User login
Research and Reviews for the Practicing Oncologist
Differences in psychosocial stressors between black and white cancer patients
For patients with cancer, acknowledgment of mental and emotional distress is critically important when developing and implementing a treatment plan. The psychosocial distress associated with cancer diagnosis and treatment can have an impact on a patient’s quality of life, influence a patient’s ability to adhere to treatment regimens, and increase cost of care.1-4 Rates of depression have been reported to range from 8%-36%, with a 29% risk of anxiety in cancer patients.5, 6 Emotional distress is linked to increased hopelessness about their cancer diagnosis, increased issues with chronic pain, and negative treatment outcomes.7 Timely screening of psychosocial distress at the first clinical visit enables providers to make appropriate referrals to resources early in their course of treatment; however, referrals to psychosocial interventions remain infrequent nationwide in the United States.8
There is some evidence of a differential impact of cancer on mental health diagnoses between racial/ethnic groups; however, results are not entirely consistent across studies. Using the Kessler Pyschological Distress Scale (K6) score, Alcala and colleagues found that cancer was more detrimental to mental health for black patients than for non-Hispanic white patients.9 Black breast cancer survivors have also been shown to be more likely to stop working during the early phases of their treatment, indicating that they and their physicians need to take steps to minimize long-term employment consequences.10 However, in a study of women with breast cancer, black women reported fewer depressive symptoms than did non-Hispanic whites.11
The American College of Surgeons’ Commission on Cancer (ACS CoC) developed a set of Continuum of Care standards in 2012, including the implementation of psychosocial distress screening for patients with cancer. Since 2015, all accredited cancer programs are now required to evaluate these patients for signs of distress during at least 1 pivotal physician visit.12 The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology has developed a tool that provides a mechanism for meeting the requirements of the ACS CoC accreditation requirements. The NCCN defines distress in cancer as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms and its treatment.”13 The recommendation of the NCCN is to provide a brief screening for psychosocial distress to identify individuals in need of additional support and to provide referrals for patients at high risk of psychosocial distress. The NCCN Distress Thermometer screening tool has been widely accepted as an effective method of identifying and characterizing distress. The NCCN tool provides a visual analogue scale for patients to rate their current distress on a scale of 1-10, as well as a problem checklist. The problem checklist includes 22 stressors addressing the practical, spiritual/religious, emotional, and physical concerns of patients. Although the NCCN tool is used widely, differences in distress scores between black and white cancer patients have not been previously described. The purpose of the study was to compare the global distress screening scores of black and white patients at an academic comprehensive cancer center in the Midwest. A second objective was to examine the distribution of individual stressors between black and white women.
Methods
Study sample
The study included all cancer patients from a cancer center in the Midwest who completed the NCCN distress thermometer during January 1, 2015-February 19, 2016. The patient population for this cancer center was primarily non-Hispanic white and non-Hispanic black, therefore, only patients identifying as non-Hispanic white and non-Hispanic black are included in this analysis. As part of routine clinical care, patients are asked to complete the NCCN distress thermometer at their first visit to the center. All patients in this analytic sample were newly diagnosed patients. Some patients also completed the NCCN screening tool at additional appointments; therefore, for patients with more than 1 completed tool, only the first distress screening was used in this analysis. Overall scores and individual stressor scores were entered into the electronic medical record by clinic staff at the time the patients were roomed for their visit. Patient demographics were collected through a reporting mechanism within the electronic medical record that allows for monitoring of the psychosocial screening process.
Variables
Race was assessed through self-report and classified as non-Hispanic white and non-Hispanic black. There were not enough patients of any other racial/ethnic group to be included in this analysis. Age was categorized as 18-40 years, 41-60 years, 61-84 years, and 85 years and older. Cancer type was grouped as follows: head and neck cancer, gastrointestinal cancer (esophagus, stomach, small intestine, colon, rectum, anus), hepatobiliary (liver, gallbladder, pancreas), sarcoma (bone and soft tissue), melanoma, nonmelanoma skin cancer, breast cancer, genitourinary (prostate, kidney, bladder), hematologic, and brain.
Two primary outcomes were assessed: overall distress, and each individual problem indicator. Overall distress was assessed using the thermometer visual analog rating (the thermometer rating of the NCCN screening tool) where possible values range from 0 (no distress) to 10 (extreme distress). The overall distress score was categorized into low distress (<4) and high distress (≥4) for analysis. The response options for individual stressors on the problem list are Yes or No for each of 17 discrete stressors: child care, housing, insurance/financial, transportation, work/school, treatment decisions, dealing with children, dealing with partner, ability to have children, family health issues, depression, fears, nervousness, sadness, worry, loss of interest, and spiritual/religious concerns. Physical complaints were not assessed in this study. Comparisons were made between white and black patients on overall distress score as well as for each individual psychosocial stressor.
Data analysis
Descriptive statistics (counts and proportions or means and standard deviations) were calculated stratified by race. Categorical variables were compared by race using chi-square or Fisher exact test. Logistic regression was used to predict high distress by race adjusting for sex, age, and cancer type. All analyses were conducted using SAS 9.4 (Cary, NJ).
This study was reviewed and approved by the Saint Louis University Institutional Review Board (protocol number 26269).
Results
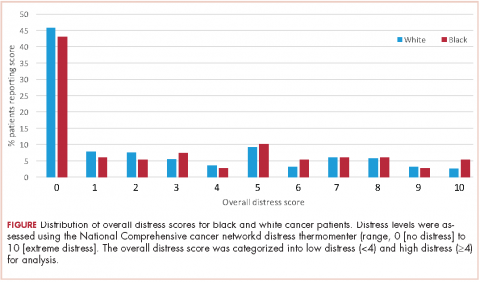
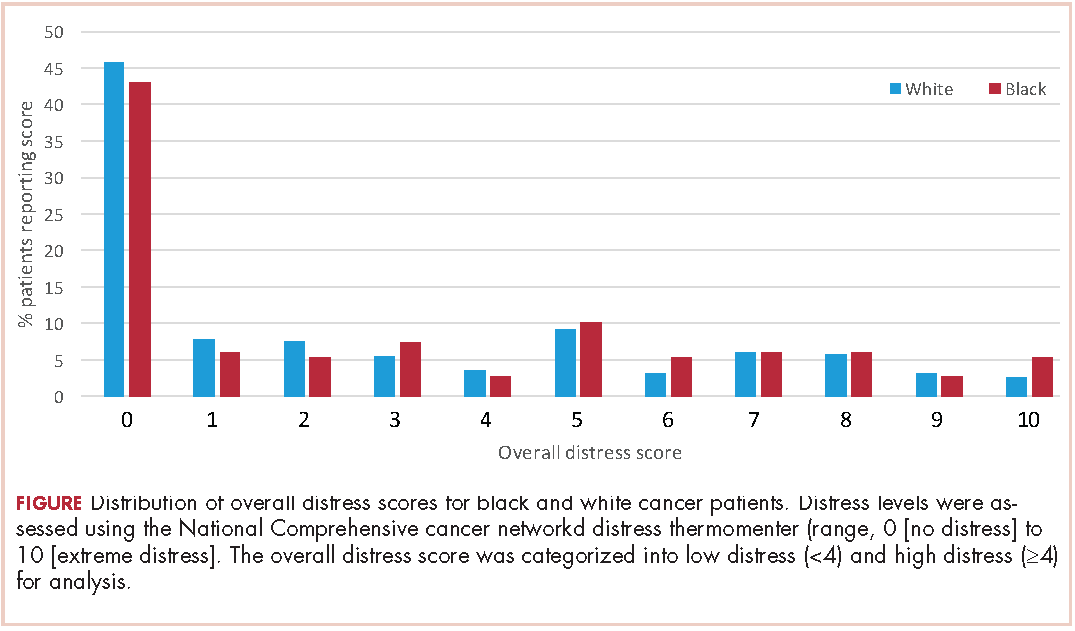
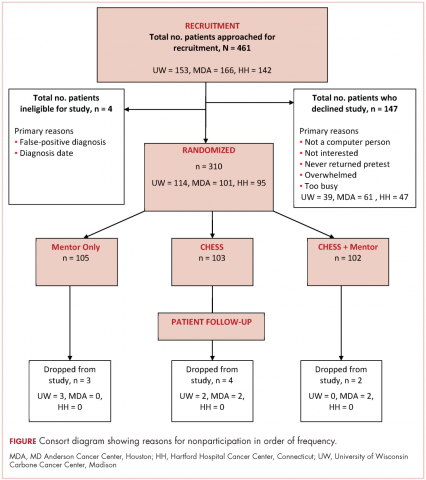
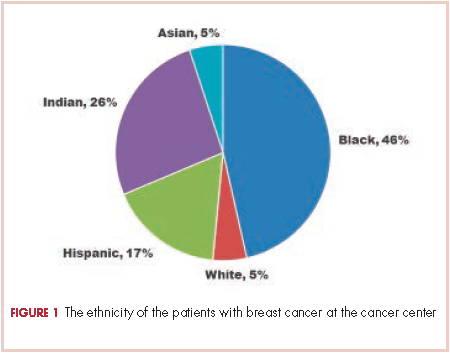
A total of 933 patients with cancer completed the NCCN distress screening tool. Of that total, 45 patients did not complete the overall distress score thermometer, but did complete the checklist of individual stressors. Those 45 patients were excluded from the logistic regression analysis for overall distress score, but included on comparisons of individual stressors. The distribution of overall distress scores by race can be seen in the Figure.
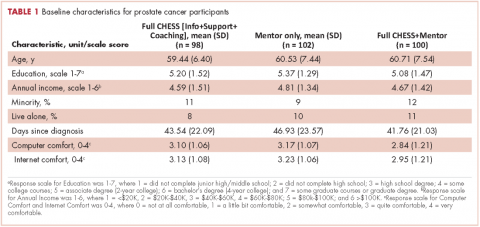
Briefly, the full sample was 16.9% black and 38.8% female. In all, 32.6% of the sample indicated high distress on the distress thermometer at their first visit. Demographics for the participants stratified by race are reported in Table 1 (see PDF).There was no statistically significant difference in the gender or age distribution between black and white patients. Cancer distribution did vary by race. Black patients were proportionally more represented in gastrointestinal cancers, hepatobiliary cancers, sarcomas, breast cancer, and genitourinary cancers. White patients were proportionally more represented in melanoma, nonmelanoma skin cancers, and hematologic cancers.
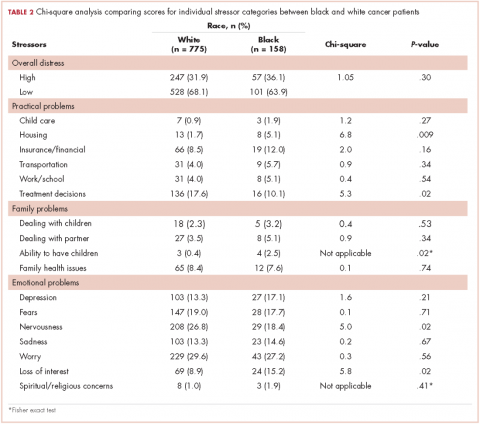
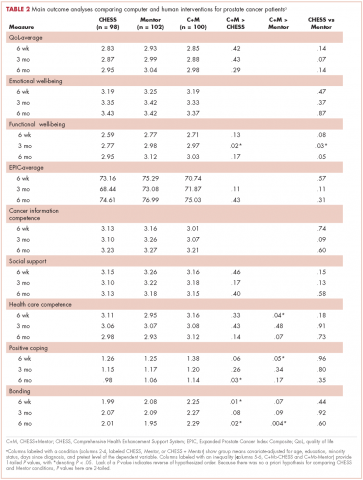
Table 2 presents bivariate comparisons on overall distress and individual stressors between black and white patients. There was no difference in the high distress between black and white patients in bivariate analysis (31.9% and 36.1%, respectively, P = .30). However, there were differences in the individual stressors identified for each racial group (Table 2). White patients, compared with black patients, more frequently identified treatment decisions (17.6% vs 10.1%, P = .02) and nervousness (26.8% vs 18.4%, P = .02) as sources of distress. Black patients, compared with white patients, more frequently identified housing (5.1% vs 1.7%, P = .009), the ability to have children (2.5% vs 0.4%, P =.02), and loss of interest (15.2% vs 8.9%, P = .02) as sources of distress. Distress scores did not differ between black and white patients for child care, insurance or financial issues, transportation, work or school, dealing with children, dealing with partners, family health issues, depression, fears, sadness, worry, or spiritual or religious concerns.
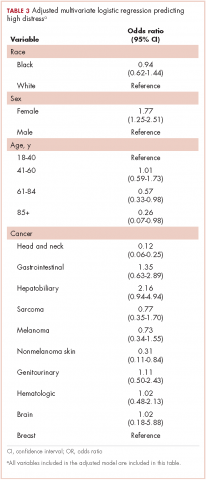
Table 3 presents the results from the logistic analysis predicting high distress. In adjusted analysis, black race did not predict high distress (OR, 0.94; 95% confidence interval [CI], 0.62-1.44). High distress was associated with sex, age, and some cancer categories. Women had 77% higher odds of high distress compared with men (OR, 1.77; 95% CI, 1.25-2.51).
Compared with patients aged 18-44 years, patients aged 61-84 had 43% lower odds of high distress (OR, 0.57; 95% CI, 0.33-0.98), and patients aged 85 and older had 74% lower odds of high distress (OR, 0.26; 95% CI, 0.07-0.98). There was no statistically significant difference between patients aged 18-40 and those aged 41-60 for high distress (OR, 1.01; 95% CI, 0.59-1.73).
Discussion
Management of patients with cancer continues to evolve. Although a tremendous amount of importance is still placed on the pathophysiology of cancer and its prescribed treatments, more emphasis is being assigned to the physical and psychosocial effects of cancer on these patients. In 2008, the Institute of Medicine published a report that examined the psychosocial health of patients with cancer.14 The report recommended that all cancer care should ensure the provision of appropriate psychosocial health services by facilitating effective communication between patients and care providers, identifying each patient’s psychosocial health needs, coordinating referrals for psychosocial services and monitoring efficacy of psychosocial interventions. The inclusion of psychosocial distress screening in all cancer programs accredited by the ACS CoC helped to prioritize the identification and treatment of psychosocial issues for all cancer patients.
The present study is the first of its kind to compare the individual stressors identified through psychosocial distress screening between black and white cancer patients. In our sample, 304 of 933 patients (32%) reported high distress, with a total score of ≥4. Previous research on overall distress difference across race/ethnicity is mixed. VanHoose and colleagues found no difference in overall distress between racial groups,15 Alcala and colleagues found higher overall distress in black patients with cancer compared with white patients with cancer,9 and Culver and colleagues found black women with breast cancer had lower overall distress compared with white women.11 We found no difference in the presence of high distress between black and white patients at our cancer center in either crude or adjusted analysis. Differences in overall distress across studies may be owing to the timing of screening. Given that overall distress may vary across time16,17 and there is no current information on whether temporal variations in distress differ by race, it is possible that the time of distress assessment may influence demonstrated differences between racial groups. For example, if different stressors affect black and white women differentially, and those stressors are associated with different points across the cancer continuum, then we might see that the magnitude of racial differences in overall stress are time dependent. Alcala and colleagues examined any cancer diagnosis across the lifespan, whereas Culver and colleagues examined multiple time points across treatment for a small group of breast cancer patients. Badr and colleagues, in a sample of head and neck cancer patients, found that distress increased across the course of treatment;18 however they did not examine variations in type of stressors related to overall distress, nor did they examine racial differences in distress. Differences in results may also be the result of differences in measurement of distress. Culver and colleagues did not examine distress using the NCCN distress thermometer, rather psychological distress was measured by a scale rating a series of “mood-descriptive adjectives” (p. 497).11 Alcala used the K-6 as a measure of psychological distress;9 therefore, demonstrated differences in overall distress between white and black women may vary across studies because of differences in measurement of the underlying distress variable. The lack of racial differences in overall distress in our study is consistent with the findings of VanHoose and colleagues,15 who also examined distress near the start of treatment and also used the NCCN distress thermometer as the measure of psychosocial distress.
We did find differences in the individual stressors between racial groups, indicating that the source of distress does vary between black and white cancer patients. Black patients more frequently reported distress secondary to housing, loss of interest and their ability to have children than did white patients. By comparison, white patients more frequently reported distress secondary to nervousness and treatment decisions than black patients. Identified differences in individual stressors may be attributable to sociocultural differences or differences in external support. It is also possible that black patients are more likely to willingly report distress related to nonpsychological factors, whereas white patients are more willing to report factors, such as nervousness, that are related to psychological disorders. Although it has been suggested that black cancer patients have more concerns about finances and work than do white cancer patients,19 we did not identify a statistically significant difference in child care, insurance or financial issues, transportation, work, or school between these 2 cohorts. This may be because the psychosocial distress screening score included in this study was performed at the time of initial diagnosis, and not further into their prescribed treatment at which point the financial worries may be more realized. Psychosocial screening scores obtained at subsequent visits were not included in the analysis because they are not routinely collected as part of clinical care in the center where this study took place. Furthermore, it is impossible to identify where a specific patient is in their treatment regimen based on their demographic data or subsequent distress scores in our data extraction tool. Further investigation into the sources of distress at different time points along the continuum of care may shed more light on this topic.
Limitations
There are several limitations to this study. First, the method of data extraction from an electronic medical record report limited the capacity to explore possible differences between the patients in our sample, such as insurance status, level of education, available social support, current employment status, stage of disease, overall prognosis and prescribed treatment regimen.
Second, there were likely patients who either did not complete a psychosocial distress screening tool or whose data were not entered into the electronic medical record for inclusion in the analysis. The present study period took place during the implementation of the NCCN tool at the center. Although the policy was to screen all new patients as part of routine care; not all patients seen at the center received the NCCN screening tool at their first visit. Owing to the mechanisms for data entry and abstraction, only information from the patients who had a completed form was able to be accessed for this study, thus a statistical comparison between those who did and did not receive the NCCN tool cannot be made. During the timeframe for this study, the head and neck, breast, genitourinary, and hematologic services completed proportionally more NCCN screening of new patients than other services in the center. This is reflected in the distributional breakdown of cancer in the overall sample of this study. It is possible that the results are more representative of differences between black and white cancer patients in the services that were more likely to properly implement NCCN screening.
Third, our patient population was derived from an urban, academic medical center and the results may not be generalizable to other patient populations.
Fourth, the NCCN distress thermometer is a single-item rating of overall global distress that is not intended to be a diagnostic indicator of psychological comorbidity and, therefore, does not distinguish between common psychological diagnoses such as depression or anxiety. However, the usefulness of the tool is to provide an impetus for referral to services that may then encompass the evaluation and diagnosis of particular psychological conditions. Further, the distress thermometer tool is designed to identify stress relating to the social aspects of cancer diagnosis and treatment and is not limited to psychological distress alone.
Strengths
Despite the limitations, there are also significant strengths to this study. The NCCN tool is a widely accepted measure for the assessment of psychosocial distress in patients with cancer. The measure is a common and routine clinical instrument,20 and has also been used widely in research.18,21-24 Given the urban, academic environment of our clinical practice, our population is more racially diverse than other settings, allowing for initial examination of disparities between white and black cancer patients.
Clinical implications
Understanding differences in common psychosocial stressor between black and white cancer patients may allow for clinicians to strategically look for different types of stressors in order to facilitate faster referrals to appropriate services. It has been established in the literature that distress is correlated to cancer-related outcomes and distress screening is now considered standard of care when treating cancer patients. Identifying differences in psychosocial stressors among black and white cancer patients is paramount to ensuring that the appropriate resources are available to assist them through their cancer journey. The differences in type of stressor, may indicate fundamental differences in the way patients perceive their disease or the social and cultural implication of a cancer diagnosis. In this study, white patients were more likely to find distress in the psychological realm (nervousness, decision-making), whereas black patients were more likely to be distressed about social issues (housing, ability to have children, and loss of interest). The referral needs of patients may be quite different, even with similar levels of overall distress. More research is necessary to further characterize sources of distress for cancer patients, how this distress impacts a patient’s physical and emotional well-being and how health care providers can better identify these issues and make the necessary referrals to support the whole patient.
1. Holland JC, Reznik I. Pathways for psychosocial care of cancer survivors. Cancer. 2005;104(11 Suppl):2624-2637.
2. Strasser F, Sweeney C, Willey J, Benisch-Tolley S, Palmer L, Bruera E. Impact of a half-day multidisciplinary symptom control and palliative care outpatient clinic in a comprehensive cancer center on recommendations, symptom intensity, and patient satisfaction: a retrospective descriptive study. J Pain Symptom Manage. 2004;27(6):481-491.
3. Carlson LE, Bultz BD. Efficacy and medical cost offset of psychosocial interventions in cancer care: making the case for economic analyses. Psychooncology. 2004;13(12):837-849.
4. Holland J, Bultz BD. The NCCN Guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw. 2007;5(1):3-7.
5. Krebber A, Buffart L, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121-130.
6. Sharp L, Carsin AE , Timmons A. Associations between cancer-related financial stress and strain and psychological well-being among individuals living with cancer. Psychooncology. 2013;22(4):745-755.
7. Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155(2):232-243.
8. Holland JC. Preliminary guidelines for the treatment of distress. Oncology. 1997;11(11A):109-114.
9. Alcala HE. Differential mental health impact of cancer across racial/ethnic groups: findings from a population-based study in California. BMC Public Health. 2014;14:930.
10. Bradley CJ, Wilk A. Racial differences in quality of life and employment outcomes in insured women with breast cancer. J Cancer Surviv. 2014;8(1):49-59.
11. Culver JL, Arena PL, Antoni MH, Carver CS. Coping and distress among women under treatment for early stage breast cancer: comparing African Americans, Hispanics and non-Hispanic whites. Psychooncology. 2002;11(6):495-504.
12. American College of Surgeons Commission on Cancer. ACSCC website. Cancer program standards: ensuring patient-centered care. 2016 edition. https://www.facs.org/quality-programs/cancer/coc/standards. Posted 2016. Accessed August 30, 2017.
13. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Distress Management. National Comprehensive Cancer Network, 2014.https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf Accessed August 30, 2017.
14. Institute of Medicine. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: The National Academies Press; 2008. https://doi.org/10.17226/11993. Accessed August 30, 2017.
15. VanHoose L, Black LL, Doty K, et al. An analysis of the distress thermometer problem list and distress in patients with cancer. Support Care Cancer. 2015;23(5):1225-1232.
16. Gessler S, Low J, Daniells E, et al. Screening for distress in cancer patients: is the distress thermometer a valid measure in the UK and does it measure change over time? A prospective validation study. Psychooncology. 2008;17(6):538-547.
17. Enns A, Waller A, Groff SL, Bultz BD, Fung T, Carlson LE. Risk factors for continuous distress over a 12-month period in newly diagnosed cancer outpatients. J Psychosoc Oncol. 2013;31(5):489-506.
18. Badr H, Gupta V, Sikora A, Posner M. Psychological distress in patients and caregivers over the course of radiotherapy for head and neck cancer. Oral Oncol. 2014;50(10):1005-1011.
19. Wang X, Cosby LG, Harris MG, Liu T. Major concerns and needs of breast cancer patients. Cancer Nurs. 1999;22(2):157-163.
20. Dabrowski M, Boucher K, Ward JH, et al. Clinical experience with the NCCN distress thermometer in breast cancer patients. J Natl Compr Canc Netw. 2007;5(1):104-11.
21. Buchmann L, Conlee J, Hunt J, Agarwal J, White S. Psychosocial distress in prevalent in head and neck cancer patients. Laryngoscope. 2013;123(6):1424-1429.
22. Agarwal J, Powers K, Pappas L, et al. Correlates of elevated distress thermometer scores in breast cancer patients. Support Care Cancer. 2013;21(8):2125-2136.
23. Johnson R, Gold MA, Wythe KF. Distress in women with gynecologic cancer. Psychooncology. 2010;19(6):665-668.
24. Kendall J, Glaze K, Oakland S, Hansen J, Parry C. What do 1281 distress screeners tell us about cancer patients in a community cancer center? Psychooncology. 2011;20(6):594-600.
For patients with cancer, acknowledgment of mental and emotional distress is critically important when developing and implementing a treatment plan. The psychosocial distress associated with cancer diagnosis and treatment can have an impact on a patient’s quality of life, influence a patient’s ability to adhere to treatment regimens, and increase cost of care.1-4 Rates of depression have been reported to range from 8%-36%, with a 29% risk of anxiety in cancer patients.5, 6 Emotional distress is linked to increased hopelessness about their cancer diagnosis, increased issues with chronic pain, and negative treatment outcomes.7 Timely screening of psychosocial distress at the first clinical visit enables providers to make appropriate referrals to resources early in their course of treatment; however, referrals to psychosocial interventions remain infrequent nationwide in the United States.8
There is some evidence of a differential impact of cancer on mental health diagnoses between racial/ethnic groups; however, results are not entirely consistent across studies. Using the Kessler Pyschological Distress Scale (K6) score, Alcala and colleagues found that cancer was more detrimental to mental health for black patients than for non-Hispanic white patients.9 Black breast cancer survivors have also been shown to be more likely to stop working during the early phases of their treatment, indicating that they and their physicians need to take steps to minimize long-term employment consequences.10 However, in a study of women with breast cancer, black women reported fewer depressive symptoms than did non-Hispanic whites.11
The American College of Surgeons’ Commission on Cancer (ACS CoC) developed a set of Continuum of Care standards in 2012, including the implementation of psychosocial distress screening for patients with cancer. Since 2015, all accredited cancer programs are now required to evaluate these patients for signs of distress during at least 1 pivotal physician visit.12 The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology has developed a tool that provides a mechanism for meeting the requirements of the ACS CoC accreditation requirements. The NCCN defines distress in cancer as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms and its treatment.”13 The recommendation of the NCCN is to provide a brief screening for psychosocial distress to identify individuals in need of additional support and to provide referrals for patients at high risk of psychosocial distress. The NCCN Distress Thermometer screening tool has been widely accepted as an effective method of identifying and characterizing distress. The NCCN tool provides a visual analogue scale for patients to rate their current distress on a scale of 1-10, as well as a problem checklist. The problem checklist includes 22 stressors addressing the practical, spiritual/religious, emotional, and physical concerns of patients. Although the NCCN tool is used widely, differences in distress scores between black and white cancer patients have not been previously described. The purpose of the study was to compare the global distress screening scores of black and white patients at an academic comprehensive cancer center in the Midwest. A second objective was to examine the distribution of individual stressors between black and white women.
Methods
Study sample
The study included all cancer patients from a cancer center in the Midwest who completed the NCCN distress thermometer during January 1, 2015-February 19, 2016. The patient population for this cancer center was primarily non-Hispanic white and non-Hispanic black, therefore, only patients identifying as non-Hispanic white and non-Hispanic black are included in this analysis. As part of routine clinical care, patients are asked to complete the NCCN distress thermometer at their first visit to the center. All patients in this analytic sample were newly diagnosed patients. Some patients also completed the NCCN screening tool at additional appointments; therefore, for patients with more than 1 completed tool, only the first distress screening was used in this analysis. Overall scores and individual stressor scores were entered into the electronic medical record by clinic staff at the time the patients were roomed for their visit. Patient demographics were collected through a reporting mechanism within the electronic medical record that allows for monitoring of the psychosocial screening process.
Variables
Race was assessed through self-report and classified as non-Hispanic white and non-Hispanic black. There were not enough patients of any other racial/ethnic group to be included in this analysis. Age was categorized as 18-40 years, 41-60 years, 61-84 years, and 85 years and older. Cancer type was grouped as follows: head and neck cancer, gastrointestinal cancer (esophagus, stomach, small intestine, colon, rectum, anus), hepatobiliary (liver, gallbladder, pancreas), sarcoma (bone and soft tissue), melanoma, nonmelanoma skin cancer, breast cancer, genitourinary (prostate, kidney, bladder), hematologic, and brain.
Two primary outcomes were assessed: overall distress, and each individual problem indicator. Overall distress was assessed using the thermometer visual analog rating (the thermometer rating of the NCCN screening tool) where possible values range from 0 (no distress) to 10 (extreme distress). The overall distress score was categorized into low distress (<4) and high distress (≥4) for analysis. The response options for individual stressors on the problem list are Yes or No for each of 17 discrete stressors: child care, housing, insurance/financial, transportation, work/school, treatment decisions, dealing with children, dealing with partner, ability to have children, family health issues, depression, fears, nervousness, sadness, worry, loss of interest, and spiritual/religious concerns. Physical complaints were not assessed in this study. Comparisons were made between white and black patients on overall distress score as well as for each individual psychosocial stressor.
Data analysis
Descriptive statistics (counts and proportions or means and standard deviations) were calculated stratified by race. Categorical variables were compared by race using chi-square or Fisher exact test. Logistic regression was used to predict high distress by race adjusting for sex, age, and cancer type. All analyses were conducted using SAS 9.4 (Cary, NJ).
This study was reviewed and approved by the Saint Louis University Institutional Review Board (protocol number 26269).
Results
A total of 933 patients with cancer completed the NCCN distress screening tool. Of that total, 45 patients did not complete the overall distress score thermometer, but did complete the checklist of individual stressors. Those 45 patients were excluded from the logistic regression analysis for overall distress score, but included on comparisons of individual stressors. The distribution of overall distress scores by race can be seen in the Figure.
Briefly, the full sample was 16.9% black and 38.8% female. In all, 32.6% of the sample indicated high distress on the distress thermometer at their first visit. Demographics for the participants stratified by race are reported in Table 1 (see PDF).There was no statistically significant difference in the gender or age distribution between black and white patients. Cancer distribution did vary by race. Black patients were proportionally more represented in gastrointestinal cancers, hepatobiliary cancers, sarcomas, breast cancer, and genitourinary cancers. White patients were proportionally more represented in melanoma, nonmelanoma skin cancers, and hematologic cancers.
Table 2 presents bivariate comparisons on overall distress and individual stressors between black and white patients. There was no difference in the high distress between black and white patients in bivariate analysis (31.9% and 36.1%, respectively, P = .30). However, there were differences in the individual stressors identified for each racial group (Table 2). White patients, compared with black patients, more frequently identified treatment decisions (17.6% vs 10.1%, P = .02) and nervousness (26.8% vs 18.4%, P = .02) as sources of distress. Black patients, compared with white patients, more frequently identified housing (5.1% vs 1.7%, P = .009), the ability to have children (2.5% vs 0.4%, P =.02), and loss of interest (15.2% vs 8.9%, P = .02) as sources of distress. Distress scores did not differ between black and white patients for child care, insurance or financial issues, transportation, work or school, dealing with children, dealing with partners, family health issues, depression, fears, sadness, worry, or spiritual or religious concerns.
Table 3 presents the results from the logistic analysis predicting high distress. In adjusted analysis, black race did not predict high distress (OR, 0.94; 95% confidence interval [CI], 0.62-1.44). High distress was associated with sex, age, and some cancer categories. Women had 77% higher odds of high distress compared with men (OR, 1.77; 95% CI, 1.25-2.51).
Compared with patients aged 18-44 years, patients aged 61-84 had 43% lower odds of high distress (OR, 0.57; 95% CI, 0.33-0.98), and patients aged 85 and older had 74% lower odds of high distress (OR, 0.26; 95% CI, 0.07-0.98). There was no statistically significant difference between patients aged 18-40 and those aged 41-60 for high distress (OR, 1.01; 95% CI, 0.59-1.73).
Discussion
Management of patients with cancer continues to evolve. Although a tremendous amount of importance is still placed on the pathophysiology of cancer and its prescribed treatments, more emphasis is being assigned to the physical and psychosocial effects of cancer on these patients. In 2008, the Institute of Medicine published a report that examined the psychosocial health of patients with cancer.14 The report recommended that all cancer care should ensure the provision of appropriate psychosocial health services by facilitating effective communication between patients and care providers, identifying each patient’s psychosocial health needs, coordinating referrals for psychosocial services and monitoring efficacy of psychosocial interventions. The inclusion of psychosocial distress screening in all cancer programs accredited by the ACS CoC helped to prioritize the identification and treatment of psychosocial issues for all cancer patients.
The present study is the first of its kind to compare the individual stressors identified through psychosocial distress screening between black and white cancer patients. In our sample, 304 of 933 patients (32%) reported high distress, with a total score of ≥4. Previous research on overall distress difference across race/ethnicity is mixed. VanHoose and colleagues found no difference in overall distress between racial groups,15 Alcala and colleagues found higher overall distress in black patients with cancer compared with white patients with cancer,9 and Culver and colleagues found black women with breast cancer had lower overall distress compared with white women.11 We found no difference in the presence of high distress between black and white patients at our cancer center in either crude or adjusted analysis. Differences in overall distress across studies may be owing to the timing of screening. Given that overall distress may vary across time16,17 and there is no current information on whether temporal variations in distress differ by race, it is possible that the time of distress assessment may influence demonstrated differences between racial groups. For example, if different stressors affect black and white women differentially, and those stressors are associated with different points across the cancer continuum, then we might see that the magnitude of racial differences in overall stress are time dependent. Alcala and colleagues examined any cancer diagnosis across the lifespan, whereas Culver and colleagues examined multiple time points across treatment for a small group of breast cancer patients. Badr and colleagues, in a sample of head and neck cancer patients, found that distress increased across the course of treatment;18 however they did not examine variations in type of stressors related to overall distress, nor did they examine racial differences in distress. Differences in results may also be the result of differences in measurement of distress. Culver and colleagues did not examine distress using the NCCN distress thermometer, rather psychological distress was measured by a scale rating a series of “mood-descriptive adjectives” (p. 497).11 Alcala used the K-6 as a measure of psychological distress;9 therefore, demonstrated differences in overall distress between white and black women may vary across studies because of differences in measurement of the underlying distress variable. The lack of racial differences in overall distress in our study is consistent with the findings of VanHoose and colleagues,15 who also examined distress near the start of treatment and also used the NCCN distress thermometer as the measure of psychosocial distress.
We did find differences in the individual stressors between racial groups, indicating that the source of distress does vary between black and white cancer patients. Black patients more frequently reported distress secondary to housing, loss of interest and their ability to have children than did white patients. By comparison, white patients more frequently reported distress secondary to nervousness and treatment decisions than black patients. Identified differences in individual stressors may be attributable to sociocultural differences or differences in external support. It is also possible that black patients are more likely to willingly report distress related to nonpsychological factors, whereas white patients are more willing to report factors, such as nervousness, that are related to psychological disorders. Although it has been suggested that black cancer patients have more concerns about finances and work than do white cancer patients,19 we did not identify a statistically significant difference in child care, insurance or financial issues, transportation, work, or school between these 2 cohorts. This may be because the psychosocial distress screening score included in this study was performed at the time of initial diagnosis, and not further into their prescribed treatment at which point the financial worries may be more realized. Psychosocial screening scores obtained at subsequent visits were not included in the analysis because they are not routinely collected as part of clinical care in the center where this study took place. Furthermore, it is impossible to identify where a specific patient is in their treatment regimen based on their demographic data or subsequent distress scores in our data extraction tool. Further investigation into the sources of distress at different time points along the continuum of care may shed more light on this topic.
Limitations
There are several limitations to this study. First, the method of data extraction from an electronic medical record report limited the capacity to explore possible differences between the patients in our sample, such as insurance status, level of education, available social support, current employment status, stage of disease, overall prognosis and prescribed treatment regimen.
Second, there were likely patients who either did not complete a psychosocial distress screening tool or whose data were not entered into the electronic medical record for inclusion in the analysis. The present study period took place during the implementation of the NCCN tool at the center. Although the policy was to screen all new patients as part of routine care; not all patients seen at the center received the NCCN screening tool at their first visit. Owing to the mechanisms for data entry and abstraction, only information from the patients who had a completed form was able to be accessed for this study, thus a statistical comparison between those who did and did not receive the NCCN tool cannot be made. During the timeframe for this study, the head and neck, breast, genitourinary, and hematologic services completed proportionally more NCCN screening of new patients than other services in the center. This is reflected in the distributional breakdown of cancer in the overall sample of this study. It is possible that the results are more representative of differences between black and white cancer patients in the services that were more likely to properly implement NCCN screening.
Third, our patient population was derived from an urban, academic medical center and the results may not be generalizable to other patient populations.
Fourth, the NCCN distress thermometer is a single-item rating of overall global distress that is not intended to be a diagnostic indicator of psychological comorbidity and, therefore, does not distinguish between common psychological diagnoses such as depression or anxiety. However, the usefulness of the tool is to provide an impetus for referral to services that may then encompass the evaluation and diagnosis of particular psychological conditions. Further, the distress thermometer tool is designed to identify stress relating to the social aspects of cancer diagnosis and treatment and is not limited to psychological distress alone.
Strengths
Despite the limitations, there are also significant strengths to this study. The NCCN tool is a widely accepted measure for the assessment of psychosocial distress in patients with cancer. The measure is a common and routine clinical instrument,20 and has also been used widely in research.18,21-24 Given the urban, academic environment of our clinical practice, our population is more racially diverse than other settings, allowing for initial examination of disparities between white and black cancer patients.
Clinical implications
Understanding differences in common psychosocial stressor between black and white cancer patients may allow for clinicians to strategically look for different types of stressors in order to facilitate faster referrals to appropriate services. It has been established in the literature that distress is correlated to cancer-related outcomes and distress screening is now considered standard of care when treating cancer patients. Identifying differences in psychosocial stressors among black and white cancer patients is paramount to ensuring that the appropriate resources are available to assist them through their cancer journey. The differences in type of stressor, may indicate fundamental differences in the way patients perceive their disease or the social and cultural implication of a cancer diagnosis. In this study, white patients were more likely to find distress in the psychological realm (nervousness, decision-making), whereas black patients were more likely to be distressed about social issues (housing, ability to have children, and loss of interest). The referral needs of patients may be quite different, even with similar levels of overall distress. More research is necessary to further characterize sources of distress for cancer patients, how this distress impacts a patient’s physical and emotional well-being and how health care providers can better identify these issues and make the necessary referrals to support the whole patient.
For patients with cancer, acknowledgment of mental and emotional distress is critically important when developing and implementing a treatment plan. The psychosocial distress associated with cancer diagnosis and treatment can have an impact on a patient’s quality of life, influence a patient’s ability to adhere to treatment regimens, and increase cost of care.1-4 Rates of depression have been reported to range from 8%-36%, with a 29% risk of anxiety in cancer patients.5, 6 Emotional distress is linked to increased hopelessness about their cancer diagnosis, increased issues with chronic pain, and negative treatment outcomes.7 Timely screening of psychosocial distress at the first clinical visit enables providers to make appropriate referrals to resources early in their course of treatment; however, referrals to psychosocial interventions remain infrequent nationwide in the United States.8
There is some evidence of a differential impact of cancer on mental health diagnoses between racial/ethnic groups; however, results are not entirely consistent across studies. Using the Kessler Pyschological Distress Scale (K6) score, Alcala and colleagues found that cancer was more detrimental to mental health for black patients than for non-Hispanic white patients.9 Black breast cancer survivors have also been shown to be more likely to stop working during the early phases of their treatment, indicating that they and their physicians need to take steps to minimize long-term employment consequences.10 However, in a study of women with breast cancer, black women reported fewer depressive symptoms than did non-Hispanic whites.11
The American College of Surgeons’ Commission on Cancer (ACS CoC) developed a set of Continuum of Care standards in 2012, including the implementation of psychosocial distress screening for patients with cancer. Since 2015, all accredited cancer programs are now required to evaluate these patients for signs of distress during at least 1 pivotal physician visit.12 The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology has developed a tool that provides a mechanism for meeting the requirements of the ACS CoC accreditation requirements. The NCCN defines distress in cancer as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms and its treatment.”13 The recommendation of the NCCN is to provide a brief screening for psychosocial distress to identify individuals in need of additional support and to provide referrals for patients at high risk of psychosocial distress. The NCCN Distress Thermometer screening tool has been widely accepted as an effective method of identifying and characterizing distress. The NCCN tool provides a visual analogue scale for patients to rate their current distress on a scale of 1-10, as well as a problem checklist. The problem checklist includes 22 stressors addressing the practical, spiritual/religious, emotional, and physical concerns of patients. Although the NCCN tool is used widely, differences in distress scores between black and white cancer patients have not been previously described. The purpose of the study was to compare the global distress screening scores of black and white patients at an academic comprehensive cancer center in the Midwest. A second objective was to examine the distribution of individual stressors between black and white women.
Methods
Study sample
The study included all cancer patients from a cancer center in the Midwest who completed the NCCN distress thermometer during January 1, 2015-February 19, 2016. The patient population for this cancer center was primarily non-Hispanic white and non-Hispanic black, therefore, only patients identifying as non-Hispanic white and non-Hispanic black are included in this analysis. As part of routine clinical care, patients are asked to complete the NCCN distress thermometer at their first visit to the center. All patients in this analytic sample were newly diagnosed patients. Some patients also completed the NCCN screening tool at additional appointments; therefore, for patients with more than 1 completed tool, only the first distress screening was used in this analysis. Overall scores and individual stressor scores were entered into the electronic medical record by clinic staff at the time the patients were roomed for their visit. Patient demographics were collected through a reporting mechanism within the electronic medical record that allows for monitoring of the psychosocial screening process.
Variables
Race was assessed through self-report and classified as non-Hispanic white and non-Hispanic black. There were not enough patients of any other racial/ethnic group to be included in this analysis. Age was categorized as 18-40 years, 41-60 years, 61-84 years, and 85 years and older. Cancer type was grouped as follows: head and neck cancer, gastrointestinal cancer (esophagus, stomach, small intestine, colon, rectum, anus), hepatobiliary (liver, gallbladder, pancreas), sarcoma (bone and soft tissue), melanoma, nonmelanoma skin cancer, breast cancer, genitourinary (prostate, kidney, bladder), hematologic, and brain.
Two primary outcomes were assessed: overall distress, and each individual problem indicator. Overall distress was assessed using the thermometer visual analog rating (the thermometer rating of the NCCN screening tool) where possible values range from 0 (no distress) to 10 (extreme distress). The overall distress score was categorized into low distress (<4) and high distress (≥4) for analysis. The response options for individual stressors on the problem list are Yes or No for each of 17 discrete stressors: child care, housing, insurance/financial, transportation, work/school, treatment decisions, dealing with children, dealing with partner, ability to have children, family health issues, depression, fears, nervousness, sadness, worry, loss of interest, and spiritual/religious concerns. Physical complaints were not assessed in this study. Comparisons were made between white and black patients on overall distress score as well as for each individual psychosocial stressor.
Data analysis
Descriptive statistics (counts and proportions or means and standard deviations) were calculated stratified by race. Categorical variables were compared by race using chi-square or Fisher exact test. Logistic regression was used to predict high distress by race adjusting for sex, age, and cancer type. All analyses were conducted using SAS 9.4 (Cary, NJ).
This study was reviewed and approved by the Saint Louis University Institutional Review Board (protocol number 26269).
Results
A total of 933 patients with cancer completed the NCCN distress screening tool. Of that total, 45 patients did not complete the overall distress score thermometer, but did complete the checklist of individual stressors. Those 45 patients were excluded from the logistic regression analysis for overall distress score, but included on comparisons of individual stressors. The distribution of overall distress scores by race can be seen in the Figure.
Briefly, the full sample was 16.9% black and 38.8% female. In all, 32.6% of the sample indicated high distress on the distress thermometer at their first visit. Demographics for the participants stratified by race are reported in Table 1 (see PDF).There was no statistically significant difference in the gender or age distribution between black and white patients. Cancer distribution did vary by race. Black patients were proportionally more represented in gastrointestinal cancers, hepatobiliary cancers, sarcomas, breast cancer, and genitourinary cancers. White patients were proportionally more represented in melanoma, nonmelanoma skin cancers, and hematologic cancers.
Table 2 presents bivariate comparisons on overall distress and individual stressors between black and white patients. There was no difference in the high distress between black and white patients in bivariate analysis (31.9% and 36.1%, respectively, P = .30). However, there were differences in the individual stressors identified for each racial group (Table 2). White patients, compared with black patients, more frequently identified treatment decisions (17.6% vs 10.1%, P = .02) and nervousness (26.8% vs 18.4%, P = .02) as sources of distress. Black patients, compared with white patients, more frequently identified housing (5.1% vs 1.7%, P = .009), the ability to have children (2.5% vs 0.4%, P =.02), and loss of interest (15.2% vs 8.9%, P = .02) as sources of distress. Distress scores did not differ between black and white patients for child care, insurance or financial issues, transportation, work or school, dealing with children, dealing with partners, family health issues, depression, fears, sadness, worry, or spiritual or religious concerns.
Table 3 presents the results from the logistic analysis predicting high distress. In adjusted analysis, black race did not predict high distress (OR, 0.94; 95% confidence interval [CI], 0.62-1.44). High distress was associated with sex, age, and some cancer categories. Women had 77% higher odds of high distress compared with men (OR, 1.77; 95% CI, 1.25-2.51).
Compared with patients aged 18-44 years, patients aged 61-84 had 43% lower odds of high distress (OR, 0.57; 95% CI, 0.33-0.98), and patients aged 85 and older had 74% lower odds of high distress (OR, 0.26; 95% CI, 0.07-0.98). There was no statistically significant difference between patients aged 18-40 and those aged 41-60 for high distress (OR, 1.01; 95% CI, 0.59-1.73).
Discussion
Management of patients with cancer continues to evolve. Although a tremendous amount of importance is still placed on the pathophysiology of cancer and its prescribed treatments, more emphasis is being assigned to the physical and psychosocial effects of cancer on these patients. In 2008, the Institute of Medicine published a report that examined the psychosocial health of patients with cancer.14 The report recommended that all cancer care should ensure the provision of appropriate psychosocial health services by facilitating effective communication between patients and care providers, identifying each patient’s psychosocial health needs, coordinating referrals for psychosocial services and monitoring efficacy of psychosocial interventions. The inclusion of psychosocial distress screening in all cancer programs accredited by the ACS CoC helped to prioritize the identification and treatment of psychosocial issues for all cancer patients.
The present study is the first of its kind to compare the individual stressors identified through psychosocial distress screening between black and white cancer patients. In our sample, 304 of 933 patients (32%) reported high distress, with a total score of ≥4. Previous research on overall distress difference across race/ethnicity is mixed. VanHoose and colleagues found no difference in overall distress between racial groups,15 Alcala and colleagues found higher overall distress in black patients with cancer compared with white patients with cancer,9 and Culver and colleagues found black women with breast cancer had lower overall distress compared with white women.11 We found no difference in the presence of high distress between black and white patients at our cancer center in either crude or adjusted analysis. Differences in overall distress across studies may be owing to the timing of screening. Given that overall distress may vary across time16,17 and there is no current information on whether temporal variations in distress differ by race, it is possible that the time of distress assessment may influence demonstrated differences between racial groups. For example, if different stressors affect black and white women differentially, and those stressors are associated with different points across the cancer continuum, then we might see that the magnitude of racial differences in overall stress are time dependent. Alcala and colleagues examined any cancer diagnosis across the lifespan, whereas Culver and colleagues examined multiple time points across treatment for a small group of breast cancer patients. Badr and colleagues, in a sample of head and neck cancer patients, found that distress increased across the course of treatment;18 however they did not examine variations in type of stressors related to overall distress, nor did they examine racial differences in distress. Differences in results may also be the result of differences in measurement of distress. Culver and colleagues did not examine distress using the NCCN distress thermometer, rather psychological distress was measured by a scale rating a series of “mood-descriptive adjectives” (p. 497).11 Alcala used the K-6 as a measure of psychological distress;9 therefore, demonstrated differences in overall distress between white and black women may vary across studies because of differences in measurement of the underlying distress variable. The lack of racial differences in overall distress in our study is consistent with the findings of VanHoose and colleagues,15 who also examined distress near the start of treatment and also used the NCCN distress thermometer as the measure of psychosocial distress.
We did find differences in the individual stressors between racial groups, indicating that the source of distress does vary between black and white cancer patients. Black patients more frequently reported distress secondary to housing, loss of interest and their ability to have children than did white patients. By comparison, white patients more frequently reported distress secondary to nervousness and treatment decisions than black patients. Identified differences in individual stressors may be attributable to sociocultural differences or differences in external support. It is also possible that black patients are more likely to willingly report distress related to nonpsychological factors, whereas white patients are more willing to report factors, such as nervousness, that are related to psychological disorders. Although it has been suggested that black cancer patients have more concerns about finances and work than do white cancer patients,19 we did not identify a statistically significant difference in child care, insurance or financial issues, transportation, work, or school between these 2 cohorts. This may be because the psychosocial distress screening score included in this study was performed at the time of initial diagnosis, and not further into their prescribed treatment at which point the financial worries may be more realized. Psychosocial screening scores obtained at subsequent visits were not included in the analysis because they are not routinely collected as part of clinical care in the center where this study took place. Furthermore, it is impossible to identify where a specific patient is in their treatment regimen based on their demographic data or subsequent distress scores in our data extraction tool. Further investigation into the sources of distress at different time points along the continuum of care may shed more light on this topic.
Limitations
There are several limitations to this study. First, the method of data extraction from an electronic medical record report limited the capacity to explore possible differences between the patients in our sample, such as insurance status, level of education, available social support, current employment status, stage of disease, overall prognosis and prescribed treatment regimen.
Second, there were likely patients who either did not complete a psychosocial distress screening tool or whose data were not entered into the electronic medical record for inclusion in the analysis. The present study period took place during the implementation of the NCCN tool at the center. Although the policy was to screen all new patients as part of routine care; not all patients seen at the center received the NCCN screening tool at their first visit. Owing to the mechanisms for data entry and abstraction, only information from the patients who had a completed form was able to be accessed for this study, thus a statistical comparison between those who did and did not receive the NCCN tool cannot be made. During the timeframe for this study, the head and neck, breast, genitourinary, and hematologic services completed proportionally more NCCN screening of new patients than other services in the center. This is reflected in the distributional breakdown of cancer in the overall sample of this study. It is possible that the results are more representative of differences between black and white cancer patients in the services that were more likely to properly implement NCCN screening.
Third, our patient population was derived from an urban, academic medical center and the results may not be generalizable to other patient populations.
Fourth, the NCCN distress thermometer is a single-item rating of overall global distress that is not intended to be a diagnostic indicator of psychological comorbidity and, therefore, does not distinguish between common psychological diagnoses such as depression or anxiety. However, the usefulness of the tool is to provide an impetus for referral to services that may then encompass the evaluation and diagnosis of particular psychological conditions. Further, the distress thermometer tool is designed to identify stress relating to the social aspects of cancer diagnosis and treatment and is not limited to psychological distress alone.
Strengths
Despite the limitations, there are also significant strengths to this study. The NCCN tool is a widely accepted measure for the assessment of psychosocial distress in patients with cancer. The measure is a common and routine clinical instrument,20 and has also been used widely in research.18,21-24 Given the urban, academic environment of our clinical practice, our population is more racially diverse than other settings, allowing for initial examination of disparities between white and black cancer patients.
Clinical implications
Understanding differences in common psychosocial stressor between black and white cancer patients may allow for clinicians to strategically look for different types of stressors in order to facilitate faster referrals to appropriate services. It has been established in the literature that distress is correlated to cancer-related outcomes and distress screening is now considered standard of care when treating cancer patients. Identifying differences in psychosocial stressors among black and white cancer patients is paramount to ensuring that the appropriate resources are available to assist them through their cancer journey. The differences in type of stressor, may indicate fundamental differences in the way patients perceive their disease or the social and cultural implication of a cancer diagnosis. In this study, white patients were more likely to find distress in the psychological realm (nervousness, decision-making), whereas black patients were more likely to be distressed about social issues (housing, ability to have children, and loss of interest). The referral needs of patients may be quite different, even with similar levels of overall distress. More research is necessary to further characterize sources of distress for cancer patients, how this distress impacts a patient’s physical and emotional well-being and how health care providers can better identify these issues and make the necessary referrals to support the whole patient.
1. Holland JC, Reznik I. Pathways for psychosocial care of cancer survivors. Cancer. 2005;104(11 Suppl):2624-2637.
2. Strasser F, Sweeney C, Willey J, Benisch-Tolley S, Palmer L, Bruera E. Impact of a half-day multidisciplinary symptom control and palliative care outpatient clinic in a comprehensive cancer center on recommendations, symptom intensity, and patient satisfaction: a retrospective descriptive study. J Pain Symptom Manage. 2004;27(6):481-491.
3. Carlson LE, Bultz BD. Efficacy and medical cost offset of psychosocial interventions in cancer care: making the case for economic analyses. Psychooncology. 2004;13(12):837-849.
4. Holland J, Bultz BD. The NCCN Guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw. 2007;5(1):3-7.
5. Krebber A, Buffart L, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121-130.
6. Sharp L, Carsin AE , Timmons A. Associations between cancer-related financial stress and strain and psychological well-being among individuals living with cancer. Psychooncology. 2013;22(4):745-755.
7. Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155(2):232-243.
8. Holland JC. Preliminary guidelines for the treatment of distress. Oncology. 1997;11(11A):109-114.
9. Alcala HE. Differential mental health impact of cancer across racial/ethnic groups: findings from a population-based study in California. BMC Public Health. 2014;14:930.
10. Bradley CJ, Wilk A. Racial differences in quality of life and employment outcomes in insured women with breast cancer. J Cancer Surviv. 2014;8(1):49-59.
11. Culver JL, Arena PL, Antoni MH, Carver CS. Coping and distress among women under treatment for early stage breast cancer: comparing African Americans, Hispanics and non-Hispanic whites. Psychooncology. 2002;11(6):495-504.
12. American College of Surgeons Commission on Cancer. ACSCC website. Cancer program standards: ensuring patient-centered care. 2016 edition. https://www.facs.org/quality-programs/cancer/coc/standards. Posted 2016. Accessed August 30, 2017.
13. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Distress Management. National Comprehensive Cancer Network, 2014.https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf Accessed August 30, 2017.
14. Institute of Medicine. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: The National Academies Press; 2008. https://doi.org/10.17226/11993. Accessed August 30, 2017.
15. VanHoose L, Black LL, Doty K, et al. An analysis of the distress thermometer problem list and distress in patients with cancer. Support Care Cancer. 2015;23(5):1225-1232.
16. Gessler S, Low J, Daniells E, et al. Screening for distress in cancer patients: is the distress thermometer a valid measure in the UK and does it measure change over time? A prospective validation study. Psychooncology. 2008;17(6):538-547.
17. Enns A, Waller A, Groff SL, Bultz BD, Fung T, Carlson LE. Risk factors for continuous distress over a 12-month period in newly diagnosed cancer outpatients. J Psychosoc Oncol. 2013;31(5):489-506.
18. Badr H, Gupta V, Sikora A, Posner M. Psychological distress in patients and caregivers over the course of radiotherapy for head and neck cancer. Oral Oncol. 2014;50(10):1005-1011.
19. Wang X, Cosby LG, Harris MG, Liu T. Major concerns and needs of breast cancer patients. Cancer Nurs. 1999;22(2):157-163.
20. Dabrowski M, Boucher K, Ward JH, et al. Clinical experience with the NCCN distress thermometer in breast cancer patients. J Natl Compr Canc Netw. 2007;5(1):104-11.
21. Buchmann L, Conlee J, Hunt J, Agarwal J, White S. Psychosocial distress in prevalent in head and neck cancer patients. Laryngoscope. 2013;123(6):1424-1429.
22. Agarwal J, Powers K, Pappas L, et al. Correlates of elevated distress thermometer scores in breast cancer patients. Support Care Cancer. 2013;21(8):2125-2136.
23. Johnson R, Gold MA, Wythe KF. Distress in women with gynecologic cancer. Psychooncology. 2010;19(6):665-668.
24. Kendall J, Glaze K, Oakland S, Hansen J, Parry C. What do 1281 distress screeners tell us about cancer patients in a community cancer center? Psychooncology. 2011;20(6):594-600.
1. Holland JC, Reznik I. Pathways for psychosocial care of cancer survivors. Cancer. 2005;104(11 Suppl):2624-2637.
2. Strasser F, Sweeney C, Willey J, Benisch-Tolley S, Palmer L, Bruera E. Impact of a half-day multidisciplinary symptom control and palliative care outpatient clinic in a comprehensive cancer center on recommendations, symptom intensity, and patient satisfaction: a retrospective descriptive study. J Pain Symptom Manage. 2004;27(6):481-491.
3. Carlson LE, Bultz BD. Efficacy and medical cost offset of psychosocial interventions in cancer care: making the case for economic analyses. Psychooncology. 2004;13(12):837-849.
4. Holland J, Bultz BD. The NCCN Guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw. 2007;5(1):3-7.
5. Krebber A, Buffart L, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121-130.
6. Sharp L, Carsin AE , Timmons A. Associations between cancer-related financial stress and strain and psychological well-being among individuals living with cancer. Psychooncology. 2013;22(4):745-755.
7. Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155(2):232-243.
8. Holland JC. Preliminary guidelines for the treatment of distress. Oncology. 1997;11(11A):109-114.
9. Alcala HE. Differential mental health impact of cancer across racial/ethnic groups: findings from a population-based study in California. BMC Public Health. 2014;14:930.
10. Bradley CJ, Wilk A. Racial differences in quality of life and employment outcomes in insured women with breast cancer. J Cancer Surviv. 2014;8(1):49-59.
11. Culver JL, Arena PL, Antoni MH, Carver CS. Coping and distress among women under treatment for early stage breast cancer: comparing African Americans, Hispanics and non-Hispanic whites. Psychooncology. 2002;11(6):495-504.
12. American College of Surgeons Commission on Cancer. ACSCC website. Cancer program standards: ensuring patient-centered care. 2016 edition. https://www.facs.org/quality-programs/cancer/coc/standards. Posted 2016. Accessed August 30, 2017.
13. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Distress Management. National Comprehensive Cancer Network, 2014.https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf Accessed August 30, 2017.
14. Institute of Medicine. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: The National Academies Press; 2008. https://doi.org/10.17226/11993. Accessed August 30, 2017.
15. VanHoose L, Black LL, Doty K, et al. An analysis of the distress thermometer problem list and distress in patients with cancer. Support Care Cancer. 2015;23(5):1225-1232.
16. Gessler S, Low J, Daniells E, et al. Screening for distress in cancer patients: is the distress thermometer a valid measure in the UK and does it measure change over time? A prospective validation study. Psychooncology. 2008;17(6):538-547.
17. Enns A, Waller A, Groff SL, Bultz BD, Fung T, Carlson LE. Risk factors for continuous distress over a 12-month period in newly diagnosed cancer outpatients. J Psychosoc Oncol. 2013;31(5):489-506.
18. Badr H, Gupta V, Sikora A, Posner M. Psychological distress in patients and caregivers over the course of radiotherapy for head and neck cancer. Oral Oncol. 2014;50(10):1005-1011.
19. Wang X, Cosby LG, Harris MG, Liu T. Major concerns and needs of breast cancer patients. Cancer Nurs. 1999;22(2):157-163.
20. Dabrowski M, Boucher K, Ward JH, et al. Clinical experience with the NCCN distress thermometer in breast cancer patients. J Natl Compr Canc Netw. 2007;5(1):104-11.
21. Buchmann L, Conlee J, Hunt J, Agarwal J, White S. Psychosocial distress in prevalent in head and neck cancer patients. Laryngoscope. 2013;123(6):1424-1429.
22. Agarwal J, Powers K, Pappas L, et al. Correlates of elevated distress thermometer scores in breast cancer patients. Support Care Cancer. 2013;21(8):2125-2136.
23. Johnson R, Gold MA, Wythe KF. Distress in women with gynecologic cancer. Psychooncology. 2010;19(6):665-668.
24. Kendall J, Glaze K, Oakland S, Hansen J, Parry C. What do 1281 distress screeners tell us about cancer patients in a community cancer center? Psychooncology. 2011;20(6):594-600.
The impact of combining human and online supportive resources for prostate cancer patients
Prostate cancer is the most common cancer among men and the second leading cause of cancer-related death in men. 1 Treatment choices for prostate cancer are perhaps more varied than for many other cancers, with surgery, external beam radiation therapy, and brachytherapy all widely used, a number of adjuvant and nonstandard therapy options available, as well as the possibility of not immediately treating the cancer – the “active surveillance” option.
Biochemical failure rates do not differ between the 3 main treatments,2 but each exposes patients to the risk of side effects, including impotence, incontinence, rectal injury, and operative mortality. Recovery can be gradual and will not always involve a return to baseline functioning.3 Quality-of-life comparisons observed covariate-controlled decreases in varying specific aspects of quality of life for each of the treatments.4
Surgery, brachytherapy, and external beam radiation therapy have each shown advantages over other treatments on at least some specific aspect, but disadvantages on others.4 Ongoing surveillance of a cancer left in place has become a more common option in part because of the disadvantages of traditional treatment and because of the growing recognition that sensitive diagnosis techniques often locate cancers that might not be life threatening. Recent reviews and reasonably long-term trials portray active surveillance as a valid alternative to surgery and radiation in many cases, with little difference in life expectancy and cancer-related quality of life, and possibly some reduction in health system cost.5-7
Prostate cancer patients cope with these uncertainties and decisions in many ways,8 often using multiple coping behaviors,9 but coping almost always includes seeking information and social support, as well as active problem-solving, to make informed treatment decisions consistent with their values.
Unfortunately, prostate patients often do not receive or use needed information. McGregor10 reported that patients were aware of their incomplete understanding of their disease and treatment options. Findings from several studies suggest that patients often perceive that clinicians inform them about the disease and treatment options but then send them home unprepared to deal with such things as incontinence or difficulties with sexual functioning.11
Similarly, previous research demonstrates the benefits of social support for prostate cancer patients who receive it, but also that overall they are underserved.12,13 Male cancer patients are generally far less likely to seek support and health information than are female patients. And when patients with prostate cancer do participate in online cancer support groups, they are more likely to exchange information, whereas breast cancer patients provide support for each other.14
Mentoring
Some responses to these knowledge and support gaps pair newly diagnosed patients with survivors willing to be a guide, coach, and a source of information, as in the American Cancer Society’s (ACS’s) Man-to-Man support groups.15 Peer mentors may have a sophisticated level of understanding from their own experiences with medical literature and the health care system, but this cannot be assumed. Another mentoring model is expert-based, exemplified by the National Cancer Institute’s (NCI’s) cancer information specialist at the Cancer Information Service (CIS) and a similar system at the ACS. These telephone services allow for responsiveness to the caller’s needs, existing knowledge, and the caller’s readiness for information. The CIS specialist can also introduce important information the caller might not have known to ask about.16
However, not all problems presented by callers can be solved in a single conversation. Callers are encouraged to call back with additional questions or when their situation changes, but speaking with the same specialist is not facilitated, so it is hard for a second call to build upon the first. Combining the expertise of the cancer information specialist with the ongoing and proactive contact and support typical of the lay guide/mentor/navigator could be more effective. Here a CIS-trained information specialist called prostate patients multiple times over the intervention period to help them deal with information seeking and interpretation. In a previous study with breast cancer patients, a mentor of this sort improved patient information competence and emotional processing.17
Interactive resources
Online resources allow cancer patients self-paced and self-directed access to information and support anonymously and at any time. However, this can be more complicated than it might at first seem. With the complexities of the prostate cancer diagnosis, the multiple treatment options, and the uncertain but potentially serious effects of the treatments themselves, the amount of potentially relevant information is quite large. Then, because individuals will value differentially the attributes of treatments, their consequences, or even notions of risk and gain, a system must be able to respond appropriately to a range of very different people. Beyond this, as prostate cancer patients move from the shock of a cancer diagnosis to the problems of interpreting its details, to making treatment decisions, to dealing with problems of recovery, and then re-establishing what is a “new normal” for them, an individual’s demands on a system vary as well. Comprehensive and integrated systems of services meet the varying needs of their users at different times and different situations.18,19 The systems approach not only makes it far easier for users to find what they need, it may also encourage them to see connections between physical, emotional, and social aspects of their illness. Versions of the system used in the present study – CHESS, or Comprehensive Health Enhancement Support System – have been effective supporting patients with AIDS and breast and lung cancers, and teens with asthma.16,20
Study goals and hypotheses
Given the success of the 2 aforementioned approaches, we wanted to compare how CHESS and ongoing contact with a human cancer information mentor in patients with prostate cancer would affect both several general aspects of quality of life and 1 specific to prostate cancer. We also examined differences in the patients’ information competence, quality of life, and social support. There was no a priori expectation that one intervention would be superior to the other, but any differences found could be important to policy decisions, given their quite-different cost and scalability.
More importantly, the primary hypothesis of the study was that patients with access to both CHESS and a mentor would experience substantially better outcomes than those with access to either intervention alone, because each had the potential to enhance the other’s benefits. For example, a patient could read CHESS material and come to the mentor much better prepared. By referring the user to specific parts of CHESS for basic information, the mentor could use calls to address more complex issues, or help interpret and evaluate difficult issues. In addition, because CHESS provides the mentor information about changes in the patient’s treatments, symptoms, and CHESS use, in the combined condition the cancer information mentor can know much more about the patient than when working alone. We also expected that the mentor would stimulate the kind of diverse use of CHESS services we have found to be most effective
Because both mentoring and CHESS have consistently produced positive quality of life effects on their own, compared to controls, there is no reasonable expectation that negative effects of a combined condition could occur and should be tested for. Thus, the study was powered for 1-tailed significance in the comparison between the combined condition and either intervention alone, a procedure used consistently in previous studies of CHESS components or combined conditions. However, since the research question comparing the 2 interventions alone had no such strong history it was tested 2-tailed.
Methods
Recruitment
Study recruitment was conducted from January 1, 2007 to September 30, 2008 at the University of Wisconsin’s Paul P Carbone Comprehensive Cancer Center in Madison, Hartford Hospital’s Helen and Harry Gray Cancer Center in Hartford, Connecticut, and The University of Texas MD Anderson Cancer Center in Houston.
A total of 461 patients were invited to participate in the study. Of those patients, 147 declined to participate, 4 were excluded, and 310 were randomized to access to CHESS only, access to a human mentor only, or access to CHESS and a mentor (CHESS+Mentor) during the 6-month intervention period, which provided adequate power (>.80) for effects of moderate size (Figure 1). Randomization was done with a computer-generated list that site study managers accessed on a patient-by-patient basis, with experimental conditions blocked within sites.
Recruitment was done by posting brochures about the study at the relevant locations and devising standardized recruitment scripts for clinical staff to use when talking to patients about the study. Staff at all sites invited patients they thought might be eligible to learn more about the study. As appropriate, staff members then reviewed informed consent and HIPAA information, explained the interventions, answered patient questions, obtained written consent, collected complete patient contact and computer access information, and provided patients the baseline questionnaires.
The standard inclusion criteria were: men older than 17 years, being able to read and understand English, and being within 2 months of a diagnosis of primary prostate cancer (stage 1 or 2) at the time of recruitment. Despite the 2-month window, few men had begun treatment before pretest. Only 9 of the 310 participants reported having already had surgery (7 prostatectomies, 2 implants), so participants may be fairly characterized as beginning the study in time to benefit from interventions during most stages of their experience with prostate cancer.
Interventions
To provide an equal baseline, all of the participants were given access to the Internet, which is becoming a de facto standard for information access. Internet access charges were paid for all participants during the 6-month intervention period, and computers were loaned to those who did not have a personal computer. All of the participants were offered training on using the computer, particularly with Google search procedures so that they could access resources on prostate cancer.
Participants assigned to the CHESS or CHESS+Mentor conditions were also offered training in using CHESS (basically a guided tour), which typically took about 30 minutes on the telephone but was occasionally done in-person.
CHESS intervention. In creating CHESS for prostate cancer patients, a combination of patient needs assessments, focus groups with patients and family members, and clinician expertise helped us identify the needs, coping mechanisms, and relevant medical information to help patients respond to the disease. An article describing development of the CHESS Prostate Cancer Module22 presents how those different services address patient needs for information, communication, and support, or build skills.
Most of these services were present in CHESS for other diseases, but several were newly created to meet needs of prostate cancer patients and partners, such as a decision map tool and a module on managing sexual problems.22 Also, patients expressed frustration at being overwhelmed by the volume of information and said they would prefer to focus only on what was most relevant, so we created an alternative navigation structure on the CHESS homepage. Using terms suggested by focus groups of prostate cancer survivors and their spouses, we devised a navigation structure called Step-by-Step that identified 6 typical sequential steps of men’s experience with prostate cancer. Clicking on a step would take a patient to a menu focused on actions and considerations specific to that disease step, links to information most relevant at that step, and suggested questions to ask oneself and one’s doctor.
Mentor intervention. The cancer information mentor who made most of the calls to patients was an experienced information specialist with the Cancer Information Service and had served as the expert for the CHESS Ask an Expert service for 6 years. She was highly knowledgeable about prostate cancer and patient information needs. Her additional training for this study focused on taking advantage of repeated contacts with the participants and how to set limits to avoid any semblance of psychological counseling. At recruitment, we made clear that a male mentor was also available if the participant would prefer to discuss sensitive topics with another man. The male mentor was experienced in the Man-to-Man program and received additional training for this role, but he was used for only 1% of all contacts.
During calls, the mentor had Internet access to a range of NCI, ACS, and other resources. She could help interpret information the participant already possessed as well as refer him to other public resources, including those on the Internet. CHESS software designers created an additional interface for the mentor that handled call scheduling and allowed her to record the topics of conversations, her responses and recommendations, and her overall ratings of patient preparedness and satisfaction. Using this interface allowed the mentor to quickly review a participant’s status and focus the conversation on issues raised by past conversations or scheduled treatment events. The mentor calls were audiorecorded and reviewed frequently by the project director during the early months of intervention and less frequently thereafter to ensure adherence to the protocol.
The mentor telephoned weekly during the first month of intervention, then twice during the second month, and once a month during the final 4 months of the intervention (ie, 10 scheduled calls, though patients could also initiate additional calls). Calls were scheduled through a combination of telephone contact and e-mail according to the patient’s preference. Call length ranged from 5 minutes to an hour, with the average about 12 minutes (the first call tended to be considerably longer, and was scheduled for 45 minutes). About 10%-15% of participants in the Mentor conditions initiated calls to the mentor to obtain additional support, and about 15% of scheduled calls in fact took place as e-mail exchanges. A few calls were missed because of scheduling difficulties, and some participants stopped scheduling the last few calls, but the average number of full calls or e-mails was 6.41 per participant.
CHESS+Mentor intervention. For the CHESS+Mentor condition, the interactions and resources used were similar to those of the Mentor-only condition, but the interface also provided the mentor with a summary of the participant’s recent CHESS use and any concerns reported to CHESS, which helped the mentor assess knowledge and make tailored recommendations. The mentor could also refer participants to specific resources within CHESS, aided by knowledge of what parts of CHESS had or had not been used.
Assessment methods
Patients were given surveys at the baseline visit to complete and mail back to research staff before randomization. Follow-up surveys were mailed to patients at 2, 6, 12, and 24 weeks post intervention access, and patients returned the surveys by mail. Patient withdrawal rates were about 3%.
Measures
Outcomes. This study included 4 measures of quality of life (an average of relevant portions of the World Health Organization’s Quality of Life (WHOQOL) measure, Emotional and Functional Well-being, and a prostate-cancer specific index, the Expanded Prostate Cancer Index Composite (EPIC). We also tested group differences on 5 more specific outcomes that were likely to be proximal rather than distal effects of the interventions: Cancer Information Competence, Health care Competence, Social Support, Bonding (with other patients), and Positive Coping.
Quality of life. Quality of life was measured by combining the psychological, social, and overall dimensions of the WHOQOL measures.23 Each of the 11 items was assessed with a 5-point scale, and the mean of those answers was the overall score.
Emotional well-being. Respondents answered 6 items of the Functional Assessment of Cancer Therapy – Prostate
Functional well-being. Respondents indicated how often they experienced each of the seven functional well-being subscale items of the FACT-Prostate.24
Prostate cancer patient functioning. We used the EPIC to measure of 3 of 4 domains of prostate cancer patient functioning: urinary, bowel, and sexual (omitting hormonal).25 The EPIC measures frequency and subjective degree of being a problem of several aspects in each domain. We then summed scores across the domains and transformed linearly to a 0-100 scale, with higher scores representing better functioning.
Cancer information competence. Five cancer information competence items, measured on a 5-point scale, assessed a participant’s perception about whether he could find and effectively and use health information, and were summed to create a single score.20
Social support. Six 5-point social support items assessed the informational and emotional support provided by friends, family, coworkers, and others, and were summed to create a single score.20
Health care competence. Five 5-point health care competence items assessed a patient’s comfort and activation level dealing with physicians and health care situations, and were summed to create a single score.20
Positive coping. Coping strategies were measured with the Brief Cope, a shorter version of the original 60-item COPE scale.26 Positive coping strategy, a predictor of positive adaptation in numerous coping contexts, was measured with the mean score of 4 scales (8 items in all): active coping, planning, positive reframing, and humor.
Bonding. Bonding with other prostate cancer patients was measured with five 5-point items about how frequently participants connected with and got information and support from other men with prostate cancer.27
User vs nonuser. Intent-to-treat analyses compared the assigned conditions. However, because CHESS use was self-selected and available at any time whereas mentor calls were scheduled and initiated by another person, the proportion actually using the interventions was quite different.
Since a participant assigned access to CHESS had to select the URL, even a single entry to the system was counted as use. Of 198 participants assigned to either the CHESS or CHESS+Mentor conditions, 43 (22%) never logged in and were classified as nonusers.
Because the mentor scheduled calls and attempted repeatedly to complete scheduled calls, the patient was in a reactive position, and the decision not to use the mentor’s services could come at the earliest at the end of a first completed call. However, after examining call notes and consulting with the mentors, it was clear that opting not to receive mentoring typically occurred at the second call. Furthermore, much (though not all) of the first call was typically taken up with getting acquainted and scheduling issues, so that defining “nonuse” as 2 or fewer completed calls was most faithful to what actually happened. Of 202 participants assigned access to a mentor, 16 (8%) were thus defined as nonusers.
Results
Overall, the participants were about 60 years of age and had some college education and middle-class incomes (Table 1). Only about 10% were minorities or lived alone, and their comfort using computers and the Internet was at or above the “quite comfortable” level. None of groups differed significantly from any other.
The 2 primary hypotheses of the study were that participants in the combined condition would manifest higher outcome scores than those with either intervention alone. Table 2 displays group means at 3 posttest intervals, controlling for theoretically chosen covariates (age, education, and minority status) and pretest levels of the dependent variable. The table also summarizes tests examining the hypotheses and the comparison of CHESS and Mentor conditions. The 4 quality-of-life scores appear first, followed by 5 more specific outcomes that are perhaps more proximal effects of these interventions.
The combined condition scored significantly higher than the CHESS-only condition on functional well-being at 3 months, on positive coping at 6 months, and on bonding at both 6 weeks and 6 months. The combined condition scored significantly higher than Mentor-only on health care competence and positive coping at 6 weeks, and on bonding at 6 months. This represents partial but scattered support for the hypotheses. And some comparisons of the combined condition with the Mentor-only condition showed reversals of the predicted relationship (although only cancer information competence at 3 months would have reached statistical significance in a 2-tailed test).
No directional hypotheses were made for the comparison of the 2 interventions (see Table 2 for the results of 2-tailed tests). Participants in the Mentor condition reported significantly higher functional well-being at 3 months, although there were 5 other comparisons in which the Mentor group scored higher at P < .10, and higher than the CHESS group on 22 of the 27 comparisons. Thus, it seemed that the Mentor condition alone might have been a somewhat stronger intervention than CHESS alone.
Discussion
We used a randomized control design to test whether combining computer-based and human interventions would provide greater benefits to prostate cancer patients than either alone, as previous research had shown for breast cancer patients.18 The computer-based resource was CHESS, a repeatedly evaluated integrated system combining information, social support, and interactive tools to help patients manage their response to disease. The human cancer information mentor intervention combined the expertise of NCI’s Cancer Information Service with the repeated contact more typical of peer mentoring. Previous research with breast cancer patients had shown both interventions to provide greater information, support, and quality-of-life benefits than Internet access alone.14 This study also compared outcomes obtained by the separate CHESS and Mentor conditions, but without predicting a direction of difference.
Tests at 6 weeks, 3 months and 6 months after intervention found instances in which prostate cancer patients assigned to the combined CHESS+Mentor condition experienced more positive quality of life or other outcomes than those assigned to CHESS or Mentor alone, but those differences were scattered rather than consistent. In the direct comparisons of the separate CHESS and Mentor conditions, significance was even rarer, but outcome scores tended to be higher in the Mentor condition than in the CHESS condition.
We noted that differential uptake of the 2 interventions (92% for Mentor vs 78% for CHESS) made interpreting the intent-to-treat analyses problematic, as the mentor’s control of the call schedule meant that far more patients in that condition actually received at least some intervention than in the CHESS condition, where patients used or did not use CHESS entirely at their own volition. This could have biased results in several ways, such as by underestimating the efficacy of the CHESS condition alone and thus inflating the contrast between CHESS alone and CHESS+Mentor. Or the combined condition might have been less different than the Mentor-only condition than intended, thus making for a conservative test of that comparison. However, post hoc analyses of only those participants who had actually used their assigned interventions (and this led to some reclassification of those originally assigned to the CHESS+Mentor condition) produced results that were little different than the intent-to-treat analysis.
Thus, although the combined condition produced some small advantages over either intervention alone, these advantages did not live up to expectations or to previous experience with breast cancer patients.17 We expected the mentor to be able to reinforce and help interpret what the participants learned from CHESS and their clinicians, and also to advise and direct these patients to be much more effective users of CHESS and other resources. Similarly, we expected that CHESS would make patients much better prepared for mentoring, so that instead of dealing with routine information matters, the mentor could go into greater detail or deal with more complex issues. Their combined effect should have been much larger than each alone, and that was not the case. Perhaps from the prostate cancer patients’ perspective, the 2 interventions seemed to offer similar resources, and a patient benefitted from one or the other but expected no additional gain from attending to both.
The 2 interventions themselves seemed nearly equally effective. The Mentor intervention was significantly stronger than CHESS in only 1 of 27 tests in the intent-to-treat analysis and 2 in the analysis limited to intervention users.
These results for prostate cancer patients are somewhat weaker than those previously reported with breast cancer patients.17 It is possible that prostate cancer patients (or men in general) are less inclined to seek health information, support, and health self-management than breast cancer patients (or women in general), perhaps becaus
Although these interventions were experienced by prostate cancer patients in their homes in natural and familiar ways, any experimental manipulation must acknowledge possible problems with external validity. More important here, our recruitment procedures may have produced self-selection to enter or not enter the study in 2 ways that limit its applicability. First, although we thought that offering Internet access to all participants would make participation more likely, the most frequent reason men gave in declining to join the study was “not a computer person.” Our participants were certainly very comfortable with computers and the Internet, and most used them frequently even before the study. Second, it seems that, except for their prostate cancer, our sample was healthy in other respects, as indicated by the low number of other health care visits or surgeries/hospitalization they reported (and “overwhelmed” and “too busy,” 2 common reasons for declining study participations could also be coming from men with more comorbidities). Thus, our sample was probably more computer literate and healthier than the general population of prostate cancer patients.
Nonetheless, for policymakers deciding what information and support interventions to put in place for prostate cancer patients (or more generally for other cancer patients as well), these results have 2 implications. First, since the combination of the mentor and CHESS produced only small advantages over either alone, the extra effort of doing both seems clearly unwarranted for prostate cancer patients. The somewhat larger advantage of the combined intervention shown for breast cancer patients in previous studiesmight warrant using the combination in some circumstances, but even that is not clear-cut.
Finding that CHESS and the cancer information mentor separately provided essentially equal benefits might seem to suggest that they can be regarded as alternatives. However, computer-based services can be provided much more cheaply and scaled up far more readily than services dependent on one-on-one contacts by a highly trained professional. This may direct health care decision makers first toward computer-based services.
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277-300.
2. Cozzarini C. Low-dose rate brachytherapy, radical prostatectomy, or external-beam radiation therapy for localized prostate carcinoma: The growing dilemma. European Urology. 2011;60(5):894-896.
3. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250-1261.
4. Ferrer F, Guedea F, Pardo Y, et al. Quality of life impact of treatments for localized prostate cancer. Radiother Oncol. 2013;108(2):306-313.
5. Cooperberg, MR, Carroll, PR, Klotz, L. Active Surveillance for prostate cancer: progress and promise. J Clin Onc. 2011;29:3669-3676.
6. Hamdy, FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
7. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-37.
8. Lavery JF, Clarke VA. Prostate cancer: patients’ spouses’ coping and marital adjustment. Psychol Health Med. 1999;4(3):289-302.
9. Folkman S, Lazarus R. If it changes it must be a process: study of emotion and coping during three stages of a college examination. J Pers Soc Psycol. 1985;48:150-170.
10. McGregor S. What information patients with localized prostate cancer hear and understand. Patient Educ Couns. 2003;49:273-278.
11. Steginga SK, Occhipinti S, Dunn J, Gardiner RA, Heathcote P, Yaxley J. (2001) The supportive care needs of men with prostate cancer (2000). Psychooncology. 2001;10(1):66-75.
12. Gregoire I, Kalogeropoulos D, Corcos J. The effectiveness of a professionally led support group for men with prostate cancer. Urologic Nurs. 1997;17(2):58-66.
13. Katz D, Koppie T, Wu D, et al. Sociodemographic characteristics and health related quality of life in men attending prostate cancer support groups. J Urol. 2002;168:2092-2096.
14. Klemm P, Hurst M, Dearholt S, Trone S. Gender differences on Internet cancer support groups. Comput Nurs. 1999;17(2):65-72.
15. Gray R, Fitch M, Phillips C, Labrecque M, Fergus K. Managing the impact of illness: the experiences of men with prostate cancer and their spouses. J Health Psychol. 2000;5(4):531-548.
16. Thomsen CA, Ter Maat J. Evaluating the Cancer Information Service: a model for health communications. Part 1. J Health Commun. 1998;3(suppl.):1-13.
17. Hawkins RP, Pingree S, Baker TB, et al. Integrating eHealth with human services for breast cancer patients. Transl Behav Med. 2011;1(1):146-154.
18. Strecher V. Internet methods for delivering behavioral and health-related interventions. Ann Rev Clin Psychol. 2007;(3):53-76.
19. Gustafson DH, Hawkins RP, McTavish F, et al. Internet-based interactive support for cancer patients: Are integrated systems better? J Commun. 2008;58(2):238-257.
20. Gustafson DH, Hawkins RP, Boberg EW, et al. CHESS: Ten years of research and development in consumer health informatics for broad populations, including the underserved. Int J Med Inform. 2002;65(3):169-177.
21. Han JY, Hawkins RP, Shaw B, Pingree S, McTavish F, Gustafson D. Unraveling uses and effects of an interactive health communication system. J Broadcast Electron Media. 2009;53(1):1-22.
22. Van Bogaert D, Hawkins RP, Pingree S, Jarrard D. The development of an eHealth tool suite for prostate cancer patients and their partners. J Support Oncol. 2012;10(5):202-208.
23. The WHOQOL Group. Development of the WHOQOL: Rationale and current status. Int J Ment Health. 1994;23:24-56.
24. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920-928.
25. Wei JT, Dunn R, Litwin M, Sandler H, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899-905.
26. Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4: 91-100.
27. Gustafson D, McTavish F, Stengle W, et al. Use and impact of eHealth System by low-income women with breast cancer. J Health Commun. 2005;10(suppl 1):219-234.
Prostate cancer is the most common cancer among men and the second leading cause of cancer-related death in men. 1 Treatment choices for prostate cancer are perhaps more varied than for many other cancers, with surgery, external beam radiation therapy, and brachytherapy all widely used, a number of adjuvant and nonstandard therapy options available, as well as the possibility of not immediately treating the cancer – the “active surveillance” option.
Biochemical failure rates do not differ between the 3 main treatments,2 but each exposes patients to the risk of side effects, including impotence, incontinence, rectal injury, and operative mortality. Recovery can be gradual and will not always involve a return to baseline functioning.3 Quality-of-life comparisons observed covariate-controlled decreases in varying specific aspects of quality of life for each of the treatments.4
Surgery, brachytherapy, and external beam radiation therapy have each shown advantages over other treatments on at least some specific aspect, but disadvantages on others.4 Ongoing surveillance of a cancer left in place has become a more common option in part because of the disadvantages of traditional treatment and because of the growing recognition that sensitive diagnosis techniques often locate cancers that might not be life threatening. Recent reviews and reasonably long-term trials portray active surveillance as a valid alternative to surgery and radiation in many cases, with little difference in life expectancy and cancer-related quality of life, and possibly some reduction in health system cost.5-7
Prostate cancer patients cope with these uncertainties and decisions in many ways,8 often using multiple coping behaviors,9 but coping almost always includes seeking information and social support, as well as active problem-solving, to make informed treatment decisions consistent with their values.
Unfortunately, prostate patients often do not receive or use needed information. McGregor10 reported that patients were aware of their incomplete understanding of their disease and treatment options. Findings from several studies suggest that patients often perceive that clinicians inform them about the disease and treatment options but then send them home unprepared to deal with such things as incontinence or difficulties with sexual functioning.11
Similarly, previous research demonstrates the benefits of social support for prostate cancer patients who receive it, but also that overall they are underserved.12,13 Male cancer patients are generally far less likely to seek support and health information than are female patients. And when patients with prostate cancer do participate in online cancer support groups, they are more likely to exchange information, whereas breast cancer patients provide support for each other.14
Mentoring
Some responses to these knowledge and support gaps pair newly diagnosed patients with survivors willing to be a guide, coach, and a source of information, as in the American Cancer Society’s (ACS’s) Man-to-Man support groups.15 Peer mentors may have a sophisticated level of understanding from their own experiences with medical literature and the health care system, but this cannot be assumed. Another mentoring model is expert-based, exemplified by the National Cancer Institute’s (NCI’s) cancer information specialist at the Cancer Information Service (CIS) and a similar system at the ACS. These telephone services allow for responsiveness to the caller’s needs, existing knowledge, and the caller’s readiness for information. The CIS specialist can also introduce important information the caller might not have known to ask about.16
However, not all problems presented by callers can be solved in a single conversation. Callers are encouraged to call back with additional questions or when their situation changes, but speaking with the same specialist is not facilitated, so it is hard for a second call to build upon the first. Combining the expertise of the cancer information specialist with the ongoing and proactive contact and support typical of the lay guide/mentor/navigator could be more effective. Here a CIS-trained information specialist called prostate patients multiple times over the intervention period to help them deal with information seeking and interpretation. In a previous study with breast cancer patients, a mentor of this sort improved patient information competence and emotional processing.17
Interactive resources
Online resources allow cancer patients self-paced and self-directed access to information and support anonymously and at any time. However, this can be more complicated than it might at first seem. With the complexities of the prostate cancer diagnosis, the multiple treatment options, and the uncertain but potentially serious effects of the treatments themselves, the amount of potentially relevant information is quite large. Then, because individuals will value differentially the attributes of treatments, their consequences, or even notions of risk and gain, a system must be able to respond appropriately to a range of very different people. Beyond this, as prostate cancer patients move from the shock of a cancer diagnosis to the problems of interpreting its details, to making treatment decisions, to dealing with problems of recovery, and then re-establishing what is a “new normal” for them, an individual’s demands on a system vary as well. Comprehensive and integrated systems of services meet the varying needs of their users at different times and different situations.18,19 The systems approach not only makes it far easier for users to find what they need, it may also encourage them to see connections between physical, emotional, and social aspects of their illness. Versions of the system used in the present study – CHESS, or Comprehensive Health Enhancement Support System – have been effective supporting patients with AIDS and breast and lung cancers, and teens with asthma.16,20
Study goals and hypotheses
Given the success of the 2 aforementioned approaches, we wanted to compare how CHESS and ongoing contact with a human cancer information mentor in patients with prostate cancer would affect both several general aspects of quality of life and 1 specific to prostate cancer. We also examined differences in the patients’ information competence, quality of life, and social support. There was no a priori expectation that one intervention would be superior to the other, but any differences found could be important to policy decisions, given their quite-different cost and scalability.
More importantly, the primary hypothesis of the study was that patients with access to both CHESS and a mentor would experience substantially better outcomes than those with access to either intervention alone, because each had the potential to enhance the other’s benefits. For example, a patient could read CHESS material and come to the mentor much better prepared. By referring the user to specific parts of CHESS for basic information, the mentor could use calls to address more complex issues, or help interpret and evaluate difficult issues. In addition, because CHESS provides the mentor information about changes in the patient’s treatments, symptoms, and CHESS use, in the combined condition the cancer information mentor can know much more about the patient than when working alone. We also expected that the mentor would stimulate the kind of diverse use of CHESS services we have found to be most effective
Because both mentoring and CHESS have consistently produced positive quality of life effects on their own, compared to controls, there is no reasonable expectation that negative effects of a combined condition could occur and should be tested for. Thus, the study was powered for 1-tailed significance in the comparison between the combined condition and either intervention alone, a procedure used consistently in previous studies of CHESS components or combined conditions. However, since the research question comparing the 2 interventions alone had no such strong history it was tested 2-tailed.
Methods
Recruitment
Study recruitment was conducted from January 1, 2007 to September 30, 2008 at the University of Wisconsin’s Paul P Carbone Comprehensive Cancer Center in Madison, Hartford Hospital’s Helen and Harry Gray Cancer Center in Hartford, Connecticut, and The University of Texas MD Anderson Cancer Center in Houston.
A total of 461 patients were invited to participate in the study. Of those patients, 147 declined to participate, 4 were excluded, and 310 were randomized to access to CHESS only, access to a human mentor only, or access to CHESS and a mentor (CHESS+Mentor) during the 6-month intervention period, which provided adequate power (>.80) for effects of moderate size (Figure 1). Randomization was done with a computer-generated list that site study managers accessed on a patient-by-patient basis, with experimental conditions blocked within sites.
Recruitment was done by posting brochures about the study at the relevant locations and devising standardized recruitment scripts for clinical staff to use when talking to patients about the study. Staff at all sites invited patients they thought might be eligible to learn more about the study. As appropriate, staff members then reviewed informed consent and HIPAA information, explained the interventions, answered patient questions, obtained written consent, collected complete patient contact and computer access information, and provided patients the baseline questionnaires.
The standard inclusion criteria were: men older than 17 years, being able to read and understand English, and being within 2 months of a diagnosis of primary prostate cancer (stage 1 or 2) at the time of recruitment. Despite the 2-month window, few men had begun treatment before pretest. Only 9 of the 310 participants reported having already had surgery (7 prostatectomies, 2 implants), so participants may be fairly characterized as beginning the study in time to benefit from interventions during most stages of their experience with prostate cancer.
Interventions
To provide an equal baseline, all of the participants were given access to the Internet, which is becoming a de facto standard for information access. Internet access charges were paid for all participants during the 6-month intervention period, and computers were loaned to those who did not have a personal computer. All of the participants were offered training on using the computer, particularly with Google search procedures so that they could access resources on prostate cancer.
Participants assigned to the CHESS or CHESS+Mentor conditions were also offered training in using CHESS (basically a guided tour), which typically took about 30 minutes on the telephone but was occasionally done in-person.
CHESS intervention. In creating CHESS for prostate cancer patients, a combination of patient needs assessments, focus groups with patients and family members, and clinician expertise helped us identify the needs, coping mechanisms, and relevant medical information to help patients respond to the disease. An article describing development of the CHESS Prostate Cancer Module22 presents how those different services address patient needs for information, communication, and support, or build skills.
Most of these services were present in CHESS for other diseases, but several were newly created to meet needs of prostate cancer patients and partners, such as a decision map tool and a module on managing sexual problems.22 Also, patients expressed frustration at being overwhelmed by the volume of information and said they would prefer to focus only on what was most relevant, so we created an alternative navigation structure on the CHESS homepage. Using terms suggested by focus groups of prostate cancer survivors and their spouses, we devised a navigation structure called Step-by-Step that identified 6 typical sequential steps of men’s experience with prostate cancer. Clicking on a step would take a patient to a menu focused on actions and considerations specific to that disease step, links to information most relevant at that step, and suggested questions to ask oneself and one’s doctor.
Mentor intervention. The cancer information mentor who made most of the calls to patients was an experienced information specialist with the Cancer Information Service and had served as the expert for the CHESS Ask an Expert service for 6 years. She was highly knowledgeable about prostate cancer and patient information needs. Her additional training for this study focused on taking advantage of repeated contacts with the participants and how to set limits to avoid any semblance of psychological counseling. At recruitment, we made clear that a male mentor was also available if the participant would prefer to discuss sensitive topics with another man. The male mentor was experienced in the Man-to-Man program and received additional training for this role, but he was used for only 1% of all contacts.
During calls, the mentor had Internet access to a range of NCI, ACS, and other resources. She could help interpret information the participant already possessed as well as refer him to other public resources, including those on the Internet. CHESS software designers created an additional interface for the mentor that handled call scheduling and allowed her to record the topics of conversations, her responses and recommendations, and her overall ratings of patient preparedness and satisfaction. Using this interface allowed the mentor to quickly review a participant’s status and focus the conversation on issues raised by past conversations or scheduled treatment events. The mentor calls were audiorecorded and reviewed frequently by the project director during the early months of intervention and less frequently thereafter to ensure adherence to the protocol.
The mentor telephoned weekly during the first month of intervention, then twice during the second month, and once a month during the final 4 months of the intervention (ie, 10 scheduled calls, though patients could also initiate additional calls). Calls were scheduled through a combination of telephone contact and e-mail according to the patient’s preference. Call length ranged from 5 minutes to an hour, with the average about 12 minutes (the first call tended to be considerably longer, and was scheduled for 45 minutes). About 10%-15% of participants in the Mentor conditions initiated calls to the mentor to obtain additional support, and about 15% of scheduled calls in fact took place as e-mail exchanges. A few calls were missed because of scheduling difficulties, and some participants stopped scheduling the last few calls, but the average number of full calls or e-mails was 6.41 per participant.
CHESS+Mentor intervention. For the CHESS+Mentor condition, the interactions and resources used were similar to those of the Mentor-only condition, but the interface also provided the mentor with a summary of the participant’s recent CHESS use and any concerns reported to CHESS, which helped the mentor assess knowledge and make tailored recommendations. The mentor could also refer participants to specific resources within CHESS, aided by knowledge of what parts of CHESS had or had not been used.
Assessment methods
Patients were given surveys at the baseline visit to complete and mail back to research staff before randomization. Follow-up surveys were mailed to patients at 2, 6, 12, and 24 weeks post intervention access, and patients returned the surveys by mail. Patient withdrawal rates were about 3%.
Measures
Outcomes. This study included 4 measures of quality of life (an average of relevant portions of the World Health Organization’s Quality of Life (WHOQOL) measure, Emotional and Functional Well-being, and a prostate-cancer specific index, the Expanded Prostate Cancer Index Composite (EPIC). We also tested group differences on 5 more specific outcomes that were likely to be proximal rather than distal effects of the interventions: Cancer Information Competence, Health care Competence, Social Support, Bonding (with other patients), and Positive Coping.
Quality of life. Quality of life was measured by combining the psychological, social, and overall dimensions of the WHOQOL measures.23 Each of the 11 items was assessed with a 5-point scale, and the mean of those answers was the overall score.
Emotional well-being. Respondents answered 6 items of the Functional Assessment of Cancer Therapy – Prostate
Functional well-being. Respondents indicated how often they experienced each of the seven functional well-being subscale items of the FACT-Prostate.24
Prostate cancer patient functioning. We used the EPIC to measure of 3 of 4 domains of prostate cancer patient functioning: urinary, bowel, and sexual (omitting hormonal).25 The EPIC measures frequency and subjective degree of being a problem of several aspects in each domain. We then summed scores across the domains and transformed linearly to a 0-100 scale, with higher scores representing better functioning.
Cancer information competence. Five cancer information competence items, measured on a 5-point scale, assessed a participant’s perception about whether he could find and effectively and use health information, and were summed to create a single score.20
Social support. Six 5-point social support items assessed the informational and emotional support provided by friends, family, coworkers, and others, and were summed to create a single score.20
Health care competence. Five 5-point health care competence items assessed a patient’s comfort and activation level dealing with physicians and health care situations, and were summed to create a single score.20
Positive coping. Coping strategies were measured with the Brief Cope, a shorter version of the original 60-item COPE scale.26 Positive coping strategy, a predictor of positive adaptation in numerous coping contexts, was measured with the mean score of 4 scales (8 items in all): active coping, planning, positive reframing, and humor.
Bonding. Bonding with other prostate cancer patients was measured with five 5-point items about how frequently participants connected with and got information and support from other men with prostate cancer.27
User vs nonuser. Intent-to-treat analyses compared the assigned conditions. However, because CHESS use was self-selected and available at any time whereas mentor calls were scheduled and initiated by another person, the proportion actually using the interventions was quite different.
Since a participant assigned access to CHESS had to select the URL, even a single entry to the system was counted as use. Of 198 participants assigned to either the CHESS or CHESS+Mentor conditions, 43 (22%) never logged in and were classified as nonusers.
Because the mentor scheduled calls and attempted repeatedly to complete scheduled calls, the patient was in a reactive position, and the decision not to use the mentor’s services could come at the earliest at the end of a first completed call. However, after examining call notes and consulting with the mentors, it was clear that opting not to receive mentoring typically occurred at the second call. Furthermore, much (though not all) of the first call was typically taken up with getting acquainted and scheduling issues, so that defining “nonuse” as 2 or fewer completed calls was most faithful to what actually happened. Of 202 participants assigned access to a mentor, 16 (8%) were thus defined as nonusers.
Results
Overall, the participants were about 60 years of age and had some college education and middle-class incomes (Table 1). Only about 10% were minorities or lived alone, and their comfort using computers and the Internet was at or above the “quite comfortable” level. None of groups differed significantly from any other.
The 2 primary hypotheses of the study were that participants in the combined condition would manifest higher outcome scores than those with either intervention alone. Table 2 displays group means at 3 posttest intervals, controlling for theoretically chosen covariates (age, education, and minority status) and pretest levels of the dependent variable. The table also summarizes tests examining the hypotheses and the comparison of CHESS and Mentor conditions. The 4 quality-of-life scores appear first, followed by 5 more specific outcomes that are perhaps more proximal effects of these interventions.
The combined condition scored significantly higher than the CHESS-only condition on functional well-being at 3 months, on positive coping at 6 months, and on bonding at both 6 weeks and 6 months. The combined condition scored significantly higher than Mentor-only on health care competence and positive coping at 6 weeks, and on bonding at 6 months. This represents partial but scattered support for the hypotheses. And some comparisons of the combined condition with the Mentor-only condition showed reversals of the predicted relationship (although only cancer information competence at 3 months would have reached statistical significance in a 2-tailed test).
No directional hypotheses were made for the comparison of the 2 interventions (see Table 2 for the results of 2-tailed tests). Participants in the Mentor condition reported significantly higher functional well-being at 3 months, although there were 5 other comparisons in which the Mentor group scored higher at P < .10, and higher than the CHESS group on 22 of the 27 comparisons. Thus, it seemed that the Mentor condition alone might have been a somewhat stronger intervention than CHESS alone.
Discussion
We used a randomized control design to test whether combining computer-based and human interventions would provide greater benefits to prostate cancer patients than either alone, as previous research had shown for breast cancer patients.18 The computer-based resource was CHESS, a repeatedly evaluated integrated system combining information, social support, and interactive tools to help patients manage their response to disease. The human cancer information mentor intervention combined the expertise of NCI’s Cancer Information Service with the repeated contact more typical of peer mentoring. Previous research with breast cancer patients had shown both interventions to provide greater information, support, and quality-of-life benefits than Internet access alone.14 This study also compared outcomes obtained by the separate CHESS and Mentor conditions, but without predicting a direction of difference.
Tests at 6 weeks, 3 months and 6 months after intervention found instances in which prostate cancer patients assigned to the combined CHESS+Mentor condition experienced more positive quality of life or other outcomes than those assigned to CHESS or Mentor alone, but those differences were scattered rather than consistent. In the direct comparisons of the separate CHESS and Mentor conditions, significance was even rarer, but outcome scores tended to be higher in the Mentor condition than in the CHESS condition.
We noted that differential uptake of the 2 interventions (92% for Mentor vs 78% for CHESS) made interpreting the intent-to-treat analyses problematic, as the mentor’s control of the call schedule meant that far more patients in that condition actually received at least some intervention than in the CHESS condition, where patients used or did not use CHESS entirely at their own volition. This could have biased results in several ways, such as by underestimating the efficacy of the CHESS condition alone and thus inflating the contrast between CHESS alone and CHESS+Mentor. Or the combined condition might have been less different than the Mentor-only condition than intended, thus making for a conservative test of that comparison. However, post hoc analyses of only those participants who had actually used their assigned interventions (and this led to some reclassification of those originally assigned to the CHESS+Mentor condition) produced results that were little different than the intent-to-treat analysis.
Thus, although the combined condition produced some small advantages over either intervention alone, these advantages did not live up to expectations or to previous experience with breast cancer patients.17 We expected the mentor to be able to reinforce and help interpret what the participants learned from CHESS and their clinicians, and also to advise and direct these patients to be much more effective users of CHESS and other resources. Similarly, we expected that CHESS would make patients much better prepared for mentoring, so that instead of dealing with routine information matters, the mentor could go into greater detail or deal with more complex issues. Their combined effect should have been much larger than each alone, and that was not the case. Perhaps from the prostate cancer patients’ perspective, the 2 interventions seemed to offer similar resources, and a patient benefitted from one or the other but expected no additional gain from attending to both.
The 2 interventions themselves seemed nearly equally effective. The Mentor intervention was significantly stronger than CHESS in only 1 of 27 tests in the intent-to-treat analysis and 2 in the analysis limited to intervention users.
These results for prostate cancer patients are somewhat weaker than those previously reported with breast cancer patients.17 It is possible that prostate cancer patients (or men in general) are less inclined to seek health information, support, and health self-management than breast cancer patients (or women in general), perhaps becaus
Although these interventions were experienced by prostate cancer patients in their homes in natural and familiar ways, any experimental manipulation must acknowledge possible problems with external validity. More important here, our recruitment procedures may have produced self-selection to enter or not enter the study in 2 ways that limit its applicability. First, although we thought that offering Internet access to all participants would make participation more likely, the most frequent reason men gave in declining to join the study was “not a computer person.” Our participants were certainly very comfortable with computers and the Internet, and most used them frequently even before the study. Second, it seems that, except for their prostate cancer, our sample was healthy in other respects, as indicated by the low number of other health care visits or surgeries/hospitalization they reported (and “overwhelmed” and “too busy,” 2 common reasons for declining study participations could also be coming from men with more comorbidities). Thus, our sample was probably more computer literate and healthier than the general population of prostate cancer patients.
Nonetheless, for policymakers deciding what information and support interventions to put in place for prostate cancer patients (or more generally for other cancer patients as well), these results have 2 implications. First, since the combination of the mentor and CHESS produced only small advantages over either alone, the extra effort of doing both seems clearly unwarranted for prostate cancer patients. The somewhat larger advantage of the combined intervention shown for breast cancer patients in previous studiesmight warrant using the combination in some circumstances, but even that is not clear-cut.
Finding that CHESS and the cancer information mentor separately provided essentially equal benefits might seem to suggest that they can be regarded as alternatives. However, computer-based services can be provided much more cheaply and scaled up far more readily than services dependent on one-on-one contacts by a highly trained professional. This may direct health care decision makers first toward computer-based services.
Prostate cancer is the most common cancer among men and the second leading cause of cancer-related death in men. 1 Treatment choices for prostate cancer are perhaps more varied than for many other cancers, with surgery, external beam radiation therapy, and brachytherapy all widely used, a number of adjuvant and nonstandard therapy options available, as well as the possibility of not immediately treating the cancer – the “active surveillance” option.
Biochemical failure rates do not differ between the 3 main treatments,2 but each exposes patients to the risk of side effects, including impotence, incontinence, rectal injury, and operative mortality. Recovery can be gradual and will not always involve a return to baseline functioning.3 Quality-of-life comparisons observed covariate-controlled decreases in varying specific aspects of quality of life for each of the treatments.4
Surgery, brachytherapy, and external beam radiation therapy have each shown advantages over other treatments on at least some specific aspect, but disadvantages on others.4 Ongoing surveillance of a cancer left in place has become a more common option in part because of the disadvantages of traditional treatment and because of the growing recognition that sensitive diagnosis techniques often locate cancers that might not be life threatening. Recent reviews and reasonably long-term trials portray active surveillance as a valid alternative to surgery and radiation in many cases, with little difference in life expectancy and cancer-related quality of life, and possibly some reduction in health system cost.5-7
Prostate cancer patients cope with these uncertainties and decisions in many ways,8 often using multiple coping behaviors,9 but coping almost always includes seeking information and social support, as well as active problem-solving, to make informed treatment decisions consistent with their values.
Unfortunately, prostate patients often do not receive or use needed information. McGregor10 reported that patients were aware of their incomplete understanding of their disease and treatment options. Findings from several studies suggest that patients often perceive that clinicians inform them about the disease and treatment options but then send them home unprepared to deal with such things as incontinence or difficulties with sexual functioning.11
Similarly, previous research demonstrates the benefits of social support for prostate cancer patients who receive it, but also that overall they are underserved.12,13 Male cancer patients are generally far less likely to seek support and health information than are female patients. And when patients with prostate cancer do participate in online cancer support groups, they are more likely to exchange information, whereas breast cancer patients provide support for each other.14
Mentoring
Some responses to these knowledge and support gaps pair newly diagnosed patients with survivors willing to be a guide, coach, and a source of information, as in the American Cancer Society’s (ACS’s) Man-to-Man support groups.15 Peer mentors may have a sophisticated level of understanding from their own experiences with medical literature and the health care system, but this cannot be assumed. Another mentoring model is expert-based, exemplified by the National Cancer Institute’s (NCI’s) cancer information specialist at the Cancer Information Service (CIS) and a similar system at the ACS. These telephone services allow for responsiveness to the caller’s needs, existing knowledge, and the caller’s readiness for information. The CIS specialist can also introduce important information the caller might not have known to ask about.16
However, not all problems presented by callers can be solved in a single conversation. Callers are encouraged to call back with additional questions or when their situation changes, but speaking with the same specialist is not facilitated, so it is hard for a second call to build upon the first. Combining the expertise of the cancer information specialist with the ongoing and proactive contact and support typical of the lay guide/mentor/navigator could be more effective. Here a CIS-trained information specialist called prostate patients multiple times over the intervention period to help them deal with information seeking and interpretation. In a previous study with breast cancer patients, a mentor of this sort improved patient information competence and emotional processing.17
Interactive resources
Online resources allow cancer patients self-paced and self-directed access to information and support anonymously and at any time. However, this can be more complicated than it might at first seem. With the complexities of the prostate cancer diagnosis, the multiple treatment options, and the uncertain but potentially serious effects of the treatments themselves, the amount of potentially relevant information is quite large. Then, because individuals will value differentially the attributes of treatments, their consequences, or even notions of risk and gain, a system must be able to respond appropriately to a range of very different people. Beyond this, as prostate cancer patients move from the shock of a cancer diagnosis to the problems of interpreting its details, to making treatment decisions, to dealing with problems of recovery, and then re-establishing what is a “new normal” for them, an individual’s demands on a system vary as well. Comprehensive and integrated systems of services meet the varying needs of their users at different times and different situations.18,19 The systems approach not only makes it far easier for users to find what they need, it may also encourage them to see connections between physical, emotional, and social aspects of their illness. Versions of the system used in the present study – CHESS, or Comprehensive Health Enhancement Support System – have been effective supporting patients with AIDS and breast and lung cancers, and teens with asthma.16,20
Study goals and hypotheses
Given the success of the 2 aforementioned approaches, we wanted to compare how CHESS and ongoing contact with a human cancer information mentor in patients with prostate cancer would affect both several general aspects of quality of life and 1 specific to prostate cancer. We also examined differences in the patients’ information competence, quality of life, and social support. There was no a priori expectation that one intervention would be superior to the other, but any differences found could be important to policy decisions, given their quite-different cost and scalability.
More importantly, the primary hypothesis of the study was that patients with access to both CHESS and a mentor would experience substantially better outcomes than those with access to either intervention alone, because each had the potential to enhance the other’s benefits. For example, a patient could read CHESS material and come to the mentor much better prepared. By referring the user to specific parts of CHESS for basic information, the mentor could use calls to address more complex issues, or help interpret and evaluate difficult issues. In addition, because CHESS provides the mentor information about changes in the patient’s treatments, symptoms, and CHESS use, in the combined condition the cancer information mentor can know much more about the patient than when working alone. We also expected that the mentor would stimulate the kind of diverse use of CHESS services we have found to be most effective
Because both mentoring and CHESS have consistently produced positive quality of life effects on their own, compared to controls, there is no reasonable expectation that negative effects of a combined condition could occur and should be tested for. Thus, the study was powered for 1-tailed significance in the comparison between the combined condition and either intervention alone, a procedure used consistently in previous studies of CHESS components or combined conditions. However, since the research question comparing the 2 interventions alone had no such strong history it was tested 2-tailed.
Methods
Recruitment
Study recruitment was conducted from January 1, 2007 to September 30, 2008 at the University of Wisconsin’s Paul P Carbone Comprehensive Cancer Center in Madison, Hartford Hospital’s Helen and Harry Gray Cancer Center in Hartford, Connecticut, and The University of Texas MD Anderson Cancer Center in Houston.
A total of 461 patients were invited to participate in the study. Of those patients, 147 declined to participate, 4 were excluded, and 310 were randomized to access to CHESS only, access to a human mentor only, or access to CHESS and a mentor (CHESS+Mentor) during the 6-month intervention period, which provided adequate power (>.80) for effects of moderate size (Figure 1). Randomization was done with a computer-generated list that site study managers accessed on a patient-by-patient basis, with experimental conditions blocked within sites.
Recruitment was done by posting brochures about the study at the relevant locations and devising standardized recruitment scripts for clinical staff to use when talking to patients about the study. Staff at all sites invited patients they thought might be eligible to learn more about the study. As appropriate, staff members then reviewed informed consent and HIPAA information, explained the interventions, answered patient questions, obtained written consent, collected complete patient contact and computer access information, and provided patients the baseline questionnaires.
The standard inclusion criteria were: men older than 17 years, being able to read and understand English, and being within 2 months of a diagnosis of primary prostate cancer (stage 1 or 2) at the time of recruitment. Despite the 2-month window, few men had begun treatment before pretest. Only 9 of the 310 participants reported having already had surgery (7 prostatectomies, 2 implants), so participants may be fairly characterized as beginning the study in time to benefit from interventions during most stages of their experience with prostate cancer.
Interventions
To provide an equal baseline, all of the participants were given access to the Internet, which is becoming a de facto standard for information access. Internet access charges were paid for all participants during the 6-month intervention period, and computers were loaned to those who did not have a personal computer. All of the participants were offered training on using the computer, particularly with Google search procedures so that they could access resources on prostate cancer.
Participants assigned to the CHESS or CHESS+Mentor conditions were also offered training in using CHESS (basically a guided tour), which typically took about 30 minutes on the telephone but was occasionally done in-person.
CHESS intervention. In creating CHESS for prostate cancer patients, a combination of patient needs assessments, focus groups with patients and family members, and clinician expertise helped us identify the needs, coping mechanisms, and relevant medical information to help patients respond to the disease. An article describing development of the CHESS Prostate Cancer Module22 presents how those different services address patient needs for information, communication, and support, or build skills.
Most of these services were present in CHESS for other diseases, but several were newly created to meet needs of prostate cancer patients and partners, such as a decision map tool and a module on managing sexual problems.22 Also, patients expressed frustration at being overwhelmed by the volume of information and said they would prefer to focus only on what was most relevant, so we created an alternative navigation structure on the CHESS homepage. Using terms suggested by focus groups of prostate cancer survivors and their spouses, we devised a navigation structure called Step-by-Step that identified 6 typical sequential steps of men’s experience with prostate cancer. Clicking on a step would take a patient to a menu focused on actions and considerations specific to that disease step, links to information most relevant at that step, and suggested questions to ask oneself and one’s doctor.
Mentor intervention. The cancer information mentor who made most of the calls to patients was an experienced information specialist with the Cancer Information Service and had served as the expert for the CHESS Ask an Expert service for 6 years. She was highly knowledgeable about prostate cancer and patient information needs. Her additional training for this study focused on taking advantage of repeated contacts with the participants and how to set limits to avoid any semblance of psychological counseling. At recruitment, we made clear that a male mentor was also available if the participant would prefer to discuss sensitive topics with another man. The male mentor was experienced in the Man-to-Man program and received additional training for this role, but he was used for only 1% of all contacts.
During calls, the mentor had Internet access to a range of NCI, ACS, and other resources. She could help interpret information the participant already possessed as well as refer him to other public resources, including those on the Internet. CHESS software designers created an additional interface for the mentor that handled call scheduling and allowed her to record the topics of conversations, her responses and recommendations, and her overall ratings of patient preparedness and satisfaction. Using this interface allowed the mentor to quickly review a participant’s status and focus the conversation on issues raised by past conversations or scheduled treatment events. The mentor calls were audiorecorded and reviewed frequently by the project director during the early months of intervention and less frequently thereafter to ensure adherence to the protocol.
The mentor telephoned weekly during the first month of intervention, then twice during the second month, and once a month during the final 4 months of the intervention (ie, 10 scheduled calls, though patients could also initiate additional calls). Calls were scheduled through a combination of telephone contact and e-mail according to the patient’s preference. Call length ranged from 5 minutes to an hour, with the average about 12 minutes (the first call tended to be considerably longer, and was scheduled for 45 minutes). About 10%-15% of participants in the Mentor conditions initiated calls to the mentor to obtain additional support, and about 15% of scheduled calls in fact took place as e-mail exchanges. A few calls were missed because of scheduling difficulties, and some participants stopped scheduling the last few calls, but the average number of full calls or e-mails was 6.41 per participant.
CHESS+Mentor intervention. For the CHESS+Mentor condition, the interactions and resources used were similar to those of the Mentor-only condition, but the interface also provided the mentor with a summary of the participant’s recent CHESS use and any concerns reported to CHESS, which helped the mentor assess knowledge and make tailored recommendations. The mentor could also refer participants to specific resources within CHESS, aided by knowledge of what parts of CHESS had or had not been used.
Assessment methods
Patients were given surveys at the baseline visit to complete and mail back to research staff before randomization. Follow-up surveys were mailed to patients at 2, 6, 12, and 24 weeks post intervention access, and patients returned the surveys by mail. Patient withdrawal rates were about 3%.
Measures
Outcomes. This study included 4 measures of quality of life (an average of relevant portions of the World Health Organization’s Quality of Life (WHOQOL) measure, Emotional and Functional Well-being, and a prostate-cancer specific index, the Expanded Prostate Cancer Index Composite (EPIC). We also tested group differences on 5 more specific outcomes that were likely to be proximal rather than distal effects of the interventions: Cancer Information Competence, Health care Competence, Social Support, Bonding (with other patients), and Positive Coping.
Quality of life. Quality of life was measured by combining the psychological, social, and overall dimensions of the WHOQOL measures.23 Each of the 11 items was assessed with a 5-point scale, and the mean of those answers was the overall score.
Emotional well-being. Respondents answered 6 items of the Functional Assessment of Cancer Therapy – Prostate
Functional well-being. Respondents indicated how often they experienced each of the seven functional well-being subscale items of the FACT-Prostate.24
Prostate cancer patient functioning. We used the EPIC to measure of 3 of 4 domains of prostate cancer patient functioning: urinary, bowel, and sexual (omitting hormonal).25 The EPIC measures frequency and subjective degree of being a problem of several aspects in each domain. We then summed scores across the domains and transformed linearly to a 0-100 scale, with higher scores representing better functioning.
Cancer information competence. Five cancer information competence items, measured on a 5-point scale, assessed a participant’s perception about whether he could find and effectively and use health information, and were summed to create a single score.20
Social support. Six 5-point social support items assessed the informational and emotional support provided by friends, family, coworkers, and others, and were summed to create a single score.20
Health care competence. Five 5-point health care competence items assessed a patient’s comfort and activation level dealing with physicians and health care situations, and were summed to create a single score.20
Positive coping. Coping strategies were measured with the Brief Cope, a shorter version of the original 60-item COPE scale.26 Positive coping strategy, a predictor of positive adaptation in numerous coping contexts, was measured with the mean score of 4 scales (8 items in all): active coping, planning, positive reframing, and humor.
Bonding. Bonding with other prostate cancer patients was measured with five 5-point items about how frequently participants connected with and got information and support from other men with prostate cancer.27
User vs nonuser. Intent-to-treat analyses compared the assigned conditions. However, because CHESS use was self-selected and available at any time whereas mentor calls were scheduled and initiated by another person, the proportion actually using the interventions was quite different.
Since a participant assigned access to CHESS had to select the URL, even a single entry to the system was counted as use. Of 198 participants assigned to either the CHESS or CHESS+Mentor conditions, 43 (22%) never logged in and were classified as nonusers.
Because the mentor scheduled calls and attempted repeatedly to complete scheduled calls, the patient was in a reactive position, and the decision not to use the mentor’s services could come at the earliest at the end of a first completed call. However, after examining call notes and consulting with the mentors, it was clear that opting not to receive mentoring typically occurred at the second call. Furthermore, much (though not all) of the first call was typically taken up with getting acquainted and scheduling issues, so that defining “nonuse” as 2 or fewer completed calls was most faithful to what actually happened. Of 202 participants assigned access to a mentor, 16 (8%) were thus defined as nonusers.
Results
Overall, the participants were about 60 years of age and had some college education and middle-class incomes (Table 1). Only about 10% were minorities or lived alone, and their comfort using computers and the Internet was at or above the “quite comfortable” level. None of groups differed significantly from any other.
The 2 primary hypotheses of the study were that participants in the combined condition would manifest higher outcome scores than those with either intervention alone. Table 2 displays group means at 3 posttest intervals, controlling for theoretically chosen covariates (age, education, and minority status) and pretest levels of the dependent variable. The table also summarizes tests examining the hypotheses and the comparison of CHESS and Mentor conditions. The 4 quality-of-life scores appear first, followed by 5 more specific outcomes that are perhaps more proximal effects of these interventions.
The combined condition scored significantly higher than the CHESS-only condition on functional well-being at 3 months, on positive coping at 6 months, and on bonding at both 6 weeks and 6 months. The combined condition scored significantly higher than Mentor-only on health care competence and positive coping at 6 weeks, and on bonding at 6 months. This represents partial but scattered support for the hypotheses. And some comparisons of the combined condition with the Mentor-only condition showed reversals of the predicted relationship (although only cancer information competence at 3 months would have reached statistical significance in a 2-tailed test).
No directional hypotheses were made for the comparison of the 2 interventions (see Table 2 for the results of 2-tailed tests). Participants in the Mentor condition reported significantly higher functional well-being at 3 months, although there were 5 other comparisons in which the Mentor group scored higher at P < .10, and higher than the CHESS group on 22 of the 27 comparisons. Thus, it seemed that the Mentor condition alone might have been a somewhat stronger intervention than CHESS alone.
Discussion
We used a randomized control design to test whether combining computer-based and human interventions would provide greater benefits to prostate cancer patients than either alone, as previous research had shown for breast cancer patients.18 The computer-based resource was CHESS, a repeatedly evaluated integrated system combining information, social support, and interactive tools to help patients manage their response to disease. The human cancer information mentor intervention combined the expertise of NCI’s Cancer Information Service with the repeated contact more typical of peer mentoring. Previous research with breast cancer patients had shown both interventions to provide greater information, support, and quality-of-life benefits than Internet access alone.14 This study also compared outcomes obtained by the separate CHESS and Mentor conditions, but without predicting a direction of difference.
Tests at 6 weeks, 3 months and 6 months after intervention found instances in which prostate cancer patients assigned to the combined CHESS+Mentor condition experienced more positive quality of life or other outcomes than those assigned to CHESS or Mentor alone, but those differences were scattered rather than consistent. In the direct comparisons of the separate CHESS and Mentor conditions, significance was even rarer, but outcome scores tended to be higher in the Mentor condition than in the CHESS condition.
We noted that differential uptake of the 2 interventions (92% for Mentor vs 78% for CHESS) made interpreting the intent-to-treat analyses problematic, as the mentor’s control of the call schedule meant that far more patients in that condition actually received at least some intervention than in the CHESS condition, where patients used or did not use CHESS entirely at their own volition. This could have biased results in several ways, such as by underestimating the efficacy of the CHESS condition alone and thus inflating the contrast between CHESS alone and CHESS+Mentor. Or the combined condition might have been less different than the Mentor-only condition than intended, thus making for a conservative test of that comparison. However, post hoc analyses of only those participants who had actually used their assigned interventions (and this led to some reclassification of those originally assigned to the CHESS+Mentor condition) produced results that were little different than the intent-to-treat analysis.
Thus, although the combined condition produced some small advantages over either intervention alone, these advantages did not live up to expectations or to previous experience with breast cancer patients.17 We expected the mentor to be able to reinforce and help interpret what the participants learned from CHESS and their clinicians, and also to advise and direct these patients to be much more effective users of CHESS and other resources. Similarly, we expected that CHESS would make patients much better prepared for mentoring, so that instead of dealing with routine information matters, the mentor could go into greater detail or deal with more complex issues. Their combined effect should have been much larger than each alone, and that was not the case. Perhaps from the prostate cancer patients’ perspective, the 2 interventions seemed to offer similar resources, and a patient benefitted from one or the other but expected no additional gain from attending to both.
The 2 interventions themselves seemed nearly equally effective. The Mentor intervention was significantly stronger than CHESS in only 1 of 27 tests in the intent-to-treat analysis and 2 in the analysis limited to intervention users.
These results for prostate cancer patients are somewhat weaker than those previously reported with breast cancer patients.17 It is possible that prostate cancer patients (or men in general) are less inclined to seek health information, support, and health self-management than breast cancer patients (or women in general), perhaps becaus
Although these interventions were experienced by prostate cancer patients in their homes in natural and familiar ways, any experimental manipulation must acknowledge possible problems with external validity. More important here, our recruitment procedures may have produced self-selection to enter or not enter the study in 2 ways that limit its applicability. First, although we thought that offering Internet access to all participants would make participation more likely, the most frequent reason men gave in declining to join the study was “not a computer person.” Our participants were certainly very comfortable with computers and the Internet, and most used them frequently even before the study. Second, it seems that, except for their prostate cancer, our sample was healthy in other respects, as indicated by the low number of other health care visits or surgeries/hospitalization they reported (and “overwhelmed” and “too busy,” 2 common reasons for declining study participations could also be coming from men with more comorbidities). Thus, our sample was probably more computer literate and healthier than the general population of prostate cancer patients.
Nonetheless, for policymakers deciding what information and support interventions to put in place for prostate cancer patients (or more generally for other cancer patients as well), these results have 2 implications. First, since the combination of the mentor and CHESS produced only small advantages over either alone, the extra effort of doing both seems clearly unwarranted for prostate cancer patients. The somewhat larger advantage of the combined intervention shown for breast cancer patients in previous studiesmight warrant using the combination in some circumstances, but even that is not clear-cut.
Finding that CHESS and the cancer information mentor separately provided essentially equal benefits might seem to suggest that they can be regarded as alternatives. However, computer-based services can be provided much more cheaply and scaled up far more readily than services dependent on one-on-one contacts by a highly trained professional. This may direct health care decision makers first toward computer-based services.
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277-300.
2. Cozzarini C. Low-dose rate brachytherapy, radical prostatectomy, or external-beam radiation therapy for localized prostate carcinoma: The growing dilemma. European Urology. 2011;60(5):894-896.
3. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250-1261.
4. Ferrer F, Guedea F, Pardo Y, et al. Quality of life impact of treatments for localized prostate cancer. Radiother Oncol. 2013;108(2):306-313.
5. Cooperberg, MR, Carroll, PR, Klotz, L. Active Surveillance for prostate cancer: progress and promise. J Clin Onc. 2011;29:3669-3676.
6. Hamdy, FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
7. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-37.
8. Lavery JF, Clarke VA. Prostate cancer: patients’ spouses’ coping and marital adjustment. Psychol Health Med. 1999;4(3):289-302.
9. Folkman S, Lazarus R. If it changes it must be a process: study of emotion and coping during three stages of a college examination. J Pers Soc Psycol. 1985;48:150-170.
10. McGregor S. What information patients with localized prostate cancer hear and understand. Patient Educ Couns. 2003;49:273-278.
11. Steginga SK, Occhipinti S, Dunn J, Gardiner RA, Heathcote P, Yaxley J. (2001) The supportive care needs of men with prostate cancer (2000). Psychooncology. 2001;10(1):66-75.
12. Gregoire I, Kalogeropoulos D, Corcos J. The effectiveness of a professionally led support group for men with prostate cancer. Urologic Nurs. 1997;17(2):58-66.
13. Katz D, Koppie T, Wu D, et al. Sociodemographic characteristics and health related quality of life in men attending prostate cancer support groups. J Urol. 2002;168:2092-2096.
14. Klemm P, Hurst M, Dearholt S, Trone S. Gender differences on Internet cancer support groups. Comput Nurs. 1999;17(2):65-72.
15. Gray R, Fitch M, Phillips C, Labrecque M, Fergus K. Managing the impact of illness: the experiences of men with prostate cancer and their spouses. J Health Psychol. 2000;5(4):531-548.
16. Thomsen CA, Ter Maat J. Evaluating the Cancer Information Service: a model for health communications. Part 1. J Health Commun. 1998;3(suppl.):1-13.
17. Hawkins RP, Pingree S, Baker TB, et al. Integrating eHealth with human services for breast cancer patients. Transl Behav Med. 2011;1(1):146-154.
18. Strecher V. Internet methods for delivering behavioral and health-related interventions. Ann Rev Clin Psychol. 2007;(3):53-76.
19. Gustafson DH, Hawkins RP, McTavish F, et al. Internet-based interactive support for cancer patients: Are integrated systems better? J Commun. 2008;58(2):238-257.
20. Gustafson DH, Hawkins RP, Boberg EW, et al. CHESS: Ten years of research and development in consumer health informatics for broad populations, including the underserved. Int J Med Inform. 2002;65(3):169-177.
21. Han JY, Hawkins RP, Shaw B, Pingree S, McTavish F, Gustafson D. Unraveling uses and effects of an interactive health communication system. J Broadcast Electron Media. 2009;53(1):1-22.
22. Van Bogaert D, Hawkins RP, Pingree S, Jarrard D. The development of an eHealth tool suite for prostate cancer patients and their partners. J Support Oncol. 2012;10(5):202-208.
23. The WHOQOL Group. Development of the WHOQOL: Rationale and current status. Int J Ment Health. 1994;23:24-56.
24. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920-928.
25. Wei JT, Dunn R, Litwin M, Sandler H, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899-905.
26. Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4: 91-100.
27. Gustafson D, McTavish F, Stengle W, et al. Use and impact of eHealth System by low-income women with breast cancer. J Health Commun. 2005;10(suppl 1):219-234.
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277-300.
2. Cozzarini C. Low-dose rate brachytherapy, radical prostatectomy, or external-beam radiation therapy for localized prostate carcinoma: The growing dilemma. European Urology. 2011;60(5):894-896.
3. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250-1261.
4. Ferrer F, Guedea F, Pardo Y, et al. Quality of life impact of treatments for localized prostate cancer. Radiother Oncol. 2013;108(2):306-313.
5. Cooperberg, MR, Carroll, PR, Klotz, L. Active Surveillance for prostate cancer: progress and promise. J Clin Onc. 2011;29:3669-3676.
6. Hamdy, FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
7. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-37.
8. Lavery JF, Clarke VA. Prostate cancer: patients’ spouses’ coping and marital adjustment. Psychol Health Med. 1999;4(3):289-302.
9. Folkman S, Lazarus R. If it changes it must be a process: study of emotion and coping during three stages of a college examination. J Pers Soc Psycol. 1985;48:150-170.
10. McGregor S. What information patients with localized prostate cancer hear and understand. Patient Educ Couns. 2003;49:273-278.
11. Steginga SK, Occhipinti S, Dunn J, Gardiner RA, Heathcote P, Yaxley J. (2001) The supportive care needs of men with prostate cancer (2000). Psychooncology. 2001;10(1):66-75.
12. Gregoire I, Kalogeropoulos D, Corcos J. The effectiveness of a professionally led support group for men with prostate cancer. Urologic Nurs. 1997;17(2):58-66.
13. Katz D, Koppie T, Wu D, et al. Sociodemographic characteristics and health related quality of life in men attending prostate cancer support groups. J Urol. 2002;168:2092-2096.
14. Klemm P, Hurst M, Dearholt S, Trone S. Gender differences on Internet cancer support groups. Comput Nurs. 1999;17(2):65-72.
15. Gray R, Fitch M, Phillips C, Labrecque M, Fergus K. Managing the impact of illness: the experiences of men with prostate cancer and their spouses. J Health Psychol. 2000;5(4):531-548.
16. Thomsen CA, Ter Maat J. Evaluating the Cancer Information Service: a model for health communications. Part 1. J Health Commun. 1998;3(suppl.):1-13.
17. Hawkins RP, Pingree S, Baker TB, et al. Integrating eHealth with human services for breast cancer patients. Transl Behav Med. 2011;1(1):146-154.
18. Strecher V. Internet methods for delivering behavioral and health-related interventions. Ann Rev Clin Psychol. 2007;(3):53-76.
19. Gustafson DH, Hawkins RP, McTavish F, et al. Internet-based interactive support for cancer patients: Are integrated systems better? J Commun. 2008;58(2):238-257.
20. Gustafson DH, Hawkins RP, Boberg EW, et al. CHESS: Ten years of research and development in consumer health informatics for broad populations, including the underserved. Int J Med Inform. 2002;65(3):169-177.
21. Han JY, Hawkins RP, Shaw B, Pingree S, McTavish F, Gustafson D. Unraveling uses and effects of an interactive health communication system. J Broadcast Electron Media. 2009;53(1):1-22.
22. Van Bogaert D, Hawkins RP, Pingree S, Jarrard D. The development of an eHealth tool suite for prostate cancer patients and their partners. J Support Oncol. 2012;10(5):202-208.
23. The WHOQOL Group. Development of the WHOQOL: Rationale and current status. Int J Ment Health. 1994;23:24-56.
24. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920-928.
25. Wei JT, Dunn R, Litwin M, Sandler H, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899-905.
26. Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4: 91-100.
27. Gustafson D, McTavish F, Stengle W, et al. Use and impact of eHealth System by low-income women with breast cancer. J Health Commun. 2005;10(suppl 1):219-234.
Metastatic eccrine carcinoma with stomach and pericardial involvement
Skin adnexal tumors (SAT) are rare tumors that make up about 1%-2% of all cutaneous malignancies. They represent a various group of benign and malignant tumors that arise from skin adnexal epithelial structures: hair follicle, pilosebaceous unit, and apocrine or eccrine sweat glands. Although this derivation provides a practical basis for classification, some tumors may exhibit a mixed or more than one line of differentiation, rendering precise classification of those neoplasms difficult, and such cases should be categorized according to prevailing phenotype. In this report, we present a patient with metastatic eccrine carcinoma. Clinical experience for metastatic disease treatment is derived from a few reports, and there are no universal treatment guidelines. Given the few reported cases and the absence of randomized clinical trials for these patients, it is important to collect clinical experiences.
Case presentation and summary
A 56-year-old African man presented with a 5-week history of multiple nontender subcutaneous skin nodules all over his body except for his palms and soles, and associated with generalized itching. He had a mass in the sole of his right foot 35 years previously in another country. The mass had recurred 15 years later and was excised again. The exact etiology of the mass was unknown to the patient. He had no other medical problems. He was on no medications and did not smoke, drink, or use recreational drugs.
His vital signs on admission were normal. Examination was significant for innumerable superficial skin nodules in the scalp, back, torso, and abdomen. The largest was in the neck and measured 4 x 2 cm. A firm right inguinal mass of 7 x 4 cm was palpable. An abdominal exam revealed large ascites but no organomegaly.
The results of laboratory tests were significant for hyponatremia 126 mEq/L (normal, 135-145), hypercalcemia of 12.2 mg/dL (8.5-10.5), with normal phosphorous of 2.5 mg/dL (2.5-4.5), parathyroid of 11.5 pg/ml (6-65), and low vitamin D level of <7 ng/ml (30-100). Other test results were: carcinoembryonic antigen (CEA), 4.36 ng/ml (0.00-2.99); alpha fetoprotein, 2.39 IU/ml (0.00-9.0); calcium 11.6 mg/dL (8.5-10.2); lactate dehydrogenase, 325 U/L (85-210); aspartate aminotransferase, 59 U/L (0-40); alanine aminotransferase 43 U/L (5-35); alkaline phosphatase, 65 u/L (50-120); albumin, 2.7 g/dL (3.8-5.2); white blood cell count, 14.1 k/uL (4.4-10.6); h
A chest and abdomen computed-tomography scan on presentation showed presence of innumerable subcutaneous and intramuscular nodules throughout the chest, abdomen, and pelvis (Figure 1).
Extensive peritoneal carcinomatosis in addition to moderate ascites and perivascular lymphadenopathy were evident in the abdomen cuts. Remarkably, multiple lytic, osseous metastases were seen with subacute pathologic fracture of right fourth rib in addition to mediastinal lymphadenopathy with small pericardial effusion in the chest cuts. The right thigh mass was described as a large lobulated solid and cystic mass. Ascitic fluid analysis was negative for malignant cells. Biopsy of one the skin nodules in the upper back showed carcinoma involving the skin with focal tubular differentiation (Figure 2).
Immunohistochemical stains were positive for p63, epithelial membrane antigen, high molecular weight keratin, and p40. The lesional cells were negative for CEA, bcl-2, Ber-Ep4, CK7, and CK20. The profile was compatible with a skin adnexal carcinoma of sweat gland origin. The groin lymph node showed eccrine acrospiroma.
The patient underwent an upper endoscopy to assess for recurrent vomiting and it revealed diffuse areas of large erythematous ulcerated nodules noted in the cardia, fundus, and body of the stomach (Figure 3). A biopsy of the gastric nodules revealed gastric mucosa with metastatic carcinoma.
After a thorough review of the literature, he was started on palliative chemotherapy 13 days after initial presentation with docetaxel 75 mg/m2, carboplatin AUC 5 (470 mg), and 5-FU (5-fluorouracil, 750 mg/m2) over 24 hours on days 1 through 5. However, on day 2 of the chemotherapy, he became hypotensive and was found to have cardiac tamponade. He underwent an emergent pericardial window procedure. Analysis of the pericardial fluid was consistent with metastatic carcinoma (Figure 4). Chemotherapy was discontinued while he remained hypotensive requiring multiple vasopressors. His clinical condition did not improve and he passed away 27 days from initial presentation.
Discussion
Sweat gland carcinomas are very rare malignant tumors of the adnexal epithelial structures of the skin, sebaceous, hair follicle, apocrine or eccrine glands that were first described by Cornil in 1865.1 They occur primarily in adult patients, with a peak incidence in fifth and sixth decades of life.2,3 The etiology is unknown, but some cases have been reported to be a consequence of radiation therapy.4 They are almost always an incidental histologic diagnosis.2,5 The tumors usually appear as single nodule, and multinodularity usually associated with both local and metastatic disease.6 There are no characteristic findings to suggest that a particular nodule may represent sweat gland carcinoma, and even if sweat gland tumor is suspected, benign counterparts are more common.
Eccrine carcinoma is the most aggressive among skin adnexal tumors. They can arise on the lower limbs, trunk, head and neck, scalp and ears, upper extremities, abdomen, and genital sites.7
The cells of eccrine sweat glands express low molecular weight keratin, epithelial membrane antigen, carcinoembryonic antigen, as well as S100 protein, smooth muscle actin, p63, calponin, cytokeratin 14, and bcl-2.8 Skin tumors with eccrine differentiation may stain for estrogen and progesterone, which has important clinical implications because those patients can be treated with hormonal therapy.9 Positivity for estrogen receptors does not differentiate cutaneous eccrine tumors from cutaneous metastases of breast cancers.8,9 Androgen receptor evaluation in these cases can help distinguish between the two.10 Human epidermal growth factor receptor 2 (HER-2) is expressed in 3.5% of skin adnexal tumors.11
The molecular pathogenesis of malignant adnexal tumors is not clear, but overexpression of tumor suppressor protein p16 has been described as a common feature in eccrine carcinomas.12
Prognostic factors for sweat gland carcinoma are difficult to identify, because of the small number of reported cases. The likely prognostic factors include size, histological type, lymph node involvement, and presence of distant metastasis. Absent of lymph node involvement correlates with 10-year disease-free survival rate of 56%, which falls to 9% if nodes are involved.13
There are no uniform guidelines for the treatment sweat gland carcinomas, and the clinical experience described in the literature is the only source of available information.
The treatment of choice of all subtypes of localized sweat gland carcinomas is wide surgical excision with broad tumor margins, given the propensity for local recurrences along with regional lymph node dissection in the presence of clinically positive nodes. Prophylactic lymph node resection does not seem to improve survival or decrease recurrence rates.7 The use of adjuvant radiotherapy to prevent local recurrence also is not well established. One report suggested radiosensitivity of these tumors, and adjuvant radiation was therefore recommended in high-risk cases (ie, large tumors of 5 cm and positive surgical margins of 1 cm) and moderate to poorly differentiated tumors with lymphovascular invasion.14 Adjuvant radiation to the involved lymph node basin is suggested in the setting of extranodal extension or extensive involvement, that is, 4 lymph nodes.15 The role of lymphadenectomy has not been adequately addressed in the literature.
The role of chemotherapy in metastatic disease is not clear, but sweat gland carcinomas are considered chemoresistant (Table). Several combinations have been used with short-term responses. In one case treated with doxorubicin, mitomycin, vincristine, and 5-FU followed by maintenance therapy, the patient achieved a complete response that lasted for 16 months.16 In another report, the treatment response was 2 years with treatment consisted of anthracyclin, cyclophosphamide, vincristine, and bloemycin.17 Other combinations used in the literature include carboplatin and paclitaxel, which led to prolonged remission.14 Cisplatin and 5-FU, or cisplatin plus cetuximab have been reported but with discouraging results.18 Results to taxanes showed conflicting results.19,20
Hormonal therapy can be effective in cases in which estrogen and progesterone receptors are expressed, which can range from 19%-30% of eccrine sweat gland carcinomas.21,22 Two cases have reported complete regression of lymph nodes in patients with metastatic disease, and in 1 patient relief from pain caused by bone metastases with durable response of around 3 years.23,24 a
Experience with targeted therapy is very limited. Sunitinib has been reported to have some activity in metastatic adnexal tumors as a second-line therapy in 2 patients, with disease control for 8 and 10 months respectively.25 Trastuzumab has been reported as having activity in 1 patient with strong HER2 expression (IHC score of 3+, denoting HER2 positivity), with complete regression of metastatic tumor. Upon progression in the same patient, a combination of lapatinib and capecitabine also showed positive response.26
In conclusion, metastatic sweat gland tumors treatment has not been standardized because of a dearth of reports in the literatures. Its early identification and complete excision gives the best chance of a cure. Neither chemotherapy nor radiation therapy has been proven to be of clinical benefit in treating metastatic disease.
1. Gates O, Warren S, Warvi WN. Tumors of sweat glands. Am J Pathol. 1943;19(4):591-631.
2. Mitts DL, Smith MT, Russell L, Bannayan GA, Cruz AB. Sweat gland carcinoma: a clinico-pathological reappraisal. J Surg Oncol. 1976;8(1):23-29.
3. Panoussopoulos D, Darom A, Lazaris AC, Misthos P, Papadimitriou K, Androulakis G. Sweat gland carcinoma with multiple local recurrences: a case report. Adv Clin Path. 1999;3(3):63-68.
4. Marone U, Caracò C, Anniciello AM, et al. Metastatic eccrine porocarcinoma : report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
5. Yildirim S, Aköz T, Akan M, Ege GA. De novo malignant eccrine spiradenoma with an interesting and unusual location. Dermatol Surg. 2001;27(4):417-420.
6. Shaw M, McKee PH, Lowe D, Black MM. Malignant eccrine poroma: a study of twenty-seven cases. Br J Dermatol. 1982;107(6):675-680.
7. De Iuliis F, Amoroso L, Taglieri L, et al. Chemotherapy of rare skin adnexal tumors: a review of literature. Anticancer Res. 2014;34(10):5263-5268.
8. Alsaad KO, Obaidat NA, Ghazarian D. Skin adnexal neoplasms – part 1: an approach to tumours of the pilosebaceous unit. J Clin Pathol. 2007;60(2):129-144.
9. Serhrouchni KI, Harmouch T, Chbani L, et al. Eccrine carcinoma : a rare cutaneous neoplasm. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3570399/. Published online February 4, 2013. Accessed October 11, 2017.
10. Shidham VB, Komorowski RA, Machhi JK. Androgen receptor expression in metastatic adenocarcinoma in females favors a breast primary. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1601970/. Published online October 4, 2006. Accessed October 11, 2017.
11. Hiatt KM, Pillow JL, Smoller BR. Her-2 expression in cutaneous eccrine and apocrine neoplasms. Mod Pathol. 2004;17(1):28-32.
12. Gu L-H, Ichiki Y, Kitajima Y. Aberrant expression of p16 and RB protein in eccrine porocarcinoma. J Cutan Pathol. 2002;29(8):473-479.
13. el-Domeiri AA, Brasfield RD, Huvos AG, Strong EW. Sweat gland carcinoma: a clinico-pathologic study of 83 patients. Ann Surg. 1971;173(2):270-274.
14. Tlemcani K, Levine D, Smith R V, et al. Metastatic apocrine carcinoma of the scalp: prolonged response to systemic chemotherapy. J Clin Oncol. 2010;28(24):e412-e414.
15. Chamberlain RS, Huber K, White JC, Travaglino-Parda R. Apocrine gland carcinoma of the axilla: review of the literature and recommendations for treatment. Am J Clin Oncol. 1999;22(2):131-135.
16. Gutermuth J, Audring H, Voit C, Trefzer U, Haas N. Antitumour activity of paclitaxel and interferon-alpha in a case of metastatic eccrine porocarcinoma. J Eur Acad Dermatol Venereol. 2004;18(4):477-479.
17. Mezger J, Remberger K, Schalhorn A, Wohlrab A, Wilmanns W. Treatment of metastatic sweat gland carcinoma by a four drug combination chemotherapy: response in two cases. Med Oncol Tumor Pharmacother. 1986;3(1):29-34.
18. Aaribi I, Mohtaram A, Ben Ameur El Youbi M, et al. Successful management of metastatic eccrine porocarcinoma. https://www.hindawi.com/journals/crionm/2013/282536/. Published 2013. Accessed October 10, 2017.
19. Shiohara J, Koga H, Uhara H, Takata M, Saida T. Eccrine porocarcinoma: clinical and pathological studies of 12 cases. J Dermatol. 2007;34(8):516-522.
20. Swanson PE, Mazoujian G, Mills SE, Campbell RJ, Wick MR. Immunoreactivity for estrogen receptor protein in sweat gland tumors. Am J Surg Pathol. 1991;15(9):835-841.
21. Busam KJ, Tan LK, Granter SR, et al. Epidermal growth factor, estrogen, and progesterone receptor expression in primary sweat gland carcinomas and primary and metastatic mammary carcinomas. Mod Pathol. 1999;12(8):786-793.
22. Sridhar KS, Benedetto P, Otrakji CL, Charyulu KK. Response of eccrine adenocarcinoma to tamoxifen. Cancer. 1989;64(2):366-370.
23. Daniel SJ, Nader R, Kost K, Hüttner I. Facial sweat gland carcinoma metastasizing to neck nodes: a diagnostic and therapeutic challenge. Arch Otolaryngol Head Neck Surg. 2001;127(12):1495-1498.
24. Battistella M, Mateus C, Lassau N, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24(2):199-203.
25. Hidaka T, Fujimura T, Watabe A, et al. Successful treatment of HER-2-positive metastatic apocrine carcinoma of the skin with lapatinib and capecitabine. Acta Derm Venereol. 2012;92(6):654-655.
26. Mandaliya H, Nordman I. Metastatic eccrine porocarcinoma: a rare case of successful treatment. Case Rep Oncol. 2016;9(2):454-456.
27. de Bree E, Volalakis E, Tsetis D, et al. Treatment of advanced malignant eccrine poroma with locoregional chemotherapy. Br J Dermatol. 2005;152(5):1051-1055.
28. Bahl A, Sharma DN, Julka PK, Das A, Rath GK. Sweat gland carcinoma with lung metastases. J Cancer Res Ther. 2(4):209-211.
29. Wang X-X, Wang H-Y, Zheng J-N, Sui J-C. Primary cutaneous sweat gland carcinoma. J Cancer Res Ther. 10(2):390-392.
Skin adnexal tumors (SAT) are rare tumors that make up about 1%-2% of all cutaneous malignancies. They represent a various group of benign and malignant tumors that arise from skin adnexal epithelial structures: hair follicle, pilosebaceous unit, and apocrine or eccrine sweat glands. Although this derivation provides a practical basis for classification, some tumors may exhibit a mixed or more than one line of differentiation, rendering precise classification of those neoplasms difficult, and such cases should be categorized according to prevailing phenotype. In this report, we present a patient with metastatic eccrine carcinoma. Clinical experience for metastatic disease treatment is derived from a few reports, and there are no universal treatment guidelines. Given the few reported cases and the absence of randomized clinical trials for these patients, it is important to collect clinical experiences.
Case presentation and summary
A 56-year-old African man presented with a 5-week history of multiple nontender subcutaneous skin nodules all over his body except for his palms and soles, and associated with generalized itching. He had a mass in the sole of his right foot 35 years previously in another country. The mass had recurred 15 years later and was excised again. The exact etiology of the mass was unknown to the patient. He had no other medical problems. He was on no medications and did not smoke, drink, or use recreational drugs.
His vital signs on admission were normal. Examination was significant for innumerable superficial skin nodules in the scalp, back, torso, and abdomen. The largest was in the neck and measured 4 x 2 cm. A firm right inguinal mass of 7 x 4 cm was palpable. An abdominal exam revealed large ascites but no organomegaly.
The results of laboratory tests were significant for hyponatremia 126 mEq/L (normal, 135-145), hypercalcemia of 12.2 mg/dL (8.5-10.5), with normal phosphorous of 2.5 mg/dL (2.5-4.5), parathyroid of 11.5 pg/ml (6-65), and low vitamin D level of <7 ng/ml (30-100). Other test results were: carcinoembryonic antigen (CEA), 4.36 ng/ml (0.00-2.99); alpha fetoprotein, 2.39 IU/ml (0.00-9.0); calcium 11.6 mg/dL (8.5-10.2); lactate dehydrogenase, 325 U/L (85-210); aspartate aminotransferase, 59 U/L (0-40); alanine aminotransferase 43 U/L (5-35); alkaline phosphatase, 65 u/L (50-120); albumin, 2.7 g/dL (3.8-5.2); white blood cell count, 14.1 k/uL (4.4-10.6); h
A chest and abdomen computed-tomography scan on presentation showed presence of innumerable subcutaneous and intramuscular nodules throughout the chest, abdomen, and pelvis (Figure 1).
Extensive peritoneal carcinomatosis in addition to moderate ascites and perivascular lymphadenopathy were evident in the abdomen cuts. Remarkably, multiple lytic, osseous metastases were seen with subacute pathologic fracture of right fourth rib in addition to mediastinal lymphadenopathy with small pericardial effusion in the chest cuts. The right thigh mass was described as a large lobulated solid and cystic mass. Ascitic fluid analysis was negative for malignant cells. Biopsy of one the skin nodules in the upper back showed carcinoma involving the skin with focal tubular differentiation (Figure 2).
Immunohistochemical stains were positive for p63, epithelial membrane antigen, high molecular weight keratin, and p40. The lesional cells were negative for CEA, bcl-2, Ber-Ep4, CK7, and CK20. The profile was compatible with a skin adnexal carcinoma of sweat gland origin. The groin lymph node showed eccrine acrospiroma.
The patient underwent an upper endoscopy to assess for recurrent vomiting and it revealed diffuse areas of large erythematous ulcerated nodules noted in the cardia, fundus, and body of the stomach (Figure 3). A biopsy of the gastric nodules revealed gastric mucosa with metastatic carcinoma.
After a thorough review of the literature, he was started on palliative chemotherapy 13 days after initial presentation with docetaxel 75 mg/m2, carboplatin AUC 5 (470 mg), and 5-FU (5-fluorouracil, 750 mg/m2) over 24 hours on days 1 through 5. However, on day 2 of the chemotherapy, he became hypotensive and was found to have cardiac tamponade. He underwent an emergent pericardial window procedure. Analysis of the pericardial fluid was consistent with metastatic carcinoma (Figure 4). Chemotherapy was discontinued while he remained hypotensive requiring multiple vasopressors. His clinical condition did not improve and he passed away 27 days from initial presentation.
Discussion
Sweat gland carcinomas are very rare malignant tumors of the adnexal epithelial structures of the skin, sebaceous, hair follicle, apocrine or eccrine glands that were first described by Cornil in 1865.1 They occur primarily in adult patients, with a peak incidence in fifth and sixth decades of life.2,3 The etiology is unknown, but some cases have been reported to be a consequence of radiation therapy.4 They are almost always an incidental histologic diagnosis.2,5 The tumors usually appear as single nodule, and multinodularity usually associated with both local and metastatic disease.6 There are no characteristic findings to suggest that a particular nodule may represent sweat gland carcinoma, and even if sweat gland tumor is suspected, benign counterparts are more common.
Eccrine carcinoma is the most aggressive among skin adnexal tumors. They can arise on the lower limbs, trunk, head and neck, scalp and ears, upper extremities, abdomen, and genital sites.7
The cells of eccrine sweat glands express low molecular weight keratin, epithelial membrane antigen, carcinoembryonic antigen, as well as S100 protein, smooth muscle actin, p63, calponin, cytokeratin 14, and bcl-2.8 Skin tumors with eccrine differentiation may stain for estrogen and progesterone, which has important clinical implications because those patients can be treated with hormonal therapy.9 Positivity for estrogen receptors does not differentiate cutaneous eccrine tumors from cutaneous metastases of breast cancers.8,9 Androgen receptor evaluation in these cases can help distinguish between the two.10 Human epidermal growth factor receptor 2 (HER-2) is expressed in 3.5% of skin adnexal tumors.11
The molecular pathogenesis of malignant adnexal tumors is not clear, but overexpression of tumor suppressor protein p16 has been described as a common feature in eccrine carcinomas.12
Prognostic factors for sweat gland carcinoma are difficult to identify, because of the small number of reported cases. The likely prognostic factors include size, histological type, lymph node involvement, and presence of distant metastasis. Absent of lymph node involvement correlates with 10-year disease-free survival rate of 56%, which falls to 9% if nodes are involved.13
There are no uniform guidelines for the treatment sweat gland carcinomas, and the clinical experience described in the literature is the only source of available information.
The treatment of choice of all subtypes of localized sweat gland carcinomas is wide surgical excision with broad tumor margins, given the propensity for local recurrences along with regional lymph node dissection in the presence of clinically positive nodes. Prophylactic lymph node resection does not seem to improve survival or decrease recurrence rates.7 The use of adjuvant radiotherapy to prevent local recurrence also is not well established. One report suggested radiosensitivity of these tumors, and adjuvant radiation was therefore recommended in high-risk cases (ie, large tumors of 5 cm and positive surgical margins of 1 cm) and moderate to poorly differentiated tumors with lymphovascular invasion.14 Adjuvant radiation to the involved lymph node basin is suggested in the setting of extranodal extension or extensive involvement, that is, 4 lymph nodes.15 The role of lymphadenectomy has not been adequately addressed in the literature.
The role of chemotherapy in metastatic disease is not clear, but sweat gland carcinomas are considered chemoresistant (Table). Several combinations have been used with short-term responses. In one case treated with doxorubicin, mitomycin, vincristine, and 5-FU followed by maintenance therapy, the patient achieved a complete response that lasted for 16 months.16 In another report, the treatment response was 2 years with treatment consisted of anthracyclin, cyclophosphamide, vincristine, and bloemycin.17 Other combinations used in the literature include carboplatin and paclitaxel, which led to prolonged remission.14 Cisplatin and 5-FU, or cisplatin plus cetuximab have been reported but with discouraging results.18 Results to taxanes showed conflicting results.19,20
Hormonal therapy can be effective in cases in which estrogen and progesterone receptors are expressed, which can range from 19%-30% of eccrine sweat gland carcinomas.21,22 Two cases have reported complete regression of lymph nodes in patients with metastatic disease, and in 1 patient relief from pain caused by bone metastases with durable response of around 3 years.23,24 a
Experience with targeted therapy is very limited. Sunitinib has been reported to have some activity in metastatic adnexal tumors as a second-line therapy in 2 patients, with disease control for 8 and 10 months respectively.25 Trastuzumab has been reported as having activity in 1 patient with strong HER2 expression (IHC score of 3+, denoting HER2 positivity), with complete regression of metastatic tumor. Upon progression in the same patient, a combination of lapatinib and capecitabine also showed positive response.26
In conclusion, metastatic sweat gland tumors treatment has not been standardized because of a dearth of reports in the literatures. Its early identification and complete excision gives the best chance of a cure. Neither chemotherapy nor radiation therapy has been proven to be of clinical benefit in treating metastatic disease.
Skin adnexal tumors (SAT) are rare tumors that make up about 1%-2% of all cutaneous malignancies. They represent a various group of benign and malignant tumors that arise from skin adnexal epithelial structures: hair follicle, pilosebaceous unit, and apocrine or eccrine sweat glands. Although this derivation provides a practical basis for classification, some tumors may exhibit a mixed or more than one line of differentiation, rendering precise classification of those neoplasms difficult, and such cases should be categorized according to prevailing phenotype. In this report, we present a patient with metastatic eccrine carcinoma. Clinical experience for metastatic disease treatment is derived from a few reports, and there are no universal treatment guidelines. Given the few reported cases and the absence of randomized clinical trials for these patients, it is important to collect clinical experiences.
Case presentation and summary
A 56-year-old African man presented with a 5-week history of multiple nontender subcutaneous skin nodules all over his body except for his palms and soles, and associated with generalized itching. He had a mass in the sole of his right foot 35 years previously in another country. The mass had recurred 15 years later and was excised again. The exact etiology of the mass was unknown to the patient. He had no other medical problems. He was on no medications and did not smoke, drink, or use recreational drugs.
His vital signs on admission were normal. Examination was significant for innumerable superficial skin nodules in the scalp, back, torso, and abdomen. The largest was in the neck and measured 4 x 2 cm. A firm right inguinal mass of 7 x 4 cm was palpable. An abdominal exam revealed large ascites but no organomegaly.
The results of laboratory tests were significant for hyponatremia 126 mEq/L (normal, 135-145), hypercalcemia of 12.2 mg/dL (8.5-10.5), with normal phosphorous of 2.5 mg/dL (2.5-4.5), parathyroid of 11.5 pg/ml (6-65), and low vitamin D level of <7 ng/ml (30-100). Other test results were: carcinoembryonic antigen (CEA), 4.36 ng/ml (0.00-2.99); alpha fetoprotein, 2.39 IU/ml (0.00-9.0); calcium 11.6 mg/dL (8.5-10.2); lactate dehydrogenase, 325 U/L (85-210); aspartate aminotransferase, 59 U/L (0-40); alanine aminotransferase 43 U/L (5-35); alkaline phosphatase, 65 u/L (50-120); albumin, 2.7 g/dL (3.8-5.2); white blood cell count, 14.1 k/uL (4.4-10.6); h
A chest and abdomen computed-tomography scan on presentation showed presence of innumerable subcutaneous and intramuscular nodules throughout the chest, abdomen, and pelvis (Figure 1).
Extensive peritoneal carcinomatosis in addition to moderate ascites and perivascular lymphadenopathy were evident in the abdomen cuts. Remarkably, multiple lytic, osseous metastases were seen with subacute pathologic fracture of right fourth rib in addition to mediastinal lymphadenopathy with small pericardial effusion in the chest cuts. The right thigh mass was described as a large lobulated solid and cystic mass. Ascitic fluid analysis was negative for malignant cells. Biopsy of one the skin nodules in the upper back showed carcinoma involving the skin with focal tubular differentiation (Figure 2).
Immunohistochemical stains were positive for p63, epithelial membrane antigen, high molecular weight keratin, and p40. The lesional cells were negative for CEA, bcl-2, Ber-Ep4, CK7, and CK20. The profile was compatible with a skin adnexal carcinoma of sweat gland origin. The groin lymph node showed eccrine acrospiroma.
The patient underwent an upper endoscopy to assess for recurrent vomiting and it revealed diffuse areas of large erythematous ulcerated nodules noted in the cardia, fundus, and body of the stomach (Figure 3). A biopsy of the gastric nodules revealed gastric mucosa with metastatic carcinoma.
After a thorough review of the literature, he was started on palliative chemotherapy 13 days after initial presentation with docetaxel 75 mg/m2, carboplatin AUC 5 (470 mg), and 5-FU (5-fluorouracil, 750 mg/m2) over 24 hours on days 1 through 5. However, on day 2 of the chemotherapy, he became hypotensive and was found to have cardiac tamponade. He underwent an emergent pericardial window procedure. Analysis of the pericardial fluid was consistent with metastatic carcinoma (Figure 4). Chemotherapy was discontinued while he remained hypotensive requiring multiple vasopressors. His clinical condition did not improve and he passed away 27 days from initial presentation.
Discussion
Sweat gland carcinomas are very rare malignant tumors of the adnexal epithelial structures of the skin, sebaceous, hair follicle, apocrine or eccrine glands that were first described by Cornil in 1865.1 They occur primarily in adult patients, with a peak incidence in fifth and sixth decades of life.2,3 The etiology is unknown, but some cases have been reported to be a consequence of radiation therapy.4 They are almost always an incidental histologic diagnosis.2,5 The tumors usually appear as single nodule, and multinodularity usually associated with both local and metastatic disease.6 There are no characteristic findings to suggest that a particular nodule may represent sweat gland carcinoma, and even if sweat gland tumor is suspected, benign counterparts are more common.
Eccrine carcinoma is the most aggressive among skin adnexal tumors. They can arise on the lower limbs, trunk, head and neck, scalp and ears, upper extremities, abdomen, and genital sites.7
The cells of eccrine sweat glands express low molecular weight keratin, epithelial membrane antigen, carcinoembryonic antigen, as well as S100 protein, smooth muscle actin, p63, calponin, cytokeratin 14, and bcl-2.8 Skin tumors with eccrine differentiation may stain for estrogen and progesterone, which has important clinical implications because those patients can be treated with hormonal therapy.9 Positivity for estrogen receptors does not differentiate cutaneous eccrine tumors from cutaneous metastases of breast cancers.8,9 Androgen receptor evaluation in these cases can help distinguish between the two.10 Human epidermal growth factor receptor 2 (HER-2) is expressed in 3.5% of skin adnexal tumors.11
The molecular pathogenesis of malignant adnexal tumors is not clear, but overexpression of tumor suppressor protein p16 has been described as a common feature in eccrine carcinomas.12
Prognostic factors for sweat gland carcinoma are difficult to identify, because of the small number of reported cases. The likely prognostic factors include size, histological type, lymph node involvement, and presence of distant metastasis. Absent of lymph node involvement correlates with 10-year disease-free survival rate of 56%, which falls to 9% if nodes are involved.13
There are no uniform guidelines for the treatment sweat gland carcinomas, and the clinical experience described in the literature is the only source of available information.
The treatment of choice of all subtypes of localized sweat gland carcinomas is wide surgical excision with broad tumor margins, given the propensity for local recurrences along with regional lymph node dissection in the presence of clinically positive nodes. Prophylactic lymph node resection does not seem to improve survival or decrease recurrence rates.7 The use of adjuvant radiotherapy to prevent local recurrence also is not well established. One report suggested radiosensitivity of these tumors, and adjuvant radiation was therefore recommended in high-risk cases (ie, large tumors of 5 cm and positive surgical margins of 1 cm) and moderate to poorly differentiated tumors with lymphovascular invasion.14 Adjuvant radiation to the involved lymph node basin is suggested in the setting of extranodal extension or extensive involvement, that is, 4 lymph nodes.15 The role of lymphadenectomy has not been adequately addressed in the literature.
The role of chemotherapy in metastatic disease is not clear, but sweat gland carcinomas are considered chemoresistant (Table). Several combinations have been used with short-term responses. In one case treated with doxorubicin, mitomycin, vincristine, and 5-FU followed by maintenance therapy, the patient achieved a complete response that lasted for 16 months.16 In another report, the treatment response was 2 years with treatment consisted of anthracyclin, cyclophosphamide, vincristine, and bloemycin.17 Other combinations used in the literature include carboplatin and paclitaxel, which led to prolonged remission.14 Cisplatin and 5-FU, or cisplatin plus cetuximab have been reported but with discouraging results.18 Results to taxanes showed conflicting results.19,20
Hormonal therapy can be effective in cases in which estrogen and progesterone receptors are expressed, which can range from 19%-30% of eccrine sweat gland carcinomas.21,22 Two cases have reported complete regression of lymph nodes in patients with metastatic disease, and in 1 patient relief from pain caused by bone metastases with durable response of around 3 years.23,24 a
Experience with targeted therapy is very limited. Sunitinib has been reported to have some activity in metastatic adnexal tumors as a second-line therapy in 2 patients, with disease control for 8 and 10 months respectively.25 Trastuzumab has been reported as having activity in 1 patient with strong HER2 expression (IHC score of 3+, denoting HER2 positivity), with complete regression of metastatic tumor. Upon progression in the same patient, a combination of lapatinib and capecitabine also showed positive response.26
In conclusion, metastatic sweat gland tumors treatment has not been standardized because of a dearth of reports in the literatures. Its early identification and complete excision gives the best chance of a cure. Neither chemotherapy nor radiation therapy has been proven to be of clinical benefit in treating metastatic disease.
1. Gates O, Warren S, Warvi WN. Tumors of sweat glands. Am J Pathol. 1943;19(4):591-631.
2. Mitts DL, Smith MT, Russell L, Bannayan GA, Cruz AB. Sweat gland carcinoma: a clinico-pathological reappraisal. J Surg Oncol. 1976;8(1):23-29.
3. Panoussopoulos D, Darom A, Lazaris AC, Misthos P, Papadimitriou K, Androulakis G. Sweat gland carcinoma with multiple local recurrences: a case report. Adv Clin Path. 1999;3(3):63-68.
4. Marone U, Caracò C, Anniciello AM, et al. Metastatic eccrine porocarcinoma : report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
5. Yildirim S, Aköz T, Akan M, Ege GA. De novo malignant eccrine spiradenoma with an interesting and unusual location. Dermatol Surg. 2001;27(4):417-420.
6. Shaw M, McKee PH, Lowe D, Black MM. Malignant eccrine poroma: a study of twenty-seven cases. Br J Dermatol. 1982;107(6):675-680.
7. De Iuliis F, Amoroso L, Taglieri L, et al. Chemotherapy of rare skin adnexal tumors: a review of literature. Anticancer Res. 2014;34(10):5263-5268.
8. Alsaad KO, Obaidat NA, Ghazarian D. Skin adnexal neoplasms – part 1: an approach to tumours of the pilosebaceous unit. J Clin Pathol. 2007;60(2):129-144.
9. Serhrouchni KI, Harmouch T, Chbani L, et al. Eccrine carcinoma : a rare cutaneous neoplasm. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3570399/. Published online February 4, 2013. Accessed October 11, 2017.
10. Shidham VB, Komorowski RA, Machhi JK. Androgen receptor expression in metastatic adenocarcinoma in females favors a breast primary. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1601970/. Published online October 4, 2006. Accessed October 11, 2017.
11. Hiatt KM, Pillow JL, Smoller BR. Her-2 expression in cutaneous eccrine and apocrine neoplasms. Mod Pathol. 2004;17(1):28-32.
12. Gu L-H, Ichiki Y, Kitajima Y. Aberrant expression of p16 and RB protein in eccrine porocarcinoma. J Cutan Pathol. 2002;29(8):473-479.
13. el-Domeiri AA, Brasfield RD, Huvos AG, Strong EW. Sweat gland carcinoma: a clinico-pathologic study of 83 patients. Ann Surg. 1971;173(2):270-274.
14. Tlemcani K, Levine D, Smith R V, et al. Metastatic apocrine carcinoma of the scalp: prolonged response to systemic chemotherapy. J Clin Oncol. 2010;28(24):e412-e414.
15. Chamberlain RS, Huber K, White JC, Travaglino-Parda R. Apocrine gland carcinoma of the axilla: review of the literature and recommendations for treatment. Am J Clin Oncol. 1999;22(2):131-135.
16. Gutermuth J, Audring H, Voit C, Trefzer U, Haas N. Antitumour activity of paclitaxel and interferon-alpha in a case of metastatic eccrine porocarcinoma. J Eur Acad Dermatol Venereol. 2004;18(4):477-479.
17. Mezger J, Remberger K, Schalhorn A, Wohlrab A, Wilmanns W. Treatment of metastatic sweat gland carcinoma by a four drug combination chemotherapy: response in two cases. Med Oncol Tumor Pharmacother. 1986;3(1):29-34.
18. Aaribi I, Mohtaram A, Ben Ameur El Youbi M, et al. Successful management of metastatic eccrine porocarcinoma. https://www.hindawi.com/journals/crionm/2013/282536/. Published 2013. Accessed October 10, 2017.
19. Shiohara J, Koga H, Uhara H, Takata M, Saida T. Eccrine porocarcinoma: clinical and pathological studies of 12 cases. J Dermatol. 2007;34(8):516-522.
20. Swanson PE, Mazoujian G, Mills SE, Campbell RJ, Wick MR. Immunoreactivity for estrogen receptor protein in sweat gland tumors. Am J Surg Pathol. 1991;15(9):835-841.
21. Busam KJ, Tan LK, Granter SR, et al. Epidermal growth factor, estrogen, and progesterone receptor expression in primary sweat gland carcinomas and primary and metastatic mammary carcinomas. Mod Pathol. 1999;12(8):786-793.
22. Sridhar KS, Benedetto P, Otrakji CL, Charyulu KK. Response of eccrine adenocarcinoma to tamoxifen. Cancer. 1989;64(2):366-370.
23. Daniel SJ, Nader R, Kost K, Hüttner I. Facial sweat gland carcinoma metastasizing to neck nodes: a diagnostic and therapeutic challenge. Arch Otolaryngol Head Neck Surg. 2001;127(12):1495-1498.
24. Battistella M, Mateus C, Lassau N, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24(2):199-203.
25. Hidaka T, Fujimura T, Watabe A, et al. Successful treatment of HER-2-positive metastatic apocrine carcinoma of the skin with lapatinib and capecitabine. Acta Derm Venereol. 2012;92(6):654-655.
26. Mandaliya H, Nordman I. Metastatic eccrine porocarcinoma: a rare case of successful treatment. Case Rep Oncol. 2016;9(2):454-456.
27. de Bree E, Volalakis E, Tsetis D, et al. Treatment of advanced malignant eccrine poroma with locoregional chemotherapy. Br J Dermatol. 2005;152(5):1051-1055.
28. Bahl A, Sharma DN, Julka PK, Das A, Rath GK. Sweat gland carcinoma with lung metastases. J Cancer Res Ther. 2(4):209-211.
29. Wang X-X, Wang H-Y, Zheng J-N, Sui J-C. Primary cutaneous sweat gland carcinoma. J Cancer Res Ther. 10(2):390-392.
1. Gates O, Warren S, Warvi WN. Tumors of sweat glands. Am J Pathol. 1943;19(4):591-631.
2. Mitts DL, Smith MT, Russell L, Bannayan GA, Cruz AB. Sweat gland carcinoma: a clinico-pathological reappraisal. J Surg Oncol. 1976;8(1):23-29.
3. Panoussopoulos D, Darom A, Lazaris AC, Misthos P, Papadimitriou K, Androulakis G. Sweat gland carcinoma with multiple local recurrences: a case report. Adv Clin Path. 1999;3(3):63-68.
4. Marone U, Caracò C, Anniciello AM, et al. Metastatic eccrine porocarcinoma : report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
5. Yildirim S, Aköz T, Akan M, Ege GA. De novo malignant eccrine spiradenoma with an interesting and unusual location. Dermatol Surg. 2001;27(4):417-420.
6. Shaw M, McKee PH, Lowe D, Black MM. Malignant eccrine poroma: a study of twenty-seven cases. Br J Dermatol. 1982;107(6):675-680.
7. De Iuliis F, Amoroso L, Taglieri L, et al. Chemotherapy of rare skin adnexal tumors: a review of literature. Anticancer Res. 2014;34(10):5263-5268.
8. Alsaad KO, Obaidat NA, Ghazarian D. Skin adnexal neoplasms – part 1: an approach to tumours of the pilosebaceous unit. J Clin Pathol. 2007;60(2):129-144.
9. Serhrouchni KI, Harmouch T, Chbani L, et al. Eccrine carcinoma : a rare cutaneous neoplasm. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3570399/. Published online February 4, 2013. Accessed October 11, 2017.
10. Shidham VB, Komorowski RA, Machhi JK. Androgen receptor expression in metastatic adenocarcinoma in females favors a breast primary. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1601970/. Published online October 4, 2006. Accessed October 11, 2017.
11. Hiatt KM, Pillow JL, Smoller BR. Her-2 expression in cutaneous eccrine and apocrine neoplasms. Mod Pathol. 2004;17(1):28-32.
12. Gu L-H, Ichiki Y, Kitajima Y. Aberrant expression of p16 and RB protein in eccrine porocarcinoma. J Cutan Pathol. 2002;29(8):473-479.
13. el-Domeiri AA, Brasfield RD, Huvos AG, Strong EW. Sweat gland carcinoma: a clinico-pathologic study of 83 patients. Ann Surg. 1971;173(2):270-274.
14. Tlemcani K, Levine D, Smith R V, et al. Metastatic apocrine carcinoma of the scalp: prolonged response to systemic chemotherapy. J Clin Oncol. 2010;28(24):e412-e414.
15. Chamberlain RS, Huber K, White JC, Travaglino-Parda R. Apocrine gland carcinoma of the axilla: review of the literature and recommendations for treatment. Am J Clin Oncol. 1999;22(2):131-135.
16. Gutermuth J, Audring H, Voit C, Trefzer U, Haas N. Antitumour activity of paclitaxel and interferon-alpha in a case of metastatic eccrine porocarcinoma. J Eur Acad Dermatol Venereol. 2004;18(4):477-479.
17. Mezger J, Remberger K, Schalhorn A, Wohlrab A, Wilmanns W. Treatment of metastatic sweat gland carcinoma by a four drug combination chemotherapy: response in two cases. Med Oncol Tumor Pharmacother. 1986;3(1):29-34.
18. Aaribi I, Mohtaram A, Ben Ameur El Youbi M, et al. Successful management of metastatic eccrine porocarcinoma. https://www.hindawi.com/journals/crionm/2013/282536/. Published 2013. Accessed October 10, 2017.
19. Shiohara J, Koga H, Uhara H, Takata M, Saida T. Eccrine porocarcinoma: clinical and pathological studies of 12 cases. J Dermatol. 2007;34(8):516-522.
20. Swanson PE, Mazoujian G, Mills SE, Campbell RJ, Wick MR. Immunoreactivity for estrogen receptor protein in sweat gland tumors. Am J Surg Pathol. 1991;15(9):835-841.
21. Busam KJ, Tan LK, Granter SR, et al. Epidermal growth factor, estrogen, and progesterone receptor expression in primary sweat gland carcinomas and primary and metastatic mammary carcinomas. Mod Pathol. 1999;12(8):786-793.
22. Sridhar KS, Benedetto P, Otrakji CL, Charyulu KK. Response of eccrine adenocarcinoma to tamoxifen. Cancer. 1989;64(2):366-370.
23. Daniel SJ, Nader R, Kost K, Hüttner I. Facial sweat gland carcinoma metastasizing to neck nodes: a diagnostic and therapeutic challenge. Arch Otolaryngol Head Neck Surg. 2001;127(12):1495-1498.
24. Battistella M, Mateus C, Lassau N, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24(2):199-203.
25. Hidaka T, Fujimura T, Watabe A, et al. Successful treatment of HER-2-positive metastatic apocrine carcinoma of the skin with lapatinib and capecitabine. Acta Derm Venereol. 2012;92(6):654-655.
26. Mandaliya H, Nordman I. Metastatic eccrine porocarcinoma: a rare case of successful treatment. Case Rep Oncol. 2016;9(2):454-456.
27. de Bree E, Volalakis E, Tsetis D, et al. Treatment of advanced malignant eccrine poroma with locoregional chemotherapy. Br J Dermatol. 2005;152(5):1051-1055.
28. Bahl A, Sharma DN, Julka PK, Das A, Rath GK. Sweat gland carcinoma with lung metastases. J Cancer Res Ther. 2(4):209-211.
29. Wang X-X, Wang H-Y, Zheng J-N, Sui J-C. Primary cutaneous sweat gland carcinoma. J Cancer Res Ther. 10(2):390-392.
Cold hemolytic anemia: a rare complication of influenza A
Autoimmune hemolytic anemia (AIHA) is characterized by the temperature at which the auto-antibody has the greatest avidity for the target red cell antigen, either warm or cold forms. It is detected by a positive direct antiglobulin test (DAT) also known as the direct Coombs test. DAT is used to determine if red cells have been coated in vivo with immunoglobulin, complement, or both.1 Some causes of a positive DAT include hemolytic transfusion reactions, hemolytic disease of the fetus and newborn, AIHA, and drug-induced immune hemolysis.
Case presentation and summary
A 58-year-old woman from Brazil with past medical history only significant for cholecystectomy and cesarean section had been visiting in United States for 2 months when she presented to an outside hospital with fever, shortness of breath, and syncope that had resulted in a foot injury. She reported she had been feeling short of breath and had a nonproductive cough and malaise for about 2 weeks before presentation with sick contacts at home. On admission it was noted that she had a hemoglobin level of 7.7 g/dL (normal, 12.0-15.5 g/dL; MCV, 94 fL), total bilirubin of 2.14 mg/dL (normal, 0.2-1.0 mg/dL), and lactate dehydrogenase of 523 U/L (normal, 81-234 U/L). There were no signs of bleeding on her examination. Her DAT was positive and moderate red blood cell agglutination was reported. During the first admission at the outside hospital she was diagnosed with influenza A and completed a full course of oseltamivir (75 mg po twice daily for 5 days). A chest X-ray was negative for infiltrates and showed that the patient’s lung fields were clear. She was transfused 2 units of packed red blood cells with response in hemoglobin up to 9.8 g/dL. The patient was treated with dexamethasone (4 mg IV Q8) as an inpatient and was discharged on a prednisone taper (40 mg, with taper by 10 mg every 3 days) with hemoglobin of 8.1 g/dL.
The patient continued to have nonproductive cough, dyspnea, fevers, chills, and generalized weakness, when she returned to the same outside hospital’s emergency department 2 days after her discharge. At that time, it was noted that she had leucocytosis (white blood cell count, 34.6 x 109 per L), a hemoglobin level of 6.8 g/dL, and her total bilirubin level was 6.9 mg/dL. Her hemodynamics were unstable and she was admitted to their intensive care unit. The results of a chest X-ray revealed right lung consolidation.
The day after this admission, her hemoglobin level fell to 4.7 g/dL, and she was transfused 2 units of packed red blood cells before being transferred to our hospital. A chest X-ray at our hospital confirmed a right lung infiltrate. Vancomycin (1,250 mg IV Q12), levaquin (750 mg IV Q24), and maxipime (1 g IV Q12) were initiated for pneumonia and the patient was transferred to our hospital’s intensive care unit. She was afebrile at 98.3°F, her pulse rate was 84 beats per minute, she was tachypneic with respiratory rate of 26 breaths per minute, her blood pressure was 98/51 mmHg, and she had an oxygen saturation of 99% on 2L oxygen via nasal cannula.
On physical examination she was noted to have scleral icterus and was in mild respiratory distress. A chest X-ray revealed a patchy opacity in the right mid to lower lung. Her initial complete blood panel revealed anemia, with hemoglobin, 6.3 g/dL; white blood cell count, 27 x 109 per L; and platelets, 533 x 109 per L. The patient was then transfused another 2 units of packed red blood cells. She was given intravenous hydration, acetaminophen, and albuterol nebulizer treatments as supportive care. She was provided with blankets to keep warm. In addition to her antibiotics, she was also given prednisone 70 mg for her respiratory symptoms.
Further tests revealed haptoglobin, <30 mg/dL (normal, 36-195 mg/dL); lactate dehydrogenase, 371 U/L (normal, 98-192 U/L); and complements C3, 90 mg/dL (normal, 79-152 mg/dL) and C4, <8 mg/dL (normal, 18-55 mg/dL). Her DAT was positive, and agglutination was seen on peripheral smear (Figure 1). This was her second positive DAT as she had positive one at the outside hospital initially. Her tests for mycoplasma pneumonia, the PCR and IgM, were negative, as were the Monospot for mononucleosis and the ANA for autoimmune disorders. Her cold agglutinin titer was 1:256 (normal, no agglutination <1:64). The patient’s repeat respiratory viral panel was negative given recent full treatment for her influenza A at the previous hospital. Her blood and urine cultures were negative.
The patient was given antibiotics (vancomycin 1,250 mg IV Q12, cefepime 2 g IV Q8, and azithromycin 500 mg daily) for her pneumonia. Her respiratory status improved, and she was transferred to general medical floors after the first day of her admission. Her total bilirubin trended down to 1.9 mg/dL. She remained on prednisone 70 mg daily.
The patient remained in the hospital for an additional 6 days before being discharged home on prednisone. She wanted to return to her home country of Brazil as soon as she was able to and said she would seek outpatient follow-up there with a hematologist. At the time of her discharge, her hemoglobin was 6.6 g/dL and her reticulocyte count, 6.0%. Figures 2 and 3 illustrate her hemoglobin and reticulocyte trend during her admission at our hospital.
Discussion
The incidence of cold AIHA or cold agglutinin disease (CAD) occurs about 4 per 1 million people and commonly affects women more often than men.2 The cause of CAD can be subdivided into primary, idiopathic, or secondary causes, which can include infections, malignancies, or benign diseases.3,4 Primary CAD is a chronic disorder that is generally seen in older women. Secondary CAD can be associated with B-cell lymphoproliferative disorders, such as Waldenstrom macroglobulinemia or chronic lymphocytic leukemia, and infectious agents such as Mycoplasma pneumoniae and mononucleosis caused by Epstein-Barr virus.
Mild hemolysis or acrocyanosis may occur with exposure to cold. The blood smear in CAD demonstrates red blood cell agglutination or clumping, polychromasia, and an absence of spherocytosis. In general, most cases require no treatment, but cytotoxic agents or rituximab can be used to treat more severe cases. Appropriate treatment for infectious causes of CAD includes supportive care aimed at the underlying disease process. In addition, it is helpful to keep the patient warm. There is no role for steroid therapy in CAD unlike in warm AIHA. However, our patient was symptomatic from her pneumonia, so we added steroids to help with her pulmonary insult.
The patient had a cold agglutinin titer of 1:256. Titers of 1:32 or higher are considered elevated by this technique. Elevated titers are generally rarely seen except in primary atypical pneumonia due to either M. pneumoniae, influenza A, influenza B, parainfluenza, and adenovirus, and in certain hemolytic anemias. Low titers of cold agglutinins have been demonstrated in malaria, peripheral vascular disease, and common respiratory diseases.
Warm AIHA is caused by IgG antibody activities at body temperature or at 98.6°F. They may or may not bind complement and are removed from circulation by the spleen. Cold AIHA is due to IgM antibodies coating red cells at lower temperatures. They bind complement and lead to red blood cell destruction of agglutinated cells. If the antibody is active at temperatures approaching 98.6°F, clinically significant intravascular and sometimes extravascular complement-mediated hemolysis occur in the liver.5
The incidence of warm AIHA occurs about 10 per 1 million people and affects women twice often as men.2 It can be primary or idiopathic, or associated with various underlying conditions, including autoimmune disorders, immunodeficiency syndromes, lymphoproliferative disorders, other malignancies, and certain drugs. In more severe cases, jaundice and splenomegaly may occur. The blood smear in warm AIHA demonstrates variable spherocytosis, polychromasia, and rare erythrophagocytosis. Treatment usually includes steroids, cytotoxic agents, and splenectomy in severe cases.
There have been few case reports describing influenza as a cause of cold agglutinin hemolytic anemia. Chen and colleagues reported a case of influenza A infection in a 22-month-old boy.6Schoindre and colleagues reported the case of a 60-year-old woman infected with influenza A H1N1 virus who died from CAD.7 Shizuma reported the case of a 67-year-old man with alcoholic cirrhosis who developed a mixed hemolytic anemia and was positive for influenza A.8Our patient presented with influenza A, which had been diagnosed by respiratory virus panel at a different hospital, and she was anemic at the time of presentation to the outside hospital, with a positive DAT test. She was treated for influenza A with a full course of osltamivir and then returned with complaints of worsening fatigue and was again noted to be anemic with the development of patchy opacities on chest X-ray. The patient was subsequently transferred to our hospital and remained anemic during the course of her treatment. She received supportive care for her underlying influenza A and had symptomatic improvement. She ultimately decided the she would like to pursue further treatment in her native country and was discharged.
In conclusion, this case represents a rare complication of a common illness. Few cases of influenza causing hemolytic anemia have been reported in the literature. There have been reports of oseltamivir causing hemolytic anemia, but our patient presented with evidence of hemolytic anemia before initiation of the medication. In all the aforementioned cases, the patients died as a result of comorbid conditions. Our patient was stable enough to be discharged from the hospital after treatment of her comorbid conditions.
Acknowledgment
The authors thank David Henry, MD, at Pennsylvania Hospital, Philadelphia, for sharing this case and for his guidance during this patient’s treatment.
1. Roback JD, Grossman BJ, Harris T, Hillyer CD. Technical manual [17th ed]. Bethesda, MD; American Association of Blood Banks; 2011.
2. Jaffee ES, Harris NL, Vardiman JW, Campo E, Arber DA. Hematopathology. St. Louis, MO; Elsevier Saunders, 2011.
3. Feizi T. Monotypic cold agglutinins in infection by Mycoplasma pneumoniae. Nature. 1967;215(5100):540-542.
4. Horwitz CA, Moulds J, Henle W, et al. Cold agglutinins in infectious mononucleosis and heterophil-antibody-negative mononucleosis-like syndromes. Blood. 1977;50(2):195-202.
5. Hsi ED, editor. Hematopathology [3rd ed]. Philadelphia, PA; Elsevier Saunders; 2012.
6. Chen H, Jia XL, Gao HM, Qian SY. Comorbid presentation of severe novel influenza A (H1N1) and Evans syndrome: a case report. Chin Med J. 2011;124(11):1743-1746.
7. Schoindre Y, Bollée G, Dumont MD, Lesavre P, Servais A. Cold agglutinin syndrome associated with a 2009 influenza A H1N1 infection. http://www.amjmed.com/article/S0002-9343(10)00482-1/fulltext. Published February 2011. Accessed October 10, 2017.
8. [Article in Japanese] Shizuma T. [A case of autoimmune hemolytic anemia caused by type A influenza infection in a patient with alcoholic liver cirrhosis]. Kansenshogaku Zasshi. 2010;84(3):296-299.
Autoimmune hemolytic anemia (AIHA) is characterized by the temperature at which the auto-antibody has the greatest avidity for the target red cell antigen, either warm or cold forms. It is detected by a positive direct antiglobulin test (DAT) also known as the direct Coombs test. DAT is used to determine if red cells have been coated in vivo with immunoglobulin, complement, or both.1 Some causes of a positive DAT include hemolytic transfusion reactions, hemolytic disease of the fetus and newborn, AIHA, and drug-induced immune hemolysis.
Case presentation and summary
A 58-year-old woman from Brazil with past medical history only significant for cholecystectomy and cesarean section had been visiting in United States for 2 months when she presented to an outside hospital with fever, shortness of breath, and syncope that had resulted in a foot injury. She reported she had been feeling short of breath and had a nonproductive cough and malaise for about 2 weeks before presentation with sick contacts at home. On admission it was noted that she had a hemoglobin level of 7.7 g/dL (normal, 12.0-15.5 g/dL; MCV, 94 fL), total bilirubin of 2.14 mg/dL (normal, 0.2-1.0 mg/dL), and lactate dehydrogenase of 523 U/L (normal, 81-234 U/L). There were no signs of bleeding on her examination. Her DAT was positive and moderate red blood cell agglutination was reported. During the first admission at the outside hospital she was diagnosed with influenza A and completed a full course of oseltamivir (75 mg po twice daily for 5 days). A chest X-ray was negative for infiltrates and showed that the patient’s lung fields were clear. She was transfused 2 units of packed red blood cells with response in hemoglobin up to 9.8 g/dL. The patient was treated with dexamethasone (4 mg IV Q8) as an inpatient and was discharged on a prednisone taper (40 mg, with taper by 10 mg every 3 days) with hemoglobin of 8.1 g/dL.
The patient continued to have nonproductive cough, dyspnea, fevers, chills, and generalized weakness, when she returned to the same outside hospital’s emergency department 2 days after her discharge. At that time, it was noted that she had leucocytosis (white blood cell count, 34.6 x 109 per L), a hemoglobin level of 6.8 g/dL, and her total bilirubin level was 6.9 mg/dL. Her hemodynamics were unstable and she was admitted to their intensive care unit. The results of a chest X-ray revealed right lung consolidation.
The day after this admission, her hemoglobin level fell to 4.7 g/dL, and she was transfused 2 units of packed red blood cells before being transferred to our hospital. A chest X-ray at our hospital confirmed a right lung infiltrate. Vancomycin (1,250 mg IV Q12), levaquin (750 mg IV Q24), and maxipime (1 g IV Q12) were initiated for pneumonia and the patient was transferred to our hospital’s intensive care unit. She was afebrile at 98.3°F, her pulse rate was 84 beats per minute, she was tachypneic with respiratory rate of 26 breaths per minute, her blood pressure was 98/51 mmHg, and she had an oxygen saturation of 99% on 2L oxygen via nasal cannula.
On physical examination she was noted to have scleral icterus and was in mild respiratory distress. A chest X-ray revealed a patchy opacity in the right mid to lower lung. Her initial complete blood panel revealed anemia, with hemoglobin, 6.3 g/dL; white blood cell count, 27 x 109 per L; and platelets, 533 x 109 per L. The patient was then transfused another 2 units of packed red blood cells. She was given intravenous hydration, acetaminophen, and albuterol nebulizer treatments as supportive care. She was provided with blankets to keep warm. In addition to her antibiotics, she was also given prednisone 70 mg for her respiratory symptoms.
Further tests revealed haptoglobin, <30 mg/dL (normal, 36-195 mg/dL); lactate dehydrogenase, 371 U/L (normal, 98-192 U/L); and complements C3, 90 mg/dL (normal, 79-152 mg/dL) and C4, <8 mg/dL (normal, 18-55 mg/dL). Her DAT was positive, and agglutination was seen on peripheral smear (Figure 1). This was her second positive DAT as she had positive one at the outside hospital initially. Her tests for mycoplasma pneumonia, the PCR and IgM, were negative, as were the Monospot for mononucleosis and the ANA for autoimmune disorders. Her cold agglutinin titer was 1:256 (normal, no agglutination <1:64). The patient’s repeat respiratory viral panel was negative given recent full treatment for her influenza A at the previous hospital. Her blood and urine cultures were negative.
The patient was given antibiotics (vancomycin 1,250 mg IV Q12, cefepime 2 g IV Q8, and azithromycin 500 mg daily) for her pneumonia. Her respiratory status improved, and she was transferred to general medical floors after the first day of her admission. Her total bilirubin trended down to 1.9 mg/dL. She remained on prednisone 70 mg daily.
The patient remained in the hospital for an additional 6 days before being discharged home on prednisone. She wanted to return to her home country of Brazil as soon as she was able to and said she would seek outpatient follow-up there with a hematologist. At the time of her discharge, her hemoglobin was 6.6 g/dL and her reticulocyte count, 6.0%. Figures 2 and 3 illustrate her hemoglobin and reticulocyte trend during her admission at our hospital.
Discussion
The incidence of cold AIHA or cold agglutinin disease (CAD) occurs about 4 per 1 million people and commonly affects women more often than men.2 The cause of CAD can be subdivided into primary, idiopathic, or secondary causes, which can include infections, malignancies, or benign diseases.3,4 Primary CAD is a chronic disorder that is generally seen in older women. Secondary CAD can be associated with B-cell lymphoproliferative disorders, such as Waldenstrom macroglobulinemia or chronic lymphocytic leukemia, and infectious agents such as Mycoplasma pneumoniae and mononucleosis caused by Epstein-Barr virus.
Mild hemolysis or acrocyanosis may occur with exposure to cold. The blood smear in CAD demonstrates red blood cell agglutination or clumping, polychromasia, and an absence of spherocytosis. In general, most cases require no treatment, but cytotoxic agents or rituximab can be used to treat more severe cases. Appropriate treatment for infectious causes of CAD includes supportive care aimed at the underlying disease process. In addition, it is helpful to keep the patient warm. There is no role for steroid therapy in CAD unlike in warm AIHA. However, our patient was symptomatic from her pneumonia, so we added steroids to help with her pulmonary insult.
The patient had a cold agglutinin titer of 1:256. Titers of 1:32 or higher are considered elevated by this technique. Elevated titers are generally rarely seen except in primary atypical pneumonia due to either M. pneumoniae, influenza A, influenza B, parainfluenza, and adenovirus, and in certain hemolytic anemias. Low titers of cold agglutinins have been demonstrated in malaria, peripheral vascular disease, and common respiratory diseases.
Warm AIHA is caused by IgG antibody activities at body temperature or at 98.6°F. They may or may not bind complement and are removed from circulation by the spleen. Cold AIHA is due to IgM antibodies coating red cells at lower temperatures. They bind complement and lead to red blood cell destruction of agglutinated cells. If the antibody is active at temperatures approaching 98.6°F, clinically significant intravascular and sometimes extravascular complement-mediated hemolysis occur in the liver.5
The incidence of warm AIHA occurs about 10 per 1 million people and affects women twice often as men.2 It can be primary or idiopathic, or associated with various underlying conditions, including autoimmune disorders, immunodeficiency syndromes, lymphoproliferative disorders, other malignancies, and certain drugs. In more severe cases, jaundice and splenomegaly may occur. The blood smear in warm AIHA demonstrates variable spherocytosis, polychromasia, and rare erythrophagocytosis. Treatment usually includes steroids, cytotoxic agents, and splenectomy in severe cases.
There have been few case reports describing influenza as a cause of cold agglutinin hemolytic anemia. Chen and colleagues reported a case of influenza A infection in a 22-month-old boy.6Schoindre and colleagues reported the case of a 60-year-old woman infected with influenza A H1N1 virus who died from CAD.7 Shizuma reported the case of a 67-year-old man with alcoholic cirrhosis who developed a mixed hemolytic anemia and was positive for influenza A.8Our patient presented with influenza A, which had been diagnosed by respiratory virus panel at a different hospital, and she was anemic at the time of presentation to the outside hospital, with a positive DAT test. She was treated for influenza A with a full course of osltamivir and then returned with complaints of worsening fatigue and was again noted to be anemic with the development of patchy opacities on chest X-ray. The patient was subsequently transferred to our hospital and remained anemic during the course of her treatment. She received supportive care for her underlying influenza A and had symptomatic improvement. She ultimately decided the she would like to pursue further treatment in her native country and was discharged.
In conclusion, this case represents a rare complication of a common illness. Few cases of influenza causing hemolytic anemia have been reported in the literature. There have been reports of oseltamivir causing hemolytic anemia, but our patient presented with evidence of hemolytic anemia before initiation of the medication. In all the aforementioned cases, the patients died as a result of comorbid conditions. Our patient was stable enough to be discharged from the hospital after treatment of her comorbid conditions.
Acknowledgment
The authors thank David Henry, MD, at Pennsylvania Hospital, Philadelphia, for sharing this case and for his guidance during this patient’s treatment.
Autoimmune hemolytic anemia (AIHA) is characterized by the temperature at which the auto-antibody has the greatest avidity for the target red cell antigen, either warm or cold forms. It is detected by a positive direct antiglobulin test (DAT) also known as the direct Coombs test. DAT is used to determine if red cells have been coated in vivo with immunoglobulin, complement, or both.1 Some causes of a positive DAT include hemolytic transfusion reactions, hemolytic disease of the fetus and newborn, AIHA, and drug-induced immune hemolysis.
Case presentation and summary
A 58-year-old woman from Brazil with past medical history only significant for cholecystectomy and cesarean section had been visiting in United States for 2 months when she presented to an outside hospital with fever, shortness of breath, and syncope that had resulted in a foot injury. She reported she had been feeling short of breath and had a nonproductive cough and malaise for about 2 weeks before presentation with sick contacts at home. On admission it was noted that she had a hemoglobin level of 7.7 g/dL (normal, 12.0-15.5 g/dL; MCV, 94 fL), total bilirubin of 2.14 mg/dL (normal, 0.2-1.0 mg/dL), and lactate dehydrogenase of 523 U/L (normal, 81-234 U/L). There were no signs of bleeding on her examination. Her DAT was positive and moderate red blood cell agglutination was reported. During the first admission at the outside hospital she was diagnosed with influenza A and completed a full course of oseltamivir (75 mg po twice daily for 5 days). A chest X-ray was negative for infiltrates and showed that the patient’s lung fields were clear. She was transfused 2 units of packed red blood cells with response in hemoglobin up to 9.8 g/dL. The patient was treated with dexamethasone (4 mg IV Q8) as an inpatient and was discharged on a prednisone taper (40 mg, with taper by 10 mg every 3 days) with hemoglobin of 8.1 g/dL.
The patient continued to have nonproductive cough, dyspnea, fevers, chills, and generalized weakness, when she returned to the same outside hospital’s emergency department 2 days after her discharge. At that time, it was noted that she had leucocytosis (white blood cell count, 34.6 x 109 per L), a hemoglobin level of 6.8 g/dL, and her total bilirubin level was 6.9 mg/dL. Her hemodynamics were unstable and she was admitted to their intensive care unit. The results of a chest X-ray revealed right lung consolidation.
The day after this admission, her hemoglobin level fell to 4.7 g/dL, and she was transfused 2 units of packed red blood cells before being transferred to our hospital. A chest X-ray at our hospital confirmed a right lung infiltrate. Vancomycin (1,250 mg IV Q12), levaquin (750 mg IV Q24), and maxipime (1 g IV Q12) were initiated for pneumonia and the patient was transferred to our hospital’s intensive care unit. She was afebrile at 98.3°F, her pulse rate was 84 beats per minute, she was tachypneic with respiratory rate of 26 breaths per minute, her blood pressure was 98/51 mmHg, and she had an oxygen saturation of 99% on 2L oxygen via nasal cannula.
On physical examination she was noted to have scleral icterus and was in mild respiratory distress. A chest X-ray revealed a patchy opacity in the right mid to lower lung. Her initial complete blood panel revealed anemia, with hemoglobin, 6.3 g/dL; white blood cell count, 27 x 109 per L; and platelets, 533 x 109 per L. The patient was then transfused another 2 units of packed red blood cells. She was given intravenous hydration, acetaminophen, and albuterol nebulizer treatments as supportive care. She was provided with blankets to keep warm. In addition to her antibiotics, she was also given prednisone 70 mg for her respiratory symptoms.
Further tests revealed haptoglobin, <30 mg/dL (normal, 36-195 mg/dL); lactate dehydrogenase, 371 U/L (normal, 98-192 U/L); and complements C3, 90 mg/dL (normal, 79-152 mg/dL) and C4, <8 mg/dL (normal, 18-55 mg/dL). Her DAT was positive, and agglutination was seen on peripheral smear (Figure 1). This was her second positive DAT as she had positive one at the outside hospital initially. Her tests for mycoplasma pneumonia, the PCR and IgM, were negative, as were the Monospot for mononucleosis and the ANA for autoimmune disorders. Her cold agglutinin titer was 1:256 (normal, no agglutination <1:64). The patient’s repeat respiratory viral panel was negative given recent full treatment for her influenza A at the previous hospital. Her blood and urine cultures were negative.
The patient was given antibiotics (vancomycin 1,250 mg IV Q12, cefepime 2 g IV Q8, and azithromycin 500 mg daily) for her pneumonia. Her respiratory status improved, and she was transferred to general medical floors after the first day of her admission. Her total bilirubin trended down to 1.9 mg/dL. She remained on prednisone 70 mg daily.
The patient remained in the hospital for an additional 6 days before being discharged home on prednisone. She wanted to return to her home country of Brazil as soon as she was able to and said she would seek outpatient follow-up there with a hematologist. At the time of her discharge, her hemoglobin was 6.6 g/dL and her reticulocyte count, 6.0%. Figures 2 and 3 illustrate her hemoglobin and reticulocyte trend during her admission at our hospital.
Discussion
The incidence of cold AIHA or cold agglutinin disease (CAD) occurs about 4 per 1 million people and commonly affects women more often than men.2 The cause of CAD can be subdivided into primary, idiopathic, or secondary causes, which can include infections, malignancies, or benign diseases.3,4 Primary CAD is a chronic disorder that is generally seen in older women. Secondary CAD can be associated with B-cell lymphoproliferative disorders, such as Waldenstrom macroglobulinemia or chronic lymphocytic leukemia, and infectious agents such as Mycoplasma pneumoniae and mononucleosis caused by Epstein-Barr virus.
Mild hemolysis or acrocyanosis may occur with exposure to cold. The blood smear in CAD demonstrates red blood cell agglutination or clumping, polychromasia, and an absence of spherocytosis. In general, most cases require no treatment, but cytotoxic agents or rituximab can be used to treat more severe cases. Appropriate treatment for infectious causes of CAD includes supportive care aimed at the underlying disease process. In addition, it is helpful to keep the patient warm. There is no role for steroid therapy in CAD unlike in warm AIHA. However, our patient was symptomatic from her pneumonia, so we added steroids to help with her pulmonary insult.
The patient had a cold agglutinin titer of 1:256. Titers of 1:32 or higher are considered elevated by this technique. Elevated titers are generally rarely seen except in primary atypical pneumonia due to either M. pneumoniae, influenza A, influenza B, parainfluenza, and adenovirus, and in certain hemolytic anemias. Low titers of cold agglutinins have been demonstrated in malaria, peripheral vascular disease, and common respiratory diseases.
Warm AIHA is caused by IgG antibody activities at body temperature or at 98.6°F. They may or may not bind complement and are removed from circulation by the spleen. Cold AIHA is due to IgM antibodies coating red cells at lower temperatures. They bind complement and lead to red blood cell destruction of agglutinated cells. If the antibody is active at temperatures approaching 98.6°F, clinically significant intravascular and sometimes extravascular complement-mediated hemolysis occur in the liver.5
The incidence of warm AIHA occurs about 10 per 1 million people and affects women twice often as men.2 It can be primary or idiopathic, or associated with various underlying conditions, including autoimmune disorders, immunodeficiency syndromes, lymphoproliferative disorders, other malignancies, and certain drugs. In more severe cases, jaundice and splenomegaly may occur. The blood smear in warm AIHA demonstrates variable spherocytosis, polychromasia, and rare erythrophagocytosis. Treatment usually includes steroids, cytotoxic agents, and splenectomy in severe cases.
There have been few case reports describing influenza as a cause of cold agglutinin hemolytic anemia. Chen and colleagues reported a case of influenza A infection in a 22-month-old boy.6Schoindre and colleagues reported the case of a 60-year-old woman infected with influenza A H1N1 virus who died from CAD.7 Shizuma reported the case of a 67-year-old man with alcoholic cirrhosis who developed a mixed hemolytic anemia and was positive for influenza A.8Our patient presented with influenza A, which had been diagnosed by respiratory virus panel at a different hospital, and she was anemic at the time of presentation to the outside hospital, with a positive DAT test. She was treated for influenza A with a full course of osltamivir and then returned with complaints of worsening fatigue and was again noted to be anemic with the development of patchy opacities on chest X-ray. The patient was subsequently transferred to our hospital and remained anemic during the course of her treatment. She received supportive care for her underlying influenza A and had symptomatic improvement. She ultimately decided the she would like to pursue further treatment in her native country and was discharged.
In conclusion, this case represents a rare complication of a common illness. Few cases of influenza causing hemolytic anemia have been reported in the literature. There have been reports of oseltamivir causing hemolytic anemia, but our patient presented with evidence of hemolytic anemia before initiation of the medication. In all the aforementioned cases, the patients died as a result of comorbid conditions. Our patient was stable enough to be discharged from the hospital after treatment of her comorbid conditions.
Acknowledgment
The authors thank David Henry, MD, at Pennsylvania Hospital, Philadelphia, for sharing this case and for his guidance during this patient’s treatment.
1. Roback JD, Grossman BJ, Harris T, Hillyer CD. Technical manual [17th ed]. Bethesda, MD; American Association of Blood Banks; 2011.
2. Jaffee ES, Harris NL, Vardiman JW, Campo E, Arber DA. Hematopathology. St. Louis, MO; Elsevier Saunders, 2011.
3. Feizi T. Monotypic cold agglutinins in infection by Mycoplasma pneumoniae. Nature. 1967;215(5100):540-542.
4. Horwitz CA, Moulds J, Henle W, et al. Cold agglutinins in infectious mononucleosis and heterophil-antibody-negative mononucleosis-like syndromes. Blood. 1977;50(2):195-202.
5. Hsi ED, editor. Hematopathology [3rd ed]. Philadelphia, PA; Elsevier Saunders; 2012.
6. Chen H, Jia XL, Gao HM, Qian SY. Comorbid presentation of severe novel influenza A (H1N1) and Evans syndrome: a case report. Chin Med J. 2011;124(11):1743-1746.
7. Schoindre Y, Bollée G, Dumont MD, Lesavre P, Servais A. Cold agglutinin syndrome associated with a 2009 influenza A H1N1 infection. http://www.amjmed.com/article/S0002-9343(10)00482-1/fulltext. Published February 2011. Accessed October 10, 2017.
8. [Article in Japanese] Shizuma T. [A case of autoimmune hemolytic anemia caused by type A influenza infection in a patient with alcoholic liver cirrhosis]. Kansenshogaku Zasshi. 2010;84(3):296-299.
1. Roback JD, Grossman BJ, Harris T, Hillyer CD. Technical manual [17th ed]. Bethesda, MD; American Association of Blood Banks; 2011.
2. Jaffee ES, Harris NL, Vardiman JW, Campo E, Arber DA. Hematopathology. St. Louis, MO; Elsevier Saunders, 2011.
3. Feizi T. Monotypic cold agglutinins in infection by Mycoplasma pneumoniae. Nature. 1967;215(5100):540-542.
4. Horwitz CA, Moulds J, Henle W, et al. Cold agglutinins in infectious mononucleosis and heterophil-antibody-negative mononucleosis-like syndromes. Blood. 1977;50(2):195-202.
5. Hsi ED, editor. Hematopathology [3rd ed]. Philadelphia, PA; Elsevier Saunders; 2012.
6. Chen H, Jia XL, Gao HM, Qian SY. Comorbid presentation of severe novel influenza A (H1N1) and Evans syndrome: a case report. Chin Med J. 2011;124(11):1743-1746.
7. Schoindre Y, Bollée G, Dumont MD, Lesavre P, Servais A. Cold agglutinin syndrome associated with a 2009 influenza A H1N1 infection. http://www.amjmed.com/article/S0002-9343(10)00482-1/fulltext. Published February 2011. Accessed October 10, 2017.
8. [Article in Japanese] Shizuma T. [A case of autoimmune hemolytic anemia caused by type A influenza infection in a patient with alcoholic liver cirrhosis]. Kansenshogaku Zasshi. 2010;84(3):296-299.
Pembrolizumab for dMMR/MSI-H tumors marks first tumor agnostic FDA approval
The United States Food and Drug Administration’s approval earlier this year of pembrolizumab marks the first tumor agnostic indication for a cancer drug.1,2 Accelerated approval was granted for the treatment of adult and pediatric patients with any unresectable or metastatic solid tumor that displays mismatch repair deficiencies (dMMR) or high levels of microsatellite instability (MSI-H) and who have progressed after previous treatment and have no satisfactory alternatives. It is also approved specifically for patients with MSI-H or dMMR colorectal cancer (CRC) that has progressed after treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.
Pembrolizumab is a programmed cell death protein-1 (PD-1) receptor inhibitor that blocks the interaction between PD-1 and its ligand, PD-L1, restoring the activity of tumor-infiltrating T cells and boosting the anti-tumor immune response. It is thought to be particularly effective in dMMR/MSI-H tumors because they have a high mutational load and therefore display an abundance of antigens on their surfaces to provoke an immune response.
Approval for the drug was based on the demonstration of durable responses in 149 patients with MSI-H or dMMR cancers across 5 uncontrolled, multicohort, multicenter, single-arm trials. In all, 90 of the patients had CRC, and the remaining 59 patients had 1 of 14 other cancer types that included endometrial, biliary, gastric or gastroesophageal, pancreatic, and breast cancers.
Patients in these trials received pembrolizumab at 1 of 2 different doses, either 200 mg every 3 weeks or 10 mg/kg every 2 weeks, until unacceptable toxicity or disease progression that was symptomatic, rapidly progressive, required urgent intervention, or coincided with a decline in performance status. Treatment was administered for a maximum of 2 years. Patients with an active autoimmune disease or a medical condition that required immunosuppression were ineligible for treatment in all 5 studies.
The median age of enrolled patients was 55 years; 56% were men; 77% white, 19% Asian, 2% black; 98% had metastatic or unresectable disease; and all had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (range, 0-5, where 0 denotes full activity and 1, restricted in physically strenuous activity but ambulatory). MSI-H and MMR status were identified prospectively using polymerase chain reaction and immunohistochemical analyses, respectively.
The primary endpoint was objective response rate (ORR), according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), as assessed by blinded independent central radiologist review, and response duration. The ORR across all five studies
The safety profile was consistent with previously reported safety data for pembrolizumab. The most common adverse events included fatigue, pruritus, diarrhea, decreased appetite, rash, pyrexia, cough, dyspnea, musculoskeletal pain, constipation, and nausea.
The prescribing information includes a “limitation of use” that states that pembroliumab’s safety and efficacy haven’t been established in pediatric patients with MSI-H cancers of the central nervous system.3 It also details warnings and precautions about immune-mediated toxicities, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and renal dysfunction, among others.
Patients should be monitored for signs and symptoms of these toxicities and treated appropriately. Treatment should be withheld and corticosteroids should be administered for grade 2 or higher pneumonitis, colitis, hepatitis, and nephritis; and corticosteroids and hormone replacement as clinically indicated for endocrinopathies. It should also be withheld for aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels >3-5 times the upper limit of normal (ULN) or total bilirubin levels >1.5-3 times ULN.
Pembrolizumab should be permanently discontinued upon grade 3, 4, or recurrent grade 2 pneumonitis, colitis, nephritis/renal dysfunction, and endocrinopathies or for AST or ALT levels >5 times ULN or total bilirubin levels >3 times ULN. For patients with liver metastases who begin treatment with grade 2 AST or ALT, treatment should be permanently discontinued following increases of more than 50%, relative to baseline, that last for at least 1 week.
Health care providers should also bear in mind that pembrolizumab can, more rarely, cause other immune-mediated toxicities, such as arthritis and exfoliative rash that may require treatment and, based on its mechanism of action, pembrolizumab can also cause fetal harm. Patients with reproductive potential should be advised of the implications. Pembrolizumab is marketed as Keytruda by Merck & Co Inc.
1. United States Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for tissue/site agnostic indication. US FDA Web site. https://www.fda.gov/drugs/informationondrugs/ approveddrugs/ucm560040.htm. Last updated May 30, 2017. Accessed July 15, 2017.
2. Merck. News Release. FDA Approves Merck’s KEYTRUDA (pembrolizumab) for Adult and Pediatric Patients with Unresectable or Metastatic, Microsatellite Instability-High (MSI-H) or Mismatch Repair De[1]cient (dMMR) Solid Tumors. http://www.mrknewsroom. com/news-release/prescription-medicine-news/fda-approvesmercks- keytruda-pembrolizumab-adult-and-pediatr. Last updated May 23, 2017. Accessed July 17, 2017.
3. Keytruda (pembrolizumab) for injection, for intravenous use. Prescribing information. Merck & Co Inc. https://www.merck.com/ product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Posted May 2017. Accessed July 15, 2017.
The United States Food and Drug Administration’s approval earlier this year of pembrolizumab marks the first tumor agnostic indication for a cancer drug.1,2 Accelerated approval was granted for the treatment of adult and pediatric patients with any unresectable or metastatic solid tumor that displays mismatch repair deficiencies (dMMR) or high levels of microsatellite instability (MSI-H) and who have progressed after previous treatment and have no satisfactory alternatives. It is also approved specifically for patients with MSI-H or dMMR colorectal cancer (CRC) that has progressed after treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.
Pembrolizumab is a programmed cell death protein-1 (PD-1) receptor inhibitor that blocks the interaction between PD-1 and its ligand, PD-L1, restoring the activity of tumor-infiltrating T cells and boosting the anti-tumor immune response. It is thought to be particularly effective in dMMR/MSI-H tumors because they have a high mutational load and therefore display an abundance of antigens on their surfaces to provoke an immune response.
Approval for the drug was based on the demonstration of durable responses in 149 patients with MSI-H or dMMR cancers across 5 uncontrolled, multicohort, multicenter, single-arm trials. In all, 90 of the patients had CRC, and the remaining 59 patients had 1 of 14 other cancer types that included endometrial, biliary, gastric or gastroesophageal, pancreatic, and breast cancers.
Patients in these trials received pembrolizumab at 1 of 2 different doses, either 200 mg every 3 weeks or 10 mg/kg every 2 weeks, until unacceptable toxicity or disease progression that was symptomatic, rapidly progressive, required urgent intervention, or coincided with a decline in performance status. Treatment was administered for a maximum of 2 years. Patients with an active autoimmune disease or a medical condition that required immunosuppression were ineligible for treatment in all 5 studies.
The median age of enrolled patients was 55 years; 56% were men; 77% white, 19% Asian, 2% black; 98% had metastatic or unresectable disease; and all had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (range, 0-5, where 0 denotes full activity and 1, restricted in physically strenuous activity but ambulatory). MSI-H and MMR status were identified prospectively using polymerase chain reaction and immunohistochemical analyses, respectively.
The primary endpoint was objective response rate (ORR), according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), as assessed by blinded independent central radiologist review, and response duration. The ORR across all five studies
The safety profile was consistent with previously reported safety data for pembrolizumab. The most common adverse events included fatigue, pruritus, diarrhea, decreased appetite, rash, pyrexia, cough, dyspnea, musculoskeletal pain, constipation, and nausea.
The prescribing information includes a “limitation of use” that states that pembroliumab’s safety and efficacy haven’t been established in pediatric patients with MSI-H cancers of the central nervous system.3 It also details warnings and precautions about immune-mediated toxicities, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and renal dysfunction, among others.
Patients should be monitored for signs and symptoms of these toxicities and treated appropriately. Treatment should be withheld and corticosteroids should be administered for grade 2 or higher pneumonitis, colitis, hepatitis, and nephritis; and corticosteroids and hormone replacement as clinically indicated for endocrinopathies. It should also be withheld for aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels >3-5 times the upper limit of normal (ULN) or total bilirubin levels >1.5-3 times ULN.
Pembrolizumab should be permanently discontinued upon grade 3, 4, or recurrent grade 2 pneumonitis, colitis, nephritis/renal dysfunction, and endocrinopathies or for AST or ALT levels >5 times ULN or total bilirubin levels >3 times ULN. For patients with liver metastases who begin treatment with grade 2 AST or ALT, treatment should be permanently discontinued following increases of more than 50%, relative to baseline, that last for at least 1 week.
Health care providers should also bear in mind that pembrolizumab can, more rarely, cause other immune-mediated toxicities, such as arthritis and exfoliative rash that may require treatment and, based on its mechanism of action, pembrolizumab can also cause fetal harm. Patients with reproductive potential should be advised of the implications. Pembrolizumab is marketed as Keytruda by Merck & Co Inc.
The United States Food and Drug Administration’s approval earlier this year of pembrolizumab marks the first tumor agnostic indication for a cancer drug.1,2 Accelerated approval was granted for the treatment of adult and pediatric patients with any unresectable or metastatic solid tumor that displays mismatch repair deficiencies (dMMR) or high levels of microsatellite instability (MSI-H) and who have progressed after previous treatment and have no satisfactory alternatives. It is also approved specifically for patients with MSI-H or dMMR colorectal cancer (CRC) that has progressed after treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.
Pembrolizumab is a programmed cell death protein-1 (PD-1) receptor inhibitor that blocks the interaction between PD-1 and its ligand, PD-L1, restoring the activity of tumor-infiltrating T cells and boosting the anti-tumor immune response. It is thought to be particularly effective in dMMR/MSI-H tumors because they have a high mutational load and therefore display an abundance of antigens on their surfaces to provoke an immune response.
Approval for the drug was based on the demonstration of durable responses in 149 patients with MSI-H or dMMR cancers across 5 uncontrolled, multicohort, multicenter, single-arm trials. In all, 90 of the patients had CRC, and the remaining 59 patients had 1 of 14 other cancer types that included endometrial, biliary, gastric or gastroesophageal, pancreatic, and breast cancers.
Patients in these trials received pembrolizumab at 1 of 2 different doses, either 200 mg every 3 weeks or 10 mg/kg every 2 weeks, until unacceptable toxicity or disease progression that was symptomatic, rapidly progressive, required urgent intervention, or coincided with a decline in performance status. Treatment was administered for a maximum of 2 years. Patients with an active autoimmune disease or a medical condition that required immunosuppression were ineligible for treatment in all 5 studies.
The median age of enrolled patients was 55 years; 56% were men; 77% white, 19% Asian, 2% black; 98% had metastatic or unresectable disease; and all had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (range, 0-5, where 0 denotes full activity and 1, restricted in physically strenuous activity but ambulatory). MSI-H and MMR status were identified prospectively using polymerase chain reaction and immunohistochemical analyses, respectively.
The primary endpoint was objective response rate (ORR), according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), as assessed by blinded independent central radiologist review, and response duration. The ORR across all five studies
The safety profile was consistent with previously reported safety data for pembrolizumab. The most common adverse events included fatigue, pruritus, diarrhea, decreased appetite, rash, pyrexia, cough, dyspnea, musculoskeletal pain, constipation, and nausea.
The prescribing information includes a “limitation of use” that states that pembroliumab’s safety and efficacy haven’t been established in pediatric patients with MSI-H cancers of the central nervous system.3 It also details warnings and precautions about immune-mediated toxicities, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and renal dysfunction, among others.
Patients should be monitored for signs and symptoms of these toxicities and treated appropriately. Treatment should be withheld and corticosteroids should be administered for grade 2 or higher pneumonitis, colitis, hepatitis, and nephritis; and corticosteroids and hormone replacement as clinically indicated for endocrinopathies. It should also be withheld for aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels >3-5 times the upper limit of normal (ULN) or total bilirubin levels >1.5-3 times ULN.
Pembrolizumab should be permanently discontinued upon grade 3, 4, or recurrent grade 2 pneumonitis, colitis, nephritis/renal dysfunction, and endocrinopathies or for AST or ALT levels >5 times ULN or total bilirubin levels >3 times ULN. For patients with liver metastases who begin treatment with grade 2 AST or ALT, treatment should be permanently discontinued following increases of more than 50%, relative to baseline, that last for at least 1 week.
Health care providers should also bear in mind that pembrolizumab can, more rarely, cause other immune-mediated toxicities, such as arthritis and exfoliative rash that may require treatment and, based on its mechanism of action, pembrolizumab can also cause fetal harm. Patients with reproductive potential should be advised of the implications. Pembrolizumab is marketed as Keytruda by Merck & Co Inc.
1. United States Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for tissue/site agnostic indication. US FDA Web site. https://www.fda.gov/drugs/informationondrugs/ approveddrugs/ucm560040.htm. Last updated May 30, 2017. Accessed July 15, 2017.
2. Merck. News Release. FDA Approves Merck’s KEYTRUDA (pembrolizumab) for Adult and Pediatric Patients with Unresectable or Metastatic, Microsatellite Instability-High (MSI-H) or Mismatch Repair De[1]cient (dMMR) Solid Tumors. http://www.mrknewsroom. com/news-release/prescription-medicine-news/fda-approvesmercks- keytruda-pembrolizumab-adult-and-pediatr. Last updated May 23, 2017. Accessed July 17, 2017.
3. Keytruda (pembrolizumab) for injection, for intravenous use. Prescribing information. Merck & Co Inc. https://www.merck.com/ product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Posted May 2017. Accessed July 15, 2017.
1. United States Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for tissue/site agnostic indication. US FDA Web site. https://www.fda.gov/drugs/informationondrugs/ approveddrugs/ucm560040.htm. Last updated May 30, 2017. Accessed July 15, 2017.
2. Merck. News Release. FDA Approves Merck’s KEYTRUDA (pembrolizumab) for Adult and Pediatric Patients with Unresectable or Metastatic, Microsatellite Instability-High (MSI-H) or Mismatch Repair De[1]cient (dMMR) Solid Tumors. http://www.mrknewsroom. com/news-release/prescription-medicine-news/fda-approvesmercks- keytruda-pembrolizumab-adult-and-pediatr. Last updated May 23, 2017. Accessed July 17, 2017.
3. Keytruda (pembrolizumab) for injection, for intravenous use. Prescribing information. Merck & Co Inc. https://www.merck.com/ product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Posted May 2017. Accessed July 15, 2017.
Brigatinib approval yields additional treatment options for crizotinib-resistant, ALK-positive NSCLC patients
The accelerated approval by the United States Food and Drug Administration (FDA) of the anaplastic lymphoma kinase (ALK) inhibitor brigatinib, marked the fourth approved drug in this class.1 The most recent approval expands the available treatment options for patients with metastatic ALK-positive non–small-cell lung cancer (NSCLC) whose disease is no longer responding to the first-line ALK inhibitor crizotinib. The FDA based its decision on the results of the phase 2 ALTA trial, in which a significant proportion of patients experienced tumor shrinkage.2
The pivotal trial was a noncomparative, 2-arm, open-label, multicenter study that was carried out during June 2014-September 2015 at 71 centers across 18 countries. Eligible patients were 18 years or older, with locally advanced or metastatic ALK-positive NSCLC, disease progression while taking crizotinib, at least 1 measurable lesion, adequate organ and hematologic function, and Eastern Cooperative Oncology Group (ECOG) performance status of ≤2 (range, 0-5, where 0 means the patient is fully active, and 2, ambulatory and capable of all self-care but not able to carry out any work activities).
Patients were excluded from the trial if they had received previous ALK inhibitor therapy, other than crizotinib, or had received crizotinib within 3 days of the first dose of brigatinib, or they had received chemotherapy, radiation therapy, or investigational drugs within 14 days or monoclonal antibody therapy within 30 days of the first dose of the study drug. Anyone with a history or the presence of pulmonary interstitial disease or drug-related pneumonitis or symptomatic central nervous system (CNS) metastases that were neurologically unstable or required an increasing dose of corticosteroids was also ineligible.
A total of 222 patients were randomized to receive one of two brigatinib doses, either 90 mg daily or 180 mg daily after a 7-day lead-in at 90 mg (the latter to help mitigate pulmonary adverse events observed in previous studies). Randomization was stratified according to baseline brain metastases (present or absent) and best investigator-assessed response to crizotinib (complete response [CR] or partial response [PR] vs other or unknown)
Chest and abdomen imaging by computed-tomography (CT) or magnetic resonance imaging (MRI) with contrast were performed to assess disease at screening and every 8 weeks through cycle 15, and then every 12 weeks until disease progression. Contrast-enhanced brain MRI was carried out at screening and repeated after baseline for the 68% of patients who had CNS metastases at the time of enrollment.
The primary endpoint was confirmed investigator-assessed objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), and secondary endpoints included CNS response, duration of response (DoR), progression-free and overall survival (PFS and OS, respectively). ORRs for the 90-mg and 180-mg doses were 48% and 53%, respectively. Responses occurred quickly and were durable in both arms; after a median follow-up of 8 months, median DoR was 13.8 months for both doses. Among the patients with brain metastases, the intracranial response rates for the two doses were 42% and 67%, respectively, notable because of the poor ability of crizotinib to penetrate the blood-brain barrier.
Other secondary outcomes also favored the 180-mg dose. Investigator-assessed PFS for the 90-mg and 180-mg doses were 9.2 months and 12.9 months, respectively, and estimated 1-year OS was 71% and 80%, respectively, the latter representing a nonstatistically significant 43% reduction in the risk of death with the 180 mg dose. There were 4 confirmed CRs in the 180-mg arm and 1 in the 90-mg arm.
The safety of brigatinib was evaluated in 219 patients who received at least 1 dose of brigatinib. Treatment was discontinued in 8% of patients in the 180-mg arm and 3% in the 90-mg arm because of adverse events (AEs). The most common AEs were nausea, diarrhea, fatigue, cough, and headache, and visual disturbances also occurred. The most common serious AEs were pneumonia and interstitial lung disease/pneumonitis.
The prescribing information details warnings and precautions about these and other potential toxicities, including hypertension, bradycardia, creatine phosphokinase (CPK) and pancreatic enzyme elevation, and hyperglycemia.3 Patients should be monitored for new or worsening respiratory symptoms, especially during the first week of initiating brigatinib treatment; blood pressure should be controlled before treatment initiation and monitored after 2 weeks and at least monthly thereafter; heart rate and blood pressure should be monitored frequently; patients should be advised to report any visual symptoms, or any unexplained muscle pain, tenderness or weakness; CPK, lipase, and amylase levels should be monitored during treatment, and fasting glucose tested before starting treatment and periodically thereafter.
Brigatinib should be withheld in any patient with new or worsening respiratory symptoms, for grade 3 hypertension despite optimal antihypertensive therapy, for symptomatic bradycardia, for patients with new or worsening visual symptoms of grade 2 or above, for grade 3 or 4 CPK or pancreatic enzyme elevation, or if adequate hyperglycemia control cannot be achieved. Treatment should be permanently discontinued for grade 3 or 4 or recurrent interstitial lung disease/pneumonitis, grade 4 or recurrent grade 3 hypertension, life-threatening bradycardia, and grade 4 visual disturbance.
Based on its mechanism of action, brigatinib can cause fetal harm and patients of reproductive potential should be advised of the risks and necessary precautions. Brigatinib is marketed as Alunbrig. It was discovered by Ariad Pharmaceuticals Inc, which was acquired by Takeda in February 2017.
1. United States Food and Drug Administration. Brigatinib. US FDA Web site. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm555841.htm. Last updated April 28, 2017. Accessed July 15, 2017
2. Kim D-W, Tiseo M, Ahn M-J, Reckamp KL, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non–small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490-2498.
3. Alunbrig (brigatinib) tablets, for oral use. Prescribing information. Ariad Pharmaceuticals Inc. https://www.alunbrig.com/assets/pi.pdf. Posted April 2017. Accessed July 15, 2017.
The accelerated approval by the United States Food and Drug Administration (FDA) of the anaplastic lymphoma kinase (ALK) inhibitor brigatinib, marked the fourth approved drug in this class.1 The most recent approval expands the available treatment options for patients with metastatic ALK-positive non–small-cell lung cancer (NSCLC) whose disease is no longer responding to the first-line ALK inhibitor crizotinib. The FDA based its decision on the results of the phase 2 ALTA trial, in which a significant proportion of patients experienced tumor shrinkage.2
The pivotal trial was a noncomparative, 2-arm, open-label, multicenter study that was carried out during June 2014-September 2015 at 71 centers across 18 countries. Eligible patients were 18 years or older, with locally advanced or metastatic ALK-positive NSCLC, disease progression while taking crizotinib, at least 1 measurable lesion, adequate organ and hematologic function, and Eastern Cooperative Oncology Group (ECOG) performance status of ≤2 (range, 0-5, where 0 means the patient is fully active, and 2, ambulatory and capable of all self-care but not able to carry out any work activities).
Patients were excluded from the trial if they had received previous ALK inhibitor therapy, other than crizotinib, or had received crizotinib within 3 days of the first dose of brigatinib, or they had received chemotherapy, radiation therapy, or investigational drugs within 14 days or monoclonal antibody therapy within 30 days of the first dose of the study drug. Anyone with a history or the presence of pulmonary interstitial disease or drug-related pneumonitis or symptomatic central nervous system (CNS) metastases that were neurologically unstable or required an increasing dose of corticosteroids was also ineligible.
A total of 222 patients were randomized to receive one of two brigatinib doses, either 90 mg daily or 180 mg daily after a 7-day lead-in at 90 mg (the latter to help mitigate pulmonary adverse events observed in previous studies). Randomization was stratified according to baseline brain metastases (present or absent) and best investigator-assessed response to crizotinib (complete response [CR] or partial response [PR] vs other or unknown)
Chest and abdomen imaging by computed-tomography (CT) or magnetic resonance imaging (MRI) with contrast were performed to assess disease at screening and every 8 weeks through cycle 15, and then every 12 weeks until disease progression. Contrast-enhanced brain MRI was carried out at screening and repeated after baseline for the 68% of patients who had CNS metastases at the time of enrollment.
The primary endpoint was confirmed investigator-assessed objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), and secondary endpoints included CNS response, duration of response (DoR), progression-free and overall survival (PFS and OS, respectively). ORRs for the 90-mg and 180-mg doses were 48% and 53%, respectively. Responses occurred quickly and were durable in both arms; after a median follow-up of 8 months, median DoR was 13.8 months for both doses. Among the patients with brain metastases, the intracranial response rates for the two doses were 42% and 67%, respectively, notable because of the poor ability of crizotinib to penetrate the blood-brain barrier.
Other secondary outcomes also favored the 180-mg dose. Investigator-assessed PFS for the 90-mg and 180-mg doses were 9.2 months and 12.9 months, respectively, and estimated 1-year OS was 71% and 80%, respectively, the latter representing a nonstatistically significant 43% reduction in the risk of death with the 180 mg dose. There were 4 confirmed CRs in the 180-mg arm and 1 in the 90-mg arm.
The safety of brigatinib was evaluated in 219 patients who received at least 1 dose of brigatinib. Treatment was discontinued in 8% of patients in the 180-mg arm and 3% in the 90-mg arm because of adverse events (AEs). The most common AEs were nausea, diarrhea, fatigue, cough, and headache, and visual disturbances also occurred. The most common serious AEs were pneumonia and interstitial lung disease/pneumonitis.
The prescribing information details warnings and precautions about these and other potential toxicities, including hypertension, bradycardia, creatine phosphokinase (CPK) and pancreatic enzyme elevation, and hyperglycemia.3 Patients should be monitored for new or worsening respiratory symptoms, especially during the first week of initiating brigatinib treatment; blood pressure should be controlled before treatment initiation and monitored after 2 weeks and at least monthly thereafter; heart rate and blood pressure should be monitored frequently; patients should be advised to report any visual symptoms, or any unexplained muscle pain, tenderness or weakness; CPK, lipase, and amylase levels should be monitored during treatment, and fasting glucose tested before starting treatment and periodically thereafter.
Brigatinib should be withheld in any patient with new or worsening respiratory symptoms, for grade 3 hypertension despite optimal antihypertensive therapy, for symptomatic bradycardia, for patients with new or worsening visual symptoms of grade 2 or above, for grade 3 or 4 CPK or pancreatic enzyme elevation, or if adequate hyperglycemia control cannot be achieved. Treatment should be permanently discontinued for grade 3 or 4 or recurrent interstitial lung disease/pneumonitis, grade 4 or recurrent grade 3 hypertension, life-threatening bradycardia, and grade 4 visual disturbance.
Based on its mechanism of action, brigatinib can cause fetal harm and patients of reproductive potential should be advised of the risks and necessary precautions. Brigatinib is marketed as Alunbrig. It was discovered by Ariad Pharmaceuticals Inc, which was acquired by Takeda in February 2017.
The accelerated approval by the United States Food and Drug Administration (FDA) of the anaplastic lymphoma kinase (ALK) inhibitor brigatinib, marked the fourth approved drug in this class.1 The most recent approval expands the available treatment options for patients with metastatic ALK-positive non–small-cell lung cancer (NSCLC) whose disease is no longer responding to the first-line ALK inhibitor crizotinib. The FDA based its decision on the results of the phase 2 ALTA trial, in which a significant proportion of patients experienced tumor shrinkage.2
The pivotal trial was a noncomparative, 2-arm, open-label, multicenter study that was carried out during June 2014-September 2015 at 71 centers across 18 countries. Eligible patients were 18 years or older, with locally advanced or metastatic ALK-positive NSCLC, disease progression while taking crizotinib, at least 1 measurable lesion, adequate organ and hematologic function, and Eastern Cooperative Oncology Group (ECOG) performance status of ≤2 (range, 0-5, where 0 means the patient is fully active, and 2, ambulatory and capable of all self-care but not able to carry out any work activities).
Patients were excluded from the trial if they had received previous ALK inhibitor therapy, other than crizotinib, or had received crizotinib within 3 days of the first dose of brigatinib, or they had received chemotherapy, radiation therapy, or investigational drugs within 14 days or monoclonal antibody therapy within 30 days of the first dose of the study drug. Anyone with a history or the presence of pulmonary interstitial disease or drug-related pneumonitis or symptomatic central nervous system (CNS) metastases that were neurologically unstable or required an increasing dose of corticosteroids was also ineligible.
A total of 222 patients were randomized to receive one of two brigatinib doses, either 90 mg daily or 180 mg daily after a 7-day lead-in at 90 mg (the latter to help mitigate pulmonary adverse events observed in previous studies). Randomization was stratified according to baseline brain metastases (present or absent) and best investigator-assessed response to crizotinib (complete response [CR] or partial response [PR] vs other or unknown)
Chest and abdomen imaging by computed-tomography (CT) or magnetic resonance imaging (MRI) with contrast were performed to assess disease at screening and every 8 weeks through cycle 15, and then every 12 weeks until disease progression. Contrast-enhanced brain MRI was carried out at screening and repeated after baseline for the 68% of patients who had CNS metastases at the time of enrollment.
The primary endpoint was confirmed investigator-assessed objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), and secondary endpoints included CNS response, duration of response (DoR), progression-free and overall survival (PFS and OS, respectively). ORRs for the 90-mg and 180-mg doses were 48% and 53%, respectively. Responses occurred quickly and were durable in both arms; after a median follow-up of 8 months, median DoR was 13.8 months for both doses. Among the patients with brain metastases, the intracranial response rates for the two doses were 42% and 67%, respectively, notable because of the poor ability of crizotinib to penetrate the blood-brain barrier.
Other secondary outcomes also favored the 180-mg dose. Investigator-assessed PFS for the 90-mg and 180-mg doses were 9.2 months and 12.9 months, respectively, and estimated 1-year OS was 71% and 80%, respectively, the latter representing a nonstatistically significant 43% reduction in the risk of death with the 180 mg dose. There were 4 confirmed CRs in the 180-mg arm and 1 in the 90-mg arm.
The safety of brigatinib was evaluated in 219 patients who received at least 1 dose of brigatinib. Treatment was discontinued in 8% of patients in the 180-mg arm and 3% in the 90-mg arm because of adverse events (AEs). The most common AEs were nausea, diarrhea, fatigue, cough, and headache, and visual disturbances also occurred. The most common serious AEs were pneumonia and interstitial lung disease/pneumonitis.
The prescribing information details warnings and precautions about these and other potential toxicities, including hypertension, bradycardia, creatine phosphokinase (CPK) and pancreatic enzyme elevation, and hyperglycemia.3 Patients should be monitored for new or worsening respiratory symptoms, especially during the first week of initiating brigatinib treatment; blood pressure should be controlled before treatment initiation and monitored after 2 weeks and at least monthly thereafter; heart rate and blood pressure should be monitored frequently; patients should be advised to report any visual symptoms, or any unexplained muscle pain, tenderness or weakness; CPK, lipase, and amylase levels should be monitored during treatment, and fasting glucose tested before starting treatment and periodically thereafter.
Brigatinib should be withheld in any patient with new or worsening respiratory symptoms, for grade 3 hypertension despite optimal antihypertensive therapy, for symptomatic bradycardia, for patients with new or worsening visual symptoms of grade 2 or above, for grade 3 or 4 CPK or pancreatic enzyme elevation, or if adequate hyperglycemia control cannot be achieved. Treatment should be permanently discontinued for grade 3 or 4 or recurrent interstitial lung disease/pneumonitis, grade 4 or recurrent grade 3 hypertension, life-threatening bradycardia, and grade 4 visual disturbance.
Based on its mechanism of action, brigatinib can cause fetal harm and patients of reproductive potential should be advised of the risks and necessary precautions. Brigatinib is marketed as Alunbrig. It was discovered by Ariad Pharmaceuticals Inc, which was acquired by Takeda in February 2017.
1. United States Food and Drug Administration. Brigatinib. US FDA Web site. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm555841.htm. Last updated April 28, 2017. Accessed July 15, 2017
2. Kim D-W, Tiseo M, Ahn M-J, Reckamp KL, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non–small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490-2498.
3. Alunbrig (brigatinib) tablets, for oral use. Prescribing information. Ariad Pharmaceuticals Inc. https://www.alunbrig.com/assets/pi.pdf. Posted April 2017. Accessed July 15, 2017.
1. United States Food and Drug Administration. Brigatinib. US FDA Web site. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm555841.htm. Last updated April 28, 2017. Accessed July 15, 2017
2. Kim D-W, Tiseo M, Ahn M-J, Reckamp KL, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non–small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490-2498.
3. Alunbrig (brigatinib) tablets, for oral use. Prescribing information. Ariad Pharmaceuticals Inc. https://www.alunbrig.com/assets/pi.pdf. Posted April 2017. Accessed July 15, 2017.
Hallmark tumor metabolism becomes a validated therapeutic target
Altered cell metabolism has long been recognized as a distinctive feature of malignant cells but, until recently, research efforts had focused on a single aspect. It has become increasingly evident that many metabolic pathways are altered in cancer cells. Improved understanding has yielded the first regulatory approval in this new class of drugs. Here, we discuss the latest developments in the therapeutic targeting of the cancer metabolism hallmark.
A cancer cell’s sweet tooth
The metabolism of cancer cells differs from that of normal cells, an observation that has spawned a dedicated field of research and new targeted drug development. The German physiologist Otto Warburg is credited as the father of the field with his observations about the way in which cancer cells derive energy from glucose.1
In normal cells, glucose is converted into pyruvate in the cytoplasm, which is then, most often, fed to the mitochondria that use oxidative phosphorylation to produce energy in the form of adenosine triphosphate (ATP). Cancer cells seem instead to favor using the pyruvate to produce lactate through glycolysis (Figure 1).
Glycolysis is usually reserved for conditions of poor oxygen availability, but although the tumor microenvironment is often hypoxic, cancer cells have been shown to use glycolysis even when oxygen is plentiful. As a result, the phenomenon is known as aerobic glycolysis, although it is most often referred to as the Warburg effect.2
Glycolysis is much less efficient than oxidative phosphorylation at producing energy, yielding only 2 ATP. In order to meet their energy demands in this way, cancer cells ramp up their glucose intake, an effect that has been exploited for the detection of cancer with positron-emission tomography.
Warburg postulated that this metabolic shift was a result of mitochondrial damage and defective oxidative phosphorylation, even going so far as to suggest that cancer was a mitochondrial disease. It has subsequently been shown that the mitochondria are mostly intact in cancer cells and that oxidative phosphorylation can still occur.3
The Warburg effect has been the subject of significant investigative efforts as researchers have attempted to better understand how this phenomenon comes about. Studies have shown that it is driven in large part by the transcription factors hypoxia inducible factor 1 alpha (HIF-1α) and c-Myc. In addition, numerous other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway, and the activation of oncogenes and inactivation of tumor suppressors, are thought to play a central role.
HIF-1α is an oxygen-sensing transcription factor that coordinates cellular responses to reduced oxygen levels by binding to specific regions, known as hypoxia response elements, on target genes in the nucleus and regulating their subsequent expression. Oxygen levels and metabolism are tightly linked, and HIF-1α sits at the intersection of the 2 since many of its target genes are involved in metabolic pathways, including many glycolytic enzymes, but it also directly inhibits oxidative phosphorylation by suppressing key enzymes in this metabolic pathway.
The expression of HIF-1α and numerous glycolytic enzymes, including lactate dehydrogenase (LDH), phosphofructokinase (PFK), hexokinase II (HKII), and pyruvate dehydrogenase kinase (PDK) is increased in many tumor types. Other molecules that are associated with glucose uptake and metabolism are also dysregulated, such as the GLUT-1 glucose transporter.2,4-6
Targeting glycolysis and glucose uptake
According to one study, glucose transporters and glycolytic enzymes are overexpressed in 24 different types of cancer, representing more than 70% of all cancer cases.7 This enables cancer cells to respond metabolically as though they are experiencing hypoxia, even when oxygen is plentiful and, indeed, when hypoxia is a concern, to mount a faster response. It also provides a tempting avenue for anticancer drug design by exploiting the dependency of cancer cells on glycolysis to survive and thrive.
Inhibitors of HKII, LDH, PFK, PDK, and GLUT-1 have been and continue to be developed. For example, 2-deoxy-D-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced by hydrogen, preventing further glycolysis; it acts as a competitive inhibitor of HKII. Dichloroacetate (DCA) activates the pyruvate dehydrogenase complex and inhibits the actions of the PDKs. Although development of DCA itself was unsuccessful, DCA derivatives continue to be pursued. WZB117 and STF-31 are novel small-molecule inhibitors of GLUT-1-mediated glucose transport. To date, where inhibitors of glycolysis have progressed into clinical trials, they have not proved successful, often limited by off-target effects and low potency.8-11
A variety of cell signaling pathways are implicated in metabolism by tightly regulating the ability of cells to gain access to and use nutrients. Through aberrations in these pathways, cancer cells can essentially go rogue, ignoring regulatory signals and taking up nutrients in an autonomous manner. One of the most frequently altered signaling pathways in human cancer, the PI3K-Akt-mTOR pathway, is also an important regulator of metabolism, coordinating the uptake of multiple nutrients, including glucose.
Akt in particular is thought to have a critical role in glucose metabolism and increased Akt pathway signaling has been shown to correlate with increased rates of glycolysis in cancer cells. Thus, Akt inhibitors could double as glycolytic or glucose transport inhibitors.12,13
A number of Akt inhibitors are being evaluated in clinical trials (Table) and results from the phase 2 LOTUS trial of ipatasertib (GDC-0068) were recently published.
Among 124 patients randomly assigned to paclitaxel in combination with either ipatasertib or placebo, there was a modest improvement in progression-free survival (PFS) in the ipatasertib arm in patients with triple-negative breast cancer (TNBC; 6 months vs 4.2 months, respectively; hazard ratio [HR], 0.60; P = .037). The effect was more pronounced, though not statistically significant, in patients with phosphatase and tensin homolog (PTEN)-low tumors (6.2 months vs 3.7 months; HR, 0.59; P = .18). The most common grade 3 and higher adverse events (AEs) were diarrhea, reduced neutrophil count, and neutropenia.14
The Warburg paradox
Although the molecular mechanisms underlying the Warburg effect have been revealed to some extent, why cancer cells would choose to use such an energy-inefficient process when they have such high energy demands, remains something of a paradox. It’s still not entirely clear, but several explanations that are not necessarily mutually exclusive have been proposed and relate to the inherent benefits of glycolysis and might explain why cancer cells favor this pathway despite its poor energy yield. First, ATP is produced much more rapidly through glycolysis than oxidative phosphorylation, up to 100 times faster. Thus, using glycolysis is a trade-off, between making less energy and making it more quickly.
Second, cancer cells require more than just ATP to meet their metabolic demands. They need amino acids for protein synthesis; nucleotides for DNA replication; lipids for cell membrane synthesis; nicotinamide adenine dinucleotide phosphate (NADPH), which helps the cancer cell deal with oxidative stress; and various other metabolites. Glycolysis branches off into other metabolic pathways that generate many of these metabolites. Among these branched pathways is the pentose phosphate pathway (PPP), which is required for the generation of ribonucleotides and is a major source for NADPH. Cancer cells have been shown to upregulate the flux of glucose into the PPP to meet their anabolic demands and counter oxidative stress.
Third, the lactic acid produced through glycolysis is actively exported from tumor cells by monocarboxylate transporters (MCTs). This creates a highly acidic tumor microenvironment, which can promote several cancer-related processes and also plays a role in tumor-induced immunosuppression, by inhibiting the activity of tumor-infiltrating T cells, reducing dendritic cell maturation, and promoting the transformation of macrophages to a protumorigenic form.2,4,6
Beyond the Warburg effect
Although the focus has been on glucose metabolism and glycolysis, it has been increasingly recognized that many different metabolic pathways are altered. Fundamental changes to the metabolism of all 4 major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – have been observed, encompassing all aspects of cellular metabolism and enabling cancer cells to meet their complete metabolic requirements. There is also evidence that cancer cells are able to switch between different metabolic pathways depending on the availability of oxygen, their energetic needs, environmental stresses, and many other factors. Certainly, there is significant heterogeneity in the metabolic changes that occur in tumors, which vary from tumor to tumor and even within the same tumor and across the lifespan of a tumor as it progresses from an early stage to more advanced or metastatic disease.
The notion of the Warburg effect as a universal phenomenon in cancer cells is now being widely disregarded. Many tumors continue to use oxidative phosphorylation, particularly slower growing tumors, to meet their energy needs. More recently a “reverse” Warburg effect was described, whereby cancer cells are thought to influence the metabolism of the surrounding stromal fibroblasts and essentially outsource aerobic glycolysis to these cells, while performing energy-efficient oxidative phosphorylation themselves (Figure 2).5,15,16
There is thought to be a “lactate shuttle” between the stromal and cancer cells. The stromal cells express high levels of efflux MCTs so that they can remove the subsequently high levels of lactate from the cytoplasm and avoid pickling themselves. The lactate is then shuttled to the cancer cells that have MCTs on their surface that are involved in lactate uptake. The cancer cells oxidize the lactate back into pyruvate, which can then be used in the tricarboxylic acid (TCA) cycle to feed oxidative phosphorylation for efficient ATP production. This hypothesis reflects a broader appreciation of the role of the microenvironment in contributing to cancer metabolism.17,18
An improved holistic understanding of cancer cell metabolism has led to the recognition of altered cancer metabolism as one of the hallmark abilities required for transformation of a normal cell into a cancerous one. It is categorized as “deregulation of bioenergetics” in the most up to date review of the cancer hallmarks.19 It has also begun to shape the therapeutic landscape as new drug targets have emerged.
IDH inhibitors first to market
A number of new metabolically-targeted treatment strategies are being developed. Most promising are small molecule inhibitors of the isocitrate dehydrogenase (IDH) enzymes. These enzymes play an essential role in the TCA cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, generating carbon dioxide and NADPH. Recurrent mutations in the IDH1 and IDH2 genes have been observed in several different types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
IDH mutations are known as neomorphic mutations because they confer a new function on the altered gene product. In this case, the mutant IDH enzyme converts alpha-ketoglutarate further into D-2-hydroxyglutarate (D-2HG). This molecule has a number of different effects that promote tumorigenesis, including fostering defective DNA repair (Figure 3).20,21
Intriguing research presented at the American Association of Cancer Research Annual Meeting revealed that IDH mutations may make cancer cells more vulnerable to poly (ADP-ribose) polymerase (PARP) inhibition, likely as a result of defects in homologous recombination pathways of DNA repair.22The pursuit of IDH as a potential therapeutic target has yielded the first regulatory approval for a metabolically targeted anticancer therapy. In August 2017, the United States Food and Drug Administration (FDA) approved enasidenib, an IDH2 inhibitor, for the treatment of relapsed or refractory AML with an IDH2 mutation. It was approved in combination with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect IDH2 mutations.
The approval was based on a single-arm trial in which responses occurred in almost a quarter of the 199 patients treated with 100 mg oral enasidenib daily. After a median follow-up of 6.6 months, 23% of the patients experienced a complete response or a complete response with partial hematologic recovery lasting a median of 8.2 months. The most common AEs were nausea, vomiting, diarrhea, elevated bilirubin levels, and reduced appetite.23
Several other IDH inhibitors are also showing encouraging efficacy. Ivosidenib is an IDH1 inhibitor and the results of a phase 1 study in patients with cholangiocarcinoma were recently presented at a leading conference. Escalating doses of ivosidenib (100 mg twice daily to 1,200 mg once daily) were administered to 73 patients (as of December 2016). The confirmed partial response (PR) rate was 6%, the rate of stable disease was 56%, and PFS at 6 months was 40%. There were no dose-limiting toxicities (DLTs) and treatment-emergent AEs included fatigue, nausea, vomiting, diarrhea, decreased appetite, dysgeusia, and QT prolongation.24
Another study of ivosidenib was presented at the 2017 annual meeting of the Society for Neuro-Oncology. In that study, patients with glioma received daily doses of ivosidenib ranging from 300 mg to 900 mg. Two patients had a minor response, 83% had stable disease, and the median PFS was 13 months. There were no DLTs and most AEs were mild to moderate and included, most commonly, headache, nausea, diarrhea, and vomiting.25
Pursuing alternative targets and repurposing drugs
Other metabolic targets that are being pursued include glutaminase, given the observation of significantly enhanced glutamine uptake in cancer cells. CB-839 is a glutaminase inhibitor that is currently being evaluated in phase 1 and 2 clinical trials. Updated clinical trial data from a phase 1 trial of CB-839 in combination with paclitaxel in patients with advanced/metastatic TNBC were presented at the San Antonio Breast Cancer Symposium last year.26
As of October 2017, 49 patients had been treated with 400 mg, 600 mg, or 800 mg CB-839 twice daily in combination with 80 mg/m2 intravenous paclitaxel weekly. Among the 44 patients evaluable for response, the rate of PR was 22% and of disease control, 59%. The one DLT was grade 3 neutropenia at the 400 mg dose. Overall AEs were mostly low grade and reversible.
In recent years, lactate has emerged as more than just a by-product of altered cancer cell metabolism. It is responsible, at least in part, for the highly acidic tumor microenvironment that fosters many of the other hallmarks of cancer. In addition, lactate promotes angiogenesis by upregulating HIF-1α in endothelial cells. Depriving tumor cells of the ability to export lactate is a potentially promising therapeutic strategy. An MCT-1 inhibitor, AZD3965, is being evaluated in early stage clinical trials.
Finally, several drugs that are renowned for their use in other disease settings are being repurposed for cancer therapy because of their potential effects on cancer cell metabolism. Ritonavir, an antiretroviral drug used in the treatment of HIV, is an inhibitor of GLUT-1 and is being evaluated in phase 1 and 2 clinical trials. Meanwhile, long-term studies of metformin, a drug that has revolutionized the treatment of diabetes, have revealed a reduction in the emergence of new cancers in diabetic patients treated who are treated with it, and the drug has been shown to improve breast cancer survival rates. Its precise anticancer effects are somewhat unclear, but it is thought to act in part by inhibiting oxidative phosphorylation. Numerous clinical trials of metformin in different types of cancer are ongoing.27,2
1. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270.
2. Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908-38926.
3. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314.
4. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
5. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68.
6. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155-171.
7. Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014-1020.
8. Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430-3440.
9. Zhang W, Zhang SL, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390-1400.
10. Talekar M, Boreddy SR, Singh A, Amiji M. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opin Biol Ther. 2014;14(8):1145-1159.
11. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
12. Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In: Cramer T, Schmitt CA, eds. Metabolism in Cancer. Cham, Switzerland: Springer International Publishing; 2016:39-72.
13. Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D (eds). Oxidative stress in cancer biology and therapy. Humana Press (copyright holder, Springer Science+Business Media, LLC); 2012:21-46.
14. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372.
15. Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813-57825.
16. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198-203.
17. Brooks GA. Cell–cell and intracellular lactate shuttles. Journal Physiol. 2009;587(23):5591-5600.
18. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272-280.
19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
20. Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373-380.
21. Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370.
22. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709-1718.
23. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
24. Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015-4015.
25. Mellinghoff IK, Touat M, Maher E, et al. ACTR-46. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(suppl_6):vi10-vi11.
26. Kalinsky K, Harding J, DeMichele A, et al. Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas.
27. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int. 2015;2015:548436.
28. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376.
Altered cell metabolism has long been recognized as a distinctive feature of malignant cells but, until recently, research efforts had focused on a single aspect. It has become increasingly evident that many metabolic pathways are altered in cancer cells. Improved understanding has yielded the first regulatory approval in this new class of drugs. Here, we discuss the latest developments in the therapeutic targeting of the cancer metabolism hallmark.
A cancer cell’s sweet tooth
The metabolism of cancer cells differs from that of normal cells, an observation that has spawned a dedicated field of research and new targeted drug development. The German physiologist Otto Warburg is credited as the father of the field with his observations about the way in which cancer cells derive energy from glucose.1
In normal cells, glucose is converted into pyruvate in the cytoplasm, which is then, most often, fed to the mitochondria that use oxidative phosphorylation to produce energy in the form of adenosine triphosphate (ATP). Cancer cells seem instead to favor using the pyruvate to produce lactate through glycolysis (Figure 1).
Glycolysis is usually reserved for conditions of poor oxygen availability, but although the tumor microenvironment is often hypoxic, cancer cells have been shown to use glycolysis even when oxygen is plentiful. As a result, the phenomenon is known as aerobic glycolysis, although it is most often referred to as the Warburg effect.2
Glycolysis is much less efficient than oxidative phosphorylation at producing energy, yielding only 2 ATP. In order to meet their energy demands in this way, cancer cells ramp up their glucose intake, an effect that has been exploited for the detection of cancer with positron-emission tomography.
Warburg postulated that this metabolic shift was a result of mitochondrial damage and defective oxidative phosphorylation, even going so far as to suggest that cancer was a mitochondrial disease. It has subsequently been shown that the mitochondria are mostly intact in cancer cells and that oxidative phosphorylation can still occur.3
The Warburg effect has been the subject of significant investigative efforts as researchers have attempted to better understand how this phenomenon comes about. Studies have shown that it is driven in large part by the transcription factors hypoxia inducible factor 1 alpha (HIF-1α) and c-Myc. In addition, numerous other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway, and the activation of oncogenes and inactivation of tumor suppressors, are thought to play a central role.
HIF-1α is an oxygen-sensing transcription factor that coordinates cellular responses to reduced oxygen levels by binding to specific regions, known as hypoxia response elements, on target genes in the nucleus and regulating their subsequent expression. Oxygen levels and metabolism are tightly linked, and HIF-1α sits at the intersection of the 2 since many of its target genes are involved in metabolic pathways, including many glycolytic enzymes, but it also directly inhibits oxidative phosphorylation by suppressing key enzymes in this metabolic pathway.
The expression of HIF-1α and numerous glycolytic enzymes, including lactate dehydrogenase (LDH), phosphofructokinase (PFK), hexokinase II (HKII), and pyruvate dehydrogenase kinase (PDK) is increased in many tumor types. Other molecules that are associated with glucose uptake and metabolism are also dysregulated, such as the GLUT-1 glucose transporter.2,4-6
Targeting glycolysis and glucose uptake
According to one study, glucose transporters and glycolytic enzymes are overexpressed in 24 different types of cancer, representing more than 70% of all cancer cases.7 This enables cancer cells to respond metabolically as though they are experiencing hypoxia, even when oxygen is plentiful and, indeed, when hypoxia is a concern, to mount a faster response. It also provides a tempting avenue for anticancer drug design by exploiting the dependency of cancer cells on glycolysis to survive and thrive.
Inhibitors of HKII, LDH, PFK, PDK, and GLUT-1 have been and continue to be developed. For example, 2-deoxy-D-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced by hydrogen, preventing further glycolysis; it acts as a competitive inhibitor of HKII. Dichloroacetate (DCA) activates the pyruvate dehydrogenase complex and inhibits the actions of the PDKs. Although development of DCA itself was unsuccessful, DCA derivatives continue to be pursued. WZB117 and STF-31 are novel small-molecule inhibitors of GLUT-1-mediated glucose transport. To date, where inhibitors of glycolysis have progressed into clinical trials, they have not proved successful, often limited by off-target effects and low potency.8-11
A variety of cell signaling pathways are implicated in metabolism by tightly regulating the ability of cells to gain access to and use nutrients. Through aberrations in these pathways, cancer cells can essentially go rogue, ignoring regulatory signals and taking up nutrients in an autonomous manner. One of the most frequently altered signaling pathways in human cancer, the PI3K-Akt-mTOR pathway, is also an important regulator of metabolism, coordinating the uptake of multiple nutrients, including glucose.
Akt in particular is thought to have a critical role in glucose metabolism and increased Akt pathway signaling has been shown to correlate with increased rates of glycolysis in cancer cells. Thus, Akt inhibitors could double as glycolytic or glucose transport inhibitors.12,13
A number of Akt inhibitors are being evaluated in clinical trials (Table) and results from the phase 2 LOTUS trial of ipatasertib (GDC-0068) were recently published.
Among 124 patients randomly assigned to paclitaxel in combination with either ipatasertib or placebo, there was a modest improvement in progression-free survival (PFS) in the ipatasertib arm in patients with triple-negative breast cancer (TNBC; 6 months vs 4.2 months, respectively; hazard ratio [HR], 0.60; P = .037). The effect was more pronounced, though not statistically significant, in patients with phosphatase and tensin homolog (PTEN)-low tumors (6.2 months vs 3.7 months; HR, 0.59; P = .18). The most common grade 3 and higher adverse events (AEs) were diarrhea, reduced neutrophil count, and neutropenia.14
The Warburg paradox
Although the molecular mechanisms underlying the Warburg effect have been revealed to some extent, why cancer cells would choose to use such an energy-inefficient process when they have such high energy demands, remains something of a paradox. It’s still not entirely clear, but several explanations that are not necessarily mutually exclusive have been proposed and relate to the inherent benefits of glycolysis and might explain why cancer cells favor this pathway despite its poor energy yield. First, ATP is produced much more rapidly through glycolysis than oxidative phosphorylation, up to 100 times faster. Thus, using glycolysis is a trade-off, between making less energy and making it more quickly.
Second, cancer cells require more than just ATP to meet their metabolic demands. They need amino acids for protein synthesis; nucleotides for DNA replication; lipids for cell membrane synthesis; nicotinamide adenine dinucleotide phosphate (NADPH), which helps the cancer cell deal with oxidative stress; and various other metabolites. Glycolysis branches off into other metabolic pathways that generate many of these metabolites. Among these branched pathways is the pentose phosphate pathway (PPP), which is required for the generation of ribonucleotides and is a major source for NADPH. Cancer cells have been shown to upregulate the flux of glucose into the PPP to meet their anabolic demands and counter oxidative stress.
Third, the lactic acid produced through glycolysis is actively exported from tumor cells by monocarboxylate transporters (MCTs). This creates a highly acidic tumor microenvironment, which can promote several cancer-related processes and also plays a role in tumor-induced immunosuppression, by inhibiting the activity of tumor-infiltrating T cells, reducing dendritic cell maturation, and promoting the transformation of macrophages to a protumorigenic form.2,4,6
Beyond the Warburg effect
Although the focus has been on glucose metabolism and glycolysis, it has been increasingly recognized that many different metabolic pathways are altered. Fundamental changes to the metabolism of all 4 major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – have been observed, encompassing all aspects of cellular metabolism and enabling cancer cells to meet their complete metabolic requirements. There is also evidence that cancer cells are able to switch between different metabolic pathways depending on the availability of oxygen, their energetic needs, environmental stresses, and many other factors. Certainly, there is significant heterogeneity in the metabolic changes that occur in tumors, which vary from tumor to tumor and even within the same tumor and across the lifespan of a tumor as it progresses from an early stage to more advanced or metastatic disease.
The notion of the Warburg effect as a universal phenomenon in cancer cells is now being widely disregarded. Many tumors continue to use oxidative phosphorylation, particularly slower growing tumors, to meet their energy needs. More recently a “reverse” Warburg effect was described, whereby cancer cells are thought to influence the metabolism of the surrounding stromal fibroblasts and essentially outsource aerobic glycolysis to these cells, while performing energy-efficient oxidative phosphorylation themselves (Figure 2).5,15,16
There is thought to be a “lactate shuttle” between the stromal and cancer cells. The stromal cells express high levels of efflux MCTs so that they can remove the subsequently high levels of lactate from the cytoplasm and avoid pickling themselves. The lactate is then shuttled to the cancer cells that have MCTs on their surface that are involved in lactate uptake. The cancer cells oxidize the lactate back into pyruvate, which can then be used in the tricarboxylic acid (TCA) cycle to feed oxidative phosphorylation for efficient ATP production. This hypothesis reflects a broader appreciation of the role of the microenvironment in contributing to cancer metabolism.17,18
An improved holistic understanding of cancer cell metabolism has led to the recognition of altered cancer metabolism as one of the hallmark abilities required for transformation of a normal cell into a cancerous one. It is categorized as “deregulation of bioenergetics” in the most up to date review of the cancer hallmarks.19 It has also begun to shape the therapeutic landscape as new drug targets have emerged.
IDH inhibitors first to market
A number of new metabolically-targeted treatment strategies are being developed. Most promising are small molecule inhibitors of the isocitrate dehydrogenase (IDH) enzymes. These enzymes play an essential role in the TCA cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, generating carbon dioxide and NADPH. Recurrent mutations in the IDH1 and IDH2 genes have been observed in several different types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
IDH mutations are known as neomorphic mutations because they confer a new function on the altered gene product. In this case, the mutant IDH enzyme converts alpha-ketoglutarate further into D-2-hydroxyglutarate (D-2HG). This molecule has a number of different effects that promote tumorigenesis, including fostering defective DNA repair (Figure 3).20,21
Intriguing research presented at the American Association of Cancer Research Annual Meeting revealed that IDH mutations may make cancer cells more vulnerable to poly (ADP-ribose) polymerase (PARP) inhibition, likely as a result of defects in homologous recombination pathways of DNA repair.22The pursuit of IDH as a potential therapeutic target has yielded the first regulatory approval for a metabolically targeted anticancer therapy. In August 2017, the United States Food and Drug Administration (FDA) approved enasidenib, an IDH2 inhibitor, for the treatment of relapsed or refractory AML with an IDH2 mutation. It was approved in combination with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect IDH2 mutations.
The approval was based on a single-arm trial in which responses occurred in almost a quarter of the 199 patients treated with 100 mg oral enasidenib daily. After a median follow-up of 6.6 months, 23% of the patients experienced a complete response or a complete response with partial hematologic recovery lasting a median of 8.2 months. The most common AEs were nausea, vomiting, diarrhea, elevated bilirubin levels, and reduced appetite.23
Several other IDH inhibitors are also showing encouraging efficacy. Ivosidenib is an IDH1 inhibitor and the results of a phase 1 study in patients with cholangiocarcinoma were recently presented at a leading conference. Escalating doses of ivosidenib (100 mg twice daily to 1,200 mg once daily) were administered to 73 patients (as of December 2016). The confirmed partial response (PR) rate was 6%, the rate of stable disease was 56%, and PFS at 6 months was 40%. There were no dose-limiting toxicities (DLTs) and treatment-emergent AEs included fatigue, nausea, vomiting, diarrhea, decreased appetite, dysgeusia, and QT prolongation.24
Another study of ivosidenib was presented at the 2017 annual meeting of the Society for Neuro-Oncology. In that study, patients with glioma received daily doses of ivosidenib ranging from 300 mg to 900 mg. Two patients had a minor response, 83% had stable disease, and the median PFS was 13 months. There were no DLTs and most AEs were mild to moderate and included, most commonly, headache, nausea, diarrhea, and vomiting.25
Pursuing alternative targets and repurposing drugs
Other metabolic targets that are being pursued include glutaminase, given the observation of significantly enhanced glutamine uptake in cancer cells. CB-839 is a glutaminase inhibitor that is currently being evaluated in phase 1 and 2 clinical trials. Updated clinical trial data from a phase 1 trial of CB-839 in combination with paclitaxel in patients with advanced/metastatic TNBC were presented at the San Antonio Breast Cancer Symposium last year.26
As of October 2017, 49 patients had been treated with 400 mg, 600 mg, or 800 mg CB-839 twice daily in combination with 80 mg/m2 intravenous paclitaxel weekly. Among the 44 patients evaluable for response, the rate of PR was 22% and of disease control, 59%. The one DLT was grade 3 neutropenia at the 400 mg dose. Overall AEs were mostly low grade and reversible.
In recent years, lactate has emerged as more than just a by-product of altered cancer cell metabolism. It is responsible, at least in part, for the highly acidic tumor microenvironment that fosters many of the other hallmarks of cancer. In addition, lactate promotes angiogenesis by upregulating HIF-1α in endothelial cells. Depriving tumor cells of the ability to export lactate is a potentially promising therapeutic strategy. An MCT-1 inhibitor, AZD3965, is being evaluated in early stage clinical trials.
Finally, several drugs that are renowned for their use in other disease settings are being repurposed for cancer therapy because of their potential effects on cancer cell metabolism. Ritonavir, an antiretroviral drug used in the treatment of HIV, is an inhibitor of GLUT-1 and is being evaluated in phase 1 and 2 clinical trials. Meanwhile, long-term studies of metformin, a drug that has revolutionized the treatment of diabetes, have revealed a reduction in the emergence of new cancers in diabetic patients treated who are treated with it, and the drug has been shown to improve breast cancer survival rates. Its precise anticancer effects are somewhat unclear, but it is thought to act in part by inhibiting oxidative phosphorylation. Numerous clinical trials of metformin in different types of cancer are ongoing.27,2
Altered cell metabolism has long been recognized as a distinctive feature of malignant cells but, until recently, research efforts had focused on a single aspect. It has become increasingly evident that many metabolic pathways are altered in cancer cells. Improved understanding has yielded the first regulatory approval in this new class of drugs. Here, we discuss the latest developments in the therapeutic targeting of the cancer metabolism hallmark.
A cancer cell’s sweet tooth
The metabolism of cancer cells differs from that of normal cells, an observation that has spawned a dedicated field of research and new targeted drug development. The German physiologist Otto Warburg is credited as the father of the field with his observations about the way in which cancer cells derive energy from glucose.1
In normal cells, glucose is converted into pyruvate in the cytoplasm, which is then, most often, fed to the mitochondria that use oxidative phosphorylation to produce energy in the form of adenosine triphosphate (ATP). Cancer cells seem instead to favor using the pyruvate to produce lactate through glycolysis (Figure 1).
Glycolysis is usually reserved for conditions of poor oxygen availability, but although the tumor microenvironment is often hypoxic, cancer cells have been shown to use glycolysis even when oxygen is plentiful. As a result, the phenomenon is known as aerobic glycolysis, although it is most often referred to as the Warburg effect.2
Glycolysis is much less efficient than oxidative phosphorylation at producing energy, yielding only 2 ATP. In order to meet their energy demands in this way, cancer cells ramp up their glucose intake, an effect that has been exploited for the detection of cancer with positron-emission tomography.
Warburg postulated that this metabolic shift was a result of mitochondrial damage and defective oxidative phosphorylation, even going so far as to suggest that cancer was a mitochondrial disease. It has subsequently been shown that the mitochondria are mostly intact in cancer cells and that oxidative phosphorylation can still occur.3
The Warburg effect has been the subject of significant investigative efforts as researchers have attempted to better understand how this phenomenon comes about. Studies have shown that it is driven in large part by the transcription factors hypoxia inducible factor 1 alpha (HIF-1α) and c-Myc. In addition, numerous other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway, and the activation of oncogenes and inactivation of tumor suppressors, are thought to play a central role.
HIF-1α is an oxygen-sensing transcription factor that coordinates cellular responses to reduced oxygen levels by binding to specific regions, known as hypoxia response elements, on target genes in the nucleus and regulating their subsequent expression. Oxygen levels and metabolism are tightly linked, and HIF-1α sits at the intersection of the 2 since many of its target genes are involved in metabolic pathways, including many glycolytic enzymes, but it also directly inhibits oxidative phosphorylation by suppressing key enzymes in this metabolic pathway.
The expression of HIF-1α and numerous glycolytic enzymes, including lactate dehydrogenase (LDH), phosphofructokinase (PFK), hexokinase II (HKII), and pyruvate dehydrogenase kinase (PDK) is increased in many tumor types. Other molecules that are associated with glucose uptake and metabolism are also dysregulated, such as the GLUT-1 glucose transporter.2,4-6
Targeting glycolysis and glucose uptake
According to one study, glucose transporters and glycolytic enzymes are overexpressed in 24 different types of cancer, representing more than 70% of all cancer cases.7 This enables cancer cells to respond metabolically as though they are experiencing hypoxia, even when oxygen is plentiful and, indeed, when hypoxia is a concern, to mount a faster response. It also provides a tempting avenue for anticancer drug design by exploiting the dependency of cancer cells on glycolysis to survive and thrive.
Inhibitors of HKII, LDH, PFK, PDK, and GLUT-1 have been and continue to be developed. For example, 2-deoxy-D-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced by hydrogen, preventing further glycolysis; it acts as a competitive inhibitor of HKII. Dichloroacetate (DCA) activates the pyruvate dehydrogenase complex and inhibits the actions of the PDKs. Although development of DCA itself was unsuccessful, DCA derivatives continue to be pursued. WZB117 and STF-31 are novel small-molecule inhibitors of GLUT-1-mediated glucose transport. To date, where inhibitors of glycolysis have progressed into clinical trials, they have not proved successful, often limited by off-target effects and low potency.8-11
A variety of cell signaling pathways are implicated in metabolism by tightly regulating the ability of cells to gain access to and use nutrients. Through aberrations in these pathways, cancer cells can essentially go rogue, ignoring regulatory signals and taking up nutrients in an autonomous manner. One of the most frequently altered signaling pathways in human cancer, the PI3K-Akt-mTOR pathway, is also an important regulator of metabolism, coordinating the uptake of multiple nutrients, including glucose.
Akt in particular is thought to have a critical role in glucose metabolism and increased Akt pathway signaling has been shown to correlate with increased rates of glycolysis in cancer cells. Thus, Akt inhibitors could double as glycolytic or glucose transport inhibitors.12,13
A number of Akt inhibitors are being evaluated in clinical trials (Table) and results from the phase 2 LOTUS trial of ipatasertib (GDC-0068) were recently published.
Among 124 patients randomly assigned to paclitaxel in combination with either ipatasertib or placebo, there was a modest improvement in progression-free survival (PFS) in the ipatasertib arm in patients with triple-negative breast cancer (TNBC; 6 months vs 4.2 months, respectively; hazard ratio [HR], 0.60; P = .037). The effect was more pronounced, though not statistically significant, in patients with phosphatase and tensin homolog (PTEN)-low tumors (6.2 months vs 3.7 months; HR, 0.59; P = .18). The most common grade 3 and higher adverse events (AEs) were diarrhea, reduced neutrophil count, and neutropenia.14
The Warburg paradox
Although the molecular mechanisms underlying the Warburg effect have been revealed to some extent, why cancer cells would choose to use such an energy-inefficient process when they have such high energy demands, remains something of a paradox. It’s still not entirely clear, but several explanations that are not necessarily mutually exclusive have been proposed and relate to the inherent benefits of glycolysis and might explain why cancer cells favor this pathway despite its poor energy yield. First, ATP is produced much more rapidly through glycolysis than oxidative phosphorylation, up to 100 times faster. Thus, using glycolysis is a trade-off, between making less energy and making it more quickly.
Second, cancer cells require more than just ATP to meet their metabolic demands. They need amino acids for protein synthesis; nucleotides for DNA replication; lipids for cell membrane synthesis; nicotinamide adenine dinucleotide phosphate (NADPH), which helps the cancer cell deal with oxidative stress; and various other metabolites. Glycolysis branches off into other metabolic pathways that generate many of these metabolites. Among these branched pathways is the pentose phosphate pathway (PPP), which is required for the generation of ribonucleotides and is a major source for NADPH. Cancer cells have been shown to upregulate the flux of glucose into the PPP to meet their anabolic demands and counter oxidative stress.
Third, the lactic acid produced through glycolysis is actively exported from tumor cells by monocarboxylate transporters (MCTs). This creates a highly acidic tumor microenvironment, which can promote several cancer-related processes and also plays a role in tumor-induced immunosuppression, by inhibiting the activity of tumor-infiltrating T cells, reducing dendritic cell maturation, and promoting the transformation of macrophages to a protumorigenic form.2,4,6
Beyond the Warburg effect
Although the focus has been on glucose metabolism and glycolysis, it has been increasingly recognized that many different metabolic pathways are altered. Fundamental changes to the metabolism of all 4 major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – have been observed, encompassing all aspects of cellular metabolism and enabling cancer cells to meet their complete metabolic requirements. There is also evidence that cancer cells are able to switch between different metabolic pathways depending on the availability of oxygen, their energetic needs, environmental stresses, and many other factors. Certainly, there is significant heterogeneity in the metabolic changes that occur in tumors, which vary from tumor to tumor and even within the same tumor and across the lifespan of a tumor as it progresses from an early stage to more advanced or metastatic disease.
The notion of the Warburg effect as a universal phenomenon in cancer cells is now being widely disregarded. Many tumors continue to use oxidative phosphorylation, particularly slower growing tumors, to meet their energy needs. More recently a “reverse” Warburg effect was described, whereby cancer cells are thought to influence the metabolism of the surrounding stromal fibroblasts and essentially outsource aerobic glycolysis to these cells, while performing energy-efficient oxidative phosphorylation themselves (Figure 2).5,15,16
There is thought to be a “lactate shuttle” between the stromal and cancer cells. The stromal cells express high levels of efflux MCTs so that they can remove the subsequently high levels of lactate from the cytoplasm and avoid pickling themselves. The lactate is then shuttled to the cancer cells that have MCTs on their surface that are involved in lactate uptake. The cancer cells oxidize the lactate back into pyruvate, which can then be used in the tricarboxylic acid (TCA) cycle to feed oxidative phosphorylation for efficient ATP production. This hypothesis reflects a broader appreciation of the role of the microenvironment in contributing to cancer metabolism.17,18
An improved holistic understanding of cancer cell metabolism has led to the recognition of altered cancer metabolism as one of the hallmark abilities required for transformation of a normal cell into a cancerous one. It is categorized as “deregulation of bioenergetics” in the most up to date review of the cancer hallmarks.19 It has also begun to shape the therapeutic landscape as new drug targets have emerged.
IDH inhibitors first to market
A number of new metabolically-targeted treatment strategies are being developed. Most promising are small molecule inhibitors of the isocitrate dehydrogenase (IDH) enzymes. These enzymes play an essential role in the TCA cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, generating carbon dioxide and NADPH. Recurrent mutations in the IDH1 and IDH2 genes have been observed in several different types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
IDH mutations are known as neomorphic mutations because they confer a new function on the altered gene product. In this case, the mutant IDH enzyme converts alpha-ketoglutarate further into D-2-hydroxyglutarate (D-2HG). This molecule has a number of different effects that promote tumorigenesis, including fostering defective DNA repair (Figure 3).20,21
Intriguing research presented at the American Association of Cancer Research Annual Meeting revealed that IDH mutations may make cancer cells more vulnerable to poly (ADP-ribose) polymerase (PARP) inhibition, likely as a result of defects in homologous recombination pathways of DNA repair.22The pursuit of IDH as a potential therapeutic target has yielded the first regulatory approval for a metabolically targeted anticancer therapy. In August 2017, the United States Food and Drug Administration (FDA) approved enasidenib, an IDH2 inhibitor, for the treatment of relapsed or refractory AML with an IDH2 mutation. It was approved in combination with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect IDH2 mutations.
The approval was based on a single-arm trial in which responses occurred in almost a quarter of the 199 patients treated with 100 mg oral enasidenib daily. After a median follow-up of 6.6 months, 23% of the patients experienced a complete response or a complete response with partial hematologic recovery lasting a median of 8.2 months. The most common AEs were nausea, vomiting, diarrhea, elevated bilirubin levels, and reduced appetite.23
Several other IDH inhibitors are also showing encouraging efficacy. Ivosidenib is an IDH1 inhibitor and the results of a phase 1 study in patients with cholangiocarcinoma were recently presented at a leading conference. Escalating doses of ivosidenib (100 mg twice daily to 1,200 mg once daily) were administered to 73 patients (as of December 2016). The confirmed partial response (PR) rate was 6%, the rate of stable disease was 56%, and PFS at 6 months was 40%. There were no dose-limiting toxicities (DLTs) and treatment-emergent AEs included fatigue, nausea, vomiting, diarrhea, decreased appetite, dysgeusia, and QT prolongation.24
Another study of ivosidenib was presented at the 2017 annual meeting of the Society for Neuro-Oncology. In that study, patients with glioma received daily doses of ivosidenib ranging from 300 mg to 900 mg. Two patients had a minor response, 83% had stable disease, and the median PFS was 13 months. There were no DLTs and most AEs were mild to moderate and included, most commonly, headache, nausea, diarrhea, and vomiting.25
Pursuing alternative targets and repurposing drugs
Other metabolic targets that are being pursued include glutaminase, given the observation of significantly enhanced glutamine uptake in cancer cells. CB-839 is a glutaminase inhibitor that is currently being evaluated in phase 1 and 2 clinical trials. Updated clinical trial data from a phase 1 trial of CB-839 in combination with paclitaxel in patients with advanced/metastatic TNBC were presented at the San Antonio Breast Cancer Symposium last year.26
As of October 2017, 49 patients had been treated with 400 mg, 600 mg, or 800 mg CB-839 twice daily in combination with 80 mg/m2 intravenous paclitaxel weekly. Among the 44 patients evaluable for response, the rate of PR was 22% and of disease control, 59%. The one DLT was grade 3 neutropenia at the 400 mg dose. Overall AEs were mostly low grade and reversible.
In recent years, lactate has emerged as more than just a by-product of altered cancer cell metabolism. It is responsible, at least in part, for the highly acidic tumor microenvironment that fosters many of the other hallmarks of cancer. In addition, lactate promotes angiogenesis by upregulating HIF-1α in endothelial cells. Depriving tumor cells of the ability to export lactate is a potentially promising therapeutic strategy. An MCT-1 inhibitor, AZD3965, is being evaluated in early stage clinical trials.
Finally, several drugs that are renowned for their use in other disease settings are being repurposed for cancer therapy because of their potential effects on cancer cell metabolism. Ritonavir, an antiretroviral drug used in the treatment of HIV, is an inhibitor of GLUT-1 and is being evaluated in phase 1 and 2 clinical trials. Meanwhile, long-term studies of metformin, a drug that has revolutionized the treatment of diabetes, have revealed a reduction in the emergence of new cancers in diabetic patients treated who are treated with it, and the drug has been shown to improve breast cancer survival rates. Its precise anticancer effects are somewhat unclear, but it is thought to act in part by inhibiting oxidative phosphorylation. Numerous clinical trials of metformin in different types of cancer are ongoing.27,2
1. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270.
2. Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908-38926.
3. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314.
4. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
5. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68.
6. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155-171.
7. Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014-1020.
8. Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430-3440.
9. Zhang W, Zhang SL, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390-1400.
10. Talekar M, Boreddy SR, Singh A, Amiji M. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opin Biol Ther. 2014;14(8):1145-1159.
11. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
12. Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In: Cramer T, Schmitt CA, eds. Metabolism in Cancer. Cham, Switzerland: Springer International Publishing; 2016:39-72.
13. Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D (eds). Oxidative stress in cancer biology and therapy. Humana Press (copyright holder, Springer Science+Business Media, LLC); 2012:21-46.
14. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372.
15. Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813-57825.
16. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198-203.
17. Brooks GA. Cell–cell and intracellular lactate shuttles. Journal Physiol. 2009;587(23):5591-5600.
18. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272-280.
19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
20. Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373-380.
21. Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370.
22. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709-1718.
23. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
24. Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015-4015.
25. Mellinghoff IK, Touat M, Maher E, et al. ACTR-46. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(suppl_6):vi10-vi11.
26. Kalinsky K, Harding J, DeMichele A, et al. Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas.
27. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int. 2015;2015:548436.
28. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376.
1. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270.
2. Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908-38926.
3. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314.
4. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
5. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68.
6. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155-171.
7. Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014-1020.
8. Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430-3440.
9. Zhang W, Zhang SL, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390-1400.
10. Talekar M, Boreddy SR, Singh A, Amiji M. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opin Biol Ther. 2014;14(8):1145-1159.
11. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
12. Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In: Cramer T, Schmitt CA, eds. Metabolism in Cancer. Cham, Switzerland: Springer International Publishing; 2016:39-72.
13. Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D (eds). Oxidative stress in cancer biology and therapy. Humana Press (copyright holder, Springer Science+Business Media, LLC); 2012:21-46.
14. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372.
15. Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813-57825.
16. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198-203.
17. Brooks GA. Cell–cell and intracellular lactate shuttles. Journal Physiol. 2009;587(23):5591-5600.
18. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272-280.
19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
20. Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373-380.
21. Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370.
22. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709-1718.
23. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
24. Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015-4015.
25. Mellinghoff IK, Touat M, Maher E, et al. ACTR-46. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(suppl_6):vi10-vi11.
26. Kalinsky K, Harding J, DeMichele A, et al. Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas.
27. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int. 2015;2015:548436.
28. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376.
David Henry's JCSO podcast, November-December 2017
In the final JCSO podcast for 2017, Dr David Henry, the Editor-in-Chief, discusses a round-up by Dr Linda Bosserman of cancer care in 2017 and an accompanying article on the therapeutic implications of some of the year’s landmark approvals. Continuing with the theme of therapeutic advances, he also highlights a review of the expanding treatment options for diverse neuroendocrine tumors. Two articles, one on setting up systems to facilitate adoption of the oncology care model in the practice setting and another on the clinical presentation, diagnosis, and management of bronchopulmonary carcinoid, provide evidence-based information and guidelines for practice-based oncology care teams. Patient and survivor care are the focus of three original research reports in which authors report on supportive medications and interventions received by prostate cancer survivors (PiCTure study); differences in psychosocial stressors between black and white cancer patients; and the impact of combining human and online supportive resources for prostate cancer patients. Also discussed are case reports on metastatic eccrine carcinoma with stomach and pericardial involvement and on cold hemolytic anemia as a rare complication of influenza A, as well as the approvals of pembrolizumab for dMMR/MSI-H tumors and brigatinib for crizotinib-resistant, ALK-positive NSCLC patients.
Listen to the podcast below.
In the final JCSO podcast for 2017, Dr David Henry, the Editor-in-Chief, discusses a round-up by Dr Linda Bosserman of cancer care in 2017 and an accompanying article on the therapeutic implications of some of the year’s landmark approvals. Continuing with the theme of therapeutic advances, he also highlights a review of the expanding treatment options for diverse neuroendocrine tumors. Two articles, one on setting up systems to facilitate adoption of the oncology care model in the practice setting and another on the clinical presentation, diagnosis, and management of bronchopulmonary carcinoid, provide evidence-based information and guidelines for practice-based oncology care teams. Patient and survivor care are the focus of three original research reports in which authors report on supportive medications and interventions received by prostate cancer survivors (PiCTure study); differences in psychosocial stressors between black and white cancer patients; and the impact of combining human and online supportive resources for prostate cancer patients. Also discussed are case reports on metastatic eccrine carcinoma with stomach and pericardial involvement and on cold hemolytic anemia as a rare complication of influenza A, as well as the approvals of pembrolizumab for dMMR/MSI-H tumors and brigatinib for crizotinib-resistant, ALK-positive NSCLC patients.
Listen to the podcast below.
In the final JCSO podcast for 2017, Dr David Henry, the Editor-in-Chief, discusses a round-up by Dr Linda Bosserman of cancer care in 2017 and an accompanying article on the therapeutic implications of some of the year’s landmark approvals. Continuing with the theme of therapeutic advances, he also highlights a review of the expanding treatment options for diverse neuroendocrine tumors. Two articles, one on setting up systems to facilitate adoption of the oncology care model in the practice setting and another on the clinical presentation, diagnosis, and management of bronchopulmonary carcinoid, provide evidence-based information and guidelines for practice-based oncology care teams. Patient and survivor care are the focus of three original research reports in which authors report on supportive medications and interventions received by prostate cancer survivors (PiCTure study); differences in psychosocial stressors between black and white cancer patients; and the impact of combining human and online supportive resources for prostate cancer patients. Also discussed are case reports on metastatic eccrine carcinoma with stomach and pericardial involvement and on cold hemolytic anemia as a rare complication of influenza A, as well as the approvals of pembrolizumab for dMMR/MSI-H tumors and brigatinib for crizotinib-resistant, ALK-positive NSCLC patients.
Listen to the podcast below.
Targeted therapies forge ahead in multiple breast cancer subtypes
As our understanding of the biology of breast cancer has improved, treatment has become increasingly personalized. Targeted therapies continue to significantly improve patient outcomes in multiple subtypes, with several recent drug approvals. Here, we discuss some of these latest developments.
A disease of many faces
Clinically speaking, breast cancers can be divided into at least 5 subtypes on the basis of the genes they express (Figure 1). The luminal subtypes make up the largest proportion and are characterized by the expression of hormone receptor (HR) genes. Luminal A tumors are negative for human epidermal growth factor receptor 2 (HER2; HER2-negative), whereas luminal B tumors often co-express the HER2 genes.1
The remainder of HER2-positive patients fall into the HER2-enriched category, in which HER2 expression is the defining characteristic. Basal-like tumors, meanwhile, represent the most heterogeneous subtype, overlapping to a large extent with tumors dubbed “triple-negative” because of their lack of either HER2 or ESR1 and PGR gene expression. The fifth subtype is known as normal breast-like and remains poorly characterized.
In recent years, there have been significant advancements in the genomic characterization of breast cancer that have begun to provide a more comprehensive understanding of the driver molecular mechanisms, which has helped to explain some of the limitations of current targeted approaches and to reveal new possible treatments, with a shift toward increasingly personalized strategies.2
HER2: what’s neu?
An estimated 18%-20% of breast tumors are HER2 positive, displaying amplification of the HER2/neu gene or overexpression of its protein product.3 Historically, HER2 positivity correlated with a highly aggressive and metastatic form of disease, conferring poor prognosis.4,5 The HER2-targeted monoclonal antibody (mAb), trastuzumab serves as a prime example of the power of personalized medicine. Evidence suggests that trastuzumab has altered the natural history of HER2-positive breast cancer, such that trastuzumab-treated patients with HER2-positive breast cancer now have a better prognosis than do patients with HER2-negative disease.6,7
Several additional HER2-targeted drugs have joined trastuzumab on the market, including other mAbs, small molecule tyrosine kinase inhibitors (TKIs), and an antibody–drug conjugate that combines the specificity of a mAb with the anti-tumor potency of a cytotoxic drug. These drugs have further improved patient outcomes in both early and advanced disease settings (Table 1).
The most recent regulatory approval was for neratinib, a potent TKI inhibiting all members of the HER protein family. On the basis of the phase 3 ExteNET study, neratinib was granted approval by the US Food and Drug Administration (FDA) for extended adjuvant treatment of patients with HER2-positive, early-stage breast cancer previously treated with trastuzumab. In a 5-year analysis of the study, invasive disease-free survival (DFS) was 90.4% with neratinib, compared with 87.9% with placebo (hazard ratio [HR], 0.74; P = .017).8,9
The tide of advancements in HER2-targeted therapy looks set to continue in the coming years as potentially practice-changing data emerges from ongoing clinical trials and, as the patent on trastuzumab has expired, a number of biosimilars, such as MYL-1401O have the potential to help patients who may not have access to trastuzumab.10
One of the biggest remaining challenges is identifying drugs that can effectively treat patients with brain metastases because the blood–brain barrier presents an impediment to the delivery of effective concentrations of anticancer drugs. Initially, it was hoped that the small molecule inhibitors lapatinib and neratinib could cross the blood–brain barrier and may be more effective in patients with brain metastases, but that hypothesis has not borne out in randomized clinical trials.11
Tucatinib (ONT-380) has shown significant promise in this respect. In a phase 1 trial, ONT-380 had significant efficacy in patients with and without central nervous system metastases; the overall response rate (ORR) in the CNS was 36%. ONT-380 is also notable for its specificity for HER2, without significant inhibition of HER1 and EGFR, which could translate into a better toxicity profile.12
Doubling down on resistant tumors
Since the success of HER2-targeted therapy is limited by the development of resistance, there has been significant interest in assessing the potential of dual HER2 blockade, exploiting the unique mechanisms of action of different drugs in combination therapy, and ensuring more complete inhibition of the HER2 pathway. Although numerous different combinations have been tested, a double antibody combination has proved most effective.
In fact, dual HER2 targeting with trastuzumab and pertuzumab in combination with chemotherapy has replaced a trastuzumab-chemotherapy regimen as the new standard of care in the metastatic setting. A 6-month improvement in progression-free survival (PFS) sealed FDA approval for the combination and in a recently published final analysis of the trial overall survival (OS) was also improved to a level unprecedented in the first-line setting.13,14The double antibody combination has also been successful in the neoadjuvant setting. Approval followed the results of the phase 2 NeoSphere trial, in which the combination was associated with a significant improvement in pathologic complete response (pCR) rate, a measure that acts as a surrogate for improved survival in the neoadjuvant setting. In a 5-year analysis of the NeoSphere trial, improved pCR did indeed translate into improved PFS and DFS.15,16
The results of the phase 3 APHINITY trial evaluating this combination in the adjuvant setting have been hotly anticipated. In a presentation at the 2017 American Society of Clinical Oncology (ASCO) meeting in June, the study authors reported that in 4,085 patients with operable HER2-positive disease, it significantly reduced the risk of disease recurrence or death compared with trastuzumab and chemotherapy alone.17
There is an ongoing effort to determine if it is possible to de-escalate treatment by removing the chemotherapy component. At least in the neoadjuvant setting, pCR rates in the chemotherapy-free arms of several studies suggest that a proportion of patients might benefit from this strategy15,18,19 and the challenge now is to identify them. To that end, the phase 2 PAMELA trial identified the HER2-enriched subtype as a strong predictor of response to neoadjuvant dual blockade (lapatinib and trastuzumab) without chemotherapy. The pCR rate was 40.6% for the combination in patients with the HER2-enriched subtype of breast cancer and only 10% in patients with non–HER2-enriched tumors.20
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.
PARP inhibitors make a dent in BRCA1/2-mutated cancers
The most renowned breast cancer genes, BRCA1 and BRCA2 are present in about 5%-10% of all breast cancers. They play a central role in the homologous recombination pathway that fixes double-strand breaks in the DNA. Genome sequencing studies have revealed that the presence of the BRCA1/2 genes and other DNA repair defects is highest among patients with the basal-like subtype of breast cancer, in particular those who have triple-negative disease.34,35
This type of breast cancer has proved stubbornly resistant to efforts to improve patient outcomes with targeted therapies. BRCA1/2 mutations and other DNA repair defects that confer a so-called BRCAness phenotype, render tumor cells dependent on other pathways for DNA repair and there has been considerable interest in therapeutically exploiting this through the development of inhibitors of the poly(ADP-ribose) polymerase (PARP) enzyme, which is involved in the repair of single-strand breaks in the DNA. The double damage to DNA repair mechanisms through PARP inhibition in patients with BRCA1/2-mutant tumors proves overwhelming to cancerous cells.
Despite more than a decade of investigation in breast cancer, PARP inhibitors have yet to yield any FDA-approved treatment options. That may be set to change imminently, following the success of olaparib (Table 3). In the first randomized phase 3 trial of a PARP inhibitor in breast cancer (OlympiAD), olaparib was compared with standard chemotherapy in patients with BRCA1/2-mutated MBC who had received up to 2 previous lines of chemotherapy. Olaparib reduced the risk of disease progression by 42% compared with standard chemotherapy and was well tolerated.36
The novel PARP inhibitor talazoparib, which is the most potent to date, is also demonstrating significant efficacy in clinical trials. The results of the phase 2 ABRAZO trial were presented at the ASCO annual meeting. Two cohorts were treated; the first included 49 patients who had responded to their last platinum-containing regimen for metastatic disease and progressed more than 8 weeks after last platinum dose and the other included 35 patients previously treated with 3 or more nonplatinum regimens for metastatic disease. ORR was 28% across the 2 cohorts; 23% and 33% in BRCA1- and BRCA2-mutant carriers, respectively; and 26% in patients with triple-negative breast cancer.37 PARP inhibition is not faring so well in early-stage triple-negative disease; a phase 3 trial of veliparib in combination with chemotherapy did not meet its primary endpoint.38
As our understanding of the biology of breast cancer has improved, treatment has become increasingly personalized. Targeted therapies continue to significantly improve patient outcomes in multiple subtypes, with several recent drug approvals. Here, we discuss some of these latest developments.
A disease of many faces
Clinically speaking, breast cancers can be divided into at least 5 subtypes on the basis of the genes they express (Figure 1). The luminal subtypes make up the largest proportion and are characterized by the expression of hormone receptor (HR) genes. Luminal A tumors are negative for human epidermal growth factor receptor 2 (HER2; HER2-negative), whereas luminal B tumors often co-express the HER2 genes.1
The remainder of HER2-positive patients fall into the HER2-enriched category, in which HER2 expression is the defining characteristic. Basal-like tumors, meanwhile, represent the most heterogeneous subtype, overlapping to a large extent with tumors dubbed “triple-negative” because of their lack of either HER2 or ESR1 and PGR gene expression. The fifth subtype is known as normal breast-like and remains poorly characterized.
In recent years, there have been significant advancements in the genomic characterization of breast cancer that have begun to provide a more comprehensive understanding of the driver molecular mechanisms, which has helped to explain some of the limitations of current targeted approaches and to reveal new possible treatments, with a shift toward increasingly personalized strategies.2
HER2: what’s neu?
An estimated 18%-20% of breast tumors are HER2 positive, displaying amplification of the HER2/neu gene or overexpression of its protein product.3 Historically, HER2 positivity correlated with a highly aggressive and metastatic form of disease, conferring poor prognosis.4,5 The HER2-targeted monoclonal antibody (mAb), trastuzumab serves as a prime example of the power of personalized medicine. Evidence suggests that trastuzumab has altered the natural history of HER2-positive breast cancer, such that trastuzumab-treated patients with HER2-positive breast cancer now have a better prognosis than do patients with HER2-negative disease.6,7
Several additional HER2-targeted drugs have joined trastuzumab on the market, including other mAbs, small molecule tyrosine kinase inhibitors (TKIs), and an antibody–drug conjugate that combines the specificity of a mAb with the anti-tumor potency of a cytotoxic drug. These drugs have further improved patient outcomes in both early and advanced disease settings (Table 1).
The most recent regulatory approval was for neratinib, a potent TKI inhibiting all members of the HER protein family. On the basis of the phase 3 ExteNET study, neratinib was granted approval by the US Food and Drug Administration (FDA) for extended adjuvant treatment of patients with HER2-positive, early-stage breast cancer previously treated with trastuzumab. In a 5-year analysis of the study, invasive disease-free survival (DFS) was 90.4% with neratinib, compared with 87.9% with placebo (hazard ratio [HR], 0.74; P = .017).8,9
The tide of advancements in HER2-targeted therapy looks set to continue in the coming years as potentially practice-changing data emerges from ongoing clinical trials and, as the patent on trastuzumab has expired, a number of biosimilars, such as MYL-1401O have the potential to help patients who may not have access to trastuzumab.10
One of the biggest remaining challenges is identifying drugs that can effectively treat patients with brain metastases because the blood–brain barrier presents an impediment to the delivery of effective concentrations of anticancer drugs. Initially, it was hoped that the small molecule inhibitors lapatinib and neratinib could cross the blood–brain barrier and may be more effective in patients with brain metastases, but that hypothesis has not borne out in randomized clinical trials.11
Tucatinib (ONT-380) has shown significant promise in this respect. In a phase 1 trial, ONT-380 had significant efficacy in patients with and without central nervous system metastases; the overall response rate (ORR) in the CNS was 36%. ONT-380 is also notable for its specificity for HER2, without significant inhibition of HER1 and EGFR, which could translate into a better toxicity profile.12
Doubling down on resistant tumors
Since the success of HER2-targeted therapy is limited by the development of resistance, there has been significant interest in assessing the potential of dual HER2 blockade, exploiting the unique mechanisms of action of different drugs in combination therapy, and ensuring more complete inhibition of the HER2 pathway. Although numerous different combinations have been tested, a double antibody combination has proved most effective.
In fact, dual HER2 targeting with trastuzumab and pertuzumab in combination with chemotherapy has replaced a trastuzumab-chemotherapy regimen as the new standard of care in the metastatic setting. A 6-month improvement in progression-free survival (PFS) sealed FDA approval for the combination and in a recently published final analysis of the trial overall survival (OS) was also improved to a level unprecedented in the first-line setting.13,14The double antibody combination has also been successful in the neoadjuvant setting. Approval followed the results of the phase 2 NeoSphere trial, in which the combination was associated with a significant improvement in pathologic complete response (pCR) rate, a measure that acts as a surrogate for improved survival in the neoadjuvant setting. In a 5-year analysis of the NeoSphere trial, improved pCR did indeed translate into improved PFS and DFS.15,16
The results of the phase 3 APHINITY trial evaluating this combination in the adjuvant setting have been hotly anticipated. In a presentation at the 2017 American Society of Clinical Oncology (ASCO) meeting in June, the study authors reported that in 4,085 patients with operable HER2-positive disease, it significantly reduced the risk of disease recurrence or death compared with trastuzumab and chemotherapy alone.17
There is an ongoing effort to determine if it is possible to de-escalate treatment by removing the chemotherapy component. At least in the neoadjuvant setting, pCR rates in the chemotherapy-free arms of several studies suggest that a proportion of patients might benefit from this strategy15,18,19 and the challenge now is to identify them. To that end, the phase 2 PAMELA trial identified the HER2-enriched subtype as a strong predictor of response to neoadjuvant dual blockade (lapatinib and trastuzumab) without chemotherapy. The pCR rate was 40.6% for the combination in patients with the HER2-enriched subtype of breast cancer and only 10% in patients with non–HER2-enriched tumors.20
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.
PARP inhibitors make a dent in BRCA1/2-mutated cancers
The most renowned breast cancer genes, BRCA1 and BRCA2 are present in about 5%-10% of all breast cancers. They play a central role in the homologous recombination pathway that fixes double-strand breaks in the DNA. Genome sequencing studies have revealed that the presence of the BRCA1/2 genes and other DNA repair defects is highest among patients with the basal-like subtype of breast cancer, in particular those who have triple-negative disease.34,35
This type of breast cancer has proved stubbornly resistant to efforts to improve patient outcomes with targeted therapies. BRCA1/2 mutations and other DNA repair defects that confer a so-called BRCAness phenotype, render tumor cells dependent on other pathways for DNA repair and there has been considerable interest in therapeutically exploiting this through the development of inhibitors of the poly(ADP-ribose) polymerase (PARP) enzyme, which is involved in the repair of single-strand breaks in the DNA. The double damage to DNA repair mechanisms through PARP inhibition in patients with BRCA1/2-mutant tumors proves overwhelming to cancerous cells.
Despite more than a decade of investigation in breast cancer, PARP inhibitors have yet to yield any FDA-approved treatment options. That may be set to change imminently, following the success of olaparib (Table 3). In the first randomized phase 3 trial of a PARP inhibitor in breast cancer (OlympiAD), olaparib was compared with standard chemotherapy in patients with BRCA1/2-mutated MBC who had received up to 2 previous lines of chemotherapy. Olaparib reduced the risk of disease progression by 42% compared with standard chemotherapy and was well tolerated.36
The novel PARP inhibitor talazoparib, which is the most potent to date, is also demonstrating significant efficacy in clinical trials. The results of the phase 2 ABRAZO trial were presented at the ASCO annual meeting. Two cohorts were treated; the first included 49 patients who had responded to their last platinum-containing regimen for metastatic disease and progressed more than 8 weeks after last platinum dose and the other included 35 patients previously treated with 3 or more nonplatinum regimens for metastatic disease. ORR was 28% across the 2 cohorts; 23% and 33% in BRCA1- and BRCA2-mutant carriers, respectively; and 26% in patients with triple-negative breast cancer.37 PARP inhibition is not faring so well in early-stage triple-negative disease; a phase 3 trial of veliparib in combination with chemotherapy did not meet its primary endpoint.38
As our understanding of the biology of breast cancer has improved, treatment has become increasingly personalized. Targeted therapies continue to significantly improve patient outcomes in multiple subtypes, with several recent drug approvals. Here, we discuss some of these latest developments.
A disease of many faces
Clinically speaking, breast cancers can be divided into at least 5 subtypes on the basis of the genes they express (Figure 1). The luminal subtypes make up the largest proportion and are characterized by the expression of hormone receptor (HR) genes. Luminal A tumors are negative for human epidermal growth factor receptor 2 (HER2; HER2-negative), whereas luminal B tumors often co-express the HER2 genes.1
The remainder of HER2-positive patients fall into the HER2-enriched category, in which HER2 expression is the defining characteristic. Basal-like tumors, meanwhile, represent the most heterogeneous subtype, overlapping to a large extent with tumors dubbed “triple-negative” because of their lack of either HER2 or ESR1 and PGR gene expression. The fifth subtype is known as normal breast-like and remains poorly characterized.
In recent years, there have been significant advancements in the genomic characterization of breast cancer that have begun to provide a more comprehensive understanding of the driver molecular mechanisms, which has helped to explain some of the limitations of current targeted approaches and to reveal new possible treatments, with a shift toward increasingly personalized strategies.2
HER2: what’s neu?
An estimated 18%-20% of breast tumors are HER2 positive, displaying amplification of the HER2/neu gene or overexpression of its protein product.3 Historically, HER2 positivity correlated with a highly aggressive and metastatic form of disease, conferring poor prognosis.4,5 The HER2-targeted monoclonal antibody (mAb), trastuzumab serves as a prime example of the power of personalized medicine. Evidence suggests that trastuzumab has altered the natural history of HER2-positive breast cancer, such that trastuzumab-treated patients with HER2-positive breast cancer now have a better prognosis than do patients with HER2-negative disease.6,7
Several additional HER2-targeted drugs have joined trastuzumab on the market, including other mAbs, small molecule tyrosine kinase inhibitors (TKIs), and an antibody–drug conjugate that combines the specificity of a mAb with the anti-tumor potency of a cytotoxic drug. These drugs have further improved patient outcomes in both early and advanced disease settings (Table 1).
The most recent regulatory approval was for neratinib, a potent TKI inhibiting all members of the HER protein family. On the basis of the phase 3 ExteNET study, neratinib was granted approval by the US Food and Drug Administration (FDA) for extended adjuvant treatment of patients with HER2-positive, early-stage breast cancer previously treated with trastuzumab. In a 5-year analysis of the study, invasive disease-free survival (DFS) was 90.4% with neratinib, compared with 87.9% with placebo (hazard ratio [HR], 0.74; P = .017).8,9
The tide of advancements in HER2-targeted therapy looks set to continue in the coming years as potentially practice-changing data emerges from ongoing clinical trials and, as the patent on trastuzumab has expired, a number of biosimilars, such as MYL-1401O have the potential to help patients who may not have access to trastuzumab.10
One of the biggest remaining challenges is identifying drugs that can effectively treat patients with brain metastases because the blood–brain barrier presents an impediment to the delivery of effective concentrations of anticancer drugs. Initially, it was hoped that the small molecule inhibitors lapatinib and neratinib could cross the blood–brain barrier and may be more effective in patients with brain metastases, but that hypothesis has not borne out in randomized clinical trials.11
Tucatinib (ONT-380) has shown significant promise in this respect. In a phase 1 trial, ONT-380 had significant efficacy in patients with and without central nervous system metastases; the overall response rate (ORR) in the CNS was 36%. ONT-380 is also notable for its specificity for HER2, without significant inhibition of HER1 and EGFR, which could translate into a better toxicity profile.12
Doubling down on resistant tumors
Since the success of HER2-targeted therapy is limited by the development of resistance, there has been significant interest in assessing the potential of dual HER2 blockade, exploiting the unique mechanisms of action of different drugs in combination therapy, and ensuring more complete inhibition of the HER2 pathway. Although numerous different combinations have been tested, a double antibody combination has proved most effective.
In fact, dual HER2 targeting with trastuzumab and pertuzumab in combination with chemotherapy has replaced a trastuzumab-chemotherapy regimen as the new standard of care in the metastatic setting. A 6-month improvement in progression-free survival (PFS) sealed FDA approval for the combination and in a recently published final analysis of the trial overall survival (OS) was also improved to a level unprecedented in the first-line setting.13,14The double antibody combination has also been successful in the neoadjuvant setting. Approval followed the results of the phase 2 NeoSphere trial, in which the combination was associated with a significant improvement in pathologic complete response (pCR) rate, a measure that acts as a surrogate for improved survival in the neoadjuvant setting. In a 5-year analysis of the NeoSphere trial, improved pCR did indeed translate into improved PFS and DFS.15,16
The results of the phase 3 APHINITY trial evaluating this combination in the adjuvant setting have been hotly anticipated. In a presentation at the 2017 American Society of Clinical Oncology (ASCO) meeting in June, the study authors reported that in 4,085 patients with operable HER2-positive disease, it significantly reduced the risk of disease recurrence or death compared with trastuzumab and chemotherapy alone.17
There is an ongoing effort to determine if it is possible to de-escalate treatment by removing the chemotherapy component. At least in the neoadjuvant setting, pCR rates in the chemotherapy-free arms of several studies suggest that a proportion of patients might benefit from this strategy15,18,19 and the challenge now is to identify them. To that end, the phase 2 PAMELA trial identified the HER2-enriched subtype as a strong predictor of response to neoadjuvant dual blockade (lapatinib and trastuzumab) without chemotherapy. The pCR rate was 40.6% for the combination in patients with the HER2-enriched subtype of breast cancer and only 10% in patients with non–HER2-enriched tumors.20
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.
PARP inhibitors make a dent in BRCA1/2-mutated cancers
The most renowned breast cancer genes, BRCA1 and BRCA2 are present in about 5%-10% of all breast cancers. They play a central role in the homologous recombination pathway that fixes double-strand breaks in the DNA. Genome sequencing studies have revealed that the presence of the BRCA1/2 genes and other DNA repair defects is highest among patients with the basal-like subtype of breast cancer, in particular those who have triple-negative disease.34,35
This type of breast cancer has proved stubbornly resistant to efforts to improve patient outcomes with targeted therapies. BRCA1/2 mutations and other DNA repair defects that confer a so-called BRCAness phenotype, render tumor cells dependent on other pathways for DNA repair and there has been considerable interest in therapeutically exploiting this through the development of inhibitors of the poly(ADP-ribose) polymerase (PARP) enzyme, which is involved in the repair of single-strand breaks in the DNA. The double damage to DNA repair mechanisms through PARP inhibition in patients with BRCA1/2-mutant tumors proves overwhelming to cancerous cells.
Despite more than a decade of investigation in breast cancer, PARP inhibitors have yet to yield any FDA-approved treatment options. That may be set to change imminently, following the success of olaparib (Table 3). In the first randomized phase 3 trial of a PARP inhibitor in breast cancer (OlympiAD), olaparib was compared with standard chemotherapy in patients with BRCA1/2-mutated MBC who had received up to 2 previous lines of chemotherapy. Olaparib reduced the risk of disease progression by 42% compared with standard chemotherapy and was well tolerated.36
The novel PARP inhibitor talazoparib, which is the most potent to date, is also demonstrating significant efficacy in clinical trials. The results of the phase 2 ABRAZO trial were presented at the ASCO annual meeting. Two cohorts were treated; the first included 49 patients who had responded to their last platinum-containing regimen for metastatic disease and progressed more than 8 weeks after last platinum dose and the other included 35 patients previously treated with 3 or more nonplatinum regimens for metastatic disease. ORR was 28% across the 2 cohorts; 23% and 33% in BRCA1- and BRCA2-mutant carriers, respectively; and 26% in patients with triple-negative breast cancer.37 PARP inhibition is not faring so well in early-stage triple-negative disease; a phase 3 trial of veliparib in combination with chemotherapy did not meet its primary endpoint.38
The effect of centralizing breast cancer care in an urban public hospital
When cancer care is centralized in a comprehensive fashion, the quality of care and the outcomes improve.1,2 Unfortunately, because of the medical insurance structure in New York City, most patients of lower socioeconomic status do not receive their cancer care in such dedicated cancer centers. In New York City, the majority of the underserved vulnerable populations – that is, those without health insurance – receive their care from the public hospital system known as NYC Health and Hospitals. Cancer care in this system is not centralized and may result in fragmented implementation of various modalities of treatment. In addition, because there is no centralized care, needs such as early screening and prevention programs are often not addressed. This problem was evident in Queens in 2000 and before when many patients with late-stage cancers were presenting for cancer care. Queens, which is one of the 5 boroughs of New York City, has more than 2.3 million residents. It has 2 public hospitals, Elmhurst Hospital Center and Queens Hospital Center (QHC). In 2001, the plan was devised for the establishment of a cancer center at QHC, mainly because of the high rate of late-stage cancers that were being seen at presentation and recognition of the need for more comprehensive care. In 2002, the Queens Cancer Center (QCC) began to see patients. QCC is a single facility that provides medical, surgical, radiation, gynecologic, and urologic oncology all in one area of the QHC.
This study is an investigation of the possible impact on care for breast cancer patients of low socioeconomic status who were treated at a comprehensive cancer center, with specific consideration of the change or improvement in treatment modalities and outcomes. Data on treatment modalities and outcomes of cancer patients who were treated at the QHC during 2000, before the QCC was set up, were compared with data of patients treated during 2008 (2008 was selected because we have 5-year survival data for those patients). The public hospital system treats all patients regardless of their ability to pay, so the majority of patients in the system are of lower socioeconomic status. In addition, 92% of the patients seen QHC are from a minority population. These are the populations that tend to have a worse prognosis and often are not given optimal treatment.3 The payer mix of patients in the public hospital system is different than that of private hospitals. Most of the patients present at the hospital with no insurance and if they are diagnosed with cancer they may be converted to emergency Medicaid. About 10% of patients will not be converted because of their document status.
Patients and methods
We used the Queens Hospital Tumor Registry to identify the patients who had been diagnosed with and treated for breast cancer in 2000 and 2008. The electronic medical records were reviewed, and in the case of the 2000-year patients, the written charts were also reviewed. The study was approved by the Mount Sinai institutional review board. It was not necessary to obtain patient consent because it was a retrospective study.
Only patients diagnosed with stage 0, I, II, or III breast cancer who received their treatment at QHC were included in the study. Patients who were seen in consultation at QHC but not treated there were excluded. Statistics were done using the 2x2 chi-squared SPSS analysis; a P value of .05 was considered significant. The survival data was analyzed using SAS.
Results
There were 24 evaluable patients in 2000 and 78 evaluable patients in 2008 who had stage 0, I, II, or III primary breast cancer and were treated at QHC. The average age of the patients in 2000 was 53.5 years and 54.7 years in 2008. The mean age for both groups was 55 years. The patients were ethnically diverse in both groups with 46% black, 17% Hispanic, 25% ethnic Asian Indian, and 6% white (Figure 1).
The payer mix in 2000 was 9 patients (37.5%) self-pay, 7 (29%) Medicaid, and 8 (33%) Medicare. In 2008, 11 patients (14%) were self-pay, 46 (59%) Medicaid, 11 (14%) Medicare, and 10 (13%) were private insurance. In 2000, there were 3 (12%) patients with stage 0 disease, 5 (21%) with stage I; 9 (37.5%) with stage II, and 7 (29%) with stage III. In 2008 there were 28 (36%) patients with stage 0 disease, 15 (19%) with stage I, 17 (22%) with stage II, and 18 (23%) with stage III (Figure 2).
None of those values are statistically different. In 2000, 2 of the 24 patients had lumpectomies (partial mastectomy) and the rest had mastectomies. In 2008, 39 (50%) patients had mastectomy and 39 (50%) had lumpectomies (Figure 3). This was a statistically significant difference.
Radiation was given to both patients with lumpectomy in the 2000 group. In the 2008 group, all patients with lumpectomies were evaluated for radiation, and 6 of them did not receive radiation for the following reasons: 3 had very small foci of ductal carcinoma in situ (DCIS) and were treated with hormone therapy and no radiation; 1 patient had a lumpectomy for stage 1 cancer and also did not get radiation therapy because of a low oncotype and very small lesion; 2 patients were older than 70 years and had DCIS and were treated with tamoxifen alone as per NCCN Guidelines for women in that age group. The rest of the patients with lumpectomies received postoperative radiation.
Hormone and HER2 (human epidermal growth factor receptor 2) status was obtained on all patients. For the 2000 patients, 71% had 1 hormone receptor–positive (estrogen receptor [ER] or progesterone receptor [PR]), 21% were triple negative (ER-PR and HER2-neu), and 42% had HER2-neu–positive tumors. For the 2008, patients 65% were positive for 1 hormone receptor (ER or PR), 28% were triple negative (ER-PR and HER2-neu), and 7% had HER2-neu-positive tumors.
All patients were offered chemotherapy and hormone therapy if appropriate, as per NCCN guidelines. If a patient’s tumor was found to be HER2-positive, then the chemotherapy regimen would include the use of trastuzumab in both groups.
The 5-year survival for the 2008 stage III patients was 73.7%, compared with 14.2% for the 2000 stage III patients. The only deaths in the 2008 group were in patients with stage III disease. In the 2000 group, 4 of the 5 patients with stage III cancer died, and 33% of patients with stage I or II either died or were lost to follow-up before 5 years. This survival difference is significant by a chi-square and Wilcoxon analysis, with a P value of .01.
In 2000, 86% of patients with cancer were termed self-pay, that is, they had no insurance and they were not converted to emergency Medicaid. In 2008, 16% of patients were self-pay, and the rest were converted to Medicaid. In 2000, fewer than 2% of patients had commercial insurance, compared with 9% in 2008.
Discussion
There have been numerous studies reporting on disparities in the treatment of patients with breast cancer based on race or socioeconomic status.4-18 Many studies have shown inferior survival for black women with breast cancer, but it is not entirely clear whether these differences are the result of the quality of medical care received or biologic differences.14,19 A moderately large study from a metropolitan medical center in Detroit showed no difference in survival in their patients based on race when all of the patients received equal treatments.15 A meta-analysis of survival in black and white breast cancer patients showed that the black women had significantly poorer outcomes.19
Findings from a recent study showed that patients of lower socioeconomic status are more likely to undergo mastectomy than breast conserving therapy.20 The study, which identified 727,927 patients with early-stage breast cancer during 1998-2011, found that the rate of breast conservation increased from 54% to 59% during that time period and that there were significant barriers to women receiving breast-conserving therapy based on their type of insurance and having a lower socioeconomic status.20
The treatment of breast cancer is best delivered in a multimodality setting, but many inner-city public hospitals do not have such a facility for their patients. QHC is the only public hospital in New York City that has established a comprehensive cancer center. The patient population of QHC is overwhelmingly of minority origin (only 5% of patients are white). In addition, it is a safety net hospital, so no patient is turned away because they cannot pay, and most patients are of lower socioeconomic status and do not have insurance. The purpose of the cancer center was to provide a single site at which our patients could receive all their treatment. It was to ensure that our patients had easy access to care and treatment during all phases of their disease trajectory and did not “fall through the cracks” of the system. Those goals were addressed by having all of the center’s physicians in one place. Physicians involved in care included medical, surgical, and radiation oncologists, a gynecologic oncologist, a genitourinary oncologist, and a thoracic surgery oncologist. The support groups organized for the cancer patients included 3 oncology social workers, an oncology navigator, a nutritionist, a pastoral care supporter, and an oncology psychologist, all located in the same area. All of the clerical and financial aspects of care were also placed within the center. This made the experience as seamless as possible for both the patients and the treating physicians. A “survivors clinic” was established so the cancer patients could be seen by integrated primary care providers to address all noncancer-related health issues such as hypertension, diabetes, or heart disease. Finally, a robust clinical oncology research team was established in the same location. The research included several protocols for new drug treatments for breast cancer from pharmaceutical companies as well as the multi-institutional oncology groups.
Part of the mission of the cancer center was to reach out into the community of Queens to provide education about early detection, cancer prevention, and other public health issues such as tobacco cessation. We established a close working relationship with the Queens Public Library System to connect with their users and dispense information about cancer care and early detection. The Queens Library system is the largest in the United States, and everyone who lives in Queens has easy access to one of its 63 branch libraries. We arranged several lectures about breast cancer awareness in some of the branch libraries. We also procured a mobile mammogram unit for free screening events at the lectures, especially in neighborhoods with a large number residents who were of lower socioeconomic status.
To study the possible effect of these changes on our patients with breast cancer, we compared 2 groups of patients. One group was from the year 2000, a year before the cancer center was opened. The other was from the year 2008, the last year we could get real 5-year survival statistics. We explored how establishing the cancer center might have changed the patients’ stage at diagnosis, care, treatment modalities such as type of surgery, and outcomes. It is difficult to compare these 2 groups because of differences in the patients’ cancers, such as their receptor status, as well as differences in treatment options between the two time periods. However, we had no other way to compare the data to see if there were any trends.
There was a migration to earlier-stage cancer at diagnosis during the 6-year period after the cancer center was opened. It is likely that the educational sessions that were done in the community contributed to this migration. We also saw an increase in the number of mammograms done, from 6,300 in 2000 to 8,800 in 2008. This increase in screening also could account for more patients being identified with earlier-stage disease and might be attributable to the community education through the outreach programs.
As a quality control method, the cancer center has been evaluated by the Commission on Cancer every 3 years. At the 2013 evaluation, we received the Gold Commendation – the highest possible recognition for having 8 out of 8 commendations – and a 3-year accreditation.
There was a notable increase in the use of lumpectomy over mastectomy after the establishment of the cancer center, possibly due to the addition of 2 surgical oncologists to the cancer center’s care team. The integration of multimodiality care for each patient may also have increased the use of breast-conserving surgery.
There was a significant increase from 2000 to 2008 in the survival of patients treated for stage III breast cancer. New drugs and new patterns of adjuvant care might have been partly responsible for that change. The establishment of the comprehensive cancer center with access to new protocols ensured that patients received state-of-the-art cancer treatment. Moreover, the facility addressed all aspects of patient care throughout the disease trajectory by including designated social workers, psychologists, a nutritionist, pastoral care, and patient and survivor support groups to ensure that patients would keep coming to the center for their therapy, with no delays and very little loss to follow-up.
Most patients without insurance were able to acquire emergency Medicaid through the cancer center. This was done by having 2 financial counselors who met with every patient and who could facilitate access to Medicaid as needed. As a result of that, the percentage of patients with no coverage went from 86% in 2000 to 16% in 2008. Before this system was set up, patients who were designated self-pay would pay a fee as low as $15 for each visit and received thousands of dollars’ worth of care. Thus, by forming a cancer center and facilitating patient access to Medicaid, we were able to save money for this public institution because of the gain in revenue from Medicaid.
Our findings suggest that the development of comprehensive cancer centers within inner-city health systems can ensure better treatment for patients of lower socioeconomic status. We present evidence that this may result in increased survival, more sophisticated surgical options, and better patient quality of life. Moreover, this can be achieved while effectively increasing revenue for the public hospitals. Correcting the inequality of access to care and better therapeutic options by setting up comprehensive cancer centers could contribute to improved parity of outcomes for underserved populations.
The author acknowledges the statistical help of Brian Altonen, MPH.
1. Kesson EM, Allardice GM, George WD, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
2. Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21(3):261-266.
3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496.
4. Wheeler SB, Hayes-Reeder KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986-993.
5. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78-93.
6. Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117:3311-3321.
7. Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169-2177.
8. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713-719.
9. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008;100:1717-1723.
19. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357-1362.
11. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121-131.
12. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31.
13. Naik AM, Joseph K, Harris M, Davis C, Shapiro R, Hiotis KL. Indigent breast cancer patients among all racial and ethnic groups present with more advanced disease compared with nationally reported date. Am J Surg. 2003;186:400-403.
14. Hersman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27: 2157-2162.
15
16. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471-473.
17. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256-1264.
18. Cross C, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US. Cancer. 2002;95:1988-1999.
19. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342-1349.
20. Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA. 2015;150:778-786.
When cancer care is centralized in a comprehensive fashion, the quality of care and the outcomes improve.1,2 Unfortunately, because of the medical insurance structure in New York City, most patients of lower socioeconomic status do not receive their cancer care in such dedicated cancer centers. In New York City, the majority of the underserved vulnerable populations – that is, those without health insurance – receive their care from the public hospital system known as NYC Health and Hospitals. Cancer care in this system is not centralized and may result in fragmented implementation of various modalities of treatment. In addition, because there is no centralized care, needs such as early screening and prevention programs are often not addressed. This problem was evident in Queens in 2000 and before when many patients with late-stage cancers were presenting for cancer care. Queens, which is one of the 5 boroughs of New York City, has more than 2.3 million residents. It has 2 public hospitals, Elmhurst Hospital Center and Queens Hospital Center (QHC). In 2001, the plan was devised for the establishment of a cancer center at QHC, mainly because of the high rate of late-stage cancers that were being seen at presentation and recognition of the need for more comprehensive care. In 2002, the Queens Cancer Center (QCC) began to see patients. QCC is a single facility that provides medical, surgical, radiation, gynecologic, and urologic oncology all in one area of the QHC.
This study is an investigation of the possible impact on care for breast cancer patients of low socioeconomic status who were treated at a comprehensive cancer center, with specific consideration of the change or improvement in treatment modalities and outcomes. Data on treatment modalities and outcomes of cancer patients who were treated at the QHC during 2000, before the QCC was set up, were compared with data of patients treated during 2008 (2008 was selected because we have 5-year survival data for those patients). The public hospital system treats all patients regardless of their ability to pay, so the majority of patients in the system are of lower socioeconomic status. In addition, 92% of the patients seen QHC are from a minority population. These are the populations that tend to have a worse prognosis and often are not given optimal treatment.3 The payer mix of patients in the public hospital system is different than that of private hospitals. Most of the patients present at the hospital with no insurance and if they are diagnosed with cancer they may be converted to emergency Medicaid. About 10% of patients will not be converted because of their document status.
Patients and methods
We used the Queens Hospital Tumor Registry to identify the patients who had been diagnosed with and treated for breast cancer in 2000 and 2008. The electronic medical records were reviewed, and in the case of the 2000-year patients, the written charts were also reviewed. The study was approved by the Mount Sinai institutional review board. It was not necessary to obtain patient consent because it was a retrospective study.
Only patients diagnosed with stage 0, I, II, or III breast cancer who received their treatment at QHC were included in the study. Patients who were seen in consultation at QHC but not treated there were excluded. Statistics were done using the 2x2 chi-squared SPSS analysis; a P value of .05 was considered significant. The survival data was analyzed using SAS.
Results
There were 24 evaluable patients in 2000 and 78 evaluable patients in 2008 who had stage 0, I, II, or III primary breast cancer and were treated at QHC. The average age of the patients in 2000 was 53.5 years and 54.7 years in 2008. The mean age for both groups was 55 years. The patients were ethnically diverse in both groups with 46% black, 17% Hispanic, 25% ethnic Asian Indian, and 6% white (Figure 1).
The payer mix in 2000 was 9 patients (37.5%) self-pay, 7 (29%) Medicaid, and 8 (33%) Medicare. In 2008, 11 patients (14%) were self-pay, 46 (59%) Medicaid, 11 (14%) Medicare, and 10 (13%) were private insurance. In 2000, there were 3 (12%) patients with stage 0 disease, 5 (21%) with stage I; 9 (37.5%) with stage II, and 7 (29%) with stage III. In 2008 there were 28 (36%) patients with stage 0 disease, 15 (19%) with stage I, 17 (22%) with stage II, and 18 (23%) with stage III (Figure 2).
None of those values are statistically different. In 2000, 2 of the 24 patients had lumpectomies (partial mastectomy) and the rest had mastectomies. In 2008, 39 (50%) patients had mastectomy and 39 (50%) had lumpectomies (Figure 3). This was a statistically significant difference.
Radiation was given to both patients with lumpectomy in the 2000 group. In the 2008 group, all patients with lumpectomies were evaluated for radiation, and 6 of them did not receive radiation for the following reasons: 3 had very small foci of ductal carcinoma in situ (DCIS) and were treated with hormone therapy and no radiation; 1 patient had a lumpectomy for stage 1 cancer and also did not get radiation therapy because of a low oncotype and very small lesion; 2 patients were older than 70 years and had DCIS and were treated with tamoxifen alone as per NCCN Guidelines for women in that age group. The rest of the patients with lumpectomies received postoperative radiation.
Hormone and HER2 (human epidermal growth factor receptor 2) status was obtained on all patients. For the 2000 patients, 71% had 1 hormone receptor–positive (estrogen receptor [ER] or progesterone receptor [PR]), 21% were triple negative (ER-PR and HER2-neu), and 42% had HER2-neu–positive tumors. For the 2008, patients 65% were positive for 1 hormone receptor (ER or PR), 28% were triple negative (ER-PR and HER2-neu), and 7% had HER2-neu-positive tumors.
All patients were offered chemotherapy and hormone therapy if appropriate, as per NCCN guidelines. If a patient’s tumor was found to be HER2-positive, then the chemotherapy regimen would include the use of trastuzumab in both groups.
The 5-year survival for the 2008 stage III patients was 73.7%, compared with 14.2% for the 2000 stage III patients. The only deaths in the 2008 group were in patients with stage III disease. In the 2000 group, 4 of the 5 patients with stage III cancer died, and 33% of patients with stage I or II either died or were lost to follow-up before 5 years. This survival difference is significant by a chi-square and Wilcoxon analysis, with a P value of .01.
In 2000, 86% of patients with cancer were termed self-pay, that is, they had no insurance and they were not converted to emergency Medicaid. In 2008, 16% of patients were self-pay, and the rest were converted to Medicaid. In 2000, fewer than 2% of patients had commercial insurance, compared with 9% in 2008.
Discussion
There have been numerous studies reporting on disparities in the treatment of patients with breast cancer based on race or socioeconomic status.4-18 Many studies have shown inferior survival for black women with breast cancer, but it is not entirely clear whether these differences are the result of the quality of medical care received or biologic differences.14,19 A moderately large study from a metropolitan medical center in Detroit showed no difference in survival in their patients based on race when all of the patients received equal treatments.15 A meta-analysis of survival in black and white breast cancer patients showed that the black women had significantly poorer outcomes.19
Findings from a recent study showed that patients of lower socioeconomic status are more likely to undergo mastectomy than breast conserving therapy.20 The study, which identified 727,927 patients with early-stage breast cancer during 1998-2011, found that the rate of breast conservation increased from 54% to 59% during that time period and that there were significant barriers to women receiving breast-conserving therapy based on their type of insurance and having a lower socioeconomic status.20
The treatment of breast cancer is best delivered in a multimodality setting, but many inner-city public hospitals do not have such a facility for their patients. QHC is the only public hospital in New York City that has established a comprehensive cancer center. The patient population of QHC is overwhelmingly of minority origin (only 5% of patients are white). In addition, it is a safety net hospital, so no patient is turned away because they cannot pay, and most patients are of lower socioeconomic status and do not have insurance. The purpose of the cancer center was to provide a single site at which our patients could receive all their treatment. It was to ensure that our patients had easy access to care and treatment during all phases of their disease trajectory and did not “fall through the cracks” of the system. Those goals were addressed by having all of the center’s physicians in one place. Physicians involved in care included medical, surgical, and radiation oncologists, a gynecologic oncologist, a genitourinary oncologist, and a thoracic surgery oncologist. The support groups organized for the cancer patients included 3 oncology social workers, an oncology navigator, a nutritionist, a pastoral care supporter, and an oncology psychologist, all located in the same area. All of the clerical and financial aspects of care were also placed within the center. This made the experience as seamless as possible for both the patients and the treating physicians. A “survivors clinic” was established so the cancer patients could be seen by integrated primary care providers to address all noncancer-related health issues such as hypertension, diabetes, or heart disease. Finally, a robust clinical oncology research team was established in the same location. The research included several protocols for new drug treatments for breast cancer from pharmaceutical companies as well as the multi-institutional oncology groups.
Part of the mission of the cancer center was to reach out into the community of Queens to provide education about early detection, cancer prevention, and other public health issues such as tobacco cessation. We established a close working relationship with the Queens Public Library System to connect with their users and dispense information about cancer care and early detection. The Queens Library system is the largest in the United States, and everyone who lives in Queens has easy access to one of its 63 branch libraries. We arranged several lectures about breast cancer awareness in some of the branch libraries. We also procured a mobile mammogram unit for free screening events at the lectures, especially in neighborhoods with a large number residents who were of lower socioeconomic status.
To study the possible effect of these changes on our patients with breast cancer, we compared 2 groups of patients. One group was from the year 2000, a year before the cancer center was opened. The other was from the year 2008, the last year we could get real 5-year survival statistics. We explored how establishing the cancer center might have changed the patients’ stage at diagnosis, care, treatment modalities such as type of surgery, and outcomes. It is difficult to compare these 2 groups because of differences in the patients’ cancers, such as their receptor status, as well as differences in treatment options between the two time periods. However, we had no other way to compare the data to see if there were any trends.
There was a migration to earlier-stage cancer at diagnosis during the 6-year period after the cancer center was opened. It is likely that the educational sessions that were done in the community contributed to this migration. We also saw an increase in the number of mammograms done, from 6,300 in 2000 to 8,800 in 2008. This increase in screening also could account for more patients being identified with earlier-stage disease and might be attributable to the community education through the outreach programs.
As a quality control method, the cancer center has been evaluated by the Commission on Cancer every 3 years. At the 2013 evaluation, we received the Gold Commendation – the highest possible recognition for having 8 out of 8 commendations – and a 3-year accreditation.
There was a notable increase in the use of lumpectomy over mastectomy after the establishment of the cancer center, possibly due to the addition of 2 surgical oncologists to the cancer center’s care team. The integration of multimodiality care for each patient may also have increased the use of breast-conserving surgery.
There was a significant increase from 2000 to 2008 in the survival of patients treated for stage III breast cancer. New drugs and new patterns of adjuvant care might have been partly responsible for that change. The establishment of the comprehensive cancer center with access to new protocols ensured that patients received state-of-the-art cancer treatment. Moreover, the facility addressed all aspects of patient care throughout the disease trajectory by including designated social workers, psychologists, a nutritionist, pastoral care, and patient and survivor support groups to ensure that patients would keep coming to the center for their therapy, with no delays and very little loss to follow-up.
Most patients without insurance were able to acquire emergency Medicaid through the cancer center. This was done by having 2 financial counselors who met with every patient and who could facilitate access to Medicaid as needed. As a result of that, the percentage of patients with no coverage went from 86% in 2000 to 16% in 2008. Before this system was set up, patients who were designated self-pay would pay a fee as low as $15 for each visit and received thousands of dollars’ worth of care. Thus, by forming a cancer center and facilitating patient access to Medicaid, we were able to save money for this public institution because of the gain in revenue from Medicaid.
Our findings suggest that the development of comprehensive cancer centers within inner-city health systems can ensure better treatment for patients of lower socioeconomic status. We present evidence that this may result in increased survival, more sophisticated surgical options, and better patient quality of life. Moreover, this can be achieved while effectively increasing revenue for the public hospitals. Correcting the inequality of access to care and better therapeutic options by setting up comprehensive cancer centers could contribute to improved parity of outcomes for underserved populations.
The author acknowledges the statistical help of Brian Altonen, MPH.
When cancer care is centralized in a comprehensive fashion, the quality of care and the outcomes improve.1,2 Unfortunately, because of the medical insurance structure in New York City, most patients of lower socioeconomic status do not receive their cancer care in such dedicated cancer centers. In New York City, the majority of the underserved vulnerable populations – that is, those without health insurance – receive their care from the public hospital system known as NYC Health and Hospitals. Cancer care in this system is not centralized and may result in fragmented implementation of various modalities of treatment. In addition, because there is no centralized care, needs such as early screening and prevention programs are often not addressed. This problem was evident in Queens in 2000 and before when many patients with late-stage cancers were presenting for cancer care. Queens, which is one of the 5 boroughs of New York City, has more than 2.3 million residents. It has 2 public hospitals, Elmhurst Hospital Center and Queens Hospital Center (QHC). In 2001, the plan was devised for the establishment of a cancer center at QHC, mainly because of the high rate of late-stage cancers that were being seen at presentation and recognition of the need for more comprehensive care. In 2002, the Queens Cancer Center (QCC) began to see patients. QCC is a single facility that provides medical, surgical, radiation, gynecologic, and urologic oncology all in one area of the QHC.
This study is an investigation of the possible impact on care for breast cancer patients of low socioeconomic status who were treated at a comprehensive cancer center, with specific consideration of the change or improvement in treatment modalities and outcomes. Data on treatment modalities and outcomes of cancer patients who were treated at the QHC during 2000, before the QCC was set up, were compared with data of patients treated during 2008 (2008 was selected because we have 5-year survival data for those patients). The public hospital system treats all patients regardless of their ability to pay, so the majority of patients in the system are of lower socioeconomic status. In addition, 92% of the patients seen QHC are from a minority population. These are the populations that tend to have a worse prognosis and often are not given optimal treatment.3 The payer mix of patients in the public hospital system is different than that of private hospitals. Most of the patients present at the hospital with no insurance and if they are diagnosed with cancer they may be converted to emergency Medicaid. About 10% of patients will not be converted because of their document status.
Patients and methods
We used the Queens Hospital Tumor Registry to identify the patients who had been diagnosed with and treated for breast cancer in 2000 and 2008. The electronic medical records were reviewed, and in the case of the 2000-year patients, the written charts were also reviewed. The study was approved by the Mount Sinai institutional review board. It was not necessary to obtain patient consent because it was a retrospective study.
Only patients diagnosed with stage 0, I, II, or III breast cancer who received their treatment at QHC were included in the study. Patients who were seen in consultation at QHC but not treated there were excluded. Statistics were done using the 2x2 chi-squared SPSS analysis; a P value of .05 was considered significant. The survival data was analyzed using SAS.
Results
There were 24 evaluable patients in 2000 and 78 evaluable patients in 2008 who had stage 0, I, II, or III primary breast cancer and were treated at QHC. The average age of the patients in 2000 was 53.5 years and 54.7 years in 2008. The mean age for both groups was 55 years. The patients were ethnically diverse in both groups with 46% black, 17% Hispanic, 25% ethnic Asian Indian, and 6% white (Figure 1).
The payer mix in 2000 was 9 patients (37.5%) self-pay, 7 (29%) Medicaid, and 8 (33%) Medicare. In 2008, 11 patients (14%) were self-pay, 46 (59%) Medicaid, 11 (14%) Medicare, and 10 (13%) were private insurance. In 2000, there were 3 (12%) patients with stage 0 disease, 5 (21%) with stage I; 9 (37.5%) with stage II, and 7 (29%) with stage III. In 2008 there were 28 (36%) patients with stage 0 disease, 15 (19%) with stage I, 17 (22%) with stage II, and 18 (23%) with stage III (Figure 2).
None of those values are statistically different. In 2000, 2 of the 24 patients had lumpectomies (partial mastectomy) and the rest had mastectomies. In 2008, 39 (50%) patients had mastectomy and 39 (50%) had lumpectomies (Figure 3). This was a statistically significant difference.
Radiation was given to both patients with lumpectomy in the 2000 group. In the 2008 group, all patients with lumpectomies were evaluated for radiation, and 6 of them did not receive radiation for the following reasons: 3 had very small foci of ductal carcinoma in situ (DCIS) and were treated with hormone therapy and no radiation; 1 patient had a lumpectomy for stage 1 cancer and also did not get radiation therapy because of a low oncotype and very small lesion; 2 patients were older than 70 years and had DCIS and were treated with tamoxifen alone as per NCCN Guidelines for women in that age group. The rest of the patients with lumpectomies received postoperative radiation.
Hormone and HER2 (human epidermal growth factor receptor 2) status was obtained on all patients. For the 2000 patients, 71% had 1 hormone receptor–positive (estrogen receptor [ER] or progesterone receptor [PR]), 21% were triple negative (ER-PR and HER2-neu), and 42% had HER2-neu–positive tumors. For the 2008, patients 65% were positive for 1 hormone receptor (ER or PR), 28% were triple negative (ER-PR and HER2-neu), and 7% had HER2-neu-positive tumors.
All patients were offered chemotherapy and hormone therapy if appropriate, as per NCCN guidelines. If a patient’s tumor was found to be HER2-positive, then the chemotherapy regimen would include the use of trastuzumab in both groups.
The 5-year survival for the 2008 stage III patients was 73.7%, compared with 14.2% for the 2000 stage III patients. The only deaths in the 2008 group were in patients with stage III disease. In the 2000 group, 4 of the 5 patients with stage III cancer died, and 33% of patients with stage I or II either died or were lost to follow-up before 5 years. This survival difference is significant by a chi-square and Wilcoxon analysis, with a P value of .01.
In 2000, 86% of patients with cancer were termed self-pay, that is, they had no insurance and they were not converted to emergency Medicaid. In 2008, 16% of patients were self-pay, and the rest were converted to Medicaid. In 2000, fewer than 2% of patients had commercial insurance, compared with 9% in 2008.
Discussion
There have been numerous studies reporting on disparities in the treatment of patients with breast cancer based on race or socioeconomic status.4-18 Many studies have shown inferior survival for black women with breast cancer, but it is not entirely clear whether these differences are the result of the quality of medical care received or biologic differences.14,19 A moderately large study from a metropolitan medical center in Detroit showed no difference in survival in their patients based on race when all of the patients received equal treatments.15 A meta-analysis of survival in black and white breast cancer patients showed that the black women had significantly poorer outcomes.19
Findings from a recent study showed that patients of lower socioeconomic status are more likely to undergo mastectomy than breast conserving therapy.20 The study, which identified 727,927 patients with early-stage breast cancer during 1998-2011, found that the rate of breast conservation increased from 54% to 59% during that time period and that there were significant barriers to women receiving breast-conserving therapy based on their type of insurance and having a lower socioeconomic status.20
The treatment of breast cancer is best delivered in a multimodality setting, but many inner-city public hospitals do not have such a facility for their patients. QHC is the only public hospital in New York City that has established a comprehensive cancer center. The patient population of QHC is overwhelmingly of minority origin (only 5% of patients are white). In addition, it is a safety net hospital, so no patient is turned away because they cannot pay, and most patients are of lower socioeconomic status and do not have insurance. The purpose of the cancer center was to provide a single site at which our patients could receive all their treatment. It was to ensure that our patients had easy access to care and treatment during all phases of their disease trajectory and did not “fall through the cracks” of the system. Those goals were addressed by having all of the center’s physicians in one place. Physicians involved in care included medical, surgical, and radiation oncologists, a gynecologic oncologist, a genitourinary oncologist, and a thoracic surgery oncologist. The support groups organized for the cancer patients included 3 oncology social workers, an oncology navigator, a nutritionist, a pastoral care supporter, and an oncology psychologist, all located in the same area. All of the clerical and financial aspects of care were also placed within the center. This made the experience as seamless as possible for both the patients and the treating physicians. A “survivors clinic” was established so the cancer patients could be seen by integrated primary care providers to address all noncancer-related health issues such as hypertension, diabetes, or heart disease. Finally, a robust clinical oncology research team was established in the same location. The research included several protocols for new drug treatments for breast cancer from pharmaceutical companies as well as the multi-institutional oncology groups.
Part of the mission of the cancer center was to reach out into the community of Queens to provide education about early detection, cancer prevention, and other public health issues such as tobacco cessation. We established a close working relationship with the Queens Public Library System to connect with their users and dispense information about cancer care and early detection. The Queens Library system is the largest in the United States, and everyone who lives in Queens has easy access to one of its 63 branch libraries. We arranged several lectures about breast cancer awareness in some of the branch libraries. We also procured a mobile mammogram unit for free screening events at the lectures, especially in neighborhoods with a large number residents who were of lower socioeconomic status.
To study the possible effect of these changes on our patients with breast cancer, we compared 2 groups of patients. One group was from the year 2000, a year before the cancer center was opened. The other was from the year 2008, the last year we could get real 5-year survival statistics. We explored how establishing the cancer center might have changed the patients’ stage at diagnosis, care, treatment modalities such as type of surgery, and outcomes. It is difficult to compare these 2 groups because of differences in the patients’ cancers, such as their receptor status, as well as differences in treatment options between the two time periods. However, we had no other way to compare the data to see if there were any trends.
There was a migration to earlier-stage cancer at diagnosis during the 6-year period after the cancer center was opened. It is likely that the educational sessions that were done in the community contributed to this migration. We also saw an increase in the number of mammograms done, from 6,300 in 2000 to 8,800 in 2008. This increase in screening also could account for more patients being identified with earlier-stage disease and might be attributable to the community education through the outreach programs.
As a quality control method, the cancer center has been evaluated by the Commission on Cancer every 3 years. At the 2013 evaluation, we received the Gold Commendation – the highest possible recognition for having 8 out of 8 commendations – and a 3-year accreditation.
There was a notable increase in the use of lumpectomy over mastectomy after the establishment of the cancer center, possibly due to the addition of 2 surgical oncologists to the cancer center’s care team. The integration of multimodiality care for each patient may also have increased the use of breast-conserving surgery.
There was a significant increase from 2000 to 2008 in the survival of patients treated for stage III breast cancer. New drugs and new patterns of adjuvant care might have been partly responsible for that change. The establishment of the comprehensive cancer center with access to new protocols ensured that patients received state-of-the-art cancer treatment. Moreover, the facility addressed all aspects of patient care throughout the disease trajectory by including designated social workers, psychologists, a nutritionist, pastoral care, and patient and survivor support groups to ensure that patients would keep coming to the center for their therapy, with no delays and very little loss to follow-up.
Most patients without insurance were able to acquire emergency Medicaid through the cancer center. This was done by having 2 financial counselors who met with every patient and who could facilitate access to Medicaid as needed. As a result of that, the percentage of patients with no coverage went from 86% in 2000 to 16% in 2008. Before this system was set up, patients who were designated self-pay would pay a fee as low as $15 for each visit and received thousands of dollars’ worth of care. Thus, by forming a cancer center and facilitating patient access to Medicaid, we were able to save money for this public institution because of the gain in revenue from Medicaid.
Our findings suggest that the development of comprehensive cancer centers within inner-city health systems can ensure better treatment for patients of lower socioeconomic status. We present evidence that this may result in increased survival, more sophisticated surgical options, and better patient quality of life. Moreover, this can be achieved while effectively increasing revenue for the public hospitals. Correcting the inequality of access to care and better therapeutic options by setting up comprehensive cancer centers could contribute to improved parity of outcomes for underserved populations.
The author acknowledges the statistical help of Brian Altonen, MPH.
1. Kesson EM, Allardice GM, George WD, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
2. Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21(3):261-266.
3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496.
4. Wheeler SB, Hayes-Reeder KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986-993.
5. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78-93.
6. Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117:3311-3321.
7. Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169-2177.
8. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713-719.
9. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008;100:1717-1723.
19. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357-1362.
11. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121-131.
12. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31.
13. Naik AM, Joseph K, Harris M, Davis C, Shapiro R, Hiotis KL. Indigent breast cancer patients among all racial and ethnic groups present with more advanced disease compared with nationally reported date. Am J Surg. 2003;186:400-403.
14. Hersman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27: 2157-2162.
15
16. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471-473.
17. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256-1264.
18. Cross C, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US. Cancer. 2002;95:1988-1999.
19. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342-1349.
20. Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA. 2015;150:778-786.
1. Kesson EM, Allardice GM, George WD, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
2. Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21(3):261-266.
3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496.
4. Wheeler SB, Hayes-Reeder KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986-993.
5. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78-93.
6. Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117:3311-3321.
7. Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169-2177.
8. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713-719.
9. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008;100:1717-1723.
19. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357-1362.
11. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121-131.
12. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31.
13. Naik AM, Joseph K, Harris M, Davis C, Shapiro R, Hiotis KL. Indigent breast cancer patients among all racial and ethnic groups present with more advanced disease compared with nationally reported date. Am J Surg. 2003;186:400-403.
14. Hersman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27: 2157-2162.
15
16. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471-473.
17. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256-1264.
18. Cross C, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US. Cancer. 2002;95:1988-1999.
19. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342-1349.
20. Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA. 2015;150:778-786.