User login
European Academy of Dermatology & Venereology (EADV): Annual Congress
EADV: Long-term apremilast results show what to expect for psoriasis
COPENHAGEN – What can physicians and their psoriasis patients realistically expect from long-term apremilast therapy?
“I think the take-home number is a 50%-plus PASI-75 response after 1 year among patients with moderate to severe plaque psoriasis,” Dr. Kristian Reich said at the annual congress of the European Academy of Dermatology and Venereology.
He presented what he considers to be the first solid data addressing this key question. The data come from a new analysis of the 52-week results of the LIBERATE trial.
“Due to the randomized withdrawal design and long-term treatment only of responders in the original pivotal phase III ESTEEM program, I think we’ve had no real understanding from clinical trials of what the long-term efficacy of apremilast [Otezla] is. Of course, with psoriasis being a chronic disease, this is really what we want to know. And we can get this data very nicely from the LIBERATE study. I should point out that although LIBERATE is a relatively small study, every single patient is included here. So I think this is a very robust analysis of 1-year efficacy data,” said Dr. Reich, managing partner of Dermatologikum Hamburg (Germany).
In contrast to the inclusive analysis performed in LIBERATE, in the pivotal ESTEEM I trial a mere 77 of 562 psoriasis patients randomized to apremilast were on the oral phosphodiesterase type 4 inhibitor for the full 52-week study period, with 61% of that superselect subgroup showing a PASI-75 response (J Am Acad Dermatol. 2015 Jul;73[1]:37-49).
LIBERATE included 250 psoriasis patients. Their baseline Psoriasis Area and Severity Index (PASI) score was about 20, with 27% body surface area involved, a body mass index of 29 kg/m2, 89 kg of body weight, and a mean Dermatology Life Quality Index (DLQI) score of 13. Seventy percent to 80% of subjects had previously used conventional systemic therapies, but no one was allowed to have prior use of biologic therapies.
Patients were randomized to one of three study arms: 16 weeks of oral apremilast at the approved dose of 30 mg twice daily plus a weekly placebo injection, subcutaneous etanercept (Enbrel) at 50 mg once weekly plus a placebo tablet, or dual placebos. At 16 weeks, all patients were switched to apremilast at 30 mg twice daily with no placebos through week 52.
The PASI-75 response rate at week 16 was 11.9% in the placebo group, 39.8% with apremilast, and 48.2% with etanercept.
“I am surprised to see in the more recent studies the high response to etanercept. This is fantastic data. Etanercept seems to ripen like old wine. It’s getting better the longer we have it,” the dermatologist commented.
The PASI-75 rates at 1 year were 46.4% with placebo/apremilast, 50.6% with apremilast throughout, and 55.4% with etanercept/apremilast.
The mean improvement in DLQI from baseline to 52 weeks – a secondary endpoint – was 6.6 points with placebo/apremilast, 8.0 with apremilast/apremilast, and 8.9 points with etanercept/apremilast. A noteworthy finding was that during the first 16 weeks apremilast brought a clinically meaningful improvement in quality of life significantly faster than etanercept did, with a significant difference seen between the two study arms even in the first 1-2 weeks.
The likely explanation for this benefit is that the mean reduction in pruritus visual analog scale scores was significantly greater with apremilast than etanercept through the first 8 weeks. Although pruritus is traditionally thought of more in the context of atopic dermatitis, it’s actually also the No. 1 complaint among psoriasis patients, according to Dr. Reich.
“This is a special thing with apremilast, that the pruritus really goes down very significantly early on. It raises an interesting question about what the role of phosphodiesterase-4 inhibition might be in pruritus. I couldn’t give you a molecular explanation, but I think because pruritus is so annoying and it definitely affects quality of life, this could be a possible explanation for why there is more rapid improvement in quality of life independent of the PASI improvement,” he said.
Switching from etanercept to apremilast didn’t result in any clinically significant safety findings through week 52. No meaningful laboratory changes occurred during 52 weeks of monitoring. There were no cases of suicidal ideation, no increase in serious infections, and only a single cardiac event. The most common apremilast-associated side effects seen in LIBERATE were loose stools in 8%-15% of patients, nausea in 10%, and headache in 13%. These adverse events were mild to moderate in nature and decreased in prevalence over time. The maximum weight loss noted over the course of 52 weeks occurred in the placebo-to-apremilast group, with a mean 1.3-kg reduction.
“I would call the LIBERATE results a validation of the earlier data showing a very clean safety profile with this drug,” Dr. Reich said.
This is reflected in the product labeling, which unlike other systemic therapies for psoriasis includes no requirements for laboratory monitoring or tuberculosis testing.
Both the LIBERATE and the ESTEEM trials were sponsored by Celgene. Dr. Reich received research grants as an investigator in both programs.
COPENHAGEN – What can physicians and their psoriasis patients realistically expect from long-term apremilast therapy?
“I think the take-home number is a 50%-plus PASI-75 response after 1 year among patients with moderate to severe plaque psoriasis,” Dr. Kristian Reich said at the annual congress of the European Academy of Dermatology and Venereology.
He presented what he considers to be the first solid data addressing this key question. The data come from a new analysis of the 52-week results of the LIBERATE trial.
“Due to the randomized withdrawal design and long-term treatment only of responders in the original pivotal phase III ESTEEM program, I think we’ve had no real understanding from clinical trials of what the long-term efficacy of apremilast [Otezla] is. Of course, with psoriasis being a chronic disease, this is really what we want to know. And we can get this data very nicely from the LIBERATE study. I should point out that although LIBERATE is a relatively small study, every single patient is included here. So I think this is a very robust analysis of 1-year efficacy data,” said Dr. Reich, managing partner of Dermatologikum Hamburg (Germany).
In contrast to the inclusive analysis performed in LIBERATE, in the pivotal ESTEEM I trial a mere 77 of 562 psoriasis patients randomized to apremilast were on the oral phosphodiesterase type 4 inhibitor for the full 52-week study period, with 61% of that superselect subgroup showing a PASI-75 response (J Am Acad Dermatol. 2015 Jul;73[1]:37-49).
LIBERATE included 250 psoriasis patients. Their baseline Psoriasis Area and Severity Index (PASI) score was about 20, with 27% body surface area involved, a body mass index of 29 kg/m2, 89 kg of body weight, and a mean Dermatology Life Quality Index (DLQI) score of 13. Seventy percent to 80% of subjects had previously used conventional systemic therapies, but no one was allowed to have prior use of biologic therapies.
Patients were randomized to one of three study arms: 16 weeks of oral apremilast at the approved dose of 30 mg twice daily plus a weekly placebo injection, subcutaneous etanercept (Enbrel) at 50 mg once weekly plus a placebo tablet, or dual placebos. At 16 weeks, all patients were switched to apremilast at 30 mg twice daily with no placebos through week 52.
The PASI-75 response rate at week 16 was 11.9% in the placebo group, 39.8% with apremilast, and 48.2% with etanercept.
“I am surprised to see in the more recent studies the high response to etanercept. This is fantastic data. Etanercept seems to ripen like old wine. It’s getting better the longer we have it,” the dermatologist commented.
The PASI-75 rates at 1 year were 46.4% with placebo/apremilast, 50.6% with apremilast throughout, and 55.4% with etanercept/apremilast.
The mean improvement in DLQI from baseline to 52 weeks – a secondary endpoint – was 6.6 points with placebo/apremilast, 8.0 with apremilast/apremilast, and 8.9 points with etanercept/apremilast. A noteworthy finding was that during the first 16 weeks apremilast brought a clinically meaningful improvement in quality of life significantly faster than etanercept did, with a significant difference seen between the two study arms even in the first 1-2 weeks.
The likely explanation for this benefit is that the mean reduction in pruritus visual analog scale scores was significantly greater with apremilast than etanercept through the first 8 weeks. Although pruritus is traditionally thought of more in the context of atopic dermatitis, it’s actually also the No. 1 complaint among psoriasis patients, according to Dr. Reich.
“This is a special thing with apremilast, that the pruritus really goes down very significantly early on. It raises an interesting question about what the role of phosphodiesterase-4 inhibition might be in pruritus. I couldn’t give you a molecular explanation, but I think because pruritus is so annoying and it definitely affects quality of life, this could be a possible explanation for why there is more rapid improvement in quality of life independent of the PASI improvement,” he said.
Switching from etanercept to apremilast didn’t result in any clinically significant safety findings through week 52. No meaningful laboratory changes occurred during 52 weeks of monitoring. There were no cases of suicidal ideation, no increase in serious infections, and only a single cardiac event. The most common apremilast-associated side effects seen in LIBERATE were loose stools in 8%-15% of patients, nausea in 10%, and headache in 13%. These adverse events were mild to moderate in nature and decreased in prevalence over time. The maximum weight loss noted over the course of 52 weeks occurred in the placebo-to-apremilast group, with a mean 1.3-kg reduction.
“I would call the LIBERATE results a validation of the earlier data showing a very clean safety profile with this drug,” Dr. Reich said.
This is reflected in the product labeling, which unlike other systemic therapies for psoriasis includes no requirements for laboratory monitoring or tuberculosis testing.
Both the LIBERATE and the ESTEEM trials were sponsored by Celgene. Dr. Reich received research grants as an investigator in both programs.
COPENHAGEN – What can physicians and their psoriasis patients realistically expect from long-term apremilast therapy?
“I think the take-home number is a 50%-plus PASI-75 response after 1 year among patients with moderate to severe plaque psoriasis,” Dr. Kristian Reich said at the annual congress of the European Academy of Dermatology and Venereology.
He presented what he considers to be the first solid data addressing this key question. The data come from a new analysis of the 52-week results of the LIBERATE trial.
“Due to the randomized withdrawal design and long-term treatment only of responders in the original pivotal phase III ESTEEM program, I think we’ve had no real understanding from clinical trials of what the long-term efficacy of apremilast [Otezla] is. Of course, with psoriasis being a chronic disease, this is really what we want to know. And we can get this data very nicely from the LIBERATE study. I should point out that although LIBERATE is a relatively small study, every single patient is included here. So I think this is a very robust analysis of 1-year efficacy data,” said Dr. Reich, managing partner of Dermatologikum Hamburg (Germany).
In contrast to the inclusive analysis performed in LIBERATE, in the pivotal ESTEEM I trial a mere 77 of 562 psoriasis patients randomized to apremilast were on the oral phosphodiesterase type 4 inhibitor for the full 52-week study period, with 61% of that superselect subgroup showing a PASI-75 response (J Am Acad Dermatol. 2015 Jul;73[1]:37-49).
LIBERATE included 250 psoriasis patients. Their baseline Psoriasis Area and Severity Index (PASI) score was about 20, with 27% body surface area involved, a body mass index of 29 kg/m2, 89 kg of body weight, and a mean Dermatology Life Quality Index (DLQI) score of 13. Seventy percent to 80% of subjects had previously used conventional systemic therapies, but no one was allowed to have prior use of biologic therapies.
Patients were randomized to one of three study arms: 16 weeks of oral apremilast at the approved dose of 30 mg twice daily plus a weekly placebo injection, subcutaneous etanercept (Enbrel) at 50 mg once weekly plus a placebo tablet, or dual placebos. At 16 weeks, all patients were switched to apremilast at 30 mg twice daily with no placebos through week 52.
The PASI-75 response rate at week 16 was 11.9% in the placebo group, 39.8% with apremilast, and 48.2% with etanercept.
“I am surprised to see in the more recent studies the high response to etanercept. This is fantastic data. Etanercept seems to ripen like old wine. It’s getting better the longer we have it,” the dermatologist commented.
The PASI-75 rates at 1 year were 46.4% with placebo/apremilast, 50.6% with apremilast throughout, and 55.4% with etanercept/apremilast.
The mean improvement in DLQI from baseline to 52 weeks – a secondary endpoint – was 6.6 points with placebo/apremilast, 8.0 with apremilast/apremilast, and 8.9 points with etanercept/apremilast. A noteworthy finding was that during the first 16 weeks apremilast brought a clinically meaningful improvement in quality of life significantly faster than etanercept did, with a significant difference seen between the two study arms even in the first 1-2 weeks.
The likely explanation for this benefit is that the mean reduction in pruritus visual analog scale scores was significantly greater with apremilast than etanercept through the first 8 weeks. Although pruritus is traditionally thought of more in the context of atopic dermatitis, it’s actually also the No. 1 complaint among psoriasis patients, according to Dr. Reich.
“This is a special thing with apremilast, that the pruritus really goes down very significantly early on. It raises an interesting question about what the role of phosphodiesterase-4 inhibition might be in pruritus. I couldn’t give you a molecular explanation, but I think because pruritus is so annoying and it definitely affects quality of life, this could be a possible explanation for why there is more rapid improvement in quality of life independent of the PASI improvement,” he said.
Switching from etanercept to apremilast didn’t result in any clinically significant safety findings through week 52. No meaningful laboratory changes occurred during 52 weeks of monitoring. There were no cases of suicidal ideation, no increase in serious infections, and only a single cardiac event. The most common apremilast-associated side effects seen in LIBERATE were loose stools in 8%-15% of patients, nausea in 10%, and headache in 13%. These adverse events were mild to moderate in nature and decreased in prevalence over time. The maximum weight loss noted over the course of 52 weeks occurred in the placebo-to-apremilast group, with a mean 1.3-kg reduction.
“I would call the LIBERATE results a validation of the earlier data showing a very clean safety profile with this drug,” Dr. Reich said.
This is reflected in the product labeling, which unlike other systemic therapies for psoriasis includes no requirements for laboratory monitoring or tuberculosis testing.
Both the LIBERATE and the ESTEEM trials were sponsored by Celgene. Dr. Reich received research grants as an investigator in both programs.
AT THE EADV CONGRESS
Key clinical point: Patients on apremilast for moderate to severe plaque psoriasis can reasonably anticipate a 50%-plus likelihood of a PASI-75 response after 52 weeks of treatment.
Major finding: A 75% or greater improvement in Psoriasis Area and Severity Index scores was documented in 50.6% of patients after 52 weeks on apremilast and in 55.4% who underwent a protocol-mandated switch from etanercept to apremilast after 16 weeks on the injectable biologic agent.
Data source: A prospective 52-week study randomizing 250 patients with moderate to severe psoriasis to 16 weeks of oral apremilast, etanercept, or placebo followed by 36 weeks of apremilast for all.
Disclosures: The LIBERATE trial was sponsored by Celgene. The presenter received a research grant from the company.
EADV: Hidradenitis suppurativa carries high cardiovascular risk
COPENHAGEN – Hidradenitis suppurativa, a common, chronic, inflammatory scarring skin disease of the hair follicles, is a red flag signaling elevated levels of multiple cardiovascular risk factors, according to a systematic review and meta-analysis.
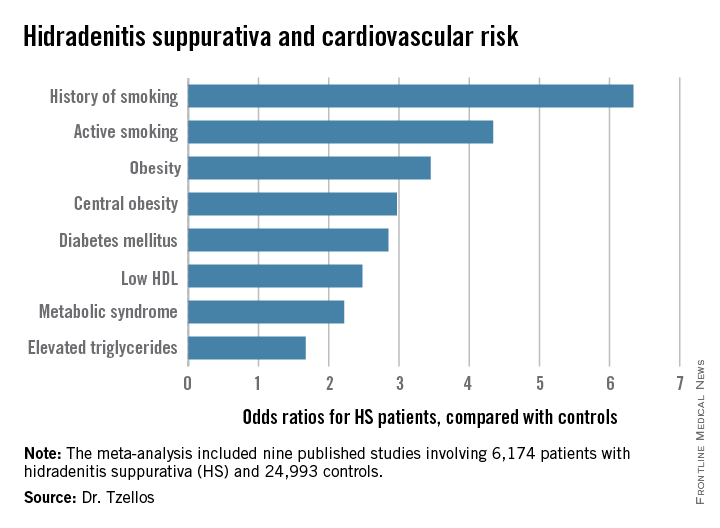
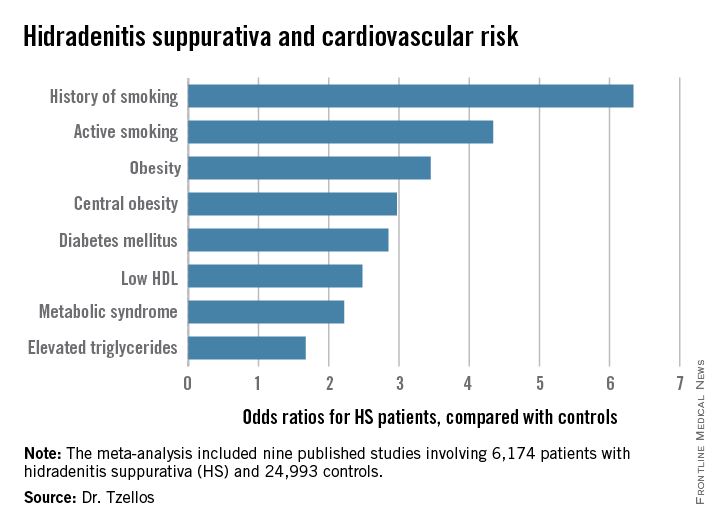
“The need for screening of hidradenitis suppurativa patients for modifiable cardiovascular risk is emphasized,” Dr. Thrasyvoulos Tzellos said in presenting the findings at the annual congress of the European Academy of Dermatology and Venereology.
For such a common and dramatically destructive disease, hidradenitis suppurativa (HS) was underresearched until recently. Investigative interest grew as the tumor necrosis factor inhibitor adalimumab (Humira) underwent development as a novel therapy for what has been traditionally a notoriously difficult to treat disease. The biologic agent received Food and Drug Administration marketing approval in October as the first and only approved treatment for HS.
Dr. Tzellos’s meta-analysis included nine published studies totaling 6,174 HS patients and 24,993 controls. Five studies were case control, and the other four were cross sectional. An indicator of the recent explosive research interest in HS can be seen in the fact that 80% of all the HS patients included in the meta-analysis come from two studies published within just the last year, one from Massachusetts General Hospital (J Am Acad Dermatol. 2014 Dec;71[6]:1144-50) and the other from Israel (Br J Dermatol. 2015 Aug;173[2]:464-70).
Not all the studies examined the same cardiovascular risk factors. For example, only six of nine studies looked at diabetes mellitus as an endpoint. Of those studies that did, diabetes occurred in 856 of 5,685 HS patients, a rate 2.85-fold higher than in controls, according to Dr. Tzellos of University Hospital of North Norway in Troms.
The only cardiovascular risk factor examined that was not significantly more common among patients with HS than controls was hypertension. The 1.57-fold increased likelihood of hypertension among HS patients didn’t achieve statistical significance.
Although patients whose HS was treated exclusively in outpatient settings had significantly higher levels of cardiovascular risk factors than did controls, risk levels were consistently higher still in patients who had been hospitalized for HS.
A meta-analysis such as this cannot address causality, leaving open the question of whether increased cardiovascular risk factors are intrinsic to HS, or the debilitating recurrent skin disease causes affected patients to take a defeatest attitude toward maintenance of a healthy lifestyle.
Dr. Tzellos reported having no financial conflicts regarding this meta-analysis, carried out with academic funding.
COPENHAGEN – Hidradenitis suppurativa, a common, chronic, inflammatory scarring skin disease of the hair follicles, is a red flag signaling elevated levels of multiple cardiovascular risk factors, according to a systematic review and meta-analysis.

“The need for screening of hidradenitis suppurativa patients for modifiable cardiovascular risk is emphasized,” Dr. Thrasyvoulos Tzellos said in presenting the findings at the annual congress of the European Academy of Dermatology and Venereology.
For such a common and dramatically destructive disease, hidradenitis suppurativa (HS) was underresearched until recently. Investigative interest grew as the tumor necrosis factor inhibitor adalimumab (Humira) underwent development as a novel therapy for what has been traditionally a notoriously difficult to treat disease. The biologic agent received Food and Drug Administration marketing approval in October as the first and only approved treatment for HS.
Dr. Tzellos’s meta-analysis included nine published studies totaling 6,174 HS patients and 24,993 controls. Five studies were case control, and the other four were cross sectional. An indicator of the recent explosive research interest in HS can be seen in the fact that 80% of all the HS patients included in the meta-analysis come from two studies published within just the last year, one from Massachusetts General Hospital (J Am Acad Dermatol. 2014 Dec;71[6]:1144-50) and the other from Israel (Br J Dermatol. 2015 Aug;173[2]:464-70).
Not all the studies examined the same cardiovascular risk factors. For example, only six of nine studies looked at diabetes mellitus as an endpoint. Of those studies that did, diabetes occurred in 856 of 5,685 HS patients, a rate 2.85-fold higher than in controls, according to Dr. Tzellos of University Hospital of North Norway in Troms.
The only cardiovascular risk factor examined that was not significantly more common among patients with HS than controls was hypertension. The 1.57-fold increased likelihood of hypertension among HS patients didn’t achieve statistical significance.
Although patients whose HS was treated exclusively in outpatient settings had significantly higher levels of cardiovascular risk factors than did controls, risk levels were consistently higher still in patients who had been hospitalized for HS.
A meta-analysis such as this cannot address causality, leaving open the question of whether increased cardiovascular risk factors are intrinsic to HS, or the debilitating recurrent skin disease causes affected patients to take a defeatest attitude toward maintenance of a healthy lifestyle.
Dr. Tzellos reported having no financial conflicts regarding this meta-analysis, carried out with academic funding.
COPENHAGEN – Hidradenitis suppurativa, a common, chronic, inflammatory scarring skin disease of the hair follicles, is a red flag signaling elevated levels of multiple cardiovascular risk factors, according to a systematic review and meta-analysis.

“The need for screening of hidradenitis suppurativa patients for modifiable cardiovascular risk is emphasized,” Dr. Thrasyvoulos Tzellos said in presenting the findings at the annual congress of the European Academy of Dermatology and Venereology.
For such a common and dramatically destructive disease, hidradenitis suppurativa (HS) was underresearched until recently. Investigative interest grew as the tumor necrosis factor inhibitor adalimumab (Humira) underwent development as a novel therapy for what has been traditionally a notoriously difficult to treat disease. The biologic agent received Food and Drug Administration marketing approval in October as the first and only approved treatment for HS.
Dr. Tzellos’s meta-analysis included nine published studies totaling 6,174 HS patients and 24,993 controls. Five studies were case control, and the other four were cross sectional. An indicator of the recent explosive research interest in HS can be seen in the fact that 80% of all the HS patients included in the meta-analysis come from two studies published within just the last year, one from Massachusetts General Hospital (J Am Acad Dermatol. 2014 Dec;71[6]:1144-50) and the other from Israel (Br J Dermatol. 2015 Aug;173[2]:464-70).
Not all the studies examined the same cardiovascular risk factors. For example, only six of nine studies looked at diabetes mellitus as an endpoint. Of those studies that did, diabetes occurred in 856 of 5,685 HS patients, a rate 2.85-fold higher than in controls, according to Dr. Tzellos of University Hospital of North Norway in Troms.
The only cardiovascular risk factor examined that was not significantly more common among patients with HS than controls was hypertension. The 1.57-fold increased likelihood of hypertension among HS patients didn’t achieve statistical significance.
Although patients whose HS was treated exclusively in outpatient settings had significantly higher levels of cardiovascular risk factors than did controls, risk levels were consistently higher still in patients who had been hospitalized for HS.
A meta-analysis such as this cannot address causality, leaving open the question of whether increased cardiovascular risk factors are intrinsic to HS, or the debilitating recurrent skin disease causes affected patients to take a defeatest attitude toward maintenance of a healthy lifestyle.
Dr. Tzellos reported having no financial conflicts regarding this meta-analysis, carried out with academic funding.
AT THE EADV CONGRESS
Key clinical point: Be vigilant in screening for modifiable cardiovascular risk factors in patients with hidradenitis suppurativa.
Major finding: Hidradenitis suppurativa patients were 2.85-fold more likely than controls to have diabetes, 2.22-fold more likely to have metabolic syndrome, and 4.34-fold more likely to be active smokers.
Data source: A meta-analysis of nine published studies totaling 6,174 hidradenitis suppurativa patients and 24,993 controls.
Disclosures: The presenter reported having no financial conflicts regarding this meta-analysis, carried out with academic funding.
EADV: Ixekizumab promising for psoriatic arthritis
COPENHAGEN – Ixekizumab not only shows considerable promise for the treatment of moderate to severe psoriasis, it looks like it may be a winner for comorbid psoriatic arthritis, too.
The investigational IgG4 humanized monoclonal antibody directed against interleukin-17A brought marked improvements in joint pain, systemic inflammatory burden, and quality of life as well as skin disease in patients with both psoriasis and self-reported psoriatic arthritis in a combined analysis of three phase III clinical trials, Dr. Alice B. Gottlieb reported at the annual congress of the European Academy of Dermatology and Venereology.
Of the 3,126 patients with moderate to severe psoriasis who participated in the 12-week, phase III UNCOVER-1, -2, and -3 trials, 751 (24%) also had self-reported psoriatic arthritis; her analysis focused on them.
She was quick to note that the UNCOVER trials were primarily psoriasis studies that relied upon patient self-report of psoriatic arthritis. Nevertheless, it seems likely that the great majority of self-reported psoriatic arthritis patients really did have the rheumatologic disease, since the mean baseline C-reactive protein (CRP) level in that group was 8.43 mg/L, a level far higher than expected in patients with psoriasis only.
In any case, more-rigorous phase III studies of ixekizumab conducted specifically in patients with formally rheumatologist-diagnosed psoriatic arthritis and treated in rheumatology practices are due to be presented at the annual meeting of the American College of Rheumatology in November. And while Dr. Gottlieb wasn’t at liberty to discuss those results, she did hint that the data will be strongly positive.
“If you’re happy about these UNCOVER findings, you’ll be ecstatic about those,” predicted Dr. Gottlieb, professor of dermatology and dermatologist in chief at Tufts Medical Center, Boston.
Also coming up at the American College of Rheumatology meeting will be the results of the first-ever head-to-head comparison of an IL-17 inhibitor versus a tumor necrosis factor–alpha blocker in psoriatic arthritis patients. While at present most physicians consider a TNF inhibitor to be the treatment of choice in patients with psoriatic arthritis, that view may change as a result of the forthcoming comparative study, according to the dermatologist.
In each of the three phase III UNCOVER studies, patients were randomized to 12 weeks of subcutaneous ixekizumab at 80 mg every 2 or 4 weeks following a 160-mg loading dose, or to placebo. At baseline, the subgroup with self-reported psoriatic arthritis had a mean Psoriasis Area and Severity Index ( PASI) 0f about 21, a self-rated joint pain severity of 50 on a 0-100 scale, a CRP of 8.43 mg/L, and a Dermatology Life Quality Index (DLQI) score of 14.
Joint pain decreased dramatically in the two ixekizumab groups as early at 2 weeks into the trial, at which point, patients on treatment every 2 weeks averaged a 13.1-point reduction from baseline, with a similar 14.1-point drop noted in those on an every 4 weeks schedule. At week 12, the mean reductions from baseline were 25.2 and 26.8 points, compared with a 1.1-point increase in joint pain among placebo-treated controls.
Inflammatory burden plunged quickly, as evidenced by mean reductions in CRP of 4.63 mg/L and 4.33 mg/L at week 1 with biweekly and monthly dosing, respectively. These reductions were then maintained through week 12.
In terms of improvement in skin symptoms, with ixekizumab dosed every 2 weeks, the PASI 75 response was 89.8% at 12 weeks, the PASI 90 response was 69.3%, and the PASI 100 response (clear skin) was 37.1%. In patients treated every 4 weeks, the rates were 81.1%, 60.8%, and 34.7%.
“There’s good news in both groups, but I think the news is even better in the every-2-weeks group,” Dr. Gottlieb commented.
The mean 12-week decrease from baseline in DLQI was 11.8 points with biweekly dosing and 10.5 with 4-week dosing, compared with 0.8 points in controls. That’s impressive given that a 5-point reduction in DLQI is deemed clinically meaningful, the dermatologist observed. At 12 weeks, 56.5% of patients on ixekizumab every 2 weeks had a DLQI of 0 or 1, as did 54% on monthly dosing and 1.5% of controls.
The ixekizumab-treated groups also showed what Dr. Gottlieb described as “dramatic” improvements – in the 4+ to 5+ point range – in both the mental and physical component scores on the SF-36, another widely used quality of life measure.
Improvements in skin and self-reported joint symptoms appeared to correlate. Patients with a PASI 50 to less-than PASI 75 response had a mean 17-point reduction in joint pain scores, while those with a PASI 75 to less-than PASI 90 response averaged a 25.1-point improvement in joint pain, patients with a PASI 90 to less-than PASI 100 skin response averaged a 27.6-point reduction, and PASI 100 responders had a mean 30.3-point reduction in joint pain scores.
“Obviously, one needs to look at this more carefully in a phase III psoriatic arthritis study. That’ll provide a more robust answer. But this gives a hint,” the dermatologist said.
The UNCOVER program was sponsored by Eli Lilly. Dr. Gottlieb serves as an adviser to Lilly and numerous other pharmaceutical companies.
COPENHAGEN – Ixekizumab not only shows considerable promise for the treatment of moderate to severe psoriasis, it looks like it may be a winner for comorbid psoriatic arthritis, too.
The investigational IgG4 humanized monoclonal antibody directed against interleukin-17A brought marked improvements in joint pain, systemic inflammatory burden, and quality of life as well as skin disease in patients with both psoriasis and self-reported psoriatic arthritis in a combined analysis of three phase III clinical trials, Dr. Alice B. Gottlieb reported at the annual congress of the European Academy of Dermatology and Venereology.
Of the 3,126 patients with moderate to severe psoriasis who participated in the 12-week, phase III UNCOVER-1, -2, and -3 trials, 751 (24%) also had self-reported psoriatic arthritis; her analysis focused on them.
She was quick to note that the UNCOVER trials were primarily psoriasis studies that relied upon patient self-report of psoriatic arthritis. Nevertheless, it seems likely that the great majority of self-reported psoriatic arthritis patients really did have the rheumatologic disease, since the mean baseline C-reactive protein (CRP) level in that group was 8.43 mg/L, a level far higher than expected in patients with psoriasis only.
In any case, more-rigorous phase III studies of ixekizumab conducted specifically in patients with formally rheumatologist-diagnosed psoriatic arthritis and treated in rheumatology practices are due to be presented at the annual meeting of the American College of Rheumatology in November. And while Dr. Gottlieb wasn’t at liberty to discuss those results, she did hint that the data will be strongly positive.
“If you’re happy about these UNCOVER findings, you’ll be ecstatic about those,” predicted Dr. Gottlieb, professor of dermatology and dermatologist in chief at Tufts Medical Center, Boston.
Also coming up at the American College of Rheumatology meeting will be the results of the first-ever head-to-head comparison of an IL-17 inhibitor versus a tumor necrosis factor–alpha blocker in psoriatic arthritis patients. While at present most physicians consider a TNF inhibitor to be the treatment of choice in patients with psoriatic arthritis, that view may change as a result of the forthcoming comparative study, according to the dermatologist.
In each of the three phase III UNCOVER studies, patients were randomized to 12 weeks of subcutaneous ixekizumab at 80 mg every 2 or 4 weeks following a 160-mg loading dose, or to placebo. At baseline, the subgroup with self-reported psoriatic arthritis had a mean Psoriasis Area and Severity Index ( PASI) 0f about 21, a self-rated joint pain severity of 50 on a 0-100 scale, a CRP of 8.43 mg/L, and a Dermatology Life Quality Index (DLQI) score of 14.
Joint pain decreased dramatically in the two ixekizumab groups as early at 2 weeks into the trial, at which point, patients on treatment every 2 weeks averaged a 13.1-point reduction from baseline, with a similar 14.1-point drop noted in those on an every 4 weeks schedule. At week 12, the mean reductions from baseline were 25.2 and 26.8 points, compared with a 1.1-point increase in joint pain among placebo-treated controls.
Inflammatory burden plunged quickly, as evidenced by mean reductions in CRP of 4.63 mg/L and 4.33 mg/L at week 1 with biweekly and monthly dosing, respectively. These reductions were then maintained through week 12.
In terms of improvement in skin symptoms, with ixekizumab dosed every 2 weeks, the PASI 75 response was 89.8% at 12 weeks, the PASI 90 response was 69.3%, and the PASI 100 response (clear skin) was 37.1%. In patients treated every 4 weeks, the rates were 81.1%, 60.8%, and 34.7%.
“There’s good news in both groups, but I think the news is even better in the every-2-weeks group,” Dr. Gottlieb commented.
The mean 12-week decrease from baseline in DLQI was 11.8 points with biweekly dosing and 10.5 with 4-week dosing, compared with 0.8 points in controls. That’s impressive given that a 5-point reduction in DLQI is deemed clinically meaningful, the dermatologist observed. At 12 weeks, 56.5% of patients on ixekizumab every 2 weeks had a DLQI of 0 or 1, as did 54% on monthly dosing and 1.5% of controls.
The ixekizumab-treated groups also showed what Dr. Gottlieb described as “dramatic” improvements – in the 4+ to 5+ point range – in both the mental and physical component scores on the SF-36, another widely used quality of life measure.
Improvements in skin and self-reported joint symptoms appeared to correlate. Patients with a PASI 50 to less-than PASI 75 response had a mean 17-point reduction in joint pain scores, while those with a PASI 75 to less-than PASI 90 response averaged a 25.1-point improvement in joint pain, patients with a PASI 90 to less-than PASI 100 skin response averaged a 27.6-point reduction, and PASI 100 responders had a mean 30.3-point reduction in joint pain scores.
“Obviously, one needs to look at this more carefully in a phase III psoriatic arthritis study. That’ll provide a more robust answer. But this gives a hint,” the dermatologist said.
The UNCOVER program was sponsored by Eli Lilly. Dr. Gottlieb serves as an adviser to Lilly and numerous other pharmaceutical companies.
COPENHAGEN – Ixekizumab not only shows considerable promise for the treatment of moderate to severe psoriasis, it looks like it may be a winner for comorbid psoriatic arthritis, too.
The investigational IgG4 humanized monoclonal antibody directed against interleukin-17A brought marked improvements in joint pain, systemic inflammatory burden, and quality of life as well as skin disease in patients with both psoriasis and self-reported psoriatic arthritis in a combined analysis of three phase III clinical trials, Dr. Alice B. Gottlieb reported at the annual congress of the European Academy of Dermatology and Venereology.
Of the 3,126 patients with moderate to severe psoriasis who participated in the 12-week, phase III UNCOVER-1, -2, and -3 trials, 751 (24%) also had self-reported psoriatic arthritis; her analysis focused on them.
She was quick to note that the UNCOVER trials were primarily psoriasis studies that relied upon patient self-report of psoriatic arthritis. Nevertheless, it seems likely that the great majority of self-reported psoriatic arthritis patients really did have the rheumatologic disease, since the mean baseline C-reactive protein (CRP) level in that group was 8.43 mg/L, a level far higher than expected in patients with psoriasis only.
In any case, more-rigorous phase III studies of ixekizumab conducted specifically in patients with formally rheumatologist-diagnosed psoriatic arthritis and treated in rheumatology practices are due to be presented at the annual meeting of the American College of Rheumatology in November. And while Dr. Gottlieb wasn’t at liberty to discuss those results, she did hint that the data will be strongly positive.
“If you’re happy about these UNCOVER findings, you’ll be ecstatic about those,” predicted Dr. Gottlieb, professor of dermatology and dermatologist in chief at Tufts Medical Center, Boston.
Also coming up at the American College of Rheumatology meeting will be the results of the first-ever head-to-head comparison of an IL-17 inhibitor versus a tumor necrosis factor–alpha blocker in psoriatic arthritis patients. While at present most physicians consider a TNF inhibitor to be the treatment of choice in patients with psoriatic arthritis, that view may change as a result of the forthcoming comparative study, according to the dermatologist.
In each of the three phase III UNCOVER studies, patients were randomized to 12 weeks of subcutaneous ixekizumab at 80 mg every 2 or 4 weeks following a 160-mg loading dose, or to placebo. At baseline, the subgroup with self-reported psoriatic arthritis had a mean Psoriasis Area and Severity Index ( PASI) 0f about 21, a self-rated joint pain severity of 50 on a 0-100 scale, a CRP of 8.43 mg/L, and a Dermatology Life Quality Index (DLQI) score of 14.
Joint pain decreased dramatically in the two ixekizumab groups as early at 2 weeks into the trial, at which point, patients on treatment every 2 weeks averaged a 13.1-point reduction from baseline, with a similar 14.1-point drop noted in those on an every 4 weeks schedule. At week 12, the mean reductions from baseline were 25.2 and 26.8 points, compared with a 1.1-point increase in joint pain among placebo-treated controls.
Inflammatory burden plunged quickly, as evidenced by mean reductions in CRP of 4.63 mg/L and 4.33 mg/L at week 1 with biweekly and monthly dosing, respectively. These reductions were then maintained through week 12.
In terms of improvement in skin symptoms, with ixekizumab dosed every 2 weeks, the PASI 75 response was 89.8% at 12 weeks, the PASI 90 response was 69.3%, and the PASI 100 response (clear skin) was 37.1%. In patients treated every 4 weeks, the rates were 81.1%, 60.8%, and 34.7%.
“There’s good news in both groups, but I think the news is even better in the every-2-weeks group,” Dr. Gottlieb commented.
The mean 12-week decrease from baseline in DLQI was 11.8 points with biweekly dosing and 10.5 with 4-week dosing, compared with 0.8 points in controls. That’s impressive given that a 5-point reduction in DLQI is deemed clinically meaningful, the dermatologist observed. At 12 weeks, 56.5% of patients on ixekizumab every 2 weeks had a DLQI of 0 or 1, as did 54% on monthly dosing and 1.5% of controls.
The ixekizumab-treated groups also showed what Dr. Gottlieb described as “dramatic” improvements – in the 4+ to 5+ point range – in both the mental and physical component scores on the SF-36, another widely used quality of life measure.
Improvements in skin and self-reported joint symptoms appeared to correlate. Patients with a PASI 50 to less-than PASI 75 response had a mean 17-point reduction in joint pain scores, while those with a PASI 75 to less-than PASI 90 response averaged a 25.1-point improvement in joint pain, patients with a PASI 90 to less-than PASI 100 skin response averaged a 27.6-point reduction, and PASI 100 responders had a mean 30.3-point reduction in joint pain scores.
“Obviously, one needs to look at this more carefully in a phase III psoriatic arthritis study. That’ll provide a more robust answer. But this gives a hint,” the dermatologist said.
The UNCOVER program was sponsored by Eli Lilly. Dr. Gottlieb serves as an adviser to Lilly and numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: The IL-17A inhibitor ixekizumab appears to be dramatically effective in psoriatic arthritis as well as in psoriasis.
Major finding: Self-reported joint pain scores in psoriatic arthritis patients were cut in half after 12 weeks of ixekizumab while remaining unmoved in placebo-treated controls.
Data source: This was an analysis of treatment outcomes in 751 self-reported psoriatic arthritis patients who participated in three 12-week phase III randomized clinical trials of ixekizumab vs. placebo.
Disclosures: The UNCOVER clinical trials program was sponsored by Eli Lilly. The presenter serves as an adviser to Lilly and numerous other pharmaceutical companies.
Investigational Biologic Rocks Psoriasis World
COPENHAGEN – An investigational biologic agent that selectively inhibits the p19 subunit of interleukin-23 was the talk of the 2015 EADV congress based upon its striking outperformance of ustekinumab in a head-to-head phase II study in psoriasis patients.
Not only did the investigational agent, known for now as BI 655066, achieve substantially higher rates of PASI 90 – clear or almost clear skin – and PASI 100, but it did so much faster and maintained those stellar results far longer off treatment than with ustekinumab (Stelara), Dr. Kim A. Papp reported at the annual congress of the European Academy of Dermatology and Venereology.
“These data make an irrefutable case for IL-23 as instrumental in the expression of psoriasis,” declared Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“What these data show is that selective blockade of IL-23 p19 clearly provides a remarkable response, whether we measure it as PASI 75, PASI 90, or PASI 100, compared with ustekinumab, which provides a very robust response itself. And when we look at the safety profile – admittedly in a small number of patients and for a short period of time – we see there are no real differences in safety signals. Obviously, we’re very encouraged to move forward with long-term phase III investigations,” Dr. Papp added.
The multicenter, double-blind phase II study included 166 patients with moderate to severe plaque psoriasis who were randomized to one of four treatment arms: BI 655066 at 90 or 180 mg given subcutaneously at weeks 0, 4, and 16; weight-based ustekinumab at 45 or 90 mg given on the same schedule; or a single 18-mg dose of BI 655066. Dr. Papp presented the results at 36 weeks, fully 20 weeks after the final dose was given.
Significantly more patients taking the investigational agent achieved PASI targets than did those taking ustekinumab, a highly effective medication. Here are the eye-popping 36-week results:
• PASI 90: 81% with BI 655066 at 180 mg, 69% with the lower dose, 30% with ustekinumab, and 7% with a single 18-mg dose of BI 655066.
• PASI 100: 54% and 43% with high- and low-dose BI 655066, respectively, 15% with ustekinumab, and 0% with single-dose BI 655066.
• PASI 75: 93% and 88% with high- and low-dose BI 655066; 55% with ustekinumab; and 19% after single-dose BI 655066.
One of the most intriguing study findings, according to Dr. Papp, is that the slope of the PASI 100 response curve in BI 655066–treated patients was still rising at week 20, a month after the final dose.
“It suggests that if you were to continue treating these patients, you might anticipate an even higher response,” the dermatologist said.
Another notable finding was the accelerated speed of response to the investigational agent: 57 days after the first dose of BI 655066, 50% of patients had a PASI 90 response.
“That means from the time you initiate therapy, you can expect half of your patients to get to PASI 90 in less than 2 months time,” Dr. Papp observed.
In contrast, it took 117 days – basically twice as long – for half of the ustekinumab group to achieve PASI 90.
Turning to the durability of response, Dr. Papp noted that it took 169 days for half of patients in the ustekinumab group to lose their PASI 90 response after treatment stopped, compared with 225 days in the BI 655066 90-mg group. That endpoint was never reached in the BI 655066 180-mg group before the study’s end.
Audience reaction to the study results was “wow.”
“Obviously, the data are terrific. Your mother couldn’t have invented better data,” declared Dr. Alice B. Gottlieb, professor of dermatology and dermatologist in chief at Tufts Medical Center, Boston.
She posed a question: Why are the BI 655066 response rates so much higher and longer lasting than with other biologics, including ustekinumab, which inhibits both IL-23 and IL-12?
“I think one key factor in the durability of the response is the pathway,” Dr. Papp replied. “Exactly why blockade of IL-23 p19 leads to this durable clinical response is unclear, but we can posit that we’re depopulating the Th-17 cells. IL-23 is necessary for Th-17 cell survival. So if you block the water, you ain’t going to grow the grass. I’m speculating here, but I think we’re removing that clone of cells, and it’s only once we start to allow them to repopulate that we then see recurrence of the disease.”
BI 655066 is being developed by Boehringer Ingelheim. Dr. Papp serves as an investigator for and consultant to Boehringer Ingelheim and numerous other pharmaceutical companies.
COPENHAGEN – An investigational biologic agent that selectively inhibits the p19 subunit of interleukin-23 was the talk of the 2015 EADV congress based upon its striking outperformance of ustekinumab in a head-to-head phase II study in psoriasis patients.
Not only did the investigational agent, known for now as BI 655066, achieve substantially higher rates of PASI 90 – clear or almost clear skin – and PASI 100, but it did so much faster and maintained those stellar results far longer off treatment than with ustekinumab (Stelara), Dr. Kim A. Papp reported at the annual congress of the European Academy of Dermatology and Venereology.
“These data make an irrefutable case for IL-23 as instrumental in the expression of psoriasis,” declared Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“What these data show is that selective blockade of IL-23 p19 clearly provides a remarkable response, whether we measure it as PASI 75, PASI 90, or PASI 100, compared with ustekinumab, which provides a very robust response itself. And when we look at the safety profile – admittedly in a small number of patients and for a short period of time – we see there are no real differences in safety signals. Obviously, we’re very encouraged to move forward with long-term phase III investigations,” Dr. Papp added.
The multicenter, double-blind phase II study included 166 patients with moderate to severe plaque psoriasis who were randomized to one of four treatment arms: BI 655066 at 90 or 180 mg given subcutaneously at weeks 0, 4, and 16; weight-based ustekinumab at 45 or 90 mg given on the same schedule; or a single 18-mg dose of BI 655066. Dr. Papp presented the results at 36 weeks, fully 20 weeks after the final dose was given.
Significantly more patients taking the investigational agent achieved PASI targets than did those taking ustekinumab, a highly effective medication. Here are the eye-popping 36-week results:
• PASI 90: 81% with BI 655066 at 180 mg, 69% with the lower dose, 30% with ustekinumab, and 7% with a single 18-mg dose of BI 655066.
• PASI 100: 54% and 43% with high- and low-dose BI 655066, respectively, 15% with ustekinumab, and 0% with single-dose BI 655066.
• PASI 75: 93% and 88% with high- and low-dose BI 655066; 55% with ustekinumab; and 19% after single-dose BI 655066.
One of the most intriguing study findings, according to Dr. Papp, is that the slope of the PASI 100 response curve in BI 655066–treated patients was still rising at week 20, a month after the final dose.
“It suggests that if you were to continue treating these patients, you might anticipate an even higher response,” the dermatologist said.
Another notable finding was the accelerated speed of response to the investigational agent: 57 days after the first dose of BI 655066, 50% of patients had a PASI 90 response.
“That means from the time you initiate therapy, you can expect half of your patients to get to PASI 90 in less than 2 months time,” Dr. Papp observed.
In contrast, it took 117 days – basically twice as long – for half of the ustekinumab group to achieve PASI 90.
Turning to the durability of response, Dr. Papp noted that it took 169 days for half of patients in the ustekinumab group to lose their PASI 90 response after treatment stopped, compared with 225 days in the BI 655066 90-mg group. That endpoint was never reached in the BI 655066 180-mg group before the study’s end.
Audience reaction to the study results was “wow.”
“Obviously, the data are terrific. Your mother couldn’t have invented better data,” declared Dr. Alice B. Gottlieb, professor of dermatology and dermatologist in chief at Tufts Medical Center, Boston.
She posed a question: Why are the BI 655066 response rates so much higher and longer lasting than with other biologics, including ustekinumab, which inhibits both IL-23 and IL-12?
“I think one key factor in the durability of the response is the pathway,” Dr. Papp replied. “Exactly why blockade of IL-23 p19 leads to this durable clinical response is unclear, but we can posit that we’re depopulating the Th-17 cells. IL-23 is necessary for Th-17 cell survival. So if you block the water, you ain’t going to grow the grass. I’m speculating here, but I think we’re removing that clone of cells, and it’s only once we start to allow them to repopulate that we then see recurrence of the disease.”
BI 655066 is being developed by Boehringer Ingelheim. Dr. Papp serves as an investigator for and consultant to Boehringer Ingelheim and numerous other pharmaceutical companies.
COPENHAGEN – An investigational biologic agent that selectively inhibits the p19 subunit of interleukin-23 was the talk of the 2015 EADV congress based upon its striking outperformance of ustekinumab in a head-to-head phase II study in psoriasis patients.
Not only did the investigational agent, known for now as BI 655066, achieve substantially higher rates of PASI 90 – clear or almost clear skin – and PASI 100, but it did so much faster and maintained those stellar results far longer off treatment than with ustekinumab (Stelara), Dr. Kim A. Papp reported at the annual congress of the European Academy of Dermatology and Venereology.
“These data make an irrefutable case for IL-23 as instrumental in the expression of psoriasis,” declared Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“What these data show is that selective blockade of IL-23 p19 clearly provides a remarkable response, whether we measure it as PASI 75, PASI 90, or PASI 100, compared with ustekinumab, which provides a very robust response itself. And when we look at the safety profile – admittedly in a small number of patients and for a short period of time – we see there are no real differences in safety signals. Obviously, we’re very encouraged to move forward with long-term phase III investigations,” Dr. Papp added.
The multicenter, double-blind phase II study included 166 patients with moderate to severe plaque psoriasis who were randomized to one of four treatment arms: BI 655066 at 90 or 180 mg given subcutaneously at weeks 0, 4, and 16; weight-based ustekinumab at 45 or 90 mg given on the same schedule; or a single 18-mg dose of BI 655066. Dr. Papp presented the results at 36 weeks, fully 20 weeks after the final dose was given.
Significantly more patients taking the investigational agent achieved PASI targets than did those taking ustekinumab, a highly effective medication. Here are the eye-popping 36-week results:
• PASI 90: 81% with BI 655066 at 180 mg, 69% with the lower dose, 30% with ustekinumab, and 7% with a single 18-mg dose of BI 655066.
• PASI 100: 54% and 43% with high- and low-dose BI 655066, respectively, 15% with ustekinumab, and 0% with single-dose BI 655066.
• PASI 75: 93% and 88% with high- and low-dose BI 655066; 55% with ustekinumab; and 19% after single-dose BI 655066.
One of the most intriguing study findings, according to Dr. Papp, is that the slope of the PASI 100 response curve in BI 655066–treated patients was still rising at week 20, a month after the final dose.
“It suggests that if you were to continue treating these patients, you might anticipate an even higher response,” the dermatologist said.
Another notable finding was the accelerated speed of response to the investigational agent: 57 days after the first dose of BI 655066, 50% of patients had a PASI 90 response.
“That means from the time you initiate therapy, you can expect half of your patients to get to PASI 90 in less than 2 months time,” Dr. Papp observed.
In contrast, it took 117 days – basically twice as long – for half of the ustekinumab group to achieve PASI 90.
Turning to the durability of response, Dr. Papp noted that it took 169 days for half of patients in the ustekinumab group to lose their PASI 90 response after treatment stopped, compared with 225 days in the BI 655066 90-mg group. That endpoint was never reached in the BI 655066 180-mg group before the study’s end.
Audience reaction to the study results was “wow.”
“Obviously, the data are terrific. Your mother couldn’t have invented better data,” declared Dr. Alice B. Gottlieb, professor of dermatology and dermatologist in chief at Tufts Medical Center, Boston.
She posed a question: Why are the BI 655066 response rates so much higher and longer lasting than with other biologics, including ustekinumab, which inhibits both IL-23 and IL-12?
“I think one key factor in the durability of the response is the pathway,” Dr. Papp replied. “Exactly why blockade of IL-23 p19 leads to this durable clinical response is unclear, but we can posit that we’re depopulating the Th-17 cells. IL-23 is necessary for Th-17 cell survival. So if you block the water, you ain’t going to grow the grass. I’m speculating here, but I think we’re removing that clone of cells, and it’s only once we start to allow them to repopulate that we then see recurrence of the disease.”
BI 655066 is being developed by Boehringer Ingelheim. Dr. Papp serves as an investigator for and consultant to Boehringer Ingelheim and numerous other pharmaceutical companies.
AT THE EADV CONGRESS
EADV: Investigational biologic rocks psoriasis world
COPENHAGEN – An investigational biologic agent that selectively inhibits the p19 subunit of interleukin-23 was the talk of the 2015 EADV congress based upon its striking outperformance of ustekinumab in a head-to-head phase II study in psoriasis patients.
Not only did the investigational agent, known for now as BI 655066, achieve substantially higher rates of PASI 90 – clear or almost clear skin – and PASI 100, but it did so much faster and maintained those stellar results far longer off treatment than with ustekinumab (Stelara), Dr. Kim A. Papp reported at the annual congress of the European Academy of Dermatology and Venereology.
“These data make an irrefutable case for IL-23 as instrumental in the expression of psoriasis,” declared Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“What these data show is that selective blockade of IL-23 p19 clearly provides a remarkable response, whether we measure it as PASI 75, PASI 90, or PASI 100, compared with ustekinumab, which provides a very robust response itself. And when we look at the safety profile – admittedly in a small number of patients and for a short period of time – we see there are no real differences in safety signals. Obviously, we’re very encouraged to move forward with long-term phase III investigations,” Dr. Papp added.
The multicenter, double-blind phase II study included 166 patients with moderate to severe plaque psoriasis who were randomized to one of four treatment arms: BI 655066 at 90 or 180 mg given subcutaneously at weeks 0, 4, and 16; weight-based ustekinumab at 45 or 90 mg given on the same schedule; or a single 18-mg dose of BI 655066. Dr. Papp presented the results at 36 weeks, fully 20 weeks after the final dose was given.
Significantly more patients taking the investigational agent achieved PASI targets than did those taking ustekinumab, a highly effective medication. Here are the eye-popping 36-week results:
• PASI 90: 81% with BI 655066 at 180 mg, 69% with the lower dose, 30% with ustekinumab, and 7% with a single 18-mg dose of BI 655066.
• PASI 100: 54% and 43% with high- and low-dose BI 655066, respectively, 15% with ustekinumab, and 0% with single-dose BI 655066.
• PASI 75: 93% and 88% with high- and low-dose BI 655066; 55% with ustekinumab; and 19% after single-dose BI 655066.
One of the most intriguing study findings, according to Dr. Papp, is that the slope of the PASI 100 response curve in BI 655066–treated patients was still rising at week 20, a month after the final dose.
“It suggests that if you were to continue treating these patients, you might anticipate an even higher response,” the dermatologist said.
Another notable finding was the accelerated speed of response to the investigational agent: 57 days after the first dose of BI 655066, 50% of patients had a PASI 90 response.
“That means from the time you initiate therapy, you can expect half of your patients to get to PASI 90 in less than 2 months time,” Dr. Papp observed.
In contrast, it took 117 days – basically twice as long – for half of the ustekinumab group to achieve PASI 90.
Turning to the durability of response, Dr. Papp noted that it took 169 days for half of patients in the ustekinumab group to lose their PASI 90 response after treatment stopped, compared with 225 days in the BI 655066 90-mg group. That endpoint was never reached in the BI 655066 180-mg group before the study’s end.
Audience reaction to the study results was “wow.”
“Obviously, the data are terrific. Your mother couldn’t have invented better data,” declared Dr. Alice B. Gottlieb, professor of dermatology and dermatologist in chief at Tufts Medical Center, Boston.
She posed a question: Why are the BI 655066 response rates so much higher and longer lasting than with other biologics, including ustekinumab, which inhibits both IL-23 and IL-12?
“I think one key factor in the durability of the response is the pathway,” Dr. Papp replied. “Exactly why blockade of IL-23 p19 leads to this durable clinical response is unclear, but we can posit that we’re depopulating the Th-17 cells. IL-23 is necessary for Th-17 cell survival. So if you block the water, you ain’t going to grow the grass. I’m speculating here, but I think we’re removing that clone of cells, and it’s only once we start to allow them to repopulate that we then see recurrence of the disease.”
BI 655066 is being developed by Boehringer Ingelheim. Dr. Papp serves as an investigator for and consultant to Boehringer Ingelheim and numerous other pharmaceutical companies.
COPENHAGEN – An investigational biologic agent that selectively inhibits the p19 subunit of interleukin-23 was the talk of the 2015 EADV congress based upon its striking outperformance of ustekinumab in a head-to-head phase II study in psoriasis patients.
Not only did the investigational agent, known for now as BI 655066, achieve substantially higher rates of PASI 90 – clear or almost clear skin – and PASI 100, but it did so much faster and maintained those stellar results far longer off treatment than with ustekinumab (Stelara), Dr. Kim A. Papp reported at the annual congress of the European Academy of Dermatology and Venereology.
“These data make an irrefutable case for IL-23 as instrumental in the expression of psoriasis,” declared Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“What these data show is that selective blockade of IL-23 p19 clearly provides a remarkable response, whether we measure it as PASI 75, PASI 90, or PASI 100, compared with ustekinumab, which provides a very robust response itself. And when we look at the safety profile – admittedly in a small number of patients and for a short period of time – we see there are no real differences in safety signals. Obviously, we’re very encouraged to move forward with long-term phase III investigations,” Dr. Papp added.
The multicenter, double-blind phase II study included 166 patients with moderate to severe plaque psoriasis who were randomized to one of four treatment arms: BI 655066 at 90 or 180 mg given subcutaneously at weeks 0, 4, and 16; weight-based ustekinumab at 45 or 90 mg given on the same schedule; or a single 18-mg dose of BI 655066. Dr. Papp presented the results at 36 weeks, fully 20 weeks after the final dose was given.
Significantly more patients taking the investigational agent achieved PASI targets than did those taking ustekinumab, a highly effective medication. Here are the eye-popping 36-week results:
• PASI 90: 81% with BI 655066 at 180 mg, 69% with the lower dose, 30% with ustekinumab, and 7% with a single 18-mg dose of BI 655066.
• PASI 100: 54% and 43% with high- and low-dose BI 655066, respectively, 15% with ustekinumab, and 0% with single-dose BI 655066.
• PASI 75: 93% and 88% with high- and low-dose BI 655066; 55% with ustekinumab; and 19% after single-dose BI 655066.
One of the most intriguing study findings, according to Dr. Papp, is that the slope of the PASI 100 response curve in BI 655066–treated patients was still rising at week 20, a month after the final dose.
“It suggests that if you were to continue treating these patients, you might anticipate an even higher response,” the dermatologist said.
Another notable finding was the accelerated speed of response to the investigational agent: 57 days after the first dose of BI 655066, 50% of patients had a PASI 90 response.
“That means from the time you initiate therapy, you can expect half of your patients to get to PASI 90 in less than 2 months time,” Dr. Papp observed.
In contrast, it took 117 days – basically twice as long – for half of the ustekinumab group to achieve PASI 90.
Turning to the durability of response, Dr. Papp noted that it took 169 days for half of patients in the ustekinumab group to lose their PASI 90 response after treatment stopped, compared with 225 days in the BI 655066 90-mg group. That endpoint was never reached in the BI 655066 180-mg group before the study’s end.
Audience reaction to the study results was “wow.”
“Obviously, the data are terrific. Your mother couldn’t have invented better data,” declared Dr. Alice B. Gottlieb, professor of dermatology and dermatologist in chief at Tufts Medical Center, Boston.
She posed a question: Why are the BI 655066 response rates so much higher and longer lasting than with other biologics, including ustekinumab, which inhibits both IL-23 and IL-12?
“I think one key factor in the durability of the response is the pathway,” Dr. Papp replied. “Exactly why blockade of IL-23 p19 leads to this durable clinical response is unclear, but we can posit that we’re depopulating the Th-17 cells. IL-23 is necessary for Th-17 cell survival. So if you block the water, you ain’t going to grow the grass. I’m speculating here, but I think we’re removing that clone of cells, and it’s only once we start to allow them to repopulate that we then see recurrence of the disease.”
BI 655066 is being developed by Boehringer Ingelheim. Dr. Papp serves as an investigator for and consultant to Boehringer Ingelheim and numerous other pharmaceutical companies.
COPENHAGEN – An investigational biologic agent that selectively inhibits the p19 subunit of interleukin-23 was the talk of the 2015 EADV congress based upon its striking outperformance of ustekinumab in a head-to-head phase II study in psoriasis patients.
Not only did the investigational agent, known for now as BI 655066, achieve substantially higher rates of PASI 90 – clear or almost clear skin – and PASI 100, but it did so much faster and maintained those stellar results far longer off treatment than with ustekinumab (Stelara), Dr. Kim A. Papp reported at the annual congress of the European Academy of Dermatology and Venereology.
“These data make an irrefutable case for IL-23 as instrumental in the expression of psoriasis,” declared Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“What these data show is that selective blockade of IL-23 p19 clearly provides a remarkable response, whether we measure it as PASI 75, PASI 90, or PASI 100, compared with ustekinumab, which provides a very robust response itself. And when we look at the safety profile – admittedly in a small number of patients and for a short period of time – we see there are no real differences in safety signals. Obviously, we’re very encouraged to move forward with long-term phase III investigations,” Dr. Papp added.
The multicenter, double-blind phase II study included 166 patients with moderate to severe plaque psoriasis who were randomized to one of four treatment arms: BI 655066 at 90 or 180 mg given subcutaneously at weeks 0, 4, and 16; weight-based ustekinumab at 45 or 90 mg given on the same schedule; or a single 18-mg dose of BI 655066. Dr. Papp presented the results at 36 weeks, fully 20 weeks after the final dose was given.
Significantly more patients taking the investigational agent achieved PASI targets than did those taking ustekinumab, a highly effective medication. Here are the eye-popping 36-week results:
• PASI 90: 81% with BI 655066 at 180 mg, 69% with the lower dose, 30% with ustekinumab, and 7% with a single 18-mg dose of BI 655066.
• PASI 100: 54% and 43% with high- and low-dose BI 655066, respectively, 15% with ustekinumab, and 0% with single-dose BI 655066.
• PASI 75: 93% and 88% with high- and low-dose BI 655066; 55% with ustekinumab; and 19% after single-dose BI 655066.
One of the most intriguing study findings, according to Dr. Papp, is that the slope of the PASI 100 response curve in BI 655066–treated patients was still rising at week 20, a month after the final dose.
“It suggests that if you were to continue treating these patients, you might anticipate an even higher response,” the dermatologist said.
Another notable finding was the accelerated speed of response to the investigational agent: 57 days after the first dose of BI 655066, 50% of patients had a PASI 90 response.
“That means from the time you initiate therapy, you can expect half of your patients to get to PASI 90 in less than 2 months time,” Dr. Papp observed.
In contrast, it took 117 days – basically twice as long – for half of the ustekinumab group to achieve PASI 90.
Turning to the durability of response, Dr. Papp noted that it took 169 days for half of patients in the ustekinumab group to lose their PASI 90 response after treatment stopped, compared with 225 days in the BI 655066 90-mg group. That endpoint was never reached in the BI 655066 180-mg group before the study’s end.
Audience reaction to the study results was “wow.”
“Obviously, the data are terrific. Your mother couldn’t have invented better data,” declared Dr. Alice B. Gottlieb, professor of dermatology and dermatologist in chief at Tufts Medical Center, Boston.
She posed a question: Why are the BI 655066 response rates so much higher and longer lasting than with other biologics, including ustekinumab, which inhibits both IL-23 and IL-12?
“I think one key factor in the durability of the response is the pathway,” Dr. Papp replied. “Exactly why blockade of IL-23 p19 leads to this durable clinical response is unclear, but we can posit that we’re depopulating the Th-17 cells. IL-23 is necessary for Th-17 cell survival. So if you block the water, you ain’t going to grow the grass. I’m speculating here, but I think we’re removing that clone of cells, and it’s only once we start to allow them to repopulate that we then see recurrence of the disease.”
BI 655066 is being developed by Boehringer Ingelheim. Dr. Papp serves as an investigator for and consultant to Boehringer Ingelheim and numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: An investigational inhibitor of the p19 subunit of interleukin-23 is generating efficacy results never before seen in psoriasis.
Major finding: The PASI 90 response rate was 81% fully 20 weeks after the third and final dose of BI 655066 at 180 mg, compared with 30% for ustekinumab.
Data source: This prospective, multicenter, double-blind study included 166 patients with moderate to severe plaque psoriasis who were randomized to various doses of ustekinumab or BI 655066 and followed for 36 weeks.
Disclosures: The study was sponsored by Boehringer Ingelheim. The presenter serves as an investigator for and consultant to Boehringer Ingelheim and numerous other pharmaceutical companies.
EADV: Comorbid spondyloarthropathy common in hidradenitis suppurativa
COPENHAGEN – Back pain is surprisingly common in patients with hidradenitis suppurativa, and more than half of affected patients showed MRI evidence of axial spondyloarthropathy, Dr. Sylke Schneider-Burrus reported at the Annual Congress of the European Academy of Dermatology and Venereology.
“Our study demonstrates that back pain and spondyloarthropathy are very common among hidradenitis suppurativa patients and that neither history nor clinical parameters provide any hints for the presence of spondyloarthropathy. Therefore, we strongly suggest that hidradenitis suppurativa patients should be evaluated for spondyloarthropathy and affected patients should be treated systemically with TNF-alpha blockers in order to avoid chronic joint alterations,” said Dr. Schneider-Burrus, a dermatologist at Charite University Hospital in Berlin.
Hidradenitis suppurativa (HS) is a chronic, recurrent, scarring, inflammatory skin disease of the hair follicles. It causes painful, purulent, foul-smelling fistulating sinuses in the axillae, groin, and perianal region.
Because several other chronic inflammatory diseases affecting epithelial tissue have been associated with increased rates of axial spondyloarthropathy – notably, Crohn’s disease, ulcerative colitis, and psoriasis – Dr. Schneider-Burrus and coinvestigators wondered whether that might true of HS as well.
She presented a survey of 100 HS patients. To her surprise, fully 71% indicated they suffer from back pain, with lower back complaints predominating.
Forty-eight HS patients with back pain consented to undergo a pelvic MRI exam. Fifteen of the 48 (32%) showed clear MRI evidence of spondyloarthropathy, including sacroiliac erosions and subchondral sclerosis, while another 12 showed active sacroiliac synovitis and other acute inflammatory changes.
No significant differences were found between HS patients with and without axial spondyloarthropathy in terms of age at onset of HS, disease duration, HS severity as reflected in Sartorius score, age at MRI, body mass index, or smoking status.
Dr. Schneider-Burrus reported serving as a paid investigator for and consultant to Novartis and AbbVie.
COPENHAGEN – Back pain is surprisingly common in patients with hidradenitis suppurativa, and more than half of affected patients showed MRI evidence of axial spondyloarthropathy, Dr. Sylke Schneider-Burrus reported at the Annual Congress of the European Academy of Dermatology and Venereology.
“Our study demonstrates that back pain and spondyloarthropathy are very common among hidradenitis suppurativa patients and that neither history nor clinical parameters provide any hints for the presence of spondyloarthropathy. Therefore, we strongly suggest that hidradenitis suppurativa patients should be evaluated for spondyloarthropathy and affected patients should be treated systemically with TNF-alpha blockers in order to avoid chronic joint alterations,” said Dr. Schneider-Burrus, a dermatologist at Charite University Hospital in Berlin.
Hidradenitis suppurativa (HS) is a chronic, recurrent, scarring, inflammatory skin disease of the hair follicles. It causes painful, purulent, foul-smelling fistulating sinuses in the axillae, groin, and perianal region.
Because several other chronic inflammatory diseases affecting epithelial tissue have been associated with increased rates of axial spondyloarthropathy – notably, Crohn’s disease, ulcerative colitis, and psoriasis – Dr. Schneider-Burrus and coinvestigators wondered whether that might true of HS as well.
She presented a survey of 100 HS patients. To her surprise, fully 71% indicated they suffer from back pain, with lower back complaints predominating.
Forty-eight HS patients with back pain consented to undergo a pelvic MRI exam. Fifteen of the 48 (32%) showed clear MRI evidence of spondyloarthropathy, including sacroiliac erosions and subchondral sclerosis, while another 12 showed active sacroiliac synovitis and other acute inflammatory changes.
No significant differences were found between HS patients with and without axial spondyloarthropathy in terms of age at onset of HS, disease duration, HS severity as reflected in Sartorius score, age at MRI, body mass index, or smoking status.
Dr. Schneider-Burrus reported serving as a paid investigator for and consultant to Novartis and AbbVie.
COPENHAGEN – Back pain is surprisingly common in patients with hidradenitis suppurativa, and more than half of affected patients showed MRI evidence of axial spondyloarthropathy, Dr. Sylke Schneider-Burrus reported at the Annual Congress of the European Academy of Dermatology and Venereology.
“Our study demonstrates that back pain and spondyloarthropathy are very common among hidradenitis suppurativa patients and that neither history nor clinical parameters provide any hints for the presence of spondyloarthropathy. Therefore, we strongly suggest that hidradenitis suppurativa patients should be evaluated for spondyloarthropathy and affected patients should be treated systemically with TNF-alpha blockers in order to avoid chronic joint alterations,” said Dr. Schneider-Burrus, a dermatologist at Charite University Hospital in Berlin.
Hidradenitis suppurativa (HS) is a chronic, recurrent, scarring, inflammatory skin disease of the hair follicles. It causes painful, purulent, foul-smelling fistulating sinuses in the axillae, groin, and perianal region.
Because several other chronic inflammatory diseases affecting epithelial tissue have been associated with increased rates of axial spondyloarthropathy – notably, Crohn’s disease, ulcerative colitis, and psoriasis – Dr. Schneider-Burrus and coinvestigators wondered whether that might true of HS as well.
She presented a survey of 100 HS patients. To her surprise, fully 71% indicated they suffer from back pain, with lower back complaints predominating.
Forty-eight HS patients with back pain consented to undergo a pelvic MRI exam. Fifteen of the 48 (32%) showed clear MRI evidence of spondyloarthropathy, including sacroiliac erosions and subchondral sclerosis, while another 12 showed active sacroiliac synovitis and other acute inflammatory changes.
No significant differences were found between HS patients with and without axial spondyloarthropathy in terms of age at onset of HS, disease duration, HS severity as reflected in Sartorius score, age at MRI, body mass index, or smoking status.
Dr. Schneider-Burrus reported serving as a paid investigator for and consultant to Novartis and AbbVie.
AT THE EADV CONGRESS
Key clinical point: Axial spondyloarthropathy is extremely common in patients with hidradenitis suppurativa.
Major finding: Seventy-one percent of surveyed hidradenitis suppurativa patients reported suffering from back pain, and 56% of affected patients showed MRI evidence of axial spondyloarthropathy.
Data source: A back pain survey of 100 patients with hidradenitis suppurativa along with pelvic MRI exams in the 48 who reported back pain.
Disclosures: The presenter reported serving as a paid investigator for and consultant to Novartis and AbbVie.
EADV: Prophylactic photodynamic therapy benefits transplant recipients
COPENHAGEN – Twice-yearly prophylactic photodynamic therapy for primary prevention of actinic keratoses and squamous cell carcinomas is a novel and effective strategy that addresses the problem of accelerated photocarcinogenesis in organ transplant recipients, according to an interim analysis of a multinational, randomized, controlled trial.
“The overall aim is to prevent squamous cell carcinoma development. Photodynamic therapy is well established for secondary prevention of further AKs, and these very early data show that it can also be used for primary prevention in very high-risk patients,” Dr. Katrine Togsverd-Bo said at the annual congress of the European Academy of Dermatology and Venereology.
Accelerated carcinogenesis on sun-exposed skin is a major concern in organ transplant recipients (OTRs). They experience early onset of multiple AKs, with field cancerization and up to a 100-fold increased risk of squamous cell carcinomas (SCCs). Moreover, their SCCs are at substantially greater risk of metastasis than SCCs occurring in the general population, noted Dr. Togsverd-Bo of Bispebjerg Hospital and the University of Copenhagen.
She presented an interim analysis of an ongoing 5-year prospective randomized trial in 50 renal transplant recipients at academic dermatology centers in Copenhagen, Oslo, and Gothenburg, Sweden. All participants had clinically normal-appearing skin at baseline, with no history of AKs or SCCs. They are undergoing twice-yearly, split-side photodynamic therapy (PDT) on the face, forearm, and hand, with the opposite side serving as the untreated control.
To date, 25 patients have completed 3 years of the study. At 3 years of prospective follow-up by blinded evaluators, 50% of patients had AKs on their untreated side, compared with 26% on the prophylactic PDT side. The collective number of AKs on untreated skin was 43, compared with just 8 AKs on PDT-treated skin. Seven patients had AKs only on their untreated side, six had AKs on both sides, and none had any AKs only on their PDT-treated side.
The twice-yearly prophylactic PDT regimen consists of a 3-hour application of 20% methyl aminolevulinate as a photosensitizer followed by applications of a conventional LED light at 37 J/cm2.
Dr. Togsverd-Bo reported having no financial conflicts regarding her study.
COPENHAGEN – Twice-yearly prophylactic photodynamic therapy for primary prevention of actinic keratoses and squamous cell carcinomas is a novel and effective strategy that addresses the problem of accelerated photocarcinogenesis in organ transplant recipients, according to an interim analysis of a multinational, randomized, controlled trial.
“The overall aim is to prevent squamous cell carcinoma development. Photodynamic therapy is well established for secondary prevention of further AKs, and these very early data show that it can also be used for primary prevention in very high-risk patients,” Dr. Katrine Togsverd-Bo said at the annual congress of the European Academy of Dermatology and Venereology.
Accelerated carcinogenesis on sun-exposed skin is a major concern in organ transplant recipients (OTRs). They experience early onset of multiple AKs, with field cancerization and up to a 100-fold increased risk of squamous cell carcinomas (SCCs). Moreover, their SCCs are at substantially greater risk of metastasis than SCCs occurring in the general population, noted Dr. Togsverd-Bo of Bispebjerg Hospital and the University of Copenhagen.
She presented an interim analysis of an ongoing 5-year prospective randomized trial in 50 renal transplant recipients at academic dermatology centers in Copenhagen, Oslo, and Gothenburg, Sweden. All participants had clinically normal-appearing skin at baseline, with no history of AKs or SCCs. They are undergoing twice-yearly, split-side photodynamic therapy (PDT) on the face, forearm, and hand, with the opposite side serving as the untreated control.
To date, 25 patients have completed 3 years of the study. At 3 years of prospective follow-up by blinded evaluators, 50% of patients had AKs on their untreated side, compared with 26% on the prophylactic PDT side. The collective number of AKs on untreated skin was 43, compared with just 8 AKs on PDT-treated skin. Seven patients had AKs only on their untreated side, six had AKs on both sides, and none had any AKs only on their PDT-treated side.
The twice-yearly prophylactic PDT regimen consists of a 3-hour application of 20% methyl aminolevulinate as a photosensitizer followed by applications of a conventional LED light at 37 J/cm2.
Dr. Togsverd-Bo reported having no financial conflicts regarding her study.
COPENHAGEN – Twice-yearly prophylactic photodynamic therapy for primary prevention of actinic keratoses and squamous cell carcinomas is a novel and effective strategy that addresses the problem of accelerated photocarcinogenesis in organ transplant recipients, according to an interim analysis of a multinational, randomized, controlled trial.
“The overall aim is to prevent squamous cell carcinoma development. Photodynamic therapy is well established for secondary prevention of further AKs, and these very early data show that it can also be used for primary prevention in very high-risk patients,” Dr. Katrine Togsverd-Bo said at the annual congress of the European Academy of Dermatology and Venereology.
Accelerated carcinogenesis on sun-exposed skin is a major concern in organ transplant recipients (OTRs). They experience early onset of multiple AKs, with field cancerization and up to a 100-fold increased risk of squamous cell carcinomas (SCCs). Moreover, their SCCs are at substantially greater risk of metastasis than SCCs occurring in the general population, noted Dr. Togsverd-Bo of Bispebjerg Hospital and the University of Copenhagen.
She presented an interim analysis of an ongoing 5-year prospective randomized trial in 50 renal transplant recipients at academic dermatology centers in Copenhagen, Oslo, and Gothenburg, Sweden. All participants had clinically normal-appearing skin at baseline, with no history of AKs or SCCs. They are undergoing twice-yearly, split-side photodynamic therapy (PDT) on the face, forearm, and hand, with the opposite side serving as the untreated control.
To date, 25 patients have completed 3 years of the study. At 3 years of prospective follow-up by blinded evaluators, 50% of patients had AKs on their untreated side, compared with 26% on the prophylactic PDT side. The collective number of AKs on untreated skin was 43, compared with just 8 AKs on PDT-treated skin. Seven patients had AKs only on their untreated side, six had AKs on both sides, and none had any AKs only on their PDT-treated side.
The twice-yearly prophylactic PDT regimen consists of a 3-hour application of 20% methyl aminolevulinate as a photosensitizer followed by applications of a conventional LED light at 37 J/cm2.
Dr. Togsverd-Bo reported having no financial conflicts regarding her study.
AT THE EADV CONGRESS
Key clinical point: Prophylactic photodynamic therapy is a new and effective strategy for primary prevention of actinic keratoses and squamous cell carcinomas in organ transplant recipients.
Major finding: At 3 years of follow-up, 25 renal transplant recipients collectively had 8 actinic keratoses on the side of their face, forearms, and hands treated with twice-yearly prophylactic photodynamic therapy, compared with 43 AKs on the untreated control side.
Data source: This is an interim 3-year analysis from an ongoing 5-year prospective multinational, randomized, controlled trial involving 50 renal transplant recipients.
Disclosures: The presenter reported having no financial conflicts regarding this ongoing study.
EADV: Latest gruesome twosome: Psoriasis spawns renal disease
COPENHAGEN – Severe psoriasis is an independent risk factor for serious renal disease – and psoriatic arthritis multiplies that risk even further, according to a Taiwanese national study.
“We think this might be related to the higher inflammatory status associated with psoriatic arthritis. For those with mild psoriasis without psoriatic arthritis, the risk was not increased,” Dr. Ching-Chi Chi said at the annual congress of the European Academy of Dermatology and Venereology.
The past decade has brought a mountain of data documenting that psoriasis, a chronic inflammatory dermatosis, is associated with increased risks of cardiovascular disease, diabetes, and other metabolic abnormalities, noted Dr. Chi, professor of dermatology at Chang Gung University in Taoyuan.
He presented a nationwide retrospective cohort study utilizing Taiwan’s national health insurance system, which covers more than 99% of the population. The study included 4,633 patients with psoriasis diagnosed by a dermatologist or rheumatologist since 2005 and 922,534 controls. A total of 453 patients were classified as having severe psoriasis based upon ever having received systemic treatment or phototherapy; the other 4,180 were classified as having mild psoriasis.
Among the controls there were 36,615 incident cases of chronic kidney disease (CKD) and 9,493 new cases of end-stage renal disease (ESRD) during the study period, translating to rates of 676 per 1,000,000 person-years and 172 per 1,000,000 person-years, respectively. In contrast, patients with severe psoriasis had an incident CKD rate of 2,160 per 1,000,000 person-years and an ESRD rate of 876 per 1,000,000 person-years.
In a Cox regression analysis adjusted for potential confounders including age, gender, comorbid cardiovascular disease, gout, hypertension, dyslipidemia, and use of NSAIDs, severe psoriasis was independently associated with a 1.9-fold increased risk of new-onset CKD and a 3-fold increased risk of ESRD.
Although mild psoriasis alone wasn’t associated with increased risk of renal disease, the 254 patients with mild psoriasis and psoriatic arthritis were at 1.3-fold increased risk of CKD and 2.5-fold increased risk of incident ESRD, compared with controls. Moreover, among the 93 patients with severe psoriasis plus psoriatic arthritis, the risks of incident CKD and ESRD were increased by 2.6- and 6.7-fold over the control subjects.
The new Taiwanese national study confirms earlier work by Dr. Joel M. Gelfand and his coinvestigators at the University of Pennsylvania, Philadelphia. Their population-based cohort study and nested cross-sectional study utilizing a huge U.K. electronic medical records database analyzed 136,529 patients with mild psoriasis, 7,354 with severe psoriasis, and nearly 690,000 matched controls and concluded that moderate to severe psoriasis is associated with an increased risk of stage 3-5 chronic kidney disease (BMJ. 2013 Oct 15;347:f5961).
He reported having no financial conflicts regarding this study.
This article was updated October 29, 2015.
COPENHAGEN – Severe psoriasis is an independent risk factor for serious renal disease – and psoriatic arthritis multiplies that risk even further, according to a Taiwanese national study.
“We think this might be related to the higher inflammatory status associated with psoriatic arthritis. For those with mild psoriasis without psoriatic arthritis, the risk was not increased,” Dr. Ching-Chi Chi said at the annual congress of the European Academy of Dermatology and Venereology.
The past decade has brought a mountain of data documenting that psoriasis, a chronic inflammatory dermatosis, is associated with increased risks of cardiovascular disease, diabetes, and other metabolic abnormalities, noted Dr. Chi, professor of dermatology at Chang Gung University in Taoyuan.
He presented a nationwide retrospective cohort study utilizing Taiwan’s national health insurance system, which covers more than 99% of the population. The study included 4,633 patients with psoriasis diagnosed by a dermatologist or rheumatologist since 2005 and 922,534 controls. A total of 453 patients were classified as having severe psoriasis based upon ever having received systemic treatment or phototherapy; the other 4,180 were classified as having mild psoriasis.
Among the controls there were 36,615 incident cases of chronic kidney disease (CKD) and 9,493 new cases of end-stage renal disease (ESRD) during the study period, translating to rates of 676 per 1,000,000 person-years and 172 per 1,000,000 person-years, respectively. In contrast, patients with severe psoriasis had an incident CKD rate of 2,160 per 1,000,000 person-years and an ESRD rate of 876 per 1,000,000 person-years.
In a Cox regression analysis adjusted for potential confounders including age, gender, comorbid cardiovascular disease, gout, hypertension, dyslipidemia, and use of NSAIDs, severe psoriasis was independently associated with a 1.9-fold increased risk of new-onset CKD and a 3-fold increased risk of ESRD.
Although mild psoriasis alone wasn’t associated with increased risk of renal disease, the 254 patients with mild psoriasis and psoriatic arthritis were at 1.3-fold increased risk of CKD and 2.5-fold increased risk of incident ESRD, compared with controls. Moreover, among the 93 patients with severe psoriasis plus psoriatic arthritis, the risks of incident CKD and ESRD were increased by 2.6- and 6.7-fold over the control subjects.
The new Taiwanese national study confirms earlier work by Dr. Joel M. Gelfand and his coinvestigators at the University of Pennsylvania, Philadelphia. Their population-based cohort study and nested cross-sectional study utilizing a huge U.K. electronic medical records database analyzed 136,529 patients with mild psoriasis, 7,354 with severe psoriasis, and nearly 690,000 matched controls and concluded that moderate to severe psoriasis is associated with an increased risk of stage 3-5 chronic kidney disease (BMJ. 2013 Oct 15;347:f5961).
He reported having no financial conflicts regarding this study.
This article was updated October 29, 2015.
COPENHAGEN – Severe psoriasis is an independent risk factor for serious renal disease – and psoriatic arthritis multiplies that risk even further, according to a Taiwanese national study.
“We think this might be related to the higher inflammatory status associated with psoriatic arthritis. For those with mild psoriasis without psoriatic arthritis, the risk was not increased,” Dr. Ching-Chi Chi said at the annual congress of the European Academy of Dermatology and Venereology.
The past decade has brought a mountain of data documenting that psoriasis, a chronic inflammatory dermatosis, is associated with increased risks of cardiovascular disease, diabetes, and other metabolic abnormalities, noted Dr. Chi, professor of dermatology at Chang Gung University in Taoyuan.
He presented a nationwide retrospective cohort study utilizing Taiwan’s national health insurance system, which covers more than 99% of the population. The study included 4,633 patients with psoriasis diagnosed by a dermatologist or rheumatologist since 2005 and 922,534 controls. A total of 453 patients were classified as having severe psoriasis based upon ever having received systemic treatment or phototherapy; the other 4,180 were classified as having mild psoriasis.
Among the controls there were 36,615 incident cases of chronic kidney disease (CKD) and 9,493 new cases of end-stage renal disease (ESRD) during the study period, translating to rates of 676 per 1,000,000 person-years and 172 per 1,000,000 person-years, respectively. In contrast, patients with severe psoriasis had an incident CKD rate of 2,160 per 1,000,000 person-years and an ESRD rate of 876 per 1,000,000 person-years.
In a Cox regression analysis adjusted for potential confounders including age, gender, comorbid cardiovascular disease, gout, hypertension, dyslipidemia, and use of NSAIDs, severe psoriasis was independently associated with a 1.9-fold increased risk of new-onset CKD and a 3-fold increased risk of ESRD.
Although mild psoriasis alone wasn’t associated with increased risk of renal disease, the 254 patients with mild psoriasis and psoriatic arthritis were at 1.3-fold increased risk of CKD and 2.5-fold increased risk of incident ESRD, compared with controls. Moreover, among the 93 patients with severe psoriasis plus psoriatic arthritis, the risks of incident CKD and ESRD were increased by 2.6- and 6.7-fold over the control subjects.
The new Taiwanese national study confirms earlier work by Dr. Joel M. Gelfand and his coinvestigators at the University of Pennsylvania, Philadelphia. Their population-based cohort study and nested cross-sectional study utilizing a huge U.K. electronic medical records database analyzed 136,529 patients with mild psoriasis, 7,354 with severe psoriasis, and nearly 690,000 matched controls and concluded that moderate to severe psoriasis is associated with an increased risk of stage 3-5 chronic kidney disease (BMJ. 2013 Oct 15;347:f5961).
He reported having no financial conflicts regarding this study.
This article was updated October 29, 2015.
AT THE EADV CONGRESS
Key clinical point: Patients with severe but not mild psoriasis are at increased risk of new-onset chronic kidney disease and end-stage renal disease.
Major finding: Severe psoriasis was independently associated with a 1.9-fold increased risk of new-onset CKD and a 3-fold increased risk of ESRD. Comorbid psoriatic arthritis further boosted those risks.
Data source: This retrospective cohort study included 4,633 consecutive patients diagnosed with psoriasis in Taiwan.
Disclosures: Dr. Chi reported having no financial conflicts regarding this government-funded study.
EADV: Long-term weight loss curbs psoriasis severity
COPENHAGEN – Long-term weight loss achieved by obese patients with psoriasis provides long-lasting reductions in psoriasis severity, Dr. Peter Jensen reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a prospective 64-week observational study that was a follow-up to his earlier 16-week randomized, controlled clinical trial. The earlier study (JAMA Dermatol. 2013 Jul;149[7]:795-801) generated enormous interest because it provided the first level I evidence that weight loss by obese psoriasis patients brings clinically meaningful improvement in their skin disease as well as achieving well-established metabolic and cardiovascular risk reduction benefits.
In the randomized trial, 60 consecutive obese patients with psoriasis and a mean baseline Psoriasis Area and Severity Index (PASI) of 5.4 and a body mass index of 34.4 kg/m2 were assigned to a dietary intervention or given general advice to eat healthy foods. The intervention entailed 8 weeks of a low-energy liquid diet featuring a daily total nutrition intake of 800-1,000 kcal in the form of fortified drinks and soups. This was followed by 8 weeks in which regular foods were reintroduced at 1,200 kcal/day. At week 16, the intervention group had lost an average of 15.4 kg more body weight than controls. Their mean PASI was 2.0 points lower as well, according to Dr. Jensen, a dermatologist at the University of Copenhagen.
In the observational follow-up study, the original control group was offered the opportunity to participate in the 16-week weight loss intervention. All patients were then prospectively followed for 48 weeks after completing the dietary intervention. Psoriasis medications weren’t changed during the study period. The questions Dr. Jensen and coinvestigators sought to answer through the follow-up study were, first, can patients keep the weight off long term without a structured maintenance program and, if so, do their PASI scores stay low or do they creep back up?
Only 32 of the original 60 patients completed the full 64-week study. Among the completers, as is typical in weight loss studies, there was a gradual weight regain. Nonetheless, at 64 weeks, patients still maintained a mean 10-kg weight loss, compared with baseline, or two-thirds of the weight loss achieved during the 16-week dietary intervention.
Most importantly from a dermatologic perspective, the positive effect upon disease severity was maintained despite the regain of one-third of the initial weight loss, with patients showing a clinically important mean 3-point reduction in PASI, compared with baseline, Dr. Jensen noted.
Audience members were enthusiastic about the study findings but asked if the results are widely applicable. In other words, was this an unusually highly motivated group of patients?
“Our experience is that people really would like to try this. It was no problem to get participation,” according to Dr. Jensen.
Study limitations included the small sample size and the fact that this was a patient cohort with relatively mild psoriasis. In the future, it would be informative to study the effects of weight loss in obese patients with higher baseline PASI scores, the dermatologist said.
The study was funded by the Danish Academy of Dermatology and various research foundations. Dr. Jensen reported having no financial conflicts.
COPENHAGEN – Long-term weight loss achieved by obese patients with psoriasis provides long-lasting reductions in psoriasis severity, Dr. Peter Jensen reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a prospective 64-week observational study that was a follow-up to his earlier 16-week randomized, controlled clinical trial. The earlier study (JAMA Dermatol. 2013 Jul;149[7]:795-801) generated enormous interest because it provided the first level I evidence that weight loss by obese psoriasis patients brings clinically meaningful improvement in their skin disease as well as achieving well-established metabolic and cardiovascular risk reduction benefits.
In the randomized trial, 60 consecutive obese patients with psoriasis and a mean baseline Psoriasis Area and Severity Index (PASI) of 5.4 and a body mass index of 34.4 kg/m2 were assigned to a dietary intervention or given general advice to eat healthy foods. The intervention entailed 8 weeks of a low-energy liquid diet featuring a daily total nutrition intake of 800-1,000 kcal in the form of fortified drinks and soups. This was followed by 8 weeks in which regular foods were reintroduced at 1,200 kcal/day. At week 16, the intervention group had lost an average of 15.4 kg more body weight than controls. Their mean PASI was 2.0 points lower as well, according to Dr. Jensen, a dermatologist at the University of Copenhagen.
In the observational follow-up study, the original control group was offered the opportunity to participate in the 16-week weight loss intervention. All patients were then prospectively followed for 48 weeks after completing the dietary intervention. Psoriasis medications weren’t changed during the study period. The questions Dr. Jensen and coinvestigators sought to answer through the follow-up study were, first, can patients keep the weight off long term without a structured maintenance program and, if so, do their PASI scores stay low or do they creep back up?
Only 32 of the original 60 patients completed the full 64-week study. Among the completers, as is typical in weight loss studies, there was a gradual weight regain. Nonetheless, at 64 weeks, patients still maintained a mean 10-kg weight loss, compared with baseline, or two-thirds of the weight loss achieved during the 16-week dietary intervention.
Most importantly from a dermatologic perspective, the positive effect upon disease severity was maintained despite the regain of one-third of the initial weight loss, with patients showing a clinically important mean 3-point reduction in PASI, compared with baseline, Dr. Jensen noted.
Audience members were enthusiastic about the study findings but asked if the results are widely applicable. In other words, was this an unusually highly motivated group of patients?
“Our experience is that people really would like to try this. It was no problem to get participation,” according to Dr. Jensen.
Study limitations included the small sample size and the fact that this was a patient cohort with relatively mild psoriasis. In the future, it would be informative to study the effects of weight loss in obese patients with higher baseline PASI scores, the dermatologist said.
The study was funded by the Danish Academy of Dermatology and various research foundations. Dr. Jensen reported having no financial conflicts.
COPENHAGEN – Long-term weight loss achieved by obese patients with psoriasis provides long-lasting reductions in psoriasis severity, Dr. Peter Jensen reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a prospective 64-week observational study that was a follow-up to his earlier 16-week randomized, controlled clinical trial. The earlier study (JAMA Dermatol. 2013 Jul;149[7]:795-801) generated enormous interest because it provided the first level I evidence that weight loss by obese psoriasis patients brings clinically meaningful improvement in their skin disease as well as achieving well-established metabolic and cardiovascular risk reduction benefits.
In the randomized trial, 60 consecutive obese patients with psoriasis and a mean baseline Psoriasis Area and Severity Index (PASI) of 5.4 and a body mass index of 34.4 kg/m2 were assigned to a dietary intervention or given general advice to eat healthy foods. The intervention entailed 8 weeks of a low-energy liquid diet featuring a daily total nutrition intake of 800-1,000 kcal in the form of fortified drinks and soups. This was followed by 8 weeks in which regular foods were reintroduced at 1,200 kcal/day. At week 16, the intervention group had lost an average of 15.4 kg more body weight than controls. Their mean PASI was 2.0 points lower as well, according to Dr. Jensen, a dermatologist at the University of Copenhagen.
In the observational follow-up study, the original control group was offered the opportunity to participate in the 16-week weight loss intervention. All patients were then prospectively followed for 48 weeks after completing the dietary intervention. Psoriasis medications weren’t changed during the study period. The questions Dr. Jensen and coinvestigators sought to answer through the follow-up study were, first, can patients keep the weight off long term without a structured maintenance program and, if so, do their PASI scores stay low or do they creep back up?
Only 32 of the original 60 patients completed the full 64-week study. Among the completers, as is typical in weight loss studies, there was a gradual weight regain. Nonetheless, at 64 weeks, patients still maintained a mean 10-kg weight loss, compared with baseline, or two-thirds of the weight loss achieved during the 16-week dietary intervention.
Most importantly from a dermatologic perspective, the positive effect upon disease severity was maintained despite the regain of one-third of the initial weight loss, with patients showing a clinically important mean 3-point reduction in PASI, compared with baseline, Dr. Jensen noted.
Audience members were enthusiastic about the study findings but asked if the results are widely applicable. In other words, was this an unusually highly motivated group of patients?
“Our experience is that people really would like to try this. It was no problem to get participation,” according to Dr. Jensen.
Study limitations included the small sample size and the fact that this was a patient cohort with relatively mild psoriasis. In the future, it would be informative to study the effects of weight loss in obese patients with higher baseline PASI scores, the dermatologist said.
The study was funded by the Danish Academy of Dermatology and various research foundations. Dr. Jensen reported having no financial conflicts.
AT THE EADV CONGRESS
Key clinical point: Long-term weight loss by obese patients with psoriasi pays dividends in terms of sustained clinically meaningful reduction in PASI scores.
Major finding: At 64 weeks of follow-up, obese patients were able to maintain two-thirds of the mean 15.4-kg weight loss achieved through a 16-week dietary intervention, and their mean PASI scores were 3 points lower than the mean score of 5.4 points at baseline.
Data source: This was a prospective observational study of 32 obese psoriasis patients who completed 48 weeks of additional follow-up after a 16-week low-energy dietary intervention.
Disclosures: The study was funded by the Danish Academy of Dermatology and various research foundations. The presenter reported having no financial conflicts.
EADV: Novel topical crisaborole shines in atopic dermatitis
COPENHAGEN – The nonsteroidal phosphodiesterase-4 inhibitor crisaborole aced all Food and Drug Administration–required efficacy and safety endpoints as a topical treatment for atopic dermatitis, according to results from a pair of pivotal phase III randomized trials.
“This is a fairly rapidly effective treatment,” explained Dr. Mark G. Lebwohl, who presented the findings at the annual congress of the European Academy of Dermatology and Venereology. “It has a favorable safety profile and has been studied in patients as young as 2 years of age. It may represent a new, safe, and efficacious treatment for patients 2 years of age and older with mild to moderate atopic dermatitis.”
Atopic dermatitis (AD) experts have long complained of a major unmet need for new, safe, and effective topical agents for AD, a condition that affects an estimated 18%-20% of children and 2%-10% of adults. Current treatment options all have drawbacks.
Topical steroids, long a treatment mainstay, are viewed by many parents with phobic mistrust of safety. And both FDA-approved topical calcineurin inhibitors carry black box warnings of possible cancer risk.
The two pivotal phase III studies, identical in design, included a total of 1,522 patients aged 2 years through adulthood with mild to moderate AD. Roughly 60% of patients had moderate disease, as defined by an Investigator’s Static Global Assessment (ISGA) score of 3 on a 0-4 scale; the other 40% had mild AD. The mean involved body surface area was 18%.
Participants were randomized two to one to crisaborole ointment 2% b.i.d. or vehicle for 28 days. Physicians assessed patients at baseline on day 1 of the study and again on days 8, 15, 22, 29, and 36. The primary endpoint was the proportion of patients on day 29 who had an ISGA of 0 or 1 – clear or almost clear – as well as at least a 2-point improvement from baseline on that scale.
In one of the trials, that endpoint was achieved in 32.8% of the crisaborole group, compared with 25.4% of controls.
“That 25% placebo response is actually fairly typical for atopic dermatitis studies,” according to Dr. Lebwohl, professor and chairman of the department of dermatology at Mount Sinai School of Medicine, New York.
In the other study, 31.4% of the crisaborole group and 18% of controls achieved the primary endpoint. In both studies, the difference was statistically significant in favor of topical crisaborole.
There were two prespecified secondary endpoints. One was time to treatment success, as defined by clear or almost clear. A “striking” significant difference between the study arms appeared as early as the first assessment, just 1 week into the trial, Dr. Lebwohl observed.
The other secondary endpoint was the FDA’s former efficacy standard, which required being clear or almost clear without the additional need for at least a 2-point ISGA improvement. That endpoint was achieved by 51.7% and 48.5% of crisaborole-treated patients in the two studies, compared with 40.6% and 29.7% of controls. Again, both differences were statistically significant.
No treatment-related serious adverse events occurred in either study. Mild application-site pain was slightly more common in the crisaborole-treated patients. But the rate of study discontinuations because of adverse events was identical between the crisaborole and control groups, at 1.2%. No differences in laboratory values, ECGs, or vital signs were noted between the two groups.
Dr. Lebwohl explained that boron is an essential element in crisaborole. The boron stimulates an increase in cyclic adenosine monophosphate levels, which in turn results in a steep reduction in production of inflammatory cytokines, including interleukins-4, -2, and -31, as well as tumor necrosis factor-alpha.
One audience member asked if it’s possible that crisaborole acts systemically rather than topically, given that patients averaged 18% body surface area involvement, and such a large area of damaged skin could conceivably allow the topical agent ready access to the circulation.
Dr. Lebwohl replied that systemic absorption of the drug was minor. “If you break down the results into patients with very low body surface areas – the lowest was 5% – those patients improved as well. So, I think it would be unlikely that this was a systemic effect.”
Anacor, which is developing the drug as a treatment for AD and other skin diseases, plans to file for marketing approval during the first half of 2016.
Anacor sponsored the two pivotal phase III randomized trials. Dr. Lebwohl declared having no financial conflicts of interest, because all funds went directly to the medical center in which he practices.
COPENHAGEN – The nonsteroidal phosphodiesterase-4 inhibitor crisaborole aced all Food and Drug Administration–required efficacy and safety endpoints as a topical treatment for atopic dermatitis, according to results from a pair of pivotal phase III randomized trials.
“This is a fairly rapidly effective treatment,” explained Dr. Mark G. Lebwohl, who presented the findings at the annual congress of the European Academy of Dermatology and Venereology. “It has a favorable safety profile and has been studied in patients as young as 2 years of age. It may represent a new, safe, and efficacious treatment for patients 2 years of age and older with mild to moderate atopic dermatitis.”
Atopic dermatitis (AD) experts have long complained of a major unmet need for new, safe, and effective topical agents for AD, a condition that affects an estimated 18%-20% of children and 2%-10% of adults. Current treatment options all have drawbacks.
Topical steroids, long a treatment mainstay, are viewed by many parents with phobic mistrust of safety. And both FDA-approved topical calcineurin inhibitors carry black box warnings of possible cancer risk.
The two pivotal phase III studies, identical in design, included a total of 1,522 patients aged 2 years through adulthood with mild to moderate AD. Roughly 60% of patients had moderate disease, as defined by an Investigator’s Static Global Assessment (ISGA) score of 3 on a 0-4 scale; the other 40% had mild AD. The mean involved body surface area was 18%.
Participants were randomized two to one to crisaborole ointment 2% b.i.d. or vehicle for 28 days. Physicians assessed patients at baseline on day 1 of the study and again on days 8, 15, 22, 29, and 36. The primary endpoint was the proportion of patients on day 29 who had an ISGA of 0 or 1 – clear or almost clear – as well as at least a 2-point improvement from baseline on that scale.
In one of the trials, that endpoint was achieved in 32.8% of the crisaborole group, compared with 25.4% of controls.
“That 25% placebo response is actually fairly typical for atopic dermatitis studies,” according to Dr. Lebwohl, professor and chairman of the department of dermatology at Mount Sinai School of Medicine, New York.
In the other study, 31.4% of the crisaborole group and 18% of controls achieved the primary endpoint. In both studies, the difference was statistically significant in favor of topical crisaborole.
There were two prespecified secondary endpoints. One was time to treatment success, as defined by clear or almost clear. A “striking” significant difference between the study arms appeared as early as the first assessment, just 1 week into the trial, Dr. Lebwohl observed.
The other secondary endpoint was the FDA’s former efficacy standard, which required being clear or almost clear without the additional need for at least a 2-point ISGA improvement. That endpoint was achieved by 51.7% and 48.5% of crisaborole-treated patients in the two studies, compared with 40.6% and 29.7% of controls. Again, both differences were statistically significant.
No treatment-related serious adverse events occurred in either study. Mild application-site pain was slightly more common in the crisaborole-treated patients. But the rate of study discontinuations because of adverse events was identical between the crisaborole and control groups, at 1.2%. No differences in laboratory values, ECGs, or vital signs were noted between the two groups.
Dr. Lebwohl explained that boron is an essential element in crisaborole. The boron stimulates an increase in cyclic adenosine monophosphate levels, which in turn results in a steep reduction in production of inflammatory cytokines, including interleukins-4, -2, and -31, as well as tumor necrosis factor-alpha.
One audience member asked if it’s possible that crisaborole acts systemically rather than topically, given that patients averaged 18% body surface area involvement, and such a large area of damaged skin could conceivably allow the topical agent ready access to the circulation.
Dr. Lebwohl replied that systemic absorption of the drug was minor. “If you break down the results into patients with very low body surface areas – the lowest was 5% – those patients improved as well. So, I think it would be unlikely that this was a systemic effect.”
Anacor, which is developing the drug as a treatment for AD and other skin diseases, plans to file for marketing approval during the first half of 2016.
Anacor sponsored the two pivotal phase III randomized trials. Dr. Lebwohl declared having no financial conflicts of interest, because all funds went directly to the medical center in which he practices.
COPENHAGEN – The nonsteroidal phosphodiesterase-4 inhibitor crisaborole aced all Food and Drug Administration–required efficacy and safety endpoints as a topical treatment for atopic dermatitis, according to results from a pair of pivotal phase III randomized trials.
“This is a fairly rapidly effective treatment,” explained Dr. Mark G. Lebwohl, who presented the findings at the annual congress of the European Academy of Dermatology and Venereology. “It has a favorable safety profile and has been studied in patients as young as 2 years of age. It may represent a new, safe, and efficacious treatment for patients 2 years of age and older with mild to moderate atopic dermatitis.”
Atopic dermatitis (AD) experts have long complained of a major unmet need for new, safe, and effective topical agents for AD, a condition that affects an estimated 18%-20% of children and 2%-10% of adults. Current treatment options all have drawbacks.
Topical steroids, long a treatment mainstay, are viewed by many parents with phobic mistrust of safety. And both FDA-approved topical calcineurin inhibitors carry black box warnings of possible cancer risk.
The two pivotal phase III studies, identical in design, included a total of 1,522 patients aged 2 years through adulthood with mild to moderate AD. Roughly 60% of patients had moderate disease, as defined by an Investigator’s Static Global Assessment (ISGA) score of 3 on a 0-4 scale; the other 40% had mild AD. The mean involved body surface area was 18%.
Participants were randomized two to one to crisaborole ointment 2% b.i.d. or vehicle for 28 days. Physicians assessed patients at baseline on day 1 of the study and again on days 8, 15, 22, 29, and 36. The primary endpoint was the proportion of patients on day 29 who had an ISGA of 0 or 1 – clear or almost clear – as well as at least a 2-point improvement from baseline on that scale.
In one of the trials, that endpoint was achieved in 32.8% of the crisaborole group, compared with 25.4% of controls.
“That 25% placebo response is actually fairly typical for atopic dermatitis studies,” according to Dr. Lebwohl, professor and chairman of the department of dermatology at Mount Sinai School of Medicine, New York.
In the other study, 31.4% of the crisaborole group and 18% of controls achieved the primary endpoint. In both studies, the difference was statistically significant in favor of topical crisaborole.
There were two prespecified secondary endpoints. One was time to treatment success, as defined by clear or almost clear. A “striking” significant difference between the study arms appeared as early as the first assessment, just 1 week into the trial, Dr. Lebwohl observed.
The other secondary endpoint was the FDA’s former efficacy standard, which required being clear or almost clear without the additional need for at least a 2-point ISGA improvement. That endpoint was achieved by 51.7% and 48.5% of crisaborole-treated patients in the two studies, compared with 40.6% and 29.7% of controls. Again, both differences were statistically significant.
No treatment-related serious adverse events occurred in either study. Mild application-site pain was slightly more common in the crisaborole-treated patients. But the rate of study discontinuations because of adverse events was identical between the crisaborole and control groups, at 1.2%. No differences in laboratory values, ECGs, or vital signs were noted between the two groups.
Dr. Lebwohl explained that boron is an essential element in crisaborole. The boron stimulates an increase in cyclic adenosine monophosphate levels, which in turn results in a steep reduction in production of inflammatory cytokines, including interleukins-4, -2, and -31, as well as tumor necrosis factor-alpha.
One audience member asked if it’s possible that crisaborole acts systemically rather than topically, given that patients averaged 18% body surface area involvement, and such a large area of damaged skin could conceivably allow the topical agent ready access to the circulation.
Dr. Lebwohl replied that systemic absorption of the drug was minor. “If you break down the results into patients with very low body surface areas – the lowest was 5% – those patients improved as well. So, I think it would be unlikely that this was a systemic effect.”
Anacor, which is developing the drug as a treatment for AD and other skin diseases, plans to file for marketing approval during the first half of 2016.
Anacor sponsored the two pivotal phase III randomized trials. Dr. Lebwohl declared having no financial conflicts of interest, because all funds went directly to the medical center in which he practices.
AT THE EADV CONGRESS
Key clinical point: Crisaborole topical ointment 2% b.i.d. appeared to be a safe and effective treatment for mild to moderate atopic dermatitis in children and adults.
Major finding: The primary combined efficacy endpoint was met by 32.8% and 31.4% of crisaborole-treated patients in two randomized trials, compared with 25.4% and 18% of vehicle-treated patients.
Data source: The two identically designed pivotal phase III clinical trials included 759 patients and 763 patients aged 2 years through adulthood with mild to moderate atopic dermatitis.
Disclosures: Anacor sponsored the two pivotal phase III randomized trials. Dr. Lebwohl declared having no financial conflicts of interest, because all funds went directly to the medical center in which he practices.