User login
Research and Reviews for the Practicing Oncologist
Posttreatment survivorship care needs of Spanish-speaking Latinas with breast cancer
After treatment, cancer patients transition to a survivorship phase, often with little information or support. Cancer survivors are at increased risk of recurrence, secondary cancers, comorbid conditions, and late treatment effects.1,2 However, many remain unaware of these risks and the options for managing them3 and face numerous unmet medical, psychosocial, and informational needs that can be addressed through survivorship care programs.4 Anxiety may increase as they lose their treatment team’s support while attempting to reestablish their lives.2 Patients need to know the long-term risks of cancer treatments, probabilities of recurrence and second cancers, effectiveness of surveillance and interventions for managing late effects and psychosocial concerns, and benefits of healthy lifestyles.2
Due to sociocultural and economic factors, Spanish-speaking Latina breast cancer survivors (SSBCS) suffer worse posttreatment health-related quality of life and more pain, fatigue, depressive symptoms, body image issues, and distress than their white counterparts.5-7 However, they are less likely to receive necessary cancer treatment, symptom management, and surveillance. For example, compared with whites, Latina breast cancer survivors receive less guideline-adherent treatment8 and follow-up care, including survivorship information.3,9 SSBCS, in particular have less access to survivorship information.10 Consequently, SSBCS are more likely to report unmet symptom management needs.11
Several breast cancer survivorship program trials have included Latinas,12,13 but their effectiveness has been demonstrated only for depressive symptoms or health worry. A comprehensive assessment of the posttreatment needs of SSBCS would provide a foundation for designing tailored survivorship interventions for this vulnerable group. This study aimed to identify the symptom management, psychosocial, and informational needs of SSBCS during the transition to survivorship from the perspectives of SSBCS and their cancer support providers and cancer physicians.
Methods
We sampled respondents within a 5-county area in Northern California to obtain multiple perspectives of the survivorship care needs of SSBCS using structured and in-depth methods: a telephone survey of SSBCS; semistructured interviews with SSBCS; semistructured interviews with cancer support providers serving SSBCS; and semistructured interviews with physicians providing cancer care for SSBCS. The study protocol was approved by the University of California San Francisco Committee on Human Research.
Sample and procedures
Structured telephone survey with SSBCS. The sample was drawn evenly from San Francisco General Hospital-University of California San Francisco primary care practices and SSBCS from a previous study who agreed to be re-contacted.14 The inclusion criteria were: completed active treatment (except adjuvant hormonal therapy) for nonmetastatic breast cancer within 10 years; living in one of the five counties; primarily Spanish-speaking; and self-identified as Latina. The exclusion criteria were: previous cancer except nonmelanoma skin cancer; terminal illness; or metastatic breast cancer. Study staff mailed potential participants a bilingual letter and information sheet, and bilingual opt-out postcard (6th grade reading level assessed by Flesch-Kincaid grade level statistic). Female bilingual-bicultural research associates conducted interviews of 20-30 minutes in Spanish after obtaining verbal consent. Participants were mailed $20. Surveys were conducted during March-November 2014.
Semistructured in-person interviews with SSBCS. Four community-based organizations (CBOs) in the targeted area providing cancer support services to Latinos agreed to recruit SSBCS for interviews. Inclusion criteria were identical to the survey. Patient navigators or support providers from CBOs contacted women by phone or in-person to invite them to an interview to assess their cancer survivorship needs. Women could choose a focus group or individual interview. With permission, names and contact information were given to study interviewers who called, explained the study, screened for eligibility, and scheduled an interview.
Recruitment was stratified by age (under or over age 50). We sampled women until saturation was achieved (no new themes emerged). Focus groups (90 minutes) were conducted at the CBOs. Individual interviews (45 minutes) were conducted in participants’ homes. Written informed consent was obtained. Participants were paid $50. Interviews were conducted during August-November, 2014, audiotaped, and transcribed.
Semistructured in-person interviews with cancer support providers and physicians. Investigators invited five cancer support providers (three patient navigators from three county hospitals, and two CBO directors of cancer psychosocial support services) and four physicians (three oncologists and one breast cancer surgeon from three county hospitals) to an in-person interview to identify SSBCS’ survivorship care needs. All agreed to participate. No further candidates were approached because saturation was achieved. We obtained written informed consent and 30-minute interviews were conducted in participants’ offices during August-October, 2014. Interviews were audiotaped and transcribed. Participants were paid $50.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Measures
Structured telephone survey. Based on cancer survivor needs assessments,15 we assessed: physical and emotional symptoms; problems with sleep and memory/concentration; concerns about mortality, family, social isolation, intimacy, appearance; and healthy lifestyles. Items were adapted and translated into Spanish if needed, using forward/backward translation with team reconciliation. These questions used the introduction, “Now I am going to ask you if you have had any problems because of your cancer. In the past month, how much have you been bothered by …” with responses rated on a scale of 1-5 (1, Not at all; 5, A lot). For example, we asked, “In the past month, how much have you been bothered by fatigue?”
Regarding healthy lifestyles, we used the introduction, “Here are some changes women sometimes want to make after cancer. Would you like help with …?” For example, we asked, “Would you like help with getting more exercise?” We asked if they wanted help getting more exercise, eating healthier, managing stress, and doing meditation or yoga (Yes/No).
Semistructured interview guide for SSBCS. Participants were asked about their emotional and physical concerns when treatment ended, current cancer needs, symptoms or late effects, and issues related to relationships, family, employment, insurance, financial hardships, barriers to follow-up care, health behaviors, and survivorship program content. Sample questions are, “Have you had any symptoms or side effects related to your cancer or treatment?” and “What kinds of information do you feel you need now about your cancer or treatment?” A brief questionnaire assessed demographics.
Semistructured interview guide for cancer support providers and physicians. Support providers and physicians were asked about informational, psychosocial, and symptom management needs of SSBCS and recommended self-management content and formats. Sample questions are, “What kinds of information and support do you wish was available to help Spanish-speaking women take care of their health after treatment ends?” and “What do you think are the most pressing emotional needs of Spanish-speaking women after breast cancer treatment ends?” A brief questionnaire assessed demographics.
Analysis
Frequencies are reported for survey items. For questions about symptoms/concerns, we report the frequency of responding that they were bothered Somewhat/Quite a bit/A lot. For healthy lifestyles, we present the frequency answering Yes.
Verbatim semistructured interview transcripts were verified against audiotapes. Using QSR NVIVO software, transcripts were coded independently by two bilingual-bicultural investigators using a constant comparative method to generate coding categories for cancer survivorship needs.16 Coders started with themes specified by the interview guides, expanded them to represent the data, and discussed and reconciled coding discrepancies. Coding was compared by type of interview participant (survivor, support provider, or physician). Triangulation of survey and semistructured interviews occurred through team discussions to verify codes, themes, and implications for interventions.
Results
Telephone survey of SSBCS
Of the telephone survey sampling frame (N = 231), 118 individuals (51%) completed the interview, 37 (16%) were ineligible, 31 (13%) could not be reached, 22 (10%) had incorrect contact information, 19 (8%) refused to participate, and 4 (2%) were deceased. Mean age of the participants was 54.9 years (SD, 12.3); all were foreign-born, with more than half of Mexican origin; and most had less than a high-school education (Table 1). All had completed active treatment, and most (68%) were within 2 years of diagnosis.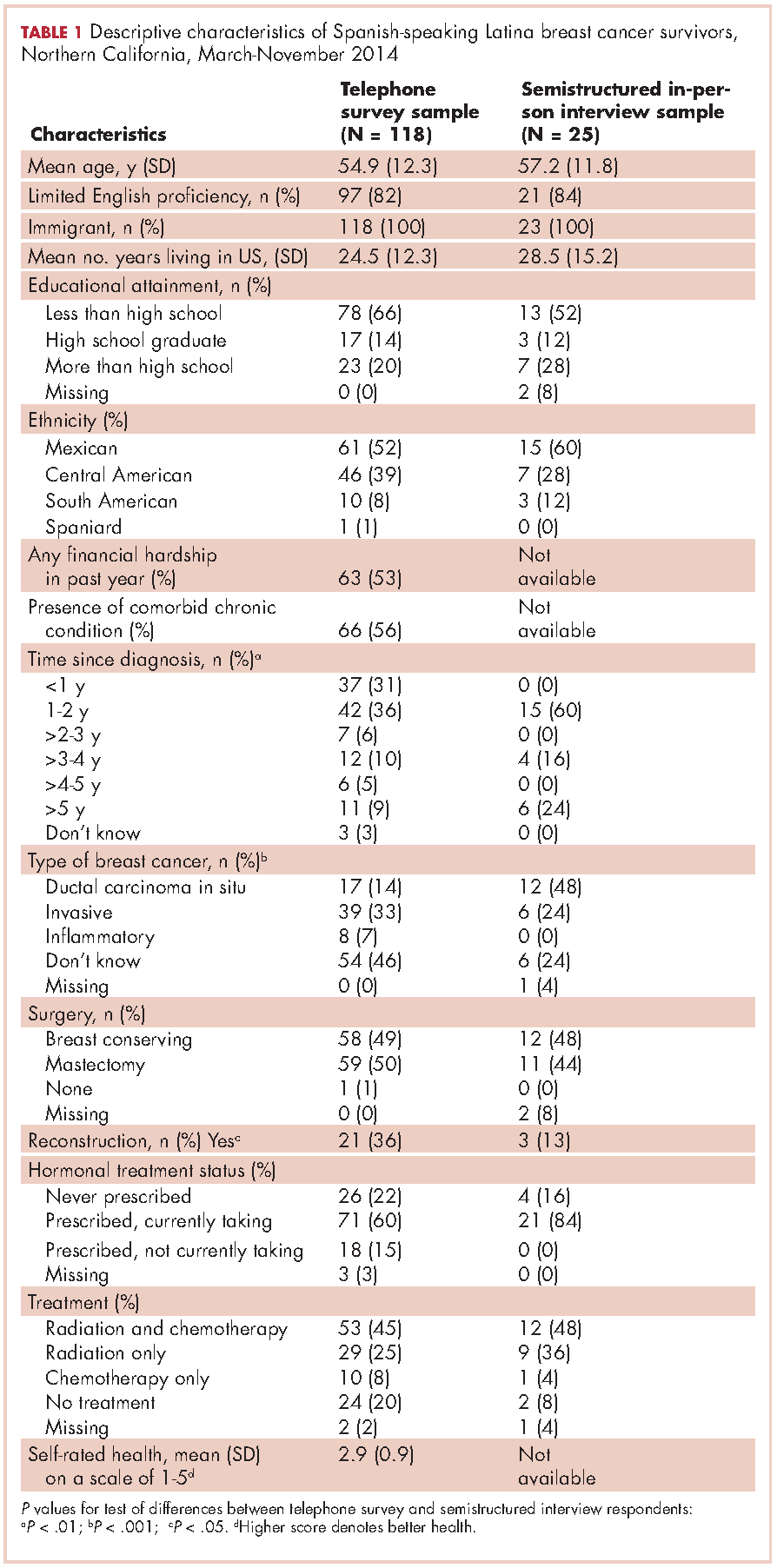
For symptom management needs (Table 2), the most prevalent (bothersome) symptoms (reported by more than 30%, in rank order) were joint pain, sleep problems, fatigue, hot flashes, numbness/tingling of extremities, and memory. Next most prevalent (reported by 20%-30%) were vaginal dryness, dry/itchy skin, dry nose/mouth, inability to concentrate, constipation, changes in urination, and shortness of breath.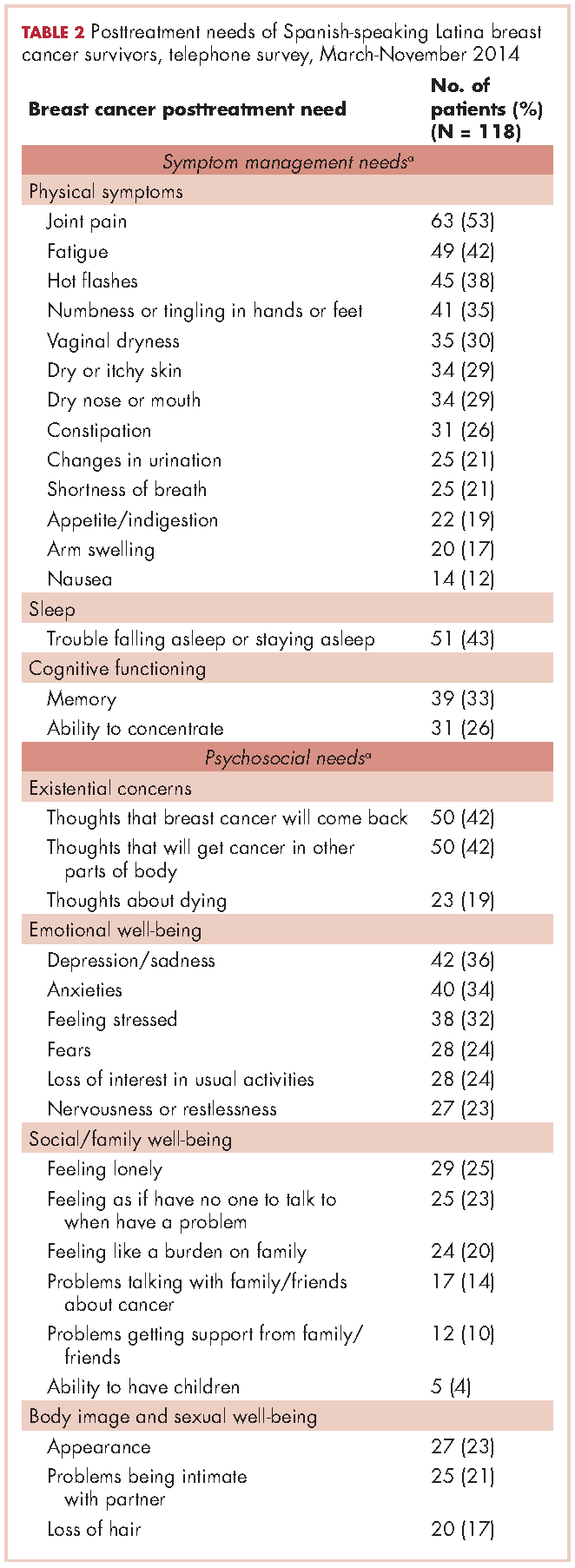

For psychosocial needs, fears of recurrence or new cancers were reported by 42%. Emotional symptoms reported by more than 30% were depression/sadness, anxieties, and feeling stressed. Next most prevalent (20%-30%) were fears, loss of interest in usual activities, and nervousness/restlessness. Social well-being concerns reported by 20%-30% of survivors were loneliness, having no one to talk to, and being a burden to their families. Body image and sexual problems reported by 20%-30% of survivors included appearance and problems being intimate with partners.
Regarding lifestyle, most of the participants said they wanted help with eating a healthier diet (74%), getting more exercise (69%), managing stress (63%), and doing yoga or meditation (55%).
Semistructured interviews
Twenty-five SSBCS completed semistructured interviews, 10 in individual interviews and 15 in one of two focus groups (one of 9 women older than 50 years; one of 6 women younger than 50). The telephone survey respondents were similar to semistructured interview respondents on all sociodemographic characteristics, but differed slightly on some clinical characteristics (Table 1). The telephone survey women had been more recently diagnosed (P < .01), were less likely to have ductal carcinoma in situ (P < .001), and more likely to have had reconstructive surgery
(P < .05).
Five cancer support providers and 4 physicians were interviewed. All support providers were Spanish-speaking Latinas with at least some college education. Cancer physicians were board certified. Two were men; two were white and two Asian; one was a breast surgeon and three were hematologists/oncologists; three spoke Spanish poorly/not at all and one spoke it fairly well.
Seven themes emerged from interviews: unmet physical symptom management needs; social support often ends when treatment ends; challenges resuming roles; sense of abandonment by health care system when treatment ends; need for formal transition from active treatment to follow-up care; fear of recurrence especially when obtaining follow-up care; and desire for information on late effects of initial treatments and side effects of hormonal treatments. We summarize results according to these themes.
Unmet physical symptom management needs. The main physical symptoms reported by survivors and physicians in interviews were arthralgia, menopausal symptoms, and neuropathy. Fatigue was reported only by survivors. Many survivors and several support providers expressed that symptoms were poorly managed and often ignored. One stated,
I have a lot of pain where I had surgery, it burns. I worry a lot about my arm because I have sacs of fluid. My doctor only says, ‘They will dissolve over the years.’ So, I don’t feel any support. (FocGrp1#6)
Survivors reported side effects of hormonal therapies, and felt that physicians downplayed these to prevent them from discontinuing medications.
Social support from family and friends often ends when treatment ends. Many survivors described a loss of support from family and friends who expected them to get back to “normal” once treatment ended. One said,
My sisters have told me to my face that there’s nothing wrong with me. So now when people ask me, I say, ‘I’m fine, thank God, I have nothing,’ even though I’m dying of pain and have all these pills to take. (Survivor#1025)
A support provider related,
The client was telling me that as she was getting closer to finishing her treatment, her husband was upset because he felt like all she was doing was focusing on the cancer. I think caregivers, family, spouses, and children out of their own sort of selfishness want this person to be well. (SuppProv#104)
A few survivors said that family bonds were strengthened after cancer and several reported lacking support because their families were in their home countries.
Challenges resuming roles, especially returning to work. Survivors, support providers, and physicians described challenges and few resources as women transition back to their normal roles. Survivors questioned their ability to return to work due to physically demanding occupations. One stated,
I would like information on how to take care of myself, how working can affect this side if I don’t take care of it. I clean houses and I need both hands. (Survivor#3012)
Survivors described how changes in memory affected daily chores and work performance. Support providers and physicians described the need for resources to aid with return to work and household responsibilities. One physician noted,
There are usually questions about how to go ahead and live their lives from that point forward. It’s a sort of reverse shock: going back to life as they know it. (Physician#004)
Support providers and physicians mentioned that women needed help with resuming intimate partner relations.
Sense of abandonment by health care system once active treatment ends. Survivors, support providers, and physicians reported a loss of support and sense of abandonment by the patients’ oncology team at the end of active treatment. One survivor stated,
Once they tell you to stop the pills, ‘You’re cured, there’s nothing wrong with you,’ the truth is that one feels, ‘Now what do I do? I have no one to help me.’ I felt very abandoned. (FocGrp1#5)
A provider said,
The support system falls apart once women complete treatment. They lose their entire support system at the medical level. They no longer have nurses checking in about symptoms and addressing anything that’s come up. They won’t have access to doctors unless they’re doing their screening. (SuppProv#101)
An oncologist, noting that this loss of support occurs when women face pressures of transitioning back to work or family obligations, commented,
So here’s a woman whose marriage is in turmoil, whose husband may even have left her during this, and now her clinic is leaving her and she’s on her own … that must be scary as hell because there’s nobody out there to support her. (Physician#002)
Need for formal transition from active treatment to follow-up care. Two themes emerged about transitioning from active treatment: transferring care from oncologists to primary care physicians (PCP); and issues of follow-up care (with oncologists or PCPs). Survivors felt lost in transitioning from specialty to primary care, or expressed apprehension seeing a PCP rather than a cancer specialist. One stated,
I have my doctor but she is not a specialist. She does what I tell her to and orders a mammogram every year. But, I don’t go to the oncologist anymore, and so I worry. With the specialists, I feel protected. (FocGrp1#5)
Physicians acknowledged the lack of a formal transition to primary care such as a survivorship care program.
Follow-up care issues were common. Physicians stressed that women needed to know how often to return for follow-up once active treatment ends and about recommended examinations and tests, especially when receiving hormonal therapy. Physicians indicated the need for patient education materials specific to patients’ treatments, for example, elevated risk of heart disease with certain chemotherapy agents. An oncologist expressed concern that PCPs are not prepared adequately about late effects and hormonal treatment side effects, and suggested providing summary notes for PCPs detailing these.
Survivors identified several barriers to follow-up care: lacking information on which symptoms merited a call to physicians; financial burden/limited health insurance; lacking appointment reminders; fear of examinations; and limited English proficiency. A survivor stated,
If you have insurance, you can make your appointment, see the doctor, and have your mammogram. I stopped taking my pills because I didn’t have insurance. I tried to get them again but they told me they would cost me a thousand dollars. (FocGrp1#5)
One oncologist suggested scheduling a follow-up appointment before patients leave treatment and calling patients who miss appointments.
Facilitators of regular follow-up care identified by survivors were physicians informing them about symptom monitoring and reporting, having a clinic contact person/navigator, being given a follow-up appointment, being assertive about one’s care, and physicians’ reinforcement of adherence to hormonal treatment and follow-up. According to support providers, a key facilitator was having a clinic contact person/navigator. Once treatment ended, support providers often served as the liaison between the patient and the physician, making them the first point of contact for symptom reporting.
Fear of recurrence especially when obtaining follow-up care. Fear of recurrence dominated survivor interviews. This fear was heightened at the time of follow-up examinations or when they experienced unusual pain. A survivor commented,
Every time I’m due for my mammogram, I can’t sleep, worrying. I lose sleep until I get the letter with my results. Then I feel at peace again. (FocGrp1#9)
Support providers discussed the need to provide reassurance to SSBCS to help them cope with fears of recurrence. Physicians expressed challenges in allaying fears of recurrence among SSBCS, requiring a lot of time when recommending follow-up mammograms.
Desire for information on late effects of treatments and side effects of hormonal therapies. All survivors expressed receiving insufficient information on potential symptoms and side effects. One stated,
Doctors only have five minutes. There has never been someone who gave me guidance like, ‘From now on you have to do this or you might get these symptoms now or in the future. (Survivor#6019)
They indicated uncertainty about what symptoms were “normal” and when symptoms merited a call to the physician. Several survivors reported being unaware that fatigue, arthralgia and neuropathy were side effects of breast cancer treatments until they reported these to physicians.
Physicians stressed the importance of women knowing about the elevated risk of future cancers, symptoms of recurrence, and seeking follow-up care if they experience symptoms that are out of the ordinary. Support providers felt that it was important to provide SSBCS with information on signs of recurrence and when to report these. However, providers expressed concern that giving women too much information might elevate their anxiety. A physician suggested,
It’s probably better to have a symptom list that’s short and relevant for the most common and catastrophic things, same thing with side effects … short to avoid overwhelming the patient. (Physician#001)
Hormonal treatments were of special concern. Survivors expressed a need for information on hormonal treatments and support providers stressed that this information is needed in simple Spanish. Several survivors indicated they stopped taking hormonal treatments due to side effects. One woman experienced severe headaches and heart palpitations, stopped taking the hormonal medication, felt better, and did not inform her physician until her next appointment. A support provider stated,
What I hear from a lot of women is that if side effects are too uncomfortable, they just stop it (hormonal treatment) without saying anything to the doctor. So more information about why they have to take it and that there is a good chance of recurrence is really important. (SuppProv#101)
Likewise, physicians indicated that SSBCS’ lack of information on hormonal treatments often resulted in nonadherence, emphasizing the need to reinforce adherence to prevent recurrence.
Conceptual framework of interventions
Based on triangulation of survey and interview results, we compiled a conceptual framework that includes needs identified, suggested components of a survivorship care intervention to address these needs, potential mediators by which such interventions could improve outcomes, and relevant outcomes (Figure). Survivorship care needs fell into four categories: symptom management, psychosocial, sense of abandonment by health care team, and healthy lifestyles. Survivorship care programs would provide skills training in symptom and stress management, and communicating with providers, family, friends, and coworkers. Mediators include increased self-efficacy, knowledge and perceived social support, ultimately leading to reduced distress (anxiety and depressive symptoms) and stress, and improved health-related quality of life.
Conclusions
Our study aimed to identify the most critical needs of SSBCS in the posttreatment survivorship phase to facilitate the design of survivorship interventions for this vulnerable group. SSBCS, cancer support providers, and cancer physicians reported substantial symptom management, psychosocial, and informational needs among this population. Results from surveys and open-ended interviews were remarkably consistent. Survivors, physicians, and support providers viewed transition out of active treatment as a time of increased psychosocial need and heightened vulnerability.
Our findings are consistent with needs assessments conducted in other breast cancer survivors. Similar to a study of rural white women with breast cancer, fear of recurrence was among the most common psychosocial concerns.17 Results of two studies that included white, African American and Latina breast cancer survivors were consistent with ours in finding that pain and fatigue were among the most persistent symptoms; in both studies, Latinas were more likely to report pain and a higher number of symptoms.7,18 The prevalence of sleep problems in our sample was identical to that reported in a sample of African American breast cancer survivors.19 Our findings of a high need for symptom management information and support, social support from family and friends, and self-management resources were similar to studies of other vulnerable breast cancer survivors.18,20
Our results suggest that it is critical for health care professionals to provide assistance with managing side effects and information to alleviate fears, and reinforce behaviors of symptom monitoring and reporting, and adherence to follow-up care and hormonal therapies. Yet this information is not being conveyed effectively and is complicated by the need to balance women’s need for information with minimizing anxiety when providing such information.
A limitation of our study is that most of our sample was Mexican origin and may not reflect experiences of Spanish-speaking Latinas of other national origin groups or outside of Northern California. Another limitation is the lack of an English-speaking comparison group, which would have permitted the identification of similarities and differences across language groups. Finally, we did not interview radiation oncologists who may have had opinions that are not represented here.
Survivorship care programs offer great promise for meeting patients’ informational and symptom management needs and improving well-being and communication with clinicians.21 Due to limited access to survivorship care information, financial hardships, and pressures from their families to resume their social roles, concerted efforts are needed to develop appropriate survivorship programs for SSBCS.22 Unique language, cultural and socioeconomic factors of Spanish-speaking Latinas require tailoring of cancer survivorship programs to best meet their needs.23 These programs need to provide psychosocial stress and symptom management assistance, simple information on recommended follow-up care, and healthy lifestyle and role reintegration strategies that account for their unique sociocultural contexts.
1. Danese MD, O’Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756-1765.
2. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academy of Sciences; 2006.
3. Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2(3):179-189.
4. Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on posttreatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270-2273.
5. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413-428.
6. Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ Rev. 2008;29(4):23-40.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Posttreatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-256.
8. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362.
9. Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29(10):1280-1289.
10. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058-1067.
11. Yoon J, Malin JL, Tisnado DM, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69-77.
12. Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors. Psychooncology. 2014;23(5):507-515.
13. Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795-806.
14. Napoles AM, Ortiz C, Santoyo-Olsson J, et al. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105(suppl 3):e55-63.
15. Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. ‘I learned to live with it’ is not good enough: challenges reported by posttreatment cancer survivors in the Livestrong surveys. Austin, TX: Livestrong;2011.
16. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Hawthorne: Aldine Publishing Company; 1967.
17. Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Womens Health (Larchmt). 2011;20(9):1307-1313.
18. Fu OS, Crew KD, Jacobson JS, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3(4):241-250.
19. Taylor TR, Huntley ED, Makambi K, et al. Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psychooncology. 2012;21(8):896-902.
20. Adams N, Gisiger-Camata S, Hardy CM, Thomas TF, Jukkala A, Meneses K. Evaluating survivorship experiences and needs among rural African American breast cancer survivors. J Cancer Educ. October 24, 2015 [Epub ahead of print].
21. Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664-1674.
22. Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724-733.
23. Napoles-Springer AM, Ortiz C, O’Brien H, Diaz-Mendez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268-280
After treatment, cancer patients transition to a survivorship phase, often with little information or support. Cancer survivors are at increased risk of recurrence, secondary cancers, comorbid conditions, and late treatment effects.1,2 However, many remain unaware of these risks and the options for managing them3 and face numerous unmet medical, psychosocial, and informational needs that can be addressed through survivorship care programs.4 Anxiety may increase as they lose their treatment team’s support while attempting to reestablish their lives.2 Patients need to know the long-term risks of cancer treatments, probabilities of recurrence and second cancers, effectiveness of surveillance and interventions for managing late effects and psychosocial concerns, and benefits of healthy lifestyles.2
Due to sociocultural and economic factors, Spanish-speaking Latina breast cancer survivors (SSBCS) suffer worse posttreatment health-related quality of life and more pain, fatigue, depressive symptoms, body image issues, and distress than their white counterparts.5-7 However, they are less likely to receive necessary cancer treatment, symptom management, and surveillance. For example, compared with whites, Latina breast cancer survivors receive less guideline-adherent treatment8 and follow-up care, including survivorship information.3,9 SSBCS, in particular have less access to survivorship information.10 Consequently, SSBCS are more likely to report unmet symptom management needs.11
Several breast cancer survivorship program trials have included Latinas,12,13 but their effectiveness has been demonstrated only for depressive symptoms or health worry. A comprehensive assessment of the posttreatment needs of SSBCS would provide a foundation for designing tailored survivorship interventions for this vulnerable group. This study aimed to identify the symptom management, psychosocial, and informational needs of SSBCS during the transition to survivorship from the perspectives of SSBCS and their cancer support providers and cancer physicians.
Methods
We sampled respondents within a 5-county area in Northern California to obtain multiple perspectives of the survivorship care needs of SSBCS using structured and in-depth methods: a telephone survey of SSBCS; semistructured interviews with SSBCS; semistructured interviews with cancer support providers serving SSBCS; and semistructured interviews with physicians providing cancer care for SSBCS. The study protocol was approved by the University of California San Francisco Committee on Human Research.
Sample and procedures
Structured telephone survey with SSBCS. The sample was drawn evenly from San Francisco General Hospital-University of California San Francisco primary care practices and SSBCS from a previous study who agreed to be re-contacted.14 The inclusion criteria were: completed active treatment (except adjuvant hormonal therapy) for nonmetastatic breast cancer within 10 years; living in one of the five counties; primarily Spanish-speaking; and self-identified as Latina. The exclusion criteria were: previous cancer except nonmelanoma skin cancer; terminal illness; or metastatic breast cancer. Study staff mailed potential participants a bilingual letter and information sheet, and bilingual opt-out postcard (6th grade reading level assessed by Flesch-Kincaid grade level statistic). Female bilingual-bicultural research associates conducted interviews of 20-30 minutes in Spanish after obtaining verbal consent. Participants were mailed $20. Surveys were conducted during March-November 2014.
Semistructured in-person interviews with SSBCS. Four community-based organizations (CBOs) in the targeted area providing cancer support services to Latinos agreed to recruit SSBCS for interviews. Inclusion criteria were identical to the survey. Patient navigators or support providers from CBOs contacted women by phone or in-person to invite them to an interview to assess their cancer survivorship needs. Women could choose a focus group or individual interview. With permission, names and contact information were given to study interviewers who called, explained the study, screened for eligibility, and scheduled an interview.
Recruitment was stratified by age (under or over age 50). We sampled women until saturation was achieved (no new themes emerged). Focus groups (90 minutes) were conducted at the CBOs. Individual interviews (45 minutes) were conducted in participants’ homes. Written informed consent was obtained. Participants were paid $50. Interviews were conducted during August-November, 2014, audiotaped, and transcribed.
Semistructured in-person interviews with cancer support providers and physicians. Investigators invited five cancer support providers (three patient navigators from three county hospitals, and two CBO directors of cancer psychosocial support services) and four physicians (three oncologists and one breast cancer surgeon from three county hospitals) to an in-person interview to identify SSBCS’ survivorship care needs. All agreed to participate. No further candidates were approached because saturation was achieved. We obtained written informed consent and 30-minute interviews were conducted in participants’ offices during August-October, 2014. Interviews were audiotaped and transcribed. Participants were paid $50.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Measures
Structured telephone survey. Based on cancer survivor needs assessments,15 we assessed: physical and emotional symptoms; problems with sleep and memory/concentration; concerns about mortality, family, social isolation, intimacy, appearance; and healthy lifestyles. Items were adapted and translated into Spanish if needed, using forward/backward translation with team reconciliation. These questions used the introduction, “Now I am going to ask you if you have had any problems because of your cancer. In the past month, how much have you been bothered by …” with responses rated on a scale of 1-5 (1, Not at all; 5, A lot). For example, we asked, “In the past month, how much have you been bothered by fatigue?”
Regarding healthy lifestyles, we used the introduction, “Here are some changes women sometimes want to make after cancer. Would you like help with …?” For example, we asked, “Would you like help with getting more exercise?” We asked if they wanted help getting more exercise, eating healthier, managing stress, and doing meditation or yoga (Yes/No).
Semistructured interview guide for SSBCS. Participants were asked about their emotional and physical concerns when treatment ended, current cancer needs, symptoms or late effects, and issues related to relationships, family, employment, insurance, financial hardships, barriers to follow-up care, health behaviors, and survivorship program content. Sample questions are, “Have you had any symptoms or side effects related to your cancer or treatment?” and “What kinds of information do you feel you need now about your cancer or treatment?” A brief questionnaire assessed demographics.
Semistructured interview guide for cancer support providers and physicians. Support providers and physicians were asked about informational, psychosocial, and symptom management needs of SSBCS and recommended self-management content and formats. Sample questions are, “What kinds of information and support do you wish was available to help Spanish-speaking women take care of their health after treatment ends?” and “What do you think are the most pressing emotional needs of Spanish-speaking women after breast cancer treatment ends?” A brief questionnaire assessed demographics.
Analysis
Frequencies are reported for survey items. For questions about symptoms/concerns, we report the frequency of responding that they were bothered Somewhat/Quite a bit/A lot. For healthy lifestyles, we present the frequency answering Yes.
Verbatim semistructured interview transcripts were verified against audiotapes. Using QSR NVIVO software, transcripts were coded independently by two bilingual-bicultural investigators using a constant comparative method to generate coding categories for cancer survivorship needs.16 Coders started with themes specified by the interview guides, expanded them to represent the data, and discussed and reconciled coding discrepancies. Coding was compared by type of interview participant (survivor, support provider, or physician). Triangulation of survey and semistructured interviews occurred through team discussions to verify codes, themes, and implications for interventions.
Results
Telephone survey of SSBCS
Of the telephone survey sampling frame (N = 231), 118 individuals (51%) completed the interview, 37 (16%) were ineligible, 31 (13%) could not be reached, 22 (10%) had incorrect contact information, 19 (8%) refused to participate, and 4 (2%) were deceased. Mean age of the participants was 54.9 years (SD, 12.3); all were foreign-born, with more than half of Mexican origin; and most had less than a high-school education (Table 1). All had completed active treatment, and most (68%) were within 2 years of diagnosis.
For symptom management needs (Table 2), the most prevalent (bothersome) symptoms (reported by more than 30%, in rank order) were joint pain, sleep problems, fatigue, hot flashes, numbness/tingling of extremities, and memory. Next most prevalent (reported by 20%-30%) were vaginal dryness, dry/itchy skin, dry nose/mouth, inability to concentrate, constipation, changes in urination, and shortness of breath.

For psychosocial needs, fears of recurrence or new cancers were reported by 42%. Emotional symptoms reported by more than 30% were depression/sadness, anxieties, and feeling stressed. Next most prevalent (20%-30%) were fears, loss of interest in usual activities, and nervousness/restlessness. Social well-being concerns reported by 20%-30% of survivors were loneliness, having no one to talk to, and being a burden to their families. Body image and sexual problems reported by 20%-30% of survivors included appearance and problems being intimate with partners.
Regarding lifestyle, most of the participants said they wanted help with eating a healthier diet (74%), getting more exercise (69%), managing stress (63%), and doing yoga or meditation (55%).
Semistructured interviews
Twenty-five SSBCS completed semistructured interviews, 10 in individual interviews and 15 in one of two focus groups (one of 9 women older than 50 years; one of 6 women younger than 50). The telephone survey respondents were similar to semistructured interview respondents on all sociodemographic characteristics, but differed slightly on some clinical characteristics (Table 1). The telephone survey women had been more recently diagnosed (P < .01), were less likely to have ductal carcinoma in situ (P < .001), and more likely to have had reconstructive surgery
(P < .05).
Five cancer support providers and 4 physicians were interviewed. All support providers were Spanish-speaking Latinas with at least some college education. Cancer physicians were board certified. Two were men; two were white and two Asian; one was a breast surgeon and three were hematologists/oncologists; three spoke Spanish poorly/not at all and one spoke it fairly well.
Seven themes emerged from interviews: unmet physical symptom management needs; social support often ends when treatment ends; challenges resuming roles; sense of abandonment by health care system when treatment ends; need for formal transition from active treatment to follow-up care; fear of recurrence especially when obtaining follow-up care; and desire for information on late effects of initial treatments and side effects of hormonal treatments. We summarize results according to these themes.
Unmet physical symptom management needs. The main physical symptoms reported by survivors and physicians in interviews were arthralgia, menopausal symptoms, and neuropathy. Fatigue was reported only by survivors. Many survivors and several support providers expressed that symptoms were poorly managed and often ignored. One stated,
I have a lot of pain where I had surgery, it burns. I worry a lot about my arm because I have sacs of fluid. My doctor only says, ‘They will dissolve over the years.’ So, I don’t feel any support. (FocGrp1#6)
Survivors reported side effects of hormonal therapies, and felt that physicians downplayed these to prevent them from discontinuing medications.
Social support from family and friends often ends when treatment ends. Many survivors described a loss of support from family and friends who expected them to get back to “normal” once treatment ended. One said,
My sisters have told me to my face that there’s nothing wrong with me. So now when people ask me, I say, ‘I’m fine, thank God, I have nothing,’ even though I’m dying of pain and have all these pills to take. (Survivor#1025)
A support provider related,
The client was telling me that as she was getting closer to finishing her treatment, her husband was upset because he felt like all she was doing was focusing on the cancer. I think caregivers, family, spouses, and children out of their own sort of selfishness want this person to be well. (SuppProv#104)
A few survivors said that family bonds were strengthened after cancer and several reported lacking support because their families were in their home countries.
Challenges resuming roles, especially returning to work. Survivors, support providers, and physicians described challenges and few resources as women transition back to their normal roles. Survivors questioned their ability to return to work due to physically demanding occupations. One stated,
I would like information on how to take care of myself, how working can affect this side if I don’t take care of it. I clean houses and I need both hands. (Survivor#3012)
Survivors described how changes in memory affected daily chores and work performance. Support providers and physicians described the need for resources to aid with return to work and household responsibilities. One physician noted,
There are usually questions about how to go ahead and live their lives from that point forward. It’s a sort of reverse shock: going back to life as they know it. (Physician#004)
Support providers and physicians mentioned that women needed help with resuming intimate partner relations.
Sense of abandonment by health care system once active treatment ends. Survivors, support providers, and physicians reported a loss of support and sense of abandonment by the patients’ oncology team at the end of active treatment. One survivor stated,
Once they tell you to stop the pills, ‘You’re cured, there’s nothing wrong with you,’ the truth is that one feels, ‘Now what do I do? I have no one to help me.’ I felt very abandoned. (FocGrp1#5)
A provider said,
The support system falls apart once women complete treatment. They lose their entire support system at the medical level. They no longer have nurses checking in about symptoms and addressing anything that’s come up. They won’t have access to doctors unless they’re doing their screening. (SuppProv#101)
An oncologist, noting that this loss of support occurs when women face pressures of transitioning back to work or family obligations, commented,
So here’s a woman whose marriage is in turmoil, whose husband may even have left her during this, and now her clinic is leaving her and she’s on her own … that must be scary as hell because there’s nobody out there to support her. (Physician#002)
Need for formal transition from active treatment to follow-up care. Two themes emerged about transitioning from active treatment: transferring care from oncologists to primary care physicians (PCP); and issues of follow-up care (with oncologists or PCPs). Survivors felt lost in transitioning from specialty to primary care, or expressed apprehension seeing a PCP rather than a cancer specialist. One stated,
I have my doctor but she is not a specialist. She does what I tell her to and orders a mammogram every year. But, I don’t go to the oncologist anymore, and so I worry. With the specialists, I feel protected. (FocGrp1#5)
Physicians acknowledged the lack of a formal transition to primary care such as a survivorship care program.
Follow-up care issues were common. Physicians stressed that women needed to know how often to return for follow-up once active treatment ends and about recommended examinations and tests, especially when receiving hormonal therapy. Physicians indicated the need for patient education materials specific to patients’ treatments, for example, elevated risk of heart disease with certain chemotherapy agents. An oncologist expressed concern that PCPs are not prepared adequately about late effects and hormonal treatment side effects, and suggested providing summary notes for PCPs detailing these.
Survivors identified several barriers to follow-up care: lacking information on which symptoms merited a call to physicians; financial burden/limited health insurance; lacking appointment reminders; fear of examinations; and limited English proficiency. A survivor stated,
If you have insurance, you can make your appointment, see the doctor, and have your mammogram. I stopped taking my pills because I didn’t have insurance. I tried to get them again but they told me they would cost me a thousand dollars. (FocGrp1#5)
One oncologist suggested scheduling a follow-up appointment before patients leave treatment and calling patients who miss appointments.
Facilitators of regular follow-up care identified by survivors were physicians informing them about symptom monitoring and reporting, having a clinic contact person/navigator, being given a follow-up appointment, being assertive about one’s care, and physicians’ reinforcement of adherence to hormonal treatment and follow-up. According to support providers, a key facilitator was having a clinic contact person/navigator. Once treatment ended, support providers often served as the liaison between the patient and the physician, making them the first point of contact for symptom reporting.
Fear of recurrence especially when obtaining follow-up care. Fear of recurrence dominated survivor interviews. This fear was heightened at the time of follow-up examinations or when they experienced unusual pain. A survivor commented,
Every time I’m due for my mammogram, I can’t sleep, worrying. I lose sleep until I get the letter with my results. Then I feel at peace again. (FocGrp1#9)
Support providers discussed the need to provide reassurance to SSBCS to help them cope with fears of recurrence. Physicians expressed challenges in allaying fears of recurrence among SSBCS, requiring a lot of time when recommending follow-up mammograms.
Desire for information on late effects of treatments and side effects of hormonal therapies. All survivors expressed receiving insufficient information on potential symptoms and side effects. One stated,
Doctors only have five minutes. There has never been someone who gave me guidance like, ‘From now on you have to do this or you might get these symptoms now or in the future. (Survivor#6019)
They indicated uncertainty about what symptoms were “normal” and when symptoms merited a call to the physician. Several survivors reported being unaware that fatigue, arthralgia and neuropathy were side effects of breast cancer treatments until they reported these to physicians.
Physicians stressed the importance of women knowing about the elevated risk of future cancers, symptoms of recurrence, and seeking follow-up care if they experience symptoms that are out of the ordinary. Support providers felt that it was important to provide SSBCS with information on signs of recurrence and when to report these. However, providers expressed concern that giving women too much information might elevate their anxiety. A physician suggested,
It’s probably better to have a symptom list that’s short and relevant for the most common and catastrophic things, same thing with side effects … short to avoid overwhelming the patient. (Physician#001)
Hormonal treatments were of special concern. Survivors expressed a need for information on hormonal treatments and support providers stressed that this information is needed in simple Spanish. Several survivors indicated they stopped taking hormonal treatments due to side effects. One woman experienced severe headaches and heart palpitations, stopped taking the hormonal medication, felt better, and did not inform her physician until her next appointment. A support provider stated,
What I hear from a lot of women is that if side effects are too uncomfortable, they just stop it (hormonal treatment) without saying anything to the doctor. So more information about why they have to take it and that there is a good chance of recurrence is really important. (SuppProv#101)
Likewise, physicians indicated that SSBCS’ lack of information on hormonal treatments often resulted in nonadherence, emphasizing the need to reinforce adherence to prevent recurrence.
Conceptual framework of interventions
Based on triangulation of survey and interview results, we compiled a conceptual framework that includes needs identified, suggested components of a survivorship care intervention to address these needs, potential mediators by which such interventions could improve outcomes, and relevant outcomes (Figure). Survivorship care needs fell into four categories: symptom management, psychosocial, sense of abandonment by health care team, and healthy lifestyles. Survivorship care programs would provide skills training in symptom and stress management, and communicating with providers, family, friends, and coworkers. Mediators include increased self-efficacy, knowledge and perceived social support, ultimately leading to reduced distress (anxiety and depressive symptoms) and stress, and improved health-related quality of life.
Conclusions
Our study aimed to identify the most critical needs of SSBCS in the posttreatment survivorship phase to facilitate the design of survivorship interventions for this vulnerable group. SSBCS, cancer support providers, and cancer physicians reported substantial symptom management, psychosocial, and informational needs among this population. Results from surveys and open-ended interviews were remarkably consistent. Survivors, physicians, and support providers viewed transition out of active treatment as a time of increased psychosocial need and heightened vulnerability.
Our findings are consistent with needs assessments conducted in other breast cancer survivors. Similar to a study of rural white women with breast cancer, fear of recurrence was among the most common psychosocial concerns.17 Results of two studies that included white, African American and Latina breast cancer survivors were consistent with ours in finding that pain and fatigue were among the most persistent symptoms; in both studies, Latinas were more likely to report pain and a higher number of symptoms.7,18 The prevalence of sleep problems in our sample was identical to that reported in a sample of African American breast cancer survivors.19 Our findings of a high need for symptom management information and support, social support from family and friends, and self-management resources were similar to studies of other vulnerable breast cancer survivors.18,20
Our results suggest that it is critical for health care professionals to provide assistance with managing side effects and information to alleviate fears, and reinforce behaviors of symptom monitoring and reporting, and adherence to follow-up care and hormonal therapies. Yet this information is not being conveyed effectively and is complicated by the need to balance women’s need for information with minimizing anxiety when providing such information.
A limitation of our study is that most of our sample was Mexican origin and may not reflect experiences of Spanish-speaking Latinas of other national origin groups or outside of Northern California. Another limitation is the lack of an English-speaking comparison group, which would have permitted the identification of similarities and differences across language groups. Finally, we did not interview radiation oncologists who may have had opinions that are not represented here.
Survivorship care programs offer great promise for meeting patients’ informational and symptom management needs and improving well-being and communication with clinicians.21 Due to limited access to survivorship care information, financial hardships, and pressures from their families to resume their social roles, concerted efforts are needed to develop appropriate survivorship programs for SSBCS.22 Unique language, cultural and socioeconomic factors of Spanish-speaking Latinas require tailoring of cancer survivorship programs to best meet their needs.23 These programs need to provide psychosocial stress and symptom management assistance, simple information on recommended follow-up care, and healthy lifestyle and role reintegration strategies that account for their unique sociocultural contexts.
After treatment, cancer patients transition to a survivorship phase, often with little information or support. Cancer survivors are at increased risk of recurrence, secondary cancers, comorbid conditions, and late treatment effects.1,2 However, many remain unaware of these risks and the options for managing them3 and face numerous unmet medical, psychosocial, and informational needs that can be addressed through survivorship care programs.4 Anxiety may increase as they lose their treatment team’s support while attempting to reestablish their lives.2 Patients need to know the long-term risks of cancer treatments, probabilities of recurrence and second cancers, effectiveness of surveillance and interventions for managing late effects and psychosocial concerns, and benefits of healthy lifestyles.2
Due to sociocultural and economic factors, Spanish-speaking Latina breast cancer survivors (SSBCS) suffer worse posttreatment health-related quality of life and more pain, fatigue, depressive symptoms, body image issues, and distress than their white counterparts.5-7 However, they are less likely to receive necessary cancer treatment, symptom management, and surveillance. For example, compared with whites, Latina breast cancer survivors receive less guideline-adherent treatment8 and follow-up care, including survivorship information.3,9 SSBCS, in particular have less access to survivorship information.10 Consequently, SSBCS are more likely to report unmet symptom management needs.11
Several breast cancer survivorship program trials have included Latinas,12,13 but their effectiveness has been demonstrated only for depressive symptoms or health worry. A comprehensive assessment of the posttreatment needs of SSBCS would provide a foundation for designing tailored survivorship interventions for this vulnerable group. This study aimed to identify the symptom management, psychosocial, and informational needs of SSBCS during the transition to survivorship from the perspectives of SSBCS and their cancer support providers and cancer physicians.
Methods
We sampled respondents within a 5-county area in Northern California to obtain multiple perspectives of the survivorship care needs of SSBCS using structured and in-depth methods: a telephone survey of SSBCS; semistructured interviews with SSBCS; semistructured interviews with cancer support providers serving SSBCS; and semistructured interviews with physicians providing cancer care for SSBCS. The study protocol was approved by the University of California San Francisco Committee on Human Research.
Sample and procedures
Structured telephone survey with SSBCS. The sample was drawn evenly from San Francisco General Hospital-University of California San Francisco primary care practices and SSBCS from a previous study who agreed to be re-contacted.14 The inclusion criteria were: completed active treatment (except adjuvant hormonal therapy) for nonmetastatic breast cancer within 10 years; living in one of the five counties; primarily Spanish-speaking; and self-identified as Latina. The exclusion criteria were: previous cancer except nonmelanoma skin cancer; terminal illness; or metastatic breast cancer. Study staff mailed potential participants a bilingual letter and information sheet, and bilingual opt-out postcard (6th grade reading level assessed by Flesch-Kincaid grade level statistic). Female bilingual-bicultural research associates conducted interviews of 20-30 minutes in Spanish after obtaining verbal consent. Participants were mailed $20. Surveys were conducted during March-November 2014.
Semistructured in-person interviews with SSBCS. Four community-based organizations (CBOs) in the targeted area providing cancer support services to Latinos agreed to recruit SSBCS for interviews. Inclusion criteria were identical to the survey. Patient navigators or support providers from CBOs contacted women by phone or in-person to invite them to an interview to assess their cancer survivorship needs. Women could choose a focus group or individual interview. With permission, names and contact information were given to study interviewers who called, explained the study, screened for eligibility, and scheduled an interview.
Recruitment was stratified by age (under or over age 50). We sampled women until saturation was achieved (no new themes emerged). Focus groups (90 minutes) were conducted at the CBOs. Individual interviews (45 minutes) were conducted in participants’ homes. Written informed consent was obtained. Participants were paid $50. Interviews were conducted during August-November, 2014, audiotaped, and transcribed.
Semistructured in-person interviews with cancer support providers and physicians. Investigators invited five cancer support providers (three patient navigators from three county hospitals, and two CBO directors of cancer psychosocial support services) and four physicians (three oncologists and one breast cancer surgeon from three county hospitals) to an in-person interview to identify SSBCS’ survivorship care needs. All agreed to participate. No further candidates were approached because saturation was achieved. We obtained written informed consent and 30-minute interviews were conducted in participants’ offices during August-October, 2014. Interviews were audiotaped and transcribed. Participants were paid $50.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Measures
Structured telephone survey. Based on cancer survivor needs assessments,15 we assessed: physical and emotional symptoms; problems with sleep and memory/concentration; concerns about mortality, family, social isolation, intimacy, appearance; and healthy lifestyles. Items were adapted and translated into Spanish if needed, using forward/backward translation with team reconciliation. These questions used the introduction, “Now I am going to ask you if you have had any problems because of your cancer. In the past month, how much have you been bothered by …” with responses rated on a scale of 1-5 (1, Not at all; 5, A lot). For example, we asked, “In the past month, how much have you been bothered by fatigue?”
Regarding healthy lifestyles, we used the introduction, “Here are some changes women sometimes want to make after cancer. Would you like help with …?” For example, we asked, “Would you like help with getting more exercise?” We asked if they wanted help getting more exercise, eating healthier, managing stress, and doing meditation or yoga (Yes/No).
Semistructured interview guide for SSBCS. Participants were asked about their emotional and physical concerns when treatment ended, current cancer needs, symptoms or late effects, and issues related to relationships, family, employment, insurance, financial hardships, barriers to follow-up care, health behaviors, and survivorship program content. Sample questions are, “Have you had any symptoms or side effects related to your cancer or treatment?” and “What kinds of information do you feel you need now about your cancer or treatment?” A brief questionnaire assessed demographics.
Semistructured interview guide for cancer support providers and physicians. Support providers and physicians were asked about informational, psychosocial, and symptom management needs of SSBCS and recommended self-management content and formats. Sample questions are, “What kinds of information and support do you wish was available to help Spanish-speaking women take care of their health after treatment ends?” and “What do you think are the most pressing emotional needs of Spanish-speaking women after breast cancer treatment ends?” A brief questionnaire assessed demographics.
Analysis
Frequencies are reported for survey items. For questions about symptoms/concerns, we report the frequency of responding that they were bothered Somewhat/Quite a bit/A lot. For healthy lifestyles, we present the frequency answering Yes.
Verbatim semistructured interview transcripts were verified against audiotapes. Using QSR NVIVO software, transcripts were coded independently by two bilingual-bicultural investigators using a constant comparative method to generate coding categories for cancer survivorship needs.16 Coders started with themes specified by the interview guides, expanded them to represent the data, and discussed and reconciled coding discrepancies. Coding was compared by type of interview participant (survivor, support provider, or physician). Triangulation of survey and semistructured interviews occurred through team discussions to verify codes, themes, and implications for interventions.
Results
Telephone survey of SSBCS
Of the telephone survey sampling frame (N = 231), 118 individuals (51%) completed the interview, 37 (16%) were ineligible, 31 (13%) could not be reached, 22 (10%) had incorrect contact information, 19 (8%) refused to participate, and 4 (2%) were deceased. Mean age of the participants was 54.9 years (SD, 12.3); all were foreign-born, with more than half of Mexican origin; and most had less than a high-school education (Table 1). All had completed active treatment, and most (68%) were within 2 years of diagnosis.
For symptom management needs (Table 2), the most prevalent (bothersome) symptoms (reported by more than 30%, in rank order) were joint pain, sleep problems, fatigue, hot flashes, numbness/tingling of extremities, and memory. Next most prevalent (reported by 20%-30%) were vaginal dryness, dry/itchy skin, dry nose/mouth, inability to concentrate, constipation, changes in urination, and shortness of breath.

For psychosocial needs, fears of recurrence or new cancers were reported by 42%. Emotional symptoms reported by more than 30% were depression/sadness, anxieties, and feeling stressed. Next most prevalent (20%-30%) were fears, loss of interest in usual activities, and nervousness/restlessness. Social well-being concerns reported by 20%-30% of survivors were loneliness, having no one to talk to, and being a burden to their families. Body image and sexual problems reported by 20%-30% of survivors included appearance and problems being intimate with partners.
Regarding lifestyle, most of the participants said they wanted help with eating a healthier diet (74%), getting more exercise (69%), managing stress (63%), and doing yoga or meditation (55%).
Semistructured interviews
Twenty-five SSBCS completed semistructured interviews, 10 in individual interviews and 15 in one of two focus groups (one of 9 women older than 50 years; one of 6 women younger than 50). The telephone survey respondents were similar to semistructured interview respondents on all sociodemographic characteristics, but differed slightly on some clinical characteristics (Table 1). The telephone survey women had been more recently diagnosed (P < .01), were less likely to have ductal carcinoma in situ (P < .001), and more likely to have had reconstructive surgery
(P < .05).
Five cancer support providers and 4 physicians were interviewed. All support providers were Spanish-speaking Latinas with at least some college education. Cancer physicians were board certified. Two were men; two were white and two Asian; one was a breast surgeon and three were hematologists/oncologists; three spoke Spanish poorly/not at all and one spoke it fairly well.
Seven themes emerged from interviews: unmet physical symptom management needs; social support often ends when treatment ends; challenges resuming roles; sense of abandonment by health care system when treatment ends; need for formal transition from active treatment to follow-up care; fear of recurrence especially when obtaining follow-up care; and desire for information on late effects of initial treatments and side effects of hormonal treatments. We summarize results according to these themes.
Unmet physical symptom management needs. The main physical symptoms reported by survivors and physicians in interviews were arthralgia, menopausal symptoms, and neuropathy. Fatigue was reported only by survivors. Many survivors and several support providers expressed that symptoms were poorly managed and often ignored. One stated,
I have a lot of pain where I had surgery, it burns. I worry a lot about my arm because I have sacs of fluid. My doctor only says, ‘They will dissolve over the years.’ So, I don’t feel any support. (FocGrp1#6)
Survivors reported side effects of hormonal therapies, and felt that physicians downplayed these to prevent them from discontinuing medications.
Social support from family and friends often ends when treatment ends. Many survivors described a loss of support from family and friends who expected them to get back to “normal” once treatment ended. One said,
My sisters have told me to my face that there’s nothing wrong with me. So now when people ask me, I say, ‘I’m fine, thank God, I have nothing,’ even though I’m dying of pain and have all these pills to take. (Survivor#1025)
A support provider related,
The client was telling me that as she was getting closer to finishing her treatment, her husband was upset because he felt like all she was doing was focusing on the cancer. I think caregivers, family, spouses, and children out of their own sort of selfishness want this person to be well. (SuppProv#104)
A few survivors said that family bonds were strengthened after cancer and several reported lacking support because their families were in their home countries.
Challenges resuming roles, especially returning to work. Survivors, support providers, and physicians described challenges and few resources as women transition back to their normal roles. Survivors questioned their ability to return to work due to physically demanding occupations. One stated,
I would like information on how to take care of myself, how working can affect this side if I don’t take care of it. I clean houses and I need both hands. (Survivor#3012)
Survivors described how changes in memory affected daily chores and work performance. Support providers and physicians described the need for resources to aid with return to work and household responsibilities. One physician noted,
There are usually questions about how to go ahead and live their lives from that point forward. It’s a sort of reverse shock: going back to life as they know it. (Physician#004)
Support providers and physicians mentioned that women needed help with resuming intimate partner relations.
Sense of abandonment by health care system once active treatment ends. Survivors, support providers, and physicians reported a loss of support and sense of abandonment by the patients’ oncology team at the end of active treatment. One survivor stated,
Once they tell you to stop the pills, ‘You’re cured, there’s nothing wrong with you,’ the truth is that one feels, ‘Now what do I do? I have no one to help me.’ I felt very abandoned. (FocGrp1#5)
A provider said,
The support system falls apart once women complete treatment. They lose their entire support system at the medical level. They no longer have nurses checking in about symptoms and addressing anything that’s come up. They won’t have access to doctors unless they’re doing their screening. (SuppProv#101)
An oncologist, noting that this loss of support occurs when women face pressures of transitioning back to work or family obligations, commented,
So here’s a woman whose marriage is in turmoil, whose husband may even have left her during this, and now her clinic is leaving her and she’s on her own … that must be scary as hell because there’s nobody out there to support her. (Physician#002)
Need for formal transition from active treatment to follow-up care. Two themes emerged about transitioning from active treatment: transferring care from oncologists to primary care physicians (PCP); and issues of follow-up care (with oncologists or PCPs). Survivors felt lost in transitioning from specialty to primary care, or expressed apprehension seeing a PCP rather than a cancer specialist. One stated,
I have my doctor but she is not a specialist. She does what I tell her to and orders a mammogram every year. But, I don’t go to the oncologist anymore, and so I worry. With the specialists, I feel protected. (FocGrp1#5)
Physicians acknowledged the lack of a formal transition to primary care such as a survivorship care program.
Follow-up care issues were common. Physicians stressed that women needed to know how often to return for follow-up once active treatment ends and about recommended examinations and tests, especially when receiving hormonal therapy. Physicians indicated the need for patient education materials specific to patients’ treatments, for example, elevated risk of heart disease with certain chemotherapy agents. An oncologist expressed concern that PCPs are not prepared adequately about late effects and hormonal treatment side effects, and suggested providing summary notes for PCPs detailing these.
Survivors identified several barriers to follow-up care: lacking information on which symptoms merited a call to physicians; financial burden/limited health insurance; lacking appointment reminders; fear of examinations; and limited English proficiency. A survivor stated,
If you have insurance, you can make your appointment, see the doctor, and have your mammogram. I stopped taking my pills because I didn’t have insurance. I tried to get them again but they told me they would cost me a thousand dollars. (FocGrp1#5)
One oncologist suggested scheduling a follow-up appointment before patients leave treatment and calling patients who miss appointments.
Facilitators of regular follow-up care identified by survivors were physicians informing them about symptom monitoring and reporting, having a clinic contact person/navigator, being given a follow-up appointment, being assertive about one’s care, and physicians’ reinforcement of adherence to hormonal treatment and follow-up. According to support providers, a key facilitator was having a clinic contact person/navigator. Once treatment ended, support providers often served as the liaison between the patient and the physician, making them the first point of contact for symptom reporting.
Fear of recurrence especially when obtaining follow-up care. Fear of recurrence dominated survivor interviews. This fear was heightened at the time of follow-up examinations or when they experienced unusual pain. A survivor commented,
Every time I’m due for my mammogram, I can’t sleep, worrying. I lose sleep until I get the letter with my results. Then I feel at peace again. (FocGrp1#9)
Support providers discussed the need to provide reassurance to SSBCS to help them cope with fears of recurrence. Physicians expressed challenges in allaying fears of recurrence among SSBCS, requiring a lot of time when recommending follow-up mammograms.
Desire for information on late effects of treatments and side effects of hormonal therapies. All survivors expressed receiving insufficient information on potential symptoms and side effects. One stated,
Doctors only have five minutes. There has never been someone who gave me guidance like, ‘From now on you have to do this or you might get these symptoms now or in the future. (Survivor#6019)
They indicated uncertainty about what symptoms were “normal” and when symptoms merited a call to the physician. Several survivors reported being unaware that fatigue, arthralgia and neuropathy were side effects of breast cancer treatments until they reported these to physicians.
Physicians stressed the importance of women knowing about the elevated risk of future cancers, symptoms of recurrence, and seeking follow-up care if they experience symptoms that are out of the ordinary. Support providers felt that it was important to provide SSBCS with information on signs of recurrence and when to report these. However, providers expressed concern that giving women too much information might elevate their anxiety. A physician suggested,
It’s probably better to have a symptom list that’s short and relevant for the most common and catastrophic things, same thing with side effects … short to avoid overwhelming the patient. (Physician#001)
Hormonal treatments were of special concern. Survivors expressed a need for information on hormonal treatments and support providers stressed that this information is needed in simple Spanish. Several survivors indicated they stopped taking hormonal treatments due to side effects. One woman experienced severe headaches and heart palpitations, stopped taking the hormonal medication, felt better, and did not inform her physician until her next appointment. A support provider stated,
What I hear from a lot of women is that if side effects are too uncomfortable, they just stop it (hormonal treatment) without saying anything to the doctor. So more information about why they have to take it and that there is a good chance of recurrence is really important. (SuppProv#101)
Likewise, physicians indicated that SSBCS’ lack of information on hormonal treatments often resulted in nonadherence, emphasizing the need to reinforce adherence to prevent recurrence.
Conceptual framework of interventions
Based on triangulation of survey and interview results, we compiled a conceptual framework that includes needs identified, suggested components of a survivorship care intervention to address these needs, potential mediators by which such interventions could improve outcomes, and relevant outcomes (Figure). Survivorship care needs fell into four categories: symptom management, psychosocial, sense of abandonment by health care team, and healthy lifestyles. Survivorship care programs would provide skills training in symptom and stress management, and communicating with providers, family, friends, and coworkers. Mediators include increased self-efficacy, knowledge and perceived social support, ultimately leading to reduced distress (anxiety and depressive symptoms) and stress, and improved health-related quality of life.
Conclusions
Our study aimed to identify the most critical needs of SSBCS in the posttreatment survivorship phase to facilitate the design of survivorship interventions for this vulnerable group. SSBCS, cancer support providers, and cancer physicians reported substantial symptom management, psychosocial, and informational needs among this population. Results from surveys and open-ended interviews were remarkably consistent. Survivors, physicians, and support providers viewed transition out of active treatment as a time of increased psychosocial need and heightened vulnerability.
Our findings are consistent with needs assessments conducted in other breast cancer survivors. Similar to a study of rural white women with breast cancer, fear of recurrence was among the most common psychosocial concerns.17 Results of two studies that included white, African American and Latina breast cancer survivors were consistent with ours in finding that pain and fatigue were among the most persistent symptoms; in both studies, Latinas were more likely to report pain and a higher number of symptoms.7,18 The prevalence of sleep problems in our sample was identical to that reported in a sample of African American breast cancer survivors.19 Our findings of a high need for symptom management information and support, social support from family and friends, and self-management resources were similar to studies of other vulnerable breast cancer survivors.18,20
Our results suggest that it is critical for health care professionals to provide assistance with managing side effects and information to alleviate fears, and reinforce behaviors of symptom monitoring and reporting, and adherence to follow-up care and hormonal therapies. Yet this information is not being conveyed effectively and is complicated by the need to balance women’s need for information with minimizing anxiety when providing such information.
A limitation of our study is that most of our sample was Mexican origin and may not reflect experiences of Spanish-speaking Latinas of other national origin groups or outside of Northern California. Another limitation is the lack of an English-speaking comparison group, which would have permitted the identification of similarities and differences across language groups. Finally, we did not interview radiation oncologists who may have had opinions that are not represented here.
Survivorship care programs offer great promise for meeting patients’ informational and symptom management needs and improving well-being and communication with clinicians.21 Due to limited access to survivorship care information, financial hardships, and pressures from their families to resume their social roles, concerted efforts are needed to develop appropriate survivorship programs for SSBCS.22 Unique language, cultural and socioeconomic factors of Spanish-speaking Latinas require tailoring of cancer survivorship programs to best meet their needs.23 These programs need to provide psychosocial stress and symptom management assistance, simple information on recommended follow-up care, and healthy lifestyle and role reintegration strategies that account for their unique sociocultural contexts.
1. Danese MD, O’Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756-1765.
2. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academy of Sciences; 2006.
3. Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2(3):179-189.
4. Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on posttreatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270-2273.
5. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413-428.
6. Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ Rev. 2008;29(4):23-40.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Posttreatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-256.
8. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362.
9. Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29(10):1280-1289.
10. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058-1067.
11. Yoon J, Malin JL, Tisnado DM, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69-77.
12. Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors. Psychooncology. 2014;23(5):507-515.
13. Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795-806.
14. Napoles AM, Ortiz C, Santoyo-Olsson J, et al. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105(suppl 3):e55-63.
15. Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. ‘I learned to live with it’ is not good enough: challenges reported by posttreatment cancer survivors in the Livestrong surveys. Austin, TX: Livestrong;2011.
16. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Hawthorne: Aldine Publishing Company; 1967.
17. Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Womens Health (Larchmt). 2011;20(9):1307-1313.
18. Fu OS, Crew KD, Jacobson JS, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3(4):241-250.
19. Taylor TR, Huntley ED, Makambi K, et al. Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psychooncology. 2012;21(8):896-902.
20. Adams N, Gisiger-Camata S, Hardy CM, Thomas TF, Jukkala A, Meneses K. Evaluating survivorship experiences and needs among rural African American breast cancer survivors. J Cancer Educ. October 24, 2015 [Epub ahead of print].
21. Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664-1674.
22. Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724-733.
23. Napoles-Springer AM, Ortiz C, O’Brien H, Diaz-Mendez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268-280
1. Danese MD, O’Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756-1765.
2. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academy of Sciences; 2006.
3. Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2(3):179-189.
4. Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on posttreatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270-2273.
5. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413-428.
6. Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ Rev. 2008;29(4):23-40.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Posttreatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-256.
8. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362.
9. Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29(10):1280-1289.
10. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058-1067.
11. Yoon J, Malin JL, Tisnado DM, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69-77.
12. Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors. Psychooncology. 2014;23(5):507-515.
13. Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795-806.
14. Napoles AM, Ortiz C, Santoyo-Olsson J, et al. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105(suppl 3):e55-63.
15. Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. ‘I learned to live with it’ is not good enough: challenges reported by posttreatment cancer survivors in the Livestrong surveys. Austin, TX: Livestrong;2011.
16. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Hawthorne: Aldine Publishing Company; 1967.
17. Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Womens Health (Larchmt). 2011;20(9):1307-1313.
18. Fu OS, Crew KD, Jacobson JS, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3(4):241-250.
19. Taylor TR, Huntley ED, Makambi K, et al. Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psychooncology. 2012;21(8):896-902.
20. Adams N, Gisiger-Camata S, Hardy CM, Thomas TF, Jukkala A, Meneses K. Evaluating survivorship experiences and needs among rural African American breast cancer survivors. J Cancer Educ. October 24, 2015 [Epub ahead of print].
21. Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664-1674.
22. Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724-733.
23. Napoles-Springer AM, Ortiz C, O’Brien H, Diaz-Mendez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268-280
Gap analysis: a strategy to improve the quality of care of head and neck cancer patients
In the United States, there will be an estimated 49,670 new cases of head and neck cancer for 2017.1 Head and neck cancer (HNC) is a term used to describe a range of tumors that originate in the area of the body spanning from the lower neck to the upper nasal cavity.2 Specifically, they are malignancies arising in the mouth, larynx, nasal cavity, sinuses, tongue, lips, and numerous glands such as the thyroid and salivary.2 To clarify, HNC, despite the encompassing name, does not include growths of the bones, teeth, skin, brain parenchyma, and eye; therefore, such tumors will not be addressed in this article.
Patients with HNC often experience fragmented and uncoordinated care that leads to delays in cancer treatment, severe distress in patients and families, and dissatisfaction with care. Literature reports that these patients face numerous stressors including aggressive cancer treatments, severe symptoms, body image concerns, loss of speech, difficulty swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis.3,4In addition, patients with HNC and their families are challenged to navigate the health care system and to overcome the difficulties of accessing services within the context of financial constraints. A multidisciplinary team (MDT) approach is the standard of care for HNC patients, as demonstrated in studies reporting better 5-year survival outcomes, increased completion of adjuvant therapy, and higher compliance with speech-language pathologist (SLP) recommendations.5, 6 Furthermore, a recent systematic review of cancer teams concluded that the MDT approach leads to improved clinical outcomes and enhanced communication between the patient and the team.7
The Institute of Medicine (IOM) stated in its 2013 report on cancer care that a high-quality care delivery system requires continuing measurement of cancer care and strategies to carry out performance improvement.8 Following the IOM premise, the cancer center at an academic medical center in Philadelphia made efforts to improve patient access to multidisciplinary services, first, by creating a multidisciplinary Cancer Appetite and Rehabilitation (CARE)clinic to address the symptoms and nutritional needs of HNC patients,9 and second, by using a gap analysis to conduct an assessment of the cancer care services provided to this cancer population. The need to conduct this assessment was generated by the desire to improve access to multidisciplinary care, with the goal of meeting standard benchmarks for completion of treatment while increasing the use of ancillary services. This article describes the process of conducting a gap analysis of cancer services for HNC patients, and includes discussion of the findings, recommendations for improving care, a description of the quality improvement interventions, and a report of the outcomes based on an interval re-assessment 18 months later.
Methods
Methods included a gap analysis, implementation of quality improvement recommendations, and re-assessment of indicators (Figure). A gap analysis “identifies differences between desired and actual practice conditions, including service delivery and quality patient outcomes as measured against evidence-based benchmarks while incorporating key stakeholder concerns and expectations.”10 The gap analysis of cancer care services offered to HNC patients was achieved through the step-by-step process described hereinafter. The implementation of quality improvement recommendations was accomplished by establishing two task force committees focused respectively on education and transitions in care coordination. Re-assessment of indicators related to timeliness of delivery of cancer treatments and collection of additional baseline data regarding supportive services.
Gap analysis
Identification of the scope of the problem. Members of the HNC multidisciplinary team raised concerns about unintended breaches in care for HNC patients that resulted not only in delay of the patients’ cancer treatments, but also in unnecessary distress for the patients and their families. As a result, the HNC team decided to conduct a gap analysis to identify the barriers in care for HNC patients, and by doing this, to determine possible solutions.
Identification of best practice care indicators. The indicators of best practice care (benchmarks) for HNC patients were identified after exhaustive review of the literature11-22(Table 1). For this gap analysis, the indicators focused on waiting time to treatment (surgery, chemotherapy, radiation therapy) and to supportive care interventions (nutrition, speech and language pathology) as follows:
- 9.2Futura StdInitial ear-nose-throat (ENT) visit to surgery: <30 days
- Biopsy to start radiation therapy (RT) for nonsurgical patients: 40 days
- Surgery to RT start: 42 days
- Surgery to nutrition consultation (outpatient), start RT to nutrition: Pretreatment
- Surgery to outpatient SLP, initial ENT visit to SLP referral, surgery to SLP referral, RT start to outpatient SLP start: Pretreatment
Measure gaps against benchmarks. To Gap analysis of measure gaps against benchmarks, the authors used the Agency for Health Care Research and Quality tool that provides a systematic method to compare current practice with best practices and determine the barriers to best practice and the feasibility of implementing best practices by the institution23 (Table 1). For this project, a process map of waiting time to treatment and supportive care interventions was created, so that real-world conditions could be measured against benchmarks.
Process map. The authors identified 67 newly diagnosed HNC patients during January-July 2014 from the surgery, radiation therapy, and nutrition departments, but only 33 patients were able to be tracked from their initial visit at the cancer center until the completion of their treatment through the electronic medical record (EMR) system. Their information was compiled in a spreadsheet based on the EMR information. Data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
To map the typical patient process, the patients were split into two groups: surgical (n = 22) and nonsurgical (n = 11). Surgical patients underwent surgery as their primary treatment and received adjuvant radiation therapy or concurrent chemotherapy. Nonsurgical patients did not require surgery other than biopsy as a part of their treatment. Most of the nonsurgical patients received chemotherapy, and 1 patient received palliative radiation therapy.
SWOT analysis. The SWOT analysis is used to chart institution performance in relation to benchmarks while describing stakeholders’ perceptions.24The stakeholder perspective for this project focused on the views of the health care providers from all disciplines regarding the quality of care provided to the HNC population. In addition, a patient survey was conducted to assess their perception of the care they received.
Clinician survey. We surveyed 25 clinicians, including physicians, advanced practice providers, nurses, and allied health professionals, from the surgical (n = 3), hospitalization (n = 6), radiation (n = 3), chemotherapy (n = 3) and supportive services teams (n = 10). The survey was conducted face to face and included 7 open-ended questions designed to gain insight about problems encountered with coordination of care, suggestions to improve coordination of care, factors in treatment delays, suggestions to decrease treatment delays, factors in excellent patient outcomes, rate overall patient care, and suggestions for improvement of service. Initial survey responses were filtered by recurring themes in each question among the different patient service teams.
Patient satisfaction survey. The sample of patients was obtained from the surgery, radiation therapy, and nutrition departments during January-July 2014. Sixty-seven initial patients were identified but only 43 were eligible for interview because they had a listed phone number. A six-question nonvalidated survey was developed by the authors to measure patient satisfaction with the scheduling process, waiting time, information provided about treatment and their medical status, emotional support, the coordination of care, and the payment process. Satisfaction was rated on a scale from 1 to 5 (1 = Poor, 2 = Fair, 3 = Satisfactory, 5 = Great).
Analysis and final report. See Results section.
Quality improvement implementation. The transitions and the education committees were created to address the gaps identified during the analysis. The transitions committee developed strategies to improve the coordination of care of HNC patients throughout their cancer treatment and the education committee elaborated new ways to enhance patient education while meeting treatment timeline standards. The implementation of the interventions was developed by the inpatient and outpatient MTD teams caring for the HNC population.
Re-assessment of indicators. During January-December 2015, a total of 58 patients diagnosed with HNC were identified. Of those, 40 patients with recurrent disease were eliminated, leaving 18 patients (10 surgical, 8 nonsurgical). Similar to the initial assessment for the gap analysis, data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
Results
Most of the patients were men (70%), white (70%), and 60% were within the 50-69 years age range at the time of diagnosis.
Clinician survey
The clinicians were surveyed and their responses analyzed by two people, the project leader and the project assistant. The most commonly identified weaknesses in care that the clinicians identified were delayed access to dental referrals, insufficient preoperative patient education, and inefficient discharge planning and/or home care coordination. Dental referrals were identified as a major cause of delay in starting radiation therapy because of scheduling issues, a lack of patient motivation, limited insurance coverage, and difficulty identifying reliable dentists in the patient’s geographic area. Clinicians also identified problems coordinating smoking cessation referrals for patients.
In addition, they identified the hospitalization and/or home care phases as areas for potential improvement. During hospitalization, patients often expressed surprise upon learning that they had a feeding tube and/or tracheostomy despite having received pre-operative education. This misunderstanding by the patient was likely related to the clinicians’ assumptions about the best timing for patient education and the amount of time needed for education before the surgical procedure. The surgical team provided patient education based on individual needs, and it has not been standardized because they felt that patients’ education needs vary from person to person. In contrast, patient education prior radiation therapy is standardized, and all patients received a comprehensive package of information that is re-enforced by direct patient education by the clinicians.
Another gap in care identified by the inpatient team was a prolonged intensive care unit (ICU) stay for the HNC patients. These patients remained in the ICU for the entirety of their stay. Not only was this causing overuse of resources, but patients also felt unprepared for an independent discharge home given the high level of care received in the ICU.
A range of suggestions were made to solve these problems. The most prevalent suggestion was to use a nurse navigator to coordinate referrals, schedule appointments, facilitate interdisciplinary communication, and to address social, financial, and transport needs for HNC patients. Several other suggestions referred to standardizing treatment procedures and pre-operative patient education.
Patient survey
Forty-three patients were identified for the patient satisfaction survey. Each patient was contacted at least three times over the course of 3 weeks. Of the 43 patients, 20 had an invalid phone number, 10 were not available for participation, and 1 declined to participate. A total of 12 patients completed the survey.
Although the sample size was small, the patients surveyed were very satisfied with their care. Of the 12 patients, 5 patients rated all of the services relevant to their treatment as a 5 (Great). Areas of particular concern for the patients included the waiting time to see a physician in the ENT clinic, the explanation/collection of charges, and the accessibility of support groups. Services rated 3 (Satisfactory) included waiting time to schedule appointments; the amount of information and patient education provided by about radiation, nutrition, physical therapy (PT), occupational therapy (OT), and SLP; and overall satisfaction with care.
Surgical patients. The Danish Head and Neck Society guidelines state that the interval between the initial visit diagnosis and surgery should be within 30 days.12A comparison of the average intervals between important treatment points for the surgical sample patients with the benchmark timing recommended in the literature are shown in Table 2. The mean time from initial visit to surgery was 28 days in the cancer center sample; 67% of patients (n = 14) had surgery within 30 days, and 33% of patients (n = 7) had surgery beyond 30 days. The interval re-assessment showed improvements in this area: the mean time from initial visit to surgery went from 28 to 18 days, and 100% of patients
n = 10) had surgery within 30 days.
Huang and colleagues have indicated that postoperative radiation therapy should ideally occur within 42 days of surgery;13 however, in the present study, 79% (n = 11) of the sample surgical patients undergoing radiation began their therapy on average more than 63 days after surgery. The interval re-assessment found the same results with 80% of patients starting radiation over 42 days after surgery although the average time lag decreased from 68 days to 53 days.
Nonsurgical patients. Huang and colleagues have indicated that for patients undergoing radiation as their primary form of treatment, an interval of 40 days between biopsy and the start of radiation is ideal.13 The average intervals between important time points of treatment for patients who did not require surgery in their treatment are shown in Table 2. The cancer center met the benchmark at baseline with an average of 38 days (n = 11 patients). The re-assessment showed improvement in this area with 100% of cases (n = 10) meeting the benchmark with an average of 32 days. Likewise, the benchmark waiting time from RT consultation to RT start of less than 30 days11 was met by the cancer center for the nonsurgical group (n = 11).
Access to supportive services
Nutrition care. Studies have shown that standard nutritional care for HNC patients should start before treatment.18,19 In the present study, the waiting time from surgery to outpatient nutrition assessment improved from 61 days to 50 days (Table 2). For patients in the surgical group, the time interval between the initial ENT visit to the outpatient nutrition assessment decreased from 85 days at baseline to 66 days at reassessment, and 82 days to 35 days, respectively, for the nonsurgical group. The time interval from surgery to nutrition assessment has not reached the recommended pretreatment benchmark, but data showed a trend of improvement from 61 days at baseline to 50 days at reassessment for patients in the group.
Patients were typically referred to outpatient nutrition at the start of radiation therapy. In the initial assessment, all patients (n = 33) had access to nutrition services, but 21% (n = 7) never spoke to the nutritionist. The re-assessment found all but one (n = 7) of the patients had been seen by a nutritionist at some point during the treatment period. The benchmark of preradiation nutrition assessment was met by 2 postsurgical patients, with the remainder of the patients being seen within 3 days of the initiation of radiation.
Speech-language pathology management. The literature recommends that patients receive SLP management before the surgery.14-17 In this gap analysis, a difference in access to SLP services was identified between inpatient and outpatient settings. On average, patients within the sample were referred to outpatient SLP over a month after their surgery. In contrast, inpatient surgical patients had access to rapid consultations with SLP (eg, 1 day after surgery for total laryngectomy, and 4 days after surgery for oropharyngeal and oral surgery patients; T Hogan, unpublished data, June 2014). Overall, the benchmark was not met, as patients were not seen by the SPL prior to treatment.
New baseline data was collected about SLP services and showed that 70% of patients had contact with the outpatient SLP at some point during their treatment. Of those, only 29% of patients saw SLP before surgery, meeting the benchmark. The baseline waiting time was an average of 15 days before surgery and 43 days after surgery. Overall, the trend is moving toward the benchmark of care.
Similarly, studies determined that the gold standard of care for nonsurgical patients is that SLPs begin pretreatment management of HNC.16Patients in the baseline sample were typically referred to outpatient SLP about a month after biopsy (presumably diagnosis), but before the start of chemo-radiation. There were no data available for the number of patients who were actually seen by the outpatient SLP before the start of chemo-radiation.
The new baseline data found that 100% of nonsurgical patients were referred to SLP, but only25% (n = 2) were seen before they started chemo-radiation therapy (an average 5 days before) and 75% (n = 6) were seen after starting chemo-radiation therapy (an average 23 days after).
SWOT analysis
The SWOT analysis included strengths, weaknesses, opportunities and threats of the care provided to HNC patients at the cancer center. The gap analysis based on the results of the clinician surveys, process mapping, and patient satisfaction survey is summarized in Table 3. Three main gaps were identified: waiting time to treatment, education, and coordination of and transitions in care.
Quality improvement actions
Interventions by the outpatient MTD team included changing the process of scheduling dental appointments, creating a new approach to outpatient nutrition by proactively meeting patients in the ENT clinic, and conducting PT and SLP assessments to patients in the chemotherapy unit while receiving their treatment. A nurse navigator position for this patient population was approved and an expedited referral system was initiated. At the same time, the inpatient team implemented a specialized HNC unit in the medical-surgical floor, developed the protocols for the management of postsurgery HNC patients, educated nursing staff, and standardized patient education to facilitate transition to the next level of care (Table 3).
Discussion
The gap analysis of services provided to HNC patients at the cancer center identified three gaps in care: delay in treatment and supportive services, nonstandardized patient education, and lack of care coordination.
All patients should have access to a timely treatment initiation. In this analysis, surgical patients encountered a delay between surgery and the start of radiation therapy, about 3 weeks beyond the recommended in the literature.12 Clinicians mentioned delays in ensuring preradiation dental consultations as a significant issue affecting the patient treatment process. Re-assessment data reported that despite interventions for early dental referrals, 80% of patients still started radiation over 6 weeks after surgery; however, the average time lag decreased from 68 days to 53 days.
RT delays in HNC patients not only affect patients’ emotional state but may also impact clinical outcomes. Treatment delays have the potential to harm patients by: allowing tumor growth that impact on the curative outcomes of RT; postponing the benefits of palliative RT on symptom relief; and causing psychological distress.25 In addition, delay in starting treatment has shown to increase the risk for local recurrence,13,26 and decrease survival.27
Higher demand for advanced RT modalities has been linked to treatment delays. Waiting times from initial RT evaluation to start RT have increased over time, from <14 days in 1989 to 31 days in 1997.11 This is explained by the complexity of the pretreatment evaluations and the increasing demand of radiation services, especially in high volume institutions.25,27A fast-track program to reduce waiting time in the treatment of HNC patients reported to be effective.22 This program includes a patient coordinator, a hotline for referral procedures, prebooked slots for ENT and RT clinics, faster pathology and imaging reports, and the establishment of an MTD team.
The clinician survey identified patient needs classified in three categories: pre-operative education, hospitalization process, and access to support services. Regarding pre-operative education, clinicians acknowledged that although patients were educated about their surgical options and possible outcomes prior to hospitalization, they often could not fully understand this information at the time of the instruction. The high need for education particularly in the pretreatment phase was documented in a needs assessment survey for HNC patients conducted at the cancer center D DeMille, RD, unpublished data, August 2013).
Studies have looked at the effectiveness of education in cancer patients. The use of teaching interventions (written information, audiotapes, videotapes, and computer programs) has proven to be valuable for educating patients prior to experiencing cancer treatments.20Further, a systematic review of preparatory education for cancer patients undergoing surgery reported that face-to-face discussions appear to be effective at improving patient outcomes with regards to increasing knowledge and decreasing anxiety.21 However, it was stated that the timing of the delivery of education is critical to be efficient. For example, an education session provided one day prior the day of surgery is not useful as it may place additional stress on a patient who is already highly anxious and decreases the likelihood for the information to be managed. It is recommended to deliver education early enough prior surgery to allow time for the patient to process the information. Also, a study reported that presurgical education on potential side effects; the assessment of patients’ needs by an SLP, physical therapist, nutritionist, and social worker; and pre-operative nutritional support decrease postoperative complications.4
The education committee was created in response to the gap on patient education. The inpatient team took the lead and provided intense education on the care of HNC patients to the nursing staff and to HNC patients and their families about postoperative care at home. Education was also extended to rehabilitation facilities caring for this cancer population at discharge from the hospital.
Clinicians identified a gap during the hospitalization process. The gap included prolonged stay of patients in the ICU postsurgery, inefficient interclinician communication, lack of standardization of postsurgical care, and difficulty communicating with external home care teams. A major intervention was implemented that included the creation of a HNC specialized unit that offered a structured setting for standardized care and communication between patients and clinicians. Dedicated units for the management of HNC patients highly enhance the quality of care provided because it enables the MTD team to work properly by clearly defining roles and responsibilities, delineating evidence-based clinical interventions, and promoting expert care for this patient population.23In addition, several key steps have been recommended to reduce the fragmentation of care for hospital patients, including developing a referral/transition tracking system, organizing and training staff members to coordinate transition/referrals, and identifying and creating agreements with key care providers.28
Early patient access to supportive services was a concern to most clinicians. HNC providers were not consulting the CARE clinic about patients’ nutritional, physical and SLP needs until the patient was having serious problems. Patient tracking found that the minority of patients met the standard of having a presurgical speech referral. Most patients had access to outpatient nutrition services during radiation therapy but the majority of patients in the sample did not attend CARE clinic. The literature strongly supports early management of HNC patients by the SPL and nutrition counselor. Van der Molen and colleagues demonstrated that a pretreatment SPL rehabilitation program is feasible and offers reasonable patient compliance despite of the burden caused by ongoing chemo-radiation therapy for HNC patients.16Similarly, early nutrition counseling for HNC patients undergoing RT has reported to decrease unintended weight loss and malnutrition compared with late nutrition intervention.19
Although there are clear gaps in care for HNC patients from the clinicians’ perspective, the patients surveyed indicated a clear satisfaction with their care at the cancer center. Almost all patients were satisfied with their relationships with clinicians in the team. Some patients mentioned complaints of insufficient pre-operative education and waiting time, but there were not significant complaints about coordination, which clinicians had identified as a major issue. This is likely explained by the small sample size and the patients’ inability to see the background interclinician communication.
A crucial suggestion to address all of these gaps in care was the implementation of a nurse navigator. With the support of hospital and cancer center administration, a nurse navigator was hired to address the needs of HNC patients throughout their disease trajectory. The team agreed that the nurse navigator should make contact with HNC patients during their initial appointment at the surgical ENT office. This initial contact allows the nurse navigator to provide support and connection to resources. Thereafter, early contact with this patient population allows the nurse navigator to follow the patient through the continuum of care from biopsy and diagnosis to survivorship. The nurse navigator facilitates communication between clinicians, patients and their families; and provides emotional support to patients while helping to manage their financial and transportation needs.29
Limitations
This is a quality improvement project with a small sample size of HNC cases. Data from this gap analysis are not statistically significant; yet, are clinically relevant in the management of the HNC population at the cancer center. Likewise, the patient sample size was small, making definitive generalizations about patient experience difficult; however, the data are helpful in highlighting possible problems for patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7-30.
2. National Cancer Institute. Head and neck cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Reviewed February 1, 2013. Accessed January 26, 2017
3. Weiderholt PA, Connor NP, Hartig GK, Harari PM. Bridging gaps in multidisciplinary head and neck cancer care: nursing coordination and case management. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl):S88-S91.
4. Dingman C, Hegedus PD, Likes C, McDowell P, McCarthy E, Zwilling C. A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol. 2008;6(3):125-131.
5. Liao C, Kang CJ, Lee LY, et al. Association between multidisciplinary team care approach and survival rates in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1544-1553.
6. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011;121(10):2131-2135.
7. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganizing cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organization of MDTs and their impact on patient outcomes. Health Pol. 2015;119(4):464-474.
8. Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. 2013. www.nationalacademies.org/hmd/Reports/2013/Delivering-High-Quality-Cancer-Care-Charting-a-New-Course-for-a-System-in-Crisis.aspx. Published September 10, 2013. Accessed May 29, 2016.
9. Granda-Cameron C, DeMille D, Lynch MP, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72-80.
10. Davis-Ajami ML, Costa L, Kulik S. Gap analysis: synergies and opportunities for effective nursing leadership. Nurs Econ. 2014;32(1):17-25.
11. Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929-936.
12. Van Harten MC, Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282-290.
13. Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J ClinOncol. 2003;21(3):555-563.
14. Lazarus CL. Management of swallowing disorders in head and neck cancer patients: optimal patterns of care. Sem Speech Lang. 2000;21(4):293-310.
15. Mayer KR. Learning to speak after laryngectomy. http://speech-language-pathology-audiology.advanceweb.com/Features/Articles/Learning-to-Speak-After-Laryngectomy.aspx. Posted October 27, 2014. Accessed January 17, 2017.
16. van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemo-radiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155-170.
17. Starmer HM, Gourin CG. Is speech language pathologist evaluation necessary in the nonoperative treatment of head and neck cancer? Laryngoscope. 2013;123(7):1571-1572.
18. [Article in French] Meuric J, Garabige V, Blanc-Vincent MP, et al. Good clinical practice in nutritional management of head and neck cancer patients. Bull Cancer. 1999;86(10):843-854.
19. van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872-877.
20. Waller A, Forshaw K, Bryant J, Mair S. Interventions for preparing patients for chemotherapy and radiotherapy: a systematic review. Supp Care Ca. 2014;22(8):2297-2308.
21. Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ Couns. 2015;98:1540-1549.
22. Toustrup K, Lambersten K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636-641.
23. Bergamini C, Locati L, Bossi P et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54-57.
24. AHRQ. Pediatric toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/pediatrictoolkit.html . Reviewed July 2016. Accessed January 26, 2017.
25. Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1-4.
26.Chen Z, King, W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16.
27. Sharma S, Bekelman J, Lin A et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with ororpharyngeal squamous cell carcinoma. Oral Oncol. 2016;56:17-24.
28. Improving chronic illness care. Reducing care fragmentation. Care coordination. http://www.improvingchroniccare.org/index.php?p=Care_Coordination&s=326. Published 2010. Accessed May 28, 2016.
29. Fillion L, de Serres M, Cook S, et al. Professional patient navigation in head and neck cancer. Sem Oncol Nurs. 2009;25(3):212-221.
In the United States, there will be an estimated 49,670 new cases of head and neck cancer for 2017.1 Head and neck cancer (HNC) is a term used to describe a range of tumors that originate in the area of the body spanning from the lower neck to the upper nasal cavity.2 Specifically, they are malignancies arising in the mouth, larynx, nasal cavity, sinuses, tongue, lips, and numerous glands such as the thyroid and salivary.2 To clarify, HNC, despite the encompassing name, does not include growths of the bones, teeth, skin, brain parenchyma, and eye; therefore, such tumors will not be addressed in this article.
Patients with HNC often experience fragmented and uncoordinated care that leads to delays in cancer treatment, severe distress in patients and families, and dissatisfaction with care. Literature reports that these patients face numerous stressors including aggressive cancer treatments, severe symptoms, body image concerns, loss of speech, difficulty swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis.3,4In addition, patients with HNC and their families are challenged to navigate the health care system and to overcome the difficulties of accessing services within the context of financial constraints. A multidisciplinary team (MDT) approach is the standard of care for HNC patients, as demonstrated in studies reporting better 5-year survival outcomes, increased completion of adjuvant therapy, and higher compliance with speech-language pathologist (SLP) recommendations.5, 6 Furthermore, a recent systematic review of cancer teams concluded that the MDT approach leads to improved clinical outcomes and enhanced communication between the patient and the team.7
The Institute of Medicine (IOM) stated in its 2013 report on cancer care that a high-quality care delivery system requires continuing measurement of cancer care and strategies to carry out performance improvement.8 Following the IOM premise, the cancer center at an academic medical center in Philadelphia made efforts to improve patient access to multidisciplinary services, first, by creating a multidisciplinary Cancer Appetite and Rehabilitation (CARE)clinic to address the symptoms and nutritional needs of HNC patients,9 and second, by using a gap analysis to conduct an assessment of the cancer care services provided to this cancer population. The need to conduct this assessment was generated by the desire to improve access to multidisciplinary care, with the goal of meeting standard benchmarks for completion of treatment while increasing the use of ancillary services. This article describes the process of conducting a gap analysis of cancer services for HNC patients, and includes discussion of the findings, recommendations for improving care, a description of the quality improvement interventions, and a report of the outcomes based on an interval re-assessment 18 months later.
Methods
Methods included a gap analysis, implementation of quality improvement recommendations, and re-assessment of indicators (Figure). A gap analysis “identifies differences between desired and actual practice conditions, including service delivery and quality patient outcomes as measured against evidence-based benchmarks while incorporating key stakeholder concerns and expectations.”10 The gap analysis of cancer care services offered to HNC patients was achieved through the step-by-step process described hereinafter. The implementation of quality improvement recommendations was accomplished by establishing two task force committees focused respectively on education and transitions in care coordination. Re-assessment of indicators related to timeliness of delivery of cancer treatments and collection of additional baseline data regarding supportive services.
Gap analysis
Identification of the scope of the problem. Members of the HNC multidisciplinary team raised concerns about unintended breaches in care for HNC patients that resulted not only in delay of the patients’ cancer treatments, but also in unnecessary distress for the patients and their families. As a result, the HNC team decided to conduct a gap analysis to identify the barriers in care for HNC patients, and by doing this, to determine possible solutions.
Identification of best practice care indicators. The indicators of best practice care (benchmarks) for HNC patients were identified after exhaustive review of the literature11-22(Table 1). For this gap analysis, the indicators focused on waiting time to treatment (surgery, chemotherapy, radiation therapy) and to supportive care interventions (nutrition, speech and language pathology) as follows:
- 9.2Futura StdInitial ear-nose-throat (ENT) visit to surgery: <30 days
- Biopsy to start radiation therapy (RT) for nonsurgical patients: 40 days
- Surgery to RT start: 42 days
- Surgery to nutrition consultation (outpatient), start RT to nutrition: Pretreatment
- Surgery to outpatient SLP, initial ENT visit to SLP referral, surgery to SLP referral, RT start to outpatient SLP start: Pretreatment
Measure gaps against benchmarks. To Gap analysis of measure gaps against benchmarks, the authors used the Agency for Health Care Research and Quality tool that provides a systematic method to compare current practice with best practices and determine the barriers to best practice and the feasibility of implementing best practices by the institution23 (Table 1). For this project, a process map of waiting time to treatment and supportive care interventions was created, so that real-world conditions could be measured against benchmarks.
Process map. The authors identified 67 newly diagnosed HNC patients during January-July 2014 from the surgery, radiation therapy, and nutrition departments, but only 33 patients were able to be tracked from their initial visit at the cancer center until the completion of their treatment through the electronic medical record (EMR) system. Their information was compiled in a spreadsheet based on the EMR information. Data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
To map the typical patient process, the patients were split into two groups: surgical (n = 22) and nonsurgical (n = 11). Surgical patients underwent surgery as their primary treatment and received adjuvant radiation therapy or concurrent chemotherapy. Nonsurgical patients did not require surgery other than biopsy as a part of their treatment. Most of the nonsurgical patients received chemotherapy, and 1 patient received palliative radiation therapy.
SWOT analysis. The SWOT analysis is used to chart institution performance in relation to benchmarks while describing stakeholders’ perceptions.24The stakeholder perspective for this project focused on the views of the health care providers from all disciplines regarding the quality of care provided to the HNC population. In addition, a patient survey was conducted to assess their perception of the care they received.
Clinician survey. We surveyed 25 clinicians, including physicians, advanced practice providers, nurses, and allied health professionals, from the surgical (n = 3), hospitalization (n = 6), radiation (n = 3), chemotherapy (n = 3) and supportive services teams (n = 10). The survey was conducted face to face and included 7 open-ended questions designed to gain insight about problems encountered with coordination of care, suggestions to improve coordination of care, factors in treatment delays, suggestions to decrease treatment delays, factors in excellent patient outcomes, rate overall patient care, and suggestions for improvement of service. Initial survey responses were filtered by recurring themes in each question among the different patient service teams.
Patient satisfaction survey. The sample of patients was obtained from the surgery, radiation therapy, and nutrition departments during January-July 2014. Sixty-seven initial patients were identified but only 43 were eligible for interview because they had a listed phone number. A six-question nonvalidated survey was developed by the authors to measure patient satisfaction with the scheduling process, waiting time, information provided about treatment and their medical status, emotional support, the coordination of care, and the payment process. Satisfaction was rated on a scale from 1 to 5 (1 = Poor, 2 = Fair, 3 = Satisfactory, 5 = Great).
Analysis and final report. See Results section.
Quality improvement implementation. The transitions and the education committees were created to address the gaps identified during the analysis. The transitions committee developed strategies to improve the coordination of care of HNC patients throughout their cancer treatment and the education committee elaborated new ways to enhance patient education while meeting treatment timeline standards. The implementation of the interventions was developed by the inpatient and outpatient MTD teams caring for the HNC population.
Re-assessment of indicators. During January-December 2015, a total of 58 patients diagnosed with HNC were identified. Of those, 40 patients with recurrent disease were eliminated, leaving 18 patients (10 surgical, 8 nonsurgical). Similar to the initial assessment for the gap analysis, data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
Results
Most of the patients were men (70%), white (70%), and 60% were within the 50-69 years age range at the time of diagnosis.
Clinician survey
The clinicians were surveyed and their responses analyzed by two people, the project leader and the project assistant. The most commonly identified weaknesses in care that the clinicians identified were delayed access to dental referrals, insufficient preoperative patient education, and inefficient discharge planning and/or home care coordination. Dental referrals were identified as a major cause of delay in starting radiation therapy because of scheduling issues, a lack of patient motivation, limited insurance coverage, and difficulty identifying reliable dentists in the patient’s geographic area. Clinicians also identified problems coordinating smoking cessation referrals for patients.
In addition, they identified the hospitalization and/or home care phases as areas for potential improvement. During hospitalization, patients often expressed surprise upon learning that they had a feeding tube and/or tracheostomy despite having received pre-operative education. This misunderstanding by the patient was likely related to the clinicians’ assumptions about the best timing for patient education and the amount of time needed for education before the surgical procedure. The surgical team provided patient education based on individual needs, and it has not been standardized because they felt that patients’ education needs vary from person to person. In contrast, patient education prior radiation therapy is standardized, and all patients received a comprehensive package of information that is re-enforced by direct patient education by the clinicians.
Another gap in care identified by the inpatient team was a prolonged intensive care unit (ICU) stay for the HNC patients. These patients remained in the ICU for the entirety of their stay. Not only was this causing overuse of resources, but patients also felt unprepared for an independent discharge home given the high level of care received in the ICU.
A range of suggestions were made to solve these problems. The most prevalent suggestion was to use a nurse navigator to coordinate referrals, schedule appointments, facilitate interdisciplinary communication, and to address social, financial, and transport needs for HNC patients. Several other suggestions referred to standardizing treatment procedures and pre-operative patient education.
Patient survey
Forty-three patients were identified for the patient satisfaction survey. Each patient was contacted at least three times over the course of 3 weeks. Of the 43 patients, 20 had an invalid phone number, 10 were not available for participation, and 1 declined to participate. A total of 12 patients completed the survey.
Although the sample size was small, the patients surveyed were very satisfied with their care. Of the 12 patients, 5 patients rated all of the services relevant to their treatment as a 5 (Great). Areas of particular concern for the patients included the waiting time to see a physician in the ENT clinic, the explanation/collection of charges, and the accessibility of support groups. Services rated 3 (Satisfactory) included waiting time to schedule appointments; the amount of information and patient education provided by about radiation, nutrition, physical therapy (PT), occupational therapy (OT), and SLP; and overall satisfaction with care.
Surgical patients. The Danish Head and Neck Society guidelines state that the interval between the initial visit diagnosis and surgery should be within 30 days.12A comparison of the average intervals between important treatment points for the surgical sample patients with the benchmark timing recommended in the literature are shown in Table 2. The mean time from initial visit to surgery was 28 days in the cancer center sample; 67% of patients (n = 14) had surgery within 30 days, and 33% of patients (n = 7) had surgery beyond 30 days. The interval re-assessment showed improvements in this area: the mean time from initial visit to surgery went from 28 to 18 days, and 100% of patients
n = 10) had surgery within 30 days.
Huang and colleagues have indicated that postoperative radiation therapy should ideally occur within 42 days of surgery;13 however, in the present study, 79% (n = 11) of the sample surgical patients undergoing radiation began their therapy on average more than 63 days after surgery. The interval re-assessment found the same results with 80% of patients starting radiation over 42 days after surgery although the average time lag decreased from 68 days to 53 days.
Nonsurgical patients. Huang and colleagues have indicated that for patients undergoing radiation as their primary form of treatment, an interval of 40 days between biopsy and the start of radiation is ideal.13 The average intervals between important time points of treatment for patients who did not require surgery in their treatment are shown in Table 2. The cancer center met the benchmark at baseline with an average of 38 days (n = 11 patients). The re-assessment showed improvement in this area with 100% of cases (n = 10) meeting the benchmark with an average of 32 days. Likewise, the benchmark waiting time from RT consultation to RT start of less than 30 days11 was met by the cancer center for the nonsurgical group (n = 11).
Access to supportive services
Nutrition care. Studies have shown that standard nutritional care for HNC patients should start before treatment.18,19 In the present study, the waiting time from surgery to outpatient nutrition assessment improved from 61 days to 50 days (Table 2). For patients in the surgical group, the time interval between the initial ENT visit to the outpatient nutrition assessment decreased from 85 days at baseline to 66 days at reassessment, and 82 days to 35 days, respectively, for the nonsurgical group. The time interval from surgery to nutrition assessment has not reached the recommended pretreatment benchmark, but data showed a trend of improvement from 61 days at baseline to 50 days at reassessment for patients in the group.
Patients were typically referred to outpatient nutrition at the start of radiation therapy. In the initial assessment, all patients (n = 33) had access to nutrition services, but 21% (n = 7) never spoke to the nutritionist. The re-assessment found all but one (n = 7) of the patients had been seen by a nutritionist at some point during the treatment period. The benchmark of preradiation nutrition assessment was met by 2 postsurgical patients, with the remainder of the patients being seen within 3 days of the initiation of radiation.
Speech-language pathology management. The literature recommends that patients receive SLP management before the surgery.14-17 In this gap analysis, a difference in access to SLP services was identified between inpatient and outpatient settings. On average, patients within the sample were referred to outpatient SLP over a month after their surgery. In contrast, inpatient surgical patients had access to rapid consultations with SLP (eg, 1 day after surgery for total laryngectomy, and 4 days after surgery for oropharyngeal and oral surgery patients; T Hogan, unpublished data, June 2014). Overall, the benchmark was not met, as patients were not seen by the SPL prior to treatment.
New baseline data was collected about SLP services and showed that 70% of patients had contact with the outpatient SLP at some point during their treatment. Of those, only 29% of patients saw SLP before surgery, meeting the benchmark. The baseline waiting time was an average of 15 days before surgery and 43 days after surgery. Overall, the trend is moving toward the benchmark of care.
Similarly, studies determined that the gold standard of care for nonsurgical patients is that SLPs begin pretreatment management of HNC.16Patients in the baseline sample were typically referred to outpatient SLP about a month after biopsy (presumably diagnosis), but before the start of chemo-radiation. There were no data available for the number of patients who were actually seen by the outpatient SLP before the start of chemo-radiation.
The new baseline data found that 100% of nonsurgical patients were referred to SLP, but only25% (n = 2) were seen before they started chemo-radiation therapy (an average 5 days before) and 75% (n = 6) were seen after starting chemo-radiation therapy (an average 23 days after).
SWOT analysis
The SWOT analysis included strengths, weaknesses, opportunities and threats of the care provided to HNC patients at the cancer center. The gap analysis based on the results of the clinician surveys, process mapping, and patient satisfaction survey is summarized in Table 3. Three main gaps were identified: waiting time to treatment, education, and coordination of and transitions in care.
Quality improvement actions
Interventions by the outpatient MTD team included changing the process of scheduling dental appointments, creating a new approach to outpatient nutrition by proactively meeting patients in the ENT clinic, and conducting PT and SLP assessments to patients in the chemotherapy unit while receiving their treatment. A nurse navigator position for this patient population was approved and an expedited referral system was initiated. At the same time, the inpatient team implemented a specialized HNC unit in the medical-surgical floor, developed the protocols for the management of postsurgery HNC patients, educated nursing staff, and standardized patient education to facilitate transition to the next level of care (Table 3).
Discussion
The gap analysis of services provided to HNC patients at the cancer center identified three gaps in care: delay in treatment and supportive services, nonstandardized patient education, and lack of care coordination.
All patients should have access to a timely treatment initiation. In this analysis, surgical patients encountered a delay between surgery and the start of radiation therapy, about 3 weeks beyond the recommended in the literature.12 Clinicians mentioned delays in ensuring preradiation dental consultations as a significant issue affecting the patient treatment process. Re-assessment data reported that despite interventions for early dental referrals, 80% of patients still started radiation over 6 weeks after surgery; however, the average time lag decreased from 68 days to 53 days.
RT delays in HNC patients not only affect patients’ emotional state but may also impact clinical outcomes. Treatment delays have the potential to harm patients by: allowing tumor growth that impact on the curative outcomes of RT; postponing the benefits of palliative RT on symptom relief; and causing psychological distress.25 In addition, delay in starting treatment has shown to increase the risk for local recurrence,13,26 and decrease survival.27
Higher demand for advanced RT modalities has been linked to treatment delays. Waiting times from initial RT evaluation to start RT have increased over time, from <14 days in 1989 to 31 days in 1997.11 This is explained by the complexity of the pretreatment evaluations and the increasing demand of radiation services, especially in high volume institutions.25,27A fast-track program to reduce waiting time in the treatment of HNC patients reported to be effective.22 This program includes a patient coordinator, a hotline for referral procedures, prebooked slots for ENT and RT clinics, faster pathology and imaging reports, and the establishment of an MTD team.
The clinician survey identified patient needs classified in three categories: pre-operative education, hospitalization process, and access to support services. Regarding pre-operative education, clinicians acknowledged that although patients were educated about their surgical options and possible outcomes prior to hospitalization, they often could not fully understand this information at the time of the instruction. The high need for education particularly in the pretreatment phase was documented in a needs assessment survey for HNC patients conducted at the cancer center D DeMille, RD, unpublished data, August 2013).
Studies have looked at the effectiveness of education in cancer patients. The use of teaching interventions (written information, audiotapes, videotapes, and computer programs) has proven to be valuable for educating patients prior to experiencing cancer treatments.20Further, a systematic review of preparatory education for cancer patients undergoing surgery reported that face-to-face discussions appear to be effective at improving patient outcomes with regards to increasing knowledge and decreasing anxiety.21 However, it was stated that the timing of the delivery of education is critical to be efficient. For example, an education session provided one day prior the day of surgery is not useful as it may place additional stress on a patient who is already highly anxious and decreases the likelihood for the information to be managed. It is recommended to deliver education early enough prior surgery to allow time for the patient to process the information. Also, a study reported that presurgical education on potential side effects; the assessment of patients’ needs by an SLP, physical therapist, nutritionist, and social worker; and pre-operative nutritional support decrease postoperative complications.4
The education committee was created in response to the gap on patient education. The inpatient team took the lead and provided intense education on the care of HNC patients to the nursing staff and to HNC patients and their families about postoperative care at home. Education was also extended to rehabilitation facilities caring for this cancer population at discharge from the hospital.
Clinicians identified a gap during the hospitalization process. The gap included prolonged stay of patients in the ICU postsurgery, inefficient interclinician communication, lack of standardization of postsurgical care, and difficulty communicating with external home care teams. A major intervention was implemented that included the creation of a HNC specialized unit that offered a structured setting for standardized care and communication between patients and clinicians. Dedicated units for the management of HNC patients highly enhance the quality of care provided because it enables the MTD team to work properly by clearly defining roles and responsibilities, delineating evidence-based clinical interventions, and promoting expert care for this patient population.23In addition, several key steps have been recommended to reduce the fragmentation of care for hospital patients, including developing a referral/transition tracking system, organizing and training staff members to coordinate transition/referrals, and identifying and creating agreements with key care providers.28
Early patient access to supportive services was a concern to most clinicians. HNC providers were not consulting the CARE clinic about patients’ nutritional, physical and SLP needs until the patient was having serious problems. Patient tracking found that the minority of patients met the standard of having a presurgical speech referral. Most patients had access to outpatient nutrition services during radiation therapy but the majority of patients in the sample did not attend CARE clinic. The literature strongly supports early management of HNC patients by the SPL and nutrition counselor. Van der Molen and colleagues demonstrated that a pretreatment SPL rehabilitation program is feasible and offers reasonable patient compliance despite of the burden caused by ongoing chemo-radiation therapy for HNC patients.16Similarly, early nutrition counseling for HNC patients undergoing RT has reported to decrease unintended weight loss and malnutrition compared with late nutrition intervention.19
Although there are clear gaps in care for HNC patients from the clinicians’ perspective, the patients surveyed indicated a clear satisfaction with their care at the cancer center. Almost all patients were satisfied with their relationships with clinicians in the team. Some patients mentioned complaints of insufficient pre-operative education and waiting time, but there were not significant complaints about coordination, which clinicians had identified as a major issue. This is likely explained by the small sample size and the patients’ inability to see the background interclinician communication.
A crucial suggestion to address all of these gaps in care was the implementation of a nurse navigator. With the support of hospital and cancer center administration, a nurse navigator was hired to address the needs of HNC patients throughout their disease trajectory. The team agreed that the nurse navigator should make contact with HNC patients during their initial appointment at the surgical ENT office. This initial contact allows the nurse navigator to provide support and connection to resources. Thereafter, early contact with this patient population allows the nurse navigator to follow the patient through the continuum of care from biopsy and diagnosis to survivorship. The nurse navigator facilitates communication between clinicians, patients and their families; and provides emotional support to patients while helping to manage their financial and transportation needs.29
Limitations
This is a quality improvement project with a small sample size of HNC cases. Data from this gap analysis are not statistically significant; yet, are clinically relevant in the management of the HNC population at the cancer center. Likewise, the patient sample size was small, making definitive generalizations about patient experience difficult; however, the data are helpful in highlighting possible problems for patients.
In the United States, there will be an estimated 49,670 new cases of head and neck cancer for 2017.1 Head and neck cancer (HNC) is a term used to describe a range of tumors that originate in the area of the body spanning from the lower neck to the upper nasal cavity.2 Specifically, they are malignancies arising in the mouth, larynx, nasal cavity, sinuses, tongue, lips, and numerous glands such as the thyroid and salivary.2 To clarify, HNC, despite the encompassing name, does not include growths of the bones, teeth, skin, brain parenchyma, and eye; therefore, such tumors will not be addressed in this article.
Patients with HNC often experience fragmented and uncoordinated care that leads to delays in cancer treatment, severe distress in patients and families, and dissatisfaction with care. Literature reports that these patients face numerous stressors including aggressive cancer treatments, severe symptoms, body image concerns, loss of speech, difficulty swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis.3,4In addition, patients with HNC and their families are challenged to navigate the health care system and to overcome the difficulties of accessing services within the context of financial constraints. A multidisciplinary team (MDT) approach is the standard of care for HNC patients, as demonstrated in studies reporting better 5-year survival outcomes, increased completion of adjuvant therapy, and higher compliance with speech-language pathologist (SLP) recommendations.5, 6 Furthermore, a recent systematic review of cancer teams concluded that the MDT approach leads to improved clinical outcomes and enhanced communication between the patient and the team.7
The Institute of Medicine (IOM) stated in its 2013 report on cancer care that a high-quality care delivery system requires continuing measurement of cancer care and strategies to carry out performance improvement.8 Following the IOM premise, the cancer center at an academic medical center in Philadelphia made efforts to improve patient access to multidisciplinary services, first, by creating a multidisciplinary Cancer Appetite and Rehabilitation (CARE)clinic to address the symptoms and nutritional needs of HNC patients,9 and second, by using a gap analysis to conduct an assessment of the cancer care services provided to this cancer population. The need to conduct this assessment was generated by the desire to improve access to multidisciplinary care, with the goal of meeting standard benchmarks for completion of treatment while increasing the use of ancillary services. This article describes the process of conducting a gap analysis of cancer services for HNC patients, and includes discussion of the findings, recommendations for improving care, a description of the quality improvement interventions, and a report of the outcomes based on an interval re-assessment 18 months later.
Methods
Methods included a gap analysis, implementation of quality improvement recommendations, and re-assessment of indicators (Figure). A gap analysis “identifies differences between desired and actual practice conditions, including service delivery and quality patient outcomes as measured against evidence-based benchmarks while incorporating key stakeholder concerns and expectations.”10 The gap analysis of cancer care services offered to HNC patients was achieved through the step-by-step process described hereinafter. The implementation of quality improvement recommendations was accomplished by establishing two task force committees focused respectively on education and transitions in care coordination. Re-assessment of indicators related to timeliness of delivery of cancer treatments and collection of additional baseline data regarding supportive services.
Gap analysis
Identification of the scope of the problem. Members of the HNC multidisciplinary team raised concerns about unintended breaches in care for HNC patients that resulted not only in delay of the patients’ cancer treatments, but also in unnecessary distress for the patients and their families. As a result, the HNC team decided to conduct a gap analysis to identify the barriers in care for HNC patients, and by doing this, to determine possible solutions.
Identification of best practice care indicators. The indicators of best practice care (benchmarks) for HNC patients were identified after exhaustive review of the literature11-22(Table 1). For this gap analysis, the indicators focused on waiting time to treatment (surgery, chemotherapy, radiation therapy) and to supportive care interventions (nutrition, speech and language pathology) as follows:
- 9.2Futura StdInitial ear-nose-throat (ENT) visit to surgery: <30 days
- Biopsy to start radiation therapy (RT) for nonsurgical patients: 40 days
- Surgery to RT start: 42 days
- Surgery to nutrition consultation (outpatient), start RT to nutrition: Pretreatment
- Surgery to outpatient SLP, initial ENT visit to SLP referral, surgery to SLP referral, RT start to outpatient SLP start: Pretreatment
Measure gaps against benchmarks. To Gap analysis of measure gaps against benchmarks, the authors used the Agency for Health Care Research and Quality tool that provides a systematic method to compare current practice with best practices and determine the barriers to best practice and the feasibility of implementing best practices by the institution23 (Table 1). For this project, a process map of waiting time to treatment and supportive care interventions was created, so that real-world conditions could be measured against benchmarks.
Process map. The authors identified 67 newly diagnosed HNC patients during January-July 2014 from the surgery, radiation therapy, and nutrition departments, but only 33 patients were able to be tracked from their initial visit at the cancer center until the completion of their treatment through the electronic medical record (EMR) system. Their information was compiled in a spreadsheet based on the EMR information. Data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
To map the typical patient process, the patients were split into two groups: surgical (n = 22) and nonsurgical (n = 11). Surgical patients underwent surgery as their primary treatment and received adjuvant radiation therapy or concurrent chemotherapy. Nonsurgical patients did not require surgery other than biopsy as a part of their treatment. Most of the nonsurgical patients received chemotherapy, and 1 patient received palliative radiation therapy.
SWOT analysis. The SWOT analysis is used to chart institution performance in relation to benchmarks while describing stakeholders’ perceptions.24The stakeholder perspective for this project focused on the views of the health care providers from all disciplines regarding the quality of care provided to the HNC population. In addition, a patient survey was conducted to assess their perception of the care they received.
Clinician survey. We surveyed 25 clinicians, including physicians, advanced practice providers, nurses, and allied health professionals, from the surgical (n = 3), hospitalization (n = 6), radiation (n = 3), chemotherapy (n = 3) and supportive services teams (n = 10). The survey was conducted face to face and included 7 open-ended questions designed to gain insight about problems encountered with coordination of care, suggestions to improve coordination of care, factors in treatment delays, suggestions to decrease treatment delays, factors in excellent patient outcomes, rate overall patient care, and suggestions for improvement of service. Initial survey responses were filtered by recurring themes in each question among the different patient service teams.
Patient satisfaction survey. The sample of patients was obtained from the surgery, radiation therapy, and nutrition departments during January-July 2014. Sixty-seven initial patients were identified but only 43 were eligible for interview because they had a listed phone number. A six-question nonvalidated survey was developed by the authors to measure patient satisfaction with the scheduling process, waiting time, information provided about treatment and their medical status, emotional support, the coordination of care, and the payment process. Satisfaction was rated on a scale from 1 to 5 (1 = Poor, 2 = Fair, 3 = Satisfactory, 5 = Great).
Analysis and final report. See Results section.
Quality improvement implementation. The transitions and the education committees were created to address the gaps identified during the analysis. The transitions committee developed strategies to improve the coordination of care of HNC patients throughout their cancer treatment and the education committee elaborated new ways to enhance patient education while meeting treatment timeline standards. The implementation of the interventions was developed by the inpatient and outpatient MTD teams caring for the HNC population.
Re-assessment of indicators. During January-December 2015, a total of 58 patients diagnosed with HNC were identified. Of those, 40 patients with recurrent disease were eliminated, leaving 18 patients (10 surgical, 8 nonsurgical). Similar to the initial assessment for the gap analysis, data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
Results
Most of the patients were men (70%), white (70%), and 60% were within the 50-69 years age range at the time of diagnosis.
Clinician survey
The clinicians were surveyed and their responses analyzed by two people, the project leader and the project assistant. The most commonly identified weaknesses in care that the clinicians identified were delayed access to dental referrals, insufficient preoperative patient education, and inefficient discharge planning and/or home care coordination. Dental referrals were identified as a major cause of delay in starting radiation therapy because of scheduling issues, a lack of patient motivation, limited insurance coverage, and difficulty identifying reliable dentists in the patient’s geographic area. Clinicians also identified problems coordinating smoking cessation referrals for patients.
In addition, they identified the hospitalization and/or home care phases as areas for potential improvement. During hospitalization, patients often expressed surprise upon learning that they had a feeding tube and/or tracheostomy despite having received pre-operative education. This misunderstanding by the patient was likely related to the clinicians’ assumptions about the best timing for patient education and the amount of time needed for education before the surgical procedure. The surgical team provided patient education based on individual needs, and it has not been standardized because they felt that patients’ education needs vary from person to person. In contrast, patient education prior radiation therapy is standardized, and all patients received a comprehensive package of information that is re-enforced by direct patient education by the clinicians.
Another gap in care identified by the inpatient team was a prolonged intensive care unit (ICU) stay for the HNC patients. These patients remained in the ICU for the entirety of their stay. Not only was this causing overuse of resources, but patients also felt unprepared for an independent discharge home given the high level of care received in the ICU.
A range of suggestions were made to solve these problems. The most prevalent suggestion was to use a nurse navigator to coordinate referrals, schedule appointments, facilitate interdisciplinary communication, and to address social, financial, and transport needs for HNC patients. Several other suggestions referred to standardizing treatment procedures and pre-operative patient education.
Patient survey
Forty-three patients were identified for the patient satisfaction survey. Each patient was contacted at least three times over the course of 3 weeks. Of the 43 patients, 20 had an invalid phone number, 10 were not available for participation, and 1 declined to participate. A total of 12 patients completed the survey.
Although the sample size was small, the patients surveyed were very satisfied with their care. Of the 12 patients, 5 patients rated all of the services relevant to their treatment as a 5 (Great). Areas of particular concern for the patients included the waiting time to see a physician in the ENT clinic, the explanation/collection of charges, and the accessibility of support groups. Services rated 3 (Satisfactory) included waiting time to schedule appointments; the amount of information and patient education provided by about radiation, nutrition, physical therapy (PT), occupational therapy (OT), and SLP; and overall satisfaction with care.
Surgical patients. The Danish Head and Neck Society guidelines state that the interval between the initial visit diagnosis and surgery should be within 30 days.12A comparison of the average intervals between important treatment points for the surgical sample patients with the benchmark timing recommended in the literature are shown in Table 2. The mean time from initial visit to surgery was 28 days in the cancer center sample; 67% of patients (n = 14) had surgery within 30 days, and 33% of patients (n = 7) had surgery beyond 30 days. The interval re-assessment showed improvements in this area: the mean time from initial visit to surgery went from 28 to 18 days, and 100% of patients
n = 10) had surgery within 30 days.
Huang and colleagues have indicated that postoperative radiation therapy should ideally occur within 42 days of surgery;13 however, in the present study, 79% (n = 11) of the sample surgical patients undergoing radiation began their therapy on average more than 63 days after surgery. The interval re-assessment found the same results with 80% of patients starting radiation over 42 days after surgery although the average time lag decreased from 68 days to 53 days.
Nonsurgical patients. Huang and colleagues have indicated that for patients undergoing radiation as their primary form of treatment, an interval of 40 days between biopsy and the start of radiation is ideal.13 The average intervals between important time points of treatment for patients who did not require surgery in their treatment are shown in Table 2. The cancer center met the benchmark at baseline with an average of 38 days (n = 11 patients). The re-assessment showed improvement in this area with 100% of cases (n = 10) meeting the benchmark with an average of 32 days. Likewise, the benchmark waiting time from RT consultation to RT start of less than 30 days11 was met by the cancer center for the nonsurgical group (n = 11).
Access to supportive services
Nutrition care. Studies have shown that standard nutritional care for HNC patients should start before treatment.18,19 In the present study, the waiting time from surgery to outpatient nutrition assessment improved from 61 days to 50 days (Table 2). For patients in the surgical group, the time interval between the initial ENT visit to the outpatient nutrition assessment decreased from 85 days at baseline to 66 days at reassessment, and 82 days to 35 days, respectively, for the nonsurgical group. The time interval from surgery to nutrition assessment has not reached the recommended pretreatment benchmark, but data showed a trend of improvement from 61 days at baseline to 50 days at reassessment for patients in the group.
Patients were typically referred to outpatient nutrition at the start of radiation therapy. In the initial assessment, all patients (n = 33) had access to nutrition services, but 21% (n = 7) never spoke to the nutritionist. The re-assessment found all but one (n = 7) of the patients had been seen by a nutritionist at some point during the treatment period. The benchmark of preradiation nutrition assessment was met by 2 postsurgical patients, with the remainder of the patients being seen within 3 days of the initiation of radiation.
Speech-language pathology management. The literature recommends that patients receive SLP management before the surgery.14-17 In this gap analysis, a difference in access to SLP services was identified between inpatient and outpatient settings. On average, patients within the sample were referred to outpatient SLP over a month after their surgery. In contrast, inpatient surgical patients had access to rapid consultations with SLP (eg, 1 day after surgery for total laryngectomy, and 4 days after surgery for oropharyngeal and oral surgery patients; T Hogan, unpublished data, June 2014). Overall, the benchmark was not met, as patients were not seen by the SPL prior to treatment.
New baseline data was collected about SLP services and showed that 70% of patients had contact with the outpatient SLP at some point during their treatment. Of those, only 29% of patients saw SLP before surgery, meeting the benchmark. The baseline waiting time was an average of 15 days before surgery and 43 days after surgery. Overall, the trend is moving toward the benchmark of care.
Similarly, studies determined that the gold standard of care for nonsurgical patients is that SLPs begin pretreatment management of HNC.16Patients in the baseline sample were typically referred to outpatient SLP about a month after biopsy (presumably diagnosis), but before the start of chemo-radiation. There were no data available for the number of patients who were actually seen by the outpatient SLP before the start of chemo-radiation.
The new baseline data found that 100% of nonsurgical patients were referred to SLP, but only25% (n = 2) were seen before they started chemo-radiation therapy (an average 5 days before) and 75% (n = 6) were seen after starting chemo-radiation therapy (an average 23 days after).
SWOT analysis
The SWOT analysis included strengths, weaknesses, opportunities and threats of the care provided to HNC patients at the cancer center. The gap analysis based on the results of the clinician surveys, process mapping, and patient satisfaction survey is summarized in Table 3. Three main gaps were identified: waiting time to treatment, education, and coordination of and transitions in care.
Quality improvement actions
Interventions by the outpatient MTD team included changing the process of scheduling dental appointments, creating a new approach to outpatient nutrition by proactively meeting patients in the ENT clinic, and conducting PT and SLP assessments to patients in the chemotherapy unit while receiving their treatment. A nurse navigator position for this patient population was approved and an expedited referral system was initiated. At the same time, the inpatient team implemented a specialized HNC unit in the medical-surgical floor, developed the protocols for the management of postsurgery HNC patients, educated nursing staff, and standardized patient education to facilitate transition to the next level of care (Table 3).
Discussion
The gap analysis of services provided to HNC patients at the cancer center identified three gaps in care: delay in treatment and supportive services, nonstandardized patient education, and lack of care coordination.
All patients should have access to a timely treatment initiation. In this analysis, surgical patients encountered a delay between surgery and the start of radiation therapy, about 3 weeks beyond the recommended in the literature.12 Clinicians mentioned delays in ensuring preradiation dental consultations as a significant issue affecting the patient treatment process. Re-assessment data reported that despite interventions for early dental referrals, 80% of patients still started radiation over 6 weeks after surgery; however, the average time lag decreased from 68 days to 53 days.
RT delays in HNC patients not only affect patients’ emotional state but may also impact clinical outcomes. Treatment delays have the potential to harm patients by: allowing tumor growth that impact on the curative outcomes of RT; postponing the benefits of palliative RT on symptom relief; and causing psychological distress.25 In addition, delay in starting treatment has shown to increase the risk for local recurrence,13,26 and decrease survival.27
Higher demand for advanced RT modalities has been linked to treatment delays. Waiting times from initial RT evaluation to start RT have increased over time, from <14 days in 1989 to 31 days in 1997.11 This is explained by the complexity of the pretreatment evaluations and the increasing demand of radiation services, especially in high volume institutions.25,27A fast-track program to reduce waiting time in the treatment of HNC patients reported to be effective.22 This program includes a patient coordinator, a hotline for referral procedures, prebooked slots for ENT and RT clinics, faster pathology and imaging reports, and the establishment of an MTD team.
The clinician survey identified patient needs classified in three categories: pre-operative education, hospitalization process, and access to support services. Regarding pre-operative education, clinicians acknowledged that although patients were educated about their surgical options and possible outcomes prior to hospitalization, they often could not fully understand this information at the time of the instruction. The high need for education particularly in the pretreatment phase was documented in a needs assessment survey for HNC patients conducted at the cancer center D DeMille, RD, unpublished data, August 2013).
Studies have looked at the effectiveness of education in cancer patients. The use of teaching interventions (written information, audiotapes, videotapes, and computer programs) has proven to be valuable for educating patients prior to experiencing cancer treatments.20Further, a systematic review of preparatory education for cancer patients undergoing surgery reported that face-to-face discussions appear to be effective at improving patient outcomes with regards to increasing knowledge and decreasing anxiety.21 However, it was stated that the timing of the delivery of education is critical to be efficient. For example, an education session provided one day prior the day of surgery is not useful as it may place additional stress on a patient who is already highly anxious and decreases the likelihood for the information to be managed. It is recommended to deliver education early enough prior surgery to allow time for the patient to process the information. Also, a study reported that presurgical education on potential side effects; the assessment of patients’ needs by an SLP, physical therapist, nutritionist, and social worker; and pre-operative nutritional support decrease postoperative complications.4
The education committee was created in response to the gap on patient education. The inpatient team took the lead and provided intense education on the care of HNC patients to the nursing staff and to HNC patients and their families about postoperative care at home. Education was also extended to rehabilitation facilities caring for this cancer population at discharge from the hospital.
Clinicians identified a gap during the hospitalization process. The gap included prolonged stay of patients in the ICU postsurgery, inefficient interclinician communication, lack of standardization of postsurgical care, and difficulty communicating with external home care teams. A major intervention was implemented that included the creation of a HNC specialized unit that offered a structured setting for standardized care and communication between patients and clinicians. Dedicated units for the management of HNC patients highly enhance the quality of care provided because it enables the MTD team to work properly by clearly defining roles and responsibilities, delineating evidence-based clinical interventions, and promoting expert care for this patient population.23In addition, several key steps have been recommended to reduce the fragmentation of care for hospital patients, including developing a referral/transition tracking system, organizing and training staff members to coordinate transition/referrals, and identifying and creating agreements with key care providers.28
Early patient access to supportive services was a concern to most clinicians. HNC providers were not consulting the CARE clinic about patients’ nutritional, physical and SLP needs until the patient was having serious problems. Patient tracking found that the minority of patients met the standard of having a presurgical speech referral. Most patients had access to outpatient nutrition services during radiation therapy but the majority of patients in the sample did not attend CARE clinic. The literature strongly supports early management of HNC patients by the SPL and nutrition counselor. Van der Molen and colleagues demonstrated that a pretreatment SPL rehabilitation program is feasible and offers reasonable patient compliance despite of the burden caused by ongoing chemo-radiation therapy for HNC patients.16Similarly, early nutrition counseling for HNC patients undergoing RT has reported to decrease unintended weight loss and malnutrition compared with late nutrition intervention.19
Although there are clear gaps in care for HNC patients from the clinicians’ perspective, the patients surveyed indicated a clear satisfaction with their care at the cancer center. Almost all patients were satisfied with their relationships with clinicians in the team. Some patients mentioned complaints of insufficient pre-operative education and waiting time, but there were not significant complaints about coordination, which clinicians had identified as a major issue. This is likely explained by the small sample size and the patients’ inability to see the background interclinician communication.
A crucial suggestion to address all of these gaps in care was the implementation of a nurse navigator. With the support of hospital and cancer center administration, a nurse navigator was hired to address the needs of HNC patients throughout their disease trajectory. The team agreed that the nurse navigator should make contact with HNC patients during their initial appointment at the surgical ENT office. This initial contact allows the nurse navigator to provide support and connection to resources. Thereafter, early contact with this patient population allows the nurse navigator to follow the patient through the continuum of care from biopsy and diagnosis to survivorship. The nurse navigator facilitates communication between clinicians, patients and their families; and provides emotional support to patients while helping to manage their financial and transportation needs.29
Limitations
This is a quality improvement project with a small sample size of HNC cases. Data from this gap analysis are not statistically significant; yet, are clinically relevant in the management of the HNC population at the cancer center. Likewise, the patient sample size was small, making definitive generalizations about patient experience difficult; however, the data are helpful in highlighting possible problems for patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7-30.
2. National Cancer Institute. Head and neck cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Reviewed February 1, 2013. Accessed January 26, 2017
3. Weiderholt PA, Connor NP, Hartig GK, Harari PM. Bridging gaps in multidisciplinary head and neck cancer care: nursing coordination and case management. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl):S88-S91.
4. Dingman C, Hegedus PD, Likes C, McDowell P, McCarthy E, Zwilling C. A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol. 2008;6(3):125-131.
5. Liao C, Kang CJ, Lee LY, et al. Association between multidisciplinary team care approach and survival rates in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1544-1553.
6. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011;121(10):2131-2135.
7. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganizing cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organization of MDTs and their impact on patient outcomes. Health Pol. 2015;119(4):464-474.
8. Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. 2013. www.nationalacademies.org/hmd/Reports/2013/Delivering-High-Quality-Cancer-Care-Charting-a-New-Course-for-a-System-in-Crisis.aspx. Published September 10, 2013. Accessed May 29, 2016.
9. Granda-Cameron C, DeMille D, Lynch MP, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72-80.
10. Davis-Ajami ML, Costa L, Kulik S. Gap analysis: synergies and opportunities for effective nursing leadership. Nurs Econ. 2014;32(1):17-25.
11. Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929-936.
12. Van Harten MC, Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282-290.
13. Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J ClinOncol. 2003;21(3):555-563.
14. Lazarus CL. Management of swallowing disorders in head and neck cancer patients: optimal patterns of care. Sem Speech Lang. 2000;21(4):293-310.
15. Mayer KR. Learning to speak after laryngectomy. http://speech-language-pathology-audiology.advanceweb.com/Features/Articles/Learning-to-Speak-After-Laryngectomy.aspx. Posted October 27, 2014. Accessed January 17, 2017.
16. van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemo-radiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155-170.
17. Starmer HM, Gourin CG. Is speech language pathologist evaluation necessary in the nonoperative treatment of head and neck cancer? Laryngoscope. 2013;123(7):1571-1572.
18. [Article in French] Meuric J, Garabige V, Blanc-Vincent MP, et al. Good clinical practice in nutritional management of head and neck cancer patients. Bull Cancer. 1999;86(10):843-854.
19. van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872-877.
20. Waller A, Forshaw K, Bryant J, Mair S. Interventions for preparing patients for chemotherapy and radiotherapy: a systematic review. Supp Care Ca. 2014;22(8):2297-2308.
21. Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ Couns. 2015;98:1540-1549.
22. Toustrup K, Lambersten K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636-641.
23. Bergamini C, Locati L, Bossi P et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54-57.
24. AHRQ. Pediatric toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/pediatrictoolkit.html . Reviewed July 2016. Accessed January 26, 2017.
25. Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1-4.
26.Chen Z, King, W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16.
27. Sharma S, Bekelman J, Lin A et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with ororpharyngeal squamous cell carcinoma. Oral Oncol. 2016;56:17-24.
28. Improving chronic illness care. Reducing care fragmentation. Care coordination. http://www.improvingchroniccare.org/index.php?p=Care_Coordination&s=326. Published 2010. Accessed May 28, 2016.
29. Fillion L, de Serres M, Cook S, et al. Professional patient navigation in head and neck cancer. Sem Oncol Nurs. 2009;25(3):212-221.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7-30.
2. National Cancer Institute. Head and neck cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Reviewed February 1, 2013. Accessed January 26, 2017
3. Weiderholt PA, Connor NP, Hartig GK, Harari PM. Bridging gaps in multidisciplinary head and neck cancer care: nursing coordination and case management. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl):S88-S91.
4. Dingman C, Hegedus PD, Likes C, McDowell P, McCarthy E, Zwilling C. A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol. 2008;6(3):125-131.
5. Liao C, Kang CJ, Lee LY, et al. Association between multidisciplinary team care approach and survival rates in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1544-1553.
6. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011;121(10):2131-2135.
7. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganizing cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organization of MDTs and their impact on patient outcomes. Health Pol. 2015;119(4):464-474.
8. Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. 2013. www.nationalacademies.org/hmd/Reports/2013/Delivering-High-Quality-Cancer-Care-Charting-a-New-Course-for-a-System-in-Crisis.aspx. Published September 10, 2013. Accessed May 29, 2016.
9. Granda-Cameron C, DeMille D, Lynch MP, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72-80.
10. Davis-Ajami ML, Costa L, Kulik S. Gap analysis: synergies and opportunities for effective nursing leadership. Nurs Econ. 2014;32(1):17-25.
11. Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929-936.
12. Van Harten MC, Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282-290.
13. Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J ClinOncol. 2003;21(3):555-563.
14. Lazarus CL. Management of swallowing disorders in head and neck cancer patients: optimal patterns of care. Sem Speech Lang. 2000;21(4):293-310.
15. Mayer KR. Learning to speak after laryngectomy. http://speech-language-pathology-audiology.advanceweb.com/Features/Articles/Learning-to-Speak-After-Laryngectomy.aspx. Posted October 27, 2014. Accessed January 17, 2017.
16. van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemo-radiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155-170.
17. Starmer HM, Gourin CG. Is speech language pathologist evaluation necessary in the nonoperative treatment of head and neck cancer? Laryngoscope. 2013;123(7):1571-1572.
18. [Article in French] Meuric J, Garabige V, Blanc-Vincent MP, et al. Good clinical practice in nutritional management of head and neck cancer patients. Bull Cancer. 1999;86(10):843-854.
19. van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872-877.
20. Waller A, Forshaw K, Bryant J, Mair S. Interventions for preparing patients for chemotherapy and radiotherapy: a systematic review. Supp Care Ca. 2014;22(8):2297-2308.
21. Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ Couns. 2015;98:1540-1549.
22. Toustrup K, Lambersten K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636-641.
23. Bergamini C, Locati L, Bossi P et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54-57.
24. AHRQ. Pediatric toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/pediatrictoolkit.html . Reviewed July 2016. Accessed January 26, 2017.
25. Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1-4.
26.Chen Z, King, W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16.
27. Sharma S, Bekelman J, Lin A et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with ororpharyngeal squamous cell carcinoma. Oral Oncol. 2016;56:17-24.
28. Improving chronic illness care. Reducing care fragmentation. Care coordination. http://www.improvingchroniccare.org/index.php?p=Care_Coordination&s=326. Published 2010. Accessed May 28, 2016.
29. Fillion L, de Serres M, Cook S, et al. Professional patient navigation in head and neck cancer. Sem Oncol Nurs. 2009;25(3):212-221.
David Henry's JCSO podcast, January-February 2017
For the January-February issue of the Journal of Community and Supportive Oncology, the Editor in Chief, Dr David Henry, highlights a thought-provoking article by JCSO Editor, Dr Thomas Strouse, on end-of-life options and legal pathways to physician-assisted dying. He also features a guide by Dr Adam Bagg on the diagnosis and classification of lymphomas, and an in-depth examination of blood-based biopsies by Jane de Lartigue. Three Original Reports hone in on patient care and support and quality of care: a report on the Florida CaPCaS study documents the experiences and needs of black men at the point of prostate cancer diagnosis; another, among Spanish-speaking Latinas with breast cancer, addresses the posttreatment survivorship care needs of that population; and a third looks at strategies to improve the quality of care among head and neck cancer patients. Among the Journal’s regular offerings, the Community Translations section features the approvals of atezolizumab as a therapy for bladder cancer (the first in more than 30 years) and lenvatinib for renal cell carcinoma, and there are two Case Report, one on a patient with breast cancer who experienced severe hyponatremia with seizures associated with single-, low-dose cyclophosphamide, and another about paraneoplastic leukemoid reaction as a poor prognostic marker in a patient with urothelial bladder carcinoma.
Listen to the podcast below.
For the January-February issue of the Journal of Community and Supportive Oncology, the Editor in Chief, Dr David Henry, highlights a thought-provoking article by JCSO Editor, Dr Thomas Strouse, on end-of-life options and legal pathways to physician-assisted dying. He also features a guide by Dr Adam Bagg on the diagnosis and classification of lymphomas, and an in-depth examination of blood-based biopsies by Jane de Lartigue. Three Original Reports hone in on patient care and support and quality of care: a report on the Florida CaPCaS study documents the experiences and needs of black men at the point of prostate cancer diagnosis; another, among Spanish-speaking Latinas with breast cancer, addresses the posttreatment survivorship care needs of that population; and a third looks at strategies to improve the quality of care among head and neck cancer patients. Among the Journal’s regular offerings, the Community Translations section features the approvals of atezolizumab as a therapy for bladder cancer (the first in more than 30 years) and lenvatinib for renal cell carcinoma, and there are two Case Report, one on a patient with breast cancer who experienced severe hyponatremia with seizures associated with single-, low-dose cyclophosphamide, and another about paraneoplastic leukemoid reaction as a poor prognostic marker in a patient with urothelial bladder carcinoma.
Listen to the podcast below.
For the January-February issue of the Journal of Community and Supportive Oncology, the Editor in Chief, Dr David Henry, highlights a thought-provoking article by JCSO Editor, Dr Thomas Strouse, on end-of-life options and legal pathways to physician-assisted dying. He also features a guide by Dr Adam Bagg on the diagnosis and classification of lymphomas, and an in-depth examination of blood-based biopsies by Jane de Lartigue. Three Original Reports hone in on patient care and support and quality of care: a report on the Florida CaPCaS study documents the experiences and needs of black men at the point of prostate cancer diagnosis; another, among Spanish-speaking Latinas with breast cancer, addresses the posttreatment survivorship care needs of that population; and a third looks at strategies to improve the quality of care among head and neck cancer patients. Among the Journal’s regular offerings, the Community Translations section features the approvals of atezolizumab as a therapy for bladder cancer (the first in more than 30 years) and lenvatinib for renal cell carcinoma, and there are two Case Report, one on a patient with breast cancer who experienced severe hyponatremia with seizures associated with single-, low-dose cyclophosphamide, and another about paraneoplastic leukemoid reaction as a poor prognostic marker in a patient with urothelial bladder carcinoma.
Listen to the podcast below.
Partnering with stakeholders using an example patient-reported outcomes project
Recently, researchers have been challenged to design methods that ensure that key constituents are partners in research, and not simply participants. Here we describe some innovative approaches we used to engage stakeholders. The approaches are drawn from a patient-centered outcomes research project, focusing on the graphic display of patient-reported outcomes (PROs) data. PROs represent patients’ perspectives on the impact of health, disease, and treatment, without interpretation by a clinician or anyone else. PROs include, among other things, patients’ assessments of their symptoms, their level of physical and psychosocial functioning, and health-related quality-of-life.1
As a first example of the key role of stakeholders in this project, input from cancer patients and clinicians, drawn from previous research, motivated us to ask whether there might be a “better way” to display PRO data when used to inform clinical practice. Specifically, even though cancer patients and clinicians endorse the importance of PRO data to promote patient-centered care, both groups report challenges using PROs in practice because of difficulty understanding what the PRO scores mean (eg, what is a good score or a bad score?; for individual patients, which scores should clinicians be concerned about?; for clinical trial PROs, what differences in PRO scores between treatments are clinically important?). The challenges in interpreting PRO data result in part from a large number of PRO measures (eg, one database includes more than 1,000 instruments)2 and no standards across PRO measures regarding how they are scored and scaled, or in how the data are presented.3 For example, on some PRO measures, higher scores represent better outcomes; on some PRO measures, lower scores represent better outcomes; and on some PRO measures, whether higher or lower scores represent better outcomes depends on the domain being measured. Further, some measures are scaled 0-100, with the extremes representing the best/worst scores possible, whereas others are normed to, for example, a population average of 50. Because of this variation, a score of 70 can have a completely different meaning depending on the PRO measure (or domain within a measure). As noted above, previous research has documented that this variation limits patients’ and clinicians’ understanding of the PRO scores, creating an important barrier to their use in practice.4-5
To address this stakeholder-driven research question, we undertook a three-part study to identify approaches for PRO data display that can be easily interpreted, regardless of scoring or scaling conventions, with the overall goal of improving patient and clinician understanding and use of PROs in oncology clinical practice. Part 1 of the study identified attributes of graphic displays of PRO data that are helpful and confusing.6 Part 2 involved developing improved PRO data presentation approaches.7 Part 3 evaluated the accuracy-of-interpretation and clarity of the developed approaches.8-10 The methods and findings of the three-part study are reported elsewhere;6-10 here, we describe the various approaches employed to engage stakeholders throughout the project.
As described above, the first reflection of stakeholder input was in the research question we asked. We then sought to identify the key stakeholder groups and ensure that they participated in each stage of the project. The relevant stakeholder groups we identified were: patients and their caregivers; health care providers (eg, oncologists, oncology nurses) who need to understand PRO data for their own consideration and for discussion with patients; and PRO researchers who develop, validate, and apply PRO measures.
Having identified these three key stakeholder groups, we sought to obtain broad representation of their perspectives. For example, we ensured that our investigative team included a cancer survivor, a cancer care provider, and PRO researchers. To supplement the stakeholder input from the investigative team, we formed a nine-member Stakeholder Advisory Board, with multiple representatives from each key constituency. We also aimed to be as broad as possible in the populations sampled for data collection. For example, we extended beyond the Johns Hopkins cancer center to include the Johns Hopkins Clinical Research Network, a consortium of academic and community health systems across the mid-Atlantic United States. Beyond the in-person data collection across the region, our study also included an internet survey of cancer patients/survivors, cancer care providers, and PRO researchers from across the United States and internationally. Taken together, these approaches improve the diversity of our sample and, thereby, the generalizability of our findings.
In addition to obtaining broad perspectives across stakeholder groups, we created genuine partnerships with the stakeholders to inform every aspect of the project. As described above, the study itself was motivated by feedback from cancer patients and clinicians regarding the challenges they experienced when trying to interpret PRO scores, and we therefore ensured that each stakeholder group contributed to the study’s design. Stakeholders also played a critical role in the conduct of the study. For example, in the first part of the study, we conducted one-on-one interviews with 50 cancer patients and 20 cancer clinicians to obtain their insights regarding attributes of current approaches for presenting PRO data that are helpful and confusing.6 At the completion of each interview, we asked participants whether they would be interested in partnering with the researchers in developing improved presentation formats in the next phase of the project. These volunteers were organized into work groups that reviewed the findings from the initial round of interviews with the investigative team, provided suggestions regarding candidate formats that could be used to improve presentation approaches, and helped pilot the internet survey.7 In this way, research participants had the opportunity to evolve into research partners, providing critically important input throughout the process.
The implementation and dissemination of findings is another area in which stakeholder partnership is particularly valuable. For example, several of our stakeholder partners have an advocacy background, which can be quite useful for conveying the project’s results in a compelling way. Other stakeholders, such as journal editors, are in a position to act directly to implement the study findings by, for example, adding best practices for presenting PRO data to their journal’s author instructions. Notably, some of the skills stakeholders bring come in addition to their role as stakeholders. For example, one of our patient stakeholders has a background in marketing, and this marketing expertise (completely separate from his patient experience) has helped the research team think about how to present data to broad audiences in a meaningful way.
In summary, this project has implemented stakeholder-driven approaches to address an important barrier to patient-centered cancer care. Several key lessons in stakeholder engagement have emerged from this experience. It is important to identify the key constituencies early on in the process. Involving stakeholders from the start enables them to play important roles in every aspect of the study, starting with study design conception. There are also innovative ways to integrate stakeholders in study conduct, such as our work groups of research participants who volunteered to partner with the research team to develop improved data presentation approaches. Implementation and dissemination is another area where stakeholders, based on their background and connections, can play a critical role. Throughout the process, it is valuable to challenge the project to obtain perspectives from as broad a range of stakeholders as possible. Finally, stakeholders have expertise beyond their stakeholder roles, and these skills can be quite valuable to the overall research agenda. In this project, our partnership with stakeholders has helped improve the presentation of PRO data to patients and providers, thereby improving the patient-centeredness of cancer care.
Acknowledgments
The PRO Data Presentation Stakeholder Advisory Board includes Neil K Aaronson, PhD (Netherlands Cancer Institute, Amsterdam); Patricia A Ganz, MD (University of California-Los Angeles and Jonsson Comprehensive Cancer Center, Los Angeles, CA); Ravin Garg, MD (Anne Arundel Medical Center, Annapolis, MD); Michael Fisch, MD (MD Anderson Cancer Center, Houston, TX); Vanessa Hoffman, MPH (Bladder Cancer Advocacy Network, Washington, DC); Bryce B Reeve, PhD (University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center, Chapel Hill, NC); Eden Stotsky-Himelfarb (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD); Ellen Stovall (National Coalition for Cancer Survivorship, Washington, DC [posthumous]); Matthew Zachary (Stupid Cancer, New York, NY).
The authors thank The Johns Hopkins Clinical Research Network site investigators and staff and, in particular, the patients and clinicians who participated in this project.
Supported by a Patient-Centered Outcomes Research Institute (PCORI) Award (R-1410-24904). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of PCORI, its board of governors or methodology committee. Drs Snyder and Smith are members of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (P30 CA 006973). The funders had no role in the study design; data collection, analysis, or interpretation; writing; or decision to submit.
1. Acquadro C, Berzon R, Dubois D, et al. Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Administration, February 16, 2001. Value Health. 2003;6(5):522-531.
2. PROQOLID, the Patient-Reported Outcome and Quality of Life Instruments Database. https://eprovide.mapi-trust.org/. Accessed November 10, 2016.
3. Brundage MD, Snyder CF. Patient-reported outcomes in clinical practice: using standards to break down barriers. Clin Invest. 2012;2(4):343-346.
4. Brundage M, Bass B, Jolie R, et al. A knowledge translation challenge: clinical use of quality of life data from cancer clinical trials. Qual Life Res. 2011;20(7):979-985.
5. Snyder CF, Jensen R, Courtin SO, et al. PatientViewpoint: a website for patient-reported outcomes assessment. Qual Life Res. 2009;18(7):793-800.
6. Brundage M, Smith KC, Little EA, Bantug ET, Snyder CF. PRO Data Presentation Stakeholder Advisory Board. Communicating patient-reported outcome scores using graphic formats: results from a mixed-methods evaluation. Qual Life Res. 2015;24(10):2457-2472.
7. Smith KC, Brundage MD, Tolbert E, Little EA, Bantug ET, Snyder C. PRO Data Presentation Stakeholder Advisory Board. Engaging stakeholders to improve presentation of patient-reported outcomes data in clinical practice. Support Care Cancer. 2016;24(10):4149-4157.
8. Snyder CF, Smith KC, Bantug ET, Tolbert EE, Blackford AL, Brundage MD. PRO Data Presentation Stakeholder Advisory Board. What do these scores mean? Presenting patient-reported outcomes data to patients and clinicians to improve interpretability. Cancer. 2017;123(10):1848-1859.
9. Brundage M, Blackford A, Tolbert E, Smith K, Bantug E, Snyder C. PRO Data Presentation Stakeholder Advisory Board. Presenting comparative study PRO results to clinicians and researchers: beyond the eye of the beholder. Qual Life Res. 2017 Nov 2 [Epub ahead of print].
10. Tolbert E, Snyder C, Bantug E, Blackford A, Brundage M. PRO Data Presentation Stakeholder Advisory Board. Graphing group-level data from research studies for presentation to patients in educational materials and decision aids. Qual Life Res. 2016;25(suppl 1):17.
Recently, researchers have been challenged to design methods that ensure that key constituents are partners in research, and not simply participants. Here we describe some innovative approaches we used to engage stakeholders. The approaches are drawn from a patient-centered outcomes research project, focusing on the graphic display of patient-reported outcomes (PROs) data. PROs represent patients’ perspectives on the impact of health, disease, and treatment, without interpretation by a clinician or anyone else. PROs include, among other things, patients’ assessments of their symptoms, their level of physical and psychosocial functioning, and health-related quality-of-life.1
As a first example of the key role of stakeholders in this project, input from cancer patients and clinicians, drawn from previous research, motivated us to ask whether there might be a “better way” to display PRO data when used to inform clinical practice. Specifically, even though cancer patients and clinicians endorse the importance of PRO data to promote patient-centered care, both groups report challenges using PROs in practice because of difficulty understanding what the PRO scores mean (eg, what is a good score or a bad score?; for individual patients, which scores should clinicians be concerned about?; for clinical trial PROs, what differences in PRO scores between treatments are clinically important?). The challenges in interpreting PRO data result in part from a large number of PRO measures (eg, one database includes more than 1,000 instruments)2 and no standards across PRO measures regarding how they are scored and scaled, or in how the data are presented.3 For example, on some PRO measures, higher scores represent better outcomes; on some PRO measures, lower scores represent better outcomes; and on some PRO measures, whether higher or lower scores represent better outcomes depends on the domain being measured. Further, some measures are scaled 0-100, with the extremes representing the best/worst scores possible, whereas others are normed to, for example, a population average of 50. Because of this variation, a score of 70 can have a completely different meaning depending on the PRO measure (or domain within a measure). As noted above, previous research has documented that this variation limits patients’ and clinicians’ understanding of the PRO scores, creating an important barrier to their use in practice.4-5
To address this stakeholder-driven research question, we undertook a three-part study to identify approaches for PRO data display that can be easily interpreted, regardless of scoring or scaling conventions, with the overall goal of improving patient and clinician understanding and use of PROs in oncology clinical practice. Part 1 of the study identified attributes of graphic displays of PRO data that are helpful and confusing.6 Part 2 involved developing improved PRO data presentation approaches.7 Part 3 evaluated the accuracy-of-interpretation and clarity of the developed approaches.8-10 The methods and findings of the three-part study are reported elsewhere;6-10 here, we describe the various approaches employed to engage stakeholders throughout the project.
As described above, the first reflection of stakeholder input was in the research question we asked. We then sought to identify the key stakeholder groups and ensure that they participated in each stage of the project. The relevant stakeholder groups we identified were: patients and their caregivers; health care providers (eg, oncologists, oncology nurses) who need to understand PRO data for their own consideration and for discussion with patients; and PRO researchers who develop, validate, and apply PRO measures.
Having identified these three key stakeholder groups, we sought to obtain broad representation of their perspectives. For example, we ensured that our investigative team included a cancer survivor, a cancer care provider, and PRO researchers. To supplement the stakeholder input from the investigative team, we formed a nine-member Stakeholder Advisory Board, with multiple representatives from each key constituency. We also aimed to be as broad as possible in the populations sampled for data collection. For example, we extended beyond the Johns Hopkins cancer center to include the Johns Hopkins Clinical Research Network, a consortium of academic and community health systems across the mid-Atlantic United States. Beyond the in-person data collection across the region, our study also included an internet survey of cancer patients/survivors, cancer care providers, and PRO researchers from across the United States and internationally. Taken together, these approaches improve the diversity of our sample and, thereby, the generalizability of our findings.
In addition to obtaining broad perspectives across stakeholder groups, we created genuine partnerships with the stakeholders to inform every aspect of the project. As described above, the study itself was motivated by feedback from cancer patients and clinicians regarding the challenges they experienced when trying to interpret PRO scores, and we therefore ensured that each stakeholder group contributed to the study’s design. Stakeholders also played a critical role in the conduct of the study. For example, in the first part of the study, we conducted one-on-one interviews with 50 cancer patients and 20 cancer clinicians to obtain their insights regarding attributes of current approaches for presenting PRO data that are helpful and confusing.6 At the completion of each interview, we asked participants whether they would be interested in partnering with the researchers in developing improved presentation formats in the next phase of the project. These volunteers were organized into work groups that reviewed the findings from the initial round of interviews with the investigative team, provided suggestions regarding candidate formats that could be used to improve presentation approaches, and helped pilot the internet survey.7 In this way, research participants had the opportunity to evolve into research partners, providing critically important input throughout the process.
The implementation and dissemination of findings is another area in which stakeholder partnership is particularly valuable. For example, several of our stakeholder partners have an advocacy background, which can be quite useful for conveying the project’s results in a compelling way. Other stakeholders, such as journal editors, are in a position to act directly to implement the study findings by, for example, adding best practices for presenting PRO data to their journal’s author instructions. Notably, some of the skills stakeholders bring come in addition to their role as stakeholders. For example, one of our patient stakeholders has a background in marketing, and this marketing expertise (completely separate from his patient experience) has helped the research team think about how to present data to broad audiences in a meaningful way.
In summary, this project has implemented stakeholder-driven approaches to address an important barrier to patient-centered cancer care. Several key lessons in stakeholder engagement have emerged from this experience. It is important to identify the key constituencies early on in the process. Involving stakeholders from the start enables them to play important roles in every aspect of the study, starting with study design conception. There are also innovative ways to integrate stakeholders in study conduct, such as our work groups of research participants who volunteered to partner with the research team to develop improved data presentation approaches. Implementation and dissemination is another area where stakeholders, based on their background and connections, can play a critical role. Throughout the process, it is valuable to challenge the project to obtain perspectives from as broad a range of stakeholders as possible. Finally, stakeholders have expertise beyond their stakeholder roles, and these skills can be quite valuable to the overall research agenda. In this project, our partnership with stakeholders has helped improve the presentation of PRO data to patients and providers, thereby improving the patient-centeredness of cancer care.
Acknowledgments
The PRO Data Presentation Stakeholder Advisory Board includes Neil K Aaronson, PhD (Netherlands Cancer Institute, Amsterdam); Patricia A Ganz, MD (University of California-Los Angeles and Jonsson Comprehensive Cancer Center, Los Angeles, CA); Ravin Garg, MD (Anne Arundel Medical Center, Annapolis, MD); Michael Fisch, MD (MD Anderson Cancer Center, Houston, TX); Vanessa Hoffman, MPH (Bladder Cancer Advocacy Network, Washington, DC); Bryce B Reeve, PhD (University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center, Chapel Hill, NC); Eden Stotsky-Himelfarb (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD); Ellen Stovall (National Coalition for Cancer Survivorship, Washington, DC [posthumous]); Matthew Zachary (Stupid Cancer, New York, NY).
The authors thank The Johns Hopkins Clinical Research Network site investigators and staff and, in particular, the patients and clinicians who participated in this project.
Supported by a Patient-Centered Outcomes Research Institute (PCORI) Award (R-1410-24904). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of PCORI, its board of governors or methodology committee. Drs Snyder and Smith are members of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (P30 CA 006973). The funders had no role in the study design; data collection, analysis, or interpretation; writing; or decision to submit.
Recently, researchers have been challenged to design methods that ensure that key constituents are partners in research, and not simply participants. Here we describe some innovative approaches we used to engage stakeholders. The approaches are drawn from a patient-centered outcomes research project, focusing on the graphic display of patient-reported outcomes (PROs) data. PROs represent patients’ perspectives on the impact of health, disease, and treatment, without interpretation by a clinician or anyone else. PROs include, among other things, patients’ assessments of their symptoms, their level of physical and psychosocial functioning, and health-related quality-of-life.1
As a first example of the key role of stakeholders in this project, input from cancer patients and clinicians, drawn from previous research, motivated us to ask whether there might be a “better way” to display PRO data when used to inform clinical practice. Specifically, even though cancer patients and clinicians endorse the importance of PRO data to promote patient-centered care, both groups report challenges using PROs in practice because of difficulty understanding what the PRO scores mean (eg, what is a good score or a bad score?; for individual patients, which scores should clinicians be concerned about?; for clinical trial PROs, what differences in PRO scores between treatments are clinically important?). The challenges in interpreting PRO data result in part from a large number of PRO measures (eg, one database includes more than 1,000 instruments)2 and no standards across PRO measures regarding how they are scored and scaled, or in how the data are presented.3 For example, on some PRO measures, higher scores represent better outcomes; on some PRO measures, lower scores represent better outcomes; and on some PRO measures, whether higher or lower scores represent better outcomes depends on the domain being measured. Further, some measures are scaled 0-100, with the extremes representing the best/worst scores possible, whereas others are normed to, for example, a population average of 50. Because of this variation, a score of 70 can have a completely different meaning depending on the PRO measure (or domain within a measure). As noted above, previous research has documented that this variation limits patients’ and clinicians’ understanding of the PRO scores, creating an important barrier to their use in practice.4-5
To address this stakeholder-driven research question, we undertook a three-part study to identify approaches for PRO data display that can be easily interpreted, regardless of scoring or scaling conventions, with the overall goal of improving patient and clinician understanding and use of PROs in oncology clinical practice. Part 1 of the study identified attributes of graphic displays of PRO data that are helpful and confusing.6 Part 2 involved developing improved PRO data presentation approaches.7 Part 3 evaluated the accuracy-of-interpretation and clarity of the developed approaches.8-10 The methods and findings of the three-part study are reported elsewhere;6-10 here, we describe the various approaches employed to engage stakeholders throughout the project.
As described above, the first reflection of stakeholder input was in the research question we asked. We then sought to identify the key stakeholder groups and ensure that they participated in each stage of the project. The relevant stakeholder groups we identified were: patients and their caregivers; health care providers (eg, oncologists, oncology nurses) who need to understand PRO data for their own consideration and for discussion with patients; and PRO researchers who develop, validate, and apply PRO measures.
Having identified these three key stakeholder groups, we sought to obtain broad representation of their perspectives. For example, we ensured that our investigative team included a cancer survivor, a cancer care provider, and PRO researchers. To supplement the stakeholder input from the investigative team, we formed a nine-member Stakeholder Advisory Board, with multiple representatives from each key constituency. We also aimed to be as broad as possible in the populations sampled for data collection. For example, we extended beyond the Johns Hopkins cancer center to include the Johns Hopkins Clinical Research Network, a consortium of academic and community health systems across the mid-Atlantic United States. Beyond the in-person data collection across the region, our study also included an internet survey of cancer patients/survivors, cancer care providers, and PRO researchers from across the United States and internationally. Taken together, these approaches improve the diversity of our sample and, thereby, the generalizability of our findings.
In addition to obtaining broad perspectives across stakeholder groups, we created genuine partnerships with the stakeholders to inform every aspect of the project. As described above, the study itself was motivated by feedback from cancer patients and clinicians regarding the challenges they experienced when trying to interpret PRO scores, and we therefore ensured that each stakeholder group contributed to the study’s design. Stakeholders also played a critical role in the conduct of the study. For example, in the first part of the study, we conducted one-on-one interviews with 50 cancer patients and 20 cancer clinicians to obtain their insights regarding attributes of current approaches for presenting PRO data that are helpful and confusing.6 At the completion of each interview, we asked participants whether they would be interested in partnering with the researchers in developing improved presentation formats in the next phase of the project. These volunteers were organized into work groups that reviewed the findings from the initial round of interviews with the investigative team, provided suggestions regarding candidate formats that could be used to improve presentation approaches, and helped pilot the internet survey.7 In this way, research participants had the opportunity to evolve into research partners, providing critically important input throughout the process.
The implementation and dissemination of findings is another area in which stakeholder partnership is particularly valuable. For example, several of our stakeholder partners have an advocacy background, which can be quite useful for conveying the project’s results in a compelling way. Other stakeholders, such as journal editors, are in a position to act directly to implement the study findings by, for example, adding best practices for presenting PRO data to their journal’s author instructions. Notably, some of the skills stakeholders bring come in addition to their role as stakeholders. For example, one of our patient stakeholders has a background in marketing, and this marketing expertise (completely separate from his patient experience) has helped the research team think about how to present data to broad audiences in a meaningful way.
In summary, this project has implemented stakeholder-driven approaches to address an important barrier to patient-centered cancer care. Several key lessons in stakeholder engagement have emerged from this experience. It is important to identify the key constituencies early on in the process. Involving stakeholders from the start enables them to play important roles in every aspect of the study, starting with study design conception. There are also innovative ways to integrate stakeholders in study conduct, such as our work groups of research participants who volunteered to partner with the research team to develop improved data presentation approaches. Implementation and dissemination is another area where stakeholders, based on their background and connections, can play a critical role. Throughout the process, it is valuable to challenge the project to obtain perspectives from as broad a range of stakeholders as possible. Finally, stakeholders have expertise beyond their stakeholder roles, and these skills can be quite valuable to the overall research agenda. In this project, our partnership with stakeholders has helped improve the presentation of PRO data to patients and providers, thereby improving the patient-centeredness of cancer care.
Acknowledgments
The PRO Data Presentation Stakeholder Advisory Board includes Neil K Aaronson, PhD (Netherlands Cancer Institute, Amsterdam); Patricia A Ganz, MD (University of California-Los Angeles and Jonsson Comprehensive Cancer Center, Los Angeles, CA); Ravin Garg, MD (Anne Arundel Medical Center, Annapolis, MD); Michael Fisch, MD (MD Anderson Cancer Center, Houston, TX); Vanessa Hoffman, MPH (Bladder Cancer Advocacy Network, Washington, DC); Bryce B Reeve, PhD (University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center, Chapel Hill, NC); Eden Stotsky-Himelfarb (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD); Ellen Stovall (National Coalition for Cancer Survivorship, Washington, DC [posthumous]); Matthew Zachary (Stupid Cancer, New York, NY).
The authors thank The Johns Hopkins Clinical Research Network site investigators and staff and, in particular, the patients and clinicians who participated in this project.
Supported by a Patient-Centered Outcomes Research Institute (PCORI) Award (R-1410-24904). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of PCORI, its board of governors or methodology committee. Drs Snyder and Smith are members of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (P30 CA 006973). The funders had no role in the study design; data collection, analysis, or interpretation; writing; or decision to submit.
1. Acquadro C, Berzon R, Dubois D, et al. Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Administration, February 16, 2001. Value Health. 2003;6(5):522-531.
2. PROQOLID, the Patient-Reported Outcome and Quality of Life Instruments Database. https://eprovide.mapi-trust.org/. Accessed November 10, 2016.
3. Brundage MD, Snyder CF. Patient-reported outcomes in clinical practice: using standards to break down barriers. Clin Invest. 2012;2(4):343-346.
4. Brundage M, Bass B, Jolie R, et al. A knowledge translation challenge: clinical use of quality of life data from cancer clinical trials. Qual Life Res. 2011;20(7):979-985.
5. Snyder CF, Jensen R, Courtin SO, et al. PatientViewpoint: a website for patient-reported outcomes assessment. Qual Life Res. 2009;18(7):793-800.
6. Brundage M, Smith KC, Little EA, Bantug ET, Snyder CF. PRO Data Presentation Stakeholder Advisory Board. Communicating patient-reported outcome scores using graphic formats: results from a mixed-methods evaluation. Qual Life Res. 2015;24(10):2457-2472.
7. Smith KC, Brundage MD, Tolbert E, Little EA, Bantug ET, Snyder C. PRO Data Presentation Stakeholder Advisory Board. Engaging stakeholders to improve presentation of patient-reported outcomes data in clinical practice. Support Care Cancer. 2016;24(10):4149-4157.
8. Snyder CF, Smith KC, Bantug ET, Tolbert EE, Blackford AL, Brundage MD. PRO Data Presentation Stakeholder Advisory Board. What do these scores mean? Presenting patient-reported outcomes data to patients and clinicians to improve interpretability. Cancer. 2017;123(10):1848-1859.
9. Brundage M, Blackford A, Tolbert E, Smith K, Bantug E, Snyder C. PRO Data Presentation Stakeholder Advisory Board. Presenting comparative study PRO results to clinicians and researchers: beyond the eye of the beholder. Qual Life Res. 2017 Nov 2 [Epub ahead of print].
10. Tolbert E, Snyder C, Bantug E, Blackford A, Brundage M. PRO Data Presentation Stakeholder Advisory Board. Graphing group-level data from research studies for presentation to patients in educational materials and decision aids. Qual Life Res. 2016;25(suppl 1):17.
1. Acquadro C, Berzon R, Dubois D, et al. Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Administration, February 16, 2001. Value Health. 2003;6(5):522-531.
2. PROQOLID, the Patient-Reported Outcome and Quality of Life Instruments Database. https://eprovide.mapi-trust.org/. Accessed November 10, 2016.
3. Brundage MD, Snyder CF. Patient-reported outcomes in clinical practice: using standards to break down barriers. Clin Invest. 2012;2(4):343-346.
4. Brundage M, Bass B, Jolie R, et al. A knowledge translation challenge: clinical use of quality of life data from cancer clinical trials. Qual Life Res. 2011;20(7):979-985.
5. Snyder CF, Jensen R, Courtin SO, et al. PatientViewpoint: a website for patient-reported outcomes assessment. Qual Life Res. 2009;18(7):793-800.
6. Brundage M, Smith KC, Little EA, Bantug ET, Snyder CF. PRO Data Presentation Stakeholder Advisory Board. Communicating patient-reported outcome scores using graphic formats: results from a mixed-methods evaluation. Qual Life Res. 2015;24(10):2457-2472.
7. Smith KC, Brundage MD, Tolbert E, Little EA, Bantug ET, Snyder C. PRO Data Presentation Stakeholder Advisory Board. Engaging stakeholders to improve presentation of patient-reported outcomes data in clinical practice. Support Care Cancer. 2016;24(10):4149-4157.
8. Snyder CF, Smith KC, Bantug ET, Tolbert EE, Blackford AL, Brundage MD. PRO Data Presentation Stakeholder Advisory Board. What do these scores mean? Presenting patient-reported outcomes data to patients and clinicians to improve interpretability. Cancer. 2017;123(10):1848-1859.
9. Brundage M, Blackford A, Tolbert E, Smith K, Bantug E, Snyder C. PRO Data Presentation Stakeholder Advisory Board. Presenting comparative study PRO results to clinicians and researchers: beyond the eye of the beholder. Qual Life Res. 2017 Nov 2 [Epub ahead of print].
10. Tolbert E, Snyder C, Bantug E, Blackford A, Brundage M. PRO Data Presentation Stakeholder Advisory Board. Graphing group-level data from research studies for presentation to patients in educational materials and decision aids. Qual Life Res. 2016;25(suppl 1):17.
Pembrolizumab is the first immune checkpoint inhibitor to receive approval for head and neck cancer
The first immune checkpoint inhibitor was approved for the treatment of head and neck cancer approved in August 2016. Pembrolizumab, which targets the programmed cell death 1 (PD-1) protein, is designed to reinstate the anti-tumor immune response to kill cancer cells and was approved for the treatment of recurrent or metastatic disease that progressed during or after platinum-containing chemotherapy.
Click on the PDF icon at the top of this introduction to read the full article.
The first immune checkpoint inhibitor was approved for the treatment of head and neck cancer approved in August 2016. Pembrolizumab, which targets the programmed cell death 1 (PD-1) protein, is designed to reinstate the anti-tumor immune response to kill cancer cells and was approved for the treatment of recurrent or metastatic disease that progressed during or after platinum-containing chemotherapy.
Click on the PDF icon at the top of this introduction to read the full article.
The first immune checkpoint inhibitor was approved for the treatment of head and neck cancer approved in August 2016. Pembrolizumab, which targets the programmed cell death 1 (PD-1) protein, is designed to reinstate the anti-tumor immune response to kill cancer cells and was approved for the treatment of recurrent or metastatic disease that progressed during or after platinum-containing chemotherapy.
Click on the PDF icon at the top of this introduction to read the full article.
Pancreatic cancer: a therapeutic challenge yet to be met
Although there are numerous hard-to-treat tumor types, pancreatic cancer, which most commonly presents as pancreatic ductal adenocarcinoma (PDA) is particularly notorious and arguably the most challenging and deadly of all cancers. It is currently ranked as the fourth most common cause of cancer-related mortality, and looks set to move up to the number 2 slot within the next 15 years.1 Here, we discuss the evolution of much-needed novel treatment strategies.
Click on the PDF icon at the top of this introduction to read the full article.
Although there are numerous hard-to-treat tumor types, pancreatic cancer, which most commonly presents as pancreatic ductal adenocarcinoma (PDA) is particularly notorious and arguably the most challenging and deadly of all cancers. It is currently ranked as the fourth most common cause of cancer-related mortality, and looks set to move up to the number 2 slot within the next 15 years.1 Here, we discuss the evolution of much-needed novel treatment strategies.
Click on the PDF icon at the top of this introduction to read the full article.
Although there are numerous hard-to-treat tumor types, pancreatic cancer, which most commonly presents as pancreatic ductal adenocarcinoma (PDA) is particularly notorious and arguably the most challenging and deadly of all cancers. It is currently ranked as the fourth most common cause of cancer-related mortality, and looks set to move up to the number 2 slot within the next 15 years.1 Here, we discuss the evolution of much-needed novel treatment strategies.
Click on the PDF icon at the top of this introduction to read the full article.
Unicentric Castleman disease disguised as a pancreatic neoplasm
Castleman disease or angiofollicular lymph node hyperplasia is an uncommon cause of an incidental abdominal mass found on imaging. The etiology of Castleman disease is relatively unknown, however, it is thought to be primarily associated with an oversecretion of interleukin-6. The oversecretion of this pro-inflammatory cytokine leads to lymph node hyperplasia. Castleman disease can be classified into 2 categories: unicentric or multicentric. Most cases of unicentric Castleman disease are asymptomatic and are found on routine imaging. It is found predominately in middle-aged persons of equal sex and is managed primarily by surgical resection. We present here a case of a peripancreatic mass diagnosed by surgical excision as Castleman disease, hyaline vascular type.
Click on the PDF icon at the top of this introduction to read the full article.
Castleman disease or angiofollicular lymph node hyperplasia is an uncommon cause of an incidental abdominal mass found on imaging. The etiology of Castleman disease is relatively unknown, however, it is thought to be primarily associated with an oversecretion of interleukin-6. The oversecretion of this pro-inflammatory cytokine leads to lymph node hyperplasia. Castleman disease can be classified into 2 categories: unicentric or multicentric. Most cases of unicentric Castleman disease are asymptomatic and are found on routine imaging. It is found predominately in middle-aged persons of equal sex and is managed primarily by surgical resection. We present here a case of a peripancreatic mass diagnosed by surgical excision as Castleman disease, hyaline vascular type.
Click on the PDF icon at the top of this introduction to read the full article.
Castleman disease or angiofollicular lymph node hyperplasia is an uncommon cause of an incidental abdominal mass found on imaging. The etiology of Castleman disease is relatively unknown, however, it is thought to be primarily associated with an oversecretion of interleukin-6. The oversecretion of this pro-inflammatory cytokine leads to lymph node hyperplasia. Castleman disease can be classified into 2 categories: unicentric or multicentric. Most cases of unicentric Castleman disease are asymptomatic and are found on routine imaging. It is found predominately in middle-aged persons of equal sex and is managed primarily by surgical resection. We present here a case of a peripancreatic mass diagnosed by surgical excision as Castleman disease, hyaline vascular type.
Click on the PDF icon at the top of this introduction to read the full article.
Paraneoplastic Isaacs syndrome leading to diagnosis of small-cell lung cancer
Paraneoplastic Isaacs syndrome is a rare disorder with distinct clinical and electromyographic characteristics. It is a consequence of neoplastic process that is not directly caused by the tumor itself, but usually mediated by immune response primarily against the tumor and neural tissues are damaged owing to bystander effect. Paraneoplastic neurologic disorders may precede cancer diagnosis. Here we report the case of 75-year-old woman who presented with numbness, tingling sensation, and weakness of lower extremities, and was diagnosed with Isaacs syndrome and subsequently small-cell lung cancer. Plasmapheresis and treatment of small-cell lung cancer produced signficant symptoms improvement. We also conduct a complete review of the published case reports and case series of Isaacs syndrome of paraneoplastic etiology, which usually has good response to carbamazepine and to specfic treatment of underlying neoplasm.
Click on the PDF icon at the top of this introduction to read the full article.
Paraneoplastic Isaacs syndrome is a rare disorder with distinct clinical and electromyographic characteristics. It is a consequence of neoplastic process that is not directly caused by the tumor itself, but usually mediated by immune response primarily against the tumor and neural tissues are damaged owing to bystander effect. Paraneoplastic neurologic disorders may precede cancer diagnosis. Here we report the case of 75-year-old woman who presented with numbness, tingling sensation, and weakness of lower extremities, and was diagnosed with Isaacs syndrome and subsequently small-cell lung cancer. Plasmapheresis and treatment of small-cell lung cancer produced signficant symptoms improvement. We also conduct a complete review of the published case reports and case series of Isaacs syndrome of paraneoplastic etiology, which usually has good response to carbamazepine and to specfic treatment of underlying neoplasm.
Click on the PDF icon at the top of this introduction to read the full article.
Paraneoplastic Isaacs syndrome is a rare disorder with distinct clinical and electromyographic characteristics. It is a consequence of neoplastic process that is not directly caused by the tumor itself, but usually mediated by immune response primarily against the tumor and neural tissues are damaged owing to bystander effect. Paraneoplastic neurologic disorders may precede cancer diagnosis. Here we report the case of 75-year-old woman who presented with numbness, tingling sensation, and weakness of lower extremities, and was diagnosed with Isaacs syndrome and subsequently small-cell lung cancer. Plasmapheresis and treatment of small-cell lung cancer produced signficant symptoms improvement. We also conduct a complete review of the published case reports and case series of Isaacs syndrome of paraneoplastic etiology, which usually has good response to carbamazepine and to specfic treatment of underlying neoplasm.
Click on the PDF icon at the top of this introduction to read the full article.
Quality of life after surgery for pleural malignant mesothelioma – methodological considerations
Background There is a dearth of literature on patient quality of life (QoL) after treatment for malignant pleural mesothelioma (MPM).
Objectives To review the literature on QoL after surgery for MPM and assess differences in quality of life between patients who have extrapleural pneumonectomy (EPP) and those who have pleurectomy and decortication (P-D).
Methods We retrieved and reviewed original research studies on quality of life after mesothelioma surgery. They had been published from January 1990 through June 2016, and included 15 articles and 12 datasets for a total of 523 patients.
Results QoL data was available for 102 EPP patients and 296 P-D patients. Two studies directly compared QoL outcomes between the 2 techniques. Symptoms, lung function parameters, and physical and social functioning were still compromised 6 months after surgery. However, P-D patients fared better than did EPP patients across QoL measures.
Limitations The amount of available literature is small, and the studies are heterogeneous.
Conclusions QoL is better for a longer period of time in patients who undergo P-D, compared with those who have EPP. Given the need for multimodality therapy for MPM and the aggressive nature of the disease, QoL outcomes should be strongly considered when choosing type of surgery for mesothelioma.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a dearth of literature on patient quality of life (QoL) after treatment for malignant pleural mesothelioma (MPM).
Objectives To review the literature on QoL after surgery for MPM and assess differences in quality of life between patients who have extrapleural pneumonectomy (EPP) and those who have pleurectomy and decortication (P-D).
Methods We retrieved and reviewed original research studies on quality of life after mesothelioma surgery. They had been published from January 1990 through June 2016, and included 15 articles and 12 datasets for a total of 523 patients.
Results QoL data was available for 102 EPP patients and 296 P-D patients. Two studies directly compared QoL outcomes between the 2 techniques. Symptoms, lung function parameters, and physical and social functioning were still compromised 6 months after surgery. However, P-D patients fared better than did EPP patients across QoL measures.
Limitations The amount of available literature is small, and the studies are heterogeneous.
Conclusions QoL is better for a longer period of time in patients who undergo P-D, compared with those who have EPP. Given the need for multimodality therapy for MPM and the aggressive nature of the disease, QoL outcomes should be strongly considered when choosing type of surgery for mesothelioma.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a dearth of literature on patient quality of life (QoL) after treatment for malignant pleural mesothelioma (MPM).
Objectives To review the literature on QoL after surgery for MPM and assess differences in quality of life between patients who have extrapleural pneumonectomy (EPP) and those who have pleurectomy and decortication (P-D).
Methods We retrieved and reviewed original research studies on quality of life after mesothelioma surgery. They had been published from January 1990 through June 2016, and included 15 articles and 12 datasets for a total of 523 patients.
Results QoL data was available for 102 EPP patients and 296 P-D patients. Two studies directly compared QoL outcomes between the 2 techniques. Symptoms, lung function parameters, and physical and social functioning were still compromised 6 months after surgery. However, P-D patients fared better than did EPP patients across QoL measures.
Limitations The amount of available literature is small, and the studies are heterogeneous.
Conclusions QoL is better for a longer period of time in patients who undergo P-D, compared with those who have EPP. Given the need for multimodality therapy for MPM and the aggressive nature of the disease, QoL outcomes should be strongly considered when choosing type of surgery for mesothelioma.
Click on the PDF icon at the top of this introduction to read the full article.
Social support needs among patients with advanced breast cancer: sensitivity trumps substance
Background The importance of social support for cancer patients has been established in previous studies. However, much of the existing research has identified associations between general measures of social support and various health indicators. Nevertheless, some research has begun to suggest the utility of more nuanced understandings of how patients receive and use social support.
Objective To examine the roles of nondirective (ie, support that accepts recipients’ feelings and is cooperative with their plans) and directive support (ie, support that prescribes “correct” choices and feelings) as well as social support needs and desires among patients with advanced breast cancer.
Methods We conducted semi-structured interviews (qualitative method) with 8 patients with stage IV breast cancer to collect qualitative information about the disease-related challenges they faced, the support they received from their families and medical teams, and the appropriateness of directive and nondirective support. In addition, we used the 14-item Hospital Anxiety and Depression Scale (HADS) to assess clinically relevant cut-offs for anxiety and depression and the 16-item Social Support Inventory to assess the provision of nondirective and directive social support to the patients (quantitative method).
Results Qualitative findings suggested that there was considerable variability among patients’ reports of social support provided by family, friends, and the medical team. From the qualitative data, patients reported directive support as more useful in times of acute need and emphasized the importance of supportive systems rather than supportive persons in providing emotional support. From the quantitative data, patients reported nondirective support as more typical of support received from both family and medical teams than directive support. On the HADS, 1 patient had a score of 9 on the anxiety subscale, above the score of 7 that is for mild anxiety. No patients scored above the criterion for mild depression, also a score of 7.
Limitations Very small sample limits the ability to generalize findings.
Conclusions The right type of support for patients with advanced breast cancer is contingent on a range of variables, which suggests that the key characteristic of support may not be any particular feature, but the nuanced adjustment of its content and style of delivery to the patient’s circumstances.
Funding Peers for Progress
Click on the PDF icon at the top of this introduction to read the full article.
Background The importance of social support for cancer patients has been established in previous studies. However, much of the existing research has identified associations between general measures of social support and various health indicators. Nevertheless, some research has begun to suggest the utility of more nuanced understandings of how patients receive and use social support.
Objective To examine the roles of nondirective (ie, support that accepts recipients’ feelings and is cooperative with their plans) and directive support (ie, support that prescribes “correct” choices and feelings) as well as social support needs and desires among patients with advanced breast cancer.
Methods We conducted semi-structured interviews (qualitative method) with 8 patients with stage IV breast cancer to collect qualitative information about the disease-related challenges they faced, the support they received from their families and medical teams, and the appropriateness of directive and nondirective support. In addition, we used the 14-item Hospital Anxiety and Depression Scale (HADS) to assess clinically relevant cut-offs for anxiety and depression and the 16-item Social Support Inventory to assess the provision of nondirective and directive social support to the patients (quantitative method).
Results Qualitative findings suggested that there was considerable variability among patients’ reports of social support provided by family, friends, and the medical team. From the qualitative data, patients reported directive support as more useful in times of acute need and emphasized the importance of supportive systems rather than supportive persons in providing emotional support. From the quantitative data, patients reported nondirective support as more typical of support received from both family and medical teams than directive support. On the HADS, 1 patient had a score of 9 on the anxiety subscale, above the score of 7 that is for mild anxiety. No patients scored above the criterion for mild depression, also a score of 7.
Limitations Very small sample limits the ability to generalize findings.
Conclusions The right type of support for patients with advanced breast cancer is contingent on a range of variables, which suggests that the key characteristic of support may not be any particular feature, but the nuanced adjustment of its content and style of delivery to the patient’s circumstances.
Funding Peers for Progress
Click on the PDF icon at the top of this introduction to read the full article.
Background The importance of social support for cancer patients has been established in previous studies. However, much of the existing research has identified associations between general measures of social support and various health indicators. Nevertheless, some research has begun to suggest the utility of more nuanced understandings of how patients receive and use social support.
Objective To examine the roles of nondirective (ie, support that accepts recipients’ feelings and is cooperative with their plans) and directive support (ie, support that prescribes “correct” choices and feelings) as well as social support needs and desires among patients with advanced breast cancer.
Methods We conducted semi-structured interviews (qualitative method) with 8 patients with stage IV breast cancer to collect qualitative information about the disease-related challenges they faced, the support they received from their families and medical teams, and the appropriateness of directive and nondirective support. In addition, we used the 14-item Hospital Anxiety and Depression Scale (HADS) to assess clinically relevant cut-offs for anxiety and depression and the 16-item Social Support Inventory to assess the provision of nondirective and directive social support to the patients (quantitative method).
Results Qualitative findings suggested that there was considerable variability among patients’ reports of social support provided by family, friends, and the medical team. From the qualitative data, patients reported directive support as more useful in times of acute need and emphasized the importance of supportive systems rather than supportive persons in providing emotional support. From the quantitative data, patients reported nondirective support as more typical of support received from both family and medical teams than directive support. On the HADS, 1 patient had a score of 9 on the anxiety subscale, above the score of 7 that is for mild anxiety. No patients scored above the criterion for mild depression, also a score of 7.
Limitations Very small sample limits the ability to generalize findings.
Conclusions The right type of support for patients with advanced breast cancer is contingent on a range of variables, which suggests that the key characteristic of support may not be any particular feature, but the nuanced adjustment of its content and style of delivery to the patient’s circumstances.
Funding Peers for Progress
Click on the PDF icon at the top of this introduction to read the full article.