleagues continue with the theme of access to care in cancer patients, but shift the focus in their systemic literature review to supportive and palliative interventions for patients with advanced disease who live in rural communities (p. e248). Compared with urban communities, the rates of late-stage cancer and mortality are higher in rural communities, where low socioeconomic status, inadequate health coverage, and less workplace flexibility and social support hamper access to care. The findings show that the interventions resulted in a reduction in physical and emotional symptoms; improvement in patient quality of life and well-being, access to health care services, and quality of care; and cost savings for patients who received care from rural- instead of urban-based hospitals. The authors emphasize the importance of technology, especially tele- and video-conferencing, in delivering palliative and supportive care to underserved communities.
Patient-reported outcomes have been described as a picture of the patient perspective on treatment and its side effects, and they are increasingly being included in clinical trials and incorporated into the delivery of quality care. Valenti and colleagues studied the impact of cancer-therapy–related adverse events (AEs) on patient quality of life (p. e256). They compared the impact on quality of life reported by cancer patients who had experienced a particular AE with the impact envisioned by participants from the general public who had not experienced AEs and found that those who had experienced AEs perceived a lower impact on quality of life compared with the general public participants who had not experienced the AEs.
Also in this issue are a pertinent and comprehensive review of the latest in breast cancer therapies, specifically targeted therapies for multiple subtypes (p. e277); two Case Reports, one on managing tonsillar carcinoma with advanced radiation and chemotherapy techniques (p. e268), another on familial essential thrombocythemia associated with JAK2 V617F mutation in siblings (p. e274); and Community Translations articles on the approvals of atezolizumab for non–small-cell lung cancer (p. e 242)and lenlidomide for multiple myeloma in the maintenance setting (p. e245).
Timely access to cancer care can translate into improved outcomes and quality of care, better patient quality of life, and cost savings for patients, practices, and society alike. There are many ways to facilitate such access, starting with educating patients and caregivers about its importance and that ensuring routine screening recommendations are followed to increase the likelihood of earlier-stage diagnosis and therefore earlier therapy initiation. But literal access – physically getting to the point of care – can also be an issue. For patients who live in rural or remote areas or those of low socioeconomic status, even in an urban area, the cost and logistics of getting to the point of care for treatment can be prohibitive, especially if the various components of care are not consolidated. Two articles in this issue focus on access to care and the impact on outcomes.
On page e263, Margaret Kemeny reports on the impact on outcomes and quality of care in patients of low socioeconomic status who were treated for breast cancer at a centralized cancer care center. The center was established at a public hospital in Queens, New York, to provide single-site, comprehensive care encompassing the clinical, supportive, and clerical-financial aspects of care during all phases of the disease trajectory. Kemeny compared the data of breast cancer patients treated at the hospital before the center was established with the five-year data of those treated at the cancer care center. She found that several factors changed, among them, that there was an increase in the number of patients diagnosed with earlier-stage breast cancer, an increase in the use of lumpectomies, and an increase in survival for patients with stage 3 disease. Not only are the findings of this study compelling, but the introduction and discussion sections provide a useful review of related literature as well as some practical “how-to” pointers for anyone thinking about setting up a similar center.
Gilbertson-White and colleagues continue with the theme of access to care in cancer patients, but shift the focus in their systemic literature review to supportive and palliative interventions for patients with advanced disease who live in rural communities (p. e248). Compared with urban communities, the rates of late-stage cancer and mortality are higher in rural communities, where low socioeconomic status, inadequate health coverage, and less workplace flexibility and social support hamper access to care. The findings show that the interventions resulted in a reduction in physical and emotional symptoms; improvement in patient quality of life and well-being, access to health care services, and quality of care; and cost savings for patients who received care from rural- instead of urban-based hospitals. The authors emphasize the importance of technology, especially tele- and video-conferencing, in delivering palliative and supportive care to underserved communities.
Patient-reported outcomes have been described as a picture of the patient perspective on treatment and its side effects, and they are increasingly being included in clinical trials and incorporated into the delivery of quality care. Valenti and colleagues studied the impact of cancer-therapy–related adverse events (AEs) on patient quality of life (p. e256). They compared the impact on quality of life reported by cancer patients who had experienced a particular AE with the impact envisioned by participants from the general public who had not experienced AEs and found that those who had experienced AEs perceived a lower impact on quality of life compared with the general public participants who had not experienced the AEs.
Also in this issue are a pertinent and comprehensive review of the latest in breast cancer therapies, specifically targeted therapies for multiple subtypes (p. e277); two Case Reports, one on managing tonsillar carcinoma with advanced radiation and chemotherapy techniques (p. e268), another on familial essential thrombocythemia associated with JAK2 V617F mutation in siblings (p. e274); and Community Translations articles on the approvals of atezolizumab for non–small-cell lung cancer (p. e 242)and lenlidomide for multiple myeloma in the maintenance setting (p. e245).
Timely access to cancer care can translate into improved outcomes and quality of care, better patient quality of life, and cost savings for patients, practices, and society alike. There are many ways to facilitate such access, starting with educating patients and caregivers about its importance and that ensuring routine screening recommendations are followed to increase the likelihood of earlier-stage diagnosis and therefore earlier therapy initiation. But literal access – physically getting to the point of care – can also be an issue. For patients who live in rural or remote areas or those of low socioeconomic status, even in an urban area, the cost and logistics of getting to the point of care for treatment can be prohibitive, especially if the various components of care are not consolidated. Two articles in this issue focus on access to care and the impact on outcomes.
On page e263, Margaret Kemeny reports on the impact on outcomes and quality of care in patients of low socioeconomic status who were treated for breast cancer at a centralized cancer care center. The center was established at a public hospital in Queens, New York, to provide single-site, comprehensive care encompassing the clinical, supportive, and clerical-financial aspects of care during all phases of the disease trajectory. Kemeny compared the data of breast cancer patients treated at the hospital before the center was established with the five-year data of those treated at the cancer care center. She found that several factors changed, among them, that there was an increase in the number of patients diagnosed with earlier-stage breast cancer, an increase in the use of lumpectomies, and an increase in survival for patients with stage 3 disease. Not only are the findings of this study compelling, but the introduction and discussion sections provide a useful review of related literature as well as some practical “how-to” pointers for anyone thinking about setting up a similar center.
Gilbertson-White and colleagues continue with the theme of access to care in cancer patients, but shift the focus in their systemic literature review to supportive and palliative interventions for patients with advanced disease who live in rural communities (p. e248). Compared with urban communities, the rates of late-stage cancer and mortality are higher in rural communities, where low socioeconomic status, inadequate health coverage, and less workplace flexibility and social support hamper access to care. The findings show that the interventions resulted in a reduction in physical and emotional symptoms; improvement in patient quality of life and well-being, access to health care services, and quality of care; and cost savings for patients who received care from rural- instead of urban-based hospitals. The authors emphasize the importance of technology, especially tele- and video-conferencing, in delivering palliative and supportive care to underserved communities.
Patient-reported outcomes have been described as a picture of the patient perspective on treatment and its side effects, and they are increasingly being included in clinical trials and incorporated into the delivery of quality care. Valenti and colleagues studied the impact of cancer-therapy–related adverse events (AEs) on patient quality of life (p. e256). They compared the impact on quality of life reported by cancer patients who had experienced a particular AE with the impact envisioned by participants from the general public who had not experienced AEs and found that those who had experienced AEs perceived a lower impact on quality of life compared with the general public participants who had not experienced the AEs.
Also in this issue are a pertinent and comprehensive review of the latest in breast cancer therapies, specifically targeted therapies for multiple subtypes (p. e277); two Case Reports, one on managing tonsillar carcinoma with advanced radiation and chemotherapy techniques (p. e268), another on familial essential thrombocythemia associated with JAK2 V617F mutation in siblings (p. e274); and Community Translations articles on the approvals of atezolizumab for non–small-cell lung cancer (p. e 242)and lenlidomide for multiple myeloma in the maintenance setting (p. e245).
Biosimilars poised to save $54 billion over the next decade
Biosimilars could reduce overall spending on biologic products by $54 billion from 2017 to 2026, according to new research from the Rand Corp.
Given the level of uncertainty surrounding the biosimilars market, however, the range of savings could be as low at $24 billion or as high as $150 billion.
“Because of limited U.S. experience with biosimilars, the key assumptions on market share and biosimilar prices are ‘best guesses’ based on anecdotes or professional opinion,” Andrew Mulcahy, PhD, a health policy researcher at Rand, and his colleagues, wrote in a perspective report.
“Whether actual cost savings end up above or below our baseline estimate hinges in large part on whether manufacturers continue to have a business case to invest in developing and marketing biosimilars,” the authors noted, citing a number of areas, including intellectual property litigation, payment, price competition, nonprice competition from reference biologic manufacturers, naming convention, and interchangeability.
Getting over these hurdles could require legislative or regulatory solutions.
“The pervasive uncertainty in the U.S. biosimilar market – including questions as to whether the market will be sustainable and lead to cost savings, as intended – presents two choices for policymakers,” Dr. Mulcahy and his colleagues wrote. “One strategy is to let the market continue to develop under current policies,” with stability coming from experience.
The alternative could be policy levers to “help steer the U.S. biosimilar market more quickly to a sustainable, competitive state,” they continued. “For example, regulators at the FDA could experiment with new approaches to provide stronger, earlier signals through guidance documents or other mechanisms on expectations surrounding interchangeability and other topics.”
The FDA appears to be moving on the latter. In an Oct. 23 blog post, FDA Commissioner Scott Gottlieb, MD, and Leah Christl, PhD, associate director for therapeutic biologics in the office of new drugs at the FDA’s Center for Drug Evaluation and Research, outlined a number of recent tools to help biosimilar adoption. The resources provide basics such as the basic definition associated with biosimilars (i.e., what is a biosimilar and a reference product, and what it means to be interchangeable), the standards of approval that biosimilars must go through, and easily accessible information on what the FDA is using to review biosimilarity.
“Next, FDA plans to embark on additional research with health care professionals to learn more about the types of information prescribers need to properly communicate with their patients about biosimilars,” Commissioner Gottlieb and Dr. Christl wrote. “An increase in market competition, offered by a growing complement of biosimilars, may lead to meaningfully reduced costs for both patients and our health care system.”
The Centers for Medicare & Medicaid Services also plays a role in developing policy to spur biosimilar adoption, Dr. Mulcahy and his colleagues wrote. They note work being done by the Medicare Payment Advisory Commission on recommendations that could address payment for physician-administered biosimilars under Part B, as well as incentives in the Part D prescription drug program to steer patients and providers toward lower cost biosimilars when appropriate. CMS changed current payment policy for biosimilars for 2018, which may have an effect.
“Beyond FDA regulation, payment, and coverage, both government and industry could play a role in educating patients and providers about the potential cost savings from biosimilars, much like both groups have done for generic drugs,” they stated. “While our study does not address whether policy action is needed now, it is likely that the answer will become clearer over the next 1 to 3 years as the market continues to develop.”
Biosimilars could reduce overall spending on biologic products by $54 billion from 2017 to 2026, according to new research from the Rand Corp.
Given the level of uncertainty surrounding the biosimilars market, however, the range of savings could be as low at $24 billion or as high as $150 billion.
“Because of limited U.S. experience with biosimilars, the key assumptions on market share and biosimilar prices are ‘best guesses’ based on anecdotes or professional opinion,” Andrew Mulcahy, PhD, a health policy researcher at Rand, and his colleagues, wrote in a perspective report.
“Whether actual cost savings end up above or below our baseline estimate hinges in large part on whether manufacturers continue to have a business case to invest in developing and marketing biosimilars,” the authors noted, citing a number of areas, including intellectual property litigation, payment, price competition, nonprice competition from reference biologic manufacturers, naming convention, and interchangeability.
Getting over these hurdles could require legislative or regulatory solutions.
“The pervasive uncertainty in the U.S. biosimilar market – including questions as to whether the market will be sustainable and lead to cost savings, as intended – presents two choices for policymakers,” Dr. Mulcahy and his colleagues wrote. “One strategy is to let the market continue to develop under current policies,” with stability coming from experience.
The alternative could be policy levers to “help steer the U.S. biosimilar market more quickly to a sustainable, competitive state,” they continued. “For example, regulators at the FDA could experiment with new approaches to provide stronger, earlier signals through guidance documents or other mechanisms on expectations surrounding interchangeability and other topics.”
The FDA appears to be moving on the latter. In an Oct. 23 blog post, FDA Commissioner Scott Gottlieb, MD, and Leah Christl, PhD, associate director for therapeutic biologics in the office of new drugs at the FDA’s Center for Drug Evaluation and Research, outlined a number of recent tools to help biosimilar adoption. The resources provide basics such as the basic definition associated with biosimilars (i.e., what is a biosimilar and a reference product, and what it means to be interchangeable), the standards of approval that biosimilars must go through, and easily accessible information on what the FDA is using to review biosimilarity.
“Next, FDA plans to embark on additional research with health care professionals to learn more about the types of information prescribers need to properly communicate with their patients about biosimilars,” Commissioner Gottlieb and Dr. Christl wrote. “An increase in market competition, offered by a growing complement of biosimilars, may lead to meaningfully reduced costs for both patients and our health care system.”
The Centers for Medicare & Medicaid Services also plays a role in developing policy to spur biosimilar adoption, Dr. Mulcahy and his colleagues wrote. They note work being done by the Medicare Payment Advisory Commission on recommendations that could address payment for physician-administered biosimilars under Part B, as well as incentives in the Part D prescription drug program to steer patients and providers toward lower cost biosimilars when appropriate. CMS changed current payment policy for biosimilars for 2018, which may have an effect.
“Beyond FDA regulation, payment, and coverage, both government and industry could play a role in educating patients and providers about the potential cost savings from biosimilars, much like both groups have done for generic drugs,” they stated. “While our study does not address whether policy action is needed now, it is likely that the answer will become clearer over the next 1 to 3 years as the market continues to develop.”
Biosimilars could reduce overall spending on biologic products by $54 billion from 2017 to 2026, according to new research from the Rand Corp.
Given the level of uncertainty surrounding the biosimilars market, however, the range of savings could be as low at $24 billion or as high as $150 billion.
“Because of limited U.S. experience with biosimilars, the key assumptions on market share and biosimilar prices are ‘best guesses’ based on anecdotes or professional opinion,” Andrew Mulcahy, PhD, a health policy researcher at Rand, and his colleagues, wrote in a perspective report.
“Whether actual cost savings end up above or below our baseline estimate hinges in large part on whether manufacturers continue to have a business case to invest in developing and marketing biosimilars,” the authors noted, citing a number of areas, including intellectual property litigation, payment, price competition, nonprice competition from reference biologic manufacturers, naming convention, and interchangeability.
Getting over these hurdles could require legislative or regulatory solutions.
“The pervasive uncertainty in the U.S. biosimilar market – including questions as to whether the market will be sustainable and lead to cost savings, as intended – presents two choices for policymakers,” Dr. Mulcahy and his colleagues wrote. “One strategy is to let the market continue to develop under current policies,” with stability coming from experience.
The alternative could be policy levers to “help steer the U.S. biosimilar market more quickly to a sustainable, competitive state,” they continued. “For example, regulators at the FDA could experiment with new approaches to provide stronger, earlier signals through guidance documents or other mechanisms on expectations surrounding interchangeability and other topics.”
The FDA appears to be moving on the latter. In an Oct. 23 blog post, FDA Commissioner Scott Gottlieb, MD, and Leah Christl, PhD, associate director for therapeutic biologics in the office of new drugs at the FDA’s Center for Drug Evaluation and Research, outlined a number of recent tools to help biosimilar adoption. The resources provide basics such as the basic definition associated with biosimilars (i.e., what is a biosimilar and a reference product, and what it means to be interchangeable), the standards of approval that biosimilars must go through, and easily accessible information on what the FDA is using to review biosimilarity.
“Next, FDA plans to embark on additional research with health care professionals to learn more about the types of information prescribers need to properly communicate with their patients about biosimilars,” Commissioner Gottlieb and Dr. Christl wrote. “An increase in market competition, offered by a growing complement of biosimilars, may lead to meaningfully reduced costs for both patients and our health care system.”
The Centers for Medicare & Medicaid Services also plays a role in developing policy to spur biosimilar adoption, Dr. Mulcahy and his colleagues wrote. They note work being done by the Medicare Payment Advisory Commission on recommendations that could address payment for physician-administered biosimilars under Part B, as well as incentives in the Part D prescription drug program to steer patients and providers toward lower cost biosimilars when appropriate. CMS changed current payment policy for biosimilars for 2018, which may have an effect.
“Beyond FDA regulation, payment, and coverage, both government and industry could play a role in educating patients and providers about the potential cost savings from biosimilars, much like both groups have done for generic drugs,” they stated. “While our study does not address whether policy action is needed now, it is likely that the answer will become clearer over the next 1 to 3 years as the market continues to develop.”
Onodera’s Prognostic Nutritional Index in soft tissue sarcoma patients as a predictor of wound complications
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2).
In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications.
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2).
In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications.
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2).
In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications.
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
David Henry's JCSO podcast, September-October 2017
In this podcast, coinciding with breast cancer awareness month, Dr David Henry highlights an article by contributor Jane de Lartigue on recent advances in the use of targeted therapies in multiple breast cancer subtypes and another by a practicing oncologist on the positive impact of centralizing breast cancer care in an urban public hospital. Patient-reported outcomes are the focus a review on PROs in palliative and supportive interventions rural cancer patients and an original research report on findings on adverse events from systemic treatment of cancer and patient-reported quality of life. Also featured are two Community Translations columns on the approvals for atezolizumab for non–small-cell lung cancer and lenalidomide as standard of care for multiple myeloma in the maintenance setting, and two case reports on familial essential thrombocythemia associated with JAK2 V617F mutation in siblings and on managing tonsillar carcinoma with advanced radiation and chemotherapy techniques.
Listen to the podcast below.
In this podcast, coinciding with breast cancer awareness month, Dr David Henry highlights an article by contributor Jane de Lartigue on recent advances in the use of targeted therapies in multiple breast cancer subtypes and another by a practicing oncologist on the positive impact of centralizing breast cancer care in an urban public hospital. Patient-reported outcomes are the focus a review on PROs in palliative and supportive interventions rural cancer patients and an original research report on findings on adverse events from systemic treatment of cancer and patient-reported quality of life. Also featured are two Community Translations columns on the approvals for atezolizumab for non–small-cell lung cancer and lenalidomide as standard of care for multiple myeloma in the maintenance setting, and two case reports on familial essential thrombocythemia associated with JAK2 V617F mutation in siblings and on managing tonsillar carcinoma with advanced radiation and chemotherapy techniques.
Listen to the podcast below.
In this podcast, coinciding with breast cancer awareness month, Dr David Henry highlights an article by contributor Jane de Lartigue on recent advances in the use of targeted therapies in multiple breast cancer subtypes and another by a practicing oncologist on the positive impact of centralizing breast cancer care in an urban public hospital. Patient-reported outcomes are the focus a review on PROs in palliative and supportive interventions rural cancer patients and an original research report on findings on adverse events from systemic treatment of cancer and patient-reported quality of life. Also featured are two Community Translations columns on the approvals for atezolizumab for non–small-cell lung cancer and lenalidomide as standard of care for multiple myeloma in the maintenance setting, and two case reports on familial essential thrombocythemia associated with JAK2 V617F mutation in siblings and on managing tonsillar carcinoma with advanced radiation and chemotherapy techniques.
Listen to the podcast below.