User login
Are ESC’s new heart failure guidelines already outdated?
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
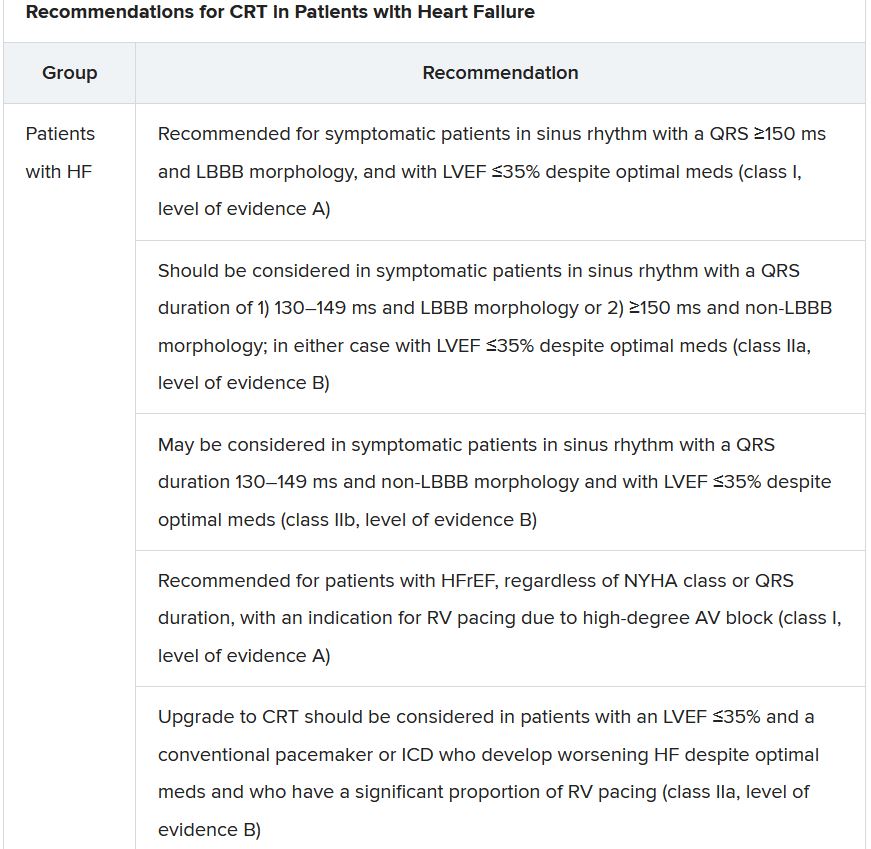
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
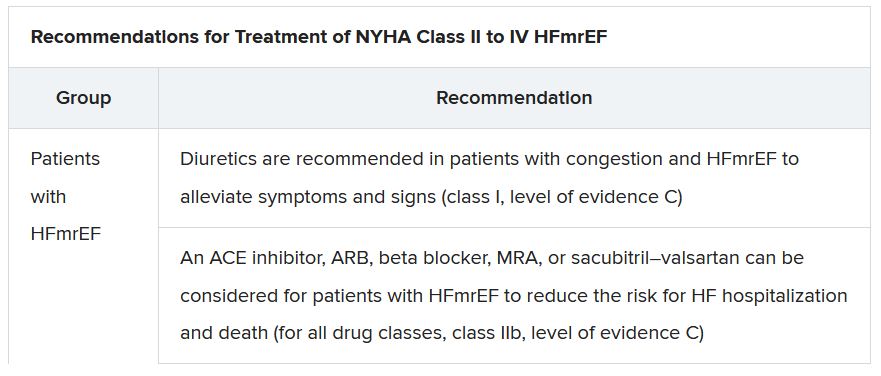
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
‘Quadpill’ bests monotherapy for initial BP lowering: QUARTET
A “quadpill” containing quarter doses of four blood pressure (BP)–lowering medications was more effective than monotherapy for initial treatment of hypertension, with similar tolerability, in the 1-year, phase 3 QUARTET randomized, active-control trial.
Clara Chow, MD, PhD, academic director of the Westmead Applied Research Centre, University of Sydney, presented the findings in a late-breaking trial session at the annual congress of the European Society of Cardiology. The study was simultaneously published in The Lancet.
The primary outcome, mean unattended office BP at 12 weeks, dropped from 142/86 mm Hg to 120/71 mm Hg in patients who received the daily quadpill – a capsule containing irbesartan, amlodipine, indapamide, and bisoprolol – and fell from 140/83 mm Hg to 127/79 mm Hg in patients who received a daily full dose of irbesartan.
This 6.9 mm Hg greater drop in systolic BP at 12 months is clinically meaningful, Dr. Chow told this news organization. “If maintained, it would be expected to confer about a 15%-20% reduction” in heart disease, stroke, and heart failure.
In the SPRINT study, she noted, the final systolic BP was 120 mm Hg in the intervention group and 134 mm Hg in the control group, and the difference was associated with a 27% reduction in the composite cardiovascular (CV) outcome.
The results of QUARTET suggest that, “even in those with stage 1 hypertension, we can safely reduce BP to a significant degree by this simple approach, compared to usual care,” Salim Yusuf, MD, DPhil, a long-time advocate of a polypill approach, said in an email.
Importantly, Dr. Chow pointed out, at 12 months, 81% of patients treated with the quadpill versus 62% of patients treated with monotherapy had BP control (<140/90 mm Hg). Patients who received monotherapy did not “catch up,” even though a higher percentage received stepped-up therapy.
The quadpill dosing strategy aligns with the latest 2018 ESC/European Society of Hypertension guidelines, which recommend starting antihypertensive treatment with more than one drug, session cochair Thomas Kahan, MD, PhD, Karolinska Institute, Danderyd Hospital, department of clinical sciences, Stockholm, commented.
“How many drugs should be in the initial step?” he asked. “Is four better than three or two, or should we have even more drugs at low doses?”
The trial was not designed to answer these questions, Dr. Chow replied. “We were really comparing [the quadpill] against what the majority of people around the planet are still doing, which is starting on one drug and slowly but surely stepping it up,” she said.
The quadpill was actually a capsule, she clarified, that contained four generic BP medications available in half doses in Australia. The half doses were cut in half and the medications were encapsulated. The control drug was prepared in an identical-looking capsule.
It is important to note that “the time to BP control was shorter in patients who received the quadpill versus monotherapy,” session cochair Felix Mahfoud, MD, internal medicine and cardiology, Saarland University Hospital, Hamburg, Germany, pointed out, because “in clinical practice we aim to get patients to BP control as quickly as possible.”
“What is new here is the use of four drugs, each given at quarter doses,” Dr. Yusuf, director of the Population Health Research Institute, McMaster University, Hamilton, Ont., said. Although a few questions remain, “this study emphasizes the importance and potential benefits and simplicity of using combination BP-lowering drugs at low doses.”
For guidelines to be changed, he observed, the findings would have to be replicated in independent studies, and the quadpill would likely have to be shown to be superior to the dual pill.
“It took about 20 years [to change guidelines] after the first evidence that combinations of two pills were preferable to single-drug combinations,” he noted.
“I hope that in most people with elevated BP, at least a two-drug combination plus a statin plus aspirin will be prescribed,” Dr. Yusuf said. “This can reduce the risk of CVD events by 50% or more – a big impact both for the individual and for populations. The quad pill may have a role in this approach.”
Four-in-one pill
Worldwide, hypertension control is poor, Dr. Chow said, because of the need for multiple medications, treatment inertia, and concerns about adverse events.
The researchers hypothesized that initial antihypertensive treatment with a four-in-one pill with quarter doses of each medication would minimize side effects, maximize BP lowering, and overcome these treatment barriers and concerns. A pilot study of this strategy published by the group in 2017 showed promise.
QUARTET randomized 591 adults with hypertension, seen at clinics in four states in Australia from June 2017 through August 2020.
Patients were either receiving no antihypertensive medication and had an unattended standard office BP of 140/90 to 179/109 mm Hg or daytime ambulatory BP greater than 135/95 mm Hg, or they were on BP-lowering monotherapy and had a BP of 130/85 to 179/109 mm Hg or daytime ambulatory BP greater than 125/80 mm Hg. Patients who were taking antihypertensive therapy entered a washout period before the trial.
The researchers randomized 291 participants to receive 150 mg irbesartan daily (usual care or control group).
The other 300 participants received a daily quadpill containing 37.5 mg irbesartan, 1.25 mg amlodipine, 0.625 mg indapamide, and 2.5 mg bisoprolol. The first three drugs are the most commonly prescribed angiotensin II receptor blocker, calcium channel blocker, and thiazide or thiazidelike diuretic in Australia, and the last drug, a beta-blocker, has a long duration of action, the study protocol explains.
Patients in both groups had similar baseline characteristics. They were a mean age of 58 years, 40% were women, and 82% were White. They also had a mean body mass index of 31 kg/m2. About 8% were current smokers, and about 54% were not taking a BP-lowering drug.
Participants had clinic visits at baseline, 6 weeks, and 12 weeks, and if they continued the study, at 26 weeks and 52 weeks.
If a patient’s blood pressure was higher than 140/90 mm Hg, clinicians could add another medication, starting with amlodipine 5 mg.
At 12 weeks, 15% of patients in the intervention group and 40% in the control group had stepped up treatment.
Despite greater up-titration in the usual care group, BP control remained higher in the quadpill group, Dr. Chow pointed out. That is, patients in the quadpill group were more likely than patients in the usual care group to have a BP less than 140/90 mm Hg (76% vs. 58% respectively; P < .0001).
Patients in the quadpill group also had lower daytime and nighttime ambulatory systolic BP.
At 12 months, among the 417 patients who continued treatment, patients in the quadpill group had a 7.7 mm Hg greater drop in systolic BP, compared with patients in the control group, (P < .001).
There were no significant differences in adverse events, which were most commonly dizziness (31% and 25%) or muscle cramps, gastrointestinal complaints, headache, or musculoskeletal complaints.
At 12 weeks, there were seven serious adverse events in the intervention group versus three in the control group. There were 12 treatment withdrawals in the intervention group versus seven in the control group (P = .27).
Remaining questions, upcoming phase 3 U.S. study
“While the [QUARTET] results are impressive, we are left with a number of questions,” Dr. Yusuf said.
Would the results be the same with a three-drug combo or even a two-drug combo at half doses? In the HOPE 3 trial, a two-drug combo at half doses provided similar results to the current study, over a much longer mean follow-up of 5.6 years, he noted.
Also, is the quadpill associated with higher rates of diabetes or higher creatinine levels in the long term? “Given that we do not have any data on long-term clinical outcomes from a four-drug combination,” Dr. Yusuf said, “caution should be utilized.”
Would the reduced risk of CVD be greater with a combination of low doses of two BP-lowering drugs plus a statin plus aspirin? That may be superior, he said, “based on recent information published on the polypill indicating a 50% relative risk reduction in CVD events.”
The related phase 2 QUARTET US trial should shed further light on a quadpill strategy. Patients with hypertension are being randomized to a daily quadpill containing 2 mg candesartan, 1.25 mg amlodipine besylate, 0.625 mg indapamide, and 2.5 mg bisoprolol, or to usual care, 8 mg candesartan daily.
Investigators plan to enroll 87 participants in the Chicago area, with estimated study completion by March 31, 2023.
The study was supported by an Australian National Health and Medical Research Council grant. The George Institute for Global Health has submitted patent applications for low–fixed-dose combination products to treat CV or cardiometabolic disease. Dr. Chow and coauthor Kris Rogers, PhD, senior biostatistician at The George Institute for Global Health, Newtown, Australia, are listed as inventors, but they do not have direct financial interests in these patent applications.
A version of this article first appeared on Medscape.com.
A “quadpill” containing quarter doses of four blood pressure (BP)–lowering medications was more effective than monotherapy for initial treatment of hypertension, with similar tolerability, in the 1-year, phase 3 QUARTET randomized, active-control trial.
Clara Chow, MD, PhD, academic director of the Westmead Applied Research Centre, University of Sydney, presented the findings in a late-breaking trial session at the annual congress of the European Society of Cardiology. The study was simultaneously published in The Lancet.
The primary outcome, mean unattended office BP at 12 weeks, dropped from 142/86 mm Hg to 120/71 mm Hg in patients who received the daily quadpill – a capsule containing irbesartan, amlodipine, indapamide, and bisoprolol – and fell from 140/83 mm Hg to 127/79 mm Hg in patients who received a daily full dose of irbesartan.
This 6.9 mm Hg greater drop in systolic BP at 12 months is clinically meaningful, Dr. Chow told this news organization. “If maintained, it would be expected to confer about a 15%-20% reduction” in heart disease, stroke, and heart failure.
In the SPRINT study, she noted, the final systolic BP was 120 mm Hg in the intervention group and 134 mm Hg in the control group, and the difference was associated with a 27% reduction in the composite cardiovascular (CV) outcome.
The results of QUARTET suggest that, “even in those with stage 1 hypertension, we can safely reduce BP to a significant degree by this simple approach, compared to usual care,” Salim Yusuf, MD, DPhil, a long-time advocate of a polypill approach, said in an email.
Importantly, Dr. Chow pointed out, at 12 months, 81% of patients treated with the quadpill versus 62% of patients treated with monotherapy had BP control (<140/90 mm Hg). Patients who received monotherapy did not “catch up,” even though a higher percentage received stepped-up therapy.
The quadpill dosing strategy aligns with the latest 2018 ESC/European Society of Hypertension guidelines, which recommend starting antihypertensive treatment with more than one drug, session cochair Thomas Kahan, MD, PhD, Karolinska Institute, Danderyd Hospital, department of clinical sciences, Stockholm, commented.
“How many drugs should be in the initial step?” he asked. “Is four better than three or two, or should we have even more drugs at low doses?”
The trial was not designed to answer these questions, Dr. Chow replied. “We were really comparing [the quadpill] against what the majority of people around the planet are still doing, which is starting on one drug and slowly but surely stepping it up,” she said.
The quadpill was actually a capsule, she clarified, that contained four generic BP medications available in half doses in Australia. The half doses were cut in half and the medications were encapsulated. The control drug was prepared in an identical-looking capsule.
It is important to note that “the time to BP control was shorter in patients who received the quadpill versus monotherapy,” session cochair Felix Mahfoud, MD, internal medicine and cardiology, Saarland University Hospital, Hamburg, Germany, pointed out, because “in clinical practice we aim to get patients to BP control as quickly as possible.”
“What is new here is the use of four drugs, each given at quarter doses,” Dr. Yusuf, director of the Population Health Research Institute, McMaster University, Hamilton, Ont., said. Although a few questions remain, “this study emphasizes the importance and potential benefits and simplicity of using combination BP-lowering drugs at low doses.”
For guidelines to be changed, he observed, the findings would have to be replicated in independent studies, and the quadpill would likely have to be shown to be superior to the dual pill.
“It took about 20 years [to change guidelines] after the first evidence that combinations of two pills were preferable to single-drug combinations,” he noted.
“I hope that in most people with elevated BP, at least a two-drug combination plus a statin plus aspirin will be prescribed,” Dr. Yusuf said. “This can reduce the risk of CVD events by 50% or more – a big impact both for the individual and for populations. The quad pill may have a role in this approach.”
Four-in-one pill
Worldwide, hypertension control is poor, Dr. Chow said, because of the need for multiple medications, treatment inertia, and concerns about adverse events.
The researchers hypothesized that initial antihypertensive treatment with a four-in-one pill with quarter doses of each medication would minimize side effects, maximize BP lowering, and overcome these treatment barriers and concerns. A pilot study of this strategy published by the group in 2017 showed promise.
QUARTET randomized 591 adults with hypertension, seen at clinics in four states in Australia from June 2017 through August 2020.
Patients were either receiving no antihypertensive medication and had an unattended standard office BP of 140/90 to 179/109 mm Hg or daytime ambulatory BP greater than 135/95 mm Hg, or they were on BP-lowering monotherapy and had a BP of 130/85 to 179/109 mm Hg or daytime ambulatory BP greater than 125/80 mm Hg. Patients who were taking antihypertensive therapy entered a washout period before the trial.
The researchers randomized 291 participants to receive 150 mg irbesartan daily (usual care or control group).
The other 300 participants received a daily quadpill containing 37.5 mg irbesartan, 1.25 mg amlodipine, 0.625 mg indapamide, and 2.5 mg bisoprolol. The first three drugs are the most commonly prescribed angiotensin II receptor blocker, calcium channel blocker, and thiazide or thiazidelike diuretic in Australia, and the last drug, a beta-blocker, has a long duration of action, the study protocol explains.
Patients in both groups had similar baseline characteristics. They were a mean age of 58 years, 40% were women, and 82% were White. They also had a mean body mass index of 31 kg/m2. About 8% were current smokers, and about 54% were not taking a BP-lowering drug.
Participants had clinic visits at baseline, 6 weeks, and 12 weeks, and if they continued the study, at 26 weeks and 52 weeks.
If a patient’s blood pressure was higher than 140/90 mm Hg, clinicians could add another medication, starting with amlodipine 5 mg.
At 12 weeks, 15% of patients in the intervention group and 40% in the control group had stepped up treatment.
Despite greater up-titration in the usual care group, BP control remained higher in the quadpill group, Dr. Chow pointed out. That is, patients in the quadpill group were more likely than patients in the usual care group to have a BP less than 140/90 mm Hg (76% vs. 58% respectively; P < .0001).
Patients in the quadpill group also had lower daytime and nighttime ambulatory systolic BP.
At 12 months, among the 417 patients who continued treatment, patients in the quadpill group had a 7.7 mm Hg greater drop in systolic BP, compared with patients in the control group, (P < .001).
There were no significant differences in adverse events, which were most commonly dizziness (31% and 25%) or muscle cramps, gastrointestinal complaints, headache, or musculoskeletal complaints.
At 12 weeks, there were seven serious adverse events in the intervention group versus three in the control group. There were 12 treatment withdrawals in the intervention group versus seven in the control group (P = .27).
Remaining questions, upcoming phase 3 U.S. study
“While the [QUARTET] results are impressive, we are left with a number of questions,” Dr. Yusuf said.
Would the results be the same with a three-drug combo or even a two-drug combo at half doses? In the HOPE 3 trial, a two-drug combo at half doses provided similar results to the current study, over a much longer mean follow-up of 5.6 years, he noted.
Also, is the quadpill associated with higher rates of diabetes or higher creatinine levels in the long term? “Given that we do not have any data on long-term clinical outcomes from a four-drug combination,” Dr. Yusuf said, “caution should be utilized.”
Would the reduced risk of CVD be greater with a combination of low doses of two BP-lowering drugs plus a statin plus aspirin? That may be superior, he said, “based on recent information published on the polypill indicating a 50% relative risk reduction in CVD events.”
The related phase 2 QUARTET US trial should shed further light on a quadpill strategy. Patients with hypertension are being randomized to a daily quadpill containing 2 mg candesartan, 1.25 mg amlodipine besylate, 0.625 mg indapamide, and 2.5 mg bisoprolol, or to usual care, 8 mg candesartan daily.
Investigators plan to enroll 87 participants in the Chicago area, with estimated study completion by March 31, 2023.
The study was supported by an Australian National Health and Medical Research Council grant. The George Institute for Global Health has submitted patent applications for low–fixed-dose combination products to treat CV or cardiometabolic disease. Dr. Chow and coauthor Kris Rogers, PhD, senior biostatistician at The George Institute for Global Health, Newtown, Australia, are listed as inventors, but they do not have direct financial interests in these patent applications.
A version of this article first appeared on Medscape.com.
A “quadpill” containing quarter doses of four blood pressure (BP)–lowering medications was more effective than monotherapy for initial treatment of hypertension, with similar tolerability, in the 1-year, phase 3 QUARTET randomized, active-control trial.
Clara Chow, MD, PhD, academic director of the Westmead Applied Research Centre, University of Sydney, presented the findings in a late-breaking trial session at the annual congress of the European Society of Cardiology. The study was simultaneously published in The Lancet.
The primary outcome, mean unattended office BP at 12 weeks, dropped from 142/86 mm Hg to 120/71 mm Hg in patients who received the daily quadpill – a capsule containing irbesartan, amlodipine, indapamide, and bisoprolol – and fell from 140/83 mm Hg to 127/79 mm Hg in patients who received a daily full dose of irbesartan.
This 6.9 mm Hg greater drop in systolic BP at 12 months is clinically meaningful, Dr. Chow told this news organization. “If maintained, it would be expected to confer about a 15%-20% reduction” in heart disease, stroke, and heart failure.
In the SPRINT study, she noted, the final systolic BP was 120 mm Hg in the intervention group and 134 mm Hg in the control group, and the difference was associated with a 27% reduction in the composite cardiovascular (CV) outcome.
The results of QUARTET suggest that, “even in those with stage 1 hypertension, we can safely reduce BP to a significant degree by this simple approach, compared to usual care,” Salim Yusuf, MD, DPhil, a long-time advocate of a polypill approach, said in an email.
Importantly, Dr. Chow pointed out, at 12 months, 81% of patients treated with the quadpill versus 62% of patients treated with monotherapy had BP control (<140/90 mm Hg). Patients who received monotherapy did not “catch up,” even though a higher percentage received stepped-up therapy.
The quadpill dosing strategy aligns with the latest 2018 ESC/European Society of Hypertension guidelines, which recommend starting antihypertensive treatment with more than one drug, session cochair Thomas Kahan, MD, PhD, Karolinska Institute, Danderyd Hospital, department of clinical sciences, Stockholm, commented.
“How many drugs should be in the initial step?” he asked. “Is four better than three or two, or should we have even more drugs at low doses?”
The trial was not designed to answer these questions, Dr. Chow replied. “We were really comparing [the quadpill] against what the majority of people around the planet are still doing, which is starting on one drug and slowly but surely stepping it up,” she said.
The quadpill was actually a capsule, she clarified, that contained four generic BP medications available in half doses in Australia. The half doses were cut in half and the medications were encapsulated. The control drug was prepared in an identical-looking capsule.
It is important to note that “the time to BP control was shorter in patients who received the quadpill versus monotherapy,” session cochair Felix Mahfoud, MD, internal medicine and cardiology, Saarland University Hospital, Hamburg, Germany, pointed out, because “in clinical practice we aim to get patients to BP control as quickly as possible.”
“What is new here is the use of four drugs, each given at quarter doses,” Dr. Yusuf, director of the Population Health Research Institute, McMaster University, Hamilton, Ont., said. Although a few questions remain, “this study emphasizes the importance and potential benefits and simplicity of using combination BP-lowering drugs at low doses.”
For guidelines to be changed, he observed, the findings would have to be replicated in independent studies, and the quadpill would likely have to be shown to be superior to the dual pill.
“It took about 20 years [to change guidelines] after the first evidence that combinations of two pills were preferable to single-drug combinations,” he noted.
“I hope that in most people with elevated BP, at least a two-drug combination plus a statin plus aspirin will be prescribed,” Dr. Yusuf said. “This can reduce the risk of CVD events by 50% or more – a big impact both for the individual and for populations. The quad pill may have a role in this approach.”
Four-in-one pill
Worldwide, hypertension control is poor, Dr. Chow said, because of the need for multiple medications, treatment inertia, and concerns about adverse events.
The researchers hypothesized that initial antihypertensive treatment with a four-in-one pill with quarter doses of each medication would minimize side effects, maximize BP lowering, and overcome these treatment barriers and concerns. A pilot study of this strategy published by the group in 2017 showed promise.
QUARTET randomized 591 adults with hypertension, seen at clinics in four states in Australia from June 2017 through August 2020.
Patients were either receiving no antihypertensive medication and had an unattended standard office BP of 140/90 to 179/109 mm Hg or daytime ambulatory BP greater than 135/95 mm Hg, or they were on BP-lowering monotherapy and had a BP of 130/85 to 179/109 mm Hg or daytime ambulatory BP greater than 125/80 mm Hg. Patients who were taking antihypertensive therapy entered a washout period before the trial.
The researchers randomized 291 participants to receive 150 mg irbesartan daily (usual care or control group).
The other 300 participants received a daily quadpill containing 37.5 mg irbesartan, 1.25 mg amlodipine, 0.625 mg indapamide, and 2.5 mg bisoprolol. The first three drugs are the most commonly prescribed angiotensin II receptor blocker, calcium channel blocker, and thiazide or thiazidelike diuretic in Australia, and the last drug, a beta-blocker, has a long duration of action, the study protocol explains.
Patients in both groups had similar baseline characteristics. They were a mean age of 58 years, 40% were women, and 82% were White. They also had a mean body mass index of 31 kg/m2. About 8% were current smokers, and about 54% were not taking a BP-lowering drug.
Participants had clinic visits at baseline, 6 weeks, and 12 weeks, and if they continued the study, at 26 weeks and 52 weeks.
If a patient’s blood pressure was higher than 140/90 mm Hg, clinicians could add another medication, starting with amlodipine 5 mg.
At 12 weeks, 15% of patients in the intervention group and 40% in the control group had stepped up treatment.
Despite greater up-titration in the usual care group, BP control remained higher in the quadpill group, Dr. Chow pointed out. That is, patients in the quadpill group were more likely than patients in the usual care group to have a BP less than 140/90 mm Hg (76% vs. 58% respectively; P < .0001).
Patients in the quadpill group also had lower daytime and nighttime ambulatory systolic BP.
At 12 months, among the 417 patients who continued treatment, patients in the quadpill group had a 7.7 mm Hg greater drop in systolic BP, compared with patients in the control group, (P < .001).
There were no significant differences in adverse events, which were most commonly dizziness (31% and 25%) or muscle cramps, gastrointestinal complaints, headache, or musculoskeletal complaints.
At 12 weeks, there were seven serious adverse events in the intervention group versus three in the control group. There were 12 treatment withdrawals in the intervention group versus seven in the control group (P = .27).
Remaining questions, upcoming phase 3 U.S. study
“While the [QUARTET] results are impressive, we are left with a number of questions,” Dr. Yusuf said.
Would the results be the same with a three-drug combo or even a two-drug combo at half doses? In the HOPE 3 trial, a two-drug combo at half doses provided similar results to the current study, over a much longer mean follow-up of 5.6 years, he noted.
Also, is the quadpill associated with higher rates of diabetes or higher creatinine levels in the long term? “Given that we do not have any data on long-term clinical outcomes from a four-drug combination,” Dr. Yusuf said, “caution should be utilized.”
Would the reduced risk of CVD be greater with a combination of low doses of two BP-lowering drugs plus a statin plus aspirin? That may be superior, he said, “based on recent information published on the polypill indicating a 50% relative risk reduction in CVD events.”
The related phase 2 QUARTET US trial should shed further light on a quadpill strategy. Patients with hypertension are being randomized to a daily quadpill containing 2 mg candesartan, 1.25 mg amlodipine besylate, 0.625 mg indapamide, and 2.5 mg bisoprolol, or to usual care, 8 mg candesartan daily.
Investigators plan to enroll 87 participants in the Chicago area, with estimated study completion by March 31, 2023.
The study was supported by an Australian National Health and Medical Research Council grant. The George Institute for Global Health has submitted patent applications for low–fixed-dose combination products to treat CV or cardiometabolic disease. Dr. Chow and coauthor Kris Rogers, PhD, senior biostatistician at The George Institute for Global Health, Newtown, Australia, are listed as inventors, but they do not have direct financial interests in these patent applications.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2021
Growing proportion of cardiac arrests in U.S. considered opioid related
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
FROM ESC 2021
STOP-DAPT 2 ACS: 1 month of DAPT proves inadequate for patients with recent ACS
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
New valvular heart disease guidelines change several repair indications
Antithrombotic recommendations also altered
New European guidelines for the management of valvular heart disease offer more than 45 revised or completely new recommendations relative to the previous version published in 2017, according to members of the writing committee who presented the changes during the annual congress of the European Society of Cardiology.
With their emphasis on early diagnosis and expansion of indications, these 2021 ESC/European Association for Cardio-Thoracic Surgery guidelines are likely to further accelerate the already steep growth in this area of interventional cardiology, according to Alec Vahanian, MD, a professor of cardiology at the University of Paris.
“Valvular heart disease is too often undetected, and these guidelines stress the importance of clinical examination and the best strategies for diagnosis as well as treatment,” he said.
Of the multiple sections and subsections, which follow the same format of the previous guidelines, the greatest number of revisions and new additions involve perioperative antithrombotic therapy, according to the document, which was published in conjunction with the ESC Congress.
Eleven new guidelines for anticoagulants
On the basis of evidence published since the previous guidelines, there are 11 completely new recommendations regarding the use of anticoagulants or antiplatelet therapies. The majority of these have received a grade I indication, which signifies “recommended” or “indicated.” These include indications for stopping or starting anticoagulants and which anticoagulants or antiplatelet drugs to consider in specific patient populations.
The next most common focus of new or revised recommendations involves when to consider surgical aortic valve repair (SAVR) relative to transcatheter aortic valve implantation (TAVI) in severe aortic stenosis. Most of these represent revisions from the previous guidelines, but almost all are also grade I recommendations.
“SAVR and TAVI are both excellent options in appropriate patients, but they are not interchangeable,” explained Bernard D. Prendergast, BMedSci, MD, director of the cardiac structural intervention program, Guy’s and St Thomas’ Hospital, London.
While the previous guidelines generally reserved TAVI for those not suitable for SAVR, the new guidelines are more nuanced.
As a rule, SAVR is generally preferred for younger patients. The reason, according to Dr. Prendergast, is concern that younger patients might outlive the expected lifespan of the prosthetic TAVI device.
No single criterion for selecting SAVR over TAVI
However, there are many exceptions and additional considerations beyond age. When both SAVR and TAVI are otherwise suitable options, but TAVI cannot be performed with a transfemoral access, Dr. Prendergast pointed out that SAVR might be a better choice.
Transfemoral access is the preferred strategy in TAVI, but Dr. Prendergast emphasized that a collaborative “heart team” should help patients select the most appropriate option. In fact, there is a grade IIb recommendation (“usefulness or efficacy is less well established”) to consider other access sites in patients at high surgical risk with contraindications for transfemoral TAVI.
Of new recommendations in the area of severe aortic stenosis, valvular repair may now be considered in asymptomatic patients with a left ventricular ejection fraction of less than 55%, according to a grade IIb recommendation. The 2017 guidelines did not address this issue.
Grade I recommendation for bioprostheses
A substantial number of the revisions involve minor clarifications without a change in the grade, but a revision regarding bioprosthetics is substantial. In the 2017 guidelines, there was a grade IIa recommendation (“weight of evidence is in favor”) to consider bioprosthetics in patients with a life expectancy less than the expected durability of the device. The 2021 guidelines have changed this to a grade I indication, adding an indication for bioprostheses in patients contraindicated for or unlikely to achieve good-quality anticoagulation.
On this same topic, there is a new grade IIb recommendation to consider bioprosthesis over alternative devices in patients already on a long-term non–vitamin K oral anticoagulant because of the high risk of thromboembolism.
There are two new recommendations in the realm of severe secondary mitral regurgitation, reported Fabien Praz, MD, an interventional cardiologist at the University of Bern (Switzerland). The first, which is perhaps the most significant, is a grade I recommendation for valve surgery in patients with severe secondary mitral regurgitation who remain symptomatic despite optimized medical therapy.
The second is a grade IIa recommendation for invasive procedures, such as a percutaneous coronary intervention, for patients with symptomatic secondary mitral regurgitation and coexisting coronary artery disease. In both cases, however, Dr. Praz emphasized language in the guidelines that calls for a collaborative heart team to agree on the suitability of these treatments.
Ultimately, none of these recommendations can be divorced from patient expectations and values, according to Friedhelm Beyersdorf, MD, chairman of the department of cardiovascular surgery at the Heart Center of the University of Freiburg (Germany).
Even if treatment is not expected to prolong life, “symptom relief on its own may justify intervention,” said Dr. Beyersdorf. However, he emphasized that “thoroughly informed” patients are an essential part of the process in selecting a treatment strategy most likely to satisfy patient goals.
Dr. Vahanian and Dr. Prendergast reported no conflicts of interest relevant to these guidelines. Dr. Praz reported a financial relationship with Edwards Lifesciences. Dr. Beyersdorf reported a financial relationship with Resuscitec.
Antithrombotic recommendations also altered
Antithrombotic recommendations also altered
New European guidelines for the management of valvular heart disease offer more than 45 revised or completely new recommendations relative to the previous version published in 2017, according to members of the writing committee who presented the changes during the annual congress of the European Society of Cardiology.
With their emphasis on early diagnosis and expansion of indications, these 2021 ESC/European Association for Cardio-Thoracic Surgery guidelines are likely to further accelerate the already steep growth in this area of interventional cardiology, according to Alec Vahanian, MD, a professor of cardiology at the University of Paris.
“Valvular heart disease is too often undetected, and these guidelines stress the importance of clinical examination and the best strategies for diagnosis as well as treatment,” he said.
Of the multiple sections and subsections, which follow the same format of the previous guidelines, the greatest number of revisions and new additions involve perioperative antithrombotic therapy, according to the document, which was published in conjunction with the ESC Congress.
Eleven new guidelines for anticoagulants
On the basis of evidence published since the previous guidelines, there are 11 completely new recommendations regarding the use of anticoagulants or antiplatelet therapies. The majority of these have received a grade I indication, which signifies “recommended” or “indicated.” These include indications for stopping or starting anticoagulants and which anticoagulants or antiplatelet drugs to consider in specific patient populations.
The next most common focus of new or revised recommendations involves when to consider surgical aortic valve repair (SAVR) relative to transcatheter aortic valve implantation (TAVI) in severe aortic stenosis. Most of these represent revisions from the previous guidelines, but almost all are also grade I recommendations.
“SAVR and TAVI are both excellent options in appropriate patients, but they are not interchangeable,” explained Bernard D. Prendergast, BMedSci, MD, director of the cardiac structural intervention program, Guy’s and St Thomas’ Hospital, London.
While the previous guidelines generally reserved TAVI for those not suitable for SAVR, the new guidelines are more nuanced.
As a rule, SAVR is generally preferred for younger patients. The reason, according to Dr. Prendergast, is concern that younger patients might outlive the expected lifespan of the prosthetic TAVI device.
No single criterion for selecting SAVR over TAVI
However, there are many exceptions and additional considerations beyond age. When both SAVR and TAVI are otherwise suitable options, but TAVI cannot be performed with a transfemoral access, Dr. Prendergast pointed out that SAVR might be a better choice.
Transfemoral access is the preferred strategy in TAVI, but Dr. Prendergast emphasized that a collaborative “heart team” should help patients select the most appropriate option. In fact, there is a grade IIb recommendation (“usefulness or efficacy is less well established”) to consider other access sites in patients at high surgical risk with contraindications for transfemoral TAVI.
Of new recommendations in the area of severe aortic stenosis, valvular repair may now be considered in asymptomatic patients with a left ventricular ejection fraction of less than 55%, according to a grade IIb recommendation. The 2017 guidelines did not address this issue.
Grade I recommendation for bioprostheses
A substantial number of the revisions involve minor clarifications without a change in the grade, but a revision regarding bioprosthetics is substantial. In the 2017 guidelines, there was a grade IIa recommendation (“weight of evidence is in favor”) to consider bioprosthetics in patients with a life expectancy less than the expected durability of the device. The 2021 guidelines have changed this to a grade I indication, adding an indication for bioprostheses in patients contraindicated for or unlikely to achieve good-quality anticoagulation.
On this same topic, there is a new grade IIb recommendation to consider bioprosthesis over alternative devices in patients already on a long-term non–vitamin K oral anticoagulant because of the high risk of thromboembolism.
There are two new recommendations in the realm of severe secondary mitral regurgitation, reported Fabien Praz, MD, an interventional cardiologist at the University of Bern (Switzerland). The first, which is perhaps the most significant, is a grade I recommendation for valve surgery in patients with severe secondary mitral regurgitation who remain symptomatic despite optimized medical therapy.
The second is a grade IIa recommendation for invasive procedures, such as a percutaneous coronary intervention, for patients with symptomatic secondary mitral regurgitation and coexisting coronary artery disease. In both cases, however, Dr. Praz emphasized language in the guidelines that calls for a collaborative heart team to agree on the suitability of these treatments.
Ultimately, none of these recommendations can be divorced from patient expectations and values, according to Friedhelm Beyersdorf, MD, chairman of the department of cardiovascular surgery at the Heart Center of the University of Freiburg (Germany).
Even if treatment is not expected to prolong life, “symptom relief on its own may justify intervention,” said Dr. Beyersdorf. However, he emphasized that “thoroughly informed” patients are an essential part of the process in selecting a treatment strategy most likely to satisfy patient goals.
Dr. Vahanian and Dr. Prendergast reported no conflicts of interest relevant to these guidelines. Dr. Praz reported a financial relationship with Edwards Lifesciences. Dr. Beyersdorf reported a financial relationship with Resuscitec.
New European guidelines for the management of valvular heart disease offer more than 45 revised or completely new recommendations relative to the previous version published in 2017, according to members of the writing committee who presented the changes during the annual congress of the European Society of Cardiology.
With their emphasis on early diagnosis and expansion of indications, these 2021 ESC/European Association for Cardio-Thoracic Surgery guidelines are likely to further accelerate the already steep growth in this area of interventional cardiology, according to Alec Vahanian, MD, a professor of cardiology at the University of Paris.
“Valvular heart disease is too often undetected, and these guidelines stress the importance of clinical examination and the best strategies for diagnosis as well as treatment,” he said.
Of the multiple sections and subsections, which follow the same format of the previous guidelines, the greatest number of revisions and new additions involve perioperative antithrombotic therapy, according to the document, which was published in conjunction with the ESC Congress.
Eleven new guidelines for anticoagulants
On the basis of evidence published since the previous guidelines, there are 11 completely new recommendations regarding the use of anticoagulants or antiplatelet therapies. The majority of these have received a grade I indication, which signifies “recommended” or “indicated.” These include indications for stopping or starting anticoagulants and which anticoagulants or antiplatelet drugs to consider in specific patient populations.
The next most common focus of new or revised recommendations involves when to consider surgical aortic valve repair (SAVR) relative to transcatheter aortic valve implantation (TAVI) in severe aortic stenosis. Most of these represent revisions from the previous guidelines, but almost all are also grade I recommendations.
“SAVR and TAVI are both excellent options in appropriate patients, but they are not interchangeable,” explained Bernard D. Prendergast, BMedSci, MD, director of the cardiac structural intervention program, Guy’s and St Thomas’ Hospital, London.
While the previous guidelines generally reserved TAVI for those not suitable for SAVR, the new guidelines are more nuanced.
As a rule, SAVR is generally preferred for younger patients. The reason, according to Dr. Prendergast, is concern that younger patients might outlive the expected lifespan of the prosthetic TAVI device.
No single criterion for selecting SAVR over TAVI
However, there are many exceptions and additional considerations beyond age. When both SAVR and TAVI are otherwise suitable options, but TAVI cannot be performed with a transfemoral access, Dr. Prendergast pointed out that SAVR might be a better choice.
Transfemoral access is the preferred strategy in TAVI, but Dr. Prendergast emphasized that a collaborative “heart team” should help patients select the most appropriate option. In fact, there is a grade IIb recommendation (“usefulness or efficacy is less well established”) to consider other access sites in patients at high surgical risk with contraindications for transfemoral TAVI.
Of new recommendations in the area of severe aortic stenosis, valvular repair may now be considered in asymptomatic patients with a left ventricular ejection fraction of less than 55%, according to a grade IIb recommendation. The 2017 guidelines did not address this issue.
Grade I recommendation for bioprostheses
A substantial number of the revisions involve minor clarifications without a change in the grade, but a revision regarding bioprosthetics is substantial. In the 2017 guidelines, there was a grade IIa recommendation (“weight of evidence is in favor”) to consider bioprosthetics in patients with a life expectancy less than the expected durability of the device. The 2021 guidelines have changed this to a grade I indication, adding an indication for bioprostheses in patients contraindicated for or unlikely to achieve good-quality anticoagulation.
On this same topic, there is a new grade IIb recommendation to consider bioprosthesis over alternative devices in patients already on a long-term non–vitamin K oral anticoagulant because of the high risk of thromboembolism.
There are two new recommendations in the realm of severe secondary mitral regurgitation, reported Fabien Praz, MD, an interventional cardiologist at the University of Bern (Switzerland). The first, which is perhaps the most significant, is a grade I recommendation for valve surgery in patients with severe secondary mitral regurgitation who remain symptomatic despite optimized medical therapy.
The second is a grade IIa recommendation for invasive procedures, such as a percutaneous coronary intervention, for patients with symptomatic secondary mitral regurgitation and coexisting coronary artery disease. In both cases, however, Dr. Praz emphasized language in the guidelines that calls for a collaborative heart team to agree on the suitability of these treatments.
Ultimately, none of these recommendations can be divorced from patient expectations and values, according to Friedhelm Beyersdorf, MD, chairman of the department of cardiovascular surgery at the Heart Center of the University of Freiburg (Germany).
Even if treatment is not expected to prolong life, “symptom relief on its own may justify intervention,” said Dr. Beyersdorf. However, he emphasized that “thoroughly informed” patients are an essential part of the process in selecting a treatment strategy most likely to satisfy patient goals.
Dr. Vahanian and Dr. Prendergast reported no conflicts of interest relevant to these guidelines. Dr. Praz reported a financial relationship with Edwards Lifesciences. Dr. Beyersdorf reported a financial relationship with Resuscitec.
FROM ESC 2021
Mediterranean diet tied to less severe erectile dysfunction
In an observational study of 250 middle-aged men with hypertension and erectile dysfunction, those whose eating patterns more closely matched a Mediterranean diet had significantly higher testosterone levels, better exercise capacity, and better erectile performance than their peers.
In addition, more closely following a Mediterranean diet – which emphasizes eating fruit, vegetables, whole grains, and olive oil, with modest consumption of dairy products and limited red meat – was associated with better coronary blood flow and less arterial stiffness, all after adjusting for age, body mass index, type 2 diabetes, statin use, and smoking.
Athanasios Angelis, MD, First Cardiology Clinic, Hippokration Hospital, School of Medicine, University of Athens, presented the study at the annual congress of the European Society of Cardiology.
“While we did not examine mechanisms,” Dr. Angelis said in a press release from the ESC, “it seems plausible that this dietary pattern may improve fitness and erectile performance by enhancing function of the blood vessels and limiting the fall in testosterone that occurs in midlife.”
“The findings suggest that the Mediterranean diet could play a role in maintaining several parameters of vascular health and quality of life and in middle-aged men with hypertension and erectile dysfunction,” he concluded.
“A Mediterranean diet may help erectile dysfunction by improving endothelial physiology,” Dr. Angelis said in an interview. “We suggest the Mediterranean diet as a basic parameter of hypertension and erectile dysfunction treatment. We advise all our patients to be careful regarding salt consumption and to try to exercise regularly.”
“Depending on the severity of the erectile dysfunction, we may suggest only lifestyle changes (e.g., quit smoking), at least for the beginning, or combination with medication,” consisting of phosphodiesterase type 5 (PDE5) inhibitors such as Viagra.
A ‘first-choice’ diet for men with ED, low T, high CVD risk?
This research “adds to the growing evidence that a Mediterranean diet is protective against erectile dysfunction,” said Joseph Whittaker, MSc, a clinical nutritionist from the University of Worcester (England) and coauthor of a related meta-analysis about dietary fat and testosterone.
This way of eating “also improves cardiovascular health, so it could become a low-risk, first choice treatment for these three pathologies (low testosterone, erectile dysfunction, increased risk of CVD), which so commonly coexist,” he wrote in an email.
“However, most of the research to date is observational,” he cautioned, which often has a “healthy user bias,” that is, the men eating a Mediterranean diet are probably health-conscious individuals, with other healthy habits such as exercise, good sleep, low stress, etc. “So, was it the diet, the healthy habits, or both?”
Randomized studies are needed to replicate the positive results of observational studies like this one, Mr. Whittaker added. In the meantime, “a Mediterranean diet will probably improve your health anyway,” he noted, “so trying it for the purposes of erectile function (before starting drugs) is a viable option.”
Previous research has shown that dietary fat and olive oil may boost testosterone levels, Mr. Whittaker noted, and nuts have also been shown to improve erectile function.
“So, the increase in healthy fats – mono- and polyunsaturated fatty acids (MUFAs and PUFAs, respectively) – on the Mediterranean diet is probably responsible for these benefits,” he speculated.
Middle-aged hypertensive men with ED
Men with hypertension are twice as likely to have erectile dysfunction as their peers with normal blood pressure, according to background information in the ESC press release.
Erectile dysfunction is thought to be a disorder of the small arteries, which lose their ability to dilate and increase blood flow. Declining testosterone levels in middle age also contribute to weakened erectile performance.
Physical fitness is linked with longer life in men with hypertension, and the Mediterranean diet is associated with lower blood pressure and fewer heart attacks and strokes in individuals at high cardiovascular risk.
Therefore, Dr. Angelis and colleagues aimed to see if greater adherence to a Mediterranean diet was associated with better exercise capacity, testosterone levels, coronary flow reserve, and erectile performance in middle-aged hypertensive men with erectile dysfunction.
Participants were a mean age of 56. They had a treadmill test to determine their exercise capacity, expressed as metabolic equivalent of tasks (METs), and a blood test to determine testosterone levels.
They replied to two questionnaires: a food questionnaire to determine a Mediterranean Diet score (range, 0-55, where higher scores indicate greater adherence to a Mediterranean diet) and a Sexual Health Inventory for Men (SHIM) questionnaire (score range, 0-25, where higher scores indicate better erectile performance).
Researchers used echocardiography to determine participants’ coronary flow reserve, a measure of the cardiovascular system’s ability to increase blood flow when needed. They used a SphygmoCor device to determine participants’ augmentation index and central pulse pressure, measures of arterial stiffness.
The men with a higher Mediterranean diet score (>29) had better erectile performance (SHIM scores > 14), as well as higher testosterone levels, higher coronary flow reserve, and less arterial stiffness than the other men.
The fitter men with greater exercise capacity (>10 METs) were more likely to adhere to a Mediterranean diet (scores > 25), and they also had better erectile performance (SHIM scores > 12), higher testosterone levels, greater coronary flow reserve, and less arterial stiffness than the other men.
The study did not receive any funding. The study authors and Mr. Whittaker have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an observational study of 250 middle-aged men with hypertension and erectile dysfunction, those whose eating patterns more closely matched a Mediterranean diet had significantly higher testosterone levels, better exercise capacity, and better erectile performance than their peers.
In addition, more closely following a Mediterranean diet – which emphasizes eating fruit, vegetables, whole grains, and olive oil, with modest consumption of dairy products and limited red meat – was associated with better coronary blood flow and less arterial stiffness, all after adjusting for age, body mass index, type 2 diabetes, statin use, and smoking.
Athanasios Angelis, MD, First Cardiology Clinic, Hippokration Hospital, School of Medicine, University of Athens, presented the study at the annual congress of the European Society of Cardiology.
“While we did not examine mechanisms,” Dr. Angelis said in a press release from the ESC, “it seems plausible that this dietary pattern may improve fitness and erectile performance by enhancing function of the blood vessels and limiting the fall in testosterone that occurs in midlife.”
“The findings suggest that the Mediterranean diet could play a role in maintaining several parameters of vascular health and quality of life and in middle-aged men with hypertension and erectile dysfunction,” he concluded.
“A Mediterranean diet may help erectile dysfunction by improving endothelial physiology,” Dr. Angelis said in an interview. “We suggest the Mediterranean diet as a basic parameter of hypertension and erectile dysfunction treatment. We advise all our patients to be careful regarding salt consumption and to try to exercise regularly.”
“Depending on the severity of the erectile dysfunction, we may suggest only lifestyle changes (e.g., quit smoking), at least for the beginning, or combination with medication,” consisting of phosphodiesterase type 5 (PDE5) inhibitors such as Viagra.
A ‘first-choice’ diet for men with ED, low T, high CVD risk?
This research “adds to the growing evidence that a Mediterranean diet is protective against erectile dysfunction,” said Joseph Whittaker, MSc, a clinical nutritionist from the University of Worcester (England) and coauthor of a related meta-analysis about dietary fat and testosterone.
This way of eating “also improves cardiovascular health, so it could become a low-risk, first choice treatment for these three pathologies (low testosterone, erectile dysfunction, increased risk of CVD), which so commonly coexist,” he wrote in an email.
“However, most of the research to date is observational,” he cautioned, which often has a “healthy user bias,” that is, the men eating a Mediterranean diet are probably health-conscious individuals, with other healthy habits such as exercise, good sleep, low stress, etc. “So, was it the diet, the healthy habits, or both?”
Randomized studies are needed to replicate the positive results of observational studies like this one, Mr. Whittaker added. In the meantime, “a Mediterranean diet will probably improve your health anyway,” he noted, “so trying it for the purposes of erectile function (before starting drugs) is a viable option.”
Previous research has shown that dietary fat and olive oil may boost testosterone levels, Mr. Whittaker noted, and nuts have also been shown to improve erectile function.
“So, the increase in healthy fats – mono- and polyunsaturated fatty acids (MUFAs and PUFAs, respectively) – on the Mediterranean diet is probably responsible for these benefits,” he speculated.
Middle-aged hypertensive men with ED
Men with hypertension are twice as likely to have erectile dysfunction as their peers with normal blood pressure, according to background information in the ESC press release.
Erectile dysfunction is thought to be a disorder of the small arteries, which lose their ability to dilate and increase blood flow. Declining testosterone levels in middle age also contribute to weakened erectile performance.
Physical fitness is linked with longer life in men with hypertension, and the Mediterranean diet is associated with lower blood pressure and fewer heart attacks and strokes in individuals at high cardiovascular risk.
Therefore, Dr. Angelis and colleagues aimed to see if greater adherence to a Mediterranean diet was associated with better exercise capacity, testosterone levels, coronary flow reserve, and erectile performance in middle-aged hypertensive men with erectile dysfunction.
Participants were a mean age of 56. They had a treadmill test to determine their exercise capacity, expressed as metabolic equivalent of tasks (METs), and a blood test to determine testosterone levels.
They replied to two questionnaires: a food questionnaire to determine a Mediterranean Diet score (range, 0-55, where higher scores indicate greater adherence to a Mediterranean diet) and a Sexual Health Inventory for Men (SHIM) questionnaire (score range, 0-25, where higher scores indicate better erectile performance).
Researchers used echocardiography to determine participants’ coronary flow reserve, a measure of the cardiovascular system’s ability to increase blood flow when needed. They used a SphygmoCor device to determine participants’ augmentation index and central pulse pressure, measures of arterial stiffness.
The men with a higher Mediterranean diet score (>29) had better erectile performance (SHIM scores > 14), as well as higher testosterone levels, higher coronary flow reserve, and less arterial stiffness than the other men.
The fitter men with greater exercise capacity (>10 METs) were more likely to adhere to a Mediterranean diet (scores > 25), and they also had better erectile performance (SHIM scores > 12), higher testosterone levels, greater coronary flow reserve, and less arterial stiffness than the other men.
The study did not receive any funding. The study authors and Mr. Whittaker have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an observational study of 250 middle-aged men with hypertension and erectile dysfunction, those whose eating patterns more closely matched a Mediterranean diet had significantly higher testosterone levels, better exercise capacity, and better erectile performance than their peers.
In addition, more closely following a Mediterranean diet – which emphasizes eating fruit, vegetables, whole grains, and olive oil, with modest consumption of dairy products and limited red meat – was associated with better coronary blood flow and less arterial stiffness, all after adjusting for age, body mass index, type 2 diabetes, statin use, and smoking.
Athanasios Angelis, MD, First Cardiology Clinic, Hippokration Hospital, School of Medicine, University of Athens, presented the study at the annual congress of the European Society of Cardiology.
“While we did not examine mechanisms,” Dr. Angelis said in a press release from the ESC, “it seems plausible that this dietary pattern may improve fitness and erectile performance by enhancing function of the blood vessels and limiting the fall in testosterone that occurs in midlife.”
“The findings suggest that the Mediterranean diet could play a role in maintaining several parameters of vascular health and quality of life and in middle-aged men with hypertension and erectile dysfunction,” he concluded.
“A Mediterranean diet may help erectile dysfunction by improving endothelial physiology,” Dr. Angelis said in an interview. “We suggest the Mediterranean diet as a basic parameter of hypertension and erectile dysfunction treatment. We advise all our patients to be careful regarding salt consumption and to try to exercise regularly.”
“Depending on the severity of the erectile dysfunction, we may suggest only lifestyle changes (e.g., quit smoking), at least for the beginning, or combination with medication,” consisting of phosphodiesterase type 5 (PDE5) inhibitors such as Viagra.
A ‘first-choice’ diet for men with ED, low T, high CVD risk?
This research “adds to the growing evidence that a Mediterranean diet is protective against erectile dysfunction,” said Joseph Whittaker, MSc, a clinical nutritionist from the University of Worcester (England) and coauthor of a related meta-analysis about dietary fat and testosterone.
This way of eating “also improves cardiovascular health, so it could become a low-risk, first choice treatment for these three pathologies (low testosterone, erectile dysfunction, increased risk of CVD), which so commonly coexist,” he wrote in an email.
“However, most of the research to date is observational,” he cautioned, which often has a “healthy user bias,” that is, the men eating a Mediterranean diet are probably health-conscious individuals, with other healthy habits such as exercise, good sleep, low stress, etc. “So, was it the diet, the healthy habits, or both?”
Randomized studies are needed to replicate the positive results of observational studies like this one, Mr. Whittaker added. In the meantime, “a Mediterranean diet will probably improve your health anyway,” he noted, “so trying it for the purposes of erectile function (before starting drugs) is a viable option.”
Previous research has shown that dietary fat and olive oil may boost testosterone levels, Mr. Whittaker noted, and nuts have also been shown to improve erectile function.
“So, the increase in healthy fats – mono- and polyunsaturated fatty acids (MUFAs and PUFAs, respectively) – on the Mediterranean diet is probably responsible for these benefits,” he speculated.
Middle-aged hypertensive men with ED
Men with hypertension are twice as likely to have erectile dysfunction as their peers with normal blood pressure, according to background information in the ESC press release.
Erectile dysfunction is thought to be a disorder of the small arteries, which lose their ability to dilate and increase blood flow. Declining testosterone levels in middle age also contribute to weakened erectile performance.
Physical fitness is linked with longer life in men with hypertension, and the Mediterranean diet is associated with lower blood pressure and fewer heart attacks and strokes in individuals at high cardiovascular risk.
Therefore, Dr. Angelis and colleagues aimed to see if greater adherence to a Mediterranean diet was associated with better exercise capacity, testosterone levels, coronary flow reserve, and erectile performance in middle-aged hypertensive men with erectile dysfunction.
Participants were a mean age of 56. They had a treadmill test to determine their exercise capacity, expressed as metabolic equivalent of tasks (METs), and a blood test to determine testosterone levels.
They replied to two questionnaires: a food questionnaire to determine a Mediterranean Diet score (range, 0-55, where higher scores indicate greater adherence to a Mediterranean diet) and a Sexual Health Inventory for Men (SHIM) questionnaire (score range, 0-25, where higher scores indicate better erectile performance).
Researchers used echocardiography to determine participants’ coronary flow reserve, a measure of the cardiovascular system’s ability to increase blood flow when needed. They used a SphygmoCor device to determine participants’ augmentation index and central pulse pressure, measures of arterial stiffness.
The men with a higher Mediterranean diet score (>29) had better erectile performance (SHIM scores > 14), as well as higher testosterone levels, higher coronary flow reserve, and less arterial stiffness than the other men.
The fitter men with greater exercise capacity (>10 METs) were more likely to adhere to a Mediterranean diet (scores > 25), and they also had better erectile performance (SHIM scores > 12), higher testosterone levels, greater coronary flow reserve, and less arterial stiffness than the other men.
The study did not receive any funding. The study authors and Mr. Whittaker have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2021
Two swings, two misses with colchicine, Vascepa in COVID-19
The anti-inflammatory agents colchicine and icosapent ethyl (Vascepa; Amarin) failed to provide substantial benefits in separate randomized COVID-19 trials.
Both were reported at the European Society of Cardiology (ESC) Congress 2021.
The open-label ECLA PHRI COLCOVID trial randomized 1,277 hospitalized adults (mean age 62 years) to usual care alone or with colchicine at a loading dose of 1.5 mg for 2 hours followed by 0.5 mg on day 1 and then 0.5 mg twice daily for 14 days or until discharge.
The investigators hypothesized that colchicine, which is widely used to treat gout and other inflammatory conditions, might modulate the hyperinflammatory syndrome, or cytokine storm, associated with COVID-19.
Results showed that the need for mechanical ventilation or death occurred in 25.0% of patients receiving colchicine and 28.8% with usual care (P = .08).
The coprimary endpoint of death at 28 days was also not significantly different between groups (20.5% vs. 22.2%), principal investigator Rafael Diaz, MD, said in a late-breaking COVID-19 trials session at the congress.
Among the secondary outcomes at 28 days, colchicine significantly reduced the incidence of new intubation or death from respiratory failure from 27.0% to 22.3% (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99) but not mortality from respiratory failure (19.5% vs. 16.8%).
The only important adverse effect was severe diarrhea, which was reported in 11.3% of the colchicine group vs. 4.5% in the control group, said Dr. Diaz, director of Estudios Clínicos Latinoamérica (ECLA), Rosario, Argentina.
The results are consistent with those from the massive RECOVERY trial, which earlier this year stopped enrollment in the colchicine arm for lack of efficacy in patients hospitalized with COVID-19, and COLCORONA, which missed its primary endpoint using colchicine among nonhospitalized adults with COVID-19.
Session chair and COLCORONA principal investigator Jean-Claude Tardif, MD, pointed out that, as clinicians, it’s fairly uncommon to combine systemic steroids with colchicine, which was the case in 92% of patients in ECLA PHRI COLCOVID.
“I think it is an inherent limitation of testing colchicine on top of steroids,” said Dr. Tardif, of the Montreal Heart Institute.
Icosapent ethyl in PREPARE-IT
Dr. Diaz returned in the ESC session to present the results of the PREPARE-IT trial, which tested whether icosapent ethyl – at a loading dose of 8 grams (4 capsules) for the first 3 days and 4 g/d on days 4-60 – could reduce the risk for SARS-CoV-2 infection in 2,041 health care and other public workers in Argentina at high risk for infection (mean age 40.5 years).
Vascepa was approved by the Food and Drug Administration in 2012 for the reduction of elevated triglyceride levels, with an added indication in 2019 to reduce cardiovascular (CV) events in people with elevated triglycerides and established CV disease or diabetes with other CV risk factors.
The rationale for using the high-dose prescription eicosapentaenoic acid (EPA) preparation includes its anti-inflammatory and antithrombotic effects, and that unsaturated fatty acids, especially EPA, might inactivate the enveloped virus, he explained.
Among 1,712 participants followed for up to 60 days, however, the SARS-CoV-2 infection rate was 7.9% with icosapent ethyl vs. 7.1% with a mineral oil placebo (P = .58).
There were also no significant changes from baseline in the icosapent ethyl and placebo groups for the secondary outcomes of high-sensitivity C-reactive protein (0 vs. 0), triglycerides (median –2 mg/dL vs. 7 mg/dL), or Influenza Patient-Reported Outcome (FLU-PRO) questionnaire scores (median 0.01 vs. 0.03).
The use of a mineral oil placebo has been the subject of controversy in previous fish oil trials, but, Dr. Diaz noted, it did not have a significant proinflammatory effect or cause any excess adverse events.
Overall, adverse events were similar between the active and placebo groups, including atrial fibrillation (none), major bleeding (none), minor bleeding (7 events vs. 10 events), gastrointestinal symptoms (6.8% vs. 7.0%), and diarrhea (8.6% vs. 7.7%).
Although it missed the primary endpoint, Dr. Diaz said, “this is the first large, randomized blinded trial to demonstrate excellent safety and tolerability of an 8-gram-per-day loading dose of icosapent ethyl, opening up the potential for acute use in randomized trials of myocardial infarction, acute coronary syndromes, strokes, and revascularization.”
During a discussion of the results, Dr. Diaz said the Delta variant was not present at the time of the analysis and that the second half of the trial will report on whether icosapent ethyl can reduce the risk for hospitalization or death in participants diagnosed with COVID-19.
ECLA PHRI COLCOVID was supported by the Estudios Clínicos Latinoamérica Population Health Research Institute. PREPARE-IT was supported by Estudios Clínicos Latinoamérica with collaboration from Amarin. Dr. Diaz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agents colchicine and icosapent ethyl (Vascepa; Amarin) failed to provide substantial benefits in separate randomized COVID-19 trials.
Both were reported at the European Society of Cardiology (ESC) Congress 2021.
The open-label ECLA PHRI COLCOVID trial randomized 1,277 hospitalized adults (mean age 62 years) to usual care alone or with colchicine at a loading dose of 1.5 mg for 2 hours followed by 0.5 mg on day 1 and then 0.5 mg twice daily for 14 days or until discharge.
The investigators hypothesized that colchicine, which is widely used to treat gout and other inflammatory conditions, might modulate the hyperinflammatory syndrome, or cytokine storm, associated with COVID-19.
Results showed that the need for mechanical ventilation or death occurred in 25.0% of patients receiving colchicine and 28.8% with usual care (P = .08).
The coprimary endpoint of death at 28 days was also not significantly different between groups (20.5% vs. 22.2%), principal investigator Rafael Diaz, MD, said in a late-breaking COVID-19 trials session at the congress.
Among the secondary outcomes at 28 days, colchicine significantly reduced the incidence of new intubation or death from respiratory failure from 27.0% to 22.3% (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99) but not mortality from respiratory failure (19.5% vs. 16.8%).
The only important adverse effect was severe diarrhea, which was reported in 11.3% of the colchicine group vs. 4.5% in the control group, said Dr. Diaz, director of Estudios Clínicos Latinoamérica (ECLA), Rosario, Argentina.
The results are consistent with those from the massive RECOVERY trial, which earlier this year stopped enrollment in the colchicine arm for lack of efficacy in patients hospitalized with COVID-19, and COLCORONA, which missed its primary endpoint using colchicine among nonhospitalized adults with COVID-19.
Session chair and COLCORONA principal investigator Jean-Claude Tardif, MD, pointed out that, as clinicians, it’s fairly uncommon to combine systemic steroids with colchicine, which was the case in 92% of patients in ECLA PHRI COLCOVID.
“I think it is an inherent limitation of testing colchicine on top of steroids,” said Dr. Tardif, of the Montreal Heart Institute.
Icosapent ethyl in PREPARE-IT
Dr. Diaz returned in the ESC session to present the results of the PREPARE-IT trial, which tested whether icosapent ethyl – at a loading dose of 8 grams (4 capsules) for the first 3 days and 4 g/d on days 4-60 – could reduce the risk for SARS-CoV-2 infection in 2,041 health care and other public workers in Argentina at high risk for infection (mean age 40.5 years).
Vascepa was approved by the Food and Drug Administration in 2012 for the reduction of elevated triglyceride levels, with an added indication in 2019 to reduce cardiovascular (CV) events in people with elevated triglycerides and established CV disease or diabetes with other CV risk factors.
The rationale for using the high-dose prescription eicosapentaenoic acid (EPA) preparation includes its anti-inflammatory and antithrombotic effects, and that unsaturated fatty acids, especially EPA, might inactivate the enveloped virus, he explained.
Among 1,712 participants followed for up to 60 days, however, the SARS-CoV-2 infection rate was 7.9% with icosapent ethyl vs. 7.1% with a mineral oil placebo (P = .58).
There were also no significant changes from baseline in the icosapent ethyl and placebo groups for the secondary outcomes of high-sensitivity C-reactive protein (0 vs. 0), triglycerides (median –2 mg/dL vs. 7 mg/dL), or Influenza Patient-Reported Outcome (FLU-PRO) questionnaire scores (median 0.01 vs. 0.03).
The use of a mineral oil placebo has been the subject of controversy in previous fish oil trials, but, Dr. Diaz noted, it did not have a significant proinflammatory effect or cause any excess adverse events.
Overall, adverse events were similar between the active and placebo groups, including atrial fibrillation (none), major bleeding (none), minor bleeding (7 events vs. 10 events), gastrointestinal symptoms (6.8% vs. 7.0%), and diarrhea (8.6% vs. 7.7%).
Although it missed the primary endpoint, Dr. Diaz said, “this is the first large, randomized blinded trial to demonstrate excellent safety and tolerability of an 8-gram-per-day loading dose of icosapent ethyl, opening up the potential for acute use in randomized trials of myocardial infarction, acute coronary syndromes, strokes, and revascularization.”
During a discussion of the results, Dr. Diaz said the Delta variant was not present at the time of the analysis and that the second half of the trial will report on whether icosapent ethyl can reduce the risk for hospitalization or death in participants diagnosed with COVID-19.
ECLA PHRI COLCOVID was supported by the Estudios Clínicos Latinoamérica Population Health Research Institute. PREPARE-IT was supported by Estudios Clínicos Latinoamérica with collaboration from Amarin. Dr. Diaz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agents colchicine and icosapent ethyl (Vascepa; Amarin) failed to provide substantial benefits in separate randomized COVID-19 trials.
Both were reported at the European Society of Cardiology (ESC) Congress 2021.
The open-label ECLA PHRI COLCOVID trial randomized 1,277 hospitalized adults (mean age 62 years) to usual care alone or with colchicine at a loading dose of 1.5 mg for 2 hours followed by 0.5 mg on day 1 and then 0.5 mg twice daily for 14 days or until discharge.
The investigators hypothesized that colchicine, which is widely used to treat gout and other inflammatory conditions, might modulate the hyperinflammatory syndrome, or cytokine storm, associated with COVID-19.
Results showed that the need for mechanical ventilation or death occurred in 25.0% of patients receiving colchicine and 28.8% with usual care (P = .08).
The coprimary endpoint of death at 28 days was also not significantly different between groups (20.5% vs. 22.2%), principal investigator Rafael Diaz, MD, said in a late-breaking COVID-19 trials session at the congress.
Among the secondary outcomes at 28 days, colchicine significantly reduced the incidence of new intubation or death from respiratory failure from 27.0% to 22.3% (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99) but not mortality from respiratory failure (19.5% vs. 16.8%).
The only important adverse effect was severe diarrhea, which was reported in 11.3% of the colchicine group vs. 4.5% in the control group, said Dr. Diaz, director of Estudios Clínicos Latinoamérica (ECLA), Rosario, Argentina.
The results are consistent with those from the massive RECOVERY trial, which earlier this year stopped enrollment in the colchicine arm for lack of efficacy in patients hospitalized with COVID-19, and COLCORONA, which missed its primary endpoint using colchicine among nonhospitalized adults with COVID-19.
Session chair and COLCORONA principal investigator Jean-Claude Tardif, MD, pointed out that, as clinicians, it’s fairly uncommon to combine systemic steroids with colchicine, which was the case in 92% of patients in ECLA PHRI COLCOVID.
“I think it is an inherent limitation of testing colchicine on top of steroids,” said Dr. Tardif, of the Montreal Heart Institute.
Icosapent ethyl in PREPARE-IT
Dr. Diaz returned in the ESC session to present the results of the PREPARE-IT trial, which tested whether icosapent ethyl – at a loading dose of 8 grams (4 capsules) for the first 3 days and 4 g/d on days 4-60 – could reduce the risk for SARS-CoV-2 infection in 2,041 health care and other public workers in Argentina at high risk for infection (mean age 40.5 years).
Vascepa was approved by the Food and Drug Administration in 2012 for the reduction of elevated triglyceride levels, with an added indication in 2019 to reduce cardiovascular (CV) events in people with elevated triglycerides and established CV disease or diabetes with other CV risk factors.
The rationale for using the high-dose prescription eicosapentaenoic acid (EPA) preparation includes its anti-inflammatory and antithrombotic effects, and that unsaturated fatty acids, especially EPA, might inactivate the enveloped virus, he explained.
Among 1,712 participants followed for up to 60 days, however, the SARS-CoV-2 infection rate was 7.9% with icosapent ethyl vs. 7.1% with a mineral oil placebo (P = .58).
There were also no significant changes from baseline in the icosapent ethyl and placebo groups for the secondary outcomes of high-sensitivity C-reactive protein (0 vs. 0), triglycerides (median –2 mg/dL vs. 7 mg/dL), or Influenza Patient-Reported Outcome (FLU-PRO) questionnaire scores (median 0.01 vs. 0.03).
The use of a mineral oil placebo has been the subject of controversy in previous fish oil trials, but, Dr. Diaz noted, it did not have a significant proinflammatory effect or cause any excess adverse events.
Overall, adverse events were similar between the active and placebo groups, including atrial fibrillation (none), major bleeding (none), minor bleeding (7 events vs. 10 events), gastrointestinal symptoms (6.8% vs. 7.0%), and diarrhea (8.6% vs. 7.7%).
Although it missed the primary endpoint, Dr. Diaz said, “this is the first large, randomized blinded trial to demonstrate excellent safety and tolerability of an 8-gram-per-day loading dose of icosapent ethyl, opening up the potential for acute use in randomized trials of myocardial infarction, acute coronary syndromes, strokes, and revascularization.”
During a discussion of the results, Dr. Diaz said the Delta variant was not present at the time of the analysis and that the second half of the trial will report on whether icosapent ethyl can reduce the risk for hospitalization or death in participants diagnosed with COVID-19.
ECLA PHRI COLCOVID was supported by the Estudios Clínicos Latinoamérica Population Health Research Institute. PREPARE-IT was supported by Estudios Clínicos Latinoamérica with collaboration from Amarin. Dr. Diaz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
EMPEROR-Preserved spouts torrent of reports on empagliflozin treatment of HFpEF
The featured report from the 6,000-patient EMPEROR-Preserved trial at the virtual annual congress of the European Society of Cardiology drew lots of attention for its headline finding: the first unequivocal demonstration that a medication, empagliflozin, can significantly reduce the rate of cardiovascular death and hospitalization for heart failure in patients with heart failure with preserved ejection fraction (HFpEF, a left ventricular ejection fraction of more than 40%), with the details simultaneously published online.
But at the same time, the EMPEROR-Preserved investigators released four additional reports with a lot more outcome analyses that also deserve some attention.
The puzzling neutral effect on renal events
Perhaps the most surprising and complicated set of findings among the main EMPEROR-Preserved outcomes involved renal outcomes.
The trial’s primary outcome was the combined rate of cardiovascular death or hospitalization for heart failure (HHF), and the results showed that treatment with empagliflozin (Jardiance) for a median of 26 months on top of standard treatment for patients with HFpEF led to a significant 21% relative risk reduction, compared with placebo-treated patients.
The trial had two prespecified secondary outcomes. One was the total number of HHF, which dropped by a significant 27%, compared with placebo. The second was the mean change in slope of estimated glomerular filtration rate (eGFR) on an annualized basis, and the empagliflozin regimen reduced the cumulative annual deficit, compared with placebo by an average of 1.36 mL/min per 1.73 m2, a significant difference.
This preservation of renal function was consistent with results from many prior studies of empagliflozin and all of the other U.S.-approved agents from the sodium-glucose cotransporter 2 inhibitor class. Preservation of renal function and a reduction in renal events has become a hallmark property of all agents in the SGLT2 inhibitor class both in patients with type 2 diabetes, as well as in those without diabetes but with heart failure with reduced ejection fraction (HFrEF) or with chronic kidney disease.
EMPEROR-Preserved threw a wrench into what had been an unbroken history of renal protection by SGLT2 inhibitors. That happened when a prespecified endpoint of the study – a composite renal outcome defined as time to first occurrence of chronic dialysis, renal transplantation, a sustained reduction of at least 40% in eGFR, or a sustained drop in eGFR of more than 10 or 15 mL/min per 1.73 m2 from baseline – yielded an unexpected neutral finding.
For this composite renal outcome, EMPEROR-Preserved showed a nonsignificant 5% reduction, compared with placebo, a result that both differed from what had been seen in essentially all the other SGLT2 inhibitor trials that had looked at this, but which also seemed at odds with the observed significant preservation of renal function that seemed substantial enough to produce a clinically meaningful benefit.
Renal effects blunted in HFpEF
The immediate upshot was a letter published by several EMPEROR-Preserved investigators that spelled out this discrepancy and came to the jolting conclusion that “eGFR slope analysis has limitations as a surrogate for predicting the effect of drugs on renal outcomes in patients with heart failure.”
The same authors, along with some additional associates, also published a second letter that noted a further unexpected twist with the renal outcome: “In prior large-scale clinical trials, the effect of SGLT2 inhibitors on heart failure and renal outcomes had consistently tracked together,” they noted, but in this case it didn’t, a discordance they said was “extraordinarily puzzling”.
This led the study’s leaders to reanalyze the renal outcomes using a different definition, one that Milton Packer, MD, who helped design the trial and oversaw several of its analyses, called “a more conventional definition of renal events,” during his presentation of these findings at the congress. The researchers swapped out a 40% drop from baseline eGFR as an event and replaced it with a 50% decline, a change designed to screen out less severe, and often transient, reductions in kidney function that have less lasting impact on health. They also added an additional component to the composite endpoint, renal death. A revised analysis using this new renal composite outcome appeared in the European Journal of Heart Failure letter.
This change cut the total number of renal events tallied in the trial nearly in half, down to 112, and showed a more robust decline in renal events with empagliflozin treatment compared with the initial analysis, although the drop remained nonsignificant. The revised analysis also showed that the overall, nonsignificant 22% relative reduction in renal events in patients on empagliflozin, compared with placebo, dwindled down to completely nonexistent in the tertile of patients with a left ventricular ejection fraction of 60% or greater. In this tertile the hazard ratio actually showed a nonsignificant point estimate of a 24% increased rate of renal events on empagliflozin, with the caveat that this subgroup now included a total of just 40 total events between the two treatment arms. (Each of the two other tertiles also had roughly the same number of total events.)
The biggest effect on renal-event reduction was in the tertile of patients with an ejection fraction of 41%-49%, in which empagliflozin treatment was linked with a significant 59% cut in renal events, compared with placebo. The analysis also showed significant heterogeneity in thus outcome between this subgroup and the other two tertiles that had higher ejection fractions and showed reduced rates of protection by empagliflozin against renal events.
This apparent blunting of a renal effect despite preservation of renal function seemed to mimic the blunting of the primary cardiovascular outcome effect that also appeared in patients with ejection fractions in the 60%-65% range or above.
“If we knew what blunted the effect of empagliflozin on heart failure outcomes at higher ejection fraction levels, we think the same explanation may also apply to the blunting of effect on renal outcomes, but right now we do not know the answer to either question,” Dr. Packer said in an interview. He’s suggested that one possibility is that many of the enrolled patients identified as having HFpEF, but with these high ejection fractions may have not actually had HFpEF, and their signs and symptoms may have instead resulted from atrial fibrillation.
“Many patients with an ejection fraction of 60%-65% and above had atrial fibrillation,” he noted, with a prevalence at enrollment in this subgroup of about 50%. Atrial fibrillation can cause dyspnea, a hallmark symptom leading to diagnosis of heart failure, and it also increases levels of N-terminal of the prohormone brain natriuretic peptide, a metric that served as a gatekeeper for entry into the trial. “Essentially, we are saying that many of the criteria that we specified to ensure that patients had heart failure probably did not work very well in patients with an ejection fraction of 65% or greater,” said Dr. Packer, a cardiologist at Baylor University Medical Center in Dallas. “We need to figure out who these patients are.”
Some experts not involved with the study voiced skepticism that the renal findings reflected a real issue.
“I’m quite optimistic that in the long-term the effect on eGFR will translate into renal protection,” said Rudolf A. de Boer, MD, PhD, a professor of translational cardiology at University Medical Center Groningen (the Netherlands), and designated discussant at the congress for the presentation by Dr. Packer.
John J.V. McMurray, MD, a professor of cardiology and a heart failure specialist at Glasgow University, speculated that the unexpected renal outcomes data may relate to the initial decline in renal function produced by treatment with SGLT2 inhibitors despite their longer-term enhancement of renal protection.
“If you use a treatment that protects the kidneys in the long-term but causes an initial dip in eGFR, more patients receiving that treatment will have an early ‘event,’ ” he noted in an interview. He also cautioned about the dangers of subgroup analyses that dice the study population into small cohorts.
“Trials are powered to look at the effect of treatment in the overall population. Everything else is exploratory, underpowered, and subject to the play of chance,” Dr. McMurray stressed.
Counting additional cardiovascular disease events allows more analyses
A third auxiliary report from the EMPEROR-Preserved investigators performed several prespecified analyses that depended on adding additional cardiovascular disease endpoints to the core tallies of cardiovascular death or HHF – such as emergent, urgent, and outpatient events that reflected worsening heart failure – and also included information on diuretic and vasopressor use because of worsening heart failure. The increased event numbers allowed the researchers to perform 30 additional analyses included in this report, according to the count kept by Dr. Packer who was the lead author.
He highlighted several of the additional results in this paper that documented benefits from empagliflozin treatment, compared with placebo:
- A significant 29% reduction in the need for admission to a cardiac care unit or intensive care unit during an HHF.
- A nonsignificant 33% reduction in the need for intravenous vasopressors or positive inotropic drugs during HHF.
- A significantly increased rate of patients achieving a higher New York Heart Association functional class. For example, after the first year of treatment patients who received empagliflozin had a 37% higher rate of functional class improvement, compared with patients who received placebo.
Dr. McMurray had his own list of key takeaways from this paper, including:
- Among patients who needed hospitalization, “those treated with empagliflozin were less sick than those in the placebo group.”
- In addition to reducing HHF empagliflozin treatment also reduced episodes of outpatient worsening as reflected by their receipt of intensified diuretic treatment, which occurred a significant 27% less often, compared with patients on placebo.
- Treatment with empagliflozin also linked with a significant 39% relative reduction in emergency or urgent-care visits that required intravenous therapy.
Empagliflozin’s performance relative to sacubitril/valsartan
The fourth additional report focused on a post hoc, cross-trial comparison of the results from EMPEROR-Preserved and from another recent trial that, like EMPEROR-Preserved, assessed in patients with HFpEF a drug previously proven to work quite well in patients with HFrEF. The comparator drug was sacubitril/valsartan (Entresto), which underwent testing in patients with HFpEF in the PARAGON-HF trial.
The primary outcome of PARAGON-HF, which randomized 4,822 patients, was reduction in cardiovascular death and in total HHF. This dropped by a relative 13%, compared with placebo, during a median of 35 months, a between-group difference that came close to but did not achieve significance (P = .06). Despite this limitation, the Food and Drug Administration in February 2021 loosened the indication for using sacubitril/valsartan in patients with heart failure and a “below normal” ejection fraction, a category that can include many patients considered to have HFpEF.
Although the researchers who ran this analysis, including Dr. Packer, who was the first author, admitted that “comparison of effect sizes across trials is fraught with difficulties,” they nonetheless concluded from their analysis that “for all outcomes that included HHF the effect size was larger for empagliflozin than for sacubitril/valsartan.”
Dr. McMurray, a lead instigator for PARAGON-HF, said there was little to take away from this analysis.
“The patient populations were different, and sacubitril/valsartan was compared against an active therapy, valsartan,” while in EMPEROR-Preserved empagliflozin compared against placebo. “Most of us believe that sacubitril/valsartan and SGLT2 inhibitors work in different but complementary ways, and their benefits are additive. You would want patients with HFpEF or HFrEF to take both,” he said in an interview.
Dr. Packer agreed with that approach and added that he would probably also prescribe a third agent, spironolactone, to many patients with HFpEF.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and Eli Lilly, which jointly market empagliflozin (Jardiance). PARAGON-HF was sponsored by Novartis, which markets sacubitril/valsartan (Entresto). Dr. Packer has received consulting fees from Boehringer Ingelheim and from numerous other companies. Dr. de Boer has research contracts with Boehringer Ingelheim as well as from Abbott, AstraZeneca, Cardior, Ionis, Novo Nordisk, and Roche, and he has been a consultant to Novartis as well as to Abbott, AstraZeneca, Gayer, and Roche. Dr. McMurray led trials of sacubitril/valsartan sponsored by Novartis, and his institution has received compensation for his participation in studies sponsored by Abbvie, AstraZeneca, Cardurion, DalCor, GlaxoSmithKline, Pfizer, and Theracos.
The featured report from the 6,000-patient EMPEROR-Preserved trial at the virtual annual congress of the European Society of Cardiology drew lots of attention for its headline finding: the first unequivocal demonstration that a medication, empagliflozin, can significantly reduce the rate of cardiovascular death and hospitalization for heart failure in patients with heart failure with preserved ejection fraction (HFpEF, a left ventricular ejection fraction of more than 40%), with the details simultaneously published online.
But at the same time, the EMPEROR-Preserved investigators released four additional reports with a lot more outcome analyses that also deserve some attention.
The puzzling neutral effect on renal events
Perhaps the most surprising and complicated set of findings among the main EMPEROR-Preserved outcomes involved renal outcomes.
The trial’s primary outcome was the combined rate of cardiovascular death or hospitalization for heart failure (HHF), and the results showed that treatment with empagliflozin (Jardiance) for a median of 26 months on top of standard treatment for patients with HFpEF led to a significant 21% relative risk reduction, compared with placebo-treated patients.
The trial had two prespecified secondary outcomes. One was the total number of HHF, which dropped by a significant 27%, compared with placebo. The second was the mean change in slope of estimated glomerular filtration rate (eGFR) on an annualized basis, and the empagliflozin regimen reduced the cumulative annual deficit, compared with placebo by an average of 1.36 mL/min per 1.73 m2, a significant difference.
This preservation of renal function was consistent with results from many prior studies of empagliflozin and all of the other U.S.-approved agents from the sodium-glucose cotransporter 2 inhibitor class. Preservation of renal function and a reduction in renal events has become a hallmark property of all agents in the SGLT2 inhibitor class both in patients with type 2 diabetes, as well as in those without diabetes but with heart failure with reduced ejection fraction (HFrEF) or with chronic kidney disease.
EMPEROR-Preserved threw a wrench into what had been an unbroken history of renal protection by SGLT2 inhibitors. That happened when a prespecified endpoint of the study – a composite renal outcome defined as time to first occurrence of chronic dialysis, renal transplantation, a sustained reduction of at least 40% in eGFR, or a sustained drop in eGFR of more than 10 or 15 mL/min per 1.73 m2 from baseline – yielded an unexpected neutral finding.
For this composite renal outcome, EMPEROR-Preserved showed a nonsignificant 5% reduction, compared with placebo, a result that both differed from what had been seen in essentially all the other SGLT2 inhibitor trials that had looked at this, but which also seemed at odds with the observed significant preservation of renal function that seemed substantial enough to produce a clinically meaningful benefit.
Renal effects blunted in HFpEF
The immediate upshot was a letter published by several EMPEROR-Preserved investigators that spelled out this discrepancy and came to the jolting conclusion that “eGFR slope analysis has limitations as a surrogate for predicting the effect of drugs on renal outcomes in patients with heart failure.”
The same authors, along with some additional associates, also published a second letter that noted a further unexpected twist with the renal outcome: “In prior large-scale clinical trials, the effect of SGLT2 inhibitors on heart failure and renal outcomes had consistently tracked together,” they noted, but in this case it didn’t, a discordance they said was “extraordinarily puzzling”.
This led the study’s leaders to reanalyze the renal outcomes using a different definition, one that Milton Packer, MD, who helped design the trial and oversaw several of its analyses, called “a more conventional definition of renal events,” during his presentation of these findings at the congress. The researchers swapped out a 40% drop from baseline eGFR as an event and replaced it with a 50% decline, a change designed to screen out less severe, and often transient, reductions in kidney function that have less lasting impact on health. They also added an additional component to the composite endpoint, renal death. A revised analysis using this new renal composite outcome appeared in the European Journal of Heart Failure letter.
This change cut the total number of renal events tallied in the trial nearly in half, down to 112, and showed a more robust decline in renal events with empagliflozin treatment compared with the initial analysis, although the drop remained nonsignificant. The revised analysis also showed that the overall, nonsignificant 22% relative reduction in renal events in patients on empagliflozin, compared with placebo, dwindled down to completely nonexistent in the tertile of patients with a left ventricular ejection fraction of 60% or greater. In this tertile the hazard ratio actually showed a nonsignificant point estimate of a 24% increased rate of renal events on empagliflozin, with the caveat that this subgroup now included a total of just 40 total events between the two treatment arms. (Each of the two other tertiles also had roughly the same number of total events.)
The biggest effect on renal-event reduction was in the tertile of patients with an ejection fraction of 41%-49%, in which empagliflozin treatment was linked with a significant 59% cut in renal events, compared with placebo. The analysis also showed significant heterogeneity in thus outcome between this subgroup and the other two tertiles that had higher ejection fractions and showed reduced rates of protection by empagliflozin against renal events.
This apparent blunting of a renal effect despite preservation of renal function seemed to mimic the blunting of the primary cardiovascular outcome effect that also appeared in patients with ejection fractions in the 60%-65% range or above.
“If we knew what blunted the effect of empagliflozin on heart failure outcomes at higher ejection fraction levels, we think the same explanation may also apply to the blunting of effect on renal outcomes, but right now we do not know the answer to either question,” Dr. Packer said in an interview. He’s suggested that one possibility is that many of the enrolled patients identified as having HFpEF, but with these high ejection fractions may have not actually had HFpEF, and their signs and symptoms may have instead resulted from atrial fibrillation.
“Many patients with an ejection fraction of 60%-65% and above had atrial fibrillation,” he noted, with a prevalence at enrollment in this subgroup of about 50%. Atrial fibrillation can cause dyspnea, a hallmark symptom leading to diagnosis of heart failure, and it also increases levels of N-terminal of the prohormone brain natriuretic peptide, a metric that served as a gatekeeper for entry into the trial. “Essentially, we are saying that many of the criteria that we specified to ensure that patients had heart failure probably did not work very well in patients with an ejection fraction of 65% or greater,” said Dr. Packer, a cardiologist at Baylor University Medical Center in Dallas. “We need to figure out who these patients are.”
Some experts not involved with the study voiced skepticism that the renal findings reflected a real issue.
“I’m quite optimistic that in the long-term the effect on eGFR will translate into renal protection,” said Rudolf A. de Boer, MD, PhD, a professor of translational cardiology at University Medical Center Groningen (the Netherlands), and designated discussant at the congress for the presentation by Dr. Packer.
John J.V. McMurray, MD, a professor of cardiology and a heart failure specialist at Glasgow University, speculated that the unexpected renal outcomes data may relate to the initial decline in renal function produced by treatment with SGLT2 inhibitors despite their longer-term enhancement of renal protection.
“If you use a treatment that protects the kidneys in the long-term but causes an initial dip in eGFR, more patients receiving that treatment will have an early ‘event,’ ” he noted in an interview. He also cautioned about the dangers of subgroup analyses that dice the study population into small cohorts.
“Trials are powered to look at the effect of treatment in the overall population. Everything else is exploratory, underpowered, and subject to the play of chance,” Dr. McMurray stressed.
Counting additional cardiovascular disease events allows more analyses
A third auxiliary report from the EMPEROR-Preserved investigators performed several prespecified analyses that depended on adding additional cardiovascular disease endpoints to the core tallies of cardiovascular death or HHF – such as emergent, urgent, and outpatient events that reflected worsening heart failure – and also included information on diuretic and vasopressor use because of worsening heart failure. The increased event numbers allowed the researchers to perform 30 additional analyses included in this report, according to the count kept by Dr. Packer who was the lead author.
He highlighted several of the additional results in this paper that documented benefits from empagliflozin treatment, compared with placebo:
- A significant 29% reduction in the need for admission to a cardiac care unit or intensive care unit during an HHF.
- A nonsignificant 33% reduction in the need for intravenous vasopressors or positive inotropic drugs during HHF.
- A significantly increased rate of patients achieving a higher New York Heart Association functional class. For example, after the first year of treatment patients who received empagliflozin had a 37% higher rate of functional class improvement, compared with patients who received placebo.
Dr. McMurray had his own list of key takeaways from this paper, including:
- Among patients who needed hospitalization, “those treated with empagliflozin were less sick than those in the placebo group.”
- In addition to reducing HHF empagliflozin treatment also reduced episodes of outpatient worsening as reflected by their receipt of intensified diuretic treatment, which occurred a significant 27% less often, compared with patients on placebo.
- Treatment with empagliflozin also linked with a significant 39% relative reduction in emergency or urgent-care visits that required intravenous therapy.
Empagliflozin’s performance relative to sacubitril/valsartan
The fourth additional report focused on a post hoc, cross-trial comparison of the results from EMPEROR-Preserved and from another recent trial that, like EMPEROR-Preserved, assessed in patients with HFpEF a drug previously proven to work quite well in patients with HFrEF. The comparator drug was sacubitril/valsartan (Entresto), which underwent testing in patients with HFpEF in the PARAGON-HF trial.
The primary outcome of PARAGON-HF, which randomized 4,822 patients, was reduction in cardiovascular death and in total HHF. This dropped by a relative 13%, compared with placebo, during a median of 35 months, a between-group difference that came close to but did not achieve significance (P = .06). Despite this limitation, the Food and Drug Administration in February 2021 loosened the indication for using sacubitril/valsartan in patients with heart failure and a “below normal” ejection fraction, a category that can include many patients considered to have HFpEF.
Although the researchers who ran this analysis, including Dr. Packer, who was the first author, admitted that “comparison of effect sizes across trials is fraught with difficulties,” they nonetheless concluded from their analysis that “for all outcomes that included HHF the effect size was larger for empagliflozin than for sacubitril/valsartan.”
Dr. McMurray, a lead instigator for PARAGON-HF, said there was little to take away from this analysis.
“The patient populations were different, and sacubitril/valsartan was compared against an active therapy, valsartan,” while in EMPEROR-Preserved empagliflozin compared against placebo. “Most of us believe that sacubitril/valsartan and SGLT2 inhibitors work in different but complementary ways, and their benefits are additive. You would want patients with HFpEF or HFrEF to take both,” he said in an interview.
Dr. Packer agreed with that approach and added that he would probably also prescribe a third agent, spironolactone, to many patients with HFpEF.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and Eli Lilly, which jointly market empagliflozin (Jardiance). PARAGON-HF was sponsored by Novartis, which markets sacubitril/valsartan (Entresto). Dr. Packer has received consulting fees from Boehringer Ingelheim and from numerous other companies. Dr. de Boer has research contracts with Boehringer Ingelheim as well as from Abbott, AstraZeneca, Cardior, Ionis, Novo Nordisk, and Roche, and he has been a consultant to Novartis as well as to Abbott, AstraZeneca, Gayer, and Roche. Dr. McMurray led trials of sacubitril/valsartan sponsored by Novartis, and his institution has received compensation for his participation in studies sponsored by Abbvie, AstraZeneca, Cardurion, DalCor, GlaxoSmithKline, Pfizer, and Theracos.
The featured report from the 6,000-patient EMPEROR-Preserved trial at the virtual annual congress of the European Society of Cardiology drew lots of attention for its headline finding: the first unequivocal demonstration that a medication, empagliflozin, can significantly reduce the rate of cardiovascular death and hospitalization for heart failure in patients with heart failure with preserved ejection fraction (HFpEF, a left ventricular ejection fraction of more than 40%), with the details simultaneously published online.
But at the same time, the EMPEROR-Preserved investigators released four additional reports with a lot more outcome analyses that also deserve some attention.
The puzzling neutral effect on renal events
Perhaps the most surprising and complicated set of findings among the main EMPEROR-Preserved outcomes involved renal outcomes.
The trial’s primary outcome was the combined rate of cardiovascular death or hospitalization for heart failure (HHF), and the results showed that treatment with empagliflozin (Jardiance) for a median of 26 months on top of standard treatment for patients with HFpEF led to a significant 21% relative risk reduction, compared with placebo-treated patients.
The trial had two prespecified secondary outcomes. One was the total number of HHF, which dropped by a significant 27%, compared with placebo. The second was the mean change in slope of estimated glomerular filtration rate (eGFR) on an annualized basis, and the empagliflozin regimen reduced the cumulative annual deficit, compared with placebo by an average of 1.36 mL/min per 1.73 m2, a significant difference.
This preservation of renal function was consistent with results from many prior studies of empagliflozin and all of the other U.S.-approved agents from the sodium-glucose cotransporter 2 inhibitor class. Preservation of renal function and a reduction in renal events has become a hallmark property of all agents in the SGLT2 inhibitor class both in patients with type 2 diabetes, as well as in those without diabetes but with heart failure with reduced ejection fraction (HFrEF) or with chronic kidney disease.
EMPEROR-Preserved threw a wrench into what had been an unbroken history of renal protection by SGLT2 inhibitors. That happened when a prespecified endpoint of the study – a composite renal outcome defined as time to first occurrence of chronic dialysis, renal transplantation, a sustained reduction of at least 40% in eGFR, or a sustained drop in eGFR of more than 10 or 15 mL/min per 1.73 m2 from baseline – yielded an unexpected neutral finding.
For this composite renal outcome, EMPEROR-Preserved showed a nonsignificant 5% reduction, compared with placebo, a result that both differed from what had been seen in essentially all the other SGLT2 inhibitor trials that had looked at this, but which also seemed at odds with the observed significant preservation of renal function that seemed substantial enough to produce a clinically meaningful benefit.
Renal effects blunted in HFpEF
The immediate upshot was a letter published by several EMPEROR-Preserved investigators that spelled out this discrepancy and came to the jolting conclusion that “eGFR slope analysis has limitations as a surrogate for predicting the effect of drugs on renal outcomes in patients with heart failure.”
The same authors, along with some additional associates, also published a second letter that noted a further unexpected twist with the renal outcome: “In prior large-scale clinical trials, the effect of SGLT2 inhibitors on heart failure and renal outcomes had consistently tracked together,” they noted, but in this case it didn’t, a discordance they said was “extraordinarily puzzling”.
This led the study’s leaders to reanalyze the renal outcomes using a different definition, one that Milton Packer, MD, who helped design the trial and oversaw several of its analyses, called “a more conventional definition of renal events,” during his presentation of these findings at the congress. The researchers swapped out a 40% drop from baseline eGFR as an event and replaced it with a 50% decline, a change designed to screen out less severe, and often transient, reductions in kidney function that have less lasting impact on health. They also added an additional component to the composite endpoint, renal death. A revised analysis using this new renal composite outcome appeared in the European Journal of Heart Failure letter.
This change cut the total number of renal events tallied in the trial nearly in half, down to 112, and showed a more robust decline in renal events with empagliflozin treatment compared with the initial analysis, although the drop remained nonsignificant. The revised analysis also showed that the overall, nonsignificant 22% relative reduction in renal events in patients on empagliflozin, compared with placebo, dwindled down to completely nonexistent in the tertile of patients with a left ventricular ejection fraction of 60% or greater. In this tertile the hazard ratio actually showed a nonsignificant point estimate of a 24% increased rate of renal events on empagliflozin, with the caveat that this subgroup now included a total of just 40 total events between the two treatment arms. (Each of the two other tertiles also had roughly the same number of total events.)
The biggest effect on renal-event reduction was in the tertile of patients with an ejection fraction of 41%-49%, in which empagliflozin treatment was linked with a significant 59% cut in renal events, compared with placebo. The analysis also showed significant heterogeneity in thus outcome between this subgroup and the other two tertiles that had higher ejection fractions and showed reduced rates of protection by empagliflozin against renal events.
This apparent blunting of a renal effect despite preservation of renal function seemed to mimic the blunting of the primary cardiovascular outcome effect that also appeared in patients with ejection fractions in the 60%-65% range or above.
“If we knew what blunted the effect of empagliflozin on heart failure outcomes at higher ejection fraction levels, we think the same explanation may also apply to the blunting of effect on renal outcomes, but right now we do not know the answer to either question,” Dr. Packer said in an interview. He’s suggested that one possibility is that many of the enrolled patients identified as having HFpEF, but with these high ejection fractions may have not actually had HFpEF, and their signs and symptoms may have instead resulted from atrial fibrillation.
“Many patients with an ejection fraction of 60%-65% and above had atrial fibrillation,” he noted, with a prevalence at enrollment in this subgroup of about 50%. Atrial fibrillation can cause dyspnea, a hallmark symptom leading to diagnosis of heart failure, and it also increases levels of N-terminal of the prohormone brain natriuretic peptide, a metric that served as a gatekeeper for entry into the trial. “Essentially, we are saying that many of the criteria that we specified to ensure that patients had heart failure probably did not work very well in patients with an ejection fraction of 65% or greater,” said Dr. Packer, a cardiologist at Baylor University Medical Center in Dallas. “We need to figure out who these patients are.”
Some experts not involved with the study voiced skepticism that the renal findings reflected a real issue.
“I’m quite optimistic that in the long-term the effect on eGFR will translate into renal protection,” said Rudolf A. de Boer, MD, PhD, a professor of translational cardiology at University Medical Center Groningen (the Netherlands), and designated discussant at the congress for the presentation by Dr. Packer.
John J.V. McMurray, MD, a professor of cardiology and a heart failure specialist at Glasgow University, speculated that the unexpected renal outcomes data may relate to the initial decline in renal function produced by treatment with SGLT2 inhibitors despite their longer-term enhancement of renal protection.
“If you use a treatment that protects the kidneys in the long-term but causes an initial dip in eGFR, more patients receiving that treatment will have an early ‘event,’ ” he noted in an interview. He also cautioned about the dangers of subgroup analyses that dice the study population into small cohorts.
“Trials are powered to look at the effect of treatment in the overall population. Everything else is exploratory, underpowered, and subject to the play of chance,” Dr. McMurray stressed.
Counting additional cardiovascular disease events allows more analyses
A third auxiliary report from the EMPEROR-Preserved investigators performed several prespecified analyses that depended on adding additional cardiovascular disease endpoints to the core tallies of cardiovascular death or HHF – such as emergent, urgent, and outpatient events that reflected worsening heart failure – and also included information on diuretic and vasopressor use because of worsening heart failure. The increased event numbers allowed the researchers to perform 30 additional analyses included in this report, according to the count kept by Dr. Packer who was the lead author.
He highlighted several of the additional results in this paper that documented benefits from empagliflozin treatment, compared with placebo:
- A significant 29% reduction in the need for admission to a cardiac care unit or intensive care unit during an HHF.
- A nonsignificant 33% reduction in the need for intravenous vasopressors or positive inotropic drugs during HHF.
- A significantly increased rate of patients achieving a higher New York Heart Association functional class. For example, after the first year of treatment patients who received empagliflozin had a 37% higher rate of functional class improvement, compared with patients who received placebo.
Dr. McMurray had his own list of key takeaways from this paper, including:
- Among patients who needed hospitalization, “those treated with empagliflozin were less sick than those in the placebo group.”
- In addition to reducing HHF empagliflozin treatment also reduced episodes of outpatient worsening as reflected by their receipt of intensified diuretic treatment, which occurred a significant 27% less often, compared with patients on placebo.
- Treatment with empagliflozin also linked with a significant 39% relative reduction in emergency or urgent-care visits that required intravenous therapy.
Empagliflozin’s performance relative to sacubitril/valsartan
The fourth additional report focused on a post hoc, cross-trial comparison of the results from EMPEROR-Preserved and from another recent trial that, like EMPEROR-Preserved, assessed in patients with HFpEF a drug previously proven to work quite well in patients with HFrEF. The comparator drug was sacubitril/valsartan (Entresto), which underwent testing in patients with HFpEF in the PARAGON-HF trial.
The primary outcome of PARAGON-HF, which randomized 4,822 patients, was reduction in cardiovascular death and in total HHF. This dropped by a relative 13%, compared with placebo, during a median of 35 months, a between-group difference that came close to but did not achieve significance (P = .06). Despite this limitation, the Food and Drug Administration in February 2021 loosened the indication for using sacubitril/valsartan in patients with heart failure and a “below normal” ejection fraction, a category that can include many patients considered to have HFpEF.
Although the researchers who ran this analysis, including Dr. Packer, who was the first author, admitted that “comparison of effect sizes across trials is fraught with difficulties,” they nonetheless concluded from their analysis that “for all outcomes that included HHF the effect size was larger for empagliflozin than for sacubitril/valsartan.”
Dr. McMurray, a lead instigator for PARAGON-HF, said there was little to take away from this analysis.
“The patient populations were different, and sacubitril/valsartan was compared against an active therapy, valsartan,” while in EMPEROR-Preserved empagliflozin compared against placebo. “Most of us believe that sacubitril/valsartan and SGLT2 inhibitors work in different but complementary ways, and their benefits are additive. You would want patients with HFpEF or HFrEF to take both,” he said in an interview.
Dr. Packer agreed with that approach and added that he would probably also prescribe a third agent, spironolactone, to many patients with HFpEF.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and Eli Lilly, which jointly market empagliflozin (Jardiance). PARAGON-HF was sponsored by Novartis, which markets sacubitril/valsartan (Entresto). Dr. Packer has received consulting fees from Boehringer Ingelheim and from numerous other companies. Dr. de Boer has research contracts with Boehringer Ingelheim as well as from Abbott, AstraZeneca, Cardior, Ionis, Novo Nordisk, and Roche, and he has been a consultant to Novartis as well as to Abbott, AstraZeneca, Gayer, and Roche. Dr. McMurray led trials of sacubitril/valsartan sponsored by Novartis, and his institution has received compensation for his participation in studies sponsored by Abbvie, AstraZeneca, Cardurion, DalCor, GlaxoSmithKline, Pfizer, and Theracos.
FROM ESC 2021
ACST-2: Carotid stenting, surgery on par in asymptomatic patients
Carotid artery stenting (CAS) and carotid endarterectomy (CEA) provided comparable outcomes over time in asymptomatic patients receiving good medical therapy in the largest trial to date of what to do with severe carotid artery narrowing that is yet to cause a stroke.
Among more than 3,600 patients, stenting and surgery performed by experienced physicians involved a 1.0% risk for causing disabling stroke or death within 30 days.
The annual rate of fatal or disabling strokes was about 0.5% with either procedure over an average 5 years’ follow-up – essentially halving the annual stroke risk had neither procedure been performed, according to Alison Halliday, MD, principal investigator of the Asymptomatic Carotid Surgery Trial-2 (ACST-2).
The results were reported Aug. 29 in a Hot Line session at the virtual annual congress of the European Society of Cardiology and published simultaneously online in The Lancet.
Session chair Gilles Montalescot, MD, Sorbonne University, Paris, noted that ACST-2 doubled the number of randomly assigned patients with asymptomatic carotid stenosis studied in previous trials, “so, a huge contribution to the evidence base in this field and apparently good news for both revascularization techniques.”
Thirty-day and 5-year outcomes
The trial was conducted in 33 countries between January 2008 and December 2020, enrolling 3,625 patients (70% were male; mean age, 70 years) with carotid stenosis of at least 60% on ultrasonography, in whom stenting or surgery was suitable but both the doctor and patient were “substantially uncertain” which procedure to prefer.
Among the 1,811 patients assigned to stenting, 87% underwent the procedure at a median of 14 days; 6% crossed over to surgery, typically because of a highly calcified lesion or a more tortuous carotid than anticipated; and 6% had no intervention.
Among the 1,814 patients assigned to surgery, 92% had the procedure at a median of 14 days; 3% crossed over to stenting, typically because of patient or doctor preference or reluctance to undergo general anesthesia; and 4% had no intervention.
Patients without complications who had stenting stayed on average 1 day less than did those undergoing surgery.
During an earlier press briefing, Dr. Halliday highlighted the need for procedural competency and said doctors had to submit a record of their CEA or CAS experience and, consistent with current guidelines, had to demonstrate an independently verified stroke or death rate of 6% or less for symptomatic patients and 3% or lower for asymptomatic patients.
The results showed the 30-day risk for death, myocardial infarction (MI), or any stroke was 3.9% with carotid stenting and 3.2% with surgery (P = .26).
But with stenting, there was a slightly higher risk for procedural nondisabling strokes (48 vs. 29; P = .03), including 15 strokes vs. 5 strokes, respectively, that left patients with no residual symptoms. This is “consistent with large, recent nationally representative registry data,” observed Dr. Halliday, of the University of Oxford (England).
For those undergoing surgery, cranial nerve palsies were reported in 5.4% vs. no patients undergoing stenting.
At 5 years, the nonprocedural fatal or disabling stroke rate was 2.5% in each group (rate ratio [RR], 0.98; P = .91), with any nonprocedural stroke occurring in 5.3% of patients with stenting vs. 4.5% with surgery (RR, 1.16; P = .33).
The investigators performed a meta-analysis combining the ACST-2 results with those of eight prior trials (four in asymptomatic and four in symptomatic patients) that yielded a similar nonsignificant result for any nonprocedural stroke (RR, 1.11; P = .21).
Based on the results from ACST-2 plus the major trials, stenting and surgery involve “similar risks and similar benefits,” Dr. Halliday concluded.
Discussant Marco Roffi, MD, University Hospital of Geneva, said, “In centers with documented expertise, carotid artery stenting should be offered as an alternative to carotid endarterectomy in patients with asymptomatic stenosis and suitable anatomy.”
While the trial provides “good news” for patients, he pointed out that a reduction in the sample size from 5,000 to 3,625 limited the statistical power and that enrollment over a long period of time may have introduced confounders, such as changes in equipment technique, and medical therapy.
Also, many centers enrolled few patients, raising the concern over low-volume centers and operators, Dr. Roffi said. “We know that 8% of the centers enrolled 39% of the patients,” and “information on the credentialing and experience of the interventionalists was limited.”
Further, a lack of systematic MI assessment may have favored the surgery group, and more recent developments in stenting with the potential of reducing periprocedural stroke were rarely used, such as proximal emboli protection in only 15% and double-layer stents in 11%.
Friedhelm Beyersdorf, MD, University Hospital of Freiburg, Germany, said that, as a vascular surgeon, he finds it understandable that there might be a higher incidence of nonfatal strokes when treating carotid stenosis with stents, given the vulnerability of these lesions.
“Nevertheless, the main conclusion from the entire study is that carotid artery treatment is extremely safe, it has to be done in order to avoid strokes, and, obviously, there seems to be an advantage for surgery in terms of nondisabling stroke,” he said.
Session chair Dr. Montalescot, however, said that what the study cannot address – and what was the subject of many online audience comments – is whether either intervention should be performed in these patients.
Unlike earlier trials comparing interventions to medical therapy, Dr. Halliday said ACST-2 enrolled patients for whom the decision had been made that revascularization was needed. In addition, 99%-100% were receiving antithrombotic therapy at baseline, 85%-90% were receiving antihypertensives, and about 85% were taking statins.
Longer-term follow-up should provide a better picture of the nonprocedural stroke risk, with patients asked annually about exactly what medications and doses they are taking, she said.
“We will have an enormous list of exactly what’s gone on and the intensity of that therapy, which is, of course, much more intense than when we carried out our first trial. But these were people in whom a procedure was thought to be necessary,” she noted.
When asked during the press conference which procedure she would choose, Dr. Halliday, a surgeon, observed that patient preference is important but that the nature of the lesion itself often determines the optimal choice.
“If you know the competence of the people doing it is equal, then the less invasive procedure – providing it has good long-term viability, and that’s why we’re following for 10 years – is the more important,” she added.
The study was funded by the UK Medical Research Council and Health Technology Assessment Programme. Dr. Halliday reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Carotid artery stenting (CAS) and carotid endarterectomy (CEA) provided comparable outcomes over time in asymptomatic patients receiving good medical therapy in the largest trial to date of what to do with severe carotid artery narrowing that is yet to cause a stroke.
Among more than 3,600 patients, stenting and surgery performed by experienced physicians involved a 1.0% risk for causing disabling stroke or death within 30 days.
The annual rate of fatal or disabling strokes was about 0.5% with either procedure over an average 5 years’ follow-up – essentially halving the annual stroke risk had neither procedure been performed, according to Alison Halliday, MD, principal investigator of the Asymptomatic Carotid Surgery Trial-2 (ACST-2).
The results were reported Aug. 29 in a Hot Line session at the virtual annual congress of the European Society of Cardiology and published simultaneously online in The Lancet.
Session chair Gilles Montalescot, MD, Sorbonne University, Paris, noted that ACST-2 doubled the number of randomly assigned patients with asymptomatic carotid stenosis studied in previous trials, “so, a huge contribution to the evidence base in this field and apparently good news for both revascularization techniques.”
Thirty-day and 5-year outcomes
The trial was conducted in 33 countries between January 2008 and December 2020, enrolling 3,625 patients (70% were male; mean age, 70 years) with carotid stenosis of at least 60% on ultrasonography, in whom stenting or surgery was suitable but both the doctor and patient were “substantially uncertain” which procedure to prefer.
Among the 1,811 patients assigned to stenting, 87% underwent the procedure at a median of 14 days; 6% crossed over to surgery, typically because of a highly calcified lesion or a more tortuous carotid than anticipated; and 6% had no intervention.
Among the 1,814 patients assigned to surgery, 92% had the procedure at a median of 14 days; 3% crossed over to stenting, typically because of patient or doctor preference or reluctance to undergo general anesthesia; and 4% had no intervention.
Patients without complications who had stenting stayed on average 1 day less than did those undergoing surgery.
During an earlier press briefing, Dr. Halliday highlighted the need for procedural competency and said doctors had to submit a record of their CEA or CAS experience and, consistent with current guidelines, had to demonstrate an independently verified stroke or death rate of 6% or less for symptomatic patients and 3% or lower for asymptomatic patients.
The results showed the 30-day risk for death, myocardial infarction (MI), or any stroke was 3.9% with carotid stenting and 3.2% with surgery (P = .26).
But with stenting, there was a slightly higher risk for procedural nondisabling strokes (48 vs. 29; P = .03), including 15 strokes vs. 5 strokes, respectively, that left patients with no residual symptoms. This is “consistent with large, recent nationally representative registry data,” observed Dr. Halliday, of the University of Oxford (England).
For those undergoing surgery, cranial nerve palsies were reported in 5.4% vs. no patients undergoing stenting.
At 5 years, the nonprocedural fatal or disabling stroke rate was 2.5% in each group (rate ratio [RR], 0.98; P = .91), with any nonprocedural stroke occurring in 5.3% of patients with stenting vs. 4.5% with surgery (RR, 1.16; P = .33).
The investigators performed a meta-analysis combining the ACST-2 results with those of eight prior trials (four in asymptomatic and four in symptomatic patients) that yielded a similar nonsignificant result for any nonprocedural stroke (RR, 1.11; P = .21).
Based on the results from ACST-2 plus the major trials, stenting and surgery involve “similar risks and similar benefits,” Dr. Halliday concluded.
Discussant Marco Roffi, MD, University Hospital of Geneva, said, “In centers with documented expertise, carotid artery stenting should be offered as an alternative to carotid endarterectomy in patients with asymptomatic stenosis and suitable anatomy.”
While the trial provides “good news” for patients, he pointed out that a reduction in the sample size from 5,000 to 3,625 limited the statistical power and that enrollment over a long period of time may have introduced confounders, such as changes in equipment technique, and medical therapy.
Also, many centers enrolled few patients, raising the concern over low-volume centers and operators, Dr. Roffi said. “We know that 8% of the centers enrolled 39% of the patients,” and “information on the credentialing and experience of the interventionalists was limited.”
Further, a lack of systematic MI assessment may have favored the surgery group, and more recent developments in stenting with the potential of reducing periprocedural stroke were rarely used, such as proximal emboli protection in only 15% and double-layer stents in 11%.
Friedhelm Beyersdorf, MD, University Hospital of Freiburg, Germany, said that, as a vascular surgeon, he finds it understandable that there might be a higher incidence of nonfatal strokes when treating carotid stenosis with stents, given the vulnerability of these lesions.
“Nevertheless, the main conclusion from the entire study is that carotid artery treatment is extremely safe, it has to be done in order to avoid strokes, and, obviously, there seems to be an advantage for surgery in terms of nondisabling stroke,” he said.
Session chair Dr. Montalescot, however, said that what the study cannot address – and what was the subject of many online audience comments – is whether either intervention should be performed in these patients.
Unlike earlier trials comparing interventions to medical therapy, Dr. Halliday said ACST-2 enrolled patients for whom the decision had been made that revascularization was needed. In addition, 99%-100% were receiving antithrombotic therapy at baseline, 85%-90% were receiving antihypertensives, and about 85% were taking statins.
Longer-term follow-up should provide a better picture of the nonprocedural stroke risk, with patients asked annually about exactly what medications and doses they are taking, she said.
“We will have an enormous list of exactly what’s gone on and the intensity of that therapy, which is, of course, much more intense than when we carried out our first trial. But these were people in whom a procedure was thought to be necessary,” she noted.
When asked during the press conference which procedure she would choose, Dr. Halliday, a surgeon, observed that patient preference is important but that the nature of the lesion itself often determines the optimal choice.
“If you know the competence of the people doing it is equal, then the less invasive procedure – providing it has good long-term viability, and that’s why we’re following for 10 years – is the more important,” she added.
The study was funded by the UK Medical Research Council and Health Technology Assessment Programme. Dr. Halliday reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Carotid artery stenting (CAS) and carotid endarterectomy (CEA) provided comparable outcomes over time in asymptomatic patients receiving good medical therapy in the largest trial to date of what to do with severe carotid artery narrowing that is yet to cause a stroke.
Among more than 3,600 patients, stenting and surgery performed by experienced physicians involved a 1.0% risk for causing disabling stroke or death within 30 days.
The annual rate of fatal or disabling strokes was about 0.5% with either procedure over an average 5 years’ follow-up – essentially halving the annual stroke risk had neither procedure been performed, according to Alison Halliday, MD, principal investigator of the Asymptomatic Carotid Surgery Trial-2 (ACST-2).
The results were reported Aug. 29 in a Hot Line session at the virtual annual congress of the European Society of Cardiology and published simultaneously online in The Lancet.
Session chair Gilles Montalescot, MD, Sorbonne University, Paris, noted that ACST-2 doubled the number of randomly assigned patients with asymptomatic carotid stenosis studied in previous trials, “so, a huge contribution to the evidence base in this field and apparently good news for both revascularization techniques.”
Thirty-day and 5-year outcomes
The trial was conducted in 33 countries between January 2008 and December 2020, enrolling 3,625 patients (70% were male; mean age, 70 years) with carotid stenosis of at least 60% on ultrasonography, in whom stenting or surgery was suitable but both the doctor and patient were “substantially uncertain” which procedure to prefer.
Among the 1,811 patients assigned to stenting, 87% underwent the procedure at a median of 14 days; 6% crossed over to surgery, typically because of a highly calcified lesion or a more tortuous carotid than anticipated; and 6% had no intervention.
Among the 1,814 patients assigned to surgery, 92% had the procedure at a median of 14 days; 3% crossed over to stenting, typically because of patient or doctor preference or reluctance to undergo general anesthesia; and 4% had no intervention.
Patients without complications who had stenting stayed on average 1 day less than did those undergoing surgery.
During an earlier press briefing, Dr. Halliday highlighted the need for procedural competency and said doctors had to submit a record of their CEA or CAS experience and, consistent with current guidelines, had to demonstrate an independently verified stroke or death rate of 6% or less for symptomatic patients and 3% or lower for asymptomatic patients.
The results showed the 30-day risk for death, myocardial infarction (MI), or any stroke was 3.9% with carotid stenting and 3.2% with surgery (P = .26).
But with stenting, there was a slightly higher risk for procedural nondisabling strokes (48 vs. 29; P = .03), including 15 strokes vs. 5 strokes, respectively, that left patients with no residual symptoms. This is “consistent with large, recent nationally representative registry data,” observed Dr. Halliday, of the University of Oxford (England).
For those undergoing surgery, cranial nerve palsies were reported in 5.4% vs. no patients undergoing stenting.
At 5 years, the nonprocedural fatal or disabling stroke rate was 2.5% in each group (rate ratio [RR], 0.98; P = .91), with any nonprocedural stroke occurring in 5.3% of patients with stenting vs. 4.5% with surgery (RR, 1.16; P = .33).
The investigators performed a meta-analysis combining the ACST-2 results with those of eight prior trials (four in asymptomatic and four in symptomatic patients) that yielded a similar nonsignificant result for any nonprocedural stroke (RR, 1.11; P = .21).
Based on the results from ACST-2 plus the major trials, stenting and surgery involve “similar risks and similar benefits,” Dr. Halliday concluded.
Discussant Marco Roffi, MD, University Hospital of Geneva, said, “In centers with documented expertise, carotid artery stenting should be offered as an alternative to carotid endarterectomy in patients with asymptomatic stenosis and suitable anatomy.”
While the trial provides “good news” for patients, he pointed out that a reduction in the sample size from 5,000 to 3,625 limited the statistical power and that enrollment over a long period of time may have introduced confounders, such as changes in equipment technique, and medical therapy.
Also, many centers enrolled few patients, raising the concern over low-volume centers and operators, Dr. Roffi said. “We know that 8% of the centers enrolled 39% of the patients,” and “information on the credentialing and experience of the interventionalists was limited.”
Further, a lack of systematic MI assessment may have favored the surgery group, and more recent developments in stenting with the potential of reducing periprocedural stroke were rarely used, such as proximal emboli protection in only 15% and double-layer stents in 11%.
Friedhelm Beyersdorf, MD, University Hospital of Freiburg, Germany, said that, as a vascular surgeon, he finds it understandable that there might be a higher incidence of nonfatal strokes when treating carotid stenosis with stents, given the vulnerability of these lesions.
“Nevertheless, the main conclusion from the entire study is that carotid artery treatment is extremely safe, it has to be done in order to avoid strokes, and, obviously, there seems to be an advantage for surgery in terms of nondisabling stroke,” he said.
Session chair Dr. Montalescot, however, said that what the study cannot address – and what was the subject of many online audience comments – is whether either intervention should be performed in these patients.
Unlike earlier trials comparing interventions to medical therapy, Dr. Halliday said ACST-2 enrolled patients for whom the decision had been made that revascularization was needed. In addition, 99%-100% were receiving antithrombotic therapy at baseline, 85%-90% were receiving antihypertensives, and about 85% were taking statins.
Longer-term follow-up should provide a better picture of the nonprocedural stroke risk, with patients asked annually about exactly what medications and doses they are taking, she said.
“We will have an enormous list of exactly what’s gone on and the intensity of that therapy, which is, of course, much more intense than when we carried out our first trial. But these were people in whom a procedure was thought to be necessary,” she noted.
When asked during the press conference which procedure she would choose, Dr. Halliday, a surgeon, observed that patient preference is important but that the nature of the lesion itself often determines the optimal choice.
“If you know the competence of the people doing it is equal, then the less invasive procedure – providing it has good long-term viability, and that’s why we’re following for 10 years – is the more important,” she added.
The study was funded by the UK Medical Research Council and Health Technology Assessment Programme. Dr. Halliday reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Angiography can wait for cardiac arrest without ST-elevation
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.
The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”
There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.
The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”
There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.
The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”
There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.