User login
European Academy of Dermatology & Venereology (EADV): Annual Congress
EADV: New long-term data on biologics for pediatric psoriasis ‘encouraging’
COPENHAGEN – The longest-ever clinical trials of etanercept and adalimumab for pediatric psoriasis show reassuring maintenance of efficacy, coupled with safety and tolerability profiles similar to what has been seen in long-term trials in adults, investigators reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Richard G. Langley presented outcomes from a 5-year open-label extension of an initial 12-week, multicenter, double-blind, randomized trial of etanercept or placebo in children and adolescents with moderate to severe chronic plaque psoriasis. The primary results were published more than 7 years ago (N Engl J Med. 2008 Jan 17;358[3]:241-51).
“This is the largest and longest follow-up of any biologic in children and adolescents to date. I think it’s encouraging data and should be reassuring to those of us who are managing this important population of pediatric patients in our conversations with parents and families,”said Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
In the original 211-patient study, 57% of patients receiving etanercept (Enbrel) once weekly at 0.8 mg/kg to a maximum of 50 mg achieved a PASI 75 response at week 12, and 27% had a PASI 90. In the 69 patients who completed the full 264 weeks of follow-up, those response rates remained essentially unchanged.
Dr. Langley was quick to point out that this was an as-observed analysis, meaning results were counted only in those patients still participating at week 264. While conceding that the two-thirds dropout rate is an important study limitation, he added: “Notwithstanding that, what matters to us in the clinic are the patients we continue to treat and how they’re responding.”
Of note, most study discontinuations didn’t result from loss of response or adverse events, they were due to withdrawal of consent by families in which the patient began the study as a cooperative child and who as time went by turned into an independent and often willful teenager, he said.
No new safety signals arose over the course of 5 years. There were no opportunistic infections, no malignancies. The most common adverse events were the same ones seen in the original short-term study: upper respiratory infections, nasopharyngitis, and headaches. There was only one serious adverse event deemed by investigators as ‘possibly related’ to etanercept therapy: a case of cellulitis.
Separately, Dr. Diamant Thaci and Dr. Kim A. Papp presented 52-week outcomes for different aspects of the pivotal phase III randomized trial which earlier in 2015 earned adalimumab (Humira) European Commission marketing approval as the first biologic agent indicated for treatment of children as young as age 4 years, as well as for adolescents. The multi-arm trial included 114 patients aged 4-17 years with moderate to severe plaque psoriasis.
Dr. Thaci reported on the 37 subjects initially randomized double-blind to 16 weeks of oral methotrexate at 0.1-0.4 mg/kg weekly. The 19 patients (51%) who were deemed nonresponders to methotrexate because of inadequate PASI response at week 16 were then switched to open-label adalimumab at 0.8 mg/kg every other week for the remainder of the 52 weeks.
After 16 weeks on adalimumab, the methotrexate nonresponders had a PASI 75 of 90% and a PASI 90 of 74%, and 79% of the subjects were rated clear or almost clear by Physician’s Global Assessment. At week 52, the PASI 75 rate was 79%, the PASI 90 rate was 58%, and 68% of patients were rated clear or almost clear, according to Dr. Thaci of University Hospital Schleswig-Holstein, in L<scaps>ü</scaps>beck, Germany.
The side effects of methotrexate and adalimumab were similar to those seen in adults. There were no serious adverse events. The infections that occurred during adalimumab therapy were “very banal things,” mostly nasopharyngitis and upper respiratory tract infections, the dermatologist said. There were no opportunistic infections, malignancies, or cases of tuberculosis during the phase III study.
This was an important analysis because it recapitulates daily clinical practice, he explained. In most of the world, when dermatologists deem it time for systemic therapy, they generally start out with methotrexate, reserving biologics for second-line therapy because of the cost.
Dr. Papp reported on 39 patients on adalimumab at 0.4 mg/kg every other week, and 38 on 0.8 mg/kg every other week throughout the 52-week study. The response rate was essentially a flat line from week 16 to week 52, with PASI 75s of 44% at week 16 and 50% at week 52 in the low-dose group, and 58% and 56% in the high-dose group. Half of patients on 0.4 mg/kg were clear or almost clear at week 52, as were 56% on 0.8 mg/kg.
There was a nonsignificant trend for an increasing infection rate with greater exposure to adalimumab, which will require evaluation during the ongoing follow-up beyond 52 weeks, said Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“I think what’s gratifying about this is we see that these children actually have a robust response and that response is maintained over a full year of treatment, which is reassuring because that reflects what we’ve seen in the adult population as well,” he said.
Dr. Papp and Dr. Thaci receive research funding and serve as scientific advisers to AbbVie, which sponsored the adalimumab trial. They also have ties to other pharmaceutical companies.
Dr. Langley has served as principal investigator for and is on the scientific advisory boards of Amgen, which sponsored the etanercept pediatric psoriasis study. He also has ties to other pharmaceutical companies.
COPENHAGEN – The longest-ever clinical trials of etanercept and adalimumab for pediatric psoriasis show reassuring maintenance of efficacy, coupled with safety and tolerability profiles similar to what has been seen in long-term trials in adults, investigators reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Richard G. Langley presented outcomes from a 5-year open-label extension of an initial 12-week, multicenter, double-blind, randomized trial of etanercept or placebo in children and adolescents with moderate to severe chronic plaque psoriasis. The primary results were published more than 7 years ago (N Engl J Med. 2008 Jan 17;358[3]:241-51).
“This is the largest and longest follow-up of any biologic in children and adolescents to date. I think it’s encouraging data and should be reassuring to those of us who are managing this important population of pediatric patients in our conversations with parents and families,”said Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
In the original 211-patient study, 57% of patients receiving etanercept (Enbrel) once weekly at 0.8 mg/kg to a maximum of 50 mg achieved a PASI 75 response at week 12, and 27% had a PASI 90. In the 69 patients who completed the full 264 weeks of follow-up, those response rates remained essentially unchanged.
Dr. Langley was quick to point out that this was an as-observed analysis, meaning results were counted only in those patients still participating at week 264. While conceding that the two-thirds dropout rate is an important study limitation, he added: “Notwithstanding that, what matters to us in the clinic are the patients we continue to treat and how they’re responding.”
Of note, most study discontinuations didn’t result from loss of response or adverse events, they were due to withdrawal of consent by families in which the patient began the study as a cooperative child and who as time went by turned into an independent and often willful teenager, he said.
No new safety signals arose over the course of 5 years. There were no opportunistic infections, no malignancies. The most common adverse events were the same ones seen in the original short-term study: upper respiratory infections, nasopharyngitis, and headaches. There was only one serious adverse event deemed by investigators as ‘possibly related’ to etanercept therapy: a case of cellulitis.
Separately, Dr. Diamant Thaci and Dr. Kim A. Papp presented 52-week outcomes for different aspects of the pivotal phase III randomized trial which earlier in 2015 earned adalimumab (Humira) European Commission marketing approval as the first biologic agent indicated for treatment of children as young as age 4 years, as well as for adolescents. The multi-arm trial included 114 patients aged 4-17 years with moderate to severe plaque psoriasis.
Dr. Thaci reported on the 37 subjects initially randomized double-blind to 16 weeks of oral methotrexate at 0.1-0.4 mg/kg weekly. The 19 patients (51%) who were deemed nonresponders to methotrexate because of inadequate PASI response at week 16 were then switched to open-label adalimumab at 0.8 mg/kg every other week for the remainder of the 52 weeks.
After 16 weeks on adalimumab, the methotrexate nonresponders had a PASI 75 of 90% and a PASI 90 of 74%, and 79% of the subjects were rated clear or almost clear by Physician’s Global Assessment. At week 52, the PASI 75 rate was 79%, the PASI 90 rate was 58%, and 68% of patients were rated clear or almost clear, according to Dr. Thaci of University Hospital Schleswig-Holstein, in L<scaps>ü</scaps>beck, Germany.
The side effects of methotrexate and adalimumab were similar to those seen in adults. There were no serious adverse events. The infections that occurred during adalimumab therapy were “very banal things,” mostly nasopharyngitis and upper respiratory tract infections, the dermatologist said. There were no opportunistic infections, malignancies, or cases of tuberculosis during the phase III study.
This was an important analysis because it recapitulates daily clinical practice, he explained. In most of the world, when dermatologists deem it time for systemic therapy, they generally start out with methotrexate, reserving biologics for second-line therapy because of the cost.
Dr. Papp reported on 39 patients on adalimumab at 0.4 mg/kg every other week, and 38 on 0.8 mg/kg every other week throughout the 52-week study. The response rate was essentially a flat line from week 16 to week 52, with PASI 75s of 44% at week 16 and 50% at week 52 in the low-dose group, and 58% and 56% in the high-dose group. Half of patients on 0.4 mg/kg were clear or almost clear at week 52, as were 56% on 0.8 mg/kg.
There was a nonsignificant trend for an increasing infection rate with greater exposure to adalimumab, which will require evaluation during the ongoing follow-up beyond 52 weeks, said Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“I think what’s gratifying about this is we see that these children actually have a robust response and that response is maintained over a full year of treatment, which is reassuring because that reflects what we’ve seen in the adult population as well,” he said.
Dr. Papp and Dr. Thaci receive research funding and serve as scientific advisers to AbbVie, which sponsored the adalimumab trial. They also have ties to other pharmaceutical companies.
Dr. Langley has served as principal investigator for and is on the scientific advisory boards of Amgen, which sponsored the etanercept pediatric psoriasis study. He also has ties to other pharmaceutical companies.
COPENHAGEN – The longest-ever clinical trials of etanercept and adalimumab for pediatric psoriasis show reassuring maintenance of efficacy, coupled with safety and tolerability profiles similar to what has been seen in long-term trials in adults, investigators reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Richard G. Langley presented outcomes from a 5-year open-label extension of an initial 12-week, multicenter, double-blind, randomized trial of etanercept or placebo in children and adolescents with moderate to severe chronic plaque psoriasis. The primary results were published more than 7 years ago (N Engl J Med. 2008 Jan 17;358[3]:241-51).
“This is the largest and longest follow-up of any biologic in children and adolescents to date. I think it’s encouraging data and should be reassuring to those of us who are managing this important population of pediatric patients in our conversations with parents and families,”said Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
In the original 211-patient study, 57% of patients receiving etanercept (Enbrel) once weekly at 0.8 mg/kg to a maximum of 50 mg achieved a PASI 75 response at week 12, and 27% had a PASI 90. In the 69 patients who completed the full 264 weeks of follow-up, those response rates remained essentially unchanged.
Dr. Langley was quick to point out that this was an as-observed analysis, meaning results were counted only in those patients still participating at week 264. While conceding that the two-thirds dropout rate is an important study limitation, he added: “Notwithstanding that, what matters to us in the clinic are the patients we continue to treat and how they’re responding.”
Of note, most study discontinuations didn’t result from loss of response or adverse events, they were due to withdrawal of consent by families in which the patient began the study as a cooperative child and who as time went by turned into an independent and often willful teenager, he said.
No new safety signals arose over the course of 5 years. There were no opportunistic infections, no malignancies. The most common adverse events were the same ones seen in the original short-term study: upper respiratory infections, nasopharyngitis, and headaches. There was only one serious adverse event deemed by investigators as ‘possibly related’ to etanercept therapy: a case of cellulitis.
Separately, Dr. Diamant Thaci and Dr. Kim A. Papp presented 52-week outcomes for different aspects of the pivotal phase III randomized trial which earlier in 2015 earned adalimumab (Humira) European Commission marketing approval as the first biologic agent indicated for treatment of children as young as age 4 years, as well as for adolescents. The multi-arm trial included 114 patients aged 4-17 years with moderate to severe plaque psoriasis.
Dr. Thaci reported on the 37 subjects initially randomized double-blind to 16 weeks of oral methotrexate at 0.1-0.4 mg/kg weekly. The 19 patients (51%) who were deemed nonresponders to methotrexate because of inadequate PASI response at week 16 were then switched to open-label adalimumab at 0.8 mg/kg every other week for the remainder of the 52 weeks.
After 16 weeks on adalimumab, the methotrexate nonresponders had a PASI 75 of 90% and a PASI 90 of 74%, and 79% of the subjects were rated clear or almost clear by Physician’s Global Assessment. At week 52, the PASI 75 rate was 79%, the PASI 90 rate was 58%, and 68% of patients were rated clear or almost clear, according to Dr. Thaci of University Hospital Schleswig-Holstein, in L<scaps>ü</scaps>beck, Germany.
The side effects of methotrexate and adalimumab were similar to those seen in adults. There were no serious adverse events. The infections that occurred during adalimumab therapy were “very banal things,” mostly nasopharyngitis and upper respiratory tract infections, the dermatologist said. There were no opportunistic infections, malignancies, or cases of tuberculosis during the phase III study.
This was an important analysis because it recapitulates daily clinical practice, he explained. In most of the world, when dermatologists deem it time for systemic therapy, they generally start out with methotrexate, reserving biologics for second-line therapy because of the cost.
Dr. Papp reported on 39 patients on adalimumab at 0.4 mg/kg every other week, and 38 on 0.8 mg/kg every other week throughout the 52-week study. The response rate was essentially a flat line from week 16 to week 52, with PASI 75s of 44% at week 16 and 50% at week 52 in the low-dose group, and 58% and 56% in the high-dose group. Half of patients on 0.4 mg/kg were clear or almost clear at week 52, as were 56% on 0.8 mg/kg.
There was a nonsignificant trend for an increasing infection rate with greater exposure to adalimumab, which will require evaluation during the ongoing follow-up beyond 52 weeks, said Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“I think what’s gratifying about this is we see that these children actually have a robust response and that response is maintained over a full year of treatment, which is reassuring because that reflects what we’ve seen in the adult population as well,” he said.
Dr. Papp and Dr. Thaci receive research funding and serve as scientific advisers to AbbVie, which sponsored the adalimumab trial. They also have ties to other pharmaceutical companies.
Dr. Langley has served as principal investigator for and is on the scientific advisory boards of Amgen, which sponsored the etanercept pediatric psoriasis study. He also has ties to other pharmaceutical companies.
EXPERT ANALYSIS FROM THE EADV CONGRESS
EADV: Pediatric psoriasis called ‘grossly undertreated’
COPENHAGEN – Children and adolescents with moderate to severe psoriasis are generally undertreated, with resultant potential psychological impairment, social stigmatization, and isolation, psoriasis experts agreed at the annual congress of the European Academy of Dermatology and Venereology.
In a session featuring new long-term safety and efficacy data from extended pediatric clinical trials of etanercept and adalimumab, prominent clinical trial investigators who presented the findings asserted that many physicians and parents are overly timid about the use of systemic treatment options in children and adolescents when such therapy is clearly warranted.
“The burden of psoriasis is particularly acute in the pediatric population. I always ask patients, ‘How is psoriasis affecting you?’ And with children, it’s affecting them and it’s also affecting their parents. If it’s affecting their self-esteem – if it’s ruining your life and you can’t control it with topical agents – I think it’s time to think of other things,” declared Dr. Richard G. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
Yet all too often that doesn’t happen, as documented in the recently reported physician portion of the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey, which included 391 dermatologists and 390 rheumatologists in North America and Western Europe (J Eur Acad Dermatol Venereol. 2015 Oct;29[10]:2002-10).
Dr. Langley was a coinvestigator in MAPP, which found that 39% of dermatologists reported treating their moderate to severe psoriasis patients with conventional oral and/or biologic agents (19.5% and 19.6%, respectively). A total of 75% indicated they prescribe topical medications as monotherapy for their patients with moderate to severe psoriasis. In children, that rate was even higher, he said, despite the fact that topical monotherapy is clearly inadequate for treatment of more severe disease.
“I think there’s a fear about using systemics among parents and among some practitioners, which was one of the top reasons in the MAPP survey that people didn’t get appropriate treatment,” he said.
That’s a shortsighted attitude, the dermatologist continued: “The risk is not just the risk of the drug, but the risk of untreated disease, because untreated disease devastates patients.”
MAPP was the largest-ever survey of physician and patient perspectives regarding psoriasis and psoriatic arthritis. The patient perspective, derived from interviews with nearly 3,500 patients, was published earlier (J Am Acad Dermatol. 2014 May;70[5]:871-81).
One impediment to more widespread use of systemic therapies for pediatric psoriasis is that it’s almost entirely off-label prescribing. In the United States, no conventional oral agents or biologics are Food and Drug Administration approved for use in psoriasis patients under age 18 years. That was the case in Europe as well until earlier this year, when adalimumab (Humira) received European marketing approval for children ages 4 years and up with severe chronic plaque psoriasis with an inadequate response to topical agents and phototherapy or in whom such therapies are contraindicated.
Dr. Kim A. Papp, who presented new 52-week safety and efficacy data from a phase III study of adalimumab in pediatric psoriasis patients, said that even when dermatologists utilize biologics in pediatric patients, the medications are typically underdosed. In the phase III pediatric adalimumab trial, for example, most participants were overweight or obese, and efficacy dropped off with increasing body mass index, as has been the case in trials of most of the biologics.
“When I look at the doses of the biologic agents that are used in treating the pediatric population and what we know about how these molecules are distributed in the body, we can safely say we are grossly underdosing children. We’re doing that because we believe somehow they’re at greater risk from exposures that are comparable to those in the adult population. And it’s not true. What we’ve seen over 20 years of using the biologics is that these agents are very safe and very effective when used appropriately in adequate doses,” according to Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
Asked which biologics he’s most comfortable with in prescribing for pediatric patients, Dr. Papp replied, “I think the best biologic therapies for pediatric patients are the ones that have been studied in the pediatric population: etanercept, ustekinumab, and adalimumab. Those three are the ones we want to choose because we at least have some guidance and some assurance in terms of expectations of response and adverse events.”
Dr. Papp and Dr. Langley have served as principal investigators of numerous clinical trials for and served as advisors to pharmaceutical companies developing dermatologic medications.
COPENHAGEN – Children and adolescents with moderate to severe psoriasis are generally undertreated, with resultant potential psychological impairment, social stigmatization, and isolation, psoriasis experts agreed at the annual congress of the European Academy of Dermatology and Venereology.
In a session featuring new long-term safety and efficacy data from extended pediatric clinical trials of etanercept and adalimumab, prominent clinical trial investigators who presented the findings asserted that many physicians and parents are overly timid about the use of systemic treatment options in children and adolescents when such therapy is clearly warranted.
“The burden of psoriasis is particularly acute in the pediatric population. I always ask patients, ‘How is psoriasis affecting you?’ And with children, it’s affecting them and it’s also affecting their parents. If it’s affecting their self-esteem – if it’s ruining your life and you can’t control it with topical agents – I think it’s time to think of other things,” declared Dr. Richard G. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
Yet all too often that doesn’t happen, as documented in the recently reported physician portion of the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey, which included 391 dermatologists and 390 rheumatologists in North America and Western Europe (J Eur Acad Dermatol Venereol. 2015 Oct;29[10]:2002-10).
Dr. Langley was a coinvestigator in MAPP, which found that 39% of dermatologists reported treating their moderate to severe psoriasis patients with conventional oral and/or biologic agents (19.5% and 19.6%, respectively). A total of 75% indicated they prescribe topical medications as monotherapy for their patients with moderate to severe psoriasis. In children, that rate was even higher, he said, despite the fact that topical monotherapy is clearly inadequate for treatment of more severe disease.
“I think there’s a fear about using systemics among parents and among some practitioners, which was one of the top reasons in the MAPP survey that people didn’t get appropriate treatment,” he said.
That’s a shortsighted attitude, the dermatologist continued: “The risk is not just the risk of the drug, but the risk of untreated disease, because untreated disease devastates patients.”
MAPP was the largest-ever survey of physician and patient perspectives regarding psoriasis and psoriatic arthritis. The patient perspective, derived from interviews with nearly 3,500 patients, was published earlier (J Am Acad Dermatol. 2014 May;70[5]:871-81).
One impediment to more widespread use of systemic therapies for pediatric psoriasis is that it’s almost entirely off-label prescribing. In the United States, no conventional oral agents or biologics are Food and Drug Administration approved for use in psoriasis patients under age 18 years. That was the case in Europe as well until earlier this year, when adalimumab (Humira) received European marketing approval for children ages 4 years and up with severe chronic plaque psoriasis with an inadequate response to topical agents and phototherapy or in whom such therapies are contraindicated.
Dr. Kim A. Papp, who presented new 52-week safety and efficacy data from a phase III study of adalimumab in pediatric psoriasis patients, said that even when dermatologists utilize biologics in pediatric patients, the medications are typically underdosed. In the phase III pediatric adalimumab trial, for example, most participants were overweight or obese, and efficacy dropped off with increasing body mass index, as has been the case in trials of most of the biologics.
“When I look at the doses of the biologic agents that are used in treating the pediatric population and what we know about how these molecules are distributed in the body, we can safely say we are grossly underdosing children. We’re doing that because we believe somehow they’re at greater risk from exposures that are comparable to those in the adult population. And it’s not true. What we’ve seen over 20 years of using the biologics is that these agents are very safe and very effective when used appropriately in adequate doses,” according to Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
Asked which biologics he’s most comfortable with in prescribing for pediatric patients, Dr. Papp replied, “I think the best biologic therapies for pediatric patients are the ones that have been studied in the pediatric population: etanercept, ustekinumab, and adalimumab. Those three are the ones we want to choose because we at least have some guidance and some assurance in terms of expectations of response and adverse events.”
Dr. Papp and Dr. Langley have served as principal investigators of numerous clinical trials for and served as advisors to pharmaceutical companies developing dermatologic medications.
COPENHAGEN – Children and adolescents with moderate to severe psoriasis are generally undertreated, with resultant potential psychological impairment, social stigmatization, and isolation, psoriasis experts agreed at the annual congress of the European Academy of Dermatology and Venereology.
In a session featuring new long-term safety and efficacy data from extended pediatric clinical trials of etanercept and adalimumab, prominent clinical trial investigators who presented the findings asserted that many physicians and parents are overly timid about the use of systemic treatment options in children and adolescents when such therapy is clearly warranted.
“The burden of psoriasis is particularly acute in the pediatric population. I always ask patients, ‘How is psoriasis affecting you?’ And with children, it’s affecting them and it’s also affecting their parents. If it’s affecting their self-esteem – if it’s ruining your life and you can’t control it with topical agents – I think it’s time to think of other things,” declared Dr. Richard G. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
Yet all too often that doesn’t happen, as documented in the recently reported physician portion of the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey, which included 391 dermatologists and 390 rheumatologists in North America and Western Europe (J Eur Acad Dermatol Venereol. 2015 Oct;29[10]:2002-10).
Dr. Langley was a coinvestigator in MAPP, which found that 39% of dermatologists reported treating their moderate to severe psoriasis patients with conventional oral and/or biologic agents (19.5% and 19.6%, respectively). A total of 75% indicated they prescribe topical medications as monotherapy for their patients with moderate to severe psoriasis. In children, that rate was even higher, he said, despite the fact that topical monotherapy is clearly inadequate for treatment of more severe disease.
“I think there’s a fear about using systemics among parents and among some practitioners, which was one of the top reasons in the MAPP survey that people didn’t get appropriate treatment,” he said.
That’s a shortsighted attitude, the dermatologist continued: “The risk is not just the risk of the drug, but the risk of untreated disease, because untreated disease devastates patients.”
MAPP was the largest-ever survey of physician and patient perspectives regarding psoriasis and psoriatic arthritis. The patient perspective, derived from interviews with nearly 3,500 patients, was published earlier (J Am Acad Dermatol. 2014 May;70[5]:871-81).
One impediment to more widespread use of systemic therapies for pediatric psoriasis is that it’s almost entirely off-label prescribing. In the United States, no conventional oral agents or biologics are Food and Drug Administration approved for use in psoriasis patients under age 18 years. That was the case in Europe as well until earlier this year, when adalimumab (Humira) received European marketing approval for children ages 4 years and up with severe chronic plaque psoriasis with an inadequate response to topical agents and phototherapy or in whom such therapies are contraindicated.
Dr. Kim A. Papp, who presented new 52-week safety and efficacy data from a phase III study of adalimumab in pediatric psoriasis patients, said that even when dermatologists utilize biologics in pediatric patients, the medications are typically underdosed. In the phase III pediatric adalimumab trial, for example, most participants were overweight or obese, and efficacy dropped off with increasing body mass index, as has been the case in trials of most of the biologics.
“When I look at the doses of the biologic agents that are used in treating the pediatric population and what we know about how these molecules are distributed in the body, we can safely say we are grossly underdosing children. We’re doing that because we believe somehow they’re at greater risk from exposures that are comparable to those in the adult population. And it’s not true. What we’ve seen over 20 years of using the biologics is that these agents are very safe and very effective when used appropriately in adequate doses,” according to Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
Asked which biologics he’s most comfortable with in prescribing for pediatric patients, Dr. Papp replied, “I think the best biologic therapies for pediatric patients are the ones that have been studied in the pediatric population: etanercept, ustekinumab, and adalimumab. Those three are the ones we want to choose because we at least have some guidance and some assurance in terms of expectations of response and adverse events.”
Dr. Papp and Dr. Langley have served as principal investigators of numerous clinical trials for and served as advisors to pharmaceutical companies developing dermatologic medications.
EXPERT ANALYSIS FROM THE EADV CONGRESS
Case series supports oral itraconazole for infantile hemangiomas
COPENHAGEN – Ever since Dr. Yuping Ran published his initial report of successful treatment of infantile hemangiomas using oral itraconazole, dermatologists from around the world have been bombarding him with the same three questions: Does it really work? Is the treatment safe? What’s the mechanism for the response?
His research has continued at a brisk clip since that preliminary publication (J Dermatol. 2015 Feb;42(2):202-6), so he was able to provide updated answers to those three key questions at the annual congress of the European Academy of Dermatology and Venereology.
Efficacy: His case series is now up to 17 treated patients, average age 3.6 months, with disfiguring or functionally significant infantile hemangiomas. Twelve of the 17 (71%) were successfully treated as defined by 80%-100% improvement that met with the approval of parents and physicians, said Dr. Ran, professor of dermatology at Sichuan University in Chengdu, China.
Treatment was given for an average of 8.8 weeks with oral itraconazole at 5 mg/kg/day. Dr. Ran’s initial serendipitous observation of a therapeutic effect on infantile hemangioma came from a baby he was treating with itraconazole capsules for a Candida albicans infection. He now uses only the oral solution, however, because it’s so much easier for parents to administer.
Safety: “We’ve monitored liver function before, during, and after treatment. It has always been within normal range,” Dr. Ran reported. Roughly 30% of infants have developed mild diarrhea on treatment. This has been readily manageable and hasn’t led to any halt in treatment.
Mechanism of benefit: In vitro studies conducted in Dr. Ran’s laboratory have shown that itraconazole inhibits growth and migration of proliferating human hemangioma epithelial cells, while ketoconazole does not. Moreover, when Dr. Ran and coworkers compared itraconazole to propranolol – a first-line medical treatment for infantile hemangiomas – itraconazole inhibited hemangioma epithelial cells far more efficiently than propranolol. Indeed, in order for propranolol to match itraconazole’s apoptotic effect, the cells had to be exposed to the beta blocker at a concentration that was 10-fold greater than that of itraconazole.
In further studies, Dr. Ran and coworkers have shown that itraconazole downregulates two key pathways in hemangioma cell growth: the hedgehog pathway and the P13K/AKT/mTOR signaling pathway. This is the likely mechanism of therapeutic effect, he said.
Dr. Ran’s studies are supported by the National Natural Science Foundation of China. He reported having no financial conflicts of interest.
COPENHAGEN – Ever since Dr. Yuping Ran published his initial report of successful treatment of infantile hemangiomas using oral itraconazole, dermatologists from around the world have been bombarding him with the same three questions: Does it really work? Is the treatment safe? What’s the mechanism for the response?
His research has continued at a brisk clip since that preliminary publication (J Dermatol. 2015 Feb;42(2):202-6), so he was able to provide updated answers to those three key questions at the annual congress of the European Academy of Dermatology and Venereology.
Efficacy: His case series is now up to 17 treated patients, average age 3.6 months, with disfiguring or functionally significant infantile hemangiomas. Twelve of the 17 (71%) were successfully treated as defined by 80%-100% improvement that met with the approval of parents and physicians, said Dr. Ran, professor of dermatology at Sichuan University in Chengdu, China.
Treatment was given for an average of 8.8 weeks with oral itraconazole at 5 mg/kg/day. Dr. Ran’s initial serendipitous observation of a therapeutic effect on infantile hemangioma came from a baby he was treating with itraconazole capsules for a Candida albicans infection. He now uses only the oral solution, however, because it’s so much easier for parents to administer.
Safety: “We’ve monitored liver function before, during, and after treatment. It has always been within normal range,” Dr. Ran reported. Roughly 30% of infants have developed mild diarrhea on treatment. This has been readily manageable and hasn’t led to any halt in treatment.
Mechanism of benefit: In vitro studies conducted in Dr. Ran’s laboratory have shown that itraconazole inhibits growth and migration of proliferating human hemangioma epithelial cells, while ketoconazole does not. Moreover, when Dr. Ran and coworkers compared itraconazole to propranolol – a first-line medical treatment for infantile hemangiomas – itraconazole inhibited hemangioma epithelial cells far more efficiently than propranolol. Indeed, in order for propranolol to match itraconazole’s apoptotic effect, the cells had to be exposed to the beta blocker at a concentration that was 10-fold greater than that of itraconazole.
In further studies, Dr. Ran and coworkers have shown that itraconazole downregulates two key pathways in hemangioma cell growth: the hedgehog pathway and the P13K/AKT/mTOR signaling pathway. This is the likely mechanism of therapeutic effect, he said.
Dr. Ran’s studies are supported by the National Natural Science Foundation of China. He reported having no financial conflicts of interest.
COPENHAGEN – Ever since Dr. Yuping Ran published his initial report of successful treatment of infantile hemangiomas using oral itraconazole, dermatologists from around the world have been bombarding him with the same three questions: Does it really work? Is the treatment safe? What’s the mechanism for the response?
His research has continued at a brisk clip since that preliminary publication (J Dermatol. 2015 Feb;42(2):202-6), so he was able to provide updated answers to those three key questions at the annual congress of the European Academy of Dermatology and Venereology.
Efficacy: His case series is now up to 17 treated patients, average age 3.6 months, with disfiguring or functionally significant infantile hemangiomas. Twelve of the 17 (71%) were successfully treated as defined by 80%-100% improvement that met with the approval of parents and physicians, said Dr. Ran, professor of dermatology at Sichuan University in Chengdu, China.
Treatment was given for an average of 8.8 weeks with oral itraconazole at 5 mg/kg/day. Dr. Ran’s initial serendipitous observation of a therapeutic effect on infantile hemangioma came from a baby he was treating with itraconazole capsules for a Candida albicans infection. He now uses only the oral solution, however, because it’s so much easier for parents to administer.
Safety: “We’ve monitored liver function before, during, and after treatment. It has always been within normal range,” Dr. Ran reported. Roughly 30% of infants have developed mild diarrhea on treatment. This has been readily manageable and hasn’t led to any halt in treatment.
Mechanism of benefit: In vitro studies conducted in Dr. Ran’s laboratory have shown that itraconazole inhibits growth and migration of proliferating human hemangioma epithelial cells, while ketoconazole does not. Moreover, when Dr. Ran and coworkers compared itraconazole to propranolol – a first-line medical treatment for infantile hemangiomas – itraconazole inhibited hemangioma epithelial cells far more efficiently than propranolol. Indeed, in order for propranolol to match itraconazole’s apoptotic effect, the cells had to be exposed to the beta blocker at a concentration that was 10-fold greater than that of itraconazole.
In further studies, Dr. Ran and coworkers have shown that itraconazole downregulates two key pathways in hemangioma cell growth: the hedgehog pathway and the P13K/AKT/mTOR signaling pathway. This is the likely mechanism of therapeutic effect, he said.
Dr. Ran’s studies are supported by the National Natural Science Foundation of China. He reported having no financial conflicts of interest.
AT THE EADV CONGRESS
Key clinical point: Oral itraconazole appears to be an effective alternative to propranolol for treatment of infantile hemangiomas.
Major finding: Twelve of 17 infants with painful, disruptive hemangiomas experienced 80%-100% improvement in response to oral itraconazole for an average of 8.8 weeks at 5 mg/kg/day.
Data source: This is an ongoing open-label case series supported by translational studies aimed at uncovering the mechanism of benefit.
Disclosures: This study was supported by the National Natural Science Foundation of China. The presenter reported having no financial conflicts of interest.
EADV: Venous leg ulcers herald increased cancer risk
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
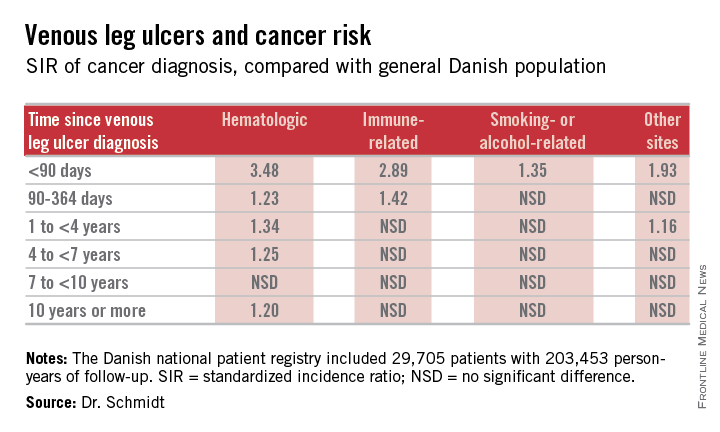
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.
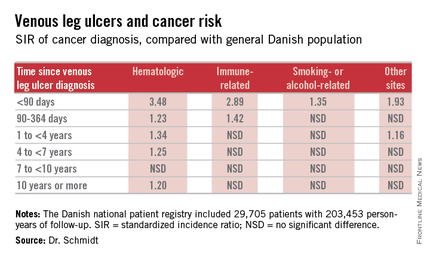
It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.

It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.

It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
AT THE EADV CONGRESS
Key clinical point: Venous leg ulceration appears to be a red flag for occult malignancy.
Major finding: During the first 89 days following diagnosis of a venous leg ulcer, affected patients are at a 3.48-fold increased risk of being diagnosed with a hematologic malignancy and a 2.89-fold greater risk of immune-related cancer compared with the general population.
Data source: This Danish nationwide cohort study compared standardized incidence ratios for various types of cancer during more than 200,000 person-years of follow-up in 29,705 patients with a venous leg ulcer vs. the general population.
Disclosures: The presenter reported having no financial conflicts of interest regarding her study, which was supported by Danish institutional funds.
EADV: Intralesional therapy for scleroderma dystrophic calcifications
COPENHAGEN – Intralesional sodium thiosulfate injections are an effective treatment for the painful and disabling dystrophic calcifications associated with systemic sclerosis, lupus, and other autoimmune diseases, as well as with nephrogenic systemic fibrosis, Dr. Jane Baumgartner-Nielsen said at the annual congress of the European Academy of Dermatology and Venereology.
“We suggest that intralesional injections of sodium thiosulfate may be considered in severe or ulcerated lesions before surgery or amputation,” said Dr. Baumgartner-Nielsen of Aarhus (Denmark) University.
Treatment of these often ulcerated cutaneous lesions has traditionally been challenging. While surgery is common, it’s problematic because wound healing is often prolonged in patients with autoimmune disease, she observed.
She presented a case series of six patients who underwent interlesional injections of sodium thiosulfate for painful and disabling dystrophic calcifications. The lesions were located on extensor surfaces or the fingertips. They were extremely painful: patients rated their pain as 9 on a 10-point scale. All six patients were women. Five had anticentromere antibody–positive systemic sclerosis; other investigators have reported that dystrophic calcifications occur in roughly 70% of such patients. The sixth patient had nephrogenic systemic fibrosis.
The six patients underwent a total of 21 injections of eight lesions. The injections were placed at the base of the calcifications. The concentration of sodium thiosulfate employed was 150 mg/mL. Dystrophic calcifications less than 5 mm in diameter on the fingertips received a single injection. Larger lesions complicated by ulceration got four injections at 4-week intervals.
The lesions decreased in size by an average of 67% at 4 weeks and 90% at 12 weeks. Complete remission was achieved by week 12 in half of patients; the other half had 80% reduction of their lesions. All patients reported dramatically less pain and improved physical function, compared with baseline. There were no serious side effects.
Audience member Dr. Alice B. Gottlieb inquired as to how painful the injections are.
“About 9 or 10 on a 10-point scale, but the pain disappears very quickly. In 30 seconds the patient is smiling again,” Dr. Baumgartner-Nielsen replied.
Dr. Gottlieb said she was interested in the intralesional therapy for her pediatric lupus patients with dystrophic calcifications. “But if there’s that much injection site pain, you might have to put the kid out,” noted Dr. Gottlieb, professor and dermatologist-in-chief at Tufts Medical Center, Boston.
Dr. Baumgartner-Nielsen reported having no financial conflicts regarding her study.
COPENHAGEN – Intralesional sodium thiosulfate injections are an effective treatment for the painful and disabling dystrophic calcifications associated with systemic sclerosis, lupus, and other autoimmune diseases, as well as with nephrogenic systemic fibrosis, Dr. Jane Baumgartner-Nielsen said at the annual congress of the European Academy of Dermatology and Venereology.
“We suggest that intralesional injections of sodium thiosulfate may be considered in severe or ulcerated lesions before surgery or amputation,” said Dr. Baumgartner-Nielsen of Aarhus (Denmark) University.
Treatment of these often ulcerated cutaneous lesions has traditionally been challenging. While surgery is common, it’s problematic because wound healing is often prolonged in patients with autoimmune disease, she observed.
She presented a case series of six patients who underwent interlesional injections of sodium thiosulfate for painful and disabling dystrophic calcifications. The lesions were located on extensor surfaces or the fingertips. They were extremely painful: patients rated their pain as 9 on a 10-point scale. All six patients were women. Five had anticentromere antibody–positive systemic sclerosis; other investigators have reported that dystrophic calcifications occur in roughly 70% of such patients. The sixth patient had nephrogenic systemic fibrosis.
The six patients underwent a total of 21 injections of eight lesions. The injections were placed at the base of the calcifications. The concentration of sodium thiosulfate employed was 150 mg/mL. Dystrophic calcifications less than 5 mm in diameter on the fingertips received a single injection. Larger lesions complicated by ulceration got four injections at 4-week intervals.
The lesions decreased in size by an average of 67% at 4 weeks and 90% at 12 weeks. Complete remission was achieved by week 12 in half of patients; the other half had 80% reduction of their lesions. All patients reported dramatically less pain and improved physical function, compared with baseline. There were no serious side effects.
Audience member Dr. Alice B. Gottlieb inquired as to how painful the injections are.
“About 9 or 10 on a 10-point scale, but the pain disappears very quickly. In 30 seconds the patient is smiling again,” Dr. Baumgartner-Nielsen replied.
Dr. Gottlieb said she was interested in the intralesional therapy for her pediatric lupus patients with dystrophic calcifications. “But if there’s that much injection site pain, you might have to put the kid out,” noted Dr. Gottlieb, professor and dermatologist-in-chief at Tufts Medical Center, Boston.
Dr. Baumgartner-Nielsen reported having no financial conflicts regarding her study.
COPENHAGEN – Intralesional sodium thiosulfate injections are an effective treatment for the painful and disabling dystrophic calcifications associated with systemic sclerosis, lupus, and other autoimmune diseases, as well as with nephrogenic systemic fibrosis, Dr. Jane Baumgartner-Nielsen said at the annual congress of the European Academy of Dermatology and Venereology.
“We suggest that intralesional injections of sodium thiosulfate may be considered in severe or ulcerated lesions before surgery or amputation,” said Dr. Baumgartner-Nielsen of Aarhus (Denmark) University.
Treatment of these often ulcerated cutaneous lesions has traditionally been challenging. While surgery is common, it’s problematic because wound healing is often prolonged in patients with autoimmune disease, she observed.
She presented a case series of six patients who underwent interlesional injections of sodium thiosulfate for painful and disabling dystrophic calcifications. The lesions were located on extensor surfaces or the fingertips. They were extremely painful: patients rated their pain as 9 on a 10-point scale. All six patients were women. Five had anticentromere antibody–positive systemic sclerosis; other investigators have reported that dystrophic calcifications occur in roughly 70% of such patients. The sixth patient had nephrogenic systemic fibrosis.
The six patients underwent a total of 21 injections of eight lesions. The injections were placed at the base of the calcifications. The concentration of sodium thiosulfate employed was 150 mg/mL. Dystrophic calcifications less than 5 mm in diameter on the fingertips received a single injection. Larger lesions complicated by ulceration got four injections at 4-week intervals.
The lesions decreased in size by an average of 67% at 4 weeks and 90% at 12 weeks. Complete remission was achieved by week 12 in half of patients; the other half had 80% reduction of their lesions. All patients reported dramatically less pain and improved physical function, compared with baseline. There were no serious side effects.
Audience member Dr. Alice B. Gottlieb inquired as to how painful the injections are.
“About 9 or 10 on a 10-point scale, but the pain disappears very quickly. In 30 seconds the patient is smiling again,” Dr. Baumgartner-Nielsen replied.
Dr. Gottlieb said she was interested in the intralesional therapy for her pediatric lupus patients with dystrophic calcifications. “But if there’s that much injection site pain, you might have to put the kid out,” noted Dr. Gottlieb, professor and dermatologist-in-chief at Tufts Medical Center, Boston.
Dr. Baumgartner-Nielsen reported having no financial conflicts regarding her study.
AT THE EADV CONGRESS
Key clinical point: Intralesional sodium thiosulfate is an effective alternative to surgery for disabling dystrophic calcifications in patients with systemic sclerosis or other autoimmune diseases.
Major finding: Fifty percent of patients with severely painful dystrophic calcifications experienced complete remission 12 weeks after their first intralesional injection of sodium thiosulfate; the remaining lesions were 80% smaller, compared with baseline.
Data source: This was a case series involving six patients with eight treated dystrophic calcifications secondary to systemic sclerosis of nephrogenic systemic fibrosis.
Disclosures: The study presenter reported having no financial conflicts of interest regarding her case series.
EADV: No cancer signal with ixekizumab
COPENHAGEN – Ixekizumab showed no hint of an increased malignancy risk of any sort in 4,208 patients with moderate to severe psoriasis who had 6,480 patient-years of exposure to the investigational interleukin-17A inhibitor in seven clinical trials.
“Patients were studied for an average of 18 months on ixekizumab. You may say that’s not enough time to assess the true risk for malignancy, and I would say you’re probably right. But 18 months isn’t shabby. From such an exposure, we can get some sense of the malignancy risk with this drug,” Dr. Bruce E. Strober asserted at the annual congress of the European Academy of Dermatology and Venereology.
That’s a key issue whenever a dermatologist prescribes a potent immune-altering anti-inflammatory medication for an indefinite period of time, he added.
In the randomized trials in which more than 2,300 patients who received 80 mg of ixekizumab every 2 or 4 weeks were compared with 739 patients assigned to etanercept (Enbrel) and 791 on placebo, malignancy rates were similarly low, at 0.1-0.3 cases per 100 patient-years across all four treatment arms, reported Dr. Strober, chair of the department of dermatology at the University of Connecticut, Farmington.
Moreover, in the long-term maintenance studies featuring 60 months of follow-up, the risks of both nonmelanoma skin cancer and other types of cancer were similarly low in patients randomized to ixekizumab at 80 mg every 2 weeks and those dosed every 4 weeks. That’s reassuring, because if a drug causes cancer one would expect to see that the more of the drug given, the higher the malignancy rate.
“You really don’t see any kind of a pattern or any difference between the various compared groups, including patients on etanercept, a drug that all of us feel very comfortable using in patients with psoriasis,” the dermatologist observed.
It’s thought that psoriasis patients have a higher background risk of malignancy than the general population due to the nature of the chronic disease, which involves immune dysregulation and chronic inflammation. In the studies to date, however, the malignancy risk in ixekizumab-treated patients is similar to those on placebo or etanercept.
“In my opinion, it’s comforting that the malignancy rates of the currently approved biologic agents for psoriasis are low and – one could argue – not above that which you would expect for the population of patients we’re treating. So I’ve always felt that monotherapy – and that’s an important point, not combination therapy with other immunosuppressive drugs – offers very little risk of de novo malignancy with the currently approved biologics,” Dr. Strober said.
Lumping together the results of the seven studies in his analysis, the incidence of squamous cell carcinoma was 0.1 cases per 100 patient-years of ixekizumab exposure, the risk of basal cell carcinoma was 0.3 cases per 100 patient-years, and the risk of malignancies other than nonmelanoma skin cancer was 0.5 per 100 patient-years of exposure to ixekizumab.
“That’s basically in line with every other biologic that I’ve examined with regard to malignancy rates: about 1 in 200 patients treated for 1 year gets a nonmelanoma skin cancer of some nature if you look at all data sources, including long-term registries and clinical trials. So it appears that this drug falls right in line with the other biologics in terms of rates of the more serious cancers, at least over the time period studied,” Dr. Strober commented.
He offered a caveat, however: “What will happen in patients given combination therapy with other immunosuppressive drugs? Or special populations, such as patients at higher risk for malignancy, be it skin cancer or other types of cancer? Or – and here’s the question we often have the most difficulty answering – what about people with a prior history of malignancy? What would be their risk of malignancy in being placed on a drug of this nature? Only time and really good registry data will allow us to answer these questions.”
Dr. Strober is a consultant to Eli Lilly, which sponsored the seven ixekizumab studies, as well as to numerous other pharmaceutical companies.
COPENHAGEN – Ixekizumab showed no hint of an increased malignancy risk of any sort in 4,208 patients with moderate to severe psoriasis who had 6,480 patient-years of exposure to the investigational interleukin-17A inhibitor in seven clinical trials.
“Patients were studied for an average of 18 months on ixekizumab. You may say that’s not enough time to assess the true risk for malignancy, and I would say you’re probably right. But 18 months isn’t shabby. From such an exposure, we can get some sense of the malignancy risk with this drug,” Dr. Bruce E. Strober asserted at the annual congress of the European Academy of Dermatology and Venereology.
That’s a key issue whenever a dermatologist prescribes a potent immune-altering anti-inflammatory medication for an indefinite period of time, he added.
In the randomized trials in which more than 2,300 patients who received 80 mg of ixekizumab every 2 or 4 weeks were compared with 739 patients assigned to etanercept (Enbrel) and 791 on placebo, malignancy rates were similarly low, at 0.1-0.3 cases per 100 patient-years across all four treatment arms, reported Dr. Strober, chair of the department of dermatology at the University of Connecticut, Farmington.
Moreover, in the long-term maintenance studies featuring 60 months of follow-up, the risks of both nonmelanoma skin cancer and other types of cancer were similarly low in patients randomized to ixekizumab at 80 mg every 2 weeks and those dosed every 4 weeks. That’s reassuring, because if a drug causes cancer one would expect to see that the more of the drug given, the higher the malignancy rate.
“You really don’t see any kind of a pattern or any difference between the various compared groups, including patients on etanercept, a drug that all of us feel very comfortable using in patients with psoriasis,” the dermatologist observed.
It’s thought that psoriasis patients have a higher background risk of malignancy than the general population due to the nature of the chronic disease, which involves immune dysregulation and chronic inflammation. In the studies to date, however, the malignancy risk in ixekizumab-treated patients is similar to those on placebo or etanercept.
“In my opinion, it’s comforting that the malignancy rates of the currently approved biologic agents for psoriasis are low and – one could argue – not above that which you would expect for the population of patients we’re treating. So I’ve always felt that monotherapy – and that’s an important point, not combination therapy with other immunosuppressive drugs – offers very little risk of de novo malignancy with the currently approved biologics,” Dr. Strober said.
Lumping together the results of the seven studies in his analysis, the incidence of squamous cell carcinoma was 0.1 cases per 100 patient-years of ixekizumab exposure, the risk of basal cell carcinoma was 0.3 cases per 100 patient-years, and the risk of malignancies other than nonmelanoma skin cancer was 0.5 per 100 patient-years of exposure to ixekizumab.
“That’s basically in line with every other biologic that I’ve examined with regard to malignancy rates: about 1 in 200 patients treated for 1 year gets a nonmelanoma skin cancer of some nature if you look at all data sources, including long-term registries and clinical trials. So it appears that this drug falls right in line with the other biologics in terms of rates of the more serious cancers, at least over the time period studied,” Dr. Strober commented.
He offered a caveat, however: “What will happen in patients given combination therapy with other immunosuppressive drugs? Or special populations, such as patients at higher risk for malignancy, be it skin cancer or other types of cancer? Or – and here’s the question we often have the most difficulty answering – what about people with a prior history of malignancy? What would be their risk of malignancy in being placed on a drug of this nature? Only time and really good registry data will allow us to answer these questions.”
Dr. Strober is a consultant to Eli Lilly, which sponsored the seven ixekizumab studies, as well as to numerous other pharmaceutical companies.
COPENHAGEN – Ixekizumab showed no hint of an increased malignancy risk of any sort in 4,208 patients with moderate to severe psoriasis who had 6,480 patient-years of exposure to the investigational interleukin-17A inhibitor in seven clinical trials.
“Patients were studied for an average of 18 months on ixekizumab. You may say that’s not enough time to assess the true risk for malignancy, and I would say you’re probably right. But 18 months isn’t shabby. From such an exposure, we can get some sense of the malignancy risk with this drug,” Dr. Bruce E. Strober asserted at the annual congress of the European Academy of Dermatology and Venereology.
That’s a key issue whenever a dermatologist prescribes a potent immune-altering anti-inflammatory medication for an indefinite period of time, he added.
In the randomized trials in which more than 2,300 patients who received 80 mg of ixekizumab every 2 or 4 weeks were compared with 739 patients assigned to etanercept (Enbrel) and 791 on placebo, malignancy rates were similarly low, at 0.1-0.3 cases per 100 patient-years across all four treatment arms, reported Dr. Strober, chair of the department of dermatology at the University of Connecticut, Farmington.
Moreover, in the long-term maintenance studies featuring 60 months of follow-up, the risks of both nonmelanoma skin cancer and other types of cancer were similarly low in patients randomized to ixekizumab at 80 mg every 2 weeks and those dosed every 4 weeks. That’s reassuring, because if a drug causes cancer one would expect to see that the more of the drug given, the higher the malignancy rate.
“You really don’t see any kind of a pattern or any difference between the various compared groups, including patients on etanercept, a drug that all of us feel very comfortable using in patients with psoriasis,” the dermatologist observed.
It’s thought that psoriasis patients have a higher background risk of malignancy than the general population due to the nature of the chronic disease, which involves immune dysregulation and chronic inflammation. In the studies to date, however, the malignancy risk in ixekizumab-treated patients is similar to those on placebo or etanercept.
“In my opinion, it’s comforting that the malignancy rates of the currently approved biologic agents for psoriasis are low and – one could argue – not above that which you would expect for the population of patients we’re treating. So I’ve always felt that monotherapy – and that’s an important point, not combination therapy with other immunosuppressive drugs – offers very little risk of de novo malignancy with the currently approved biologics,” Dr. Strober said.
Lumping together the results of the seven studies in his analysis, the incidence of squamous cell carcinoma was 0.1 cases per 100 patient-years of ixekizumab exposure, the risk of basal cell carcinoma was 0.3 cases per 100 patient-years, and the risk of malignancies other than nonmelanoma skin cancer was 0.5 per 100 patient-years of exposure to ixekizumab.
“That’s basically in line with every other biologic that I’ve examined with regard to malignancy rates: about 1 in 200 patients treated for 1 year gets a nonmelanoma skin cancer of some nature if you look at all data sources, including long-term registries and clinical trials. So it appears that this drug falls right in line with the other biologics in terms of rates of the more serious cancers, at least over the time period studied,” Dr. Strober commented.
He offered a caveat, however: “What will happen in patients given combination therapy with other immunosuppressive drugs? Or special populations, such as patients at higher risk for malignancy, be it skin cancer or other types of cancer? Or – and here’s the question we often have the most difficulty answering – what about people with a prior history of malignancy? What would be their risk of malignancy in being placed on a drug of this nature? Only time and really good registry data will allow us to answer these questions.”
Dr. Strober is a consultant to Eli Lilly, which sponsored the seven ixekizumab studies, as well as to numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: In studies conducted in psoriasis patients to date, ixekizumab doesn’t raise any malignancy concerns.
Major finding: The risk of malignancies of any type was 0.9 cases per 100 patient-years of exposure to the investigational interleukin-17A inhibitor ixekizumab, which is in line with rates associated with the currently approved biologic agents.
Data source: This was an analysis of treatment-emergent malignancies among participants in seven clinical psoriasis trials representing a total of 6,480 patient-years of exposure to ixekizumab.
Disclosures: The presenter of this analysis is a consultant to Eli Lilly, which sponsored the seven ixekizumab studies, as well as to numerous other pharmaceutical companies.
EADV: Secukinumab shows sustained high efficacy in SCULPTURE through 3 years
COPENHAGEN – Secukinumab maintained consistently high and previously unheard of long-term Psoriasis Area and Severity Index 90 and PASI 100 response rates in psoriasis patients through 3 years of prospective follow-up in an extension of the phase III SCULPTURE trial.
The key to maintaining these high response rates was to give secukinumab (Cosentyx) on a fixed schedule every 4 weeks. SCULPTURE tested the hypothesis that retreatment of initial responders only as needed based upon early evidence of relapse off treatment might be a reasonable alternative strategy. The answer turns out to be emphatically no.
“We can see from the 3-year data that retreatment as needed is probably not a good strategy over the long term. We see four times fewer patients with PASI 90s and 10 times fewer patients with PASI 100s if they are treated as needed. From the patient point of view, quality of life and satisfaction are not optimal with retreatment as needed,” Dr. Diamant Thaci said at the annual congress of the European Academy of Dermatology and Venereology.
The 3-year extension of SCULPTURE also provided reassuring safety data, he noted.
“It’s interesting that with long-term treatment out to 3 years, we don’t see any new safety signals. In fact, the signals we saw in the preapproval studies are slightly diluted here, and now that secukinumab is approved, we’re not seeing them as problems in real life clinical practice,” said Dr. Thaci of University Hospital in Lübeck, Germany.
The 52-week SCULPTURE results were published earlier this year (J Am Acad Dermatol. 2015 Jul;73[1]:27-36). Dr. Thaci’s update extended the findings from 52 weeks through 3 years.
The double-blind phase of the core SCULPTURE study began with 966 patients with moderate to severe plaque psoriasis and a baseline PASI score of about 23. After 12 weeks on subcutaneous secukinumab, the 842 subjects who had achieved 75% or more improvement from their baseline PASI score were randomized to one of four study arms: secukinumab at either 300 mg or 150 mg, administered either every 4 weeks or by retreatment as needed when an individual’s response dropped below PASI 75.
Picking up where the published 52-week results left off, Dr. Thaci focused special attention on the PASI 90 outcomes out to 3 years because he said he believes PASI 90 will become generally accepted as the new treatment target, now that secukinumab is on the market and other supereffective biologic agents are advancing through the developmental pipeline.
At 52 weeks, 68.5% of patients on fixed-dose secukinumab at 300 mg every 4 weeks had a PASI 90 response. At 3 years, after an additional 2 years on this schedule, the PASI 90 rate was still hovering around the two-thirds mark at 63.8%. In contrast, patients on retreatment with 300 mg as needed had a PASI 90 rate of 14.8% at 52 weeks and 13% at 3 years.
Although the Food and Drug Administration recommended the 300-mg dose when it approved secukinumab as a first-in-class biologic in January, the agency added that 150 mg may be acceptable in some patients. But the 150-mg dose was clearly inferior long term in the SCULPTURE extension. At 52 weeks, 50.3% of patients on secukinumab at 150 mg every 4 weeks had a PASI 90 response; at 3 years the rate was 36.8%. With retreatment as needed using 150 mg, the 52-week and 3-year PASI 90 rates were 12% and 11.8%, Dr. Thaci reported.
The PASI 100 (clear skin) response rate at 52 weeks with fixed-dose secukinumab at 300 mg was 43.8%. At 3 years it was 42.6%.
“These excellent responders kept their excellent response by continuing treatment for 3 years,” the dermatologist noted.
The PASI 100 rates with retreatment as needed were 5.3% and 3.8%.
In terms of safety through 3 years of treatment, rates of the adverse events of greatest interest with the use of immunomodulatory therapies – Candida infections, malignancies, serious infections, and major adverse cardiovascular events – were similarly low across all four treatment arms, ranging from 0 to 1.6 events per 100 subject-years. Thus, there was no safety advantage in using the retreatment as needed strategy.
“In the past we were all talking about the risk of Candida infection caused by inhibiting [interleukin]-17. But if you treat patients in real life clinical practice, you may see a signal of Candida infection in the beginning, yet in the long term you don’t see Candida as a significant side effect in our daily practice – and in this trial the incidence decreases,” Dr. Thaci said.
One audience member rose to criticize the way the analysis of the long-term extension of SCULPTURE was structured – namely, that only patients still on secukinumab at the 3-year mark were included in the results. This “as-observed” method inflates the efficacy results, he said. How many patients dropped out over time and weren’t counted? he asked.
Dr. Thaci replied that roughly 90% of patients in the trial at 52 weeks continued on secukinumab out to 3 years.
“It’s not a massive dropout. I wish it were a nonresponder imputation analysis, but in long-term studies, it’s very difficult to show that analysis because the vast majority of the trials that have been presented in the past have been ‘as observed.’ But I don’t think you’ll see a big difference between this as-observed analysis and a nonresponder imputation analysis. This is a very fair and convincing analysis,” he said.
Secukinumab is marketed by Novartis, which sponsored the study. Dr. Thaci serves as an investigator and consultant to Novartis and other pharmaceutical companies.
COPENHAGEN – Secukinumab maintained consistently high and previously unheard of long-term Psoriasis Area and Severity Index 90 and PASI 100 response rates in psoriasis patients through 3 years of prospective follow-up in an extension of the phase III SCULPTURE trial.
The key to maintaining these high response rates was to give secukinumab (Cosentyx) on a fixed schedule every 4 weeks. SCULPTURE tested the hypothesis that retreatment of initial responders only as needed based upon early evidence of relapse off treatment might be a reasonable alternative strategy. The answer turns out to be emphatically no.
“We can see from the 3-year data that retreatment as needed is probably not a good strategy over the long term. We see four times fewer patients with PASI 90s and 10 times fewer patients with PASI 100s if they are treated as needed. From the patient point of view, quality of life and satisfaction are not optimal with retreatment as needed,” Dr. Diamant Thaci said at the annual congress of the European Academy of Dermatology and Venereology.
The 3-year extension of SCULPTURE also provided reassuring safety data, he noted.
“It’s interesting that with long-term treatment out to 3 years, we don’t see any new safety signals. In fact, the signals we saw in the preapproval studies are slightly diluted here, and now that secukinumab is approved, we’re not seeing them as problems in real life clinical practice,” said Dr. Thaci of University Hospital in Lübeck, Germany.
The 52-week SCULPTURE results were published earlier this year (J Am Acad Dermatol. 2015 Jul;73[1]:27-36). Dr. Thaci’s update extended the findings from 52 weeks through 3 years.
The double-blind phase of the core SCULPTURE study began with 966 patients with moderate to severe plaque psoriasis and a baseline PASI score of about 23. After 12 weeks on subcutaneous secukinumab, the 842 subjects who had achieved 75% or more improvement from their baseline PASI score were randomized to one of four study arms: secukinumab at either 300 mg or 150 mg, administered either every 4 weeks or by retreatment as needed when an individual’s response dropped below PASI 75.
Picking up where the published 52-week results left off, Dr. Thaci focused special attention on the PASI 90 outcomes out to 3 years because he said he believes PASI 90 will become generally accepted as the new treatment target, now that secukinumab is on the market and other supereffective biologic agents are advancing through the developmental pipeline.
At 52 weeks, 68.5% of patients on fixed-dose secukinumab at 300 mg every 4 weeks had a PASI 90 response. At 3 years, after an additional 2 years on this schedule, the PASI 90 rate was still hovering around the two-thirds mark at 63.8%. In contrast, patients on retreatment with 300 mg as needed had a PASI 90 rate of 14.8% at 52 weeks and 13% at 3 years.
Although the Food and Drug Administration recommended the 300-mg dose when it approved secukinumab as a first-in-class biologic in January, the agency added that 150 mg may be acceptable in some patients. But the 150-mg dose was clearly inferior long term in the SCULPTURE extension. At 52 weeks, 50.3% of patients on secukinumab at 150 mg every 4 weeks had a PASI 90 response; at 3 years the rate was 36.8%. With retreatment as needed using 150 mg, the 52-week and 3-year PASI 90 rates were 12% and 11.8%, Dr. Thaci reported.
The PASI 100 (clear skin) response rate at 52 weeks with fixed-dose secukinumab at 300 mg was 43.8%. At 3 years it was 42.6%.
“These excellent responders kept their excellent response by continuing treatment for 3 years,” the dermatologist noted.
The PASI 100 rates with retreatment as needed were 5.3% and 3.8%.
In terms of safety through 3 years of treatment, rates of the adverse events of greatest interest with the use of immunomodulatory therapies – Candida infections, malignancies, serious infections, and major adverse cardiovascular events – were similarly low across all four treatment arms, ranging from 0 to 1.6 events per 100 subject-years. Thus, there was no safety advantage in using the retreatment as needed strategy.
“In the past we were all talking about the risk of Candida infection caused by inhibiting [interleukin]-17. But if you treat patients in real life clinical practice, you may see a signal of Candida infection in the beginning, yet in the long term you don’t see Candida as a significant side effect in our daily practice – and in this trial the incidence decreases,” Dr. Thaci said.
One audience member rose to criticize the way the analysis of the long-term extension of SCULPTURE was structured – namely, that only patients still on secukinumab at the 3-year mark were included in the results. This “as-observed” method inflates the efficacy results, he said. How many patients dropped out over time and weren’t counted? he asked.
Dr. Thaci replied that roughly 90% of patients in the trial at 52 weeks continued on secukinumab out to 3 years.
“It’s not a massive dropout. I wish it were a nonresponder imputation analysis, but in long-term studies, it’s very difficult to show that analysis because the vast majority of the trials that have been presented in the past have been ‘as observed.’ But I don’t think you’ll see a big difference between this as-observed analysis and a nonresponder imputation analysis. This is a very fair and convincing analysis,” he said.
Secukinumab is marketed by Novartis, which sponsored the study. Dr. Thaci serves as an investigator and consultant to Novartis and other pharmaceutical companies.
COPENHAGEN – Secukinumab maintained consistently high and previously unheard of long-term Psoriasis Area and Severity Index 90 and PASI 100 response rates in psoriasis patients through 3 years of prospective follow-up in an extension of the phase III SCULPTURE trial.
The key to maintaining these high response rates was to give secukinumab (Cosentyx) on a fixed schedule every 4 weeks. SCULPTURE tested the hypothesis that retreatment of initial responders only as needed based upon early evidence of relapse off treatment might be a reasonable alternative strategy. The answer turns out to be emphatically no.
“We can see from the 3-year data that retreatment as needed is probably not a good strategy over the long term. We see four times fewer patients with PASI 90s and 10 times fewer patients with PASI 100s if they are treated as needed. From the patient point of view, quality of life and satisfaction are not optimal with retreatment as needed,” Dr. Diamant Thaci said at the annual congress of the European Academy of Dermatology and Venereology.
The 3-year extension of SCULPTURE also provided reassuring safety data, he noted.
“It’s interesting that with long-term treatment out to 3 years, we don’t see any new safety signals. In fact, the signals we saw in the preapproval studies are slightly diluted here, and now that secukinumab is approved, we’re not seeing them as problems in real life clinical practice,” said Dr. Thaci of University Hospital in Lübeck, Germany.
The 52-week SCULPTURE results were published earlier this year (J Am Acad Dermatol. 2015 Jul;73[1]:27-36). Dr. Thaci’s update extended the findings from 52 weeks through 3 years.
The double-blind phase of the core SCULPTURE study began with 966 patients with moderate to severe plaque psoriasis and a baseline PASI score of about 23. After 12 weeks on subcutaneous secukinumab, the 842 subjects who had achieved 75% or more improvement from their baseline PASI score were randomized to one of four study arms: secukinumab at either 300 mg or 150 mg, administered either every 4 weeks or by retreatment as needed when an individual’s response dropped below PASI 75.
Picking up where the published 52-week results left off, Dr. Thaci focused special attention on the PASI 90 outcomes out to 3 years because he said he believes PASI 90 will become generally accepted as the new treatment target, now that secukinumab is on the market and other supereffective biologic agents are advancing through the developmental pipeline.
At 52 weeks, 68.5% of patients on fixed-dose secukinumab at 300 mg every 4 weeks had a PASI 90 response. At 3 years, after an additional 2 years on this schedule, the PASI 90 rate was still hovering around the two-thirds mark at 63.8%. In contrast, patients on retreatment with 300 mg as needed had a PASI 90 rate of 14.8% at 52 weeks and 13% at 3 years.
Although the Food and Drug Administration recommended the 300-mg dose when it approved secukinumab as a first-in-class biologic in January, the agency added that 150 mg may be acceptable in some patients. But the 150-mg dose was clearly inferior long term in the SCULPTURE extension. At 52 weeks, 50.3% of patients on secukinumab at 150 mg every 4 weeks had a PASI 90 response; at 3 years the rate was 36.8%. With retreatment as needed using 150 mg, the 52-week and 3-year PASI 90 rates were 12% and 11.8%, Dr. Thaci reported.
The PASI 100 (clear skin) response rate at 52 weeks with fixed-dose secukinumab at 300 mg was 43.8%. At 3 years it was 42.6%.
“These excellent responders kept their excellent response by continuing treatment for 3 years,” the dermatologist noted.
The PASI 100 rates with retreatment as needed were 5.3% and 3.8%.
In terms of safety through 3 years of treatment, rates of the adverse events of greatest interest with the use of immunomodulatory therapies – Candida infections, malignancies, serious infections, and major adverse cardiovascular events – were similarly low across all four treatment arms, ranging from 0 to 1.6 events per 100 subject-years. Thus, there was no safety advantage in using the retreatment as needed strategy.
“In the past we were all talking about the risk of Candida infection caused by inhibiting [interleukin]-17. But if you treat patients in real life clinical practice, you may see a signal of Candida infection in the beginning, yet in the long term you don’t see Candida as a significant side effect in our daily practice – and in this trial the incidence decreases,” Dr. Thaci said.
One audience member rose to criticize the way the analysis of the long-term extension of SCULPTURE was structured – namely, that only patients still on secukinumab at the 3-year mark were included in the results. This “as-observed” method inflates the efficacy results, he said. How many patients dropped out over time and weren’t counted? he asked.
Dr. Thaci replied that roughly 90% of patients in the trial at 52 weeks continued on secukinumab out to 3 years.
“It’s not a massive dropout. I wish it were a nonresponder imputation analysis, but in long-term studies, it’s very difficult to show that analysis because the vast majority of the trials that have been presented in the past have been ‘as observed.’ But I don’t think you’ll see a big difference between this as-observed analysis and a nonresponder imputation analysis. This is a very fair and convincing analysis,” he said.
Secukinumab is marketed by Novartis, which sponsored the study. Dr. Thaci serves as an investigator and consultant to Novartis and other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: Three-year data from a phase III trial provide reassurance regarding the long-term safety and efficacy of secukinumab for psoriasis.
Major finding: The high level of improvement seen in the 52-week core study of secukinumab, at the dose of 300 mg every 4 weeks, in patients with psoriasis was largely maintained with 2 more years of therapy, with a 63.8% PASI-90 rate at 3 years.
Data source: A prospective 2-year extension of a 1-year, randomized double-blind trial of 842 psoriasis patients, who initially achieved at least a PASI-75 response to secukinumab.
Disclosures: The study was sponsored by Novartis. The presenter serves as a paid investigator and consultant for Novartis and other pharmaceutical companies.
EADV: Spotlight on alexithymia in psoriasis
COPENHAGEN – Alexithymia – difficulty in recognizing and describing one’s emotions – is exceptionally common among psoriasis patients and may represent a novel therapeutic target, according to Dr. Carle Paul.
“We found a significant association between alexithymia and more severe psoriasis, anxiety, depression, decreased quality of life, harmful alcohol consumption, and work impairment,” reported Dr. Paul, professor and chairman of the department of dermatology at the University of Toulouse (France).
Alexithymia, a personality construct sometimes referred to as “emotional blindness,” was first described by psychologists in the 1970s. The previous glaring lack of data on the prevalence and consequences of alexithymia in psoriasis patients served as the impetus for the ongoing EPIDEPSO study (Epidemiological Study in Patients With Recently Diagnosed Psoriasis), a prospective 1-year international, epidemiologic, noninterventional observational study involving 719 adults with moderate to severe plaque psoriasis of less than 10 years’ duration. Dr. Paul presented the baseline findings at the at the annual congress of the European Academy of Dermatology and Venereology.
The first noteworthy finding was the strikingly high prevalence of alexithymia in this group of psoriasis patients: 39% of the 719 patients had alexithymia, as defined by a score of 61 or more on the validated, 20-item Toronto Alexithymia Scale.
Patients with alexithymia had slightly but significantly more severe psoriasis as evidenced by their mean Psoriasis Area and Severity Index score of 11.5, compared with 9.8 in unaffected patients. Hand psoriasis was more common in alexithymic patients by a margin of 51%-39%, although the prevalence of psoriasis of the face and neck was similar in the two groups.
Alexithymia was associated with significantly higher rates of several forms of psychiatric comorbidity and problems in living as assessed by validated tests. One comorbid condition stood out above the rest.
“The most striking feature is the very close relationship between alexithymia and anxiety,” according to Dr. Paul. “Among alexithymic patients, 50% had moderate to severe anxiety, as measured by the Hospital Anxiety and Depression Scale–A, whereas in psoriasis patients without alexithymia, this proportion was 12%.”
“Alexithymia identifies a patient population with a high burden of psoriasis, but at the same time, they have difficulty in expressing their emotions and feelings to their doctor. And I think this may explain the fact that some psoriasis patients have difficulties in interacting with doctors, because even though they have a high psoriasis burden, they cannot express how much they suffer from the disease,” the dermatologist continued.
Audience members at this standing-room-only EADV session on new research findings in psoriasis were clearly intrigued by the novel findings about a psychological condition unfamiliar to most. They wanted to know if the Toronto Alexithymia Scale is suitable for use in everyday clinical practice. The answer is yes, Dr. Paul replied, but the practical importance of identifying the large subgroup of patients with alexithymia has yet to be determined.
“We want to find out if we can modify alexithymia with interventions, but we don’t have the prospective data yet,” he added.
Even if alexithymia turns out to be a fixed characteristic not amenable to intervention, however, EPIDEPSO has shown that it could be useful as a red flag because it keeps company with several psychiatric conditions, which are anxiety, depression, and alcohol abuse.
What about causality? others asked. Does alexithymia cause anxiety and depression, or does the emotional toll of psoriasis promote anxiety and depression, which then causes alexithymia? Dr. Paul responded that it’s impossible to say at this point because these initial EPIDEPSO data are cross-sectional; however, the 1-year follow-up may yield insight.
EPIDEPSO is funded by Janssen. Dr. Paul reported receiving a research grant from the company to conduct the study.
COPENHAGEN – Alexithymia – difficulty in recognizing and describing one’s emotions – is exceptionally common among psoriasis patients and may represent a novel therapeutic target, according to Dr. Carle Paul.
“We found a significant association between alexithymia and more severe psoriasis, anxiety, depression, decreased quality of life, harmful alcohol consumption, and work impairment,” reported Dr. Paul, professor and chairman of the department of dermatology at the University of Toulouse (France).
Alexithymia, a personality construct sometimes referred to as “emotional blindness,” was first described by psychologists in the 1970s. The previous glaring lack of data on the prevalence and consequences of alexithymia in psoriasis patients served as the impetus for the ongoing EPIDEPSO study (Epidemiological Study in Patients With Recently Diagnosed Psoriasis), a prospective 1-year international, epidemiologic, noninterventional observational study involving 719 adults with moderate to severe plaque psoriasis of less than 10 years’ duration. Dr. Paul presented the baseline findings at the at the annual congress of the European Academy of Dermatology and Venereology.
The first noteworthy finding was the strikingly high prevalence of alexithymia in this group of psoriasis patients: 39% of the 719 patients had alexithymia, as defined by a score of 61 or more on the validated, 20-item Toronto Alexithymia Scale.
Patients with alexithymia had slightly but significantly more severe psoriasis as evidenced by their mean Psoriasis Area and Severity Index score of 11.5, compared with 9.8 in unaffected patients. Hand psoriasis was more common in alexithymic patients by a margin of 51%-39%, although the prevalence of psoriasis of the face and neck was similar in the two groups.
Alexithymia was associated with significantly higher rates of several forms of psychiatric comorbidity and problems in living as assessed by validated tests. One comorbid condition stood out above the rest.
“The most striking feature is the very close relationship between alexithymia and anxiety,” according to Dr. Paul. “Among alexithymic patients, 50% had moderate to severe anxiety, as measured by the Hospital Anxiety and Depression Scale–A, whereas in psoriasis patients without alexithymia, this proportion was 12%.”
“Alexithymia identifies a patient population with a high burden of psoriasis, but at the same time, they have difficulty in expressing their emotions and feelings to their doctor. And I think this may explain the fact that some psoriasis patients have difficulties in interacting with doctors, because even though they have a high psoriasis burden, they cannot express how much they suffer from the disease,” the dermatologist continued.

Audience members at this standing-room-only EADV session on new research findings in psoriasis were clearly intrigued by the novel findings about a psychological condition unfamiliar to most. They wanted to know if the Toronto Alexithymia Scale is suitable for use in everyday clinical practice. The answer is yes, Dr. Paul replied, but the practical importance of identifying the large subgroup of patients with alexithymia has yet to be determined.
“We want to find out if we can modify alexithymia with interventions, but we don’t have the prospective data yet,” he added.
Even if alexithymia turns out to be a fixed characteristic not amenable to intervention, however, EPIDEPSO has shown that it could be useful as a red flag because it keeps company with several psychiatric conditions, which are anxiety, depression, and alcohol abuse.
What about causality? others asked. Does alexithymia cause anxiety and depression, or does the emotional toll of psoriasis promote anxiety and depression, which then causes alexithymia? Dr. Paul responded that it’s impossible to say at this point because these initial EPIDEPSO data are cross-sectional; however, the 1-year follow-up may yield insight.
EPIDEPSO is funded by Janssen. Dr. Paul reported receiving a research grant from the company to conduct the study.
COPENHAGEN – Alexithymia – difficulty in recognizing and describing one’s emotions – is exceptionally common among psoriasis patients and may represent a novel therapeutic target, according to Dr. Carle Paul.
“We found a significant association between alexithymia and more severe psoriasis, anxiety, depression, decreased quality of life, harmful alcohol consumption, and work impairment,” reported Dr. Paul, professor and chairman of the department of dermatology at the University of Toulouse (France).
Alexithymia, a personality construct sometimes referred to as “emotional blindness,” was first described by psychologists in the 1970s. The previous glaring lack of data on the prevalence and consequences of alexithymia in psoriasis patients served as the impetus for the ongoing EPIDEPSO study (Epidemiological Study in Patients With Recently Diagnosed Psoriasis), a prospective 1-year international, epidemiologic, noninterventional observational study involving 719 adults with moderate to severe plaque psoriasis of less than 10 years’ duration. Dr. Paul presented the baseline findings at the at the annual congress of the European Academy of Dermatology and Venereology.
The first noteworthy finding was the strikingly high prevalence of alexithymia in this group of psoriasis patients: 39% of the 719 patients had alexithymia, as defined by a score of 61 or more on the validated, 20-item Toronto Alexithymia Scale.
Patients with alexithymia had slightly but significantly more severe psoriasis as evidenced by their mean Psoriasis Area and Severity Index score of 11.5, compared with 9.8 in unaffected patients. Hand psoriasis was more common in alexithymic patients by a margin of 51%-39%, although the prevalence of psoriasis of the face and neck was similar in the two groups.
Alexithymia was associated with significantly higher rates of several forms of psychiatric comorbidity and problems in living as assessed by validated tests. One comorbid condition stood out above the rest.
“The most striking feature is the very close relationship between alexithymia and anxiety,” according to Dr. Paul. “Among alexithymic patients, 50% had moderate to severe anxiety, as measured by the Hospital Anxiety and Depression Scale–A, whereas in psoriasis patients without alexithymia, this proportion was 12%.”
“Alexithymia identifies a patient population with a high burden of psoriasis, but at the same time, they have difficulty in expressing their emotions and feelings to their doctor. And I think this may explain the fact that some psoriasis patients have difficulties in interacting with doctors, because even though they have a high psoriasis burden, they cannot express how much they suffer from the disease,” the dermatologist continued.

Audience members at this standing-room-only EADV session on new research findings in psoriasis were clearly intrigued by the novel findings about a psychological condition unfamiliar to most. They wanted to know if the Toronto Alexithymia Scale is suitable for use in everyday clinical practice. The answer is yes, Dr. Paul replied, but the practical importance of identifying the large subgroup of patients with alexithymia has yet to be determined.
“We want to find out if we can modify alexithymia with interventions, but we don’t have the prospective data yet,” he added.
Even if alexithymia turns out to be a fixed characteristic not amenable to intervention, however, EPIDEPSO has shown that it could be useful as a red flag because it keeps company with several psychiatric conditions, which are anxiety, depression, and alcohol abuse.
What about causality? others asked. Does alexithymia cause anxiety and depression, or does the emotional toll of psoriasis promote anxiety and depression, which then causes alexithymia? Dr. Paul responded that it’s impossible to say at this point because these initial EPIDEPSO data are cross-sectional; however, the 1-year follow-up may yield insight.
EPIDEPSO is funded by Janssen. Dr. Paul reported receiving a research grant from the company to conduct the study.
AT THE EADV CONGRESS
Key clinical point: Alexithymia is strikingly common among psoriasis patients and is associated with multiple psychiatric comorbidities and problems in living.
Major finding: Thirty-nine percent of a large cohort of psoriasis patients met the criteria for alexithymia, an inability to identify and describe one’s emotions. Affected patients had markedly higher rates of anxiety, depression, problem drinking, and impairments of quality of life and work productivity.
Data source: The EPIDEPSO study, an ongoing 1-year prospective, observational international study involving 719 adults with moderate to severe psoriasis of less than 10 years’ duration.
Disclosures: The EPIDEPSO study is funded by Janssen, which provided the presenter with a research grant.
EADV: Best treatments for great saphenous vein reflux
COPENHAGEN – Superior 5-year outcomes for great saphenous vein reflux were achieved with conventional surgery and endovenous laser ablation as compared with ultrasound-guided foam sclerotherapy in a randomized trial, Dr. Simone van der Velden reported at the annual congress of the European Academy of Dermatology and Venereology.
The multicenter study included 224 randomized legs belonging to symptomatic patients with a target great saphenous vein diameter of at least 5 mm. If deemed necessary, patients could undergo one re-treatment at 3 or 12 months after their initial procedure. At 5 years of follow-up, 86% of the treated legs were available for long-term evaluation, noted Dr. van der Velden of Erasmus University Medical Center in Rotterdam, the Netherlands.
The primary endpoint was obliteration or absence of the treated great saphenous vein segment. This was achieved with conventional surgery in 85% of treated cases, in 77% of legs treated with endovenous laser ablation (EVLA), and in 23% with ultrasound-guided foam sclerotherapy (UGFS).
Absence of above-the-knee greater saphenous vein reflux – a secondary endpoint – was achieved in 85% of the conventional surgery group and in 82% of the EVLA group, both of which were significantly better results than the 41% response with UGFS.
Another secondary endpoint was grade II neovascularization. Here again, both conventional surgery and EVLA outperformed UGFS, with rates of 17%, 13%, and 4%, respectively. In contrast, there was no significant difference between the three treatment groups in terms of the presence of refluxing tributaries above or below knee level, she continued.
Scores on the disease-specific Chronic Venous Insufficiency quality of life Questionnaire (CIVIQ) deteriorated over time in the UGFS group, improved in the EVLA-treated patients, and remained stable in the conventional surgery group.
Conventional surgery was performed under general anesthesia and entailed high ligation of the saphenofemoral junction and phlebectomy of tributaries. In contrast, EVLA was done under local tumescent anesthesia using a 940-nm laser. The laser fiber was introduced at knee level, positioned 1-2 cm below the saphenofemoral junction, and delivered an energy of roughly 60 Joules/cm2.
For UGFS, operators utilized a foam comprising 1 mL of sodium tetradecyl sulfate per 3 mL of air. A maximum of 10 mL of foam could be injected per treatment session, depending upon the diameter of the great saphenous vein and length of the refluxing trunk. Phlebectomies in this group were performed only in the event of patient complaints.
Of note, patients in the minimally invasive UGFS group required re-treatment three times more often than did those in the other two study arms.
Dr. van der Velden said she has heard from some UGFS partisans that she and her coinvestigators may have undertreated patients in that study arm because they didn’t routinely perform phlebectomies of the tributaries, and the average amount of foam they injected, about 4.5 mL, was on the low side.
The study was sponsored by Erasmus University. Dr. van der Velden reported having no financial conflicts of interest.
COPENHAGEN – Superior 5-year outcomes for great saphenous vein reflux were achieved with conventional surgery and endovenous laser ablation as compared with ultrasound-guided foam sclerotherapy in a randomized trial, Dr. Simone van der Velden reported at the annual congress of the European Academy of Dermatology and Venereology.
The multicenter study included 224 randomized legs belonging to symptomatic patients with a target great saphenous vein diameter of at least 5 mm. If deemed necessary, patients could undergo one re-treatment at 3 or 12 months after their initial procedure. At 5 years of follow-up, 86% of the treated legs were available for long-term evaluation, noted Dr. van der Velden of Erasmus University Medical Center in Rotterdam, the Netherlands.
The primary endpoint was obliteration or absence of the treated great saphenous vein segment. This was achieved with conventional surgery in 85% of treated cases, in 77% of legs treated with endovenous laser ablation (EVLA), and in 23% with ultrasound-guided foam sclerotherapy (UGFS).
Absence of above-the-knee greater saphenous vein reflux – a secondary endpoint – was achieved in 85% of the conventional surgery group and in 82% of the EVLA group, both of which were significantly better results than the 41% response with UGFS.
Another secondary endpoint was grade II neovascularization. Here again, both conventional surgery and EVLA outperformed UGFS, with rates of 17%, 13%, and 4%, respectively. In contrast, there was no significant difference between the three treatment groups in terms of the presence of refluxing tributaries above or below knee level, she continued.
Scores on the disease-specific Chronic Venous Insufficiency quality of life Questionnaire (CIVIQ) deteriorated over time in the UGFS group, improved in the EVLA-treated patients, and remained stable in the conventional surgery group.
Conventional surgery was performed under general anesthesia and entailed high ligation of the saphenofemoral junction and phlebectomy of tributaries. In contrast, EVLA was done under local tumescent anesthesia using a 940-nm laser. The laser fiber was introduced at knee level, positioned 1-2 cm below the saphenofemoral junction, and delivered an energy of roughly 60 Joules/cm2.
For UGFS, operators utilized a foam comprising 1 mL of sodium tetradecyl sulfate per 3 mL of air. A maximum of 10 mL of foam could be injected per treatment session, depending upon the diameter of the great saphenous vein and length of the refluxing trunk. Phlebectomies in this group were performed only in the event of patient complaints.
Of note, patients in the minimally invasive UGFS group required re-treatment three times more often than did those in the other two study arms.
Dr. van der Velden said she has heard from some UGFS partisans that she and her coinvestigators may have undertreated patients in that study arm because they didn’t routinely perform phlebectomies of the tributaries, and the average amount of foam they injected, about 4.5 mL, was on the low side.
The study was sponsored by Erasmus University. Dr. van der Velden reported having no financial conflicts of interest.
COPENHAGEN – Superior 5-year outcomes for great saphenous vein reflux were achieved with conventional surgery and endovenous laser ablation as compared with ultrasound-guided foam sclerotherapy in a randomized trial, Dr. Simone van der Velden reported at the annual congress of the European Academy of Dermatology and Venereology.
The multicenter study included 224 randomized legs belonging to symptomatic patients with a target great saphenous vein diameter of at least 5 mm. If deemed necessary, patients could undergo one re-treatment at 3 or 12 months after their initial procedure. At 5 years of follow-up, 86% of the treated legs were available for long-term evaluation, noted Dr. van der Velden of Erasmus University Medical Center in Rotterdam, the Netherlands.
The primary endpoint was obliteration or absence of the treated great saphenous vein segment. This was achieved with conventional surgery in 85% of treated cases, in 77% of legs treated with endovenous laser ablation (EVLA), and in 23% with ultrasound-guided foam sclerotherapy (UGFS).
Absence of above-the-knee greater saphenous vein reflux – a secondary endpoint – was achieved in 85% of the conventional surgery group and in 82% of the EVLA group, both of which were significantly better results than the 41% response with UGFS.
Another secondary endpoint was grade II neovascularization. Here again, both conventional surgery and EVLA outperformed UGFS, with rates of 17%, 13%, and 4%, respectively. In contrast, there was no significant difference between the three treatment groups in terms of the presence of refluxing tributaries above or below knee level, she continued.
Scores on the disease-specific Chronic Venous Insufficiency quality of life Questionnaire (CIVIQ) deteriorated over time in the UGFS group, improved in the EVLA-treated patients, and remained stable in the conventional surgery group.
Conventional surgery was performed under general anesthesia and entailed high ligation of the saphenofemoral junction and phlebectomy of tributaries. In contrast, EVLA was done under local tumescent anesthesia using a 940-nm laser. The laser fiber was introduced at knee level, positioned 1-2 cm below the saphenofemoral junction, and delivered an energy of roughly 60 Joules/cm2.
For UGFS, operators utilized a foam comprising 1 mL of sodium tetradecyl sulfate per 3 mL of air. A maximum of 10 mL of foam could be injected per treatment session, depending upon the diameter of the great saphenous vein and length of the refluxing trunk. Phlebectomies in this group were performed only in the event of patient complaints.
Of note, patients in the minimally invasive UGFS group required re-treatment three times more often than did those in the other two study arms.
Dr. van der Velden said she has heard from some UGFS partisans that she and her coinvestigators may have undertreated patients in that study arm because they didn’t routinely perform phlebectomies of the tributaries, and the average amount of foam they injected, about 4.5 mL, was on the low side.
The study was sponsored by Erasmus University. Dr. van der Velden reported having no financial conflicts of interest.
AT THE EADV CONGRESS
Key clinical point: Long-term outcomes for treatment of great saphenous vein reflux were significantly better with conventional surgery or endovenous laser ablation than with ultrasound-guided foam sclerotherapy.
Major finding: Obliteration or absence of the treated great saphenous vein segment was achieved with conventional surgery in 85% of treated cases, with endovenous laser ablation (EVLA) in 77% of legs treated, and with ultrasound-guided foam sclerotherapy (UGFS) in 23%.
Data source: This multicenter clinical trial with 5-year follow-up included 224 legs randomized to one of three popular treatments for great saphenous varicose veins.
Disclosures: The study was sponsored by Erasmus University. The presenter reported having no financial conflicts of interest.
EADV: Another promising topical for atopic dermatitis
COPENHAGEN – A novel topical nonsteroidal inhibitor of phosphodiesterase-4 showed a favorable efficacy to side effect ratio in a phase II study of adolescents and adults with mild to moderate atopic dermatitis, Dr. Jon M. Hanifin reported at the annual congress of the European Academy of Dermatology and Venereology.
“For topical agents, I think a 30% improvement with few side effects is a desirable balance,” observed Dr. Hanifin of Oregon Health and Sciences University, Portland.
The investigational agent, known for now as OPA-15406, is formulated as a twice-daily ointment. It is highly selective for the phosphodiesterase-4 (PDE-4) B subtype.
Dr. Hanifin noted that these are “exciting times” in the development of new topical therapies for atopic dermatitis (AD). In addition to the successful phase II study of OPA-15406 he presented, a highlight of the EADV congress was the strongly positive pivotal phase III data presented for crisaborole, another nonsteroidal topical PDE-4 inhibitor, albeit with a different mechanism of action.
For Dr. Hanifin these developments are particularly satisfying personally because 33 years ago as a young investigator – before the term ‘translational science’ had come into vogue – he and his research team made the seminal observation that increased phosphodiesterase activity is “a basic biochemical characteristic relevant to skin immunocellular regulation in atopic disease” (J Allergy Clin Immunol. 1982 Dec;70[6]:452-7).
The 8-week, multicenter, randomized, double-blind phase II OPA-15406 dose-ranging study included 121 patients, 70% with moderate AD, the rest with mild disease. About 20% were adolescents, the rest adults. Their mean baseline Eczema Area Severity Index (EASI) score was 9.5, with a self-reported pruritus score of 61 on a 0-100 scale. Participants were randomized to twice-daily application of OPA-15406 at 0.3% or 1% or the vehicle ointment as a control.
A significant treatment effect was seen within the first week. At 1 week, the mean EASI score was reduced by 31% from baseline in the 1% OPA-15406 group, 15% with the 0.3% formulation, and 6% with vehicle. The active treatment remained significantly more effective than vehicle throughout the 8-week period.
Another measure of efficacy – an Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 along with at least a 2-point improvement on the 0-5 scale at week 4 – was met by 21% of patients on 1% OPA-15406, 15% on the 0.3% formulation, and 2.7% on vehicle. By the less stringent standard of an IGA of 0 or 1 plus at least a 1-point reduction at week 4, the success rates were 30%, 24%, and 10%.
Dr. Hanifin said the 1% formulation is the one likely to advance to phase III studies, given its superior efficacy. This was particularly evident with regard to itch. At the first evaluation, after just 1 week of treatment, pruritus scores showed a mean 30% reduction with 1% OPA-15406 versus no significant change in controls. He added that his clinical impression is that many patients experience a significant improvement in itching within the first 24 hours on the 1% formulation, an observation that deserves formal study.
Scores on the Dermatology Life Quality Index and Children’s Dermatology Life Quality Index were significantly better in the active therapy arms than with vehicle as early as week 1.
The adverse events findings were “not very exciting,” according to the dermatologist. No treatment-related serious adverse events occurred. The two most common adverse events leading to treatment discontinuation – worsening AD and pruritus – occurred more often in the vehicle-treated controls.
Session cochair Dr. Jacek Szepietowski called the pruritus results particularly impressive.
“It’s very difficult to do successful clinical trials of topicals for atopic dermatitis because the vehicle alone, especially if it’s an ointment, can have favorable effects which increase over time both on EASI and pruritus,” commented Dr. Szepietowski, professor and head of the department of dermatology, venereology, and allergology at the Medical University of Wroclaw (Poland).
The study was sponsored by Otsuka Pharmaceuticals. Dr. Hanifin reported serving as a consultant to and paid investigator for the company.
COPENHAGEN – A novel topical nonsteroidal inhibitor of phosphodiesterase-4 showed a favorable efficacy to side effect ratio in a phase II study of adolescents and adults with mild to moderate atopic dermatitis, Dr. Jon M. Hanifin reported at the annual congress of the European Academy of Dermatology and Venereology.
“For topical agents, I think a 30% improvement with few side effects is a desirable balance,” observed Dr. Hanifin of Oregon Health and Sciences University, Portland.
The investigational agent, known for now as OPA-15406, is formulated as a twice-daily ointment. It is highly selective for the phosphodiesterase-4 (PDE-4) B subtype.
Dr. Hanifin noted that these are “exciting times” in the development of new topical therapies for atopic dermatitis (AD). In addition to the successful phase II study of OPA-15406 he presented, a highlight of the EADV congress was the strongly positive pivotal phase III data presented for crisaborole, another nonsteroidal topical PDE-4 inhibitor, albeit with a different mechanism of action.
For Dr. Hanifin these developments are particularly satisfying personally because 33 years ago as a young investigator – before the term ‘translational science’ had come into vogue – he and his research team made the seminal observation that increased phosphodiesterase activity is “a basic biochemical characteristic relevant to skin immunocellular regulation in atopic disease” (J Allergy Clin Immunol. 1982 Dec;70[6]:452-7).
The 8-week, multicenter, randomized, double-blind phase II OPA-15406 dose-ranging study included 121 patients, 70% with moderate AD, the rest with mild disease. About 20% were adolescents, the rest adults. Their mean baseline Eczema Area Severity Index (EASI) score was 9.5, with a self-reported pruritus score of 61 on a 0-100 scale. Participants were randomized to twice-daily application of OPA-15406 at 0.3% or 1% or the vehicle ointment as a control.
A significant treatment effect was seen within the first week. At 1 week, the mean EASI score was reduced by 31% from baseline in the 1% OPA-15406 group, 15% with the 0.3% formulation, and 6% with vehicle. The active treatment remained significantly more effective than vehicle throughout the 8-week period.
Another measure of efficacy – an Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 along with at least a 2-point improvement on the 0-5 scale at week 4 – was met by 21% of patients on 1% OPA-15406, 15% on the 0.3% formulation, and 2.7% on vehicle. By the less stringent standard of an IGA of 0 or 1 plus at least a 1-point reduction at week 4, the success rates were 30%, 24%, and 10%.
Dr. Hanifin said the 1% formulation is the one likely to advance to phase III studies, given its superior efficacy. This was particularly evident with regard to itch. At the first evaluation, after just 1 week of treatment, pruritus scores showed a mean 30% reduction with 1% OPA-15406 versus no significant change in controls. He added that his clinical impression is that many patients experience a significant improvement in itching within the first 24 hours on the 1% formulation, an observation that deserves formal study.
Scores on the Dermatology Life Quality Index and Children’s Dermatology Life Quality Index were significantly better in the active therapy arms than with vehicle as early as week 1.
The adverse events findings were “not very exciting,” according to the dermatologist. No treatment-related serious adverse events occurred. The two most common adverse events leading to treatment discontinuation – worsening AD and pruritus – occurred more often in the vehicle-treated controls.
Session cochair Dr. Jacek Szepietowski called the pruritus results particularly impressive.
“It’s very difficult to do successful clinical trials of topicals for atopic dermatitis because the vehicle alone, especially if it’s an ointment, can have favorable effects which increase over time both on EASI and pruritus,” commented Dr. Szepietowski, professor and head of the department of dermatology, venereology, and allergology at the Medical University of Wroclaw (Poland).
The study was sponsored by Otsuka Pharmaceuticals. Dr. Hanifin reported serving as a consultant to and paid investigator for the company.
COPENHAGEN – A novel topical nonsteroidal inhibitor of phosphodiesterase-4 showed a favorable efficacy to side effect ratio in a phase II study of adolescents and adults with mild to moderate atopic dermatitis, Dr. Jon M. Hanifin reported at the annual congress of the European Academy of Dermatology and Venereology.
“For topical agents, I think a 30% improvement with few side effects is a desirable balance,” observed Dr. Hanifin of Oregon Health and Sciences University, Portland.
The investigational agent, known for now as OPA-15406, is formulated as a twice-daily ointment. It is highly selective for the phosphodiesterase-4 (PDE-4) B subtype.
Dr. Hanifin noted that these are “exciting times” in the development of new topical therapies for atopic dermatitis (AD). In addition to the successful phase II study of OPA-15406 he presented, a highlight of the EADV congress was the strongly positive pivotal phase III data presented for crisaborole, another nonsteroidal topical PDE-4 inhibitor, albeit with a different mechanism of action.
For Dr. Hanifin these developments are particularly satisfying personally because 33 years ago as a young investigator – before the term ‘translational science’ had come into vogue – he and his research team made the seminal observation that increased phosphodiesterase activity is “a basic biochemical characteristic relevant to skin immunocellular regulation in atopic disease” (J Allergy Clin Immunol. 1982 Dec;70[6]:452-7).
The 8-week, multicenter, randomized, double-blind phase II OPA-15406 dose-ranging study included 121 patients, 70% with moderate AD, the rest with mild disease. About 20% were adolescents, the rest adults. Their mean baseline Eczema Area Severity Index (EASI) score was 9.5, with a self-reported pruritus score of 61 on a 0-100 scale. Participants were randomized to twice-daily application of OPA-15406 at 0.3% or 1% or the vehicle ointment as a control.
A significant treatment effect was seen within the first week. At 1 week, the mean EASI score was reduced by 31% from baseline in the 1% OPA-15406 group, 15% with the 0.3% formulation, and 6% with vehicle. The active treatment remained significantly more effective than vehicle throughout the 8-week period.
Another measure of efficacy – an Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 along with at least a 2-point improvement on the 0-5 scale at week 4 – was met by 21% of patients on 1% OPA-15406, 15% on the 0.3% formulation, and 2.7% on vehicle. By the less stringent standard of an IGA of 0 or 1 plus at least a 1-point reduction at week 4, the success rates were 30%, 24%, and 10%.
Dr. Hanifin said the 1% formulation is the one likely to advance to phase III studies, given its superior efficacy. This was particularly evident with regard to itch. At the first evaluation, after just 1 week of treatment, pruritus scores showed a mean 30% reduction with 1% OPA-15406 versus no significant change in controls. He added that his clinical impression is that many patients experience a significant improvement in itching within the first 24 hours on the 1% formulation, an observation that deserves formal study.
Scores on the Dermatology Life Quality Index and Children’s Dermatology Life Quality Index were significantly better in the active therapy arms than with vehicle as early as week 1.
The adverse events findings were “not very exciting,” according to the dermatologist. No treatment-related serious adverse events occurred. The two most common adverse events leading to treatment discontinuation – worsening AD and pruritus – occurred more often in the vehicle-treated controls.
Session cochair Dr. Jacek Szepietowski called the pruritus results particularly impressive.
“It’s very difficult to do successful clinical trials of topicals for atopic dermatitis because the vehicle alone, especially if it’s an ointment, can have favorable effects which increase over time both on EASI and pruritus,” commented Dr. Szepietowski, professor and head of the department of dermatology, venereology, and allergology at the Medical University of Wroclaw (Poland).
The study was sponsored by Otsuka Pharmaceuticals. Dr. Hanifin reported serving as a consultant to and paid investigator for the company.
AT THE EADV CONGRESS
Key clinical point: The pipeline for nonsteroidal topical therapies for pediatric and adult atopic dermatitis shows great promise.
Major finding: Atopic dermatitis patients showed a mean 31% reduction in Eczema Area Severity Index scores after 1 week on topical 1% OPA-15406 ointment, compared with a 6% decrease on vehicle alone.
Data source: This phase II multicenter, double-blind, 8-week randomized trial included 121 adolescents and adults with mild to moderate atopic dermatitis.
Disclosures: The study was sponsored by Otsuka Pharmaceuticals. The presenter reported serving as a consultant and paid investigator for the company.